Abstract
Acute myeloid leukemia (AML) presents therapeutic challenges in older adults because of high-risk leukemia biology conferring chemoresistance, and poor functional status resulting in increased therapy-related toxicities. Recent FDA approval of 8 new drugs for AML has increased therapeutic armamentarium and also provides effective low-intensity treatment options. Rational therapy selection strategies that consider individual’s risk of therapy-related toxicities and probability of disease control can maximize benefits of available treatments. Studies have demonstrated that fitness level, measured by geriatric assessment can predict therapy-related toxicities, whereas cytogenetic and mutation results correlate with the probability of responses to standard chemotherapy. We are approaching an era when we move from “one size fits all” approach to personalized therapy selection based on geriatric assessment, genetic and molecular profiling.
Keywords: Acute myeloid leukemia, older adults, therapy selection, geriatric assessment, genetic profiling, molecular mutation
Introduction
The therapeutic landscape of acute myeloid leukemia (AML) has changed with the recent discoveries of novel and targeted therapies. The FDA approval of 8 new AML drugs in a two-year time is unprecedented and is the result of decades of work in leukemia biology and therapeutics. The availability of multiple therapies requires us to develop rational therapy selection strategies to maximize the benefit of therapies and minimize the risk of toxicities for an individual patient. This review will focus on selection of an upfront chemotherapy option for older adults aged ≥60 years with AML, other than acute promyelocytic leukemia.
The fundamental challenges in selecting a therapy option for an older adult include difficulty in predicting the risk of chemotherapy-related toxicities and identifying the probability of achieving remission and long-term disease control.[1] Older adults often have multiple comorbidities and poor functional status that increases the risk of toxicities.[2] AML in older adults is frequently associated with high-risk genetic and molecular features and chemotherapy resistance.[3, 4] Thus, older adults face a double threat of higher toxicity and lower efficacy. These issues may be overcome, to some extent, with the incorporation of geriatric assessment, and genetic and molecular features of AML in selecting therapy. Therapy selection should also consider patient’s preferences and goals of care.[5] For fit older adults, goals of care may include achievement of complete remission, consolidation with allogeneic stem cell transplant and long-term survival. For older adults with poor functional or cognitive status, goals of care may include reduction in therapy-related toxicities, improvement in quality of life, disease control and extension of survival to the extent possible.
Therapy options for initial management of AML
Available therapy options for AML may be divided into intensive or low-intensity chemotherapy. Intensive chemotherapy options include cytarabine and anthracycline (“7+3”) with or without gemtuzumab or midostaurin, or CPX-351 (liposomal preparation of cytarabine and daunorubicin in fixed 5:1 molar ratio) (Table 1). Low-intensity chemotherapy options include hypomethylating agent (HMA) such as azacitidine or decitabine, venetoclax in combination with HMA or low-dose cytarabine (LDAC), glasdegib in combination with LDAC, or single-agent gemtuzumab ozogamicin. Other off-label options in use include single-agent ivosidenib (IDH1 inhibitor) or enasidenib (IDH2 inhibitor), or HMA in combination with targeted or novel agents such as FLT3 inhibitors, or IDH 1 or IDH2 inhibitors. Additional promising therapies not currently approved but undergoing phase III trials for upfront use are listed in table 2.
Table 1.
Landmark trials of newly approved agents for management of acute myeloid leukemia
| Study drugs/arms | Study population | Phase, N | CR, CRi or CRp | OS | Comments |
|---|---|---|---|---|---|
| Intensive therapy options for upfront use | |||||
| 7+3 with vs. without gemtuzumab ozogamicin[34] | 50–70 years with de-novo AML | Phase 3, n=280 | 81% vs. 75%, p=0.2 | 53% vs. 42% at 2 years, p=0.04* | Higher EFS benefit for good-and intermediate-risk AML |
| 7+3 with vs. without midostaurin[38] | <60 years old with FLT3 ITD or TKD mutation | Phase 3, n=717 | 59% vs. 54% CR, p=0.1 | 51% vs. 44% at 4 years, p=0.009 | Similar benefit across FLT3 subtypes, data reflect outcomes in younger adults |
| CPX-351 vs. 7+3[25] | 60–75 years with secondary or therapy-related AML, or AML MRC | Phase 3, n=309 | 48% vs. 33%, p=0.02 | 31% vs. 12% at 2 years, p=0.003 | No CR/CRi or OS benefit in patients with prior HMA exposure (subset analysis)† |
| Low-intensity therapy options for upfront use | |||||
| Venetoclax and hypomethylating agent[77] | Mostly ≥75 years or comorbidities precluding intensive induction | Phase 1b, n=145 | 67% | 17 months; 46% at 2 years | 60% response rate in high- risk AML, 71% in IDH½ mutated AML |
| Venetoclax and LDAC[87, 88] | Mostly ≥75 years or comorbidities precluding intensive induction | Phase ½, n=82 | 42% (54% CR/CRi and 32% MRD response for those treated with 600 mg venetoclax) | 10 months median, 27% at 2 years | 30% response rate in TP53 mutated AML, 72% in IDH½ mutated AML, 44% in FLT3 mutated AML |
| Glasdegib and LDAC vs. LDAC[80] | ≥75 years or comorbidities precluding intensive induction | Randomized phase 2, n= 132 (16 MDS patients) | 24% vs. 5% CR/CRi | 8 vs. 5 months, p=0.002 | OS 4 vs. 2 months in poor- risk patients |
| Gemtuzumab ozogamicin vs. BSC including hydroxyurea[79] | ≥61 years and unfit for intensive induction | Phase 3, n=237 | 27% with gemtuzumab | 24% vs. 10% at 1 year, p=0.005 | 24% of patients ≥81 years‡ |
| Newly approved agents for relapsed or refractory AML | |||||
| Ivosidenib[52] | ≥18 years (median 68 years) with IDH1 mutation (39% with secondary or therapy related AML; 31% with poor-risk AML) | Phase 1, n=258 | 30% | 9 months, median OS | Lower response rate with higher co-mutational burden, with receptor tyrosine kinase pathway mutations, multiple lines of therapies¶ |
| Enasidenib[53] | ≥18 years (median 67 years) with IDH2 mutation (27% with AML MRC, 33% with poor-risk AML) | Phase ½, n=239 | 27% | 9 months, median OS | Lower response rate with higher co-mutational burden, with RAS pathway mutations¶ |
| Gilteritinib[43, 44] | ≥18 years (41% ≥65 years) with FLT3 mutation | Phase III trial, interim analysis of 138 patients | 21% | Not available | No CR/CRi in 12 FLT3 TKD mutated patients |
AML acute myeloid leukemia, BSC best supportive care, CR complete remission, CRi CR with incomplete count recovery, CRp CR with incomplete platelet recovery, EFS event-free survival, HMA hypomethylating agent, LDAC low-dose cytarabine, MRC myelodysplasia-related changes, N number of patients in the trial, OS overall survival
Addition of gemtuzumab improved event-free survival (41% vs. 17% at 2 years), primary endpoint of the study including among subgroups of patients with NPM1 mutated AML and FLT3 ITD mutated AML. OS benefit not seen in subgroup analysis except for FLT3 ITD mutated patients.
For FLT3 mutated patients, CPX-351, compared to 7+3, resulted in a higher CR/CRi rate (68% vs. 27%) without statistically significant increase in OS (median OS, 10 vs. 5 months).
The OS benefit with GO was consistent across most subgroups, and was especially apparent in patients with high CD33 expression status, in those with favorable/intermediate cytogenetic risk profile, and in women.
Variant allele frequency of IDH1 or IDH2 mutation does not affect responses. Also, single co-occurring mutation does not affect responses. Both ivosidenib and enasidenib can achieve molecular remission in a subset of patients who achieve CR.
Table 2.
