Abstract
To assess the effect of hyaluronan on meniscus injury and repair, we had 35 mature New Zealand White rabbits undergo bilateral meniscus injury and repair (19 in the peripheral region, and 16 in the inner region). A longitudinal tear was created in the medial meniscus and repaired with horizontally placed nylon sutures. The left knee joint received intraarticular injections of hyaluronan 1 week after surgery and once a week for 5 weeks. The right knees were injected with phosphate-buffered saline (the carrier vehicle of the hyaluronan). Twelve weeks after repair, tears in the peripheral region showed gross and histologic evidence of healing, with no difference between the vehicle- and hyaluronan-treated menisci. Biochemically, the ratio of reducible collagen cross-links in the hyaluronan-treated menisci was significantly higher than in the vehicle-treated menisci, indicating greater level of collagen remodeling. Biomechanically the vehicle- and hyaluronan-treated menisci demonstrated similarly high tearing load and fracture toughness. In the inner region, poor healing response was observed grossly and histologically in both treatment groups. Water content in the hyaluronan-treated menisci was significantly lower than in the vehicle-treated menisci, indicating a lower level of swelling. Hyaluronan treatment stimulated collagen remodeling in the peripheral region and inhibited swelling of the meniscus repaired in the inner region.
Meniscus tears within the peripheral vascular region have the ability to heal.4,5,9,12,24 Clinical results after arthroscopic meniscus repair have shown completely healed lesions; however, less successful results have also been reported, even in the peripheral region.7,11,19,27 These poor results may be caused by a slow or immature healing response of the meniscus after repair in the peripheral region. On the other hand, the inner region of the meniscus has no blood supply and a low healing potential.4,5 Previous studies have shown that meniscus repairs in the inner region do not heal and thereby have provided the rationale for partial meniscectomy.4,5,13,22 In an effort to extend the level of repair into the avascular region, several techniques have been developed to enhance the potential for healing, such as vascular access channels,5,16,22 synovial flap,16,27 or fibrin clot6,23 with autogenous cultured marrow cells.33
Hyaluronan, a component of proteoglycan aggregate,20,21 has the ability to increase proteoglycan synthesis,1 to prevent the release of glycosaminoglycans from the cartilage matrix,31 and to stimulate tissue inhibitor of metalloproteinase-1 in articular chondrocytes.41 Intraarticular injection of hyaluronan has been shown to decrease the clinical symptoms in the early stages of osteoarthritis.25,29,34 In addition to its effects on cartilage tissue, hyaluronan has the ability to stimulate meniscal collagen remodeling and inhibit swelling after partial meniscectomy,38 to inhibit meniscal matrix degeneration in the ACL-deficient knee,37 to enhance cell migration in ligament healing,40 and to stimulate wound healing8 by upregulating the expression of transforming growth factor-β.10 Our hypothesis was that hyaluronan enhances meniscus healing after injury and repair. Thus, the purpose of this study was to assess the effect of hyaluronan on meniscus repair in the peripheral and inner regions using a rabbit model.
MATERIALS AND METHODS
Thirty-five New Zealand White rabbits, 7 to 8 months old and weighing 3.5 to 4.0 kg, were operated on. These rabbits were skeletally mature with closed epiphyses, as confirmed by roentgenograms. The rabbits were divided into two surgical groups depending on the area of the meniscus to be injured and repaired. The peripheral region of each medial meniscus was injured and repaired in 19 rabbits, and the inner region of each medial meniscus was injured and repaired in 16 rabbits. Each animal was anesthetized with an intramuscular injection of ketamine (80 to 100 mg/kg) and xylazine (7 to 10 mg/kg). Both hindlimbs were shaved and disinfected with povidone-iodine solution. Identical surgical techniques were used in both knees of each rabbit as