Abstract
Ovarian cancer remains a deadly diagnosis with an 85% recurrence rate and a 5-year survival rate of only 46%. The poor outlook of this disease has improved little over the past 50 years owing to the lack of early detection, chemoresistance and the complex tumor microenvironment. Within the peritoneal cavity, the presence of ascites stimulates ovarian tumors with shear stresses. The stiff environment found within the tumor extracellular matrix and the peritoneal membrane are also implicated in the metastatic potential and epithelial to mesenchymal transition (EMT) of ovarian cancer. Though these mechanical cues remain highly relevant to the understanding and treatment of ovarian cancers, our current knowledge of their biological processes and their clinical relevance is deeply lacking. Seminal studies on ovarian cancer mechanotransduction have demonstrated close ties between mechanotransduction and ovarian cancer chemoresistance, EMT, enhanced cancer stem cell populations, and metastasis. This review summarizes our current understanding of ovarian cancer mechanotransduction and the gaps in knowledge that exist. Future investigations on ovarian cancer mechanotransduction will greatly improve clinical outcomes via systematic studies that determine shear stress magnitude and its influence on ovarian cancer progression, metastasis, and treatment.
I. INTRODUCTION
Ovarian cancer is the fifth leading cause of cancer related deaths in females1 and remains a deadly diagnosis with 54%2 of patients dying from their initial or recurrent diagnosis. While significant advancements in treatment therapies and success rates have been observed in some cancers, there has been no significant progress in ovarian cancer treatment over the past 50 years.3,4 Much of this failure arises from the lack of early detection capabilities, with 60%–70% of all patients diagnosed at advanced stages (III or IV),1,5–8 and an 85% recurrence rate.9 Ovarian cancer is categorized by the cell of origin, with approximately 90% originating from epithelial cells. Epithelial ovarian cancers arise from either an ovarian surface epithelial stem cell or a fimbrial stem cell that becomes entrapped within the ovary cortex. This entrapped cell then forms a cortical inclusion cyst that is driven to high-grade serous carcinoma from the aberrant niche environment.3,10,11 The readers are requested to refer to the review by Ng and Barker for the detailed origin of ovarian cancers.10 Epithelial ovarian cancer is classified into histological subgroups, where serous carcinoma makes up 70% of all tumors.11 The serous histological subtype is grouped into a two-tier system based on the prevalence of mitotic rate and atypical nuclei.3,12 90% of all serous epithelial ovarian cancer is of “high grade,” making it the most prevalent type of ovarian cancer characterized by TP53 mutations, rapid tumor growth, and high recurrence.11,12 The recurrent disease is often chemoresistant and has a median survival of 12–24 months.9 Detection of ascites within the peritoneal cavity is associated with most stages of ovarian cancer. According to the American Joint Committee on Cancer (AJCC) and International Federation of Gynecology and Obstetrics (FIGO), stage IC, IIB, III, and IV ovarian cancers are all categorized by the presence of cancer in the peritoneal cavity.13,14 The detection of malignant ascites is an integral step in the clinical assessment of ovarian cancer.15 Furthermore, malignant ascitic fluid is a major contributor to ovarian cancer progression and poor prognoses,16 and is consequently closely monitored by oncologists. Many of these statistics arise from factors within the tumor microenvironment; therefore, it is critical to consider their role when striving to understand and devise treatment strategies to improve patient outcomes. This review will address the contribution of specific cues from the tumor microenvironment to the disease progression and the impact of these findings on our understanding of ovarian cancers.
A. The ovarian cancer mechanical microenvironment
Located within the peritoneal cavity, the ovaries exist within the abdominal space where the cellular and acellular content are tightly regulated by the anatomy of the peritoneal membrane. The peritoneal membrane consists of five layers: endothelial cells, endothelial basement membrane, interstitial space, submesothelial basement membrane, and mesothelial cells.17 These tight layers inhibit cells and large protein molecules such as albumin from migrating into the peritoneal cavity. In healthy individuals, the peritoneal membrane modulates a net oncotic pressure out of the cavity17 filtering 50–100 ml of fluid into the lymphatic vessels every hour,18 with post-menopausal women carrying an average of 2.3 ml of intraperitoneal fluid at any given time.19 However, in a diseased state, this intraperitoneal fluid is not readily drained and a backup of liquid, termed ascites, may begin to amass in some patients.
Approximately, 36.7% of all ovarian cancer patients develop ascites,20–22 defined as a minimum of 25 ml of fluid accumulation23 within the peritoneal cavity. The retention of ascitic fluid in diseased patients is predicted to stem from an increase in the permeability of the capillaries through the peritoneal membrane, lymphatic obstruction of normal drainage, and the net oncotic pressure into the cavity.16–18,24 Ovarian cancer cells and cellular aggregates that are shed into the peritoneal cavity can physically block the homeostatic lymphatic drainage system.25 This theory of ascitic fluid retention in ovarian cancers has been around for more than 60 years;24,26,27 yet, the exact mechanisms have yet to be proven.16
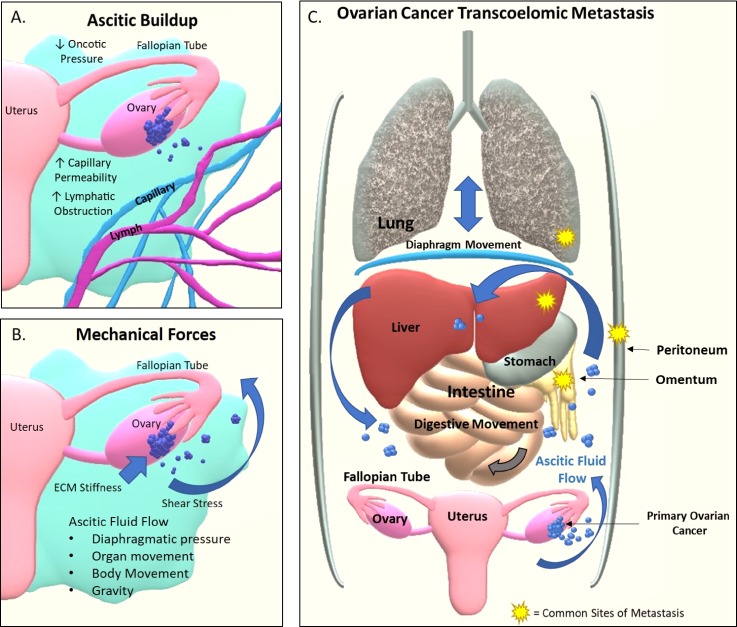
The presence of ascitic fluid has been shown to aid in metastasis28 and chemoresistance.29,30 It also mechanically stimulates the cancer with hydrostatic compression and shear forces. The ascitic fluid flow is triggered by gravity, changes in diaphragmatic pressure from breathing, surrounding organ movement aiding digestion, and bodily movements like walking.31 The continuous barrage of turbulent fluid flow stimulates a variety of mechanotransduction signaling pathways and further exfoliates ovarian tumor cells and cellular aggregates from the ovarian surface epithelium into the peritoneal cavity. After their escape into the ascites, these free-floating cancer cells and cellular clusters often self-assemble and aggregate to form spheroids, thereby overcoming anoikis.25,32 Once ovarian cancer cells have disseminated within the ascites, they have access to the most common metastatic sites of ovarian cancers: the peritoneum, the greater omentum, the right subphrenic region, the lung, and the liver.31–33 The presence of ascites and forces associated with them facilitate transcoelomic metastasis, the most common form of ovarian cancer metastasis.28,31 Figure 1 details the mechanical forces relevant to ovarian cancers, the ascitic buildup, and the transcoelomic metastatic process in ovarian cancers.
FIG. 1.
The microenvironment of ovarian cancer facilitates transcoelomic metastasis. (a) The buildup of ascites is triggered by the primary tumor which causes increased capillary permeability, lymphatic obstruction of drainage, and an overall decrease in oncotic pressure out of the peritoneal cavity. (b) The ovarian cancer cells experience the surrounding ECM stiffness within the primary tumor, spheroid cell aggregates within the ascites, and potential metastatic sites. Shear stress stimulates the ovarian cancer cells via interstitial fluid flow within the primary tumor and ascitic fluid flow triggered by gravity, bodily movements, change in the diaphragmatic pressure from breathing, and organ movements from functions such as digestion. (c) Transcoelomic metastasis starts with the exfoliation and detachment of cancer cells from the primary tumor site caused by shear stress within the ascites. Cancer cells within ascites evade the immune system and detached cells form spheroids to avoid anoikis. Ovarian cancer spheroids are then carried by the ascitic current to metastatic sites where implantation, invasion, and growth facilitate the formation of new tumors.
