Abstract
Peroxisome proliferator-activated receptor gamma (PPARγ) is a nuclear transcription factor belonging to the superfamily of ligand-activated nuclear receptors. It is activated by diverse endogenous lipid metabolites as well as by exogenous ligands such as the thiazolidinediones. Its activity regulates cellular metabolism, proliferation, differentiation, and inflammation, the latter in part through trans-repression of pro-inflammatory cytokines. PPARγ is highly expressed in alternatively activated alveolar macrophages (AMs), a primary host cell for airborne Mycobacterium tuberculosis (M.tb). Our previous in vitro study identified the importance of PPARγ activation through the mannose receptor (CD206) on human macrophages in enabling M.tb growth. The aim of the current study was to investigate the role of PPARγ in vivo during M.tb infection using a macrophage-specific PPARγ knock out mouse model with special emphasis on the lung environment. Our data show that the absence of PPARγ in lung macrophages reduces the growth of virulent M.tb, enhances pro-inflammatory cytokines and reduces granulomatous infiltration. These findings demonstrate that PPARγ activation, which down-regulates macrophage pro-inflammatory responses, impacts the lung’s response to M.tb infection, thereby supporting PPARγ’s role in tuberculosis (TB) pathogenesis.
Keywords: PPARγ, M. tuberculosis, macrophage
1. Introduction1
In mammals, there are three peroxisome proliferator-activated receptors (PPARs): PPARα (also called NR1C1), PPARβ/δ (also called NR1C2) and PPARγ (also called NR1C3). PPARγ functions as a nuclear transcription factor that is highly expressed in alveolar macrophages (AMs) and important for AM differentiation [1]. Upon activation by a ligand, PPARγ forms heterodimers with the nuclear receptor retinoid X receptor (RXR) which bind to specific DNA-response elements in target genes known as PPAR response elements (PPREs) to control the expression of several gene networks involved in lipid metabolism, maintenance of metabolic homeostasis and inflammation [2, 3].
PPARγ binds and responds to diverse lipid metabolites, some of them related to immune responses, including 15-deoxy-Δ12,14-prostaglandin J2, 15-hydroxyeicosatetraenoic acid, 13-hydroxyoctadecadienoic acid, and some oxidized phospholipids [3–6]. Its activity has been linked to several diseases such as asthma, rheumatoid arthritis and atherosclerosis [7]. PPARγ is highly expressed in AMs which play a key role in tuberculosis (TB) pathogenesis since Mycobacterium tuberculosis (M.tb) is acquired by the respiratory route and AMs are the first immune cells that encounter M.tb.
We have demonstrated earlier that PPARγ is activated during M.tb phagocytosis by human macrophages via the mannose receptor (CD206) [8]. Its activation leads to reduction in the pro-inflammatory response and PPARγ silencing leads to decreased bacterial growth [8] while its activation enhances M.tb growth [9]. Similarly Almeida et al. demonstrated that BCG infection enhances the expression of PPARγ through TLR2 in mouse macrophages and regulates lipid body formation and PGE2 production [10]. Thus, PPARγ activation generates a host cell that is more susceptible to M.tb and we hypothesize that PPARγ in lung macrophages plays an important role in modulating immune responses during M.tb infection. The aim of this current study was to investigate the role of PPARγ in vivo during M.tb infection using the mouse aerosol M.tb infection model. Our data demonstrate that absence of PPARγ in macrophages decreases bacterial burden, increases pro-inflammatory cytokines and reduces total lung inflammation. These findings along with previously published work demonstrate the importance of PPARγ activation in promoting M.tb infection during the innate immune response.
2.Materials and Methods
2.1 Chemicals, reagents and bacterial strains
All chemicals used were of the highest purity available from Sigma-Aldrich (St. Louis, Mo) unless otherwise specified. Dulbecco’s PBS (no Ca2+, no Mg2+), Dulbecco’s Modified Eagle’s media (DMEM) and heat-inactivated fetal bovine serum (hi-FBS) were purchased from Life Technologies (Carlsbad, CA).
2.2 Mice
All mice were kept under controlled conditions in a specific pathogen-free environment, with sterile food and water ad libitum, and used in accordance with protocols approved by the Institutional Animal Care and Use Committee at The Ohio State University.
2.3 Generation of myeloid-specific PPARγ targeted mice
BALB/c mice (LysM-cre and PPARγ floxed) were kindly provided by Dr. Ajay Chawla (UCSF) [11] and crossed in house to generate mice with PPARγ deficiency in myeloid cells. Conditionally targeted and littermate controls were generated by crossing PPARγflox/flox, Cre+ mice with PPARγflox/flox, Cre− mice. Animals were genotyped for the presence of the Cre alleles from tail snip. PCR genotyping was performed by Platinum™ Taq DNA Polymerase (Life technologies) following the manufacturer instructions using a set of custom primers (forward 5’-3’ TgC CCA AgA AgA AgA ggA Agg T and reverse 5’-3’ AAA TCA gTg CgT TCg AAC gCT AgA) which provides a presumptive genotype predicting conditional targeting (PPARγflox/flox, Cre+) and littermate control (PPARγflox/flox, Cre−) [11]. Efficiency of deletion was quantified by Immunoblot analysis for PPARγ in control and Mac-PPARγ KO mice. Total of 10 μg of protein from whole cell lysates from AMs were subjected to electrophoresis and immunoblotted for PPARγ antibody (Cell Signaling Technology, Beverly, MA) (Supplemental Fig 1).
