Abstract
Background:
We have a limited understanding of the biological underpinnings of symptoms in heart failure (HF), particularly in response to left ventricular assist device (LVAD) implantation.
Objective:
Quantify the degree to which symptoms and biomarkers change in parallel from prior to implantation through the first 6 months after LVAD implantation in advanced HF.
Methods:
This was a prospective cohort study of 101 patients receiving an LVAD for the management of advanced HF. Data on symptoms (dyspnea, early & subtle symptoms (HF Somatic Perception Scale), pain severity (Brief Pain Inventory), wake disturbance (Epworth Sleepiness Scale), depression (Patient Health Questionnaire) and anxiety (Brief Symptom Inventory)) and peripheral biomarkers of myocardial stretch, systemic inflammation and hyper-volumetric mechanical stress were measured prior to implantation with a commercially-available LVAD, and again at 30, 90 and 180 days after LVAD implantation. Latent growth curve and parallel process modeling were used to describe changes in symptoms and biomarkers and the degree to which they change in parallel in response to LVAD implantation.
Results:
In response to LVAD implantation, changes in myocardial stretch were closely associated with changes in early & subtle physical symptoms as well as depression, and changes in hyper-volumetric stress were closely associated with changes in pain severity and wake disturbances. Changes in systemic inflammation were not closely associated with changes in physical or affective symptoms in response to LVAD implantation.
Conclusions:
These findings provide new insights into the many ways in which symptoms and biomarkers provide concordant or discordant information about LVAD response.
Introduction
In the management of advanced heart failure (HF), implantation of a left ventricular assist device (LVAD) is a common strategy as a bridge to transplantation or as destination therapy.1 It is well-known that adults living with advanced HF experience considerable burden from physical symptoms like dyspnea and pain as well as affective symptoms like depression and anxiety.2 In general, physical and affective symptoms improve in response to LVAD implantation.3,4 There remains, however, a general disconnect between what can be measured objectively about HF pathogenesis and the level of symptoms experienced by patients living with HF; that is, several groups have provided evidence of the limited association between objective markers of HF (i.e. clinical characteristics, echocardiographic values, right heart catheterization parameters, exercise tolerance tests results and common biomarkers) and symptoms.5–12 Additionally, relationships between biomarkers of HF pathogenesis and symptoms are different comparing patients with advanced to patients with moderate HF.13
Because there is no one metric used to judge LVAD responsiveness, providers must integrate both objective and subjective data to personalize their appraisal of therapeutic response. But, discordance between symptoms and objective metrics is common and is associated with worse clinical outcomes in HF.14 Moreover, there is current concern over the way in which biomarkers are used in advanced HF,15 and common-but-catch-all patient-reported outcomes like quality of life in the context of advanced HF are poorly understood by providers and patients alike.16,17 Hence, there is more information needed to understand how specific symptoms and biomarkers change in response to LVAD, both independently and collectively, and gain insight into the complexity of LVAD response.
The purpose of this paper was to quantify the relationship between changes in physical and affective symptoms and changes in biomarkers of HF pathogenesis in response to LVAD implantation. We first characterized change in symptoms and biomarkers separately, and then quantified the degree to which symptoms and biomarkers change in parallel from prior to implantation through the first 6 months after LVAD implantation.
Methods
This was a prospective cohort study focused on symptom and biological responses to LVAD implantation among adults with advanced HF (the Profiling Biobehavioral Responses to Mechanical Support in Advanced Heart Failure (PREMISE) study).18 Participants in the PREMISE study were ≥21 years of age and undergoing implantation of a commercially-available continuous-flow LVAD as a bridge to heart transplantation or as destination therapy as the pre-implant strategy as designated by a multidisciplinary advanced HF selection committee. All participants met criteria for Interagency Registry for Mechanically Assisted Circulatory Support profiles 1–4.19 Patients were not eligible if they had a heart transplantation or an LVAD prior to enrollment, diagnosis of a major psychiatric illness or documented major cognitive impairment such as Alzheimer’s disease. Participants were recruited through an advanced HF center between April, 2012 and December, 2015. The study was reviewed and approved by our institutional review board; written informed consent was obtained from all participants.
Data Collection
Participants were consented for this study prior to LVAD implantation by a member of the research team who was not directly involved with patient care. Self-report symptom data and blood samples were collected a median of 5 days pre-implant, and again at 30, 90 and 180 days after LVAD implantation. Clinical data, including HF parameters, laboratory values and co-morbid conditions, were extracted from a detailed review of medical records.
Symptoms
Dyspnea was measured using the HF Somatic Perception Scale (HFSPS).20 The HFSPS asks about how much the participant was bothered by common HF symptoms during the last week. Response options range from 0 (did not have symptom) followed by degree of bother ranging from 1 (not at all bothersome) to 5 (extremely bothersome). The 6-item HFSPS subscale for dyspnea was used in this analysis (range 0–30; higher scores indicate worse dyspnea). Cronbach’s alpha on the dyspnea scale was 0.89 in this study. The HFSPS also has a 7-item subscale for what was called “early & subtle” symptoms by the measure architect.20 Early & subtle symptoms include having an upset stomach, early satiety, fatigue and cough (subscale range 0–35; higher scores indicate worse symptoms).20 Cronbach’s alpha on the early & subtle scale was 0.74 in this study. The HFSPS was chosen over alternative measures because of both the utility and predictive validity of the dyspnea and early & subtle sub-scales.20
The Brief Pain Inventory (BPI) was used as an assessment of pain severity.21 The BPI consists of 4 questions about pain severity (i.e. worst, least, average and current pain intensity), referring to pain that occurs anywhere in the body but excluding everyday minor aches. A pain severity score was then calculated (ranging from 1–10; 0 = no pain, and 10 = pain as bad as they could imagine). Cronbach’s alpha was 0.90 in this study.
