Abstract
Prostate cancer remains the second leading cause of male cancer deaths in the United States and most western countries. Prostatic acinar adenocarcinoma is the most commonly diagnosed form of prostate cancer. Small cell neuroendocrine carcinoma is less frequently identified at the time of initial diagnosis, but this highly aggressive form of prostate cancer is increasingly observed in patients who have failed first and second line hormone therapy. Thus, developing and exploring models of neuroendocrine prostate cancer (NePC) is of increasing importance. Here we review the relevant xenograft tumor and genetically engineered mouse models of NePC, with the aim of addressing salient features and clinical relevance.
Keywords: Neuroendocrine prostate cancer, castration-resistant prostate cancer, xenograft tumors, genetically engineered mice
INTRODUCTION
Prostate cancer is the second most common cancer in men worldwide (Ferlay, et al. 2010). With greater than 90% of prostate cancers initially diagnosed as acinar adenocarcinomas (Fine 2012; Humphrey 2012), neuroendocrine carcinomas of the prostate (also described as small cell neuroendocrine carcinomas) are rare at the time of initial diagnosis, with only approximately 0.5%−2% of prostate cancers identified as such (Helpap, et al. 1999; Humphrey 2012; Stein, et al. 2008; Tan, et al. 2014; Wang and Epstein 2008). Unlike acinar adenocarcinomas that, per the 2004 World Health Organization (WHO) classification, are composed of variably differentiated glandular structures, express prostate specific antigen (PSA) and the androgen receptor (AR), and are subject to Gleason scoring (Eble, et al. 2004; Humphrey 2004), neuroendocrine prostate cancers (NePCs) are characterized by sheets of highly atypical cells that do not form glandular structures and are immunopositive for markers of neuroendocrine differentiation, such as chromogranin, synaptophysin, CD56, and/or neuron specific enolase (NSE) (Beltran, et al. 2014; Epstein, et al. 2014; Fine 2012; Helpap and Kollermann 1999; Helpap et al. 1999; Humphrey 2012; Komiya, et al. 2009; Stein et al. 2008; Wang and Epstein 2008). The majority of cells in NePCs are immunonegative for PSA and AR (Beltran et al. 2014; Eble et al. 2004; Epstein et al. 2014; Fine 2012; Helpap and Kollermann 1999; Helpap et al. 1999; Humphrey 2012; Stein et al. 2008; Wang and Epstein 2008), and these tumors have often lost Rb, p53, and/or PTEN tumor suppressor activities (Beltran et al. 2014; Tan et al. 2014). Approximately 50% of NePCs exhibit ERG gene rearrangements (Beltran et al. 2014; Mosquera, et al. 2013) and overexpression or amplification of the N-Myc and Aurora kinase A genetic loci is common (Beltran 2014; Beltran, et al. 2011; Beltran et al. 2014; Mosquera et al. 2013). NePCs are also reported to have a high proliferative index, with more than 50% of tumor cells being immunohistochemically positive for Ki67 (Beltran et al. 2014; Epstein et al. 2014).
Although the diagnosis of NePC at the time of initial cancer identification is rare, these tumors are more frequently found in patients who have previously received both first and second line androgen ablation therapy (Epstein et al. 2014; Humphrey 2012; Mosquera et al. 2013). It has been suggested that androgen ablation therapy may select for cells with a neuroendocrine phenotype since these cells are predominantly castration-resistant (Beltran et al. 2014; Epstein et al. 2014; Tan et al. 2014). Given the fact that NePCs are largely negative for AR (Beltran et al. 2014), NePCs present a therapeutic challenge (Beltran et al. 2014; Fine 2012; Humphrey 2012; Stein et al. 2008). Compounding this challenge is the fact that NePCs are also often diagnosed at an advanced stage with visceral metastases present (Epstein et al. 2014; Humphrey 2012; Stein et al. 2008) and associated with shortened survival times (Epstein et al. 2014; Humphrey 2012; Marcus, et al. 2012; Stein et al. 2008). Confounding the diagnosis is the fact that patients with NePCs may have seemingly incongruently low PSA elevations (Beltran et al. 2014; Epstein et al. 2014), and NePCs can coexist with the acinar adenocarcinomas (Eble et al. 2004; Epstein et al. 2014; Fine 2012; Humphrey 2012; Stein et al. 2008; Wang and Epstein 2008). Therefore, novel therapeutics are needed for this clinically significant and challenging variant of prostate cancer.
Importantly, there is no consensus regarding the cell of origin of NePCs. Multiple lineages have been proposed. One theory is that these tumors arise from “dedifferentiation” of acinar adenocarcinoma cells, essentially resulting in an “epithelial to neuroendocrine” transition (Beltran et al. 2014; Helpap and Kollermann 1999; Helpap et al. 1999; Stein et al. 2008). It has also been suggested that NePCs arise secondary to neoplastic transformation of a multipotential epithelial cell or stem cell within the prostate (Helpap and Kollermann 1999; Helpap et al. 1999; Stein et al. 2008). Given the fact that neuroendocrine cells are a normal component of the prostatic epithelium and can be identified immunohistochemically within acinar adenocarcinomas, some speculate that NePCs are the result of transformation of prostate specific neuroendocrine cells that share a common origin with luminal and basal prostatic epithelial cells (Beltran et al. 2014; Helpap and Kollermann 1999; Helpap et al. 1999). Finally, a proposal that appears to have fallen out favor is that NePCs originate from non-prostate specific neuroendocrine cells of the diffuse neuroendocrine system (formerly called the amino precursor and decarboxylation [APUD] system) that reside within the prostate (Helpap and Kollermann 1999; Helpap et al. 1999; Pearse 1969).
Animal models of prostatic NePC are essential for understanding the biology of NePC and developing more effective therapies. Multiple mouse models of NePC exist, and herein, we will review the major xenograft and genetically engineered mouse models of NePC. We will describe the xenograft models and detail the lesions that the genetically engineered mice develop, their disease progression, and how genetic manipulation of some of these mice has led to a greater understanding of NePC.
XENOGRAFT MODELS OF NEPC
There are at least seven well-characterized xenograft models of NePC: LUCAP 49, WISH-PC2, UCRU-PR-2, WM-4A, MDA PCA 144–13, LTL352, and LTL370. A summary of the major characteristics of these seven xenograft tumor models can be found in Table 1.
Table 1:
Features of the xenograft models of NePC
| Xenograft Tumor Name | Source | Immunohistochemical Characteristics | Able to Grow in Castrated Animals? | Able to Metastasize? | Other | Selected References |
|---|---|---|---|---|---|---|
| LUCAP 49 | Omental metastasis of a NePC |
Immunopositive for:
Synaptophysin, NSE, and CD57. Focally positive for
Chromogranin Immunonegative for: AR, PSA, and PAP |
Yes | Do not metastasize | High proliferative index. Cells demonstrate loss of heterozygosity of the short arm of chromosome 8p | (True, et al. 2002) |
| WISH-PC2 | Resected NePC |
Immunopositive for:
Chromogranin, Synaptophysin, and NSE Immunonegative for: AR, PSA, PAP, PSCA, PMSA, CD19, CD20, CD22, and MDR1 |
Yes, but tumor growth rate is increased in the presence of androgens | Occasionally, with metastases identified in the liver, lung, and lymph node. Metastatic potential increased following irradiation. | Express a mutated form of p53. Tumors develop after orthotopic injection into the prostate, liver, and bone. | (Agemy, et al. 2008; Pinthus, et al. 2000) |
| UCRU-PR-2 | Biopsy of a NePC |
Immunopositive for: NSE,
Epithelial Membrane Antigen, Carcinoembryonic
Antigen Immunonegative for: AR, ER, PSA, PAP, Keratin |
Unknown, but presumed to be able to grow in castrated mice, since tumor cells do not express the AR | Do not metastasize, and tumor cells injected intravenously do not form lung tumors | Tumors secrete POMC derived hormones. Implantation site affects invasiveness | (Jelbart, et al. 1988; Jelbart, et al. 1989; van Haaften-Day, et al. 1987) |
| WM-4A | Resected prostate tumor with a mixed phenotype |
Immunopositive for:
Chromogranin Immunonegative for: PSA |
Unknown | Metastasize following irradiation | Tumors are sensitive to radiation therapy | (Agemy et al. 2008) |
| MDA PCA 144 | Resected prostate tumor with a mixed phenotype |
Immunopositive for:
Synaptophysin, Chromogranin, CD56. Focally positive for
Cytokeratin Immunonegative for: AR, PSA, PAP, AMACR |
Unknown, but presumed to be able to grow in castrated mice, since tumor cells do not express the AR | Do not metastasize | High proliferative index. Cells do not express Rb or cyclin D1. | (Aparicio, et al. 2011; Tzelepi, et al. 2012) |
| LTL352 and LTL370 | Resected urethral metastasis of a NePC (LTL352). Resected penile metastasis of a NePC (LTL370) |
Immunopositive for:
Synaptophysin and Chromogranin Immunonegative for: AR and PSA |
Yes | Reported to metastasize | Tumors express PTEN and do not express ERG or SPINK1 | (Lin et al. 2014) |
LUCAP 49
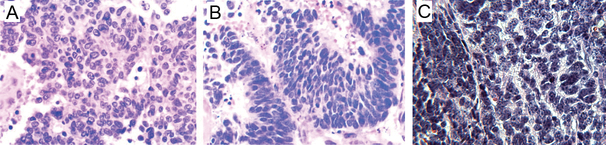
The LUCAP 49 xenograft model was first described by True et al (True, et al. 2002) and was derived from a metastasis of a prostate carcinoma that had received radiation therapy. The prostate tumor was histologically and immunohistochemically consistent with a NePC, although a small component (less than 5%) of the primary tumor was an acinar carcinoma (True et al. 2002). Subcutaneous xenograft tumors were established via serial passage of an omental metastasis in Fox Chase CB.17 Severe Combined Immune Deficiency (SCID) mice (True et al. 2002). Although the cells were unable to survive passage in vitro, at the time of publication of the initial report by True et al, tumors had been successfully serially passaged as xenografts in the CB.17 SCID mice for over four years (Clegg, et al. 2003; True et al. 2002). Histologically, the xenograft tumors have the same NePC morphology as the original prostate tumor (Figure 1 A and B). Molecular and pathological features of these tumors are consistent with clinical NePCs such as lack of PSA, prostatic acid phosphatase (PAP), and AR expression, immunopositivity for the neuroendocrine markers synaptophysin and NSE and the neural marker CD57, and demonstration of a perinuclear configuration of low molecular weight keratin immunoreactivity characteristic of neuroendocrine cells. These tumors are only focally immunopositive for chromogranin (Clegg et al. 2003; True et al. 2002) and demonstrate loss of heterozygosity of the short arm of chromosome 8p. The xenograft tumors have a high proliferative index, with greater than 75% of nuclei immunopositive for Ki67. The high proliferative index is also reflected in the short tumor doubling time—approximately 6.5 days. There are no reports of these tumors metastasizing. However, the tumors are castration-resistant; tumors are able to grow in castrated male mice, and the growth rate is reportedly unaffected by castration of intact male tumor baring mice (True et al. 2002).
