Abstract
Introduction:
Digital microfluidics (DMF) is an emerging technology with the appropriate metrics for application to newborn and high-risk screening for inherited metabolic disease and other conditions that present in the newborn.
Areas covered:
This review traces the development of electrowetting-based DMF technology over the past ten years toward the fulfillment of its promise to provide an inexpensive platform to conduct enzymatic assays and targeted biomarker assays at the bedside. The high-throughput DMF platform, referred to as SEEKER®, was recently authorized by the United States Food and Drug Administration (FDA) to screen newborns for four lysosomal storage disorders (LSDs) and is deployed in newborn screening programs in the United States. The development of reagents and methods to screen for LSDs and results from screening centers are reviewed. Preliminary results from a more compact DMF device, to perform disease-specific test panels applicable in the neonatal intensive care unit from small volumes of blood, are also reviewed. Literature for this review was sourced using principal author and subject searches in PubMed.
Expert commentary:
Newborn screening is a vital and highly successful public health program. DMF technology adds value to the current testing platforms that will benefit apparently healthy newborns with underlying genetic disorders and infants at-risk for conditions that present with symptoms in the newborn period.
Keywords: digital microfluidics, lysosomal storage disorders, newborn screening, dried blood spots, near patient testing, hyperbilirubinemia, hypoglycemia
1. Introduction
The theory of digital microfluidics and its applications have been reviewed [1]. The principles and development of electrowetting-based digital microfluidic devices to provide a practical and effective platform upon which to base a wide variety of clinically useful tests was reviewed by Pollack, et al. several years ago [2]. This review article described the “lab-on-a-chip” system, conceived of and developed by Advanced Liquid Logic, Inc., that permitted the manipulation of micro-droplets on a disposable printed circuit board (PCB) with built-in reservoirs for samples, reagents, and waste by software control, such that all the steps required for an enzymatic assay, for example, were performed programmatically. The versatility of this platform to perform assays of interest to the newborn screening (NBS) community was explored at about this time [3]. There was considerable interest in expanding the range of NBS testing in dried blood spots (DBS) to include certain lysosomal storage disorders (LSDs) that were considered treatable [4,5]. Screening for LSDs was initially suggested by Chamoles, et al., who developed several fluorogenic enzymatic assays to test DBS samples in 96-well microtiter plates using a benchtop microfluorometer [6,7,8]. Chamoles used existing fluorogenic substrates that were already in use in diagnostic laboratories and translated those to the microtiter plate format. Subsequently, tandem mass spectrometry (MS/MS) methods for LSD enzyme measurement that used non-fluorometric synthetic substrates were developed [9,10,11]. The initial translation of fluorometric enzyme assays for selected LSDs onto the DMF platform [12] took advantage of the fact that up to eight assays on each of twelve samples could potentially be performed within a single run on the same platform. A prototype instrument was developed and used in a pilot NBS program in Illinois to prospectively screen for three LSDs [13]. However, it was the development of a cartridge capable of performing at least 5 assays on up to 48 samples that launched high-throughput NBS for LSDs using the DMF platform [14]. The state of Missouri became the first NBS program in the United States to prospectively screen all newborns for four LSDs using the SEEKER DMF platform with novel reagents supplied by the manufacturer [15]. The fact that it is still doing so with no significant problems, changes or improvements testifies to the robustness of the platform. The acquisition of Advanced Liquid Logic by Illumina [16] resulted in a hiatus of approximately two years in further clinical DMF assay development until a new company, Baebies Inc., was founded in late 2014. In February 2017, SEEKER became the first and currently remains the only FDA authorized platform to screen newborns for LSDs [17]. It was also the first DMF device to be cleared by the FDA for clinical applications. Future development of this technology is expected to expand the menu of available assays for high-throughput NBS. The technology is also being miniaturized to support rapid, comprehensive diagnostic testing of critical neonatal conditions such as hyperbilirubinemia, hypothyroidism, hypoglycemia, and hypercoagulation near the patient using small sample volumes.
2. Principles of Electrowetting Digital Microfluidic Technology
2.1. System Overview
Digital microfluidic platforms may be operated either on an open plate or on a two-plate “sandwich” format [18]. Figure 1 outlines the principles of electrowetting-based DMF as it applies to the two-plate format. Essentially, a cartridge consisting of a PCB with discrete electrodes is coated with a dielectric and hydrophobic material. The top plate is made from a clear plastic with both a conductive and hydrophobic coating on the inner surface. These two plates form a sandwich that is filled with an oil to prevent evaporation of the aqueous droplets. The droplets are manipulated on the PCB surface by applying voltage to the electrodes. The droplet is attracted to an activated electrode by the principle of electrowetting, and is moved to an adjacent electrode by applying a voltage to it while simultaneously switching off the voltage on the one previously activated [19]. In this manner, droplets can be moved along pathways defined by the pattern of electrodes on the PCB using electrical impulses controlled by a computer program [1,2]. The droplet volume is determined by the area of the electrode and is typically hundreds of nanoliters. It is possible to merge and split droplets with perfect control. For example, a “sample” droplet can be merged with a “reagent” droplet to perform a chemical or biochemical reaction, then with a third droplet to quench the reaction after a defined time. The mixed droplet is then moved to a fixed location where a detector is placed to measure a fluorescent or absorbance signal from the reaction product, and finally the droplet is disposed into a waste reservoir. The design of the “flow” in spatially multiplexed assays takes this into consideration in order to minimize carryover, which can be analytically quantified and characterized if necessary. Therefore, multiple droplets can traverse the same electrodes while cross-contamination is minimized without impacting results and clinical interpretation [2].
Figure 1.
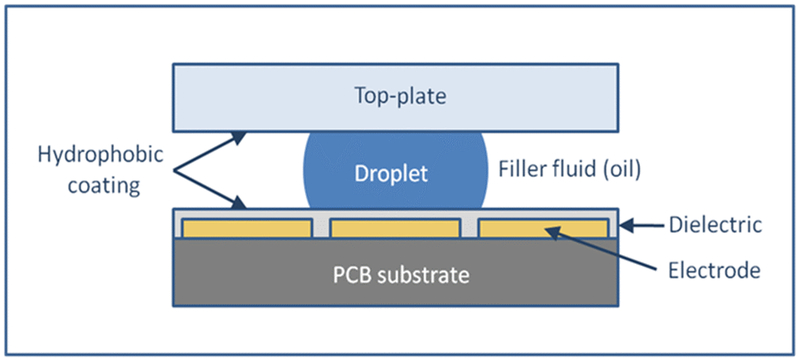
Principles of electrowetting-based digital microfluidics. Electrodes on a printed circuit board surface are turned ON and OFF to manipulate the droplets within an open or oil-filled chamber.
Supplemental link to video: https://www.baebies.com/electrowetting-droplet-operations-on-a-digital-microfluidic-cartridge/
2.2. Other DMF Formats
Electrowetting based DMF has also been applied to transport droplets on three-dimensional (3D) structures with various curvatures and vertical or twisted configurations [20, 21, 22]. In one 3D DMF iteration, electrodes are embedded in both the top and bottom plate of the cartridge [23]. Similar to the 2D DMF cartridge shown in Figure 1, the 3D DMF cartridge is also filled with oil and horizontal droplet transport operations are used to facilitate droplet transport, mixing and merging. Vertical droplet transport is achieved by applying a square pulse signal to electrodes on the top plate; removing voltage from the bottom plate subsequently enables the hanging droplet to move from the bottom to the top electrode. Potential application of this technology for cell-based assays and drug discovery has been proposed, but not yet demonstrated [23].
Several digital microfluidics systems not based on electrowetting have also been described, including dielectrophoresis, magnetic actuation, acoustic, surface acoustic, and optical liquid actuation methods. An in-depth description of these diverse DMF systems is outside the scope of this review, but can be found elsewhere [24, 25].
3. Benefits of Digital Microfluidics
There are many benefits to the DMF platform concept. First, the overall simplicity of the device starkly contrasts with conventional benchtop equipment or with other microfluidic systems that require mechanical pumps or valves, fixed channels or tubing. In practice, each cartridge is loaded with filler oil, reagents and samples – a process that takes a few minutes at most - then placed into a machine that houses the electronic and detector components. Activating a computer program starts the assay sequence that includes the functional fluidic steps required - setting up of incubation temperatures, detection of the reaction product, generation and reporting of the results - all of which are completed entirely without operator intervention [2].
