Abstract
Aims
To examine the prevalence and person-level predictors of undiagnosed Type 2 diabetes among adults with elevated HbA1c values.
Methods
We identified adults without diabetes who had a first elevated HbA1c (index HbA1c ≥ 48 mmol/mol; ≥ 6.5%) between January 2014 and December 2015, and classified them by Type 2 diabetes diagnosis status at 1 year following this result. Multilevel modelling techniques were used to examine the association of individual demographic, clinical and utilization characteristics with remaining undiagnosed. We quantified differences in early Type 2 diabetes care between diagnosed and undiagnosed individuals.
Results
Of the 18 356 adults with a first elevated index HbA1c, 30.2% remained undiagnosed with Type 2 diabetes 1 year later. Individuals with lower index HbA1c values [adjusted odds ratio (aOR) 5.95, 95% confidence interval (CI) 5.21–6.78 for 48 to < 53 mmol/mol (6.5% to 7.0%); referent 53 to < 64 mmol/mol (7.0% to <8.0%)], who were ≥ 70 years old (aOR 1.40, 95% CI 1.24–1.59; referent 50–59 years), and who had a prior prediabetes diagnosis (aOR 1.35, 95% CI 1.24–1.47; referent no prediabetes) had increased odds of remaining undiagnosed. After adjusting for age, race and index HbA1c, remaining undiagnosed was associated with lower odds of initiating metformin (aOR 0.06, 95% CI 0.05–0.07).
Conclusions
Almost one-third of adults with an elevated HbA1c value were not diagnosed with Type 2 diabetes within 1 year. Undiagnosed Type 2 diabetes, in turn, was associated with differences in early care. Strategies that leverage the electronic health record to facilitate earlier diagnosis may help reduce delays and allow for early intervention towards the goal of improved outcomes.
Introduction
Most people with Type 2 diabetes experience delays in clinical diagnosis of 4–7 years following the onset of hyperglycaemia [1,2]. Although the undiagnosed period is often asymptomatic, it is a missed opportunity for early intervention to treat hyperglycaemia, implement lifestyle changes, and address cardiovascular risk factors. In fact, up to one-quarter of people already have diabetes-related microvascular changes by the time a clinical diagnosis is made [1,3,4]. Achieving early glycaemic control is particularly critical in preventing such disease-related complications [5]. The United Kingdom Prospective Diabetes Study (UKPDS) demonstrated that better early glycaemic control conveyed a substantially lower risk of microvascular complications and myocardial infarctions, a risk reduction that persisted for decades after diagnosis compared with people without initial tight control [5]. Understanding the current prevalence and person-level correlates of a delayed Type 2 diabetes diagnosis is crucial to decreasing such diagnostic delays and optimizing early care.
Inadequate care access and under-screening contribute to the prevalence of undiagnosed diabetes [6–8]. However, missed or delayed Type 2 diabetes diagnoses still occur among insured individuals, even when evidence of hyperglycaemia is available in the electronic health record (EHR) [9,10]. For example, in a 2002 cross-sectional analysis of 1426 adults with EHR-documented evidence of hyperglycaemia, only 79% had diagnostic codes indicating a diagnosis of diabetes [9]. Furthermore, a 2010 chart review of people seen at a Veterans Affairs Medical Center (a system with a well-established EHR) revealed an average delay of 3.7 years between initial EHR evidence of hyperglycaemia and clinical diagnosis [10]. Our work provides an updated look at the prevalence of delayed diagnoses and builds on these two small studies by examining the person-level characteristics and early care differences that are associated with delayed Type 2 diabetes diagnoses.
In this study, we assessed the prevalence of undiagnosed Type 2 diabetes 1 year following an elevated HbA1c, examined factors associated with remaining undiagnosed, and examined differences in receipt of three diabetes-specific care activities based on diagnosis status.
Research design and methods
Study design and setting
We conducted a retrospective, longitudinal cohort analysis of Kaiser Permanente Northern California (KPNC) EHR data to examine the prevalence of undiagnosed Type 2 diabetes 1 year following EHR-documented hyperglycaemia (defined as HbA1c ≥ 48 mmol/mol; ≥ 6.5%) among adult KPNC members. We examined person-level correlates of remaining undiagnosed during this year, as well as differences in receipt of American Diabetes Association (ADA)-recommended care between undiagnosed and diagnosed individuals.
Study population
KPNC is an integrated healthcare system that serves 4.2 million members and has a well-established, Epic®-based EHR. KPNC EHR data were used to identify adults (age ≥ 21 years) with no evidence of prior diabetes who had a first diabetes-consistent HbA1c (index HbA1c ≥ 48mmol/mol; ≥ 6.5%) between 1 January 2014 and 31 December 2015. For KPNC members, all HbA1c testing is performed within KPNC and available in the EHR. We defined a prior diabetes diagnosis as any encounter before the index HbA1c with an ICD-9/10 [11] diagnostic code for diabetes, the presence of diabetes on the EHR-based problem list, or a prior prescription for a diabetes-related medication (other than metformin). We excluded members with any ICD-9/10 codes specific to Type 1 or gestational diabetes. To identify newly diagnosed Type 2 diabetes (rather than new KPNC members with prevalent Type 2 diabetes), we required continuous KPNC membership and at least one outpatient visit in the year before the index HbA1c. We also excluded individuals who did not remain KPNC members during the year following their index HbA1c.
