Abstract
Background
Biologic disease‐modifying anti‐rheumatic drugs (biologics) are highly effective in treating rheumatoid arthritis (RA), however there are few head‐to‐head biologic comparison studies. We performed a systematic review, a standard meta‐analysis and a network meta‐analysis (NMA) to update the 2009 Cochrane Overview. This review is focused on the adults with RA who are naive to methotrexate (MTX) that is, receiving their first disease‐modifying agent.
Objectives
To compare the benefits and harms of biologics (abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, tocilizumab) and small molecule tofacitinib versus comparator (methotrexate (MTX)/other DMARDs) in people with RA who are naive to methotrexate.
Methods
In June 2015 we searched for randomized controlled trials (RCTs) in CENTRAL, MEDLINE and Embase; and trials registers. We used standard Cochrane methods. We calculated odds ratios (OR) and mean differences (MD) along with 95% confidence intervals (CI) for traditional meta‐analyses and 95% credible intervals (CrI) using a Bayesian mixed treatment comparisons approach for network meta‐analysis (NMA). We converted OR to risk ratios (RR) for ease of interpretation. We also present results in absolute measures as risk difference (RD) and number needed to treat for an additional beneficial or harmful outcome (NNTB/H).
Main results
Nineteen RCTs with 6485 participants met inclusion criteria (including five studies from the original 2009 review), and data were available for four TNF biologics (adalimumab (six studies; 1851 participants), etanercept (three studies; 678 participants), golimumab (one study; 637 participants) and infliximab (seven studies; 1363 participants)) and two non‐TNF biologics (abatacept (one study; 509 participants) and rituximab (one study; 748 participants)).
Less than 50% of the studies were judged to be at low risk of bias for allocation sequence generation, allocation concealment and blinding, 21% were at low risk for selective reporting, 53% had low risk of bias for attrition and 89% had low risk of bias for major baseline imbalance. Three trials used biologic monotherapy, that is, without MTX. There were no trials with placebo‐only comparators and no trials of tofacitinib. Trial duration ranged from 6 to 24 months. Half of the trials contained participants with early RA (less than two years' duration) and the other half included participants with established RA (2 to 10 years).
Biologic + MTX versus active comparator (MTX (17 trials (6344 participants)/MTX + methylprednisolone 2 trials (141 participants))
In traditional meta‐analyses, there was moderate‐quality evidence downgraded for inconsistency that biologics with MTX were associated with statistically significant and clinically meaningful benefit versus comparator as demonstrated by ACR50 (American College of Rheumatology scale) and RA remission rates. For ACR50, biologics with MTX showed a risk ratio (RR) of 1.40 (95% CI 1.30 to 1.49), absolute difference of 16% (95% CI 13% to 20%) and NNTB = 7 (95% CI 6 to 8). For RA remission rates, biologics with MTX showed a RR of 1.62 (95% CI 1.33 to 1.98), absolute difference of 15% (95% CI 11% to 19%) and NNTB = 5 (95% CI 6 to 7). Biologics with MTX were also associated with a statistically significant, but not clinically meaningful, benefit in physical function (moderate‐quality evidence downgraded for inconsistency), with an improvement of HAQ scores of ‐0.10 (95% CI ‐0.16 to ‐0.04 on a 0 to 3 scale), absolute difference ‐3.3% (95% CI ‐5.3% to ‐1.3%) and NNTB = 4 (95% CI 2 to 15).
We did not observe evidence of differences between biologics with MTX compared to MTX for radiographic progression (low‐quality evidence, downgraded for imprecision and inconsistency) or serious adverse events (moderate‐quality evidence, downgraded for imprecision). Based on low‐quality evidence, results were inconclusive for withdrawals due to adverse events (RR of 1.32, but 95% confidence interval included possibility of important harm, 0.89 to 1.97). Results for cancer were also inconclusive (Peto OR 0.71, 95% CI 0.38 to 1.33) and downgraded to low‐quality evidence for serious imprecision.
Biologic without MTX versus active comparator (MTX 3 trials (866 participants)
There was no evidence of statistically significant or clinically important differences for ACR50, HAQ, remission, (moderate‐quality evidence for these benefits, downgraded for imprecision), withdrawals due to adverse events,and serious adverse events (low‐quality evidence for these harms, downgraded for serious imprecision). All studies were for TNF biologic monotherapy and none for non‐TNF biologic monotherapy. Radiographic progression was not measured.
Authors' conclusions
In MTX‐naive RA participants, there was moderate‐quality evidence that, compared with MTX alone, biologics with MTX was associated with absolute and relative clinically meaningful benefits in three of the efficacy outcomes (ACR50, HAQ scores, and RA remission rates). A benefit regarding less radiographic progression with biologics with MTX was not evident (low‐quality evidence). We found moderate‐ to low‐quality evidence that biologic therapy with MTX was not associated with any higher risk of serious adverse events compared with MTX, but results were inconclusive for withdrawals due to adverse events and cancer to 24 months.
TNF biologic monotherapy did not differ statistically significantly or clinically meaningfully from MTX for any of the outcomes (moderate‐quality evidence), and no data were available for non‐TNF biologic monotherapy.
We conclude that biologic with MTX use in MTX‐naive populations is beneficial and that there is little/inconclusive evidence of harms. More data are needed for tofacitinib, radiographic progression and harms in this patient population to fully assess comparative efficacy and safety.
Keywords: Adult; Humans; Abatacept; Abatacept/therapeutic use; Adalimumab; Adalimumab/therapeutic use; Antibodies, Monoclonal; Antibodies, Monoclonal/therapeutic use; Antirheumatic Agents; Antirheumatic Agents/therapeutic use; Arthritis, Rheumatoid; Arthritis, Rheumatoid/drug therapy; Bayes Theorem; Biological Products; Biological Products/therapeutic use; Etanercept; Etanercept/therapeutic use; Infliximab; Infliximab/therapeutic use; Methotrexate; Methotrexate/therapeutic use; Methylprednisolone; Methylprednisolone/therapeutic use; Network Meta‐Analysis; Piperidines; Piperidines/therapeutic use; Pyrimidines; Pyrimidines/therapeutic use; Pyrroles; Pyrroles/therapeutic use; Randomized Controlled Trials as Topic; Rituximab; Rituximab/therapeutic use
Plain language summary
Biologics for rheumatoid arthritis (RA) in people not previously treated with methotrexate (MTX)
Review question
We studied the benefits and harms of biologics or tofacitinib on people with rheumatoid arthritis (RA) who have not previously been treated with methotrexate (MTX), in trials done until June 2015. Data was available for four TNF biologics (adalimumab, etanercept, golimumab, infliximab) and two non‐TNF biologics (abatacept, rituximab).
What is RA and what are biologics/tofacitinib?
In RA, the immune system, which normally fights infection, attacks the joint lining making it inflamed. Without treatment, the inflammation can lead to joint damage and disability. Biologics or tofacitinib are medications that can reduce joint inflammation/damage and improve symptoms.
The review shows that in people with RA:
‐ Biologics (abatacept, adalimumab, etanercept, golimumab, infliximab, rituximab) in combination with MTX probably improve signs and symptoms of RA (tender or swollen joints), the chances of RA remission (disappearance of symptoms) and probably slightly improve functional ability. We downgraded our confidence in the results because of concerns about the inconsistency of some results.
‐ Biologics in combination with MTX may make little or no difference in the risk of serious adverse events or withdrawals due to adverse events. We downgraded our confidence in the results because of concerns about the inconsistency of some results and the lack of data.
‐ We often do not have precise information about side effects and complications. Because of the lack of data, we are uncertain of the effect of biologics on the risk of cancer.
‐TNF biologics (adalimumab, etanercept, golimumab) alone (not in combination with MTX) probably make little or no difference in signs and symptoms of RA or chances of RA remission (no data for non‐TNF biologics alone).
Best estimate of what happens to people with RA when taking biologics:
ACR50 (American College of Rheumatology 50: number of tender or swollen joints, pain and disability) :
Biologic + MTX versus MTX: 56 people out of 100 who were on a biologic (in combination with MTX) experienced improvement in RA compared to 40 people out of 100 who were on MTX (16% improvement).
Biologic monotherapy (TNF biologics) versus MTX: 35 people out of 100 who were on a biologic experienced improvement in RA compared to 37 people out of 100 who were on MTX (2% reduction)
Remission (DAS <1.6 or DAS28 < 2.6)
Biologic + MTX versus MTX: 37 people out of 100 who took a biologic (in combination with MTX) had their RA symptoms disappear compared to 22 people out of 100 who were on MTX (15% improvement).
Biologic monotherapy (TNF biologics) versus MTX: 22 people out of 100 who took a biologic had their RA symptoms disappear compared to 20 people out of 100 who were on MTX (2% improvement).
Progression of disease damage as measured on X‐rays (scale 0 to 448)
Biologic + MTX versus MTX: people who took a biologic (in combination with MTX) showed radiographic progression of 0.45 points compared to those on MTX who showed progression of 3 points (0.5% reduction).
There were no studies for biologic monotherapy.
Drug withdrawal due to adverse events
Biologic + MTX versus MTX: 7 people out of 100 who took a biologic (in combination with MTX) withdrew from the study due to adverse events compared to 5 out of 100 participants who took MTX (2% more).
Biologic monotherapy (TNF biologics) versus MTX: 6 people out of 100 who took a biologic withdrew from the study due to adverse events compared to 6 out of 100 participants who took MTX (0% difference).
Serious adverse events
Biologic + MTX versus MTX: 11 participants out of 100 who took a biologic (in combination with MTX) reported serious adverse events compared to 10 participants out of 100 on MTX (1% more serious adverse events).
Biologic monotherapy (TNF biologics) versus MTX: 3 participants out of 100 who took a biologic reported serious adverse events compared to 7 participants out of 100 on MTX (4% fewer serious adverse events).
Cancer
The same number of people (1 out of 100) reported cancer for biologic (both alone and in combination with MTX) and the comparator MTX. However, there were few events of cancer so caution in this interpretation is needed.
Background
Description of the condition
Rheumatoid Arthritis (RA) is a chronic inflammatory arthritis characterized by inflammation of the synovial lining of the joints, tendons and periarticular structures, with main disease features of joint pain, swelling and joint destruction (Lee 2001). RA affects 0.5% to 1.0% of the population (Kvien 2004) and frequently leads to health‐related quality of life (HRQoL) deficits (Kvien 2005; Lubeck 2004), functional limitation, and in people with refractory disease, untreated disease or longer disease duration, or both, to joint destruction, severe disability and disfigurement (Odegard 2005; Yelin 2007).
Pharmacological treatment options for RA include non‐steroidal anti‐inflammatory drugs (NSAIDs), glucocorticoids, traditional disease‐modifying anti‐rheumatic drugs (DMARDs), biologic DMARDs and oral small molecules (e.g. tofacitinib). Traditional DMARDs (referred to as DMARDs from here on), most commonly methotrexate (MTX), but also including sulfasalazine, hydroxychloroquine, leflunomide, cyclosporine etc., either alone or in combination, are usually the first choice drug for people with early or established RA; when these fail, biologics (alone or with DMARDs) or DMARD combinations or tofacitinib are treatment options (Singh 2012; Singh 2016a; Smolen 2014). DMARDs are the cornerstone of RA management since their use is associated with improvement in pain and physical function and a reduction in radiographic progression (Finckh 2006; Pincus 2002) and disability (Cash 1994; Strand 2008).
Description of the interventions
Biologics are newer treatment options available for treatment of RA. Biologics are medications that are made in live cell systems. Biologics are frequently categorized based on their mechanism of action. Two broad categories are biologics that do or do not inhibit tumor necrosis factor (TNF), a key cytokine in RA (Scott 2006). Further classification is based on whether the biologic is a receptor or an antibody (for TNF inhibitors ), or the specific cytokine/pathway/cell they inhibit (non‐TNF biologic). The currently approved biologics for the treatment of RA are:
-
TNF biologics
-
monoclonal antibodies against the TNF (TNF antibody biologic)
-
Soluble TNF receptor
etanercept (Enbrel, approved 1998) (FDA 1998a), that binds free‐circulating TNF so that it does not bind to the cellular receptor.
-
-
Non‐TNF biologics
-
anti‐CD28 therapy:
abatacept (Orencia, approved 2005) (FDA 2005)
-
anti‐B‐cell therapy:
rituximab (Rituxan/Mabthera, approved 1997 for lymphoma and 2006 for RA) (FDA 1997;Drugs 2006)
-
anti‐interleukin (IL)‐6 therapy:
tocilizumab (Actemra, approved 2010) (FDA 2010)
-
anti‐IL‐1 therapy:
anakinra (Kineret, approved 2001) (FDA 2001)
-
Tofacitinib (XELJANZ), an oral small molecule drug, (FDA 2012), was approved in 2012 in the USA. Biologics or tofacitinib provide clinically important improvements in pain, function and HRQoL in people not responding to traditional DMARDs such as methotrexate (MTX) (Boyce 2016; Strand 2008). Although biosimilars (generic medications for biologics) are available in the USA, Europe and other regions, these were not available at the literature review cut‐off. Therefore, our network meta‐analysis and systematic review includes biologics or tofacitinib.
How the intervention might work
Systemic and joint inflammation in RA is mediated by the activation of several potential targets including T‐cells (Cope 2008), B‐cells (Buggati 2014), macrophages (Szekanecz 2007), and other immune cells (Woolley 2003) in response to an environmental trigger/antigen, associated with expression of chemokines, metalloproteinases and inflammatory cytokines (TNF‐alpha, IL‐1, IL‐6 etc.) (Brennan 2008; Choy 2001) and activation of host cells such as fibroblasts, osteoclasts and chondrocytes leading to bone and cartilage destruction, a hallmark of RA (Brennan 2008; Connell 2006). Treatment guidelines published recently ( Saag 2008; Singh 2012; Singh 2016a; Smolen 2014) and consensus statements (Furst 2008; Furst 2010; Furst 2012) highlight the current evidence and the role of biologics or tofacitinib in the management of RA.
Why it is important to do this overview
Biologics are used in a variety of scenarios, most commonly when someone has a sub‐optimal response (DMARD/MTX‐inadequate responders (MTX‐IR)) or intolerance to traditional DMARDs such as MTX (Singh 2016a). Tofacitinib is also used in this patient population. It is not well known what role, if any, these drugs can play in the treatment of people with RA who are MTX‐naive, although this is not a current treatment option in many countries due to their greater costs. It is not clear whether or not they are more effective than traditional DMARDs and how the harms compare to traditional DMARDs in people with RA who are MTX‐naive.
Existing Cochrane systematic reviews of biologics have included trials that evaluated the benefits and harms of single biologics compared with either placebo, MTX or other DMARDs. But to inform choice of biologic, data about the comparative benefits and harms of different biologics is needed. Ideally, evaluation of comparative effectiveness requires head‐to‐head comparison studies, but when these are scant (Gabay 2013; Schiff 2008a; Weinblatt 2013), indirect comparisons that use a common comparator may be informative (Song 2003). Use of all available data from both direct and indirect comparisons is the essence of network meta‐analysis (NMA). Our review differs from the usual systematic reviews, in that it is not intended to examine only one intervention for RA but aims to systematically review and simultaneously compare the existing randomized trials of biologics or tofacitinib for RA and, while doing so, consider both direct and indirect evidence using a network meta‐analysis (NMA) (Becker 2008; Puhan 2014).
Our previous overview and NMA of biologics for RA was performed in 2009 (Singh 2009) and is ready for an update. Due to feasibility issues, an a priori decision was made to examine use of biologics or tofacitinib in four RA populations separately:
methotrexate‐naive (people who have not previously been treated with methotrexate; MTX‐naive) (this publication);
methotrexate/disease‐modifying anti‐rheumatic drug incomplete (inadequate) responder (MTX/DMARD‐IR, that is, people whose treatment with MTX/DMARDs failed due to lack of efficacy (primary or secondary), adverse event, patient preference etc. or a combination of these reasons), assessing the effect of biologic + MTX/DMARD (Singh 2016b);
methotrexate/disease‐modifying anti‐rheumatic drug incomplete (inadequate) responder (MTX/DMARD‐IR), assessing the effect of biologic monotherapy (Singh 2016c); and
biologic‐experienced (people whose treatment with biologic failed due to lack of efficacy (primary or secondary), adverse event, cost, patient preference etc. or a combination of these reasons (Singh 2017).
Objectives
To compare the benefits and harms of biologics (abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, tocilizumab) and small molecule tofacitinib versus comparator (methotrexate (MTX)/other DMARDs) in people with RA who are naive to methotrexate.
Methods
Criteria for considering reviews for inclusion
NOTE: this update uses individual studies, not reviews, for the basis of all analyses.
Randomized controlled trials (RCTs) of biologics or tofacitinib for RA in people who are MTX‐naive.
Types of studies
Our 2009 review only included studies that examined the efficacy/safety of standard‐dose biologics. for the 2015 update we expanded our inclusion criteria to include studies with any dose of biologic, provided they had clinically relevant outcomes. We included all nine approved biologics for RA (TNF biologics (adalimumab, certolizumab pegol, etanercept, golimumab, infliximab) and non‐TNF biologics (abatacept, anakinra, rituximab, tocilizumab)) and also searched for trials of tofacitinib.
Types of participants
Adults 18 years or older, with RA meeting the 1987 American College of Rheumatology (ACR) classification criteria for RA (Arnett 1988) or the 2010 ACR/European League Against Rheumatism (EULAR) classification criteria for RA (Aletaha 2010), who are MTX‐naive.
Types of interventions
TNF biologics: adalimumab, certolizumab pegol, etanercept, golimumab, infliximab; or non‐TNF biologics: abatacept, anakinra, rituximab, tocilizumab; or tofacitinib used alone or in combination with traditional DMARD/other biologic compared to placebo alone or to placebo plus traditional DMARDs or biologics or combinations of DMARDs.
Types of outcome measures
Primary/major outcomes
We pre‐specified seven outcomes, ACR50, Health Assessment Questionnaire (HAQ), RA disease remission, radiographic progression, withdrawals due to adverse events, serious adverse events (SAEs) and cancer.
ACR50, defined as 50% improvement in both tender and swollen joint counts and 50% improvement in at least three of the following five variables: patient global assessment, physician global assessment, pain score, function measurement with instruments such as HAQ score, and acute phase reactant (erythrocyte sedimentation rate (ESR) or C‐reactive protein (CRP) (Chung 2006; Felson 1995). We chose ACR50, as clinical and statistical evidence supports this as the preferred endpoint for contemporary RA clinical trials (Ghogomu 2014). We specified that we would assess outcomes at the longest duration of follow‐up. Most RA trials assess benefits and harms outcomes between four and six months, with some trials assessing longer‐term outcomes.
Function measured by HAQ score or modified HAQ calculated as score changes (Fries 1980; Pincus 1983) and the proportion achieving minimal clinically important difference on HAQ 0.22 or less (Wells 1993).
RA disease remission defined as DAS less than 1.6 or DAS28 less than 2.6 (Fransen 2005; Prevoo 1996).
Radiographic progression, as measured by Larsen/Sharp/modified Sharp scores (Larsen 1977; Sharp 1971; Van der Heijde 1989).
Withdrawals due to adverse events (Ioannidis 2004)
Serious adverse events (SAEs) (Ioannidis 2004)
Cancer
We recognize that RCTs included in this overview are limited in their ability to assess long term safety, since rare or delayed effects will not be detected. We therefore also searched websites of various regulatory agencies, including the US Food and Drug Administration (FDA), Health Canada and European Medicines Agency (EMA) to summarize warnings related to each of the biologics.
Search methods for identification of reviews
NOTE: this update uses individual studies, not reviews, for the basis of all analyses.
We conducted a search starting at the end date of last search and up to June 2015 (one search and analysis update to February 2014 and a second one to June 2015) for the 2015 update. A Cochrane Information Specialist (TR) conducted an updated search for the 2015 update to identify individual studies in multiple databases, namely: the Cochrane Central Register of Controlled Trials (CENTRAL; in The Cochrane Library, 2015, Issue 1), MEDLINE (via OVID 1946 to 11 February 2015), and Embase (via OVID 1947 to 11 February 2015). We considered the 31 studies in the 2009 version (Singh 2009), which contained all people with RA, including those that were MTX‐naive. We searched trials registers, including clinicaltrials.gov and the WHO trials register for ongoing studies, who.int/ictrp/en/.
Data collection and analysis
Selection of reviews
Two abstractors (SN/TC) reviewed the results of the search (titles and abstracts), and obtained the full texts of articles identified as relevant for this update.
Data extraction and management
Two pairs of abstractors (SN/TC; TC/JS), within each pair, independently extracted data from the reviews using a predefined data extraction form created as a Microsoft Excel® spreadsheet for the 2015 update and independently abstracted additional data for all doses (SN/JS) and additional outcomes, since the original review only included standard doses of biologics and not all outcomes were the same. TC double‐checked all data for accuracy after the initial abstraction.
We resolved disagreements by discussion with JS or GW, as appropriate. We obtained additional information from the original RCTs where necessary, from the online supplementary materials or by contacting study authors. AM and JS designed the spreadsheets.
Assessment of methodological quality of included reviews
NOTE: this update uses individual studies, not reviews, for the basis of all analyses.
Two abstractors (JS/TC; SN/TC) independently evaluated the risk of bias of included studies and overall quality of the evidence as summarized below.
Risk of bias of included trials
Two abstractors (JS/ETG) independently assessed risk of bias for each included trial using the Cochrane 'Risk of bias' tool. We assessed the risk of bias on each of the following criteria: random sequence generation, allocation concealment, presence of blinding (participants, personnel, and outcome assessors) in the studies, incomplete outcome data, and selective outcome reporting (Higgins 2011). The risk of bias was assessed as recommended: low risk, high risk, or unclear risk (either lack of information or uncertainty over the potential for bias). We resolved disagreements by discussion between the review authors.
Quality of evidence
Two review authors (JS and AM) independently assessed the overall quality of the evidence for each outcome using the GRADE approach (Guyatt 2008). The GRADE approach improves reliability in comparison to intuitive judgments about the certainty of a body of evidence (Mustafa 2013). The GRADE system specifies four levels of quality of evidence.
High quality for randomized trials; or double‐upgraded observational studies.
Moderate quality for downgraded randomized trials; or upgraded observational studies.
Low quality for double‐downgraded randomized trials; or observational studies.
Very low quality for triple‐downgraded randomized trials; or downgraded observational studies; or case series/case reports.
Randomized trial evidence could be downgraded by one or two levels depending on the presence of five factors.
Serious (‐1) or very serious (‐2) limitation to study quality
Important inconsistency (‐1)
Some (‐1) or major (‐2) uncertainty about directness
Imprecise or sparse data (‐1)
High probability of reporting bias (‐1)
Data synthesis
Statistical analyses
We performed the standard and NMA analyses including important factors such as the route of biologic (intravenous versus subcutaneous), dose (low dose (LD) versus standard dose (SD) versus high dose (HD)) and concomitant MTX/DMARD, for the 2015 update. We also performed pre‐specified analyses for subgroups by trial and RA disease duration, since they might contribute to differences in benefits and harms of biologics. In order to handle rare events in direct comparison meta‐analyses, we used Peto's odds ratios as the effect measure. For other outcomes, we used odds ratio (OR) or mean difference (MD) as effect measures. We considered P values less than 0.05 and 95% confidence intervals (CI) or credible intervals (CrI) that did not include 1 to be statistically significant.
The standard meta‐analysis (direct comparisons) determined the effectiveness of treatments directly compared to each other and was performed using Review Manager 5 (RevMan5) (RevMan 2014). We used the I2 statistic for quantifying heterogeneity of the results in individual studies (Higgins 2003), since heterogeneity is a common issue encountered while performing meta‐analyses ( Higgins 2002; Thompson 1999). This statistic combines the Chi2 statistic and the number of studies contributing to each summary estimate in the figure. In all the forest plots presenting effect measure data per treatment, we applied the random‐effects model as the default option (DerSimonian 2007) for illustrative purposes. We estimated the number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH), with 95% CIs on the basis of the derived OR comparing treatment to control and considering the overall event rate in the placebo group as a proxy for the community baseline event rate. This method enables direct translation into clinical practice (Osiri 2003), using Visual Rx with the overall (pooled) number of responders within the available studies as proxy for the expected rate of responders in a given RA population (Cates 2009).
We conducted a network meta analysis (NMA) based on a Bayesian mixed treatment comparison (MTC) approach, using the WinBUGS statistical software for the Bayesian analysis (MRC Biostatistics Unit, Cambridge, UK) (Spiegelhalter 2003). We performed a Markov Chain Monte Carlo (MCMC) simulation with at least 5000 or more iterations (as needed) to derive the corresponding 95% CrIs. We used informative priors for the variance parameters (Turner 2012). Where considered more suitable, we used vague priors for basic parameters. Assessment of model fit for the NMA was based on deviance information criterion (DIC) and comparison of residual deviance (Spiegelhalter 2003). We assessed trace plots and the Brooks‐Gelman‐Rubin statistic to ensure that convergence was reached (Spiegelhalter 2003). We applied the continuity correction for zero event cells to make non‐zero cells where needed. In order to assess inconsistency (conflict between direct and indirect evidence (Wells 2009), we compared deviance and deviance information criteria (DIC) statistics in fitted consistency and inconsistency models (Dias 2011) and examined the inconsistency plot. We chose between the random‐effects model and the fixed‐effect model based on the assessment of the DIC and comparison of residual deviance to number of unconstrained data points.
We used OR as effect measure for dichotomous outcomes, that is, the number of participants achieving ACR50, remission, serious adverse events, and withdrawals due to adverse events; and MD for continuous outcomes such as HAQ and radiographic progression. For cancer data, we anticipated that events would be rare (Bradburn 2007; Sweeting 2004). In order to handle these expected sparse data, we applied an empirical Bayes (treatment arm‐based) approach (Salanti 2008). AK and AH performed data analyses, under the supervision of GW.
Sub‐group analyses/planned comparisons
In addition to the biologic + MTX versus comparator and biologic alone (monotherapy) versus comparator analysis, we conducted the following a priori subgroup analyses using the standard meta‐analysis or NMA.
By type of biologic: TNF biologics versus non‐TNF biologics
By type of biologic, receptor versus antibody: medications targeting TNF receptor (etanercept) versus monoclonal antibodies against TNF (adalimumab, certolizumab pegol, golimumab, infliximab) versus non‐TNF biologic
-
By biologic dose: high‐dose (HD) versus standard‐dose (SD) versus low‐dose (LD) biologic. We expanded the definitions of standard dose of each biologic from 2009 to include the newer biologics and tofacitinib, as follows:
abatacept intravenous: every four weeks intravenously at 500 mg dose in people weighing less than 60 kg, 750 mg in people weighing 60 kg to 100 kg and 1000 mg in people weighing more than 100 kg, after the initial dosing regimen of baseline, two‐ and four‐week infusions;
abatacept subcutaneous: 125 mg subcutaneous weekly;
adalimumab: 40 mg subcutaneous every two weeks;
anakinra: 100 mg subcutaneous every day;
certolizumab pegol: 400 mg initially and at weeks 2 and 4, followed by 200 mg every other week (for maintenance dosing, 400 mg every four weeks can be considered);
etanercept: 25 mg subcutaneous twice weekly or 50 mg subcutaneous once weekly;
golimumab: 50 mg administered by subcutaneous injection once a month;
infliximab: 3 mg/kg intravenous every eight weeks after initial dosing at 0, 2 and 6 weeks;
rituximab: two 1000 mg intravenous doses two weeks apart;
tocilizumab intravenous: starting dose is 4 mg per kg every four weeks followed by an increase to 8 mg per kg every four weeks based on clinical response;
tocilizumab subcutaneous: 162 mg administered subcutaneously every other week, followed by an increase to every week based on clinical response for people weighing less than 100 kg, and 162 mg administered subcutaneously every week for people weighing 100 kg or more;
tofacitinib: 5 mg orally twice a day, or 10 mg once daily.
The following subgroup analyses, specified a priori, were also performed using NMA.
Trial duration: short duration (six months or less), intermediate duration (between six and 12 months) or long duration (more than 12 months)
RA disease duration: early RA (mean/median duration of less than two years) (Boers 2001), established RA (mean/median duration 2 to 10 years) or late RA (mean/median duration more than 10 years) (Barlow 1999).
Results
Description of included reviews
NOTE: this update uses individual studies, not reviews, for the basis of all analyses.
Figure 1 shows the overall study selection process. We identified a total of 19 trials from 8771 titles (including five of the 31 RCTs from the Cochrane Reviews in our original 2009 overview), including 6485 MTX‐naive participants. Data for analyses were available from all trials. In 17 trials the comparator was MTX, while MTX + methylprednisolone was the comparator in two studies (Durez 2007; Nam 2014a). Therefore, we refer to the comparator as "MTX" for simplicity throughout the Results, Abstract, Plain language summary, Discussion and tables.
1.
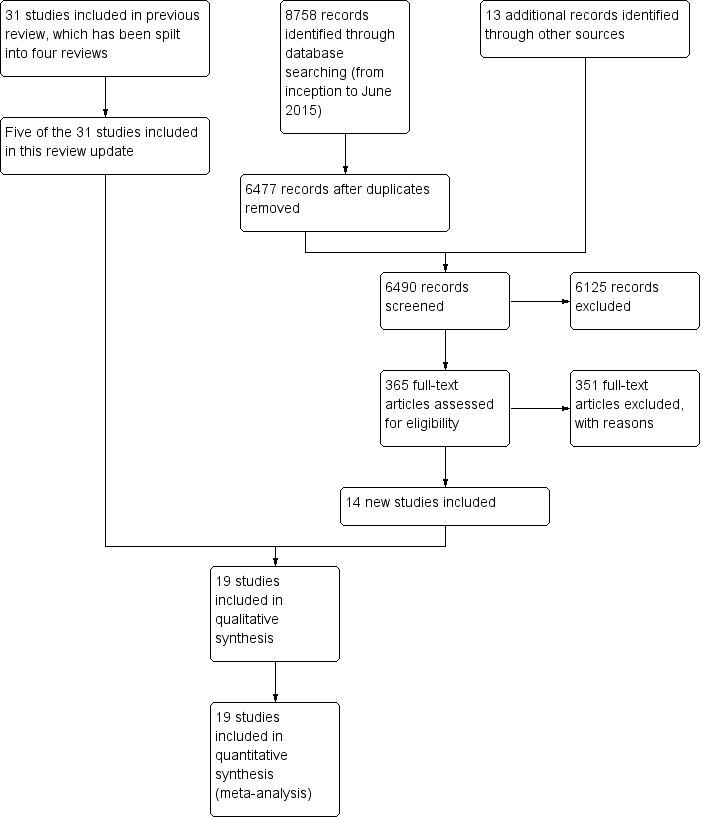
Study flow diagram
Characteristics of included studies are provided in Table 1. Study sample size ranged from 20 to 1049 participants. Nine studies were performed in participants with established RA (2 to 10 years), nine studies were performed in participants with early RA (less than two years) and one study included participants with unclear disease duration. These studies included four TNF biologics (adalimumab, etanercept, golimumab and infliximab) and two non‐TNF biologics (abatacept and rituximab).
1. Characteristics of included studies.
| Study name | Biologic(s) | Biologic dose(s) | Number of study arms | Non‐biologic comparator | Concomitant use of MTX | Trial duration | RA duration | Biologic‐naive | Total number of participants |
| Bejarano 2008 | Adalimumab | SD | 2 | MTX + PL | Yes | 13 months | Established | Yes | 148 |
| Bejarano 2010 | Infliximab | SD | 2 | MTX + PL | Yes | 8 years | Established | No | 20 |
| Breedveld 2006 | Adalimumab (+/‐ MTX) | SD | 3 | MTX + PL | Yes | 24 months | Established | Yes | 799 |
| Detert 2013 | Adalimumab | SD | 2 | MTX + PL | Yes | 12 months | Early | Yes | 172 |
| Durez 2007 | Infliximab | SD | 2 | MP + MTX | Yes | 12 months | Early | No | 29 |
| Emery 2008 (COMET) | Etanercept | SD | 2 | MTX + PL | Yes | 12 months | Established | Yes | 542 |
| Emery 2009 (GO‐BEFORE) | Golilumab (+/‐ MTX) | SD, HD | 4 | MTX + PL | Yes | 12 months | Established | No | 637 |
| Kavanaugh 2013 (OPTIMA) | Adalimumab | SD | 2 | MTX + PL | Yes | 6 months | Established | No | 1032 |
| Marcora 2006 | Etanercept | SD | 2 | MTX | No | 6 months | Unknown | Yes | 26 |
| Nam 2014a | Infliximab | SD | 2 | MP + MTX | Yes | 6 months | Early | Yes | 112 |
| Nam 2014b | Etanercept | SD | 2 | MTX + PL | Yes | 12 months | Early | Yes | 110 |
| Quinn 2005 | Infliximab | SD | 2 | MTX + PL | Yes | 12 months | Early | Yes | 20 |
| Rantalaiho 2014 | Infliximab | SD | 2 | MTX + PL | Yes | 24 months | Early | Yes | 93 |
| Soubrier 2009 | Adalimumab | SD | 2 | MTX + PL | Yes | 12 months | Established | No | 65 |
| St Clair 2004 (ASPIRE) | Infliximab | SD, HD | 3 | MTX + PL | Yes | 12 months | Early | Yes | 1049 |
| Tak 2012 | Rituximab | SD, LD | 3 | MTX + PL | Yes | 24 months | Early | Yes | 748 |
| Takeuchi 2014 | Adalimumab | SD | 2 | MTX + PL | Yes | 6 months | Early | Yes | 334 |
| Tam 2012 | Infliximab | SD | 2 | MTX | Yes | 12 months | Established | Yes | 40 |
| Westhovens 2009 | Abatacept | SD | 2 | MTX + PL | Yes | 24 months | Established | No | 509 |
HD: high dose; LD: low dose; MTX: methotrexate; PL: placebo; SD = standard dose
Eighteen trials included a biologic + MTX as the intervention of interest. Only one trial studied a biologic monotherapy (etanercept) (Marcora 2006). Most (n = 14) studies had a duration of 6 to 13 months, while five studies had a duration of 24 months or greater. We found no tofacitinib studies eligible for inclusion, since in the only study that enrolled participants who were MTX‐naive (Lee 2014), more than one‐third of the participants were not naive to other traditional DMARDs and data were not reported separately.
Three hundred and fifty‐one studies were excluded, and the main reasons for exclusion were wrong drug exposure, duplicate studies or abstracts. One hundred and twenty‐six of the 351 excluded studies were included in the other split updates. See additional details in Appendix 1. Ongoing trials listed in trial registers are provided in Appendix 2.
Methodological quality of included reviews
For the 2015 update, two reviewers abstracted these study characteristics from the published reports of the individual trials.
Risk of bias of included trials in the 2015 update
Detailed 'Risk of bias' assessments for each trial including the reasons for each judgment are available at the Cochrane Musculoskeletal website Risk of bias A and Risk of bias B.
Allocation (selection bias)
All trials were described as randomized, however, only eight of 19 (42%) reported adequate sequence generation and we assessed them as low risk, while 11 (58%) did not describe the method used and we assessed them as unclear risk. We assessed allocation concealment as low risk in seven (37%) trials, unclear in 11 (58%) trials, and high risk in one (5%) trial.
Blinding (performance and detection bias)
We judged a total of eight (42%) trials at low risk of performance bias, and seven (37%) at unclear risk of bias. In four (21%) trials, participants were not blinded and these trials were at high risk of performance bias.
We assessed low risk of detection bias in nine (47%) trials, high risk of detection bias in three (16%) and unclear risk in seven (37%) trials.
Incomplete outcome data (attrition bias)
We judged more trials (10 out of 19; 53%) at low risk of attrition bias and nine (47%) trials were at high risk of attrition bias because more than 20% of participants withdrew/dropped out.
Selective reporting (reporting bias)
We judged four (21%) trials at low risk of bias and fifteen (79%) trials as unclear risk, since the study protocols were not available and we did not have enough information in the study report to assess selective reporting.
Other potential sources of bias
We assessed major baseline imbalance and 17 (89%) trials had low risk of bias, and two (11%) had high risk of bias.
Effect of interventions
In comparison to the original 2009 version, the 2015 update has several new key aspects:
instead of six biologics, we included all nine biologics and tofacitinib in our search although only studies from six biologics met the criteria for this review;
we included cancer and serious adverse events as outcomes;
we included all doses of biologics and analyzed by dose;
we analyzed outcomes by whether MTX/other DMARDs were used concomitantly or not; and
we used a Bayesian approach rather than a frequentist approach for analyses and reported odds ratios and 95% CrI.
For the 2015 version, we extracted all relevant data from the included RCTs. We pre‐specified 7 outcomes, ACR50, HAQ, RA disease remission, radiographic progression, withdrawals due to adverse events, serious adverse events (SAEs) and cancer. Analyses and comparisons for all pre‐specified outcomes were performed where data were available.
We followed the principles below while describing results to keep this review as comprehensive as possible.
We first present the odds ratios for the biologic + MTX and biologic alone (monotherapy) versus comparator, followed by pre‐specified comparisons (e.g. TNF versus non‐TNF biologic), followed by subgroup analyses, where data were available.
In the odds ratio analyses, only the last set of odds ratios compare the biologic by dose; other analyses prior to the dose analysis include all doses and provide comparison by a different characteristic of interest, for example, the type of biologic.
In the main analyses, when not specified, the biologic is in standard dose. We specify high dose and low dose in every instance when considered.
For biologics that are approved for only one route of administration (i.e. intravenous or subcutaneous only), we do not specify the route. The mention of the drug without the route implies that the only approved route for the drug was: subcutaneous (SC) for adalimumab, certolizumab pegol, etanercept and golimumab; intravenous (IV) for infliximab, and rituximab. Since only two biologics are approved for both subcutaneous and intravenous use (tocilizumab and abatacept), we specify these routes when describing results for abatacept. For other biologics, when not specified, the approved route of use is implicit. We recognize that some trials prior to approval of biologics used a different route in many cases (e.g. intravenous for golimumab).
We refer to the lack of statistical significance at P < 0.05 as 'not associated' or 'not significantly associated'. We also have additional comments about clinical significance, where applicable.
'Summary of findings' table
The 'Summary of findings' table presents both the direct estimates of biologics versus MTX with the quality of evidence followed by estimates from the NMA with the quality of evidence (Table 2). Absolute and risk ratio differences are provided for each estimate. We converted from OR in the NMA to RR in the 'Summary of findings' table and Abstract for ease of interpretation for clinicians.
2. 'Summary of findings' table for biologics vs comparator in MTX/other DMARD‐naive people.
| Comparison | Direct evidence | Network meta‐analysis | ||||||
| Outcome: ACR50 |
No. of participants (studies) |
RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |
| Biologics + MTX | versus comparator | 5720 (14 studies) |
1.40 (1.30 to 1.49) | 16% (13% to 20%) NNTB = 7 (6 to 8) |
⊕⊕⊕⊖ moderate (downgraded for inconsistency)1 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) |
0.94 (0.73 to 1.22) | ‐2% (‐11% to 7%) NNTB = n/a |
⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 1.00 (0.82 to 1.21) | 0% (‐8% to 9%) NNTB = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 4463 (12 studies) |
1.44 (1.34 to 1.54) | 17% (13% to 21%) NNTB = 6 (5 to 8) |
⊕⊕⊕⊕ high3 | 1.42 (1.30 to 1.54) | 18% (13% to 22%) NNTB = 6 (5 to 8) |
⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) |
1.27 (1.14 to 1.42) | 13% (7% to 19%) NNTB = 8 (6 to 14) |
⊕⊕⊕⊕ high3 | 1.31 (1.11 to 1.52) | 13% (5% to 22%) NNTB = 8 (5 to 22) |
⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
|
Outcome: HAQ score 0‐3 (higher = worse): a measure of function |
No. of participants (studies) |
Direct evidence | Network meta‐analysis | |||||
| MD (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | MD (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 3872 (13 studies) |
‐0.10 (‐0.16 to ‐0.04) | ‐3.3% (‐5.3% to ‐1.3%) NNTB = 4 (2 to 15) |
⊕⊕⊕⊖ moderate (downgraded for inconsistency)5 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 557 (2 studies) |
0.09 (‐0.24 to 0.41) | 3% (‐8% to 13.7%) NNTB = n/a |
⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 0.17 (‐0.19 to 0.54) | 5.7% (‐6.3% to 18%) NNTB = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 2615 (11 studies) |
‐0.09 (‐0.26 to 0.07) | ‐3% (‐8.7% to 2.3%) NNTB = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,6 | ‐0.08 (‐0.25 to 0.07) | ‐2.7% (‐8.3% to 2.3%) NNTB = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) |
‐0.22 (‐0.26 to ‐0.18) | ‐7.3% (‐8.7% to ‐6%) NNTB = 2 (2 to 3) |
⊕⊕⊕⊖ moderate (downgraded for inconsistency)7 | ‐0.22 (‐0.55 to 0.11) | ‐7.3% (‐18.3% to 3.7%) NNTB = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
|
Outcome: Remission (defined as DAS < 1.6 or DAS28 < 2.6) |
No. of participants (studies) |
Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 5128 (15 studies) |
1.62 (1.33 to 1.98) | 15% (11% to 19%) NNTB = 5 (6 to 7) |
⊕⊕⊕⊖ moderate (downgraded for inconsistency)8 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) |
1.08 (0.83 to 1.41) | 2% (‐3% to 8%) NNTB = n/a |
⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 1.02 (0.74 to 1.39) | 1% (‐7% to 11%) NNTB = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 4463 (12 studies) |
1.55 (1.22 to 1.96) | 14% (9% to 19%) NNTB = 7 (5 to 10) |
⊕⊕⊕⊖ moderate (downgraded for inconsistency)9 | 1.62 (1.40 to 1.86) | 18% (12% to 23%) NNTB = 7 (5 to 10) |
⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) |
2.10 (1.45 to 3.04) | 19% (15% to 24%) NNTB = 6 (4 to 9) |
⊕⊕⊕⊖ moderate (downgraded for inconsistency)10 | 1.85 (1.46 to 2.28) | 24% (13% to 35%) NNTB = 6 (4 to 10) |
⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
|
Outcome: Radiographic progression on Sharp/Van der Heijde modification (0‐448 points) |
No. of participants (studies) |
Direct evidence | Network meta‐analysis | |||||
| MD (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | MD (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 2256 (5 studies) |
‐2.56 (‐6.03 to 0.92) | ‐0.57% (‐1.35% to 0.21%) NNTB = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,11 | n/a | ||
| TNF biologic + MTX | versus comparator | 1747 (4 studies) |
‐3.18 (‐6.80 to 0.43) | ‐0.71% (‐1.52% to 959.82%) NNTB = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,12 | ‐3.73 (‐5.78 to ‐1.62) | ‐0.83% (‐1.29% to ‐0.36%) NNTB = 3 (3 to 7) |
⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 509 (1 study) |
‐0.43 (‐2.04 to 1.18) | ‐0.22% (‐0.46% to 0.26%) NNTB = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and inconsistency)2,11 | ‐0.42 (‐4.22 to 3.41) | ‐0.09% (‐0.94% to 0.76%) NNTB = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Outcome: Withdrawals due to adverse events |
No. of participants (studies) |
Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 5800 (14 studies) |
1.32 (0.89 to 1.97) | 2% (0% to 4%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for inconsistency and imprecision )1,2 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) |
1.14 (0.62 to 2.10) | 0% (‐4% to 4%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.93 (0.41 to 1.90) | 0% (‐2% to 3%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 4543 (12 studies) |
1.60 (1.10 to 2.32) | 3% (1% to 4%) NNTH = 35 (17 to 183) |
⊕⊕⊕⊖ moderate (downgraded for imprecision)14 | 1.68 (1.16 to 2.56) | 3% (1% to 5%) NNTH = 31 (14 to 138) |
⊕⊕⊕⊖ moderate (downgraded for indirectness)4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) |
0.56 (0.31 to 1.01) | ‐2% (‐5% to 1%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.56 (0.25 to 1.29) | ‐2% (‐3% to 1%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Outcome: Serious adverse events |
No. of participants (studies) |
Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 4850 (12 studies) |
1.05 (0.87 to 1.26) | 1% (‐1% to 3%) NNTH = n/a |
⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 319 (1 study) |
0.46 (0.16 to 1.29) | ‐4% (‐8% to 1%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.52 (0.16 to 1.30) | ‐5% (‐9% to 3%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 3593 (10 studies) |
1.14 (0.92 to 1.42) | 1% (‐1% to 3%) NNTH = n/a |
⊕⊕⊕⊖ moderate (downgraded for imprecision)2 | 1.16 (0.90 to 1.51) | 2% (‐1% to 4%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Non‐TNF biologic + MTX | versus comparator | 1257 (2 studies) |
0.87 (0.64 to 1.18) | ‐1% (‐5% to 2%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.87 (0.57 to 1.34) | ‐1% (‐4% to 3%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
|
Outcome: Cancer (note: Peto OR used but can interpret as RR due to low event rate) |
No. of participants (studies) |
Direct evidence | Network meta‐analysis | |||||
| RR (95% CI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | RR (95% CrI) | Absolute risk difference, NNTB | Quality of evidence (GRADE) | |||
| Biologics + MTX | versus comparator | 4611 (11 studies) |
0.71 (0.38 to 1.33) | 0% (0% to 0%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | n/a | ||
| TNF biologic alone (without MTX) | versus comparator | 850 (2 studies) |
0.79 (0.24 to 2.61) | 0% (‐2% to 1%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.94 (0.25 to 3.18) | 0% (‐1% to 2%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| TNF biologic + MTX | versus comparator | 3863 (10 studies) |
0.77 (0.35 to 1.69) | 0% (0% to 0%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.81 (0.36 to 1.73) | 0% (‐1% to 0%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
| Non‐TNF biologic + MTX | versus comparator | 748 (1 study) |
0.62 (0.22 to 1.77) | 0% (‐3% to 1%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for serious imprecision)13 | 0.64 (0.20 to 2.12) | 0% (‐1% to 1%) NNTH = n/a |
⊕⊕⊖⊖ low (downgraded for imprecision and indirectness)2,4 |
Comparator = MTX and/or DMARD
GRADE Working Group grades of evidence High quality (⊕⊕⊕⊕): we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality (⊕⊕⊕⊖): we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality (⊕⊕⊖⊖): our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality (⊕⊖⊖⊖): we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
CI: confidence interval; CrI; credible interval; DAS: Disease Activity Score; DMARD: disease‐modifying anti‐rheumatic drug; MTX: methotrexate; n/a: not available; NNTB/NNTH: number needed to treat for an additional beneficial/harmful outcome; OR: odds ratio; RR: risk ratio; TNF: tumor necrosis factor
1Downgraded for inconsistency: I2= 51%. 2Downgraded for imprecision: 95% CI estimate includes both null effect and appreciable benefit or harm. 3No evidence of imprecision or inconsistency. Number of events > 300. 4Downgraded for indirectness/intransitivity due to differing participant characteristics (established vs late RA; types of failures); differing biologic doses and co‐interventions; and differing comparators. 5Downgraded for inconsistency: I2= 93%. 6Downgraded for inconsistency: I2= 90%. 7Downgraded for inconsistency: I2= 95%. 8Downgraded for inconsistency: I2= 75%. 9Downgraded for inconsistency: I2= 81%. 10Downgraded for inconsistency: I2= 65%. 11Downgraded for inconsistency: I2= 97%. 12Downgraded for inconsistency: I2= 96%. 13Downgraded twice for serious imprecision ‐ few events (< 300) and 95% CI estimate includes both null effect and appreciable benefit or harm. 14Downgraded for imprecision ‐ few events (< 300)
Direct estimates were fairly consistent with the NMA estimates for all seven outcomes. There was moderate‐quality evidence (downgraded for inconsistency) that biologics were associated with superior clinically meaningful and statistically significant improvements versus comparator in ACR50 and RA disease remission and physical function as measured by the HAQ did show a statistically significant difference but was not clinically meaningful. There was low‐quality evidence (downgraded for inconsistency and imprecision) that radiographic progression was not clinically meaningfully or statistically significantly reduced in those on biologics versus MTX. Based on moderate‐quality evidence, results for serious adverse events showed no statistically significant or clinically meaningful differences. Based on low‐quality evidence, results for withdrawals due to adverse and cancer were inconclusive, since the estimates included null effect as well as possibility of important harm.
Findings separately by TNF biologic and non‐TNF biologic
In standard meta‐analysis, based on high‐quality evidence, biologic + MTX was also associated with statistically significant and clinically meaningful higher odds of ACR50 compared to the comparator in both TNF biologic and non‐TNF biologic subgroups with risk ratio (RR) of 1.44 (95% CI 1.34 to 1.54) and 1.27 (95% CI 1.14 to 1.42) and absolute difference 17% (95% CI 13% to 21%) and 13% (95% CI 7% to 19%), and NNTB = 6 (95% CI 5 to 8) and = 8 (95% CI 6 to 14), respectively. Results were similar for the NMA.
In standard meta‐analysis, based on low‐quality evidence, compared to MTX, TNF biologic + MTX was associated with lower HAQ scores with better HAQ score improvement with mean difference of ‐0.09 (95% CI ‐0.26 to 0.07), which was neither statistically significant nor clinically meaningful. Based on moderate‐quality evidence, non‐TNF biologic + MTX was associated with better HAQ scores with statistically significant and clinically meaningful HAQ score improvement with a mean difference of ‐0.22 (95% CI ‐0.26 to ‐0.18) and an absolute difference of ‐7.3% (95% CI ‐8.7% to ‐6%) compared to MTX. Results did not show evidence of a clinically meaningful or statistically significant difference in TNF biologic monotherapy versus MTX.
In standard meta‐analysis, based on moderate‐quality evidence, TNF biologic + MTX showed a statistically significant and clinically meaningful higher rate of remission with RR of 1.55 (95% CI 1.22 to 1.96) and absolute difference 14% (95% CI 9% to 19%) and NNTB = 7 (95% CI 5 to 10), as did non‐TNF biologic + MTX with RR 2.10 (95% CI 1.45 to 3.04), absolute difference 19% (95% CI 15% to 24%) and NNTB = 6 (95% CI 4 to 9). Results were similar in the NMA.
In standard meta‐analysis, based on low‐quality evidence, TNF biologic + MTX showed a non‐statistically significant improvement in radiographic progression versus MTX with risk ratio of ‐3.18 (95% CI ‐6.80 to 0.43), absolute difference ‐0.71% (95% CI ‐1.52% to 959%). In NMA, this comparison was statistically significant with a difference of ‐3.73 (95% CrI ‐5.78 to ‐1.62), absolute difference , ‐0.83% (95% CI ‐1.29% to ‐0.36%) and NNTB = 3 (95%CI, 3 to 7), but the clinical significance of this difference was unclear. Non‐TNF biologic + showed a much lower non‐statistically significant improvement with an RR of ‐0.40 (95% CI ‐2.04 to 1.18), absolute difference ‐0.22% (95% CI ‐0.46% to 0.26%), which was also not significantly different in the NMA.
In standard meta‐analysis, based on moderate‐quality evidence, there was a clinically meaningful and statistically significant difference in increase in withdrawals due to adverse events in TNF biologic + MTX versus MTX with a RR of 1.60 (95% CI 1.10 to 2.32), absolute difference 3% (95% CI 1% to 4%) and NNTH = 35 (95% CI 17 to 183). Based on low‐quality evidence, there was no evidence of a clinically meaningful and statistically significant difference in this outcome among the non‐TNF biologic + MTX versus MTX, RR of 0.56 (95% CI 0.31 to 1.01), absolute difference ‐2% (95% CI ‐5% to 1%). Results were similar in the NMA.
In standard meta‐analysis, based on moderate‐quality evidence, there was no evidence of a statistically significant or clinically meaningful difference in serious averse events for TNF biologic + MTX versus MTX with a RR of 1.14 (95% CI 0.92 to 1.42), absolute difference 1% (95% CI ‐1% to 3%). Based on low‐quality evidence, there was no evidence of a clinically meaningful and statistically significant difference in the non‐TNF biologic + MTX versus MTX with a RR of 0.87 (95% CI 0.64 to 1.18), absolute difference ‐1% (95% CI ‐5% to 2%).
In standard meta‐analysis, based on low‐quality evidence, results were inconclusive for the risk of cancer for statistically significant or clinically meaningful difference for biologic + MTX versus MTX with a Peto's OR of 0.71 (95% CI 0.38 to 1.33) and an absolute difference of 0% (95% CI 0% to 0%). Results were also inconclusive for TNF biologic monotherapy versus MTX (there were no data for non‐TNF monotherapy). Results were similar in the NMA.
Number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH)
For ACR50, HAQ and remission, NNTB for biologics + MTX were 7, 4 and 5, respectively. NNTB ranged from 6 to 8 for ACR50 among TNF and non‐TNF biologic subgroups (both in combination with MTX). For HAQ, NNTB was 2 in the non‐TNF biologic (+ MTX) subgroup. NNTBs ranged from 6 to 7 among TNF and non‐TNF biologic subgroups for remission (both in combination with MTX). NNTBs for radiographic progression were not calculable for the direct comparisons but was 3 for the TNF biologic + MTX subgroup versus comparator in the NMA.
For the harms outcomes, only withdrawals due to adverse events provided an NNTH of 35 for the direct comparison for TNF biologic + MTX subgroup and 31 for the same subgroup in the NMA.
Since there were no statistically significant differences between TNF biologic monotherapy versus MTX (active comparator), NNTB and NNTH could not be calculated. All studies of biologic monotherapy were for TNF biologic monotherapy only, with none for non‐TNF biologic monotherapy.
Main analysis: comparison of the biologics with regard to benefit and harm
Primary/major benefit outcome: ACR50
Fourteen studies with 6153 participants reported ACR50. Of these, all studies included at least one arm with participants on a biologic with concomitant MTX. An example of a network diagram for MTX‐naive is shown in Figure 2 for ACR50.
2.
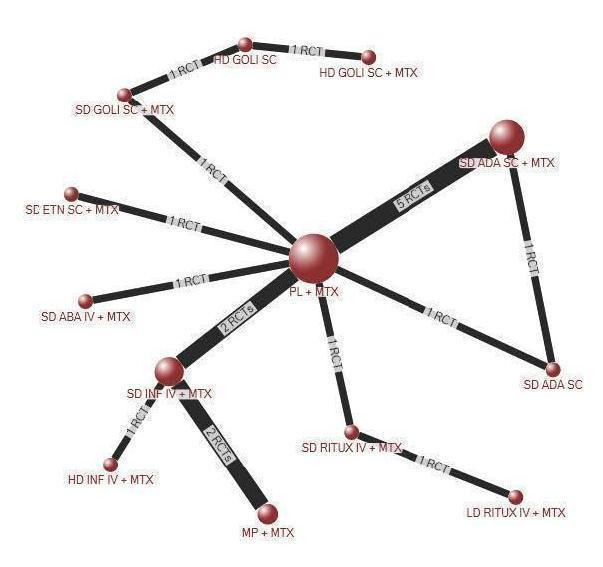
Network diagram: ACR50 in people with rheumatoid arthritis who were MTX/other DMARD‐naive
Odds ratios using standard meta‐analyses
Biologic + MTX versus active comparator (mostly MTX) (14 studies)
Biologics + MTX were associated with a clinically meaningfully and statistically significantly higher odds of achieving an ACR50 response, OR 1.95 (95% CI 1.71 to 2.23), absolute difference of 16% (95% CI 13% to 20%) and NNTB = 7 (95% CI 6 to 8) with an I2 of 22% indicating heterogeneity that might not be important; (Figure 3) (moderate‐quality evidence).
3.
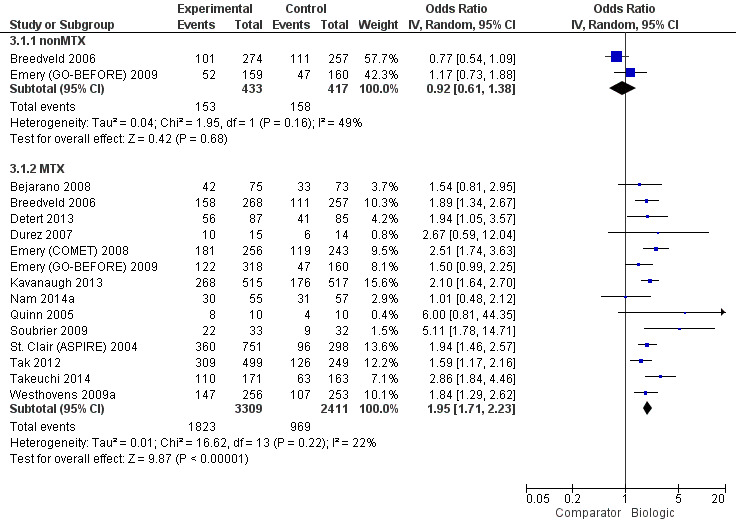
ACR50: biologic (with and without concomitant MTX) versus comparator
Biologic without MTX versus active comparator (2 studies)
There was no evidence to indicate an effect of biologics without MTX compared to MTX, for achieving an ACR50 response, with an OR 0.92 (95% CI 0.61 to 1.38), absolute difference of ‐2% (95% CI ‐11% to 7%) with an I2 of 49% indicating moderate heterogeneity; (Figure 3) (moderate‐quality evidence).
Odds ratios by biologic type and dose using NMA
The overall rate of ACR50 by the type of biologic and the dose were as follows (14 studies, 6153 participants).
-
Type of biologic, TNF versus non‐TNF biologic:
biologic + MTX: compared to TNF biologic, non‐TNF biologic was not associated with any statistically significant or clinically meaningful difference in the odds of ACR50, OR: 0.84 (95% CrI 0.57 to 1.23);
biologic alone without MTX: no studies were available for analysis.
-
Type of biologic, etanercept versus TNF antibody biologic versus non‐TNF biologic:
biologic + MTX: compared to monoclonal TNF antibody biologic, neither non‐TNF biologic nor etanercept were associated with any statistically significant or clinically meaningful differences in ACR50 rates, OR: 0.86 (95% CrI 0.56 to 1.28) and OR: 1.27 (95% CrI 0.72 to 2.25), respectively;
biologic alone without MTX: no studies were available for analysis.
