Abstract
Exposure to environmental pollutants is associated with a greater risk for metabolic diseases including cardiovascular disease. Pollutant exposure can also alter gut microbial populations that may contribute to metabolic effects and progression of inflammatory diseases. Short-chain fatty acids (SCFAs), produced from gut fermentation of dietary carbohydrates, such as inulin, exert numerous effects on host energy metabolism and are linked to a reduced risk of diseases. The hypothesis was that exposure to dioxin-like pollutants modulate gut microbial viability and/or fermentation processes. An inulin-utilizing isolate was collected from murine feces, characterized and used in subsequent experiments. Exposure to polychlorinated biphenyl, PCB 126 impeded bacterial viability of the isolate at concentrations of 20 and 200 μM. PCB 126 exposure also resulted in a significant loss of intracellular potassium following exposure, indicating cell membrane disruption of the isolate. Furthermore, total fecal microbe samples from mice were harvested, resuspended and incubated for 24 hours in anaerobic media containing inulin with or without PCB 126. HPLC analysis of supernatants revealed that PCB 126 exposure reduced succinic acid production, but increased propionate production, both of which can influence host glucose and lipid metabolism. Overall, the presented evidence supports the idea that pollutant exposure may contribute to alterations in host metabolism through gut microbiota-dependent mechanisms, specifically through bacterial fermentation processes or membrane disruption.
Keywords: dioxin, gut microbiota, polychlorinated biphenyls, fiber, inulin
Introduction
Exposure to environmental pollutants can illicit numerous deleterious health effects. Polychlorinated biphenyls are a class of persistent organic pollutants that increase development of chronic inflammatory diseases such as diabetes and atherosclerosis, primarily through inflammatory mechanisms [1]. Due to their lipophilic nature, they accumulate in the adipose tissue of living organisms and bioaccumulate along the food chain. Thus, one of the primary routes of human exposure is through the ingestion of contaminated foods including fatty meats, fish, and dairy [1]. This exposure route is unique in that it has the ability to directly impact the gastrointestinal tract and gut microbiota.
The gut microbiota can be impacted by outside influences including diet and environmental exposures [2]. Over the past decade, greater appreciation and understanding has come about regarding the gut microbiota and its influence on overall host health and wellbeing. The gut microbiota exerts impacts on neurological, metabolic, and inflammatory bowel diseases, highlighting the diverse and wide-reaching impact of our intestinal bacterial residents [3]. Fermentation is an innate aspect of gut microbial metabolism and the metabolites produced can have diverse impacts on host health. The primary end products of bacterial saccharolytic fermentation of non-digestible carbohydrates are short chain fatty acids (SCFAs). These SCFA are formed when carbohydrates escape digestion and absorption in the small intestine and thus persist into more distal regions of the digestive tract where they are metabolized by the residing gut microbiota [4]. The primary SCFA formed by bacterial fermentation are acetate, propionate, and butyrate, although there are numerous other fermentation acids produced. SCFA exert effects both locally, within the intestine, and systemically, and they serve a variety of biological purposes [4]. For example, butyrate serves as a preferential energy source for the colonic epithelial cells, facilitating the maintenance of an intestinal barrier through regulation of tight junction proteins such as ZO-1, occludins, and claudins [4, 5]. A strong intestinal barrier is critical for preventing leakage of bacteria and toxins into the systemic circulation, which may contribute to chronic inflammatory responses that can influence the development of disease [4, 6]. Additionally, SCFA concentrations are sensed by specific G-protein coupled receptors (GPRs), GPR41 and GPR43, which are involved in numerous systemic processes including the regulation of glucose and lipid metabolism [4, 5].
The prebiotic inulin is a polymer of fructans containing linear chains of fructosyl groups that are linked by β(2–1) glycosidic bonds and terminated with an alpha-D(1–2) glucopyranoside ring group on the reducing end [7]. The chain length of inulin varies from having two to more than 100 fructose units, which results in differences in fermentation by gut microbiota [7]. In preclinical and clinical models, inulin has been demonstrated to have beneficial effects on several disease states including cardiovascular disease and diabetes [8–10]. Inulin is fermented by certain members of the gut microbial community to produce short chain fatty acids including acetate, propionate, and butyrate, which is believed to be one of the mechanisms of action in which inulin exerts host health effects [11]. In the human nutrition community, inulin, is commonly referred to as a dietary fiber based on its inability to be metabolized by mammalian enzymes and thus is not absorbed by the human body [12]. Due to the observed health effects of inulin consumption, it is commonly added to numerous food products to increase the dietary fiber content while adding slight non-caloric sweetness.
Our lab has demonstrated previously that PCB 126 exposure decreases gut microbial diversity, reduces specific bacterial populations, and impacts overall host metabolism in a mouse model [13]. However, little is known about the mechanisms by which PCBs impact the gut microbiota and how this may translate to effects for the host. Therefore, the objective of this study was to examine the impacts of PCB 126 exposure on gut microbial viability and fermentation utilizing the prebiotic inulin as a substrate.
Materials and Methods
Materials and Chemicals
Inulin from chicory was purchased from Sigma Aldrich (St. Louis, MO, USA). 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) was obtained from AccuStandard Inc. (New Haven, CT, USA).
Animals and Fecal Collection
Male C56BL6/J mice were fed a standard chow diet ad libitum, housed at 22 °C with 50% humidity, and exposed to a 12-h light/ 12-h dark cycle. When fecal samples were needed for in vitro experiments, fresh fecal pellets were collected from mice and immediately placed in a sterile microcentrifuge tube. Samples were quickly transferred in an insulated container at 37 °C for immediate experiment setup to ensure bacterial viability. For fermentation experiments, fecal cell suspensions were made from pooled samples of three mice and repeated a total of three times. The University of Kentucky animal care and use committee approved all animal procedures.