Examined outcomes
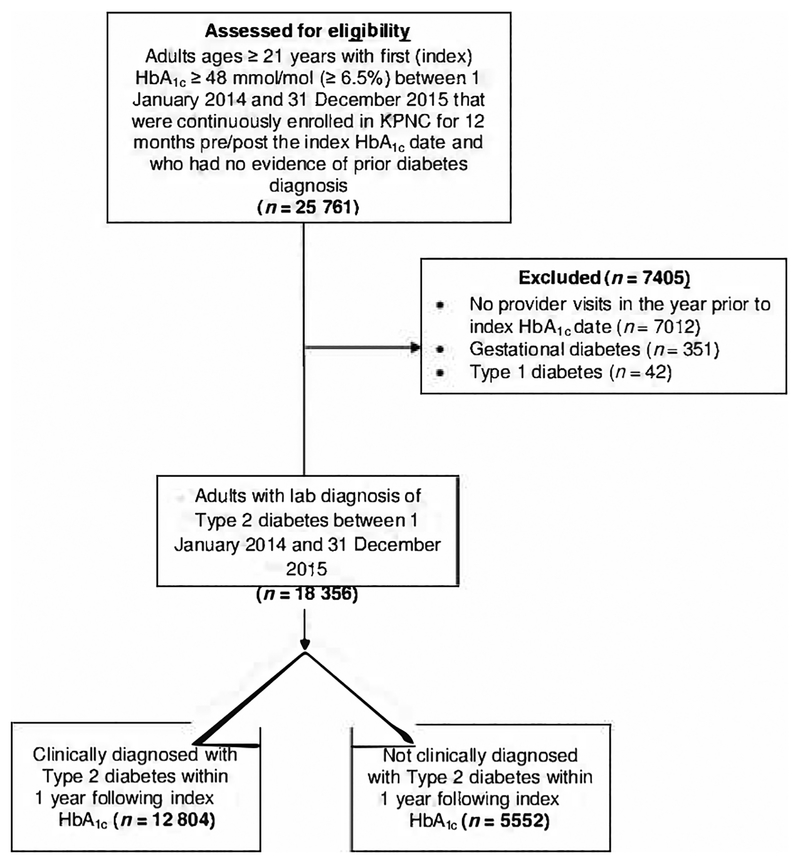
The primary study outcome was individuals’ diagnosis status 1 year after their index HbA1c value (Fig. 1). Individuals were designated as having undiagnosed Type 2 diabetes if they had no encounters (outpatient, inpatient, telephone or secure electronic message) with an associated ICD-9/10 diagnostic code for diabetes and did not have diabetes added to their EHR-based problem list during the year following their index HbA1c date.
FIGURE 1.
Identifying adults with undiagnosed Type 2 diabetes during the year following Electronic Health Record-documented hyperglycaemia
We defined two HbA1c measures to capture subsequent testing: (i) ‘confirmatory HbA1c’, defined as at least one repeated value within 3 months of the index value; and (ii) ‘any follow-up HbA1c’, defined as at least one repeat HbA1c value within 3–12 months following the index value.
We examined differences in three types of ADA-recommended care during the year following the index HbA1c: (i) diabetes-related health education (including both weight management and diabetes self-management), (ii) a retinal exam, and (iii) initiation of metformin. Each of these outcomes was defined as a dichotomous variable. Metformin initiation was defined as an individual filling a prescription at a KPNC pharmacy (nearly all medications are filled internally).
Person-level predictors
Demographic covariates included age, gender, ethnicity/race, English proficiency and neighbourhood socio-economic status. Clinical characteristics included index HbA1c value, BMI, prior prediabetes ICD-9/10 codes, and the presence of comorbid conditions, specifically hypertension, hyperlipidaemia, chronic renal disease, cardiovascular disease and depression (defined using ICD-9/10 codes during the 2 years prior to the index HbA1c). Because use of oral corticosteroids could contribute to hyperglycaemia, use of these agents during the year prior to the index HbA1c was noted. Finally, we examined individuals’ primary care provider (PCP) contact in the year prior to their index HbA1c value (defined as any vs. none). We also examined whether the index HbA1c value was ordered by the PCP or another provider, as well as the PCPs’ years of practice (defined as years since medical school graduation). Since individual-level data on educational attainment and income were not available, we quantified each person’s neighbourhood socio-economic status using the Neighbourhood Deprivation Index (NDI) [12]. Specifically, each person’s geocoded address was linked with census data from the American Community Survey (ACS) and the NDI was computed from census tract-level variables representing income, education, employment, and housing using a previously validated algorithm [13,14].
