Abstract
Background:
Morphomic studies have demonstrated a correlation between sarcopenia and clinical outcomes in septic patients. However, tendon morphomics has not yet been studied in this context. The purpose of the present study was to evaluate tendon morphology in septic patients through analytic morphomics. We hypothesized that morphomic analyses would reveal concomitant muscle and tendon wasting in sepsis patients. The results of this study may help to implement different rehabilitation modalities for critically ill patients.
Materials and Methods:
The volume and fat content of bilateral psoas muscles and tendons were measured on abdominal CT scans of 25 ICU septic and 25 control trauma patients admitted to the University of Michigan between 2011 and 2012. Univariate and multivariate analyses were performed to determine the relationship between psoas muscle and tendon morphometric data, and the association with clinical variables such as smoking and comorbidities.
Results:
Average psoas muscle volume was 12.21 ±5.6 cm3 for control patients and 9.318 ±3.3 cm3 in septic patients (p=0.0023). The average psoas muscle/fat ratio for septic patients was 0.0288 ±0.071 cm3, compared to 0.0107 ± 0.008 cm3 in the control group (p= 0.075). Average tendon volume in the septic population (0.508 ±0.191 cm3) was not different than the control cohort (0.493 ± 0.182 cm3) (p=0.692).
Conclusions:
Our results demonstrate significantly smaller psoas muscle volume in septic patients than in age, gender and BMI-matched trauma patients but no demonstrable change in tendon morphology between patient groups. These findings begin to define the boundaries of clinical application within the field of morphomics.
Keywords: Morphomics, Tendon, Sarcopenia, Sepsis, ICU-acquired weakness
Introduction
Muscle wasting in the acutely ill has become a well-established and extensively-studied phenomenon[1-4] that is estimated to occur in 50-100% of septic ICU patients[5-9]. The severity of sarcopenia has been shown to be both a marker for overall patient health status as well as a predictor of patient outcomes[6,10-13]. The biochemical mechanisms behind the physiology of muscle wasting in acute illness are complex and multifactorial [1-4,14,15]. Acute systemic illness leads to inflammation and metabolic derangements resulting in dysregulation of protein metabolism with increased protein degradation and decreased protein synthesis[3]. Moreover, the loss of muscle mass has also been repeatedly associated with increased intramuscular fatty accumulation[16-18].
While the clinical implications of muscle wasting and ICU-acquired weakness are well established, tendon morphology in these acutely ill patients has thus far been unexplored. The tendon attached to the terminal aspect of a sarcopenic muscle (i.e., the psoas major muscle) are subject to the same inflammatory cytokines and metabolic derangements of acute illness and it is therefore reasonable to surmise that tendon tissues would also be adversely affected. In addition, cell sclerasix lineage found in high densities in tendon tissues, have been implicated in both muscle fibrosis and atrophy[19]. Findings of tendon degradation or atrophy in septic patients could have significant implications with development of ICU mobility protocols or rehabilitative efforts. For example, muscle health is often optimized with movement, weight bearing and exercise, while tendon injury is typically treated with weight bearing relief, immobilization and rest. If both muscle and tendon are found to be adversely affected in an acutely ill patient, one might consider reformatting therapeutic or rehabilitative efforts.
Morphomics has recently emerged as a technique for measuring morphological characteristics of biological systems in 3 dimensional space [20-23]. This measurement tool was first utilized as a predictor of patient outcomes based on the quantification of trunk muscle area using computed tomography (CT) scans. Specifically, the psoas major muscle volume has since been shown to be a predictor of patient functional status, postoperative morbidity, length of stay, cost of care, and overall mortality for surgical patients[10,24-26]. Morphomics has subsequently been used in a variety of clinical contexts with applications across the pediatric[27], geriatric[28], oncologic[29-32], endocrinologic[33], and nutrition[26] literature. Muscle volume, muscle density, visceral fat, subcutaneous fat, body fat content, vessel calcification, trabecular and cortical bone density have all been studied with this method. However, morphomic analysis of tendons have not yet been examined.
The purpose of the present study was to evaluate tendon morphology septic patients through analytic morphomics. Through evaluation of psoas major muscle and psoas major tendon in both septic and healthy patients, we set to examine for concomitant muscle and tendon wasting in acute illness. We hypothesized that morphomic analyses would reveal concomitant muscle and tendon wasting in sepsis patients. The results of this study may help to implement earlier or different rehabilitation modalities for critically ill patients.