follows. A medial parapatellar incision and arthrotomy were performed. The patella was dislocated laterally and the knee placed in full flexion. In the region medial to the incision, the joint capsule was separated from the synovial tissues and the medial collateral ligament was exposed. A longitudinal incision was made just anterior to the medial collateral ligament to separate the synovial tissues in front of the medial meniscus and the femur. These synovial tissues were cut transversely, turned over, and grasped to pull out the medial meniscus. Using this surgical procedure, we exposed the medial meniscus without release of the medial collateral ligament.38
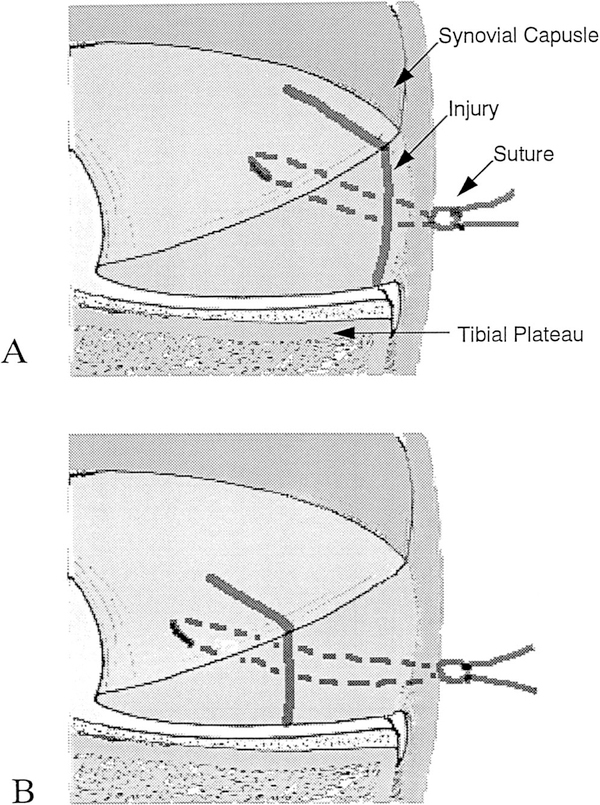
To assess meniscus repair in the peripheral region, we used a No. 11 surgical blade to create a 5- to 7-mm longitudinal tear in the anterior half of the medial meniscus. The tear was made within 2 mm of the meniscosynovial junction (Fig. 1A). To assess meniscus repair in the inner region, we created a 4- to 5-mm longitudinal tear at 50% to 70% of the meniscus width from the free edge of the anterior medial meniscus (Fig. 1B). Immediately after injury, the tear was repaired with two interrupted horizontally placed 5–0 monofilament nylon nonabsorbable sutures (5–0 Ethilon, Ethicon Inc., Somerville, New Jersey). Great care was taken not to injure the articular cartilage surface and the cruciate ligaments.
Figure 1.
Coronal section demonstrating meniscus injury and repair in the peripheral region (A) and in the inner region (B).
After meniscus repair, the joint was manipulated through its full range of motion and irrigated with sterile saline. The capsule, with synovial tissues, was closed with 4–0 monofilament polypropylene sutures, and the skin was closed with 3–0 nylon sutures. After surgery, the rabbits were allowed cage activity (60 × 60 × 40 cm) and the limbs were not immobilized. The rabbits were given a daily intramuscular injection of analgesics (buprenorphine hydrochloride 0.01 to 0.02 mg/kg) for 3 days and of antibiotics (enrofloxacin 1.0 to 1.3 mg/kg) for 7 days after surgery.
In each rabbit, the left knee was injected intraarticularly with 0.3 ml of hyaluronan from highly purified rooster comb (1.0% sodium hyaluronan [molecular weight = 8 × 105] Seikagaku Corp., Tokyo, Japan)38,42 1 week after surgery and once a week for 5 weeks, in a protocol similar to that used clinically.25,29,34 The right knees were injected with phosphate-buffered saline (the carrier vehicle of the hyaluronan). During intraarticular injection the animals were anesthetized intramuscularly with small doses of ketamine and xylazine. All rabbits were sacrificed at 12 weeks after surgery with an intracardiac injection of a mixed solution of pentobarbital sodium, phenytoin sodium, ethyl alcohol, and propylene glycol.
Gross Morphologic Study
Thirteen medial menisci injured and repaired in the peripheral region and eight menisci injured and repaired in the inner region were evaluated after releasing the sutures and were graded morphologically.36 The samples for histologic and vascular studies were not used for gross morphologic studies. The appearance of the meniscus at the repair site was classified as healed (stable bonding at the repair site without evidence of any residual defect of the surface), incompletely healed (stable bonding at the repair site but with evidence of some partial-thickness defect of the surface), or not healed (no evidence of bonding at the repair site, with a full-thickness defect present). The examinations was performed by two observers blinded as to which menisci had been hyaluronan-treated to exclude observer bias.
Histologic Study
Menisci from four rabbits repaired in the peripheral region and from six rabbits repaired in the inner region were fixed in 10% buffered formalin with 1% cetylpyridinium chloride and embedded in paraffin without releasing the sutures. Six-micron thick coronal sections were cut and stained with hematoxylin and eosin for cellular detail and safranin O/fast green for the presence of glycosaminoglycans. The specimens were studied by light microscopy and polarized light for collagen architecture.
