Abstract
The prognosis of patients with myelodysplastic syndromes (MDS) following failure of hypomethylating agent (HMA) therapy is poor. Allogeneic hematopoietic cell transplantation (HCT) can be effective in curing patients who have failed therapy with HMA. However, published results have not addressed the outcomes with HCT in this setting. We identified 125 MDS patients who had been treated with HMA and underwent subsequent HCT. Among these, 68 were considered HMA failures, and 57 responders. Failure was defined as progression to higher grade MDS or acute myeloid leukemia (AML), lack of hematological improvement after at least 4 HMA cycles, or loss of response after initial improvement. Response was defined as showing at least hematological improvement. Outcomes were compared using Cox regression. Overall, 73 of 125 HMA-treated patients (58%) had died by the time of last contact. Median follow-up of survivors, measured from HCT, was 41.9 (range, 2.7–98.5) months. The estimated probability of relapse at 3 years was 56.6% and 34.2% among failing and responding patients, respectively (hazard ratio [HR] 2.1, 95% CI, 1.2–3.66, p< 0.01). The estimated probability of relapse-free survival (RFS) at 3 years was 23.8% and 42% in failing and responding patients, respectively (HR for relapse/death 1.88, 95% CI, 1.19–2.95, p< 0.01). The risk of non-relapse mortality was similar for both groups (HR 1.12, 95% CI, 0.52–2.39, p= 0.77). Failure of treatment with HMA was associated with higher risk of post-HCT relapse than observed in patients responding to HMA. Prospective trials are needed to evaluate the efficacy of novel conditioning regimens and post-HCT maintenance strategies in patients who have failed HMA pre-HCT.
Keywords: MDS, allogeneic transplantation, hypomethylating agents, azacitidine, decitabine
INTRODUCTION
The hypomethylating agents (HMA) azacitidine (AZA) and 2-deoxy-azacytidine [decitabine (DEC)] have shown clinical activity in myelodysplastic syndromes (MDS) [1, 2]. They are nucleoside analogues with direct cytotoxicity and the ability to interfere within epigenetic regulation processes. Although AZA treatment was associated with overall survival (OS) benefit when compared to conventional care in MDS patients, the median prolongation of survival was on the order of only nine months [3], and in most patients the life expectancy is reduced by more than 90% in comparison to controls. Hematopoietic cell transplantation (HCT) remains the only therapeutic approach with curative potential in this setting [4].
Retrospective analyses have confirmed a very poor prognosis for patients who failed to respond or whose disease progressed on HMA [5, 6]. Salvage options are limited, but include low or higher dose chemotherapy, investigational agents, HCT or supportive care. While HCT may be the treatment associated with the best outcome based on retrospective analyses, only a third of patients with HMA failure experienced prolonged relapse-free survival [5]. While prior studies have evaluated the use of hypomethylating therapy before HCT [7, 8], no studies to date have focused on the population of patients who have failed HMA.
The aim of the current study was to compare post-HCT outcomes among patients who failed HMA to outcomes of patients who responded to HMA prior to HCT, with a focus on the risk of relapse.
PATIENTS AND METHODS
Patients
Between June 2004 (FDA approval of AZA), and December 2013, 125 patients with MDS or chronic myelomonocytic leukemia (CMML) who had been treated with HMA underwent HCT at the Fred Hutchinson Cancer Research Center (Fred Hutch). The diagnosis was confirmed according to World Health Organization (WHO) 2008 criteria [9]. The disease risk was assessed using the international prognostic scoring system (IPSS) [10], as well as the revised IPSS (IPSS-R) [11]. All patients or their legal guardians had given informed consent to use medical information for research purposes as approved by the Institutional Review Board of the FHCRC in accordance with the Declaration of Helsinki.
In addition to the 5-group cytogenetic classification by Schanz et al. [12] that has been incorporated into the IPSS-R, we also identified patients with monosomal karyotype as defined elsewhere [13]. MDS was considered “secondary” if preceded by cytotoxic therapy for hematological or non-hematological disorders.
Definition of HMA Failures and Responders
Response to HMA was determined using the International Working Group (IWG) 2006 criteria [14]. Treatment failure was defined as loss of response after initial improvement, progression to higher risk MDS or AML, or no hematological improvement after at least 4 HMA cycles.