Ovarian cancer cells isolated from ascites are rich in cancer stem cells (CSCs).34,35 CSCs are defined as a small subset of cancer cells, with the capability of self-renewal, multilineage differentiation, tumor initiation, metastasis, and chemoresistance to conventional or targeted chemotherapies and radiotherapies. Ovarian CSCs are typically identified through expression of specific markers such as CD133, ALDH1A, CD24, CD117, CD44,36–39 and micro ribonucleic acid (miRNA), as well as functional phenotypes such as self-renewal, production of heterogeneous progenies, and enhanced tumor formation capabilities.36 CSCs are typically enriched after chemotherapy as residual cells that lead to tumor relapse in patients. The presence of ascites increases the drug efflux mechanisms within the ovarian cancer cells including ABC transporter genes: MDR1a, MDR1b, and BCRP.34,40 The upregulation of these transporter genes provides ovarian cancer cells the necessary mechanisms to survive chemotherapy and renew tumor growth post-treatment. Additionally, ascites have been shown to enhance epithelial to mesenchymal transition (EMT) in ovarian cancer cells.8,41,42 During EMT, a stationary epithelial cell transforms into a mesenchymal cell capable of motility. This transition is an important precursor for metastasis and chemoresistance.43,44 Currently, the role of mechanical cues within the ovarian tumor microenvironment that leads to these outcomes is not well defined. Therefore, the effects of mechanotransduction in the ovarian cancer microenvironment need to be investigated in the context of disease progression and chemoresistance. It is likely that future findings could greatly improve patient treatment and outcome. The known contribution of mechanical cues towards tumor progression and metastasis within the ovarian cancer microenvironment is reviewed in Secs. II B, II D, and III.
II. 2D AND 3D IN VITRO MODELS OF OVARIAN CANCER MECHANOTRANSDUCTION
To study the physiologically relevant forces of shear stress and extracellular matrix (ECM) stiffness, many research groups have developed bioreactors capable of systematic and controlled force stimulation that independently explore the effects of mechanical stimuli on ovarian cancer. Here, we detail the published studies that have investigated the effects of mechanical stimuli on ovarian cancer and their overall findings.
A. Shear stress estimates in ovarian cancers
Accurate in vivo shear force estimates within patient ascites and the corresponding shear stress values on ovarian cancer are not known. It has been predicted that shear stress values within ascites are low, with relatively little to no support in either experimental or mathematical modeling.29,31,45 Computer simulated models are required to improve our understanding of the physiological stresses that occur within the peritoneal cavity. A diseased patient's musculoskeletal/organ movements cause a change in the shape of the peritoneal cavity which in turn causes fluid movement within the ascites. This fluid movement is directly correlated to the levels of shear stress experienced by both free floating and attached ovarian cancer spheroids. These complex multistep interactions can be modeled with the help of finite element analysis and fluid dynamic modeling systems. The interstitial fluid velocity ranging from 0.2 to 0.8 μm/s has been reported in neoplastic tissues,46 but no direct measurements of ovarian specific tissues exist. Moreover, the wall shear stress in a computational simulation of gastrointestinal models45 ranges from 0.14 to 11 dyn/cm2,45,47 and has been used as an estimate for shear stress ranges on ovarian tumors. In contrast, circulating tumor cells experience a large range of shear stresses from venous (0.5–4.0 dyn/cm2) and arterial blood flow (4.0–30.0 dyn/cm2).48 Given the paucity of research on the physiological role of shear stress and specific values relevant to ovarian cancer, there is a critical unmet need for systematic studies that determine shear stress magnitude and its influence on ovarian cancer progression, metastasis, and treatment.
B. Shear stress models specific to ovarian cancer
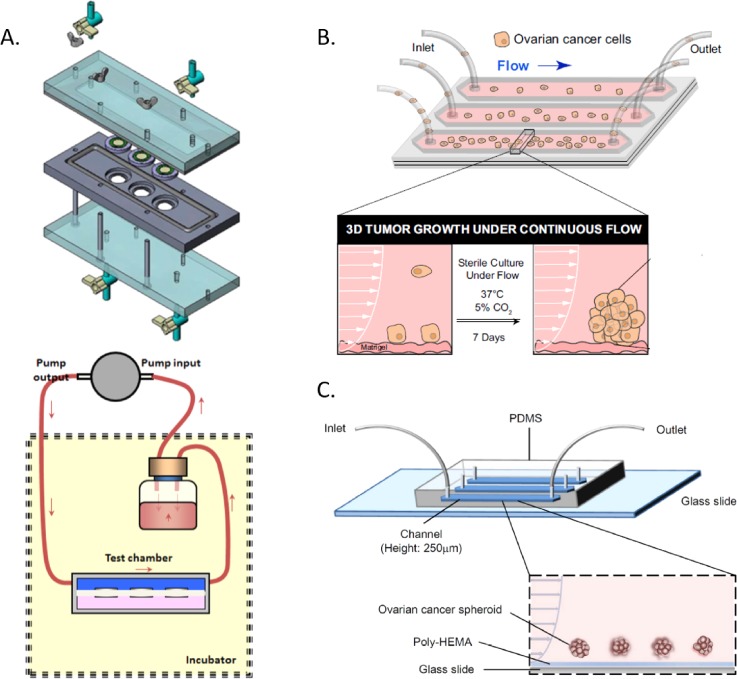
The study of shear stress on cells has been considerably investigated with both commercially available and custom-made lab bioreactors.29,49–51 However, only a few published studies have investigated shear stress stimulation of ovarian cancer cells in 2D or 3D culture models. To answer the question of how fluid flow induced wall shear stress affects the cytoskeleton of ovarian cancer and regulates its penetration and spread to the peritoneum, Avraham-Chakim et al. fabricated a custom-made 2D shear stress device45 [shown in Fig. 2(a)]. OVCAR3 cells, representative of high grade serous ovarian cancer,52 were cultured in monolayers and then exposed to shear stress of 0.5–1.5 dyn/cm2 for 30 min. Morphological analysis revealed that the shear stimulated OVCAR3 cells elongated significantly, increased stress fiber formation, and generated a cytoskeletal network of microtubules with increasing shear stress. Shear stress experienced by ovarian cancer cells induced cell motility and targeting these specific cytoskeletal pathways may benefit ovarian cancer treatment.45
FIG. 2.
Selected bioreactors and devices utilized for ovarian cancer shear stress investigations. (a) Flow chamber schematic of the 2D ovarian cancer cell culture using a closed circuit pump design for shear stress stimulation.45 (b) 2D/3D hybrid design where ovarian cancer cells flow into the microfluidic chamber, adhere to the Matrigel basement layer, and continue to grow under a shear stress stimulus for 7 days.31 (c) Microfluidic device design where ovarian cancer spheroids do not adhere to the poly-HEMA basement layer and are stimulated with shear stress within the channel for 24 h.29 Reproduced with permission from Ip et al., Sci. Rep. 6, (2016). Copyright 2016 Nature Publishing Group,29 Avraham-Chakim et al., PLoS One 8, e60965 (2013). Copyright 2013 PLOS,45 and Rizvi et al., Proc. Natl. Acad. Sci. 110, E1974–E1983 (2013). Copyright 2013 National Academy of Sciences.31
Seeking to replicate the initial dissemination of ovarian cancer cells into the peritoneal cavity, Hyler et al. devised an experiment to test low levels of shear stress on five cell lines of variable metastatic potential. Three murine ovarian cell lines ranging from benign to highly aggressive mouse ovarian cancer epithelial cells (MOSE), OCE1 (benign human), and SKOV3 (human ovarian clear cell adenocarcinoma) cell lines were exposed to fluid shear stress ranging from 0.13 to 0.32 dyn/cm2 on a rotator plate for up to 12 days.53 Fluid shear stress was shown to increase the capacity for spheroid formation in cell lines with a higher metastatic phenotype, increase the number of actin-containing protrusions and vinculin-containing focal adhesions for all cell types, as well as show nuclear change with an increase in multi-lobed nuclei and the number of tetraploid chromosomes in benign cell populations.