Second, the volumes of sample and reagent solutions are miniscule compared with those required by typical benchtop assay methods. This is particularly advantageous in situations where sample quantity is limited, as is usually the case with newborns, where ten or more tests, each of which requires a separate DBS punch, are often performed. The DMF platform has the potential to perform hundreds of discrete assays from the extract of a single DBS punch, which contains just 3.2 μl of whole blood.
Third, the ability to perform enzymatic reactions within discrete droplets permits optimization of the reaction conditions in terms of pH, buffer and reagent concentration. This is not practical using other methods, including MS/MS, where compromises must be made to optimize sample flow. In the case of LSD enzymatic assays, for example, the concentration of enzyme substrate within the droplet usually exceeds the Km even in cases where the Km is high, which is difficult to achieve with conventional benchtop assays.
A fourth advantage of the DMF assay platform is the greatly reduced time to generate results. Mixing can be performed rapidly by transporting and shaking droplets back and forth [29] and the small thermal mass of these droplets allows rapid heating and cooling [30]. Enzymatic assays can generate a result in typically less than one hour because the reaction rates within a droplet are high whereas conventional benchtop assays can require much longer incubation times [5–7]. DMF has been shown to routinely generate 48 results for each of five different LSD enzymatic assays performed on a single cartridge within 3.5 h, including time to load the cartridge with samples and reagents [14]. Near patient tests obviously dramatically reduce time to results by performing the test at the patient’s location, but DMF can also take advantage of cartridges with pre-loaded reagents that require only the addition of a single drop of the physiological fluid, whether it is blood, saliva or urine, and generate results within minutes of sample loading.
A fifth advantage of the DMF platform is scalability. The sample volume in NBS programs can be over 1,000 per day. Coping with such high sample volumes can be achieved by use of multiple workstations. For example, the Missouri NBS program uses two SEEKER workstations, each with four instruments controlled by one computer and occupying less than four linear feet of bench space per workstation. Alternative methods to scale up might use stacking of cartridges within a single instrument, or increasing the number of sample inputs on the cartridge. Point-of-care test cartridges can expand scale by performing multiple tests of varying types (enzymatic, molecular, immunologic) on the same cartridge using a single sample.
In addition to the aforementioned convenience factors, DMF is highly cost-effective compared with competing benchtop methods and lab-on-chip systems, largely owing to the use of disposable cartridges, low capital instrument cost with virtually no maintenance requirements, low power consumption and low reagent use and disposal costs. In the case of LSD enzyme assays, the competitive platform for high-throughput assays is a tandem mass spectrometer, for which the capital investment and ongoing maintenance costs are far in excess of those required for DMF [31].
4. Limitations of Digital Microfluidics
While digital microfluidics is compatible with several common biochemical assay formats (enzyme activity assays, immunoassays, nucleic acid amplification, etc.), current iterations of the technology are not capable of performing high throughput metabolite analysis such as amino acid and acylcarnitine profiling. Such metabolite profiling is an essential component of newborn screening and is currently performed using mass spectrometry. The successful application of DMF platforms to prepare samples for analysis by mass spectrometry in the fields of synthetic chemistry, peptidomics, proteomics, cell biology and biochemistry has been reviewed [18]. In most examples, the analysis by MS is performed off-line to the DMF platform. Although some efforts have been made to directly couple DMF with nano ESI MS, including analytes of interest for newborn screening and biochemical genetics, the realization of a robust commercially available device is not imminent [18]. In the DMF platform described in this report, the main challenge would be the selective sampling of a droplet while avoiding contamination from the filler oil.
5. Development of Assays for Newborn Screening
5.1. Background
The historical development of screening newborns for inherited metabolic disorders (IMD) has been previously reviewed [32,33]. Briefly, NBS has evolved from a simple bacterial inhibition assay for phenylketonuria (PKU), introduced in 1963, to testing for a few additional conditions such as galactosemia and congenital hypothyroidism added over the next 35 years or so. The introduction of the first multiplex test in the 1990s, using MS/MS to identify multiple biomarkers and thus screen for an additional 30 or more IMDs with a single test [34,35], led to a marked increase in interest in newborn screening as a means of identifying infants at risk for IMDs and treating them prior to the onset of symptoms that could otherwise be devastating [36].
NBS for lysosomal storage disorders (LSDs), a group of approximately 40 IMDs that were not previously accessible to NBS, gained momentum in the early 2000s with the development of fluorometric enzymatic assays including alpha-iduronidase, alpha-galactosidase and acid alpha-glucosidase, the enzymes deficient in Hurler Syndrome (MPS I), Fabry Disease and Pompe Disease (GSD-II), respectively [6–8]. The development of enzyme replacement therapies for these and other LSDs generated even more interest in NBS to find patients that could most benefit from early treatment [5]. Accordingly, NBS for Pompe disease started in Taiwan, which has a relatively high incidence of this LSD compared with other ethnic groups, using an adaptation of the benchtop microfluorometric assay [37]. Results showed convincingly that NBS for LSDs was viable, and outcomes for cases of infantile Pompe disease were much better when treatment was provided soon after birth [38,39]. In the meantime, a method to screen for galactosylceramidase (GALC), the enzyme deficient in Krabbe disease, using MS/MS with a non-fluorogenic synthetic substrate, was introduced [9]. Cases of early onset Krabbe disease reportedly were treated successfully by bone marrow transplantation [40] and the family of one such case subsequently lobbied successfully to introduce NBS for Krabbe in New York State.
A somewhat frenzied period of both further assay development by tandem mass spectrometry, including claims of multiplexing LSD enzyme tests [10,41], and the introduction of treatment options for more LSDs ensued [42]. Confronted with these new challenges, most state NBS programs referred to a review body, the Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC) for guidance. The SACHDNC recommends that every newborn screening program include a Uniform Screening Panel (RUSP) of at least 31 core disorders and 26 secondary disorders (as of February 2014) [43]. This Committee, charged with the responsibility of adding new conditions to the RUSP based on evidence review, initially rejected applications for LSDs but later approved Pompe (2015) and Hurler (2016) for inclusion to the RUSP. As of November 2017 there were 34 core disorders on the RUSP [43].
In spite of this consensus system, several states have been successfully lobbied by special interest groups to screen for as many as five other conditions, mostly LSDs not on the RUSP, including the neighboring states of Illinois (IL) and Missouri (MO) [4,5]. By 2013, there were two competing systems in development for multiple LSD screening – tandem mass spectrometry using novel synthetic substrates [10,41] and DMF using new fluorogenic substrates [14]. In 2013, MO began prospectively screening newborns for four LSDs (Fabry, Pompe, Hurler and Gaucher diseases) using DMF and was the first state program to report results for these four conditions [15]. The development of the DMF platform for this pilot study, plus more recent developments to expand the repertoire of NBS tests, are detailed below.
5.2. Development of enzymatic assays
In principle, enzymatic assays are the most straightforward type of assay to perform on the DMF platform because all that is required is to mix sample with reagent, stop the reaction after a defined time and then detect the product. An enzymatic DMF method for blood glucose was reported in 2004 [44] and the development of LSD enzymatic assays using fluorogenic reagents began in 2010 and proceeded as a collaborative venture between Advanced Liquid Logic, Inc. (now Baebies, Inc.) with the State of North Carolina Public Health Laboratory and the Biochemical Genetics laboratory at Duke University, affiliated with the Pediatric Medical Genetics Division (currently with the Duke Health System Clinical Laboratory) with initial funding by NICHD. In order to show proof-of-principle for multiplex LSD enzyme screening on a DMF platform [3], the cartridge shown in Figure 2 (left panel) was employed. Fluorogenic substrates for acid alpha-glucosidase (GAA), alpha-galactosidase (GLA), alpha-L-iduronidase (IDUA) and beta-glucuronidase (GUSB), for Pompe, Fabry and Hurler diseases and MPS VII deficiency, respectively, were exposed to droplets of the extract from a single 3-mm punch from a DBS. The fluorescence values for each reaction droplet were plotted against incubation time and suggested that <6 h incubation time would still be sufficient to produce enough signal from the reaction products.
Figure 2.