Assessment of Transplant Outcomes
The day of engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) of ≥ 0.5 × 109/L. Primary graft failure was defined as not reaching an ANC of 0.5 × 109/L by day 28 (day 55 in case of cord blood HCT). Secondary graft failure was defined as a progressive decline in peripheral neutrophil counts after initial recovery. In addition, donor CD3+ T cells <5% on day 28 or donor T-cell decline to <5% after previous evidence of engraftment were considered evidence of primary or secondary graft failure, respectively [15]. The analyses were performed on days 28, 56, 84, 180 and 365, and then as clinically indicated.
Survival time was the time from HCT until death or date of last contact. Relapse-free survival was the time from HCT until death, relapse, or date of last contact. All patients were scheduled for marrow aspiration and biopsy on day 28, day 84, and 1 year post-HCT with morphology, flow cytometric, and cytogenetic analyses. Relapse was defined as the recurrence of any cytogenetic abnormality or immunophenotypic markers that were present pre-HCT or by a recurrence of dysplasia or increased bone marrow myeloblast count detected by morphology. Acute and chronic graft vs. host disease (GvHD) were diagnosed, graded, and treated as previously described [16, 17]. In patients who died following relapse, relapse was considered the cause of death, regardless of the proximal cause of death. Causes of death were attributed using previously described criteria [18].
Statistical Analysis
Estimates of overall survival (OS) and progression-free survival (PFS) probabilities were obtained using the method of Kaplan and Meier [19]. Probabilities of relapse, non-relapse mortality (NRM), and GvHD were summarized using cumulative incidence estimates [20], where death without relapse was considered a competing risk for relapse, and relapse a competing risk for NRM. Death and relapse without GvHD were considered competing risks for GvHD.
Cox regression models were fit in order to compare the cause-specific hazards of failure between treatment failures and responders for each of the above endpoints. Variables considered for inclusion into each regression model included gender, age at HCT, secondary nature of MDS, IPSS-R, AML evolution, minimal identifiable disease by cytogenetics at HCT [21], conditioning regimen intensity, and presence of monosomal karyotype [22] at diagnosis. No adjustments were made for multiple comparisons.
RESULTS
Patient and Transplant Characteristics
Patient characteristics are summarized in Table 1. Patients who failed HMA were more likely to have a higher stage WHO classification, have evidence of disease at time of HCT, and more likely to have evolved to AML than the patients who did not fail HMA. The median age at HCT was 61 (range, 30–76) years among HMA failures, and 61 (range, 34–77) years in HMA responders. Patients who failed to respond received a median of 5 (range, 1–20) HMA cycles compared to 4 (range, 1–40) cycles in responders. The cytogenetic risk profiles at diagnosis were similar for failures and responders, as were the proportions of patients with secondary MDS, at 24% and 23%, respectively. A monosomal karyotype [13] was detected in 14 patients who failed to respond (21%) and 17 patients (30%) who responded.
Table 1.
Patient and Disease Characteristics
| Characteristic | Patients | HMA Failures |
HMA Responders |
P-value |
|---|---|---|---|---|
| Patients, no. (%) | 125 | 68 (54) | 57 (46) | |
| Male / female, no. (%) | 75 (60) / 50 (40) | 41 (60) / 27 (40) | 34 (60) / 23 (40) | 1 |