Statistical analysis
To estimate the adjusted prevalence of undiagnosed Type 2 diabetes in the population, we performed mixed-effects logistic regression with PCP and KPNC medical facility as random effects to account for clustering of individuals by PCP and medical facility. Chi-square and t-tests were performed to determine whether clinically diagnosed and undiagnosed groups differed with respect to their demographic factors, clinical characteristics and prior PCP contact at baseline. Chi-square tests were also used to compare differences in confirmatory and follow-up HbA1c tests between groups.
Mixed-effects logistic regression [15] with PCP and KPNC medical facility included as random effects was used to identify independent predictors of remaining undiagnosed at 1 year following the index HbA1c. The random effects were included to account for clustering of individuals by PCP and medical facility. The covariates included as fixed effects in this model were selected based on research team discussions and the existing literature [16–19] and included: age (categorical variable), index HbA1c (a categorical variable), gender, ethnicity/race, NDI, comorbidities, (including depression), recent oral corticosteroid prescription, prior prediabetes diagnosis, BMI (categorical variable), the provider who ordered the index HbA1c and the PCP’s years of practice.
To estimate the adjusted odds of receiving the examinined diabetes care during the year following the index HbA1c, we employed mixed-effects logistic regression models with PCP and medical facility included as random effects. Based on existing literature and care guidelines, index HbA1c, age, and ethnicity/race were included as fixed effects in these models [19–21].
Additional analyses
We repeated all the aforementioned analyses in two subpopulations. First, we excluded undiagnosed individuals who had a confirmatory HbA1c < 48 mmol/mol (<6.5%) (i.e. ‘unconfirmed Type 2 diabetes’). Second, we limited the population to individuals with ‘milder’ hyperglycaemia (index HbA1c 48 to < 53 mmol/mol; 6.5% to < 7.0%), to better assess the association between initial disease severity and differences in early care.
We also examined how the predictors of remaining undiagnosed differed by age (< 50, 50–70 and ≥ 70 years). All analyses were conducted using SAS Enterprise Guide 4.3.
Results
A total of 18 356 people had an index HbA1c ≥ 48 mmol/mol (≥ 6.5%) (Fig. 1). Of this group, 30.2% (N = 5552) remained undiagnosed in the 12 months following their index HbA1c. Accounting for correlation in individuals’ outcomes within PCP and within KPNC facility, the estimated prevalence of undiagnosed diabetes in this population was 28% (95% confidence interval 26%–31%).
Undiagnosed individuals were older [61 (sd 13) years for undiagnosed vs. 57 (13) years for diagnosed; P < 0.001] and differed significantly from diagnosed individuals by gender, ethnicity/race and NDI (P < 0.001 for all comparisons) (Table 1).
Table 1.
Population characteristics at baseline by clinical diagnosis status
| Diagnosed N =12 804 (69.8%) |
Undiagnosed N = 5552 (30.2%) |
P-value | |||
|---|---|---|---|---|---|
| Characteristics | n | % | n | % | |
| Age; years | < 0.001 | ||||
| 21–29 | 216 | 1.7 | 51 | 0.9 | |
| 30–39 | 1027 | 8.0 | 291 | 5.2 | |
| 40–49 | 2516 | 19.7 | 779 | 14.0 | |
| 50–59 | 3894 | 30.4 | 1475 | 26.6 | |
| 60–69 | 3094 | 24.2 | 1513 | 27.3 | |
| ≥ 70 | 2057 | 16.1 | 1443 | 26.0 | |
| Male | 6465 | 50.5 | 2585 | 46.6 | < 0.001 |
| Race* | < 0.001 | ||||
| White | 4804 | 37.5 | 1867 | 33.6 | |
| Asian | 2881 | 22.5 | 1470 | 26.5 | |
| Latino | 2760 | 21.6 | 991 | 17.8 | |
| Black | 1289 | 10.1 | 734 | 13.2 | |
| Other | 1070 | 8.4 | 490 | 8.8 | |
| Neighborhood Deprivation Index | < 0.001 | ||||
| Least deprived | 2718 | 21.5 | 1312 | 23.8 | |
| Most deprived | 2636 | 20.8 | 1114 | 20.2 | |