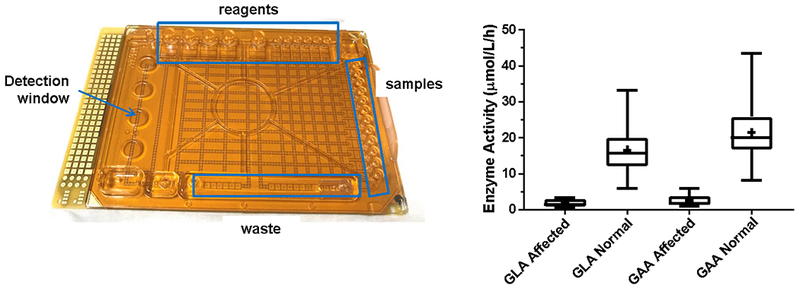
Left panel: experimental DMF cartridge for performing enzymatic assays by fluorometry. There are 12 sample inputs (right edge), 8 reagent inputs (top and bottom edge) plus additional reservoirs for stop buffer and waste. Individual droplets are dispensed, mixed and manipulated entirely by software. Right panel: results of enzymatic assays for the enzymes deficient in Fabry (GLA) disease and Pompe (GAA) disease by DMF using a 1 h incubation time. Results for known affected patients (GLA: n=6; GAA: n=7) are compared with presumed unaffected controls (n=105) and show no overlap. Data modified and reprinted with permission from the American Association of Clinical Chemistry [12].
5.3. DMF Assays for the Pompe and Fabry enzymes (GAA and GLA)
From this first joint project, the three collaborating institutions compared bench-based, fluorometric enzymatic analysis to DMF fluorometric assays. The comparison was performed on 60 de-identified newborn DBS samples, 10 confirmed Pompe affected DBS and 11 Fabry affected DBS samples. Each bench assay used two 3-mm DBS punches and an incubation time of 20 h, while the DMF assay used a single punch for both assays and an incubation time of 6 h. Repeating the DMF assays using a more sensitive detector enabled a reduction in the incubation time to 1 h [12]. Assay results were generally comparable, although mean enzymatic activity for GAA using microfluidics was approximately 3 times higher than that obtained using bench-based methods, attributable to higher substrate concentration. Clear separation was observed between the normal and affected samples at both 6 h and 1 h incubation times using DMF. The results from the 1 h incubation study are shown in Figure 2 (right panel), from which it was concluded that 1 h would become the standard incubation time for DMF LSD enzyme assays.
This study also determined that there was no discernible effect from hemoglobin on the fluorescence values determined by DMF under these conditions, which was previously reported to exhibit an optical quenching effect on fluorescence in this type of assay [45]. This important advantage of DMF was ascribed to the much-reduced path length of a droplet (<0.3 mm) compared with the column of liquid in a microplate.
5.4. DMF assay for Hunter Syndrome (Iduronate-2-sulfatase, or IDS deficiency)
Hunter Syndrome is one of the LSDs that has been treated successfully in early infancy [46] and is being considered as a potential target for NBS. The use of 4-methylumbelliferyl-α-L-iduronide-2-sulfate as a fluorescence substrate to measure the activity of IDS requires the sequential action of a second enzyme, α-L-iduronidase, to convert the product of the sulfatase, 4-methylumbelliferyl-α-L-iduronic acid, into iduronic acid and the fluorescent end-product, 4-methylumbelliferone (4-MU). Previous fluorometric assays for IDS used partially purified iduronidase from rabbit liver or bovine testis and sequentially performed the two reactions over a 24 h incubation period to accommodate the difference in pH optima for the 2 enzymes [47]. An improved homogeneous assay for IDS was accomplished on the DMF platform by combining both the enzymes in a single reaction mix using sulfatase (from the DBS sample) and pure, recombinant iduronidase with a 1 h incubation time [48]. This novel assay was able to completely differentiate DBS samples from known affected patients with Hunter (n=6) from unaffected controls (n=105), and represented an important step towards the validation of DMF assays for LSDs [49].
5.5. DMF Assays for Gaucher (GBA) and Hurler Syndrome (IDUA)
A further development in DMF assays for LSDs was the translation of fluorometric methods for glucocerebrosidase (GBA) and alpha-iduronidase (IDUA) – the enzymes deficient in Gaucher disease and Hurler syndrome, respectively – from the benchtop to the DMF platform. The comparison of methods was applied to 10 confirmed cases of Gaucher, 7 Hurler, and 100 newborn healthy controls [50]. The assays for IDUA were comparable and clearly separated the affected samples from controls, whereas the DMF assay for GBA was superior to the benchtop method. As with the other LSD assays described previously, the DMF assays were performed simultaneously from a single DBS extract with an incubation time of 1 h. The next phase of DMF development was to develop a LSD assay method suitable for prospective NBS for up to 5 LSDs.
5.6. Multiple LSD Assay Development for NBS on the DMF Platform
The foregoing LSD methods were developed on the platform shown in Figure 2 – a prototype cartridge capable of performing up to eight assays on just twelve DBS sample extracts. In spite of this limitation, this prototype was utilized to screen for three LSD enzymes, corresponding to Gaucher, Fabry and Pompe diseases, in a pilot program conducted in the state of Illinois [13]. From a total of 8,012 DBS samples screened, seven cases of Fabry disease and two cases of Gaucher disease were confirmed. In order to achieve the sample throughput required by NBS laboratories, a disposable, single use DMF cartridge was developed with 48 input reservoirs for DBS extracts, 4-MU calibrants, extraction buffer and quality control (QC) spot extracts, 5 input reservoirs for enzymatic substrates in assay buffers, 5 input reservoirs for stop buffers, and one output reservoir (waste reservoir) to collect all of the droplets after incubation and detection (Fig. 3, top left panel).
Figure 3.
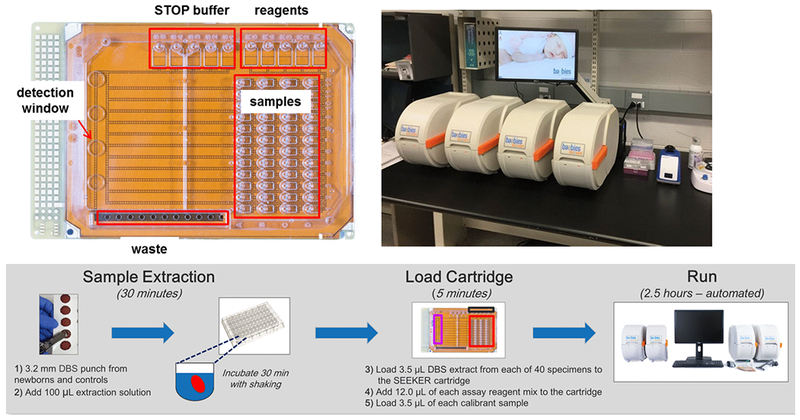
High-throughput DMF cartridge (upper left) capable of analyzing 48 samples for up to 5 enzyme assays with a SEEKER workstation (upper right) comprised of 4 analyzer units connected to a PC. Sample workflow , summarized in the lower panel, shows times required for each stage after sample punching. Two sample batches are easily completed on each workstation by one FTE within a standard 8 h shift. Workstation photo provided with permission from Eleanor Stanley, Michigan Department of Health and Human Services.
The scale conforms to that of a standard 96-well microtiter plate to facilitate the transfer of DBS extracts from standard 96-well plates using a multi-channel pipet. The residual enzymes are first extracted from a DBS in 96-well plates using aqueous medium (100 μL) from one 3 mm DBS punch per sample. A volume of 3.5 μL of the extract from each sample is loaded onto the cartridge, and 12 μL of each reagent is loaded in the appropriate reagent wells. The total time to load the filler oil between the top and bottom plates, all the samples, calibrators and reagents is less than 5 minutes.
Using this new system, assays for five LSD enzymes (indicative of Pompe, Gaucher, Hunter, Hurler and Fabry diseases) were performed on a single cartridge accepting 44 unique DBS extracts within a total run time of 3.5 h, including DBS sample extraction and loading [14]. The volume of each droplet in this platform is just 100 nL. Precision and linearity of the assays were determined by analyzing quality control DBS samples, while clinical performance was determined by analyzing 600 presumed normal DBS and known affected samples (12 for Pompe, 7 for Fabry and 10 each for Hunter, Gaucher and Hurler). Overall coefficient of variation (CV) ranged from 6 to 25% for instrument to instrument and 1.5 to 17% for multi-day precision analyses. Linearity correlation coefficients were ≥0.98 for all assays. The multiple enzymatic assay format performed from a single DBS punch was able to discriminate presumed normal from known affected samples for all 5 LSDs.