-
Biologic dose, SD versus LD versus HD biologic:
biologic + MTX: compared to SD biologic, HD and LD biologic were not associated with any statistically significant or clinically meaningful differences in the odds of ACR50 at OR: 1.00 (95% CrI 0.74 to 1.33) and OR: 0.80 (95% CrI 0.53 to 1.20), respectively; LD was not statistically significantly less likely than HD to be associated with ACR50, OR: 0.80 (95% CrI 0.50 to 1.33);
biologic alone without MTX: compared to SD biologic, HD biologic was associated with no statistically significant or clinically meaningful differences in odds of ACR50 at OR: 1.80 (95% CrI 0.98 to 3.30).
Main analyses using NMA
Fourteen RCTs (ten 2‐arm, three 3‐arm, and one 4‐arm trial) enrolling 6153 participants provided data for all dose analyses (Appendix 3). Five SD biologics + MTX (adalimumab, rituximab, infliximab, etanercept, abatacept intravenous) were superior to placebo + MTX for ACR50 rates, with OR ranging from 1.84 to 2.52; HD infliximab + MTX was also superior to placebo + MTX. Five biologics in SDs + MTX (adalimumab, rituximab, infliximab, etanercept, abatacept) were superior to SD adalimumab monotherapy for ACR50 with OR ranging 2.08 to 3.08; HD infliximab + MTX was associated with 2.69‐times odds compared to SD adalimumab monotherapy.
Subgroup analyses by RA disease duration (early versus established versus late RA)
Early RA (RA disease duration less than two years)
There were not enough data to perform NMA.
Established RA (disease duration 2 to 10 years)
Compared to placebo + MTX, SD adalimumab subcutaneous + MTX and SD etanercept + MTX, were associated with statistically significantly higher OR of ACR50 of 2.07 and 2.52, respectively. Compared to SD adalimumab subcutaneous, SD adalimumab subcutaneous + MTX and SD etanercept + MTX were associated with statistically significantly higher OR of ACR50: 2.58 and 3.13, respectively (3689 participants, 7 studies) (Appendix 4).
Late RA (disease duration more than 10 years)
There were not enough data to perform NMA.
Subgroup analyses by trial duration
Trial duration, six months or less
Compared to placebo + MTX, SD adalimumab subcutaneous + MTX was associated with 2.41‐times higher odds of ACR50 (2240 participants, 5 studies) (Appendix 5).
Trial duration, between six and 12 months
Compared to placebo + MTX, SD infliximab + MTX was associated with 1.94‐times higher odds of ACR50 (2106 participants, 5 studies) (Appendix 6).
Trial duration, between six and 12 months
Compared to placebo + MTX, SD adalimumab + MTX was associated with 1.78‐times higher odds of ACR50. Compared to SD adalimumab subcutaneous, SD adalimumab subcutaneous + MTX was associated with 2.40‐times higher OR of ACR50 (1695 participants, 3 studies) (Appendix 7).
Primary/major benefit outcome‐ HAQ
14 studies with 4,172 participants reported data on HAQ scores on a 0 to 3 scale. Of these, 13 studies included at least one arm with participants on a biologic with concomitant MTX and 1 study had only biologic without MTX).
Mean difference using standard meta‐analyses
Biologic + MTX versus active comparator
Compared to MTX, biologic + MTX use was associated with a statistically significant mean difference (MD) in HAQ scores ‐0.10 (95% CI‐0.16 to ‐0.04, absolute difference ‐3.3% (95% CI ‐5.3% to ‐1.3%), NNTB = 4 (95% CI 2 to 15) with I2 = 94%) indicating considerable heterogeneity and the difference may not be clinically meaningful; (Figure 4) (moderate‐quality evidence). When we excluded three studies contributing to high heterogeneity (Durez 2007; St Clair 2004; Tak 2012), biologic + MTX use was associated with a statistically significant and potentially clinically meaningful mean difference in HAQ scores ‐0.20 versus comparator (mostly MTX) (95% CI‐0.25, ‐0.15), I2 = 17%, indicating heterogeneity that might not be important.
4.
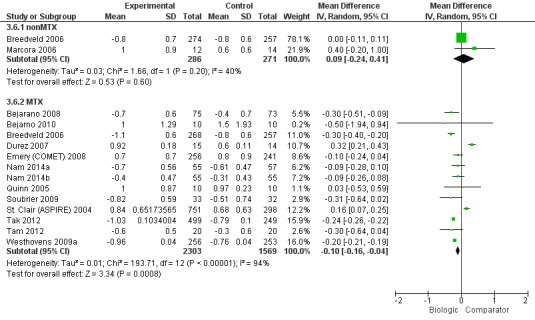
HAQ: biologic (with and without concomitant MTX) versus comparator
Biologic without MTX versus active comparator
Compared to MTX, biologics without MTX was not associated with evidence of any statistically significant mean difference in HAQ scores 0.09 (95% CI ‐0.24 to 0.41, absolute difference 3% (95% CI ‐8% to 13.7%), I2 = 40%) indicating moderate heterogeneity; this difference may not be clinically meaningful; (Figure 4) (moderate‐quality evidence).
Odds ratios by biologic type and dose using NMA
The overall HAQ scores by the type of biologic and the dose was as follows (4,172 participants, 14 studies).
-
Type of biologic, TNF versus non‐TNF biologic:
biologic + MTX: compared to TNF biologic, non‐TNF biologic did not show a significant or clinically meaningful difference in HAQ scores, MD: ‐0.14 (95% CrI ‐0.50 to 0.23);
biologic alone without MTX: there were no studies to perform this analysis.
-
Type of biologic, etanercept versus TNF antibody biologic versus non‐TNF biologic:
biologic + MTX: compared to monoclonal TNF antibody biologic, neither non‐TNF biologic nor etanercept were associated with any statistically significant or clinically meaningful differences in HAQ scores, MD: ‐0.13 (95% CrI ‐0.54 to 0.29) and MD: ‐0.01 (95% CrI ‐0.42 to 0.43), respectively. Compared to non‐TNF biologic, etanercept was not associated with a statistically significant or clinically meaningful difference in HAQ scores, MD: 0.13 (95% CrI ‐0.40 to 0.66).
biologic alone without MTX: Compared to monoclonal TNF antibody biologic, etanercept was not associated with a statistically significant or clinically meaningful difference in HAQ scores, MD: 0.30 (95% CrI ‐0.61 to 1.20).
-
Biologic dose, SD versus LD versus HD biologic:
biologic + MTX: compared to SD biologic, LD biologic was not associated with any statistically significant difference in HAQ scores, MD: ‐0.06 (95% CrI ‐0.45 to 0.33). HD biologic was not associated with any statistically significant or clinically meaningful difference in HAQ scores compared to SD biologic, MD: 0.20 (95% CrI ‐0.20 to 0.60) and LD biologic, MD: 0.26 (95% CrI ‐0.29 to 0.80).
biologic alone without MTX: There were no studies to perform this analysis.
Main analyses using NMA
Fourteen studies (eleven 2‐arm and three 3‐arm trials) with 4172 participants provided HAQ data in MTX‐naive participants (Appendix 8). Compared to MTX, SD adalimumab + MTX was associated with statistically significantly better HAQ score, the mean difference being ‐0.30 (95% CI ‐0.59 to ‐0.02), which was also clinically meaningful.
Subgroup analyses by RA disease duration (early versus established versus late RA)
Early RA (RA disease duration less than two years)
Four 2‐arm and two 3‐arm trials provided HAQ data. There were no statistically significant differences between various treatments (2,068 participants, 6 studies) (Appendix 9).
Established RA (disease duration 2 to 10 years)
Six 2‐arm and one 3‐arm trial provided HAQ data. There were no statistically significant differences between various treatments (2,078 participants, 7 studies) (Appendix 10).
Late RA (disease duration more than 10 years)
There were not enough data to perform NMA.
Subgroup analyses by trial duration
Trial duration, six months or less
All studies that provided HAQ data were two‐arm trials. There were no statistically significant differences between various treatments (243 participants, 4 studies) (Appendix 11).
Trial duration, between six and 12 months
Five 2‐arm and one 3‐arm trials provided HAQ data. There were no statistically significant differences between various treatments (2214 participants, 6 trials) (Appendix 12).
Trial duration, between six and 12 months
Two 2‐arm and two 3‐arm trials provided HAQ data. There were no statistically significant differences between various treatments (1715 participants, 4 studies) (Appendix 13).
Primary/major benefit outcome: Remission
Fifteen studies with 5561 participants reported data on remission (defined as DAS less than 1.6 or DAS28 less than 2.6). Of these, all studies included at least one arm with participants on a biologic with concomitant MTX.
Odds ratios using standard meta‐analysis
Biologic + MTX versus active comparator
The odds of remission with biologic + MTX was statistically significantly and clinically meaningfully higher, OR 2.23 (95% CI 1.82 to 2.73), absolute difference 15% (95% CI 11% to 19%), NNTB = 5 (95% CI 6 to 7); I2 of 41%, indicating moderate heterogeneity (Figure 5) (moderate‐quality evidence)
5.
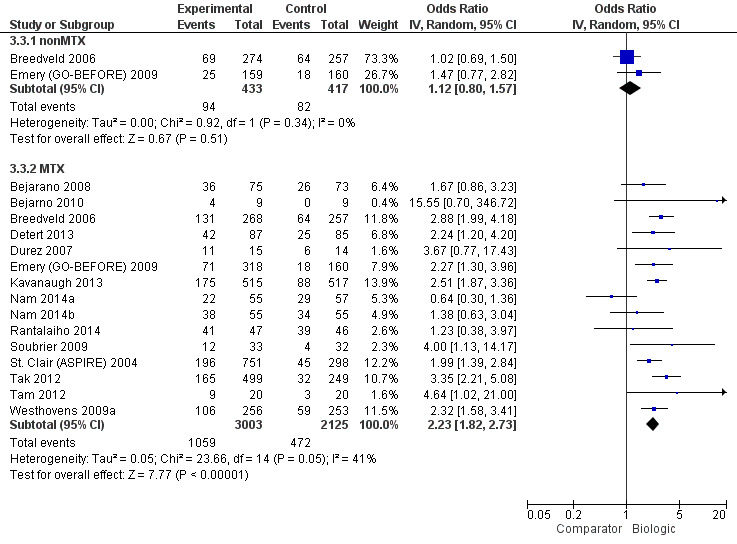
Remission: biologic (with and without concomitant MTX) versus comparator
Biologic without MTX versus active comparator
There was no evidence of a statistically significantly and clinically meaningfully difference, OR 1.12 (95% CI 0.08 to 1.57), absolute difference 2% (95% CI ‐3% to 8%), I2 of 0%, indicating heterogeneity that might not be important (Figure 5) (moderate‐quality evidence).
Odds ratios by biologic type and dose using NMA
The odds ratios (95% CrI) of remission by the type of biologic and the dose were as follows (5561 participants, 15 studies).
-
Type of biologic, TNF versus non‐TNF biologic:
biologic + MTX: compared to non‐TNF biologic + MTX, TNF biologic + MTX was not associated with any statistically significant or clinically meaningful difference in the odds of remission, OR: 1.30 (95% CrI 0.79 to 2.19);
biologic alone without MTX: there were no data to perform this analysis.
-
Type of biologic, etanercept versus TNF antibody biologic versus non‐TNF biologic:
biologic + MTX: compared to monoclonal antibody TNF biologic, monoclonal antibody TNF biologic + MTX was not associated with any statistically significant or clinically meaningful difference in the odds of remission, OR: 1.26 (95% CrI 0.76 to 2.15). There were no statistically significant or clinically meaningful differences between etanercept + MTX and monoclonal antibody TNF biologic + MTX, OR: 0.62 (95% CrI 0.24 to 1.67);
biologic alone without MTX: there were no data to perform this analysis.
-
Biologic dose, SD versus LD versus HD biologic:
biologic + MTX: compared to SD biologic monotherapy, HD biologic and LD biologic monotherapy were associated with no statistically significant or clinically meaningful difference in odds of remission, OR: 1.13 (95% CrI 0.68 to 1.78) and OR: 1.29 (95% CrI 0.69 to 2.50).
biologic alone without MTX: compared to SD biologic, HD biologic was associated with no statistically significant or clinically meaningful difference in odds of remission, OR: 1.72 (95% CrI 0.65 to 4.38).
Main analyses using NMA
Fifteen studies (eleven 2‐arm, three 3‐arm, and one 4‐arm trial) with 5561 participants provided remission data in MTX‐naive participants (Appendix 14). Compared to MTX, several biologics were associated with higher odds of disease remission:
SD infliximab + MTX, OR 1.82;
SD adalimumab + MTX, OR 2.55;
SD abatacept intravenous + MTX, OR 2.33;
SD golimumab subcutaneous + MTX, OR 2.69;
SD rituximab + MTX, OR 3.22;
LD rituximab + MTX, OR 3.55;
HD infliximab + MTX, OR 2.80.
Compared to SD abatacept subcutaneous, the following combinations with MTX were each associated with higher odds of RA disease remission:
SD adalimumab + MTX, OR 2.69;
SD golimumab, OR 2.85;
SD rituximab + MTX, OR 3.40;
LD rituximab + MTX, OR 3.75;
HD infliximab + MTX, OR 2.96.
Subgroup analyses by RA disease duration (early versus established versus late RA)
Early RA (RA disease duration less than two years)
There were five 2‐arm and three 3‐arm trials. Compared to MTX + placebo, SD rituximab + MTX and LD rituximab + MTX were associated with statistically significantly higher odds of remission, OR: 3.20 (95% CrI 1.24 to 8.66) and OR: 3.54 (95% CrI 1.35 to 9.45), respectively (2313 participants, 7 studies) (Appendix 15).
Established RA (disease duration 2 to 10 years)
There were six 2‐arm trials and one each of 3‐arm and 4‐arm trials. Compared to MTX + placebo, SD infliximab + MTX, SD adalimumab + MTX, SD abatacept + MTX and SD golimumab + MTX were associated with statistically significantly higher odds of remission, with ORs ranging from 2.32 to 7.01 (3248 participants, 8 studies) (Appendix 16).
Late RA (disease duration more than 10 years)
There were no studies for late RA.
Subgroup analyses by trial duration
Trial duration, six months or less
There were five 2‐arm trials and one 4‐arm trial. Compared to MTX + placebo, the following were associated with higher odds of remission: methylprednisolone + MTX, OR: 8.44; SD adalimumab + MTX, OR: 2.67; and SD golimumab + MTX, OR: 2.70 (2058 participants, 6 studies) (Appendix 17).
Trial duration, between six and 12 months
There were three 2‐arm trials and one 3‐arm trial. None of the comparisons were statistically significant (1697 participants, 4 studies) (Appendix 18).
Trial duration, between six and 12 months
There were three 2‐arm and two 3‐arm trials. Compared to MTX, SD rituximab + MTX and LD rituximab + MTX were associated with higher odds of remission, ORs ranging from 2.42 to 3.55. Compared to SD adalimumab, SD adalimumab + MTX and LD rituximab + MTX were associated with higher odds of remission, OR: 2.63 and 3.85, respectively (1806 participants, 5 studies) (Appendix 19).
Primary/major benefit outcome: Radiographic progression
Five studies with 2256 participants reported data on radiographic progression. Of these, all studies had at least one arm with participants on a biologic with concomitant MTX.
Odds ratios using standard meta‐analyses
Biologic + MTX versus active comparator
The use of biologic + MTX therapy was not statistically significantly associated with less radiographic progression compared to MTX with a mean difference of ‐2.56 (95% CI ‐6.03 to 0.92) Sharp or modified Sharp units (0 to 448 points), absolute difference ‐0.57% (95% CI ‐1.35% to 0.21%) (Figure 6) with a considerable degree of heterogeneity, with I2 of 97%.; this change was not clinically meaningful (low‐quality evidence).
6.
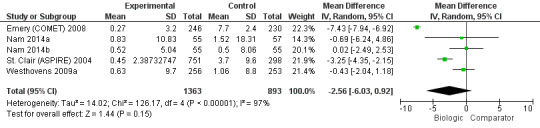
Radiographic progression: biologic (+MTX) versus comparator
Biologic without MTX versus active comparator
There were no studies of biologic therapy without MTX.
Odds ratios by biologic type and dose using NMA
The overall radiographic progression by the type of biologic and the dose was as follows (2256 participants, 5 studies).
-
Type of biologic, TNF versus non‐TNF biologic:
biologic + MTX: compared to TNF biologic + MTX, non‐TNF biologic + MTX was associated with no statistically significant or clinically meaningful difference in radiographic progression, MD: 3.32 (95% CrI ‐1.08 to 7.63);
biologic alone without MTX: there were no data to perform this analysis.
-
Type of biologic, etanercept versus TNF antibody biologic versus non‐TNF biologic:
biologic + MTX: compared to etanercept + MTX, monoclonal antibody + MTX or non‐TNF biologic + MTX were not associated with any statistically significant or clinically meaningful difference in radiographic progression, MD: 1.87 (95% CrI ‐2.34 to 6.05) and MD: 4.10 (95% CrI ‐0.64 to 8.68);
biologic alone without MTX: there were no data to perform this analysis.
-
Biologic dose, SD versus LD versus HD biologic:
biologic + MTX: compared to SD biologic + MTX, LD biologic + MTX, MD: ‐0.05 (95% CrI ‐3.34 to 3.20) was not associated with statistically significant or clinically meaningful difference in radiographic progression;
biologic alone without MTX: there were no data to perform this analysis.
Main analyses using NMA
None of the biologics were statistically significantly different from each other or DMARDs (Appendix 20).
Subgroup analyses by RA disease duration (early versus established versus late RA)
Early RA (RA disease duration less than two years)
There were not enough data to perform NMA.
Established RA (disease duration 2 to 10 years)
There were not enough data to perform NMA.
Late RA (disease duration more than 10 years)
There were not enough data to perform NMA.
Subgroup analyses by trial duration
Trial duration, six months or less
There were not enough data to perform NMA.
Trial duration, between six and 12 months
Three 2‐arm trials and one 3‐arm trials provided data. Compared to MTX + placebo, three treatments were associated with slower radiographic progression that was statistically significant with a mean difference of:
SD etanercept + MTX, MD: ‐5.50 (95% CrI ‐7.09 to ‐3.82);
SD infliximab + MTX, MD: ‐3.30 (95% CrI ‐5.46 to ‐1.15); and
HD infliximab + MTX, MD: ‐3.20 (95% CrI ‐5.35 to ‐1.06)
(2144 participants, 4 studies) (Appendix 21).
Trial duration, between six and 12 months
There were not enough data to perform NMA.
Primary/major harm outcome: Withdrawals due to adverse events
Fourteen studies with 6233 participants reported withdrawals due to adverse events (AE). Of these, all studies included at least one arm with participants on a biologic with concomitant MTX.
Odds ratios using standard meta‐analyses
Biologic + MTX versus active comparator
Results were inconclusive for a statistically significantly or clinically meaningfully difference in withdrawals due to AEs with biologic + MTX compared to MTX with an OR: 1.35 (95% CI 0.89 to 2.06; where the 95% confidence interval includes possibility of important harm), absolute difference 2% (95% CI 0% to 4%), I2 of 55%, representing moderate to substantial heterogeneity; Figure 7 (low‐quality evidence).
7.
Withdrawals due to adverse events: biologic (with and without concomitant MTX) versus comparator
Biologic + MTX versus active comparator
Results were inconclusive for a statistically significantly and clinically meaningfully difference in withdrawals due to AEs in the biologic without MTX compared to MTX, OR: 1.14 (95% CI 0.58 to 2.24), absolute difference (95% CI 0% (‐4% to 4%), with a I2 of 9%, representing heterogeneity that might not be important. Figure 7 (low‐quality evidence).
Odds ratios by biologic type and dose using NMA
Overall withdrawals due to AEs differed by the type of biologic and the dose as follows (6233 participants, 14 trials).
-
Type of biologic, TNF versus non‐TNF biologic:
biologic + MTX: compared to TNF biologic, non‐TNF biologic showed clinically meaningfully and statistically significantly lower odds of withdrawals due to AEs, OR: 0.32 (95% CrI 0.12 to 0.80);
biologic alone without MTX: there were no studies to compare.
-
Type of biologic, etanercept versus TNF antibody biologic versus non‐TNF biologic:
biologic + MTX: compared to monoclonal antibodies against TNF, non‐TNF biologic was associated with clinically meaningful and statistically significantly lower odds of withdrawals due to AEs, OR: 0.27 (95% CrI 0.12 to 0.61), except for etanercept, OR: 0.44 (95% CrI 0.20 to 1.14). We also found compared to TNF biologic, non‐TNF biologic was not associated with any statistically significant or clinically meaningful difference in odds of withdrawals due to AEs, OR: 1.66 (95% CrI 0.60 to 5.02);
biologic alone without MTX: there were no studies to compare.
-
Biologic dose, SD versus LD versus HD biologic:
biologic + MTX: compared to SD biologic, odds of withdrawal due to AEs with HD or LD biologic were not statistically significantly or clinically meaningfully different from SD biologic (HD: OR: 1.41 (95% CrI 0.94 to 2.12), LD: OR: 0.56 (95% CrI 0.23 to 1.24) However, compared to HD biologic, LD biologic was associated with a statistically significant and clinically meaningful lower odds of withdrawals due to AEs, OR: 0.40 (95% CrI 0.15 to 0.97);
biologic alone without MTX: compared to HD biologic, LD biologic was associated with a statistically significant and clinically meaningful lower odds of withdrawals due to AEs, OR: 0.22 (95% CrI 0.03 to 0.92), although data for this analysis were sparse.
Main analyses using NMA
Compared to MTX, SD adalimumab + MTX, SD infliximab + MTX, HD golimumab + MTX, and HD infliximab + MTX, were associated with higher odds of withdrawals due to AEs, with ORs ranging 1.74 to 3.68. Compared to SD rituximab + MTX, HD golimumab + MTX and HD infliximab + MTX were associated with higher OR of 9.97 and 6.51. Compared to LD rituximab + MTX, HD golimumab + MTX and HD infliximab + MTX were associated with higher OR of 8.45 and 5.57. Compared to HD golimumab, HD golimumab + MTX was associated with higher odds, 8.13 (95% CrI 1.80 to 67.37) (6233 participants, 14 studies) (Appendix 22).
Subgroup analyses by RA disease duration (early versus established versus late RA)
Early RA (RA disease duration less than two years)
There were four 2‐arm, and two 3‐arm trials. Compared to MTX + placebo, both SD infliximab + MTX and HD infliximab + MTX were associated with higher odds of withdrawals due to AEs, OR: 3.34 and OR: 3.38, respectively. Compared to SD infliximab + MTX, SD rituximab + MTX and LD rituximab + MTX were associated with lower odds of withdrawals due to AEs, OR: 0.11 and OR: 0.13, respectively (2416 participants, 6 trials) (Appendix 23).
Established RA (disease duration 2 to 10 years)
There were four 2‐arm, one 3‐arm and one 4‐arm trials. HD golimumab subcutaneous was associated with statistically significantly higher odds of withdrawals due to AEs compared with MTX + placebo, OR: 3.78; SD etanercept + MTX, OR: 4.84; and HD golimumab subcutaneous, OR: 8.17 (3667 participants, 6 trials) (Appendix 24).
Late RA (disease duration more than 10 years)
There were not enough data to perform NMA.
Subgroup analyses by trial duration
Trial duration, six months or less
There were three 2‐arm trials and one 4‐arm trial. Compared to MTX + placebo, HD golimumab subcutaneous + MTX was associated with 3.71‐times higher odds of withdrawals due to AEs. Compared to HD golimumab subcutaneous monotherapy, HD golimumab subcutaneous + MTX was associated with 8.06‐times higher odds of withdrawals due to AEs (2175 participants, 4 trials) (Appendix 25).
Trial duration, between six and 12 months
There were four 2‐arm and two 3‐arm trials. Compared to MTX + placebo, both SD infliximab + MTX and HD infliximab + MTX were associated with higher odds of withdrawals due to AEs, OR: 3.32 and OR: 3.36, respectively (3029 participants, 6 trials) (Appendix 26).
Trial duration, between six and 12 months
There were no data.
Primary/major harm outcome: Serious adverse events
Twelve studies with 5169 participants reported serious adverse events (SAEs). Of these, all studies included at least one arm with participants on a biologic with concomitant MTX.
Odds ratios using standard meta‐analyses
Biologic + MTX versus active comparator
The use of biologic + MTX therapy was associated with no evidence of a statistically significant or clinically meaningful difference in the odds of SAEs versus MTX, OR: 1.06 (95% CI 0.86 to 1.31), absolute difference 1% (95% CI ‐1% to 3%), I2 of 8%, representing heterogeneity which might not be important (Figure 8) (moderate‐quality evidence).
8.
Serious adverse events: biologic (with and without concomitant MTX) versus comparator
Biologic without MTX versus active comparator
The use of biologic without MTX therapy was associated with no evidence of a statistically significant or clinically meaningful difference in the odds of SAEs versus MTX (based on one study), OR: 0.44 (95% CI 0.15 to 1.30) absolute difference ‐4% (95% CI ‐8% to 1%), with no I2, since data were derived from one study (low‐quality evidence).
Odds ratios by biologic type and dose using NMA
The overall SAEs differed by the type of biologic and the dose as follows (5009 participants, 12 studies).
-
Type of biologic, TNF versus non‐TNF biologic:
biologic + MTX: compared to TNF biologic, the odds of SAEs were not statistically significantly or clinically meaningfully different with non‐TNF biologic, OR: 0.73 (95% CrI 0.42 to 1.28);
biologic alone without MTX: there were no studies comparing biologic monotherapy regimens to each other.
-
Type of biologic, etanercept versus TNF antibody biologic versus non‐TNF biologic:
biologic + MTX: the odds of SAEs were not statistically significantly or clinically meaningfully different with the comparators as follows: non‐TNF biologic, OR: 0.77 (95% CrI 0.45 to 1.34) and etanercept, OR: 3.40 (95% CrI 0.82 to 18.68). Compared to MTX + non‐TNF biologic, MTX + TNF biologic was associated with statistically significantly higher odds of SAEs, OR: 4.41 (95% CrI 1.01 to 25.18), which may be clinically meaningful;
biologic alone without MTX: there were no studies comparing biologic monotherapies to each other.
-
biologic dose, SD versus LD versus HD biologic:
biologic + MTX: the odds of SAEs were not statistically significantly or clinically meaningfully different of the following compared to SD biologic as follows: HD biologic, OR: 1.02 (95% CrI 0.65 to 1.61); and LD biologic, OR: 0.94 (95% CrI 0.53 to 1.67);
biologic alone without MTX: there were no studies comparing biologic monotherapies to each other by dose.
Main analyses using NMA
Twelve RCTs (nine 2‐arm, two 3‐arm, and one 4‐arm trial) enrolling 5009 participants (Appendix 27) provided data for all dose analyses for SAEs. None of the biologics were significantly different from each other or MTX, except that HD golimumab was associated with lower odds of SAEs compared to SD etanercept + MTX.
Subgroup analyses by RA disease duration (early versus established versus late RA)
Early RA (RA disease duration less than two years)
There were five 2‐arm and two 3‐arm trials. There were no statistically significant differences between biologic and comparators or between biologic + MTX and comparators (MTX + placebo in most cases) (2618 participants, 7 studies) (Appendix 28).
Established RA (disease duration 2 to 10 years)
There were four 2‐arm and one 4‐arm trial. There were no statistically significant differences between biologic and comparators or between biologic + MTX and comparators (MTX + placebo in most cases) (2391 participants, 5 studies) (Appendix 29).
Late RA (disease duration more than 10 years)
There were no data to analyze.
Subgroup analyses by trial duration
Trial duration, six months or less
There were four 2‐arm and one 4‐arm trial. There were no statistically significant differences between biologic and comparators or between biologic + MTX and comparators (MTX + placebo in most cases) (2240 participants, 5 studies) (Appendix 30).
Trial duration, between six and 12 months
There were two 2‐arm and one 3‐arm trial. There were no statistically significant differences between biologic and comparators or between biologic + MTX and comparators (1668 participants, 3 studies) (Appendix 31).
Trial duration, between six and 12 months
There were two 2‐arm and one 3‐arm trial. There were no statistically significant differences (989 participants, 3 studies) (Appendix 32).
Primary/major harm outcome: Cancer
Eleven studies with 5044 participants reported cancer. Of these, all studies included at least one arm with participants on a biologic with concomitant MTX.
Odds ratios using standard meta‐analyses
Biologic + MTX versus active comparator
The use of biologic + MTX therapy was inconclusive for evidence of a statistically significant or clinically meaningful difference in the odds of cancer, compared to MTX, Peto OR: 0.71 (95% CI 0.38 to 1.33), absolute difference 0% (95% CI 0% to 0%) with an I2 of 0% representing no heterogeneity (Figure 9) (low‐quality evidence).
9.
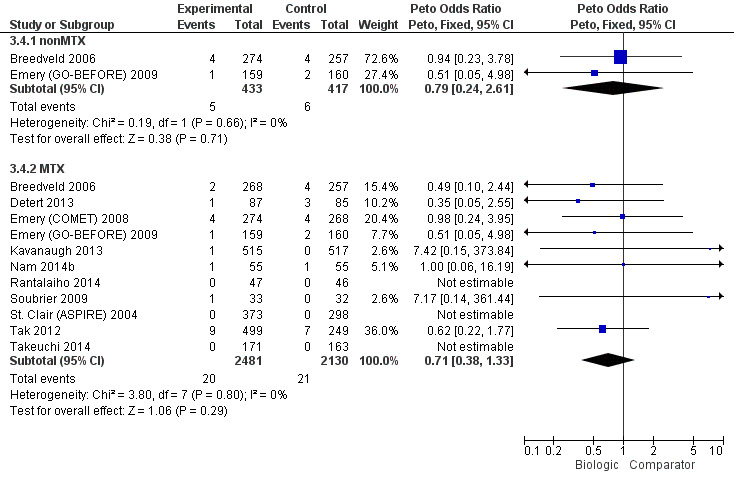
Cancer: biologic (with and without concomitant MTX) versus comparator
Biologic without MTX versus active comparator
The use of biologic without MTX therapy was inconclusive for evidence of a statistically significant or clinically meaningful difference in the odds of cancer, compared to MTX, Peto OR: 0.79 (95% CI 0.24 to 2.61), absolute difference 0% (95% CI ‐2% to 1%) with an I2 of 0%, representing no heterogeneity (Figure 9) (low‐quality evidence).
Odds ratios by biologic type and dose using NMA
The overall rate of cancer by the type of biologic and the dose were as follows (5044 participants, 11 studies).
-
Type of biologic, TNF versus non‐TNF biologic:
biologic + MTX: compared to TNF inhibitors, the odds of cancer with non‐TNF biologic were not statistically significantly or clinically meaningfully different, Peto OR: 0.80 (95% CrI 0.20 to 3.28);
biologic alone without MTX: no comparisons were available.
-
Type of biologic, etanercept versus TNF antibody biologic versus non‐TNF biologic:
biologic + MTX: compared to monoclonal TNF antibodies, the odds of cancer were not statistically significantly or clinically meaningfully different with etanercept, Peto OR: 1.43 (95% CrI 0.25 to 8.68) or non‐TNF biologic, Peto OR: 0.90 (95% CrI 0.20 to 4.33);
biologic alone without MTX: no comparisons were available.
-
Biologic dose, SD versus LD versus HD biologic:
biologic + MTX: compared to SD biologic + MTX, the odds of cancer were not statistically significantly or clinically meaningfully different with LD biologic + MTX, Peto OR: 1.50 (95% CrI 0.42 to 5.07). No comparisons were available for HD biologic + MTX versus SD biologic + MTX;
biologic alone without MTX: compared to SD biologic, the odds of cancer were not statistically significantly or clinically meaningfully different with HD biologic, Peto OR: 0.41 (95% CrI 0.01 to 6.10). No comparisons were available for LD biologic versus SD biologic.
Main analyses using NMA
Eleven RCTs (eight 2‐arm and three 3‐arm trials) enrolling 5044 participants (Appendix 33): we found no statistically significant differences comparing various biologics to MTX or to each other.
Subgroup analyses by RA disease duration (early versus established versus late RA)
Early RA (RA disease duration less than two years)
There were not enough data to perform NMA.
Established RA (disease duration 2 to 10 years)
No statistically significant differences in cancer rates were noted between treatments.
Late RA (disease duration more than 10 years)
There were not enough data to perform NMA.
Subgroup analyses by trial duration
Trial duration, six months or less
No statistically significant differences in cancer rates were noted between treatments (2081 participants, 5 studies (four 2‐arm and one 3‐arm trial)) (Appendix 35).
Trial duration, between six and 12 months
There were not enough data to perform NMA.
Trial duration, more than 12 months
There were not enough data to perform NMA.
Summary of safety warnings from regulatory agencies
Evidence from RCTs is limited in informing participants and physicians about uncommon or rare adverse events. In Appendix 36, we summarize warnings from the FDA, EMA and Health Canada, the regulatory agencies in the USA, Europe and Canada, respectively.
Discussion
Summary of main results
ACR50
In standard meta‐analysis, based on moderate‐quality evidence, biologic use + MTX was associated with a statistically significant and clinically meaningful higher odds of ACR50 compared to MTX with an odds ratio of 1.95 (95% CI 1.71 to 2.23) or a RR of 1.40 (95% CI 1.30 to 1.49), absolute difference 16% (95% CI 13% to 20%) and NNTB = 7 (95% CI 6 to 8). TNF biologic monotherapy did not show a clinically meaningful or statistically significant difference from MTX (there were no data for non‐TNF monotherapy). Results were similar in the NMA.
In standard meta‐analysis, based on high‐quality evidence, biologic + MTX was also associated with statistically significant and clinically meaningful higher odds of ACR50 compared to the comparator in both TNF biologic and non‐TNF biologic subgroups with RR of 1.44 (95% CI 1.34 to 1.54) and 1.27 (95% CI 1.14 to 1.42) and absolute difference 17% (95% CI 13% to 21%) and 13% (95% CI 7% to 19%), and NNTB = 6 (95% CI 5 to 8) and = 8 (95% CI 6 to 14), respectively. Results were similar for the NMA.
In subgroup analyses using the NMA, with respect to the type of biologics and biologics by dose, TNF biologics did not show evidence of a statistically significant or clinically meaningful difference from non‐TNF biologics and similar results were seen when comparing biologics doses.
Function assessed by HAQ
In standard meta‐analysis, there was moderate‐quality evidence that biologics use + MTX was associated with statistically significant HAQ score improvement with a mean difference of ‐0.10 (95% CI ‐0.16 to ‐0.04) and an absolute difference of ‐3.3% (95% CI ‐5.3% to ‐1.3%) when compared to MTX, but the difference did not seem to be clinically meaningful. TNF biologic monotherapy did not show a statistically significant or clinically meaningful difference from MTX (there were no data for non‐TNF monotherapy). Results were similar in the NMA.
In standard meta‐analysis, based on low‐quality evidence, compared to MTX, TNF biologic + MTX was associated with lower HAQ scores with better HAQ score improvement with mean difference of ‐0.09 (95% CI ‐0.26 to 0.07), which was neither statistically significant nor clinically meaningful. Based on moderate‐quality evidence, non‐TNF biologic + MTX was associated with better HAQ scores with statistically significant and clinically meaningful HAQ score improvement with a mean difference of ‐0.22 (95% CI ‐0.26 to ‐0.18) and an absolute difference of ‐7.3% (95% CI ‐8.7% to ‐6%) compared to MTX. Results did not show evidence of a clinically meaningful or statistically significant difference in TNF biologic monotherapy versus MTX.
In NMA, none of the subgroups (TNF or non‐TNF biologic) showed statistically significant or clinically meaningful differences. We noted no evidence of clinically meaningful and statistically significant differences in HAQ scores by the type of biologic (TNF versus non‐TNF biologics or receptor versus antibody TNF biologic) or in biologic‐dose analysis.
Remission
In standard meta‐analysis, based on moderate‐quality evidence, biologic use + MTX was associated with a statistically significant and clinically meaningful higher odds of remission compared to MTX, with OR of 2.23 (95% CI 1.82 to 2.73) or a RR of 1.62 (95% CI 1.33 to 1.98), absolute difference 15% (95% CI 11% to 19%) and NNTB = 5 (95% CI 6 to 7). TNF biologic monotherapy did not show evidence of a statistically significant or clinically meaningful difference from MTX (there were no data for non‐TNF monotherapy). Results were similar in the NMA.
In standard meta‐analysis, based on moderate‐quality evidence, TNF biologic + MTX showed a statistically significant and clinically meaningful higher rate of remission with risk ratio of 1.55 (95% CI 1.22 to 1.96) and absolute difference 14% (95% CI 9% to 19%) and NNTB = 7 (95% CI 5 to 10), as did non‐TNF biologic + MTX with RR 2.10 (95% CI 1.45 to 3.04), absolute difference 19% (95% CI 15% to 24%) and NNTB = 6 (95% CI 4 to 9). Results were similar in the NMA.
In subgroup analyses using the NMA, there was no evidence of statistically significant or clinically meaningful differences among biologic groups or in biologic‐dose analysis.
Radiographic progression
In standard meta‐analysis, based on low‐quality evidence, biologic use + MTX showed a non‐statistically significant difference in radiographic progression versus MTX with a mean difference of ‐2.56 (95% CI ‐6.03 to 0.92) and absolute difference ‐0.57% (95% CI ‐1.35% to 0.21%) on a scale of 0 to 448 points; this difference does not seem to be clinically meaningful. There were no data for TNF‐biologic or non‐TNF biologic monotherapy.
In standard meta‐analysis, based on low‐quality evidence, TNF biologic + MTX showed a non‐statistically significant improvement in radiographic progression versus MTX with mean difference of ‐3.18 (95% CI ‐6.80 to 0.43), absolute difference ‐0.71% (95% CI ‐1.52% to 959%). In NMA, this comparison was statistically significant with a mean difference of ‐3.73 (95% CrI ‐5.78 to ‐1.62), absolute difference ‐0.83% (95% CI ‐1.29% to ‐0.36%) and NNTB = 3 (95%CI, 3 to 7), but the clinical significance of this difference was unclear. Non‐TNF biologic + MTX showed a much lower non‐statistically significant improvement with a mean difference of ‐0.43 (95% CI ‐2.04 to 1.18), absolute difference ‐0.22% (95% CI ‐0.46% to 0.26%), which was also not significantly different in the NMA.
In subgroup NMA, no evidence of clinically meaningful or statistically significant differences were noted by the type of biologic (TNF versus non‐TNF biologic), receptor versus antibody TNF biologic, or biologic dose (SD versus HD versus LD).
Withdrawals due to adverse events
In standard meta‐analysis, based on low‐quality evidence, results were inconclusive for biologic use + MTX for any clinically meaningful or statistically significant difference in withdrawal due to adverse events rates compared to MTX, since the 95% CI included the possibility of important harm with an OR of 1.35 (95% CI 0.89 to 2.06) or a RR of 1.32 (95% CI 0.89 to 1.97) and absolute difference of 2% (95% CI 0% to 4%). Results were inconclusive for TNF biologic monotherapy versus MTX (there were no data for non‐TNF monotherapy). Results were similar in the NMA.
In standard meta‐analysis, based on moderate‐quality evidence, there was a clinically meaningful and statistically significant increase in withdrawals due to adverse events in TNF biologic + MTX versus MTX with a RR of 1.60 (95% CI 1.10 to 2.32), absolute difference 3% (95% CI 1% to 4%) and NNTH = 35 (95% CI 17 to 183). Based on low‐quality evidence, there was no evidence of a clinically meaningful and statistically significant difference in this outcome among the non‐TNF biologic + MTX versus MTX, RR of 0.56 (95% CI 0.31 to 1.01), absolute difference ‐2% (95% CI ‐5% to 1%). Results were similar in the NMA.
In subgroup analyses using the NMA, compared to TNF biologic, non‐TNF biologic showed a clinically meaningful and statistically significant lower odds ratio of withdrawals due to adverse events, OR 0.32 (95% CrI 0.12 to 0.80). Compared to HD biologic with concomitant MTX, LD biologic with concomitant MTX was associated with statistically significantly lower withdrawals due to adverse events, OR: 0.40 (95% CrI 0.15 to 0.97), which also seemed to be clinically meaningful.
Serious adverse events (SAEs)
In standard meta‐analysis, based on moderate‐quality evidence, the odds of SAEs did not show evidence of a statistically significant or clinically meaningful difference in participants comparing biologics + MTX to MTX alone; OR was 1.06 (95% CI 0.86 to 1.31), RR was 1.05 (95% CI 0.87 to 1.26) and absolute difference of 1% (95% CI ‐1% to 3%). Based on low‐quality evidence, TNF biologic monotherapy did not show evidence of a clinically meaningful or statistically significant difference from MTX (there were no data for non‐TNF monotherapy). Results were similar in the NMA.
In standard meta‐analysis, based on moderate‐quality evidence, there was no evidence of a statistically significant or clinically meaningful difference in the TNF biologic + MTX versus MTX with a RR of 1.14 (95% CI 0.92 to 1.42), absolute difference 1% (95% CI ‐1% to 3%). Based on low‐quality evidence, there was no evidence of a clinically meaningful and statistically significant difference in the non‐TNF biologic + MTX versus MTX with a RR of 0.87 (95% CI 0.64 to 1.18), absolute difference ‐1% (95% CI ‐5% to 2%).
In subgroup NMA, in one comparison TNF biologic + MTX was associated with statistically significantly higher odds of SAEs compared to non‐TNF biologic + MTX, with OR of 4.41 (95% CrI 1.01 to 25.18), which may be clinically meaningful. Other subgroup comparisons did not show evidence of a clinically meaningful or statistically significant difference.
Cancer
In standard meta‐analysis, based on low‐quality evidence, results were inconclusive for the risk of cancer for statistically significant or clinically meaningful difference for biologic + MTX versus MTX with a Peto's OR of 0.71 (95% CI 0.38 to 1.33) and an absolute difference of 0% (95% CI 0% to 0%). Results were also inconclusive for TNF biologic monotherapy versus MTX (there were no data for non‐TNF monotherapy). Results were similar in the NMA.
In subgroup NMA, no evidence of clinically meaningful or statistically significant differences were noted by the type of biologic (TNF versus non‐TNF biologic; receptor versus antibody TNF biologic), biologic dose (SD versus HD versus LD), RA disease duration or trial duration.
Overall completeness and applicability of evidence
ACR50 and withdrawals due to adverse events were reported by almost all of the studies included in this overview and NMA, and data from several studies were available for many other outcomes. Some important outcomes, including radiographic scores and cancer, were reported either by few RCTs (e.g. radiographic scores) or had a low event rate (e.g. cancer), or both, which limited our ability to draw firm conclusions about them for biologics overall and to compare them between biologics. The evidence report is up to date and current, with this 2015 update. Due to the rarity of trials with direct comparisons, this study provides comparisons of biologics to MTX and indirect comparisons of biologics to each other.
Quality of the evidence
For the 2015 update, the quality of included trials was reasonably good. However poor reporting of the conduct of the included trials was a major issue as all were described as double‐blind randomized but less than 50% reported adequate sequence generation, allocation concealment and blinding. Forty‐two percent reported adequate sequence generation, 37% of trials were judged to be at low risk for allocation concealment, 42% trials were judged at low risk of performance bias (blinding), 47% at low risk of detection bias (blinding), 53% trials had low risk of attrition bias and 89% trials had low risk of major baseline imbalance. Selective reporting bias could not be assessed since for several trials, we could not find published protocols.
The overall quality of the direct evidence for most outcomes (ACR50, HAQ, remission, serious adverse events) was downgraded to moderate due to inconsistency of effect. Direct evidence was low quality for radiographic outcomes, withdrawals due to adverse events and cancer, due to imprecision or indirectness or serious imprecision. The quality of evidence from the NMA for all outcomes was low to moderate quality due to imprecision and indirectness. We did not detect publication bias. Wide credible intervals were only found in some subgroup analyses.
Potential biases in the overview process
Our review has several limitations. Lack of reporting of many important outcomes from RCTs or few events (radiographic scores, cancer, etc.), or both, limited our ability to analyze and compare these outcomes between biologics and comparators and between biologics (TNF versus non‐TNF biologic; by dose etc.).
With the introduction of multiple biologics, whose benefits have yet to be compared to one another, it is unclear whether one or more biologics might be more beneficial or safer, or better tailored to different subgroups of participants suffering from RA. To our knowledge there are only few head‐to‐head comparisons of benefit and safety of various biologics in people with RA. Gabay 2013; Schiff 2008a; and Weinblatt 2013 are the most well‐known trials. We included direct comparator studies in our analyses; however, the majority of the studies compared biologic to MTX and two trials to MTX with methylprednisolone. In the absence of such direct comparisons, NMA results that incorporate indirect comparisons can provide useful information although still need to be interpreted with caution.
Indirect comparisons (incorporated into NMA estimates) have several limitations. RCTs differ in patient population characteristics, most prominently in prior failed therapy, biologic dose, concomitant use of DMARDs, mean RA disease duration, and trial duration. To overcome this limitation, we analyzed in the categories of previous DMARD history (MTX‐naive, MTX‐experienced, DMARD‐experienced and TNF‐experienced), biologic dose (SD, HD, LD), and concomitant MTX (yes/no). We observed several novel findings when these analyses were performed, which make results of this NMA and overview comprehensive. We also performed a‐priori‐specified subgroup analyses by the duration of RA (early, established, late) and duration of the trial (less than six, 6 to 12 and more than 12 months) as a surrogate of biologic exposure. These analyses also provide interesting results in specific subpopulations of people with RA. However, subgroup analyses are subject to low power and type II error, that is, missing significant results, by chance, due to lower number of studies and participants. Therefore, these results must be interpreted with caution.
Additionally, some studies presented data on safety for all doses of the biologic together, not just the recommended dose and in some cases presented data for the entire study duration, including the open‐label phase. This limited our ability to get the data for the randomized phase or by dose.
One must be careful in interpreting the odds ratios that may look slightly different from each other numerically, but not statistically. It is important to consider the 95% confidence intervals while interpreting these numbers. Due to a large number of comparisons and challenge interpreting these tables, we summarized all statistically significant odds ratios, to the extent possible, in the main text. This should make it easier for readers to interpret the results.
There is also possibility of type‐I error, due to multiple comparisons and up to five differences per 100 comparisons may be due to chance. However, given the limited data for most outcomes, short trial duration and rarity of harms outcomes, our main concern with most of these analyses is type‐II error, that is, missing an important difference due to small number of events, not type‐I error.
Two abstractors abstracted all data independently for this updated 2015 version and the original 2009 version. This we believe minimizes errors in data abstractions, and biases due to this error. Abstract and titles were also reviewed in duplicate independently, to avoid errors, as part of systematic review.
Agreements and disagreements with other studies or reviews
Our NMA and overview was performed using a comprehensive strategy, accounting for many potential factors that differ between trials and trial arms. Therefore, direct comparison of results to most published NMAs is not possible, particularly those that have not stratified participants by these important characteristics.
In general, many results agree with several similar analyses in the past. However, since our review has several more studies than included in the previous reviews/overviews, some estimates differ, as expected. Additionally, our review includes all available studies for biologics, while most previous reviews have included anti‐TNF biologics with few exceptions, and many used RCT data from non‐standardized doses.
Thompson 2011 conducted a meta‐analysis of TNF biologics versus MTX in people with RA with early disease (not necessarily all MTX‐naive) and found that the risk of serious infections and cancer with TNF biologics were similar to MTX with an OR of 1.28 (95% CI 0.82 to 2.00) and 1.08 (95% CI 0.50 to 2.32), respectively. Similarly, we found no difference in rates of serious adverse events with biologics versus comparator (MTX/other DMARDs + placebo in most cases) with moderate quality of evidence, but results for the risk of cancer were inconclusive with low quality of evidence.
In a meta‐analysis of RCTs of anti‐TNF biologics in people with RA who were MTX‐naive (Alonso‐Ruiz 2008), the RR of achieving ACR20 and ACR50 for biologic monotherapy versus MTX was RR 1.6 (95% CI 1.4 to 1.7) and RR 1.0 (95% CI 0.9 to 1.1) versus comparator. The pattern of these risk ratios are very similar to those reported in our study, although with better power and more studies, we found that the rates of ACR50 were higher in people receiving biologics compared to MTX/other DMARDs with an OR of 1.85 (95% CrI 1.56 to 2.19) or a RR of 1.36 (95% CrI 1.25 to 1.48).
Several systematic reviews and NMAs could not be compared to our study due to significant differences in patient population, since they focused on MTX/other DMARD‐failure population and either did not include RCTs from MTX/other DMARD‐naive population, or when included, these constituted a small proportion of all RCTs (Bongartz 2006; Bongartz 2009; Donahue 2008; Gartlehner 2006; Lee 2008; Leombruno 2008; Nam 2010; Nixon 2007; Orme 2012). Desai 2012 studied treatment discontinuation, an outcome different than the outcome we included.
Authors' conclusions
Implications for practice.
In the presence of very few direct head‐to‐head comparator trials of biologics in people with RA, practitioners are faced with a dilemma when choosing between biologics, for people who are MTX‐naive (where this is possible and desirable). This review summarizes the direct and indirect comparator data for biologics, as monotherapy or in combination with MTX in this patient population. This review and NMA can provide guidance for the individual and the healthcare providers, especially when making a decision about starting biologic in MTX‐naive people and choosing which one. Our finding that biologic with MTX use in people with RA who are MTX‐naive is associated with statistically significant and clinically meaningful benefits in terms of achievement of ACR50, remission and HAQ scores, implies that biologics in combination with MTX could be a reasonable alternative to MTX for some people with active RA; however potential costs and side effects of biologic must be balanced against this and treatment options individualized for each person. However, they are much more costly and in most countries, the use of a biologic is predicated on having demonstrated a failure to respond to MTX and/or other DMARDs. We also found that in those who were MTX‐naive, that there was no evidence that the benefit of biologic monotherapy was better than MTX, an important finding. This result further supports the current practice of using MTX first in people with RA who are MTX‐naive.
Implications for research.
We believe that more RCTs of direct head‐to‐head comparisons of biologic agents in people with RA are needed. These RCTs should examine the relative benefit and safety of biologics for various stages of the disease (early, established and late RA), various levels of functional limitation (mild, moderate and severe limitation) and in people with variable exposure to other therapies (traditional DMARD‐naive, traditional DMARD‐failure, biologic‐failure, multiple biologic failure). More long‐term observational studies and assessments of biologic registry data are needed to determine the longer‐term benefits and harms of different treatment strategies for RA.
What's new
| Date | Event | Description |
|---|---|---|
| 15 September 2015 | New search has been performed | Updated, description: new search with 14 new studies |
| 15 September 2015 | New citation required and conclusions have changed | New citation: conclusions changed. Description: original review of biologics in RA split into four by patient population:
This review will focus on people with RA who are MTX‐naive. |
History
Review first published: Issue 5, 2017
| Date | Event | Description |
|---|---|---|
| 1 March 2010 | Amended | Odds ratios have been used in the network meta‐analyses. See 'Published notes' for details. |
| 25 February 2010 | Amended | CMSG ID: C187‐R |
Notes
We used risk ratios in the Abstract, 'Summary of findings' table and Plain language summary for ease of interpretation. Throughout the rest of the review and NMA, we used odds ratios derived from the NMA.
Acknowledgements
We thank Shahrzad Noorbaloochi and Tyler Cullis at Boston University for contributing to abstract/title review and data abstraction, and TC for double‐checking the data; Tamara Rader of University of Ottawa for performing the searches.
This work was supported in part by resources provided to the University of Alabama at Birmingham (UAB) Cochrane NMA Satellite by the Rheuamtology division at UAB.
JS is supported by the resources and the use of facilities at the VA Medical Center at Birmingham, Alabama, USA.
RB is funded by an Australian National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship.
