Abstract
Background
MDR-TB is a major threat to global TB control. In 2015, 580,000 were treated for MDR-TB worldwide. The worldwide roll-out of GeneXpert MTB/RIF® has improved diagnosis of MDR-TB; however, in many countries laboratories are unable to assess drug resistance and clinical predictors of MDR-TB could help target suspected patients. In this study, we aimed to determine the clinical factors associated with MDR-TB in Bamako, Mali.
Methods
We performed a cross-sectional study of 214 patients with presumed MDR-TB admitted to University of Bamako Teaching Hospital, Point-G between 2007 and 2016. We calculated crude and adjusted odds ratios for MDR-TB disease diagnosis using SPSS.
Results
We found that age ≤40years (OR = 2.56. 95% CI: 1.44–4.55), two courses of prior TB treatment (OR = 3.25,95% CI: 1.44–7.30), TB treatment failure (OR = 3.82,95% CI 1.82–7.79), sputum microscopy with 3+ bacilli load (OR = 1.98, 95% CI: 1.13–3.48) and a history of contact with a TB patient (OR = 2.48, 95% CI: 1.11–5.50) were significantly associated with confirmation of MDR-TB disease. HIV was not a risk factor for MDR-TB (aOR = 0.88, 95% CI: 0.34–1.94).
Conclusion
We identified several risk factors that could be used to identify MDR-TB suspects and prioritize them for laboratory confirmation. Prospective studies are needed to understand factors associated with TB incidence and clinical outcomes of TB treatment and disease.
Keywords: Multi-Drug Resistant Tuberculosis, Risk factors, Mali
Background
Tuberculosis (TB) is a major global health problem. Despite progress made in the diagnosis and treatment, mortality associated with TB remains high. TB was classified by WHO in 2016 as the most deadly infectious disease with 5,000 deaths per day (WHO, 2019). Multi-Drug Resistant Tuberculosis (MDR-TB) defined as Mycobacterium tuberculosis strain resistant to both isoniazid and rifampicin represents a threat to global TB control. In 2015, among the 10.4 million new cases of TB disease, 480,000 were confirmed to be MDR-TB and 100,000 were found to have rifampicin-resistant TB (RR-TB) and treated with second-line TB drugs (WHO, 2019). Also contributing is HIV/AIDS, a well-known risk factor for TB disease, TB drug resistance, and TB related death (Wells et al., 2007). Among the 1.8 million TB deaths in 2015, 22% were HIV coinfected and 35% of HIV deaths were due to TB (WHO, 2019). The emergence of MDR-TB has been sustained by the lack of diagnostics, non-adherence to first-line treatment, retreatment failure, and poor second-line therapy success in many countries (McBryde et al., 2017). Several risk factors have been associated with MDR-TB. History of prior TB treatment, HIV infection, contact with a known TB patient, receipt of more than two treatment courses, the larger burden of bacilli on sputum microscopy, lung cavitation, and bilateral lung disease (Diandé et al., 2009; Workicho et al., 2017; Chuchottaworn et al., 2015). Since 2010, the World Health Organization (WHO) recommended GeneXpert MTB/RIF® for rapid detection of rifampicin resistance and diagnosing MDR-TB in more than 90% of those tested (WHO, 2016). In Mali, the prevalence of MDR-TB was 3.4% and 66.3% respectively in new and previously treated TB patients (Diarra et al., 2016). The microbiological confirmation of MDR-TB is based on labor-intensive, costly, and time-consuming culture and drug susceptibility testing (DST) methods that require extensive laboratory infrastructure and are not routinely available in countries with limited resources. These limitations often result in overuse of unnecessary second-line TB drugs for individuals with susceptible TB disease and non-tuberculosis mycobacteria (NTM). We aimed to determine clinical factors associated with microbiologic confirmation of MDR-TB disease among patients suspected of having MDR TB by clinical criteria in Bamako, Mali. This knowledge is important for clinicians to identify individuals who are most likely to have confirmed MDR-TB in this setting.
Methods
Study design
We conducted a cross-sectional study of patients suspected of having MDR TB between January 2007 and December 2016 in Bamako, Mali.
Setting and subjects
The study was conducted at the national reference Unit for Multidrug-Resistant Tuberculosis (MDR-TB) of the Department of Pneumo-Phtisiology at the University of Bamako Teaching Hospital Of Point-G in Bamako. We studied patients suspected of MDR-TB and admitted to the hospital for confirmation and treatment. Between 2007 and 2014 patients’ admission criteria were chronic TB which was defined as sputum microscopy positive after 10-months of consecutive or discontinued first-line TB treatment. Patients with chronic TB were classified as either re-treatment failure or relapse. Re-treatment failure was defined as microscopy positive sputum after 5 months of category II TB treatment and relapse a microscopy positive sputum at least 2-months after category II treatment completion. Category II TB treatment consisted of 8-Months of rifampicin (R), isoniazid (I), pyrazinamide (Z), ethambutol (E) and streptomycin (S). Streptomycin and pyrazinamide were stopped at month-2 and 3, respectively (2RHZES/1RHZE/5RHE). Since 2014, the molecular diagnostic platform, GeneXpert MTB/RIF® was available for TB diagnostics and care in Mali. In addition to chronic TB, patients with category I (2RHZE/4RH) treatment failure (microscopy positive sputum at month 5) were also tested for rifampicin resistance (RR) and included if they qualified for MDR-TB treatment.