Figure 1:
A) Photomicrograph of the neuroendocrine prostate tumor from which the LuCAP 49 xenograft tumor was derived (H&E, 400× magnification). Reprinted from The American Journal of Pathology, Volume 161, True LD, Buhler K, Quinn J, Williams E, Nelson PS, Clegg N, Macoska JA, Norwood T, Liu A, Ellis W, et al, A neuroendocrine/small cell prostate carcinoma xenograft-LuCaP 49, Pages 705–715, Copyright 2002 with permission from Elsevier. B) Photomicrograph of a LuCAP 49 xenograft tumor. Note that the xenograft tumor and the patient’s original prostate tumor have a similar histologic appearance consistent with NePCs (H&E, 400× magnification). Reprinted from The American Journal of Pathology, Volume 161, True LD, Buhler K, Quinn J, Williams E, Nelson PS, Clegg N, Macoska JA, Norwood T, Liu A, Ellis W, et al, A neuroendocrine/small cell prostate carcinoma xenograft-LuCaP 49, Pages 705–715, Copyright 2002 with permission from Elsevier. C) Representative image of a typical TRAMP mouse neuroendocrine prostate tumor exhibiting high nuclear to cytoplasmic ratio, anisocytosis, and anisokaryosis (H&E, 400× magnification).
WISH-PC2
The WISH-PC2 xenograft model was developed by Pinthus et al (Pinthus, et al. 2000). The line was derived from a transurethrally resected prostate tumor that had been treated with androgen ablation therapy (goserelin and bicalutamide). Although the patient’s tumor was initially diagnosed as an acinar adenocarcinoma, the tumor that was resected and served as the source for the xenograft was histologically consistent with a NePC (Pinthus et al. 2000). Xenografts were initially established as subcutaneous tumors in CB.17/Icr Beige or NOD SCID mice and Balb/c nude mice. The xenograft tumors resemble typical NePCs and are immunopositive for chromogranin, synaptophysin, and NSE and express a mutated form of p53 and the anti-apoptotic protein BCL-2. Tumor cells have a high proliferative index, as determined by Ki67 immunostaining and exhibit DNA aneuploidy. Cells lack AR, PSA, PAP, prostate stem cell antigen (PSCA), prostate specific membrane antigen (PSMA), CD19, CD20, CD22, and multidrug resistance 1 (MDR1) (Pinthus et al. 2000). Although these tumors are immunonegative for AR and able to grow in castrated male mice, the tumor growth rate is affected by the presence of androgens, with slightly faster growth rates noted when mice are supplemented with testosterone. The tumor volume doubling time in the absence of androgens, however, remains relatively fast, ranging from 13.5 to 18 days, depending on whether the tumors originated from subcutaneously injected tumor cells or implanted tumor sections (Pinthus et al. 2000). The subcutaneous xenograft tumors are able to metastasize to a limited extent, with metastases occasionally identified in the lymph node, lung, or liver (Pinthus et al. 2000). However, the metastatic potential of WISH-PC2 xenografts is augmented by irradiation, with metastases identified in the adrenal gland, brown fat, and perirenal tissue (Agemy, et al. 2008). Tumor cells injected orthotopically into the prostate, liver, and bone are able to grow into discrete tumors, with bone lesions having both an osteolytic and osteoblastic component (Pinthus et al. 2000).
UCRU-PR-2
The UCRU-PR-2 xenograft model was first described by van Haaften-Day et al (van Haaften-Day, et al. 1987). The patient from whom this xenograft line was derived was initially diagnosed with a prostatic acinar carcinoma that progressed, following androgen-deprivation therapy (bilateral castration), to a NePC. The tissue used to establish the xenograft line was obtained from a biopsy of the NePC. The xenografts were established as subcutaneous tumors in Balb/c nude mice via serial transplantation. Histologically, the xenograft tumors have the same NePC morphology as the primary prostate tumor. The xenograft tumors do not express AR, estrogen receptor (ER), PSA, PAP, or keratin but express NSE, epithelial membrane antigen, and carcinoembryonic antigen (Jelbart, et al. 1989; van Haaften-Day et al. 1987). These tumors also secrete a number of proopiomelanocortin (POMC) derived hormones in vivo, including adrenocorticotrophic hormone (ACTH), somatostatin, and β-endorphin (Jelbart, et al. 1988). Interestingly, although these xenograft tumors maintain their NePC phenotype regardless of the implantation site, implantation site affects the tumor invasiveness; tumors grafted underneath the renal capsule or within skeletal muscle exhibit local tissue invasion while tumors inoculated into the subcutaneous fat or peritoneum lack this feature. Tumors do not develop when tissue is implanted under the capsule of the liver or spleen, established xenograft tumors do not metastasize, and intravenous injection does not result in lung tumors. The average subcutaneous tumor volume doubling time is approximately 14.7 days (Jelbart et al. 1989).
WM-4A
The WM-4A xenograft tumor model was developed by Agemy et al (Agemy et al. 2008) from a prostate tumor that had been treated with radiation and complete androgen deprivation therapy. The prostate tumor contained areas consistent with an acinar adenocarcinoma and other areas compatible with a NePC. The patient’s tumor was immunopositive for chromogranin, synaptophysin, and CD57 and focally immunoreactive against PSA and PAP. The xenograft model was developed in CB.17/Icr beige SCID male mice. Xenograft tumors express chromogranin but not PSA. Although the xenograft tumors themselves are sensitive to radiation, irradiation appears to promote metastasis in this model, with irradiated xenograft tumors metastasizing to the adrenal gland, perirenal fat, and brown fat (Agemy et al. 2008).
MDA PCA 144
The MDA PCA 144 xenograft tumor model was established by Aparicio et al (Aparicio, et al. 2011). The xenograft tumor line was derived from a prostate tumor that had been treated with radiation therapy, androgen-deprivation therapy (leuprolide), carboplatin, docetaxel, cisplatin, and etoposide. The histological appearance of the resected tumor was mixed, containing areas of acinar adenocarcinoma, small cell neuroendocrine carcinoma (SCNC), and large cell neuroendocrine carcinoma. Areas of SCNC were immunopositive for chromogranin, synaptophysin, and the neural cell adhesion marker CD56 and immunonegative for AR, PSA, and PAP. To establish the xenograft model, a number of fragments from the resected prostate tumor were implanted into the subcutaneous tissue of male CB.17 SCID mice, and four of the xenografts were consistent with a prostatic SCNC (MDA PCA 144 lines 11, 13, 20, 23). Cells of these MDA PCA 144 xenografts have the typical histological appearance of a SCNC and resemble a clinical NePC, being immunonegative for the AR, PSA, PAP, and alpha-methylacyl-CoAracemase (AMACR). These xenografts are immunopositive for synaptophysin, chromogranin, and CD56, focally immunopositive for cytokeratin and have a high proliferative index as determined by Ki67 immunohistochemistry and by the prominence of mitotic figures (Aparicio et al. 2011). Extensive analysis was done on the MDA PCA 144–13 xenograft, and it was determined that its cells do not express Rb or cyclin D1, and they upregulate mitotic genes such as ubiquitin-conjugating enzyme E2C (UBE2C). Additionally, MDA PCA 144–13 cells demonstrate nuclear p53 immunostaining (Tzelepi, et al. 2012).
