Abstract
Background:
In people with atrial fibrillation (AF), periods of sinus rhythm present an opportunity to detect pro-thrombotic atrial remodeling through measurement of P-wave indices (PWIs)—prolonged P-wave duration, abnormal P-wave axis, advanced inter-atrial block, and abnormal P-wave terminal force in lead V1. We hypothesized that addition of PWIs to the CHA2DS2-VASc score would improve its ability to predict AF-related ischemic stroke.
Methods:
We included 2229 Atherosclerosis Risk in Communities (ARIC) study and 700 Multi-Ethnic Study of Atherosclerosis (MESA) participants with incident AF who were not on anticoagulants within 1 year of AF diagnosis. PWIs were obtained from study visit ECGs before development of AF. AF was ascertained using study visit ECGs and hospital records. Ischemic stroke cases were based on physician adjudication of hospital records. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) of PWIs for ischemic stroke. Improvement in 1-year stroke prediction was assessed by C-statistic, categorical net reclassification improvement (NRI), and relative integrated discrimination improvement (IDI).
Results:
Abnormal P-wave axis was the only PWI associated with increased ischemic stroke risk (HR, 1.84; 95% CI, 1.33-2.55) independent of CHA2DS2-VASc variables and that resulted in meaningful improvement in stroke prediction. The beta estimate was approximately twice that of the CHA2DS2-VASc variables, thus abnormal P-wave axis was assigned 2 points to create the P2-CHA2DS2-VASc score. This improved the C-statistic (95% CI) from 0.60 (0.51-0.69) to 0.67 (0.60-0.75) in ARIC and 0.68 (0.52-0.84) to 0.75 (0.60-0.91) in MESA (validation cohort). In ARIC and MESA, the categorical NRI (95% CI) were 0.25 (0.13-0.39) and 0.51 (0.18-0.86), respectively, and the relative IDI (95% CI) were 1.19 (0.96-1.44) and 0.82 (0.36-1.39), respectively.
Conclusions:
Abnormal P-wave axis—an ECG correlate of left atrial abnormality—improves ischemic stroke prediction in AF. Compared with CHA2DS2-VASc, the P2-CHA2DS2-VASc is a better prediction tool for AF-related ischemic stroke.
Keywords: Atrial Fibrillation, Stroke, CHA2DS2-VASc score
Introduction
Atrial fibrillation (AF) is associated with a 5-fold increased risk of stroke.1 Current practice guidelines recommend risk stratification with the CHA2DS2-VASc (congestive heart failure, hypertension, age, diabetes, stroke, vascular disease, sex) score to identify appropriate candidates for systemic anticoagulation to prevent stroke.2,3 Unfortunately, emerging clinical evidence has highlighted limitations in the predictive value of the CHA2DS2-VASc score.4–9 Use of markers that more directly reflect the underlying mechanisms of AF-related thromboembolism may help improve the current risk stratification paradigm.
There has been increasing recognition of the contribution of pro-thrombotic remodeling of the atrial architecture to thromboembolism risk.6,10 Abnormal atrial conduction measured through analysis of P-wave morphology—P-wave indices (PWIs)—has been associated with atrial remodeling11–14 and stroke.15–21 In people with AF, periods of sinus rhythm present an opportunity to detect underlying atrial remodeling through measurement of PWIs. We hypothesized that addition of PWIs to the CHA2DS2-VASc score would augment stroke risk prediction in individuals with AF. We tested this hypothesis in the Atherosclerosis Risk in Communities (ARIC) study and validated our findings in the Multi-Ethnic Study of Atherosclerosis (MESA), two large prospective community-based cohort studies in the United States.
Methods
Data Availability
Some access restrictions apply to the data underlying the findings. The consent signed by study participants does not allow the public release of their data. Data from the ARIC study can be accessed by contacting the ARIC Coordinating Center (aricpub@unc.edu). Data from the MESA study can be accessed by contacting the MESA data coordinating center (chsccweb@u.washington.edu).
Study Population
The ARIC study was designed to evaluate risk factors, etiology, and clinical manifestations of atherosclerotic coronary heart disease in the general population. Between the years of 1987 and 1989, 15792 men and women aged 45-64 years were recruited and enrolled from four United States communities (Washington County, MD; Forsyth County, NC; Jackson, MS; and suburban Minneapolis, MN). After the baseline examination, participants have completed 5 follow-up study visits, the most recent in 2016-17. In between study visits, participants (or proxy) have been contacted annually by telephone (semi-annually since 2012) to ascertain information on hospitalizations and deaths. Active community-wide surveillance of local hospitals has been performed to identify additional hospitalizations and cardiovascular events. Further details regarding outcome ascertainment procedures, study design, and population statistics have been previously described.22 Approval for the study was obtained from the institutional review board on human research at each participating institution and all participants provided informed consent.
The present analysis utilized data obtained from the baseline study visit in 1987-89 through 2013. We excluded participants with missing ECG data (n=242), missing P-wave indices at baseline (n=45), prevalent AF (n=37), and those who were not white or black from all study sites and nonwhite from Minneapolis and Washington County (due to small sample size; n=103) resulting in a baseline cohort of 15365 participants. We then identified 2625 cases of incident AF after the baseline study visit. Due to the potential bias introduced by anticoagulant use when studying stroke risk, participants with anticoagulant use within 1 year of AF diagnosis (n=172) were excluded. We also excluded those without follow-up beyond AF date (n=224) resulting in a final cohort of 2229 participants with incident AF.
Two-dimensional (2D) and three-dimensional (3D) echocardiograms were performed during visit 5 (2011-2013). 2D echocardiograms were performed on 6,538 participants during visit 5. As previously done, exclusions were made for race (n=42) resulting in 6,496 participants. Of these, 6,008 had interpretable ECGs with PWI values. Of these, 5,830 had interpretable 2D echocardiogram data. 3D echocardiograms were performed on 3,035 participants during visit 5. Exclusions were made for race (n=16), poor image quality (n=1779), severe valvular heart disease or history of valve surgery (n=64), and missing body mass index or body surface area data (n=34) resulting in a final analysis cohort of 1,142 participants with 3D echocardiograms.
The MESA study was designed to investigate the prevalence, natural history, and correlates of subclinical cardiovascular disease. Between the years of 2000 and 2002, 6814 men and women aged 45-85 without prevalent cardiovascular disease were recruited and enrolled from six United States communities (Baltimore City and Baltimore County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, NY; and St. Paul, MN). After the initial baseline study visit, there have been five additional follow-up visits, the most recent ongoing in 2016-18. Participants are contacted on an annual basis to identify new hospitalizations and medical diagnoses. Death certificates and medical records are then reviewed for purposes of outcome ascertainment. Further details on study protocol and procedures have been previously described.23 Approval for the study was obtained from the institutional review board on human research at each participating institution and all participants provided informed consent.
The present analysis utilized data obtained during the baseline visit in 2000-02 through 2014. We excluded participants with prevalent AF (n=66) or missing ECG or P-wave indices at baseline (n=49), and identified 876 cases of incident AF. We then excluded those without follow-up beyond the date of AF diagnosis (n=117), oral anticoagulant use within 1 year of AF diagnosis (n=54), and those with invalid P-wave axis measurements (n=5) resulting in a final cohort of 700 participants with incident AF.