Molecular changes associated with the metastatic cascade due to continuous shear force was investigated within a microfluidic device designed by Rizvi et al.31 [Fig. 2(b)]. High grade serous ovarian cancer OVCAR5 cells in suspension were placed under continuous flow for 7 days above a Matrigel basement layer used to model a stromal bed. The shear stress varied with the location within the device, with the flow velocity ranging from 0 mm/s on the edge of the device to approximately 10 mm/s throughout the device center, where majority of cell attachment was located. The cells that attached under these shear conditions formed micronodules and showed increased EMT biomarkers, including decreased proliferation, upregulation of epidermal growth factor receptor (EGFR), decreased E-cadherin expression, and an associated increase in vimentin expression without any change in integrin α5.31 The flow-induced EMT was predicted to influence the chemoresistance of cells and the effectiveness of targeted inhibitors. These predictions were sequentially validated by Ip et al.29
Expanding upon the previous findings of Rizvi et al., Ip et al. sought to identify the role of CSC in ovarian cancer chemoresistance. SKOV3, a p53 mutant clear cell adenocarcinoma cell line, was first grown into spheroids before being placed under extremely low shear conditions (0.02 and 0.002 dyn/cm2) in a microfluidic shear device29 [Fig. 2(c)]. Shear stress was applied to the spheroids atop a poly(2-hydroxyethyl methacrylate) (Poly-HEMA) layer, to prevent adherence, for 24 hours, before sequential analysis was performed. The lack of adherence to the basement layer provided 3D stimulation mimicking that of the ascitic environment. The shear stimulated SKOV3 spheroids were found to have enriched with CSCs with the expression of Oct-4, CD117, ABCG2, and P-gp. Concurrently, EMT was enhanced through the upregulation of gene and protein expression of Snail, Slug, and N-cadherin, and downregulation of E-cadherin.29 Apart from substantiating the work of Rizvi et al., they found that the shear stress stimulated cells were chemoresistant to cisplatin and paclitaxel treatment, as previously hypothesized.31 The CSC phenotypes and chemoresistance were attributed to the PI3K/Akt signaling pathway, where LY294002, a specific inhibitor to PI3K, abated the previously observed enhanced CSC marker expression. Sequential chemotherapy treatment was not performed with the PI3K/Akt inhibitor. Overall, these findings emphasize the impact that shear stress stimulus has on chemoresistance and recurrence through CSC populations within patient ascites. These findings also bring to light the importance of the PI3K/Akt pathway suggesting it as an essential target within CSC and chemoresistant phenotypes in ovarian cancer, though additional work should be done to validate these findings in additional cell lines.
The spread of ovarian cancer to distant metastatic sites through tumor cells that have intravasated to the circulation and then extravasate and colonize a new tumor site was investigated by Egan et al.54 and Giavazzi et al.55 Egan et al. utilized a simple cone and plate viscometer setup to test the protection potential of platelets when sheared under venous and arterial stresses with A2780 (endometrioid histotype) ovarian cancer cells. This setup was designed to test the viability of circulating tumor cells under physiological shear stresses within arterial and venous circulation. This is an important point of concern once tumor cell extravasation has occurred; however, it is a less predominant form of ovarian cancer metastasis. Shear rates of 1.5 and 12 dyn/cm2 were explored for 10 minutes with and without platelet incorporation. The amount of lactate dehydrogenase (LDH) was measured for the indication of cancer cell membrane damage. The results demonstrated a significant reduction in LDH when platelets were present under shear stress, implying the prolonged non-destructive circulation of cancer cells under in vivo conditions.54
Beyond circulation survival, the ability to adhere and extravasate is necessary for circulating tumor cells to metastasize. The rolling and attachment capability of circulating tumor cells was investigated by Giavazzi et al. where a 2D/3D hybrid approach was developed from a parallel plate apparatus. This experimental design was developed to determine ovarian cancer cell affinity to adherence and rolling on a 2D culture of human umbilical vein endothelial cells (HUVEC). This design contained OVCAR3 cells within a fluidic suspension and the shear stress ranged from 0.3 to 3.0 dyn/cm2 to more closely replicate venous blood flow for a duration of 12 min. Only a small proportion of their experiments pertained to OVCAR3 cells, but results showed that little interaction occurred between the resting HUVEC surface layer and OVCAR3 cells, while minimal attachment and rolling occurred on IL-1 activated HUVECs.55 These findings implicate that specific adhesion mechanisms are necessary for ovarian tumor cell attachment and extravasation, while the cell type also plays a critical role in which attachment or rolling mechanisms are utilized. A compact summary of the shear stress mechanotransduction studies on ovarian cancer is detailed in Table I with schematics of select bioreactors shown in Fig. 2.
TABLE I.
Ovarian cancer specific shear stress investigations and major findings.
| 2D/3D culture | Device design | Shear stress and duration | Cell type | Findings | Citation |
|---|---|---|---|---|---|
| 2D/3D hybrid | Parallel plate | 0.3–3.0 dyn/cm2 12 min | OVCAR3 | • Little interaction with HUVEC resting cells • Some attachment and rolling on IL-1 activated HUVEC cells | Giavazzi et al.55 |
| HUVEC monolayer | |||||
| 2D | Rotator plate | 0.13–0.32 dyn/cm2 12 days | MOSE-E | • Increased spheroid formation• Formation of actin-containing protrusions• Increase in vinculin-containing focal adhesions• Change in nuclear structure associated with aneuploidy | Hyler et al.53 |
| MOSE-L | |||||
| MOSE-LTICν | |||||
| OCE1 | |||||
| SKOV3 | |||||
| 2D | Custom | 0.5, 1.0, 1.5 dyn/cm2 30 min | OVCAR3 | • Cell elongation• Formation of stress fibers• Formation of cytoskeletal microtubule network | Avraham-Chakim et al.45 |
| 2D/3D hybrid | Custom microfluidic | Range of 0 to >10 mm/s 7 days | OVCAR5 | • Increased EMT • Increased EGFR, vimentin, p27Kip1 • Decreased E-cadherin, CDC2 | Rizvi et al.31 |
| 3D | Cone and plate viscometer | 1.5, 12 dyn/cm2 10 min | A2780 | • Reduced lactate dehydrogenase (LDH) release with platelet co-culture under shear | Egan et al.54 |
| 3D | Custom microfluidic | 0.02, 0.002 dyn/cm2 24 h | SKOV3 | • Enhancement of CSC markers: Oct-4, CD117, ABCG2, P-gp• Increased EMT• Enhanced chemoresistance• PI3K/Akt signaling pathway involvement | Ip et al.29 |
C. Alternative shear bioreactors for examining ovarian cancer mechanotransduction
