Abstract
Background
Pharmacotherapy remains an important modality for the treatment of neuropathic pain. However, as monotherapy current drugs are associated with limited efficacy and dose‐related side effects. Combining two or more different drugs may improve analgesic efficacy and, in some situations, reduce overall side effects (e.g. if synergistic interactions allow for dose reductions of combined drugs).
Objectives
This review evaluated the efficacy, tolerability and safety of various drug combinations for the treatment of neuropathic pain.
Search methods
We identified randomised controlled trials (RCTs) of various drug combinations for neuropathic pain from CENTRAL, MEDLINE, EMBASE and handsearches of other reviews and trial registries. The most recent search was performed on 9 April 2012.
Selection criteria
Double‐blind, randomised studies comparing combinations of two or more drugs (systemic or topical) to placebo and/or at least one other comparator for the treatment of neuropathic pain.
Data collection and analysis
Data extracted from each study included: proportion of participants a) reporting ≥ 30% pain reduction from baseline OR ≥ moderate pain relief OR ≥ moderate global improvement; b) dropping out of the trial due to treatment‐emergent adverse effects; c) reporting each specific adverse effect (e.g. sedation, dizziness) of ≥ moderate severity. The primary comparison of interest was between study drug(s) and one or both single‐agent comparators. We combined studies if they evaluated the same drug class combination at roughly similar doses and durations of treatment. We used RevMan 5 to analyse data for binary outcomes.
Main results
We identified 21 eligible studies: four (578 participants) evaluated the combination of an opioid with gabapentin or pregabalin; two (77 participants) evaluated an opioid with a tricyclic antidepressant; one (56 participants) of gabapentin and nortriptyline; one (120 participants) of gabapentin and alpha‐lipoic acid, three (90 participants) of fluphenazine with a tricyclic antidepressant; three (90 participants) of an N‐methyl‐D‐aspartate (NMDA) blocker with an agent from a different drug class; five (604 participants) of various topical medications; one (313 participants) of tramadol with acetaminophen; and another one (44 participants) of a cholecystokinin blocker (L‐365,260) with morphine. The majority of combinations evaluated to date involve drugs, each of which share some element of central nervous system (CNS) depression (e.g. sedation, cognitive dysfunction). This aspect of side effect overlap between the combined agents was often reflected in similar or higher dropout rates for the combination and may thus substantially limit the utility of such drug combinations. Meta‐analysis was possible for only one comparison of only one combination, i.e. gabapentin + opioid versus gabapentin alone. This meta‐analysis involving 386 participants from two studies demonstrated modest, yet statistically significant, superiority of a gabapentin + opioid combination over gabapentin alone. However, this combination also produced significantly more frequent side effect‐related trial dropouts compared to gabapentin alone.
Authors' conclusions
Multiple, good‐quality studies demonstrate superior efficacy of two‐drug combinations. However, the number of available studies for any one specific combination, as well as other study factors (e.g. limited trial size and duration), preclude the recommendation of any one specific drug combination for neuropathic pain. Demonstration of combination benefits by several studies together with reports of widespread clinical polypharmacy for neuropathic pain surely provide a rationale for additional future rigorous evaluations. In order to properly identify specific drug combinations which provide superior efficacy and/or safety, we recommend that future neuropathic pain studies of two‐drug combinations include comparisons with placebo and both single‐agent components. Given the apparent adverse impact of combining agents with similar adverse effect profiles (e.g. CNS depression), the anticipated development and availability of non‐sedating neuropathic pain agents could lead to the identification of more favourable analgesic drug combinations in which side effects are not compounded.
Plain language summary
Drug combinations for chronic neuropathic pain in adults
Neuropathic pain – due to nerve disease or damage – is often treated by pain medications which have limited effect and/or dose‐related side effects when given alone. Combinations of more than one drug are often used with the goal of achieving better pain relief or fewer side effects (if the pain relieving effects of the combined drugs are more additive than the side effects), or both. Despite evidence that over 45% of individuals suffering from neuropathic pain take two or more drugs for their pain, we could find only 21 high‐quality studies of various different systemic and topical drug combinations. Given the wide possible variety of different drug combinations and the small number of studies, results for neuropathic pain from this review are insufficient to suggest the value of any one specific drug combination. However, the publication of multiple high‐quality studies suggesting the superiority of some drug combinations, together with evidence that drug combinations are widely used in clinical practice, underline the importance of conducting more combination studies with improved methodology.
Background
Description of the condition
Neuropathic pain has recently been redefined by the International Association for the Study of Pain (IASP) as “pain caused by a lesion or disease of the somatosensory system” (Jensen 2011) and comprises a wide variety of different central (e.g. post‐stroke thalamic pain, spinal cord injury pain) and peripheral (e.g. diabetic neuropathy, postherpetic neuralgia) disorders. Depending on estimation methods, recent reports on the prevalence of neuropathic pain have varied from 5% (Bouhassira 2008) to 8% (Torrance 2006).
Description of the intervention
In addition to a wide variety of non‐pharmacological approaches and interventional techniques for the treatment of neuropathic pain, pharmacological therapy remains an important component of neuropathic pain management (Gilron 2006). To address limitations in the efficacy and tolerability of neuropathic pain drugs as monotherapies clinicians often resort to concurrent administration of more than one pharmacological agent, i.e. 'polypharmacy' or 'combination pharmacotherapy'. In acute pain and migraine, combinations of analgesics used simultaneously provide additive pain relief (Moore 2012a), and combination analgesics are among the most effective drugs in acute pain (Moore 2011).
How the intervention might work
Evidence suggests that, even among individuals with seemingly singular neuropathic conditions (e.g. postherpetic neuralgia), substantial diversity exists with respect to various clinical manifestations, sensory examination features and presumably underlying pain mechanisms (e.g. see Baron 2009a; Maier 2010). This mechanistic diversity may be one reason for limited analgesic efficacy of pharmacologic agents as monotherapy, i.e. incomplete suppression of multiple nociceptive mechanisms. Also, dose‐related drug side effects (e.g. somnolence, dizziness) may limit the tolerability of higher, more efficacious doses of analgesic drugs. Thus, combining drugs with different pharmacological mechanisms may result in greater efficacy by simultaneously suppressing multiple pain mechanisms (Gilron 2005a). Furthermore, the potential for favourable additive or synergistic interactions between different analgesics may allow for lower doses of individual drugs which may provide a better safety/tolerability profile (as long as there is no additivity or synergy for adverse effects). It must be emphasised that, in most situations, a clinically useful two‐drug (A + B) combination should be superior to either drug alone (i.e. A + B > A and A + B > B) and should contain agents both of which are efficacious on their own (i.e. A > placebo and B > placebo). Furthermore, if both drugs share common adverse effects (e.g. sedation), what is necessary for a drug combination to be useful is that pain‐relieving effects are more additive than are adverse effects, i.e. synergy for pain reduction is not absolutely necessary (Gilron 2005a). Combination pharmacotherapy in acute pain results in an additive, not synergistic, effect (Moore 2012a); there was no evidence for synergy, if defined as interaction or co‐operation of two or more drugs to produce a combined effect greater than the sum of their separate effects.
Why it is important to do this review
Identification of favourable analgesic combinations will promote their more widespread use with the end result of improving population effectiveness of neuropathic pain pharmacotherapy. Identification of analgesic combinations associated with an unfavourable therapeutic ratio (i.e. balance between analgesia and side effects) will discourage their subsequent use and ultimately reduce the population toxicity, and improve the cost‐effectiveness of neuropathic pain pharmacotherapy. Finally, given these important objectives of analgesic combination trials, detailed review and consideration of methodology of combination trials conducted to date will serve to guide future improvements in the continued evaluation of promising analgesic combinations.
Objectives
The objectives of this review were to evaluate the efficacy (primary), tolerability (secondary) and safety (secondary) of various drug combinations for the treatment of neuropathic pain.
Methods
Criteria for considering studies for this review
Types of studies
We sought double‐blind, randomised controlled trials (RCTs) comparing combinations of two or more drugs to placebo and/or at least one other comparator for the treatment of neuropathic pain. We graded all considered studies for quality as per the 'Risk of bias' tool.
Types of participants
We included studies involving adult participants of 18 years and older with a diagnosis of neuropathic pain.
Types of interventions
We included interventions involving a combination of two or more different drugs.
Types of outcome measures
We included participant‐reported measure(s) of pain intensity or pain relief using validated methods.
Primary outcomes
The primary outcome we sought was the proportion of participants reporting ≥ 30% pain reduction from baseline OR ≥ moderate pain relief OR ≥ moderate global improvement. The selection of ≥ 30% pain reduction from baseline was based upon analyses demonstrating a relationship between this degree of pain reduction (≥ 30%) and concurrent patient ratings of 'much improved' or 'very much improved' using a patient global impression of change scale (Farrar 2001). The most recent guidance favours an even more stringent definition of at least 50% pain relief (Moore 2010b) because distribution of pain relief tends to be bimodal, and because high levels of pain relief are strongly associated with improved fatigue, sleep, depression, work ability and quality of life. This outcome tends not to have been reported in older studies, and to be unavailable without access to data at the level of the individual patient.
Secondary outcomes
The secondary outcomes we sought were as follows:
Proportion of participants dropping out of the study due to treatment‐emergent adverse effects.
Proportion of participants reporting each specific adverse effect (e.g. sedation, dizziness) of ≥ moderate severity.
Search methods for identification of studies
Electronic searches
We searched the following databases for studies (most recent search conducted 9 April 2012):
the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 3 2012);
MEDLINE (1947 to April 9, 2012); and
EMBASE (via Ovid, 1980 to April 9, 2012).
In addition to the pre‐planned literature search, we further searched the clinical trials.gov and controlled‐trials.com trial databases for completed pharmaceutical industry trials which posted their results on the clinicalstudyresults.org website.
We performed a snowballing search to increase the accuracy of the protocol defined search (Greenhalgh 2005).
We developed a search strategy for MEDLINE and also adapted this for the other databases (see Appendix 1).
Searching other resources
We also searched the reference lists of over 50 published systematic reviews on the subject of neuropathic pain pharmacotherapy for eligible studies.
Data collection and analysis
Selection of studies
We selected studies as per the criteria listed above.
Data extraction and management
We extracted the following data from each study included: study drug name(s), dose(s), route(s) and study/treatment duration; proportion of participants a) reporting ≥ 30% pain reduction from baseline OR ≥ moderate pain relief OR ≥ moderate global improvement; b) dropping out of the study due to treatment‐emergent adverse effects; c) reporting each specific adverse effect (e.g. sedation, dizziness) of ≥ moderate severity.
Assessment of risk of bias in included studies
We graded all considered studies for quality as per the 'Risk of bias' tool (Higgins 2011). It should be noted that the protocol, and thus the methodology, of this review preceded more recent considerations of bias such as imputation methods for treatment responder analyses (Moore 2012b).
Measures of treatment effect
The primary comparison of interest was between study drug(s) and one or both single‐agent comparators. We also searched for comparisons of each two‐drug combination and any other placebo and/or active treatment comparators. We combined studies if they evaluated the same drug class combination at roughly similar doses and durations of treatment. We used RevMan 5 (RevMan 2011) to analyse study data for binary outcomes.
Unit of analysis issues
For studies involving more than one active treatment group, we would divide the control treatment group among the active treatment arms.
Dealing with missing data
Analyses were based upon intention‐to‐treat (ITT). We considered randomised participants receiving assigned treatments and providing at least 50% of the required outcome data in the ITT population.
Assessment of heterogeneity
We only combined studies evaluating similar conditions for analysis so as to avoid clinical heterogeneity. We planned to use visual data assessment with L'Abbé plots (L'Abbé 1987) and to calculate the I² statistic to explore statistical heterogeneity when the I² was greater than 50%.
Assessment of reporting biases
In this review we extracted dichotomous data which are independent of other results that study authors may have chosen to report. The review did not include any evaluation of publication bias.
Data synthesis
We planned to use a fixed‐effect model for any meta‐analyses conducted.
Subgroup analysis and investigation of heterogeneity
We would group according to specific combinations of drug classes (e.g. opioids and anticonvulsants).
Sensitivity analysis
We planned to use sensitivity analyses to evaluate the robustness of a particular result by repeating primary analyses without studies which were considered to be outliers with respect to study quality, drug dose/duration or pain measurement scales.
Results
Description of studies
See the 'Characteristics of included studies' and the 'Characteristics of excluded studies' tables for further information.
We identified 107 relevant citations for this review but found only 21 neuropathic pain RCTs that fulfilled the review inclusion criteria.
Results of the search
On 9 April 2012 we identified 1168 citations, which two of the review authors (IG, LEC) independently screened based on the title and the abstract. The first screening for obvious exclusions yielded 132 records that we further reviewed in more detail. We retrieved and reviewed 107 articles in full text and finally 21 fulfilled the inclusion criteria (see Figure 1).
1.
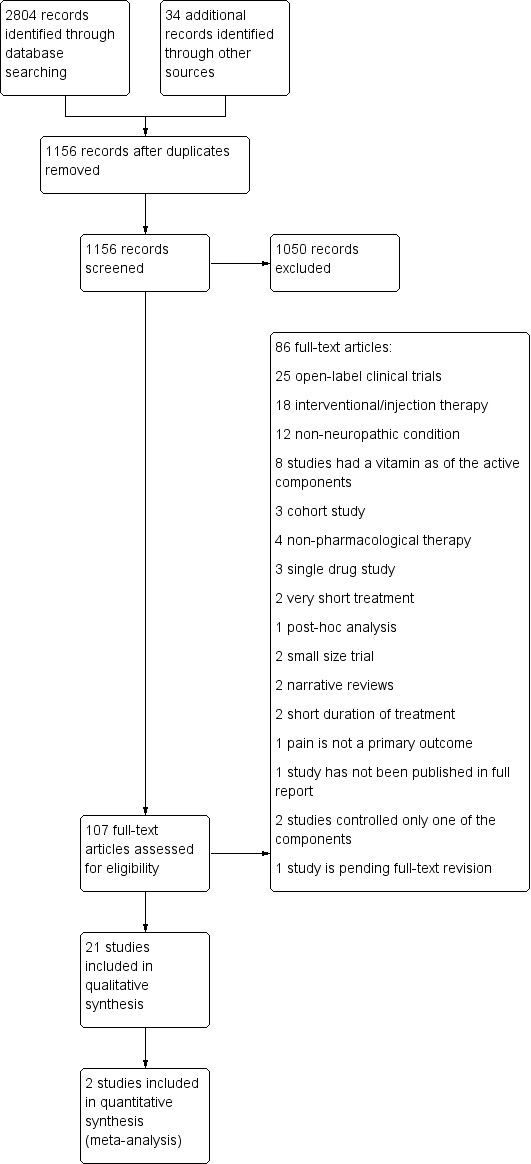
Study flow diagram.
Our search for studies registered on the clinicaltrials.gov and controlled‐trials.com databases only yielded one recently completed relevant study (http://clinicaltrials.gov/ct2/show/NCT00944697) and another ongoing trial (http://clinicaltrials.gov/ct2/show/NCT00516503); results from these studies were not available at the time of this review.
Study selection
We identified 21 studies that fulfilled the inclusion criteria of the review. Four studies (578 participants) evaluated the combination of an opioid with gabapentin or pregabalin (Caraceni 2004; Gilron 2005b; Hanna 2008; Zin 2010); two studies (77 participants) evaluated the combination of an opioid with a tricyclic antidepressant (Khoromi 2007; Mercadante 2002); one study (56 participants) evaluated the combination of gabapentin and nortriptyline (Gilron 2009); three studies (90 participants) evaluated the combination of fluphenazine with a tricyclic antidepressant (Gomez‐Perez 1985; Gomez‐Perez 1996; Graff‐Radford 2000); three studies (90 participants) evaluated the combination of an N‐methyl‐D‐aspartate (NMDA) blocker with an agent from a different drug class (Amr 2010; Eichenberger 2008; Tonet 2008); five studies (604 participants) evaluated combinations of various topical medications (Agrawal 2009; Barton 2011; Lynch 2003; Lynch 2005; McCleane 2000); one study (313 participants) evaluated the combination of tramadol with acetaminophen (Freeman 2007), another one (44 participants) evaluated the combination of a cholecystokinin blocker (L‐365,260) with morphine (McCleane 2003) and a recent trial evaluated the combination of alpha lipoic acid and gabapentin for burning mouth syndrome (Lopez‐D'alessandro 2011). Full characteristics are presented in the 'Characteristics of included studies' table.
Study design
Twelve studies (Agrawal 2009; Amr 2010; Barton 2011; Caraceni 2004; Freeman 2007; Graff‐Radford 2000; Hanna 2008; Lopez‐D'alessandro 2011; Lynch 2005; McCleane 2000; Tonet 2008; Zin 2010) used a parallel design and nine (Eichenberger 2008; Gilron 2005b; Gilron 2009; Gomez‐Perez 1985; Gomez‐Perez 1996; Khoromi 2007; Lynch 2003; McCleane 2003; Mercadante 2002) used a cross‐over design. None of the cross‐over trials conducted analyses involving first period data only, likely due to inadequate statistical power.