Media and Anaerobic Technique
Growth medium was prepared, and cultures were transferred using the Hungate anaerobic method. Growth medium contained (per 1 L) 240 mg KH2PO4, 240 mg K2HPO4, 480 mg NaCl, 480 mg (NH4)2SO4, 100 mg MgSO4 7H2O, 64 mg CaCl2 2H2O, and 600 mg cysteine hydrochloride [14]. Media was adjusted to a pH of 6.7 by adding NaOH, autoclaved to remove O2, and cooled under CO2, at which point 4.0 g Na2CO2 was added. Media was then anaerobically transferred to Hungate tubes, capped with rubber stoppers and aluminum seals, and autoclaved for sterilization. Inulin was prepared anaerobically and added aseptically at a concentration of 4 g/L unless otherwise indicated. Cultures were routinely transferred with anaerobic technique using a tuberculin syringe and incubated at 37*C.
Inulin-Fermenter Enumeration, Isolation, and Characterization
Fecal samples (175 mg) were placed in anaerobic chamber (Coy Labs, Grass Lake, MI; 95% CO2, 5% H2), suspended in 1 mL dilution buffer (PBS), and subjected to 10-fold serial dilution in dilution buffer using sterile anaerobic techniques. Samples (200 μL) from the dilutions were plated on basal medium agar (15 mg/mL) containing 4g/L of inulin for bacterial isolation. Dilution plates were incubated in an anaerobic chamber for 72h at 37 °C. Following incubation, colonies were isolated and grown in liquid growth media (4 g/L inulin) at 37 °C for 24–48h. Cultures were then characterized by Gram staining and light microscopy. Phylogenetic identity was determined via 16S rRNA sequencing. Genomic DNA was extracted (QiaAMP DNA Mini Stool Kit; Qiagen, CA, USA) and amplified by PCR using PuReTaq™ Ready-to-Go™ PCR beads (GE Healthcare, Buckinghamshire, United Kingdom) and universal 16S primers (10 pmol; Integrated DNA Technologies, Coralville, IA, USA; Forward-5′-AGAGTTTGATCCTGGCTCAG-3′, Reverse – 3′-ACGGCTACCTTGTTACGACTT-5′). The PCR cycles were: denaturing (94 °C; 5 min), 35 cycles (94 °C, 0.5 min; 55 °C, 1 min; 72 °C, 1 min), and final extension (72 °C; 5 min). PCR products were sequenced by the University of Kentucky, sequencing core facility (Lexington, KY, USA) using an ABI 3730 DNA Analyzer (Applied Biosystems, CT, USA). The closest phylogenetic relatives were identified using a BLAST search of GenBank [15]. The 16S sequences were aligned and a phylogenetic dendrogram was assembled using the Neighbor-Joining method in Geneious (version 10.2.5). The bacterial isolate was further characterized for substrate utilization using an API 20A kit (BioMeriux, Marcy I’Etoile, France).
Growth Experiments
Growth experiments were conducted in growth media (9mL, pH 6.7) in Hungate tubes. Stock solutions of PCB 126 were solubilized in DMSO and the same amounts of DMSO as in PCB-treated cultures were added to control cultures. The levels of DMSO in cultures was less than 0.05%. PCB 126 (0.02 μM) or control (DMSO) was added to growth media prior to inoculation with JB12 cultures. The dose of 0.2 μM was chosen as a level that has been documented a in exposed human populations. Each tube was inoculated with (1% v/v) from stationary phase (16 h) cultures and incubated at 37 °C. Optical densities were determined via spectrophotometry (600 nm) to quantify bacterial growth.
The effect of increasing concentrations of PCB 126 on the growth of the inulin-fermenting isolate was conducted via broth dilution (10-fold increments). Isolates were transferred and maintained in growth media (9 mL, pH 6.7) containing 4 g/L of inulin that was prepared using sterile anaerobic technique. PCB 126 was added to growth media via a Hamilton syringe in 10-fold dilutions (0.02, 0.2, 2, 20, 200 μM). Each tube was inoculated with a stationary phase (1% v/v) from 16h cultures and incubated at 37 °C. Optical densities were recorded 24h after inoculation and the degree to which growth was observed was documented. All growth experiments were conducted in triplicate.
Intracellular Potassium Quantification
Intracellular potassium was quantified using a method described in Flythe et. al.2007 [16]. Growing cultures were energized with glucose for 25min, at which point PCB 126 or vehicle control (DMSO) were introduced. Concentrations and preparations of PCB 126 and DMSO were as described above. At each timepoint, cells were separated from supernatant via centrifugation (13000g, 1 min) through silicon oil using a 50:50 mixture of Dexter Hysol 550 and 560. Cell pellets were removed with dog nail trimmers, and subsequently digested in 3 N HNO3 (25 °C for 72h). Insoluble cell material was removed via centrifugation (13,000g, 2 min) and the potassium concentrations were determined using flame photometry (Cole-Parmer 2655–00 Digital Flame Analyzer; Cole-Parmer Instruments, IL, USA).
Fecal Cell Suspensions:
Fecal samples (175 mg) were placed in an anaerobic chamber and suspended in 9mL of basal medium[14]. Samples were centrifuged (340g, 5 min) to remove major undigested material. Supernatants were collected and centrifuged (25,000g, 10 min) to pellet bacteria. Bacterial pellets were resuspended in 9mL of growth medium and split into 3 tubes each containing 2 mL of bacterial suspension. PCB 126 (0.02, 0.2 or 2μM final conc.) or vehicle control (DMSO) and inulin (4 g/L or 10 g/L final conc.) were added to the bacterial suspensions. The preparation of PCB 126 and DMSO were the same as described above. The pH and optical density (OD; absorbance at 600 nm) were quantified to ensure normalization. Samples were incubated for 0h or 48h at 37 °C, centrifuged (21,000g, 2 min) to collect supernatant for fermentation end product quantification, and frozen stored at −20 °C until analysis.
Fermentation End Product Quantification
Fermentation end product quantification was conducted as discussed previously [14]. Supernatant samples were thawed and clarified in a microcentrifuge (21,000g, 2 min) for short-chain fatty acid (SCFA) quantifications. SCFAs were quantified using as Summit HPLC (Dionex; CA, USA). Extracts (100 μL) were injected into an anion-exchange column (Amine HP-87H; Bio-Rad, Hercules, CA) at 50 °C, separated isocratically with 5 mM sulfuric acid (0.4 mL/min flow rate), and subsequently detected via refractive index and UV absorption at 210 nm.
