Abstract
Background
Pressure ulcers, also known as bedsores, decubitus ulcers and pressure injuries, are localised areas of injury to the skin or the underlying tissue, or both. Dressings are widely used to treat pressure ulcers and promote healing, and there are many options to choose from including alginate, hydrocolloid and protease‐modulating dressings. Topical agents have also been used as alternatives to dressings in order to promote healing.
A clear and current overview of all the evidence is required to facilitate decision‐making regarding the use of dressings or topical agents for the treatment of pressure ulcers. Such a review would ideally help people with pressure ulcers and health professionals assess the best treatment options. This review is a network meta‐analysis (NMA) which assesses the probability of complete ulcer healing associated with alternative dressings and topical agents.
Objectives
To assess the effects of dressings and topical agents for healing pressure ulcers in any care setting. We aimed to examine this evidence base as a whole, determining probabilities that each treatment is the best, with full assessment of uncertainty and evidence quality.
Search methods
In July 2016 we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses, guidelines and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
Published or unpublished randomised controlled trials (RCTs) comparing the effects of at least one of the following interventions with any other intervention in the treatment of pressure ulcers (Stage 2 or above): any dressing, or any topical agent applied directly to an open pressure ulcer and left in situ. We excluded from this review dressings attached to external devices such as negative pressure wound therapies, skin grafts, growth factor treatments, platelet gels and larval therapy.
Data collection and analysis
Two review authors independently performed study selection, risk of bias assessment and data extraction. We conducted network meta‐analysis using frequentist mega‐regression methods for the efficacy outcome, probability of complete healing. We modelled the relative effectiveness of any two treatments as a function of each treatment relative to the reference treatment (saline gauze). We assumed that treatment effects were similar within dressings classes (e.g. hydrocolloid, foam). We present estimates of effect with their 95% confidence intervals for individual treatments compared with every other, and we report ranking probabilities for each intervention (probability of being the best, second best, etc treatment). We assessed the certainty (quality) of the body of evidence using GRADE for each network comparison and for the network as whole.
Main results
We included 51 studies (2947 participants) in this review and carried out NMA in a network of linked interventions for the sole outcome of probability of complete healing. The network included 21 different interventions (13 dressings, 6 topical agents and 2 supplementary linking interventions) and was informed by 39 studies in 2127 participants, of whom 783 had completely healed wounds.
We judged the network to be sparse: overall, there were relatively few participants, with few events, both for the number of interventions and the number of mixed treatment contrasts; most studies were small or very small. The consequence of this sparseness is high imprecision in the evidence, and this, coupled with the (mainly) high risk of bias in the studies informing the network, means that we judged the vast majority of the evidence to be of low or very low certainty. We have no confidence in the findings regarding the rank order of interventions in this review (very low‐certainty evidence), but we report here a summary of results for some comparisons of interventions compared with saline gauze. We present here only the findings from evidence which we did not consider to be very low certainty, but these reported results should still be interpreted in the context of the very low certainty of the network as a whole.
It is not clear whether regimens involving protease‐modulating dressings increase the probability of pressure ulcer healing compared with saline gauze (risk ratio (RR) 1.65, 95% confidence interval (CI) 0.92 to 2.94) (moderate‐certainty evidence: low risk of bias, downgraded for imprecision). This risk ratio of 1.65 corresponds to an absolute difference of 102 more people healed with protease modulating dressings per 1000 people treated than with saline gauze alone (95% CI 13 fewer to 302 more). It is unclear whether the following interventions increase the probability of healing compared with saline gauze (low‐certainty evidence): collagenase ointment (RR 2.12, 95% CI 1.06 to 4.22); foam dressings (RR 1.52, 95% CI 1.03 to 2.26); basic wound contact dressings (RR 1.30, 95% CI 0.65 to 2.58) and polyvinylpyrrolidone plus zinc oxide (RR 1.31, 95% CI 0.37 to 4.62); the latter two interventions both had confidence intervals consistent with both a clinically important benefit and a clinically important harm, and the former two interventions each had high risk of bias as well as imprecision.
Authors' conclusions
A network meta‐analysis (NMA) of data from 39 studies (evaluating 21 dressings and topical agents for pressure ulcers) is sparse and the evidence is of low or very low certainty (due mainly to risk of bias and imprecision). Consequently we are unable to determine which dressings or topical agents are the most likely to heal pressure ulcers, and it is generally unclear whether the treatments examined are more effective than saline gauze.
More research is needed to determine whether particular dressings or topical agents improve the probability of healing of pressure ulcers. The NMA is uninformative regarding which interventions might best be included in a large trial, and it may be that research is directed towards prevention, leaving clinicians to decide which treatment to use on the basis of wound symptoms, clinical experience, patient preference and cost.
Plain language summary
Which dressings or topical agents are the most effective for healing pressure ulcers?
Dressings and topical agents for treating pressure ulcers
Review question
We reviewed the evidence about the effects of dressings and topical agents (such as ointments, creams and gels) on pressure ulcer healing. There are many different dressings and topical agents available, and we wanted to find out which were the most effective.
Background
Pressure ulcers, also known as bedsores, decubitus ulcers and pressure injuries, are wounds involving the skin and sometimes the tissue that lies underneath. Pressure ulcers can be painful, may become infected and affect people's quality of life. People at risk of developing pressure ulcers include those with limited mobility ‐ such as older people and people with short‐term or long‐term medical conditions ‐ and people with spinal cord injuries. In 2004 the total yearly cost of treating pressure ulcers in the UK was estimated as being GBP 1.4 to 2.1 billion, which was equivalent to 4% of the total National Health Service expenditure.
Topical agents such as ointments, creams or gels are applied to unhealed pressure ulcers and left in place to treat the wound; they may be covered with a dressing. Some of these treatments have been compared with each other in trials, usually comparing two treatments at a time. We used a method called 'network meta‐analysis' to bring together all the trial results of different treatments in a reliable way. We hoped that this method, which compares all treatment options, would help us find out which was the best treatment for healing pressure ulcers.
Study characteristics
In July 2016 we searched for randomised controlled trials looking at dressings and topical agents for treating pressure ulcers and that gave results for complete wound healing. We found 51 studies involving a total of 2947 people. Thirty‐nine of these studies, involving 2127 people, gave results we could bring together in a network meta‐analysis comparing 21 different treatments. Most participants in the trials were older people; three of the 39 trials involved participants with spinal cord injuries.
Key results
Generally, the studies we found did not have many participants and results were often inconclusive. This problem carried over into the network meta‐analysis and made the findings unclear. As a result, it was unclear whether one topical agent or dressing was better than another. Some findings for individual comparisons may be slightly more reliable. Protease‐modulating dressings, foam dressings or collagenase ointment may be better at healing than gauze; but even this evidence is not certain enough to be an adequate guide for treatment choices.
Certainty of the evidence
We judged the certainty of the evidence to be very low or low. The next step might be to do more research of better quality to see which dressings or topical agents could best heal pressure ulcers.
This plain language summary is up to date as of July 2016.
Summary of findings
Summary of findings for the main comparison. NMA evidence for individual network: proportion with complete healing ‐ interventions versus saline gauze.
| NMA evidence for individual network: proportion with complete healing ‐ interventions versus saline gauze | ||||
|
Patient or population: people with pressure ulcers
Intervention: dressing or topical agent
Comparator: saline gauze Settings: hospital, community or care home, or combinations | ||||
|
Contrasts: interventions versus saline gauze |
Relative effect (95% CI) |
Anticipated absolute effects* (95% CI) ‐ from median of saline gauze control groups in direct evidence |
Certainty (quality) of the evidence (GRADE) | |
| Median CGR | With interventions | |||
| Alginate dressings | RR 1.09 (0.11 to 10.57) | 157 per 1000 | 171 per 1000 (17 to 1000) | ⊕⊝⊝⊝ Very low1 |
|
14 more people healed per 1000 (140 fewer to 1000 more) | ||||
| Sequential hydrocolloid alginate dressings | RR 0.50 (0.12 to 1.98) | 157 per 1000 | 78 per 1000 (1.9 to 31.2) | ⊕⊝⊝⊝ Very low1 |
|
79 fewer people healed per 1000 (138 fewer to 155 more) | ||||
| Basic wound contact dressings | RR 1.30 (0.65 to 2.58) | 157 per 1000 | 204 per 1000 (102 to 407) | ⊕⊕⊝⊝ Low2 |
|
47 more people healed per 1000 (55 fewer to 250 more) | ||||
| Collagenase ointment | RR 2.12 (1.06 to 4.22) | 157 per 1000 | 333 per 1000 (166 to 663) | ⊕⊕⊝⊝ Low3 |
|
176 more people healed per 1000 (9 more to 506 more) | ||||
| Dextranomer | RR 4.76 (0.86 to 26.39) | 157 per 1000 | 747 per 1000 (135 to 1000) | ⊕⊝⊝⊝ Very low4 |
|
590 more people healed per 1000 (22 fewer to 1000 more) | ||||
| Foam dressings | RR 1.52 (1.03 to 2.26) | 157 per 1000 | 239 per 1,000 (162 to 353) | ⊕⊕⊝⊝ Low5 |
|
82 more people healed per 1,000 (5 more to 196 more) | ||||
| Hydrocolloid dressing with/without alginate | RR 1.22 (0.06 to 24.74) | 157 per 1000 | 192 per 1,000 (9 to 1000) | ⊕⊝⊝⊝ Very low1 |
|
35 more people healed per 1,000 (148 fewer to 1000 more) | ||||
| Hydrocolloid dressings | RR 1.43 (1.00 to 2.05) | 157 per 1000 | 225 per 1000 (157 to 322) | ⊕⊝⊝⊝ Very low6 |
|
68 more people healed per 1000 (from 0 fewer to 165 more) | ||||
| Hydrogel | RR 1.55 (1.02 to 2.36) | 157 per 1000 | 243 per 1000 (160 to 371) | ⊕⊝⊝⊝ Very low6 |
|
86 more people healed per 1000 (from 3 more to 214 more) | ||||
| Iodine‐containing dressings | RR 1.08 (0.58 to 2.03) | 157 per 1000 | 170 per 1000 (91 to 316) | ⊕⊝⊝⊝ Very low1 |
|
13 more people healed per 1000 (from 66 fewer to 159 more) | ||||
| Phenytoin | RR 1.27 (0.58 to 2.80) | 157 per 1000 | 199 per 1000 (91 to 440) | ⊕⊝⊝⊝ Very low7 |
|
42 more people healed per 1000 (from 66 fewer to 283 more) | ||||
| Protease‐modulating dressings | RR 1.65 (0.92 to 2.94) | 157 per 1000 | 259 per 1,000 (144 to 462) | ⊕⊕⊕⊝ Moderate8 |
|
102 more people healed per 1000 (from 13 fewer to 305 more) | ||||
| Polyvinylpyrrolidone + zinc oxide | RR 1.31 (0.37 to 4.62) | 157 per 1000 | 206 per 1,000 (58 to 732) | ⊕⊕⊝⊝ Low2 |
|
49 more people healed per 1000 (from 99 fewer to 575 more) | ||||
| Combination silicone foam dressings | RR 1.93 (0.38 to 9.98) | 157 per 1000 | 303 per 1,000 (60 to 1,000) | ⊕⊝⊝⊝ Very low1 |
|
146 more people healed per 1000 (from 97 fewer to 1,000 more) | ||||
| Soft polymer dressings | RR 1.35 (0.55 to 3.27) | 157 per 1000 | 212 per 1,000 (86 to 517) | ⊕⊝⊝⊝ Very low1 |
|
55 more people healed per 1000 (from 71 fewer to 360 more) | ||||
| Sugar + egg white | RR 0.70 (0.03 to 15.62) | 157 per 1000 | 110 per 1000 (5 to 1,000) | ⊕⊝⊝⊝ Very low1 |
|
47 fewer people healed per 1000 (from 152 fewer to 1000 more) | ||||
| Tripeptide copper gel | RR 3.90 (1.04 to 14.63) | 157 per 1000 | 612 per 1000 (163 to 1000) | ⊕⊝⊝⊝ Very low9 |
|
455 more people healed per 1000 (6 more to 1000 more) | ||||
| Vapour‐permeable dressings | RR 1.45 (0.74 to 2.81) | 157 per 1000 | 228 per 1000 (118 to 440) | ⊕⊝⊝⊝ Very low1 |
|
71 more people healed per 1000 (from 39 fewer to 283 more) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparator group and the relative effect of the intervention (and its 95% CI). CGR: control group risk; CI: confidence interval; RR: risk ratio | ||||
| GRADE Working Group grades of evidence High certainty (quality): we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty (quality): we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty (quality): our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty (quality): we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
1Majority of evidence at high risk of bias (downgraded once); imprecision: very wide CI (crosses 0.75 and 1.25) (downgraded twice). 2Imprecision: very wide CI (crosses 0.75 and 1.25) (downgraded twice). 3Majority of evidence at high risk of bias (downgraded once); imprecision: wide CI and direct evidence on collagenase from three studies, 11 events (downgraded once). 4Majority of evidence at high risk of bias (downgraded once): imprecision: wide CI (crosses 1.25) and direct evidence on dextranomer from one study, seven participants and four events (downgraded twice). 5Majority of evidence at high risk of bias (downgraded once); imprecision: wide CI (downgraded once). 6Majority of evidence at high risk of bias (downgraded once); inconsistency: heterogeneity in direct evidence (downgraded once); imprecision: wide CI (downgraded once). 7Majority of evidence at high risk of bias (downgraded once); inconsistency: significant difference between direct and indirect estimates (downgraded once); imprecision: very wide CI (crossed 0.75 and 1.25). 8Imprecision: wide CI (crosses 1.25); (direct evidence for protease‐modulating dressing: four studies, 76 participants, 31 events) (downgraded once). 9Majority of evidence at high risk of bias (downgraded once): imprecision: wide CI (crosses 1.25) and direct evidence on tripeptide copper gel from one study, six participants and five events (downgraded twice).
Background
Description of the condition
Pressure ulcers, also known as pressure injuries, bedsores, decubitus ulcers or pressure sores, are localised areas of injury to the skin, the underlying tissue or both. They often occur over bony prominences such as the sacrum (base of the spine) and heel (Vanderwee 2007), and are caused by external forces such as pressure, or shear, or a combination of both (EPUAP‐NPUAP‐PPPIA 2014; NPUAP 2016; Dumville 2015a; Dumville 2015b; Keogh 2013; Walker 2014).
Risk factors for pressure ulcer development have been summarised into three main categories: a lack of mobility; poor perfusion (e.g. diabetes and vascular disease) and low skin status (Coleman 2013); the latter category includes the presence of stage 1 pressure ulcers or incontinence or both, which also increases the risk of ulceration by producing a detrimental environment for the skin (Brandeis 1994).
Pressure ulcers vary in severity. One of the most widely recognised systems for categorising pressure ulcers is that of the National Pressure Ulcer Advisory Panel (NPUAP). Their international classification recognises four categories or stages of pressure ulcer and two categories of unclassifiable pressure injury. Stage 1 ulcers involve intact skin, but Stages 2 to 4 describe progressively deeper wounds with larger degrees of skin and tissue loss: Stage 2 pressure ulcers have partial‐thickness skin loss and exposed dermis; Stage 3 refers to full‐thickness skin loss and exposed fat tissue; and Stage 4 ulcers have full‐thickness skin and tissue loss, with exposed fascia, muscle, tendon, ligament, cartilage or bone. The two categories of unclassifiable pressure injury are reserved for wounds for which wound depth or extent, or both, cannot be accurately determined; unclassifiable pressure ulcers are generally severe and would be grouped clinically with Stage 3 or Stage 4 ulcers (EPUAP‐NPUAP‐PPPIA 2014) (see Appendix 1 for further details of grading).
Prevalence
Pressure ulcers are one of the most common types of complex wound. Prevalence estimates differ according to the type of population assessed, the data collection methods used and period of data collection and whether Stage 1 ulcers were included).
One large European study estimated a hospital pressure ulcer prevalence (Stage 2 and above) of 10.5% (Vanderwee 2007) whilst a US study estimated a prevalence of 9.0% (Stage 2 and above) across acute‐care, long‐term care and rehabilitation settings (the highest prevalence of 26% was in long‐term acute‐care settings (VanGilder 2009)). In the UK, national pressure ulcer data are collected across community and acute settings (although data collection is not yet universal) as part of the National Health Service (NHS) Safety Thermometer initiative (Power 2012). About 4.4% of patients across these settings were estimated to have a pressure ulcer (Stage 2 to Stage 4) in November 2014 (NHS Quality Observatory 2015).
We note that all the prevalence figures quoted above are for at‐risk populations currently receiving medical care. The point prevalence of pressure ulceration in the total adult population was recently estimated as 0.31 per 1000 population (including Stage 1) (Hall 2014).
Treatments for pressure ulcers
There are two main strategies in the treatment of pressure ulcers, namely relief of pressure ‐ commonly using specialist support surfaces (McInnes 2011; NICE 2014) ‐ together with management of the wound environment using wound dressings. Other general strategies include patient education, pain management, optimising circulation/perfusion, optimising nutrition and the treatment of clinical infection (EPUAP‐NPUAP‐PPPIA 2014; NICE 2014). Pressure ulcers are normally expected to show signs of healing within two weeks, but this may not occur and there can be deterioration (EPUAP‐NPUAP‐PPPIA 2014).
Impact of pressure ulcers on patients and financial costs
Pressure ulcers have a large impact on those affected; the ulcers can be painful, and may become seriously infected or malodorous. It has been shown that after adjustment for age, sex and co‐morbidities people with pressure ulcers have a lower health‐related quality of life than those without pressure ulcers (Essex 2009).
The financial cost of treating pressure ulcers in the UK has been estimated to range from GBP 1214 for a Stage 1 ulcer to GBP 14,108 for a Stage 4 ulcer. Costs are mainly dominated by health professional time, and for more severe ulcers, by the incidence of complications including hospital admission/length of stay (Dealey 2012). In 2004, the total annual cost of treating pressure ulcers in the UK was estimated as GBP 1.4 to 2.1 billion, which was equivalent to 4% of the total NHS expenditure (Bennett 2004). Pressure ulcers have been shown to increase length of hospital stay and associated hospital costs (Allman 1999). Figures from the USA suggest that for half a million hospital stays in 2006, 'pressure ulcer' was noted as a diagnosis; for adults, the total hospital cost for these stays was USD 11 billion (Russo 2008). Costs to the Australian healthcare system for treating pressure ulceration have been estimated at AUD 285 million annually (Graves 2005).
Description of the intervention
This review includes RCTs of any dressings or topical agents applied directly onto or into wounds and left in situ, as opposed to products used to irrigate, wash or cleanse wounds and those that are only in contact with wounds for a short period.
Dressings
The classification of dressings usually depends on the key material used in their construction, and whether additional substances are added to the dressing. Several attributes of an ideal wound dressing have been described (BNF 2016; Bradley 1999), including the ability of the dressing to:
absorb and contain exudate without leakage or strike‐through, in order to maintain a wound that is moist but not macerated;
achieve freedom from particulate contaminants or toxic chemicals left in the wound;
provide thermal insulation, in order to maintain the optimum temperature for healing;
allow permeability to water, but not bacteria;
optimise the pH of the wound;
minimise wound infection and avoid excessive slough;
avoid wound trauma on dressing removal;
accommodate the need for frequent dressing changes;
provide pain relief; and
be comfortable.
There are numerous and diverse dressings available for treating pressure ulcers and their properties are described below.
Absorbent dressings are applied directly to the wound and may be used as secondary absorbent layers in the management of heavily exuding wounds. Examples include Primapore (Smith & Nephew), Mepore (Mölnlycke) and absorbent cotton gauze (BP 1988).
Alginate dressings are highly absorbent fabrics/yarns that come in the form of calcium alginate or calcium sodium alginate and can be combined with collagen. The alginate forms a gel when in contact with the wound surface; this can be lifted off at dressing removal, or rinsed away with sterile saline. Bonding to a secondary viscose pad increases absorbency. Examples include: Curasorb (Covidien), SeaSorb (Coloplast) and Sorbsan (Unomedical).
Capillary‐action dressings consist of an absorbent core of hydrophilic fibres held between two low‐adherent contact layers. Examples include: Advadraw (Advancis) and Vacutex (Protex).
Films, i.e. permeable film and membrane dressings are permeable to water vapour and oxygen, but not to water or micro‐organisms. Examples include Tegaderm (3M) and OpSite (Smith & Nephew).
Foam dressings contain hydrophilic polyurethane foam and are designed to absorb wound exudate and maintain a moist wound surface. There are a variety of versions and some include additional absorbent materials, such as viscose and acrylate fibres, or particles of superabsorbent polyacrylate, which are silicone‐coated for non‐traumatic removal. Examples include: Allevyn (Smith & Nephew), Biatain (Coloplast) and Tegaderm (3M).
Honey‐impregnated dressings contain medical‐grade honey that is purported to have antimicrobial and anti‐inflammatory properties and can be used for acute or chronic wounds. Examples include: Medihoney (Medihoney) and Activon Tulle (Advancis).
Hydrocolloid dressings are usually composed of an absorbent hydrocolloid matrix on a vapour‐permeable film or foam backing. Examples include: Granuflex (ConvaTec) and NU DERM (Systagenix). Fibrous alternatives that resemble alginates and are not occlusive have also been developed: Aquacel (ConvaTec).
Iodine‐impregnated dressings release free iodine, which is thought to act as a wound antiseptic when exposed to wound exudate. Examples include Iodoflex (Smith & Nephew) and Iodozyme (Insense).
Low‐adherence dressings and wound contact materials usually consist of cotton pads that are placed directly in contact with the wound. They can be non‐medicated (e.g. paraffin gauze dressing, saline gauze dressing) or medicated (e.g. containing povidone iodine or chlorhexidine). Examples include paraffin gauze dressing, BP 1993 and Xeroform (Covidien) dressing ‐ a non‐adherent petrolatum blend with 3% bismuth tribromophenate on fine mesh gauze.
Odour‐absorbent dressings contain charcoal and are used to absorb wound odour. Often this type of wound dressing is used in conjunction with a secondary dressing to improve absorbency. An example is CarboFLEX (ConvaTec).
Other antimicrobial dressings are composed of a gauze or low‐adherent dressing impregnated with an ointment thought to have antimicrobial properties. Examples include: chlorhexidine gauze dressing (Smith & Nephew) and Cutimed Sorbact (BSN Medical).
Protease‐modulating matrix dressings alter the activity of proteolytic enzymes in chronic wounds. Examples include: Promogran (Systagenix).
Silver‐impregnated dressings are used to treat infected wounds, as silver ions are thought to have antimicrobial properties. Silver versions of most dressing types are available, including silver impregnated dressings (e.g. silver hydrocolloid etc). Examples include: Acticoat (Smith & Nephew) and Urgosorb Silver (Urgo).
Soft polymer dressings are composed of a soft silicone polymer held in a non‐adherent layer; these are moderately absorbent. Examples include: Mepitel (Mölnlycke) and Urgotul (Urgo).
Topical agents
Topical agents are defined as hydrogels. ointments and creams that are placed in contact with the wound and left in situ; they may be covered with a secondary dressing.The following types of topical agents are considered as interventions in this review:
Cadexomer‐iodine paste consists of a water‐soluble, modified starch polymer containing iodine. It releases free iodine when exposed to wound exudate. The free iodine acts as an antiseptic on the wound surface, and the cadexomer absorbs wound exudate and encourages de‐sloughing. Examples include: Iodosorb (Smith & Nephew) ointment and powder.
Collagenase‐containing ointment is an enzymatic debriding ointment. Collagenase is thought to digest collagen in necrotic tissue and to contribute to granulation and epithelisation.
Hydrogels consist of a starch polymer and up to 96% water. They can absorb wound exudate or rehydrate a wound depending on the wound moisture levels. Hydrogels are often considered to be dressings, but are also topical in nature. They are supplied in either flat sheets, an amorphous hydrogel or as beads. Examples include: ActiformCool (Activa and Aquaflo (Covidien).
Phenytoin topical is thought to promote wound healing by a number of mechanisms, including stimulation of fibroblast proliferation, facilitation of collagen deposition and antibacterial activity.
Silver sulfadiazine cream is a topical antimicrobial cream that is used to treat and prevent infection in wounds by damaging bacterial cell membranes. Examples include Flamazine (Smith & Nephew) and Silvadene (Pfizer).
Products containing growth factors, platelet‐rich plasma or other platelet‐derived products and colony‐stimulating factors are outside the scope of this review.
How the intervention might work
Animal experiments conducted over 40 years ago suggested that acute wounds heal more quickly when their surfaces are kept moist rather than left to dry and scab (Winter 1962; Winter 1963a; Winter 1963b). A moist environment is thought to provide optimal conditions for the cells involved in the healing process, as well as allowing autolytic debridement (removal of dead tissue by natural processes), which is thought to be an important part of the healing pathway (Cardinal 2009).
The desire to maintain a moist wound environment is a key driver for the use of wound dressings and related topical agents. Whilst a moist environment at the wound site has been shown to aid the rate of epithelialisation in superficial wounds, excess moisture at the wound site can cause maceration of the surrounding skin (Cutting 2002), and it has also been suggested that dressings that permit fluid to accumulate might predispose wounds to infection (Hutchinson 1991). Wound treatments vary in their level of absorbency, so that a very wet wound can be treated with an absorbent dressing (such as a foam dressing) to draw excess moisture away and avoid skin damage, whilst a drier wound can be treated with a more occlusive dressing or a hydrogel to maintain a moist environment.
Some dressings are now also formulated with an 'active' ingredient (e.g. silver, honey or protease modulators).
Why it is important to do this review
The diversity of dressings and related materials available to health professionals for treating pressure ulcers makes evidence‐based decision‐making difficult when determining the optimum treatment regimen for a particular patient (Gillespie 2012; NICE 2014). With increasingly sophisticated technology being applied to wound care, practitioners need to know the relative effectiveness and cost‐effectiveness of these sometimes expensive dressings. Even where cost is not an issue, the most effective treatment may not be available (e.g. in some developing countries) or may be difficult or to use, so that information on the second and third best treatments is important too (Salanti 2011).
Current evidence syntheses include four Cochrane Reviews (Dumville 2015a; Dumville 2015b; Keogh 2013; Walker 2014), two other systematic reviews (Reddy 2008; Smith 2013), and two recent clinical guidelines (EPUAP‐NPUAP‐PPPIA 2014; NICE 2014). Each of these consists of a series of pairwise comparisons. No review finds clear evidence of any effect of one dressing compared to another in terms of assessed outcome measures, including complete wound healing.
In the absence of an overview or network meta‐analysis, decision‐makers have to consider the findings of multiple pairwise randomised controlled trials (RCTs) simultaneously and qualitatively to judge, in the face of uncertainty, which dressing they might decide to use. It is extremely difficult to do this effectively, and this difficulty is compounded when the evidence comprises single small trials, about which decision‐makers may have little confidence.
Network meta‐analysis (NMA) is the simultaneous comparison of linked, multiple, competing treatments in a single statistical model (Caldwell 2005; Chaimani 2013a; Lu 2004; Salanti 2008). NMA utilises evidence from 'direct' (head‐to‐head or 'pairwise') comparisons (e.g. trials directly comparing treatments A and B), 'indirect' comparisons (e.g. the combination of trials comparing A with C and trials comparing B with C), and a synthesis of both when available. When pooling relative effect estimates, NMAs preserve within‐trial randomisation (Grant 2013; Thorlund 2012; Tu 2012).
Where there are relevant common comparators across trials that allow treatments to be linked and form a network of evidence, NMA produces a set of effect estimates for each treatment relative to every other, whether or not they have been compared in head‐to‐head trials. In this way NMA allows us to obtain estimates for comparisons for which there is no (direct) trial evidence. Even when direct evidence is available there may not be much of it, so pooling it with data from indirect comparisons generally gives more robust evidence and reduces uncertainty in the estimates of effect (Higgins 1996; Thorlund 2012). From the NMA analysis, it is possible to evaluate the probability of each treatment being the best for a specific outcome: these probabilities reflect the precision surrounding the effect estimates (Caldwell 2014; Salanti 2011).
A glossary of NMA terms is given in Appendix 2.
This review comprised a network meta‐analysis (NMA) for the outcome of pressure ulcer healing, for alternative dressings and topical agents for the treatment of pressure ulcers of Stage 2 and above. The NMA enabled us to compare pairs of dressings/topical agents, taking into account direct and indirect evidence simultaneously, and explicitly determining the uncertainty in effect estimates. The ranking process allowed us to examine the evidence base as a whole, identifying the support of the evidence for each treatment, having consideration for indirect evidence (where it existed) and fully reflecting evidence uncertainties. We also explored assumptions made in the analysis.
Objectives
To assess the effects of dressings and topical agents for healing pressure ulcers in any care setting. We aimed to examine this evidence base as a whole, determining probabilities that each treatment is the best, with full assessment of uncertainty and evidence quality.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs), irrespective of language of report. We did not identify any cross‐over trials, but we would have included them only if they reported outcome data at the end of the first treatment period and prior to cross‐over. We excluded studies using quasi‐random methods of allocation (such as alternation). We highlighted trials in which three or more interventions were randomised.
Types of participants
We included studies that recruited people with a diagnosis of pressure ulcer, Stage 2 and above (EPUAP‐NPUAP‐PPPIA 2014), managed in any care setting. We excluded studies that only recruited people with Stage 1 ulcers as these are not open wounds requiring dressings.
We accepted study authors' definitions of what they classed as Stage 2 or above, unless it was clear that they included wounds with unbroken skin. Where authors used grading scales other than NPUAP, we attempted to map to the NPUAP scale.
We included studies that recruited participants with pressure ulcers of Stage 2 severity or higher alongside people with Stage 1 pressure ulcers or other types of complex wound (e.g. leg and/or foot ulcers), or both, provided the allocation of participants was stratified by type of wound or pressure ulcer severity at randomisation and provided the results for people with eligible pressure ulcers (that is Stage 2 or higher) were presented separately (or became available from the study authors). Where studies included participants with Stage 1 ulcers or other types of complex wounds, but these made up 25% or less of the total study population we included all study data.
Types of interventions
Interventions of direct interest (decision set)
The interventions in this section were all those that can be directly applied as dressings or topical agents to open pressure ulcers. We presented results for these interventions and included them in summary tables. In the context of a network of competing treatments, there are no 'comparators'.
We considered trials for which at least one of the interventions was (1) any dressing, including impregnated dressings or saline‐moistened dressings or combination dressings or (2) any topical agent applied directly to an open pressure ulcer and left in situ. Combination dressings are when two or more dressings are applied sequentially over time (e.g., hydrocolloid for four weeks followed by alginate for four weeks), or a product contains two or more types of dressing material (e.g., a multilayer product comprising silicone polymer and hydrocolloid). The treatment of interest had to be the only systematic difference between treatment groups. We did not take into account secondary dressings.
Some of the interventions we considered were as follows:
Basic wound contact dressings (includes low‐adherence (including paraffin gauze) or absorbent dressings (of any absorbency))
Saline‐moistened gauze (all degrees of moistness)
Hydrogel dressing (includes hydrogel sheet or hydrogel application (amorphous) or sodium hyaluronate)
Vapour‐permeable films and membranes (includes adhesive film (semi‐permeable) or adhesive film with absorbent pad)
Soft polymer dressings (with/without absorbent pad or cellulose)
Hydrocolloid dressing (with/without adhesive border or matrix hydrocolloid)
Fibrous (spun) hydrocolloid
Foam dressings (all absorbencies)
Alginate dressings
Capillary action dressings
Alginate dressing with charcoal
Other charcoal‐containing dressing
Honey sheet dressing or topical honey
Cadexomer iodine ointments
Iodine‐containing dressings
Soft polymer dressing (with silver)
Hydrocolloid (with silver)
Foam dressings (with silver)
Alginate dressings (with silver)
Silver sulfadiazine cream
Protease‐modulating matrix dressings
Collagenase‐containing ointment
Topical phenytoin
Topical zinc oxide
No dressing (wound left exposed)
Other treatments considered by the review team (with additional clinical advice where required) to be dressings or topical agents applied directly to the wound and left in‐situ.
The following interventions were not part of the decision set: treatments in which dressings are attached to external devices such as negative pressure wound therapies, skin grafts, growth factor treatments, platelet gels and larval therapy.
We grouped together dressings in the same class (e.g. alginates) (BNF 2016). This was regardless of a particular brand's stated absorbency, size, concentration of active component or the degree of moistness. Thus, where studies only compared two dressings from the same class (for example, two alginates or two foam dressings), we excluded such studies from the review as they contributed no information about the effectiveness of the class.
We included any RCT in which other concurrent therapies were given (e.g. antibiotics, debridement), provided that these treatments were delivered in a standardised way across the trial arms of the individual trial (such that the treatment of interest was the only systematic difference). We did not treat separately comparisons with and without concurrent therapies, that is, we considered intervention 1 + concurrent therapy versus intervention 2 + concurrent therapy to be the same as intervention 1 versus intervention 2.
One of the assumptions underpinning NMA is that interventions in the network are exchangeable, that is, participants in the network could, in principle, be randomised to any of the treatments being compared. For example, a person with a pressure ulcer could be equally likely to be randomised to an alginate dressing, a polyurethane foam dressing, honey or saline gauze. Depending on the wound requirements for the dressing (e.g. highly absorbent), this may not always be a good assumption for individual wounds, but across the population in the trials may be reasonable.
Supplementary intervention set
Some of the trial interventions were not included in the decision set (see above) but were included in a supplementary intervention set if they linked two or more decision set interventions: such supplementary interventions were of value solely because they allowed inferences to be drawn about the treatments of interest. In our individual network, the supplementary intervention set included radiant heat and skin substitute.
Terminology
For the rest of this review, we use the term 'comparison' to mean two interventions compared in a single study or in a pairwise meta‐analysis of direct data. We use the term 'contrast' to mean two interventions compared across all studies in an NMA. This may be either direct or indirect evidence or both. We use the following terms: 'direct contrast' for interventions linked directly in the network; 'indirect contrast' when the two interventions are linked solely via indirect NMA evidence; and 'mixed treatment contrast' when either direct or indirect evidence or both are involved. Direct evidence may be informed by more than one study comparing the two interventions. Indirect estimates may be calculated using a 'node‐splitting' approach, in which the NMA is run after excluding the direct evidence for a particular contrast.
We also use the term 'core intervention' to mean interventions that form part of at least one loop and 'peripheral interventions' to mean interventions that are not part of a loop and are only connected in a peripheral way.
Types of outcome measures
We reported outcome measures at the last time point available (assumed to be length of follow‐up if not specified) or the time point specified in the methods as being of primary interest (if this was different from the latest time point available). Initially, we noted when studies reported results at other time points or whether they included Kaplan‐Meier plots, or both.
Primary outcomes
The primary outcome for this review was complete wound healing.
We regarded the following as providing the most relevant measures of outcome for the analyses:
the proportion of wounds healed (frequency of complete healing: arm‐level data);
time to complete healing (survival data: study‐level data).
We accepted authors' definitions of what constituted a healed wound.
Secondary outcomes
We did not consider any secondary outcomes, however they are reported in other relevant reviews (Dumville 2015a; Dumville 2015b; Keogh 2013; Walker 2014).
Search methods for identification of studies
Four existing Cochrane Reviews were relevant to this NMA (Dumville 2015a; Dumville 2015b; Keogh 2013; Walker 2014), and the protocol for this NMA complemented the protocols for these four reviews (an author on these four reviews is also a review author here). We automatically included trials from these reviews in this NMA if they reported complete healing outcomes; we planned to use the extracted data from these reviews where possible, supplementing if necessary which was required as some reviews had not been completed.
We conducted searches to identify relevant trials not covered by the four Cochrane Reviews as well as recently published trials. We cross‐checked the identified trials against those in the 2014 NICE guideline and the 2013 US Agency for Healthcare Research and Quality (AHRQ) guideline on treating pressure ulcers to further locate any additional trials (AHRQ 2013; NICE 2014); we also checked the references of 24 systematic reviews identified by our search.
Electronic searches
We searched the following electronic databases to identify reports of relevant randomised clinical trials:
the Cochrane Wounds Specialised Register (searched 12 July 2016);
the Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Library) (2016, Issue 6);
Ovid MEDILINE (1946 to 12 July 2016);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (12 July 2016);
Ovid Embase (1974 to 12 July 2016);
EBSCO CINAHL Plus (1937 to 12 July 2016).
The search strategies for CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 3. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase randomised trials filter terms developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL search with the randomised trials filter terms developed by the Scottish Intercollegiate Guidelines Network (SIGN 2017). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov)
WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/Default.aspx)
EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
Searching other resources
We searched for other potentially eligible trials or ancillary publications in the reference lists of retrieved included studies as well as relevant systematic reviews, meta‐analyses, guidelines and health technology assessment reports.
Data collection and analysis
Data collection and analysis were carried out according to methods stated in the published protocol (Westby 2015), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Two review authors independently assessed the titles and abstracts of the citations retrieved by the searches for relevance. After this initial assessment, we obtained full‐text copies of all studies considered to be potentially relevant. Two review authors independently checked the full papers for eligibility; disagreements were resolved by discussion and, where required, the input of a third review author. We did not contact study authors. We recorded all reasons for exclusion of the studies for which we had obtained full copies. We completed a PRISMA flowchart to summarise this process (Liberati 2009).
Where studies were reported in multiple publications/reports we obtained all publications. Such a study was included only once in the review, but we extracted data from all reports to ensure maximal relevant data were obtained.
Data extraction and management
We extracted the following information from each included study:
interventions being compared, including any ineligible interventions randomised to additional trial groups;
duration of the intervention;
details of any co‐interventions;
the unit of randomisation (e.g. participant or ulcer);
the number of ulcers per person;
the unit of analysis (including any selection methods for people with multiple ulcers);
the number of participants in each arm;
the hazard ratio and its 95% confidence interval (CI) (or any data that would allow its calculation (Tierney 2007)) for comparisons between arms);
the number of participants that healed in each arm, both at the latest time point or (if different) at another time specified as of primary interest in the study's methods section;
all other follow‐up times reported;
we noted if a Kaplan Meier plot was displayed;
missing data rates per arm, and reasons for 'missingness', including the number of people dying.
Data on potential effect modifiers
We were not aware of any population‐specific effect modifiers for this research question: there was no existing evidence to suggest that one type of dressing worked better than another for certain subgroups, for example, people with different depths of tissue damage.
However, we extracted data that allowed us to determine for each included study factors that may act as effect modifiers (in this context):
type of funding (e.g. industry, academic, government); this was dichotomised into non‐for‐profit and other;
risk of bias (see Assessment of risk of bias in included studies).
Other data
We also extracted the following data regarding patient and study characteristics at baseline for each intervention arm if possible:
care setting;
age of participants;
duration of pressure ulcer(s);
severity/grade of pressure ulcer;
nature of pressure ulcer wounds (e.g. sloughy, necrotic, infected);
size of pressure ulcer(s).
Assessment of risk of bias in included studies
Cochrane risk of bias assessment
We assessed risk of bias for each included study for the complete healing outcome. There is only one outcome in this review (complete wound healing) and so risk of bias assessments at the outcome level apply to the whole study.
Two review authors independently assessed included studies using the Cochrane risk of bias tool (Higgins 2011b) with involvement of a third author where consensus could not be reached. We also determined an all‐domain risk of bias (see below).
Additionally, we reported separately an overall risk of bias for each direct comparison meta‐analysis and for each contrast in the NMA (see next section).
Overall risk of bias and linking to GRADE assessment
In order to link these Cochrane ratings to the GRADE assessment for risk of bias of the evidence (downgrading 0, 1 or 2 times), we used a two‐stage process. Firstly, we obtained an all‐domain risk of bias for each study and then used this to produce an overall risk of bias for each comparison.
All‐domain risk of bias for each study
We summarised data for each of the key domains of selection bias, detection bias, attrition bias, reporting bias and other bias, assigning one of four ratings: low, unclear, high and very high. For example, selection bias was informed by sequence generation, allocation concealment and comparability of baseline characteristics.
In an adaption of the GRADE approach (Guyatt 2011a), we produced an all‐domain risk of bias, with four ratings defined as:
'very high' ‐ two or more key domains with a high risk of bias or a single domain with very high levels of uncertainty (e.g. very high degree of differential missing data);
'high' ‐ high risk of bias for any one domain or we judged the risk of bias to be 'almost high' across more than one domain;
'low' ‐ low risk of bias for each of the key domains;
'unclear' ‐ insufficient information for at least one key domain (with the other domains being at low risk of bias).
Then we grouped together the low and unclear all‐domain risk‐of‐bias ratings.
We included this all‐domain risk of bias in the summary 'Risk of bias' figure, by adding two further columns: red in both of the last two columns indicated 'very high' all‐domain risk of bias; red in the penultimate column (but not the last column) indicated 'high' risk of bias; and the combined low/unclear group was marked green in the penultimate column, with the last column remaining blank.
Overall risk of bias for a direct comparison
Wherever more than one study was pooled in a pairwise meta‐analysis, we assigned an overall risk of bias for that comparison, by calculating a weighted average all‐domain risk of bias across studies; weights were those produced in the meta‐analysis (based on the inverse variance). We assigned numerical values to the all‐domain ratings for each study: low/unclear (1), high (2) and very high (3) and calculated the weighted average.
We used the weighted average to give a rating of overall risk of bias for that comparison: low, high and very high, and aligned these ratings respectively with the GRADE categories of no limitations (not downgraded on risk of bias), serious limitations (downgraded once) and very serious limitations (downgraded twice) (Guyatt 2011a; Salanti 2014).
We superimposed the overall risk of bias for each direct comparison (on the basis of the direct meta‐analysis) on the network diagram, using colours to represent different ratings. We used these overall risks of bias to calculate the risk of bias for each mixed treatment contrast (see below).
Overall risk of bias for each mixed treatment contrast in the network
An NMA comprises a set of interventions linked via a series of comparisons ('direct contrasts'). Each direct contrast contributes data to the evidence for all other contrasts in the network to which that contrast is linked indirectly (and becomes indirect evidence). The contribution of each piece of indirect evidence to a mixed treatment contrast depends on its point estimate, precision and relative location within the network, and on that of any direct evidence or other indirect evidence (Chaimani 2013b; Salanti 2014). A recently published tool, Krahn 2013, allows such contributions to be determined for each contrast in the network informed by direct and indirect evidence. We summarised the percentage contribution of each direct contrast to each network estimate in a matrix with columns and rows corresponding to the direct and mixed treatment contrasts respectively.
The overall risk of bias for each mixed treatment contrast is a composite measure of the risks of bias for all the contributing direct contrasts (that is, the sum of the all‐domain risks of bias for all the direct contrasts, each weighted by their percentage contributions to the mixed treatment contrast).
We calculated the overall risk of bias for the entire network using percentage contributions to the whole network for each direct contrast.
Measures of treatment effect
Relative treatment effects
For each contrast in the NMA, we presented the risk ratio with its 95% CI. We used raw data from individual studies, taking the number of ulcers healed at the latest time point, unless otherwise stated.
We also recorded separately the time‐to‐healing outcome for studies that reported this.
Relative treatment ranking
We presented the relative treatment ranking as a cumulative probability at each rank and as a Surface Under the Cumulative RAnking (SUCRA) value for each treatment (see Data synthesis ‐ methods for indirect and mixed comparisons and Appendix 2).
Unit of analysis issues
We expected the main unit of analysis issues to occur when participants had more than one wound per person. In these cases, we treated the participant as the unit of analysis when the number of wounds assessed appeared to be equal to the number of participants (e.g. one wound per person). This included studies in which participants were randomised to treatments and there was more than one wound per person, but results were reported for one selected wound; we considered whether there was risk of bias in the selection process.
Where studies randomised at the participant level, used the allocated treatment on multiple wounds per participant, and measured and analysed outcomes at the wound level (e.g. wound healing), we expected there to be unit of analysis issues if the data were not correctly analysed. In practice, there was insufficient information to approximate the correct analyses (in accordance with Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions, using information adapted from Higgins 2011c), so we assessed risk of unit‐of‐analysis bias, taking into account the number of people randomly assigned to each intervention; and the average (mean) number of wounds per person.
Dealing with missing data
It is common to have data missing from trial reports. Excluding participants post‐randomisation, or ignoring those participants who withdrew from the trial or were lost to follow‐up, compromises the randomisation and potentially introduces bias into the trial. Where data were missing for the primary outcome of proportion of ulcers healed, we assumed participants did not have the outcome (i.e. they were considered in the denominator but not the numerator). We examined this assumption in a sensitivity analysis, using a complete case analysis instead.
Assessment of heterogeneity
Assessment of clinical and methodological heterogeneity within treatment comparisons
We assessed the presence of clinical heterogeneity within each pairwise direct comparison (i.e. the degree to which studies varied in terms of participant, intervention and outcome characteristics) by comparing information extracted for included studies.
Assessment of transitivity across treatment contrasts
'Transitivity' refers to the situation in which an intervention effect measured using an indirect contrast is valid and equivalent to the intervention effect measured using a direct contrast. Where there are differences in (known or unknown) effect modifiers across contrasts, the transitivity assumption may not be met which may generate statistical inconsistency in the network (Grant 2013; Jansen 2013). We did not identify any potential effect modifiers from the literature, so there was no evidence that the transitivity assumption was not met. There were also limited underlying theoretical reasons to consider effect modification for these treatments.
If we had had sufficient data we planned to explore the effect of the funding source and differences in risk of bias as possible effect modifiers across the network. However, there was insufficient variation in these factors.
Assessment of reporting biases
We assessed for the presence of publication bias using a contour‐enhanced funnel plot, provided there were at least 10 included studies (Peters 2008; Salanti 2014).
Data synthesis
General methods
We performed analyses in a frequentist framework using the statistical software STATA (STATA 2013). This is a change from the protocol, in which we had proposed a Bayesian framework using the statistical software WinBUGS for most of the analyses (Dias 2016; Lunn 2000; Lunn 2009; Spiegelhalter 2003; WinBUGS 2015), and STATA to calculate contributions of direct contrasts to the NMA results. One major advantage of the Bayesian framework would have been to confer flexibility by explicitly considering the duration of follow‐up across studies by modelling the hazard function (Dias 2016; Saramago 2014; Soares 2014). However, there was insufficient variation in follow‐up duration and fewer than 20% of the studies reported time‐to‐event data, in six contrasts without loops, so we could not justify modelling the outcome data in this way. We therefore conducted analyses using the proportion healed, and we pooled risk ratios, ignoring differences in follow‐up, This lack of need to model time, together with recent software developments in STATA for NMA (especially the contributions matrix routine, important for GRADE analysis), led to a decision to use a frequentist approach in STATA for all analyses (Chaimani 2013a; Chaimani 2013b; Chaimani 2015; Gasparrini 2015; Salanti 2014).
We have given a brief description of the STATA analytical routines used in Appendix 4, together with routines that enabled us to display the output visually (Chaimani 2013b). Where there were zero events in any one arm of a trial, we added 0.5 to the numerator and 1 to the denominator for each arm in the trial, in accordance with the general approach taken by STATA.
Methods for standard meta‐analysis
We performed pairwise meta‐analyses in a frequentist framework, both within the STATA software and also using Review Manager 5 (RevMan 5) (RevMan 2014) for convenience in producing forest plots. For RevMan, we used both inverse variance weighting and a random‐effects model (for consistency with the NMA methods). Results for the two sets of software were compared and found to be identical in most cases; where there were differences we reported both sets of results. Differences were due to how zero cells are dealt with.
Methods for network meta‐analysis
We initially used the STATA software to produce a network diagram based on all included studies in order to inform the analysis plan (Chaimani 2013b). We then excluded from the analysis two‐arm studies in which one of the interventions could be described as 'standard care' or 'mixed care' involving the choice of more than one treatment because they crossed intervention categories. We also excluded from the analysis studies that had one intervention of direct interest (e.g. hydrocolloid) compared with one ineligible intervention (e.g. radiant heat), unless we found, after examining the network diagram, that the ineligible intervention linked two or more interventions of direct interest.
We performed multivariate network meta‐analysis using STATA routines. This took into account correlations between the effect sizes from multi‐arm studies (Chaimani 2013a; Chaimani 2013b; White 2012). We used a consistency model (which assumes that there is agreement between direct and indirect sources of evidence) and assumed a random‐effects model. The NMA results were reported for 'mixed treatment contrasts', which means the evidence synthesis involved both direct evidence and indirect evidence from across the whole network. The output was reported as pooled risk ratios, with their 95% CIs.
We evaluated the surface under the cumulative ranking curve (SUCRA) and obtained mean ranks (Salanti 2011) for each treatment. Both these measures are based on an assessment of the probability of each treatment being best, second best, etc. In general, the probability that a particular treatment ranks best represents the likelihood of it being considered the most effective (within the pool of treatments analysed) reflecting the evidence of effectiveness and the precision surrounding the estimates. It is expressed as a proportion, where a value of 1 means that the evidence determines that a particular treatment is the best with certainty and 0 is the certainty that it is not the best. The SUCRA is a numerical summary of the distribution of ranks for each treatment (probability of being best, second best, etc) and provides a hierarchy of the treatments that accounts both for the location and the variance of all relative treatment effects. The larger the SUCRA value, the better the rank of the treatment.
We conducted two NMAs: one for individual treatments and one in which dressings interventions were grouped in broader categories, with clinical guidance. We had planned the second (grouped) network as a sensitivity analysis at the protocol stage, but later decided to conduct this analysis in parallel with the individual treatment NMA, because we expected the group analysis to provide valuable and complementary clinical information. The results of the group analysis are presented in Appendix 5.
Assessment of statistical heterogeneity
We assessed statistically the presence of heterogeneity within each pairwise comparison using the I² (Higgins 2003) and tau² statistics from the RevMan 5 analyses; I² measures the percentage of variability that cannot be attributed to random error and tau² measures the extent of heterogeneity among the intervention effects observed in different studies. We also took into account the overlap of CIs and the variability in the point estimates.
Assessment of statistical inconsistency
We assessed inconsistency in two main ways: determining local inconsistencies (around particular contrasts in the network) and assessing inconsistency for the network as a whole. These tests are often underpowered so we assessed at the 90% significance level.
Local approaches to evaluating inconsistency
To evaluate the presence of inconsistency locally we considered two main approaches.
Firstly, we used a loop‐specific approach. This method evaluated the consistency assumption in each closed loop of the network separately as the difference between direct and indirect estimates for a specific contrast in the loop (inconsistency factor: IF). We assumed a common heterogeneity estimate within each loop. We report results as the ratio of risk ratios (RoRR) with its 90% CI ‐ the natural logarithm of the RoRR is the same as IF (Appendix 2). The magnitude and 90% CIs were used to draw inferences about the presence of inconsistency in each loop. If the CI excluded 1, statistically there was significant inconsistency. We also considered whether the CI included 2 or more (or 0.5 or less). This means that the direct estimate could be twice as large (half as big) as the indirect estimate, which is an indication of potential inconsistency (Chaimani 2013b). We also report the IF assuming a common heterogeneity estimate for the whole network (Veroniki 2013).
Secondly, we considered a "node splitting" approach (Dias 2010; Salanti 2014) This method was applied, singly, to each direct contrast (called a "node" by Dias 2010). The STATA routine calculated an indirect estimate using the rest of the network, by running the NMA after excluding the direct evidence for that contrast. The indirect estimates were then compared with the respective direct estimates, again calculating a RoRR with its 90% CI for each contrast.
Finally, we compared NMA results using inconsistency versus consistency assumptions for each contrast.
Global approaches to evaluating inconsistency
We evaluated consistency in the entire network simultaneously, by extending the analysis to include an inconsistency model that omitted consistency equations (Dias 2013). The latter used a design‐by‐treatment interaction model, which allowed for different designs (2‐arm trials (A‐X); 2‐arm trials without A, and 3‐arm trials, where A is the base treatment). This approach produced a set of inconsistency parameters. After fitting the inconsistency model, the null hypothesis of consistency is tested for the set of inconsistency parameters using a global Wald test. This test may lack power and we considered a significance level of P < 0.1 (Higgins 2012; White 2012).
Investigation of heterogeneity and inconsistency
If there had been sufficient studies available, we would have performed network meta‐regression or subgroup analyses using funding source and risk of bias as possible sources of inconsistency or heterogeneity, or both. This was not possible.
Sensitivity analysis
We had intended to re‐analyse the network with studies removed that were considered to be at high risk of bias for any one or more of selection, attrition or detection bias, however, due to the sparseness of the data available and the generally poor methodological quality of the studies, this analysis had to be restricted to removing studies with two or more domains at high risk of bias ("very high risk of bias") (Appendix 6).
We conducted a sensitivity analysis to assess the impact of imputing missing outcome data on the network estimates, via assessment of risk of attrition bias (as defined in Appendix 6), testing the assumption of imputation of no event for missing data by conducting a complete case analysis.
Quality assessment of evidence (GRADE) generated from the NMA and 'Summary of findings' table
We summarise the findings according to GRADE principles (Schünemann 2011a; Schünemann 2011b).
The quality of the data included in any synthesis model is key to determining the validity of the results and of inferences made. We explored the application of GRADE methodology to network meta‐analysis, focusing on the approach of Salanti 2014. We assessed evidence quality (certainty) in two main ways, firstly, for each contrast and secondly, for the network as a whole, in order to assess the quality of the ranking order. We assessed GRADE factors as follows:
Risk of bias: we considered contributions for each particular contrast, and used them to assess the overall risk of bias for that contrast (see Assessment of risk of bias in included studies section, Risk of bias for each contrast in the network). We assessed overall risk of bias per contrast and also for the network as a whole.
Indirectness: we defined this as without limitations in GRADE because we had not identified any effect modifiers.