2.4 M.tb preparation for in vitro infection
Lyophilized M.tb H37Rv (ATCC 25618) was obtained from American Tissue Culture Collection (Manassas, VA), reconstituted, and used as described previously [12, 13]. For each experiment aliquots of M.tb frozen stocks were plated on Difco™ Mycobacteria 7H11 agar plates (BD, Difco™) with 0.5% of glycerol and 5% Middlebrook OADC enrichment and bacteria were grown for 9–14 days at 37°C, 5% CO2 and 95% relative humidity.
Single cell suspensions of M.tb H37Rv were prepared as we previously described [12]. Briefly, bacteria were scraped from agar plates, suspended in cell culture media, briefly vortexed (five, 1 sec pulses) with two glass beads (3 mm), and allowed to settle for 30 min. The upper bacterial suspension (devoid of clumps) was then transferred to a second tube and let rest for an additional 5 min to obtain the final single cell suspension. Bacterial concentrations were determined by counting using a Petroff-Hausser chamber and subsequent plating for colony-forming units (CFUs). The concentration of bacteria was 1–2 × 108 bacteria/ml and the degree of clumping was ≤10%. Bacteria prepared in this fashion are ≥90% viable by CFU assay.
2.5 M.tb preparation for in vivo infection
M.tb H37Rv was grown in Proskauer Beck medium containing 0.01% Tween 80 to mid-log phase and stored at −70°C in 2 ml aliquots [14]. Bacterial concentrations determined by plating for CFUs were 1–2 × 108 bacteria/ml.
2.6 Whole lung macrophage isolation
Mice were euthanized by CO2 asphyxiation. Following perfusion through the heart with 10ml of PBS containing 50U/ml of heparin, the lungs were removed and placed in DMEM supplemented with 10% hi-FBS, 10mM HEPES buffer, 2X minimal non-essential amino acids, 2mM L-glutamine and penicillin/streptomycin solution (1 U/ml penicillin, and 1 μg/ml streptomycin in 0.9% NaCl) (cDMEM). The lung lobes were dissociated into single cell suspensions by enzymatic degradation with collagenase XI and type IV bovine pancreatic DNase for 30 min at 37°C, 5% CO2 and 95% relative humidity followed by a mechanical dissociation process (gentleMACS dissociator, Miltenyi Biotec inc.). cDMEM was added to dilute enzymatic activity and the lung pieces were pressed through sterile 70μm nylon mesh screens (BD Biosciences; San Jose, CA) to obtain single cell suspensions. Residual red blood cells were lysed using 2ml of lysis buffer (0.15 M NH4Cl, 1mM KHCO3) for 3 min at room temperature followed by washing with cDMEM. Cells were centrifuged at 200g for 7 min at 4°C and re-suspended in cDMEM. On average, 8.8 x106 and 9.9 x106 lung macrophages were recovered from each control and PPARγ KO mouse, respectively. Viable cells were counted using Trypan blue exclusion (97% viability).
2.7 Alveolar macrophage isolation
Mice were euthanized by CO2 asphyxiation, tracheas were cannulated and AMs were harvested by performing bronchoalveolar lavage (BAL) 10 times with 0.5mL of phosphate buffered saline solution (PBS; Life Technologies) each [14]. Cells were centrifuged at 200g for 7 min at 4°C and re-suspended in cDMEM. Viable cells were counted using Trypan blue exclusion (99% viability). On average, >95% of BAL cells were AMs by Giemsa stain. This yielded an average of 9.5 x104 and 8 x104 AMs from each control and PPARγ KO mouse, respectively. AMs from 10–15 mice were pooled for each experiment.
2.8 Peritoneal macrophage isolation
Mice were euthanized by CO2 asphyxiation and macrophages were harvested by rinsing the peritoneal cavity with 10mL of PBS. Cells were centrifuged at 200g for 7 min at 4°C and re-suspended in cDMEM. Viable cells were counted using Trypan blue exclusion (99% viability).
2.9 Bone marrow-derived macrophage (BMDM) isolation
Mice were euthanized by CO2 asphyxiation and BMDMs were obtained from cultures of bone marrow stem cells, as previously described [15]. In brief, the marrow was flushed from femurs, the marrow plugs were disrupted into single-cell suspensions and cells were cultured at a cell density of 1 × 106 cells/mL in 100-mm polystyrene tissue culture dishes in Iscove's Modified Dulbecco's Media (IMDM) containing 10% hi-FBS, 20% L cell-conditioned medium, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C, 5% CO2 and 95% relative humidity. After 5 days of incubation, cells were collected and adhered to 24 well culture plates for 2h in IMDM containing 10% hi-FBS. Cells were washed 3 times and rested overnight at 37°C, 5% CO2 and 95% relative humidity.
2.10 M.tb infection of macrophages in vitro and bacterial burden
The macrophages harvested as described above were placed in cDMEM and cultured at a cell density of 4 × 106 cells/mL. Cells were adhered to a standard 24 well culture plate for 2h and washed 3 times to remove non-adherent cells. After resting overnight at 37°C, 5% CO2 and 95% relative humidity, macrophages were infected with a single cell suspension of M.tb at a multiplicity of infection (MOI) of 5:1. At the indicated times, macrophages were lysed as previously described [16] and M.tb burden was determined by plating serial dilutions onto OADC-supplemented 7H11 agar. M.tb CFUs were counted after 3 weeks at 37°C.
