Abstract
Purpose
To increase awareness, outline strategies, and offer guidance on the recommended management of immune-related adverse events in patients treated with immune checkpoint inhibitor (ICPi) therapy.
Methods
A multidisciplinary, multi-organizational panel of experts in medical oncology, dermatology, gastroenterology, rheumatology, pulmonology, endocrinology, urology, neurology, hematology, emergency medicine, nursing, trialist, and advocacy was convened to develop the clinical practice guideline. Guideline development involved a systematic review of the literature and an informal consensus process. The systematic review focused on guidelines, systematic reviews and meta-analyses, randomized controlled trials, and case series published from 2000 through 2017.
Results
The systematic review identified 204 eligible publications. Much of the evidence consisted of systematic reviews of observational data, consensus guidelines, case series, and case reports. Due to the paucity of high-quality evidence on management of immune-related adverse events, recommendations are based on expert consensus.
Recommendations
Recommendations for specific organ system-based toxicity diagnosis and management are presented. While management varies according to organ system affected, in general, ICPi therapy should be continued with close monitoring for grade 1 toxicities, with the exception of some neurologic, hematologic, and cardiac toxicities. ICPi therapy may be suspended for most grade 2 toxicities, with consideration of resuming when symptoms revert to grade 1 or less. Corticosteroids may be administered. Grade 3 toxicities generally warrant suspension of ICPis and the initiation of high-dose corticosteroids (prednisone 1 to 2 mg/kg/d or methylprednisolone 1 to 2 mg/kg/d). Corticosteroids should be tapered over the course of at least 4 to 6 weeks. Some refractory cases may require infliximab or other immunosuppressive therapy. In general, permanent discontinuation of ICPis is recommended with grade 4 toxicities, with the exception of endocrinopathies that have been controlled by hormone replacement. Additional information is available at www.asco.org/supportive-care-guidelines and www.asco.org/guidelineswiki.
THE BOTTOM LINE
Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline
Guideline Question
How should clinicians manage immune-related adverse events (irAEs) in adult patients with cancer treated with immune checkpoint blockade antibodies?
Target Population
Adult patients with cancer receiving treatment with immune checkpoint blockade inhibitors alone.
Target Audience
Health care practitioners, including oncologists, medical specialists, emergency medicine, family practitioners, nurses, and pharmacists, who provide care to patients with cancer as well as patients receiving immune checkpoint inhibitors (ICPis) and their caregivers.
Methods
An Expert Panel was convened to develop clinical practice guideline recommendations based on a systematic review of the medical literature.
Recommendations
The following are general recommendations that should be followed irrespective of affected organs. For organ-specific management, see Tables 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10. (Note: Definition of grades are found in each table and, for the most part, follow the Common Terminology Criteria for Adverse Events [version 5.0]).3
It is recommended that clinicians manage toxicities as follows:
Patient and family caregivers should receive timely and up-to-date education about immunotherapies, their mechanism of action, and the clinical profile of possible irAEs prior to initiating therapy and throughout treatment and survivorship.
There should be a high level of suspicion that new symptoms are treatment related.
In general, ICPi therapy should be continued with close monitoring for grade 1 toxicities, with the exception of some neurologic, hematologic, and cardiac toxicities.
Hold ICPis for most grade 2 toxicities and consider resuming when symptoms and/or laboratory values revert to grade 1 or less. Corticosteroids (initial dose of 0.5 to 1 mg/kg/d of prednisone or equivalent) may be administered.
Hold ICPis for grade 3 toxicities and initiate high-dose corticosteroids (prednisone 1 to 2 mg/kg/d or methylprednisolone IV 1 to 2 mg/kg/d). Corticosteroids should be tapered over the course of at least 4 to 6 weeks. If symptoms do not improve with 48 to 72 hours of high-dose corticosteroid, infliximab may be offered for some toxicities.
When symptoms and/or laboratory values revert to grade 1 or less, rechallenging with ICPis may be offered; however, caution is advised, especially in those patients with early-onset irAEs. Dose adjustments are not recommended.
In general, grade 4 toxicities warrant permanent discontinuation of ICPis, with the exception of endocrinopathies that have been controlled by hormone replacement.
All recommendations in this guideline are based on expert consensus, benefits outweigh harms, moderate strength of recommendation.
ASCO believes that cancer clinical trials are vital to inform medical decisions and improve cancer care and that all patients should have the opportunity to participate.
INTRODUCTION
Immune checkpoint inhibitors (ICPis) have revolutionized the treatment of many different types of cancers. These inhibitors work by blocking pathways called checkpoints. These checkpoint pathways are mechanisms for the human immune system to control the immune response. The immune checkpoint proteins cytotoxic T-lymphocyte-associated-4 (CTLA-4) and programmed cell death protein 1 (PD-1) are receptors expressed on the surface of cytotoxic T cells that interact with their ligands CD80/CD86 in the case of CTLA-4 and programmed death-ligand 1 (PD-L1) in the case of PD-1. These pathways can be co-opted to help cancer cells to evade cytotoxic T-cell-mediated death.1 ICPis work by preventing the receptors and ligands from binding to each other, thereby disrupting signaling.1
Currently, there are several ICPis approved by the US Food and Drug Administration. Ipilimumab, an anti–CTLA-4 antibody, was the first agent approved for use in patients with advanced melanoma.2 Pembrolizumab and nivolumab target PD-1 and have been approved for melanoma, metastatic non-small-cell lung cancer (NSCLC), head and neck squamous cancers, urothelial carcinoma, gastric adenocarcinoma, and mismatch-repair-deficient solid tumors as well as for classic Hodgkin lymphoma.2 Nivolumab is approved for use for hepatocellular carcinoma and patients with renal cell carcinoma. The combination of ipilimumab and nivolumab for patients with advanced melanoma has also received US Food and Drug Administration approval.2 Most recently, PD-L1 antibodies atezolizumab (approved for use in urothelial cancers and NSCLC), durvalumab (approved for use in urothelial cancers), and avelumab (approved for use in Merkel cell carcinoma and urothelial carcinoma) have also been developed to block the PD-1 pathway. The indications for use continue to expand at a rapid pace. Development of novel ICPi agents and combinations continue to be evaluated for multiple indications. Thus, this field is rapidly changing.
Despite the often durable clinical benefits of the immune checkpoint blockade therapy, ICPi use is associated with a spectrum of adverse effects related to the mechanism of action that is quite different from other systemic therapies such as cytotoxic chemotherapy. The adverse effects can affect multiple organs of the body and are most commonly seen in the skin; GI tract; lungs; and endocrine, thyroid, adrenal, pituitary, musculoskeletal, renal, nervous, hematologic, cardiovascular, and ocular systems, and there should be a high level of suspicion that any changes are treatment-related (Appendix Fig A1, online only). ICPi therapy can usually continue in the presence of mild immune-related adverse events (irAEs) with close monitoring. However, moderate to severe irAEs may be associated with severe declines in organ function and quality of life, and fatal outcomes have been reported; hence, these toxicities require early detection and proper management. Use of ICPis in patients with preexisting autoimmune disease or history of prior organ transplant requires an especially thoughtful discussion of potential risks and benefits.
In recognition of an increasing need for guidance, ASCO and the National Comprehensive Cancer Network partnered to develop guidelines on the management of irAEs. Organizational representation from the Society for Immunotherapy of Cancer, the American Society of Hematology, and the Oncology Nursing Society and informal collaboration with the Friends of Cancer Research and the Parker Institute helped to ensure coordination of efforts and a harmonization of recommended care options for this patient population. With the increasing use of immunotherapy in cancer treatment regimens, it is imperative that clinicians are knowledgeable about the symptoms associated with these agents, their recommended management, and how best to monitor for them.
GUIDELINE QUESTION
This clinical practice guideline addresses one overarching clinical question: How should clinicians manage irAEs in adult patients with cancer treated with immune checkpoint blockade antibodies?
METHODS
Guideline Development Process
A multidisciplinary, multi-organizational panel of experts in medical oncology, dermatology, gastroenterology, rheumatology, pulmonology, endocrinology, urology, neurology, hematology, emergency medicine, nursing, trialist, and advocacy was convened to develop the clinical practice guideline (Appendix Table A1, online only). The Expert Panel met in person, via teleconference, and webinar and corresponded through e-mail. Based on the consideration of the evidence, the authors were asked to contribute to the development of the guideline, provide critical review, and finalize the guideline recommendations. Members of the Expert Panel were responsible for reviewing and approving the penultimate version of guideline, which was then circulated for external review and submitted to Journal of Clinical Oncology for editorial review and consideration for publication. All ASCO guidelines are ultimately reviewed and approved by the Expert Panel and the ASCO Clinical Practice Guideline Committee prior to publication. All funding for the administration of this project was provided by ASCO.
ASCO guidelines are based on systematic reviews of the literature. A protocol for each systematic review defines parameters for a targeted literature search. Additional parameters include relevant study designs, literature sources, types of reports, and prespecified inclusion and exclusion criteria for literature identified. The protocol for this guideline was reviewed and approved by the ASCO Clinical Practice Guidelines Committee’s Supportive Care Guideline Advisory Group.
Study eligibility was guided by the population, intervention, comparator, and outcome (PICO) framework as described in the Cochrane Handbook for Systematic Reviews of Interventions. In addition, the review took into account specific timing, setting, and study design as appropriate. The PICO criteria for the studies that were included in this review are as follows:
Population: Adult patients with cancer receiving treatment with immune checkpoint blockade inhibitors alone (not in combination with chemotherapy)
Intervention: Corticosteroids; immunosuppressive therapy; dose modification or discontinuation of therapy; organ-specific management, including hormone replacement, disease-modifying antirheumatic drugs (DMARDs), plasmapheresis, hospitalization, consultation to subspecialties, and best supportive care
Comparator: No intervention or best supportive care
Outcomes: Hospitalization, discontinuations of immunotherapy due to AE, AE-related morbidity or mortality, organ dysfunction based on organ system affected, required treatment due to irAEs, retreatment with immunotherapy, recovery from AEs, and health-related quality of life
The searches were designed and conducted by a team of expert medical librarians at Doctor Evidence in established clinical and medical bibliographic databases by using a range of Medical Subject Headings, EMTREE, and free-text terms based on the PICO criteria. All searches were peer reviewed by a senior Doctor Evidence (DOC) librarian. Bibliographic sources included MEDLINE In-Process via PubMed, Embase via OvidSP, and Cochrane Central Register of Control Trial via Wiley.
All study selection and screening were conducted using the DOC Library software platform (Doctor Evidence). DOC Library is a Web-based platform featuring duplicate removal, keyword emphasis (coloring or bolding of keywords), and search and ranking functionalities and can assign and manage reasons for exclusion. Before screening began, duplicate studies and those that did not meet language or date restrictions were excluded. Screening guidelines based on the protocol were then developed by consensus between methodology staff and the lead librarian and checked by a senior methodologist.
The screening procedure was conducted based on a two-step process: (1) title/abstract screening and (2) full-text screening. At both stages, the reasons for exclusion were documented. Full-text screening was conducted by two reviewers. Discrepancies between reviewers were resolved by an independent third reviewer. Articles were excluded from the systematic review if they were editorials, commentaries, letters, news articles, narrative reviews, or published in a non-English language.
The guideline recommendations are crafted, in part, by using the Guidelines Into Decision Support (GLIDES) methodology. In addition, a guideline implementability review was conducted. Based on the implementability review, revisions were made to the draft to clarify recommended actions for clinical practice.
Detailed information about the methods used to develop this guideline is available in the Methodology Supplement at www.asco.org/supportive-care-guidelines, including an overview (eg, panel composition, development process, revision dates), literature search and data extraction, recommendation development process, and quality assessment.
The ASCO Expert Panel and guidelines staff will work with co-chairs to keep abreast of any substantive updates to the guideline. Based on formal review of the emerging literature, ASCO will determine the need to update. The Methodology Supplement (available at www.asco.org/supportive-care-guidelines) provides additional information about the signals approach to guideline updating.
This is the most recent information as of the publication date. Visit the ASCO Guidelines Wiki at www.asco.org/guidelineswiki to submit new evidence.
All abbreviations used in this Guideline can be found in Appendix Table A3, online only.
Guideline Disclaimer
The Clinical Practice Guidelines and other guidance published herein are provided by the American Society of Clinical Oncology, Inc. (ASCO) to assist providers in clinical decision making. The information herein should not be relied upon as being complete or accurate, nor should it be considered as inclusive of all proper treatments or methods of care or as a statement of the standard of care. With the rapid development of scientific knowledge, new evidence may emerge between the time information is developed and when it is published or read. The information is not continually updated and may not reflect the most recent evidence. The information addresses only the topics specifically identified therein and is not applicable to other interventions, diseases, or stages of diseases. This information does not mandate any particular course of medical care. Further, the information is not intended to substitute for the independent professional judgment of the treating provider, as the information does not account for individual variation among patients. Recommendations reflect high, moderate, or low confidence that the recommendation reflects the net effect of a given course of action. The use of words like “must,” “must not,” “should,” and “should not” indicates that a course of action is recommended or not recommended for either most or many patients, but there is latitude for the treating physician to select other courses of action in individual cases. In all cases, the selected course of action should be considered by the treating provider in the context of treating the individual patient. Use of the information is voluntary. ASCO provides this information on an “as is” basis and makes no warranty, express or implied, regarding the information. ASCO specifically disclaims any warranties of merchantability or fitness for a particular use or purpose. ASCO assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of this information, or for any errors or omissions.
Guideline and Conflicts of Interest
The Expert Panel was assembled in accordance with ASCO’s Conflict of Interest Policy Implementation for Clinical Practice Guidelines (“Policy,” found at http://www.asco.org/rwc). All members of the Expert Panel completed ASCO’s disclosure form, which requires disclosure of financial and other interests, including relationships with commercial entities that are reasonably likely to experience direct regulatory or commercial impact as a result of promulgation of the guideline. Categories for disclosure include employment; leadership; stock or other ownership; honoraria, consulting or advisory role; speaker’s bureau; research funding; patents, royalties, other intellectual property; expert testimony; travel, accommodations, expenses; and other relationships. In accordance with the Policy, the majority of the members of the Expert Panel did not disclose any relationships constituting a conflict under the Policy.
RESULTS
A total of 38 systematic reviews and 166 primary studies met the eligibility criteria of the systematic review. Much of the evidence consisted of systematic reviews of observational data, consensus guidelines, case series, and case reports. Due to the limitations of the available evidence, the guideline relied on informal consensus for the recommendations. Use of formal consensus methodology was deemed unnecessary, favoring open discussion that allowed for articulation of views and opinions instead. Dissenting opinions, when raised, are noted.
RECOMMENDATIONS
Clinical Question
How should clinicians manage irAEs in adult patients with cancer treated with immune checkpoint blockade antibodies? All recommendations in this guideline are expert consensus based, with benefits outweighing harms, and a moderate strength of recommendation.
1.0. Skin Toxicities
Please refer to Table 1 for a complete set of recommendations, definition of grades, and additional considerations.
Table 1.
Management of Skin irAEs in Patients Treated With ICPis
| 1.0 Skin Toxicities | |
| 1.1 Rash/inflammatory dermatitis | |
| Definition: Erythema multiforme minor (a targetoid reaction in the skin and mucous membranes usually triggered by infections, such as herpes simplex viruses, but can be associated with an immune-related drug eruption and if progresses to erythema multiforme major, it and can be a harbinger of SCAR, such as SJS), lichenoid (resembling the flat-topped, polygonal, and sometimes scaly or hypertrophic lesions of lichen-planus), eczematous (inflammatory dermatitis characterized by pruritic, erythematous, scaly, or crusted papules or plaques on the skin, which is vulnerable to superinfection, psoriasiform [resembling the well-demarcated, erythematous, and scaly papules and plaques of psoriasis], morbilliform [a nonpustular, nonbullous measles-like exanthematous rash of the skin often referred to as "maculopapular" and without systemic symptoms or laboratory abnormalities, excluding occasional isolated peripheral eosinophilia, palmoplantar erythrodysesthesia [hand-foot syndrome; redness, numbness, burning, itching, and superficial desquamation of the palms and soles], neutrophilic dermatoses [eg, Sweet syndrome], and others) | |
| Diagnostic work-up | |
| Pertinent history and physical examination | |
| Rule out any other etiology of the skin problem, such as an infection, an effect of another drug, or a skin condition linked to another systemic disease or unrelated primary skin disorder | |
| If needed, a biologic checkup, including a blood cell count and liver and kidney tests | |
| Directed serologic studies if an autoimmune condition is suspected, such as lupus or dermatomyositis: a screening antinuclear antibody test, SS-A/Anti-Ro, SS-B/Anti-La if predominantly photodistributed/photosensitivity, antihistone, double-stranded DNA, and other relevant serologies. Consider expanding serologic studies or diagnostic work-up if other autoimmune conditions are considered based on signs, symptoms | |
| Skin biopsy | |
| Consider clinical monitoring with use of serial clinical photography | |
| Review full list of patient medications to rule out other drug-induced cause for photosensitivity | |
| Grading | Management |
| Grading according to CTCAE is a challenge for skin. Instead, severity may be based on BSA, tolerability, morbidity, and duration. | |
| G1: Symptoms do not affect the quality of life or controlled with topical regimen and/or oral antipruritic | Continue ICPi Treat with topical emollients and/or mild-moderate potency topical corticosteroids Counsel patients to avoid skin irritants and sun exposure |
| G2: Inflammatory reaction that affects quality of life and requires intervention based on diagnosis | Consider holding ICPi and monitor weekly for improvement. If not resolved, interrupt treatment until skin AE has reverted to grade 1 Consider initiating prednisone (or equivalent) at dosing 1 mg/kg, tapering over at least 4 weeks In addition, treat with topical emollients, oral antihistamines, and medium- to high-potency topical corticosteroids |
| G3: As G2 but with failure to respond to indicated interventions for a G 2 dermatitis | Hold ICPi therapy and consult with dermatology to determine appropriateness of resuming Treat with topical emollients, oral antihistamines, and high-potency topical corticosteroids Initiate (methyl)prednisolone (or equivalent) 1–2 mg/kg, tapering over at least 4 weeks |
| G4: All severe rashes unmanageable with prior interventions and intolerable | Immediately hold ICPi and consult dermatology to determine appropriateness of resuming ICPi therapy upon resolution of skin toxicity and once corticosteroids are reduced to prednisone (or equivalent) ≤ 10 mg Systemic corticosteroids: IV (methyl)prednisolone (or equivalent) dosed at 1–2 mg/kg with slow tapering when the toxicity resolves Monitor closely for progression to severe cutaneous adverse reaction Should admit patient immediately with direct oncology involvement and with an urgent consult by dermatology Consider alternative antineoplastic therapy over resuming ICPis if the skin irAE does not resolve to G1 or less; if ICPIs are the patient's only option, consider restarting once these adverse effects have resolved to a G1 level |
| 1.2 Bullous dermatoses | |
| Definition: Including bullous pemphigoid or other autoimmune bullous dermatoses, bullous drug reaction | |
| Diagnostic work-up | |
| Physical examination | |
| Rule out any other etiology of the skin problem, such as an infection, an effect of another drug, or a skin condition linked to another systemic disease | |
| If needed, a biologic checkup, including a blood cell count, liver, and kidney tests; consider serum antibody tests to rule out bullous pemphigoid or, under the guidance of dermatology, sending patient serum for indirect immunofluorescent testing to rule out other autoimmune blistering diseases | |
| Referral to dermatology for blisters that are not explained by infectious or transient other causes (eg, herpes simplex, herpes zoster, bullous impetigo, bullous insect bite, friction or pressure blister) | |
| Consider skin biopsy (both hematoxylin and eosin evaluation of lesional skin and direct immunofluorescence evaluation of perilesional skin) | |
| Grading | Management |
| G1: Asymptomatic, blisters covering < 10% BSA and no associated erythema | If blisters are < 10% BSA, asymptomatic, and noninflammatory (such as the case with friction blisters or pressure blisters), cessation of ICPi is not necessary, and only observation and/or local wound care is warranted. When symptomatic bullae or erosions, which are deroofed vesicles or bullae, are observed on the skin or mucosal surfaces, the cutaneous irAE is by definition considered at least G2 See G2 management recommendations |
| G2: Blistering that affects quality of life and requires intervention based on diagnosis not meeting criteria for grade > 2 Blisters covering Ί0%-30% BSA |
Hold ICPi therapy and consult with dermatology for work-up and to determine appropriateness of resuming Attention given to general local wound care, which includes plain petrolatum ointment and bandages or plain petrolatum ointment gauze and bandage over any open erosions, which are left over on the skin after the blister has popped or if the roof of the blister easily sloughs off Counsel patients to avoid skin irritants and overexposure to sun, wear protective clothing, use sunscreens Work-up for autoimmune bullous disease as above Initiate class 1 high-potency topical corticosteroid (eg, clobetasol, betamethasone or equivalent) and reassess every 3 days for progression or improvement Low threshold to initiate treatment with prednisone (or equivalent) at 0.5–1 mg/kg dosing and taper over at least 4 weeks Monitor patients with G2 irAEs closely for progression to involvement of greater BSA and/or mucous membrane involvement. Consider following patients closely using serial photography Primer on monitoring for complicated cutaneous adverse drug reactions: • Review of systems: Skin pain (like a sunburn), fevers, malaise, myalgias, arthralgias, abdominal pain, ocular discomfort or photophobia, sores or discomfort in the nares, sores or discomfort in the oropharynx, odynophagia, hoarseness, dysuria, sores or discomfort in the vaginal area for women or involving the meatus of the penis for men, sores in the perianal area, or pain with bowel movements • Physical examination: Include vital signs and a full skin examination specifically evaluating all skin surfaces and mucous membranes (eyes, nares, oropharynx, genitals, and perianal area). Assess for lymphadenopathy, facial or distal extremity swelling (may besigns of DIHS/DRESS). Assess for pustules orblisters or erosions in addition to areas of “dusky erythema," which may feel painful to palpation. To assess for a positive Nikolsky sign, place a gloved finger tangentially over erythematous skin and apply friction parallel to the skin surface. Nikolsky sign is positive if this results in detached or sloughing epidermis demonstrating poor attachment of the epidermis to the dermis, which is the case in some autoimmune disorders (eg, pemphigus) and SJS/TEN |
| G3: Skin sloughing covering > 30% BSA with associated pain and limiting self-care ADL | Hold ICPi therapy and consult with dermatology to determine appropriateness of resuming Administer IV(methyl)prednisolone (or equivalent) 1–2 mg/kg, tapering over at least 4 weeks If bullous pemphigoid is diagnosed, it may be possible to avoid long-term use of systemic corticosteroids and treat with rituximab, as an alternative approach to treating the irAE Seek infectious disease consultation if patient might have secondary cellulitis or if patient has other infection risk factors, such as neutropenia, etc. |
| G4: Blisters covering > 30% BSA with associated fluid or electrolyte abnormalities | Permanently discontinue ICPi Admit patient immediately and place under supervision of a dermatologist Administer IV (methyl)prednisolone (or equivalent) 1–2 mg/kg with tapering over at least 4 weeks when the toxicity resolves If bullous pemphigoid is diagnosed, it may be possible to avoid long-term use of systemic corticosteroids and treat with rituximab as an alternative approach to treating the irAE Seek infectious disease consultation if patient might have secondary cellulitis or if patient has other infection risk factors, such as neutropenia, etc |
| 1.3 SCARs, including SJS, TEN, acute generalized exanthematous pustulosis, and DRESS/DIHS | |
| Definition: Severe changes in either structure or functions of skin, the appendages or the mucous membranes due to a drug | |
| Diagnostic work-up | |
| Total body skin examination with attention to examining all mucous membranes as well as complete review of systems | |
| Rule out any other etiology of the skin problem, such as an infection, an effect of another drug, or a skin condition linked to another systemic disease | |
| A biologic checkup, including a CBC with differential test, and liver and kidney function tests, including urinalysis, in addition to the blood work; if the patient is febrile, blood cultures should be considered as well | |
| Skin biopsies to assess for full-thickness epidermal necrosis, as is seen in SJS/TEN, as well as other possible etiologies like paraneoplastic pemphigus or other autoimmune blistering dermatoses or other drug reactions, such as acute generalized exanthematous pusulosis | |
| Consider following patients closely using serial clinical photography | |
| If mucous membrane involvement or blistering is observed on the skin, consider early admission to a burn center for further monitoring and management | |
| Primer on monitoring for complicated cutaneous adverse drug reactions: | |
| Review of systems: Skin pain (like a sunburn), fevers, malaise, myalgias, arthralgias, abdominal pain, ocular discomfort or photophobia, sores or discomfort in the nares, sores or discomfort in the oropharynx, odynophagia, hoarseness, dysuria, sores or discomfort in the vaginal area for women or involving the meatus of the penis for men, sores in the perianal area, or pain with bowel movements | |
| Physical examination: Include vital signs and a full skin examination specifically evaluating all skin surfaces and mucous membranes (eyes, nares, oropharynx, genitals, and perianal area). Assess for lymphadenopathy, facial or distal extremity swelling (may be signs of DIHS/DRESS). Assess for pustules or blisters or erosions in addition to areas of “dusky erythema”, which may feel painful to palpation. To assess for a positive Nikolsky sign, place a gloved finger tangentially over erythematous skin and apply friction parallel to the skin surface. Nikolsky sign is positive if this results in detached or sloughing epidermis demonstrating poor attachment of the epidermis to the dermis, which is the case in some autoimmune disorders (eg, pemphigus) and SJS/TEN | |
| Grading | Management |
| All grades | In cases of suspected SJS or any mucous membrane involvement, discontinue ICPi treatment and monitor closely for improvement, regardless of grade |
| G1: NA | For SCARs, there isnoG1 category; if lower BSA is involved with bullae or erosions, there should remain a high concern that this reaction will progress to G3 or G4 |
| G2: Morbilliform (“maculopapular”) exanthem covering 10%– 30% BSA with systemic symptoms, lymphadenopathy, or facial swelling | Hold ICPi and monitor patients closely every 3 days with G2 irAEs for progression to involvement of greater BSA and/or mucous membrane involvement Consider following patients closely using serial photography Initiate therapy with topical emollients, oral antihistamines, and medium- to high- strength topical corticosteroids Consider initiation of prednisone (or equivalent) 0.5–1 mg/kg tapered over at least 4 weeks |
| G3: Skin sloughing covering < 10% BSA with mucosal involvement associated signs (eg, erythema, purpura, epidermal detachment, mucous membrane detachment) | Hold ICPi therapy and consult with dermatology Treat skin with topical emollients and other petrolatum emollients, oral antihistamines, and high-strength topical corticosteroids; dimethicone may also be offered as an alternative to petrolatum Administer IV (methyl)prednisolone (orequivalent) 0.5–1 mg/kg and convert to oral corticosteroids on response, wean over at least 4 weeks Admit to burn and/or consult wound services with attention to supportive care, including fluid and electrolyte balance, minimizing insensible water losses, and preventing infection Given the immune mechanism of action of these medicines, use of immune suppression is warranted and should be offered For mucous membrane involvement of SJS orTEN, appropriateconsulting services should be offered to guide management in preventing sequelae from scarring (eg, ophthalmology; ear, nose, and throat; urology; gynecology; etc, as appropriate) |
| G4: Skin erythema and blistering/sloughing covering ≥ 10% BSA with associated signs (eg, erythema, purpura, epidermal detachment, mucous membrane detachment) and/or systemic symptoms and concerning associated blood workabnormalities(eg, liverfunctiontestelevations in the setting of DRESS/DIHS) | Permanently discontinue ICPi Admit patient immediately to a burn unit or ICU with consulted dermatology and wound care services Consider further consultations based on management of mucosal surfaces (eg, ophthalmology; urology; gynecology; ear, nose, and throat surgery; etc) Initiate IV (methyl)prednisolone (or equivalent) 1–2 mg/kg, tapering when toxicity resolves to normal IVIG or cyclosporine may also be considered in severe or corticosteroid- unresponsive cases Consider pain/palliative consultation and/or admission in patients presenting with DRESS manifestations |
| Additional considerations: The usual prohibition of corticosteroids for SJS is not relevant here, as the underlying mechanism is a T-cell immunodirected toxicity. Adequate suppression is necessary with corticosteroids or other agents and may be prolonged in cases of DRESS/DIHS | |
| All recommendations are expert consensus based, with benefits outweighing harms, and strength of recommendations are moderate | |
Abbreviations: ADL, activities of daily living; BSA, body surface area; CTCAE, Common Terminology Criteria for Adverse Events; DIHS, drug-induced hypersensitivity syndrome; DRESS, drug reaction with eosinophilia and systemic symptoms; G, grade; ICPi, immune checkpoint inhibitor; ICU, intensive care unit; irAE, immune-related adverse event; IV, intravenous; IVIG, intravenous immunoglobulin; NA, not applicable; SCAR, severe cutaneous adverse reactions; SJS, Stevens-Johnson syndrome; TENS, toxic epidermal necrolysis.
1.1. RASH/INFLAMMATORY DERMATITIS
Recommendation 1.1a – Diagnostic Work-up.
It is recommended that for all grades, the diagnostic work-up should include the following:
Pertinent history and physical examination.
Rule out any other etiology of the skin problem, such as an infection, an effect of another drug, or a skin condition linked to another systemic disease or unrelated primary skin disorder.
A biologic checkup, including a blood cell count, liver, and kidney tests, may be performed if needed.
Directed serologic studies if an autoimmune condition is suspected, such as lupus or dermatomyositis: a screening antinuclear antibody (ANA) test, SS-A/Anti-Ro, and SS-B/Anti-La if the rash is predominantly photodistributed or demonstrating photosensitivity. Consider expanding serologic studies or diagnostic work-up if other autoimmune conditions are considered.
Skin biopsy, clinical photography may be performed when indicated.
Review full list of patient medications to rule out other drug- induced cause for photosensitivity.
Recommendation 1.1b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue to offer ICPi.
Should treat skin with topical emollients (if predominately dry skin is observed) and/or mild to moderate potency (hydrocortisone 2.5% or equivalent to triamcinolone 0.1% or equivalent) topical corticosteroids (signs of inflammation/redness with or without itching).
Should counsel patients to avoid skin irritants and sun exposure. It is recommended that clinicians manage grade 2 toxicities, including intermittent pruritus, as follows:
May hold ICPi and monitor weekly for improvement. If not resolved, interrupt treatment until skin AE has reverted to grade 1 or less and consider dermatology referral.
Should treat skin with topical emollients, oral antihistamines, and medium- to high-potency topical corticosteroids.
In addition, consider initiating prednisone (or equivalent) at dosing 1 mg/kg tapering over at least 4 weeks, depending on primary skin lesions observed on examination.
It is recommended that clinicians manage grade 3 toxicities, including constant pruritus, as follows:
Should hold ICPi therapy and consult with dermatology, if available, to determine appropriateness of resuming.
Should treat skin with topical emollients, oral antihistamines, and high-potency topical corticosteroids.
Initiate intravenously (IV) (methyl)prednisolone (or equivalent) dosed at 1 to 2 mg/kg and taper over at least 4 weeks.
If not resolved, refer to dermatology.
It is recommended that clinicians manage grade 4 toxicities as follows:
Should immediately hold ICPi and consult dermatology to determine appropriateness of resuming ICPi therapy upon resolution of skin toxicity and once corticosteroids are reduced to prednisone (or equivalent) 10 mg or less.
Should administer IV (methyl)prednisolone (or equivalent) dosed at 1 to 2 mg/kg, with slow tapering when the toxicity resolves.
Should monitor closely for progression to severe cutaneous adverse reaction (SCAR).
Should admit patient immediately with direct oncology involvement and with an urgent consult by dermatology.
Consider alternative antineoplastic therapy over resuming ICPis if the skin irAE does not resolve to grade 1 or less. If ICPis are the patient’s only option, consider restarting once these adverse effects have resolved to a grade 1 level.
1.2. Bullous Dermatoses
Recommendation 1.2a – Diagnostic Work-up.
It is recommended that for all grades of irAEs the diagnostic work-up should include the following:
Comprehensive physical examination, including evaluation of all mucous membranes.
Rule out any other etiology of the skin problem, such as an infection, an effect of another drug, or a skin condition linked to another systemic disease or unrelated primary skin disorder.
If needed, a biologic checkup may be performed, including a blood cell count, liver and kidney tests, hepatitis antibody tests, and tuberculosis (TB) testing.
Referral to dermatology for blisters that are not explained by infectious/transient other causes (eg, herpes simplex, herpes zoster infections, pressure/friction bullae).
Skin biopsy (lesional biopsy of inflamed skin or the edge of a bulla or vesicle) for hematoxylin and eosin histology and biopsy of a perilesional or “near-inflamed” area for direct immunofluorescence testing.
If the biopsy demonstrates a subepidermal blister and/or the direct immunofluorescence testing is suspicious or positive for a diagnosis of bullous pemphigoid (BP), or in cases where skin biopsies are not possible, consider serum testing to further evaluate tense bullae (BP 230 and BP 130 enzyme-linked immunosorbent assay serum testing). If negative, under the guidance of dermatology, sending the patient serum for indirect immunofluorescent testing to rule out other autoimmune blistering diseases could be considered.
Recommendation 1.2b – Management.
If blisters are < 10% body surface area (BSA), asymptomatic, and noninflammatory (eg, the case with friction blisters or pressure blisters), cessation of ICPi is not necessary, and only observation and/or local wound care is warranted. Once a blister or erosion, which is essentially a deroofed blister, is observed on examination, with associated erythema or symptoms, the reaction should be considered due to ICPi therapy and graded at 2 or above. It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi therapy and consult with dermatology (or skin care team, which may include general surgeon) to determine appropriateness of resuming ICPi and initiate general local skin/wound care, which includes plain petrolatum ointment and bandages or plain petrolatum ointment gauze and bandage over any open erosions that are left over on the skin after the blister has popped or if the roof of the blister easily sloughs off.
Should counsel patients to avoid skin irritants and overexposure to sun, wear protective clothing, and use sunscreens.
Should order work-up for autoimmune bullous disease as above.
Initiate class 1 high-potency topical corticosteroid (eg, clobetasol, betamethasone or equivalent) and reassess every 3 days for progression or improvement.
Lower threshold to initiate treatment with prednisone (or equivalent) at 0.5 to 1 mg/kg dosing and taper over at least 4 weeks.
Monitor patients with grade 2 irAEs closely for progression to involvement of greater BSA and/or mucous membrane involvement. Consider following patients closely using serial photography.
- Primer on monitoring for complicated cutaneous adverse drug reactions:
- Review of systems: skin pain (“like a sunburn”), fevers, malaise, myalgias, arthralgias, abdominal pain, ocular discomfort or photophobia, sores or discomfort in the nares, sores or discomfort in the oropharynx, odynophagia, hoarseness, dysuria, sores or discomfort in the vaginal area for women or involving the meatus of the penis for men, sores in the perianal area, or pain with bowel movements.
- Physical examination: include vital signs and a full skin examination, specifically evaluating all skin surfaces and mucous membranes (eyes, nares, oropharynx, genitals, and perianal area). Assess for lymphadenopathy, facial or distal extremity swelling (may be signs of drug-induced hypersensitivity syndrome [DIHS]/drug reaction with eosinophilia and systemic symptoms [DRESS]). Assess for pustules or blisters or erosions in addition to areas of “dusky erythema,” which may feel painful to palpation. To assess for a positive Nikolsky sign, place a gloved finger tangentially over erythematous skin and apply friction parallel to the skin surface. Nikolsky sign is positive if this results in detached or sloughing epidermis, demonstrating poor attachment of the epidermis to the dermis, which is the case in some autoimmune disorders (eg, pemphigus) and Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN).