Inclusion and exclusion criteria
We included patients with presumed MDR-TB and hospitalized at the MDR-TB unit of University of Bamako Teach Hospital between 2007 and 2016. We only included those who were18 years or older at admission, received second-line TB drugs for a minimum of 30 consecutive days and who were tested for drug resistance by culture and DST, GeneXpert MTB/RIF® or both methods. Patients with incomplete demographic information and those that did have drug resistance testing results available were excluded.
Data collection procedures
The MDR-TB Unit’s registry was used to identify patients with presumed MDR-TB hospitalized during the study period. Two investigators reviewed each record separately to verify the eligibility criteria and consent signature obtained at admission. Baseline data included demographic information (age, sex, profession, marital status, and permanent address); medical history (coexisting diseases, medications prior to admission); clinical data (weight, temperature, heart rate, respiratory rate, symptoms, chest X-ray); tobacco and alcohol consumption. Laboratory data included sputum, whole blood, and serum HIV test results.
Drug resistance testing (DST)
Sputum samples were collected over two consecutive days at the hospital and sent to the University Clinical Research Center (UCRC/SEREFO) Biosafety Laboratory Level-3. Samples were processed following a standard laboratory algorithm. Sputa were decontaminated, digested using N-acetyl-L-Cysteine 4% NaOH solution, and then concentrated through centrifugation at 3000 g. Pellets were tested by fluorescent smear microscopy using Auramine Rhodamine coloration to identify the presence of Acid Fast Bacilli (AFB). Inoculations were done on both liquid Microscopy Growth Incubator Tube (MGIT®) and Solid Media (Middlebrook 7H11) for six weeks. Positive cultures were identified by Gene probe AccuProbe® GenProbe, San Diego, CA, USA or Capilia for differentiation between Mycobacterium tuberculosis complex (MTBC) and non-tuberculosis mycobacteria (NTM). All samples confirmed MTBC were tested for drug susceptibility using antibiotic susceptibility testing pre-prepared media (AST) containing four major first-line TB drugs, streptomycin, isoniazid, rifampicin, ethambutol (MGIT AST/SIRE®). Patients were confirmed MDR-TB if they were resistant to both rifampicin (R) and isoniazid (I). From 2014 to 2016, in addition to culture/DST, patients with rifampicin resistant (RR-TB) by GeneXpert MTB/RIF® also qualified for MDR-TB treatment as recommended by World Health Organization (WHO). Culture/DST and GeneXpert MTB/RIF® results were used to classified patients as MDR-TB (rifampicin + isoniazid or rifampicin-resistant, respectively) and non-MDR-TB (all other patients).
Hematology and serology for human immunodeficiency virus (HIV)
Blood samples were tested at baseline to determine blood cell counts using Fascount®. Serum was used to test HIV antibodies using Determine® HIV1/2 Rapid Test and HIV ELISA (Genscreen Ultra Ag_Ac) and confirmation with Western Blot (New Lav Blot I and II). HIV-Infected individuals were simultaneously evaluated for combination antiretroviral therapy (ART) in collaboration with the Infectious Diseases Department.
Data analysis and statistical tests
Data were captured electronically, de-identified, all dates removed, and coded (MDR-001, MDR-002, etc.) to preserve confidentiality prior to any data analysis. Data were analyzed using Microsoft SPSS 25.0 software package version 2017. Comparison between means was calculated using independent t-test. Crude and adjusted odds ratios and 95% confidence intervals were calculated for baseline characteristics. In the multivariate regression analysis, we included all variables in the model regardless of p-values in the bivariate analysis. Differences were considered statistically significant if calculated p-values were less than 5% (p <0.05).
Ethical considerations
The protocol was approved by the Ethics committee of the Faculty of Medicine and Pharmacy of the University of Sciences, Techniques and Technologies of Bamako (USTTB), Mali. Written informed consent was obtained from all the subjects at the time of admission for the use of their data for research purposes.
Results
In total, 253 patients were admitted to the MDR TB Care Unit at the Department of Pneumo-phtisiology of Point-G University Teaching Hospital between January 2007 and December 2016. Among them, 39 were excluded from the study due to either incomplete demographic information or missing baseline sputum results. Two hundred fourteen (214) patients with sputum culture and DST results were included in this analysis. Based on our definition of MDR-TB described above, 134 (62.62%) were microbiologically confirmed as having MDR-TB (MDR TB group) and 80 (37.38) were not confirmed to have MDR-TB (Non-MDR-TB group) (Figure 1).
Figure 1.