The performance metrics of this DMF platform convinced the MO State NBS program to adopt the platform to fulfill its mandate to screen all newborns for Pompe, Fabry, Hurler and Gaucher diseases. When full population pilot screening started in January 2013, MO became the first state program in the United States to prospectively screen all newborns for these 4 LSDs [15]. Missouri has an annual birth rate of about 78,000. To accommodate this workload, the MO program uses two workstations, each consisting of four DMF instruments controlled by a single computer (Figure 3, top right panel) to analyze up to 640 patient DBS specimens in each 8 h shift. This throughput significantly exceeds the average daily volume of about 375 tests (including repeat analyses for abnormal results). The eight DMF instruments in MO have been in continuous operation for over 5 years without requiring any maintenance and with only a single instrument malfunction due to blowout of a fuse [51] . The workflow for assay performance shown in Figure 3 (bottom panel) is based on feedback from the MO program. The program reported its findings after the first 6 months of screening [15] showing the successful detection of multiple cases of Pompe and Fabry disease, plus at least one with each of the other conditions, with an acceptable false positive rate for each condition. More recently, the efficacy of DMF technology for LSD newborn screening has been established with over 32 confirmed cases of Pompe disease, 5 confirmed cases of Gaucher disease, 2 confirmed cases of Hurler disease and 94 confirmed cases of Fabry disease identified over 4 years of continuous testing in MO [52].
5.7. Comparison of Methods for Multiple LSD Screening
Although screening for multiple LSDs is not standard practice in most NBS programs, the addition of Pompe disease in 2015 and Hurler syndrome in 2016 to the RUSP has certainly generated momentum toward this. In addition to the DMF platform for multiple LSD screening, tandem mass spectrometry enzyme tests for multiple LSDs have been described [10,41]. Recently, it became possible for the first time to compare prospective NBS results from MO (DMF) with those from the IL screening program, which uses MS/MS, for the same four LSDs (Fabry, Gaucher, Pompe and Hurler). This comparison revealed that in the prospective NBS setting, MS/MS had no clear advantage over DMF [53]. On the contrary, DMF performance in terms of positive predictive value appeared to be better, especially for Fabry. In a rebuttal, it was argued that differences were due to differences in cut-off values, set by each program to define the threshold that should include all true positives for a given condition [54]. This view was again challenged on the basis that for enzymatic assays, regardless of the method used, cut-offs are at the low end of the assay range and inevitably will detect “false positives” that predispose to low enzyme activities due to inherent genetic, environmental and other factors, which is reflected in the screening results [55]. Screening for LSDs introduces new challenges for NBS programs that are as yet unsolved but will almost certainly rely on post-analytical methods to improve the value of the initial screening test [56]. In summary, both MS/MS and DMF are viable platforms for high-throughput multiple LSD screening, but DMF has major cost and convenience advantages that are irrefutable [31,53].
5.8. Expanding DMF NBS assays to other conditions
Thus far, the dialog has focused on LSD assays. In this section, we describe recent developments toward expanding the range of DMF to include other conditions that benefit from early identification through NBS. We reasoned that because several of the current assay methods in NBS programs are based on fluorometry for such conditions as biotinidase, hypothyroidism and galactosemia, for example, it would be logical to investigate the transfer of such assays to the DMF platform with a view toward simplifying the overall process of NBS. In another collaborative effort between Duke Health System Biochemical Genetics Laboratory, Advanced Liquid Logic and the North Carolina State Public Health Laboratory, a method to translate the NBS test for biotinidase was developed [57]. The enzymatic assay uses 4-methylumbelliferyl biotin, similar to a fluorogenic substrate currently used in benchtop NBS analyzers for biotinidase screening [58], as the fluorogenic substrate. Biotinidase deficiency assays were performed on Centers for Disease Control (CDC) proficiency test (PT) samples, and normal (n = 200) and deficient (n = 7) newborn DBS specimens using the same platform and extraction protocol, sample volumes, and incubation times as previously described for DMF-based LSD enzyme tests [14]. Enzymatic activity analysis of biotinidase deficiency revealed distinct separation between normal and affected DBS specimens using DMF (Figure 4) and PT results that matched the expected activity [58].
Figure 4.
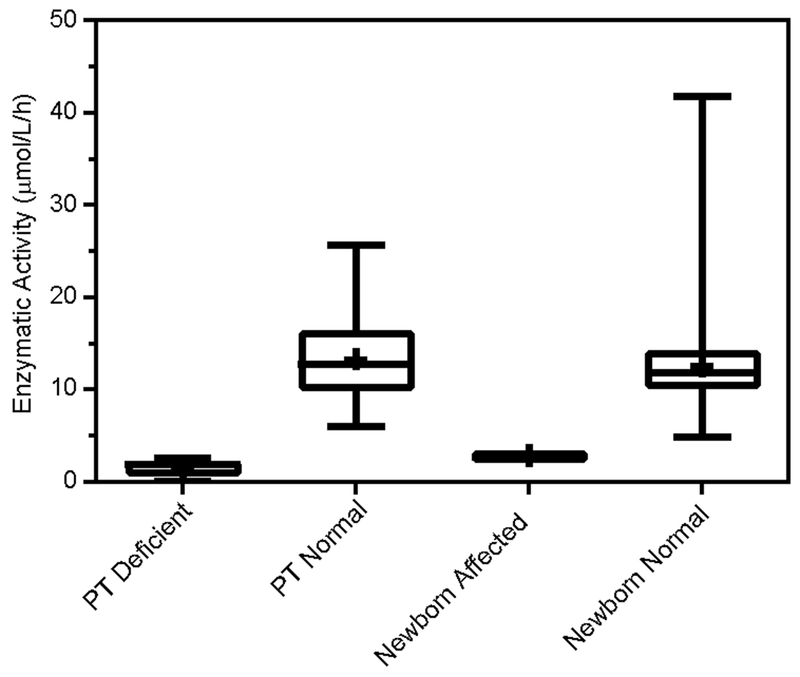
Results from DMF enzymatic assay for biotinidase showing complete separation of affected newborn samples (n=7) from confirmed unaffected (control) newborn DBS samples (n=200) as well as expected results from proficiency test (PT) samples obtained from CDC. Data modified and reprinted from [58] with permission from Elsevier.
A demonstration of the ability to combine assays for biotinidase with tests for galactosemia, Pompe and Hurler syndrome on the same DMF cartridge was recently reported [59]. These four conditions are on the RUSP and are time-critical for diagnosis; early onset forms of these diseases can have devastating consequences if undiagnosed and untreated in the early neonatal period.
More recently, in a further demonstration of the versatility of the DMF platform, 10 distinct enzyme assays were combined in a single run [60]. The conditions targeted were Pompe, Hurler, Gaucher, Fabry, Biotinidase, Sanfilippo, Sly, GM1 gangliosidosis, and alpha and beta mannosidoses. The concept for a 10-plex enzyme assay on the same cartridge was presented, in which a dual fluorophore detector system permits combining two reagents in each of 5 reagent input wells that release different fluorophores when exposed to their targeted enzyme. One set of fluorogenic substrates is based on 4MU (detected using excitation and emission wavelengths of 360 nm and 460 nm, respectively) and the other set based on resorufin (detected using excitation and emission wavelengths of 570 nm and 590 nm, respectively). The principles of this concept were proven by comparison with data from individual assays. For higher throughput of multiple enzymatic assays of this type, a cartridge capable of analyzing 96 samples and an analyzer with a built-in dual detector would be desirable.
In summary, the DMF high-throughput platform is a user-friendly, low-cost and versatile system for performing multiple NBS tests on the extract from a single specimen. The test menu can be customized to suit the requirements of any NBS program and the time required to generate results is much reduced compared with alternative platforms. The ergonomics of the DMF high-throughput platform favor its adoption as the primary platform for all enzymatic assays used in newborn screening centers.
6. Digital Microfluidics for Diagnostic Testing
6.1. Introduction
The development of clinically useful assays on a digital microfluidic platform was previously reviewed [2]. In addition to the enzymatic assays described in the foregoing section, immunoassays that use magnetic beads as a solid phase and nucleic acid assays that enable ultrafast PCR amplification have been effectively accomplished. Several different sample types, including whole blood, saliva, sweat and urine have been successfully analyzed on DMF platforms. The adaptability of DMF to incorporate diverse assay methods and sample types makes the technology suitable for a wide range of clinical diagnostic assays, including hematologic, renal, and neuromuscular. The inherent flexibility of the technology also makes it possible to consolidate multiple types of assays that currently require separate instrument systems onto a single instrument.