It is recommended that clinicians manage grade 3 toxicities as follows:
Should hold ICPi therapy and consult with dermatology to determine appropriateness of resuming.
Should administer IV (methyl)prednisolone (or equivalent) at 1 to 2 mg/kg dosing tapered over at least 4 weeks.
If BP is diagnosed, it maybe possible to avoid long-term use of systemic corticosteroids and treat with rituximab as an alternative approach to treating the irAE.
Seek infectious disease consultation if patient might have secondary cellulitis or if patient has other infection risk factors, such as neutropenia, etc.
It is recommended that clinicians manage grade 4 toxicities as follows:
Should permanently discontinue ICPi.
Should admit patient immediately and place under supervision of a dermatologist.
Should administer IV (methyl)prednisolone (or equivalent) 1 to 2 mg/kg/d. When toxicity improves to grade 2 or less, start corticosteroid taper. Taper should be at least 4 weeks.
If BP is diagnosed, it maybe possible to avoid long-term use of systemic corticosteroids and treat with rituximab as an alternative approach to treating the irAE.
Seek infectious disease consultation if patient might have secondary cellulitis or if patient has other infection risk factors, such as neutropenia, etc.
1.3. Severe Cutaneous Adverse Reactions
Recommendation 1.3a - Diagnostic work-up.
Severe cutaneous adverse reactions, or SCARs, include, but are not limited to, SJS/TEN and DRESS (also called DIHS).
It is recommended that for all grades of irAEs, the diagnostic work-up should include the following:
Total body skin examination with attention to ALL mucous membranes as well as a complete review of systems.
Rule out any other etiology of the skin problem, such as an infection, an effect of another drug, or a skin condition linked to another systemic disease.
A biologic checkup, including a CBC with differential test, and liver and kidney function tests, including urinalysis, in addition to the blood work. If the patient is febrile, blood cultures should be considered as well.
Skin biopsies to assess for full-thickness epidermal necrosis, as is seen in SJS/TEN, as well as other possible etiologies like paraneoplastic pemphigus or other autoimmune blistering dermatoses, or other drug reactions, such as acute generalized exanthematous pusulosis.
Consider following patients closely using serial clinical photography.
If mucous membrane involvement or blistering is observed on the skin, consider early admission to a burn center for further monitoring and management.
- Primer on monitoring for complicated cutaneous adverse drug reactions:
- Review of systems: skin pain (“like a sunburn”), fevers, malaise, myalgias, arthralgias, abdominal pain, ocular discomfort or photophobia, sores or discomfort in the nares, sores or discomfort in the oropharynx, odynophagia, hoarseness, dysuria, sores or discomfort in the vaginal area for women or involving the meatus of the penis for men, sores in the perianal area, or pain with bowel movements.
- Physical examination: include vital signs and a full skin examination specifically evaluating all skin surfaces and mucous membranes (eyes, nares, oropharynx, genitals, and perianal area). Assess for lymphadenopathy, facial or distal extremity swelling (may be signs of DIHS/DRESS). Assess for pustules or blisters or erosions in addition to areas of dusky erythema, which may feel painful to palpation. To assess for a positive Nikolsky sign, place a gloved finger tangentially over erythematous skin and apply friction parallel to the skin surface. Nikolsky sign is positive if this results in detached or sloughing epidermis, demonstrating poor attachment of the epidermis to the dermis, which is the case in some autoimmune disorders (eg, pemphigus) and SJS/TEN.
Recommendation 1.3b – Management.
In cases of suspected SJS or any mucous membrane involvement, it is recommended that clinicians should discontinue ICPi treatment and refer to dermatology. It would not be advisable to restart ICPi unless “cleared” by a dermatologist if SJS/TEN is suspected.
For SCARs, there is no grade 1 category. If lower BSA is involved with bullae or erosions, there should remain a high concern that this reaction will progress to grade 3 or 4.
It is recommended that clinicians manage grade 2 toxicities as follows:
Hold ICPi and monitor patients closely every 3 days with grade 2 irAEs for progression to involvement of greater BSA and/or mucous membrane involvement.
Consider following patients closely by using serial photography.
Initiate therapy with topical emollients, oral antihistamines, and medium- to high-strength topical corticosteroids.
Consider initiation of prednisone or equivalent at 0.5 to 1 mg/kg tapered over at least 4 weeks.
It is recommended that clinicians manage grade 3 toxicities as follows:
Should hold ICPi therapy and consult with dermatology.
Should treat skin with topical emollients and other petrolatum emollients, oral antihistamines, and high-strength topical corticosteroids. Dimethicone may also be offered as an alternative to petrolatum.
Administer IV (methyl)prednisolone (or equivalent) at doses of 1 to 2 mg/kg and taper over at least 4 weeks.
Admit to burn and/or consult wound services with attention to supportive care, including fluid and electrolyte balance, minimizing insensible water losses, and preventing infection.
Given the immune mechanism of action of these medicines, use of immune suppression, such as with systemic corticosteroids, is warranted and should be offered, though the use of systemic corticosteroids has been more controversial for the treatment of SJS/TEN, in general. For DRESS/DIHS, high-dose and usually prolonged courses of systemic corticosteroids is first-line therapy following cessation of the offending drug.
For mucous membrane involvement of SJS or TEN, appropriate consulting services should be offered to guide management in preventing sequelae from scarring (eg, ophthalmology; ear, nose, and throat; urology; gynecology; etc, as appropriate).
Seek infectious disease consultation if patient might have secondary cellulitis or if patient has other infection risk factors, such as neutropenia, etc.
It is recommended that clinicians manage grade 4 toxicities as follows:
Should permanently discontinue ICPi.
Should admit patient immediately with consideration to a burn unit or ICU in the case of SJS/TEN and consult dermatology.
Administer IV (methyl)prednisolone or equivalent 1 to 2 mg/kg with tapering when the toxicity resolves to normal.
May consider IV immunoglobulin (IVIG) or cyclosporine as an alternative or in corticosteroid-refractory cases.
Seek infectious disease consultation if patient might have secondary cellulitis or if patient has other infection risk factors, such as neutropenia, etc.
Qualifying statement.
The usual prohibition of corticosteroids for SJS is not relevant here, as the underlying mechanism is a T-cell immunodirected toxicity. Adequate suppression is necessary with corticosteroids or other agents and may be prolonged in cases of DRESS/DIHS. Additionally, patients with DRESS/DIHS may experience other autoimmune diseases as long-term sequelae, such as thyroid disease and recurrences as systemic corticosteroids are tapered or discontinued are not uncommon. Thus, continued monitoring should be considered for these patients. It is generally advisable to avoid rechallenge with an offending drug when a patient experiences SCARs, such as SJS/TEN or DRESS/DIHS. It is advisable to consider alternate antineoplastic therapies because rechallenge in these cases may result in an even more severe SCAR.
Discussion.
The dramatic and durable responses seen with ICPis are often at the cost of increased toxicities due to unrestrained activity of T cells.4 Among the diverse irAEs, cutaneous toxicities such as rash, pruritus, and vitiligo are by far the most common and the earliest to occur.5 Although most cutaneous toxicities are transient, they can cause significant morbidity and impairment of patients’ health-related quality of life.
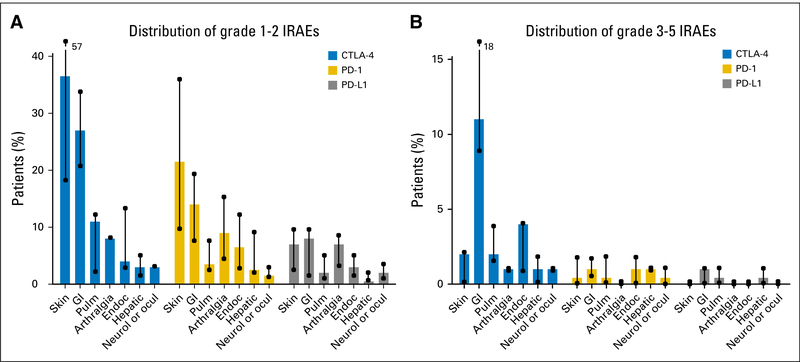
Cutaneous toxicities are reported in 30% to 50% of patients treated with ICPis.6 Our understanding of cutaneous toxicities stems mostly from the ipilimumab experience wherein the overall incidence ranges from 37% to 70% for all-grade and 1% to 3% for grade 3 or higher cutaneous toxicities.7,8 Cutaneous toxicities are less frequently reported with anti–PD-1 agents (17% to 37%); however, the incidence of grade 3 or higher toxicities is the same as with ipilimumab.
Cutaneous toxicities pose a myriad of challenges. Rash is the most common cutaneous toxicity reported with ICPis. They span a variety of inflammatory conditions, including spongiotic, psoriasiform, and lichenoid dermatitides, mimicking eczema, psoriasis, and lichen planus, respectively. The clinical presentations vary with focal to diffuse distributions, including flexural, inverse, and erythrodermic variants. Pruritus can be severe and is the most common associated symptom. Vitiligo presents as well-demarcated depigmented macules and patches, reported exclusively in patients with melanoma. Besides varying clinical presentation, the time to onset varies greatly among these rashes, as vitiligo can appear months after treatment initiation; however, the inflammatory dermatoses usually present within the first one to two cycles of treatment. This mandates constant vigilance for signs and symptoms of cutaneous toxicities. In addition, these irAEs are increasingly recognized as a contributing factor to treatment noncompliance, discontinuation, or dose modification. As targeted systemic therapies are available for eczema and psoriasis, correlating the inflammatory patterns of the cutaneous toxicities with the inflammatory patterns that they mimic may lead to more efficacious treatments, fewer drug interruptions and dose modifications, and increased compliance and efficacy of the immune ICPis. However, classification of rashes has not been undertaken prospectively, and histologic characterization of the cutaneous toxicities is lacking.
Interestingly, emerging data suggest that development of cutaneous toxicity, especially rash and vitiligo, may correlate with response to ICPi therapy in patients with metastatic melanoma. In a retrospective analysis of 148 patients with melanoma treated with nivolumab plus peptide vaccine or nivolumab, survival benefit was reported in patients who developed rash (n = 64) or vitiligo (n = 19).9 Overall survival (OS) was significantly longer in patients who developed rash (hazard ratio [HR], 0.423; 95% CI, 0.243 to 0.735; P = .001) and vitiligo (HR, 0.184; 95% CI, 0.036 to 0.94; P = .012). Objective response rate (ORR) was also significantly higher in patients with rash (P = .03) or vitiligo (P = .009). In a prospective study evaluating pembrolizumab in treatment of patients with melanoma, ORR was higher in patients who developed vitiligo than in those who did not (71% v 28%; P = .002).10 Similarly, in a phase I study of ipilimumab for patients with melanoma, rash was the most common irAE reported among responders.11 Furthermore, in a meta-analysis of 27 studies12 in patients with melanoma treated with various immunotherapeutic agents, vitiligo was significantly associated with both progression-free survival (HR, 0.51; 95% CI, 0.32 to 0.82; P = .005) and OS (HR, 0.25; 95% CI, 0.10 to 0.61; P = .003). These findings from large clinical development programs suggest that cutaneous irAEs may be a surrogate for clinical benefit, and it would be important to correctly identify these skin changes so that the ICPi therapy is not discontinued in these cases with good prognoses. In addition, many cutaneous toxicities may be managed without the discontinuation of therapy. With early diagnosis and prompt management of cutaneous toxicities, patients may be able to stay on ICPi therapy, which could be crucial for improved treatment outcomes. However, little is known about the underlying mechanisms and the relationship between cutaneous toxicity and clinical outcome in patients with advanced cancer other than melanoma. This lack of knowledge presents challenges for prompt diagnosis and hampers the development of strategies to mitigate or minimize the occurrence of cutaneous toxicities in patients treated with ICPis. With increasing use of ICPis in the clinic, characterization and development of sensitive and robust markers of cutaneous toxicity is a priority.
2.0. GI Toxicities
Please refer to Table 2, for a complete set of recommendations, definition of grades, and additional considerations.
Table 2.
Management of GI irAEs in Patients Treated With ICPis
| 2.0 GI Toxicities | |
| 2.1 Colitis | |
| Definition: A disorder characterized by inflammation of the colon | |
| Diagnostic work-up | |
| G2 | |
| Work-up of blood (CBC, comprehensive metabolic panel, TSH, ESR, CRP), stool (culture, Clostridium difficile, parasite, CMV or other viral etiology, ova and parasite) should be performed | |
| Consider testing for lactoferrin (for patient stratification to determine who needs more urgent endoscopy) and calprotectin (to follow up on disease activity) | |
| Screening laboratories (HIV, hepatitisAand B, and blood quantiferon forTB) to prepare patients to start infliximab should be routinelydone in patients at high risk for those infections and appropriately selected patients based on infectious disease expert's evaluation | |
| Imaging (eg, CT scan ofabdomen and pelvis and GIendoscopywith biopsy) should be considered as there is evidence showing thatthe presence of ulceration in the colon can predict a corticosteroid-refractory course, which may require early infliximab | |
| Consider repeating endoscopy for patients who do not respond to immunosuppressive agents; repeating endoscopy for disease monitoring can be considered when clinically indicated and when planning to resume therapy | |
| G3–4 | |
| All the work-up listed for G2 (blood, stool, imaging, and scope with biopsy) should be completed immediately | |
| Consider repeating endoscopy for patients who do not respond to immunosuppressive agents; repeating endoscopy for disease monitoring should only be considered when clinically indicated and when planning to resume ICPi | |
| Grading (based on CTCAE for diarrhea, as most often used clinically) | Management |
| All patients | Counsel all patients to be aware of and inform their health care provider immediately if they experience: Abdominal pain, nausea, cramping, blood or mucus in stool or changes in bowel habits Fever, abdominal distention, obstipation, constipation For G2 or higher, consider permanently discontinuing CTLA-4 agents and may restart PD-1, PD- L1 agents if patient can recover to G1 or less; concurrent immunosuppressant maintenance therapy should be considered only if clinically indicated in individual cases |
| G1: Increaseoffewerthan fourstools perdayover baseline; mild increase in ostomy output compared with baseline | Continue ICPi; alternatively, ICPi may be held temporarilyand resumed iftoxicitydoes notexceed G1 Monitor for dehydration and recommend dietary changes Facilitate expedited phone contact with patient/caregiver May obtain gastroenterology consult for prolonged G1 cases |
| G2: Increase offour to six stools perday over baseline; moderate increase in ostomy output compared with baseline | Should hold ICPi temporarily until patient’s symptoms recover to G1; can consider permanently discontinuing CTLA-4 agents and may restart PD-1, PD-L1 agents if patientcan recovertoG1 or less Concurrent immunosuppressant maintenance therapy (< 10 mg prednisone equivalent dose) may be offered only if clinically indicated in individual cases Mayalso include supportive care with medications such as Imodium if infection has been ruled out Should consult with gastroenterology for G2 or higher Administer corticosteroids, unless diarrhea is transient, starting with initial dose of 1 mg/kg/day prednisone or equivalent When symptoms improve to G1 or less, taper corticosteroids over at least 4–6 weeks before resuming treatment, although resuming treatment while on low-dose corticosteroid may also be an option after an evaluation of the risks and benefits >EGD/colonoscopy, endoscopy evaluation should be highly recommended for cases grade ≥ 2 to stratify patients for early treatment with infliximab based on the endoscopic findings and to determine the safety of resuming PD-1, PD-L1 therapy Stool inflammatory markers can be considered (lactoferrin and calprotectin) in cases of G2 or higher to differentiate functional v inflammatory diarrhea, and use calprotectin to monitor treatment response if provider prefers Repeat colonoscopy is optional for cases of G2 or higher for disease activity monitoring to achieve complete remission, especially if there is a plan to resume ICPi |
| G3: Increase of seven or more stools per day over baseline, incontinence, hospitalization indicated, severe increase in ostomy output compared with baseline, limiting self-care ADL | Should consider permanently discontinuing CTLA-4 agents and may restart PD-1, PD-L1 agents if patient can recover to G1 or less. Administer corticosteroids (initial dose of 1–2 mg/kg/d prednisone or equivalent) Consider hospitalization or outpatient facility for patients with dehydration or electrolyte imbalance If symptoms persist ≥ 3–5 days or recur after improvement, consider administering IV corticosteroid or noncorticosteroid (eg, infliximab) Consider colonoscopy in cases where patients have been on immunosuppression and may be at risk for opportunistic infections as an independent cause for diarrhea (ie, CMV colitis) and for those who are anti-TNF or corticosteroid refractory |
| G4: Life-threatening consequences; urgent intervention indicated | Permanently discontinue treatment Should admit patientwhen clinically indicated; patients managed as outpatients should bevery closely monitored Administer 1–2 mg/kg/d methylprednisolone or equivalent until symptoms improve to G1, and then start taper over 4–6 weeks Consider early infliximab 5–10 mg/kg if symptoms refractory to corticosteroid within 2–3 days Consider lowerGI endoscopy if symptoms are refractory despite treatment or there is concern of new infections |
| Additional considerations | |
| The use of vedolizumab may be considered in patients refractory to infliximab and/or contraindicated to TNF-α blocker. The decision should be made on an individual basis from gastroenterology and oncology evaluation. This is based on case series showing promising results13–15 | |
| Patients with hepatitis and irAE colitis are rare, and management should include permanently discontinuing ICPi and offering other immunosuppressant agents that work systemically for both conditions | |
| Currently, enteritis alone as the cause of diarrhea is uncommon and requires small bowel biopsy as the evaluation tool. It may be managed similar as colitis, including corticosteroid and/or infliximab, etc | |
| 2.2 Hepatitis | |
| Definition: A disorder characterized by a viral pathologic process involving the liver parenchyma | |
| Diagnostic work-up | |
| Monitor patient for abnormal liver blood tests: AST, ALT, and bilirubin prior to each infusion and/or weekly if G1 liver function test elevations. No treatment is recommended for G1 liver function test abnormality | |
| For G2 or higher: | |
| Work-up for other causes of elevated liver enzymes should be tested, viral hepatitis, alcohol history, iron study, thromboembolic event, liver ultrasound, crosssectional imaging for potential liver metastasis from primary malignancy. If suspicion for primary autoimmune hepatitis is high, can consider ANAs, antismooth muscle antibodies, antineutrophil cytoplasmic antibodies. If patients with elevated alkaline phosphatase alone, γ-glutamyl transferase should be tested. For isolated elevation of transaminases, consider checking CK for other etiologies | |
| Grading | Management |
| All patients | Counsel all patients to be aware of and inform their health care provider immediately if they experience: Yellowing of skin or whites of the eyes Severe nausea or vomiting Pain on the right side of the abdomen Drowsiness Dark urine (tea colored) Bleeding or bruising more easily than normal Feeling less hungry than usual |
| G1: Asymptomatic (AST or ALT > ULN to 3.0 × ULN and/or total bilirubin > ULN to 1.5 × ULN) | Continue ICPi with close monitoring; consider alternate etiologies Monitor laboratories one to two times weekly Manage with supportive care for symptom control |
| G2: Asymptomatic (AST or ALT > 3.0 to ≤ 5 × ULN and/or total bilirubin > 1.5 to ≤ 3 × ULN) | Hold ICPi temporarily and resume if recover to G1 or less on prednisone ≤ 10 mg/d For grade 2 hepatic toxicity with symptoms, may administer corticosteroid 0.5–1 mg/kg/d prednisone or equivalent if the abnormal elevation persists with significant clinical symptoms in 3–5 days Increase frequency of monitoring to every 3 days Infliximab might not be the most appropriate treatment option in the situation of immune-mediated hepatitis given the potential risk of idiosyncratic liver failure (Note: No clear evidence shows the liver toxicity from infliximab from other studies) In follow-up, may resume ICPi treatment followed by taper only when symptoms improve to G1 or less and corticosteroid ≤ 10 mg/d; taper over at least 1 month Patients should be advised to stop unnecessary medications and any known hepatotoxic drugs |
| G3: Symptomatic liver dysfunction, fibrosis by biopsy, compensated cirrhosis, reactivation of chronic hepatitis (AST or ALT 5–20 × ULN and/or total bilirubin 3–10 × ULN) | Permanently discontinue ICPi Immediately start corticosteroid 1–2 mg/kg methylprednisolone or equivalent If corticosteroid refractory or no improvement after 3 days, consider mycophenolate mofetil or azathioprine (if using azathioprine should test for thiopurine methyltransferase deficiency) Laboratories at daily or every other day; consider inpatient monitoring for patients with AST/ALT > 8 × ULN and/or elevated TB 3 × ULN Increase frequency of monitoring to every 1–2 days Infliximab might not be the most appropriate treatment option in the situation of immune-mediated hepatitis given the potential risk of liver failure (Note: No clear evidence shows that the liver toxicity from infliximab from other studies); alternatives include non–TNF-α agents as systemic immunosuppressants If no improvement is achieved with corticosteroids or for patients on combination therapy with a novel agent, with standard chemotherapy, or with targeted therapy, refer to hepatologist for further pathologic evaluation of hepatitis Corticosteroid taper can be attempted around 4–6 weeks; re-escalate if needed; optimal duration unclear |
| G4: Decompensated liverfunction (eg, ascites, coagulopathy, encephalopathy, coma; AST orALT > 20 × ULN and/or total bilirubin > 10 × ULN) | Permanently discontinue ICPi Administer 2 mg/kg/d methylprednisolone equivalents If corticosteroid refractory or no improvement after 3 days, consider mycophenolate mofetil Monitor laboratories daily; consider inpatient monitoring Avoid the use of infliximab in the situation of immune-mediated hepatitis Hepatology consult if no improvement was achieved with corticosteroid Corticosteroid taper can be attempted around 4–6 weeks when symptoms improve to G1 or less; re-escalate if needed; optimal duration unclear Consider transfer to tertiary care facility if necessary |
| All recommendations are expert consensus based, with benefits outweighing harms, and strength of recommendations is moderate. | |
Abbreviations: ADL, activities of daily living; ANA, antinuclearantibody; CK, creatine kinase; CMV, cytomegalovirus; CRP, C-reactive protein; CT, computed tomography; CTCAE, Common Terminology Criteria for Adverse Events; CTLA-4, cytotoxicT-cell lymphocyte-4; EGD, esophagogastroduodenoscopy; ESR, erythrocyte sedimentation rate; G, grade; ICPi, immune checkpoint inhibitor; irAE, immune-related adverse event; IV, intravenous; PD-1; programmed death 1; PD-L1, programmed death ligand 1; TB, tuberculosis; TNF, tumor necrosis factor; TSH, thyroid-stimulating hormone; ULN, upper limit of normal.
2.1. Colitis
Recommendation 2.1a – Diagnostic work-up.
No specific diagnostic work-up is recommended for grade 1 adverse events.
It is recommended that the diagnostic work-up should include the following for grade 2 toxicity:
Work-up of blood (CBC, comprehensive metabolic panel, thyroid-stimulating hormone [TSH], erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]), stool (culture, Clostridium difficile, parasite, cytomegalovirus [CMV] or other viral etiology, ova and parasite) should be performed.
May test for lactoferrin for patient stratification to determine who needs urgent endoscopy, and calprotectin may be offered to follow up on disease activity.
Screening laboratories (HIV, hepatitis A and B, and blood quantiferon for TB) to prepare patients to start infliximab should be routinely done in patients at high risk for those infections and in appropriately selected patients based on infectious disease expert’s evaluation.
Imaging with computed tomography (CT) scan of abdomen and pelvis and GI endoscopy with biopsy maybe performed as there is evidence showing that the presence of ulceration in the colon can predict a corticosteroid-refractory course, which may require early infliximab. Infliximab or other tumor necrosis factor (TNF)-blocking agent should not be delayed while awaiting the results of these screening tests.
Repeat endoscopy may be offered to patients who do not respond to immunosuppressive agents. Repeating endoscopy for disease monitoring should only be offered when clinically indicated and when planning to resume therapy.
It is recommended that the diagnostic work-up should include the following for grade 3 to 4 toxicity:
All the work-up listed for G2 (blood, stool, imaging, and scope with biopsy) should be completed immediately.
Repeat endoscopy may be offered for patients who do not respond to immunosuppressive agents. Repeating endoscopy for disease monitoring should only be offered when clinically indicated and when planning to resume ICPi.
Recommendation 2.1b – Management.
It is recommended that clinicians counsel all patients to be aware of and inform their health care provider immediately if they experience:
Abdominal pain, nausea, cramping, blood or mucus in stool, or changes in bowel habits
Fever, abdominal distention, obstipation, constipation
It is recommended that clinicians manage grade 1 toxicities as follows:
May continue ICPi. Alternatively, ICPi may be held temporarily and resumed if toxicity does not exceed grade 1.
Should monitor for dehydration and recommend dietary changes.
Should facilitate expedited phone contact with patient/caregiver.
May obtain gastroenterology consult for prolonged grade 1 cases.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold the ICPi until patient’s symptoms recover to grade 1 or less. Can consider permanently discontinuing CTLA-4 agents and may restart PD-1, PD-L1 agents if patient can recover to grade 1 or less.
Concurrent immunosuppressant maintenance therapy (< 10 mg prednisone equivalent dose) may be offered only if clinically indicated in individual cases.
May also include supportive care with medications such as Imodium if infection has been ruled out.
Should consult with gastroenterology for grade 2 or higher.
Should administer corticosteroids, unless diarrhea is transient, starting with an initial dose of 1 mg/kg/d prednisone or equivalent.
When symptoms improve to grade 1 or less, should taper corticosteroids over at least 4 to 6 weeks before resuming treatment, although resuming treatment while on low-dose corticosteroid may also be an option after an evaluation of the risks and benefits.
Should offer esophagogastroduodenoscopy/colonoscopy, endoscopy evaluation for cases of grade 2 or higher to stratify patients for early treatment with infliximab based on the endoscopic findings and to determine the safety of resuming PD-1, PD-L1 therapy.
Testing for stool inflammatory markers, lactoferrin, or calprotectin may be offered in cases of grade 2 or higher to differentiate functional versus inflammatory diarrhea. Calprotectin testing may also be offered to monitor treatment response.
Repeat colonoscopy is optional and may be offered for cases of grade 2 or higher for disease activity monitoring to achieve complete remission, especially if there is a plan to resume ICPi.
Qualifying statement.
Starting infliximab before colonoscopy is reasonable if negative infectious stool work-up is confirmed. However, prompt access to colonoscopy is advised to justify the dose and duration of infliximab. Once infliximab is indicated, patients most often have grade 2 and higher diarrhea/colitis, and most are hospitalized.
It is recommended that clinicians manage grade 3 toxicities as follows:
Should consider permanently discontinuing CTLA-4 agents and may restart PD-1, PD-L1 agents if patient can recover to grade 1 or less.
Should administer corticosteroids (initial dose of 1 to 2 mg/kg/d prednisone or equivalent).
Should refer to hospitalization or outpatient facility for patients with dehydration or electrolyte imbalance.
If symptoms persist ≥ 3 to 5 days or recur after improvement, may administer IV corticosteroid or noncorticosteroid (eg, infliximab).
May offer colonoscopy in cases where patients have been on immunosuppression and may be at risk for opportunistic infections as an independent cause for diarrhea (ie, CMV colitis) and for those who are anti-TNF or corticosteroid refractory.
It is recommended that clinicians manage grade 4 toxicities as follows:
Should permanently discontinue all ICPi treatment.
Should admit patient when clinically indicated. Patients managed as outpatients should be very closely monitored.
Should administer IV corticosteroid until symptoms improve to grade 1 and then start taper over 4 to 6 weeks.
May offer early infliximab 5 to 10 mg/kg if symptoms are refractory to corticosteroid within 2 to 3 days.
May offer lower GI endoscopy if symptoms are refractory despite treatment or there is concern of new infections.
Qualifying statement.
The use of vedolizumab may be offered to patients refractory to infliximab and/or contraindicated to TNF-α blocker. The decision should be made on an individual basis from gastroenterology and oncology evaluation. This is based on case series showing promising results.13–15
2.2. Hepatitis
Recommendation 2.2a – Diagnostic work-up.
It is recommended that work-up should include the following:
Monitor patient for abnormal liver blood tests: AST, ALT, and bilirubin prior to each infusion and/or weekly if there are grade 1 liver function test elevations. No treatment is recommended for grade 1 liver function test abnormality.
For grade 2 or higher toxicity:
Work-up for other causes of elevated liver enzymes, viral hepatitis, alcohol history, iron study, thromboembolic event, liver ultrasound, cross-sectional imaging for potential liver metastasis. If suspicion for primary autoimmune hepatitis is high, can consider ANA, anti–smooth muscle antibodies, and antineutrophil cytoplasmic antibodies. If patients with elevated alkaline phosphatase alone, γ-glutamyl transferase should be tested. For isolated elevation of transaminases, consider checking creatine kinase (CK) for other etiologies.
Recommendation 2.2b – Management.
It is recommended that clinicians counsel all patients to be aware of and inform their health care provider immediately if they experience:
Yellowing of skin or whites of the eyes
Severe nausea or vomiting
Pain on the right side of the abdomen
Drowsiness
Dark urine (tea colored)
Bleeding or bruising more easily than normal
Feeling less hungry than usual
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue to offer ICPi, but with close monitoring.
Should rule out alternate etiologies.
Should monitor laboratories one to two times weekly.
Should offer supportive care for symptom control.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi treatment temporarily and resume if recover to grade 1 or less on prednisone ≤ 10 mg/d.
For grade 2 hepatic toxicity with symptoms, may administer corticosteroid 0.5 to 1 mg/kg/d prednisone or equivalent if the abnormal elevation persists with significant clinical symptoms in 3 to 5 days.
Should increase frequency of monitoring to every 3 days.
Infliximab might not be the most appropriate treatment option in the situation of immune-mediated hepatitis given the potential risk of idiosyncratic liver failure (Note: No clear evidence shows liver toxicity from infliximab from other studies).
In follow-up, may resume ICPi treatment followed by taper only when symptoms improve to grade 1 or less on corticosteroid ≤ 10 mg/d. Taper over at least 1 month.
Patients should be advised to stop unnecessary medications and any known hepatotoxic drugs.
It is recommended that clinicians manage grade 3 toxicities as follows:
Should permanently discontinue treatment with ICPi.
Should immediately administer corticosteroid 1 to 2 mg/kg methylprednisolone or equivalent.
If corticosteroid refractory or no improvement after 3 days, may offer mycophenolate mofetil or azathioprine (if using azathioprine, should test for thiopurine methyltransferase deficiency).
Should order laboratories daily or every other day; may offer inpatient monitoring for patients with AST/ALT more than eight times the upper limit of normal (ULN) and/or elevated TB three times ULN.
Should increase frequency of monitoring to every 1 to 2 days.
Infliximab might not be the most appropriate treatment option in the situation of immune-mediated hepatitis given the potential risk of liver failure (Note: No clear evidence shows liver toxicity from infliximab from other studies). Alternatives include non–TNF-α agents as systemic immunosuppressants.
If no improvement is achieved with corticosteroids or for patients on combination therapy with a novel agent, with standard chemotherapy, or with targeted therapy, refer to hepatologist for further pathologic evaluation of hepatitis.
Corticosteroid taper should be attempted over a period of 4 to 6 weeks, re-escalate if needed, optimal duration unclear.
It is recommended that clinicians manage grade 4 toxicities as follows:
Should permanently discontinue treatment with ICPi.
Should administer 2 mg/kg/d methylprednisolone equivalents.
If corticosteroid refractory or no improvement after 3 days, may offer mycophenolate mofetil.
Should monitor laboratories daily; inpatient monitoring may be offered.
Should not offer infliximab in the situation of immune-mediated hepatitis.
Should refer to hepatology if no improvement is achieved with corticosteroid.
Corticosteroid taper should be attempted over a period of 4 to 6 weeks when symptoms improve to grade 1 or less, reescalate if needed, optimal duration unclear.
Consider transfer to tertiary care facility if necessary.
Discussion.
GI toxicities are some of the most common complications reported with ICPi use. While the frequency of colitis reported in the literature ranges from 8% to 27%, the incidence of diarrhea is as high as 54% in patients treated with anti–CTLA-4 antibodies,16,17 especially in patients who receive anti–CTLA-4 and anti–PD-1 combination therapy.18 GI toxicity is less common with anti–PD-1 monotherapy, with the incidence of diarrhea reported to be ≤ 19%.16 In a recent meta-analysis of patients with cancer treated with ICPis, the relative risk (RR) of all-grade diarrhea and colitis was 1.64 (95% CI, 1.19 to 2.26; P = .002) and 10.35 (95% CI, 5.78 to 18.53; P < .001), the RR of high-grade diarrhea and colitis is reported to be 4.46 (95% CI, 1.46 to 13.57; P = .008) and 15.81 (95% CI, 6.34 to 39.42; P < .001), respectively.19 On the contrary, RR of upper-GI symptoms (eg, vomiting) was not significant. Frequency of intestinal perforation has been described at approximately 1%.16,20
The most common clinical presentations of immune-related GI toxicities vary from very frequent and/or loose stools to colitis symptoms (eg, mucous in the stools, abdominal pain, fever, rectal bleeding).18 The onset of these GI symptoms is most often in the range of 5 to 10 weeks after initiation of ICPi but can occur or recur months after discontinuation of immunotherapy.5,18 While clinical factors associated with ICPi-induced colitis have not been well established, nonsteroidal anti-inflammatory drug (NSAID) use is reported to be associated with an increase in ICPi-induced enterocolitis,21 and care should be taken with NSAID use in this setting. There is a lot of similarity between ICPi-induced colitis and inflammatory bowel disease (eg, clinical presentations,22,23 radiologic findings).19 CT findings of ICPi-induced colitis include mesenteric vessel engorgement; bowel wall thickening; and fluid-filled colonic distention; and on positron emission tomography/CT scan, diffuse colonic wall thickening is observed.19 The distribution of colitis has been reported to involve descending colon more often than other parts of the colon.16,19 On the other hand, pathology from patients with colitis demonstrated changes that were more than what classic inflammatory bowel disease shows.19,24 The histologic picture is often characterized by marked mixed inflammatory cell infiltrates in the lamina propria, consisting of neutrophils, lymphocytes, plasma cells, and eosinophils.16,21,24 Inflammatory changes also tend to be more diffuse (75%).16
For patients with mild diarrhea symptoms (grade 1), usually conservative observation and maintenance of hydration are recommended rather than more-aggressive evaluation. Once diarrhea symptoms are grade 2 or higher or with apparent colitis symptoms, corticosteroid at 1 to 2 mg/kg is still the first-line treatment option if the stool infectious work-up is negative. Endoscopic evaluation can be considered if clinically deemed critical. If the symptoms are not improving after 3 to 5 days of corticosteroid treatment, stronger immunosuppressive agents (eg, TNF-α blocker infliximab, anti-integrin α4β7 antibody vedolizumab) have been shown in multiple case reports and case series to be very effective at achieving clinical remission and successful corticosteroid taper for patients who are corticosteroid refractory.14 No significant adverse effects or negative effect on the overall survival on the patient’s outcomes were identified.
