Abstract
The increasing prevalence of older adults living with HIV has raised growing concerns about a possible rise in the incidence of neurocognitive disorders due to HIV and other age-related factors. In typical aging, subjective cognitive impairment (SCI) among individuals with normal neurocognitive functioning may be an early manifestation of an incipient neurocognitive disorder. The current study examined the frequency and correlates of SCI in 188 HIV-infected adults without performance-based neurocognitive deficits or a current psychiatric disorder and 133 HIV seronegative comparison participants. All participants completed the Prospective and Retrospective Memory Questionnaire and Profile of Mood States Confusion/Bewilderment scale. Consistent with the diagnostic criteria proposed by Jessen et al. (2014), participants were classified with SCI if their scores on either of the self-reported measures was greater than 1.5 SD above the normative mean. A logistic regression controlling for current mood complaints and lifetime history of substance use disorders revealed that HIV infection increased the odds of SCI (odds ratio = 4.5 [1.6,15.4], p=004). Among HIV+ individuals, SCI was associated with lower performance-based learning and delayed memory scores (Cohen’s d range .41-.42.) and poorer global everyday functioning (odds ratio = 8.5 [2.6, 15.9]), but not HIV disease severity (ps>.10). In a sample of individuals without neurocognitive impairment or elevated mood symptoms, HIV disease was associated with a nearly fivefold increased odds of SCI compared to seronegative individuals, which may indicate an increased risk for developing major neurocognitive disorders as these HIV+ individuals age.
Keywords: subjective cognitive impairment, HIV, everyday functioning, neurocognitive disorders
INTRODUCTION
In the era of combined antiretroviral therapies (cART), there has been an increase in the number of older adults who are newly infected or living with HIV disease (1). The increasing age of the HIV+ population has sparked questions regarding the risk for age-related health conditions (e.g., cardiovascular disease) that can accompany longer life expectancies (2). Specifically, age-related changes in the brains of HIV+ individuals have raised concerns regarding alterations of the onset and trajectory of non-HIV-associated neurocognitive disorders (e.g., Alzheimer’s disease) (3,4). In parallel, older HIV+ adults are at increased risk for HIV-associated neurocognitive disorders (HAND) (4), which is associated with poorer everyday functioning (5), health behaviors (e.g., medication management) (6), and healthcare engagement (7). Given these clinical outcomes and changes in the prevalence and trajectory of cognitive aging, it is becoming increasingly important to detect and characterize pathological aging early in HIV disease.
Borrowing from the typically aging literature, one potentially fruitful method of detecting the early effects of neurocognitive disorders is to measure subjectively reported cognitive declines prior to the onset of objective deficits, defined as subjective cognitive impairment (SCI) (8). SCI is conceptualized as an early manifestation of incipient neurocognitive disorders (for review, see (9)). Compared to those without SCI, individuals with SCI reliably show higher rates of incident dementia (10,11) and more rapid declines in cognition (12). Additionally, among seronegative samples there is evidence that individuals with SCI have elevated rates of biomarkers associated with Alzheimer’s disease including phosphorylated tau and amyloid- β (13,14,15), supporting the notion that SCI may closely parallel biological changes that pre-date objective neurocognitive declines. Similarly, neuroimaging studies of neurocognitively normal samples have shown that individuals with SCI evidence alterations in brain activity including notable functional changes in frontal, temporal, and cerebellar networks (16,17,18). Broadly, investigations among typically aging groups support SCI as an important factor associated with pathology even in the absence of clinically detectable cognitive impairment.
The cognitive aging literature in HIV has many parallels to the seronegative SCI literature. For instance, alterations in brain structure and function of HIV+ individuals are evident despite intact neurocognitive functioning (19,20,21). In addition, HIV+ individuals show elevated rates of mild cognitive impairment (MCI) (22), which represents the most sensitive set of criteria for objectively observable early cognitive impairment. In fact, the timeline of pathological aging associated with dementia, involving progressive stages starting with biomarker accumulation and brain atrophy with subsequent cognitive decline and clinical diagnoses (23) may parallel the aging trajectory seen in HIV disease (24,25). Another reason for examining early signs of pathology in HIV center around questions of accelerated aging trajectories in HIV-infected persons (26,27,28). For example, younger HIV+ individuals demonstrate rates of HAND similar to the rates of neurocognitive disorders observed in much older seronegative samples (29,30), possibly indicating accelerated neurocognitive aging (31). However, investigations into early signs of aging in HIV have predominantly focused on the manifest diagnostic aspects of HAND, such as Asymptomatic Neurocognitive Disorder (ANI) (32). As such, applying SCI criteria to an HIV sample is a logical next step for assessing risk of neurocognitive disorders earlier in the aging trajectory in this population.
Previous studies of cognitive complaints in HIV have shown mixed findings, with some reporting higher rates of subjectively reported cognitive symptoms in HIV disease (33) and others indicating no serostatus differences (34). However, many studies that have reported elevated rates of subjectively reported cognitive problems in HIV have failed to fully account for important cofactors that might otherwise explain such complaints, such as mood disorder or objective neurocognitive impairment. Notably, SCI research criteria put forth by Jessen et al. (8) require consideration of objective cognitive impairment and mood symptoms. It is important to control for objective neuropsychological performance and mood variables (e.g., depression) when investigating SCI since these factors have been shown to increase reporting of cognitive (35) and everyday functioning (36) symptoms, which may bias estimates of subjective impairment. To date, no investigation has applied newly developed diagnostic criteria for subjective cognitive impairment to address these issues among individuals infected with HIV.