Methods
This study was approved by the University of Michigan Institutional Review Board, allowing access to any CT scan performed retrospectively on patients for trauma and nontrauma indications within the University of Michigan Health System. Informed consent was waived by the IRB.
Patient Enrollment:
A sepsis patient cohort was selected from a group of adult patients admitted to the University of Michigan Surgical Intensive Care Unit with sepsis between July 2011 and June 2012. Patients with CT scan of the abdomen and pelvis obtained 0-25 days following ICU admission were included in the study. Patients were excluded if there was incomplete or temporally inappropriate imaging. Control patients were selected from a group of blunt trauma patients admitted from January 2011 to September 2012. Blunt trauma patients were included in the control cohort as these individuals are likely to represent a healthy member of the general population as well as have an abdominal CT scan upon admission.
On first examination of our database, a total of 35 septic patients met inclusion criteria. Thirty-five blunt trauma patients admitted to UMHS with comprehensive CT imaging within 24 hours of admission were included as control patients. Patients were excluded from the control cohort if they had a personal history of sepsis or if they were victim of penetrating or musculoskeletal trauma that would preclude accurate morphometric measurements.
The characteristics of the initial patient cohorts (n=35) are presented in Table 1. Morphomic analysis in revealed dramatic differences in both psoas muscle volume and psoas muscle fat content as displayed in Table 2. However, the patient groups were very different with regards to both age and gender, two variables established as predictors in morphomic analysis[24,34]. For example, men and younger individuals are known to have larger psoas muscles. Some studies have also shown a positive correlation between BMI and muscle attenuation caused by fat accumulation, but other analyses have shown the exact opposite[17,35]. Because of this conflicting information, it was deemed most prudent to also BMI-match our patient groups as well in effort to avoid this potential confounder. This change in protocol was established in order to make accurate morphomic comparisons between cohorts by eliminating possible confounding factors including age, gender and BMI. Our final patient cohort then included 25 ICU patients and 25 age, gender and BMI-matched adult blunt trauma patients.
Table 1.
Summary of patient demographic and clinical data with n = 35 in each group.
| Control patients (n = 35) | Sepsis patients (n = 35) | P value | |
|---|---|---|---|
| Men | 23 (65.7%) | 18 (51.4%) | 0.3319 |
| Age | 42.6 ± 17.94 | 59.25 ± 18.57 | 0.0003* |
| BMI (kg/m2) | 26.31 ± 7.17 | 27.60 ± 6.34 | 0.4280 |
| Smoking history | 7 (20.0%) | 20 (57.1%) | 0.0029* |
| No. of comorbidities | |||
| 0 | 16 (45.7%) | 6 (17.1%) | |
| 1 | 13 (37.1%) | 16 (45.7%) | |
| 2 + | 6 (17.1%) | 13 (37.1%) | |
Patient cohorts were not age or gender matched. Malignancy, diabetes, COPD, kidney disease, heart disease, liver disease, hypertension, and hyperlipidemia were included as comorbidities in this analysis.
Denotes statistical significance.
Table 2.
Summary of psoas muscle and tendon morphometric data from both control and sepsis patient groups (n = 35).
| Control patients | Sepsis patients | P value | |
|---|---|---|---|
| Average psoas muscle volume (cm3) | 13.10 ± 4.98 | 8.70 ± 3.2 | <0.0001* |
| Average psoas fat content (%) | 0.87 ± 0.70 | 2.84 ± 5.43 | <0.0364* |
| Average psoas major tendon volume (cm3) | 0.487 ± 0.149 | 0.518 ± 0.149 | 0.387 |
Patient cohorts were not age or gender matched.
Denotes statistical significance. Psoas muscle volume was significantly larger in the control patient cohort. There was no difference in psoas major tendon volume between these groups.
Age, gender, BMI, comorbidity, and laboratory data were collected via chart review of the electronic medical record for all 50 patients. Malignancy, diabetes, COPD, kidney disease, heart disease, liver disease, hypertension, and hyperlipidemia were included as comorbidities in this analysis.