2.11 In vivo M.tb infection and bacterial burden
Mice were infected with a low dose (LDA, 10–100 CFU/mouse lung) or mid-dose (MDA, 500–1000 CFU/mouse lung) M.tb aerosol using an Inhalation Exposure System (Glas-col, Terre Haute, IN) [14, 17]. Initial M.tb dose (day 0 of infection) and M.tb burden were determined by plating serial dilutions of tissue homogenates onto OADC-supplemented 7H11 agar. M.tb CFUs were counted after 3 weeks at 37°C.
2.12 Histology and histomorphometry
The procedures were performed as we have described [14]. Briefly, lung lobes from individual mice were inflated with 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin or for acid fast bacilli by a modified Ziehl-Neelsen stain. Slides were digitally scanned using an Aperio ScanScope (Vista, CA). The analysis was performed qualitatively and quantitatively. For quantitative analysis: slides comprising five lung sections from 2 mice per genotype and time point were evaluated by a board-certified veterinary anatomic pathologist (KMDL) in blinded fashion. Granulomas were defined as focal aggregates of mixed inflammatory cells (macrophages, lymphocytes ± neutrophils). The extent of the alveolitis (0: absent; 1: minimal; 2: slight; 3: moderate; 4: marked; 5: severe), as well as the characterization of foamy macrophages (0: absent; 1: rare macrophages scattered through the alveolitis; 2: small clusters of macrophages distributed throughout the alveolitis; 3: macrophages in equal proportion to neutrophils; 4: alveolitis primarily composed of macrophages) and degenerate neutrophils (0: absent; 1: rare neutrophils scattered throughout the alveolitis; 2: small clusters of neutrophils distributed throughout the alveolitis; 3: neutrophils in equal proportions to macrophages; 4: alveolitis primarily composed of neutrophils) comprising the alveolitis, was graded on a nominal scale. Lymphocytic cuffs were defined as partial or complete concentric layers of one or more lymphocytes within perivascular or peribronchiolar interstitium, and were graded on a nominal scale (0: absent; 1: minimal; 2: slight; 3: moderate; 4: marked; 5: severe). For qualitative analysis: slides comprising ten lung sections from five mice per genotype and time point were evaluated (by EG) in blinded fashion. The total infiltrated area vs. the total tissue area was determined (μm2) using the Aperio Image Analysis software. The granulomatous infiltration in the lung was defined as the percentage ratio obtained after dividing the infiltrated area by the total area of the lung sections, and then multiplying it by 100.
2.13 Cytokine ELISAs
Cytokine concentrations were measured by ELISA (R&D Systems, Minneapolis, MN) following the manufacturer instructions. The following kits were used: TNFα (DY410), IL-6 (DY406) and IL-10 (DY417).
2.14 Cytokine Gene expression studies by TaqMan Quantitative Real-time PCR (qRT-PCR)
Macrophages were collected into TRIzol reagent (Life Technologies, Carlsbad, CA, USA) and RNA was isolated from macrophages following the manufacturer instructions and established methods [18]. RNA purity and quantity were analyzed by NanoDrop 1000 Spectrophotometer (Thermo Scientific). Total RNA (100 ng) was reverse transcribed to cDNA using SuperScript III Reverse Transcriptase33 (Life Technologies, Carlsbad, CA, USA) following the manufacturer’s recommendations and established methods [18]. qRT-PCR was performed using mouse (TNFα, IL-6, IL-1β, IL-10) TaqMan® Gene Expression Systems (Applied Biosystems). Negative controls included no reverse transcriptase and no template reactions. All samples were run in triplicate using the CFX96 Real-Time System (Bio-Rad) and analyzed by the 2−ΔΔCT method [19]. Expression of each gene was normalized to 18s as a housekeeping gene (ΔCt).
2.15 Statistics
All experiments were performed at least 3 times in triplicate. Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, San Diego, CA). Multi-group comparisons were analyzed by one-way ANOVA (analysis of variance) followed by Bonferroni or Tukey’s post-test to test the significance of group differences between more than two groups. Pairwise comparisons used the Student’s t-test or Mann Whitney tests. Statistical significance in all analyses was defined as *p<0.05, **p<0.01, ***p<0.001.
3 Results
3.1 Loss of PPARγ confers improved control of M.tb intracellular growth in mouse lung macrophages in vitro
Several reports have indicated that PPARγ activation blocks the expression of several pro-inflammatory cytokines, such as TNFα and IL-6, as well as oxidant generation and has been shown to play an important role in M.tb clearance [8, 9, 20]. Moreover, survival of M.tb is increased in alternatively activated macrophages [21–24] and we have previously reported that PPARγ plays a role in promoting M.tb growth in human macrophages [8], at least in part through transcriptional regulation of apoptosis (unpublished observations). Therefore, we investigated whether PPARγ KO macrophages are able to better control M.tb intracellular survival in vitro and whether the lung environment uniquely impacts the effects of PPARγ during M.tb infection.