6.2. Testing for G6PD enzyme activity
The utility of DMF for glucose-6-phosphate dehydrogenase (G6PD) enzyme testing at the point of care was recently reported [61]. G6PD deficiency is the most common human enzyme deficiency and leads to an impaired regeneration of reduced glutathione due to lower levels of NADPH produced through the dysfunctional pentose phosphate pathway, which makes red blood cells susceptible to oxidative stress and resulting hemolytic anemia. In the newborn, G6PD deficiency is associated with high incidence of severe hyperbilirubinemia and puts the child at risk for bilirubin induced neurotoxicity. The World Health Organization therefore recommends G6PD screening for newborns in regions with the highest frequency of G6PD deficiency (primarily African, Asian and Mediterranean nations) [62]. In this report, a fluorescent readout of NADH and an intricate, automated sample preparation protocol was utilized in the DMF G6PD assay. The authors report a high correlation of their DMF assay (using whole blood sample input) to a diagnostic microtiter plate assay that required extensive washing, filtration and lysis of the whole blood sample prior to analysis (Figure 5, left panel).
Figure 5.
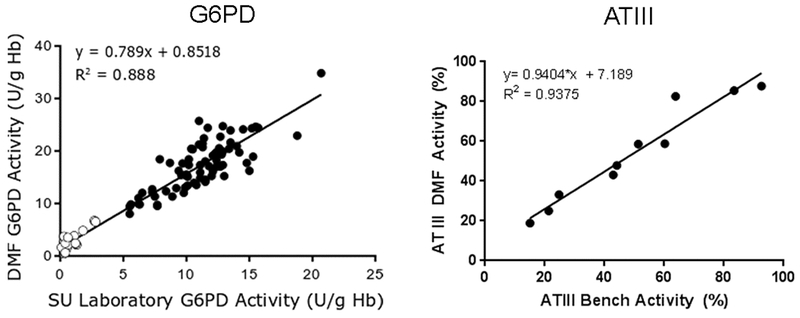
Comparison of G6PD enzyme activity results of same set of samples measured using the DMF assay (Y axis) and the Stanford Medical Center standard reference method (X axis) are shown in the left panel. Open circles denote deficient samples while solid circles denote normal samples. ATIII functional activity results of samples measured using the DMF assay (Y axis) are compared with the Boston Children’s Hospital standard reference method (X axis) in the right panel. Data modified from [74] and [63] by permission of Oxford University Press.
The automated DMF protocol for whole blood processing and red blood cell lysis negates the requirement for auxiliary equipment (centrifuge, pipettes, etc.) and eliminates most of the hands-on effort required for testing. This is significant because these features enable the DMF G6PD assay to be performed outside of a central laboratory or in low resource settings that lack the necessary equipment for sample processing. Potential applications of near patient DMF G6PD testing will be discussed in the next section.
6.3. Testing for hypercoagulation
DMF technology has also been applied to demonstrate assays for thrombosis, or clotting disorders [63,64]. The comprehensive assessment of thrombosis status requires as many as a dozen different tests and several milliliters of blood. Magnetic bead immunoassays for anti-thrombin III (ATIII), protein C, protein S and factor VIII antigens, as well as anti-cardiolipin and anti-β2-glycoprotein IgG antibodies were developed on a DMF platform using sample droplet volumes of 300 nl for each assay. Results for ATIII are shown in Figure 5 (right panel) and demonstrate comparable performance of the DMF ‘on-chip ELISAs’ to standard sandwich ELISA methods. Functional assays for ATIII, protein C, Factor VIII and plasminogen also were recently adapted to a DMF platform using novel fluorescent substrates and compared well to standard laboratory methods. Importantly, the sample volume required for the four functional assays was nearly 100-fold lower using the DMF format compared to microtiter plate assays (25 μl vs. 2 ml). Further studies are required to determine the efficacy of these assays in clinical settings.
6.4. Nucleic acid tests
Nucleic acid testing is used clinically to diagnose certain disease states and to provide guidance on therapy. The application of DMF for nucleic acid testing was first reported in 2003 [65] and was recently reviewed [66]. DMF technology has since been applied to nucleic acid amplification assays for several other clinically important targets, including cytomegalovirus (CMV) [67] and bacterial targets such as methicillin-resistant Staphylococcus aureus (MRSA), Mycoplasma pneumoniae and Candida albicans [68–70]. The DMF platform enables automated sample preparation (DNA extraction and purification) and thermal manipulation on a single cartridge. Sample droplets are shuttled rapidly between two temperature zones (95°C and 60°C) to amplify target sequences, which are detected in real–time on the cartridge. This system was able to detect as few as 3 copies of CMV DNA template (in a 50 μl saliva sample) with a cycle time of about half an hour. Thus this platform obviates the necessity to use several items of equipment, including a thermocycler, to achieve the same goal using standard laboratory methods.
The first DMF system to be authorized by the FDA for diagnostic testing is the ePlex® Respiratory Pathogen (RP) panel of nucleic acid assays; this system was developed by Genmark Diagnostics in collaboration with Advanced Liquid Logic [71,72]. This self-contained reaction cartridge employs electrowetting fluid manipulation technology to simultaneously identify seventeen of the most common viral and bacterial organisms associated with upper respiratory infections, including influenza. All reagents and buffers are stored within the disposable cartridge, which automates sample preparation, DNA extraction and PCR amplification. Additional panels for multiplexed blood culture identification and gastrointestinal pathogen identification, all of which would use the same core instrumentation, are currently in development.
7. Digital Microfluidics for Near Patient Applications
Point-of-care tests are a category of simple diagnostic medical tests that are performed near the patient with a goal of obtaining results quickly so that the treatment plan can be determined, or adjusted through follow-up monitoring. The most common point-of-care tests are blood glucose, blood gas, blood electrolytes, pregnancy tests and infectious disease tests, which are typically performed using portable or hand-held instruments that require small volumes of readily available samples (urine, saliva finger stick blood, etc.) and produce results in a short time (minutes). DMF technology is compatible with many of these requirements. The small sample volume format of DMF has the added benefit of minimizing blood loss resulting from recurrent testing; an especially important benefit for sick or preterm newborns. In this section, we review the development of this concept, which is now in development stage and near to fulfillment. The first iteration of a near patient DMF platform was recently described [73] and consists of a small footprint instrument with a detachable mini-tablet user interface that utilizes single-use disposable assay cartridges. All buffers and reagents for the assay(s) are stored within the cartridge. Similar to larger format DMF instruments, this miniature DMF platform is capable of performing multiple enzyme formats (enzyme activity, immunoassays and molecular assays) and can multiplex several unique assays onto a single cartridge run. Hands-on effort for these tests involves only loading the sample (~ 50 μl of whole blood, urine or saliva) and initiating the cartridge from the tablet user interface. Notably, automated protocols are developed for plasma separation performed on the cartridge. Droplets of whole blood are added to an agglutination agent that promotes clumping of the red blood cells; the plasma portion of the sample subsequently responds to electrowetting while the solid portion does not. This difference in phase motility is exploited to isolate plasma for assays that require it.
The near patient DMF platform is intended for single patient testing of multiple (4 or more) analytes from a very small sample volume (~50 μl). The multiplex format enables the comprehensive assessment of disease states such as hypoglycemia and thyroid function from much smaller volumes of blood and with much shorter turnaround times than comparable tests performed in a central laboratory setting. The first published reports of near patient DMF platform are focused on pediatric and neonatal disorders and include panels for neonatal hyperbilirubinemia (total serum bilirubin, direct bilirubin, G6PD and albumin) [74] and severe neonatal hypoglycemia (glucose, beta hydroxybutyrate, free fatty acids, insulin, cortisol, and growth hormone) [75]. For the hyperbilirubinemia panel, two different sample sources (plasma and red blood cells) are prepared on the same DMF cartridge from a single sample input (whole blood) and analyzed with a total run time of under 15 minutes, including sample preparation. For the hypoglycemia panel, a combination of immunoassays and enzyme assays are performed on the same DMF cartridge with a single sample input.
Rapid assays suited to NBS of time critical newborn disorders such as galactosemia have also been developed for whole blood samples on the near patient DMF platform [76]. The application of this technology to single-sample newborn testing near the patient is a major paradigm shift from the centralized model previously described for high-throughput NBS in a central laboratory. Near patient NBS has already been largely implemented in the United States with physiological monitoring for congenital hearing loss and critical congenital heart disease but not yet for biochemical testing. The ability to screen for severe, time critical neonatal conditions before a newborn is discharged from the hospital has the potential to improve the standard of care for newborns. This development could be especially important for countries that do not have a centralized public health laboratory or testing facility and thus do not collect newborn DBS samples.