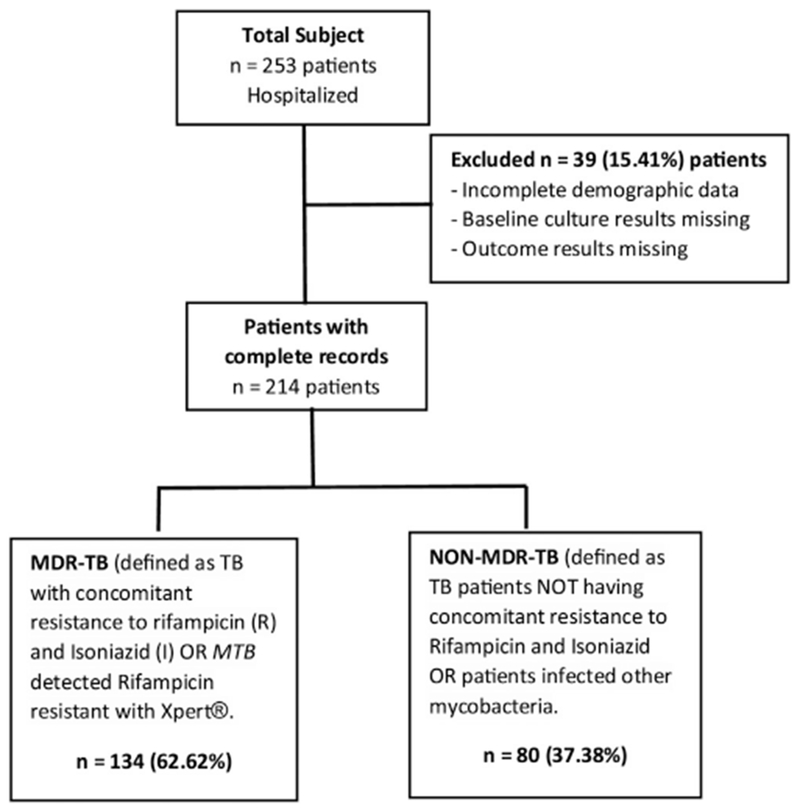
Flow Chart showing patients’ recruitment and stratification in the study categorization for study analysis.
Patients’ demographic characteristics and medical history
The average age was 39.31 ± 14.64 (range 18–83) years; 62.6% (134/214) were less than 40 years old. Patients were predominantly male (163/214; 76.2%) and 77.1% were married (165/214) (Tables 1 and 2). The majority of the patients (81.31%) were living in Mali when they had TB while 18.69% were visiting Mali (85% from Cote d’Ivoire).
Table 1.
Study population characteristics.
| Socio-demographic characteristics | N (%) |
|---|---|
| Gender (N = 214) | |
| Female | 51 (23.8) |
| Male | 163 (76.2) |
| Age in years (N = 214) | |
| ≤40 | 134 (62.6) |
| >40 | 80 (37.4) |
| Occupation (N = 214) | |
| Student/teacher | 28 (13.08) |
| Merchant | 46 (21.50 |
| Farmer/fisher | 39 (18.22) |
| Housewife | 42 (19.63) |
| Killed worker/employee | 15 (7.01) |
| Labor/artist | 44 (20.56) |
| Current marital status (N = 214) | |
| Single | 48 (22.4) |
| Married | 165 (77.1) |
| Divorced | 1 (0.5) |
| Address (N = 214) | |
| Bamako | 98 (45.8) |
| Region | 76 (35.5) |
| Living outside Mali | 40 (18.70) |
| Tobacco (N = 214) | |
| Yes | 68 (31.8) |
| No | 146 (68.2) |
| HIV | |
| Positive | 24 (11.2) |
| Negative | 190 (88.8) |
| Sputum Smear result | |
| Acid Fast Bacilli <3+ | 48 (35.82) |
| Acid Fast Bacilli 3+ | 86 (64.18) |
| Number of previous TB treatment (N = 214) | |
| First episode | 9 (4.2) |
| Episode 2 or 3 | 176 (82.2) |
| Episode ≥4 | 29 (13.6) |
| Had contact with TB Patient before being sick (N = 214) | |
| Yes | 41 (19.2) |
| No | 173 (80.8) |
| Result of last treatment (N = 214) | |
| Failure | 173 (80.3) |
| Relapse | 14 (19.2) |
| Treatment interruption (N = 214) | |
| Yes | 43 (20.1) |
| No | 171 (79.9) |
| Comorbidity (N = 214) | |
| No | 205 (95.8) |
| Diabetes | 6 (2.8) |
| Others* | 3 (1.4) |
| Alcohol consumption (N = 214) | |
| Yes | 18 (8.4) |
| No | 196 (91.6) |
Hypertension (1 case), Pott’s disease (1 case) and adrenal insufficiency (1 case).
Table 2.