Statistics
Each experiment was performed in triplicate. Data were input into GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) for statistical analyses and graphing. Growth inhibition, intracellular potassium, and fermentation acid data were analyzed by Student’s t-test to calculate comparisons of means between treatment groups. All data are presented as means ± SEM and results were considered statistically significant with an observed p-value <0.05.
Results
Identification of an inulin fermenting isolate
An inulin fermenting microorganism (JB12) was isolated mouse feces. The isolate formed smooth, white opaque colonies approximately 1mm in diameter on basal medium agar with 4 g/L inulin. Culture purity was confirmed through repeated isolation streak. Sequence homology (16S rRNA) revealed that the closest characterized relative of JB12 was Acutalibacter muris (96% identity), a member of the Ruminococcaceae (Figure 1). JB12 was Gram-positive, and, unlike A. muris, it formed central endospores. In addition to inulin, JB12 metabolized glucose, saccharose, maltose, salicin, xylose, cellobiose, mannose, and trehalose (Table 1) [17–19].
Figure 1.
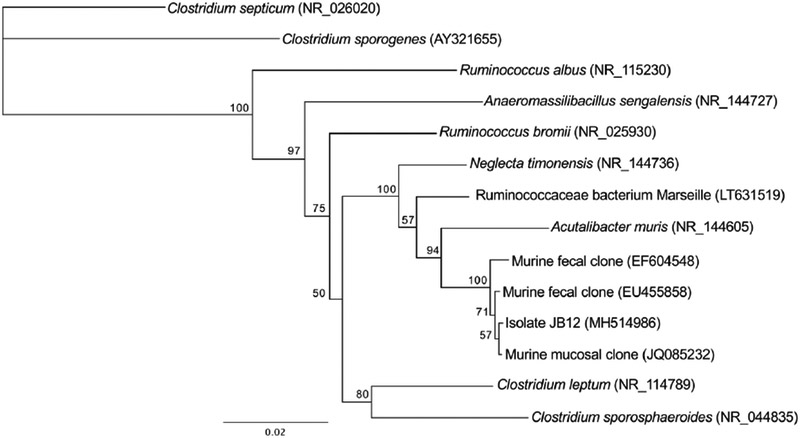
A phylogenetic dendrogram of isolate JB12 and closely related relatives. Relationships were based on 16S rRNA sequences. The tree was constructed using the neighbor-joining method in Geneious (version 10.2.5) and bootstrap values are expressed as a percentage of 1000 replications. The scale represents a 2% difference in nucleotide sequence.
Table 1:
Substrate utilization profile of isolate JB12 and close relatives
| Substrate | Isolate JB12 | Acutalibacter muris (Strain Kb18) | Anaeromassilibacillus senegalensis (strain mt9t) | Clostridium leptum (ATCC 29065) |
|---|---|---|---|---|
| Indole | − | − | − | n.d. |
| Urea | − | − | − | n.d. |
| Glucose | + | − | − | − (w) |
| Mannitol | − | − | − | n.d. |
| Lactose | + | − | − | − |
| Saccharose | + | − | − | − |
| Maltose | + | − | + | + |
| Salicin | + | − | n.d. | − |
| Xylose | + | − | n.d. | − (w) |
| Arabinose | − | − | n.d. | − |
| Gelatin | − | − | n.d. | − |
| Esculin | − | + | n.d. | − (w) |
| Glycerol | − | − | n.d. | n.d. |
| Cellobiose | + | − | n.d. | − |
| Mannose | + | − | − | − |
| Melezitose | − | − | n.d. | n.d. |
| Raffinose | − | − | n.d. | − |
| Sorbitol | − | − | n.d. | − |
| Rhamnose | − | − | n.d. | − |
| Trehalose | + | − | n.d. | − (w) |
| Catalase | − | − | − | − |
| Spores | + | n.d. | + | + |
| Gram Reaction | + | n.d. | − | + |
| Coccus | − | − | − | − |
Effect of PCB 126 on bacterial growth
To elucidate the effects of PCB 126 on an isolate JB12, experiments examining growth effects were conducted. For growth curve experiments, PCB 126 (0.02 μM) or control (DMSO) was added to growth media (9 mL) and subsequently inoculated with a stationary phase (16h culture; 1% v/v) of JB12 cultures. Cultures were incubated at 37°C and growth was quantified over time via optical densities (600 nm). JB12 grew rapidly in the growth medium with inulin as the substrate and reached an optical density of approximately 2.5 (DMSO) and 1.5 OD600 (PCB 126) after 5h. (data not shown).
To examine the effects of PCB 126 dose on JB12 growth, increasing doses of PCB 126 were added to tubes containing basal media which were subsequently inoculated with a stationary phase (16h culture; 1% v/v) culture of JB12 and incubated at 37°C for 24h. 24h after inoculation, growth was evaluated via optical density. We observed a significant reduction of bacterial growth at the two highest PCB 126 concentrations (20 μM and 200 μM) compared to control (P<0.05) (Figure 2). Apparent reduction in bacterial growth at lower PCB concentrations less than 20 μM were not significant.
Figure 2.