Appendices
Appendix 1. Characteristics of excluded studies
| Study | Reason for exclusion |
| Abe 2006 | Wrong drug exposure |
| Axelsen 2015 | Duplicate of Hørslev‐Petersen 2014 |
| Bae 2013 | Wrong drug exposure |
| Bathon 2000 | Duplicate of Genovese 2002 |
| Bonafede 2015 | Not a RCT |
| Bingham 2015 | Wrong drug exposure |
| Burmester 2013 | Comparing two routes of administration of a biologic |
| Burmester 2015 | Wrong drug exposure |
| Chen 2009 | Wrong drug exposure |
| Cheng 2014 | Conference abstract |
| Choy 2012 | Wrong drug exposure |
| Cohen 2002 | Wrong drug exposure |
| Cohen 2003 | Wrong drug exposure |
| Cohen 2004 | Wrong drug exposure |
| Cohen 2006 | Wrong drug exposure |
| Combe 2006 | Wrong drug exposure |
| Conaghan 2013 | Wrong drug exposure |
| Conaghan 2014 | Sub‐study of Huizinga 2015 |
| Dougados 2014 | Duplicate of Huizinga 2015 |
| Doyle 2013 | Wrong drug exposure |
| Durez 2004 | Wrong drug exposure |
| Emery 2006 | Wrong drug exposure |
| Emery 2010 | Wrong drug exposure |
| Emery 2014a | Duplicate |
| Emery 2014b | Conference abstract |
| Ericksson 2015 | Follow‐up at two years of Van Vollenhoven 2012a NCT00764725 |
| Fleischmann 2003 | Wrong drug exposure |
| Fleischmann 2009 | Wrong drug exposure |
| Fleischmann 2013 | Conference abstract |
| Furst 2003 | Wrong drug exposure |
| Furst 2007 | Wrong drug exposure |
| Furst 2015 | Open label study |
| Gabay 2013 | Wrong drug exposure |
| Gashi 2014 | Comparing two doses of a biologic |
| Genovese 2004 | Wrong drug exposure |
| Genovese 2005 | Wrong drug exposure |
| Genovese 2008 | Wrong drug exposure |
| Genovese 2011 | Wrong drug exposure |
| Genovese 2014 | Wrong drug exposure |
| Gherge 2014 | Conference abstract |
| Goekoop‐Ruiterman 2007 | Wrong drug exposure |
| Haraoui 2014 | Duplicate of Pope 2014 |
| Heimans 2014 | Wrong drug exposure |
| Hobbs 2015 | Wrong drug exposure |
| Hørslev‐Petersen 2014 | Open label study |
| Iannone 2014 | Open label study |
| Johnsen 2006 | Wrong drug exposure |
| Jones 2010 | Wrong drug exposure |
| Kaine 2012 | Wrong drug exposure |
| Kameda 2011 | Wrong drug exposure |
| Kavanaugh 2014 | Conference abstract |
| Kay 2008 | Wrong drug exposure |
| Kennedy 2014 | Wrong drug exposure |
| Keystone 2004a | Wrong drug exposure |
| Keystone 2004b | Wrong drug exposure |
| Keystone 2008 | Wrong drug exposure |
| Keystone 2014 | Conference abstract |
| Kim 2007 | Wrong drug exposure |
| Kim 2012 | Wrong drug exposure |
| Kim 2013 | Wrong drug exposure |
| Kivitz 2014 | Vaccine response study |
| Koroleva 2014a | Conference abstract |
| Koroleva 2014b | Conference abstract |
| Kremer 2003 | Wrong drug exposure |
| Kremer 2005 | Wrong drug exposure |
| Kremer 2006 | Wrong drug exposure |
| Kremer 2011 | Wrong drug exposure |
| Kremer 2009 | Wrong drug exposure |
| Kremer 2015 | Cross over study design |
| Lan 2004 | Wrong drug exposure |
| Landewe 2015 | Conference abstract |
| Lipsky 2000 | Wrong drug exposure |
| Lisbona 2008 | Wrong drug exposure |
| Lisbona 2010 | Wrong drug exposure |
| Machado 2014 | Wrong drug exposure |
| Maini 1999 | Wrong drug exposure |
| Manders 2015 | Trial participants switched therapy within one year/before completion of study period |
| Mathias 2000 | Wrong drug exposure |
| McInnes 2015 | Compares lipid levels in those randomized to CZP or PL, in MTX‐IR patients |
| Moreland 2002 | Wrong drug exposure |
| Navarro 2014 | Conference abstract |
| Nishimoto 2009 | Wrong drug exposure |
| O'Dell 2013 | Wrong drug exposure |
| Oakley 2014 | Conference abstract |
| Pavelka 2013 | Sub‐group analysis of Smolen 2013 NCT00565409 |
| Rau 2004 | Wrong drug exposure |
| Rigby 2011 | Duplicate of Tak 2011 |
| Rubbert‐Roth 2010 | Wrong drug exposure |
| Schiff 2004 | Wrong drug exposure |
| Schiff 2008a | Wrong drug exposure |
| Schiff 2014 | Wrong drug exposure |
| Smolen 2008 | Wrong drug exposure |
| Smolen 2009 | Wrong drug exposure |
| Smolen 2013 | Wrong drug exposure |
| Smolen 2014 | Participants in the control group also received the intervention |
| Smolen 2015 | Wrong drug exposure |
| Sonomoto 2014 | Open label study |
| Strand 2012 | Wrong drug exposure |
| Tak 2011 | Wrong drug exposure |
| Tanaka 2011 | Wrong drug exposure |
| Tanaka 2012 | Wrong drug exposure |
| Taylor 2004 | Wrong drug exposure |
| Taylor 2006 | Wrong drug exposure |
| Van der Heidje 2013 | Wrong drug exposure |
| Van der Kooij 2009 | Wrong drug exposure |
| Van Vollenhoven 2009 | Wrong drug exposure |
| Van Vollenhoven 2012a | Duplicate of Van Vollenhoven 2009 |
| Van Vollenhoven 2012b | Wrong drug exposure |
| Vital 2015 | B cell depletion study |
| Weinblatt 1999 | Wrong drug exposure |
| Weinblatt 2003 | Wrong drug exposure |
| Weinblatt 2006 | Wrong drug exposure |
| Weinblatt 2007 | Wrong drug exposure |
| Weinblatt 2008 | Wrong drug exposure |
| Weinblatt 2012 | Wrong drug exposure |
| Weinblatt 2013a | Wrong drug exposure |
| Weinblatt 2013b | Wrong drug exposure |
| Weinblatt 2014 | Results at 1 year of Weinblatt 2013b GO‐FURTHER trial, NCT00973479 |
| Weisman 2003 | Wrong drug exposure |
| Weisman 2007 | Wrong drug exposure |
| Westhovens 2006 | Wrong drug exposure |
| Westhovens 2014 | Conference abstract |
| Yamamoto 2014a | Wrong drug exposure |
| Yamanaka 2014 | Post hoc analysis of Takeuchi 2014 |
| Yazici 2012 | Wrong drug exposure |
| Zhang 2006 | Wrong drug exposure |
| CZP: certolizumab pegol; MTX‐IR: methotrexate inadequate responder; PL: placebo; RCT: randomised controlled trial | |
Appendix 2. Ongoing trials from the WHO trial register and Clinicaltrials.gov
| NCT Number | Title |
| ACTRN12605000784617 | A phase IIIb multi‐center, randomized, double‐blind study to evaluate remission and joint damage progression in methotrexate naive early erosive RA subjects treated with abatacept plus methotrexate compared with methotrexate |
| ACTRN12605000785606 | A phase II study of abatacept versus placebo to assess the prevention of rheumatoid arthritis (RA) in adult patients with undifferentiated arthritis who are at high risk for the development of RA |
| ACTRN12606000248561 | A phase 1 randomised double blind, placebo‐controlled, single dose, dose escalation study of kb002, a chimeric monoclonal antibody which binds to granulocyte macrophage‐colony stimulating factor (gm‐csf), in patients with rheumatoid arthritis |
| ACTRN12608000397314 | Multi‐national open‐label study to evaluate the safety, tolerability and efficacy of tocilizumab versus tocilizumab plus non‐biologic disease modifying antirheumatic drugs in patients with active rheumatoid arthritis |
| ACTRN12609000747224 | Extension phase of the multi‐national open‐label study to evaluate the safety, tolerability and efficacy of tocilizumab in patients with active rheumatoid arthritis on background non‐biologic disease‐modifying anti‐rheumatic drugs (DMARDs) who have an inadequate response to current non‐biologic DMARD and/or anti tumor necrosis factor (anti‐TNF) therapy |
| ACTRN12610000284066 | A longitudinal study of patients with rheumatoid arthritis starting biological therapy; assessment of joint inflammation by use of ultrasonography |
| ACTRN12611000972921 | The hunter Humira and endothelial function in early rheumatoid arthritis trial |
| ACTRN12611001202954 | A comparison of arthroscopic synovial biopsy based targeted biologic therapy versus conventional therapy in rheumatoid arthritis (RA) |
| ACTRN12614000903684 | A randomized, single‐blind, single‐dose, 3‐arm, parallel group study to determine the pharmacokinetic similarity of abp 710 and infliximab (Remicade 'registered trademark') in healthy adult subjects |
| ACTRN12615000557538 | Hunter heart‐RA‐2 (HHRA‐2) study: a randomised controlled trial evaluating the effects of Humira upon cardiovascular risk as measured by endothelial function in patients with rheumatoid arthritis who test positive for anti‐CCP antibodies as well as those who test negative for anti‐CCP antibodies |
| ChiCTR‐CCC‐10001054 | Circulating dickkopf‐1 (DKK‐1) is correlated with bone erosion and inflammation in rheumatoid arthritis |
| ChiCTR‐IIR‐16008693 | Pharmacokinetics, safety and tolerability study of single dose of abatacept 125mg administered subcutaneously |
| ChiCTR‐INR‐16009546 | The efficacy and safety of low dose il‐2 combined il‐6 antagonist therapy in Chinese over‐treated patients with rheumatoid arthritis |
| ChiCTR‐TRC‐09000383 | Efficacy and safety of recombinant human il‐1 receptor antagonist in Chinese patients with rheumatoid arthritis: a double‐blind, randomized, placebo‐controlled trial |
| ChiCTR‐TRC‐10001060 | Efficacy and safety of infliximab in Chinese patients with rheumatoid arthritis: a double‐blind, randomized, placebo‐controlled trial |
| CTRI/2008/091/000295 | A clinical trial to study the safety and effectiveness of a monoclonal antibody in combination with methotrexate in patients with active rheumatoid arthritis |
| CTRI/2012/05/002660 | A clinical study to demonstrate safety and efficacy data to support the development of R‐TPR‐015 (1422015) in patients with active rheumatoid arthritis on stable dose of methotrexate |
| CTRI/2013/05/003678 | A randomized controlled study to evaluate pharmacokinetic, pharmacodynamic (efficacy) and safety of rituximab (Zydus) and rituximab (Roche) in patients with rheumatoid arthritis |
| CTRI/2013/09/003963 | Study to compare the safety and efficacy of etanercept of Intas biopharmaceuticals ltd against Enbrel® in patients with active rheumatoid arthritis |
| CTRI/2013/10/004040 | A study to evaluate efficacy, tolerability and safety of adalimumab (Zydus) and adalimumab (Reference) in patients with rheumatoid arthritis |
| CTRI/2014/04/004571 | A clinical trial to study the effects of two drugs, R‐TPR‐021 / Humira® in patients with active rheumatoid arthritis on a stable dose of methotrexate |
| CTRI/2014/07/004742 | Phase III clinical trial comparing efficacy and safety of BCD‐020 (CJSC BIOCAD, Russia) and Mabthera® (f. Hoffmann‐la Roche ltd, Switzerland) in patients with rheumatoid arthritis. |
| CTRI/2014/09/004954 | A clinical trial to study the effects of three anti‐cd20 monoclonal antibodies in patients with moderate to severe active, seropositive rheumatoid arthritis with an inadequate response to methotrexate based therapy |
| CTRI/2015/01/005398 | A study to determine pharmacodynamics (effect of drug in the body) and to compare pharmacokinetics (how drug behaves in the body), safety and tolerability of single dose of Lupin’s Rituximab with Roche’s Rituximab following I.V. infusion in patients with rheumatoid arthritis |
| CTRI/2016/02/006625 | A clinical trial to evaluate efficacy and safety of BMO‐2 and adalimumab in patients with active rheumatoid arthritis |
| CTRI/2016/04/006884 | A clinical study to evaluate the efficacy, safety, immunogenicity, and pharmacokinetics of subcutaneous injection of adalimumab (test product, Hetero) and reference medicinal product (reference product, Abbvie) concomitantly administered with methotrexate in patients with rheumatoid arthritis |
| CTRI/2016/05/006899 | A study to compare the biosimilar of etanercept (coded as YLB113) made by YLBiologics with Enbrel (originator’s etanercept) in patients suffering from rheumatoid arthritis with respect to its efficacy, safety and antibody formation |
| CTRI/2016/07/007097 | Multi‐centre, randomized, double‐blind, two‐arm, parallel group, comparative clinical study to evaluate pharmacokinetic, efficacy and safety of etanercept in patients with active rheumatoid arthritis |
| DRKS00011083 | Clinical study of an anthroposophic treatment strategy for early rheumatoid arthritis, compared to conventional long‐term therapy |
| EUCTR2004‐000563‐96‐HU | A 24‐month, randomized, double‐blind, two period study to evaluate the efficacy and safety of the combination of etanercept and methotrexate and methotrexate alone in subjects with active early rheumatoid arthritis: combination of methotrexate and etanercept |
| EUCTR2004‐000922‐59‐SE | A phase III, multi‐center, randomized, double‐blind, placebo‐controlled comparative study of abatacept or infliximab in combination with methotrexate in controlling disease activity in subjects with rheumatoid arthritis having an inadequate clinical response to methotrexate |
| EUCTR2004‐002620‐18‐DE | A multi‐national randomized, double‐blind, exploratory study of abatacept versus placebo in preventing the development of rheumatoid arthritis in adult subjects with undifferentiated inflammatory arthritis at high risk for the development of rheumatoid arthritis |
| EUCTR2004‐002993‐49‐HU | A phase III multicentre, double blind, placebo‐controlled, parallel group 52‐week study to assess the efficacy and safety of 2 dose regimens of lyophilised cdp870 given subcutaneously as additional medication to methotrexate in the treatment of signs and symptoms and preventing structural damage in patients with active rheumatoid arthritis who have an incomplete response to methotrexate |
| EUCTR2004‐003295‐10‐GB | A multicenter, randomized, double‐blind, placebo‐controlled trial of golimumab, a fully human anti‐TNFalfa monoclonal antibody, administered subcutaneously, in methotrexate‐naïve subjects with active rheumatoid arthritis |
| EUCTR2004‐003296‐36‐DE | A multicenter, randomized, double‐blind, placebo‐controlled trial of golimumab, a fully human anti‐TNFalfa monoclonal antibody, administered subcutaneously, in subjects with active rheumatoid arthritis despite methotrexate therapy |
| EUCTR2004‐003299‐12‐FI | A multicenter, randomized, double‐blind, placebo‐controlled trial of golimumab, a fully human anti‐TNFalfa monoclonal antibody, administered subcutaneously, in subjects with active ankylosing spondylitis |
| EUCTR2004‐003733‐14‐FI | A study investigating whether tocilizumab (study drug) prevents joint damage, and how safe it is, in patients with moderate to severe rheumatoid arthritis randomly divided to groups receiving treatment with tocilizumab and methotrexate or methotrexate and placebo |
| EUCTR2004‐003741‐40‐AT | A randomized, double‐blind, parallel group study of the safety and reduction of signs and symptoms during treatment with MRA versus placebo, in combination with methotrexate, in patients with moderate to severe active rheumatoid arthritis |
| EUCTR2004‐003771‐37‐HU | A double‐blind, randomized, placebo controlled, dose escalation, multi‐center phase I/II trial of humax‐cd20, a fully human monoclonal anti‐cd20 antibody, in patients with active rheumatoid arthritis who have previously failed one or more disease modifying anti‐rheumatic drugs. ‐ humax‐cd20 in active rheumatoid arthritis, phase I/II |
| EUCTR2004‐005210‐37‐DE | A randomized, double‐blind, placebo‐controlled, parallel group study of the safety and reduction of signs and symptoms during treatment with MRA versus placebo, in combination with traditional DMARD therapy in patients with moderate to severe active rheumatoid arthritis and an inadequate response to current DMARD therapy |
| EUCTR2005‐000492‐18‐IT | Insulin resistance and endothelial dysfunction TNF‐alpha dependent in patients with rheumatoid arthritis or metabolic syndrome |
| EUCTR2005‐000674‐43‐GB | An open label study of the effect of treatment with rituximab on resistant rheumatoid arthritis: clinical, radiological, synovial and immunological outcomes ‐ rituximab in rheumatoid arthritis |
| EUCTR2005‐000784‐26‐GB | A phase IIIb multi‐center, randomized, double‐blind study to evaluate remission and joint damage progression in methotrexate naive early erosive RA subjects treated with abatacept plus methotrexate compared with methotrexate revised protocol 04 |
| EUCTR2005‐000884‐25‐DE | A randomized, double‐blind, placebo‐controlled, parallel group study of the safety and reduction of signs and symptoms during treatment with MRA versus placebo, in combination with methotrexate in patients with moderate to severe active rheumatoid arthritis and an inadequate response to previous anti‐TNF therapy. |
| EUCTR2005‐001138‐33‐LT | A randomized, double‐blind, double‐dummy, parallel group study of the safety and efficacy of MRA monotherapy, versus methotrexate (MTX) monotherapy, in patients with active rheumatoid arthritis. |
| EUCTR2005‐001549‐41‐HU | A randomised, double‐blind study comparing the safety and efficacy of etanercept with sulphasalazine in subjects with ankylosing spondylitis ‐ ASCEND |
| EUCTR2005‐001633‐14‐DK | Randomised, multi‐center, open‐label, parallel‐group study comparing adalimumab (Humira) 40 mg s.c. EOW versus infliximab (Remicade®) 3 mg/kg i.v. every 6. week in RA patients with unsustainable clinical response to infliximab 3 mg/kg every 8. week ‐ The SWITCH Study |
| EUCTR2005‐001742‐16‐GB | A multicenter, randomized, double‐blind, placebo‐controlled trial of golimumab, a fully human anti‐TNFalfa monoclonal antibody, administered subcutaneously in subjects with active rheumatoid arthritis and previously treated with biologic anti‐TNFalfa agent(s) ‐ go‐after |
| EUCTR2005‐001889‐13‐SE | Randomised controlled trial evaluating strategies to optimize disease activity control in RA patients treated with infliximab in clinical practice |
| EUCTR2005‐002326‐63‐LT | A phase III multi–center, double–blind, placebo–controlled, parallel group 24–week study to assess the efficacy and safety of two dose regimens of liquid certolizumab pegol as additional medication to methotrexate |
| EUCTR2005‐002392‐32‐IE | A randomised, placebo controlled, double‐blind, parallel group, international study to evaluate the safety and efficacy of rituximab (Mabthera/Rituxan) in combination with methotrexate, compared to methotrexate monotherapy, in patients with active rheumatoid arthritis |
| EUCTR2005‐002395‐15‐FI | A randomized, phase 3, controlled, double‐blind, parallel‐group, multicenter study to evaluate the safety and efficacy of rituximab in combination with methotrexate (MTX) compared to MTX alone, in methotrexate‐naïve patients with active rheumatoid arthritis |
| EUCTR2005‐002396‐33‐ES | A randomised, double‐blind, international study to evaluate the efficacy and safety of various re‐treatment regimens of rituximab in combination with methotrexate in RA patients with an inadequate response to methotrexate |
| EUCTR2005‐002423‐13‐DE | Long‐term extension study of safety during treatment with tocilizumab (MRA) in patients completing treatment in WA17822 |
| EUCTR2005‐002909‐23‐ES | Long‐term extension study of safety during treatment with tocilizumab (MRA) in patients completing treatment in MRA core studies |
| EUCTR2005‐003632‐22‐ES | A study of the pharmacokinetic and pharmacodynamic activity of rituximab in combination with methotrexate (MTX) in synovial tissue and in peripheral blood of patients with rheumatoid arthritis |
| EUCTR2005‐004530‐40‐AT | Induction of remission in RA patients at low disease activity by additional infliximab‐therapy |
| EUCTR2005‐004582‐41‐GB | Safety and efficacy of combination treatment with rituximab and leflunomide in patients with active rheumatoid arthritis ‐ rituximab and leflunomide in RA |
| EUCTR2005‐005013‐37‐GB | A multi‐centre randomised double dummy double blind study comparing two regimens of combination induction therapy in early DMARD naive rheumatoid arthritis: the IDEA study (infliximab as induction therapy in early rheumatoid arthritis) ‐ IDEA |
| EUCTR2005‐005358‐27‐GB | Efficacy of rituximab (Mabthera) in active ankylosing spondylitis: a clinical and magnetic resonance imaging study |
| EUCTR2006‐000363‐28‐GB | Differentiating the mechanism of action of anti TNF‐alpha agents ‐ data study |
| EUCTR2006‐000854‐32‐AT | Rituximab in rheumatoid arthritis in patients who failed therapy with TNF‐blockers |
| EUCTR2006‐001000‐37‐DE | Efficacy and safety of rituximab in patients with rheumatoid arthritis ‐ FIRST |
| EUCTR2006‐001428‐38‐GB | Remission induction in very early rheumatoid arthritis (RIVERA): a comparison of etanercept plus methotrexate plus steroid with standard therapy ‐ RIVERA |
| EUCTR2006‐001553‐10‐BE | A 26‐week, phase II, multi‐center, randomized, double‐blind, placebo‐controlled study to assess the response to treatment (ACR50) and to determine a biomarker profile in responders to ACZ885 (anti‐interleukin‐1beta monoclonal antibody) plus MTX as compared to MTX alone in early rheumatoid arthritis patients |
| EUCTR2006‐003843‐22‐IT | Prospective study on intensive early rheumatoid arthritis treatment with adalimumab: induction of remission and maintenance ‐ 'CURE' A phase IV multicenter, randomized, double‐blind study |
| EUCTR2006‐004139‐31‐BE | A multicenter, randomized, double‐period, double‐blind study to determine the optimal protocol for treatment initiation with methotrexate and adalimumab combination therapy in patients with early rheumatoid arthritis ‐ OPTIMA |
| EUCTR2006‐004673‐98‐HU | Efficacy of rituximab treatment in patients with rheumatoid arthritis having inadequate response to TNF blocker |
| EUCTR2006‐005137‐38‐FR | A 3 month, randomised, open label, parallel group, descriptive study to explore and compare perceptions and satisfaction for two different delivery mechanisms for etanercept (etanercept autoinjector and the etanercept prefilled syringe) |
| EUCTR2006‐005157‐29‐FR | Effet du methotrexate sur la relation dose ‐ effet de l'infliximab dans la spondylarthrite ankylosante ‐ SPAXIM |
| EUCTR2006‐005386‐19‐BE | Cytokines and inflammatory proteins gene expression study in synovial biopsies from rheumatoid arthritis patients refractory to anti‐TNF therapy treated with rituximab ‐ anti TNF resistant RA / RTX / mini |
| EUCTR2006‐005640‐81‐GB | A placebo controlled study of the effect of extended treatment with rituximab on resistant rheumatoid arthritis: ‐ EXXTRA |
| EUCTR2006‐006127‐40‐GB | Cerebral blood flow following TNF‐alpha antagonism in rheumatoid arthritis ‐ a pilot study ‐ TNF/cbf in RA |
| EUCTR2006‐006186‐16‐NL | Improved: induction therapy with methotrexate and prednisone in rheumatoid or very early arthritic disease a randomized clinical trial in patients with recent‐onset arthritis to compare the efficacy of DMARD combination therapy including prednisone with combination therapy including adalimumab, a TNF‐blocking agent ‐ IMPROVED |
| EUCTR2006‐006275‐21‐GB | A randomised, pragmatic, open‐label study of adalimumab versus etanercept for rheumatoid arthritis. ‐ adalimumab versus etanercept for RA |
| EUCTR2006‐006591‐37‐BE | A 3 month, randomised, open label, parallel group, descriptive study to explore and compare perceptions and satisfaction for two different delivery mechanisms for etanercept (etanercept auto‐injector and the etanercept prefilled syringe) |
| EUCTR2006‐006746‐33‐DE | Re‐treatment with rituximab in patients with rheumatoid arthritis who have had an inadequate response to not more than one a TNF (extension study to ML19070) ‐ efficacy of re‐therapy in anti‐TNFalpha IR |
| EUCTR2007‐000082‐38‐DK | The OPERA Study. Optimized treatment algorithm in early rheumatoid arthritis: Methotrexate and intra‐articular glucocorticosteroid plus adalimumab or placebo in the treatment of early rheumatoid arthritis. A Randomised, double‐blind and placebo‐controlled, two arms, parallel group study of the additive effect of adalimumab concerning inflammatory control and inhibition of erosive development. ‐ The OPERA Study |
| EUCTR2007‐000593‐24‐GB | An open‐label, observational study of the effects of anti‐TNF therapy on peripheral blood and synovial biomarkers in patients with active rheumatoid arthritis |
| EUCTR2007‐000828‐40‐FR | A phase IIIb, multi‐centre, double‐blind randomized, placebo‐controlled, parallel group 52‐week study to evaluate safety and efficacy of the PEGylated anti‐TNFα Fab′fragment, certolizumab pegol, administered concomitantly with stable‐dose DMARDs in patients with moderate to low disease activity rheumatoid arthritis |
| EUCTR2007‐000896‐41‐HU | A randomized, double‐blind study comparing the safety and efficacy of once‐weekly administration of etanercept 50 mg, etanercept 25 mg, and placebo in combination with methotrexate in subjects with moderately active rheumatoid arthritis |
| EUCTR2007‐001190‐28‐GB | Randomised controlled trial of tumour‐necrosis‐factor inhibitors against combination intensive therapy with conventional disease modifying anti‐rheumatic drugs in established rheumatoid arthritis ‐ TACIT |
| EUCTR2007‐001420‐12‐BE | A randomised, double‐blind (with open comparator etanercept limb), placebo‐controlled, phase IIb, multicentre study to evaluate the efficacy of 4 doses of azd9056 administered for 6 months on the signs and symptoms of rheumatoid arthritis |
| EUCTR2007‐001585‐33‐LT | A randomized, placebo controlled, multicenter clinical study investigating efficacy of rituximab (Mabthera®/Rituxan®) in the inhibition of joint structural damage assessed by magnetic resonance imaging in patients with rheumatoid arthritis and inadequate response to methotrexate ‐ the R.A. Score study |
| EUCTR2007‐001625‐10‐HU | An open‐label, randomized study to evaluate the radiographic efficacy and safety of Enbrel™ (etanercept) added to methotrexate in comparison with usual treatment in subjects with moderate rheumatoid arthritis disease activity ‐ EXTRA |
| EUCTR2007‐001754‐11‐IT | Pilot study to evaluate the effect of rituximab in combination with MTX in the inhibition of progression of synovitis, bone marrow edema, and erosions evaluated by magnetic resonance imaging (MRI) in the hand of patients with rheumatoid arthritis |
| EUCTR2007‐002066‐35‐HU | A phase 2B, randomized, double‐blind, placebo controlled active comparator, multicenter study to compare 5 dose regimens of CP‐690,550 and adalimumab versus placebo, administered for 6 months in the treatment of subjects with active rheumatoid arthritis |
| EUCTR2007‐002536‐29‐FR | A randomised, double‐blind, placebo controlled, multi‐centre phase II study of atacicept in anti‐ TNF alfa‐naïve patients with moderate to severely active rheumatoid arthritis and an inadequate response to methotrexate ‐ atacicept in anti‐TNF alfa‐naïve subjects with RA |
| EUCTR2007‐003096‐39‐IT | Effects of etanercept on endothelial function and carotid intima‐media thickness in patients with active ankylosing spondylitis: a 52‐weeks, randomized, double blind, placebo‐controlled study ‐ crest |
| EUCTR2007‐003288‐36‐NL | A phase 4, multicenter, open‐label, assessor‐blinded, switch study of the efficacy and safety of infliximab (Remicade) in patients with active rheumatoid arthritis who are responding inadequately to etanercept (Enbrel) of adalimumab (Humira) |
| EUCTR2007‐003358‐27‐DE | Phase III, multi‐center, randomized, double blind, placebo‐controlled study for treatment of juvenile ankylosing spondylitis with adalimumab ‐ Humira Study |
| EUCTR2007‐003623‐20‐ES | Estudio de los efectos de la terapia anti‐célula b (rituximab) sobre la inmunopatología del tejido sinovial y las células b de sangre periférica en artritis reumatoide (estudio tesice‐ar). Study of the b‐cell‐targeted therapy (rituximab) effects on the synovial tissue inmunopathology and peripheral blood b cells in rheumatoid arthritis (tesice‐ar study) |
| EUCTR2007‐003647‐75‐NL | A randomised, double‐blind, placebo controlled, multi‐centre, exploratory, pilot, phase II trial of 150 mg atacicept given subcutaneously in combination with rituximab in subjects with rheumatoid arthritis. ‐ atacicept in combination with rituximab in subjects with rheumatoid arthritis |
| EUCTR2007‐004694‐26‐BE | A comparative study of a 6‐month infliximab (Remicade) or placebo regimen in undifferentiated arthritis at high risk for the development of rheumatoid arthritis: clinical, radiological (MRI) and synovial benefit |
| EUCTR2007‐005464‐26‐GB | Development of heart and blood vessel problems in patients with conditions which cause long‐term, widespread, inflammation in the body |
| EUCTR2007‐005905‐23‐DE | A multi‐center, randomized, double‐blind, placebo‐controlled study comparing 80 mg of adalimumab with placebo, and demonstrating the non‐inferiority of monthly 80 mg adalimumab dosing compared with 40 mg adalimumab every other week dosing |
| EUCTR2007‐006657‐63‐FI | Study comparing the effect on disease activity when reducing or discontinuing etanercept in subjects with rheumatoid arthritis (RA) |
| EUCTR2007‐007539‐14‐CZ | A randomised, double‐blind, placebo‐controlled, phase IIb dose‐ranging study (with open‐label etanercept treatment group) to investigate efficacy, safety and pharmacokinetics of azd5672 administered for 12 weeks to rheumatoid arthritis patients receiving methotrexate |
| EUCTR2008‐000105‐11‐DE | Effectiveness after 4 and 24 weeks and safety of tocilizumab in patients with active RA ‐ TAMARA ‐ tocilizumab and DMARDs: achievements in rheumatoid arthritis |
| EUCTR2008‐000587‐17‐GB | Multi‐national open‐label study to evaluate the safety, tolerability and efficacy of tocilizumab in patients with active rheumatoid arthritis on background non‐biologic DMARDs who have an inadequate response to current non‐biologic DMARD and/or anti‐TNF therapy |
| EUCTR2008‐001241‐26‐HU | A randomized, placebo‐controlled, double‐blind, dose escalation study to evaluate the efficacy, safety and tolerability of the study drug bt971 in patients with rheumatoid arthritis receiving concomitant methotrexate |
| EUCTR2008‐002381‐55‐ES | Evaluación de la eficacia de rituximab en pacientes con artritis reumatoide a través de la medición, por resonancia magnética de mano, de los parámetros clínicos de la enfermedad. Estudio resonar. Efficacy of rituximab in patients with rheumatoid arthritis, by measurement of disease parameters through magnetic resonance of the hand. RESONAR study |
| EUCTR2008‐002623‐85‐NL | A 3‐phase study to evaluate sustained remission and productivity outcomes in subjects with early rheumatoid arthritis initiated on treatment with etanercept plus methotrexate |
| EUCTR2008‐002631‐33‐GB | Randomized, placebo controlled, double blind, multi‐center phase II proof‐of‐concept study to assess the efficacy of ain457 in patients with moderate to severe ankylosing spondylitis |
| EUCTR2008‐003011‐12‐GB | Prospective randomised double‐blind placebo controlled study assessing the efficacy of tocilizumab with synovial analysis in patients with rheumatoid arthritis ‐ TOCRA |
| EUCTR2008‐004126‐16‐FI | Local open‐label study to evaluate the safety and efficacy of tocilizumab in patients with active rheumatoid arthritis on background non‐biologic DMARDs who have an inadequate response to current non‐biologic DMARDs |
| EUCTR2008‐004931‐39‐PL | A study to determine the safety, efficacy, and pharmacokinetics of 80 mg, 160 mg, and 320 mg ald518 versus placebo administered as multiple intravenous infusions to patients with active rheumatoid arthritis who have had an inadequate response to methotrexate |
| EUCTR2008‐005212‐40‐SE | Pain mechanisms and fatigue in rheumatoid arthritis (RA) and healthy volunteers. Can antirheumatic and biological therapy affect pain processing and fatigue in RA? |
| EUCTR2008‐005320‐81‐AT | A 2‐year open‐label second extension study to evaluate the safety, tolerability and efficacy of canakinumab (acz885) an anti‐interleukin‐1ß monoclonal antibody in patients with active rheumatoid arthritis |
| EUCTR2008‐005450‐20‐BE | The cost‐effectiveness of abatacept, rituximab or anti‐TNF alpha for patients with rheumatoid arthritis. ‐ Dutch Rheumatoid Arthritis Monitoring (DREAM) Targetted Immune Modulator Evaluation (TIME) |
| EUCTR2008‐005525‐11‐ES | Estudio randomizado, controlado con placebo, doble ciego y con grupos paralelos para comparar la seguridad y la reducción de la actividad de la enfermedad con la combinación de rituximab (Mabthera) y tocilizumab (roactemra) frente al tratamiento con tocilizumab en pacientes con artritis reumatoide activa con respuesta incompleta a metotrexato. A randomized, placebo controlled, double‐blind, parallel group study to compare the safety and efficacy of the combination of rituximab (Mabthera) and tocilizumab (Actemra) versus tocilizumab therapy in patients with active rheumatoid arthritis with an incomplete response to methotrexate |
| EUCTR2008‐006256‐22‐FR | Evaluation by high resolution micro computerized tomography of bone microarchitecture changes in patients with rheumatoid arthritis under anti‐TNF therapy |
| EUCTR2008‐006885‐27‐NL | Efficacy and safety of adalimumab (Humira®) in patients with peripheral spondyloarthritis without ankylosing spondylitis or psoriatic arthritis |
| EUCTR2008‐006924‐68‐AT | Extension phase of the multi‐national open‐label study to evaluate the safety, tolerability and efficacy of tocilizumab in patients with active rheumatoid arthritis on background non‐biologic DMARDs who have an inadequate response to current non‐biologic DMARD and/or anti‐TNF therapy. |
| EUCTR2008‐006936‐37‐HU | A randomized, parallel, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of ILV‐094 administered subcutaneously to subjects with active rheumatoid arthritis on a stable background of methotrexate |
| EUCTR2008‐008338‐35‐CZ | Phase 3 randomized, double‐blind, active comparator, placebo‐controlled study of the efficacy and safety of 2 doses of CP 690,550 in patients with active rheumatoid arthritis on background methotrexate |
| EUCTR2009‐010582‐23‐DE | A golimumab phase 3b, multicenter, switch assessment of subcutaneous and intravenous efficacy in rheumatoid arthritis patients who have inadequate disease control despite treatment with etanercept (Enbrel®) or adalimumab (Humira®) |
| EUCTR2009‐010955‐29‐NL | Prevention of clinically manifest rheumatoid arthritis by B cell directed therapy in the earliest phase of the disease |
| EUCTR2009‐011105‐17‐IT | A single‐arm, open‐label study of early improvement of anemia and fatigue during treatment with tocilizumab (TCZ) in combination with non biologic DMARDs, in adult patients with moderate to severe active rheumatoid arthritis |
| EUCTR2009‐011137‐26‐DE | An open‐label study assessing the addition of subcutaneous golimumab (GLM) to conventional disease‐modifying antirheumatic drug (DMARD) therapy in biologic‐naïve subjects with rheumatoid arthritis (part 1), followed by a randomized study assessing the value of combined intravenous and subcutaneous GLM administration aimed at inducing and maintaining remission (part 2) |
| EUCTR2009‐011520‐53‐SK | Evaluation of adherence and persistence to tocilizumab in combination with methotrexate or tocilizumab monotherapy in patients with moderate to severe active rheumatoid arthritis in local environment |
| EUCTR2009‐011591‐30‐GB | An open‐label non‐randomized extension study to evaluate the safety and tolerability of ain457 (anti interleukin‐17 monoclonal antibody) in patients with moderate to severe ankylosing spondylitis |
| EUCTR2009‐011719‐19‐FR | Phase 3, multicenter, randomized, double‐blind, placebo‐controlled study to evaluate efficacy and safety of certolizumab pegol in subjects with active axial spondyloarthritis |
| EUCTR2009‐012185‐32‐IT | Open label, multicentric phase IIIb study to evaluate the effect of tocilizumab in combination with DMARDs in the inhibition of progression of synovitis, bone marrow edema, and erosions evaluated by dedicated magnetic resonance imaging (MRI) in the hand of patients with rheumatoid arthritis (RA) |
| EUCTR2009‐012204‐42‐IE | A randomized, double‐blind, placebo‐controlled, parallel group study to investigate the ability of gsk706769 to maintain clinical efficacy after withdrawal of Enbrel in patients with rheumatoid arthritis |
| EUCTR2009‐012218‐30‐PT | A randomized, double‐blind, placebo‐controlled study to assess the efficacy of tocilizumab (TCZ) + non‐biological DMARD in reducing synovitis as measured by magnetic resonance imaging (MRI) at 12 weeks after initiation of treatment in patients with moderate to severe rheumatoid arthritis (RA) with inadequate response to non‐biological DMARDs |
| EUCTR2009‐012759‐12‐GB | A multi‐center, randomized, double‐blind, parallel group study of the safety, disease remission and prevention of structural joint damage during treatment with tocilizumab (TCZ), as a monotherapy and in combination with methotrexate (MTX), versus methotrexate in patients with early, moderate to severe rheumatoid arthritis |
| EUCTR2009‐013316‐12‐NL | A multi‐center, randomized, double blind, placebo controlled study to evaluate remission in DMARD and biological naïve early rheumatoid arthritis (RA) subjects treated with tocilizumab (TCZ) plus tight control methotrexate (MTX) treatment, TCZ monotherapy or tight control MTX monotherapy. ‐ U‐ACT‐EARLY |
| EUCTR2009‐013758‐33‐SE | Multicenter study with a 16‐week double‐blind, placebo‐controlled (during the initial 2 weeks) randomized period, followed by a 24‐week open label extension to assess magnetic resonance image‐verified early response to certolizumab pegol in subjects with active rheumatoid arthritis |
| EUCTR2009‐015515‐40‐NL | Prevention of the progression of very early symptoms into ankylosing spondylitis: a placebo controlled trial with etanercept |
| EUCTR2009‐015653‐20‐NL | Prospective study on the effects of etanercept treatment in patients with rheumatoid arthritis who are naïve for TNF‐alpha blocking therapy and patients who do not respond (anymore) to prior treatment with other anti‐TNF‐alpha medication |
| EUCTR2009‐015740‐42‐DE | A phase 3, multicenter, randomized, open, prospective, controlled, parallel‐group study of reduction of therapy in patients with rheumatoid arthritis in ongoing remission RETRO |
| EUCTR2009‐015845‐21‐GB | A multi‐center, randomized, blinded, parallel‐group study of the reduction of signs and symptoms during monotherapy treatment with tocilizumab 8 mg/kg intravenously versus adalimumab 40 mg subcutaneously in patients with rheumatoid arthritis |
| EUCTR2009‐015950‐39‐DE | Rituximab‐treatment in addition to leflunomide in patients with active rheumatoid arthritis |
| EUCTR2009‐016789‐10‐NL | Efficacy of the H1N1 flu (swine flu) vaccination in patients with rheumatoid arthritis treated with rituximab |
| EUCTR2009‐017325‐19‐FI | The effect of six months adalimumab treatment on sick leaves and retirement in patients with rheumatoid arthritis who are at risk of losing their ability to work |
| EUCTR2009‐017443‐34‐GB | A ph II/III seamless, multi‐center, randomized, double‐blind, placebo‐controlled study of the reduction in signs and symptoms and inhibition of structural damage during treatment with tocilizumab versus placebo in patients with ankylosing spondylitis who have failed non‐steroidal anti‐inflammatory drugs and are naïve to TNF antagonist therapy |
| EUCTR2009‐017488‐40‐GB | A randomized, double‐blind, parallel‐group placebo‐controlled study of the safety and reduction of signs and symptoms during treatment with tocilizumab (TCZ) versus placebo in patients with ankylosing spondylitis who have had an inadequate response to previous TNF antagonist therapy |
| EUCTR2010‐018331‐18‐GB | A 52 week, single center, open‐label study to evaluate neutrophil function and survival effects of tocilizumab (TCZ) in patients with active rheumatoid arthritis (RA) on background non‐biologic DMARDs who have an inadequate response to current non‐biologic DMARD and/or anti‐TNF therapy |
| EUCTR2010‐018375‐22‐ES | A randomized, double‐blind, parallel group study of the safety and effect on clinical outcome of tocilizumab sc versus tocilizumab iv, in combination with traditional disease modifying anti‐rheumatoid arthritis drugs (DMARDs), in patients with moderate to severe active rheumatoid arthritis. Estudio randomizado, doble ciego, con grupos de tratamiento paralelos, para evaluar la seguridad y el efecto sobre el resultado clínico de tocilizumab sc frente a tocilizumab iv en combinación con fármacos antirreumáticos modificadores de la enfermedad (FAMEs) tradicionales, en pacientes con artritis reumatoide activa moderada a severa |
| EUCTR2010‐019694‐15‐BE | Act‐alone: an open‐label, single‐arm study to describe glucocorticoid use in rheumatoid arthritis patients treated with tocilizumab in daily clinical practice and to evaluate systematic glucocorticoid dose reduction once low disease activity is reached |
| EUCTR2010‐019873‐13‐BE | Comparative study of the clinical response and cardiorespiratory endurance in early rheumatoid arthritis patients treated with tociluzimab or methotrexate addendum protocol: global gene expression profiles in synovial biopsies from early rheumatoid arthritis patients treated with tocilizumab or methotrexate ‐TOMERA |
| EUCTR2010‐019935‐37‐FI | A pragmatic, randomized, parallel group study of the effect on disease remission, work productivity, and tolerability of tocilizumab in combination with DMARDs and individually designed best practice DMARD therapy in patients with early, moderate to severe rheumatoid arthritis |
| EUCTR2010‐020738‐24‐GB | To see whether for patients with established rheumatoid arthritis that have already achieved a good response to tumour necrosis factor inhibitor (TNF inhibitor) treatment, whether the treatment be tapered to a minimum dose without affecting the control of disease activity |
| EUCTR2010‐020839‐39‐GB | Efficacy and safety of cdp6038 in patients with rheumatoid arthritis with an unsuccessful response to anti‐TNF therapy |
| EUCTR2010‐020913‐10‐GB | An open label, pilot, multi‐centre, step‐down, randomised controlled trial to examine whether etanercept 25mg once weekly is effective in maintaining a clinical response in patients with ankylosing spondylitis who have responded to 50mg once weekly |
| EUCTR2010‐021020‐94‐DE | A randomized, double‐blind, parallel‐group, placebo‐ and active calibrator‐controlled study assessing the clinical benefit of sar153191 subcutaneous (sc) on top of methotrexate (MTX) in patients with active rheumatoid arthritis (RA) who have failed previous TNF‐a anatagonists |
| EUCTR2010‐022049‐88‐DE | "Efficacy and safety study of a sequential therapy of tocilizumab (TCZ) and, if initially inadequately responded to tocilizumab (TCZ), followed by rituximab (RTX) in DMARD‐ir patients with rheumatoid arthritis (MIRAI)" ‐ MIRAI |
| EUCTR2010‐022378‐15‐DE | A clinical study to explore the therapeutic effects of different doses of the new drug veltuzumab, a drug of biologic origin, and placebo, in patients with rheumatoid arthritis |
| EUCTR2010‐023910‐30‐GB | A prospective, single‐centre, randomised study evaluating the clinical, imaging and immunological depth of remission achieved by very early versus delayed etanercept in patients with rheumatoid arthritis (VEDERA) |
| EUCTR2010‐023956‐99‐HU | Phase IIB rheumatoid arthritis dose ranging study for BMS‐945429 in subjects who are not responding to methotrexate |
| EUCTR2011‐000215‐79‐FR | Tociluzimab effect on endothelial function in patients with rheumatoid arthritis ‐ TEFRA |
| EUCTR2011‐001626‐15‐ES | A study of Roactemra/Actemra (tocilizumab) in combination with methotrexate in patients with rheumatoid arthritis with inadequate response to prior treatment with methotrexate and low disease activity with the combination de Roactemra/Actemra y methotrexate |
| EUCTR2011‐001729‐25‐DE | Study designed to demonstrate the efficacy and safety of certolizumab pegol in combination with methotrexate in the treatment of subjects suffering from early, progressive active rheumatoid arthritis |
| EUCTR2011‐001863‐39‐AT | A study of safety and efficacy of tocilizumab (TCZ) in combination with methotrexate (MTX) versus tocilizumab monotherapy in patients with mild to moderate rheumatoid arthritis, who have not adequately responded to their current treatment with MTX |
| EUCTR2011‐002275‐41‐CZ | A study of two different adalimumab formulations in adults with rheumatoid arthritis |
| EUCTR2011‐002325‐22‐GB | Study of ixekizumab in participants with active ankylosing spondylitis (AS) |
| EUCTR2011‐002363‐15‐IS | A clinical trial with the aim to explore infusion reactions from tocilizumab given either in 31 or 60 minutes to patients with moderate to severe rheumatoid arthritis |
| EUCTR2011‐004017‐17‐GB | Tocilizumab and remission in early rheumatoid arthritis |
| EUCTR2011‐004171‐36‐CZ | A study comparing sait101 to Mabthera® in subjects with severe rheumatoid arthritis (RA) |
| EUCTR2011‐004468‐31‐GB | Evaluating the long‐term safety and efficacy effects of ct‐p13 together with methotrexate in patients with arthritis |
| EUCTR2011‐005021‐48‐HU | A study to investigate and compare the efficacy, safety, tolerability and pharmacodynamic (biochemical and physiological effects of the drug) of tl011 and Mabthera® (rituximab) in patients with severe, active rheumatoid arthritis treated with methotrexate (MTX) |
| EUCTR2011‐005204‐15‐AT | Could ultrasound help to identify the patients with rheumatoid arthritis, in those the treatment with biological DMARDs could be stopped? |
| EUCTR2011‐005260‐20‐GB | Roactemra® (tocilizumab) plus methotrexate (MTX) in stable dosage in comparison with Roactemra® plus reducing (tapering) MTX dosages in patients with severe rheumatoid arthritis (RA) that have inadequate responded to a trial of two disease modifying anti‐rheumatic drugs (DMARDs), including MTX and have not been previously treated with a biologic agent, such as a TNF inhibitor |
| EUCTR2011‐005448‐87‐HU | A study of the maintenance of efficacy of etanercept plus DMARD(s) compared with DMARD(s) alone in subjects with rheumatoid arthritis after achieving an adequate response with etanercept plus DMARD(s) |
| EUCTR2011‐005649‐10‐DE | An exploratory clinical study to investigate mavrilimumab, an antibody being developed for the treatment of moderate to severe rheumatoid arthritis, an inflammatory condition that affects the joints versus a different antibody whose mechanism works by inhibiting tumor necrosis factor |
| EUCTR2011‐006001‐10‐IT | Efficacy of rituximab at the dose of 500 mg e.v., two infusions two weeks apart, versus rituximab at the usual dose of 1000 mg, two infusions two weeks apart, in patients affected by rheumatoid arthritis, who had been previously treated with rituximab at the standard dose for at least two cycles obtaining a good clinical response |
| EUCTR2011‐006040‐79‐DK | The efficacy and safety of adding tocilizumab to methotrexate and intra‐articular glucocorticosteroid treatment in early rheumatoid arthritis. |
| EUCTR2011‐006125‐14‐HU | A multicenter, open‐label, single arm, long term extension study of WA19926 to describe safety during treatment with tocilizumab in patients with early, moderate to severe rheumatoid arthritis ‐ function LTE |
| EUCTR2012‐000139‐21‐AT | Multi‐center biomarker trial to predict therapeutic responses of patients with rheumatoid arthritis to a specific biologic mode of action |
| EUCTR2012‐001760‐30‐IT | Evaluation effects of treatment with an inhibitor of the receptor of a protein (interleukin‐6 il‐6)involved in inflammatory process, on the clinical response and on the changes from baseline in the biomarkers in patients with rheumatoid arthritis (RA)not responding adequately to disease‐modifying antirheumatic drugs (DMARDs) and/or to a first biological agent |
| EUCTR2012‐002009‐23‐HU | Clinical trial to demonstrate that treatments with gp2015 and Enbrel® are comparable in patients with rheumatoid arthritis |
| EUCTR2012‐002322‐73‐HU | A phase 3 study in moderate to severe rheumatoid arthritis |
| EUCTR2012‐002535‐28‐GB | A randomised, open labelled study in anti‐TNFa inadequate responders to investigate the mechanisms for response ‐ resistance to rituximab versus tocilizumab in RA (r4‐ra) |
| EUCTR2012‐003057‐29‐CZ | A multi‐centre, randomised, double‐blind multiple dose study of increasing doses of xmab5871 in patients with rheumatoid arthritis |