Crude Odd Ratio (OR) for demographic and clinical characteristics for MDR-TB.
| Baseline characteristics (n = 214) | MDR-TB n = 134 (%) | Non MDR-TB n = 80 (%) | Odds Ratio, 95% (CI) | P-Value |
|---|---|---|---|---|
| Gender | ||||
| Female | 32 (23.88) | 19 (23.75) | ||
| Male | 102 (76.12) | 61 (76.25) | 0.99 (0.52–1.90) | 0.98 |
| Age | ||||
| ≤40 years | 95 (70.90) | 39 (48.75) | 2.56 (1.44–4.55) | <0.01* |
| >40 years | 39 (29.10) | 41 (51.25) | ||
| Marital status | n = 133 | |||
| Married | 100 (75.19) | 65 (80.25) | 0.70 (0.35–1.39) | 0.31 |
| Single | 33 (24.81) | 15 (18.75) | ||
| HIV | ||||
| Negative | 120 (89.55) | 70 (87.5) | ||
| Positive | 14 (10.45) | 10 (12.5) | 0.82 (0.34–1.94) | 0.65 |
| Smoking | ||||
| No | 93 (69.40) | 53 (66.25) | ||
| Yes | 41 (30.60) | 27 (33.75) | 0.86 (0.48–1.56) | 0.63 |
| Last treatment result | ||||
| Relapse | 15 (11.20) | 26 (32.50) | ||
| Failure | 119 (88.80) | 54 (67.50) | 3.82 (1.87–7.79) | <0.01* |
| Number of previous treatment | ||||
| ≤2 Series of treatment | 123 (91.79) | 62 (77.50) | 3.25 (1.44–7.30) | <0.01* |
| >2 Series of treatment | 11 (8.21) | 18 (22.50) | ||
| Sputum Smear result | ||||
| Acid Fast Bacilli <3+ | 48 (35.82) | 42 (52.50) | ||
| Acid Fast Bacilli 3+ | 86 (64.18) | 38 (47.50) | 1.98 (1.13–3.48) | 0.02* |
| Hemoptysis | ||||
| No | 120 (89.55) | 73 (91.25) | ||
| Yes | 14 (10.45) | 7 (8.75) | 1.22 (0.47–3.15) | 0.71 |
| Alcohol | ||||
| No | 123 (91.80) | 73 (91.25) | ||
| Yes | 11 (8.20) | 7 (8.75) | 1.03 (0.67–1.51) | 1.000 |
| Contact with TB patient | ||||
| No | 102 (76.12) | 71 (88.75) | ||
| Yes | 32 (23.88) | 9 (11.25) | 2.48 (1.11–5.50) | 0.02* |
| History of treatment interruption | ||||
| No | 107 (79.85) | 64 (80.00) | ||
| Yes | 27 (20.15) | 16 (20.00) | 1.01 (0.51–2.02) | 0.98 |
| Impaired physical condition | ||||
| No | 60 (44.78) | 21 (26.25) | ||
| Yes | 74 (55.22) | 59 (73.75) | 0.44 (0.24–0.80) | <0.01* |
n = Number of subjects included in the test CI = Confidence Interval.
Clinical symptoms, physical exam, and hematological parameters
The most common were chronic cough (92.0% (212/214)), weight loss (96.7% (207/214)); night sweats (100% (214/214)). Impairment of physical condition was noted in 62.1% (133/214); hemoptysis in 10.3% (22/214); and peripheral lymph nodes on examination in 2.3% (5/214). The average weight was 50.97 ± 9.16kilograms (Kgs); the average respiratory rate was 28.6 ± 8.3 cycles per minute; average body temperature was 36.86 ± 0.84 °C and the average heart rate was 97.1 ± 15.3 beats per minute. History of close contact with a TB patient was reported in 19.16% (41/214) and TB therapy interruption occurred during first-line treatment in 20.1% (43/214). Co-morbidities were observed in 4.21% (9/214) of the patients with the majority (56% (5/9)) having diabetes. The average White Blood Cells (WBC) was 8968.1 ± 3743.8 cells per millimeter cubic (Cells/mm3), average hemoglobin level was 11.6 ± 2.1 gram per deciliter (g/dL), and the mean platelet count was 470.1 ± 169.7 cells per mm3.
