Abstract
Background:
Given the potential for recovery in recent onset non-ischemic cardiomyopathy (ROCM), the timing and need for ICDs remains controversial. We examined the utilization of ICDs and the impact on survival for subjects with ROCM
Methods and Results:
An NHLBI sponsored registry enrolled 373 subjects with ROCM, all with an LVEF ≤0.40 and ≤six months of symptoms. The mean age was 45±14 years, 38% were female, 21% black, 75% NYHA II/III, and the mean LVEF was 0.24 ± 0.08. Survival was comparable for subjects with an ICD within one month of entry (n=43, 1/2/3 year % survival=97/97/92) and those with no ICD at one month (n=330, % survival=98/97/95, p=0.30) and between those with and without an ICD at 6 months (ICD, n=73, 1/2/3 year % survival =98/98/95; no ICD, n=300, % survival =98/96/95, p=0.95). There were only 6 sudden cardiac deaths (SCD) noted (% survival free from SCD=99/98/97) and these occurred in 1.9% of subjects without ICD and 0.9% of those with a device (p=0.50).
Conclusions:
In a multicenter cohort of ROCM the risk of SCD was low at 1% per year. Early ICD placement did not impact survival and can be deferred while assessing potential for myocardial recovery.
Introduction
In the United States, sudden cardiac death (SCD) is responsible for up to 450,000 deaths per year (1, 2). In subjects with chronic systolic heart failure from either ischemic or non-ischemic cardiomyopathy, the current guidelines are explicit with respect to the use of implantable cardioverter-defibrillators (ICDs) (3). In contrast to a wealth of data in chronic heart failure (4, 5, 6), for patients with newly diagnosed non-ischemic cardiomyopathy of recent onset (ROCM), the risk of SCD and the benefits of ICDs are not well established. Given the potential for myocardial recovery in ROCM (7, 8, 9) significant controversy exists with respect to both the benefits and timing of ICD implantation.
The dynamic nature of ROCM, with recovery of left ventricular function expected in a subset, has led to their exclusion from previous ICD trials, and there is limited data on the risk of SCD in this population. Guidelines on the use of ICDs for primary prevention in ROCM generally advocate deferring implantation of devices for a period of time (10), without clear data on the risks of delay. We examined the risk of SCD and the use of ICDs in a multicenter prospective cohort study of patients with ROCM.
Methods:
IMAC2 (Intervention in Myocarditis and Acute Cardiomyopathy), was an NHLBI funded multicenter registry investigating predictors of left ventricular recovery in subjects with non-ischemic cardiomyopathy of recent onset. Three hundred seventy three subjects were enrolled at 16 centers between May 2002 and December 2008. Informed consent was obtained from all subjects, and the protocol was approved by the institutional review boards of all participating centers. All subjects had a left ventricular ejection fraction ≤0.40, an evaluation consistent with idiopathic dilated cardiomyopathy or myocarditis, and were within six months of the onset of symptoms at the time of enrollment. Myocardial biopsy was not required based on current guidelines (11).
All subjects had an echocardiogram at entry and at six months post enrollment and LVEF was calculated by a core laboratory. Subjects were followed for up to 60 months. Cause of death and hospitalizations were adjudicated by an independent endpoints and events committee. Statistical analysis was performed using SPSS 18.0. Continuous variables were compared by ANOVA and categorical variables by chi square analysis. For analysis of the impact of early intervention, outcomes for subjects receiving an ICD prior to or within 30 days of enrollment (ICD early, n=43) were compared to those with no ICD at 30 days (no ICD early, n=330). For analysis of the risk of delay of ICD until recovery assessment, outcomes for subjects with an ICD by 6 month reassessment (ICD at 6 months, n=73) were compared to those with no ICD (no ICD at 6 months, n=300). Survival and survival free from SCD were compared by Kaplan-Meier log rank analysis.
Results:
IMAC Cohort:
Three hundred seventy three subjects were enrolled between May 2002 and December 2008. The cohort was 38% women (n=143), 21% (n=80) black, and the mean age was 45 ± 14 years. At entry, the LVEF was 0.24 ± 0.08 (median 0.23, interquartile range (ÍQR)= 0.14 to 0.30), symptom duration 2.2 ± 1.7 months, heart rate 83 ± 17, blood pressure 112±19/ 71±13 mm Hg, and the percent of subjects NYHA functional class I/I I/I II/IV was 18 / 46 / 29 / 7 respectively. At enrollment, 91% were on an ACE inhibitor or ARB, 82% a beta blocker, and 27% an aldosterone receptor antagonist. Forty four subjects (12%) had an endomyocardial biopsy (EMB) which revealed cellular inflammation in 15 (4.0 %) and myocarditis in only 10 (2.6%). The mean length of follow-up was 2.2 ± 1.4 years, median 2.0, IQR 0.9 to 3.5 years.
