Abstract
Background
Pseudomonas aeruginosa is the commonest micro‐organism associated with respiratory infections in cystic fibrosis. Retrospective studies have suggested that using an aggressive policy of intravenous anti‐pseudomonal antibiotics at regular intervals, irrespective of symptoms, increases survival.
Objectives
To determine whether there is evidence that an elective (regular) versus symptomatic intravenous antibiotic regimen is associated with an improvement in clinical status and survival rates in people with cystic fibrosis. To identify any adverse effects associated with the use of elective intravenous antibiotics, including an increase in the development of resistant organisms.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register which comprises references identified from comprehensive electronic database searches, handsearches of relevant journals and abstract books of conference proceedings.
Date of the most recent search of the Group's Cystic Fibrosis Trials Register: 15 March 2012.
Selection criteria
All randomised or quasi‐randomised controlled trials describing the use of elective compared with symptomatic intravenous antibiotic policies for any duration or dose regimen. Elective versus symptomatic intravenous antibiotic regimens against any organisms were considered. People with cystic fibrosis of any age or disease severity were included.
Data collection and analysis
Both authors independently assessed trial eligibility and quality; both extracted the data.
Main results
Searches identified four studies. Two studies reporting results from a total of 79 participants were included in the review. Differences in study design and objectives meant that data could not be pooled for meta‐analysis. Neither study demonstrated significant differences in outcome measures between intervention and comparison groups.
Authors' conclusions
Studies are insufficient to identify conclusive evidence favouring a policy of elective intravenous antibiotic administration, despite its widespread use, neither are the potential risks adequately evaluated. The results should be viewed with caution, as participant numbers are small. Clearly there is a need for a well‐designed, adequately‐powered, multicentred randomised controlled trial to evaluate these issues.
This review will no longer be regularly updated. Searches will still be undertaken on a two‐yearly basis by the Cochrane Cystic Fibrosis & Genetic Disorders Group. If, in future, relevant trials are identified, the review will be updated again.
Plain language summary
Elective (regular) versus symptomatic intravenous antibiotic therapy for cystic fibrosis
Chronic infection of the airways by Pseudomonas aeruginosa in people with cystic fibrosis is associated with deterioration in respiratory function. Intravenous antibiotics are the standard therapy for pulmonary exacerbations caused by this micro‐organism. Many centres advocate the use of elective (regular) three‐monthly antibiotics to reduce the frequency of exacerbations and therefore slow the deterioration of lung function. Alternatively, intravenous antibiotics are only prescribed when symptoms indicate. Elective therapy may encourage multi‐resistance to antibiotics. This review aimed to identify randomised and quasi‐randomised controlled trials that evaluated the results of the two different approaches. No clear conclusions were identified. This review will no longer be regularly updated. Searches will still be undertaken on a two‐yearly basis by the Cochrane Cystic Fibrosis & Genetic Disorders Group. If, in future, relevant trials are identified, the review will be updated again.
Background
Cystic fibrosis (CF) is an inherited disease that is commonest in Caucasian populations. It is inherited by the autosomal recessive mode. Although prognosis continues to improve, it remains an important life‐limiting disorder. The commonest cause of death is respiratory failure resulting from recurrent and chronic respiratory infection and inflammation.
Pseudomonas aeruginosa (P. aeruginosa) is the commonest micro‐organism associated with respiratory infections in CF. The colonisation rate with P. aeruginosa varies considerably between clinics, depending on their infection prevention routine and early treatment policies. The onset of chronic P. aeruginosa infection may be substantially delayed by cohort isolation and early intensive treatment with inhaled colistin and oral ciprofloxacin from initial isolation of P. aeruginosa (Valerius 1991). The progression of lung damage in people with CF with recurrent or chronic P. aeruginosa infection is highly variable, but average life expectancy is reduced compared with those who remain uninfected (Fitzsimmons 1993).
The aim of treatment of chronic P. aeruginosa infection is to maintain good lung function, improve quality of life and to reduce mortality. There is no international consensus on the management of chronic P. aeruginosa infection in people with CF. Two main strategies are widely practised. The elective regimen is to administer regular courses of intravenous (IV) antibiotics ‐ usually three‐monthly, irrespective of clinical state (Pedersen 1987; Szaff 1983). The alternative regimen is prompt treatment of acute exacerbations (Bauernfeind 1996), as determined by clinical or radiological findings or deterioration in lung function parameters (symptomatic regimen). Intravenous antibiotics are usually given for about 14 days, but the duration may be modified depending on clinical response. Choice of antibiotics is generally determined by sputum sensitivies and centre policies. The use of single versus combination IV antibiotic therapy remains controversial and is the subject of another Cochrane Review (Elphick 2005). Many centres also use long‐term nebulised antibiotics in people with CF who are chronically infected with P. aeruginosa, which may influence the outcome.