Wake disturbances were measured using the 8-item Epworth Sleepiness Scale (ESS).22 The ESS asks participants to rate how likely they would be to fall asleep in 8 situations (e.g. sitting and reading, watching television, sitting and talking to someone); response options range from 0 (would never fall asleep) to 3 (high chance). The ESS (range 0–24 with higher values indicating worse wake disturbances) correlates significantly with sleep latency measures.22 Cronbach’s alpha was 0.87 in this study.
Depression was measured using the 9-Item Patient Health Questionnaire (PHQ9).23 The PHQ9 scores each of the 9 related DSM-IV criteria providing four response options ranging from 0 (not at all) to 3 (nearly every day). The PHQ9 total score ranges from 0 to 27 with higher scores indicating worse depressive symptoms.23 Cronbach’s alpha was 0.81 in this study.
Anxiety was measured using the Brief Symptom Inventory (BSI).24 The BSI asks about feelings during the past seven days and provides five response options ranging from 0 (no) to 4 (extreme). Subscale scores (ranging from 0 to 4) are calculated by adding the ratings and dividing the total by the number of items in the subscale (6 items for anxiety), with higher scores indicating higher anxiety. Cronbach’s alpha was 0.84 in this study.
Biomarkers
We used a multi-marker strategy25 that included metrics of myocardial stretch, systemic inflammation and hyper-volumetric stress. We quantified amino terminal pro-B-type natriuretic peptide (NTproBNP) as a measure of myocardial stretch (Cusabio Technology, Houston, TX) as NTproBNP is reduced after LVAD implantation.26 We quantified soluble tumor necrosis factor α receptor 1 (sTNFαR1) as a metric of systemic inflammation (R&D Systems, Minneapolis, MN) as sTNFαR1 is increased after LVAD implantation.27 Finally, we quantified the soluble form of suppression of tumorigenicity-2 (sST2), which is the soluble receptor for interleukin-33 and a metric of hyper-volumetric stress in HF (Critical Diagnostics, San Diego, CA) because it is elevated in advanced HF and also reduced in response to LVAD implantation.28 NTproBNP, sTNFαR1 and sST2 also have different cross-sectional relationships with physical symptoms comparing patients with advanced HF to those with moderate HF.13
Statistical Analysis
Means and standard deviations or counts and proportions were used to describe the sample. Latent growth curve modeling29 was performed to quantify change in symptoms and biomarkers independently across four time points from pre-implant to 180 days post-implant. We performed multiphase growth modeling30 to capture pre-implant values as well as the two major phases of change observed in most measures; initial improvements observed between pre-implant and 30 days post implant (i.e. slope 1 or Δ1), and subsequent improvements between 30 and 180 days post implant (i.e. slope 2 or Δ2). In figures 1 and 2, pre-implant values are presented in means and standard errors; phases of change are presented as mean slope, standard error of the slope, and the significance of change as well as Cohen’s d to quantify the magnitude of change (≈ 0.2–0.3 is a small effect, ≈ 0.4–0.5 is a moderate effect, and ≥ 0.8 is a large).31 Parallel process modeling was then completed to quantity the degree of similarity/dissimilarity between changes in symptoms and changes in biomarkers in response to LVAD implantation; although minimal, missing data were handled using maximum likelihood estimation. Parallel process modeling32,33 is an extension of growth curve modeling that entails quantifying two growth curves (i.e. one for the symptom and one of the biomarker) and random effects between intercepts and slopes of the two growth curves. Because this approach is based on structural equation modeling, fit statistics are used to judge similarity between change in symptoms and change in biomarkers over time. Thresholds for acceptable fit, and therefore closely associated change in this study, were considered a) a non-significant chi-square test, b) comparative fit index (CFI) ≥0.95, c) Tucker-Lewis index (TLI) ≥0.95, d) root mean square error of approximation (RMSEA) ≤0.06, and e) a standardized root mean square residual (SRMR) of ≤ 0.08.34 Values of all of these metrics close to the cutoffs are needed to conclude that there is a relatively good fit. There is no standard approach for sample size considerations in growth modeling particularly in fields where there is a limited evidence base. With four symptom and four biomarker measurements, however, our n-to-items ratio exceeded sample size recommendations for related approaches (10:1).35 All statistical analyses were performed using StataMP v15 (College Station, Texas) and Mplus v8 (Los Angeles, California).
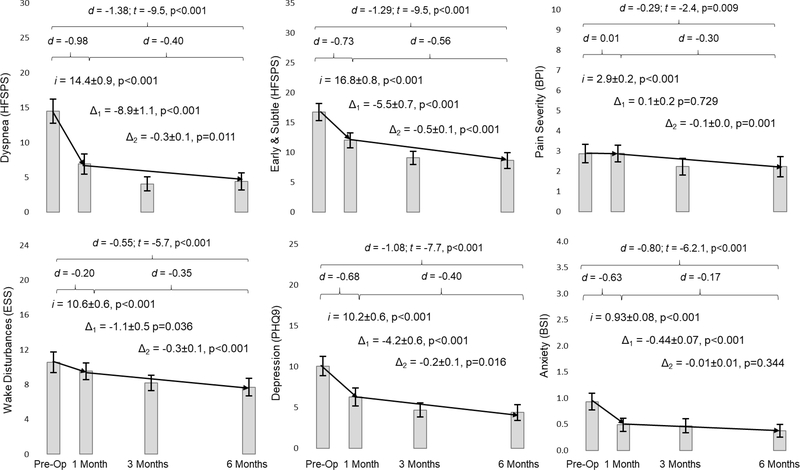
Figure 1: Changes in Physical and Affective Symptoms in Response to Left Ventricular Assist Device Implantation.