Measurement of P-Wave Indices
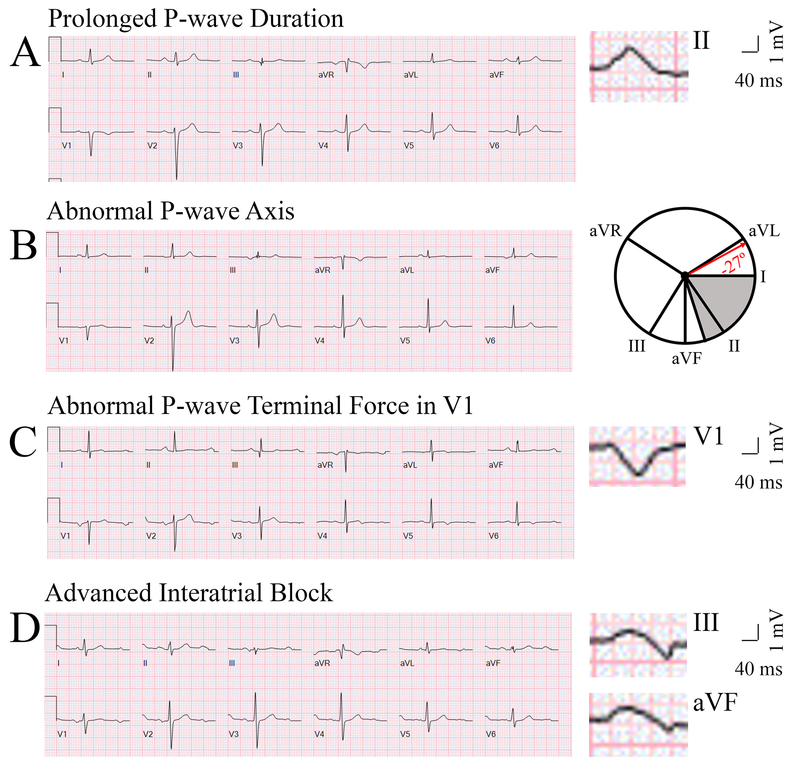
Measurement of PWIs in ARIC17 and MESA23 was done in a consistent manner and has been described. We evaluated P-wave axis, P-wave duration, advanced inter-atrial block (aIAB), and P-wave terminal force in lead V1 using a standard 12-lead ECG (Figure 1). P-wave axis is a measure of the net direction of atrial depolarization. It is determined by measuring net positive or negative P-wave deflections on all six limb leads and calculating the net direction of electrical activity using the hexaxial reference system (Figure 1). It is a standard, computer-generated ECG index that was reported on all ECGs in ARIC and MESA. Abnormal P-wave axis (aPWA) was defined as any value outside 0-75°. P-wave duration is a reflection of the time required for right and left atrial depolarization. It was measured from the conclusion of the T-P segment (P wave onset) to return to baseline (PR interval). For biphasic P-waves, P-wave duration encompassed both positive and negative deflections from baseline. Prolonged P-wave duration (PPWD) was present if the maximum P-wave duration in any lead was >120 ms on a standard 12-lead ECG. aIAB is an indicator of inter-atrial conduction block in Bachman’s bundle such that the left atrium is activated superiorly. It was defined as PPWD + biphasic P-wave morphology in leads III and aVF with biphasic morphology or notched morphology in lead II. P-wave terminal force in lead V1 is a measure of left atrial activation. It is determined by multiplying the duration (ms) and the depth (μV) of the downward deflection (terminal portion) of the P-wave in lead V1. Abnormal P-wave terminal force in lead V1 (aPTFV1) was defined as ≤ −4000 μV*ms. All P-wave indices were computed from the closest sinus rhythm ECG prior to AF diagnosis.
Figure 1.
Representative electrocardiogram tracings of abnormal P-wave indices—Prolonged P-wave duration (A), Abnormal P-wave axis (B), Abnormal P-wave terminal force in V1 (C), and Advanced Interatrial block (D). The maximal P-wave duration on panel A is seen in lead II (136 ms). The P-wave axis on panel B is −27°. The grey area on the hexaxial reference system (lead I 0°, lead II 60°, aVF 90°, aVR −150°, aVL −30°) in panel B represents normal P-wave axis (0-75°). The P-wave terminal force on panel C is −9632 μV*ms (amplitude −112 μV, duration 86 ms). The maximal P-wave duration on panel D is seen in lead III (136 ms). Biphasic P-waves can be seen in III and aVF.
Stroke Classification
Details on stroke identification and specific classification criteria for ischemic stroke in the ARIC24 and MESA25 cohorts have been described. In ARIC, potential cases of stroke were identified from review of hospital records and death certificates. Classification of stroke was then adjudicated by physicians with assistance of a computerized algorithm utilizing validated criteria from the National Survey of Stroke by the National Institute of Neurological Disorders. Strokes were classified as definite or probable thrombotic stroke, definite or probable cardioembolic stroke, definite or probable subarachnoid hemorrhage, definite or probable brain hemorrhage, and possible stroke of undetermined type. The primary endpoint in our study was ischemic stroke, which included all thrombotic and cardioembolic strokes (definite and probable).
In MESA, potential cases of stroke were identified from review of medical records and death certificates. Stroke was defined as a focal neurologic deficit lasting 24 hours or until death or if the deficit lasted <24 hours and there was a clinically relevant lesion on brain imaging. Cases with focal neurological deficits secondary to brain trauma, tumor, infections, or other nonvascular cause were excluded. Cases were physician adjudicated by members of the MESA study events committee. Strokes were sub-classified as subarachnoid hemorrhage, intra-parenchymal hemorrhage, other hemorrhage, brain infarction or other stroke. We included all non-hemorrhagic strokes in our analysis. Due to the relatively low number of stroke events, we elected to use a composite primary endpoint of stroke and transient ischemic attack (TIA) for the MESA analysis. In MESA, TIA was defined as one or more documented episodes of focal neurologic deficit lasting 30 seconds to 24 hours without brain imaging documenting stroke.
Assessment of Covariates
The covariates included in our analysis—age, sex, heart failure26,27, hypertension28,29, diabetes29,30, myocardial infarction27,31, stroke, TIA32, and peripheral arterial disease27,33 —were derived from data obtained during participant interviews, clinical examinations, and review of medical records as previously described. Prevalent (at baseline visit) and incident (during follow-up) variables were included. Precise definitions were comparable in ARIC and MESA. All covariates in ARIC and MESA were ascertained at the time of AF diagnosis or at the most recent study visit exam prior to AF diagnosis.
AF Ascertainment
AF cases were ascertained by review of ECGs during study visits and hospital discharge records. AF associated with cardiothoracic surgery was not considered. Further details on specific ascertainment procedures in ARIC34 and MESA35 have been previously described.
Anticoagulation Status
Use of anticoagulants was captured through participant interviews during each study visit in MESA and ARIC and annually in ARIC after 2006. During the time period of our analysis in both ARIC and MESA, Coumadin was the only anticoagulant captured during participant interviews.
Echocardiography
2D and 3D echocardiography analysis protocols have been previously described.36,37 Briefly, two-dimensional echocardiograms were performed using dedicated Philips iE33 Ultrasound systems with Vision 2011. Three-dimensional echocardiograms were performed using a dedicated Philips X3-1 transducer. Echocardiograms were analyzed at the Echocardiography Reading Center (ERC; Brigham and Women’s Hospital, Boston, MA) in accordance with American Society of Echocardiography recommendations.
N Terminal Pro B-Type Natriuretic Peptide (NT-proBNP) Levels
NT-ProBNP levels were measured from participant plasma samples obtained during ARIC visit 5 using an electrochemiluminescent immunoassay on an automated Cobas e411 analyzer (Roche Diagnostics).
Statistical Analysis
We used the ARIC sample to derive a novel risk score for AF-related ischemic stroke prediction by incorporating PWIs with the CHA2DS2-VASc score variables. We used the MESA sample to validate our risk score.
In the ARIC sample, we used Cox proportional hazards models to calculate hazard ratios (HRs) and 95% confidence intervals (95% CI) of abnormal PWIs for ischemic stroke. Person-years at risk were calculated from the date of AF ascertainment until the date of ischemic stroke, death not due to stroke, loss to follow-up, or end of follow-up, whichever occurred first.
