Abstract
Background
Self‐management education may help patients with cystic fibrosis and their families to choose, monitor and adjust treatment requirements for their illness, and also to manage the effects of illness on their lives. Although self‐management education interventions have been developed for cystic fibrosis, no previous systematic review of the evidence of effectiveness of these interventions has been conducted.
Objectives
To assess the effects of self‐management education interventions on improving health outcomes for patients with cystic fibrosis and their caregivers
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register (date of the last search: 22 August 2013).
We also searched databases through EBSCO (CINAHL; Psychological and Behavioural Sciences Collection; PsychInfo; SocINDEX) and Elsevier (Embase) and handsearched relevant journals and conference proceedings (date of the last searches: 01 February 2014 ).
Selection criteria
Randomised controlled trials, quasi‐randomised controlled trials or controlled clinical trials comparing different types of self‐management education for cystic fibrosis or comparing self‐management education with standard care or no intervention.
Data collection and analysis
Two authors assessed trial eligibility and risk of bias. Three authors extracted data.
Main results
Four trials (involving a total of 269 participants) were included. The participants were children with cystic fibrosis and their parents or caregivers in three trials and adults with cystic fibrosis in one trial. The trials compared four different self‐management education interventions versus standard treatment: (1) a training programme for managing cystic fibrosis in general; (2) education specific to aerosol and airway clearance treatments; (3) disease‐specific nutrition education; and (4) general and disease‐specific nutrition education. Training children to manage cystic fibrosis in general had no statistically significant effects on weight after six to eight weeks, mean difference ‐7.74 lb (i.e. 3.51 kg) (95% confidence interval ‐35.18 to 19.70). General and disease‐specific nutrition education for adults had no statistically significant effects on: pulmonary function (forced expiratory volume at one second), mean difference ‐5.00 % (95% confidence interval ‐18.10 to 8.10) at six months and mean difference ‐5.50 % (95% confidence interval ‐18.46 to 7.46) at 12 months; or weight, mean difference ‐ 0.70 kg (95% confidence interval ‐6.58 to 5.18) at six months and mean difference ‐0.70 kg (95% confidence interval ‐6.62 to 5.22) at 12 months; or dietary fat intake scores, mean difference 1.60 (85% confidence interval ‐2.90 to 6.10) at six months and mean difference 0.20 (95% confidence interval ‐4.08 to 4.48) at 12 months. There is some limited evidence to suggest that self‐management education may improve knowledge in patients with cystic fibrosis but not in parents or caregivers. There is also some limited evidence to suggest that self‐management education may result in positively changing a small number of behaviours in both patients and caregivers.
Authors' conclusions
The available evidence from this review is of insufficient quantity and quality to draw any firm conclusions about the effects of self‐management education for cystic fibrosis. Further trials are needed to investigate the effects of self‐management education on a range of clinical and behavioural outcomes in children, adolescents and adults with cystic fibrosis and their caregivers.
Plain language summary
Self‐management education for cystic fibrosis
We set out to review the effects of self‐management education for cystic fibrosis on a range of health outcomes in individuals of all ages with cystic fibrosis and their caregivers. Our search for available evidence identified four trials, and all four compared a form of self‐management education to standard treatment. The precise focus of self management differed between trials and included a training programme for managing cystic fibrosis, education on chest treatments, education on nutrition specific to cystic fibrosis, and education on general and disease‐specific nutrition. Self‐management education had no positive effects on lung function, weight, or intake of fatty food. There is some evidence to suggest that self‐management education improves knowledge about cystic fibrosis and its management in patients with this condition and some self‐management behaviours in patients and caregivers. However, due to the small number of trials in this review, and because of concerns about the quality of these trials, we are unable to reach any firm conclusions about the effects of self‐management education for cystic fibrosis. We recommend that further trials are conducted to evaluate the effects of self‐management education interventions.
Background
Description of the condition
Cystic fibrosis (CF) is the most common life‐limiting, autosomal recessively inherited disease in Caucasian populations with an estimated incidence of 1 per 3000 births per annum (Walters 2007). Most individuals are diagnosed in their first year of life and many countries now have newborn screening programmes. The disease manifests as pancreatic insufficiency, leading to malabsorption and failure to thrive and impaired mucociliary clearance, leading to recurrent chest infections and bronchiectasis. Advances in the treatment of this disease have resulted in a marked increase in survival rates over the past three decades and individuals can now be expected to live into their fourth decade (Dodge 2007). Nonetheless, CF remains a progressive disease involving a complex regimen of daily treatment including high fat, high calorie dietary intake, pancreatic enzyme replacement, vitamin supplementation, chest physiotherapy, nebulized medication, and antibiotic therapy in the event of respiratory infection. This daily regimen places considerable responsibility on patients and family members (especially parents of children and adolescents) to implement treatment requirements in an effort to optimise health and slow down disease progression.
Description of the intervention
The role that individuals with CF and family members play in the active management of their care is now seen as important for increasing the likelihood of positive health outcomes (Savage 2007; Sawicki 2007; Williams 2007). A number of self‐management education interventions for patients with CF and their families, or both, have been developed since the 1990s (e.g. Bartholomew 1991; Bartholomew 1997; Downs 2006). Self‐management can be described as helping patients and their families to choose, monitor and adjust treatment requirements for their illness, and also manage the effects of illness on their lives. The aim is to help them achieve the best possible health, and to fit treatment requirements into their everyday activities around a flexible management plan. The role of health care professionals is to support patients and families in this task (Newman 2004).
How the intervention might work
In order to make a difference, self‐management education interventions should help patients and families to solve problems, set goals, and then plan changes in the ways they behave, so that they are motivated to manage their illness in the best possible way toward optimum health outcomes (Lorig 2003; Schreurs 2003). Traditionally, patient education programmes typically provided disease‐specific knowledge aimed at increasing compliance with medical treatment and healthcare professional advice (Lorig 2002). In contrast, self‐management education interventions should equip patients and families with knowledge, confidence, and skills to take responsibility for daily decisions concerning their health and to take effective control over managing the demands of chronic illness in ways that are flexible and relevant to their lives (Lorig 2002). Self‐management education should work in ways that position patients and their families as 'experts' working in partnership with health care professionals (Department of Health 2001).
Why it is important to do this review
Although self‐management education interventions for patients with CF or family members, or both, continue to be developed and advocated, there remains uncertainty over the effects of these interventions and to date no previous systematic review of the evidence has been conducted. This is an updated version of a previously published review (Savage 2011).
Objectives
To assess the effects of self‐management education interventions on improving health outcomes for patients with CF and their caregivers.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials, quasi‐randomised controlled trials and controlled clinical trials. Both published and unpublished studies were considered and no language restrictions were applied.
Types of participants
Individuals of all ages with a diagnosis of CF (diagnosed clinically and by sweat or genetic testing) or family members, or both.
Types of interventions
Self‐management education programmes designed to help patients, of any age group, or family members, or both, to solve problems, set goals, and to plan how best to manage treatment requirements of CF in their daily lives. Education programmes were only included if a focus on self‐management was explicitly specified in the aims of the programme or the content of the programme, or both. Programmes involving any structured educational or instructional approach were considered, e.g. web‐based learning; computer‐aided programme; video or audiotapes; written materials; one‐to‐one or group educational sessions. The interventions included, but were not limited to, self‐management education designed to assist patients or their caregivers or both with dietary management including pancreatic enzyme replacement and vitamin supplementation, physiotherapy techniques and exercises; and medication management.
The following comparisons were considered:
a self‐management education intervention versus another self‐management educational intervention;
a self‐management education intervention versus no intervention;
a self‐management education intervention versus 'standard treatment'.
Types of outcome measures
Primary outcomes
-
Pulmonary function (analysed as per cent predicted)
forced expiratory volume at one second (FEV1)
forced vital capacity (FVC)
residual volume/total lung capacity (RV/TLC)
forced expiratory flow 25‐75% (FEF25‐75%)
-
Indices of nutritional health or growth
change in height
change in weight
body mass index (BMI)
z score
any other indices of nutritional health
Secondary outcomes
Self‐management behaviour: any measure of the abilities of the patient (or family member, or both) to fit treatment requirements for CF into their everyday activities. For the purpose of this systematic review, we included measures of self‐management skills (e.g. monitoring symptoms, monitoring calorie intake, regulating pancreatic enzymes according to fat content of food, performance of breathing techniques; goal setting and planning care; communicating about illness or aspects of care). We also included measures of independence, self‐efficacy, coping, problem solving.
Adherence to CF treatment requirements: any measure of the patient's or family member's, or both, adherence including pill counts, self‐report forms, diaries, electronic monitoring, prescription refill history.
Knowledge: any measure of the patient's or family member's, or both, knowledge of CF and its management.
Health‐related quality of life: generic or disease‐specific, or both; physical, psychological, social, cognitive, school functioning.
Utilisation of health services: e.g. number of acute hospitalisations, average length of hospital stay, clinic appointments (scheduled and unscheduled), number of visits to general practitioner, number of respiratory exacerbations requiring systemic antibiotics.
Search methods for identification of studies
Electronic searches
We identified relevant trials from the Group's Cystic Fibrosis Trials Register facilitated by the Trials Search co‐ordinator using the terms: *education* OR family/community based support program OR behaviour.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (Clinical Trials) (updated each new issue of The Cochrane Library), quarterly searches of MEDLINE, a search of EMBASE to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work was identified by searching the book of abstracts of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group Module.
Date of the last search of the Cochrane Cystic Fibrosis and Genetic Disorders Group Cystic Fibrosis Trials Register: 22 August 2013.
We also undertook separate searches of the following databases: CINAHL with full text (EBSCO) (Appendix 1); Psychological and Behavioural Sciences Collection (EBSCO) (Appendix 2); PsycINFO (EBSCO) (Appendix 3); SocINDEX (EBSCO) (Appendix 4); Embase (Elsevier) (Appendix 5). No language restrictions were applied to separate searches of databases.
Date of the last search of each of these databases: 01 February 2014.
Searching other resources
Reference lists of relevant trials identified were examined for additional citations. Specialists in the field and authors of the included trials were contacted to identify possible unpublished data.
Data collection and analysis
Selection of studies
To identify potentially eligible trials, two authors (ES, PB) independently screened the titles and abstracts of all reports gleaned through the search strategy. Where it was not possible to tell from the title and abstract whether a study was potentially eligible for inclusion, the authors retrieved full text copies of the studies. We applied no language restrictions to our search strategy. We planned to have any papers written in a foreign language translated prior to evaluating eligibility for inclusion if this could not be determined from the title and abstract (if available in the English language), or if an abstract was not available. We identified one non‐Engligh paper (French) (David 2008). One author (MNiC), who is fluent in this language, translated this paper. Two authors (ES, PB) independently read full text copies of all trials appearing to meet the inclusion criteria to determine their eligibility for inclusion in the review. We resolved any disagreements by discussion. If resolution was not possible, we planned to consult the other members of review team to adjudicate and reach consensus, however, this was not necessary.
Data extraction and management
For all trials that met the inclusion criteria, one author (ES) extracted data, two authors (PB, DF) independently cross‐checked these. We resolved discrepancies by discussion. If needed, we planned to consult the other members of review team to resolve any disagreements. We used a standardised form adapted from the checklist of items in Table 7.3a in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), to extract data from each trial:
general information (e.g. title, authors, citation and contact details);
methods (trial design, randomisation process and other concerns about bias, study duration);
participants (total number and flow of participants through trial, reasons for attrition, sample size estimations, settings, severity of illness, age and sex, details on co‐morbidity);
interventions (description of intervention including its content, mode of delivery, duration, setting, number of groups, treatment of controls);
outcomes (primary and secondary outcomes relevant to this review, measures used, time points of data collection, intention to treat analysis);
results (for each outcome ‐ sample size, number of missing participants, summary data for each intervention group, estimate of effect, and subgroup analyses);
miscellaneous (funding source, key conclusions by study authors, references to other relevant articles).
A third author (DF) cross‐checked data on number of participants, mean scores and standard deviations (SD) entered into RevMan for each outcome against the data extraction forms and published records (RevMan 2011). We contacted trial authors for information either missing or unclear in published records. Where possible, we grouped outcome data into those measured at 1 to 6 months, 7 to 12 months, 13 to 18 months, 19 to 24 months and 6 monthly intervals after these time points if applicable.
Assessment of risk of bias in included studies
Two authors (ES, PB) assessed each of the included trials for risk of bias and disagreements were resolved through discussion without the need to consult other members of the review team. We assessed the risk of bias using the six specific domain‐based evaluation criteria as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1 (Higgins 2011b). These were sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias. For 'other sources of bias' we assessed efforts at minimizing cross‐contamination bias (i.e. unofficial delivery of any aspect of the intervention to the 'control' group) including selecting intervention and control groups from different CF centres, asking participants in the 'control' group not to access or use self‐management education material from sources such as the Internet, other CF families, CF organisations; asking participants in intervention group not to discuss the intervention with others until the end of the study; asking the control group what information about managing CF they have accessed during the course of the study. In evaluating the risk of bias for each of the six domains within each study included in the review, we made a judgement of 'low risk', 'high risk' or 'unclear risk' on the following basis:
'low risk' of bias if the description of a domain indicated that it was adequately addressed;
'high risk' of bias if the description of a domain indicated that it was not adequately addressed;
'unclear risk' of bias if insufficient detail about a domain was reported.
Measures of treatment effect
To assess differences between groups, we recorded post‐treatment mean difference (MD) values with 95% confidence intervals (CI) as our treatment effect measure for continuous variables. For dichotomous outcome data, we planned to assess treatment effects by calculating risk ratios (RR) with 95% confidence intervals (CIs). However, the trials included in this review only reported continuous outcome data.
Unit of analysis issues
For longitudinal measurements, we analysed data at each assessment time‐point post treatment.
Dealing with missing data
To allow an intention‐to‐treat analysis, we planned to seek data on the number of participants with each outcome event, by allocated treated group, irrespective of adherence and whether or not the individual was later thought to be ineligible or otherwise excluded from treatment or follow‐up. We contacted primary authors of trials to clarify data where necessary or to advise on data missing from published papers. We have listed the authors who replied to our requests for further information in the Acknowledgements section.
Assessment of heterogeneity
We planned to pool the results of studies only if they were judged to be sufficiently similar in terms of populations, interventions and outcomes. We planned to measure the inconsistency of trial results using I2 statistic to determine if variation in outcomes across trials was due to heterogeneity rather than occurring by chance (Deeks 2011). The I2 statistic quantifies heterogeneity in terms of overlapping percentage intervals: 0% to 40% (might not be important); 30% to 60% (may represent moderate heterogeneity); 50% to 90% (may represent substantial heterogeneity); and 75% to 100% (considerable heterogeneity) (Deeks 2011).
Assessment of reporting biases
We planned to assess funnel plot asymmetry for publication biases and other causes. However, this was not possible because tests for funnel plot asymmetry are not recommended unless there are at least 10 trials included in a meta‐analysis (Sterne 2011).
Data synthesis
If we had identified studies as being clinically (e.g. similar age groups) or methodologically (e.g. similar interventions) homogenous but statistically heterogeneous, we planned to conduct a random‐effects meta‐analysis. However, we did not conduct any meta‐analysis in this review since studies were either clinically or methodologically diverse (or both). Conducting a meta‐analysis on data from diverse studies runs the risk of obscuring genuine differences in effect (Deeks 2011). For future updates of this review, we will continue to plan for meta‐analysis if appropriate. A narrative synthesis of the data is currently presented.
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity and included a sufficient number of trials, we planned to conduct subgroup analyses to investigate possible reasons for variations in results across trials. For subgroup analyses, we planned to make comparisons between subsets of participants, subsets of interventions, subsets of settings in which interventions were delivered, and subsets of personnel delivering interventions. We planned to stratify studies into:
participant age‐group subsets (infants and toddlers up to two years, pre‐school children aged 2 years to 5 years, primary school children aged 6 years to 12 years, adolescents aged 13 years to 17 years, adults aged 18 years and over);
intervention type (e.g. web‐based learning, computer‐aided programme, written materials, etc) and duration;
settings in which intervention was conducted (e.g. home, hospital, school);
personnel delivering intervention (e.g. dietitians, nurses, physicians, physiotherapists, CF advocacy or voluntary groups).
In future updates of this review, we will continue to adopt this plan for subgroup analysis and investigation of heterogeneity.
Sensitivity analysis
If appropriate, we planned to conduct sensitivity analysis to determine the influence on effect size of: published and unpublished trials; risk of bias as outlined above; length and size of studies. However, there were insufficient studies to perform this analysis.
Results
Description of studies
Results of the search
A total of 208 records were identified through our search strategy as potentially relevant for inclusion. Of these, 62 citations reporting on 34 studies were identified by a search of the Cystic Fibrosis and Genetic Disorders Group’s CF Trials Register. An additional 145 records were identified from our search of individual databases. One additional record was identified in a newsletter published in Cystic Fibrosis Worldwide. Of the 208 records examined, a total of 11 records reporting on four trials were identified as meeting the inclusion criteria (Cottrell 1996; Downs 2006; Stapleton 2001; Watson 2008). Eightadditional records (on six studies) were identified as potentially eligible for inclusion and are awaiting classification (Bergman 2007; Cannon 1999; Jessup 2008; Johnson 2001; Van der Gieesen 2006; Wainwright 2009). One record was identified as of an ongoing trial (Huang 2009). The remaining 186 records were excluded.
Included studies
The four included trials were published in peer‐reviewed journals (Cottrell 1996; Downs 2006; Stapleton 2001; Watson 2008). Multiple records for three of the trials were identified: one was reported in three journal articles (Stapleton 2001); one was reported in four conference proceeding abstracts and one journal article (Watson 2008); and one was reported in an unpublished thesis and in a journal article (Cottrell 1996). For multiple records, data were extracted from the most recent publication and then from earlier publications as necessary.