Preliminary results of agents undergoing phase III trial for upfront management of acute myeloid leukemia in older adults unfit for intensive chemotherapy
| Study drugs/arms | Phase, N | Response rate | Median OS | Ongoing phase III trial |
|---|---|---|---|---|
| Guadecitabine, (2 different doses)[57] | Randomized phase 2, n=107 | 53% CR/CRi, no difference among high-risk AML or sAML | 10 months, no difference among high-risk AML or sAML | Guadecitabine vs. treatment choice (NCT02920008) |
| Ten-day decitabine[50] | Phase 2, n=53 | 64% CR/CRi (74–75% CR/CRi among sAML or tAML and high-risk AML)* | 55 weeks | 10-day decitabine vs. 7+3 (NCT02172872) |
| Pracinostat and azacitidine[56] | Phase 2, n=50 | 52% CR/CRi/MLFS | 62% at 1-year, median OS 13 months in high- risk AML | Azacitidine with or without pracinostat (NCT03151408) |
| Pevonedistat and azacitidine[55] | Phase 1b, n=64 | 50% CR/CRi/PR, no difference in de novo vs sAML, and int vs high risk AML; 80% CR/PR in TP53 mutated AML (n=5) | 7 months | Azacitidine with or without pevonedistat in low-blast AML, CMML, high-risk MDS (NCT03268954) |
| Glasdegib with LDAC, decitabine or 7+3[89] | Phase 1b, n=52 including 7 MDS | CR/CRi 9%, 29% and 54% | 4, 11 and 35 months | Azacitidine or 7+3, with or without glasdegib (NCT03416179) |
| Ivosidenib or enasidenib and azacitidine[84] in IDH mutated† | Phase 1b/2, n=17 (ongoing) | 53% CR/CRi/PR | NA | Azacitidine with or without ivosidenib (NCT03173248) |
| Azacitidine and nivolumab[90]‡ | Phase 2, n=10 (ongoing) | 55% CR/CRp | NA | 4-arm phase II/III trials, azacitidine alone, with nivolumab or midostaurin, or decitabine and cytarabine (NCT03092674) |
| Uproleselan (GMI-1271) and 7+3[54]¶ | Part of a phase 2 trial, n= 25 | 72% CR/CRi, 69% among sAML | 52% at 1 year; median OS of 10 months for sAML | 7+3 with or without uproleselan (phase II/III trial, NCT03701308) |
AML acute myeloid leukemia, CMML chronic myelomonocytic leukemia, CR complete remission, CRi CR with incomplete count recovery, CRp CR with incomplete platelet recovery, MDS myelodysplastic syndrome, MLFS morphologic leukemia-free state, N number of patients in the trial, NA not available, OS overall survival, PR partial remission, sAML secondary AML, tAML therapy-related AML
Another study[51] also demonstrated a high response rate among patients with AML and myelodysplastic syndrome with high-risk cytogenetic (67%) and TP53 mutation (100%) with 10-day decitabine.
An ongoing phase 1 trial of single-agent ivosidenib in newly diagnosed IDH1 mutated AML patients demonstrated a CR/CR with incomplete hematological recovery (CRh) of 41% among 34 patients treated with a dose of 500 mg daily.[91] Early results of a phase 1b/II sub-study from the BEAT AML Master Trial demonstrated a CR/CRi rate of 43% with enasidenib monotherapy in 23 older adults with newly diagnosed AML.[92] Another phase 1 trial of ivosidenib or enasidenib in combination with intensive chemotherapy in IDH mutated AML (n=134) demonstrated high rates of CR/CRi/CRp among de novo and sAML patients treated with ivosidenib (93% and 46%) and enasidenib (73% and 63%). MRD negative rates were 89% and 58% for IDH1 and IDH2 mutated patients, respectively.[93]
Nivolumab in combination with idarubicin and intermediate-dose cytarabine in newly diagnosed AML or high-risk MDS patients aged 18–65 years resulted in a CR/CRi of 77%, MRD negativity rate of 53% and median OS of 18 months (versus 13 months in historical cohort, p=0.2).[94]
No grade ¾ mucositis was seen with uproleselan and 7+3.
Geriatric assessment: predicting chemotherapy tolerance
Geriatric assessment examines multiple health domains including comorbidities, physical function, cognition, presence of depression or geriatric syndromes (e.g. falls, delirium, urinary or stool incontinence), malnutrition, polypharmacy and social isolation.[6–8] Studies in patients with various solid and hematological malignancies have demonstrated that geriatric assessment can predict chemotherapy-related toxicities and overall survival (OS).[6, 9, 10] Serious treatment-related toxicities can worsen physical function, cognition and quality of life of older adults, and increase early mortality and hence, attempts should be made to avoid toxicities. A phase III randomized multicenter trial in advanced non–small-cell lung cancer demonstrated a reduction in toxicity with geriatric assessment-guided therapy selection compared to treatments based on age and performance status.[11] Identification of specific health impairments can also allow opportunities to tailor supportive care interventions such as physical therapy, nutritional support, or treatment of depression or geriatric syndromes to improve functional status.[7, 12] Based on these reasons, the International Society of Geriatric Oncology,[8] American Society of Clinical Oncology,[7] National Comprehensive Cancer Network guidelines [13] and Cancer and Aging Research Group [14] recommend integrating geriatric assessment for therapeutic decision-making and supportive care planning.
Klepin et al., and other groups have demonstrated the feasibility of geriatric assessment before initiation of treatment in AML,[15] and its ability to uncover physical and cognitive [16] impairments even among patients considered fit by standard oncological evaluation.[15, 17] Feasibility of geriatric assessment has also been demonstrated in multicenter trials of patients treated with HMA[17, 18] or intensive chemotherapy (for example, in trials conducted through the Alliance in Clinical Trials in Oncology). Geriatric assessment can predict the risk of chemotherapy-related toxicities and OS in AML.[2] Three domains of geriatric assessment, cognition, physical function and comorbidity burden may be particularly important.[2, 19, 20] Impaired cognition (hazard ratio, HR 2.5, 95% confidence interval, CI 1.2–5.5) and impaired physical function measured by short physical performance battery (HR 2.2, 95% CI 1.1–4.6) were associated with a higher risk of mortality among older adults treated with intensive chemotherapy.[2] A hematopoietic cell transplant comorbidity index (HCT CI) predicted higher risk of mortality in 1100 newly diagnosed adults with AML, aged 20–89 years (median 60 years), who were predominantly treated with intensive chemotherapy. The probability of one-year OS decreased with increasing score on HCT CI (70–74% for a score of 0–2, and 30–50% for a score of ≥3).[21] Taken together, these studies indicate that older adults who are physically fit and do not have cognitive impairment or high comorbidity burden (e.g. a score of ≥3 on HCT CI) can tolerate intensive chemotherapy. Conversely, unfit patients are likely to have significant toxicities from intensive chemotherapy, poor quality of life, and poor OS. Hence, geriatric assessment should include measures of cognition (mini-mental state exam, MMSE or Montreal Cognitive Assessment, MOCA), physical function (instrumental activities of daily living, IADL or short physical performance battery, SPPB) and comorbidities (HCT CI) at the least. Further details regarding comprehensive geriatric assessment and screening tests for frailty assessment, care models and supportive care management are described in recent reviews.[6, 7]
While various components of geriatric assessment are prognostic, prospective trials using geriatric assessment-guided treatments have not been published yet. Key trials that have resulted in approval of drugs provide information on study participants’ age, performance status and comorbidities but not detailed geriatric assessment. This fact indicates that future trials in older patients should utilize and report the results of geriatric assessment. The limitation of other definitions to identify older patients unfit for intensive chemotherapy is highlighted by the European Leukemia Net (ELN) recommendations that indicate, “firm criteria to consider older patients unfit for intensive induction therapy cannot be provided.” Both the ELN[22] and National Comprehensive Cancer Network (NCCN) guidelines[23] recommend taking into consideration poor performance status, significant comorbidities and adverse cytogenetics or molecular mutations to decide against intensive chemotherapy. The NCCN guidelines[23] highlight that “comprehensive geriatric assessments are complementary to assessment of comorbid conditions and are emerging as better predictive tools of functional status.”