Cancer induced ascites or malignant ascites are not unique to ovarian cancer. Other cancers, including colon, pancreatic, gastrointestinal tract, lung, and breast, feature tumor cells in ascites and pleural effusion.17,56 Tumor cells within the ascites are often found at late stages of cancer progression. Previous studies have investigated the impact of shear stress stimulus on a variety of cancer types due to ascitic shear stresses, heightened interstitial fluid flow, and high shear conditions experienced by circulating tumor cells.57 For a review of shear stress studies on cancer, the readers are kindly referred to Mitchell and King.57
Work on breast cancer has shown shear stress to affect: adherence to the endothelium58 due to an increase in the expression of EMT characteristics,51 acidic microenvironment development,59 cancer stem cell populations,60 migration,61,62 involvement of caveolin-1 through the FAK/Src, ROCK/pMLC,52 and PI3K/Akt/mTOR63 pathways, and glycoprotein IIb/IIIa and αvβ3 integrin in PI3K/Akt and NF-kB signaling.64 Glioma cells exposed to shear stress showed migratory activity dependent on matrix metalloproteinase (MMP) activation and expression,65 while prostate cancer cells showed YAP1 dependent motility.66 Shear stress stimuli on bladder, colon, and pancreatic cancers have shown enhanced axial spreading,67 sensitization to TRAIL-induced apoptosis,49 involvement of Wnt/β-catenin, mitogen-activated protein kinase (MAPK), and NF-κB pathways,68 and the necessity of mucin 16 for pancreatic cell adherence.69
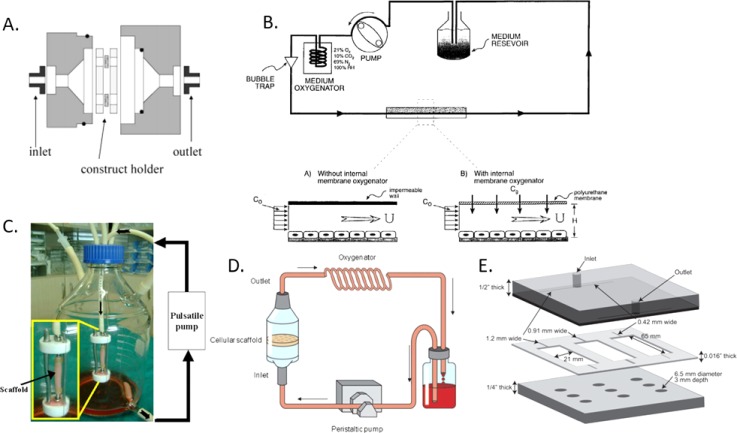
Given that the ascitic environment has been investigated for other tumor cell types, these findings may be of interest to future investigations in ovarian cancer mechanotransduction. The specific pathway findings such as involvement with PI3K, Akt, ROCK, and NF-κB must be considered, as PI3K/Akt pathway contributions under shear stress have already been identified.29 Additionally, CSC populations, migration potential, and metastatic potential should all be scrutinized under shear stress because of the concurrent findings between cell types. However, novel studies on ovarian cancer cells, including those derived from primary and metastatic tumors and ascites, are still needed to confirm these similarities and identify the unique characteristics and potential target pathways for ovarian cancer mechanotransduction. Distinct bioreactor designs will arise depending on specific biological questions. Shear bioreactors have been implemented in cell culture for the past quarter-century. Their designs have ranged from 2D microfluidic devices to large scale 3D perfusion bioreactors. With these devices, researchers have been able to test shear forces on cells seeded on a wide variety of surfaces and scaffolds. However, each shear stress bioreactor device also comes with a specific set of design limitations that must be taken into consideration when devising an experiment. For example, some devices have a limited working shear stress range. In the case of ovarian cancer, it is currently hypothesized that most shear stresses experienced in the peritoneal cavity are below 1 dyn/cm2.29 Some bioreactors may not be suitable for providing this type of shear stress value, especially in a manner that is both consistent and reproducible. Bioreactors such as the orbital shaker and the cone and plate viscometer will have intrinsic variable shear stress and may produce shear ranges outside that of suitable physiological values. Other bioreactors may only support 2D culture, making it impossible to incorporate any type of 3D scaffold within them. Table II details some popular shear bioreactor designs and schematics of select bioreactors are shown in Fig. 3. The application of these devices to ovarian cancer investigations may be suitable for future research.
TABLE II.
Prominent shear stress bioreactors: Shear stress bioreactors with design relevance for future investigations in ovarian cancer research.
| 2D/3D culture | Material and device | Stimulant type | Shear stress | Cell type | Citation |
|---|---|---|---|---|---|
| 2D | Flat plate | Laminar flow | 0.01–21 dyn/cm2 | Rat hepatocytes cocultured with 3T3-J2 fibroblasts | Tilles et al.71 |
| 2D | Cone and plate | Laminar flow | 5 dyn/cm2 | Human endothelial cells | Dai et al.75 |
| 2D | Orbital shaker | Laminar flow | 5–14 dyn/cm2 | Endothelial cells | Dardik et al.76 |
| 2D | Tubular poly(ethylene glycol) (PEG) microfluidic device | Laminar flow | 0.5 dyn/cm2 | PC3 prostate cancer cells | Lee et al.66 |
| 3D | Poly(lactide-co-caprolactone) (PLCL) tubular perfusion bioreactor | Laminar porous flow | Flow rate: 130 ml/min, P = 25 mmHg 1 Hz pulse | Rabbit aortic smooth muscle cells | Jeong et al.72 |
| 3D | Polyester-urethane foam perfusion bioreactor | Laminar porous flow | 0.046–0.56 dyn/cm2 | Bovine articular chondrocytes | Raimondi et al.70 |
| 3D | Porous poly(l-lactic acid)/poly(l-lactic-co-glycolic acid) (PLLA/PLGA) scaffold perfusion bioreactor | Laminar porous flow | 1–10 dyn/cm2 | Human foreskin fibroblasts | Lesman et al.73 |
| 3D | Alginate scaffold perfusion bioreactor | Laminar porous flow | 1–13 dyn/cm2 | Human umbilical vein endothelial cells | Rotenberg et al.49 |
| 3D | Collagen type I gel microfluidic device | Laminar flow or oscillatory shear | 2–20 dyn/cm2 | Porcine aortic valve endothelial cells | Mahler et al.74 |
FIG. 3.
Relevant shear stress bioreactors for future studies on ovarian cancer mechanotransduction. (a) Custom 3D porous scaffold shear bioreactor device; cells were seeded on a 1 mm thick biodegradable polyester-urethane foam and perfused with medium.70 (b) 2D flat plate design; cells were seeded on a glass slide and experienced uniform fluid shear.71 (c) 3D shear bioreactor utilizing a PLCL tubular scaffold; cells were seeded onto a particulate leached PLCL scaffold and perfused with medium.72 (d) Porous perfusion scaffold bioreactor; cells were seeded onto a perfused particle leached PLLA/PLGA scaffold.73 (e) 3D microfluidic device providing three unique shear rates; cells seeded on a Collagen Type I scaffold experienced shear over the surface of the scaffold.74 Reproduced with permission from Raimondi et al., Biorheology 43, 215–222 (2006). Copyright 2006 IOS Press,70 Tilles et al., Biotechnol. Bioeng. 73, 379–389 (2001). Copyright 2001 John Wiley & Sons,71 Lesman et al., Biotechnol. Bioeng. 105, 645–654 (2010). Copyright 2010 John Wiley & Sons,73 Jeong et al., Biomaterials 26, 1405–1411 (2005). Copyright 2005 Elsevier,72 and Mahler et al., Biotechnol. Bioeng. 111, 2326–2337 (2014). Copyright 2014 Elsevier.74
D. ECM stiffness within the ovarian cancer mechanical microenvironment
An additional prominent feature of a cell's mechanical microenvironment is the rigidity of its ECM. Cells can perceive the surrounding stiffness of their microenvironment and its modulation has been shown to heavily influence phenotype,77,78 protein expression,79,80 and differentiation.81,82 For cancer cells, the stiffness of their surrounding ECM can influence metastasis, invasion, proliferation, and chemoresistance.83–86 Numerous studies have proven that stiffer substrates enhance the metastatic phenotypes of cancer cells.87–91 However, within the field of ovarian cancer, studies have resulted in contradictory findings.