-
Inconsistency:
At the level of the contrast, inconsistency could only be assessed where there was both direct and indirect evidence. We took into consideration heterogeneity in the direct evidence for that contrast (see Data synthesis, Assessment of statistical heterogeneity) and inconsistency, as described above (see Data synthesis, Local approaches to evaluating inconsistency). We assessed GRADE inconsistency as 'serious limitations' if there was heterogeneity in the direct estimate or inconsistency in the network with respect to that contrast. We attributed 'very serious limitations' to the contrast if there was severe heterogeneity or severe inconsistency or limitations with both heterogeneity and inconsistency, as agreed by two review authors.
At the level of the network, we considered the global Wald test for inconsistency (see Data synthesis, Assessment of statistical inconsistency). Tests of this nature are typically underpowered, so a P value less than 0.1 was considered significant. Additionally, if several contrasts showed direct and indirect results that would have led to different clinical decisions, we assigned inconsistency.
-
Imprecision: currently, NMA GRADE methods do not consider the optimal information size (OIS) approaches used for systematic reviews of pairwise interventions (Guyatt 2011b) and imprecision is based solely on the CI in relation to minimum important difference (MID) values or the null (Salanti 2014), or both. However, in the type of sparse networks typically found in wounds research, the small sample size and ensuing Type I and Type II errors are potentially more of an issue (Dumville 2012; Soares 2014). We firstly considered whether the network was sparse, taking into account the total number of participants, the total number of events and the number of interventions and contrasts in the NMA. If we considered the network not to be sparse, we applied the methods of Salanti 2014. If we considered the network to be sparse, we used the following approach adapted from the Salanti 2014 guidance:
At the level of the contrast ‐ we considered the CI for the individual contrast in relation to the GRADE 'default' minimum important difference (MID) values of RR = 1.25 and 0.75. If the CI crossed both of these MIDs, we downgraded twice for imprecision. If the CI crossed one MID, we downgraded once, regardless of whether the null was crossed. For contrasts involving peripheral interventions, for which large effects were found, we additionally took into account the amount of direct evidence involving this intervention, considering (in an analogous way to simple meta‐analysis) whether the evidence was 'fragile' because of small numbers of events (Guyatt 2011b).
At the level of the network, we took into consideration the overlap of the rankograms/the magnitude of the SUCRA estimates and the sparseness of the network.
We assessed publication bias by plotting a contour‐enhanced funnel plot, which allowed visual assessment of asymmetry for either a particular contrast (all one colour) or for the network as a whole. We did this for the former only if there were 10 studies or more.
We have presented the main results of the review in a 'Summary of findings' table, reporting the results for a representative set of contrasts, with one row for each intervention versus saline gauze. Such tables present key information concerning the certainty (formerly, quality) of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data (Schünemann 2011a). 'Summary of findings' tables also include an overall grading of the evidence using the GRADE approach. The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest.
For calculating absolute risk differences for the probability of healing we used a 'control group risk', calculated as the median of the probability of healing for saline gauze across all studies with these interventions.
Results
Description of studies
Results of the search
The search generated 1038 records: we obtained 381 full papers Figure 1); 305 studies were excluded with reasons (Characteristics of excluded studies). We included 51 studies described in 74 reports. Two protocols of studies were also identified (ISRCTN57842461; ChiCTR‐TRC‐13003959), which appear to be ongoing (see Characteristics of ongoing studies).
1.
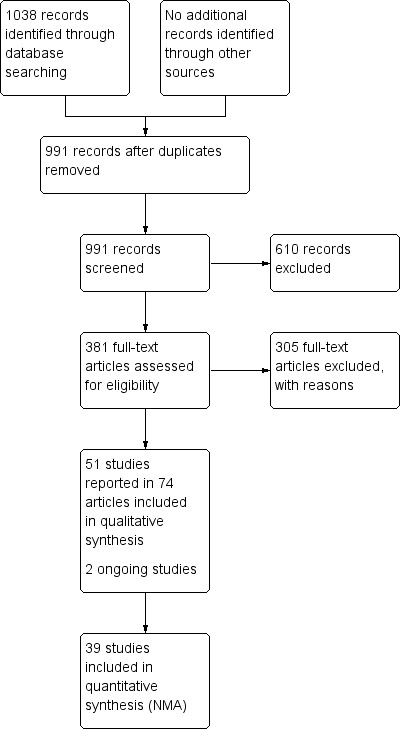
Study flow diagram
We also searched reference lists from identified systematic reviews and for two recent guidelines, but found no extra studies outside the electronic searching.
Included studies
This review distinguishes three sets of included studies: (i) all studies that meet the inclusion criteria ('all included studies'); (ii) the subset of (i) for which all studies have interventions that are joined into the network ('the individual network') (see Effects of interventions) and (iii) the subset of (i) for which all studies are joined in a network in which interventions are grouped ('the group network') (see Appendix 5). In this section we have given a brief summary for the individual network. Further details of each set of included studies are given in Table 2.
1. Summary characteristics of included studies.
Fifty‐one studies, involving 2947 participants, met the inclusion criteria for the whole review. Most of these studies could be linked to form a network of interventions, but 12 were not linked into the network; further details, and the results for the comparisons reported in these 12 studies are given in Appendix 7. The joined network (Figure 2) included 39 studies (Aguilo Sanchez 2002; Alm 1989; Bale 1997a; Banks 1994b; Banks 1994a; Banks 1994c; Barrois 1992; Belmin 2002; Brod 1990; Brown‐Etris 1996; Brown‐Etris 1997; Brown‐Etris 2008; Burgos 2000b; Colwell 1993; Darkovich 1990; Graumlich 2003; Hollisaz 2004; Hondé 1994; Kaya 2005; Kraft 1993; Matzen 1999; Meaume 2003; Motta 1999; Muller 2001; Neill 1989a; Oleske 1986; Parish 1979; Payne 2009; Piatkowski 2012; Price 2000; Romanelli 2001; Seeley 1999; Serena 2010; Sopata 2002; Thomas 1997a; Thomas 1998; Thomas 2005; Xakellis 1992; Zeron 2007). The median (range) study size was 41 (10 to 168).
2.
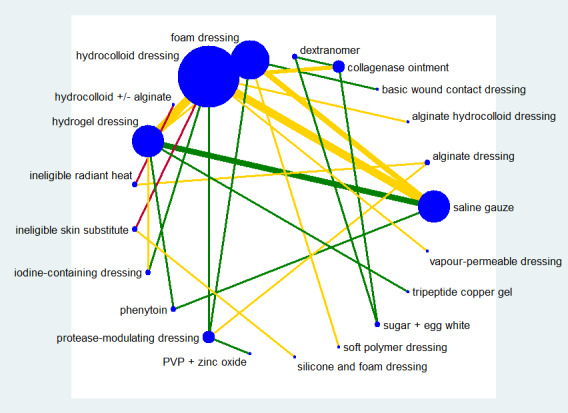
Network diagram ‐ individual interventions, by risk of bias (3 categories)
Key: green = low/unclear; yellow = high; red = very high overall risk of bias for the contrast. The number of studies for each contrast is given in Table 3.
The majority of the 39 studies had only two randomised interventions (37), randomised people rather than ulcers or clusters (34), included at least some of the participants from a hospital setting (20), and were not funded by industry (7) or funding was not stated (17). The median follow‐up time was eight weeks; range 10 days to 6 months. Most studies included participants with a mean age more than 65 years (33) and had ulcers that were mainly Stage 2 (15), Stage 3 (10) or Stages 2 and 3 (7). Sixteen studies included participants with ulcers of less than three months' duration; two had more than three months' duration and the rest (21) were unclear on duration. Further details are given in Table 2. We considered the clinical characteristics to be sufficiently similar across the studies to combine in the analysis, particularly since we had not defined clinical effect modifiers.
Excluded studies
We excluded 305 studies from this review (see Characteristics of excluded studies) The most common reasons for exclusion were 67 with a non‐RCT study design; ineligible outcomes in 120 studies (including 64 with healing outcomes that were not reported as the time to complete healing or the probability of complete healing) and 57 had an ineligible patient population. Eleven studies were excluded because they had two interventions in the same class and 36 other studies had ineligible interventions in both randomised arms, or had treatments that could not be classified as a single intervention.
Risk of bias in included studies
Risk of bias for all included studies is summarised in Figure 3. In order to represent 'very high' risk of bias, we have used two columns ‐ so very high risk of bias occurs when the cell is red in the final column (see Assessment of risk of bias in included studies).
3.
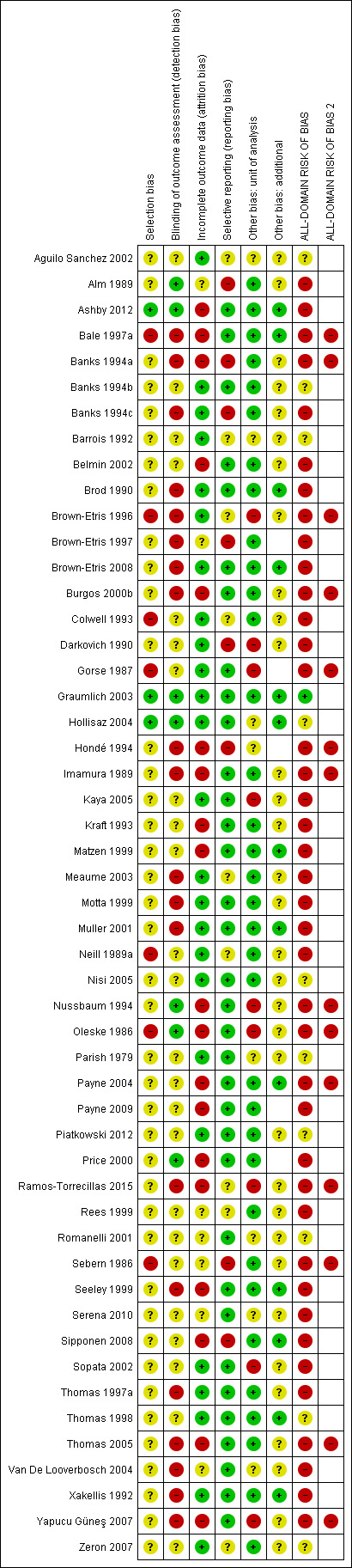
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
We judged only one of the 51 studies (2%) to be at low risk of bias (Graumlich 2003) and ten (20%) to have unclear risk of bias (Aguilo Sanchez 2002; Banks 1994b; Barrois 1992; Hollisaz 2004; Nisi 2005*; Parish 1979; Piatkowski 2012; Romanelli 2001; Thomas 1998; Zeron 2007). We judged 14 (27%) studies to be at very high risk of bias, that is, to have high risk of bias for two or more domains (Bale 1997a; Banks 1994a; Brown‐Etris 1996; Burgos 2000b; Gorse 1987*; Hondé 1994; Imamura 1989*; Nussbaum 1994*; Oleske 1986; Payne 2004*; Ramos‐Torrecillas 2015*; Sebern 1986*; Thomas 2005; Yapucu Güneş 2007*). We assessed the rest of the studies at high risk of bias. We grouped the low and unclear categories together.
*Studies marked with an asterisk were not included in the individual network.
Effects of interventions
See: Table 1
In this section, we present the results for the individual NMA. Results for the group network are given in Appendix 5.
We report the results in two ways. Firstly, we give risk ratios (RR) with their 95% CIs for each intervention compared with every other intervention in the network (NMA effect estimates); all results are presented in a forest plot, but we focus on a representative set of comparisons versus a reference intervention (saline gauze for the individual network). Secondly, we summarise findings for the network as a whole, giving the rank order for all the interventions in the network and the probability that a particular intervention is the best, second best, etc treatment.
We report the results alongside the assessment of evidence quality. To do this we applied various statistical techniques and tests, including methods for determining risk of bias in the whole network, examining whether the results for each comparison in the network were consistent with one another, and considering the uncertainty in various measures (e.g. the CI around the RR). For the latter, we downgraded evidence twice if the 95% CI crossed both of the two GRADE 'default' values (RR = 1.25 and RR = 0.75) and once if the 95% CI crossed one of these values. Additionally, if there was a large effect and there were very few events in the direct evidence for a particular intervention, we downgraded the evidence further ('fragility'; see Data synthesis, Quality assessment). We also conducted some sensitivity analyses to test assumptions made in the analysis. Much of the assessment of evidence quality is reported in Appendices, but is summarised in 'Summary of findings' tables for the comparisons with the reference intervention.
Interventions and comparisons
The individual network comprised 21 interventions: 13 eligible dressings (foam, hydrocolloid, alginate, protease‐modulating, iodine‐containing, soft polymer, vapour‐permeable, silicone‐foam combination, two alginate‐hydrocolloid combination or sequential dressings, saline gauze, polyvinylpyrrolidone plus zinc oxide and basic wound contact); six topical agents (hydrogel, dextranomer, collagenase ointment, phenytoin, tripeptide copper gel, and sugar plus egg white) and two supplementary linking interventions (skin substitute and radiant heat dressing).
Two studies were three‐arm trials: Hollisaz 2004 (hydrogel, phenytoin and saline gauze); and Parish 1979 (dextranomer, collagenase ointment, and sugar plus egg white). The total number of comparisons was 43, encompassing a total of 2127 participants, who experienced a total of 783 events (complete healing) ‐ this is 72% of the participants included in all studies in the review before we excluded studies for not fitting into the network. There were 27 different direct contrasts and eight triangular loops, including one that was exclusive to one of the three‐arm trials (Parish 1979).
In the network diagram (Figure 2), node (circle) size reflects weighting according to the number of studies reporting each intervention and the thickness of the edge lines reflects weighting according to the inverse variance of the direct treatment effect estimates for the particular contrast (Chaimani 2013b). We identified seven interventions as 'core interventions' (i.e. part of at least one loop: foam dressing, hydrocolloid dressing, hydrogel, iodine‐containing dressing, phenytoin, protease‐modulating dressing and saline gauze). The other interventions were only connected in a peripheral way.
Risk of bias for the individual network
We report risk of bias in three ways (see Methods: Assessment of risk of bias in included studies):
For each study, as the all‐domain risk of bias ‐ taking into account selection bias, detection bias, attrition bias, reporting bias and other bias
For each direct comparison of two interventions, as an overall risk of bias ‐ taking into account the all‐domain risk of bias for the studies (1 above) and the weighting in the meta‐analysis for that comparison
For each contrast in the network (any pair of interventions in the network) as the overall risk of bias ‐ taking into account the risk of bias for each direct comparison (2 above) and their percentage contributions to the network estimate. We also calculated the overall risk of bias in the network as a whole.
All‐domain risk of bias for each study is shown in Figure 3. We judged one study to be at low risk of bias (Graumlich 2003) and nine at unclear risk of bias (Aguilo Sanchez 2002; Banks 1994b; Barrois 1992; Hollisaz 2004; Parish 1979; Piatkowski 2012; Romanelli 2001; Thomas 1998; Zeron 2007). Seven were at very high risk of bias (Bale 1997a; Banks 1994a; Brown‐Etris 1996; Burgos 2000b; Hondé 1994; Oleske 1986; Thomas 2005) and the rest we assessed to be at high risk of bias. We grouped the low and unclear categories together.
We have indicated the overall risk of bias for each direct comparison in Figure 2, using colour for three risk of bias ratings: low/unclear (green), high (yellow), very high (red). There is a relatively large amount of direct evidence at high or very high risk of bias.
For each contrast in the network, we calculated the overall risk of bias as described in Appendix 8, and the 'Risk of bias' ratings are shown beside the results in Figure 4.
4.
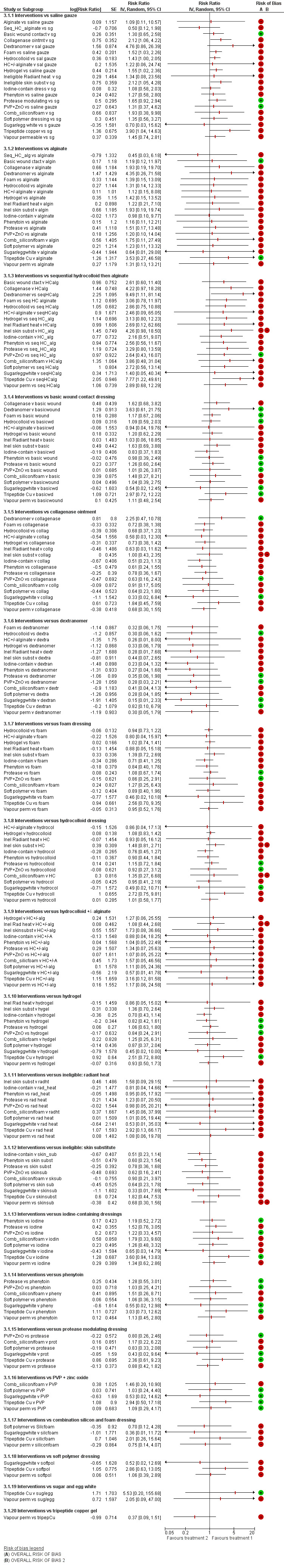
NMA results: individual intervention 1 versus individual intervention 2 Key for overall risk of bias for the contrast: green = low/unclear; one red = high; two reds = very high
Network meta‐analysis results
The NMA generated results for 210 mixed treatment contrasts (i.e. all possible pairwise combinations of the interventions). The data were sparse and there was much uncertainty.
Figure 4 shows all NMA results, with the all‐domain risk of bias shown alongside the forest plot contrasts.
As a consequence of the sparseness in the network, no contrast had precise estimates, all CIs were wide or very wide and we downgraded all evidence at least once for imprecision, some because of 'fragility' (Figure 4). The majority of the evidence for each contrast was informed by studies at high or very high risk of bias. Across all the mixed treatment contrasts, there was only one that we assessed to have moderate‐certainty evidence (downgraded once only): protease‐modulating dressing versus saline gauze. Evidence for all other contrasts was of low or very low certainty, and the moderate‐certainty evidence should also be interpreted in the light of the very low‐certainty evidence for the network from which it was derived.
As a summary, we presented the evidence for the individual mixed treatment contrasts using a representative set of each intervention versus saline gauze (Table 1) and Figure 4, first subgroup of results); we did not include the ineligible interventions (radiant heat and skin substitute) in the 'Summary of findings' table. Further details of information used for GRADE assessment can be found in Appendix 8 and Appendix 9).
It is not clear whether protease‐modulating dressings increase the probability of pressure ulcer healing, compared with saline gauze dressings (RR 1.65; 95% CI 0.92 to 2.94, moderate certainty evidence). This corresponds to an absolute risk difference of 102 more people healed per 1000 (95% CI 13 fewer to 305 more), for a saline gauze median probability of healing of 157 per 1000. We downgraded the evidence once for imprecision (low risk of bias).
For each of four contrasts, it is unclear whether the intervention increases the probability of healing compared with saline gauze dressings: collagenase ointment (RR 2.12; 95% CI 1.06 to 4.22); foam dressing (RR 1.52; 95% CI 1.03 to 2.26); basic wound contact dressing (RR 1.30; 95% CI 0.65 to 2.58) and polyvinylpyrrolidone (PVP) plus zinc oxide (RR 1.31; 95% CI 0.37 to 4.62) (Figure 4). In each of these four contrasts, the evidence was graded as low certainty; downgraded either once for imprecision and once for risk of bias (collagenase ointment and foam dressing) or twice for imprecision and not for risk of bias (basic wound contact dressing and PVP plus zinc oxide).
It is unclear whether there is a difference in the probability of healing associated with the following interventions compared with saline gauze for the remaining 13 contrasts because the evidence is of very low certainty (downgraded mainly for risk of bias (once) and imprecision (twice)): alginate dressings, sequential hydrocolloid alginate dressings, dextranomer, hydrocolloid dressing with/without alginate filler (given if the wound was highly exudative), hydrocolloid dressings, hydrogel, iodine‐containing dressings, phenytoin, silicone‐foam dressings, soft polymer dressings, sugar plus egg white, tripeptide copper gel and vapour‐permeable dressings. Two contrasts were informed by very few participants in the direct evidence, with seven participants (4 events) receiving dextranomer and six participants (5 events) receiving tripeptide copper gel; we therefore downgraded imprecision twice to allow for the fragility this invoked. There was also heterogeneity or inconsistency, or both, for some contrasts.
Ranking of treatments
The NMA produced a large number of estimates. An alternative way of presenting and interpreting data from the whole NMA was to summarise using rankograms: data for each intervention were shown as the probability that each intervention is the best, second best, third best treatment, etc. These probabilities are based on uncertainty, reflecting the effectiveness from the network contrasts and the precision around the estimates. The closer the probability of a rank to 100% (or 0%) and the narrower the distribution across different ranks, the greater the confidence in the ranking. Results are given in Figure 5 and Appendix 10 and summarised here, but must be interpreted in the light of the considerable uncertainty and sparseness in the network and the individual estimates, giving potentially misleading results (see quality assessment below). Numerically, dextranomer and tripeptide copper gel had the highest probabilities of being the best treatments (41% and 25%, respectively), and the sequential hydrocolloid alginate dressings and sugar plus egg white were most likely to be the worst treatments (35% and 32%, respectively). No intervention had more than 50% probability of being the best treatment and the rankograms for each treatment show considerable overlap. However, these rankings are likely to be artificially high: the direct evidence for dextranomer and tripeptide copper involves one study each with, respectively, seven participants (4 events) and six participants (5 events). The NMA results for these peripheral interventions have wide CIs and large point estimates. Consequently, these interventions have a finite probability of having a very large effect estimate (at their upper confidence limit), in turn leading to an artificially high probability of being the best treatment.
5.
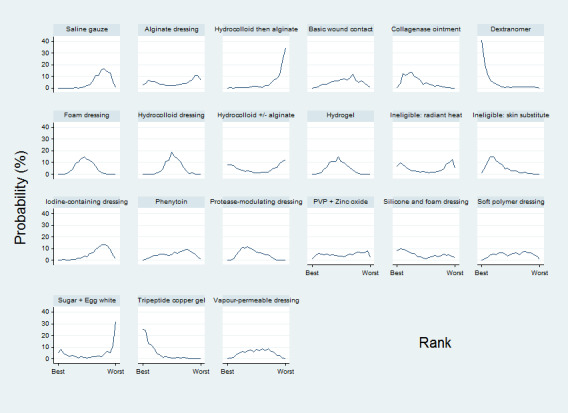
Rankograms for each intervention ‐ individual network
Comparison of results from standard meta‐analysis with NMA findings
We compared the NMA results with the direct comparison (pairwise) results for the proportion completely healed for the 27 different comparisons informing the individual network (Table 3). Six of these comparisons had two or more direct comparison studies (Analysis 1.1; Analysis 1.2). The direct comparison evidence shows heterogeneity for the comparisons of hydrocolloid dressing versus saline gauze dressing; hydrogel versus saline gauze dressing and hydrogel versus hydrocolloid dressing. Direct comparison evidence results for the time‐to‐healing outcome are reported in Appendix 11 for six comparisons in seven studies. The results for the direct comparison evidence and the NMA are shown in Table 3: there is too much uncertainty (wide CIs) to determine whether there are differences.
2. Direct comparisons for individual interventions ‐ proportion healed ‐ compared with NMA results.
| Contrast/comparison | Number of studies (participants) |
RR (95% CI) direct evidence Random‐effects (inverse variance) Heterogeneity statistics |
NMA results (consistency assumption) RR (95% CI) |
|
Hydrocolloid dressing versus saline gauze dressing (Alm 1989; Colwell 1993; Neill 1989a; Xakellis 1992) |
4 (279) | 1.89 (0.91 to 3.93) Tau² = 0.35; P = 0.01; I² = 73% |
1.43 (1.00 to 2.05) |
|
Hydrogel versus saline gauze dressing (Hollisaz 2004; Matzen 1999; Thomas 1998) |
3 (110) | 2.44 (0.64 to 9.27) Tau² = 0.90; P = 0.03; I² = 71% |
1.55 (1.02 to 2.36) |
|
Foam dressings versus saline gauze dressing (Kraft 1993; Oleske 1986; Payne 2009) |
3 (93) | 1.51 (0.78 to 2.90) P = 0.41; I² = 0% |
1.52 (1.03 to 2.26) |
| Phenytoin versus saline gauze dressing (Hollisaz 2004) | 1 (40) | 3.02 (0.97 to 9.35) | 1.27 (0.58 to 2.80) |
|
Hydrogel versus hydrocolloid dressings (Brod 1990; Brown‐Etris 1996; Darkovich 1990; Motta 1999) |
4 (322) | 1.11 (0.74 to 1.67) Tau² = 0.08; P = 0.11; I² = 51% |
1.08 (0.83 to 1.42) |
|
Foam dressing versus hydrocolloid dressing (Aguilo Sanchez 2002; Bale 1997a; Banks 1994a; Banks 1994c; Seeley 1999; Thomas 1997a) |
6 (292) | 1.05 (0.81 to 1.36) Tau² = 0.00; P = 0.67; I² = 0% (Stata: 1.05 (0.73 to 1.23)) |
1.07 (0.82 to 1.38) |
|
Collagenase ointment versus hydrocolloid dressing (Burgos 2000b; Muller 2001) |
2 (61) | 1.51 (0.93 to 2.43) P = 0.61; I² = 0% |
1.48 (0.81 to 2.69) |
|
Iodine‐containing dressing versus hydrocolloid dressing (Barrois 1992) |
1 (76) | 0.90 (0.41 to 1.96) | 0.76 (0.45 to 1.27) |
|
Protease‐modulating dressing versus hydrocolloid dressing (Graumlich 2003) |
1 (65) | 1.03 (0.64 to 1.66) | 1.15 (0.72 to 1.84) |
|
Vapour‐permeable dressing versus hydrocolloid dressing (Brown‐Etris 2008) |
1 (72) | 1.01 (0.69 to 1.47) | 1.01 (0.58 to 1.77) |
|
Hydrocolloid dressing 4 weeks then alginate dressing 4 weeks versus hydrocolloid dressing (Belmin 2002) |
1 (110) | 0.35 (0.10 to 1.25) | 0.35 (0.09 to 1.33) |
|
Ineligible intervention: skin substitute versus hydrocolloid dressing (Hondé 1994) |
1 (168) | 1.48 (0.95 to 2.32) | 1.48 (0.81 to 2.71) |
| Foam dressing versus hydrogel (Sopata 2002) | 1 (38) | 1.11 (0.80 to 1.54) | 0.98 (0.71 to 1.36) |
|
Tripeptide copper versus hydrogel (Romanelli 2001) |
1 (12) | 2.50 (0.76 to 8.19) | 2.51 (0.72 to 8.80) |
|
Iodine‐containing dressing versus hydrogel (Kaya 2005) |
1 (49) | 0.64 (0.43 to 0.97) | 0.70 (0.43 to 1.14) |
| Phenytoin versus hydrogel (Hollisaz 2004) | 1 (39) | 0.71 (0.41 to 1.24) | 0.82 (0.42 to 1.61) |
|
Foam dressing versus protease‐modulating dressing (Piatkowski 2012) |
1 (10) | 0.82 (0.49 to 1.38) | 0.93 (0.57 to 1.49) |
| Alginate dressing versus protease‐modulating dressing (Brown‐Etris 1997) | 1 (36) | 0.67 (0.08 to 5.75) | 0.30 (0.07 to 1.25) |
|
PVP + zinc oxide versus protease‐modulating dressing (Zeron 2007) |
1 (24) | 0.80 (0.28 to 2.27) | 0.80 (0.26 to 2.46) |
|
Soft polymer dressing versus foam dressing (Meaume 2003) |
1 (38) | 0.89 (0.45 to 1.75) | 0.89 (0.40 to 1.96) |
|
Combination silicone‐foam dressing versus ineligible intervention: skin substitute (Serena 2010) |
1 (74) | 0.91 (0.22 to 3.77) | 0.90 (0.21 to 3.97) |
|
Hydrocolloid with/without alginate filler versus ineligible intervention: radiant heat (Thomas 2005) |
1 (41) | 0.92 (0.41 to 2.06) | 0.92 (0.37 to 2.27) |
|
Collagenase ointment versus dextranomer (Parish 1979) |
1 (12) | 0.35 (0.05 to 2.26) (Stata 0.44 (0.10 to 2.02)) |
0.44 (0.09 to 2.13) |
|
Collagenase ointment versus sugar + egg white (Parish 1979) |
1 (10) | 3.00 (0.15 to 59.89) (Stata 3.00 (0.15 to 59.79)) |
3.00 (0.15 to 61.59) |
|
Dextranomer versus sugar + egg white (Parish 1979) |
1 (12) | 6.75 (0.44 to 102.80) | 6.75 (0.43 to 105.99) |
|
Foam dressing versus basic wound contact dressing (Banks 1994b) |
1 (50) | 1.17 (0.79 to 1.72) | 1.17 (0.67 to 2.06) |
|
Alginate dressing versus ineligible intervention: radiant heat (Price 2000) |
1 (58) | 0.82 (0.15 to 4.55) | 0.82 (0.14 to 4.77) |
1.1. Analysis.
Comparison 1 Direct evidence: individual interventions, number with complete healing, Outcome 1 Interventions vs saline gauze.
1.2. Analysis.
Comparison 1 Direct evidence: individual interventions, number with complete healing, Outcome 2 Interventions vs hydrocolloid.
Certainty/quality assessment of the evidence across the whole network
The weighted average risk of bias across the network was high (Appendix 8). There did not appear to be much inconsistency in the network (see Appendix 9) and there were relatively few contrasts with conflicting results for direct and indirect or NMA estimates, so across the network we did not downgrade for inconsistency. We downgraded the evidence twice for imprecision: in addition to the sparseness (and probably as a consequence of it), there is substantial overlap of the individual rankograms (see Appendix 10); the mean rank was no smaller than 3.6 and no larger than 18.6 (out of 21) for any intervention, with no SUCRA value being zero or 1 (indicating uncertainty). A contour‐enhanced funnel plot is shown in Figure 6. There may be a small studies effect, but this was too unclear for downgrading. Overall, we classed the evidence for the whole network as being of very low certainty (downgraded once on risk of bias and twice on imprecision).
6.
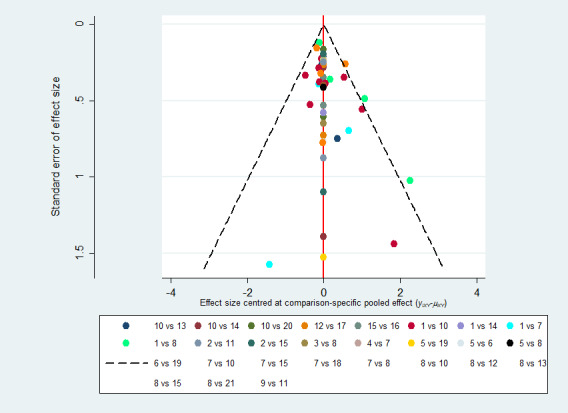
Funnel plot ‐ individual network
Key to interventions: 1: saline gauze; 2: alginate dressing; 3: sequential hydrocolloid alginate dressings; 4: basic wound contact dressing; 5: collagenase ointment; 6: dextranomer; 7: foam dressing; 8: hydrocolloid dressing; 9: hydrocolloid +/‐ alginate (hydrocolloid dressing with/without alginate filler); 10: hydrogel dressing; 11: ineligible radiant heat; 12: ineligible skin substitute; 13: iodine‐containing dressing; 14: phenytoin; 15: protease‐modulating dressing; 16: PVP + zinc oxide 17: silicone + foam dressing; 18: soft polymer dressing; 19: sugar + egg white; 20: tripeptide copper gel; 21: vapour‐permeable dressing
Overall, we have little confidence in the findings in this network, either in terms of the effect estimates or in the ranking of interventions.
Sensitivity analyses
We carried out the following pre‐specified sensitivity analyses to examine the above inconsistencies: excluding studies at very high risk of bias; and assuming an available case analysis rather than imputing no event for missing values. The sensitivity analyses are discussed in Appendix 12. Neither sensitivity analysis had much impact on the effect estimates or the rankograms. There appeared to be less inconsistency in the sensitivity analysis that excluded studies at very high risk of bias, but this possible improvement was at the expense of precision and resulted a smaller network, and so the original analysis was preserved. An additional post‐hoc sensitivity analysis (Appendix 12) examined the original assumption of combining topical agents and dressings in the same NMA, by restricting the network to studies comparing any two eligible dressings ‐ similar results were found for the contrasts versus saline gauze, and the imprecision in the overall network continued to give uncertainty.
Group network findings
We mapped individual interventions onto the group categories (Appendix 5), grouping together dressings into the following pre‐specified categories: basic wound dressings, advanced dressings and antimicrobial dressings (as described in the BNF 2016), and keeping specialist dressings (e.g. protease‐modulating matrix dressings) and the different topical agents as separate categories. The group network included 22 studies (of 51 included) in 946 participants, encompassing 10 different interventions in 12 direct contrasts and these informed 45 mixed treatment contrasts. The median (range) study size was 38.5 (10 to 100). We had hoped that grouping interventions might increase the power in the network, but fewer than half of the included studies formed the group network (see Appendix 5) and only 32% of the participants were involved; only three contrasts were informed by more than one study.
The group NMA generated results for 45 mixed treatment contrasts. The network was dominated by the advanced dressing versus basic dressing contrast and the rest of the data were sparse. Figure 7 shows all group NMA results, with the all‐domain risk of bias shown alongside the forest plot contrasts. The results and the certainty of the evidence are summarised for a representative set of contrasts (each intervention versus basic dressing) in Table 4. Evidence was of low or very low certainty, with the exception of one contrast, for which we assessed the evidence to be of moderate certainty. As for the individual network, this moderate‐certainty evidence should be interpreted in the light of the very low‐certainty evidence for the network as a whole.
7.
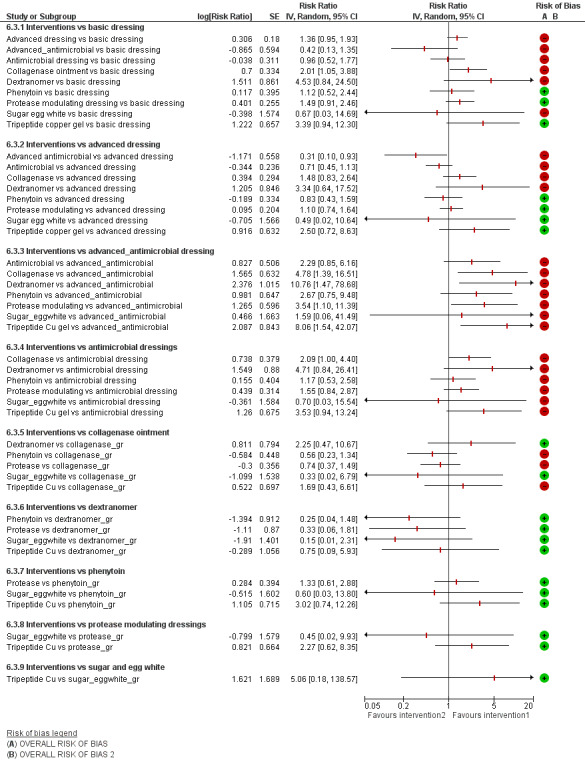
Intervention 1 versus intervention 2 ‐ group network Key for overall risk of bias for the contrast: green = low/unclear; one red = high; two reds = very high
3. NMA evidence for group network: proportion with complete healing ‐ interventions versus basic dressings.
| NMA evidence for group network: proportion with complete healing ‐ interventions versus basic dressings | ||||
|
Patient or population: people with pressure ulcers
Intervention: dressing or topical agent
Comparison: basic dressing Settings: hospital, community or care home, or combinations | ||||
|
Contrasts: interventions versus basic dressing |
Relative effect (95% CI) |
Anticipated absolute effects* (95% CI) ‐ from median of basic dressing control groups in direct evidence |
Certainty (quality) of the evidence (GRADE) | |
| Median CGR | With interventions | |||
| Advanced dressings versus basic dressing | RR 1.36 (0.95 to 1.93) | 191 per 1000 | 260 per 1000 (181 to 369) | ⊕⊝⊝⊝ Very low1 |
|
69 more people healed per 1000 (from 10 fewer to 178 more) | ||||
|
Advanced + antimicrobial dressing versus basic dressing |
RR 0.42 (0.13 to 1.35) | 191 per 1000 | 80 per 1000 (25 to 258) | ⊕⊝⊝⊝ Very low2 |
|
111 fewer people healed per 1000 (from 67 more to 166 fewer) | ||||
| Antimicrobial dressing versus basic dressing | RR 0.96 (0.52 to 1.77) | 191 per 1000 | 183 per 1000 (99 to 338) | ⊕⊝⊝⊝ Very low2 |
|
8 fewer people healed per 1000 (from 92 fewer to 147 more) | ||||
| Collagenase ointment versus basic dressing | RR 2.01 (1.05 to 3.88) | 191 per 1000 | 384 per 1000 (201 to 741) | ⊕⊕⊝⊝ Low3 |
|
193 more people healed per 1000 (from 10 more to 550 more) | ||||
| Dextranomer versus basic dressing | RR 4.53 (0.84 to 24.50) | 191 per 1000 | 865 per 1000 (160 to 1000) | ⊕⊝⊝⊝ Very low4 |
|
674 more people healed per 1000 (from 31 fewer to 1,000 more) | ||||
| Phenytoin versus basic dressing | RR 1.12 (0.52 to 2.44) | 191 per 1000 | 214 per 1000 (99 to 466) | ⊕⊝⊝⊝ Very low5 |
|
23 more people healed per 1000 (from 92 fewer to 275 more) | ||||
| Protease‐modulating dressing versus basic dressing | RR 1.49 (0.91 to 2.46) | 191 per 1000 | 285 per 1000 (174 to 470) | ⊕⊕⊕⊝ Moderate6 |
|
94 more people healed per 1000 (from 17 fewer to 279 more) | ||||
| Sugar + egg white versus basic dressing | RR 0.67 (0.03 to 14.69) |
191 per 1000 | 128 per 1000 (6 to 1000) | ⊕⊝⊝⊝ Very low2 |
|
63 fewer people healed per 1000 (from 185 fewer to 1000 more) | ||||
| Tripeptide copper gel versus basic dressing | RR 3.39 (0.94 to 12.30) | 191 per 1000 | 647 per 1000 (180 to 1000) | ⊕⊕⊝⊝ Low7 |
|
456 more people healed per 1000 (from 11 fewer to 1000 more) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CGR: control group risk; CI: confidence interval; RR: risk ratio | ||||
| GRADE Working Group grades of evidence High certainty (quality): We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty (quality): We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty (quality): Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty (quality): We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
1Majority of evidence at high risk of bias (downgraded once); inconsistency: heterogeneity in direct evidence (downgraded once); imprecision: wide CI (downgraded once). 2Majority of evidence at high risk of bias (downgraded once); imprecision: very wide CI (crosses 0.75 and 1.25) (downgraded twice). 3Majority of evidence at high risk of bias (downgraded once); imprecision: wide CI (crosses 1.25) and direct evidence on collagenase from three studies, 11 events (downgraded once). 4Majority of evidence at high risk of bias (downgraded once): imprecision: wide CI (crosses 1.25) and direct evidence on dextranomer from one study, seven participants and four events (downgraded twice). 5Inconsistency: significant difference between direct and indirect estimates (downgraded once); imprecision: very wide CI (crossed 0.75 and 1.25). 6Imprecision: wide CI (crosses 1.25) (downgraded once). 7Imprecision: wide CI (crosses 1.25) and direct evidence on tripeptide copper gel from one study, six participants and five events (downgraded twice).
Rankograms for the group network are shown in Figure 8. There was more of a distinction between interventions, but still overlap of rankograms and the improvement in precision came at the expense of increased inconsistency and possible publication bias (Figure 9). Overall we downgraded the evidence certainty three times for the network as a whole, because of risk of bias (once), imprecision (once) and inconsistency and publication bias (once). As in the individual network, dextranomer and tripeptide copper had high ranks and this was again likely to be an artificial result. Further details of the group network are given in Appendix 5.
8.
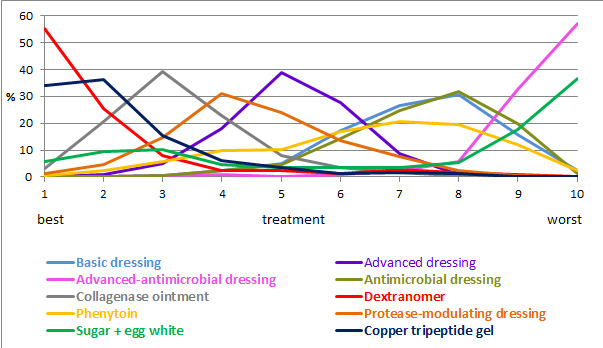
Rankograms combined ‐ group network
9.
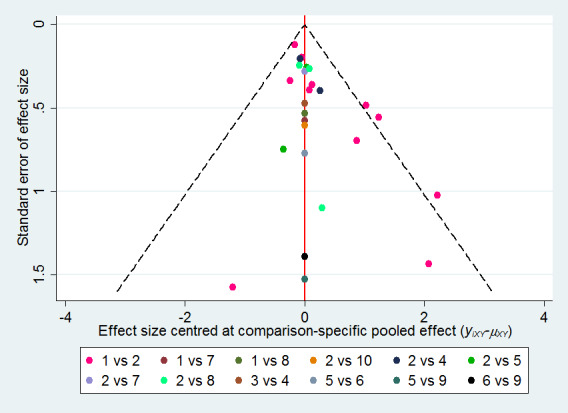
Funnel plot ‐ group network
Key to interventions: 1: basic dressing; 2: advanced dressing; 3: advanced or antimicrobial dressing; 4: antimicrobial dressing; 5: collagenase ointment; 6: dextranomer; 7: phenytoin; 8: protease‐modulating dressing; 9: sugar + egg white; 10: tripeptide copper gel
Discussion
Summary of main results
We have successfully conducted a network meta‐analysis of dressings and topical agents for healing pressure ulcers. Alongside the analysis we have applied a new method of GRADE assessment (Salanti 2014), which allows us to view the results in the light of our certainty in their findings. Using this approach, we found the majority of the evidence to be of low or very low certainty, and was mainly downgraded for risk of bias and imprecision (see Quality of the evidence). This level of uncertainty within the totality of the dataset impacts on all subsequent interpretation of its outputs.
This review includes 51 RCTs involving a total of 2964 participants, comparing 39 different dressings or topical agents for the healing of pressure ulcers. Most of the studies were in older participants, but four included participants with spinal cord injuries and one was in younger people said to be chronically ill or physically disabled. Seventeen (33%) studies included participants mainly with Stage 2 pressure ulcers and 15 (29%) mainly had Stage 3 pressure ulcers; 13 studies investigated treatment of ulcers with a mean duration of less than three months.
We treated each topical agent as a separate intervention, but initially grouped dressings by class as described in the BNF 2016 (e.g. alginates, hydrocolloids). The network involved 39 studies in 2116 participants, encompassing 21 different interventions in 27 direct contrasts and these informed 210 mixed treatment contrasts.
We reported the evidence in two ways, firstly, as effect estimates for each of 210 NMA mixed treatment contrasts , and secondly as rank order of interventions. We summarised the set of effect estimates using contrasts versus saline gauze.
Overall findings reflect the uncertainty of the component evidence and the sparseness of the network, and even moderate ratings should be interpreted in the context of the network uncertainty. For network contrasts involving saline gauze, it is not clear whether protease‐modulating dressings result in more healing (RR 1.65, 95% CI 0.92 to 2.94; moderate certainty evidence). It is unclear whether four interventions increase the probability of healing compared with saline gauze dressings: collagenase ointment RR 2.12 (95% CI 1.06 to 4.22); foam dressing RR 1.52 (95% CI 1.03 to 2.26); basic wound contact dressing RR 1.30 (95% CI 0.65 to 2.58) and PVP plus zinc oxide RR 1.31 (95% CI 0.37 to 4.62) (all low certainty evidence). It is worth noting that the contrasts for the latter two interventions had CIs consistent with both a clinically important benefit and a clinically important harm, and the other two contrasts had both high risk of bias and some imprecision. The remaining contrasts were all very low‐certainty evidence, with all being imprecise, often with CIs consistent with both a clinically important increase and a clinically important decrease in the probability of healing.
Relative to the median control group risk (probability) (CGR) of healing for saline gauze of 157 per 1000, the absolute risk differences for the above comparisons in the individual network were: protease‐modulating dressings: 102 more people healed per 1000 (13 fewer to 305 more); foam dressings: 82 more per 1000 (5 more to 196 more); collagenase ointment 176 more per 1000 (9 more to 506 more); basic wound contact dressing: 47 more per 1000 (55 fewer to 250 more); polyvinylpyrrolidone plus zinc oxide: 49 more per 1000 (99 fewer to 575 more). Thus, uncertainty notwithstanding, the effect is relatively small and fairly large numbers of wounds remain unhealed.
For the network as a whole, the evidence was of very low certainty, reflecting the general uncertainty surrounding the mixed treatment contrasts, as described above. There was considerable uncertainty in the ranking of interventions and no intervention had more than 50% probability of being the best treatment.
Overall completeness and applicability of evidence
The network is sparse, in terms of the total number of participants, the total number of wounds healed, the number of studies per contrast, the size of the constituent studies and the duration of follow‐up: 21 of 27 direct contrasts were informed by only one study and the average number of events per mixed treatment contrast was around four. The median (range) study size was 41 (10 to 168) and several studies had zero events. The duration of follow‐up was relatively short for most studies (median 8 weeks): only 3/39 studies in the network had a follow‐up duration of 16 weeks or more.
In parallel we conducted a second NMA, grouping together some classes of dressings. We had hoped that the group network would provide more power in the analysis, but in practice too many data were excluded from the network, and the network was also unbalanced, being dominated by the advanced dressing versus basic dressing contrast, which involved about 55% of the participants in the group network. The group network provided equally uncertain evidence and the findings are not discussed further here, but are reported in Appendix 5 for the interested reader.
There may have been small‐study effects, and the contour‐enhanced funnel plot appeared to show some asymmetry. The Chaimani 2013b methodological paper demonstrated that small‐study effects can materially affect the rank order of effectiveness. STATA code is available to adjust for small‐study effects in ranking, however, we did not investigate this approach because the evidence was of such low certainty for reasons of risk of bias, imprecision and inconsistency. Additionally, Kibret 2014 suggested in a simulation study in a Bayesian setting that an unequal number of studies per comparison may result in biased estimates of treatment rank probabilities.
In the absence of evidence for effect modifiers, we can make observations about the population covered and the trial duration, only approximating the applicability of the evidence. In particular, there were eight studies with a follow‐up time of less than six weeks, which may be too short to properly investigate healing, and the reporting of time‐to‐event data was insufficient to understand how the hazard of healing changes over time. Whilst treatments may have impacted on the speed of wound healing as well as the number of healing events per se, this requires further exploration, which would be better supported by increased collection and analysis of time‐to‐healing data in wound care trials. We note that the two small three‐arm trials, which may have shown some incongruent results, were in younger people with spinal cord injuries or chronic illnesses/physical disabilities. Overall, our view is that the results can probably be applied more generally, within the constraints of the uncertainty of the evidence and also the comparisons for which trial data exist. There are many different dressing and topical treatment choices and, whilst several key treatments are represented by trial data, others are addressed only in pilot studies and there may be treatments that are yet to be evaluated in a trial or for which data remain unpublished. We could only assess publication bias in a limited way.
The NMA focused on complete wound healing as the key outcome ‐ this has repeatedly been found to be the most important outcome to patients and health professionals (Cullum 2016; Kelly 2015). Dressings and topical agents are generally low risk treatments so we did not consider adverse events. Other outcomes that might have been useful include those related to the management properties of dressings such as ease of use, exudate management and pain on removal. We did not consider these in the NMA for practical reasons: such outcomes are reported inconsistently with data that rarely allow meta‐analysis. Given that the quality assessment of healing data was based on study‐level issues like small samples and flawed methodology, we can suppose the quality of other outcome data would have been equally sparse and likely uncertain.
Quality of the evidence
We have explored the application of a new approach to GRADE analysis, alongside NMA in STATA (Chaimani 2013b; Salanti 2014). We applied the GRADE approach separately to effect estimates for different contrasts and to the ranking of interventions, but the two aspects are closely inter‐related and, in this review, are a consequence of the sparse network and the high risk of bias through much of the network. The effect estimates were exemplified by contrasts of interventions versus saline gauze.
For the effect estimates' assessment, most of the evidence was of very low certainty (very low quality). The GRADE meaning of 'low‐certainty evidence' is that "our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect". 'Very low‐certainty evidence' means "We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect". 'Moderate‐certainty evidence' means "We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different" (Balshem 2011). Exceptions to an assignment of very low certainty were found for contrasts with protease‐modulating dressings (moderate certainty); collagenase ointment, basic wound contact dressing, foam dressing and PVP plus zinc oxide (low‐certainty evidence), We downgraded evidence certainty mainly because of risk of bias and imprecision, although there was inconsistency for the contrasts hydrogel and hydrocolloid versus saline gauze and phenytoin versus saline gauze. Having said this, we are uncertain about the inconsistency assessment because of wide CIs around the test parameters. The majority of the comparisons with saline gauze had high risk of bias. However, a few contrasts had evidence solely downgraded on the basis of wide confidence intervals, that is, random error (protease‐modulating dressings, basic wound contact dressing and PVP plus zinc oxide, each in comparisons with saline gauze). The sparseness of the network led to widespread imprecision in the effect estimates. Although we rated the evidence for one contrast as moderate‐certainty, this result should be interpreted in the context of the network as a whole and not taken as an implication for practice.
Across the network as a whole, the evidence was of very low certainty. There was overall high risk of bias and overlap of the ranking probability distributions, and no clearcut results. The evidence was of such poor quality that we consider it inappropriate to focus on which treatments had the highest probabilities of healing (see also Potential biases in the review process).
Potential biases in the review process
This was a sparse network and there may have been small‐study effects which impacted on the network (see Overall completeness and applicability of evidence). The STATA routines have largely been developed for and tested on larger networks, and our work has contributed to modifications for sparse networks in the netweight routine. Other STATA routines can be modified by the user to take into account small‐study effects, but we did not explore these approaches because there was too much uncertainty in the network for us to be confident of interpreting the results. Instead, we used the standard routines for NMA and adapted the recent approach to GRADE (Salanti 2014) to bring in sparseness when assessing evidence certainty.
The recent GRADE approach has not been applied in many NMA reviews so far, and so could give potential for bias. We judge that it is a useful approach for many of the GRADE factors, however, there is one area in which we consider imprecision is underestimated: the GRADE method does not currently have a way of assessing optimum information size and 'fragility' of the confidence intervals when there are large effect estimates with wide CIs; such effects can result when the direct evidence for a particular intervention derives from very small studies peripheral to the network. Wide CIs can lead some interventions to have a finite probability of having a very large effect estimate, in turn leading to an artificially high probability of being the best treatment. For example, there were only seven participants who actually received dextranomer, yet this intervention was the most highly ranked, and effect estimates versus other treatments were largest for dextranomer. Numerically, when we consider the direct evidence for dextranomer versus collagenase ointment, for example, a missed healing diagnosis for just one person treated with collagenase could change the risk ratio by 50%. This, in turn, could affect the ranking and effect estimates of other contrasts with dextranomer. It was important to capture this potential bias in the review process, and we therefore produced a modification to the GRADE process to enable the 'sample size' of the direct evidence to be considered in a way analogous to the GRADE 'fragility' effects in pairwise meta‐analysis (Guyatt 2011b). Our approach does not change the magnitude of the effect estimate or ranking order, rather it allows us to represent our uncertainty around these values.
A further effect of the sparseness of the network may have been to hide any inconsistencies. The various statistical tests for inconsistency were generally not significant, but this may have been due to a lack of sensitivity of the tests and the wide CIs around the measures. Despite this, we found inconsistencies in the network for contrasts involving phenytoin. We cannot be sure that there are no other inconsistencies, but this may not matter given the already identified large uncertainties.
We have made some assumptions: firstly, to include dressings and topical agents of various types in the same NMA. This implies that dressings and topical agents fulfil the same role and are exchangeable (i.e. that the participants/wounds receiving topical agents are similar to those receiving dressings). We did a post‐hoc sensitivity analysis, which included only trials comparing two dressings, to investigate this assumption. It gave similar effect estimates and CIs for individual contrasts.
Finally, application of the GRADE approach to this NMA has given a rating of moderate‐certainty evidence for only one contrast in the whole NMA, and we recognise that by using a representative set of comparisons and by applying GRADE rules of thumb, however carefully, we may have inadvertently emphasised the importance of one intervention. This is a limitation of the approach. Instead the evidence on protease‐modulating dressings should be set in the context of the uncertainty in the network as a whole.
Agreements and disagreements with other studies or reviews
We have been unable to identify any network meta‐analyses directed at healing pressure ulcers and incorporating both dressings and topical agents. The AHRQ guideline reviewed the evidence for dressings in a series of pairwise comparisons and stated that overall, they did not find substantial evidence to support certain local wound applications over others (AHRQ 2013). The most recent NICE guideline on the prevention and management of pressure ulcers (NICE 2014) considered all RCT evidence on dressings and separately all RCT evidence on topical agents. NICE recommendations are to not use saline gauze dressings and for the health professional and adult to discuss the type of dressing to use, taking into account pain and tolerance, position of the ulcer, amount of exudate and frequency of dressing change. These recommendations rely heavily on consensus decisions, weakly supported by the evidence, and as such, agree with the findings of this review.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to judge whether any one dressing or topical treatment increases the probability of pressure ulcer healing compared with others (and neither is there sufficient evidence to judge whether there is a negative relative impact on wound healing or no relative impact). None of the interventions with moderate‐ or low‐quality evidence appear to result in a higher proportion of wounds healed. It is important to note that many trials in this review were small and at high risk of bias. Based on current evidence, decision‐makers may wish to make wound dressing choices on the basis of wound symptoms, clinical experience, patient preference and cost.