| English proficiency (yes) | 11 223 | 88.0 | 4878 | 88.1 | 0.838 |
| Index HbA1c value (%) | < 0.001 | ||||
| 48 to < 53 mmol/mol (6.5 to < 7%) | 6781 | 53.0 | 5121 | 92.2 | |
| 53 to < 64 mmol/mol (7 to < 8%) | 2639 | 20.6 | 337 | 6.1 | |
| 64 to < 75 mmol/mol (8 to < 9%) | 870 | 6.8 | 35 | 0.6 | |
| 75 to < 86 mmol/mol (9 to < 10%) | 582 | 4.5 | 21 | 0.4 | |
| ≥ 86 mmol/mol (≥ 10%) | 1932 | 15.1 | 38 | 0.7 | |
| Preceding diagnoses (yes) | |||||
| Hypertension | 7058 | 55.1 | 3231 | 58.2 | < 0.001 |
| Hyperlipidaemia | 7344 | 57.4 | 3178 | 57.2 | 0.884 |
| Cardiovascular disease | 3480 | 27.2 | 2009 | 36.2 | < 0.001 |
| Chronic renal disease | 1080 | 8.4 | 555 | 10.0 | < 0.001 |
| Prediabetes | 6432 | 50.2 | 3618 | 65.2 | < 0.001 |
| Depression | 1550 | 12.1 | 624 | 11.2 | 0.095 |
| Recent corticosteroid (yes) | 1215 | 9.5 | 648 | 11.7 | < 0.001 |
| BMI; kg/m2 | < 0.001 | ||||
| Normal (18.5–24.9) | 1201 | 9.8 | 833 | 15.5 | |
| Overweight (25.0–29.9) | 3230 | 26.3 | 1653 | 30.8 | |
| Obese (≥ 30.0) | 7861 | 64.0 | 2885 | 53.7 | |
| In-person PCP encounters during year prior to index HbA1c | |||||
| At least one encounter | 10 299 | 80.4 | 4683 | 84.4 | < 0.001 |
| Any PCP encounters during year prior to index HbA1c (%) | |||||
| At least one encounter | 11 240 | 87.8 | 5028 | 90.6 | < 0.001 |
| HbA1c ordered by PCP | 10 482 | 81.9 | 4443 | 80.1 | 0.005 |
| PCP years of practice | |||||
| < 10 | 1816 | 14.5 | 660 | 12.2 | < 0.001 |
| 10 to < 20 | 5081 | 40.7 | 2119 | 39.1 | |
| ≥ 20 | 5589 | 44.8 | 2639 | 48.7 | |
White, non-Hispanic white; Black, non-Hispanic black; Asian, non-Hispanic Asian; Hispanic, Latino.
Undiagnosed people had lower mean index HbA1c values [50 mmol/mol, 6.7% (sd 0.5) for undiagnosed vs. 62 mmol/mol, 7.8% (1.9) for diagnosed; P < 0.001], with 92.2% of undiagnosed individuals having an index HbA1c < 53 mmol/mol (< 7.0%) (compared with 53% of diagnosed individuals; P < 0.001). Fewer undiagnosed individuals had no in-person PCP contact during the year prior to the index HbA1c (15.7% for undiagnosed vs. 19.6% for diagnosed; P < 0.001).
Overall, few individuals had a confirmatory HbA1c value (12.1% for undiagnosed vs. 27.6% for diagnosed; P < 0.001). Of the 5552 undiagnosed individuals, only 10.2% (n = 565) had a confirmatory HbA1c < 48 mmol/mol (6.5%) (compared with 21.6% for diagnosed individuals; P < 0.001), and only 40.5% of undiagnosed individuals had any follow-up HbA1c testing (compared with 76.6% of diagnosed individuals; P < 0.001). The mean number of follow-up HbA1c tests was 0.5 (sd 0.7) for undiagnosed individuals and 1.1 (0.9) for diagnosed individuals (P < 0.001).
For the results of adjusted analyses, we report adjusted odds ratios (aORs) followed by the 95% confidence interval (95% CI). People in the oldest age group (≥ 70 years) were most likely to remain undiagnosed (aOR 1.40, 95% CI 1.24–1.59; referent 50–59 years) (Table 2). Black individuals were also more likely to remain undiagnosed compared with white individuals (aOR 1.26, 95% CI 1.10–1.45). Individuals with with an index HbA1c < 53 mmol/mol (< 7.0%) [aOR 5.95, 95% CI 5.21–6.78; referent 53 to < 64 mmol/mol (7.0% to < 8.0%)] or prior prediabetes (aOR 1.35, 95% CI 1.24–1.47; referent no prior prediabetes) were also more likely to remain undiagnosed. Those with recent oral corticosteroid use had higher odds of remaining undiagnosed compared with those without (aOR 1.27, 95% CI 1.12–1.43). Individuals with previously diagnosed hyperlipidaemia (aOR 0.72, 95% CI 0.66–0.78) or hypertension (aOR 0.90, 95% CI 0.82–0.98), and those with a BMI in the obese range (aOR 0.71, 95% CI 0.62–0.80; referent BMI < 25 kg/m2) were less likely to remain undiagnosed. Also, individuals with PCPs who had < 10 years’ experience were less likely to remain undiagnosed compared with those with PCPs with ≥ 20 years of experience (aOR 0.90, 95% CI 0.81–1.01).
Table 2.