Vascular Study
Two animals from each group (meniscus repair in the peripheral region and in the inner region) were anesthetized with intramuscular ketamine and xylazine. In each rabbit, the abdominal descending aorta was exposed and cannulated with a polyethylene catheter. Filtered india ink (150 ml) was perfused into the aorta of each animal with continuous manual pressure and the animals were sacrificed. The modified Spalteholz technique was used for tissue clearing. The menisci, with sutures, were fixed in 10% buffered formalin with 1% cetylpyridinium chloride for 3 days and washed in running water for 8 hours. After dehydration by passing through graded changes of 70% to 100% ethanol, specimens were immersed in methyl salicylate until tissue clearing was advanced. Photographs of the upper view of the menisci were taken with light microscopy.
Biochemical Study
Biochemical characterization of the medial menisci included measurements of hydration, total glycosaminoglycans, and reducible collagen cross-links. Samples were harvested from the anterior half of the medial menisci, that is, the repaired area, without synovial or ligamentous tissues. Biochemical measurements were made not for the repair tissue only, but for the anterior half of the medial meniscus as well.
Hydration
Hydration levels in the repaired menisci from seven animals in each group were determined by measuring wet and dry weights of these tissues and calculating the percentage of water content.
Total Glycosaminoglycans
Total glycosaminoglycan concentration in the repaired menisci from seven animals in each group was determined by measuring the concentrations of hexosamine contained in the tissues. Next, 2 to 4 mg of dry tissue was hydrolyzed in 6N hydrochloric acid at 100°C for 5 hours. Released amino sugars (hexosamine) were quantified as described previously.3 Results are expressed as micrograms of hexosamine per milligram of dry tissue.
Reducible Collagen Cross-Links
The reducible collagen cross-links, dihydroxy-lysinonorleucine (DHLNL) and hydroxylysinonorleucine (HLNL) were quantified3 to assess collagen remodeling in the repaired menisci from five animals in each group (two samples were too small for us to measure collagen cross-links). Samples of lyophilized tissue were reduced with tritium-labeled sodium borohydride and then hydrolyzed in 6N hydrochloric acid. Isolation of the cross-links was accomplished by cation-exchange high-pressure liquid chromatography, and quantitation was achieved with in-line liquid scintillation spectometry. Results are expressed as the ratio of the reducible collagen cross-links (DHLNL:HLNL).
Biomechanical Study
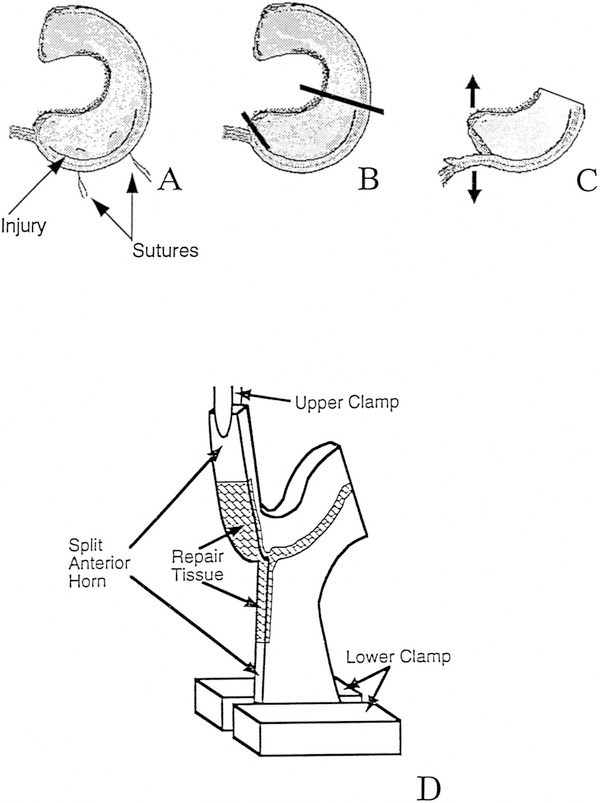
A biomechanical testing technique modified from Roeddecker et al.35 was used to determine the average tearing load and fracture toughness (rate of energy dissipation per fracture area) of the repaired meniscus in the peripheral region from five animals (Fig. 2). After opening the knee joint, the meniscus was washed and soaked in 10 ml of phosphate-buffered saline containing proteinase inhibitors15 (1mM phenylmethylsulfonyl fluoride, 2mM disodium ethylenediaminetetraacetatic acid, 5mM benzamidine-hydrogen chloride, and 10mM N-ethyl maleimide). The surface of each specimen was painted with a solution containing 25 μl phosphate-buffered saline and proteinase inhibitors and 5 μl of india ink to determine the injured and repaired region. Excess ink solution was removed by gentle blotting with a paper tissue that was premoistened with the proteinase inhibitor solution.