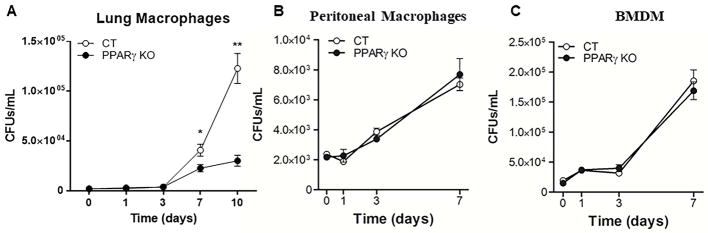
Isolated whole lung macrophages and AMs, as well as isolated peritoneal macrophages and BMDMs, were incubated with M.tb H37Rv for different time periods and bacterial survival was determined by CFU assay. Lung macrophages demonstrated enhanced growth of M.tb relative to peritoneal or BMDMs from wild type mice (lines with open circles in Fig. 1 graphs, mean growth increase over 7 days of 26.0 ± 2.9, 3.0 ± 0.3, and 9.9 ± 0.4 for lung, peritoneal, and BMDM macrophages, respectively). Importantly, the results showed that M.tb intracellular growth is significantly decreased in lung macrophages from PPARγ KO mice compared to macrophages from wild type mice (Fig. 1A, Table 1), and this phenotype is already observed in AMs at 3 days p.i. (3.1x104 ± 7.1x103 CFU recovered from wild type AMs and 2.4x104 ± 5.2x103 from PPARγ KO AMs 3 days after infection, mean ± SEM, n=3, not significant). Interestingly, bacterial survival was equal in PPARγ KO and wild type peritoneal and BMDMs, when infected under the same set of conditions (Fig. 1B&C). Thus, these data provide evidence that M.tb-induced PPARγ is an important regulator of M.tb growth in macrophages, specifically within the lung compartment.
Figure 1. Intracellular growth of M.tb in different macrophage populations in vitro.
(A) Whole lung, (B) peritoneal and (C) BMDM macrophages were isolated from control (CT) and PPARγ KO mice, then infected with virulent M.tb H37Rv (MOI 5) for 2 h and washed. Intramacrophage growth of M.tb was assessed by CFU assay at the indicated time points. Results are mean ± SEM of three independent experiments, note the different scales used for each graph. p-value *< 0.05, ** < 0.005.
Table 1. Intracellular growth of M.tb in different macrophage populations in vitro.
Whole lung, peritoneal and BMDM macrophages were isolated from control and PPARγ KO mice, then infected with virulent M.tb H37Rv (MOI 5) for 2 h and washed. Intramacrophage growth of M.tb was assessed by CFU assay at 1 and 7 days post infection.
| Lung | Peritoneal | BMDMs | ||||
|---|---|---|---|---|---|---|
| Control | PPARγ KO | Control | PPARγ KO | Control | PPARγ KO | |
| Growth Increase | 26.0 ± 2.9 | 13.9 ± 1.8 * | 3.0 ± 0.3 | 3.6 ± 0.5 | 9.9 ± 0.4 | 9.5 ± 0.1 |
Results are the mean ± SEM growth increase from Day 1 to Day 7.
p-value *< 0.05.
3.2 M.tb infection enhances the production of pro-inflammatory cytokines and decreases IL-10 in PPARγ KO lung macrophages in vitro
Lack of PPARγ has been shown to generate a Th1-like inflammatory response in AMs [25]. Furthermore, our lab has previously shown that infection with virulent M.tb up-regulates PPARγ expression and increases COX2 expression, IL-8 and PGE2 production in human macrophages [8]. These studies suggest that PPARγ functions as an important “molecular switch” in regulating macrophage immune responses to M.tb. Based on these findings, we investigated the production of cytokines in lung macrophages from wild type and PPARγ KO lung macrophages.
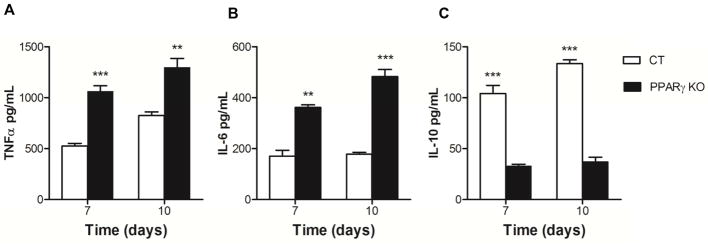
Isolated whole lung macrophages were incubated with M.tb for different time periods, and cell free culture supernatants were analyzed for TNFα, IL-6 and IL-10 secretion by ELISA. Our results demonstrated that M.tb infection of macrophages from PPARγ KO mice enhances TNFα and IL-6 release at 7 and 10 days p.i. in lung macrophages compared to wild type control mice (Fig. 2A&B). In contrast, secretion of the immunomodulatory cytokine IL-10 was reduced in PPARγ KO mice at the same time points (Fig. 2C). These results indicate that the absence of PPARγ in lung macrophages during M.tb infection promotes a more pro-inflammatory response which may contribute to better control of M.tb growth, particularly during the early stage of infection in the lung.
Figure 2. PPARγ deficiency regulates cytokine response in lung macrophages in vitro.
Whole lung macrophages were isolated from control (CT) and PPARγ KO mice and subsequently infected with virulent M.tb H37Rv (MOI 5) for 7 and 10 days. Cell free culture supernatants were used to determine concentration of (A) TNFα, (B) IL-6, and (C) IL-10 by ELISA. Results are mean ± SEM from two independent experiments. p-value ** < 0.005, ***<0.001.
3.3 Absence of PPARγ in lung macrophages reduces the growth of virulent M.tb in vivo
Based on our finding of better control of M.tb intracellular growth in lung macrophages in vitro in PPARγ KO lung macrophages, we next determined whether the KO mice would similarly reduce M.tb growth in vivo. The bacillary loads were determined in the lungs at several time points after in vivo infection in the medium dose aerosol (MDA) infection model to enable more robust analyses at earlier time points. Our data showed that the KO mice had a significant reduction in M.tb growth at 21 days p.i. (6.39 x 105 CFUs in Wt and 2.99 x 105 CFUs in PPARγ KO; Table 2). The low dose aerosol (LDA) infection model data showed the same tendency although not statistically significant (data not shown).