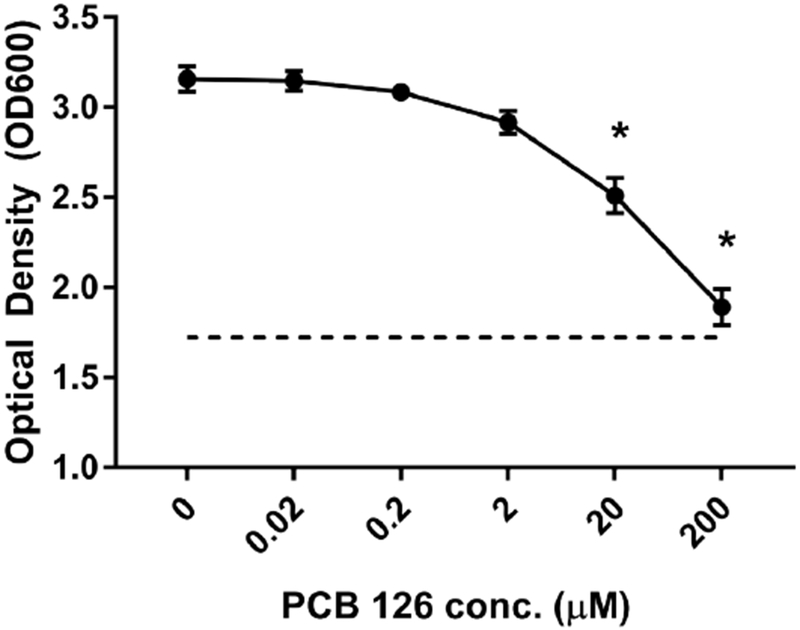
PCB 126 is an inhibitor to an inulin-utilizing bacteria. Growing cultures of isolate JB12 were exposed to increasing concentrations of PCB 126 (0.02, 0.2, 2, 20, 200 μM) and optical density was quantified after 24 hours of incubation (n=3). PCB 126 impeded bacterial growth at concentrations of 20 and 200 μM (p<0.05). All data are presented as means ± SEM. *Statistically different compared to 0 μM PCB 126 control (p<0.05; Student’s t-test).
Disruption of bacterial cell membrane by PCB 126
When cell suspensions of the inulin-fermenting isolate were energized with glucose, they accumulated intracellular potassium. When PCB 126 was added to the cell suspension, energized cells continuously lost intracellular potassium while a control that received only vehicle continued to accumulate intracellular potassium (Figure 3). Significantly lower levels of intracellular-potassium observed at 35, 45, 55, and 65 minutes compared to vehicle control.
Figure 3.
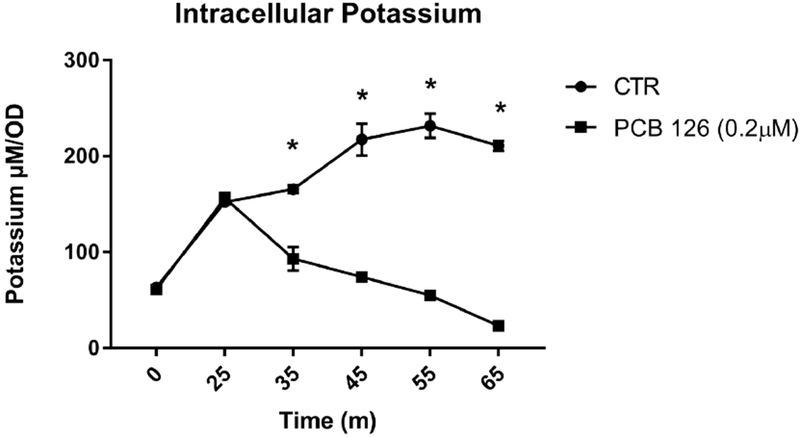
PCB 126 treatment significantly decreases levels of intracellular potassium over time in energized inulin-fermenting strain JB12. Growing cultures were energized with glucose at time 0 and PCB126 and vehicle control (DMSO) were introduced at 25m (n=3). Samples at each timepoint were subject to centrifugation to remove insoluble cell material and potassium concentration was determined using flame photometry. PCB 126 significantly reduced intracellular potassium levels at 35, 45, 55, and 65 minutes compared to vehicle control. All data are presented as means ± SEM. * Significantly different compared to vehicle control (DMSO) at specific timepoint (p<0.05; Student’s t-test).
PCB 126-induced modulation of fermentation acid production from inulin substrate
Fecal cell suspensions were grown and maintained using inulin as a substrate (4g/L or 10g/L) and exposed to PCB 126 at varying doses or DMSO control for 48h. Examination of the fermentation acid production from inulin at 4g/L revealed that the highest concentrations of PCB 126 (0.2 μM and 2 μM) reduced production of succinate (Figure 4). Additionally, PCB 126 exposure significantly increased propionate concentrations in the 2μM exposed cells. The cells that were not exposed to PCB 126 produced 46 mM total fermentation acids, including 11 mM propionate, 12 mM butyrate, and 21 mM acetate. To further understand the effects of PCB 126 on fermentation acid production, we performed a second experiment in which the inulin substrate concentration was increased to 10g/L. At this increased concentration of inulin, PCB 126 exerted no significant effects on fermentation acid production at any of the exposure levels (Figure 5).
Figure 4.
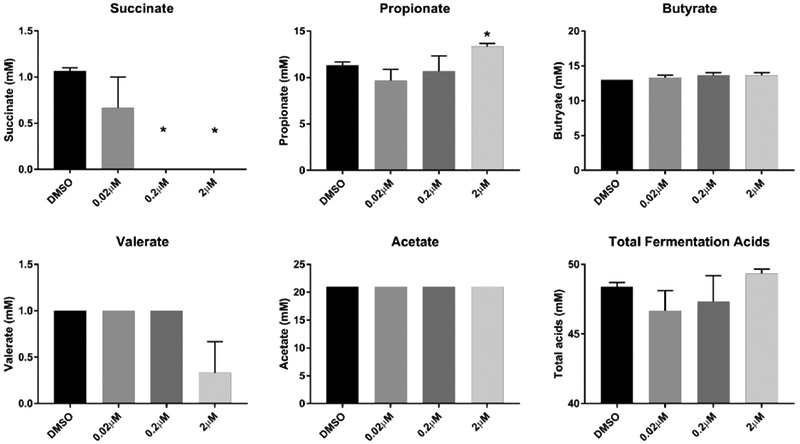
PCB 126 treatment differentially modulates fermentative SCFA from moderate concentrations of the dietary fiber inulin. Bacterial cell suspensions were maintained on inulin substrate (4 g/L) and subsequently exposed to PCB 126 (0.02 μM, 0.2 μM, 2 μM) or vehicle control (DMSO) for 48h (n=3). HPLC was utilized to quantify fermentation acids in the media after incubation. PCB 126 significantly reduced production of succinate at 0.2 μM and 2μM exposure concentrations and significantly increased propionate production at the 2 μM concentration. All data are presented as means ± SEM. * Significantly different compared to vehicle control (DMSO) (p<0.05; Student’s t-test).