Figure 2.
Biomechanical testing of meniscus after peripheral injury and repair. After releasing the suture (A), the meniscus was split from the anterior horn to the beginning of the repaired tissue (B), thus producing two free ends (C). The posterior half of the meniscus was removed, and the two free ends of the anterior portion were inserted into clamps of a materials testing machine (D).
After releasing the suture with a No. 12 surgical blade, the meniscus was split with a No. 11 surgical blade from the anterior horn to the beginning of the repaired tissue (Fig. 2B). This produced two free ends (Fig. 2C), which were inserted into a materials testing machine (Dynastat, IMASS Inc., Accord, Massachusetts) with clamps tightened to a force of 10 to 20 N. During testing, the repaired tissue was split and the tissue was kept moist by irrigation with phosphate-buffered saline with proteinase inhibitors (Fig. 2D). Biomechanical testing was performed by tearing the menisci at 5 mm/min,35 in a mode I opening configuration, in which the gripped portions of the peripheral and inner regions of the meniscus were pulled in opposite directions, causing the repaired area to “open up.” The tearing loads were measured, and the portion within 25% of the peak load was analyzed. The meniscus thickness along the tear path was measured at four points with a current-sensing contact micrometer. The fracture toughness was computed by normalizing the load to the average meniscus thickness.
Biomechanical testing was not performed on meniscus samples repaired in the inner region because of the poor healing response in all sample groups.
Statistical Analysis
Quantitative data from biochemical and biomechanical measurements are presented as mean plus or minus standard error of the mean (SEM). These values were subjected to statistical analysis using analysis of variance. Data from gross morphologic gradings were analyzed by Fisher’s exact test with the level of significance at P = 0.05.
RESULTS
Gross Morphologic Study
Twelve weeks after repair, the meniscus tears in the peripheral region demonstrated gross morphologic evidence of healing. On the basis of the criteria used, 62% (8 of 13) were found to be healed and 39% (5 of 13) were incompletely healed in the vehicle-treated menisci, while 69% (9 of 13) were found to be healed and 31% (4 of 13) were incompletely healed in the hyaluronan-treated menisci. In both the vehicle- and hyaluronan-treated groups, no specimens were not healed. There was no significant difference in gross morphologic healing rates between the vehicle- and hyaluronan-treated menisci repaired in the peripheral region.
After repair in the inner region, a poor healing response was observed. Using the same criteria, 88% (7 of 8) of inner-region tears were found to be not healed and 13% (1 of 8) were incompletely healed in the vehicle-treated menisci, while 75% (6 of 8) were not healed and 25% (2 of 8) were incompletely healed in the hyaluronan-treated menisci. In both groups, no specimens were healed. There was no significant difference in gross morphologic healing rates between the vehicle- and hyaluronan-treated menisci repaired in the inner region.
Histologic and Vascular Study
After meniscus repair in the peripheral region, the histologic evidence of meniscus healing with a small surface gap was demonstrated in three of four vehicle-treated menisci and in three of four hyaluronan-treated menisci (arrow in Fig. 3A). In these menisci, normal glycosaminoglycan distribution was demonstrated by safranin O staining (Fig. 3A). One of four menisci in each group demonstrated a gap that was deeper than half the thickness of the repaired region. By vascular study, a perimeniscal capillary plexus was shown to reach the repaired region in both treatment groups (arrow in Fig. 3B). When comparing the vehicle- and hyaluronan-treated menisci repaired in the peripheral region, there were no obvious differences in the repairs as shown by hematoxylin and eosin staining, safranin O staining, polarized light, and vascular studies.
Figure 3.
Histologic (safranin O/fast green) and vascular examination of medial menisci after meniscus repair in the peripheral region and treatment with hyaluronan. A, a coronal section of a specimen showing meniscus healing with a small surface gap (arrow) and normal distribution of glycosaminoglycans after meniscus repair. B, an upper view of a specimen showing that perimeniscal capillary plexus reached the injured/repaired region (arrow).