Nineteen of the included studies evaluated a two‐drug combination and only two studies (Barton 2011; Tonet 2008) evaluated a three‐drug combination. Three studies compared the combination of interest to placebo only (Barton 2011; Freeman 2007; Gomez‐Perez 1985). Nine double‐drug studies compared their combination to placebo and each of the two single agents alone (Agrawal 2009; Eichenberger 2008; Gilron 2005b; Graff‐Radford 2000; Khoromi 2007; Lopez‐D'alessandro 2011; Lynch 2003; Lynch 2005; McCleane 2000; McCleane 2003). One study compared the combination to the medications alone with no placebo arm (Gilron 2009). Five studies looked at the effect of the combination compared with only one of the two single‐agent components (Amr 2010; Caraceni 2004; Hanna 2008; Mercadante 2002; Zin 2010). One study compared the combination to a completely different single‐agent drug (Gomez‐Perez 1996). Only one study compared the effect of a three‐drug combination to two of those drugs (Tonet 2008) and that study did not include a placebo arm.
Outcomes
Only 10 out of 21 studies reported the primary outcome, as defined in the protocol for this review (percentage of pain relief) (Eichenberger 2008; Freeman 2007; Gilron 2005b; Gilron 2009; Gomez‐Perez 1985; Gomez‐Perez 1996; Hanna 2008; Khoromi 2007; Lynch 2005; Zin 2010). Most of the studies reported pain intensity scores (Agrawal 2009; Amr 2010; Caraceni 2004; Eichenberger 2008; Freeman 2007; Gilron 2005b; Gilron 2009; Graff‐Radford 2000; Hanna 2008; Khoromi 2007; Lynch 2003; Lynch 2005; McCleane 2000; McCleane 2003; Mercadante 2002; Tonet 2008; Zin 2010). Patient global impression of change was reported in seven studies (Freeman 2007; Gilron 2005b; Gilron 2009; Hanna 2008; Khoromi 2007; Lopez‐D'alessandro 2011; Zin 2010). A number reported the Short Form of the McGill Pain Questionnaire (SF‐MPQ) (Agrawal 2009; Freeman 2007; Gilron 2005b; Gilron 2009; Graff‐Radford 2000; Hanna 2008; Lynch 2003; Lynch 2005) and/or the Brief Pain Inventory (BPI) (Freeman 2007; Gilron 2005b; Gilron 2009; Hanna 2008). Only three studies measured co‐analgesia requirement (Caraceni 2004; McCleane 2003; Tonet 2008). Five studies reported the 36‐item Short‐Form General Health Survey (SF‐36) (Freeman 2007; Gilron 2005b; Gilron 2009; Khoromi 2007; Zin 2010) and four reported sleep interference (Freeman 2007; Hanna 2008; Mercadante 2002; Zin 2010). Seven studies reported a mood state scale (Freeman 2007; Gilron 2005b; Gilron 2009; Graff‐Radford 2000; Khoromi 2007; Lopez‐D'alessandro 2011; Mercadante 2002; Zin 2010). Additionally, four studies measured the serum levels of the studied drugs (Gilron 2009; Graff‐Radford 2000; Lynch 2003; Lynch 2005) and only seven studies reported neuropathy symptoms as well as pain scores (Barton 2011; Caraceni 2004; Lynch 2005; Gomez‐Perez 1985; Gomez‐Perez 1996; McCleane 2000; Zin 2010). Three studies reported use of sensory tests (Agrawal 2009; Eichenberger 2008; Lynch 2005).
Pain conditions
Painful diabetic neuropathy was explored in 11 studies (Agrawal 2009; Freeman 2007; Gilron 2005b; Gilron 2009; Gomez‐Perez 1985; Gomez‐Perez 1996; Hanna 2008; Lynch 2003; Lynch 2005; Tonet 2008; Zin 2010); postherpetic neuralgia in seven studies (Gilron 2005b; Gilron 2009; Graff‐Radford 2000; Lynch 2003; Lynch 2005; Tonet 2008; Zin 2010); neuropathic cancer pain in three studies (Barton 2011; Caraceni 2004; Mercadante 2002); chronic sciatica in one study (Khoromi 2007); spinal cord injury pain in one study (Amr 2010); another study was developed in patients with burning mouth syndrome (Lopez‐D'alessandro 2011) and peripheral nerve injury was the pain condition in three studies (Lynch 2003; Lynch 2005; Tonet 2008). Only one study included participants with complex regional pain syndrome (CRPS) type II (Tonet 2008); in one study, the diagnosis was made based on constituent symptoms (McCleane 2000); and one study included "intractable neuropathic pain of mixed aetiology unresponsive to currently available tricyclic antidepressants, anticonvulsants, opioids and non steroidal anti‐inflammatory drugs" (McCleane 2003).
Previous and concomitant pain treatment
We evaluated the analgesic profile previous to participation in the trial for the included studies: patients continued taking at least two additional analgesics during a trial of neuropathic pain in cancer (NPC) (Caraceni 2004); another study in patients with NPC excluded those that were already taking the evaluated drug (antidepressants) (Mercadante 2002); of note, the baseline opioid requirement of the included patients was not stated. In a recent study in chemotherapy‐induced peripheral neuropathy, the participants could not be concurrently treated with antidepressants or anticonvulsants (Barton 2011).
Two studies, one evaluating patients with phantom limb pain (Eichenberger 2008) and another one of topical analgesia (Lynch 2005) included participants taking a wide range of number and type of co‐analgesics: patients were taking from no medications to three additional drugs, apart from those evaluated in the trial.
The majority of the patients (39/57) in one trial were not taking any concomitant drug or taking acetaminophen or anti‐inflammatories only (Gilron 2005b). In a similar trial 28/56 patients were free of analgesics before the trial and 20/56 were taking acetaminophen or anti‐inflammatories only (Gilron 2009). One study allowed the concomitant use of any analgesic except for extended‐release opioids, which were the studied drug (Hanna 2008). In one study that evaluated patients with sciatica, most of the participants were taking non‐steroidal anti‐inflammatory drugs or opioids before the trial (Khoromi 2007). One study evaluating topical analgesics allowed the use of any other analgesic during the trial; however, the report does not show the concomitant analgesia profile of the participants (Lynch 2003).
One study excluded patients who failed treatment with anti‐inflammatories or antidepressants (McCleane 2000); another study from the same research group included patients that were unresponsive to any other analgesic scheme (McCleane 2003); however, the concomitant analgesic profile of the participants was not stated in the publication of those studies. The only study that evaluated the combination of three drugs (Tonet 2008) excluded opioid users; however the participants were rescued with 30 mg codeine.
Previous analgesics were stopped before the trial in three studies (Agrawal 2009; Freeman 2007; Zin 2010). Finally, the information concerning concomitant analgesia is not stated in five studies (Amr 2010; Gomez‐Perez 1985; Gomez‐Perez 1996; Graff‐Radford 2000; Lopez‐D'alessandro 2011).
Excluded studies
We excluded all studies that used a neuraxial approach (Aldrete 2006; Amr 2011; Autio 2004; Blonna 2004; Braun 1982; Bush 1991; Devulder 1999; Dureja 2010; Glynn 1996; Karppinen 2001; Kotani 2000; Lauretti 2002; Pirbudak 2003; Rodriguez 1999; Schechtmann 2010; Siddall 2000) and targeted injection therapies (Amjad 2005; Eker 2012; Lemos 2008; Martinez 1990; Stajcic 1990), given that they have been recently reviewed elsewhere (Manchikanti 2010; Patel 2009) and because our search strategy was not designed to detect all studies of injection therapies. We excluded two studies combining transcutaneous electrical stimulation plus systemic analgesia (Alvaro 1999; Barbarisi 2010). One study for CRPS patients included a low proportion of CRPS type II (documented nerve injury) (Gustin 2010). One study adding immunoglobulins to carbamazepine in patients suffering from trigeminal neuralgia has published the protocol (Goebel 2003). We also excluded studies evaluating the effectiveness of combined treatments including vitamins (Abbas 1997; Fliege 1966; Goldberg 2009; Kottschade 2009; Lagalla 2002; Levin 2009; Tian 2005). For other reasons for exclusion (e.g. methodological flaws) see the 'Characteristics of excluded studies' table.
Risk of bias in included studies
See 'Risk of bias' table in Figure 2 for summary information.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Two of the review authors (LC and IG) assessed each study independently for quality using the 'Risk of bias' tool (ROB) (Higgins 2011). Ten out 21 studies had at least four out of seven items qualified as low risk of bias. Most of the studies did not adequately describe methods of blinding of outcomes assessors. Since most study medications were associated with recognisable adverse effects (e.g. sedation), methods to prevent and/or evaluate (e.g. blinding questionnaires) quality of blinding of outcomes assessors were not adequately addressed. Details are in the 'Characteristics of included studies' table. 'Risk of bias' tables are presented (Figure 2; Figure 3).
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Random sequence generation and allocation concealment (selection bias)
Only six out of 21 studies reported the method used to generate a random sequence and to keep the allocation concealed (Gilron 2005b; Gilron 2009; Hanna 2008; Khoromi 2007; Lynch 2005; Zin 2010) and seven additional studies (Amr 2010; Barton 2011; Caraceni 2004; Eichenberger 2008; Freeman 2007; Lopez‐D'alessandro 2011; McCleane 2000) appropriately reported one or the other item.
Blinding
All studies except one reported how participants were blinded; however, only four of the 21 adequately described methods for blinding the outcomes assessors (see Figure 2).
Incomplete outcome data
Eight of the 21 studies did not provide information about trial dropouts (Agrawal 2009; Amr 2010; Lopez‐D'alessandro 2011; Lynch 2003; Lynch 2005; McCleane 2000; McCleane 2003; Mercadante 2002). We qualified attrition bias as 'low risk' for studies where the dropout rate was below 20% (Bhandari 2005). We qualified studies with higher dropout rate but including ITT analysis as 'unclear' or 'high risk of bias'.
Selective reporting
Although few studies indicated pre‐trial registration on a clinical trial registry, all reported at least one of the outcomes that are considered to be clinically relevant (Dworkin 2005).
Other potential sources of bias
We assessed the 'other bias' item as high risk in studies where the follow‐up was shorter than eight weeks (Moore 2010a; Moore 2010b) and/or the study had fewer than 50 participants per arm or period of treatment in parallel or cross‐over studies, respectively (Moore 1998).
Effects of interventions
Please see Table 1 for more information.
1. Methodology of included analgesic combination trials.
| Trial comparisons | ||||
| Trial ID | Placebo‐controlled | Combination versus only 1 component | Combination versus both components | Combination versus other |
| Agrawal 2009 | ╋ | ╋ | ||
| Amr 2010 | ╋ | |||
| Caraceni 2004 | ╋ | |||
| Eichenberger 2008 | ╋ | ╋ | ||
| Freeman 2007 | ╋ | ╋ | ||
| Gilron 2005b | ╋ | ╋ | ||
| Gilron 2009 | ╋ | |||
| Gomez‐Perez 1985 | ╋ | |||
| Gomez‐Perez 1996 | ╋ | |||
| Graff‐Radford 2000 | ╋ | ╋ | ||
| Hanna 2008 | ╋ | |||
| Khoromi 2007 | ╋ | ╋ | ||
| Lynch 2003 | ╋ | ╋ | ||
| Lynch 2005 | ╋ | ╋ | ||
| McCleane 2000 | ╋ | ╋ | ||
| McCleane 2003 | ╋ | |||
| Mercadante 2002 | ╋ | |||
| Tonet 2008 | •• | |||
| Zin 2010 | ╋ | |||
•• Comparison of amitriptyline + carbamazepine + ketamine versus amitriptyline + carbamazepine + placebo.
'‐' No difference between combination and single‐agent component.
We included 21 studies with a total of 1972 participants in this review; 966 participants were exposed to a combination of drugs.
Tricyclic antidepressants combined with morphine
Two clinical trials (Khoromi 2007; Mercadante 2002) were identified. Using a cross‐over design, Khoromi 2007 evaluated the combination of nortriptyline and controlled‐released morphine versus each medication alone and versus active placebo (benztropine) for chronic lumbar root pain; only 28 out 61 participants completed the four nine‐week periods of the study. This study reported no significant differences in the primary outcome, average leg pain, across any of the four study treatments: 13/32 participants during the morphine alone period, 12/31 during the nortriptyline alone period, 18/28 during the combination treatment and 9/37 in the placebo period reported at least moderate pain relief. The most frequent reported side effects were constipation, dry mouth, drowsiness and fatigue. Dropouts due to side effects were as follows: 4/41 participants in the morphine period, 2/34 in the nortriptyline period, 4/34 in the combination period and 1/39 in the placebo period.
Mercadante 2002 also used a cross‐over design to evaluate the effect of adding amitriptyline or placebo in patients that were already on morphine therapy for neuropathic cancer pain; 15/16 participants completed the two one‐week periods of treatments. No significant differences were reported between groups for the primary outcome, global pain intensity. Primary outcomes, as defined in the protocol for this review, were not reported as such in this study. Additionally, no differences in opioid requirement were observed. Only one participant dropped out from the study during the amitriptyline period due to severe confusion and drowsiness. Significant differences in side effects against adding amitriptyline to morphine treatment were reported, such as drowsiness, confusion and dry mouth.
We did not combine these two studies in a meta‐analysis because they involved diverse pain conditions (e.g. lumbar radiculopathy versus neuropathic cancer pain) and outcome measures.
Gabapentin or pregabalin combined with opioids
Four clinical trials (Caraceni 2004; Gilron 2005b; Hanna 2008; Zin 2010) were identified. Caraceni 2004 used a parallel design to evaluate the effectiveness of adding titrated doses of gabapentin in patients that were receiving opioid therapy for neuropathic cancer pain; a significant proportion (> 10% per group) of the participants were also receiving non‐steroidal anti‐inflammatories, antidepressants and/or steroid therapy. Primary outcomes, as defined in the protocol for this review, were not reported as such in this study. Twenty‐one out of 79 participants in the gabapentin group and 9/41 in the placebo group dropped out of the study due to the need for prohibited therapy. Reduction in the primary outcome, average pain score, was statistically superior for the gabapentin group. The number of participants that discontinued the study due to side effects was 6/79 in the combination arm and 3/41 in the opioid alone group.
Gilron 2005b used a four‐period cross‐over design to evaluate the effectiveness of titrated doses of gabapentin and morphine versus each drug alone and versus active placebo (lorazepam) in population with post‐herpetic neuralgia or painful diabetic neuropathy. Forty‐one out of 57 participants completed all four treatment periods. The trial’s primary outcome, mean daily pain score at maximal tolerated dose, was significantly lower for the combination versus placebo and versus either drug alone; 27/44 participants during the gabapentin alone period, 13/42 during the placebo period, 35/44 during the morphine period and 32/41 during the gabapentin + opioid period reported at least moderate pain relief. Forty‐eight participants received gabapentin and 3/4 withdrawals were due to treatment‐emergent side effects; 49 participants received morphine and 5/5 withdrawals were due to treatment‐emergent side effects; 47 participants received gabapentin + morphine and all withdrawals were due to treatment‐emergent side effects; 44 participants received placebo and 0/1 withdrawal was due to treatment‐emergent side effects. The combination increased the frequency of constipation compared with gabapentin alone and that of dry mouth compared with morphine alone.
Hanna 2008 used a parallel‐design clinical trial to compare the effectiveness of adding increasing doses of controlled‐release oxycodone versus placebo in participants that were already using varying doses of gabapentin for painful diabetic neuropathy; 48 out of 328 participants were concomitantly taking amitriptyline. Two hundred and forty‐nine out of 338 randomised participants completed the study. Based on the primary outcome, 'Box Scale‐11' pain scores, the study showed a significant effect favouring the oxycodone treatment over placebo using last observation carried forward (LOCF) imputation for withdrawal, which overestimates treatment effect (Moore 2012b); 52/169 participants in the gabapentin alone group versus 68/169 in the gabapentin + controlled‐release oxycodone group reported good or very good pain relief. A significant difference in increasing opioid‐related side effects, such as constipation, nausea and vomiting, was observed in the combination treatment group as well as dizziness, fatigue and somnolence; 9/169 participants in the gabapentin alone versus 27/169 in the combined treatment group dropped out from the study due to adverse events.
Zin 2010 conducted a parallel‐design clinical trial, where participants were randomised to low‐dose oxycodone (10 mg/day) or placebo for one week in a double‐blind fashion which continued on during subsequent open‐label titration of pregabalin (75 to 600 mg/day). Sixty‐two participants were randomised and 51 finished the study. The study did not demonstrate a significant difference between groups based in the primary outcome (2/10 difference in pain intensity). Responder rates based on ≥ 50% pain were reached by 15/26 in the pregabalin/oxycodone and 19/29 in the pregabalin/placebo group. Four participants in the oxycodone/pregabalin versus none in the placebo/pregabalin group discontinued the treatment due to side effects.
A meta‐analysis is reported including Gilron 2005b and Hanna 2008. Although both trials evaluated the combination of gabapentin with an opioid, several differences should be noted: 1) Gilron 2005b evaluated morphine whereas Hanna 2008 evaluated oxycodone and 2) Gilron 2005b used a cross‐over design to compare a morphine‐gabapentin combination to each monotherapy and active placebo (lorazepam), whereas Hanna 2008 used a parallel design to compare an oxycodone‐gabapentin to placebo‐gabapentin (i.e. no comparison was made between oxycodone‐gabapentin and oxycodone). It should be noted that Analysis 1.1 (efficacy) is based on fewer patients than Analysis 1.2 (tolerability) since available data for Analysis 1.1 only allowed for a completer analysis for Gilron 2005b, which will make the data look better than in an intention‐to‐treat analysis. Therefore, some caution is necessary when interpreting the results of this meta‐analysis. Caraceni 2004 was not included in the analysis given that the authors did not report the primary outcome for this review. Zin 2010 reported not 30% but 50% of responder rate; furthermore, this study used a very small (10 mg) and fixed dose of oxycodone; this dosage is well below the minimum 40 mg recommended for neuropathic pain (Furlan 2010).
