Abstract
Exome sequencing (ES) has revolutionized molecular diagnosis in children with genetic disease over the past decade. However, exome sequencing in the inpatient setting has traditionally been discouraged, in part due to an increased risk of providers failing to retrieve and act upon results, as many patients are discharged before results return. The development of rapid turn-around-times (TATs) for genomic testing has begun to shift this paradigm. Rapid exome sequencing (rES) is increasingly being used as a diagnostic tool for critically-ill infants with likely genetic disease and presents significant challenges to execute. We implemented a program, entitled the Rapid Inpatient Genomic Testing (RIGhT) project, to identify critically-ill children for whom a molecular diagnosis is likely to change inpatient management. Two important goals of the RIGhT project were to provide appropriate genetic counseling, and to develop protocols to ensure efficient test coordination- both of which relied heavily on laboratory and clinic based genetic counselors (GCs). rES was performed on 27 inpatient trios from October 2016 to August 2018; laboratory and clinical GCs encountered significant challenges in the coordination of this testing. The GCs involved retrospectively reviewed these cases and identified three common challenges encountered during pre-test counseling and coordination. The aim of this paper is to define these challenges using illustrative case examples that highlight the importance of including GCs to support rES programs.
Keywords: Genetic counseling, rapid, genomic testing, pediatric, incidental findings, secondary findings, exome sequencing, critical care, informed consent, utilization management
Introduction
Many institutions have restrictions related to genomic testing due to increased order error rates (Valenstein 2008), high test costs with poor reimbursement (Dickerson 2014), and poor results retrieval rates (Casalino 2009). Laboratory stewardship (utilization management) efforts can improve these aspects of genomic testing coordination in the inpatient setting, which is even more at risk for errors compared to ambulatory clinics (Mathias 2016). The value of inpatient genetic testing is also diminished when compared to ambulatory clinics, as long turn-around-times (TATs) increase the likelihood a patient will be discharged prior to results returning and will not be acted upon (Casalino 2009, Mathias 2016).
Rapid exome sequencing (rES) is increasingly being used as a diagnostic tool for critically-ill infants with a high pre-test probability of genetic disease (Farnaes 2018, Petrikin 2015, Petrikin 2018, Stark 2018, Willig 2015). The nature of rES requires modified clinical and non-clinical work flows compared to traditional genetic testing (Stark 2018). Therefore, a thoughtful and robust approach that includes all stakeholders is necessary to implement successful rES programs.
Our pediatric tertiary care institution provides access to 17 board certified geneticists, 11 clinical GCs, and has an active laboratory test stewardship program that includes 4 laboratory GCs (lab GCs). Our hospital has 407 beds, 91 of which are in an intensive care unit (ICU), and serves the largest region of any pediatric academic medical center in the United States. These resources influenced the development of a program, which we entitled the Rapid Inpatient Genomic Testing (RIGhT) project, to offer clinical rES to eligible patients. The RIGhT project began in October 2016 with the goal to identify critically-ill children with a likely genetic disorder in whom a rapid molecular diagnosis would impact inpatient management. A small RIGhT team was selected to ensure efficient communication and coordination of testing, which has previously been described as beneficial to rES (Stark 2018).
Clinical genetic counselors (GCs) can provide valuable support to the coordination, interpretation, and education of genomic testing (Bennett 2003, Biesecker 2001, Ciarleglio 2003, Wang 2004). Exome sequencing involves significantly more considerations than other, historically more common genetic tests within pediatric and neonatal intensive care units. Exome sequencing also requires that families decide on opting-in or opting-out of receiving secondary findings within 59 medically actionable genes, as defined by American College of Medical Genetics (ACMG) (Kalia 2016). These factors significantly increase the complexity of pretest counseling for exome sequencing. In addition, the parents of critically ill children are often facing significant psychosocial stressors that GCs are trained to address. Lab GCs embedded within institutional stewardship programs are especially well-suited to ensure coordination of appropriate and valuable genomic testing for patients (Conta 2017, Kotzer 2014). While literature is limited, the value of GCs involved in pediatric rapid exome sequencing (rES) testing has been described (Stark 2018).
Historically, clinical GCs have not been involved in inpatient testing coordination at our institution. Pre- and post-test counseling as well as follow-up was the responsibility of the consulting geneticist. We chose to specifically include lab and clinical GCs as a part of the RIGhT team to provide consistency in counseling and test coordination for rES. This is in alignment with policy statements for genomic testing (ACMG Board of Directors 2013). The RIGhT project GCs identified challenges on a case-by-case basis, which were discussed at monthly RIGhT project meetings. Over time, it became clear there were common challenges encountered, which were subsequently classified by unanimous agreement of the GCs for this study. We describe these challenges with a focus on the essential role clinical and laboratory GCs have on the counseling and coordination of rES in this setting to inform appropriate implementation of rES programs at other institutions.