Analysis:
Using the McKesson imaging software system, CT scans with IV contrast of both sepsis and trauma patients were studied. Specifically, the bilateral psoas muscles and psoas major tendons were analyzed. The right psoas major muscle was first identified at the level of vertebral body of L4. Depending on CT slice thickness, the cross-sectional area of the psoas muscle was traced on 2-4 cuts to calculate psoas muscle volume spanning 1cm thick slices (cm3). While maintaining this muscle volume selection, fat volume was measured. Fat volume was defined as the summated volume of pixels that have attenuation coefficients between −50 and −250 Hounsfield units, the known attenuation range for fat[36-38]. This allowed for software analysis of total volume of fat within the measured psoas volume, or, muscle fat content (cm3). Fat volume:total volume ratio was next calculated to provide overall percentage of fat as a surrogate for muscle atrophy. This process was repeated for the left psoas muscle. Differences in psoas muscle size and muscle fat content between left and right sides in individual patients were observed. For this reason, unilateral volume values were averaged to determine final patient values.
Next, the right psoas major tendon was identified at the level at which it directly opposes the anterior acetabulum. Volume measurements of the tendon were performed and recorded in a fashion identical to that described above. These measurements were performed bilaterally and averaged, as above.
All CT scan measurements were performed by an 18 year-experienced musculoskeletal radiologist who was blinded to patient age and cohort. Average psoas muscle volume at the L4 vertebral body, psoas muscle fat content, and psoas major tendon volume were measured bilaterally for each patient.
Statistical design:
Continuous variables were summarized using means and standard deviations and categorical variables were summarized as percentages. The relationship between patient characteristics, psoas muscle volume, fat content, and tendon volume was compared between patient groups by independent samples t-tests or univariate analysis of variance (ANOVA) as appropriate. Multivariable regression analysis was used to assess the relationship between the outcomes and sepsis while controlling for age, gender, and known confounders such as smoking and comorbidities. Significance was set at α<0.05 and all analyses were conducted using STATA13.
Results
A total of 50 patients were included in this study with volumetric data from 100 psoas muscles and 100 psoas tendons. A summary of patient demographic and clinical data is presented in Table 3. The control (trauma) and sepsis patient groups were age, gender, and BMI-matched. 56% of control patients and 52% of the sepsis cohort were men. The average age in the control group was 49.71 years compared to 53.48 years in the sepsis cohort (p=0.377). The BMI in the control group averaged to 26.97 kg/m2 versus 28.19 kg/m2 in the sepsis group (p=0.587). A brief clinical summary of the sepsis patient cohort is presented in Table 4. The majority of ICU patients were diagnosed with intraabdominal sepsis (n=19, 75%). Average length of stay in the ICU was 22.5 days ± 16.7. The average day at which the CT scan was obtained was 5.68 ± 6.55.
Table 3.
Summary of patient demographic and clinical data.
| Control patients (n = 25) | Sepsis patients (n = 25) | P value | |
|---|---|---|---|
| Men | 14 (56%) | 13 (52%) | 0.77 |
| Age | 49.71 ± 15.62 | 53.48 ± 14.29 | 0.3816 |
| BMI (kg/m2) | 26.97 ± 7.78 | 28.19 ± 6.99 | 0.587 |
| Smoking history | 6 (24%) | 12 (48%) | 0.07 |
| No. of comorbidities | |||
| 0 | 11 (44%) | 5 (20%) | 0.032 |
| 1 | 9 (36%) | 6 (24%) | |
| 2 + | 5 (20%) | 14 (56%) | |
The control and sepsis groups were matched by age, gender, and BMI. Malignancy, diabetes, COPD, kidney disease, heart disease, liver disease, hypertension, and hyperlipidemia were included as comorbidities in this analysis.
Table 4.
ICU hospitalization summary of sepsis patient cohort.
| Etiology of sepsis | |
|---|---|
| Intra-abdominal | 19 (76%) |
| Pulmonary | 3 (12%) |
| Soft tissue | 3 (12%) |
| ICU d at CT scan (HD#) | 5.68 ± 6.55 |
| Total length of stay (d) | 22.5 ± 16.7 |
| ICU length of stay (d) | 5.96 ± 4.61 |
| Days on ventilator | 2.64 ± 4.09 |
| Death during admission | 3 (12%) |
| Death within 30 d of ICU admission | 1 (4%) |
Data presented as mean ± SD for continuous variables and n (%) for categorical variables.