Implications for research.
There is a lack of high‐quality research evidence regarding whether particular wound dressings or topical treatments have a beneficial impact on wound healing, even compared with basic dressings. This lack of evidence is disturbing in view of the high personal and health service burden of pressure ulcers (and indeed several other types of wounds), and also in view of the many potential participants who could be invited to take part in trials. The network meta‐analysis (NMA) exposes the generally poor quality of randomised controlled trials of pressure ulcer dressings, suggesting a need for radical improvements in the planning and conduct of trials in this field.
Given the high uncertainty across several competing interventions, any investment in future research must maximise its value to decision‐makers. Any future evaluation of interventions for healing pressure ulcers could focus on the dressings or topical agents that health professionals use most widely, with consideration given to protease‐modulating dressings. Any future research should consider time to healing: quicker healing may be as important to people with pressure ulcers as whether healing occurs.
There may be value in asking decision‐makers (including people with pressure ulcers) what they feel are the most important issues, for example, type of dressing, purpose of the dressing/topical agent (including possible evaluation of broader groups of dressings e.g. advanced or basic), or duration that a dressing remains in situ, as well as which outcomes are most important. At a more fundamental level, decision‐makers and funders should decide where research resources are best invested, for example, pressure ulcer treatment or prevention. Such planning means that research resources can be focused to address priorities. Where trials are conducted, good practice guidelines must be followed in their design, implementation and reporting, in particular outcome assessors should be blinded. Studies should be adequately powered and have sufficient follow‐up time to allow healing to occur.
Acknowledgements
The authors would like to acknowledge the contribution of Cochrane Wounds editor, Joan Webster, the peer referees Anne‐Marie Bagnall, Gill Worthy, Emma Ladds, Zena Moore, Linda Faye Lehman and Janet Yarrow and the copy editors Jenny Bellorini and Denise Mitchell. The authors are also grateful to Adolfo Maria Tambella for providing translation services.
Appendices
Appendix 1. Pressure ulcer grading
One of the most widely recognised systems for categorising pressure ulcers is that of the National Pressure Ulcer Advisory Panel (NPUAP). Their international classification recognises four categories or stages of pressure ulcer and two categories of unclassifiable pressure injury, in which wound depth and/or extent, or both, cannot be accurately determined; unclassifiable pressure ulcers are generally severe and would be grouped clinically with Stage 3 or Stage 4 ulcers (EPUAP‐NPUAP‐PPPIA 2014; NPUAP 2016):
Category/Stage 1: Non‐blanchable erythema of intact skin: Intact skin with a localized area of non‐blanchable erythema, which may appear differently in darkly pigmented skin. Presence of blanchable erythema or changes in sensation, temperature, or firmness may precede visual changes. Color changes do not include purple or maroon discoloration; these may indicate deep tissue pressure injury.
Category/Stage 2: Partial‐thickness skin loss with exposed dermis: Partial‐thickness loss of skin with exposed dermis. The wound bed is viable, pink or red, moist, and may also present as an intact or ruptured serum‐filled blister. Adipose (fat) is not visible and deeper tissues are not visible. Granulation tissue, slough and eschar are not present. These injuries commonly result from adverse microclimate and shear in the skin over the pelvis and shear in the heel. This stage should not be used to describe moisture associated skin damage (MASD) including incontinence associated dermatitis (IAD), intertriginous dermatitis (ITD), medical adhesive related skin injury (MARSI), or traumatic wounds (skin tears, burns, abrasions).
Category/Stage 3: Full‐thickness skin loss: Full‐thickness loss of skin, in which adipose (fat) is visible in the ulcer and granulation tissue and epibole (rolled wound edges) are often present. Slough and/or eschar may be visible. The depth of tissue damage varies by anatomical location; areas of significant adiposity can develop deep wounds. Undermining and tunneling may occur. Fascia, muscle, tendon, ligament, cartilage and/or bone are not exposed. If slough or eschar obscures the extent of tissue loss this is an Unstageable Pressure Injury.
Category/Stage 4: Full‐thickness skin and tissue loss: Full‐thickness skin and tissue loss with exposed or directly palpable fascia, muscle, tendon, ligament, cartilage or bone in the ulcer. Slough and/or eschar may be visible. Epibole (rolled edges), undermining and/or tunneling often occur. Depth varies by anatomical location. If slough or eschar obscures the extent of tissue loss this is an Unstageable Pressure Injury.
The two additional categories of unclassifiable wounds are:
Unstageable/unclassified ‐ Obscured full‐thickness skin and tissue loss: Full‐thickness skin and tissue loss in which the extent of tissue damage within the ulcer cannot be confirmed because it is obscured by slough or eschar. If slough or eschar is removed, a Stage 3 or Stage 4 pressure injury will be revealed. Stable eschar (i.e. dry, adherent, intact without erythema or fluctuance) on the heel or ischemic limb should not be softened or removed.
Deep Tissue Pressure Injury ‐ Persistent non‐blanchable deep red, maroon or purple discoloration: Intact or non‐intact skin with localized area of persistent non‐blanchable deep red, maroon, purple discoloration or epidermal separation revealing a dark wound bed or blood filled blister. Pain and temperature change often precede skin color changes. Discoloration may appear differently in darkly pigmented skin. This injury results from intense and/or prolonged pressure and shear forces at the bone‐muscle interface. The wound may evolve rapidly to reveal the actual extent of tissue injury, or may resolve without tissue loss. If necrotic tissue, subcutaneous tissue, granulation tissue, fascia, muscle or other underlying structures are visible, this indicates a full thickness pressure injury (Unstageable, Stage 3 or Stage 4). Do not use DTPI to describe vascular, traumatic, neuropathic, or dermatologic conditions.
Photographs of the different PU stages are included in the US Agency for Healthcare Research and Quality guideline (AHRQ 2013).
Appendix 2. Glossary of NMA terms
Arm‐specific outcomes/arm‐level data: raw outcome data (e.g. mean (SD) or risk) for each arm of the trial (see treatment contrast).
Assumptions for NMA: in common with all meta‐analysis, the true treatment effect across trials is assumed to be described by a fixed‐effect or random‐effects model. Additionally, transitivity is assumed and, concurrently, exchangeability and consistency.
Baseline risk: the absolute risk of the outcome in the 'control' group. This is affected by the presence of prognostic factors. Some authors have used the baseline risk as a proxy effect modifier, but in general the effect estimate (RR/OR/HR) is independent of the baseline risk; on the other hand, the absolute risk difference depends on baseline risk.
Bayesian approach: the explicit quantitative use of external evidence in the design, monitoring, analysis, interpretation of a health‐care evaluation. In the Bayesian paradigm, prior beliefs about parameters in the models are specified and factored into the estimation. Posterior distributions of model parameters are then derived from the prior information and the observed data. In NMA, it is common to use non‐informative priors for effect estimates.
Coherence/consistency: the direct effect estimate (e.g. mean difference or log odds ratio) is the same as the sum of the indirect effect estimates.
Connected network: a group of linked interventions, such that every trial in the network has at least one intervention in common with at least one other trial. Sometimes individual comparisons are not connected to the rest of the network (disconnected network) and can sometimes be joined in by extending the network to include supplementary interventions.
Contour‐enhanced funnel plot: contour‐enhanced funnel plots show areas of statistical significance, and they can help in distinguishing publication bias from other possible reasons for asymmetry. In a network of interventions, each study estimates the relative effect of different interventions, so asymmetry in the funnel plot cannot be judged. To account for this, an adaptation of the funnel plot can be used, in which the standard error is plotted against an adjusted effect size for each study: the adjusted effect size for a comparison is the study‐specific effect size minus the mean for the meta‐analysis for that comparison.
Contrast/comparison/study‐level data: outcome data for the comparison (e.g. mean difference, odds ratio).
Credible interval (CrI): the 95% credible interval is the range within which the mean value lies with posterior probability of 95%.
Decision space/decision set: the interventions in the decision set are the focal treatments of interest to systematic review authors.
Direct evidence/direct comparison/direct contrast: head‐to‐head comparison of two treatments, for example, A versus B (see indirect evidence).
Edge: line representing a direct contrast on a network diagram.
Effect modifier: effect modification occurs when the effect of A versus B (as the RR/OR/HR for binary outcomes) is significantly different in two or more subgroups, and this leads to heterogeneity, either within trials or between trials, or both. Factors that give rise to subgroup effects are called effect modifiers, and it is important to identify potential effect modifiers and allow for them in the analysis. The identification of significant effect modifiers may lead to stratification (separate analyses for each subgroup) or to a decision not to combine data from different trials in a meta‐analysis. In general, trials have different distributions of effect modifiers (e.g. proportion of people with and without diabetes), leading to inconsistency between trials in the treatment effect. This is often magnified when there is a network of different contrasts.
Exchangeability: it is assumed that treatments in a NMA are exchangeable, so, if treatment B had been given to participants in the indirect A versus C trials and if A had been given in the B versus C indirect trials, then the true AB differences in these indirect studies would be identical to the true AB difference in direct A versus B trials, or at least from the same common distribution. Furthermore, if participants in other trials within the wider linked network (e.g. D versus E trials) were given A and B, the AB differences would also be the same or from the same distribution. This assumption breaks down when there are effect modifiers.
Fixed‐effect: the true treatment effect is assumed to be constant across trials (fixed‐effect) ‐ see also random‐effects and transitivity.
Global inconsistency: inconsistency across a network is described as global inconsistency. It can be evaluated statistically by fitting models that allow and do not allow for inconsistency. See also: Inconsistency/incoherence:
Heterogeneity in a NMA: participants are not randomised to different trials. Therefore, there may be systematic differences in study characteristics or the distribution of participant characteristics across trials. If these characteristics influence the treatment effects (i.e. are effect modifiers), then there are systematic differences in treatment effects across trials, which is called between‐trial heterogeneity. There may also be within‐trial heterogeneity if there are subgroups of an effect modifier for which results are reported separately. In a NMA, the term, 'heterogeneity' applies to variation in effect modifiers within a single contrast (e.g. A versus B); the term, 'inconsistency' refers to the imbalance in effect modifiers between contrasts.
Heterogeneity variance parameter (tau²): in a random‐effects model we assume there is heterogeneity for each pairwise comparison (e.g. A versus B) with variance (tau²AB), but in a NMA we often assume that there is a common heterogeneity amongst all the contrasts in the network; this common heterogeneity has a variance (tau²), which is called the 'heterogeneity variance parameter'. It can be compared with empirical distributions of heterogeneity values typically found in meta‐analyses (Salanti 2014; Turner 2012).
Inconsistency/incoherence: this occurs when the effect estimate derived from an indirect contrast is not the same as the effect estimate derived from a direct contrast. For example, in a network of three interventions, there is inconsistency if dAB(direct) ǂ dAB(indirect), where dAB(indirect) = dAC(direct) ‐ dBC(direct); the effect estimates are given as mean differences or log(odds ratios/risk ratios/hazard ratios). Note that in order to investigate inconsistency there must be both indirect and direct evidence (loops in the network). See also global inconsistency.
Inconsistency factor: this is the absolute difference between the direct and indirect estimates on the log scale (or the logarithm of the ratio of the two odds/hazard ratios) for one of the contrasts in a loop. A statistically low powered z‐test and a 90% or 95% confidence interval (CI) of the inconsistency is computed to determine whether this difference is significant.
Indirect evidence/indirect comparison/indirect contrast: comparison of two treatments, for example, A versus B, obtained from combinations of other comparisons (e.g. trials comparing A versus C and trials comparing B with C) (see direct evidence).
Indirect comparison meta‐analysis: meta‐analysis of a set of treatments that are linked via common comparator(s), but none are compared directly; evidence is combined in a single internally consistent model.
Leverage: this is the effective number of parameters of the model, which is calculated differently for fixed‐effect and random‐effects models, with the latter having greater complexity.
Likelihood (function): the likelihood function is a tool for inferring the underlying distribution of the observed data. To do this, we propose a model to represent the data ‐ often a parametric distribution is assumed (e.g. binomial) ‐ and unknown parameters of that distribution are determined, given the data, by maximising the likelihood (the larger the likelihood, the closer the model fit).
Loop (of evidence): combination of direct and indirect evidence, such that the interventions in the network diagram can be linked to form a closed loop.
Meta‐analysis: a statistical synthesis of the results from two or more separate studies. Methods involve calculating a weighted average of effect estimates from the separate studies.
Mixed treatment comparison meta‐analysis: another name for network meta‐analysis.
Model: a statistical model is a (simplified) mathematical representation of the system we wish to learn about, and which generates our observed data. The model will usually depend on some known factors, such as other variables measured alongside the data, and some unknown parameters that we wish to determine. Then having determined the unknown parameters, the model should be able to simulate data that are an approximation of the real data, allowing us to make inferences from the data.
Multi‐arm trial: individual trial that compares more than two interventions.
Network: trials must be linked in a network of interventions, such that every trial in the network has at least one intervention in common with at least one other trial.
Network diagram: graphical representation of the interventions in the network. It consists of nodes representing the interventions and edges representing the contrasts. The amount of available information can be presented by 'weighting' the nodes and edges using different node sizes and line thicknesses according to the number of studies reporting that treatment or contrast respectively. Other types of weighting are discussed in Chaimani 2013b.
Network meta‐analysis (NMA): NMA is the simultaneous combination of data from randomised comparisons of multiple competing treatments (A versus B, A versus C, A versus D, B versus D, and so on), to deliver an internally consistent set of estimates while respecting the randomisation in the evidence. The use of indirect estimates can provide information on contrasts for which no trials exist. It can also improve the precision of the direct estimate by reducing the width of the CIs compared with the direct evidence alone.
Node: intervention represented on a network diagram, usually by a circle of weighted size.
Node splitting: a method of assessing inconsistency. A 'leave‐one comparison‐out' approach, often called 'node splitting,' is applied, with each direct contrast being excluded from the network and then estimating the difference between this direct evidence and the indirect evidence from the network.
Pairwise meta‐analysis: meta‐analysis of one or more trials of direct comparisons (e.g. A versus B) ‐ see direct evidence.
Prognostic factors: population or study characteristics that affect the risk of the outcome. In a sufficiently large randomised trial that is free from bias, prognostic factors are distributed evenly between intervention groups and do not affect the effect estimate (RR/OR/HR for binary outcomes) unless they are effect modifiers, but they do affect the baseline risk and absolute risk difference.
Random‐effects: trial‐specific treatment differences are assumed to be from a common distribution ‐ see also fixed‐effect and transitivity.
Ranking: ordering of treatments according to their relative effectiveness.
Rankogram: graph of probability versus rank order for a particular treatment. Rankograms are based on the uncertainty in the effect estimates. So if two treatments A and B are each compared with the reference intervention and the CIs for the effect overlap, then each treatment will have some probability of being the most effective. If there is no overlap and A is better than B, then the probability of A being the best will be 1.
Sparse data: data with wide CIs because of few events as a consequence of small studies or short follow‐up periods.
Study‐level data: see contrast.
SUCRA: Surface Under the Cumulative RAnking. This is a measure of the probability that the given treatment is the best. Thus, a SUCRA would be 1 (or 100%) when a treatment was certain to be the best and 0 (0%) when a treatment was certain to be the worst.
Supplementary set (of interventions): interventions added to the network to provide additional evidence on relative treatment effects of the decision set. This may be to connect an otherwise unconnected network of treatments, to increase the precision of the treatment effect estimates or to help address between‐trial heterogeneity.
Transitivity: NMA requires a transitivity assumption, such that there is no imbalance in the distribution of effect modifiers across the different types of treatment contrasts (see also exchangeability).
'Unadjusted' meta‐analysis: meta‐analysis of all the treatment arms for a particular treatment (e.g. all A arms). This breaks the randomisation and should not be done.
References include: Caldwell 2005; Caldwell 2014; Chaimani 2013a; Chaimani 2013b; Cipriani 2013; Dias 2013; Dias 2016; Grant 2013; Jansen 2013; Lu 2004; Salanti 2008; Salanti 2011; Salanti 2014; Soares 2014; Thorlund 2012; Tu 2012; White 2012.
Appendix 3. Search strategies
The Cochrane Central Register of Controlled Trials (CENTRAL)
#1MeSH descriptor: [Bandages] explode all trees #2MeSH descriptor: [Alginates] explode all trees #3MeSH descriptor: [Hydrogels] explode all trees #4MeSH descriptor: [Honey] explode all trees #5MeSH descriptor: [Silver] explode all trees #6MeSH descriptor: [Silver Sulfadiazine] explode all trees #7MeSH descriptor: [Charcoal] explode all trees #8MeSH descriptor: [Silicones] explode all trees #9(dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or "non adherent" or hydrocolloid* or "sodium hyaluronate" or alginat* or hydrogel* or silver* or honey* or matrix or iodine* or "protease modulat*" or "capillary action" or charcoal or silicon* or polymer*):ti,ab,kw #10((odour or odor) near/3 absorb*):ti,ab,kw #11(primapore or curasorb or seasorb or sorbsan or advadraw or vacutex or tegaderm or opsite or allevyn or biatain or medihoney or activon tulle or granuflex or "nu derm" or aquacel or iodoflex or iodozyme or xeroform or carboflex or cutimed sorbact or promogran or acticoat or "urgosorb silver" or mepitel or urgotul):ti,ab,kw #12{or #1‐#11} #13MeSH descriptor: [Metronidazole] explode all trees #14metronidazole:ti,ab,kw #15MeSH descriptor: [Anti‐Bacterial Agents] explode all trees #16MeSH descriptor: [Administration, Topical] explode all trees #17{and #15‐#16} #18(topical near/2 (antibiotic* or antimicrobial* or antibacterial*)):ti,ab,kw #19MeSH descriptor: [Iodophors] explode all trees #20{and #16, #19} #21((topical near/2 iodin*) or ("cadexomer iodine")):ti,ab,kw #22MeSH descriptor: [Collagenases] explode all trees #23{and #16, #22} #24(topical near/2 collagen*):ti,ab,kw #25MeSH descriptor: [Phenytoin] explode all trees #26{and #16, #25} #27(topical near/2 phenytoin):ti,ab,kw #28MeSH descriptor: [Zinc Oxide] explode all trees #29{and #16, #28} #30(topical near/2 zinc):ti,ab,kw #31(iodosorb or actiformcool or aquaflo or flamazine or silvadene):ti,ab,kw #32MeSH descriptor: [Ointments] explode all trees #33(ointment* or lotion* or cream* or powder* or gel or gels):ti,ab,kw #34(topical next (agent* or preparation* or therap* or treatment*)):ti,ab,kw #35{or #13‐#14, #17‐#18, #20‐#21, #23‐#24, #26‐#27, #29‐#34} #36{or #12, #35} #37MeSH descriptor: [Pressure Ulcer] explode all trees #38(pressure next (ulcer* or sore* or injur*)):ti,ab,kw #39(decubitus next (ulcer* or sore*)):ti,ab,kw #40((bed next sore*) or bedsore*):ti,ab,kw #41{or #37‐#40} #42{and #36, #41} in Trials
Ovid MEDLINE
1 exp Bandages/ 2 exp Alginates/ 3 exp Hydrogels/ 4 exp Honey/ 5 exp Silver/ 6 exp Silver Sulfadiazine/ 7 exp Charcoal/ 8 exp Silicones/ 9 (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or "non adherent" or hydrocolloid* or "sodium hyaluronate" or alginat* or hydrogel* or silver* or honey* or matrix or iodine* or "protease modulat*" or "capillary action" or charcoal or silicon* or polymer*).tw. 10 ((odour or odor) adj3 absorb*).tw. 11 (primapore or curasorb or seasorb or sorbsan or advadraw or vacutex or tegaderm or opsite or allevyn or biatain or medihoney or activon tulle or granuflex or "nu derm" or aquacel or iodoflex or iodozyme or xeroform or carboflex or cutimed sorbact or promogran or acticoat or "urgosorb silver" or mepitel or urgotul).tw. 12 or/1‐11 13 exp Metronidazole/ 14 metronidazole.tw. 15 exp Administration, Topical/ 16 exp Anti‐Bacterial Agents/ 17 and/15‐16 18 (topical adj2 (antibiotic* or antimicrobial* or antibacterial*)).tw. 19 exp Iodophors/ 20 and/15,19 21 ((topical adj2 iodin*) or "cadexomer iodine").tw. 22 exp Collagenases/ 23 and/15,22 24 (topical adj2 collagen*).tw. 25 exp Phenytoin/ 26 and/15,25 27 (topical adj2 phenytoin).tw. 28 exp Zinc Oxide/ 29 and/15,28 30 (topical adj2 zinc).tw. 31 (iodosorb or actiformcool or aquaflo or flamazine or silvadene).tw. 32 exp Ointments/ 33 (ointment* or lotion* or cream* or powder* or gel or gels).tw. 34 (topical adj (agent* or preparation* or therap* or treatment*)).tw. 35 or/13‐14,17‐18,20‐21,23‐24,26‐27,29‐34 36 or/12,35 37 exp Pressure Ulcer/ 38 (pressure adj (ulcer* or sore* or injur*)).tw. 39 (decubitus adj (ulcer* or sore*)).tw. 40 (bedsore* or bed sore*).tw. 41 or/37‐40 42 and/36,41 43 randomized controlled trial.pt. 44 controlled clinical trial.pt. 45 randomi?ed.ab. 46 placebo.ab. 47 clinical trials as topic.sh. 48 randomly.ab. 49 trial.ti. 50 or/43‐49 51 exp animals/ not humans.sh. 52 50 not 51 53 and/42,52
Ovid Embase
1 exp "bandages and dressings"/ 2 exp honey/ 3 exp hydrogel/ 4 exp Calcium Alginate/ 5 (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or "non adherent" or hydrocolloid* or "sodium hyaluronate" or alginat* or hydrogel* or silver* or honey* or matrix or iodine* or "protease modulat*" or "capillary action" or charcoal or silicon* or polymer*).ti,ab. 6 ((odour or odor) adj3 absorb*).ti,ab. 7 (primapore or curasorb or seasorb or sorbsan or advadraw or vacutex or tegaderm or opsite or allevyn or biatain or medihoney or activon tulle or granuflex or "nu derm" or aquacel or iodoflex or iodozyme or xeroform or carboflex or cutimed sorbact or promogran or acticoat or "urgosorb silver" or mepitel or urgotul).ti,ab. 8 or/1‐7 9 exp metronidazole/ 10 metronidazole.ti,ab. 11 topical drug administration/ 12 exp Antibiotic Agent/ 13 and/11‐12 14 (topical adj2 (antibiotic* or antimicrobial* or antibacterial*)).ti,ab. 15 exp cadexomer iodine/ 16 and/11,15 17 "cadexomer iodine".ti,ab. 18 exp silver/ or exp sulfadiazine silver/ 19 and/11,18 20 exp collagenase/ 21 and/11,20 22 (topical adj2 collagen*).ti,ab. 23 phenytoin/ 24 and/11,23 25 (topical adj2 phenytoin).ti,ab. 26 exp zinc oxide/ 27 and/11,26 28 (topical adj2 zinc).ti,ab. 29 (iodosorb or actiformcool or aquaflo or flamazine or silvadene).ti,ab. 30 exp ointment/ 31 (ointment* or lotion* or cream* or powder* or gel or gels).ti,ab. 32 (topical adj (agent* or preparation* or therap* or treatment*)).ti,ab. 33 or/9‐10,13‐14,16‐17,19,21‐22,24‐25,27‐32 34 or/8,33 35 exp decubitus/ 36 (pressure adj (ulcer* or sore* or injur*)).tw. 37 (decubitus adj (ulcer* or sore*)).tw. 38 (bedsore* or bed sore*).tw. 39 or/35‐38 40 and/34,39 41 Randomized controlled trials/ 42 Single‐Blind Method/ 43 Double‐Blind Method/ 44 Crossover Procedure/ 45 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab. 46 (doubl* adj blind*).ti,ab. 47 (singl* adj blind*).ti,ab. 48 or/41‐47 49 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 50 human/ or human cell/ 51 and/49‐50 52 49 not 51 53 48 not 52 54 and/40,53
EBSCO CINAHL Plus
S57 S43 AND S56 S56 S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 S55 TI allocat* random* or AB allocat* random* S54 MH "Quantitative Studies" S53 TI placebo* or AB placebo* S52 MH "Placebos" S51 TI random* allocat* or AB random* allocat* S50 MH "Random Assignment" S49 TI randomi?ed control* trial* or AB randomi?ed control* trial* S48 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S47 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S46 TI clinic* N1 trial* or AB clinic* N1 trial* S45 PT Clinical trial S44 MH "Clinical Trials+" S43 S37 AND S42 S42 S38 OR S39 OR S40 OR S41 S41 TI decubitus or AB decubitus S40 TI ( bed sore* or bedsore* ) or AB ( bed sore* or bedsore* ) S39 TI ( pressure ulcer* or pressure sore* ) or AB ( pressure ulcer* or pressure sore* ) S38 (MH "Pressure Ulcer+") S37 S13 OR S36 S36 S14 OR S15 OR S18 OR S19 OR S21 OR S22 OR S24 OR S25 OR S27 OR S28 OR S30 OR S31 OR S33 OR S34 OR S35 S35 TI (topical N3 agent* or topical N3 preparation* or topical N3 therap* and topical N3 treatment*) OR AB (topical N3 agent* or topical N3 preparation* or topical N3 therap* and topical N3 treatment*) S34 TI (ointment* or lotion* or cream* or powder* or gel or gels) OR AB (ointment* or lotion* or cream* or powder* or gel or gels) S33 (MH "Ointments") S32 TI (iodosorb or actiformcool or aquaflo or flamazine or silvadene) OR AB (iodosorb or actiformcool or aquaflo or flamazine or silvadene) S31 TI (topical N2 zinc) OR AB (topical N2 zinc) S30 S16 AND S29 S29(MH "Zinc Oxide") S28TI (topical N2 phenytoin) OR AB (topical N2 phenytoin) S27S16 AND S26 S26(MH "Phenytoin+") S25 TI (topical N2 collagen*) OR AB (topical N2 collagen*) S24 S16 AND S23 S23 (MH "Collagen") S22 TI "cadexomer iodine" OR AB "cadexomer iodine" S21 S16 AND S20 S20 (MH "Iodophors+") S19 TI (topical N2 (antibiotic* or antimicrobial* or antibacterial*)) OR AB (topical N2 (antibiotic* or antimicrobial* or antibacterial*)) S18 S16 AND S17 S17 (MH "Antiinfective Agents+") S16 (MH "Administration, Topical+") S15 TI metronidazole OR AB metronidazole S14 (MH "Metronidazole") S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 S12 AB (primapore or curasorb or seasorb or sorbsan or advadraw or vacutex or tegaderm or opsite or allevyn or biatain or medihoney or activon tulle or granuflex or "nu derm" or aquacel or iodoflex or iodozyme or xeroform or carboflex or cutimed sorbact or promogran or acticoat or "urgosorb silver" or mepitel or urgotul) S11 TI (primapore or curasorb or seasorb or sorbsan or advadraw or vacutex or tegaderm or opsite or allevyn or biatain or medihoney or activon tulle or granuflex or "nu derm" or aquacel or iodoflex or iodozyme or xeroform or carboflex or cutimed sorbact or promogran or acticoat or "urgosorb silver" or mepitel or urgotul) S10 TI odor N3 absorb* or AB odor N3 absorb* S9 TI odour N3 absorb* or AB odour N3 absorb* S8 AB (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or "non adherent" or hydrocolloid* or "sodium hyaluronate" or alginat* or hydrogel* or silver* or honey* or matrix or iodine* or "protease modulat*" or "capillary action" or charcoal or silicon* or polymer*) S7 TI (dressing* or pad or pads or gauze or tulle or film or bead or foam* or non‐adherent or "non adherent" or hydrocolloid* or "sodium hyaluronate" or alginat* or hydrogel* or silver* or honey* or matrix or iodine* or "protease modulat*" or "capillary action" or charcoal or silicon* or polymer*) S6 (MH "Silver") or (MH "Silver Sulfadiazine") S5 (MH "Honey") S4 (MH "Charcoal") S3 (MH "Silicones") S2 (MH "Alginates") S1 (MH "Bandages and Dressings+")
Appendix 4. STATA routines
We used the following specialist NMA STATA routines in addition to the standard STATA meta‐analysis routines of metan and mvmeta.
network meta consistency ‐ a multivariate network meta‐analysis routine; 'consistency' means assuming the heterogeneity variance is the same for all contrasts
network meta inconsistency ‐ multivariate network meta‐analysis routine: 'inconsistency' means account is taken of different study designs (e.g. pairwise/3‐arm trials) ‐ heterogeneity is assumed to be different amongst contrasts
intervalplot ‐ output of all NMA results (follows network meta routines)
netleague ‐ gives a 'league table' of results (follows network meta routines)
network rank ‐ produces ranking of interventions (follows network meta routines)
netweight ‐ calculates all direct pairwise summary effect sizes with their variances, creates the design matrix, and estimates the percentage contribution of each direct comparison to the network summary estimates and in the entire network.
ifplot ‐ identifies all triangular and quadratic loops in a network of interventions and estimates the respective inconsistency factors and their uncertainties
We also used STATA routines to visually display the data
network plot ‐ produces a network diagram, can be modified to add risk of bias
network rank ‐ produces rankograms
network forest ‐ plots results grouped by study design
netfunnel ‐ plots a comparison‐adjusted funnel plot for assessing small‐study effects within a network of interventions
Appendix 5. Group Network
Studies included in the group network
Individual interventions were mapped onto the group categories as shown in Table 10. Based on an assumption that dressings within certain categories can be used interchangeably, we grouped the individual interventions into the following pre‐specified categories: basic wound dressings, advanced dressings and antimicrobial dressings (as described in the BNF 2016), and we kept the different types of specialist dressings (e.g. protease‐modulating matrix dressings) and the different topical agents as separate categories.
5. Mapping from individual to group interventions.
| Individual intervention |
Group intervention (studies in final network) |
| Basic wound contact dressing | Basic dressing (12 studies) |
| Saline gauze dressing | |
| Polyvinylpyrrolidone | |
| Hydrocolloid dressing | Advanced dressing (19 studies) |
| Foam dressing | |
| Hydrogel dressing | |
| Soft polymer dressing | |
| Alginate dressing | |
| Vapour‐permeable dressing | |
| Combination silicone foam dressing | |
| Hydrocolloid with/without alginate | |
| Standard care as described in Ashby 2012 | |
| Honey | Antimicrobial dressing (3 studies) |
| Iodine‐containing dressing | |
| Ethoxy diaminoacridine + nitrofurazone dressing | |
| Resin salve | |
| Hydrocolloid or hydrocolloid silver dressing | Advanced or antimicrobial dressing (1 study) |
| Protease‐modulating dressing | Protease‐modulating dressing (4 studies) |
| Collagenase ointment | Collagenase ointment (3 studies) |
| Dextranomer | Dextranomer (1 study) |
| Phenytoin topical | Phenytoin topical (1 study) |
| Sugar + egg white | Sugar + egg white (1 study) |
| Tripeptide copper gel | Tripeptide copper gel (1 study) |
We excluded 15 of the 51 included studies from the group analysis because they compared interventions in the same group: 14 had advanced dressings in both arms (Aguilo Sanchez 2002; Bale 1997a; Banks 1994a; Banks 1994c; Belmin 2002; Brod 1990; Brown‐Etris 1996; Brown‐Etris 2008; Darkovich 1990; Motta 1999; Muller 2001; Seeley 1999; Sopata 2002; Thomas 1997a); and one had two antimicrobial dressings (Yapucu Güneş 2007). Three additional studies not joined into the individual network were also isolated from the group network (Imamura 1989; Payne 2004; Van De Looverbosch 2004). Two studies previously excluded from the individual network were included initially in the group network (Ashby 2012; Sipponen 2008), but only the Sipponen 2008 study was included in the network. We excluded the Ashby 2012 study and nine others from the network because they were joined to an ineligible intervention that did not link two or more interventions in the network (Gorse 1987; Hondé 1994; Nisi 2005; Nussbaum 1994; Price 2000; Ramos‐Torrecillas 2015; Rees 1999; Serena 2010; Thomas 2005). We also excluded the Sebern 1986 study from the group network, as for the individual network.
Interventions and comparisons
The group network comprised 22 studies (which is less than half of the 51 included studies) (Alm 1989; Banks 1994b; Barrois 1992; Brown‐Etris 1997; Burgos 2000b; Colwell 1993; Graumlich 2003; Hollisaz 2004; Kaya 2005; Kraft 1993; Matzen 1999; Muller 2001; Neill 1989a; Oleske 1986; Parish 1979; Payne 2009; Piatkowski 2012; Romanelli 2001; Sipponen 2008; Thomas 1998; Xakellis 1992; Zeron 2007), The median (range) study size was 38.5 (10 to 100). Only three contrasts were informed by more than one study.
The network comprised 10 interventions. Two studies were three‐arm trials (Hollisaz 2004; Parish 1979), so the total number of comparisons was 26, encompassing a total of 959 participants, experiencing 362 events (complete healing). There were eight direct contrasts and three triangular loops (one of which was exclusive to the Parish 1979 study). This is only 32.5% of the participants in the 51 included studies. The network diagram is shown in Figure 16. Only three contrasts were informed by more than one study:advanced dressing versus basic dressing (11 studies); antimicrobial dressing versus advanced dressing (2); and collagenase ointment versus advanced dressing (2).
10.
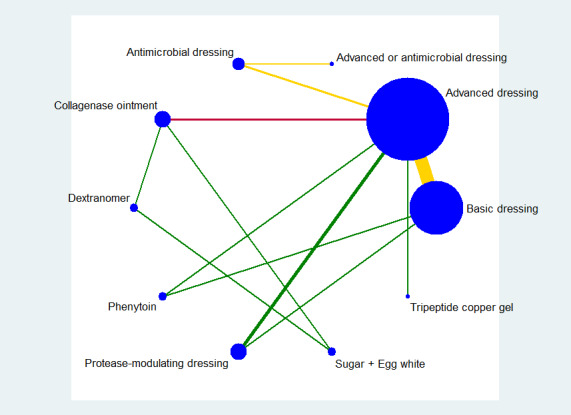
Key: green = low/unclear risk of bias; yellow = high risk of bias; red = very high overall risk of bias for the contrast. The number of studies for each contrast is given in Table 11.
4. Direct evidence for grouped interventions ‐ proportion healed.
| Contrast/comparison | Number of studies (participants) |
RR (95% CI) direct evidence Random‐effects (inverse variance) Heterogeneity statistics |
NMA results (consistency assumption) RR (95% CI) |
|
Advanced dressings versus basic dressings (Alm 1989; Banks 1994b; Colwell 1993; Hollisaz 2004; Kraft 1993; Matzen 1999; Neill 1989a; Oleske 1986; Payne 2009; Thomas 1998; Xakellis 1992) |
11 (532) | 1.55 (1.10 to 2.19) Tau² = 0.13; P = 0.02; I² = 52% |
1.36 (0.95 to 1.93) |
|
Phenytoin versus basic dressing (Hollisaz 2004) |
1 (40) | 3.02 (0.97 to 9.35) | 1.12 (0.52 to 2.44) |
|
Protease‐modulating dressing versus basic dressing (Zeron 2007) |
1 (24) | 1.25 (0.44 to 3.55) | 1.49 (0.91 to 2.46) |
|
Antimicrobial dressings versus advanced dressings (Barrois 1992; Kaya 2005) |
2 (125) | 0.69 (0.48 to 0.99) Tau² = 0.00; P = 0.46; I² = 0% |
0.71 (0.45 to 1.13) |
| Collagenase ointment versus advanced dressings (Burgos 2000b; Muller 2001) | 2 (61) | 1.51 (0.93 to 2.43) Tau² = 0.00; P = 0.61 ; I² = 0% |
1.48 (0.83 to 2.64) |
| Phenytoin versus advanced dressing (Hollisaz 2004) | 1 (39) | 0.71 (0.41 to 1.24) | 0.83 (0.43 to 1.59) |
|
Protease‐modulating dressing versus advanced dressing (Brown‐Etris 1997; Graumlich 2003; Piatkowski 2012) |
3 (112) | 1.13 (0.80 to 1.60) Tau² = 0.00; P = 0.84 ; I² = 0% (Stata: 1.12 (0.79 to 1.59)) |
1.10 (0.74 to 1.64) |
| Tripeptide copper gel versus advanced dressing (Romanelli 2001) | 1 (12) | 2.50 (0.76 to 8.19) | 2.50 (0.72 to 8.63) |
|
Antimicrobial dressing versus advanced antimicrobial dressing (Sipponen 2008) |
1 (37) | 2.29 (0.91 to 5.77) | 2.29 (0.85 to 6.16) |
| Collagenase versus dextranomer (part of 3‐arm trial) (Parish 1979) | 1 (12) | 0.35 (0.05 to 2.26) (Stata 0.44 (0.10 to 2.02)) |
0.44 (0.09 to 2.11) |
|
Collagenase ointment versus sugar + egg white (part of 3‐arm trial) (Parish 1979) |
1 (10) | 3.00 (0.15 to 59.89) (Stata 3.00 (0.15 to 59.79)) |
3.00 (0.15 to 61.18) |
|
Dextranomer versus sugar + egg white (part of 3‐arm trial) (Parish 1979) |
1 (12) | 6.75 (0.44 to 102.80) | 6.75 (0.43 to 105.22) |
| TOTAL | 22 (959) |
The characteristics of studies and participants in the group network are described in Table 2.
Risk of bias
We have summarised the all‐domain risk of bias for each study in the group network in Figure 17. We judged one study to be at low risk of bias (Graumlich 2003) and eight at unclear risk of bias (Banks 1994b; Barrois 1992; Hollisaz 2004; Parish 1979; Piatkowski 2012; Romanelli 2001; Thomas 1998; Zeron 2007). We judged three studies to be at very high risk of bias, that is, to have high risk of bias for two or more domains (Banks 1994a; Burgos 2000b; Oleske 1986). The rest of the studies we assessed to be at high risk of bias. We grouped the low and unclear categories together in the network.
11.
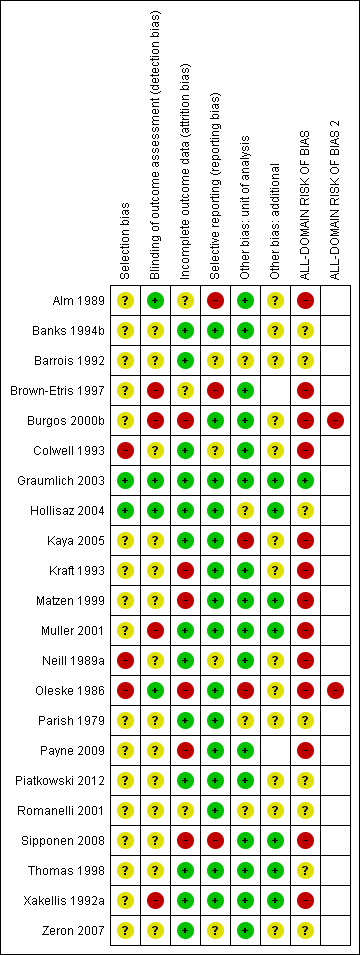
Risk of bias summary ‐ group network: review authors' judgements about each risk of bias item for each included study
For each direct comparison, the overall risk of bias is shown colour coded in Figure 16. There is a relatively large amount of evidence at high or very high risk of bias.
For each contrast in the network, we calculated the overall risk of bias as described in Appendix 8, and the risk of bias ratings are also shown beside the results in Figure 7.
Network meta‐analysis results
The group NMA generated results for 45 mixed treatment contrasts. The network was dominated by the contrast advanced dressing versus basic dressing and the rest of the data were sparse.
Figure 7 shows all NMA results, with the all‐domain risk of bias shown alongside the forest plot contrasts.
As in the individual network, the evidence for the majority of contrasts was informed by studies at high risk of bias, and CIs were wide or very wide, such that we downgraded all evidence at least once for imprecision. There was also heterogeneity or inconsistency, or both, for some contrasts. Consequently, evidence was of low or very low certainty, with the exception of one contrast, for which we assessed the evidence to be of moderate certainty. As for the individual network, this moderate‐certainty evidence should be interpreted in the light of the very low‐certainty evidence for the network as a whole.
We report the representative set of contrasts of each intervention versus basic dressings (Table 4 and Figure 7 first subgroup). Further details of the GRADE assessment can be found in Appendix 8 and Appendix 9.
It is not clear whether protease‐modulating dressings increase the probability of healing compared with basic dressings (RR 1.49; 95% CI 0.91 to 2.46, moderate quality evidence). This corresponds to an absolute risk difference of 94 more people healed per 1000 (95% CI 17 fewer to 279 more). We downgraded the evidence once for imprecision (low risk of bias).
For each of two contrasts (collagenase ointment and tripeptide copper gel) it is unclear whether the intervention increases the probability of healing compared with basic dressings (collagenase: RR 2.01, 95% CI 1.05 to 3.88 and tripeptide copper gel: RR 3.39, 95% CI 0.94 to 12.30); Figure 7). This was low certainty evidence, downgraded once for risk of bias and once for imprecision for collagenase ointment, and twice for imprecision for tripeptide copper gel (for which the direct evidence involved only six participants experiencing five events (complete healing)).
It is unclear whether the remaining six interventions affect the probability of healing compared with basic dressings (advanced dressings, advanced and antimicrobial dressings; antimicrobial dressings, dextranomer, phenytoin and sugar plus egg white) because the evidence is of very low certainty (downgraded mainly for risk of bias (once) and imprecision (twice), although two contrasts (phenytoin and advanced dressings versus basic dressings) had inconsistency.
Ranking of treatments
The rank probability data are shown in Figure 18 and Table 12. The rankograms have maximum probabilities more sharply defined in the group network compared with the individual network for the treatments advanced dressings, advanced‐antimicrobial dressings, collagenase ointment and dextranomer, but there is still overlap of the rankograms for different treatments (Figure 8). The mean rank was 2.0 for dextranomer and 2.3 for tripeptide copper gel, and two treatments had a mean rank of 10 (out of 10): advanced‐antimicrobial dressings and sugar plus egg white. However, no SUCRA value was 0 or 1, indicating uncertainty in the group network.
12.
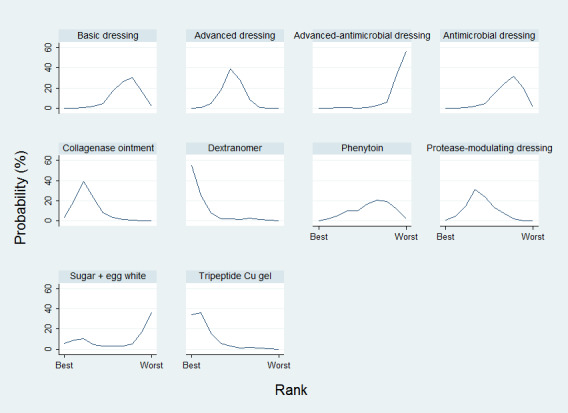
Group network ‐ rankograms
6. Ranks of interventions ‐ group network.
| Group intervention | Mean rank | SUCRA | Probability at maximum | Rank at maximum probability |
| Basic dressing | 7.3 | 0.3 | 30.6 | 8 |
| Advanced dressing | 5.2 | 0.5 | 38.8 | 5 |
| Advanced ‐ antimicrobial dressing | 9.4 | 0.1 | 57.0 | 10 |
| Antimicrobial dressing | 7.4 | 0.3 | 31.9 | 8 |
| Collagenase ointment | 3.3 | 0.7 | 39.4 | 3 |
| Dextranomer | 2 | 0.9 | 55.3 | 1 |
| Phenytoin | 6.5 | 0.4 | 20.5 | 7 |
| Protease‐modulating dressing | 4.6 | 0.6 | 31.2 | 4 |
| Sugar + egg white | 7 | 0.3 | 36.5 | 10 |
| Tripeptide copper gel | 2.3 | 0.9 | 36.2 | 2 |
As with the individual network, the results must be interpreted in the light of the considerable uncertainty in the network and individual estimates, which can give misleading results. Numerically, dextranomer and tripeptide copper gel had the highest probabilities of being the best treatments (55% and 34%, respectively), but these high rankings are likely to be an artificial result. . Across all treatments there was very low certainty in the ranking of interventions (see quality assessment below).
Comparison of results from standard meta‐analysis versus NMA findings
We compared the NMA results with the direct comparison (pairwise) results for the proportion completely healed for the eight different comparisons informing the group network (Table 11). Three comparisons had two or more direct comparison studies (Analysis 2.1). The direct evidence shows some heterogeneity for the comparison of advanced dressing versus basic dressing (I² = 52%, P = 0.02 and some variation in the point estimates). Appendix 11 shows direct evidence results for the time‐to‐healing outcome for three comparisons in five studies.
2.1. Analysis.
Comparison 2 Direct evidence group intervention, number with complete healing, Outcome 1 Intervention 1 vs intervention 2.
Certainty/quality assessment of the network
Overall we downgraded the evidence certainty three times for the network as a whole, because of risk of bias (once), imprecision (once) and inconsistency and publication bias (once): the weighted average risk of bias across the network was high (Appendix 8). For inconsistency, the global Wald test was borderline significant at the 90% significance level (P value was 0.095) (see Appendix 9), however, there were relatively few contrasts with conflicting results for direct and indirect estimates. We downgraded the evidence once for imprecision: there is some overlap of the individual rankograms and no SUCRA value was zero or 1, suggesting uncertainty around treatment estimates and ranking in this network. A contour‐enhanced funnel plot (Figure 9) suggested there may be some asymmetry in the plot (which may be a consequence of publication bias).
Overall, we have little confidence in the findings in this group network, either in terms of the effect estimates or in the ranking of interventions.
Sensitivity analyses ‐ group network
We did not pre‐specify sensitivity analyses for the group network, mainly because the group network itself is based on the assumption that a variety of dressings can be grouped as advanced dressings or basic dressings (as defined by the BNF 2016). We attempted to investigate this assumption by examining the network contrasts for the individual network that compared two advanced dressings, expecting the effect estimates to be close to 1 if the assumption was valid. Results can be seen in Figure 4. Most point estimates were fairly close to 1, but CIs were usually wide or very wide around the estimates. Without exception, the risk of bias for each contrast was either high or very high, and the CI crossed at least one GRADE default MID. Thus, there is no clear evidence either to support or refute the group assumption.
A post‐hoc sensitivity analysis (Appendix 12) examined the original assumption of combining topical agents and dressings in the same NMA, by restricting the group NMA to dressings. There may have been less imprecision in the network as a whole, but results for the contrasts with basic dressings were similar to those in the full group network.
Appendix 6. Assessment of risk of bias
1. Was the allocation sequence randomly generated? (Part of 'Selection bias')
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random‐number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed? (Part of 'Selection bias')
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study? (Performance bias for blinding of participants and caregivers; detection bias for outcome assessors)
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Either participants or some key study personnel were not blinded, and the non‐blinding was likely to introduce bias.
Unclear
Either of the following.
Insufficient information provided to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed? (Attrition bias)
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to induce clinically relevant bias in observed effect size.
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting? (Outcome reporting bias)
Low risk of bias
Either of the following.
The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study's pre‐specified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes of the study were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important additional risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 7. Studies included in the individual network
The 51 included studies compared 39 different interventions in 59 comparisons, summarised in Table 13.
7. Interventions in the included studies.
| Intervention | Number of included studies | Number of participants in included studies | In joined network? | Number of studies in joined individual network | Number of participants in joined individual network |
| Alginate dressings | 2 | 38 | Y | 2 | 38 |
| Basic wound contact dressings | 2 | 33 | Y | 1 | 24 |
| Collagenase‐containing ointment | 3 | 35 | Y | 3 | 35 |
| Combination dressing: non‐adherent + saline gauze + foam | 1 | 16 | N | ||
| Combination dressing: silicone + foam | 1 | 44 | Y | 1 | 44 |
| Dextranomer | 1 | 7 | Y | 1 | 7 |
| Enamel matrix protein | 1 | 6 | N | ||
| Ethoxy‐diaminoacridine plus nitrofurazone dressing | 1 | 26 | N | ||
| Foam dressings | 13 | 266 | Y | 13 | 266 |
| Gauze saline dressings | 11 | 245 | Y | 10 | 233 |
| Honey | 1 | 25 | N | ||
| Hydrocolloid dressings | 22 | 791 | Y | 21 | 715 |
| Hydrocolloid or hydrocolloid silver dressing | 1 | 16 | N | ||
| Hydrocolloid with or without alginate filler (hydrocolloid +/‐ alginate) | 1 | 20 | Y | 1 | 20 |
| Hydrocolloid‐alginate sequential dressings | 1 | 57 | Y | 1 | 57 |
| Hydrogel | 12 | 335 | Y | 10 | 279 |
| Ineligible intervention: graft + conventional dressing | 1 | 18 | N | ||
| Ineligible intervention: growth factor + hyaluronic acid | 1 | 40 | N | ||
| Ineligible intervention: growth factor | 2 | 152 | N | ||
| Ineligible intervention laser | 1 | 7 | N | ||
| Ineligible intervention: NPWT (only included in group analysis) |
1 | 6 | N | ||
| Ineligible intervention: povidone iodine + paraffin soaked gauze | 1 | 40 | N | ||
| Ineligible intervention: radiant heat | 2 | 53 | Y | 2 | 53 |
| Ineligible intervention: skin substitute | 2 | 110 | Y | 2 | 110 |
| Ineligible intervention: ultrasound + ultraviolet | 1 | 6 | N | ||
| Ineligible intervention: whirlpool + chloramine dressing | 1 | 52 | N | ||
| Iodine‐containing dressings | 2 | 62 | Y | 2 | 62 |
| Lysosyme ointment | 1 | 69 | N | ||
| Phenytoin topical | 1 | 21 | Y | 1 | 21 |
| Polyvinylpyrrolidone + zinc oxide | 1 | 12 | Y | 1 | 12 |
| Propylene glycol alginate | 1 | 5 | N | ||
| Protease‐modulating dressings | 5 | 116 | Y | 4 | 76 |
| Resin salve | 1 | 16 | N | ||
| Soft polymer dressing | 1 | 18 | Y | 1 | 18 |
| Standard care (only included in group analysis) | 1 | 6 | N | ||
| Sugar + egg white | 1 | 5 | Y | 1 | 5 |
| Sugar + povidone iodine | 1 | 72 | N | ||
| Tripeptide copper + Opsite | 1 | 6 | Y | 1 | 6 |
| Vapour‐permeable dressings | 2 | 57 | Y | 1 | 35 |
One study (Ashby 2012) had a range of advanced dressings as the comparator, so was ineligible for the individual intervention NMA (but was eligible for the group NMA). Thus there were 50 eligible studies for the individual NMA, comparing 37 different interventions in 58 comparisons with 2952 participants.There were nine interventions eligible for the individual network, which, on their own, did not meet the inclusion criteria of the review, but were compared with interventions that did meet the inclusion criteria ‐ we have called these 'ineligible interventions'.
The network diagram for all interventions in the individual NMA is shown in Figure 19. Ten interventions (including one ineligible intervention) were isolated from the network and are shown in red in Figure 19. Two further ineligible interventions (radiant heat and skin substitute) linked two of the eligible interventions and so were included in the joined network; the other six ineligible interventions (shown in blue) did not link eligible interventions and their studies were therefore omitted from thejoined network. Two of these omitted studies were three‐arm trials each with two ineligible interventions (Nussbaum 1994; Ramos‐Torrecillas 2015). Therefore, ten studies could not be included in the joined network (Gorse 1987; Imamura 1989; Nisi 2005; Nussbaum 1994; Payne 2004; Ramos‐Torrecillas 2015; Rees 1999; Sipponen 2008; Van De Looverbosch 2004; Yapucu Güneş 2007). The results for the isolated studies in which both interventions were eligible are reported in Analysis 5.1. Another study (Sebern 1986) only partially reported their results and was not considered further.
13.
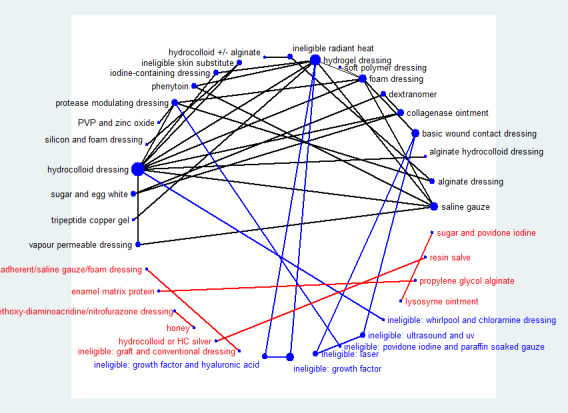
Network diagram ‐ all interventions Key: red = isolated interventions; blue = ineligible interventions joined to only one eligible intervention. Line and node weights not to scale
5.1. Analysis.
Comparison 5 Direct evidence ‐ non‐network comparisons, Outcome 1 Intervention 1 vs intervention 2.
Thus 39 studies were included in the individual network (Aguilo Sanchez 2002; Alm 1989; Bale 1997a; Banks 1994b; Banks 1994a; Banks 1994c; Barrois 1992; Belmin 2002; Brod 1990; Brown‐Etris 1996; Brown‐Etris 1997; Brown‐Etris 2008; Burgos 2000b; Colwell 1993; Darkovich 1990; Graumlich 2003; Hollisaz 2004; Hondé 1994; Kaya 2005; Kraft 1993; Matzen 1999; Meaume 2003; Motta 1999; Muller 2001; Neill 1989a; Oleske 1986; Parish 1979; Payne 2009; Piatkowski 2012; Price 2000; Romanelli 2001; Seeley 1999; Serena 2010; Sopata 2002; Thomas 1997a; Thomas 1998; Thomas 2005; Xakellis 1992; Zeron 2007).