| EUCTR2012‐003194‐25‐LT | Bioequivalence trial of Mabioncd20® (Mabion SA) compared to reference product: Mabthera® (rituximab, Roche) in patients with rheumatoid arthritis |
| EUCTR2012‐003536‐23‐CZ | To evaluate the safety of sar153191 (REGN88) and tocilizumab added to other RA drugs in patients with RA who are not responding to or intolerant of anti‐TNF therapy (Saril‐RA‐Ascertain) |
| EUCTR2012‐003644‐71‐ES | Randomized, double‐blind, placebo‐controlled trial of etanercept plus methotrexate in monoclonal antibody (MAB) anti‐TNF failure |
| EUCTR2012‐003876‐38‐DE | Clinical study to find out if the biologically similar medicine gp2013 is safe in patients with rheumatoid arthritis who have been treated with Rituxan® or Mabthera® in the past |
| EUCTR2012‐004482‐40‐ES | Evaluation of a protocol for the reduction of doses in patients with rheumatoid arthritis (RA) in clinical remission in treatment with biological therapies |
| EUCTR2012‐005026‐30‐HU | A study comparing SB4 to Enbrel® in subjects with moderate to severe rheumatoid arthritis despite methotrexate therapy |
| EUCTR2012‐005275‐14‐NO | Remission in rheumatoid arthritis – assessing withdrawal of disease‐modifying antirheumatic drugs |
| EUCTR2012‐005733‐37‐CZ | A study comparing SB2 to Remicade® in subjects with moderate to severe rheumatoid arthritis |
| EUCTR2013‐000337‐13‐DE | Prediction of response to certolizumab pegol treatment with MRI of the brain. A multi‐center, randomized double‐blind controlled study prediction of response to certolizumab‐pegol in rheumatoid arthritis (PRECEPRA) |
| EUCTR2013‐000342‐19‐NL | A clinical trial where patients with rheumatoid arthritis are treated with the study drug tocilizumab, subcutaneous (injection in the skin), with or without other non‐biological anti‐rheumatic drugs, to study the safety and efficacy of the drug |
| EUCTR2013‐000525‐31‐GB | A randomized, double‐blind, phase 3 Study of ABP 501 efficacy and safety compared to adalimumab in subjects with moderate to severe rheumatoid arthritis |
| EUCTR2013‐001569‐17‐IT | A national, open‐label, single‐arm, phase IIIb study to evaluate the efficacy of weekly tocilizumab subcutaneous, administered as monotherapy or in combination with other non‐biological medicinal products in rheumatoid arthritis (RA) patients |
| EUCTR2013‐002007‐34‐FI | Safety and efficacy study of tocilizumab injected under the skin in patients with active rheumatoid arthritis (RA) and inadequate response to disease modifying antirheumatic drugs |
| EUCTR2013‐002150‐79‐BE | A study to evaluate the efficacy and safety of tocilizumab subcutaneous in RA patients |
| EUCTR2013‐002429‐52‐ES | Study to evaluate the efficacy, safety and tolerability of subcutaneous (SC) tocilizumab (TCZ) in subjects with rheumatoid arthritis |
| EUCTR2013‐002777‐22‐GB | Targeted Ultrasound in Rheumatoid Arthritis (TURA) |
| EUCTR2013‐003177‐99‐SE | A clinical study to evaluate the safety of two different doses of tofacitinib for the treatment of rheumatoid arthritis |
| EUCTR2013‐003413‐18‐GB | Arthritis prevention with abatacept |
| EUCTR2013‐004051‐20‐ES | Not controlled study to assess the efficacy of tocilizumab in patients with moderate or severe rheumatoid arthritis who are candidates to be treated with a biological therapy as monotherapy |
| EUCTR2013‐004148‐49‐LT | Randomized study of pf‐06438179 and infliximab in combination with methotrexate in subjects with moderately to severely active rheumatoid arthritis |
| EUCTR2013‐004555‐21‐AT | A randomized, controlled, double‐blind, parallel‐group, phase 3 study to compare the pharmacokinetics, efficacy and safety between ct‐p10, Rituxan and Mabthera in patients with rheumatoid arthritis |
| EUCTR2013‐005543‐90‐HU | A randomized, double‐blind study to compare pharmacokinetics and pharmacodynamics, efficacy and safety of ABP 798 with rituximab in subjects with moderate to severe rheumatoid arthritis |
| EUCTR2014‐000109‐11‐DE | Study to assess the efficacy and safety of FKB327 compared with Humira®, when each is administered in combination with methotrexate in patients with rheumatoid arthritis |
| EUCTR2014‐000110‐61‐CZ | Study to assess the long‐term efficacy and safety of FKB327 compared with Humira®, when each is administered in combination with methotrexate in patients with rheumatoid arthritis |
| EUCTR2014‐002374‐36‐SE | A study with dose de‐escalation of conventional or biologic treatments in early rheumatoid arthritis in patients with low disease activity |
| EUCTR2014‐002945‐23‐GB | A 24‐week randomized, open‐label, parallel‐group, active‐controlled, exploratory, proof‐of‐mechanism imaging study investigating the efficacy of 150 mg of namilumab administered subcutaneously vs adalimumab in patients with moderate to severe early rheumatoid arthritis inadequately responding to methotrexate ‐ a phase 2 study of namilumab vs anti‐tumor necrosis factor in patients with rheumatoid arthritis |
| EUCTR2014‐003255‐54‐CZ | A study evaluating the effects of rgb‐03 and Mabthera combined with methotrexate in patients with rheumatoid arthritis |
| EUCTR2014‐003307‐30‐HU | Multiple dose study of ucb4940 as add‐on to certolizumab pegol in subjects with rheumatoid arthritis |
| EUCTR2014‐003453‐34‐EE | Study of a new drug’s effect in people with rheumatoid arthritis who have not responded sufficiently well to treatment with methotrexate |
| EUCTR2014‐003529‐16‐GB | Stratification of biologic therapies for rheumatoid arthritis by pathobiology |
| EUCTR2014‐004558‐33‐Outside‐EU/EEA | A multicenter, open‐label study of the safety, efficacy, and pharmacokinetics of the human anti‐TNF monoclonal antibody adalimumab in children with polyarticular juvenile rheumatoid arthritis |
| EUCTR2014‐004673‐16‐DE | Randomized, blinded, controlled study to compare the efficacy of treatment with tocilizumab with or without glucocorticoids in rheumatoid arthritis |
| EUCTR2014‐004704‐29‐ES | This trial is designed to determine what effects the human body has on the investigational medicine, ABP 710, and what effects the body has on the investigational medicine after you have been given it, and if this is comparable to what is seen for the licensed medicine, infliximab, in patients with moderate or severe rheumatoid arthritis (RA). This study will assess if the investigational medicine is safe and effective in treating moderate or severe RA compared to the licensed medicine |
| EUCTR2014‐004868‐38‐GR | A study comparing the use of etanercept and methotrexate, used either alone or in combination, for maintaining remission in rheumatoid arthritis |
| EUCTR2014‐004904‐31‐NL | A clinical study to investigate the infliximab serum concentration of Remsima™ (infliximab biosimilar) after switching from Remicade (infliximab) in subjects with Crohn’s disease (CD), ulcerative colitis (UC) or rheumatoid arthritis (RA) in stable remission |
| EUCTR2014‐005368‐13‐HU | A study comparing sait101 to Mabthera® or Rituxan® in patients with rheumatoid arthritis (RA) |
| EUCTR2015‐000581‐58‐CZ | A randomized,biomarker trial to predict therapeutic responses of patients with rheumatoid arthritis to a specific biologic mode of action |
| EUCTR2015‐001246‐28‐BE | Ultrasound scores as imaging biomarkers of early response to subcutaneous tocilizumab in association with methotrexate in early rheumatoid arthritis (TOVERA study) |
| EUCTR2015‐001894‐41‐HU | Multicenter study to evaluate efficacy and safety of certolizumab pegol in subjects with active inflammation in the spine with no damage on x‐rays |
| EUCTR2015‐002284‐42‐FI | INTENT: immunogenicity in patients failing response on anti‐TNF |
| EUCTR2015‐002466‐22‐Outside‐EU/EEA | A randomized, multi‐center, blinded, placebo‐controlled study with an open label run‐in period to evaluate the efficacy, safety, and pharmacokinetics of daily, single, subcutaneous injections of r‐methuil‐1ra (anakinra) in polyarticular‐course juvenile rheumatoid arthritis |
| EUCTR2015‐002809‐12‐HU | A study to compare ylb113 and Enbrel for the treatment of rheumatoid arthritis |
| EUCTR2015‐003433‐10‐CZ | ADMYRA Trial: clinical trial to compare treatment with GP2017 and Humira® in patients with rheumatoid arthritis |
| EUCTR2015‐004386‐91‐PL | Study to explore and compare the effects of a new drug in combination with methotrexate therapy in people with early and established rheumatoid arthritis. |
| EUCTR2015‐004858‐17‐NL | Remission induction in very early rheumatoid arthritis |
| EUCTR2015‐005307‐83‐CZ | Study of the efficacy and safety of Olokizumab in patients with moderately to severely active rheumatoid arthritis inadequately controlled by methotrexate therapy |
| EUCTR2016‐000933‐37‐HU | Study to compare abt‐494 to abatacept in subjects with rheumatoid arthritis on stable dose of conventional synthetic disease‐modifying antirheumatic drugs (csDMARDs) who have an inadequate response or intolerance to biologic DMARDs (select‐choice) |
| EUCTR2016‐002125‐11‐LV | Evaluating efficacy, pharmacokinetics and safety between subcutaneous CT‐P13 and intravenous CT‐P13 in patients with active rheumatoid arthritis |
| EUCTR2016‐002852‐26‐HU | MSB11022 in moderately to severely active rheumatoid arthritis |
| EUCTR2016‐002908‐15‐NL | Redo study: research into the effects of lower doses rituximab in patients with rheumatoid arthritis |
| IRCT201206266302N3 | Comparative analysis of Altebrel® (aryogen) with Enbrel® |
| IRCT2014090319025N1 | Efficacy of Mabasia (adalimumab) in rheumatoid arthritis |
| IRCT2015030321315N1 | The effect of adalimumab on treatment of rheumatoid arthritis |
| ISRCTN14909030 | Rituximab in rheumatoid arthritis: is a reduced dose every 6 months equally effective as the regular dose if the patient has low or very low disease activity? |
| ISRCTN15819795 | Effect of anakinra (soluble interleukin‐1 receptor antagonist) as combination therapy: second UK combination therapy in early rheumatoid arthritis |
| ISRCTN23348591 | A placebo controlled study of the effect of extended treatment with rituximab on resistant rheumatoid arthritis: clinical and radiological outcomes |
| ISRCTN27093749 | Rituximab in rheumatoid arthritis in patients who failed therapy with tumour necrosis factor‐blockers: a multi‐centre clinical observational real‐life study (phase IIIb) |
| ISRCTN29665463 | A placebo‐controlled trial of anti‐TNFa chimeric monoclonal antibody (infliximab, Remicade) in the modification of vascular disease markers in active rheumatoid arthritis |
| ISRCTN36745608 | A controlled randomised double‐blind multicentre study comparing two therapy strategies in disease modifying anti‐rheumatic drug‐naive early rheumatoid arthritis patients over 48 weeks: induction therapy with adalimumab and methotrexate over 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate monotherapy |
| ISRCTN39045408 | Anti‐tumour necrosis factor (anti‐TNF) therapy over two years increases body fat mass in early rheumatoid arthritis |
| ISRCTN44880063 | Differentiating the mechanism of action of anti‐TNF alpha agents |
| ISRCTN46017566 | Arthritis prevention in the pre‐clinical phase of rheumatoid arthritis with abatacept |
| ISRCTN48638981 | A multicentre randomised double‐blind placebo‐controlled study comparing two regimens of combination induction therapy in early disease‐modifying anti‐rheumatic drug naïve rheumatoid arthritis |
| ISRCTN49682259 | Remission induction in very early rheumatoid arthritis: a comparison of etanercept plus methotrexate plus steroid with standard therapy |
| ISRCTN51200229 | Randomised double blind trial of safety of anti‐tumour necrosis factor (anti‐TNF) chimeric monoclonal antibody (infliximab) in combination with methotrexate compared to methotrexate alone in patients with rheumatoid arthritis on standard disease modifying anti‐rheumatic drugs |
| ISRCTN57761809 | Effect of anti‐tumour necrosis factor alpha (TNFa) therapy on blood vessel health in patients with rheumatoid arthritis |
| ISRCTN62900439 | Leflunomide or methotrexate plus subcutaneous tumour necrosis factor‐alpha (TNF‐alpha) blocking agents in rheumatoid arthritis |
| ISRCTN70800019 | Effects on tocilizumab drug therapy on fat tissue proteins in rheumatoid arthritis |
| ISRCTN75505683 | Remission induction study in early rheumatoid arthritis (RA) |
| ISRCTN82317088 | Changes in bone density and bone turnover in patients with rheumatoid arthritis treated with rituximab, a b cell depleting antibody |
| ISRCTN89222125 | Switching to alternative tumour‐necrosis factor (TNF)‐blocking drugs or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF‐blocking drug |
| ISRCTN95861172 | Randomised efficacy and discontinuation study of etanercept and adalimumab (RED SEA): a pragmatic open label study in rheumatoid arthritis |
| ISRCTN97686858 | A randomized, controlled study of intra‐articular injections of etanercept or glucocorticosteroids in patients with rheumatoid arthritis |
| JPRN‐JapicCTI‐111620 | A randomized, double‐blind, phase I/II study of CT‐P13 compared with Remicade in patients with rheumatoid arthritis |
| JPRN‐JapicCTI‐142505 | Phase III study of MRA‐SC 162 mg/week |
| JPRN‐JapicCTI‐142621 | Chs‐0214 phase III trial |
| JPRN‐UMIN000000512 | Efficacy of tacrolimus in rheumatoid arthritis patients who have been treated unsuccessfully with infliximab and methotrexate |
| JPRN‐UMIN000001240 | The efficacy of tocilizumab to patients with rheumatoid arthritis refractory to anti‐TNF agents: the open trial |
| JPRN‐UMIN000001407 | The efficacy and safety of the new biologic agents (humanized anti‐human interlukin‐6 receptor monoclonal antibody) on abnormal lipid metabolism and atherosclerosis for rheumatoid arthritis patients in Japan |
| JPRN‐UMIN000001798 | Prevention of cartilage destruction in rheumatoid arthritis by etanercept (PRECEPT study) |
| JPRN‐UMIN000002110 | Discontinuation of infliximab therapy after acquisition of low disease activity by infliximab in rheumatoid arthritis study: RRR (remission induction by Remicade) study |
| JPRN‐UMIN000002246 | Study for predictors of effectiveness in tocilizumab therapy (PETITE) |
| JPRN‐UMIN000002340 | Comparison of effects between higher dosages of infliximab and switching to other biologics for rheumatoid arthritis patients with less responsiveness to infliximab therapy (Chamlet) |
| JPRN‐UMIN000002421 | Multicenter, open‐label parallel‐groups study comparing tocilizumab versus conventional treatment in rheumatoid arthritis with the complication of AA amyloidosis |
| JPRN‐UMIN000002687 | Enbrel clinical outcome in RA patients for growing evidence |
| JPRN‐UMIN000002744 | Success of tocilizumab in RA patients with remission induction and sustained efficacy after discontinuation |
| JPRN‐UMIN000003344 | Induction of the remission by use of infliximab in RA |
| JPRN‐UMIN000003880 | Keeping cartilaginous quality by adalimumab in patient with rheumatoid arthritis in Kansai area |
| JPRN‐UMIN000004412 | Corticosteroid‐sparing effect of Actemra in patients with rheumatoid arthritis refractory to anti‐TNF agents, methotrexate and corticosteroid |
| JPRN‐UMIN000005113 | Evaluation of the clinical remission and its sustainment after discontinuation of infliximab in patients with rheumatoid arthritis who receive "programmed" treatment in randomized controlled trial |
| JPRN‐UMIN000005590 | Maintenance of remission by tocilizumab mono‐therapy after remission obtained by combination with methotrexate in patients with rheumatoid arthritis |
| JPRN‐UMIN000006914 | Postmarketing surveillance for investigating success in achieving clinical and functional remission and sustaining efficacy with tocilizumab in biologics naive RA patients |
| JPRN‐UMIN000006956 | Efficacy and safety of tocilizumab in RA patients in daily clinical practice: an retrospective observational study |
| JPRN‐UMIN000007019 | Efficacy and safety of tocilizumab mono‐therapy in patients with adult‐onset Still's disease |
| JPRN‐UMIN000007086 | An observational study for investigating success in achieving clinical, structural and functional remission and sustaining efficacy with tocilizumab |
| JPRN‐UMIN000007380 | Comparison of the effects of single high‐dose methotrexate and methotrexate‐tocilizumab therapy on rheumatoid arthritis |
| JPRN‐UMIN000007432 | Prospective research of infliximab treatment in active RA patients refractory to anti‐interleukin six receptor monoclonal antibody |
| JPRN‐UMIN000007786 | Effect examination of infliximab to the effect insufficient example and the example of effect decrease of the 1st TNF inhibitor in rheumatoid arthritis |
| JPRN‐UMIN000007806 | The feasibility study of accelated infliximab infusion during maintenance phase |
| JPRN‐UMIN000008185 | Pilot study on the efficacy and safety of LD tocilizumab therapy in elderly RA patients |
| JPRN‐UMIN000008281 | Dose‐escalation study of infliximab or methotrexate based on the disease activity in patients with rheumatoid arthritis treated with infliximab |
| JPRN‐UMIN000008404 | Extension of tocilizumab dose intervals in patients with low to moderate disease activity rheumatoid arthritis using il‐6 serum level as starting criteria |
| JPRN‐UMIN000008572 | The Kitasato Institute non‐inferiority trial of etanercept and tacrolimus, the combined therapy with methotrexate in rheumatoid arthritis patients |
| JPRN‐UMIN000008756 | Abatacept‐based approach to cure of RA |
| JPRN‐UMIN000008812 | Efficacy and safety of tocilizumab mono‐therapy in patients with large vessel vasculitis (LVV; giant cell arteritis or Takayasu arteritis) and polymyalgia rheumatica (PMR) |
| JPRN‐UMIN000008889 | Cohort study of infectious disease risk management in rheumatoid arthritis patients receiving tocilizumab |
| JPRN‐UMIN000009425 | A validity inspection study of the treat‐to‐target strategy with golimumab for the treatment of rheumatoid arthritis patient |
| JPRN‐UMIN000009435 | Analysis of factors for bio‐free remission due to the tight control by Remicade in rheumatoid arthritis patients. Birdie study |
| JPRN‐UMIN000009887 | Associations between the initial concentration of serum TNF alpha and effects due to increasing a dose of infliximab, and between effects of infliximab and the concentration of serum il‐6 |
| JPRN‐UMIN000010033 | To investigate the efficacy of tocilizumab in RA patients with moderate disease activity under biologic therapy |
| JPRN‐UMIN000011520 | Keep persistent efficacy by abstaining from biological treatment after numerical SDAI remission with adalimumab (KANSAI study) |
| JPRN‐UMIN000011584 | A longitudinal, prospective, multicenter observational study in patients with rheumatoid arthritis receiving tocilizumab |
| JPRN‐UMIN000012005 | Identification of bio‐markers predicting the therapeutic effects of tocilizumab in rheumatoid arthritis |
| JPRN‐UMIN000012073 | Effects of subcutaneous actemra and MTX blending in RA |
| JPRN‐UMIN000012306 | Observational study for investigating the ability of recuperation of work/ house work state with tocilizumab (Actemra) subcutaneous treatment in biologics‐naive RA patients |
| JPRN‐UMIN000012690 | Study of actemra remission induction of RA and sequential maintenance of remission by reasonable cost treatment |
| JPRN‐UMIN000013750 | Study on effects of cytokine targeted therapy on periodontal condition in patients with rheumatoid arthritis |
| JPRN‐UMIN000014311 | Examination of the clinical remission and functional remission in infliximab using the increase‐in‐quantity protocol to TNF‐alpha inhibitory drug resistance rheumatoid arthritis |
| JPRN‐UMIN000014484 | Saitama Actemra study for QoL in patients with rheumatoid arthritis |
| JPRN‐UMIN000014670 | The effect of tocilizumab on synovitis of rheumatoid arthritis. Analysis by musculoskeletal ultrasonography |
| JPRN‐UMIN000014934 | A study for effectiveness and safety of tocilizumab therapy in rheumatoid arthritis patients with renal insufficiency |
| JPRN‐UMIN000015175 | Head‐to‐head comparison of subcutaneous tocilizumab versus abatacept for rheumatoid arthritis: prospective, randomized trial |
| JPRN‐UMIN000015297 | The feasibility study of accelerated infliximab infusion from initial administration |
| JPRN‐UMIN000015482 | Maintenance of remission with 6‐week interval of tocilizumab in RA patients who have been in remission |
| JPRN‐UMIN000015616 | Biologic mater clinical performance test for ADA and TCZ efficacy prediction |
| JPRN‐UMIN000016844 | The clinical study for seeking strategy how to treat rheumatoid arthritis by TNF inhibitors |
| JPRN‐UMIN000016950 | Clinical outcome in patients with rheumatoid arthritis switched to tumor necrosis factor blockers after tocilizumab or abatacept |
| JPRN‐UMIN000017230 | Correlation between efficacy of the biological therapy (tocilizumab) and levels of oxidative stress markers in Japanese patients with rheumatoid arthritis (inadequate responders to existing therapies) |
| JPRN‐UMIN000017495 | Establish the suitable strategy of maintenance therapy for rheumatoid arthritis patient with methotrexate and adalimumab |
| JPRN‐UMIN000017577 | Tapering and withdrawal of methotrexate(MTX) or tocilizumab(TCZ), after achievement of RA remission in concomitant use of MTX and TCZ,a randomized control study. |
| JPRN‐UMIN000017947 | Efficacy and change of serum il‐6 levels in patients with rheumatoid arthritis treated with tocilizumab |
| JPRN‐UMIN000018659 | Inhibitory effects of tocilizumab on serum oxidative stress in patients with rheumatoid arthritis ‐comparison with other biologic agents‐ |
| JPRN‐UMIN000020799 | Efficacy of infliximab as a second bio in patients with refractory rheumatoid arthritis |
| JPRN‐UMIN000020833 | The efficacy of iguratimod, and adding adalimumab in patients with active rheumatoid arthritis: an open label multicenter randomized parallel study |
| JPRN‐UMIN000021004 | Effectiveness and safety of tocilizumab therapy for rheumatoid arthritis patients |
| JPRN‐UMIN000021048 | Longitudinal study about the impact of treatment with tumor necrosis factor (TNF) inhibitors on tuberculin skin test (TST) reaction in patients with rheumatoid arthritis (RA) |
| JPRN‐UMIN000021247 | Tocilizumab treatment with reducing and stopping methotrexate in patients with rheumatoid arthritis in stable low disease activity‐state |
| JPRN‐UMIN000021492 | To investigate the safety of switch from infliximab biosimilar 1 in rheumatoid arthritis patients |
| JPRN‐UMIN000021929 | Optimization of infliximab withdrawal strategy for rheumatoid arthritis |
| JPRN‐UMIN000023006 | Usefullness of infliximab as a second tumor necrosis factor inhibitor in patients with rheumatoid arthritis with inadequate response to tumor necrosis factor inhibitor |
| JPRN‐UMIN000024025 | The clinical impact of methotrexate dose reduction at combination therapy with adalimumab plus methotrexate in rheumatoid arthritis; ALIBABA study |
| JPRN‐UMIN000024071 | Serious infections after tocilizumab administration in patients with rheumatoid arthritis: a retrospective study using an adverse drug reaction database ‐analysis of clinical symptoms and laboratory test data in serious infections |
| KCT0000089 | Identification of the best treatment strategy in Korean patients with early rheumatoid arthritis |
| NCT00000433 | Blocking tumor necrosis factor in ankylosing spondylitis |
| NCT00001862 | TNRF:Fc to treat eye inflammation in juvenile rheumatoid arthritis |
| NCT00001901 | Etanercept to treat Wegener's granulomatosis |
| NCT00001954 | Etanercept therapy for Sjögren's syndrome |
| NCT00006070 | Etanercept (Enbrel) to treat pain and swelling after third molar extraction |
| NCT00006292 | Infliximab for the treatment of early rheumatoid arthritis |
| NCT00012506 | The safety and efficacy of a tumor necrosis factor receptor fusion protein on uveitis associated with juvenile rheumatoid arthritis |
| NCT00029042 | Infliximab to treat children with juvenile rheumatoid arthritis |
| NCT00034060 | The role of cytokines on growth hormone suppression in premenopausal women with rheumatoid arthritis and the effect of treatment with etanercept |
| NCT00036374 | A study of the safety and effectiveness of infliximab (Remicade) in patients with juvenile rheumatoid arthritis |
| NCT00036387 | A study of the safety and effectiveness of infliximab (Remicade) in patients with rheumatoid arthritis. |
| NCT00037648 | Juvenile rheumatoid arthritis |
| NCT00037700 | Evaluation of the efficacy of combination treatment with anakinra and pegsunercept in improving rheumatoid arthritis |
| NCT00048568 | A phase III study of abatacept (BMS‐188667) in patients with active rheumatoid arthritis and inadequate response to methotrexate |
| NCT00048581 | Phase III study of BMS‐188667 (CTLA4IG) in patients with rheumatoid arthritis who are currently failing anti‐TNF therapy or who have failed anti‐TNF therapy in the past |
| NCT00048932 | A phase III study of bms‐188667 in subjects with active rheumatoid arthritis |
| NCT00069329 | Anakinra to treat patients with neonatal onset multisystem inflammatory disease |
| NCT00074438 | Study to assess the efficacy and safety of rituximab in patients with rheumatoid arthritis |
| NCT00075075 | Infliximab to treat non‐infectious scleritis |
| NCT00078806 | Safety and efficacy study of etanercept (Enbrel®) in children with systemic onset juvenile rheumatoid arthritis |
| NCT00094341 | Preference of rheumatoid arthritis (RA) patients of Enbrel® (etanercept) auto‐injector versus Enbrel® pre‐filled syringes |
| NCT00094900 | Interleukin‐1 trap to treat autoinflammatory diseases |
| NCT00095147 | Abatacept and infliximab in combination with methotrexate in subjects with rheumatoid arthritis |
| NCT00095173 | BMS‐188667 in children and adolescents with juvenile rheumatoid arthritis |
| NCT00101829 | Anti‐CD20 antibody therapy for Sjögren's syndrome |
| NCT00106522 | A study to assess the effect of tocilizumab + methotrexate on signs and symptoms in patients with moderate to severe active rheumatoid arthritis currently on methotrexate therapy |
| NCT00106535 | A study to assess the effect of tocilizumab + methotrexate on prevention of structural joint damage in patients with moderate to severe active rheumatoid arthritis (RA) |
| NCT00106548 | A study to assess the effect of tocilizumab + methotrexate on signs and symptoms in patients with moderate to severe active rheumatoid arthritis |
| NCT00106574 | A study to assess the effect of tocilizumab + DMARD therapy on signs and symptoms in patients with moderate to severe active rheumatoid arthritis |
| NCT00109408 | A study to assess the safety and efficacy of tocilizumab in patients with active rheumatoid arthritis |
| NCT00111410 | Evaluating the effect of anakinra (r‐methuil‐1ra) on vaccine antibody response in subjects with rheumatoid arthritis (RA) |
| NCT00115219 | Evaluating efficacy and safety of etanercept 50 mg twice weekly (biw) in rheumatoid arthritis (RA) subjects who are sub‐optimal responders to etanercept 50 mg once weekly (qw) |
| NCT00121043 | Evaluating Kineret® (anakinra) in rheumatoid arthritis (RA) subjects using a self‐reported questionnaire |
| NCT00122382 | Remission and joint damage progression in early rheumatoid arthritis |
| NCT00124449 | Study of abatacept versus placebo to assess the prevention of rheumatoid arthritis (RA) in adult patients |
| NCT00132418 | Study of Enbrel in rheumatoid arthritis (RA) subjects with comorbid disorders |
| NCT00135720 | Study of etanercept (Enbrel) in the treatment of pemphigus vulgaris |
| NCT00144508 | Phase III comparative study(open‐label) of MRA for rheumatoid arthritis(RA) |
| NCT00144521 | Comparative study (double‐blind) of MRA for rheumatoid arthritis (RA) |
| NCT00144560 | Drug‐drug interaction study of MRA in patient with rheumatoid arthritis (RA) |
| NCT00152386 | A placebo controlled study to assess efficacy and safety of certolizumab pegol in the treatment of rheumatoid arthritis |
| NCT00160602 | A study of liquid certolizumab pegol as additional medication to methotrexate in the treatment of signs and symptoms of rheumatoid arthritis and in prevention of joint damage in patients with active rheumatoid arthritis |
| NCT00160641 | A study of the safety and effectiveness of liquid certolizumab pegol in the treatment of signs and symptoms of rheumatoid arthritis and in prevention of joint damage in patients with active rheumatoid arthritis |
| NCT00160693 | Open label long‐term safety study of certolizumab pegol (CZP) for patients with rheumatoid arthritis |
| NCT00162266 | Abatacept with methotrexate‐ phase IIb |
| NCT00162279 | The study of abatacept in combination with etanercept |
| NCT00175877 | A study of the safety and effectiveness of lyophilized certolizumab pegol in the treatment of signs and symptoms of rheumatoid arthritis and in prevention of joint damage in patients with active rheumatoid arthritis |
| NCT00195494 | Study comparing etanercept and methotrexate versus methotrexate alone in rheumatoid arthritis |
| NCT00195663 | Efficacy and safety of adalimumab and methotrexate (MTX) versus MTX monotherapy in subjects with early rheumatoid arthritis |
| NCT00195702 | Efficacy and safety of adalimumab in patients with active rheumatoid arthritis treated concomitantly with methotrexate |
| NCT00202852 | A placebo‐controlled, double‐blinded, randomized trial of Remicade in Korean patients with rheumatoid arthritis despite methotrexate |
| NCT00207714 | An efficacy and safety study of CNTO 148 subcutaneous injection compared with placebo in patients with active rheumatoid arthritis |
| NCT00216177 | Comparison of adalimumab and infliximab treatment of rheumatoid arthritis |
| NCT00228839 | A Pediatric Phase I Pharmacokinetic Study Using Anti Tumor Necrosis Factor Antibody (Infliximab) for Treatment of Acute Graft Versus Host Disease |
| NCT00233558 | Open‐label steroid reduction study of adalimumab with methotrexate in patients with active rheumatoid arthritis |
| NCT00234845 | Adalimumab in combination with methotrexate vs methotrexate alone in early rheumatoid arthritis |
| NCT00234897 | Efficacy of Humira in subjects with active rheumatoid arthritis |
| NCT00234936 | Quality of life study with adalimumab in rheumatoid arthritis |
| NCT00235859 | Adalimumab administered in Korean rheumatoid arthritis subjects treated with methotrexate |
| NCT00236028 | A safety and efficacy study for infliximab (Remicade) with methotrexate in patients with early rheumatoid arthritis |
| NCT00243412 | A study of the safety and efficacy of rituximab in patients with moderate to severe rheumatoid arthritis receiving methotrexate |
| NCT00244556 | Study comparing Enbrel (etanercept) plus methotrexate versus Enbrel alone in active rheumatoid arthritis despite current methotrexate therapy |
| NCT00249041 | Enbrel liquid immunogenicity protocol |
| NCT00252668 | Study evaluating the combination of etanercept and methotrexate in rheumatoid arthritis subjects |
| NCT00254293 | Study to assess steady‐state trough concentrations, safety, and immunogenicity of abatacept after subcutaneous (sc) administration to subjects with rheumatoid arthritis (RA) |
| NCT00259610 | Treatment of early aggressive rheumatoid arthritis (TEAR) |
| NCT00261118 | Rituximab in active ulcerative colitis |
| NCT00264537 | A study of the safety and efficacy of golimumab in subjects with rheumatoid arthritis that are methotrexate‐naive |
| NCT00264550 | An efficacy and safety study of golimumab in patients with active rheumatoid arthritis despite methotrexate therapy |
| NCT00266227 | A study of retreatment with rituximab in patients with rheumatoid arthritis receiving background methotrexate |
| NCT00269867 | Infliximab plus methotrexate for the treatment of rheumatoid arthritis |
| NCT00279734 | Vaccination study of abatacept (BMS‐188667) for normal healthy volunteers |
| NCT00279760 | Phase I/II multiple‐dose LEA29Y vs CTLAG4IG vs placebo in rheumatoid arthritis |
| NCT00282308 | A study to evaluate the effects of rituximab on immune responses in subjects with active rheumatoid arthritis receiving background methotrexate |
| NCT00283712 | Use of infliximab for the treatment of pemphigus vulgaris |
| NCT00291915 | Multicenter randomized prospective trial comparing methotrexate alone or in combination with adalimumab in early arthritis |
| NCT00293202 | Safety and efficacy study of the effect of etanercept in hemodialysis patients |
| NCT00298272 | Safety and tolerability of Rituxan with methotrexate and etanercept or methotrexate and adalimumab in patients with active rheumatoid arthritis |
| NCT00299104 | A study to evaluate rituximab in combination with methotrexate in methotrexate‐naive patients with active rheumatoid arthritis |
| NCT00299130 | A study to evaluate the safety and efficacy of rituximab in combination with methotrexate compared to methotrexate alone in patients with active rheumatoid arthritis |
| NCT00299546 | A study of the safety and efficacy of golimumab (CNTO 148) in subjects with active rheumatoid arthritis previously treated with biologic anti‐TNFa agent(s) |
| NCT00317538 | Open‐label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after incomplete response to etanercept |
| NCT00327275 | The effects of a 16‐week individualized, intensive strength training program in patients with rheumatoid arthritis |
| NCT00345748 | A study of abatacept in Japanese patients with active rheumatoid arthritis while receiving methotrexate |
| NCT00361335 | A study of safety and effectiveness of golimumab in participants with active rheumatoid arthritis despite methotrexate therapy |
| NCT00363350 | Rituximab treatment in Sjögren's syndrome |
| NCT00365001 | A drug interaction study between tocilizumab, methotrexate and simvastatin on patients with rheumatoid arthritis |
| NCT00393471 | Study comparing etanercept plus methotrexate to either etanercept or methotrexate alone in rheumatoid arthritis |
| NCT00394589 | Re³ (re‐cube: retain Remicade® response) |
| NCT00396747 | A comparison of methotrexate alone or combined to infliximab or to pulse methylprednisolone in early rheumatoid arthritis: a magnetic resonance imaging study |
| NCT00396812 | Rituximab for the treatment of early rheumatoid arthritis (RA) |
| NCT00405275 | Rheumatoid arthritis: comparison of active therapies in patients with active disease despite methotrexate therapy |
| NCT00409838 | A phase III study of abatacept in patients with rheumatoid arthritis and an inadequate response to methotrexate |
| NCT00420199 | A phase IIIb study of BMS‐188667 in subjects with active rheumatoid arthritis and inadequate response to methotrexate |
| NCT00420927 | Study of the optimal protocol for methotrexate and adalimumab combination therapy in early rheumatoid arthritis |
| NCT00422227 | Study comparing etanercept with usual DMARD therapy in subjects with rheumatoid arthritis in the Asia Pacific region |
| NCT00422383 | A study of retreatment with Mabthera (rituximab) in combination with methotrexate in patients with rheumatoid arthritis (RA) |
| NCT00424502 | A study of Mabthera (rituximab) in patients with rheumatoid arthritis who have had an inadequate response to a TNF‐blocker |
| NCT00425932 | Impact of rituximab on MRI evidence of disease activity in patients with moderate to severe rheumatoid arthritis |
| NCT00426543 | Effect of b‐cell depletion in patients with primary Sjögren's syndrome |
| NCT00432406 | Tumor necrosis factors (TNF)‐ blockade for psoriatic arthritis |
| NCT00442611 | A study to evaluate the safety and efficacy of abatacept in patients with diffuse systemic sclerosis (scleroderma) |
| NCT00443430 | Trial of early aggressive drug therapy in juvenile idiopathic arthritis |
| NCT00443950 | Study evaluating the efficacy and safety of etanercept in Chinese subjects with rheumatoid arthritis |
| NCT00445770 | Study evaluating the efficacy and safety of etanercept and methotrexate in Japanese subjects with rheumatoid arthritis |
| NCT00459706 | Study comparing perceptions and satisfaction for two different delivery mechanisms for etanercept |
| NCT00462072 | Centocor microarray study of patients |
| NCT00463580 | A study of infliximab for treatment resistant major depression |
| NCT00468377 | Safety and efficacy study of re‐treatment with rituximab (Mabthera/Rituxan) in patients with active rheumatoid arthritis who respond poorly to anti‐TNFa therapies |
| NCT00468546 | A study to evaluate the safety and efficacy of Mabthera (rituximab) in combination with methotrexate (MTX) in participants with active rheumatoid arthritis who failed on anti‐tumor necrosis factor alpha therapy |
| NCT00480272 | Prospective study on intensive early rheumatoid arthritis treatment |
| NCT00484237 | A study evaluating 10 mg and 25 mg doses of etanercept in patients with rheumatoid arthritis |
| NCT00502996 | A non‐comparative study to assess the safety of Mabthera (rituximab) in patients with rheumatoid arthritis. |
| NCT00503425 | A study of Mabthera (rituximab) in participants with rheumatoid arthritis who have had an inadequate response to disease‐modifying antirheumatic drugs (DMARD) and/or anti‐tumor necrosis factor (anti‐TNF) therapy |
| NCT00514982 | Medical treatment of colitis in patients with Hermansky‐Pudlak Syndrome |
| NCT00520572 | A 6‐month randomised, double‐blind, open arm comparator, phase IIb, with azd9056, in patients with rheumatoid arthritis (RA) |
| NCT00522184 | Intra‐articular injection of etanercept in patient suffering from rheumatoid arthritis: a double‐blind randomized study |
| NCT00523692 | Remission induction in very early rheumatoid arthritis |
| NCT00531817 | A study of tocilizumab in combination with DMARDs in patients with moderate to severe rheumatoid arthritis |
| NCT00533897 | Phase IIIb subcutaneous missed dose study |
| NCT00534313 | Safety and efficacy of abatacept versus placebo in participants with psoriatic arthritis |
| NCT00535782 | A study of the effect of tocilizumab on markers of atherogenic risk in patients with moderate to severe rheumatoid arthritis |
| NCT00537667 | The spectra study |
| NCT00538902 | Safety and efficacy study of adalimumab in adult Chinese rheumatoid arthritis subjects treated with methotrexate |
| NCT00544154 | Efficacy and safety of CDP870 and methotrexate compared to methotrexate alone in subjects with rheumatoid arthritis |
| NCT00548834 | Efficacy and safety of CDP870 versus placebo in the treatment of the signs and symptoms of rheumatoid arthritis |
| NCT00550446 | A phase 2 study for patients with a physician's diagnosis of rheumatoid arthritis |
| NCT00555542 | An analysis of peripheral blood t cell subsets on rheumatoid arthritis |
| NCT00559585 | Methotrexate‐inadequate response study |
| NCT00565331 | Rituximab for prevention of rejection after renal transplantation |
| NCT00565409 | Study comparing etanercept in combination with methotrexate in subjects with rheumatoid arthritis |
| NCT00578305 | A study of rituximab (Mabthera®/Rituxan®) in patients with rheumatoid arthritis and inadequate response to methotrexate |
| NCT00578565 | Rituximab in rheumatoid arthritis lung disease |
| NCT00580229 | A safety analysis of oral prednisone as a pre‐treatment for rituximab in rheumatoid arthritis. |
| NCT00580840 | Dosing flexibility study in patients with rheumatoid arthritis |
| NCT00595413 | Atacicept in anti‐tumor necrosis factor alpha‐naive subjects with rheumatoid arthritis (AUGUST II) |
| NCT00647270 | Study comparing 80 mg of adalimumab with placebo, and demonstrating the non‐inferiority of monthly 80 mg adalimumab dosing compared with 40 mg adalimumab every other week dosing |
| NCT00647491 | A study of adalimumab in adult Japanese subjects with rheumatoid arthritis |
| NCT00647920 | Study of adalimumab administered as subcutaneous injections in adult Chinese rheumatoid arthritis subjects treated with methotrexate |
| NCT00649545 | Study of the human anti‐TNF monoclonal antibody in patients with active rheumatoid arthritis |
| NCT00649922 | Assessment of the effect of adalimumab on response to influenza virus and pneumococcal vaccines in subjects with rheumatoid arthritis |
| NCT00650026 | Early access program of the safety of human anti‐TNF monoclonal antibody adalimumab in subjects with active rheumatoid arthritis |
| NCT00650156 | Pharmacokinetic and safety study with adalimumab in Chinese subjects with mild rheumatoid arthritis |
| NCT00650390 | Open label study to assess efficacy and safety of the fully human anti‐TNF‐alpha monoclonal antibody adalimumab |
| NCT00654368 | CAMEO: Canadian methotrexate and etanercept outcome study |
| NCT00660647 | Optimized treatment algorithm for patients with early rheumatoid arthritis (RA) |
| NCT00664521 | Atacicept in combination with rituximab in subjects with rheumatoid arthritis |
| NCT00674362 | Rheumatoid arthritis (RA) moderate to low disease activity study |
| NCT00678782 | Evaluation of the efficacy and safety of intra‐articular etanercept in patients with refractory knee joint synovitis |
| NCT00683345 | Fatigue and interleukin‐1 (IL‐1) blockade in primary Sjögrens syndrome |
| NCT00686868 | Study to evaluate sc route of administration of ofatumumab in RA patients |
| NCT00688103 | Efficacy and safety of etanercept in active RA despite methotrexate therapy in japan |
| NCT00689728 | A study for patients with rheumatoid arthritis on methotrexate (MTX) with an inadequate response to TNF‐inhibitor therapy |
| NCT00691028 | Efficacy and safety of increased dose of ta‐650 (infliximab) in patients with rheumatoid arthritis |
| NCT00696059 | Humira in rheumatoid arthritis ‐ do bone erosions heal? |
| NCT00706797 | Study evaluating efficacy/safety of etanercept + methotrexate compared to usual treatment in moderate RA subjects |
| NCT00711503 | Anti‐interleukin‐1 in diabetes action |
| NCT00713544 | A proof of concept and dose ranging study in patients with rheumatoid arthritis |
| NCT00714493 | RESTART C0168Z05 rheumatoid arthritis study |
| NCT00716248 | Bucillamine study of holding remission after infliximab dose‐off |
| NCT00717236 | Certolizumab pegol for the treatment of patients with active rheumatoid arthritis |
| NCT00720798 | An extension study of tocilizumab (myeloma receptor antibody (MRA)) in patients completing treatment in tocilizumab core studies |
| NCT00721123 | A long‐term extension study of tocilizumab (myeloma receptor antibody (MRA)) in patients with rheumatoid arthritis |
| NCT00727987 | A safety and efficacy study of golimumab (CNTO 148) in patients with active rheumatoid arthritis despite methotrexate therapy |
| NCT00732875 | A trial of anti‐TNF chimeric monoclonal antibody (CA2) in Korean patients with active rheumatoid arthritis despite methotrexate (extension part)(study P05645)(completed) |
| NCT00740948 | Tolerance and efficacy of rituximab in Sjögren's disease |
| NCT00753454 | Open label extension for patients coming from the dosing flexibility study in patients with rheumatoid arthritis (RA) |
| NCT00754559 | A study to assess efficacy with respect to clinical improvement in disease activity and safety of tocilizumab in patients with active rheumatoid arthritis. |
| NCT00764725 | Comparison of MTX+anti‐TNF to MTX+conventional DMARDs in patients with early rheumatoid arthritis (RA) who failed MTX alone (SWEFOT) |
| NCT00768053 | Evaluation of EULAR‐RAID score in rheumatoid arthritis patients |
| NCT00771251 | A safety and efficacy study of golimumab (CNTO148) in patients with active rheumatoid arthritis (RA) |
| NCT00773461 | A study of tocilizumab in combination with DMARD therapy in patients with active rheumatoid arthritis |
| NCT00780793 | Spacing of TNF‐blocker injections in rheumatoid arthritis study |
| NCT00783536 | A multicenter study to compare the efficacy and safety of the combination of etanercept and methotrexate in treatment of rheumatoid arthritis |
| NCT00789724 | Anakinra to prevent post‐infarction remodeling |
| NCT00791921 | Efficacy confirmation trial of CDP870 without coadministration of methotrexate (MTX) in Japanese rheumatoid arthritis (RA) |
| NCT00791999 | Efficacy confirmation trial of CDP870 as add‐on medication to methotrexate (MTX) in Japanese rheumatoid arthritis (RA) |
| NCT00794898 | Efficacy of Remicade in the treatment of active rheumatoid arthritis despite methotrexate (study p03027) |
| NCT00796705 | Switching anti‐TNF‐alpha agents in rheumatoid arthritis (RA) |
| NCT00808210 | A study to evaluate ocrelizumab in combination with methotrexate compared with infliximab plus methotrexate in patients with active rheumatoid arthritis currently responding inadequately to etanercept or adalimumab |
| NCT00808509 | A pilot study of the feasibility of discontinuation of adalimumab in stable rheumatoid arthritis patients in clinical remission |
| NCT00810199 | A study of tocilizumab and methotrexate treatment strategies (adding tocilizumab to methotrexate versus switching to tocilizumab) in patients with active rheumatoid arthritis with inadequate response to prior methotrexate treatment |
| NCT00810277 | A study of tocilizumab in patients with rheumatoid arthritis who have an inadequate response to current non‐biologic DMARDs |
| NCT00814866 | Bone resorption, osteoclastogenesis and adalimumab |
| NCT00837434 | Anti‐TNF agents for the treatment of rheumatoid arthritis |
| NCT00843778 | Follow‐up of rheumatoid arthritis (RA) moderate to low disease activity study |
| NCT00844714 | Cardiovascular risk markers in patients with rheumatoid arthritis: effect of rituximab therapy |
| NCT00845832 | A study of combination treatment with Mabthera (rituximab) and Roactemra (tocilizumab) versus Roactemra in patients with rheumatoid arthritis with an incomplete response to methotrexate |
| NCT00848354 | Open‐label study comparing etanercept to conventional disease modifying antirheumatic drug (DMARD) therapy |
| NCT00850343 | Long‐term treatment study of certolizumab pegol without coadministration of methotrexate in Japanese rheumatoid arthritis (RA) patients |
| NCT00851318 | Long‐term treatment study of certolizumab pegol (cdp870) as add‐on medication to methotrexate in Japanese rheumatoid arthritis (RA) patients |
| NCT00853385 | A phase 3 study comparing 2 doses of CP‐690,550 and the active comparator, Humira (adalimumab) versus Placebo for treatment of rheumatoid arthritis |
| NCT00858780 | Study comparing the effect on disease activity when reducing or discontinuing etanercept in subjects with RA |
| NCT00868751 | Single patient use of tocilizumab in systemic onset juvenile idiopathic arthritis |
| NCT00870467 | A study of adalimumab in Japanese subjects with rheumatoid arthritis |
| NCT00887341 | A study comparing infusion rates of tocilizumab in patients with moderate to severe rheumatoid arthritis |
| NCT00891020 | A study of tocilizumab in patients with moderate to severe active rheumatoid arthritis who have an inadequate response to or are unable to tolerate biologic and non‐biologic disease‐modifying antirheumatic drugs (DMARDs) |
| NCT00901550 | The Chinese university of Hong Kong early arthritis study |
| NCT00908089 | Tnf‐blocking therapy in combination with disease‐modifying antirheumatic drugs in early rheumatoid arthritis |
| NCT00913458 | Study evaluating etanercept plus methotrexate in early rheumatoid arthritis |
| NCT00920478 | Targeting synovitis in early rheumatoid arthritis |
| NCT00929864 | Abatacept versus adalimumab head‐to‐head |
| NCT00948610 | Sleep and immunity in rheumatoid arthritis: Remicade substudy |
| NCT00963703 | Treatment of TNFa naive patients with poor prognosis rheumatoid arthritis |
| NCT00965653 | A study of subcutaneously administered tocilizumab in patients with rheumatoid arthritis |
| NCT00973479 | An effectiveness and safety study of intravenous golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate therapy |