In this analysis, gabapentin plus opioid (48% moderate or good pain relief) was significantly, yet modestly, better than gabapentin alone (37%), with a risk ratio (RR) of 1.3 (95% confidence interval (CI) 1.04 to 1.61) and with a number needed to treat for one patient to benefit (NNTB) of 9.5 (95% CI 5.0 to 86) (Analysis 1.1; Figure 4). More participants dropped out of the study because of side effects with gabapentin plus opioid (15%) than with gabapentin alone (6%); the RR was 2.8 (95% CI 1.5 to 5.2) and number needed to treat to harm (NNTH) was 10 (6.5 to 25) (Analysis 1.2; Figure 5).
1.1. Analysis.

Comparison 1: Anticonvulsants and opioids versus anticonvulsants alone, Outcome 1: At least moderate/good pain relief
4.

Forest plot of comparison: 1 Anticonvulsants and opioids versus anticonvulsants alone, outcome: 1.1 At least moderate/good pain relief.
1.2. Analysis.

Comparison 1: Anticonvulsants and opioids versus anticonvulsants alone, Outcome 2: Proportion of patients who dropped out due to side effects
5.

Forest plot of comparison: 1 Anticonvulsants and opioids versus anticonvulsants alone, outcome: 1.2 Proportion of patients who dropped out due to side effects.
Gabapentin combined with nortriptyline
We identified only one clinical trial in this category that fulfilled the inclusion criteria of this review (Gilron 2009). Gilron et al used a three‐period, cross‐over design to compare the effectiveness of the combination of titrated doses of nortriptyline and gabapentin versus each drug alone in a mixed neuropathic pain population of participants with painful diabetic polyneuropathy or postherpetic neuralgia. Forty‐five out of 56 participants completed all three treatment periods. The primary outcome, mean daily pain at maximum tolerated dose, was significantly lower during combination treatment versus either drug alone; 30/46 participants during the gabapentin alone period, 38/50 during the nortriptyline period and 42/50 during the combination treatment period reported at least moderate pain relief. Severe dry mouth was reported more frequently during the combination and nortriptyline treatment periods, compared with the gabapentin treatment period; 5/54 participants that received gabapentin withdrew due to treatment‐emergent side effects. The same situation occurred in only 1/52 of the participants during the combined treatment. No participants in the nortriptyline period dropped out due to treatment‐emergent side effects.
Tricyclic antidepressants combined with fluphenazine
Three clinical trials were identified (Gomez‐Perez 1985; Gomez‐Perez 1996; Graff‐Radford 2000) for the review. Graff‐Radford 2000 reported a parallel‐design trial evaluating the effectiveness of combining titrated doses of amitriptyline (up to 200 mg) and fluphenazine (up to 3 mg) versus each drug alone and active placebo (glycopyrrolate) in patients with postherpetic neuralgia. Based on the pain intensity by visual analogue scale (VAS) a significant reduction from baseline was observed with amitriptyline‐fluphenazine combination and amitriptyline alone but not fluphenazine alone or placebo. However, no significant changes from baseline were reported with any of the four treatments according to McGill Pain Questionnaire scores. Primary outcomes, as defined in the protocol for this review, were not reported as such in this study. Only 1/49 participants dropped out of the study due to side effects. The participant had been allocated to the amitriptyline only group. Sleepiness was more frequently reported in the fluphenazine group and the incidence of dry mouth was higher in the amitriptyline group.
Gomez‐Perez reported two cross‐over design studies (Gomez‐Perez 1985; Gomez‐Perez 1996) in participants with painful diabetic neuropathy. Both studies evaluated the efficacy of combining titrated doses of nortriptyline (up to 60 mg/day) and fluphenazine (up to 3 mg/day) versus placebo (Gomez‐Perez 1985) or titrated doses of carbamazepine (up to 600 mg/day) (Gomez‐Perez 1996). The mean per cent of change of pain and paraesthesia (considering a value of 100% at baseline) were the reported study outcomes. The combination of drugs produced at least 50% pain reduction in the placebo‐controlled study (Gomez‐Perez 1985); 17/18 participants during the combined treatment period versus 4/18 during the placebo treatment period reported at least 30% of pain relief. Six out of 24 participants dropped out of this study, but none of the six dropouts were due to side effects of the medications; dry mouth and dizziness were more prevalent in the combined treatment. In the other study (Gomez‐Perez 1996) no significant differences, in terms of effectiveness, were reported between groups. Only two out of 16 participants dropped out due upper gastrointestinal bleeding and non‐adherence to treatment.
N‐methyl‐D‐aspartate (NMDA) receptor blockers combined with other analgesic drugs
Three clinical trials have evaluated the combination of an N‐methyl‐D‐aspartate (NMDA) receptor blocker with other analgesic drugs for neuropathic pain (Amr 2010; Eichenberger 2008; Tonet 2008).
Amr 2010 conducted a parallel‐design clinical trial comparing the effectiveness of combining seven days of intravenous infusions of 80 mg of ketamine and gabapentin 300 mg orally three times a day versus a saline infusion plus gabapentin in participants with spinal cord injury pain. All participants received midazolam prior to the ketamine infusion. Based on the primary outcome, a 0 to 100 visual analogue scale (VAS) pain score, the score was significantly lower within the first week of the ketamine infusion up to the third week of follow‐up. Primary outcomes, as defined in the protocol for this review, were not reported as such in this study. Short‐lasting delusions were reported in the ketamine‐gabapentin group only; two participants in the combined treatment group and one participant in the gabapentin group reported dizziness. The number of participants, if any, that dropped out from the study due to side effects is not stated.
Eichenberger 2008 reported a two‐day per period cross‐over study that compared the combination of intravenous infusions of ketamine (0.4 mg/kg) and calcitonin (200 IE) versus the infusions of each drug alone versus placebo for participants with chronic phantom limb pain. Based on the primary outcome, a 0 to 10 VAS up to the 48 hours of each period of treatment, a higher rate of responders was reported for the combined treatment and the infusion of ketamine alone compared to calcitonin alone or placebo. Primary and secondary outcomes, as defined in the protocol for this review, were not reported as such in this study. Zero participants discontinued the study due to side effects
Tonet 2008 conducted a parallel‐design clinical trial that compared the combination of amitriptyline (25 mg/day), carbamazepine (200 mg TID) and oral ketamine (10 mg three times daily) versus the combination of amitriptyline, carbamazepine and placebo for patients with neuropathic pain of different aetiologies. Based on the primary outcome, a 0 to 10 numerical rating scale for pain intensity and pain relief, no significant differences were recorded between groups. Primary outcomes, as defined in the protocol for this review, were not reported as such in this study. In the trimodal treatment group 5/15 participants were excluded due to treatment‐emergent side effects and the same occurred in 2/15 that received the bimodal treatment.
Miscellaneous analgesic combination trials
Freeman 2007 conducted a parallel‐design trial that compared the combination of acetaminophen and tramadol (325 mg + 37.5 mg respectively) versus placebo for participants with painful diabetic neuropathy. Based on the primary outcome, mean change of average daily pain scores, the combined treatment was statistically superior to placebo. In the combination group 90/160 participants versus 58/153 participants in the placebo group reported at least 30% of pain reduction; 13/160 in the combination group versus 10/153 in the placebo group dropped out of the study due to side effects. A significant increase in the incidence of nausea (11.9% versus 3.3%), dizziness (6.3% versus 1.3%) and somnolence (6.3 versus 1.3) was reported in the acetaminophen‐tramadol group.
McCleane 2003 conducted a cross‐over study involving three two‐week treatment periods that compared the combination of the cholecystokinin‐2 antagonist, L‐365,260 (30 or 120 mg daily) and morphine 20 mg twice daily versus morphine alone in chronic neuropathic pain. Thirty‐nine out of 44 participants completed the study. Based on the primary outcome, 11‐point Likert scale and categorical scale pain scores, no significant differences were detected between treatments. The author evaluated using a standardised scale from "become more intense to completely relieved". No data were reported for dropouts due to adverse effects.
Lopez‐D'alessandro 2011 developed a four‐arm study in the only clinical trial developed for burning mouth syndrome that fulfilled our inclusion criteria. The participants received for two months of gabapentin alone (300 mg/day) or alpha lipoic acid (600 mg/day) or the combination of these treatments or cellulose starch. The primary outcome, based on a four‐level categorical evaluation including worsening, no change, improvement and total recovery, demonstrated a significant benefit from the combination over the interventions alone or placebo. Pain intensity or pain relief scores were not reported. The number of patients who discontinued the treatment was not reported.
Topical analgesics
Five clinical trials evaluated combinations of topical analgesics (Agrawal 2009; Barton 2011; Lynch 2003; Lynch 2005; McCleane 2000).
Agrawal 2009 conducted a parallel trial that evaluated a combination of glyceryl trinitrate spray (0.4 mg/actuation/night) and oral valproate (20 mg/kg/day) versus each treatment alone versus placebo for painful diabetic neuropathy. Based on the trial outcomes, mean VAS pain scores, short form McGill pain questionnaire, present pain intensity and 10‐point Likert scale, all active treatments showed a significant difference compared with placebo. Differences between the combination and glyceryl trinitrate spray alone were not significant for any outcome and differences between the combination and oral valproate were only significant based on VAS pain scores. Primary outcomes, as defined in the protocol for this review, were not reported as such in this study. A negligible number of side effects were observed, even in the combined treatment group, but no dropouts were reported.
Lynch 2003 conducted a two‐day, four‐period, cross‐over trial comparing the combination of topical amitriptyline (1%) and topical ketamine (0.5%) versus each single agent versus placebo in participants with chronic neuropathic pain. Eighteen out of 21 participants completed the study. Based on the outcomes, VAS for pain intensity and pain relief as well as Short Form of the McGill Pain Questionnaire (SF‐MPQ), no significant differences were reported between groups. Primary outcomes, as defined in the protocol for this review, were not reported as such in this study. No significant side effects were noticed for dropping out from the study.
Subsequently, Lynch 2005 conducted a parallel study comparing the combination of topical amitriptyline (2%) and topical ketamine (1%) versus each single agent versus placebo in a mixed group of participants with postherpetic neuralgia, painful diabetic neuropathy and post‐traumatic neuropathic pain with allodynia. Eighty out of 92 participants completed the study. Based on the primary outcome, an 11‐point numerical rating scale for pain, no differences were detected between groups. Six of 22 participants in the amitriptyline group, 4/22 in ketamine, 9/23 in combination treatment and 7/25 in the placebo group exhibited a pain reduction > 30% according to the NRS‐PI. Participants that withdrew due to side effects were 2/25 in the placebo, 2/22 in ketamine and 1/22 in the amitriptyline group.
McCleane 2000 conducted a parallel, four‐week clinical trial that compared the combination of topical doxepin (3.3%) and topical capsaicin (0.025%) versus each single agent versus placebo in chronic neuropathic pain. One hundred and fifty‐one out of 200 participants completed the study. Based on the primary outcome, a 0 to 10 VAS pain score, significant improvements from baseline were observed with all active treatments. However, no statistically significant differences were reported between any of the three active treatments. Primary outcomes, as defined in the protocol for this review, were not reported as such in this study. The number of participants that dropped out from the study due to side effects is not stated.
Barton 2011 conducted a four‐week, parallel‐design clinical trial in cancer patients who developed moderate or severe pain secondary to chemotherapy. The effectiveness of a compound gel that included ketamine, baclofen and amitriptyline was compared with placebo. One hundred and fifty of 208 patients completed the study. Based on the evaluation of unpleasant sensory symptoms such as cramping, shooting, burning and tingling, this trial demonstrated a significant benefit of the gel over placebo. No significant side effects were reported. More benefit was reported for symptoms in the upper extremities compared to lower extremities. Motor and autonomic subscales were also evaluated and favoured the use of the compound over placebo.
Discussion
Summary of main results
Given the evidence that at least 45% of participants with neuropathic pain concurrently receive two or more drugs to treat their pain (Tarride 2006), it is somewhat surprising that we were only able to identify 107 relevant citations for this review and only 21 high‐quality neuropathic pain randomised controlled trials (RCTs) that evaluated the strategy of combination pharmacotherapy. Even these tended to be relatively small, typically of rather short duration for chronic pain, and sometimes had high withdrawal rates, all of which can contribute to overestimation of treatment effects (Moore 2010b). Only one eligible study evaluated a combination of the two most widely used classes of neuropathic pain drugs, i.e. antidepressants and anticonvulsants. Three studies evaluated opioid‐anticonvulsant combinations and only two studies evaluated opioid‐antidepressant combinations. The remaining dozen studies evaluated combinations involving other drugs including NMDA receptor antagonists, fluphenazine and other miscellaneous agents, including topically applied drugs. Meta‐analysis was possible for only one comparison of only one combination, i.e. gabapentin + opioid versus gabapentin alone. This meta‐analysis involving 386 participants from two studies (Gilron 2005b; Hanna 2008) demonstrated statistically significant superiority of a gabapentin + opioid combination over gabapentin alone, but with more frequent side effect‐related trial dropouts compared to gabapentin alone. The magnitude of the effect was not large, and may have been smaller if it had been possible to perform other than a completer analysis from data from the multiple cross‐over study (Gilron 2005b).
Overall completeness and applicability of evidence
Evaluation and utility of combination pharmacotherapy for neuropathic pain obviously needs to be considered on a combination‐specific basis, although it may be reasonable to consider combinations of slightly different drugs which involve agents from common drug classes collectively (e.g. 'gabapentinoid' + 'tricyclic antidepressant' combination). That being said, the dearth of studies currently available for each studied drug class combination precludes any well‐founded conclusions about most combinations.
The only combination with more than one study that we found suitable for pooling in a meta‐analysis was the gabapentin + opioid combination. In one study, Gilron 2005b reported that the efficacy of the gabapentin + morphine combination was superior to each of the two drugs alone and to active placebo alone (low‐dose lorazepam). However, in the other study, Hanna 2008 employed an 'add‐on' design whereby patients already receiving gabapentin were randomised to receive either oxycodone or placebo. Therefore, while pooling these studies may provide more robust evidence for the comparison of gabapentin + opioid versus gabapentin alone, only the Gilron 2005b study provides evidence for the comparison of gabapentin + opioid versus opioid alone. It should also be emphasised that, while the gabapentin + opioid combination was superior to gabapentin alone for analgesic efficacy, it was also associated with more frequent study dropouts due to treatment‐emergent adverse effects.
The search strategy for this review was not designed to capture all studies of drug combinations administered by targeted injection (e.g. epidural or transforaminal nerve block), so any such studies were excluded from this review. Therefore, we point interested readers to other relevant reviews of these interventions (see Manchikanti 2010; Patel 2009).
Quality of the evidence
The 21 included studies were of reasonably good quality with mostly low risks of bias related to treatment randomisation and blinding. Frequent risks of bias in many of these studies were related to small sample size (< 50 participants) (Moore 1998; Nuesch 2010) and/or short trial duration (< 8 weeks) (Moore 2010a; Moore 2010b). Reports of investigations which were insufficiently blinded and/or uncontrolled were excluded as shown in the 'Characteristics of excluded studies' table. Some studies used imputation methods (like last observation carried forward ‐ LOCF) for some pain outcomes, and these are known to confer large positive biases on results (Moore 2012b); we avoided outcomes where this may have been a problem.
Potential biases in the review process
Restriction of this review to double‐blind RCTs limits the potential for bias, though the small size of most of the studies, their relatively short duration and the high levels of withdrawals in some studies could all be sources of bias leading to greater treatment effect for combination therapies. Lack of access to negative studies which remain unpublished could be a source of publication bias that our search strategy could not overcome. While unpublished studies are unlikely, even one modest‐sized study showing zero effect would be sufficient to overcome the positive result in our meta‐analysis (Moore 2008).
Agreements and disagreements with other studies or reviews
A systematic review in cancer pain (Bennett 2011) included clinical trials and observational studies focused on adding antidepressants or anticonvulsants to opioid therapy, given the considerable incidence of neuropathic pain in this population. They identified five RCTs and three observational studies; we included in our review two of these studies that satisfied our methodological inclusion criteria and were conducted in a neuropathic cancer pain population. A recent meta‐analysis (Finnerup 2010), including only 10/19 of the studies that we identified, stressed the paucity of combination studies and modest efficacy of combination as reported by already published clinical trials. However, the most recent version of the European (EFNS) Practice Guideline for neuropathic pain (Attal 2010) gave a Level A to antidepressant‐gabapentin or gabapentin‐opioid combinations based on three Class I studies that were included in our review. Fourteen of 19 clinical trials that we selected for this review were described/included in this guideline. The combination tramadol/acetaminophen was recommended for pain exacerbations. In the conclusions of the guideline, combination therapy is recommended in the event of partial response to monotherapy, however, larger studies were strongly recommended. Finally, a considerably more inclusive qualitative review was recently published (Vorobeychik 2011). This review included a variety of randomised, double‐blind and open‐label studies, as well as observational studies.
Authors' conclusions
Implications for practice.
Multiple, good‐quality studies demonstrate the superior efficacy of two‐drug combinations. However, the number of available studies for any one specific combination as well as other study factors (e.g. limited trial size and duration) preclude the recommendation of any one specific drug combination for neuropathic pain. Pooled analysis of two studies comparing gabapentin + opioid to gabapentin alone suggested modest analgesic superiority but also reduced tolerability, leaving the overall benefit of this combination unclear. Given that combination pharmacotherapy may increase the risk of toxicity (particularly when the combined drugs produce common adverse effects such as sedation), practical use of analgesic drug combinations requires vigilant risk‐benefit assessment during combination treatment. One common clinical approach to minimising combination toxicity is to use sequential combination therapy, i.e. to start treatment with monotherapy and pursue 'add‐on' combination therapy in cases of partial treatment response. However, this approach may lead to a different dose‐ratio for the combination than might be achieved with simultaneous combination therapy and, possibly, different results (Gilron 2005a).