Utilization of ICD:
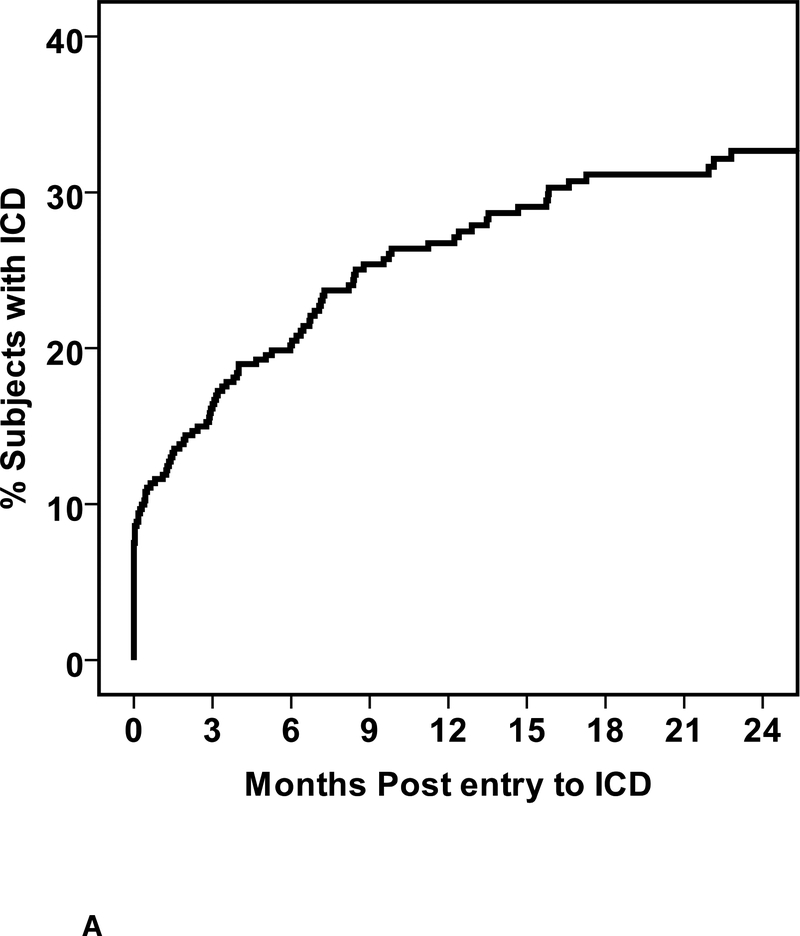
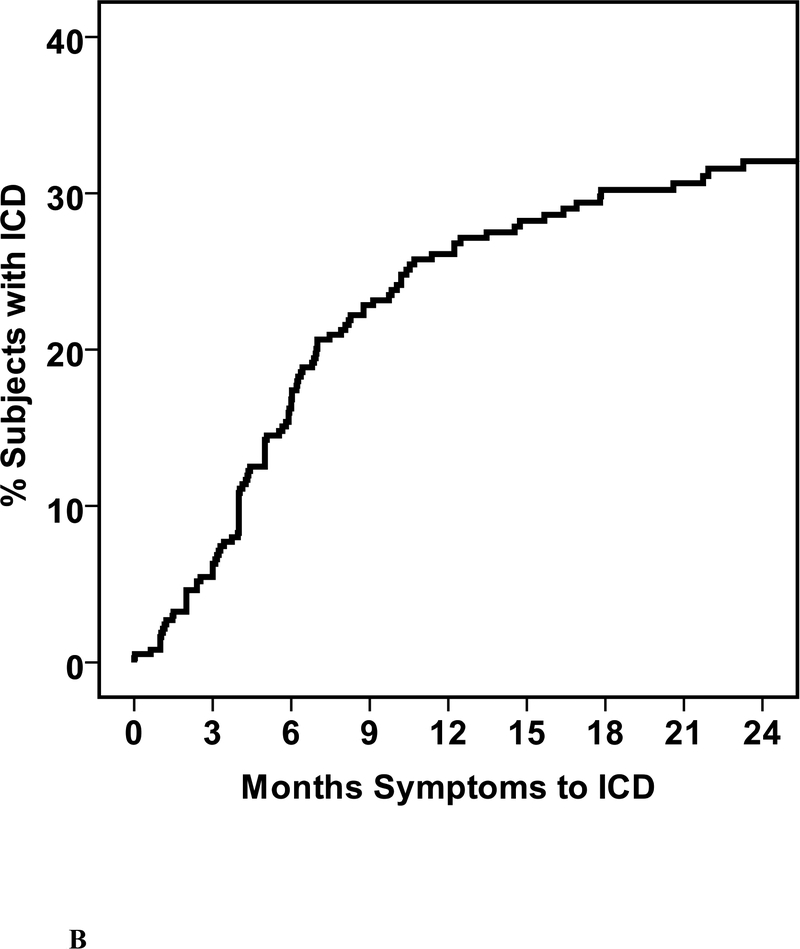
Of the cohort, 109 (29%) received an ICD including 72 who received an ICD alone and 37 cardiac resynchronization therapy with ICD (CRT-D). Twenty eight devices were implanted prior to enrollment, while 81 were placed during follow up (mean time post enrollment to implantation 203±225 days, median 120, IQR 43 to 275 days (figure 1A). For all 109 subjects with an ICD or CRT-D, the mean time from initial symptoms to device implant was 7.6 ±7.2 months, median 5.8, IQR 3.4 to 9.8 months (Figure 1B). Three subjects received external wearable cardioverter defibrillators. For one subject this was replaced with an ICD at 38 days post enrollment, while for the remaining two the wearable defibrillator was discontinued and not replaced with an ICD. This included a subject with fulminant myocarditis requiring support with a left ventricular assist device, recovered to a normal LVEF and was explanted. An external wearable defibrillator was worn for 3 months post explant then discontinued.
1. Time to ICD Implantation.
a. Days from enrollment into IMAC2 registry to device implant b. Months from initial cardiac symptoms prior to implant
Early devices:
Forty three subjects received a device early (29 ICD alone and 14 CRT-D) either prior to enrollment (n=28) or within the first 30 days (n=15). The mean duration of symptoms for subjects implanted early was 3.2 ± 1.8 months (median 3.3, IQR 2.0 to 4.4 months). When compared to subjects with no ICD at 30 days (n=330), subjects with early ICD placement had a longer duration of symptoms, and were more likely to be male and to be receiving anti-arrhythmic therapy (table 1).
Table 1.