| NCT00977106 | Torpedo study: a study on rapid effect of tocilizumab in patients with rheumatoid arthritis with an inadequate response to disease‐modifying antirheumatic drugs (DMARDs) or anti‐TNF |
| NCT00979771 | A study to investigate the ability of GSK706769 to maintain clinical remission after withdrawal of Enbrel in rheumatoid arthritis patients |
| NCT00989235 | Substudy ‐ low dose of abatacept in subjects with rheumatoid arthritis |
| NCT00993317 | A study of cdp870 as add‐on meditation to methotrexate (MTX) in patients with rheumatoid arthritis |
| NCT00993668 | Assessing the use of certolizumab pegol in adult subjects with rheumatoid arthritis on the antibody response when receiving influenza virus and pneumococcal vaccines |
| NCT00996606 | A study of tocilizumab in combination with disease‐modifying anti‐rheumatic drugs (DMARDs) in participants with moderate to severe active rheumatoid arthritis with an inadequate response to DMARDs |
| NCT01000441 | Rotation or change of biotherapy after first anti‐TNF treatment failure for rheumatoid arthritis |
| NCT01001832 | Efficacy, pharmacokinetics, safety, and immunogenicity study of abatacept administered subcutaneously to treat rheumatoid arthritis in Japanese patients |
| NCT01002781 | Efficacy and safety of tocilizumab in adult's still disease |
| NCT01004432 | Golimumab in rheumatoid arthritis participants with an inadequate response to etanercept (Enbrel) or adalimumab (Humira) |
| NCT01007435 | A study of tocilizumab as monotherapy and in combination with methotrexate versus methotrexate in patients with early moderate to severe rheumatoid arthritis |
| NCT01009879 | Human tumor necrosis factor alpha (TNFa)‐induced pre‐B cell bone marrow emigrants |
| NCT01010503 | A study of tocilizumab with or without methotrexate in patients with rheumatoid arthritis |
| NCT01021735 | Optimal management of rheumatoid arthritis patients requiring biologic therapy |
| NCT01033656 | Treatment of refractory adult‐onset Still's disease with anakinra: a randomized study |
| NCT01034137 | A study of tocilizumab and methotrexate in combination or as monotherapy in treatment‐naïve patients with early rheumatoid arthritis |
| NCT01034397 | A study of tocilizumab plus non‐biological DMARD in patients with moderate to severe rheumatoid arthritis and an inadequate response to non‐biological DMARDs |
| NCT01044498 | A study of tocilizumab in combination with an oral contraceptive in patients with rheumatoid arthritis |
| NCT01072058 | Heart function in rheumatoid arthritis and ankylosing spondylitis pre and post‐TNF blocker |
| NCT01086033 | A 3‐year study following up patients with moderate to severe rheumatoid arthritis treated with Humira in Greece |
| NCT01088165 | The influence of adalimumab on cardiovascular and metabolic risk in psoriasis |
| NCT01101555 | Repeat dose subcutaneous Rheumatoid arthritis efficacy study |
| NCT01116427 | A cooperative clinical study of abatacept in multiple sclerosis |
| NCT01117129 | A study of efficacy of rituximab (Mabthera/Rituxan) in patients with rheumatoid arthritis using magnetic resonance imaging of the hand (RESONAR) |
| NCT01119859 | A study of tocilizumab (roactemra/Actemra) versus adalimumab in patients with rheumatoid arthritis |
| NCT01120366 | Success of tocilizumab in RA patients with remission induction and sustained efficacy after discontinuation |
| NCT01123070 | Tl011 in severe, active rheumatoid arthritis patients |
| NCT01126541 | SMART Study: a study of re‐treatment with Mabthera (rituximab) in patients with rheumatoid arthritis who have failed on anti‐TNF alfa therapy |
| NCT01142726 | Efficacy and safety study of abatacept subcutaneous plus methotrexate in inducing remission in adults with very early rheumatoid arthritis |
| NCT01147341 | Can TNF‐alpha incomplete secondary responders attain a safe and efficacious response switching to Cimzia |
| NCT01162421 | A Canadian study to evaluate early use of adalimumab after methotrexate failure in early rheumatoid arthritis |
| NCT01163617 | The usability and injection time of the Physiolis syringe and autoinjector in rheumatoid arthritis patients |
| NCT01163747 | A study of the effects of Roactemra/Actemra on vaccination in patients with rheumatoid arthritis on background methotrexate (VISARA) |
| NCT01173120 | Methotrexate ‐ inadequate response device sub‐study |
| NCT01185288 | A study to determine the effect of methotrexate (MTX) dose on clinical outcome and ultrasonographic signs in subjects with moderately to severely active rheumatoid arthritis (RA) treated with adalimumab (MUSICA) |
| NCT01185301 | Study to determine the effects of different doses of methotrexate (MTX) when taken with adalimumab in subjects with early rheumatoid arthritis (RA) |
| NCT01185522 | An observational study of the impact of Roactemra/Actemra on fatigue in patients with rheumatoid arthritis (PEPS) |
| NCT01194414 | A study to compare subcutaneous versus intravenous administration of Roactemra/Actemra (tocilizumab) in participants with moderate to severe active rheumatoid arthritis |
| NCT01197066 | Open‐label, extension study of CDP870 in patients with rheumatoid arthritis |
| NCT01197144 | Pain modulation in rheumatoid arthritis (RA) ‐ influence of adalimumab |
| NCT01211834 | Efficacy and safety of tocilizumab in combination with DMARDs in patients with moderate to severe rheumatoid arthritis |
| NCT01212094 | Double blind combination of rituximab by intravenous and intrathecal injection versus placebo in patients with low‐inflammatory secondary progressive multiple sclerosis (RIVITALISE) |
| NCT01213017 | The effect of certolizumab pegol on MRI synovitis and bone edema in rheumatoid arthritis patients |
| NCT01216631 | Seronegative oligoarthritis of the knee study (SOKS) |
| NCT01217086 | Program evaluating the autoimmune disease investigational drug ct‐p13 in RA patients (PLANETRA) |
| NCT01221636 | Pharmacokinetic study to compare the blood levels of low vs high metal manufacture of abatacept |
| NCT01225393 | A study to evaluate the efficacy and safety of MLTA3698A in combination with a disease‐modifying anti‐rheumatic drug (DMARD) compared with adalimumab in combination with a DMARD in patients with active rheumatoid arthritis |
| NCT01232569 | A study of Roactemra/Actemra (tocilizumab) given subcutaneously in combination with traditional DMARDs in patients with moderate to severe active rheumatoid arthritis |
| NCT01235598 | Magnetic resonance image verified early response to certolizumab pegol in subjects with active rheumatoid arthritis (RA) |
| NCT01242488 | Efficacy and safety of CDP6038 in patients with rheumatoid arthritis with an unsuccessful response to anti‐tumor necrosis factor (anti‐TNF) therapy |
| NCT01244958 | Addition of rituximab to leflunomide in patients with active rheumatoid arthritis |
| NCT01245361 | A 6‐months infliximab or placebo study in UA at high risk of RA: clinical, radiological and synovial benefit |
| NCT01245439 | A study of Roactemra/Actemra (tocilizumab) in patients with moderate to severe rheumatoid arthritis |
| NCT01245452 | Study of the response and cardiorespiratory endurance in early RA patients treated with tocilizumab or methotrexate |
| NCT01248780 | Study of subcutaneous golimumab in Chinese patients with active rheumatoid arthritis despite methotrexate therapy |
| NCT01251120 | A study of Roactemra/Actemra (tocilizumab) in combination with DMARDs versus current best practice DMARD therapy in patients with rheumatoid arthritis |
| NCT01255761 | A comparison of two assessment tools in predicting treatment success of Cimzia in rheumatoid arthritis subjects |
| NCT01258712 | Study of tocilizumab in combination with methotrexate for treatment of moderate to severe rheumatoid arthritis patients |
| NCT01264770 | Evaluation of efficacy and safety of Fostamatinib monotherapy compared with adalimumab monotherapy in patients with rheumatoid arthritis (RA) |
| NCT01270035 | Efficacy and safety of adalimumab 80 mg every other week with methotrexate |
| NCT01270087 | The effect of adalimumab (Humira) on vascular abnormalities in rheumatoid arthritis. A pilot study |
| NCT01270997 | Randomized double‐blind parallel trial to evaluate equivalence in efficacy and safety of hd203 and Enbrel in RA patients |
| NCT01272908 | A study of Mabthera (rituximab) in patients with rheumatoid arthritis who have failed on one prior anti‐TNF therapy (reset) |
| NCT01274182 | Gp2013 in the treatment of RA patients refractory to or intolerant of standard therapy |
| NCT01283971 | A study of Roactemra/Actemra (tocilizumab) versus adalimumab in combination with methotrexate (MTX) in patients with moderate to severe active rheumatoid arthritis and an inadequate response to treatment with only one tumor necrosis factor (TNF)‐inhibitor |
| NCT01292265 | A 12 week study to assess changes in joint inflammation using ultrasonography in patients with rheumatoid arthritis (RA) |
| NCT01295151 | SWITCH clinical trial for patients with rheumatoid arthritis who have failed an initial TNF‐blocking drug |
| NCT01295814 | Efficacy study of adalimumab to treat interstitial cystitis |
| NCT01303874 | Etanercept and methotrexate in patients to induce remission in early arthritis (empire) |
| NCT01308255 | Infliximab as induction therapy in early rheumatoid arthritis (idea) |
| NCT01313208 | Moderate rheumatoid arthritis (RA) with etanercept (Enbrel) |
| NCT01313520 | A study to evaluate the effectiveness of infliximab and changes in hand and wrist magnetic resonance imaging (MRI) in participants with active rheumatoid arthritis (RA) (p08136) |
| NCT01326962 | A study of Roactemra/Actemra (tocilizumab) in patients with rheumatoid arthritis who have an inadequate response to DMARDs or anti‐TNF |
| NCT01331837 | A study of tocilizumab in comparison to etanercept in participants with rheumatoid arthritis and cardiovascular disease risk factors |
| NCT01333878 | Impact of subcutaneous abatacept in rheumatoid arthritis assessing inhibition of structural damage |
| NCT01338103 | Treatment of pemphigus patients with rituximab 1000mgx2 and assessment of immune status via Cylex |
| NCT01350804 | Efficacy at 24 weeks and safety, tolerability and long term efficacy of secukinumab (ain457) in patients with active rheumatoid arthritis (RA) and an inadequate response to anti‐tumor necrosis factor α (Anti‐TNFα) agents (CAIN457f2309 and CAIN457f2309e1) |
| NCT01351480 | Benefits of injectable abatacept using magnetic resonance imaging (MRI) in rheumatoid arthritis (RA) patients |
| NCT01362153 | A pharmacokinetic (pk) and pharmacodynamic (pd) study of golimumab in patients with rheumatoid arthritis (RA) |
| NCT01369017 | Effect of interleukin‐1 receptor antagonist on inhalation of 20,000 EU clinical CTR reference endotoxin in normal volunteers |
| NCT01373151 | Phase IIB rheumatoid arthritis dose ranging study for BMS‐945429 in subjects who are not responding to methotrexate |
| NCT01374971 | Rheumatoid arthritis treatment and biopsy study assessing certolizumab pegol (Cimzia) |
| NCT01382160 | Serum concentration of adalimumab as a predictive factor of clinical outcomes in rheumatoid arthritis (AFORA) |
| NCT01390441 | A study of the pharmacokinetics and safety of mk‐8808 (MK‐8808‐002) |
| NCT01390545 | Velvet, a dose range finding trial of veltuzumab in subjects with moderate to severe rheumatoid arthritis |
| NCT01394913 | Comparison of two etanercept regimens (Reumatocept® versus Enbrel®) for treatment of rheumatoid arthritis |
| NCT01396317 | Study of tocilizumab to treat polymyalgia rheumatica |
| NCT01399697 | A study of Roactemra/Actemra (tocilizumab) in combination with methotrexate versus Roactemra/Actemra monotherapy in patients with rheumatoid arthritis and an inadequate response to methotrexate |
| NCT01405326 | Restore Working Ability in rheumatoid arthritis |
| NCT01426815 | Exploration of TNF‐alpha blockade with golimumab in the induction of clinical remission in patients with early peripheral spondyloarthritis (SPA) according to ASAS‐criteria |
| NCT01439204 | Pharmacokinetic study to compare the blood levels of abatacept manufactured at Lonza biologics to the blood levels of abatacept manufactured at the Devens, Massachusetts (MA) facility of Bristol‐Myers Squibb |
| NCT01443364 | Open label study to assess the predictability of early response to certolizumab pegol in patients with rheumatoid arthritis |
| NCT01451203 | Efficacy confirmation study of cdp870 in early rheumatoid arthritis |
| NCT01468077 | A study in patients with moderate to severe active rheumatoid arthritis comparing different infusion durations of Roactemra/Actemra (tocilizumab) treatment |
| NCT01491815 | Active conventional therapy compared to three different biologic treatments in early rheumatoid arthritis with subsequent dose reduction |
| NCT01500278 | Study to assess the short‐ and long‐term efficacy of certolizumab pegol plus methotrexate compared to adalimumab plus methotrexate in subjects with moderate to severe rheumatoid arthritis (RA) inadequately responding to methotrexate |
| NCT01502423 | A crossover study of the safety and tolerability of two formulations of adalimumab |
| NCT01519791 | A multi‐center, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of certolizumab pegol in combination with methotrexate in the treatment of disease modifying antirheumatic drugs (DMARD)‐naïve adults with early active rheumatoid arthritis |
| NCT01521923 | A multi‐center, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of certolizumab pegol in combination with methotrexate in the treatment of disease modifying antirheumatic drugs (DMARD)‐naïve adults with early active rheumatoid arthritis |
| NCT01526057 | A pharmacokinetic/pharmacodynamic study comparing pf‐05280586 to rituximab in subjects with active rheumatoid arthritis with an inadequate response to TNF inhibitors (reflections b328‐01) |
| NCT01534884 | Demonstrate the equivalence of ct‐p10 to Mabthera with respect to the pharmacokinetic profile in patients with rheumatoid arthritis |
| NCT01548768 | RHYTHM (formerly Escape II Myocardium) |
| NCT01557374 | Toward the lowest effective dose of abatacept or tocilizumab |
| NCT01561313 | Crossover study of safety and tolerability of two formulations of adalimumab |
| NCT01566201 | Effects of interleukin‐1 inhibition on vascular and left ventricular function in rheumatoid arthritis patients with coronary artery disease |
| NCT01567358 | Study of ni‐071 in comparison with Remicade in patients with rheumatoid arthritis |
| NCT01571219 | An extension study to demonstrate long‐term efficacy and safety of CT‐P13 when co‐administered with methotrexate in patient with rheumatoid arthritis who were treated with infliximab (Remicade or CT‐P13) in study CT‐P13 3.1 |
| NCT01578850 | Study conducted in subjects with rheumatoid arthritis who have moderate to severe disease activity despite methotrexate therapy with or without other non biologic disease modifying antirheumatic drugs (DMARDs)for at least 12 weeks prior to screening |
| NCT01587989 | A study of Roactemra/Actemra (tocilizumab) with or without methotrexate in patients with mild to moderate rheumatoid arthritis with an inadequate response to methotrexate |
| NCT01590966 | Scintigraphic detection of the biodistribution of tumor necrosis factor with a radiolabeled anti‐TNF in patients with active rheumatoid arthritis and active axial and peripheral spondyloarthritis |
| NCT01602302 | Ultrasound and withdrawal of biological DMARDs in rheumatoid arthritis |
| NCT01609205 | Doppler evaluation in RA patients after adalimumab |
| NCT01635686 | Comparison the safety and pharmacokinetic characteristics of DWP422 25 mg with those of Enbrel 25MG PFS inj. after subcutaneous injection in healthy male volunteers |
| NCT01638715 | A randomized, multi‐center biomarker trial to predict therapeutic responses of patients with rheumatoid arthritis to a specific biologic mode of action |
| NCT01643928 | Rheumatoid arthritis extension trial for subjects who have participated in other PF‐05280586 trials (reflections b328‐04) |
| NCT01649804 | A long‐term safety extension study of WA19926 in participants with rheumatoid arthritis |
| NCT01657513 | TNF‐alfa inhibitors and antibody production in patients with psoriasis |
| NCT01661140 | A study of Roactemra/Actemra (tocilizumab) in combination with methotrexate in patients with severe active rheumatoid arthritis, comparing tapering versus maintaining the methotrexate dosage |
| NCT01664598 | An extension study of wa19926 of the long‐term safety of Roactemra/Actemra (tocilizumab) in patients with early moderate to severe rheumatoid arthritis |
| NCT01665430 | A long‐term extension study to wa19926 of Roactemra/Actemra (tocilizumab) in patients with early, moderate to severe rheumatoid arthritis |
| NCT01668966 | A long term extension study of wa19926 (nct01649804) of tocilizumab (Roactemra/Actemra) in participants with early moderate to severe rheumatoid arthritis |
| NCT01682512 | Efficacy, pharmacokinetics, and safety of bi 695500 in patients with rheumatoid arthritis |
| NCT01690299 | Phase 3b safety and efficacy study of apremilast to treat moderate to severe plaque‐plaque psoriasis |
| NCT01696929 | An open‐label trial of tocilizumab in schizophrenia |
| NCT01710358 | A study in moderate to severe rheumatoid arthritis |
| NCT01712178 | A study in rheumatoid arthritis (RA) patients to compare two formulations of adalimumab for pharmacokinetic, pharmacodynamic and safety |
| NCT01715831 | A long‐term safety extension study of tocilizumab in Brazilian participants with RA having completed the studies ml21530 and ma21488 |
| NCT01715896 | A study of mavrilimumab versus anti tumor necrosis factor in subjects with rheumatoid arthritis |
| NCT01717859 | Musculoskeletal ultrasound in predicting early dose titration with tocilizumab |
| NCT01724268 | Corticosteroids and anti TNF in methotrexate inadequate responder rheumatoid arthritis patient |
| NCT01730456 | A long‐term extension study of Roactemra/Actemra (tocilizumab) in patients with early moderate to severe rheumatoid arthritis who completed study WA19926 |
| NCT01734993 | A long‐term extension study of WA22762 to evaluate safety and efficacy of subcutaneous tocilizumab in participants with moderate to severe rheumatoid arthritis (RA) |
| NCT01752335 | Effect of monoclonal anti‐il6 antibody (tocilizumab) on the cardiovascular risk in patients with rheumatoid arthritis |
| NCT01752855 | Study in rheumatoid arthritis for subjects who completed preceding study M13‐390 with adalimumab |
| NCT01758198 | Abatacept post‐marketing clinical study in Japan |
| NCT01759030 | Study of safety and efficacy of BCD‐020 comparing to Mabthera in patients with rheumatoid arthritis |
| NCT01764997 | An evaluation of sarilumab plus methotrexate compared to etanercept plus methotrexate in RA patients not responding to adalimumab plus methotrexate |
| NCT01765374 | Study of sonographic efficacy of rituximab in rheumatoid arthritis |
| NCT01768572 | To evaluate the safety of SAR153191 (REGN88) and tocilizumab added to other RA drugs in patients with RA who are not responding to or intolerant of anti‐TNF therapy (SARIL‐RA‐ASCERTAIN) |
| NCT01772316 | A long‐term extension study of wa22763 and na25220 of subcutaneous Roactemra/Actemra (tocilizumab) in patients with moderate to severe rheumatoid arthritis |
| NCT01782235 | Efficacy of tocilizumab in primary Sjögren's syndrome |
| NCT01783015 | Study of etanercept in subjects with rheumatoid arthritis who have had an inadequate response to adalimumab or infliximab plus methotrexate |
| NCT01793519 | Stopping TNF alpha inhibitors in rheumatoid arthritis |
| NCT01794117 | Anakinra for inflammatory pustular skin diseases |
| NCT01835613 | Evaluation effects of treatment with il‐6r inhibitor on clinical response and biomarkers in patients with rheumatoid arthritis (RA) not responding to DMARDs and/or a first biological agent |
| NCT01842386 | Rituximab for anti‐cytokine autoantibody‐associated diseases |
| NCT01844895 | Methotrexate‐inadequate response autoinjector device sub study |
| NCT01846975 | Introducing a single iv abatacept treatment in RA patients currently receiving weekly sc abatacept to simulate a holiday |
| NCT01855789 | A study of the impact of methotrexate (MTX) discontinuation on the efficacy of subcutaneous tocilizumab with methotrexate in participants with moderate to severe active rheumatoid arthritis |
| NCT01864265 | Prediction of response to certolizumab pegol treatment by functional MRI of the brain |
| NCT01873443 | Long‐term efficacy and safety of ct‐p10 in patients with RA |
| NCT01875991 | Preference between two autoinjectors in patients with rheumatoid arthritis and plaque psoriasis treated with etanercept |
| NCT01878318 | A study of the effect of Roactemra/Actemra (tocilizumab) in combination with methotrexate on articular damage in the hand in patients with moderate to severe rheumatoid arthritis who have an inadequate response to non‐biological DMARDs |
| NCT01890473 | Study to characterize the pharmacokinetics of a single dose of sc abatacept 125 mg using the bd autoinjector or the prefilled syringe |
| NCT01893996 | Study of adalimumab to lower cardiovascular risk in RA patients with well controlled joint disease |
| NCT01895309 | A study comparing sb4 to Enbrel® in subjects with moderate to severe rheumatoid arthritis despite methotrexate therapy |
| NCT01901185 | Study to evaluate the ability of subjects with rheumatoid arthritis or psoriatic arthritis to effectively use a reusable autoinjector to self‐inject etanercept |
| NCT01927263 | A phase 3 study of ni‐071 in patients with rheumatoid arthritis |
| NCT01927757 | Evaluating etanercept use in patients with moderate to severe rheumatoid arthritis who have lost response to adalimumab |
| NCT01936181 | A study comparing SB2 to Remicade® in subjects with moderate to severe rheumatoid arthritis despite methotrexate therapy |
| NCT01941095 | A study of subcutaneously administered Roactemra/Actemra (tocilizumab) in patients with rheumatoid arthritis |
| NCT01941940 | A study to evaluate efficacy of tocilizumab administered as monotherapy or in combination with methotrexate and/or other disease modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis (RA) participants |
| NCT01951170 | An open‐label study of Roactemra/Actemra (tocilizumab) in patients with moderate to severe active rheumatoid arthritis |
| NCT01954979 | A phase i study of abatacept in the treatment of patients with steroid refractory chronic graft versus host disease (CGVHD) |
| NCT01962974 | A golimumab phase 3b, multicenter, assessment of intravenous efficacy in rheumatoid arthritis subjects who have diminished disease control despite treatment with infliximab (Remicade®) |
| NCT01969409 | Autoantibody reduction therapy in patients with idiopathic pulmonary fibrosis |
| NCT01970475 | Efficacy and safety study of ABP 501 compared to adalimumab in subjects with moderate to severe rheumatoid arthritis |
| NCT01987479 | The safety and efficacy of Roactemra/Actemra alone or in combination with non‐biologic antirheumatics in rheumatoid arthritis patients |
| NCT01999868 | Efficacy of ustekinumab followed by abatacept for the treatment of psoriasis vulgaris |
| NCT02001987 | A study of Roactemra/Actemra (tocilizumab) in tocilizumab‐naive patients with rheumatoid arthritis with inadequate response to non‐biologic disease‐modifying antirheumatic drugs (DMARDs) or biologic therapy |
| NCT02010216 | A study of Roactemra/Actemra (tocilizumab) in adult patients with rheumatoid arthritis (SVOBODA Programme) |
| NCT02018042 | An open‐label single‐arm clinical trial to evaluate the efficacy of abatacept in moderate to severe patch type alopecia areata |
| NCT02019472 | A study comparing sirukumab (CNTO 136) monotherapy with adalimumab (Humira®) monotherapy in the treatment of active rheumatoid arthritis |
| NCT02019602 | A multicenter, postmarketing study evaluating the transfer of Cimzia from the mother to the infant via the placenta |
| NCT02027298 | Abatacept for patients with inflammatory arthritis associated with Sjögren's syndrome: an open‐label phase II study |
| NCT02035800 | Bone resorption, osteoclastogenesis and adalimumab |
| NCT02053727 | Abatacept vs placebo in RA patients with hepatitis B on entecavir background |
| NCT02056184 | Targeted ultrasound in rheumatoid arthritis |
| NCT02067910 | Efficacy and safety of abatacept in patients with primary Sjögren's syndrome |
| NCT02079532 | A study of Mabthera (rituximab) in patients with rheumatoid arthritis who have had an inadequate response to a single anti‐TNF inhibitor |
| NCT02090101 | Study evaluating the influence of lv5fu2 bevacizumab plus anakinra association on metastatic colorectal cancer |
| NCT02092467 | Safety study of tofacitinib versus tumor necrosis factor (TNF) inhibitor in subjects with rheumatoid arthritis |
| NCT02092961 | Randomised double‐blind, placebo‐controlled, parallel group study in patients with active rheumatoid arthritis: magnetic resonance imaging sub‐study |
| NCT02097264 | A trial investigating the mechanism of action of NNC0109‐0012 (anti‐IL‐20 MAB) through synovial biopsies in subjects with rheumatoid arthritis and an inadequate response to methotrexate |
| NCT02097524 | Single‐dose study to describe the pharmacodynamics (pd) and safety of sarilumab (regn88/sar153191) and tocilizumab in adults with rheumatoid arthritis (RA) |
| NCT02097745 | A study of the efficacy and safety of re‐treatments with rituximab in patients with active rheumatoid arthritis who have had an inadequate response to anti‐TNFa therapies |
| NCT02109289 | Etanercept in rheumatoid arthritis and vascular inflammation |
| NCT02114931 | Long‐term safety and efficacy of ABP 501 in subjects with moderate to severe rheumatoid arthritis |
| NCT02115750 | Comparison of CHS‐0214 to Enbrel (etanercept) in patients with rheumatoid arthritis (RA) |
| NCT02116504 | Anti‐biopharmaceutical immunization: prediction and analysis of clinical relevance to minimize the risk of immunization in rheumatoid arthritis patients or juvenile idiopathic arthritis patients |
| NCT02132234 | Effects of biological treatment on blood pressure and endothelial function in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis |
| NCT02137226 | BI 695501 compared to adalimumab in patients with active rheumatoid arthritis |
| NCT02141997 | A study to investigate the safety and efficacy of ABT‐122 given with methotrexate in subjects with active rheumatoid arthritis who have an inadequate response to methotrexate |
| NCT02148640 | The NOR‐SWITCH study |
| NCT02148718 | Rapidity of response to adalimumab treatment in patients with Crohn's disease |
| NCT02149121 | Pk similarity prospective phase 3 study in patients with rheumatoid arthritis |
| NCT02150473 | The effect of adalimumab plus methotrexate (MTX) versus placebo plus MTX on cartilage in (RA) patients |
| NCT02151851 | A study of certolizumab pegol as additional therapy in Chinese patients with active rheumatoid arthritis |
| NCT02154425 | A multicenter, postmarketing study evaluating the concentration of Cimzia® in mature breast milk of lactating mothers |
| NCT02167139 | A study comparing SB5 to Humira® in subjects with moderate to severe rheumatoid arthritis despite methotrexate therapy |
| NCT02175056 | A dose‐block randomized, placebo controlled (double‐blind), active controlled(open‐label), dose‐escalation study |
| NCT02187055 | An efficacy and safety study evaluating tofacitinib with and without methotrexate compared to adalimumab with methotrexate |
| NCT02198651 | A phase 4 trial assessing the impact of residual inflammation detected via imaging techniques, drug levels and patient characteristics on the outcome of dose tapering of adalimumab in clinical remission rheumatoid arthritis (RA) subjects (PREDICTRA) |
| NCT02222493 | A study of pf‐06438179 (infliximab‐Pfizer) and infliximab in combination with methotrexate in subjects with active rheumatoid arthritis |
| NCT02232880 | Treatment of resistant hypertension by prevention of t‐cell co‐stimulation |
| NCT02236481 | Clinical study to evaluate the efficacy of anakinra in patients with rheumatoid arthritis and diabetes |
| NCT02242474 | Anti‐TNF use during elective foot and ankle surgery in patients with rheumatoid arthritis |
| NCT02260791 | A study to compare FKB327 efficacy and safety with Humira® in rheumatoid arthritis patients |
| NCT02287922 | A phase IIb study for alx‐0061 monotherapy in subjects with rheumatoid arthritis |
| NCT02293590 | Rice: remission by intra‐articular injection plus certolizumab |
| NCT02296775 | Comparative pharmacokinetic, pharmacodynamic, safety and efficacy study of three anti‐cd20 monoclonal antibodies in patients with moderate to severe rheumatoid arthritis |
| NCT02304354 | Relationship between t lymphocytes depletion and clinical response to rituximab in rheumatoid arthritis (Lyritux) |
| NCT02308163 | A study to evaluate safety and efficacy of asp015k in patients with rheumatoid arthritis (RA) who had an inadequate response to DMARDs |
| NCT02319642 | An open‐label extension study of certolizumab pegol in Chinese patients with rheumatoid arthritis who enrolled in RA0044 |
| NCT02332590 | Efficacy and safety of sarilumab and adalimumab monotherapy in patients with rheumatoid arthritis (SARIL‐RA‐MONARCH) |
| NCT02353780 | Mechanistic studies of b‐ and t‐cell function in RA patients treated with TNF antagonists, tocilizumab, or abatacept |
| NCT02357069 | A study comparing lbec0101 to Enbrel® in subjects with active rheumatoid arthritis despite methotrexate therapy |
| NCT02371096 | Comparative pharmacokinetic trial of rgb‐03 and Mabthera |
| NCT02373813 | Study of etanercept monotherapy vs methotrexate monotherapy for maintenance of rheumatoid arthritis remission |
| NCT02374021 | Treatments against RA and effect on FDG‐PET/CT |
| NCT02376335 | B‐cell depleting therapy (rituximab) as a treatment for fatigue in primary biliary cirrhosis |
| NCT02378506 | Study to assess the immunogenicity, safety, and efficacy of high capacity process etanercept in rheumatoid arthritis subjects |
| NCT02393378 | Namilumab vs adalimumab in participants with moderate to severe early rheumatoid arthritis inadequately responding to methotrexate |
| NCT02404558 | Single‐dose study to describe the safety of sarilumab and tocilizumab in patients with rheumatoid arthritis |
| NCT02405780 | A study to compare FKB327 long‐term safety, efficacy and immunogenicity with Humira® in rheumatoid arthritis patients |
| NCT02429934 | Abatacept for SLE arthritis |
| NCT02430909 | Multiple dose study of ucb4940 as add‐on to certolizumab pegol in subjects with rheumatoid arthritis |
| NCT02433184 | Very early versus delayed etanercept in patients with RA |
| NCT02451839 | An observational study of the effectiveness of adalimumab on health and disability outcomes in New Zealand patients with immune‐mediated inflammatory diseases (VITALITY) |
| NCT02466581 | Dose reduction for early rheumatoid arthritis patients with low disease activity |
| NCT02468791 | MabionCD20® compared to Mabthera® in patients with rheumatoid arthritis |
| NCT02480153 | A study of PF‐06410293 (adalimumab‐Pfizer) and adalimumab (Humira) In combination with methotrexate in subjects with active rheumatoid arthritis (REFLECTIONS B538‐02) |
| NCT02481180 | Tolerance, pharmacokinetics and preliminary efficacy of t0001 in RA (rheumatoid arthritis) |
| NCT02495129 | Study of pharmacodynamic effects of vay736 in patients with primary Sjögren's syndrome |
| NCT02504268 | Effects of abatacept in patients with early rheumatoid arthritis |
| NCT02514772 | Gp2013 treatment in patients with active rheumatoid arthritis, previously treated with Rituxan® or Mabthera® |
| NCT02526992 | Evaluation by HR‐pqct of bone microarchitecture changes in patients with rheumatoid arthritis under anti‐TNF therapy |
| NCT02547493 | Vaccination against pneumococcal in naïve abatacept rheumatoid arthritis patients |
| NCT02557100 | Study to assess changes in the immune profile in adults with early rheumatoid arthritis |
| NCT02565810 | An multicentre clinical study to evaluate the usability and safety of the pre‐filled pen and pre‐filled syringe of SB5 in subjects with rheumatoid arthritis |
| NCT02573012 | Study to compare the efficacy of tocilizumab with or without glucocorticoid discontinuation in rheumatoid arthritis participants |
| NCT02616380 | Real‐world outcome of adalimumab on rheumatoid arthritis patients in Taiwan |
| NCT02629159 | A study comparing ABT‐494 to placebo and to adalimumab in subjects with rheumatoid arthritis who are on a stable dose of methotrexate and who have an inadequate response to methotrexate |
| NCT02631538 | Safety and efficacy study of subcutaneous belimumab and intravenous rituximab co‐administration in subjects with primary Sjögren's syndrome |
| NCT02638259 | Comparative efficacy and safety study of gp2015 and Enbrel® in patients with rheumatoid arthritis |
| NCT02640612 | Long‐term assessment of safety and efficacy of BI 695501 in patients with rheumatoid arthritis |
| NCT02652273 | Inhibition of co‐stimulation in rheumatoid arthritis |
| NCT02659150 | Effect of subcutaneous Actemra on inflamed atherosclerotic plaques in patients with rheumatoid arthritis |
| NCT02682823 | Tocilizumab real‐life human factors (RLHFS) validation study |
| NCT02683564 | Bow015 (infliximab‐epirus) and infliximab in patients with active rheumatoid arthritis: the uniform study |
| NCT02693210 | A study to evaluate the efficacy and safety of Mabthera alone and in combination with either cyclophosphamide or methotrexate in patients with rheumatoid arthritis |
| NCT02714634 | Clinical trial evaluating methotrexate + biologic versus methotrexate, salazopyrine and hydroxychloroquine in patients with rheumatoid arthritis and insufficient response to methotrexate |
| NCT02714881 | Lipids, inflammation, and CV risk in RA |
| NCT02715908 | A study to evaluate the long‐term safety and efficacy of lbec0101 in subjects with active rheumatoid arthritis despite methotrexate (MTX) |
| NCT02722044 | Usability of an AI for M923 in subjects with moderate to severe RA |
| NCT02722694 | A phase 3 study of abatacept in Chinese patients with active rheumatoid arthritis and inadequate response to methotrexate |
| NCT02731560 | Rituximab (rtx) for disease modifying anti rheumatic drug (DMARD) non‐responders in Pakistan: the Pakistan rituximab study (PaRiS) |
| NCT02743390 | Effects of the TNF‐alpha inhibitIon on hemodynamic parameters in resistant hypertension |
| NCT02744196 | Clinical trial to evaluate efficacy and safety of Acellbia® (JSC "Biocad") with methotrexate in first line biological therapy of patients with active rheumatoid arthritis |
| NCT02744755 | Clinical trial to compare treatment with GP2017 and Humira® in patients with rheumatoid arthritis |
| NCT02746380 | A study comparing LBAL to Humira® in subjects with active rheumatoid arthritis despite methotrexate therapy |
| NCT02760407 | Evaluation of the effectiveness and safety of two dosing regimens of Olokizumab (OKZ), compared to placebo and adalimumab, in subjects with rheumatoid arthritis (RA) who are taking methotrexate but have active disease |
| NCT02762838 | Comparative clinical trial of efficacy and safety of BCD‐055 and Remicade® in combination with methotrexate in patients with active rheumatoid arthritis |
| NCT02765074 | Filling bone erosions: a longitudinal multicentric HR‐PQCT study of subcutaneous tocilizumab in rheumatoid arthritis |
| NCT02770794 | Optimization of infliximab withdrawal strategy for rheumatoid arthritis |
| NCT02778906 | Abatacept reversing subclinical inflammation as measured by MRI in ACPA positive arthralgia |
| NCT02779114 | Retro (reduction of therapy in RA patients in ongoing remission) |
| NCT02780583 | Treatment of macrophage activation syndrome (mas) with anakinra |
| NCT02792699 | Study to assess if ABP798 is safe & effective in treating moderate to severe rheumatoid arthritis compared to rituximab |
| NCT02805010 | Pharmacokinetics, safety and tolerability study of single dose of abatacept 125mg administered subcutaneously |
| NCT02819726 | A study to compare the pharmacokinetics, pharmacodynamics, safety, and efficacy of sait101 versus Mabthera® versus Rituxan® in patients with rheumatoid arthritis (RA) |
| NCT02833350 | Safety and efficacy study of GDC‐0853 compared with placebo and adalimumab in participants with rheumatoid arthritis (RA) |
| NCT02837146 | Ultrasound as imaging biomarker of early response to tocilizumab and methotrexate in very early rheumatoid arthritis |
| NCT02840175 | Treatment tapering in JIA with inactive disease |
| NCT02843789 | Evolution of adipokines and body composition in rheumatoid arthritis patients receiving tocilizumab therapy |
| NCT02862574 | GS‐5745 as add‐on therapy to a tumor necrosis factor inhibitor and methotrexate regimen in adults with moderately to severely active rheumatoid arthritis |
| NCT02889796 | Filgotinib in combination with methotrexate in adults with moderately to severely active rheumatoid arthritis who have an inadequate response to methotrexate |
| NCT02908217 | Safety and efficacy of tocilizumab versus placebo in polymyalgia rheumatica with glucocorticoid dependence semaphore |
| NCT02915159 | A study to assess the efficacy and safety of abatacept in adults with active primary Sjögren's syndrome |
| NCT02935387 | Remission induction in very early rheumatoid arthritis |
| NCT02937701 | Study to assess if abp710 is safe & effective in treating moderate to severe rheumatoid arthritis compared to infliximab |
| NCT02986139 | Assess the injection site pain associated with a new etanercept formulation in adult subjects with RA or PSA |
| NCT02990806 | A phase 3 study of ni‐071 in patients with rheumatoid arthritis (RADIANCE) |
| NTR1011 | Hypothesis generating study to identify the changes in synovial tissue early after initiation of infliximab therapy |
| NTR1088 | Sevra‐trial safety and efficacy of vaccination with t cell‐dependent and t cell‐independent primary and recall antigens in patients with rheumatoid arthritis treated with anti TNF‐a antibodies (adalimumab) and anti B cell therapy (rituximab). |
| NTR1137 | An open‐label pilot study on the effects of trivalent inactivated influenza vaccination (Influvac®) in rheumatoid arthritis patients treated with rituximab |
| NTR1210 | Exploratory trial on intra‐articular etanercept treatment in inflammatory arthritis |
| NTR144 | Strategies in early arthritis management |
| NTR1605 | (English) Cost‐effectiveness of new medicines (Mabthera and Orencia) compared to a second TNF blocking medicine, for patients with inadequate effect of a first TNF blocking medicine. (Dutch) Onderzoek naar de kosteneffectiviteit van nieuwe medicijnen (Mabthera en Orencia) vergeleken met een tweede TNF blokerend middel, voor patienten met onvoldoende effect van een eerste behandeling met TNF blokkerende middelen |
| NTR2911 | Tocilizumab met biopten cohort. Tocilizumab with biopsy cohort |
| NTR3216 | Onderzoek naar non inferioriteit van afbouw en stop behandelstrategieën van adalimumab of etanercept bij patiënten met reumatoïde artritis: kosten besparen tegen welke prijs? |
| NTR3327 | Influence of rituximab on endothelial dysfunction in RA |
| NTR3509 | Therapeutic drug monitoring: toward tailored dosing of adalimumab in rheumatoid arthritis |
| NTR383 | The efficacy and safety of intra‐articular injections with the TNF‐a antagonist infliximab in patients with chronic or recurrent arthritis of the knee |
| NTR3903 | Dose‐to‐target of etanercept treatment in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis |
| NTR5279 | The effect of switching treatment from innovator infliximab to infliximab biosimilar on efficacy, safety and immunogenicity in patients with rheumatoid arthritis, spondyloarthritis or psoriatic arthritis in daily clinical care |
| NTR801 | IMPROVED: Induction therapy with methotrexate and prednisone in rheumatoid or very early arthritic disease |
| NTR851 | Prospective study on the effects of rituximab on synovial tissue of patients with rheumatoid arthritis |
| NTR859 | Identification of predictive factors in synovial samples for the clinical response to TNF‐alpha blockade in rheumatoid arthritis |
| SLCTR/2008/008 | Efficacy of low dose rituximab with methotrexate compared to leflunomide with methotrexate in patients with refractory rheumatoid arthritis: a randomized double blind controlled clinical trial |
Appendix 3. ACR50: main analysis
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| MP + MTX | PL + MTX | 1.52 (0.63, 3.70) | 1.25 (0.75, 1.76) | 0.10 (‐0.10, 0.31) |
| SD ADA SC | 0.82 (0.51, 1.33) | 0.89 (0.64, 1.17) | ‐0.05 (‐0.15, 0.07) | |
| SD ADA SC + MTX | 2.17 (1.70, 2.84) | 1.46 (1.31, 1.64) | 0.19 (0.13, 0.25) | |
| SD INF IV + MTX | 1.88 (1.20, 3.30) | 1.38 (1.11, 1.71) | 0.16 (0.05, 0.29) | |
| SD RITUX IV + MTX | 1.71 (1.00, 2.95) | 1.32 (1.00, 1.64) | 0.13 (0.00, 0.26) | |
| SD ABA IV + MTX | 1.84 (1.08, 3.14) | 1.37 (1.05, 1.68) | 0.15 (0.02, 0.28) | |
| SD ETN SC + MTX | 2.52 (1.46, 4.38) | 1.55 (1.23, 1.85) | 0.23 (0.09, 0.34) | |
| SD GOLI SC + MTX | 1.61 (0.88, 2.98) | 1.29 (0.93, 1.65) | 0.12 (‐0.03, 0.26) | |
| LD RITUX IV + MTX | 1.48 (0.86, 2.54) | 1.23 (0.92, 1.56) | 0.10 (‐0.04, 0.23) | |
| HD GOLI SC | 1.17 (0.63, 2.17) | 1.09 (0.74, 1.47) | 0.04 (‐0.11, 0.19) | |
| HD GOLI SC + MTX | 1.38 (0.74, 2.55) | 1.19 (0.83, 1.56) | 0.08 (‐0.07, 0.23) | |
| HD INF IV + MTX | 2.20 (1.37, 3.81) | 1.47 (1.19, 1.78) | 0.19 (0.08, 0.32) | |
| SD ADA SC | MP + MTX | 0.54 (0.20, 1.47) | 0.71 (0.44, 1.27) | ‐0.15 (‐0.38, 0.09) |
| SD ADA SC + MTX | 1.43 (0.57, 3.57) | 1.17 (0.82, 1.97) | 0.09 (‐0.13, 0.31) | |
| SD INF IV + MTX | 1.24 (0.60, 2.66) | 1.10 (0.83, 1.71) | 0.05 (‐0.12, 0.23) | |
| SD RITUX IV + MTX | 1.12 (0.40, 3.15) | 1.06 (0.68, 1.83) | 0.03 (‐0.22, 0.27) | |
| SD ABA IV + MTX | 1.22 (0.43, 3.35) | 1.09 (0.71, 1.88) | 0.05 (‐0.20, 0.29) | |
| SD ETN SC + MTX | 1.66 (0.58, 4.64) | 1.24 (0.82, 2.11) | 0.12 (‐0.12, 0.36) | |
| SD GOLI SC + MTX | 1.07 (0.36, 3.08) | 1.03 (0.64, 1.81) | 0.02 (‐0.24, 0.27) | |
| LD RITUX IV + MTX | 0.97 (0.34, 2.71) | 0.99 (0.63, 1.72) | ‐0.01 (‐0.25, 0.24) | |
| HD GOLI SC | 0.77 (0.26, 2.21) | 0.88 (0.52, 1.57) | ‐0.06 (‐0.32, 0.19) | |
| HD GOLI SC + MTX | 0.90 (0.30, 2.64) | 0.95 (0.58, 1.69) | ‐0.02 (‐0.28, 0.23) | |
| HD INF IV + MTX | 1.46 (0.61, 3.55) | 1.18 (0.83, 1.92) | 0.09 (‐0.11, 0.30) | |
| SD ADA SC + MTX | SD ADA SC | 2.65 (1.65, 4.30) | 1.65 (1.26, 2.27) | 0.24 (0.12, 0.34) |
| SD INF IV + MTX | 2.29 (1.21, 4.80) | 1.55 (1.10, 2.30) | 0.20 (0.05, 0.37) | |
| SD RITUX IV + MTX | 2.08 (1.01, 4.28) | 1.48 (1.01, 2.20) | 0.18 (0.00, 0.34) | |
| SD ABA IV + MTX | 2.25 (1.09, 4.57) | 1.54 (1.05, 2.25) | 0.20 (0.02, 0.36) | |
| SD ETN SC + MTX | 3.08 (1.48, 6.34) | 1.74 (1.22, 2.51) | 0.27 (0.10, 0.43) | |
| SD GOLI SC + MTX | 1.96 (0.91, 4.28) | 1.45 (0.95, 2.18) | 0.16 (‐0.02, 0.34) | |
| LD RITUX IV + MTX | 1.80 (0.88, 3.67) | 1.39 (0.93, 2.07) | 0.14 (‐0.03, 0.31) | |
| HD GOLI SC | 1.42 (0.65, 3.08) | 1.23 (0.77, 1.91) | 0.08 (‐0.10, 0.27) | |
| HD GOLI SC + MTX | 1.68 (0.77, 3.66) | 1.34 (0.86, 2.05) | 0.13 (‐0.06, 0.31) | |
| HD INF IV + MTX | 2.69 (1.37, 5.55) | 1.66 (1.17, 2.42) | 0.24 (0.08, 0.40) | |
| SD INF IV + MTX | SD ADA SC + MTX | 0.87 (0.52, 1.58) | 0.94 (0.74, 1.18) | ‐0.03 (‐0.16, 0.11) |
| SD RITUX IV + MTX | 0.79 (0.43, 1.42) | 0.90 (0.67, 1.14) | ‐0.06 (‐0.21, 0.08) | |
| SD ABA IV + MTX | 0.85 (0.46, 1.52) | 0.93 (0.70, 1.17) | ‐0.04 (‐0.19, 0.10) | |
| SD ETN SC + MTX | 1.16 (0.63, 2.11) | 1.06 (0.82, 1.28) | 0.04 (‐0.11, 0.16) | |
| SD GOLI SC + MTX | 0.74 (0.38, 1.43) | 0.88 (0.62, 1.14) | ‐0.07 (‐0.24, 0.08) | |
| LD RITUX IV + MTX | 0.68 (0.37, 1.22) | 0.84 (0.61, 1.08) | ‐0.09 (‐0.24, 0.05) | |
| HD GOLI SC | 0.54 (0.27, 1.04) | 0.75 (0.50, 1.02) | ‐0.15 (‐0.31, 0.01) | |
| HD GOLI SC + MTX | 0.63 (0.32, 1.23) | 0.82 (0.56, 1.08) | ‐0.11 (‐0.27, 0.05) | |
| HD INF IV + MTX | 1.02 (0.58, 1.82) | 1.01 (0.79, 1.24) | 0.00 (‐0.13, 0.14) | |
| SD RITUX IV + MTX | SD INF IV + MTX | 0.91 (0.41, 1.81) | 0.96 (0.67, 1.29) | ‐0.02 (‐0.21, 0.14) |
| SD ABA IV + MTX | 0.98 (0.45, 1.93) | 0.99 (0.70, 1.32) | ‐0.01 (‐0.19, 0.16) | |
| SD ETN SC + MTX | 1.34 (0.60, 2.68) | 1.12 (0.82, 1.47) | 0.07 (‐0.12, 0.23) | |
| SD GOLI SC + MTX | 0.86 (0.37, 1.79) | 0.94 (0.63, 1.28) | ‐0.04 (‐0.24, 0.14) | |
| LD RITUX IV + MTX | 0.79 (0.36, 1.55) | 0.90 (0.62, 1.22) | ‐0.06 (‐0.25, 0.11) | |
| HD GOLI SC | 0.62 (0.27, 1.30) | 0.79 (0.51, 1.13) | ‐0.12 (‐0.31, 0.07) | |
| HD GOLI SC + MTX | 0.73 (0.32, 1.53) | 0.87 (0.57, 1.21) | ‐0.08 (‐0.28, 0.10) | |
| HD INF IV + MTX | 1.17 (0.69, 1.87) | 1.07 (0.86, 1.29) | 0.04 (‐0.09, 0.15) | |
| SD ABA IV + MTX | SD RITUX IV + MTX | 1.08 (0.50, 2.29) | 1.03 (0.73, 1.46) | 0.02 (‐0.17, 0.20) |
| SD ETN SC + MTX | 1.48 (0.68, 3.18) | 1.17 (0.86, 1.62) | 0.09 (‐0.09, 0.27) | |
| SD GOLI SC + MTX | 0.94 (0.42, 2.13) | 0.97 (0.66, 1.41) | ‐0.01 (‐0.21, 0.18) | |
| LD RITUX IV + MTX | 0.86 (0.50, 1.50) | 0.93 (0.72, 1.21) | ‐0.04 (‐0.17, 0.10) | |
| HD GOLI SC | 0.68 (0.30, 1.55) | 0.83 (0.53, 1.24) | ‐0.10 (‐0.29, 0.11) | |
| HD GOLI SC + MTX | 0.81 (0.36, 1.83) | 0.90 (0.60, 1.33) | ‐0.05 (‐0.25, 0.15) | |
| HD INF IV + MTX | 1.29 (0.63, 2.82) | 1.11 (0.83, 1.57) | 0.06 (‐0.11, 0.25) | |
| SD ETN SC + MTX | SD ABA IV + MTX | 1.37 (0.64, 2.94) | 1.13 (0.84, 1.55) | 0.07 (‐0.10, 0.25) |
| SD GOLI SC + MTX | 0.88 (0.39, 1.98) | 0.94 (0.64, 1.35) | ‐0.03 (‐0.23, 0.16) | |
| LD RITUX IV + MTX | 0.80 (0.38, 1.72) | 0.90 (0.63, 1.28) | ‐0.05 (‐0.24, 0.13) | |
| HD GOLI SC | 0.63 (0.28, 1.44) | 0.80 (0.52, 1.19) | ‐0.11 (‐0.30, 0.09) | |
| HD GOLI SC + MTX | 0.75 (0.33, 1.68) | 0.87 (0.58, 1.27) | ‐0.07 (‐0.26, 0.13) | |
| HD INF IV + MTX | 1.20 (0.59, 2.58) | 1.08 (0.81, 1.49) | 0.04 (‐0.13, 0.22) | |
| SD GOLI SC + MTX | SD ETN SC + MTX | 0.64 (0.28, 1.45) | 0.83 (0.58, 1.16) | ‐0.11 (‐0.30, 0.09) |
| LD RITUX IV + MTX | 0.59 (0.27, 1.26) | 0.80 (0.57, 1.10) | ‐0.13 (‐0.30, 0.06) | |
| HD GOLI SC | 0.46 (0.20, 1.06) | 0.71 (0.47, 1.02) | ‐0.19 (‐0.37, 0.01) | |
| HD GOLI SC + MTX | 0.55 (0.24, 1.24) | 0.77 (0.52, 1.09) | ‐0.15 (‐0.34, 0.05) | |
| HD INF IV + MTX | 0.87 (0.42, 1.92) | 0.95 (0.73, 1.28) | ‐0.03 (‐0.20, 0.15) | |
| LD RITUX IV + MTX | SD GOLI SC + MTX | 0.92 (0.40, 2.06) | 0.96 (0.65, 1.43) | ‐0.02 (‐0.22, 0.18) |
| HD GOLI SC | 0.72 (0.39, 1.33) | 0.85 (0.61, 1.16) | ‐0.08 (‐0.22, 0.07) | |
| HD GOLI SC + MTX | 0.85 (0.47, 1.57) | 0.93 (0.68, 1.25) | ‐0.04 (‐0.18, 0.11) | |
| HD INF IV + MTX | 1.37 (0.63, 3.10) | 1.14 (0.83, 1.67) | 0.08 (‐0.11, 0.27) | |
| HD GOLI SC | LD RITUX IV + MTX | 0.79 (0.35, 1.79) | 0.88 (0.57, 1.34) | ‐0.06 (‐0.25, 0.14) |
| HD GOLI SC + MTX | 0.93 (0.41, 2.10) | 0.97 (0.63, 1.44) | ‐0.02 (‐0.22, 0.18) | |
| HD INF IV + MTX | 1.49 (0.73, 3.23) | 1.19 (0.88, 1.70) | 0.10 (‐0.07, 0.28) | |
| HD GOLI SC + MTX | HD GOLI SC | 1.18 (0.64, 2.19) | 1.09 (0.79, 1.53) | 0.04 (‐0.11, 0.19) |
| HD INF IV + MTX | 1.89 (0.87, 4.37) | 1.35 (0.94, 2.08) | 0.16 (‐0.03, 0.35) | |
| HD INF IV + MTX | HD GOLI SC + MTX | 1.60 (0.74, 3.66) | 1.23 (0.88, 1.85) | 0.12 (‐0.07, 0.31) |
| Random‐effects model | Residual deviance | 34.01 vs 33 data‐points | ||
| Deviance information criteria | 233.176 | |||
| Fixed‐effect model | Residual deviance | 34.92 vs 33 data‐points | ||
| Deviance information criteria | 232.343 | |||
| ABA: abatacept ADA: adalimumab CrI: credible interval ETN: etanercept GOLI: golimumab HD: high dose INF: infliximab IV: intravenous LD: low dose MP: methylprednisolone MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 4. Subgroup analysis: ACR50, established RA