Compared with lower-GI toxicities, upper-GI toxicity, characterized by dysphagia, nausea/vomiting, and epigastric pain, is much less common. Pathology can present as patchy chronic duodenitis or chronic gastritis with rare granulomas.21,25 It can coexist with lower-GI toxicity or as an isolated condition. The treatment strategy is similar to colitis: corticosteroid followed by TNF-α blockers for refractory cases based on case studies.21,25,26
Hepatotoxicity has been reported to occur in 2% to 10% of patients treated with ipilimumab, nivolumab, and pembrolizumab monotherapy.27,160–162 Combination treatment with ipilimumab and nivolumab has resulted in a reported 25% to 30% all-grade hepatitis and approximately 15% incidence of grade 3 toxicity. Onset develops predominately within the first 6 to 12 weeks after treatment initiation.28 The mainstay of treatment is prednisone or equivalent at 1 to 2 mg/kg with frequent monitoring of liver tests. However, other etiologies that could contribute to liver dysfunction have to be thoroughly evaluated and ruled out. For corticosteroid refractory cases, mycophenolate mofetil has been reported in a case study with some success.29 The TNF-α blocker infliximab is not recommended given the concern of liver toxicity, despite lack of evidence. Other alternative immunosuppressive agents still need further data proof for efficacy and safety. The patient with preexisting hepatitis who experiences ICPi-induced colitis is rare but represents a management challenge. Available options are more limited and should include permanent cessation of anti–CTLA-4 and possibly other ICPi treatment.
Acute pancreatitis has also been reported in the literature, but it is rare.22 Routine monitoring of amylase/lipase in asymptomatic patients is not recommended unless pancreatitis is clinically suspected. In the absence of symptoms, corticosteroid treatment is not indicated for modest elevations in serum amylase and lipase.2
In terms of resumption of ICPi after toxicities have occurred, the recommendation is different based on the grade level of toxicities. For grade 4 toxicities, ICPi treatment should be permanently discontinued. In patients with grade 3 or less toxicities who improve from their irAE after adequate treatment, the risk of recurrent toxicities with rechallenge appears to vary with organ toxicity and type of ICPi therapy. Only a small proportion of patients with ICPi-related colitis are reported to experience recurrences with anti–PD-1 resumption alone.30–32 Toxicities such as hepatitis and pancreatitis also have some risk of recurrence.30 These most often occur early and are generally low grade and manageable with standard treatments. Nonetheless, care should be taken to ensure that proper monitoring and management strategies are implemented.30
3.0. Lung Toxicity
Please refer to Table 3 a complete set of recommendations, definition of grades, and additional considerations.
Table 3.
Management of Lung irAEs in Patients Treated With ICPis
| 3.0 Lung Toxicities | |
| 3.1 Pneumonitis | |
| Definition: Focal or diffuse inflammation of the lung parenchyma (typically identified on CT imaging) | |
| No symptomatic, pathologic, or radiographic features are pathognomonic for pneumonitis | |
| Diagnostic work-up | |
| Should include the following: CXR, CT, pulse oximetry | |
| For G2 or higher, may include the following infectious work-up: nasal swab, sputum culture and sensitivity, blood culture and sensitivity, urine culture and sensitivity | |
| Grading | Management |
| G1: Asymptomatic, confined to one lobe of the lung or < 25% of lung parenchyma, clinical or diagnostic observations only | Hold ICPi with radiographic evidence of pneumonitis progression May offer one repeat CT in 3–4 weeks; in patients who have had baseline testing, may offer a repeat spirometry/DLCO in 3–4 weeks May resume ICPi with radiographic evidence ofimprovement or resolution. If no improvement, should treat as G2 Monitor patients weekly with history and physical examination and pulse oximetry; may also offer CXR |
| G2: Symptomatic, involves more than one lobe of the lung or 25%–50% of lung parenchyma, medical intervention indicated, limiting instrumental ADL | Hold ICPi until resolution to G1 or less Prednisone 1–2 mg/kg/d and taper by 5–10 mg/wk over 4–6 weeks Consider bronchoscopy with BAL Consider empirical antibiotics Monitor every 3 days with history and physical examination and pulse oximetry, consider CXR; no clinical improvement after 48–72 hours of prednisone, treat as G3 |
| G3: Severe symptoms, hospitalization required, involves all lung lobes or > 50% of lung parenchyma, limiting self-care ADL, oxygen indicated G4: Life-threatening respiratory compromise, urgent intervention indicated (intubation) |
Permanently discontinue ICPi Empirical antibiotics; (methyl)prednisolone IV 1–2 mg/kg/d; no improvement after 48 hours, may add infliximab 5 mg/kg or mycophenolate mofetil IV 1 g twice a day or IVIG for 5 days or cyclophosphamide; taper corticosteroids over 4–6 weeks Pulmonary and infectious disease consults if necessary Bronchoscopy with BAL ± transbronchial biopsy Patients should be hospitalized for further management |
| Additional considerations GI and Pneumocystis prophylaxis with PPI and Bactrim may be offered to patients on prolonged corticosteroid use (> 12 weeks), according to institutional guidelines34–37 Consider calcium and vitamin D supplementation with prolonged corticosteroid use The role of prophylactic fluconazole with prolonged corticosteroid use (> 12 weeks) remains unclear, and physicians should proceed according to institutional guidelines33 Bronchoscopy + biopsy; if clinical picture is consistent with pneumonitis, no need for biopsy | |
| All recommendations are expert consensus based, with benefits outweighing harms, and strength of recommendations are moderate. | |
Abbreviations: ADL, activities of daily living; BAL, bronchoalveolar lavage; CT, computed tomography; CXR, chest x-ray; DLCO, diffusing capacity of lung for carbon monoxide; G, grade; ICPi, immune checkpoint inhibitor; irAE, immune-related adverse event; IV, intravenous; IVIG, intravenous immunoglobulin; PPI, proton pump inhibitor.
3.1. Pneumonitis
Recommendation 3.1a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
Chest x-ray (CXR), CT, pulse oximetry
For grade 2 or higher, may include the following infectious work-up: nasal swab, sputum culture and sensitivity, blood culture and sensitivity, and urine culture and sensitivity
Recommendation 3.1b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should hold ICPi with radiographic evidence of pneumonitis progression.
May offer one repeat CT in 3 to 4 weeks. In patients who have had baseline testing (Appendix Table A2, online only), may offer a repeat spirometry/diffusing capacity of lung for carbon monoxide in 3 to 4 weeks.
May resume ICPi with radiographic evidence of improvement or resolution. If no improvement, should treat as grade 2.
Should monitor patients weekly with history and physical examination and pulse oximetry; may also offer CXR.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold immunotherapy until resolution to grade 1 or less.
Should administer prednisone 1 to 2 mg/kg/d and taper by 5 to 10 mg/wk over 4 to 6 weeks per institutional guidelines.
May offer bronchoscopy with bronchoalveolar lavage.
May prescribe empirical antibiotics.
Should monitor every 3 days with history and physical examination and pulse oximetry; may also offer CXR. No clinical improvement after 48 to 72 hours of prednisone, should treat as grade 3.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should permanently discontinue ICPi.
Should prescribe empirical antibiotics and administer (methyl)prednisolone IV 1 to 2 mg/kg/d. No improvement after 48 hours, may add infliximab 5 mg/kg or mycophenolate mofetil IV 1 g twice a day or IVIG for 5 days or cyclophosphamide. Taper corticosteroids over 4 to 6 weeks.
Should consult pulmonary and infectious disease if necessary.
Should offer bronchoscopy with bronchoalveolar lavage with or without transbronchial biopsy.
Patients should be hospitalized for further management.
Qualifying statement.
The role of prophylactic fluconazole with prolonged corticosteroid use (> 12 weeks) remains unclear, and physicians should proceed according to institutional guidelines.33
Discussion.
ICPi-related pneumonitis is an uncommon but potentially serious toxicity. The reported incidence of pneumonitis in studies investigating anti–PD-1/PD-L1 is variable and ranges from 0% to 10%,38,163 with an overall incidence of 2.7% reported in a recent meta-analysis of 20 studies with PD-1 inhibition.39 The toxicity seems to be less common with anti–CTLA-4 treatment, with pneumonitis reported in < 1% of trial participants receiving ipilimumab.40–44 A higher incidence was seen in patients who received combination therapy than those who received monotherapy (10% v 3%, respectively; P < .001),38 and patients treated with combination immunotherapy may be less likely to experience resolution of the irAE compared with patients treated with monotherapy.40,45 In patients who improve from their irAE, rechallenging with anti–PD-(L)1 therapy was associated with recurrent or new irAEs in half of patients and was more common in early-onset irAEs.46 The majority of such patients were managed successfully, but two deaths have been reported.46
The evidence on whether the risk of ICPi-related pneumonitis and pneumonitis-related deaths varies by tumor type remains equivocal. The odds of all-grade pneumonitis was higher in patients with NSCLC than in those with melanoma (odds ratio [OR], 1.43; 95% CI, 1.08 to 1.89; P = .005) according to the Nishino et al39 meta-analysis. Similarly, patients with renal cell carcinoma were also significantly more likely to experience all-grade pneumonitis than patients with melanoma (OR, 1.59; 95% CI, 1.32 to 1.92; P < .001).39 In contrast, other studies have reported similar rates of grades 3 to 4 pneumonitis across tumor types but with more treatment-related deaths due to pneumonitis seen in patients with NSCLC.40,47–49 In a multicenter, large retrospective analysis, pneumonitis was reported to develop in both former/current smokers (56%) and never smokers (44%).38 Recent evidence also demonstrated no significant difference in the rates of irAEs, including pneumonitis, between patients who received thoracic radiotherapy in addition to checkpoint inhibitors.50
The median onset of ICPi-related pneumonitis can vary, with a range of 2 to 24 months and a median time to onset of approximately 3 months reported in the literature.38 However, onset does occur earlier with combination therapy versus monotherapy.40 Clinical symptoms can include dyspnea (53%), cough (35%), fever (12%), and chest pain (7%).51 Hypoxia may occur and progress rapidly, leading to respiratory failure.40,52
Ground-glass opacities or patchy nodular infiltrates, predominantly in the lower lobes, are common manifestations on chest imaging.53 Radiologic abnormalities vary but are often reported to be focal and very different from the diffuse pneumonitis associated with targeted agents.53 Naidoo et al38 recently reported on five distinct radiologic subtypes: chronic obstructive pneumonia like, ground-glass opacities, hypersensitivity type, interstitial type, and pneumonitis not otherwise specified.
When the clinical picture is consistent with pneumonitis, biopsy is generally unnecessary. However, transbronchial biopsy may have a role in assisting to rule out other etiologies like lymphangitic spread of tumor or infection. The decision to perform lung biopsy in the evaluation of immune-related pulmonary reactions is based on the probability that this examination will yield a specific diagnosis, leading to a change in management. Yet, there is no specific pathology to confirm immune-related pneumonitis. Ultimately, the decision to proceed with biopsy should be taken after a careful risk-benefit analysis, with the optimal technique, number, size, and location of biopsies depending upon the suspected diagnosis, the anatomic distribution of the disease process, and the availability of pulmonologists.
In addition to typical findings of pneumonitis, sarcoid-like granulomatous reactions, including subpleural micronodular opacities and hilar lymphadenopathy, as well as pleural effusions have been associated with both CTLA-4– and PD-1/PD-L1–targeted therapies.40,42,54–57 Clinical manifestations are diverse and often patient-specific and can include cough, wheezing, fatigue, chest pain, or no symptoms at all. With varying clinical presentation, it is prudent for clinicians to be aware of the possibility of such immune-related pulmonary reactions, as they may mimic disease progression on imaging and examination. Biopsy may assist in confirming the diagnosis.
4.0. Endocrine Toxicities
Please refer to Table 4 for a complete set of recommendations, definition of grades, and additional considerations.
Table 4.
Management of Endocrine irAEs in Patients Treated With ICPis
| 4.0 Endocrine Toxicity | |
| Counsel patients to inform their health care provider immediately if they experience any changes in their health since their last visit, especially any of the following: Headaches that will not go away or unusual headache patterns Vision changes Rapid heartbeat Increased sweating Extreme tiredness or weakness Muscle aches Weight gain or weight loss Dizziness or fainting Feeling more hungry or thirsty than usual Hair loss Changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness Feeling cold Constipation Voice gets deeper Urinating more often than usual Nausea or vomiting Abdominal pain | |
| 4.1 Thyroid | |
| 4.1.1 Primary hypothyroidism | |
| Definition: Elevated TSH, normal or low FT4 | |
| Diagnostic work-up | |
| TSH and FT4 every 4–6 weeks as part of routine clinical monitoring on therapy or for case detection in symptomatic patients | |
| Grading | Management |
| G1: TSH < 10 mIU/L and asymptomatic | Should continue ICPi with close follow-up and monitoring of TSH, FT4 |
| G2: Moderate symptoms; able to perform ADL; TSH persistently > 10 mIU/L | May hold ICPi until symptoms resolve to baseline Consider endocrine consultation Prescribe thyroid hormone supplementation in symptomatic patients with any degree of TSH elevation or in asymptomatic patients with TSH levels that persist > 10 mIU/L (measured 4 weeks apart) Monitor TSH even/6–8 weeks while titrating hormone replacement to normal TSH FT4 can be used in the short term (2 weeks) to ensure adequacy of therapy in those with frank hypothyroidism where the FT4 was initially low Once adequately treated, should monitor thyroid function (at least TSH) every 6 weeks while on active ICPi therapy or as needed for symptoms to ensure appropriate replacement; repeat testing annually or as indicated by symptoms once stable |
| G3–4: Severe symptoms, medically significant or life- threatening consequences, unable to perform ADL | Hold ICPi until symptoms resolve to baseline with appropriate supplementation Endocrine consultation May admit for IV therapy if signs of myxedema (bradycardia, hypothermia) Thyroid supplementation and reassessment as in G2 |
| Additional considerations | |
| For patients without risk factors, full replacement can be estimated with an ideal body weight-based dose of approximately 1.6 μg/kg/d | |
| For elderly or fragile patients with multiple comorbidities, consider titrating up from low dose, starting at 25–50 μg | |
| Extreme elevations of TSH can be seen in the recovery phase of thyroiditis and can be watched in asymptomatic patients to determine whether there is recovery to normal within 3–4 weeks | |
| Under guidance of endocrinology, consider tapering hormone replacement and retesting in patients with a history of thyroiditis (initial thyrotoxic phase) | |
| Adrenal dysfunction, if present, must always be replaced before thyroid hormone therapy is initiated | |
| 4.1.2 Hyperthyroidism | |
| Definition: Suppressed TSH and high normal or elevated FT4 and/or triiodothyronine | |
| Diagnostic work-up | |
| Monitor TSH, FT4 every 4–6 weeks from the start of therapy or as needed for case detection in symptomatic patients | |
| Consider TSH receptor antibodies if there are clinical features and suspicion of Grave disease (eg, ophthalmopathy) | |
| Close monitoring of thyroid function every 2–3 weeks after diagnosis to catch transition to hypothyroidism in patients with thyroiditis and hyperthyroidism | |
| Grading | Management |
| G1: Asymptomatic or mild symptoms | Can continue ICPi with close follow-up and monitoring of TSH, FT4 every 2–3 weeks until it is clear whether there will be persistent hyperthyroidism (see below) or hypothyroidism (see 4.1.1) |
| G2: Moderate symptoms, able to perform ADL | Consider holding ICPi until symptoms return to baseline Consider endocrine consultation β-Blocker (eg, atenolol, propranolol) for symptomatic relief Hydration and supportive care Corticosteroids are not usually required to shorten duration For persistent hyperthyroidism (> 6 weeks) or clinical suspicion, work-up for Graves disease (TSI or TRAb) and consider thionamide (methimazole or PTU Refer to endocrinology for Graves disease |
| G3–4: Severe symptoms, medically significant or life- threatening consequences, unable to perform ADL | Hold ICPi until symptoms resolve to baseline with appropriate therapy Endocrine consultation β-Blocker (eg, atenolol, propranolol) for symptomatic relief For severe symptoms or concern for thyroid storm, hospitalize patient and initiate prednisone 1–2 mg/kg/d or equivalent tapered over 1–2 weeks; consider also use of SSKI or thionamide (methimazole or PTU). |
| Additional considerations Thyroiditis is transient and resolves in a couple of weeks to primary hypothyroidism or normal. Hypothyroidism can be treated as above. Graves disease is generally persistent and is due to increased thyroid hormone production that can be treated with antithyroid medical therapy. Physical examination findings of ophthalmopathy or thyroid bruit are diagnostic of Graves and should prompt early endocrine referral. | |
| 4.2 Adrenal - primary adrenal insufficiency | |
| Definition: Adrenal gland failure leading to low morning cortisol, high morning ACTH, as well as hyponatremia and hyperkalemia with orthostasis and volume depletion due to loss of aldosterone | |
| Diagnostic work-up for patients in whom adrenal insufficiency is suspected: Evaluate ACTH (AM), cortisol level (AM) Basic metabolic panel (Na, K, CO2, glucose) Consider ACTH stimulation test for indeterminate results If primary adrenal insufficiency (high ACTH, low cortisol) is found biochemically: Evaluate for precipitating cause of crisis such as infection Perform an adrenal CT for metastasis/hemorrhage | |
| Grading | Management |
| G1: Asymptomatic or mild symptoms | Consider holding ICPi until patient is stabilized on replacement hormone Endocrine consultation Replacement therapy with prednisone (5–10 mg daily) or hydrocortisone (10–20 mg orally every morning, 5–10 mg orally in early afternoon) May require fludrocortisone (0.1 mg/d) for mineralocorticoid replacement in primary adrenal insufficiency Titrate dose up or down as symptoms dictate |
| G2: Moderate symptoms, able to perform ADL | Consider holding ICPi until patient is stabilized on replacement hormone Endocrine consultation Initiate outpatient treatment at two to three times maintenance (if prednisone, 20 mg daily; if hydrocortisone, 20–30 mg in the morning, and 10–20 mg in the afternoon) to manage acute symptoms. Taper stress-dose corticosteroids down to maintenance doses over 5–10 days Maintenance therapy as in G1. |
| G3–4: Severe symptoms, medically significant or life- threatening consequences, unable to perform ADL | Hold ICPi until patient is stabilized on replacement hormone Endocrine consultation See in clinic or, for after hours, make an emergency department referral for normal saline (at least 2 L) and IV stress-dose corticosteroids on presentation(hydrocortisone 100 mg or dexamethasone 4 mg (if the diagnosis is not clear and stimulation testing will be needed) Taper stress-dose corticosteroids down to maintenance doses over 7–14 days after discharge Maintenance therapy as in G1 |
| Additional considerations Primary and secondary adrenal insufficiency can be distinguished by the relationship between ACTH and cortisol. If the ACTH is low with low cortisol, then management is as per 4.3. Patients on corticosteroids for management of other conditions will have low morning cortisol as a result of iatrogenic, secondary adrenal insufficiency. ACTH will also be low in these patients. A diagnosis of adrenal insufficiency is challenging to make in these situations (see next section on hypophysitis). Emergent therapy for someone with suspected adrenal insufficiency is best done with dexamethasone as a stimulation test can still be performed. If the diagnosis is already confirmed, can use hydrocortisone 100 mg. All patients need education on stress dosing and a medical alert bracelet for adrenal insufficiency to trigger stress-dose corticosteroids by EMS. Endocrine consultation prior to surgery or any procedure for stress-dose planning. | |
| 4.3 Pituitary - hypophysitis | |
| Definition: Inflammation of the pituitarywith varying effects on hormone function. Mostcommonly presenting with central adrenal insufficiency. May also have central hypothyroidism, diabetes insipidus, and hypogonadism. | |
| Diagnostic work-up Diagnosis: Low ACTH with a low cortisol. Low or normal TSH with a low FT4. Hypernatremia and volume depletion with diabetes insipidus. Low testosterone or estradiol with low LH and FSH. Testing: Evaluate ACTH, cortisol (AM), TSH, FT4, electrolytes Consider evaluating LH, FSH, and testosterone levels in males or estrogen in premenopausal females with fatigue, loss of libido, and mood changes Consider MRI of the brain with or without contrast with pituitary/sellar cuts in patients with multiple endocrine abnormalities ± new severe headaches or complaints of vision changes | |
| Grading | Management |
| G1: Asymptomatic or mild symptoms | Considering holding ICPi until patient is stabilized on replacement hormones Hormonal supplementation as needed, using dosing as above for primary hypothyroidism and adrenal insufficiency (eg, hydrocortisone 10–20 mg orally in the morning, 5–10 mg orally in early afternoon; levothyroxine by weight) Testosterone or estrogen therapy as needed in those without contraindications Endocrine consultation Always start corticosteroids several days before thyroid hormone to prevent precipitating adrenal crisis Follow FT4 for thyroid hormone replacement titration (TSH is not accurate) |
| G2: Moderate symptoms, able to perform ADL | Consider holding ICPi until patient is stabilized on replacement hormones Endocrine consultation Hormonal supplementation as in G1 |
| G3–4: Severe symptoms, medically significant or life- threatening consequences, unable to perform ADL | Hold ICPi until patient is stabilized on replacement hormones Endocrine consultation Hormonal supplementation as in G1 Consider initial pulse dose therapy with prednisone 1–2 mg/kg oral daily (or equivalent) tapered over at least 1–2 weeks |
| Additional considerations Be aware of the need to start corticosteroids first when planning hormone replacement therapy for multiple deficiencies All patients need instruction on doubling doses for illness (stress dosing) and a medical alert bracelet for adrenal insufficiency to trigger stress-dose corticosteroids by EMS Corticosteroid use can cause isolated central adrenal insufficiency Work-up cannot be done with a simple AM cortisol in a patient on corticosteroids for other conditions Laboratory confirmation of adrenal insufficiency should not be attempted until treatment with corticosteroids for other disease is ready to be discontinued For long-term exposure, consult endocrinology for recovery and weaning protocol using hydrocortisone. | |
| 4.4 Diabetes | |
| Definition:T2DM is a combination of insulin resistance and insufficiency that may require oral or insulin therapy. It may be newonsetor exacerbated during therapy for nonimmunologic reasons, such as corticosteroid exposure. Autoimmune T1DM results from islet cell destruction and is often acute onset, with ketosis and an insulin requirement | |
| Diagnostic work-up Monitor patients for hyperglycemia or other signs and symptoms of new or worsening DM, including measuring glucose at baseline and with each treatment cycle during induction for 12 weeks, then every 3–6 weeks thereafter. To guide the work-up in new-onset hyperglycemia, clinicians should consider a patient's medical background, exposure history, and risk factors for each subtype of DM. Laboratory evaluation in suspected T1DM should include testing for ketosis in urine and an assessment of the anion gap on a metabolic panel. Anti-glutamic acid decarboxylase, anti-islet cell, oranti-insulin antibodies are highly specific forautoimmune diabetes. Insulin and C-peptide levels can also assist in the diagnosis. | |
| Grading | Management |
| Gl: Asymptomatic or mild symptoms; fasting glucose value > UlN (160 mg/dL); fasting glucose value > ULN (8.9 mmol/L); no evidence of ketosis or laboratory evidence of T1DM | Can continue ICPi with close clinical follow-up and laboratory evaluation May initiate oral therapy for those with new-onset T2DM Screen for T1DM ifappropriate, forexample, acuteonsetwith prior normal values or clinical concern for ketosis |
| G2: Moderate symptoms, able to perform ADL, fasting glucose value > 160–250 mg/dL; fasting glucose value > 8.9–13.9 mmol/L, ketosis or evidence ofT1DMatany glucose level | May hold ICPi until glucose control is obtained Titrate oral therapy or add insulin for worsening control in T2DM Should administer insulin for T1DM (or as default therapy if there is confusion about type) Urgentendocrine consultation forany patient with T1DM; in theabsence of endocrinology, internal medicine may suffice Consider admission for T1DM if early outpatient evaluation is not available or signs of ketoacidosis are present |
| G3–4: Severe symptoms, medically significant or life- threatening consequences, unable to perform ADL G3: > 250–500 mg/dL (> 13.9–27.8 mmol/L) G4: > 500 mg/dL (> 27.8 mmol/L) |
Hold ICPi until glucose control is obtained on therapywith reduction oftoxicity to G1 or less Urgent endocrine consultation for all patients Initiate insulin therapy for all patients Admit for inpatient management: Concerns for developing DKA, Symptomatic patients regardless of diabetes type, New-onset T1DM unable to see endocrinology |
| Additional considerations Insulin therapy can be used as the default in any case with hyperglycemia. Long-acting therapy alone is not usuallysufficient forT1DM, where halfof daily requirements are usuallygiven in divided doses as prandial coverage and half as long acting. Insulin doses will be lower in T1DM because of preserved sensitivity (total daily requirement can be estimated at 0.3–0.4 units/kg/d). In T2DM, sliding-scale coverage with meals over a few days provides data to estimate a patient’s daily requirements and can be used to more rapidly titrate basal needs. All recommendations are expert consensus based, with benefits outweighing harms, and strength of recommendations are moderate. | |
Abbreviations: ACTH, adrenocorticotropic hormone; ADL, activities of daily living; CT, computed tomography; DKA, diabetic ketoacidosis; DM, diabetes mellitus; EMS, emergency medical services; FSH, follicle-stimulating hormone; FT4, free thyroxine; G, grade; ICPi, immune checkpoint inhibitor; irAE, immune-related adverse event; LH, luteinizing hormone; MRI, magnetic resonance imaging; PTU, propylthiouracil; SSKI, potassium iodide;T1DM, type 1 diabetes mellitus;T2DM, type 2 diabetes mellitus;TRAb, thyroid-stimulating hormone receptor antibody; TSH, thyroid-stimulating hormone; TSI, thyroid-stimulating immunoglobulin; ULN, upper limit of normal.
Recommendation 4.0 – Endocrine general.
It is recommended that clinicians counsel patients to inform their health care provider and team immediately if they experience any changes in their health since their last visit, especially any of the following:
Headaches that will not go away or unusual headache patterns
Vision changes
Rapid heartbeat
Increased sweating
Extreme tiredness or weakness
Muscle aches
Weight gain or weight loss
Dizziness or fainting
Feeling more hungry or thirsty than usual
Hair loss
Changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
Feeling cold
Constipation
Voice gets deeper
Urinating more often than usual
Nausea or vomiting
Abdominal pain
4.1. Thyroid
4.1.1. Primary Hypothyroidism
Recommendation 4.1.1a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
Testing for TSH and free thryoxine (FT4) every 4 to 6 weeks as part of routine clinical monitoring on therapy or for case detection in symptomatic patients.
Recommendation 4.1.1b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue to offer ICPi with close follow-up and monitoring of TSH, FT4.
It is recommended that clinicians manage grade 2 toxicities as follows:
May hold ICPi until symptoms resolve to baseline.
May consult endocrinology.
Should prescribe thyroid hormone supplementation in symptomatic patients with any degree of TSH elevation or in asymptomatic patients with TSH levels that persist > 10 mIU/L (measured 4 weeks apart).
Should monitor TSH every 6 to 8 weeks while titrating hormone replacement to normal TSH.
FT4 can be used in the short term (2 weeks) to ensure adequacy of therapy in those with frank hypothyroidism where the FT4 was initially low.
Once adequately treated, should monitor thyroid function (at least TSH) every 6 weeks while on active therapy or as needed for symptoms to ensure appropriate replacement. Repeat testing annually or as indicated by symptoms once stable.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should hold ICPi until symptoms resolve to baseline with appropriate supplementation.
Should consult endocrinology.
May admit for IV therapy if signs of myxedema (bradycardia, hypothermia).
Should prescribe thyroid supplementation and offer reassessment as in grade 2.
4.1.2. Hyperthyroidism
Recommendation 4.1.2a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
Monitor TSH and FT4 every 4 to 6 weeks from the start of therapy or as needed for case detection in symptomatic patients.
Test for TSH receptor antibodies if there are clinical features and suspicion of Grave disease (eg, ophthalmopathy).
Should closely monitor thyroid function every 2 to 3 weeks after diagnosis to catch transition to hypothyroidism in patients with thyroiditis and hyperthyroidism.
Recommendation 4.1.2b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue to offer ICPi with close follow-up and monitoring of TSH and FT4 every 2 to 3 weeks until it is clear whether there will be persistent hyperthyroidism (see below) or hypothyroidism (see 4.1.1).
It is recommended that clinicians manage grade 2 toxicities as follows:
May hold ICPi until symptoms return to baseline.
May consult endocrinology.
Should offer a β-blocker (eg, atenolol, propranolol) for symptomatic relief.
Should offer hydration and supportive care.
Should note that corticosteroids are not usually required to shorten duration.
For persistent hyperthyroidism (> 6 weeks) or clinical suspicion, clinicians should work up for Graves disease (thyroid-stimulating immunoglobulin or TSH receptor antibody) and consider thionamide (methimazole or propylthiouracil).
Should refer to endocrinology for Graves disease.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should hold ICPi until symptoms resolve to baseline with appropriate therapy.
Should consult endocrinology.
Should offer a β-blocker (eg, atenolol, propranolol) for symptomatic relief.
For severe symptoms or concern for thyroid storm, should hospitalize patient and initiate prednisone 1 to 2 mg/kg/d or equivalent tapered over 1 to 2 weeks. May also use saturated solution of potassium iodide or thionamide (methimazole or propylthiouracil).
4.2. Adrenal – Primary Adrenal Insufficiency
Recommendation 4.2a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following for patients in whom adrenal insufficiency is suspected:
Evaluate adrenocorticotropic hormone (ACTH; AM), cortisol level (AM).
Basic metabolic panel (Na, K, CO2, glucose).
Consider ACTH stimulation test for indeterminate results.
For evidence of primary adrenal insufficiency (high ACTH, low cortisol), evaluate for a precipitating cause of crisis, such as infection, and perform an adrenal CT scan for metastasis/hemorrhage.
Recommendation 4.2b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
May hold ICPi until patient is stabilized on replacement hormone.
Should consult endocrinology.
Should offer replacement therapy with prednisone (5 to 10 mg daily) or hydrocortisone (10 to 20 mg orally in the morning, 5 to 10 mg orally in early afternoon)
May prescribe fludrocortisone (0.1 mg/d) for mineralocorticoid replacement in primary adrenal insufficiency.
Should titrate dose up or down as symptoms dictate.
It is recommended that clinicians manage grade 2 toxicities as follows:
May hold ICPi until patient is stabilized on replacement hormone.
Should consult endocrinology.
Should initiate outpatient treatment at two to three times maintenance (eg, if prednisone, 20 mg daily; if hydrocortisone, 20 to 30 mg in the morning and 10 to 20 mg in the afternoon) to manage acute symptoms.
Should taper stress-dose corticosteroids down to maintenance doses over 5 to 10 days.
Should offer maintenance therapy as in grade 1.
It is recommended that clinicians manage grade 3 to 4 tox-icities as follows:
Should hold ICPi until patient is stabilized on replacement hormone.
Should consult endocrinology.
Should see in clinic or, for after hours, make an emergency department referral for normal saline (at least 2 L) and IV stress-dose corticosteroids on presentation (hydrocortisone 100 mg or dexamethasone 4 mg if the diagnosis is not clear and stimulation testing will be needed).
Should taper stress-dose corticosteroids down to maintenance doses over 7 to 14 days after discharge.
Should offer maintenance therapy as in grade 1.
Qualifying statement.
Primary and secondary adrenal insufficiency can be distinguished by the relationship between ACTH and cortisol. If the ACTH is low with low cortisol, then management is as per 4.3. Emergent therapy for someone with suspected adrenal insufficiency is best done with dexamethasone, as a stimulation test can still be performed. If the diagnosis is already confirmed, can use hydrocortisone 100 mg.
4.3. Pituitary – Hypophysitis
Recommendation 4.3a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
Evaluate ACTH, cortisol (am), TSH, FT4, and electrolytes.
Consider evaluating luteinzing hormone, follicle-stimulating hormone, and testosterone levels in males or estrogen in premenopausal females with fatigue, loss of libido, and mood changes.
Consider magnetic resonance imaging (MRI) of brain with or without contrast with pituitary/sellar cuts in patients with multiple endocrine abnormalities with or without new severe headaches or complaint of vision changes.
Recommendation 4.3b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
May hold ICPi until patient is stabilized on replacement hormones.
Should offer hormonal supplementation as needed, using dosing as specified for primary hypothyroidism and adrenal insufficiency (eg, hydrocortisone 10 to 20 mg orally in the morning, 5 to 10 mg orally in early afternoon; levothyroxine by weight).
Testosterone or estrogen therapy as needed in those without contraindications.
Should consult endocrinology.
Should always start corticosteroids several days before thyroid hormone to prevent precipitating adrenal crisis.
Should follow FT4 for thyroid hormone replacement titration (TSH is not accurate).
It is recommended that clinicians manage grade 2 toxicities as follows:
May hold ICPi until patient is stabilized on replacement hormones.
Should consult endocrinology.
Should offer hormonal supplementation as in grade 1.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should hold ICPi until patient is stabilized on replacement hormones.
Should consult endocrinology.
Should offer hormonal supplementation as in grade 1.
May administer initial pulse dose therapy with prednisone 1 to 2 mg/kg oral daily (or equivalent) tapered over at least 1 to 2 weeks.
Qualifying statement.
Be aware of the need to start corticosteroids first when planning hormone replacement therapy for multiple deficiencies. All patients need instruction on doubling doses for illness (stress dosing) and a medical alert bracelet for adrenal insufficiency to trigger stress-dose corticosteroids by emergency medical services.
4.4. Diabetes
Recommendation 4.4a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
Monitor patients for hyperglycemia or other signs and symptoms of new or worsening diabetes mellitus (DM), including measuring glucose at baseline and with each treatment cycle during induction for 12 weeks, then every 3 to 6 weeks thereafter.
To guide the work-up in new-onset hyperglycemia, clinicians should consider a patient’s medical background, exposure history, and risk factors for each subtype of DM.
Laboratory evaluation in suspected type 1 DM (T1DM) should include testing for ketosis in urine and an assessment of the anion gap on a metabolic panel. Anti–glutamic acid decarboxylase, anti–islet cell, or anti–insulin antibodies are highly specific for autoimmune diabetes. Insulin and C-peptide levels can also assist in the diagnosis.
Recommendation 4.4b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
May continue to offer ICPi with close clinical follow-up and laboratory evaluation.
May initiate oral therapy for those with new-onset type 2 DM (T2DM).
Should screen for T1DM if appropriate, for example, acute onset with prior normal values or clinical concern for ketosis.
It is recommended that clinicians manage grade 2 toxicities as follows:
May hold ICPi until glucose control is obtained.
Should titrate oral therapy or add insulin for worsening control in T2DM.
Should administer insulin for T1DM (or as default therapy if there is confusion about type).
Should seek urgent endocrine consultation for any patient with T1DM. In the absence of endocrinology, internal medicine may suffice.
May admit for T1DM if early outpatient evaluation is not available or signs of ketoacidosis are present.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should hold ICPi until glucose control is obtained on therapy with reduction of toxicity to grade 1 or less.
Should seek urgent endocrine consultation for all patients.
Should initiate insulin therapy for all patients.
Should admit for inpatient management for any of the following: concern for developing diabetic ketoacidosis (DKA), symptomatic patients regardless of diabetes type, new onset T1DM unable to see endocrinology.
Discussion.