Clinical Characteristics by Early Device (ICD or ICD/CRT prior to or within 30 days of entry):
| No Device Early (n=330) | Device Early (n=43) | p value | |
|---|---|---|---|
| Age (years) | 45 +/− 14 | 44 +/− 13 | 0.42 |
| Symptoms prior to Enrollment (Months) | 2.1 +/− 1.6 | 3.1 +/− 1.8 | <0.001*** |
| Women (n, %) | 39.8 | 22.6 | 0.02* |
| NYHA I/II/III/IV (%) | 19/46/29/7 | 14/50/29/7 | 0.95 |
| African American (%) | 21.9 | 20.5 | 0.93 |
| ACE inhibitor (%) | 81.5 | 88.4 | 0.27 |
| Beta Blocker (%) | 80.9 | 90.7 | 0.12 |
| Anti-Arrhythmic (%) | 5.2 | 17.9 | <0.001*** |
| LVEF baseline (%) | 0.24 + 0.08 | 0.23 + 0.09 | 0.67 |
| LVEF 6 months (%) | 0.41 + 0.12 | 0.36 + 0.14 | 0.02* |
| LVEDD (cm) | 6.3 + 1.0 | 6.4 + 1.1 | 0.31 |
| LBBB (%) | 21.0 | 7.5 | 0.04 |
| Atrial Fibrillation (%) | 9.9 | 2.5 | 0.12 |
| Months symptoms to device**** | 10.5 + 7.9 (n=63) | y 3.2 + 1.8 (n=43) | <0.001 |
p<0.05
p<0.01
p<0.001
subjects with device only
Devices prior to 6 month reassessment:
Of the entire cohort, 73 (19.6%) had an ICD by the 6 months reassessment, while 300 (80.6%) did not. When compared to subjects with a device by 6 month assessment, those without devices had a shorter duration of symptoms, lower NHYA class, smaller LVEDD, and a significantly higher mean LVEF (Table 2). Thirty six subjects without an ICD at the 6 month reassessment subsequently received either an ICD (n=22) or CRT-D (n=14) during the course of follow up. The mean time from initial symptoms to implantation in this subset was 15.0 ± 8.1 months (median 12.2, IQR 9.8 to 17.8 months).
Table 2.
Clinical Characteristics by Device (ICD or ICD/CRT) at 6 month follow up:
| No Device at 6 months (n=300) | Device at 6 months (n=73) | p value | |
|---|---|---|---|
| Age (years) | 45 + 14 | 45 + 12 | 0.74 |