The median survival rate of people with CF doubled between 1969 and 1990, from 14 to 28 years (FitzSimmons 1996) and is still improving (Dodge 1997). Contributory factors to this encouraging development may be cohort isolation and segregation policies, vigorous attention to nutritional status and new therapeutic agents. Recent reports suggest that the most marked increase in survival probability coincided with introducing a policy of elective IV antibiotics every three months (Friederiksen 1996). However, the multiple use of potent antibiotics may increase the chances of adverse effects and lead to an increased emergence of resistant organisms (Levy 1998).
Description of the condition
Cystic fibrosis (CF) is an inherited disease that is commonest in Caucasian populations. It is inherited by the autosomal recessive mode. Although prognosis continues to improve, it remains an important life‐limiting disorder. The commonest cause of death is respiratory failure resulting from recurrent and chronic respiratory infection and inflammation.
Pseudomonas aeruginosa (P. aeruginosa) is the commonest micro‐organism associated with respiratory infections in CF. The colonisation rate with P. aeruginosa varies considerably between clinics, depending on their infection prevention routine and early treatment policies. The onset of chronic P. aeruginosa infection may be substantially delayed by cohort isolation and early intensive treatment with inhaled colistin and oral ciprofloxacin from initial isolation of P. aeruginosa (Valerius 1991). The progression of lung damage in people with CF with recurrent or chronic P. aeruginosa infection is highly variable, but average life expectancy is reduced compared with those who remain uninfected (Fitzsimmons 1993).
Description of the intervention
The aim of treatment of chronic P. aeruginosa infection is to maintain good lung function, improve quality of life and to reduce mortality. There is no international consensus on the management of chronic P. aeruginosa infection in people with CF. Two main strategies are widely practised. The elective regimen is to administer regular courses of intravenous (IV) antibiotics ‐ usually three‐monthly, irrespective of clinical state (Pedersen 1987; Szaff 1983). The alternative regimen is prompt treatment of acute exacerbations (Bauernfeind 1996), as determined by clinical or radiological findings or deterioration in lung function parameters (symptomatic regimen). Intravenous antibiotics are usually given for about 14 days, but the duration may be modified depending on clinical response. Choice of antibiotics is generally determined by sputum sensitivies and centre policies. The use of single versus combination IV antibiotic therapy remains controversial and is the subject of another Cochrane Review (Elphick 2005). Many centres also use long‐term nebulised antibiotics in people with CF who are chronically infected with P. aeruginosa, which may influence the outcome.
Why it is important to do this review
The median survival rate of people with CF doubled between 1969 and 1990, from 14 to 28 years (FitzSimmons 1996) and is still improving (Dodge 1997). Contributory factors to this encouraging development may be cohort isolation and segregation policies, vigorous attention to nutritional status and new therapeutic agents. Recent reports suggest that the most marked increase in survival probability coincided with introducing a policy of elective IV antibiotics every three months (Friederiksen 1996). However, the multiple use of potent antibiotics may increase the chances of adverse effects and lead to an increased emergence of resistant organisms (Levy 1998).
Objectives
To determine whether the use of elective (regular) IV antibiotics compared with symptomatic IV antibiotics is associated with an improvement in clinical status and survival rates in people with CF.
To identify any adverse effects associated with the use of elective IV antibiotics.
To identify whether the use of elective IV antibiotics leads to an increase in the development of resistant organisms.
Methods
Criteria for considering studies for this review
Types of studies
Randomised (RCTs) or quasi‐randomised controlled trials, published or unpublished.
Types of participants
Children and adults with CF, diagnosed clinically and by sweat or genetic testing. People with CF with all stages of lung disease have been included.
Types of interventions
Elective IV antibiotic course compared with symptomatic IV course.
Types of outcome measures
Primary outcomes
Changes in lung function (forced expiratory volume at one second (FEV1) and forced vital capacity (FVC)) from baseline
Number of acute exacerbations
Changes in Shwachman or Chrispin‐Norman scores
Number of deaths
Number of adverse effects e.g. renal or auditory impairment or sensitivity reactions
Development of resistant organisms and changes in bacteriological status
Secondary outcomes
Changes from baseline in quality of life
Changes in nutritional status
Drop out rate and reason
Cost (including indirect cost) of therapy
Search methods for identification of studies
Electronic searches
Relevant studies were identified from the Group's Cystic Fibrosis Trials Register using the terms: (antibiotics OR macrolide) AND (intravenous or not stated) AND (continuous OR intermittent OR regular OR prophylaxis OR elective OR symptomatic [freetext]).
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), quarterly searches of MEDLINE, a search of EMBASE to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the Cystic Fibrosis Trials Register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group Module.
Date of the most recent search of the Group's Cystic Fibrosis Trials Register: 15 March 2012.
Data collection and analysis
Selection of studies
Both authors assessed study eligibility. They applied inclusion criteria to all potential studies. Authors resolved any disagreements as to which studies to include by negotiation.
