Abstract
Purpose of review:
This review demonstrates the growing body of evidence connecting DNA methylation to prior exposure. It highlights the potential to use DNA methylation patterns as a feasible, stable, and accurate biomarker of past exposure, opening new opportunities for environmental and gene-environment interaction studies among existing banked samples.
Recent findings:
We present the evidence for association between past exposure, including prenatal exposures, and DNA methylation measured at a later time in the life course. We demonstrate the potential utility of DNA methylation-based biomarkers of past exposure using results from multiple studies of smoking as an example. Multiple studies show the ability to accurately predict prenatal smoking exposure based on DNA methylation measured at birth, in childhood, and even adulthood. Separate sets of DNA methylation loci have been used to predict past personal smoking exposure (postnatal) as well. Further, it appears that these two types of exposures, prenatal and previous personal exposure, can be isolated from each other. There is also a suggestion that quantitative methylation scores may be useful for estimating dose. We highlight the remaining needs for rigor in methylation biomarker development including analytic challenges as well as the need for development across multiple developmental windows, multiple tissue types, and multiple ancestries.
Summary:
If fully developed, DNA methylation-based biomarkers can dramatically shift our ability to carry out environmental and genetic-environmental epidemiology using existing biobanks, opening up unprecedented opportunities for environmental health.
Keywords: DNA methylation, biomarker, past exposure, environmental exposure, prenatal smoking, EWAS, epigenomic
Introduction
Heritability analyses for most complex disorders show that at least some portion of disease liability is due to environmental factors[1], often a large component of risk. The specific health consequences of environmental exposures have been well established for many toxicants and outcomes[2, 3]. Yet, many environmental risk factors have not yet been discovered, despite evidence that they play a role in disease. Environmental epidemiology’s goal of identification and characterization of non-heritable risk factors is critical, as these factors provide actionable insights about modifiable causes of disease that can lead to better prediction, prevention, treatment, and policy.
A major limitation to further discovery in environmental epidemiology has been the need for timing-specific exposure information and prospective outcome data. This is a great challenge, particularly for exposures influencing risk on outcomes years to decades later, and for exposures that are difficult to measure or occur prior to feasible study enrollment, such as prenatal or preconception exposures. Some prospective cohort studies do begin prior to pregnancy, or early in pregnancy, and follow new babies through life (e.g.,[4–9]). However, these study designs take years to accumulate outcomes, often with attrition or low enrollment numbers given the timing of enrollment and the length of commitment. Retrospective measurement of exposure is notoriously difficult, given the potential for recall bias in self-report, the lack of information in administrative data such as electronic health records, particularly for toxicants, and the short half-lives of many toxicants – such that biomarker measurement weeks or years later is irrelevant to amounts of exposure at the time of vulnerability.
Thus, there is a critical need in environmental epidemiology for measurement tools that can accurately capture past exposure, particularly prenatal and early life exposures. One emerging area of promise is the ability to measure toxicant content of shed baby teeth, available at middle childhood, but able to inform exposures that occurred in utero[10, 11]. While this is a promising avenue, it does require availability of baby teeth and is to date, relatively expensive with few labs able to perform detailed measurement. Among the other emerging options is the potential for blood, or other readily available tissue samples, to provide past exposure proxy information. This could be transformational for environmental epidemiology and genetic epidemiology. If one can use biosamples already in biobanks, such as UK biobank[12] or the vast genetic consortia banks (e.g.,[13]) to estimate prior exposure with accuracy, there would be ample power to ask environmental exposure questions not previously possible and to truly integrate genetic and environmental information in these large sample sets.
One promising possibility for a blood (or convenience tissue)-based biomarker of past exposure that could enable environmental and gene-environmental work in existing biosamples is the potential for DNA methylation patterns to mark prior exposure. As we show in this review, there is now a substantial body of evidence that DNA methylation measured in blood, and other tissues, is associated with prior exposure, and that this association may be strong enough to enable an accurate predictor of exposure that is timing and toxicant specific. More work must be done to establish such biomarkers for specific exposure, but here we show evidence from discovery epigenome-wide association studies (EWAS) for several exposures and timing, paving the way for such biomarker development. Such discoveries must be further evaluated in prediction models to establish their biomarker utility. As an example, we elaborate on the work done with the association between prenatal smoking exposure and DNA methylation patterns, which has moved from EWAS discovery to biomarker development. The results show promising accuracy, reproducibility, specificity to exposure, and persistence over many years. We also discuss DNA methylation patterns as a cumulative exposure biomarker, or biomarker of aging, through what has been termed “DNA methylation clocks”. Through this review, we hope to present these findings as examples of the opportunities that exist for environmental and genetic-environmental epidemiology through DNA methylation-based biomarkers and call for more work to be done in the field to realize this potential.
Suitability of DNA methylation as a biomarker of past exposure
DNA methylation is a type of epigenetic mark with several inherent properties that make it well suited for exposure biomarker purposes. DNA methylation involves the covalent addition of a methyl or hydroxylmethyl group to cytosine nucleotides in human DNA, and thus, it is relatively stable and not easily degraded with long-term storage. It also does not require any burdensome up front sample collection or processing methods. These properties are particularly important when considering new methods to extract past exposure information from existing biobanks and repositories. While chemically stable, DNA methylation is a dynamic process that can be modified by environmental context and over time; a critical feature of any exposure biomarker. It provides a mechanism for cells and organisms to respond to their environment without changing the DNA sequence. Finally, because DNA methylation is quantitative in nature, it may capture “biological dose” and/or effects of exposure mixtures.