Trial design
All four trials were of parallel design. Three trials were conducted in a single centre (Cottrell 1996; Stapleton 2001; Watson 2008). One trial was multicentre involving CF clinics of three public hospitals (Downs 2006).Two trials were undertaken in Australia (Downs 2006; Stapleton 2001); one in the USA (Cottrell 1996) and one in the UK (Watson 2008).
Participants
A total of 368 participants were recruited and randomised across the four trials: 139 children with CF; 155 caregivers of children; and 74 adults with CF. A total of 269 participants completed the trials: 104 children; 117 parents/carergivers (Cottrell 1996; Downs 2006; Stapleton 2001); and 48 adults (Watson 2008).
1. Children
Children with CF were included in three trials, aged 8 years to 18 years in one trial (Cottrell 1996), and 6 years to 11 years in two trials (Downs 2006; Stapleton 2001).
2. Caregivers/parents
Caregivers of children were included in three trials (Cottrell 1996; Downs 2006; Stapleton 2001). Caregivers were explicitly stated as parents in one trial (Cottrell 1996) and as the adult most responsible for managing children’s nutrition in another trial (Stapleton 2001). It was not explicitly stated who the caregivers were in one trial (Downs 2006).
3. Adults
Adults with CF were aged 16 years to 43 years. None of the adults were waiting on a "heart/lung transplant list or were pregnant or lactating" at the time of taking part in the trial (Watson 2008).
Interventions
1. A self‐management education intervention versus another self‐management educational intervention
No included trial made this comparison.
2. A self‐management education intervention versus no intervention
No included trial made this comparison.
3. A self‐management education intervention versus 'standard treatment'
The four included trials made this comparison.
a. Self‐management training programme versus standard treatment
One trial evaluated the effects of a self‐management training programme in reducing the impact of CF on children and parents (Cottrell 1996). The programme was delivered in two six‐hour group sessions in a hospital setting, facilitated by a registered nurse or psychologist. In the group sessions, knowledge of the nature of CF, principles of self‐management, and strategies for managing CF‐related problems were addressed. Skills training included problem solving and stress management.
b. Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment
One trial evaluated the effects of an education programme ('Airways') on the self‐management of aerosol and airway clearance treatments (Downs 2006). The ‘Airways’ programme was home based using written material containing child friendly information and behavioural exercises. Over a period of 10 weeks, children and their caregivers completed weekly exercises, each lasting approximately 20 minutes. The knowledge content of the programme drew on disciplines of medicine, physiotherapy, psychology and education. Self‐management skills addressed in the programme were assessment, treatment implementation, decision making, and strategies to overcome barriers to treatment.
c. Nutrition self‐management education versus standard treatment
Two trials made this comparison focusing on either disease‐specific nutrition education (Stapleton 2001) or general and disease‐specific nutrition education (Watson 2008).
i. Sub‐comparison: Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment
One trial evaluated the effects of a nutrition education programme ('Go and Grow with CF') on disease‐specific nutrition knowledge and self‐management skills (Stapleton 2001). The 'Go and Grow with CF' programme was home based using child‐friendly written material on nutrition management. Over a period of 10 weeks, children and their caregivers completed weekly exercises, each lasting approximately 60 minutes. The programme included supplementary introductory and concluding workshops for separate groups of children and caregivers in a hospital setting, facilitated by dietitians. Knowledge content of the programme included disease‐specific nutrition topics: enzymes; energy and fat; malabsorption; vitamins and minerals; growth; snacks; and salt. Self‐management skills addressed in the programme were goal setting in small incremental steps to increase self efficacy, and self‐monitoring adherence to daily goals.
ii. Sub‐comparison: General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment
One trial evaluated the effects of a nutrition education programme ('Eat Well with CF') on general and disease‐specific knowledge and self‐management skills training (Watson 2008). The 'Eat Well for CF' programme was home‐based using written materials. Over a period of 10 weeks, participants completed weekly activities, each lasting approximately 30 minutes. Knowledge content of the programme included general and disease‐specific nutrition topics: energy intake; digestion; pancreatic enzyme replacement; managing appetite; exercise; dietary fibre; reading food labels; body image. Self‐management skills included goal setting in small incremental steps to establish new behaviours.The programme included supplementary group workshops (introductory weeks 5 and 10) in a hospital setting and weekly telephone calls, facilitated by dietitians. During the course of the trial, microbiological segregation was introduced following which workshops could no longer be held. Consequently, the trial was terminated.
Outcomes
Only outcomes of trials comparing 'Self‐management education intervention versus standard treatment' are reported since no trials were identified for the remaining two comparisons groups considered in this review.
3. Self‐management education intervention versus 'standard treatment'
a. Self‐management training programme versus standard treatment
In the one trial that made this comparison, one primary and four secondary outcomes relevant to this review were assessed (Cottrell 1996). Only our pre‐defined outcomes that were reported in the included trials are listed below.
Primary outcomes
2. Indices of nutritional health or growth
Change in weight was measured in pounds using participants' home scales (Cottrell 1996).
Secondary outcomes
1. Self‐management behaviour
The number and frequency of both children's and parent's behaviours in relation to managing digestive and pulmonary system problem areas were assessed using previously established questionnaires. The number of self‐management behaviours that were done 'at least sometimes' were recorded out of a total of 21 digestive system problem areas and a total of 26 pulmonary system problem areas. The maximum total score for self‐management behaviour was 47. For each of the 47 self‐management behaviours, frequency of performance was rated on a four‐point scale ranging from 0 = never to 4 = always. An average of the response scores provided an overall frequency score ranging between zero and four (Cottrell 1996).
2. Adherence
Medications and aerosol treatment taken by children as well as the number of chest physiotherapy sessions were assessed using a self‐report diary. It was unclear whether children or parents completed the diary. Percentage 'compliance' was computed for each aspect of treatment by comparing reported 'compliance' with the schedule prescribed by physicians (Cottrell 1996).
3. Knowledge
Knowledge was assessed using an established ‘CF Knowledge Survey’ consisting of multiple‐choice questions for children, adolescents, and parents. The percentage of correct answers from each participant was recorded (Cottrell 1996).
4. Health‐related quality of life
Children’s quality of life was measured using the ‘quality of well‐being scale’ comprising three sub scales of functioning (mobility, physical activity, social activity) and twenty two problems/symptoms that could impair function. The total quality of life score ranged from zero (dead) to one (optimal functioning) (Cottrell 1996).
All outcomes were assessed at baseline and at six‐ to eight‐week follow‐up. Results for all outcomes were expressed as means and SDs (Cottrell 1996).
b. Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment
In the one trial that made this comparison, three secondary outcomes relevant to this review were assessed (Downs 2006).
Secondary outcomes
1. Self‐management behaviours
Self‐management behaviours of caregivers relating to aerosol and airway clearance treatments were assessed. Caregivers completed a newly developed one‐week diary card constructed around three self‐management sub scales: assessment; treatment; and communication. The unit of measure was a fractional score with one being the best possible score (Downs 2006).
Self‐management responsiveness of caregivers to airway clearance treatment during children’s unwell days was recorded. The performance of longer and additional airway clearance treatment was considered to be responsive to the child's treatment needs. A mean responsiveness score for all unwell days was calculated (Downs 2006).
Self‐efficacy of caregivers to manage airway clearance treatments was assessed using an established ‘self‐efficacy scale’ with five being the best possible score (Downs 2006).
2. Adherence
Adherence to aerosol and airway clearance treatment was reported by caregivers in a one‐week diary and was measured as a percentage of prescribed treatments taken by children (Downs 2006).
3. Knowledge
Children's knowledge on airway clearance treatment was assessed using a newly developed questionnaire with 23 being the best possible score (Downs 2006).
In this trial, adherence, self‐management behaviours (assessment, treatment, communication) and self‐efficacy were assessed at baseline, at immediate post test, and at 6‐ and 12‐month follow‐up. Self‐management responsiveness was assessed at baseline and at post test. Knowledge was assessed at baseline, post test and at 12‐month follow‐up. Results for all outcomes were expressed as means and SDs (Downs 2006).
c. Nutrition self‐management education versus standard treatment
i. sub‐comparison: Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment
In the one trial that made this sub‐comparison, two secondary outcomes relevant to this review were assessed (Stapleton 2001).
Secondary outcomes
1. Self‐management behaviours
Self‐management skills of both children and caregivers were assessed using scenarios designed to yield open responses categorised as appropriate or inappropriate. For children, scenarios related to signs of malabsorption and communicating to caregivers about nutritional management; the highest possible scores being 23 for appropriate responses and five for inappropriate responses. For caregivers, scenarios related to malabsorption and assessment of what age they expected their children to manage their own pancreatic enzyme replacement therapy. The highest possible scores for caregivers were 61 for appropriate responses and 41 for inappropriate responses (Stapleton 2001).
3. Knowledge
Nutritional and enzyme knowledge was assessed using similar but separate newly developed questionnaires for children and caregivers. Each correct response was allocated a score of one. For children, the best possible score was 37. For caregivers, the best possible score was 42 (Stapleton 2001).
All outcomes in this trial were assessed at baseline, at immediate post‐test, and at 12 month follow‐up. Results for all outcomes were reported as mean score change and standard error (SE) values from baseline (Stapleton 2001). On request, the author provided unpublished data on mean differences and SEs for intervention effects.
ii. sub‐comparison: General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment
In the one trial that made this sub‐comparison, two primary outcomes and three secondary outcomes relevant to this review were assessed (Watson 2008).
Primary outcomes
1. Pulmonary function
Watson assessed FEV1 analysed as per cent predicted (Watson 2008).
2. Indices of nutritional health or growth
Change in weight was assessed in kilograms using the same medical weighing scale for all participants (Watson 2008).
Dietary fat intake was assessed using a 17‐item self‐reported food frequency questionnaire, yielding a maximum score of sixty three points as the best possible score (Watson 2008).
Secondary outcomes
1. Self‐management behaviour
Self‐efficacy of adults to cope with a special diet was assessed using a newly developed measure, with 27 being the best possible score (Watson 2008).
3. Knowledge
Disease‐specific and general nutrition knowledge were assessed using separate questionnaires adapted from previously established questionnaires designed for adults; the highest possible scores being 55 for disease‐specific knowledge and 21 for general knowledge (Watson 2008).
4. Health‐related quality of life
Quality of life was assessed using an established disease‐specific measure for adults comprised of nine CF‐specific domains (physical functioning, social functioning, treatment issues, chest symptoms, emotional responses, concerns for the future, interpersonal relationships, body image, career issues). The best possible health‐related quality of life score that could be attained was 100 (Watson 2008).
All outcomes in this trial were assessed at baseline, and at 6‐ and 12‐month follow‐up. Results for all outcomes were expressed as means and SDs with the exception of quality of life, which were presented as differences in scores for each domain between intervention and control group (Watson 2008).
Excluded studies
Of the 208 records examined, 140 records were excluded following a review of title and abstracts because they were: review papers; reported on practice initiatives and were not studies; reported on instrument development; were clearly not education interventions; or did not include participants with CF. An additional 46 records (reporting on 25 studies) were excluded following review of abstracts and related full text publications because they were not RCTs, quasi‐RCTs or CCTs or did not explicitly address self‐management education in the aims or content of the programme. Details of the 25 excluded studies are presented in the Characteristics of excluded studiestable.
Missing data
The principal authors of the four included trials were contacted for information missing from published records. Missing data in the four trials related mainly to criteria for assessing risk of bias Three authors provided additional information (Stapleton 2001; Downs 2006; Watson 2008). Details of missing data are provided in the Characteristics of included studies table.
Studies awaiting classification
Six studies await classification, five of which were published as abstracts (Cannon 1999; Jessup 2008; Johnson 2001; Van der Gieesen 2006; Wainwright 2009). The remaining study was published in the Cystic Fibrosis Worldwide Newsletter targeting a lay and professional audience (Bergman 2007). All studies are awaiting classification because insufficient details on characteristics of the studies are available, and data on outcomes could not be extracted from publications in the format required for analysis. The principal authors of five studies have been contacted for further information (Bergman 2007; Jessup 2008; Johnson 2001; Van der Gieesen 2006; Wainwright 2009), two of whom have responded (Johnson 2001; Van der Gieesen 2006). Efforts to locate contact details on any of the authors concerning one study have failed (Cannon 1999). Information available to date on the six studies is presented in the Characteristics of studies awaiting classification table.
Risk of bias in included studies
Based on the six domain‐based evaluation criteria recommended in the Cochrane Handbook for Systematic Reviews of Interventions 5.1 (Higgins 2011b), none of the four included trials were judged as adequately meeting all criteria (Figure 1; Figure 2).
1.
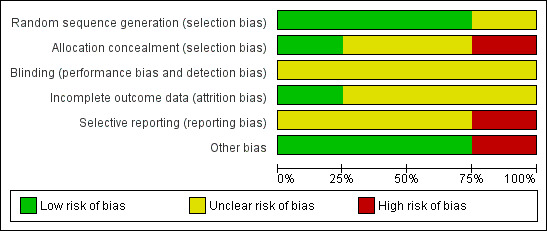
Risk of bias graph: review authors' judgements about each risk of bias criterion presented as percentages across all included studies.
2.
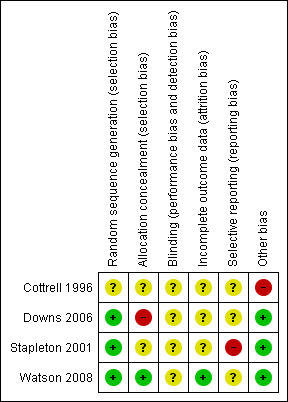
Risk of bias summary: review authors' judgements about each risk of bias criterion for each included study.
Please refer to the risk of bias tables for each individual trial located within the Characteristics of included studies table.
Allocation
Sequence generation was judged to be low risk in three trials (Downs 2006; Stapleton 2001; Watson 2008) and unclear in one trial (Cottrell 1996). Allocation concealment was judged to be unclear in two trials (Cottrell 1996; Stapleton 2001), high risk in one trial (Downs 2006), and low risk in one trial (Watson 2008).
Blinding
Details on blinding were unclear in the four trials (Cottrell 1996; Downs 2006; Stapleton 2001; Watson 2008). Blinding of participants is not possible for any of the interventions considered, however in two trials outcome assessors were blinded to at least some of the outcomes (Downs 2006; Stapleton 2001).
Incomplete outcome data
For incomplete outcome data, one trial was judged to be low risk (Watson 2008) and unclear in three trials (Cottrell 1996; Downs 2006; Stapleton 2001).
Selective reporting
One trial was judged to be high risk in terms of being 'free of selective reporting' (Stapleton 2001) and unclear in three trials (Cottrell 1996; Downs 2006; Watson 2008).
Other potential sources of bias
For the criterion on ‘free from other bias’, three trials were deemed to be low risk (Downs 2006; Stapleton 2001; Watson 2008), and high risk in one trial (Cottrell 1996).
Effects of interventions
Only the effects of 'a self‐management education intervention versus standard treatment' are reported since no trials made the remaining two comparisons considered in this review. Only our pre‐defined outcomes that have been reported on within the included trials are listed below. A summary of effects of interventions is presented in Table 1.