LTL352 and LTL370
The LTL352 and LTL370 xenografts were established by Lin et al (Lin, et al. 2014). These two xenografts were derived from biopsies of a urethral metastasis of a NePC (LTL352) and a penile metastasis of a NePC (LTL370). No information was provided by the authors regarding whether these patients had been treated with androgen-deprivation therapy, radiation therapy, and or chemotherapy prior to the biopsy. Fragments of tissue were implanted into the subrenal capsule of testosterone-supplemented male NOD SCID (NOD.CB17-Prkdcscid/J) mice (Lin et al. 2014). The xenografts resemble NePCs histologically and exhibit an expression pattern typical of these tumors. Neoplastic cells are immunopositive for synaptophysin and chromogranin, and immunonegative for AR and PSA. The tumors are castration-resistant, being able to grow in mice after androgen-deprivation. These tumors do not express ERG or serine protease inhibitor Kazal-type 1 (SPINK1) but do express PTEN. As with other NePC xenografts, these tumors have a rapid doubling time, with tumors doubling in size in approximately 10 to 12 days. These NePC xenografts reportedly metastasize, but the authors do not describe the frequencies or locations of the metastases (Lin et al. 2014).
EXPERIMENTAL MANIPULATIONS
In addition to the described xenograft tumor models that spontaneously exhibit neuroendocrine differentiation, experimental manipulations can induce cells within more traditional xenograft prostate cancer lines to transition to a neuroendocrine phenotype. For example, castration of mice baring the LTL331 prostate adenocarcinoma subrenal capsule xenograft causes the tumors to transition to a NePC phenotype (LTL331R). While, LTL331 xenografts express AR and PSA, LTL331R tumors express synaptophysin, chromogranin, and CD56 and do not express AR or PSA (Lin et al. 2014). Additionally, androgen-deprivation can increase the number of cells that express markers of neuroendocrine differentiation within PC-310 (Jongsma, et al. 2000, 2002; Noordzij, et al. 1996), PC-295 (Jongsma, et al. 1999; Noordzij et al. 1996), and CWR22 xenograft tumors (Huss, et al. 2004). Interestingly, ionizing radiation stimulates a population of cells within LNCAP xenograft tumors to express chromogranin (Deng, et al. 2011). Taken together, these models demonstrate that commonly used therapies (androgen-deprivation and irradiation) can stimulate cells within more “classical” prostatic carcinomas to develop a neuroendocrine phenotype.
SUMMARY OF XENOGRAFT MODELS
Herein, we have reviewed seven of the xenograft models of established NePC. These xenografts serve as clinically relevant tumor models for human NePC. Importantly, five of the xenograft tumor lines were derived from patients that had been treated with androgen-deprivation therapy, radiation therapy, and/or chemotherapy (Agemy et al. 2008; Aparicio et al. 2011; Pinthus et al. 2000; True et al. 2002; van Haaften-Day et al. 1987). The treatment statuses of the patients from whom LTL352 and LTL370 were derived were not available (Lin et al. 2014). Thus, the majority of these xenograft tumors serve as appropriate models for NePC that arise post-treatment. Additionally, these tumors model human NePC molecularly, with all of these xenograft tumors demonstrating at least one marker of neuroendocrine differentiation (see Table 1) and lacking expression of AR and/or prostate epithelial specific markers, such as PSA (Agemy et al. 2008; Aparicio et al. 2011; Jelbart et al. 1989; Lin et al. 2014; Pinthus et al. 2000; True et al. 2002; van Haaften-Day et al. 1987). Finally, these xenograft tumors are able to model the rapid growth rate that is characteristic of many NePCs. High proliferative indices as determined by Ki67 immunohistochemistry are features of LUCAP 49 (True et al. 2002), WISH-PC2 (Pinthus et al. 2000), and MDA PCA 144 (Aparicio et al. 2011) xenograft tumors. Similarly, the average tumor doubling time for UCRU-PR-2 is approximately 2 weeks (Jelbart et al. 1989), and LTL352 and LTL370 tumors double in approximately 10 to 12 days (Lin et al. 2014).
Although these xenografts models are valid in vivo models for NePC, they are not without limitations. For example, even though xenograft models allow one to study the behavior of a human prostate tumor in vivo, the ability to study metastatic potential is limited (Sausville and Burger 2006). Although WISH-PC2 xenografts demonstrate the ability to metastasize spontaneously, they metastasize rarely and never to bone (Pinthus et al. 2000). LTL352 and LTL370 reportedly metastasize, but the locations and frequencies of the metastases were not detailed in the original description of these models (Lin et al. 2014). This is an important limitation, given the metastatic nature of NePCs. With the exception of LTL331R, a NePC xenograft that develops after castration of mice baring LTL331 prostate adenocarcinoma xenograft tumors (Lin et al. 2014), another disadvantage is that NePC xenograft tumors only allow for investigation of the characteristics and behaviors of established post-treatment NePCs, not the molecular mechanisms involved in the development of a NePC. Moreover, although the majority of NePCs observed in the clinic arise in patients that have previously received androgen-deprivation therapy, some NePCs occur in treatment naïve patients. Therefore, new models are needed for “treatment naïve” de novo NePCs, since the currently available xenograft tumors reflect post-treatment NePCs with biologic behaviors and molecular alterations distinct from “treatment naïve” NePCs. Additionally, the effects of the human immune system on tumor growth, invasion, and metastasis cannot be studied since the tumors grow in a mouse microenvironment (Sausville and Burger 2006). Finally, it is unclear how many of the established xenograft tumor lines are readily available given the extensive handling required to propagate and maintain them. Thus, despite the utility of the established NePC xenograft tumor lines, additional xenograft models of NePC are needed to further explore this clinically challenging variant of prostate cancer.
GENETICALLY ENGINEERED MOUSE MODELS OF NEPC
A number of genetically engineered mouse models of NePC exist. The salient features of these models can be found in Table 2. All of these models autochthonously develop prostate tumors that histologically resemble human NePC and express at least one marker of neuroendocrine differentiation (most commonly synaptophysin or chromogranin). In most of these models, prostate carcinomas with neuroendocrine phenotypes (i.e, neuroendocrine carcinomas) are induced via the expression of one or both of the simian virus 40 (SV40) early genes (the large and small T antigens) in prostate epithelial cells (Gabril, et al. 2005; Gabril, et al. 2002; Gingrich, et al. 1999; Gingrich, et al. 1996; Greenberg, et al. 1995; Kasper, et al. 1998; Masumori, et al. 2001; Perez-Stable, et al. 1996; Perez-Stable, et al. 1997; Reiner, et al. 2007) or in prostate neuroendocrine cells (Garabedian, et al. 1998). This results in prostate tumorigenesis because the large T antigen inhibits the activities of the tumor suppressors p53 and Rb (Gingrich et al. 1996; Greenberg et al. 1995), and the small T antigen interacts with protein phosphatase 2A (Gingrich et al. 1996; Greenberg et al. 1995; Pallas, et al. 1990). Conditionally knocking-out p53 and Rb in prostate epithelial cells has been used as an alternative way of producing neuroendocrine prostate carcinomas in mice (Zhou, et al. 2006).