We constructed 2 models for the association of each PWI and CHA2DS2-VASc variable with incident ischemic stroke in ARIC. Model 1 was an unadjusted model. Model 2 was adjusted for remaining CHA2DS2-VASc variables: age, sex, heart failure, hypertension, diabetes, previous stroke/TIA, previous myocardial infarction, and peripheral arterial disease.
To estimate the predictive value of PWIs for stroke, we constructed 6 models for 1-year stroke risk which were used in our cohort of participants with incident AF. Model A was constructed with the CHA2DS2-VASc score variables. Models B to E were constructed by adding aPWA, PPWD, aPTFV1, and aIAB to Model A, respectively. Model F was constructed by adding all 4 PWIs to Model A. We evaluated model performance by calculating the C-statistic, categorical net reclassification improvement (NRI), and relative integrated discrimination improvement (IDI) using Model A as the benchmark for comparison. Reclassification categories were defined as <1%, 1–2%, and >2% 1-year risk of stroke. We used the Hosmer-Lemeshow chi-squared statistic to evaluate model calibration and also compared observed to predicted stroke rates for score categories
To create our risk score for 1-year stroke prediction, we screened our results for PWIs that resulted in meaningful improvement in risk reclassification and model discrimination. To assign a point value for the candidate PWI, we compared the coefficient of the PWI with the coefficient of CHA2DS2-VASc variables in the same Cox proportional hazards model for ischemic stroke. We validated the new score in MESA.
To estimate the odds of structural heart disease for abnormal PWIs, we conducted a cross-sectional analysis in participants from visit 5 who underwent 2D and 3D echocardiograms. We utilized logistic regression models to calculate odds ratios. Model a was unadjusted. Model b was additionally adjusted for age, sex, and race. Data are presented as odds ratios (95% CI) for categorical variables and difference (95% CI) for continuous variables.
Finally, we calculated the C-statistic of the CHA2DS2-VASc score for 5-year ischemic stroke in participants without AF with abnormal PWIs and participants with AF. For participants without AF, we used our baseline cohort (15,365 participants at visit 1). Baseline ECGs were used to evaluate PWIs. For participants with AF, we utilized our cohort of participants with incident AF (2229 participants with incident AF). Participants were followed until their first ischemic stroke, death, or censorship.
The proportional hazards assumption was assessed with scaled Schoenfeld residuals for both graphical and numerical tests, time interaction terms, and inspection of log negative log survival curves. Model assumptions were not violated in any model. Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC) and STATA 13.0, College Station, TX: StataCorp LP. To evaluate whether differences between baseline characteristics were statistically significant, a Student’s t-test was used for continuous variables and a chi-squared test was used for categorical variables. All P values reported were 2-sided, and statistical significance threshold was chosen as 0.05.
Results
In the ARIC sample, we identified 163 ischemic strokes over a mean follow-up time of 5.4 years after AF diagnosis. There were 47 ischemic strokes within the first year after AF diagnosis. In the MESA sample, we identified 31 cases of stroke/TIA over a mean follow-up time of 3.3 years after AF diagnosis. There were 10 or fewer cases of stroke/TIA within the first year after AF diagnosis. Due to the Centers for Medicare and Medicaid Services sparse cell suppression policy which stipulates that no cell (e.g. admittances, discharges, patients, services) 10 or less may be displayed, we are unable to report the exact number of events in the first year after AF diagnosis. The mean (SD) time period from ECG detection of aPWA to AF diagnosis in ARIC and MESA was 8.0 (5.3) and 4.4 (3.0) years, respectively.
The baseline characteristics of participants at the time of AF diagnosis in each study sample are listed in Table 1. In the ARIC sample, participants who developed stroke were more likely to be women and to have prevalent heart failure, hypertension, myocardial infarction, stroke/TIA, peripheral arterial disease, aPWA, PPWD, aPTFV1, and aIAB. There were no statistically significant differences in the CHADS2 and CHA2DS2VASc scores between participants who did and did not develop stroke. In the MESA sample, participants who developed stroke were on average older, more likely to be women, and more likely to have prevalent hypertension, heart failure, stroke/TIA, and aPWA than MESA participants who did not develop stroke. Participants who developed stroke or TIA in MESA had higher CHADS2 and CHA2DS2-VASc scores compared to those who did not.
Table 1:
Clinical Characteristics at time of Atrial Fibrillation diagnosis, Atherosclerosis Risk in Communities (ARIC) Study and Multi-Ethnic Study of Atherosclerosis (MESA)
| ARIC | MESA | |||||
|---|---|---|---|---|---|---|
| All | No Stroke | Stroke | All | No Stroke/TIA | Stroke/TIA | |
| Characteristic | N=2229 | N=2066 | N=163 | N=700 | N=669 | N=31 |
| Age (Years) | 73 +/− 8 | 73 +/− 8 | 69 +/− 8‡ | 76 +/− 8 | 76 +/− 8 | 79 +/− 6† |
| Female Sex | 1039 (47) | 955 (46) | 84 (52) | 315 (45) | 299 (45) | 16 (52) |
| Diabetes | 679 (30) | 622 (30) | 57 (35) | 129 (18) | || | || |
| Hypertension | 1661 (75) | 1533 (74) | 128 (79) | 476 (68) | 452 (68) | 24 (77) |
| Previous MI | 524 (24) | 485 (23) | 39 (24) | 40 (6) | 40 (6) | || |
| Heart Failure | 840 (38) | 774 (37) | 66 (41) | 55 (8) | || | || |
| PAD | 205 (9.0) | 181 (9) | 24 (15)† | 15 (2) | 15 (2) | || |
| Past Stroke/TIA | 326 (15) | 297 (14) | 29 (18) | 45 (6) | || | || |
| CHADS2 | 2.1 +/− 1.3 | 2.0 +/− 1.3 | 2.1 +/− 1.4 | 1.6 +/− 1.0 | 1.6 +/− 1.0 | 2.1 +/− 1.2‡ |
| CHA2DS2VASc | 3.6 +/− 1.7 | 3.6 +/− 1.7 | 3.7 +/− 1.9 | 3.0 +/− 1.3 | 3.0 +/− 1.3 | 3.6 +/− 1.4† |
| Race | ||||||
| Black | 414 (19) | 375 (18) | 39 (24) | 142 (20) | || | || |
| White | 1815 (81) | 1691 (82) | 124 (76) | 342 (49) | 323 (48) | 19 (61) |
| Chinese | § | § | § | 94 (13) | 92 (14) | || |
| Hispanic | § | § | § | 122 (17) | 115 (17) | || |
| aPWA | 529 (24) | 472 (23) | 57 (35)‡ | 82 (12) | 74 (11) | || |
| PWA (degrees) | 51 +/− 26 | 51 +/− 26 | 49 +/− 30 | 50 +/− 29 | 50 +/− 28 | 40 +/− 47 |
| PPWD | 921 (41) | 851 (41) | 70 (43) | 157 (22) | || | || |
| PWD (ms) | 113 +/− 18 | 112 +/− 18 | 115 +/− 21 | 110 +/− 22 | 110 +/− 20 | 99 +/− 41 |
| aPTFV1 | 670 (30) | 617 (30) | 53 (33) | 160 (23) | || | || |
| PTFV1 (μV*ms) | 2492 +/− 2240 | 2471 +/− 2218 | 2762 +/− 2491 | 2675 +/− 2205 | 2704 +/− 2222 | 2059 +/− 1707 |
| aIAB | 99 (4.4) | 81 (3.9) | 18 (11)‡ | § | § | § |
| Current Smoker | 465 (21) | 434 (21) | 31(19) | 53 (7) | || | || |
| COPD | 248 (11) | 224 (11) | 24 (15) | 34 (5) | || | || |
Data are presented as n(%) or mean +/− standard deviation.