Morphometric analysis results summarized in Table 5. Early sepsis and control patient groups were age, gender and BMI matched. The vast majority of the subjects had IV contrast imaging (n=42, 84%). There was a statistically significant difference in average psoas muscle volume between control patients and septic patients (12.2 vs 9.3, p<0.029). Average psoas muscle fat content in septic patients was 0.0288 ±0.0626 (2.88 ± 6.26%) compared to the control group average of 0.0107 ±0.007 (1.07 ± 0.7%). The muscle fat content in the septic group was more than twice that found in control patients. However, this difference was not found to be statistically significant (p=0.156).
Table 5.
Summary of psoas muscle and tendon morphometric data from both control and sepsis patient groups.
| Control patients |
Sepsis patients |
P value | |
|---|---|---|---|
| Average psoas muscle volume (cm3) | 12.2 ± 5.5 | 9.3 ± 3.3 | 0.029* |
| Average psoas fat content (%) | 1.07 ± 0.7 | 2.88 ± 6.26 | 0.156 |
| Average psoas major tendon volume (cm3) | 0.493 ± 0.166 | 0.508 ± 0.171 | 0.824 |
The control and sepsis groups were matched by age, gender, and BMI.
Denotes statistical significance. Psoas muscle volume was significantly larger in the control patient cohort. There was no difference in psoas major tendon volume between these groups.
There were no observed differences of psoas major tendon volume between patient groups. The average volume of the psoas major tendon in the septic patient population was 0.508 ± 0.171 cm3 compared to 0.493 ±0.166 cm3 in the trauma cohort (p=0.758).
Importantly, the number of comorbidities (coded as 0=0 comorbidities, 1=1 comorbidity, 2= 2 or more) was not associated with any of the outcomes in this sample including psoas muscle volume, psoas muscle fat content, and tendon volume. A multiple regression analysis was conducted for each of the outcomes. Septic/control, age, gender, smoking status and number of comorbidities were included in each model as adjustments. The results indicated psoas muscle volume was significantly reduced by 2.43 cm3 (p<0.045) for the septic group when compared to the control group while adjusting for age, gender, smoking and comorbidities.
Discussion
Computed tomography has been established as the primary means of conducting morphomic analysis [21,24,34,39,40]. Advantages of CT imaging include its wide availability and reproducibility using standard anatomic landmarks. CT scans are regularly obtained for a number of indications and were readily available in our patient cohort. Although ultrasonography is sometimes utilized to examine tendon morphology[41], it remains quite user dependent. Furthermore, uninjured tendons are not routinely evaluated by ultrasounds and as such, these images were not available for our retrospective analysis.
In this study, our results demonstrate significantly smaller psoas muscle volume in septic patients than in control trauma patients. While we cannot definitively conclude that these differences are not related to patient baseline characteristics, we have limited baseline confounders by age, gender and BMI-matching our control cohort. As mentioned above, blunt trauma patients were included as controls because they represent healthy members of the general population who also frequently have an abdominal CT scan upon admission. As such, this population was derived from a cohort with the majority of patients without many baseline comorbidities. The average number of comorbidities in sepsis group was 1.98 compared to only 0.8 in the control group. This difference in comorbidities may reflect increased rates of malignancy or COPD, for example. Even with this consideration, multiple regression analysis demonstrated no significant association between the number of comorbidities and any of the outcomes in this sample. These findings combined with age, sex and BMI-matching suggest that patient baseline comorbidities and characteristics did not contribute to overall psoas muscle size.
Muscle wasting in acute illness reportedly takes between 2-4 weeks to become clinically apparent. In our patient cohort however, morphometric analysis was performed on CT scans obtained an average of only 5.7 days after ICU admission. Earlier muscle atrophy in this patient cohort may be attributed to a number of factors including infectious or inflammatory processes starting days earlier than actual ICU admission. A 2006 study by Khan et al. set out to characterize the prevalence and time of onset of neuromuscular dysfunction in patients with severe sepsis[8]. In this study, the majority of patients demonstrated changes in nerve conduction studies (NCS) very early in the course of severe sepsis (i.e., <7 days after admission). These NCS changes were also predictive of the development of acquired neuromuscular dysfunction and mortality in ICU patients. A more recent study by Axer et al. demonstrated septic patients to have electrophysiologic evidence of peripheral nerve axonal damage during the first week after onset of sepsis[42]. These findings suggest that neuromuscular pathology may start earlier in the time course than previously described.