Mean values of each symptom are presented as rectangles and the whisker bars represent the 95% confidence interval at each time point. The two diagonal lines for each graph represent the initial change between pre-implant and 30 days post implant (Δ1) and the subsequent change (Δ2) through 180 days after implantation. Latent growth estimates of pre-implant values (i for intercept) and both the initial and subsequent rates of change (Δ1 and Δ2) are presented along with the standard error and related p-values. Finally, effect sizes overall and for each phase of change are presented in Cohen’s d (d) along with the statistical significance of change over 180 days after LVAD implantation. Abbreviations: BPI = Brief Pain Inventory; BSI = Brief Symptom Inventory; ESS = Epworth Sleepiness Scale; HFSPS = Heart Failure Somatic Perception Scale; PHQ9 = 9-item Patient Health Questionnaire.
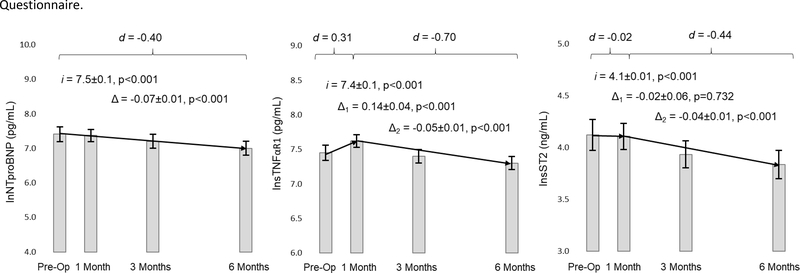
Figure 2: Changes in Biomarkers in Response to Left Ventricular Assist Device Implantation.
Mean values of each biomarker are presented as the filled rectangle and the whisker bars represent the 95% confidence interval for each time point. Horizontal lines for each graph represent overall change (Δ) in the case of NTproBNP or the initial change between pre-implant and 30 days post implant (Δ1) and the subsequent change (Δ2) through 180 days after implantation in the case of sTNFαR1 and sST2. Latent growth modeling estimates of pre-implant values (i for intercept) and phases of change are presented along with the standard error and related p-values. Finally, effect sizes overall or for each phase of change are presented in Cohen’s d (d). Raw values are presented to assist with interpretation. The natural log of biomarker levels is what was used in the analysis to approximate normality. Abbreviations: ln = natural logarithm; NTproBNP = amino terminal pro-B-type natriuretic peptide; sST2 = soluble form of suppression of tumorigenicity-2; sTNFαR1 = soluble tumor necrosis factor alpha receptor 1.
Results
The sample (n=101) was predominantly male and Caucasian, and slightly more than two-thirds of participants received an LVAD with bridge to transplantation as the pre-implant strategy (Table 1). As a whole, the sample had the characteristics of advanced HF including poor contractility, high filling pressures and reduced function; many required inotropic support.
Table 1:
Pre-implant characteristics of the sample
| Characteristic (mean ± SD or n (%)) | Sample (n=101) |
|---|---|
| Patient age (in years) | 53.1 ± 13.9 |
| Female | 20 (19.8%) |
| Caucasian | 83 (82.2%) |
| Body Mass Index (kg/m2) | 28.9 ± 5.4 |
| Ischemic Etiology | 34 (33.7%) |
| Comorbidities: | |
| Type II Diabetes Mellitus | 41 (40.6%) |
| Sleep Apnea/Sleep Disordered Breathing | 46 (45.5%) |
| Hypertension | 52 (51.5%) |
| Pulmonary Hypertension | 34 (33.7%) |
| Atrial Fibrillation | 49 (48.5%) |
| Chronic Kidney Disease Stage 3 | 37 (36.6%) |
| Chronic Obstructive Pulmonary Disease | 22 (21.8%) |
| Ne York Heart Association Class III/IV | 97 (96.0%) |
| Center Ventricular Ejection Fraction (%) | 20.5 ± 2.8 |
| Center Ventricular Internal Diastolic Diameter (cm) | 7.4 ± 1.1 |
| Pulmonary Capillary edge Pressure (mm Hg) | 23.4 ± 8.6 |
| Right Atrial Pressure (mm Hg) | 9.3 ± 4.8 |
| Cardiac Index (by Fick Equation) (L/min/m2) | 1.9 ± 0.5 |
| V02 max (L/min) | 15.0 ± 4.5 |
| Serum Sodium (mEq/L) | 134.4 ± 4.1 |
| Serum Hemoglobin (%) | 12.1 ± 2.0 |
| Blood Urea Nitrogen/Creatinine Ratio | 22.5 ± 8.3 |
| Continuous Inotropic Support | 72 (71.3%) |
| Intra-Aortic Balloon Pump | 43 (42.6%) |
| Bridge to Transplant | 68 (67.3%) |
Changes in symptoms are presented in Figure 1. There were large and significant improvements in dyspnea (p<0.001) and early & subtle symptoms (p<0.001) in response to LVAD implantation with the vast majority of improvement occurring within the first 30 days after implantation followed by continued significant improvement through 180 days. There was a small but significant improvement in wake disturbances at 30 days (p=0.036) followed by continued significant improvement through 180 days (p<0.001). Pain severity took longer to improve in response to LVAD with only a small but significant improvement at 180 days compared with pre-implant pain (p=0.009). Overall, there were large improvements in depression (p<0.001) and anxiety (p<0.001) over the course of 180 days; the greatest improvements in affective symptoms occurred within the first 30 days after LVAD implantation.