Genetic and molecular profiling: predicting efficacy
Multiple large studies demonstrate the prognostic value of cytogenetic risk categories in AML including in older adults specifically.[4, 24] Patients with high-risk AML are less likely to obtain benefit from intensive chemotherapy such as 7+3. For instance, in the phase III randomized trial assessing the role of dose-escalation of anthracycline as a part of 7+3 in older adults, the probability of complete remission (CR) rate (82% vs. 60–65% vs. 34–56%) and two-year OS (60% vs. 31–34% vs. 4–19%) significantly differed between good-, intermediate- and high-risk AML.[24] Older adults with secondary AML or treatment-related AML are less likely to benefit from intensive chemotherapy; the CR and two-year OS rates with 7+3 were 40% and 12% respectively in a recent phase III trial comparing 7+3 versus CPX-351.[25]
The presence of high-risk mutations such as TP53, SRSF2, ASXL1 or secondary-type mutations confers lower benefit from intensive chemotherapy.[3, 26, 27] The presence of secondary-type mutations such as SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2 identifies a group of de novo AML in older adult that behaves like a secondary AML. Approximately half of patients with these mutations do not achieve a CR after intensive chemotherapy.[3] The presence of ASXL1 or SRSF2 mutations and particularly concurrent presence of both mutations are associated with poor prognosis.[26] Overall, the presence of either high-risk cytogenetic or high-risk mutations confers chemotherapy resistance. In such high-risk patients, the use of intensive therapy is associated with low rates of CR, and dismal OS. For these reasons, the NCCN guidelines[23] recommend to use karyotype and several molecular markers for risk stratification and to guide therapy. The ELN guidelines[22] recommend that the results from cytogenetics be obtained preferably within 5 to 7 days, and NPM1 and FLT3 mutational screening within 48 to 72 hours. This may require the use of circulating blasts for genetic testing or quickly performing a bone marrow aspirate and biopsy.
Clinico-genetic risk stratification and therapy selection
Multidisciplinary team approach and development of geriatric leukemia program are important aspects of caring older adults with AML.[1] Early integration of geriatricians, palliative care specialists, physical therapists, social workers, and other specialists can identify health impairments, optimize functional status and management of complex comorbidities, develop supportive care interventions throughout the course of treatment, and provide useful insights regarding patients’ goals of care. Palliative care is significantly underutilized and early integration of palliative care should be a goal.[28] Collaboration with genetic and molecular laboratories should be established to expedite the results of genetic and molecular analyses. Such results should be made available as early as 5–7 days of specimen collection and should guide therapy selection. Awaiting the results of genetic and molecular test for a few days before initiating therapy is not associated with worsening of outcomes in stable older adults with AML.[29] While waiting for genetic and molecular results, patients who are unfit, or have multiple comorbidities, fevers, organ dysfunction or other concerns may need to be admitted in the hospital for close monitoring and treatment of leukemic complications. The BEAT AML trial has established the feasibility of rapid precision medicine approach in older adults with newly diagnosed AML. This trial opened with 3 arms but currently has 11 treatment arms with 7 novel agents, which are assigned based on cytogenetic and molecular characteristics of AML. The initial report of this trial demonstrated that 210 out of 268 patients received treatment assignment, about 95% of whom received assignment within 7 days. Early death and disease progression are uncommon outside of MLL rearranged AML, promising efficacy has been observed in one phase 2 sub-study (enasidenib +/− HMA: 43% CR/CRi rate), and three additional studies have completed phase 1b dose escalation for combined novel agent + HMA therapy.[30]
Selection of specific chemotherapy regimen requires consideration of patient’s fitness level, measured preferably by geriatric assessment, cytogenetic and molecular features of AML, and patients’ preferences including a possibility of financial toxicities (figure 1). Patients who have high-risk cytogenetic features or mutations are less likely to achieve CR and long-term disease control with intensive chemotherapy. Unfit patients with poor functional status, or multiple comorbidities are at a higher risk of significant toxicities, frequent hospitalization, decline in functional status[31] and quality of life,[32] and higher early mortality.[33] For these reasons, outside of clinical trials of novel therapies in combination with standard induction, intensive chemotherapy should be limited to fit patients with good- or intermediate-risk AML or patients meeting indication for CPX-351, who desire long-term disease control and accept a risk of significant toxicities. At the University of Nebraska Medical Center, we are investigating the feasibility and role of genetic results and geriatric assessment-guided therapy selection in older adults with AML (NCT03226418). In our trial, patients with significant physical or cognitive impairment (measured by ADL, IADL, SPPB and MOCA) or HCT CI of ≥3 (≥5 in therapy-related AML, to allow the use of CPX-351) do not receive intensive chemotherapy. Additionally, patients’ preferences of desired level of disease control, acceptability of toxicities of specific treatment, characteristics and burden of treatment (e.g. inpatient versus outpatient administration) can guide therapy selection.[5]
Figure 1. Selection of therapy for older adults with newly diagnosed acute myeloid leukemia (AML), other than acute promyelocytic leukemia.
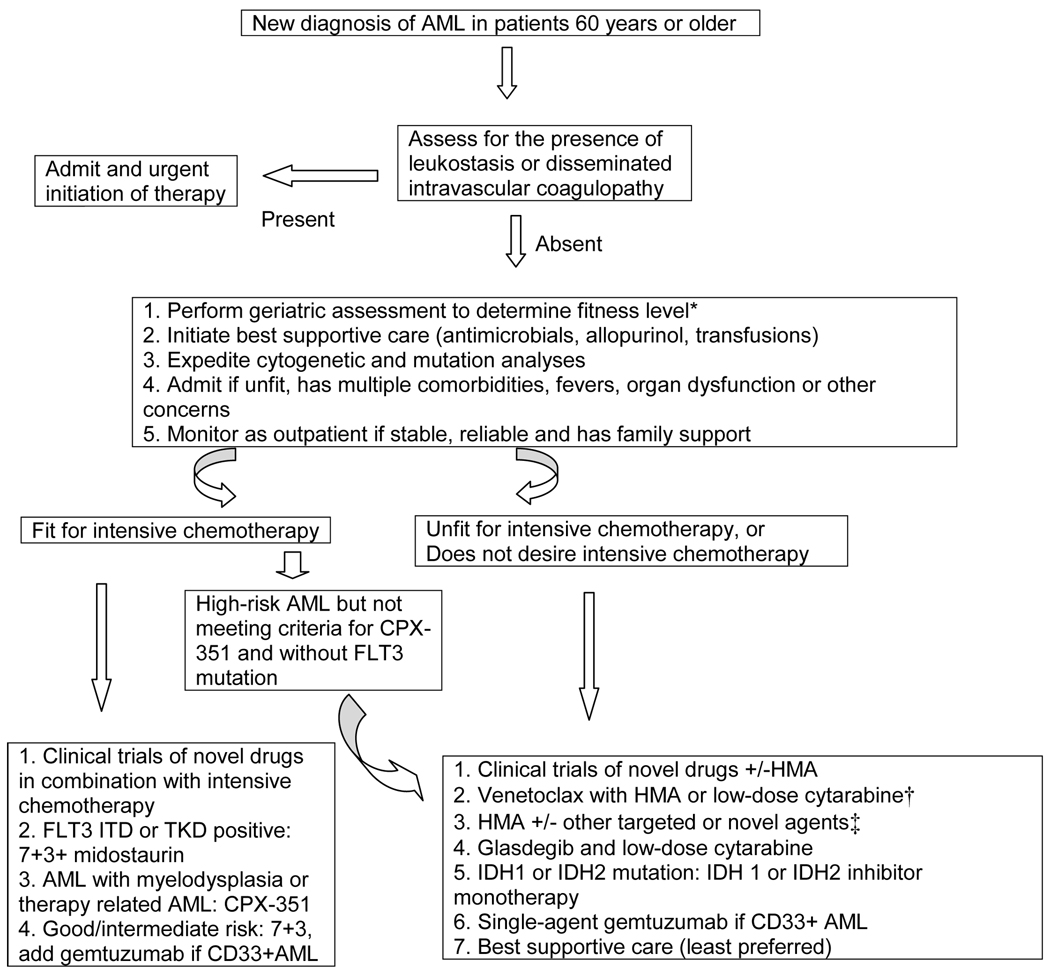
HMA indicate hypomethylating agent.