| Symptoms prior to Enrollment (Months) | 2.1 + 1.7 | 2.8 + 1.7 | 0.003** |
| Women (n, %) | 39.7 | 27.9 | 0.14 |
| NYHA I/II/III/IV (%) | 18/48/29/5 | 18/38/30/14 | 0.049* |
| African American (%) | 19.5 | 20.0 | 0.91 |
| ACE inhibitor (%) | 81.3 | 86.3 | 0.32 |
| Beta Blocker (%) | 80.0 | 90.4 | 0.04* |
| Anti-Arrhythmic (%) | 4.7 | 12.3 | 0.02* |
| LVEF baseline (%) | 0.24 + 0.08 | 0.22 + 0.08 | 0.04* |
| LVEF 6 months (%) | 0.42 + 0.11 | 0.34 + 0.13 | <0.001*** |
| LVEDD (cm) | 6.2 + 1.0 | 6.6 + 1.0 | 0.009** |
| LBBB (%) | 20.4 | 15.4 | 0.36 |
| Atrial Fibrillation (%) | 10.5 | 6.5 | 0.06 |
| Months symptoms to device**** | 15.0 + 8.1 (n=36) | 4.0 + 2.0 (n=73) | <0.001 |
p<0.05
p<0.01
p<0.001
subjects with device only
Overall Survival and the Impact of Device::
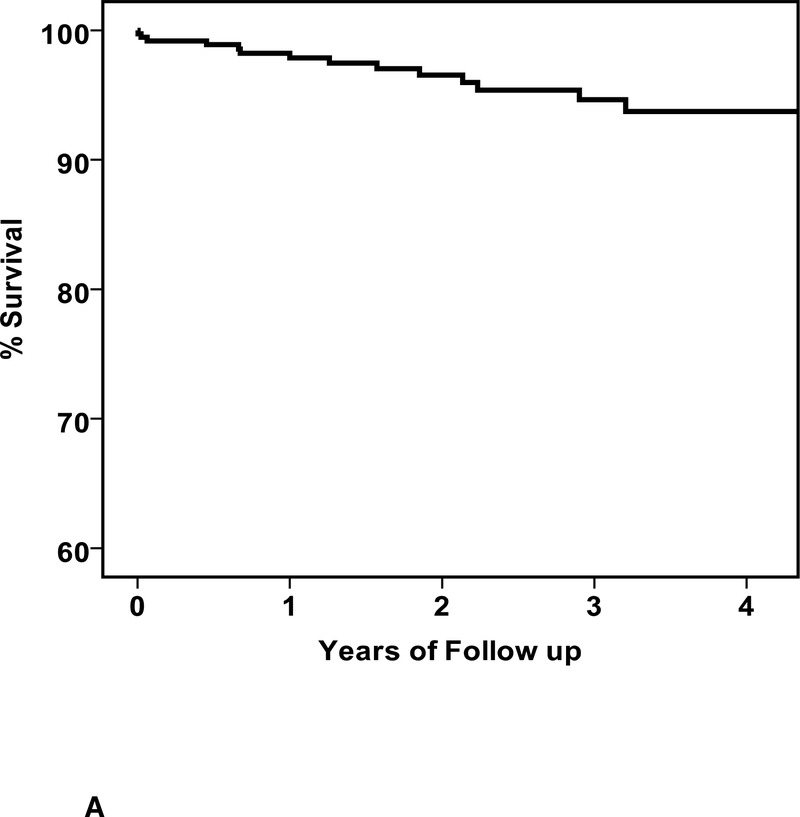
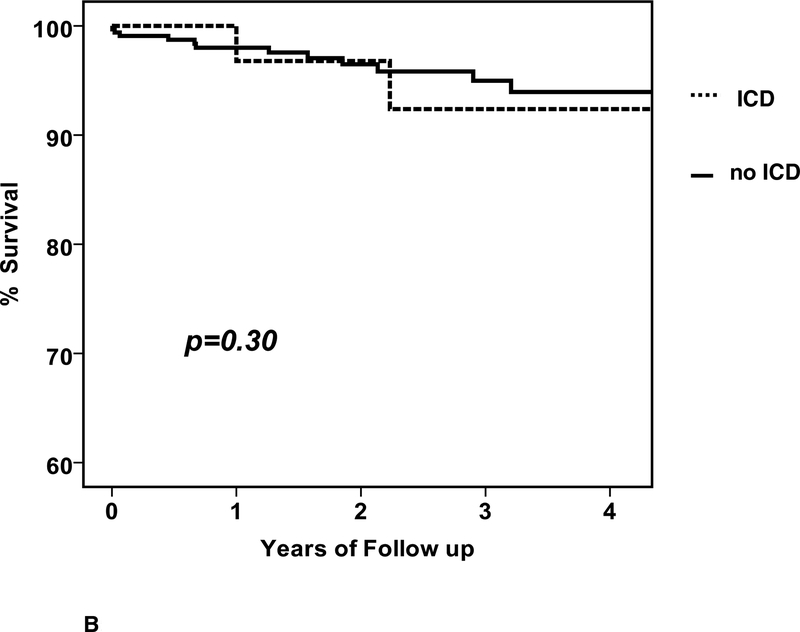
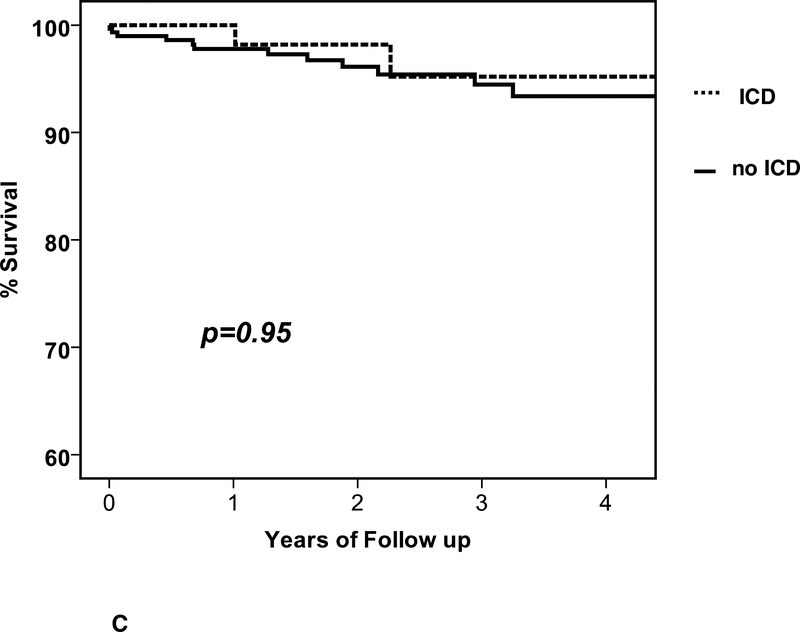
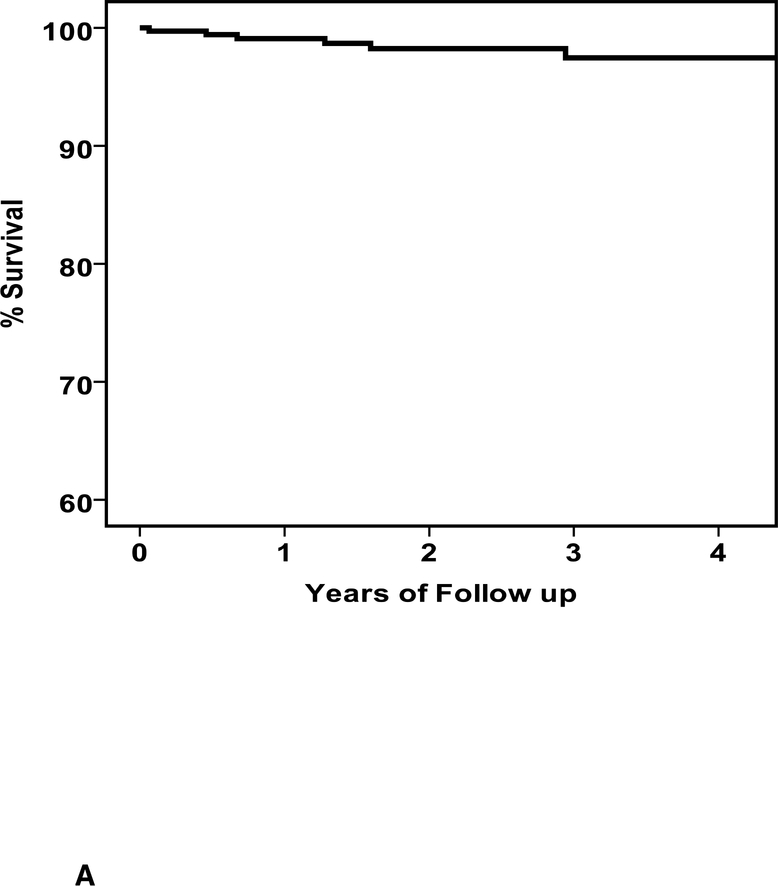
During follow up, there were 15 deaths and the clinical characteristics are listed in Table 3. Of the subjects that died there were 15 men and no women (p=0.002), the mean age was 45 ± 15 years, baseline LVEF was 0.19±0.08 and LVEDD was 6.4±1.0 cm. The mean time from entry to death was 549±482 days (median 460, IQR 165 to 815 days). The overall percent survival at 1, 2 and 3 years was 98, 97 and 95%, respectively (figure 2A). Of the 15 deaths, 10 were in subjects without an ICD (3.8%) and 5 in subjects with an ICD (4.6%).When comparing survival in patients with an ICD early, no significant differences were apparent (early ICD, n=43, 1/2/3 year % survival=97/97/92; no early ICD, n=330, survival = 98/97/95, p=0.30, figure 2B), nor was any difference apparent when comparing patients with an ICD by 6 months (ICD at 6 months, n=73, survival=98/98/95; no ICD at 6 months, n=300, survival=98/96/95, p=0.95, figure 2C).