Table 2. Absence of PPARγ in lung macrophages reduces the growth of M.tb in vivo.
Control and PPARγ KO mice were infected with a MDA infection of M.tb H37Rv for 1, 14 and 21 days. Bacterial load in the lungs was determined by CFU assay.
| Time (days) | Control | PPARγ KO |
|---|---|---|
| 1 | 2.54 ± 0.42 x 102 | 1.96 ± 0.30 x 102 |
| 14 | 2.28 ± 0.36 x 104 | 1.60 ± 0.24 x 104 |
| 21 | 6.39 ± 0.84 x 105 | 2.99 ± 0.40 x 105 ** |
Results are expressed as mean ± SEM, obtained from groups of five mice per time point and two independent experiments.
p-value ** < 0.005.
3.4 PPARγ KO mice have reduced infiltration and dispersed granulomatous infiltration
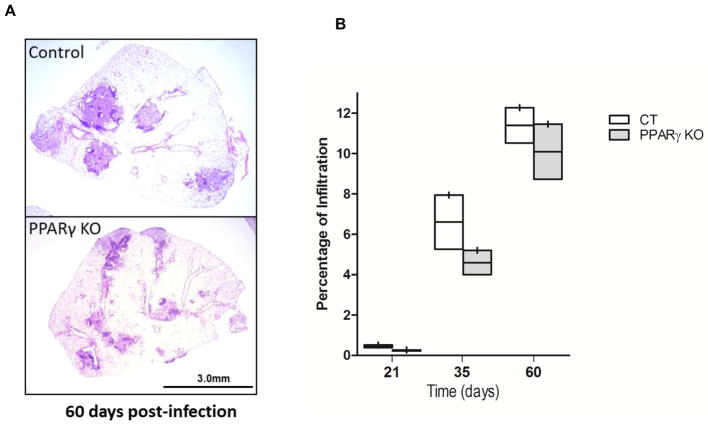
Since granulomatous infiltration plays a critical role in controlling mycobacterial dissemination, we examined the magnitude of granulomatous infiltration in the lung, defined as the ratio obtained after dividing the infiltrated area by the total area of the lung section, and then multiplying it by 100. The results in the LDA infection model showed that at 60 days p.i., granulomatous infiltration was decreased in PPARγ KO mice compared to control mice (Fig. 3A). Quantification of the infiltration indicated a consistent reduction in granulomatous infiltration in the PPARγ KO mice at all time points tested. Although this did not reach significance with a two-tailed t-test, the results at 21 days were significantly different with a one-tailed t-test (Fig 3B). Similar results were obtained using the MDA infection model at 21 days p.i. (data not shown).
Figure 3. PPARγ KO mice have reduced and dispersed granulomatous infiltration.
Control and PPARγ KO mice were infected with low dose aerosol (LDA) M.tb H37Rv for the indicated time. The lungs were harvested, processed and H&E stained. (A) Representative H&E stained lung section at day 60 p.i. showing decreased size of inflammatory nodules with more dispersion in PPARγ KO lungs. (B) Percentage of pulmonary infiltration was obtained by dividing the area of granulomatous infiltration by the total area of the lung lobes and multiplying x 100. Graph shown represents ten lung images (5 mice with 2 lung lobes/mouse/genotype/time point). The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Data are not statistically significant. N=2 independent experiments.
A more detailed analysis of lung inflammation composition revealed moderate differences in cell infiltrates in PPARγ KO compared to wild type mice (Table 3). The presence and extent of lymphocytic cuffs (composed primarily of lymphocytes and plasma cells) within the peribronchiolar/perivascular interstitium was higher in PPARγ KO mice compared to control mice, in both LDA and MDA models (Table 3). The presence/extent of alveolitis was also slightly higher in PPARγ KO compared to wild type controls, for both the LDA and MDA models, and the amount of degenerate neutrophils admixed with macrophages was lower in the KO compared to wild type. Thus, in the absence of PPARγ, there were fewer granulomas, and the granulomatous lesions that were present contained more lymphocyteic cuffs, more alveolitis, and fewer neutrophils.
Table 3. Histological analysis of M.tb infected wt and PPARγ KO mice.
The presence of lymphocytic cuffs in the peribronchiolar/perivascular interstitium, the presence and extent of alveolitis, and amount of degenerate neutrophils, which were admixed with macrophages, in the alveolitis was determined at the indicated times as described in the materials and methods. Briefly, five lung sections from two mice per genotype were evaluated in blinded fashion.
| LDA | MDA | |||
|---|---|---|---|---|
| Time(Days) | wt | KO | wt | KO |
| Lymphocytic cuffs | ||||
| 9 | - | - | 0.25 | 0.375 |
| 14 | - | - | 0.25 | 0.5 |
| 21 | 0.7 | 1.05 | 1.375 | 1.5 |
| 35 | 1.7 | 1.8 | - | - |
| 60 | 1.9 | 2.2 | - | - |
| Alveolitis | ||||
| 21 | - | - | 1 | 1.13 |
| 60 | 2.25 | 2.3 | - | - |
| Degenerate neutrophils admixed with macrophages | ||||
| 21 | - | - | 2.4 | 1.3 |
The numbers indicate the ordinal scale as defined in the methods. The – indicates not determined.