Predictors remaining undiagnosed with Type 2 diabetes at one-year following index HbA1c*
| Variable | aOR (95% CI) | P-value |
|---|---|---|
| Index age; years (referent: 50–59) | ||
| 21–29 | 0.98 (0.66–1.47) | 0.927 |
| 30–39 | 1.14 (0.95–1.37) | 0.167 |
| 40–49 | 0.99 (0.87–1.12) | 0.819 |
| 60–69 | 1.12 (1.01–1.25) | 0.036 |
| 70+ | 1.40 (1.24–1.59) | < 0.001 |
| Index HbA1c (referent: 53 to < 64 mmol/mol; 7 to < 8%) | ||
| 48 to < 53 mmol/mol (6.5 to < 7%) | 5.95 (5.21–6.78) | < 0.001 |
| 64 to < 75 mmol/mol (8 to < 9%) | 0.33 (0.22–0.48) | < 0.001 |
| 75 to < 86 mmol/mol (9 to < 10%) | 0.27 (0.17–0.44) | < 0.001 |
| ≥ 86 mmol/mol (≥ 10%) | 0.15 (0.10–0.21) | < 0.001 |
| Gender (referent: male) | 0.93 (0.86–1.01) | 0.167 |
| Race (referent: white) | ||
| Black | 1.26 (1.10–1.45) | 0.001 |
| Asian | 1.07 (0.95–1.20) | 0.282 |
| Latino | 1.03 (0.92–1.16) | 0.624 |
| Other | 1.17 (1.01–1.36) | 0.034 |
| Neighborhood Deprivation Index (referent: 1st quartile) | ||
| 2nd quartile | 1.01 (0.91–1.13) | 0.800 |
| 3rd quartile | 0.91(0.82–1.02) | 0.117 |
| 4th quartile | 1.01 (0.89–1.14) | 0.882 |
| Preceding diagnoses (referent: no) | ||
| Chronic renal disease | 0.93 (0.81–1.07) | 0.097 |
| Cardiovascular disease | 1.05 (0.95–1.15) | 0.358 |
| Hyperlipidaemia | 0.72 (0.66–0.78) | < 0.001 |
| Hypertension | 0.90 (0.82–0.98) | 0.014 |
| Prediabetes | 1.35 (1.24–1.47) | < 0.001 |
| Depression | 0.90 (0.80–1.01) | 0.082 |
| Recent corticosteroid (referent: no) | 1.27 (1.12–1.43) | < 0.001 |
| BMI (referent: normal) | ||
| Overweight | 0.81 (0.71–0.92) | 0.001 |
| Obese | 0.71 (0.62–0.80) | < 0.001 |
| HbA1c ordering provider (referent: PCP) | ||
| Non-PCP | 1.46 (1.32–1.62) | < 0.001 |
| PCP years practice (referent: ≥ 20 years) | ||
| < 10 | 0.90 (0.81–1.01) | < 0.001 |
| 10 to < 20 | 0.74 (0.63–0.87) | 0.069 |
Model included random effects for PCP and medical facility.
Repeating the analysis after the exclusion of undiagnosed individiduals with a confirmatory HbA1c < 48 mmol/mol (<6.5%) did not significantly change any of the identified predictors of remaining undiagnosed.
In each of the examined age strata, people with index HbA1c values < 53 mmol/mol (< 7%) had increased odds of remaining undiagnosed compared with those with index HbA1c values between 53 and < 64 mmol/mol (7% and < 8%) (Tables S1–S3). For people aged < 50 years and 50 to < 70 years, being black and having a prior prediabetes diagnosis still increased the likelihood of remaining undiagnosed (Tables S1 and S2). However, for people ≥ 70 years, the likelihood of being undiagnosed was no longer associated with being black or prior prediabetes (Table S3).
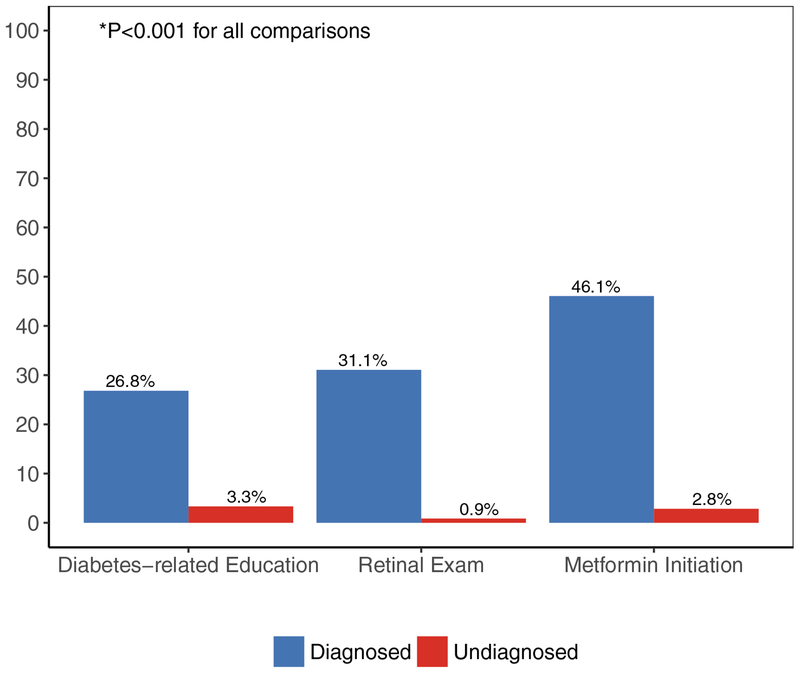
Undiagnosed individuals were less likely to receive diabetes-related education, a retinal exam, and be started on metformin during the year following their index HbA1c values (Fig. 2). After adjustment for age, index HbA1c and ethnicity/race, undiagnosed people were less likely to receive each examined type of care: diabetes-related education (aOR 0.08, 95% CI 0.06–0.09), retinal exam (aOR 0.02, 95% CI 0.02–0.03) and metformin initiation (aOR 0.06, 95% CI 0.05–0.07).