Implications for research.
Demonstration of combination benefits by several individual studies together with reports of widespread clinical polypharmacy for neuropathic pain surely provide a rationale for additional future rigorous evaluations of analgesic drug combinations. Examination of the studies included in this review may serve to guide future improvements in the evaluation of drug combinations for neuropathic pain. It should be noted that, of the 18 included two‐drug combination studies (N.B. one of the 19 studies compared a triple combination to two of its components), only nine compared the combination to both single‐agent components. One problem with this incomplete design is that observed differences between the combination of drugs 'A + B' versus drug 'A' alone could potentially be due strictly to differences in efficacy between drugs 'A' and 'B' and, thus, additional comparison of 'A + B' also with 'B' alone is crucial for the comprehensive evaluation of the combination. Furthermore, nine of the included studies were not placebo‐controlled and five of those failed to demonstrate a difference between the combination of interest and its comparator(s). It should be noted that these five negative studies are essentially inconclusive since, without a demonstrable difference between active treatment and placebo, it is not possible to confirm whether those studies had the assay sensitivity to detect a treatment effect (Dworkin 2010; Max 1991). Therefore, we recommend that future trials of two‐drug combinations include comparisons with placebo and both single‐agent components, as well as reporting outcomes (such as at least 50% pain intensity reduction with tolerable adverse effects) linked to improved functioning over the longer term (at least 12 weeks). In addition to identifying specific drug combinations which provide additional benefit over monotherapy, other objectives to be incorporated into future analgesic combination trials include identification of optimal dose‐ratio for a given combination, cost‐effectiveness comparisons for combination versus monotherapy and the therapeutic benefits of concurrent versus sequential 'add‐on' combination therapy (Gilron 2005a).
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 1, 2011 Review first published: Issue 7, 2012
| Date | Event | Description |
|---|---|---|
| 7 June 2017 | Review declared as stable | See Published notes. |
| 27 June 2012 | Amended | Contact details updated. |
Notes
Assessed for updating in 2017
We performed a full search in May 2017 but we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Assessed for updating in 2020
At June 2020 we are not aware of any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be assessed for updating in five years. If appropriate we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Acknowledgements
Nuffield Division of Anaesthetics, Oxford University
Oxford Pain Relief Trust
Queen's University, Kingston, Ontario, Canada
Royal College of Physicians and Surgeons of Canada
IASP John Bonica Trainee Fellowship to LEC
Appendices
Appendix 1. MEDLINE search strategy
1. Facial Neuralgia/
2. Neuralgia/
3. Causalgia/
4. Hereditary Sensory and Autonomic Neuropathies/
5. neuropath$ or neuralgi$ or radiculopathy
6. or/1‐5
7. pain$
8. 6 and 7
9. (combin$ or cotreat$ or co‐treat$ or coadministr$ or co‐administr$ or synerg$ or isobol$ or add on$ or add‐on$)
10. 8 and 9
The search above was combined with the following trial design search filter which was developed for MEDLINE.
Cochrane highly sensitive search strategy for identifying randomised trials in MEDLINE: sensitivity‐ and, precision‐maximising version (2008 revision); OVID format:
1. randomised controlled trial.pt. 2. controlled clinical trial.pt. 3. randomised.ab. 4. placebo.ab. 5. clinical trials as topic.sh. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. Animals.sh. not (humans.sh. and animals.sh.) 10. 8 not 9"
Data and analyses
Comparison 1. Anticonvulsants and opioids versus anticonvulsants alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 At least moderate/good pain relief | 2 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.04, 1.61] |
| 1.2 Proportion of patients who dropped out due to side effects | 2 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [1.47, 5.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agrawal 2009.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel design for 12 weeks | |
| Participants | At least > 4/10 and > 3 months painful diabetic neuropathy; 87 participants were screened, 83 randomised and 80 completed the study. Mean age: 59; proportion of female/male was similar. Analgesics for diabetic neuropathy were stopped at least 2 weeks before the trial. | |
| Interventions | Glyceryl trinitrate spray (GTN): 0.4 mg on each leg before going to bed combined with sodium valproate 20 mg/kg/day orally versus placebo + GTN spray versus sodium valproate + placebo spray versus placebo tablets + placebo spray. Supplementary analgesia was not allowed. | |
| Outcomes | This study reported 0 to 10 VAS pain score, Short Form McGill pain questionnaire (SF‐MPQ) and present pain intensity in a 10‐point Likert scale. All outcomes were recorded at baseline and every 4 weeks up to week 12. | |
| Notes | Small size trial: only 20 participants per arm. Only 3/83 dropouts. The study also explored electrophysiological response to treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo tablets used were similar in colour, size and texture as well as the placebo spray was identical in colour and odour with that of GTN" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Clinical evaluation, nerve conduction study and pain scoring were done by another member of the team who was completely ignorant about the administration of the drug" |
| Incomplete outcome data (attrition bias) | Low risk | Minimal missing outcome data |
| Selective reporting (reporting bias) | Low risk | Not apparent selective outcome reporting. Trial registration number is not stated. |
| Other bias | High risk | Fewer than 50 participants per arm of treatment |
Amr 2010.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, active‐controlled, parallel design for 4 weeks | |
| Participants | > 6 months spinal cord injury pain; 40 participants randomised and completed the trial. Mean age: 48.6 years. 7/40 were female. The information about previous or concomitant analgesic treatments through the trial is not stated. | |
| Interventions | 80 mg of ketamine was diluted in 500 ml of saline to be administered for 5 hours daily for 1 week and then once a week for 1 month. Gabapentin 300 mg was administered orally 3 times a day. One group received the ketamine infusion plus gabapentin and the other saline infusion plus gabapentin. | |
| Outcomes | They reported 0 to 100 VAS pain score and drug‐related side effects profile | |
| Notes | A minimum baseline pain score for inclusion in the trial was not reported. Small size trial: only 20 participants per arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not stated |
| Allocation concealment (selection bias) | Low risk | "The envelopes, infusion bottles containing either ketamine or placebo, and coding of these materials were prepared by an anesthesiologist in cooperation with the hospital’s pharmacy. This anesthesiologist did not participate in the study, evaluate the patients or the data, or report the findings". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The sealed envelopes were opened by a blinded chief nurse not participating in the study or data collection for the patients to indicate the group in which they were assigned". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed ketamine‐related side effects were also assessing pain outcomes, they might be biased |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropout rate is not stated |
| Selective reporting (reporting bias) | Low risk | Not apparent selective outcome reporting. Trial registration number is not stated. |
| Other bias | High risk | Fewer than 50 participants per arm of treatment and < 8 weeks duration of trial |
Barton 2011.
| Study characteristics | ||
| Methods | Randomised and double‐blind, parallel, placebo‐controlled, clinical trial during 4 weeks | |
| Participants | Participants that had received neurotoxic chemotherapy and developed numbness, tingling or pain for at least 1 month. Pain intensity > 4/10 was required for inclusion. Participants could not be concurrently treated with any agent with suspected efficacy for neuropathy, such as anticonvulsants or tricyclic antidepressants. | |
| Interventions | Participants were randomised to receive 1.31 g of a compounded gel containing 10 mg of baclofen, 40 mg of amitriptyline HCL, and 20 mg of ketamine versus an identical appearing placebo gel. The gel was applied 2 times/day during 4 weeks. | |
| Outcomes | The primary end point for the study was the change in the sensory neuropathy subscale as measured by the European Organization for Research and Treatment of Cancer QLQ‐CIPN20 (CIPN‐20); profile of mood states, brief pain inventory, and the sensory neuropathy subsection of the NCI common terminology criteria. Side effect profile was also evaluated. | |
| Notes | There were 5 participants who withdrew from the study before starting study medication. There were 26 participants in the baclofen arm and 27 in the placebo arm who did not provide primary endpoint data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was done using dynamic allocation to balance marginal distributions of the stratification factors". |
| Allocation concealment (selection bias) | Low risk | "Drug assignments to individual patients were accessible only by the North Central Treatment Group randomization office, study pharmacists, and the study statisticians". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants were randomised to receive 1.31 g of a compounded gel containing 10 mg of baclofen, 40 mg of amitriptyline HCL, and 20 mg of ketamine versus an identical appearing placebo gel". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants completed questionnaires at baseline and at 4 weeks. Participants rated the severity of these symptoms on a 0 to 10 scale, with 10 being the most severe. Adverse events were evaluated through the patient‐reported questions mentioned above as well as being graded through the NCI Common Terminology Criteria, version 3.0". |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are balanced across groups: "There were 26 participants in the baclofen arm and 27 in the placebo arm who did not provide primary endpoint data. In the baclofen arm, 11 refused due to experiencing an adverse event and 15 refused for nonspecified reasons. In the placebo arm, eight refused due to an adverse event, one patient died, and 18 refused for nonspecified reasons". |
| Selective reporting (reporting bias) | Unclear risk | "The study was registered according to current US federal regulations". The instruments for evaluation of the primary and secondary endpoints are valid and clinically relevant. |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
Caraceni 2004.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel design study for 10 days only | |
| Participants | Neuropathic pain (≥ 5/10) secondary to tumoral infiltration or compression of neural structures. 691 patients were screened, 121 randomised and 89 completed the trial. Mean age: 59 (SD 11) years. 68/121 were female. Average global pain score was 7.0 (SD 1.4) in the gabapentin group and 7.7 (SD 1.3) in the placebo. Previous analgesic (opioids and non‐steroidal anti‐inflammatory drugs) and adjuvant therapies (i.e. steroids, antidepressants, anticonvulsants, anxiolytics or muscle relaxants) were unchanged throughout the study. One extra dose of opioid medication was available as needed (prn) and it was prescribed at visit 1 based on the previous opioid regimen. Patients needing more than one daily prn. opioid dose during the treatment phase were withdrawn from study. Patients were comparable in their analgesic requirement (based on oral morphine‐equivalent pre‐trial analgesic requirement). The majority of the patients were taking during the trial anti‐inflammatories and steroids. The trial showed a higher analgesic requirement in the placebo versus the gabapentin group. | |
| Interventions | Titrated doses of gabapentin (600 to 1800 mg/day); dose was increased if pain score ≥ 3/10. Placebo capsules were titrated in the same fashion. Previous analgesics were allowed unchanged during the trial. One extra pill of opioid was allowed per day; however, more than one pill request was a reason for exclusion from the study. | |
| Outcomes | The primary outcome was average follow‐up pain score and secondary outcomes were subjective rating scale scores for lancinating pain, burning pain, dysaesthesias, presence/absence of allodynia and use of prn. analgesics | |
| Notes | The duration of pain before starting the study was not reported. 21/79 discontinued in the gabapentin + opioid due to the need for prohibited therapy versus 9/41 in the opioid alone arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not stated |
| Allocation concealment (selection bias) | Low risk | "...random sequence by the pharmacy department of the sponsor’s laboratories. All study participants were blinded to allocation sequence". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study medications were provided as identical capsules containing 300 mg of gabapentin or placebo in numbered containers". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed gabapentin‐related side effects were also assessing pain outcomes, they might be biased |
| Incomplete outcome data (attrition bias) | Unclear risk | "The main analysis was performed on the intent‐to‐treat (ITT) population". Substantial dropout rate and we do not know the trial outcomes for these dropouts. |
| Selective reporting (reporting bias) | Low risk | Not apparent selective outcome reporting. Trial registration number is not stated. |
| Other bias | High risk | < 8 weeks duration of trial |
Eichenberger 2008.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, cross‐over design study for 2 days only | |
| Participants | Phantom limb pain (≥ 3/10) due to surgical or traumatic amputation. 20 participants were included and 17 completed the study. 5/20 patients were female. Median age: 57. Median duration phantom limb pain: 10.9 years. The baseline mean pain score was 4.3/10. Medications: 5/20 patients were not taking analgesics before the trial; 3/20 were taking opioids only; 4/20 were taking anti‐inflammatories only; 2/20 were taking an anticonvulsant combined with opioid or antidepressant; 5/20 were taking a combination of antidepressant plus anti‐inflammatories, opioids or anticonvulsants; finally 4/20 patients were combining 3 analgesics for neuropathic pain during the trial (apart of the evaluated medications). | |
| Interventions | Intravenous infusion of 200 IE of calcitonin versus racemic ketamine 0.4 mg/kg (only 10/20 patients received ketamine alone) versus a combination of the previous interventions versus saline. All medications were diluted in 20 ml and were injected over one hour using an infusion pump. Washout period 48 hours. | |
| Outcomes | The primary outcome was VAS pain 30 minutes after start the infusion, at the end of the infusion and then every 4 hours up to 48 hours; maximal pain experienced during the 48 hours after each session was recorded. Response to therapy was defined as reduction of at least 50% in pain intensity after the end of the infusion. Pain thresholds after electrical, thermal and pressure stimulation were recorded before and during each infusion. | |
| Notes | The baseline VAS mean score was 3.7 only. A carry‐over analysis was not performed. Small size trial: 20 participants only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomization, which was performed by drawing lots..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...neither the investigator performing the experiment nor the patients were aware of the solutions infused..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed ketamine‐related side effects were also assessing pain outcomes, they might be biased (this even acknowledged by authors) |
| Incomplete outcome data (attrition bias) | High risk | 50% of the population did not received ketamine |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported. Trial registration number is not stated. |
| Other bias | High risk | Fewer than 50 participants per arm of treatment and < 8 weeks duration of trial |
Freeman 2007.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group design for 66 days | |
| Participants | Pain model: > 4/10 distal symmetric painful diabetic neuropathy in the lower extremities on a daily basis for the past 3 months. 313 patients were randomised; 129/160 in the tramadol/APAP arm and 109/153 in the placebo arm completed the study. 41% were female. Mean age: 55.7 (SD 10.32). Average daily pain at baseline was 7.13 and 7.12 in the tramadol/APAP and placebo groups, respectively. Medications: the study excluded patients who failed to the studied medications, previous failures to more than 2 analgesic treatments as well as those using capsaicin, steroids, antidepressants (except for SSRIs prescribed for depression) or anticonvulsants. Topical local anaesthetics and anti‐inflammatories were stopped a couple of days before trial. | |
| Interventions | 10‐day titration period + 8 weeks maintenance period. 37.5 mg tramadol/325 mg APAP tablets or placebo tablets titrated from 1 to 4 tablets prn. Supplemental acetaminophen was allowed during titration. Maintenance period: 1 to 2 tablets as needed 4 times a day. No supplemental analgesics were permitted. | |
| Outcomes | Primary and secondary outcomes: average daily pain, sleep interference and number of study medication. BPI, SF‐MPQ, SF‐36, patient global impression of change. | |
| Notes | No tramadol or acetaminophen alone groups. 75/313 participants dropped out from the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not stated |
| Allocation concealment (selection bias) | Low risk | "Randomization schedules were prepared for each study center and were balanced with randomly permuted blocks". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...37.5 mg tramadol/325 mg APAP tablets or placebo tablets that were matched in appearance..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...subjects called the Interactive Voice‐Response system every night to report average daily pain, sleep interference, and the number of tablets of study medication taken that day". |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data are balanced across the 2 groups and intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | Outcome measured are standard in pain clinical trials. Registry # NCT‐00210847. |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gilron 2005b.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, active placebo‐controlled, 4‐period cross‐over design for 5 weeks each period | |
| Participants | Pain model(s): at least moderate painful diabetic neuropathy [PDN] (median age: 60 and duration of pain 4.5 ± 3.8 years) and post‐herpetic neuralgia [PHN] (median age: 68 and duration of pain 4.6 ± 5.2 years). 86 patients were screened, 57 randomised and 41 completed all 4 treatment periods. 25/57 of the participants were female. Baseline pain score (0 to 10) were: 5.5 ± 1.5 (PDN) and 5.0 ± 1.3 (PHN). Medications: patients were allowed to continue taking an stable dose of non‐opioid analgesia except for gabapentin. | |
| Interventions | Patients received 25 days of titrated dose, 1 week of maximum tolerated dose, 4‐day tapering and 7 days washout. Morphine period: ER morphine 30 mg BID + lactose placebos TID; max. daily dose 120 mg. Gabapentin period: lactose placebos BID + gabapentin 400 mg TID; max. daily dose: 3600 mg. Gabapentin‐morphine period: ER morphine 15 mg BID + gabapentin 300 mg TID; max. daily dose: morphine 60 mg and gabapentin 2400 mg. Placebo period: lorazepam 0.2 mg BID + lorazepam 0.1 mg TID; max. daily dose: 1.6 mg. Gabapentin was allowed through the study. | |
| Outcomes | Primary outcome: mean intensity of pain during the maximum tolerated dose period. Secondary: rate of adverse effects, BPI, SF‐MPQ, BDI, SF‐36, Mini‐Mental State examination, patient global impression of change. | |
| Notes | A carry‐over effect was noticed for the next treatment when morphine was compared with placebo. 16/57 (28%) participants did not complete the 4 periods of treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "At the commencement of the trial, a pharmacist at the Kingston General Hospital in Kingston, Ontario, Canada, prepared a concealed allocation schedule randomly assigning the four sequences, in blocks of four, to a consecutive series of numbers". |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled central allocation. See above. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Medications were placed in blue and gray gelatin capsules by the investigational pharmacist in order to maintain double‐blind conditions". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed treatment‐related side effects were also assessing pain outcomes, they might be biased |