The present study aims to extend previous findings by examining rates of SCI in a large sample of HIV+ and HIV− comparison participants who have been screened for objective neurocognitive disorders and current mood disorders. SCI will be classified as having selfreported problems as measured by standardized, normatively-adjusted measures of memory and global cognition. In our first aim, it is hypothesized that HIV+ individuals will have significantly higher rates of SCI compared to HIV− individuals. As a secondary aim, we hypothesize that SCI will be related to important outcomes of everyday functioning (e.g., mild declines in activities of daily living) within the HIV+ group while controlling for mood, objective neuropsychological performance, and disease severity. Our final aim is to explore possible clinicodemographic and objective neuropsychological performance variables that relate to SCI within the HIV+ group, wherein we hypothesize that global neuropsychological performance will be significantly related to SCI among individuals with HIV disease, while no significant associations are expected between SCI and demographic or HIV disease severity variables.
METHOD
Participants
The study sample included 321 participants aged 18 years and older who were recruited from the San Diego community and local HIV clinics with the aim of recruiting seronegative participants with comparable rates of non-HIV-related characteristics (e.g., hepatitis C virus). HIV serostatus for 188 HIV-seropositive (HIV+) and 133 HIV-seronegative comparison individuals (HIV-) was confirmed using standard Western blot and/or a point-of-care test (MedMira Inc., Nova Scotia, Canada). Participants were excluded from this study if they had a current diagnosis of Major Depressive Disorder (MDD), Generalized Anxiety Disorder, or current substance abuse or dependence per the Diagnostic and Statistical Manual of Mental Disorders (37) as operationalized by the Composite International Diagnostic Interview, version 2.1. Additionally, participants were excluded if on the day of testing they had a Breathalyzer test positive for alcohol, or a urine toxicology screening test that was positive for illicit drugs (except for marijuana). Using methods described previously (see (38) and (22)), participants were also excluded if they met criteria for neurocognitive impairment per the Frascati criteria for HIV-associated neurocognitive disorders (HAND; see (39)) or mild cognitive impairment (MCI; see (22)). Additional exclusion criteria consisted of self-reported psychotic (e.g., schizophrenia) or neurological disease commonly associated with cognitive impairment (e.g., traumatic brain injury with a loss of consciousness > 5 min, seizure disorder, multiple sclerosis), or an estimated verbal IQ lower than 70 (as determined by the Wechsler Test of Adult Reading).
Materials and Procedure
After providing written, informed consent, all participants completed psychiatric, neuropsychological, and standardized medical research evaluations, for which they received nominal financial compensation.
Subjective Cognitive Impairment Measures.
All participants were administered the Prospective and Retrospective Memory Questionnaire (PRMQ) (40) and the Profile of Mood States (POMS) (41), from which designations of SCI were formulated.
Prospective and Retrospective Memory Questionnaire.
The PRMQ is a 16-item, self-report inventory that measures the frequency with which perceived memory difficulties occur in everyday life on a 5-point Likert-type scale that ranges from 1 (“never”) to 5 (“very often”). The PRMQ includes eight retrospective memory (e.g., “How often do you forget something that you were told a few minutes before?”) and eight prospective memory (e.g., “How often do you forget appointments if you are not prompted by someone else or by a reminder, such as a diary or a calendar?”) items. The current study utilized normative data published by Crawford et al. (42), which provides population-based normative T-scores based on raw PRMQ total scores. Note that, age and gender were not associated with PRMQ total scores in the Crawford et al. (42) normative sample or in the current study sample (ps > .05). Prior research supports the internal consistency (Cronbach’s alphas ≥.80), factor structure (42), and predictive validity (40,43) of the PRMQ.
Profile of Mood States.
The POMS is a 65-item self-report evaluation of current mood states, in which participants rate various adjectives (e.g., “forgetful”) on a 5-point Likert-type scale ranging from 0 (not at all) to 4 (extremely) for the week prior to evaluation. For this study, the Confusion/Bewilderment subscale (7 items; score range 0–28) was used as an index of subjective cognitive complaints (e.g., “Forgetful,” “Can’t Concentrate,” and “Confused”). The POMS Confusion/Bewilderment subscale has adequate psychometric properties (41) and has been utilized previously as an individual measure of self-reported cognitive difficulties in HIV disease (44). Raw Confusion/Bewilderment subscale scores were converted to normatively adjusted z-scores which are corrected for age and gender (41). Additionally, a composite score of the 5 non-cognitive POMS subscales (i.e., tension/anxiety, depression/dejection, anger/hostility, vigor/activity, fatigue/intertia) was computed by calculating a sample-z score derived from the sum of these 5 raw subscale scores. This non-cognitive POMS composite was intended as a covariate indexing overall level of current self-reported distress (e.g., “other complaints”). This non-cognitive POMS composite was intended as a covariate indexing overall level of current self-reported distress (e.g., “other complaints”).
Subjective Cognitive Impairment.
SCI was defined as having a normed z-score on either the POMS Confusion/Bewilderment subscale or the PRMQ falling > 1.5 standard deviations above the normative mean. These SCI criteria are consistent with those proposed by Jessen et al. (8), whereby either or both scores may be impaired to warrant a diagnosis of SCI. We elected to use a 1.5 SD cutoff as our primary outcome threshold to parallel commonly suggested clinically significant cutoffs for other aging/disease populations (e.g., Parkinson’s disease) (45). As shown in Table I, across the entire study sample (N=321) the median normative T-score (higher scores representing more cognitive complaints) for the PRMQ was 43 (25th% = 37, 75th% = 51) and for the POMS confusion/bewilderment scale was 46 (41, 55). The two individual SCI measures were strongly correlated (rs = .59, p < .001). Impairment rates on the two scales were broadly comparable at each respective level of impairment (see Table I), although McNemar’s tests of homogeneity revealed that POMS and PRMQ scales were statistically inconsistent at a 1.0 SD cutoff (χ2 = 5.12; p = .024), but consistent using the 1.5 SD (χ2 = 0.62; p = .433).
Table I.