8. Conclusions
Over the past decade, digital microfluidic technology has moved out of concept phase and into practical, sustainable use for newborn screening of lysosomal storage disorders and for the detection of common sources of upper respiratory tract infection. The impact of this technology is easily tracked in the newborn screening setting: over 130 babies have already been identified with a confirmed lysosomal storage disorder and been given the chance for a healthier life through early treatment [52]. DMF is poised to take its place as a valuable and versatile testing platform for NBS. Furthermore, DMF technology will contribute to care of the neonate presenting with illness or prematurity at birth that requires transfer to the neonatal intensive care unit for evaluation, where the need for rapid testing and results is particularly acute.
9. Expert Commentary
The field of newborn screening for inherited metabolic disorders has been a major contributor to improvement of human health across the world. It has evolved rapidly during the past ten years and seems poised to continue evolving. The driving forces for further expansion include the rapidly increasing pace of development of new therapeutic strategies for previously untreatable conditions, such as enzyme replacement, stem cell transplant, substrate inhibition, chaperones, gene replacement and combinations thereof. The development of screening tests lags behind that of new treatments, and is separating into three major components – enzymatic, analyte-specific and molecular.
The primary requirement for a screening test platform is a robust and reliable “turnkey” system to deliver meaningful results within 24 h of receipt of the specimen (dried blood spots collected on filter paper in the birthing center) at the testing facility. The majority of conditions are currently targeted using tandem mass spectrometry (MS/MS), by analysis of amino acids, acylcarnitines and other disease-specific biomarkers. Enzymatic assays are currently performed on various platforms, most of which utilize fluorometric assays. The SEEKER platform, that was designed to screen for up to four LSDs using fluorometric enzymatic methods, can demonstrably absorb these well-established fluorometric methods especially in view of its capability to multiplex at least two assays within a droplet by means of different fluorophores and additional detectors. DMF is particularly attractive because it also has the fastest turnaround from specimen receipt to result of any of the current NBS screening platforms. The bulk of the screening tests that are envisaged in the near term could therefore be covered by MS/MS and DMF. MS/MS should also play an increasing role in the application of second-tier testing to improve the positive predictive value of primary newborn screening tests. Although numerous examples have been published, limited use is being made of this capability at present.
Molecular testing is already established for second-tier testing in NBS for conditions such as cystic fibrosis, for which the primary screening test lacks specificity. It is also currently used to screen for primary immune defects, including SCID, and will soon be required to screen for spinal muscular atrophy (SMA) and possibly for other conditions for which a suitable biochemical test is not available. The expansion of molecular testing to whole exome sequencing from dried blood spots (DBS) is already practical, and has the potential to encompass a much wider range of heritable conditions than the current panel of recommended conditions. The obvious drawbacks to wholesale genetic screening of newborns include cost, inconclusive results, delays in interpretation and result reporting, lack of autonomy, and many other issues that raise serious ethical considerations. Similar arguments apply to other untargeted methodologies, such as metabolomics and proteomics that have been proposed to expand NBS.
On the other hand, newer DMF devices are close to commercial development that can screen for high-risk conditions near the patient from a single drop of blood and thereby add a valuable additional dimension to NBS. By avoiding delays incurred by specimen (DBS) collection, transport to a central laboratory, testing and result reporting, near patient testing would enable targeted screening for conditions that pose the greatest risk of harm in the early neonatal period. Such conditions include galactosemia (risk of brain damage by ingestion of milk products), defects of fatty acid oxidation (risk of hypoglycemia) and SCID (risk of exposure to live vaccines).
In developing countries that lack a centralized public health system or testing facility, NBS is either unavailable or is limited to hospitals or birthing centers that can afford the technology and typically charge their clients a fee for this service. In these settings, the DMF platform may represent the best, or perhaps even the only option for population NBS because it has significant advantages of low cost, simplicity, transportability and robustness that other NBS technologies, including MS/MS, lack.
Near patient testing for conditions such as for hyperbilirubinemia, hypoglycemia and coagulopathies in sick neonates transferred to neonatal intensive care has until now been unavailable. Testing for these and other severe underlying conditions using a simple DMF device at the bedside has been demonstrated and is already in trial phase; this is an area that we would expect to see significant expansion for DMF and competing technologies during the coming years.
10. Five-year view
It is most likely that during the next few years, more NBS programs will choose to adopt DMF for LSD screening from dried blood spots, and more LSD conditions will be added to the recommended uniform screening panel. The incorporation of fluorometric assays currently performed on other platforms onto a DMF platform also seems a likely development. In the future, high-throughput testing on the DMF platform has the potential to screen for many childhood conditions for which early identification and treatment improves the standard of care. DMF also presents an opportunity for countries that currently have either no or very limited capability to test newborns to establish a screening program rapidly and with minimal costs, by enabling testing at the birthing centers or at regional clinics, and perhaps even using a mobile laboratory, since DMF has minimal power and infrastructure requirements. The technology will soon be applied to single patient, multiple analyte testing near the patient for diagnosis and monitoring of time-critical conditions using FDA approved DMF devices. In this scenario, actionable, laboratory equivalent results can be obtained using much smaller volumes of sample and with significantly faster time to result than is possible with standard clinical laboratory tests. Smaller, inexpensive versions of these platforms also have high potential to bring rapid, precise, low sample volume testing to low resource settings that lack the infrastructure to support central laboratory testing.
interest in its commercialization. The authors have no other relevant affiliations of financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Key Issues.
Electro-wetting based digital microfluidics (DMF) is a versatile, low-cost technology for practical development of biological assays with colorimetric or fluorometric end-points.
The SEEKER is the only version of this platform that is commercially available for enzyme activity measurement and has been FDA cleared to screen newborns for up to four lysosomal storage diseases.
The SEEKER has been used for full population screening in a newborn screening program continuously since January 2013 with excellent results and no technical problems.
Results from the DMF platform compare very favorably with those generated by tandem mass spectrometry when used to screen prospectively for the same enzyme targets.
DMF generates results faster than any other method currently used for lysosomal storage disorder screening in newborn screening programs.
DMF is expandable to cover many more conditions - thus far up to twelve has been achieved - without consuming more sample or other precious resources.
New DMF devices for near patient testing for time-critical metabolic conditions are close to commercial development.
Point-of-care testing in the neonatal intensive care unit of very premature and sick neonates is also practicable using DMF and will provide valuable clinical information much faster than current methods allow.
Acknowledgments
Funding
This paper was funded by NIH – National Heart, Lung, and Blood Institute (HHSN268201000001C and R43HL125484); National Institute of Child Health and Human Development (R44HD057713, R44HD072853, R44HD092154); National Institute on Deafness and Other Communication Disorders (R44DC012967).
Footnotes
Declaration of Interest
DS Millington is a shareholder of Baebies, Inc. and serves on their scientific advisory board. S Norton, R Singh, R Sista, V Srinivasan and VK Pamula are employees and shareholders of Baebies, Inc. Baebies Inc. has a proprietary position with respect to the technology discussed here and has a financial interest in its commercialization. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Reference annotations
* Of interest
** Of considerable interest
- [1].Choi K, Ng A, Fobel AHC, Wheeler AR. Digital Microfluidics. Annu Rev Anal Chem 2012, 5: 413–430. [DOI] [PubMed] [Google Scholar]
- [2].Pollack MG, Pamula VK, Srinivasan V, et al. Applications of electrowetting-based digital microfluidics in clinical diagnostics. Expert Rev Mol Diagn. 2011;11(4):393–407. [DOI] [PubMed] [Google Scholar]
- [3].Millington DS, Sista R, Eckhardt A, et al. Digital microfluidics: a future technology in the newborn screening laboratory? Semin Perinatol. 2010;34(2):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marsden D, Levy H. Newborn screening of lysosomal storage disorders. Clin Chem. 2010;56(7):1071–1079. [DOI] [PubMed] [Google Scholar]
- [5].Zhou H, Fernhoff P, Vogt RF. Newborn bloodspot screening for lysosomal storage disorders. J Pediatr. 2011;159(1):7–13 e1. [DOI] [PubMed] [Google Scholar]
- [6].Chamoles NA, Blanco M, Gaggioli D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001;308(1-2):195–196. [DOI] [PubMed] [Google Scholar]
- [7].Chamoles NA, Blanco M, Gaggioli D. Diagnosis of alpha-L-iduronidase deficiency in dried blood spots on filter paper: the possibility of newborn diagnosis. Clin Chem. 2001;47(4):780–781. [PubMed] [Google Scholar]; ** First fluorometric LSD enzyme assay compatible with dried blood spot samples.