There are several advantages to using DNA methylation as a biomarker of exposure relative to prospectively or retrospectively collected exposure data, metabolites, gene expression, or objective wearable devices. More traditional exposure ascertainment methods can pose several problems. Prospective collection of exposure data is ideal but is costly and can be inefficient for diseases with lower prevalence rates or those with long lag times between exposure and development of disease. Retrospective collection of exposure data is subject to recall bias or misclassification and is impossible to collect for certain exposures (e.g. metals toxicants). The emergence of objective wearable devices can overcome many of these issues but have only recently come online, and thus, don’t enable utilization of existing large-scale biobanks. Use of molecular biomarkers of exposure has been mainstream for decades. For some exposures, metabolites have been the gold standard measurement tool to collect accurate highly reliable information about exposure. For example, cotinine, a major metabolite of nicotine, is widely recognized as the optimal collection metric to obtain smoking status[14, 15]. Untargeted metabolomic assays also have the potential to capture exposure mixtures and quantities. However, one of the major limitations to using metabolites as biomarkers of past exposure is their short half-life. The half-life of most metabolites, including cotinine, is on the order of hours to days[16–18]. Metabolites collected from untargeted assays can also be sensitive to dietary intake differences and sample collection protocols that may vary within and across large biobanks. Laboratory and analytic methods to best address these issues are still under development. Exposure-related transcriptome changes have also been observed. Isolating high quality RNA suitable for gene expression profiling can be challenging in an epidemiologic and biobank resource setting because it is less stable than DNA and more subject to degradation with longer-term storage or suboptimal collection protocols. New molecular biomarkers that are long-lived, specific, stable, and that can be reliably measured in existing banked samples are needed; as evidenced in detail below, DNA methylation meets these criteria.
DNA methylation is associated with past exposure, across multiple domains
With the emergence of affordable genome-scale epigenetic technologies it is now feasible to measure DNA methylation in a large number of samples and perform epigenome-wide association studies (EWAS) to discover methylation differences, at specific CpG sites in the genome, associated with particular exposures or outcomes[19]. This technological advance, coupled with a strong interest in identifying molecular changes related to environmental exposures has led to a rapid increase in environmental epigenomics studies. A wide-range of exposures have now been linked to epigenetic changes in studies where both types of data were measured at the same time; these have been extensively reviewed elsewhere[20–22]. In this review, we focus on EWAS showing DNA methylation patterns, measured across the lifespan, reflect past exposures. As summarized in Table 1, methylation changes have been linked to past exposure, across a wide-range of environmental domains.
Table 1.
Summary of discovery EWAS and look-up replication studies showing exposure-related DNA methylation patterns are present and can be detected long after an exposure occurred.
| Exposure Domain | Tissue | Scale and purpose | Reference | Notes | ||
|---|---|---|---|---|---|---|
| DNAm measure | ||||||
| Behavior/Lifestyle | ||||||
| Smoking | Prenatal | Birth | Placenta | Genome-scale discovery | [82] | |
| Cord blood | EWAS discovery | [27] | ||||
| EWAS discovery | [25] | |||||
| Meta-EWAS discovery | [23] | |||||
| Infancy | Blood spots: Guthrie cards | Lookup of birth EWAS meta-results | [29] | |||
| Birth & 18 months | Cord & peripheral blood | Candidate gene, replication & DNAm persistence | [51] | Showed longitudinal persistence of prenatal smoking DNAm marks | ||
| Childhood | Buccal epithelium | Targeted, gene discovery | [83] | |||
| Peripheral blood | EWAS discovery | [30] | ||||
| Peripheral blood | Lookup of birth EWAS in older children | [23, 31] | ||||
| Birth, childhood, adolescence | Cord blood, peripheral blood | EWAS discovery in cord blood, lookup results in blood collected later in life | [24] | Showed longitudinal persistence of DNAm marks at age 7, and 17 even after adjusting for postnatal and personal exposure | ||
| Peripheral blood | Replication of adolescent EWAS results in an independent sample across 3 timepoints | [26] | Adjusted for postnatal self and parental smoking | |||
| Adolescence | Peripheral blood | EWAS discovery in adolescent blood | [26] | Adjusted for postnatal personal and parental smoking | ||
| Adult | Later adulthood | Buccal epithelium | EWAS discovery | [48] | ||
| Peripheral blood | EWAS discovery | [48] | ||||
| Peripheral blood | Meta-EWAS discovery | [46] | Show methylation trajectories in former smokers do not return to non-smoker levels even after 30yrs | |||
| Peripheral blood | EWAS discovery and replication in different Race | [47] | ||||
| Alcohol | Prenatal | Birth | Cord blood | Meta-EWAS discovery | [28] | |
| Adult | Later adulthood | Peripheral blood | Meta-EWAS discovery, current use prediction | [49] | ||
| Nutrition & Supplementation | ||||||
| Folic acid | Prenatal | Adults, at age 47 | Saliva | EWAS discovery | [32] | Exposure from randomized folic acid supplementation trial - 1960’s |
| Maternal diet | Peri-conception | Childhood | Peripheral blood | Candidate gene discovery | [33, 34] | Seasonally driven nutrition differences - The Gambia |
| Infancy | Peripheral blood | Candidate gene discovery | [35, 36] | Seasonally driven nutrition differences – The Gambia | ||
| Methyl-group donor intake | Prenatal | Infancy | Buccal | Candidate gene, discovery | [37] | |
| Birth | Cord blood | Candidate gene, discovery | [38] | |||
| Docosahexaenoic acid (DHA) | Prenatal | Neonates | Guthrie blood cards | EWAS discovery | [39] | From DOMInO randomized controlled trial for DHA supplementation |
| Childhood | Peripheral blood | Lookup at later time point for persistent changes | ||||
| Short-term high fat overfeeding diet | Adulthood | Adults, 6–8 weeks later | Skeletal muscle | Targeted gene, discovery | [50] | Diet-related DNA methylation levels were still present months after returning to a normal diet |
| Adversity | ||||||
| Dietary (Famine) | Prenatal | Adults, at age 60 | Peripheral blood | Candidate gene discovery | [42, 84] | Data from the Dutch Hunger Winter study |
| Adulthood | Peripheral blood | Candidate gene, replication | [85] | Bangladesh sample | ||
| Stress (Maltreatment) | Childhood | Later Childhood | Saliva | Candidate gene discovery | [43] | Showed 6 month longitudinal changes in DNA methylation among 35 year olds with severe maltreatment exposure |
| Toxicants | ||||||
| Air pollutants (Nitrogen dioxide) | Prenatal | Birth | Cord blood | EWAS discovery | [40] | |
| Childhood | Peripheral blood | Lookup of birth EWAS results in older children | [40] | Associations with prenatal NO2 levels not explained by postnatal NO2 exposure near time of sample collection. | ||
| Lead | Cumulative Lifelong | Adulthood | Peripheral Blood | DNAm global burden (LINE-1) | [86] | |
| Mercury | Prenatal | Birth & childhood | Cord & peripheral blood | DNAm global burden (LC-MS/MS) | [41] | |
| Health conditions | ||||||
| Maternal early pregnancy BMI | Prenatal | Birth | Cord blood | Meta-EWAS discovery | [44] | |
| Prenatal | Adolescence | Peripheral blood | Lookup of meta- EWAS results in adolescence | [44] | ||
| Maternal Eating Disorder | Prenatal | Birth | Cord blood | Global genome DNAm burden | [45] | This could reflect dietary deficiency exposure |
BMI, body mass index
DNAm, DNA methylation
EWAS, epigenome-wide association study
Prenatal exposure to smoking and alcohol.