*Older adults who have poor physical function measured by instrumental activities of daily living or short physical performance battery, moderate to severe cognitive impairment (e.g. Montreal Cognitive Assessment of <25) or high comorbidity burden (e.g. a score of ≥3 on hematopoietic cell transplant comorbidity index) may be considered unfit or poor candidate for intensive chemotherapy. †Venetoclax in combination with hypomethylating agent, given its high efficacy, may be the preferred low-intensity option for patients who can tolerate the combination. ‡Other novel and targeted agents in current use include ivosidenib (IDH1 mutated AML), enasidenib (IDH2 mutated AML), gemtuzumab ozogamicin (CD33+ AML) or FLT3 inhibitors.
Treatment of AML in fit older adults
Good or intermediate risk AML:
Fit older adults with good-risk AML treated with standard 7+3 achieve a high complete remission rate (up to 82%) and OS (60% at 2 years).[24] Among good-risk AML, intensive chemotherapy may be able to control disease for a long time without the use of allogeneic hematopoietic cell transplant (HCT). The probability of CR (60–65%) and OS (31–34% at 2 years) is generally considered acceptable among patients with intermediate-risk AML as well.[24] Intensive chemotherapy can achieve remission faster, may achieve minimal residual disease negative status and allow use of HCT in patients with intermediate-risk AML. As more effective treatment options are approved, and longer follow-up data are available with newer treatments, the use of intensive chemotherapy for intermediate-risk AML may decline in the future. Conversely, the use of geriatric assessment may allow selection of older adults who have lower risk of toxicities and early mortality, thus reducing harm with intensive chemotherapy.
The addition of fractionated doses of gemtuzumab ozogamicin to 7+3 increases event-free survival (EFS) (41% vs. 17% at 2 years), particularly among good- and intermediate-risk AML,[34] hence is recommended for good- and intermediate-risk CD33+ AML. A phase 3 trial in NPM1-mutated AML demonstrated that the addition of gemtuzumab ozogamicin to intensive chemotherapy (idarubicin, cytarabine, etoposide and arsenic trioxide) increased induction-related mortality (7% vs 3%, p=0.02), and decreased risk of relapse (p=0.02) but did not affect event-free survival. This trial utilized etoposide and arsenic trioxide in addition to idarubicin and cytarabine, and induction therapy consisted of two cycles of intensive chemotherapy.[35] Such differences may explain the discrepancy in the results of this trial.
In patients with intermediate-risk AML, who are planned for HCT, concern exists that the use of gemtuzumab ozogamicin may increase the risk of veno-occlusive disease. Hence, an interval of 2–3 months has been suggested between the last dose of gemtuzumab ozogamicin and HCT.[36, 37] This may mean use of gemtuzumab ozogamicin with induction only. A recent analysis of the ALFA-0701 trial demonstrated similar post-transplant survival among patients who did (n=32) versus did not (n=53) receive gemtuzumab ozogamicin before HCT. Of 85 patients in the study, 3 patients in the gemtuzumab ozogamicin arm and 2 in the control arm (both of whom received gemtuzumab ozogamicin as follow-up therapy) developed veno-occlusive disease.[37]
FLT3 ITD or TKD mutated AML:
The addition of midostaurin to 7+3 increases OS among FLT3 ITD or TKD mutated AML among adults younger than 60 years (4-year OS 51% vs. 44%).[38] A phase II trial has demonstrated safety of midostaurin in combination with intensive chemotherapy in adults up to the age of 70 years (34% ≥60 years).[39] Hence, fit older adults are recommended to receive midostaurin in combination with 7+3 for FLT3 mutated AML. Although posaconazole reduces risk of fungal infection over fluconazole and improves survival in AML,[40] drug interaction exists between midostaurin and posaconazole (strong CYP3A4 inhibitor). Isavuconazole is a moderate CYP3A4 inhibitor and may be a safer alternative.[41] Nonetheless, co-administration of midostaurin with strong CYP3A4 inhibitors, without midostaurin dose adjustment, resulted in shorter time to toxicities but no increase in midostaurin-related toxicities. Additionally, increase in dose intensity was associated with improvement in remission and survival.[42] Close monitoring of QTc interval while on midostaurin is also important in older adults, who frequently have cardiac diseases and are on other drugs (e.g. ondansetron and fluoroquinolones) that can prolong QTc interval.
For FLT3 mutated patients, CPX-351 resulted in higher response rate than the standard 7+3 (68% vs. 27%, p=0.01)[25] but a combination of CPX-351 and FLT3 inhibitor has not been studied yet. The addition of gemtuzumab ozogamicin to 7+3 increases EFS in FLT3 ITD mutated patients;[34] however, a combination of 7+3 to midostaurin is generally preferred over combination with gemtuzumab ozogamicin in such patients. Currently, a combination of both midostaurin and gemtuzumab to 7+3 cannot be recommended because of lack of safety data.
Newer FLT3 inhibitors have shown promising results and are undergoing further evaluation in phase III trials. Gilteritinib was recently approved for relapsed/refractory AML based on response rate on an interim analysis of a phase III trial.[43, 44] Preliminary results of an ongoing study in newly diagnosed AML also demonstrate high response rate with gilteritinib in combination with 7+3 (90–100% CR or CR with incomplete count recovery, CRi).[45] A phase 1 trial of quizartinib in combination with 7+3 resulted in a CR/CRi/complete recovery with incomplete platelet recovery (CRp) rate of 74% among newly diagnosed AML patients with or without FLT3 mutation.[46] Quizartinib improved OS (27% vs. 20% at 1 year) over standard salvage chemotherapy in a phase III trial,[47] and is expected to be approved in the near future for relapsed/refractory AML. A preliminary result of study using crenolanib in combination with 7+3 also indicated an improvement in 18-month OS (50–100% vs. 20–40% for historical control) among newly diagnosed patients with concurrent FLT3 and other driver mutation such as RUNX1, WT1 and NPM1 with DNMT3A.[48] Multiple phase III trials are ongoing among patients with newly diagnosed FLT3-mutated AML. These studies will compare crenolanib versus midostaurin in combination with 7+3 in younger patients (18–60 years) (NCT03258931), quizartinib versus placebo in combination with standard chemotherapy in adults up to the age of years (NCT02668653) and gilteritinib versus placebo maintenance post-remission after induction and consolidation therapy (NCT02927262) or after HCT (NCT02997202). The results of these trials will provide further evidence to select specific chemoregimen for FLT3 mutated AML.
Secondary or therapy-related AML:
The use of CPX-351, compared to standard 7+3, improves CR/CRi (48% vs. 33%), and two-year OS (31% vs. 12%) among older adults with secondary AML, AML with myelodysplasia and therapy-related AML (AML with a history of myeloproliferative disorder other than chronic myelomonocytic leukemia was excluded). Although early mortality rates were lower, and rate of HCT was higher with CPX-351, statistical significance could not be reached. For those patients, who underwent HCT, the post-transplant mortality was significantly lower with CPX-351; however, the overall risk of grade ≥3 toxicities is otherwise similar to 7+3.[25] For fit older adults, who are agreeable to intensive chemotherapy followed by HCT, CPX-351 is preferred treatment given the availability of phase III data. Whether CPX-351 is superior to low-intensity chemotherapy is a matter of debate.[49] Several newer treatments including ten-day decitabine,[50, 51] venetoclax and HMA,[52] ivosidenib,[52] enasidenib[53] and newer agents such as uproleselan (GMI-1271) and 7+3,[54] pevonedistat and azacitidine,[55] pracinostat and azacitidine[56] or guadecitabine[57] have shown promising results in patients with high-risk AML including those with secondary AML, therapy-related AML, TP53 mutated AML or AML with myelodysplasia-related changes. Preliminary results of a phase 1b trial with venetoclax in combination with intensive chemotherapy (5-day cytarabine and 2-day idarubicin) in induction treatment naïve older adults demonstrated a CR/CRi of 95% in de novo AML, 42% in secondary/therapy-related AML, 46% in AML with high-risk cytogenetic and 33% in TP53 mutated AML.[58] As further data emerge, the role of these newer treatments in these high-risk diseases may be better established.