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC | PL + MTX | 0.80 (0.46, 1.43) | 0.87 (0.59, 1.22) | ‐0.05 (‐0.16, 0.09) |
| SD ADA SC + MTX | 2.07 (1.51, 3.02) | 1.46 (1.25, 1.71) | 0.18 (0.10, 0.27) | |
| SD ABA IV + MTX | 1.85 (1.00, 3.43) | 1.39 (1.00, 1.77) | 0.15 (0.00, 0.30) | |
| SD ETN SC + MTX | 2.52 (1.37, 4.70) | 1.58 (1.19, 1.94) | 0.23 (0.08, 0.36) | |
| SD GOLI SC + MTX | 1.63 (0.82, 3.25) | 1.31 (0.88, 1.74) | 0.12 (‐0.05, 0.28) | |
| HD GOLI SC | 1.17 (0.59, 2.36) | 1.10 (0.70, 1.55) | 0.04 (‐0.12, 0.21) | |
| HD GOLI SC + MTX | 1.38 (0.70, 2.76) | 1.20 (0.79, 1.65) | 0.08 (‐0.08, 0.25) | |
| SD ADA SC + MTX | SD ADA SC | 2.58 (1.51, 4.60) | 1.67 (1.22, 2.45) | 0.23 (0.10, 0.35) |
| SD ABA IV + MTX | 2.30 (1.00, 5.24) | 1.58 (1.00, 2.51) | 0.20 (0.00, 0.38) | |
| SD ETN SC + MTX | 3.13 (1.36, 7.20) | 1.80 (1.17, 2.79) | 0.28 (0.07, 0.45) | |
| SD GOLI SC + MTX | 2.03 (0.83, 4.85) | 1.50 (0.90, 2.41) | 0.17 (‐0.04, 0.37) | |
| HD GOLI SC | 1.46 (0.60, 3.53) | 1.26 (0.72, 2.11) | 0.09 (‐0.12, 0.29) | |
| HD GOLI SC + MTX | 1.72 (0.71, 4.16) | 1.38 (0.81, 2.28) | 0.13 (‐0.08, 0.33) | |
| SD ABA IV + MTX | SD ADA SC + MTX | 0.89 (0.43, 1.76) | 0.95 (0.66, 1.25) | ‐0.03 (‐0.21, 0.13) |
| SD ETN SC + MTX | 1.22 (0.59, 2.40) | 1.08 (0.79, 1.37) | 0.05 (‐0.13, 0.20) | |
| SD GOLI SC + MTX | 0.79 (0.36, 1.65) | 0.90 (0.59, 1.22) | ‐0.06 (‐0.25, 0.12) | |
| HD GOLI SC | 0.56 (0.26, 1.20) | 0.75 (0.47, 1.08) | ‐0.14 (‐0.32, 0.05) | |
| HD GOLI SC + MTX | 0.67 (0.30, 1.41) | 0.82 (0.53, 1.15) | ‐0.10 (‐0.29, 0.08) | |
| SD ETN SC + MTX | SD ABA IV + MTX | 1.36 (0.57, 3.27) | 1.14 (0.79, 1.65) | 0.07 (‐0.13, 0.28) |
| SD GOLI SC + MTX | 0.88 (0.35, 2.22) | 0.94 (0.60, 1.45) | ‐0.03 (‐0.25, 0.19) | |
| HD GOLI SC | 0.63 (0.25, 1.60) | 0.79 (0.48, 1.26) | ‐0.11 (‐0.32, 0.11) | |
| HD GOLI SC + MTX | 0.75 (0.30, 1.89) | 0.87 (0.54, 1.35) | ‐0.07 (‐0.29, 0.15) | |
| SD GOLI SC + MTX | SD ETN SC + MTX | 0.65 (0.26, 1.62) | 0.83 (0.54, 1.23) | ‐0.11 (‐0.32, 0.11) |
| HD GOLI SC | 0.46 (0.19, 1.18) | 0.70 (0.43, 1.08) | ‐0.19 (‐0.39, 0.04) | |
| HD GOLI SC + MTX | 0.55 (0.22, 1.39) | 0.76 (0.48, 1.15) | ‐0.15 (‐0.35, 0.08) | |
| HD GOLI SC | SD GOLI SC + MTX | 0.72 (0.36, 1.42) | 0.84 (0.58, 1.20) | ‐0.08 (‐0.24, 0.08) |
| HD GOLI SC + MTX | 0.85 (0.43, 1.66) | 0.92 (0.65, 1.29) | ‐0.04 (‐0.20, 0.12) | |
| HD GOLI SC + MTX | HD GOLI SC | 1.18 (0.60, 2.32) | 1.09 (0.76, 1.60) | 0.04 (‐0.12, 0.2) |
| Random‐effects model | Residual deviance | 17.84 vs 17 data‐points | ||
| Deviance information criteria | 126.687 | |||
| Fixed‐effect model | Residual deviance | 18.05 vs 17 data‐points | ||
| Deviance information criteria | 125.82 | |||
| ABA: abatacept ADA: adalimumab CrI: credible interval ETN: etanercept GOLI: golimumab HD: high dose INF: infliximab IV: intravenous MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 5. Subgroup analysis 2: ACR50, trial duration ≤ 6 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC + MTX | PL + MTX | 2.41 (1.74, 3.66) | 1.59 (1.35, 1.91) | 0.22 (0.14, 0.31) |
| SD GOLI SC + MTX | 1.64 (0.80, 3.29) | 1.33 (0.87, 1.82) | 0.12 (‐0.05, 0.29) | |
| HD GOLI SC | 1.18 (0.58, 2.38) | 1.11 (0.69, 1.60) | 0.04 (‐0.12, 0.21) | |
| HD GOLI SC + MTX | 1.40 (0.69, 2.80) | 1.22 (0.78, 1.71) | 0.08 (‐0.08, 0.25) | |
| SD GOLI SC + MTX | SD ADA SC + MTX | 0.68 (0.29, 1.44) | 0.84 (0.53, 1.16) | ‐0.10 (‐0.30, 0.09) |
| HD GOLI SC | 0.49 (0.21, 1.03) | 0.70 (0.41, 1.01) | ‐0.18 (‐0.36, 0.01) | |
| HD GOLI SC + MTX | 0.58 (0.25, 1.22) | 0.77 (0.47, 1.09) | ‐0.13 (‐0.33, 0.05) | |
| HD GOLI SC | SD GOLI SC + MTX | 0.72 (0.36, 1.44) | 0.84 (0.56, 1.22) | ‐0.08 (‐0.24, 0.09) |
| HD GOLI SC + MTX | 0.85 (0.43, 1.70) | 0.92 (0.63, 1.33) | ‐0.04 (‐0.20, 0.13) | |
| HD GOLI SC + MTX | HD GOLI SC | 1.18 (0.59, 2.38) | 1.10 (0.74, 1.66) | 0.04 (‐0.12, 0.20) |
| Random‐effects model | Residual deviance | 12.3 vs 12 data‐points | ||
| Deviance information criteria | 85.785 | |||
| Fixed‐effect model | Residual deviance | 13.04 vs 12 data‐points | ||
| Deviance information criteria | 85.321 | |||
| ADA: adalimumab CrI: credible interval GOLI: golimumab HD: high dose MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 6. Subgroup analysis 2: ACR50, trial duration 6‐12 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| MP + MTX | PL + MTX | 0.71 (0.11, 5.35) | 0.81 (0.18, 1.91) | ‐0.08 (‐0.36, 0.37) |
| SD INF IV + MTX | 1.94 (1.03, 5.77) | 1.38 (1.02, 1.98) | 0.16 (0.01, 0.39) | |
| SD ABA IV + MTX | 1.84 (0.71, 4.79) | 1.35 (0.81, 1.86) | 0.15 (‐0.08, 0.36) | |
| SD ETN SC + MTX | 2.52 (0.98, 6.54) | 1.51 (0.99, 1.99) | 0.22 (‐0.01, 0.40) | |
| HD INF IV + MTX | 2.24 (1.00, 6.38) | 1.45 (1.00, 2.00) | 0.20 (0.00, 0.40) | |
| SD INF IV + MTX | MP + MTX | 2.82 (0.49, 17.20) | 1.70 (0.81, 7.32) | 0.25 (‐0.14, 0.53) |
| SD ABA IV + MTX | 2.63 (0.28, 20.99) | 1.65 (0.62, 7.72) | 0.23 (‐0.27, 0.57) | |
| SD ETN SC + MTX | 3.60 (0.38, 28.45) | 1.86 (0.73, 8.62) | 0.30 (‐0.19, 0.63) | |
| HD INF IV + MTX | 3.21 (0.44, 22.82) | 1.79 (0.78, 8.04) | 0.27 (‐0.16, 0.59) | |
| SD ABA IV + MTX | SD INF IV + MTX | 0.95 (0.21, 2.75) | 0.98 (0.51, 1.45) | ‐0.01 (‐0.34, 0.22) |
| SD ETN SC + MTX | 1.30 (0.28, 3.76) | 1.10 (0.62, 1.58) | 0.06 (‐0.27, 0.28) | |
| HD INF IV + MTX | 1.16 (0.41, 2.57) | 1.06 (0.71, 1.39) | 0.03 (‐0.19, 0.20) | |
| SD ETN SC + MTX | SD ABA IV + MTX | 1.37 (0.35, 5.21) | 1.12 (0.67, 1.95) | 0.07 (‐0.23, 0.35) |
| HD INF IV + MTX | 1.21 (0.36, 5.03) | 1.08 (0.68, 1.94) | 0.05 (‐0.22, 0.35) | |
| HD INF IV + MTX | SD ETN SC + MTX | 0.89 (0.26, 3.77) | 0.96 (0.62, 1.62) | ‐0.03 (‐0.28, 0.28) |
| Random‐effects model | Residual deviance | 11.35 vs 11 data‐points | ||
| Deviance information criteria | 75.5 | |||
| Fixed‐effect model | Residual deviance | 11.62 vs 11 data‐points | ||
| Deviance information criteria | 75.511 | |||
| ABA: abatacept CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous MP: methylprednisolone MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 7. Subgroup analysis 2: ACR50, trial duration > 12 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC | PL + MTX | 0.74 (0.37, 1.44) | 0.84 (0.53, 1.20) | ‐0.07 (‐0.22, 0.09) |
| SD ADA SC + MTX | 1.78 (1.01, 3.03) | 1.31 (1.00, 1.60) | 0.14 (0.00, 0.26) | |
| SD RITUX IV + MTX | 1.71 (0.84, 3.53) | 1.29 (0.91, 1.65) | 0.13 (‐0.04, 0.29) | |
| LD RITUX IV + MTX | 1.48 (0.72, 3.06) | 1.21 (0.83, 1.58) | 0.10 (‐0.08, 0.26) | |
| SD ADA SC + MTX | SD ADA SC | 2.40 (1.20, 4.66) | 1.55 (1.09, 2.34) | 0.21 (0.04, 0.36) |
| SD RITUX IV + MTX | 2.30 (0.88, 6.30) | 1.53 (0.94, 2.56) | 0.20 (‐0.03, 0.42) | |
| LD RITUX IV + MTX | 1.99 (0.76, 5.43) | 1.44 (0.87, 2.43) | 0.17 (‐0.06, 0.39) | |
| SD RITUX IV + MTX | SD ADA SC + MTX | 0.96 (0.40, 2.44) | 0.98 (0.67, 1.40) | ‐0.01 (‐0.22, 0.20) |
| LD RITUX IV + MTX | 0.83 (0.35, 2.12) | 0.93 (0.62, 1.34) | ‐0.04 (‐0.25, 0.17) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 0.86 (0.42, 1.79) | 0.94 (0.69, 1.28) | ‐0.04 (‐0.20, 0.13) |
| Random‐effects model | Residual deviance | 7.554 vs 8 data‐points | ||
| Deviance information criteria | 60.272 | |||
| Fixed‐effect model | Residual deviance | 7.305 vs 8 data‐points | ||
| Deviance information criteria | 59.607 | |||
| ADA: adalimumab CrI: credible interval IV: intravenous LD: low dose MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 8. HAQ: main analysis
| Treatment | Reference | MD (95% CrI) |
| MP + MTX | MTX | ‐0.17 (‐0.63, 0.27) |
| SD ADA SC | 0.00 (‐0.42, 0.42) | |
| SD ETN SC | 0.40 (‐0.33, 1.14) | |
| SD INF IV + MTX | ‐0.03 (‐0.37, 0.25) | |
| SD ADA SC + MTX | ‐0.30 (‐0.59, ‐0.02) | |
| SD ETN SC + MTX | ‐0.10 (‐0.43, 0.24) | |
| SD ABA IV + MTX | ‐0.20 (‐0.65, 0.25) | |
| SD RITUX IV + MTX | ‐0.24 (‐0.68, 0.21) | |
| LD RITUX IV + MTX | ‐0.24 (‐0.69, 0.21) | |
| HD INF IV + MTX | 0.12 (‐0.32, 0.54) | |
| SD ADA SC | MP + MTX | 0.17 (‐0.43, 0.80) |
| SD ETN SC | 0.57 (‐0.28, 1.45) | |
| SD INF IV + MTX | 0.14 (‐0.21, 0.45) | |
| SD ADA SC + MTX | ‐0.13 (‐0.65, 0.41) | |
| SD ETN SC + MTX | 0.07 (‐0.46, 0.65) | |
| SD ABA IV + MTX | ‐0.03 (‐0.64, 0.63) | |
| SD RITUX IV + MTX | ‐0.07 (‐0.68, 0.58) | |
| LD RITUX IV + MTX | ‐0.07 (‐0.67, 0.59) | |
| HD INF IV + MTX | 0.29 (‐0.24, 0.83) | |
| SD ETN SC | SD ADA SC | 0.41 (‐0.44, 1.25) |
| SD INF IV + MTX | ‐0.03 (‐0.58, 0.46) | |
| SD ADA SC + MTX | ‐0.30 (‐0.72, 0.12) | |
| SD ETN SC + MTX | ‐0.10 (‐0.63, 0.44) | |
| SD ABA IV + MTX | ‐0.20 (‐0.81, 0.41) | |
| SD RITUX IV + MTX | ‐0.24 (‐0.84, 0.37) | |
| LD RITUX IV + MTX | ‐0.24 (‐0.85, 0.38) | |
| HD INF IV + MTX | 0.12 (‐0.50, 0.70) | |
| SD INF IV + MTX | SD ETN SC | ‐0.44 (‐1.25, 0.35) |
| SD ADA SC + MTX | ‐0.71 (‐1.50, 0.09) | |
| SD ETN SC + MTX | ‐0.50 (‐1.31, 0.33) | |
| SD ABA IV + MTX | ‐0.60 (‐1.46, 0.26) | |
| SD RITUX IV + MTX | ‐0.64 (‐1.49, 0.22) | |
| LD RITUX IV + MTX | ‐0.64 (‐1.50, 0.22) | |
| HD INF IV + MTX | ‐0.28 (‐1.14, 0.56) | |
| SD ADA SC + MTX | SD INF IV + MTX | ‐0.27 (‐0.66, 0.17) |
| SD ETN SC + MTX | ‐0.07 (‐0.49, 0.42) | |
| SD ABA IV + MTX | ‐0.17 (‐0.67, 0.41) | |
| SD RITUX IV + MTX | ‐0.21 (‐0.72, 0.37) | |
| LD RITUX IV + MTX | ‐0.21 (‐0.71, 0.36) | |
| HD INF IV + MTX | 0.15 (‐0.25, 0.60) | |
| SD ETN SC + MTX | SD ADA SC + MTX | 0.21 (‐0.23, 0.65) |
| SD ABA IV + MTX | 0.10 (‐0.43, 0.63) | |
| SD RITUX IV + MTX | 0.06 (‐0.47, 0.59) | |
| LD RITUX IV + MTX | 0.06 (‐0.46, 0.59) | |
| HD INF IV + MTX | 0.43 (‐0.10, 0.93) | |
| SD ABA IV + MTX | SD ETN SC + MTX | ‐0.10 (‐0.67, 0.46) |
| SD RITUX IV + MTX | ‐0.14 (‐0.70, 0.42) | |
| LD RITUX IV + MTX | ‐0.14 (‐0.70, 0.41) | |
| HD INF IV + MTX | 0.22 (‐0.33, 0.75) | |
| SD RITUX IV + MTX | SD ABA IV + MTX | ‐0.04 (‐0.67, 0.60) |
| LD RITUX IV + MTX | ‐0.04 (‐0.67, 0.59) | |
| HD INF IV + MTX | 0.32 (‐0.32, 0.91) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 0.00 (‐0.44, 0.45) |
| HD INF IV + MTX | 0.36 (‐0.26, 0.96) | |
| HD INF IV + MTX | LD RITUX IV + MTX | 0.36 (‐0.27, 0.96) |
| Random‐effects model | Total Residual deviance | 30.22 vs 31 data‐points |
| Deviance information criteria | ‐69.557 | |
| Fixed‐effect model | Total Residual deviance | 43.38 vs 31 data‐points |
| Deviance information criteria | ‐60.97 | |
| ABA: abatacept ADA: adalimumab CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous LD: low dose MD: mean difference MP: methylprednisolone MTX: methotrexate RITUX: rituximab SC: subcutaneous SD: standard dose | ||
Appendix 9. Subgroup analysis: HAQ, early RA
| Treatment | Reference | MD (95% CrI) |
| MP + MTX | MTX + PL | ‐0.04 (‐1.58, 1.51) |
| SD INF IV + MTX | 0.09 (‐1.01, 1.17) | |
| SD ETN SC + MTX | ‐0.09 (‐1.62, 1.46) | |
| SD RITUX IV + MTX | ‐0.24 (‐1.75, 1.28) | |
| LD RITUX IV + MTX | ‐0.24 (‐1.77, 1.29) | |
| HD INF IV + MTX | 0.19 (‐1.26, 1.61) | |
| SD INF IV + MTX | MP + MTX | 0.13 (‐0.98, 1.20) |
| SD ETN SC + MTX | ‐0.05 (‐2.23, 2.14) | |
| SD RITUX IV + MTX | ‐0.20 (‐2.37, 1.97) | |
| LD RITUX IV + MTX | ‐0.20 (‐2.35, 1.98) | |
| HD INF IV + MTX | 0.23 (‐1.58, 2.01) | |
| SD ETN SC + MTX | SD INF IV + MTX | ‐0.18 (‐2.04, 1.72) |
| SD RITUX IV + MTX | ‐0.33 (‐2.17, 1.55) | |
| LD RITUX IV + MTX | ‐0.33 (‐2.18, 1.57) | |
| HD INF IV + MTX | 0.10 (‐1.33, 1.54) | |
| SD RITUX IV + MTX | SD ETN SC + MTX | ‐0.15 (‐2.32, 2.01) |
| LD RITUX IV + MTX | ‐0.15 (‐2.32, 2.02) | |
| HD INF IV + MTX | 0.28 (‐1.83, 2.37) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 0.00 (‐1.51, 1.53) |
| HD INF IV + MTX | 0.43 (‐1.64, 2.49) | |
| HD INF IV + MTX | LD RITUX IV + MTX | 0.43 (‐1.66, 2.50) |
| Random‐effects model | Residual deviance | 13.9 vs 14 data‐points |
| Deviance information criteria | ‐39.969 | |
| Fixed‐effect model | Residual deviance | 25.44 vs 14 data‐points |
| Deviance information criteria | ‐30.231 | |
| Note: | ||
| Total participants | 2068 | |
| Total studies | 6 | |
| 2‐arm | 4 | |
| 3‐arm | 2 | |
| CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous LD: low dose MD: mean difference MP: methylprednisolone MTX: methotrexate PL: placebo RITUX: rituximab SC: subcutaneous SD: standard dose | ||
Appendix 10. Subgroup analysis: HAQ, established RA
| Treatment | Reference | MD (95% CrI) |
| SD ADA SC | MTX + PL | 0.00 (‐0.48, 0.48) |
| SD ADA SC + MTX | ‐0.30 (‐0.61, 0.01) | |
| SD INF IV + MTX | ‐0.31 (‐0.87, 0.19) | |
| SD ETN SC + MTX | ‐0.10 (‐0.61, 0.43) | |
| SD ABA IV + MTX | ‐0.20 (‐0.70, 0.31) | |
| SD ADA SC + MTX | SD ADA SC | ‐0.30 (‐0.78, 0.17) |
| SD INF IV + MTX | ‐0.31 (‐1.04, 0.35) | |
| SD ETN SC + MTX | ‐0.10 (‐0.80, 0.62) | |
| SD ABA IV + MTX | ‐0.20 (‐0.89, 0.50) | |
| SD INF IV + MTX | SD ADA SC + MTX | ‐0.01 (‐0.65, 0.56) |
| SD ETN SC + MTX | 0.20 (‐0.40, 0.81) | |
| SD ABA IV + MTX | 0.10 (‐0.48, 0.69) | |
| SD ETN SC + MTX | SD INF IV + MTX | 0.21 (‐0.49, 0.99) |
| SD ABA IV + MTX | 0.11 (‐0.57, 0.87) | |
| SD ABA IV + MTX | SD ETN SC + MTX | ‐0.10 (‐0.83, 0.63) |
| Random‐effects model | Residual deviance | 13.06 vs 15 data‐points |
| Deviance information criteria | ‐31.935 | |
| Fixed‐effect model | Residual deviance | 12.09 vs 15 data‐points |
| Deviance information criteria | ‐33.894 | |
| Note: | ||
| Total participants | 2078 | |
| Total studies | 7 | |
| 2‐arm | 6 | |
| 3‐arm | 1 | |
| ABA: abatacept ADA: adalimumab CrI: credible interval ETN: etanercept INF: infliximab IV: intravenous MD: mean difference MTX: methotrexate PL: placebo SC: subcutaneous SD: standard dose | ||
Appendix 11. Subgroup analysis 2: HAQ, trial duration ≤ 6 months
| Treatment | Reference | MD (95% CrI) |
| MP + MTX | MTX + PL | ‐0.22 (‐3.80, 3.33) |
| SD ETN SC | 0.40 (‐2.17, 2.97) | |
| SD INF IV + MTX | ‐0.31 (‐2.82, 2.19) | |
| SD ADA SC + MTX | ‐0.31 (‐2.84, 2.22) | |
| SD ETN SC | MP + MTX | 0.62 (‐3.76, 5.05) |
| SD INF IV + MTX | ‐0.09 (‐2.60, 2.45) | |
| SD ADA SC + MTX | ‐0.10 (‐4.44, 4.28) | |
| SD INF IV + MTX | SD ETN SC | ‐0.71 (‐4.31, 2.89) |
| SD ADA SC + MTX | ‐0.71 (‐4.31, 2.91) | |
| SD ADA SC + MTX | SD INF IV + MTX | 0.00 (‐3.56, 3.63) |
| Random‐effects model | Residual deviance | 8.01 vs 8 data‐points |
| Deviance information criteria | ‐3.373 | |
| Fixed‐effect model | Residual deviance | 8.012 vs 8 data‐points |
| Deviance information criteria | ‐3.369 | |
| Note: | ||
| Total participants | 243 | |
| Total studies | 4 | |
| 2‐arm | 4 | |
| ADA: adalimumab CrI: credible interval ETN: etanercept INF: infliximab IV: intravenous MD: mean difference MP: methylprednisolone MTX: methotrexate PL: placebo SC: subcutaneous SD: standard dose | ||
Appendix 12. Subgroup analysis 2: HAQ, trial duration 6‐12 months
| Treatment | Reference | MD (95% CrI) |
| MP + MTX | MTX + PL | ‐0.21 (‐1.28, 0.78) |
| SD INF IV + MTX | 0.11 (‐0.54, 0.69) | |
| SD ETN SC + MTX | ‐0.10 (‐0.68, 0.48) | |
| SD ABA IV + MTX | ‐0.20 (‐1.03, 0.62) | |
| HD INF IV + MTX | 0.20 (‐0.60, 0.94) | |
| SD INF IV + MTX | MP + MTX | 0.32 (‐0.49, 1.16) |
| SD ETN SC + MTX | 0.11 (‐1.05, 1.36) | |
| SD ABA IV + MTX | 0.01 (‐1.27, 1.36) | |
| HD INF IV + MTX | 0.40 (‐0.71, 1.55) | |
| SD ETN SC + MTX | SD INF IV + MTX | ‐0.21 (‐1.03, 0.69) |
| SD ABA IV + MTX | ‐0.31 (‐1.31, 0.75) | |
| HD INF IV + MTX | 0.08 (‐0.68, 0.86) | |
| SD ABA IV + MTX | SD ETN SC + MTX | ‐0.10 (‐1.12, 0.91) |
| HD INF IV + MTX | 0.29 (‐0.70, 1.23) | |
| HD INF IV + MTX | SD ABA IV + MTX | 0.40 (‐0.77, 1.50) |
| Random‐effects model | Residual deviance | 12.03 vs 13 data‐points |
| Deviance information criteria | ‐40.422 | |
| Fixed‐effect model | Residual deviance | 11.12 vs 13 data‐points |
| Deviance information criteria | ‐42.312 | |
| Note: | ||
| Total participants | 2214 | |
| Total studies | 6 | |
| 2‐arm | 5 | |
| 3‐arm | 1 | |
| ABA: abatacept CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous MD: mean difference MP: methylprednisolone MTX: methotrexate PL: placebo SC: subcutaneous SD: standard dose | ||
Appendix 13. Subgroup analysis 2: HAQ, trial duration > 12 months
| Treatment | Reference | MD (95% CrI) |
| SD ADA SC | MTX + PL | 0.00 (‐1.58, 1.56) |
| SD ADA SC + MTX | ‐0.30 (‐1.50, 0.88) | |
| SD INF IV + MTX | ‐0.52 (‐2.63, 1.58) | |
| SD RITUX IV + MTX | ‐0.24 (‐1.93, 1.45) | |
| LD RITUX IV + MTX | ‐0.24 (‐1.89, 1.42) | |
| SD ADA SC + MTX | SD ADA SC | ‐0.30 (‐1.88, 1.28) |
| SD INF IV + MTX | ‐0.52 (‐3.07, 2.07) | |
| SD RITUX IV + MTX | ‐0.24 (‐2.53, 2.08) | |
| LD RITUX IV + MTX | ‐0.24 (‐2.51, 2.06) | |
| SD INF IV + MTX | SD ADA SC + MTX | ‐0.22 (‐2.60, 2.18) |
| SD RITUX IV + MTX | 0.06 (‐1.99, 2.15) | |
| LD RITUX IV + MTX | 0.06 (‐1.98, 2.11) | |
| SD RITUX IV + MTX | SD INF IV + MTX | 0.28 (‐2.40, 2.93) |
| LD RITUX IV + MTX | 0.28 (‐2.36, 2.92) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 0.00 (‐1.67, 1.68) |
| Random‐effects model | Residual deviance | 9.734 vs 10 data‐points |
| Deviance information criteria | ‐25.052 | |
| Fixed‐effect model | Residual deviance | 9.012 vs 10 data‐points |
| Deviance information criteria | ‐26.494 | |
| Note: | ||
| Total participants | 1715 | |
| Total studies | 4 | |
| 2‐arm | 2 | |
| 3‐arm | 2 | |
| ADA: adalimumab CrI: credible interval INF: infliximab IV: intravenous LD: low dose MD: mean difference MTX: methotrexate PL: placebo RITUX: rituximab SC: subcutaneous SD: standard dose | ||
Appendix 14. Remission: main analysis
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| MP + MTX | MTX | 1.90 (0.75, 5.06) | 1.54 (0.80, 2.52) | 0.14 (‐0.05, 0.38) |
| SD ADA SC | 0.95 (0.51, 1.78) | 0.96 (0.58, 1.49) | ‐0.01 (‐0.11, 0.12) | |
| SD INF IV + MTX | 1.82 (1.13, 3.49) | 1.50 (1.09, 2.18) | 0.13 (0.02, 0.29) | |
| SD ADA SC + MTX | 2.55 (1.74, 3.76) | 1.81 (1.45, 2.24) | 0.21 (0.12, 0.31) | |
| SD ABA IV + MTX | 2.33 (1.18, 4.64) | 1.73 (1.13, 2.42) | 0.19 (0.03, 0.36) | |
| SD ADA + MTX | 2.26 (0.98, 5.26) | 1.70 (0.99, 2.54) | 0.18 (0.00, 0.39) | |
| SD ETN SC + MTX | 1.39 (0.54, 3.62) | 1.26 (0.61, 2.18) | 0.07 (‐0.10, 0.30) | |
| SD GOLI SC + MTX | 2.69 (1.18, 6.23) | 1.87 (1.13, 2.70) | 0.23 (0.03, 0.43) | |
| SD RITUX IV + MTX | 3.22 (1.56, 6.70) | 2.04 (1.36, 2.77) | 0.27 (0.09, 0.44) | |
| LD RITUX IV + MTX | 3.55 (1.72, 7.32) | 2.13 (1.45, 2.84) | 0.29 (0.12, 0.46) | |
| HD GOLI SC | 1.49 (0.63, 3.50) | 1.32 (0.70, 2.15) | 0.08 (‐0.08, 0.29) | |
| HD GOLI SC + MTX | 1.93 (0.84, 4.49) | 1.55 (0.87, 2.40) | 0.14 (‐0.03, 0.35) | |
| HD INF IV + MTX | 2.80 (1.55, 5.70) | 1.91 (1.35, 2.64) | 0.24 (0.09, 0.40) | |
| SD ADA SC | MP + MTX | 0.50 (0.16, 1.53) | 0.62 (0.31, 1.36) | ‐0.15 (‐0.40, 0.08) |
| SD INF IV + MTX | 0.96 (0.45, 2.28) | 0.98 (0.66, 1.76) | ‐0.01 (‐0.20, 0.17) | |
| SD ADA SC + MTX | 1.34 (0.47, 3.73) | 1.18 (0.70, 2.34) | 0.07 (‐0.18, 0.29) | |
| SD ABA IV + MTX | 1.23 (0.37, 3.89) | 1.12 (0.59, 2.31) | 0.05 (‐0.23, 0.31) | |
| SD ADA + MTX | 1.19 (0.32, 4.16) | 1.11 (0.53, 2.35) | 0.04 (‐0.27, 0.32) | |
| SD ETN SC + MTX | 0.73 (0.18, 2.81) | 0.82 (0.34, 1.92) | ‐0.07 (‐0.37, 0.23) | |
| SD GOLI SC + MTX | 1.42 (0.39, 4.89) | 1.21 (0.61, 2.52) | 0.08 (‐0.22, 0.36) | |
| SD RITUX IV + MTX | 1.70 (0.49, 5.58) | 1.32 (0.71, 2.70) | 0.13 (‐0.17, 0.39) | |
| LD RITUX IV + MTX | 1.88 (0.55, 6.10) | 1.39 (0.75, 2.80) | 0.15 (‐0.15, 0.41) | |
| HD GOLI SC | 0.78 (0.21, 2.73) | 0.86 (0.38, 1.90) | ‐0.06 (‐0.34, 0.22) | |
| HD GOLI SC + MTX | 1.02 (0.28, 3.57) | 1.01 (0.48, 2.18) | 0.00 (‐0.29, 0.29) | |
| HD INF IV + MTX | 1.47 (0.55, 4.17) | 1.24 (0.75, 2.39) | 0.09 (‐0.14, 0.32) | |
| SD INF IV + MTX | SD ADA SC | 1.91 (0.92, 4.93) | 1.56 (0.95, 3.01) | 0.14 (‐0.02, 0.33) |
| SD ADA SC + MTX | 2.69 (1.44, 5.02) | 1.89 (1.24, 3.06) | 0.22 (0.09, 0.33) | |
| SD ABA IV + MTX | 2.47 (0.97, 6.19) | 1.80 (0.98, 3.27) | 0.20 (‐0.01, 0.39) | |
| SD ADA + MTX | 2.39 (0.84, 6.85) | 1.77 (0.89, 3.37) | 0.19 (‐0.04, 0.42) | |
| SD ETN SC + MTX | 1.47 (0.47, 4.63) | 1.31 (0.57, 2.77) | 0.08 (‐0.14, 0.33) | |
| SD GOLI SC + MTX | 2.85 (1.01, 8.07) | 1.94 (1.01, 3.62) | 0.23 (0.00, 0.46) | |
| SD RITUX IV + MTX | 3.40 (1.31, 8.94) | 2.12 (1.18, 3.81) | 0.28 (0.06, 0.48) | |
| LD RITUX IV + MTX | 3.75 (1.43, 9.77) | 2.21 (1.25, 3.94) | 0.30 (0.08, 0.50) | |
| HD GOLI SC | 1.57 (0.54, 4.52) | 1.37 (0.64, 2.76) | 0.09 (‐0.12, 0.32) | |
| HD GOLI SC + MTX | 2.03 (0.72, 5.81) | 1.61 (0.79, 3.13) | 0.15 (‐0.06, 0.38) | |
| HD INF IV + MTX | 2.96 (1.27, 7.72) | 1.98 (1.16, 3.65) | 0.24 (0.05, 0.44) | |
| SD ADA SC + MTX | SD INF IV + MTX | 1.40 (0.64, 2.52) | 1.21 (0.79, 1.72) | 0.08 (‐0.11, 0.22) |
| SD ABA IV + MTX | 1.29 (0.48, 2.82) | 1.16 (0.64, 1.76) | 0.06 (‐0.17, 0.25) | |
| SD ADA + MTX | 1.24 (0.42, 3.18) | 1.13 (0.58, 1.84) | 0.05 (‐0.20, 0.28) | |
| SD ETN SC + MTX | 0.76 (0.23, 2.15) | 0.84 (0.36, 1.54) | ‐0.06 (‐0.30, 0.18) | |
| SD GOLI SC + MTX | 1.48 (0.50, 3.75) | 1.24 (0.66, 1.96) | 0.09 (‐0.16, 0.32) | |
| SD RITUX IV + MTX | 1.78 (0.64, 4.01) | 1.36 (0.78, 2.03) | 0.14 (‐0.11, 0.33) | |
| LD RITUX IV + MTX | 1.96 (0.70, 4.43) | 1.42 (0.82, 2.10) | 0.17 (‐0.09, 0.35) | |
| HD GOLI SC | 0.81 (0.27, 2.10) | 0.88 (0.41, 1.53) | ‐0.05 (‐0.28, 0.18) | |
| HD GOLI SC + MTX | 1.06 (0.36, 2.71) | 1.04 (0.51, 1.73) | 0.01 (‐0.23, 0.24) | |
| HD INF IV + MTX | 1.55 (0.77, 2.74) | 1.27 (0.86, 1.70) | 0.11 (‐0.06, 0.24) | |
| SD ABA IV + MTX | SD ADA SC + MTX | 0.92 (0.42, 2.01) | 0.95 (0.59, 1.41) | ‐0.02 (‐0.20, 0.17) |
| SD ADA + MTX | 0.89 (0.35, 2.25) | 0.94 (0.53, 1.47) | ‐0.03 (‐0.24, 0.20) | |
| SD ETN SC + MTX | 0.55 (0.20, 1.54) | 0.70 (0.33, 1.25) | ‐0.14 (‐0.34, 0.11) | |
| SD GOLI SC + MTX | 1.06 (0.43, 2.63) | 1.03 (0.60, 1.56) | 0.01 (‐0.20, 0.23) | |
| SD RITUX IV + MTX | 1.26 (0.56, 2.90) | 1.12 (0.72, 1.62) | 0.06 (‐0.14, 0.25) | |
| LD RITUX IV + MTX | 1.39 (0.61, 3.17) | 1.17 (0.77, 1.66) | 0.08 (‐0.12, 0.27) | |
| HD GOLI SC | 0.58 (0.23, 1.49) | 0.73 (0.37, 1.23) | ‐0.13 (‐0.32, 0.10) | |
| HD GOLI SC + MTX | 0.76 (0.30, 1.90) | 0.86 (0.47, 1.37) | ‐0.07 (‐0.27, 0.16) | |
| HD INF IV + MTX | 1.10 (0.55, 2.48) | 1.05 (0.72, 1.54) | 0.02 (‐0.15, 0.22) | |
| SD ADA + MTX | SD ABA IV + MTX | 0.97 (0.33, 2.87) | 0.98 (0.53, 1.76) | ‐0.01 (‐0.26, 0.25) |
| SD ETN SC + MTX | 0.60 (0.18, 1.94) | 0.73 (0.33, 1.45) | ‐0.12 (‐0.36, 0.16) | |
| SD GOLI SC + MTX | 1.15 (0.40, 3.38) | 1.08 (0.60, 1.88) | 0.03 (‐0.22, 0.29) | |
| SD RITUX IV + MTX | 1.38 (0.52, 3.75) | 1.18 (0.71, 1.97) | 0.08 (‐0.16, 0.31) | |
| LD RITUX IV + MTX | 1.52 (0.56, 4.13) | 1.23 (0.75, 2.05) | 0.10 (‐0.14, 0.33) | |
| HD GOLI SC | 0.64 (0.21, 1.90) | 0.76 (0.38, 1.44) | ‐0.11 (‐0.34, 0.15) | |
| HD GOLI SC + MTX | 0.82 (0.28, 2.43) | 0.90 (0.47, 1.63) | ‐0.05 (‐0.29, 0.21) | |
| HD INF IV + MTX | 1.20 (0.49, 3.25) | 1.10 (0.70, 1.88) | 0.05 (‐0.17, 0.28) | |
| SD ETN SC + MTX | SD ADA + MTX | 0.61 (0.17, 2.22) | 0.74 (0.32, 1.61) | ‐0.11 (‐0.38, 0.18) |
| SD GOLI SC + MTX | 1.20 (0.36, 3.86) | 1.10 (0.58, 2.09) | 0.04 (‐0.24, 0.32) | |
| SD RITUX IV + MTX | 1.42 (0.47, 4.27) | 1.20 (0.69, 2.20) | 0.09 (‐0.18, 0.34) | |
| LD RITUX IV + MTX | 1.57 (0.52, 4.66) | 1.25 (0.73, 2.27) | 0.11 (‐0.16, 0.36) | |
| HD GOLI SC | 0.66 (0.19, 2.18) | 0.78 (0.37, 1.60) | ‐0.10 (‐0.36, 0.18) | |
| HD GOLI SC + MTX | 0.85 (0.26, 2.79) | 0.91 (0.46, 1.81) | ‐0.04 (‐0.31, 0.24) | |
| HD INF IV + MTX | 1.24 (0.44, 3.72) | 1.12 (0.67, 2.09) | 0.05 (‐0.20, 0.31) | |
| SD GOLI SC + MTX | SD ETN SC + MTX | 1.93 (0.54, 6.90) | 1.47 (0.71, 3.32) | 0.15 (‐0.14, 0.42) |
| SD RITUX IV + MTX | 2.31 (0.70, 7.76) | 1.61 (0.83, 3.52) | 0.20 (‐0.09, 0.45) | |
| LD RITUX IV + MTX | 2.55 (0.78, 8.44) | 1.68 (0.88, 3.66) | 0.22 (‐0.06, 0.47) | |
| HD GOLI SC | 1.06 (0.29, 3.85) | 1.04 (0.45, 2.49) | 0.01 (‐0.27, 0.28) | |
| HD GOLI SC + MTX | 1.38 (0.38, 4.96) | 1.22 (0.56, 2.84) | 0.07 (‐0.22, 0.34) | |
| HD INF IV + MTX | 2.02 (0.66, 6.69) | 1.51 (0.80, 3.37) | 0.17 (‐0.10, 0.41) | |
| SD RITUX IV + MTX | SD GOLI SC + MTX | 1.20 (0.40, 3.59) | 1.09 (0.64, 1.93) | 0.04 (‐0.22, 0.30) |
| LD RITUX IV + MTX | 1.32 (0.44, 3.94) | 1.14 (0.68, 2.00) | 0.07 (‐0.20, 0.32) | |
| HD GOLI SC | 0.55 (0.25, 1.20) | 0.71 (0.42, 1.11) | ‐0.14 (‐0.31, 0.04) | |
| HD GOLI SC + MTX | 0.72 (0.33, 1.55) | 0.83 (0.52, 1.28) | ‐0.08 (‐0.26, 0.10) | |
| HD INF IV + MTX | 1.04 (0.38, 3.14) | 1.02 (0.63, 1.85) | 0.01 (‐0.23, 0.27) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 1.10 (0.56, 2.15) | 1.05 (0.76, 1.44) | 0.02 (‐0.14, 0.18) |
| HD GOLI SC | 0.46 (0.15, 1.40) | 0.65 (0.32, 1.20) | ‐0.19 (‐0.42, 0.08) | |
| HD GOLI SC + MTX | 0.60 (0.20, 1.79) | 0.76 (0.40, 1.35) | ‐0.13 (‐0.37, 0.14) | |
| HD INF IV + MTX | 0.87 (0.35, 2.43) | 0.93 (0.61, 1.56) | ‐0.04 (‐0.25, 0.21) | |
| HD GOLI SC | LD RITUX IV + MTX | 0.42 (0.14, 1.28) | 0.62 (0.31, 1.14) | ‐0.21 (‐0.44, 0.06) |
| HD GOLI SC + MTX | 0.54 (0.18, 1.64) | 0.73 (0.39, 1.28) | ‐0.15 (‐0.39, 0.12) | |
| HD INF IV + MTX | 0.79 (0.31, 2.21) | 0.90 (0.59, 1.47) | ‐0.06 (‐0.27, 0.19) | |
| HD GOLI SC + MTX | HD GOLI SC | 1.30 (0.58, 2.91) | 1.18 (0.71, 1.99) | 0.06 (‐0.12, 0.24) |
| HD INF IV + MTX | 1.89 (0.68, 5.81) | 1.44 (0.81, 2.97) | 0.15 (‐0.09, 0.39) | |
| HD INF IV + MTX | HD GOLI SC + MTX | 1.45 (0.53, 4.39) | 1.23 (0.72, 2.37) | 0.09 (‐0.15, 0.34) |
| Random‐effects model | Residual deviance | 38.64 vs 35 data‐points | ||
| Deviance information criteria | 232.262 | |||
| Fixed‐effect model | Residual deviance | 40.19 vs 35 data‐points | ||
| Deviance information criteria | 231.958 | |||
| ABA: abatacept ADA: adalimumab CrI: credible interval ETN: etanercept GOLI: golimumab HD: high dose INF: infliximab IV: intravenous LD: low dose MP: methylprednisolone MTX: methotrexate OR: odds ratio RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 15. Subgroup analysis: Remission, early RA
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| MP + MTX | MTX + PL | 1.49 (0.38, 4.45) | 1.25 (0.50, 1.95) | 0.10 (‐0.20, 0.35) |
| SD INF IV + MTX | 1.46 (0.67, 3.13) | 1.24 (0.77, 1.75) | 0.09 (‐0.09, 0.28) | |
| SD ADA + MTX | 2.26 (0.79, 6.50) | 1.52 (0.86, 2.14) | 0.20 (‐0.05, 0.42) | |
| SD ETN SC + MTX | 1.39 (0.44, 4.40) | 1.21 (0.56, 1.94) | 0.08 (‐0.17, 0.35) | |
| SD RITUX IV + MTX | 3.20 (1.24, 8.66) | 1.73 (1.13, 2.25) | 0.28 (0.05, 0.46) | |
| LD RITUX IV + MTX | 3.54 (1.35, 9.45) | 1.79 (1.19, 2.29) | 0.30 (0.07, 0.47) | |
| HD INF IV + MTX | 2.48 (0.99, 6.11) | 1.58 (1.00, 2.10) | 0.22 (0.00, 0.41) | |
| SD INF IV + MTX | MP + MTX | 0.98 (0.42, 2.86) | 0.99 (0.69, 1.97) | 0.00 (‐0.20, 0.22) |
| SD ADA + MTX | 1.52 (0.35, 9.05) | 1.21 (0.62, 3.14) | 0.10 (‐0.24, 0.47) | |
| SD ETN SC + MTX | 0.94 (0.20, 5.81) | 0.97 (0.42, 2.65) | ‐0.02 (‐0.37, 0.39) | |
| SD RITUX IV + MTX | 2.15 (0.54, 12.31) | 1.37 (0.79, 3.49) | 0.18 (‐0.14, 0.53) | |
| LD RITUX IV + MTX | 2.38 (0.59, 13.57) | 1.42 (0.82, 3.57) | 0.20 (‐0.12, 0.55) | |
| HD INF IV + MTX | 1.66 (0.53, 7.12) | 1.25 (0.78, 2.89) | 0.12 (‐0.14, 0.43) | |
| SD ADA + MTX | SD INF IV + MTX | 1.55 (0.42, 5.82) | 1.22 (0.64, 2.16) | 0.11 (‐0.20, 0.39) |
| SD ETN SC + MTX | 0.95 (0.24, 3.89) | 0.98 (0.43, 1.88) | ‐0.01 (‐0.32, 0.31) | |
| SD RITUX IV + MTX | 2.19 (0.65, 7.86) | 1.39 (0.83, 2.34) | 0.19 (‐0.10, 0.44) | |
| LD RITUX IV + MTX | 2.42 (0.71, 8.65) | 1.44 (0.86, 2.41) | 0.21 (‐0.08, 0.46) | |
| HD INF IV + MTX | 1.69 (0.71, 4.18) | 1.27 (0.85, 1.89) | 0.13 (‐0.08, 0.32) | |
| SD ETN SC + MTX | SD ADA + MTX | 0.62 (0.13, 2.89) | 0.80 (0.36, 1.63) | ‐0.12 (‐0.45, 0.24) |
| SD RITUX IV + MTX | 1.41 (0.35, 6.03) | 1.14 (0.68, 2.07) | 0.08 (‐0.23, 0.38) | |
| LD RITUX IV + MTX | 1.57 (0.37, 6.56) | 1.17 (0.71, 2.12) | 0.10 (‐0.21, 0.40) | |
| HD INF IV + MTX | 1.09 (0.27, 4.34) | 1.04 (0.60, 1.91) | 0.02 (‐0.28, 0.33) | |
| SD RITUX IV + MTX | SD ETN SC + MTX | 2.30 (0.53, 10.57) | 1.42 (0.78, 3.12) | 0.20 (‐0.14, 0.51) |
| LD RITUX IV + MTX | 2.55 (0.58, 11.78) | 1.47 (0.81, 3.20) | 0.22 (‐0.12, 0.52) | |
| HD INF IV + MTX | 1.77 (0.41, 7.66) | 1.30 (0.69, 2.84) | 0.14 (‐0.20, 0.45) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 1.10 (0.43, 2.80) | 1.03 (0.75, 1.42) | 0.02 (‐0.17, 0.21) |
| HD INF IV + MTX | 0.77 (0.20, 2.82) | 0.91 (0.55, 1.48) | ‐0.06 (‐0.34, 0.23) | |
| HD INF IV + MTX | LD RITUX IV + MTX | 0.70 (0.18, 2.60) | 0.88 (0.54, 1.41) | ‐0.08 (‐0.35, 0.21) |
| Random‐effects model | Residual deviance | 17.42 vs 16 data‐points | ||
| Deviance information criteria | 107.304 | |||
| Fixed‐effect model | Residual deviance | 18.28 vs 16 data‐points | ||
| Deviance information criteria | 107.435 | |||
| Note: | ||||
| Total participants | 2313 | |||
| Total studies | 7 | |||
| 2‐arm | 5 | |||
| 3‐arm | 2 | |||
| ADA: adalimumab CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous LD: low dose MP: methylprednisolone MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 16. Subgroup analysis: Remission, established RA
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC | MTX + PL | 0.95 (0.55, 1.64) | 0.96 (0.60, 1.48) | ‐0.01 (‐0.07, 0.08) |
| SD INF IV + MTX | 7.01 (1.84, 32.99) | 3.46 (1.58, 6.40) | 0.42 (0.11, 0.70) | |
| SD ADA SC + MTX | 2.55 (1.81, 3.55) | 2.02 (1.58, 2.55) | 0.17 (0.10, 0.25) | |
| SD ABA IV + MTX | 2.32 (1.29, 4.20) | 1.90 (1.23, 2.77) | 0.15 (0.04, 0.29) | |
| SD GOLI SC + MTX | 2.67 (1.25, 5.78) | 2.08 (1.20, 3.30) | 0.18 (0.03, 0.37) | |
| HD GOLI SC | 1.48 (0.67, 3.32) | 1.37 (0.71, 2.42) | 0.06 (‐0.05, 0.23) | |
| HD GOLI SC + MTX | 1.91 (0.89, 4.24) | 1.65 (0.91, 2.79) | 0.11 (‐0.02, 0.29) | |
| SD INF IV + MTX | SD ADA SC | 7.44 (1.72, 37.88) | 3.62 (1.48, 7.89) | 0.42 (0.10, 0.71) |
| SD ADA SC + MTX | 2.69 (1.56, 4.57) | 2.11 (1.38, 3.32) | 0.18 (0.08, 0.26) | |
| SD ABA IV + MTX | 2.46 (1.11, 5.46) | 1.98 (1.08, 3.62) | 0.16 (0.02, 0.31) | |
| SD GOLI SC + MTX | 2.82 (1.12, 7.24) | 2.17 (1.09, 4.17) | 0.19 (0.02, 0.38) | |
| HD GOLI SC | 1.56 (0.60, 4.11) | 1.43 (0.65, 2.97) | 0.07 (‐0.07, 0.25) | |
| HD GOLI SC + MTX | 2.02 (0.79, 5.25) | 1.73 (0.83, 3.48) | 0.12 (‐0.04, 0.31) | |
| SD ADA SC + MTX | SD INF IV + MTX | 0.36 (0.07, 1.45) | 0.58 (0.32, 1.27) | ‐0.25 (‐0.56, 0.08) |
| SD ABA IV + MTX | 0.33 (0.06, 1.41) | 0.55 (0.27, 1.24) | ‐0.26 (‐0.59, 0.08) | |
| SD GOLI SC + MTX | 0.38 (0.07, 1.81) | 0.60 (0.27, 1.41) | ‐0.23 (‐0.58, 0.14) | |
| HD GOLI SC | 0.21 (0.04, 1.02) | 0.40 (0.16, 1.01) | ‐0.35 (‐0.67, 0.01) | |
| HD GOLI SC + MTX | 0.27 (0.05, 1.30) | 0.48 (0.21, 1.18) | ‐0.30 (‐0.63, 0.06) | |
| SD ABA IV + MTX | SD ADA SC + MTX | 0.91 (0.47, 1.80) | 0.94 (0.58, 1.45) | ‐0.02 (‐0.16, 0.14) |
| SD GOLI SC + MTX | 1.05 (0.46, 2.43) | 1.03 (0.58, 1.70) | 0.01 (‐0.16, 0.21) | |
| HD GOLI SC | 0.58 (0.25, 1.40) | 0.68 (0.34, 1.24) | ‐0.11 (‐0.25, 0.07) | |
| HD GOLI SC + MTX | 0.75 (0.33, 1.78) | 0.82 (0.44, 1.44) | ‐0.06 (‐0.21, 0.13) | |
| SD GOLI SC + MTX | SD ABA IV + MTX | 1.15 (0.44, 3.03) | 1.10 (0.57, 2.04) | 0.03 (‐0.17, 0.25) |
| HD GOLI SC | 0.63 (0.24, 1.72) | 0.72 (0.34, 1.45) | ‐0.09 (‐0.27, 0.11) | |
| HD GOLI SC + MTX | 0.82 (0.31, 2.19) | 0.87 (0.43, 1.69) | ‐0.04 (‐0.23, 0.17) | |
| HD GOLI SC | SD GOLI SC + MTX | 0.55 (0.27, 1.14) | 0.66 (0.38, 1.09) | ‐0.12 (‐0.27, 0.03) |
| HD GOLI SC + MTX | 0.71 (0.36, 1.45) | 0.80 (0.49, 1.28) | ‐0.07 (‐0.22, 0.08) | |
| HD GOLI SC + MTX | HD GOLI SC | 1.30 (0.62, 2.70) | 1.21 (0.70, 2.11) | 0.05 (‐0.09, 0.19) |
| Random‐effects model | Residual deviance | 18.13 vs 19 data‐points | ||
| Deviance information criteria | 122.691 | |||
| Fixed‐effect model | Residual deviance | 18.28 vs 19 data‐points | ||
| Deviance information criteria | 121.942 | |||
| Note: | ||||
| Total participants | 3248 | |||
| Total studies | 8 | |||
| 2‐arm | 6 | |||
| 3‐arm | 1 | |||
| 4‐arm | 1 | |||
| ABA: abatacept ADA: adalimumab CrI: credible interval GOLI: golimumab HD: high dose INF: infliximab IV: intravenous MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 17. Subgroup analysis 2: Remission, trial duration ≤ 6 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| MP + MTX | MTX + PL | 8.44 (1.18, 71.50) | 3.78 (1.14, 7.10) | 0.46 (0.03, 0.78) |
| SD INF IV + MTX | 5.35 (1.00, 34.69) | 3.11 (1.00, 6.51) | 0.35 (0.00, 0.71) | |
| SD ADA SC + MTX | 2.67 (1.47, 5.77) | 2.10 (1.36, 3.37) | 0.18 (0.06, 0.36) | |
| SD ADA + MTX | 2.27 (0.88, 5.98) | 1.88 (0.90, 3.40) | 0.14 (‐0.02, 0.37) | |
| SD GOLI SC + MTX | 2.70 (1.07, 6.93) | 2.11 (1.06, 3.69) | 0.18 (0.01, 0.40) | |
| HD GOLI SC | 1.49 (0.56, 3.95) | 1.38 (0.61, 2.73) | 0.06 (‐0.07, 0.26) | |
| HD GOLI SC + MTX | 1.93 (0.76, 5.10) | 1.68 (0.79, 3.14) | 0.11 (‐0.04, 0.33) | |
| SD INF IV + MTX | MP + MTX | 0.64 (0.22, 1.80) | 0.85 (0.51, 1.39) | ‐0.09 (‐0.31, 0.12) |
| SD ADA SC + MTX | 0.32 (0.04, 2.66) | 0.56 (0.28, 1.93) | ‐0.27 (‐0.64, 0.20) | |
| SD ADA + MTX | 0.27 (0.03, 2.42) | 0.50 (0.19, 1.80) | ‐0.31 (‐0.69, 0.18) | |
| SD GOLI SC + MTX | 0.32 (0.03, 2.86) | 0.56 (0.23, 1.97) | ‐0.27 (‐0.66, 0.22) | |
| HD GOLI SC | 0.17 (0.02, 1.60) | 0.37 (0.13, 1.39) | ‐0.39 (‐0.75, 0.09) | |
| HD GOLI SC + MTX | 0.23 (0.02, 2.06) | 0.45 (0.17, 1.63) | ‐0.34 (‐0.71, 0.14) | |
| SD ADA SC + MTX | SD INF IV + MTX | 0.51 (0.07, 3.18) | 0.68 (0.30, 2.22) | ‐0.16 (‐0.56, 0.24) |
| SD ADA + MTX | 0.42 (0.05, 2.98) | 0.61 (0.22, 2.06) | ‐0.20 (‐0.61, 0.22) | |
| SD GOLI SC + MTX | 0.50 (0.06, 3.48) | 0.68 (0.26, 2.26) | ‐0.16 (‐0.58, 0.26) | |
| HD GOLI SC | 0.28 (0.03, 1.95) | 0.45 (0.15, 1.60) | ‐0.28 (‐0.67, 0.13) | |
| HD GOLI SC + MTX | 0.36 (0.05, 2.49) | 0.54 (0.19, 1.87) | ‐0.23 (‐0.63, 0.18) | |
| SD ADA + MTX | SD ADA SC + MTX | 0.85 (0.25, 2.55) | 0.89 (0.37, 1.78) | ‐0.04 (‐0.28, 0.21) |
| SD GOLI SC + MTX | 1.00 (0.29, 2.97) | 1.00 (0.43, 1.93) | 0.00 (‐0.25, 0.25) | |
| HD GOLI SC | 0.55 (0.16, 1.69) | 0.65 (0.25, 1.42) | ‐0.12 (‐0.34, 0.11) | |
| HD GOLI SC + MTX | 0.72 (0.21, 2.18) | 0.80 (0.32, 1.64) | ‐0.07 (‐0.30, 0.17) | |
| SD GOLI SC + MTX | SD ADA + MTX | 1.19 (0.31, 4.58) | 1.12 (0.46, 2.78) | 0.04 (‐0.25, 0.31) |
| HD GOLI SC | 0.65 (0.17, 2.57) | 0.73 (0.27, 1.99) | ‐0.08 (‐0.34, 0.18) | |
| HD GOLI SC + MTX | 0.85 (0.22, 3.31) | 0.89 (0.35, 2.32) | ‐0.03 (‐0.30, 0.24) | |
| HD GOLI SC | SD GOLI SC + MTX | 0.55 (0.22, 1.36) | 0.66 (0.33, 1.24) | ‐0.11 (‐0.29, 0.06) |
| HD GOLI SC + MTX | 0.72 (0.29, 1.77) | 0.80 (0.42, 1.47) | ‐0.07 (‐0.25, 0.11) | |
| HD GOLI SC + MTX | HD GOLI SC | 1.30 (0.52, 3.29) | 1.22 (0.62, 2.43) | 0.05 (‐0.12, 0.22) |
| Random‐effects model | Residual deviance | 13.72 vs 14 data‐points | ||
| Deviance information criteria | 91.46 | |||
| Fixed‐effect model | Residual deviance | 13.64 vs 14 data‐points | ||
| Deviance information criteria | 91.099 | |||
| Note: | ||||
| Total participants | 2058 | |||
| Total studies | 6 | |||
| 2‐arm | 5 | |||
| 4‐arm | 1 | |||
| ADA: adalimumab CrI: credible interval GOLI: golimumab HD: high dose INF: infliximab IV: intravenous MP: methylprednisolone MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 18. Subgroup analysis 2: Remission, trial duration 6‐12 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| MP + MTX | MTX + PL | 0.39 (0.04, 2.98) | 0.48 (0.06, 1.86) | ‐0.16 (‐0.30, 0.26) |
| SD INF IV + MTX | 1.52 (0.55, 4.09) | 1.31 (0.64, 2.12) | 0.09 (‐0.11, 0.34) | |
| SD ABA IV + MTX | 2.33 (0.84, 6.33) | 1.65 (0.88, 2.44) | 0.20 (‐0.04, 0.43) | |
| SD ETN SC + MTX | 1.39 (0.41, 4.64) | 1.24 (0.51, 2.26) | 0.07 (‐0.16, 0.36) | |
| HD INF IV + MTX | 2.53 (0.93, 6.85) | 1.72 (0.95, 2.50) | 0.22 (‐0.01, 0.44) | |
| SD INF IV + MTX | MP + MTX | 3.89 (0.65, 27.17) | 2.69 (0.80, 16.60) | 0.24 (‐0.10, 0.45) |
| SD ABA IV + MTX | 5.99 (0.63, 63.99) | 3.40 (0.80, 26.15) | 0.35 (‐0.10, 0.61) | |
| SD ETN SC + MTX | 3.58 (0.33, 43.76) | 2.56 (0.52, 21.27) | 0.22 (‐0.24, 0.54) | |
| HD INF IV + MTX | 6.49 (0.85, 57.16) | 3.53 (0.93, 24.88) | 0.36 (‐0.03, 0.60) | |
| SD ABA IV + MTX | SD INF IV + MTX | 1.54 (0.37, 6.33) | 1.26 (0.59, 2.74) | 0.10 (‐0.22, 0.40) |
| SD ETN SC + MTX | 0.91 (0.19, 4.38) | 0.95 (0.36, 2.32) | ‐0.02 (‐0.35, 0.33) | |
| HD INF IV + MTX | 1.67 (0.62, 4.51) | 1.31 (0.79, 2.28) | 0.12 (‐0.10, 0.33) | |
| SD ETN SC + MTX | SD ABA IV + MTX | 0.59 (0.12, 2.83) | 0.75 (0.30, 1.72) | ‐0.13 (‐0.44, 0.24) |
| HD INF IV + MTX | 1.09 (0.26, 4.55) | 1.04 (0.53, 2.07) | 0.02 (‐0.30, 0.34) | |
| HD INF IV + MTX | SD ETN SC + MTX | 1.83 (0.38, 8.55) | 1.38 (0.62, 3.42) | 0.15 (‐0.22, 0.46) |
| Random‐effects model | Residual deviance | 9.119 vs 9 data‐points | ||
| Deviance information criteria | 62.314 | |||
| Fixed‐effect model | Residual deviance | 9.107 vs 9 data‐points | ||
| Deviance information criteria | 62.28 | |||
| Note: | ||||
| Total participants | 1697 | |||
| Total studies | 4 | |||
| 2‐arm | 3 | |||
| 3‐arm | 1 | |||
| ABA: abatacept CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous MP: methylprednisolone MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 19. Subgroup analysis 2: Remission, trial duration > 12 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC | MTX + PL | 0.92 (0.36, 2.24) | 0.95 (0.45, 1.62) | ‐0.02 (‐0.17, 0.19) |
| SD INF IV + MTX | 2.25 (0.68, 9.21) | 1.61 (0.77, 2.91) | 0.19 (‐0.08, 0.50) | |
| SD ADA SC + MTX | 2.42 (1.11, 4.66) | 1.66 (1.07, 2.25) | 0.21 (0.02, 0.36) | |
| SD RITUX IV + MTX | 3.21 (1.20, 8.66) | 1.87 (1.13, 2.67) | 0.28 (0.04, 0.48) | |
| LD RITUX IV + MTX | 3.55 (1.32, 9.47) | 1.94 (1.20, 2.73) | 0.30 (0.06, 0.50) | |
| SD INF IV + MTX | SD ADA SC | 2.44 (0.58, 13.68) | 1.68 (0.72, 4.48) | 0.21 (‐0.12, 0.55) |
| SD ADA SC + MTX | 2.63 (1.02, 6.33) | 1.75 (1.01, 3.32) | 0.23 (0.00, 0.39) | |
| SD RITUX IV + MTX | 3.49 (0.93, 14.00) | 1.97 (0.96, 4.46) | 0.29 (‐0.02, 0.55) | |
| LD RITUX IV + MTX | 3.85 (1.02, 15.17) | 2.05 (1.01, 4.59) | 0.32 (0.01, 0.57) | |
| SD ADA SC + MTX | SD INF IV + MTX | 1.07 (0.21, 4.09) | 1.03 (0.51, 2.14) | 0.02 (‐0.35, 0.33) |
| SD RITUX IV + MTX | 1.44 (0.25, 6.35) | 1.17 (0.55, 2.42) | 0.09 (‐0.31, 0.42) | |
| LD RITUX IV + MTX | 1.59 (0.27, 7.02) | 1.21 (0.59, 2.50) | 0.11 (‐0.29, 0.43) | |
| SD RITUX IV + MTX | SD ADA SC + MTX | 1.32 (0.42, 4.83) | 1.13 (0.65, 1.94) | 0.07 (‐0.20, 0.35) |
| LD RITUX IV + MTX | 1.47 (0.46, 5.32) | 1.17 (0.69, 1.99) | 0.09 (‐0.18, 0.36) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 1.10 (0.42, 2.86) | 1.04 (0.71, 1.54) | 0.02 (‐0.19, 0.23) |
| Random‐effects model | Residual deviance | 13.97 vs 12 data‐points | ||
| Deviance information criteria | 80.022 | |||
| Fixed‐effect model | Residual deviance | 15.28 vs 12 data‐points | ||
| Deviance information criteria | 80.498 | |||
| Note: | ||||
| Total participants | 1806 | |||
| Total studies | 5 | |||
| 2‐arm | 3 | |||
| 3‐arm | 2 | |||
| ADA: adalimumab CrI: credible interval INF: infliximab IV: intravenous LD: low dose MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 20. Radiographic progression: main analysis
| Treatment | Reference | MD (95% CrI) |
| MP + MTX | MTX + PL | ‐2.63 (‐14.60, 9.55) |
| SD ETN SC + MTX | ‐4.00 (‐9.38, 1.64) | |
| SD INF IV + MTX | ‐3.32 (‐10.98, 4.43) | |
| SD ABA IV + MTX | ‐0.44 (‐8.20, 7.31) | |
| HD INF IV + MTX | ‐3.17 (‐10.90, 4.50) | |
| SD ETN SC + MTX | MP + MTX | ‐1.38 (‐14.62, 11.89) |
| SD INF IV + MTX | ‐0.71 (‐10.12, 8.62) | |
| SD ABA IV + MTX | 2.19 (‐12.20, 16.56) | |
| HD INF IV + MTX | ‐0.58 (‐12.77, 11.43) | |
| SD INF IV + MTX | SD ETN SC + MTX | 0.70 (‐8.89, 10.11) |
| SD ABA IV + MTX | 3.54 (‐6.06, 13.05) | |
| HD INF IV + MTX | 0.84 (‐8.77, 10.09) | |
| SD ABA IV + MTX | SD INF IV + MTX | 2.85 (‐8.09, 13.82) |
| HD INF IV + MTX | 0.10 (‐7.58, 7.76) | |
| HD INF IV + MTX | SD ABA IV + MTX | ‐2.74 (‐13.79, 8.14) |
| Random‐effects model | 11.23 Residual deviance | vs 11 data‐points |
| Deviance information criteria | 29.311 | |
| Fixed‐effect model | 42.46 Residual deviance | vs 11 data‐points |
| Deviance information criteria | 59.485 | |
| ABA: abatacept CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous MD: mean difference MP: methylprednisolone MTX: methotrexate PL: placebo SC: subcutaneous SD: standard dose | ||
Appendix 21. Subgroup analysis 2: Radiographic progression, trial duration 6‐12 months
| Treatment | Reference | MD (95% CrI) |
| SD ETN SC + MTX | MTX + PL | ‐5.50 (‐7.09, ‐3.82) |
| SD INF IV + MTX | ‐3.30 (‐5.46, ‐1.15) | |
| SD ABA IV + MTX | ‐0.43 (‐2.82, 1.96) | |
| HD INF IV + MTX | ‐3.20 (‐5.35, ‐1.06) | |
| SD INF IV + MTX | SD ETN SC + MTX | 2.21 (‐0.58, 4.84) |
| SD ABA IV + MTX | 5.08 (2.14, 7.92) | |
| HD INF IV + MTX | 2.30 (‐0.46, 4.95) | |
| SD ABA IV + MTX | SD INF IV + MTX | 2.88 (‐0.36, 6.10) |
| HD INF IV + MTX | 0.10 (‐1.84, 2.04) | |
| HD INF IV + MTX | SD ABA IV + MTX | ‐2.78 (‐5.97, 0.46) |
| Random‐effects model | Residual deviance | 17.38 vs 9 data‐points |
| Deviance information criteria | 26.794 | |
| Fixed‐effect model | Residual deviance | 40.49 vs 19 data‐points |
| Deviance information criteria | 49.323 | |
| Total participants | 2144 | |
| Total studies | 4 | |
| 2‐arm | 3 | |
| 3‐arm | 1 | |
| ABA: abatacept CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous MD: mean difference MTX: methotrexate PL: placebo SC: subcutaneous SD: standard dose | ||
Appendix 22. Withdrawals due to adverse events: main analysis
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC | MTX | 1.32 (0.60, 2.91) | 1.31 (0.61, 2.72) | 0.01 (‐0.01, 0.06) |
| SD ADA SC + MTX | 1.74 (1.00, 3.07) | 1.69 (1.00, 2.86) | 0.03 (0.00, 0.07) | |
| SD INF IV + MTX | 2.10 (1.09, 4.01) | 2.02 (1.08, 3.66) | 0.04 (0.00, 0.09) | |
| SD ETN SC + MTX | 0.89 (0.45, 2.12) | 0.89 (0.46, 2.04) | 0.00 (‐0.02, 0.04) | |
| SD ABA IV + MTX | 0.78 (0.26, 2.38) | 0.79 (0.26, 2.27) | ‐0.01 (‐0.03, 0.05) | |
| SD GOLI SC + MTX | 2.73 (0.73, 12.02) | 2.56 (0.73, 8.67) | 0.06 (‐0.01, 0.27) | |
| SD RITUX IV + MTX | 0.38 (0.12, 1.09) | 0.39 (0.12, 1.09) | ‐0.02 (‐0.04, 0.00) | |
| LD RITUX IV + MTX | 0.44 (0.15, 1.25) | 0.45 (0.15, 1.24) | ‐0.02 (‐0.04, 0.01) | |
| HD GOLI SC | 0.45 (0.05, 2.80) | 0.46 (0.05, 2.63) | ‐0.02 (‐0.04, 0.06) | |
| HD GOLI SC + MTX | 3.68 (1.04, 15.81) | 3.34 (1.04, 10.44) | 0.09 (0.00, 0.33) | |
| HD INF IV + MTX | 2.46 (1.10, 5.58) | 2.33 (1.09, 4.84) | 0.05 (0.00, 0.13) | |
| SD ADA SC + MTX | SD ADA SC | 1.31 (0.61, 2.85) | 1.29 (0.63, 2.71) | 0.01 (‐0.03, 0.05) |
| SD INF IV + MTX | 1.60 (0.55, 4.33) | 1.56 (0.58, 3.99) | 0.03 (‐0.04, 0.08) | |
| SD ETN SC + MTX | 0.67 (0.24, 2.19) | 0.68 (0.26, 2.11) | ‐0.01 (‐0.07, 0.03) | |
| SD ABA IV + MTX | 0.59 (0.15, 2.31) | 0.60 (0.16, 2.21) | ‐0.02 (‐0.08, 0.04) | |
| SD GOLI SC + MTX | 2.07 (0.44, 10.95) | 1.96 (0.46, 8.14) | 0.05 (‐0.04, 0.26) | |
| SD RITUX IV + MTX | 0.28 (0.07, 1.06) | 0.29 (0.08, 1.06) | ‐0.03 (‐0.09, 0.00) | |
| LD RITUX IV + MTX | 0.33 (0.09, 1.24) | 0.35 (0.09, 1.23) | ‐0.03 (‐0.09, 0.01) | |
| HD GOLI SC | 0.34 (0.03, 2.46) | 0.35 (0.04, 2.33) | ‐0.03 (‐0.09, 0.05) | |
| HD GOLI SC + MTX | 2.81 (0.62, 14.39) | 2.57 (0.64, 9.87) | 0.07 (‐0.03, 0.32) | |
| HD INF IV + MTX | 1.86 (0.61, 5.75) | 1.78 (0.63, 5.07) | 0.04 (‐0.03, 0.13) | |
| SD INF IV + MTX | SD ADA SC + MTX | 1.21 (0.50, 2.80) | 1.20 (0.52, 2.60) | 0.01 (‐0.05, 0.07) |
| SD ETN SC + MTX | 0.51 (0.21, 1.44) | 0.53 (0.23, 1.41) | ‐0.03 (‐0.07, 0.02) | |
| SD ABA IV + MTX | 0.45 (0.13, 1.55) | 0.47 (0.14, 1.50) | ‐0.03 (‐0.08, 0.03) | |
| SD GOLI SC + MTX | 1.57 (0.37, 7.65) | 1.51 (0.39, 5.72) | 0.03 (‐0.05, 0.25) | |
| SD RITUX IV + MTX | 0.22 (0.06, 0.72) | 0.23 (0.07, 0.73) | ‐0.05 (‐0.09, ‐0.01) | |
| LD RITUX IV + MTX | 0.25 (0.07, 0.83) | 0.27 (0.08, 0.83) | ‐0.04 (‐0.09, ‐0.01) | |
| HD GOLI SC | 0.26 (0.03, 1.74) | 0.27 (0.03, 1.68) | ‐0.04 (‐0.09, 0.04) | |
| HD GOLI SC + MTX | 2.14 (0.52, 10.03) | 2.00 (0.54, 6.90) | 0.06 (‐0.04, 0.31) | |
| HD INF IV + MTX | 1.42 (0.53, 3.80) | 1.38 (0.55, 3.36) | 0.02 (‐0.04, 0.11) | |
| SD ETN SC + MTX | SD INF IV + MTX | 0.42 (0.17, 1.31) | 0.44 (0.18, 1.29) | ‐0.04 (‐0.09, 0.01) |
| SD ABA IV + MTX | 0.37 (0.10, 1.40) | 0.39 (0.11, 1.36) | ‐0.04 (‐0.10, 0.02) | |
| SD GOLI SC + MTX | 1.30 (0.30, 6.62) | 1.27 (0.32, 4.93) | 0.02 (‐0.07, 0.24) | |
| SD RITUX IV + MTX | 0.18 (0.05, 0.63) | 0.19 (0.05, 0.64) | ‐0.06 (‐0.11, ‐0.02) | |
| LD RITUX IV + MTX | 0.21 (0.06, 0.72) | 0.22 (0.07, 0.74) | ‐0.06 (‐0.11, ‐0.01) | |
| HD GOLI SC | 0.21 (0.02, 1.53) | 0.23 (0.02, 1.48) | ‐0.06 (‐0.11, 0.03) | |
| HD GOLI SC + MTX | 1.77 (0.42, 8.77) | 1.67 (0.45, 6.00) | 0.05 (‐0.05, 0.30) | |
| HD INF IV + MTX | 1.16 (0.57, 2.58) | 1.15 (0.59, 2.34) | 0.01 (‐0.04, 0.09) | |
| SD ABA IV + MTX | SD ETN SC + MTX | 0.88 (0.21, 3.22) | 0.88 (0.22, 3.06) | 0.00 (‐0.05, 0.05) |
| SD GOLI SC + MTX | 3.05 (0.62, 15.53) | 2.85 (0.64, 11.38) | 0.06 (‐0.02, 0.28) | |
| SD RITUX IV + MTX | 0.42 (0.10, 1.47) | 0.43 (0.11, 1.46) | ‐0.02 (‐0.06, 0.01) | |
| LD RITUX IV + MTX | 0.50 (0.12, 1.68) | 0.51 (0.13, 1.66) | ‐0.02 (‐0.06, 0.02) | |
| HD GOLI SC | 0.50 (0.05, 3.52) | 0.50 (0.05, 3.30) | ‐0.02 (‐0.06, 0.06) | |
| HD GOLI SC + MTX | 4.15 (0.89, 20.05) | 3.74 (0.89, 13.75) | 0.09 (‐0.01, 0.33) | |
| HD INF IV + MTX | 2.76 (0.84, 7.83) | 2.60 (0.85, 6.84) | 0.05 (‐0.01, 0.14) | |
| SD GOLI SC + MTX | SD ABA IV + MTX | 3.51 (0.61, 22.37) | 3.25 (0.63, 16.78) | 0.06 (‐0.02, 0.28) |
| SD RITUX IV + MTX | 0.48 (0.10, 2.21) | 0.49 (0.10, 2.17) | ‐0.01 (‐0.07, 0.02) | |
| LD RITUX IV + MTX | 0.56 (0.12, 2.60) | 0.57 (0.13, 2.54) | ‐0.01 (‐0.07, 0.02) | |
| HD GOLI SC | 0.56 (0.05, 4.99) | 0.57 (0.05, 4.65) | ‐0.01 (‐0.07, 0.07) | |
| HD GOLI SC + MTX | 4.75 (0.87, 29.63) | 4.25 (0.88, 20.74) | 0.09 (‐0.01, 0.34) | |
| HD INF IV + MTX | 3.14 (0.78, 12.27) | 2.95 (0.80, 10.74) | 0.06 (‐0.01, 0.14) | |
| SD RITUX IV + MTX | SD GOLI SC + MTX | 0.14 (0.02, 0.75) | 0.15 (0.03, 0.76) | ‐0.08 (‐0.29, ‐0.01) |
| LD RITUX IV + MTX | 0.16 (0.03, 0.87) | 0.18 (0.04, 0.87) | ‐0.08 (‐0.29, 0.00) | |
| HD GOLI SC | 0.17 (0.02, 0.77) | 0.18 (0.02, 0.79) | ‐0.07 (‐0.25, ‐0.01) | |
| HD GOLI SC + MTX | 1.35 (0.47, 4.03) | 1.30 (0.52, 3.44) | 0.03 (‐0.08, 0.17) | |
| HD INF IV + MTX | 0.89 (0.17, 4.27) | 0.90 (0.22, 3.85) | ‐0.01 (‐0.23, 0.10) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 1.17 (0.34, 4.08) | 1.17 (0.35, 3.99) | 0.00 (‐0.02, 0.03) |
| HD GOLI SC | 1.18 (0.11, 10.03) | 1.18 (0.11, 9.31) | 0.00 (‐0.03, 0.08) | |
| HD GOLI SC + MTX | 9.97 (1.90, 60.09) | 8.74 (1.85, 41.81) | 0.11 (0.02, 0.35) | |
| HD INF IV + MTX | 6.51 (1.73, 26.09) | 6.00 (1.69, 22.81) | 0.07 (0.02, 0.16) | |
| HD GOLI SC | LD RITUX IV + MTX | 1.01 (0.09, 8.66) | 1.01 (0.10, 8.02) | 0.00 (‐0.03, 0.08) |
| HD GOLI SC + MTX | 8.45 (1.62, 50.37) | 7.43 (1.58, 34.71) | 0.11 (0.02, 0.35) | |
| HD INF IV + MTX | 5.57 (1.53, 22.12) | 5.15 (1.49, 19.12) | 0.07 (0.02, 0.15) | |
| HD GOLI SC + MTX | HD GOLI SC | 8.13 (1.80, 67.37) | 7.03 (1.70, 55.97) | 0.10 (0.02, 0.31) |
| HD INF IV + MTX | 5.54 (0.73, 58.34) | 5.12 (0.75, 51.46) | 0.07 (‐0.02, 0.15) | |
| HD INF IV + MTX | HD GOLI SC + MTX | 0.66 (0.13, 3.06) | 0.69 (0.18, 2.78) | ‐0.04 (‐0.29, 0.09) |
| Random‐effects model | Residual deviance | 33.71 vs 33 data‐points | ||
| Deviance information criteria | 179.39 | |||
| Fixed‐effect model | Residual deviance | 34.36 vs 33 data‐points | ||
| Deviance information criteria | 178.816 | |||
| ABA: abatacept ADA: adalimumab CrI: credible interval ETN: etanercept GOLI: golimumab HD: high dose INF: infliximab IV: intravenous LD: low dose MTX: methotrexate OR: odds ratio RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 23. Subgroup analysis: Withdrawals due to adverse events, early RA
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA + MTX | MTX + PL | 1.08 (0.33, 3.39) | 1.08 (0.34, 3.20) | 0.00 (‐0.02, 0.05) |
| SD INF IV + MTX | 3.34 (1.33, 9.36) | 3.12 (1.31, 7.99) | 0.06 (0.01, 0.16) | |
| SD RITUX IV + MTX | 0.38 (0.11, 1.19) | 0.39 (0.12, 1.19) | ‐0.02 (‐0.03, 0.00) | |
| LD RITUX IV + MTX | 0.44 (0.13, 1.38) | 0.45 (0.13, 1.36) | ‐0.01 (‐0.03, 0.01) | |
| HD INF IV + MTX | 3.38 (1.24, 9.99) | 3.16 (1.23, 8.32) | 0.06 (0.01, 0.18) | |
| SD INF IV + MTX | SD ADA + MTX | 3.12 (0.72, 14.66) | 2.92 (0.74, 12.66) | 0.06 (‐0.02, 0.16) |
| SD RITUX IV + MTX | 0.35 (0.07, 1.84) | 0.36 (0.07, 1.82) | ‐0.02 (‐0.07, 0.01) | |