1. Summary of the Effects of Interventions.
| Self‐management training programme versus standard treatment ( Cottrell 1996 ) | ||
|
Effects of intervention (Statistically significant results favouring intervention group are presented in bold) |
||
| PRIMARY OUTCOME (Children) | ||
|
Indices of nutritional health or growth Change in weight |
Assessment time points: 6‐ to 8‐weeks follow up No statistically significant difference between groups in change in weight: MD ‐7.74 lb (95% CI ‐35.18 to 19.70) (in kg this is equivalent to: MD 3.51 kg (95% CI ‐15.96 to 8.94) (Analysis 1.1). |
|
| SECONDARY OUTCOMES (Children) | ||
|
Self‐management behaviours Number of digestive and pulmonary system behaviours Frequency of digestive and pulmonary system behaviours |
Assessment time points: 6‐ to 8‐weeks follow up Statistically significantly greater number of digestive behaviours in the standard treatment group than training group, MD ‐5.30 (95% CI ‐9.29 to ‐1.31) (Analysis 1.2). No statistically significant difference between groups in the number of pulmonary system behaviours, MD ‐1.00 (95% CI ‐6.31 to 4.31) (Analysis 1.3). No statistically significant differences between groups for digestive system, pulmonary system, or both systems combined, MD ‐0.35 (95% CI ‐1.05 to 0.35); MD ‐0.28 (95% CI ‐0.90 to 0.34); and MD ‐0.18 (95% CI ‐0.81 to 0.45) respectively (Analysis 1.4; Analysis 1.5; Analysis 1.6) |
|
|
Adherence Percentages of medications, aerosol treatments, and chest physiotherapy taken. |
Assessment time points: 6‐ to 8‐ weeks follow up No statistically significant differences between groups in the % of prescribed treatments taken by children: medications MD 2.00% (95% CI ‐16.31 to 20.31); aerosol treatments, MD 13.00% (95% CI ‐20.11 to 46.11), and chest physiotherapy, MD ‐8.00 % (95% CI ‐46.13 to 30.13) (Analysis 1.7; Analysis 1.8; Analysis 1.9). |
|
| Knowledge |
Assessment time points:6‐ to 8‐weeks follow up Statsitically significant greater knowledge scores about CF and its management in the training group than the standard treatment groups, MD 19.25% (95% CI 7.57 to 30.93) (Analysis 1.10). |
|
|
Health‐related quality of life Quality of well‐being |
Assessment time points:6‐ to 8‐weeks follow up No statistically significant difference between groups, MD ‐0.02 (95% CI ‐0.09 to 0.05) (Analysis 1.11). |
|
| SECONDARY OUTCOMES (Parents) | ||
|
Self‐management behaviours Number of digestive and pulmonary system behaviours Frequency of digestive and pulmonary system behaviours |
Assessment time points: 6‐ to 8‐weeks follow up No statistically significant differences between groups for digestive system, pulmonary system, or both systems combined, MD ‐1.00 (95% CI ‐3.47 to 1.47), MD 0.40 (95% CI ‐2.73 to 3.53), and MD ‐0.60 (95% CI ‐5.20 to 4.00) respectively (Analysis 1.12; Analysis 1.13; Analysis 1.14). No statistically significant differences between groups for digestive system, pulmonary system, or both systems combined, MD ‐0.09 (95% CI ‐0.65 to 0.47); MD 0.02 (95% CI ‐0.45 to 0.49); and MD 0.00 (95% CI ‐0.44 to 0.44) respectively (Analysis 1.15; Analysis 1.16; Analysis 1.17). |
|
| Knowledge |
Assessment time points:6‐ to 8‐weeks follow up No statistically significant difference between groups, MD 2.11% (95% CI ‐6.65 to 10.87) (Analysis 1.18). |
|
| Self‐management education on aerosol and airway treatment clearance education ('Airways') versus standard treatment (Downs 2006). | ||
|
Effects of intervention (Statistically significant results favouring intervention group are presented in bold) |
||
| SECONDARY OUTCOMES (Children) | ||
| Adherence |
Assessment time points: Post test, 6‐months follow up, 12‐months follow up Statistically significant greater % of prescribed aerosol treatments taken by 'Airways' group at each time point (Analysis 2.1): Post testMD 29.70% (95% CI 14.29 to 45.11);6‐months follow upMD 21.00% (95% CI 5.59 to 36.41);12‐months follow upMD 17.50% (95% CI 5.50 to 29.50) . Statistically significant greater % of prescribed airway clearance treatments taken by 'Airways' group significantly greater at 6‐months follow‐up only (Analysis 2.2): Post test MD 19.00% (95% CI ‐0.62 to 38.62); 6‐months follow upMD 21.60% (CI 95% 7.04 to 36.16);12‐months follow upMD 15.10% (95% CI ‐3.18 to 33.38). |
|
| Knowledge |
Assessment time points: Post test, 12‐months follow up Significantly greater knowledge scores in the 'Airways' group at each time point (Analysis 2.3): Post testMD 3.80 (95% CI 2.29 to 5.31);12‐months follow upMD 4.60 (95% CI 2.83 to 6.37). |
|
| SECONDARY OUTCOMES (Caregivers) | ||
|
Self‐management behaviours Assessment behaviour Treatment behaviour Communication behaviour Responsiveness to airway clearance treatments on children's unwell days Self‐efficacy to manage airway clearance treatments |
Assessment time points: Post test, 6‐months follow up, 12‐months follow up Statistically significant greater assessment behaviour scores in the 'Airways' group post test only (Analysis 2.4): Post testMD 0.17 (95% CI 0.02 to 0.32);6‐months follow up MD 0.15 (95% CI ‐0.01 to 0.31); 12‐months follow up MD 0.08 (95% CI ‐0.06 to 0.22). Statistically significant greater treatment behaviour scores in the 'Airways' group at 6 month only (Analysis 2.5): Post test MD 0.07 (95% CI ‐0.01 to 0.15); 6‐months follow upMD 0.12 (95% CI 0.06 to 0.18);12‐months follow upMD 0.04 (95% CI ‐0.04 to 0.12). Statistically significant greater communication behaviour scores in the 'Airways' group at post test and 6 months (Analysis 2.6): Post test,MD 0.29 (95% CI 0.13 to, 0.45);6‐months follow upMD 0.20 (95% CI 0.03 to 0.37);12‐months follow up MD 0.11 (95% CI ‐0.06 to 0.28). No statistically significant difference between groups in responsiveness (Analysis 2.7): Post test MD 0.23 (95% CI ‐0.08 to 0.54) (not assessed at 6‐ or 12‐month follow ups). No statistically significant differences between groups in self‐efficacy (Analysis 2.8): Post test MD 0.06 (95% CI ‐0.25 to 0.37); 6‐months follow up MD 0.23 (95% CI ‐0.08 to 0.54); 12‐months follow up MD 0.25 (95% CI ‐0.00 to 0.50). |
|
|
Nutrition self‐management education versus standard treatment i. Sub‐comparison: Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment (Stapleton 2001). | ||
| Effects of intervention (Statistically significant results favouring intervention group are presented in bold) | ||
| SECONDARY OUTCOMES (Children) | ||
|
Self‐management behaviours Appropriateness of nutrition and enzyme self‐management |
Assessment time points: Post test, 12‐months follow up No statistically significant differences between groups in changes in nutrition and enzyme appropriate self‐management response scores (Analysis 3.1): Post test MD 0.60 (95% CI ‐1.34 to 2.54); 12‐months follow up MD ‐0.50 (95% CI ‐2.16 to 1.16). Statistically significant lower inappropriate nutrition and enzyme inappropriate self‐management response score in the 'Go and Grow' group at post test only (Analysis 3.2): Post testMD 0.80 (95% CI 0.10 to 1.50);12‐months follow up MD 0.40 (95% CI ‐0.58 to 1.38). |
|
| Knowledge |
Assessment time points: Post test, 12‐months follow up Statistically significant greater nutrition knowledge scores in the 'Go and Grow' group at post test only (Analysis 3.3): Post testMD 3.10 (95% CI 0.06 to 6.14);12‐months follow upMD ‐0.30 (95% CI ‐3.56 to 2.96). |
|
| SECONDARY OUTCOMES (Caregivers) | ||
|
Self‐management behaviours Appropriateness of nutrition and enzyme self‐management |
Assessment time points: Post test, 12‐months follow up No statistically significant differences between groups in nutrition and enzyme appropriate self‐management response scores (Analysis 3.4): Post test MD 0.40 (95% CI ‐0.98 to 1.78); 12‐months follow up MD 0.00 (95% CI ‐1.68 to 1.68). No statistically significant differences between groups in nutrition and enzyme inappropriate self‐management response scores (Analysis 3.5): Post test MD 0.60 (95% CI ‐1.37 to 2.57);12‐months follow up MD ‐0.80 (95% CI ‐3.73 to 2.13). |
|
| Knowledge |
Assessment time points: Post test, 12‐months follow up No statistically significant differences between groups in nutrition knowledge scores (Analysis 3.6): Post test MD 0.10 (95% CI ‐1.56 to 1.76); 12‐months follow up MD ‐0.60 (95% CI ‐2.13 to 0.93). |
|
| ii. Sub‐comparison: General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment ( Watson 2008) | ||
|
Effects of intervention (Statistically significant results favouring intervention group are presented in bold) |
||
| PRIMARY OUTCOME (Adults) | ||
|
Pulmonary Function FEV1 |
Assessment time points: 6‐months follow up, 12‐months follow up No statistically significant differences between groups in per cent predicted FEV1 (Analysis 4.1): 6‐months follow up MD ‐5.00 % (95% CI ‐18.10 to 8.10); 12‐months follow up MD ‐5.50 % (95% CI ‐18.46 to 7.46). |
|
|
Indices of nutritional health or growth Change in weight Dietary fat intake |
Assessment time points: 6‐months follow‐up, 12‐months follow up No statistically significant differences between groups in changes in weight (Analysis 4.2): 6‐months follow up MD ‐ 0.70 kg (95% C1 ‐6.58 to 5.18); 12‐months follow up MD ‐0.70 kg (95% C1 ‐6.62 to 5.22). No statistically significant differences between groups in self‐reported dietary fat intake scores (Analysis 4.3): 6‐months follow up MD 1.60 (85% C1‐2.90 to 6.10); 12‐months follow up MD 0.20 (95% CI ‐4.08 to 4.48). |
|
| SECONDARY OUTCOMES (Adults) | ||
|
Self‐management behaviour Self‐efficacy |
Assessment time points: 6‐months follow‐up, 12‐months follow up Statistically significant greater self‐efficacy scores in the 'Eat Well with CF' group (Analysis 4.4):6‐months follow upMD 4.20 (95% CI 1.51 to 6.89); 12‐months follow upMD 3.30 (95% CI 0.56 to 6.04). |
|
|
Knowledge Disease‐specific nutrition knowledge General nutrition knowledge |
Assessment time points: 6‐months follow up, 12‐months follow up Statistically significant greater disease‐specific nutrition knowledge scores in the 'Eat Well with CF' group at 6 months only (Analysis 4.5): 6‐months follow upMD 5.20 (95% CI 2.61 to 7.79);12‐months follow up MD 2.90 (95% CI ‐0.22 to 6.02). No statistically significant differences between groups in general nutrition knowledge scores (Analysis 4.6): 6‐months follow up MD 0.70 (95% CI ‐1.20 to 2.60); 12‐months follow up MD 0.90 (95% CI ‐09.93 to 2.73). |
|
| Health‐related quality of life | Data for analysis in the format required for the review could not be extracted from published papers or unpublished data available on this trial. | |
CF: cystic fibrosis CI: confidence intervals FEV1: forced expiratory volume at one second kg: kilogram lb: pound MD: mean difference
3. A self‐management education intervention versus 'standard treatment'
a. Self‐management training programme versus standard treatment
One trial made this comparison (Cottrell 1996).
Primary outcomes
i. Children
2. Indices of nutritional health or growth
At six‐ to eight‐week follow‐up, there was no statistically significant difference in change in weight between children in the training group and those in the standard treatment group, MD ‐7.74 lb (95% CI ‐35.18 to 19.70), which in kg, is equivalent to, MD 3.51 kg (95% CI ‐15.96 to 8.94) (Analysis 1.1).
1.1. Analysis.
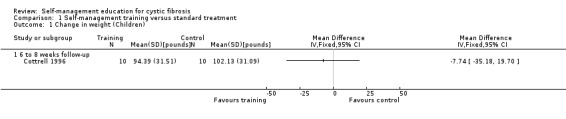
Comparison 1 Self‐management training versus standard treatment, Outcome 1 Change in weight (Children).
Secondary outcomes
i. Children
1. Self‐management behaviours
a. Number of digestive and pulmonary system behaviours
At the six‐ to eight‐ week follow‐up, the number of digestive system behaviours was significantly greater in the standard treatment group than training group, MD ‐5.30 (95% CI ‐9.29 to ‐1.31) (Analysis 1.2).
1.2. Analysis.
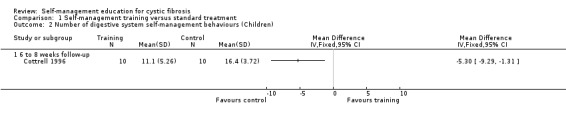
Comparison 1 Self‐management training versus standard treatment, Outcome 2 Number of digestive system self‐management behaviours (Children).
There was no statistically significant difference between groups in the number of pulmonary system behaviours, MD ‐1.00 (95% CI ‐6.31 to 4.31) (Analysis 1.3). The mean difference between the training and the standard treatment groups in the total number of behaviours performed for both digestive and pulmonary systems could not be calculated because published data were missing on the control group.
1.3. Analysis.
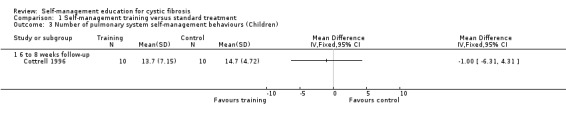
Comparison 1 Self‐management training versus standard treatment, Outcome 3 Number of pulmonary system self‐management behaviours (Children).
b. Frequency of digestive and pulmonary system behaviours
At six‐ to eight‐week follow‐up, there were no statistically significant differences between training and standard treatment groups in the frequency scores on performing behaviours for digestive system, pulmonary system, or total frequency scores of both systems combined, MD ‐0.35 (95% CI ‐1.05 to 0.35), MD ‐0.28 (95% CI ‐0.90 to 0.34), and MD ‐0.18 (95% CI ‐0.81 to 0.45) respectively (Analysis 1.4; Analysis 1.5; Analysis 1.6).
1.4. Analysis.
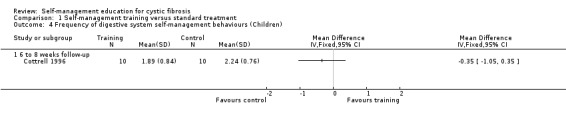
Comparison 1 Self‐management training versus standard treatment, Outcome 4 Frequency of digestive system self‐management behaviours (Children).
1.5. Analysis.
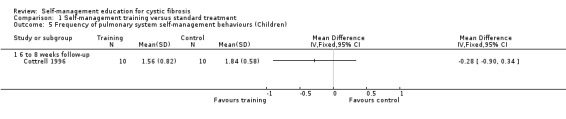
Comparison 1 Self‐management training versus standard treatment, Outcome 5 Frequency of pulmonary system self‐management behaviours (Children).
1.6. Analysis.

Comparison 1 Self‐management training versus standard treatment, Outcome 6 Total frequency of digestive and pulmonary self‐management behaviours (Children).
2. Adherence
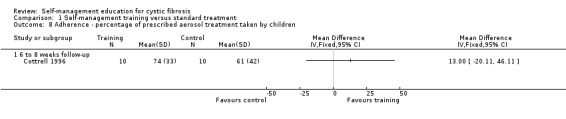
At six‐ to eight‐week follow‐up, there were no statistically significant differences between the training and standard treatment groups in the percentage of prescribed medications, aerosol treatments, and chest physiotherapy taken by children, MD 2.00% (95% CI ‐16.31 to 20.31), MD 13.00% (95% CI ‐20.11 to 46.11), and MD ‐8.00 % (95% CI ‐46.13 to 30.13) respectively (Analysis 1.7; Analysis 1.8; Analysis 1.9).
1.7. Analysis.
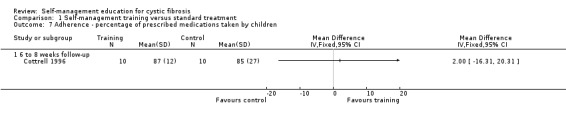
Comparison 1 Self‐management training versus standard treatment, Outcome 7 Adherence ‐ percentage of prescribed medications taken by children.
1.8. Analysis.
Comparison 1 Self‐management training versus standard treatment, Outcome 8 Adherence ‐ percentage of prescribed aerosol treatment taken by children.
1.9. Analysis.
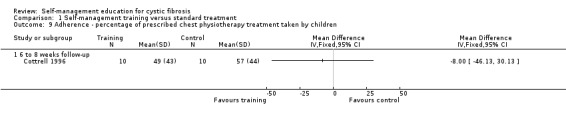
Comparison 1 Self‐management training versus standard treatment, Outcome 9 Adherence ‐ percentage of prescribed chest physiotherapy treatment taken by children.
3. Knowledge
At six‐ to eight‐week follow‐up, the percentage of correct answers on knowledge about CF and its management were significantly greater in children in the training group than the standard treatment groups, MD 19.25% (95% CI 7.57 to 30.93) (Analysis 1.10).
1.10. Analysis.
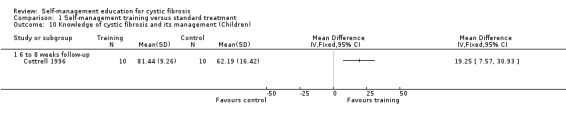
Comparison 1 Self‐management training versus standard treatment, Outcome 10 Knowledge of cystic fibrosis and its management (Children).
4. Health‐related quality of life
At six‐ to eight‐week follow‐up, there was no statistically significant difference in children's quality of well‐being scores between training and standard treatment groups, MD ‐0.02 (95% CI ‐0.09 to 0.05) Analysis 1.11).
1.11. Analysis.
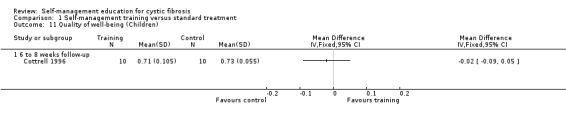
Comparison 1 Self‐management training versus standard treatment, Outcome 11 Quality of well‐being (Children).
ii. Parents
1. Self‐management behaviours
a. Number of digestive and pulmonary system behaviours
At the six‐ to eight‐week follow‐up, there were no statistically significant differences between training and standard treatment groups in the number of behaviours performed for digestive system, pulmonary system, or total number of behaviours for both systems combined, MD ‐1.00 (95% CI ‐3.47 to 1.47), MD 0.40 (95% CI ‐2.73 to 3.53), and MD ‐0.60 (95% CI ‐5.20 to 4.00) respectively (Analysis 1.12; Analysis 1.13; Analysis 1.14).
1.12. Analysis.
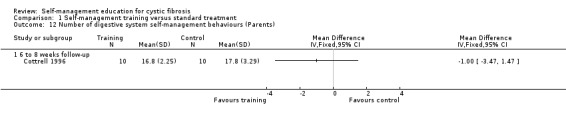
Comparison 1 Self‐management training versus standard treatment, Outcome 12 Number of digestive system self‐management behaviours (Parents).
1.13. Analysis.
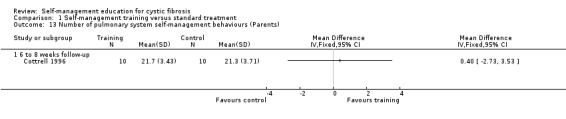
Comparison 1 Self‐management training versus standard treatment, Outcome 13 Number of pulmonary system self‐management behaviours (Parents).
1.14. Analysis.

Comparison 1 Self‐management training versus standard treatment, Outcome 14 Total number of digestive and pulmonary self‐management behaviours (Parents).
b. Frequency of digestive and pulmonary system behaviours
At six‐ to eight‐week follow‐up, there were no statistically significant differences between training and standard treatment groups in the frequency scores on performing behaviours for digestive system, pulmonary system, or total frequency of both systems combined, MD ‐0.09 (95% CI ‐0.65 to 0.47); MD 0.02 (95% CI ‐0.45 to 0.49); and MD 0.00 (95% CI ‐0.44 to 0.44) respectively (Analysis 1.15; Analysis 1.16; Analysis 1.17).
1.15. Analysis.
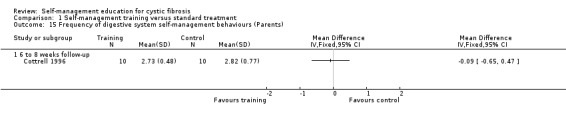
Comparison 1 Self‐management training versus standard treatment, Outcome 15 Frequency of digestive system self‐management behaviours (Parents).