Several EWAS have identified site-specific changes in DNA methylation levels at birth related to prenatal exposure to maternal smoking[23–27] and alcohol use[28] (Table 1). Several genomic regions have shown suggestive differences in cord blood DNA methylation levels related to maternal drinking habits during early pregnancy[28]. However, studies of prenatal alcohol exposure and DNA methylation are limited by sample size and window of pregnancy timing. Additional genome-wide significant findings may emerge with increased sample sizes and/or more resolved alcohol exposure metrics in the future. For prenatal smoking exposure, site-specific changes in DNA methylation have been detected in peripheral blood obtained from infants[29], older children[23, 24, 26, 30, 31] and adolescents[24]. Associations between later life blood DNA methylation and prenatal smoking exposure persist even after adjusting for postnatal and personal smoking exposures[24, 26]. Smoking and drinking are thought to have similar social determinants and correlated patterns of use; however, the associated DNA methylation findings published to date have not been consistent across these exposures, indicating DNA methylation signatures may be exposure-specific and not merely capturing a social determinant construct[23, 28, 31].
Nutrition and supplementation.
As shown in Table 1, a number of studies have observed DNA methylation changes in samples collected - from birth through adulthood - related to differences in peri- and prenatal exposure to nutrient intake and nutritional supplements [32–39]. Differences in maternal nutrient intake during peri-conception and pregnancy through diet and food availability have been linked to DNA methylation changes, at specific genes, in blood and buccal samples obtained from their offspring at birth, infancy, and childhood[34–38]. A number of studies have leveraged data from cohorts dating back to the 1960’s when the first randomized control trials were carried out to assess the impact of folic acid and/or docosahexaenoic acid (DHA) supplementation on birth and child outcomes. Saliva DNA methylation profiles collected in 47-year old adult offspring of the Aberdeen Folic Acid Supplementation Trial (AFAST) participants showed differences related to whether their mothers received folic acid supplementation during pregnancy or were in the placebo group[32]. A randomized controlled trial for Docosahexaenoic acid (DHA), an omega-3 fatty acid, observed differentially methylated genomic regions among infants whose mothers received DHA relative to those that did not receive the supplement. Furthermore, the methylation differences were also shown to be present in peripheral blood samples collected at 5 years of age[39].
Prenatal toxicant exposures.
In the past year, DNA methylation changes have been linked to air pollutant exposure in the prenatal time period (Table 1). More specifically, a multi-study EWAS meta-analysis identified CpG loci showing significant methylation changes in cord blood, at birth, related to prenatal nitrogen dioxide (NO2) exposure levels. Interestingly, prenatal NO2 associated methylation changes were also observed in peripheral blood obtained from older children. The NO2 exposure levels at the time of blood sample collection in the older children were substantially lower than those the children experienced during pregnancy, arguing that their presence in childhood samples was not likely due to continued postnatal exposure or current NO2 exposure status[40]. More evidence in this area is likely to transpire as additional studies with unified prenatal air pollutant and DNA methylation data emerge. In addition to site-specific changes in DNA methylation, a significant global decrease in the total genomic amount of 5-hydroxymethyl, a specific type of DNA methylation, was observed in birth and early childhood blood samples among children with elevated prenatal exposure to mercury[41].
Prenatal exposure to adversity.
Several social adversity exposures have been associated with long-term changes in DNA methylation (Table 1), although, they have mainly focused on candidate genes. For example, candidate-gene based work, from the historic Dutch Hunger Winter study, revealed DNA methylation levels at the IGF2 gene locus differ significantly between individuals with prenatal exposure to the 1944–45 famine relative to their unexposed same-sex siblings[42]. These changes were detected in blood samples provided 60 years after their prenatal exposure to famine. Exposure to severe maltreatment during early childhood has also been linked to methylation changes in saliva. Significant decreases in DNA methylation at the NR3C1 gene locus were observed among preschool age children exposed to stress/maltreatment in the six months prior to biospecimen collection compared to unexposed children with similar economic status[43].
Maternal conditions in pregnancy.
There is also evidence that exposure to adverse maternal health conditions during pregnancy are related to methylation changes at birth through adolescence (Table 1). A meta-analysis of 19 cohorts reported 86 site-specific changes in DNA methylation, in cord blood, related to maternal body mass index (BMI) at the start of pregnancy[44]. Of those, 72 sites showed a similar association, direction, and magnitude of effect in peripheral blood samples obtained in adolescence[44]. DNA methylation levels among infants born to women with an active eating disorder during pregnancy differed from those whose mothers had an active eating disorder (ED) prior to conception and non-ED controls[45]
Adult exposures and later measurement.