Appendix 8. Contributions and risk of bias in the individual and group networks
7.1. Individual network
The percentage contributions to the mixed treatment contrasts from each direct contrast are shown in Figure 20 for the individual network contrasts versus saline gauze. We calculated the risk of bias for each contrast in the NMA and results for each mixed treatment contrast are shown in the last column of the table in Figure 20 and represented on the forest plot (see Assessment of risk of bias in included studies).
14.
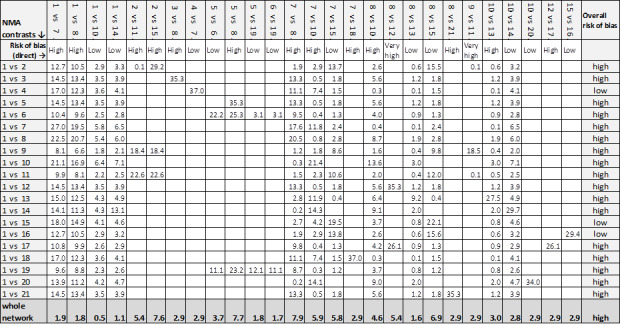
Contributions matrix ‐ interventions versus saline gauze (independent network)
Key: 1 = saline gauze dressing; 2 = alginate dressing; 3 = sequential hydrocolloid alginate dressings; 4 = basic wound contact dressing; 5 = collagenase ointment; 6 = dextranomer; 7 = foam dressing; 8 = hydrocolloid dressing; 9 = hydrocolloid +/‐ alginate (hydrocolloid with/without alginate filler); 10 = hydrogel dressing; 11 = ineligible intervention: radiant heat; 12 = ineligible intervention: skin substitute; 13 = iodine‐containing dressing; 14 = phenytoin; 15 = protease‐modulating dressing; 16 = PVP + zinc oxide; 17 = silicone + foam dressing; 18 = soft polymer dressing; 19 = sugar + egg white; 20 = tripeptide copper gel; 21 = vapour‐permeable dressing.
The contributions to the whole network from each direct contrast are given in the last row of the table in Figure 20. The overall risk of bias was high and the largest contributions to the network were from the direct contrasts: foam versus hydrocolloid; collagenase ointment versus sugar plus egg white; and protease‐modulating dressings versus alginate dressings (but all contributions were still 10% or less).
7.2. Group network
The percentage contributions to the mixed treatment contrasts from each direct contrast are shown in Table 14 for the group network contrasts versus basic dressings. We calculated the risk of bias for each contrast in the NMA and results for each mixed treatment contrast are shown in the last column of Table 14.
8. Contributions matrix ‐ group network.
|
NMA
Contrasts ⇩ |
Contributions from each direct evidence contrast | Overall risk of bias |
| 1 vs 2 | 74.5% 1 vs 2 + 5.5% 1 vs 7 + 7.2% 1 vs 8 + 5.5% 2 vs 7 + 7.2% 2 vs 8 | high |
| 1 vs 3 | 27.1% 1 vs 2 + 2.0% 1 vs 7 + 2.6% 1 vs 8 + 31.8% 2 vs 4 + 2.0% 2 vs 7 + 2.6% 2 vs 8 + 31.8% 3 vs 4 |
high |
| 1 vs 4 | 39.8% 1 vs 2 + 2.9% 1 vs 7 + 3.9% 1 vs 8 + 46.6% 2 vs 4 + 2.9% 2 vs 7 + 3.9% 2 vs 8 |
high |
| 1 vs 5 | 39.8% 1 vs 2 + 2.9% 1 vs 7 + 3.9% 1 vs 8 + 46.6% 2 vs 5 + 2.9% 2 vs 7 + 3.9% 2 vs 8 | high |
| 1 vs 6 | 26.1% 1 vs 2 + 1.9% 1 vs 7 + 2.5% 1 vs 8 + 30.6% 2 vs 5 + 1.9% 2 vs 7 + 2.5% 2 vs 8 + 26.8% 5 vs 6 + 3.8% 5 vs 9 + 3.8% 6 vs 9 | high |
| 1 vs 7 | 37.8% 1 vs 2 + 13.4% 1 vs 7 + 3.7% 1 vs 8 + 41.4% 2 vs 7 + 3.7% 2 vs 8 | low |
| 1 vs 8 | 40.8% 1 vs 2 + 3.0% 1 vs 7 + 9.3% 1 vs 8 + 3.0% 2 vs 7 + 43.8% 2 vs 8 | low |
| 1 vs 9 | 23.6% 1 vs 2 + 1.7% 1 vs 7 + 2.3% 1 vs 8 + 27.6% 2 vs 5 + 1.7% 2 vs 7 + 2.3% 2 vs 8 + 13.2% 5 vs 6 + 14.3% 5 vs 9 + 13.2% 6 vs 9 | high |
| 1 vs 10 | 39.8% 1 vs 2 + 2.9% 1 vs 7 + 3.9% 1 vs 8 + 2.9% 2 vs 7 + 3.9% 2 vs 8 + 46.9% 2 vs 10 | low |
|
Whole network |
8.7% 1 vs 2 + 2.1% 1 vs 7 + 1.6% 1 vs 8 + 14.9% 2 vs 4 + 19.6% 2 vs 5 + 7.3% 2 vs 7 + 8.2% 2 vs 8 + 8.4% 2 vs 10 + 8.4% 3 vs 4 + 10.5% 5 vs 6 + 5.2% 5 vs 9 + 5.1% 6 vs 9 | high |
Key to interventions: 1 = basic dressing; 2 = advanced dressing; 3 = advanced +/‐ antimicrobial dressing; 4 = antimicrobial dressing; 5 = collagenase ointment; 6 = dextranomer; 7 = phenytoin; 8 = protease‐modulating dressing; 9 = sugar + egg white; 10 = tripeptide copper gel
Risk of bias for direct contrasts: Low ‐ 1 vs 7; 1 vs 8; 2 vs 7; 2 vs 8; 2 vs 10; 5 vs 6; 5 vs 9; 6 vs 9. High risk of bias: 1 vs 2; 2 vs 4; 2 vs 5; 3 vs 4
Contributions to the whole network are given in the last row. The overall risk of bias was high and the largest contributions to the network were for the direct contrasts: collagenase ointment versus advanced dressings (19.6%); antimicrobial dressings versus advanced dressings (14.9%) and dextranomer versus collagenase ointment (10.5%).
Appendix 9. Inconsistency
8.1 Individual network
8.1.1. Inconsistency for each contrast (local inconsistency)
Firstly, we examined inconsistency factors, comparing results from the direct evidence with those from the indirect evidence for each contrast informed by a loop. We reported results as the ratio of risk ratios (RoRR), with its 90% confidence interval (CI) for the seven loops (Table 15), assuming a common heterogeneity estimate within each loop. At the 90% significance level, there appeared to be inconsistency in the saline gauze‐hydrogel‐phenytoin loop (RoRR 3.90, 90% CI 1,19 to 12.77). The results also suggested some non‐significant potential inconsistency in all other loops (because the 90% CI crosses 2 or 0.5), except for the loop comprising foam, hydrocolloid and hydrogel. In particular, the loop comprising saline gauze‐foam‐hydrogel had potential inconsistency (RoRR 1.64, 90% CI 0.27 to 9.99); this loop also had a fairly high tau² (0.512), suggesting heterogeneity within that loop. We also examined inconsistency factors using the assumption of a common heterogeneity estimate across the network (Veroniki 2013) (Table 15): for the individual network, tau² (network) was 0.043, and the RoRR for the saline gauze‐hydrogel‐phenytoin loop was 3.75 (90% CI 1.14 to 12.29), that is, similar to the original assumption. For this analysis, the 90% CI crossed 0.5 or 2.0 for all loops except foam‐hydrocolloid‐hydrogel.
9. Inconsistency factors ‐ individual network.
| Common heterogeneity estimate within each loop |
Common heterogeneity estimate for network: tau² (network) = 0.0435 |
||||
| Loop | Ratio of RR (90% CI) | P value |
Loop heterogeneity tau² (loop) |
Ratio of RR (90% CI) | P value |
| Saline gauze ‐ hydrogel ‐ phenytoin | 3.90 (1.19 to 12.77) | 0.059 | 0.000 | 3.75 (90%CI 1.14 to 12.29) | 0.067 |
| Saline gauze ‐ foam ‐ hydrogel | 1.64 (0.27 to 9.99) | 0.651 | 0.512 | 1.21 (90%CI 0.56 to 2.63) | 0.682 |
| Hydrocolloid ‐ hydrogel ‐ iodine containing dressing |
1.26 (0.44 to 3.61) | 0.721 | 0.084 | 1.28 (90%CI 0.59 to 2.75) | 0.602 |
| Foam ‐ hydrocolloid ‐ protease‐modulating | 1.25 (0.66 to 2.35) | 0.562 | 0 | 1.24 (90%CI 0.66 to 2.35) | 0.572 |
| Foam ‐ hydrocolloid ‐ hydrogel | 1.13 (0.7 to 1.83) | 0.675 | 0.016 | 1.16 (90%CI 0.77 to 1.75) | 0.548 |
| Saline gauze ‐ foam ‐ hydrocolloid | 1.04 (0.45 to 2.41) | 0.936 | 0.084 | 1.04 (90%CI 0.55 to 1.96) | 0.919 |
| Saline gauze ‐ hydrocolloid ‐ hydrogel | 1.01 (0.33 to 3.11) | 0.993 | 0.244 | 1.09 (90%CI 0.62 to 1.89) | 0.808 |
–Secondly, a node‐splitting approach was taken. The results following node‐splitting for indirect and direct NMA estimates are shown in Table 16, together with the ratio of risk ratios (RoRR) (indirect/direct) with its 90% CI (the 90% significance level was chosen for this test because of its lack of power). The 'indirect' estimate is the result when the NMA is run in the absence of the direct evidence for that contrast. This is only meaningful if the two interventions in the contrast are joined indirectly through the rest of the network; therefore, we report node splitting results for only 12 (of 27) direct contrasts. We made the following observations:
10. Inconsistency: node splitting ‐ individual network.
| Contrast |
Direct evidence RR (95% CI) |
Indirect evidence RR (95% CI) |
RR direct/RR indirect (90% CI) |
P value | tau² |
| Foam versus saline gauze | 1.52 (0.73 to 3.16) | 1.54 (0.95 to 2.49) | 0.99 (90% CI 0.47 to 2.05) | 0.973 | 0.22 |
| Hydrocolloid versus saline gauze | 1.41 (0.88 to 2.26) | 1.49 (0.87 to 2.56) | 0.95 (90% CI 0.53 to 1.69) | 0.876 | 0.22 |
| Hydrogel versus saline gauze | 1.67 (0.86 to 3.22) | 1.52 (0.91 to 2.54) | 1.10 (90% CI 0.57 to 2.12) | 0.820 | 0.22 |
| Phenytoin versus saline gauze | 3.01 (0.93 to 9.71) | 0.29 (0.05 to 1.51) | 10.06 (90% CI 1.35 to 75.13) | 0.059 | 0.15 |
| Hydrocolloid versus foam | 0.95 (0.68 to 1.32) | 0.91 (0.57 to 1.44) | 1.04 (90% CI 0.65 to 1.68) | 0.881 | 0.23 |
| Hydrogel versus foam | 0.90 (0.51 to 1.58) | 1.10 (0.72 to 1.68) | 0.81 (90% CI 0.45 to 1.47) | 0.568 | 0.23 |
| Protease‐modulating dressing versus foam | 1.22 (0.61 to 2.41) | 0.94 (0.46 to 1.92) | 1.29 (90% CI 0.56 to 2.94) | 0.614 | 0.22 |
| Hydrogel versus hydrocolloid | 1.10 (0.76 to 1.59) | 1.07 (0.68 to 1.68) | 1.02 (90% CI 0.63 to 1.67) | 0.935 | 0.24 |
| Iodine‐containing dressing versus hydrocolloid | 0.90 (0.36 to 2.19) | 0.68 (0.35 to 1.33) | 1.31 (90% CI 0.51 to 3.32) | 0.638 | 0.22 |
| Protease‐modulating dressing versus hydrocolloid | 1.02 (0.53 to 1.97) | 1.32 (0.63 to 2.76) | 0.78 (90% CI 0.34 to 1.77) | 0.614 | 0.22 |
| Iodine‐containing dressing versus hydrogel | 0.64 (0.35 to 1.16) | 0.84 (0.32 to 2.15) | 0.77 (90% CI 0.30 to 1.95) | 0.639 | 0.22 |
| Phenytoin versus hydrogel | 0.71 (0.38 to 1.34) | 7.18 (0.68 to 75.47) | 0.10 (90% CI 0.01 to 0.74) | 0.059 | 0.15 |
Results for two contrasts suggested inconsistency at the 90% confidence level: phenytoin versus saline gauze (RoRR 10.06, 90% CI 1.35 to 75.13) and phenytoin versus hydrogel (RoRR 0.10, 90% CI 0.01 to 0.74). However, the CIs were wide.
There was potential for inconsistency for four other contrasts (with the CI including either 0.5 or 2, but not both): hydrogel versus foam (RoRR 0.81, 90% CI 0.45 to 1.47); protease‐modulating dressing versus foam (RoRR 1.29, 90% CI 0.56 to 2.94); protease‐modulating dressing versus hydrocolloid (RoRR 0.78, 90% CI 0.34 to 1.77) and iodine‐containing dressing versus hydrogel (RoRR 0.77, 90% CI 0.30 to 1.95).
However, all the CIs were wide and there was uncertainty around whether there was inconsistency or not.
We also compared inconsistency versus consistency assumptions for each contrast, examining any differences between different designs (3‐arm and 2‐arm), and between inconsistency and consistency NMA results (Table 17). We reported results only for contrasts with two 'core' interventions; there were no differences between models for 'peripheral' contrasts. Only one contrast had more than one type of design (hydrogel versus saline gauze) and there appeared to be large non‐significant differences in the results for different designs. One contrast had non‐significant differences between the NMA results using inconsistency and consistency assumptions (phenytoin versus saline gauze).
11. Inconsistency and consistency NMA results.
| Contrast | Design 1 (pairwise) | NMA results for design 1 Inconsistency assumption RR (95% CI) | Design 2 (3‐arm) | (NMA results for design 2 Inconsistency assumption RR (95% CI) | NMA results Consistency assumption RR (95% CI) |
| Foam versus saline gauze | 7 vs 1 (3 studies) | 1.53 (0.71 to 2.22) | NA | NA | 1.52 (1.03 to 1.85) |
| Hydrocolloid versus saline gauze | 8 vs 1 (4 studies) | 1.50 (0.9 to 1.92) | NA | NA | 1.43 (1.00 to 1.70) |
| Hydrogel versus saline gauze | 10 vs 1 (2 studies; heterogeneity) |
1.16 (0.51 to 1.73) | 10 vs 1 vs 14 (one 3‐arm study) |
4.22 (1.26 to 7.65) | 1.55 (1.02 to 1.91) |
| Phenytoin versus saline gauze | NA | NA | 14 vs 1 vs 10 (one 3‐arm study) | 3.02 (0.86 to 5.56) | 1.28 (0.58 to 1.88) |
| Hydrocolloid versus collagenase | 8 vs 5 (2 studies) | 0.68 (0.35 to 0.95) | NA | NA | 0.68 (0.37 to 0.91) |
| Hydrocolloid versus foam | 8 vs 7 (6 studies) | 0.96 (0.67 to 1.13) | NA | NA | 0.94 (0.73 to 1.07) |
| Hydrogel versus foam | 10 vs 7 (1 study) | 0.90 (0.48 to 1.22) | NA | NA | 1.02 (0.74 to 1.2) |
| Protease‐modulating versus foam | 15 vs 7 (1 study) | 1.22 (0.58 to 1.76) | NA | NA | 1.08 (0.67 to 1.36) |
| Hydrogel versus hydrocolloid | 10 vs 8 (4 studies) | 1.11 (0.74 to 1.35) | NA | NA | 1.09 (0.83 to 1.24) |
| Iodine‐containing dressing versus hydrocolloid |
13 vs 8 (1 study) | 0.90 (0.35 to 1.43) | NA | NA | 0.76 (0.45 to 0.97) |
| Protease‐modulating versus hydrocolloid |
15 vs 8 (1 study) | 1.03 (0.5 to 1.46) | NA | NA | 1.15 (0.72 to 1.45) |
| Iodine‐containing dressing versus hydrogel |
13 vs 10 (1 study) | 0.64 (0.33 to 0.9) | NA | NA | 0.70 (0.43 to 0.88) |
| Phenytoin versus hydrogel | NA | NA | 14 vs 10 vs 1 (one 3‐arm study) | 0.71 (0.33 to 1.04) | 0.82 (0.42 to 1.14) |
8.1.2. Inconsistency in the network as a whole
We conducted both consistency and inconsistency analyses. The latter showed that the six inconsistency parameters (IP) for the individual network were as follows:
(1) IP = 0: design is the 3‐arm trial comparing saline gauze versus hydrogel versus phenytoin (2) IP = 0: design is 2‐arm trials comparing foam and hydrocolloid (3) IP = 0: design is 2‐arm trials comparing foam and hydrogel (4) IP = 0: design is 2‐arm trials comparing hydrocolloid and hydrogel (5) IP = 0: design is 2‐arm trials comparing hydrogel and protease‐modulating dressings (6) IP = 0: design is 2‐arm trials comparing hydrogel and iodine‐containing dressings The global Wald test for inconsistency gave: Chi²(6) = 3.59 and P value 0.7314.
8.2 Group network
8.2.1. Inconsistency for each contrast (local inconsistency)
Inconsistency factors are reported as the RoRR with its 90% CI in Table 18. There are two loops, basic dressing ‐ advanced dressing ‐ phenytoin; and basic dressing ‐ advanced dressing ‐ protease‐modulating dressing. At the 90% significance level, there did not appear to be inconsistency in these loops, but the results suggested some non‐significant potential inconsistency in both loops (because the 90% CI crossed 2): for the loop containing phenytoin, the RoRR was 3.04 (0.71 to 13.06) and for the loop containing protease‐modulating dressing the RoRR was 1.36 (0.39 to 4.73).
12. Inconsistency factors ‐ group network.
| Loop | Ratio of RR (90% CI) | P value | Loop heterogeneity ‐ tau² |
| Basic dressing ‐ advanced dressing ‐ phenytoin | 3.04 (0.71 to 13.06) | 0.210 | 0.085 |
| Basic dressing ‐ advanced dressing ‐ protease‐modulating dressing |
1.36 (0.39 to 4.73) | 0.682 | 0.091 |
The results following node‐splitting are shown in Table 19 for five (of 11) direct contrasts. The results suggested that there may be inconsistency at the 90% confidence level for two contrasts: phenytoin versus basic dressing (RoRR 12.51, 90% CI 1.87 to 83.55; P = 0.029) and phenytoin versus advanced dressing (RoRR 0.08, 90% CI 0.01 to 0.53; P = 0.029). However, the CIs were wide.
13. Inconsistency: node splitting ‐ group network.
| Contrast | Direct evidence RR (95% CI) | Indirect evidence RR (95% CI) | RR direct/RR indirect (90% CI) | P value | Tau² |
| Advanced dressing versus basic dressing | 1.41 (0.96 to 2.09) | 1.10 (0.33 to 3.73) | 1.28 (90% CI 0.44 to 3.76) | 0.705 | 0.221791 |
| Phenytoin versus basic dressing | 3.02 (0.97 to 9.38) | 0.24 (0.06 to 1.02) | 12.51 (90% CI 1.87 to 83.55) | 0.029 | 0.048414 |
| Protease‐modulating dressing versus basic dressing |
1.25 (0.4 to 3.87) | 1.60 (0.87 to 2.95) | 0.78 (90% CI 0.27 to 2.3) | 0.707 | 0.221734 |
| Phenytoin versus advanced dressing | 0.71 (0.41 to 1.25) | 8.94 (0.96 to 83.43) | 0.08 (90% CI 0.01 to 0.53) | 0.029 | 0.048424 |
| Protease‐modulating dressing versus advanced dressing |
1.13 (0.71 to 1.79) | 0.88 (0.27 to 2.92) | 1.28 (90% CI 0.44 to 3.76) | 0.706 | 0.221781 |
We also compared inconsistency versus consistency assumptions for each contrast, examining any differences between different designs (3‐arm and 2‐arm), and between inconsistency and consistency NMA results (Table 20). Results were reported only for contrasts with two 'core' interventions; there were no differences between models for 'peripheral' contrasts. Only one contrast had more than one type of design (advanced dressings versus basic dressings) and there appeared to be large non‐significant differences in the results for different designs. One contrast had non‐significant differences between the NMA results using inconsistency and consistency assumptions (phenytoin versus basic dressings).
14. Inconsistency and consistency NMA results ‐ group network.
| Contrast | Design 1 (pairwise) | NMA results for design 1 Inconsistency assumption RR (95% CI) | Design 2 (3‐arm) | NMA results for design 2 Inconsistency assumption RR (95% CI) | NMA results Consistency assumption RR (95% CI) |
| Advanced dressing versus basic dressing | 2 vs 1 (10 studies; heterogeneity) |
1.21 (0.88 to 1.67) | 2 vs 1 vs 7 (1 study) |
4.22 (1.41 to 12.68) | 1.36 (0.95 to 1.93) |
| Phenytoin versus basic dressing | NA | NA | 7 vs 1 vs 2 (1 study) |
3.02 (0.96 to 9.44) | 1.12 (0.52 to 2.44) |
| Protease‐modulating dressing versus basic dressing |
8 vs 1 (1 study) | 1.25 (0.44 to 3.59) | NA | NA | 1.49 (0.91 to 2.46) |
| Phenytoin versus advanced dressing | NA | NA | 7 vs 2 vs 1 (1 study) |
0.71 (0.4 to 1.27) | 0.83 (0.43 to 1.59) |
| Protease‐modulating dressing versus advanced dressing |
8 vs 2 (3 studies) | 1.12 (0.78 to 1.62) | NA | NA | 1.10 (0.74 to 1.64) |
8.2.2. Inconsistency in the network as a whole
There were two inconsistency parameters (IP) for the group network:
IP = 0: design is the 3‐arm trial comparing basic dressing, advanced dressing and phenytoin IP = 0: design is the 2‐arm trial comparing advanced dressing and protease‐modulating dressing
The global Wald test for inconsistency gave: Chi²(2) = 4.66 and P‐value 0.0975. This is borderline significant at the 90% confidence level.
Appendix 10. Ranking interventions
Data for each intervention were shown as the probability that each intervention is the best, second best, third best treatment, etc. (see Figure 5, and Table 21). There was substantial overlap of the individual rankograms, illustrated in Figure 21, which intentionally shows the confusion, together with some indication that dextranomer and tripeptide copper gel may be the best treatments and that the worst treatments may be the sequential hydrocolloid‐alginate dressings and sugar plus egg white. Across all treatments there was considerable uncertainty in the ranking of interventions and no intervention had more than 50% probability of being the best treatment. This, together with the mean rank being no higher than 3.6 and no lower than 18.6 (out of 21), and no SUCRA value being 0 or 1, reinforces our view of the considerable uncertainty around treatment estimates in this network.
15.
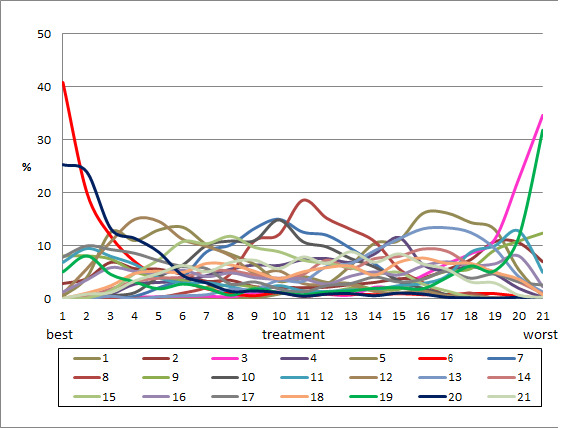
Rankograms combined ‐ individual network Key to interventions: 1: saline gauze; 2: alginate dressing; 3: sequential hydrocolloid alginate dressings; 4: basic wound contact dressing; 5: collagenase ointment; 6: dextranomer; 7: foam dressing; 8: hydrocolloid dressing; 9: hydrocolloid +/‐ alginate (hydrocolloid dressing with/without alginate filler); 10: hydrogel dressing; 11: ineligible radiant heat; 12: ineligible skin substitute; 13: iodine‐containing dressing; 14: phenytoin; 15: protease‐modulating dressing; 16: PVP + zinc oxide 17: silicone + foam dressing; 18: soft polymer dressing; 19: sugar + egg white; 20: tripeptide copper gel; 21: vapour‐permeable dressing
15. Ranks of interventions ‐ individual network.
| Intervention | Mean rank | SUCRA | Probability at maximum | Rank at maximum probability |
| Saline gauze | 16.3 | 0.2 | 16.2 | 17 |
| Alginate dressing | 12.4 | 0.4 | 10.8 | 19 |
| Sequential hydrocolloid alginate dressings | 18.6 | 0.1 | 34.6 | 21 |
| Basic wound contact dressing | 12.4 | 0.4 | 11.6 | 15 |
| Collagenase ointment | 6.9 | 0.7 | 13.5 | 6 |
| Dextranomer | 3.5 | 0.9 | 40.8 | 1 |
| Foam dressing | 10.3 | 0.5 | 15.0 | 10 |
| Hydrocolloid dressing | 11.6 | 0.5 | 18.6 | 11 |
| Hydrocolloid with/without alginate filler | 11.9 | 0.5 | 12.4 | 21 |
| Hydrogel | 9.9 | 0.6 | 14.9 | 10 |
| Ineligible intervention ‐ radiant heat | 11.4 | 0.5 | 12.8 | 20 |
| Ineligible intervention ‐ skin substitute | 6.6 | 0.7 | 15.0 | 4 |
| Iodine‐containing dressing | 15.3 | 0.3 | 13.4 | 17 |
| Phenytoin | 12.6 | 0.4 | 9.4 | 16 |
| Protease‐modulating dressing | 9.3 | 0.6 | 11.8 | 8 |
| PVP + zinc oxide | 11.8 | 0.5 | 8.1 | 20 |
| Silicone + foam dressing | 8.9 | 0.6 | 10.0 | 2 |
| Soft polymer dressing | 11.9 | 0.5 | 7.7 | 16 |
| Sugar + egg white | 14.4 | 0.3 | 31.8 | 21 |
| Tripeptide copper gel | 3.7 | 0.9 | 25.3 | 1 |
| Vapour‐permeable dressing | 11.4 | 0.5 | 8.9 | 13 |
Appendix 11. Time to event data: direct evidence
The duration of follow‐up ranged from 3 to 26 weeks, but the distribution was insufficient to allow modelling of time dependence in the network.
Seven studies (Alm 1989; Brod 1990; Graumlich 2003; Muller 2001; Payne 2009; Thomas 2005; Xakellis 1992) reported time‐to‐event data. We calculated the hazard ratio using the method and spreadsheet from Tierney 2007; one study (Xakellis 1992) reported the hazard ratio directly, adjusted for exudate level. The time‐to‐healing data are shown in Analysis 3.1 and summary statistics for the time‐to‐healing and the proportion healed are compared in Table 22 for the studies that report both healing outcomes.
3.1. Analysis.
Comparison 3 Direct evidence: individual interventions, time‐to‐healing data, Outcome 1 Time‐to‐healing (survival analysis).
16. Direct evidence: comparison of time‐to‐event outcomes and dichotomous data.
| Contrast | Study | Risk ratio (95% CI) | Hazard ratio (95% CI) | Median times to healing |
| Hydrocolloid versus saline gauze |
Alm 1989 (6 weeks) |
3.43 (1.32 to 8.89) | 1.88 (0.80 to 4.45) | |
| Xakellis 1992 (26 weeks) | 1.04 (0.82 to 1.32) | 1.67 (0.81 to 3.45) | 9 days versus 11 days (P = 0.12; unadjusted); hydrocolloid time to healing consistently shorter across time period | |
| Meta‐analysis of 2 studies in 95 participants (Alm 1989; Xakellis 1992) ‐ selected |
Meta‐analysis: 1.72 (0.54 to 5.47) I² = 82%, P = 0.02 |
Meta‐analysis: 1.75 (95% CI 1.00 to 3.05 I² = 0%, P = 0.84 |
||
| Hydrogel versus hydrocolloid |
Brod 1990
(43 participants) (8 weeks) |
1.11 (0.74 to 1.67) | 1.30 (0.54 to 3.13) | 32 days versus 42 days (P = 0.56); Kaplan‐Meier curves crossing |
| Protease‐modulating dressing versus hydrocolloid |
Graumlich 2003
(65 participants) (8 weeks) |
1.03 (0.64 to 1.66) | 1.34 (0.67 to 2.65) | 4 weeks and 7 weeks (estimated from Kaplan‐Meier plot); protease‐modulating dressing had more healing from 5 weeks |
| Collagenase ointment versus hydrocolloid |
Muller 2001
(24 participants) (16 weeks ‐ probably) |
1.57 (0.95 to 2.61) | 2.58 (1.00 to 6.65) | |
| Hydrocolloid +/‐ alginate versus ineligible: radiant heat |
Thomas 2005
(41 participants) (12 weeks) |
0.92 (0.41 to 2.06) | 0.64 (0.23 to 1.77) | > 90 days and 70 days (estimated from Kaplan‐Meier plot); radiant heat had consistently more healing at all time points |
| Foam versus saline gauze |
Payne 2009
(36 participants) (4 weeks) |
1.33 (0.62 to 2.88) | 1.12 (0.42 to 3.01) |
In the individual network, two studies in 95 participants suggested that the time to healing may have been quicker for hydrocolloid versus saline gauze (HR 1.75, 95% CI 1.00 to 3.05); there was no heterogeneity (unlike the risk ratio). One study in 24 participants suggested healing may have been quicker for collagenase ointment compared with hydrocolloid (HR 2.58, 95% CI 1.00 to 2.65). In the other studies, the CI showed much uncertainty.
There was some suggestion of a time dependent effect because there were qualitative and quantitative differences between the HR and the RR: for shorter studies (4‐6 weeks), the HR gave a smaller effect than the RR, but for the medium and longer term studies the HR gave a larger effect than the RR, suggesting that wounds that heal do so relatively quickly.
For the group network, time‐to‐healing data are shown in Analysis 4.1. Three studies in 131 participants suggested that the time to healing may have been quicker for advanced dressing versus basic dressing (HR 1.57, 95% CI 0.97 to 2.55); there was no heterogeneity. This compared with the same three studies analysed as the proportion healed: RR (random‐effects) 1.48 (95% CI 0.79 to 2.77); I² = 66%, P = 0.05.
4.1. Analysis.
Comparison 4 Direct evidence: group interventions, time‐to‐healing data, Outcome 1 Time‐to‐healing (survival analysis).
Appendix 12. Sensitivity analyses
We conducted pre‐specified sensitivity analyses for the individual network and a post‐hoc sensitivity analysis for both networks.
11.1. Sensitivity analysis by risk of bias (individual network)
The planned sensitivity analysis for risk of bias was to restrict the network to those studies at low or unclear risk of bias. Only 12 studies with 13 interventions remained and these formed three isolated loops.
Instead we conducted a sensitivity analysis which excluded studies that had high risk of bias for two or more domains (very high risk of bias) ‐ we excluded seven studies from the joined network (Bale 1997a; Banks 1994a; Brown‐Etris 1996; Burgos 2000b; Payne 2004; Ramos‐Torrecillas 2015; Thomas 2005); one further study (Serena 2010) was no longer joined into the network. This left 31 studies with 35 comparisons, including 18 interventions and 1513 participants (i.e. 51% of the participants in the full network and 72% of the joined network participants).
The NMA results for interventions versus saline gauze are shown in Table 23 alongside the original data. There were only minor differences. The mean rank order was similar to the original data (Table 23) and the rankograms similarly indicated much imprecision.
17. NMA results and ranks for original and sensitivity analyses.
| Risk ratio (95% CI) intervention versus saline gauze | Mean rank (of 21 interventions unless otherwise stated) | |||||
| Intervention | Original |
Sensitivity analysis 1. Very high risk of bias studies excluded |
Sensitivity analysis 2. Complete case |
Original saline 16.3 |
Sensitivity analysis 1. Very high risk of bias excluded (rank of 18) saline = 14.1 |
Sensitivity analysis 2. Complete case saline = 16.3 |
| Alginate dressing | 1.10 (0.11 to 10.57) | 1.14 (0.11 to 11.45) | 1.08 (0.11 to 10.69) | 12.4 | 11 | 12.6 |
| Sequential hydrocolloid alginate dressing | s0.50 (0.12 to 1.99) | 0.52 (0.12 to 2.20) | 0.51 (0.12 to 2.1) | 18.6 | 15.8 | 18.5 |
| Basic wound contact dressing | 1.30 (0.65 to 2.59) | 1.33 (0.61 to 2.93) | 1.44 (0.76 to 2.73) | 12.4 | 10.6 | 11.1 |
| Collagenase ointment | 2.11 (1.06 to 4.21) | 2.35 (1.02 to 5.44) | 2.01 (0.98 to 4.12) | 6.9 | 5.2 | 7.4 |
| Dextranomer | 4.75 (0.86 to 26.34) | 5.29 (0.87 to 32.26) | 4.51 (0.79 to 25.88) | 3.5 | 2.9 | 3.8 |
| Foam dressing | 1.52 (1.03 to 2.26) | 1.56 (1 to 2.43) | 1.45 (0.97 to 2.15) | 10.3 | 9 | 11.2 |
| Hydrocolloid dressing | 1.43 (1 to 2.05) | 1.50 (0.99 to 2.26) | 1.47 (1.02 to 2.12) | 11.6 | 9.7 | 11.1 |
| Hydrocolloid with/without alginate filler | 1.23 (0.06 to 24.86) | not in network | 1.33 (0.06 to 27.37) | 11.9 | not in network | 11.5 |
| Hydrogel dressing | 1.55 (1.02 to 2.36) | 1.74 (1.09 to 2.77) | 1.56 (1.02 to 2.37) | 9.9 | 7.5 | 9.8 |
| Ineligible: radiant heat | 1.34 (0.08 to 23.53) | 1.39 (0.07 to 25.76) | 1.62 (0.09 to 29.28) | 11.4 | not in network | 10.2 |
| Ineligible: skin substitute | 2.12 (1.05 to 4.28) | not in network | 1.92 (0.91 to 4.02) | 6.6 | 9.8 | 7.6 |
| Iodine‐containing dressing | 1.08 (0.58 to 2.02) | 1.19 (0.6 to 2.38) | 1.13 (0.58 to 2.18) | 15.3 | 12.1 | 14.7 |
| Phenytoin | 1.28 (0.58 to 2.81) | 1.66 (0.71 to 3.89) | 1.3 (0.57 to 2.96) | 12.6 | 8.6 | 12.4 |
| Protease‐modulating dressing | 1.64 (0.92 to 2.93) | 1.71 (0.89 to 3.26) | 1.63 (0.89 to 2.97) | 9.3 | 7.9 | 9.4 |
| PVP + ZnO | 1.32 (0.37 to 4.64) | 1.37 (0.36 to 5.19) | 1.30 (0.35 to 4.78) | 11.8 | 10.2 | 11.9 |
| Combined silicone foam dressing | 1.93 (0.37 to 9.92) | not in network | 1.74 (0.33 to 9.32) | 8.9 | not in network | 9.7 |
| Soft polymer dressing | 1.35 (0.56 to 3.27) | 1.39 (0.53 to 3.63) | 1.29 (0.52 to 3.23) | 11.9 | 10.2 | 12.3 |
| Sugar + egg white | 0.70 (0.03 to 15.6) | 0.78 (0.03 to 18.33) | 0.67 (0.03 to 15.11) | 14.4 | 12 | 14.6 |
| Tripeptide copper gel | 3.88 (1.03 to 14.56) | 2.78 (0.90 to 8.58) | 3.89 (1.01 to 15) | 3.7 | 4.8 | 3.8 |
| Vapour‐permeable dressing | 1.44 (0.74 to 2.8) | 1.51 (0.70 to 3.25) | 1.48 (0.72 to 3.04) | 11.4 | 9.5 | 11 |
The global Wald test for inconsistency was not significant ‐ the P value was 0.761, which was very similar to the original analysis (P = 0.731). The point estimates for node splitting gave smaller ratios of RRs than the original for the contrasts previously showing inconsistencies: phenytoin versus saline gauze RoRR 1.97 (90% CI 0.33 to 11.87) and phenytoin versus hydrogel RoRR 0.96 (95% CI 0.47 to 1.94). These RoRRs were no longer significant at the 90% confidence level.
11.2. Sensitivity analysis by missing data assumption (individual network)
We conducted a sensitivity analysis using a different assumption regarding missing data: the original assumption was an intention‐to‐treat analysis with imputation that missing data had no event. This sensitivity analysis used an available case analysis assumption: data were reported for 22 of the 39 studies (Bale 1997a; Banks 1994b; Banks 1994a; Banks 1994c; Barrois 1992; Brod 1990; Brown‐Etris 1996; Darkovich 1990; Graumlich 2003; Hondé 1994; Kraft 1993; Matzen 1999; Meaume 2003; Muller 2001; Payne 2009; Price 2000; Seeley 1999; Sopata 2002; Thomas 1997a; Thomas 1998; Thomas 2005; Xakellis 1992). For this analysis, the 39 studies had data on 1838 participants (i.e. 87%).
The NMA results for the sensitivity analysis are shown in Table 23 alongside the original data. Differences were only small. The mean rank order was similar to the original data (Table 23). The Wald test for inconsistency was not significant ‐ the P value was 0.638, which was similar to the original analysis (P = 0.731).
The global Wald test for inconsistency was not significant ‐ the P value was 0.638, which was similar to the original analysis (P = 0.731). The point estimates for node splitting gave similar RoRRs.
11.3 Post‐hoc sensitivity analysis ‐ dressings only (both individual and group networks)
For the individual network, we also investigated, post‐hoc, our original assumption that topical agents could be used in place of dressings, by examining only the network of studies involving two or more dressings (or hydrogel). There were no three‐arm trials remaining and the 30 studies compared 12 interventions in a total of 1627 participants experiencing 641 events, with 16 direct contrasts and 66 mixed treatment contrasts. The NMA rankings were similarly imprecise (data not shown).
For the group network, 17 studies compared five interventions in a total of 798 participants experiencing 304 events, with five direct contrasts and 10 mixed treatment contrasts. This network was still sparse in terms of total participants, but, on average, there were more events per contrast. The post‐hoc sensitivity analysis had less overlap of rankograms (data not shown) than the full group network, and the mean rank was closer to a whole number; one SUCRA value was 0 and another was 0.9. The mean ranks were: protease‐modulating 1.4; advanced 1.9; basic 3.1; antimicrobial 3.7: advanced‐antimicrobial 4.9. For the comparisons with basic dressing, effect estimates were similar to those in Table 4 but CIs were still wide.
Data and analyses
Comparison 1. Direct evidence: individual interventions, number with complete healing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interventions vs saline gauze | 10 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Hydrocolloid vs saline gauze | 4 | 279 | Risk Ratio (IV, Random, 95% CI) | 1.89 [0.91, 3.93] |
| 1.2 Hydrogel vs saline gauze | 3 | 110 | Risk Ratio (IV, Random, 95% CI) | 2.44 [0.64, 9.27] |
| 1.3 Foam vs saline gauze | 3 | 93 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.78, 2.90] |
| 2 Interventions vs hydrocolloid | 13 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Hydrogel vs hydrocolloid | 4 | 322 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.74, 1.67] |
| 2.2 Foam vs hydrocolloid | 6 | 292 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.81, 1.36] |
| 2.3 Collagenase ointment vs hydrocolloid | 2 | 61 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.93, 2.43] |
| 2.4 Protease‐modulating dressing vs hydrocolloid | 1 | 65 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.64, 1.66] |
Comparison 2. Direct evidence group intervention, number with complete healing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intervention 1 vs intervention 2 | 18 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Advanced dressing vs basic dressing | 11 | 532 | Risk Ratio (IV, Random, 95% CI) | 1.55 [1.10, 2.19] |
| 1.2 Antimicrobial dressing vs advanced dressing | 2 | 125 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.48, 0.99] |
| 1.3 Collagenase ointment vs advanced dressing | 2 | 61 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.93, 2.43] |
| 1.4 Protease‐modulating dressing vs advanced dressing | 3 | 112 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.80, 1.60] |
Comparison 3. Direct evidence: individual interventions, time‐to‐healing data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time‐to‐healing (survival analysis) | 7 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Hydrocolloid versus saline gauze | 2 | 95 | Hazard Ratio (Fixed, 95% CI) | 1.75 [1.00, 3.05] |
| 1.2 Hydrogel versus hydrocolloid | 1 | 43 | Hazard Ratio (Fixed, 95% CI) | 1.30 [0.54, 3.13] |
| 1.3 Protease‐modulating versus hydrocolloid | 1 | 65 | Hazard Ratio (Fixed, 95% CI) | 1.34 [0.67, 2.65] |
| 1.4 Collagenase ointment versus hydrocolloid | 1 | 24 | Hazard Ratio (Fixed, 95% CI) | 2.59 [1.01, 6.62] |
| 1.5 Foam versus saline gauze | 1 | 36 | Hazard Ratio (Fixed, 95% CI) | 1.13 [0.42, 3.00] |
| 1.6 Hydrocolloid +/‐ alginate versus ineligible: radiant heat | 1 | 41 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.23, 1.77] |
Comparison 4. Direct evidence: group interventions, time‐to‐healing data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time‐to‐healing (survival analysis) | 5 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Advanced dressing versus basic dressing | 3 | Hazard Ratio (Fixed, 95% CI) | 1.57 [0.97, 2.55] | |
| 1.2 Protease‐modulating dressing versus advanced dressing | 1 | Hazard Ratio (Fixed, 95% CI) | 1.34 [0.67, 2.65] | |
| 1.3 Advanced dressings versus collagenase ointment | 1 | Hazard Ratio (Fixed, 95% CI) | 0.27 [0.11, 0.67] |
Comparison 5. Direct evidence ‐ non‐network comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intervention 1 vs intervention 2 | 4 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Sugar + povidone iodine vs lysosyme | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Enamel matrix protein vs propylene glycol alginate | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Honey vs ethoxy‐diaminoacridine +nitrofurazone dressings | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Resin salve vs hydrocolloid or hydrocolloid silver dressing | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aguilo Sanchez 2002.
| Methods | RCT; unit of randomisation unclear (unclear if > 1 wound per person) Funding: not stated. Setting: not stated Duration of follow‐up about 7 weeks Unit of analysis: unclear | |
| Participants | ˜24 participants with pressure ulcers. PU Stage: not stated (PU classification: not stated) Age: not stated. Duration of ulcer: not stated. Ulcer size: not stated Wound characteristics at baseline: infection not reported; slough not reported; necrosis not reported; exudate not reported Comment: PU grade not stated | |
| Interventions | Group 1: hydrocolloid dressing ‐ Comfeel Plus: hydrocolloid‐alginate, combination of 2 groups randomised to treatment in the debridement and granulation phases; n = 12 (probably). Grouped intervention category: advanced dressing Group 2: foam dressing ‐ Biatain Adhesive (combination of 2 groups randomised to treatment in the debridement and granulation phases); n = 12 (probably). Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at about 7 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: unclear who the outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none ‐ i.e. no missing data |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias unit of analysis | Unclear risk | Unit of randomisation unclear and unit of analysis unclear ‐ assumed the participant was analysed ("cases"); no details on the ratio of ulcers:participants |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear Reasons: unclear selection bias; unclear blinding; unclear unit of analysis; unclear subgroup Comments: unclear risk of bias on unit of analysis; time to event may have been reported ‐ unclear |
Alm 1989.
| Methods | RCT; ulcers randomised (> 1 wound per person, all followed) Funding: not stated. Setting: hospital inpatients Duration of follow‐up 6 weeks (also reported at 12 for time to event weeks) Unit of analysis: ulcer | |
| Participants | 50 participants with pressure ulcers. PU Stage: not stated and no indication apart from mean depth (PU classification: not stated) Age: mean 83.6 (SD 9.2) and 83.4 (SD 9.4). Duration of ulcer: 4.6 (SD10.9) and 4.8 (SD 6.5). Ulcer size: median (range?) 2.02 (0.95, 3.10) and 2.44 (0.97, 3 .24) Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: "considerable amount of debris" | |
| Interventions | Group 1: hydrocolloid dressing ‐ Comfeel Ulcus (not in BNF): 1 week washout with saline gauze; then hydrocolloid sheet and, if appropriate, hydrocolloid paste (7) and powder (1 ulcer); dressings changed when necessary; n = total 50 (number per group not reported). Grouped intervention category: advanced dressing Group 2: gauze saline dressing ‐ saline wet (1 week washout with saline gauze; then saline gauze changed twice/day); n = total 50 (number per group not reported). Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: complete healing not reported; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but of unclear importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data: not reported by group and very unclear overall ‐ possibly 9/50 (18%) missing (1 died, 2 protocol violations, 2 results missing, 3 discontinued for surgery, 1 adverse event) |
| Selective reporting (reporting bias) | High risk | Inadequate – reported incompletely (e.g. P value > 0.05) |
| Other bias unit of analysis | Low risk | Unit of randomisation ulcer and unit of analysis ulcer ‐ 6/50 participants had 2 pressure ulcers (2 participants had 1 ulcer assigned to each group); ulcer:person = 60/56 overall = 1.12 |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias; unclear missing data; unclear if PU grade sufficient; main outcome results estimated Comments: very poorly reported study; PU stage not stated; main outcomes estimated; ulcers randomised and analysed, so no unit of analysis errors; stated to be some baseline differences in ulcer duration, but degree and importance unclear |
Ashby 2012.
| Methods | RCT; participants randomised (> 1 wound per person, other selection of wound) Funding: non‐industry funding ‐ MRC grant. NPWT units supplied by Kinetic Concepts Inc, but they had no input to the trial. Setting: hospital and community Duration of follow = up to 26 weeks (6 months) Unit of analysis: person (1 ulcer/person) | |
| Participants | 12 participants with pressure ulcers. PU Stage: 3 (n = 7); 4 (n = 5) overall; data per group not stated (PU classification: NPUAP) Age: median (IQR) 67.5 (54.5 to 82.0) years. Duration of ulcer: median (IQR): overall ‐ 4.0 months (2.2 to 28.5). Ulcer size: median: 3.0 cm wide x 5.0 cm long x 4 cm deep (overall) Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: deepest wound selected if more than 1 per person (but not stated if this occurred) | |
| Interventions | Group 1: standard care (all advanced dressings): hydrocolloid (fibrous hydrocolloid) dressing, a foam dressing or an alginate dressing (all non‐silver); n = 6. Grouped intervention category: advanced dressing Group 2: ineligible intervention ‐ negative pressure wound therapy (PU was filled with either VAC WhiteFoamW or Granufoam dressings and VAC applied); n = 6). Grouped intervention category: ineligible ‐ NPWT | |
| Outcomes | Primary outcomes: proportion completely healed at 26 (6 months) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment adequate ‐ central randomisation with contact details or list held independently. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 1/6 (17%) withdrew from treatment and received other treatment; 0/6 died (PU slow to heal). Group 2 ‐ 6/6 (100%) withdrew from treatment and received other treatment. 2/6 (33%) died during the trial (1 recurrence of black slough, 1 ulcer too small to continue treatment, 1 foam embedded in granulation tissue, 1 deterioration, 1 participant refusal, 1 difficulty with applying treatment) i.e. differential missing data rates; high differential rate – likely to change effect estimate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Low risk | The study appears to be free of other sources of bias |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: differential missing data due to death; also differential switching to other treatments Comments: attrition bias (death); small trial, but more comorbidities in NPWT group |
Bale 1997a.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: not stated. Setting: hospital inpatients Duration of follow‐up 4 (30 days) weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 60 participants with pressure ulcers. PU Stage: II/III (acceptable); 71% and 79% Stage II (PU classification: Stirling) Age: median 74 years and 73 years. Duration of ulcer: not stated. Ulcer size: < 5 cm² (32% and 48%), 5 to < 10 (19% and 21%), 10 to < 20 (29% and 14%), > 20 (19% and 17%) Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate low‐moderate levels Comment: same number of ulcers as participants in table; exudate: none (32% and 28%), slight (58% and 31%), moderate (10% and 41%); 5‐centre trial | |
| Interventions | Group 1: hydrocolloid dressing ‐ Granuflex; n = 31. Grouped intervention category: advanced dressing Group 2: foam dressing ‐ Allevyn Adhesive; n = 29. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 4 (30 days) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ not stated. Allocation concealment inadequate ‐ evidence that researchers knew the sequence. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Other evidence for no blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 22/31 (71%) withdrew (8 discharged, 2 died, 2 adverse incident, 2 participant request, 2 dressing unsuitable, 2 wound deteriorated, 1 lack of progress, 2 dressing rolling). Group 2 ‐ 18/29 withdrew (62%) (5 discharged, 6 died, 3 adverse incident, 2 participant request, 1 dressing unsuitable, 1 wound deteriorated) i.e. similar rate missing in both groups; high rate – more than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Low risk | The study appears to be free of other sources of bias |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Comments: allocation concealment inadequate ‐ "allocated sequentially using an open randomisation list"; ulcer size larger for hydrocolloid group. Not blinded: performance assessed at dressing change; attrition bias |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Banks 1994a.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: industry funded ‐ CV Laboratories Ltd (foam manufacturer) and Calgon Vestal Laboratories (HC manufacturer). Setting: community Duration of follow‐up 6 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 40 participants with pressure ulcers. PU Stage: II and III (Stages I, IV, V excluded); proportions not stated (PU classification: not stated) Age: median (range): 73 (46‐93) years and 71 (40‐100) years. Duration of ulcer: median (range): 21 (5‐252) days and 56 (3‐365) days; P < 0.08. Ulcer size: median (range): 0.74 (0.16‐8.19) cm² and 0.67 (0.03‐9.7) cm²; mean 1.51 (SD1.86) cm² and 1.47 (SD 2.26) cm² Wound characteristics at baseline: no wounds infected; slough not reported; no wounds necrotic; exudate unclear Comment: exuding wounds but level not stated. Inclusion criteria: shallow/moist pressure sore involving loss of skin tissue | |
| Interventions | Group 1: hydrocolloid dressing ‐ Granuflex: concurrent standard pressure‐relieving devices and cushions in community as appropriate; n = 20. Grouped intervention category: advanced dressing Group 2: foam dressing ‐ Spyrosorb (not in BNF) (necessary by the treating health professional); n = 20). Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 6 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but of unclear importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 10/20 (50%) withdrawn (2 wound deteriorated, 2 overgranulation, 2 discomfort, 4 unrelated to wound (2 died, 2 had respite care)). Group 2 ‐ 2/20 (10%) (2 for reasons unrelated to wound (1 died, 1 admitted to hospital)) i.e. differential missing data rates; high differential rate – likely to change effect estimate |
| Selective reporting (reporting bias) | High risk | Inadequate – outcome included in methods section but not results |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: unclear selection bias, not blinded, attrition bias Comments: some difference in duration of ulcers; time‐to‐event data reported only as not significant; Grade II assumed to be acceptable (loss of skin tissue) |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Banks 1994b.
| Methods | RCT; participants randomised (> 1 wound per person, unclear how assessed) Funding: unclear ‐ authors at wound healing research unit. Setting: hospital and community Duration of follow‐up 12 weeks (also reported at 1, 2, 6 weeks) Unit of analysis: person (unclear if > 1 ulcer analysed) | |
| Participants | 50 participants with pressure ulcers. PU Stage: II (non‐blanching erythema +/‐ superficial damage) and III (PU classification: Torrance) Age: 68% over 75 years. Duration of ulcer: ascertained but not reported. Not available for 28%. Ulcer size: 16 and 19 ≤ 1 cm², 3 and 3 > 1 cm² and ≤ 2.5 cm²; 7 and 2 > 2.5 cm² Wound characteristics at baseline: no wounds infected; not reported; no wounds necrotic; exudate not reported Comment: number ulcers/person not stated, but some had > 1 ulcer | |
| Interventions | Group 1: foam dressing ‐ Lyofoam; n = 26. Grouped intervention category: advanced dressing Group 2: basic wound contact dressing ‐ N‐A Dressing; n = 24). Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment adequate ‐ independent 3rd party allocates and retains schedule. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear ‐ vague |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 7/26 (27%) (2 died, 5 withdrew; 2 reasons NS, 2 improved, 1 deteriorated). Group 2 ‐ 9/24 (38%) (2 died, 7 withdrew, 2 reason NS, 1 improved, 4 deteriorated) i.e. similar rate missing in both groups; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ stated that protocol allowed > 1 per wound person, but no evidence that this happened |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear Comments: trial co‐ordinator was outcome assessor, unclear if blinded; imbalance at baseline ‐ not clear if problem. More large ulcers for intervention 1 |
Banks 1994c.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: industry funded ‐ CV Laboratories Ltd (foam manufacturer) and Calgon Vestal Laboratories (HC manufacturer). Setting: hospital inpatients Duration of follow‐up 6 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 29 participants with pressure ulcers. PU Stage: II and III (involving loss of skin) proportions not stated (PU classification: not stated) Age: median (range): 74 (40‐95) years and 73 (40‐88) years. Duration of ulcer: median (range): 5.5 (2‐365) days and 7 (2‐14) days. Ulcer size: median (range): 2.4 (0.1‐25.8) and 1.4 (0.5‐14.3) cm² Wound characteristics at baseline: no wounds infected; slough not reported; no wounds necrotic; exudate moderate levels | |
| Interventions | Group 1: hydrocolloid dressing ‐ Granuflex: Granuflex E; additional support therapy for immobile participants; n = 16. Grouped intervention category: advanced dressing Group 2: foam dressing ‐ Spyrosorb (not in BNF) (additional support therapy for immobile participants); n = 13). Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 6 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 4/16 (25%) (3 wound deterioration, 1 wound/dressing‐related problems). Group 2 ‐ 3/13 (23%) (1 wound deterioration, 1 wound/dressing‐related problems, 1 discharged from hospital) i.e. similar rate missing in both groups; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | High risk | Inadequate – outcome included in methods section but not results |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, not blinded, baseline differences Comments: wound area showed no significant difference, but median 2.4 versus 1.4; Grade II assumed to be acceptable (loss of skin tissue) |
Barrois 1992.