Endocrine adverse events with immune checkpoint therapy present a unique clinical challenge for the non-endocrinologist who faces the possibility of central as well as primary endocrine dysfunction in a patient with symptoms or abnormal laboratories. Diverse therapies and combinations have varied rates of targeting individual organs, for example, hypophysitis is most commonly seen when ipilimumab is used,58–61 and primary ovarian failure has not yet been reported.62 However, with sporadic autoimmune disease known for all endocrine organs, we anticipate the possibility of any condition as the use becomes more widespread. In a recent systematic review and meta-analysis that included 7,551 patients in 38 randomized trials, the overall incidence of clinically significant endocrinopathies was approximately 10% of patients treated with checkpoint inhibitors.62
Clinical measure of both the primary hormone and the pituitary hormone are needed to localize disease. For example, a low morning cortisol suggests adrenal insufficiency but not whether the problem is pituitary or adrenal. We would look for hypophysitis if a simultaneously measured ACTH is low, whereas in primary adrenal insufficiency (eg, Addison), the ACTH will be elevated. The same applies for systems where we typically screen with the pituitary hormone—low TSH suggests hyperthyroidism if the thyroid hormone level (FT4 is typically sufficient) is elevated and central hypothyroidism if FT4 is low. Drawing both hormones is especially important when hypophysitis is suspected because TSH can be at low-normal levels but lack function with pituitary disease.
Distinguishing primary from secondary hormonal problems is necessary because there are treatment implications. Perhaps most importantly for preventing harm is recognizing that hypophysitis often causes both central hypothyroidism and secondary adrenal insufficiency.62 If thyroid hormone is replaced first when cortisol is low, the increase in cortisol metabolism can trigger an adrenal crisis. Fludrocortisone is needed in addition to hydrocortisone in most cases of primary adrenal insufficiency, which involves the loss of mineralocorticoid as well as glucocorticoid, leading to more-profound blood pressure and electrolyte abnormalities.63 Monitoring is also affected by localization, as pituitary hormones are not reliable indicators of status with central disease. TSH, therefore, is not helpful in monitoring therapy with levothyroxine in central hypothyroidism, and FT4 should be used instead.64
Diagnosis of endocrine dysfunction is complicated by any acute illness and the administration of medications that have an effect on pituitary function, including many therapies that patients with cancer are on, such as narcotics and megestrol acetate. Most relevant for the patients administered ICPis is the effect of corticosteroids, given for many irAEs, which will directly suppress ACTH and may cause persistent central adrenal insufficiency when stopped. Cortisol levels should not be routinely measured while patients are on corticosteroid therapy because of variable assay effects from synthetic corticosteroids, low endogenous levels from the exposure, and the fact that the patient is on supraphysiologic doses and therefore treated for any underlying adrenal insufficiency that might have developed. If a diagnosis is needed, for example, after acute treatment of presumed adrenal crisis is initiated, ACTH stimulation testing may be performed on dexamethasone, which is not measured by most assays. Endogenous levels can be directly measured 24 hours after the last dose of physiologic hydrocortisone replacement to assess for functional recovery. High-dose corticosteroids can also cause a low TSH and a pattern similar to nonthyroidal illness, neither of which are thought to benefit from therapy.65,66 Especially in difficult cases, endocrinology consult is recommended.
The response of the oncologist to the development of endocrine dysfunction may be different from other irAEs because organ failure can be managed with hormone replacement. It is not a given that the patient benefits from stopping cancer therapy to get immunosuppressive therapy to reverse the autoimmune disease. For example, there is no good evidence at this time that high-dose corticosteroids improve the rate of pituitary hormone recovery.59,61 Therefore, a clinical judgment is needed to balance benefits, such as the possibility of improved headache, with risks, such as corticosteroid adverse effects, on glycemic control and delay of therapy.
Rare cases of T1DM present an analogous challenge to the clinician in the need to distinguish these from the much more common cases of worsening glycemic control attributable to insulin resistance and T2DM. The acute risks of DKA in T1DM require vigilance on the part of treating oncologists, despite the very low occurrence rate. New-onset hyperglycemia in a patient without risk factors for T2DM (eg, preexisting disease, corticosteroid exposure) should raise the level of concern for T1DM. Acute onset of polyuria, polydipsia, weight loss, and lethargy are characteristic presenting features of T1DM. Urine ketones and acid base status can be evaluated as screening for DKA and the need for inpatient evaluation. Antibodies, insulin, and C-peptide levels can be sent to support diagnosis, although the initiation of therapy should not be delayed pending results. Insulin should be used to treat hyperglycemia in anyone where the diagnosis is in question. Endocrinology consultation is appropriate where the diagnosis of T1DM is suspected even without evidence of DKA.
5.0. Musculoskeletal Toxicities
Please refer to Table 5 for a complete set of recommendations, definition of grades, and additional considerations.
Table 5.
Management of Musculoskeletal irAEs in Patients Treated With ICPis
| 5.0 Musculoskeletal Toxicities | |
| 5.1 Inflammatory arthritis | |
| Definition: A disorder characterized by inflammation of the joints | |
| Clinical symptoms: Joint pain accompanied by joint swelling; inflammatory symptoms, such as stiffness after inactivity or in the morning, lasting > 30 minutes to 1 hour; improvement of symptoms with NSAIDs orcorticosteroids but notwith opioids orother pain medications may also be suggestive of inflammator/ arthritis. | |
| Diagnostic work-up | |
| G1 | |
| Complete rheumatologic history and examination of all peripheral joints for tenderness, swelling, and range of motion; examination of the spine Consider plain x-ray/imaging to exclude metastases and evaluate joint damage (erosions), if appropriate Consider autoimmune blood panel including ANA, RF, and anti-CCP, and anti-inflammatory markers (ESR and CRP) if symptoms persist; if symptoms are suggestive of reactive arthritis or affect the spine, consider HLA B27 testing | |
| G2 | |
| Complete history and examination as above; laboratory tests as above Consider US ± MRI of affected joints if clinically indicated (eg, persistent arthritis unresponsive to treatment, suspicion for differential diagnoses such as metastatic lesions or septic arthritis) Consider early referral to a rheumatologist, if there is joint swelling (synovitis) or if symptoms of arthralgia persist > 4 weeks | |
| G3–4 | |
| As for G2 Seek rheumatologist advice and review | |
| Monitoring: Patients with inflammatory arthritis should be monitored with serial rheumatologic examinations, including inflammatory markers, every 4–6 weeks after treatment is instituted. | |
| Grading | Management |
| All grades | Clinicians should follow reports of new joint pain to determine whether inflammatory arthritis is present; question whether symptom new since receiving ICPi |
| G1: Mild pain with inflammation, erythema, or joint swelling | Continue ICPi Initiate analgesia with acetaminophen and/or NSAIDs |
| G2: Moderate pain associated with signs of inflammation, erythema, or joint swelling, limiting instrumental ADL | Hold ICPi and resume upon symptom control and on prednisone ≤ 10 mg/d Escalate analgesia and consider higher doses of NSAIDS as needed If inadequately controlled, initiate prednisone or prednisolone 10–20 mg/d or equivalent for 4–6 weeks If improvement, slow taper according to response during the next 4–6 weeks; if no improvement after initial 4–6 weeks, treat as G3 If unable to lower corticosteroid dose to < 10 mg/d after 3 months, consider DMARD Consider intra-articular corticosteroid injections for large joints Referral to rheumatology |
| G3–4: Severe pain associated with signs of inflammation, erythema, or joint swelling; irreversible joint damage; disabling; limiting self-care ADL | Hold ICPi temporarily and may resume in consultation with rheumatology, if recover to G1 or less Initiate oral prednisone 0.5–1 mg/kg If failure of improvement after 4 weeks or worsening in meantime, consider synthetic or biologic DMARD Synthetic: methotrexate, leflunomide Biologic: consider anticytokine therapy such as TNF-α or IL-6 receptor inhibitors. (Note: As caution, IL-6 inhibition can cause intestinal perforation; while this is extremely rare, it should not be used in patients with colitis.) Test for viral hepatitis B, C, and latent/active TB test prior to DMARD treatment Referral to rheumatology. |
| Additional considerations Early recognition is critical to avoid erosive joint damage. Corticosteroids can be used as partof initial therapy in inflammatory arthritis, butdue to likely prolonged treatment requirements, physicians should considerstarting corticosteroid-sparing agents earlier than one would with other irAEs Oligoarthritis can be treated early on with intra-articular corticosteroids; consider early referral. Consider PCP prophylaxis for patients treated with high dose of corticosteroids for > 12 weeks, as per local guidelines. | |
| 5.2 Myositis | |
| Definition: A disorder characterized by muscle inflammation with weakness and elevated muscle enzymes (CK). Muscle pain can be present in severe cases. Can be life threatening if respiratory muscles or myocardium are involved | |
| Diagnostic work-up Complete rheumatologic and neurologic history regarding differential diagnosis; rheumatologic and neurologic examination, including muscle strength; and examination of the skin for findings suggestive ofdermatomyositis. Muscle weakness is more typical of myositis than pain. Consider preexisting conditions that can cause similar symptoms. Blood testing to evaluate muscle inflammation CK, transaminases (AST, ALT), LDH, and aldolase can also be elevated Troponin to evaluate myocardial involvement and other cardiac testing, such as echocardiogram, as needed Inflammatory markers (ESR and CRP) Consider EMG, imaging (MRI), and/or biopsy on an individual basis when diagnosis is uncertain and overlap with neurologic syndromes, such as myasthenia gravis, is suspected Consider paraneoplastic autoantibody testing for myositis and neurologic conditions, such as myasthenia gravis Monitoring: CK, ESR, CRP | |
| G1: Complete examination and laboratory work-up as above | |
| G2: Complete history and examination as above; autoimmune myositis blood panel; EMG, MRI of affected joints Early referral to a rheumatologist or neurologist | |
| G3–4: As for G2 Urgent referral to a rheumatologist or neurologist | |
| Grading | Management |
| G1: Mild weakness with or without pain | Continue ICPi If CK is elevated and patient has muscle weakness, may offer oral corticosteroids, and treat as G2 Offeranalgesia with acetaminophen or NSAIDs ifthereare no contraindications |
| G2: Moderate weakness with or without pain, limiting age-appropriate instrumental ADL | Hold ICPi temporarilyand may resume upon symptom control, if CKis normal and prednisone dose < 10 mg; if worsens, treat as per G3 NSAIDs as needed Referral to rheumatologist or neurologist If CK is elevated three times or more), initiate prednisone or equivalent at 0.5–1 mg/kg May require permanent discontinuation of ICPi in most patients with G2 symptoms and objective findings (elevated enzymes, abnormal EMG, abnormal muscle MRI or biopsy) |
| G3–4: Severe weakness with or without pain, limiting self-care ADL | Hold ICPi until G1 or less while off immune suppression and permanently discontinue if any evidence of myocardial involvement Consider hospitalization for severe weakness Referral to rheumatologist or neurologist Initiate prednisone 1 mg/kg or equivalent. Consider 1–2 mg/kg of methylprednisolone IV or higher-dose bolus if severe compromise (weakness severely limiting mobility, cardiac, respiratory, dysphagia) Consider plasmapheresis Consider IVIG therapy Consider other immunosuppressant therapy, such as methotrexate, azathioprine, or mycophenolate mofetil, if symptoms and CK levels do not improve or worsen after 4–6 weeks; rituximab is used in primary myositis but caution is advised given its long biologic duration |
| Additional considerations: Caution is advised with rechallenging | |
| 5.3 Polymyalgia-like syndrome | |
| Definition: Characterized by marked pain and stiffness in proximal upper and/or lower extremities and no signs of true muscle inflammation such as CK elevation or EMG findings of myositis. No true muscle weakness, difficulty in active motion related to pain | |
| Diagnostic work-up | |
| G1 | |
| Complete rheumatologic history regarding differential diagnosis and examination of all joints and skin Check for symptoms of temporal arteritis, such as headache or visual disturbances; refer to ophthalmologist if present, and consider temporal artery biopsy ANA, RF, anti-CCP CK to evaluate differential diagnosis of myositis Inflammatory markers (ESR, CRP) Monitoring: ESR, CRP | |
| G2: Complete history and examination as above; autoimmune tests as required for differential diagnosis; early referral to a rheumatologist | |
| G3–4: As for G2; see rheumatologist advice and review | |
| Grading | Management |
| G1: Mild stiffness and pain | Continue ICPi Initiate analgesia with acetaminophen and/or NSAIDs if there are no contraindications |
| G2: Moderate stiffness and pain, limiting age-appropriate instrumental ADL | Consider holding ICPi and resuming upon symptom control, prednisolone < 10 mg; if worsens, treat as per G3 Initiate prednisone 20 mg/d or equivalent; if symptoms improve, start to taper dose after 3–4 weeks If no improvementor need for higherdosages after4weeks, escalate to G3 Consider referral to rheumatology |
| G3–4: Severe stiffness and pain, limiting self-care ADL | Hold ICPi and may resume, in consultation with rheumatology, if recover to G1 or less; however, note that cases of toxicity returning upon rechallenge have been reported. Referral to rheumatology Should initiate prednisone 20 mg/d or equivalent. If no improvement or need for higher dosages for prolonged time, may offer a corticosteroid-sparing agent such as methotrexate or IL-6 inhibition with tocilizumab (Note: As caution, IL-6 inhibition can cause intestinal perforation; while this is extremely rare, it should not be used in patients with colitis or GI metastases). Consider admission for pain control |
| All recommendations are expert consensus based, with benefits outweighing harms, and strength of recommendations are moderate. | |
Abbreviations: ADL, activities of daily living; ANA, antinuclear antibodies; CCP, citrullinated protein antibody; CK, creatine kinase; CRP, C-reactive protein; DMARD, disease-modifying antirheumatic drug; EMG, electromyography; ESR, erythrocyte sedimentation rate; ICPi, immune checkpoint inhibitor; IL, interleukin; irAE, immune-related adverseevent; IV, intravenous; IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; NSAID, nonsteroidal anti-inflammatory drug; PCP, Pneumocystis pneumonia; RF, rheumatoid factor; TNF, tumor necrosis factor.
5.1. Inflammatory Arthritis
Recommendation 5.1a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following for grade 1:
Complete rheumatologic history and examination of all peripheral joints for tenderness, swelling, and range of motion. Examination of the spine.
Consider plain x-ray/imaging to exclude metastases and evaluate joint damage (erosions), if appropriate.
Consider autoimmune blood panel, including ANA, rheumatoid factor (RF), and anti–citrullinated protein antibody (anti-CCP), and inflammatory markers (ESR and CRP) if symptoms persist. If symptoms are suggestive of reactive arthritis or affect the spine, consider HLA B27 testing.
It is recommended that the diagnostic work-up should include the following for grade 2:
Complete history and examination as above; laboratory tests as above.
Consider ultrasound with or without MRI of affected joints if clinically indicated (eg, persistent arthritis unresponsive to treatment, suspicion for differential diagnoses such as metastatic lesions or septic arthritis).
Consider early referral to a rheumatologist if there is joint swelling (synovitis) or if symptoms of arthralgia persist > 4 weeks.
It is recommended that the diagnostic work-up should include the following for grades 3 to 4:
As for grade 2.
Seek rheumatologist advice and review.
It is recommended that all patients with inflammatory arthritis be monitored with serial rheumatologic examinations, including inflammatory markers, every 4 to 6 weeks after treatment is instituted.
Recommendation 5.1b – Management.
It is recommended that clinicians should follow reports of new joint pain to determine whether inflammatory arthritis is present. Clinicians should question whether symptoms are new since receiving ICPi.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue to offer ICPi.
Should initiate analgesia with acetaminophen and/or NSAIDs.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi and resume upon symptom control and on prednisone ≤ 10 mg/d.
Should escalate analgesia and consider higher doses of NSAIDs as needed.
If inadequately controlled, should initiate oral prednisone 10 to 20 mg/d or equivalent for 4 to 6 weeks.
If improvement, slow taper according to response during the next 4 to 6 weeks. If no improvement after initial 4 to 6 weeks, treat as grade 3.
If unable to lower prednisone dose to < 10 mg/d after 3 months, may offer DMARD.
May offer intra-articular corticosteroid injections for large joints.
Should refer to rheumatology.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should hold ICPi and may resume in consultation with rheumatology, if recover to grade 1 or less.
Should initiate oral prednisone 0.5 to 1 mg/kg.
If failure of improvement after 4 weeks or worsening in meantime, may offer synthetic or biologic DMARD:
Synthetic: methotrexate, leflunomide.
Biologic: consider anticytokine therapy, such as TNF-α or interleukin-6 (IL-6) receptor inhibitors. (Note: As caution, IL-6 inhibition can cause intestinal perforation. While this is extremely rare, it should not be used in patients with colitis).
Should test for viral hepatitis B, C, and latent/active TB test prior to DMARD treatment.
Should refer to rheumatology.
5.2. Myositis
Recommendation 5.2a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
Complete rheumatologic and neurologic history regarding differential diagnosis; rheumatologic and neurologic examination, including muscle strength; and examination of the skin for findings suggestive of dermatomyositis. Muscle weakness is more typical of myositis than pain. Consider preexisting conditions that can cause similar symptoms.
Blood testing to evaluate muscle inflammation.
CK, transaminases (AST, ALT), lactate dehydrogenase (LDH), and aldolase can also be elevated.
Troponin to evaluate myocardial involvement and other cardiac testing, such as echocardiogram, as needed.
Inflammatory markers (ESR and CRP).
Consider electromyography (EMG), imaging (MRI), and/or biopsy on an individual basis when diagnosis is uncertain and overlap with neurologic syndromes, such as myasthenia gravis, is suspected.
Consider paraneoplastic autoantibody testing for myositis and neurologic conditions, such as myasthenia gravis.
It is recommended that the following should be included for monitoring:
CK, ESR, CRP
It is recommended that the diagnostic work-up should include the following for grade 1:
Complete examination and laboratory work-up as specified in Section 5.2a
It is recommended that the diagnostic work-up should include the following for grade 2:
Complete history and examination as above; autoimmune myositis and neurologic panel; EMG, MRI of affected proximal limbs as needed. Consider muscle biopsy if diagnosis is uncertain.
Early referral to a rheumatologist or neurologist.
It is recommended that the diagnostic work-up should include the following for grade 3:
As for grade 2
Urgent referral to a rheumatologist or neurologist
Recommendation 5.2b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue to offer ICPi.
If CK is elevated and patient has muscle weakness, may offer oral corticosteroids and treat as grade 2.
Should offer analgesia as needed for pain with acetaminophen or NSAIDs if there are no contraindications.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi and may resume upon symptom control, if CK is normal, and prednisone dose < 10 mg; if worsens, treat as per grade 3. Permanently discontinue if there is evidence of myocardial involvement.
Should offer NSAIDs as needed.
Referral to rheumatologist or neurologist.
If CK is elevated (three times or more), should initiate prednisone or equivalent at 0.5 to 1 mg/kg.
May require permanent discontinuation of ICPi therapy in most patients with grade 2 symptoms and objective findings (elevated enzymes, abnormal EMG, abnormal muscle MRI or biopsy).
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should hold ICPi until grade 1 or less while off immune suppression and permanently discontinue if any evidence of myocardial involvement.
Consider hospitalization for severe weakness.
Referral to rheumatologist or neurologist.
Should initiate prednisone 1 mg/kg or equivalent. Consider 1 to 2 mg/kg of methylprednisolone IV or higher-dose bolus if severe compromise (weakness severely limiting mobility, cardiac, respiratory, dysphagia).
May offer plasmapheresis.
May offer IVIG therapy.
May offer other immunosuppressant therapy, such as methotrexate, azathioprine, or mycophenolate mofetil, if symptoms and laboratory findings do not improve or worsen after 4 to 6 weeks. Rituximab is used in primary myositis, but caution is advised given its long biologic duration.
5.3. Polymyalgia-Like Syndrome
Recommendation 5.3a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following for grade 1:
Complete rheumatologic history regarding differential diagnosis and examination of all joints and skin.
Check for symptoms of temporal arteritis, such as headache or visual disturbances; refer to ophthalmologist. If temporal arteritis is suspected, consider temporal artery biopsy.
ANA, RF, anti-CCP.
CK to evaluate differential diagnosis of myositis.
Inflammatory markers (ESR, CRP).
It is recommended that the following should be included for monitoring:
ESR, CRP
It is recommended that the diagnostic work-up should include the following for grade 2:
Complete history and examination as above
Autoimmune tests as above and others as required for differential diagnosis
Early referral to a rheumatologist
It is recommended that the diagnostic work-up should include the following for grades 3 to 4:
As for grade 2
Seek rheumatologist advice and review
Recommendation 5.3b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue to offer ICPi.
Should initiate analgesia with acetaminophen and/or NSAIDs if there are no contraindications.
It is recommended that clinicians manage grade 2 toxicities as follows:
May hold ICPi and resume upon symptom control, prednisone < 10 mg; if worsens, treat as per grade 3.
Should initiate prednisone 20 mg/d or equivalent. If symptoms improve, start to taper dose after 3 to 4 weeks.
If no improvement or need for higher dosages after 4 weeks, escalate to grade 3.
Consider referral to rheumatology.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should hold ICPi and may resume, in consultation with rheumatology, if recover to grade 1 or less. However, note that cases of toxicity returning upon rechallenge have been reported.
Referral to rheumatology.
Should initiate prednisone 20 mg/d or equivalent. If no improvement or need for higher dosages for prolonged time, consider a corticosteroid-sparing agent such as methotrexate or IL-6 inhibition with tocilizumab. (Note: As caution, IL-6 inhibition can cause intestinal perforation. While this is extremely rare, it should not be used in patients with colitis or GI metastases).
Consider admission for pain control.
Discussion.
Musculoskeletal symptoms such as arthralgia and myalgia are common in patients receiving ICPi therapy, as reported in up to 40% of those treated in clinical trials.67,68 More-severe inflammatory AEs are not as frequent but can have an important effect on patients’ quality of life because of their effect on function and daily activities.69 The most common musculoskeletal and rheumatic irAEs are arthritis, polymyalgia-like syndromes, and myositis. These events can occur with either CTLA-4 or anti–PD-1/PD-L1 antagonists, but seem to be more frequent with the latter class of drugs and when these agents are used in combination.67
The clinical presentation of patients with immune-related arthritis secondary to ICPi can vary and affect large and/or small joints.68 Some patients present with oligoarthritis of large joints, such as knees, ankles, or wrists. These patients can also have other features commonly seen with reactive arthritis, such as conjunctivitis or urethritis, and occasionally complain of back pain or cervical pain suggestive of sacroiliitis. Other patients present with symmetrical polyarthritis resembling rheumatoid arthritis and can have autoantibodies such as RF present in their seras. Many patients also develop sicca symptoms, with dry eyes and dry mouth; autoantibodies, such anti-SSA and anti-SSB, have occasionally been found, but most patients tend to be seronegative. Of interest, arthritis can occur at any time during treatment. Some patients have developed arthritis for the first time many months after initiation of ICPi therapy.70 Most common differential diagnoses include other causes of joint pain, including degenerative joint disease or osteoarthritis and soft tissue rheumatic disorders, such as rotator cuff tendinitis, crystal arthropathies (gout and pseudogout), and septic arthritis. Diagnostic evaluation should include serum inflammatory markers (ESR, CRP), evaluation of autoantibodies (ANA, RF, and anti-CCP), and imaging as needed (x-rays, ultrasound, and/or MRI). Inflammatory markers are usually very elevated in patients with ICPi-induced arthritis and are useful to differentiate these events from other rheumatic syndromes. NSAIDs alone are usually not sufficient to control symptoms, and corticosteroids and synthetic or biologic DMARDs might be required.71,72 Intra-articular corticosteroid injections are an option if only one or two joints are affected.
Patients receiving ICPis can develop severe myalgia in their proximal upper and lower extremities, with severe fatigue resembling polymyalgia rheumatica.73 These patients can also have arthralgia but typically do not have definite synovitis, although ultrasound or MRI might show a mild effusion in the shoulder joints. Patients experiencing a polymyalgia-like syndrome have pain but not true weakness. Differential diagnoses include inflammatory myositis, fibromyalgia, statin-induced myopathy, and other types of arthritis or soft tissue rheumatic syndromes. RF and anti-CCP are negative, and inflammatory markers are highly elevated. CK levels should generally be within normal limits, differentiating this condition from myositis. Imaging with MRI and EMG should not show any evidence of myopathy or muscle inflammation.
Myositis is a rare complication of ICPis but can be severe and fatal. It is more common with PD-1/PD-L1 inhibitors than with ipilimumab.68,74 It can present as reactivation of preexisting paraneoplastic polymyositis or dermatomyositis or as a de novo myositis. The main symptom of inflammatory myositis is weakness, primarily in the proximal extremities, with difficulties in standing up, lifting arms, and moving around. In severe cases, patients can complain of myalgia as well. Patients with de novo myositis do not develop the typical rash seen with paraneoplastic dermatomyositis. Myositis can have a fulminant necrotizing course with rhabdomyolysis and can involve vital skeletal muscle, such as the myocardium, in which case it requires urgent treatment to avoid fatal complications.75,76 Laboratory tests include measurement of muscle enzymes, especially CK, which often is markedly elevated, and inflammatory markers; autoantibody panels for myositis can be considered, although there is no evidence that any specific autoantibodies have a role in ICPi-associated myositis. Other diagnostic tests that may be useful include EMG, which can show muscle fibrillations indicative of myopathy, and/or MRI, which shows increased intensity and edema in affected muscles. Finally, biopsy can be performed to confirm the diagnosis. Differential diagnoses include generalized fatigue, polymyalgia rheumatica, fibromyalgia, adverse events from concomitant therapies (eg, statins, corticosteroids), and muscle dystrophies. These other disorders (other than some muscle dystrophies or drug-induced myopathy) have normal CK. The cornerstone of initial treatment is high-dose corticosteroids that should be administered as a bolus in severe cases. Plasmapheresis should be considered in cases with poor response to corticosteroids or in life-threatening situations. The use of other immunosuppressants and IVIG may also be indicated, as they are used for treatment of polymyositis/dermatomyositis. However, their efficacy in ICPi-induced myositis is not clearly documented.
A number of other rheumatic disorders have been occasionally documented as case reports of patients receiving ICPis.77 These include vasculitis and lupus-like syndromes, among others. Management and treatment principles are similar to those reported for other ICPi-induced rheumatic syndromes.
Patients with preexisting autoimmune rheumatic conditions may be at higher risk of toxicity as either irAEs or flares of their preexisting disease.78 Many of these patients, nevertheless, can continue ICPi therapy or be rechallenged after their AEs are properly managed, so having preexisting autoimmune disease does not represent an absolute contraindication for treatment. Close monitoring and multidisciplinary management is required for these patients, as they frequently need concomitant treatment of their preexisting autoimmune disease once they develop an AE. Management principles are similar to those described for irAE.
6.0. Renal Toxicities
Please refer to Table 6 for a complete set of recommendations, definition of grades, and additional considerations.
Table 6.
Management of Renal irAEs in Patients Treated With ICPis
| 6.0 Renal Toxicities | |
| Nephritis and renal dysfunction: diagnosis and monitoring For any suspected immune-mediated adverse reactions, exclude other causes Monitor patients for elevated serum creatinine prior to every dose Routine urinalysis is not necessary, other than to rule out UTIs, etc; nephrology may consider further If no potential alternative cause of AKI identified, then one should forego biopsy and proceed directly with immunosuppressive therapy Swift treatment of autoimmune component important | |
| 6.1 Nephritis | |
| Definition: Inflammation of the kidney affecting the structure | |
| Grading | Management |
| G1: Creatinine level increase of > 0.3 mg/dL; creatinine 1.5–2.0 × over baseline | Consider temporarily holding ICPi, pending consideration of potential alternative etiologies (recent IV contrast, medications, fluid status) and baseline renal function. A change that is still < 1.5 ULN could be meaningful |
| G2: Creatinine 2–3 × above baseline | Hold ICPi temporarily Consult nephrology Evaluate for other causes (recent IV contrast, medications, fluid status, etc); if other etiologies ruled out, administer 0.5–1 mg/kg/d prednisone equivalents If worsening or no improvement: 1 to 2 mg/kg/d prednisone equivalents and permanently discontinue treatment If improved to G1 or less, taper corticosteroids over 4–6 weeks If no recurrence of chronic renal insufficiency, discuss resumption of ICPI with patient after taking into account the risks and benefits. |
| G3: Creatinine > 3 × baseline or > 4.0 mg/dL; hospitalization indicated | Permanently discontinue ICPi |
| G4: Life-threatening consequences; dialysis indicated | Consult nephrology Evaluate for other causes (recent IV contrast, medications, fluid status, etc) Administercorticosteroids (initial dose of 1–2 mg/kg/d prednisone or equivalent) |
| Additional considerations Monitor creatinine weekly Reflex kidney biopsy should be discouraged until corticosteroid treatment has been attempted | |
| 6.2 Symptomatic nephritis: follow-up | |
| Grading | Management |
| G1 | If improved to baseline, resume routine creatinine monitoring |
| G2 | If improved to G1, taper corticosteroids over at least 3 weeks before resuming treatment with routine creatinine monitoring If elevations persist > 7 days or worsen and no other cause found, treat as G3 |
| G3 | If improved to G1, taper corticosteroids over at least 4 weeks If elevations persist > 3–5 days orworsen, consider additional immunosuppression (eg, mycophenolate) |
| G4 | If improved to G1, taper corticosteroids over at least 4 weeks Ifelevations persist > 2–3 days orworsen, consideradditional immunosuppression (eg, mycophenolate) |
| All recommendations are expert consensus based, with benefits outweighing harms, and strength of recommendations are moderate. | |
Abbreviations: AKI, acute kidney injury; G, grade; ICPi, immune checkpoint inhibitor; irAE, immune-related adverse event; IV, intravenous; ULN, upper limit of normal; UTI, urinary tract infection.
6.1. Nephritis
Recommendation 6.1a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
For any suspected immune-mediated adverse reactions, should exclude other causes.
Monitor patients for elevated serum creatinine prior to every dose.
Qualifying Statement.
Routine urinalysis is not necessary other than to rule out urinary tract infections, etc. Nephrology may be considered. If no potential alternative cause of acute kidney injury (AKI) is identified, then one should forego biopsy and proceed directly with immunosuppressive therapy. The swift treatment of the autoimmune component is important.
Recommendation 6.1b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
May hold treatment temporarily, pending consideration of potential alternative etiologies (recent IV contrast, medications, fluid status) and baseline renal function. (Note: A change that is still < 1.5 ULN could be meaningful).
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold treatment temporarily.
Should consult nephrology.
Should evaluate for other causes (recent IV contrast, medications, fluid status, etc). If other etiologies are ruled out, should administer 0.5 to 1 mg/kg/d prednisone equivalents.
If worsening or no improvement, should administer 1 to 2 mg/kg/d prednisone or equivalent and permanently discontinue ICPi.
If improved to grade 1 or less, taper corticosteroids over 4 to 6 weeks.
If no recurrence of chronic renal insufficiency, discuss resumption of ICPi with patient after taking into account the risks and benefits.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should permanently discontinue ICPi.
Should consult nephrology.
Should evaluate for other causes (recent IV contrast, medications, fluid status, etc).
Should administer corticosteroids (initial dose of 1 to 2 mg/kg/d prednisone or equivalent).
6.2. Symptomatic Nephritis
Recommendation 6.2a – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
If improved to baseline, should resume routine creatinine monitoring.
It is recommended that clinicians manage grade 2 toxicities as follows:
If improved to grade 1, should taper corticosteroids over at least 3 weeks before resuming treatment with routine creatinine monitoring.
If elevations persist > 7 days or worsen and no other cause found, should treat as grade 3.
It is recommended that clinicians manage grade 3 toxicities as follows:
If improved to grade 1, should taper corticosteroids over at least 4 weeks.
If elevations persist > 3 to 5 days or worsen, may offer additional immunosuppression (eg, mycophenolate).
It is recommended that clinicians manage grade 4 toxicities as follows:
If improved to grade 1, should taper corticosteroids over at least 4 weeks.
If elevations persist > 2 to 3 days or worsen, may offer additional immunosuppression (eg, mycophenolate).
Discussion.
AKI is an uncommon complication of checkpoint inhibitor immunotherapy. The estimated incidence of any-grade AKI was 1% to 2% in patients treated with a single agent (ipilimumab, nivolumab, pembrolizumab) and 4.5% in those treated with the combination of nivolumab plus ipilimumab. The incidence of grade 3 or 4 AKI was < 1% with single agents and 1.6% with the combination of nivolumab plus ipilimumab.79,80 While initial studies had quoted a small incidence of AKI with ICPi use, emerging data suggest a higher incidence rate of AKI (range, 9.9% to 29%) with ICPi. The vast majority of this extra toxicity is stage I based on AKI network criteria80 and typically involves electrolyte disturbances rather than declines in renal function.
In a retrospective series of 13 patients who underwent kidney biopsy at seven centers, renal toxicity was diagnosed a median of 91 days after initiation of checkpoint inhibitor immunotherapy (range, 21 to 245 days). The median peak serum creatinine was 4.5 mg/dL. Pathology from the kidney biopsies revealed acute tubulointerstitial nephritis in 12 patients and thrombotic microangiography in one patient. Two of 13 patients required transient hemodialysis, and two remained on hemodialysis at the time of publication.81 Checkpoint inhibitor immunotherapy was discontinued in all 13 patients. Eleven patients were treated with corticosteroids, and among these 11, nine improved. One patient with thrombotic microangiopathy did not improve, despite glucocorticoids, and another patient transiently improved but then worsened. Two additional patients did not receive immunosuppression and did not recover renal function.
Checkpoint inhibitor therapy appears to be safe in patients with baseline renal impairment from a nonimmune basis (eg, prior nephrectomy, old age, hypertension); however, patients with a renal allograft are at high risk of rejecting the transplanted kidney and requiring dialysis. Limited data suggest that the risk of renal allograft rejection with anti–CTLA-4 antibodies82 may be less than the near-universal rejection seen with PD-1 pathway blockers.82–85 Although some patients may be able to be treated with PD-1 pathway blockers with preservation of their allografts by having adjustments in their immunosuppressive agents,86 this approach should only be considered with multidisciplinary input from the renal transplant nephrology team.
Patients should have their renal function (serum creatinine) checked prior to every dose of checkpoint inhibitor therapy. For those with new elevations in creatinine, one should consider holding therapy while other potential causes are evaluated (eg, recent IV radiographic contrast administration, dehydration, other medicines, urinary tract infection) and if identified, treat appropriately. Patients without other obvious causes or who do not respond to alternative treatment measures should be presumed to have immune-related renal toxicity and treated empirically according to the algorithm.
7.0. Nervous System Toxicities
Please refer to Table 7 for a complete set of recommendations, definition of grades, and additional considerations.
Table 7.