To examine the impact of compliant versus rigid ECM stiffness, McGrail et al. first differentiated human mesenchymal stem cells (MSCs) into either adipocytes or osteoblasts via substrate stiffness. The resulting cell monolayers had differential innate stiffness values, E = 0.9 kPa or E = 2.6 kPa, respectively. These cell layers were then used to analyze ovarian cancer cell preference for adherence and migration patterns. Ovarian cancer cells were found to be more adherent to softer adipocyte substrates with enhanced migratory capacity, as well as being more proliferative and chemoresistant, despite predictions. The Rho-ROCK signaling pathway was crucial to these phenotypic observations. EMT traits were observed on the soft adipocyte cultures where SKOV3 cells exerted traction force and showed an elongated morphology indicating a mesenchymal phenotype. When results were compared to the less metastatic cell line OVCAR3, enhanced adhesion, proliferation, chemoresistance, and migration on the soft substrates were not observed. The OVCAR3 cells only displayed a slight increase in traction forces. Treatment with lysophosphatidic acid (LPA), an activator of Rho and ROCK, induced motility on stiff substrates and collapse of the cells on soft substrates due to hypercontractility. The specific inhibition of ROCK by small-molecule inhibitors Y27632 and H1152 lead to rigidity independent mobility of the ovarian cancer cells. These findings demonstrated the importance of substrate stiffness on ovarian cancer cell phenotype, differing metastatic potentials between cell lines, and the incorporation of the Rho/ROCK pathway in ovarian cancer mechanotransduction.92
To evaluate the importance of investigating cellular-ECM interactions in a 3D environment, varying stiffness 3D constructs were studied by Zhang et al.,93 Loessner et al.,94 and Guo et al.95 The work of Zhang and Loessner both utilized PEG constructs. Zhang et al. investigated hydrogels with three stiffnesses and found that the epithelial ovarian papillary serous cyst-adenocarcinoma cell line,96 HO8910, grew the fastest, formed multicellular spheroids, and adhered preferentially to the medium hydrogel stiffness, of 12 kPa.93 The PEG gel investigated by Loessner et al. incorporated both MMP cleavable sites and arginylglycylaspartic acid (RGD) motifs to enhance cell attachment and allow cell motility. The 3D cultures formed spheroids and exhibited higher chemoresistance in 3D vs 2D culture. Enhanced proliferation was found in the 2D cultures and OV-MZ-6 3D cultures (a serous adenocarcinoma ovarian cancer cell line).97 Within 3D culture, cells increased the expression of α3/α5/α1 integrin surface receptors as well as MMP9 production. Greater proliferation was found on RGD or MMP functionalized hydrogels compared to the PEG gels alone, and less proliferation was found on stiffer hydrogel constructs.94 The contradictory finding of enhanced proliferation and cell aggregation within the stiffer constructs was observed in an investigation by Guo et al.95 As the 3D culture material used in this study consisted of crosslinked egg whites as opposed to PEG hydrogels, the conclusions from this study are not directly comparable94 to those of Zhang and Loessner et al.
Overall, these findings point towards a preference of softer substrates for ovarian cancer growth and metastatic advancement. A detailed layout of the experiments and conclusions for ovarian cancer stiffness effects can be found in Table III. Given the minimal number of ovarian cancer ECM stiffness investigations and contradictory evidence, further studies are needed to deepen our understanding of the role of substrate stiffness in ovarian cancer mechanotransduction.
TABLE III.
Ovarian cancer specific stiffness investigations and major findings.
| 2D/3D culture | Material | Stiffness (kPa) | Cell type | Findings | References |
|---|---|---|---|---|---|
| 3D | PEG hydrogel with RGD and MMP degradable motifs | 12.01, 0.241 | OV-MZ-6 SKOV3 | • 3D culture • Spheroid formation • Higher chemoresistance • Increased expression: a3/a5/b1 integrins and MMP9 • Less proliferation in stiffer gels • Greater proliferation in RGD or MMP functionalized hydrogels • 2D culture • Enhanced proliferation | Loessner et al.94 |
| 3D | PEG crosslinked poly(vinyl ether-co-maleic acid) hydrogel | 2.19–105.1 | HO8910 | • Multicellular spheroid formation• Gel with 12.02 kPa stiffness • Fastest cell growth • Best cell adherence | Zhang et al.93 |
| 2D | Human mesenchymal stem cells differentiated to soft and stiff adipocytes and osteoblast monolayers on polyacrylamide substrates | Adipocytes (E = 0.9) | SKOV3 OVCAR3 | • SKOV3 on soft substrate • Increased adherence to softer substrates • More proliferative and chemoresistant • Enhanced EMT and traction forces • Elongated morphology• OVCAR3 on soft substrate • Slight increase in traction forces• Rho/ROCK dependent phenotypes | McGrail et al.92 |
| Osteoblasts (E = 2.6) | |||||
| Polyacrylamide: 2.83, 34.88 | |||||
| 3D | Egg white and poly[(methyl vinyl ether)-alt-(maleic acid)] | G′ range | SKOV3 | • Enhanced proliferation in stiffer samples• Greater cell aggregation in stiffer samples | Guo et al.95 |
| 0.00121–0.06328 | |||||
| G″ range | |||||
| 0.00043–0.01362 |
It may be beneficial to consider the prominent pathways affected by ECM stiffness in other cancer malignancies as potential starting points of investigation in ovarian cancers. Some prominent pathways modulated by substrate stiffness in cancer include YAP/TAZ, Rho/ROCK, Cav1, and FAK/PI3K/Akt. The transcription factors YAP (Yes-associated protein) and TAZ (transcriptional coactivator with a PDZ-binding motif) have been shown to be heavily associated with ECM stiffness, cell spreading, and stress fiber activity.98–100 Additionally, YAP/TAZ is implicated in many important cancer hallmarks including proliferation, metastasis, and stem cell-like behavior.101,102 As ovarian cancer experiences an environment with variable stiffness, the YAP/TAZ pathway is a point of interest for future mechanotransduction studies. The Rho/Rock pathway has already been tied to stiffness effects on ovarian cancer cells92 and it has been established as a well-known factor in both mechanotransduction and cancer progression for a variety of tumor types.103–107 Caveolin-1 has been shown to be essential for stiffness sensing, and thus when silenced, tumor cells are able to proliferate and migrate independent of the rigidity of the surrounding ECM.108 However, these claims appear dependent on the cancer cell type, as confounding evidence has been demonstrated regarding their contribution to tumor growth and metastasis.109–111 Ovarian cancer studies concerning Cav-1 have shown it to be downregulated in both primary cells and immortalized cell lines, indicating its likely action as a tumor suppressor.112–115 However, these studies have yet to correlate Cav-1 to ECM stiffness. Upregulation of the FAK-PI3K/Akt pathway has been attributed to enhanced ovarian cancer migration and invasion.116 It is also a known pathway in mechanotransduction activation through stiffness modulation.117 Therefore, future ovarian cancer studies must study the activation of this pathway in conjunction with ECM stiffness.
Most mechanotransduction pathways involving stiffness are highly integrated, thereby making them quite complex, and as a result, difficult to study. However, the correlation that ECM stiffness has with cancer metastasis also makes it a promising avenue for new and innovative ovarian cancer treatments. As a complete examination of cancer mechanotransduction pathways is beyond the scope of this review, additional details on the influence of ECM stiffness can be found in the works by Pathak and Kumar,87 Spill et al.,101 and Chin et al.118
III. RELATING IN VITRO MECHANOTRANSDUCTION RESULTS TO IN VIVO PATIENT OUTCOMES
The exploration of mechanotransduction within ovarian cancer is still in its infancy. However, current findings reiterate the urgency of expanding this field for furthering the development of drug targets within metastasis, chemoresistance, and tumor recurrence pathways. The overlap of clinical and laboratory based findings consistently hint at the important role of mechanotransduction in the progression of ovarian cancer.
The direct impact of mechanotransduction on ovarian cancer and its associated pathways remains vastly unknown both in vitro and in vivo. Clinical research has shown that side populations of ovarian cancer cells found within the ascites can display the characteristics of both EMT and stem cell-like behavior.119–121 EMT is an important part of ovarian cancer progression, in which free floating spheroids attach to the mesothelium, disseminate and metastasize to surrounding tissues.122,123 Expression of CD44 and CA125, high levels of IL-6, CXR4, and CXCL12 and the amplification of PIK3CA, Akt and bone morphogenetic protein (BMP) pathways have been associated with ovarian cancer EMT.17,124,125 The review by Tan et al. provides an in-depth look at epithelial ovarian cancer metastasis.28 Recent clinical studies and xenograft research have shown that side populations of ovarian cancer within the ascites display characteristics of CSCs.126,127 These ovarian CSCs have heightened chemoresistance, the ability to asymmetrically proliferate, and the capacity to self-renew.
Research done in vivo on the ascites of ovarian cancer patients has shown that the formation of non-adherent spheroids within the ascites may be correlated to the recurrence of the disease. These non-adherent spheroids express high levels of CSC markers EpCAM, STAT3, and Oct4, as well as CA125.34 The upregulation of ovarian stem cell markers CD44 and CD177/c-Kit has been shown to be attributed to side populations within the ascites.128,129 The ABC transporter protein ABCG2/BCRP1 has also been shown to have a high expression in ovarian cancer cells found within the ascites.36,37,129 From these investigations, it is evident that the ascites facilitate an enhanced expression of chemoresistance, stem cell-like behavior, and metastasis in ovarian cancer. Preliminary findings seem to suggest that mechanotransduction plays an important role in this shift of phenotype, as evident through the commonality of markers and pathways modulated both in vitro and in vivo. However, further proof is necessary to corroborate these findings and develop new targets for the next generation of ovarian cancer treatments. Future studies will integrate the in vitro and in vivo data to direct research into treatment regimens that take mechanotransduction into consideration.