Data extraction and management
Each author independently extracted data using standard data acquisition forms. They aimed to group outcome data into those measured at 12 months and annually thereafter. If outcome data were recorded at other time periods, the authors considered examining these as well.
Assessment of risk of bias in included studies
Each author assessed the methodological quality of each study using the method as described by Schulz (Schulz 1995). In particular, they examined the generation of allocation sequence, the concealment of treatment allocation schedule, whether the study was blinded, whether intention‐to‐treat analysis was used or was possible from the available data and if the number of participants lost to follow‐up or subsequently excluded from the study was recorded.
Measures of treatment effect
For binary outcome measures, in order to allow an intention‐to‐treat analysis, the authors collected data on the number of participants with each outcome event, by allocated treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up. They planned to calculate a pooled estimate of the treatment effect for each outcome across studies, (using the pooled relative risk as a treatment effect estimate), however, to date, only data from one trial are available for analysis. For continuous outcomes, the authors recorded either mean change from baseline in each group or mean post‐treatment or intervention values and standard deviation or standard error for each group. They planned to calculate a pooled estimate of treatment effect by calculating the weighted mean difference, however, to date, only data from one trial are available for analysis.
Assessment of heterogeneity
If the authors include data from further studies in future updates of this review, they propose to measure the degree of heterogeneity between studies using the I2 statistic (Higgins 2003).
Results
Description of studies
Results of the search
Four studies were identified from the searches (Brett 1992; De Boeck 1999; Elborn 2000; Nikolaizik 2005).
Included studies
Two studies met the inclusion criteria including a total of 79 participants (Brett 1992; Elborn 2000). Both studies were randomised using the minimisation method (Pocock 1975), and full papers of both were published. Minimisation is a method of allocation used to provide comparison groups that are closely similar for several variables. This method is commonly used in smaller studies, or those where small numbers of participants are recruited from several centres.
The two included studies had different objectives. One was a direct comparison of elective versus symptomatic IV antibiotics (Elborn 2000), while the other was designed to look at the value of serum IgG titres against P. aeruginosa and involved randomising participants to elective four‐monthly antibiotics, or observing participants and treating symptomatically (Brett 1992). Despite differences in methodology, both authors agreed that the two studies should be included.
The Brett study included 19 participants over a one‐year period (Brett 1992). Seven of these participants were randomised to receive elective (four‐monthly) antibiotics and the remaining 12 participants to receive symptomatic antibiotics. The Elborn study recruited 60 participants (Elborn 2000). Thirty‐two of these were randomised to receive elective (three‐monthly) antibiotics and 28 to receive symptomatic antibiotics. The duration of the study was three years, with further data published in abstract form five years after randomisation. There were no interval data reported before the end of either study.
Elborn enrolled participants aged eight years or older, with a mean age of 18 years in both treatment groups, 42% of his cohort were under the age of 16 years (Elborn 2000). The participants in the Brett study were aged between 6 and 29 years, with a mean of 14 years in one treatment group and 13 years in the other group (Brett 1992).
Severity of lung disease varied between the studies. The participants in the Brett study had early signs of chronic respiratory infection (Brett 1992). They had a moderate increase in serum IgG titres against P. aeruginosa plus isolation of the organism from respiratory cultures. Participants were excluded if they had extremely high IgG titres and if they were already on corticosteroids or nebulised anti‐pseudomonal antibiotics. Previous studies would suggest that these criteria exclude those with chronic and irreversible infection (Brett 1986; Brett 1987; Brett 1988). The mean baseline FEV1 in the participants in the Brett study was 75% in the symptomatic group and 67% in the elective group. The participants in the Elborn study had lower baseline FEV1 measurements with a mean of 63% in the symptomatic group and 59% in the elective group. Elborn selected people with CF who were chronically infected with P. aeruginosa (i.e. 3 or more isolations in 12 months) (Elborn 2000). He excluded those with a history of hypersensitivity to anti‐pseudomonal agents, those who were already on a regular treatment regimen of intermittent IV antibiotics, and those who had had less than two or more than four exacerbations during the previous year requiring IV antibiotics (Elborn 2000). Exclusion of those who had very frequent and also infrequent IV antibiotics meant that the difference in the number of courses between the two groups was very small. Participants on nebulised antibiotics were not excluded, but on entry both groups were receiving similar concomitant treatments such as nebulised antibiotics, oral anti‐staphylococcal antibiotics and regular inhaled bronchodilators (Elborn 2000).
The frequency of use of elective antibiotic administration varied. Elborn used an elective three‐monthly regimen of intravenous therapy for 10 to 14 days. This resulted in four courses per annum versus three courses per year in the symptomatic group (Elborn 2000). Brett electively treated participants on a four‐monthly regimen (each course being of 14 days duration) until serum IgG levels had dropped back down into the normal range. This resulted in an average of 2.8 courses per year in the elective group versus 1.09 in the symptomatic group (Brett 1992). In both studies the elective groups received rescue intravenous antibiotic therapy if symptoms indicated (Brett 1992; Elborn 2000).