P-value <0.05 from χ2 tests or t tests for comparisons of those who did or did not experience an incident or recurrent stroke or TIA (MESA) during follow-up
P-value <0.01 from χ2 tests or t tests for comparisons of those who did or did not experience an incident or recurrent stroke or TIA (MESA) during follow-up
indicates variable unavailable in ARIC/MESA database
indicates suppressed due to small cell size in accordance with the Centers for Medicare & Medicaid sparse cell suppression policy.
Abbreviations: Myocardial Infarction (MI), Peripheral Arterial Disease (PAD), Transient Ischemic Attack (TIA), Abnormal P-wave Axis (aPWA), Prolonged P-wave Duration (PPWD), Abnormal P-wave Terminal Force in V1 (aPTFV1), Advanced Interatrial Block (aIAB)
Table 2 lists the results of our Cox proportional hazards models that were used to estimate the association between abnormal PWIs and ischemic stroke in the ARIC cohort. aPWA and aIAB were associated with increased risk of ischemic stroke in the unadjusted model (model 1). These associations remained significant after adjustment for the individual CHA2DS2-VASc variables (model 2). PPWD and aPTFV1 were not associated with increased risk of ischemic stroke in either model.
Table 2.
Association of P-wave Indices with Ischemic Stroke in Participants with Atrial Fibrillation, Atherosclerosis Risk in Communities (ARIC) Study
| Model 1* | Model 2* | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| Abnormal P-wave Axis | 1.92 (1.40-2.66) | <0.0001 | 1.88 (1.36-2.61) | 0.0001 |
| Prolonged P-wave Duration | 1.04 (0.76-1.42) | 0.82 | 0.97 (0.71-1.33) | 0.83 |
| Abnormal P-wave Terminal Force in V1 | 1.27 (0.91-1.76) | 0.15 | 1.08 (0.77-1.51) | 0.67 |
| Advanced Interatrial Block | 3.22 (1.98-5.27) | <0.0001 | 2.93 (1.78-4.81) | <0.0001 |
| Age | 1.01 (0.98-1.03) | 0.67 | 0.99 (0.97-1.02) | 0.59 |
| Male sex | 0.79 (0.58-1.07) | 0.13 | 0.90 (0.65-1.23) | 0.49 |
| Stroke/Transient Ischemic Attack | 1.57 (1.05-2.35) | 0.03 | 1.40 (0.93-2.10) | 0.10 |
| Heart Failure | 1.66 (1.21-2.28) | 0.002 | 1.48 (1.07-2.05) | 0.02 |
| Hypertension | 2.00 (1.37-2.92) | 0.0003 | 1.80 (1.22-2.66) | 0.003 |
| Diabetes | 1.72 (1.24-2.38) | 0.001 | 1.49 (1.07-2.08) | 0.02 |
| Myocardial Infarction | 1.20 (0.84-1.72) | 0.32 | 1.01 (0.69-1.46) | 0.97 |
| Peripheral Arterial Disease | 1.94 (1.26-2.99) | 0.003 | 1.89 (1.22-2.93) | 0.004 |
Model 1: Unadjusted Cox proportional hazards model. Model 2: Cox proportional hazards model adjusted for remaining CHA2DS2VASc variables of age, sex, stroke/transient ischemic attack, heart failure, hypertension, diabetes, myocardial infarction and peripheral artery disease
Abbreviations: Confidence Interval (CI), Hazard Ratio (HR)
Table 3 lists our analysis of stroke risk prediction model discrimination, risk reclassification, and calibration. Model A, which was constructed with the CHA2DS2-VASc variables, served as our benchmark for stroke prediction. aPWA was the only PWI which resulted in meaningful improvement in discrimination and risk reclassification. For 1-year stroke risk, the addition of aPWA (model B) to model A improved the C-statistic (95% CI) from 0.659 (0.574-0.743) to 0.738 (0.663-0.814) corresponding to categorical NRI (95% CI) of 0.266 (0.084-0.442) and relative IDI (95%) of 0.773 (0.474-1.131). The addition of all PWIs to model A (model F) improved the C-statistic (95% CI) from 0.659 (0.574-0.743) to 0.749 (0.678-0.820) corresponding to categorical NRI (95% CI) of 0.374 (0.215-0.521) and relative IDI (95%) of 1.04 (0.652-1.52). All models were well calibrated for 1-year stroke risk.
Table 3.
Performance of Predictive Models for 1-Year Ischemic Stroke Risk in Participants with Atrial Fibrillation, Atherosclerosis Risk in Communities (ARIC) Study
| Model‡ | C-statistic (95% CI) | x2 (P-value)* | NRI (95% CI)† | Relative IDI (95% CI) |
|---|---|---|---|---|
| A | 0.659 (0.574, 0.743) | 4.7 (0.86) | ||
| B | 0.738 (0.663, 0.814) | 10.4 (0.32) | 0.266 (0.084, 0.442) | 0.773 (0.474, 1.131) |
| C | 0.659 (0.575, 0.743) | 4.7 (0.86) | 0.001 (−0.001, 0.002) | 0.001 (−0.001, 0.002) |
| D | 0.668 (0.586, 0.750) | 3.1 (0.96) | −0.052 (−0.151, 0.026) | 0.067 (−0.010, 0.138) |
| E | 0.666 (0.581, 0.751) | 8.5 (0.48) | 0.072 (−0.040, 0.184) | 0.239 (0.050, 0.498) |
| F | 0.749 (0.678, 0.820) | 5.1 (0.82) | 0.374 (0.215, 0.521) | 1.04 (0.652, 1.52) |
Hosmer-Lemeshow chi-squared statistic
For categorical NRI, we used the following categories for 1-year stroke risk: <1%, 1-<2%, and ≥2%. (Based on 47 cases)
Model A: age, sex, stroke/transient ischemic attack, heart failure, hypertension, diabetes, myocardial infarction and peripheral artery disease
Model B: Model A + abnormal P-wave axis
Model C: Model A + prolonged P-wave duration
Model D: Model A + abnormal P-wave terminal force in V1
Model E: Model A + advanced inter-atrial block
Model F: Model A + abnormal P-wave axis, prolonged P-wave duration, abnormal P-wave terminal force in V1, and advanced inter-atrial block
Abbreviations: Category-based Net Reclassification Index (NRI), Integrative Discrimination Improvement (IDI)
Based on our findings that aPWA was the only PWI that significantly improved prediction of ischemic stroke, we constructed a new risk score by incorporating aPWA into the CHA2DS2-VASc score. In model B, the beta estimates of aPWA and CHA2DS2-VASc score, modeled as a continuous variable, for 1-year stroke risk were 0.582 and 0.262, respectively. Thus, aPWA was assigned a value of 2 points to create the P2-CHA2DS2-VASc score— aPWA (2 points), age (1 point for 65-74, 2 points for ≥75), sex (1 point for female), heart failure (1 point), hypertension (1 point), diabetes (1 point), previous myocardial infarction/peripheral artery disease (1 point), prevalent stroke/TIA (2 points).
In the ARIC sample, the CHA2DS2-VASc score had modest discrimination for 1-year stroke risk when assessed by C-statistic (0.60, 95% CI 0.51-0.69). The P2-CHA2DS2-VASc demonstrated superior model discrimination assessed by C-statistic (0.67, 95% CI 0.60-0.75) and IDI (1.19, 95% CI 0.96-1.44). This corresponded to a substantial categorical NRI (0.25, 95% CI 0.13-0.39) (Table 4). Compared to its performance in ARIC, in the MESA sample, the CHA2DS2-VASc had better discrimination assessed by C-statistic (0.68, 95% CI 0.52-0.84). The P2-CHA2DS2-VASc again demonstrated superior discrimination properties assessed by C-statistic (0.75, 95% CI 0.60-0.91) and relative IDI (0.82, 95% CI 0.36-1.39). This corresponded to a substantial categorical NRI (0.51, 95% CI 0.18-0.86) (Table 4).