IV. CONCLUSION AND FUTURE DIRECTIONS
Over the last 20 years, a new narrative has begun to emerge implicating mechanotransduction in the metastasis of ovarian cancer and the promotion of a CSC-like side population within the ascites. A gap in our understanding of ovarian cancer pathology is evident; one that must be bridged before treatment of the disease can be improved. It is well known that isolation in the peritoneal cavity allows ovarian cancer to progress into more advanced stages of disease, as well as disseminate to distant parts of the body. Correspondingly, the peritoneal cavity is a dynamic space, one that continuously changes shape and stimulates ovarian cancer cells with high levels of shear stress. In vitro models that can simulate the microenvironment are necessary to explore the effects of mechanotransduction on ovarian cancer in detail. With in vitro mechanical stress bioreactors, stresses can be isolated, explored, and used as a platform to test drug efficacy.
When designing bioreactors for ovarian cancer mechanotransduction investigations, there are several additional factors that should be considered. Beyond force stimulation and application duration, the other cell types present in ascites may be an additional avenue of investigation. The cell type distribution within ascites typically consists of 37% lymphocytes, 29% mesothelial cells, 32% macrophages and <0.1% adenocarcinoma cells.12 Investigations using a coculture of ovarian cancer, stromal, and immune cell types should be performed concurrently with force stimulus found within the peritoneal cavity. With this combinatory approach, it will be possible to gain a more complete picture of the cancer microenvironment and ascertain potential avenues of treatment. Additionally, non-cell factors such as chemotaxis,130 3D culture94,131–136, and hypoxia137 should be considered for future investigations, in conjunction with mechanical cues to create a microenvironment that can more fully recapitulate in vivo conditions. The study of ovarian cancer mechanotransduction promises to improve patient treatment through future investigations that utilize designs pertinent to the specific microenvironment.
The field of mechanotransduction in ovarian cancer is still growing. Future investigations are needed to accurately model the forces present in the peritoneal cavity. Computer aided simulations modeling shear stress in the ascites and direct measurements of tissue stiffness will provide a strong foundation for all future exploration into the mechanobiology of this field. Limited experiments have been performed to show how ECM stiffness may affect ovarian cancer, consequently, more robust studies are needed to show the role of stiffness in ovarian cancer biology. The few studies modeling shear stresses on ovarian cancer have shown promising results, where the promotion of EMT, chemoresistance and CSC surface markers is evident. These results have a wide impact on the future of ovarian oncology and the potential process for drug screening. Mechanotransduction might yet prove to be the key to improving the clinical outcomes in ovarian cancers.
ACKNOWLEDGMENTS
This material is based upon work supported by the DOD OCRP Early Career Investigator Award No. W81XWH-13-1-0134 and DOD Pilot Award No. W81XWH-16-1-0426. This research was supported by grants from the Rivkin Center for Ovarian Cancer and the Michigan Ovarian Cancer Alliance (MIOCA). C.M.N. was supported by the National Science Foundation Graduate Research Fellowship under Grant No. 1256260.
The authors declare no potential conflicts of interest.
References
- 1. Siegel R. L., Miller K. D., and Jemal A., “Cancer statistics, 2016,” Cancer J. Clin. 66, 7–30 (2016). 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.See https://ocrfa.org/patients/about-ovarian-cancer/treatment/staging-and-grading/stage-4/ for Stage IV, Ovarian Cancer Research Fund Alliance, 2018; accessed 17 January 2018.
- 3. Nik N. N., Vang R., Shih I.-M., and Kurman R. J., “Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma,” Annu. Rev. Pathol. Mech. Dis. 9, 27–45 (2014). 10.1146/annurev-pathol-020712-163949 [DOI] [PubMed] [Google Scholar]
- 4. Matei D. et al. , “Imatinib mesylate in combination with docetaxel for the treatment of patients with advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis,” Cancer 113, 723–732 (2008). 10.1002/cncr.23605 [DOI] [PubMed] [Google Scholar]
- 5. Thibault B., Castells M., Delord J.-P., and Couderc B., “Ovarian cancer microenvironment: Implications for cancer dissemination and chemoresistance acquisition,” Cancer Metastasis Rev. 33, 17–39 (2014). 10.1007/s10555-013-9456-2 [DOI] [PubMed] [Google Scholar]
- 6. Dinkelspiel H. E. et al. , “Long-term mortality among women with epithelial ovarian cancer,” Gynecol. Oncol. 138, 421–428 (2015). 10.1016/j.ygyno.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.See https://seer.cancer.gov/statfacts/html/ovary.html for Ovarian cancer–Cancer stat facts, National Cancer Institute; accessed 25 January 2018.
- 8. Yeung T. L. et al. , “Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: Cell and molecular processes in cancer metastasis,” Am. J. Physiol.—Cell Physiol. 309, C444–C456 (2015). 10.1152/ajpcell.00188.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foley O., Alejandro Rauh-Hain J., and Del Carmen M. G., “Recurrent epithelial ovarian cancer: An update on treatment,” Cancer Network 27, 288–294 (2013). [PubMed] [Google Scholar]
- 10. Ng A. and Barker N., “Ovary and fimbrial stem cells: Biology, niche and cancer origins,” Nat. Rev. Mol. Cell Biol. 16, 625–638 (2015). 10.1038/nrm4056 [DOI] [PubMed] [Google Scholar]
- 11. Erickson B. K., Conner M. G., and C. N. Landen, Jr. , “The role of the fallopian tube in the origin of ovarian cancer,” Am. J. Obstet. Gynecol. 209, 409–414 (2013). 10.1016/j.ajog.2013.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaldawy A. et al. , “Low-grade serous ovarian cancer: A review,” Gynecol. Oncol. 143, 433 (2016). 10.1016/j.ygyno.2016.08.320 [DOI] [PubMed] [Google Scholar]
- 13.PDQ Adult Treatment Editorial Board, “Ovarian epithelial, fallopian tube, and primary peritoneal cancer treatment (PDQ®): Health professional version,” in PDQ Cancer Information Summaries ( National Cancer Institute, US, 2002). [PubMed] [Google Scholar]
- 14. Helm C. W. and Edwards R., Ovarian Cancer Staging: TNM and FIGO Classifications for Ovarian Cancer ( Medscape, 2017). [Google Scholar]
- 15. Javadi S., Ganeshan D. M., Qayyum A., Iyer R. B., and Bhosale P., “Ovarian cancer, the revised FIGO staging system, and the role of imaging,” Am. J. Roentgenol. 206, 1351–1360 (2016). 10.2214/AJR.15.15199 [DOI] [PubMed] [Google Scholar]
- 16. Cohen M. and Petignat P., “The bright side of ascites in ovarian cancer,” Cell Cycle 13, 2319 (2014). 10.4161/cc.29951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kipps E., Tan D. S. P., and Kaye S. B., “Meeting the challenge of ascites in ovarian cancer: New avenues for therapy and research,” Nat. Rev. Cancer 13, 273–282 (2013). 10.1038/nrc3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin-Hirsch P., Preston N., and Tomlinson A., “Scientific impact paper No. 45: Management of ascites in ovarian cancer patients,” Obstet. Gynaecol. 17, 70–71 (2015). [Google Scholar]
- 19. Yoshikawa T. et al. , “Peritoneal fluid accumulation in healthy men and postmenopausal women: Evaluation on pelvic MRI,” Am. J. Roentgenol. 200, 1181–1185 (2013). 10.2214/AJR.12.9645 [DOI] [PubMed] [Google Scholar]
- 20. Ayantunde A. A. and Parsons S. L., “Pattern and prognostic factors in patients with malignant ascites: A retrospective study,” Ann. Oncol. 18, 945–949 (2007). 10.1093/annonc/mdl499 [DOI] [PubMed] [Google Scholar]