- [8].Chamoles NA, Niizawa G, Blanco M, et al. Glycogen storage disease type II: enzymatic screening in dried blood spots on filter paper. Clin Chim Acta. 2004;347(1-2):97–102. [DOI] [PubMed] [Google Scholar]
- [9].Li Y, Brockmann K, Turecek F, et al. Tandem mass spectrometry for the direct assay of enzymes in dried blood spots: application to newborn screening for Krabbe disease. Clin Chem. 2004;50(3):638–640. [DOI] [PubMed] [Google Scholar]
- [10].Li Y, Scott CR, Chamoles NA, et al. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50(10):1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang D, Eadala B, Sadilek M, et al. Tandem mass spectrometric analysis of dried blood spots for screening of Mucopolysaccharidosis I in newborns. Clin Chem. 2005;51(5):898–900. [DOI] [PubMed] [Google Scholar]
- [12].Sista RS, Eckhardt AE, Wang T, et al. Digital microfluidic platform for multiplexing enzyme assays: implications for lysosomal storage disease screening in newborns. Clin Chem. 2011;57(10):1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Multiplexed LSD enzyme testing on a DMF platform.
- [13].Burton B, Charrow J, Angle B, et al. A pilot newborn screening program for lysosomal storage disorders (LSD) in Illinois. Mol Genet Metab. 2012;105(2):S23–S24. [Google Scholar]
- [14].Sista RS, Wang T, Wu N, et al. Multiplex newborn screening for Pompe, Fabry, Hunter, Gaucher, and Hurler diseases using a digital microfluidic platform. Clin Chim Acta. 2013;424:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hopkins P, Campbell C, Klug T, et al. Lysosomal storage disorder screening implementation: Findings from the first six month of full population pilot testing in Missouri. J Pediatr. 2015;166:172–177. [DOI] [PubMed] [Google Scholar]
- [16].Illumina acquires Advanced Liquid Logic, leader in digital microfluidics solutions. Business Wire [Internet]. 2013. July 12 [cited 2018 March 30]; Available from: https://www.businesswire.com/news/home/20130723006279/en/Illumina-Acquires-Advanced-Liquid-Logic-Leader-Digital..
- [17].FDA News Release [Internet]. Silver Spring (MD): U.S. Food & Drug Administration; c2018. FDA permits marketing of first newborn screening system for detection of four, rare metaboic disorders. 2017. February 3 [cited 2018 March 30]; Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm539893.htm. [Google Scholar]
- [18].Kirby AE, Wheeler AR. Digital Microfluidics: An Emerging Sample Preparation Platform for Mass Spectrometry. Anal Chem. 2013, 85:6178–6184. [DOI] [PubMed] [Google Scholar]
- [19].Pollack MG, Shenderov AD, Fair RB. Electrowetting-based actuation of droplets for integrated microfluidics. Lab Chip. 2002;2(2):96–101. [DOI] [PubMed] [Google Scholar]
- [20].Yang H, Fan S-K, Lin C-P, Wu C-T & Hsu W 3D droplet transportation by EWOD actuations on flexible polymer films. Paper presented at ASME 2005 International Mechanical Engineering Congress and Exposition: Microelectromechanical Systems , Florida, USA. American Society of Mechanical Engineers ( 10.1115/IMECE2005-80744) (2005, November). [DOI] [Google Scholar]
- [21].Fan S-K, Yang H & Hsu W Droplet-on-a-wristband: chip-to-chip digital microfluidic interfaces between replaceable and flexible electrowetting modules. Lab Chip 2011; 11:343–347. [DOI] [PubMed] [Google Scholar]
- [22].Abdelgawad M, Freire SLS, Yang H & Wheeler AR All-terrain droplet actuation. Lab Chip 2008, 8:672–677. [DOI] [PubMed] [Google Scholar]
- [23].Hong J, Kim J.Won D-W., Kim J, Lee SJ. Three-dimensional digital microfluidic manipulations of droplets in oil medium. Sci. Reports. 2015; 5:10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kokalj T, Pérez-Ruiz E, Lammertyn J. Building bio-assays with magnetic particles on a digital microfluidic platform. N Biotechnol. 2015;32(5):485–503. [DOI] [PubMed] [Google Scholar]
- [25].Jebrail MJ, Bartsch MS, Patel KD.Digital microfluidics: a versatile tool for applications in chemistry, biology and medicine. Lab Chip. 2012;12(14):2452–2563. [DOI] [PubMed] [Google Scholar]
- [26].Abdulwahab S, Ng AHC, Chamberlain D, Ahmado H, et al. Towards a personalized approach to aromatase inhibitor therapy: a digital microfluidic platform for rapid analysis of estradiol in core-needle-biopsies. Lab Chip. 2017; 17(9):1594–1602. [DOI] [PubMed] [Google Scholar]
- [27].Ng AHC, Fobel R, Fobel C, Lamanna J. A digital microfluidic system for serological immunoassays in remote settings. Sci Transl Med. 2018;10:438. [DOI] [PubMed] [Google Scholar]
- [28].Lonning PE. Comment on “Towards a personalized approach to aromatase inhibitor therapy: a digital microfluidic platform for rapid analysis of estradiol in core-needle-biopsies.” Lab Chip 2017; 17(18):3186–3187. [DOI] [PubMed] [Google Scholar]
- [29].Paik P, Pamula VK, Fair RB. Rapid droplet mixers for digital microfluidic systems. Lab Chip. 2003;3(4):253–259. [DOI] [PubMed] [Google Scholar]
- [30].Paik PY, Pamula VK, Chakrabarty. Adaptive cooling of integrated circuits using digital microfluidics. IEEE Trans. Very Large Scale Integr. (VLSI) Syst. 2008;16(4):432–443.. [Google Scholar]
- [31].Peake RW, Marsden DL, Bodamer OA, et al. Newborn screening for lysosomal storage disorders: quo vadis? Clin Chem. 2016;62(11):1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pitt JJ. Newborn screening. Clin Biochem Rev. 2010;31(2):57–68. [PMC free article] [PubMed] [Google Scholar]
- [33].Wilcken B, Wiley V. Newborn screening. Pathology. 2008;40(2):104–115. [DOI] [PubMed] [Google Scholar]
- [34].Millington DS, Kodo N, Norwood DL, et al. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13(3):321–324. [DOI] [PubMed] [Google Scholar]
- [35].Rashed MS, Bucknall MP, Little D, et al. Screening blood spots for inborn errors of metabolism by electrospray tandem mass spectrometry with a microplate batch process and a computer algorithm for automated flagging of abnormal profiles. Clin Chem. 1997;43(7):1129–1141. [PubMed] [Google Scholar]
- [36].Schulze A, Lindner M, Kohlmuller D, et al. Expanded newborn screening for inborn errors of metabolism by electrospray ionization-tandem mass spectrometry: results, outcome, and implications. Pediatrics. 2003;111(6 Pt 1):1399–1406. [DOI] [PubMed] [Google Scholar]
- [37].Chien YH, Chiang SC, Zhang XK, et al. Early detection of Pompe disease by newborn screening is feasible: results from the Taiwan screening program. Pediatrics. 2008;122(1):e39–45. [DOI] [PubMed] [Google Scholar]; * First prospective newborn screening results for Pompe disease.