Several studies have reported long-lasting DNA methylation patterns in later adulthood biospecimens related to past earlier adulthood exposures. Similar to prenatal exposures, most findings to date are for behavioral and lifestyle types of exposures including smoking and alcohol use (Table 1). This is likely due to lack of unified exposure and methylation data in the same samples for other, more difficult, to obtain exposures. In world-wide population samples, meta-EWAS have identified thousands of loci where peripheral blood methylation levels differ by current, former, and never smoker status[46–48]. Joehanes et al, found that methylation values among former smokers that quit smoking 30 years prior to collection of methylation measurements in blood samples, still had not reached levels comparable to individuals that never smoked[46]; thus, DNA methylation changes associated with past exposures can be long-lived. Further, smoking-related methylation values appear to capture additional valuable information about past exposures: time-since quitting and number of pack-years smoked[46–48]. This has important implications for the potential to use DNA methylation signatures to serve not only as a simple dichotomous exposure biomarker but also as a biomarker that can be used to determine specific windows and doses of exposure. Similar differences in methylation related to smoking status, time since quitting, and pack-years have also been documented in buccal samples[48], another highly accessible and available tissue source. However, a comparison of DNA methylation patterns among hundreds of former drinkers compared to never drinkers, ~4 years after alcohol cessation, showed only marginal differences between the two exposure groups[49]. Epigenetic changes related to nutrition in adults have also been observed (Table 1). Males exposed to a short-term high fat overfeeding diet showed epigenetic changes that persisted for 6–8 weeks after the men resumed their normal diets[50].
Longitudinal DNAm data.
To date, three studies have reported repeated measures of DNA methylation and associations with exposure information; two were focused on DNA methylation signatures of prenatal smoking exposure and the third examined the effects of maltreatment. Longitudinal analysis of methylation profiles at prenatal smoking-associated CpG sites showed similar differences in DNAm related to prenatal smoking status at 18 months [51], 7, and 17 years of age[24] even after accounting for any postnatal smoking exposures in the older children[24]. However, in adolescence, there were 3 CpG sites that showed reversion back to methylation levels observed among adolescence with no prenatal exposed to maternal smoking[52]. This suggests that signatures of prenatal exposure developed solely in cord blood samples may fail to account for important differences in methylation stability in the postnatal period. Thus, the development of a robust epigenetic biomarker of past exposure will need to take this into account and evaluate methylation patterns at multiple post-exposure time points. The third study examined baseline and longitudinal changes in saliva methylation levels over a period of 6 months, among preschool age children, to assess the effects of maltreatment (at baseline) on methylation at NR3C1[43]. Children with no history of maltreatment showed little variation in methylation across the 2 time points. However, children with a history of maltreatment had significantly higher levels of methylation at baseline and significantly decreased methylation 6 months later. This suggests looking for differences in methylation variation among exposed and unexposed individuals, as opposed to mean methylation shifts, may be a fruitful and important avenue for future studies.
Cumulative exposures, aging, and epigenetic “clocks”
In addition to serving as a biomarker for discrete intervals of exposure, DNA methylation signatures have also been reported to capture continuous cumulative levels of exposures including toxicant and behavioral. For example, measures of global DNA methylation levels in LINE-1 elements were significantly decreased among men with increased cumulative exposure to lead, as assayed via patella bone K-Xray which is a well-established traditional biomarker of long-term lead exposure[53]. In addition, several studies of adult smokers have consistently demonstrated DNA methylation patterns at specific sites accurately reflect the cumulative amount and duration of current and prior smoking[46–48].
A number of DNA methylation “clocks” have been developed to reflect gestational[54–56], pediatric[57], and adult[58–63] chronologic ages, a type of demographic exposure, that can also be thought of as a cumulative exposure. These methylation clocks have been widely used to predict a number of adverse health outcomes demonstrating the utility of DNA methylation exposure biomarkers in epidemiology studies, more broadly[64–67]. For example, the adult-derived epigenetic clock has been shown to better predict all-cause mortality than examination of traditional risk factors or chronological age[68].
Biomarkers require predictive modeling beyond EWAS discovery analyses
EWAS findings continue to emerge and provide valuable insights into the biologic targets of environmental exposures. However, the main output from EWAS isn’t directly informative or useful as a predictive biomarker. Results are typically per-CpG, rather than a collective “signature”. Further, discovery analyses typically rely on general associations between exposed versus unexposed samples. A predictive modeling approach is needed to develop a useful biomarker. Accuracy parameters such as sensitivity, specificity, and area under the ROC curve (AUC) are more relevant for biomarker development[69, 70]. Further, a collection of CpGs associated with the particular exposure will necessarily have better predictive properties than a single CpG. Selection of this collective list, modeling of the prediction algorithm, and evaluation of prediction performance is necessary. This approach has been taken in the development of epigenetic clocks described above. Choices for CpG selection include simply taking all CpGs meeting a particular statistical threshold in EWAS, or building machine-learning models using techniques such as support vector machines or elastic net[71]. Prediction algorithms can then include all CpGs equally, or weighted by their association with the exposure, or other characteristics. The output may be a probabilistic exposure membership (dichotomous, with associated probability), or a methylation-based exposure “score”[52, 72].
Prenatal smoking as an example
For the most well-studied and replicated exposure – prenatal smoking - work in this area has already begun and can be used as an exemplary model for the field to be extended to other types of exposures. The first site-specific differences in DNA methylation related to prenatal exposure to smoking were reported in 2012 by Joubert et al[27], where EWAS revealed 26 CpG sites with exposure-associated DNA methylation differences achieving genome-wide significance. Not long after, studies emerged replicating the findings in additional birth samples and adding a hand full of new loci[24–26]. Many also showed similar DNA methylation patterns associated with prenatal smoking exposure, but when measured in blood samples from older children, ranging in age from 5–17 years[24, 26, 30, 31], even after accounting for parental and personal postnatal smoking exposures[24, 26].