| Incomplete outcome data (attrition bias) | Unclear risk | Substantial dropout rate |
| Selective reporting (reporting bias) | Low risk | Protocol appears at clinical trials.gov and the outcomes are identical |
| Other bias | High risk | Fewer than 50 participants per period of treatment and < 8 weeks duration of trial |
Gilron 2009.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, active‐controlled, 3‐period cross‐over design for 6 weeks each period | |
| Participants | Pain model(s): > 4/10 painful diabetic neuropathy (median age: 61 years and duration of pain 5.2 ± 3.4 years) and post‐herpetic neuralgia (median age: 68 years and duration of pain 2.8 ± 4.3 years). 73 participants were screened, 56 randomised and 45 completed all 3 treatment periods. 21/56 were female. Patients taking, and perceiving benefit from, sustained‐release opioids, non‐steroidal anti‐inflammatory drugs or paracetamol were allowed to continue these drugs at a steady dose for the entire study. However, procedural pain treatments (e.g. nerve blocks or acupuncture) were forbidden. | |
| Interventions | Patients received 24 days of increasing doses of the studied medications, 1 week of maximum tolerated dose, 4‐day dose tapering and 1‐week washout. Nortriptyline period: nortriptyline 10 mg BID + lactose placebos TID; max. daily dose 100 mg. Gabapentin period: lactose placebos BID + gabapentin 400 mg TID; max. daily dose: 3600 mg. Gabapentin‐nortriptyline period: nortriptyline BID + gabapentin 400 mg TID; max. daily dose: nortriptyline 100 mg and gabapentin 3600 mg. | |
| Outcomes | Primary outcome: 0 to 10 pain intensity at the maximum tolerated dose. Secondary outcomes: maximum tolerated dose of study drug, serum concentration of study drugs, BPI, patient reported nocturnal pain, SF‐MPQ, SF‐36 guessing questionnaires, and bodyweight and global impression of change. | |
| Notes | No placebo arm. 19.6% of the participants did not complete the 3 periods of treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A trial pharmacist prepared a concealed allocation schedule by computer randomisation of these three sequences, in blocks of three, to a consecutive number series". |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled central allocation. See above. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Drugs were given as yellow and orange capsules to maintain double‐blinding". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed treatment‐related side effects were also assessing pain outcomes, they might be biased |
| Incomplete outcome data (attrition bias) | Low risk | Non significant dropout rate (< 20%). No patients were excluded from the analysis because of missing data. |
| Selective reporting (reporting bias) | Low risk | Protocol was published and outcomes measures are identical |
| Other bias | High risk | < 8 weeks of duration |
Gomez‐Perez 1985.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, 2‐period, cross‐over design for 30 days each period | |
| Participants | Pain model: painful diabetic neuropathy. Mean age: 55 years (30 to 73). 24 participants were randomised and 18/24 completed the 2 periods of treatment. 9/18 were women. Medications: information about concomitant analgesia is not stated. | |
| Interventions | Nortriptyline 10 mg + fluphenazine 0.5 mg: the starting dose was 1 tablet TID for 2 weeks and it was increased 2 tablets TID for another 15 days. Identical tablets and increasing scheme were applied during the placebo period. | |
| Outcomes | The initial level of pain and paraesthesia were given a 100% value and changes were considered positive or negative per cent deviations (mean per cent of change). VAS change from baseline was used in the outcomes assessor’s office as well as a side effects record. | |
| Notes | Baseline pain intensity or minimum pain intensity for inclusion in the study was not reported. Small sample size. No carry‐over effect analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The active drugs, 10 mg of nortriptyline and 0.5 mg of fluphenazine, and the placebo, an inactive substance, were supplied as identical tablets under a code unknown to clinicians" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Substantial dropout rate |
| Selective reporting (reporting bias) | Low risk | Not apparent selective outcome reporting. Trial was not registered. |
| Other bias | High risk | Fewer than 50 participants per period and < 8 weeks duration |
Gomez‐Perez 1996.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, active‐controlled, 2‐period, cross‐over design for 30 days each period | |
| Participants | Pain model: > 6 months of moderate/severe painful diabetic neuropathy. Mean age: 43.1 (nortriptyline + fluphenazine group) and 51.5 (carbamazepine group) years). 9/16 patients were female. Information about concomitant analgesia is not stated. | |
| Interventions | Nortriptyline 10 mg + fluphenazine 0.5 mg: the starting dose was 1 tablet at bedtime; the second day an additional was added at lunch and a third one at breakfast the third day. 12 days later the dose was doubled. Subsequently, a washout period of 2 to 4 weeks until symptoms returned to baseline. Carbamazepine: titrated doses from 100 to 600 mg. | |
| Outcomes | Primary outcomes: mean per cent of change of pain and paraesthesia and side effects profile | |
| Notes | Small sample size (16 in total). Dropouts: 2/16. No carry‐over effect analyses. Side effects: 8/16 (N + F) and 3/16 on carbamazepine group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An identical placebo tablet of the comparing drug was given simultaneously with the active drug following the same pattern of administration". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | Dropout rate was not significant |
| Selective reporting (reporting bias) | Low risk | Not apparent selective outcome reporting. The trial was not registered. |
| Other bias | High risk | Fewer than 50 participants per period and < 8 weeks duration |
Graff‐Radford 2000.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, active placebo‐controlled, parallel design for 8 weeks | |
| Participants | Pain model: > 6 months post‐herpetic neuralgia. Mean age: 72.9 (SD 10.1); duration of pain 33.4 months (SD 29.5)). 92 participants were screened, 50 randomised and 49 completed the study. 22/49 of the participants were female. Baseline pain score (0 to 100) was 55.22 (SD 16.34); baseline MPQ: 23.22 (SD 13.23). Information about pre‐trial analgesia is not stated. | |
| Interventions | Patients received increasing doses (every week) of amitriptyline (12.5 to 200 mg), fluphenazine (1 to 3 mg), the medications combined or active placebo (glycopyrrolate) | |
| Outcomes | This study reported visual analogue scale, McGill Pain Questionnaire, amitriptyline serum levels, MMPI, Beck Depression Inventory, Spielberg State Trait Anxiety Inventory and side effects profile | |
| Notes | No description of the minimum pain intensity to be included. Only 1/49 dropouts (amitriptyline group due to sedation). Study granted by the National Institute of Health Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not stated ("...were randomly assigned to one of four treatment groups...") |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All patients received two different capsules. One capsule (blue) was either amitriptyline or cellulose". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | Dropout rate was not significant |
| Selective reporting (reporting bias) | Low risk | Not apparent selective outcome reporting |
| Other bias | High risk | Fewer than 50 participants per arm of treatment trial |
Hanna 2008.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, active placebo‐controlled, parallel, add‐on, LOCF‐analysis, design study for 12 weeks | |
| Participants | Pain model: painful diabetic neuropathy. Median age: 60.1 (SD 10.24). 406 patients were screened, 338 randomised and 249 completed the study. 118/328 (36%) of the participants were female. At least moderate pain was required for inclusion; baseline pain scores (0 to 10) were: 6.4 ± 1.76 (oxycodone group) and 6.5 ± 1.71 (placebo). Medications pre‐trial: the study excluded any patients treated with a long‐acting opioid in the previous month or who had previously used oxycodone in combination with gabapentin. Stable dose of concomitant analgesics were allowed. Paracetamol was allowed as rescue medication. | |
| Interventions | Subjects on maximum tolerated dose of gabapentin (48% on < 1200 mg/day) were randomly assigned to Controlled‐Released oxycodone or placebo. Oxycodone was started at 5 mg BID and titrated during the 12 weeks up to max. 80 mg BID. Acetaminophen (one gram) was the rescue medication. NSAIDs and TCAs were continued in stable dose. 6.4% of the participants were taking concomitantly TCAs, gabapentin and oxycodone. | |
| Outcomes | The primary outcome of the study: Box Scale‐11 (BS‐11) pain scores (mean reduction in pain scores). Secondary outcomes: acetaminophen request and sleep quality. Exploratory analyses: BPI, SF MPQ, Euro Qol ‐5D questionnaire, and subject resource utilisation. | |
| Notes | This study was sponsored by Mundipharma Research Limited. 89/338 (26.3%) dropouts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed using a validated interactive voice response system that automated the assignment of treatment groups to randomisation numbers in accordance with a randomisation schedule". |
| Allocation concealment (selection bias) | Low risk | "Randomisation data were kept strictly confidential". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, and all personnel involved in the study, including investigators, site personnel and sponsor’s staff, were blinded to the medication codes until the time of unblinding". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Substantial dropout rate and we do not know the trial outcomes for these dropouts. Missing these data COULD affect the results and lead to bias. |
| Selective reporting (reporting bias) | Low risk | Not apparent selective outcome reporting |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Khoromi 2007.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, active placebo‐controlled, 4‐period cross‐over design for 9 weeks each period | |
| Participants | Pain model: chronic sciatica (> 4/10 pain intensity). Median age: 52.5 (range 30 to 64) and duration of pain 5 (0.3 to 37) years). 61 patients were screened, 55 randomised and 28 completed all 4 treatment periods. 14/28 of the completers were female. Baseline pain score (average leg) was: 4.9 ± 2.43. Medications: before the beginning of the trial, 22/28 of the completers were taking NSAIDs, 8 were taking opioids, 3 anticonvulsants, one antidepressants, 3 muscle relaxants and 15 were taking other drugs. | |
| Interventions | During 5 weeks of dose escalation and 2 weeks of maintenance at the highest tolerated dose patients received BID sustained‐released morphine (15 to 90 mg; mean 62 mg), nortriptyline (25 to 100 mg; mean 84 mg), their combination (morphine 49 mg and NT 55 mg) or benztropine‐active placebo (0.25 to 1 mg); subsequently, 2 weeks of tapering; next period started one pain score reached > 4/10. Opioids and antidepressants were not allowed. NSAIDs and acetaminophen were used as rescue medications. | |
| Outcomes | The primary outcomes: mean scores for average leg pain during the maintenance weeks. Pain diaries consigned 0 to 10 pain score at bedtime, average back, leg and overall pain, worst back, leg and overall. Secondary: global pain relief scores, Oswestry low back pain disability questionnaire, BDI, SF‐36 and general health status instrument. | |
| Notes | A carry‐over effect was not noticed between treatments. 27/55 (49%) participants did not complete the 4 periods of treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned by random numbers within blocks of four to one of four treatment sequences specified by a Latin square". |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed by the NIH Pharmaceutical Development Service". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "During the MS Contin treatment period, each blue pill contained MS Contin 15 mg and each pink pill contained inert placebo"... |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The rate of guessing by the nurses was above the rate for chance only (> 25%), but did not reach a high percentage |
| Incomplete outcome data (attrition bias) | High risk | Substantial dropout rate; however, missing outcome data were balanced across groups |
| Selective reporting (reporting bias) | Low risk | Not apparent selective outcome reporting. The expected outcomes for a pain clinical trial were reported. |
| Other bias | High risk | Fewer than 50 participants per period of treatment trial |
Lopez‐D'alessandro 2011.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, clinical trial with 60 days follow‐up | |
| Participants | 120 patients were selected among those patients with burning mouth syndrome (BMS) who had been treated at the service between March 2003 and March 2008, without responding to the applied treatments. A total of 120 patients with idiopathic BMS of more than 3 months duration that wanted to participate voluntarily were included. Polypharmacy patients using more than 3 systemically daily drugs, those ones taking psychotropic and antihypertensives drugs as well as patients with serious psychiatric conditions previously diagnosed were excluded. Patients with deficiencies of folic acid, vitamin B, carriers of anemias of any kind and patients with Sjögren syndrome were also excluded. The pre‐trial analgesic profile of the participants is not described in the publication. | |
| Interventions | 6 treatment cycles were determined: cycles A, B, C, D, E and F, so that cycle A (n = 20) corresponded to 600 mg/day of alpha lipoic acid (ALA), the cycle B (n = 20) 300 mg/day of gabapentin (GABA), the cycle C (n = 20) to the combination of both drugs and the cycles D (n = 20), E (n = 20) and F (n = 20) were 100 mg/day of starch and cellulose (placebo). The support staff of our service made a draw with 6 balls to link the groups with the cycles of treatment. After the draw, the 3 groups were combined with cycles D, E and F to be treated with placebo, thus forming a single group for these patients, Group D (n = 60) or control group. | |
| Outcomes | To evaluate the changes that occurred with the taking of the different drugs, it was established that the improvements (positive changes) involved the passage of a certain level or numerical category of burning to a lower one, the deteriorations (negative changes) involved an increase of a certain level of burning to a higher one and the total resolution indicated the total absence of burning, that is to say the transition from any higher value to zero value. In this way 4 categories were obtained for the analysis of the results: Category 1: with negative changes (deterioration), Category 2: no changes; Category 3: with positive changes (improvements) and Category 4: with total recovery. | |
| Notes | Pain intensity was not recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The support staff of our service made a draw with 6 balls to link the groups with the cycles of treatment. After the draw, the three groups were combined with cycles D, E and F to be treated with placebo, thus forming a single group for these patients..." |
| Allocation concealment (selection bias) | Unclear risk | "...allocation that was always masked to both patients and researcher, through the use of capsules of similar size and appearance so that just the support staff was the one who recorded the information..." For low risk of bias allocation concealment is required the use of identical appearance containers or to describe the use of a central allocation place (pharmacy). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...through the use of capsules of similar size and appearance so that just the support staff..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed treatment‐related side effects were also assessing pain outcomes, they might be biased |
| Incomplete outcome data (attrition bias) | Unclear risk | "All subjects were evaluated through an Intention‐to‐Treat Analysis which would take into account all patients although they could discontinue the treatment". |
| Selective reporting (reporting bias) | Unclear risk | The study was not registered (to compare protocol and publication outcomes), however the outcomes selected are clinically relevant |
| Other bias | Unclear risk | Fewer than 50 participants per arm of treatment |
Lynch 2003.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, 4‐period cross‐over design, 2 days trial plus 7 days of open‐label follow‐up for responders | |
| Participants | Pain model: at least moderate chronic neuropathic pain. Mean age: 58.7 (SD 12.9) and duration of spontaneous pain 43.8 months). 21 participants were randomised and 20 completed the study, however, analyses were conducted in 18 due to incomplete data. 12/20 of the completers were female. Baseline pain score ranged between 4.4 (SD 2.0) and 5.0 (2.3). Pre‐trial analgesic profile: subjects were permitted to continue using previous analgesics including NSAIDs, opioids, antidepressants, and anticonvulsants. | |
| Interventions | Participants received 5 ml of topical treatment: 1% amitriptyline, 0.5% ketamine, the combination and placebo q6h for periods of 2 days. Creams were identical. A return to baseline pain was required between treatments. | |
| Outcomes | Reported outcomes: McGill Pain Questionnaire, VAS present pain intensity, 0 to 10 pain relief, blood levels of medications and side effects | |
| Notes | 3/21 (14.2%) participants did not complete the 4 periods of treatment. Small size trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All topical formulations were prepared by the study pharmacist using the same vehicle and were identical in consistency, color, and volume". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessor was not biased by the side effects, given that the incidence of those ones was negligible |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Not apparent selective outcome reporting. Trial registration is not reported. |
| Other bias | High risk | Fewer than 50 participants per period and < 8 weeks duration of trial |
Lynch 2005.
| Study characteristics | ||
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled, parallel design for 3 weeks | |
| Participants | Pain model: chronic, > 3 months and moderate‐severe, neuropathic pain. Median age: 51 and duration of spontaneous pain: 3 to 264 months. 140 participants were screened, 92 participants were randomised and 80 completed the study; 45/92 were female. baseline pain score ranged between 6.66 (SD 1.22) and 7.38 (1.23). Participants were permitted to continue using pre‐study oral analgesics including non‐steroidal anti‐inflammatory drugs, opioids, antidepressants (including amitriptyline and other tricyclics) and anticonvulsants. | |
| Interventions | Participants received 4 ml of topical treatment: 2% amitriptyline, 1% ketamine, the combination or placebo TID for 3 weeks. Creams were identical. Blood samples were takes for assay of amitriptyline and ketamine. | |
| Outcomes | The primary outcomes were 11‐point NRS for pain intensity (daily measures) and SF‐MPQ (first visit and at the end of the 3 weeks). Secondary measures: evoked pain: dynamic tactile allodynia, pinprick hyperalgesia, pinprick hyperaesthesia, pain disability index, 0 to 10 patient satisfaction and plasma concentrations. | |
| Notes | 12/92 (13%) dropout from the study mostly due to adverse events (5/12) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to one of four treatment groups using a computer‐generated randomization list". |
| Allocation concealment (selection bias) | Low risk | "The study medication containers were numbered sequentially. The manufacturing site (Pharmaform LLC, Austin, TX) was separate from the study site". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All topical formulations were identical in consistency, color, and volume". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear if the outcomes assessor was involved in the clinical evaluation. This issue can introduce bias. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Outcomes reported are normally used for pain clinical trials |
| Other bias | High risk | Fewer than 50 participants per period and < 8 weeks duration of trial |
McCleane 2000.