METHODS
RIGhT Project Process
We established a standardized protocol for the execution of rES testing at our institution. To ensure that rES was being ordered appropriately, we formed a committee (the RIGhT team) comprised of both clinical and laboratory members that included the Medical Director of the Division of Medical Genetics, 4 board certified geneticists, 1 board certified pediatric pathologist, 2 clinical GCs, 1 lab GC, and 1 administrative assistant. The RIGhT team is a subcommittee of our institution’s Laboratory Test Stewardship Committee, as part of our hospital’s Utilization Review.
After initial genetics consult by the neonatal intensive care unit, the consulting geneticist emailed their rES request to the lab GC for initial review of eligibility and potential coordination challenges (Figure 1). We used a standardized format for these requests that emphasized key eligibility factors, including: (1) recommendation for rES by the consulting geneticist, (2) high suspicion of monogenic disease, (3) patient located in an ICU at time of consult. Trio analysis with both biological parents was initially required by the reference lab, but a non-trio option became available on a case-by-case basis with reference lab approval. Alternate testing, such as non-rapid ES or expedited panel testing, was available and coordinated for patients as-needed, per institutional test stewardship policies, and most often without clinical GC support.
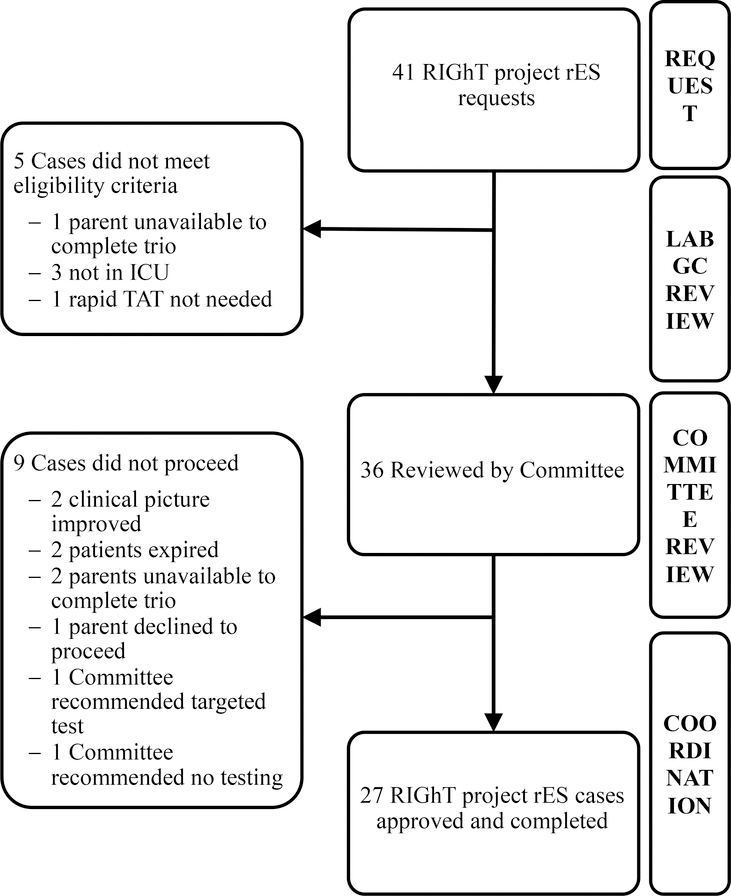
Figure 1.
Case selection flowchart for RIGhT project. Of the 41 requested, 27 rES trios were completed. Lab GCs performed review of eligibility prior to Committee review and coordination.
After initial lab GC review, eligible requests were then emailed to the RIGhT Committee, comprised of the 4 pediatric medical geneticists on the RIGhT team with combined specialty expertise in mosaic disorders, dysmorphology, epilepsy genetics, and biochemical genetics. Each member of the RIGhT Committee emailed individual responses back to the lab GC with approval/disapproval and any other recommendations within twenty-four hours. The lab GC then notified the consulting geneticist, clinical GC, and clinical administrative staff of the Committee’s determination within one business day of request. If unanimously approved, the appropriate clinician (geneticist in 2 cases, genetics fellow in 3 cases, and clinical GC in 22 cases) provided pre-test counseling to parents and coordinated sample collection for trio analysis. The lab GC coordinated pre-accessioning of testing with the reference lab and sample send-out, consistent with institutional laboratory stewardship procedures. After test results were completed, the lab GC also facilitated communication to the consulting geneticist, who was responsible for interpreting and returning results to the ICU team and patient’s family.
The RIGhT team participates in monthly meetings to retrospectively review challenges and outcomes of cases to continuously improve the project’s impact. Clinical and lab GCs heavily influence this process, as they are front-line providers in the coordination of testing and pre-test counseling. RIGhT patient cases were reviewed by the team and discussions about difficulties encountered were often identified as part of this review.