After repair in the inner region, a poor healing response was seen histologically in five of six vehicle-treated menisci and in five of six hyaluronan-treated menisci. Glycosaminoglycans were observed by safranin O staining in the region adjacent to the repaired area (Fig. 4A). By vascular study, a perimeniscal capillary plexus, synovial vasculature, or both were separated from the repaired region in these specimens (arrow in Fig. 4B). One of six menisci in each group demonstrated a synovial infiltration that created a membranous tissue in the gap. After meniscus repair in the inner region, there were no obvious differences between the vehicle- and hyaluronan-treated menisci, as shown by hematoxylin and eosin staining, safranin O staining, polarized light, and vascular studies.
Figure 4.
Histologic (safranin O/fast green) and vascular examination of medial menisci after meniscus repair in the inner region and treatment with hyaluronan. A, a coronal section of a specimen showing no healing response (arrow) but the presence of glycosaminoglycans after meniscus repair. The asterisk marks a hole made by a suture string. B, an upper view of a specimen showing synovial vascularity separated from the injured/repaired region (arrow).
Biochemical Study
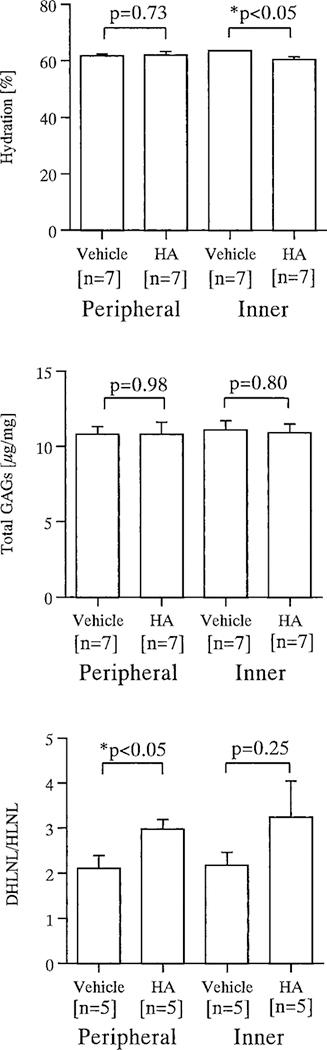
Hydration was not significantly different between the vehicle- and hyaluronan-treated menisci after repair in the peripheral region: 61.7% ± 0.7% versus 62.1% ± 1.2%, respectively. On the other hand, after repair in the inner region, hydration of the vehicle-treated menisci was greater than that of hyaluronan-treated menisci: 63.6% ± 0.3% versus 60.5% ± 1.0%, respectively (P < 0.05). This difference indicated less swelling in the hyaluronan-treated menisci (Fig. 5).
Figure 5.
Biochemical characterization of the medial menisci from vehicle- and hyaluronan-treated knees after meniscus repair in the peripheral and inner regions. Top, water content (based on 7 specimens in each group); Middle, total glycosaminoglycans (based on 7 specimens in each group); Bottom, the ratio of reducible collagen cross-links (DHLNL: HLNL) (based on 5 specimens in each group). Data are illustrated as mean ± standard error. Asterisks denote significant (P < 0.05) differences between vehicle- and hyaluronan (HA)-treated menisci.
The amount of glycosaminoglycan demonstrated was not significantly different between the vehicle-treated (10.8 ± 0.5 μg/mg) and the hyaluronan-treated menisci (10.8 ± 0.8 μg/mg) after repair in the peripheral region. Likewise, there was no significant difference between the vehicle-treated (11.1 ± 0.6 μg/mg) and the hyaluronan-treated menisci (10.9 ± 0.6 μg/mg) after repair in the inner region (Fig. 5).
After meniscus repair in the peripheral region, the ratio of reducible collagen cross-links (DHLNL:HLNL) in the vehicle-treated menisci was 2.11 ± 0.28. The hyaluronan-treated menisci revealed a significantly higher ratio (2.98 ± 0.21) (P < 0.05), demonstrating a greater remodeling of the collagen architecture than in the vehicle-treated menisci. After repair in the inner region, this ratio in the hyaluronan-treated menisci (3.24 ± 0.81) was relatively high, but not statistically significant, when compared with the vehicle-treated menisci (2.18 ± 0.28) (P = 0.25) (Fig. 5).