Table 4.
Performance of P2-CHA2DS2VASc score for 1-Year Ischemic Stroke Risk in Participants with Atrial Fibrillation, Atherosclerosis Risk in Communities (ARIC) Study and Multi-Ethic Study of Atherosclerosis (MESA)
| C-statistic (95% CI) | NRI (95% CI)* | Relative IDI (95% CI) | ||
|---|---|---|---|---|
| ARIC | CHA2DS2VASc† | 0.60 (0.51-0.69) | ||
| P2-CHA2DS2VASc‡ | 0.67 (0.60-0.75) | 0.25 (0.13, 0.39) | 1.19 (0.96, 1.44) | |
| MESA | CHA2DS2VASc† | 0.68 (0.52-0.84) | ||
| P2-CHA2DS2VASc‡ | 0.75 (0.60-0.91) | 0.51 (0.18, 0.86) | 0.82 (0.36, 1.39) |
For categorical NRI, we used the following categories for stroke risk: <1%, 1-<2%, and ≥2%
age (1 point for >65, 2 points for >75), sex (1 point for female), Heart Failure (1 point), hypertension (1 point), diabetes (1 point), previous myocardial infarction/peripheral artery disease (1 point), prevalent stroke/transient ischemic attack (2 points)
CHA2DS2VASc + abnormal P-wave axis (2 points)
Abbreviations: Category-based Net Reclassification Improvement (NRI), Integrated Discrimination Improvement (IDI)
Table 5 lists our analysis of 1-year stroke risk reclassification using the P2-CHA2DS2-VASc score compared to the CHA2DS2-VASc score. In ARIC participants who developed stroke within 1 year of AF diagnosis, 14% were correctly reclassified to higher risk categories while 6% were incorrectly classified to lower risk categories. In those who did not develop stroke, 20.9% were correctly reclassified to lower risk categories while 5.3% were incorrectly reclassified to higher risk categories. In MESA participants who developed stroke within 1 year of AF diagnosis, 33.3% were correctly reclassified to higher risk categories and no participants were incorrectly reclassified to lower risk categories. In those who did not develop stroke, 8.5% were incorrectly reclassified to higher risk categories while 26.3% were correctly reclassified to lower risk categories.
Table 5.
Improvement in Reclassification of 1-Year Stroke Risk in the Atherosclerosis Risk in Communities (ARIC) study.
| CHA2DS2VASc‡ | P2-CHA2DS2VASc§ |
CHA2DS2VASc‡ | P2-CHA2DS2VASc§ |
||||||||
| <1% | 1-2% | >2% | Total | <1% | 1-2% | >2% | Total | ||||
| <1% | 19 | 3* | 0* | 22 | <1% | 290 | 22† | 0† | 312 | ||
| 1-2% | 3† | 9 | 4* | 16 | 1-2% | 167* | 686 | 94† | 947 | ||
| >2% | 0† | 0† | 9 | 9 | >2% | 0* | 290* | 633 | 923 | ||
| Total | 22 | 12 | 13 | 47 | Total | 457 | 998 | 727 | 2182 | ||
| Participants with stroke in 1 year | Participants without stroke in 1 year | ||||||||||
Favorable reclassification
Unfavorable reclassification
age (1 point for >65, 2 points for >75), sex (1 point for female), Heart Failure (1 point), hypertension (1 point), diabetes (1 point), previous myocardial infarction/peripheral artery disease (1 point), prevalent stroke/transient ischemic attack (2 points)
CHA2DS2VASc + abnormal P-wave axis (2 points)
Abbreviations: Category-based Net Reclassification Index (NRI), Relative Integrative Discrimination Improvement (IDI), TIA (transient ischemic attack)
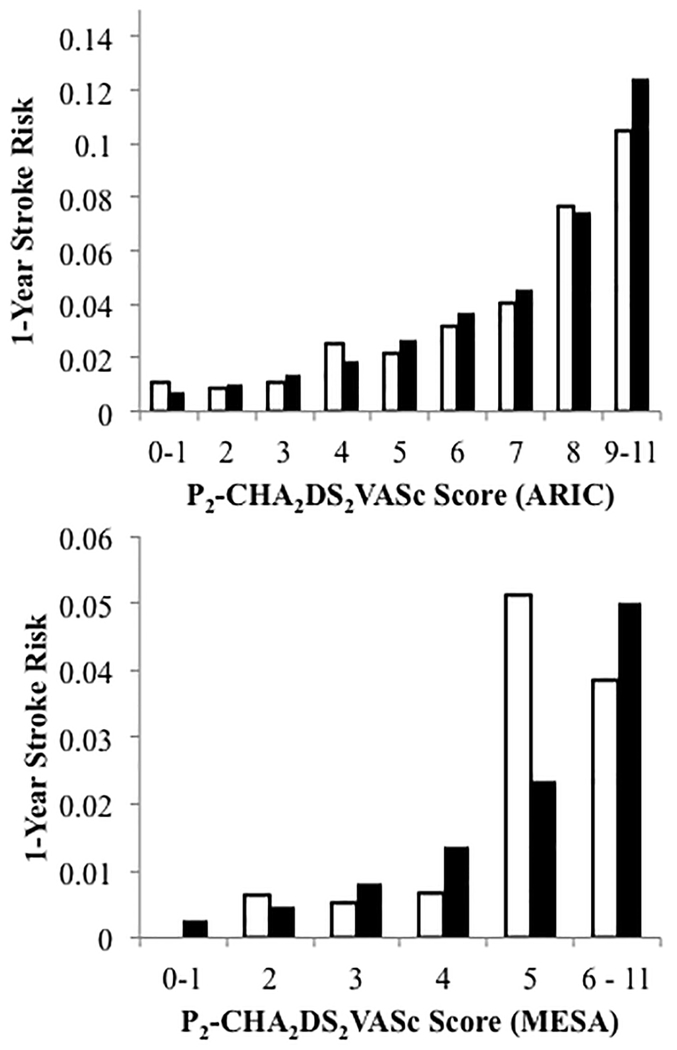
Figure 2 depicts the observed and predicted 1-year stroke risk for P2-CHA2DS2-VASc score categories in ARIC and MESA. The score was well calibrated in ARIC. Given the low number of events in MESA, some variation between observed and predicted risk is to be expected. A 2% annual stroke risk, which is the threshold for anticoagulation according to the 2014 AHA/ACC/HRS2 practice guidelines, corresponded to a score of 4-5.
Figure 2.
Observed (white bars) and predicted (black bars) 1-year stroke risk for P2-CHA2DS2-VASc score categories in the Atherosclerosis Risk in Communities (ARIC) Study and the Multi-Ethnic Study of Atherosclerosis (MESA).
The association of PWIs with structural heart disease is displayed in Table 6. After adjustment for age, sex, and race, all abnormal PWIs were independently associated with left atrial enlargement, greater LV mass, greater LV end diastolic dimension. PPWD, aPWA, and aIAB were associated with higher N-terminal pro b-type natriuretic peptide levels. PPWD, aPTFV1, and aPWA were associated with lower LV ejection fraction. aPWA and PPWD were associated with lower left atrial emptying fraction. aPWA was associated with lower left atrial global longitudinal strain.