Drug dosages were not reported. Data were not provided on the proportion of antibiotic courses administered in hospital. This would have a bearing on clinical improvement because of other inpatient services, such as physiotherapy and dietetic input. In both studies, the choice of anti‐pseudomonal antibiotic was based on sputum cultures and sensitivities.
Excluded studies
The remaining two studies were excluded as one was a comparison of an IV preparation with an inhaled antibiotic and the an elective regimen was not compared with a symptomatic regimen (Nikolaizik 2005); the second trial was randomised on antibiotic regimen, with the elective and symptomatic regimens analysed as a subgroup only (De Boeck 1999).
Risk of bias in included studies
Methodological quality was assessed based on a method described by Schulz (Schulz 1995). Allocation concealment and generation of the randomisation sequence were categorized as adequate, unclear or inadequate. Intention‐to‐treat analysis was categorized as adequate, unclear or inadequate. RCTs were categorized according to whether double blinding had been reported or not.
Both of the included studies were RCTs, concealment of allocation and generation of the randomisation sequence was unclear in both studies (Brett 1992; Elborn 2000). Intention‐to‐treat analysis was unclear in the Brett study (Brett 1992), but in the study by Elborn it was stated that the analysis was conducted according to the principle of intention‐to‐treat and was therefore classed as adequate (Elborn 2000). Double blinding was not applicable due to the nature of the included studies.
Effects of interventions
Primary outcomes
1. Changes in lung function from baseline
Lung function was reported at one year in the Brett study (Brett 1992) and at three years in the Elborn study (Elborn 2000). In the Brett study, FEV1 remained constant in the symptomatic group and improved by 11% in the elective group, these differences were not statistically significant. FVC was not reported in the Brett study (Brett 1992). There was no significant change in FEV1, mean difference (MD) 2.40 (95% confidence interval (CI) ‐6.23 to 11.03) or FVC, MD 1.10 (95% CI ‐8.22 to 10.42) from baseline (Elborn 2000) (Analysis 1.1; Analysis 1.2).
1.1. Analysis.
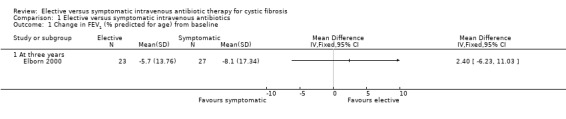
Comparison 1 Elective versus symptomatic intravenous antibiotics, Outcome 1 Change in FEV1 (% predicted for age) from baseline.
1.2. Analysis.
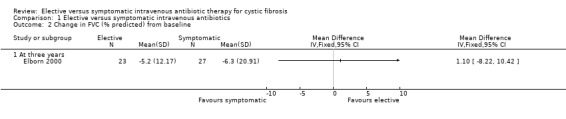
Comparison 1 Elective versus symptomatic intravenous antibiotics, Outcome 2 Change in FVC (% predicted) from baseline.
2. Number of acute exacerbations
In the Elborn study, 40% of IV antibiotic courses in the elective group were for symptomatic exacerbations (Elborn 2000). The number of acute exacerbations in the elective group was not reported in the Brett study (Brett 1992).
3. Shwachman / Chrispin‐Norman scores
There was no significant difference in the change in Shwachman score between the two groups in the Elborn study, MD 1.80 (95% CI ‐4.12 to 7.72) (Elborn 2000) (Analysis 1.3). Neither was there a significant difference in the Chrispin‐Norman score between the two groups, MD 0.20 (95% CI ‐2.13 to 2.53) (Elborn 2000) (Analysis 1.4). Brett only commented on these parameters on entry to the study (Brett 1992).
1.3. Analysis.
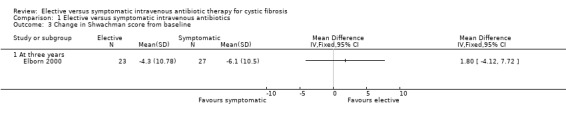
Comparison 1 Elective versus symptomatic intravenous antibiotics, Outcome 3 Change in Shwachman score from baseline.
1.4. Analysis.
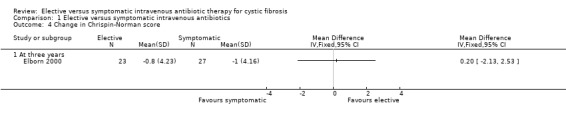
Comparison 1 Elective versus symptomatic intravenous antibiotics, Outcome 4 Change in Chrispin‐Norman score.