Table 2:
Features of the genetically engineered mouse models of NePC
| Genetically Engineered Mouse Model | Genetic Manipulation | Pathologic Progression | Castration Resistant Disease? | Immunohistochemistry | Metastatic Potential | Other | Selected References |
|---|---|---|---|---|---|---|---|
| TRAMP | Rat probasin promoter driving the SV40 large and small T antigens | PIN by 6 weeks. Well-differentiated adenocarcinoma by 18 weeks. Neuroendocrine carcinoma by 24 weeks | Yes | Immunopositive for: Synaptophysin, Immunonegative for: Cytokeratin 8 and E-cadherin. Variable to no expression of the AR | Yes: adrenal gland, kidney, liver, lung, lymph nodes | Lesion development is affected by mouse background strain. Mice can develop extra-prostatic lesions that interfere with research. | (Gingrich, et al. 1999; Greenberg, et al. 1995; Kaplan-Lefko, et al. 2003) |
| 12T-10 LPB-Tag | Large probasin promoter (LPB) driving SV40 Large T antigen | PIN when mice are 2–5 months old. Carcinoma in mice 6 months of age and older. Neuroendocrine carcinomas in mice older than 8 months | Not evaluated, presumed based on the fact that the tumors do not express the AR |
Immunopositive for:
Chromogranin A, weakly immunopositive for
Cytokeratin. Immunonegative for: AR |
Yes: liver, lung, lymph node | Allograft tumors metastasize. | (Masumori, et al. 2001) |
| 12T-7f LPB-Tag/PB-hepsin | Large probasin promoter (LPB) driving SV40 Large T antigen/rat probasin promoter driving hepsin overexpression | 21 week old mice demonstrate prostate adenocarcinomas with some areas resembling neuroendocrine carcinomas | Not evaluated | Immunopositive for: Synaptophysin | Yes: bone, liver, lung | Metastatic lesions express the AR. | (Klezovitch, et al. 2004) |
| FG-Tag | Fetal globin promoter driving the SV40 large and small T antigens | PIN in 16–20 week old mice. Carcinomas with epithelial and neuroendocrine features in 16 to 32 week old mice | Yes |
Immunopositive for:
Chromogranin A, Synaptophysin, Cytokeratin 8. Consistent low AR
expression Immunonegative for: E-cadherin, Mouse dorsolateral prostate secretory protein, Connexin 32 |
Yes: adrenal gland, bone, kidney, lung, and lymph node | Fetal globin promoter is not prostate specific. Mice can develop a number of extra-prostatic lesions. | (Perez-Stable, et al. 1996; Perez-Stable, et al. 1997; Reiner, et al. 2007) |
| PSP-TGMAP | Prostate secretory protein of 94 amino acids (PSP94) promoter/enhancer region driving SV40 large and small T antigens | Epithelial hyperplasia in 10-week-old mice. PIN in 12 to 19 week old mice. Well-differentiated adenocarcinomas in 24 to 32 week old mice. Poorly differentiated prostatic carcinomas without apparent glandular architecture have been described in mice as young as 16 weeks of age. | Yes | Expresses markers of neuroendocrine differentiation, such as Chromogranin A (as determined by cDNA microarray) | Yes: lymph nodes | Three different lines of mice exist. TG183–2 mice have the highest incidence of neuroendocrine carcinoma development. High transgene copy number has been associated with the development of extra-prostatic expression and lesions. | (Gabril, et al. 2005; Gabril, et al. 2002) |
| PSP-KIMAP | The SV40 large and small T antigens are knocked-in at the PSP94 promoter/enhancer locus | PIN is present age 6–7 weeks, and well-differentiated adenocarcinomas are apparent by 10–12 weeks of age. Well-differentiated and moderately differentiated adenocarcinomas are the most common cancer types in mice older than 12 weeks. Neuroendocrine carcinomas have been found in mice that are older than 1 year. | No | Immunopositive for: Chromogranin A | Yes: liver, lungs, and lymph nodes | Tumors with neuroendocrine differentiation are rare in this model. | (Duan, et al. 2005; Gabril et al. 2005) |
| CR2-Tag | Cryptdin-2 promoter driving the the SV40 large and small T antigens | PIN in 8 to 10 week old mice with microinvasion by 12 to 16 weeks. Neuroendocrine carcinomas by 24 weeks of age. | Yes |
Immunopositive for:
Chromogranin A, Synaptophysin Immunonegative for: AR |
Yes: bone marrow, liver, lung, and or lymph nodes | The cryptidin-2 promoter is not prostate specific. | (Garabedian, et al. 1998) |
| p53PE−/−; RbPE−/− | Conditionally knocking-out p53 and Rb from the epithelium of all lobes of the mouse prostate | PIN by 8 weeks of age. Poorly differentiated prostate carcinoma with neuroendocrine features by approximately 32 weeks of age | Yes |
Immunopositive for:
Synaptophysin, Cytokeratin 8, AR Immunonegative for: Cytokeratin 5 |
Yes: adrenal gland, liver, lung, and lymph node | Half of p53PE−/−; RbPE+/− mice will develop prostate carcinomas with systemic metastases similar to those of the p53PE−/−; RbPE−/− mice by approximately 72 weeks of age. | (Zhou, et al. 2006) |
GENETICALLY ENGINEERED MOUSE MODELS UTILIZING THE SV40 T ANTIGENS
TRAMP Model
The most well-known transgenic mouse model of NePC is the transgenic adenocarcinoma of the mouse prostate (TRAMP) model. In the TRAMP model, the rat probasin promoter (a 426 base pair long fragment of the promoter and 28 base pairs from the 5 prime untranslated region) drives the expression the SV40 large and small T antigens in prostatic epithelial cells (Gingrich et al. 1999; Gingrich et al. 1996; Greenberg et al. 1995; Irshad and Abate-Shen 2013). The rat probasin promoter is an androgen and zinc dependent promoter, and transgene expression should be highest in the dorsal, lateral, and ventral lobes of the prostate (Gingrich et al. 1996; Greenberg et al. 1995). Prostate lesion development commences at puberty (approximately 6 weeks of age) with the appearance of low-grade prostatic intraepithelial neoplasia (PIN). By approximately 10–16 weeks of age, the prostates of most TRAMP mice exhibit high grade PIN that progresses by approximately 18 weeks to well-differentiated adenocarcinomas. By approximately 24 weeks of age, most TRAMP mice will have poorly differentiated carcinomas with neuroendocrine features (ie, neuroendocrine carcinomas, Figure 1C) (Gingrich et al. 1999; Kaplan-Lefko, et al. 2003). These neuroendocrine carcinomas metastasize readily, with metastases identifiable in the adrenal gland, kidney, liver, lung, lymph nodes, and, rarely, the vertebrae with spinal cord compression (Gingrich et al. 1999; Gingrich et al. 1996; Kaplan-Lefko et al. 2003).
TRAMP mouse neuroendocrine carcinomas are immunopositive for synaptophysin and the SV40 large T antigen and have a high proliferative index as determined by Ki67. The neuroendocrine carcinomas are largely immunonegative for the epithelial markers cytokeratin 8 and E-cadherin. These carcinomas have variable to no AR expression (Kaplan-Lefko et al. 2003). TRAMP mouse tumorigenesis is largely castration-resistant, with approximately 80% mice castrated at 12 weeks developing neuroendocrine carcinomas with metastases by 24 weeks of age (Gingrich, et al. 1997; Kaplan-Lefko et al. 2003).
Interestingly, the TRAMP mouse strain affects lesion development and progression. For example, while extensive tumor burdens result in most C57BL/6 TRAMP × FVB F1 mice being euthanized prior to 33 weeks of age, C57BL/6 TRAMP mice appear to develop their tumor burden at a slower rate and are frequently able to survive until approximately 36–40 weeks of age. Neuroendocrine carcinomas in C57BL/6 TRAMP mice can invade the seminal vesicle and urethra whereas these tumors C57BL/6 TRAMP × FVB F1 mice tend to spare the seminal vesicles (Gingrich et al. 1999; Kaplan-Lefko et al. 2003). C57BL/6 TRAMP mice may also have a lower incidence of neuroendocrine carcinoma development (Chiaverotti, et al. 2008).
Much like with human NePC, there is no consensus regarding the cell lineage of TRAMP neuroendocrine carcinomas. Two basic theories exist. The first is that these neuroendocrine carcinomas originate from bipotential stem cells that are capable of expressing both epithelial (E-cadherin) and neuroendocrine (synaptophysin) markers and not from epithelial cells within PIN lesions (Chiaverotti et al. 2008). The second theory is that the neuroendocrine carcinomas arise from an “epithelial to neuroendocrine” transition (Kaplan-Lefko et al. 2003). Given the fact that there is debate regarding the origin of human NePCs, this controversy does not negate the use of TRAMP mice as a model of NePC.
In addition to being used to evaluate the efficacy of novel therapeutics, TRAMP mice have been extensively manipulated in order to investigate prostate cancer progression and metastasis. For example, TRAMP mice that are heterozygous for the Pten tumor suppressor (TRAMP/Pten−/+) have larger prostate tumors and shorter survival times than TRAMP mice that are wild type for Pten. These two lines of TRAMP mice have similar rates of visceral metastasis. Together, this indicates an important role for Pten in prostate cancer progression but not metastasis (Kwabi-Addo, et al. 2001). This is clinically relevant, given the frequency of Pten loss in human NePC (Tan et al. 2014). TRAMP mice that lack the ubiquitin ligase Siah2 (TRAMP/Siah2−/−) develop fewer neuroendocrine prostate carcinomas and fewer visceral metastases than TRAMP with wild-type Siah2, suggesting a role for Siah2 in the acquisition of a neuroendocrine phenotype and metastasis (Qi, et al. 2010).
Despite its utility as a model of NePC, the TRAMP mouse model is not without limitations. For example, TRAMP mice can develop a number of extra-prostatic transgene associated tumors that complicate research studies and necessitate early removal (Berman-Booty, et al. 2014). These include epithelial-stromal (phyllodes-like) tumors of the seminal vesicles (Tani, et al. 2005), renal tubulo-acinar carcinomas (Suttie, et al. 2005), neuroendocrine tumors of the urethra (Suttie et al. 2005), anaplastic midbrain tumors (Berman-Booty et al. 2014), and poorly differentiated submandibular salivary gland adenocarcinomas (Berman-Booty et al. 2014). Thus, although the TRAMP mouse is one of the best-characterized genetically engineered models of NePC demonstrating a lesion progression and an immunophenotype similar to human NePC, it is not without shortcomings.