Figure 5.
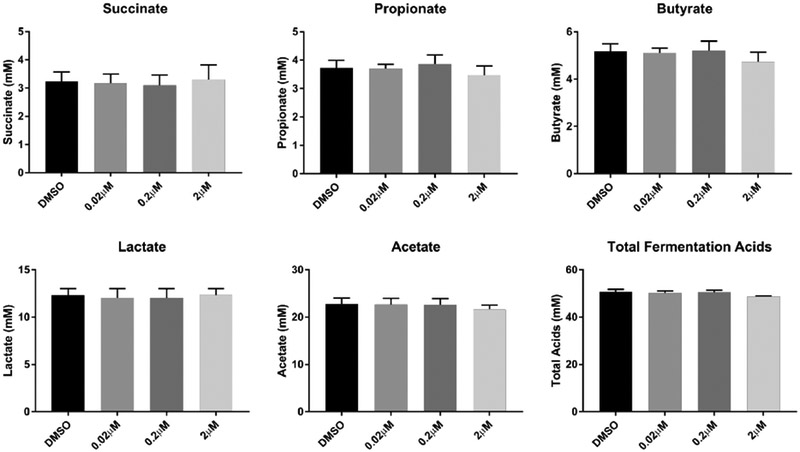
Increased inulin provision abolishes effects of PCB 126 treatment on fermentative SCFA production by isolated fecal bacterial. Bacterial cell suspensions were maintained on inulin substrate (10g/L) and subsequently exposed to PCB 126 (0.02 μM, 0.2 μM, 2 μM) or vehicle control (DMSO) for 48h (n=3). HPLC was utilized to quantify fermentation acids in the media after incubation. PCB 126 had no significant effect on fermentation acid production from a higher inulin substrate concentration (10 g/L). There was a trend towards lower total fermentation acid production (p=0.055) in bacterial cultures exposed to higher levels of PCB 126. All data are presented as means ± SEM. * Significantly different compared to vehicle control (DMSO) (p<0.05; Student’s t-test)
Discussion
The objective of this study was to examine the impacts of PCB 126 exposure on gut fermentation utilizing the prebiotic inulin as a substrate. Previous results indicated that the dioxin-like PCB altered the population sizes of a variety of phylogenetic groups in the hindgut when mice received the toxicant in vivo [13]. The current study follows up by mechanistically examining the effects of PCB 126 on bacteria isolated from murine feces. The effects on uncultivated hindgut bacterial fermentation ex vivo are also shown. Bacterial fermentation is a key metabolic process allowing bacteria to extract energy from compounds that the host takes in [4, 5]. Products of fermentation of indigestible carbohydrates, such as inulin, include various fermentation acids or short chain fatty acids. SCFA are common, easily-quantifiable products of bacterial fermentation and thus are a good measure to examine potential pollutant-induced effects [4, 5].
Inulin was chosen as the host-indigestible carbohydrate substrate due to its increased popularity in the food and supplement industry as well as the strong basis of scientific evidence linking inulin consumption to beneficial host health outcomes [11, 20–22]. United States citizens ingest 1–4g of inulin-type fructans per day, which can be anticipated to rise with increasing popularity [23]. Administration of inulin has been demonstrated to reduce adiposity and parameters associated with metabolic syndrome [8, 10, 22]. The mechanisms behind the beneficial actions of inulin are believed to include alterations in gastrointestinal peptide and short chain fatty acid production, regulation of the immune system, and modulation of lipid metabolism [22]. For example, Weitkunat et. al. found that gnotobiotic mice colonized with a human microbiota and fed an inulin-enriched diet had significantly greater amounts of acetate, propionate and butyrate in the cecum as well as elevated levels of acetate and propionate in the plasma of the portal vein compared to mice fed cellulose [24]. Inulin feeding also altered hepatic genes associated with lipogenesis and fatty acid elongation, which was hypothesized to be due to the differences in short chain fatty acid levels. These data highlight the fermentative capability of inulin and the role that fermentation products (SCFA) play on overall host health, making inulin a relevant substrate to study the effects of pollutant exposure.
An inulin-utilizing microorganism was isolated from feces in order to examine the inhibitory effects of PCB 126 at a physiological level. The isolate (JB12) was most closely related to Acutalibacter muris which was previously isolated from murine feces [19]. Isolate JB12 grew rapidly in the growth medium when inulin was provided as the substrate, but growth slowed in an apparent stationary phase in the presence of PCB 126 at 0.2 μM. However, the growth appeared to be diauxic because greater optical densities were observed at 24h (Figure 2). At 24h, there was a dose-response inhibitory effect of PCB 126 on the culture. Diauxic, or biphasic, growth is not surprising because inulin is not truly a single substrate. It is composed of oligofructose of different chain lengths, which are fermented at different rates and to different extents [25]. When polymers of different chain lengths are fermented as though they were different substrates, classical diauxic growth could occur [26]. It was also observed that concentrations greater than 20 μM of the polychlorinated biphenyl reduced the growth of JB12. These results are consistent with the previous in vivo data that showed PCB 126 exposure disrupted bacterial populations and decreased bacterial diversity [13]. However, the antimicrobial mechanism of action of PCB 126 on gut bacteria was unknown. Previously, research was conducted on the effects of PCBs and other environmental pollutants on soil microbes [27]. It has been discussed that there is no convincing evidence that PCBs exert genotoxicity in bacteria, such as Salmonella enterica [28]. Additionally, there is some evidence that PCBs may be membrane active within specific soil microbial populations [27]; therefore, we examined the effect of PCB 126 on the intracellular potassium of isolate JB12. Potassium is the main intracellular cation in bacterial cells and serves important roles such as acting as a cofactor for certain enzymes [29]. Quantification of intracellular potassium is a method that allows examination of potential membrane disruptions that that result in the leakage or loss of this intracellular cation. We observed that upon exposure to PCB 126, the bacterial cells rapidly lost potassium, which is consistent with a loss of cell membrane integrity. This result indicates that bacterial cell membrane disruption is a potential antimicrobial mechanism of action by which polychlorinated biphenyls could disrupt gut microbial communities.