- 21. Auersperg N., Ota T., and Mitchell G. W. E., “Early events in ovarian epithelial carcinogenesis: Progress and problems in experimental approaches,” Int. J. Gynecol. Cancer 12, 691–703 (2002). 10.1046/j.1525-1438.2002.01152.x [DOI] [PubMed] [Google Scholar]
- 22. Cvetkovic D., “Early events in ovarian oncogenesis,” Reprod. Biol. Endocrinol. 1, 68 (2003). 10.1186/1477-7827-1-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pedersen J. S., Bendtsen F., and Møller S., “Management of cirrhotic ascites,” Ther. Adv. Chronic Dis. 6, 124–137 (2015). 10.1177/2040622315580069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagy J. A., Herzberg K. T., Dvorak J. M., and Dvorak H. F., “Pathogenesis of malignant ascites formation: Initiating events that lead to fluid accumulation,” Cancer Res. 53, 2631–2643 (1993). [PubMed] [Google Scholar]
- 25. Puiffe M. L. et al. , “Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer,” Neoplasia 9, 820–829 (2007). 10.1593/neo.07472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feldman G. B., Knapp R. C., Order S. E., and Hellman S., “The role of lymphatic obstruction in the formation of ascites in a murine ovarian carcinoma,” Cancer Res. 32, 1663–1666 (1972). [PubMed] [Google Scholar]
- 27. Holm-Nielsen P., “Pathogenesis of ascites in peritoneal carcinomatosis,” Acta Pathol. Microbiol. Scand. Banner 33, 10–21 (1953). 10.1111/j.1699-0463.1953.tb04805.x [DOI] [PubMed] [Google Scholar]
- 28. Tan D. S., Agarwal R., and Kaye S. B., “Mechanisms of transcoelomic metastasis in ovarian cancer,” Lancet Oncol. 7, 925–934 (2006). 10.1016/S1470-2045(06)70939-1 [DOI] [PubMed] [Google Scholar]
- 29. Ip C. K. M. et al. , “Stemness and chemoresistance in epithelial ovarian carcinoma cells under shear stress,” Sci. Rep. 6, 26788 (2016) 10.1038/srep26788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mo L. et al. , “Ascites increases expression/function of multidrug resistance proteins in ovarian cancer cells,” PLoS One 10, e0131579 (2015). 10.1371/journal.pone.0131579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rizvi I. et al. , “Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules,” Proc. Natl. Acad. Sci. 110, E1974–E1983 (2013). 10.1073/pnas.1216989110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lengyel E., “Ovarian cancer development and metastasis,” Am. J. Pathol. 177, 1053–1064 (2010). 10.2353/ajpath.2010.100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Healy J. C. and Reznek R. H., “The peritoneum, mesenteries and omenta: Normal anatomy and pathological processes,” Eur. Radiol. 8, 886–900 (1998). 10.1007/s003300050485 [DOI] [PubMed] [Google Scholar]
- 34. Latifi A. et al. , “Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: Molecular phenotype of chemoresistant ovarian tumors,” PLoS One 7, e46858 (2012). 10.1371/journal.pone.0046858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He Q. et al. , “Isolation and characterization of cancer stem cells from high-grade serous ovarian carcinomas,” Cell. Physiol. Biochem. 33, 173–184 (2014). 10.1159/000356660 [DOI] [PubMed] [Google Scholar]
- 36. Szotek P. P. et al. , “Ovarian cancer side population defines cells with stem cell-like characteristics and mullerian inhibiting substance responsiveness,” Proc. Natl. Acad. Sci. U. S. A. 103, 11154–11159 (2006). 10.1073/pnas.0603672103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu L., McArthur C., and Jaffe R. B., “Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant,” Br. J. Cancer 102, 1276–1283 (2010). 10.1038/sj.bjc.6605626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rizzo S. et al. , “Ovarian cancer stem cell–like side populations are enriched following chemotherapy and overexpress EZH2,” Mol. Cancer Ther. 10, 325–335 (2011). 10.1158/1535-7163.MCT-10-0788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burgos-Ojeda D., Rueda B. R., and Buckanovich R. J., “Ovarian cancer stem cell markers: Prognostic and therapeutic implications,” Cancer Lett. 322, 1–7 (2012). 10.1016/j.canlet.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mo L. et al. , “Syngeneic murine ovarian cancer model reveals that ascites enriches for ovarian cancer stem-like cells expressing membrane GRP78,” Mol. Cancer Ther. 14, 747–756 (2015). 10.1158/1535-7163.MCT-14-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carduner L. et al. , “Ascites-induced shift along epithelial-mesenchymal spectrum in ovarian cancer cells: Enhancement of their invasive behavior partly dependant on αv integrins,” Clin. Exp. Metastasis 31, 675–688 (2014). 10.1007/s10585-014-9658-1 [DOI] [PubMed] [Google Scholar]
- 42. Leng R., Liao G., Wang H., Kuang J., and Tang L., “Rac1 expression in epithelial ovarian cancer: Effect on cell EMT and clinical outcome,” Med. Oncol. 32, 1–12 (2015) 10.1007/s12032-014-0329-5. [DOI] [PubMed] [Google Scholar]
- 43. Ahmed N., Abubaker K., Findlay J., and Quinn M., “Epithelial mesenchymal transition and cancer stem cell-like phenotypes facilitate chemoresistance in recurrent ovarian cancer,” Curr. Cancer Drug Targets 10, 268–278 (2010). 10.2174/156800910791190175 [DOI] [PubMed] [Google Scholar]
- 44. Arend R. C., Londoño-Joshi A. I., J. M. Straughn, Jr. , and Buchsbaum D. J., “The Wnt/β-catenin pathway in ovarian cancer: A review,” Gynecol. Oncol. 131, 772–779 (2013). 10.1016/j.ygyno.2013.09.034 [DOI] [PubMed] [Google Scholar]
- 45. Avraham-Chakim L. et al. , “Fluid-flow induced wall shear stress and epithelial ovarian cancer peritoneal spreading,” PLoS One 8, e60965 (2013). 10.1371/journal.pone.0060965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jain R. K., Martin J. D., and Stylianopoulos T., “The role of mechanical forces in tumor growth and therapy,” Annu. Rev. Biomed. Eng. 16, 321–346 (2014). 10.1146/annurev-bioeng-071813-105259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jeffrey B., Udaykumar H. S., and Schulze K. S., “Flow fields generated by peristaltic reflex in isolated guinea pig ileum: Impact of contraction depth and shoulders,” Am. J. Physiol.–Gastrointestinal Liver Physiol. 285, G907–G918 (2003). 10.1152/ajpgi.00062.2003 [DOI] [PubMed] [Google Scholar]
- 48. Mitchell M. J. and King M. R., “Fluid shear stress sensitizes cancer cells to receptor-mediated apoptosis via trimeric death receptors,” New J. Phys. 15, 015008 (2013). 10.1088/1367-2630/15/1/015008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rotenberg M. Y., Ruvinov E., Armoza A., and Cohen S., “A multi-shear perfusion bioreactor for investigating shear stress effects in endothelial cell constructs,” Lab Chip 12, 2696–2703 (2012). 10.1039/c2lc40144d [DOI] [PubMed] [Google Scholar]
- 50. Moss M. S., Sisken B., Zimmer S., and Anderson K. W., “Adhesion of nonmetastatic and highly metastatic breast cancer cells to endothelial cells exposed to shear stress,” Biorheology 36, 359–371 (1999). [PubMed] [Google Scholar]
- 51. Xiong N. et al. , “Involvement of caveolin-1 in low shear stress-induced breast cancer cell motility and adhesion: Roles of FAK/Src and ROCK/p-MLC pathways,” Biochim. Biophys. Acta 1864, 12–22 (2017). 10.1016/j.bbamcr.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 52. Domcke S., Sinha R., Levine D. A., Sander C., and Schultz N., “Evaluating cell lines as tumour models by comparison of genomic profiles,” Nat. Commun. 4, 2126 (2013) 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hyler A. R. et al. , “Fluid shear stress impacts ovarian cancer cell viability, subcellular organization, and promotes genomic instability,” PLoS One 13, e0194170 (2018). 10.1371/journal.pone.0194170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Egan K., Cooke N., and Kenny D., “Living in shear: Platelets protect cancer cells from shear induced damage,” Clin. Exp. Metastasis 31, 697–704 (2014). 10.1007/s10585-014-9660-7 [DOI] [PubMed] [Google Scholar]