Biomechanical Study
After meniscus repair in the peripheral region, the average tearing load in the vehicle-treated menisci (3.84 ± 0.58 N) and the hyaluronan-treated menisci (4.24 ± 0.60 N) did not demonstrate a significant difference (P = 0.65). The fracture toughness also demonstrated no significant difference between the vehicle- and hyaluronan-treated menisci (3.79 ± 0.62 kJ/m2 versus 4.06 ± 0.52 kJ/m2) (P = 0.74) (Fig. 6).
Figure 6.
Biomechanical characterization of the medial menisci from vehicle- and hyaluronan-treated knees after meniscus repair in the peripheral region. Top, average load; Bottom, fracture toughness. Data are illustrated as mean ± standard error.
DISCUSSION
In the present study, the healing response of the menisci after injury and repair in two different regions, peripheral and inner, was investigated. Previous studies have shown that the peripheral region of the meniscus contains vasculature proceeding from the perimeniscal capillary plexus.4,5,12 Therefore, meniscus injuries in this region have the ability to heal.9,24 In our rabbit model of meniscus repair in the peripheral region, 12 weeks after meniscus injury and repair 62% of the vehicle-treated menisci and 69% of the hyaluronan-treated menisci were healed on gross morphologic assessment according to the criteria by Scott et al.36 Histologically, 75% of menisci in each group demonstrated a satisfactory healing response. These results support previous basic research4,9,24 and clinical studies7,13,18,19,27,36 that reveal the peripheral region of the meniscus has a healing potential.
Although there were no significant gross morphologic or histologic differences between the vehicle- and hyaluronan-treated menisci after repair in the peripheral region, the ratio of reducible collagen cross-links in the hyaluronan-treated menisci was significantly higher than that in the vehicle-treated menisci (P < 0.05). Dihyroxylysino-norleucine is the major reducible collagen cross-link in embryonic and healing tissue,2,14 and during early collagen remodeling the amount of DHLNL produced is increased relative to HLNL, although both cross-links are present in higher concentrations in healing tissues than in normal tissues. Therefore, the higher ratio of DHLNL to HLNL demonstrates a greater remodeling of the collagen architecture. In our recent study of partial meniscectomy,38 a higher concentration of DHLNL was also found in hyaluronan-treated menisci, which was associated with a newly remodeled translucent tissue after partial meniscectomy.
Biomechanical properties of repaired menisci have been evaluated in dogs,26,32 goats,33 and rabbits.35 After meniscus repair in the peripheral region, the maximum tensile strength of the repaired tissue reaches 80% of the contralateral control,26 and compressive force-displacement behavior of the repaired menisci reveals a nearly normal pattern.32 In our study, the average tearing load and fracture toughness were compared after repair in the peripheral region and were not distinguishably different between the vehicle- and hyaluronan-treated menisci.
In the menisci repaired in the inner region, gross morphologic examination showed that 88% of the vehicle-treated menisci and 75% of the hyaluronan-treated menisci were not healed, while the remainder were incompletely healed. No specimens were shown to be healed. Histologically, 83% in each treatment group demonstrated no healing tissue. These results support previous basic research17,24,28,30,39,43 and clinical studies7,23,27 that reveal the inner region of the meniscus has an extremely low healing potential. In the present study, a major healing response was not detected in either the vehicle- or hyaluronan-treated menisci. However, the hyaluronan-treated menisci demonstrated lower water content (P < 0.05) and a relatively high ratio of reducible collagen cross-links (P < 0.25) when compared with the vehicle-treated menisci. These results suggest hyaluronan may inhibit swelling and increase collagen remodeling after meniscus repair in the inner region. However, the difference in hydration data between the vehicle-treated (63.6% ± 0.3%) and hyaluronan-treated menisci (60.5% ± 1.0%) was small, although statistically significant; therefore it is unclear whether the difference would be clinically significant.
In summary, after meniscus repair in the peripheral region, hyaluronan appears to enhance collagen remodeling biochemically. In the inner region, hyaluronan has the ability to inhibit swelling, although the repaired region cannot be healed successfully. These positive effects of hyaluronan on meniscus repair may demonstrate a capability to enhance the clinical outcome after meniscus injury and repair. Clinical applications of hyaluronan after meniscus repair are currently being used in Asia. More basic-science investigations and longer-term studies should be performed to determine the positive or negative effects of such procedures.
ACKNOWLEDGMENTS
This research was performed with support from NIH Grant AR07484; the Seikagaku Corporation, Tokyo, Japan; and the Malcolm and Dorothy Coutts Institute of Joint Reconstruction and Research, San Diego, California. We also thank Richard D. Coutts, MD, Yuichi Wada, MD, PhD, Kohei Kobayashi, MD, PhD, Choji Shimizu, MD, and Kenji Takahashi, MD, PhD, for their suggestions and Erika Iverson, Robert Healey, Karen Bowden, Thira Maris, and Michael Furniss for their technical assistance.