4. Number of deaths
In the Elborn study there were 4 deaths out of 32 participants in the elective group compared to none out of 28 participants in the symptomatic group at three years (Elborn 2000). Thus, there was no significant difference between the two groups, RR 7.91 (95%CI 0.44 to 140.73) (Analysis 1.5). Participants recruited to the study were followed up for five years and it was reported that four further deaths occurred in the elective group and one in the symptomatic group, P < 0.04 (Fisher's exact test) (Elborn 2000). All the deaths were due to cardiopulmonary failure secondary to overwhelming lung infection (Elborn 2000). Brett did not report any deaths in either group (Brett 1992).
1.5. Analysis.
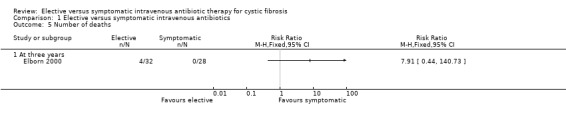
Comparison 1 Elective versus symptomatic intravenous antibiotics, Outcome 5 Number of deaths.
5. Adverse effects
None were reported in either study (Brett 1992; Elborn 2000).
6. Development of resistant organisms & changes in bacteriological status
In the Brett study, proportions of sputum isolates containing mucoid P. aeruginosa increased from 14% to 39% in the symptomatic group (P < 0.1) but remained essentially unchanged in the elective group. Resistance in particular was not commented upon (Brett 1992). Elborn did not find any significant difference in the incidence of new cases of resistant bacteria between the groups during the study period, or new antimicrobial species such as Burkolderia cepacia and Stenotrophomonas maltophilia (Elborn 2000). Neither study commented on the occurrence of fungal infection (Brett 1992; Elborn 2000).
Secondary outcomes
1. Changes from baseline in quality of life
Formal quality of life analysis was not performed in either study, but certain issues were addressed by both (Brett 1992; Elborn 2000). For the Brett study, due to the nature of the study design, regular venepuncture caused distress among some participants (Brett 1992). Both Elborn and Brett commented on the disruption to family life due to regular courses of intravenous antibiotics (Brett 1992; Elborn 2000) .
2. Changes in nutritional status
Brett did not comment on this outcome (Brett 1992). Elborn recorded data on height z scores, MD ‐0.16 (95% CI ‐0.46 to 0.14) (Analysis 1.6), or weight z scores, MD ‐0.09 (95% CI ‐0.36 to 0.18) (Elborn 2000) (Analysis 1.7). There was no significant difference in these parameters between the two groups. Neither was there a significant difference in the change in weight or height (% predicted) from baseline, MD 0.80 (95% CI ‐3.59 to 5.19) (Elborn 2000) (Analysis 1.8).
1.6. Analysis.
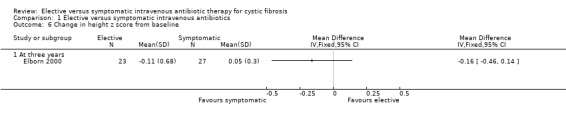
Comparison 1 Elective versus symptomatic intravenous antibiotics, Outcome 6 Change in height z score from baseline.
1.7. Analysis.
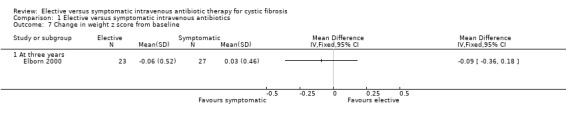
Comparison 1 Elective versus symptomatic intravenous antibiotics, Outcome 7 Change in weight z score from baseline.
1.8. Analysis.
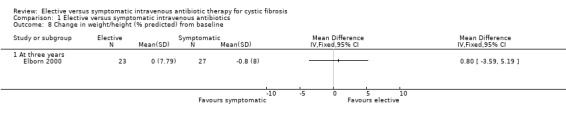
Comparison 1 Elective versus symptomatic intravenous antibiotics, Outcome 8 Change in weight/height (% predicted) from baseline.
3. Dropout rate and reason
Brett did not have any dropouts during the one‐year study period (Brett 1992). At three years, Elborn did not find any significant difference in dropout rate, RR 4.38 (95%CI 0.54 to 35.24) (Analysis 1.9). There were 5 out of 32 participant withdrawals in the elective group and only 1 out of 28 in the symptomatic group (Elborn 2000). In the elective group, three of the withdrawals were due to inconvenience of regular antibiotic treatments; one was due to the participant undergoing heart‐lung transplantation; and one following pregnancy. The reason for the withdrawal in the symptomatic group was not stated, but was at the participant's own request.
1.9. Analysis.
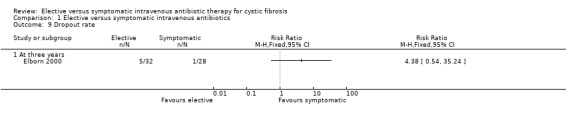
Comparison 1 Elective versus symptomatic intravenous antibiotics, Outcome 9 Dropout rate.