Table 6:
Association Between Structural Heart Disease and Abnormal P-wave Indices
| Structural Heart Disease Variable* | Model† | aPWA | PPWD | aPTFV1 | aIAB |
|---|---|---|---|---|---|
| Left atrial volume index>34 ml/m2 | Model a | 1.74 (1.50-2.01) § | 2.16 (1.86-2.50) § | 1.57 (1.36-1.82) § | 2.18 (1.61-2.97) § |
| Model b | 1.69 (1.46-1.96) § | 1.93 (1.66-2.24) § | 1.49 (1.29-1.74) § | 1.79 (1.31-2.44) § | |
| Left ventricular mass index >115 g/m2 for men, >95 g/m2 for women | Model a | 1.20 (1.01-1.41) ‡ | 1.94 (1.65-2.29) § | 1.69 (1.43-1.99) § | 2.35 (1.70-3.26) § |
| Model b | 1.19 (1.01-1.41) ‡ | 2.06 (1.74-2.43) § | 1.67 (1.42-1.98) § | 2.39 (1.71-3.33) § | |
| Left ventricular end diastolic diameter >5.8 cm for men, >5.2 cm for women | Model a | 1.43 (1.03-1.98) ‡ | 2.45 (1.75-3.42) § | 1.72 (1.24-2.38) § | 2.26 (1.23-4.14) § |
| Model b | 1.43 (1.03-1.99) ‡ | 2.75 (1.96-3.87) § | 1.75 (1.26-2.43) § | 2.71 (1.46-5.01) § | |
| Left ventricular ejection fraction < 52% for men, <54% for female | Model a | 1.28 (1.05-1.57) ‡ | 1.34 (1.10-1.64) § | 1.46 (1.19-1.79) § | 1.21 (0.75-1.96) |
| Model b | 1.20 (0.98-1.48) | 1.20 (0.98-1.48) | 1.33 (1.08-1.63) § | 1.08 (0.66-1.76) | |
| NT-Pro BNP pg/ml, difference (95% CI) | Model a | 203.6 (156.1 to 251.2) § | 92.2 (45.6 to 138.9) § | 52.0 (3.51 to 100.5) ‡ | 279 (160 to 399) § |
| Model b | 197.1 (149.7 to 244.5) § | 67.8 (20.6 to 115.0) § | 38.9 (−9.68 to 87.5) | 230 (111 to 350) § | |
| LAGLS %, difference (95% CI) | Model a | −1.65 (0.87, 2.43) § | −1.10 (0.33, 1.86) § | 0.15 (−0.94, 0.63) | −1.28 (−0.84, 3.41) |
| Model b | −1.40 (0.63, 2.16) § | −0.53 (−0.25, 1.30) | 0.46 (−1.23, 0.31) | −0.38 (−1.73, 2.49) | |
| LAEF %, difference (95% CI) | Model a | −3.23 (−4.69, −1.78) § | −2.29 (−3.72, −0.85) § | −0.49 (−1.95, 0.97) | −3.18 (−7.15, 0.80) |
| Model b | −2.85 (−4.29, −1.40) § | −1.57 (−3.03, −0.10) ‡ | −0.03 (−1.48, 1.42) | −1.97 (−5.95, 2.00) |
Data displayed as odds ratio (95% CI) unless otherwise stated. Difference is the difference in number of units (pg/ml for NT-Pro BNP and % for LAGLS and LAEF) between participants with an abnormal PWI and participants with a normal PWI. For example, compared to participants with normal PWA, those with abnormal PWA have higher NT-pro BNP, and lower LAGLS and LVEF
Logistic regression models. Model a is unadjusted. Model b is adjusted for age, sex, and race.
P-value<0.05
P-value<0.01
Abbreviations: Abnormal P-wave axis (aPWA), Prolonged P-wave duration (PPWD), Abnormal P-wave terminal force in V1 (aPTFV1), Advanced interatrial block (aIAB), N-terminal pro b-type natriuretic peptide (NT-Pro BNP), Left atrial global longitudinal strain (LAGLS), Left atrial emptying fraction (LAEF)
The predictive value of the CHA2DS2-VASc score for 5-year ischemic stroke risk is listed in supplemental table 1. The CHA2DS2-VASc score had a C-statistic of 0.682 (0.617-0.748) and 0.636 (0.577-0.695) in participants without AF with any abnormal PWI and participants with AF, respectively.
Discussion
In this study, we demonstrated that aPWA and aIAB were independently associated with AF-related ischemic stroke. We found that use of aPWA improved ischemic stroke prediction over and above the CHA2DS2-VASc score variables. We derived and validated the P2-CHA2DS2-VASc score—aPWA (2 points), age (1 point for 65-74, 2 points for ≥75), sex (1 point for female), heart failure (1 point), hypertension (1 point), diabetes (1 point), previous myocardial infarction/peripheral artery disease (1 point), prevalent stroke/TIA (2 points)—for prediction of AF-related stroke using data from 2 large community-based prospective cohort studies. We found that this scoring system had superior discrimination compared to the CHA2DS2-VASc score and resulted in significant improvement in ischemic stroke-risk reclassification. Of note, while aIAB was independently associated with AF-related stroke, it did not improve risk prediction in our models. This was likely due to its low prevalence in our population.
Fibrotic atrial remodeling and enlargement are critical components of the proarrhythmic and prothrombotic substrate underlying development of AF and AF-related stroke.10 Measurement of PWIs forms the basis for ECG-based detection of this substrate.11–14 Our analysis supports an association between abnormal PWIs and adverse cardiac remodeling including left atrial enlargement, an independent risk factor for development of AF and cardioembolic/cryptogenic stroke.38,39 Abnormal PWIs—PPWD40,41, aIAB16,42,43, , aPTFV117,18,40, and aPWA15,44—have been found to be independently associated with AF and stroke in the general population, even after adjustment for AF. However, the clinical utility of PWIs has not been well established.
The CHA2DS2-VASc score is recommended for AF-related stroke prediction despite emerging evidence that has highlighted limitations in its discriminatory capacity. In a meta-analysis of 8 clinical studies, the CHA2DS2-VASc score demonstrated only modest discrimination (C-statistic 0.675, 95% CI 0.656-0.694) in non-anticoagulated AF patients.9 Furthermore, individuals with low risk scores of 0-1 have been found to still have clinically significant stroke risk. A study of 73,242 low-risk AF patients from the National Health Insurance Research Database in Taiwan reported that the annual stroke rates of patients with CHA2DS2-VASc scores of 0 and 1 were 1.09% and 1.72%, respectively.5 Analysis of 186,750 AF patients from the same database indicated that males with a score of 1 had annual stroke rates ranging from 1.96% (95% CI, 1.56-2.42) to as high as 3.5% (95% CI, 3.27-3.74%).4 Limitations in the predictive value of the CHA2DS2-VASc score may arise from the fact that it only measures atherosclerotic risk factors and does not completely or directly reflect mechanisms of AF-related thromboembolism. Our study is the first to incorporate ECG-based markers of atrial remodeling (PWIs) into a clinically applicable risk score that outperformed the CHA2DS2-VASc score for prediction of AF-related stroke.
A large component of the improvement in stroke risk reclassification achieved by the P2-CHA2DS2-VASc score was derived from correctly identifying low risk individuals. There is disagreement between the 2012 ESC3 and the 2014 AHA/ACC/HRS2 practice guidelines on the precise definition of low risk. Specifically, the 2012 ESC guidelines recommend anticoagulation for CHA2DS2VASc score of 1 whereas the ACC/AHA/HRS guidelines recommend anticoagulation for a score of 2 and equivocate at a score of 1. Use of the P2-CHA2DS2VASc score may address this clinical equipoise and aid in scenarios where the perceived risk/benefit ratio of anticoagulation therapy for prevention of AF-related stroke is unclear.
Considering PWA in stroke prediction has a practical advantage: it is automatically reported on the 12-lead ECG printout. Commercially available ECG recorders do not routinely report the other PWIs—PPWD, aIAB, aPTFV1. Calculation of these indices requires ECG digitalization and specialized analytic software to allow for precise measurements making them impractical for everyday clinical use, particularly in the primary care setting. In contrast, P-wave axis is a routine, automated index that is reported on the 12-lead ECG printout by most commercially available ECG recorders. Thus, the P2-CHA2DS2-VASc score is a feasible score for use in the primary care setting.