To better understand how these findings may impact the gut microbiota as a whole, we examined the effects of PCB 126 on fermentation acid production in a fecal microbial cell suspension. PCB 126 addition (0.2 μM and 2 μM) to the fecal cell suspension completely inhibited succinate production and increased propionate production (2 μM). This is important for the host due because succinate is a substrate in intestinal gluconeogenesis. Impaired intestinal gluconeogenesis was shown to impact systemic glucose tolerance [30]. Furthermore, microbiota-derived succinate was shown to improve glucose and insulin tolerance in wild-type mice [31]. It has been documented that PCB 126 disrupts glucose tolerance, yielding a diabetic phenotype in mouse models, thus this new finding of microbial-derived succinate loss with PCB 126 may play a role in this previously observed phenotype [32]. An increase in propionate was also observed in the fecal cell suspensions exposed to PCB 126, which may have implications in alterations in host hepatic metabolism. In support of this data, previous in vivo evidence indicated an increase in propionate producers including Clostridiales [33] and Akkermansia [34] in cecum samples of mice exposed to PCB 126 [13]. Propionate can affect hepatic de novo lipogenesis as well as lipolysis and in humans an increased level of propionate has been observed in individuals with non-alcoholic fatty liver disease [35]. It is well documented that exposure to dioxin-like pollutants increases hepatic lipid content and exacerbated non-alcoholic fatty liver disease [36, 37]. Therefore, the effects observed on propionate production have the potential to contribute to these well-known hepatic consequences of pollutant exposure.
The modulatory effects of PCB 126 on fermentation acid production were not observed when the inulin concentration was increased to 10 g/L. This is not surprising because all of the microorganisms in the gut microbiome were not sensitive to PCB 126 at the concentrations tested. At higher inulin concentrations, lactate was a major end product. Lactic acid production is common when easily fermented carbohydrate is in excess. Many Firmicutes (e.g. streptococci) and Proteobacteria (e.g. E. coli) possess a 1,6- fructose bisphosphate (FBP)-dependent lactate dehydrogenase (LDH) [38]. Rapid membrane transport of sugars leads to an increase in cellular FBP, which triggers LDH and homolactic metabolism [38]. Abundant lactate production regardless of PCB 126 suggests that one or more fructanolytic lactic acid bacterial species were not sensitive to PCB 126. It is important to note that increased lactate production from fructans fermentation is not always beneficial. For example, in horses, excess lactate production and resultant drop in pH in the hindgut can increase intestinal permeability and contribute to the development of laminitis, resulting in required euthanasia [14]. Our present data do not warrant a suggestion for excess consumption of inulin, but rather serves to highlight that the bacterial populations that are able to grow at higher concentrations of inulin are not sensitive to PCB 126. These data indicate that PCBs might be selectively toxic to specific gut microbial populations.
Although this present study is novel and adds to the growing body of literature examining the impacts of pollutant exposure on gut microbial populations, there are a few limitations that should be noted and addressed in subsequent studies. First, the mechanistic aspects of this study focus on a single isolate and thus the translatability of our findings to other microbial species is limited but should be examined in future experiments. Additionally, it has been reported that serum concentrations of PCBs can be found at approximately 3μM in exposed individuals [39]. Due to the primary route of exposure (i.e. food consumption), it could be expected then that the intestinal environment sees similar or higher levels. Thus, the 0.02μM, 0.2μM and 2μM concentrations used for most experiments in this manuscript are physiologically relevant, especially in heavily exposed populations. However, the highest concentrations in utilized in the inhibition experiment (Figure 2) are supraphysiological. Use of these higher levels, while not found in human populations, is important to help characterize and better understand the microbial impact of PCB 126. Furthermore, the experiments conducted in the present study are representative of acute exposures while humans often see more chronic exposures due to the primary route of exposure of PCBs (i.e. food) and their participation in enterohepatic circulation. Thus, gastrointestinal microbial populations are continually exposed to these pollutants which could elicit a different and perhaps more detrimental response over time. Finally, it appears that some microbiota are effected by exposure to PCB 126 while others are not. This is the first study to show impacts of a polychlorinated biphenyl compound on fermentation by gut microbiota. It remains to be seen if this interaction of the native gut microorganisms with an environmental toxicant is beneficial to the host.
Conclusion
PCB 126 decreased the fermentative ability and viability of an inulin-metabolizing murine fecal bacterium. Potassium efflux indicated that the mechanism of action was membrane perturbation. The fermentation of washed fecal microorganisms were also impacted by PCB 126 ex vivo when the inulin concentration was low. However, the toxicant was not inhibitory to fermentation when the inulin concentration was greater. These results indicate that not all species (e.g. the lactic acid bacteria that flourish under carbohydrate excess conditions) were equally sensitive, a conclusion which is consistent with previous results by our research group using culture-independent methods [13].
Highlights:
Exposure to polychlorinated biphenyl PCB 126 impeded bacterial viability of an inulin-fermenting isolate.
PCB 126 induced membrane disruptions in an inulin-fermenting isolate.
Reductions in succinate and increases in propionate production were observed in fecal cell suspensions exposed to PCB 126.
Increased inulin substrate provision abolished PCB 126-induced alterations in fermentation acid production.
Acknowledgments
Funding: This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [P42ES007380] and the University of Kentucky Agricultural Experiment Station. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. MF was supported by the USDA, Agricultural Research Service National Program NP-215, Grass, Forage and Rangeland Agroecosystems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no competing financial interests.