Radiographic fat content of the psoas major muscles in the septic patients was found to be twice that of the control population in our study. Increased intramuscular fatty infiltration is consistent with the natural disease course in muscle atrophy[16-18] and further lends support to the presence of muscle atrophy in the early sepsis population. Most of the quantitative CT literature on intramuscular fat has been completed in rotator cuff analyses. However, quantitative CT has also been used to investigate site-specific adipose deposition in different body compartments and to assess differential fat deposition and lean muscle mass[36-38]. Computed tomography was the original gold standard for intramuscular fat content measurement, though MRI is now used as another method of measuring intramuscular adiposity[43].
Our laboratory has recently defined the role of fascial tissues in aberrant wound healing after trauma[44]. In adulthood, muscle and tendon repair is guided by progenitor cells of the scleraxis cell lineage, found in high densities in both fascia and tendon tissues. These scleraxis cell types have been implicated in promoting muscle atrophy and fibrosis[19]. Given the small diameter of fasica compared to myofibers in a muscle, we chose to quantify regions with high percentage of scleraxis (i.e., the psoas tendon) in the setting of acute illness to evaluate for concomitant tissue wasting. Our results demonstrate that psoas tendon size was not significantly different between sepsis patients when compared with controls. This critical conclusion is perhaps the most important finding in this study and specifically, its implications for future studies in the field of morphomics. Also subject to the same inflammatory cytokines, gross tendon morphology appears to remain unaffected in our patient population. Thus, tendon morphology is unlikely to be a useful metric in CT morphomic studies of the future.
There are several limitations of this study that must be discussed. The primary limitation of this study is that the results reflect the state of the muscle tendon unit at only a single point in time. Because of the constraints in retrospective design, sequential morphomic measurements were unable to be obtained in our cohort. Only three septic patients had more than one abdominal CT during their ICU hospitalization. Furthermore, our limited cohort did not allow for subgroup analysis with consideration of ICU day at CT scan. Muscle wasting in sepsis would be ideally studied in many individual patients through analysis of serial CT scans throughout course of sepsis in order to establish a time course of atrophy. In this way, a muscle wasting pattern could be clearly delineated and described. However, unfortunately this optimal patient population was not available for study in our retrospective analysis.
It should further be emphasized that this study is limited in its focus on tendon morphology rather than tendon pathology. Our study does not examine tendon strength, stiffness and function. These parameters might be best assessed in real-time with different imaging methodologies and are beyond the scope of morphomic analyses. While psoas tendon volume is an important measure of morphology, entire tendon dimensions, stretch, and position might also be considered. Tendon length was unable to be measured in our patients as the gradual coalescence of fibers at the musculotendinous junction rendered exact tendon dimensions unclear. Similarly, retrospective constraints did not allow confirmation of neutral leg position at the time of imaging, and thus variable tension on the tendons in the study patients cannot be excluded.
Conclusion
In this study, morphometric analysis reveals psoas muscle wasting in sepsis patients though there was no evidence to suggest the presence of concomitant tendon atrophy. With these findings, we begin to define the boundaries of clinical application within the field of morphomics. Specifically, computed tomography is unable to elucidate any changes in tendon health in the context of sepsis.
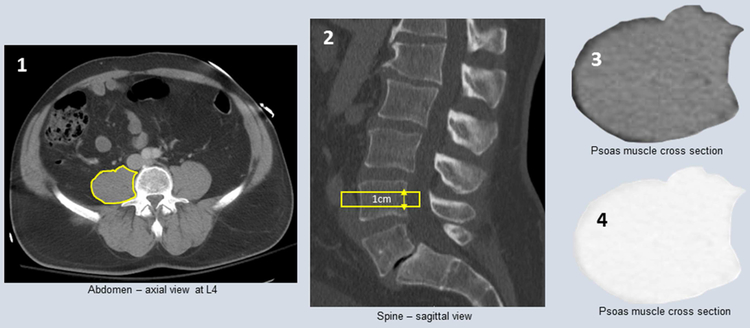
Figure 1: Right psoas muscle measurements.