Median (quartile) raw and normative scores, impairment rates, and correlations of Prospective and Retrospective Memory Questionnaire (PRMQ) and Profile of Mood States (POMS) in the entire study sample of HIV+ and HIV− individuals (N=321).
| Variable | PRMQ | POMS C/B |
|---|---|---|
| Total Raw Score (of 80 PRMQ, of 28 POMS C/B) | 33 (27,40) | 4 (2,8) |
| Normative T-Score | 43 (37,51) | 46 (41,55) |
| Impairment Rates (% of sample) | ||
| > 1.0 | 10.4 | 14.3 |
| > 1.5 | 7.5 | 8.7 |
| rs correlation raw (p-value) | .59 (< .001) | |
| rs correlation T-Score (p-value) | .59 (< .001) | |
Note. Data represent median (25th percentile, 75th percentile) or valid population percentage values. Higher raw and T-Scores scores indicate more cognitive complaints on each respective scale. POMS = Profile of Mood States Confusion/Bewilderment (C/B) Subscale; PRMQ = Prospective and Retrospective Memory Questionnaire.
rs = correlation between PRMQ and POMS scores
Manifest Everyday Functioning.
To examine the association between SCI and everyday functioning, a dichotomous (dependent, independent) composite score was computed using four domains of everyday functioning. Dependence was defined as 2 or more of the following everyday functioning domains impaired: (1) unemployment (not due to elective retirement), (2) ≥ 2 endorsed declines in instrumental activities of daily living (iADLs) on the Heaton modification of the Lawton and Brody (46,47) ADL Scale (i.e., finance management, purchasing groceries, cooking, using transportation, shopping, medication management, and social activity planning; (3) ≥ 2 endorsed declines in basic activities of daily living (bADLs) on the Heaton modification of the Lawton and Brody ADL scale (i.e., housekeeping/cleaning, laundry, home repairs, bathing, and dressing); or (4) scores < 90 on the Karnofsky Scale of Performance Status (48). This approach has been applied previously in HIV samples (5).
Neuropsychological Evaluation.
Participants were administered a comprehensive neuropsychological evaluation to examine neurocognitive correlates of SCI among HIV+ individuals and to serve as an a priori covariate for the everyday functioning analyses. Domains and associated measures included: (1) delayed memory, Logical Memory II subtest from the Wechsler Memory Scale, 3rd edition (WMS-III) (49) and the long-delay free recall trial of the California Verbal Learning Test, 2nd edition (CVLT-II) (50); (2) learning, Logical Memory I subtest from the WMS-III and trials 1–5 on the CVLT-II; (3) executive functions, Trailmaking Test, Part B (51) and the total moves score from the Tower of London Test (Drexel Version) (52); (4) attention, WMS-III Digit Span and CVLT-II Trial 1; (5) speed of information processing, Trailmaking Test, Part A and the Total Execution Time from the Tower of London Test; and (6) motor skills, dominant and nondominant hand time to completion on Grooved Pegboard (53). The two normatively-adjusted T-scores within each domain were averaged to create six composite domain T-scores. In addition, a global neuropsychological T-score was derived using the average of all 12 neuropsychological measures, which was included as a covariate for analyses of everyday functioning outcomes.
Statistical Analyses
The primary analysis was conducted using a logistic regression with HIV serostatus as the independent variable (i.e., predictor) and SCI status as the dependent variable (i.e., criterion). Any variable listed in Table I was included as a covariate in the primary logistic regression if it: 1) significantly differed between HIV+ and HIV− groups; and 2) was also significantly related to the outcome of SCI in the entire sample using a critical alpha of .05. In addition, given the possibility that general over-reporting of symptoms/complaints could otherwise explain elevated rates of SCI in HIV, we decided a priori to include the POMS non-cognitive complaints summary variable as a covariate. For our second aim, we conducted a logistic regression within the HIV+ group examining SCI as the primary predictor and the everyday functioning composite as the criterion variable. Drawing from the existing literature, this model included a priori selected covariates of lifetime MDD, global mean neuropsychological test T scores, and AIDS status. Planned post hoc analyses of the association between SCI and individual domains comprising the manifest everyday functioning domain were performed using individual chi-square tests. Finally, we examined possible demographic, objective neuropsychological performance, mood, medical, and disease severity variables to determine whether important clinicodemographic and objective neuropsychological performance variables relate to SCI within the HIV+ group using individual one-way ANOVA or Wilcoxon rank sum tests (for non-normally distributed variables).
RESULTS
Clinicodemographic Differences Across HIV Serostatus Groups
As shown in Table II, the HIV+ group was relatively older, contained a higher proportion of men, reported fewer years of education, obtained lower global neuropsychological T-scores, and higher rates of lifetime histories of GAD, MDD, substance use disorder, and hepatitis c virus (all ps < .05; see Table II). Among these variables, only lifetime MDD and substance use disorder were also significantly related to SCI. Since it was determined a priori to include an additional covariate of generic complaints on the POMS, we elected to include only POMS non-cognitive complaints scores as a measure of current mood complaints in lieu of the historical diagnosis of MDD. Nevertheless, including lifetime MDD instead of POMS non-cognitive complaints in the primary model did not change the overall pattern of findings or magnitude of effect sizes. As such, the logistic regression included covariates of lifetime substance use disorder and POMS non-cognitive complaints.
Table II.