Table 3:
Clinical characteristics of 15 subjects who died during follow up. SD=standard deviation, M=male, NA=not available, N=no, Y =yes
| subject | age | sex | LVEDDcm baseline | LVEF baseline | 6 month LVEF | ICD or CRT-D | days to ICD | Hospitalization (reason) | days to first Hospitalization | sudden death | days tc death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | M | 6.1 | 0.12 | 0.40 | N | Heart Failure | 54 | N | 779 | |
| 2 | 39 | M | 6.7 | 0.22 | 0.22 | ICD | entry | Heart Failure | 1,644 | N | 1,656 |
| 3 | 77 | M | 6.5 | 0.22 | NA | N | N | N | 1 | ||
| 4 | 58 | M | NA | 0.13 | 0.49 | N | N | N | 246 | ||
| 5 | 26 | M | 5.6 | 0.35 | 0.44 | CRT-D | entry | Heart Failure | 26 | N | 815 |
| 6 | 44 | M | 6.6 | 0.10 | 0.20 | N | N | Y | 460 | ||
| 7 | 41 | M | 4.4 | 0.37 | 0.67 | N | N | Y | 1,059 | ||
| 8 | 56 | M | 7.0 | 0.10 | NA | N | N | Y | 23 | ||
| 9 | 68 | M | 8.8 | 0.15 | 0.38 | ICD | 286 | N | N | 1,170 | |
| 10 | 27 | M | 7.0 | 0.15 | 0.15 | ICD | entry | N | N | 365 | |
| 11 | 52 | M | 6.3 | 0.15 | 0.26 | ICD | 181 | Heart Failure | 553 | Y | 574 |
| 12 | 45 | M | 5.3 | 0.19 | NA | N | N | N | 8 | ||
| 13 | 22 | M | 6.2 | 0.15 | NA | N | Heart Failure | 164 | Y | 165 | |
| 14 | 30 | M | 6.8 | 0.17 | 0.38 | N | N | N | 676 | ||
| 15 | 46 | M | 6.0 | 0.23 | 0.35 | N | N | Y | 242 | ||
| Mean | 45 | 6.4 | 0.19 | 0.36 | 549 | ||||||
| SD | 15 | 1.0 | 0.08 | 0.15 | 482 |
2. Survival in IMAC2.
a. Overall survival, entire cohort: n=373, % survival at 1/2/3 year=98/97/95 b. Overall survival by ICD early: ICD early (n=43) % survival 1/2/3 year =97/97/92; no ICD early (n=330) % survival=98/97/95, p=0.30 c. Overall survival by ICD at 6 months: ICD at 6 months (n=73) % survival 1/2/3 year = 98/98/95; no ICD at 6 months (n-300) % survival 98/96/95, p=0.95
Sudden Death and Impact of Device:
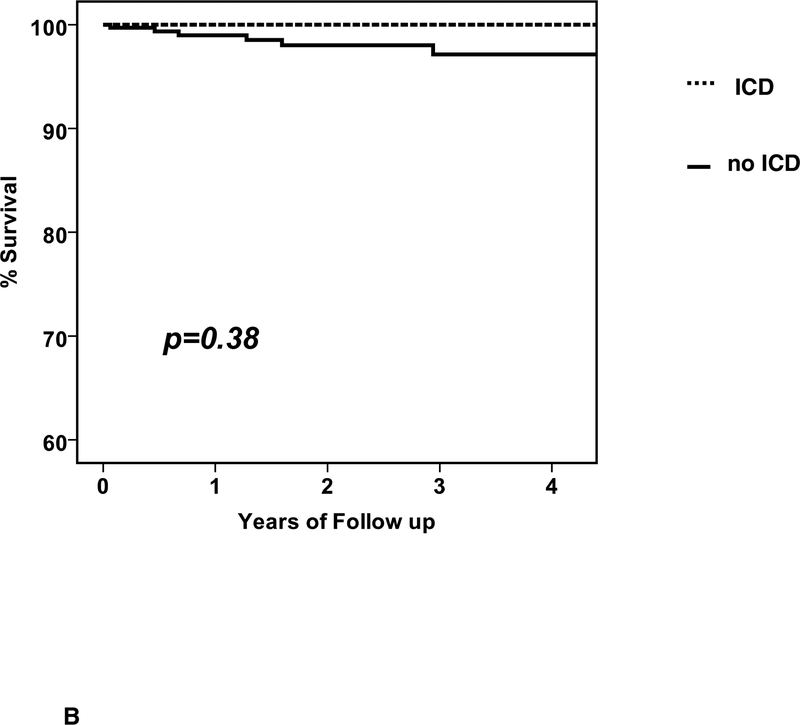
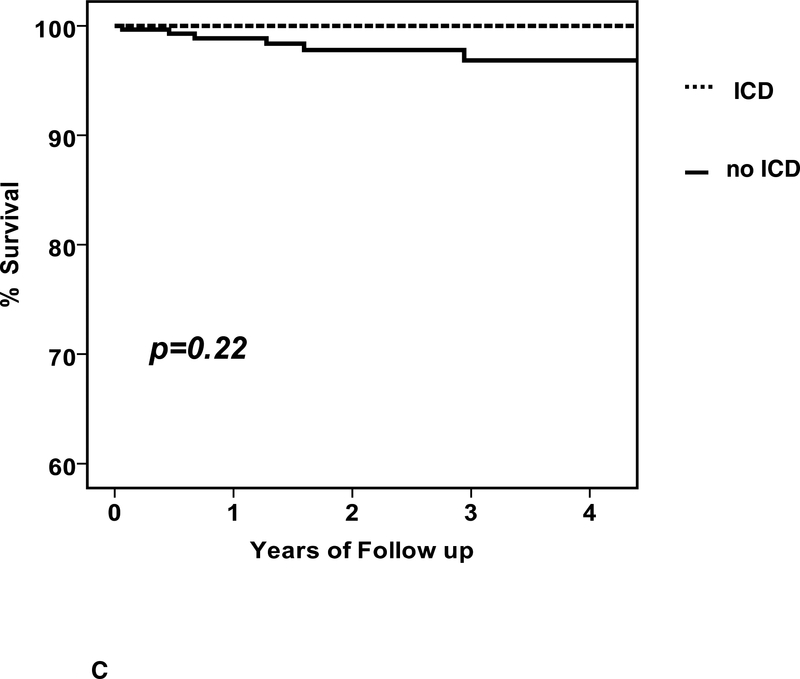
Six subjects had sudden death (6 men, no women, age 44 ±12 years) at a mean 420± 371days (median 351, range 23 to 1059). The survival free from sudden death was 99, 98 and 97% (figure 3A). The 6 subjects with sudden death included 5 without an ICD (1.9%) and one who received an ICD (0.9%) just after 6 month reassessment and experienced sudden death one year later. Subgroup analysis for survival free from SCD was limited due to the small number of events (figure 3).
3. Survival free from sudden cardiac death (SCD).
a. Survival free from SCD, entire cohort: n=373, survival free from SCD at 1/2/3 year= 99/98/97 b. Survival free from SCD by ICD early: with ICD early (n=43) % survival free from SCD at 1/2/3 year =100/100/100; no ICD early (n=330) % survival=99/98/97, p =0.38 (note: the events in the “no ICD early” cohort includes one subject who received an ICD just after 6 months and experienced sudden death one year later) c. Survival free from SCD by ICD at 6 months: with ICD at 6 months (n=73) % survival 1/2/3 year = 100/100/100; no ICD at 6 months (n=300) % survival 99/98/97, p=0.22 (note: the events in the “no ICD at six months” cohort includes one subject who received an ICD just after 6 months and experienced sudden death one year later)
Hospitalizations for Arrhythmias:
During the course of follow up, 152 cardiac hospitalizations occurred in 87 patients (23%). The most frequent reason for hospitalization was heart failure which was the etiology in 77 hospitalizations (51% of all hospitalizations) in 49 subjects (13% of the cohort). In contrast there were only 11 hospitalizations for ventricular tachycardia (VT) (7% of all hospitalizations) in 8 patients (2%). Overall there were 14 subjects with either VT or SCD (4%). There were 23 hospitalizations for supraventricular arrhythmias (15% of hospitalizations) in 14 subjects (4% of cohort) most of which were related to atrial fibrillation.
Discussion:
Clinicians evaluating subjects with ROCM frequently must wrestle with the question of the need and timing of ICD placement. Current guidelines recommend reassessment of LV function several months after the diagnosis of ROCM with ICD implantation only in subjects with persistent LV dysfunction. This current investigation found early ICD placement was not associated with improved survival and that delay of ICD placement until after a six month reassessment was not associated with any decrease in survival. This supports the concept that for subjects with ROCM every effort should be made to determine a subject’s potential for recovery prior to committing them to a permanent ICD.
These findings appear at odds with previous reports of the benefits of ICD placement in newly diagnosed non-ischemic cardiomyopathy. In 2005, the Center for Medicare and Medicaid Services published coverage guidelines recommending that implantation of an ICD be deferred in patients with new onset cardiomyopathy of less than 9 months duration (12). Soon afterwards, a single center report compared the event rate (ICD treated arrhythmia) in subjects with recent onset cardiomyopathy (less than 9 months, n=52) with those with more chronic disease (n=79) and concluded that the benefits of ICD implantation were similar (13). This analysis was limited by the use of surrogate endpoints (ICD treated arrhythmias) and was notable for a high rate of “lethal” arrhythmias (defined as ventricular fibrillation or ventricular flutter in 13.5%) in the recent onset cohort despite low overall mortality (1.9%).
The DEFINITE trial in 438 non-ischemic subjects with an LVEF≤ 0.35 (5) revealed a trend towards improved survival in patients with an ICD (p<0.08) including a significant reduction in sudden death (p<0.05). Subset analysis was performed comparing recent onset (less than 3 months and less than 9 months duration) with more chronic disease and reported similar benefits (14). Indeed most of the ICD benefit appeared to be in the recent onset group and was not evident in the more chronic subset, leading the authors to speculate that the percentage of non-arrhythmic death increases with duration of illness resulting in less benefit from the ICD over time. The recent onset subset from this trial was quite different from the current study as reversible cardiomyopathies were excluded. The mortality in the recent onset subset from DEFINITE was 11.2% at 2.5 years compared to 5% for the IMAC cohort for the same interval. Given the exclusion of reversible cardiomyopathies and the much higher mortality rate, the “recent onset” subset in DEFINITE appears be more chronic and functionally limited than the subjects in the current analysis.