FIGURE 2.
Proportion of people who received select diabetes-related care during the year following their index HbA1c value by clinical diagnosis status.
Among the subpopulation of individuals with index HbA1c values < 53 mmol/mol (< 7.0%), we observed similar significant differences in the receipt of the examined care activities during the year following the index HbA1c, including lower odds of metformin initiation (aOR 0.06, 95% CI 0.05–0.07) for undiagnosed individuals (Fig. S1 and Table S4).
Conclusions
In this longitudinal analysis of data from a large, integrated healthcare system, 30.2% of people with an EHR-documented elevated HbA1c were not clinically diagnosed in the 12 months following this index value. Lower index HbA1c, older age and a prior prediabetes diagnosis were associated with greater odds of remaining undiagnosed. We also quantified the expected association between undiagnosed diabetes and less subsequent recommended diabetes-related care, including diabetes-related education, retinal exams and metformin therapy.
The high prevalence of undiagnosed diabetes suggests the existence of explanations beyond missed or unreviewed lab results [22]. Several provider behaviours may contribute to these delayed diagnoses. First, providers may not formally document a diagnosis unless they are initiating pharmacological treatment, because ordering a prescription medication requires an associated ICD-9/10 code. If this is the case, then people with lower index HbA1c values, who may be less likely to be prescribed medications, would be less likely to have a documented diagnosis. This documentation practice may also explain the observed associations between undiagnosed diabetes with older age and prior prediabetes diagnoses. Given the less-stringent HbA1c targets recommended for older adults, providers may be less likely to start pharmacological treatment and, therefore, would be less likely to document a new clinical diagnosis [21]. Based on recommendations for regular follow-up testing, people with established prediabetes diagnoses may have diabetes detected earlier and have lower index HbA1c values, and, therefore, may be less likely to start pharmacological treatment and have a formal diagnosis documented [22]. Second, some providers may place higher priority on a confirmatory diagnostic test, particularly in the setting of lower levels of hyperglycaemia (e.g. lower index HbA1c). Although this makes theoretical sense, it may contribute to the observed diagnostic delays given the low rates of repeat testing. Only 12.1% of undiagnosed individuals had a confirmatory HbA1c and only 40.5% had any follow-up HbA1c testing at all during the 12 months following their index HbA1c. Finally, the association between lower index HbA1c values and remaining undiagnosed may be further explained by provider disagreement or misinterpretation of guidelines that define an HbA1c value ≥ 48 mmol/mol (≥ 6.5%) as diagnostic, but an HbA1c < 53 mmol/mol (< 7.0%) as the therapeutic goal. Providers’ familiarity with guidelines may also explain the lower likelihood of remaining undiagnosed for people with less-experienced PCPs, as the addition of HbA1c as a diagnostic test was relatively recent.
The increased chance of remaining undiagnosed among black adults is consistent with past work that has demonstrated that non-white populations are more likely to experience diagnostic delays [19,20]. These ethnicity/race-based disparities are often attributed to differential access to care and screening, both barriers that should be minimized for insured KPNC members with already-documented hyperglycaemia. Still, these differences in diagnosis might reflect different levels of interaction and engagement with the healthcare system. Further work is needed to explore this possibility, as well as other potential drivers of these disparities in clinical diagnosis, including differences in Type 2 diabetes clinical presentation and initial treatment preferences [7]. Regardless of the cause, healthcare system-level strategies are needed to improve the timeliness of clinical diagnosis among black individuals.