1.16. Analysis.
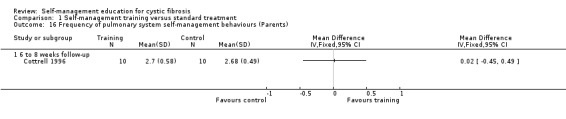
Comparison 1 Self‐management training versus standard treatment, Outcome 16 Frequency of pulmonary system self‐management behaviours (Parents).
1.17. Analysis.

Comparison 1 Self‐management training versus standard treatment, Outcome 17 Total frequency of digestive and pulmonary system self‐management behaviours (Parents).
3. Knowledge
At the six‐ to eight‐week follow‐up, there was no statistically significant difference between intervention and control groups in the percentage of correct knowledge answers about CF and its management, MD 2.11% (95% CI ‐6.65 to 10.87) (Analysis 1.18).
1.18. Analysis.
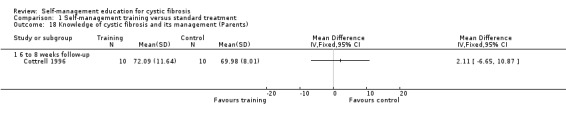
Comparison 1 Self‐management training versus standard treatment, Outcome 18 Knowledge of cystic fibrosis and its management (Parents).
b. Self‐management education on aerosol and airway treatment clearance education ('Airways') versus standard treatment
One trial made this comparison (Downs 2006).
Secondary outcomes
i. Children
2. Adherence
At post test, the six‐month and 12‐month follow‐up, the percentage of prescribed aerosol treatments taken by children was significantly greater in the 'Airways' group than standard treatment group, MD 29.70% (95% CI 14.29 to 45.11), MD 21.00% (95% CI 5.59 to 36.41), and MD 17.50% (95% CI 5.50 to 29.50) respectively (Analysis 2.1).
2.1. Analysis.
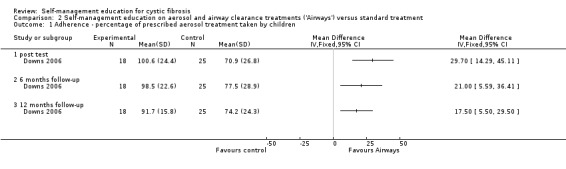
Comparison 2 Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment, Outcome 1 Adherence ‐ percentage of prescribed aerosol treatment taken by children.
At the six‐month follow‐up, the percentage of prescribed airway clearance treatments taken by children was significantly greater in the 'Airways' group than standard treatment group, MD 21.60% (CI 95% 7.04 to 36.16). The difference was not statistically significant at immediate post test and the 12‐month follow‐up, MD 19.00% (95% CI ‐0.62 to 38.62), and MD 15.10% (95% CI ‐3.18 to 33.38) respectively (Analysis 2.2).
2.2. Analysis.
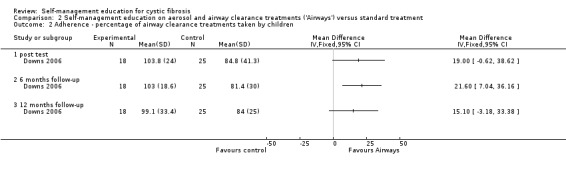
Comparison 2 Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment, Outcome 2 Adherence ‐ percentage of airway clearance treatments taken by children.
3. Knowledge
At immediate post test and the 12‐month follow‐up, children's knowledge scores were significantly greater in the 'Airways' group than standard treatment group, MD 3.80 (95% CI 2.29 to 5.31), and MD 4.60 (95% CI 2.83 to 6.37) respectively. This outcome was not assessed at the six‐month follow‐up (Analysis 2.3).
2.3. Analysis.
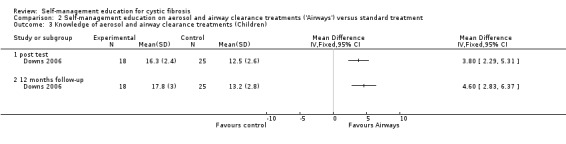
Comparison 2 Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment, Outcome 3 Knowledge of aerosol and airway clearance treatments (Children).
ii. Caregivers
1. Self‐management behaviours
a. Assessment behaviour
At immediate post test, caregiver assessment behaviour scores were significantly greater in the 'Airways' group than standard treatment group, MD 0.17 (95% CI 0.02 to 0.32). The differences were not statistically significant at the six‐month or 12‐month follow‐up: MD 0.15 (95% CI ‐0.01 to 0.31); and MD 0.08 (95% CI ‐0.06 to 0.22) respectively (Analysis 2.4).
2.4. Analysis.
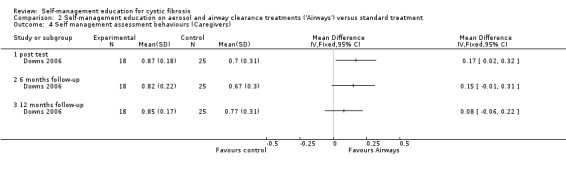
Comparison 2 Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment, Outcome 4 Self management assessment behaviours (Caregivers).
b. Treatment behaviour
At immediate post test, there was no statistically significant difference between 'Airways' and standard treatment groups in caregiver treatment behaviour scores, MD 0.07 (95% CI ‐0.01 to 0.15). At six‐month follow‐up, caregiver treatment behaviour scores were significantly greater in the 'Airways' group than standard treatment group, MD 0.12 (95% CI 0.06 to 0.18). At 12‐month follow‐up, the difference in scores between groups was not statistically significant, MD 0.04 (95% CI ‐0.04 to 0.12) (Analysis 2.5).
2.5. Analysis.
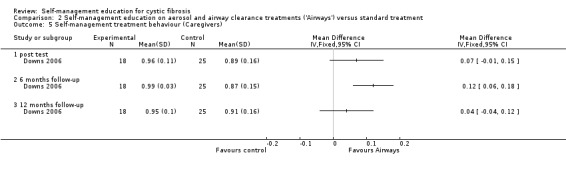
Comparison 2 Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment, Outcome 5 Self‐management treatment behaviour (Caregivers).
c. Communication behaviour
At immediate post test and the six‐month follow‐up, caregiver communication behaviour scores were significantly greater in the 'Airways' group than standard treatment group, MD 0.29 (95% CI 0.13 to, 0.45), and MD 0.20 (95% CI 0.03 to 0.37) respectively. The difference in scores between groups was not statistically significant at the 12‐month follow‐up, MD 0.11 (95% CI ‐0.06 to 0.28) (Analysis 2.6).
2.6. Analysis.
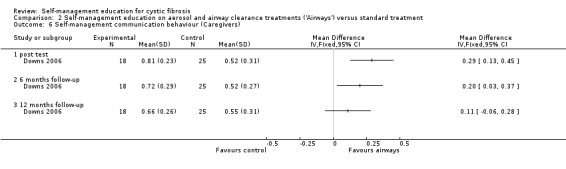
Comparison 2 Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment, Outcome 6 Self‐management communication behaviour (Caregivers).
d. Responsiveness to airway clearance treatments on children's unwell days
At immediate post test, there was no statistically significant difference between 'Airways' and standard treatment groups in caregiver scores for responsiveness to airway clearance treatments on children's unwell days, MD 0.23 (95% CI ‐0.08 to 0.54). This outcome was not assessed at follow‐up time points (Analysis 2.7).
2.7. Analysis.
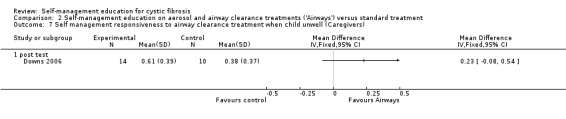
Comparison 2 Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment, Outcome 7 Self management responsiveness to airway clearance treatment when child unwell (Caregivers).
e. Self‐efficacy to manage airway clearance treatments
At immediate post test, six‐month and 12‐month follow‐up, there were no statistically significant differences between intervention and control groups in caregiver self‐efficacy scores, MD 0.06 (95% CI ‐0.25 to 0.37), MD 0.23 (95% CI ‐0.08 to 0.54), and MD 0.25 (95% CI ‐0.00 to 0.50) respectively (Analysis 2.8).
2.8. Analysis.
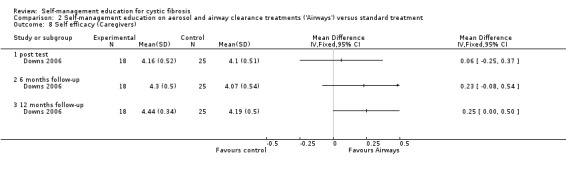
Comparison 2 Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment, Outcome 8 Self efficacy (Caregivers).
c. Nutrition self‐management education versus standard treatment
i. Sub‐comparison: Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment
One trial made this sub‐comparison (Stapleton 2001).
Secondary outcomes
i. Children
1. Self‐management behaviours
Data for analysis in the format required for this review could not be extracted from published records of the trial. These data became available from the principal author on request.
a. Appropriateness of nutrition and enzyme self‐management
At immediate post test and 12‐month follow‐up, there were no statistically significant differences between 'Go and Grow' and standard treatment groups in changes in nutrition and enzyme appropriate self‐management response scores, MD 0.60 (95% CI ‐1.34 to 2.54), and MD ‐0.50 (95% CI ‐2.16 to 1.16) respectively (Analysis 3.1).
3.1. Analysis.
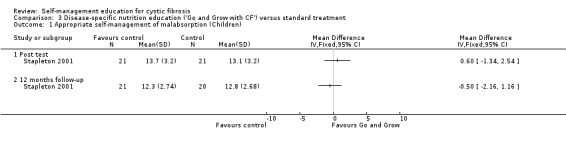
Comparison 3 Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment, Outcome 1 Appropriate self‐management of malabsorption (Children).
At immediate post test, children's nutrition and enzyme inappropriate self‐management response scores were significantly lower in the 'Go and Grow' group than standard treatment group, MD 0.80 (95% CI 0.10 to 1.50). The difference was not statistically significant at the 12‐month follow‐up, MD 0.40 (95% CI ‐0.58 to 1.38) (Analysis 3.2).
3.2. Analysis.
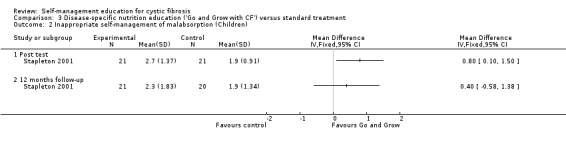
Comparison 3 Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment, Outcome 2 Inappropriate self‐management of malabsorption (Children).
3. Knowledge
At immediate post test, children's nutrition knowledge scores were significantly greater in the 'Go and Grow' group than standard treatment group, MD 3.10 (95% CI 0.06 to 6.14). The difference between groups was not statistically significant at the 12‐month follow‐up, MD ‐0.30 (95% CI ‐3.56 to 2.96) (Analysis 3.3).
3.3. Analysis.
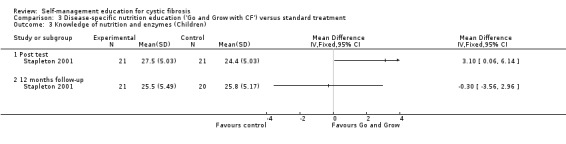
Comparison 3 Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment, Outcome 3 Knowledge of nutrition and enzymes (Children).
ii. Caregivers
1. Self‐management behaviours
a. Appropriateness of nutrition and enzyme self‐management
At immediate post test and the 12‐month follow‐up, there were no statistically significant differences between 'Go and Grow' and standard treatment groups in caregivers nutrition and enzyme appropriate self‐management response scores, MD 0.40 (95% CI ‐0.98 to 1.78), and MD 0.00 (95% CI ‐1.68 to 1.68) respectively (Analysis 3.4).
3.4. Analysis.
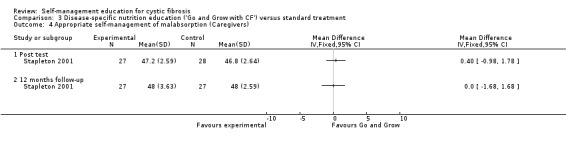
Comparison 3 Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment, Outcome 4 Appropriate self‐management of malabsorption (Caregivers).
At immediate post test and the 12‐month follow‐up, there were no statistically significant differences between 'Go and Grow' and standard treatment groups in caregivers nutrition and enzyme inappropriate self‐management response scores, MD 0.60 (95% CI ‐1.37 to 2.57) and MD ‐0.80 (95% CI ‐3.73 to 2.13) respectively (Analysis 3.5).
3.5. Analysis.
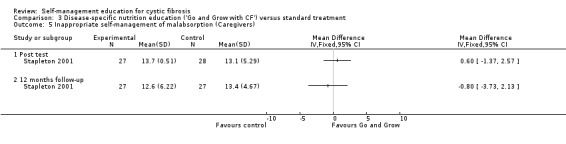
Comparison 3 Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment, Outcome 5 Inappropriate self‐management of malabsorption (Caregivers).
3. Knowledge
At immediate post test and the 12‐month follow‐up, there were no statistically significant differences between 'Go and Grow' and standard treatment groups in caregivers' nutrition knowledge scores, MD 0.10 (95% CI ‐1.56 to 1.76), and MD ‐0.60 (95% CI ‐2.13 to 0.93) respectively (Analysis 3.6).
3.6. Analysis.
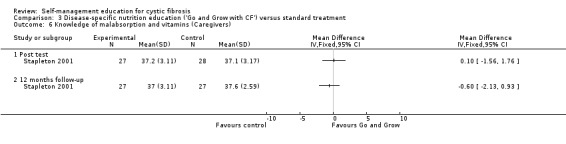
Comparison 3 Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment, Outcome 6 Knowledge of malabsorption and vitamins (Caregivers).
ii. Sub‐comparison: General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment
One trial made this sub‐comparison in adults with CF (Watson 2008).
Primary Outcomes
1. Pulmonary function (analysed as per cent predicted):
a. forced expiratory volume at one second (FEV1)
At the six‐month and 12‐month follow‐up, there were no statistically significant differences between ' Eat Well with CF' and standard treatment groups in per cent predicted FEV1, MD ‐5.00 % (95% CI ‐18.10 to 8.10), and MD ‐5.50 % (95% CI ‐18.46 to 7.46) respectively (Analysis 4.1).
4.1. Analysis.
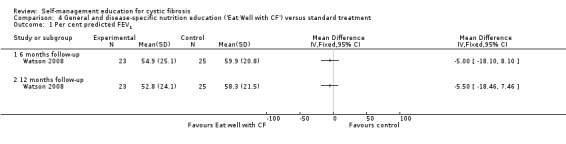
Comparison 4 General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment, Outcome 1 Per cent predicted FEV1.
2. Indices of nutritional health or growth
a. Change in weight
At the six‐month and 12‐month follow‐up, there were no statistically significant differences between 'Eat Well with CF' and standard treatment groups in changes in weight, MD ‐ 0.70 kg (95% C1 ‐6.58 to 5.18), MD ‐0.70 kg (95% C1 ‐6.62 to 5.22) respectively (Analysis 4.2) .
4.2. Analysis.
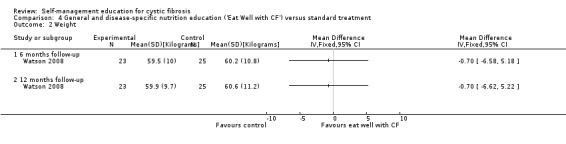
Comparison 4 General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment, Outcome 2 Weight.
b. Dietary fat intake
At the 6‐month and 12‐month follow‐up, there were no statistically significant differences between 'Eat Well with CF' and standard treatment groups in self‐reported dietary fat intake scores, MD 1.60 (85% C1‐2.90 to 6.10), and MD 0.20 (95% CI ‐4.08 to 4.48) respectively (Analysis 4.3).
4.3. Analysis.
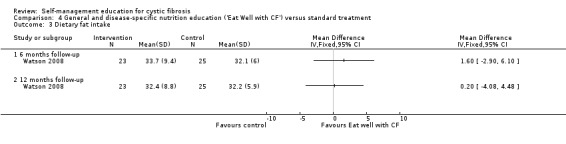
Comparison 4 General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment, Outcome 3 Dietary fat intake.
Secondary outcomes
1. Self‐management behaviour
a. Self‐efficacy
At the six‐month and 12‐month follow‐up, self‐efficacy scores were significantly greater in the 'Eat Well with CF' than the standard treatment group, MD 4.20 (95% CI 1.51 to 6.89), and MD 3.30 (95% CI 0.56 to 6.04) respectively (Analysis 4.4).
4.4. Analysis.
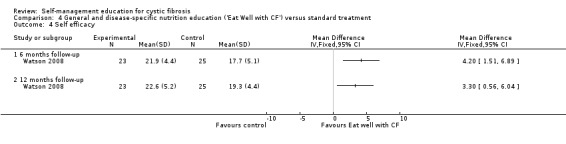
Comparison 4 General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment, Outcome 4 Self efficacy.
3. Knowledge
At the six‐month follow‐up, disease‐specific nutrition knowledge scores were significantly greater in the 'Eat Well with CF' group than standard treatment group, MD 5.20 (95% CI 2.61 to 7.79). The difference between groups was not statistically significant at the 12‐month follow‐up, MD 2.90 (95% CI ‐0.22 to 6.02) (Analysis 4.5) .
4.5. Analysis.
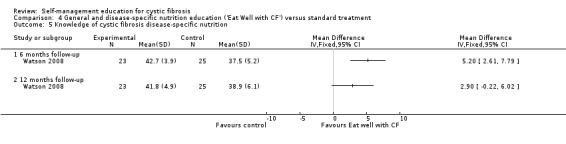
Comparison 4 General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment, Outcome 5 Knowledge of cystic fibrosis disease‐specific nutrition.
At the six‐month and 12‐month follow‐up, there were no statistically significant differences between 'Eat Well with CF' and standard treatment group in general nutrition knowledge scores, MD 0.70 (95% CI ‐1.20 to 2.60), and MD 0.90 (95% CI ‐09.93 to 2.73) respectively (Analysis 4.6).