| LD RITUX IV + MTX | 0.41 (0.08, 2.16) | 0.42 (0.08, 2.12) | ‐0.02 (‐0.07, 0.02) | |
| HD INF IV + MTX | 3.15 (0.69, 15.71) | 2.95 (0.71, 13.18) | 0.06 (‐0.02, 0.18) | |
| SD RITUX IV + MTX | SD INF IV + MTX | 0.11 (0.02, 0.50) | 0.12 (0.03, 0.52) | ‐0.08 (‐0.17, ‐0.02) |
| LD RITUX IV + MTX | 0.13 (0.03, 0.58) | 0.14 (0.03, 0.60) | ‐0.07 (‐0.17, ‐0.02) | |
| HD INF IV + MTX | 1.01 (0.42, 2.41) | 1.01 (0.45, 2.18) | 0.00 (‐0.07, 0.09) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 1.17 (0.32, 4.26) | 1.17 (0.32, 4.18) | 0.00 (‐0.02, 0.02) |
| HD INF IV + MTX | 8.93 (1.93, 45.48) | 8.18 (1.88, 38.25) | 0.08 (0.02, 0.19) | |
| HD INF IV + MTX | LD RITUX IV + MTX | 7.69 (1.65, 38.22) | 7.05 (1.61, 32.28) | 0.08 (0.02, 0.19) |
| Random‐effects model | Residual deviance | 12.76 vs 14 data‐points | ||
| Deviance information criteria | 71.072 | |||
| Fixed‐effect model | Residual deviance | 12.73 vs 14 data‐points | ||
| Deviance information criteria | 70.632 | |||
| Note: | ||||
| Total participants | 2416 | |||
| Total studies | 6 | |||
| 2‐arm | 4 | |||
| 3‐arm | 2 | |||
| ADA: adalimumab CrI: credible interval HD: high dose INF: infliximab IV: intravenous LD: low dose MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RITUX: rituximab RR: risk ratio SD: standard dose | ||||
Appendix 24. Subgroup analysis: Withdrawals due to adverse events, established RA
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC | MTX + PL | 1.28 (0.59, 2.71) | 1.26 (0.60, 2.51) | 0.01 (‐0.02, 0.07) |
| SD ADA SC + MTX | 1.62 (0.92, 2.78) | 1.57 (0.92, 2.58) | 0.03 (0.00, 0.07) | |
| SD ETN SC + MTX | 0.78 (0.36, 1.70) | 0.79 (0.37, 1.64) | ‐0.01 (‐0.03, 0.03) | |
| SD ABA IV + MTX | 0.80 (0.27, 2.31) | 0.81 (0.28, 2.18) | ‐0.01 (‐0.04, 0.05) | |
| SD GOLI SC + MTX | 2.82 (0.77, 12.17) | 2.58 (0.78, 8.25) | 0.08 (‐0.01, 0.31) | |
| HD GOLI SC | 0.46 (0.05, 2.93) | 0.47 (0.06, 2.70) | ‐0.03 (‐0.05, 0.07) | |
| HD GOLI SC + MTX | 3.78 (1.09, 15.66) | 3.32 (1.09, 9.67) | 0.11 (0.00, 0.37) | |
| SD ADA SC + MTX | SD ADA SC | 1.27 (0.60, 2.65) | 1.25 (0.63, 2.50) | 0.01 (‐0.04, 0.06) |
| SD ETN SC + MTX | 0.61 (0.21, 1.84) | 0.63 (0.23, 1.78) | ‐0.02 (‐0.08, 0.03) | |
| SD ABA IV + MTX | 0.62 (0.17, 2.36) | 0.64 (0.18, 2.23) | ‐0.02 (‐0.09, 0.05) | |
| SD GOLI SC + MTX | 2.20 (0.50, 11.82) | 2.04 (0.52, 8.26) | 0.06 (‐0.04, 0.30) | |
| HD GOLI SC | 0.36 (0.04, 2.68) | 0.37 (0.04, 2.49) | ‐0.04 (‐0.10, 0.06) | |
| HD GOLI SC + MTX | 2.96 (0.69, 15.28) | 2.63 (0.71, 9.81) | 0.10 (‐0.03, 0.36) | |
| SD ETN SC + MTX | SD ADA SC + MTX | 0.48 (0.19, 1.26) | 0.50 (0.21, 1.24) | ‐0.04 (‐0.09, 0.01) |
| SD ABA IV + MTX | 0.49 (0.15, 1.66) | 0.51 (0.16, 1.60) | ‐0.04 (‐0.09, 0.04) | |
| SD GOLI SC + MTX | 1.74 (0.43, 8.42) | 1.64 (0.45, 5.88) | 0.05 (‐0.06, 0.29) | |
| HD GOLI SC | 0.28 (0.03, 1.99) | 0.30 (0.03, 1.88) | ‐0.05 (‐0.11, 0.05) | |
| HD GOLI SC + MTX | 2.33 (0.60, 10.88) | 2.11 (0.62, 6.93) | 0.09 (‐0.04, 0.35) | |
| SD ABA IV + MTX | SD ETN SC + MTX | 1.02 (0.27, 3.82) | 1.02 (0.28, 3.57) | 0.00 (‐0.05, 0.07) |
| SD GOLI SC + MTX | 3.59 (0.79, 18.91) | 3.25 (0.80, 13.13) | 0.09 (‐0.01, 0.32) | |
| HD GOLI SC | 0.59 (0.06, 4.38) | 0.60 (0.06, 4.01) | ‐0.01 (‐0.06, 0.08) | |
| HD GOLI SC + MTX | 4.84 (1.10, 25.03) | 4.19 (1.10, 15.89) | 0.12 (0.01, 0.38) | |
| SD GOLI SC + MTX | SD ABA IV + MTX | 3.55 (0.65, 21.41) | 3.20 (0.68, 15.21) | 0.09 (‐0.03, 0.32) |
| HD GOLI SC | 0.58 (0.05, 4.83) | 0.59 (0.06, 4.45) | ‐0.01 (‐0.08, 0.08) | |
| HD GOLI SC + MTX | 4.81 (0.91, 28.67) | 4.15 (0.92, 18.54) | 0.12 (‐0.01, 0.38) | |
| HD GOLI SC | SD GOLI SC + MTX | 0.16 (0.02, 0.76) | 0.19 (0.02, 0.78) | ‐0.10 (‐0.30, ‐0.01) |
| HD GOLI SC + MTX | 1.34 (0.48, 3.87) | 1.28 (0.54, 3.16) | 0.03 (‐0.10, 0.19) | |
| HD GOLI SC + MTX | HD GOLI SC | 8.17 (1.85, 64.91) | 6.82 (1.72, 51.09) | 0.13 (0.03, 0.35) |
| Random‐effects model | Residual deviance | 14.15 vs 15 data‐points | ||
| Deviance information criteria | 91.568 | |||
| Fixed‐effect model | Residual deviance | 13.9 vs 15 data‐points | ||
| Deviance information criteria | 90.89 | |||
| Note: | ||||
| Total participants | 3667 | |||
| Total studies | 6 | |||
| 2‐arm | 4 | |||
| 3‐arm | 1 | |||
| 4‐arm | 1 | |||
| ABA: abatacept ADA: adalimumab CrI: credible interval ETN: etanercept GOLI: golimumab HD: high dose IV: intravenous MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 25. Subgroup analysis 2: Withdrawals due to adverse events, trial duration ≤ 6 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA + MTX | MTX + PL | 1.49 (0.70, 2.95) | 1.47 (0.70, 2.83) | 0.01 (‐0.01, 0.04) |
| SD GOLI SC + MTX | 2.78 (0.71, 13.07) | 2.66 (0.72, 10.41) | 0.04 (‐0.01, 0.21) | |
| HD GOLI SC | 0.46 (0.05, 2.90) | 0.47 (0.05, 2.78) | ‐0.01 (‐0.03, 0.04) | |
| HD GOLI SC + MTX | 3.71 (1.03, 16.68) | 3.46 (1.03, 12.51) | 0.06 (0.00, 0.25) | |
| SD GOLI SC + MTX | SD ADA + MTX | 1.88 (0.41, 10.56) | 1.82 (0.43, 8.46) | 0.03 (‐0.03, 0.20) |
| HD GOLI SC | 0.31 (0.03, 2.33) | 0.32 (0.03, 2.25) | ‐0.02 (‐0.06, 0.03) | |
| HD GOLI SC + MTX | 2.52 (0.59, 13.57) | 2.38 (0.61, 10.29) | 0.05 (‐0.02, 0.24) | |
| HD GOLI SC | SD GOLI SC + MTX | 0.16 (0.02, 0.83) | 0.18 (0.02, 0.84) | ‐0.05 (‐0.20, ‐0.01) |
| HD GOLI SC + MTX | 1.33 (0.44, 4.18) | 1.30 (0.47, 3.68) | 0.02 (‐0.07, 0.14) | |
| HD GOLI SC + MTX | HD GOLI SC | 8.06 (1.73, 65.65) | 7.30 (1.66, 57.20) | 0.08 (0.02, 0.24) |
| Random‐effects model | Residual deviance | 10.15 vs 10 data‐points | ||
| Deviance information criteria | 55.097 | |||
| Fixed‐effect model | Residual deviance | 10.3 vs 10 data‐points | ||
| Deviance information criteria | 54.868 | |||
| Note: | ||||
| Total participants | 2175 | |||
| Total studies | 4 | |||
| 2‐arm | 3 | |||
| 4‐arm | 1 | |||
| ADA: adalimumab CrI: credible interval GOLI: golimumab HD: high dose MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 26. Subgroup analysis 2: Withdrawals due to adverse events, trial duration 6‐12 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC | MTX + PL | 1.32 (0.47, 3.73) | 1.30 (0.48, 3.34) | 0.01 (‐0.02, 0.10) |
| SD ADA SC + MTX | 1.71 (0.62, 4.77) | 1.66 (0.63, 4.10) | 0.03 (‐0.02, 0.14) | |
| SD INF IV + MTX | 3.32 (1.21, 10.13) | 2.99 (1.20, 7.59) | 0.09 (0.01, 0.25) | |
| SD ETN SC + MTX | 0.90 (0.42, 2.84) | 0.91 (0.43, 2.64) | 0.00 (‐0.03, 0.07) | |
| SD ABA IV + MTX | 0.80 (0.23, 2.74) | 0.81 (0.24, 2.55) | ‐0.01 (‐0.04, 0.07) | |
| HD INF IV + MTX | 3.36 (1.18, 10.50) | 3.02 (1.16, 7.70) | 0.09 (0.01, 0.27) | |
| SD ADA SC + MTX | SD ADA SC | 1.30 (0.48, 3.57) | 1.27 (0.51, 3.22) | 0.02 (‐0.05, 0.10) |
| SD INF IV + MTX | 2.52 (0.60, 11.66) | 2.30 (0.63, 9.08) | 0.08 (‐0.04, 0.24) | |
| SD ETN SC + MTX | 0.68 (0.20, 3.39) | 0.70 (0.23, 3.16) | ‐0.02 (‐0.10, 0.06) | |
| SD ABA IV + MTX | 0.60 (0.12, 2.97) | 0.62 (0.14, 2.78) | ‐0.02 (‐0.11, 0.06) | |
| HD INF IV + MTX | 2.55 (0.58, 12.10) | 2.32 (0.62, 9.14) | 0.08 (‐0.04, 0.25) | |
| SD INF IV + MTX | SD ADA SC + MTX | 1.94 (0.46, 8.92) | 1.81 (0.51, 6.93) | 0.06 (‐0.07, 0.23) |
| SD ETN SC + MTX | 0.53 (0.16, 2.63) | 0.55 (0.18, 2.48) | ‐0.03 (‐0.14, 0.05) | |
| SD ABA IV + MTX | 0.47 (0.09, 2.27) | 0.49 (0.11, 2.15) | ‐0.04 (‐0.15, 0.05) | |
| HD INF IV + MTX | 1.97 (0.45, 9.14) | 1.83 (0.50, 6.99) | 0.06 (‐0.07, 0.24) | |
| SD ETN SC + MTX | SD INF IV + MTX | 0.27 (0.07, 1.26) | 0.30 (0.10, 1.24) | ‐0.09 (‐0.25, 0.02) |
| SD ABA IV + MTX | 0.24 (0.05, 1.16) | 0.27 (0.06, 1.14) | ‐0.10 (‐0.26, 0.01) | |
| HD INF IV + MTX | 1.02 (0.38, 2.60) | 1.02 (0.44, 2.22) | 0.00 (‐0.11, 0.13) | |
| SD ABA IV + MTX | SD ETN SC + MTX | 0.88 (0.16, 3.53) | 0.89 (0.17, 3.28) | 0.00 (‐0.08, 0.07) |
| HD INF IV + MTX | 3.72 (0.75, 13.88) | 3.33 (0.77, 10.45) | 0.10 (‐0.02, 0.27) | |
| HD INF IV + MTX | SD ABA IV + MTX | 4.24 (0.83, 22.80) | 3.76 (0.84, 17.35) | 0.10 (‐0.01, 0.27) |
| Random‐effects model | Residual deviance | 13.88 vs 14 data‐points | ||
| Deviance information criteria | 81.811 | |||
| Fixed‐effect model | Residual deviance | 14.15 vs 14 data‐points | ||
| Deviance information criteria | 81.916 | |||
| Note: | ||||
| Total participants | 3029 | |||
| Total studies | 6 | |||
| 2‐arm | 4 | |||
| 3‐arm | 2 | |||
| ABA: abatacept ADA: adalimumab CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous MTX: methotrexate OR: odds ratio PL: placebo RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 27. Serious adverse events: main analysis
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| MP + MTX | MTX | 0.79 (0.21, 2.95) | 0.81 (0.23, 2.53) | ‐0.02 (‐0.07, 0.13) |
| SD ADA SC + MTX | 0.99 (0.63, 1.58) | 0.99 (0.65, 1.50) | 0.00 (‐0.03, 0.04) | |
| SD INF IV + MTX | 1.33 (0.70, 2.59) | 1.29 (0.72, 2.28) | 0.03 (‐0.03, 0.11) | |
| SD ABA IV + MTX | 0.99 (0.41, 2.35) | 0.99 (0.44, 2.10) | 0.00 (‐0.05, 0.10) | |
| SD ETN SC + MTX | 3.68 (0.86, 20.72) | 2.97 (0.87, 8.05) | 0.18 (‐0.01, 0.56) | |
| SD GOLI SC + MTX | 0.91 (0.31, 2.61) | 0.92 (0.33, 2.30) | ‐0.01 (‐0.06, 0.11) | |
| SD RITUX IV + MTX | 0.75 (0.35, 1.60) | 0.76 (0.37, 1.52) | ‐0.02 (‐0.06, 0.05) | |
| LD RITUX IV + MTX | 0.86 (0.41, 1.83) | 0.87 (0.43, 1.70) | ‐0.01 (‐0.05, 0.06) | |
| HD GOLI SC | 0.42 (0.11, 1.44) | 0.44 (0.12, 1.39) | ‐0.05 (‐0.08, 0.03) | |
| HD GOLI SC + MTX | 0.91 (0.31, 2.64) | 0.92 (0.33, 2.32) | ‐0.01 (‐0.06, 0.11) | |
| HD INF IV + MTX | 1.29 (0.63, 2.65) | 1.25 (0.65, 2.32) | 0.02 (‐0.03, 0.11) | |
| SD ADA SC + MTX | MP + MTX | 1.25 (0.31, 5.05) | 1.23 (0.37, 4.63) | 0.02 (‐0.14, 0.08) |
| SD INF IV + MTX | 1.67 (0.54, 5.29) | 1.59 (0.59, 4.78) | 0.04 (‐0.08, 0.11) | |
| SD ABA IV + MTX | 1.24 (0.26, 5.96) | 1.22 (0.30, 5.27) | 0.01 (‐0.14, 0.13) | |
| SD ETN SC + MTX | 4.68 (0.64, 39.87) | 3.64 (0.69, 18.15) | 0.19 (‐0.05, 0.58) | |
| SD GOLI SC + MTX | 1.14 (0.21, 6.28) | 1.12 (0.25, 5.47) | 0.01 (‐0.15, 0.14) | |
| SD RITUX IV + MTX | 0.94 (0.21, 4.24) | 0.94 (0.25, 3.91) | 0.00 (‐0.15, 0.08) | |
| LD RITUX IV + MTX | 1.08 (0.24, 4.86) | 1.07 (0.28, 4.42) | 0.00 (‐0.15, 0.10) | |
| HD GOLI SC | 0.52 (0.08, 3.18) | 0.54 (0.10, 2.98) | ‐0.03 (‐0.18, 0.06) | |
| HD GOLI SC + MTX | 1.14 (0.20, 6.21) | 1.13 (0.24, 5.39) | 0.01 (‐0.15, 0.14) | |
| HD INF IV + MTX | 1.62 (0.43, 6.25) | 1.55 (0.48, 5.50) | 0.04 (‐0.10, 0.13) | |
| SD INF IV + MTX | SD ADA SC + MTX | 1.34 (0.60, 2.98) | 1.30 (0.63, 2.61) | 0.03 (‐0.04, 0.12) |
| SD ABA IV + MTX | 1.00 (0.37, 2.65) | 1.00 (0.40, 2.36) | 0.00 (‐0.07, 0.10) | |
| SD ETN SC + MTX | 3.72 (0.80, 21.85) | 2.99 (0.82, 8.75) | 0.18 (‐0.02, 0.56) | |
| SD GOLI SC + MTX | 0.91 (0.28, 2.89) | 0.92 (0.31, 2.53) | ‐0.01 (‐0.08, 0.12) | |
| SD RITUX IV + MTX | 0.75 (0.31, 1.81) | 0.77 (0.33, 1.70) | ‐0.02 (‐0.08, 0.05) | |
| LD RITUX IV + MTX | 0.86 (0.36, 2.06) | 0.87 (0.39, 1.91) | ‐0.01 (‐0.07, 0.07) | |
| HD GOLI SC | 0.42 (0.10, 1.55) | 0.45 (0.11, 1.49) | ‐0.05 (‐0.10, 0.04) | |
| HD GOLI SC + MTX | 0.91 (0.28, 2.91) | 0.92 (0.31, 2.55) | ‐0.01 (‐0.08, 0.12) | |
| HD INF IV + MTX | 1.30 (0.55, 3.03) | 1.26 (0.58, 2.64) | 0.02 (‐0.05, 0.12) | |
| SD ABA IV + MTX | SD INF IV + MTX | 0.74 (0.25, 2.19) | 0.77 (0.28, 1.99) | ‐0.03 (‐0.12, 0.08) |
| SD ETN SC + MTX | 2.78 (0.57, 17.16) | 2.29 (0.61, 7.21) | 0.15 (‐0.06, 0.54) | |
| SD GOLI SC + MTX | 0.68 (0.19, 2.37) | 0.71 (0.22, 2.12) | ‐0.03 (‐0.13, 0.09) | |
| SD RITUX IV + MTX | 0.56 (0.20, 1.51) | 0.59 (0.24, 1.45) | ‐0.05 (‐0.14, 0.04) | |
| LD RITUX IV + MTX | 0.65 (0.24, 1.73) | 0.67 (0.27, 1.63) | ‐0.04 (‐0.13, 0.05) | |
| HD GOLI SC | 0.32 (0.07, 1.27) | 0.34 (0.08, 1.24) | ‐0.07 (‐0.16, 0.02) | |
| HD GOLI SC + MTX | 0.68 (0.20, 2.35) | 0.71 (0.22, 2.11) | ‐0.03 (‐0.13, 0.10) | |
| HD INF IV + MTX | 0.97 (0.48, 1.95) | 0.97 (0.52, 1.79) | 0.00 (‐0.07, 0.07) | |
| SD ETN SC + MTX | SD ABA IV + MTX | 3.75 (0.68, 25.21) | 2.99 (0.72, 10.77) | 0.17 (‐0.04, 0.56) |
| SD GOLI SC + MTX | 0.92 (0.23, 3.65) | 0.92 (0.26, 3.19) | ‐0.01 (‐0.12, 0.12) | |
| SD RITUX IV + MTX | 0.75 (0.24, 2.41) | 0.77 (0.27, 2.25) | ‐0.02 (‐0.12, 0.06) | |
| LD RITUX IV + MTX | 0.86 (0.28, 2.75) | 0.87 (0.31, 2.53) | ‐0.01 (‐0.11, 0.08) | |
| HD GOLI SC | 0.42 (0.09, 1.94) | 0.45 (0.10, 1.84) | ‐0.05 (‐0.15, 0.05) | |
| HD GOLI SC + MTX | 0.92 (0.23, 3.62) | 0.93 (0.26, 3.18) | ‐0.01 (‐0.12, 0.12) | |
| HD INF IV + MTX | 1.31 (0.43, 4.03) | 1.27 (0.47, 3.51) | 0.02 (‐0.09, 0.13) | |
| SD GOLI SC + MTX | SD ETN SC + MTX | 0.24 (0.03, 1.47) | 0.31 (0.07, 1.40) | ‐0.18 (‐0.57, 0.04) |
| SD RITUX IV + MTX | 0.20 (0.03, 1.04) | 0.26 (0.07, 1.04) | ‐0.19 (‐0.58, 0.00) | |
| LD RITUX IV + MTX | 0.23 (0.04, 1.19) | 0.29 (0.09, 1.17) | ‐0.18 (‐0.57, 0.02) | |
| HD GOLI SC | 0.11 (0.01, 0.76) | 0.15 (0.03, 0.79) | ‐0.22 (‐0.60, ‐0.02) | |
| HD GOLI SC + MTX | 0.24 (0.03, 1.49) | 0.31 (0.08, 1.41) | ‐0.18 (‐0.57, 0.04) | |
| HD INF IV + MTX | 0.35 (0.05, 1.78) | 0.42 (0.13, 1.66) | ‐0.15 (‐0.54, 0.06) | |
| SD RITUX IV + MTX | SD GOLI SC + MTX | 0.82 (0.22, 3.05) | 0.84 (0.26, 2.83) | ‐0.01 (‐0.13, 0.07) |
| LD RITUX IV + MTX | 0.95 (0.26, 3.48) | 0.95 (0.30, 3.19) | 0.00 (‐0.13, 0.09) | |
| HD GOLI SC | 0.47 (0.12, 1.59) | 0.49 (0.13, 1.54) | ‐0.04 (‐0.14, 0.03) | |
| HD GOLI SC + MTX | 1.00 (0.34, 2.95) | 1.00 (0.37, 2.69) | 0.00 (‐0.09, 0.09) | |
| HD INF IV + MTX | 1.42 (0.40, 5.16) | 1.37 (0.44, 4.48) | 0.03 (‐0.10, 0.13) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 1.15 (0.53, 2.50) | 1.14 (0.56, 2.33) | 0.01 (‐0.05, 0.07) |
| HD GOLI SC | 0.56 (0.12, 2.41) | 0.58 (0.13, 2.27) | ‐0.03 (‐0.10, 0.06) | |
| HD GOLI SC + MTX | 1.22 (0.33, 4.49) | 1.20 (0.35, 3.88) | 0.01 (‐0.07, 0.14) | |
| HD INF IV + MTX | 1.73 (0.61, 4.97) | 1.65 (0.64, 4.29) | 0.04 (‐0.04, 0.14) | |
| HD GOLI SC | LD RITUX IV + MTX | 0.49 (0.11, 2.09) | 0.51 (0.12, 1.97) | ‐0.04 (‐0.12, 0.05) |
| HD GOLI SC + MTX | 1.06 (0.29, 3.90) | 1.06 (0.31, 3.39) | 0.00 (‐0.09, 0.13) | |
| HD INF IV + MTX | 1.50 (0.53, 4.30) | 1.45 (0.57, 3.71) | 0.03 (‐0.06, 0.13) | |
| HD GOLI SC + MTX | HD GOLI SC | 2.14 (0.62, 8.36) | 2.03 (0.64, 7.41) | 0.04 (‐0.03, 0.14) |
| HD INF IV + MTX | 3.08 (0.73, 13.81) | 2.84 (0.76, 11.82) | 0.07 (‐0.02, 0.17) | |
| HD INF IV + MTX | HD GOLI SC + MTX | 1.42 (0.40, 5.13) | 1.37 (0.44, 4.47) | 0.03 (‐0.10, 0.13) |
| Random‐effects model | Residual deviance | 27.66 vs 28 data‐points | ||
| Deviance information criteria | 171.724 | |||
| Fixed‐effect model | Residual deviance | 28.69 vs 28 data‐points | ||
| Deviance information criteria | 171.488 | |||
| ABA: abatacept ADA: adalimumab CrI: credible interval ETN: etanercept GOLI: golimumab HD: high dose INF: infliximab IV: intravenous LD: low dose MP: methylprednisolone MTX: methotrexate OR: odds ratio RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 28. Subgroup analysis: Serious adverse events, early RA
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| MP + MTX | MTX | 0.79 (0.18, 3.37) | 0.80 (0.20, 2.78) | ‐0.02 (‐0.08, 0.16) |
| SD INF IV + MTX | 1.34 (0.60, 2.92) | 1.30 (0.63, 2.51) | 0.03 (‐0.04, 0.13) | |
| SD ADA SC + MTX | 0.70 (0.30, 1.79) | 0.72 (0.33, 1.67) | ‐0.03 (‐0.07, 0.06) | |
| SD ETN SC + MTX | 3.69 (0.77, 23.15) | 2.95 (0.79, 8.62) | 0.18 (‐0.02, 0.58) | |
| SD RITUX IV + MTX | 0.75 (0.29, 1.91) | 0.77 (0.31, 1.76) | ‐0.02 (‐0.07, 0.07) | |
| LD RITUX IV + MTX | 0.86 (0.34, 2.19) | 0.87 (0.36, 1.98) | ‐0.01 (‐0.06, 0.09) | |
| HD INF IV + MTX | 1.29 (0.53, 3.17) | 1.26 (0.55, 2.66) | 0.02 (‐0.04, 0.15) | |
| SD INF IV + MTX | MP + MTX | 1.69 (0.50, 5.93) | 1.60 (0.56, 5.30) | 0.04 (‐0.09, 0.13) |
| SD ADA SC + MTX | 0.89 (0.17, 5.18) | 0.89 (0.21, 4.72) | ‐0.01 (‐0.18, 0.09) | |
| SD ETN SC + MTX | 4.79 (0.56, 48.00) | 3.66 (0.61, 21.26) | 0.19 (‐0.07, 0.59) | |
| SD RITUX IV + MTX | 0.95 (0.17, 5.42) | 0.95 (0.21, 4.84) | 0.00 (‐0.18, 0.10) | |
| LD RITUX IV + MTX | 1.09 (0.19, 6.12) | 1.08 (0.24, 5.41) | 0.01 (‐0.17, 0.12) | |
| HD INF IV + MTX | 1.64 (0.37, 7.44) | 1.56 (0.43, 6.37) | 0.04 (‐0.12, 0.16) | |
| SD ADA SC + MTX | SD INF IV + MTX | 0.52 (0.17, 1.84) | 0.55 (0.20, 1.73) | ‐0.05 (‐0.16, 0.05) |
| SD ETN SC + MTX | 2.79 (0.48, 20.47) | 2.28 (0.53, 8.16) | 0.15 (‐0.08, 0.56) | |
| SD RITUX IV + MTX | 0.56 (0.17, 1.89) | 0.59 (0.20, 1.76) | ‐0.05 (‐0.16, 0.06) | |
| LD RITUX IV + MTX | 0.64 (0.19, 2.18) | 0.67 (0.23, 1.98) | ‐0.04 (‐0.15, 0.08) | |
| HD INF IV + MTX | 0.97 (0.41, 2.34) | 0.97 (0.45, 2.07) | 0.00 (‐0.09, 0.10) | |
| SD ETN SC + MTX | SD ADA SC + MTX | 5.31 (0.84, 39.41) | 4.08 (0.86, 15.36) | 0.21 (‐0.01, 0.60) |
| SD RITUX IV + MTX | 1.08 (0.27, 3.60) | 1.08 (0.30, 3.24) | 0.00 (‐0.09, 0.10) | |
| LD RITUX IV + MTX | 1.24 (0.32, 4.15) | 1.22 (0.35, 3.65) | 0.01 (‐0.08, 0.12) | |
| HD INF IV + MTX | 1.87 (0.49, 6.11) | 1.76 (0.53, 5.05) | 0.05 (‐0.06, 0.17) | |
| SD RITUX IV + MTX | SD ETN SC + MTX | 0.20 (0.03, 1.25) | 0.26 (0.06, 1.22) | ‐0.20 (‐0.60, 0.02) |
| LD RITUX IV + MTX | 0.23 (0.03, 1.43) | 0.30 (0.07, 1.37) | ‐0.19 (‐0.59, 0.03) | |
| HD INF IV + MTX | 0.35 (0.05, 2.14) | 0.43 (0.11, 1.90) | ‐0.16 (‐0.56, 0.09) | |
| LD RITUX IV + MTX | 1.15 (0.45, 2.95) | 1.14 (0.48, 2.69) | 0.01 (‐0.06, 0.09) | |
| HD INF IV + MTX | SD RITUX IV + MTX | 1.72 (0.48, 6.34) | 1.64 (0.52, 5.28) | 0.04 (‐0.06, 0.17) |
| HD INF IV + MTX | LD RITUX IV + MTX | 1.50 (0.42, 5.43) | 1.44 (0.46, 4.52) | 0.03 (‐0.08, 0.16) |
| Random‐effects model | Residual deviance | 16.68 vs 16 data‐points | ||
| Deviance information criteria | 100.878 | |||
| Fixed‐effect model | Residual deviance | 17.38 vs 16 data‐points | ||
| Deviance information criteria | 100.951 | |||
| ADA: adalimumab CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous LD: low dose MP: methylprednisolone MTX: methotrexate OR: odds ratio RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 29. Subgroup analysis: Serious adverse events, established RA
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC + MTX | MTX | 1.19 (0.68, 2.12) | 1.17 (0.70, 1.96) | 0.01 (‐0.03, 0.07) |
| SD ABA IV + MTX | 0.99 (0.42, 2.34) | 0.99 (0.44, 2.12) | 0.00 (‐0.05, 0.09) | |
| SD GOLI SC + MTX | 0.91 (0.31, 2.59) | 0.91 (0.34, 2.30) | ‐0.01 (‐0.06, 0.10) | |
| HD GOLI SC | 0.42 (0.11, 1.42) | 0.44 (0.12, 1.37) | ‐0.05 (‐0.08, 0.03) | |
| HD GOLI SC + MTX | 0.90 (0.30, 2.58) | 0.91 (0.32, 2.29) | ‐0.01 (‐0.06, 0.10) | |
| SD ABA IV + MTX | SD ADA SC + MTX | 0.83 (0.29, 2.30) | 0.85 (0.32, 2.09) | ‐0.01 (‐0.09, 0.09) |
| SD GOLI SC + MTX | 0.76 (0.23, 2.49) | 0.78 (0.25, 2.23) | ‐0.02 (‐0.10, 0.10) | |
| HD GOLI SC | 0.35 (0.09, 1.32) | 0.38 (0.10, 1.29) | ‐0.06 (‐0.13, 0.02) | |
| HD GOLI SC + MTX | 0.76 (0.22, 2.50) | 0.78 (0.25, 2.23) | ‐0.02 (‐0.10, 0.10) | |
| SD GOLI SC + MTX | SD ABA IV + MTX | 0.92 (0.24, 3.52) | 0.92 (0.27, 3.11) | ‐0.01 (‐0.11, 0.11) |
| HD GOLI SC | 0.42 (0.09, 1.85) | 0.45 (0.10, 1.77) | ‐0.04 (‐0.14, 0.04) | |
| HD GOLI SC + MTX | 0.91 (0.23, 3.52) | 0.92 (0.26, 3.10) | ‐0.01 (‐0.11, 0.11) | |
| HD GOLI SC | SD GOLI SC + MTX | 0.46 (0.12, 1.59) | 0.49 (0.13, 1.54) | ‐0.04 (‐0.13, 0.03) |
| HD GOLI SC + MTX | 0.99 (0.34, 2.96) | 0.99 (0.37, 2.71) | 0.00 (‐0.09, 0.09) | |
| HD GOLI SC + MTX | HD GOLI SC | 2.14 (0.62, 8.28) | 2.04 (0.64, 7.42) | 0.04 (‐0.03, 0.13) |
| Random‐effects model | Residual deviance | 10.55 vs 12 data‐points | ||
| Deviance information criteria | 71.193 | |||
| Fixed‐effect model | Residual deviance | 10.13 vs 12 data‐points | ||
| Deviance information criteria | 70.371 | |||
| ABA: abatacept ADA: adalimumab CrI: credible interval GOLI: golimumab HD: high dose IV: intravenous MTX: methotrexate OR: odds ratio RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 30. Subgroup analysis: Serious adverse events, trial duration ≤ 6 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC + MTX | MTX | 0.96 (0.54, 1.75) | 0.96 (0.56, 1.66) | 0.00 (‐0.04, 0.05) |
| SD GOLI SC + MTX | 0.90 (0.27, 2.95) | 0.91 (0.29, 2.57) | ‐0.01 (‐0.06, 0.12) | |
| HD GOLI SC | 0.42 (0.10, 1.55) | 0.44 (0.11, 1.49) | ‐0.04 (‐0.08, 0.04) | |
| HD GOLI SC + MTX | 0.90 (0.27, 2.96) | 0.91 (0.29, 2.59) | ‐0.01 (‐0.06, 0.12) | |
| SD GOLI SC + MTX | SD ADA SC + MTX | 0.94 (0.24, 3.50) | 0.95 (0.26, 3.04) | 0.00 (‐0.07, 0.12) |
| HD GOLI SC | 0.43 (0.09, 1.81) | 0.45 (0.10, 1.72) | ‐0.04 (‐0.10, 0.04) | |
| HD GOLI SC + MTX | 0.93 (0.25, 3.48) | 0.94 (0.27, 3.02) | 0.00 (‐0.07, 0.12) | |
| HD GOLI SC | SD GOLI SC + MTX | 0.46 (0.11, 1.76) | 0.48 (0.12, 1.70) | ‐0.03 (‐0.14, 0.03) |
| HD GOLI SC + MTX | 1.00 (0.30, 3.32) | 1.00 (0.33, 3.02) | 0.00 (‐0.10, 0.09) | |
| HD GOLI SC + MTX | HD GOLI SC | 2.14 (0.57, 9.09) | 2.05 (0.60, 8.11) | 0.03 (‐0.03, 0.14) |
| Random‐effects model | Residual deviance | 12.67 vs 12 data‐points | ||
| Deviance information criteria | 71.031 | |||
| Fixed‐effect model | Residual deviance | 14.37 vs 12 data‐points | ||
| Deviance information criteria | 71.625 | |||
| ADA: adalimumab CrI: credible interval GOLI: golimumab HD: high dose MTX: methotrexate OR: odds ratio RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 31. Subgroup analysis 2: Serious adverse events, trial duration 6‐12 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD INF IV + MTX | MTX | 1.36 (0.48, 3.85) | 1.33 (0.50, 3.19) | 0.02 (‐0.04, 0.16) |
| SD ABA IV + MTX | 0.99 (0.32, 3.00) | 1.00 (0.34, 2.63) | 0.00 (‐0.05, 0.12) | |
| SD ETN SC + MTX | 3.75 (0.76, 24.29) | 3.11 (0.78, 11.06) | 0.16 (‐0.02, 0.52) | |
| HD INF IV + MTX | 1.31 (0.47, 3.68) | 1.28 (0.49, 3.09) | 0.02 (‐0.04, 0.15) | |
| SD ABA IV + MTX | SD INF IV + MTX | 0.73 (0.16, 3.33) | 0.75 (0.19, 2.91) | ‐0.02 (‐0.16, 0.10) |
| SD ETN SC + MTX | 2.77 (0.42, 23.12) | 2.34 (0.48, 11.31) | 0.13 (‐0.09, 0.50) | |
| HD INF IV + MTX | 0.97 (0.35, 2.64) | 0.97 (0.40, 2.34) | 0.00 (‐0.10, 0.09) | |
| SD ETN SC + MTX | SD ABA IV + MTX | 3.80 (0.54, 32.89) | 3.13 (0.59, 16.13) | 0.15 (‐0.06, 0.51) |
| HD INF IV + MTX | 1.32 (0.29, 6.02) | 1.29 (0.33, 5.00) | 0.02 (‐0.10, 0.15) | |
| HD INF IV + MTX | SD ETN SC + MTX | 0.35 (0.04, 2.30) | 0.41 (0.08, 2.04) | ‐0.13 (‐0.50, 0.09) |
| Random‐effects model | Residual deviance | 7.06 vs 7 data‐points | ||
| Deviance information criteria | 46.793 | |||
| Fixed‐effect model | Residual deviance | 7.085 vs 7 data‐points | ||
| Deviance information criteria | 46.847 | |||
| ABA: abatacept CrI: credible interval ETN: etanercept HD: high dose INF: infliximab IV: intravenous MTX: methotrexate OR: odds ratio RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 32. Subgroup analysis 2: Serious adverse events, trial duration > 12 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC + MTX | MTX | 1.17 (0.33, 4.27) | 1.15 (0.37, 3.11) | 0.02 (‐0.09, 0.24) |
| SD INF IV + MTX | 1.28 (0.23, 7.23) | 1.23 (0.26, 4.46) | 0.03 (‐0.11, 0.35) | |
| SD RITUX IV + MTX | 0.75 (0.27, 2.14) | 0.77 (0.29, 1.88) | ‐0.03 (‐0.10, 0.11) | |
| LD RITUX IV + MTX | 0.86 (0.30, 2.45) | 0.88 (0.33, 2.08) | ‐0.02 (‐0.09, 0.13) | |
| SD INF IV + MTX | SD ADA SC + MTX | 1.09 (0.13, 9.33) | 1.07 (0.18, 5.96) | 0.01 (‐0.25, 0.34) |
| SD RITUX IV + MTX | 0.64 (0.12, 3.28) | 0.68 (0.17, 2.79) | ‐0.05 (‐0.27, 0.12) | |
| LD RITUX IV + MTX | 0.73 (0.14, 3.75) | 0.76 (0.19, 3.12) | ‐0.03 (‐0.26, 0.14) | |
| SD RITUX IV + MTX | SD INF IV + MTX | 0.58 (0.08, 4.29) | 0.63 (0.13, 3.64) | ‐0.06 (‐0.37, 0.13) |
| LD RITUX IV + MTX | 0.67 (0.09, 4.90) | 0.71 (0.14, 4.05) | ‐0.04 (‐0.36, 0.16) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 1.15 (0.40, 3.25) | 1.13 (0.45, 2.80) | 0.01 (‐0.09, 0.12) |
| Random‐effects model | Residual deviance | 7.115 vs 7 data‐points | ||
| Deviance information criteria | 44.854 | |||
| Fixed‐effect model | Residual deviance | 7.118 vs 7 data‐points | ||
| Deviance information criteria | 44.858 | |||
| ADA: adalimumab CrI: credible interval INF: infliximab IV: intravenous LD: low dose MTX: methotrexate OR: odds ratio RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 33. Cancer: main analysis
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC | MTX | 1.13 (0.22, 5.31) | 1.13 (0.22, 5.17) | 0.00 (‐0.01, 0.03) |
| SD ADA SC + MTX | 0.76 (0.23, 2.33) | 0.76 (0.23, 2.32) | 0.00 (‐0.01, 0.01) | |
| SD INF IV + MTX | 1.02 (0.02, 65.27) | 1.02 (0.02, 51.84) | 0.00 (‐0.01, 0.20) | |
| SD ETN SC + MTX | 0.97 (0.23, 4.29) | 0.97 (0.23, 4.21) | 0.00 (‐0.01, 0.02) | |
| SD GOLI SC + MTX | 0.48 (0.02, 6.01) | 0.48 (0.02, 5.82) | 0.00 (‐0.01, 0.03) | |
| SD RITUX IV + MTX | 0.40 (0.07, 1.80) | 0.40 (0.07, 1.79) | 0.00 (‐0.01, 0.01) | |
| LD RITUX IV + MTX | 0.85 (0.21, 3.23) | 0.85 (0.22, 3.18) | 0.00 (‐0.01, 0.02) | |
| HD GOLI SC + MTX | 0.48 (0.03, 5.68) | 0.48 (0.03, 5.52) | 0.00 (‐0.01, 0.03) | |
| SD ADA SC + MTX | SD ADA SC | 0.67 (0.13, 3.49) | 0.67 (0.14, 3.47) | 0.00 (‐0.03, 0.01) |
| SD INF IV + MTX | 0.94 (0.01, 80.23) | 0.94 (0.01, 63.96) | 0.00 (‐0.03, 0.20) | |
| SD ETN SC + MTX | 0.87 (0.10, 7.28) | 0.87 (0.11, 7.17) | 0.00 (‐0.03, 0.02) | |
| SD GOLI SC + MTX | 0.43 (0.01, 8.57) | 0.43 (0.02, 8.32) | 0.00 (‐0.03, 0.03) | |
| SD RITUX IV + MTX | 0.34 (0.04, 3.27) | 0.34 (0.04, 3.25) | 0.00 (‐0.03, 0.01) | |
| LD RITUX IV + MTX | 0.75 (0.10, 6.36) | 0.76 (0.10, 6.27) | 0.00 (‐0.03, 0.02) | |
| HD GOLI SC + MTX | 0.43 (0.02, 8.44) | 0.43 (0.02, 8.20) | 0.00 (‐0.03, 0.03) | |
| SD INF IV + MTX | SD ADA SC + MTX | 1.39 (0.02, 103.70) | 1.39 (0.02, 81.40) | 0.00 (‐0.01, 0.20) |
| SD ETN SC + MTX | 1.27 (0.21, 8.58) | 1.27 (0.21, 8.42) | 0.00 (‐0.01, 0.02) | |
| SD GOLI SC + MTX | 0.64 (0.02, 9.91) | 0.64 (0.02, 9.64) | 0.00 (‐0.01, 0.03) | |
| SD RITUX IV + MTX | 0.52 (0.07, 3.52) | 0.52 (0.07, 3.48) | 0.00 (‐0.01, 0.01) | |
| LD RITUX IV + MTX | 1.12 (0.18, 6.58) | 1.12 (0.18, 6.48) | 0.00 (‐0.01, 0.02) | |
| HD GOLI SC + MTX | 0.64 (0.03, 9.27) | 0.64 (0.03, 9.04) | 0.00 (‐0.01, 0.03) | |
| SD ETN SC + MTX | SD INF IV + MTX | 0.93 (0.01, 67.47) | 0.93 (0.02, 66.48) | 0.00 (‐0.20, 0.02) |
| SD GOLI SC + MTX | 0.44 (0.00, 55.00) | 0.44 (0.00, 52.63) | 0.00 (‐0.20, 0.03) | |
| SD RITUX IV + MTX | 0.37 (0.00, 27.52) | 0.37 (0.01, 27.33) | 0.00 (‐0.20, 0.01) | |
| LD RITUX IV + MTX | 0.81 (0.01, 61.16) | 0.81 (0.01, 60.36) | 0.00 (‐0.20, 0.02) | |
| HD GOLI SC + MTX | 0.43 (0.00, 47.80) | 0.43 (0.00, 46.89) | 0.00 (‐0.20, 0.03) | |
| SD GOLI SC + MTX | SD ETN SC + MTX | 0.49 (0.02, 9.58) | 0.49 (0.02, 9.30) | 0.00 (‐0.02, 0.03) |
| SD RITUX IV + MTX | 0.41 (0.04, 3.33) | 0.41 (0.04, 3.30) | 0.00 (‐0.02, 0.01) | |
| LD RITUX IV + MTX | 0.88 (0.12, 6.43) | 0.88 (0.12, 6.34) | 0.00 (‐0.02, 0.02) | |
| HD GOLI SC + MTX | 0.50 (0.02, 8.15) | 0.50 (0.02, 7.94) | 0.00 (‐0.02, 0.03) | |
| SD RITUX IV + MTX | SD GOLI SC + MTX | 0.80 (0.04, 23.51) | 0.81 (0.04, 23.36) | 0.00 (‐0.03, 0.01) |
| LD RITUX IV + MTX | 1.79 (0.10, 51.11) | 1.79 (0.10, 50.34) | 0.00 (‐0.03, 0.02) | |
| HD GOLI SC + MTX | 0.99 (0.05, 27.09) | 0.99 (0.05, 26.72) | 0.00 (‐0.02, 0.02) | |
| LD RITUX IV + MTX | SD RITUX IV + MTX | 2.16 (0.45, 12.82) | 2.15 (0.45, 12.69) | 0.00 (0.00, 0.02) |
| HD GOLI SC + MTX | 1.24 (0.05, 24.10) | 1.24 (0.05, 23.38) | 0.00 (‐0.01, 0.03) | |
| HD GOLI SC + MTX | LD RITUX IV + MTX | 0.56 (0.03, 9.61) | 0.56 (0.03, 9.33) | 0.00 (‐0.02, 0.03) |
| Random‐effects model | Residual deviance | 19.83 vs 25 data‐points | ||
| Deviance information criteria | 98.38 | |||
| Fixed‐effect model | Residual deviance | 19.89 vs 25 data‐points | ||
| Deviance information criteria | 98.272 | |||
| ADA: adalimumab CrI: credible interval ETN: etanercept GOLI: golimumab HD: high dose INF: infliximab IV: intravenous LD: low dose MTX: methotrexate OR: odds ratio RD: risk difference RITUX: rituximab RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 34. Subgroup analysis: Cancer, established RA
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC | MTX | 1.28 (0.24, 7.33) | 1.27 (0.24, 7.04) | 0.00 (‐0.01, 0.04) |
| SD ADA SC + MTX | 1.01 (0.24, 4.38) | 1.01 (0.24, 4.29) | 0.00 (‐0.01, 0.02) | |
| SD ETN SC + MTX | 1.00 (0.17, 5.44) | 1.00 (0.18, 5.25) | 0.00 (‐0.01, 0.03) | |
| SD GOLI SC + MTX | 0.50 (0.03, 6.25) | 0.50 (0.03, 6.04) | 0.00 (‐0.01, 0.04) | |
| HD GOLI SC | 0.50 (0.03, 5.68) | 0.50 (0.03, 5.49) | 0.00 (‐0.01, 0.03) | |
| SD ADA SC + MTX | SD ADA SC | 0.79 (0.13, 4.65) | 0.79 (0.14, 4.59) | 0.00 (‐0.04, 0.01) |
| SD ETN SC + MTX | 0.78 (0.07, 8.78) | 0.78 (0.07, 8.51) | 0.00 (‐0.04, 0.03) | |
| SD GOLI SC + MTX | 0.38 (0.01, 8.84) | 0.38 (0.01, 8.49) | ‐0.01 (‐0.05, 0.03) | |
| HD GOLI SC | 0.39 (0.01, 7.71) | 0.39 (0.01, 7.49) | ‐0.01 (‐0.05, 0.03) | |
| SD ETN SC + MTX | SD ADA SC + MTX | 0.99 (0.10, 8.92) | 0.99 (0.10, 8.64) | 0.00 (‐0.02, 0.04) |
| SD GOLI SC + MTX | 0.49 (0.02, 8.80) | 0.49 (0.02, 8.48) | 0.00 (‐0.02, 0.04) | |
| HD GOLI SC | 0.50 (0.02, 7.99) | 0.50 (0.02, 7.72) | 0.00 (‐0.02, 0.03) | |
| SD GOLI SC + MTX | SD ETN SC + MTX | 0.50 (0.02, 10.88) | 0.50 (0.02, 10.47) | 0.00 (‐0.04, 0.04) |
| HD GOLI SC | 0.50 (0.02, 9.65) | 0.50 (0.02, 9.37) | 0.00 (‐0.04, 0.03) | |
| HD GOLI SC | SD GOLI SC + MTX | 1.00 (0.05, 20.51) | 1.00 (0.05, 20.18) | 0.00 (‐0.03, 0.03) |
| Random‐effects model | Residual deviance | 11.45 vs 12 data‐points | ||
| Deviance information criteria | 52.311 | |||
| Fixed‐effect model | Residual deviance | 11.63 vs 12 data‐points | ||
| Deviance information criteria | 52.347 | |||
| ADA: adalimumab CrI: credible interval ETN: etanercept GOLI: golimumab HD: high dose MTX: methotrexate OR: odds ratio RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 35. Subgroup analysis 2: Cancer, trial duration < 6 months
| Treatment | Reference | OR (95% CrI) | RR (95% CrI) | RD % (95% CrI) |
| SD ADA SC + MTX | MTX | 1.04 (0.24, 4.62) | 1.04 (0.24, 4.57) | 0.00 (‐0.01, 0.01) |
| SD GOLI SC + MTX | 0.41 (0.01, 5.97) | 0.42 (0.01, 5.83) | 0.00 (‐0.01, 0.02) | |
| HD GOLI SC | 0.40 (0.01, 6.24) | 0.40 (0.01, 6.08) | 0.00 (‐0.01, 0.03) | |
| SD GOLI SC + MTX | SD ADA SC + MTX | 0.39 (0.01, 8.06) | 0.39 (0.01, 7.84) | 0.00 (‐0.02, 0.03) |
| HD GOLI SC | 0.37 (0.01, 8.57) | 0.37 (0.01, 8.32) | 0.00 (‐0.02, 0.03) | |
| HD GOLI SC | SD GOLI SC + MTX | 0.99 (0.02, 42.56) | 0.99 (0.02, 41.79) | 0.00 (‐0.02, 0.02) |
| Random‐effects model | Residual deviance | 9.036 vs 11 data‐points | ||
| Deviance information criteria | 40.215 | |||
| Fixed‐effect model | Residual deviance | 9.2 vs 11 data‐points | ||
| Deviance information criteria | 40.214 | |||
| ADA: adalimumab CrI: credible interval GOLI: golimumab HD: high dose MTX: methotrexate OR: odds ratio RD: risk difference RR: risk ratio SC: subcutaneous SD: standard dose | ||||
Appendix 36. Summary of safety warnings from regulatory agencies
Abatacept
No recent warnings have been issued with regard to abatacept. On the product label of abatacept, the FDA warns against known safety implications reporting, "In controlled clinical trials, patients receiving concomitant abatacept and TNF antagonist therapy experienced more infections (63%) and serious infections (4.4%) compared to patients treated with only TNF antagonists (43% and 0.8%, respectively). Concurrent administration of a TNF antagonist with abatacept has been associated with an increased risk of serious infections and no statistically significant additional benefit over use of the TNF antagonists alone" (FDA 2007). Furthermore, the FDA reports that, "rare occurrences of anaphylaxis or anaphylactoid reactions have been observed in two of 2,688 patients treated with abatacept in clinical trials" (FDA 2017). Trials have also shown that, "COPD patients treated with abatacept developed adverse events more frequently than those treated with placebo, including COPD exacerbations, cough, rhonchi, and dyspnea" (FDA 2017).
The effects of abatacept on pregnant women, pediatric patients, and the development of malignancies is "not yet fully understood" (FDA 2017). The European Medicines Agency (EMA) reports the adverse reactions in patients treated with abatacept, ranking the occurrences of such reactions as very common (≤ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); and very rare (< 1/10,000). EMA 2009a reports increase in blood pressure, abnormal liver function test (transaminases increased) and headaches are very common adverse reactions. Dizziness, cough, rash including dermatitis, diarrhea, nausea, dyspepsia, abdominal pain, lower respiratory tract infection (including bronchitis), urinary tract infection, herpes simplex, upper respiratory tract infection, hypertension, flushing, fatigue and asthenia are common (EMA 2009a). Overall, "the most commonly reported adverse events (occurring in 10% or more of patients) were headaches, upper respiratory tract infection, nasopharyngitis, and nausea. The adverse events most commonly resulting in clinical intervention were due to infection" (FDA 2017).
Adalimumab
The updated FDA label for adalimumab reports "Serious infections, sepsis, tuberculosis and cases of opportunistic infections, including fatalities, have been reported with the use of TNF blocking agents including Humira® (adalimumab)" (FDA 2017a). "Other serious infections seen in clinical trials include pneumonia, pyelonephritis, septic arthritis and septicaemia. Hospitalization or fatal outcomes associated with infections have been reported" (EMA 2009b). Furthermore, hepatitis B reactivation has been shown to be associated with adalimumab treatment (Health Canada 2006a). The FDA reports, "As observed with other TNF blocking agents, tuberculosis associated with the administration of Humira® in clinical trials has been reported" (FDA 2017a).
In rare instances, adalimumab has been associated with, "new onset or exacerbation of clinical symptoms and/or radiographic evidence of demyelinating disease including multiple sclerosis" (EMA 2009b). Furthermore, "In the controlled portions of clinical trials of some TNF‐blocking agents, including Humira, more cases of malignancies have been observed among patients receiving those TNF blockers compared to control patients" (FDA 2017a).
"Some of these hepatosplenic T‐cell lymphomas have occurred in young adult patients on concomitant treatment with azathioprine or 6‐mercaptopurine used for Crohn’s disease". Thus, the risk of the development of hepatosplenic T‐cell lymphoma cannot be excluded for patients treated with adalimumab (EMA 2009b). Though the causal relationship of hematological reactions and the use of adalimumab remain unclear as of 2008, the FDA label states, "Rare reports of pancytopenia including aplastic anemia have been reported with TNF blocking agents". Furthermore, the FDA reports "Treatment with Humira® (adalimumab) may result in the formation of autoantibodies and, rarely, in the development of a lupus‐like syndrome" (FDA 2017a).
Anakinra
Anakinra leads to an increased rate of infections (2%) versus placebo (less than 1%). Following the EMA standard of classification of frequency of the occurrence of "undesirable effects" mentioned above, neutropenia and serious infection requiring hospitalization were common (between 1/10 and 1/100) and headaches and injection site reactions were very common occurring in 1/10 or more patients treated with anakinra (EMA 2004). "A… clinical trial sponsored by Amgen Inc. showed a higher incidence of serious infection and of neutropenia in anakinra and etanercept combination group than patients receiving Enbrel (etanercept) alone and higher than observed in previous trials where Kineret (anakinra) was used alone (EMA 2003), therefore, the use of etanercept and anakinra is not recommended as it leads to safety complications)". Furthermore, the FDA reports that "Hypersensitivity reactions associated with Kineret (anakinra) administration are rare" (FDA 2016). Moreover, the FDA reports the effects of anakinra on the hematologic conditions of patients stating that, "In placebo‐controlled studies with Kineret® (anakinra), treatment was associated with small reductions in the mean values for total white blood count, platelets, and absolute neutrophil count (ANC), and a small increase in the mean eosinophil differential percentage" (FDA 2016). With regard to the development of malignancies for patients treated with anakinra, trials show that, "twenty‐one malignancies of various types were observe in 2531 RA patients treated in clnical trials with Kineret® for up to 50 months. The observed rates and incidences were similar to those expected for the population studied." (FDA 2016).
Etanercept
In the post‐marketing reports of etanercept, "Infections, including serious infections leading to hospitalization or death, have been observed in patients treated with Enbrel® (etanercept)" (FDA 2016a). Furthermore, "Data from clinical trials and preclinical studies suggest that the risk of reactivation of latent tuberculosis infection is lower with Enbrel® than with TNF‐blocking monoclonal antibodies. Nonetheless, post‐marketing cases of tuberculosis reactivation have been reported for TNF blockers, including Enbrel® (etanercept). Patients receiving Enbrel® should be monitored closely for signs and symptoms of active tuberculosis. The possibility of tuberculosis should be considered, especially in patients who have travelled to countries with a high prevalence of tuberculosis or had close contact with a person with active tuberculosis. All patients treated with Enbrel® should have a thorough history taken prior to initiating therapy" (FDA 2016a). This finding is also stated in an important health warning issued by Health Canada in 2006 (Health Canada 2006a).
Furthemore, etanercept has been associated with the risk of histoplasmosis and other invasive fungal infections. Health Canada 2009 states, "...although no histoplasmosis infections were reported among 17,696 patients from the United States and Canada who were treated with Enbrel®, in 38 clinical trials and four cohort studies involving all authorized indications, post marketing cases of serious and sometimes fatal fungal infections, including histoplasmosis, have been reported with TNF blockers, including Enbrel®." The FDA also outlines the risk of nervous system complications stating, "nervous system complications such as multiple sclerosis, seizures, or inflammation of the nerves of the eyes have occurred in rare cases" (FDA 2016a).
Infections, including serious infections leading to hospitalization or death, have been observed in patients treated with Enbrel® (etanercept) (FDA 2016a). The FDA reports on the risk of malignancies for patients on etanercept treatment, stating "Patients have been observed in clinical trials with Enbrel® for over five years. Among 4462 rheumatoid arthritis patients treated with Enbrel® in clinical trials for a mean of 27 months (approximately 10,000 patient‐years of therapy), lymphomas were observed for a rate of 0.09 cases per 100 patient‐years. This is 3‐fold higher than the rate of lymphomas expected in the general population based on the Surveillance, Epidemiology, and End Results Database. Rare reports of pancytopenia including aplastic anemia, some with a fatal outcome, have been reported in patients treated with Enbrel®" (FDA 2016a). The FDA also reports, "Treatment with Enbrel® may result in the formation of autoantibodies and, rarely, in the development of a lupus‐like syndrome or autoimmune hepatitis which may resolve following withdrawal of Enbrel®" (FDA 2016a).
The use of etanercept has also been associated with the relapse of hepatitis B (Health Canada 2006a).
Infliximab
In its recent revised report on infliximab, the EMA reports on the risk of infusion reactions and hypersensitivity, stating, "An infusion‐related reaction was defined in clinical studies as any adverse event occurring during an infusion or within 1 to 2 hours after an infusion. In clinical studies, approximately 20% of infliximab‐treated patients compared with approximately 10% of placebo‐treated patients experienced an infusion‐related effect. Approximately 3% of patients discontinued treatment due to infusions reactions" (EMA 2009a). Infliximab is also associated with the relapse of hepatitis B as reported by Health Canada in 2006 (Health Canada 2006a). "Opportunistic infections have been reported in patients treated with infliximab, suggesting that host defence against infection is compromised. It should be noted that suppression of TNF‐alpha may also mask symptoms of infection such as fever." There is also a possible association between infliximab and heptosplenix T‐Cell lymphoma in pediatric and young adult patients with Crohn’s disease (Health Canada 2006b).
"In a study designed to evaluate Remicade® (infliximab) in congestive heart failure (CHF), 150 patients with moderate to severe (NYHA class II‐IV) CHF were treated with three infusions of Remicade 5mg/ kg, or placebo over six weeks. Higher incidences of mortality and hospitalization for worsening heart failure were seen in those patients treated with Remicade®, especially those treated with the higher dose of 10mg/kg. At present 7 out of 101 patients treated with Remicade® have died compared to no deaths among 49 patients on placebo" (EMA 2001). In a May 2009 revision of the Remicade label, the FDA warns, "Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving TNF‐ blocking agents. Among opportunistic infections, tuberculosis, histoplasmosis, aspergillosis, candidiasis, coccidioidomycosis, listeriosis, and pneumocystosis were the most commonly reported. Patients have frequently presented with disseminated rather than localized disease, and are often taking concomitant immunosuppressants such as methotrexate or corticosteroids with Remicade®" (FDA 2015a). In an investigation of neurological events, EMA reports "Infliximab and other agents that inhibit TNF‐alpha have been associated in rare cases with optic neuritis, seizure and new onset or exacerbation of clinical symptoms and/or radiographic evidence of central nervous system demyelinating disorders, including multiple sclerosis, and peripheral demyelinating disorders, including Guillain‐Barré syndrome" (EMA 2009a).
Evidence of infliximab associated with lymphoma states that "In the controlled portions of clinical trials of all the TNF‐blocking agents, more cases of lymphoma have been observed among patients receiving a TNF blocker compared with control patients… In the combined clinical trial population for rheumatoid arthritis, Crohn's disease, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and plaque psoriasis, lymphomas were observed for a rate of 0.10 cases per 100 patient‐years of follow‐up, which is approximately 4‐fold higher than expected in the general population. Patients with Crohn's disease, rheumatoid arthritis or plaque psoriasis, particularly patients with highly active disease and/or chronic exposure to immunosuppressant therapies, may be at a higher risk (up to several fold) than the general population for the development of lymphoma, even in the absence of TNF‐blocking therapy" (FDA 2015a).
TNF‐blockers as a group
In 2008, the FDA issued a safety alert regarding anti‐TNF biologics, which stated that the risk of pulmonary and disseminated histoplasmosis, coccidioidomycosis, blastomycosis and other opportunistic infections were not consistently recognized in patients taking tumor necrosis factor‐alpha blockers (TNF blockers including etanercept, adalimumab, infliximab or certolizumab), which resulted in the delay of proper antifungal treatment and at times led to death (FDA 2008a). The FDA reviewed 240 reports of histoplasmosis, an infection caused by the fungus Histoplasma capsulatum, in patients being treated with Enbrel, Humira, or Remicade. The majority of the reports involved people in the Ohio River and Mississippi River valleys (the fungus is commonly found in those areas). In at least 21 of the reports, histoplasmosis was initially not recognized by healthcare professionals, and antifungal treatment was delayed. Twelve of those patients died. The FDA recommended that for patients at risk of histoplasmosis and other invasive fungal infections, clinicians should consider empiric antifungal treatment until the pathogen(s) are identified.
Rituximab
The FDA provides its most recent safety information for Rituxan® (rituximab) from 2014. Rituxan was found to be associated with progressive multifocal leukoencephalopathy. FDA and Genentech notified: "A third case of progressive multifocal leukoencephalopathy (PML) has been reported in a patient with rheumatoid arthritis treated with Rituxan.” In view of this event, Genentech has advised physicians to have high index of suspicion for PML stated as “Physicians should consider PML in any patient being treated with Rituxan who presents with new onset neurologic manifestations. Consultation with a neurologist, brain MRI, and lumbar puncture should be considered as clinically indicated. In patients who develop PML, Rituxan should be discontinued.” (FDA 2014). Another event associated with Rituxan was notified in a FDA label from 2008. In this label, the possible safety complications of Rituxan® use included "tumor lysis syndrome which necessitates clinicians to administer prophylaxis and monitor patients renal function, hepatitis B reactivation with fulminant hepatitis, which can sometimes (be) fatal and the risk of progressive multifocal leukoencephalopathy" (Drugs 2006).
FDA and Genentech informed healthcare professionals of important emerging safety information about Rituxan®. "Two patients died after being treated with Rituxan® for systemic lupus erythematosus (SLE). Rituxan® is approved for the above indication and is prescribed off‐label for other serious diseases and conditions such as SLE. The cause of death was progressive multifocal leukoencephalopathy, a viral infection of the brain (that is caused by reactivated JC virus which is present in about 80% of adults" (FDA 2006). Further risks include "cardiac arrhythmias and angina" which can be life threatening, and "bowel obstruction and perforation" (FDA 2016a). Health Canada 2006a also provided warnings of bowel obstruction and perforation, "Reports of abdominal pain, bowel obstruction, and perforation, in some cases leading to death, have been observed in patients receiving Rituxan®. The majority of reports, including all deaths, have occurred in patients receiving Rituxan in combination with chemotherapy for NHL ( (non‐Hodgkin’s Lymphoma)) indication. A causal relationship has not been established".
Tofacitinib
In 2015, the FDA issued a Risk Evaluation and Mitigation Strategy (REMS) warning related to tofacitinib highlighting the "Risk of serious infections, malignancies, decreases in peripheral lymphocyte counts, neutrophil counts, hemoglobin, and increases in lipid parameters in peripheral blood with XELJANZ (tofacitinib)" (FDA 2015).
Contributions of authors
JS ‐ study concept JS, GW ‐ protocol development JS, PT, GW, ETG ‐ protocol editing JS, GW, ETG ‐ data extraction JS, AM ‐ study quality rating AH, GW, JS, AM‐ data analysis JS ‐ first draft of the review and NMA All authors ‐ revision of the manuscript, and approval of the final version
Sources of support
Internal sources
-
The Oak Foundation, Switzerland.
provide support for The Parker Institute: Musculoskeletal Statistics Unit.
-
Birmingham VA Medical Center, Other.
JAS is also supported by the resources and the use of facilities at the VA Medical Center at Birmingham, Alabama, USA.
External sources
-
Jasvinder Singh, USA.
This work was supported in part by resources provided to the UAB Cochrane NMA Satellite by the Rheumatology division at University of Alabama at Birmingham (UAB). JAS is supported by grants from the Agency for Health Quality and Research Center for Education and Research on Therapeutics (AHRQ CERTs) U19 HS021110, National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) P50 AR060772 and U34 AR062891, National Institute of Aging (NIA) U01 AG018947, National Cancer Institute (NCI) U10 CA149950, and research contract CE‐1304‐6631 from the Patient Centered Outcomes Research Institute (PCORI). JAS is also supported by the resources and the use of facilities at the VA Medical Center at Birmingham, Alabama, USA.