Comparing risk factors for patients with and without MDR-TB
Comparison of characteristics between patients with and without MDR-TB and crude odd radios with 95% confidence intervals through bivariate analyses are shown in Table 2. There was no association between sex and MDR-TB diagnosis OR = 0.99, 95% CI (0.52–1.90), p = 0.98. The average age of MDR-TB and non-MDR-TB patients were 36.6 ± 13.25 and 43.9 ± 15.76years respectively. MDR-TB patients were significantly younger with a mean difference of −7.25 ± 2.01 years 95% CI (−11.22, −3.29); p = 0.0003. Comparing the two groups regarding age ≤ 40 years and > 40 years we found an OR 2.56 (1.44–4.55), p = 0.001. The average weight of MDR-TB was 51.56 ± 9.40 versus 49.98 ± 8.73 Kilograms for non-MDR-TB (independent-Sample t-test average difference was, 1.59 ± 1.29, 95% CI (−0.96 to 4.13), p = 0.22. HIV co-infection was 10.45% (14/134) among MDR-TB and 12.50% (10/80) in Non-MDR-TB with no significant association found OR = 0.82, 95% CI: (0.34–1.94), p = 0.65. Previous treatment failure was 88.81% (119/134) among MDR-TB and 67.5% (64/80) in Non MDR-TB, OR = 3.82, 95% CI (1.87–7.79), p = 0.0002. Receipt of ≤2 prior courses of TB treatment was reported among 123 (91.79%) with MDR-TB and 62 (77.50%) in non-MDR-TB, OR = 3.25 (1.44–7.30) p = 0.004. Sputum smear microscopy positive with 3+ bacilli was reported in 64.18% (84/134) of MDR-TB and 47.5% (38/80) of Non MDR-TB, OR = 1.98, 95% CI (1.13–3.48), p = 0.02. Tobacco consumption was 30.60% (41/134) among MDR-TB and 33.75% (27/80) in Non MDR-TB; OR = 0.86, 95% CI (0.48–1.56), p = 0.63; close contact with TB patient was found 32 (23.88%) of MDR-TB patients versus 9 (11.25%) for Non-MDR-TB, OR = 2.48 (1.11–5.50), 0.02*. Impaired physical condition was noted in 55.22% (74/134) with MDR-TB and 73.75% (59/80) with Non MDR-TB, OR = 0.44, 95% CI (0.24–0.80), p = 0.006. The number of MDR-TB confirmations was increasing during the last 3 years due to the use of GeneXpert MTB/RIF® (Figure 2).
Figure 2.
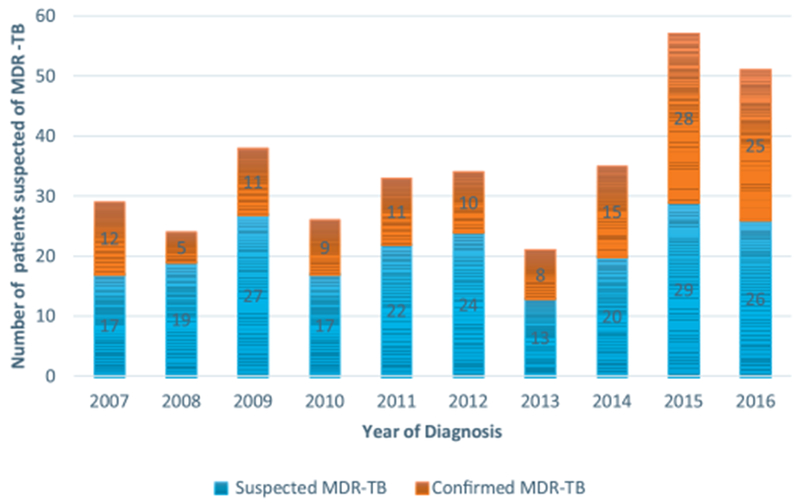
Evolution of MDR-TB diagnosis over time among suspected.Despite that the number of suspected patients referred did not vary significantly in the first seven years, the number of MDR-TB remained on treatment has increased over time. During the last 3 years, more than 90% of referred patients have been diagnosed as MDR-TB cases. The use of GeneXpert MTB/RIF® in routine care starting in 2014 has positively impacted health professionals’ decision in the management of drug-resistant tuberculosis in Mali.
In multivariate regression analyses, factors significantly associated with confirmed MDR TB diagnosis were age ≤ 40 Years OR = 2.69 (1.310–5.560), p = 0.007; previous TB therapy failure OR = 4.43 (1.985–9.890), p <0.001; number of prior TB treatment courses ≤ 2 OR = 2.38 (1.052–5.413), p = 0.03; close contact with TB patient OR = 3.07 (1.244–7.573), p = 0.01. However, impaired physical condition was significantly associated with Non-MDR-TB, OR = 0.383 (0.192–0.764), p = 0.006 (Table 3).
Table 3.
Adjusted Odds Ratios (aOR) for all significant and not significant parameters were calculated for MDR-TB versus Non-MDR-TB. Age ≤ 40 years, treatment episodes ≤ 2, previous treatment failure, close contact with TB patient, an impaired physical condition was independent risk factors for MDR-TB.
| Variables | aOR (95%C.I) | P-Value |
|---|---|---|
| Sex | 0.8 (0.3–1.8) | 0.56 |
| Age ≤ 40 Years | 2.7 (1.3–5.6) | <0.01* |
| Married | 1.6 (0.7–3.7) | 0.32 |
| HIV Positive | 0.9 (0.3–2.4) | 0.80 |
| Number of TB treatment ≤ 2 episodes | 2.4 (1.1–5.4) | 0.04* |
| Previous treatment failure | 4.4 (2.0–10.0) | <0.01* |
| Smoking | 0.8 (0.4–1.7) | 0.56 |
| Alcohol consumption | 1.1 (0.4–3.7) | 0.82 |
| Close contact with TB patient | 3.1 (1.2–7.6) | 0.02* |
| Bacilloscopy 3+ | 1.8 (1.0–3.5) | 0.06 |
| History of TB treatment interruption | 0.9 (0.4–2.0) | 0.75 |
| Impaired physical condition | 0.38 (0.2–0.76) | <0.01* |
| Hemoptysis | 1.0 (0.3–3.01) | 0.10 |
= statistically significant.