Participants
As of August 3, 2018, 41 rES cases had been requested and 27 rES cases had been approved and coordinated (Figure 1). The demographics of these patients are included in Table I. All patients were located in an ICU at time of genetics consult and their testing included parental samples in all cases. In 3 cases, an additional sample from a sibling was included (quad analysis).
Table I.
Patient demographics and opt-in or opt-out choice for secondary findings. Median age of the patient, average length of stay in the ICU, average age of parents, and average turn-around-time of verbal results from time of rES request, as well as biological sex of patients is described. There was no significant difference between opt-in and opt-out rates for any category (data not shown).
| Patient Characteristics | Opted-in (N=21) | Opted-out (N=6) |
|---|---|---|
| Median Age of patient at time of rES request (min, max, average) | 17 days (1 day, >15 years, 11 months) | 14 days (1 day, >1 year, 3 months) |
| Sex (female:male) | 52%:48% | 33%:67% |
| Average length of stay in ICU prior to rES request (min, max, median) | 15 days (1 day, 4 months, 4 days) | 16 days (0 days, 59 days, 8 days) |
| Average age of parents (min, max, median) | 32 years (18 years, 52 years, 31 years) | 29 years (22 years, 37 years, 28 years) |
| Average TAT from rES request to verbal results (min, max, median) | 8 days (5 days, 16 days, 7 days) | 10 days (6 days, 21 days, 9 days) |
Data Analysis
All data were collected and reviewed as approved by Seattle Children’s HSPP (Institutional Review Board Office) STUDY00000553. Retrospective review of monthly RIGhT team meeting minutes for challenges discussed during case review, as well as chart review of the 27 rES cases from October 2016 to August 2018 was completed. In August 2018, the 3 RIGhT team GCs (S.V.C.C., M.C.S., and J.M.T.) independently reviewed all 27 cases for identification of recurring challenges collected from the monthly RIGhT team case review meeting minutes, comprehensive patient chart review, and GC recall of the case. Themes were then further classified into discrete themes by unanimous consensus of all 3 GCs on the RIGhT team.
RESULTS
There were no counseling challenges identified for 13 cases. Just over half (n=14) of the 27 cases were found to have a specific genetic counseling challenge, as follows: Coordination (n=7 cases), Incidental and Secondary Findings (n=4 cases), and Informed Consent (n=4 cases). (Table II). Of these 14 cases, 1 case was identified to have both coordination and informed consent challenges. The themes were further assessed using case examples.
Table II.
Counseling challenges retrospectively identified for rES patients. Challenges and their overarching theme were determined by unanimous consensus of the three RIGhT team GCs. Challenges were identified in 14 of 27 cases, with 1 case represented in both the coordination and secondary and incidental findings themes.
| Theme of Challenge (N cases) | |||
|---|---|---|---|
| Coordination (7) | Secondary and Incidental Findings (4) | Informed Consent (4) | |
| Patient details (N patients) | Delays in coordination of first RIGhT project patient due to unfamiliarity with process. (1) Delay due to parent located remotely. (2) Family rescheduled pre-test counseling appointment multiple times. (1) Send out delayed due to sample not being drawn urgently by ICU. (1) rES recommended on Friday. Coordination ahead of full RIGhT committee approval was needed to avoid weekend delay. Additional buccal sample was required for concurrent mitochondrial DNA analysis. (1) |
Secondary finding resulted post-mortem. (2) Incidental finding of adult-onset condition complicated transplant decision. (1) Incidental finding resulted after discharge. (1) |
Proband was at age of assent but temporarily incapacitated. (1) Parents consented on day 1 post-partum; difficulty tracking discussion. (1) Extremely low health literacy. (1) Geneticist did not fully discuss testing recommendation with family prior to GC visit leading to counseling challenges. (1) |
Theme 1: Coordination
GC’s played a critical role in coordinating rES as part of the RIGhT project. Of the 27 patients, 5 had significant coordination challenges. The first RIGhT case successfully coordinated had the longest TAT (21 days) due to inexperience of all providers involved at the beginning of the project (Table I). The importance of a rES team experienced with the subtleties of rES is evident.
Case example 1:
A neonate with bradycardia on extracorporeal membrane oxygenation (ECMO) was urgently considered for pacemaker placement, cardiac transplant, and cardiac biopsy, among other procedures. A biochemical geneticist was consulted Friday morning and was hopeful that rES samples would be shipped same-day to prevent delay in testing due to the weekend, if approved. Although full RIGhT committee approval had not yet been obtained in time, the lab GC initiated coordination of rES testing anyway, allowing samples to be shipped same-day. The basis for this decision by the GC included fragility of the patient, knowledge of hospital shipping deadlines, and knowledge that the committee was likely to approve unanimously. Because of this decision, compound heterozygous variants in a gene associated with cardiomyopathy and conduction defects were communicated to the team only 6 days after rES request.