The differences we observed in receipt of ADA-recommended care by diagnosis status, although expected, help to quantify the early intervention opportunities that may be missed when diagnoses are delayed. Regardless of formal Type 2 diabetes diagnosis status, all the examined individuals arguably have some level of impaired glucose tolerance and could benefit from health education and the initiation of metformin therapy [5,23,24]. For any of these individuals who may technically have prediabetes, support for behaviour change and metformin therapy could help to prevent or delay the onset of Type 2 diabetes. For many of these individuals who do have Type 2 diabetes, the HbA1c reduction achieved with metformin therapy could be enough to achieve the level of early glycaemic control that is associated with decreased long-term micro- and macrovascular complication risks [5,23,24]. Further, initiation of metformin soon after diagnosis and while the HbA1c is low may help to preserve β-cell function, prolonging the effectiveness of metformin and decreasing the risk of future disease-related complications [25]. We plan to follow this cohort over time to assess what happens to those who remained undiagnosed at 1 year and to explore the relationships between diagnosis timing and early care with longer-term health outcomes. Finally, the persistence of these care differences within a subpopulation of people with ‘milder’ initial hyperglycaemia (index HbA1c < 53 mmol/mol; < 7.0%) suggests that these practice differences are not just driven by disease severity, but reflect healthcare-, provider- or person-level variations in care.
Our results must be interpreted within the context of the study design. Eligible people were all members of a single healthcare system, potentially limiting the generalizability of the findings to other populations. Still, past work has demonstrated that the demographic characteristics and diabetes prevalence among KPNC members are representative of the general population and insured populations in Northern California, except at the extremes of incomes [26]. Second, we relied solely on HbA1c values to identify the cohort and may have missed some individuals with diagnostic fasting or random glucose values. However, the use of the HbA1c provided a reliable marker of hyperglycaemia (does not require verification of fasting state or the presence of symptoms) and reflects current diabetes screening practices [27]. Third, providers may have documented diabetes diagnoses in ways we did not capture (e.g. within the text of encounter notes). Similarly, we were not able to capture diabetes-attributable symptoms that may have influenced providers’ diagnostic decisions. Finally, this study cannot address causation. We can only comment on observed associations between person-level characteristics and remaining undiagnosed.
One proposed solution to the high prevalence of undiagnosed diabetes in the USA has been to increase screening in high-risk adults, resulting in national-level changes in screening recommendations [28]. Our findings demonstrate that delays exist even after screening occurs. In this study, almost one-third of adults with EHR-documented hyperglycaemia were not clinically diagnosed within 1 year. Although a better understanding of provider decision-making regarding the diagnosis and documentation of new diabetes is needed, the prevalence of undiagnosed diabetes raises questions regarding our current use of the EHR [29,30]. Although EHRs provide easy access to available HbA1c data, this may not be sufficient to trigger the documentation and subsequent care processes for people with newly diagnosed Type 2 diabetes. Ensuring timely Type 2 diabetes diagnoses may require EHR advances that more explicitly and automatically connect available test results to diabetes diagnoses and prompt early intervention towards the goal of improved Type 2 diabetes outcomes.
Supplementary Material
Table S1. Predictors of remaining undiagnosed at one year among adults aged 21 to < 50 years.
Table S2. Predictors of remaining undiagnosed at one year among adults aged 50 to < 70 years
Table S3. Predictors of remaining undiagnosed at one year among adults aged ≥ 70 years.
Table S4. Adjusted odds of American Diabetes Association-recommended diabetes-related care for undiagnosed individuals compared with diagnosed individuals in sub-population of individuals with index HbA1c values of 48 to < 53 mmol/mol (6.5 to < 7%).
Figure S1. Proportion of people with index HbA1c values of 48 to < 53 mmol/mol (6.5 to < 7%) who had received select diabetes-related care during the year following their index HbA1c by clinical diagnosis status.
What’s new?
Although undiagnosed Type 2 diabetes is usually asymptomatic, diagnostic delays result in missed opportunities for early interventions that may improve peoples’ long-term health.
In this study, we examine the prevalence and predictors of undiagnosed Type 2 diabetes among adults with documented hyperglycaemia.
In a population of 18 356 adults with a first elevated HbA1c, individuals with milder hyperglycaemia, prior prediabetes and those of older age or of black race had higher odds of remaining undiagnosed at 1 year.
After accounting for HbA1c and age at diagnosis, remaining undiagnosed was associated with missed early interventions, including metformin initiation and formal diabetes-related education to support behaviour change.
Acknowledgements
This work was funded by a grant from the Kaiser Permanente Northern California Community Benefit fund. RWG’s time was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K24DK109114).
Footnotes
Early results from this work were presented as posters at the 2017 American Diabetes Association Scientific Sessions and the 2017 Academy Health Annual Research Meeting.