Management of Nervous System irAEs in Patients Treated With ICPis
| 7.0 Nervous System Toxicities | |
| 7.1 Myasthenia gravis | |
| Definition: Fatigable or fluctuating muscle weakness, generally more proximal than distal. Frequently has ocular and/or bulbar involvement (ptosis, extraocular movement abnormalities resulting in double vision, dysphagia, dysarthria, facial muscle weakness). May have neckand/or respiratory muscle weakness. (Note: May occurwith myositis and/or myocarditis. Respiratory symptoms may require evaluation to ruleout pneumonitis, myocarditis. Miller Fishervariantof Guillain- Barré syndrome (ophthalmoparesis) and the oculobulbar myositis (ptosis, ophthalmoparesis, dysphagia, neck and respiratory weakness) with ICPi may have overlapping symptoms. | |
| Diagnostic work-up AChR and antistriated muscle antibodies in blood; if AChR antibodies are negative, consider muscle specific kinase and lipoprotein-related 4 antibodies in blood Pulmonary function assessment with NIF and VC CPK, aldolase, ESR, CRP for possible concurrent myositis Consider MRI of brain and/or spine, depending on symptoms to rule out CNS involvement by disease or alternate diagnosis If respiratory insufficiency or elevated CPK, troponin T, perform cardiac examination with ECG and TTE for possible concomitant myocarditis Neurologic consultation Electrodiagnositic studies, including neuromuscularjunction testing with repetitive stimulation and/orjitter studies, NCS to exclude neuropathy, and needle EMG to evaluate for myositis | |
| Grading | Management |
| All grades | All grades warrant work-up and intervention given potential for progressive myasthenia gravis to lead to respiratory compromise |
| No G1 | |
| G2: Some symptoms interfering with ADL MGFA severity class 1 (ocular symptoms and findings only) and MGFA severity class 2 (mild generalized weakness) | Hold ICPi and may resume in G2 patients (MGFA 1 and 2) only if symptoms resolve87 Should consult neurology Pyridostigmine starting at 30 mg orally three times a day and gradually increase to maximum of 120 mg orally four times a day as tolerated and based on symptoms Administer corticosteroids (prednisone, 1–1.5 mg/kg orally daily) if symptoms G2; wean based on symptom improvement |
| G3–4: Limiting self-care and aids warranted, weakness limiting walking, ANY dysphagia, facial weakness, respiratory muscle weakness, or rapidly progressive symptoms, or MGFA severityclass 3–4 moderate to severe generalized weakness to myasthenic crisis | Permanently discontinue ICPi Admit patient, may need ICU-level monitoring Neurology consult Continue corticosteroids and initiate IVIG 2 g/kg IV over 5 days (0.4 g/kg/d) or plasmapheresis for 5 days Frequent pulmonary function assessment Daily neurologic review |
| Additional considerations Avoid medications that can worsen myasthenia: β-blockers, IV magnesium, fluoroquinolones, aminoglycosides, and macrolides Initially a 5-day course of plasmapheresis or a 2 g/kg course of IVIG over 5 days 1–2 mg/kg methylprednisolone daily, wean based on symptom improvement Pyridostigmine, wean based on improvement ICPi-associated myasthenia gravis may be monophasic, and additional corticosteroid-sparing agents may not be required | |
| 7.2 Guillain-Barré syndrome | |
| Definition: Progressive, most often symmetrical muscle weakness with absent or reduced deep tendon reflexes. Often starts with sensory symptoms/neuropathic pain localized to lower back and thighs. May involve extremities (typically ascending weakness but not always), facial, respiratory, and bulbar and oculomotor nerves. May have dysregulation of autonomic nerves. | |
| Diagnostic work-up Neurologic consultation MRI of spine with or without contrast (rule out compressive lesion and evaluate for nerve root enhancement/thickening) Lumbar puncture: CSF typically has elevated protein and often elevated WBCs; even though this is not typically seen in classic Guillain-Barré syndrome, cytology should be sent with any CSF sample from a patient with cancer. Serum antiganglioside antibody tests for Guillain-Barré syndromeand its subtypes (eg, anti-GQlb for Miller Fishervariantassociated with ataxia and ophthalmoplegia) Electrodiagnostic studies to evaluate polyneuropathy Pulmonary function testing (NIF/NC) Frequent neurochecks | |
| Grading | Management |
| All grades | Warrant work-up and intervention given potential for progressive Guillain-Barré syndrome to lead to respiratory compromise Note: There is no G1 toxicity |
| G1: Mild, none | NA |
| G2: Moderate, some interference with ADL, symptoms concerning to patient G3–4: Severe, limiting self-care and aids warranted, weakness limiting walking, ANY dysphagia, facial weakness, respiratory muscle weakness, or rapidly progressive symptoms |
Discontinue ICPi Admission to inpatient unit with capability of rapid transfer to ICU-level monitoring Start IVIG (0.4 g/kg/d for 5 days for a total dose of 2 g/kg) or plasmapheresis. Corticosteroids are usually not recommended for idiopathic Guillain-Barré syndrome; however, in ICPi-related forms, a trial is reasonable (methylprednisolone 2–4 mg/kg/d), followed by slow corticosteroid taper Pulse corticosteroid dosing (methylprednisolone 1 g/d for 5 days) may also be considered for G3–4 along with IVIG or plasmapheresis Frequent neurochecks and pulmonary function monitoring Monitor for concurrent autonomic dysfunction Nonopioid management of neuropathic pain Treatment of constipation/ileus |
| Additional considerations Slow prednisone taper after corticosteroid pulse plus IVIG or plasmapheresis May require repeat IVIG courses Caution with rechallenging for severe cases | |
| 7.3 Peripheral neuropathy | |
| Definition: Can present as asymmetric or symmetric sensory, motor, or sensory motor deficit. Focal mononeuropathies, including cranial neuropathies (eg, facial neuropathies/Bell palsy) may be present. Numbness and paresthesias may be painful or painless. Hypo- or areflexia or sensory ataxia may be present. | |
| Diagnostic work-up | |
| G1 Screen for reversible neuropathy causes: diabetic screen, B12, folate, TSH, HIV, consider serum protein electrophoresis, and other vasculitic and autoimmune screen Neurologic consultation Consider MRI of spine with or without contrast | |
| G2: in addition to above MRI spine advised/MRI of brain if cranial nerve Consider EMG/NCS Consider neurology consultation | |
| G3–4: go to Guillain-Barré syndrome algorithm | |
| Grading | Management |
| G1: Mild, no interference with function and symptoms not concerning to patient. Note: Any cranial nerve problem should be managed as moderate | Low threshold to hold ICPi and monitor symptoms for a week If to continue, monitor very closely for any symptom progression |
| G2: Moderate, some interference with ADL, symptoms concerning to patient (ie, pain but no weakness or gait limitation) |
Hold ICPi and resume once return to G1 Initial observation OR initiate prednisone 0.5–1 mg/kg (if progressing from mild) Neurontin, pregabalin, or duloxetine for pain |
| G3–4: Severe, limiting self-care and aids warranted, weakness limiting walking or respiratory problems (ie, leg weakness, foot drop, rapidly ascending sensory changes) Severe may be Guillain-Barré syndrome and should be managed as such | Permanently discontinue ICPi Admit patient Neurologic consultation Initiate IV methylprednisolone 2–4 mg/kg and proceed as per Guillain-Barré syndrome management |
| 7.4 Autonomic neuropathy | |
| Definition: Nerves that control involuntary bodily functions are damaged. This may affect blood pressure, temperature control, digestion, bladder function, and sexual function. A case of severe enteric neuropathy with ICPi has been reported. Can present with GI difficulties such as new severe constipation, nausea, urinary problems, sexual difficulties, sweating abnormalities, sluggish pupil reaction, and orthostatic hypertension. | |
| Diagnostic work-up An evaluation by neurologist or relevant specialist, depending on organ system, with testing that may include Screen for other causes of autonomic dysfunction: diabetic screen, adrenal insufficiency, HIV, parproteinemia, amyloidosis, botulism, consider chronic diseases such as Parkinson's and other autoimmune screen •Orthostatic vital signs •Consider electrodiagnostic studies to evaluate for concurrent polyneuropathy •Consider paraneoplastic autoimmune dysautonomia antibody testing (eg, anti-ganglionic acetylcholine receptor, antineuronal nuclearantibodytype 1 [ANNA-1], and N-type voltage gated calcium channel antibodies) | |
| Grading | Management |
| G1: Mild, no interference with function and symptoms not concerning to patient | Low threshold to hold ICPi and monitor symptoms for a week; if to continue, monitor very closely for any symptom progression |
| G2: Moderate, some interference with ADL, symptoms concerning to patient | Hold ICPi and resume once return to G1 Initial observation OR initiate prednisone 0.5–1 mg/kg (if progressing from mild) Neurologic consultation |
| G3–4: Severe, limiting self-care and aids warranted | Permanently discontinue ICPi Admit patient Initiate methylprednisolone 1 g daily for 3 days followed by oral corticosteroid taper Neurologic consultation |
| 7.5 Aseptic meningitis | |
| Definition: may present with headache, photophobia, and neck stiffness; often afebrile but may be febrile. There may be nausea/vomiting. Mental status should be normal (distinguishes from encephalitis). | |
| Diagnostic work-up MRI of brain with or without contrast + pituitary protocol AM cortisol, ACTH to rule out adrenal insufficiency Consider lumbar puncture: measure opening pressure; check cell count and protein glucose; and perform Gram stain, culture, PCR for HSV, and other viral PCRs depending on suspicion, cytology May see elevated WBC count with normal glucose, normal culture, and Gram stain; may see reactive lymphocytes or histiocytes on cytology | |
| Grading | Management |
| G1: Mild, no interference with function and symptoms not Hold ICPi and discuss resumption with patient only after taking into account the risks concerning to patient. Note: Any cranial nerve problem should be managed as moderate. G2: Moderate, some interference with ADL, symptoms concerning to patient (ie, pain but no weakness or gait limitation) G3–4: Severe, limiting self-care and aids warranted |
Hold ICPi and discuss resumption with patient only after taking into account the risks and benefits Consider empirical antiviral (IV acyclovir) and antibacterial therapy until CSF results Once bacterial and viral infection are negative, may closely monitor off corticosteroids or consider oral prednisone 0.5–1 mg/kg or IV methylprednisolone 1 mg/kg if moderate/severe symptoms |
| 7.6 Encephalitis | |
| Definition: As for aseptic meningitis, need to exclude infectious causes, especially viral (ie, HSV). Confusion, altered behavior, headaches, seizures, short-term memory loss, depressed level of consciousness, focal weakness, speech abnormality | |
| Diagnostic work-up Neurologic consultation MRI of brain with orwithout contrast may reveal T2/fluid-attenuated inversion recovery changes typical of what is seen in autoimmune encephalopathies or limbic encephalitis or may be normal Lumbar puncture: check cell count and protein glucose and perform Gram stain, culture, PCR for HSV and other viral PCRs depending on suspicion, cytology, oligoclonal bands, autoimmune encephalopathy, and paraneoplastic panels. May see elevated WBC count with lymphocytic predominance and/or elevated protein EEG to evaluate for subclinical seizures Blood: metabolic, CBC, ESR, CRP, ANCA (if suspect vasculitic process), thyroid panel including TPO and thyroglobulin Rule out concurrent anemia/thrombocytopenia, which can present with severe headaches and confusion | |
| Grading | Management |
| G1: Mild, no interference with function and symptoms not concerning to patient. Note: Any cranial nerve problem should be managed as moderate. G2: Moderate, some interference with ADL, symptoms concerning to patient (ie, pain but no weakness or gait limitation) G3–4: Severe, limiting self-care and aids warranted |
Hold ICPi and discuss resumption with patient only after taking into account the risks
and benefits As above for aseptic meningitis, suggest concurrent IV acyclovir until PCR results obtained and negative Trial of methylprednisolone 1–2 mg/kg If severe or progressing symptoms or oligoclonal bands present, consider pulse corticosteroids methylprednisolone 1 g IV daily for 3–5 days plus IVIG 2 g/kg over 5 days If positive for autoimmune encephalopathy antibody and limited or no improvement, consider rituximab or plasmapheresis in consultation with neurology |
| 7.7 Transverse myelitis | |
| Definition: Acute or subacute weakness or sensory changes bilateral, often with increased deep tendon reflexes | |
| Diagnostic work-up Neurologic consultation MRI of spine (with thin axial cuts through the region of suspected abnormality) and MRI of brain Lumbar puncture: cell count, protein, glucose, oligoclonal bands, viral PCRs, cytology, onconeural antibodies Blood: B12, HIV, RPR, ANA, Ro/La, TSH, aquaporin-4 IgG Evaluation for urinary retention, constipation | |
| Grading | Management |
| G1: Mild, no interference with function and symptoms not concerning to patient. Note: Any cranal nerve problem should be managed as moderate. G2:Moderate, someinterferencewith ADL, symptoms concerning to patient (ie, pain but no weakness or gait limitation) G3–4: Severe, limiting self-care and aids warranted |
Permanently discontinue ICPi Methylprednisolone 2 mg/kg Strongly consider higher doses of 1 g/d for 3–5 days Strongly consider IVIG |
| All recommendations are expert consensus based, with benefits outweighing harms, and strength of recommendations are moderate. | |
Abbreviations: AChR, acetylcholine receptor; ACTH, adrenocorticotropic hormone; ADL, activities of daily living; ANCA, antineutrophil cytoplasmic antibodies; CPK, creatine phosphokinase; CRP, C-reactive protein; EMG, electromyography; ESR, erythrocyte sedimentation rate; HSV, herpes simplex virus; ICPi, immune checkpoint inhibitor; ICU, intensive care unit; IgG, immunoglobulin G; IV, intravenous; IVIG, intravenous immunoglobulin; irAE, immune-related adverse event; MGFA, Myasthenia Gravis Foundation of America; MRI, magnetic resonance imaging; NA, not applicable; NCS, nerve conduction study; NIF, negative inspiratory force; PCR, polymerase chain reaction; RPR, rapid plasma reagin, TPO, thyroid peroxidase; TSH, thyroid-stimulating hormone; TTE, transthoracic echocardiogram; VC, vital capacity.
7.1. Myasthenia Gravis
Recommendation 7.1a – Diagnostic work-up.
It is recommended that the diagnostic work-up for all grades should include the following:
Acetylcholine receptor (AChR) and antistriated muscle antibodies in blood. If AChR antibodies are negative, consider muscle-specific kinase and lipoprotein-related 4 antibodies in blood.
Pulmonary function assessment with negative inspiratory force and vital capacity.
Creatine phosphokinase (CPK), aldolase, ESR, and CRP for possible concurrent myositis.
Consider MRI of brain and/or spine, depending on symptoms, to rule out CNS involvement by disease or alternate diagnosis.
If respiratory insufficiency or elevated CPK, troponin T, perform cardiac examination with ECG and transthoracic echocardiogram (TTE) for possible concomitant myocarditis.
Neurology consultation.
Electrodiagnositic studies, including neuromuscular junction testing with repetitive stimulation and/or jitter studies, nerve conduction study (NCS), to exclude neuropathy, and needle EMG to evaluate for myositis.
Recommendation 7.1b – Management.
All grades warrant work-up and intervention given potential for progressive myasthenia gravis to lead to respiratory compromise. (Note: There is no grade 1 toxicity).
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi and may resume in grade 2 patients (Myasthenia Gravis Foundation of America 1 and 2) only if symptoms resolve.87
Should consult neurology.
Should offer pyridostigmine starting at 30 mg PO three times a day and gradually increase to a maximum of 120 mg orally four times day as tolerated and based on symptoms.
May go directly to corticosteroids (prednisone 1 to 1.5 mg/kg PO daily) if symptoms grade 2. Wean based on symptom improvement.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should permanently discontinue ICPi.
Should admit patient, may need intensive care unit monitoring.
Consult neurology.
Continue corticosteroids and initiate IVIG 2 g/kg over 5 days or plasmapheresis for 5 days.
Should offer frequent pulmonary function assessment.
Should offer daily neurologic evaluation.
Qualifying statement.
Avoid medications that can worsen myasthenia, such as β-blockers, IV magnesium, fluoroquinolones, aminoglycosides, and macrolides. ICPi-associated myasthenia gravis may be monophasic; therefore, additional corticosteroidsparing agents may not be required.
7.2. Guillain-Barré Syndrome
Recommendation 7.2a – Diagnostic work-up.
It is recommended that the diagnostic workup should include the following:
Neurology consultation.
MRI of spine with and without contrast (rule out compressive lesion and evaluate for nerve root enhancement/thickening).
Lumbar puncture: CSF typically has elevated protein and often elevated WBCs; even though this is not typically seen in classic Guillain-Barré syndrome (GBS), cytology should be sent with any CSF sample from a patient with cancer.
Serum antiganglioside antibody tests for GBS and its subtypes (eg, anti-GQ1b for Miller Fisher variant associated with ataxia and ophthalmoplegia).
Electrodiagnostic studies to evaluate polyneuropathy.
Pulmonary function testing (negative inspiratory force/vital capacity).
Frequent neurochecks.
Recommendation 7.2b – Management.
All grades warrant work-up and intervention given the potential for progressive GBS to lead to respiratory compromise. (Note: There is no grade 1 toxicity).
It is recommended that clinicians manage all-grade toxicities as follows:
Should discontinue ICPi.
Admission to inpatient unit with capability for rapid transfer to intensive care unit-level monitoring.
Corticosteroids are not usually recommended for idiopathic GBS; however, in ICPi-related forms, a trial is reasonable. Should start IVIG 0.4 g/kg/d for 5 days for a total dose of 2 g/kg or plasmapheresis plus concurrent corticosteroids (methylprednisolone 2 to 4 mg/kg/d).
Should offer frequent neurochecks and pulmonary function monitoring.
Should monitor for concurrent autonomic dysfunction.
Nonopioid management of neuropathic pain.
Treatment of constipation/ileus.
7.3. Peripheral Neuropathy
Recommendation 7.3a – Diagnostic work-up.
It is recommended that the diagnostic work-up for grade 1 should include the following:
Screen for reversible neuropathy causes: diabetic screen, B12, folate, TSH, HIV. Consider serum protein electrophoresis and other vasculitic and autoimmune screen.
Consider MRI of spine with or without contrast.
Consider neurology consultation.
It is recommended that the diagnostic work-up for grade 2 should include the following, in addition to what is recommended for grade 1:
MRI of spine advised; MRI of brain if cranial nerve.
Consider EMG/NCS.
Consider neurology consultation
It is recommended that the diagnostic work-up for grades 3 to 4 should follow that of GBS.
Recommendation 7.3b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should have a low threshold to hold ICPi and monitor symptoms for a week. If to continue, should monitor very closely for any symptom progression.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi and resume once return to grade 1.
Should offer initial observation OR may initiate prednisone 0.5 to 1 mg/kg (if progressing from mild).
Should offer neurontin, pregabalin, or duloxetine for pain.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should permanently discontinue ICPi.
Should admit patient.
Should consult neurology.
Should initiate IV methylprednisolone 2 to 4 mg/kg and proceed as per GBS management.
7.4. Autonomic Neuropathy
Recommendation 7.4a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
An evaluation by neurologist or relevant specialist, depending on organ system, with testing that may include:
Screening for other causes of autonomic dysfunction: diabetic screen, adrenal insufficiency, HIV, paraproteinemia, amyloidosis, botulism. Consider chronic diseases such as Parkinson and other autoimmune screening.
Orthostatic vital signs.
Consider electrodiagnostic studies to evaluate for concurrent polyneuropathy.
Consider paraneoplastic autoimmune dysautonomia antibody testing (eg, anti-ganglionic acetylcholine receptor, antineuronal nuclear antibody type 1 [ANNA-1], and N-type voltage gated calcium channel antibodies).
Recommendation 7.4b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should have a low threshold to hold ICPi and monitor symptoms for a week. If to continue, should monitor very closely for any symptom progression.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi and resume once return to grade 1.
Should offer initial observation OR may initiate prednisone 0.5 to 1 mg/kg (if progressing from mild).
Should consult neurology.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should permanently discontinue ICPi.
Should admit patient.
Should initiate methylprednisolone 1 g daily for 3 days followed by oral corticosteroid taper.
Should consult neurology.
7.5. Aseptic Meningitis
Recommendation 7.5a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
MRI of brain with or without contrast and pituitary protocol.
Cortisol (AM), adrenocorticotropic hormone (ACTH) to rule out adrenal insufficiency.
Consider lumbar puncture: measure opening pressure; check cell count and protein glucose; and perform Gram stain, culture, and polymerase chain reaction (PCR) for herpes simplex virus and other viral PCRs depending on suspicion, cytology.
May see elevated WBC count with normal glucose, normal culture, and Gram stain. May see reactive lymphocytes or histiocytes on cytology.
Recommendation 7.5b – Management.
It is recommended that clinicians manage all-grade toxicities as follows:
Should hold ICPi.
May offer empirical antiviral (IV acyclovir) and antibacterial therapy until CSF results.
Once bacterial and viral infection are negative, may closely monitor off corticosteroids or consider oral prednisone 0.5 to 1 mg/kg or IV methylprednisolone 1 mg/kg if moderate/severe symptoms.
7.6. Encephalitis
Recommendation 7.6a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
Neurologic consultation.
MRI of brain with or without contrast may reveal T2/fluid-attenuated inversion recovery changes typical of what is seen in autoimmune encephalopathies or limbic encephalitis or may be normal.
Lumbar puncture: check cell count and protein glucose and perform Gram stain, culture, PCR for herpes simplex virus, and other viral PCRs depending on suspicion, cytology, oligoclonal bands, autoimmune encephalopathy, and paraneoplastic panels.
May see elevated WBC count with lymphocytic predominance and/or elevated protein.
EEG to evaluate for subclinical seizures.
Blood: metabolic; CBC; ESR; CRP; antineutrophil cytoplasmic antibodies (if suspect vasculitic process); and thyroid panel, including thyroid peroxidase and thyroglobulin.
Rule out concurrent anemia/thrombotic thrombocytopenic purpura (TTP) as cause of encephalopathy; check peripheral smear.
Recommendation 7.6b – Management.
It is recommended that clinicians manage all-grade toxicities as follows:
Should hold ICPi.
As above for aseptic meningitis, suggest concurrent IV acyclovir until PCR results obtained and negative.
Should offer a trial of methylprednisolone 1 to 2 mg/kg.
If severe or progressing symptoms or oligoclonal bands present, may offer pulse corticosteroid methylprednisolone 1 g IV daily for 3 to 5 days plus IVIG 2 g/kg over 5 days.
If positive for autoimmune encephalopathy or paraneoplastic antibody and limited or no improvement, may offer rituximab or plasmapheresis in consultation with neurology.
7.7. Transverse Myelitis
Recommendation 7.7a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
Neurologic consultation
MRI of spine (with thin axial cuts through the region of suspected abnormality) and MRI of brain done with and without contrast
Lumbar puncture: cell count, protein, glucose, oligoclonal bands, viral PCRs, cytology, onconeural antibodies
Blood: B12, HIV, rapid plasma reagin, ANA, Ro/La, TSH, aquaporin-4 immunoglobulin G
Evaluation for urinary retention and constipation
Recommendation 7.7b – Management.
It is recommended that clinicians manage all-grade toxicities as follows:
Should permanently discontinue ICPi.
Should administer methylprednisolone 2 mg/kg.
Should strongly consider higher doses of 1 g/d for 3 to 5 days.
Should strongly consider IVIG.
Discussion.
ICPi-related neurologic toxicities were originally reported with 1% incidence; however, more-recent analyses suggest that they are more common.88–90 An analysis of 59 trials totaling 9,208 patients reported the overall incidence of neurologic irAEs to be 3.8% in patients receiving anti–CTLA-4 antibodies, 6.1% in patients receiving anti–PD-1 antibodies, and 12.0% in patients receiving a combination ofboth.88 However, the incidence of grade 3 and 4 irAEs was < 1% across all ICPis. A review of patients who received ICPi therapy for melanoma at the Royal Marsden Hospital found the rate of neurologic irAEs to be 2.4%.90 The EORTC 18071 trial reported neurologic irAEs at a rate of 4% in the adjuvant ipilimumab arm.91 Most neurologic events are mild; headache and peripheral sensory neuropathy are the most commonly encountered symptoms.88 Severe neurologic irAEs grade 3 or higher occur in < 1% of patients and may involve the peripheral nervous system or CNS. They encompass a broad spectrum of neurologic syndromes, including myasthenia gravis/myasthenic syndrome, aseptic meningitis, encephalitis, sensory motor neuropathy or Guillain-Barré-like syndromes, painful sensory neuropathy, enteric neuropathy, transverse myelitis, and posterior reversible encephalopathy syndrome.
The first step in management is to rule out CNS progression of cancer, seizure activity, infection, and metabolic derangement as causes of neurologic symptoms. Consultation with a neurologist is advised for all neurologic irAEs grade 2 or higher to help to determine the type and severity of neurologic impairment and guide selection and interpretation of further neurologic tests and management. In patients presenting with headache (which, in isolation, could suggest aseptic meningitis), it is important to evaluate for new confusion, altered behavior, aphasia, seizure-like activity, or short-term memory loss, any of which might suggest encephalitis. The distinction is important because suspected encephalitis triggers a distinct work-up and management from aseptic meningitis, including autoimmune encephalitis and paraneoplastic antibody evaluation and consideration of pulse-dose corticosteroids.92,93
For most neurologic irAEs, diagnostic work-up should include MRI of the brain and/or spine with and without contrast and CSF analysis, including cytology, to rule out leptomeningeal metastasis. CSF analysis is helpful in cases of clinical suspicion of encephalitis, aseptic meningitis, and sensory motor neuropathy or GBS, revealing lymphocytic pleocytosis and elevated protein in many cases. Abnormal leptomeningeal enhancement on neuroimaging may occur in aseptic meningitis, encephalitis, and sensory motor neuropathy, underscoring the importance of checking CSF cytology, which should be negative. NCSs and EMG may assist in diagnosis of sensory symptoms or weakness. Autonomic neuropathy may occur along with other neuropathy symptoms and should be screened for. EEG is helpful for ruling out seizure activity in cases of encephalopathy. Paraneoplastic neurologic syndromes and autoimmune encephalopathies should also be considered.92
For mild (grade 1) neurologic symptoms, checkpoint inhibitor therapy may be continued under close observation. For grade 2 or higher neurologic symptoms, checkpoint inhibitor therapy should be held until the nature of the irAE and symptom progression is defined. In the event of significant neurologic toxicity of grade 2 or higher, a corticosteroid equivalent of methylprednisolone 1 to 4 mg/kg, depending on the symptoms, should be started. For more-severe grade 3 or higher toxicity, immunotherapy should be discontinued. Symptom control may require escalation of corticosteroid therapy to pulse-dose methylprednisolone (1 g daily for 5 days) in addition to IVIG, or plasma exchange (PEX). Pyridostigmine may be helpful for myasthenia gravis in addition to corticosteroids.
8.0. Hematologic Toxicities
Please refer to Table 8 for a complete set of recommendations, definition of grades, and additional considerations.
Table 8.
Management of Hematologic irAEs in Patients Treated With ICPis
| 8.0 Hematologic Toxicities | |
| 8.1 Autoimmune hemolytic anemia | |
| Definition:A condition in which RBCs are destroyed and removed from the blood stream before their normal lifespan is over. Symptoms includeweakness, paleness, jaundice, dark-colored urine, fever, inability to do physical activity, and heart murmur. | |
| Diagnostic work-up History and physical examination (with special consideration of history of new drugs and insect, spider, or snake bites) Blood chemistry, CBC with evidence of anemia, macrocytosis, evidence of hemolysis on peripheral smear; LDH, haptoglobin, bilirubin, reticulocyte count, free Hgb DIC panel, which could include PTINR infectious causes Autoimmune serology Paroxysmal nocturnal hemoglobinuria screening Direct and indirect bilirubin; LDH; direct agglutinin test; and if no obvious cause, bone marrow analysis, cytogenetic analysis to evaluate for myelodysplastic syndromes Evaluation for viral/bacterial (mycoplasma, etc) causes of hemolysis studies Protein electrophoresis, cryoglobulin analysis Work-up for bone marrow failure syndrome if refractory, including B12, folate, copper, parvovirus, FE, thyroid, infection Glucose-6-phosphate dehydrogenase Evaluation of common drug causes (ribavirin, rifampin, dapsone, interferon, cephalosporins, penicillins, NSAIDs, quinine/quinidine, fludarabine, ciprofloxacin, lorazepam, diclofenac, etc) Assessment of methemoglobinemia | |
| Grading | Management |
| G1: Hgb < LLN to 10.0 g/dL; < LLN to 6.2 mmol/L; < LLN to 100 g/L | Continue ICPi with close clinical follow-up and laboratory evaluation |
| G2: Hgb < 10.0 to 8.0 g/dL; < 6.2 to 4.9 mmol/L; < 100 to 80 g/L | Hold ICPi and strongly consider permanent discontinuation Administer 0.5–1 mg/kg/d prednisone equivalents |
| G3: Hgb < 8.0 g/dL; < 4.9 mmol/L; < 80 g/L; transfusion indicated | Permanently discontinue ICPi Should use clinical judgment and consider admitting the patient Hematology consult Prednisone 1–2 mg/kg/d (oral or IV depending on symptoms/speed of development) If worsening or no improvement, 1–2 mg/kg/d prednisone equivalents and permanently discontinue ICPi treatment Consider RBC transfusion per existing guidelines; do not transfuse more than the minimum number of RBC units necessary to relieve symptoms of anemia or to return a patient to a safe Hgb range (7–8 g/dL in stable, noncardiac inpatients) Should offer patients supplementation with folic acid 1 mg once daily |
| G4: Life-threatening consequences, urgent intervention indicated | Permanently discontinue ICPi Admit patient Hematology consult IV prednisone corticosteroids 1–2 mg/kg/d If no improvement or if worsening while on corticosteroids or severe symptoms on presentation, initiate other immunosuppressive drugs, such as rituximab, IVIG, cyclosporin A, and mycophenolate mofetil RBC transfusion per existing guidelines; discuss with blood bank team prior to transfusions that a patient with possible ICPi serious AE is in house. |
| Additional considerations: Monitor Hgb levels on a weekly basis until the corticosteroid tapering process is complete; thereafter, less-frequent testing is needed164 | |
| 8.2 Acquired TTP | |
| Definition: A disorder characterized by the presence of microangiopathic hemolytic anemia, thrombocytopenic purpura, fever, renal abnormalities, and neurologic abnormalities, such as seizures, hemiplegia, and visual disturbances. It is an acute or subacute condition. | |
| Diagnostic work-up History with specific questions related to drug exposure (eg, chemotherapy, sirolimus, tacrolimus, opana ER antibiotics, quinine) Physical examination, peripheral smear ADAMTS13 activity level and inhibitor titer LDH, haptoglobin, reticulocyte count, bilirubin, urinalysis to rule out other causes PT, activated PTT, fibrinogen Blood group and antibody screen, direct antiglobulin test, CMV serology Consider CT/MRI brain, echocardiogram, ECG Viral studies Note: This disorder is usually associated with a severe drop in platelets and hemolysis/anemia precipitously | |
| Grading | Management |
| All grades | The first step in the management of TTP is a high index of suspicion for the diagnosis and timely recognition; hematology consult should immediately be called, as delay in identification is associated with increased mortality/morbidity. Initially, the patient should be stabilized and any critical organ dysfunction stabilized |
| G1: Evidence of RBC destruction (schistocytosis) without anemia, renal insufficiency, or thrombocytopenia clinically G2: Evidence of RBC destruction (schistocytosis) without clinical consequence with G2 anemia and thrombocytopenia G3: Laboratory findings with clinical consequences (G3 thrombocytopenia, anemia, renal insufficiency > 2) G4: Life-threatening consequences (eg, CNS hemorrhage or thrombosis/embolism or renal failure) |
Hold ICPi and discuss resumption with patient only after taking into account the risks and benefits, noting that there are currently no data to recommend restarting ICPi therapy Hematology consult Administer 0.5–1 mg/kg/d prednisone Hold ICPi and discuss resumption with patient only after taking into account the risks and benefits, noting that there are currently no data to recommend restarting ICPi therapy Hematology consult In conjunction with hematology, initiate PEX according to existing guidelines with further PEX dependent on clinical progress94–96 Administer methylprednisolone 1 g IV daily for 3 days, with the first dose typically administered immediately after the first PEX May offer rituximab |
| 8.3 Hemolytic uremic syndrome | |
| Definition: Adisorder characterized byaform ofthrombotic microangiopathywith renal failure, hemolytic anemia, and severe thrombocytopenia. Signs and symptoms of hemolytic uremic syndrome can include: Bloody diarrhea Decreased urination or blood in the urine Abdominal pain, vomiting, and occasionally fever Pallor Small, unexplained bruises or bleeding from the nose and mouth Fatigue and irritability Confusion or seizures High blood pressure Swelling of the face, hands, feet, or entire body | |
| Diagnostic work-up History and physical examination (special consideration for new history of high-risk drugs, hypertension, or cardiac causes) CBC with indices Blood smear morphology. Note that the presence of schistocytes on smear is critical for diagnosis. Serum creatinine ADAMTS13 (to rule out TTP) Homocysteine/methylmalonic acid Complement testing C3, C4, CH50 (complement inhibitory antibodies for suspected familial) Evaluate reticulocyte count and mean corpuscular volume Evaluation of infectious cause, including screening for EBV, CMV, HHV6 Evaluation for nutritional causes of macrocytosis (B12 and folate) Pancreatic enzymes Evaluation for diarrheal causes, shiga toxin, Escherichia coli 0157, etc Direct antibody test (Coombs test), haptoglobin, LDH, and other etiologies of anemia Evaluation for common drugs causing hemolysis (tacrolimus, cyclosporine, sirolimus, etc) Evaluation for concurrent confusion | |
| Grading | Management |
| G1–2: Evidence of RBC destruction (schistocytosis) without clinical consequences ofanemia, thrombocytopenia grade 2 G3: Laboratory findings with clinical consequences (eg, renal insufficiency, petechiae) G4: Life-threatening consequences (eg, CNS thrombosis/ embolism or renal failure) |
Continue ICPi with close clinical follow-up and laboratory evaluation Supportive care Permanently discontinue ICPi Begin therapy with eculizumab therapy 900 mg weekly for four doses, 1,200 mg week 5, then 1,200 mg every 2 weeks Red blood transfusion according to existing guidelines |
| 8.4 Aplastic anemia | |
| Definition: Condition in which the body stops producing enough new blood cells | |
| Diagnostic work-up History and physical examination (close attention to medications, exposure to radiation, toxins, recent viral infections) CBC, smear, reticulocyte count Viral studies, including CMV, HHV6, EBV, parvovirus Nutritional assessments including B12, folate, iron, copper, ceruloplasmin, vitamin D Serum LDH, renal function Work-up for infectious causes Identify marrow hypo/aplasia Bone marrow biopsy and aspirate analysis Peripheral blood analysis, including neutrophil count, proportion of GPI-negative cells by flow for PNH Flow cytometry to evaluate loss of GPI-anchored proteins Type and screen patient for transfusions and notify blood bank that all transfusions need to be irradiated and filtered | |
| Grading | Management |
| G1: Nonsevere, > 0.5 polymorphonuclear cells × 109/L hypocellular marrow, with marrow cellularity < 25%, peripheral platelet count > 20,000, reticulocyte count > 20,000 | Hold ICPi and provide growth factorsupport and close clinical follow-up, and laboratoryevaluation Supportive transfusions as per local guidelines |
| G2: Severe, hypocellular marrow < 25% and two of the following: ANC < 500, peripheral platelet < 20,000, and reticulocyte < 20,000 | Hold ICPi and provide growth factor support and close clinical laboratory evaluations daily Administer ATG + cyclosporine; HLA typing and evaluation for bone marrow transplantation if patient is candidate; all blood products should be irradiated and filtered Supportive care with granulocyte colony-stimulating factor may be added in addition |
| G3–4: Very severe, ANC < 200, platelet count < 20,000, reticulocyte count < 20,000, plus hypocellular marrow < 25% | Hold ICPi and monitor weekly for improvement; if not resolved, discontinue treatment until AE has reverted to G1 Hematology consult, growth factor support Horse ATG plus cyclosporine If no response, repeat immunosuppression with rabbit ATG plus cyclosporine, cyclophosphamide For refractory patients, consider eltrombopag plus supportive care |
| 8.5 Lymphopenia | |
| Definition: An abnormally low level of lymphocytes in PB; for adults, counts of < 1,500/mm3 | |
| Diagnostic work-up History and physical examination (special attention for lymphocyte-depleting therapy such as fludarabine, ATG, corticosteroids, cytotoxic chemotherapy, radiation exposure, etc, as well as history of autoimmune disease, family history of autoimmune disease) Evaluation of nutritional state as cause Spleen size CBC with differential, peripheral smear and reticulocyte counts CXR for evaluation of presence of thymoma Bacterial cultures and evaluation for infection (fungal, viral, bacterial specifically CMV/HIV) | |
| Grading | Management |
| G1–2: 500–1,000 PB lymphocyte count | Continue ICPi |
| G3: 250–499 PB lymphocyte count | Continue ICPi, checking CBC weekly for monitoring, initiation of CMV screening |
| G4: < 250 PB lymphocyte count | Consider holding ICPi Initiate Mycobacterium avium complex prophylaxis and Pneumocystis jirovecii prophylaxis, CMV screening. HIV/hepatitis screening if not already done May consider EBV testing if evidence of lymphadenopathy/hepatitis, fevers, hemolysis consistent with lymphoproliferative disease |
| 8.6 Immune thrombocytopenia | |
| Definition: An autoimmune disorder characterized by immunologic destruction of otherwise normal platelets | |
| Diagnostic work-up History and physical examination (special attention for lymphocyte-depleting therapy, such as fludarabine, ATG, corticosteroids, cytotoxic therapy) Family history of autoimmunity or personal history of autoimmune disease History of viral illness CBC Peripheral blood smear, reticulocyte count Bone marrow evaluation only if abnormalities in the above test results and further investigation is necessary for a diagnosis Patients with newly diagnosed immune thrombocytopenia should undergo testing for HIV, hepatitis C virus, hepatitis B virus, and Helicobacter pylori Direct antigen test should be checked to rule out concurrent Evan syndrome Nutritional evaluation Bone marrow evaluation if other cell lines affected and concern for aplastic anemia | |
| Grading | Management |
| G1: Platelet count < 100/μL | Continue ICPi with close clinical follow up and laboratory evaluation |
| G2: Platelet count < 75/μL | Hold ICPi but monitor for improvement; if not resolved, interrupt treatment until AE has reverted to G1 Administer prednisone 1 mg/kg/d (dosage range, 0.5–2 mg/kg/d) orally for 2–4 weeks after which time this medication should be tapered over 4–6 weeks to the lowest effective dose IVIG may be used in conjunction with corticosteroids ifa more-rapid increase in plateletcount is required. |
| G3: Platelet count < 50/μL G4: Platelet count < 25/μL |
Hold ICPi but monitor for improvement; if not resolved, interrupt treatment until AE has reverted to G1 Hematology consult Prednisone 1–2 mg/kg/d (oral or IV depending on symptoms) If worsening or no improvement, 1–2 mg/kg/d prednisone equivalents and permanently discontinue treatment IVIG used with corticosteroids when a more-rapid increase in platelet count is required If IVIG is used, the dose should initially be 1 g/kg as a one-time dose. This dosage may be repeated if necessary If previous treatment with corticosteroids and/or IVIG unsuccessful, subsequent treatment may include rituximab, thrombopoietin receptor agonists, or more-potent immunosuppression (From American Society of Hematology guideline on immune thrombocytopenia97; consult for further details) |
| 8.7 Acquired hemophilia | |
| Definition: Disorder caused by the development of autoantibodies (inhibitors) directed against plasma coagulation factors | |
| Diagnostic work-up Full blood count to assess platelet number, fibrinogen, PT, PTT, INR; the typical finding in patients with acquired hemophilia A is a prolonged activated PTT with a normal PT MRI, CT, and ultrasonography may be indicated to localize, quantify, and serially monitor the location and response of bleeding Medication review to assess for alternative causes Determination of Bethesda unit level of inhibitor | |
| Grading | Management |
| G1: Mild, 5%–40% ofnormal factoractivityin blood, 0.05–0.4 lU/mL of whole blood | Hold ICPi and discuss resumption with patient only after taking into account the risks and benefits Administer 0.5–1 mg/kg/d prednisone Transfusion support as required Treatment of bleeding disorders with hematology consult |
| G2: Moderate, 1%–5% of normal factor activity in blood, 0.01–.05 IU/mL of whole blood | Hold ICPi and discuss resumption with patient only after taking into account the risks and benefits Hematology consult Administration of factor replacement (choice based on Bethesda unit of titer) Administer 1 mg/kg/d prednisone ± rituximab (dose, 375 mg/m2 weekly for 4 weeks) and/or cyclophosphamide (dose, 1–2 mg/kg/d); choice of rituximab vcyclophosphamide is patient specific and should be done with assistance of hematology consult; prednisone, rituximab, and cyclophosphamide should be given for at least 5 weeks Factors should be provided to increase level during bleeding episodes, with choice of factor based on presence or absence of inhibitor |
| G3–4: Severe, < 1% of normal factor activity in blood, < 0.01 IU/mL of whole blood | Permanently discontinue ICPi Admit patient Hematology consult Administration of factor replacement, choice based on Bethesda unit level of inhibitor Bypassing agents may be used (factor VII, factor VIII inhibitor bypass activity); caution should be taken in the elderly and those with coronary artery disease Prednisone 1–2 mg/kg/d (oral or IV depending on symptoms) ± rituximab (dose, 375 mg/m2 weekly for 4 weeks) and/or cyclophosphamide (dose, 1–2 mg/kg/d). Transfusion support as required for bleeding If worsening or no improvement add cyclosporine or immunosuppression/immunoadsorption |
| Additional considerations: Acquired hemophilia A requires specialist clinical and laboratory expertise. Consult and/or transferto a specialist center is often appropriate. If consultation with or transfer to a hemophilia center is not immediately possible, then investigation and treatment should be initiated while a liaison is being established.98 | |
| All recommendations are expert consensus based, with benefits outweighing harms, and strength of recommendations are moderate. | |
Abbreviations: AE, adverse event; ANC, antineutrophil cytoplasmic antibodies; ATG, antithymocyte globulin; CMV, cytomegalovirus; CT, computed tomography; DIC, disseminated intravascular coagulation; EBV, Epstein-Barr virus; G, grade; GPI, glycosylphosphatidylinositol; Hgb, hemoglobin; HHV6, human herpesvirus 6; ICPi, immune checkpoint inhibitor; INR, international normalized ratio; irAE, immune-related adverse event; IV, intravenous; IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; LLN, lower limit of normal; MRI, magnetic resonance imaging; NSAID, nonsteroidal anti-inflammatory drug; PB, peripheral blood; PEX, plasma exchange; PNH, paroxusmal nocturnal hemoglobinuria; PT, prothrombin time; PTT, partial thromboplastin time; TTP, thrombotic thrombocytopenic purpura.