Case 1 describes the flexibility and case-by-case assessment required for most effective rES coordination. In this case, a neonate and their parents were provided pre-test counseling ahead of official approval by the RIGhT Committee. This was at the discretion of the lab GC who was familiar with previous committee determinations for similar patients. Clear contracting between the lab GC, clinical GC, the geneticist and the family were needed to ensure compliant coordination. The family consented to proceed with sample collection with full understanding that testing was awaiting final approval. In this case, the patient received a diagnosis 6 days after the rES request was communicated.
Coordinating consent and sample collection for both biological parents can be difficult. Three cases were delayed due to challenges with parental coordination. In 1 case, pre-test counseling had to be rescheduled multiple times to ensure both parents were concurrently available. Two other cases were delayed due to a parent located out of the state; the parent in one of these cases was able to travel to the institution for sample collection, while the other case required coordination for the parent to be collected remotely.
Factors influencing the approach of rES coordination include fragility of the patient, planned procedures (e.g., transplant considerations), post-transfusion status, sample shipping schedule, and analytic schedule of the reference laboratory, among others. The lab and clinical GCs familiar with the subtleties of rES play an important role in assessing and facilitating the best approach for timely testing in the acute setting.
Theme 2: Secondary and Incidental findings
The ACMG published guidelines regarding reporting of secondary findings in exome testing and recommendation of opt-in, opt-out availability (Kalia 2016). The policy statement defines “secondary findings” as variants known to be disease-causing in 59 medically actionable genes, which can be analyzed as part of ES for patients who opt-in. In contrast, incidental findings are defined as genomic variants reported in genes unrelated to the patient’s phenotype and found incidentally to the indication for testing, apart from the 59 genes recommended by ACMG (Kalia 2016). For example, a deletion variant in the PMP22 gene, associated with Hereditary Neuropathy with Liability to Pressure Palsies (HNPP), may be reported incidentally to the primary indication of rES testing for a neonate with respiratory distress, who is currently asymptomatic for HNPP. Genetic counseling is recommended to fully discuss these considerations (Doyle 2015, Green 2013, Markel & Yashar 2004), and this was a core responsibility of the RIGhT team clinical GCs.
There is limited literature regarding why patients opt-in or out of secondary findings in the setting of acute pediatric inpatient testing (Clift 2015, Cornelis 2016). These decisions are based on many factors, including their ability to cope with their child’s acute medical condition (Bergner 2014, Clift 2015, Cornelis 2016, Roche 2015, Sapp 2014). This is important to consider when providing rES pre-test counseling to parents who are in the midst of coping with their child’s hospitalization, given the median age of our patients were 17 days of age when rES was requested (Table I). In a large retrospective study, a major reference lab that offers ES published that their opt-out rate dropped from 25% to 8% between June of 2013 and March of 2017 (Siegler 2017). The study by Siegler et al. did not identify causative factors for this shift in opt-out rate and there is no professional standard for optimal opt-out rate. In our cohort, 6/27 (22%) families opted-out of learning secondary findings (Table I). The current literature stresses the importance of choice and personal values when choosing whether or not to receive secondary findings (Clift 2015, Cornelis 2016, Sapp 2014). In discussing secondary findings with the parents of our patients undergoing rES, we identified similar themes.
Case examples 2 & 3:
r’ES was recommended for 2 unrelated neonates with multiple congenital anomalies. Parents in both cases chose to opt-in to learning secondary findings. Unfortunately, both patients died prior to results reporting. rES identified no variants associated with the patients’ phenotype, however did identify pathogenic variants within RET and BRCA2, respectively. These secondary finding variants were inherited from a healthy mother in each case.
Case example 4:
A neonate presented with cardiomyopathy and multiple congenital anomalies. The patient’s testing identified likely pathogenic compound heterozygous variants associated with an autosomal recessive type of hypomyelinating leukodystrophy. This result did not explain the patient’s primary indication and indicated a risk for a disorder with onset predicted to occur in early childhood.
Cases 2 and 3 highlight the importance of comprehensive pretest counseling and contracting regarding results. As illustrated in these cases, both patients did not receive diagnostic results for their presenting symptoms and passed away prior to return of results. However, in both cases the child was found to have an adult-onset condition unrelated to the indication for testing that was inherited from a healthy parent and had to be reported after the death of a child. This can be extremely difficult from a genetic counseling standpoint and one that occurs more frequently with rES in the pediatric ICU setting.
Another challenge in pursuing rES in this pediatric setting is illustrated in case 4. ES interpretation relies heavily on the phenotype of the patient (Pena 2018). This is challenging when interpreting data for an infant who has not yet developed their full phenotype and has a rapidly evolving clinical course. In Case 4, an incidental finding did not explain the patient’s primary features, but was reported due to a possible association with the patient’s MRI findings, unrelated to their clinical course. Providing comprehensive post-test counseling in this case was imperative, as pre-test counseling focused on uncovering the etiology for the child’s cardiomyopathy in the hopes to provide insight to treatment decisions. The incidental finding was difficult for the family and care team to incorporate into their decision making about cardiac transplant. This was an unanticipated consequence of pursuing rES for this child, and their family benefitted from additional post-test counseling by the clinical GC to incorporate these results into their personal goals of care.