Changes in biomarkers in response to LVAD implantation are presented in Figure 2. There was a small-to-moderate and significant reduction in NTproBNP at 180 days after LVAD implantation (p<0.001). There was a moderate and significant increase in sTNFαR1 in the first 30 days after implantation (p<0.001) followed by a moderate and significant reduction between 30 and 180 days after implantation (p=0.001). There was no change in sST2 at 30 days (p=0.732) followed by a moderate reduction between 30 and 180 days after implantation (p<0.001).
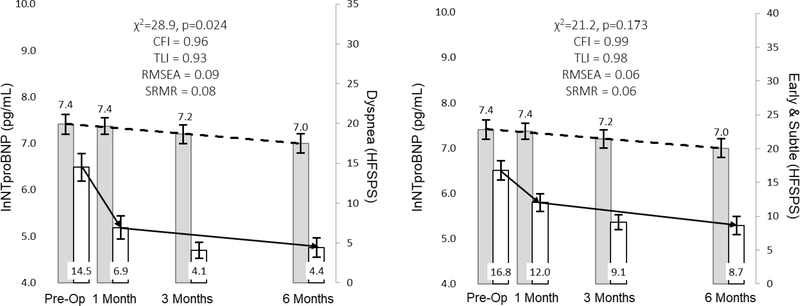
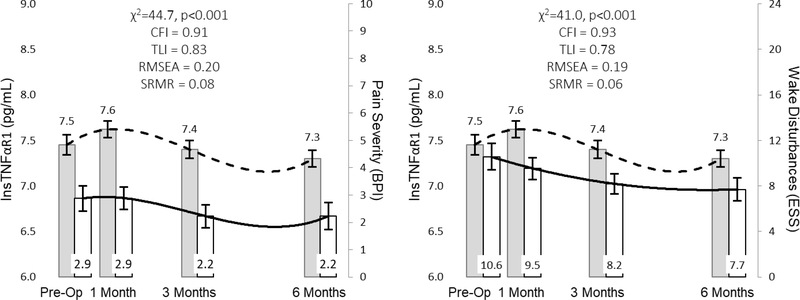
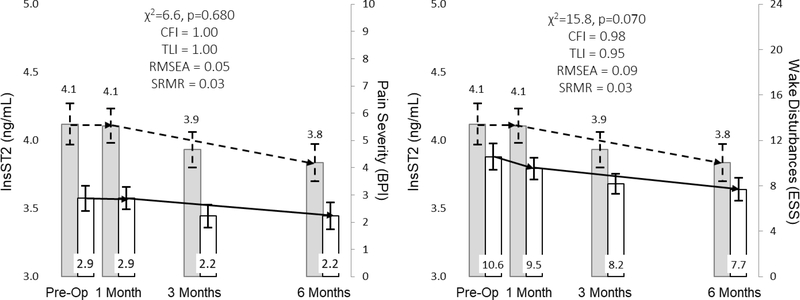
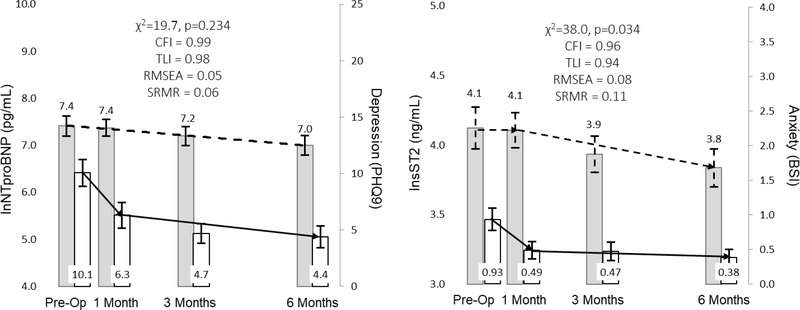
The relationship between NTproBNP and dyspnea and early & subtle symptoms are represented in Figure 3. In response to LVAD implantation, change in myocardial stretch is more closely associated with change in early & subtle symptoms as opposed to dyspnea. The relationships between sTNFαR1 and pain severity and wake disturbances are presented in Figure 4. In response to LVAD implantation, change in systemic inflammation was not closely associated with changes in either pain severity or wake disturbances. The relationships between sST2 and both pain severity and wake disturbances are presented in Figure 5. Change in hyper-volumetric stress is closely associated with change in both pain severity and wake disturbances. The relationship between NTproBNP and depression as well as the relationship between sST2 and anxiety are presented in Figure 6. Change in myocardial stretch is closely associated with change in depression whereas change in hyper-volumetric stress is not closely associated with change in anxiety. There were no other examples of close association in comparisons between symptoms and biomarkers over time (data not shown).
Figure 3: Parallel Changes in Myocardial Stretch and Physical Symptoms in Response to Left Ventricular Assist Device Implantation.