| Methods | RCT (abstract); participants randomised (unclear if > 1 wound per person) Funding: not stated. Setting: not stated Duration of follow‐up 8 weeks Unit of analysis: person (unclear if > 1 ulcer analysed) | |
| Participants | 76 participants with pressure ulcers. PU Stage: not stated (PU classification: not stated) Age: not stated. Duration of ulcer: not stated. Ulcer size: mean 15 cm² overall Wound characteristics at baseline: infection not reported; slough not reported; all wounds necrotic; exudate not reported Comment: implies 1 ulcer per person; "multicentre good practice trial" | |
| Interventions | Group 1: hydrocolloid dressing ‐ Granuflex; n = 38. Grouped intervention category: advanced dressing Group 2: iodine containing dressing ‐ povidone iodine soaked gauze (tulle impregnated with PI); n = 38. Grouped intervention category: antimicrobial dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 2/38 (5%) (2 dropped out due to deterioration). Group 2 ‐ 5/38 (13%) (5 dropped out due to deterioration in the wound) i.e. similar rate missing in both groups; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias unit of analysis | Unclear risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ probably 1 ulcer per person |
| Other bias additional | Unclear risk | PU classification unclear |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear Comments: unclear selection bias, unclear whether ulcer or person is unit of analysis. Grade of PU not stated (but open necrotic pressure sores/ulceration) |
Belmin 2002.
| Methods | RCT; participants randomised (> 1 wound per person, other selection of wound) Funding: industry funded ‐ Urgo (manufacturers of intervention 2). Setting: hospital inpatients Duration of follow‐up 8 weeks Unit of analysis: person (selected ulcer) | |
| Participants | 110 participants with pressure ulcers. PU Stage: III and IV; stage III proportions = group 1: 82.7% and group 2: 71.4% (PU classification: Yarkony) Age: 82.2 (SD 7.9) years and 84.8 (SD 7.1) years . Duration of ulcer: 7.7 weeks and 7.2 weeks. Ulcer size: mean 12.6 (SD 8.0) cm² and 14.7 (SD 10.4) cm² (NS) Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM Extra Thin: note different HC; hydrocolloid paste for deep ulcers. Prior treatment with mainly HC; n = 53. Grouped intervention category: advanced dressing Group 2: sequential dressing ‐ hydrocolloid‐alginate (Urgosorb (4 weeks) then Algoplaque (4 weeks); hydrocolloid paste for deep ulcers in first 4 weeks only. Prior treatment mainly HC); n = 57. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ other. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ all analysed, though 16/53 (30%) did not complete treatment (8 died and 8 withdrew (2 transfer to another unit, 3 local infection, 3 PU impairment)). Group 2 ‐ all analysed, though 17/57 (30%) did not complete treatment (11 died and 6 withdrew (1 transfer to another unit, 1 worsening health status, 1 local infection, 3 PU impairment)) i.e. all analysed but non‐completers ‐ similar rate in each group; high rate – more than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (selected ulcer) ‐ one ulcer selected |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Comments: unclear selection bias (block randomised), different hydrocolloids and pastes used; unclear who assessed healing ‐ nurses not blinded, assessor of wound area was blinded; baseline differences: diabetes, hypertension significantly higher for sequential; proportion of grade IV ulcers higher in sequential |
Brod 1990.
| Methods | RCT (letter to journal); participants randomised (unclear if > 1 wound per person) Funding: industry funded ‐ Acme/Chaston division, National Patent Development Corp (manufacturer poly HEMA). Setting: care home Duration of follow‐up 8 weeks Unit of analysis: person (unclear if > 1 ulcer analysed) | |
| Participants | 43 participants with pressure ulcers. PU Stage: II and III (description available); stratified then randomised; proportions not stated (PU classification: not stated) Age: median 86 years and 82 years. Duration of ulcer: not stated, but comparable. Ulcer size: median 2.5 cm² and 1.9 cm² (P = 0.09) Wound characteristics at baseline: infection not reported; slough not reported; some wounds necrotic; exudate not reported Comment: if necrosis, wounds were debrided first | |
| Interventions | Group 1: hydrogel dressing ‐ poly HEMA: Hydron dressing; n = 27. Grouped intervention category: advanced dressing Group 2: hydrocolloid dressing ‐ DuoDERM; n = 16. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded to interventions – clear description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 2/27 (7%) (both died). Group 2 ‐ 3/16 (19%) (1 died, 2 did not complete treatment (1 poor response, 1 adverse event)) i.e. differential missing data rates; low differential rate – unlikely to change effect estimate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ one ulcer implied (e.g. "52% of group 1 had complete healing of the study ulcer") |
| Other bias additional | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, not blinded Comments: unblinded research nurse who had no clinical responsibilities |
Brown‐Etris 1996.
| Methods | RCT; ulcers randomised (> 1 wound per person, other selection of wound) Funding: not stated. Setting: care home and hospital and community Duration of follow‐up 10 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | ||
| Interventions | Group 1: hydrogel dressing ‐ Transorbent dressing; n = 77. Grouped intervention category: advanced dressing Group 2: hydrocolloid dressing ‐ DuoDERM CGF (not BNF); n = 63. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 10 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 19/77 (25%) (11 unable to follow, 5 died, 3 other; overall 19 participants did not complete first 3 weeks of trial or missed 2 sequential visits ). Group 2 ‐ 12/63 (19%) (4 unable to follow, 5 died, 3 other; overall 19 participants did not complete first 3 weeks of trial or missed 2 sequential visits) i.e. similar rate missing in both groups; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias unit of analysis | High risk | Unit of randomisation ulcer and unit of analysis person (1 ulcer/person) ‐ ulcers randomised (stratified), but one II, III or IV ulcer was selected (implied at the beginning), at the discretion of the (unblinded) investigator at each centre |
| Other bias additional | Unclear risk | Some discrepancy between text and table in the number of participants |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: selection bias (baseline differences), not blinded, ulcer selected by investigator Comments: allocation concealment ‐ each centre randomised independently. Says wounds randomised and stratified by surface area and stage, but later says one ulcer was selected (implied at the beginning), at the discretion of the investigator. Baseline differences in the proportion with Grade III/IV ulcers (more in foam group) and duration of ulcer shorter in hydrocolloid group. Some discrepancy between text and table in the number of participants |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Brown‐Etris 1997.
| Methods | RCT (abstract); participants randomised (unclear if > 1 wound per person) Funding: non‐industry funding ‐ authors worked for health care agency. Setting: unclear Duration of follow‐up 8 weeks Unit of analysis: person (unclear if > 1 ulcer analysed) | |
| Participants | 36 participants with pressure ulcers. PU Stage: II, III and IV (proportions not stated) (PU classification: not stated) Age: not stated. Duration of ulcer: not stated. Ulcer size: not stated Wound characteristics at baseline: infection not reported; slough not reported; necrosis not reported; exudate not reported Comment: few details (abstract) | |
| Interventions | Group 1: protease‐modulating dressing ‐ Fibracol (90% collagen, 10% alginate (from suppliers' website)); n = 24. Grouped intervention category: protease‐modulating dressing Group 2: alginate dressing ‐ Kaltostat; n = 12. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data: Group 1 ‐ 116 total enrolled, 80 evaluable and interim analysis on 36 (not stated). Group 2 ‐ 116 total enrolled, 80 evaluable and interim analysis on 36 (not stated) i.e. missing data, but unclear |
| Selective reporting (reporting bias) | High risk | Inadequate – outcome included in methods section but not results |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ one ulcer implied (e.g. "participants stratified before randomisation according to pressure ulcer location and size") |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, not blinded Comments: interim analysis ‐ but planned, so acceptable |
Brown‐Etris 2008.
| Methods | RCT; participants randomised (> 1 wound per person, other selection of wound) Funding: industry funded ‐ 3M grant (manufacturers of Tegaderm). Setting: care home and community Duration of follow‐up 8 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 72 participants with pressure ulcers. PU Stage: II (59.5% and 65%; P = 0.59), and shallow III (PU classification: not stated) Age: mean 72.7 (SD 18.61) years and 78.3 (SD 14.70) years. Duration of ulcer: median (range): 32.0 days (2‐635) and 21.0 days (1‐291); P = 0.169. Ulcer size: mean (SD): 2.5 (4.86) and 1.5 (1.69) cm² Wound characteristics at baseline: no wounds infected; slough not reported; some wounds necrotic; exudate low‐moderate levels Comment: < 25% necrotic | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM CGF; n = 37. Grouped intervention category: advanced dressing Group 2: vapour‐permeable dressing ‐ Tegaderm Absorbent Clear; n = 35). Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none i.e. no missing data (no details) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ if > 1, authors selected highest grade PU then largest ulcer |
| Other bias additional | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, not blinded |
Burgos 2000b.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: industry funded ‐ supported by Laboratorios Knoll (manufacturer of collagenase ointment). Setting: hospital inpatients Duration of follow‐up 12 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 37 participants with pressure ulcers. PU Stage: III only (PU classification: not stated) Age: mean 78.6 (SD 10.4) years and 81.9 (SD 12.7) years. Duration of ulcer: 2.6 (SD 1.9) months and 3.2 (SD 2.0) months P = 0.44; 89% and 83% previously treated. Ulcer size: approx 22 and 20.5 cm² (estimated from graph) Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: same number of ulcers as participants in table | |
| Interventions | Group 1: hydrocolloid dressing ‐ Varihesive (not in BNF): ulcers cleaned with saline; Varihesive paste used for deep ulcers/high exudate for HC group only; n = 19. Grouped intervention category: advanced dressing Group 2: collagenase‐containing ointment ‐ Iruxol (not BNF) (ulcers cleaned with saline); n = 18. Grouped intervention category: collagenase ointment | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ other. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Other evidence for no blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: 6 participants excluded overall (4 protocol violations) ‐ not given by group. Additionally, discontinuations: Group 1: 6 (32%) (because of death due to unrelated cause, deterioration in general condition, discharge from hospital, protocol violations, lack of efficacy). Group 2: 8 (44%) (because of deaths due to unrelated cause, discharge from hospital, transfer to another centre), i.e. similar rate missing in both groups; high rate – more than control event rate. "Eight (44.4%) and six (31.6%) patients in the collagenase and hydrocolloid groups, respectively, discontinued the study prematurely. Reasons for discontinuation in the collagenase group were: death due to unrelated cause (n = 3), discharge from the hospital (n = 3) and transfer to another centre (n = 3). Reasons for discontinuation in the hydrocolloid group included death due to unrelated cause (n = 1), deterioration of the patient’s general condition (n = 1), discharge from the hospital (n = 1), protocol violation (n = 2) and lack of efficacy (n = 1)", i.e. discrepancy between total number missing and sum of reasons for group 2 ‐ but 44% corresponds to 8 participants. |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ same number of ulcers as participants in table |
| Other bias additional | Unclear risk | Paste used for hydrocolloid group only |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Comments: randomisation conducted by department of biometry of sponsor; said to be not blinded; paste used for hydrocolloid group only; difference between interventions for people leaving study prematurely |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Colwell 1993.
| Methods | RCT; ulcers randomised (> 1 wound per person, all followed) Funding: industry funded ‐ Convatec (manufacturer of hydrocolloid). Setting: hospital inpatients Duration of follow‐up 12 weeks Unit of analysis: ulcer | |
| Participants | 70 participants with pressure ulcers. PU stage: II (69% and 44%) and III (PU classification: NS). Age: mean (range): 68 (18‐100) years and 68 (29‐92) years. Duration of ulcer: 55% and 59% < 1 month; 45% and 41% 1‐3 months. Ulcer size: surface area: 2.29 cm² and 2.37 cm² Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: tertiary care centre; "each patient's ulcers were randomised to 1 of 2 treatments" and discussion states ulcers randomised. 94 participants enrolled, but analysis on 70 participants with 97 ulcers | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM CGF (not BNF); n = 33. Grouped intervention category: advanced dressing Group 2: gauze saline dressing ‐ saline moist; n = 37. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ not stated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ Overall 24/94 (26%) (12 died from causes unrelated to PU, 5 discharged from hospital, 5 lost to follow‐up, 1 colonised with MRSA, 1 participant's ulcer progressed to Stage 4. Equivalent number dropped from each group). Group 2 ‐ Overall 24/94 (26%) (12 died from causes unrelated to PU, 5 discharged from hospital, 5 lost to follow‐up, 1 colonised with MRSA, 1 participant's ulcer progressed to Stage 4. Equivalent number dropped from each group) i.e. overall rate only; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias unit of analysis | Low risk | Unit of randomisation ulcer and unit of analysis ulcer ‐ approx 1.5 ulcer:person ratio = 48/33 and 49/37 |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: selection bias (baseline imbalance), available case only, baseline imbalance Comments: results and number of ulcers not reported for those that dropped out of the study, so available case analysis only. Significantly more grade III ulcers for the saline gauze dressing vs hydrocolloid (56% vs 31%). Ulcers randomised and analysed so no unit of analysis issues |
Darkovich 1990.
| Methods | RCT; unit of randomisation unclear (> 1 wound per person, all followed) Funding: not stated. Setting: hospital and care home Duration of follow‐up 8.5 (60 days) weeks Unit of analysis: ulcer | |
| Participants | 90 participants with pressure ulcers. PU Stage: I and II (54% and 56%) (results separate); stage I is ulceration or skin breakdown limited to superficial epidermal and dermal layer ‐ probably corresponds to grade II? (PU classification: Enis and Sarmiento). Age: overall mean: 75 years (range 30‐98); mean in acute care 69 years, in care homes 83 years. Duration of ulcer: not stated. Ulcer size: hydrogel: mean 11.0 (range 0.2‐100) cm²; hydrocolloid: mean 9.2 (0.4‐63.75) cm² Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: it says wounds randomised, but also says people with multiple wounds had same treatments; 67/49 (1.4) and 62/41 (1.5) wounds per person | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM; n = 49 overall. Grouped intervention category: advanced dressing Group 2: hydrogel dressing ‐ Biofilm (not in BNF); n = 41 overall. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8.5 (60 days) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear ‐ no information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 4/67 (6%) excluded from the authors' analysis (3 wounds' size increased by more than 10% per day and 1 decreased by more than 25% per day). Group 2 ‐ 2/62 (3%) excluded from the authors' analysis (1 wound's size increased by more than 10% per day and 1 decreased by more than 25% per day). i.e. similar rate missing in both groups; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | High risk | Inadequate – reported incompletely |
| Other bias unit of analysis | High risk | Unit of randomisation unclear and unit of analysis ulcer ‐ Overall ulcer:person ratio = 67/49 and 62/41 (1.52) |
| Other bias additional | Unclear risk | Extraction from a graph |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high/very high Reasons: unclear selection bias, unit of analysis issues; extraction from a graph Comments: baseline difference: 11.0 versus 9.2 cm² mean wound area; number of ulcers reported for grade II only on graph. May be best to report overall (see definition of stage I). Unit of analysis issues; 6/90 participants excluded as outliers |
Gorse 1987.
| Methods | RCT; wards randomised (> 1 wound per person, all followed) Funding: not stated. Setting: hospital inpatients Duration of follow‐up approx 11 (assumed from mean + SD) weeks Unit of analysis: ulcer | |
| Participants | 52 participants with pressure ulcers. PU Stage: II (87% and 79%) and III (with acceptable definition) (PU classification: not stated) Age: mean (SD): 72.0 (12.8) years and 68.4 (13.5) years; proportion ≥ 65 years: 75% and 56%. Duration of ulcer: not stated. Ulcer size: not stated Wound characteristics at baseline: some wounds infected; slough not reported; some wounds necrotic; exudate not reported Comment: infection at baseline: 9% and 23%; proportion with necrotic wounds not stated | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM; n = 27. Grouped intervention category: advanced dressing Group 2: ineligible intervention ‐ whirlpool + chloramine dressing (gauze dampened with Dakin's solution + whirlpool hydrotherapy 3 times/week); n = 25. Grouped intervention category: ineligible ‐ whirlpool | |
| Outcomes | Primary outcomes: proportion completely healed at approx 11 (assumed from mean + SD) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none i.e. no missing data (clearly stated) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | High risk | Unit of randomisation ward and unit of analysis ulcer ‐ each ward assigned one or other treatment regimen |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: selection bias (large baseline differences); unit of analysis issues ‐ ward randomised, ulcer analysed; unclear blinding Comments: baseline differences for: proportion of ulcers in over 65 age group (greater for hydrocolloid), proportion of grade II ulcers (87% and 79%), proportion infected ulcers (9% and 23%) |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Graumlich 2003.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: mixed industry and non‐industry ‐ Biocore Medical Technologies supplied the collagen + grant from Retirement Research Foundation. Setting: care home Duration of follow‐up 8 weeks (also reported at 1 and 4 weeks) Unit of analysis: person (1 ulcer/person) | |
| Participants | 65 participants with pressure ulcers. PU Stage: 2 (77% and 83%) and 3 (PU classification: NPUAP) Age: 80.6 (SD 12.2) years and 82.0 (SD 9.9) years. Duration of ulcer: median (IQR): 6.5 (2.0, 12.0) weeks and 3.0 (1.6, 8.0) weeks (not statistically significant). Ulcer size: median (IQR) 1.74 (0.5, 4.36) and 1.21 (0.63, 3.38); not statistically significant Wound characteristics at baseline: infection not reported; no wounds sloughy; no wounds necrotic; exudate not reported Comment: wounds with eschar (not slough) or necrosis excluded (but re‐included after debridement) | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM: twice‐weekly. Standard nursing care. No ancillary non‐protocol treatments; n = 30. Grouped intervention category: advanced dressing Group 2: protease‐modulating dressing (cleansed with saline then sprinkled with collagen particles in thin continuous layer; covered with dry gauze. Standard nursing care. No ancillary non‐protocol treatments); n = 35. Grouped intervention category: protease‐modulating dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment adequate ‐ central randomisation with contact details or list held independently. Baseline comparability adequate ‐ no suggestion of problems. Rating: low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 5/30 (17%) (1 withdrew consent, 3 died, 2 hospitalised). Group 2 ‐ 6/35 (17%) (2 died, 1 hospitalised, 2loss to follow‐up). i.e. similar rate missing in both groups; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Low risk | Adequate ‐ well‐conducted study |
| ALL‐DOMAIN RISK OF BIAS | Low risk | Rating: low Comments: some differences at baseline (size and duration) but not statistically significant |
Hollisaz 2004.
| Methods | RCT; participants randomised (> 1 wound per person, all followed) Funding: non‐industry funding ‐ Jaonbazan Medical and Engineering Research Center (Iranian government body for spinal chord injury war victims). Setting: care home and community with spinal injury Duration of follow‐up 8 weeks Unit of analysis: ulcer | |
| Participants | 52 participants with pressure ulcers. PU Stage: I (33%; 36%) and II (58%, 64%) (stratified and results separate). Shea I defined as "Limited to epidermis, exposing dermis; includes a red area" (PU classification: Shea). Age: for all participants (mixed wounds): mean 36.6 (SD 6.0) years ‐ no difference between groups. Duration of ulcer: for all participants (mixed wounds): 7.6 (SD 5.6) weeks, 5.8 (SD 8.0) weeks, 5.3 (SD 5.4) weeks; P > 0.10. Ulcer size: for all participants (mixed wounds): mean 7.26 cm² (SD 15.4), 5.12 cm² (SD 3.63), 10.27 cm² (SD 15.32); P > 0.10. Wound characteristics at baseline: infection not reported; slough not reported; no wounds necrotic; exudate not reported Comment: spinal chord injury; all male and young war victims; wounds debrided first if necessary | |
| Interventions | Group 1: hydrogel dressing ‐ hydrocolloid adhesive dressing (description "hydrocolloid adhesive dressings absorb water and low molecular weight components from ulcer secretions, so they swell to produce a jelly"). No concomitant antibiotic, steroid or antisuppressant treatments allowed. No debridement needed during treatment. All other concomitant treatments the same; n = 16. Grouped intervention category: advanced dressing Group 2: phenytoin topical ‐ phenytoin topical (no concomitant antibiotic, steroid or antisuppressant treatments allowed. No debridement needed during treatment. All other concomitant treatments the same); n = 19. Grouped intervention category: phenytoin topical Group 3: saline wet ‐ no concomitant antibiotic, steroid or antisuppressant treatments allowed. No debridement needed during treatment. All other concomitant treatments the same (no concomitant antibiotic, steroid or antisuppressant treatments allowed. No debridement needed during treatment. All other concomitant treatments the same; n = 17. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | Sequence generation adequate ‐ random number tables. Allocation concealment adequate ‐ central randomisation with contact details or list held independently. Baseline comparability adequate ‐ no suggestion of problems. Rating: low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none. Group 3 ‐ none i.e. no missing data (clearly stated) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Unclear risk | Unit of randomisation person and unit of analysis ulcer ‐ probably participants randomised; if > 1 ulcer then same treatment within participant; < 1.2 ulcer:person = 18/16, 21/19 and 19/17 |
| Other bias additional | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear/low Reasons: unit of analysis issues (small) Comments: slight unit of analysis issues (but number of ulcers very close to number of participants) |
Hondé 1994.
| Methods | RCT; participants randomised (> 1 wound per person, other selection of wound) Funding: industry funded ‐ funded by Synthelabo Recherche (manufacturers of Inerpan). Setting: hospital inpatients Duration of follow‐up 8 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 168 participants with pressure ulcers. PU Stage: 1 grade I (excluded from analysis), 187 II to IV (II: 54% and 64%; III: 40% and 30%; IV: 5.7% and 6.2%) (PU classification: Shea) Age: mean 83.5 (SD 7.8; range 64‐101) years and mean 80.4 (SD 8.2, range 63‐98) years. Duration of ulcer: not stated. Ulcer size: mean surface area: 6.85 cm² and 8.99 cm² Wound characteristics at baseline: infection not reported; slough not reported; unclear necrotic; exudate unclear Comment: study says, "in cases of multiple ulcers, only one sore per patient was evaluated" | |
| Interventions | Group 1: hydrocolloid dressing ‐ Comfeel (unspecified); n = 88. Grouped intervention category: advanced dressing Group 2: ineligible intervention ‐ skin substitute (amino acid copolymer (leucine and methyl glutamate) ‐ Interpam); n = 80. Grouped intervention category: ineligible intervention ‐ skin substitute | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ vague statement about central randomisation. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 24/88 (27%) (6 withdrew because of local complications (mainly necrosis), 18 withdrew for reasons unconnected with treatment (mainly death, transfer to another ward, discharge from hospital)). Group 2 ‐ 14/80 (17.5%) (4 withdrew because of local complications (mainly necrosis), 10 withdrew for reasons unconnected with treatment (mainly death, transfer to another ward, discharge from hospital)) i.e. differential missing data rates; high differential rate – likely to change effect estimate |
| Selective reporting (reporting bias) | High risk | Inadequate ‐ analysis methods differed from those of other trials |
| Other bias unit of analysis | Unclear risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ study says, "in cases of multiple ulcers, only one sore per patient was evaluated". Not stated how many this applied to |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: not blinded, attrition bias, unclear selection bias Comments: allocation concealment: according to a randomisation list prepared by Biometry group (does not say what happened to list). Open label trial, "investigators asked to give an assessment of treatment performance (healed)". Time to event analysis using Wilcoxon. Age and grade of PU differences at baseline |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Imamura 1989.
| Methods | RCT (translation); participants randomised (only 1 wound per person) Funding: unclear. Setting: hospital inpatients Duration of follow‐up 8 weeks (also reported at 1, 2, 4, 6 weeks) Unit of analysis: person (1 ulcer/person) | |
| Participants | 141 participants with pressure ulcers. PU Stage: I (23% and 21%), II and III (44% and 38%) and IV (34% and 41%) (PU classification: not stated) Age: not stated/translated. Duration of ulcer: not stated/translated. Ulcer size: not stated Wound characteristics at baseline: unclear infection; slough not reported; necrosis not reported; exudate not reported Comment: number with change in infection status reported, but unclear what sort of change | |
| Interventions | Group 1: topical ‐ sugar plus povidone iodine: sugar 70 g/100 g and povidone iodine 3 g/100 g; ointment applied directly on the wound or applied on a sheet of gauze and then applied on the wound once or twice a day; n = 72. Grouped intervention category: sugar plus povidone iodine Group 2: other topical ‐ lysozyme ointment (5 g/100 g ointment applied directly on the wound or on a sheet of gauze and then on the wound once or twice a day); n = 69.Grouped intervention category: lysosyme ointment | |
| Outcomes | Primary outcomes: complete healing not reported; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ random number tables. Allocation concealment adequate ‐ central randomisation with contact details or list held independently. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Other evidence for no blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 27/72 (38%) (withdrew (1 because of adverse effects)). Group 2 ‐ 29/69 (42%) (withdrew (1 because of adverse effects)). i.e. similar rate missing in both groups; high rate – more than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Comments: unclear selection bias: baseline differences for proportion of Stage 4 ulcers (34% vs 41%); translated as 'not blinded'; attrition bias |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Kaya 2005.
| Methods | RCT; participants randomised (> 1 wound per person, all followed) Funding: non‐industry funding ‐ declaration of interest: none. Setting: hospital with spinal chord injury Duration of follow‐up unclear weeks Unit of analysis: ulcer | |
| Participants | 27 participants with pressure ulcers. PU Stage: 1 (24% and 25% of ulcers), 2 (68% and 71%) and 3 (results separate, but best to combine) (PU classification: NPUAP) Age: mean (SD): 35.3 (14.6), range 16‐56 years and 29.7 (6.4), range 17‐39 years. Duration of ulcer: not stated. Ulcer size: mean (SD): 4.13 (2.73; range: 2‐13) cm²; reporting of control group unclear: range 2‐35 cm² Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: spinal chord injury (78% complete, 22% incomplete SCI); 15 participants/25 ulcers and 12 participants/24 ulcers | |
| Interventions | Group 1: hydrogel dressing ‐ Elastogel (not in BNF); n = 15. Grouped intervention category: advanced dressing Group 2: iodine containing dressing ‐ povidone iodine soaked gauze; n = 12. Grouped intervention category: antimicrobial dressing | |
| Outcomes | Primary outcomes: complete healing not reported; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear ‐ no information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 0. Group 2 ‐ 0; i.e. no missing data (no details) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | High risk | Unit of randomisation person and unit of analysis ulcer ‐ for combination of stages I and II and III, ulcer:person ratio = 25/15 (1.7) and 24/12 (2.0) |
| Other bias additional | Unclear risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Comments: unclear selection bias, unclear blinding; unit of analysis issues |
Kraft 1993.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: industry funded ‐ Calgon Vestal Laboratories, manufacturer of foam dressing. Setting: hospital and care home with spinal injury Duration of follow‐up 24 weeks (also reported at 3, 6, 12 (graph) weeks) Unit of analysis: person (1 ulcer/person) | |
| Participants | 38 participants with pressure ulcers. PU Stage: II (58% overall) and III (PU classification: Enterstomal Therapy) Age: overall mean: 76, range 28‐78 years. Duration of ulcer: 58% for 2 months or less; range 0‐5 years. Ulcer size: not stated Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: 33/38 were people with spinal chord injury | |
| Interventions | Group 1: foam dressing ‐ Epi‐Lock (not in BNF); n = 24. Grouped intervention category: advanced dressing Group 2: gauze saline dressing ‐ saline moist; n = 14. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 24 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ not stated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 11/24 (45%) and (5 staff‐requested removal, 1 participant‐requested removal, 1 special bed treatment, 4 reactions to treatment). Group 2 ‐ 6/14 (43%) (2 died, 1 staff‐requested removal, 1 participant‐requested removal, 1 surgery, 1 reaction to treatment). i.e. similar rate missing in both groups; high rate – more than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, attrition bias Comments: all assessed by same rater (a registered nurse), but no information on what she knew |
Matzen 1999.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: not stated. Setting: community Duration of follow‐up 12 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 32 participants with pressure ulcers. PU Stage: III and IV: median for both groups was IV (PU classification: not stated) Age: median (range): 82 (32‐97) years and 84 (46‐89) years. Duration of ulcer: not stated. Ulcer size: not stated Wound characteristics at baseline: no wounds infected; slough not reported; unclear necrotic; exudate not reported | |
| Interventions | Group 1: hydrogel dressing ‐ amorphous hydrocolloid (hydrogel, Coloplast) ‐ in Cochrane Review as hydrogel; n = 17. Grouped intervention category: advanced dressing Group 2: gauze saline dressing ‐ saline gauze; n = 15. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 9/17 (53%) (5 other illness, 2 deaths, 1 missing schedule, 1 wish to cease participation). Group 2 ‐ 11/15 (73%) (6 insufficient effect of treatment, 3 other illness, 1 death, 1 wish to cease participation) i.e. differential missing data rates; high differential rate – likely to change effect estimate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Comments: unclear selection bias, attrition bias; unlikely that outcome assessor blinded, but not clear who it was |
Meaume 2003.
| Methods | RCT; participants randomised (unclear if > 1 wound per person) Funding: not stated. Setting: care home Duration of follow‐up 8 weeks Unit of analysis: person (unclear if > 1 ulcer analysed) | |
| Participants | 38 participants with pressure ulcers. PU Stage: 2 (PU classification: EPUAP) Age: mean age 83.8 years, range 74.9‐95.1 and 82.5 years, range 66.4‐91.9 . Duration of ulcer: at least 4 weeks; NICE guideline: mean (range) 8.3 (1‐24) weeks and 13.0 (1‐52) weeks. Ulcer size: not reported (table 2 missing); NICE guideline: mean 4.9 (0.7‐25.3) cm² and 5.4 (0.2‐26.0) Wound characteristics at baseline: no wounds infected; some wounds sloughy; no wounds necrotic; exudate not reported Comment: red‐yellow wounds in the red‐yellow‐black system (no necrosis, but some slough) | |
| Interventions | Group 1: soft polymer dressing ‐ Mepilex Border; n = 18. Grouped intervention category: advanced dressing Group 2: foam dressing ‐ Tielle; n = 20. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ envelopes not said to be opaque. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded to interventions – clear description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 1/18 ? (6%) (unclear if other withdrawals) (1 died during the study (so missing), 1 had hip fracture). Group 2 ‐ 1/20? (5%) (unclear about withdrawals) (1 died (but unclear when and not listed by authors as missing); 1 developed symptoms of heart disorder). i.e. similar rate missing in both groups; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ implies 1 per person |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Comments: unclear selection bias ‐ allocation concealment: envelopes not said to be opaque; also says block size unknown to investigators and predetermined list; not blinded; unclear re missing data and appropriate tables not available |
Motta 1999.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: industry funded ‐ educational grant from Acryl Med (manufacturer of hydrogel). Setting: community Duration of follow‐up 8 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 10 participants with pressure ulcers. PU Stage: II (30%) and III (PU classification: not stated) Age: 'average' 60 (range 34‐76) years. Duration of ulcer: 'average' 49.8 days. Ulcer size: Group 1 IPD: mean (SD) area 10.2 cm² (SD 10.6), median 6.67 cm² (range 0.75‐24); Group 2: mean(SD) 1.94 cm² (SD 1.48), median 2 cm² Wound characteristics at baseline: infection not reported; slough not reported; necrosis not reported; exudate low‐moderate levels Comment: exudate levels assumed from text | |
| Interventions | Group 1: hydrogel dressing ‐ Flexigel (not in BNF); n = 5. Grouped intervention category: advanced dressing Group 2: hydrocolloid dressing ‐ DuoDERM CGF (not BNF); n = 5. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Other evidence for no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 0. Group 2 ‐ 0. i.e. no missing data (no details) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Comments: circumstantial evidence for lack of blinding (e.g. parameters relating to dressing performance scored at each dressing change, and participants receiving wound care treatment in a home healthcare environment); hydrogel group had 4/5 grade III ulcers and hydrocolloid had 2/5; ulcer area: mean 10.2 cm² and 1.9 cm² |
Muller 2001.
| Methods | RCT; participants randomised (> 1 wound per person, all followed) Funding: non‐industry funding ‐ cost effectiveness study stated to have an unrestricted grant from Knoll AG (manufacturers of collagenase); original trial states no support from either manufacturer. Setting: hospital inpatients Duration of follow‐up probably 16 weeks Unit of analysis: person (all ulcers analysed as a whole) | |
| Participants | 24 participants with pressure ulcers. PU Stage: IV (PU classification: not stated) Age: mean (range) 72.4 (65‐78) years and 74.6 (68‐79) years. Duration of ulcer: not stated. Ulcer size: not stated Wound characteristics at baseline: infection not reported; slough not reported; no wounds necrotic; exudate not reported Comment: debridement to remove all necrotic tissue; 2/24 participants had 2 ulcers i.e. approx 1 ulcer/person. All participants were female. Heel ulcers only | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM: complete debridement first. New necrosis led to a change to alginate or collagenase (4/12; 33%); n = 12. Grouped intervention category: advanced dressing Group 2: collagenase‐containing ointment ‐ Novuxol (not BNF) (Novuxol + paraffin gauze secondary dressing. Complete debridement first. New necrosis led to a change to alginate or collagenase (1/12; 8%)); n = 12. Grouped intervention category: collagenase ointment | |
| Outcomes | Primary outcomes: proportion completely healed at probably 16 weeks; time to complete healing reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded to interventions – clear description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 1/12 (8%); 4/12 (33%) changed treatment (1 failed to comply with weekly inspection, so dropped; changed treatment for new necrosis). Group 2 ‐ 1/12 (8%) changed treatment (changed treatment for new necrosis) i.e. differential switching data rates; switching rate low ‐ less than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ reported incompletely as ‘significant’ or P value < 0.05 |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (all ulcers analysed as a whole) ‐ 2/24 (8%) participants had 2 ulcers ‐ but participants analysed; ratio ulcers:participants = 13/12 (1.08) in each group |
| Other bias additional | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high/very high Comments: not blinded; outcome assessor was 'physician each week', who also oversaw the changing of dressings (so not blinded) |
Neill 1989a.
| Methods | RCT; ulcers randomised (> 1 wound per person, all followed) Funding: industry funded ‐ 3M Company. Setting: hospital and care home Duration of follow‐up 8 weeks Unit of analysis: ulcer | |
| Participants | 87 participants with pressure ulcers. PU Stage: II (60% and 76%) and III (% of available cases) (PU classification: Shea) Age: not stated. Duration of ulcer: not stated. Ulcer size: mean (SD): 8.3 (9.9), range 0.4‐43.9 cm² and 7.6 (8.6), range 0.2‐35.2 cm² Wound characteristics at baseline: some wounds infected; some wounds sloughy; some wounds necrotic; exudate not reported Comment: 32/42 (76%) and 32/45 (71%) had infected wounds at baseline. Initially 81% and 62% wounds necrotic but treated before randomised treatments given | |
| Interventions | Group 1: hydrocolloid dressing ‐ Tegasorb (not in BNF): dressing scheduled to be changed every 7 days; if there was necrotic tissue it was debrided; n = 100 ulcers randomised (total), number of participants not stated, but available cases 87 total. Grouped intervention category: advanced dressing Group 2: gauze saline dressing ‐ saline wet‐to‐damp (dressing scheduled to be changed every 8 h; if there was necrotic tissue it was debrided); n = 100 ulcers randomised (total), number of participants not stated, but available cases 87 total. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ “randomised”. Allocation concealment inadequate ‐ alternation. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ overall 13/100 (13%) ulcers excluded from the analysis (intercurrent medical events (n = 11) and 2 had protocol violations). Group 2 ‐ overall 13/100 (13%) ulcers excluded from the analysis (intercurrent medical events (n = 11) and 2 had protocol violations) i.e. overall rate only; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias unit of analysis | Low risk | Unit of randomisation ulcer and unit of analysis ulcer ‐ 22/87 (25%) participants had 2 ulcers |
| Other bias additional | Unclear risk | 25% had 2 ulcers ‐ not treated as paired data |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high/very high Reasons: high selection bias; unclear blinding, some unit of analysis issues Comments: some baseline differences in grade of ulcer 60% and 76% grade II and HC size was larger, with more necrotic tissue; if participants had 2 ulcers, then alternation; blinding not stated, overall 13/100 missing data; number of ulcers per group not stated, so available case used; 25% had 2 ulcers ‐ not treated as paired data |
Nisi 2005.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: not stated. Setting: hospital inpatients Duration of follow‐up 8 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 80 participants with pressure ulcers. PU Stage: 2‐4 (proportion not stated) (PU classification: NPUAP) Age: mean 45 (range 35‐85) years, overall. Duration of ulcer: not stated. Ulcer size: not stated Wound characteristics at baseline: no wounds infected; slough not reported; no wounds necrotic; exudate unclear Comment: debridement to remove infection and necrosis; some exudate but level not stated | |
| Interventions | Group 1: protease‐modulating dressing ‐ Promogran: hydropolymer secondary dressing; preparation phase included hydrogel; n = 40. Grouped intervention category: protease modulating dressing Group 2: ineligible intervention ‐ povidone iodine + paraffin‐soaked gauze (50% povidone iodine wash then viscose‐rayon gauze soaked in white Vaseline + hydropolymer secondary dressing; phase 1 included hydrogel); n = 40. Grouped intervention category: ineligible ‐ basic dressing + antiseptic | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 0 (all appear to be covered). Group 2 ‐ 0 i.e. no missing data (no details) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear Reasons: unclear selection bias, unclear blinding Comments: times of healing given, so potential for time to event, but not reported |
Nussbaum 1994.
| Methods | RCT; participants randomised (> 1 wound per person, all followed) Funding: non‐industry funding ‐ study was funded by the John Labatt Seed Fund Award. Setting: hospital with spinal chord injury Duration of follow‐up could choose (IPD) e.g. Results given at 8 (reviewer choice) weeks (also reported at various times from IPD graph weeks). Unit of analysis: ulcer | |
| Participants | 20 participants with pressure ulcers. PU Stage: not stated (PU classification: not stated) Age: mean (range): 36 (15‐46) years; 42.2 (26‐59) years; 42 (30‐61) years. Duration of ulcer: > 6 weeks 67%, 100%, 100%, < 1 week 33%, 0%, 0%. Ulcer size: not stated Wound characteristics at baseline: infection not reported; slough not reported; necrosis not reported; exudate not reported Comment: people with spinal chord injury (younger people) | |
| Interventions | Group 1: basic wound contact dressing ‐ paraffin gauze (Jelonet); n = 9. Grouped intervention category: basic dressing Group 2: ineligible intervention ‐ ultrasound + UV (US/UV + Jelonet); n = 5. Grouped intervention category: ineligible ‐ ultrasound + UV Group 3: laser ‐ laser + Jelonet (laser + Jelonet; n = 6). Grouped intervention category: ineligible ‐ laser | |
| Outcomes | Primary outcomes: proportion completely healed at could choose (IPD) e.g. Results given at 8 (reviewer choice) weeks; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 3/9 (33%) (2 elected to have wounds surgically repaired and withdrew; 1 transferred to acute hospital). Group 2 ‐ 0. Group 3 ‐ 1/6 (17%) (1 transferred to acute hospital). i.e. differential missing data rates; high differential rate – likely to change effect estimate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | High risk | Unit of randomisation person and unit of analysis ulcer ‐ IPD reported per ulcer (but only 2/16 (12.5%) participants had 2 ulcers); ≤ 1.2 ulcer:person = 9/9, 6/5 and 7/6 |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: unclear selection bias, attrition bias, unit of analysis issues Comments: PU grade not reported. Baseline characteristics: laser group had 2/6 deeper ulcers (6‐10 mm), other ulcers all shallower; control group had 2/6 acute ulcers |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Oleske 1986.
| Methods | RCT; nursing module (cluster)s randomised (> 1 wound per person, all followed) Funding: non‐industry funding ‐ supported by Rush‐Presbyterian‐St Lukes Medical Center and Chicago Community Trust. Setting: hospital inpatients Duration of follow‐up 1.5 (12 days) weeks Unit of analysis: ulcer | |
| Participants | 15 participants with pressure ulcers. PU Stage: I (22% and 50%) and II, results separately for II. Inclusion criteria state all should have break in skin (PU classification: Enis and Sarmiento) Age: overall mean (SD): 69 (6), range 52‐93 years. Duration of ulcer: not stated. Ulcer size: mean 3.5 (SD 1.2), range 1.7‐5.0 cm²; mean 7.9 (SD 7.3), range 1.2‐22.7cm² Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: nursing modules on 4 participating units were randomised (no info on cluster size) | |
| Interventions | Group 1: foam dressing ‐ self adhesive PU dressing; n = 7 (5 grade II). Grouped intervention category: advanced dressing Group 2: gauze saline dressing ‐ other (normal saline dressing); n = 8 (5 grade II). Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 1.5 (12 days) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ other. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 1/16 dropped from analysis but group unclear (1 unanticipated transfer to nursing home). Group 2 ‐ 1/16 dropped from analysis but group unclear (1 unanticipated transfer to nursing home). i.e. overall rate only; high rate ‐ comparable with control event rate |
| Selective reporting (reporting bias) | Low risk | Inadequate – reported incompletely (e.g. P value > 0.05) |
| Other bias unit of analysis | High risk | Unit of randomisation nursing module (cluster) and unit of analysis ulcer ‐ 4/15 (27%) participants had 2 ulcers each (2 participants had different treatments for their 2 ulcers); < 1.3 ulcer:person ratio = 9/7 and 10/8 |
| Other bias additional | Unclear risk | Results not adjusted for clustering. Unclear if grades I and II are subgroups in this classification |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: inadequate selection bias (baseline characteristics), attrition bias, unit of analysis issues Comments: results not adjusted for clustering. Unclear if grades I and II are subgroups in this classification. Differences at baseline in proportion grade II (7/9 and 5/10 ulcers) and size of PU (mean 3.5 and 7.9 cm²) |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Parish 1979.
| Methods | RCT; participants randomised (> 1 wound per person, all followed) Funding: not stated. Setting: care home Duration of follow‐up 4 weeks Unit of analysis: results for both people and ulcers | |
| Participants | 17 participants with pressure ulcers. PU Stage: not stated (PU classification: not stated) Age: range 28‐59 years, 29‐57 years and 32‐70 years. Duration of ulcer: not stated. Ulcer size: collagenase: 10.24 cm²; dextranomer: 20.25 cm² and sugar + egg white 5.76 cm² Wound characteristics at baseline: infection not reported; slough not reported; necrosis not reported; exudate not reported Comment: assumed that all ulcers in a participant had to heal before a participant was healed | |
| Interventions | Group 1: collagenase‐containing ointment ‐ collaganese: ointment applied with wooden applicator and covered with a dry dressing; n = 5. Grouped intervention category: collagenase ointment Group 2: dextranomer ‐ dextranomer (dextranomer beads poured into the ulcer and covered with dry dressing); n = 7. Grouped intervention category: dextranomer Group 3: sugar + egg white ‐ sugar + egg white applied to the area 4 times/d (sugar + egg white applied to the area 4 times/d; n = 5. Grouped intervention category: sugar + egg white | |
| Outcomes | Primary outcomes: proportion completely healed at 4 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none. Group 3 ‐ none i.e. no missing data (no details) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Unclear risk | Unit of randomisation person and unit of analysis results for both people and ulcers ‐ we used the results for the participants, but unclear what was meant by healing => unclear risk of bias |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear Reasons: unclear selection bias, unclear unit of analysis issues Comments: says participants and investigators were blinded and nurses looked after participants => implies outcome assessors were investigators. Baseline differences said to be not statistically significant in area of ulcer |
Payne 2004.
| Methods | RCT; participants randomised (> 1 wound per person, largest selected) Funding: industry funded ‐ sponsored by Smith & Nephew Inc, makers of Dermagraft. Setting: community outpatients Duration of follow‐up 26 weeks (also reported at 12 weeks) Unit of analysis: person (1 ulcer/person) | |
| Participants | 34 participants with pressure ulcers. PU Stage: III (PU classification: not stated) Age: mean (SD): 69.1 (18.5) years and 69.4 (16.5) years. Duration of ulcer: mean (range): 29.2 (4.0‐104.0) weeks and 30.2 (6‐95.3) weeks. Ulcer size: mean (range): 21.1 (3.5‐1.2) and 19.8 (5.2‐60.7) cm² Wound characteristics at baseline: no wounds infected; slough not reported; no wounds necrotic; exudate not reported Comment: after debridement, no infection or necrotic tissue | |
| Interventions | Group 1: combination intervention ‐ other: non‐adherent + saline gauze + foam (Allevyn) dressing; n = 16. Grouped intervention category: mixed advanced and basic dressings Group 2: ineligible intervention ‐ graft + conventional dressing (Dermagraft + intervention 1 dressings); n = 18. Grouped intervention category: ineligible ‐ graft + basic and advanced | |
| Outcomes | Primary outcomes: proportion completely healed at 26 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ "sealed envelopes". Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 11/16 (69%) (1 death due to unrelated cause, other withdrawals related to morbidity). Group 2 ‐ 13/18 (72%) (3 deaths due to unrelated causes, other withdrawals related to morbidity) i.e. similar rate missing in both groups; high rate – more than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ largest ulcer selected |
| Other bias additional | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: unclear selection bias, high attrition bias Comments: high levels of missing data (70%). Says it was single blind, so this could be the outcome assessor |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Payne 2009.
| Methods | RCT; participants randomised (> 1 wound per person, largest selected) Funding: industry funded ‐ funded by Smith & Nephew (manufacturers of PU foam). Setting: care home and hospital and community Duration of follow‐up 4 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 36 participants with pressure ulcers. PU Stage: 2 (PU classification: NPUAP) Age: median 74.0 years and 71.5 years; mean (SD): 72.5 (14.3) years and 73.3 (12.4) years. Duration of ulcer: median 3.5 weeks and 2.0 weeks. Ulcer size: median 1.8 cm² and 1.4 cm² Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate low‐moderate levels Comment: multicentre (2 hospital inpatient wards, 1 hospital outpatients, 1 community, 1 care home) | |
| Interventions | Group 1: foam dressing ‐ Allevyn Thin: no secondary dressing; n = 20. Grouped intervention category: advanced dressing Group 2: gauze saline dressing ‐ saline soaked (secondary dressing as required); n = 16. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 4 weeks; time to complete healing reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ other. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear ‐ vague |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 6/20 (30%) (3 died, 1 developed wound infection, 1 developed an abscess unrelated to the study wound, 1 ineligible for other reasons). Group 2 ‐ 3/16 (19%) (2 died, 1 asked to be discharged) i.e. differential missing data rates; high differential rate – likely to change effect estimate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ largest ulcer selected |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, attrition bias Comments: "randomisation schedule"; may be a difference in duration of wound at baseline (3.5 and 2.0 weeks) |
Piatkowski 2012.
| Methods | RCT; participants randomised (> 1 wound per person, largest selected) Funding: industry funded ‐ educational grant from Lohmann & Rauscher GmbH (manufacturer of both interventions). Author employee. Setting: hospital inpatients Duration of follow‐up 3 weeks (also reported at 2 weeks) Unit of analysis: person (1 ulcer/person) | |
| Participants | 10 participants with pressure ulcers. PU Stage: 3 (PU classification: EPUAP) Age: mean (range): 67.0 (59‐71) years and 63.0 (52‐68) years. Duration of ulcer: at least 4 weeks . Ulcer size: median (range) diameter: 11.4 (5.2‐19.6) cm and 9.3 (4.3‐21.0) cm. Wound characteristics at baseline: no wounds infected; no wounds sloughy; no wounds necrotic; exudate not reported | |
| Interventions | Group 1: protease‐modulating dressing ‐ Suprasorb C: with Suprasorb P as secondary dressing; n = 5. Grouped intervention category: protease‐modulating dressing Group 2: foam dressing ‐ Suprasorb P (not in BNF); n = 5. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 3 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 0. Group 2 ‐ 0 i.e. no missing data (clearly stated) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ largest ulcer selected |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear Reasons: unclear selection bias Comments: differences at baseline probably unimportant ‐ slightly bigger diameter for the collagen group |
Price 2000.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: not stated ‐ clear statement of no funding. Setting: hospital and community Duration of follow‐up 6 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 58 participants with pressure ulcers. PU Stage: III (92% and 80%) and IV (PU classification: not stated) Age: mean (SD): 69.76 (16.2) years and 75.72 (16.8) years. Duration of ulcer: not stated. Ulcer size: mean (SD): 9.8 (12.0) cm² and 7.3 (7.0) cm², median 4.18 cm² and 5.10 cm² Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: range of settings from hospitals to their own homes. Same number of ulcers as participants in tables 1 and 2 | |
| Interventions | Group 1: alginate dressing ‐ type not stated (standard care); n = 26 (missing data added). Grouped intervention category: advanced dressing Group 2: ineligible intervention ‐ radiant heat; n = 32 (missing data added). Grouped intervention category: ineligible ‐ radiant heat | |
| Outcomes | Primary outcomes: proportion completely healed at 6 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment adequate ‐ serially‐numbered opaque sealed envelopes. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 1/26 (4%); Group 2 ‐ 7/32 (22%). Reasons for 'missingness' across both groups: 3 died, 3 experienced general deterioration, 1 experienced device‐related deterioration and 1 asked to withdraw: i.e. differential missing data rates; high differential rate – likely to change effect estimate |
| Selective reporting (reporting bias) | Low risk | Adequate – outcome measured but not necessarily analysed for a good reason |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias (baseline differences); attrition bias Comments: time to event recorded for 75%, 50%, 25% healed but not 100% ‐ probably available, but few events. Differences at baseline in diabetes, urinary incontinence, neurological disorders, BMI (direction not stated), proportion of stage III (92% and 80%) |
Ramos‐Torrecillas 2015.
| Methods | RCT; participants randomised (> 1 wound per person, all followed) Funding: non‐industry funding. Setting: hospital inpatients Duration of follow‐up 5 (36 days) weeks Unit of analysis: ulcer | |
| Participants | 124 ulcers, participants with pressure ulcers. PU Stage: 2 and 3 (control: 96%, group A: 85.3%, group B: 100% and group C: 60%) (PU classification: EPUAP) Age: overall mean (SD): 82.5 (4.7) years, range 64‐90 years. Duration of ulcer: mean (SD): control 6.2 (1.5) months; group A 4.8 (1.1) months, group B 5.0 (1.6) months and group C 4 (1.1) months. Ulcer size: not stated Wound characteristics at baseline: no wounds infected; slough not reported; no wounds necrotic; exudate not reported Comment: one long‐stay hospital and 3 'geriatric centres' in Granada, Spain | |
| Interventions | Group 1: hydrogel dressing ‐ Intrasite Gel: saline cleansing, hydrogel and PU (secondary) dressing; n = 25 ulcers. Grouped intervention category: advanced dressing Group 2: ineligible intervention ‐ growth factor gel (combining 2 GF groups (1 and 2 doses) + hydrogel; % estimated from graph (8% and 32% respectively); n = 59 ulcers. Grouped intervention category: ineligible ‐ growth factor gel Group 3: growth factor gel + hyaluronic acid ‐ platelet GF + HA + hydrogel (platelet GF + HA + hydrogel; n = 40 ulcers;. Grouped intervention category: ineligible ‐ growth factor gel + HA | |
| Outcomes | Primary outcomes: proportion completely healed at 5 (36 days) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 15/115 (13%) overall (loss to follow‐up). Group 2 ‐ 15/115 (13%) overall (loss to follow‐up). Group 3 ‐ 15/115 (13%) overall (loss to follow‐up). i.e. overall rate only; high rate ‐ comparable with control event rate |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias unit of analysis | High risk | Unit of randomisation person and unit of analysis ulcer ‐ multiple PUs per person treated with the same interventions. 140 ulcers in 100 persons across both groups. Unit of analysis issue |
| Other bias additional | Unclear risk | Data extracted from graph |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: unclear selection bias, not blinded, unit of analysis issues, data extracted from graph Comments: some baseline differences (e.g. group C had more Grade II ulcers) |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Rees 1999.
| Methods | RCT; participants randomised (> 1 wound per person, likely slowest healing wound selected) Funding: industry funded ‐ funded by Johnson & Johnson Inc. Setting: unclear Duration of follow‐up 16 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 124 participants with pressure ulcers. PU Stage: 3 and 4 (PU classification: NPUAP) Age: mean (SD) group 1: 50 (13.6) years; group 2: 48 (13.1) years; group 3: 49 (12.5) years and group 4: 51 (18.3) years. Duration of ulcer: median (IQR) Group 1: 30 (43) weeks; group 2: 22 (32) weeks; group 3: 33 (40) weeks and group 4: 22 (52) weeks. Ulcer size: ulcer volume median (IQR): group 1: 19.6 (21.9) cm²; group 2: 16.6 (15.1) cm²; group 3: 17.2 (19.7) cm² and group 4: 17.6 (33.8) cm² Wound characteristics at baseline: no wounds infected; no wounds sloughy; no wounds necrotic; exudate not reported Comment: probably a community‐based study; ulcer selected that was likely to be the slowest healing; debridement to remove necrotic material and fibrin (slough) | |
| Interventions | Group 1: hydrogel dressing ‐ carboxymethylcellulose vehicle gel (as placebo) + saline gauze; n = 31. Grouped intervention category: advanced dressing
Group 2: ineligible intervention ‐ 100 µg / g of growth factor in sodium carboxymethylcellulose vehicle gel + saline gauze Group 3: ineligible intervention ‐ 300 µg / g of growth factor in vehicle gel + saline gauze Group 4: ineligible intervention ‐ 100 µg / g of growth factor in vehicle gel, twice daily + saline gauze Results available separately ‐ numbers calculated from % ‐ but results from groups 2‐4 were combined ( n = 93). Grouped intervention category: ineligible ‐ growth factor gel |
|
| Outcomes | Primary outcomes: proportion completely healed at 16 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data: Group 1 ‐ unclear but may be 0. Group 2 ‐ unclear but may be 1 (1 participant with 100 microg bid discontinued). i.e. similar rate missing in both groups; unclear rate |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ ulcer selected that was likely to be the slowest healing |
| Other bias additional | Unclear risk | Results calculated from percentages |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias; results calculated from percentages Comments: number of missing data unclear, assumed 0. Slight differences in duration of ulcer |
Romanelli 2001.