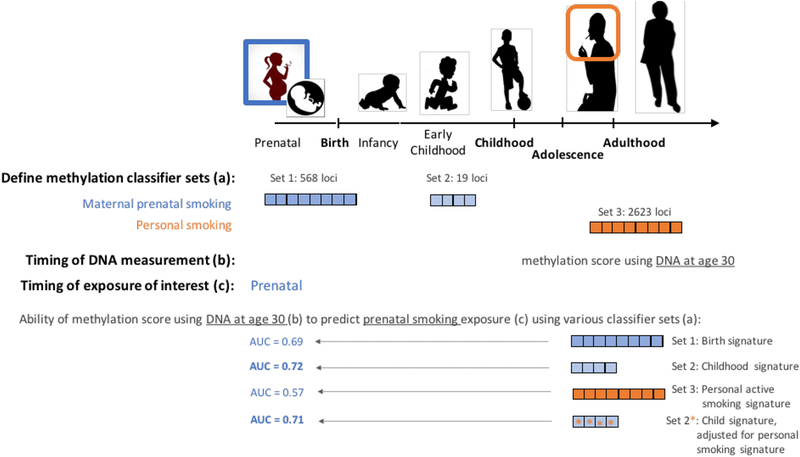
Ladd-Acosta et al[31] were the first to use predictive modeling to evaluate how well DNA methylation levels, measured in blood samples from 5-year old children, at the originally reported 26 CpG sites associated with prenatal smoking exposure, could predict prenatal exposure to smoking from childhood, rather than cord blood. Their support vector machine classifier, with 10-fold cross validation, predicted the children’s exposure to sustained active maternal smoking in pregnancy with 87% accuracy when compared to maternal report of smoking during pregnancy (Table 2). Receiver operating characteristic (ROC) curves also showed the specificity of the model was high; prediction of prenatal smoking exposure using permuted random sets of 26 loci never achieved greater than 60% accuracy and the prenatal smoking classifier was not able to predict exposure to maternal alcohol or medication use with higher than 56% accuracy[31]. The following year, Reese et al[72] developed a single numeric methylation score, based on DNA methylation measured in blood, and showed good correspondence to prenatal cotinine levels consistent with sustained exposure to active maternal smoking. In an independent test set of cord blood samples, the methylation score was able to predict prenatal exposure to sustained smoking with 91% overall accuracy[72] (Table 2). A recent cord blood methylation meta-analysis, spanning 13 world-wide studies and 6,685 samples, showed consistency with previous findings and expanded the set of loci significantly associated with prenatal smoking from dozens to 2,965 CpG sites[23]. Nominally significant differences in methylation were also observed in older children (n=3,187) for every CpG site identified at birth[23]. More recently, Richmond et al[52], developed a methylation-based smoking score using meta-EWAS findings and evaluated its ability to predict prenatal smoking exposure in an independent set of blood samples collected 30 years after pregnancy (Figure 1; Table 2). The first score they derived was based on 568 loci that reached genome-wide significance in cord blood at birth (associated with prenatal smoking exposure) and a second score was based on 19 sites detected in blood from older children at genome-wide significance (associated with prenatal smoking)[23]. Given the age of the participants at time of blood collection and methylation measurements, it is possible that the offspring themselves smoked; therefore, the authors also computed a methylation score for personal (postnatal) smoking exposure using 2,623 sites identified as significantly associated with current smoking status in a large adult smoking meta-analysis[46]. As shown in Figure 1 and Table 2, the classification accuracy of the prenatal exposure methylation score, based on 30-year old adult blood specimens, was highest when using the 19 locus methylation score method that had been derived using middle childhood methylation data (AUC=0.72). Somewhat unexpectedly, the cord blood derived score had a lower overall prediction accuracy (AUC = 0.69). This highlights the importance of including childhood samples in discovery EWAS and for including loci identified in childhood samples in prenatal biomarker development, if later life biosamples are the intended use. Importantly, they also showed current smoking exposure scores can’t predict prenatal smoking exposure with high accuracy (AUC = 0.57). Thus, these classifiers appear specific to prenatal exposure. This is consistent with previous observations that there is some, but not complete, overlap of loci associated with prenatal smoking exposure and personal adolescent or adult smoking exposures[26, 46].
Table 2.
DNA methylation-based biomarkers of exposure to smoking.
| Study | Persistence | Tissue | Model | Predictive accuracy | Notes | ||
|---|---|---|---|---|---|---|---|
| DNAm measure | |||||||
| Reese et al (2017)[72] | Prenatal | Birth | Days-months | Cord blood | Score: linear combination of 28 loci and LASSO regression coefficients | AUC1 = 0.90 | |
| Ladd-Acosta et al (2016)[31] | Early Childhood (Age 5) | 5 years | Peripheral blood | SVM classifier: 26 loci | AUC1 = 0.87 | ||
| Richmond et al (2018)[52] | Adulthood (Age 30) | ~30 years | Peripheral blood | Score: weighted† sum of 568 loci* |
AUC1 = 0.69 | ||
| Adulthood (Age 30) | ~30 years | Peripheral blood | Score: weighted† sum of 19** loci |
AUC1 = 0.72 | |||
| Adulthood (Age 30) | Peripheral blood | Score: weighted† sum of 2623*** loci |
AUC1 = 0.57 | Shows current personal smoking score is not a good predictor of prenatal smoking exposure. | |||
| Shenker etl (2013)[73] | Adult | Later Adulthood | Peripheral blood | GLM using 4 loci | AUC2 = 0.83 AUC3 = 0.97 |
DNAm biomarker better predictor than cotinine (AUC=0.47) DNAm correlated with time since quitting and duration of smoking |
DNAm, DNA methylation
GLM, generalized linear model
SVM, support vector machine
Predicting prenatally exposed versus unexposed
Predicting former vs never smokers
Predicting current versus never smokers
568 DNAm loci originally identified in cord blood
DNAm loci originally identified in blood during middle childhood
DNAm loci identified in adult blood as predictive of current smoking
Weights determined by: per CpG effect size/average effect size for all measured CpG sites.