4. Cost
Brett did not report on this outcome (Brett 1992). Elborn highlighted the significant cost implication of a policy of elective antibiotic therapy, but no actual economic analysis was undertaken (Elborn 2000).
Discussion
The life expectancy of people with CF has improved enormously over the last 30 years. This systematic review aimed to establish whether there is evidence that the use of an elective (regular) regimen of IV antibiotics compared with symptomatic IV antibiotics is associated with decreased morbidity and improved survival in people with CF. It is therefore disappointing that only two studies met the inclusion criteria for this review, and that the total number of participants involved was small. Due to this, any results reported should be interpreted with caution. As a result of the difference in design of the two studies, it was not possible to perform a meta‐analysis of the data. The studies used different outcome measures and were of different durations. The selection criteria for the two studies meant that the participants in the Brett study would not have been selected for the Elborn study and vice versa. The disease severity of the cohorts was different resulting in data that were incompatible for pooling.
Morbidity due to P. aeruginosa is important in CF and leads to progressive deterioration in lung function. People with CF who are chronically colonised often require repeated courses of antibiotics for the management of pulmonary exacerbations. In the Elborn study, there was little difference in the total number of IV courses between the elective and symptomatic groups, therefore any real difference attributable to the elective group would need to be quite large before it would be noticeable (Elborn 2000). This aspect should be considered in the design of any future studies. Whilst there is anecdotal evidence of improvement in lung function with an elective three‐monthly regimen of antibiotics as opposed to symptomatic IVs, we have found no substantial evidence to support this from RCTs.
Adverse events have not been evaluated in any great detail. Neither study reported in their methods which adverse effects were sought. These factors need to be addressed in order to make informed decisions about policy changes. In the Elborn study, there were a significantly higher proportion of deaths in the electively treated group at five years. However, this did not appear to be caused by the emergence of virulent strains of bacteria due to induced antibiotic resistance (Elborn 2000). It is disturbing that there is no obvious explanation for this apparent excess of deaths and if this is a feature of further studies explanations need to be sought.
With the advent of nebulised and oral anti‐pseudomonal agents, it is even more unclear what place elective IV antibiotics have in management protocols. Studies with clear outcome measures are needed to properly evaluate this widely used treatment policy for chronic colonisation with P. aeruginosa in CF. The main question would be whether regular IV antibiotics keep the lungs of people with CF healthier for longer than symptomatic IV antibiotics, and if so, at what cost.
Authors' conclusions
Implications for practice.
We have found no conclusive evidence to show that an elective regimen of intravenous antibiotics is more effective than a symptomatic regimen in reducing deterioration in respiratory function in CF. Neither is there statistically significant evidence to suggest that an elective regimen encourages the formation of antibiotic resistant micro‐organisms and hence increased mortality. These results need to be interpreted with caution, as the total number of participants in these studies was small. This review will no longer be regularly updated. Searches will still be undertaken on a two‐yearly basis by the Cochrane Cystic Fibrosis & Genetic Disorders Group. If, in future, relevant trials are identified, the review will be updated again.
Implications for research.
There have been insufficient RCTs of elective versus symptomatic administration of intravenous antibiotics in people with CF to answer important questions about long‐term outcomes. Studies should be carried out to evaluate the effectiveness of routine elective intravenous antibiotics on long‐term lung function, adverse effects and mortality in people with CF. The most widely used elective regimen is that of three‐monthly administration and this should be compared with standard symptomatic intravenous treatment in a multicentre, adequately‐powered and well‐designed RCT to address these important issues.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2017 | Amended | Contact details updated. |
| 11 July 2012 | Review declared as stable | This review will no longer be regularly updated. Searches will still be undertaken on a two‐yearly basis by the Cochrane Cystic Fibrosis & Genetic Disorders Group. If, in future, relevant trials are identified, the review will be updated again. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 13 April 2015 | Amended | Contact details updated. |
| 18 May 2012 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified 11 references to three studies all of which were ineligible for inclusion in the review. |
| 18 May 2012 | New citation required but conclusions have not changed | No new trials have been included in this review, minor changes to the text have been made throughout. |
| 16 June 2010 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified 11 references. One was an additional reference (full paper) to an already excluded trial (Nikolaizik 2005) and the remaining 10 were not eligible for inclusion within the review. |
| 17 July 2008 | New search has been performed | The search of the Group's Cystic Fibrosis Trials Register identified no new references for inclusion in the review. The trial previously listed in 'Studies awaiting assessment' has now been moved to the 'Excluded studies' section of the review (De Boeck 1999). |
| 17 July 2008 | Amended | Converted to new review format. |
| 1 November 2006 | New search has been performed | The search of the Group's Cystic Fibrosis Trials Register identified two new references to a single study, but this study was not eligible for inclusion in the review. |
| 2 May 2005 | New search has been performed | The search of the Group's Cystic Fibrosis Trials Register identified no new studies to be included in the review. |
| 3 May 2004 | New search has been performed | The search identified no new studies to be included in the review. |
| 1 January 2003 | New search has been performed | Additional data supplied by primary authors ‐ S. Elborn and R. Prescott (Elborn 2000) were included within the review. The 'Results' and 'Conclusions' sections of the review were not affected by this new data. |
| 24 August 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors thank Dr R. Prescott and Dr S. Elborn for kindly providing additional data (Elborn 2000). We also thank Professor De Boeck for providing information on a trial now listed in 'Excluded studies' (De Boeck 1999).