| Methods | RCT (abstract); participants randomised (unclear if > 1 wound per person) Funding: not stated. Setting: not stated Duration of follow‐up 8 weeks Unit of analysis: unclear | |
| Participants | 12 participants with pressure ulcers. PU Stage: 2 and 3 (proportions not stated) (PU classification: EPUAP) Age: not stated. Duration of ulcer: not stated. Ulcer size: not stated Wound characteristics at baseline: infection not reported; slough not reported; necrosis not reported; exudate not reported | |
| Interventions | Group 1: hydrogel dressing ‐ DuoDERM Hydrogel (not in BNF): with OpSite Flexigrid secondary dressing; n = 6. Grouped intervention category: advanced dressing Group 2: topical ‐ tripeptide‐copper gel + OpSite; n = 6. Grouped intervention category: tripeptide‐copper | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data: Group 1 ‐ 0 (implied). Group 2 ‐ 0 (implied) i.e. unclear if data missing; unclear rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Unclear risk | Unit of randomisation person and unit of analysis unclear ‐ 1 ulcer per person implied |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear Reasons: unclear selection bias, unclear attrition, unclear blinding (abstract); preliminary results Comments: abstract ‐ few details |
Sebern 1986.
| Methods | RCT; ulcers randomised (> 1 wound per person, all followed) Funding: mixed industry and non‐industry ‐ part supported by Research Grant Award to the University Nursing dept from Sigma Theta Tau and part funded by 3M Medical Division. Setting: home care population Duration of follow‐up 8 weeks Unit of analysis: ulcer | |
| Participants | 48 participants with pressure ulcers. PU Stage: II and III (41% and 70% grade III) (PU classification: Shea). All participants had chronic illness (focal cerebral disorders, spinal chord disorders, neurological disorders, cardiac disease, diabetes) Age ‐ mean (SD): group 1: 76.3 (SD 17.6) years; group 2: 72.4 (SD 17.8) years. Duration of ulcer: not stated. Ulcer size: group 1: grade II median (range) 1.9 (0.1‐32.9) cm²; grade III 6.1 (0.3‐33.0) cm². Group 2: grade II 3.4 (0.6‐23.9) cm², grade III 4.5 (0.5‐47.1) cm² | |
| Interventions | Group 1: vapour‐permeable dressing: polyurethane adhesive dressing; vapour‐permeable; n = unclear number randomised, but overall 48 participants in analysed population. Grouped intervention category: advanced dressing Group 2: gauze saline dressing ‐ wet‐to‐dry; n = unclear number randomised, but overall 48 participants in analysed population. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: complete healing not reported; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ other. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear ‐ vague |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data: Group 1 ‐ 13/50 (26%) ulcers missing (number participants missing not reported) (Overall, the "Most frequent causes of dropout were: death, hospitalisation, and inability to comply with protocol for pressure relief" ‐ no more information). Group 2 ‐ 10/50 (20%) ulcers missing (number participants missing not reported) (Overall, the "Most frequent causes of dropout were: death, hospitalisation, and inability to comply with protocol for pressure relief" ‐ no more information) i.e. similar rate missing in both groups; unclear rate |
| Selective reporting (reporting bias) | High risk | Comment: inadequate – reported incompletely (results given only for grade II ulcers and "not significantly different" for grade III ulcers) |
| Other bias unit of analysis | Low risk | Unit of randomisation ulcer and unit of analysis ulcer; > 6 people had 2 or more ulcers; 6 people had 2 ulcers assigned to different treatments; 77/48 (1.6) ulcers: people in available case analysis. |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: selection bias (baseline differences), unit of analysis issues; selective outcome reporting bias Comments: sequential list of 100 random numbers was used to assign the treatment: unclear where list kept. Outcome assessor was project director who made weekly visits to assess the wound and review the protocol for wound care ‐ implies not blinded; baseline differences: proportion of stage II different (59% vs 30%) and size of ulcer differences but numbers only reported for stage II |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Seeley 1999.
| Methods | RCT; participants randomised (> 1 wound per person, largest selected) Funding: not stated. Setting: care home and outpatients Duration of follow‐up 8 weeks Unit of analysis: person (selected ulcer) | |
| Participants | 40 participants with pressure ulcers. PU Stage: II (11 and 15%) and III (PU classification: AHCPR) Age: mean (SD): 76.7 (19.5) years and 75.7 (18.6) years. Duration of ulcer: median: 10 weeks and 9 weeks. Ulcer size: mean(SD): 4.61 (5.56) cm² and 6.84 (8.19) cm² Wound characteristics at baseline: no wounds infected; some wounds sloughy; necrosis not reported; exudate not reported Comment: slough: 4/19 (21%) and 5/20 (25%) | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM CGF (not BNF); n = 20. Grouped intervention category: advanced dressing Group 2: foam dressing ‐ Allevyn Adhesive; n = 20. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Other evidence for no blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 6/20 (30%) (2 adverse effects (both due to dressing), 1 death, 2 increased ulcer size, 1 unable to tolerate dressing). Group 2 ‐ 8/20 (40%) (1 participant request, 3 loss to follow‐up, 2 adverse effects (1 related to dressing), 1 death, 1 infection). i.e. similar rate missing in both groups; high rate ‐ comparable with control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (selected ulcer) ‐ largest ulcer selected |
| Other bias additional | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, not blinded, some attrition bias Comments: stratified randomisation (by size); unlikely to be blinded ‐ assessors were clinical investigators who changed dressings. Attrition bias borderline high (because of reasons for missingness) |
Serena 2010.
| Methods | RCT (abstract); not stated randomised (unclear if > 1 wound per person) Funding: not stated. Setting: not stated Duration of follow‐up 12 weeks Unit of analysis: person (unclear if > 1 ulcer analysed) | |
| Participants | 74 participants with pressure ulcers. PU Stage: 3 (PU classification: NPUAP) Age: not stated. Duration of ulcer: mean (SD): 71 (59) weeks and 84 (139) weeks. Ulcer size: mean (SD): 8.1 (76.1) cm² and 9.8 (12.5) cm² Wound characteristics at baseline: infection not reported; slough not reported; necrosis not reported; exudate not reported Comment: debridement throughout trial | |
| Interventions | Group 1: combination intervention ‐ "primary nonadherent silicone dressing and foam dressing"; n = 44. Grouped intervention category: advanced dressing Group 2: ineligible intervention ‐ skin substitute (Apligraf (bilayered living cell‐based treatment)); n = 30. Grouped intervention category: ineligible ‐ skin substitute | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data: Group 1 ‐ none stated. Group 2 ‐ none stated i.e. unclear if data missing; unclear rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Unclear risk | Unit of randomisation not stated and unit of analysis person (unclear if > 1 ulcer analysed) ‐ implies 1 per person |
| Other bias additional | Unclear risk | Unclear if the trial was stopped early because of the results |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, possibly terminated early, unclear blinding and attrition ‐ abstract. Comments: conclusions say "although this study was terminated early… trials of longer duration are required". It is unclear if this means the trial was stopped early because of the results. Baseline difference in ulcer size and duration (larger for the bilayer) |
Sipponen 2008.
| Methods | RCT; participants randomised (> 1 wound per person, all followed) Funding: industry funded ‐ study authors have now founded a company to manufacturer intervention 1. Setting: hospital inpatients Duration of follow‐up 26 weeks (6 months) Unit of analysis: results for both people and ulcers | |
| Participants | 37 participants with pressure ulcers. PU Stage: 2 (39% and 45%), 3 (50% and 45%) and 4 (11% and 9%) (PU classification: EPUAP) Age: per protocol: mean (SD) 80 (10) years and 74 (8) years; range 58‐98 years and 60‐88 years. Duration of ulcer: not stated. Ulcer size: width mean(SD): 3.2 (2.4) cm and 4.2 (2.8) cm Wound characteristics at baseline: some wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: 27/21 and 18/16 ulcers per person (18 (86%) and 14 (88%) participants had only 1 ulcer); number of ulcers infected not stated | |
| Interventions | Group 1: resin salve ‐ resin salve: Norway spruce salve mixed with butter between gauze; n = 21. Grouped intervention category: antimicrobial Group 2: hydrocolloid or hydrocolloid silver dressing ‐ Aquacel + Aquacel Ag (Aquacel Ag if infected wounds (NS proportion)); n = 16). Grouped intervention category: advanced ‐ antimicrobial | |
| Outcomes | Primary outcomes: proportion completely healed at 26 (6 months) weeks; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ other. Allocation concealment unclear ‐ other. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear ‐ vague |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 8/21 (38%) (3 deaths, 2 admissions to operative treatment, 1 allergic skin reaction, 1 misdiagnosis, 1 participant‐based refusal without any specific cause). Group 2 ‐ 7/16 (44%) (4 deaths, 2 participant‐based refusal without any specific cause, 1 participant‐based refusal because of randomisation to control group) i.e. similar rate missing in both groups; high rate – more than control event rate |
| Selective reporting (reporting bias) | High risk | Inadequate ‐ other. Time to event outcome excluded dropouts |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis results for both people and ulcers ‐ 3/21 (14%) and 2/16 (12.5%) participants had > 1 ulcer; study analysis seemed to require that all ulcers in a person should heal; ulcers:person ratio = 27/21 (1.3) and 18/16 (1.1) |
| Other bias additional | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, attrition bias, time to event outcome excluded drop outs, so risk of outcome reporting bias for that outcome only Comments: randomisation in permuted blocks of 4. Randomisation list in closed envelopes. Independent physicians in each hospital assessed wound ‐ this is probably enough for blinding. Time to event outcome excluded dropouts |
Sopata 2002.
| Methods | RCT; participants randomised (> 1 wound per person, all followed) Funding: non‐industry funding ‐ declaration of interest: none. Setting: hospital inpatients Duration of follow‐up 8 weeks Unit of analysis: ulcer | |
| Participants | 34 participants with pressure ulcers. PU Stage: II (non‐blanching erythema and superficial damage ‐ may be closer to NPUAP I; 33% and 30%) and III (PU classification: Torrance) Age: mean (SD): 58.7 (14.1) years and 58.5 (16.9) years. Range overall: 24–88 years. Duration of ulcer: mean (SD): 2.45 (1.60) weeks and 2.46 (0.24) weeks. Ulcer size: mean (SD): 8.28 (13.90) cm² and 11.04 (11.65) cm². Range: 0.41‐98.78 and 0.68‐51.05 cm² Wound characteristics at baseline: some wounds infected; slough not reported; no wounds necrotic; exudate not reported Comment: participants were people with advanced cancer in palliative care department; 38/34 ulcers per person; 9/17 (53%) and 10/17 (59%) participants had infected wounds | |
| Interventions | Group 1: hydrogel dressing ‐ Aquagel (not in BNF); n = 17. Grouped intervention category: advanced dressing Group 2: foam dressing ‐ Lyofoam; n = 17. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 3/17 (18%) (3 died ). Group 2 ‐ 2/17 (12%) (2 died) i.e. similar rate missing in both groups; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | High risk | Unit of randomisation person and unit of analysis ulcer ‐ ulcer:person ratio = 20/17 (1.2) and 18/17 (1.1) |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Comments: unclear selection bias, unclear subgroup ‐ grade II Torrance may be closer to NPUAP stage I, could be subgroup issue. Slightly larger wounds for foam. Slight unit of analysis issue |
Thomas 1997a.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: not stated. Setting: community Duration of follow‐up 6 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 99 participants stratified by wound. PU Stage: II and III (61% and 54% grade II) (PU classification: Stirling) Age: 78.6 (SD 14.3) years, 80.1 (SD 10.2) years. Duration of ulcer: 9 and 8 at < 1 month; 18 and 21 at 1‐3 months, 21 and 20 at > 3 months. Ulcer size: not stated Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: text says "for each wound type, patients were allocated to 2 treatment groups" => implied stratification | |
| Interventions | Group 1: hydrocolloid dressing ‐ Granuflex: cleansed using 0.9% saline as necessary; n = 49. Grouped intervention category: advanced dressing Group 2: foam dressing ‐ Tielle (cleansed using 0.9% saline as necessary); n = 50. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 6 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ "sealed envelopes". Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 1/49 (2%) and some may have died (reason not stated; overall 5 participants died). Group 2 ‐ 2/50 (4%) and some may have died (reason not stated; overall 5 participants died) i.e. similar rate missing in both groups; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, not blinded Comments: difference in proportion of grade II ulcers (61% and 54%) |
Thomas 1998.
| Methods | RCT; participants randomised (> 1 wound per person, other selection of wound) Funding: industry funded ‐ grant from Carrington labs Inc (hydrogel manufacturers). Setting: care home and community Duration of follow‐up 10 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 41 participants with pressure ulcers. PU Stage: II (50% and 43%), III (38% and 50%) and IV (13% and 7%) (PU classification: not stated) Age: mean (SD): 79 (9) years and 72 (13) years. Duration of ulcer: not stated. Ulcer size: mean (SD): 8.9 (9.3) cm² and 5.9 (6.0) cm² Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported | |
| Interventions | Group 1: hydrogel dressing ‐ Carrosyn Gel Wound Dressing (contains Acemannan hydrogel ‐ from aloe vera); n = 22. Grouped intervention category: advanced dressing Group 2: gauze saline dressing ‐ saline moist; n = 19. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 10 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear ‐ vague |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 6/22 (27%) (4 died (not attributed to treatment), 1 showed deterioration and was terminated from study, 1 participant hospitalised). Group 2 ‐ 5/19 (26%) (2 died (not attributed to treatment), 1 showed deterioration and was terminated from study, 1 participant hospitalised, 1 protocol violation) i.e. similar rate missing in both groups; low rate ‐ less than control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ 1 per person; NS how selected |
| Other bias additional | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear Reasons: unclear selection bias; unclear blinding Comments: baseline difference in ulcer size (8.9 cm² and 5.9 cm², but not significant); unclear if outcome assessors were blinded ‐ "study nurses who evaluated weekly" |
Thomas 2005.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: not stated. Setting: care home and outpatients Duration of follow‐up 12 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 41 participants with pressure ulcers. PU Stage: III (55% and 52%) or IV (PU classification: not stated) Age: mean (SD): 77.0 (11.5) years and 74.1 (13.8) years. Duration of ulcer: not stated. Ulcer size: mean (SD): 12.1 (18.2) cm² and 11.0 (9.5) cm² Wound characteristics at baseline: no wounds infected; slough not reported; necrosis not reported; exudate not reported Comment: one ulcer evaluated per person | |
| Interventions | Group 1: hydrocolloid with or without alginate ‐ DuoDERM with or without Sorbasan: calcium alginate filler given as needed if the wound was highly exudative. Dressing changed every 7 d; n = 20. Grouped intervention category: advanced dressing Group 2: ineligible intervention ‐ radiant heat (dressing change every 7 d); n = 21. Grouped intervention category: ineligible ‐ radiant heat | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ "opaque envelopes". Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded to interventions – deduced from interventions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 4/20 (20%) (1 died, 3 hospitalised). Group 2 ‐ 6/21 (29%) (2 died, 2 hospitalised, 2 dropped out for non‐study‐related reasons) i.e. similar rate missing in both groups; high rate ‐ comparable with control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ unclear if selected |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: unclear selection bias, not blinded, attrition bias Comments: outcome assessed at each visit after removing dressing ‐ not blinded |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Van De Looverbosch 2004.
| Methods | RCT (abstract); participants randomised (unclear if > 1 wound per person) Funding: industry funded ‐ Molnlycke Health Care sponsored the study. Setting: not stated Duration of follow‐up 8 weeks Unit of analysis: person (unclear if > 1 ulcer analysed) | |
| Participants | 11 participants with pressure ulcers. PU Stage: II only (no subcutaneous involvement) (PU classification: not stated) Age: mean 87.7 years and 88.2 years; 75 years and over. Duration of ulcer: more than 1 month. Ulcer size: not stated Wound characteristics at baseline: infection not reported; slough not reported; necrosis not reported; exudate not reported | |
| Interventions | Group 1: topical ‐ enamel matrix protein; n = 6. Grouped intervention category: enamel matrix protein Group 2: topical ‐ propylene glycol alginate (vehicle ‐ propylene glycol alginate); n = 5. Grouped intervention category: propylene glycol alginate | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded ("open label") and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data: Group 1 ‐ none stated. Group 2 ‐ none stated i.e. unclear if data missing; unclear rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Unclear risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ implies 1 per person |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, not blinded Comments: comparable in age, more women in control group |
Xakellis 1992.
| Methods | RCT; participants randomised (> 1 wound per person, ulcer chosen at random) Funding: non‐industry funding ‐ explicit statement that not industry funded. Supported by The Family Health Foundation of America. Setting: care home Duration of follow‐up 26 weeks (6 months) protocol Unit of analysis: person (1 ulcer/person) | |
| Participants | 39 participants with pressure ulcers. PU Stage: II (100% and 90%) and III (Shea ‐ must have a break in the skin for inclusion) (PU classification: Shea) Age: mean (SD): 77.3 (16.9) years and 83.5 (10.6) years. Duration of ulcer: not stated. Ulcer size: median (range): 0.66 (0.12‐13.4) cm² and 0.38 (0.04‐24.6) cm² Wound characteristics at baseline: infection not reported; slough not reported; some wounds necrotic; exudate mixed levels Comment: necrotic tissue: 2/18 (11%) and 7/21 (33%) but debridement used before and throughout, so unclear whether successful. Exudate: level not stated, but 9/18 (50%) and 7/21 (33%) had exudate at baseline. Exudate and necrosis were independent predictors of healing | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM; n = 18. Grouped intervention category: advanced dressing Group 2: gauze saline dressing ‐ saline wet‐to‐moist; n = 21. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 26 weeks (6 months); time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded to interventions – clear description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ 2/18 (11%) (1 hospitalised, 1 withdrew consent). Group 2 ‐ 3/21 (14%) (3 died) i.e. similar rate missing in both groups; low rate – unlikely to alter the effect estimate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ ulcer chosen at random (by coin toss) |
| Other bias additional | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high Reasons: unclear selection bias, not blinded |
Yapucu Güneş 2007.
| Methods | RCT; participants randomised (> 1 wound per person, all followed) Funding: not stated. Setting: hospital inpatients Duration of follow‐up 5 weeks Unit of analysis: ulcer | |
| Participants | 27 participants with pressure ulcers. PU Stage: II and III (96% III in both groups) (PU classification: AHCRQ) Age: mean (SD): 65.80 (6.30) years and 66.56 (5.53) years. Duration of ulcer: not stated. Ulcer size: not stated Wound characteristics at baseline: unclear infection; slough not reported; necrosis not reported; exudate not reported Comment: staging used AHRQ guidelines (probably NPUAP). Infection implied (control said to be a treatment for infected ulcers). 50+ ulcers (1 participant excluded and not stated no. of ulcers), 27 participants; all ulcers assessed | |
| Interventions | Group 1: honey ‐ unprocessed gauze impregnated (dressing): semi‐permeable adhesive secondary dressing; n = 15. Grouped intervention category: antimicrobial Group 2: combination dressing ‐ ethoxy‐diaminoacridine plus nitrofurazone dressings; n = 12. Grouped intervention category: antimicrobial | |
| Outcomes | Primary outcomes: proportion completely healed at 5 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded to interventions – clear description |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: Group 1 ‐ 0. Group 2 ‐ 1/12 (8%) (1 died) i.e. similar rate missing in both groups; high rate ‐ comparable with control event rate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ reported incompletely as ‘significant’ or P value < 0.05 |
| Other bias unit of analysis | High risk | Unit of randomisation person and unit of analysis ulcer ‐ ulcer:person ratio: 25/15 (1.7) and 26/12 (2.2) |
| Other bias additional | Unclear risk | Only available case analysis reported |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high Reasons: unclear selection bias, not blinded, attrition bias, unit of analysis issues |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
Zeron 2007.
| Methods | RCT; participants randomised (only 1 wound per person) Funding: unclear ‐ product supplied by Aspid. Setting: hospital inpatients Duration of follow‐up 3 weeks Unit of analysis: person (1 ulcer/person) | |
| Participants | 24 participants with pressure ulcers. PU Stage: 2 and 3 (PU classification: NPUAP) Age: mean 79.8 years and 78.3 years. Duration of ulcer: not stated. Ulcer size: diameter mean (SD): 3.4 (1.2) cm and 2.9 (1.3) cm Wound characteristics at baseline: infection not reported; slough not reported; necrosis not reported; exudate not reported Comment: IPD reported | |
| Interventions | Group 1: protease‐modulating dressing ‐ Fibroquel: collagen plus polyvinylpyrrolidone + zinc oxide paste cleansing; n = 12. Grouped intervention category: protease‐modulating dressing Group 2: polyvinylpyrrolidone (PVP + zinc oxide paste cleansing); n = 12. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 3 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ random number tables. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none. i.e. no missing data (clearly stated) |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias unit of analysis | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias additional | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear Reasons: unclear selection bias, unclear who outcome assessor was, unclear reporting of numbers healed (but not a problem) Comments: healing data not reported explicitly, but deduced from IPD on ulcer size (number with zero size) |
AHRQ: US Agency for Healthcare Research and Quality BNF: British National Formulary HC: hydrocolloid IPD: individual participant data NPWT: negative pressure wound therapy NS: not stated RCT: randomized controlled trial UV: ultraviolet
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbott 1968 | Ineligible outcomes |
| Agren 1985 | Ineligible outcomes |
| Ahmad 2008 | Ineligible intervention |
| Alvarez 1999 | Ineligible outcomes |
| Alvarez 2000a | Ineligible outcomes |
| Alvarez 2000b | Ineligible outcomes |
| Alvarez 2002 | Ineligible outcomes |
| Alvarez Vázquez 2014 | Ineligible patient population |
| Aminian 1999 | Ineligible type of healing outcome |
| Amione 2005 | Comparison of two interventions in the same class |
| Anitua 2008 | Ineligible patient population |
| Anonymous 1982 | Ineligible study design |
| Anonymous 2000 | Ineligible study design |
| Anzai 1989 | Ineligible patient population |
| Avanzi 1998a | Ineligible outcomes |
| Avanzi 1998b | Ineligible outcomes |
| Avanzi 2000a | Ineligible outcomes |
| Avanzi 2000b | Ineligible outcomes |
| Avanzi 2000c | Ineligible outcomes |
| Avanzi 2001 | Ineligible outcomes |
| Baade 1965 | Ineligible intervention |
| Baatenburg de Jong 2004 | Ineligible patient population |
| Baker 1981 | Ineligible study design |
| Bale 1997b | Ineligible outcomes |
| Bale 1997c | Comparison of two interventions in the same class |
| Bale 1998a | Ineligible patient population |
| Bale 1998b | Ineligible outcomes |
| Bale 2004 | Ineligible outcomes |
| Banks 1997a | Ineligible outcomes |
| Banks 1997b | Ineligible indication |
| Barnes 1992 | Comparison of two interventions in the same class |
| Bazzigaluppi 1991 | Ineligible study design |
| Becker 1984 | Ineligible type of healing outcome |
| Beele 2010 | Ineligible patient population |
| Berard 1986 | Ineligible study design |
| Bigolari 1991 | Ineligible patient population |
| Bito 2012 | Mixed intervention |
| Blanco Blanco 2002 | Ineligible indication |
| Blum 1973 | Ineligible patient population |
| Boxer 1969 | Ineligible outcomes |
| Boykin 1989 | Ineligible study design |
| Brady 1987 | Ineligible study design |
| Brem 2000 | Ineligible study design |
| Brett 2003 | Ineligible outcomes |
| Brown‐Etris 1999a | Ineligible type of healing outcome |
| Brown‐Etris 1999b | Mixed intervention |
| Burgos 2000 | Comparison of two interventions in the same class |
| Burke 1998 | Ineligible type of healing outcome |
| Capillas Pérez 2000 | Ineligible patient population |
| Carusone 2001 | Ineligible indication |
| Casali 1997 | Ineligible study design |
| Chang 1998 | Ineligible outcomes |
| Chen 2004 | Ineligible intervention |
| Cheneworth 1994 | Ineligible study design |
| Chirwa 2010 | Ineligible patient population |
| Chuangsuwanich 2011a | Ineligible type of healing outcome |
| Chuangsuwanich 2011b | Ineligible outcomes |
| Chuangsuwanich 2013 | Ineligible outcomes |
| Colin 1996a | Ineligible type of healing outcome |
| Colin 1996b | Ineligible type of healing outcome |
| Colonna 2004 | Ineligible study design |
| Cooper 2008 | Ineligible patient population |
| Coutts 2000 | Ineligible outcomes |
| D'Aniello 1998 | Ineligible outcomes |
| Dat 2014 | Ineligible study design |
| Day 1995 | Comparison of two interventions in the same class |
| De Laat 2005 | Ineligible outcomes |
| De Laat 2011 | Ineligible type of healing outcome |
| Dealey 1997 | Ineligible outcomes |
| Dealey 1998 | Ineligible study design |
| Dealey 2008 | Editorial |
| Dierick 2004a | Ineligible outcomes |
| Dierick 2004b | Ineligible type of healing outcome |
| Dobrzanski 1990 | Comparison of two interventions in the same class |
| Durović 2008 | Ineligible type of healing outcome |
| Dwivedi 2016 | Ineligible type of healing outcome |
| El Zayat 1989 | Ineligible study design |
| Ellis 2002 | Ineligible type of healing outcome |
| Ellis 2003 | Ineligible type of healing outcome |
| Engdahl 1980 | Ineligible study design |
| Esch 1989 | Ineligible type of healing outcome |
| Farsaei 2014 | Ineligible patient population |
| Fear 1992 | Ineligible outcomes |
| Feldman 2005 | Ineligible type of healing outcome |
| Felzani 2011 | Ineligible type of healing outcome |
| Flanagan 1995 | Ineligible study design |
| Ford 2002 | Mixed intervention |
| Fowler 1983 | Ineligible study design |
| Franek 2011 | Mixed intervention |
| Franek 2012 | Mixed intervention |
| Franken 1999 | Ineligible type of healing outcome |
| Fulco 2015 | Ineligible type of healing outcome |
| Fønnebø 2008 | Ineligible study design |
| García González 2002 | Ineligible outcomes |
| Garrett 1969 | Ineligible outcomes |
| Gerding 1992 | Ineligible patient population |
| Gilligan 2014 | Ineligible intervention |
| Goldmeier 1997 | Ineligible type of healing outcome |
| Gostishchev 1983 | Ineligible study design |
| Greer 1999 | Ineligible type of healing outcome |
| Gregory 1997 | Ineligible intervention |
| Guthrie 1989 | Ineligible type of healing outcome |
| Hamilton Hislop 1962 | Ineligible study design |
| Hampton 1998 | Ineligible patient population |
| Harada 1996 | Ineligible type of healing outcome |
| Harding 1996 | Ineligible outcomes |
| Harding 2000 | Ineligible study design |
| Helaly 1988 | Ineligible patient population |
| Heuckeroth 2013 | Ineligible study design |
| Heyer 2013 | Ineligible study design |
| Hinz 1986 | Ineligible patient population |
| Hirshberg 2001 | Ineligible intervention |
| Hock 1997 | Comparison of two interventions in the same class |
| Hofman 1994 | Ineligible type of healing outcome |
| Horch 2005 | Ineligible study design |
| Hsu 2000 | Ineligible study design |
| Hu 2009 | Ineligible patient population |
| Ishibashi 1991 | Ineligible patient population |
| Ishibashi 1996 | Ineligible patient population |
| Janssen 1989 | Ineligible patient population |
| Jercinovic 1994 | Ineligible intervention |
| Johnson 1992 | Mixed intervention |
| Kallianinen 2000 | Ineligible intervention |
| Karap 2008 | Ineligible outcomes |
| Kerihuel 2010 | Ineligible type of healing outcome |
| Kerstein 2004 | Ineligible study design |
| Kim 1996 | Ineligible patient population |
| Kloth 2000a | Mixed intervention |
| Kloth 2000b | Ineligible study design |
| Kloth 2001 | Mixed intervention |
| Kloth 2002 | Mixed intervention |
| Knudsen 1982 | Ineligible type of healing outcome |
| Kohr 2000 | Ineligible outcomes |
| Kordestani 2008 | Ineligible study design |
| Kucan 1981 | Ineligible outcomes |
| Kuflik 2001 | Ineligible patient population |
| Kuisma 1987 | Ineligible indication |
| Kukita 1990 | Ineligible type of healing outcome |
| Kurring 1994 | Ineligible study design |
| Kurzuk‐Howard 1985 | Ineligible study design |
| Landi 2003 | Ineligible intervention |
| Langer 1996 | Ineligible intervention |
| Lazareth 2012 | Ineligible patient population |
| Lechner 1991 | No results |
| Lee 1975 | Ineligible type of healing outcome |
| Lee 2014 | Ineligible patient population |
| LeVasseur 1991 | Ineligible study design |
| Li 2016 | Dressings/topical agents not the only difference between interventions (nursing care was also different) |
| Lin 1997 | Ineligible study design |
| Lindsay 2011 | Ineligible study design |
| Lingner 1984 | Ineligible study design |
| Liu 2012 | Ineligible type of healing outcome |
| Liu 2013 | Ineligible study design |
| Ljungberg 1998 | Ineligible type of healing outcome |
| Llewellyn 1996 | Ineligible outcomes |
| Lopez‐Jimenez 2003 | Ineligible outcomes |
| Lum 1996 | Ineligible type of healing outcome |
| Macario 2002 | Ineligible study design |
| Manzanero‐Lopez 2004 | Protocol only and review still not published |
| Martin 1996 | Ineligible outcomes |
| Meaume 1996a | Ineligible type of healing outcome |
| Meaume 1996b | Ineligible type of healing outcome |
| Meaume 2005 | Ineligible patient population |
| Mian 1992 | Ineligible study design |
| Milne 2012 | Confounded ‐ selection into phase 2 of trial on basis of results |
| Mizuhara 2012 | Mixed intervention |
| Mo 2015 | Ineligible patient population |
| Moberg 1983 | Mixed intervention |
| Mody 2008 | Ineligible type of healing outcome |
| Moody 1991 | Ineligible study design |
| Moody 2002 | Ineligible study design |
| Moore 2011 | Ineligible patient population |
| Morimoto 2015 | Ineligible study design |
| Motta 1991 | Ineligible study design |
| Motta 2004 | Ineligible patient population |
| Mouës 2004 | Ineligible patient population |
| Mouës 2007 | Ineligible patient population |
| Mulder 1989a | Ineligible patient population |
| Mulder 1989b | Ineligible patient population |
| Mulder 1993a | Ineligible type of healing outcome |
| Mulder 1993b | Ineligible type of healing outcome |
| Mustoe 1994 | Ineligible intervention |
| Myers 1990 | Ineligible type of healing outcome |
| Münter 2006 | Ineligible patient population |
| Nasar 1982 | Ineligible type of healing outcome |
| NCT02299557 | Ineligible patient population |
| Neill 1989b | Ineligible study design |
| Niezgoda 2004 | Ineligible type of healing outcome |
| Niimura 1990 | Ineligible patient population |
| Niimura 1991 | Ineligible patient population |
| Nixon 1998 | Ineligible intervention |
| Ohura 2004 | Mixed intervention |
| Olivar 1999 | Ineligible intervention |
| Ovington 1999 | Ineligible study design |
| Ozdemir 2011 | Ineligible type of healing outcome |
| Panahi 2015 | Ineligible patient population |
| Payne 2001 | Ineligible intervention |
| Perez 2000 | Ineligible type of healing outcome |
| Peschardt 1997 | Ineligible type of healing outcome |
| Picard 2015 | Ineligible patient population |
| Pierce 1994 | Ineligible outcomes |
| Pullen 2002 | Ineligible outcomes |
| Quelard 1985 | Ineligible intervention |
| Ramsay 1979 | Ineligible study design |
| Rhodes 1979 | Ineligible study design |
| Rhodes 2001 | Ineligible type of healing outcome |
| Roberts 1959 | Ineligible indication |
| Robson 1992a | Ineligible type of healing outcome |
| Robson 1992b | Ineligible intervention |
| Robson 1992c | Ineligible intervention |
| Robson 1994 | Ineligible intervention |
| Romanelli 2008 | Ineligible patient population |
| Romanelli 2009 | Ineligible patient population |
| Rooman 1991 | Ineligible patient population |
| Routkovsky‐Norval 1996 | Comparison of two interventions in the same class |
| Saha 2012 | Ineligible type of healing outcome |
| Saidkhani 2016 | Ineligible study design |
| Sayag 1996 | Ineligible type of healing outcome |
| Saydak 1990 | Ineligible study design |
| Scevola 2010 | Ineligible outcomes |
| Scott 1999 | Ineligible study design |
| Seaman 2000 | Comparison of two interventions in the same class |
| Sebern 1989 | Ineligible outcomes |
| Serra 2005 | Ineligible study design |
| Settel 1969 | Ineligible type of healing outcome |
| Shamimi Nouri 2008 | Ineligible outcomes |
| Shannon 1988 | Ineligible study design |
| Sherman 2000 | Ineligible study design |
| Shirakawa 2005 | Ineligible study design |
| Shojaei 2008 | Ineligible outcomes |
| Shrivastava 2011 | Ineligible patient population |
| Sibbald 2011 | Ineligible patient population |
| Small 2002 | Mixed intervention |
| Smietanka 1981 | Ineligible study design |
| Souliotis 2016 | Ineligible type of healing outcome |
| Stepan 2014 | Ineligible study design |
| Stephen 2016 | Ineligible type of healing outcome |
| Stoker 1990 | Ineligible study design |
| Strong 1985 | Ineligible type of healing outcome |
| Subbanna 2007 | Ineligible type of healing outcome |
| Takahashi 2006 | Ineligible study design |
| Teot 2008 | Ineligible outcomes |
| Teot 1997 | Ineligible type of healing outcome |
| Tewes 1993 | Ineligible study design |
| Thomas 1993 | Ineligible outcomes |
| Thomas 1997b | Ineligible outcomes |
| Toba 1997 | Ineligible type of healing outcome |
| Tolentino 2011 | Ineligible study design |
| Toriyabe 2004 | Ineligible study design |
| Torra i Bou 1999 | Ineligible outcomes |
| Trial 2010 | Ineligible outcomes |
| Tricco 2015 | Ineligible study design |
| Unglaub 2004 | Ineligible type of healing outcome |
| Valentini 2015 | Ineligible type of healing outcome |
| Van Leen 2004 | Ineligible study design |
| Varma 1973 | Ineligible outcomes |
| Vernassiere 2005 | Ineligible patient population |
| Wagstaff 2014 | Comparison of two interventions in the same class |
| Wallace 2009 | Ineligible study design |
| Wang 2014 | Ineligible intervention |
| Wanner 2003 | Ineligible type of healing outcome |
| Watts 1994 | Ineligible outcomes |
| Waycaster 2011 | Ineligible type of healing outcome |
| Waycaster 2013 | Confounded ‐ selection into phase 2 of trial on basis of results |
| Weheida 1991 | Ineligible patient population |
| Weststrate 1999 | Ineligible study design |
| Whitney 1999 | Mixed intervention |
| Whitney 2001 | Mixed intervention |
| Wild 2009 | Ineligible outcomes |
| Wild 2012 | Ineligible outcomes |
| Winter 1990 | Ineligible patient population |
| Woo 2009 | Ineligible outcomes |
| Worsley 1991 | Ineligible patient population |
| Yastrub 2004 | Ineligible type of healing outcome |
| Yastrub 2005 | Ineligible type of healing outcome |
| Young 1973 | Ineligible study design |
| Young 1997 | Ineligible type of healing outcome |
| Yura 1984 | Ineligible patient population |
| Zhou 2001 | Ineligible intervention |
| Zuloff‐Shani 2010 | Ineligible study design |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐13003959.
| Trial name or title | ChiCTR‐TRC‐13003959 |
| Methods | RCT pilot study; Duration 3 months |
| Participants | 30 eligible participants with pressure ulcers randomised in a ratio of 1:1 |
| Interventions | Treatment group: indirect moxibustion for 30 min before application of a dressing, 1 session daily, 5 sessions weekly for 4 weeks Control group will only receive a dressing, applied in the same way as in the treatment group |
| Outcomes | Primary outcomes: wound surface area (WSA) and proportion of ulcers healed within trial period |
| Starting date | registered 7/12/2013 |
| Contact information | |
| Notes | Protocol only |
ISRCTN57842461.
| Trial name or title | ISCRCTN57842461 study reported to be registered |
| Methods | RCT; participants randomised Duration 8 weeks |
| Participants | 820 participants with at least 1 grade II pressure ulcer will be recruited from primary health care and home care centres |
| Interventions | Polyurethane foam and hydrocolloid dressings |
| Outcomes | Primary outcome: percentage of wounds healed after 8 weeks. Secondary outcomes will include cost‐effectiveness, as evaluated by cost per healed ulcer and cost per treated participant and safety evaluated by adverse events |
| Starting date | Not stated |
| Contact information | |
| Notes | Protocol only; trial not on ClinicalTrials.gov |
RCT: randomised controlled trial
Differences between protocol and review
The protocol states that where studies have 25% or fewer Stage 1 ulcers, or 25% or fewer other complex wound types, we would include all study data initially and test the assumption in sensitivity analysis. However, we decided that it was more appropriate to exclude these studies. We also planned to carry out a sensitivity analysis in the absence of studies for which there were more than 75% but less than 100% of eligible ulcers. However, this sensitivity analysis was not done. A post‐hoc subgroup analysis was added, restricting the interventions to dressings (and hydrogel).
The major change from the protocol was to analyse the data in a frequentist rather than Bayesian framework, so STATA was used for all analyses rather than WinBUGS, as we originally proposed.
We did not contact study authors.
Contributions of authors
Maggie Westby: designed and coordinated the review; extracted data; checked the quality of data extraction; analysed and interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; contributed to writing and editing the review; made an intellectual contribution to the review; contacted an expert statistician; approved the final review prior to submission and is a guarantor of the review.
Jo Dumville: conceived, designed and coordinated the review; analysed and interpreted data; checked quality assessment; contributed to writing and editing the review; made an intellectual contribution to the review; approved the final review prior to submission; secured funding; and performed previous work that was the foundation of the current review.
Marta Soares: analysed and interpreted data; performed statistical analysis; checked the quality of the statistical analysis; contributed to writing or editing the review; made an intellectual contribution to the review; advised on the review; approved the final review prior to submission and performed previous work that was the foundation of the current review.
Nikki Stubbs: contributed to writing or editing the review; made an intellectual contribution to the review; advised on the review and approved the final review prior to submission.
Gill Norman: extracted data; checked the quality of data extraction; undertook and checked quality assessment; contributed to writing and editing the review; made an intellectual contribution to the review and approved the final review prior to submission.
Contributions of the editorial base
Nicky Cullum (Co‐ordinating Editor): edited the protocol and the review; advised on methodology, interpretation and content; approved the final protocol and review prior to submission.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process, advised on content; edited the protocol and the review.
Reetu Child (Information Specialist): designed the search strategy and ran the search; edited the search methods section.
Ursula Gonthier (Editorial Assistant): edited the plain language summary and reference sections.
Sources of support
Internal sources
Division of Nursing, Midwifery and Social Work, School of Health Sciences, University of Manchester, UK.
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane Programme Grant funding (NIHR Cochrane Programme Grant 13/89/08 – High Priority Cochrane Reviews in Wound Prevention and Treatment) to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester, UK.
Jo Dumville was partly funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester. The funder had no role in the design of the studies, data collection and analysis, decision to publish, or preparation of the manuscript. However, the review may be considered to be affiliated to the work of the NIHR CLAHRC Greater Manchester. The views expressed herein are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health.
Declarations of interest
Maggie Westby: my employment at the University of Manchester is funded by National Institute for Health Research (NIHR) and focuses on high priority Cochrane Reviews in the prevention and treatment of wounds.
Jo Dumville: I receive research funding from the National Institute for Health Research (NIHR) for the production of systematic reviews focusing on high priority Cochrane Reviews in the prevention and treatment of wounds. This work was also partly funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester.
Marta Soares: none known.
Nikki Stubbs: funding from pharmaceutical companies has supported training and educational events, and payments have been received by the author for non‐product‐related educational sessions. These have been unrelated to the subject matter of the review and have never been in support or in pursuit of the promotion of products.
Gill Norman: my employment at the University of Manchester is funded by the National Institute for Health Research (NIHR) and focuses on high priority Cochrane Reviews in the prevention and treatment of wounds.
New
References
References to studies included in this review
Aguilo Sanchez 2002 {published data only}
- Aguilo Sanchez S, Figueiras Mareque L, Quintilla Gatnau A, Veiga Bogo L. Assessment of the efficacy of a hidropolomeric [sic] dressing in three dimensions and a hydrocolloid with calcium alginate in the treatment of pressure ulcers. 12th Conference of the European Wound Management Association; 2002 May 23‐25; Granada, Spain. 2002:113.
Alm 1989 {published data only}
- Alm A, Hornmark AM, Fall PA, Linder L, Bergstrand B, Ehrnebo M, et al. Care of pressure sores: a controlled study of the use of a hydrocolloid dressing compared with wet saline gauze compresses. Acta Dermato‐Venereologica 1989;149(Suppl):1‐10. [PubMed] [Google Scholar]
Ashby 2012 {published data only}
- Ashby R, Dumville J, Soares M, McGinnis E, Stubbs N, Adderley U, et al. A pilot trial of topical negative pressure therapy (TNP) for the treatment of grade III/IV pressure ulcers. EWMA Journal 2010;10(2):202. [Google Scholar]
- Ashby R, Dumville J, Soares MO, Adderley U, McGinnis E, Stubbs N, et al. A pilot study of negative pressure wound therapy for the treatment of grade III/IV pressure ulcers. International Nursing Research Conference; 2011 May 16‐18; Harrogate, UK. 2011:95.
- Ashby RL, Dumville JC, Soares MO, McGinnis E, Stubbs N, Torgerson DJ, et al. A pilot randomised controlled trial of negative pressure wound therapy to treat grade III/IV pressure ulcers. Trials 2012;13(119):available from trialsjournal.biomedcentral.com/articles/10.1186/1745‐6215‐13‐119. [DOI: 10.1186/1745-6215-13-119] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bale 1997a {published data only}
- Bale S, Squires D, Varnon T, Walker A, Benbow M, Harding KG. A comparison of two dressings in pressure sore management. Journal of Wound Care 1997;6(10):463‐6. [DOI] [PubMed] [Google Scholar]
- Benbow M. A multicentre pilot study to investigate the physical performance of a novel Allevyn Adhesive dressing. In: Suggett A, Cherry G, Mani R, Eagelstein W editor(s). Evidence‐based Wound Care. International Congress and Symposium Series Number 227. Royal Society of Medicine Press Limited, 1998:49‐53. [Google Scholar]
- Shutler S, Stock J, Bale S, Harding KG, Squires D, Wilson I, et al. A multi‐centre comparison of a hydrocellular adhesive dressing (Allevyn Adhesive) and a hydrocolloid dressing (Granuflex) in the management of stage 2 and 3 pressure sores. Fifth European Conference on Advances in Wound Management; 1995, November 21‐24, Harrogate, UK. 1996:poster.
Banks 1994a {published data only}
- Banks V, Bale SE. Comparing two dressings for exuding pressure sores in community patients. Journal of Wound Care 1994;3(4):175‐8. [DOI] [PubMed] [Google Scholar]
- Banks V, Riggs R, Bale S, Harding KG. Comparison of an absorbent semi‐permeable polyurethane dressing (Spyrosorb/Mitraflex) and a hydrocolloid dressing (Granuflex E) for the treatment of moderately exuding stage II and III pressure sores in community patients. Third European Conference on Advances in Wound Management; 1993 October 19‐22; Harrogate, UK. 1994:159.
Banks 1994b {published data only}
- Banks V, Bale S. Superficial pressure sores: comparing two regimes. Journal of Wound Care 1994;3(1):8‐10. [DOI] [PubMed] [Google Scholar]
- Banks V, Bale S, Harding K. A comparative study to evaluate the effectiveness of Lyofoam A in the treatment of superficial pressure sores. Third European Conference on Advances in Wound Management; 1993 October 19‐22; Harrogate, UK. 1994:21‐4.
Banks 1994c {published data only}
- Banks V, Bale S, Harding K. The use of two dressings for moderately exuding pressure sores. Journal of Wound Care 1994;3(3):132‐4. [DOI] [PubMed] [Google Scholar]
- Riggs R, Banks V, Bale S, Harding K. Comparison of an absorbent semi‐permeable polyurethane dressing (Spyrosorb/Mitraflex) and a hydrocolloid dressing (Granuflex E) for the treatment of moderately exuding pressure sores in hospitalised patients. Third European Conference on Advances in Wound Management; 1993 October 19‐22; Harrogate, UK. 1994:159.
Barrois 1992 {published data only}
- Barrois B. Comparison of Granuflex and medicated paraffin gauze in pressure sores. Second European Conference on Advances in Wound Management; 1992 October 20‐23; Harrogate, UK. 1993:209‐10.
- Huchon D. Comparison between Granuflex and paraffin‐impregnated medical gauze in the treatment of pressure sores. Euroservices Communication: Going into the '90s: the Pharmacist and Wound Care 1992:23‐6.
Belmin 2002 {published data only}
- Belmin J, Meaume S, Rabus MT, Bohbot S, Investigators of the Sequential Treatment of the Elderly with Pressure Sores (STEPS) Trial. Sequential treatment with calcium alginate dressings and hydrocolloid dressings accelerates pressure ulcer healing in older subjects: a multicenter randomized trial of sequential versus nonsequential treatment with hydrocolloid dressings alone. Journal of the American Geriatrics Society 2002;50(2):269‐74. [DOI] [PubMed] [Google Scholar]
- Meaume S. A randomized controlled study to compare a sequential treatment using alginate‐CMC and hydrocolloid dressing or hydrocolloid dressing alone in decubitus ulcer management. Ninth European Conference on Advances in Wound Management; 1999 November 9‐11; Harrogate, UK. 1999:11.
- Meaume S, Faucher N, Henry O, Belmin J. A randomised controlled study to compare a sequential treatment using alginate‐CMC and hydrocolloid dressing to hydrocolloid dressing alone in decubitus ulcers management. 12th Conference of the European Wound Management Association; 2002 May 23‐25; Granada, Spain. 2002:123.
- Rabus MT, Dare F, Mouel LE, Meaume S. Sequential treatment using alginate‐CMC and hydrocolloid dressing in pressure ulcers: results of a controlled study (STEPS study). Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:159.
Brod 1990 {published data only}
- Brod M, McHenry E, Plasse TF, Fedorczyk D, Trout JR. A randomized comparison of poly‐hema and hydrocolloid dressings for treatment of pressure sores. Archives of Dermatology 1990;126(7):969‐70. [DOI] [PubMed] [Google Scholar]
Brown‐Etris 1996 {published data only}
- Brown‐Etris M, Fowler E, Papen J, Stanfield J, Harris A, Tintle T, et al. Comparison and evaluation of the performance characteristics, usability and effectiveness on wound healing of Transorbent versus Duoderm CGF. Fifth European Conference on Advances in Wound Management; 1995 November 21‐24; Harrogate, UK. 1996:151‐5.
Brown‐Etris 1997 {published data only}
- Brown‐Etris M, Bateman D, Sheilds D, Punchello M, Gokoo CF, Scott R, et al. Final report of a clinical study designed to evaluate and compare a collagen alginate dressing and a calcium‐sodium alginate dressing in pressure ulcers. European Wound Management Association Conference; 1997 April 27‐29; Milan, Italy. 1997:21.
Brown‐Etris 2008 {published data only}
- Brown‐Etris M, Milne C, Orsted H, Gates JL, Netsch D, Punchello M, et al. A prospective, randomized, multisite clinical evaluation of a transparent absorbent acrylic dressing and a hydrocolloid dressing in the management of Stage II and shallow Stage III pressure ulcers. Advances in Skin and Wound Care 2008;21(4):169‐74. [DOI] [PubMed] [Google Scholar]
- Brown‐Etris M, Punchello M, O'Connor T, Milne C, Orsted H, Couture N, et al. A randomized comparative clinical evaluation of a transparent absorbent acrylic dressing and a hydrocolloid dressing on stage II and III pressure ulcers. Journal of Wound, Ostomy, and Continence Nursing 2006;33(Suppl 1):S53. [Google Scholar]
Burgos 2000b {published data only}
- Burgos A, Gimenez J, Moreno E, Lamberto E, Ultrera M, Urraca EM, et al. Cost, efficacy, efficiency and tolerability of collagenase ointment versus hydrocolloid occlusive dressing in the treatment of pressure ulcers. Clinical Drug Investigation 2000;19(5):357‐65. [Google Scholar]
Colwell 1993 {published data only}
- Colwell JC, Foreman MD, Trotter JP. A comparison of the efficacy and cost‐effectiveness of two methods of managing pressure ulcers. Decubitus 1993;6(4):28‐36. [PubMed] [Google Scholar]
Darkovich 1990 {published data only}
- Darkovich SL, Brown‐Etris M, Spencer M. Biofilm hydrogel dressing: a clinical evaluation in the treatment of pressure sores. Ostomy/Wound Management 1990;29:47‐60. [PubMed] [Google Scholar]
Gorse 1987 {published data only}
- Gorse GJ, Messner RL. Improved pressure sore healing with hydrocolloid dressings. Archives of Dermatology 1987;123(6):766‐71. [PubMed] [Google Scholar]
Graumlich 2003 {published data only}
- Graumlich JF, Blough LS, McLaughlin RG, Milbrandt JC, Calderon CL, Agha SA, et al. Healing pressure ulcers with collagen or hydrocolloid: a randomized, controlled trial. Journal of the American Geriatrics Society 2003;51(2):147‐54. [DOI] [PubMed] [Google Scholar]
Hollisaz 2004 {published data only}
- Hollisaz MT, Khedmat H, Yari F. A randomized clinical trial comparing hydrocolloid, phenytoin and simple dressings for the treatment of pressure ulcers [ISRCTN33429693]. BMC Dermatology 2004;4(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hondé 1994 {published data only}
- Hondé C, Derks C, Tudor D. Local treatment of pressure sores in the elderly: amino acid copolymer membrane versus hydrocolloid dressing. Journal of the American Geriatrics Society 1994;42(11):1180‐3. [DOI] [PubMed] [Google Scholar]
Imamura 1989 {published data only}
- Imamura Y. The clinical effect of KT‐136 (sugar and povidone‐iodine ointment) on decubitus ulcers: a comparative study with lysozyme ointment. Japanese Pharmacology and Therapeutics 1989;17(Suppl 1):255‐80. [Google Scholar]
Kaya 2005 {published data only}
- Kaya AZ, Turani N, Akyüz M. The effectiveness of a hydrogel dressing compared with standard management of pressure ulcers. Journal of Wound Care 2005;14(1):42‐4. [DOI] [PubMed] [Google Scholar]
Kraft 1993 {published data only}
- Kraft MR, Lawson L, Pohlmann B, Reid Lokos C, Barder L. A comparison of Epi‐Lock and saline dressings in the treatment of pressure ulcers. Decubitus 1993;6(6):42‐8. [PubMed] [Google Scholar]
Matzen 1999 {published data only}
- Matzen S, Peschardt A, Alsbjørn B. A new amorphous hydrocolloid for the treatment of pressure sores: a randomised controlled study. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery 1999;33(1):13‐5. [DOI] [PubMed] [Google Scholar]
Meaume 2003 {published data only}
- Meaume S, Looverbosch D, Heyman H, Romanelli M, Ciangherotti A, Charpin S. A study to compare a new self‐adherent soft silicone dressing with a self‐adherent polymer dressing in stage II pressure ulcers. Ostomy/Wound Management 2003;49(9):44‐6, 48. [PubMed] [Google Scholar]
- Meaume S, Looverbosch D, Heyman H, Romanelli M, Ciangherotti, Charoin S. Randomised open multicentric study comparing Mepilex Border with Tielle in patients with grade II pressure ulcers [Etude ouverte randomisée multicentrique comparant Mepilex Border à Tielle chez des patients porteurs d’escarres de stade II]. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:47.