Mean values of biomarkers (leftward y-axes) and symptoms (rightward y-axes) are presented as the filled rectangle and the whisker bars represent the 95% confidence interval for each time point. Horizontal lines (dashed lines for biomarkers, solid lines for symptoms) for each graph represent overall change between pre-implant and 180 days post implant. Thresholds for acceptable fit for CFI and TLI are ≥ 0.95, RMSEA = ≤0.06 and SRMR ≤ 0.08. Abbreviations: CFI = comparative fit indices; HFSPS = heart failure somatic perception scale; lnNTproBNP = natural log of amino terminal pro-B-type natriuretic peptide; RMSEA = root mean square errors of approximation; SRMR = standardized root mean square residual; TLI = Tucker-Lewis indices.
Figure 4: Parallel Changes in Systemic Inflammation and both Pain Severity and Wake Disturbances in Response to Left Ventricular Assist Device Implantation.
Mean values of biomarkers (leftward y-axes) and symptoms (rightward y-axes) are presented as the filled rectangle and the whisker bars represent the 95% confidence interval for each time point. Horizontal lines (dashed lines for biomarkers, solid lines for symptoms) for each graph represent overall change between pre-implant and 180 days post implant. Thresholds for acceptable fit for CFI and TLI are ≥ 0.95, RMSEA = ≤0.06, and SRMR ≤ 0.08. Abbreviations: BPI = Brief Pain Inventory; CFI = comparative fit indices; ESS = Epworth Sleepiness Scale; lnsTNFαR1 = natural log of soluble tumor necrosis factor alpha receptor 1; RMSEA = root mean square errors of approximation; SRMR = standardized root mean square residual; TLI = Tucker-Lewis indices.
Figure 5: Parallel Changes in Hyper-volumetric Stress and both Pain Severity and Wake Disturbances in Response to Left Ventricular Assist Device Implantation.
Mean values of biomarkers (leftward y-axes) and symptoms (rightward y-axes) are presented as the filled rectangle and the whisker bars represent the 95% confidence interval for each time point. Horizontal lines (dashed lines for biomarkers, solid lines for symptoms) for each graph represent overall change between pre-implant and 180 days post implant. Thresholds for acceptable fit for CFI and TLI are ≥ 0.95, RMSEA = ≤0.06, and SRMR ≤ 0.08. Abbreviations: BPI = Brief Pain Inventory; CFI = comparative fit indices; ESS = Epworth Sleepiness Scale; lnsST2 = natural log of soluble form of suppression of tumorigenicity-2; RMSEA = root mean square errors of approximation; SRMR = standardized root mean square residual; TLI = Tucker-Lewis indices.
Figure 6: Parallel Changes in Myocardial Stretch and Depression, and Hyper-volumetric Stress and Anxiety in Response to Left Ventricular Assist Device Implantation.
Mean values of biomarkers (leftward y-axes) and symptoms (rightward y-axes) are presented as the filled rectangle and the whisker bars represent the 95% confidence interval for each time point. Horizontal lines (dashed lines for biomarkers, solid lines for symptoms) for each graph represent overall change between pre-implant and 180 days post implant. Thresholds for acceptable fit for CFI and TLI are ≥ 0.95, RMSEA = ≤0.06, and SRMR ≤ 0.08. Abbreviations: BPI = Brief Pain Inventory; CFI = comparative fit indices; lnsT2 = natural log of soluble form of suppression of tumorigenicity-2; PHQ9 = 9-item Patient Health Questionnaire; RMSEA = root mean square errors of approximation; SRMR = standardized root mean square residual; TLI = Tucker-Lewis indices.
Discussion
Our understanding of the biological underpinnings of symptoms in HF is quite limited. In this sample of 101 adults undergoing LVAD implantation for the management of advanced HF, we observed several ways in which changes in symptoms were closely associated with changes in peripheral biomarkers of HF pathogenesis. Specifically, we observed that changes in myocardial stretch as measured by NTproBNP were closely related to changes in early & subtle physical symptoms (e.g. having an upset stomach, early satiety, fatigue or cough) as well as depression, and that changes in hyper-volumetric stress as measured by sST2 were closely related to changes in both pain severity and wake disturbances. We also observed several ways in which changes in symptoms were seemingly unrelated to changes in peripheral HF biomarkers. For example, changes in symptoms were not related to changes in systemic inflammation as measured by sTNFαR1, and changes in dyspnea were not closely related to changes in myocardial stretch. These finding contribute to a growing body of HF symptom science and also point toward several future directions of clinical research.