Competing interests
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care 1992; 15: 815–819. [DOI] [PubMed] [Google Scholar]
- 2.Porta M, Curletto G, Cipullo D, Rigault de la Longrais R, Trento M, Passera P et al. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care 2014; 37: 1668–1674. [DOI] [PubMed] [Google Scholar]
- 3.Spijkerman AM, Dekker JM, Nijpels G, Adriaanse MC, Kostense PJ, Ruwaard D et al. Microvascular complications at time of diagnosis of type 2 diabetes are similar among diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the hoorn screening study. Diabetes Care 2003; 26: 2604–2608. [DOI] [PubMed] [Google Scholar]
- 4.Koopman RJ, Mainous AG 3rd, Liszka HA, Colwell JA, Slate EH, Carnemolla MA et al. Evidence of nephropathy and peripheral neuropathy in US adults with undiagnosed diabetes. Ann Fam Med 2006; 4: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Geiss LS, Cheng YJ, Beckles GL, Gregg EW, Kahn HS. The missed patient with diabetes: how access to health care affects the detection of diabetes. Diabetes Care 2008; 31: 1748–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casagrande SS, Cowie CC, Genuth SM. Self-reported prevalence of diabetes screening in the U.S., 2005–2010. Am J Prev Med 2014; 47: 780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiefer MM, Silverman JB, Young BA, Nelson KM. National patterns in diabetes screening: data from the National Health and Nutrition Examination Survey (NHANES) 2005–2012. J Gen Intern Med 2015; 30: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman D. Outpatient diagnostic errors: unrecognized hyperglycemia. Eff Clin Pract 2002; 5: 11–16. [PubMed] [Google Scholar]
- 10.Fraser LA, Twombly J, Zhu M, Long Q, Hanfelt JJ, Narayan KM et al. Delay in diagnosis of diabetes is not the patient’s fault. Diabetes Care 2010; 33: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Classifications. Available at http://www.who.int/classifications/icd/en/ Last accessed 19 September 2017.
- 12.Grant RW, Pabon-Nau L, Ross KM, Youatt EJ, Pandiscio JC, Park ER. Diabetes oral medication initiation and intensification: patient views compared with current treatment guidelines. Diabetes Educ 2011; 37: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J et al. The development of a standardized neighborhood deprivation index. J Urban Health 2006; 83: 1041–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoddard PJ, Laraia BA, Warton EM, Moffet HH, Adler NE, Schillinger D et al. Neighborhood deprivation and change in BMI among adults with type 2 diabetes: the Diabetes Study of Northern California (DISTANCE). Diabetes Care 2013; 36: 1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis, 2nd edn Hoboken, NJ: Wiley, 2011. [Google Scholar]
- 16.Roche MM, Wang PP. Factors associated with a diabetes diagnosis and late diabetes diagnosis for males and females. J Clin Trans Endocrinol 2014; 1: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuels TA, Cohen D, Brancati FL, Coresh J, Kao WH. Delayed diagnosis of incident type 2 diabetes mellitus in the ARIC study. Am J Manag Care 2006; 12: 717–724. [PubMed] [Google Scholar]
- 18.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015; 314: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 19.Moody A, Cowley G, Ng Fat L, Mindell JS. Social inequalities in prevalence of diagnosed and undiagnosed diabetes and impaired glucose regulation in participants in the Health Surveys for England series. BMJ Open 2016; 6: e010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt KJ, Gebregziabher M, Egede LE. Racial and ethnic differences in cardio-metabolic risk in individuals with undiagnosed diabetes: National Health and Nutrition Examination Survey 1999–2008. J Gen Intern Med 2012; 27: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Glycemic targets. Section 6. Standards of medical care in diabetes--2017. Diabetes Care. 2017; 40(Suppl 1): S48–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007; 8: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association. 5. Prevention or delay of Type 2 diabetes. Diabetes Care 2017; 40(Suppl 1): S44–S47. [DOI] [PubMed] [Google Scholar]
- 24.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JB, Conner C, Nichols GA. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 2010; 33: 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon NP. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2011 California Health Interview Survey. Oakland, CA: Kaiser Permanente Division of Research, 2015. Available at https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2011.pdf Last accessed 21 November 2017. [Google Scholar]
- 27.Mehta S, Mocarski M, Wisniewski T, Gillespie K, Narayan KMV, Lang K. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioural interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care 2017; 5: e000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siu AL. Screening for abnormal blood glucose and Type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015; 163: 861–868. [DOI] [PubMed] [Google Scholar]
- 29.Schiff GD, Bates DW. Can electronic clinical documentation help prevent diagnostic errors? N Engl J Med 2010; 362: 1066–1069. [DOI] [PubMed] [Google Scholar]
- 30.Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med 2008; 23: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Predictors of remaining undiagnosed at one year among adults aged 21 to < 50 years.
Table S2. Predictors of remaining undiagnosed at one year among adults aged 50 to < 70 years
Table S3. Predictors of remaining undiagnosed at one year among adults aged ≥ 70 years.
Table S4. Adjusted odds of American Diabetes Association-recommended diabetes-related care for undiagnosed individuals compared with diagnosed individuals in sub-population of individuals with index HbA1c values of 48 to < 53 mmol/mol (6.5 to < 7%).
Figure S1. Proportion of people with index HbA1c values of 48 to < 53 mmol/mol (6.5 to < 7%) who had received select diabetes-related care during the year following their index HbA1c by clinical diagnosis status.