12T-10 LPB-Tag Model
The 12T-10 LPB-Tag model is a derivative of the LADY transgenic mouse model. The LADY model is genetically engineered to have a portion of the rat probasin promoter (LPB: a 11,500 base pair long fragment of the promoter and 28 base pairs from the 5 prime untranslated region) (Yan, et al. 1997) drive the prostate specific expression of a mutant SV40 which only produces the large T antigen (Irshad and Abate-Shen 2013; Kasper et al. 1998; Masumori et al. 2001). Unlike most LADY mice that develop adenocarcinomas that metastasize infrequently (Irshad and Abate-Shen 2013; Kasper et al. 1998), 12T-10 LPB-Tag mice develop neuroendocrine carcinomas originating from the dorsal, lateral, or ventral lobes of the prostate. The initial lesion these mice develop is PIN, which typically appears when the mice are between 2 and 5 months of age. Interesting, aggregates of cells with an immunohistochemical profile consistent with neuroendocrine cells (immunopositive for chromogranin A) are reported to be found commonly within high grade PIN lesions in mice 5 months of age and older (Masumori et al. 2001). Prostates from mice older than 6 months of age typically have invasive foci and evidence of carcinoma, although some mice develop microinvasion and carcinomas as early as 4 months of age. Invasive foci and earlier carcinomas can have a mixed phenotype with some areas histologically consistent with adenocarcinomas and other areas typical of neuroendocrine carcinomas. More extensive invasive foci and carcinomas in mice older than 8 months often have a histologic appearance consistent with a neuroendocrine carcinoma (Masumori et al. 2001). Like the human NePCs that they are modeling, these tumors are immunopositive for chromogranin A and largely immunonegative for AR, although they are also weakly immunopositive for cytokeratin, (Masumori et al. 2001). Tumors most commonly metastasize to the liver, lung, and lymph node, with over 80% of mice 9 months of age and older having metastases. Metastatic lesions have the same histologic and immunohistochemical profile as the primary neuroendocrine prostate tumors (Masumori et al. 2001). When neuroendocrine carcinomas from 12T-10 LPB-Tag mice are implanted into the subcutaneous tissue of athymic nude mice, the allograft tumors (NE-10 allograft) maintain the original tumor’s neuroendocrine phenotype and metastasize to the liver and lung (Masumori et al. 2001; Masumori, et al. 2004).
Although the 12T-10 LPB-Tag model is the only LPB-Tag mouse line capable of spontaneously developing neuroendocrine prostate carcinomas, prostatic carcinomas with neuroendocrine metastases develop in 12T-7f LPB-Tag mice that overexpress hepsin, a serine protease (Irshad and Abate-Shen 2013; Klezovitch, et al. 2004). These mice are generated by crossing 12T-7f LPB-Tag mice with mice that overexpress hepsin under the control of the rat probasin promoter (PB-hepsin). By approximately 21 weeks of age, the resulting 12T-7f LPB-Tag/PB-hepsin mice develop PIN and prostate carcinomas with some areas that histologically resemble neuroendocrine carcinomas. Tumor metastases are found in bone, liver, and lung. Metastases are immunopositive for synaptophysin, thus confirming their neuroendocrine differentiation, and express AR (Klezovitch et al. 2004). Given the fact that 12T-7f LPB-Tag/PB-hepsin neuroendocrine tumors express AR, this model may not be completely clinically relevant.
Fetal Globin-T antigen (FG-Tag) Model
Prostate tumors with epithelial and neuroendocrine characteristics have been described in one line of male mice expressing the SV40 T antigens under the control of the fetal globin promoter (FG-Tag model), a global promoter (Perez-Stable et al. 1996; Perez-Stable et al. 1997). Prostate tumors are identifiable in 16 to 32 week old FG-Tag mice. These tumors likely originate from within PIN lesions, which are found in 16 to 20 week old mice. Consistent with the mixed histological appearance, cells within the prostate tumors express epithelial and neuroendocrine markers, namely cytokeratin 8 and chromogranin A. Tumor cells do not express mouse dorsolateral prostate secretory protein or connexin 32 (Perez-Stable et al. 1997). Later work with these mice resulted in further elucidation of the immunohistochemical characteristics, namely, that tumor cells are immunopositive for synaptophysin and immunonegative for E-cadherin. FG-Tag mouse prostate tumors are believed to originate from p63 expressing basal epithelial cells, since the T antigen has been immunohistochemically localized to these cells prior to PIN and tumor development (Reiner et al. 2007).
FG-Tag mouse prostate tumors are able to metastasize to the adrenal gland, bone, kidney, lung, and perirenal lymph node. Mice that are homozygous for the transgene have a higher incidence of tumor development than hemizygous mice. Prostate tumor development in FG-Tag mice appears to be castration-resistant, with approximately 50% of male mice castrated at age 4 to 6 weeks developing prostate tumors by 20 to 28 weeks of age (Perez-Stable et al. 1997). Despite their androgen-independent behavior, tumors consistently express a low level of the AR, with highest AR expression levels in the tumor cells surrounding blood vessels (Reiner et al. 2007).
Despite the fact that FG-Tag mice develop prostate tumors, the fetal globin promoter is not prostate specific, so mice can develop neoplastic lesions in other organs as well. For example, subcutaneous, pericardial, and periadrenal hibernomas arise in other lines of male FG-Tag mice, and adrenocortical tumors have been found in female FG-Tag mice (Perez-Stable et al. 1996). Additionally, male mice that develop prostate tumors have also been reported to develop adrenal tumors, hibernomas, and seminomas (Perez-Stable et al. 1997). Therefore, although FG-Tag mice develop castration-resistant prostate tumors that share some features with human NePCs given the potentially mixed phenotype (immunopositivity for both neuroendocrine and epithelial markers) and development of extra-prostatic lesions, this model is not without its limitations.
PSP94 gene-directed transgenic mouse adenocarcinoma in the prostate (PSP–TGMAP) Model
Poorly differentiated prostate carcinomas with a neuroendocrine phenotype typify late stage tumors in the PSP94 gene-directed transgenic mouse adenocarcinoma in the prostate (PSP-TGMAP) model of prostate cancer. In this model, expression of the SV40 T antigens is driven by a 3.84 kb long section of the prostate secretory protein of 94 amino acids (PSP94) promoter/enhancer region, which is reportedly prostate specific (Gabril et al. 2002). Three lines (lines TG183–2, TG186–3, TG186–9) were initially established. Lines TG186 differ from line TG183 in that the transgene for line TG186 includes exons 1 and 2 as well of part of the first intron of the PSP94 gene following the promoter/enhancer region (Gabril et al. 2002). Prostate tumorigenesis begins with prostate epithelial hyperplasia when the mice are approximately 10 weeks of age, followed by PIN in 12 to 19 week old mice. Well-differentiated adenocarcinomas are typically identifiable in 24 to 32 week old mice. Poorly differentiated prostatic carcinomas without apparent glandular architecture have been described in TG183–2 mice as young as 16 weeks of age. These tumors have also been identified in TG186–9 mice that are 28 weeks of age and older (Gabril et al. 2002). Poorly differentiated carcinomas show elevated expression of genes involved in neuroendocrine differentiation, including chromogranin A. Thus, these tumors have been described as having a neuroendocrine phenotype (Gabril et al. 2005). TG183–2 prostate tumors most frequently metastasize to the renal lymph nodes (Gabril et al. 2002). TG183–2 prostate tumors also appear to be castration-resistant, with 67% of mice castrated between 20 and 26 weeks of age having prostate carcinomas one month post-castration (Gabril et al. 2002).
One of the disadvantages of this model is that the rate of lesion development varies between lines. For example, although line TG183–2 mice develop poorly differentiated carcinomas with neuroendocrine features, they develop PIN and well-differentiated adenocarcinomas at a slower rate than line TG186–3 and TG186–9 mice. Therefore, care must be taken when selecting the mouse line to use. Transgene copy number also varies between lines of mice and having a high transgene copy number has been associated with the development of extra-prostatic lesions, which can complicate studies and necessitate early removal. For example, line TG186–9 mice have the highest copy number and are most prone to extra-prostatic tumor development (Gabril et al. 2002).
Interestingly, when the SV40 T antigens are targeted (“knocked in”) to the PSP94 gene locus, the resulting mice (PSP94 knockin mouse adenocarcinoma in the prostate [PSP-KIMAP] mice) display a different phenotype than when the transgene is randomly integrated into the genome, as with PSP-TGMAP mice (Duan, et al. 2005). For example, PIN is evident in PSP-KIMAP mice by age 6–7 weeks, and well-differentiated adenocarcinomas are apparent by 10–12 weeks of age. Well-differentiated and moderately differentiated adenocarcinomas are the most commonly found cancer types in PSP-KIMAP ranging from 12 weeks to approximately 52 weeks. Tumor metastases to the liver, lungs, and lymph nodes can be identified in mice older than 1 year of age. Metastases express AR. Unlike TG183–2 tumors, PSP-KIMAP tumors appear to be sensitive to castration (Duan et al. 2005). PSP-KIMAP mice rarely develop prostatic neuroendocrine carcinomas (as identified by histological appearance and immunoreactivity against chromogranin A), and, when present, these tumors have only been identified in mice older than 1 year of age (Duan et al. 2005; Gabril et al. 2005). Studies with PSP-TGMAP and PSP-KIMAP mice seem to suggest that the way that the transgene is integrated into the genome affects lesion development (Duan et al. 2005; Gabril et al. 2005).