Footnotes
Presented at the 44th annual meeting of the Orthopaedic Research Society, New Orleans, Louisiana, March 1998.
No author or related institution has received any financial benefit from research in this study. See “Acknowledgments” for funding information.
REFERENCES
- 1.Aibe K, Ryu J, Sano S: Effects of hyaluronic acid on cartilage metabolism in free chondrocytes. J Orthop Sci 1: 268–276, 1996 [Google Scholar]
- 2.Amiel D, Billings E Jr, Akeson WH: Ligament structure, chemistry, and physiology, in Daniel DM, Akeson WH, O’Connor JJ (eds): Knee Liga-ments: Structure, Function, Injury, and Repair. New York, Raven Press, 1990, pp 77–91 [Google Scholar]
- 3.Amiel D, Frank C, Harwood F, et al. : Tendons and ligaments: A morphological and biochemical comparison. J Orthop Res 1: 257–265, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Arnoczky SP, Warren RF: The microvasculature of the meniscus and its response to injury: An experimental study in the dog. Am J Sports Med 11: 131–141, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Arnoczky SP, Warren RF: Microvasculature of the human meniscus. Am J Sports Med 10: 90–95, 1982 [DOI] [PubMed] [Google Scholar]
- 6.Arnoczky SP, Warren RF, Spivak JM: Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J Bone Joint Surg 70A: 1209–1217, 1988 [PubMed] [Google Scholar]
- 7.Asahina S, Muneta T, Yamamoto H: Arthroscopic meniscal repair in conjunction with anterior cruciate ligament reconstruction: Factors affecting the healing rate. Arthroscopy 12: 541–545, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Burd DAR, Greco RM, Regauer S, et al. : Hyaluronan and wound healing: A new perspective. Br J Plastic Surg 44: 579–584, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Cabaud HE, Rodkey WG, Fitzwater JE: Medial meniscus repairs: An experimental and morphologic study. Am J Sports Med 9:129–134, 1981 [DOI] [PubMed] [Google Scholar]
- 10.Cabrera RC, Siebert JW, Eidelman Y, et al. : The in vivo effect of hyaluronan associated protein-collagen complex on wound repair. Biochem Mol Biol Int 37: 151–158, 1995 [PubMed] [Google Scholar]
- 11.Cannon WD Jr, Vittori JM: The incidence of healing in arthroscopic meniscal repairs in anterior cruciate ligament-reconstructed knees versus stable knees. Am J Sports Med 20: 176–181, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Danzig L, Resnick D, Gonsalves M, et al. : Blood supply to the normal and abnormal menisci of the human knee. Clin Orthop 172: 271–276, 1983 [PubMed] [Google Scholar]
- 13.DeHaven KE, Black KP, Griffiths HJ: Open meniscus repair. Technique and two to nine year results. Am J Sports Med 17: 788–795, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Forrest L, Shuttleworth A, Jackson DS, et al. : A comparison between the reducible intermolecular crosslinks of the collagens from mature dermis and young dermal scar tissue of the guinea pig. Biochem Biophys Res Commun 46: 1776–1781, 1972 [DOI] [PubMed] [Google Scholar]
- 15.Frank EH, Grodzinsky AJ, Koob TJ, et al. : Streaming potentials: A sensitive index of enzymatic degradation in articular cartilage. J Orthop Res 5: 497–508, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Gershuni DH, Skyhar MJ, Danzig LA, et al. : Experimental models to promote healing of tears in the avascular segment of canine knee menisci. J Bone Joint Surg 71A: 1363–1370, 1989 [PubMed] [Google Scholar]
- 17.Ghadially FN, Wedge JH, Lalonde JMA: Experimental methods of repairing injured menisci. J Bone Joint Surg 68B: 106–110, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Hamberg P, Gillquist J, Lysholm J: Suture of new and old peripheral meniscus tears. J Bone Joint Surg 65A: 193–197, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Hanks GA, Gause TM, Sebastianelli WJ, et al. : Repair of peripheral meniscal tears: Open versus arthroscopic technique. Arthroscopy 7: 72–77, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Hardingham TE, Muir H: The specific interaction of hyaluronic acid with cartilage proteoglycans. Biochim Biophys Acta 279: 401–405, 1972 [DOI] [PubMed] [Google Scholar]
- 21.Hascall VC, Heinegard D: Aggregation of cartilage proteoglycan. I. The role of hyaluronic acid. J Biol Chem 249: 4232–4241, 1974 [PubMed] [Google Scholar]