| Study characteristics | ||
| Methods | This trial was a single‐centre, randomised, double‐blind, active and placebo‐controlled, parallel design for 4 weeks | |
| Participants | Pain model: neuropathic pain unresponsive or intolerant to codeine, NSAIDs or TCAs. Mean age/duration of pain in months: placebo 45.4 (SD 13.6)/57.9 (SD 54.6); doxepin 47.8 (SD 17.2)/59.6 (SD 62.3); capsaicin 47.8 (SD 27.8)/59.4 (SD 47.9); combination 43.6 (SD 12.9)/74.9 (SD 66.3). 200 participants were randomised and 151 completed the study. 88/151 were female. Baseline pain score ranged between 7.11 and 7.47. All patients had pain that was unresponsive to simple or compound codeine‐containing analgesics or non‐steroidal anti‐inflammatory drugs. All patients had tried oral TCAs for their pain and had either been unresponsive or intolerant. |
|
| Interventions | Participants received TID topically: 3.3% doxepin, 0.025% capsaicin, the combination and placebo. Creams were identical. | |
| Outcomes | Primary outcome: 0 to 10 average 24‐hour pain intensity on a weekly basis, willingness to continue in the study and side effects profile | |
| Notes | 49/200 dropout from the study; reasons are unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomly allocated, using a computer generated random number list..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All study creams were white, odourless, had a similar nongreasy texture and they were contained in identical screw top containers marked with the appropriate randomization letter..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed drug‐related side effects were also assessing pain outcomes, they might be biased |
| Incomplete outcome data (attrition bias) | High risk | Significant dropout rate and data are not presented |
| Selective reporting (reporting bias) | High risk | Data are not reported |
| Other bias | High risk | < 8 weeks duration of trial |
McCleane 2003.
| Study characteristics | ||
| Methods | This trial was a single‐centre, randomised, double‐blind, placebo‐controlled, add‐on, cross‐over design for 3 periods of 2 weeks each | |
| Participants | Pain model: severe chronic neuropathic pain unresponsive or intolerant conventional analgesia. Mean age: 46.8 years (SD 10.3). 52 patients were screened, 44 randomised and 39 completed the 3 periods of treatments. 23/39 completers were female. The study included patients unresponsive to tricyclic antidepressants, anticonvulsants, opioids and non‐steroidal anti‐inflammatory drugs. | |
| Interventions | 2 weeks before starting the double‐blind periods, patients started oral morphine 20 mg BID. "If subjects were experiencing pain relief with their morphine or getting intolerable side effects from its use, they were withdrawn from the study”; at day 0, participants were randomised to placebo, L‐365,260 30 mg daily or L‐365,260 120 mg divided in 3 doses. | |
| Outcomes | Outcomes explored: 0 to 11 Likert scale and categorical pain score. Sedation score. Likert scale for sleep. ECG, renal and liver function, full blood count, return of unused medication. Morphine and rescue medication intake. | |
| Notes | No changes were observed in any of the measured pain parameters during the periods of treatment. 39/44 participants finished the 3 periods of treatment. No data were published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not stated |
| Allocation concealment (selection bias) | High risk | Unclear who did the randomisation and whether study personnel were able to predict treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Placebo and L‐ 365,260 capsules were identical in appearance..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed drug‐related side effects were also assessing pain outcomes, they might be biased. Also, SEs not reported. |
| Incomplete outcome data (attrition bias) | High risk | Data are not presented |
| Selective reporting (reporting bias) | High risk | Data are not presented |
| Other bias | High risk | Fewer than 50 participants per period and < 8 weeks duration of trial |
Mercadante 2002.
| Study characteristics | ||
| Methods | This trial was a single‐centre, randomised, double‐blind, placebo‐controlled, add‐on, cross‐over design for 2 periods of 1 week each | |
| Participants | Pain model: patients on morphine therapy due to neuropathic cancer pain. Mean age: 67.1 years. 16 patients were randomised and 15 completed the 2 periods of treatment. 6/16 patients were female. Participants with at least moderate pain and stable doses of morphine in the last 2 days were included. Participants that were users of treatment failure of antidepressants were not included in the study. | |
| Interventions | Patients received 25 mg of amitriptyline or equivalent drops of placebo at night for 3 days plus 50 mg for the following 4 days. Patients were in stable dose of morphine. | |
| Outcomes | Outcomes reported: pain intensity in a 0 to 10 scale. Adverse effects, mood and sleep were recorded in 0 to 3 scales. Patient’s preference was recorded at the end of the study. | |
| Notes | Small size trial (16) and small period of treatment (only 1 week) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not stated |
| Allocation concealment (selection bias) | Unclear risk | Not enough information to determine if the allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed drug‐related side effects were also assessing pain outcomes, they might be biased |
| Incomplete outcome data (attrition bias) | Low risk | Very low dropout rate |
| Selective reporting (reporting bias) | Low risk | Pain score is standard in pain clinical trials. The trial does not state if it was registered. |
| Other bias | High risk | Fewer than 50 participants per period and < 8 weeks duration of trial |
Tonet 2008.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, active‐controlled, parallel design for 1 month | |
| Participants | Pain model: neuropathic pain including participants diagnosed with sciatica, PHN, PDN, CRPS I, CRPS II and post‐traumatic nerve injury. Mean age: group 1, 55.1 ± 15.5 and group 2, 48.5 ± 15.6). The number of participants screened is unclear, 30 participants were randomised and 23 completed the trial. 8/30 of the randomised were female. The minimum pain intensity for inclusion is unclear, however, the baseline pain score (0 to 10) were: 7.7 ± 1.6 (group 1) and 7.1 ± 1.8 (group 2). Opioid users were excluded from the study. Codeine was used for analgesic rescue. | |
| Interventions | The group 1 received oral ketamine 10 mg TID + amitriptyline 25 mg/day + carbamazepine 600 mg/day; the group 2 received oral placebo + amitriptyline 25 mg/day + carbamazepine 600 mg/day; amitriptyline was started at 12.5 mg for 3 days and then increased up to 25 mg. Increasing doses of carbamazepine (every 3 days) were administered, starting at 100 mg TID up to 600 mg/day. Codeine was allowed as rescue medication at a maximum dose of 30 mg q6h. | |
| Outcomes | A numerical 0 to 10 rating scale was used for pain intensity and pain relief. Side effects were recorded too. | |
| Notes | 7/30 participants dropout from the study due to side effects of the medications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Ketamine and placebo bottles were identical in solution". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed drug‐related side effects were also assessing pain outcomes, they might be biased |
| Incomplete outcome data (attrition bias) | Unclear risk | Substantial dropout rate |
| Selective reporting (reporting bias) | Low risk | Outcomes reported are standard in pain clinical trials |
| Other bias | High risk | Fewer than 50 participants per arm and < 8 weeks duration of trial |
Zin 2010.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind (for oxycodone only), placebo‐controlled, parallel design for 5 weeks | |
| Participants | Pain models: adult participants diagnosed with postherpetic neuralgia (pain duration > 3 months and < 5 years) or painful diabetic neuropathy (pain duration > 1 year and < 5 years); pain intensity ≥ 4/10. Mean age: pregabalin/oxycodone: 70.27 and pregabalin/placebo: 66.8. 134 participants were screened, 62 randomised and 51 completed the trial. 27/62 patients randomised were female. Baseline pain scores were 6.85/10 in the pregabalin/oxycodone group and 6.73 in the pregabalin/placebo group. Medications that might possibly affect painful symptoms including opioid analgesics, anticonvulsants (e.g. gabapentin, pregabalin, sodium valproate, lamotrigine), tricyclic antidepressants, capsaicin, benzodiazepines and skeletal muscle relaxants were gradually withdrawn during the study‐washout period. Paracetamol was allowed as rescue medication. | |
| Interventions | randomised, double‐blind and fixed dose of oxycodone or placebo mixture for 1 week; subsequently, participants received titrated doses of pregabalin (75 to 600 mg/day) during 4 weeks. Acetaminophen was allowed as rescue medication. | |
| Outcomes | Primary outcome: a 2 cm drop in the pain intensity score and a pain score < 4 cm. Pain reduction > 50% was considered a pain responder. Secondary outcomes: sleep interference score, neuropathic pain scale, SF‐36, Profile of Mood States, Trial Making Test B, Patient Global Impression of Change and Clinical Global Impression of Change. | |
| Notes | 3/4 dropouts secondary to adverse effects were before starting pregabalin. Significant differences in the duration of PDN between groups being longer in the pregabalin/oxycodone. A small and fixed dose of oxycodone was used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned to 1 of 2 treatment groups using a computer‐generated randomization number". |
| Allocation concealment (selection bias) | Low risk | "The randomization sequence list was controlled independently by the clinical pharmacist". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "the oxycodone and placebo mixture were identical to ensure that study blinding was maintained..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the same assessors who noticed drug‐related side effects were also assessing pain outcomes, they might be biased |
| Incomplete outcome data (attrition bias) | Low risk | Non‐significant dropout rate. All analyses were conducted on an ITT basis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported are standard and recommended for pain clinical trials |
| Other bias | High risk | Fewer than 50 participants per period and < 8 weeks duration of trial |
ALA: alpha lipoic acid; APAP: acetaminophen; BDI: Beck Depression Inventory; BID: twice a day; BMS: burning mouth syndrome; BPI: Brief Pain Inventory; CRPS: complex regional pain syndrome; ECG: electrocardiogram; GABA: gabapentin; ITT: intention‐to‐treat; LOCF: last observation carried forward; MMPI: Minnesota Multiphasic Personality Inventory; NSAID: non‐steroidal anti‐inflammatory drug; NT: nortriptyline; prn: as needed; PDN: Painful Diabetic Neuropathy; PHN: postherpetic neuralgia; SD: standard deviation; SE: standard error; SF‐MPQ: Short Form of the McGill Pain Questionnaire; SSRI: selective serotonin re‐uptake inhibitor; TCA: tricyclic antidepressant; TID: three times a day; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbas 1997 | Vitamins are not considered analgesics |
| Achar 2010 | Open‐label study |
| Aldrete 2006 | Non‐neuropathic pain condition |
| Alvaro 1999 | Non pharmacological therapy; out of the scope of this review |
| Amjad 2005 | Invasive/injection therapy out of the scope of this review |
| Amr 2011 | Invasive/injection therapy out of the scope of this review |
| Arai 2010 | Open‐label study |
| Argoff 2004 | Open‐label study |
| Atiyat 2000 | Single cohort study |
| Autio 2004 | Invasive/injection therapy out of the scope of this review |
| Barbarisi 2010 | Non pharmacological therapy; out of the scope of this review |
| Baron 2009b | Open‐label study |
| Battla 1981 | Cohort study |
| Bertolotto 2012 | Open‐label study |
| Bestard 2011 | Open‐label study |
| Blonna 2004 | Invasive/injection therapy and open‐label study |
| Braun 1982 | Invasive/injection therapy out of the scope of this review |
| Bush 1991 | Invasive/injection therapy out of the scope of this review |
| Canovas 2009 | Open‐label study |
| De Benedittis 1992 | Head to head comparison of single agents |
| Deshpande 2006 | Secondary analysis of Gilron 2005 |
| Devulder 1999 | Non‐neuropathic pain condition |
| Dureja 2010 | Invasive/injection therapy out of the scope of this review |
| Eardley 2010 | Open‐label study |
| Eker 2012 | Invasive/injection therapy out of the scope of this review |
| Ertas 1998 | 2 drugs were mixed but only 1 has an active mechanism of action (Levodopa) |
| Fliege 1966 | Vitamins are not considered analgesics |
| Fromm 1984 | Add‐on therapy trial. Only one medication (baclofen) was randomised |
| Galer 2004 | Non‐neuropathic pain condition |
| Galer 2005 | Non‐neuropathic pain condition |
| Gatti 2009 | Open‐label study |
| Gerson 1977 | Open‐label study |
| Glantz 2004 | Open‐label study |
| Glynn 1996 | Invasive/injection therapy out of the scope of this review |
| Gobel 1995 | Open‐label study |
| Goebel 2003 | Study was published as a protocol only |
| Goldberg 2009 | Vitamins are not considered analgesics |
| Guo 2007 | Open‐label study |
| Gustin 2010 | 20 patients diagnosed with complex regional pain syndrome (CRPS). However, only 5/20 had CRPS type II (documented nerve injury) |
| Irving 2012 | The drug that was co‐administered/combined with capsaicin was not controlled |
| Karppinen 2001 | Invasive/injection therapy out of the scope of this review |
| Keskinbora 2007 | Open‐label study |
| Ko 2010 | Open‐label study |
| Kochar 1998 | Open‐label study |
| Kotani 2000 | Invasive/injection therapy out of the scope of this review |
| Kottschade 2009 | Vitamins are not considered analgesics. Abstract/poster. |
| Kukushkin 1996 | Clinical individual experience/narrative review |
| Lagalla 2002 | Vitamins are not considered analgesics |
| Lampl 2010 | Observational study |
| Langohr 1982 | Open‐label study |
| Lauretti 2002 | Invasive/injection therapy out of the scope of this review |
| Lemos 2008 | Invasive/injection therapy out of the scope of this review |
| Levin 2009 | Vitamins are not considered analgesics |
| Martinez 1990 | Invasive/injection therapy out of the scope of this review |
| McCleane 1998 | Non‐neuropathic pain condition |
| Mendel 1986 | Small size trial (6 participants, only) |
| Mercadante 1998 | Open‐label study |
| Mercadante 2000 | The treatment was for only 30 minutes |
| Minotti 1998 | Non‐neuropathic pain condition |
| Palangio 2000 | Patients with neuropathic pain were a minority in this trial |
| Patarica‐Huber 2011 | The study was randomised but apparently open‐label (no information regarding blinding process in the publication) |
| Pieri 2007 | Open‐label study |
| Pirbudak 2003 | Invasive/injection therapy out of the scope of this review |
| Rabben 1999 | Single‐dose infusion |
| Rehm 2010 | Open‐label study |
| Rodriguez 1999 | Open‐label study |
| Romano 2009 | Nociceptive and neuropathic pain conditions mixed in the same trial |
| Russo 2006 | Narrative review |
| Ruts 2007 | This trial included paediatric patients |
| Schechtmann 2010 | Invasive/injection therapy out of the scope of this review |
| Shaibani 2012 | The study looks like a combination study, however, quinidine has no analgesic properties and it was administered to optimise the pharmacokinetic profile of desmethorphan only |
| Shlay 1998 | Acupuncture plus pharmacological treatment |
| Siddall 2000 | Invasive/injection therapy out of the scope of this review |
| Silver 2007 | This trial included paediatric population |
| Simpson 2001 | A small number of patients completed the study |
| Stajcic 1990 | Invasive/injection therapy out of the scope of this review |
| Sullivan 2009 | Pain intensity was not the primary outcome |
| Takahashi 2010 | Open‐label study |
| Tanenberg 2011 | Open‐label study |
| Tian 2005 | Chinese medicine plus vitamin trial |
| Venancio‐Ramirez 2004 | Open‐label study |
| Wang 2007 | Combination including non‐pharmacological therapy |
| Ward 1981 | Authors concluded that patients had no neuropathic pain |
| Winkler 1999 | Vitamins are not considered analgesics |
Differences between protocol and review
In addition to the pre‐planned literature search, we further searched the clinical trials.gov and controlled‐trials.com trial databases for completed pharmaceutical industry trials which posted their results on the clinicalstudyresults.org website. We performed a snowballing search to increase the accuracy of the protocol‐defined search (Greenhalgh 2005). Given recent updates to the neuropathic pain literature, we also made some revisions to the background section.
Contributions of authors
The title was registered by IG. The protocol was developed and written by IG, PW and RAM. IG and LC assessed inclusion of papers and extracted data. LC and IG assumed responsibility for the full review, and write up of the review. PW and RAM contributed to the final draft and approved the final version. LC and IG will be responsible for updating the review.
Sources of support
Internal sources
-
UK Cochrane Centre, UK
UK sabbatical residence for IG
External sources
Canadian Institutes of Health Grant #MCT‐94187 and #MSH‐55041 to IG, Canada
IASP J.J. Bonica Trainee Fellowship to LC, USA
Declarations of interest
In the past five years, IG and RAM have consulted for various pharmaceutical companies. In the past five years, IG and RAM have received lecture fees from pharmaceutical companies that market analgesics and other healthcare interventions. IG and RAM have received research support from charities, government and industry sources at various times, but no such support was received for this work.
Two of the studies included in this review were authored by one of the review authors (IG).