| Median age at diagnosis, (range), years | 60.2 (29.5 – 76.2) | 59.8 (29.5 – 74.7) | 60.4 (33.3 – 76.2) | 0.93 |
| Median age at HCT, (range), years | 61.4 (30.4 – 77.2) | 61.4 (30.4 – 76.2) | 61.4 (33.9 – 77.2) | 0.93 |
| Diagnosis, no. (%) | 0.017 | |||
| RCMD | 36 (29) | 25 (37) | 11 (19) | |
| RARS | 2 (2) | 2 (3) | - | |
| Del 5q | 1 (1) | 1 (1) | - | |
| MDS-U | 8 (6) | 1 (1) | 7 (12) | |
| RCUD | 9 (7) | 3 (4) | 6 (11) | |
| RAEB-1 | 23 (18) | 9 (14) | 14 (25) | |
| RAEB-2 | 44 (35) | 25 (37) | 19 (33) | |
| CMML | 2 (2) | 2 (3) | - | |
| Etiology, no. (%) | 1 | |||
| Primary | 96 (77) | 52 (76) | 44 (77) | |
| Secondary | 29 (23) | 16 (24) | 13 (23) | |
| Disease duration | 0.12 | |||
| Median time between diagnosis (range) and HCT, months | 9.7 (0.8 – 81.8) | 11 (0.8 – 66.2) | 8.3 (3.2 – 81.8) | |
| Cytogenetics at diagnosis [12], no. (%) | 0.59 | |||
| Very good and good | 58 (46) | 34 (50) | 24 (42) | |
| Intermediate | 23 (18) | 11 (16) | 12 (21) | |
| Poor | 14 (11) | 7 (10) | 7 (12) | |
| Very poor | 28 (22) | 14 (21) | 14 (25) | |
| Unknown | 2 (2) | 2 (3) | 0 | |
| IPSS risk at diagnosis, no. (%) | 0.6 | |||
| Low | 9 (7) | 6 (9) | 3 (5) | |
| lntermediate-1 | 50 (40) | 28 (41) | 22 (39) | |
| lntermediate-2 | 48 (38) | 24 (35) | 24 (42) | |
| High | 16 (13) | 8 (12) | 8 (14) | |
| Not evaluable* | 2 (2) | 2 (3) | - | |
| IPSS-R risk at diagnosis, no. (%) | 0.29 | |||
| Very low | 5 (4) | 4 (6) | 1 (2) | |
| Low | 20 (16) | 11 (16) | 9 (16) | |
| Intermediate | 29 (23) | 16 (24) | 13 (23) | |
| High | 42 (34) | 25 (37) | 17 (30) | |
| Very high | 27 (22) | 10 (15) | 17 (30) | |
| Not evaluable* | 2 (2) | 2 (3) | - | |
| HMA received, no (%) | 0.18 | |||
| Azacitidine | 99 (79) | 58 (85) | 41 (72) | |
| Decitabine | 19 (15) | 7 (10) | 12 (21) | |
| Both agents | 7 (6) | 3 (4) | 4 (7) | |
| Disease status at HCT**, no. (%) | <0.001 | |||
| Complete remission | 39 (31) | 16 (24) | 23 (40) | |
| Marrow CR | 42 (34) | 13 (19) | 29 (51) | |
| Partial remission | 7 (6) | 7 (10) | - | |
| Stable disease | 15 (12) | 10 (15) | 5 (9) | |
| Disease progression or relapse | 22 (18) | 22 (32) | - | |
| AML evolution before HCT, no. (%) | <0.001 | |||
| Yes | 35 (28) | 32 (47) | 3 (5) | |
| No | 90 (72) | 36 (53) | 54 (95) |
1 missing cytogenetics, 1 missing peripheral blood cell counts.
International Working Group 2006 classification [14].
Abbreviations: HMA = hypomethylating agents; HCT = allogeneic hematopoietic cell transplantation; WHO = World Health Organization; RCMD = refractory cytopenia with multilineage dysplasia; RARS = refractory anemia with ring sideroblasts; Del 5q = myelodysplastic syndrome associated with isolated deletion 5q; MDS-U = myelodysplastic syndrome unclassified; RCUD = refractory cytopenia with unilineage dysplasia; RAEB = refractory anemia with excess blasts; CMML = chronic myelomonocytic leukemia; IPSS = International Prognostic Scoring System; IPSS-R = Revised International Prognostic Scoring System; CR = complete remission; AML = acute myeloid leukemia.
Patients were prepared for HCT with various conditioning regimens, categorized on the basis of treatment components and dose intensities (Table 2). The two cohorts were balanced in regards to regimen intensity, donor, and stem cell source. GvHD prophylaxis consisted of mycophenolate mofetil (MMF) and cyclosporine (CSP) or tacrolimus (TAC) in 68 patients (54%), plus sirolimus in 7 patients (6%); methotrexate (MTX) and CSP or TAC in 40 patients (32%), plus sirolimus in 2 patients (2%); and post-transplant cyclophosphamide with or without CSP or TAC in 8 patients (6%).
Table 2.