References:
- 1.Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Reviews on environmental health. 2006;21(1):1–23. PubMed PMID: 16700427. [DOI] [PubMed] [Google Scholar]
- 2.Barko PC, McMichael MA, Swanson KS, Williams DA. The Gastrointestinal Microbiome: A Review. J Vet Intern Med. 2018;32(1):9–25. Epub 2017/11/25. doi: 10.1111/jvim.14875. PubMed PMID: 29171095; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nature reviews Microbiology. 2013;11(4):227–38. doi: 10.1038/nrmicro2974. PubMed PMID: 23435359. [DOI] [PubMed] [Google Scholar]
- 4.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of lipid research. 2013;54(9):2325–40. doi: 10.1194/jlr.R036012. PubMed PMID: 23821742; PubMed Central PMCID: PMC3735932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165(6): 1332–45. doi: 10.1016/j.cell.2016.05.041. PubMed PMID: 27259147. [DOI] [PubMed] [Google Scholar]
- 6.Janssen AW, Kersten S. The role of the gut microbiota in metabolic health. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(8):3111–23. doi: 10.1096/fj.14-269514. PubMed PMID: 25921831. [DOI] [PubMed] [Google Scholar]
- 7.Roberfroid M Dietary fiber, inulin, and oligofructose: a review comparing their physiological effects. Crit Rev Food Sci Nutr. 1993;33(2):103–48. Epub 1993/01/01. doi: 10.1080/10408399309527616. PubMed PMID: 8257475. [DOI] [PubMed] [Google Scholar]
- 8.Catry E, Bindels LB, Tailleux A, Lestavel S, Neyrinck AM, Goossens JF, et al. Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut. 2018;67(2):271–83. Epub 2017/04/06. doi: 10.1136/gutjnl-2016-313316. PubMed PMID: 28377388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Zhang S, Henning Sm, Lee R, Hsu M, Grojean, et al. Cholesterol-lowering effects of dietary pomegranate extract and inulin in mice fed an obesogenic diet. The Journal of nutritional biochemistry. 2017;52:62–9. Epub 2017/11/25. doi: 10.1016/j.jnutbio.2017.10.003. PubMed PMID: 29172112. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Yu H, Xiao X, Hu L, Xin F, Yu X. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ. 2018;6:e4446 Epub 2018/03/07. doi: 10.7717/peerj.4446. PubMed PMID: 29507837; PubMed Central PMCID: PMCPMC5835350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis SA, Conceicao LL, Rosa DD, Dias MM, Peluzio Mdo C. Mechanisms used by inulin-type fructans to improve the lipid profile. Nutr Hosp. 2014;31(2):528–34. Epub 2015/01/27. doi: 10.3305/nh.2015.31.2.7706. PubMed PMID: 25617533. [DOI] [PubMed] [Google Scholar]
- 12.Cheng W, Lu J, Li B, Lin W, Zhang Z, Wei X, et al. Effect of Functional Oligosaccharides and Ordinary Dietary Fiber on Intestinal Microbiota Diversity. Frontiers in microbiology. 2017;8:1750 Epub 2017/10/06. doi: 10.3389/fmicb.2017.01750. PubMed PMID: 28979240; PubMed Central PMCID: PMCPMC5611707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petriello MC, Hoffman JB, Vsevolozhskaya O, Morris AJ, Hennig B . Dioxin-like PCB 126 increases intestinal inflammation and disrupts gut microbiota and metabolic homeostasis. Environ Pollut. 2018;242, Part A:1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlow BE, Lawrence LM, Kagan IA, Flythe MD . Inhibition of fructan-fermenting equine faecal bacteria and Streptococcus bovis by hops (Humulus lupulus L.) beta-acid. Journal of applied microbiology. 2014;117(2):329–39. doi: 10.1111/jam.12532. PubMed PMID: 24775300. [DOI] [PubMed] [Google Scholar]
- 15.Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, et al. GenBank. Nucleic Acids Res. 2013;41(Database issue):D36–42. Epub 2012/11/30. doi: 10.1093/nar/gks1195. PubMed PMID: 23193287; PubMed Central PMCID: PMCPMC3531190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flythe MD, Russell JB. Effect of acidic pH on the ability of Clostridium sporogenes MD1 to take up and retain intracellular potassium. FEMS Microbiol Lett. 2007;267(1):46–50. Epub 2007/01/20. doi: 10.1111/j.1574-6968.2006.00535.x. PubMed PMID: 17233675. [DOI] [PubMed] [Google Scholar]
- 17.Guilhot E, Alou MT, Lagier JC, Labas N, Couderc C, Delerce J, et al. Genome sequence and description of Anaeromassilibacillus senegalensis gen. nov., sp. nov., isolated from the gut of patient with kwashiorkor. New Microbes New Infect. 2017;17:54–64. Epub 2017/03/16. doi: 10.1016/j.nmni.2017.01.009. PubMed PMID: 28289546; PubMed Central PMCID: PMCPMC5338722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore WEC, Johnson JL, and Holdeman LV. Emendation of Bacteroidaceae and Butyrivibrio and descriptions of Desulfomonas gen. nov. and ten new species in the genera Desulfomonas, Butyrivibrio, Eubacterium, Clostridium, and Ruminococcus. International journal of systematic and evolutionary microbiology. 1976;1976(26):238–52. doi: 10.1099/00207713-26-2-238. [DOI] [Google Scholar]
- 19.Lagkouvardos I, Pukall R, Abt B, Foesel BU, Meier-Kolthoff JP, Kumar N, et al. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol. 2016;1(10):16131 Epub 2016/09/28. doi: 10.1038/nmicrobiol.2016.131. PubMed PMID: 27670113. [DOI] [PubMed] [Google Scholar]
- 20.Fava F, Tuohy KM. Gut microbiota: Inulin regulates endothelial function: a prebiotic smoking gun? Nat Rev Gastroenterol Hepatol. 2017;14(7):392–4. Epub 2017/06/01. doi: 10.1038/nrgastro.2017.68. PubMed PMID: 28559592. [DOI] [PubMed] [Google Scholar]