In HF, myocardial stretch-secretion coupling from congestion is the dominant mechanism for elevated NTproBNP.36,37 Since LVADs work primarily by unloading the ventricle and enhancing cardiac output, it is not surprising that reductions in NTproBNP were closely associated with concomitant improvements in early & subtle symptoms that include those related to both vascular congestion (e.g. cough and early satiety) and reduced blood flow (e.g. fatigue). One reason why reductions in NTproBNP were not closely associated with concomitant improvements in dyspnea may be that the largest improvement in any symptom in response to LVAD involved dyspnea and in contrast the smallest improvement in any biomarker involved NTproBNP. van den Broek and colleagues have shown that in HF, depression is not associated significantly with NTproBNP.38 But, there are several common pathophysiological pathways between depression and HF including autonomic dysfunction and inflammation that explain, at least in part, why we observed close associations between change in depression and change in NTproBNP in response to LVAD implantation.39,40
In response to mechanical stress or injury, interleukin-33 and the cellular receptors thereof interact in mechanisms that are cardioprotective (i.e. reduce myocardial fibrosis, hypertrophy and apoptosis).41 As the soluble receptor of interleukin-33, sST2 acts as a decoy receptor in HF pathogenesis and antagonizes what otherwise would be cardioprotective processes. Since sST2 is relevant to cardiomyocytes and fibroblasts, elevated levels in HF are viewed as indices of hyper-volumetric stress and also fibrosis, in addition to inflammation (since sST2 is a receptor of a cytokine).28 To the best of our knowledge, our finding of close associations between sST2 and pain severity and wake disturbances in response to LVAD implantation make a novel contribution to the field. In mice, interleukin-33 has been shown to mediate inflammatory hyper-nociception, a process that is mitigated by sST2.42 In advanced HF in general, pain frequently occurs in multiple sites and is not related to cardiac pain;43 but, the exact mechanisms of pain in HF have not been explicated. Since sST2 is a marker of mechanical stress, fibrosis and inflammation, these mechanisms and the regulators thereof may be helpful in exploring the origins of pain in HF in more detailed future studies. After LVAD implantation, there may be tradeoffs in the location and even origins of pain including non-cardiac pain, but overall pain severity was not improved significantly until 90 days after LVAD compared with pre-implant pain. Although not much is written about pain after LVAD, palliative care may be a way to optimize residual and post-operative pain management.44
Our group has shown previously that in a cross-sectional fashion sST2 is not associated significantly with wake disturbances in HF.13 In this study, we observed that improvements in wake disturbances were closely associated with concomitant improvements in hyper-volumetric stress. Others have shown that in HF the major determinants of wake disturbances include poor sleep quality and worse functional class.45 Although not specifically tested in this study, it may be that reductions in volume overload improve sleep quality and subsequently reduce wake disturbances. Future mechanistic studies examining the interplay between sleep quality and wake disturbances, as well as the biological underpinnings thereof, are needed. We also observed that the large reductions in anxiety were not closely associated with concomitant reductions in sST2. The exact mechanism of anxiety in HF is not clear,46 although hypothalamic-pituitary-adrenal axis dysfunction has been proposed.47 Future research into the biological underpinnings of anxiety and HF should focus on the hypothalamic-pituitary-adrenal axis.
Given the pattern of initial worsening of systemic inflammation and the marked initial improvements in most symptoms, that lack of a strong association between sTNFαR1 and symptoms was not completely counterintuitive. Others have shown that the systemic inflammatory response was present for approximately two months post-surgery, and that higher levels of TNFα after LVAD are associated with other important events like bleeding.48,49 Hence, sTNFαR1 may continue to be an important maker of systemic inflammation in HF and adverse outcomes; but, it may not necessarily be an important marker in understanding changes in symptoms post LVAD.
Given these collective insights, clinicians might expect to see similar changes in myocardial stretch and both early & subtle symptoms and depression but not dyspnea. Hence, residual dyspnea after LVAD may be a function of other issues such as unmitigated comorbid conditions. Further, patients with enduring pain or wake disturbances after LVAD may also have hyper-volumetric stress. In that way, these residual symptoms after LVAD may be a sign of unresolved congestion. Further, several important symptoms like dyspnea and pathogenic processes like systemic inflammation may be best understood in isolation and not by integration into a symptom biology perspective.
Limitations
A limitation to this and other non-experimental studies is the inability to comment on the causal nature of relationships or specific mechanisms; at this point in the state of the science of HF symptom biology, however, our findings serve as novel and additive contributions and point towards several areas of future study explicated throughout the discussion. Additionally, this was a single center study of a sample with several homogenous characteristics including race and gender and our findings may not be generalizable. Future work of ours and other groups must extend to multiple centers and aim for better patient representation, as well as the influence of multiple chronic conditions (e.g. sleep disordered breathing) that are ubiquitous in advanced HF on symptom biology.
Conclusion
In response to LVAD implantation, changes in myocardial stretch were closely associated with changes in early & subtle physical symptoms as well as depression. Changes in hyper-volumetric stress were closely associated with changes in pain severity and wake disturbances. These findings contribute to the growing body of HF symptom biology research.
Acknowledgments
Sources of Funding: This project was supported by the National Institutes of Health/ National Institute of Nursing Research (1R01NR013492). Research reported in this paper also was supported by the National Center for Advancing Translational Sciences (UL1TR000128). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Contributor Information
Christopher S. Lee, Boston College William F. Connell School of Nursing, Chestnut Hill, MA.
James O. Mudd, Oregon Health & Science University Knight Cardiovascular Institute, Portland, OR.
Karen S. Lyons, Boston College William F. Connell School of Nursing, Chestnut Hill, MA.
Quin E. Denfeld, Oregon Health & Science University School of Nursing, Portland, OR.
Corrine Y. Jurgens, Stony Brook University School of Nursing, Stony Brook, NY.
Bradley E. Aouizerat, New York University Department of Oral and Maxillofacial Surgery, New York, NY.
Jill M. Gelow, Providence Health, Portland, OR.
Christopher V. Chien, University of North Carolina REX Healthcare, Raleigh, NC.
Emily Aarons, Boston College William F. Connell School of Nursing, Chestnut Hill, MA.
Kathleen L. Grady, Feinberg School of Medicine, Northwestern University, Chicago, IL.