The need to incorporate reversibility potential in ICD decisions has been noted (15), and IMAC2 investigators appeared to have selectively used ICDs in subjects with a longer duration of symptoms, larger LVEDD, and evidence of less recovery at 6 months. Less remodelling (smaller LVEDD) has been associated with greater recovery potential (16) and was the best clinical predictor of recovery in the IMAC2 registry (9). The previous Intervention in Myocarditis and Acute Cardiomyopathy trial (IMAC1) was a multi-center investigation of the use of immune globulin in subjects with a recent onset non-ischemic cardiomyopathy or myocarditis (17). IMAC1 evaluated myocardial gene expression as a biomarker predictor of recovery and these investigations demonstrated that low levels of Fas expression, a membrane receptor initiating apoptosis, identified subjects more likely to recover (18). In the absence of biopsy guided therapeutic strategies EMB is rarely performed in ROCM and therefore this biomarker is not generally available. For IMAC2 biopsy was performed in only 44 of 373 subjects including those obtained at the time of LVAD placement. In this highly select cohort, EMB demonstrated myocarditis in only 10 of the 44. Cardiac magnetic resonance (cMR) may be of greater clinical utility, as late gadolinium enhancement (LGE) is consistent with myocardial scar (19) and predicts a subset at greater risk of adverse events (20). Evidence of LGE by cMR may identify subjects with ROCM with greater potential benefit from an ICD (21).
While the overall survival for the IMAC2 registry was excellent, the rate of SCD of 1 % per year in this relatively young cohort without previous cardiac disease remains a concern. Given the low event rate and the size of the cohort, this study was underpowered to evaluate the impact of early ICD implantation specifically for SCD. Of the six episodes of SCD, two occurred prior to the six month reassessment and included one high risk subject who had been implanted with an LVAD, recovered and died 2 months after explantation. The remaining events were all after 6 months, and included one in a subject who had received an ICD at the 6 month reassessment the previous year. Given the low risk of SCD, a randomized trial examining the use of early ICD therapy would be challenging. There is clearly a need to better risk stratify patients with ROCM in order to delineate the high risk subset who might benefit from early implantation.l
A temporary external life vest has been proposed as an important therapy for high risk patients suspected to have a short term risk of SCD (22), but its use in ROCM remains unknown. It was used rarely in IMAC2 for high risk subjects. Proving efficacy of an external life vest in patients with non-ischemic cardiomyopathy in general and in ROCM in particular will be challenging given low event rates, and while they may be appropriate for high risk patients, their use is expected to remain highly selective.
Limitations:
Survival analysis was limited by the small number of events, with only 15 deaths (1.8 per 100 patient-years) and 6 sudden deaths (0.7 per 100 patient-years). In addition, the potential for cross over into the group who received ICD therapy would likely diminish the ability to detect differences between groups. Finally, given the absence of randomization the decision to implant an ICD was left to the discretion of the physician, and this may have resulted in selection bias towards greater use of ICDs in higher risk patients. All these factors limit the ability of the current investigation to evaluate the impact of early intervention with an ICD in ROCM. However, the low sudden death rate itself in ROCM and the fact that a delay in ICD implantation till after 6 months reassessment did not appear to create a great hazard are the most important findings of the current investigation and should facilitate future assessment of the risks and benefits of early ICD therapy
One additional limitation of the current investigation is that the outcomes analysis only addressed death or hospitalization and did not assess ICD therapy (appropriate/ inappropriate shocks and or anti-tachycardia pacing). The study does not address whether there were significant life saving treatments in the ICD subset which did not result in hospitalization. The cohort itself is heterogeneous and includes subjects with reversible inflammatory cardiomyopathies. However, the vast majority of subjects were classified as idiopathic dilated cardiomyopathy, and outcomes assessment remains similar when limited to this subset.
Conclusion:
In patients with ROCM, early ICD placement provided no survival advantage, and delay of ICD until a 6 month assessment of recovery potential presented no hazard. The overall risk of sudden cardiac death is low, but at 1% per year remains of concern in a young cohort with no previous cardiac disease. For subjects with ROCM, development of conventional and novel predictors of myocardial recovery will be essential to risk stratification and may allow more targeted use of ICDs in subjects with the greatest potential benefit.
Acknowledgments
Investigation supported by NHLBI contracts R01HL075038 and HL69912 Word count 4680
APPENDIX: IMAC Investigators
University of Pittsburgh Medical Center (80) Dennis M McNamara, MD, Karen Janosko MSN,MBA, Charles McTiernan, Barry London, MD-PhD PhD, Karen Hanley-Yanez BS, John Gorcsan MD, Hidekazu Tanaka MD, Mathew Suffoletto, MD. Cleveland Clinic (51) Randall C Starling, MD; Cynthia Oblak, CCRN. Mayo Clinic (41) Leslie T. Cooper, MD, Annette McNallan, RN, LuAnne Koenig, RN. Jefferson Heart Institute at Jefferson Medical College (34) Paul Mather, MD, Natalie Pierson, BSN, Sharon Rubin, MD, Yanique Bell, RN and Alicia Ervin, RN. Penn State Hershey Medical Center (26) John Boehmer, MD; Patricia Frey, RN, BSN. University of Rochester (21) Jeffrey Alexis MD, Janice Schrack, RN, Pam LaDuke. Methodist Hospital, Houston (20) Guillermo Torre-Amione, MD-PhD, Jeannie Arredondo. University of Florida College of Medicine (20) Daniel F. Pauly, MD-PhD, Pamela C. Smith, LPN. McGill SMBD Jewish General (18) Richard Sheppard, MD; Stephanie Fuoco, RN, BSN. Johns Hopkins Hospital (15) Ilan S. Wittstein, MD; Elayne Breton, RN. Wake Forest University Baptist Medical Center (12) Vinay Thohan, MD; Deborah Wesley, RN, BSN. Massachusetts General Hospital (11) G. William Dec, MD; Diane Cocca-Spofford, RN, BSN, MHA. University of Texas Southwestern Medical Center (11) David W. Markham. MD-MSc, Lynn Fernandez, RN, Colleen Debes, RN. Newark Beth Israel Medical Center (6) Mark J. Zucker MD; Laura Adams, RN. University of Toronto (4) Peter Liu MD, Judith Renton, RN. University of California at Irvine (3) Jagat Narula, MD-PhD, Byron Allen, MD, Elizabeth Westberg, RN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
There are no disclosures.