- 21.Trautwein EA, Rieckhoff D, Erbersdobler HF. Dietary inulin lowers plasma cholesterol and triacylglycerol and alters biliary bile acid profile in hamsters. The Journal of nutrition. 1998;128(11):1937–43. Epub 1998/11/10. PubMed PMID: 9808646. [DOI] [PubMed] [Google Scholar]
- 22.Weitkunat K, Stuhlmann C, Postel A, Rumberger S, Fankhanel M, Woting A, et al. Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Scientific reports. 2017;7(1):6109 Epub 2017/07/25. doi: 10.1038/s41598-017-06447-x. PubMed PMID: 28733671; PubMed Central PMCID: PMCPMC5522422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Loo J, Coussement P, de Leenheer L, Hoebregs H, Smits G. On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit Rev Food Sci Nutr. 1995;35(6):525–52. Epub 1995/11/01. doi: 10.1080/10408399509527714. PubMed PMID: 8777017. [DOI] [PubMed] [Google Scholar]
- 24.Weitkunat K, Schumann S, Petzke KJ, Blaut M, Loh G, Klaus S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. The Journal of nutritional biochemistry. 2015;26(9):929–37. Epub 2015/06/03. doi: 10.1016/j.jnutbio.2015.03.010. PubMed PMID: 26033744. [DOI] [PubMed] [Google Scholar]
- 25.Harlow BE, Kagan IA, Lawrence LM, Flythe MD. Effects of Inulin Chain Length on Fermentation by Equine Fecal Bacteria and Streptococcus bovis. Journal of Equine Veterinary Science. 2016;48:113–20. [Google Scholar]
- 26.Gottschalk G Bacterial Metabolism. New York: Springer-Verlag; 1986. [Google Scholar]
- 27.Zoradova-Murinova S, Dudasova H, Lukacova L, Certik M, Silharova K, Vrana B, et al. Adaptation mechanisms of bacteria during the degradation of polychlorinated biphenyls in the presence of natural and synthetic terpenes as potential degradation inducers. Applied microbiology and biotechnology. 2012;94(5):1375–85. Epub 2011/12/14. doi: 10.1007/s00253-011-3763-8. PubMed PMID: 22159613. [DOI] [PubMed] [Google Scholar]
- 28.Robertson LW, Hansen LG. PCBs : recent advances in environmental toxicology and health effects. Lexington, Ky: University Press of Kentucky; 2001. xxx, 461 p. p. [Google Scholar]
- 29.Epstein W The roles and regulation of potassium in bacteria. Prog Nucleic Acid Res Mol Biol. 2003;75:293–320. Epub 2003/11/08. PubMed PMID: 14604015. [DOI] [PubMed] [Google Scholar]
- 30.Penhoat A, Fayard L, Stefanutti A, Mithieux G, Rajas F. Intestinal gluconeogenesis is crucial to maintain a physiological fasting glycemia in the absence of hepatic glucose production in mice. Metabolism: clinical and experimental. 2014;63(1):104–11. Epub 2013/10/19. doi: 10.1016/j.metabol.2013.09.005. PubMed PMID: 24135501. [DOI] [PubMed] [Google Scholar]
- 31.De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell metabolism. 2016;24(1):151–7. Epub 2016/07/15. doi: 10.1016/j.cmet.2016.06.013. PubMed PMID: 27411015. [DOI] [PubMed] [Google Scholar]
- 32.Baker NA, Karounos M, English V, Fang J, Wei Y, Stromberg A, et al. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environmental health perspectives. 2013;121(1):105–10. doi: 10.1289/ehp.1205421. PubMed PMID: 23099484; PubMed Central PMCID: PMC3553436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchandin H, Teyssier C, Campos J, Jean-Pierre H, Roger F, Gay B, et al. Negativicoccus succinicivorans gen. nov., sp. nov., isolated from human clinical samples, emended description of the family Veillonellaceae and description of Negativicutes classis nov., Selenomonadales ord. nov. and Acidaminococcaceae fam. nov. in the bacterial phylum Firmicutes. International journal of systematic and evolutionary microbiology. 2010;60(Pt 6):1271–9. Epub 2009/08/12. doi: 10.1099/ijs.0.013102-0. PubMed PMID: 19667386. [DOI] [PubMed] [Google Scholar]
- 34.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. International journal of systematic and evolutionary microbiology. 2004;54(Pt 5):1469–76. Epub 2004/09/25. doi: 10.1099/ijs.0.02873-0. PubMed PMID: 15388697. [DOI] [PubMed] [Google Scholar]
- 35.Da Silva HE, Teterina A, Comelli EM, Taibi A, Arendt BM, Fischer SE, et al. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Scientific reports. 2018;8(1):1466 Epub 2018/01/25. doi: 10.1038/s41598-018-19753-9. PubMed PMID: 29362454; PubMed Central PMCID: PMCPMC5780381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahlang B, Perkins JT, Petriello MC, Hoffman JB, Stromberg AJ, Hennig B. A Compromised Liver Alters Polychlorinated Biphenyl-Mediated Toxicity. Toxicology. 2017. doi: 10.1016/j.tox.2017.02.001. PubMed PMID: 28163111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wahlang B, Prough RA, Falkner KC, Hardesty JE, Song M, Clair HB, et al. Polychlorinated Biphenyl-Xenobiotic Nuclear Receptor Interactions Regulate Energy Metabolism, Behavior, and Inflammation in Non-alcoholic-Steatohepatitis. Toxicological sciences : an official journal of the Society of Toxicology. 2016;149(2):396–410. doi: 10.1093/toxsci/kfv250. PubMed PMID: 26612838; PubMed Central PMCID: PMC4751229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell JB, Bond DR, Cook GM. The fructose diphospate/phosphate regulation of carbohydrate metabolism in low G + C Gram-positive anaerobes. Res Microbiol. 1996;147:528–34. [DOI] [PubMed] [Google Scholar]
- 39.Wassermann M, Wassermann D, Cucos S, Miller HJ. World PCBs map: storage and effects in man and his biologic environment in the 1970s. Ann N Y Acad Sci. 1979;320:69–124. Epub 1979/05/31. PubMed PMID: 110205. [DOI] [PubMed] [Google Scholar]