3.5 PPARγ KO mice have enhanced pro-inflammatory cytokine production in response to M.tb infection in vivo in both LDA and MDA models
PPARγ is highly expressed in AMs [25–28] and several reports have indicated that PPARγ serves as a negative regulator of macrophage activation by dampening the expression of pro-inflammatory cytokines and decreasing the oxidative burst [26, 29]. To assess whether the reduced M.tb growth seen in PPARγ KO compared to control mice was associated with a change in the cytokine production in the lung, cytokine mRNA expression of TNFα, IL-6, IL-1β and IL-10 from whole lung macrophages was analyzed in the both LDA and MDA aerosol infection models.
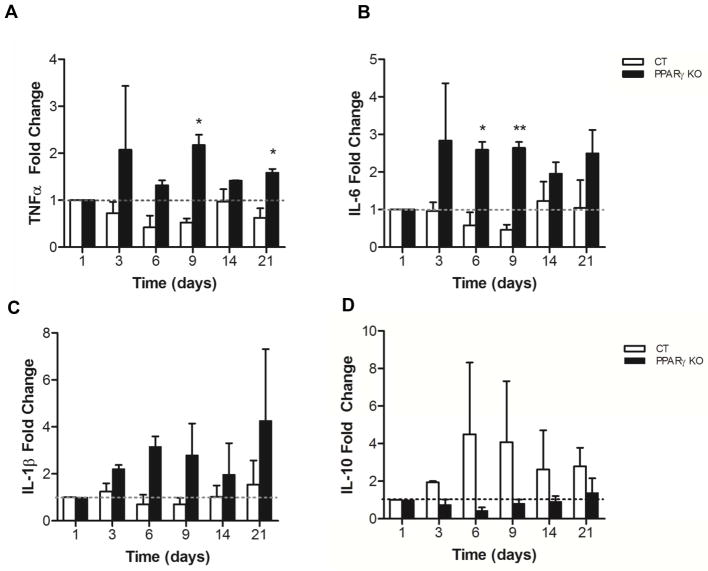
In the MDA infection model, cytokine expression in whole lung macrophages revealed an increase in mRNA expression of pro-inflammatory cytokines post-infection in the PPARγ KO mice compared to control mice (Fig. 4). In contrast, IL-10 was decreased in PPARγ KO mice consistent with the in vitro data. The LDA infection model data showed the same tendency (data not shown).
Figure 4. PPARγ KO mice have enhanced pro-inflammatory cytokine production and reduced IL-10 in response to an MDA M.tb infection.
Control (CT) and PPARγ KO mice were infected with an MDA infection of M.tb H37Rv. After the indicated times whole lung macrophages were isolated and the expression of (A) TNFα, (B) IL-6, (C) IL-1β and (D) IL-10 was determined by qRT-PCR using specific TaqMan primers. Data represent the mean ± SEM from five mice and are expressed as fold change. N = 2; p-value *< 0.05, ** < 0.005
4 Discussion
PPARγ serves as a global transcription factor in regulating metabolism and inflammation, the latter of which includes activities that negatively regulate macrophage activation such as repression of pro-inflammatory cytokines [3]. Previous work from our lab [8] and others [9] has identified an important role for PPARγ in promoting M.tb survival and growth in macrophages in in vitro models. Given that PPARγ is highly expressed in AMs [25, 27, 28, 30], is important for AM development, and regulates AM phenotype [1] its role in potentially promoting M.tb growth in lungs following aerosol M.tb infection is particularly relevant to pathogenesis. Herein we utilize a myeloid-specific PPARγ KO mouse to present evidence that absence of PPARγ in macrophages reduces M.tb growth in both whole lung and AMs but not macrophages from other compartments, and regulates the nature of the inflammatory response and granulomatous inflammation following aerosol M.tb infection.
The lungs are constantly exposed to aerosol pathogens and particulates that are drawn into the alveolar space while breathing. The AM represents the first line of defense against inhaled pathogens. The immune response to inhaled pathogens is finely regulated in the alveolar environment to preserve proper air exchange function of the lungs [31, 32]. While inflammation is required for efficient killing of inhaled pathogens, this inflammation must be dampened to avoid excessive tissue damage and lung dysfunction. Several reports have indicated that PPARγ serves as a negative regulator of macrophage activation and inflammation by altering the expression of many inflammatory genes [33, 34], modulating macrophage differentiation and activation through trans-repression of the transcription factors NF-κB, AP-1, and STAT1, which blocks the expression of many pro-inflammatory cytokines, such as TNFα or IL-6 [26], and attenuating the respiratory burst [29]. Additionally, activation of PPARγ by its ligands can inhibit the expression of iNOS, matrix metalloproteinase 9 and macrophage scavenger receptor 1 on various cell types, including monocytes and macrophages [26]. We have demonstrated earlier that PPARγ activation is increased by M.tb infection and knockdown of PPARγ in human macrophages significantly controls M.tb growth [8]. In support of this notion Mahajan et al. demonstrated that stimulation of PPARγ significantly enhances bacterial growth in macrophages [9].