Discussion
This cross-sectional study estimated associations between patient baseline characteristics and confirmation of MDR-TB. MDR-TB has been increasing with the Africa region having the highest prevalence as of 2015. Early detection and treatment are top priories to fight MDR/RR-TB (World Health Organization, 2019). Identifying clinical factors that determine high risk for MDR-TB is also a key step. The introduction of GeneXpert MTB/RIF® technology has significantly increased the detection of MDR-TB patients, and more quickly and efficiently because it does not require sophisticated laboratory facilities like the conventional DST/SIRE®. In addition to its simplicity many studies have also evaluated and reported its high accuracy in detecting MDR-TB with 100% specificity in some cases compared to the reference (Guenaoui et al., 2016; Pandey et al., 2017). Our results have shown that MDR-TB confirmed patients were younger (age ≤ 40 years, OR = 2.56 at 95% CI (1.44–4.55), p = 0.0013. The difference in mean age between MDR-TB and Non-MDR-TB was statistically significant (p = 0.0003). Despite that the general population in Mali is younger with 55% of the population being less than 19 years (Cellule de Planification et de Statistiques, 2014). A similar association has been observed in several studies in African and Asian regions (Workicho et al., 2017; Chuchottaworn et al., 2015; Elmi et al., 2016; Gupta et al., 2014; Wondemagegn et al., 2015). These results highlight the fact that much attention should be paid to younger people during their first line TB treatment, and screening for drug resistance TB should prioritize that population while older ages may be suspicious for NTM infections. Male gender was predominant in both MDR-TB and Non-MDR-TB (76.12% versus 76.25%) but no association was found between the two groups. The predominance of men in tuberculosis has been commonly reported elsewhere (Chuchottaworn et al., 2015; Elmi et al., 2016; Mulisa et al., 2015; Gao et al., 2016; Ahmad et al., 2012). HIV co-infection was not associated with MDR-TB OR = 0.82, 95% CI (0.34–1.94), p = 0.65. Nevertheless, similar to studies in other high TB and HIV burden countries such as Thailand, Malaysia, and Uganda; we found that HIV was not a factor associated with confirmation of MDR-TB (Chuchottaworn et al., 2015; Elmi et al., 2016; Lukoye et al., 2013). Several other studies, including a systematic review in Europe and Ethiopia, have reported an association between HIV and MDR-TB (Workicho et al., 2017; Mulisa et al., 2015; Mesfin et al., 2014; Faustini et al., 2006). Treatment failure to the patients’ last course of TB therapy was associated with MDR-TB confirmation (OR = 3.82, 95% CI (1.87–7.79), p = 0.0002. The majority of the Non-MDR-TB had more than one TB treatment; we, therefore, compared two or fewer series of treatments to more than two treatments. Two or less previous treatments was significantly associated with MDR-TB compared to more than two series; OR = 3.25 (p = 0.004). This finding was supported by Muluken Dessalegn et al. who reported a higher aOR for TB patients who had a history of TB treatment compared to those who experience category II regimen (Dessalegn et al., 2016). Our results may be limited by the definition of MDR-TB that combined patients confirmed with culture and DST as well as those tested with GeneXpert MTB/RIF® alone which detect about 95 to 98% of MDR-TB. Until recently after WHO recommendations, in Mali like many resource-limited countries, TB treatment was categorized and provided to patients based on the number of episodes of a cough that have been diagnosed with sputum smear acid-fast-bacilli (AFB) positive. Maiga et al. in 2012 have reported a prevalence of 12% of NTM pulmonary infections in patients with chronic sputum smear AFB-positive cough who were being treated as MDR-TB for their third or more episodes (Maiga et al., 2012). The association between MDR-TB and previous treatment has been one of the most consistent independent risk factors for MDR-TB (Wondemagegn et al., 2015; Mulisa et al., 2015). Indeed, our findings aligned with observations of an earlier MDR-TB report that most of the patients develop drug resistance during their first or second TB treatment course. These observations raise the possibility that patients under first-line TB treatment might not correctly take drugs (dose, time and precaution) (Workicho et al., 2017; Chuchottaworn et al., 2015; Mulisa et al., 2015; Gao et al., 2016; Ahmad et al., 2012; Lukoye et al., 2013; Faustini et al., 2006; Dessalegn et al., 2016; Mekonnen et al., 2015; Gomes et al., 2014). Future studies should be directed to understand the impact of patient behavior/knowledge or caregivers’ attitudes on secondary drug resistance development. Our bivariate analysis showed that patients with sputum smear microscopy positive 3+ were more likely to have MDR-TB, OR=1.98, 95% CI (1.13–3.48), p = 0.01 which has been reported in many studies (Chuchottaworn et al., 2015; Wondemagegn et al., 2015; Mulisa et al., 2015; Sander, 2019). However, the association did not remain after the multivariate analysis. This contradictory finding may be due to bias with our definition of Non-MDR-TB that includes patients with Non-Tuberculosis Mycobacteria (NTM). Patients with NTM usually have a chronic cough and high bacteria load in their sputum.