Declarations of interest
JS ‐ JS has received research grants from Takeda and Savient and consultant fees from Savient, Takeda, Regeneron, Merz, Iroko, Bioiberica, Crealta and Allergan pharmaceuticals, WebMD, UBM LLC and the American College of Rheumatology. JS serves as the principal investigator for an investigator‐initiated study funded by Horizon pharmaceuticals through a grant to DINORA, Inc., a 501 (c)(3) entity. JS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms‐length funding from 36 companies; a member of the American College of Rheumatology's (ACR) Annual Meeting Planning Committee (AMPC); Chair of the ACR Meet‐the‐Professor, Workshop and Study Group Subcommittee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee. AH ‐ none AM ‐ none EG ‐ none RB ‐ RB is a Principal Investigator and Chair of the Management Committee of the Australian Rheumatology Association Database (ARAD). The Australian Rheumatology Association receives ongoing unrestricted educational grants from Abbvie, AstraZeneca, Bristol‐Myers Squibb, Celgene, Pfizer and Sanofi to support ARAD LM ‐ none MSA ‐ research grant from Pfizer. Consultant fees from Pfizer PT ‐ grants/honoraria from Bristol Myers, Chiltern International, and UCB GW ‐ research grant and consultant fee from Bristol‐Myers Squibb
Joint senior author
Joint senior author
New
References
References to included reviews
Bejarano 2008
- Bejarano V, Quinn M, Conaghan PG, Reece R, Keenan A, Walker D, et al. Effect of the early use of the anti–tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthritis and Rheumatism 2008;59(10):1467–147. [DOI] [PubMed] [Google Scholar]
Bejarano 2010
- Bejarano V, Conaghan PG, Quinn M, Saleem B, Emery P. Benefits 8 years after a remission induction regime with an infliximab and methotrexate combination in early rheumatoid arthritis. Rheumatology 2010;49:1971–4. [DOI] [PubMed] [Google Scholar]
Breedveld 2006
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, Vollenhoven R, et al. The PREMIER Study. A multicenter, randomized, double‐blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis and Rheumatism 2006;54(1):26‐37. [DOI] [PubMed] [Google Scholar]
Detert 2013
- Detert J, Bastian H, Listing J, Weiß A, Wassenberg S, Liebhaber A, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD‐naïve patients with early rheumatoid arthritis: HIT HARD, an investigator‐initiated study. Annals of the Rheumatic Diseases 2013;72:844–50. [DOI] [PubMed] [Google Scholar]
Durez 2007
- Durez P, Malghem J, Nzeusseu Toukap A, Depresseux G, Lauwerys BR, Westhovens R, et al. Treatment of early rheumatoid arthritis. A randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis and Rheumatism 2007;56(12):3919–27. [DOI] [PubMed] [Google Scholar]
Emery 2008
- Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double‐blind, parallel treatment trial. Lancet 2008;372:375‐82. [DOI] [PubMed] [Google Scholar]
Emery 2009
- Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, et al. Golimumab, a human anti–tumor necrosis factor monoclonal antibody, injected subcutaneously every four weeks in methotrexate‐naive patients with active rheumatoid arthritis. Twenty‐four–week results of a phase III, multicenter, randomized, double‐blind, placebo‐controlled study of golimumab before methotrexate as first‐line therapy for early‐onset rheumatoid arthritis. Arthritis and Rheumatism 2009;60(8):2272–83. [DOI] [PubMed] [Google Scholar]
Kavanaugh 2013
- Kavanaugh A, Fleischmann RM, Emery P, Kupper H, Redden L, Guerette B, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26‐week results from the randomised, controlled OPTIMA study. Annals of the Rheumatic Diseases 2013;72:64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcora 2006
- Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomized phase 2 trial of anti‐tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. American Journal of Clinical Nutrition 2008;84:1463‐72. [DOI] [PubMed] [Google Scholar]
Nam 2014a
- Nam JL, Villeneuve E, Hensor EM, Conaghan PG, Keen HI, Buch MH, et al. Remission induction comparing infliximab and high‐dose intravenous steroid, followed by treat‐to‐target: a double‐blind, randomised, controlled trial in new‐onset, treatment‐naive, rheumatoid arthritis (the IDEA study). Annals of the Rheumatic Diseases 2014;73(1):75‐85. [DOI] [PubMed] [Google Scholar]
Nam 2014b
- Nam JL, Villeneuve E, Hensor EM, Wakefield RJ, Conaghan PG, Green MJ, et al. A randomised controlled trial of etanercept and methotrexate to induce remission in early inflammatory arthritis: the EMPIRE trial. Annals of the Rheumatic Diseases 2014;73(6):1027‐36. [DOI] [PubMed] [Google Scholar]
Quinn 2005
- Quinn MA, Conaghan PG, O’Connor PJ, Karim Z, Greenstein A, Brown A, et al. Very early treatment with infliximab in addition to methotrexate in early, poor‐prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal. Results from a twelve‐month randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism 2005;52(1):27‐35. [DOI] [PubMed] [Google Scholar]
Rantalaiho 2014
- Rantalaiho V, Kautiainen H, Korpela M, Hannonen P, Kaipiainen‐Seppanen O, Mottonen T, et al. Targeted treatment with a combination of traditional DMARDs produces excellent clinical and radiographic long‐term outcomes in early rheumatoid arthritis regardless of initial infliximab. The 5‐year follow‐up results of a randomised clinical trial, the NEO‐RACo trial. Annals of the Rheumatic Diseases 2014;73(11):1954‐61. [DOI] [PubMed] [Google Scholar]
Soubrier 2009
- Soubrier M, Puechal X, Sibilia J, Mariette X, Meyer O, Combe B. Evaluation of two strategies (initial methotrexate monotherapy vs its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Rheumatology 2009;49:1429–34. [DOI] [PubMed] [Google Scholar]
St Clair 2004
- Clair WE, Heidje DMFM, Smolen JS, Maini RM, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis a randomized, controlled trial. Arthritis and Rheumatism 2004;50(11):3432–43. [DOI] [PubMed] [Google Scholar]
Tak 2012
- Tak PP, Rigby W, Rubbert‐Roth A, Peterfy C, Vollenhoven RF, Stohl W, et al. Sustained inhibition of progressive joint damage with rituximab plus methotrexate in early active rheumatoid arthritis: 2‐year results from the randomised controlled trial IMAGE. Annals of the Rheumatic Diseases 2012;71:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takeuchi 2014
- Takeuchi T, Yamanaka H, Ishiguro N, Miyasaka N, Mukai M, Matsubara T, et al. Adalimumab, a human anti‐TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Annals of the Rheumatic Diseases 2014;73(3):536‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tam 2012
- Tam L‐S, Shang Q, Li EK, Wang S, Li R‐J, Lee K‐L, et al. Infliximab is associated with improvement in arterial stiffness in patients with early rheumatoid arthritis ‐ a randomized trial. The Journal of Rheumatology 2012;39:2267‐75. [DOI] [PubMed] [Google Scholar]
Westhovens 2009
- Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate‐naive patients with early rheumatoid arthritis and poor prognostic factors. Annals of the Rheumatic Diseases 2009;68:1870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to excluded reviews
Abe 2006
- Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, et al. A multicenter, double‐blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. The Journal of Rheumatology 2006;33:37‐44. [PubMed] [Google Scholar]
Axelsen 2015
- Axelsen MB, Eshed I, Horslev‐Petersen K, Stengaard‐Pedersen K, Hetland ML, Moller J, et al. A treat‐to‐target strategy with methotrexate and intra‐articular triamcinolone with or without adalimumab effectively reduces MRI synovitis, osteitis and tenosynovitis and halts structural damage progression in early rheumatoid arthritis: results from the OPERA randomised controlled trial. Annals of the Rheumatic Diseases 2015;74(5):867‐75. [DOI] [PubMed] [Google Scholar]
Bae 2013
- Bae SC, Gun SC, Mok CC, Khandker R, Nab HW, Koenig AS, et al. Improved health outcomes with etanercept versus usual DMARD therapy in an Asian population with established rheumatoid arthritis. BMC Musculoskeletal Disorders 2013;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bathon 2000
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. New England Journal of Medicine 2000;343(22):1586‐93. [DOI] [PubMed] [Google Scholar]
Bingham 2015
- Bingham CO 3rd, Rizzo W, Kivitz A, Hassanali A, Upmanyu R, Klearman M. Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: results of a randomised controlled trial (VISARA). Annals of the Rheumatic Diseases 2015;74(5):818‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonafede 2015
- Bonafede M, Johnson BH, Tang DH, Shah N, Harrison DJ, Collier DH. Etanercept‐methotrexate combination therapy initiators have greater adherence and persistence than triple therapy initiators with rheumatoid arthritis. Arthritis Care and Research (Hoboken) 2015;67(12):1656‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burmester 2013
- Burmester GR, Blanco R, Charles‐Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP‐690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381(9865):451‐60. [DOI] [PubMed] [Google Scholar]
Burmester 2015
- Burmester GR, Kivitz AJ, Kupper H, Arulmani U, Florentinus S, Goss SL, et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Annals of the Rheumatic Diseases 2015;74(6):1037‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2009
- Chen DY, Chou SJ, Hsieh TY, Chen YH, Chen HH, Hsieh CW, et al. Randomized, double‐blind, placebo‐controlled, comparative study of human anti‐TNF antibody adalimumab in combination with methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis. Journal of the Formosan Medical Association 2009;108:310‐9. [DOI] [PubMed] [Google Scholar]
Cheng 2014
- Cheng T, Wang M, Chen Z, Eisenberg RA, Zhang Y, Zou Y, et al. Tartrate‐resistant acid phosphatase 5b is a potential biomarker for rheumatoid arthritis: a pilot study in Han Chinese. Chinese Medical Journal 2014;127(16):2894‐9. [PubMed] [Google Scholar]
Choy 2012
- Choy E, McKenna F, Vencovsky J, Valente R, Goel N, Vanlunen B, et al. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology 2012;51:1226‐34. [DOI] [PubMed] [Google Scholar]
Cohen 2002
- Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin‐1 receptor antagonist, in combination with methotrexate: results of a twenty‐four‐week, multicenter, randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatology 2002;46:614‐24. [DOI] [DOI] [PubMed] [Google Scholar]
Cohen 2003
- Cohen M, Wolfe R, Mai T, Lewis D. A randomized, double blind, placebo controlled trial of a topical cream containing glucosamine sulfate, chondroitin sulfate, and camphor for osteoarthritis of the knee. Journal of Rheumatology 2003;30(3):523‐8. [PubMed] [Google Scholar]
Cohen 2004
- Cohen SB, Moreland LW, Cush JJ, Greenwald MW, Block S, Shergy WJ, et al. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Annals of the Rheumatic Diseases 2004;63:1062‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2006
- Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy: results of a multicenter, randomized, double‐blind, placebo‐controlled, phase III trial evaluating primary efficacy and safety at twenty‐four weeks. Arthritis and Rheumatism 2006;54(9):2793‐806. [DOI] [PubMed] [Google Scholar]
Combe 2006
- Combe B, Codreanu C, Fiocco U, Gaubitz M, Geusens PP, Kvien TK, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double‐blind comparison. Annals of the Rheumatic Diseases 2006;65:1357‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conaghan 2013
- Conaghan PG, Durez P, Alten RE, Burmester GR, Tak PP, Klareskog L, et al. Impact of intravenous abatacept on synovitis, osteitis and structural damage in patients with rheumatoid arthritis and an inadequate response to methotrexate: the ASSET randomised controlled trial. Annals of the Rheumatic Diseases 2013;72:1287‐94. [DOI] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conaghan 2014
- Conaghan PG, Peterfy C, Olech E, Kaine J, Ridley D, Dicarlo J, et al. The effects of tocilizumab on osteitis, synovitis and erosion progression in rheumatoid arthritis: results from the ACT‐RAY MRI substudy. Annals of the Rheumatic Diseases 2014;73(5):810‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dougados 2014
- Dougados M, Kissel K, Conaghan PG, Mola EM, Schett G, Gerli R, et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab‐based treatment strategies in rheumatoid arthritis: the ACT‐RAY study. Annals of the Rheumatic Diseases 2014;73(5):803‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2013
- Doyle MK, Rahman MU, Frederick B, Birbara CA, Vries D, Toedter G, et al. Effects of subcutaneous and intravenous golimumab on inflammatory biomarkers in patients with rheumatoid arthritis: results of a phase 1, randomized, open‐label trial. Rheumatology (Oxford) 2013;52(7):1214‐9. [DOI] [PubMed] [Google Scholar]
Durez 2004
- Durez P, Nzeusseu Toukap A, Lauwerys BR, Manicourt DH, Verschueren P, Westhovens R. A randomised comparative study of the short term clinical and biological effects of intravenous pulse methylprednisolone and infliximab in patients with active rheumatoid arthritis despite methotrexate treatment. Arthritis and Rheumatism 2004;63:1069‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emery 2006
- Emery P, Fleischmann R, Filipowicz‐Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double‐blind, placebo‐controlled, dose‐ranging trial. Arthritis and Rheumatology 2006;54:1390‐400. [DOI] [PubMed] [Google Scholar]
Emery 2010
- Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo‐controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab's Efficacy in MTX iNadequate rEsponders (SERENE)). Annals of the Rheumatic Diseases 2010;69:1629‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emery 2014a
- Emery P, Hammoudeh M, FitzGerald O, Combe B, Martin‐Mola E, Buch MH, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. New England Journal of Medicine 2014;371(19):1781‐92. [DOI] [PubMed] [Google Scholar]
Emery 2014b
- Emery P, Fleischmann RM, Hsia EC, Xu S, Zhou Y, Baker D. Efficacy of golimumab plus methotrexate in methotrexate‐naïve patients with severe active rheumatoid arthritis. Clinical Rheumatology 2014;33(9):1239‐46. [DOI] [PubMed] [Google Scholar]
Emery 2014c
- Emery P, Hammoudeh M, FitzGerald O, Combe B, Martin‐Mola E, Buch MH, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. New England Journal of Medicine 2014;371(19):1781‐92. [DOI] [PubMed] [Google Scholar]
Ericksson 2015
- Eriksson JK, Karlsson JA, Bratt J, Petersson IF, Vollenhoven RF, Ernestam S, et al. Cost‐effectiveness of infliximab versus conventional combination treatment in methotrexate‐refractory early rheumatoid arthritis: 2‐year results of the register‐enriched randomised controlled SWEFOT trial. Annals of the Rheumatic Diseases 2015;74(6):1094‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleischmann 2003
- Fleischmann RM, Schechtman J, Bennett R, Handel ML, Burmester GR, Tesser J, et al. Anakinra, a recombinant human interleukin‐1 receptor antagonist (r‐metHuIL‐1ra), in patients with rheumatoid arthritis: a large, international, multicenter, placebo‐controlled trial. Arthritis and Rheumatism 2003;48(4):927‐34. [DOI] [PubMed] [Google Scholar]
Fleischmann 2009
- Fleischmann R, Vencovsky J, Vollenhoven RF, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease‐modifying antirheumatic therapy: the FAST4WARD study. Annals of the Rheumatic Diseases 2009;68:805‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleischmann 2013
- Fleischmann R, Weinblatt ME, Schiff M, Khanna D, Maldonado MA, Nadkarni A, et al. 2‐year results from the Ample (Abatacept versus adalimumab comparison in biologic‐naïve RA patients with background methotrexate) trial: changes in patient‐reported outcomes in response to subcutaneous abatacept or adalimumab in rheumatoid arthritis. Arthritis and Rheumatism 2013;65(Suppl 10):S577. [Google Scholar]
Furst 2003
- Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor‐alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). Journal of Rheumatology 2003;30:2563‐71. [PubMed] [Google Scholar]
Furst 2007
- Furst DE, Gaylis N, Bray V, Olech E, Yocum D, Ritter J, et al. Open‐label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Annals of the Rheumatic Diseases 2007;66(7):893‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furst 2015
- Furst DE, Shaikh SA, Greenwald M, Bennett B, Davies O, Luijtens K, et al. Two dosing regimens of certolizumab pegol in patients with active rheumatoid arthritis. Arthritis Care & Research 2015;67(2):151‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabay 2013a
- Gabay C, Emery P, Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double‐blind, controlled phase 4 trial. Lancet 2013;381(9877):1541‐50. [DOI] [PubMed] [Google Scholar]
Gashi 2014
- Gashi AA, Rexhepi S, Berisha I, Kryeziu A, Ismaili J, Krasniqi G. Treatment of rheumatoid arthritis with biologic DMARDS (rituximab and etanercept). Medical Archives 2014;68(1):51‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Genovese 2002
- Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two‐year radiographic and clinical outcomes. Arthritis and Rheumatism 2002;46(6):1443‐50. [DOI] [PubMed] [Google Scholar]
Genovese 2004
- Genovese MC, Cohen S, Moreland L, Lium D, Robbins S, Newmark R, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis and Rheumatology 2004;50:1412‐9. [DOI] [PubMed] [Google Scholar]
Genovese 2005
- Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. New England Journal of Medicine 2005;353(11):1114‐23. [DOI] [PubMed] [Google Scholar]
Genovese 2008
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, Silva NA, Alecock E, et al. Interleukin‐6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease‐modifying antirheumatic drugs: the tocilizumab in combination with traditional disease‐modifying antirheumatic drug therapy study. Arthritis and Rheumatology 2008;58:2968‐80. [DOI] [PubMed] [Google Scholar]
Genovese 2011
- Genovese MC, Covarrubias A, Leon G, Mysler E, Keiserman M, Valente R, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb non‐inferiority study in patients with an inadequate response to methotrexate. Arthritis and Rheumatology 2011;63:2854‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Genovese 2014
- Genovese MC, Fleischmann R, Furst D, Janssen N, Carter J, Dasgupta B, et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study. Annals of the Rheumatic Diseases 2014;73(9):1607‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gherge 2014
- Gherge AM, Ramiro S, Landewé R, Mihai C, Heidje D. THU0229 Association of radiographic damage with physical function in patients with rheumatoid arthritis – results from the RAPID1 Trial. Annals of the Rheumatic Diseases 2014;73(Suppl 2):261. [Google Scholar]
Goekoop‐Ruiterman 2007
- Goekoop‐Ruiterman YP, Vries‐Bouwstra JK, Allaart CF, Zeben D, Kerstens PJ, Hazes JM, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Annals of Internal Medicine 2007;146:406‐15. [DOI] [PubMed] [Google Scholar]
Haraoui 2014
- Haraoui B, Thorne JC, Keystone EC, Poulin‐Costello M, Trottier E, Vieira A, et al. Clinical and radiographic outcomes with etanercept and etanercept and methotrexate in patients with rheumatoid arthritis: two‐year results from the Canadian methotrexate and etanercept outcome study (CAMEO). Arthritis and Rheumatism 2014;65(Suppl 10):1464. [Google Scholar]
Heimans 2014
- Heimans L, Wevers‐de Boer KV, Visser K, Goekoop RJ, Oosterhout M, Harbers JB, et al. A two‐step treatment strategy trial in patients with early arthritis aimed at achieving remission: the IMPROVED study. Annals of the Rheumatic Diseases 2014;73(7):1356‐61. [DOI] [PubMed] [Google Scholar]
Hobbs 2015
- Hobbs K, Deodhar A, Wang B, Bitman B, Nussbaum J, Chung J, et al. Randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of etanercept in patients with moderately active rheumatoid arthritis despite DMARD therapy. Springerplus 2015;4:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huizinga 2015
- Huizinga TW, Conaghan PG, Martin‐Mola E, Schett G, Amital H, Xavier RM, et al. Clinical and radiographic outcomes at 2 years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT‐RAY study. Ann Rheum Dis 2015;74(1):35‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hørslev‐Petersen 2014
- Hørslev‐Petersen K, Hetland ML, Junker P, Pødenphant J, Ellingsen T, Ahlquist P, et al. Adalimumab added to a treat‐to‐target strategy with methotrexate and intra‐articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator‐initiated, randomised, double‐blind, parallel‐group, placebo‐controlled trial. Annals of the Rheumatic Diseases 2014;73(4):654‐61. [DOI] [PubMed] [Google Scholar]
Iannone 2014
- Iannone F, Montagna G, Bagnato G, Gremese E, Giardina A, Lapadula G. Safety of etanercept and methotrexate in patients with rheumatoid arthritis and hepatitis C virus infection: a multicenter randomized clinical trial. Journal of Rheumatology 2014;41(2):286‐92. [DOI] [PubMed] [Google Scholar]
Johnsen 2006
- Johnsen AK, Schiff MH, Mease PJ, Moreland LW, Maier AL, Coblyn JS, et al. Comparison of 2 doses of etanercept (50 vs 100 mg) in active rheumatoid arthritis: a randomized double blind study. Journal of Rheumatology 2006;33:659‐64. [PubMed] [Google Scholar]
Jones 2010
- Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez‐Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Annals of the Rheumatic Diseases 2010;69:88‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaine 2012
- Kaine J, Gladstein G, Strusberg I, Robles M, Louw I, Gujrathi S, et al. Evaluation of abatacept administered subcutaneously in adults with active rheumatoid arthritis: impact of withdrawal and reintroduction on immunogenicity, efficacy and safety (phase Iiib ALLOW study). Annals of the Rheumatic Diseases 2012;71:38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kameda 2011
- Kameda H, Kanbe K, Sato E, Ueki Y, Saito K, Nagaoka S, et al. Continuation of methotrexate resulted in better clinical and radiographic outcomes than discontinuation upon starting etanercept in patients with rheumatoid arthritis: 52‐week results from the JESMR study. Journal of Rheumatology 2011;38(8):1585‐92. [DOI] [PubMed] [Google Scholar]
Kavanaugh 2014
- Kavanaugh A, Mease PJ, Strand V, Purcaru O, Curtis JR. Effect of certolizumab pegol on workplace and household productivity in US patients with rheumatoid arthritis with or without prior anti‐tumor necrosis factor exposure: results from the predict study. Annals of the Rheumatic Diseases 2014;73(Suppl 2):927‐8. [Google Scholar]
Kay 2008
- Kay J, Matteson EL, Dasgupta B, Nash P, Durez P, Hall S, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double‐blind, placebo‐controlled, dose‐ranging study. Arthritis and Rheumatism 2008;58(4):964‐75. [DOI] [PubMed] [Google Scholar]
Kennedy 2014
- Kennedy WP, Simon JA, Offutt C, Horn P, Herman A, Townsend MJ, et al. Efficacy and safety of pateclizumab (anti‐lymphotoxin‐alpha) compared to adalimumab in rheumatoid arthritis: a head‐to‐head phase 2 randomized controlled study (The ALTARA Study). Arthritis Research & Therapy 2014;16(5):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keystone 2004a
- Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti‐tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo‐controlled, 52‐week trial. Arthritis & Rheumatology 2004;50(5):1400‐11. [DOI] [PubMed] [Google Scholar]
Keystone 2004b
- Keystone EC, Schiff MH, Kremer JM, Kafka S, Lovy M, DeVries T, et al. Once‐weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatology 2004;50:353‐63. [DOI] [DOI] [PubMed] [Google Scholar]
Keystone 2008
- Keystone E, Burmester GR, Furie R, Loveless JE, Emery P, Kremer J, et al. Improvement in patient‐reported outcomes in a rituximab trial in patients with severe rheumatoid arthritis refractory to anti–tumor necrosis factor therapy. Arthritis and Rheumatism 2008;59(6):785–93. [DOI] [PubMed] [Google Scholar]
Keystone 2014
- Keystone EC, Anisfeld A, Ogale S, Devenport JN, Curtis JR. Continued benefit of tocilizumab plus disease‐modifying antirheumatic drug therapy in patients with rheumatoid arthritis and inadequate clinical responses by week 8 of treatment. Journal of Rheumatology 2014;41(2):216‐26. [DOI] [PubMed] [Google Scholar]
Kim 2007
- Kim HY, Lee SK, Song YW, Yoo DH, Koh EM, Yoo B, et al. A randomized, double‐blind, placebo‐controlled, phase III study of the human anti‐tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexate. APLAR Journal of Rheumatology 2007;10:9‐16. [Google Scholar]
Kim 2012
- Kim HY, Hsu PN, Barba M, Sulaiman W, Robertson D, Vlahos B, et al. Randomized comparison of etanercept with usual therapy in an Asian population with active rheumatoid arthritis: the APPEAL trial. International Journal of Rheumatic Diseases 2012;15(2):188‐96. [DOI] [PubMed] [Google Scholar]
Kim 2013
- Kim J, Ryu H, Yoo DH, Park SH, Song GG, Park W, et al. A clinical trial and extension study of infliximab in Korean patients with active rheumatoid arthritis despite methotrexate treatment. Journal of Korean Medical Science 2013;28:1716‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kivitz 2014
- Kivitz A, Olech E, Borofsky M, Zazueta BM, Navarro‐Sarabia F, Radominski SC, et al. Subcutaneous tocilizumab versus placebo in combination with disease‐modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care Research 2014;66(11):1653‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koroleva 2014a
- Koroleva MV, Raskina TA. Effect of rituximab therapy on life quality with rheumatoid arthritis patients. Osteoporosis International 2014;25:S365‐6. [Google Scholar]
Koroleva 2014b
- Koroleva MV, Raskina TA. The effect of rituximab therapy on bone mineral density with rheumatoid arthritis patients. Osteoporosis International 2014;25:S374. [Google Scholar]
Kremer 2003
- Kremer JM, Westhovens R, Leon M, Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. New England Journal of Medicine 2003;349:1907‐15. [DOI] [PubMed] [Google Scholar]
Kremer 2005
- Kremer JM, Dougados M, Emery P, Durez P, Sibilia J, Shergy W, et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept twelve‐month results of a phase IIb, double‐blind, randomized, placebo‐controlled trial. Arthritis and Rheumatism 2005;52(8):2263‐71. [DOI] [PubMed] [Google Scholar]
Kremer 2006
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud‐Mendoza C, et al. Effects of abatacept in patients with methotrexate‐resistant active rheumatoid arthritis: a randomized trial. Annals of Internal Medicine 2006;144(12):865‐76. [DOI] [PubMed] [Google Scholar]
Kremer 2009
- Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double‐blind, placebo‐controlled phase IIa trial of three dosage levels of CP‐690,550 versus placebo. Arthritis and Rheumatism 2009;60(7):1895‐905. [DOI] [PubMed] [Google Scholar]
Kremer 2011
- Kremer JM, Blanco R, Brzosko M, Burgos‐Vargas R, Halland AM, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double‐blind treatment phase of a randomized placebo‐controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis and Rheumatology 2011;63(3):609‐21. [DOI] [PubMed] [Google Scholar]
Kremer 2015
- Kremer JM, Kivitz AJ, Simon‐Campos JA, Nasonov EL, Tony HP, Lee SK, et al. Evaluation of the effect of tofacitinib on measured glomerular filtration rate in patients with active rheumatoid arthritis: results from a randomised controlled trial. Arthritis Research & Therapy 2015;17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lan 2004
- Lan JL, Chou SJ, Chen DY, Chen YH, Hsieh TY, Young M Jr. A comparative study of etanercept plus methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis: a 12‐week, double‐blind, randomized, placebo‐controlled study. Journal of the Formosan Medical Association 2004;103(8):618‐23. [PubMed] [Google Scholar]
Landewe 2015
- Landewe R, Smolen JS, Florentinus S, Chen S, Guerette B, Heijde D. Existing joint erosions increase the risk of joint space narrowing independently of clinical synovitis in patients with early rheumatoid arthritis. Arthritis Research Therapy 2015;17:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipsky 2000
- Lipsky PE, Heijde DM, Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. New England Journal of Medicine 2000;343(22):1594‐602. [DOI] [PubMed] [Google Scholar]
Lisbona 2008
- Lisbona MP1, Maymo J, Perich J, Almirall M, Pérez‐García C, Carbonell J. Etanercept reduces synovitis as measured by magnetic resonance imaging in patients with active rheumatoid arthritis after only 6 weeks. Journal of Rheumatology 2008;35(3):394‐7. [PubMed] [Google Scholar]
Lisbona 2010
- Lisbona MP, Maymó J, Perich J, Almirall M, Carbonell J. Rapid reduction in tenosynovitis of the wrist and fingers evaluated by MRI in patients with rheumatoid arthritis after treatment with etanercept. Annals of the Rheumatic Diseases 2010;69(6):1117‐22. [DOI] [PubMed] [Google Scholar]
Machado 2014
- Machado DA, Guzman RM, Xavier RM, Simon JA, Mele L, Pedersen R, et al. Open‐label observation of addition of etanercept versus a conventional disease‐modifying antirheumatic drug in subjects with active rheumatoid arthritis despite methotrexate therapy in the Latin American region. Journal of Clinical Rheumatology 2014;20(1):25‐33. [DOI] [PubMed] [Google Scholar]
Maini 1999
- Maini R, Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354(9194):1932‐9. [DOI] [PubMed] [Google Scholar]
Manders 2015
- Manders SH, Kievit W, Adang E, Brus HL, Moens HJ, Hartkamp A, et al. Cost‐effectiveness of abatacept, rituximab, and TNFi treatment after previous failure with TNFi treatment in rheumatoid arthritis: a pragmatic multi‐centre randomised trial. Arthritis Research Therapy 2015;17:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathias 2000
- Mathias SD, Colwell HH, Miller DP, Moreland LW, Buatti M, Wanke L. Health‐related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clinical Therapeutics 2000;22(1):128‐39. [DOI] [PubMed] [Google Scholar]
McInnes 2015
- McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, et al. Effect of interleukin‐6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo‐controlled study. Annals of the Rheumatic Diseases 2015;74(4):694‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreland 2002
- Moreland LW, Alten R, Bosch F, Appelboom T, Leon M, Emery P, et al. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose‐finding, double‐blind, placebo‐controlled clinical trial evaluating CTLA‐4Ig and LEA29Y eighty‐five days after the first infusion. Arthritis and Rheumatism 2002;46(6):1470‐9. [DOI] [PubMed] [Google Scholar]
Nam 2010b
- Nam JL, Winthrop KL, Vollenhoven RF, Pavelka K, Valesini G, Hensor EM, et al. Current evidence for the management of rheumatoid arthritis with biological disease‐modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Annals of the Rheumatic Diseases 2010;69(6):976‐86. [DOI] [PubMed] [Google Scholar]
Navarro 2014
- Navarro Coy NC, Brown S, Bosworth A, Davies CT, Emery P, Everett CC, et al. The ‘Switch’ study protocol: a randomised controlled trial of switching to an alternative tumour‐necrosis factor (TNF)‐inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF‐inhibitor drug. BMC Musculoskeletal Disorders 2014;15:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishimoto 2009
- Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL‐6 receptor inhibition therapy. Modern Rheumatology 2009;19(1):12‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Dell 2013
- O'Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, et al. Therapies for active rheumatoid arthritis after methotrexate failure. New England Journal of Medicine 2013;369(4):307‐18. [DOI] [PubMed] [Google Scholar]
Oakley 2014
- Oakley S, Esmaili N, Major G, Mathers D, Ratnarajah S, Kallen J, et al. A randomised controlled trial evaluating the effect of humira upon endothelial function in ACPA positive rheumatoid arthritis: an interim analysis. Arthritis and Rheumatology 2014;66:S211. [Google Scholar]
Pavelka 2013
- Pavelka K, Szekanecz Z, Damjanov N, Majdan M, Nasonov E, Mazurov V, et al. Induction of response with etanercept‐methotrexate therapy in patients with moderately active rheumatoid arthritis in Central and Eastern Europe in the PRESERVE study. Clinical Rheumatology 2013;32(9):1275‐81. [DOI] [PubMed] [Google Scholar]
Pope 2014
- Pope JE, Haraoui B, Thorne JC, Vieira A, Poulin‐Costello M, Keystone EC. The Canadian methotrexate and etanercept outcome study: a randomised trial of discontinuing versus continuing methotrexate after 6 months of etanercept and methotrexate therapy in rheumatoid arthritis.. Annals of the Rheumatic Diseases 2014;73:2144‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rau 2004
- Rau R, Simianer S, Riel PL, Putte LB, Krüger K, Schattenkirchner M, et al. Rapid alleviation of signs and symptoms of rheumatoid arthritis with intravenous or subcutaneous administration of adalimumab in combination with methotrexate. Scandinavian Journal of Rheumatology 2004;33(3):145‐53. [DOI] [PubMed] [Google Scholar]
Rigby 2011
- Rigby W, Ferraccioli G, Greenwald M, Zazueta‐Montiel B, Fleischmann R, Wassenberg S, et al. Effect of rituximab on physical function and quality of life in patients with rheumatoid arthritis previously untreated with methotrexate. Arthritis Care and Research 2011;63(5):711‐20. [DOI] [PubMed] [Google Scholar]
Rubbert‐Roth 2010
- Rubbert‐Roth A, Tak PP, Zerbini C, Tremblay JL, Carreño L, Armstrong G, et al. Efficacy and safety of various repeat treatment dosing regimens of rituximab in patients with active rheumatoid arthritis: results of a Phase III randomized study (MIRROR). Rheumatology 2010;49(9):1683‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiff 2004
- Schiff MH, DiVittorio G, Tesser J, Fleischmann R, Schechtman J, Hartman S, et al. The safety of anakinra in high‐risk patients with active rheumatoid arthritis: six‐month observations of patients with comorbid conditions. Arthritis and Rheumatism 2004;50(6):1752‐60. [DOI] [PubMed] [Google Scholar]
Schiff 2008b
- Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi‐centre, randomised, double‐blind, placebo‐controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Annals of the Rheumatic Diseases 2008;8:1096‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiff 2014
- Schiff M, Weinblatt ME, Valente R, Heijde D, Citera G, Elegbe A, et al. Head‐to‐head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two‐year efficacy and safety findings from AMPLE trial. Annals of the Rheumatic Diseases 2014;73:86‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smolen 2008
- Smolen JS, Beaulieu A, Rubbert‐Roth A, Ramos‐Remus C, Rovensky J, Alecock E, et al. Effect of interleukin‐6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double‐blind, placebo‐controlled, randomised trial. Lancet 2008;371(9617):987‐97. [DOI] [PubMed] [Google Scholar]
Smolen 2009
- Smolen JS, Han C, Heijde DM, Emery P, Bathon JM, Keystone E, et al. Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: the impacts of remission and tumour necrosis factor blockade. Annals of the Rheumatic Diseases 2009;68(6):823‐7. [DOI] [PubMed] [Google Scholar]
Smolen 2013
- Smolen JS, Nash P, Durez P, Hall S, Ilivanova E, Irazoque‐Palazuelos F, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381(9870):918‐29. [DOI] [PubMed] [Google Scholar]
Smolen 2014d
- Smolen JS, Emery P, Fleischmann R, Vollenhoven RF, Pavelka K, Durez P, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 2014;383(9914):321‐32. [DOI] [PubMed] [Google Scholar]
Smolen 2014e
- Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2013 update. Annals of the Rheumatic Diseases 2014;73(3):492‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smolen 2015
- Smolen JS, Emery P, Ferraccioli GF, Samborski W, Berenbaum F, Davies OR, et al. Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double‐blind, randomised, placebo‐controlled trial. Annals of the Rheumatic Diseases 2015;74(5):843‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonomoto 2014
- Sonomoto K, Yamaoka K, Kubo S, Hirata S, Fukuyo S, Maeshima K, et al. Effects of tofacitinib on lymphocytes in rheumatoid arthritis: relation to efficacy and infectious adverse events. Rheumatology 2014;53(5):914‐8. [DOI] [PubMed] [Google Scholar]
Strand 2008a
- Strand V, Singh JA. Improved health‐related quality of life with effective disease‐modifying antirheumatic drugs: evidence from randomized controlled trials. American Journal of Managed Care 2008;14(4):234‐54. [PubMed] [Google Scholar]
Strand 2012
- Strand V, Rentz AM, Cifaldi MA, Chen N, Roy S, Revicki D. Health‐related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. Journal of Rheumatology 2012;39(1):63‐72. [DOI] [PubMed] [Google Scholar]
Tak 2011
- Tak PP, Rigby W, Rubbert‐Roth A, Peterfy C, Vollenhoven RF, Stohl W, et al. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Annals of the Rheumatic Diseases 2011;71(3):351‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanaka 2011
- Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Tofacitinib Study Investigators. Phase II study of tofacitinib (CP‐690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Research 2011;63(8):1150‐8. [DOI] [PubMed] [Google Scholar]
Tanaka 2012
- Tanaka Y, Harigai M, Takeuchi T, Yamanaka H, Ishiguro N, Yamamoto K, et al. Golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis: results of the GO‐FORTH study. Annals of the Rheumatic Diseases 2012;71:817‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2004
- Taylor PC, Steuer A, Gruber J, Cosgrove DO, Blomley MJ, Marsters PA, et al. Comparison of ultrasonographic assessment of synovitis and joint vascularity with radiographic evaluation in a randomized, placebo‐controlled study of infliximab therapy in early rheumatoid arthritis. Arthritis and Rheumatism 2004;50(4):1107‐16. [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor PC, Steuer A, Gruber J, McClinton C, Cosgrove DO, Blomley MJ, et al. Ultrasonographic and radiographic results from a two‐year controlled trial of immediate or one‐year‐delayed addition of infliximab to ongoing methotrexate therapy in patients with erosive early rheumatoid arthritis. Arthritis and Rheumatism 2006;54(1):47‐53. [DOI] [PubMed] [Google Scholar]
Van der Heidje 2013
- Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP‐690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve‐month data from a twenty‐four‐month phase III randomized radiographic study. Arthritis and Rheumatism 2013;65(3):559‐70. [DOI] [PubMed] [Google Scholar]
Van der Kooij 2009
- Kooij SM, Cessie S, Goekoop‐Ruiterman YP, Vries‐Bouwstra JK, Zeben D, Kerstens PJ, et al. Clinical and radiological efficacy of initial vs delayed treatment with infliximab plus methotrexate in patients with early rheumatoid arthritis. Annals of the Rheumatic Diseases 2009;68(7):1153‐8. [DOI] [PubMed] [Google Scholar]
Van Vollenhoven 2009
- Vollenhoven RF, Ernestam S, Geborek P, Petersson IF, Coster L, Waltbrand E, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1‐year results of a randomised trial. Lancet 2009;374(9688):459‐66. [DOI] [PubMed] [Google Scholar]
Van Vollenhoven 2012a
- Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. New England Journal of Medicine 2012;367:508‐19. [DOI] [PubMed] [Google Scholar]
Van Vollenhoven 2012b
- Vollenhoven RF, Geborek P, Forslind K, Albertsson K, Ernestam S, Petersson IF, et al. Conventional combination treatment versus biological treatment in methotrexate‐refractory early rheumatoid arthritis: 2 year follow‐up of the randomised, non‐blinded, parallel‐group Swefot trial. Lancet 2012;379:1712‐20. [DOI] [PubMed] [Google Scholar]
Vital 2015
- Vital EM, Dass S, Buch MH, Rawstron AC, Emery P. An extra dose of rituximab improves clinical response in rheumatoid arthritis patients with initial incomplete B cell depletion: a randomised controlled trial. Annals of the Rheumatic Diseases 2015;74(6):1195‐201. [DOI] [PubMed] [Google Scholar]
Weinblatt 1999
- Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. New England Journal of Medicine 1999;340:253‐9. [DOI] [PubMed] [Google Scholar]
Weinblatt 2003
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis and Rheumatology 2003;48:35‐45. [DOI] [PubMed] [Google Scholar]
Weinblatt 2006
- Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease‐modifying antirheumatic drugs: a one‐year randomized, placebo‐controlled study. Arthritis and Rheumatology 2006;54:2807‐16. [DOI] [PubMed] [Google Scholar]
Weinblatt 2007
- Weinblatt M, Schiff M, Goldman A, Kremer J, Luggen M, Li T, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Annals of the Rheumatic Diseases 2007;66:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinblatt 2008
- Weinblatt ME, Schiff MH, Ruderman EM, Bingham CO 3rd, Li J, Louie J, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week. Arthritis and Rheumatism 2008;58(7):1921–30. [DOI] [PubMed] [Google Scholar]
Weinblatt 2012
- Weinblatt ME, Fleischmann R, Huizinga TW, Emery P, Pope J, Massarotti EM, et al. Efficacy and safety of certolizumab pegol in a broad population of patients with active rheumatoid arthritis: results from the REALISTIC phase IIIb study. Rheumatology 2012;51:2204‐14. [DOI] [PubMed] [Google Scholar]
Weinblatt 2014
- Weinblatt ME, Westhovens R, Mendelsohn AM, Kim L, Lo KH, Sheng S, et al. Radiographic benefit and maintenance of clinical benefit with intravenous golimumab therapy in patients with active rheumatoid arthritis despite methotrexate therapy: results up to 1 year of the phase 3, randomised, multicentre, double blind, placebo controlled GO‐FURTHER trial. Annals of the Rheumatic Diseases 2014;73(12):2152‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisman 2003
- Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, et al. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti‐tumor necrosis factor‐alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clinical Therapeutics 2003;25:1700‐21. [DOI] [PubMed] [Google Scholar]
Weisman 2007
- Weisman MH, Paulus HE, Burch FX, Kivitz AJ, Fierer J, Dunn M, et al. A placebo‐controlled, randomized, double‐blinded study evaluating the safety of etanercept in patients with rheumatoid arthritis and concomitant comorbid diseases. Rheumatology 2007;46:1122‐5. [DOI] [PubMed] [Google Scholar]
Westhovens 2006
- Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo‐controlled trial. Arthritis and Rheumatology 2006;54:1075‐86. [DOI] [PubMed] [Google Scholar]
Westhovens 2014
- Westhovens R, Weinblatt ME, Han C, Kim L, Mack M, Lu J, et al. Health‐related quality of life of patients with rheumatoid arthritis achieving DAS28 remission, improvement in physical function and no radiographic progression. Annals of the Rheumatic Diseases 2014;73:479‐80. [Google Scholar]
Yamamoto 2014a
- Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and safety of certolizumab pegol without methotrexate co‐administration in Japanese patients with active rheumatoid arthritis: the HIKARI randomized, placebo‐controlled trial. Modern Rheumatology 2014;24(4):552‐60. [DOI] [PubMed] [Google Scholar]
Yamanaka 2014
- Yamanaka H, Ishiguro N, Takeuchi T, Miyasaka N, Mukai M, Matsubara T, et al. Recovery of clinical but not radiographic outcomes by the delayed addition of adalimumab to methotrexate‐treated Japanese patients with early rheumatoid arthritis: 52‐week results of the HOPEFUL‐1 trial. Rheumatology 2014;53(5):904‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yazici 2012
- Yazici Y, Curtis JR, Ince A, Baraf H, Malamet RL, Teng LL, et al. Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease‐modifying antirheumatic drugs: the ROSE study. Annals of the Rheumatic Diseases 2012;71:198‐205. [DOI] [PubMed] [Google Scholar]
Zhang 2006
- Zhang F, Hou Y, Huang F, Wu D, Bao C, Ni L, et al. Infliximab versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a preliminary study from China. APLAR Journal of Rheumatology 2006;9:127‐30. [Google Scholar]
Additional references
Aletaha 2010
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis & Rheumatology 2010;62(9):2569‐81. [DOI] [PubMed] [Google Scholar]
Alonso‐Ruiz 2008
- Alonso‐Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, Calabozo M, Quintana A. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and meta analysis of efficacy and safety. BMC Musculoskeletal Disorders 2008;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnett 1988
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 1988;31(3):315‐24. [DOI] [PubMed] [Google Scholar]
Barlow 1999
- Barlow JH, Cullen LA, Rowe IF. Comparison of knowledge and psychological well‐being between patients with a short disease duration (< or = 1 year) and patients with more established rheumatoid arthritis (> or = 10 years duration). Patient Education and Counseling 1999;38:195‐203. [DOI] [PubMed] [Google Scholar]
Becker 2008
- Becker LA, Oxman AD. Chapter 22: Overviews of Reviews. In: Higgins JP, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from handbook.cochrane.org.
Boers 2001
- Boers M. Treatment of early disease. Rheumatic Diseases Clinics of North America 2001;27:405‐14, x. [DOI] [PubMed] [Google Scholar]
Bongartz 2006
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti‐TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta‐analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295(19):2275‐85. [DOI] [PubMed] [Google Scholar]
Bongartz 2009
- Bongartz T, Warren FC, Mines D, Matteson EL, Abrams KR, Sutton AJ. Etanercept therapy in rheumatoid arthritis and the risk of malignancies: a systematic review and individual patient data meta‐analysis of randomised controlled trials. Annals of the Rheumatic Diseases 2009;68(7):1177‐83. [DOI] [PubMed] [Google Scholar]
Boyce 2016
- Boyce EG, Vyas D, Rogan E, Valle‐Oseguera CS, O’Dell KM. Impact of tofacitinib on patient outcomes in rheumatoid arthritis – review of clinical studies. Patient Related Outcome Measures 2016;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradburn 2007
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26:53‐77. [DOI] [PubMed] [Google Scholar]
Brennan 2008
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. Journal of Clinical Investigation 2008;118(11):3537‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buggati 2014
- Bugatti S, Vitolo B, Caporali R, Montecucco C, Manzo A. B cells in rheumatoid arthritis: from pathogenic players to disease biomarkers. BioMed Research International 2014;2014:681678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cash 1994
- Cash JM, Klippel JH. Second‐line drug therapy for rheumatoid arthritis. New England Journal of Medicine 1994;330(19):1368‐75. [DOI] [PubMed] [Google Scholar]
Cates 2009 [Computer program]
- Dr Chris Cates' EBM website. Available from www.Nntonline.Net/visualrx/. Visual Rx NNT Calculator version 3. Dr Chris Cates' EBM website. Available from www.Nntonline.Net/visualrx/, Accessed 13 February 2009.
Choy 2001
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. New England Journal of Medicine 2001;344(12):907‐16. [DOI] [PubMed] [Google Scholar]
Chung 2006
- Chung CP, Thompson JL, Koch GG, Amara I, Strand V, Pincus T. Are American College of Rheumatology 50% response criteria superior to 20% criteria in distinguishing active aggressive treatment in rheumatoid arthritis clinical trials reported since 1997? A meta‐analysis of discriminant capacities. Annals of the Rheumatic Diseases 2006;65(12):1602‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Connell 2006
- Connell L, McInnes IB. New cytokine targets in inflammatory rheumatic diseases. Best Practice and Research Clinical Rheumatology 2006;20(5):865‐78. [DOI] [PubMed] [Google Scholar]
Cope 2008
- Cope AP. T cells in rheumatoid arthritis. Arthritis Research and Therapy 2008;10 Suppl 1:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 2007
- DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemporary Clinical Trials 2007;28(2):105‐14. [DOI] [PubMed] [Google Scholar]
Desai 2012
- Desai RJ, Hansen RA, Rao JK, Wilkins TM, Harden EA, Yuen A, et al. Mixed treatment comparison of the treatment discontinuations of biologic disease‐modifying antirheumatic drugs in adults with rheumatoid arthritis. Annals of Pharmacotherapy 2012;46(11):1491‐505. [DOI] [PubMed] [Google Scholar]
Dias 2011
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. 1–39 (2011). Available at www.nicedsu.org.uk Accessed 1May2017. [PubMed]
Donahue 2008
- Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas BL, et al. Systematic review: comparative effectiveness and harms of disease‐modifying medications for rheumatoid arthritis. Annals of Internal Medicine 2008;148(2):124‐34. [DOI] [PubMed] [Google Scholar]
Drugs 2006
- Drug Information Online. Drugs.com. FDA Approves First Rheumatoid Arthritis Indication for Rituxan. www.drugs.com/news/fda‐approves‐first‐rheumatoid‐arthritis‐indication‐rituxan‐1745.html 03/01/2006.
EMA 2001
- European Medicines Agency. EMA Public Statement on infliximab (Remicade): increased incidence of mortality and hospitalization for worsening congestive heart failure. www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/12/WC500018442.pdf.
EMA 2003
- European Medicines Agency. EMA public statement: Increased risk of serious infection and neutropenia in patients treated concurrently with Kineret (anakinra) and Enbrel (etanercept). www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/12/WC500018427.pdf.
EMA 2004
- European Medicines Agency. EPARs for authorized medicinal products for human use: Anakinra: European Public Assessment Report. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_the_public/human/000363/WC500042440.pdf First published 26/01/2009; Last updated 31/03/2016.
EMA 2009a
- European Medicines Agency. Remicade (inflixmab). www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_the_public/human/000240/WC500050883.pdf First published 10/08/2009; Last updated21/03/2012.
EMA 2009b
- European Medicines Agency. EPARs for authorized medicinal products for human use: Humira: European Public Assessment Report. www.emea.europa.eu/humandocs/Humans/EPAR/humira/humira.htm First published 23/11/2009; Last updated 21/02/2017.
FDA 1997
- Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products ‐ Rituxan. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=103705 1997. [Google Scholar]
FDA 1998
- Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products ‐ Remicade. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=103772 1998.
FDA 1998a
- Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products ‐ Enbrel. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=103795 1998.
FDA 2001
- Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products ‐ Kineret. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=103950 2001.
FDA 2002
- Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products ‐ Humira. www.accessdata.fda.gov/drugsatfda_docs/label/2002/adalabb123102lb.htm 2002.
FDA 2005
- Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products ‐ Orencia. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=125118 2005.
FDA 2006
- Food, Drug Administration. Rituxan® (rituximab). www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126519.htm.
FDA 2008
- Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products ‐ Cimzia. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=125160 2008.
FDA 2008a
- Food, Drug Administration. FDA: Manufacturers of TNF‐blocker drugs must highlight risk of fungal infections. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124185.htm#.
FDA 2009
- Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products ‐ Simponi. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=125289 2009.
FDA 2010
- Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products ‐ Actemra. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=125276 2010.
FDA 2012
- Food, Drug Administration. Drugs@FDA: FDA Approved Drug Products ‐ Xeljanz. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=203214 2012.
FDA 2014
- Food, Drug Administration. Rituxan (rituximab) Label. www.accessdata.fda.gov/drugsatfda_docs/label/2014/103705s5432lbl.pdf 2014.
FDA 2015
- Food, Drug Administration. Risk evaluation and mitigation strategy (REMS). NDA 203,214 XELJANZ® (tofacitinib). www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM330290.pdf.
FDA 2015a
- Food, Drug Administration. Remicade® (infliximab) Label. www.accessdata.fda.gov/drugsatfda_docs/label/2015/103772s5374lbl.pdf 2015.
FDA 2016
- Food, Drug Administration. Kineret (anakinra) Label. www.accessdata.fda.gov/drugsatfda_docs/label/2016/103950s5175lbl.pdf 2016.
FDA 2016a
- Food, Drug Administration. Enbrel® (etanercept) Label. www.accessdata.fda.gov/drugsatfda_docs/label/2016/103795s5552lbl.pdf 2016.
FDA 2017
- Food, Drug Administration. Orencia (abatacept) Label. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125118s211lbl.pdf 2017.
FDA 2017a
- Food, Drug Administration. Humira (adalimumab) Label. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125057s399lbl.pdf 2017.
Felson 1995
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1995;38(6):727‐35. [DOI] [PubMed] [Google Scholar]
Finckh 2006
- Finckh A, Liang MH, Herckenrode CM, Pablo P. Long‐term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta‐analysis. Arthritis and Rheumatism 2006;55(6):86‐72. [DOI] [PubMed] [Google Scholar]
Fransen 2005
- Fransen J, Riel PL. The Disease Activity Score and the EULAR response criteria. Clinical and Experimental Rheumatology 2005;23(5 Suppl 39):S93‐9. [PubMed] [Google Scholar]
Fries 1980
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis and Rheumatism 1980;23(2):137‐45. [DOI] [PubMed] [Google Scholar]
Furst 2008
- Furst DE, Keystone EC, Kirkham B, Fleischmann R, Mease P, Breedveld FC, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2008. Annals of the Rheumatic Diseases 2008;67 Suppl 3(Suppl 3):iii2‐25. [DOI] [PubMed] [Google Scholar]
Furst 2010
- Furst DE, Keystone EC, Fleischmann R, Mease P, Breedveld FC, Smolen JS, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases 2009. Annals of the Rheumatic Diseases 2010;69:Suppl 1:i2‐29. [DOI] [PubMed] [Google Scholar]
Furst 2012
- Furst DE, Keystone EC, Braun J, Breedveld FC, Burmester GR, Benedetti F, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases 2011. Annals of the Rheumatic Diseases 2012;71(Suppl 2):i2‐45. [DOI] [PubMed] [Google Scholar]
Gabay 2013
- Gabay C, Emery P, Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double‐blind, controlled phase 4 trial. Lancet 2013;381(9877):1541‐50. [DOI] [PubMed] [Google Scholar]
Gartlehner 2006
- Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. The Journal of Rheumatology 2006;33(12):2398‐408. [PubMed] [Google Scholar]
Ghogomu 2014
- Ghogomu EA, Maxwell LJ, Buchbinder R, Rader T, Pardo Pardo J, Johnston RV, et al. Updated method guidelines for Cochrane Musculoskeletal Group systematic reviews and metaanalyses. The Journal of Rheumatology 2014;41(2):194‐205. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Health Canada 2006a
- Health Canada. Important safety information on anti‐TNFα therapy: Enbrel (etanercept), Humira (adalimumab), and Remicade (infliximab) ‐ for health professionals. http://healthycanadians.gc.ca/recall‐alert‐rappel‐avis/hc‐sc/2006/14389a‐eng.php.
Health Canada 2006b
- Health Canada. Possible association of Remicade (infliximab) with hepatosplenic T‐cell lymphoma in pediatric and young adult patients with Crohn's disease ‐ for health professionals. http://healthycanadians.gc.ca/recall‐alert‐rappel‐avis/hc‐sc/2006/14201a‐eng.php.
Health Canada 2009
- Health Canada. Association of Enbrel (etanercept) with histoplasmosis and other invasive fungal infections ‐ for health professionals. http://www.healthycanadians.gc.ca/recall‐alert‐rappel‐avis/hc‐sc/2009/14545a‐eng.php.
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [DOI] [PubMed] [Google Scholar]
Kvien 2004
- Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics 2004;22(2 Suppl 1):1‐12. [DOI] [PubMed] [Google Scholar]
Kvien 2005
- Kvien TK, Uhlig T. Quality of life in rheumatoid arthritis. Scandinavian Journal of Rheumatology 2005;34(5):333‐41. [DOI] [PubMed] [Google Scholar]
Larsen 1977
- Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiologica: Diagnosis 1977;4:481‐91. [DOI] [PubMed] [Google Scholar]
Lee 2001
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001;358(9285):903‐11. [DOI] [PubMed] [Google Scholar]
Lee 2008
- Lee YH, Woo JH, Rho YH, Choi SJ, Ji JD, Song GG. Meta‐analysis of the combination of TNF inhibitors plus MTX compared to MTX monotherapy, and the adjusted indirect comparison of TNF inhibitors in patients suffering from active rheumatoid arthritis. Rheumatology International 2008;28(6):553‐9. [DOI] [PubMed] [Google Scholar]
Lee 2014
- Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. New England Journal of Medicine 2014;370(25):2377‐86. [DOI] [PubMed] [Google Scholar]
Leombruno 2008
- Leombruno JP, Einarson TR, Keystone EC. The safety of anti‐tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure‐adjusted pooled analyses of serious adverse events. Annals of the Rheumatic Diseases 2009;68(7):1136‐45. [DOI] [PubMed] [Google Scholar]
Lubeck 2004
- Lubeck DP. Patient‐reported outcomes and their role in the assessment of rheumatoid arthritis. Pharmacoeconomics 2004;22(2 Suppl 1):27‐38. [DOI] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42. [DOI] [PubMed] [Google Scholar]
Nam 2010
- Nam JL, Winthrop KL, Vollenhoven RF, Pavelka K, Valesini G, Hensor EM, et al. Current evidence for the management of rheumatoid arthritis with biological disease‐modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Annals of the Rheumatic Diseases 2010;69(6):976‐86. [DOI] [PubMed] [Google Scholar]
Nixon 2007
- Nixon RM, Bansback N, Brennan A. Using mixed treatment comparisons and meta‐regression to perform indirect comparisons to estimate the efficacy of biologic treatments in rheumatoid arthritis. Statistics in Medicine 2007;26(6):1237‐54. [DOI] [PubMed] [Google Scholar]
Odegard 2005
- Odegard S, Finset A, Kvien TK, Mowinckel P, Uhlig T. Work disability in rheumatoid arthritis is predicted by physical and psychological health status: a 7‐year study from the Oslo RA register. Scandinavian Journal of Rheumatology 2005;34(6):441‐7. [DOI] [PubMed] [Google Scholar]
Orme 2012
- Orme ME, Macgilchrist KS, Mitchell S, Spurden D, Bird A. Systematic review and network meta‐analysis of combination and monotherapy treatments in disease‐modifying antirheumatic drug‐experienced patients with rheumatoid arthritis: analysis of American College of Rheumatology criteria scores 20, 50, and 70. Biologics: targets & therapy 2012;6:429‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osiri 2003
- Osiri M, Suarez‐Almazor ME, Wells GA, Robinson V, Tugwell P. Number needed to treat (NNT): implication in rheumatology clinical practice. Annals of the Rheumatic Diseases 2003;62:316‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pincus 1983
- Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis and Rheumatism 1983;26(11):1346‐53. [DOI] [PubMed] [Google Scholar]
Pincus 2002
- Pincus T, Ferraccioli G, Sokka T, Larsen A, Rau R, Kushner I, et al. Evidence from clinical trials and long‐term observational studies that disease‐modifying anti‐rheumatic drugs slow radiographic progression in rheumatoid arthritis: updating a 1983 review. Rheumatology 2002;41(12):1346‐56. [DOI] [PubMed] [Google Scholar]
Prevoo 1996
- Prevoo ML, Gestel AM, T Hof MA, Rijswijk MH, Putte LB, Riel PL. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. British Journal of Rheumatology 1996;35(11):1101‐5. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saag 2008
- Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease‐modifying antirheumatic drugs in rheumatoid arthritis. Arthritis & Rheumatology 2008;59(6):762‐84. [DOI] [PubMed] [Google Scholar]
Salanti 2008
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17(3):279‐301. [DOI] [PubMed] [Google Scholar]
Schiff 2008a
- Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi‐centre, randomised, double‐blind, placebo‐controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Annals of the Rheumatic Diseases 2008;8:1096‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2006
- Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. New England Journal of Medicine 2006;355(7):704‐12. [DOI] [PubMed] [Google Scholar]
Sharp 1971
- Sharp JT, Lidsky MD, Collins LC, Moreland J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis and Rheumatism 1971;14(6):706‐20. [DOI] [PubMed] [Google Scholar]
Singh 2009
- Singh JA, Christensen R, Wells GA, Suarez‐Almazor ME, Buchbinder R, Lopez‐Olivo MA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007848.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2012
- Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Research 2012;64(5):625‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2016a
- Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis & Rheumatology 2016;68(1):1‐26. [DOI] [PubMed] [Google Scholar]
Singh 2016b
- Singh, Jasvinder A, Hossain, Alomgir, Tanjong G, Elizabeth, et al. Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease‐modifying anti‐rheumatic drugs: a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2016, issue 5. [DOI: 10.1002/14651858.CD012183; CD012183] [DOI] [PMC free article] [PubMed]
Singh 2016c
- Singh JA, Hossain A, Tanjong Ghogomu E, Mudano AS, Tugwell P, Wells GA. Biologic or tofacitinib monotherapy for rheumatoid arthritis in people with traditional disease‐modifying anti‐rheumatic drug (DMARD) failure: a Cochrane Systematic Review and network meta‐analysis (NMA). Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD012437] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2017
- Singh JA, Hossain A, Tanjong Ghogomu E, Mudano AS, Maxwell LJ, Buchbinder R, Lopez‐Olivo MA, Suarez‐Almazor ME, Tugwell P, Wells GA. Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD012591] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smolen 2014
- Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2013 update. Annals of the Rheumatic Diseases 2014;73(3):492‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2003
- Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. BMJ 2003;326:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spiegelhalter 2003
- Spiegelhalter D, Thomas A, Best N. WinBUGS user manual. Version 1.4, January 2003.. MRC Biostat. Unit 2. faculty.washington.edu/jmiyamot/p548/spiegelhalter%20winbugs%20user%20manual.pdf. 2003.
Strand 2008
- Strand V, Singh JA. Improved health‐related quality of life with effective disease‐modifying antirheumatic drugs: evidence from randomized controlled trials. American Journal of Managed Care 2008;14(4)(4):234‐54. [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23:1351‐75. [DOI] [PubMed] [Google Scholar]
Szekanecz 2007
- Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Current Opinion in Rheumatology 2007;19(3):289‐95. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [DOI] [PubMed] [Google Scholar]
Thompson 2011
- Thompson AE, Rieder SW, Pope JE. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: a meta‐analysis of randomized controlled trials. Arthritis & Rheumatology 2011;63(6):1479‐85. [DOI] [PubMed] [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Heijde 1989
- Heijde DM, Riel PL, Nuver‐Zwart IH, Gribnau FW, Putte LB. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet 1989;1(8646):1036‐8. [DOI] [PubMed] [Google Scholar]
Weinblatt 2013
- Weinblatt ME, Schiff M, Valente R, Heijde D, Citera G, Zhao C, et al. Head‐to‐head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis & Rheumatology 2013;65(1):28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 1993
- Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient's perspective. The Journal of Rheumatology 1993;20(3):557‐60. [PubMed] [Google Scholar]
Wells 2009
- Wells GA, Sultan SA, Chen L, Khan M, Coyle D. Indirect evidence: indirect treatment comparisons in meta‐analysis. Canadian Agency for Drugs and Technologies in Health. Ottawa 2009.
Woolley 2003
- Woolley DE. The mast cell in inflammatory arthritis. New England Journal of Medicine 2003;348(17):1709‐11. [DOI] [PubMed] [Google Scholar]
Yelin 2007
- Yelin E. Work disability in rheumatic diseases. Current Opinion in Rheumatology 2007;19(2):91‐6. [DOI] [PubMed] [Google Scholar]