Demographic and clinical information for HIV− and HIV+ groups
| Variable | HIV−(n = 133) | HIV+(n = 188) |
|---|---|---|
| Age (years)* | 43.1 (1.3) | 46.1 (0.8) |
| Gender (% men)* | 68.4 | 87.2 |
| Education (years)* | 14.5 (0.2) | 13.7 (0.2) |
| Ethnicity | ||
| Caucasian (%) | 59.4 | 62.2 |
| African American (%) | 20.3 | 19.7 |
| Hispanic (%) | 16.5 | 14.9 |
| Other (%) | 3.8 | 3.2 |
| Estimated verbal IQ (WTAR) | 106.4 (0.8) | 105.5 (0.7) |
| Neuropsychological Global T-score* | 54.0 (0.4) | 52.7 (0.3) |
| HIV Dementia Scale (of 16)♮ | 15.0 (0.1) | 14.6 (0.2) |
| Major Depressive Disordera (%)* | 30.1 | 50.0 |
| Generalized Anxiety Disordera (%)* | 3.0 | 11.7 |
| Substance Use Disorderb (%)* | 54.1 | 71.8 |
| POMS Depression/Dejection Scale (of 60) Medical | 6.1 (0.7) | 6.7 (0.6) |
| Hepatitis C Virus (%)* | 8.5 | 19.7 |
| Estimated duration of HIV infection (years) | -- | 13.5 (0.6) |
| Current CD4 count (cells/μ L) | -- | 560.5 (20.8) |
| Nadir CD4 count (cells/μ L) | -- | 207.2 (12.5) |
| AIDS (%) | -- | 56.4 |
| ARV status (% prescribed) | -- | 84.6 |
| Plasma HIV RNA (% detectable) | -- | 27.3 |
| Among those prescribed ARV | -- | 16.7 |
Note. Data represent mean (standard error) or valid population percentage values. WTAR = Wechsler Test of Adult Reading; POMS = Profile of Mood States; HCV = hepatitis C virus; CD4 = cluster of differentiation; AIDS = acquired immunodeficiency syndrome; ARV = antiretroviral.
Lifetime diagnosis.
Any lifetime diagnosis of dependence or abuse of alcohol or illicit substances.
p < .05
p < .10
SCI Rates Across HIV Serostatus Groups
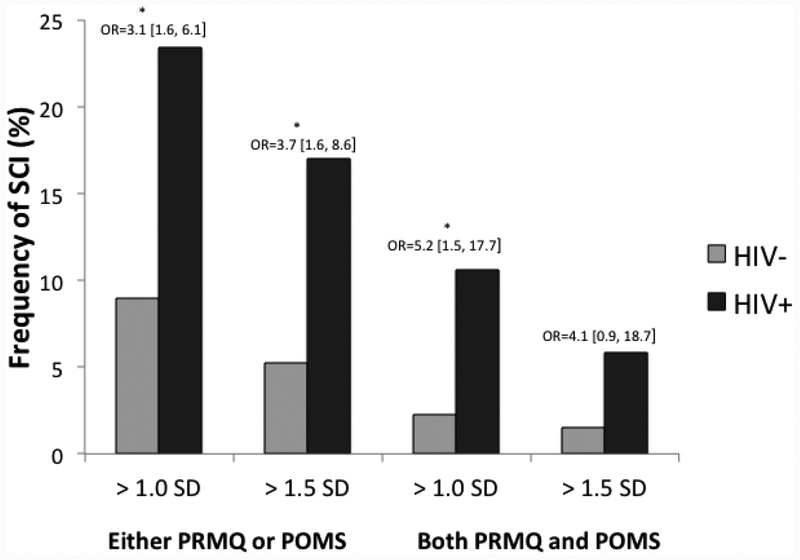
The primary logistic regression with HIV serostatus (HIV+, HIV-) predicting SCI controlling for lifetime substance use disorder and POMS non-cognitive complaints was significant, χ2(3) = 95.83, p < .001. In this model, HIV serostatus (χ2(1) = 8.17, p = .004, odds ratio = 4.5 [1.6, 15.4]), non-cognitive complaints (χ2(1) = 81.68, p < .001, unit OR = 6.8 [4.1, 12.7]), and lifetime substance use disorder (χ2(1) = 8.2, p = .004, odds ratio = 4.6 [1.6, 15.9]) were all significant independent predictors of SCI. Specifically, HIV+ persons, individuals with more severe non-cognitive complaints and those with lifetime histories of substance use disorder were more likely to meet criteria for SCI. Figure 1 shows that the univariate (unadjusted) rates of SCI were significantly higher among the HIV+ compared to HIV− individuals across all of the criteria used to define SCI (all ps < .05, OR range 3.1 – 5.2) with the exception of both PRMQ and POMS impaired at 1.5 SD, which fell at the level of a trend (χ2(1) = 4.3, p = .081, odds ratio = 4.1 [0.9, 18.7]).
Figure 1.
Frequency of Subjective Cognitive Impairment (SCI) in HIV+ (n=188) and HIV− (n=133) individuals as determined by different approaches to classification using the Prospective and Retrospective Memory Questionnaire (PRMQ Total) and Profile of Mood States (POMS Confusion/Bewilderment; age- and gender-adjusted). Odds ratios are unadjusted at the univariate level.
Everyday Functioning Associations with SCI in HIV
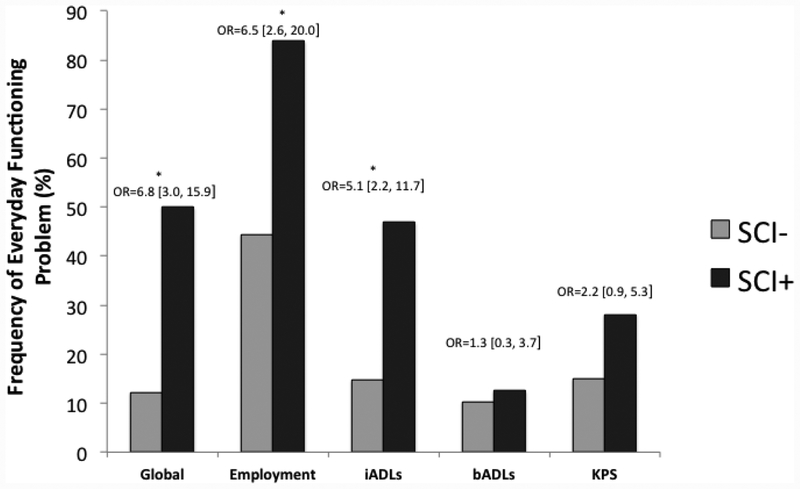
As displayed in Figure 2, within the HIV+ group, the overall logistic regression with the dichotomous composite score of impairment in everyday functioning predicted by SCI, lifetime MDD, global neuropsychological T-scores, and AIDS status was significant, χ2(4) = 40.8, p < .001. Within this model, SCI was significantly related to everyday functioning dependence (χ2(1) = 23.18, p < .001, odds ratio = 8.5 [3.5, 21.8]). Lifetime MDD (χ2(1) = 4.4, p = .036, odds ratio = 2.3 [1.1, 5.1]) and AIDS status (χ2(1) = 10.2, p = .001, odds ratio = 3.7 [1.6, 9.1]) also were significant independent predictors of everyday functioning dependence. Post hoc pair-wise chi-square comparisons of each dichotomous outcome revealed that individuals with SCI were more likely to be unemployed (χ2(1) = 17.4, p < .001, odds ratio = 6.5 [2.4, 17.8]) and have declines in instrumental activities of daily living (χ2(1) = 14.5, p < .001, odds ratio = 5.1 [2.2, 11.6]). SCI was not related to basic activities of daily living or Karnofsky performance scores (ps > .10).