- [38].Chien YH, Hwu WL, Lee NC. Pompe disease: early diagnosis and early treatment make a difference. Pediatr Neonatol. 2013;54(4):219–227. [DOI] [PubMed] [Google Scholar]
- [39].Chien YH, Lee NC, Thurberg BL, et al. Pompe disease in infants: improving the prognosis by newborn screening and early treatment. Pediatrics. 2009;124(6):e1116–1125. [DOI] [PubMed] [Google Scholar]
- [40].Krivit W, Shapiro EG, Peters C, et al. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N Engl J Med. 1998;338(16):1119–1126. [DOI] [PubMed] [Google Scholar]
- [41].Orsini JJ, Martin MM, Showers AL, et al. Lysosomal storage disorder 4+1 multiplex assay for newborn screening using tandem mass spectrometry: application to a small-scale population study for five lysosomal storage disorders. Clin Chim Acta. 2012;413(15–16):1270–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ries M Enzyme replacement therapy and beyond—in memoriam Roscoe O. Brady, M.D. (1923–2016). J Inherit Metab Dis. 2017;40:343–356. [DOI] [PubMed] [Google Scholar]
- [43].Recommended Uniform Screening Panel [Internet]. Rockville (MD): Health Resources & Services Administration; c2018. Health Resources and Services Administration ACHDNC Recommended Uniform Screening Panel. 2016. November [cited 2018 March 30]. Available from: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html. [Google Scholar]
- [44].Srinivasan V, Pamula VK, Fair RB. Droplet-based microfluidic lab-on-a-chip for glucose detection. Anal Chim Acta. 2004;507:145–150. [Google Scholar]
- [45].Oemardien LF, Boer AM, Ruijter GJ, et al. Hemoglobin precipitation greatly improves 4-methylumbelliferone-based diagnostic assays for lysosomal storage diseases in dried blood spots. Mol Genet Metab. 2011;102(1):44–48. [DOI] [PubMed] [Google Scholar]
- [46].Giugliani R, Brusius-Facchin AC, Moura de Souza CF, et al. Diagnosis and therapy options in mucopolysaccharidosis II (Hunter Syndrome). Expert Opin Orphan Drugs. 2015;3(2):141–150. [Google Scholar]
- [47].Voznyi YV, Keulemans JL, van Diggelen OP. A fluorimetric enzyme assay for the diagnosis of MPS II (Hunter disease). J Inherit Metab Dis. 2001;24(6):675–680. [DOI] [PubMed] [Google Scholar]
- [48].Tolun AA, Graham C, Shi Q, et al. A novel fluorometric enzyme analysis method for Hunter syndrome using dried blood spots. Mol Genet Metab. 2012;105(3):519–521. [DOI] [PubMed] [Google Scholar]
- [49].Sista R, Eckhardt AE, Wang T, et al. Rapid, single-step assay for Hunter syndrome in dried blood spots using digital microfluidics. Clin Chim Acta. 2011;412(19-20):1895–1897. [DOI] [PubMed] [Google Scholar]
- [50].Sista RS, Wang T, Wu N, et al. Rapid assays for Gaucher and Hurler diseases in dried blood spots using digital microfluidics. Mol Genet Metab. 2013;109(2):218–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hopkins P 4-plex LSD screening using digital microfluidics in Missouri; over 4 years of experience. Webinar presentation at: Newborn Screening Translational Research Network Joint Pompe phone call; 2017. April 6.
- [52].Hopkins PV, Klug T, Vermette L, et al. Incidence of four lysosomal storage disorders from four years of newborn screening in Missouri. Published online May 29, 2018. doi: 10.1001/jamapediatrics.2018.0263. [DOI] [PMC free article] [PubMed]; ** Four year follow-up for prospective newborn screening of 4 LSDs using the DMF platform.
- [53].Millington DS, Bali DM. Misinformation regarding tandem mass spectrometric vs fluorometric assays to screen newborns for LSDs. Mol Genet Metab Rep. 2017;11:72–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gelb MH, Scott CR, Turecek F, et al. Comparison of tandem mass spectrometry to fluorimetry for newborn screening of LSDs. Mol Genet Metab Rep 2017;11: 80–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Millington DS. Response to Gelb et al. : “Comparison of tandem mass spectrometry to fluorimetry for newborn screening of LSDs”. Mol Genet Metab Rep. 2017;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hall PL, Marquardt G, McHugh DM, et al. Postanalytical tools improve performance of newborn screening by tandem mass spectrometry. Genet Med. 2014;16(12):889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wastell H, Dale G, Bartlett K. A sensitive fluorimetric rate assay for biotinidase using a new derivative of biotin, biotinyl-6-aminoquinoline. Anal Biochem. 1984;140(1):69–73. [DOI] [PubMed] [Google Scholar]
- [58].Graham C, Sista RS, Kleinert J, et al. Novel application of digital microfluidics for the detection of biotinidase deficiency in newborns. Clin Biochem. 2013;46(18):1889–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]; * First DMF assay compatible with metabolic disorder newborn screening.
- [59].Nuffer M, Graham C, Nelson L, et al. Multiplexing current and emrgin enzymatic assays for Pompe, Mucopolysaccharidosis Type I, Biotinidase Deficiency and Galactosemia disorders on a digital microfluidic cartridge. Poster session presented at: International Society of Neonatal Screening. 10th European Regional Meeting; 2016 Sept 11-14; The Hague, the Netherlands. [Google Scholar]
- [60].Singh R, Chopra S, Graham C, et al. Demonstration of a digital microfluidic platform for the high throughput analysis of 12 discrete fluorimetric enzyme assays using a single newborn dried blood spot punch. Poster session presented at: WORLD Symposium on Lysosomal Diseases. 14th Annual Meeting; 2018 Feb 5-9; San Diego, CA. [Google Scholar]
- [61].Bhutani VK, Kaplan M, Glader B, et al. Point-of-care quantitative measure of glucose-6-phosphate dehydrogenase enzyme deficiency. Pediatrics. 2015;136(5):e1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** First demonstration of glucose 6 phosphate dehydrogenase (G6PD) enzyme testing using a near patient DMF platform.
- [62].WHO Working Group. Glucose-6-phosphate dehydrogenase deficiency.. Bull World Health Organ. 1989;67(6):601–611. [PMC free article] [PubMed] [Google Scholar]
- [63].Emani S, Nelson LT, Norton S, et al. Enzymatic functional assays of coagulation using small sample volumes. Lab Med. 2017;49(1):47–54. [DOI] [PubMed] [Google Scholar]
- [64].Emani S, Sista R, Loyola H, et al. Novel microfluidic platform for automated lab-on-chip testing of hypercoagulability panel. Blood Coagul Fibrinolysis. 2012;23(8):760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pollack M, Paik P, Shendrov, et al. Investigation of electrowetting-based microfluidics for real-time PCR applications. Proceedings of the 7th International Conference on Miniaturized Chemical and Biochemical Analysis Systems (MicroTAS’03); 2003 Oct 5-9; Squaw Valley, VA 2003;p. 619–622. [Google Scholar]
- [66].Coelho B, Veigas B, Fortunato E, et al. Digital microfluidics for nucleic acid amplification. Sensors (Basel). 2017;17(7):1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nelson L, Pati S, Bopanna S, et al. Towards point-of-care nucleic acid testing for congenital cytomegalovius infection in newborns. Poster session presented at: International Congenital CMV Conference. 5th Annual Conference; 2015 April 20-24; Brisbane, Australia. [Google Scholar]
- [68].Sista R, Hua Z, Thwar P, et al. Development of a digital microfluidic platform for point of care testing. Lab Chip. 2008;8(12):2091–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Development of a near patient electrowetting DMF platform.
- [69].Schell WA, Benton JL, Smith PB, et al. Evaluation of a digital microfluidic real-time PCR platform to detect DNA of Candida albicans in blood. Eur J Clin Microbiol Infect Dis. 2012; 31(9): 2237–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wulff-Burchfield E, Schell WA, Eckhardt AE, et al. Microfluidic platform versus conventional real-time polymerase chain reaction for the detection of Mycoplasma pneumoniae in respiratory specimens. Diagn Micr Infec Dis. 2010; 67(1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kayyem JF, Ford S, Pamula V, et al. , inventors; Advanced Liquid Logic Inc, Genmark Diagnostics Inc, assignee Instrument and cartridge for performing assays in a closed sample preparation and reaction system employing electrowetting fluid manipulation. United States patent US 20160129437A1 2016. May 12.
- [72].U.S. Food & Drug Administration 510k K163636. Silver Spring (MD): U.S. Food & Drug Administration; 2017. June 9 [cited 2018 March 30]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf16/K163636.pdf. [Google Scholar]
- [73].Ng R, Nuffer M, Basmajian M, et al. A near patient platofrm for comprehensive hyperbilirubinemia testing in newborns using low blood volume. Poster session presented at: Hot Topics in Neonatology; 2017 Dec 3-5; Washington, D.C. [Google Scholar]
- [74].Ng R, Nuffer M, Basmajian M, et al. Comprehensive, near patient hyperbilirubinemia testing in newborns using low blood volumes. Poster session accepted for: Pediatric Academic Societies Annual Meeting; 2018 May 5-8; Toronto, Canada. [Google Scholar]
- [75].Cotten CM, Coyne J, Nelson L, et al. Neonatal hypoglycemia: rapid assessment using a novel microfluidic platform. Poster session accepted for: Pediatric Academic Societies Annual Meeting; 2018 May 5-8; Toronto, Canada. [Google Scholar]
- [76].Chopra S, Mohsen A-W, Singh R, et al. Newborn screening for time-critical metabolic disorders–a new paradigm to test at the point of care. Paper presented at: Pediatric Academic Societies Annual Meeting; 2017 May 6-9; San Fransisco, CA. [Google Scholar]