1) Psoas muscle identified at the L4 vertebrae 2) Cross sectional area of muscle is traced on 2-4 slices to measure 1cm thick section of muscle 3) McKesson software calculates volume of muscle 4) Threshold changed to calculate volume of fat within defined muscle
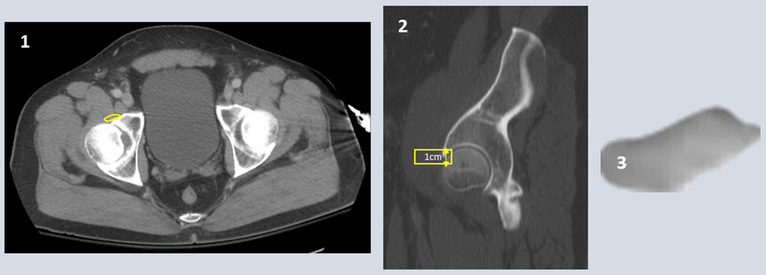
Figure 2: Right psoas major tendon measurements.
1) Psoas major tendon identified at the anterior acetabulum 2) Cross sectional area of tendon is traced on 2-4 slices to measure 1cm length of tendon 3) McKesson software calculates volume of defined tendon
Acknowledgements
BL funded by NIH, NIGMS K08GM109105, NIH R01GM123069, American Association of Plastic Surgery Research Fellowship, Plastic Surgery Foundation/AAPS Pilot Research Award, ACS Clowes Award
Footnotes
Financial Disclosure Statement: The authors have no financial disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Callahan LA, Supinski GS. Sepsis-induced myopathy. Critical Care Medicine 2009;37:S354–67. doi: 10.1097/CCM.0b013e3181b6e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 2014;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- [3].Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle 2010;1:147–57. doi: 10.1007/s13539-010-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mofarrahi M, Sigala I, Guo Y, Godin R, Davis EC, Petrof B, et al. Autophagy and skeletal muscles in sepsis. PLoS ONE 2012;7:e47265. doi: 10.1371/journal.pone.0047265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vincent J-L, Norrenberg M. Intensive care unit-acquired weakness: framing the topic. Critical Care Medicine 2009;37:S296–8. doi: 10.1097/CCM.0b013e3181b6f1e1. [DOI] [PubMed] [Google Scholar]
- [6].Peterson SJ, Braunschweig CA. Prevalence of Sarcopenia and Associated Outcomes in the Clinical Setting. Nutrition in Clinical Practice 2016;31:40–8. doi: 10.1177/0884533615622537. [DOI] [PubMed] [Google Scholar]
- [7].De Jonghe B, Cook D, Sharshar T, Lefaucheur JP, Carlet J, Outin H. Acquired neuromuscular disorders in critically ill patients: a systematic review. Groupe de Reflexion et d'Etude sur les Neuromyopathies En Reanimation. Intensive Care Med 1998;24:1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khan J, Harrison TB, Rich MM, Moss M. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology 2006;67:1421–5. doi: 10.1212/01.wnl.0000239826.63523.8e. [DOI] [PubMed] [Google Scholar]
- [9].Witt NJ, Zochodne DW, Bolton CF, Grand'Maison F, Wells G, Young GB, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest 1991;99:176–84. [DOI] [PubMed] [Google Scholar]
- [10].Shibahashi K, Sugiyama K, Kashiura M, Hamabe Y. Decreasing skeletal muscle as a risk factor for mortality in elderly patients with sepsis: a retrospective cohort study. J Intensive Care 2017;5:1308. doi: 10.1186/s40560-016-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Martin CM, Hill AD, Burns K, Chen LM. Characteristics and outcomes for critically ill patients with prolonged intensive care unit stays. Critical Care Medicine 2005;33:1922–7-quiz1936. [DOI] [PubMed] [Google Scholar]
- [12].Latronico N, Shehu I, Seghelini E. Neuromuscular sequelae of critical illness. Curr Opin Crit Care 2005;11:381–90. [DOI] [PubMed] [Google Scholar]