Figure 2.
Rates of everyday functioning impairment for a global composite of everyday functioning, unemployment, instrumental activities of daily living (iADLs), basic activities of daily living (bADLs), and Karnofsky Performance Status (KPS) for individuals with and without subjective cognitive impairment (SCI) within the HIV+ sample (n=188). Odds ratios are unadjusted at the univariate level.
Note. Global = Impairment on 2 or everyday functioning domains (unemployment, iADLs, bADLs, KPS); iADLs = instrumental activities of daily living (e.g., financial and medication management), operationalized as declines on > 2 activities; bADLs = basic activities of daily living (e.g., bathing and dressing), operationalized as declines on ≥ 2 activities; KPS = Karnofsky performance status (i.e., range 0 to 100 clinician rating of overall functioning), operationalized as score < 90.
Neuropsychological, clinicodemographic, and medical associations of SCI in HIV
Neuropsychological Associations with SCI.
As seen in Table III, SCI was associated with lower performance-based learning T-scores (F(1,186) = 5.23, p = .023, d = .42) and delayed memory T-scores (F(1,186) = 4.61, p = .033, d = .41) among HIV+ individuals. SCI was not associated with any other neuropsychological variables (all ps > .10, all d’s < .35).
Table III.
Mean (standard error) demographic, neurocognitive, and medical outcomes for HIV+ individuals with subjective cognitive decline (SCI) and without SCI as defined by either Profile of Mood States (POMS) or Prospective and Retrospective Memory Questionnaire (PRMQ) impaired at 1.5 SD cutoffs.
| Variable | SCI (n = 32) | No SCI (n = 156) | P | Cohen’s d |
|---|---|---|---|---|
| Age (years) | 45.0 (1.9) | 46.3 (0.9) | .708 | .12 |
| Gender (% men) | 81.3 | 88.5 | .287 | .30 |
| Education (years) | 13.3 (0.4) | 13.8 (0.2) | .535 | .22 |
| Ethnicity (% Caucasian) | 50.0 | 64.7 | .160 | .13 |
| Estimated verbal IQ (WTAR) | 105.4 (1.9) | 105.6 (0.8) | .940 | .02 |
| Neuropsychological Global T-score | 52.0 (0.8) | 52.9 (0.4) | .212 | .20 |
| Learning T-score* | 51.9 (1.6) | 55.5 (0.6) | .023 | .42 |
| Memory T-score* | 51.6 (1.5) | 54.8 (0.6) | .025 | .41 |
| Attention T-score | 50.2 (1.4) | 50.5 (0.6) | .684 | .04 |
| Speed of Information Processing T-score | 52.6 (1.2) | 52.2 (0.5) | .769 | .06 |
| Executive Functions T-score | 52.9 (1.2) | 54.3 (0.6) | .311 | .20 |
| Motor Dexterity T-score | 52.6 (1.3) | 49.8 (0.7) | .112 | .34 |
| Major Depressive Disordera (%)♮ | 65.6 | 46.8 | .080 | .42 |
| Generalized Anxiety Disordera (%) | 15.6 | 10.9 | .544 | .22 |
| Substance Use Disordera (%)* Medical | 90.6 | 68.0 | .009 | .84 |
| Hepatitis C Virus (%) | 17.2 | 20.3 | .716 | .10 |
| Estimated duration of HIV infection (years) | 147.7 (16.3) | 164.3 (8.0) | .383 | .18 |
| Current CD4 count (cells/μL) | 536.9 (51.3) | 565.3 (22.8) | .579 | .10 |
| Nadir CD4 count (cells/μL) | 212.4 (24.9) | 206.1 (14.2) | .466 | .04 |
| AIDS (%) | 62.5 | 55.1 | .441 | .16 |
| ARV status (% prescribed) | 78.1 | 85.9 | .286 | .29 |
| Plasma HIV RNA (% detectable) | 25.8 | 27.6 | .834 | .05 |
| Among those prescribed ARV | 8.0 | 18.3 | .171 | .52 |
Note. Data represent mean (standard error) or valid population percentage values. Cohen’s d values for binary outcomes represent effect sizes converted from odds ratios. WTAR = Wechsler Test of Adult Reading; MIST = Memory for Intentions Screening Test; POMS = Profile of Mood States; HCV = hepatitis C virus; CD4 = cluster of differentiation; AIDS = acquired immunodeficiency syndrome; ARV = antiretroviral; iADL = instrumental activities of daily living; bADL = basic activities of daily living.
Lifetime diagnosis.
Any lifetime diagnosis of dependence on alcohol or illicit substances.
p < .05
p < .10
Demographics Associated with SCI.
Within the HIV+ group, SCI was not significantly associated with any demographic variables including age, gender, education, ethnicity, and estimated verbal IQ (all ps > .10, see Table III).
Clinical and Medical Associations with SCI.
Within the HIV+ group, SCI was associated with a previous lifetime history of substance use disorder (χ2(1) = 8.0, p = .005, odds ratio = 4.6 [1.3, 15.7]) and with lifetime MDD at a trend level (χ2(1) = 3.8, p = .080, odds ratio = 2.2 [0.9, 4.8]), but not with lifetime GAD (χ2(1) = 0.5, p = .544, odds ratio = 1.5 [0.5, 4.5]). SCI was not significantly associated with hepatitis C virus or any HIV disease severity variables (all ps > .10).