References
- 1.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938 [doi]. [DOI] [PubMed] [Google Scholar]
- 2.Metra M, Ponikowski P, Dickstein K, et al. Advanced chronic heart failure: A position statement from the study group on advanced heart failure of the heart failure association of the european society of cardiology. Vol 9 ; 2007:684–94. 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Reynard AK, Butler RS, Mckee MG, Starling RC, Gorodeski EZ. Frequency of depression and anxiety before and after insertion of a continuous flow left ventricular assist device. Am J Cardiol. 2014;114(3). doi: 10.1016/j.amjcard.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Brouwers C, Denollet J, de Jonge N, Caliskan K, Kealy J, Pedersen SS. Patient-reported outcomes in left ventricular assist device therapy: A systematic review and recommendations for clinical research and practice. Circulation.Heart failure. 2011;4(6):714. doi: 10.1161/CIRCHEARTFAILURE.111.962472. [DOI] [PubMed] [Google Scholar]
- 5.Denfeld QE, Mudd JO, Hasan W, et al. Exploring the relationship between beta-adrenergic receptor kinase-1 and physical symptoms in heart failure. Heart Lung. 2018. 47(4):281–284. doi: 10.1016/j.hrtlng.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CS, Hiatt SO, Denfeld QE, Chien CV, Mudd JO, Gelow JM. Gender-specific physical symptom biology in heart failure. J Cardiovasc Nurs. 2015;30(6):517–521. doi: 10.1097/JCN.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denfeld QE, Mudd JO, Gelow JM, Chien C, Hiatt SO, Lee CS. Physical and psychological symptom biomechanics in moderate to advanced heart failure. J Cardiovasc Nurs. 2015;30(4):346–350. doi: 10.1097/JCN.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guglin M, Patel T, Darbinyan N. Symptoms in heart failure correlate poorly with objective haemodynamic parameters. Int J Clin Pract. 2012;66(12):1224–1229. doi: 10.1111/j.1742-1241.2012.03003.x [doi]. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj A, Rehman SU, Mohammed AA, et al. Quality of life and chronic heart failure therapy guided by natriuretic peptides: Results from the ProBNP outpatient tailored chronic heart failure therapy (PROTECT) study. Am Heart J. 2012;164(5):799.e1. doi: 10.1016/j.ahj.2012.08.015 [doi]. [DOI] [PubMed] [Google Scholar]
- 10.Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. J Card Fail. 2006;12(6):439–445. doi: S1071–9164(06)00213–2 [pii]. [DOI] [PubMed] [Google Scholar]
- 11.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients’ perceptions of the effects of heart failure on their quality of life. J Card Fail. 2006;12(2):87–92. doi: S1071–9164(05)01322–9 [pii]. [DOI] [PubMed] [Google Scholar]
- 12.Shah MR, Hasselblad V, Stinnett SS, et al. Dissociation between hemodynamic changes and symptom improvement in patients with advanced congestive heart failure. Eur J Heart Fail. 2002;4(3):297–304. doi: S1388984201002021 [pii]. [DOI] [PubMed] [Google Scholar]
- 13.Authors. Comparative symptom biochemistry between moderate and advanced heart failure. (Under Review). [DOI] [PMC free article] [PubMed]
- 14.Lee CS, Hiatt SO, Denfeld QE, Mudd JO, Chien C, Gelow JM. Symptom-hemodynamic mismatch and heart failure event risk. J Cardiovasc Nurs. 2015;30(5):394–402. doi: 10.1097/JCN.0000000000000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzhauser L, Kim G, Sayer G, Uriel N. The effect of left ventricular assist device therapy on cardiac biomarkers: Implications for the identification of myocardial recovery. Curr Heart Fail Rep. 2018. doi: 10.1007/s11897-018-0399-3 [doi]. [DOI] [PubMed] [Google Scholar]
- 16.Nieminen MS, Dickstein K, Fonseca C, et al. The patient perspective: Quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol. 2015;191:256–264. doi: 10.1016/j.ijcard.2015.04.235. [DOI] [PubMed] [Google Scholar]
- 17.Adams EE, Wrightson ML. Quality of life with an LVAD: A misunderstood concept. Heart & Lung. 2018;47(3):177–183. doi: 10.1016/j.hrtlng.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Lee CS, Mudd JO, Gelow JM, et al. Background and design of the profiling biobehavioral responses to mechanical support in advanced heart failure study.(report). J Cardiovasc Nurs. 2014;29(5):405. doi: 10.1097/JCN.0b013e318299fa09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. The Journal of Heart and Lung Transplantation. 2015;34(12):1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Jurgens CY, Lee CS, Riegel B. Psychometric analysis of the heart failure somatic perception scale as a measure of patient symptom perception. J Cardiovasc Nurs. 2017;32(2):140–147. doi: 10.1097/JCN.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleeland CS, Ryan KM. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: The epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derogatis LR, Melisaratos N. The brief symptom inventory: An introductory report. Psychol Med. 1983;13(3):595–605. doi: 10.1017/S0033291700048017. [DOI] [PubMed] [Google Scholar]
- 25.Allen LA, Felker GM. Multi-marker strategies in heart failure: Clinical and statistical approaches. Heart Fail Rev. 2010;15(4):343–349. doi: 10.1007/s10741-009-9144-z [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemperman H, van den Berg M, Kirkels H, de Jonge N. B-type natriuretic peptide (BNP) and N-terminal proBNP in patients with end-stage heart failure supported by a left ventricular assist device. Clinical chemistry. 2004;50(9):1670–1672. doi: 10.1373/clinchem.2003.030510. [DOI] [PubMed] [Google Scholar]