Transplant Characteristics
| Characteristic, no. (%) | HMA Failures (N=68) | HMA Responders (N=57) |
P value |
|---|---|---|---|
| High intensity conditioning | 41 | 28 | 0.28 |
| BU 16/CY | 18 (26) | 8 (14) | |
| TREO 42/FLU/TBI 2 Gy | 7 (10) | 10 (18) | |
| RAB/FLU/TBI 2 Gy | 5 (7) | 3 (5) | |
| BU 16/FLU | 4 (6) | 5 (9) | |
| RDB/BC8SA/FLU/TBI 2 Gy | 3 (4) | 2 (4) | |
| RAB/FLU/TBI 2 Gy/CY | 2 (3) | - | |
| TBI 12–13.2 Gy/CY | 1 (1) | - | |
| RAB/FLU/TBI/LI 14 Gy | 1 (1) | - | |
| Low intensity conditioning | 27 | 29 | 0.28 |
| FLU/TBI 2–3 Gy | 16 (24) | 14 (25) | |
| FLU/TBI 4–4.5 Gy | 5 (7) | 11 (19) | |
| CY/FLU/TBI 2–4 Gy | 4 (6) | 3 (6) | |
| CLOFA/TBI 2 Gy | 2 (3) | - | |
| BU 8/FLU | - | 1 (2) | |
| Donors | 0.61 | ||
| HLA-matched siblings | 18 (26) | 19 (33) | |
| Haploidentical | 2 (3) | - | |
| Matched unrelated | 31 (46) | 27 (47) | |
| Mismatched unrelated | 10 (15) | 6 (11) | |
| Cord | 7 (10) | 5 (9) | |
| Source of stem cells | 0.91 | ||
| Peripheral blood | 54 (79) | 47 (82) | |
| Marrow | 7 (10) | 5 (9) | |
| Cord | 7 (10) | 5 (9) |
Abbreviations: BU = busulfan (16 mg/kg or 8 mg/kg); CY = cyclophosphamide; FLU = fludarabine; TREO = treosulfan 42 gm/m2; TBI = total body irradiation; Gy=Gray; RAB = radiolabeled antibodies; RDB = radiolabeled dota-biotin; BC8SA = streptavidin-conjugated antiCD45 antibodies; LI = localized irradiation; CLOFA = clofarabine; HLA = human leukocyte antigen.
HMA Failures and Responders
Among the 125 patients who had received at least one cycle of HMA before HCT, 68 (54%) were classified as HMA failures and 57 (46%) as responders. AZA was given at 75 mg/m2/day for 7 days every 28 days. DEC was administrated at 20 mg/m2/day for 5 days every 28 days. Ninety-nine patients (79%) were treated with AZA, 19 patients (15%) with DEC, and 7 patients (6%) received both.
Among the 68 patients who experienced treatment failure, HMA therapy was given as a first-line approach in 64 (94%). Four patients (6%) received HMA as salvage therapy after induction-type chemotherapy. Thirty-one patients who failed first-line HMA were subsequently treated with induction-type chemotherapy before HCT. The decision to initiate induction-type chemotherapy was primarily driven by an increase in bone marrow myeloblast percentage. Among these 31 patients, 26 achieved CR or marrow CR and directly underwent HCT, 5 patients showed no response or disease progression. The other 33 patients who failed first-line HMA went directly to HCT (Figure 1).
Figure 1.
Pre HCT treatments.
Among the 68 patients who experienced treatment failure, 7 (10%) lost the response after initial improvement, 53 (78%) progressed to higher risk MDS or AML, and 8 (12%) had no hematological improvement after at least 4 HMA cycles. Twenty-six of 68 patients (38%) received less than 4 HMA cycles, all of them because of progression to higher risk MDS or AML.
Among 57 responding patients, 4 (7%) had failed to respond to induction-type chemotherapy before HMA. The remaining 53 patients (93%) received HMA as first-line treatment. The best responses to HMA included CR or marrow CR in 44 patients (77%) and PR in 1 patient (2%). Responders included 3 patients (5%) who had progressed to AML before being treated with HMA.