Our data showed no association between hemoptysis and MDR-TB. In contrast, an association was found in Thailand (Chuchottaworn et al., 2015). Smoking, 30.60% was not associated with MDR-TB; OR 0.86, 95% CI (0.48–1.56), p = 0.63. A similar observation was found in Ethiopia (Dessalegn et al., 2016) but was associated with MDR-TB in Nepal (Mekonnen et al., 2015).
In the study, there was an association between having close contact with a knownTB patient and MDR-TB OR = 2.48,95% CI (1.11–5.50), p = 0.022 which has been reported in several articles (Mulisa et al., 2015; Ahmad et al., 2012; Flora et al., 2013). However, it maybe interesting to know the drug susceptibility profile of their contacts. Impairment of physical condition was significantly less likely to be associated with confirmation of MDR-TB, OR=0.44, 95% CI (0.24–0.80), p = 0.006. This may be related to the virulence attenuation of MDR-TB bacteria compared to drug-susceptible strains. The bacteria genome mutation has a cost on its fitness which has been supported by several studies, for instance, the acquisitionof Isoniazid resistance that impacts the mycobacteria virulence which also impacts on disease severity (Nieto et al., 2016).
Our results may be limited by the fact that the Non-MDR-TB group was a mixture of culture negative, Non-Tuberculosis Mycobacteria (NTM) or first-line TB drug-susceptible TB, which are an inherently heterogeneous population and perhaps requiring different conditions.
Our study reports on clinical characteristics associated with microbiologic confirmation of MDR-TB disease. Younger age, failure of prior TB treatment, lower number of prior TB therapy courses, history of close contact with a TB patient and better physical condition were all found to be independent factors associated with microbiologically confirmed MDR-TB. Based on these findings, we recommend clinicians prioritize patients with such characteristics for optimal MDR TB therapies in resource-limited settings.
Acknowledgments
Funding
Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health under Award Number D43TW010350 and R01AI110386. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to acknowledge all SEREFO/UCRC; department of Pneumo-phtisiology of the University Teaching Hospital of Point G staff, who contributed to patient recruitment, sample processing, and data collection; Elizabeth N. Christian and Daniel Young at Northwestern University who did the administrative work of the supporting funding.
Footnotes
Name of the institution where the work was done: University Clinical Research Center (UCRC)-SEREFO Laboratory, USTTB, Bamako, Mali.
Conflict of interest
The authors have no conflict of interest
References
- Ahmad AM, Akhtar S, Hasan R, Khan JA, Hussain SF, Rizvi N. Risk factors for multidrug-resistant tuberculosis in urban Pakistan: a multicenter case-control study. Int J Mycobacteriol 2012;1:137–42. [DOI] [PubMed] [Google Scholar]
- Cellule de Planification et de Statistiques. Enquête Demographique et de Sante au Mali 2012–2013. 2014. P20.
- Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, et al. HIV infection and multidrug-resistant tuberculosis—the perfect storm. J Infect Dis 2007;196 (Suppl. 1):S86–S107. [DOI] [PubMed] [Google Scholar]
- Chuchottaworn C, Thanachartwet V, Sangsayunh P, Than TZM, Sahassananda D, Surabotsophon M, et al. Risk factors for multidrug-resistant tuberculosis among patients with pulmonary tuberculosis at the central chest institute of Thailand. PLoS One 2015;10(10)e0139986, doi: 10.1371/journal.pone.0139986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessalegn M, Daniel E, Behailu S, Wagnew M, Nyagero J. Predictors of multidrug-resistant tuberculosis among adult patients at Saint Peter Hospital Addis Ababa, Ethiopia. Pan Afr Med J 2016;25(Suppl. 2):5, doi: 10.11604/pamj.supp.2016.25.2.9203. http://www.panafrican-medjournal.com/content/series/25/2/5/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diandé S, Sangaré L, Kouanda S, Dingtoumda BI, Mourfou A, Ouédraogo F, et al. Risk factors for multidrug-resistant tuberculosis in four centers in Burkina Faso, West Africa. Microb Drug Resist Dis 2009;15(3), doi: 10.1089/mdr.2009.0906 Mary Ann Liebert, Inc. [DOI] [PubMed] [Google Scholar]
- Diarra B, Goita D, Tounkara S, Sanogo M, Baya B, Togo ACG, et al. Tuberculosis drug resistance in Bamako, Mali, from 2006 to 2014. BMC Infect Dis 2016;16(714), doi: 10.1186/s12879-016-2060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmi OS, Hasan H, Abdullah S, Mat Jeab MZ, Zilfalil BA, Nyaing NN. Treatment outcomes of patients with multidrug-resistant tuberculosis (MDR-TB) compared with non-MDR-TB infections in Peninsular Malaysia. Malays J Med Sci 2016;23(4):17–25, doi: 10.21315/mjms2016.23.4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug-resistant tuberculosis in Europe: a systematic review. Thorax 2006;61:158–63, doi: 10.1136/thx.2005.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora MS, Amin MN, Karim MR, Afroz S, Islam S, Alam A, et al. Risk factors for multidrug-resistant tuberculosis in Bangladeshi population: a case-control study. Bangladesh Med Res Counc Bull 2013;39:34–41. [DOI] [PubMed] [Google Scholar]