DISCUSSION
The aims of the current study were to: (1) determine whether HIV+ individuals demonstrate elevated rates of subjectively rated neurocognitive impairment compared to seronegatives, (2) investigate the association between SCI and manifest everyday functioning outcomes within HIV+ persons, and (3) explore possible clinicodemographic and objective neuropsychological performance variables that might relate to SCI within an HIV+ sample. Results indicated that when applying SCI research criteria to a sample without current neurocognitive impairments or mood disorders, HIV+ individuals evidenced rates of SCI that were nearly five times higher than those of HIV− individuals. These positive findings were observed despite our restrictive exclusion criteria and a conservative approach to including covariates (e.g., including non-cognitive complaints); moreover, elevated rates of SCI were apparent in HIV using several different clinical cutoffs. Among HIV+ individuals, SCI was associated with deficits in everyday functioning and objective neuropsychological functioning in the domains of learning and memory, but not HIV disease severity. Taken together, findings highlight a subset of the HIV+ population that may be at increased risk for developing major neurocognitive disorders as they continue to age (10, 11, 12).
The current results indicate that HIV+ individuals show higher rates of clinically elevated cognitive symptoms in everyday life, despite the absence of clinically detectable impairment on objective testing. Given our exclusion of current mood disorders and use of non-cognitive complaints as a covariate, these findings suggest that HIV-associated self-perceived cognitive deficits were not simply an artifact of negative affect, which can significantly affect the accuracy of self-reports (35). Results are particularly important given that SCI is a risk factor for future declines and incident major neurocognitive disorders in typically aging samples (10,11). As such, HIV+ individuals with SCI may be at increased risk for future declines, although additional studies are obviously needed to examine the predictive utility of SCI in estimating risk of incident HAND. HIV+ individuals with SCI represent cases that may have meaningful alterations in their everyday cognition, but are able to adequately harness their cognitive resources to perform normally in the laboratory; thus, these individuals might be placed into “neurocognitively normal” groups in most research studies using traditional HAND diagnostic criteria. In the current study, SCI was associated with moderately poorer objective neuropsychological performance in the domains of verbal learning and memory (i.e., list and story recall) among HIV+ individuals, indicating that SCI criteria may be capturing not only perceived problems in everyday cognitive performance but also subtle changes in actual neurocognitive performance that are below clinical thresholds. To this end, recently proposed criteria for HAND have suggested consideration of self-reported impairments (DSM-5) (54), which indeed significantly alter rates of HAND in HIV+ samples (38). Taken together, these findings suggest that SCI warrants consideration in future studies of HIV and neurocognition.
The present study also extends findings from previous applications of diagnostic schemes borrowed from the typically aging literature aimed at addressing questions of cognitive aging in HIV. For example, HIV+ individuals are at sevenfold greater odds of having Mild Cognitive Impairment (22), which is a prodromal major cognitive disorder commonly associated with progression to dementia (e.g., Alzheimer’s disease) in typically aging samples. The current application of SCI criteria to an HIV sample is the next logical investigation into earlier stages of cognitive aging in HIV by demonstrating that perceived neurocognitive changes are also frequent in HIV disease, even prior to those detected even by the most sensitive available neurocognitive criteria (i.e., MCI). Current findings address growing concerns about how HIV+ individuals may have increased risk for HAND or non-HIV-associated neurocognitive disorders as they age (55), and indicate that a risk factor for dementia found in typically aging populations is indeed elevated among HIV+ individuals. Though it remains to be seen whether SCI is a precursor to HAND or to non-HIV-associated neurocognitive disorders (or just a false alarm). Additionally, the 17% rate of SCI among this HIV+ sample in their 40s and 50s is almost identical to rates of SCI found among typically aging adults in their 60s and 70s (10). Thus, the current findings support the notion that HIV+ individuals may experience accelerated rates of neurocognitive aging even among relatively younger samples (28,31). Given previous calls for examination into possibly accelerated pathological and cognitive aging in HIV (28), the current data indicate that subtle self-perceived cognitive difficulties may exist at rates commensurate with those seen in much older neurocognitively intact typically aging samples (see also (22)).
Importantly, HIV-associated SCI is not benign in terms of its clinical impact. In this study, elevated self-reported cognitive symptoms showed a robust association with daily living outcomes (e.g., declines in instrumental activities of daily living). It is notable that the relationship between SCI and dependence in everyday functioning was independent of mood, disease severity, and objective neurocognitive performance. SCI was specifically related to higher rates of unemployment and self-reported declines in instrumental (but not basic) activities of daily living. The association with unemployment is particularly notable since it is an external indicator of everyday functioning problems that does not have shared-methods with the diagnosis of SCI (i.e., self-report). In fact, SCI was associated with an over six-fold increased risk for being unemployed, and a substantive majority (85%) of HIV+ individuals with SCI were unemployed. It therefore appears that SCI may be a particularly important predictor of employment status in the setting of neurocognitive normality among HIV+ individuals. These findings may also give credence to previous literature implicating high rates of unemployment in the HIV population (e.g., 45% unemployment rate) as a possible risk factor for cognitive dysfunction due to reduced cognitive reserve over the lifespan (for review, see 56). Considering that older adults with HAND are at disproportionate risk for having impairments in everyday functioning (5) and employment (57), identification of novel predictors of everyday functioning (such as SCI) will become increasingly important as the HIV+ population ages (58). Future work in SCI may wish to examine its associations with performance-based measures of functional capacity, health behaviors (e.g., medication adherence), and healthcare engagement. Such inquiries may identify individuals who would benefit from either compensatory strategy training programs (e.g., for individuals with self-reported everyday functioning deficits) versus cognitive rehabilitation training (e.g., for those with SCI).