Overall Outcome
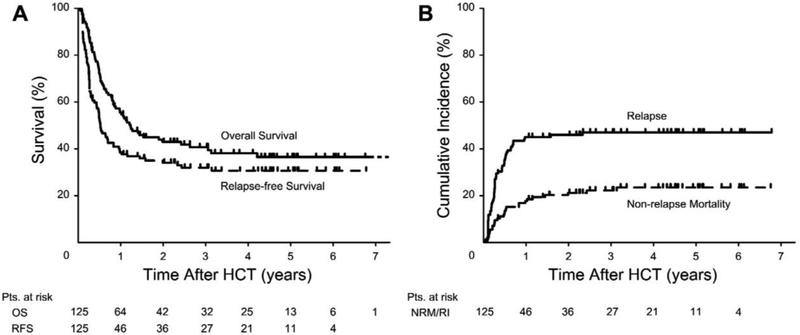
Overall, 73 patients (58.4%) had died by the time of last contact, including 46 patients who had relapsed post-HCT. The median time between HCT and relapse was 2.8 (range, 0.2–27.6) months. Twenty-seven patients died from non-relapse causes. Median follow-up from HCT among the 52 survivors was 41.9 (range, 2.7–98.5) months. OS and RFS at 3 years were 40.8% and 32.1%, and relapse and NRM 46.4% and 21.5%, respectively (Figure 2). Eight patients received a second HCT, 2 for graft failure, and 6 as salvage after relapse.
Figure 2.
A)Unadjusted Kaplan-Maier curves for overall survival and relapse-free survival; B) cumulative incidence of relapse and non-relapse mortality.
Abbreviations: OS = overall survival; RFS = relapse-free survival; HCT = allogeneic hematopoietic cell transplantation.
The estimated probability of grades II-IV (III-IV) acute GvHD was 59.8% (13%). The 3-year estimate of chronic GvHD was 43.1%.
Comparison of HMA Failures and Responders
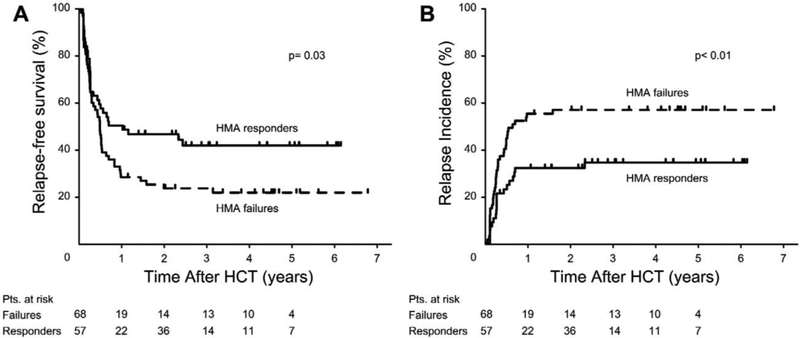
The estimated probability of relapse at 3 years was 56.6% for HMA failures and 34.2% among responders (Figure 3A). After adjusting for covariates, the risk of relapse was significantly higher among HMA failures (HR 2.1, 95% CI, 1.2–3.66, p< 0.009). The estimated probability of RFS at 3 years was 23.8% among HMA failures, and 42% among responders (Figure 3B). The adjusted risk of relapse/death was significantly increased among patients with HMA failure compared to responders (HR 1.88, 95% CI, 1.19–2.95, p< 0.006). However, the risk of NRM was similar among HMA failures and responders (HR 1.12, 95% CI, 0.52–2.39, p= 0.77). The risk of overall mortality was higher among HMA failures than among responders after adjusting for covariates, but the difference was not statistically significant (HR 1.51, 95% CI, 0.93–2.44, p= 0.09). The results of multivariable Cox regression models are summarized in Table 3. Low intensity regimens and monosomal karyotype were associated with a significantly increased risk of death/relapse. Age and monosomal karyotype were associated with a significantly increased risk of death.
Figure 3.
A)Unadjusted Kaplan-Maier curves for relapse-free survival; B) cumulative incidence of relapse.
Abbreviations: HMA = hypomethylating agents; HCT = allogeneic hematopoietic cell transplantation.
Table 3.