PWA is also reproducible. Data on the short-term reliability and stability of P-wave indices are available from a subset of 63 ARIC participants. In this study, two 12-lead ECGs were obtained from participants during two study visits spaced two weeks apart. The absolute within-visit and between-visit difference of P-wave axis was 5.1 +/− 5.8 and 6.6+/−7.2 degrees, respectively. The reported intraclass correlation coefficient (ICC) was 0.78. P wave duration and P-wave terminal force were not as reliable with reported ICCs of 0.58 and 0.46, respectively.45
Notably, we found that stroke prediction by the CHA2DS2VASc score in people without AF but with abnormal PWI was comparable to people with AF. The CHA2DS2VASc score has been shown to have predictive value in the absence of detected AF.46 It is unclear if individuals without AF will benefit from anticoagulation guided by CHA2DS2VASc score given the relatively lower proportion of cardioembolism compared to people with AF. However, people without AF who have underlying prothrombotic atrial remodeling may benefit from anticoagulation as stroke in this population may be cardioembolic.15 Thus, PWIs may have a role in stroke risk prediction and guiding anticoagulation therapy in people without AF in addition to enhancing stroke risk prediction in people with AF. If our findings are validated in other studies, further research should be conducted to determine whether anticoagulation can reduce stroke risk in people without AF but with abnormal PWIs and other markers of atrial remodeling.
The principal strengths of our study include the use of 2 large community-based, racially diverse, prospective cohorts with long follow-up duration, extensive measurement of covariates, and rigorous physician-adjudication of stroke. Some limitations should be noted. First, AF was identified primarily from hospital discharge records. Thus, we were unable to account for subclinical AF or AF managed exclusively in ambulatory clinics. However, AF incidence in the ARIC study is consistent with other population-based studies and utilizing hospital discharge records for the purposes of AF detection has been previously validated.34,47–49 Second, due to low number of stroke events in the MESA study, we used a composite endpoint of stroke/TIA for purposes of score validation. Third, anticoagulant use was not captured annually in MESA or ARIC (until 2006). Therefore, we likely were unable to exclude all participants on anticoagulants within 1 year of AF diagnosis. However, the use of anticoagulants for AF-related stroke prevention in the United States did not become widespread until after 2006, when risk stratification using the CHADS2 score was recommended by practice guidelines.50 Thus, we do not suspect that this will have significantly biased our results. Fourth, anticoagulants other than Coumadin were not captured in ARIC. However, novel oral anticoagulants were not introduced in United States markets until 2010, which is towards the end of our analysis period. Thus, aside from enoxaparin, Coumadin was likely the primary ambulatory anticoagulant used in the United States during our analysis period. Fifth, our analysis was based on PWIs obtained prior to AF diagnosis. Thus, the clinical utility of the P2-CHA2DS2-VASc score is limited in patients with persistent or permanent AF who have no available sinus ECGs and unclear in patients with AF whose only available sinus rhythm ECGs were obtained after AF diagnosis. Finally, as inherent in all observational studies, although we adjusted for potential confounders in our analyses, we could not exclude residual confounding by imperfectly measured and unmeasured factors.
Conclusion
In summary, aPWA was associated with an increased risk of AF-related ischemic stroke. When included with CHA2DS2-VASc score variables to create the P2-CHA2DS2-VASc score, aPWA helped to improve prediction of AF-related ischemic stroke in 2 large prospective community-based cohorts. Further research is needed to clarify the biological correlates of aPWA as well its accuracy and precision in detecting a pro-thrombotic atrial substrate.
Supplementary Material
Clinical Perspective.
What Is New?
Recent studies have highlighted limitations in the predictive value of the CHA2DS2-VASc score
We found that in people with atrial fibrillation, abnormal P-wave indices during sinus rhythm are associated with stroke independent of CHA2DS2-VASc variables
We derived the P2-CHA2DS2-VASc score—abnormal P-wave axis (2 points), age (1 point for 65-74, 2 points for ≥75), sex (1 point for female), heart failure (1 point), hypertension (1 point), diabetes (1 point), previous myocardial infarction/peripheral artery disease (1 point), prevalent stroke/transient ischemic attack (2 points)—for prediction of atrial fibrillation-related stroke using data from 2 large community-based prospective cohort studies.
What Are the Clinical Implications?
Use of markers reflecting left atrial remodeling, such as P-wave indices, are important for stroke risk prediction in people with atrial fibrillation
If our findings are validated in other independent cohorts, use of the P2-CHA2DS2-VASc score may help improve atrial fibrillation-related stroke-risk assessment in the general population
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA and the ARIC study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. AA is supported by an American Heart Association grant 16EIA26410001. LYC is supported by R01HL126637 and R01HL141288. MR is supported by NHLBI T32HL007779.
Funding Sources
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This research in the Multi-Ethnic Study of Atherosclerosis was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 and grant HL127659 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from National Center for Advancing Translational Sciences.
Footnotes
Disclosures
None
References
- 1.Katsnelson M, Koch S, Rundek T. Stroke Prevention in Atrial Fibrillation. J Atr Fibrillation. 2007;3:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary. Circulation. 2014;130:2071 LP–2104. [DOI] [PubMed] [Google Scholar]
- 3.John Camm A, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Knuuti J, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Vardas P, Al-Attar N, Alfieri O, Angelini A, Blömstrom-Lundqvist C, Colonna P, De Sutter J, Ernst S, Goette A, Gorenek B, Hatala R, Heidbuchel H, Heldal M, Kristensen SD, Kolh P, Le Heuzey JY, Mavrakis H, Mont L, Filardi PP, Ponikowski P, Prendergast B, Rutten FH, Schotten U, Van Gelder IC, Verheugt FWA. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 4.Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Lip GYH, Chen SA. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc Score (Beyond Sex) receive oral anticoagulation? J Am Coll Cardiol 2015;65:635–642. [DOI] [PubMed] [Google Scholar]
- 5.Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Lip GYH, Chen SA. Using the CHA2DS2-VASc score for refining stroke risk stratification in “low-risk” Asian patients with Atrial fibrillation. J Am Coll Cardiol 2014;64:1658–1665. [DOI] [PubMed] [Google Scholar]
- 6.Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, Markl M, Ng J, Shah SJ. Evaluating the atrial myopathy underlying atrial fibrillation: Identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015;132:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Staa TP, Setakis E, Di Tanna GL, Lane DALG. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost 2011;9:39–48. [DOI] [PubMed] [Google Scholar]
- 8.Lip G, Nieuwlaat R, Pisters R, Lane D, Crijns H. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 9.Chen JY, Zhang AD, Lu HY, Guo J, Wang FF, Li ZC. CHADS2 versus CHA2DS2-VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: A systematic review and meta-analysis. J Geriatr Cardiol 2013;10:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet [Internet]. 2009;373:155–166. [DOI] [PubMed] [Google Scholar]
- 11.Goyal SB, Spodick DH. Electromechanical dysfunction of the left atrium associated with interatrial block. Am Heart J. 2001;142:823–827. [DOI] [PubMed] [Google Scholar]
- 12.Ariyarajah V, Mercado K, Apiyasawat S, Puri P, Spodick DH. Correlation of left atrial size with P-wave duration in interatrial block. Chest. 2005;128:2615–2618. [DOI] [PubMed] [Google Scholar]
- 13.Jin L, Weisse A, Hernandez F, Jordan T. Significance of electrocardiographic isolated abnormal terminal P-wave force (left atrial abnormality). An echocardiographic and clinical correlation. Arch Intern Med 1988;148:1545–9. [PubMed] [Google Scholar]
- 14.Tsao CW, Josephson ME, Hauser TH, O’Halloran TD, Agarwal A, Manning WJ, Yeon SB. Accuracy of electrocardiographic criteria for atrial enlargement: validation with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2008;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maheshwari A, Norby FL, Soliman EZ, Koene RJ, Rooney MR, O’Neal WT, Alonso A, Chen LY. Abnormal P-Wave Axis and Ischemic Stroke: The ARIC Study (Atherosclerosis Risk In Communities).Stroke;48:2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neal WT, Kamel H, Zhang ZM, Chen LY, Alonso A, Soliman EZ. Advanced interatrial block and ischemic stroke: The Atherosclerosis Risk in Communities Study. Neurology. 2016;87:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC. Ethnic distribution of ecg predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the atherosclerosis risk in communities (ARIC) study. Stroke. 2009;40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamel H, O’Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the atherosclerosis risk in communities study. Ann Neurol 2015;78:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamel H, Bartz TM, Longstreth WT, Okin PM, Thacker EL, Patton KK, Stein PK, Gottesman RF, Heckbert SR, Kronmal R a., Elkind MSV, Soliman EZ. Association Between Left Atrial Abnormality on ECG and Vascular Brain Injury on MRI in the Cardiovascular Health Study. Stroke. 2015;46:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorbar M, Levrault R, Phadke JG, Spodick DH. Interatrial block as a predictor of embolic stroke. Am J Cardiol 2005;95:667–668. [DOI] [PubMed] [Google Scholar]
- 21.Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Nazarian S, Okin PM. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:2786–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The ARIC Investigators. The Atherosclerosis Risk In Communities (ARIC) Study: Design and Objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV., Folsom AR, Greenland P, Jacobs DR, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 24.Rosamond WD, Folsom AR, Chambless LE, Wang C, Mcgovern PG, Howard G, Copper LS, Shahar E. Stroke Incidence and Survival Among Middle-Aged Adults. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki R, Xie J, Cheung N, Lamoureux E, Klein R, Klein BEK, Cotch MF, Sharrett AR, Shea S, Wong TY. Retinal microvascular signs and risk of stroke: The Multi-Ethnic Study of Atherosclerosis (MESA). Stroke. 2012;43:3245–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vardeny O, Gupta DK, Claggett B, Burke S, Shah A, Loehr L, Rasmussen-Torvik L, Selvin E, Chang PP, Aguilar D, Solomon SD. Insulin Resistance and Incident Heart Failure. JACC Hear Fail 2013;1:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MESA Manual of Operations: Identification of Possible Events. 2004.