- 27.Grosman-Rimon, Billia Liza, Fuks Fillo, Jacobs Avi, McDonald Ira, Cherney Michael, Rao David Z., Vivek. New therapy, new challenges: The effects of long-term continuous flow left ventricular assist device on inflammation. International Journal of Cardiology. 2015;215:424–430. doi: 10.1016/j.ijcard.2016.04.133. [DOI] [PubMed] [Google Scholar]
- 28.Tseng CCS, Huibers MMH, Gaykema LH, et al. Soluble ST2 in end-stage heart failure, before and after support with a left ventricular assist device. Eur J Clin Invest. 2018;48(3):10.1111/eci.12886. Epub 2018 Feb 2. doi: 10.1111/eci.12886 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burant CJ. Latent growth curve models. The International Journal of Aging and Human Development. 2016;82(4):336–350. doi: 10.1177/0091415016641692. [DOI] [PubMed] [Google Scholar]
- 30.Grimm KJ, Ram N, Hamagami F. Nonlinear growth curves in developmental research. Child Dev. 2011;82(5):1357–1371. doi: 10.1111/j.1467-8624.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen J Statistical power analysis for the behavioral sciences. Hillsdale, N.J.: L. Erlbaum Associates; 1988:567 http://www.loc.gov/catdir/enhancements/fy0731/88012110-d.html. [Google Scholar]
- 32.Bravo AJ, Kelley ML, Swinkels CM, Ulmer CS. Work stressors, depressive symptoms and sleep quality among US navy members: A parallel process latent growth modelling approach across deployment. J Sleep Res. 2018;27(3):e12624. doi: 10.1111/jsr.12624 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brick LAD, Yang S, Harlow LL, Redding CA, Prochaska JO. Longitudinal analysis of intervention effects on temptations and stages of change for dietary fat using parallel process latent growth modeling. J Health Psychol. 2016:1359105316679723. doi: 10.1177/1359105316679723 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 35.Maccallum RC, Widaman KF, Zhang S, Hong S, Appelbaum MI(. Sample size in factor analysis. Psychol Methods. 1999;4(1):84–99. doi: 10.1037/1082-989X.4.1.84. [DOI] [Google Scholar]
- 36.de Bold AJ, Bruneau BG, Kuroski de Bold ML Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovasc Res. 1996;31(1):7–18. doi: 0008-6363(95)00121-2 [pii]. [PubMed] [Google Scholar]
- 37.Ogawa T, Linz W, Stevenson M, et al. Evidence for load-dependent and load-independent determinants of cardiac natriuretic peptide production. Circulation. 1996;93(11):2059–2067. [DOI] [PubMed] [Google Scholar]
- 38.van den Broek Krista C., PhD deFilippi, Christopher R, Christenson MD, Robert H, Seliger PhD, Stephen L, Gottdiener, John S, Kop MD, Willem J., PhD. Predictive value of depressive symptoms and B-type natriuretic peptide for new-onset heart failure and mortality. The American Journal of Cardiology , 2011;107(5):723–729. doi: 10.1016/j.amjcard.2010.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: Epidemiology, mechanisms, and diagnosis. Cardiovascular psychiatry and neurology. 2013;2013:695925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mbakwem A, Aina F, Amadi C. Depression in patients with heart failure: Is enough being done? Cardiac failure review. 2016;2(2):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pascual-Figal Domingo A., MD|Januzzi, James L,MD. The biology of ST2: The international ST2 consensus panel. American Journal of Cardiology, The. 2015;115(7):7B. doi: 10.1016/j.amjcard.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 42.Verri Waldiceu A., Guerrero Ana T. G., Fukada Sandra Y., et al. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(7):2723–2728. doi: 10.1073/pnas.0712116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodlin Sarah J., Wingate MD, Sue, Albert RN, Nancy M, Pressler RN, Susan J, Houser RN, Janet, Kwon RN, Jennifer, Chiong MPH, Jun, Storey MD, Porter C, Quill MD, Timothy, Teerlink MD, John R. Investigating pain in heart failure patients: The pain assessment, incidence, and nature in heart failure (PAIN-HF) study. Journal of Cardiac Failure. 2012;18(10):776–783. doi: 10.1016/j.cardfail.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Wordingham SE, McIlvennan CK, Fendler TJ, et al. Palliative care clinicians caring for patients before and after continuous flow-left ventricular assist device. Journal of Pain and Symptom Management. 2017;54(4):601–608. doi: 10.1016/j.jpainsymman.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Riegel B, Ratcliffe SJ, Sayers SL, et al. Determinants of excessive daytime sleepiness and fatigue in adults with heart failure. Clinical Nursing Research. 2012;21(3):271–293. doi: 10.1177/1054773811419842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bordoni B, Marelli F, Morabito B, Sacconi B. Depression and anxiety in patients with chronic heart failure. Future Cardiology. 2018;14(2):115–119. doi: 10.2217/fca-2017-0073. [DOI] [PubMed] [Google Scholar]
- 47.Vongmany J, Hickman LD, Lewis J, Newton PJ, Phillips JL. Anxiety in chronic heart failure and the risk of increased hospitalisations and mortality: A systematic review. European Journal of Cardiovascular Nursing. 2016;15(7):478–485. [DOI] [PubMed] [Google Scholar]
- 48.Jeske W Syed D, Escalante V. Coglianese E Schwartz J Walenga J. Inflammatory cytokines are upregulated in patients with implanted ventricular assist devices. Blood. 2014(124). [Google Scholar]
- 49.Tabit, Coplan Corey E.|, Chen Mitchell J., Jeevanandam Phetcharat, Uriel Valluvan, Liao Nir, James K Tumor necrosis factor-alpha levels and non-surgical bleeding in continuous-flow left ventricular assist devices. Journal of Heart and Lung Transplantation. 2017;37(1):107–115. doi: 10.1016/j.healun.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]