Motta 1999 {published data only}
- Motta G, Dunham L, Dye T, Mentz J, O'Connell‐Gifford E, Smith E. Clinical efficacy and cost‐effectiveness of a new synthetic polymer sheet wound dressing. Ostomy/Wound Management 1999;45(10):41, 44‐6, 48. [PubMed] [Google Scholar]
Muller 2001 {published data only}
- Muller E, Leen MW, Bergemann R. Economic evaluation of collagenase‐containing ointment and hydrocolloid dressing in the treatment of pressure ulcers. PharmacoEconomics 2001;19(12):1209‐16. [DOI] [PubMed] [Google Scholar]
- Leen MW. Collagenase treatment of chronic ulcers. Wound Repair and Regeneration 1998;6(5):483. [Google Scholar]
Neill 1989a {published data only}
- Neill KM, Conforti C, Kedas A, Burris JF. Pressure sore response to a new hydrocolloid dressing. Wounds 1989;1:173‐85. [Google Scholar]
Nisi 2005 {published data only}
- Nisi G, Brandi C, Grimaldi L, Calabrò M, D'Aniello C. Use of a protease‐modulating matrix in the treatment of pressure sores. Chirurgia Italiana 2005;57(4):465‐8. [PubMed] [Google Scholar]
Nussbaum 1994 {published data only}
- Nussbaum EL, Biemann I, Mustard B, Michlovitz S, Buinewicz BR. Comparison of ultrasound/ultraviolet‐C and laser for treatment of pressure ulcers in patients with spinal cord injury. Physical Therapy 1994;74(9):812‐23; discussion 24. [DOI] [PubMed] [Google Scholar]
Oleske 1986 {published data only}
- Oleske DM, Smith XP, White P, Pottage J, Donovan MI. A randomized clinical trial of two dressing methods for the treatment of low‐grade pressure ulcers. Journal of Enterostomal Therapy 1986;13(3):90‐8. [DOI] [PubMed] [Google Scholar]
Parish 1979 {published data only}
- Parish LC, Collins E. Decubitus ulcers: a comparative study. Cutis 1979;23(1):106‐10. [PubMed] [Google Scholar]
Payne 2004 {published data only}
- Payne WG, Wright TE, Ochs D, Mannari RJ, Robson MC, Edington H, et al. An exploratory study of dermal replacement therapy in the treatment of stage III pressure ulcers. Journal of Applied Research 2004;4(1):12‐23. [Google Scholar]
Payne 2009 {published data only}
- Payne WG, Posnett J, Alvarez O, Brown‐Etris M, Jameson G, Wolcott R, et al. A prospective, randomized clinical trial to assess the cost‐effectiveness of a modern foam dressing versus a traditional saline gauze dressing in the treatment of stage II pressure ulcers. Ostomy/Wound Management 2009;55(2):50‐5. [PubMed] [Google Scholar]
Piatkowski 2012 {published data only}
- Piatkowski A, Ulrich D, Seidel D, Abel M, Pallua N, Andriessen A. RCT comparing collagen and foam dressings in pressure ulcers evaluating their influence on healing time angiogenesis and pro‐inflammatory cells. EWMA Journal 2011;11(2 Suppl):42. [Google Scholar]
- Piatkowski A, Ulrich D, Seidel D, Abel M, Pallua N, Andriessen A. Randomised, controlled pilot to compare collagen and foam in stagnating pressure ulcers. Journal of Wound Care 2012;21(10):505‐11. [DOI] [PubMed] [Google Scholar]
- Piatkowski A, Ulrich D, Seidel S, Abel M, Pallua N, Andriessen A. Randomized controlled pilot comparing collagen and foam dressings in pressure ulcer patients evaluating their influence on healing time, angiogenesis and proinflammatory cells. 15ème Conférence Nationale des Plaies et Cicatrisations ‐ prévention et traitement; 2011 January 16‐18; Paris, France. 2011:79.
Price 2000 {published data only}
- Price P, Bale S, Crook H, Harding KG. The effect of a radiant heat dressing in the management of patients with Stage III and IV pressure ulcers. 13th Annual Symposium on Advanced Wound Care and 10th Annual Medical Research Forum on Wound Repair; 2000 April 1‐4; Dallas (TX). 2000:D14.
- Price P, Bale S, Crook H, Harding KG. The effect of a radiant heat dressing on pressure ulcers. Journal of Wound Care 2000;9(4):201‐5. [DOI] [PubMed] [Google Scholar]
Ramos‐Torrecillas 2015 {published data only}
- Ramos‐Torrecillas J, Garcia‐Martinez O, Luna‐Bertos E, Ocana‐Peinado FM, Ruiz C. Effectiveness of platelet‐rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biological Research for Nursing 2015;17(2):152‐8. [DOI] [PubMed] [Google Scholar]
Rees 1999 {published data only}
- Rees RS, Robson MC, Smiell JM, Perry BH. A randomized, double‐blind, placebo‐controlled study of Becaplermin (recombinant human platelet‐derived growth factor‐BB) gel in the treatment of pressure ulcers. Wound Repair and Regeneration 1998;6(3):246. [DOI] [PubMed] [Google Scholar]
- Rees RS, Robson MC, Smiell JM, Perry BH. Becaplermin gel in the treatment of pressure ulcers: a randomized double‐blind placebo‐controlled study. Wound Repair and Regeneration 1998;6(5):478. [DOI] [PubMed] [Google Scholar]
- Rees RS, Robson MC, Smiell JM, Perry BH, the Pressure Ulcer Study Group. Becaplermin gel in the treatment of pressure ulcers: a phase II randomized, double‐blind, placebo‐controlled study. Wound Repair and Regeneration 1999;7(3):141‐7. [DOI] [PubMed] [Google Scholar]
Romanelli 2001 {published data only}
- Romanelli M, Semeraro RM, Siani S. A pilot study on the use of a tripeptide‐copper gel on pressure ulcers: preliminary results. 11th Conference of the European Wound Management Association; 2001 May 17‐19; Dublin, Ireland. 2001:82.
Sebern 1986 {published data only}
- Sebern MD. Pressure ulcer management in home health care: efficacy and cost effectiveness of moisture vapor permeable dressing. Archives of Physical Medicine and Rehabilitation 1986;67(10):726‐9. [DOI] [PubMed] [Google Scholar]
Seeley 1999 {published data only}
- Seeley J, Jensen JL, Hutcherson J. A randomized clinical study comparing a hydrocellular dressing to a hydrocolloid dressing in the management of pressure ulcers. Ostomy/Wound management 1999;45(6):39‐44, 46. [PubMed] [Google Scholar]
Serena 2010 {published data only}
- Serena T, Wolcott R, DiSalvo M, Hurley D. Bilayered living cell‐based treatment for pressure ulcers: results of a prospective, randomized, multi‐center, open‐label clinical trial. Symposium on Advanced Wound Care and the Wound Healing Society; 2010 April 17‐20; Orlando (FL). 2010:S38. [Abstract CR‐070]
Sipponen 2008 {published data only}
- Sipponen A, Jokinen JJ, Lohi J, Sipponen P. Efficiency of resin salve from Norway spruce in treatment of pressure ulcers and chronic surgical and traumatic wounds. 19th Conference of the European Wound Management Association; 2009 May 20‐22; Helsinki, Finland. 2009:41.
- Sipponen A, Jokinen JJ, Sipponen P, Papp A, Sarna S, Lohi J. Beneficial effect of resin salve in treatment of severe pressure ulcers: a prospective, randomized and controlled multicentre trial. British Journal of Dermatology 2008;158(5):1055‐62. [DOI] [PubMed] [Google Scholar]
Sopata 2002 {published data only}
- Sopata M, Luczak J, Ciupinska M. Effect of bacteriological status on pressure ulcer healing in patients with advanced cancer. Journal of Wound Care 2002;11(3):107‐10. [DOI] [PubMed] [Google Scholar]
Thomas 1997a {published data only}
- Banks V, Fear‐Price M, Orpin J, Hagelstein S, Colgate G, Humphreys J, et al. A comparative open multi‐centre trial of Tielle hydropolymer dressing and Granuflex improved formulation. Fifth European Conference on Advances in Wound Management; 1995 November 21‐24, Harrogate, UK. 1996:163‐7.
- Thomas S, Banks V, Fear‐Price M, Hagelstein S, Harding KG, Orpin J, et al. A comparison of two dressings in the management of chronic wounds. Journal of Wound Care 1997;6(8):383‐6. [DOI] [PubMed] [Google Scholar]
Thomas 1998 {published data only}
- Thomas DR, Goode PS. Acemannan hydrogel versus saline dressings for pressure ulcers: a randomized controlled trial. Journal of Investigative Medicine 1998;46(7):283A. [PubMed] [Google Scholar]
- Thomas DR, Goode PS, LaMaster K, Tennyson T. Acemannan hydrogel dressing versus saline dressing for pressure ulcers. A randomized, controlled trial. Advances in Wound Care 1998;11(6):273‐6. [PubMed] [Google Scholar]
Thomas 2005 {published data only}
- Thomas DR, Diebold MR, Eggemeyer LM. A controlled, randomized, comparative study of a radiant heat bandage on the healing of stage 3‐4 pressure ulcers: a pilot study. Journal of the American Medical Directors Association 2005;6(1):46‐9. [DOI] [PubMed] [Google Scholar]
Van De Looverbosch 2004 {published data only}
- Looverbosch D, Heyman H, Dierick AM. Explorative, comparative study with enamel matrix protein in pressure ulcer stage II. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:X014.
Xakellis 1992 {published data only}
- Xakellis GC, Chrischilles EA. Hydrocolloid versus saline‐gauze dressings in treating pressure ulcers: a cost‐effectiveness analysis. Archives of Physical Medicine and Rehabilitation 1992;73(5):463‐9. [PubMed] [Google Scholar]
Yapucu Güneş 2007 {published data only}
- Yapucu Güneş U, Eşer I. Effectiveness of a honey dressing for healing pressure ulcers. Journal of Wound Ostomy and Continence Nursing 2007;34(2):184‐90. [DOI] [PubMed] [Google Scholar]
Zeron 2007 {published data only}
- Zeron HM, Krotzsch Gomez FE, Hernandez Munoz RE. Pressure ulcers: a pilot study for treatment with collagen polyvinylpyrrolidone. International Journal of Dermatology 2007;46(3):314‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbott 1968 {published data only}
- Abbott DF, Exton‐Smith AN, Millard PH, Temperley JM. Zinc sulphate and bedsores. British Medical Journal 1968;2(5607):763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agren 1985 {published data only}
- Agren MS, Stromberg HE. Topical treatment of pressure ulcers. Scandinavian Journal of Plastic and Reconstructive Surgery 1985;19(1):97‐100. [DOI] [PubMed] [Google Scholar]
Ahmad 2008 {published data only}
- Ahmad ET. High voltage pulsed galvanic stimulation: effect of treatment durations on healing of chronic pressure ulcers. Indian Journal of Physiotherapy and Occupational Therapy 2008;2(3):1‐5. [PMC free article] [PubMed] [Google Scholar]
Alvarez 1999 {published data only}
- Alvarez OM, Fernandez‐Obregon A, Davis SC, Cazzaniga AL, Pacheco H. A prospective randomized clinical trial of papain‐urea for the debridement of pressure ulcers. 31st Annual Wound, Ostomy and Continence Conference; 1999 June 19‐23; Minneapolis (MN). 1999:488.
Alvarez 2000a {published data only}
- Alvarez OM, Fernandez‐Obregon A, Rogers RS, Bergamo L, Masso J, Black M. Chemical debridement of pressure ulcers: a prospective, randomized, comparative trial of collagenase and papain/urea formulations. Wounds 2000;12(2):15‐25. [Google Scholar]
Alvarez 2000b {published data only}
- Alvarez OM, Fernandez‐Obregon A, Hoboken NJ, Rogers RR, Masso J, Davis SC, et al. Enzymatic debridement of pressure ulcers: a prospective randomized comparative clinical trial. 13th Annual Symposium on Advanced Wound Care and 10th Annual Medical Research Forum on Wound Repair; 2000 April 1‐4; Dallas (TX). 2000:C69.
Alvarez 2002 {published data only}
- Alvarez OM, Fernandez‐Obregon A, Rogers RS, Bergamo L, Masso J, Black M. A prospective, randomized, comparative study of collagenase and papain‐urea for pressure ulcer debridement. Wounds 2002;14(8):293‐301. [Google Scholar]
Alvarez Vázquez 2014 {published data only}
- Alvarez Vázquez JC, Estany Gestal A, Álvarez Suárez T, Mosquera JB, Castro Prado J, Gutiérrez Moeda E, et al. Prevention of deterioration of the cutaneous integrity in the sacral area through the application of an atraumatic self‐adherent foam dressing [Prevención del deterioro de la integridad cutánea en el sacro mediante la aplicación de una espuma de adhesión atraumática]. Metas de Enfermería 2014;17(2):14‐20. [Google Scholar]
Aminian 1999 {published data only}
- Aminian R, Shams M, Karim‐Aghaee B, Soveyd M, Omrani GR. The role of the autologous platelet‐derived growth factor in the management of decubitus ulcer. Archives of Iranian medicine 1999;2(2):98‐101. [Google Scholar]
Amione 2005 {published data only}
- Amione P, Ricci E, Topo F, Izzo L, Pirovano R, Rega V, et al. Comparison of Allevyn Adhesive and Biatain Adhesive in the management of pressure ulcers. Journal of Wound Care 2005;14(8):365‐70. [DOI] [PubMed] [Google Scholar]
Anitua 2008 {published data only}
- Anitua E, Aguirre JJ, Algorta J, Ayerdi E, Cabezas AI, Orive G, et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. Journal of Biomedical Materials Research. Part B: Applied Biomaterials 2008;84(2):415‐21. [DOI] [PubMed] [Google Scholar]
Anonymous 1982 {published data only}
- Anonymous. Does metronidazole help leg ulcers and pressure sores?. Drug and Therapeutics Bulletin 1982;20(3):9‐10. [PubMed] [Google Scholar]
Anonymous 2000 {published data only}
- Anonymous. Vacuum‐assisted closure for chronic wound healing. Tecnologica 2000;MAP (Medical Advisory Panel) Supplement:19‐20. [PubMed] [Google Scholar]
Anzai 1989 {published data only}
- Anzai T, Shiratori A, Otomo E, Honda S, Yamamoto T, Nishikawa T, et al. Evaluation of clinical utility of NI‐009 on various cutaneous ulcers: comparative study with base. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicine) 1989;5(12):2585‐612. [Google Scholar]
Avanzi 1998a {published data only}
- Avanzi A, Martinelli R, Accardi S, Giraudi C, Peroli P, Rowan S. An adhesive hydrocellular dressing versus a hydrocolloid dressing in the treatment of 2nd and 3rd degree pressure sores. Eighth European Conference on Advances in Wound Management; 1998 April 26‐28; Madrid, Spain. 1998:116.
Avanzi 1998b {published data only}
- Avanzi R, Martinelli R, Accardi S, Giraudi C, Peroli P, Andriessen A, et al. Adhesive hydrocellular dressing versus hydrocolloid dressing in the treatment of stage II and III pressure sores. European Wound Management Association Conference; 1998 November; Harrogate, UK. 1998:11.
Avanzi 2000a {published data only}
- Avanzi A, Martinelli M, Accardi S, Giraudi C, Peroli P, Andriessen A. Adhesive hydrocellular dressing vs hydrocolloid dressing in the treatment of 2nd and 3rd degree pressure sores. 10th Conference of the European Wound Management Association; 2000 May 18‐20; Stockholm, Sweden. 2000:29.
Avanzi 2000b {published data only}
- Avanzi A, Martinelli R, Accardi S, Giraudi C, Peroli P, Rowan S, et al. Adhesive hydrocellular dressing vs hydrocolloid dressing in the treatment of pressure sores. First World Wound Healing Congress; 2000 September 10‐13; Melbourne, Australia. 2000:90.
Avanzi 2000c {published data only}
- Avanzi A, Martinelli M, Accardi S, Giraudi C, Peroli P, Andriessen A, et al. Adhesive hydrocellular dressing vs hydrocolloid dressing in the treatment of 2nd and 3rd degree pressure sores. A prospective, controlled randomised comparative multi‐centre clinical evaluation. Wound Repair and Regeneration 2000;8:A406. [Google Scholar]
Avanzi 2001 {published data only}
- Avanzi A, Martinelli M, Accardi S, Giraudi C, Peroli P, Andreissen A, et al. Adhesive hydrocellular dressing vs hydrocolloid dressing in the management of stage 2 and stage 3 pressure ulcers. 11th European Tissue Repair Society Annual Conference; 2001 September 5‐8; Cardiff, Wales. 2001:43.
Baade 1965 {published data only}
- Baade S. Local application of an anabolically active steroid to promote wound healing. Die Medizinische Welt 1965;32:1828‐30. [PubMed] [Google Scholar]
Baatenburg de Jong 2004 {published data only}
- Baatenburg de Jong H, Admiraal H. Comparing cost per use of 3M Cavilon No Sting Barrier Film with zinc oxide oil in incontinent patients. Journal of Wound Care 2004;13(9):398‐400. [DOI] [PubMed] [Google Scholar]
Baker 1981 {published data only}
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers. A comparison with standard treatment in general practice. Practitioner 1981;225(1354):569‐73. [PubMed] [Google Scholar]
Bale 1997b {published data only}
- Bale S, Banks V, Hagelstein S, Harding KG. A randomized, controlled parallel‐group clinical trial to compare two amorphous hydrogels in the debridement of pressure sores. Sixth European Conference on Advances in Wound Management; 1996 October 1‐4; Amsterdam, the Netherlands. 1997:244‐8.
Bale 1997c {published data only}
- Bale S, Crook H. The preliminary results of a comparative study on the performance characteristics of a new hydrogel versus an existing hydrogel on necrotic pressure ulcers. European Wound Management Association Conference; 1997 April 27‐29; Milan, Italy. 1997:66‐8.
Bale 1998a {published data only}
- Bale S, Hagelstein S, Banks V, Harding KG. Costs of dressings in the community. Journal of Wound Care 1998;7(7):327‐30. [DOI] [PubMed] [Google Scholar]
- Harding KG, Bale S, Banks V, Orpin J. A cost effectiveness study using Allevyn hydrocellular dressings. Fourth European Conference on Advances in Wound Management; 1994 September 6‐9; Copenhagen, Denmark. 1995:57‐9.
Bale 1998b {published data only}
- Bale S, Banks V, Haglestein S, Harding KG. A comparison of two amorphous hydrogels in the debridement of pressure sores. Journal of Wound Care 1998;7(2):65‐8. [DOI] [PubMed] [Google Scholar]
Bale 2004 {published data only}
- Bale S, Tebbie N, Price P. A topical metronidazole gel used to treat malodorous wounds. British Journal of Nursing 2004;13(11):S4‐11. [DOI] [PubMed] [Google Scholar]
Banks 1997a {published data only}
- Banks V, Bale S, Harding K, Harding EF. Evaluation of a new polyurethane foam dressing. Journal of Wound Care 1997;6(6):266‐9. [DOI] [PubMed] [Google Scholar]
- Banks V, Bale S, Harding KG, Harding EF. A randomized, stratified, controlled, parallel‐group clinical trial of a new polyurethane foam dressing (Lyofoam Extra) versus a hydrocellular dressing in the treatment of moderate to heavily exuding wounds. Sixth European Conference on Advances in Wound Management; 1996 October 1‐4; Amsterdam, the Netherlands. 1997:235‐3.
- Banks V, Bale S, Harding KG, Harding EF. An interim analysis of a randomized stratified controlled parallel‐group clinical trial of a new polyurethane foam dressing versus a hydrocellular dressing in the treatment of moderate to heavily exuding wounds. Fifth European Conference on Advances in Wound Management; 1995, November 21‐24; Harrogate, UK. 1996:177‐80.
- Banks V, Fear M, Orpin J, Hagelstein S, Thomas N, Colgate G, et al. A comparative open multi‐center trial of hydropolymer dressing and hydrocolloid. Ninth Annual Symposium on Advanced Wound Care and Sixth Annual Medical Research Forum on Wound Repair; 1996 April 20‐24; Atlanta (GA). 1996:113.
- Banks V, Hagelstein S, Bale S, Harding KG. A comparison of a new polyurethane dressing versus a hydrocellular dressing in the treatment of moderate to heavily exudating wounds. Ninth Annual Symposium on Advanced Wound Care and Sixth Annual Medical Research Forum on Wound Repair; 1996 April 20‐24; Atlanta (GA). 1996:113.
Banks 1997b {published data only}
- Banks, Fear M, Hagelstein S, Bale S, Thomas S, Harding KG. A randomized controlled community comparison of Duoderm Extra Thin with an adhesive film as a secondary dressing. Sixth European Conference on Advances in Wound Management; 1996 October 1‐4; Amsterdam, the Netherlands. 1997:238‐41.
Barnes 1992 {published data only}
- Barnes J, Burns K. Management of pressure sores: a comparison of Granuflex hydrocolloid dressing and Granuflex E hydrocolloid dressing. Euroservices Communication: Going into the '90s: the Pharmacist and Wound Care 1992:19‐21.
Bazzigaluppi 1991 {published data only}
- Bazzigaluppi F, Biraghi MG, Fano M, Moschin AM, Toscani P, Cervone C, et al. Cadexomer iodine in the treatment of cutaneous ulcers: open, multicentric trial. Gazetta Medica Italiana 1991;150(11):471‐80. [Google Scholar]
Becker 1984 {published data only}
- Becker L, Goodemote C. Treating pressure sores with or without antacid. American Journal of Nursing 1984;84(3):351‐2. [PubMed] [Google Scholar]
Beele 2010 {published data only}
- Beele H, Meuleneir F, Nahuys M, Percival SL. A prospective randomised open label study to evaluate the potential of a new silver alginate/carboxymethylcellulose antimicrobial wound dressing to promote wound healing. International Wound Journal 2010;7(4):262‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berard 1986 {published data only}
- Berard P, Montandon S, Jedynak D, Saubier EC. Trial of a hydrocolloid in occlusive dressings in the treatment of skin wounds, "Duoderm". Revista de Enfermeria 1986;9(1):17‐20. [PubMed] [Google Scholar]
Bigolari 1991 {published data only}
- Bigolari M, Mosca P, Astengo F, Biraghi M, Balestreri R. Randomized clinical trial with Cadexomer iodine in decubitus ulcers [Studio clinico randomizzato con cadexomero iodico nelle ulcere da decubito]. Gazzetta Medica Italiana 1991;150(5):177‐85. [Google Scholar]
Bito 2012 {published data only}
- Bito S, Mizuhara A, Oonishi S, Takeuchi K, Suzuki M, Akiyama K, et al. Randomised controlled trial evaluating the efficacy of wrap therapy for wound healing acceleration in patients with NPUAP stage II and III pressure ulcer. BMJ Open 2012;2:e000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blanco Blanco 2002 {published data only}
- Blanco Blanco J, Ballester Torralba J, Rueda Lopez J, Torra i Bou JE. Comparative study of the use of a heel protecting bandage and a special hydrocellular dressing in the prevention of pressure ulcers in elderly participants. 12th Conference of the European Wound Management Association; 2002 May 23‐25; Granada, Spain. 2002:114.
Blum 1973 {published data only}
- Blum G. Therapeutic results with Iruxol in ulcus cruris, decubitus ulcer and burns. Schweizerische Rundschau für Medizin Praxis 1973;62(26):820‐6. [PubMed] [Google Scholar]
Boxer 1969 {published data only}
- Boxer AM, Gottesman N, Bernstein H, Mandl I. Debridement of dermal ulcers and decubiti with collagenase. Geriatrics 1969;24(7):75‐86. [PubMed] [Google Scholar]
Boykin 1989 {published data only}
- Boykin A, Winland‐Brown J. Pressure sores: nursing management. National League for Nursing Publications 1989;15(2232):252‐6. [Google Scholar]
Brady 1987 {published data only}
- Brady SM. Management of pressure sores with occlusive dressings in a select population. Nursing Management 1987;18:47‐50. [PubMed] [Google Scholar]
Brem 2000 {published data only}
- Brem H, Kirsner RS. Use of Graftskin (APLIGRAF) in the treatment of pressure ulcers and acute wounds. Wounds 2000;12(5 (Suppl)):72A‐77A. [Google Scholar]
Brett 2003 {published data only}
- Brett DW, Alvarez OM, Fernandez‐Obregon AC. March 2003 Letters to the Editor. Wounds 2003;15(3):available from: www.woundsresearch.com/article/1368. [Google Scholar]
Brown‐Etris 1999a {published data only}
- Brown‐Etris M, Punchello M, Shields D, Bateman D, Stanfield J, Edvalson J, et al. Final report: a comparison clinical study to evaluate the efficacy of 20% hypertonic wound gel vs collagenase ointment for the debridement of nonviable tissue in dermal wounds. 31st Annual Wound, Ostomy and Continence Conference; 1999 June 19‐23; Minneapolis (MN). 1999:245.
Brown‐Etris 1999b {published data only}
- Brown‐Etris M, Punchello M, Shields D, Barnes H, Galluzzi K, Whalen A, et al. Interim analysis of a randomized prospective multi‐centred 20‐week comparison of two wound management systems on pressure ulcers. 31st Annual Wound, Ostomy and Continence Conference; 1999 June 19‐23; Minneapolis (MN). 1999.
Burgos 2000 {published data only}
- Burgos A, Gimenez J, Moreno E, Campos J, Ardanaz J, Talaero C, et al. Collagenase ointment application at 24‐ versus 48‐hour intervals in the treatment of pressure ulcers. A randomised multicentre study. Clinical Drug Investigation 2000;19(6):399‐407. [Google Scholar]
Burke 1998 {published data only}
- Burke DT, Ho CH, Saucier MA. Hydrotherapy effects on pressure ulcer healing. Archives of Physical Medicine and Rehabilitation 1997;78(9):1053. [DOI] [PubMed] [Google Scholar]
- Burke DT, Ho CH, Saucier MA, Stewart G. Effects of hydrotherapy on pressure ulcer healing. American Journal of Physical Medicine and Rehabilitation 1998;77(5):394‐8. [DOI] [PubMed] [Google Scholar]
Capillas Pérez 2000 {published data only}
- Capillas Pérez R, Cabre Aguilar V, Gil Colome AM, Gaitano Garcia A, Torra i Bou JE. Comparison of the effectiveness and cost of treatment with humid environment as compared to traditional cure. Clinical trial on primary care patients with venous leg ulcers and pressure ulcers. Revista de Enfermería 2000;23(1):17‐24. [PubMed] [Google Scholar]
Carusone 2001 {published data only}
- Carusone A, Carella GR, Coppini R, Lorenzo E, Mancuso M, Mazzon S, et al. Effectiveness evaluation of a specific topic treatment for pressure sores prevention in high risk patients using transcutaneous oxymetry. 11th Conference of the European Wound Management Association; 2001 May 17‐19; Dublin, Ireland. 2001:75.
Casali 1997 {published data only}
- Casali B, Bonati PA, DelGin G, Ascari C. The use of a blood platelet lysate in the treatment of pressure sores. Experimental observations. European Wound Management Association Conference; 1997 April 27‐29; Milan, Italy. 1997.
Chang 1998 {published data only}
- Chang KW, Alsagoff S, Ong KT, Sim PH. Pressure ulcers: randomised controlled trial comparing hydrocolloid and saline gauze dressings. Medical Journal of Malaysia 1998;53(4):428‐31. [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen Q. Effect of egg albumen combined with infrared ray irradiation to treat patients with bedsore induced by fecal incontinence. Chinese Nursing Research 2004;18(9A):1550‐1. [Google Scholar]
Cheneworth 1994 {published data only}
- Cheneworth CC, Hagglund KH, Valmassoi B, Brannon C. Portrait of practice: healing heel ulcers. Advances in Wound Care 1994;7(2):44‐8. [PubMed] [Google Scholar]
Chirwa 2010 {published data only}
- Chirwa Z, Bhengu T, Litiane K. The cost effectiveness of using calcium alginate silver matrix in the treatment of wounds. EWMA Journal 2010;10(2):257. [Abstract P299] [Google Scholar]
Chuangsuwanich 2011a {published data only}
- Chuangsuwanich A, Chortrakarnkij P, Karnwanpum J. Cost‐effectiveness analysis in comparing alginate silver dressing with silver zinc sulfadiazine cream in treatment of pressure ulcers. EWMA Journal 2011;11(2 Suppl):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chuangsuwanich 2011b {published data only}
- Chuangsuwanich A, Charnsanti O, Lohsiriwat V, Kangwanpoom C, Thong‐In N. The efficacy of silver mesh dressing compared with silver sulfadiazine cream for the treatment of pressure ulcers. Chotmaihet Thangphaet (Journal of the Medical Association of Thailand) 2011;94(5):559‐65. [PubMed] [Google Scholar]
Chuangsuwanich 2013 {published data only}
- Chuangsuwanich A, Chortrakarnkij P, Kangwanpoom J. Cost‐effectiveness analysis in comparing alginate silver dressing with silver zinc sulfadiazine cream in the treatment of pressure ulcers. Archives of Plastic Surgery 2013;40(5):589‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colin 1996a {published data only}
- Colin D, Kurring PA, Yvon C. Managing sloughy pressure sores. Journal of Wound Care 1996;5(10):444‐6. [DOI] [PubMed] [Google Scholar]
Colin 1996b {published data only}
- Colin D, Kurring PA, Quinlan D, Yvon C. The clinical investigation of an amorphous hydrogel compared with a dextranomer paste dressing in the management of sloughy pressure sores. Fifth European Conference on Advances in Wound Management; 1995 November 21‐24; Harrogate, UK. 1996:155‐9.
Colonna 2004 {published data only}
- Colonna MR, Amadeo G, Giofre C, Strano A, Stagno D'Alcontres F. Are nanocristalline silver dressings effective in reducing both secretions and bacterial colonization in necrotic infected wounds?. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:91.
Cooper 2008 {published data only}
- Cooper P, Gray D, Russell F. Comparing Tena Wash Mousse with Clinisan Foam Cleanser: the results of a comparative study. Wounds UK 2008;4(3):12‐21. [Google Scholar]
Coutts 2000 {published data only}
- Coutts P, Sibbald RG, Inman K, Maltby‐Stephens N. The use of an adhesive hydrocellular foam compared to a hydrocolloid dressing for the treatment of Stage II and III pressure ulcers. 13th Annual Symposium on Advanced Wound Care and 10th Annual Medical Research Forum on Wound Repair; 2000 April 1‐4; Dallas, (TX). 2000:D7.
D'Aniello 1998 {published data only}
- D'Aniello C. Hydrocolloid dressing preparation to surgery for 3rd degree pressure sores. Eighth Annual Meeting of the European Tissue Repair Society; 1998 August 27‐30; Copenhagen, Denmark. 1998:A463.
Dat 2014 {published data only}
- Dat AD, Poon F, Pham KB, Doust J. Aloe vera for treating acute and chronic wounds. Sao Paulo Medical Journal 2014;132(6):382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Day 1995 {published data only}
- Day A, Dombranski S, Farkas C, Foster C, Godin J, Moody M, et al. Managing sacral pressure ulcers with hydrocolloid dressings: results of a controlled, clinical study. Ostomy/Wound management 1995;41(2):52‐4, 56, 58 passim. [PubMed] [Google Scholar]
Dealey 1997 {published data only}
- Dealey C, Keogh A, Dealey C, Keogh A. A randomized, controlled, parallel‐group clinical trial of a new polyurethane foam island dressing versus a hydrocolloid island dressing in the treatment of grade II and III pressure sores. Sixth European Conference on Advances in Wound Management; 1995 November 21‐24; Harrogate, UK. 1997:94‐5.
Dealey 1998 {published data only}
- Dealey C. Obtaining the evidence for clinically effective wound care. British Journal of Nursing 1998;7(20):1236, 1238, 1240 passim. [DOI] [PubMed] [Google Scholar]
Dealey 2008 {published data only}
- Dealey C. Review: evidence of the effectiveness of hydrocolloids for healing pressure ulcers is limited. Evidence‐Based Nursing 2008;11(4):115. [DOI] [PubMed] [Google Scholar]
De Laat 2005 {published data only}
- Laat EH, Scholte op Reimer WJ, Achterberg T. Pressure ulcers: diagnostics and interventions aimed at wound‐related complaints: a review of the literature. Journal of Clinical Nursing 2005;14(4):464‐72. [DOI] [PubMed] [Google Scholar]
De Laat 2011 {published data only}
- Laat EH, Boogaard MH, Spauwen PH, Kuppevelt DH, Goor H, Schoonhoven L. Faster wound healing with topical negative pressure therapy in difficult‐to‐heal wounds: a prospective randomized controlled trial. Annals of Plastic Surgery 2011;67(6):626‐31. [DOI] [PubMed] [Google Scholar]
Dierick 2004a {published data only}
- Dierick AM, Looverbosch D, Heyman H. Explorative, comparative study with enamel matrix protein in pressure ulcer stage II. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:15.
Dierick 2004b {published data only}
- Dierick AM, Looverbosh D, Heyman H, Looverbosh D. An open, randomised, explorative, phase II, investigation comparing enamel matrix derivative proteins (EMD) and control in patients with pressure ulcer stage II. Wound Repair and Regeneration 2004;12(2):A26. [Google Scholar]
Dobrzanski 1990 {published data only}
- Dobrzanski S, Kelly CM, Gray JI, Gregg AJ, Cosgrove CA. Granuflex dressings in treatment of full thickness pressure sores. Professional Nurse 1990;5(11):594‐9. [PubMed] [Google Scholar]
Durović 2008 {published data only}
- Durović A, Maric D, Brdareski Z, Jevtic M, Durdevic S. The effects of polarized light therapy in pressure ulcer healing. Vojnosanitetski Pregled (Military‐Medical and Pharmaceutical Review) 2008;65(12):906‐12. [DOI] [PubMed] [Google Scholar]
Dwivedi 2016 {published data only}
- Dwivedi MK, Srivastava RN, Bhagat AK, Agarwal R, Baghel K, Jain A, et al. Pressure ulcer management in paraplegic patients with a novel negative pressure device: a randomised controlled trial. Journal of Wound Care 2016;25(4):199‐207. [DOI] [PubMed] [Google Scholar]
Ellis 2002 {published data only}
- Ellis S, Leaper D. Observed effects of local radiant heat on the quality and quantity of bacteria in full thickness pressure ulcers. 12th Conference of the European Wound Management Association; 2002 May 23‐25; Granada, Spain. 2002:146.
Ellis 2003 {published data only}
- Ellis SL, Finn P, Noone M, Leaper DJ. Eradication of methicillin‐resistant Staphylococcus aureus from pressure sores using warming therapy. Surgical Infections 2003;4(1):53‐5. [DOI] [PubMed] [Google Scholar]
El Zayat 1989 {published data only}
- Zayat SG. Preliminary experience with topical phenytoin in wound healing in a war zone. Military Medicine 1989;154(4):178‐80. [PubMed] [Google Scholar]
Engdahl 1980 {published data only}
- Engdahl E. Clinical evaluation of Debrisan® on pressure sores. Current Therapeutic Research, Clinical and Experimental 1980;28(3):377‐80. [Google Scholar]
Esch 1989 {published data only}
- Esch B, Raptis G. Treatment of decubitus ulcer with Crilanomer and Dextranomer. Zeitschrift fur Hautkrankheiten 1989;64(12):1135‐8. [PubMed] [Google Scholar]
Farsaei 2014 {published data only}
- Farsaei S, Khalili H, Farboud ES, Karimzadeh I, Beigmohammadi MT. Efficacy of topical atorvastatin for the treatment of pressure ulcers: a randomized clinical trial. Pharmacotherapy 2014;34(1):19‐27. [DOI] [PubMed] [Google Scholar]
Fear 1992 {published data only}
- Fear M, Thomas S. Wound cleansing properties of Debrisan and Scherisorb gel: interim results of a clinical trial. First European Conference on Advances in Wound Management; 1991 September 4‐6; Cardiff, UK. 1992:162‐4.
Feldman 2005 {published data only}
- Feldman D, Jennings A, Andino R. Preliminary evaluation of an electrical stimulation bandage (POSiFECT dressing). Wound Repair and Regeneration 2006;14(1):A29. [Abstract 242] [Google Scholar]
Felzani 2011 {published data only}
- Felzani G, Spoletini I, Convento A, Lorenzo B, Rossi P, Miceli M. Effect of lysine hyaluronate on the healing of decubitus ulcers in rehabilitation patients. Advances in Therapy 2011;28(5):439‐45. [DOI] [PubMed] [Google Scholar]
Flanagan 1995 {published data only}
- Flanagan M. The efficacy of a hydrogel in the treatment of wounds with non‐viable tissue. Journal of Wound Care 1995;4(6):264‐7. [DOI] [PubMed] [Google Scholar]
Ford 2002 {published data only}
- Ford CN, Reinhard ER, Yeh D, Syrek D, Las Morenas A, Bergman SB, et al. Interim analysis of a prospective, randomized trial of vacuum‐assisted closure versus the healthpoint system in the management of pressure ulcers. Annals of Plastic Surgery 2002;49(1):55‐61. [DOI] [PubMed] [Google Scholar]
Fowler 1983 {published data only}
- Fowler EM. Clinical trial report: new, once‐daily pressure sore dressing speeds healing. Registered Nurse 1983;46(4):56‐7. [PubMed] [Google Scholar]
Franek 2011 {published data only}
- Franek A, Kostur R, Taradaj J, Blaszczak E, Szlachta Z, Dolibog P, et al. Effect of high voltage monophasic stimulation on pressure ulcer healing: results from a randomized controlled trial. Wounds 2011;23(1):15‐23. [Google Scholar]
Franek 2012 {published data only}
- Franek A, Kostur R, Polak A, Taradaj J, Szlachta Z, Blaszczak E, et al. Using high‐voltage electrical stimulation in the treatment of recalcitrant pressure ulcers: results of a randomized, controlled clinical study. Ostomy/Wound Management 2012;58(3):30‐44. [PubMed] [Google Scholar]
Franken 1999 {published data only}
- Franken K, Neuman HA, Andersen KE, Larsen AM. The preliminary results of a comparative study of performance characteristics and safety of a foam dressing Biatain Adhesive versus Tielle on pressure sores. Ninth European Conference on Advances in Wound Management; 1999 November 9‐11; Harrogate, UK. 1999:8.
Fulco 2015 {published data only}
- Fulco I, Erba P, Valeri RC, Vournakis J, Schaefer DJ. Poly‐N‐acetyl glucosamine nanofibers for negative‐pressure wound therapies. Wound Repair and Regeneration 2015;23(2):197‐202. [DOI] [PubMed] [Google Scholar]
Fønnebø 2008 {published data only}
- Fønnebø V. Well‐established ointment from folk medicine heals pressure ulcers better than conventional treatment. Focus on Alternative and Complementary Therapies 2008;13(3):183‐4. [Google Scholar]
García González 2002 {published data only}
- García González RF, Verdu Soriano J, Gago Fornells M, Rueda Lopez J, Segovia Gomez T, Peters Gutierrez FS. The effect of non‐irritant film barriers on the control of maceration in the care of pressure ulcers using adhesive hydrocolloid dressings. 12th Conference of the European Wound Management Association; 2002 May 23‐25; Granada, Spain. 2002:224.
Garrett 1969 {published data only}
- Garrett TA. Bacillus subtilis protease: a new topical agent for debridement. Clinical Medicine 1969;76(May):11‐5. [Google Scholar]
Gerding 1992 {published data only}
- Gerding GA, Browning JS. Oxyquinoline‐containing ointment vs. standard therapy for Stage I and Stage II skin lesions. Dermatology Nursing 1992;4(5):389‐98. [PubMed] [Google Scholar]
Gilligan 2014 {published data only}
- Gilligan AM, Waycaster C. Cost‐effectiveness of becaplermin gel on wound closure in the treatment of pressure ulcers. Value in Health 2014;17(3):A249. [Google Scholar]
Goldmeier 1997 {published data only}
- Goldmeier S. The use of CTM with essential fatty acid in decubits ulcers of cardiac patients [sic] [Comparaçäo dos triglicerídeos cadeia média com ácidos graxos essenciais, com o polivinilpirrolidona‐iodo no tratamento das úlceras de decúbito em pacientes cardiopatas]. Revista Paulista de Enfermagem 1997;16(1/3):30‐4. [Google Scholar]
Gostishchev 1983 {published data only}
- Gostishchev VK, Vasilkova ZF, Khanin AG, Vavilova GS, Lebedskoj AG. Debrisan in the treatment of purulent wounds. Vestnik Khirurgii Imeni I. I. Grekova 1983;131(9):56‐9. [PubMed] [Google Scholar]
Greer 1999 {published data only}
- Greer SE, Longaker MT, Margiotta M. Preliminary results from a multicenter, randomized, controlled study of the use of subatmospheric pressure dressing for pressure ulcer healing. Wound Repair and Regeneration 1999;7(4):A255. [Google Scholar]
Gregory 1997 {published data only}
- Gregory JE, Cox DA, Borchers V, Pfister C. A controlled trial of RH‐TGF‐B3 in decubitus ulcers. Seventh Annual Meeting of the European Tissue Repair Society; 1997 August 23‐26; Köln, Germany. 1997:3.
Guthrie 1989 {published data only}
- Guthrie M, Diakiw J, Zaydon AC, Clark M, Rinaldi E. A randomized double‐blind clinical study of dermagran dual therapeutic system in the treatment of decubitus ulcers. Wounds 1989;1(3):142‐54. [Google Scholar]
Hamilton Hislop 1962 {published data only}
- Hamilton Hislop HH, Pritchard JG. A clinical trial of creams for the prevention and treatment of pressure sores in geriatric patients. British Journal of Clinical Practice 1962;16(6):409‐12. [PubMed] [Google Scholar]
Hampton 1998 {published data only}
- Hampton S. Treatment of macerated and excoriated peri‐wound areas. Seventh European Conference on Advances in Wound Management; 1997 November 18‐20; Harrogate, UK. 1998:46.
Harada 1996 {published data only}
- Harada S, Ohara K, Takemura T, Niimura M, Nishikawa T, Kukita A. Clinical evaluation of M‐1011G (lysozyme hydrochloride ointment‐gauze) in patients with decubitus ulcer: a multi‐centered comparative study with lysozyme hydrochloride ointment. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicine) 1996;12(2):321‐39. [Google Scholar]
Harding 1996 {published data only}
- Harding KG, Bale S, Stock J, Shutler S, Squires D, Wilson I, et al. A multi‐center comparison of a hydrocellular adhesive dressing and a hydrocolloid dressing in the management of stage II and III pressure ulcers. Ninth Annual Symposium on Advanced Wound Care and 6th Annual Medical Research Forum on Wound Repair; 1996 April 20‐24; Atlanta (GA). 1996:114.
Harding 2000 {published data only}
- Harding KG, Cutting K, Price P. The cost‐effectiveness of wound management protocols of care. British Journal of Nursing 2000;9(19):Tissue Viability Supplement: S6, S8, S10 passim. [DOI] [PubMed] [Google Scholar]
Helaly 1988 {published data only}
- Helaly P, Vogt E, Schneider G, Ballanzin D, Balmer A, Benninger R, et al. Wound healing impairment and topical enzymatic therapy: a multicentric double‐blind study. Schweizerische Rundschau für Medizin Praxis 1988;77(52):1428‐34. [PubMed] [Google Scholar]
Heuckeroth 2013 {published data only}
- Heuckeroth L, Palm R. How effective are silver wound dressings? Wound healing of critically colonized and infected wounds. Pflege Zeitschrift 2013;66(6):362‐5. [PubMed] [Google Scholar]
Heyer 2013 {published data only}
- Heyer K, Augustin M, Protz K, Herberger K, Spehr C, Rustenbach SJ. Effectiveness of advanced versus conventional wound dressings on healing of chronic wounds: systematic review and meta‐analysis. Dermatology 2013;226(2):172‐84. [DOI] [PubMed] [Google Scholar]
Hinz 1986 {published data only}
- Hinz J, Hautzinger H, Stahl KW. Rationale for and results from a randomised, double‐blind trial of tetrachlorodecaoxygen anion complex in wound healing. Lancet 1986;1(8485):825‐8. [DOI] [PubMed] [Google Scholar]
Hirshberg 2001 {published data only}
- Hirshberg J, Coleman J, Marchant B, Rees RS. TGF‐beta3 in the treatment of pressure ulcers: a preliminary report. Advances in Skin and Wound Care 2001;14(2):91‐5. [DOI] [PubMed] [Google Scholar]
Hock 1997 {published data only}
- Hock S, Piaskowski P. A comparative randomized study on the performance of Comfeel SeaSorb dressing and Kaltostat on pressure sores. Sixth European Conference on Advances in Wound Management; 1996 October 1‐4; Amsterdam, the Netherlands. 1997:95.
Hofman 1994 {published data only}
- Hofman D, Burgess B, Cherry GW, Robinson BJ, Ryan TJ. Management of pressure sores and leg ulcers with Duoderm hydroactive gel. Fourth Annual Meeting of the European Tissue Repair Society; 1994 August 25‐28; Oxford, UK. 1994:198.
Horch 2005 {published data only}
- Horch RE, Kopp J, Bach AD. The influence of Suprasorb P and Suprasorb C on proteases and their inhibitors in pressure sores. Zeitschrift für Wundheilung 2005;5:213. [Google Scholar]
Hsu 2000 {published data only}
- Hsu YC, Chang HH, Chen MF, Chen JC. Therapeutic effect of sheng‐ji‐san on pressure ulcers. American Journal of Chinese Medicine 2000;28(3‐4):391‐9. [DOI] [PubMed] [Google Scholar]
Hu 2009 {published data only}
- Hu KX, Zhang HW, Zhou F, Yao G, Shi JP, Wang LF, et al. Observation on the therapeutic effects of negative‐pressure wound therapy on the treatment of complicated and refractory wounds. Zhonghua Shao Shang Za Zhi (Chinese Journal of Burns) 2009;25(4):249‐52. [PubMed] [Google Scholar]
Ishibashi 1991 {published data only}
- Ishibashi Y, Niimura M, Nishikawa T, Imamura S, Ogawa H, Ikeda S, et al. Clinical results of multicenter comparative study with lyophilized porcine dermis (KT‐104) and solcoseryl ointment (SS‐094) on intractable skin ulcers: a prospective randomized trial. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicine) 1991;7(12):2811‐28. [Google Scholar]
Ishibashi 1996 {published data only}
- Ishibashi Y, Soeda S, Oura T, Nishikawa T, Niimura M, Nakajima H, et al. Clinical effects of KCB‐1, a solution of recombinant human basic fibroblast growth factor, on skin ulcers: A phase III study comparing with sugar and povidone iodine ointment. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicine) 1996;12(10):2159‐87. [Google Scholar]
Janssen 1989 {published data only}
- Janssen PA, Janssen H, Cauwenbergh G, Doncker P, Beule K, Lewi P, et al. Use of topical ketanserin in the treatment of skin ulcers: a double‐blind study. Journal of the American Academy of Dermatology 1989;21(1):85‐90. [DOI] [PubMed] [Google Scholar]
Jercinovic 1994 {published data only}
- Jercinovic A, Karba R, Vodovnic L, Stefanovska A, Kroselj P, Turk R, et al. Low frequency pulsed current and pressure ulcer healing. IEEE Transactions on Rehabilitation Engineering 1994;2(4):225‐33. [Google Scholar]
Johnson 1992 {published data only}
- Johnson A. Dressings for deep wounds. Nursing Times 1992;88(4):55‐6, 58. [PubMed] [Google Scholar]
Kallianinen 2000 {published data only}
- Kallianinen LK, Hirshberg J, Marchant B, Rees RS. Role of platelet‐derived growth factor as an adjunct to surgery in the management of pressure ulcers. Plastic and Reconstructive Surgery 2000;106(6):1243‐8. [DOI] [PubMed] [Google Scholar]
Karap 2008 {published data only}
- Karap Z. Significant difference in pain levels of delayed‐healing wounds during applying an ionic silver hydrofibre dressing. EWMA Journal 2008;8(2 (Suppl) ):244. [Abstract P281] [Google Scholar]
Kerihuel 2010 {published data only}
- Kerihuel JC. Effect of activated charcoal dressings on healing outcomes of chronic wounds. Journal of Wound Care 2010;19(5):208, 210‐2, 214‐5. [DOI] [PubMed] [Google Scholar]
Kerstein 2004 {published data only}
- Kerstein MD. Unexpected economics of ulcer care protocols. Southern Medical Journal 2004;97(2):135‐6. [DOI] [PubMed] [Google Scholar]
Kim 1996 {published data only}
- Kim YC, Shin JC, Park CI, Oh SH, Choi SM, Kim YS. Efficacy of hydrocolloid occlusive dressing technique in decubitus ulcer treatment: a comparative study. Yonsei Medical Journal 1996;37(3):181‐5. [DOI] [PubMed] [Google Scholar]
Kloth 2000a {published data only}
- Kloth LC, Berman JE, Nett MJ, Dumit‐Minkel S, Warzel J. A randomized controlled trial using a heated dressing on full‐thickness pressure ulcers. 13th Annual Symposium on Advanced Wound Care and 10th Annual Medical Research Forum on Wound Repair; 2000 April 1‐4; Dallas (TX). 2000:10.
Kloth 2000b {published data only}
- Kloth LC, Berman JE, Dumit‐Minkel S, Sutton CH, Papanek PE, Wurzel J. Effects of a normothermic dressing on pressure ulcer healing. Advances in Skin and Wound Care 2000;13(2):69‐74. [PubMed] [Google Scholar]
Kloth 2001 {published data only}
- Kloth LC, Berman JE, Nett MJ, Papanek PE. Twelve week randomized trial to evaluate noncontact normothermic wound therapy on full‐thickness pressure ulcers. Wound Repair and Regeneration 2001;9(5):399. [DOI] [PubMed] [Google Scholar]
Kloth 2002 {published data only}
- Kloth LC, Berman JE, Nett M, Papanek PE, Dumit‐Minkel S. A randomized controlled clinical trial to evaluate the effects of noncontact normothermic wound therapy on chronic full‐thickness pressure ulcers. Advances in Skin and Wound Care 2002;15(6):270‐6. [DOI] [PubMed] [Google Scholar]
Knudsen 1982 {published data only}
- Knudsen L, Solvhoj L, Christensen B. The use of a haemodialysate in the treatment of decubital ulcer: a double‐blind randomized clinical study. Current Therapeutic Research, Clinical and Experimental 1982;32(3):498‐504. [Google Scholar]
Kohr 2000 {published data only}
- Kohr R, Whittle H, MacLean B, Garrett M. A qualitative comparison of three sacral pressure ulcer dressings. 13th Annual Symposium on Advanced Wound Care and 10th Annual Medical Research Forum on Wound Repair; 2000 April 1‐4 ; Dallas (TX). 2000:D10‐11.
Kordestani 2008 {published data only}
- Kordestani S, Shahrezaee M, Tahmasebi MN, Hajimahmodi H, Haji Ghasemali D, Abyaneh MS. A randomised controlled trial on the effectiveness of an advanced wound dressing used in Iran. Journal of Wound Care 2008;17(7):323‐7. [DOI] [PubMed] [Google Scholar]
Kucan 1981 {published data only}
- Kucan JO, Robson MC, Heggers JP, Ko F. Comparison of silver sulfadiazine, povidone‐iodine and physiologic saline in the treatment of chronic pressure ulcers. Journal of the American Geriatrics Society 1981;29(5):232‐5. [DOI] [PubMed] [Google Scholar]
Kuflik 2001 {published data only}
- Kuflik A, Stillo JV, Sanders D, Roland K, Sweeney T, Lemke PM. Petrolatum versus RESURFIX ointment in the treatment of pressure ulcers. Ostomy/Wound Management 2001;47(2):52‐3, 55‐6. [PubMed] [Google Scholar]
Kuisma 1987 {published data only}
- Kuisma I, Tamelander G. Mucopolysaccharide polysulphate cream in the prevention of pressure sores: a double blind study. Annals of Clinical Research 1987;19(6):374‐7. [PubMed] [Google Scholar]
Kukita 1990 {published data only}
- Kukita A, Oura T, Aoki T, Harada S, Takeda K, Tashiro M, et al. Clinical evaluation of NI‐009 on various cutaneous ulcers: comparative study with Elase‐C ointment. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicine) 1990;6(4):817‐48. [Google Scholar]
Kurring 1994 {published data only}
- Kurring PA, Roberts CD, Quinlan D. Evaluation of a hydrocellular dressing in the management of exuding wounds in the community. British Journal of Nursing 1994;3(20):1049‐53. [DOI] [PubMed] [Google Scholar]
Kurzuk‐Howard 1985 {published data only}
- Kurzuk‐Howard G, Simpson L, Palmieri A. Decubitus ulcer care: a comparative study. Western Journal of Nursing Research 1985;7(1):58‐79. [DOI] [PubMed] [Google Scholar]
Landi 2003 {published data only}
- Landi F, Aloe L, Russo A, Cesari M, Onder G, Bonini S, et al. Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Annals of Internal Medicine 2003;139(8):635‐41. [DOI] [PubMed] [Google Scholar]
Langer 1996 {published data only}
- Langer SW. Testing the efficacy of a material for the relief of pressure in the prevention and treatment of decubitus ulcers. Krankenpflege Journal 1996;34(6):263‐5. [PubMed] [Google Scholar]
Lazareth 2012 {published data only}
- Lazareth I, Meaume S, Sigal‐Grinberg ML, Combemale P, Guyadec T, Zagnoli A. Efficacy of a silver lipidocolloid dressing on heavily colonised wounds: a republished RCT. Journal of Wound Care 2012;21(2):96‐102. [DOI] [PubMed] [Google Scholar]
Lechner 1991 {published data only}
- Lechner JL, Ksiazekl S, Chang J, Rozek SL, Smith JM. Carrington dermal wound gel versus current treatments for the healing of decubitus ulcers and other dermal wounds. 26th Annual ASHP Midyear Clinical Meeting; 1991 December 8‐12; New Orleans (LA). 1992:294.