Multivariable Cox Regression Models
| Variable | Hazard Ratio | 95% Lower Cl | 95% Upper Cl | P-Value | |
|---|---|---|---|---|---|
| RELAPSE INCIDENCE (Observations = 125; Events = 57) | |||||
| Group | |||||
| HMA Failures | 2.10 | 1.2 | 3.66 | 0.009 | |
| HMA Responders | 1 | ||||
| Conditioning Regimen | |||||
| Low | 1.81 | 1.07 | 3.06 | 0.03 | |
| High | 1 | ||||
| NON-RELAPSE MORTALITY (Observations = 125; Events = 27) | |||||
| Group | |||||
| HMA Failures | 1.12 | 0.52 | 2.39 | 0.77 | |
| HMA Responders | 1 | ||||
| RELAPSE-FREE SURVIVAL (Observations = = 124; Events = 83) | |||||
| Group | |||||
| HMA Failures | 1.88 | 1.19 | 2.95 | 0.006 | |
| HMA Responders | 1 | ||||
| Conditioning Regimen | |||||
| Low | 1.67 | 1.08 | 2.57 | 0.02 | |
| High | 1 | ||||
| Monosomal Karyotype | |||||
| Yes | 1.83 | 1.15 | 2.93 | 0.01 | |
| No | 1 | ||||
| OVERALL SURVIVAL (Observations = 124; Events = 73) | |||||
| Group | |||||
| HMA Failures | 1.51 | 0.93 | 2.44 | 0.09 | |
| HMA Responders | 1 | ||||
| Age at Transplant | 1.03 | 1.01 | 1.06 | 0.02 | |
| Monosomal Karyotype | |||||
| Yes | 1.96 | 1.19 | 3.24 | 0.01 | |
| No | 1 | ||||
Abbreviations: HR = hazard ratio; CI = confidence interval; HMA = hypomethylating agents.
DISCUSSION
This analysis showed inferior transplant outcome in patients who failed pre-HCT HMA therapy when compared to HMA responders. Treatment with HMA can induce hematological improvement or remission in a proportion of patients. However, responses are typically of limited duration, and most patients show disease progression within 2 years [3]. Clinical studies show that 40% to 60% of patients do not achieve clinically relevant responses to HMA therapy [3, 23]. Resistance to HMA may depend on biological characteristics of the disease, such as the presence of specific gene mutations, or up-regulation of anti-apoptotic BCL2 family genes [24].
The management of MDS patients who fail to respond to HMA is challenging. Induction chemotherapy as used for AML has been associated with overall response rates (ORR) of 50%–60% [25]. However, the ORR to AML-type chemotherapy appears to be substantiallylower when given after HMA failure. Moreover, the risk of treatment-related mortality is a major limitation to the application of AML-type chemotherapy in this setting. Other salvage options include low-dose conventional chemotherapeutic agents (e.g. low-dose cytarabine, hydroxyurea, mercaptopurine, or melphalan), best supportive care, investigational treatments, and HCT.
Two major studies investigated the results of salvage treatments in patients who failed HMA. The MD Anderson team presented data on 290 patients with low or intermediate-1 risk disease by IPSS [6]. After HMA failure, 83 patients (29%) were treated with cytarabine-based regimens or additional HMA cycles; 91 patients (31%) received investigational compounds; 26 patients (9%) underwent HCT; 90 patients (31%) received best supportive care only. The ORR was 18% with cytarabine-based therapy, and 16% with investigational compounds. Higher ORR was observed after HCT (69%), but patient selection may have biased outcomes. Overall, the administration of any kind of salvage therapy was associated with improved OS compared to best supportive care, but the gain derived from those strategies tended to be small.
In a French study, information on salvage after HMA failure was available in 270 of 435 high-risk MDS patients (62%) [5]. Among these, 122 received best supportive care, and OS was approximately 4 months. AML-type chemotherapy was given to 35 younger patients or patients with more aggressive disease. Similar to the report by Jabbour et al. [6], the ORR among these 35 patients was 14%, and median OS was 8.9 months. Among 18 patients receiving low-dose chemotherapy, none responded, and the median OS was 7 months. Among 44 patients receiving a second attempt with HMA alone or in combination with histone deacetylase (HDAC) inhibitors, single agent thalidomide or its derivates, or non-registered drugs, the median OS was approximately 13 months. The heterogeneity of this cohort and the mix of treatment strategies renders a comparison to other reports difficult. As indicated before, the 37 patients who received HCT had a median OS of 19.5 months.
HMA have been widely employed as a debulking strategy or maintenance therapy before HCT [7, 8]. Results from prospective clinical trials designed to compare HMA to AML-type chemotherapy before HCT are pending. The present analysis showed that patients who fail to achieve responses to treatment with HMA or progress after initial response to HMA before HCT experience a significantly higher post-HCT incidence of relapse. In contrast, patients who are responding to HMA at time of HCT have a significantly lower rate of relapse. These data should be considered when deciding on the optimal timing of HCT, and patients who are candidates for HCT should be referred early in their treatment course rather than waiting for failure of HMA. Delaying HCT for patients who have responded to HMA may lead to inferior outcomes with HCT if patients progress.