Theme 3: Informed Consent
The severity of illness and speed with which decisions must be made in the ICU setting presents unique challenges for informed consent. Informed consent enables patients to make informed decisions about their medical care. It is a critical component of pretest counseling in rES, due to the impact of test results in patient management, financial implications of testing, and decisions about receiving secondary findings (ACMG Board of Directors 2013, Markel & Yashar 2004). The ACMG have developed guidelines that outline specific content to incorporate into the informed consent process. This includes the types of results that will or will not be reported, potential benefits and risks, limitations, potential implications for family members, and policies surrounding re-contact as new knowledge is gained (ACMG Board of Directors 2013). Additionally, the American Academy of Pediatrics and ACMG published recommendations regarding genomic testing in children that address the importance of assent (2013). Pediatric assent allows a pediatric patient to agree to the medical treatment plan even though they are legally unable to provide consent, with the goal of protecting one’s freedom of choice and autonomy (American Academy of Pediatrics 1995, American Academy of Pediatrics 2016). In instances when a pediatric patient is incapacitated and there is no documented advanced directed, substitutive judgment is a technique that can be used when assent may be appropriate. Substitutive judgment allows a proxy decision-maker (e.g., a parent/guardian) to make medical decisions on behalf of the patient. This decision should be based on what the patient would want, and not the interests of the parent/guardian (Emanuel & Emanuel 1992).
Case example 5:
A previously healthy patient in their mid-teens presented with acute onset renal failure. Diagnostic work-up included rES. Due to additional complications during treatment of renal failure including encephalopathy and seizures, the patient was not able to provide assent. Therefore, they were unable to participate in the informed consent process and could not provide assent for rES nor ACMG secondary findings. Due to the time-sensitive nature of this patient’s case, an informed consent decision could not be deferred, so the GC instructed the parents to fully consider the self-interest of the patient, using substitutive judgment. The parents felt that their child would have chosen to learn about secondary findings, and the decision to opt-in for secondary findings was made.
Case 5 highlights a complex scenario of medical decision-making for a critically and acutely ill adolescent. rES was recommended for this teenage patient while they were deemed temporarily incapable of participating in the informed consent process. Due to the time sensitive nature of medical care, the informed consent discussion could not wait. The decision to opt-in or opt-out of secondary findings for rES must be made during the informed consent process due to the rapid TAT of results. In this case it was clear that the teen’s assent could be waived based on their temporary incapacitation and acute presentation. However, should the parent in this case be allowed to make the decision, without the teen’s assent, to opt-in or opt-out of secondary findings when this information is unrelated to the patient’s current clinical care?
The GC for case 5 considered many alternative methods for obtaining or deferring informed consent and presented the family with all available options. Both parents agreed that the patient would typically be able to make informed decisions when healthy and the option to defer secondary findings was discussed. If secondary findings were declined, the patient would have the option to change this decision if/when the exome is reanalyzed in the future. However, it was clear that the family felt very strongly about opting-in to secondary findings for their child. This prompted a shift in counseling to discuss the concept of substitutive judgment. After careful consideration and discussion, the parents fully considered the self-interests of the patient and chose to opt-in to learning secondary findings. They felt that secondary findings would help prepare the patient for the possibility of another acute medical crisis. The complexities of informed consent and assent are well-illustrated by this case and exemplify the need for clinical GCs involved in clinical rES testing.
Discussion
At our institution, ES was rarely coordinated in the inpatient setting due to stewardship policies that recommended outpatient testing and TATs of ES on the order of months. As such, inpatient pre-test counseling was historically not provided by GCs. However, rES trio testing requires complex coordination and thorough pre-test counseling, complicated by psychosocial considerations for parents of critically-ill infants (Bergner 2014, Clift 2015, Cornelis 2016, Frankel 2016, Roche 2015, Sapp 2014).
Prior to the RIGhT project, rES created challenges for our geneticists who were less familiar with coordinating trio testing and providing appropriate pre-test counseling. Another rES study that provided GC support previously reported that, on average, the geneticist spent 30 minutes with the family if results were negative and 90 minutes with the family if a positive diagnosis was made (Stark 2018). As part of establishing this project at our institution, it was calculated that our RIGhT team clinical GCs spent an average of 45 minutes providing pre-test counseling, based on data from the initial 18 RIGhT project cases. This would be an overwhelming additional responsibility for our geneticists. The need for GCs was highlighted by one of our geneticists who provided feedback to our institutional leadership prior to clinical GC support that, “I am myself then left trying to consent… this is far less than ideal. Unless I get GC support to consent I will not be able to do [rES] cases.”