4.6. Analysis.
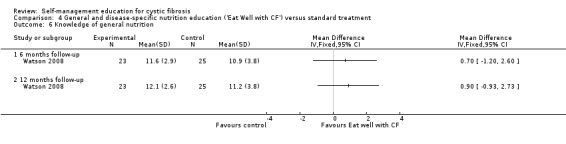
Comparison 4 General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment, Outcome 6 Knowledge of general nutrition.
4. Health‐related quality of life
Data for analysis in the format required for the review could not be extracted from published papers or unpublished data available on this trial.
Discussion
Summary of main results
There have been few trials investigating the effects of self‐management education for patients with CF and their caregivers. Four trials were included in our review involving 269 participants: children with CF and their parents or caregivers in three trials (Cottrell 1996; Downs 2006; Stapleton 2001); and adults with CF in one trial (Watson 2008). The precise focus of self‐management differed between trials and included a training programme for managing CF (Cottrell 1996), education specific to aerosol and airway clearance treatments (Downs 2006), disease‐specific nutrition education (Stapleton 2001), and general and disease‐specific nutrition education (Watson 2008). The four trials differed in the range of outcomes assessed, and in the number of assessment time points. Primary outcomes relevant to our review were assessed across two trials: pulmonary functioning (FEV1) (Watson 2008); weight (Cottrell 1996; Watson 2008); and dietary fat intake (Watson 2008). Secondary outcomes relevant to our review were assessed in one or more of the four trials, and included self‐management behaviours, adherence, knowledge, and quality of life. Utilisation of health services was not assessed in any of the trials.
The evidence presented in this review is limited due the small number of trials included, small sample sizes with inadequate or unknown power calculations to detect effect size of outcomes measured, and unclear or high risk of bias in trials (see Characteristics of included studies). The results reported in this review must therefore be interpreted cautiously. The assessment of bias in trials is important before interpreting the clinical significance of trial results by examining the confidence intervals around observed effect sizes (Davies 2009). The included trials had a number of methodological limitations; therefore, the clinical significance of the trial results is uncertain.
Results from one trial suggest that a self‐management training intervention can increase children's knowledge about CF in the short term (Cottrell 1996). However, the sample size of children in the trial was very small (n = 20) and this is reflected in the wide confidence intervals around the estimate of intervention effect regarding knowledge (see Effects of interventions). There were no statistically significant differences between the self‐management training group and those receiving standard care for the remaining outcomes assessed in the trial, which included parental knowledge, self‐management behaviours of children and parents, children's adherence to chest treatments, and children's quality of life (Cottrell 1996).
Unlike the self‐management training programme (Cottrell 1996), which focused on a broad range of CF areas such as digestive problems, respiratory problems, and medications, the remaining three trials each focused on a specific area of CF management. Results from the trial on aerosol and airway clearance treatments suggest that a 10‐week self‐management education intervention using child‐friendly information and behavioural exercises can increase and sustain children's knowledge about these treatments over time (Downs 2006). The results also suggest that adherence to chest treatments can increase following a self‐management education intervention. The sample size of 43 children and their caregivers in the trial was small, however, and this is reflected in the wide confidence intervals around the estimates of intervention effect regarding children's knowledge and adherence (see Effects of interventions). Evidence from this trial suggests that education can result in immediate or short‐term changes in some self‐management behaviours of caregivers in relation to aerosol and airway clearance treatments, namely, assessment, communication and treatment behaviours (Downs 2006). However, the differences between groups were very small (see Effects of interventions), and it is unclear if the differences in these behaviours are of any clinical significance.
There is evidence to suggest that a 10‐week programme on disease‐specific nutrition education can result in an immediate increase in children's nutrition and enzyme knowledge (Stapleton 2001). However, the confidence interval around the estimate of intervention effect on knowledge was wide (see Effects of interventions), reflecting the small sample size of children in the trial (n = 41). For caregivers, there were no statistically significant differences between groups in either knowledge or self‐management behaviours at the end of the intervention or at the 12‐month follow‐up. The benefits of disease‐specific nutrition education to children over caregivers need to be considered within the context of learning material used by children and caregivers together being in a format appropriate for children of primary school age (Stapleton 2001). This child‐centred approach to education was also evident in the trial regarding self‐management of aerosol and airway clearance treatments (Downs 2006). Therefore, the effects of self‐management education using written materials directed at caregivers in relation to nutrition or chest treatments remains unknown from our review.
Only one trial sampled adults with CF (Watson 2008). Results from this trial suggest that a home‐based behavioural nutrition education programme can result in a short‐term increase in disease‐specific nutrition knowledge, and enhanced self‐efficacy (confidence) in 'coping' with nutritional requirements for CF in the short and long term. However, the sample size in the trial was very small (n = 48), and this is reflected in the wide confidence intervals around the estimate of intervention effects on disease‐specific nutrition knowledge, and self‐efficacy respectively (see Effects of interventions). Differences between groups in general nutrition knowledge were not statistically significant. There were no statistically significant differences between groups in pulmonary function (FEV1), changes in weight, or dietary fat intake. In the Watson trial 74 adults with CF were recruited; however, the trial was discontinued with a final sample of 48 patients because of microbiological segregation policies that prohibited group education (Watson 2008). This situation illustrates a challenge that can arise when including group workshops as part of an education programme for patients with CF because of concerns about cross‐infection. The trial authors have since piloted an interactive web‐based meeting to replace group workshops in a nutrition education programme but data about its effects are not available as yet.
Overall completeness and applicability of result
Only four trials eligible for inclusion in this review were identified. Because of the small number of trials and respective small sample sizes, results cannot be generalised to subgroup populations of patients with CF (children or adolescents or adults) or to their parents or caregivers. We planned to do subgroup analysis to make comparisons between subsets of participants according to age groups. However, subgroup analysis was not possible because of the small number of similar age groups across trials. In two trials, children were aged 6 to 11 years (Downs 2006; Stapleton 2001). Adolescents were included in one trial (Cottrell 1996) and adults were included in one trial (Watson 2008).
From the current available evidence, it is not known what components of self‐management education interventions would work best for various subgroups of individuals with CF in terms of improving health outcomes. While knowledge is typically included as a component of health education programmes, knowledge gains are unlikely to translate into changes in behaviour. Self‐management education needs to include behavioural components that are problem‐based focusing on skills of goal setting, action planning, and self‐monitoring (Lorig 2003; Schreurs 2003). There was little consistency across the trials regarding behavioural skills taught and none of the trials included the range of skills recommended for self‐management education (Lorig 2003; Schreurs 2003). Goal setting was included in two trials (Stapleton 2001; Watson 2008) and self‐monitoring was included in one trial (Stapleton 2001). None of the four trials described action planning as part of the intervention content.
Behavioural changes for integrating the complex demands of CF management into a person’s daily life are likely to need ongoing reinforcement. The interventions across the four trials were short in duration (6 to 10 weeks) and did not include top‐up or booster sessions. Therefore, the potential benefits of ongoing reinforcement to changing behaviours for self‐management of CF remain unknown.
The mode of delivery in the four trials reviewed was standardised, the most common being home‐based pen and paper exercises (Downs 2006Stapleton 2001; Watson 2008). Standardised programmes are likely to have maximum effectiveness in a proportion of those to whom they are delivered because learning styles and preferences for mode of delivery can vary among individuals and families.
Evidence on the effects of self‐management education on outcomes relevant to this review is incomplete. The four trials differed in the range of outcomes assessed, and the number of post‐intervention assessment time points. Data on primary outcomes were presented across two trials only, and included indices of nutritional growth (weight, dietary fat intake), and pulmonary functioning (FEV1). Data on three secondary outcomes were available for analysis in one or more of the trials. However, the precise focus of assessment for similar outcomes differed between trials including the type of self‐management behaviours assessed, aspects of CF treatments assessed for adherence, and the type of CF knowledge assessed. Quality of life of patients with CF was assessed in two trials but data were available for analysis from one trial only. No data on utilisation of health services were presented in any of the trials.
Quality of the evidence
The evidence on which this review is based draws on data from four trials involving 269 participants, and which included children with CF and their parents or caregivers, and adults with CF. Based on our assessment of the risk of bias in the included trials, the quality of the evidence presented in this review is limited. There were concerns over the integrity of the randomisation process in all trials: generation of allocation sequence was unclear in one trial (Cottrell 1996); method of allocation concealment was judged to be low risk in one trial only (Watson 2008). Insufficient detail about blinding was provided in the four included trials. Only one trial was judged to be low risk for the criterion on incomplete outcome data (Watson 2008). Insufficient detail about missing data were provided in three of the trials (Cottrell 1996; Downs 2006; Stapleton 2001). There were concerns over the quality of reporting in all four trials: it was unclear if additional outcomes were pre‐specified in the trial protocols but not reported. Apart from one trial (Cottrell 1996), there were no concerns about the remaining three trials in terms of being 'free of other bias'. In conclusion, the available evidence from this review is of insufficient quantity and quality to draw any firm conclusions about the effects of self‐management education for CF.
Potential biases of the review process
Our objective was to include trials on educational interventions that explicitly addressed self‐management for CF in the aims of the programme or content of the programme. We are confident that through our search strategy, we have identified all trials conducted on self‐management education for CF within the years covered in this review (1990 to February 2011). The selection of trials, risk of bias assessment, and data extraction were conducted by two authors. Disagreements were resolved by discussion. We were successful in obtaining additional information from the authors of three trials (Downs 2006; Stapleton 2001; Watson 2008). Our inclusion of only those trials that explicitly referred to self‐management in the aims or content of interventions has resulted in the exclusion of one trial that evaluated the effectiveness of a CD‐ROM educational programme (STARBRIGHT) aimed at helping children and adolescents fit the demands of CF care into their everyday lives (Davis 2004). This educational programme included coping skills training to deal with stressors and problems that children and adolescents may encounter in their daily lives because of CF. In future updates of this review, it is planned to include education programmes that have behavioural components such as coping, provided that the programme is designed to help patients and their caregivers integrate CF management into their daily lives.
Agreement or disagreements with other studies or review
The evidence from the four trials included in our review are broadly in agreement with some results from other Cochrane reviews on self‐management education programmes across a range of specific chronic illnesses including: epilepsy in children (Stokes 2007) and adults (Bradley 2008; Shaw 2007); asthma in adults (Gibson 2002); type‐2 diabetes in adults (Deakin 2005); and chronic obstructive airway disease (COPD) in adults (Effing 2007). Previous Cochrane reviews relating to respiratory conditions suggest that self‐management education has no statistically significant effect on pulmonary function in adults (Effing 2007; Gibson 2002) which is in agreement with our review. Similar to our review, evidence from Cochrane reviews relating to epilepsy suggests that self‐management education may improve disease‐related knowledge in children (Stokes 2007) and adult patients (Bradley 2008; Shaw 2007). Likewise, there is some previous evidence to suggest that self‐management education may improve certain behavioural outcomes (Bradley 2008Deakin 2005; Shaw 2007). Results from two previous Cochrane reviews indicate that self‐management education has a statistically significant effect on quality of life in adults with COPD (Effing 2007) and asthma (Gibson 2002). There is no evidence available from our review regarding quality of life in adult patients with CF, since data for analysis in the format required for the review could not be extracted from published papers or unpublished data available on the trial (Watson 2008). Similar to other Cochrane reviews, we included 'utilisation of health services' as a secondary outcome of interest. However, this outcome was not assessed in any of the four trials in our review. This contrasts with previous Cochrane reviews that have presented evidence indicating that self‐management education has a statistically significant effect on reducing hospital admissions in adult patients with COPD (Effing 2007) and asthma (Gibson 2002); and in reducing emergency room visits in children with epilepsy (Stokes 2007).
Apart from two Cochrane reviews on self‐management education for epilepsy (Shaw 2007; Stokes 2007), the evidence presented in other Cochrane reviews has been drawn from a greater number of trials than included in our review (Bradley 2008; Deakin 2005; Effing 2007; Gibson 2002). However, similar to our review, there were concerns about the risk of bias in trials. Apart from one Cochrane review (Deakin 2005), meta‐analysis was not conducted in other Cochrane reviews on specific chronic illnesses (Bradley 2008; Effing 2007; Gibson 2002; Shaw 2007; Stokes 2007), due to heterogeneity in study samples, interventions, outcome measures and assessment time points. Likewise, in our review, the heterogeneity of trials precluded meta‐analysis. To date, there remains uncertainty about the effects of self‐management education on a range of health outcomes due to insufficient evidence, and previous Cochrane reviewers have consistently recommended the need for more RCTs with longer follow‐up, before firm conclusions can be made. Our review concurs with these conclusions and recommendations regarding further research.
Authors' conclusions
Implications for practice.
Due to the limited quantity and quality of trials included in this review, as well as the clinical and methodological heterogeneity of these trials, there is insufficient evidence to clearly recommend or refute the use of self‐management education for CF in routine clinical practice. There is some limited evidence to suggest that self‐management education may improve knowledge in patients with CF but not in parents or caregivers. There is also some limited evidence that self‐management education may result in positively change a small number of behaviours in both patients and caregivers. However, improvements in knowledge and behaviours were mostly short lived and were not maintained over time. To sustain the potential benefits of self‐management education, top‐up or booster sessions may be needed.
Implications for research.
Well‐designed RCTs are needed to evaluate the effects of self‐management education for CF. The behavioural component of self‐management education programmes needs to be strengthened to include the skills of goal setting, action planning, and self‐monitoring aimed at helping patients and families to fit treatment requirements into their everyday activities around flexible management plans. Behavioural change is a gradual process and future self‐management programmes need to be developed from a behavioural framework that takes account of a person’s position in the change process such as the 'Transtheoretical Model of Change' (also known as 'Stages of Change' model).
Further trials are needed to evaluate the effects of self‐management education in relation to: intervention types (e.g. web‐based learning, computer‐aided programmes, written materials); content of intervention (e.g. learning material about CF; skills training; behavioural training); format of interventions (e.g. individual, group, patient‐caregiver pairs); duration of interventions including top‐up or booster sessions, intervention settings (e.g. home, hospital, school); and personnel delivering interventions (e.g. dietitians, nurses, physicians, physiotherapists, CF advocacy or voluntary groups). Consideration also needs to be given to the effects of tailoring interventions to the personal preferences of participants. The potential for control group contamination in educational interventions needs to be addressed by designing trials that seek to avoid contamination or if this is unavoidable, the performance of power calculations to determine sample size needs to take account of this.
There is a need for consistency in the type of outcomes measured and follow‐up time points. Of particular importance is the need to investigate the long‐term effects of self‐management education on pulmonary function and nutritional growth and health since these outcomes are critical indicators of physical health and long‐term survival in people with CF.
A wide range of age groups and in sufficient numbers need to be enrolled into trials so that data on subgroups can be analysed separately. Therefore, future trials need to be adequately powered to detect small differences between intervention and control groups. An important consideration here is whether small differences are of any clinical importance to patients. Therefore, researchers conducting future trials need to define the minimum clinically important difference that is worth detecting between intervention and control groups for the primary outcome of interest.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2017 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 7, 2011
| Date | Event | Description |
|---|---|---|
| 25 July 2014 | New citation required but conclusions have not changed | New citations have been added to studies excluded, ongoing or awaiting classification. No new studies have been added to the 'Included studies' section of the review. |
| 25 July 2014 | New search has been performed | New searches have been carried out for this review update. |
| 25 April 2007 | New citation required and major changes | Substantive amendment |
Acknowledgements
We would like to thank the Cochrane Cystic Fibrosis and Genetic Disorders Group especially Tracey Remmington and Nikki Jahnke who provided support at various stages of developing and writing the review. We thank the Group also for identifying potentially eligible papers through database searches. We would like to thank the following authors for providing information about their trials including those awaiting classification: Jenny Downs; Susan Johnson; Denise Stapleton; Lianne van der Giesson; Helen Watson.
We would also like to acknowledge: The Health Research Board, Ireland, for funding this review through a Cochrane Fellowship awarded to Eileen Savage.