AMs have been described as alternatively activated or M2-type macrophages, the latter are differentiated by IL-4 or IL-13 stimulation. Characteristics of AMs include high expression of PPARγ which can drive expression of the C-type lectin mannose receptor (CD206), the latter is an important receptor for M.tb recognition on macrophages [rev in [35]]. We have demonstrated that ligation of the mannose receptor by M.tb or its cell wall mannosylated lipoarabinomannan (ManLAM) leads to a signaling pathway that activates PPARγ and limits TNFα production [8]. Here we assessed the relative contribution of PPARγ to M.tb growth in different types of macrophages isolated from wild type and PPARγ KO mice. Notably, macrophages from the lung environment (alveolar and whole lung macrophages) of PPARγ KO mice were able to control M.tb growth whereas macrophages from the peritoneum or bone marrow were not (Fig.1 and Table 1). Consistent with our earlier finding using PPARγ knockdown in human macrophages [8], we observed increased production of TNFα and IL-6 in PPARγ KO lung macrophages in response to M.tb (Fig 2. A &B). We also observed a decreased level of the immunoregulatory cytokine IL-10 (Fig 2. C) in lung macrophages of PPARγ KO mice, similar to previous reports that PPARγ is important for IL-10 production [36, 37]. Thus the absence of PPARγ led to a fundamental shift to a more robust pro-inflammatory response in these cells.
Development of a granulomatous response is a hallmark for M.tb control in the tissues. Well-formed granulomas allow for control of M.tb growth with killing but also serve as a reservoir for M.tb persistence [38, 39]. We used both a LDA and MDA model of M.tb infection to study the effects of PPARγ on granulomatous infiltration in the lungs. Based on the effects of PPARγ in in vitro experiments in macrophages, we reasoned that the effects of PPARγ in vivo would be seen relatively early during the innate immune response to M.tb. It is difficult to assess responses in the LDA model at early time points given the small amount of bacteria. Thus the MDA model offered us the opportunity to better assess responses prior to 21 days p.i. Our data show that PPARγ KO mice have a modest but decreased and less circumscribed granulomatous response compared to wild type mice (Fig. 3). The reduced amount of granulomatous inflammation seen may be a cause or consequence of the reduced bacterial burden seen in PPARγ KO mice (Table 2). These results are consistent with the in vitro macrophage data that PPARγ KO lung macrophages control M.tb growth better than wild type macrophages (Fig. 1), and the timing in vivo suggests that reduced responses seen with PPARγ KO macrophages may have consequences on adaptive immunity. Indeed, lung macrophages from PPARγ KO mice exhibited increased pro-inflammatory gene expression compared to wild type mice (Fig. 4). As with the in vitro data, we observed a significantly low level of IL-10 in lung macrophages from PPARγ KO mice suggesting that PPARγ may regulate inflammation through induction of IL-10 and repression of pro-inflammatory cytokines.
4.1 Conclusions
This report represents the first description of the role of PPARγ in regulating the nature of the immune response and M.tb growth in an aerosol model of infection. Overall, our data indicate that high expression of PPARγ in AMs (as opposed to macrophages in other tissue compartments) drives a quantitatively important effect on host susceptibility to infection, particularly during the innate immune phase of infection. PPARγ is critical for proper development and function of lung macrophages [1], and the resultant macrophage phenotype likely contributes to the increased susceptibility to M.tb. In addition, PPARγ has pleiotropic effects on metabolism and infection [40]. Thus, the effects of PPARγ likely go beyond what is described in this report and await further study. One could envision that PPARγ antagonists could potentially be used to prevent primary M.tb infection based on this study, whereas PPARγ agonists (which are used in the treatment of diabetes mellitus) could have a beneficial effect as an adjunct host-directed therapy to reduce inflammation during active TB disease.
Supplementary Material
AMs were isolated from control (CT) and PPARγ KO (KO) mice. Protein lysates were analyzed by Western blot and indicate near abolishment of PPARγ expression. The two bands in the PPARγ blot are PPARγ isoforms 1 and 2.
Acknowledgments
We thank the Comparative Pathology and Mouse Phenotyping Shared Resource Services at The Ohio State University for sectioning and processing the lung tissue samples. We thank Uchenna Mbawuike and Ian Oglesbee for their help with the mouse colony. Finally, we acknowledge the support of the BSL3 program at Ohio State University.
Funding: This work was supported by the National Institutes of Health [grants NIAID R01 AI 059639-06 to LSS, and P30 CA016058 to The Ohio State Comprehensive Cancer Center]. The NIH had no involvement in the study design; collection, analysis, and interpretation of data; writing the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Abbreviations: Peroxisome proliferator-activated receptor (PPAR); retinoid X receptor (RXR); PPAR response element (PPRE); tuberculosis (TB); Mycobacterium tuberculosis (M.tb), colony-forming units (CFUs); Dulbecco’s Modified Eagle’s media (DMEM); heat-inactivated fetal bovine serum (hi-FBS); bronchoalveolar lavage (BAL); Iscove's Modified Dulbecco's Media (IMDM); low dose aerosol (LDA); mid-dose aerosol (MDA); alveolar macrophage (AM); bone marrow-derived macrophage (BMDM), multiplicity of infection (MOI) .