- 22.Henning CE, Lynch MA, Clark JR: Vascularity for healing of meniscus repairs. Arthroscopy 3: 13–18, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Henning CE, Lynch MA, Yearout KM, et al. : Arthroscopic meniscal repair using an exogenous fibrin clot. Clin Orthop 252: 64–72, 1990 [PubMed] [Google Scholar]
- 24.Huang TL, Lin GT, O’Connor S, et al. : Healing potential of experimental meniscal tears in the rabbit: Preliminary results. Clin Orthop 267: 299–305, 1991 [PubMed] [Google Scholar]
- 25.Jones AC, Pattrick M, Doherty S, et al. : Intra-articular hyaluronic acid compared to intra-articular triamcinolone hexacetonide in inflammatory knee osteoarthritis. Osteoarthritis Cartilage 3: 269–273, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Kawai Y, Fukubayashi T, Nishino J: Meniscal suture. An experimental study in the dog. Clin Orthop 243: 286–293, 1989 [PubMed] [Google Scholar]
- 27.Kimura M, Shirakura K, Hasegawa A, et al. : Second look arthroscopy after meniscal repair. Clin Orthop 314: 185–191, 1995 [PubMed] [Google Scholar]
- 28.King D: The healing of semilunar cartilages. J Bone Joint Surg 18: 333–342, 1936 [Google Scholar]
- 29.Lohmander LS, Dalen N, Englund G, et al. : Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: A randomized, double blind, placebo controlled multicentre trial. Ann Rheum Dis 55: 424–431, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MD, Ritchie JR, Gomez BA, et al. : Meniscal repair: An experimental study in the goat. Am J Sports Med 23: 124–128, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Miyauchi S, Machida A, Onaya J, et al. : Alterations of proteoglycan synthesis in rabbit articular cartilage induced by intra-articular injection of papain. Osteoarthritis Cartilage 1: 253–262, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Newman AP, Anderson DR, Daniels AU, et al. : Mechanics of the healed meniscus in a canine model. Am J Sports Med 17: 164–175, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Port J, Jackson DW, Lee TQ, et al. : Meniscal repair supplemented withexogenous fibrin clot and autogenous cultured marrow cells in the goat model. AmJSportsMed 24: 547–555, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Puhl W, Bernau A, Greiling H, et al. : Intra-articular sodium hyaluronate in osteoarthritis of the knee: A multicenter, double-blind study. Osteoarthritis Cartilage 1: 233–241, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Roeddecker K, Muennich U, Nagelschmidt M: Meniscal healing: A biomehanical study. J Surg Res 56: 20–27, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Scott GA, Jolly BL, Henning CE: Combined posterior incision and arthroscopic intra-articular repair of the meniscus: An examination of factors affecting healing. J Bone Joint Surg 68A: 847–861, 1986 [PubMed] [Google Scholar]
- 37.Sonoda M, Harwood FL, Amiel ME, et al. : The effects of hyaluronan on the meniscus in the anterior cruciate ligament deficient knee. J Orthop Sci, in press, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Sonoda M, Harwood FL, Wada Y, et al. : The effects of hyaluronan on the meniscus and on the articular cartilage after partial meniscectomy. Am J Sports Med 25: 755–762, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Veth RPH, Den Heeten GJ, Jansen HWB, et al. : Repair of the meniscus: An experimental investigation in rabbits. Clin Orthop 175:258–262, 1983 [PubMed] [Google Scholar]
- 40.Wiig ME, Amiel D, VandeBerg J, et al. : The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: An experimental study in rabbits. J Orthop Res 8: 425–434, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Yasui T, Akatsuka M, Tobetto K, et al. : Effects of hyaluronan on the production of stromelysin and tissue inhibitor of metalloproteinase-1 (TIMP-1) in bovine articular chondrocyte. Biomed Res 13:343–348, 1992 [Google Scholar]
- 42.Yoshioka M, Shimizu C, Harwood FL, et al. : The effects of hyaluronan during the development of osteoarthritis. Osteoarthritis Cartilage 5: 251–260, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Arnold JA, Williams T, et al. : Repairs by trephination and suturing of longitudinal injuries in the avascular area of the meniscus in goats. Am J Sports Med 23: 35–41, 1995 [DOI] [PubMed] [Google Scholar]