- [13].Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. Jama 2010;304:1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fanzani A, Conraads VM, Penna F, Martinet W. Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia Sarcopenia Muscle 2012;3:163–79. doi: 10.1007/s13539-012-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nedergaard A, Karsdal MA, Sun S, Henriksen K. Serological muscle loss biomarkers: an overview of current concepts and future possibilities. J Cachexia Sarcopenia Muscle 2012;4:1–17. doi: 10.1007/s13539-012-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Anderson DE, D'Agostino JM, Bruno AG, Demissie S, Kiel DP, Bouxsein ML. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. The Journals of Gerontology Series a: Biological Sciences and Medical Sciences 2013;68:317–23. doi: 10.1093/gerona/gls168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. Journal of Applied Physiology 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- [18].Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. The Journals of Gerontology Series a: Biological Sciences and Medical Sciences 2005;60:324–33. [DOI] [PubMed] [Google Scholar]
- [19].Mendias CL, Gumucio JP, Davis ME, Bromley CW, Davis CS, Brooks SV. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve 2012;45:55–9. doi: 10.1002/mus.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mayhew TM, Lucocq JM. From gross anatomy to the nanomorphome: stereological tools provide a paradigm for advancing research in quantitative morphomics. J Anat 2015;226:309–21. doi: 10.1111/joa.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Canvasser LD, Mazurek AA, Cron DC, Terjimanian MN, Chang ET, Lee CS, et al. Paraspinous muscle as a predictor of surgical outcome. Journal of Surgical Research 2014;192:76–81. doi: 10.1016/j.jss.2014.05.057. [DOI] [PubMed] [Google Scholar]
- [22].Englesbe MJ Quantifying the eyeball test: sarcopenia, analytic morphomics, and liver transplantation. Liver Transpl 2012;18:1136–7. doi: 10.1002/lt.23510. [DOI] [PubMed] [Google Scholar]
- [23].Zhang F, Wang L-P, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature 2007;446:633–9. [DOI] [PubMed] [Google Scholar]
- [24].Miller AL, Min LC, Diehl KM, Cron DC, Chan C-L, Sheetz KH, et al. Analytic morphomics corresponds to functional status in older patients. Journal of Surgical Research 2014;192:19–26. doi: 10.1016/j.jss.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Englesbe MJ, Terjimanian MN, Lee JS, Sheetz KH, Harbaugh CM, Hussain A, et al. Morphometric Age and Surgical Risk. Journal of the American College of Surgeons 2013;216:976–85. doi: 10.1016/j.jamcollsurg.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Friedman J, Lussiez A, Sullivan J, Wang S, Englesbe M. Implications of sarcopenia in major surgery. Nutrition in Clinical Practice 2015;30:175–9. doi: 10.1177/0884533615569888. [DOI] [PubMed] [Google Scholar]
- [27].Harbaugh CM, Zhang P, Henderson B, Derstine BA, Holcombe SA, Wang SC, et al. Personalized medicine: Enhancing our understanding of pediatric growth with analytic morphomics. J Pediatr Surg 2017;0. doi: 10.1016/j.jpedsurg.2017.01.030. [DOI] [PubMed] [Google Scholar]
- [28].Benjamin AJ, Buschmann MM, Schneider A, Derstine BA, Friedman JF, Wang SC, et al. Can comprehensive imaging analysis with analytic morphomics and geriatric assessment predict serious complications in patients undergoing pancreatic surgery? J Gastrointest Surg 2017;66:7–8. doi: 10.1007/s11605-017-3392-3. [DOI] [PubMed] [Google Scholar]
- [29].Sabel MS, Lee J, Wang A, Lao C, Holcombe S, Wang S. Morphomics predicts response to ipilimumab in patients with stage IV melanoma. J Surg Oncol 2015;112:333–7. doi: 10.1002/jso.24003. [DOI] [PubMed] [Google Scholar]
- [30].Wang C, Vainshtein JM, Veksler M, Rabban PE, Sullivan JA, Wang SC, et al. Investigating the clinical significance of body composition changes in patients undergoing chemoradiation for oropharyngeal cancer using analytic morphomics. Springerplus 2016;5:429. doi: 10.1186/s40064-016-2076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chughtai K, Song Y, Zhang P, Derstine B, Gatza E, Friedman J, et al. Analytic morphomics: a novel CT imaging approach to quantify adipose tissue and muscle composition in allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2015;51:446–50. doi: 10.1038/bmt.2015.267. [DOI] [PubMed] [Google Scholar]