Figure 1. DNA methylation biomarkers, regardless of timing of sample collection, can be used to predict prenatal smoking.
As reported in Richmond et al[52], adult biosamples can accurately predict prenatal smoking, even after accounting for post-natal (own) smoking. Predefined sets of CpG DNA methylation loci can be used for prediction. Derived reference sets from infant cord blood and from middle childhood blood are available (top). The CpG set derived from childhood samples achieves slightly better prediction parameters (bottom).
Finally, separate DNA methylation patterns have been shown to predict prior adult personal smoking exposure. A 4-CpG model using predictive generalized linear models has been shown to predict prior personal smoking status among adults[73]. The 4-locus model was highly accurate in an independent test sample with an AUC = 0.83[73] (Table 2). Furthermore, they showed DNA methylation is a better long-term biomarker of exposure than cotinine. The prediction model using cotinine levels was able to accurately predict former adulthood smoking in only 47% of the samples compared to 83% when DNAm was used as a biomarker of personal smoking history[73] (Table 2). While associations between DNAm levels and specific dose, duration, and time since quitting have been observed in adults[46–48], these more detailed exposure classes have not been pursued in published predictive analyses to date.
Need for additional evidence
The smoking exposure examples demonstrate the potential for DNA methylation-based biomarkers of prior exposure. Multiple studies show the ability to accurately predict prenatal exposure based on DNA methylation measured at birth, in childhood, and even adulthood. Separate sets of DNA methylation loci can be used to accurately predict past personal adult exposure as well. Further, it appears that these two types of exposures, prenatal and previous personal exposure, can be isolated from each other. There is also a suggestion that quantitative methylation scores may be useful for estimating dose. If fully developed, such biomarkers, across multiple exposures and DNA measurement windows, can dramatically shift our ability to carry out environmental and genetic-environmental epidemiology using existing biobanks. However, much more work must be done. First, studies must move from site-by-site discovery EWAS approaches to classification approaches. The field must establish best practices for selecting CpGs that create accurate and generalizable classifiers. Multiple feature selection algorithms are available, and multiple metrics of predictive accuracy exist. The influence of QC pipelines on accuracy must also be considered, as has been done in other omics classifier work[74]. Perhaps most importantly, the accuracy and utility of DNA methylation biomarkers of exposure must be explored across ancestries and tissue matrices. Because DNA methylation at many CpG sites is, in part, genetically controlled[75, 76], it is likely that DNA methylation signatures of exposure may vary by ancestry. Additionally, the effects of environmental exposures on the epigenome can be influenced by underlying genotypes[77–81]. Genetic heterogeneity is likely to be particularly important among genes that establish, maintain, and regulate DNA methylation as well as for genes involved in exposure metabolism and detoxification. Thus, studies that assess potential genetic modification of epigenetic signatures of exposure are also needed. Tissue type will also play a critical role. While it is not necessary that a biomarker be on the causal path of an exposure to the ultimate health outcome of interest, it may still be true that different DNA methylation sites show predictive accuracy in different cell types. This is because the base level and variability of DNA methylation varies by cell type, and thus the opportunity for additional variation that captures exposure is likely to be heterogeneous across tissue types. This has already been established for epigenetic clocks, where patterns from single tissue types do not fully overlap in their age prediction accuracy[60]. These caveats to not diminish enthusiasm for this potentially influential area for epidemiology, but do call attention to the rigorous work ahead.
Conclusions:
The ability to obtain measures of environmental exposures in existing samples and biobanks will enable new large-scale analyses to investigate modifiable environmental risk factors for disease as well as their interaction with genes. Both inherent properties and empiric evidence support the potential for DNA methylation to serve as a stable, long-term biomarker of past exposures across a range of environmental domains. Predictive models and methylation based exposure scores are emerging and have shown high accuracy in their ability to predicting former prenatal and adulthood personal smoking exposures. To fully realize the potential of DNA methylation as exposure biomarkers, continued large-scale EWAS and development of predictive models, across time points, tissue types, and ancestry are needed.
Footnotes
Conflict of Interest
M. Daniele Fallin and Christine Ladd-Acosta each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Wang K, et al. , Classification of common human diseases derived from shared genetic and environmental determinants. Nat Genet, 2017. 49(9): p. 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samet JM, Tobacco smoking: the leading cause of preventable disease worldwide. Thorac Surg Clin, 2013. 23(2): p. 103–12. [DOI] [PubMed] [Google Scholar]
- 3.NTP monograph on health effects of low-level lead. NTP Monogr, 2012(1): p. xiii, xv–148. [PubMed] [Google Scholar]
- 4.Wang G, et al. , Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA, 2014. 311(6): p. 587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newschaffer CJ, et al. , Infant siblings and the investigation of autism risk factors. J Neurodev Disord, 2012. 4(1): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oken E, et al. , Cohort profile: project viva. Int J Epidemiol, 2015. 44(1): p. 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaddoe VW, et al. , The Generation R Study: Design and cohort profile. Eur J Epidemiol, 2006. 21(6): p. 475–84. [DOI] [PubMed] [Google Scholar]
- 8.Magnus P, et al. , Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol, 2016. 45(2): p. 382–8. [DOI] [PubMed] [Google Scholar]
- 9.Boyd A, et al. , Cohort Profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol, 2013. 42(1): p. 111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claus Henn B, et al. , Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environ Res, 2018. 161: p. 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andra SS, Austin C, and Arora M, Tooth matrix analysis for biomonitoring of organic chemical exposure: Current status, challenges, and opportunities. Environ Res, 2015. 142: p. 387–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudlow C, et al. , UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med, 2015. 12(3): p. e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross-Disorder Group of the Psychiatric Genomics, C., et al. , Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet, 2013. 45(9): p. 984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benowitz NL, Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect, 1999. 107 Suppl 2: p. 349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benowitz NL, Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev, 1996. 18(2): p. 188–204. [DOI] [PubMed] [Google Scholar]
- 16.Lee DH and Jacobs DR Jr., Methodological issues in human studies of endocrine disrupting chemicals. Rev Endocr Metab Disord, 2015. 16(4): p. 289–97. [DOI] [PubMed] [Google Scholar]
- 17.Johns LE, et al. , Exposure assessment issues in epidemiology studies of phthalates. Environ Int, 2015. 85: p. 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen JM, Human exposure to toxic metals: factors influencing interpretation of biomonitoring results. Sci Total Environ, 1995. 166: p. 89–135. [DOI] [PubMed] [Google Scholar]
- 19.Rakyan VK, et al. , Epigenome-wide association studies for common human diseases. Nat Rev Genet, 2011. 12(8): p. 529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakulski KM and Fallin MD, Epigenetic epidemiology: promises for public health research. Environ Mol Mutagen, 2014. 55(3): p. 171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burris HH and Baccarelli AA, Environmental epigenetics: from novelty to scientific discipline. J Appl Toxicol, 2014. 34(2): p. 113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortessis VK, et al. , Environmental epigenetics: prospects for studying epigenetic mediation of exposure-response relationships. Hum Genet, 2012. 131(10): p. 1565–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joubert BR, et al. , DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. Am J Hum Genet, 2016. 98(4): p. 680–96.*Largest epigenome-wide association study for prenatal smoking exposure to date, consisting of 6,685 samples from 13 studies. Identified thousands of loci showing DNA methylation changes in cord blood, at birth, related to in utero exposure to smoking.