Declarations of Interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schneider C, et al. Induction of the nuclear receptor PPAR-gamma by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol. 2014;15(11):1026–37. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 2.Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10(5):365–76. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 3.Nagy L, et al. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol Rev. 2012;92(2):739–89. doi: 10.1152/physrev.00004.2011. [DOI] [PubMed] [Google Scholar]
- 4.Forman BM, et al. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 5.Forman BM, Chen J, Evans RM. The peroxisome proliferator-activated receptors: ligands and activators. Ann N Y Acad Sci. 1996;804:266–75. doi: 10.1111/j.1749-6632.1996.tb18621.x. [DOI] [PubMed] [Google Scholar]
- 6.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94(9):4312–7. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiss M, Czimmerer Z, Nagy L. The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: From physiology to pathology. J Allergy Clin Immunol. 2013;132(2):264–86. doi: 10.1016/j.jaci.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 8.Rajaram MV, et al. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185(2):929–942. doi: 10.4049/jimmunol.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahajan S, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. J Immunol. 2012;188(11):5593–5603. doi: 10.4049/jimmunol.1103038. [DOI] [PubMed] [Google Scholar]
- 10.Almeida PE, et al. Mycobacterium bovis bacillus Calmette-Guerin infection induces TLR2-dependent peroxisome proliferator-activated receptor gamma expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J Immunol. 2009;183(2):1337–1345. doi: 10.4049/jimmunol.0900365. [DOI] [PubMed] [Google Scholar]
- 11.Odegaard JI, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlesinger LS, et al. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 13.Schlesinger LS, Horwitz MA. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1(CD35), CR3(CD11b/CD18), and CR4(CD11c/CD18) and interferon gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991;147:1983–1994. [PubMed] [Google Scholar]
- 14.Guirado E, et al. Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect. 2006;8(5):1252–9. doi: 10.1016/j.micinf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Rajaram MV, et al. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177(9):6317–6324. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- 16.Olakanmi O, Britigan BE, Schlesinger LS. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect Immun. 2000;68(10):5619–5627. doi: 10.1128/iai.68.10.5619-5627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vesosky B, Flaherty DK, Turner J. Th1 cytokines facilitate CD8-T-cell-mediated early resistance to infection with Mycobacterium tuberculosis in old mice. Infect Immun. 2006;74(6):3314–3324. doi: 10.1128/IAI.01475-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guirado E, et al. Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model. MBio. 2015;6(1):e02537–14. doi: 10.1128/mBio.02537-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Almeida PE, et al. PPARgamma Expression and Function in Mycobacterial Infection: Roles in Lipid Metabolism, Immunity, and Bacterial Killing. PPAR Res. 2012;2012:383829. doi: 10.1155/2012/383829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber T, et al. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183(2):1301–1312. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahnert A, et al. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006:631–647. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- 23.Day J, Friedman A, Schlesinger LS. Modeling the immune rheostat of macrophages in the lung in response to infection. Proc Natl Acad Sci U S A. 2009;106(27):11246–11251. doi: 10.1073/pnas.0904846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verreck F, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malur A, et al. Deletion of PPAR gamma in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J Immunol. 2009;182(9):5816–5822. doi: 10.4049/jimmunol.0803504. [DOI] [PubMed] [Google Scholar]
- 26.Ricote M, et al. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 27.Asada K, et al. Antiinflammatory roles of peroxisome proliferator-activated receptor gamma in human alveolar macrophages. Am J Respir Crit Care Med. 2004;169(2):195–200. doi: 10.1164/rccm.200207-740OC. [DOI] [PubMed] [Google Scholar]
- 28.Reddy RC, et al. Deactivation of murine alveolar macrophages by peroxisome proliferator-activated receptor-gamma ligands. Am J Physiol Lung Cell Mol Physiol. 2004;286(3):L613–9. doi: 10.1152/ajplung.00206.2003. [DOI] [PubMed] [Google Scholar]
- 29.von Knethen A, Brune B. Activation of peroxisome proliferator-activated receptor gamma by nitric oxide in monocytes/macrophages down-regulates p47phox and attenuates the respiratory burst. J Immunol. 2002;169(5):2619–2626. doi: 10.4049/jimmunol.169.5.2619. [DOI] [PubMed] [Google Scholar]
- 30.Ricote M, et al. Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1998;95(13):7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14(2):81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 32.Arnett E, et al. Host pathogen biology for airborne Mycobacterium tuberculosis. In: Hickey A, Misra A, Fourie P, editors. Drug delivery systems for tuberculosis preveniton and treatment. John Wiley & Sons, Ltd; Chichester, UK: 2016. pp. 11–47. [Google Scholar]
- 33.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 34.Ricote M, et al. The peroxisome proliferator-activated receptor(PPARgamma) as a regulator of monocyte/macrophage function. J Leukoc Biol. 1999;66(5):733–739. doi: 10.1002/jlb.66.5.733. [DOI] [PubMed] [Google Scholar]
- 35.Rajaram MV, et al. Macrophage immunoregulatory pathways in tuberculosis. Semin Immunol. 2014;26(6):471–485. doi: 10.1016/j.smim.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira AE, et al. PPAR-gamma/IL-10 axis inhibits MyD88 expression and ameliorates murine polymicrobial sepsis. J Immunol. 2014;192(5):2357–65. doi: 10.4049/jimmunol.1302375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon YS, et al. PPARgamma activation following apoptotic cell instillation promotes resolution of lung inflammation and fibrosis via regulation of efferocytosis and proresolving cytokines. Mucosal Immunol. 2015;8(5):1031–46. doi: 10.1038/mi.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guirado E, Schlesinger LS. Modeling the Mycobacterium tuberculosis Granuloma - the Critical Battlefield in Host Immunity and Disease. Front Immunol. 2013;4:98. doi: 10.3389/fimmu.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12(5):352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 40.Ahmadian M, et al. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19(5):557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AMs were isolated from control (CT) and PPARγ KO (KO) mice. Protein lysates were analyzed by Western blot and indicate near abolishment of PPARγ expression. The two bands in the PPARγ blot are PPARγ isoforms 1 and 2.