Cryptdin-2-T Antigen (CR2-Tag) Model
When the mouse Cryptdin-2 promoter (6500 base pairs of the promoter and 34 base pairs of the 5 prime untranslated region) is used to drive the expression of the SV40 T antigens in prostate neuroendocrine cells, prostate tumorigenesis, culminating in neuroendocrine carcinomas, results (Garabedian et al. 1998). Lesion development in the Cryptdin-2 T antigen (CR2-Tag) model begins with the appearance of PIN in 8 to 10 week old mice. By age 12 to 16 weeks, microinvasion is evident within PIN lesions, and by 24 weeks of age, the majority of CR2-Tag mice will have poorly differentiated, anaplastic prostatic carcinomas lacking glandular architecture with metastases evident in the bone marrow, liver, lung, and or lymph nodes. Targeting of the SV40 T antigens by the CR2 promoter to prostatic neuroendocrine cells and confirmation of the neuroendocrine carcinoma phenotype is determined by the presence of cells within PIN and microinvasive lesions that express both neuroendocrine markers (chromogranin A and synaptophysin) and the SV40 T antigens (Garabedian et al. 1998). Expression studies have also found that cells from tumor baring prostates express chromogranin A and B, in addition to a number of other neural and endocrine biomarkers (Hu, et al. 2002). Prostate tumorigenesis in CR2-Tag mice is castration-resistant, since mice castrated at 4 weeks of age develop similarly sized tumors as their intact littermates. Additionally, tumor cells are immunonegative for AR (Garabedian et al. 1998).
CR2-Tag mice have been used to elucidate the roles of matrix metalloproteinases (MMPs) in neuroendocrine prostate cancer progression. For example, CR2-Tag mice deficient for MMP-2 (CR2-Tag/MMP-2−/− mice) develop smaller prostate tumors, reduced tumor neovascularization, fewer foci of invasion, and fewer lung metastases than CR2-Tag mice with wild-type MMP-2. In contrast, CR2-Tag mice deficient for MMP-9 (CR2-Tag/MMP-9−/− mice) exhibit a more invasive phenotype than CR2-Tag mice with wild-type MMP-9. These studies suggest different roles for MMP-2 and MMP-9 in neuroendocrine prostate cancer (Littlepage, et al. 2010).
Summary of Models that Utilize the SV40 T Antigens
A detailed review of the features of the above mouse models clearly demonstrates that utilization of the SV40 T antigens is one of the most robust ways to induce prostate carcinomas with neuroendocrine features as evidenced by the fact that all of these models develop neuroendocrine carcinomas with systemic metastases (Irshad and Abate-Shen 2013). Use of the SV40 T antigens results in rapid tumorigenesis, since neuroendocrine carcinomas are found in many of these models by the time the mice are approximately 6-months-old (Gabril et al. 2002; Garabedian et al. 1998; Gingrich et al. 1999; Perez-Stable et al. 1997). However the incidence of neuroendocrine tumors varies between models. For example, although the majority of end stage tumors in most of these models are neuroendocrine carcinomas (Irshad and Abate-Shen 2013), the incidence of poorly differentiated carcinomas with neuroendocrine features in PSP-TGMAP (line TG183–2) may be as low as 25% (Gabril et al. 2002), and PSP-KIMAP mice rarely develop neuroendocrine carcinomas (Duan et al. 2005; Gabril et al. 2005). Importantly, with the exception of PSP-KIMAP (Duan et al. 2005) and 12T-7f LPB-Tag/PB-hepsin mice (Klezovitch et al. 2004), most tumors that develop secondary to expression of the SV40 T antigens appear to be castration-resistant (Gabril et al. 2002; Garabedian et al. 1998; Gingrich et al. 1997; Masumori et al. 2001; Perez-Stable et al. 1997) as determined by continued tumor development in castrated mice or lack of tumor cell AR expression. Castration-resistance is an important and clinically relevant feature of these genetically engineered mouse models.
In addition to the models described above, there are other mouse models that employ the SV40 T antigen for oncogenesis, but the tumors that develop do not strictly fit the criteria of “neuroendocrine carcinomas.” For example, when the expression of the SV40 T antigens is controlled by a promoter composed of the 5 prime flanking region and part of the first exon of the rat prostatic steroid binding protein [C3(1)], poorly differentiated prostate carcinomas can be found in the male mice [C3-(1)-Tag] after 8 month of age. However, despite the poorly differentiated and anaplastic histological appearance of cells within these carcinomas, these tumors have not been immunohistochemically confirmed as neuroendocrine (Maroulakou, et al. 1994).
CONDITIONAL KNOCKOUT MODELS OF NEUROENDOCRINE PROSTATE CANCER
P53 and Rb Conditional Knockout
Simultaneously, conditionally knocking-out p53 and Rb (p53PE−/−; RbPE−/−) from the epithelium of all lobes of the mouse prostate results in the development of prostate carcinomas with neuroendocrine differentiation. Loss of p53 and Rb is limited to the prostate secondary to the prostate specific expression of Cre recombinase under the control of ARR2PB promoter, a modified rat probasin promoter (Zhou et al. 2006). The average p53PE−/−; RbPE−/− mouse will develop PIN at approximately 8 weeks of age followed by a poorly differentiated prostatic carcinoma with neuroendocrine features by approximately 32 weeks of age (range 24 to 50 weeks). These tumors metastasize readily to the adrenal gland, liver, lung, and lymph node. Interestingly, cells within the primary tumors and metastases vary with regard to their reactivity against synaptophysin, cytokeratin 8, and AR, with up to 80% of cells immunopositive for each of these biomarkers and approximately 50–90% of the cells immunopositive for all three biomarkers. Tumor cells are immunonegative for cytokeratin 5 (Zhou et al. 2006).
Although prostate tumors from p53PE−/−; RbPE−/− mice express AR, tumorigenesis appears to be castration-resistant. Mice castrated at approximately 8 weeks of age develop prostate tumors with a similar histological appearance and frequency as their intact counterparts by approximately 22 weeks of age. Additionally, castration of 22-week-old mice with prostate tumors does not decrease tumor cell proliferation, although AR expression may be reduced (Zhou et al. 2006).
Interestingly, half of mice that lack prostate specific expression of both alleles of p53 and one allele of Rb (p53PE−/−; RbPE+/− mice) will develop prostate carcinomas with systemic metastases similar to those of the p53PE−/−; RbPE−/− mice by approximately 72 weeks of age. Many of the tumors from these mice will have lost expression of the remaining wild type allele of Rb. This is in contrast to mice with prostate specific inactivation of either p53 (p53PE−/− mice) or Rb (RbPE−/− mice) and mice that lack prostate specific expression of Rb but maintain one p53 allele (p53PE+/−; RbPE−/− mice). These mice develop PIN that does not progress to carcinoma (Zhou et al. 2006). This suggests that both p53 and Rb must be lost for neuroendocrine prostate tumor development and that homozygous loss of p53 may facilitate loss of the second allele of Rb.
SUMMARY OF GENETICALLY ENGINEERED MOUSE MODELS OF NEPC
Herein we have described a number of currently available genetically engineered mouse models of NePC. Interestingly, since all of the genetically engineered models discussed herein exhibit functional loss of p53 and Rb, these models illustrate the apparent importance of p53 and Rb loss in the development of a neuroendocrine phenotype. However, it is not clear whether loss of p53 and Rb is “necessary and sufficient” for neuroendocrine carcinoma development. For example, although work with the p53PE−/−; RbPE−/− mice (Zhou et al. 2006) suggests that loss of Rb in the background of complete p53 loss is “necessary and sufficient” to induce prostatic neuroendocrine carcinomas since both p53PE−/−; RbPE−/− and p53PE−/−; RbPE+/− mice develop neuroendocrine prostate cancers, while p53PE+/−; RbPE−/−, p53PE−/−, and RbPE−/− mice do not (Zhou et al. 2006), studies with TgAPT121 mice indicate otherwise (Hill, et al. 2005). TgAPT121 mice express a mutant SV40 large T antigen that only abrogates the activity of Rb family members (Rb, p107, and p130) and spares p53 (Hill et al. 2005). These mice develop PIN and well-differentiated adenocarcinomas. When further manipulated so they lose p53, the resulting TgAPT121; p53−/− mice do not develop neuroendocrine prostate tumors. This implies that the loss of function of other tumor suppressors and or gain of function of other oncogenes is required for neuroendocrine prostate cancer development (Hill et al. 2005). Further research is needed to determine the exact molecular alterations necessary for neuroendocrine prostate cancer development and growth.
One of the advantages of using genetically engineered mouse models to study NePC is that the tumors autochthonously develop within the prostate and, since lesions typically progress through a number of stages (including pre-neoplastic) before culminating in NePC, the molecular mechanisms involved in most stages of prostatic NePC development can be studied. Another advantage of these genetically engineered mouse models is that most of these models produce castration-resistant disease with solid organ and or lymph node metastases (Gabril et al. 2002; Garabedian et al. 1998; Gingrich et al. 1997; Masumori et al. 2001; Perez-Stable et al. 1997; Zhou et al. 2006) – an important feature of human NePC. Additionally, the mice have intact immune systems (Becher and Holland 2006), allowing for studies into the effect of the immune system on cancer development and regression.