References:
- 1.Zheng Z, Croft J, Giles W, and Mensah G. Sudden Cardiac Death in the United States, 1989 to 1998. Circulation. 2001; 104:2158–2163 [DOI] [PubMed] [Google Scholar]
- 2.Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011; 57:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009. Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009; 53:e1–e90. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS et al. Prophylactic Implantation of a Defibrillator in Patients with Myocardial Infarction and Reduced Ejection Fraction. N Engl J Med. 2002; 346:877–83 [DOI] [PubMed] [Google Scholar]
- 5.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic Defibrillator Implantation in Patients with Nonischemic Dilated Cardiomyopathy. N Engl J Med. 2004; 350:2151–8 [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an Implantable Cardioverter - Defibrillator for Congestive Heart Failure. N Engl J Med. 2005; 352:225–37 [DOI] [PubMed] [Google Scholar]
- 7.Steimle AE, Stevenson LW, Fonarow GC, Hamilton MA, Moriguchi JD. Prediction of improvement in recent onset cardiomyopathy after referral for heart transplantation. J Am Coll Cardiol. 1994; 23(3):553–559. [DOI] [PubMed] [Google Scholar]
- 8.McNamara DM, Holubkov R, Starling RC, Dec GW, Loh E, Torre-Amione G, et al. for the IMAC Investigators. A Controlled Trial of Intravenous Immune Globulin in Recent Onset Dilated Cardiomyopathy. Circulation 2001; 103:2254–2259, [DOI] [PubMed] [Google Scholar]
- 9.McNamara DM, Starling RC, Cooper LT, Boehmer JP, Mather PJ, Janosko KM, et al. for the IMAC Investigators. Clinical and Demographic Predictors of Outcomes in Recent Onset Dilated Cardiomyopathy: Results of IMAC2, J Am Coll Cardiol 2011; 58(11):1112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClellan M, Tunis SR. Medicare coverage of ICDs. N Engl J Med. 2005; 352:222–4. [DOI] [PubMed] [Google Scholar]
- 11.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007; 116:2216–33. [DOI] [PubMed] [Google Scholar]
- 12.Phurrough SE, Salive ME, Baldwin JF, Chin J. Coverage decision memorandum for implantable cardioverter defibrillators. Washington DC: Centers for Medicare and Medicaid Services, 2005. [Google Scholar]
- 13.Makati KJ, Fish AE, England HH, Tighiouart H, Estes NA 3rd, Link MS. Equivalent arrhythmic risk in patients recently diagnosed with dilated cardiomyopathy compared with patients diagnosed for 9 months or more. Heart Rhythm. 2006; 3:397–403 [DOI] [PubMed] [Google Scholar]
- 14.Kadish A, Schaechter A, Subacius H, Thattassery E, Sanders W, Anderson KP, et al. Patients with recently diagnosed nonischemic cardiomyopathy benefit from implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006; 47:2477–82. [DOI] [PubMed] [Google Scholar]
- 15.Marchlinski FE, Jessup M. Timing the Implantaion of Implantable Cardioverter-Defibrillators in Nonischemic Cardiomyopathy. J Am Coll Cardiol. 2006; 47:2483–85. [DOI] [PubMed] [Google Scholar]
- 16.Simon MA, Primack BA, Teuteberg J, Kormos RL, Bermudez C, Toyoda Y, et al. Left ventricular remodeling and myocardial recovery on mechanical circulatory support. J Card Fail. 2010; 16:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNamara DM , Holubkov R , Starling RC et al. “Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy”. Circulation 2001; 103:2254.-. [DOI] [PubMed] [Google Scholar]
- 18.Sheppard R, Bedi M, Kubota T, Semigran MJ, Dec W, Holubkov R, et al. Myocardial expression of fas and recovery of left ventricular function in patients with recent-onset cardiomyopathy. J Am Coll Cardiol. 2005; 46:1036–42. [DOI] [PubMed] [Google Scholar]
- 19.Strauss DG, Selvester RH, Lima JA, Arheden H, Miller JM, Gerstenblith G, et al. ECG Quantification of Myocardial Scar in Cardiomyopathy Patients with or Without Conduction Defects - Correlation with Cardiac Magnetic Resonance and Arrhythmogenesis. Circ Arrhythmia Electrophysiol. 2008; 1:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, et al. Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance Heralds and Adverse Prognosis in Nonischemic Cardiomyopathy. J Am Coll Cardiol. 2008; 51:2414–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, et al. Myocardial Fibrosis Predicts Appropriate Device Therapy in Patients With Implantable Cardioverter-Defibrillators for Primary Prevention of Sudden Cardiac Death. J Am Coll Cardiol. 2011; 57:821–8 [DOI] [PubMed] [Google Scholar]
- 22.Klein HU, Meltendorf U, Reek S, Smid J, Kuss S, Cygankiewicz I, et al. Bridging a Temporary High Risk of Sudden Arrhythmic Death. Experience with the Wearable Cardioverter Defibrillator (WCD). PACE. 2010; 33:353–367 [DOI] [PubMed] [Google Scholar]