8.1. Autoimmune Hemolytic Anemia
Recommendation 8.1a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
History and physical examination (with special consideration of history of new drugs and insect, spider, or snake bites).
Blood chemistry, CBC with evidence of anemia, macrocytosis, evidence of hemolysis on peripheral smear. LDH, haptoglobin, bilirubin, reticulocyte count, free hemoglobin.
Disseminated intravascular coagulation panel, which could include Prothrombin Time and International Normalized Ratio (PT/INR), infectious causes.
Autoimmune serology.
Paroxysmal nocturnal hemoglobinuria screening.
Direct and indirect bilirubin; LDH; direct agglutinin test; and if no obvious cause, bone marrow analysis, cytogenetic analysis to evaluate for myelodysplastic syndromes.
Evaluation for viral/bacterial (mycoplasma, etc) causes of hemolysis studies.
Protein electrophoresis, cryoglobulin analysis.
Work-up for bone marrow failure syndrome if refractory, including B12, folate, copper, parvovirus, iron, thyroid, infection.
Glucose-6-phosphate dehydrogenase.
Evaluation of common drug causes (ribavirin, rifampin, dapsone, interferon, cephalosporins, penicillins, NSAIDs, quinine/quinidine, fludarabine, ciprofloxacin, lorazepam, diclofenac, etc).
Assessment of methemoglobinemia.
Recommendation 8.1b – Management.
It is recommended that clinicians manage all grade 1 toxicities as follows:
Should continue to offer ICPi with close clinical follow-up and laboratory evaluation.
It is recommended that clinicians manage all grade 2 toxicities as follows:
Should hold ICPi and strongly consider permanent discontinuation.
Should administer 0.5 to 1 mg/kg/d prednisone equivalents.
It is recommended that clinicians manage all grade 3 toxicities as follows:
Should permanently discontinue ICPi.
Should use clinical judgment and consider admitting the patient.
Should consult hematology.
Should administer prednisone 1 to 2 mg/kg/d (oral or IV depending on symptoms/speed of development).
If worsening or no improvement, should administer 1 to 2 mg/kg/d prednisone equivalents and permanently discontinue ICPi treatment.
May offer RBC transfusion per existing guidelines. Do not transfuse more than the minimum number of RBC units necessary to relieve symptoms of anemia or to return a patient to a safe hemoglobin range (7 to 8 g/dL in stable, noncardiac inpatients).
Should offer patients supplementation with folic acid 1 mg once daily.
It is recommended that clinicians manage all grade 4 toxicities as follows:
Should permanently discontinue ICPi.
Should admit patient.
Should consult hematology.
Should administer IV prednisone 1 to 2 mg/kg/d.
If no improvement or if worsening while on corticosteroids or severe symptoms on presentation, should initiate other immunosuppressive drugs, such as rituximab, IVIG, cyclosporin A, and mycophenolate mofetil.
Should offer RBC transfusion per existing guidelines. Discuss with blood bank team prior to transfusions that a patient with a possible ICPi severe AE is in house.
8.2. Acquired Thrombotic Thrombocytopenic Purpura
Recommendation 8.2a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
History with specific questions related to drug exposure (eg, chemotherapy, sirolimus, tacrolimus, opana ER antibiotics, quinine).
Physical examination, peripheral smear.
ADAMTS13 activity level and inhibitor titer.
LDH, haptoglobin, reticulocyte count, bilirubin.
Prothrombin time (PT), activated partial thromboplastin time (PTT), fibrinogen.
Blood group and antibody screen, direct antiglobulin test, CMV serology.
Consider CT scan/MRI of brain, echocardiogram, ECG.
Viral studies.
Note: This disorder is usually associated with a severe drop in platelets and hemolysis/anemia precipitously.
Recommendation 8.2b – Management.
It is recommended that clinicians manage all-grade toxicities as follows:
The first step in the management of TTP is a high index of suspicion for the diagnosis and timely recognition. Hematology consult should immediately be called, as delay in identification is associated with increased mortality/morbidity.
Initially, the patient should be stabilized and any critical organ dysfunction stabilized.
It is recommended that clinicians manage grade 1 to 2 toxicities as follows:
Should hold ICPi and discuss resumption with patient only after taking into account the risks and benefits, noting that currently, there are no data to recommend restarting ICPi therapy.
Should consult hematology.
Should administer 0.5 to 1 mg/kg/d prednisone.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should hold ICPi and discuss resumption with patient only after taking into account the risks and benefits, noting that currently there are no data to recommend restarting ICPi therapy.
Should consult hematology.
In conjunction with hematology, should initiate PEX according to existing guidelines, with further PEX dependent on clinical progress.94–96
Should administer methylprednisolone 1 g IV daily for 3 days, with the first dose typically administered immediately after the first PEX.
May offer rituximab.
8.3. Hemolytic Uremic Syndrome
Recommendation 8.3a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
History and physical examination (special consideration for new history of high-risk drugs, hypertension, or cardiac causes).
CBC with indices.
Blood smear morphology. Note that the presence of schistocytes on smear is critical for diagnosis.
Serum creatinine.
ADAMTS13 (to rule out TTP).
Homocysteine/methylmalonic acid.
Complement testing C3, C4, CH50 (complement inhibitory antibodies for suspected familial).
Evaluate reticulocyte count and mean corpuscular volume.
Evaluation of infectious cause, including screening for Epstein-Barr virus (EBV), CMV, human herpesvirus 6.
Evaluation for nutritional causes of macrocytosis (B12 and folate).
Pancreatic enzymes.
Evaluation for diarrheal causes, shiga toxin, Escherichia coli 0157, etc
Direct antibody test (Coombs test), haptoglobin, LDH, and other etiologies of anemia.
Evaluation for common drugs causing hemolysis (tacrolimus, cyclosporine, sirolimus, etc).
Evaluation for concurrent confusion.
Recommendation 8.3b – Management.
It is recommended that clinicians manage grade 1 to 2 toxicities as follows:
Should continue to offer ICPi with close clinical follow-up and laboratory evaluation.
Should offer supportive care.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Permanently discontinue ICPi.
Begin eculizumab therapy 900 mg weekly for four doses, 1,200 mg on week 5, then 1,200 mg every 2 weeks.
Red blood transfusion according to existing guidelines.
8.4. Aplastic Anemia
Recommendation 8.4a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
History and physical examination (close attention to medications, exposure to radiation, toxins, recent viral infections).
CBC, smear, reticulocyte count.
Viral studies, including CMV, human herpesvirus 6, EBV, parvovirus.
Nutritional assessments including B12, folate, iron, copper, ceruloplasmin, vitamin D.
Serum LDH, renal function.
Work-up for infectious causes.
Identify marrow hypo/aplasia.
Bone marrow biopsy and aspirate analysis.
Peripheral blood analysis, including neutrophil count, proportion of glycosylphosphatidylinositol-negative cells.
Flow cytometry to evaluate loss of glycosylphosphatidylinositol-anchored proteins.
Type and screen patient for transfusions and notify blood bank that all transfusions need to be irradiated and filtered.
Recommendation 8.4b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should hold ICPi and provide growth factor support, close clinical follow-up, and laboratory evaluation.
Supportive transfusions as per local guidelines.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi and provide growth factor support and close clinical laboratory evaluations daily.
Should administer ATG + cyclosporine. HLA typing and evaluation for bone marrow transplantation if patient is candidate. All blood products should be irradiated and filtered.
May also offer supportive care with granulocyte colony-stimulating factor.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should hold ICPi and monitor weekly for improvement. If not resolved, should discontinue treatment until AE has reverted to grade 1.
Should consult hematology.
Should offer horse ATG plus cyclosporine.
If no response, should repeat immunosuppression with rabbit ATG plus cyclosporine, alemtuzumab.
For refractory patients, may offer eltrombopag plus supportive care.
8.5. Lymphopenia
Recommendation 8.5a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
History and physical examination (special attention for lymphocyte-depleting therapy such as fludarabine, ATG, corticosteroids, cytotoxic chemotherapy, radiation exposure, etc, as well as history of autoimmune disease, family history of autoimmune disease).
Evaluation of nutritional state as cause.
Spleen size.
CBC with differential and reticulocyte counts
CXR for evaluation of presence of thymoma.
Bacterial cultures and evaluation for infection (fungal, viral, bacterial, specifically CMV/HIV).
Recommendation 8.5b – Management.
It is recommended that clinicians manage grade 1 to 2 toxicities as follows:
Should continue to offer ICPi.
It is recommended that clinicians manage grade 3 toxicities as follows:
Continue ICPi, checking CBC weekly for monitoring, initiation of CMV screening.
It is recommended that clinicians manage grade 4 toxicities as follows:
Should hold ICPi and discuss resumption with patient only after taking into account the risks and benefits.
Initiate Mycobacterium avium complex prophylaxis and Pneumocystis jirovecii pneumonia prophylaxis, CMV screening. HIV/hepatitis screening if not already done.
May consider EBV testing if evidence of lymphadenopathy/hepatitis, fevers, hemolysis consistent with lymphoproliferative disease.
8.6. Immune Thrombocytopenia
Recommendation 8.6a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
History and physical examination (special attention for lymphocyte-depleting therapy, such as fludarabine, ATG, corticosteroids, cytotoxic therapy).
Family history of autoimmunity or personal history of autoimmune disease.
History of viral illness.
CBC.
Peripheral blood smear, reticulocyte count.
Bone marrow evaluation only if abnormalities in the above test results and further investigation is necessary for a diagnosis.
Patients with newly diagnosed immune thrombocytopenia should undergo testing for HIV, hepatitis C virus, hepatitis B virus, and Helicobacter pylori.
Direct antigen test should be checked to rule out concurrent Evan syndrome.
Nutritional evaluation.
Bone marrow evaluation if other cell lines affected and concern for aplastic anemia.
Recommendation 8.6b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue ICPi with close clinical follow-up and laboratory evaluation.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi but monitor for improvement. If not resolved, should interrupt treatment until AE has reverted to grade 1.
Should administer prednisone 1 mg/kg/d (dosage range, 0.5 to 2 mg/kg/d) orally for 2 to 4 weeks after which time this medication should be tapered over 4 to 6 weeks to the lowest effective dose.
IVIG may be used in conjunction with corticosteroids if a more-rapid increase in platelet count is required.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should hold ICPi but monitor for improvement. If not resolved, should interrupt treatment until AE has reverted to grade 1.
Should consult hematology.
Should administer prednisone 1 to 2 mg/kg/d (oral or IV depending on symptoms).
If worsening or no improvement, should administer 1 to 2 mg/kg/d prednisone equivalents and permanently discontinue treatment.
IVIG may be used with corticosteroids when a more-rapid increase in platelet count is required.
If IVIG is used, the dose should initially be 1 g/kg as a onetime dose. This dosage may be repeated if necessary.
If previous treatment with corticosteroids and/or IVIG has been unsuccessful, subsequent treatment may include splenectomy, rituximab, thrombopoietin receptor agonists, or more-potent immunosuppression.
Adapted from the American Society of Hematology guideline on immune thrombocytopenia97; consult for further details.
8.7. Acquired Hemophilia
Recommendation 8.7a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
Full blood count to assess platelet number, fibrinogen, PT, PTT, international normalized ratio. The typical finding in patients with acquired hemophilia A is a prolonged activated PTT with a normal PT.
MRI, CT, and ultrasonography may be indicated to localize, quantify, and serially monitor the location and response of bleeding.
Medication review to assess for alternative causes.
Determination of Bethesda unit level of inhibitor.
Recommendation 8.7b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should hold ICPi and discuss resumption with patient only after taking into account the risks and benefits.
Should administer 0.5 to 1 mg/kg/d prednisone.
Transfusion support as required.
May treat bleeding episodes in consultation with a hematologist and/or hemophilia center experienced in the treatment of inhibitors.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi and discuss resumption with patient only after taking into account the risks and benefits.
Should consult hematology.
Should administer 1 mg/kg/d prednisone ± rituximab (dose, 375 mg/m2 weekly for 4 weeks) and/or cyclophosphamide (dose, 1 to 2 mg/kg/d). Choice of rituximab versus cyclophosphamide is patient specific and should be done with assistance of hematology consult. Prednisone, rituximab, and cyclophosphamide should be given for at least 5 weeks.
It is recommended that clinicians manage grade 3 to 4 toxicities as follows:
Should permanently discontinue ICPi.
Should admit patient.
Should consult hematology.
Administration of factor replacement, choice based on Bethesda unit level of inhibitor.
Bypassing agents may be used (factor VII, factor VIII inhibitor bypass activity). Caution should be taken in the elderly and those with coronary artery disease.
Prednisone 1 to 2 mg/kg/d (oral or IV depending on symptoms) ± rituximab (dose, 375 mg/m2 weekly for 4 weeks) and/or cyclophosphamide (dose, 1 to 2 mg/kg/d).
Transfusion support as required for bleeding.
If worsening or no improvement, should add cyclosporine or immunosuppression/immunoadsorption.
Qualifying statement.
Acquired hemophilia A requires specialist clinical and laboratory expertise. Consult and/or transfer to a specialist center is often appropriate. If consultation with or transfer to a hemophilia center is not immediately possible, then investigation and treatment should be initiated while a liaison is being established.98
Discussion.
Review of literature for hematologic toxicities of checkpoint inhibitors revealed evidence of toxicity but relatively little in the form of comprehensive evaluation. A recent review has incorporated a systematic review of all phase I to III prospective clinical trials for Food and Drug Administration–approved ICPis and collated the incidence of common toxicities (unpublished data, J. Holter Chakrabarty, 2017).
Anemia (grades 1 to 4) occurs in approximately 11% of patients, with grades 3/4 at approximately 5.4% (1.1% to 17%).99‘100 If anemia progresses to pancytopenia or multiple cell lines are affected,101 evaluation for pure red cell aplasia,102 autoantibodies,103 aplastic anemia, and myelodysplasia must be considered. Toxicities between checkpoint inhibitors appear relatively similar. The majority of patients respond to withdrawal and are managed successfully with corticosteroids, IVIG, and growth factor support. Hemolytic anemia has been described as having development of autoantibodies103 and can commonly be treated by withholding ICPi, corticosteroids, and IVIG.
Thromobocytopenia is also relatively uncommon, occurring in approximately 8% (1% to 28%) of patients for all grades and 4.3% (3% to 6%) for grades 3/4.99,104 Evaluation for causes of thrombocytopenia must be undertaken, including evaluation of TTP, disseminated intravascular coagulation, myelodysplastic syndrome, as well as immune-mediated thrombocytopenia related to ICPi. Corticosteroids have been shown to be effective with transfusion support as required.
Factor-related acquired bleeding disorders have been described with factor VIII.105,106 Involvement of hematologic expertise should be considered, including evaluation for antibody titer formation and choice of factor replacement. At low titer levels, simple factor replacement and corticosteroids may be effective; however, at high Bethesda unit levels > 5, bypassing agents such as factor VIII inhibitor bypass activity or factor VII may be required. Care in elderly patients when using these agents should be considered
In most cases of mild hematologic toxicities, ICPi can be safely continued. However, cases of more-severe hemolytic anemia, pure red cell anemia, aplastic anemia, severe thrombocytopenia, or coagulation factor deficiencies have been described. In these cases, corticosteroids should be started and supportive care measures instituted. Of note, lymphopenia is not an uncommon event, and the degree of lymphopenia should be assessed with CD4 count and appropriate prophylaxis/assessment started for Pneumocystis and CMV undertaken.
Checkpoint inhibitors have been used in both organ and hematopoetic stem-cell transplantation. In both, caution is advised, and immediate involvement with subspecialty care is advised secondary to increased toxicities that have been seen in these populations.107
9.0. Cardiovascular Toxicities
Please refer to Table 9 for a complete set of recommendations, definition of grades, and additional considerations.
Table 9.
Management of Cardiovascular irAEs in Patients Treated With ICPis
| 9.0 Cardiovascular Toxicities | |
| 9.1 Myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure and vasculitis | |
| Definition: Signs and symptoms may include chest pain, arrhythmia, palpitations, peripheral edema, progressive or acute dyspnea, pleural effusion, fatigue | |
| Diagnostic work-up At baseline ECG Consider troponin, especially in patient treated with combination immune therapies Upon signs/symptoms (consider cardiology consult) ECG Troponin BNP Echocardiogram CXR Additional testing to be guided by cardiology and may include Stress test Cardiac catherization Cardiac MRI | |
| Grading | Management |
| G1: Abnormal cardiac biomarker testing, including abnormal ECG G2: Abnormal screening tests with mild symptoms G3: Moderately abnormal testing or symptoms with mild activity G4: Moderate to severe decompensation, IV medication or intervention required, life-threatening conditions |
All grades warrant work-up and intervention given potential for cardiac compromise Consider the following: Hold ICPi and permanently discontinue after G1 High-dose corticosteroids (1–2 mg/kg of prednisone) initiated rapidly (oral or IV depending on symptoms) Admit patient, cardiology consultation Management of cardiac symptoms according to ACC/AHA guidelines and with guidance from cardiology Immediate transfer to a coronary care unit for patients with elevated troponin or conduction abnormalities In patients without an immediate response to high-dose corticosteroids, consider early institution of cardiac transplant rejection doses of corticosteroids (methylprednisolone 1 g every day) and the addition of either mycophenolate, infliximab, or antithymocyte globulin |
| Qualifying statement: Treatment recommendations are based on anecdotal evidence and the life-threatening nature of cardiovascular complications. Holding checkpoint inhibitor therapy is recommended for all grades of complications. The appropriateness of rechallenging remains unknown. Note that infliximab has been associated with heart failure and is contraindicated at high doses in patients with moderate-severe heart failure.108 | |
| 9.2 Venous thromboembolism | |
| Definition: A disorder characterized by occlusion of a vessel by a thrombus that has migrated from a distal site via the blood stream. Clinical signs and symptoms are variable and may include pain, swelling, increased skin vein visibility, erythema, and cyanosis accompanied by unexplained fever for DVT and dyspnea, pleuritic pain, cough, wheezing, or hemoptysis for PE | |
| Diagnostic work-up Evaluation of signs and symptoms of PE or DVT may include Clinical prediction rule to stratify patients with suspected venous thromboembolism Venous ultrasound for suspected DVT CTPA for suspected PE Can also consider D-dimer for low-risk patients based on risk stratification by clinical prediction rule for DVT/PE when CT or Doppler are not available or appropriate Ventilation/perfusion scan is also an option when CTPA is not appropriate Consider other testing, including ECG, CXR, BNP and troponin levels, and arterial blood gas | |
| Grading | Management |
| G1: Venous thrombosis (eg, superficial thrombosis) | Continue ICPi Warm compress Clinical surveillance |
| G2: Venous thrombosis (eg, uncomplicated DVT), medical intervention indicated G3: Thrombosis (eg, uncomplicated PE [venous], nonembolic cardiac mural [arterial] thrombus), medical intervention indicated |
Continue ICPi Management according to CHEST, ACC, and/or AHA guidelines and consider consult from cardiology or other relevant specialties LMWH is suggested over VKA, dabigatran, rivaroxaban apixaban, or edoxaban for initial and long-term treatment IVheparin is an acceptable alternative for initial use, and oral anticoagulants are acceptable for the long term |
| G4: Life-threatening (eg, PE, cerebrovascular event, arterial insufficiency), hemodynamic or neurologic instability, urgent intervention indicated |
Permanently discontinue ICPi Admit patient and management according to CHEST, ACC, and/or AHA guidelines and with guidance from cardiology Respiratory and hemodynamic support LMWH is suggested over VKA, dabigatran, rivaroxaban, apixaban, or edoxaban for initial and long-term treatment IVheparin is an acceptable alternative for initial use, and oral anticoagulants are acceptable for the long term Further clinical management as indicated based on symptoms |
| Additional considerations While it may be impossible to determine the etiology of thromboembolic disease in patients with advanced cancer and the role, ifany, that ICPi treatment plays, it is reasonable to remove the potential inciting agents given the severity and life-threatening potential of G4 complications. Clinicians are to use clinical judgment and take into account the risks and benefits when deciding whether to discontinue ICPi treatment. Anticoagulant therapy duration should continue for a minimum of 9–12 months to indefinitely in the setting of active cancer unless patient is asymptomatic, doing well, or in remission.109,110 | |
| All recommendations are expert consensus based, with benefits outweighing harms, and strength of recommendations are moderate. | |
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; BNP, brain natriuretic peptide; CT, computed tomography; CTPA, computed tomography pulmonary angiography; CXR, chest x-ray; DVT, deep vein thrombosis; ICPi, immune checkpoint inhibitor; irAE, immune-related adverse event; IV, intravenous; LMWH, low-molecular-weight heparin; MRI, magnetic resonance imaging; PE, pulmonary embolism; VKA, vitamin K agonist.
9.1. Myocarditis, Pericarditis, Arrhythmias, Impaired Ventricular Function With Heart Failure and Vasculitis
Recommendation 9.1a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following: At baseline:
ECG
Consider troponin, especially in patient treated with combination immune therapies
Upon signs/symptoms (consider cardiology consult):
ECG
Troponin
Brain natriuretic peptide (BNP)
Echocardiogram
CXR
Additional testing to be guided by cardiology and may include:
Stress test
Cardiac catherization
Cardiac MRI
Recommendation 9.1b – Management.
It is recommended that clinicians manage all-grade toxicities as follows, as all grades warrant work-up and intervention given potential for cardiac compromise:
Should hold ICPi and permanently discontinue after grade 1.
Should administer high-dose corticosteroids (1 to 2 mg/kg of prednisone) initiated rapidly (oral or IV depending on symptoms).
Should admit patient and consult cardiology.
Should manage cardiac symptoms according to American College of Cardiology (ACC)/AHA guidelines and with guidance from cardiology.
May offer immediate transfer to a coronary care unit for patients with elevated troponin or conduction abnormalities.
In patients without an immediate response to high-dose corticosteroids, may offer early institution of cardiac transplant rejection doses of corticosteroids (methylprednisolone 1 g every day) and the addition of either mycophenolate, infliximab, or ATG.
Qualifying statement.
Treatment recommendations are based on anecdotal evidence and the life-threatening nature of cardiovascular complications. Holding checkpoint inhibitor therapy is recommended for all grades of complications. The appropriateness of rechallenging remains unknown. Note that infliximab has been associated with heart failure and is contraindicated at high doses in patients with moderate-severe heart failure.108
9.2. Venous Thromboembolism
Recommendation 9.2a – Diagnostic work-up.
It is recommended that the diagnostic work-up should include the following:
An evaluation of signs and symptoms of pulmonary embolism (PE) or deep vein thrombosis (DVT), which may include a clinical prediction rule to stratify patients with suspected venous thromboembolism, venous ultrasound for suspected DVT, and CT pulmonary angiography for suspected PE.
May also offer D-dimer for low-risk patients based on risk stratification by clinical prediction rule for DVT/PE when CT or Doppler are not available or appropriate.
Ventilation/perfusion scan is also an option when CT pulmonary angiography is not appropriate.
May make use of other testing, including ECG, CXR, BNP and troponin levels, and arterial blood gas.
Recommendation 9.2b – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue to offer ICPi.
Should offer warm compress.
Should offer clinical surveillance.
It is recommended that clinicians manage grade 2 to 3 toxicities as follows:
Should continue to offer ICPi.
Should manage according to CHEST, ACC, and/or AHA guidelines and consider consult from cardiology or other relevant specialties.
Low-molecular-weight heparin is suggested over vitamin K agonist, dabigatran, rivaroxaban, apixaban, or edoxaban for initial and long-term treatment.
IV heparin is an acceptable alternative for initial use, and oral anticoagulants are acceptable for the long term.
It is recommended that clinicians manage grade 4 toxicities as follows:
Should permanently discontinue ICPi.
Should admit patient and manage according to CHEST, ACC, and/or AHA guidelines and with guidance from cardiology.
Should seek respiratory and hemodynamic support.
Low-molecular-weight heparin is suggested over vitamin K agonist, dabigatran, rivaroxaban, apixaban, or edoxaban for initial and long-term treatment.
IV heparin is an acceptable alternative for initial use, and oral anticoagulants are acceptable for the long term.
Should offer further clinical management as indicated based on symptoms.
Qualifying statement.
While it may be impossible to determine the etiology of thromboembolic disease in patients with advanced cancer and the role, if any, that ICPi treatment plays, it is reasonable to remove the potential inciting agents given the severity and life-threatening potential of grade 4 complications. Clinicians are to use clinical judgment and take into account the risks and benefits when deciding whether to discontinue ICPi treatment. Anticoagulant therapy duration should continue for a minimum of 9 to 12 months to indefinitely in the setting of active cancer unless the patient is asymptomatic, doing well, or in remission.109,110
Discussion.
Cardiovascular complications of ICPi therapy are rare but potentially life-threatening and/or of devastating clinical consequences. They have been reported with all currently approved agents.111 However, due to their rarity and involvement of major organs leading to rapidly fatal consequences, data are sparse and generally have included case reports or small case series.112 Cardiovascular irAEs occur in < 0.1% of patients receiving these therapies based on a review of pharmaceutical safety data-bases.75 The risk may be increased when combination therapy is used. In these safety data, combination therapy of ipilimumab and nivolumab had greater rates of cardiovascular complications than nivolumab alone (0.28% v 0.06%).75 Mortality is high, with death frequently secondary to refractory arrhythmia or cardiogenic shock.75,113,114
One review of compiled case reports and case series by Jain et al111 found that the onset of cardiovascular irAEs can be as soon as 2 weeks and as long as 32 weeks after initiation of therapy, with a median onset of 10 weeks after initiation. Based on results of myocardial biopsies, these complications are thought to be caused by lymphocytic infiltration of the myocardium and myocardial conduction system.75 Pathology has shown lymphocytic infiltration in the tumor specimens.
A wide range of cardiovascular complications have been reported. Pathologic review shows occurrences of myocarditis; myocardial fibrosis; cardiomyopathy; heart failure; conduction abnormalities, including heart block; and cardiac arrest.111 Pericarditis and pericardial effusions have been described as well.35,115 There has also been a case report of irAE-associated acute coronary syndrome.116
Immune-mediated myocarditis may result in heart failure or arrhythmia. The myocarditis may be fulminant, progressive, and life-threatening.75,117 Acute heart failure may occur secondary to decreased cardiac function and diminished ejection fraction.75,114
Conduction abnormalities can include complete heart block75,114 and arrhythmias. A variety of dysrhythmias may occur from the more benign (supraventricular tachycardias) to more fatal and can lead to sudden death (ventricular tachycardias).75,76,112–114,118–120
Presentation of cardiovascular complications of checkpoint inhibitors could include arrhythmia, palpitations, chest pain, or signs and symptoms of heart failure (shortness of breath, peripheral edema, pleural effusion, fatigue). Severe cases can present with cardiogenic shock or sudden death. Patients can also present with fatigue, malaise, myalgia, and/or weakness alone or in combination with more-specific cardiovascular symptoms. Symptoms can often be masked by other irAEs (eg, pneumonitis, hypothyroidism) or symptoms related to disease (eg, pulmonary symptoms).
Initial evaluation of patients with potential cardiovascular toxicity should include ECG, troponin, BNP, and CXR. Reported cases have invariably had elevations of troponin, CK, and CK-MB.112 BNP will also be elevated in cases with decrease ejection fraction. Diagnostic evaluation should consider the possibility of other etiologies of the patient’s symptoms and could include, for example, cardiac stress testing, heart catheterization, or cardiac MRI. Due to the possibility of arrhythmia and progression to life-threatening arrhythmias or heart block, continuous telemetry monitoring should be instituted. Typically, many of these patients will often be admitted to an inpatient unit and worked up there given the severity of the symptoms. Patients with mild shortness of breath of unclear etiology should get typical outpatient testing (ECG, BNP, troponin).
Echocardiogram to evaluate for cardiac function should be performed in symptomatic patients. Echocardiogram may reveal decreased left or right ventricular ejection fraction (with global or regional abnormalities). Cardiac MRI can demonstrate evidence of myocarditis but is less sensitive than endomyocardial biopsy.112,117 Endomyocardial biopsy should be considered for patients who are unstable or failed to respond to initial therapy or in whom the diagnosis is in doubt. Typically, initial diagnostic testing reveals issues, and treatment is often administered empirically before confirmatory pathologic testing is obtained.
There is no clear evidence regarding the efficacy or value of routine baseline or serial ECGs or troponin measurements in patients receiving checkpoint inhibitor therapy. Some centers obtain baseline testing, and others continue this through the initial period of therapy. Some centers stratify management based on magnitude of troponin changes.112 Baseline information can potentially be useful when patients present acutely with nonspecific symptoms and have equivocal diagnostic testing.