Appendices
Appendix 1. Search Strategy: CINAHL with Full Text (EBSCO)
| Date of Search | 02 February 2014 |
| Years Covered | January 1990 to January 2014 |
| Complete Strategy |
|
| Language Restrictions | None |
Appendix 2. Search Strategy: Psychological and Behavioural Sciences Collection (EBSCO)
| Date of Search | 02 February 2014 |
| Years Covered | January 1990 to January 2014 |
| Complete Strategy |
|
| Language Restrictions | None |
Appendix 3. Search Strategy: PsychoInfo (EBSCO)
| Date of Search | 02 February 2014 |
| Years Covered | January 1990 to January 2014 |
| Complete Strategy |
|
| Language Restrictions | None |
Appendix 4. Search Strategy: SocINDEX (EBSCO)
| Date of Search | 03 February 2014 |
| Years Covered | January 1990 to January 2014 |
| Complete Strategy |
|
| Language Restrictions | None |
Appendix 5. Search Strategy: Embase (Elsevier)
| Date of Search | 03 February 2014 |
| Years Covered | January 1990 to January 2014 |
| Complete Strategy |
|
| Language Restrictions | None |
Data and analyses
Comparison 1. Self‐management training versus standard treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in weight (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of digestive system self‐management behaviours (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of pulmonary system self‐management behaviours (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Frequency of digestive system self‐management behaviours (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Frequency of pulmonary system self‐management behaviours (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Total frequency of digestive and pulmonary self‐management behaviours (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adherence ‐ percentage of prescribed medications taken by children | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adherence ‐ percentage of prescribed aerosol treatment taken by children | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adherence ‐ percentage of prescribed chest physiotherapy treatment taken by children | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Knowledge of cystic fibrosis and its management (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Quality of well‐being (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Number of digestive system self‐management behaviours (Parents) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Number of pulmonary system self‐management behaviours (Parents) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Total number of digestive and pulmonary self‐management behaviours (Parents) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Frequency of digestive system self‐management behaviours (Parents) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Frequency of pulmonary system self‐management behaviours (Parents) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Total frequency of digestive and pulmonary system self‐management behaviours (Parents) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Knowledge of cystic fibrosis and its management (Parents) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 6 to 8 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Self‐management education on aerosol and airway clearance treatments ('Airways') versus standard treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence ‐ percentage of prescribed aerosol treatment taken by children | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adherence ‐ percentage of airway clearance treatments taken by children | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Knowledge of aerosol and airway clearance treatments (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Self management assessment behaviours (Caregivers) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Self‐management treatment behaviour (Caregivers) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Self‐management communication behaviour (Caregivers) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Self management responsiveness to airway clearance treatment when child unwell (Caregivers) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Self efficacy (Caregivers) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Disease‐specific nutrition education ('Go and Grow with CF') versus standard treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Appropriate self‐management of malabsorption (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Inappropriate self‐management of malabsorption (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Knowledge of nutrition and enzymes (Children) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Appropriate self‐management of malabsorption (Caregivers) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Inappropriate self‐management of malabsorption (Caregivers) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Knowledge of malabsorption and vitamins (Caregivers) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Post test | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. General and disease‐specific nutrition education ('Eat Well with CF') versus standard treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Per cent predicted FEV1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Weight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dietary fat intake | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Self efficacy | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Knowledge of cystic fibrosis disease‐specific nutrition | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Knowledge of general nutrition | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 6 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cottrell 1996.
| Methods | RCT, parallel design. | |
| Participants | 34 families were enrolled in the study. 20 children (aged 8 to 18 years, mean 13.5) and their parents (18 families) completed the study through to follow‐up assessment (10 children and 10 parents in intervention group, 10 children and 10 parents in control group). Gender: intervention group (6 males, 4 females); control group (4 males, 6 females). Mean (SD) disease severity as rated independently by 3 CF centre physicians on a 10‐point scale from very severe (1) to no involvement (10): intervention group ‐ pulmonary system = 7.03 (1.614), digestive system = 6.21 (1.438); control group ‐ pulmonary system = 7.08 (2.162); digestive system = 6.94 (1.37). Differences between groups were not statistically significant. The study was conducted with families from the CF centre of a children's hospital in Columbus, Ohio, USA. |
|
| Interventions | Self‐management training for CF. Content: knowledge on the nature of CF (anatomy, physiology, pathophysiology, pharmacology) and principles of self‐management (prevention, early warning signs of illness exacerbations, self‐management strategies for problems with salt loss, maldigestion, respiratory problems, medications and principles of communication); skills on problem solving and stress management techniques. Mode of delivery: group sessions (2 x 6 hours). Children and parents were grouped together for delivery of knowledge component. Separate groups for children and parents to tailor material respectively. Group sessions delivered by registered nurse or psychologist. Duration: time frame between 6‐hour sessions not stated. Setting: CF centre, Children's Hospital, Columbus, Ohio, USA. |
|
| Outcomes | Children: weight (lb); quality of well‐being; self‐management behaviours; compliance to prescribed medications, aerosol treatment, and chest physiotherapy; knowledge of CF and its management. Parents: self‐management behaviours; knowledge of CF and its management. Assessment time points: baseline and 6‐8 weeks post intervention. |
|
| Notes | The performance of power analysis and sample‐size estimation was not reported. The trials authors stated that: "The limited number of subjects in the present study reduced the power of statistical procedures" (Cottrell 1996: page 116). Mothers of all children in intervention and control groups (n = 18) participated, and 2 fathers participated, 1 in each group. For the 20 children who completed the study, the trial authors reported that groups were equivalent at baseline on years since diagnosis and disease severity. Although weight was reported in pounds (Ib), this review has reported equivalent calculations in kilograms (kg). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned to a self‐management or control group" (Cottrell 1996: page 110). No further details are provided by the trial authors. |
| Allocation concealment (selection bias) | Unclear risk | No details are provided by the trial authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details are provided by the trial authors. However, the nature of the intervention prohibits blinding of trial participants. It is unclear if the CF centre physicians who "completed the post treatment record form on each participant" (Cottrell 1996: page 113) were blinded. It is unclear if the providers of care, outcome assessors of self‐management behaviours and quality of well‐being (involving interviews with participants) and data analysts were different to the personnel delivering the programme, and if so, whether they were blinded from knowing which group participants were randomised to. |
| Incomplete outcome data (attrition bias) All outcome | Unclear risk | Of the 34 participants enrolled, "2 families withdrew due to illness of a family member, 2 withdrew because of family vacations and 10 did not return diaries" (Cottrell 1996: page 110). It is unclear if the 4 participants who withdrew had been randomised following enrolment. It is unclear which groups the 10 non‐respondent families were allocated to. The trial authors provide no details on why 10 families dropped out. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the published and unpublished records are reported. It is unclear if additional outcomes were pre‐specified in the study protocol but not reported. |
| Other bias | High risk | The mean (SD) baseline weight measurement for children in the intervention group was 89.5 (29.29) lb and for children in the control group was 101.4 (32.17) lb. This difference indicates a clinically important baseline imbalance in the case of CF. |
Downs 2006.
| Methods | Multicentre RCT, parallel design. | |
| Participants | 62 children (aged 6 to 11 years) and their caregivers were recruited and randomised to either an intervention (n = 33) or control (n = 29) group. 43 child/caregiver pairs completed the study through to 12‐month follow‐up assessment (18 in intervention group; 25 in control group). Gender: intervention group (8 males, 10 females); Control group (16 males, 9 females). Mean age (SD): intervention group 8.4 (1.8) years; control group 8.4 (1.5) years. For inclusion, children had to be fluent in English, have no learning difficulties, be currently performing ACT as part of a home management programme but not taking part in other CF self‐management programmes. The study was conducted with children and caregivers from the CF centres of 3 public children's hospitals in Australia (Western Australia, South Australia and New South Wales). |
|
| Interventions | Self‐management education on aerosol and airway clearance treatments ('Airways'). Content: knowledge integrated from disciplines of medicine physiotherapy, psychology and education; self‐management skills on practical assessment, treatment implementation and decision making, strategies to overcome barriers to treatment. Mode of delivery: pen and paper programme consisted of 10 'chapters' completed by child and caregiver dyads; each chapter provided child friendly information and behavioural exercises, and took approximately 20 minutes to complete. Duration: 10 weeks. Setting: home. |
|
| Outcomes | Children: adherence to aerosol treatment and to airway clearance treatment; knowledge about airway clearance treatment. Caregivers: self‐management behaviours; responsiveness of airway clearance treatment; performance when child unwell; self‐efficacy to manage chest treatments. Assessment time points: baseline; post‐intervention; 6‐ and 12‐month follow‐up (children's knowledge was not assessed at 6‐month follow‐up). |
|
| Notes | Power calculations from available self‐efficacy data were performed. The trial authors stated that: "A 15% improvement (0.6 points) in self‐efficacy on a Likert scale was considered to be clinically significant and 64 caregiver child dyads were required giving a power at an alpha level of 0.05" (Downs 2006: page 21) The trial authors reported that children in intervention and control groups were equivalent on gender, age and FEV1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors report that "Following recruitment at each participating centre, participants were stratified according to the child's age (six to eight and nine to eleven years) and allocated to the control of intervention group using randomised permuted blocks" (Downs 2006: page 21). Information provided by the principal author on request states that "children and their caregiver were seen sequentially and the allocation from a random numbers table was also sequential". |
| Allocation concealment (selection bias) | High risk | No details are provided by the trial authors in the published record. Information provided by the principal author on request states that allocation was not concealed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details are provided by the trial authors in the published record. Information provided by the principal author on request states that "The children and primary caregivers (the subjects) completed questionnaires for the outcomes reported in the paper and they could not have been blind to the treatment (an education program) they had received". Outcome on pulmonary function and indices of growth were "measured by blinded assessors". It is unclear if providers of care or data analysts were blinded from knowing which group participants were randomised to. |
| Incomplete outcome data (attrition bias) All outcome | Unclear risk | The published diagram showing the flow of participants at each stage of the study states that "65 families recruited to the study and randomised to the intervention or control group" (Downs 2006: page 22). The number allocated to each group is reported as: 33 to intervention and 29 to control. The reason for the disparity in participant numbers (62 in intervention and control groups vs 65 allocated to groups) was not published but was sought from the principal author who advised that 3 participants recruited to the study withdrew prior to allocation. Of the 33 allocated to the intervention group: 7 did not complete baseline assessment; and 8 withdrew prior to or subsequent to commencing the programme. Of the 29 allocated to the control group: 4 did not complete baseline assessment. The trial authors state that "Attrition after recruitment related to failure to complete the pre‐ or post‐intervention questionnaires, withdrawal from the intervention or miscellaneous factors" (Downs 2006: page 22). It is unclear why participants failed to complete questionnaire or withdrew. Intention‐to‐treat analysis was conducted by combining data from participants in the per‐protocol analysis with the 8 participants in the intervention group who withdrew from the study. Outcome data analysed (relevant to this review) were adherence, children's knowledge of airway clearance treatment, and caregivers responsiveness of airway clearance treatment when child was unwell. The trial authors state that "Pre‐intervention scores were imputed into the missing post‐intervention and follow‐up assessment data" (Downs 2006: page 23). |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the published record are stated by the trial authors as being 'per‐protocol analysis'. It is unclear if additional outcomes were pre‐specified in the study protocol but not reported. |
| Other bias | Low risk | No other potential source of bias identified. |
Stapleton 2001.
| Methods | RCT, parallel design. | |
| Participants | 59 children aged 2 to 11 years and their caregivers were enrolled and randomised in the study. Of these, children aged 6 to 11 years only were randomly allocated to an intervention (n = 22) or control (n = 21) group. Caregivers of the participating children aged 6 to 11 years and of younger children aged 2 to 5 years were randomly allocated to the intervention (n = 28) or control (n = 28) group. 3 caregivers each had 2 children with CF. 41 children aged 6 to 11 years (21 in intervention group, 20 in control group) and 54 caregivers of children aged 2 to 11 years (27 in intervention group, 27 in control group) completed the study through to 12 month follow‐up assessment. Gender: intervention group (11 males, 10 females); control group (11 males, 10 females). For inclusion, children had to be aged between 2 and 11 years at baseline data collection and have pancreatic insufficiency. Children were excluded if they had pancreatic sufficiency, short gut syndrome, liver disease requiring drug therapy, on enteral tube feeding, or had marked language delay. The study was conducted with children and caregivers from the CF clinics of 2 children's hospitals in Perth, Western Australia. |
|
| Interventions | Disease‐specific nutrition education ('Go and Grow with CF') Content: knowledge on disease‐specific nutrition topics (enzymes, energy and fat, malabsorption, vitamins and minerals, growth, snacks, and salt); self‐management skills on goal setting in small incremental steps to increase self efficacy; and self‐monitoring adherence to daily goals. Mode of delivery: written material focusing on weekly activities completed by child and caregiver dyads, taking approximately 60 minutes each week. Supplementary introductory and concluding workshops, and monthly telephone calls delivered by a dietitian. Duration: 10 weeks. Setting: home (weekly written activities) and hospital (workshops). |
|
| Outcomes | Children: knowledge of nutrition and enzymes; self‐management skills to deal with malabsorption. Parents: knowledge of malabsorption and vitamins; self‐management to deal with malabsorption. Assessment time points: baseline, post‐intervention, and 12‐month follow‐up. |
|
| Notes | Sample‐size calculations were performed based on changes in percentage of ideal body weight. The trial authors stated that: "It was determined that 20 subjects per group would be sufficient to detect a mean difference in percentage of ideal body weight of 2.5%, with a statistical power of 85% and Type I error = 0.05. In order to account for the possibility of drop‐outs the number of subjects recruited was based on the maximum number of children eligible for the clinical trial" (Stapleton 2001: page 166). Weight was not measured as an outcome of this trial. The trial authors state that children aged 2 to 11 years were randomly assigned to an intervention or control group. However, caregivers only of children younger than 6 years were randomised and these children were not directly involved in the trial. Information on gender of children allocated to groups is unpublished and was provided by principal author on request. Information provided by the principal author on request states that participants had "similar characteristics prior to the intervention in terms of age, pulmonary function, height and weight, gender, genotype, PERT, socio‐economic status, illness and activity". Study was funded in part by The Merck, Sharp and Dohme Research Foundation, Jannsen‐Cilag Pty Ltd., Roche products Ltd., Solvay Pharmaceuticals, the Princess Margaret Hospital for Children Allied Health Trust Fund, and Curtin University of Technology. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors state that "children and their carers were randomly assigned (using a random number table) to participate in the intervention group" (Stapleton 2001: page 165). Information provided by the principal author on request states that "random allocation sequence (random number table) was applied...with 30 being randomly assigned to the intervention program and 29 randomly assigned to the control group". |
| Allocation concealment (selection bias) | Unclear risk | No details are provided by the trial authors in the published records. Information provided by the principal author on request states that "Allocation sequence of participants was unknown by personnel collecting data". No further details are provided by the trial authors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details are provided by the trial authors in the published records. However, the nature of the intervention prohibits blinding of trial participants. Information provided by the principal author on request states that "Personnel collecting the data were not informed of group allocation and were not involved in the intervention program and were advised to immediately direct any and all enquiries and comments to the investigators without engaging in any related discussion. The success of blinding was not evaluated, but the investigators were in frequent and close contact with personnel to reaffirm the importance of blinding". It is unclear if providers of care or data analysts were blinded from knowing which group participants were randomised to. |
| Incomplete outcome data (attrition bias) All outcome | Unclear risk | The trial authors state that following enrolment and random allocation into intervention or control groups, the "carer of one child chose for their family not to participate in the 'Go and Grow with CF' intervention program due to time constraints, and their data were excluded from the analysis" (Stapleton 2001: page 165). It is unclear what data were collected and subsequently excluded from analysis. 1 child and caregiver from the control group relocated following post‐intervention assessment and were unavailable for follow‐up assessment at 12 months. |
| Selective reporting (reporting bias) | High risk | The trial authors state that the "differences between groups for the children's and carers' self‐management scores were not statistically significant" (Stapleton 2001: page 166). No further detail on these scores are provided by the authors in the published records of the study. The trial authors selectively reported on data specific to communication aspects of self‐management stating that at "time 2, a greater number of children in the intervention group compared to the control group reported communicating with their carers when they experienced signs of possible malabsorption (intervention group: Time 1, 9 and Time 2, 12 out of 21; control group: Time 1, 14 and Time 2, 10 out of 21)" (Stapleton 2001: page 166). It is unclear if additional outcomes were pre‐specified in the study protocol but not reported. (Note: Data on self‐management skills scores for each assessment point were provided by the principal author on request.) |
| Other bias | Low risk | No other potential source of bias identified. |
Watson 2008.