Other high-risk AML:
Older adults with high-risk AML, even if fit and eligible for intensive chemotherapy, have a low likelihood of achieving CR (56% for high-risk genetic, 34% for monosomal karyotype) and low two-year OS (19% for high-risk genetic, 4% for monosomal karyotype) when treated with 7+3.[24] The addition of gemtuzumab does not improve outcomes in these patients.[34, 59, 60] A phase III randomized trial demonstrated that azacitidine results in similar or higher OS (10 vs. 6 months ) compared to conventional care regimens (intensive chemotherapy, low-dose cytarabine or best supportive care) or intensive chemotherapy specifically (subgroup analysis) in older adults in general. Patients with high-risk cytogenetics and AML with myelodysplasia-related changes favored azacitidine.[61] Another phase 3 trial compared azacitidine to a combination of fludarabine and cytarabine following granulocyte-colony stimulating factor (G-CSF) priming. Preliminary results demonstrated similar CR rates (21% vs. 27%), and a non-significant trend towards lower MRD-negativity rate (15% vs. 26%, p=0.28) with azacitidine, compared to intensive therapy.[62] Welch et al[51] demonstrated a high response rate among patients with AML and myelodysplastic syndrome with high-risk cytogenetic (67%) and TP53 mutation (100%), when treated with 10-day decitabine, hence 10-day decitabine has been used by many oncologists particularly for patients with TP53 mutation. A randomized phase II trial, however, failed to demonstrate an improvement in CR/CRi/CRp (40% vs. 43%) with 10-day versus 5-day decitabine. One-year OS was 25% for both groups, and median OS did not differ for TP-53 mutated AML based on the duration of decitabine.[63] A phase III trial aims to compare the results of 10-day decitabine versus 7+3 followed by HCT among older adults with AML (NCT02172872). In an exploratory analysis, the combination of venetoclax and HMA resulted in CR/CRi rate of 52–56% among patients with high-risk AML.[52] Interim analysis of another trial of venetoclax and 10-day decitabine demonstrated high CR/CRi and MRD rates in newly diagnosed AML (92% and 52%) and secondary AML (71% and 40%).[64] Taken together, in older adults with high-risk AML, low intensity options such as HMA in combination with venetoclax are preferred over standard intensive chemotherapy regimens. Over time, as novel therapies are integrated to standard intensive chemotherapy, the treatment paradigm may shift.
Treatment of AML in unfit older adults
Unfit patients are often excluded from many clinical trials, especially those that use intensive chemotherapy, hence high-quality data for this specific population remain sparse. This frequently limits our understanding of how to optimally manage such patients and calls for large prospective trials for this specific group of patients. Until such trial results are available, questions include how to identify unfit patients, whether to select intensive or low-intensity chemotherapy and what specific regimen to use? Age and performance status by themselves can predict early mortality to some extent.[33] Several prognostic tools[65–67] (discussed elsewhere[68]) have been developed to predict probability of early mortality. As discussed previously, we prefer the use of geriatric assessment to identify prognostically important health impairments and to develop supportive care interventions. Unfit patients are generally ineligible for and unlikely to benefit from intensive chemotherapy,[61, 69] have higher risk of early mortality[33] and are at a higher risk of decline in functional status,[31] and quality of life after intensive chemotherapy.[32] Unfit patients should be enrolled in clinical trials when possible or treated with low-intensity options. An argument may possibly be made to consider intensive chemotherapy for good-risk AML (e.g. core-binding factor or NPM1 mutated AML without other high-risk features) in older adults who are judged to likely tolerate intensive chemotherapy to fair extent despite other health impairments.
A few multicenter[70] or population-based studies[71] have indicated an improvement in survival or quality of life with the use of intensive chemotherapy; however, these studies were retrospective, the control arm received less effective chemotherapy, and a large retrospective study[72] does not support this findings. For example, in the Swedish AML Registry study,[71] the control arm received therapy such as hydroxyurea, supportive care or LDAC, all less effective than HMA or HMA in combination with newer treatments. Additionally, intensive chemotherapy is rarely used in community centers (<1% in one large study of the US community oncology practices[73]), and many older adults do not even receive chemotherapy at all. For example, in a large NCDB study, one-third of adults aged 71–80 did not receive chemotherapy during the years 2003–2011.[74] As discussed above, the randomized trials[61, 75] have demonstrated HMA to be as effective as conventional care regimens including intensive chemotherapy.
For the aforementioned reasons, HMA such as azacitidine or decitabine (for 5 or 10 days)[51, 61, 69, 76] were considered as preferred agents until recently for many unfit older adults outside of clinical trials. Azacitidine alone results in a response rate of 28% (CR/CRi) and a median OS of 10 months.[61] Ten-day decitabine has been shown by some groups to increase the complete remission rate to approximately 40–50% and median OS of approximately 1 year;[50, 51] however, a randomized phase II trial did not confirm a benefit of 10-day over 5-day decitabine.[63] The integration of novel drugs to HMA can improve response rates and OS, which further argues against the use of intensive chemotherapy in such patients. With the recent approval of venetoclax, a combination of HMA with venetoclax[52] represents a good option for this patient population. The combination of venetoclax to HMA increases the overall response rate (CR, CRi or partial remission) to 63%[52, 77] or higher.[78] Remission can be durable and associated with MRD negative status in some cases.[78] However, venetoclax is associated with myelosuppression and risk of serious infections. Venetoclax requires significant dose reduction when combined with posaconazole; a dose of 50–100 mg has been used.[52] Although the trials leading to approval of venetoclax and HMA required an age of ≥75 years or significant comorbidities, all enrolled patients had Eastern Cooperative Oncology Group (ECOG) PS of 0–2, hence the combination should be cautiously used in patients with poor physical function.[77]
In adults in their 70s or 80s, single-agent gemtuzumab ozogamicin improves response rate (27% CR/CRi) and OS (median OS 5 months) over best supportive care. Gemtuzumab ozogamicin is generally well tolerated, and remission can be achieved after one cycle, faster than the results achieved with HMA. Hence, for patients who do not want to present to hospital for frequent administration of HMA, gemtuzumab ozogamicin is a good option.[79] Glasdegib and LDAC[80] is another option at centers that prefer the use of LDAC. For patients, who are not candidates for any of the aforementioned therapies or do not desire chemotherapy, best supportive care with or without hydroxyurea may be reasonable.
FLT3 mutated AML in unfit patients:
FLT3 inhibitors have been studied in combination with HMA. In 27 older patients with FLT3 ITD mutated AML, the combination of sorafenib and azacitidine resulted in an overall response rate of 78% and median OS of 8.3 months.[81] A preliminary result of a phase 2/3 trial comparing gilteritinib with or without azacitidine versus azacitidine alone (NCT02752035) demonstrate a CR/CRi rate of 67% with gilteritinib in combination with azacitidine.[82] Thus, HMA in combination with a FLT3 inhibitor represent reasonable options for unfit patients. Although a relatively high response rate was seen in FLT3 mutated patients who received venetoclax and HMA combination, the number of patients is small to establish the role of combination for this specific patient population.[52] The trials using ivosidenib[83] and enasidenib[53] had only small number of patients with FLT3 mutated AML to conclude about their role in patients with both FLT3 and IDH1 or 2 mutations.