Treatment recommendations are based on anecdotal evidence and the life-threatening nature of cardiovascular complications of irAEs due to either malignant arrhythmia or the possibility of fulminant myocarditis with heart failure. Holding checkpoint inhibitor therapy is recommended for all grades of complications, including grade 1 (asymptomatic biomarker elevations), with reinstitution of treatment almost never happening.111,112
For patients with mild to moderate symptoms (grades 2 to 3), systemic prednisone or methylprednisolone is indicated at 1 to 2 mg/kg/day.6,75,113 Those with more severe disease (grades 3 to 4), including clinical decompensation, highly abnormal testing, fulminant disease, cardiogenic shock, and acute heart failure, or with life-threatening arrhythmia should be considered for moreaggressive therapy, as should those who fail to respond to initial corticosteroid dosing within 3 to 5 days. This could include therapy with higher doses of corticosteroids (methylprednisolone at 1 g daily) and the possible addition of mycophenolate, infliximab, or ATG.75,111–113,117 Management of symptoms of arrhythmia and heart failure should be as per national cardiology guidelines and clinical judgment.112
Although some diseases are fulminant and progress to death, with appropriate therapy and holding of checkpoint inhibitors, cardiac contractility and conduction abnormalities can improve.119 There have not been sufficient cases in the literature to determine the proportion expected to progress or improve. Given the potential severity of the symptoms, the patient’s disease status must be taken into account before excessive support measures are performed (eg, defibrillator, resuscitation, balloon pump).
The evidence on how to distinguish among risk factors in patients with cancer treated with ICPi therapy is limited. Furthermore, determining the true cause of thromboembolic disease in such patients is difficult, if not impossible, given the thrombogenicity of both the disease and the treatment. Treating physicians are urged to use clinical judgment in the management of these patients.
10.0. Ocular Toxicities
Please refer to Table 10 for a complete set of recommendations, definition of grades, and additional considerations.
Table 10.
Management of Ocular irAEs in Patients Treated With ICPis
| 10.0 Ocular Toxicities | |
| Counsel all patients to inform their health care provider immediately if they experience any of the following ocular symptoms Blurred vision Change in color vision Photophobia Distortion Scotomas Visual field changes Double vision Tenderness Pain with eye movement Eyelid swelling Proptosis | |
| Evaluation, under the guidance of ophthalmology Check vision in each eye separately Color vision Red reflex Pupil size, shape, and reactivity Fundoscopic examination Inspection of anterior part of eye with penlight | |
| Prior conditions Exclude patients with history of active uveitis History of recurrent uveitis requiring systemic immunosuppression or continuous local therapy | |
| Additional considerations Ocular irAEs are many times seen in the context of other organ irAEs High level of clinical suspicion as symptoms may not always be associated with severity Best to treat after ophthalmologist eye examination | |
| 10.1 Uveitis/iritis | |
| Definition: Inflammation of the middle layer of the eye | |
| Diagnostic work-up: as per above | |
| Grading | Management |
| G1: Asymptomatic | Continue ICPi Refer to ophthalmology within 1 week Artificial tears |
| G2: Medical intervention required, anterior uveitis | Hold ICPi temporarily until after ophthalmology consult Urgent ophthalmology referral Topical corticosteroids, cycloplegic agents, systemic corticosteroids May resume ICPi treatment once off systemic corticosteroids, which are purely indicated for ocular adverse effects or once corticosteroids for other concurrent systemic irAEs are reduced to ≤ 10 mg; continued topical/ocular corticosteroids are permitted when resuming therapy to manage and minimize local toxicity Re-treat after return to G1 or less |
| G3: Posterior or panuveitis | Permanently discontinue ICPi Urgent ophthalmology referral. Systemic corticosteroids and intravitreal/periocular/topical corticosteroids |
| G4: 20/200 or worse | Permanently discontinue ICPi Emergent ophthalmology referral Systemic corticosteroids (IV prednisone 1–2 mg/kg or methylprednisolone 0.8–1.6 mg/kg) and intravitreal/periocular/topical corticosteroids per ophthalmologist opinion |
| Additional considerations: Consider use of infliximab or other TNF-α blockers in cases that are severe and refractory to standard treatment121,122 | |
| 10.2 Episcleritis | |
| Definition: Inflammatory condition affecting the episcleral tissue between the conjunctiva and the sclera that occurs in the absence of an infection | |
| Diagnostic work-up: As per 10.0 | |
| Grading | Management |
| G1: Asymptomatic | Continue ICPi Refer to ophthalmology within 1 week Artificial tears |
| G2: Vision 20/40 or better | Hold ICPi therapy temporarily until after ophthalmology consult Urgent ophthalmology referral Topical corticosteroids, cycloplegic agents, systemic corticosteroids |
| G3: Symptomatic and vision worse than 2/40 | Permanently discontinue ICPi Urgent ophthalmology referral Systemic corticosteroids and topical corticosteroids with cycloplegic agents |
| G4: 20/200 or worse | Permanently discontinue ICPi Emergent ophthalmology referral Systemic corticosteroids and topical corticosteroids with cycloplegic agents |
| Additional considerations: Consider use of infliximab or other TNF-α blockers in cases that are severe and refractory to standard treatment121,122 | |
| 10.3 Blepharitis | |
| Definition: Inflammation of the eyelid that affects the eyelashes or tear production | |
| Diagnostic work-up: As per 10.0 | |
| Grading | Management |
| No formal grading system | Warm compresses and lubrication drops Continue therapy unless persistent and serious |
| All recommendations are expert consensus based, with benefits outweighing harms, and strength of recommendations are moderate. | |
Abbreviations: ICPi, immune checkpoint inhibitor; irAE, immune-related adverse event; IV, intravenous, TNF, tumor necrosis factor.
Recommendation 10.0 – Diagnostic work-up for all ocular toxicities.
It is recommended that clinicians counsel all patients to inform their health care provider immediately if they experience any of the following ocular symptoms:
Blurred vision
Change in color vision
Photophobia
Distortion
Scotomas
Visual field changes
Double vision
Tenderness
Pain with eye movement
Eyelid swelling
Proptosis
It is recommended that the diagnostic work-up should include the following, under the guidance of ophthalmology:
Check vision in each eye separately
Color vision
Red reflex
Pupil size, shape, and reactivity
Fundoscopic examination
Inspection of anterior part of eye with penlight
Qualifying statement.
Clinicians should be aware that ocular irAEs are many times seen in the context of other organ irAEs, and there should be a high level of clinical suspicion as symptoms may not always be associated with severity. It is best to treat ocular irAEs after ophthalmologist eye examination.
10.1. Uveitis/Iritris
Recommendation 10.1 – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue to offer ICPi.
Should refer to ophthalmology within 1 week.
Should offer artificial tears.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi temporarily until after ophthalmology consult.
Should make an urgent ophthalmology referral.
Should administer topical corticosteroids, cycloplegic agents, systemic corticosteroids.
May resume ICPi treatment once off systemic corticosteroids, which are purely indicated for ocular adverse effects or once corticosteroids for other concurrent systemic irAEs are reduced to ≤ 10 mg. Continued topical/ocular corticosteroids are permitted when resuming therapy to manage and minimize local toxicity. Should re-treat after return to grade 1 or less.
It is recommended that clinicians manage grade 3 toxicities as follows:
Should permanently discontinue ICPi.
Should make an urgent ophthalmology referral.
Should administer systemic corticosteroids and intravitreal/ periocular/topical corticosteroids.
It is recommended that clinicians manage grade 4 toxicities as follows:
Should permanently discontinue ICPi.
Should make an emergent ophthalmology referral.
Should administer systemic corticosteroids (IV prednisone 1 to 2 mg/kg or methylprednisolone 0.8 to 1.6 mg/kg and intravitreal/periocular/topical corticosteroids per ophthalmologist opinion)
May use infliximab in cases that are severe and refractory to standard treatment.
10.2. Episcleritis
Recommendation 10.2 – Management.
It is recommended that clinicians manage grade 1 toxicities as follows:
Should continue to offer ICPi.
Should refer to ophthalmology within 1 week.
Should offer artificial tears.
It is recommended that clinicians manage grade 2 toxicities as follows:
Should hold ICPi until after ophthalmology consult.
Should make an urgent ophthalmology referral.
Should administer topical corticosteroids, cycloplegic agents, systemic corticosteroids.
It is recommended that clinicians manage grade 3 toxicities as follows:
Should permanently discontinue ICPi.
Should make an urgent ophthalmology referral.
Should administer systemic corticosteroids and topical corticosteroids with cycloplegic agents.
It is recommended that clinicians manage grade 4 toxicities as follows:
Should permanently discontinue ICPi.
Should make an emergent ophthalmology referral.
Should administer systemic corticosteroids and topical corticosteroids with cycloplegic agents.
10.3. Blepharitis
Recommendation 10.3 – Management.
It is recommended that clinicians manage all-grade toxicities as follows:
Should offer warm compresses and lubrication drops.
Should continue to offer ICPi unless irAE is persistent and serious.
Discussion.
In the context of irAEs as a result of ICPi therapy in cancer, ocular toxicities are considered uncommon and less complex in their management compared with other immune-related toxicities.
A variety of ocular events have been reported with CTLA-4–, anti–PD-1–, and anti–PD-L1–inhibiting agents, including uveitis, iritis, episcleritis, and blepharitis. Like in other irAEs, the principal mechanism of the encountered toxicity is inflammatory and the principal management is immunosuppression with corticosteroids.
The overall incidence of uveitis with ICPis, including ipilimumab,123,124 anti–PD-1 antibodies,125–127 and anti–PD-L1 agents,165,166 is up to 1%, although the incidence maybe higher in patients receiving combination ICPis.128 Presenting symptoms include blurred vision and photophobia, change in color vision, and distortion as well as physical signs like tenderness, swelling, and pain with eye movement, among others. The practitioner should be aware that symptoms of uveitis may not indicate severity of the syndrome and thus should seek consultation with ophthalmology and slit lamp examination. Rarely, a panuveitis is induced that can lead to exudative retinal detachment and can be vision threatening. Milder forms of uveitis respond to temporary holding of ICPis and topical corticosteroids, and any symptomatic presentation should prompt urgent ophthalmology evaluation. Typical management includes topical corticosteroids and often the addition of cycloplegic agents, and in rare cases, systemic corticosteroid administration is necessary.
Episcleritis is a rare, but clinically important event, occurring in < 1% of treated patients.124 The management is similar to the one recommended for uveitis, and any visual compromise (vision < 20/40) should prompt urgent ophthalmology referral to assess the need for more-specific interventions. We recommend ophthalmology referral for all cases of episcleritis even if asymptomatic, and holding immune checkpoint therapy until such evaluation is completed. Artificial tears, topical corticosteroids, and cycloplegic agents are typically used and highly effective in managing this toxicity, but in rare cases, systemic corticosteroids may be required. In case of recurrent events or a grade 4 presentation (vision 20/200 or worse), permanent discontinuation of ICPi is advised. Infliximab may be considered for severe and treatment-refractory cases, although the data on this intervention rely on case reports only.
Blepharitis is equally rare as other ocular toxicities and is encountered < 1% of patients treated with ICPis. This toxicity is managed with warm compresses and artificial tears for lubrication. Disruption of therapy is not typically necessary but maybe advised by the consulting ophthalmologist if symptoms are severe and treatment refractory.
In most cases of ocular toxicities, ICPi can be safely continued as most presenting grades are mild and manageable with topical corticosteroids. However, ocular toxicity is commonly associated with other systemic immune-related events, and systemic corticosteroids are often used in these patients to manage the moreprominent toxicities outside the eye. Modification and possible cessation of ICPi may need to be considered in these cases as well as in cases of higher grade, treatment-refractory, or recurrent ocular toxicity.
DISCUSSION
While identifying patients at an increased risk for irAEs would help to determine the need for surveillance and prompt, aggressive treatment, the evidence of who is at an elevated risk remains unclear. Patients with preexisting autoimmune diseases, such as ulcerative colitis, Crohn disease, lupus, and active rheumatoid arthritis, are usually not offered therapy with checkpoint inhibitors and typically have been excluded from clinical trials involving these agents. However, data suggest that they may be safely treated.31,129 Indeed, a systematic review of case reports of patients with preexisting autoimmune diseases treated with ICPis found that 40% of patients did not experience an irAE or exacerbation of their autoimmune disease, despite many having active disease.130 Ultimately, cautious use of ICPi therapy may be acceptable with close monitoring for recurrence of the underlying autoimmune condition.
The pattern of toxicity based on tumor type and location has not been well established. Some reports have claimed higher incidences of pneumonitis in patients with NSCLC compared with melanoma,39 but other analyses found no statistically significantly differential effects according to cancer type.131–133 Treatment-naive patients are reported to have a higher incidence of pneumonitis compared with those previously treated.134 Other evidence is also emerging on patient-related modifiers of risk. Personal ecologic factors, such as the patient’s microbiome, may also play a role in the susceptibility to specific irAEs, such as enterocolitis.77‘135‘136 Further studies are needed to investigate whether a patient’s biologic profile predisposes to the occurrence of irAEs.137
Possible treatment-specific risks for increased irAEs include dose of therapy, individual checkpoint inhibitor (CTLA-4 v PD-1), and combination checkpoint blockade. Model-based pooled estimates from 498 trial patients who received ipilimumab monotherapy at 0.3, 3, or 10 mg/kg doses indicated that higher doses produce higher rates of irAEs.138 Grade 3 or higher irAEs are reported to occur more frequently in patients receiving anti–CTLA-4 monotherapy (ipilimumab, 15% to 42%) than in those receiving anti–PD-1 (nivolumab, 8%; pembrolizumab, 5% to 10%) or anti–PD-L1 (atezolizumab, 5% to 7%; durvalumab, 2%; avelumab, 1% to 2%) monotherapy.139 Evidence also exists for the elevated risk with combination therapy. A recent meta-analysis revealed the OR of all-grade pneumonitis was 3.7 (95% CI, 1.6 to 8.5; P = .002), with an anti–CTLA-4 and anti–PD-1 therapy combination (ipilimumab and nivolumab) versus anti–CTLA-4 monotherapy.131 Combination anti–CTLA-4 and anti–PD-1 therapy also significantly increased the risk of grade 3 and 4 rash and fatigue.140–142 As the use of ICPi therapy increases and incidences of irAEs are further collected, the understanding of which patient is at an elevated risk is sure to become clearer. In the meantime, clinicians should maintain a high level of suspicion for immune-related toxicities with checkpoint inhibitors, with early recognition and treatment of upmost importance in mitigating the severity of irAEs.143
While treatment with ICPis is sometimes well tolerated, the potential for life-disabling irAEs that are severe and/or irreversible exists.137 A recent meta-analysis of approximately 6,000 patients with solid tumors reported a statistically significant increased risk of fatal AEs for patients treated with ipilimumab (pooled Peto OR, 2.3; 95% CI, 1.4 to 3.6; P < .001).144 Among the specific causes of fatal AEs, ipilimumab was associated with an increased risk of fatal GI toxicity (OR, 4.5; 95% CI, 1.5 to 13.6).144
The decision to resume ICPi therapy after resolution of toxicity is complicated because the optimal duration of ICPi therapy is not defined. Early trials of ICPi used 1 year of therapy; later trials used 2 years of therapy or continued ICPi treatment until disease progression or patient intolerance. Recent evidence suggests that patients who discontinued induction immunotherapy due to AEs did just as well as those who continued treatment uninterrupted.145 In a pooled analysis of randomized trials of patients with advanced melanoma who received nivolumab plus ipilimumab combination therapy, Schadendorf et al145 found an ORR of approximately 60% in patients who discontinued compared with approximately 50% in those who completed induction therapy. Progression-free survival was also similar between the two groups. While these data are intriguing, prospective evidence is still required to gain a better understanding of the merits, liability, and optimal duration of ongoing anti–PD-1 therapy after discontinuing induction therapy due to irAEs.146 A patient’s tumor response status is an important factor in deciding whether to resume ICPi. If a patient has achieved objective response to initial ICPi, there is a reasonable likelihood that the response will be durable and that resumption of therapy (with attendant risk of recurrence of toxicity) may not be advisable. Conversely, for patients who have not yet responded or whose response is deemed inadequate, consideration of resumption of ICPi therapy after resolution of toxicity is reasonable.
Whether the appearance of irAEs is associated with efficacy parameters still remains unclear.147 After adjusting for differences in number of nivolumab doses received, baseline LDH, and tumor PD-L1 expression, one analysis found that the ORR was significantly better in patients who experienced irAEs of any grade compared with those who did not, with the greatest benefit seen in patients who reported three or more irAEs.148 No significant difference in ORR on the basis of the occurrence of grade 3 to 4 irAEs was observed.148
There are important studies under way that are evaluating the efficacy of various strategies in mitigating toxicities while maintaining efficacy, such as alternative dosing strategies or increasing the interval between treatment infusions.146 Until such evidence becomes available, dose reductions of ICPi therapy should be avoided. Rather, therapeutic adjustments by way of temporary interruption or permanent discontinuation of treatment are recommended.
Guidance on the management of toxicities related to ICPi therapy is in demand. This guideline and its recommendations is intended to arm the clinician with strategies and best practices to rapidly recognize, diagnose, coordinate with other medical subspecialties, and manage these sets of unique toxicities.
PATIENT AND CLINICIAN COMMUNICATION
As immunotherapeutic treatment of cancer continues to evolve with single agents and in new combinations, it is imperative that patients and family caregivers receive timely and up-to-date education about immunotherapies, their mechanism of action, and the clinical profile of possible irAEs. Patient and caregiver education should occur prior to initiating therapy and continue throughout treatment and survivorship. It should be emphasized that immunotherapy works differently than traditional chemotherapy and that these treatments elicit unique therapeutic responses and corresponding irAEs.149 This response can be unique to each patient, and irAEs may occur across the treatment trajectory from the start of treatment and into survivorship. Most notably, the ability to influence immune response even after discontinuation of the immunotherapeutic agent is a unique feature, and important education point for patients and their caregivers. As such, patients should be encouraged to alert all health care providers that they are receiving or have received an immunotherapeutic agent and to report any changes in health status to each provider. This is important as patients are often seen by multiple providers, and each provider should be aware of the potential for irAEs.
In most cases, irAEs can be managed with treatment interruption and/or supportive care and for some patients, will involve a multidisciplinary team (eg, endocrinologist, pulmonologist, gastroenterologist) to address specific symptoms.150 Patients and caregivers need to know that AEs can often be managed effectively, especially when they are identified early. In addition, education addressing the safe handling of medications, infection control, and safe sexual practices is important to supporting optimal management of irAEs.149
Using a questionnaire or standard assessment may assist the provider and patient to recognize possible irAEs. In addition, health care professionals should ask patients about any new symptoms or changes in their health, no matter how small they may seem. Minor changes in how a patient is feeling may indicate early signs of an AE, and patients may not attribute the change to their cancer treatment.151 Consistent assessment and documentation at each encounter will also enable the clinical team to note changes that may occur over time. Close monitoring throughout treatment is important as minimal changes in a patient’s baseline status may indicate an early irAE. Wallet cards detailing symptoms to watch for and how to notify their health care provider maybe an effective tool in empowering patients and their caregivers to recognize and manage irAEs and maybe useful to other health care providers (eg, emergency department staff) caring for patients with a history of immunotherapy.150 The Oncology Nursing Society has an immunotherapy wallet card available for patients and providers (Fig 1). Copies of the card or additional information can be obtained by e-mail at clinical@ons.org.
Fig 1.
Example of an immunotherapy wallet card. Reprinted courtesy of the Oncology Nursing Society. All rights reserved.
For recommendations and strategies to optimize patient-clinician communication, see Patient-Clinician Communication: American Society of Clinical Oncology Consensus Guideline.152
HEALTH DISPARITIES
Although ASCO clinical practice guidelines represent expert recommendations on the best practices in disease management to provide the highest level of cancer care, it is important to note that many patients have limited access to medical care. Racial and ethnic disparities in health care contribute significantly to this problem in the United States. Patients with cancer who are members of racial/ethnic minorities suffer disproportionately from comorbidities, experience more substantial obstacles to receiving care, are more likely to be uninsured, and are at greater risk of receiving care of poor quality than other Americans.153–156 Many other patients lack access to care because of their geographic location and distance from appropriate treatment facilities. Moreover, with evidence to suggest that patients with a higher mutation burden are at an increased likelihood of responding to ICPis157,158 African American patients with lung cancer may be affected as the burden of somatic mutations appears to be different in such patients.157 Awareness of these disparities in access to care should be considered in the context of this clinical practice guideline, and health care providers should strive to deliver the highest level of cancer care to these vulnerable populations.
While concerns about racial disparities in access to trials of new cancer drugs have been raised, including trials of anti–PD-1s, whether these disparities extend to patients in real-world practice has only recently been investigated.159 In a retrospective analysis of electronic health records of 4,643 patients treated with anti–PD-1s, investigators found that racial distributions differed for anti–PD-1–treated patients compared with non-anti–PD-1-treated patients in a cohort of patients with advanced NSCLC (P < .01) but not in a cohort of patients with metastatic renal cell carcinoma (P = .84) or advanced melanoma (P = .96). In bivariate analyses of patients with advanced NSCLC, the use of anti–PD-1 treatment was associated with race, male sex, stage II at diagnosis, squamous histology, smoking history, and line of therapy (all P < .05).159 Adjusted models showed that there were no significant differences in likelihood of receiving anti–PD-1s when comparing black and white patients undergoing systemic therapy for NSCLC.159
MULTIPLE CHRONIC CONDITIONS
Creating evidence-based recommendations to inform treatment of patients with additional chronic conditions, a situation in which the patient may have two or more such conditions—referred to as multiple chronic conditions (MCC)—is challenging. Patients with MCC are a complex and heterogeneous population, making it difficult to account for all the possible permutations to develop specific recommendations for care. In addition, the best available evidence for treating index conditions, such as cancer, is often from clinical trials whose study selection criteria may exclude patients with MCC, such as preexisting autoimmune diseases, to avoid potential interaction effects or confounding of results associated with MCC. As a result, the reliability of outcome data from these studies may be limited, thereby creating constraints for expert groups to make recommendations for care in this heterogeneous patient population.
As many patients for whom guideline recommendations apply present with MCCs, including preexisting autoimmune diseases, any treatment plan needs to take into account the complexity and uncertainty created by the presence of MCCs and highlights the importance of shared decision making regarding guideline use and implementation. Therefore, in consideration of recommended care for the target index condition, clinicians should review all other chronic conditions present in the patient and take those conditions into account when formulating the management and follow-up plan.
In light of the above considerations, practice guidelines should provide information on how to apply the recommendations for patients with MCCs, perhaps as a qualifying statement for recommended care. This may mean that some or all of the recommended care options are modified or not applied, as determined by best practice in consideration of any MCCs.
EXTERNAL REVIEW
The draft set of recommendations was submitted to an external reviewer with content expertise to obtain direct feedback. A public open comment period was also held from October 30 through November 14, 2017. A total of 17 respondents, who had not previously reviewed the recommendations, either agreed or agreed with slight modifications to the vast majority of the recommendations. Expert Panel members reviewed comments from all sources and determined whether to maintain original draft recommendations, revise with minor language changes, or consider major recommendation revisions. All changes were incorporated prior to Clinical Practice Guideline Committee review and approval.
GUIDELINE IMPLEMENTATION
ASCO guidelines are developed for implementation across health settings. Barriers to implementation include the need to increase awareness of the guideline recommendations among frontline practitioners and survivors of cancer and caregivers and to provide adequate services in the face of limited resources. The guideline Bottom Line Box facilitates implementation of recommendations. This guideline will be distributed widely through the ASCO Practice Guideline Implementation Network. ASCO guidelines are posted on the ASCO Web site and most often published in Journal of Clinical Oncology and Journal of Oncology Practice. Dissemination is also expected through ASCO Communications (which will likely include www.asco.org, media outreach, ASCO e-mails/news releases, www.cancer.net, ASCO Connection [member magazine], social media, and other member communications; may also include ASCO University, depending on the program’s needs).
ASCO believes that cancer clinical trials are vital to inform medical decisions and improve cancer care and that all patients should have the opportunity to participate.
Supplementary Material
Acknowledgment
The Expert Panel thanks Kerry Lynn Reynolds, MD, Jeffrey Kirshner, MD, Jeremy Kortmansky, MD, and the Clinical Practice Guidelines Committee for their thoughtful reviews and insightful comments on this guideline.
All Appendices and Acknowledgment material (including the table of Expert Panel members) are online only. It will appear on the JCO Web site but not in the print version.
Appendix
Fig A1.
Distribution of (A) grade 1 to 2 and (B) grade 3 to 5 immune-related adverse events (irAEs) for all tumor types in the main clinical trials with anti- cytotoxic T-cell lymphocyte-4 (anti–CTLA-4), anti-programmed death 1 (PD-1), or anti–PD ligand 1 (PD-L1) antibodies as single therapies. The values quoted are the median (range) irAE rates for the set of clinical trials as a whole. Adapted from European Journal of Cancer, Vol 54, J.M. Michot et al, Immune-Related Adverse Events With Immune Checkpoint Blockade: A Comprehensive Review, 139–149, Copyright 2016, with permission from Elsevier. Endoc, endocrinology; Neurol, neurology; ocul, ocular; Pulm, pulmonary.
Table A1.
Management of Immune-Related Adverse Events Guideline Expert Panel Membership
| Name and Designation | Affiliation/Institution | Role/Area of Expertise |
|---|---|---|
| Julie Brahmer, MD, MSc, Cochair | Johns Hopkins Kimmel Cancer Center | Thoracic oncology |
| John A. Thompson, MD, Cochair | Seattle Cancer CareAlliance, UniversityofWashington, and the Fred Hutchinson Cancer Research Center | Melanoma and kidney |
| Bryan J. Schneider, MD | University of Michigan Health System | Thoracic oncology |
| Natasha Leighl, MD | Princess Margaret Cancer Centre | Thoracic oncology |
| Jedd Wolchok, MD, PhD | Memorial Sloan Kettering Cancer Center | Melanoma |
| Jeffrey S. Weber, MD, PhD | New York University Langone Medical Center | Melanoma |
| Marc S. Ernstoff, MD | Roswell Park Cancer Institute | Melanoma, Society for Immunotherapy of Cancer (SITC) representative |
| Igor Puzanov, MD | Roswell Park Cancer Institute | Melanoma, SITC representative |
| Loretta Nastoupil, MD | MD Anderson Cancer Center | Hematology |
| Jennifer Holter Chakrabarty, MD | University of Oklahoma, Stephenson Cancer Center | Hematology, American Society of Hematology representative |
| Michael B. Atkins, MD | Georgetown Lombardi Comprehensive Cancer Center | Genitourinary |
| David F. McDermott, MD | Beth Israel Deaconess Medical Center | Genitourinary |
| Ian Chau, MD | The Royal Marsden Hospital and Institute of Cancer Research | GI |
| Yinghong Wang, MD | MD Anderson Cancer Center | GI |
| Maria E. Suarez-Almazor, MD | MD Anderson Cancer Center | Rheumatology |
| Jennifer Gardner, MD | Seattle Cancer Care Alliance, University of Washington | Dermatology |
| Cristina Reichner, MD | Georgetown University | Pulmonology |
| Aung Naing, MD | MD Anderson Cancer Center | Medical Oncology, Trialist |
| Jenna Mammen, MD, PhD | Johns Hopkins University | Endocrinology |
| Alexander Spira, MD, PhD | Virginia Cancer Specialists and US Oncology Research | Medical oncology |
| Jeffrey M. Caterino, MD, MPH | The Ohio State University Wexner Medical Center | Emergency medicine |
| Bianca Santomasso, MD, PhD | Memorial Sloan Kettering Cancer Center | Neuro-oncology |
| Sigrun Hallmeyer, MD | Oncology Specialists SC | Medical oncology, Practice Guidelines Implementation Network (PGIN) representative |
| Tanyanika Phillips, MD | CHRISTUS St Frances Cabrini Cancer Center | Medical Oncology, PGIN representative |
| Pamela Ginex, EdD, RN | Oncology Nursing Society | Oncology nursing, Oncology Nursing Society (ONS) representative |
| Kelly Brassil, PhD, RN | MD Anderson Cancer Center | Oncology nursing, ONS representative |
| Laura Porter, MD | Patient advocate | |
| Carole Seigel | Patient advocate | |
| Christina Lacchetti | American Society of Clinical Oncology | Staff, health research methodologist |
Table A2.
Commonly Conducted Testing at Baseline Prior to ICPi Therapy*
| Testing |
|---|
| Clinical |
| Physical examination, including physical stature, weight, body mass index, heart rate, and blood pressure Comprehensive history, including autoimmune, organ-specific disease, endocrinopathy, neuropathy, and infectious disease Questioning ofgeneral health, including appetite, bowel habits, and asthenia. Preexisting symptoms involving bowel movements, dyspnea, cough, rash, headaches, and arthralgia should be noted. |
| Laboratory |
| CBC + differential test Complete metabolic panel that may include serum electrolytes (Na, K, Ca, CO2), liver function (AST, ALT, alkaline phosphatase, γ-glutamyl transferase), creatinine, creatine kinase, total bilirubin Glucose Lactate dehydrogenase and aldolase Thyroid-stimulating hormone, free thyroxine Luteinizing hormone, follicle-stimulating hormone, and testosterone levels in males or estrogen in premenopausal females with fatigue, loss of libido, and mood changes Urinalysis Surveillance for latent tuberculosis Virology including HIV, hepatitis C virus and hepatitis B virus, Epstein-Barr virus, cytomegalovirus Troponin Spirometry/diffusing capacity of lung for carbon monoxide |
| Imaging |
| Chest x-ray Computed tomography ECG |
Other testing may also be necessary based on patient's history and preexisting comorbidities and/or risk factors.
Table A3.