- 24.Richmond RC, et al. , Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet, 2015. 24(8): p. 2201–17.*Repeated biosampling in children, from birth to age 17, enabled examination of long-term persistence of prenatal smoking associated methylation changes in the same individuals over time. Significant differences in methylation were observed at multiple loci even after adjusting for postnatal household and personal exposures.
- 25.Markunas CA, et al. , Identification of DNA methylation changes in newborns related to maternal smoking during pregnancy. Environ Health Perspect, 2014. 122(10): p. 1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KW, et al. , Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ Health Perspect, 2015. 123(2): p. 193–9.*Shows DNA methylation changes related to prenatal exposure can be detected in adolescence. Also replicated findings in an independent sample at birth and ages 7 and 17.
- 27.Joubert BR, et al. , 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect, 2012. 120(10): p. 1425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharp GC, et al. , Maternal alcohol consumption and offspring DNA methylation: findings from six general population-based birth cohorts. Epigenomics, 2018. 10(1): p. 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hannon E, et al. , Elevated polygenic burden for autism is associated with differential DNA methylation at birth. Genome Med, 2018. 10(1): p. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breton CV, et al. , Prenatal tobacco smoke exposure is associated with childhood DNA CpG methylation. PLoS One, 2014. 9(6): p. e99716.*Provides evidence that DNA methylation patterns present in child DNA reflect prenatal exposure to smoking
- 31.Ladd-Acosta C, et al. , Presence of an epigenetic signature of prenatal cigarette smoke exposure in childhood. Environ Res, 2016. 144(Pt A): p. 139–148.**Reported prenatal smoking associated methylation patterns, originally detected in an independent birth sample, are also present in childhood. They were the first to report prenatal exposure to smoking can be accurately (AUC=0.87) predicted using DNA methylation patterns in the blood of 5 year old children.
- 32.Richmond RC, et al. , The long-term impact of folic acid in pregnancy on offspring DNA methylation: follow-up of the Aberdeen Folic Acid Supplementation Trial (AFAST). Int J Epidemiol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steegers-Theunissen RP, et al. , Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One, 2009. 4(11): p. e7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dominguez-Salas P, et al. , Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun, 2014. 5: p. 3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silver MJ, et al. , Independent genomewide screens identify the tumor suppressor VTRNA2–1 as a human epiallele responsive to periconceptional environment. Genome Biol, 2015. 16: p. 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waterland RA, et al. , Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet, 2010. 6(12): p. e1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pauwels S, et al. , Maternal intake of methyl-group donors affects DNA methylation of metabolic genes in infants. Clin Epigenetics, 2017. 9: p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pauwels S, et al. , Dietary and supplemental maternal methyl-group donor intake and cord blood DNA methylation. Epigenetics, 2017. 12(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Dijk SJ, et al. , Effect of prenatal DHA supplementation on the infant epigenome: results from a randomized controlled trial. Clin Epigenetics, 2016. 8: p. 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruzieva O, et al. , Epigenome-Wide Meta-Analysis of Methylation in Children Related to Prenatal NO2 Air Pollution Exposure. Environ Health Perspect, 2017. 125(1): p. 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardenas A, et al. , Prenatal Exposure to Mercury: Associations with Global DNA Methylation and Hydroxymethylation in Cord Blood and in Childhood. Environ Health Perspect, 2017. 125(8): p. 087022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobi EW, et al. , DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet, 2009. 18(21): p. 4046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parent J, et al. , Dynamic stress-related epigenetic regulation of the glucocorticoid receptor gene promoter during early development: The role of child maltreatment. Dev Psychopathol, 2017. 29(5): p. 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharp GC, et al. , Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum Mol Genet, 2017. 26(20): p. 4067–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazmi N, et al. , Maternal eating disorders affect offspring cord blood DNA methylation: a prospective study. Clin Epigenetics, 2017. 9: p. 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joehanes R, et al. , Epigenetic Signatures of Cigarette Smoking. Circ Cardiovasc Genet, 2016. 9(5): p. 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MK, et al. , DNA methylation and smoking in Korean adults: epigenome-wide association study. Clin Epigenetics, 2016. 8: p. 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan ES, et al. , Cigarette smoking behaviors and time since quitting are associated with differential DNA methylation across the human genome. Hum Mol Genet, 2012. 21(13): p. 3073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu C, et al. , A DNA methylation biomarker of alcohol consumption. Mol Psychiatry, 2018. 23(2): p. 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobsen SC, et al. , Effects of short-term high-fat overfeeding on genome-wide DNA methylation in the skeletal muscle of healthy young men. Diabetologia, 2012. 55(12): p. 3341–9. [DOI] [PubMed] [Google Scholar]
- 51.Novakovic B, et al. , Postnatal stability, tissue, and time specific effects of AHRR methylation change in response to maternal smoking in pregnancy. Epigenetics, 2014. 9(3): p. 377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richmond RC, et al. , DNA methylation as a marker for prenatal smoke exposure in adults. Int J Epidemiol, 2018.**Showed methylation scores obtained from DNA collected at age 30, can predict prenatal exposure to smoking with 72% accuracy. Also showed loci associated with postnatal personal smoking are not good predictors of prenatal smoking exposure (AUC=0.57), suggesting methylation patterns differ by exposure window.