- 28.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G. Arterial Stiffness and the Development of Hypertension. Hypertension. 1999;34:201 LP–206. [DOI] [PubMed] [Google Scholar]
- 29.Bertoni A, Goff D, D’Agostino R, Liu K, Hundley, Lima J, Polak J, Saad M, Szklo M, Tracy R, Siscovick D. Diabetic Cardiomyopathy and Subclinical Cardiovascular Disease. Diabetes Care. 2006;29:588–594. [DOI] [PubMed] [Google Scholar]
- 30.Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower Heart Rate Variability Is Associated With the Development of Coronary Heart Disease in Individuals With Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study . Diabetes. 2002;51:3524–3531. [DOI] [PubMed] [Google Scholar]
- 31.Muntner P, Colantonio LD, Cushman M, Jr DCG, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd-jones DM, Safford MM. Validation of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations. 2014;311:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toole JF, Lefkowitz DS, Chambless LE, Wijnberg L, Paton CC, Heiss G. Self-reported transient ischemic attack and stroke symptoms: methods and baseline prevalence. The ARIC Study, 1987–1989. Am J Epidemiol 1996;144:849–56. [DOI] [PubMed] [Google Scholar]
- 33.Wattanakit K, Folsom AR, Selvin E, Weatherley BD, Pankow JS, Brancati FL, Hirsch AT. Risk factors for peripheral arterial disease incidence in persons with diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2005;180:389–397. [DOI] [PubMed] [Google Scholar]
- 34.Alonso A, SK A, Soliman E, Ambrose M, AM C, Prineas R, Folsom A. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heckbert S, Wiggins K, Blackshear C, Yang Y, Ding J, Liu J, McKnight B, Alonso A, Austin T, Benjamin E, Curtis L, Sotoodehnia N, Correa A. Pericardial fat volume and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis and Jackson Heart Study. Obes (Silver Spring). 2017;25:1115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonçalves A, Hung C-L, Claggett B, Nochioka K, Cheng S, Kitzman DW, Shah AM, Solomon SD. Left Atrial Structure and Function Across the Spectrum of Cardiovascular Risk in the Elderly: The Atherosclerosis Risk in Communities Study. Circ Cardiovasc Imaging. 2016;9:e004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: The atherosclerosis risk in communities stud. Circ Cardiovasc Imaging. 2014;7:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaghi S, Moon YP, Mora-McLaughlin C, Willey JZ, Cheung K, Di Tullio MR, Homma S, Kamel H, Sacco RL, Elkind MSV. Left Atrial Enlargement and Stroke Recurrence. Stroke. 2015;46:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel D a, Lavie CJ, Milani RV, Shah S, Gilliland Y. Clinical implications of left atrial enlargement: a review. Ochsner J. 2009;9:191–196. [PMC free article] [PubMed] [Google Scholar]
- 40.He J, Tse G, Korantzopoulos P, Letsas KP, Ali-Hasan-Al-Saegh S, Kamel H, Li G, Lip GYH, Liu T. P-Wave Indices and Risk of Ischemic Stroke: A Systematic Review and Meta-Analysis. Stroke. 2017;48:2066–2072. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen JB, Kühl JT, Pietersen A, Graff C, Lind B, Struijk JJ, Olesen MS, Sinner MF, Bachmann TN, Haunsø S, Nordestgaard BG, Ellinor PT, Svendsen JH, Kofoed KF, Køber L, Holst AG. P-wave duration and the risk of atrial fibrillation: Results from the Copenhagen ECG Study. Heart Rhythm. 2015;12:1887–1895. [DOI] [PubMed] [Google Scholar]
- 42.O’Neal WT, Zhang ZM, Loehr LR, Chen LY, Alonso A, Soliman EZ. Electrocardiographic Advanced Interatrial Block and Atrial Fibrillation Risk in the General Population. Am J Cardiol 2016;117:1755–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tse G, Wong CW, Gong M, Wong WT, Bazoukis G, Wong SH, Li G, Wu WKK, Tse LA, Lampropoulos K, Xia Y, Liu T, Baranchuk A. Predictive value of inter-atrial block for new onset or recurrent atrial fibrillation: A systematic review and meta-analysis. Int J Cardiol 2018;250:152–156. [DOI] [PubMed] [Google Scholar]
- 44.Maheshwari A, Norby F, Soliman E, Koene R, Rooney M, O’Neal W, Alonso A, Chen L. Refining Prediction of Atrial Fibrillation Risk in the General Population With Analysis of P-Wave Axis (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2017;120:1980–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snyder ML, Soliman EZ, Whitsel EA, Gellert KS, Heiss G. Short-term repeatability of electrocardiographic P wave indices and PR interval. J Electrocardiol 2014;47:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell LB, Southern DA, Galbraith D, Ghali WA, Knudtson M, Wilton SB. Prediction of stroke or TIA in patients without atrial fibrillation using CHADS2 and CHA2DS2-VASc scores. Heart. 2014;100:1524 LP–1530. [DOI] [PubMed] [Google Scholar]
- 47.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TSM. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 48.Benjamin E, Levy D, Vaziri S, D’Agostino R, Belanger A, Wolf P. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 49.Psaty B, Manolio T, Kuller L, Kronmal R, Cushman M, Fried L, White R, Furberg C, Rautaharju P. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 50.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Heuzey J Le, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Moralis J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Some access restrictions apply to the data underlying the findings. The consent signed by study participants does not allow the public release of their data. Data from the ARIC study can be accessed by contacting the ARIC Coordinating Center (aricpub@unc.edu). Data from the MESA study can be accessed by contacting the MESA data coordinating center (chsccweb@u.washington.edu).