A major disadvantage of all of the genetically engineered mouse models is that simultaneous loss of the p53 and Rb activity, either through the use of the SV40 T antigens (Duan et al. 2005; Gabril et al. 2002; Garabedian et al. 1998; Greenberg et al. 1995; Klezovitch et al. 2004; Masumori et al. 2001; Perez-Stable et al. 1997) or secondary to conditionally knocking-out both of these genes from the prostatic epithelium (Zhou et al. 2006), is the genetic alteration that results in neuroendocrine tumor development. Although loss of both p53 and Rb is a common finding in human NePCs (Tan et al. 2014), it is unlikely that these two major tumor suppressors are lost simultaneously or as the initial event leading to tumorigenesis. Therefore, models that lose both p53 and Rb functionality as the initiating event may not accurately model the molecular events that occur during the development of human NePC. The p53PE−/−; RbPE+/− mouse model is the one that most closely resembles non-simultaneous loss of p53 and Rb (Zhou et al. 2006), although the timing of the loss of the second allele of Rb during tumorigenesis is unknown. In addition to the non-clinically relevant simultaneous loss of p53 and Rb activity induced by the SV40 T antigens, the clinical applicability of mouse models that use these viral oncogenes is further challenged by the fact that the SV40 T antigens interact with a number of cellular targets in addition to p53 and Rb, and these interactions may be irrelevant to human prostate cancer. Specifically, the large T antigen inhibits p107 and p130, two other members of the Rb family of proteins, and binds the chaperone Hsc70 and the transcriptional co-activators CBP, p300, and p400, while the small T antigen interacts with protein phosphatase 2A (Ahuja, et al. 2005; Ali and DeCaprio 2001; Pipas 2009). Additionally, all of these genetically engineered mice develop neuroendocrine carcinomas spontaneously, that is without therapeutic interventions such as androgen-deprivation. Therefore, instead of modeling the more common “post-treatment” NePC, these mice model primary (treatment naïve) NePCs. Finally, prostate lesion development in some of these models, specifically the TRAMP (Berman-Booty et al. 2014), FG-Tag (Perez-Stable et al. 1996), and PSP-TGMAP (Gabril et al. 2002) models, can be confounded by the development of extra-prostatic lesions and tumors.
CONCLUSIONS AND FUTURE DIRECTIONS
Herein we reviewed the major xenograft and genetically engineered mouse models of NePC. These models underscore the aggressive nature of NePCs. However, current mouse models of NePC only partially mimic the salient features of clinical disease. Thus, careful consideration of the biological behaviors and characteristics of the available models is needed in order to ensure that the model of choice is appropriate for the research question posed. For example, it may be inappropriate to use one of the xenograft models to study how NePC develops in the background of PIN or to use TRAMP mice to try to model castration-sensitive disease. Additionally, the prostate tumorigenesis of the established genetically engineered mouse models may not reflect that of “post-treatment” NePCs, since these mice develop neuroendocrine carcinomas without therapeutic intervention (such as androgen-deprivation) and castration does not significantly alter their phenotype.
Xenografts that are derived from de novo “treatment naïve” NePC as well as more xenografts that reliably metastasize are clearly needed. Conversely, genetically engineered models that develop neuroendocrine carcinomas after androgen ablation therapy are needed in order to model the more common form of NePC seen in the clinic. Genetically engineered mice with truly sequential loss of function of multiple tumor suppressors (such as p53 and Rb) or gain of function of multiple oncogenes (through the use of inducible promoters) would also allow for more accurate modeling of human NePC. Given the increasing prevalence of NePC in the clinical setting, development of new models that address the limitations of the current models are of increasing urgency.
ACKNOWLEDGEMENTS
The authors thank Michael Augello for his critical review of this manuscript and Elizabeth Schade for her assistance with Figure 1.
GRANT SUPPORT
Funding for this work was provided by the following sources: NIH grant K01 OD010463 to LDBB and NIH grant R01 CA 159945 and the PA Cure Foundation to KEK.
Footnotes
DECLARATION OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES:
- Agemy L, Harmelin A, Waks T, Leibovitch I, Rabin T, Pfeffer MR & Eshhar Z 2008. Irradiation enhances the metastatic potential of prostatic small cell carcinoma xenografts. Prostate 68 530–539. [DOI] [PubMed] [Google Scholar]
- Ahuja D, Saenz-Robles MT & Pipas JM 2005. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 24 7729–7745. [DOI] [PubMed] [Google Scholar]
- Ali SH & DeCaprio JA 2001. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol 11 15–23. [DOI] [PubMed] [Google Scholar]
- Aparicio A, Tzelepi V, Araujo JC, Guo CC, Liang S, Troncoso P, Logothetis CJ, Navone NM & Maity SN 2011. Neuroendocrine prostate cancer xenografts with large-cell and small-cell features derived from a single patient’s tumor: morphological, immunohistochemical, and gene expression profiles. Prostate 71 846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher OJ & Holland EC 2006. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer Res 66 3355–3358, discussion 3358–3359. [DOI] [PubMed] [Google Scholar]
- Beltran H 2014. The N-myc Oncogene: Maximizing its Targets, Regulation, and Therapeutic Potential. Mol Cancer Res 12 815–822. [DOI] [PubMed] [Google Scholar]
- Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, et al. 2011. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 1 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G, Huang J, True L, Gleave ME, Soule H, et al. 2014. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res 20 2846–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman-Booty LD, Thomas-Ahner JM, Bolon B, Oglesbee MJ, Clinton SK, Kulp SK, Chen CS & Perle KM 2014. Extra-prostatic Transgene-associated Neoplastic Lesions in Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) Mice. Toxicol Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR & Balmain A 2008. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol 172 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg N, Ferguson C, True LD, Arnold H, Moorman A, Quinn JE, Vessella RL & Nelson PS 2003. Molecular characterization of prostatic small-cell neuroendocrine carcinoma. Prostate 55 55–64. [DOI] [PubMed] [Google Scholar]
- Deng X, Elzey BD, Poulson JM, Morrison WB, Ko SC, Hahn NM, Ratliff TL & Hu CD 2011. Ionizing radiation induces neuroendocrine differentiation of prostate cancer cells in vitro, in vivo and in prostate cancer patients. Am J Cancer Res 1 834–844. [PMC free article] [PubMed] [Google Scholar]
- Duan W, Gabril MY, Moussa M, Chan FL, Sakai H, Fong G & Xuan JW 2005. Knockin of SV40 Tag oncogene in a mouse adenocarcinoma of the prostate model demonstrates advantageous features over the transgenic model. Oncogene 24 1510–1524. [DOI] [PubMed] [Google Scholar]
- Eble JN, Sauter G, Epstein JI & Sesterhenn IA Eds 2004. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs.: IARC Press. [Google Scholar]
- Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, Robinson BD, Troncoso P & Rubin MA 2014. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 38 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C & Parkin DM 2010. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127 2893–2917. [DOI] [PubMed] [Google Scholar]
- Fine SW 2012. Variants and unusual patterns of prostate cancer: clinicopathologic and differential diagnostic considerations. Adv Anat Pathol 19 204–216. [DOI] [PubMed] [Google Scholar]
- Gabril MY, Duan W, Wu G, Moussa M, Izawa JI, Panchal CJ, Sakai H & Xuan JW 2005. A novel knock-in prostate cancer model demonstrates biology similar to that of human prostate cancer and suitable for preclinical studies. Mol Ther 11 348–362. [DOI] [PubMed] [Google Scholar]
- Gabril MY, Onita T, Ji PG, Sakai H, Chan FL, Koropatnick J, Chin JL, Moussa M & Xuan JW 2002. Prostate targeting: PSP94 gene promoter/enhancer region directed prostate tissue-specific expression in a transgenic mouse prostate cancer model. Gene Ther 9 1589–1599. [DOI] [PubMed] [Google Scholar]
- Garabedian EM, Humphrey PA & Gordon JI 1998. A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc Natl Acad Sci U S A 95 15382–15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Foster BA & Greenberg NM 1999. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis 2 70–75. [DOI] [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ & Greenberg NM 1997. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res 57 4687–4691. [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM & Greenberg NM 1996. Metastatic prostate cancer in a transgenic mouse. Cancer Res 56 4096–4102. [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ & Rosen JM 1995. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A 92 3439–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpap B & Kollermann J 1999. Undifferentiated carcinoma of the prostate with small cell features: immunohistochemical subtyping and reflections on histogenesis. Virchows Arch 434 385–391. [DOI] [PubMed] [Google Scholar]
- Helpap B, Kollermann J & Oehler U 1999. Neuroendocrine differentiation in prostatic carcinomas: histogenesis, biology, clinical relevance, and future therapeutical perspectives. Urol Int 62 133–138. [DOI] [PubMed] [Google Scholar]