Several study limitations warrant discussion. One of the primary limitations of this study is the relatively strict exclusion criteria of current mood disorders, which may reduce the generalizability of the current findings to the nearly half of persons living with HIV who are actively anxious or depressed (59). While the conservative nature of these exclusion criteria allowed us to more carefully assess the correspondence between HIV disease and SCI independent of comorbid factors, future studies may attempt to characterize and define SCI in the setting of MDD. Furthermore, our diagnoses of SCI used subjective complaints of global cognition (POMS confusion/bewilderment) or declarative memory (PRMQ), which limits inferences made into how specific non-memory cognitive domains (e.g., executive dysfunction) might contribute to SCI in HIV (60). Relatedly, the POMS and PRMQ both ask participants to rate their current symptoms which does not explicitly assess declines from previous levels of functioning as proposed by Jessen et al. (8). Although our use of normatively-adjusted scores on these SCI measures partially helps to somewhat circumvent this limitation, future studies should include items that assess for declines from previous highest levels of functioning across both memory and non-memory domains. Finally, future studies would benefit from examining possible biomarker or imaging correlates of SCI within HIV disease, which may lend further support to the hypothesis that SCI parallels neurobiological changes prior to the onset of objectively observable declines.
ACKNOWLEDGEMENTS
The authors have no financial conflicts of interest related to this work. This study was supported by NIH grants R01-MH073419 and P30-MH62512. The authors are grateful to the UC San Diego HIV Neurobehavioral Research Program (HNRP) Group (I. Grant, PI) for their infrastructure support of the parent R01. In particular, we thank Donald Franklin, Dr. Erin Morgan, Clint Cushman, and Stephanie Corkran for their assistance with data processing, Marizela Verduzco for her assistance with study management, Drs. Scott Letendre and Ronald J. Ellis for their assistance with the neuromedical aspects of the parent project, and Dr. J. Hampton Atkinson and Jennifer Marquie Beck and their assistance with participant recruitment and retention. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The authors thank the study volunteers for their participation.
Funding:
This study was funded by NIH grants R01-MH073419 and P30-MH62512.
Footnotes
Conflict of Interest:
David P. Sheppard declares that he has no conflict of interest. Steven Paul Woods declares that he has no conflict of interest. Paul J. Massman declares that he has no conflict of interest. Paul E. Gilbert declares that he has no conflict of interest.
Ethical approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent:
Informed consent was obtained from all individual participants included in the study.
References
- 1). Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem van A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015. July;15(7):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Blanco JR, Jarrin I, Vallejo M, Berenguer J, Solera C, Rubio R, et al. Definition of advanced age in HIV infection: looking for an age cut-off. AIDS Res Hum Retroviruses. 2012. September;28(9):1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Alisky JM. The coming problem of HIV-associated Alzheimer’s disease. Med Hypotheses. 2007;69(5):1140–3. [DOI] [PubMed] [Google Scholar]
- 4). Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004. September 14;63(5):822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Morgan EE, Iudicello JE, Weber E, Duarte NA, Riggs PK, Delano-Wood L, et al. Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr. 2012. November 1;61(3):341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, et al. Medication and finance management among HIV-infected adults: the impact of age and cognition. J Clin Exp Neuropsychol. 2011. February;33(2):200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Jacks A, Wainwright DA, Salazar L, Grimes R, York M, Strutt AM, et al. Neurocognitive deficits increase risk of poor retention in care among older adults with newly diagnosed HIV infection. AIDS. 2015. August 24;29(13):1711–4. [DOI] [PubMed] [Google Scholar]
- 8). Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014. November;10(6):844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Studart A, Nitrini R. Subjective cognitive decline: The first clinical manifestation of Alzheimer’s disease? Dement Neuropsychol. 2016. September;10(3):170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014. December;130(6):439–51. [DOI] [PubMed] [Google Scholar]
- 11). Ronnlund M, Sundstrom A, Adolfsson R, Nilsson L-G. Subjective memory impairment in older adults predicts future dementia independent of baseline memory performance: Evidence from the Betula prospective cohort study. Alzheimers Dement. 2015. November;11(11):1385–92. [DOI] [PubMed] [Google Scholar]
- 12). Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010. January;6(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Hessen E, Nordlund A, Stalhammar J, Eckerstrom M, Bjerke M, Eckerstrom C, et al. T-Tau is Associated with Objective Memory Decline Over Two Years in Persons Seeking Help for Subjective Cognitive Decline: A Report from the Gothenburg-Oslo MCI Study. J Alzheimers Dis. 2015;47(3):619–28. [DOI] [PubMed] [Google Scholar]
- 14). Hessen E, Eckerstrom M, Nordlund A, Selseth Almdahl I, Stalhammar J, Bjerke M, et al. Subjective Cognitive Impairment Is a Predominantly Benign Condition in Memory Clinic Patients Followed for 6 Years: The Gothenburg-Oslo MCI Study. Dement Geriatr Cogn Dis Extra. 2017. April;7(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012. February;69(2):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch Gen Psychiatry. 2011. August;68(8):845–52. [DOI] [PubMed] [Google Scholar]
- 17). Hohman TJ, Beason-Held LL, Lamar M, Resnick SM. Subjective cognitive complaints and longitudinal changes in memory and brain function. Neuropsychology. 2011. January;25(1):125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Rodda JE, Dannhauser TM, Cutinha DJ, Shergill SS, Walker Z. Subjective cognitive impairment: increased prefrontal cortex activation compared to controls during an encoding task. Int J Geriatr Psychiatry. 2009. August;24(8):865–74. [DOI] [PubMed] [Google Scholar]