IDH1 or IDH2 mutated AML in unfit patients:
Off-label use of single-agent ivosidenib[83] and enasidenib[53] may be reasonable frontline therapy in older patients given oral route of administration and overall good safety profile, and are among options suggested by the 2018 National Comprehensive Cancer Network AML guidelines.[23] Differentiation syndrome, leukocytosis with transient increase in circulating blasts and QT prolongation are important side effects of IDH1 and IDH2 inhibitor that need close monitoring and early intervention. Unlike with the use of all trans retinoic acid, differentiation syndrome with IDH ½ inhibitors may be delayed, is less predictable and may occur without leukocytosis.[83] Although ivosidenib has interactions with posaconazole, the concurrent use of posaconazole was allowed in the ivosidenib trial.[83] The combination of ivosidenib to azacitidine has also shown a response rate (CR, CRi or partial remission) of 54% in 11 patients with IDH1 mutated AML in an ongoing phase III trial.[84] Although the risk of grade 3–4 toxicities were higher than single-agent ivosidenib, overall the combination of ivosidenib and HMA is well tolerated, and the risk of differentiation syndrome appears to be lower than single agent on preliminary analysis. Preliminary results also showed a high response rate of 59% (CR/CRi) in 17 IDH1 or IDH2 mutated AML with the use of venetoclax and HMA,[52] thus showing a promise of this combination for IDH1 or IDH2 mutated patients.
Conclusion and future perspectives
Studies and interventions are required to overcome barriers to optimize the benefits of available treatments. Such barriers include low utilization of chemotherapy[74] and allogeneic hematopoietic cell transplantation (only 5.5% adults aged 61–75 years with intermediate and high-risk AML received transplant)[85]. Allogeneic hematopoietic cell transplantation is an important modality to achieve long-term disease control in select patients.[86] HLA typing and pre-transplant evaluation should be expedited in potentially transplant-eligible patients. With the approval of effective low-intensity treatment, multiple stakeholders should collaborate to improve the receipt of chemotherapy. Other barriers include high out-of-pocket expenses and societal cost of newer therapies, difficulties in timely accessing oral chemotherapy, and lack of familiarity of providers who do not treat AML on a routine basis in managing toxicities of newer therapies.
Many trials conducted in the past do not provide enough information on the impact of treatment on functional status such as ability to perform instrumental activities of daily living and functional independence, which are of interest to older patients. A greater understanding of patients’ preferences and values can be crucial in selecting a therapy that meets patients’ goal of care.[5] Data on rates of minimal residual disease clearance, and impact of co-occurring mutations on achievement of remission are important but not readily available for some therapies. We are still awaiting final read out of some of the clinical trials and long-term follow up data of newer treatments. Data from many of the ongoing phase III trials will provide crucial information and point out differences between various treatments, thus further guiding therapy selection. We expect multiple combinatorial trials of approved agents and approval of newer agents in the future, which will continue to change the therapeutic landscape of treatment of AML. While selecting and sequencing therapies may present some challenges to providers, the availability of multiple options for a fatal disease such as AML is certainly a great problem to have.
Highlights.
Older adults with acute myeloid leukemia (AML) often have high-risk disease, and poor functional status.
Recent FDA approval of 8 new drugs for AML has increased therapeutic armamentarium.
Geriatric assessment can predict therapy-related toxicities.
Cytogenetic and mutation results correlate with the probability of remission.
We discuss use of geriatric assessment and genetic profiling to individualize treatment.
Acknowledgements:
Funding: This work was supported by the National Institute of General Medical Sciences, 1 U54 GM115458, which funds the Great Plains Institutional Development Award (IDeA) Clinical Translational Research (CTR) Network, and the Fred and Pamela Buffett Cancer Center Support Grant from the National Cancer Institute (P30 CA036727). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest: VRB reports receiving consulting fees from Pfizer, CSL Behring, Agios, Incyte, Partner Therapeutics and Abbvie, and research funding from Incyte, Tolero Pharmaceuticals, Inc, and National Marrow Donor Program.
References
- [1].Bhatt VR, Gundabolu K, Koll T, Maness LJ. Initial therapy for acute myeloid leukemia in older patients: principles of care. Leuk Lymphoma 2018;59:29–41. [DOI] [PubMed] [Google Scholar]
- [2].Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 2013;121:4287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015;125:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood 2001;98:1312–20. [DOI] [PubMed] [Google Scholar]
- [5].Bhatt VR. Understanding patients’ values and priorities in selecting cancer treatments: Developing a therapy preference scale. J Geriatr Oncol 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- [6].Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood 2018;131:515–24. [DOI] [PubMed] [Google Scholar]
- [7].Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018;36:2326–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy--a systematic review. Leuk Res 2014;38:275–83. [DOI] [PubMed] [Google Scholar]
- [10].Hurria A, Mohile S, Gajra A, Klepin H, Muss H, Chapman A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol 2016;34:2366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Corre R, Greillier L, Le Caer H, Audigier-Valette C, Baize N, Berard H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08–02 Study. J Clin Oncol 2016;34:1476–83. [DOI] [PubMed] [Google Scholar]
- [12].Mohile SG, Velarde C, Hurria A, Magnuson A, Lowenstein L, Pandya C, et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J Natl Compr Canc Netw 2015;13:1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dotan E, Walter LC, Baumgartner J, Browner IS, Burhenn P, Cohen HJ et al. NCCN Clinical Practice Guidelines in Oncology Older Adult Oncology version 2.2019 Available at https://www.nccn.org/professionals/physician_gls/pdf/seniorpdf. Accessed on 01/18/20198.
- [14].Gajra A, Loh KP, Hurria A, Muss H, Maggiore R, Dale W, et al. Comprehensive Geriatric Assessment-Guided Therapy Does Improve Outcomes of Older Patients With Advanced Lung Cancer. J Clin Oncol 2016. 34:4047–8. [DOI] [PubMed] [Google Scholar]
- [15].Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Ellis LR, et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. J Am Geriatr Soc 2011;59:1837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- [17].Deschler B, Ihorst G, Platzbecker U, Germing U, Marz E, de Figuerido M, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hupfer V, Grishina O, Schmoor C, Schlenk RF, Salih HR, Crysandt M, et al. Validation of a Frailty Score Predicting Survival of Elderly, Non-Fit AML Patients Receiving Hypomethylating Therapy: Results of the Decider Trial. Blood 2018;132:720. [Google Scholar]
- [19].Wedding U, Rohrig B, Klippstein A, Fricke HJ, Sayer HG, Hoffken K. Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol 2006;132:665–71. [DOI] [PubMed] [Google Scholar]
- [20].Hshieh TT, Jung WF, Grande LJ, Chen J, Stone RM, Soiffer RJ, et al. Prevalence of Cognitive Impairment and Association With Survival Among Older Patients With Hematologic Cancers. JAMA Oncol 2018;4:686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sorror ML, Storer BE, Fathi AT, Gerds AT, Medeiros BC, Shami P, et al. Development and Validation of a Novel Acute Myeloid Leukemia-Composite Model to Estimate Risks of Mortality. JAMA Oncol 2017;3:1675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel . Blood 2017;129:424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].O’Donnell MR, Tallman MS, Abboud CN, Appelbaum FR, Bhatt VR, Bixby D, et al. NCCN Clinical Practice Guidelines in Oncology, Acute Myeloid Leukemia, version 3.2018 Available at https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed on 12/17/2018.