Data and analyses
Comparison 1. Elective versus symptomatic intravenous antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 (% predicted for age) from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At three years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in FVC (% predicted) from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At three years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in Shwachman score from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At three years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in Chrispin‐Norman score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At three years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of deaths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 At three years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in height z score from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At three years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change in weight z score from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 At three years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in weight/height (% predicted) from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At three years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Dropout rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 At three years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brett 1992.
| Methods | 1‐year trial, parallel design with 2 treatment arms. Allocation of participants by minimisation after stratification for age, sex, Shwachman and Chrispin‐Norman scores. | |
| Participants | 19 participants with a modest increase in serum IgG titres against P. aeruginosa plus P. aeruginosa isolated from respiratory cultures. Participants were excluded if they had very high titres, corticosteroid treatment or nebulised anti‐pseudomonal antibiotics. | |
| Interventions | On entry to trial, combination 2‐week course of IV antibiotic therapy repeated every 4 months until IgG titres returned to control range, versus standard treatment. | |
| Outcomes | On entry to trial and after 1 year, parameters measured were: Serum IgG titre; FEV1 (% predicted); white cell count; % neutrophils; serum IgG; % of cultures positive for mucoid and non‐mucoid P. aeruginosa; and number of courses of anti‐pseudomonal treatment per participant. | |
| Notes | No dropouts recorded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was done by minimisation after stratification for age (0‐10 years, 11‐20 years, >20 years), sex (male, female), Shwachman score (<70, 70‐85, 86‐100) and Chrispin‐Norman score (0‐10, 11‐20, >20). |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible given the intervention. |
Elborn 2000.
| Methods | Multicentred 3‐year trial, parallel design with two treatment arms. Allocation of treatment by the method of minimisation using age, severity based on chest radiographic score and treatment centre. | |
| Participants | 60 participants with CF, aged 8 years or over and with P. aeruginosa isolated on 3 or more occasions in the last year. Participants excluded if there was a positive past history of hypersensitivity reactions to anti‐pseudomonal agents, if they were on a regular treatment regimen of IV antibiotics, or if they had less than 2 or more than 4 exacerbations during the previous year requiring IV antibiotics. | |
| Interventions | Elective IV antibiotics every 3 months versus IV antibiotics only when symptoms indicated. | |
| Outcomes | On entry to trial and after 3 years, parameters measured were: FEV1 (% predicted); FVC (% predicted); Shwachman score; Chrispin‐Norman score; height and weight SD scores; weight/height (% predicted); and number of deaths. | |
| Notes | 5 dropouts recorded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation of treatment was by the method of minimisation using age, severity based on chest radiographic score, and treatment centre. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible given the intervention. |
CF: cystic fibrosis FEV1: forced expiratory volume at one second FVC: forced vital capacity IV: intravenous P. aeruginosa: Pseudomonas aeruginosa SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| De Boeck 1999 | Randomised on antibiotic regimen, elective and symptomatic regimens were analysed as a subgroup only. |
| Nikolaizik 2005 | Comparison of IV preparation with inhaled antibiotic, regimen not elective versus symptomatic. |
Contributions of authors
Dr Breen conceived the review and drafted the protocol. Dr Breen and Dr Aswani independently assessed trial eligibility and extracted data. Both authors drafted the original review. Both authors completed the updates of the review. Dr Breen acts as guarantor of the review.
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Brett 1992 {published data only}
- Brett MM, Simmonds EJ, Ghoneim ATM, Littlewood JM. The value of serum IgG titres against Pseudomonas aeruginosa in the management of early pseudomonal infection in cystic fibrosis. Archives of Disease in Childhood 1992;67(9):1086‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elborn 2000 {published data only}
- Elborn JS, Prescott R, Stack B, Shale DJ, Pantin C, Ali N, et al. A comparison of the clinical outcome of symptomatic and elective regular intravenous antibiotics in the treatment of cystic fibrosis [abstract]. Thorax 1997;52(Suppl 6):A4. [Google Scholar]
- Elborn JS, Prescott RJ, Stack BH, Goodchild MC, Bates J, Pantin C, et al. Elective versus symptomatic antibiotic treatment in cystic fibrosis patients with chronic Pseudomonas infection of the lungs [abstract]. Pediatric Pulmonology 2000;30(5):434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborn JS, Prescott RJ, Stack BHR. The British Thoracic Society study of elective versus symptomatic treatment of Pseudomonas aeruginosa infection in patients with cystic fibrosis: 5 year results [abstract]. Proceedings of the 13th International Cystic Fibrosis Congress 2000 June 4‐8; Stockholm. 2000:172.