- 19). Becker JT, Bajo R, Fabrizio M, Sudre G, Cuesta P, Aizenstein HJ, et al. Functional connectivity measured with magnetoencephalography identifies persons with HIV disease. Brain Imaging Behav. 2012. September;6(3):366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, et al. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol. 2009. March;65(3):316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013. March 26;80(13):1186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Sheppard DP, Iudicello JE, Bondi MW, Doyle KL, Morgan EE, Massman PJ, et al. Elevated rates of mild cognitive impairment in HIV disease. J Neurovirol. 2015. October;21(5):576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Lee SL, Thomas P, Fenech M. Genome instability biomarkers and blood micronutrient risk profiles associated with mild cognitive impairment and Alzheimer’s disease. Mutat Res. 2015. June;776:54–83. [DOI] [PubMed] [Google Scholar]
- 24). Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005. March 4;19(4):407–11. [DOI] [PubMed] [Google Scholar]
- 25). Cohen RA, Seider TR, Navia B. HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther. 2015;7(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Goodkin K, Miller EN, Cox C, Reynolds S, Becker JT, Martin E, et al. Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV. 2017;4(9):e411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Kuhn T, Kaufmann T, Doan NT, Westlye LT, Jones J, Nunez RA, et al. An augmented aging process in brain white matter in HIV. Hum Brain Mapp. 2018. February 27; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014. July;69(7):833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Woods SP, Dawson MS, Weber E, Grant I, HIV Neurobehavioral Research Center (HNRC) Group. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. J Clin Exp Neuropsychol. 2010. April;32(4):398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Avci G, Loft S, Sheppard DP, Woods SP, HIV Neurobehavioral Research Program (HNRP) Group. The effects of HIV disease and older age on laboratory-based, naturalistic, and selfperceived symptoms of prospective memory: does retrieval cue type and delay interval matter? Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2016;23(6):716–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Sheppard DP, Iudicello JE, Morgan EE, Kamat R, Clark LR, Avci G, et al. Accelerated and accentuated neurocognitive aging in HIV infection. J Neurovirol. 2017. June;23(3):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 2014. November;13(11):1139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004. January 1;18 Suppl 1 :S11–18. [PubMed] [Google Scholar]
- 34). Kamkwalala A, Hulgan T, Newhouse P. Subjective memory complaints are associated with poorer cognitive performance in adults with HIV. AIDS Care. 2017. May;29(5):654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Vance DE, Ross LA, Downs CA. Self-reported cognitive ability and global cognitive performance in adults with HIV. J Neurosci Nurs. 2008. February;40(1):6–13. [DOI] [PubMed] [Google Scholar]
- 36). Blackstone K, Moore DJ, Heaton RK, Franklin DR, Woods SP, Clifford DB, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012. January;18(1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.) American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 38). Tierney SM, Sheppard DP, Kordovski VM, Faytell MP, Avci G, Woods SP. A comparison of the sensitivity, stability, and reliability of three diagnostic schemes for HIV-associated neurocognitive disorders. J Neurovirol. 2017. June;23(3):404–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007. October 30;69(18):1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Smith G, Della Sala S, Logie RH, Maylor EA. Prospective and retrospective memory in normal ageing and dementia: a questionnaire study. Memory. 2000. September;8(5):311–21. [DOI] [PubMed] [Google Scholar]
- 41.) McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- 42). Crawford JR, Smith G, Maylor EA, Della Sala S, Logie RH. The Prospective and Retrospective Memory Questionnaire (PRMQ): Normative data and latent structure in a large non-clinical sample. Memory. 2003. May;11(3):261–75. [DOI] [PubMed] [Google Scholar]
- 43). Zeintl M, Kliegel M, Rast P, Zimprich D. Prospective memory complaints can be predicted by prospective memory performance in older adults. Dement Geriatr Cogn Disord. 2006;22(3):209–15. [DOI] [PubMed] [Google Scholar]
- 44). Moore RC, Paolillo EW, Heaton A, Fazeli PL, Jeste DV, Moore DJ. Clinical utility of the UCSD Performance-Based Skills Assessment-Brief (UPSA-B) in adults living with HIV: Associations with neuropsychological impairment and patient-reported everyday functioning difficulties. PLoS ONE. 2017;12(8):e0183614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010. September 21;75(12):1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 47). Heaton RK, Marcotte TD, Rivera-Mindt M, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. [DOI] [PubMed] [Google Scholar]
- 48.) Karnofsky DA, Burchenal JH. The clinical evaluation of chemo-therapeutic agents in cancer In: Maclead CM, ed. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. p. 191–205. [Google Scholar]
- 49.) Corporation Psychological. WAIS-III and WMS-III technical manual. San Antonio, TX: Author; 1997. [Google Scholar]
- 50.) Delis DC, Kramer JH, Kaplan E, Ober BA. The California verbal learning test. 2nd ed. Antonio San: The Psychological Corporation; 2000. [Google Scholar]
- 51.) Army Individual Test Battery. Manual of directions and scoring. Washington, DC:War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 52.) Culbertson WC, Zillmer EA. The Tower of London, Drexel University, research version: examiner’s manual. North Tonawanda: Multi-Health Systems; 1999. [Google Scholar]
- 53.) Kløve H Grooved pegboard. Indiana: Lafayette Instruments; 1963. [Google Scholar]
- 54.) American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edn Author, Washington DC; 2013. [Google Scholar]
- 55). Wendelken LA, Valcour V. Impact of HIV and aging on neuropsychological function. J Neurovirol. 2012. August;18(4):256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Vance DE, Cody SL, Yoo-Jeong M, Jones GLD, & Nicholson WC. The Role of Employment on Neurocognitive Reserve in Adults With HIV: A Review of the Literature. J Assoc Nurses AIDS Care. 2015. July;26(4):316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Kordovski VM, Woods SP, Verduzco M, Beltran J. The effects of aging and HIV disease on employment status and functioning. Rehabil Psychol. 2017. November;62(4):591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Woods SP, Weber E, Weisz BM, Twamley EW, Grant I, HNRP Group. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabil Psychol. 2011. February;56:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59). Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001. August;58(8):721–8. [DOI] [PubMed] [Google Scholar]
- 60). Kamat R, Doyle KL, Iudicello JE, Morgan EE, Morris S, Smith DM, et al. Neurobehavioral Disturbances During Acute and Early HIV Infection. Cogn Behav Neurol. 2016. March;29:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]