LC has no conflicts to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Agrawal 2009 {published data only}
- Agrawal RP, Goswami J, Jain S, Kochar DK. Management of diabetic neuropathy by sodium valproate and glyceryl trinitrate spray: a prospective double-blind randomised placebo-controlled study. Diabetes Research & Clinical Practice 2009;83(3):371-8. [DOI] [PubMed] [Google Scholar]
Amr 2010 {published data only}
- Amr YM. Multi-day low dose ketamine infusion as adjuvant to oral gabapentin in spinal cord injury related chronic pain: a prospective, randomised, double blind trial. Pain Physician 2010;13(3):245-9. [PubMed] [Google Scholar]
Barton 2011 {published data only}
- Barton DL, Wos EJ, Qin R, Mattar BI, Green NB, Lanier KS, et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Supportive Care in Cancer 2011;19(6):833-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caraceni 2004 {published data only}
- Caraceni A, Zecca E, Bonezzi C, Arcuri E, Yaya TR, Maltoni M, et al. Gabapentin for neuropathic cancer pain: a randomised controlled trial from the Gabapentin Cancer Pain Study Group. Journal of Clinical Oncology 2004;22(14):2909-17. [DOI] [PubMed] [Google Scholar]
Eichenberger 2008 {published data only}
- Eichenberger U, Neff F, Sveticic G, Bjorgo S, Petersen-Felix S, Arendt-Nielsen L, et al. Chronic phantom limb pain: the effects of calcitonin, ketamine, and their combination on pain and sensory thresholds. Anesthesia & Analgesia 2008;106(4):1265-73. [DOI] [PubMed] [Google Scholar]
Freeman 2007 {published data only}
- Freeman R, Raskin P, Hewitt DJ, Vorsanger GJ, Jordan DM, Xiang J, et al. Randomised study of tramadol/acetaminophen versus placebo in painful diabetic peripheral neuropathy. Current Medical Research & Opinion 2007;23(1):147-61. [DOI] [PubMed] [Google Scholar]
Gilron 2005b {published data only}
- Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. New England Journal of Medicine 2005;352(13):1324-34. [DOI] [PubMed] [Google Scholar]
Gilron 2009 {published data only}
- Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009;374(9697):1252-61. [DOI] [PubMed] [Google Scholar]
Gomez‐Perez 1985 {published data only}
- Gomez-Perez FJ, Rull JA, Dies H, Rodriquez-Rivera JG, Gonzalez-Barranco J, Lozano C. Nortriptyline and fluphenazine in the symptomatic treatment of diabetic neuropathy. A double-blind cross-over study. Pain 1985;23(4):395-400. [DOI] [PubMed] [Google Scholar]
Gomez‐Perez 1996 {published data only}
- Gomez-Perez FJ, Choza R, Rios JM, Reza A, Huerta E, Aguilar CA, Rull JA. Nortriptyline-fluphenazine vs. carbamazepine in the symptomatic treatment of diabetic neuropathy. Archives of Medical Research 1996;27(4):525-9. [PubMed] [Google Scholar]
Graff‐Radford 2000 {published data only}
- Graff-Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clinical Journal of Pain 2000;16(3):188-92. [DOI] [PubMed] [Google Scholar]
Hanna 2008 {published data only}
- Hanna M, O'Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. European Journal of Pain 2008;12(6):804-13. [DOI] [PubMed] [Google Scholar]
Khoromi 2007 {published data only}
- Khoromi S, Cui L, Nackers L, Max MB. Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain 2007;130(1-2):66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez‐D'alessandro 2011 {published data only}
- Lopez-D'alessandro E, Escovich L. Combination of alpha lipoic acid and gabapentin, its efficacy in the treatment of Burning Mouth Syndrome: a randomised, double-blind, placebo controlled trial. Medicina Oral, Patologia Oral y Cirugia Bucal 2011;16(5):e635-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lynch 2003 {published data only}
- Lynch ME, Clark AJ, Sawynok JA. A pilot study examining topical amitriptyline, ketamine, and a combination of both in the treatment of neuropathic pain. Clinical Journal of Pain 2003;19(5):323-8. [DOI] [PubMed] [Google Scholar]
Lynch 2005 {published data only}
- Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical 2% amitriptyline and 1% ketamine in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Anesthesiology 2005;103(1):140-6. [DOI] [PubMed] [Google Scholar]
McCleane 2000 {published data only}
- McCleane G. Topical application of doxepin hydrochloride, capsaicin and a combination of both produces analgesia in chronic human neuropathic pain: a randomised, double-blind, placebo-controlled study. British Journal of Clinical Pharmacology 2000;49(6):574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCleane 2003 {published data only}
- McCleane GJ. A randomised, double blind, placebo controlled crossover study of the cholecystokinin 2 antagonist L-365,260 as an adjunct to strong opioids in chronic human neuropathic pain. Neuroscience Letters 2003;338(2):151-4. [DOI] [PubMed] [Google Scholar]
Mercadante 2002 {published data only}
- Mercadante S, Arcuri E, Tirelli W, Villari P, Casuccio A. Amitriptyline in neuropathic cancer pain in patients on morphine therapy: a randomised placebo-controlled, double-blind crossover study. Tumori 2002;88(3):239-42. [DOI] [PubMed] [Google Scholar]
Tonet 2008 {published data only}
- Tonet C, Sakata RK, Issy AM, Garcia JBS, Marcelino ANM. Evaluation of oral ketamine for neuropathic pain [Avaliação da cetamina oral para dor neuropática]. Revista Brasileira de Medicina 2008;65(7):214-8. [Google Scholar]
Zin 2010 {published data only}
- Zin CS, Nissen LM, O'Callaghan JP, Duffull SB, Smith MT, Moore BJ. A randomised, controlled trial of oxycodone versus placebo in patients with postherpetic neuralgia and painful diabetic neuropathy treated with pregabalin. Journal of Pain 2010;11(5):462-71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbas 1997 {published data only}
- Abbas ZG, Swai AB. Evaluation of the efficacy of thiamine and pyridoxine in the treatment of symptomatic diabetic peripheral neuropathy. East African Medical Journal 1997;74(12):803-8. [PubMed] [Google Scholar]
Achar 2010 {published data only}
- Achar A, Chatterjee G, Ray TG, Naskar B. Comparative study of clinical efficacy with amitriptyline, pregabalin, and amitriptyline plus pregabalin combination in postherpetic neuralgia. Indian Journal of Dermatology, Venereology & Leprology 2010;76(1):63-5. [DOI] [PubMed] [Google Scholar]
Aldrete 2006 {published data only}
- Aldrete JA, Guevara U, Arenoso HJ, Ceraso OL. Efficacy and tolerability of epidural steroids vs low doses of steroids plus metamizol plus propoxyphene administered paravertebrally for postaminectomy syndrome patients [Eficacia y tolerabilidad de esteroides epidurales vs. dosis bajas de esteroides mas metamizol mas D-propoxifeno administrados por vía paravertebral en pacientes con síndrome post-laminectomía]. Revista de la Sociedad Espanola del Dolor 2006;13(7):454-61. [Google Scholar]
Alvaro 1999 {published data only}
- Alvaro M, Kumar D, Julka IS. Transcutaneous electrostimulation: emerging treatment for diabetic neuropathic pain. Diabetes Technology & Therapeutics 1999;1(1):77-80. [DOI] [PubMed] [Google Scholar]
Amjad 2005 {published data only}
- Amjad M, Mashhood AA. The efficacy of local infiltration of triamcinolone acetonide with lignocaine compared with lignocaine alone in the treatment of postherpetic neuralgia. Journal of the College of Physicians & Surgeons - Pakistan 2005;15(11):683-5. [PubMed] [Google Scholar]
Amr 2011 {published data only}
- Amr YM. Effect of addition of epidural ketamine to steroid in lumbar radiculitis: one-year follow-up. Pain Physician 2011;14(5):475-81. [PMID: ] [PubMed] [Google Scholar]
Arai 2010 {published data only}
- Arai YC, Matsubara T, Shimo K, Suetomi K, Nishihara M, Ushida T, et al. Low-dose gabapentin as useful adjuvant to opioids for neuropathic cancer pain when combined with low-dose imipramine. Journal of Anesthesia 2010;24(3):407-10. [DOI] [PubMed] [Google Scholar]
Argoff 2004 {published data only}
- Argoff CE, Galer BS, Jensen MP, Oleka N, Gammaitoni AR. Effectiveness of the lidocaine patch 5% on pain qualities in three chronic pain states: assessment with the Neuropathic Pain Scale. Current Medical Research & Opinion 2004;20:Suppl 8. [DOI] [PubMed] [Google Scholar]
Atiyat 2000 {published data only}
- Atiyat B. Triple-target regimen for treatment of chronic pain following post herpic neuralgia (CPPHN): a prospective trial at Jordan University Hospital (JUH) pain unit. Bahrain Medical Bulletin 2000;22(4):167-9. [Google Scholar]
Autio 2004 {published data only}
- Autio RA, Karppinen J, Kurunlahti M, Haapea M, Vanharanta H, Tervonen O. Effect of periradicular methylprednisolone on spontaneous resorption of intervertebral disc herniations. Spine 2004;29(15):1601-7. [DOI] [PubMed] [Google Scholar]
Barbarisi 2010 {published data only}
- Barbarisi M, Pace MC, Passavanti MB, Maisto M, Mazzariello L, Pota V, et al. Pregabalin and transcutaneous electrical nerve stimulation for postherpetic neuralgia treatment. Clinical Journal of Pain 2010;26(7):567-72. [DOI] [PubMed] [Google Scholar]
Baron 2009b {published data only}
- Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of combination therapy with 5% lidocaine medicated plaster and pregabalin in post-herpetic neuralgia and diabetic polyneuropathy. Current Medical Research & Opinion 2009;25(7):1677-87. [DOI] [PubMed] [Google Scholar]
Battla 1981 {published data only}
- Battla H, Silverblatt CW. Clinical trial of amitriptyline and fluphenazine in diabetic peripheral neuropathy. Southern Medical Journal 1981;74(4):417-8. [DOI] [PubMed] [Google Scholar]
Bertolotto 2012 {published data only}
- Bertolotto F, Massone A. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs in R&D 2012;12(1):29-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bestard 2011 {published data only}
- Bestard JA, Toth CC. An open-label comparison of nabilone and gabapentin as adjuvant therapy or monotherapy in the management of neuropathic pain in patients with peripheral neuropathy. Pain Practice: the official journal of World Institute of Pain 2011;11(4):353-68. [PMID: ] [DOI] [PubMed] [Google Scholar]
Blonna 2004 {published data only}
- Blonna D, Calvi V, Collo G, Marmotti A, Castoldi F. Gabapentin in the conservative treatment of radiculopathy. Minerva Ortopedica e Traumatologica 2004;55(1):15-22. [Google Scholar]
Braun 1982 {published data only}
- Braun H, Huberty R. Therapy of lumbar sciatica. A comparative clinical study of a corticoid-free monosubstance and a corticoid-containing combination drug. Medizinische Welt 1982;33(13):490-1. [PubMed] [Google Scholar]
Bush 1991 {published data only}
- Bush K, Hillier S. A controlled study of caudal epidural injections of triamcinolone plus procaine for the management of intractable sciatica. Spine 1991;16(5):572-5. [DOI] [PubMed] [Google Scholar]
Canovas 2009 {published data only}
- Canovas Martinez L, Gomez Gutierrez I, Castro Bande M, Peralta Espinosa E, Prieto Gutierrez JM, Segado Jimenez I. Analgesic efficacy of the association of duloxetine plus pregabalin in neuropathic pain: experience in 60 patients. Revista de la Sociedad Espanola del Dolor 2009;16(7):381-5. [Google Scholar]
De Benedittis 1992 {published data only}
- De Benedittis G, Besana F, Lorenzetti A. A new topical treatment for acute herpetic neuralgia and post-herpetic neuralgia: the aspirin/diethyl ether mixture. An open-label study plus a double-blind controlled clinical trial.. Pain 1992;48(3):383-90. [DOI] [PubMed] [Google Scholar]
Deshpande 2006 {published data only}
- Deshpande MA, Holden RR, Gilron I. The impact of therapy on quality of life and mood in neuropathic pain: what is the effect of pain reduction? Anesthesia & Analgesia 2006;102(5):1473-9. [DOI] [PubMed] [Google Scholar]
Devulder 1999 {published data only}
- Devulder J, Deene P, De LM, Van BM, Brusselmans G, Rolly G. Nerve root sleeve injections in patients with failed back surgery syndrome: a comparison of three solutions. Clinical Journal of Pain 1999;15(2):132-5. [DOI] [PubMed] [Google Scholar]
Dureja 2010 {published data only}
- Dureja GP, Usmani H, Khan M, Tahseen M, Jamal A. Efficacy of intrathecal midazolam with or without epidural methylprednisolone for management of post-herpetic neuralgia involving lumbosacral dermatomes. Pain Physician 2010;13(3):213-21. [PubMed] [Google Scholar]
Eardley 2010 {published data only}
- Eardley W, Toth C. An open-label, non-randomised comparison of venlafaxine and gabapentin as monotherapy or adjuvant therapy in the management of neuropathic pain in patients with peripheral neuropathy. Journal of Pain Research 2010;3:33-49. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eker 2012 {published data only}
- Eker HE, Cok OY, Aribogan A, Arslan G. Management of neuropathic pain with methylprednisolone at the site of nerve injury. Pain Medicine (Malden, Mass.) 2012;13(3):443-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ertas 1998 {published data only}
- Ertas M, Sagduyu A, Arac N, Uludag B, Ertekin C. Use of levodopa to relieve pain from painful symmetrical diabetic polyneuropathy. Pain 1998;75(2-3):257-9. [DOI] [PubMed] [Google Scholar]
Fliege 1966 {published data only}
- Fliege K, Mistler O. Treatment of chronic painful conditions with a diazethylthiamine (fat-soluble vitamin B 1) combination preparation. [German]. Medizinische Klinik 1966;61(52):2080-2. [PubMed] [Google Scholar]
Fromm 1984 {published data only}
- Fromm GH, Terrence CF, Chattha AS. Baclofen in the treatment of trigeminal neuralgia: double-blind study and long-term follow-up. Annals of Neurology 1984;15(3):240-4. [DOI] [PubMed] [Google Scholar]
Galer 2004 {published data only}
- Galer BS, Gammaitoni AR, Oleka N, Jensen MP, Argoff CE. Use of the lidocaine patch 5% in reducing intensity of various pain qualities reported by patients with low-back pain. Current Medical Research & Opinion 2004;20:Suppl 12. [DOI] [PubMed] [Google Scholar]
Galer 2005 {published data only}
- Galer BS, Lee D, Ma T, Nagle B, Schlagheck TG. MorphiDex (morphine sulfate/dextromethorphan hydrobromide combination) in the treatment of chronic pain: three multicenter, randomised, double-blind, controlled clinical trials fail to demonstrate enhanced opioid analgesia or reduction in tolerance. Pain 2005;115(3):284-95. [DOI] [PubMed] [Google Scholar]
Gatti 2009 {published data only}
- Gatti A, Sabato AF, Occhioni R, Colini Baldeschi G, Reale C. Controlled-release oxycodone and pregabalin in the treatment of neuropathic pain: results of a multicenter Italian study. European Neurology 2009;61(3):129-37. [DOI] [PubMed] [Google Scholar]
Gerson 1977 {published data only}
- Gerson GR, Jones RB, Luscombe DK. Studies on the concomitant use of carbamazepine and clomipramine for the relief of post-herpetic neuralgia. Postgraduate Medical Journal 1977;53:Suppl 9. [PubMed] [Google Scholar]
Glantz 2004 {published data only}
- Glantz L, Godovic G, Lekar M, Kramer M, Eidelman LA. Efficacy of transdermal nitroglycerin combined with etodolac for the treatment of chronic post-thoracotomy pain: an open-label prospective clinical trial. Journal of Pain & Symptom Management 2004;27(3):277-81. [DOI] [PubMed] [Google Scholar]
Glynn 1996 {published data only}
- Glynn C, O'Sullivan K. A double-blind randomised comparison of the effects of epidural clonidine, lignocaine and the combination of clonidine and lignocaine in patients with chronic pain. Pain 1996;64(2):337-43. [DOI] [PubMed] [Google Scholar]
Gobel 1995 {published data only}
- Gobel H, Stadler T. Treatment of pain due to postherpetic neuralgia with tramadol - results of an open, parallel pilot study vs clomipramine with or without levomepromazine. Clinical Drug Investigation 1995;10(4):208-14. [Google Scholar]
Goebel 2003 {published data only}
- Goebel A, Moore A, Weatherall R, Roewer N, Schedel R, Sprotte G. Intravenous immunoglobulin in the treatment of primary trigeminal neuralgia refractory to carbamazepine: a study protocol [ISRCTN33042138]. BMC Neurology 2003;3(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 2009 {published data only}
- Goldberg H, Scussel Jr, Cohen JC, Rzetelna H, Mezitis SGE, Nunes FP, et al. Neural compression-induced neuralgias: clinical evaluation of the effect of nucleotides associated with vitamin B12 [Neuralgias decorrentes de compressão neural: avaliação clínica da ação de nucleotídeos associados à vitamina B12]. Revista Brasileira de Medicina 2009;66(11):380-15. [Google Scholar]
Guo 2007 {published data only}
- Guo W-J, Xiao Z-Y, Yang Y-X. Effectiveness of transdermal fentanyl combined with clodine for pain control of acute herpes zoster [Chinese]. Journal of Dalian Medical University 2007;29(3):255-6. [Google Scholar]
Gustin 2010 {published data only}
- Gustin SM, Schwarz A, Birbaumer N, Sines N, Schmidt AC, Veit R, et al. NMDA-receptor antagonist and morphine decrease CRPS-pain and cerebral pain representation. Pain 2010;151(1):69-76. [DOI] [PubMed] [Google Scholar]
Irving 2012 {published data only}
- Irving GA, Backonja M, Rauck R, Webster LR, Tobias JK, Vanhove GF. NGX-4010, a capsaicin 8% dermal patch, administered alone or in combination with systemic neuropathic pain medications, reduces pain in patients with postherpetic neuralgia. Clinical Journal of Pain 2012;28(2):101-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karppinen 2001 {published data only}
- Karppinen J, Malmivaara A, Kurunlahti M, Kyllönen E, Pienimäki T, Nieminen P, et al. Periradicular infiltration for sciatica: a randomised controlled trial. Spine 2001;26(9):1059-67. [DOI] [PubMed] [Google Scholar]