- [24].Lowenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 2009;361:1235–48. [DOI] [PubMed] [Google Scholar]
- [25].Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J Clin Oncol 2018;36:2684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med 2016;374:2209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Gorlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood 2016;128:686–98. [DOI] [PubMed] [Google Scholar]
- [28].El-Jawahri AR, Abel GA, Steensma DP, LeBlanc TW, Fathi AT, Graubert TA, et al. Health care utilization and end-of-life care for older patients with acute myeloid leukemia. Cancer 2015;121:2840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sekeres MA, Elson P, Kalaycio ME, Advani AS, Copelan EA, Faderl S, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood 2009;113:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Burd A, Levine RL, Shoben A, Mims AS, Borate U, Stein EM, et al. Initial Report of the Beat AML Umbrella Study for Previously Untreated AML: Evidence of Feasibility and Early Success in Molecularly Driven Phase 1 and 2 Studies. Blood 2018;132:559.29853538 [Google Scholar]
- [31].Klepin HD, Tooze JA, Pardee TS, Ellis LR, Berenzon D, Mihalko SL, et al. Effect of Intensive Chemotherapy on Physical, Cognitive, and Emotional Health of Older Adults with Acute Myeloid Leukemia. J Am Geriatr Soc 2016;64:1988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].LeBlanc TW, Wolf SP, El-Jawahri A, Davis DM, Locke SC, Abernethy A. Symptom Burden, Quality of Life, and Distress in Acute Myeloid Leukemia Patients Receiving Induction Chemotherapy: Results of a Prospective Electronic Patient-Reported Outcomes Study. Blood 2015;126:4496. [Google Scholar]
- [33].Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood 2006;107:3481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 2012;379:1508–16. [DOI] [PubMed] [Google Scholar]
- [35].Schlenk RF, Paschka P, Krzykalla J, Weber D, Kapp-Schwoerer S, Gaidzik VI, et al. Gemtuzumab Ozogamicin in NPM1-Mutated Acute Myeloid Leukemia (AML): Results from the Prospective Randomized AMLSG 09–09 Phase-III Study. Blood 2018;132:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Magwood-Golston JS, Kessler S, Bennett CL. Evaluation of gemtuzumab ozogamycin associated sinusoidal obstructive syndrome: Findings from an academic pharmacovigilance program review and a pharmaceutical sponsored registry. Leuk Res 2016;44:61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pautas C, Raffoux E, Lambert J, Legrand O, Benner RJ, Vandendries ER, et al. Outcomes Following Hematopoietic Stem Cell Transplantation in Patients Treated with Chemotherapy with or without Gemtuzumab Ozogamicin for Acute Myeloid Leukemia. Blood 2018;132:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med 2017;377:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schlenk R, Döhner K, Salih H, Kündgen A, Fiedler W, Salwender H-J, et al. Midostaurin in Combination with Intensive Induction and As Single Agent Maintenance Therapy after Consolidation Therapy with Allogeneic Hematopoietic Stem Cell Transplantation or High-Dose Cytarabine (NCT01477606). Blood 2015;126:322. [Google Scholar]
- [40].Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007;356:348–59. [DOI] [PubMed] [Google Scholar]
- [41].Bose P, McCue D, Wiederhold NP, Kadia TM, Borthakur G, Ravandi F, et al. Isavuconazole (ISAV) As Primary Anti-Fungal Prophylaxis in Acute Myeloid Leukemia or Myelodysplastic Syndrome: An Open-Label, Prospective Study. Blood 2018;132:2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ouatas T, Duval V, Sinclair K, Berkowitz N. Concomitant Use of Midostaurin with Strong CYP3A4 Inhibitors: An Analysis from the Ratify Trial. Blood 2017;130:3814. [Google Scholar]
- [43].Perl AE, Cortes JE, Strickland SA, Ritchie EK, Neubauer A, Martinelli G, et al. An open-label, randomized phase III study of gilteritinib versus salvage chemotherapy in relapsed or refractory FLT3 mutation-positive acute myeloid leukemia. Journal of Clinical Oncology 2017;35:TPS7067. [Google Scholar]
- [44].Gilteritinib Prescribing Information Available at: https://astellas.us/docs/xospata.pdf. Accessed on December 4, 2018.
- [45].Pratz KW, Cherry M, Altman JK, Cooper B, Cruz JC, Jurcic JG, et al. Updated Results from a Phase 1 Study of Gilteritinib in Combination with Induction and Consolidation Chemotherapy in Subjects with Newly Diagnosed Acute Myeloid Leukemia (AML). Blood 2018;132:564. [Google Scholar]
- [46].Altman JK, Foran JM, Pratz KW, Trone D, Cortes JE, Tallman MS. Phase 1 study of quizartinib in combination with induction and consolidation chemotherapy in patients with newly diagnosed acute myeloid leukemia. Am J Hematol 2018;93:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cortes JE, Khaled SK, Martinelli G, Perl AE, Ganguly S, Russell NH, et al. Efficacy and Safety of Single-Agent Quizartinib (Q), a Potent and Selective FLT3 Inhibitor (FLT3i), in Patients (pts) with FLT3-Internal Tandem Duplication (FLT3-ITD)-Mutated Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML) Enrolled in the Global, Phase 3, Randomized Controlled Quantum-R Trial. Blood 2018;132:563. [Google Scholar]
- [48].Goldberg AD, Collins RH, Stone RM, Walter RB, Karanes C, Vigil CE, et al. Addition of Crenolanib to Induction Chemotherapy Overcomes the Poor Prognostic Impact of Co- Occurring Driver Mutations in Patients with Newly Diagnosed FLT3-Mutated AML. Blood 2018;132:1436. [Google Scholar]
- [49].Boddu PC, Kantarjian HM, Ravandi F, Garcia-Manero G, Verstovsek S, Jabbour EJ, et al. Characteristics and outcomes of older patients with secondary acute myeloid leukemia according to treatment approach. Cancer 2017;123:3050–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Blum W, Garzon R, Klisovic RB, Schwind S, Walker A, Geyer S, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A 2010;107:7473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Welch JS, Petti AA, Miller CA, Fronick CC, O’Laughlin M, Fulton RS, et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N Engl J Med 2016;375:2023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol 2018;19:216–28. [DOI] [PubMed] [Google Scholar]
- [53].Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017;130:722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].DeAngelo DJ, Jonas BA, Liesveld JL, Bixby DL, Advani AS, Marlton P, et al. Uproleselan (GMI-1271), an E-Selectin Antagonist, Improves the Efficacy and Safety of Chemotherapy in Relapsed/Refractory (R/R) and Newly Diagnosed Older Patients with Acute Myeloid Leukemia: Final, Correlative, and Subgroup Analyses. Blood 2018;132:331. [Google Scholar]
- [55].Swords RT, Coutre S, Maris MB, Zeidner JF, Foran JM, Cruz J, et al. Pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood 2018;131:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Garcia Manero G, Atallah E, Khaled SK, Arellano M, Patnaik MM, Odenike O, et al. A Phase 2 Study of Pracinostat and Azacitidine in Elderly Patients with Acute Myeloid Leukemia (AML) Not Eligible for Induction Chemotherapy: Response and Long-Term Survival Benefit. Blood 2016;128:100. [Google Scholar]
- [57].Kantarjian HM, Roboz GJ, Kropf PL, Yee KWL, O’Connell CL, Tibes R, et al. Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: phase 2 results from a multicentre, randomised, phase ½ trial. Lancet Oncol 2017;18:1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wei AH, Chua CC, Tiong IS, Fong CY, Ting SB, Macraild S, et al. Molecular Patterns of Response and Outcome in the Chemotherapy and Venetoclax in Elderly AML Trial (CAVEAT study). Blood 2018;132:333. [Google Scholar]
- [59].Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 2014;15:986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Onco 2012;30:3924–31. [DOI] [PubMed] [Google Scholar]