| Methods | RCT, parallel design. | |
| Participants | 74 adults were enrolled and stratified by disease severity into low or high risk disease. Equal numbers of adults were randomly allocated into intervention (n = 37) and control (n = 37) group. 48 adults completed the study through to 12‐month follow‐up assessment (23 in intervention group, 25 in control group). Gender: intervention group (12 males, 11 females); control group (14 males, 11 females). Mean (range) age: intervention group 26.4 (17.2 ‐ 43.2) years; control group 24.2 (16.9 ‐ 38.1) years. Disease status: intervention group ‐ mean BMI (kg/m2) = 21.3; pancreatic insufficiency (n = 21); Psuedomonas aeruginosa in sputum (n = 18); non‐Psuedomonas (n = 5); homozyous DF508 (n = 13); heterozygous DF508 (n = 7); other (n = 3); control group ‐ mean BMI (kg/m2) = 21.1; pancreatic insufficiency (n = 22); Psuedomonas aeruginosa in sputum (n = 21); non‐Psuedomonas (n = 4); homozyous DF508 (n = 16); heterozygous DF508 (n = 8); Other (n = 1). For inclusion, participants had to be older than 16 years, able to understand written English, not partaking in other research. Participants were excluded if they were on heart/lung transplant list or were pregnant or lactating. The study was conducted with adults from the CF clinic of Papworth Hospital, Cambridge, UK. The duration of the study was from January 2003 to August 2005. |
|
| Interventions | General and disease‐specific nutrition education ('Eat Well with CF'). Content: knowledge on general and disease‐specific nutrition topics (energy intake, digestion, pancreatic enzyme replacement, managing appetite, exercise, dietary fibre, reading food labels, body image); self‐management skills on goal setting in small incremental steps to establish new behaviours. Mode of delivery: written material focusing on weekly activities, taking approximately 30 minutes each week; supplementary workshops (introductory, weeks 5 and 10) and weekly telephone calls delivered by a dietitian. Duration: 10 weeks. Setting: home (weekly written activities) and hospital (workshops). |
|
| Outcomes | Weight (kg); pulmonary function (FEV1); self‐efficacy; knowledge of nutrition (general & disease‐specific); dietary fat intake; health‐related quality of life. Assessment time points: baseline; 6‐ and 12‐month follow‐up. |
|
| Notes | The primary outcome measure of an increase in weight after 12 months was used to calculate the required sample size. For this, data on weight gain in patients attending the CF clinic of the study centre from 1998 to 2000 were reviewed. The trial authors stated that: "By using the 'Eat Well with CF' programme it was anticipated that subjects mean (SD) weight would increase by 3 (3) kg after 12 months. With 80% power and two‐sided significance of 5% and allowing for 15% dropout or loss to follow‐up, the recruitment target was 46 participants per group" (Watson 2008: page 848). The trial authors define high disease risk as participants with < 30% predicted FEV1, on enteral feeding, or with diabetes. Microbiological segregation was introduced during the course of the study which prohibited the use of group workshops. Consequently the study could not continue and therefore target levels of recruitment could not be achieved. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors state that a "minimisation method of randomisation was used to ensure that the same number of patients were allocated to each group" (Watson 2008: page 848). |
| Allocation concealment (selection bias) | Low risk | No details are provided by the trial authors in the published records. Information provided by the principal author on request states that "an independent randomiser was used who was part of the R and D [Research and Development] department of the hospital". and which was "supervised by the project statistician...independently of the investigator". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial authors state that "The study could not be blinded to either the subjects or the investigators because of the nature of the intervention" (Watson 2008: 848). Information provided by the principal author on request states that "no blinding" of outcome assessors took place. It is unclear if providers of care or data analysts were blinded from knowing which group participants were randomised to. |
| Incomplete outcome data (attrition bias) All outcome | Low risk | Of the 74 adults enrolled with equal numbers in the intervention (n = 37) and control (n = 37) groups, 48 were included in the "completer analysis" at 12 months follow‐up (23 in intervention group, and 25 in control group). Incomplete outcome data are reported for each assessment point for intervention and control groups as follows: Intervention group: baseline data are reported as missing from 3 of the 37 allocated to group due to relocation (n = 1) and non‐return of questionnaires (n = 2). At 6 months follow‐up, data from a further 6 participants are reported as missing due to withdrawal from the study (n = 3), defaulting from follow‐up (n = 2) or death (n = 1). At 12 months follow‐up, data from a further 5 participants are reported as missing due to defaulting from follow‐up (n = 4) or death (n = 1). The number of participants in the intervention group included in the "completer analysis" is reported as 23. Control group: baseline data are reported as missing from 3 of the 37 allocated to group due to relocation (n = 1) and non‐return of questionnaires (n = 2). At 6 months follow‐up, data from a further 2 participants are reported as missing due to relocation (n = 1) or death (n = 1). At 12 months follow‐up, data from a further 7 participants are reported as missing due to defaulting from follow‐up (n = 6) or death (n = 1). The number of participants in the control group included in the "completer analysis" is reported as 23. Missing outcome data are balanced in numbers across both groups with similar reasons for missing data across both groups. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the published record are reported. It is unclear if additional outcomes were pre‐specified in the study protocol but not reported. |
| Other bias | Low risk | No other potential source of bias identified. |
ACT: airway clearance techniques CF: cystic fibrosis FEV1: forced expiratory volume at 1 second PERT: pancreatic enzyme replacement therapy RCT: randomised controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bartholomew 1997 | Not a RCT or quasi‐RCT or CCT. |
| Bartholomew 2000 | Not a RCT or a quasi‐RCT or a CCT. |
| Bosworth 1997 | No explicit focus on self‐management education in the aims or content of the programme. Home versus hospital treatment of intravenous antibiotics and chest physiotherapy. |
| Byron 2000 | No explicit focus on self‐management education in the aims or content of the program. Home‐visit program. |
| Chernoff 2002 | No explicit focus on self‐management education in the aims or content of the program. Community based support programme. |
| Christian 2006 | No explicit focus on self‐management education in the aims or content of the program. Life skill program focusing on psychological adjustment, functional and physiological health. |
| Cox 1994 | Not a RCT or quasi‐RCT or CCT. |
| David 2008 | Not a RCT or quasi‐RCT or CCT. |
| Davis 2004 | No explicit focus on self‐management education in the aims or content of the program. Educational program focusing on knowledge about cystic fibrosis and coping strategies. |
| de Jong 1994 | No explicit focus on self‐management education in the aims or content of the program. Home exercise training program. |
| Goldbeck 2001 | Not a RCT or quasi‐RCT or CCT. |
| Hains 2001 | Not a RCT or quasi‐RCT or CCT. |
| Hourigan 2013 | No explicit focus on self‐management education in the aims or content of the program. Behavioural parent‐training intervention for improving nutrition. |
| Klijn 2004 | No explicit focus on self‐management education in the aims or content of the program. Anaerobic training program. |
| Moorcroft 2004 | No explicit focus on self‐management education in the aims or content of the program. Unsupervised home based exercise program. |
| Orenstein 2002 | No explicit focus on self‐management education in the aims or content of the program. Exercise training program. |
| Power 2006 | No explicit focus on self‐management education in the aims or content of the program. Behavioural and nutrition treatment program. |
| Quittner 2000 | No explicit focus on self‐management education in the aims or content of the program. Focus on treatment adherence using family learning program or behavioural family system therapy. |
| Quittner 2011 | No explicit focus on self‐management education in the aims or content of the program. Behavioural adherence program with a primary focus on medication adherence titled I change adherence and raise expectations (iCARE). |
| Quittner 2012 | No explicit focus on self‐management education in the aims or content of the program. Cell phone (CFfone) social networking intervention to promote treatment adherence in adolescents. |
| Singer 1991 | Not a RCT or quasi‐RCT or CCT. |
| Stark 1996 | Not a RCT or quasi‐RCT or CCT. |
| Stark 2009 | No explicit focus on self‐management education in the aims or content of the program. Behavioural plus nutrition intervention program. |
| Trapp 1998 | No explicit focus on self‐management education in the aims or content of the program. Hospital self‐administration of medication program. |
| Turchetta 2004 | Not a RCT or quasi‐RCT or CCT. |
| Tuzin 1998 | Not a RCT of quasi‐RCT or CCT. |
CCT: controlled clinical trial RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Bergman 2007.
| Methods | RCT, parallel design. |
| Participants | 67 patients with CF aged 21 years or less. |
| Interventions | Web‐based interactive chronic illness program titled CF:DOC which was designed in part to promote self‐management skills. Half the group were randomised into either an intervention or control group. Control group received 'usual care'. |
| Outcomes | Change in weight for age; change in height for age; self‐care behaviours. |
| Notes | Study was published in the Cystic Fibrosis Worldwide Newsletter in 2007 For updated review, the principal author was contacted again for information. A response is awaited. |
Cannon 1999.
| Methods | Not explicit. |
| Participants | 10 children aged 6‐13 years. |
| Interventions | Educational messages delivered through in‐home video‐conferencing plus routine clinic education. Control group received routine clinic education. |
| Outcomes | Pulmonary function tests; indices of nutritional status and growth (weight, triceps skin fold thickness, mid‐arm muscle circumference); knowledge. |
| Notes | Study published in an abstract. Efforts to locate contact details on any one of the authors have failed. |
Jessup 2008.
| Methods | RCT, 3‐arm design. |
| Participants | 19 adolescents and adults. |
| Interventions | Mentorship plus IT‐based self‐monitoring on CF patients' self‐efficacy and self‐management behaviours of 6 months duration followed by a 'washout period'. Participants were allocated to an intervention group or mentorship only group or control group. |
| Outcomes | No detail provided other than 'behavioural changes'. |
| Notes | Study published in 3 abstracts. Principal author contacted for information. A response is awaited. |
Johnson 2001.
| Methods | RCT, delayed treatment design. |
| Participants | Children with CF and type 1 diabetes. |
| Interventions | Clinic‐based Adherence Intervention for Diabetes & CF (staged self‐management intervention). |
| Outcomes | |
| Notes | Information provided by principal author. Study has been conducted in Australia with a sample of children with type 1 diabetes. It is planned to conduct this study in the USA with a sample of children with CF. |
Van der Gieesen 2006.
| Methods | RCT, parallel design. |
| Participants | 37 children with cystic fibrosis aged 7‐13 years; 11 were allocated to an intervention group and 20 were allocated to control group. |
| Interventions | Board game 'Airway' to increase knowledge about CF lung disease including treatment. |
| Outcomes | Knowledge. |
| Notes | Study published in an abstract. For updated review, principal author contacted again who replied indicating that there was still no published information about the study available. |
Wainwright 2009.
| Methods | RCT, parallel design. |
| Participants | 46 adolescents and young adults with CF aged 12 to 19 years. Participants were allocated into 3 groups: standard care group (controls, n = 15); standard care plus phone mentoring group (n = 16); or standard care plus phone mentoring plus IT tool group (n = 15). |
| Interventions | Phone mentoring compared to phone mentoring plus IT Tool to facilitate electronic self‐reporting of symptoms. Mentoring focused on facilitating self‐management. |
| Outcomes | Self efficacy; quality of life; pulmonary function by spirometry; weight z scores; height z scores. |
| Notes | Study was published in abstract form. Principal author contacted for further information. A response is awaited. |
CF: cystic fibrosis RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Huang 2009.
| Trial name or title | Texting to promote chronic disease management. |
| Methods | RCT. |
| Participants | Individuals with CF aged 14 to 22 years. Individuals with type 1 diabetes, or inflammatory bowel disease for at least 6 months also included. |
| Interventions | Web‐ and text‐based self‐management information, tips, strategies and questions versus usual care. |
| Outcomes | Health knowledge, disease health knowledge, health literacy, quality of life. |
| Starting date | 2009. |
| Contact information | Huang Jeannie, University of California, San Diego, USA. |
| Notes | No published data identified in updated search strategy. |
CF: cystic fibrosis RCT: randomised controlled trial
Differences between protocol and review
Order of secondary outcomes changed to place health‐related quality of life further down.
Search strategy was revised and appendices for each database searched are included.
The author Dawn Farrel has been added.
Contributions of authors
E Savage: conception and design of the review, coordinating the review, writing the protocol, search strategy development, undertaking searches and retrieving papers for the review, screening retrieved papers against the inclusion criteria, extracting data from included papers, appraising quality of papers, writing to authors of papers for additional information, data analysis and interpretation, data management for the review, entering data into RevMan, writing the review, sourcing funding for the review, taking principal responsibility for updating the review.
P Beirne: design of review, critically evaluating protocol, screening retrieved papers against inclusion criteria, appraising quality of papers, data interpretation, providing general advice on the conducting a systematic review.
D Farrell: assisted with search strategy and with retrieval of papers. Cross‐checked data extraction. Cross‐checked data entry of results for each outcome to Revman against published papers and data extraction form. Providing administrative and technical support for all aspects of the review.
M Ni Chronin: providing clinical advice on disease management of cystic fibrosis, critically evaluating protocol, providing general advice on the review and interpretation of results.
A Duff: providing advice on self‐management education interventions for patients with cystic fibrosis, critically evaluating protocol, providing general advice on the review and interpretation of results.
T Fitzgerald: providing statistical support for data analysis and interpretation.
Sources of support
Internal sources
University College Cork, Ireland.
External sources
Health Research Board, Ireland.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Cottrell 1996 {published and unpublished data}
- Cottrell CK. The effects of self‐management training in children/adolescents with cystic fibrosis. Unpublished doctoral dissertation,Ohio University, Ohio, US 1994.
- Cottrell CK, Young GA, Creer TL, Holroyd KA, Kotses H. The development and evaluation of a self‐management program for cystic fibrosis. Pediatric Asthma, Allergy & Immunology 1996;10(3):109‐18. [Google Scholar]
Downs 2006 {published and unpublished data}
- Downs JA, Roberts CM, Blackmorel AM, Souef PN, Jenkins SC. Benefits of an education programme on the self‐management of aerosol and airway clearance treatments for children with cystic fibrosis. Chronic Respiratory Diseases 2006;3(1):19‐27. [DOI] [PubMed] [Google Scholar]
Stapleton 2001 {published and unpublished data}
- Stapleton DR, Gurrin LC, Zubrick SR, Silburn SR, Sherridd JL, Sly PD. The effect of 'Go and Grow with CF' on nutrition and pancreatic enzyme knowledge of children with cystic fibrosis. Australian Journal of Nutrition and Dietetics 2001;58(3):164‐8. [Google Scholar]
- Stapleton DR, Gurrin LC, Zubrick SR, Silburn SR, Sherriff JL, Sly PD. What do children with cystic fibrosis and their parents know about nutrition and pancreatic enzymes?. Journal of the American Dietetic Association 2000;100(12):1494‐1500. [DOI] [PubMed] [Google Scholar]
- Stapleton DR, Tunnecliffe L, McGuiness D, Sherriff J, Sly P. Development of a nutrition and behaviour intervention program: Go and Grow with CF. Australian Journal of Nutrition and Dietetics 1998;55(3):130‐7. [Google Scholar]
Watson 2008 {published and unpublished data}
- Watson H, Bilton D, Truby H. A randomised controlled trial of a behavioral nutrition education programme "Eat Well with CF" for adults with CF [abstract]. Journal of Cystic Fibrosis 2006;5(Suppl 1):S73. [Google Scholar]
- Watson H, Bilton D, Truby H. A randomized controlled trial of a new behavioural home‐based nutrition education program, "Eat Well with CF" in adults with cystic fibrosis. Journal of the American Dietetic Association 2008;108(5):847‐52. [DOI] [PubMed] [Google Scholar]
- Watson H, Bilton D, Truby H. Evaluating the process of a behavioural nutrition education programme "Eat Well with CF" for adults with CF [abstract]. Journal of Cystic Fibrosis 2006;5(Suppl 1):S73. [Google Scholar]
- Watson H, Truby H, Haworth CS, Bilton D. Pilot study to evaluate a home based behavioural education programme for adults [abstract]. Journal of Cystic Fibrosis 2004;3(Suppl):S281. [Google Scholar]
- Watson HM, Haworth CS. Pilot testing of an interactive web‐based meeting to replace group workshops in a nutrition education programme "Eat Well with CF" for adults with cystic fibrosis (CF) [abstract]. Journal of Cystic Fibrosis 2008;7(Suppl):S96. [Google Scholar]
References to studies excluded from this review
Bartholomew 1997 {published data only}
- Bartholomew LK, Czyzewski DI, Parcel GS, Swank PR, Sockrider MM, Mariotto MJ, et al. Self‐management of cystic fibrosis: Short‐term outcomes of the cystic fibrosis family education program. Health Education & Behaviour 1997;24(5):652‐66. [DOI] [PubMed] [Google Scholar]
- Bartholomew LK, Sockrider MM, Seilheimer DK, Czyzewski DI, Parcel GS, Spinelli SH. Performance objectives for the self‐management of cystic fibrosis. Patient Education Counselling 1993;22(1):15‐25. [DOI] [PubMed] [Google Scholar]
- Parcel GS, Swank PR, Mariotto MJ, Bartholomew LK, Czyzewski DI, Sockrider MM, et al. Self‐management of cystic fibrosis: a structural model for educational and behavioral variables. Social Science and Medicine 1994;38(9):1307‐15. [DOI] [PubMed] [Google Scholar]
Bartholomew 2000 {published data only}
- Bartholomew LK, Czyxewski DI, Swank PR, McCormick L, Parcel GS. Maximizing the impact of the cystic fibrosis family education program: factors related to program diffusion. Family & Community Health 2000;22(4):27‐47. [Google Scholar]
Bosworth 1997 {published data only}
- Bosworth DG, Neilson DW. Effectiveness of home versus hospital care in the routine treatment of cystic fibrosis. Pediatric Pulmonology 1997;24(1):42‐7. [DOI] [PubMed] [Google Scholar]
Byron 2000 {published data only}