- [32].Bayar MA, Antoun S, Lanoy E Statistical approaches for evaluating body composition markers in clinical cancer research. Expert Review of Anticancer Therapy 2017;17:311–8. doi: 10.1080/14737140.2017.1298446. [DOI] [PubMed] [Google Scholar]
- [33].Vaughn VM, Cron DC, Terjimanian MN, Gala ZS, Wang SC, Su GL, et al. Analytic morphomics identifies predictors of new-onset diabetes after liver transplantation. Clin Transplant 2015;29:458–64. doi: 10.1111/ctr.12537. [DOI] [PubMed] [Google Scholar]
- [34].Ranganathan K, Terjimanian M, Lisiecki J, Rinkinen J, Mukkamala A, Brownley C, et al. Temporalis muscle morphomics: the psoas of the craniofacial skeleton. Journal of Surgical Research 2014; 186:246–52. doi: 10.1016/j.jss.2013.07.059. [DOI] [PubMed] [Google Scholar]
- [35].Schafer AL, Vittinghoff E, Lang TF, Sellmeyer DE, Harris TB, Kanaya AM, et al. Fat Infiltration of Muscle, Diabetes, and Clinical Fracture Risk in Older Adults. The Journal of Clinical Endocrinology & Metabolism 2010;95:E368–72. doi: 10.1210/jc.2010-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stojanovska J, Kazerooni EA, Sinno M, Gross BH, Watcharotone K, Patel S, et al. Increased epicardial fat is independently associated with the presence and chronicity of atrial fibrillation and radiofrequency ablation outcome. Eur Radiol 2015;25:2298–309. doi: 10.1007/s00330-015-3643-1. [DOI] [PubMed] [Google Scholar]
- [37].Katznelson L, Rosenthal DI, Rosol MS, Anderson EJ, Hayden DL, Schoenfeld DA, et al. Using quantitative CT to assess adipose distribution in adult men with acquired hypogonadism. American Journal of Roentgenology 1998;170:423–7. doi: 10.2214/ajr.170.2.9456958. [DOI] [PubMed] [Google Scholar]
- [38].Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterology 2005;100:1072–81. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- [39].Pienta MJ, Zhang P, Derstine BA, Enchakalody B, Weir WB, Grenda T, et al. Analytic Morphomics Predict Outcomes After Lung Transplantation. Ann Thorac Surg 2018;105:399–405. doi: 10.1016/j.athoracsur.2017.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Benjamin AJ, Buschmann MM, Schneider A, Derstine BA, Friedman JF, Wang SC, et al. Can Comprehensive Imaging Analysis with Analytic Morphomics and Geriatric Assessment Predict Serious Complications in Patients Undergoing Pancreatic Surgery? J Gastrointest Surg 2017;21:1009–16. doi: 10.1007/s11605-017-3392-3. [DOI] [PubMed] [Google Scholar]
- [41].Kulig K, Chang Y-J, Winiarski S, Bashford GR. Ultrasound-based tendon micromorphology predicts mechanical characteristics of degenerated tendons. Ultrasound Med Biol 2016;42:664–73. doi: 10.1016/j.ultrasmedbio.2015.11.013. [DOI] [PubMed] [Google Scholar]
- [42].Axer H, Grimm A, Pausch C, Teschner U, Zinke J, Eisenach S, et al. The impairment of small nerve fibers in severe sepsis and septic shock. Crit Care 2016;20:606. doi: 10.1186/s13054-016-1241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Somerson JS, Hsu JE, Gorbaty JD, Gee AO. Classifications in Brief: Goutallier Classification of Fatty Infiltration of the Rotator Cuff Musculature. Clin Orthop Relat Res 2015;474:1328–32. doi: 10.1007/s11999-015-4630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Agarwal S, Loder SJ, Cholok D, Peterson J, Li J, Breuler C, et al. Scleraxis-Lineage Cells Contribute to Ectopic Bone Formation in Muscle and Tendon. Stem Cells 2017;35:705–10. doi: 10.1002/stem.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]