Abbreviations
| Acronym | Definition |
|---|---|
| ABG | arterial blood gas |
| ACC | American College of Cardiology |
| ACTH | adrenocorticotropic hormone |
| ADL | activities of daily living |
| AE | adverse event |
| AHA | acquired hemophilia A |
| AHA | American Heart Association |
| AI | adrenal insufficiency |
| ALT | alanine aminotransferase |
| ANA | antinuclear antibodies |
| ANCA | antineutrophil cytoplasmic antibodies |
| ANNA-1 | anti-neuronal nuclear antibody 1 |
| APTT | activated partial thromboplastic time |
| ASCO | American Society of Clinical Oncology |
| ASH | American Society of Hematology |
| ASMA | anti-smooth muscle antibodies |
| AST | aspartate aminotransferase |
| ATG | antithymocyte globulin |
| BAL | bronchoalveolar lavage |
| BNP | brain natriuretic peptide |
| BSA | body surface area |
| CAR-T | chimeric antigen receptor T-cell |
| CBC | complete blood count |
| CMP | comprehensive metabolic panel |
| CMV | cytomegalovirus |
| CNS | central nervous system |
| CPK | creatine phosphokinsase |
| CRP | C-reactive protein |
| CSF | cerebrospinal fluid |
| CT | computed tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CTLA-4 | cytotoxic T-cell lymphocyte-4 |
| CTPA | computed tomographic pulmonary angiography |
| CXR | chest x-ray |
| DAT | direct antiglobulin test |
| DIC | disseminated intravascular coagulation |
| DEB | diepoxybutane |
| DKA | diabetic ketoacidosis |
| DM | diabetes mellitus |
| DVT | deep vein thrombosis |
| EBV | Epstein-Barr virus |
| EEG | electroencephalogram |
| EGD | esophagogastroduodenoscopy |
| EMG | electromyography |
| EMS | emergency medical services |
| ENT | ears, nose, and throat |
| ESR | erythrocyte sedimentation rate |
| FLAIR | fluid-attenuated inversion recovery |
| FSH | follicle-stimulating hormone |
| FT4 | free thyroxine |
| G1 | grade 1 |
| G2 | grade 2 |
| G3 | grade 3 |
| G4 | grade 4 |
| GBS | Guillain-Barré syndrome |
| GCSF | granulocyte colony-stimulating factor |
| GI | gastrointestinal |
| GPI | glycosylphosphatidylinositol |
| HCV | hepatitis C virus |
| Hgb | hemoglobin |
| HHV6 | human herpesvirus 6 |
| HIV | human immunodeficiency virus |
| HRCT | high-resolution computed tomography |
| HSV | herpes simplex virus |
| HUS | hemolytic uremic syndrome |
| IA | inflammatory arthritis (continued in next column) |
| ICPi | mmune checkpoint inhibitor |
| ICU | intensive care unit |
| igG | mmunogloblulin G |
| irAE | immune-related adverse event |
| ITP | diopathic thrombocytopenic purpura |
| IV | intravenous |
| IVIG | ntravenous immunoglobulin |
| LEMS | Lambert-Eaton Myasthenic Syndrome |
| LFTs | iver function tests |
| LH | utenizing hormone |
| LLN | ower limit of normal |
| LMWH | ow molecular weight heparin |
| MCV | mean corpuscular volume |
| MDS | myelodysplastic syndromes |
| MGFA | myasthenia gravis foundation of America |
| MMF | mycophenolate mofetil |
| MRI | magnetic resonance imaging |
| NCS | nerve conduction study |
| NIF | negative inspiratory force |
| O&P | ova and parasite |
| PCP | Pneumocystis pneumonia |
| PCR | polymerase chain reaction |
| PD-1 | programmed death 1 |
| PD-L1 | programmed death ligand 1 |
| PE | pulmonary embolism |
| PEX | plasma exchange |
| PMNs | polymorphonuclear cells |
| PNH | paroxysmal nocturnal hemoglobinuria |
| PPI | proton pump inhibitor |
| PT | prothrombin time |
| PTU | propylthiouracil |
| RBC | red blood cell |
| RPR | rapid plasma reagin |
| T1DM | type 1 diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
| TB | tuberculosis |
| TID | three times a day |
| TPO | thyroid peroxidase |
| TSH | thyroid stimulating hormone |
| TSI | thyroid stimulation immunoglobulin |
| TTE | transthoracic echocardiogram |
| TTP | thrombotic thrombocytopenic purpura |
| ULN | upper limit of normal |
| US | ultrasound |
| UTIs | urinary tract infections |
| V/Q | ventilation-perfusion lung scan |
| VC | vital capacity |
| VKA | vitamin K antagonists |
| VTE | venous thromboembolism |
| WBC | white blood cell |
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at jco.org.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Julie R. Brahmer
Consulting or Advisory Role: Bristol-Myers Squibb, Eli Lilly, Celgene, Syndax, Janssen Pharmaceuticals, Merck
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst), Incyte (Inst), Five Prime Therapeutics (Inst), Janssen Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck
Other Relationship: Bristol-Myers Squibb, Merck
Christina Lacchetti
No relationship to disclose
Bryan J. Schneider
Consulting or Advisory Role: Genentech, Roche
Research Funding: MedImmune (Inst), Genentech (Inst), Roche (Inst), OncoMed (Inst), Bristol-Myers Squibb (Inst), Macrogenics (Inst)
Michael B. Atkins Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Genentech, Pfizer, Novartis, X4 Pharma, Bristol-Myers Squibb, Nektar, Merck, Exelixis, Acceleron Pharma, Peleton, Eisai, Celldex, Alexion Pharmaceuticals, AstraZeneca, MedImmune, Glactone Pharma, Agenus, Idera, Argos Therapeutics, Array BioPharma, Boehringer Ingelheim, Aduro Biotech
Kelly J. Brassil
Research Funding: Genentech, Roche Jeffrey M. Caterino
Stock or Other Ownership: Motive Medical Intelligence
Research Funding: JD Pharmaceuticals, AstraZeneca
Ian Chau
Honoraria: Taiho Pharmaceutical, Pfizer, Eli Lilly, Amgen, Gilead Sciences
Consulting or Advisory Role: Sanofi, Eli Lilly, Bristol-Myers Squibb, MSD Oncology, Bayer AG, Roche, Genentech, Five Prime Therapeutics
Research Funding: Janssen-Cilag (Inst), Sanofi (Inst), Merck Serono (Inst), Eli Lilly (Inst)
Travel, Accommodations, Expenses: MSD, Merck Serono, Sanofi, Eli Lilly, Bristol-Myers Squibb
Marc S. Ernstoff
Stock or Other Ownership: GE Healthcare
Consulting or Advisory Role: Omniseq, Celyad, Lion Biotechnologies, Bristol-Myers Squibb, EMD Serono
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), Alkermes (Inst), Merrimack (Inst)
Travel, Accommodations, Expenses: EMD Serono
Jennifer M. Gardner
No relationship to disclose
Pamela Ginex
Honoraria: Physician Education Resource
Consulting or Advisory Role: Genentech, Roche
Sigrun Hallmeyer
Employment: Oncology Specialists, Advocate Medical
Group Leadership: Oncology Specialists, Advocate Lutheran General Hospital
Honoraria: Bristol-Myers Squibb, Pfizer
Consulting or Advisory Role: Cardinal Health, Bristol-Myers Squibb, KBL Biologics
Speakers’ Bureau: Bristol-Myers Squibb, Pfizer
Research Funding: Russell Research Institute (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Cardinal Health
Jennifer Holter Chakrabarty
No relationship to disclose
Natasha B. Leighl
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Merck Sharp & Dohme, Bristol-Myers Squibb, Pfizer
Jennifer S. Mammen
Stock or Other Ownership: Johnson & Johnson, Bristol-Myers Squibb
David F. McDermott
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech, Roche, Pfizer, Exelixis, Novartis, X4 Pharma, Array BioPharma
Research Funding: Prometheus Laboratories (Inst)
Aung Naing
Consulting or Advisory Role: Novartis, CytomX Therapeutics
Research Funding: National Cancer Institute, EMD Serono, MedImmune, Healios, Atterocor, Amplimmune, ARMO BioSciences, Karyopharm Therapeutics, Incyte, Novartis, Regeneron
Travel, Accommodations, Expenses: ARMO BioSciences
Loretta J. Nastoupil
Honoraria: Celgene, TG Therapeutics, Gilead Science, AbbVie, Pharmacyclics
Research Funding: TG Therapeutics, Janssen Pharmaceuticals, Celgene, AbbVie, Genentech, Roche, Karus Therapeutics
Tanyanika Phillips
No relationship to disclose
Laura D. Porter
No relationship to disclose
Igor Puzanov
Consulting or Advisory Role: Amgen, Roche, Genentech, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Amgen, Merck
Cristina A. Reichner
No relationship to disclose
Bianca D. Santomasso
Honoraria: Juno Therapeutics, Kite Pharma
Consulting or Advisory Role: Juno Therapeutics, Kite Pharma, Incysus Travel, Accommodations, Expenses: Juno Therapeutics, Kite Pharma
Carole Seigel
No relationship to disclose
Alexander Spira
Honoraria: Novartis, Roche, Clovis Oncology, ARIAD Pharmaceuticals
Consulting or Advisory Role: Roche, Genentech, ARIAD Pharmaceuticals, Clovis Oncology
Research Funding: Roche (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), Clovis Oncology (Inst), MedImmune (Inst), Novartis (Inst), Newlink Genetics (Inst), Incyte (Inst), AbbVie (Inst), BeiGene (Inst), Merck KgaA (Inst), Merck (Inst), Merrimack (Inst), CytomX Therapeutics (Inst)
Travel, Accommodations, Expenses: Roche, ARIAD Pharmaceuticals
Maria E. Suarez-Almazor
Consulting or Advisory Role: Bristol-Myers Squibb, Endo Pharmaceuticals, Pfizer, Eli Lilly
Research Funding: Pfizer
Yinghong Wang
No relationship to disclose
Jeffrey S. Weber
Stock or Other Ownership: Altor BioScience, Celldex, CytomX Therapeutics
Honoraria: Bristol-Myers Squibb, Merck, Genentech, AbbVie, AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Eisai, Alto BioScience, Lion Biotechnologies, Amgen, Roche, Ichor Medical Systems, Celldex, cCam Biotherapeutics, Pieris Pharmaceuticals, CytomX Therapeutics, Nektar, Novartis, Medivation, Sellas Life Sciences, WindMIL
Consulting or Advisory Role: Celldex, Ichor Medical Systems, cCam Biotherapeutics, Lion Biotechnologies, Pieris Pharmaceuticals, Altor BioScience, Bristol-Myers Squibb, Merck, Genentech, Roche, Amgen, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo, AbbVie, Eisai, CytomX Therapeutics, Nektar, Medivation, Sellas Life Sciences, WindMIL
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), GlaxoSmithKline (Inst), Genentech (Inst), Astellas Pharma (Inst), Incyte (Inst), Roche (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Named on a patent submitted by Moffitt Cancer Center for an ipilimumab biomarker, named on a patent from Biodesix for a PD-1 antibody biomarker
Travel, Accommodations, Expenses: Bristol-Myers Squibb, GlaxoSmithKline, Daiichi Sankyo, Pieris Pharmaceuticals, cCam Biotherapeutics, Lion Biotechnologies, Roche, Celldex, Amgen, Merck, AstraZeneca, Genentech, Novartis
Jedd D. Wolchok
Stock or Other Ownership: Potenza Therapeutics, Tizona Therapeutics, Serametrix, Adaptive Biotechnologies, Trieza Therapeutics, BeiGene
Honoraria: Regeneron
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, MedImmune, Polynoma, Polaris, Genentech, BeiGene, Sellas Life Sciences, Eli Lilly, Potenza Therapeutics, Tizona Therapeutics, Amgen, Chugai Pharmaceuticals, Ono Pharmaceuticals, Ascentage Pharma
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), Genentech (Inst), Roche (Inst)
Patents, Royalties, Other Intellectual Property: Co-inventor on an issued patent for DNA vaccines for treatment of cancer in companion animals, co-inventor on a patent for use of oncolytic Newcastle Disease virus
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Chugai Pharmaceuticals, Roche, Janssen Pharmaceuticals
John A. Thompson
Consulting or Advisory Role: Seattle Genetics, Nektar, Celldex, Calithera Biosciences, Boehringer Ingelheim
Research Funding: Bristol-Myers Squibb (Inst), Roche (Inst), Seattle Genetics (Inst), Pfizer (Inst), Agensys (Inst), Five Prime Therapeutics (Inst), Corvus Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Merck (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim
ADDITIONAL RESOURCES
More information, including a Data Supplement with additional evidence tables, a Methodology Supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources, is available at www.asco.org/supportive-care-guidelines and www.asco.org/guidelinswiki. Patient information is available at www.cancer.net. Visit www.asco.org/guidelineswiki to provide comments on the guideline or to submit new evidence.
Contributor Information
Julie R. Brahmer, Johns Hopkins Kimmel Cancer Center, Baltimore, MD
Christina Lacchetti, American Society of Clinical Oncology, Alexandria, VA.
Bryan J. Schneider, University of Michigan Health System, Ann Arbor, MI
Michael B. Atkins, Georgetown Lombardi Comprehensive Cancer Center; Washington, DC
Kelly J. Brassil, Anderson Cancer Center, Houston, TX
Jeffrey M. Caterino, The Ohio State University Wexner Medical Center, Columbus, OH
Ian Chau, The Royal Marsden Hospital and Institute of Cancer Research, London and Surrey, United Kingdom.
Marc S. Ernstoff, Roswell Park Cancer Institute, Buffalo
Jennifer M. Gardner, Seattle Cancer Care Alliance and University of Washington, Seattle, WA
Pamela Ginex, Oncology Nursing Society, Pittsburgh, PA.
Sigrun Hallmeyer, Oncology Specialists SC, Park Ridge, IL.
Jennifer Holter Chakrabarty, University of Oklahoma, Stephenson Cancer Center, Oklahoma City, OK.
Natasha B. Leighl, Princess Margaret Cancer Centre, Toronto, Ontario, Canada
Jennifer S. Mammen, Johns Hopkins University, Baltimore, MD
David F. McDermott, Beth Israel Deaconess Medical Center, Boston, MA
Aung Naing, Anderson Cancer Center, Houston, TX.
Loretta J. Nastoupil, Anderson Cancer Center, Houston, TX
Tanyanika Phillips, CHRISTUS St Frances Cabrini Cancer Center, Alexandria, LA..
Laura D. Porter, Colon Cancer Alliance; Washington, DC
Igor Puzanov, Roswell Park Cancer Institute, Buffalo.
Cristina A. Reichner, Georgetown University; Washington, DC
Bianca D. Santomasso, Memorial Sloan Kettering Cancer Center, New York, NY
Carole Seigel, Massachusetts General Hospital Cancer Center, Boston, MA.
Alexander Spira, Virginia Cancer Specialists and US Oncology Research, Fairfax, VA.
Maria E. Suarez-Almazor, Anderson Cancer Center, Houston, TX
Yinghong Wang, Anderson Cancer Center, Houston, TX.
Jeffrey S. Weber, New York University Langone Medical Center, New York, NY
Jedd D. Wolchok, Memorial Sloan Kettering Cancer Center, New York, NY
John A. Thompson, Seattle Cancer Care Alliance, University of Washington, and the Fred Hutchinson Cancer Research Center, Seattle, WA
REFERENCES
- 1.Dine J, Gordon R, Shames Y, et al. : Immune checkpoint inhibitors: An innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurs 4:127–135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow M, Wolchok J: Toxicities Associated With Checkpoint Inhibitor Immunotherapy. In: UpToDate, Atkins MB (Ed), UpToDate, Waltham, MA, 2017. [Google Scholar]
- 3.National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) 5.0.
- 4.Weber JS, Yang JC, Atkins MB, et al. : Toxic- ities of immunotherapy for the practitioner. J Clin Oncol 33:2092–2099, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber JS, Kahler KC, Hauschild A: Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 30: 2691–2697, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Villadolid J, Amin A: Immune checkpoint inhibitors in clinical practice: Update on management of immune-related toxicities. Transl Lung Cancer Res 4:560–575, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggermont AM, Chiarion-Sileni V, Grob JJ: Correction to Lancet Oncol 2015; 16: 522–30. [DOI] [PubMed] [Google Scholar]; Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol 16:e262, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, et al. : Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman-Keller M, Kim Y, Cronin H, et al. : Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22:886–894, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hua C, Boussemart L, Mateus C, et al. : Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 152:45–51, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Weber JS, O’Day S, Urba W, et al. : Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 26:5950–5956, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Teulings HE, Limpens J, Jansen SN, et al. : Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J Clin Oncol 33:773–781, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Hsieh AHC, Ferman M, Brown MP, et al. : Vedolizumab: A novel treatment for ipilimumab-induced colitis. BMJ Case Reports, 2016. http://casereports.bmj.com/content/2016/bcr-2016-216641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergqvist V, Hertervig E, Gedeon P, et al. : Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 66:581–592, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diana P, Mankongppaisarnrung C, Charabaty A: Vedolizumab: A novel approach to the treatment of immune checkpoint inhibitors-induced enterocolitis, World Congress of GastroenterologyACG2017 Annual Scientific Meeting, Orlando, FL, October 16, 2017 [Google Scholar]
- 16.Gupta A, De Felice KM, Loftus EV Jr, et al. : Systematic review: Colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther 42:406–417, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Cabanillas G: Immune related adverse events and their treatment in melanoma patients receiving ipilimumab. J Clin Oncol 35, 2017. (suppl; abstr e14598) [Google Scholar]
- 18.Kumar V, Chaudhary N, Garg M, et al. : Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 8:49, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Rahman O, Elhalawani H, Fouad M: Risk of gastrointestinal complications in cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Immunotherapy 7, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Kwon ED, Drake CG, Scher HI, et al. : Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): A multicentre, randomised, doubleblind, phase 3 trial. Lancet Oncol 15:700–712, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marthey L, Mateus C, Mussini C, et al. : Cancer immunotherapy with anti–CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohn’s Colitis 10:395–401, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cramer P, Bresalier RS: Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Curr Gastroenterol Rep 19:3, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Chen JH, Pezhouh MK, Lauwers GY, et al. : Histopathologic features of colitis due to immunotherapy with anti–PD-1 antibodies. Am J Surg Pathol 41:643–654, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Berman D, Parker SM, Chasalow SD, et al. : Potential immune biomarkers of gastrointestinal toxicities and efficacy in patients with advanced melanoma treated with ipilimumab with or without prophylactic budesonide. J Clin Oncol 26:3022, 2008. (suppl 15 ) [Google Scholar]
- 25.Verschuren EC, van den Eertwegh AJ, Wonders J, et al. Clinical. endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenteral Hepatol 14: 836–842, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Jain A, Lipson EJ, Sharfman WH, et al. : Colonic ulcerations may predict steroid-refractory course in patients with ipilimumab-mediated enterocolitis. World J Gastroenterol 23:2023–2028, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber J: Ipilimumab: Controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother 58:823–830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziemer M, Koukoulioti E, Simon JC, et al. : Managing immune checkpoint-inhibitor-induced severe autoimmune-like hepatitis by liver-directed topical steroids. J Hepatol 66: 657–659, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Tripathi A, Kaymakcalan MD, LeBoeuf NR, et al. : Programmed cell death-1 pathway inhibitors in genitourinary malignancies: Specific side-effects and their management. Curr Opin Urol 26:548–555, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Pollack MH, Betof A, Dearden H, et al. : Safety of resuming anti–PD-1 in patients with immune-related adverse events (irAEs) during combined anti–CTLA-4 and anti–PD1 in metastatic melanoma. Ann Oncol 29:250–255 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menzies AM, Johnson DB, Ramanujam S, et al. : Anti–PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28: 368–376, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Weber JS, Kudchadkar RR, Gibney GT, et al. : Phase I/II trial of PD-1 antibody nivolumab with peptide vaccine in patients naive to or that failed ipilimumab. J Clin Oncol 31:9011, 2013 [Google Scholar]
- 33.Kyi C, Hellmann MD, Wolchok JD, et al. : Opportunistic infections in patients treated with immunotherapy for cancer. J Immunother Cancer 2:19, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haanen JBAG, Carbonnel F, Robert C, et al. : Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv119–iv142, 2017. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 35.Champiat S, Lambotte O, Barreau E, et al. : Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol 27:559–574, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Spain L, Diem S, Larkin J: Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44:51–60, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Postow MA. Managing Immune Checkpoint-Blocking Antibody Side Effects . Am Soc Clin Oncol Educ Book 76–83, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Naidoo J, Wang X, Woo KM, et al. : Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. 35:709–717, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishino M, Giobbie-Hurder A, Hatabu H, et al. : Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol 2:1607–1616, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Chuzi S, Tavora F, Cruz M, et al. : Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res 9:207–213, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barjaktarevic IZ, Qadir N, Suri A, et al. : Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest 143:858–861, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Tirumani SH, Ramaiya NH, Keraliya A, et al. : Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 3:1185–1192, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolchok JD, Neyns B, Linette G, et al. : Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 11:155–164, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Margolin K, Ernstoff MS, Hamid O, et al. : Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol 13:459–465, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Postow MA, Chesney J, Pavlick AC, et al. : Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006–2017, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santini FC, Rizvi H, Wilkins O, et al. : Safety of retreatment with immunotherapy after immune-related toxicity in patients with lung cancers treated with anti-PD(L)-1 therapy. J Clin Oncol 35: 2017. (suppl 9012) [Google Scholar]
- 47.Gettinger SN, Horn L, Gandhi L, et al. : Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33: 2004–2012, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garon EB, Rizvi NA, Hui R, et al. : Pem- brolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Nagata S, Ueda N, Yoshida Y, et al. : Severe interstitial pneumonitis associated with the administration of taxanes. J Infect Chemother 16:340–344, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Hwang WL, Niemierko A, Hwang KL, et al. : Clinical outcomes in patients with metastatic lung cancer treated with PD-1/PD-L1 inhibitors and thoracic radiotherapy. JAMA Oncol. Published online September 27, 2017. doi: 10.1001/jamaoncol.2017.3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassel JC, Heinzerling L, Aberle J, et al. : Combined immune checkpoint blockade (anti–PD-1/anti–CTLA-4): Evaluation and managementofadverse drug reactions. Cancer Treat Rev 57:36–49, 2017 [DOI] [PubMed] [Google Scholar]
- 52.Friedman CF, Proverbs-Singh TA, Postow MA: Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol 2:1346–1353, 2016 [DOI] [PubMed] [Google Scholar]
- 53.O’Kane GM, Labbé C, Doherty MK, et al. : Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist 22:70–80, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bashey A, Medina B, Corringham S, et al. : CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 113:1581–1588, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montaudié H, Pradelli J, Passeron T, et al. : Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br J Dermatol 176:1060–1063, 2017 [DOI] [PubMed] [Google Scholar]
- 56.Danlos FX, Pagés C, Baroudjian B, et al. : Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest 149:e133–e136, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Reuss JE, Kunk PR, Stowman AM, et al. : Sarcoidosis in the setting of combination ipilimumab and nivolumab immunotherapy: A case report & review of the literature. J Immunother Cancer 4:94, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blansfield JA, Beck KE, Tran K, et al. : Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother 28:593–598, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faje AT, Sullivan R, Lawrence D, et al. : Ipilimumab-induced hypophysitis: A detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab 99: 4078–4085, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Ryder M, Callahan M, Postow MA, et al. : Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr Relat Cancer 21:371–381, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Min L, Hodi FS, Giobbie-Hurder A, et al. : Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res 21:749–755, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. : Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol, 2017. Published online September 28, 2017. doi: 10.1001/jamaoncol.2017.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bornstein SR, Allolio B, Arlt W, et al. : Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 101:364–389, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fleseriu M, Hashim IA, Karavitaki N, et al. : Hormonal replacement in hypopituitarism in adults: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 101:3888–3921, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Persani L, Bonomi M, Radin R, et al. : Diagnostic and therapeutic challenges of acquired thyrotropic deficiency. Ann Endocrinol (Paris) 73: 138–140, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Fliers E, Bianco AC, Langouche L, et al. : Thyroid function in critically ill patients. Lancet Diabetes Endocrinol 3:816–825, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suarez-Almazor ME, Kim ST, Abdel-Wahab N, et al. : Review: Immune-related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol 69:687–699, 2017 [DOI] [PubMed] [Google Scholar]
- 68.Cappelli L, Gutierrez AK, Shah AA, et al. : Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: A systematic literature review. Arthritis Care Res 69:1751–1763, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calabrese L, Velcheti V: Checkpoint immunotherapy: Good for cancer therapy, bad for rheumatic diseases. Ann Rheum Dis 76:1–3, 2016 [DOI] [PubMed] [Google Scholar]
- 70.Cappelli LC, Gutierrez AK, Baer AN, et al. : Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 76:43–50, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cappelli LC, Naidoo J, Bingham CO III, et al. : Inflammatory arthritis due to immune checkpoint inhibitors: Challenges in diagnosis and treatment. Immunotherapy 9:5–8, 2017 [DOI] [PubMed] [Google Scholar]
- 72.Kim ST, Tayar J, Trinh VA, et al. : Successful treatmentofarthritis induced by checkpoint inhibitors with tocilizumab: A case series. Ann Rheum Dis 76: 2061–2064, 2017 [DOI] [PubMed] [Google Scholar]
- 73.Belkhir R, Burel SL, Dunogeant L, et al. : Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis 76:1747–1750, 2017 [DOI] [PubMed] [Google Scholar]
- 74.Shah M, Taylor J, Abdel-Wahab N, et al. : Myositis as a complication of checkpoint blockade at a comprehensive cancer center. Presented at Arthritis College of Rheumatology/ARHP Annual Meeting, San Diego, CA, November 3–8, 2017 [Google Scholar]
- 75.Johnson DB, Balko JM, Compton ML, Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375:1749–1755, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laubli H, Balmelli C, Bossard M, et al. : Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer 3:11, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdel-Wahab N, Shah M, Suarez-Almazor ME: Adverse events associated with immune checkpoint blockade in patients with cancer: A systematic review of case reports. PLoS One 11:e0160221, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abdel-Wahab N, Shah M, Lopez-Olivo MA, et al. : Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: A systematic review. Ann Intern Med 168:121–130, 2017 [DOI] [PubMed] [Google Scholar]
- 79.Sznol M, Ferrucci PF, Hogg D, et al. : Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 35:3815–3822, 2017 [DOI] [PubMed] [Google Scholar]
- 80.Wanchoo R, Karam S, Uppal NN, et al. : Adverse renal effects of immune checkpoint inhibitors: A narrative review. Am J Nephrol 45:160–169, 2017 [DOI] [PubMed] [Google Scholar]
- 81.Cortazar FB, Marrone KA, Troxell ML, et al. : Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90:638–647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lipson EJ, Bagnasco SM, Moore J Jr, et al. : Tumor regression and allograft rejection after administration of anti–PD-1. N Engl J Med 374:896–898, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alhamad T, Venkatachalam K, Linette GP, et al. : Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant 16:1332–1333, 2016 [DOI] [PubMed] [Google Scholar]
- 84.Spain L, Higgins R, Gopalakrishnan K, et al. : Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol 27:1135–1137, 2016 [DOI] [PubMed] [Google Scholar]
- 85.Boils CL, Aljadir DN, Cantafio AW: Use of the PD-1 pathway inhibitor nivolumab in a renal transplant patient with malignancy. Am J Transplant 16: 2496–2497, 2016 [DOI] [PubMed] [Google Scholar]
- 86.Barnett R, Barta VS, Jhaveri KD: Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab. N Engl J Med 376:191–192, 2017 [DOI] [PubMed] [Google Scholar]
- 87.Suzuki S, Ishikawa N, Konoeda F, et al. : Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 89:1127–1134, 2017 [DOI] [PubMed] [Google Scholar]
- 88.Cuzzubbo S, Javeri F, Tissier M, et al. : Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer 73:1–8, 2017 [DOI] [PubMed] [Google Scholar]
- 89.Kao JC, Liao B, Markovic SN, et al. : Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol 74:1216–1222, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spain L, Walls G, Julve M, et al. : Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: A single centre experience and review of the literature. Ann Oncol 28: 377–385, 2017 [DOI] [PubMed] [Google Scholar]
- 91.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. : Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase3 trial. Lancet Oncol 16:522–530, 2015 [DOI] [PubMed] [Google Scholar]
- 92.Williams TJ, Benavides DR, Patrice KA, et al. : Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol 73:928–933, 2016 [DOI] [PubMed] [Google Scholar]
- 93.Feng S, Coward J, McCaffrey E, et al. : Pembrolizumab-induced encephalopathy: A review of neurological toxicities with immune checkpoint inhibitors. J Thorac Oncol 12:1626–1635, 2017 [DOI] [PubMed] [Google Scholar]
- 94.George JN: How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood 116: 4060–4069, 2010 [DOI] [PubMed] [Google Scholar]
- 95.Joly BS, Coppo P, Veyradier A: Thrombotic thrombocytopenic purpura. Blood 129:2836–2846, 2017 [DOI] [PubMed] [Google Scholar]
- 96.Sayani FA, Abrams CS: How I treat refractory thrombotic thrombocytopenic purpura. Blood 125: 3860–3867, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neunert C, Lim W, Crowther M, et al. : The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 117:4190–4207, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Collins PW, Percy CL: Advances in the understanding of acquired haemophilia A: Implications forclinical practice. BrJ Haematol 148:183–194,2010 [DOI] [PubMed] [Google Scholar]
- 99.Sharma P, Callahan MK, Bono P, et al. : Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 17:1590–1598, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weber JS, D’Angelo SP, Minor D, et al. : Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti- CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16: 375–384, 2015 [DOI] [PubMed] [Google Scholar]
- 101.Inadomi K, Kumagai H, Arita S, et al. : Bi- cytopenia possibly induced by anti-PD-1 antibody for primary malignant melanoma of the esophagus: A case report. Medicine (Baltimore) 95:e4283, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nair R, Gheith S, Nair SG: Immunotherapy-associated hemolytic anemia with pure red-cell aplasia. N Engl J Med 374:1096–1097, 2016 [DOI] [PubMed] [Google Scholar]
- 103.Cooling LL, Sherbeck J, Mowers JC, et al. : Development of red blood cell autoantibodies following treatment with checkpoint inhibitors: A new class of anti-neoplastic, immunotherapeutic agents associated with immune dysregulation. Immunohe- matology 33:15–21, 2017 [PubMed] [Google Scholar]
- 104.Shiuan E, Beckermann KE, Ozgun A, et al. : Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer 5:8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Delyon J, Mateus C, Lambert T: Hemophilia A induced by ipilimumab. N Engl J Med 365:1747–1748, 2011 [DOI] [PubMed] [Google Scholar]
- 106.Lozier J: More on hemophilia A induced by ipilimumab. N Engl J Med 366:280–281, 2012 [DOI] [PubMed] [Google Scholar]
- 107.Merryman RW, Armand P: Immune checkpoint blockade and hematopoietic stem cell transplant. Curr Hematol Malig Rep 12:44–50, 2017 [DOI] [PubMed] [Google Scholar]
- 108.Kwon HJCT, Coté TR, Cuffe MS, et al. : Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med 138: 807–811, 2003 [DOI] [PubMed] [Google Scholar]
- 109.Kearon C, Akl EA, Ornelas J, et al. : Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149:315–352, 2016 [DOI] [PubMed] [Google Scholar]
- 110.Lyman GH, Khorana AA, Kuderer NM, et al. : Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 31:2189–2204, 2013 [DOI] [PubMed] [Google Scholar]
- 111.Jain V, Bahia J, Mohebtash M, et al. : Cardiovascular complications associated with novel cancer immunotherapies. Curr Treat Options Cardiovasc Med 19:36, 2017 [DOI] [PubMed] [Google Scholar]
- 112.Wang DY, Okoye GD, Neilan TG, et al. : Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep 19:21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tay RY, Blackley E, McLean C, et al. : Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br J Cancer 117:921–924, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heinzerling L, Ott PA, Hodi FS, et al. : Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 4:50, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yun S, Vincelette ND, Mansour I, et al. : Late onset ipilimumab-induced pericarditis and pericardial effusion: A rare but life threatening complication. Case Rep Oncol Med 2015:794842, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tomita Y, Sueta D, Kakiuchi Y, et al. : Acute coronary syndrome as a possible immune-related adverse event in a lung cancer patient achieving a complete response to anti-PD-1 immune checkpoint antibody. Ann Oncol 28:2393–2895, 2017 [DOI] [PubMed] [Google Scholar]
- 117.Arangalage D, Delyon J, Lermuzeaux M, et al. : Survival after fulminant myocarditis induced by immune-checkpoint inhibitors. Ann Intern Med 167: 683–684, 2017 [DOI] [PubMed] [Google Scholar]
- 118.Behling J, Kaes J, Münzel T, et al. : New-onset third-degree atrioventricular block because of autoimmune-induced myositis under treatment with anti-programmed cell death-1 (nivolumab) for metastatic melanoma. Melanoma Res 27:155–158, 2017 [DOI] [PubMed] [Google Scholar]
- 119.Reddy N, Moudgil R, Lopez-Mattei JC, et al. : Progressive and reversible conduction disease with checkpoint inhibitors. Can J Cardiol 33:1335. e13–1335.e15, 2017 [DOI] [PubMed] [Google Scholar]
- 120.Roth ME, Muluneh B, Jensen BC, et al. : Left ventricular dysfunction after treatment with ipilimumab for metastatic melanoma. Am J Ther 23: e1925–e1928, 2016 [DOI] [PubMed] [Google Scholar]
- 121.Pasadhika S, Rosenbaum JT: Update on the use of systemic biologic agents in the treatment of noninfectious uveitis. Biologics 8:67–81, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Doctor P, Sultan A, Syed S, et al. : Infliximab for the treatment of refractory scleritis. Br J Ophthalmol 94:579–583, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Voskens C, Cavallaro A, Erdmann M, et al. : Anti-cytotoxic T-cell lymphocyte antigen-4-induced regression of spinal cord metastases in association with renal failure, atypical pneumonia, vision loss, and hearing loss. J Clin Oncol 30:e356–e357, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Attia P, Phan GQ, Maker AV, et al. : Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anticytotoxic T-lymphocyte antigen-4. J Clin Oncol 23: 6043–6053, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Robert C, Schachter J, Long GV, et al. : Pem- brolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532, 2015 [DOI] [PubMed] [Google Scholar]
- 126.Ribas A, Hamid O, Daud A, et al. : Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315:1600–1609, 2016 [DOI] [PubMed] [Google Scholar]
- 127.Topalian SL, Sznol M, McDermott DF, et al. : Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Patnaik A, Socinski MA, Gubens MA, et al. : Phase 1 study of pembrolizumab (pembro; MK-3475) plus ipilimumab (IPI) as second-line therapy for advanced non-small cell lung cancer (NSCLC): KEYNOTE-021 cohort D. J Clin Oncol 33:8011–8011, 2015 [Google Scholar]
- 129.Johnson DB, Sullivan RJ, Ott PA: Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune conditions. JAMA Oncol 2: 234–240, 2016 [DOI] [PubMed] [Google Scholar]
- 130.Abdel-Wahab N, Shah M, Suarez-Almazor M: Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune diseases: Asystematic reviewofcase reports. World Congress of Gastroenterology ACG2017 Annual Scientific Meeting Washington, DC, November 11–16, 2016 [Google Scholar]
- 131.Abdel-Rahman O, Fouad M: Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Ther Adv Respir Dis 10:183–193, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang S, Liang F, Zhu J, et al. : Risk of pneumonitis associated with programmed cell death 1 inhibitors in cancer patients: A meta-analysis. Mol Cancer Ther 16:1588–1595, 2017 [DOI] [PubMed] [Google Scholar]
- 133.Minkis K, Garden BC, Wu S, et al. : The risk of rash associated with ipilimumab in patients with cancer: A systematic review of the literature and meta-Analysis. J Am Acad Dermatol 69:e121–e128, 2013 [DOI] [PubMed] [Google Scholar]
- 134.Khunger M, Rakshit S, Pasupuleti V, et al. : Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: A systematic review and meta-analysis of trials. Chest 152:271–281, 2017 [DOI] [PubMed] [Google Scholar]
- 135.Chaput N, Lepage P, Coutzac C, et al. : Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 28:1368–1379, 2017 [DOI] [PubMed] [Google Scholar]
- 136.Dubin K, Callahan MK, Ren B, et al. : Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 7:10391, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Michot JM, Bigenwald C, Champiat S, et al. : Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 54: 139–148, 2016 [DOI] [PubMed] [Google Scholar]
- 138.Feng Y, Roy A, Masson E, et al. : Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res 19:3977–3986, 2013 [DOI] [PubMed] [Google Scholar]
- 139.Davies M, Duffield EA: Safety of checkpoint inhibitors for cancer treatment: Strategies for patient monitoring and management of immune-mediated adverse events. ImmunoTargets Ther 6:51–71, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jin C, Zhang X, Zhao K, et al. : The efficacy and safety of nivolumab in the treatment of advanced melanoma: A meta-analysis of clinical trials. Onco Targets Ther 9:1571–1578, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hodi FS, Chesney J, Pavlick AC, et al. : Two-year overall survival rates from a randomised phase 2 trial evaluating the combination of nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma. Lancet Oncol 17:1558–1568, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weber JS, Postow M, Lao CD, et al. : Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist 21: 1230–1240, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang S, Liang F, Li W, et al. : Riskoftreatment-related mortality in cancer patients treated with ipi- limumab: A systematic review and meta-analysis. Eur J Cancer 83:71–79, 2017 [DOI] [PubMed] [Google Scholar]
- 145.Schadendorf D, Wolchok JD, Hodi FS, et al. : Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J Clin Oncol 35:3807–3814, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carlino MS, Sandhu S: Safety and efficacy implications of discontinuing combination ipilimumab and nivolumab in advanced melanoma. J Clin Oncol 35:3792–3793, 2017 [DOI] [PubMed] [Google Scholar]
- 147.Fay AP, Moreira RB, Nunes Filho PRS, et al. : The management of immune-related adverse events associated with immune checkpoint blockade. Expert Rev Qual Life Cancer Care 1:89–97, 2016 [Google Scholar]
- 148.Weber JS, Hodi FS, Wolchok JD, et al. : Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 35:785–792, 2017 [DOI] [PubMed] [Google Scholar]
- 149.Bayer V, Amaya B, Baniewicz D, et al. : Cancer immunotherapy: An evidence-based overview and implications for practice. Clin J Oncol Nurs 21:13–21, 2017 [DOI] [PubMed] [Google Scholar]
- 150.Rubin KM: Understanding immune checkpoint inhibitors for effective patient care. Clin J Oncol Nurs 19:709–717, 2015 [DOI] [PubMed] [Google Scholar]
- 151.Vazquez A: Hypophysitis: Nursing manage-mentofimmune-related adverse events. Clin J Oncol Nurs 21:154–156, 2017 [DOI] [PubMed] [Google Scholar]
- 152.Gilligan T, Coyle N, Frankel RM, et al. : Patient-clinician communication: American Society of Clinical Oncology consensus guideline. J Clin Oncol 35: 3618–3632, 2017 [DOI] [PubMed] [Google Scholar]
- 153.Jones K, Siegel B, Mead H, et al. : Racial and Ethnic Disparities in U.S. Health Care: A Chartbook. New York, NY, Commonwealth Fund, 2008 [Google Scholar]
- 154.National Cancer Institute: SEER Cancer Statistics Review, 1975–2013 http://seer.cancer.gov/csr/1975_2013/
- 155.American Cancer Society: Cancer Facts & Figures for African Americans 2016–2018. Atlanta, GA, American Cancer Society, 2016 [Google Scholar]
- 156.US Cancer Statistics Working Group: United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-based Report, 2017. http://www.cdc.gov/uscs
- 157.Ramalingam SS: Lung cancer: Disparities and implications for immunotherapy. 8th AACR Conference on The Science of Health Disparities in Racial/Ethnic Minorities and the Medically Underserved, Atlanta, GA, November 13–16, 2015 [Google Scholar]
- 158.Cogdill AP, Andrews MC, Wargo JA: Hallmarks of response to immune checkpoint blockade. Br J Cancer 117:1–7, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.O’Connor J, Seidl-Rathkopf K, Torres AZ, et al. : Racial disparities in the use of programmed death-1 checkpoint inhibitors. J Clin Oncol 35:3068, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: Summary of findings from completed trials in advanced melanoma. J Clin Oncol 29(15: supp 1), 2011 [Google Scholar]
- 161.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 387:1909–1920, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nanda R, Chow LQM, Dees EC, et al. Pem- brolizumab in patients with advanced triple-negative breast cancer: Phase 1b KEYNOTE-012 study. J Clin Oncol 34:2460–2467, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Naidoo J, Page DB, Li BT et al. : Toxicities of the anti–PD-1 and anti–PD-L1 immune checkpoint antibodies. Ann Oncol 26:2375–91, 2015. Erratum in: Ann Oncol 27: 1362, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Go RS, Winters JL, Kay NE: How I treat auto- immunehemolyticanemia. Blood, 129:2971–2979,2017 [DOI] [PubMed] [Google Scholar]
- 165.United States prescribing information for avelumab available online at https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5cd725a1-2fa4-408a-a651−57a7b84b2118 (Accessed on Feb 7,2018).
- 166.United States prescribing information for ate- zolizumab available online at https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6fa682c9-a312-4932-9831-f286908660ee (Accessed on Feb 7, 2018).</References.>
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.