- Byron M, Burton J, Tostevin M, Madge S. A home visit programme to improve health status and psychosocial functioning of families with child with CF [abstract]. Pediatric Pulmonology 2000;30(Suppl 20):336. [Google Scholar]
Chernoff 2002 {published data only}
- Chernoff RG, Ireys HT, DeVet KA, Kim YJ. A randomized, controlled trial of a community‐based support program for families of children with chronic illness; paediatric outcome. Archives of Pediatric & Adolescent Medicine 2002;156(6):533‐9. [DOI] [PubMed] [Google Scholar]
Christian 2006 {published data only}
- Christian B. Functional disability and quality of life of school‐age children with cystic fibrosis [abstract]. Pediatric Pulmonology 2004;38(Suppl 27):356. [] [Google Scholar]
- Christian B. The impact of chronic illness on quality of life in children with cystic fibrosis [abstract]. Pediatric Pulmonology 2006;41 Suppl 29:398. [] [Google Scholar]
- Christian B. The interface between physical activity, growth, and pulmonary function with the psychosocial impact of cystic fibrosis in children [abstract]. Pediatric Pulmonology 2005;40(Suppl 28):370. [] [Google Scholar]
- Christian B, D'Auria J, Belyea M. Loneliness in school age children with cystic fibrosis [abstract]. Pediatric Pulmonology 2003;Suppl 25:366. [] [Google Scholar]
- Christian BJ, D'Auria JP. Building life skills for children with cystic fibrosis: effectiveness of an intervention. Nursing Research 2006;55(5):300‐7. [DOI] [PubMed] [Google Scholar]
- Christian BJ, D'Auria JP, Belyea MJ, Retsch‐Bogart GZ. Psychosocial status of school‐age children with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 1999;28(S19):329. [Google Scholar]
Cox 1994 {published data only}
- Cox JE. Self‐care in the classroom for children with chronic illness: a case study of a student with cystic fibrosis. Elementary School Guidance & Counselling 1994;29(2):121. [Google Scholar]
David 2008 {published data only}
- David V, Iguenane J, Ravilly S, Berville C, Douaud P, Chailleux D, et al. Therapeutic education in cystic fibrosis's children: skills, objectives and guides. Archives de Pédiatrie 2008;15(5):750‐2. [DOI] [PubMed] [Google Scholar]
Davis 2004 {published data only}
- Davis MA, Quittner AL, Stack C. Controlled evaluation of the STARBRIGHT Explorer Series CD‐Rom program for children and adolescents with cystic fibrosis [abstract]. Pediatric Pulmonology 2002;Suppl 24:351. [] [DOI] [PubMed] [Google Scholar]
- Davis MA, Quittner AL, Stack CM, Yang MC. Controlled evaluation of the STARBRIGHT CD‐ROM program for children and adolescents with Cystic Fibrosis. Journal of Pediatric Psychology 2004;29(4):259‐67. [] [DOI] [PubMed] [Google Scholar]
de Jong 1994 {published data only}
- Jong W, Grevink RG, Roorda RJ, Kaptein AA, Schans CP. Effect of a home exercise training program in patients with cystic fibrosis. Chest 1994;105(5):463‐8. [DOI] [PubMed] [Google Scholar]
Goldbeck 2001 {published data only}
- Goldbeck L, Babka C. Development and evaluation of a multi‐family psychoeducational program for cystic fibrosis. Patient Education and Counselling 2001;44(2):187‐92. [DOI] [PubMed] [Google Scholar]
Hains 2001 {published data only}
- Hains AA, Davies WH, Behrens D, Freeman, ME, Biller, JA. Effectiveness of a cognitive behavioural intervention for young adults with cystic fibrosis. Journal of Clinical Psychology in Medical Settings 2001;8(4):325‐35. [Google Scholar]
Hourigan 2013 {published data only}
- Hourigan SE, Helms SW, Christon LM, Southam‐Gerrow MA. Improving nutrition in toddlers with cystic fibrosis: feasibility of a behavioral parent‐training intervention. Clinical Practice in Pediatric Psychology 2013;1(3):235‐49. [Google Scholar]
Klijn 2004 {published data only}
- Klijn PHC, Oudshoorn A, Ent CK, Net J, Kimpen JL, Helders PJM. Effects of anaerobic training in children with cystic fibrosis. Chest 2004;125(4):1299‐1305. [DOI] [PubMed] [Google Scholar]
Moorcroft 2004 {published data only}
- Moorcroft AJ, Dodd ME, Morris J, Webb AK. Individualised unsupervised exercise training in adults with cystic fibrosis: a 1 year randomised controlled trial. Thorax 2004;59(12):1074‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orenstein 2002 {published data only}
- Orenstein DM, Hovell MF, Mulvihill M, Keating KK, Hofstetter R, Kelsey S, et al. Strength vs aerobic training in children with cystic fibrosis. Chest 2004;126(4):1204‐14. [DOI] [PubMed] [Google Scholar]
Power 2006 {published data only}
- Powers SW. Nutrition in early childhood: focus on parent interaction. Pediatric Pulmonology 2003;36(Suppl 25):138‐139. [Google Scholar]
- Powers SW, Byars KC, Mitchell MJ, Patton SR, Schindler T, Zeller MH. A randomized pilot study of behavioural treatment to increase calorie intake in toddlers with cystic fibrosis. Children's Health Care 2003;32(4):297‐311. [Google Scholar]
- Powers SW, Jones JS, Ferguson KS, Heidemann M, Henry R, Piazza‐Waggoner C, et al. Behavioral treatment for toddlers and preschoolers with cystic fibrosis produces recommended energy intake and normal rates of growth [abstract]. Pediatric Pulmonology 2004;38(Suppl 27):361. [] [Google Scholar]
- Powers SW, Jones JS, Ferguson KS, Henry R, Heidemann M, Patton SR, et al. Overcoming barriers to evidence‐based nutrition treatment for preschoolers such as distance from the cystic fibrosis center and multiple food allergies [abstract]. Pediatric Pulmonology 2004;38(Suppl 27):341. [] [Google Scholar]
- Powers SW, Jones JS, Ferguson KS, Piazza‐Waggoner C, Daines C, Acton JD. Randomized clinical trial of behavioral and nutrition treatment to improve energy intake and growth in toddlers and preschoolers with cystic fibrosis. Pediatrics 2005;116(6):1442‐50. [] [DOI] [PubMed] [Google Scholar]
- Powers SW, Piazza‐Waggoner C, Jones J, Daines C, Acton J. Impact of behavioral and nutrition treatment for toddlers and preschoolers with CF on energy intake and growth maintains for 12 and 18 months [abstract]. Pediatric Pulmonology 2005;40(Suppl 28):372. [] [Google Scholar]
- Powers SW, Piazza‐Waggoner C, Jones JS, Ferguson KS, Daines C, Acton JD. Examining clinical trial results with single‐subject analysis: an example involving behavioural and nutrition treatment for young children with cystic fibrosis. Journal of Pediatric Psychology 2006;31(6):574‐81. [DOI] [PubMed] [Google Scholar]
- Powers SW, Schindler T, Schwarber L, Deeks CM, Byars KC, Arthur S, et al. Behavioural treatment to improve nutrition in toddlers with cystic fibrosis. Pediatric Pulmonolgy 1999;28(Suppl 19):329. [Google Scholar]
Quittner 2000 {published data only}
- Quittner AL, Drotar D, Levers‐Landis CE, Seidner D, Slocum N, Jacobsen J. Adherence to medical treatments in adolescents with cystic fibrosis: the development and evaluation of family‐based intervention. In: Drotar, D editor(s). In: Promoting Adherence to Medical Treatment in Childhood Chronic Illness: Concepts, Methods, and Interventions. London, NJ: Lawrence Erlbaum Associates, 2000:383‐407. [Google Scholar]
Quittner 2011 {published data only}
- Alpern AN, McLean KA, Marciel KK, Zhang J, Riekert KA, Quittner AL. Effects of clinical supervision on treatment fidelity in ICare [abstract]. Pediatric Pulmonology 2012;47(Suppl 35):433. [Google Scholar]
- Eakin MN, Bilderback A, Ridge A, Quittner AL, Zhang J, Riekert KA. Out‐of‐pocket costs for medication in the I change adherence and raise expectations (ICARE) study [abstract]. Pediatric Pulmonology 2011;46(Suppl 34):420. [Google Scholar]
- Marciel KK, Hernandez C, Kimberg CI, Zhang J, Rickert KA, Quittner AI. Developmental differences in health‐related quality of of life and associations between treatments and patient burden in the I change adherence and raise expectations (ICARE) study [abstract]. Pediatric Pulmonology 2011;46(Suppl 34):419. [Google Scholar]
- Marciel KK, Kimberg CI, Riekert KA, Swenson A, Quittner AL. Participation of multidisciplinary team members in a behavioural intervention to improve adherence in adolescents with CF [abstract]. Pediatric Pulmonology 2010;45(Suppl 33):437. [Google Scholar]
- Quittner A, Kimberg C, Marciel K, Zhang J, Riekert K. Randomized, controlled trial of a behavioral adherence intervention for adolescents with cystic fibrosis: I change adherence and raise expectations (iCARE) [abstract]. Chest 2011;140(4 Suppl):908. [Google Scholar]
Quittner 2012 {published data only}
- Blackwell LS, Romero SL, Romero CV, McLean KA, Dawkins K, Hoag J, et al. CFfone: a social networking site for adolescents and young adults with CF [abstract]. Pediatric Pulmonology 2012;47(Suppl 35):430. [Google Scholar]
- Marciel KK, Saiman L, Quittell LM, Dawkins K, Quittner AL. Cell phone intervention to improve adherence: cystic fibrosis care team, patient, and parent perspectives. Pediatric Pulmonology 2010;45(2):157‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittner AL, Romero SL, Blackwell LS, Marciel KK, Romero CV, Dawkins K, et al. Effect of CFfone on knowledge of disease management, psychological well‐being, and health‐related quality of life in adolescents and young adults with CF [abstract]. Journal of Cystic Fibrosis 2012;11(Suppl 1):S137. [Google Scholar]
- Quittner AL, Romero SL, Blackwell LS, Romero CV, Marciel KK, Dawkins K, et al. Preliminary results on the efficacy of an online social network for adolescents with CF: age and disease severity group comparisons. Pediatric Pulmonology 2012;47(Suppl 35):388. [Google Scholar]
Singer 1991 {published data only}
- Singer LT, Nofer JA, Benson‐Szekely LJ, Brooks LJ. Behavioural assessment and management of food refusal in children with cystic fibrosis. Developmental and Behavioural Pediatrics 1991;12(2):115‐20. [PMC free article] [PubMed] [Google Scholar]
Stark 1996 {published data only}
- Stark LJ, Bowen AM, Tyc VL, Evans S, Passero MA. A behavioural approach to increasing calorie consumption in children with cystic fibrosis. Journal of Pediatric Psychology 1990;15(3):309‐26. [DOI] [PubMed] [Google Scholar]
- Stark LJ, Knapp LG, Bowen AM, Powers SW, Jelalian E, Evans S, et al. Increasing calorie consumption in children with cystic fibrosis: replication with 2‐year follow‐up. Journal of Applied Behavior Analysis 1993;26(4):435‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LJ, Mulvihill MM, Powers SW, Jelalian E, Keating K, Creveling S, et al. Behavioral intervention to improve calorie intake of children with cystic fibrosis:Treatment versus wait list control. Journal of Pediatric Gastroenterogy and Nutrition 1996;22(3):240‐53. [DOI] [PubMed] [Google Scholar]
- Stark LJ, Powers SW, Jelalian E, Rape RN, Miller DL. Modifying problematic mealtime interactions of children with cystic fibrosis and their parents via behavioral parent training. Journal of Pediatric Psychology 1994;19(6):751‐68. [DOI] [PubMed] [Google Scholar]
Stark 2009 {published data only}
- Janicke DM, Mitchell MJ, Quittner AL, Piazza‐Waggoner C, Stark LJ. The impact of behavioural intervention on family interactions at mealtimes in pediatric cystic fibrosis. Children's Health Care 2008;37:49‐66. [Google Scholar]
- Opipari‐Arrigan L, Powers SW, Quittner AL, Stark LJ. Mealtime problems predict outcome in clinical trial to improve nutrition in children with CF. Pediatric Pulmonology 2010;45(1):78‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LJ, Mackner LM, Kessler JH, Opipari LC, Quittner AL. Preliminary findings for calcium intake in children with cystic fibrosis following behavioral intervention for calorie intake. Children's Health Care 2002;31(2):107‐18. [Google Scholar]
- Stark LJ, Opipari LC, Spieth LE, Jelalian E, Quittner AL, Higgins L, et al. Contribution of behaviour therapy to dietary treatment in cystic fibrosis: A randomized controlled study with two year follow up. Behavior Therapy 2003;34(2):237‐58. [Google Scholar]
- Stark LJ, Opipari‐Arrigan L, Quittner AL, Bean J, Powers SW. The effects of an intensive behavior and nutrition intervention compared to standard of care on weight outcomes in CF. Pediatric Pulmonology 2011;46(4):31‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LJ, Quittner AL, Opipari LC, Jelalian E, Jones E, Powers SW, Higgins L, Maguiness K, Seidner D, Hoover J, Eigen H, Duggan CP, Stallings, VA. The contribution of behavior therapy to enhancing adherence in school‐age children with CF: the example of diet. Pediatric Pulmonology 1998;26(Suppl 17):110‐1. [Google Scholar]
- Stark LJ, Quittner AL, Powers SW, Opipari‐Arrigan L, Bean JA, Duggan C, et al. Randomized clinical trial of behavioural intervention and nutrition education to improve calorie intake and weight in children with cystic fibrosis. Archives of Pediatric & Adolescent Medicine 2009;163(10):915‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trapp 1998 {published data only}
- Trapp M, Barton S, Morgan H, Lockyer L. Self‐administration of drugs for cystic fibrosis. Professional Nurse 1998;14(3):199‐203. [PubMed] [Google Scholar]
Turchetta 2004 {published data only}
- Turchetta A, Salerno T, Lucidi V, Libera F, Cutrera R, Bush A. Usefulness of a program of hospital‐supervised physical training in patients with cystic fibrosis. Pediatric Pulmonology 2004;38(2):115‐8. [DOI] [PubMed] [Google Scholar]
Tuzin 1998 {published data only}
- Tuzin BJ, Mulvihill MM, Kilbourn KM, Bertran DA, Buono M, Hovell MF, et al. Increasing physical activity of children with cystic fibrosis: A home‐based family intervention. Pediatric Excercise Science 1998;10(1):57‐68. [Google Scholar]
References to studies awaiting assessment
Bergman 2007 {published data only}
- Bergmann D, Farmer G, Moss R. CF.DOC ‐ A Web‐based Interactive Model of Care. Cystic Fibrosis Worldwide 2007;9:22‐5. [Google Scholar]
Cannon 1999 {published data only}
- Cannon C, Benitez J, Scarbary J, Taylor K, Guill M. In‐home videoconferencing for cystic fibrosis patient education. Pediatric Pulmonology 1999;18(S19):333. [Google Scholar]
Jessup 2008 {published data only}
- Jessup M, Busch J, Fitzpatrick P, Turner P, Cameron‐Tucker H, Joseph L, et al. Pathways home project; a pilot study of chronic disease self management in cystic fibrosis [abstract]. Respirology 2007;12(S4):A155. [Google Scholar]
- Jessup M, Busch J, Turner P, Cummings E, Cameron‐Tucker H, Fitzpatrick P, et al. Pathways Home Project: a pilot study of chronic disease self‐management in cystic fibrosis (CF) [abstract]. Journal of Cystic Fibrosis 2008;7:S103. [Google Scholar]
- Jessup MM, Hauser J, Cameron‐Tucker H, Cummings E, Turner P, Blizzard L, Reid D. Facilitating self‐management in adolescents and adults with cystic fibrosis: a pilot study [abstract]. Pediatric Pulmonology 2011;46(Suppl 34):405. [Google Scholar]
Johnson 2001 {unpublished data only}
- Johnson S. Clinical‐based adherence intervention for diabetes and CF. Unpublished project cited in EBSCO accessed at http://web.ebscohost.com/ehost/detail?vid=1&hid=110&sid=987d42a5‐9153‐4415‐8e July 2008 2001.
Van der Gieesen 2006 {published data only}
- Giessen L, Oude Sogtoen S, Hop W, Tiddens H. Playing the board game "Airway" with children increases knowledge about CF lung disease [abstract]. Journal of Cystic Fibrosis 2006;5(Suppl 1):S78j. [Google Scholar]
Wainwright 2009 {published data only (unpublished sought but not used)}
- Wainwright C, Saddington H, Busch J, Bizzard L, Cameron‐Tucker H, Cheney J, et al. Improving self‐efficacy in adolescents and young adults with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2009;8(Suppl 2):S93. [Google Scholar]
References to ongoing studies
Huang 2009 {published data only}
- Huang J. Texting to promote chronic disease management. ClinicalTrials.Gov at http://clinicaltrials.gov/show/NCT01253733 2009.
Additional references
Bartholomew 1991
- Bartholomew LK, Parcel GS, Seilheimer DK, Czyzewski D, Spinelli SH, Congdon B. Development of a health education program to promote the self‐management of cystic fibrosis. Health Education Quarterly 1991;18(4):429‐43. [DOI] [PubMed] [Google Scholar]
Bradley 2008
- Bradley PM, Landsay B. Care delivery and self‐management strategies for adults with epilepsy. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006244.pub2] [DOI] [PubMed] [Google Scholar]
Davies 2009
- Davies H, Crombie, I. What are confidence intervals and p‐values?. www.medicine.ox.ac.uk/bandolier/painres/download/whatis/What_are_Conf_Inter.pdf (accessed 07 March 2011).
Deakin 2005
- Deakin TA, McShane CE, Cade JE, Williams R. Group based training for self‐management strategies in people with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003417.pub2] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins J, Altman D. Chapter 9 Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Department of Health 2001
- Department of Health. The Expert Patient: A New Approach to Chronic Disease Management for the 21st Century. London: Department of Health, 2001. [Google Scholar]
Dodge 2007
- Dodge J, Lewis P, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947‐2003. European Respiratory Journal 2007;29:522‐526. [DOI] [PubMed] [Google Scholar]
Effing 2007
- Effing T, Monninkhof EM, Palen J, Herwaarden CLA, Partidge MR, Walters EH, et al. Self‐management education for patients with chronic obstructive disease. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002990.pub2] [DOI] [PubMed] [Google Scholar]
Gibson 2002
- Gibson PG, Powell H, Wilson A, Abramson MJ, Haywood P, Bauman A, et al. Self‐management education and regular practitioner review for adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001117] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lorig 2002
- Lorig K. Partnership between expert patients and physicians. Lancet 2002;359(9309):814‐5. [DOI] [PubMed] [Google Scholar]
Lorig 2003
- Lorig KR, Holman H. Self‐management education:history, outcomes, and mechanisms. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine 2003;26(1):1‐7. [DOI] [PubMed] [Google Scholar]
Newman 2004
- Newman S, Steed L, Mulligan K. Self‐management interventions for chronic illness. Lancet 2004;364(9444):1523‐37. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Savage 2007
- Savage E, Callery P. Clinic consultations with children and parents on the dietary management of cystic fibrosis. Social Science and Medicine 2007;64(2):363‐74. [DOI] [PubMed] [Google Scholar]
Sawicki 2007
- Sawicki GS, Sellers DE, McGuffie K, Robinson W. Adults with cystic fibrosis report important and unmet needs for disease information. Journal of Cystic Fibrosis 2007;6(6):411‐6. [DOI] [PubMed] [Google Scholar]
Schreurs 2003
- Schreurs KM, Colland VT, Kuijer RG, Ridder DT, Elderen T. Development, content, and process evaluation of a short self‐management intervention in patients with chronic diseases requiring self‐care behaviours. Patient Education and Counselling 2003;51(2):133‐41. [DOI] [PubMed] [Google Scholar]
Shaw 2007
- Shaw EJ, Strokes T, Camosso‐Stefinovic J, Baker R, Baker GA, Jacoby A. Self‐management education for adults with epilepsy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004723.pub3] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne J, Egger M, Moher D. Addressing reporting biases. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook forSystematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Stokes 2007
- Stokes T, Shaw EJ, Camosso‐Stefinovic J, Baker R, Baker GA, Jacoby A. Self‐management education for children with epilepsy. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004724.pub3] [DOI] [PubMed] [Google Scholar]
Walters 2007
- Walters S, Metha A. Epidemiology of cystic fibrosis. In: Hodson M. Geddes D. Bush A editor(s). Cystic Fibrosis. 3rd Edition. London: Hodder Arnold, 2007:433‐441. [Google Scholar]
Williams 2007
- Williams B, Mukhopadhyay S, Dowell J, Coyle J. From child to adult: an exploration of shifting family roles and responsibilities in managing physiotherapy for cystic fibrosis. Social Science and Medicine 2007;65(10):2135‐46. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Savage 2011
- Savage E, Beirne PV, Ni Chroinin M, Duff A, Fitzgerald T, Farrell D. Self‐management education for cystic fibrosis. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007641.pub2] [DOI] [PubMed] [Google Scholar]