- [61].Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015;126:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Paiva B, Martínez-Cuadron D, Bergua Burgues JMM, Vives S, Algarra JL, Tormo M, et al. Role of Measurable Residual Disease (MRD) in Redefining Complete Response (CR) in Elderly Patients with Acute Myeloid Leukemia (AML): Results from the Pethema-Flugaza Phase III Clinical Trial. Blood 2018;132:433. [Google Scholar]
- [63].Short NJ, Kantarjian HM, Loghavi S, Huang X, Qiao W, Borthakur G, et al. Five-Day Versus Ten-Day Schedules of Decitabine in Older Patients with Newly Diagnosed Acute Myeloid Leukemia: Results of a Randomized Phase II Study. Blood 2018;132:84. [Google Scholar]
- [64].Maiti A, DiNardo CD, Cortes JE, Borthakur G, Pemmaraju N, Benton CB, et al. Interim Analysis of Phase II Study of Venetoclax with 10-Day Decitabine (DEC10-VEN) in Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood 2018;132:286. [Google Scholar]
- [65].Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol 2009;145:598–605. [DOI] [PubMed] [Google Scholar]
- [66].Krug U, Rollig C, Koschmieder A, Heinecke A, Sauerland MC, Schaich M, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet 2010;376:2000–8. [DOI] [PubMed] [Google Scholar]
- [67].Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol 2011;29:4417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Walter RB, Estey EH. Management of older or unfit patients with acute myeloid leukemia. Leukemia 2015;29:770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kantarjian H, Ravandi F, O’Brien S, Cortes J, Faderl S, Garcia-Manero G, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 2010;116:4422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sorror ML, Storer BE, Elsawy M, Fathi AT, Brunner AM, Gerds AT, et al. Intensive Versus Non-Intensive Induction Therapy for Patients (Pts) with Newly Diagnosed Acute Myeloid Leukemia (AML) Using Two Different Novel Prognostic Models. Blood 2016;128:216. [Google Scholar]
- [71].Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009;113:4179–87. [DOI] [PubMed] [Google Scholar]
- [72].Quintas-Cardama A, Ravandi F, Liu-Dumlao T, Brandt M, Faderl S, Pierce S, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012;120:4840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ma E, Bonthapally V, Chawla A, Lefebvre P, Swords R, Lafeuille MH, et al. An Evaluation of Treatment Patterns and Outcomes in Elderly Patients Newly Diagnosed With Acute Myeloid Leukemia: A Retrospective Analysis of Electronic Medical Records From US Community Oncology Practices. Clin Lymphoma Myeloma Leuk 2016;16:625–36 [DOI] [PubMed] [Google Scholar]
- [74].Bhatt VR, Shostrom V, Gundabolu K, Armitage JO. Utilization of initial chemotherapy for newly diagnosed acute myeloid leukemia in the United States. Blood Adv 2018;2:1277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine Prolongs Overall Survival Compared With Conventional Care Regimens in Elderly Patients With Low Bone Marrow Blast Count Acute Myeloid Leukemia. Journal of Clinical Oncology 2010;28:562–9. [DOI] [PubMed] [Google Scholar]
- [76].Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012;30:2670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pollyea DA, Stevens BM, Winters A, Minhajuddin M, Gutman JA, Purev E, et al. Venetoclax (Ven) with Azacitidine (Aza) for Untreated Elderly Acute Myeloid Leukemia (AML) Patients (Pts) Unfit for Induction Chemotherapy: Single Center Clinical Experience and Mechanistic Insights from Correlative Studies. Blood 2017;130:181-.28515093 [Google Scholar]
- [79].Amadori S, Suciu S, Selleslag D, Aversa F, Gaidano G, Musso M, et al. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients With Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. J Clin Oncol 2016;34:972–9. [DOI] [PubMed] [Google Scholar]
- [80].Cortes JE, Heidel FH, Heuser M, Fiedler W, Smith BD, Robak T, et al. A Phase 2 Randomized Study of Low Dose Ara-C with or without Glasdegib (PF-04449913) in Untreated Patients with Acute Myeloid Leukemia or High-Risk Myelodysplastic Syndrome. Blood 2016;128:99-. [Google Scholar]
- [81].Ohanian M, Garcia-Manero G, Levis M, Jabbour E, Daver N, Borthakur G, et al. Sorafenib Combined with 5-azacytidine in Older Patients with Untreated FLT3-ITD Mutated Acute Myeloid Leukemia. Am J Hematol 2018. [DOI] [PubMed]
- [82].Esteve J, Schots R, Bernal Del Castillo T, Lee J-H, Wang ES, Dinner S, et al. Multicenter, Open-Label, 3-Arm Study of Gilteritinib, Gilteritinib Plus Azacitidine, or Azacitidine Alone in Newly Diagnosed FLT3 Mutated (FLT3mut+) Acute Myeloid Leukemia (AML) Patients Ineligible for Intensive Induction Chemotherapy: Findings from the Safety Cohort. Blood 2018;132:2736. [Google Scholar]
- [83].DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med 2018;378:2386–98. [DOI] [PubMed] [Google Scholar]
- [84].DiNardo CD, Stein AS, Stein EM, et al. Mutant IDH (MIDH) inhibitors, ivosidenib or enasidenib, with azacitidine (AZA) in patients with acute myeloid leukemia (AML). Abstract #S1562. Presented at the EHA 23rd Congress, June 17, 2018; Stockholm, Sweden. [Google Scholar]
- [85].Bhatt VR, Chen B, Gyawali B, Lee SJ. Socioeconomic and health system factors associated with lower utilization of hematopoietic cell transplantation in older patients with acute myeloid leukemia. Bone Marrow Transplant 2018;53:1288–94. [DOI] [PubMed] [Google Scholar]
- [86].Levin-Epstein R, Oliai C, Schiller G. Allogeneic Hematopoietic Stem Cell Transplantation for Older Patients With Acute Myeloid Leukemia. Curr Treat Options Oncol 2018;19:63. [DOI] [PubMed] [Google Scholar]
- [87].Wei A, Strickland SA, Hou J-Z, Fiedler W, Lin TL, Walter RB, et al. Venetoclax with Low-Dose Cytarabine Induces Rapid, Deep, and Durable Responses in Previously Untreated Older Adults with AML Ineligible for Intensive Chemotherapy. Blood 2018;132:284. [Google Scholar]
- [88].Venetoclax Prescribing Information Available at: https://www.rxabbvie.com/pdf/venclexta.pdf. Accessed on December 4, 2018.
- [89].Savona MR, Pollyea DA, Stock W, Oehler VG, Schroeder MA, Lancet J, et al. Phase Ib Study of Glasdegib, a Hedgehog Pathway Inhibitor, in Combination with Standard Chemotherapy in Patients with AML or High-Risk MDS. Clin Cancer Res 2018;24:2294–303. [DOI] [PubMed] [Google Scholar]
- [90].Daver N, Garcia-Manero G, Basu S, Cortes J, Ravandi F, Jabbour E, et al. Nivolumab (Nivo) in Combination with Azacytidine (AZA) in Relapsed and Frontline Elderly Acute Myeloid Leukemia (AML). Clinical Lymphoma, Myeloma and Leukemia 2017;17:S9. [Google Scholar]
- [91].Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib (AG-120) Induced Durable Remissions and Transfusion Independence in Patients with IDH1-Mutant Untreated AML: Results from a Phase 1 Dose Escalation and Expansion Study. Blood 2018;132:561. [Google Scholar]
- [92].Stein EM, Shoben A, Borate U, Baer MR, Stock W, Patel PA, et al. Enasidenib Is Highly Active in Previously Untreated IDH2 Mutant AML: Early Results from the Beat AML Master Trial. Blood 2018;132:287. [Google Scholar]
- [93].Stein EM, DiNardo CD, Fathi AT, Mims AS, Pratz KW, Savona MR, et al. Ivosidenib or Enasidenib Combined with Induction and Consolidation Chemotherapy in Patients with Newly Diagnosed AML with an IDH1 or IDH2 Mutation Is Safe, Effective, and Leads to MRD-Negative Complete Remissions. Blood 2018;132:560. [Google Scholar]
- [94].Assi R, Kantarjian HM, Daver NG, Garcia-Manero G, Benton CB, Thompson PA, et al. Results of a Phase 2, Open-Label Study of Idarubicin (I), Cytarabine (A) and Nivolumab (Nivo) in Patients with Newly Diagnosed Acute Myeloid Leukemia (AML) and High-Risk Myelodysplastic Syndrome (MDS). Blood 2018;132:905. [Google Scholar]