Keskinbora 2007 {published data only}
- Keskinbora K, Pekel AF, Aydinli I. Gabapentin and an opioid combination versus opioid alone for the management of neuropathic cancer pain: a randomised open trial. Journal of Pain & Symptom Management 2007;34(2):183-9. [DOI] [PubMed] [Google Scholar]
Ko 2010 {published data only}
- Ko SH, Kwon HS, Yu JM, Baik SH, Park IB, Lee JH, et al. Comparison of the efficacy and safety of tramadol/acetaminophen combination therapy and gabapentin in the treatment of painful diabetic neuropathy. Diabetic Medicine 2010;27(9):1033-40. [DOI] [PubMed] [Google Scholar]
Kochar 1998 {published data only}
- Kochar DK, Agarwal RP, Joshi A, Kumawat BL. Herpes zoster and post-herpetic neuralgia--a clinical trial of aspirin in chloroform for anodyne. Journal of the Association of Physicians of India 1998;46(4):337-40. [PubMed] [Google Scholar]
Kotani 2000 {published data only}
- Kotani N, Kushikata T, Hashimoto H, Kimura F, Muraoka M, Yodono M, et al. Intrathecal methylprednisolone for intractable postherpetic neuralgia. New England Journal of Medicine 2000;343(21):1514-9. [DOI] [PubMed] [Google Scholar]
Kottschade 2009 {published data only}
- Kottschade LA, Sloan JA, Mazurczak MA, Johnson DB, Murphy B, Rowland KM, et al. The use of vitamin E for prevention of chemotherapy-induced peripheral neuropathy: a phase III double-blind, placebo controlled study. Journal of Clinical Oncology 2009;Conference:9532. [Google Scholar]
Kukushkin 1996 {published data only}
- Kukushkin ML, Ivanova AF, Ovechkin AM, Gnezdilov AV, Reshetniak VK. Differential combined drug therapy of phantom pain syndrome after amputation of extremity [Russian]. Anesteziologiia i Reanimatologiia 1996;Jul-Aug(4):39-42. [PubMed] [Google Scholar]
Lagalla 2002 {published data only}
- Lagalla G, Logullo F, Di Bella P, Provinciali L, Ceravolo MG. Influence of early high-dose steroid treatment on Bell's palsy evolution. Neurological Sciences 2002;23(3):107-12. [DOI] [PubMed] [Google Scholar]
Lampl 2010 {published data only}
- Lampl C, Schweiger C, Haider B, Lechner A. Pregabalin as mono- or add-on therapy for patients with refractory chronic neuropathic pain: a post-marketing prescription-event monitoring study. Journal of Neurology 2010;257(8):1265. [DOI] [PubMed] [Google Scholar]
Langohr 1982 {published data only}
- Langohr HD, Stöhr M, Petruch F. An open and double-blind cross-over study on the efficacy of clomipramine (Anafranil) in patients with painful mono- and polyneuropathies. European Neurology 1982;21(5):309-17. [DOI] [PubMed] [Google Scholar]
Lauretti 2002 {published data only}
- Lauretti GR, Rodrigues ADM, Gomes JMA, Dos Reis MP. Epidural ketamine versus epidural clonidine as therapeutic for refractory neuropathic chronic pain. Revista Brasileira de Anestesiologia 2002;52(1):34-40. [Google Scholar]
Lemos 2008 {published data only}
- Lemos L, Flores S, Oliveira P, Almeida A. Gabapentin supplemented with ropivacain block of trigger points improves pain control and quality of life in trigeminal neuralgia patients when compared with gabapentin alone. Clinical Journal of Pain 2008;24(1):64-75. [DOI] [PubMed] [Google Scholar]
Levin 2009 {published data only}
- Levin OS, Moseĭkin IA. Vitamin B complex (milgamma) in the treatment of vertebrogenic lumbosacral radiculopathy [Russian]. Zhurnal Nevrologii i Psikhiatrii Imeni S.S.Korsakova 2009;109(10):30-5. [PubMed] [Google Scholar]
Martinez 1990 {published data only}
- Martinez GC, Abarca B, Alvarado CL, Almonte C, Acevedo M, Leyton R, et al. Bell's palsy: evaluation of steroidal treatment: peripheral streptomycin/lidocaine injections versus lidocaine alone in the treatment of idiopathic trigeminal neuralgia. A double blind controlled trial. Journal of Craniomaxillofacial Surgery 1990;18:243-6. [DOI] [PubMed] [Google Scholar]
McCleane 1998 {published data only}
- McCleane GJ, McLaughlin M. The addition of GTN to capsaicin cream reduces the discomfort associated with application of capsaicin alone. A volunteer study. Pain 1998;78(2):149-51. [DOI] [PubMed] [Google Scholar]
Mendel 1986 {published data only}
- Mendel CM, Klein RF, Chappell DA, Dere WH, Gertz BJ, Karam JH, et al. A trial of amitriptyline and fluphenazine in the treatment of painful diabetic neuropathy. JAMA 1986;255(5):637-9. [PubMed] [Google Scholar]
Mercadante 1998 {published data only}
- Mercadante S, Casuccio A, Genovese G. Ineffectiveness of dextromethorphan in cancer pain. Journal of Pain & Symptom Management 1998;16(5):317-22. [PubMed] [Google Scholar]
Mercadante 2000 {published data only}
- Mercadante S, Arcuri E, Tirelli W, Casuccio A. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: a randomised, controlled, double-blind, crossover, double-dose study. Journal of Pain & Symptom Management 2000;20(4):246-52. [DOI] [PubMed] [Google Scholar]
Minotti 1998 {published data only}
- Minotti V, De Angelis V, Righetti E, Celani MG, Rossetti R, Lupatelli M, et al. Double-blind evaluation of short-term analgesic efficacy of orally administered diclofenac, diclofenac plus codeine, and diclofenac plus imipramine in chronic cancer pain. Pain 1998;74(2-3):133-7. [DOI] [PubMed] [Google Scholar]
Palangio 2000 {published data only}
- Palangio M, Damask MJ, Morris E, Doyle RT Jr, Jiang JG, Landau CJ, et al. Combination hydrocodone and ibuprofen versus combination codeine and acetaminophen for the treatment of chronic pain. Clinical Therapeutics 2000;22(7):879-92. [DOI] [PubMed] [Google Scholar]
Patarica‐Huber 2011 {published data only}
- Patarica-Huber E, Boskov N, Pjevic M. Multimodal approach to therapy-related neuropathic pain in breast cancer. Journal of B.U.ON: official journal of the Balkan Union of Oncology 2011;16(1):40-5. [PMID: ] [PubMed] [Google Scholar]
Pieri 2007 {published data only}
- Pieri M. Treatment of post-herpetic neuralgia with a combination of tramadol, paracetamol, gabapentin and local anaesthetic: clinical trial on 26 patients [Italian]. Trends in Medicine 2007;7(3):181-8. [Google Scholar]
Pirbudak 2003 {published data only}
- Pirbudak L, Karakurum G, Oner U, Gulec A, Karadasli H. Epidural corticosteroid injection and amitriptyline for the treatment of chronic low back pain associated with radiculopathy. Pain Clinic 2003;15(3):247-53. [Google Scholar]
Rabben 1999 {published data only}
- Rabben T, Skjelbred P, Oye I. Prolonged analgesic effect of ketamine, an N-methyl-D-aspartate receptor inhibitor, in patients with chronic pain. Journal of Pharmacology & Experimental Therapeutics 1999;289(2):1060-6. [PubMed] [Google Scholar]
Rehm 2010 {published data only}
- Rehm S, Binder A, Baron R. Post-herpetic neuralgia: 5% lidocaine medicated plaster, pregabalin, or a combination of both? A randomised, open, clinical effectiveness study. Current Medical Research & Opinion 2010;26(7):1607-19. [DOI] [PubMed] [Google Scholar]
Rodriguez 1999 {published data only}
- Rodriguez Hernandez R, Flores Lopez D. Assessment of pain intensity in patients with diabetic polineuropathy treated with peridural 2% lidocaine methylprednisolone acetate vs peridural 2% lidocaine. Anestesia en Mexico 1999;11(2):65-9. [Google Scholar]
Romano 2009 {published data only}
- Romano CL, Romano D, Bonora C, Mineo G. Pregabalin, celecoxib, and their combination for treatment of chronic low-back pain. Journal of Orthopaedics & Traumatology 2009;10(4):185-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Russo 2006 {published data only}
- Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Medical Hypotheses 2006;66(2):234-46. [DOI] [PubMed] [Google Scholar]
Ruts 2007 {published data only}
- Ruts L, Koningsveld R, Jacobs BC, Doorn PA. Determination of pain and response to methylprednisolone in Guillain-Barre syndrome. Journal of Neurology 2007;254(10):1318-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schechtmann 2010 {published data only}
- Schechtmann G, Lind G, Winter J, Meyerson BA, Linderoth B. Intrathecal clonidine and baclofen enhance the pain-relieving effect of spinal cord stimulation: a comparative placebo-controlled, randomised trial. Neurosurgery 2010;67(1):173-81. [DOI] [PubMed] [Google Scholar]
Shaibani 2012 {published data only}
- Shaibani AI, Pope LE, Thisted R, Hepner A. Efficacy and safety of dextromethorphan/quinidine at two dosage levels for diabetic neuropathic pain: a double-blind, placebo-controlled, multicenter study. Pain Medicine (Malden, Mass.) 2012;13(2):243-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shlay 1998 {published data only}
- Shlay JC, Chaloner K, Max MB, Flaws B, Reichelderfer P, Wentworth D, et al. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: a randomised controlled trial. Terry Beirn Community Programs for Clinical Research on AIDS. JAMA 1998;280(18):1590-5. [DOI] [PubMed] [Google Scholar]
Siddall 2000 {published data only}
- Siddall PJ, Molloy AR, Walker S, Mather LE, Rutkowski SB, Cousins MJ. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesthesia & Analgesia 2000;91(6):1493-8. [DOI] [PubMed] [Google Scholar]
Silver 2007 {published data only}
- Silver M, Blum D, Grainger J, Hammer AE, Quessy S. Double-blind, placebo-controlled trial of lamotrigine in combination with other medications for neuropathic pain. Journal of Pain & Symptom Management 2007;34(4):446-54. [DOI] [PubMed] [Google Scholar]
Simpson 2001 {published data only}
- Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. Journal of Clinical Neuromuscular Disease 2001;3(2):53-62. [DOI] [PubMed] [Google Scholar]
Stajcic 1990 {published data only}
- Stajcic Z, Juniper RP, Todorovic L. Peripheral streptomycin/lidocaine injections versus lidocaine alone in the treatment of idiopathic trigeminal neuralgia. A double blind controlled trial. Journal of Cranio-Maxillo-Facial Surgery 1990;18(6):243-6. [DOI] [PubMed] [Google Scholar]
Sullivan 2009 {published data only}
- Sullivan FM, Swan IR, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al. A randomised controlled trial of the use of aciclovir and/or prednisolone for the early treatment of Bell's palsy: the BELLS study. Health Technology Assessment (Winchester, England) 2009;13(47):iii-v. [DOI] [PubMed] [Google Scholar]
Takahashi 2010 {published data only}
- Takahashi H, Shimoyama N. A prospective open-label trial of gabapentin as an adjuvant analgesic with opioids for Japanese patients with neuropathic cancer pain. International Journal of Clinical Oncology 2010;15(1):46-51. [DOI] [PubMed] [Google Scholar]
Tanenberg 2011 {published data only}
- Tanenberg RJ, Irving GA, Risser RC, Ahl J, Robinson MJ, Skljarevski V, et al. Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomised, noninferiority comparison. Mayo Clinic Proceedings 2011;86(7):615-26. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tian 2005 {published data only}
- Tian Z-W, Song X-F, Li W-L, Feng J. Chinese medicine plus mecobalamin for postherpetic neuralgia in patients with refractory herpes zoster. Zhongguo Linchuang Kangfu 2005;9(17):24-5. [Google Scholar]
Venancio‐Ramirez 2004 {published data only}
- Venancio-Ramirez L, Hernandez-Santos JR, Tenopala-Villegas S, Torres-Huerta JC, Rivera-Leon G, Canseco-Aguilar C. Comparison of oxcarbazepine and gabapentin at standard dose in treatment of pain for postherpetic neuropathy [Comparacion de oxcarbazepina y gabapentina a dosis estandar en el tratamiento del dolor por neuropatia postherpetica]. Revista Mexicana de Anestesiologia 2004;27(3):129-33. [Google Scholar]
Wang 2007 {published data only}
- Wang XP, Mok MS, Li YI, Cai JY. Combined therapy of Super Lizer and Durogesic patch in elderly patients with herpes zoster and diabetes mellitus. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11(13):2589-92. [Google Scholar]
Ward 1981 {published data only}
- Ward J, Armstrong W, Preston E. Pain in the diabetic leg: a trial of aspirin and dipyridamole in diabetic neuropathy. Pharmatherapeutica 1981;2(10):642. [Google Scholar]
Winkler 1999 {published data only}
- Winkler G, Pál B, Nagybéganyi E, Ory I, Porochnavec M, Kempler P. Effectiveness of different benfotiamine dosage regimens in the treatment of painful diabetic neuropathy. Arzneimittel-Forschung 1999;49(3):220-4. [DOI] [PubMed] [Google Scholar]
Additional references
Attal 2010
- Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, et al. European Federation of Neurological Societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. European Journal of Neurology 2010;17(9):1113-e88. [DOI] [PubMed] [Google Scholar]
Baron 2009a
- Baron R. Neuropathic pain: a clinical perspective. Handbook of Experimental Pharmacology 2009;194:3-30. [DOI] [PubMed] [Google Scholar]
Bennett 2011
- Bennett MI. Effectiveness of antiepileptic or antidepressant drugs when added to opioids for cancer pain: systematic review. Palliative Medicine 2011;25(5):553-9. [DOI] [PubMed] [Google Scholar]
Bhandari 2005
- Bhandari M, Haynes RB. How to appraise the effectiveness of treatment. World Journal of Surgery 2005;29(5):570-5. [DOI] [PubMed] [Google Scholar]
Bouhassira 2008
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136(3):380-7. [DOI] [PubMed] [Google Scholar]
Dworkin 2005
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1-2):9-19. [DOI] [PubMed] [Google Scholar]
Dworkin 2010
- Dworkin RH, Turk DC, Peirce-Sandner S, Baron R, Bellamy N, Burke LB, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010;149(2):177-93. [DOI] [PubMed] [Google Scholar]
Farrar 2001
- Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94(2):149-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
Finnerup 2010
- Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain 2010;150(3):573-81. [DOI] [PubMed] [Google Scholar]
Furlan 2010
- Furlan AD, Reardon R, Weppler C, National Opioid Use Guideline Group. Opioids for chronic noncancer pain: a new Canadian practice guideline. Canadian Medical Association Journal 2010;182(9):923-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilron 2005a
- Gilron I, Max MB. Combination pharmacotherapy for neuropathic pain: current evidence and future directions. Expert Review of Neurotherapeutics 2005;5(6):823-30. [DOI] [PubMed] [Google Scholar]
Gilron 2006
- Gilron I, Watson CP, Cahill CM, Moulin DE. Neuropathic pain: a practical guide for the clinician. Canadian Medical Association Journal 2006;175(3):265-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenhalgh 2005
- Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. British Medical Journal (Clinical Research Ed.) 2005;331(7524):1064-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343(18 October):10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2011
- Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, et al. A new definition of neuropathic pain. Pain 2011;152(10):2204-5. [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L’Abbé KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine 1987;107:224–33. [DOI] [PubMed] [Google Scholar]
Maier 2010
- Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 2010;150(3):439-50. [DOI] [PubMed] [Google Scholar]
Manchikanti 2010
- Manchikanti L, Datta S, Gupta S, Munglani R, Bryce DA, Ward SP, et al. A critical review of the American Pain Society clinical practice guidelines for interventional techniques: part 2. Therapeutic interventions. Pain Physician 2010;13(4):E215-64. [PubMed] [Google Scholar]
Max 1991
- Max MB, Laska EM. Single dose analgesic comparisons. In: Max MB, Portenoy RK, Laska EM, editors(s). The Design of Analgesic Clinical Trials. 1 edition. Vol. 18. New York: Raven Press, 1991:55-95. [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything--large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209-16. [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors(s). Systematic Reviews in Pain Research: Methodology Refined. 1 edition. Seattle: IASP Press, 2008:15-23. [Google Scholar]
Moore 2010a
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain - establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386-9. [DOI] [PubMed] [Google Scholar]
Moore 2011
- Moore RA, Derry S, McQuay HJ, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008659.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2012a
- Moore RA, Derry CJ, Derry S, Straube S, McQuay HJ. A conservative method of testing whether combination analgesics produce additive or synergistic effects using evidence from acute pain and migraine. European Journal of Pain 2012;16(4):585-91. [DOI] [PubMed] [Google Scholar]
Moore 2012b
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265-8. [DOI] [PubMed] [Google Scholar]
Nuesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2009
- Patel VB, Manchikanti L, Singh V, Schultz DM, Hayek SM, Smith HS. Systematic review of intrathecal infusion systems for long-term management of chronic non-cancer pain. Pain Physician 2009;12(2):345-60. [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Tarride 2006
- Tarride JE, Collet JP, Choiniere M, Rousseau C, Gordon A. The economic burden of neuropathic pain in Canada. Journal of Medical Economics 2006;9(1-4):55-68. [Google Scholar]
Torrance 2006
- Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. Journal of Pain 2006;7(4):281-9. [DOI] [PubMed] [Google Scholar]
Vorobeychik 2011
- Vorobeychik Y, Gordin V, Mao J, Chen L. Combination therapy for neuropathic pain: a review of current evidence. CNS Drugs 2011;25(12):1023-34. [DOI] [PubMed] [Google Scholar]