The RIGhT project has been a successful intervention for our eligible ICU patients, thanks in large part to the inclusion of lab and clinical GCs. Lab GCs involved in the approval process for rES have prevented inappropriate patients from being considered in 5 cases, allowing for more expedient coordination of alternative testing.
It was thought that a small, specialty RIGhT team would provide the most effective care to patients given the unique challenges of coordination and consent for rES trio testing for critically-ill infants and their parents. Limiting coordination to a small group of 3 GCs has been successful in cultivating rES testing expertise in pre-test counseling and case-by-case analysis and flexibility for effective rapid coordination. Case 1 highlights many of the outcomes that can be positively influenced by a small group of GCs expert in rES testing. Lab GCs have previously been shown to have a positive effect on results retrieval (Conta 2017, Kotzer 2014). In the RIGhT project, lab GCs have been essential to facilitating communication between the lab and consulting geneticist who are often no longer on call or even away at a satellite clinic, given our institution’s coverage of the region.
Clinical GCs have been especially impactful in ensuring appropriate pre-test counseling for this challenging population. Genetic testing in the neonatal period has different psychosocial implications for families than testing in the childhood or adult period. Previous research has identified that perceived child vulnerability, parent-child bonding and self/partner blame are disproportionally affected during the neonatal period compared to other stages of life (Frankel 2016). Logistically, consent for rES happens quickly with limited to no anticipation. Therefore, families have little time to consider and process the benefits, limitations, and option of learning secondary findings. The decision to opt-in or opt-out could be presented as a binary choice by less savvy providers, but case 5 illustrates the complexities associated with assent and the importance of comprehensive genetic counseling in this situation. GCs are most capable of discussing all available options to ensure families are best supported in their decision-making.
In our experience, some parents seem to take comfort in deferring decisions about secondary findings, as they have the option to revisit this decision when/if reanalysis of the rES data is performed in the future. Conversely, other parents have expressed desire to opt-in to secondary findings to prevent potential unanticipated health concerns in the future. Many have expressed wanting to know secondary finding information, but reconsidered after discussing potential life/long-term care/disability and autonomy considerations. All rES patients in our cohort received comprehensive pre-test counseling that allowed families to fully explore factors related to decision-making for opting-in or opting-out of secondary findings. We are comfortable with the current opt-out rate of our patients (22%) despite its inconsistency with the average opt-out rate previously reported by Siegler et al. (8%), as opt-out rates should reflect personal choice of the family that may differ by population.
This may also suggest that there are additional complexities associated with the decision-making processes of parents who have a critically-ill child, often with an unknown prognosis that may be supported by GCs. It has previously been shown that parent understanding and stress has improved with additional GC support prior to rES. GC involvement proved an increase in testing uptake in parents and anecdotally, commented on the acute nature of interactions and importance of genetic counseling (Frankel 2016). GCs are best-suited to ensure appropriate contracting occurs, especially for cases in which the child passes away and results of secondary findings need to be returned to parents.
While conclusions are limited to the experience of our institution, rES in a pediatric ICU setting highlights unique challenges surrounding test coordination, secondary and incidental findings, and pre-test counseling consent/assent. These themes should be thoughtfully considered when developing rES programs, as they illustrate the challenges inherent to this patient population and testing methodology. Genetic counselors, both clinical- and lab-based, are best-suited to meet these challenges. They provide ideal support for coordination and family education of rES testing that aligns with practice guidelines and positively impacts efficiency of testing. Additionally, a small, expert GC team is most effective for implementation, ongoing support, and quality improvement.
Our goals for the future include improving post-test counseling education and elucidating the family perspective. Clinical GC full time equivalent (FTE) is not currently allocated to be routinely involved in post-test counseling discussions in the inpatient setting at our institution. Upon review, we have determined that only 56% (n=15) of patients received comprehensive post-test counseling, either by the geneticist or the clinical GC involved, while still inpatient. Of the 12 patients who did not receive counseling while still inpatient, 75% (n=9) did not receive appropriate outpatient post-test counseling follow up. We of course recognize this gap in care as a priority, as the importance of post-test counseling after genomic testing is accepted amongst all stakeholders (Mackley 2017). We would like to distribute additional clinical GC FTE to be regularly involved in this process and to incorporate an automatic referral to outpatient GC clinic for any patient who is discharged or who passes away prior to receiving test results. Finally, parents’ perspectives of clinical rES utility and the psychosocial impacts in this setting have not been fully explored. This perspective is essential for appropriate genetic counseling as rES becomes more widely used. As this project moves forward, we hope to formally assess GC and family perspectives of rES.
Conclusions
Both laboratory and clinical genetic counselors are essential to the clinical implementation of rapid exome sequencing in the pediatric and neonatal intensive care setting. Given the increasing utilization of rES in pediatric setting, hospitals are likely to have increasing need for genetic counselors.