- 53.Leggett RW, An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect, 1993. 101(7): p. 598–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simpkin AJ, Suderman M, and Howe LD, Epigenetic clocks for gestational age: statistical and study design considerations. Clin Epigenetics, 2017. 9: p. 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knight AK, et al. , An epigenetic clock for gestational age at birth based on blood methylation data. Genome Biol, 2016. 17(1): p. 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bohlin J, et al. , Prediction of gestational age based on genome-wide differentially methylated regions. Genome Biol, 2016. 17(1): p. 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alisch RS, et al. , Age-associated DNA methylation in pediatric populations. Genome Res, 2012. 22(4): p. 623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Q, et al. , DNA methylation levels at individual age-associated CpG sites can be indicative for life expectancy. Aging (Albany NY), 2016. 8(2): p. 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weidner CI, et al. , Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol, 2014. 15(2): p. R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horvath S, DNA methylation age of human tissues and cell types. Genome Biol, 2013. 14(10): p. R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hannum G, et al. , Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell, 2013. 49(2): p. 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garagnani P, et al. , Methylation of ELOVL2 gene as a new epigenetic marker of age. Aging Cell, 2012. 11(6): p. 1132–4. [DOI] [PubMed] [Google Scholar]
- 63.Bocklandt S, et al. , Epigenetic predictor of age. PLoS One, 2011. 6(6): p. e14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marioni RE, et al. , DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol, 2015. 16: p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knight AK, Conneely KN, and Smith AK, Gestational age predicted by DNA methylation: potential clinical and research utility. Epigenomics, 2017. [DOI] [PubMed] [Google Scholar]
- 66.Horvath S, et al. , An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol, 2016. 17(1): p. 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horvath S, et al. , Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci U S A, 2014. 111(43): p. 15538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen BH, et al. , DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY), 2016. 8(9): p. 1844–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bossuyt PM, et al. , STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ, 2015. 351: p. h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phan JH, Kothari S, and Wang MD, omniClassifier: a Desktop Grid Computing System for Big Data Prediction Modeling. ACM BCB, 2014. 2014: p. 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhuang J, Widschwendter M, and Teschendorff AE, A comparison of feature selection and classification methods in DNA methylation studies using the Illumina Infinium platform. BMC Bioinformatics, 2012. 13: p. 59.**Developed a methylation score in cord blood at birth that reflects prenatal exposure to sustained smoking, The score was able to predict exposure status in an independent birth sample with 90% accuracy.
- 72.Reese SE, et al. , DNA Methylation Score as a Biomarker in Newborns for Sustained Maternal Smoking during Pregnancy. Environ Health Perspect, 2017. 125(4): p. 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shenker NS, et al. , DNA methylation as a long-term biomarker of exposure to tobacco smoke. Epidemiology, 2013. 24(5): p. 712–6.*First to show generalized linear model, using methylation levels at 4 CpG sites, can accurately predict previous personal smoking history among adults.
- 74.Xu J, et al. , The FDA’s Experience with Emerging Genomics Technologies-Past, Present, and Future. AAPS J, 2016. 18(4): p. 814–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andrews SV, et al. , Cross-tissue integration of genetic and epigenetic data offers insight into autism spectrum disorder. Nat Commun, 2017. 8(1): p. 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith AK, et al. , Methylation quantitative trait loci (meQTLs) are consistently detected across ancestry, developmental stage, and tissue type. BMC Genomics, 2014. 15: p. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ren JC, et al. , MTHFR Gene Polymorphism Is Associated With DNA Hypomethylation and Genetic Damage Among Benzene-Exposed Workers in Southeast China. J Occup Environ Med, 2018. 60(4): p. e188–e192. [DOI] [PubMed] [Google Scholar]
- 78.Zhang GH, et al. , Do mutations in DNMT3A/3B affect global DNA hypomethylation among benzene-exposed workers in Southeast China?: Effects of mutations in DNMT3A/3B on global DNA hypomethylation. Environ Mol Mutagen, 2017. 58(9): p. 678–687. [DOI] [PubMed] [Google Scholar]
- 79.Declerck K, et al. , Interaction between prenatal pesticide exposure and a common polymorphism in the PON1 gene on DNA methylation in genes associated with cardio-metabolic disease risk-an exploratory study. Clin Epigenetics, 2017. 9: p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aarabi M, et al. , High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Hum Mol Genet, 2015. 24(22): p. 6301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sipahi L, et al. , Longitudinal epigenetic variation of DNA methyltransferase genes is associated with vulnerability to post-traumatic stress disorder. Psychol Med, 2014. 44(15): p. 3165–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suter M, et al. , Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics, 2011. 6(11): p. 1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Breton CV, et al. , Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med, 2009. 180(5): p. 462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heijmans BT, et al. , Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A, 2008. 105(44): p. 17046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finer S, et al. , Is famine exposure during developmental life in rural Bangladesh associated with a metabolic and epigenetic signature in young adulthood? A historical cohort study. BMJ Open, 2016. 6(11): p. e011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wright RO, et al. , Biomarkers of lead exposure and DNA methylation within retrotransposons. Environ Health Perspect, 2010. 118(6): p. 790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]