- Gao J, Ma Y, Du J, Zhu G, Tan S, Fu Y, et al. Later emergence of acquired drug resistance and its effect on treatment outcome in patients treated with Standard Short-Course Chemotherapy for tuberculosis. BMC Pulmon Med 2016;16:26, doi: 10.1186/s12890-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M, Correia A, Mendonça D, Duarte R. Risk factors for drug-resistant tuberculosis. J Tuberc Res 2014;2:111–8, doi: 10.4236/jtr.2014.23014. [DOI] [Google Scholar]
- Guenaoui K, Harir N, Ouardi A, Zeggai S, Sellam F, Bekri F, et al. Use of GeneXpert Mycobacterium tuberculosis/rifampicin for rapid detection of rifampicin-resistant Mycobacterium tuberculosis strains of clinically suspected multi-drug resistance tuberculosis cases. Ann Transl Med 2016;4(9):168, doi: 10.21037/atm.2016.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Nagaraja MR, Kumari P, Singh G, Raman R, Singh SK, et al. Association of MDR-TB isolateswith clinical characteristics of patients from Northern region of India. Indian J Med Microbiol 2014;32:270–6. [DOI] [PubMed] [Google Scholar]
- Lukoye D, Adatu F, Musisi K, Kasule GW, Were W, Odeke R, et al. Anti-tuberculosis drug resistance among new and previously treated sputum smear-positive tuberculosis patients in Uganda: results of the first national survey. PLoS One 2013;8(8)e70763, doi: 10.1371/journal.pone.0070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiga M, Siddiqui S, Diallo S, Diarra B, Traore’ B, Shea YR, et al. Failure to recognize nontuberculous mycobacteria leads to misdiagnosis of chronic pulmonary tuberculosis. PLoS One 2012;7(5)e36902, doi: 10.1371/journal.pone.0036902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryde ES, Meehan MT, Doan TN, Ragonnet R, Marais BJ, Guernier V, et al. The risk of global epidemic replacement with drug-resistant Mycobacterium tuberculosis strains. Int J Infect Dis 2017;56:14–20. [DOI] [PubMed] [Google Scholar]
- Mekonnen F, Tessema B, Moges F, Gelaw A, Eshetie S, Kumera G. Multidrug-resistant tuberculosis: prevalence and risk factors in districts of metema and west armachiho, Northwest Ethiopia. BMC Infect Dis 2015;15(461), doi: 10.1186/s12879-015-1202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesfin YM, Hailemariam D, Biadglign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PLoS One 2014;9(1)e82235, doi: 10.1371/journal.pone.0082235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulisa G, Workneh T, Hordofa N, Suaudi M, Abebe G, Jarso G, et al. Multidrug-resistant Mycobacterium tuberculosis and associated risk factors in Oromia Region of Ethiopia. Int J Infect Dis 2015;39:57–61. [DOI] [PubMed] [Google Scholar]
- Nieto RLM, Mehaffy C, Creissen E, Troudt J, Troy A, Bielefeldt-Ohmann H, et al. Virulence of Mycobacterium tuberculosis after acquisition of isoniazid resistance: individual nature of katG mutants and the possible role of AhpC. PLoS One 2016;11(11)e0166807 ,doi: 10.1371/journal.pone.0166807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Pant ND , Rijal KR, Shrestha B, Kattel S, Banjara MR, et al. Diagnostic accuracy of GeneXpert MTB/RIF assay in comparison to conventional drug susceptibility testing method for the diagnosis of multidrug-resistant tuberculosis. PLoS One 2017;12(1)e0169798, doi: 10.1371/journal.pone.0169798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander MS. Sputum bacterial load predicts multidrug-resistant tuberculosis in retreatment patients: a case-control study. Int J Tuberc Lung Dis 2019;20(6) 793–9, doi: 10.5588/ijtld.15.0259 Q 2016 The Union. [DOI] [PubMed] [Google Scholar]
- WHO Global Tuberculosis Report. 2016 http://www.who.int/tb/publications/global-report/en/.
- WHO/HTM/TB/2016.19 Xpert MTB/RIF assay for the diagnosis ofTB Meeting Report; 2016.
- Wondemagegn M, Mekonnen D, Yimer M, Admassu A, Abera B. Risk factors for multidrug-resistant tuberculosis patients in Amhara National Regional State. Afr Health Sci 2015;15(June (2)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulhalik Workicho, Wondwosen Kassahun, Fessahaye Alemseged. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients: a case-control study. Infect Drug Resist 2017;10:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, Multi-drug resistant tuberculosis, Update October 2016. [PubMed]