- Elborn JS, Prescott RJ, Stack BHR, Goodchild MC, Bates J, Pantin C, et al. Elective versus symptomatic antibiotic treatment in cystic fibrosis patients with chronic Pseudomonas infection of the lungs. Thorax 2000;55(5):355‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
De Boeck 1999 {published and unpublished data}
- Boeck K, Sauer K, Vandeputte S. Meropenem versus ceftazidime plus tobramycin for pulmonary disease in CF patients [abstract]. Netherlands Journal of Medicine 1999;54(Suppl):S39. [Google Scholar]
Nikolaizik 2005 {published data only}
- Nikolaizik WH, Vietzke D, Ratjen F. A pilot study to compare tobramycin 80 mg injectable preparation with 300 mg solution for inhalation in cystic fibrosis patients. Canadian Respiratory Journal 2008; Vol. 15, issue 5:259‐62. [DOI] [PMC free article] [PubMed]
- Nikolaizik WH, Vietzke D, Ratjen F. Comparison of tobramycin 80 mg (IV‐Preparation) and 300 mg solution for inhalation in cystic fibrosis patients [abstract]. European Respiratory Journal 2005;26(Suppl 49):620s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaizik WH, Vietzke D, Ratjen F. Comparison of tobramycin 80mg (IV‐preparation) and 300mg solution inhaled twice daily for chronic P. aeruginosa infection [abstract]. Journal of Cystic Fibrosis 2005;4 Suppl:S53. [Google Scholar]
Additional references
Bauernfeind 1996
- Bauernfeind A, Marks MI, Strandvik B. Cystic Fibrosis Pulmonary Infections; Lessons from around the World. Basel: Verlag, 1996. [Google Scholar]
Brett 1986
- Brett MM, Ghonheim ATM, Littlewood JM. Serum antibodies to Pseudomonas aeruginosa in cystic fibrosis. Archives of Disease in Childhood 1986;61(11):1114‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brett 1987
- Brett MM, Ghonheim ATM, Littlewood JM. Serum IgG antibodies in patients with cystic fibrosis with early Pseudomonas aeruginosa infection. Archives of Disease in Childhood 1987;62(4):357‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brett 1988
- Brett MM, Ghonheim ATM, Littlewood JM. Prediction and diagnosis of early Pseudomonas aeruginosa infection in cystic fibrosis in a follow‐up study. Journal of Clinical Microbiology 1988;26(8):1565‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodge 1997
- Dodge JA, Morison S, Lewis PA, Coles EC, Geddes D, Russell, et al. Incidence, population, and survival of cystic fibrosis in the UK, 1968‐95. Archives of Disease in Childhood 1997;77(6):493‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elphick 2005
- Elphick HE, Tan A. Single versus combination intravenous antibiotic therapy for people with cystic fibrosis (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002007.pub2] [DOI] [PubMed] [Google Scholar]
Fitzsimmons 1993
- Fitzsimmons SC. The changing epidemiology of cystic fibrosis. Journal of Pediatrics 1993;122(1):1‐9. [DOI] [PubMed] [Google Scholar]
FitzSimmons 1996
- FitzSimmons SC. The Cystic Fibrosis Foundation Patient Registry Report 1996. Pedriatric Pulmonology 1996;21:267‐75. [Google Scholar]
Friederiksen 1996
- Frederiksen B, Lanng S, Koch C, Høiby N. Improved survival in the Danish Center‐treated cystic fibrosis patients: Results of aggressive treatment. Pediatric Pulmonology 1996;21(3):153‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 1998
- Levy SB. Antimicrobial resistance: bacteria on the defence. BMJ 1998;317(7159):612‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 1987
- Pedersen SS, Jensen T, Hoiby N, Koch N. Management of Pseudomonas aeruginosa lung infection in Danish cystic fibrosis patients. Acta Paediatrica Scandinavica 1987;76(6):955‐61. [DOI] [PubMed] [Google Scholar]
Pocock 1975
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometric 1975;31(1):103‐15. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Szaff 1983
- Szaff N, Flensberg EW. Pulmonary Pathology. In: Hodson ME, Norman AP editor(s). Cystic Fibrosis. London: Balliere‐Tindall, 1983:31‐51. [Google Scholar]
Valerius 1991
- Valerius NH, Koch C, Høiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet 1991;338(8769):725‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Breen 2007
- Breen L, Aswani N. Elective versus symptomatic intravenous antibiotic therapy for cystic fibrosis. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD002767] [DOI] [PubMed] [Google Scholar]