In conclusion, HMA failure is associated with higher risk of relapse after HCT. Additional studies are needed to determine biomarkers associated with response to HMA and develop novel interventions for patients who are likely to have no response to HMA. Both novel conditioning regimens and post-HCT maintenance strategies should be considered for patients who undergo HCT following HMA failure.
HIGHLIGHTS.
We compared outcomes of 68 MDS patients with HMA failure before HCT with 57 responders
HMA agents failure was independently associated to worse progression-free survival
HMA agents failure was associated to a significantly higher 3-year relapse incidence
ACKNOWLEDGMENTS
We thank Elizabeth Soll and Peg Boyle for updating and maintaining the database, all physicians and physician assistants involved in the clinical care of patients transplanted at our Center, the referring physicians, and all patients who agreed to participate in clinical research. We thank Bonnie Larson and Helen Crawford for their help with manuscript preparation. We thank Prof. Benedetto Bruno, and Prof. Mario Boccadoro for their suggestions and support throughout this project.
This work was supported in part by the National Institutes of Health (grants HL084054, HL036444, CA018029, CA015704, and HL088021). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Grant support: National Institutes of Health, grants HL084054, HL36444, CA18029, CA15704, and HL088021.
Financial Disclosure Statement
Bart Scott: Celgence honoraria, advisory committee and research support. There are no other conflicts of interest or financial disclosures associated with this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. [DOI] [PubMed] [Google Scholar]
- 2.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. Journal of Clinical Oncology. 2006;24:3895–3903. [DOI] [PubMed] [Google Scholar]
- 3.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncology. 2009;10:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malcovati L, Hellstrom-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122:2943–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prébet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. Journal of Clinical Oncology. 2011;29:3322–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabbour EJ, Garcia-Manero G, Strati P, et al. Outcome of patients with low-risk and intermediate-1-risk myelodysplastic syndrome after hypomethylating agent failure: a report on behalf of the MDS Clinical Research Consortium. Cancer. 2015;121:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL. Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biology of Blood and Marrow Transplantation. 2012;18:1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damaj G, Duhamel A, Robin M, et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Societe Francaise de Greffe de Moelle et de Therapie-Cellulaire and the Groupe-Francophone des Myelodysplasies. Journal of Clinical Oncology. 2012;30:4533–4540. [DOI] [PubMed] [Google Scholar]
- 9.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes [erratum appears in Blood 1998 Feb 1;91(3):1100]. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 11.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schanz J, Tuchler H, Sole F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. Journal of Clinical Oncology. 2012;30:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breems DA, van Putten WL, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. Journal of Clinical Oncology. 2008;26:4791–4797. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical applications and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. [DOI] [PubMed] [Google Scholar]
- 15.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- 17.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biology of Blood and Marrow Transplantation. 2005;11:945–956. [DOI] [PubMed] [Google Scholar]
- 18.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biology of Blood and Marrow Transplantation. 2007;13:1469–1476. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 20.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in Medicine. 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 21.Festuccia M, Deeg HJ, Gooley TA, et al. Minimal identifiable disease and the role of conditioning intensity in hematopoietic cell transplantation for myelodysplastic syndrome and acute myelogenous leukemia evolving from myelodysplastic syndrome. Biology of Blood and Marrow Transplantation. 2016;22:1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itzykson R, Thepot S, Eclache V, et al. Prognostic significance of monosomal karyotype in higher risk myelodysplastic syndrome treated with azacitidine. Leukemia. 2011;25:1207–1209. [DOI] [PubMed] [Google Scholar]
- 23.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. Journal of Clinical Oncology. 2002;20:2429–2440. [DOI] [PubMed] [Google Scholar]
- 24.Cluzeau T, Robert G, Mounier N, et al. BCL2L10 is a predictive factor for resistance to azacitidine in MDS and AML patients. Oncotarget. 2012;3:490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estey E, Thall P, Beran M, Kantarjian H, Pierce S, Keating M. Effect of diagnosis (refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, or acute myeloid leukemia [AML]) on outcome of AML-type chemotherapy. Blood. 1997;90:2969–2977. [PubMed] [Google Scholar]