- Hill R, Song Y, Cardiff RD & Van Dyke T 2005. Heterogeneous tumor evolution initiated by loss of pRb function in a preclinical prostate cancer model. Cancer Res 65 10243–10254. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ippolito JE, Garabedian EM, Humphrey PA & Gordon JI 2002. Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice. J Biol Chem 277 44462–44474. [DOI] [PubMed] [Google Scholar]
- Humphrey PA 2004. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol 17 292–306. [DOI] [PubMed] [Google Scholar]
- Humphrey PA 2012. Histological variants of prostatic carcinoma and their significance. Histopathology 60 59–74. [DOI] [PubMed] [Google Scholar]
- Huss WJ, Gregory CW & Smith GJ 2004. Neuroendocrine cell differentiation in the CWR22 human prostate cancer xenograft: association with tumor cell proliferation prior to recurrence. Prostate 60 91–97. [DOI] [PubMed] [Google Scholar]
- Irshad S & Abate-Shen C 2013. Modeling prostate cancer in mice: something old, something new, something premalignant, something metastatic. Cancer Metastasis Rev 32 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelbart ME, Russell PJ, Fullerton M, Russell P, Funder J & Raghavan D 1988. Ectopic hormone production by a prostatic small cell carcinoma xenograft line. Mol Cell Endocrinol 55 167–172. [DOI] [PubMed] [Google Scholar]
- Jelbart ME, Russell PJ, Russell P, Wass J, Fullerton M, Wills EJ & Raghavan D 1989. Site-specific growth of the prostate xenograft line UCRU-PR-2. Prostate 14 163–175. [DOI] [PubMed] [Google Scholar]
- Jongsma J, Oomen MH, Noordzij MA, Van Weerden WM, Martens GJ, van der Kwast TH, Schroder FH & van Steenbrugge GJ 1999. Kinetics of neuroendocrine differentiation in an androgen-dependent human prostate xenograft model. Am J Pathol 154 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma J, Oomen MH, Noordzij MA, Van Weerden WM, Martens GJ, van der Kwast TH, Schroder FH & van Steenbrugge GJ 2000. Androgen deprivation of the PC-310 [correction of prohormone convertase-310] human prostate cancer model system induces neuroendocrine differentiation. Cancer Res 60 741–748. [PubMed] [Google Scholar]
- Jongsma J, Oomen MH, Noordzij MA, Van Weerden WM, Martens GJ, van der Kwast TH, Schroder FH & van Steenbrugge GJ 2002. Different profiles of neuroendocrine cell differentiation evolve in the PC-310 human prostate cancer model during long-term androgen deprivation. Prostate 50 203–215. [DOI] [PubMed] [Google Scholar]
- Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA & Greenberg NM 2003. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate 55 219–237. [DOI] [PubMed] [Google Scholar]
- Kasper S, Sheppard PC, Yan Y, Pettigrew N, Borowsky AD, Prins GS, Dodd JG, Duckworth ML & Matusik RJ 1998. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest 78 319–333. [PubMed] [Google Scholar]
- Klezovitch O, Chevillet J, Mirosevich J, Roberts RL, Matusik RJ & Vasioukhin V 2004. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell 6 185–195. [DOI] [PubMed] [Google Scholar]
- Komiya A, Suzuki H, Imamoto T, Kamiya N, Nihei N, Naya Y, Ichikawa T & Fuse H 2009. Neuroendocrine differentiation in the progression of prostate cancer. Int J Urol 16 37–44. [DOI] [PubMed] [Google Scholar]
- Kwabi-Addo B, Giri D, Schmidt K, Podsypanina K, Parsons R, Greenberg N & Ittmann M 2001. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc Natl Acad Sci U S A 98 11563–11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Wyatt AW, Xue H, Wang Y, Dong X, Haegert A, Wu R, Brahmbhatt S, Mo F, Jong L, et al. 2014. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res 74 1272–1283. [DOI] [PubMed] [Google Scholar]
- Littlepage LE, Sternlicht MD, Rougier N, Phillips J, Gallo E, Yu Y, Williams K, Brenot A, Gordon JI & Werb Z 2010. Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res 70 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DM, Goodman M, Jani AB, Osunkoya AO & Rossi PJ 2012. A comprehensive review of incidence and survival in patients with rare histological variants of prostate cancer in the United States from 1973 to 2008. Prostate Cancer Prostatic Dis 15 283–288. [DOI] [PubMed] [Google Scholar]
- Maroulakou IG, Anver M, Garrett L & Green JE 1994. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci U S A 91 11236–11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumori N, Thomas TZ, Chaurand P, Case T, Paul M, Kasper S, Caprioli RM, Tsukamoto T, Shappell SB & Matusik RJ 2001. A probasin-large T antigen transgenic mouse line develops prostate adenocarcinoma and neuroendocrine carcinoma with metastatic potential. Cancer Res 61 2239–2249. [PubMed] [Google Scholar]
- Masumori N, Tsuchiya K, Tu WH, Lee C, Kasper S, Tsukamoto T, Shappell SB & Matusik RJ 2004. An allograft model of androgen independent prostatic neuroendocrine carcinoma derived from a large probasin promoter-T antigen transgenic mouse line. J Urol 171 439–442. [DOI] [PubMed] [Google Scholar]
- Mosquera JM, Beltran H, Park K, MacDonald TY, Robinson BD, Tagawa ST, Perner S, Bismar TA, Erbersdobler A, Dhir R, et al. 2013. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 15 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordzij MA, van Weerden WM, de Ridder CM, van der Kwast TH, Schroder FH & van Steenbrugge GJ 1996. Neuroendocrine differentiation in human prostatic tumor models. Am J Pathol 149 859–871. [PMC free article] [PubMed] [Google Scholar]
- Pallas DC, Shahrik LK, Martin BL, Jaspers S, Miller TB, Brautigan DL & Roberts TM 1990. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell 60 167–176. [DOI] [PubMed] [Google Scholar]
- Pearse AG 1969. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem 17 303–313. [DOI] [PubMed] [Google Scholar]
- Perez-Stable C, Altman NH, Brown J, Harbison M, Cray C & Roos BA 1996. Prostate, adrenocortical, and brown adipose tumors in fetal globin/T antigen transgenic mice. Lab Invest 74 363–373. [PubMed] [Google Scholar]
- Perez-Stable C, Altman NH, Mehta PP, Deftos LJ & Roos BA 1997. Prostate cancer progression, metastasis, and gene expression in transgenic mice. Cancer Res 57 900–906. [PubMed] [Google Scholar]
- Pinthus JH, Waks T, Schindler DG, Harmelin A, Said JW, Belldegrun A, Ramon J & Eshhar Z 2000. WISH-PC2: a unique xenograft model of human prostatic small cell carcinoma. Cancer Res 60 6563–6567. [PubMed] [Google Scholar]
- Pipas JM 2009. SV40: Cell transformation and tumorigenesis. Virology 384 294–303. [DOI] [PubMed] [Google Scholar]
- Qi J, Nakayama K, Cardiff RD, Borowsky AD, Kaul K, Williams R, Krajewski S, Mercola D, Carpenter PM, Bowtell D, et al. 2010. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell 18 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner T, de Las Pozas A, Parrondo R & Perez-Stable C 2007. Progression of prostate cancer from a subset of p63-positive basal epithelial cells in FG/Tag transgenic mice. Mol Cancer Res 5 1171–1179. [DOI] [PubMed] [Google Scholar]
- Sausville EA & Burger AM 2006. Contributions of human tumor xenografts to anticancer drug development. Cancer Res 66 3351–3354, discussion 3354. [DOI] [PubMed] [Google Scholar]
- Stein ME, Bernstein Z, Abacioglu U, Sengoz M, Miller RC, Meirovitz A, Zouhair A, Freixa SV, Poortmans PH, Ash R, et al. 2008. Small cell (neuroendocrine) carcinoma of the prostate: etiology, diagnosis, prognosis, and therapeutic implications--a retrospective study of 30 patients from the rare cancer network. Am J Med Sci 336 478–488. [DOI] [PubMed] [Google Scholar]
- Suttie AW, Dinse GE, Nyska A, Moser GJ, Goldsworthy TL & Maronpot RR 2005. An investigation of the effects of late-onset dietary restriction on prostate cancer development in the TRAMP mouse. Toxicol Pathol 33 386–397. [DOI] [PubMed] [Google Scholar]
- Tan HL, Sood A, Rahimi HA, Wang W, Gupta N, Hicks J, Mosier S, Gocke CD, Epstein JI, Netto GJ, et al. 2014. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res 20 890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani Y, Suttie A, Flake GP, Nyska A & Maronpot RR 2005. Epithelial-stromal tumor of the seminal vesicles in the transgenic adenocarcinoma mouse prostate model. Vet Pathol 42 306–314. [DOI] [PubMed] [Google Scholar]
- True LD, Buhler K, Quinn J, Williams E, Nelson PS, Clegg N, Macoska JA, Norwood T, Liu A, Ellis W, et al. 2002. A neuroendocrine/small cell prostate carcinoma xenograft-LuCaP 49. Am J Pathol 161 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzelepi V, Zhang J, Lu JF, Kleb B, Wu G, Wan X, Hoang A, Efstathiou E, Sircar K, Navone NM, et al. 2012. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clin Cancer Res 18 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaften-Day C, Raghavan D, Russell P, Wills EJ, Gregory P, Tilley W & Horsfall DJ 1987. Xenografted small cell undifferentiated cancer of prostate: possible common origin with prostatic adenocarcinoma. Prostate 11 271–279. [DOI] [PubMed] [Google Scholar]
- Wang W & Epstein JI 2008. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol 32 65–71. [DOI] [PubMed] [Google Scholar]
- Yan Y, Sheppard PC, Kasper S, Lin L, Hoare S, Kapoor A, Dodd JG, Duckworth ML & Matusik RJ 1997. Large fragment of the probasin promoter targets high levels of transgene expression to the prostate of transgenic mice. Prostate 32 129–139. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Flesken-Nikitin A, Corney DC, Wang W, Goodrich DW, Roy-Burman P & Nikitin AY 2006. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res 66 7889–7898. [DOI] [PubMed] [Google Scholar]