Lee 1975 {published data only}
- Lee LK, Ambrus JL. Collagenase therapy for decubitus ulcers. Geriatrics 1975;30(5):91‐8. [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee RL, Leung PH, Wong TK. A randomized controlled trial of topical tea tree preparation for MRSA colonized wounds. International Journal of Nursing Sciences 2014;1(1):7‐14. [Google Scholar]
LeVasseur 1991 {published data only}
- LeVasseur SA, Helme RD. A double‐blind clinical trial to compare the efficacy of an active based cream F14001 against a placebo non‐active based cream for the treatment of pressure ulcers in a population of elderly subjects. Journal of Advanced Nursing 1991;16(8):952‐6. [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li Y, Yao M, Wang X, Zhao Y. Effects of gelatin sponge combined with moist wound‐healing nursing intervention in the treatment of phase III bedsore. Experimental and Therapeutic Medicine 2016;11(6):2213‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 1997 {published data only}
- Lin JY, Wu XH. The effect of bactroban cream for treating bedsores. Contemporary Nurse 1997;2:38. [Google Scholar]
Lindsay 2011 {published data only}
- Lindsay S, Clark R, Cullen B. Silver dressings: do they work?. Journal of Wound, Ostomy, and Continence nursing 2011;38(3 Suppl):S88. [Google Scholar]
Lingner 1984 {published data only}
- Lingner C, Rolstad BS, Wetherill K, Danielson S. Clinical trial of a moisture vapor‐permeable dressing on superficial pressure sores. Journal of Enterostomal Therapy 1984;11(4):147‐9. [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu H‐H, Liu S. Comparison of the effectiveness of different dressings on grade 2 pressure ulcers. Fourth Congress of the World Union of Wound Healing Societies; 2012 September 2‐6; Yokohama, Japan. 2012.
Liu 2013 {published data only}
- Liu X, Meng Q, Song H, Zhao T. A traditional Chinese herbal formula improves pressure ulcers in paraplegic patients: a randomized, parallel‐group, retrospective trial. Experimental and Therapeutic Medicine 2013;5(6):1693‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ljungberg 1998 {published data only}
- Ljungberg S. Comparison of dextranomer paste and saline dressings for management of decubital ulcers. Clinical Therapeutics 1998;20(4):737‐43. [DOI] [PubMed] [Google Scholar]
Llewellyn 1996 {published data only}
- Llewellyn M, Baggott JE, Thomas N, Bale S. Comparison of a new alginate containing hydrocolloid vs traditional granuflex dressing on exuding pressure sores. Second Joint Meeting of the Wound Healing Society and the European Tissue Repair Society; 1996 May 15‐17; Boston (MA). 1996:130.
Lopez‐Jimenez 2003 {published data only}
- Lopez‐Jimenez E, Romero S, Hahn TW. A crossover study evaluating an adhesive foam dressing for heel ulcers. 13th Conference of the European Wound Management Association; 2003 May 22‐24; Pisa, Italy. 2003:211. [Poster 80]
Lum 1996 {published data only}
- Lum C, Cheung W, Chow PM, Woo J, Hui E, Or KH. Use of a hydrogel in pressure sore management: a randomized, controlled clinical trial. Fifth European Conference on Advances in Wound Management; 1995 November 21‐24; Harrogate, UK. 1996:283.
Macario 2002 {published data only}
- Macario A, Dexter F. Is noncontact normothermic wound therapy cost effective for the treatment of stages 3 and 4 pressure ulcers?. Wounds 2002;14(3):93‐106. [Google Scholar]
Manzanero‐Lopez 2004 {published data only}
- Manzanero‐Lopez E, Perez‐Luis B. Evaluation of a new dressing in the management of pressure sores. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:170.
Martin 1996 {published data only}
- Martin SJ, Corrado OJ, Kay EA. Enzymatic debridement for necrotic wounds. Journal of Wound Care 1996;5(7):310‐1. [DOI] [PubMed] [Google Scholar]
Meaume 1996a {published data only}
- Meaume S, Bonnefoy M, Guihur B, Montalembert C, Remy F, Bohbot S. Decubitus ulcers in the aged. Local care and results of a randomized multicenter study. Soins 1996;October(609):40‐5. [PubMed] [Google Scholar]
Meaume 1996b {published data only}
- Meaume S, Sayag J, Bonnefoy M, Brasseur C, Drunat O, Sebbane G, et al. Calcium alginate dressing (Algosteril) in the management of full‐thickness pressure sores: a controlled, randomized study on elderly patients. Fifth European Conference on Advances in Wound Management; 1995 November 21‐24; Harrogate, UK. 1996:19.
Meaume 2005 {published data only}
- Meaume S, Vallet D, Morere MN, Taot L. Evaluation of a silver‐releasing hydroalginate dressing in chronic wounds with signs of local infection. Journal of Wound Care 2005;14(9):411‐9. [DOI] [PubMed] [Google Scholar]
- Meaume S, Vallet D, Morere MN, Teot L. Evaluation of a silver‐releasing hydroalginate dressing in chronic wounds with signs of local infection. Zeitschrift für Wundheilung 2006;11(5):236‐45. [DOI] [PubMed] [Google Scholar]
Mian 1992 {published data only}
- Mian E, Martini P, Beconcini D, Mian M. Healing of open skin surfaces with collagen foils. International Journal of Tissue Reactions 1992;14(Suppl):27‐34. [PubMed] [Google Scholar]
Milne 2012 {published data only}
- Milne CT, Ciccarelli A, Lassy M. A comparison of collagenase to hydrogel dressings in maintenance debridement and wound closure. Wounds 2012;24(11):317‐22. [PubMed] [Google Scholar]
- Milne CT, Ciccarelli AO. A comparison of collagenase to hydrogel dressing in wound debridement, maintenance, debridement and wound healing. Symposium on Advanced Wound Care and the Wound Healing Society; 2010 April 17‐20; Orlando, Florida. 2010:S70. [Abstract CS‐077]
- Waycaster C, Milne CT. Clinical and economic benefit of enzymatic debridement of pressure ulcers compared to autolytic debridement with a hydrogel dressing. Journal of Medical Economics 2013;16(7):976‐86. [DOI] [PubMed] [Google Scholar]
Mizuhara 2012 {published data only}
- Mizuhara A, Bito S, Ohnishi S, Takeuchi K, Kobayashi K, Akiyama K. The therapeutic effectiveness of wrap therapy: a study comparing wrap therapy to standard therapy per current guidelines. Fourth Congress of the World Union of Wound Healing Societies; 2012 September 2‐6; Yokohama, Japan. 2012.
Mo 2015 {published data only}
- Mo X, Cen J, Gibson E, Wang R, Percival SL. An open multicenter comparative randomized clinical study on chitosan. Wound Repair and Regeneration 2015;23(4):518‐24. [DOI] [PubMed] [Google Scholar]
Moberg 1983 {published data only}
- Moberg S, Hoffman L, Grennert ML, Holst A. A randomized trial of cadexomer iodine in decubitus ulcers. Journal of the American Geriatrics Society 1983;31(8):462‐5. [DOI] [PubMed] [Google Scholar]
Mody 2008 {published data only}
- Mody GN, Nirmal IA, Duraisamy S, Perakath B. A blinded, prospective, randomized controlled trial of topical negative pressure wound closure in India. Ostomy/Wound Management 2008;54(12):36‐46. [PubMed] [Google Scholar]
Moody 1991 {published data only}
- Moody M. Tissue viability: calcium alginate: a dressing trial. Nursing Standard 1991;13(Suppl):3‐6. [PubMed] [Google Scholar]
Moody 2002 {published data only}
- Moody M. Relieve the pressure. Elderly care 2002;11(4):12‐14. [PubMed] [Google Scholar]
Moore 2011 {published data only}
- Moore C, Young J. Effectiveness of silver in wound care treatment. Physical Therapy Reviews 2011;16(3):201‐9. [Google Scholar]
Morimoto 2015 {published data only}
- Morimoto N, Kakudo N, Matsui M, Ogura T, Hara T, Suzuki K, et al. Exploratory clinical trial of combination wound therapy with a gelatin sheet and platelet‐rich plasma in patients with chronic skin ulcers: study protocol. BMJ Open 2015;5(5):e007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Motta 1991 {published data only}
- Motta GJ. The effectiveness of Dermagran topical therapy for treating chronic wounds in nursing facility residents. Ostomy/Wound Management 1991;36:35‐8. [PubMed] [Google Scholar]
Motta 2004 {published data only}
- Motta GJ, Milne CT, Corbett LQ. Impact of antimicrobial gauze on bacterial colonies in wounds that require packing. Ostomy/Wound Management 2004;50(8):48‐62. [PubMed] [Google Scholar]
Mouës 2004 {published data only}
- Mouës CM, Bemd GJ, Heule F, Hovius SE. A prospective randomized trial comparing vacuum therapy to conventional moist gauze therapy. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:6. [Abstract A001]
- Mouës CM, Bemd GJ, Meerding WJ, Hovius SE. Cost analysis comparing vacuum‐assisted closure wound therapy to conventional moist gauze therapy. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:87. [Abstract A008]
- Mouës CM, Bemd GC, Meerding WJ, Hovius SE. An economic evaluation of the use of TNP on full‐thickness wounds. Journal of Wound Care 2005;14(5):224‐7. [DOI] [PubMed] [Google Scholar]
- Mouës CM, Vos MC, Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair and Regeneration 2004;12(1):11‐7. [DOI] [PubMed] [Google Scholar]
- Mouës CM, Vos MC, Bemd GJCM, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy. 13th Conference of the European Wound Management Association; 2003 May 22‐24; Pisa, Italy. 2003:69.
Mouës 2007 {published data only}
- Mouës CM, Bemd GJ, Heule F, Hovius SE. Comparing conventional gauze therapy to vacuum‐assisted closure wound therapy: a prospective randomised trial. Journal of Plastic and Reconstructive Aesthetic Surgery 2007;60(6):672‐81. [DOI] [PubMed] [Google Scholar]
Mulder 1989a {published data only}
- Mulder G, Kissil M, Mahr JJ. Bacterial growth under occlusive and non‐occlusive wound dressings. Wounds 1989;1(1):63‐9. [Google Scholar]
Mulder 1989b {published data only}
- Mulder GD, Walker A. Preliminary observations on clotting under three hydrocolloid dressings. Journal of the Royal Society of Medicine 1989;82(12):739‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulder 1993a {published data only}
- Mulder GD, Altman M, Seeley JE, Tintle T. Prospective randomized study of the efficacy of hydrogel, hydrocolloid, and saline solution‐moistened dressings on the management of pressure ulcers. Wound Repair and Regeneration 1993;1(4):213‐8. [DOI] [PubMed] [Google Scholar]
Mulder 1993b {published data only}
- Mulder GD. A prospective controlled randomized study of the efficacy of Clearsite, DuoDERM, and wet‐to‐dry gauze dressing in the management of pressure ulcers. Second European Conference on Advances in Wound Management; 1992 October 20‐23; Harrogate, UK. 1993:45.
Münter 2006 {published data only}
- Münter KC, Beele H, Russell L, Basse PB, Groechenig E, Crespi A, et al. The CONTOP study: improved healing of delayed healing ulcers with sustained silver‐releasing foam dressing versus other silver dressings. 16th Conference of the European Wound Management Association; 2006 May 18‐20; Prague, Czech Republic. 2006:210. [Abstract P068]
- Münter KC, Beele H, Russell L, Crespi A, Grochenig E, Basse P, et al. The CONTOP study: a large‐scale, comparative, randomised study in patients treated with a sustained silver‐releasing foam dressing. European Wound Management Association Conference; 2005 September 15‐17; Stuttgart, Germany. 2005:292. [Poster 193]
- Münter KC, Beele H, Russell L, Crespi A, Gruchenig E, Basse P, et al. Effect of a sustained silver‐releasing dressing on ulcers with delayed healing: the CONTOP study. Journal of Wound Care 2006;15(5):199‐206. [DOI] [PubMed] [Google Scholar]
- Russell L, Nebbioso G, Münter KC, Beele H, Basse PB, Dienst H. The CONTOP Study: a hydro‐activated silver containing foam dressing versus standard care. Second World Union of Wound Healing Societies Meeting, 2004 July 8‐13, Paris, France. 2004:89.
- Scalise A, Forma O, Happe M, Hahn TW. The CONTOP study: real life experiences from an international study comparing a silver containing hydro‐activated foam dressing with standard wound care. 13th Conference of the European Wound Management Association. 2003:212. [Poster 81]
Mustoe 1994 {published data only}
- Mustoe TA, Cutler NR, Allman RM, Goode PS, Deuel TF, Prause JA, et al. A phase II study to evaluate recombinant platelet‐derived growth factor‐BB in the treatment of stage 3 and 4 pressure ulcers. Archives of Surgery 1994;129(2):213‐9. [DOI] [PubMed] [Google Scholar]
Myers 1990 {published data only}
- Myers SA, Takiguch S, Slavish S, Rose CL. Consistent wound care and nutritional support in treatment. Decubitus 1990;3(3):16‐28. [PubMed] [Google Scholar]
Nasar 1982 {published data only}
- Nasar MA, Morley R. Cost‐effectiveness in treating deep pressure sores and ulcers. Practitioner 1982;226:307‐10. [PubMed] [Google Scholar]
NCT02299557 {published data only}
- NCT02299557. A double blind study to examine the effect of oxymetazoline gel on anal pressure and incontinence in spinal cord injury patients. clinicaltrials.gov/show/NCT02299557 (first received 17 November 2014).
Neill 1989b {published data only}
- Neill K. Clarification of research: comparative evaluation of a hydrocolloid dressing formulation and wet‐to‐damp gauze dressings in the management of pressure sores. Decubitus 1989;2(2):7. [PubMed] [Google Scholar]
Niezgoda 2004 {published data only}
- Niezgoda JA. A comparison of vacuum assisted closure therapy to moist wound care in the treatment of pressure ulcers: preliminary results of a multicenter trial. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:53.
Niimura 1990 {published data only}
- Niimura M, Nishikawa T, Yamamoto K, Ogawa H, Ohara K, Ogawa N. Dose finding study of dibutyryl cyclic AMP ointment in skin ulcers: a double‐blind randomized clinical trial. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicine) 1990;6(8):1577‐98. [Google Scholar]
Niimura 1991 {published data only}
- Niimura M, Ishibashi Y, Imamura S, Hori Y, Nishikawa T, Ogawa H, et al. Clinical study of dibutyryl cyclic AMP ointment (DT‐5621) in chronic skin ulcers: well‐controlled comparative study with lysozyme ointment. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicine) 1991;7(3):677‐92. [Google Scholar]
Nixon 1998 {published data only}
- Bridel‐Nixon J, McElvenny D, Brown J, Mason S. A randomized controlled trial using a double‐triangular sequential design: methodology and management issues. Conference of the European Wound Management Association; 1997 April 27‐29; Milan, Italy. 1997:65‐6.
- Bridel‐Nixon J, McElvenny D, Brown J, Mason S. Findings from a double‐triangular sequential‐design randomized clinical trial of a dry polymer gel pad. Conference of the European Wound Management Association; 1997 April 27‐29; Milan, Italy. 1997:20‐1.
- Nixon J, McElvenny D, Mason S, Brown J, Bond S. A sequential randomised controlled trial comparing a dry visco‐elastic polymer pad and standard operating table mattress in the prevention of post‐operative pressure sores. International Journal of Nursing Studies 1998;35(4):193‐203. [DOI] [PubMed] [Google Scholar]
Ohura 2004 {published data only}
- Ohura T, Sanada H, Mino Y. Clinical activity‐based cost effectiveness of traditional versus modern wound management in patients with pressure ulcers. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:18.
Olivar 1999 {published data only}
- Olivar AE, Martinez PG, Lobato BE, Isaza MS, Jativa ED, Rios LA. Efficiency of a protocol for the treatment of cutaneous wounds in an elderly care centre in Madrid. Eighth European Conference on Advances in Wound Management; 1998 April 26‐28; Madrid, Spain. 1999:61‐3.
Ovington 1999 {published data only}
- Ovington L. Dressings and ajunctive therapies: AHCPR Guidelines revisited. Ostomy/Wound management 1999;45(1A):94S‐108S. [PubMed] [Google Scholar]
Ozdemir 2011 {published data only}
- Ozdemir F, Kasapoglu MK, Oymak F, Murat S. Efficiency of magnetic field treatment on pressure sores in bedridden patients. Balkan Medical Journal 2011;28(3):274‐8. [Google Scholar]
Panahi 2015 {published data only}
- Panahi Y, Izadi M, Sayyadi N, Rezaee R, Jonaidi‐Jafari N, Beiraghdar F, et al. Comparative trial of Aloe vera/olive oil combination cream versus phenytoin cream in the treatment of chronic wounds. Journal of Wound Care 2015;24(10):459‐60. [DOI] [PubMed] [Google Scholar]
Payne 2001 {published data only}
- Payne WG, Ochs DE, Meltzer DD, Hill DP, Mannari RJ, Robson LE, et al. Long‐term outcome study of growth factor‐treated pressure ulcers. American Journal of Surgery 2001;181(1):81‐6. [DOI] [PubMed] [Google Scholar]
Perez 2000 {published data only}
- Perez RC, Aguilar VC, Colome AM, Garcia AG, Torra i Bou JE. A comparison of the effectiveness and cost of moist environment dressings treatment as compared to traditional dressings treatment. Revista de Enfermeria 2000;23(1):17‐24. [PubMed] [Google Scholar]
- Torra i Bou JE, Perez RC, Aguilar VC, Colome AM, Garcia AG. Comparison of the cost of traditional treatment methods versus moist wound healing in the treatment of chronic wounds. Eighth European Conference on Advances in Wound Management; 1998 April 26‐28; Madrid, Spain. 1999:14.
Peschardt 1997 {published data only}
- Peschardt A, Alsbjorn B, Gottrup F. A comparative randomized study on performance characteristics of a new hydrogel versus saline gauze for the treatment of pressure sores. Sixth European Conference on Advances in Wound Management; 1995 November 21‐24; Harrogate, UK. 1997:283.
Picard 2015 {published data only}
- Picard F, Hersant B, Bosc R, Meningaud J‐P. Should we use platelet‐rich plasma as an adjunct therapy to treat 'acute wounds', 'burns', and 'laser therapies'? A review and a proposal of a quality criteria checklist for further studies. Wound Repair and Regeneration 2015;23(2):163‐70. [DOI] [PubMed] [Google Scholar]
Pierce 1994 {published data only}
- Pierce GF, Tarpley JE, Allman RM, Goode PS, Serdar CM, Morris B, et al. Tissue repair processes in healing chronic pressure ulcers treated with recombinant platelet‐derived growth factor BB. American Journal of Pathology 1994;145(6):1399‐1410. [PMC free article] [PubMed] [Google Scholar]
Pullen 2002 {published data only}
- Pullen R, Popp R, Volkers P, Fusgen I. Prospective randomized double‐blind study of the wound‐debriding effects of collagenase and fibrinolysin/deoxyribonuclease in pressure ulcers. Age and Ageing 2002;31(2):126‐30. [DOI] [PubMed] [Google Scholar]
Quelard 1985 {published data only}
- Quelard B, Cordier ME, Regent MC, Tenette M. Comparative study to determine the relative efficiency of two types of treatment of decubitus ulcers of sacro and ischial tuberosities: topical ozone treatment versus the traditional methods. Annales médicales de Nancy et de l'Est 1985;24:329‐34. [Google Scholar]
Ramsay 1979 {published data only}
- Ramsay BM. Pressure sores in para‐ and tetraplegic patients. Nursing Times 1979;75(9):361‐4. [PubMed] [Google Scholar]
Rhodes 1979 {published data only}
- Rhodes B, Daltrey D, Chattwood JG. The treatment of pressure sores in geriatric patients: a trial of sterculia powder. Nursing Times 1979;75(9):365‐8. [PubMed] [Google Scholar]
Rhodes 2001 {published data only}
- Rhodes RS, Heyneman CA, Culbertson VL, Wilson SE, Phatak HM. Topical phenytoin treatment of stage II decubitus ulcers in the elderly. Annals of Pharmacotherapy 2001;35(6):675‐81. [DOI] [PubMed] [Google Scholar]
Roberts 1959 {published data only}
- Roberts GW. Silicone emulsion for bedsores. Lancet 1959;273(7084):1207‐8. [Google Scholar]
Robson 1992a {published data only}
- Robson MC, Phillips LG, Lawrence WT, Bishop JB, Youngerman JS, Hayward PG, et al. The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores. Annals of Surgery 1992;216(4):401‐6; discussion 406‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robson 1992b {published data only}
- Robson MC, Phillips LG, Thomason A, Altrock BW, Pence PC, Heggers JP, et al. Recombinant human platelet‐derived growth factor‐BB for the treatment of chronic pressure ulcers. Annals of Plastic Surgery 1992;29(3):193‐201. [DOI] [PubMed] [Google Scholar]
Robson 1992c {published data only}
- Robson MC, Phillips LG, Thomason A, Robson LE, Pierce GF. Platelet‐derived growth factor BB for the treatment of chronic pressure ulcers. Lancet 1992;339(8784):23‐5. [DOI] [PubMed] [Google Scholar]
Robson 1994 {published data only}
- Robson MC, Abdullah A, Burns BF, Phillips LG, Garrison L, Cowan W, et al. Safety and effect of topical recombinant human interleukin‐1B in the management of pressure sores. Wound Repair and Regeneration 1994;2(3):177‐81. [DOI] [PubMed] [Google Scholar]
Romanelli 2008 {published data only}
- Romanelli M, Dimi V, Bertone MS, Mazzatenta C, Martini P. Efficacy and tolerability of 'Fitostimoline antibiotico' soaked gauzes in the topical treatment of cutaneous sores, ulcers and burns, complicated with bacterial contamination: an open‐label, controlled, randomised, multicentre, parallel group. Gazzetta Medica Italiana 2008;167(6):251‐60. [Google Scholar]
Romanelli 2009 {published data only}
- Palao i Domenech R, Romanelli M, Tsiftsis DD, Slonkova V, Jortikka A, Johannesen N, et al. Effect of an ibuprofen‐releasing foam dressing on wound pain: a real‐life RCT. Journal of Wound Care 2008;17(8):342, 344‐8. [DOI] [PubMed] [Google Scholar]
- Romanelli M, Dini V, Polignano R, Bonadeo P, Maggio G. Ibuprofen slow‐release foam dressing reduces wound pain in painful exuding wounds: preliminary findings from an international real‐life study. Journal of Dermatological Treatment 2009;20(1):19‐26. [DOI] [PubMed] [Google Scholar]
Rooman 1991 {published data only}
- Rooman RP, Janssen H. Ketanserin promotes wound healing: clinical and preclinical results. Progress in Clinical and Biological Research 1991;365:115‐28. [PubMed] [Google Scholar]
Routkovsky‐Norval 1996 {published data only}
- Routkovsky‐Norval C, Meaume S, Goldfarb JM, Provost C, Preauchat A. Randomized comparative study of two hydrocolloid dressings in the treatment of decubitus ulcers. Revue de Gériatrie 1996;21(3):213‐8. [Google Scholar]
Saha 2012 {published data only}
- Saha A, Chattopadhyay S, Azam M, Sur P. The role of honey in healing of bedsores in cancer patients. South Asian Journal of Cancer 2012;1(2):66‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saidkhani 2016 {published data only}
- Saidkhani V, Asadizaker M, Khodayar MJ, Latifi SM. The effect of nitric oxide releasing cream on healing pressure ulcers. Iranian Journal of Nursing and Midwifery Research 2016;21(3):322‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sayag 1996 {published data only}
- Sayag J, Meaume S, Bohbot S. Healing properties of calcium alginate dressings. Journal of Wound Care 1996;5(8):357‐62. [PubMed] [Google Scholar]
Saydak 1990 {published data only}
- Saydak SJ. A pilot test of two methods for the treatment of pressure ulcers. Journal of Enterostomal Therapy 1990;17(3):140‐2. [DOI] [PubMed] [Google Scholar]
Scevola 2010 {published data only}
- Scevola S, Nicoletti G, Brenta F, Isernia P, Maestri M, Faga A. Allogenic platelet gel in the treatment of pressure sores: a pilot study. International Wound Journal 2010;7(3):184‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 1999 {published data only}
- Scott D, Sholler KA, Beck K, Weaver MC. The use of a papain‐urea debriding ointment in combination with surgical/sharp debridement. 31st Annual Wound, Ostomy and Continence Conference; 1999 June 19‐23; Minneapolis (MN) 1999.
Seaman 2000 {published data only}
- Seaman S, Herbster S, Muglia J, Murray M, Rick C. Simplifying modern wound management for nonprofessional caregivers. Ostomy/Wound Management 2000;46(8):18‐27. [PubMed] [Google Scholar]
Sebern 1989 {published data only}
- Sebern MD. Cost and efficacy of pressure ulcer management in a metropolitan visiting nurse association. Decubitus 1989;2(3):58‐9. [PubMed] [Google Scholar]
Serra 2005 {published data only}
- Serra N, Torres OG, Romo MI, Llovera JM, Vigil Escalera LJ, Soto MA, et al. Hydro‐colloidal dressings which release hydro‐active silver. Revista de Enfermeria 2005;28(2):13‐8. [PubMed] [Google Scholar]
Settel 1969 {published data only}
- Settel E. Decubitus ulcer. A double blind study of an old problem and a new treatment. Medical Times 1969;97(4):220‐30. [PubMed] [Google Scholar]
Shamimi Nouri 2008 {published data only}
- Shamimi Nouri K, Karimian R, Nasli E, Kamali K, Chaman R, Farhadi M, et al. Topical application of Semelil (ANGIPARS™) in treatment of pressure ulcers: a randomized clinical trial. Daru 2008;16(Suppl 1):54‐7. [Google Scholar]
Shannon 1988 {published data only}
- Shannon ML, Miller BM. Pressure sore treatment: a case in point. Geriatric Nursing 1988;9(3):154‐7. [DOI] [PubMed] [Google Scholar]
Sherman 2000 {published data only}
- Sherman RA. Maggot debridement therapy for treating non‐healing wounds. Wound Healing Society Educational Symposium; 2000 June 4‐6; Toronto, Canada. 2000:84.
Shirakawa 2005 {published data only}
- Shirakawa M, Isseroff RR. Topical negative pressure devices: use for enhancement of healing chronic wounds. Archives of Dermatology 2005;141(11):1449‐53. [DOI] [PubMed] [Google Scholar]
Shojaei 2008 {published data only}
- Shojaei H, Sokhangoei Y, Soroush MR. Low level laser therapy in the treatment of pressure ulcers in spinal cord handicapped veterans living in Tehran. Iranian Journal of Medical Sciences 2008;33:44‐8. [Google Scholar]
Shrivastava 2011 {published data only}
- Shrivastava R. Clinical evidence to demonstrate that simultaneous growth of epithelial and fibroblast cells is essential for deep wound healing. Diabetes Research and Clinical Practice 2011;92(1):92‐9. [DOI] [PubMed] [Google Scholar]
Sibbald 2011 {published data only}
- Sibbald RG, Coutts P, Woo KY. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing‐clinical trial results. Advances in Skin and Wound Care 2011;24 (2 ):78‐84. [DOI] [PubMed] [Google Scholar]
Small 2002 {published data only}
- Small N, Mulder M, Mackenzie MJ, Nel M. A comparative analysis of pressure sore treatment modalities in community settings. Curationis 2002;25(1):74‐82. [DOI] [PubMed] [Google Scholar]
Smietanka 1981 {published data only}
- Smietanka MA, Opit LJ. A trial of a transparent adhesive dressing ('Op Site') in the treatment of decubitus ulcer. Australian Nurses' Journal 1981;10(8):40‐2. [PubMed] [Google Scholar]
Souliotis 2016 {published data only}
- Souliotis K, Kalemikerakis I, Saridi M, Papageorgiou M, Kalokerinou A. A cost and clinical effectiveness analysis among moist wound healing dressings versus traditional methods in home care patients with pressure ulcers. Wound Repair and Regeneration 2016;24(3):596‐601. [DOI] [PubMed] [Google Scholar]
Stepan 2014 {published data only}
- Stepan J, Ehrlichova J, Hladikova M. Efficacy and safety of symphytum herb extract cream in the treatment of pressure ulcers. Zeitschrift für Gerontologie und Geriatrie 2014;47(3):228‐35. [DOI] [PubMed] [Google Scholar]
Stephen 2016 {published data only}
- Stephen S, Agnihotri M, Kaur S. A randomized, controlled trial to assess the effect of topical insulin versus normal saline in pressure ulcer healing. Ostomy/Wound Management 2016;62(6):16‐23. [PubMed] [Google Scholar]
Stoker 1990 {published data only}
- Stoker F. A major contributor. Evaluation of Comfeel pressure relieving dressing. Professional Nurse 1990;5(12):644‐53. [PubMed] [Google Scholar]
Strong 1985 {published data only}
- Strong V, Mermel VM. A comparative study of the effectiveness of a nonpetroleum and a petroleum moisturizing agent on healing of stage I and stage II skin lesions. Journal of Enterostomal Therapy 1985;12(6):195‐202. [DOI] [PubMed] [Google Scholar]
Subbanna 2007 {published data only}
- Subbanna PK, Shanti MF, George J, Tharion G, Neelakantan N, Durai S, et al. Topical phenytoin solution for treating pressure ulcers: a prospective, randomized, double‐blind clinical trial. Spinal Cord 2007;45(11):739‐43. [DOI] [PubMed] [Google Scholar]
Takahashi 2006 {published data only}
- Takahashi J, Yokota O, Fujisawa Y, Sasaki K, Ishizu H, Aoki T, et al. An evaluation of polyvinylidene film dressing for treatment of pressure ulcers in older people. Journal of Wound Care 2006;15(10):449‐50, 452‐4. [DOI] [PubMed] [Google Scholar]
Teot 1997 {published data only}
- Teot L. A multi‐centre randomized comparative study of Aquacel versus a traditional dressing regimen for the management of pressure sores. Sixth European Conference on Advances in Wound Management; 1996 October 1‐4; Amsterdam, the Netherlands. 1997:283.
Teot 2008 {published data only}
- Teot L, Trial C, Lavigne JP. Results of RCT on the antimicrobial effectiveness of a new silver alginate wound dressing. EWMA Journal 2008;8(Suppl 2):54. [Abstract 69] [DOI] [PubMed] [Google Scholar]
Tewes 1993 {published data only}
- Tewes M. Prevention and treatment of pressure sores: a neglected research subject? An overview of clinically controlled studies in the period 1987‐91. Vard i Norden 1993;13(2):4‐7. [DOI] [PubMed] [Google Scholar]
Thomas 1993 {published data only}
- Thomas S, Fear M. Comparing two dressings for wound debridement. Journal of Wound Care 1993;2(5):272‐4. [DOI] [PubMed] [Google Scholar]
Thomas 1997b {published data only}
- Thomas S, Banks V, Fear M, Hagelstein S, Bale S, Harding KG. A study to compare two film dressings used as secondary dressings. Journal of Wound Care 1997;6(7):333‐6. [DOI] [PubMed] [Google Scholar]
Toba 1997 {published data only}
- Toba K, Sudoh N, Nagano K, Eto M, Mizuno Y, Nakagawa H, et al. Randomized prospective trial of gentian violet with dibutyryl cAMP and povidone‐iodine with sugar as treatment for pressure sores infected with methicillin‐resistant Staphylococcus aureus in elderly patients. Nippon Ronen Igakkai Zasshi (Japanese Journal of Geriatrics) 1997;34(7):577‐82. [DOI] [PubMed] [Google Scholar]
Tolentino 2011 {published data only}
- Tolentino AC, Takemoto ML, Fernandes RA, Takemoto MM, Santos PM. Cost‐effectiveness analysis of three wound dressings for the treatment of pressure ulcers from the public hospital perspective. Value in Health 2011;14(3):A83. [Abstract PMD22] [Google Scholar]
Toriyabe 2004 {published data only}
- Toriyabe S. A film‐dressing therapy for pressure ulcers at NPUAP stages lll/lV. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:154.
Torra i Bou 1999 {published data only}
- Torra i Bou JE. Randomized, comparative clinical trial on the debriding effect of Purilon gel versus Intrasite gel on pressure ulcers. Ninth European Conference on Advances in Wound Management; 1999 November 9‐11; Harrogate, UK. 1999:23.
Trial 2010 {published data only}
- Trial C, Darbas H, Lavigne JP, Sotto A, Simoneau G, Tillet Y, et al. Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: a RCT. Journal of Wound Care 2010;19(1):20‐6. [DOI] [PubMed] [Google Scholar]
Tricco 2015 {published data only}
- Tricco AC, Antony J, Vafaei A, Khan PA, Harrington A, Cogo E, et al. Seeking effective interventions to treat complex wounds: an overview of systematic reviews. BMC Medicine 2015;13(89):Available from bmcmedicine.biomedcentral.com/articles/10.1186/s12916‐015‐0288‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unglaub 2004 {published data only}
- Unglaub F, Ulrich D, Pallua N. Effect of promogran matrix on healing rate and elastase and plasmin activity in patients with pressure sores. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:55.
Valentini 2015 {published data only}
- Valentini SR, Nogueira AC, Fenelon VC, Sato F, Medina AN, Santana RG, et al. Insulin complexation with hydroxypropyl‐beta‐cyclodextrin: spectroscopic evaluation of molecular inclusion and use of the complex in gel for healing of pressure ulcers. International Journal of Pharmaceutics 2015;490(1‐2):229‐39. [DOI] [PubMed] [Google Scholar]
Van Leen 2004 {published data only}
- Leen MW. A prospective randomized study in recalcitrant pressure sores with poly hydrated ionogens is feasible. Second World Union of Wound Healing Societies Meeting; 2004 July 8‐13; Paris, France. 2004:poster.
Varma 1973 {published data only}
- Varma AO, Bugatch E, German FM. Debridement of dermal ulcers with collagenase. Surgery, Gynecology and Obstetrics 1973;136(2):281‐2. [PubMed] [Google Scholar]
Vernassiere 2005 {published data only}
- Vernassiere C, Cornet C, Trechot P, Alla F, Truchetet F, Cuny JF, et al. Study to determine the efficacy of topical morphine on painful chronic skin ulcers. Journal of Wound Care 2005;14(6):289‐93. [DOI] [PubMed] [Google Scholar]
Wagstaff 2014 {published data only}
- Wagstaff MJ, Driver S, Coghlan P, Greenwood JE. A randomized, controlled trial of negative pressure wound therapy of pressure ulcers via a novel polyurethane foam. Wound Repair and Regeneration 2014;22(2):205‐11. [DOI] [PubMed] [Google Scholar]
Wallace 2009 {published data only}
- Wallace M. Review: evidence on the effectiveness of honey for healing wounds is limited. Evidence‐Based Nursing 2009;12(2):53. [DOI] [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang C, Huang S, Zhu T, Sun X, Zou Y, Wang Y. Efficacy of photodynamic antimicrobial therapy for wound flora and wound healing of pressure sore with pathogen infection. National Medical Journal of China 2014;94(31):2455‐9. [PubMed] [Google Scholar]
Wanner 2003 {published data only}
- Wanner MB, Schwarzl F, Strub B, Zaech GA, Pierer G. Vacuum‐assisted wound closure for cheaper and more comfortable healing of pressure sores: a prospective study. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery 2003;37(1):28‐33. [DOI] [PubMed] [Google Scholar]
Watts 1994 {published data only}
- Watts C, Lee S. Comparison of Allevyn cavity wound dressing to saline‐moistened gauze. Third European Conference on Advances in Wound Management; 1993 October 19‐22; Harrogate, UK. 1994:159.
Waycaster 2011 {published data only}
- Waycaster C. Cost‐effectiveness of collagenase versus hydrogel dressings for chronic‐wound debridement in a long‐term care setting. Value in Health 2011;14(3):A54. [Abstract PSS9] [Google Scholar]
Waycaster 2013 {published data only}
- Waycaster C, Milne C. Economic and clinical benefit of collagenase ointment compared to a hydrogel dressing for pressure ulcer debridement in a long‐term care setting. Wounds 2013;25(6):141‐7. [PubMed] [Google Scholar]
Weheida 1991 {published data only}
- Weheida SM, Nagubib HH, El‐Banna HM, Marzouk S. Comparing the effects of two dressing techniques on healing of low grade pressure ulcers. Journal of the Medical Research Institute 1991;12(2):259‐78. [Google Scholar]
Weststrate 1999 {published data only}
- Weststrate JT, Bruining HA. Use of a hydrocolloid dressing to save nursing time. Eighth European Conference on Advances in Wound Management; 1998 April 26‐28; Madrid, Spain. 1999:57.
Whitney 1999 {published data only}
- Whitney J, Higa L, Savadalena G, Mich M. Effect of radiant heat treatment on pressure ulcer healing: preliminary findings. 31st Annual Wound, Ostomy and Continence Conference; 1999 June 19‐23; Minneapolis (MN). 1999:478.
Whitney 2001 {published data only}
- Whitney JD, Salvadalena G, Higa L, Mich M. Treatment of pressure ulcers with noncontact normothermic wound therapy: healing and warming effects. Journal of Wound, Ostomy, and Continence Nursing 2001;28(5):244‐52. [DOI] [PubMed] [Google Scholar]
Wild 2009 {published data only}
- Wild T, Bruckner M, Payrich M, Schwarz C, Eberlein T. Prospective randomized study for eradication of MRSA with polyhexanid containing cellulose dressing compare with polyhexanid wound solution. old.ewma.org/fileadmin/user_upload/EWMA/pdf/conference_abstracts/2009/Poster/P_145.pdf (accessed 13 March 2017).
Wild 2012 {published data only}
- Wild T, Bruckner M, Payrich M, Schwarz C, Eberlein T, Andriessen A. Eradication of methicillin‐resistant Staphylococcus aureus in pressure ulcers comparing a polyhexanide‐containing cellulose dressing with polyhexanide swabs in a prospective randomized study. Advances in Skin and Wound Care 2012;25(1):17‐22. [DOI] [PubMed] [Google Scholar]
Winter 1990 {published data only}
- Winter A, Hewitt H. Testing a hydrocolloid. Nursing Times 1990;86(50):59‐62. [PubMed] [Google Scholar]
Woo 2009 {published data only}
- Woo KY, Sibbald RG. Topical treatment of pain in pressure ulcers: a systematic review. Journal of Wound, Ostomy, and Continence Nursing 2009;36(3 (Suppl)):S58. [Google Scholar]
Worsley 1991 {published data only}
- Worsley M. Comparing efficacies: hydrocolloid dressing (Comfeel ulcer dressing) vs non‐adherent dressing (Betadine + melonin dressing). Nursing Standard 1991;5(25):5‐6. [Google Scholar]
Yastrub 2004 {published data only}
- Yastrub DJ. Relationship between type of treatment and degree of wound healing among institutionalized geriatric patients with stage II pressure ulcers. Care Management Journals 2004;5(4):213‐8. [DOI] [PubMed] [Google Scholar]
Yastrub 2005 {published data only}
- Yastrub DJ. Relationship between type of treatment and degree of wound healing among institutionalized geriatric patients with stage II pressure ulcers. Clinical Excellence for Nurse Practitioners 2005;9(2):89‐94. [DOI] [PubMed] [Google Scholar]
Young 1973 {published data only}
- Young CG, Oden PW. Treatment of decubitus ulcers in paraplegic patients: a comparison of three topical agents: absorbable [sic] gelatin sponge, gelatin powder, and enzyme ointment. Southern Medical Journal 1973;66(12):1375‐8. [PubMed] [Google Scholar]
Young 1997 {published data only}
- Young T, Williams C, Benboz M, Collier M, Banks V, Jones H. A study of two hydrogels used in the management of pressure sores. Sixth European Conference on Advances in Wound Management; 1996 October 1‐4; Amsterdam, the Netherlands. 1997:103‐6.
Yura 1984 {published data only}
- Yura J, Ando M, Ishikawa S, Ohashi M, Ishigaki M, Ueda H, et al. Clinical evaluation of silver sulfadiazine in the treatment of decubitus ulcer or chronic dermal ulcers: double‐blind study comparing to placebo. Chemotherapy 1984;32(4):208‐22. [Google Scholar]
Zhou 2001 {published data only}
- Zhou DP, Lu LQ, Mao XL. Insulin and hyperosmotic glucose solution external used for treating pressure sore. Hunan Yi Ke Da Xue Xue Bao (Bulletin of Hunan Medical University) 2001;26(5):475‐6. [PubMed] [Google Scholar]
Zuloff‐Shani 2010 {published data only}
- Zuloff‐Shani A, Adunsky A, Even‐Zahav A, Semo H, Orenstein A, Tamir J, et al. Hard to heal pressure ulcers (stage III‐IV): efficacy of injected activated macrophage suspension (AMS) as compared with standard of care (SOC) treatment controlled trial. Archives of Gerontology and Geriatrics 2010;51(3):268‐72. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐TRC‐13003959 {published data only}
- ChiCTR‐TRC‐13003959. Moxibustion for the promotion of wound healing of pressure ulcers: randomized controlled pilot study. www.chictr.org.cn/showprojen.aspx?proj=5608 (first received 7 December 2013).
ISRCTN57842461 {published data only}
- ISRCTN57842461. To compare the polyurethane foam dressing (hydrocellular) and the hydrocolloid dressing in patients with pressure ulcers (stage II) in primary care. www.isrctn.com/ISRCTN57842461 (first received 19 June 2012).
Additional references
AHRQ 2013
- Saha S, Smith ME, Totten A, Fu R, Wasson N, Rahman B, et al. US Agency for Healthcare Research and Quality (AHRQ). Pressure ulcer treatment strategies: comparative effectiveness review 90. May 2013. effectivehealthcare.ahrq.gov/ehc/products/308/1492/Pressure‐ulcer‐treatment‐executive‐130508.pdf (accessed 21 December 2016). [PubMed]
Allman 1999
- Allman RM, Goode PS, Burst N, Bartolucci AA, Thomas DR. Pressure ulcers, hospital complications, and disease severity: impact on hospital costs and length of stay. Advances in Wound Care 1999;12:22‐30. [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64:401‐6. [DOI] [PubMed] [Google Scholar]
Bennett 2004
- Bennett G, Dealey C, Posnett J. The cost of pressure ulcers in the UK. Age and Ageing 2004;33(3):230‐5. [DOI] [PubMed] [Google Scholar]
BNF 2016
- British Medical Association, British Royal Pharmaceutical Society of Great Britain. British National Formulary (BNF). www.medicinescomplete.com/mc/bnf/current/ (accessed 21 December 2016).
Bradley 1999
- Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D. Systematic reviews of wound care management: (2) dressings and topical agents used in the healing of chronic wounds. Health Technology Assessment 1999;3(17 (part 2)):1‐35. [PubMed] [Google Scholar]
Brandeis 1994
- Brandeis GH, Ooi WL, Hossain M, Morris JN, Lipsitz LA. A longitudinal study of risk factors associated with the formation of pressure ulcers in nursing homes. Journal of the American Geriatric Society 1994;42:388‐93. [DOI] [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades A, Higgins J. Simultaneous comparison of multiple treatments: combining direct and indirect evidence as well. BMJ 2005;331(7521):897‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caldwell 2014
- Caldwell DM. An overview of conducting systematic reviews with network meta‐analysis. Systematic Reviews 2014;3:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardinal 2009
- Cardinal M, Eisenbud DE, Armstrong DG, Zelen C, Driver V, Attinger C, et al. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair and Regeneration 2009;17(3):306‐11. [DOI] [PubMed] [Google Scholar]
Chaimani 2013a
- Chaimani A, Salanti G. Background document: stream 2 (statistical issues in NMA). methods.cochrane.org/sites/methods.cochrane.org.cmi/files/public/uploads/CMIMG%20Stream%202%20document%202013%2010%2001.pdf (accessed 19 December 2016).
Chaimani 2013b
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PloS One 2013;8(10):e76654. [DOI: 10.1371/journal.pone.0076654] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2015
- Chaimani A, Salanti G. Visualizing assumptions and results in network meta‐analysis: the network graphs package. Stata Journal 2015;15(4):905‐50. [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta‐analysis. Annals of Internal Medicine 2013;159:130‐7. [DOI] [PubMed] [Google Scholar]
Coleman 2013
- Coleman S, Gorecki C, Nelson EA, Closs SJ, Defloor T Halfens R. Patient risk factors for pressure ulcer development: Systematic review. International Journal of Nursing Studies 2013;50:974–1003. [DOI] [PubMed] [Google Scholar]
Cullum 2016
- Cullum N, Buckley H, Dumville J, Hall J, Lamb K, Madden M, et al. Wounds research for patient benefit: a 5‐year programme of research. Programme Grants for Applied Research 2016;4:1‐299. [PubMed] [Google Scholar]
Dealey 2012
- Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. Journal of Wound Care 2012;21:261‐2, 264, 266. [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. [DOI] [PubMed] [Google Scholar]
Dias 2013
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Medical Decision Making 2013;33:641–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2016
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta‐analysis of randomised controlled trials. Last updated September 2016. www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%202Sep2016v2.pdf (accessed 21 December 2016). [PubMed]
Dumville 2012
- Dumville JC, Soares MO, O'Meara S, Cullum N. Systematic review and mixed treatment comparison: dressings to heal diabetic foot ulcers. Diabetologia 2012;55:1902‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumville 2015a
- Dumville JC, Stubbs N, Keogh SJ, Walker RM, Liu Z. Hydrogel dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011226] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumville 2015b
- Dumville JC, Keogh SJ, Liu Z, Stubbs N, Walker RM, Fortnam M. Alginate dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011277] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPUAP‐NPUAP‐PPPIA 2014
- European Pressure Ulcer Advisory Panel (EPUAP) National Pressure Ulcer Advisory Panel (NPUAP), Pan Pacific Pressure Injury Alliance (PPPIA). Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. October 2014. www.npuap.org/wp‐content/uploads/2014/08/Updated‐10‐16‐14‐Quick‐Reference‐Guide‐DIGITAL‐NPUAP‐EPUAP‐PPPIA‐16Oct2014.pdf (accessed 21 December 2016).
Essex 2009
- Essex HN, Clark M, Sims J, Warriner A, Cullum N. Health‐related quality of life in hospital inpatients with pressure ulceration: assessment using generic health‐related quality of life measures. Wound Repair and Regeneration 2009;17:797‐805. [DOI] [PubMed] [Google Scholar]
Gasparrini 2015
- Gasparrini 2015. Multivariate and univariate meta‐analysis and meta‐regression. cran.r‐project.org/web/packages/mvmeta/mvmeta.pdf (accessed 15 December 2016).
Gillespie 2012
- Gillespie BM, Chaboyer W, Niuewenhoven P, Rickard CM. Drivers and barriers of surgical wound management in a large healthcare organisation: results of an environmental scan. Wound Practice and Research 2012;20(2):90‐102. [Google Scholar]
Grant 2013
- Grant ES, Calderbank‐Batista T. Network meta‐analysis for complex social interventions: problems and potential. Journal of the Society for Social Work and Research 2013;4(4):406‐20. [Google Scholar]
Graves 2005
- Graves N, Birrell F, Whitby M. Modelling the economic losses from pressure ulcers among hospitalized patients in Australia. Wound Repair and Regeneration 2005;13:462‐7. [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64:407‐15. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines: 6. rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Hall 2014
- Hall J, Buckley HL, Lamb KA, Stubbs N, Saramago P, Dumville JC, et al. Point prevalence of complex wounds in a defined United Kingdom population. Wound Repair and Regeneration 2014;22(6):694‐700. [DOI: 10.1111/wrr.12230] [DOI] [PubMed] [Google Scholar]
Higgins 1996
- Higgins JP, Whitehead A. Borrowing strength from external trials in a meta‐analysis. Statistics in Medicine 1996;15:2733–49. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3:98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutchinson 1991
- Hutchinson JJ, Lawrence JC. Wound infections under occlusive dressings. Journal of Hospital Infection 1991;17(2):83‐94. [DOI] [PubMed] [Google Scholar]
Jansen 2013
- Jansen JP, Naci H. Is network meta‐analysis as valid as standard pairwise meta‐analysis? It all depends on the distribution of effect modifiers. BMC Medicine 2013;11:159‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2015
- Kelly J, Clifton E, Fearns N, Harbour J, Heller‐Murphy S, Herbert P, et al. Antimicrobial wound dressings (AWDs) for chronic wounds. Healthcare Improvement Scotland. Health Technology Assessment. December 2015. www.healthcareimprovementscotland.org/our_work/technologies_and_medicines/shtg_‐_hta/hta13_antimicrobial_dressings.aspx (accessed 21 December 2016).
Keogh 2013
- Keogh SJ, Nelson EA, Webster J, Jolly J, Ullman AJ, Chaboyer WP. Hydrocolloid dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010364] [DOI] [Google Scholar]
Kibret 2014
- Kibret T, Richer D, Beyene J. Bias in identification of the best treatment in a Bayesian network meta‐analysis for binary outcome: a simulation study. Clinical Epidemiology 2014;6:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krahn 2013
- Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta‐analyses. BMC Medical Research Methodology 2013;13:35‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2004
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 2004;23(20):3105–24. [DOI] [PubMed] [Google Scholar]
Lunn 2000
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS ‐ a Bayesian modelling framework: concepts, structure, and extensibility. Statistics and Computing 2000;10:325‐37. [Google Scholar]
Lunn 2009
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Statistics in Medicine 2009;28:3049–67. [DOI] [PubMed] [Google Scholar]
McInnes 2011
- McInnes E, Dumville JC, Jammali‐Blasi A, Bell‐Syer SE. Support surfaces for treating pressure ulcers. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009490] [DOI] [PubMed] [Google Scholar]
NHS Quality Observatory 2015
- NHS Quality Observatory. National Safety Thermometer Data 2015. www.safetythermometer.nhs.uk/index.php?option=com_dashboards&view=classic&Itemid=126 (accessed 22 January 2015).
NICE 2014
- National Institute of Health and Care Excellence (NICE). Pressure ulcers: prevention and management. Clinical guideline [CG179]. April 2014. www.nice.org.uk/guidance/cg179 (accessed 1 April 2015).
NPUAP 2016
- National Pressure Ulcer Advisory Panel (NPUAP). Change in terminology from pressure ulcer to pressure injury. April 2016. www.npuap.org/national‐pressure‐ulcer‐advisory‐panel‐npuap‐announces‐a‐change‐in‐terminology‐from‐pressure‐ulcer‐to‐pressure‐injury‐and‐updates‐the‐stages‐of‐pressure‐injury/ (accessed 18 May 2017).
Peters 2008
- Peters J, Sutton A, Jones D, Abrams K, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991‐6. [DOI] [PubMed] [Google Scholar]
Power 2012
- Power M, Stewart K, Brotherton A. What is the NHS Safety Thermometer?. Clinical Risk 2012;18:163‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddy 2008
- Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA 2008;300:2647‐62. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russo 2008
- Russo A, Steiner C, Spector W, US Healthcare Cost and Utilization Project. Hospitalizations related to pressure ulcers among adults 18 years and older, 2006. www.hcup‐us.ahrq.gov/reports/statbriefs/sb64.jsp (accessed 21 December 2016). [PubMed]
Salanti 2008
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17:279–301. [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades A, Ioannidis J. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64:163‐71. [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta‐analysis. PLoS One 2014;9:e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saramago 2014
- Saramago P, Chuang L‐H, Soares MO. Network meta‐analysis of (individual patient) time to event data alongside (aggregate) count data. BMC Medical Research Methodology 2014;14:105. [DOI: 10.1186/1471-2288-14-105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
SIGN 2017
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html (accessed 13 June 2017).
Smith 2013
- Smith ME, Totten A, Hickam DH, Fu R, Wasson N, Rahman B, et al. Pressure ulcer treatment strategies: a systematic comparative effectiveness review. Annals of Internal Medicine 2013;159:39‐50. [DOI] [PubMed] [Google Scholar]
Soares 2014
- Soares MO, Dumville JC, Ades AE, Welton NJ. Treatment comparisons for decision making: facing the problems of sparse and few data. Journal of the Royal Statistical Society Series A 2014;177(1):259–79. [Google Scholar]
Spiegelhalter 2003
- Spiegelhalter D, Abrams K, Myles J. Bayesian Approaches to Clinical Trials and Health‐care Evaluation. Chichester: Wiley, 2003. [Google Scholar]
STATA 2013 [Computer program]
- StataCorp LLC. Stata data analysis and statistical software. Version 13. College Station, TX: StataCorp LLC, 2013.
Thorlund 2012
- Thorlund K, Mills EJ. Sample size and power considerations in network meta‐analysis. Systematic Reviews 2012;1:41‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;14:16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tu 2012
- Tu Y‐K, Faggion CM. A primer on network meta‐analysis for dental research. International Scholarly Research Notices Dentistry 2012, issue Article ID 276520:available from www.hindawi.com/journals/isrn/2012/276520/. [DOI: 10.5402/2012/276520] [DOI] [PMC free article] [PubMed]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanderwee 2007
- Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. Journal of Evaluation in Clinical Practice 2007;13(2):227‐35. [DOI] [PubMed] [Google Scholar]
VanGilder 2009
- VanGilder C, Amlung S, Harrison P, Meyer S. Results of the 2008‐2009 International Pressure Ulcer Prevalence Survey and a 3‐year, acute care, unit‐specific analysis. Ostomy Wound Management 2009;55(11):39‐45. [PubMed] [Google Scholar]
Veroniki 2013
- Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. International Journal of Epidemiology 2013;42:332‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2014
- Walker RM, Keogh SJ, Higgins NS, Whitty JA, Thalib L, Gillespie BM, et al. Foam dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD011332] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3:111‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
WinBUGS 2015 [Computer program]
- Medical Research Council Biostatistics Unit. WinBUGS. Version 1.4.3. Cambridge, UK: Medical Research Council Biostatistics Unit, 2015.
Winter 1962
- Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293‐4. [DOI] [PubMed] [Google Scholar]
Winter 1963a
- Winter GD, Scales JT. Effect of air drying and dressings on the surface of a wound. Nature 1963;197:91‐2. [DOI] [PubMed] [Google Scholar]
Winter 1963b
- Winter GD. Effect of air exposure and occlusion on experimental human skin wounds. Nature 1963;200:378‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Westby 2015
- Westby MJ, Dumville JC, Soares MO, Stubbs N, Norman G, Foley CN. Dressings and topical agents for treating pressure ulcers. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011947] [DOI] [PMC free article] [PubMed] [Google Scholar]