Acknowledgements
The authors would like to acknowledge the patients and their families, Michael Astion, Jessie Conta, Margaret Adam, Christina Lam, Heather Mefford, Katrina Dipple, Gail Deutsch, Robert DiGeronimo, Zeenia Billimoria, Katie Fogus, Jennifer Dines, M. Kenneth Ndugga-Kabuye, and the Seattle Children’s Hospital Lab Stewardship Committee for their help in developing and supporting the RIGhT project. J.T.B. is supported by the Burroughs Welcome Fund Career Award for Medical Scientists and the Arnold Lee Smith Endowed Professorship for Research Faculty Development. A.S.F. was supported by postdoctoral training grant 5T32GM007454 from the National Institute of General Medical Sciences of the National Institutes of Health.
Footnotes
Conflicts of Interest
Authors Sarah V. Clowes Candadai, Megan C. Sikes, Jenny M. Thies, Amanda S. Freed, and James T. Bennett declare that they have no conflict of interest.
Compliance with Ethical Standards
Human Studies and Informed Consent
No animal studies were carried out by the authors for this article. No identifying information is included for patients and no human experiments were carried out as part of this clinical project. All data were collected and reviewed as approved by Seattle Children’s HSPP (Institutional Review Board Office) STUDY00000553.
References
- ACMG Board of Directors. Points to consider for informed consent for genome/exome sequencing. (2013). Genetics in Medicine, 15(9), 748–749. [DOI] [PubMed] [Google Scholar]
- Amendola L, Lautenbach D, Scollon S, Bernhardt B, Biswas S, East K, et al. (2015). Illustrative case studies in the return of exome and genome sequencing results. Personalized Medicine, 12(3), 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Ethical and policy issues in genetic testing and screening of children. (2013). Pediatrics, 131(3), 620–2. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Bioethics. Informed Consent, Parental Permission and Assent in Pediatric Practice. Pediatrics. 1995;95(2):314–317. Reaffirmed October 2006. [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Bioethics. Informed Consent in Decision-Making in Pediatric Practice. Pediatrics. 2016; 138(2):e20161484. [DOI] [PubMed] [Google Scholar]
- Bennett R, Hampel H, Mandell J, & Marks J (2003). Genetic counselors: Translating genomic science into clinical practice. Journal of Clinical Investigation, 112(9), 1274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner A, Bollinger J, Raraigh K, Tichnell C, Murray B, Blout C, et al. Informed consent for exome sequencing research in families with genetic disease: the emerging issue of incidental findings. Am J Med Genet Part A. 2014;164A(11):2745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker B, Peters K, Baty B, & Baker D (2001). Process studies in genetic counseling: Peering into the black box. American Journal of Medical Genetics, 106(3), 191–198. [DOI] [PubMed] [Google Scholar]
- Botkin J, Belmont J, Berg J, Berkman B, Bombard Y, Holm I, et al. (2015). Points to Consider: Ethical, Legal, and Psychosocial Implications of Genetic Testing in Children and Adolescents. The American Journal of Human Genetics, 97(1), 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino L, Dunham D, Chin M, et al. (2009). Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med,169(12):1123–1129. [DOI] [PubMed] [Google Scholar]
- Christenhusz G, Devriendt K, & Dierickx K (2013). Disclosing incidental findings in genetics contexts: A review of the empirical ethical research. European Journal of Medical Genetics, 56(10), 529–540. [DOI] [PubMed] [Google Scholar]
- Ciarleglio L, Bennett R, Williamson J, Mandell J, & Marks J (2003). Genetic counseling throughout the life cycle. Journal of Clinical Investigation,112(9), 1280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift K, Halverson C, Fiksdal A, Kumbamu A, Sharp R, & Mccormick J (2015). Patients’ views on incidental findings from clinical exome sequencing. Applied & Translational Genomics, 4, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conta J, Hess C, & Riley J (2017). Genetic Counselor Role in Hospital Test Utilization In Practical Genetic Counseling for the Laboratory (p. Practical Genetic Counseling for the Laboratory, Chapter 10). Oxford University Press. [Google Scholar]
- Cornelis C, Tibben A, Dondorp W, Van Haelst M, Bredenoord A, Knoers N, et al. (2016). Whole-exome sequencing in pediatrics: Parents’ considerations toward return of unsolicited findings for their child. European Journal of Human Genetics, 24(12), 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson J, Cole B, Conta J, Wellner M, Wallace S, Jack R, et al. (2014). Improving the Value of Costly Genetic Reference Laboratory Testing With Active Utilization Management. Archives of Pathology & Laboratory Medicine, 138(1), 110–113. [DOI] [PubMed] [Google Scholar]
- Doyle D, Awwad L, Austin R, Baty I, Bergner J, Brewster C, et al. (2016). 2013 Review and Update of the Genetic Counseling Practice Based Competencies by a Task Force of the Accreditation Council for Genetic Counseling. Journal of Genetic Counseling, 25(5), 868–879. [DOI] [PubMed] [Google Scholar]
- Emanuel E, Emanuel L (1992). Proxy decision making for incompetent patients. An ethical and empirical analysis. JAMA, 267(15):2067–71. [PubMed] [Google Scholar]
- Farnaes L, Hildreth A, Sweeney N, Clark M, Chowdhury S, Nahas S, et al. (2018). Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genomic Medicine, 3(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel L, Pereira S, & McGuire A (2016). Potential Psychosocial Risks of Sequencing Newborns. Pediatrics, 137, S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Berg J, Grody W, Kalia S, Korf B, Martin C, et al. (2013) ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet. Med. 15, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia S, Adelman K, Bale S, Chung W, Eng C, Evans J, et al. (2016). Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): A policy statement of the American College of Medical Genetics and Genomics. Genetics in Medicine, 19(2), 249–255. [DOI] [PubMed] [Google Scholar]
- Kotzer K, Riley J, Conta J, Anderson C, Schahl K, & Goodenberger M (2014). Genetic testing utilization and the role of the laboratory genetic counselor. Clinica Chimica Acta,427, 193–195. [DOI] [PubMed] [Google Scholar]
- Mackley M, Fletcher B, Parker M, Watkins H, & Ormondroyd E (2017). Stakeholder views on secondary findings in whole-genome and whole-exome sequencing: a systematic review of quantitative and qualitative studies. Genet Med 19:283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markel D, & Yashar B (2004). The Interface Between the Practice of Medical Genetics and Human Genetic Research: What Every Genetic Counselor Needs to Know. Journal of Genetic Counseling, 13(5), 351–368. [DOI] [PubMed] [Google Scholar]
- Mathias P, Conta J, Konnick E, Sternen D, Stasi S, Cole B, et al. (2016). Preventing Genetic Testing Order Errors With a Laboratory Utilization Management Program. American Journal of Clinical Pathology, 146(2), 221–226. [DOI] [PubMed] [Google Scholar]
- Pena L, Jiang Y, Schoch K, Spillmann R, Walley N, Stong N, et al. (2018). Looking beyond the exome: a phenotype-first approach to molecular diagnostic resolution in rare and undiagnosed diseases. Genet Med., 20:464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrikin J, Willig L, Smith L, & Kingsmore S (2015). Rapid whole genome sequencing and precision neonatology. Seminars in Perinatology, 39(8), 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrikin J, Cakici J, Clark M, Willig L, Sweeney N, Farrow E, et al. (2018). The NSIGHT1-randomized controlled trial: Rapid whole-genome sequencing for accelerated etiologic diagnosis in critically ill infants. NPJ Genomic Medicine, 3(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M, & Berg I (2015). Incidental Findings with Genomic Testing: Implications for Genetic Counseling Practice. Current Genetic Medicine Reports, 3(4), 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatello M, & Appelbaum P (2015). Honey, I Sequenced the Kids: Preventive Genomics and the Complexities of Adolescence. The American Journal of Bioethics, 15(7), 19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp J, Dong D, Stark C, Ivey L, Hooker G, Biesecker L, & Biesecker B (2014). Parental attitudes, values, and beliefs toward the return of results from exome sequencing in children. Clinical Genetics, 85(2), 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler M (2017, September). Patient Preferences for receiving ACMG Secondary Findings by Clinical Exome Sequencing. Poster session presented at the NSGC 36th Annual Conference, Columbus, OH Available at https://www.genedx.com/wp-content/uploads/2017/09/Poster_NSGC2017_MelisaSiegler.pdf [Google Scholar]
- Stark Z, Lunke S, Brett G, Tan N, Stapleton R, Kumble S, et al. (2018). Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med, e-published ahead of print. [DOI] [PubMed] [Google Scholar]
- Valenstein P, Walsh M, & Stankovic A (2008). Accuracy of Send-Out Test Ordering: A College of American Pathologists Q-Probes Study of Ordering Accuracy in 97 Clinical Laboratories. Archives of Pathology & Laboratory Medicine, 132(2), 206–10. [DOI] [PubMed] [Google Scholar]
- Vissers L, Van Nimwegen K, Schieving J, Kamsteeg E, Kleefstra T, Yntema H, et al. (2017). A clinical utility study of exome sequencing versus conventional genetic testing in pediatric neurology. Genetics in Medicine, 19(9), 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Gonzalez R, & Merajver S (2004). Assessment of genetic testing and related counseling services: Current research and future directions. Social Science & Medicine, 58(7), 1427–1442. [DOI] [PubMed] [Google Scholar]
- Willig P, Smith S, Saunders C, Thiffault M, Miller N, Soden S, et al. (2015). Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: A retrospective analysis of diagnostic and clinical findings. The Lancet Respiratory Medicine, 3(5), 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]