- 55. Giavazzi R., Foppolo M., Dossi R., and Remuzzi A., “Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions,” J. Clin. Invest. 92, 3038–3044 (1993). 10.1172/JCI116928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cavazzoni E., Bugiantella W., Graziosi L., Franceschini M. S., and Donini A., “Malignant ascites: Pathophysiology and treatment,” Int. J. Clin. Oncol. 18, 1–9 (2013). 10.1007/s10147-012-0396-6 [DOI] [PubMed] [Google Scholar]
- 57. Mitchell M. J. and King M. R., “Computational and experimental models of cancer cell response to fluid shear stress,” Front. Oncol. 3, 44 (2013) 10.3389/fonc.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gomes N., Legrand C., and Lafeve F. F., “Shear stress induced release of von Willebrand factor and thrombospondin-1 in Uvec extracellular matrix enhances breast tumour cell adhesion,” Clin. Exp. Metastasis 22, 215–223 (2005). 10.1007/s10585-005-7359-5 [DOI] [PubMed] [Google Scholar]
- 59. Kawai Y., Kaidoh M., Yokoyama Y., and Ohhashi T., “Cell surface F1/Fo ATP synthase contributes to interstitial flow-mediated development of the acidic microenvironment in tumor tissues,” Am. J. Physiol.—Cell Physiol. 305, C1139–C1150 (2013). 10.1152/ajpcell.00199.2013 [DOI] [PubMed] [Google Scholar]
- 60. Triantafillu U. L., Park S., Klaassen N. L., Raddatz A. D., and Kim Y., “Fluid shear stress induces cancer stem cell-like phenotype in MCF7 breast cancer cell line without inducing epithelial to mesenchymal transition,” Int. J. Oncol. 50, 993–1001 (2017) 10.3892/ijo.2017.3865. [DOI] [PubMed] [Google Scholar]
- 61. Polacheck W. J., Charest J. L., and Kamm R. D., “Interstitial flow influences direction of tumor cell migration through competing mechanisms,” Proc. Natl. Acad. Sci. 108, 11115–11120 (2011). 10.1073/pnas.1103581108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haessler U., Teo J. C. M., Foretay D., Renaud P., and Swartz M. A., “Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber,” Integr. Biol. 4, 401–409 (2012). 10.1039/C1IB00128K [DOI] [PubMed] [Google Scholar]
- 63. Yang H. et al. , “Mechanosensitive caveolin-1 activation-induced PI3K/Akt/mTOR signaling pathway promotes breast cancer motility, invadopodia formation and metastasis in vivo,” Oncotarget 7, 16227–16247 (2016) 10.18632/oncotarget.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhao F. et al. , “Roles for GP IIb/IIIa and [alpha]v[beta]3 integrins in MDA-MB-231 cell invasion and shear flow-induced cancer cell mechanotransduction,” Cancer Lett. 344, 62–73 (2014). 10.1016/j.canlet.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 65. Qazi H., Shi Z.-D., and Tarbell J. M., “Fluid shear stress regulates the invasive potential of glioma cells via modulation of migratory activity and matrix metalloproteinase expression,” PLoS One 6, e20348 (2011). 10.1371/journal.pone.0020348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee H. J. et al. , “Fluid shear stress activates YAP1 to promote cancer cell motility,” Nat. Commun. 8, 14122 (2017). 10.1038/ncomms14122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chotard-Ghodsnia R. et al. , “Morphological analysis of tumor cell/endothelial cell interactions under shear flow,” J. Biomech. Kidlington 40, 335–344 (2007). 10.1016/j.jbiomech.2006.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Avvisato C. L. et al. , “Mechanical force modulates global gene expression and β-catenin signaling in colon cancer cells,” J. Cell Sci. 120, 2672–2682 (2007). 10.1242/jcs.03476 [DOI] [PubMed] [Google Scholar]
- 69. Chen S. H., Dallas M. R., Balzer E. M., and Konstantopoulos K., “Mucin 16 is a functional selectin ligand on pancreatic cancer cells,” FASEB J. 26, 1349–1359 (2011) 10.1096/fj.11-195669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Raimondi M. T. et al. , “The effect of hydrodynamic shear on 3D engineered chondrocyte systems subject to direct perfusion,” Biorheology 43, 215–222 (2006). [PubMed] [Google Scholar]
- 71. Tilles A. W., Baskaran H., Roy P., Yarmush M. L., and Toner M., “Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor,” Biotechnol. Bioeng. 73, 379–389 (2001). 10.1002/bit.1071 [DOI] [PubMed] [Google Scholar]
- 72. Jeong S. I. et al. , “Mechano-active tissue engineering of vascular smooth muscle using pulsatile perfusion bioreactors and elastic PLCL scaffolds,” Biomaterials 26, 1405–1411 (2005). 10.1016/j.biomaterials.2004.04.036 [DOI] [PubMed] [Google Scholar]
- 73. Lesman A., Blinder Y., and Levenberg S., “Modeling of flow-induced shear stress applied on 3D cellular scaffolds: Implications for vascular tissue engineering,” Biotechnol. Bioeng. 105, 645–654 (2010). 10.1002/bit.22555 [DOI] [PubMed] [Google Scholar]
- 74. Mahler G. J., Frendl C. M., Cao Q., and Butcher J. T., “Effects of shear stress pattern and magnitude on mesenchymal transformation and invasion of aortic valve endothelial cells,” Biotechnol. Bioeng. 111, 2326–2337 (2014). 10.1002/bit.25291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dai G. et al. , “Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature,” Proc. Natl. Acad. Sci. U. S. A. 101, 14871–14876 (2004). 10.1073/pnas.0406073101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dardik A. et al. , “Differential effects of orbital and laminar shear stress on endothelial cells,” J. Vasc. Surg. 41, 869–880 (2005). 10.1016/j.jvs.2005.01.020 [DOI] [PubMed] [Google Scholar]
- 77. Yim E. K. F., Darling E. M., Kulangara K., Guilak F., and Leong K. W., “Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells,” Biomaterials 31, 1299–1306 (2010). 10.1016/j.biomaterials.2009.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang N. and Ingber D. E., “Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension,” Biophys. J. 66, 2181–2189 (1994). 10.1016/S0006-3495(94)81014-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yamamura N., Sudo R., Ikeda M., and Tanishita K., “Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells,” Tissue Eng. 13, 1443–1453 (2007). 10.1089/ten.2006.0333 [DOI] [PubMed] [Google Scholar]
- 80. Santos L. et al. , “Extracellular stiffness modulates the expression of functional proteins and growth factors in endothelial cells,” Adv. Healthcare Mater. 4, 2056–2063 (2015). 10.1002/adhm.201500338 [DOI] [PubMed] [Google Scholar]
- 81. Wells R. G., “The role of matrix stiffness in regulating cell behavior,” Hepatology 47, 1394–1400 (2008). 10.1002/hep.22193 [DOI] [PubMed] [Google Scholar]
- 82. Mao A. S., Shin J.-W., and Mooney D. J., “Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation,” Biomaterials 98, 184–191 (2016). 10.1016/j.biomaterials.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cassereau L., Miroshnikova Y. A., Ou G., Lakins J., and Weaver V. M., “A 3D tension bioreactor platform to study the interplay between ECM stiffness and tumor phenotype,” J. Biotechnol. 193, 66–69 (2015). 10.1016/j.jbiotec.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Seewaldt V., “ECM stiffness paves the way for tumor cells,” Nat. Med. 20, 332–333 (2014). 10.1038/nm.3523 [DOI] [PubMed] [Google Scholar]
- 85. Shieh A. C., “Biomechanical forces shape the tumor microenvironment,” Ann. Biomed. Eng. 39, 1379–1389 (2011). 10.1007/s10439-011-0252-2 [DOI] [PubMed] [Google Scholar]
- 86. Zustiak S. P. et al. , “Three-dimensional matrix stiffness and adhesive ligands affect cancer cell response to toxins,” Biotechnol. Bioeng. 113, 443–452 (2016). 10.1002/bit.25709 [DOI] [PubMed] [Google Scholar]
- 87. Pathak A. and Kumar S., “Biophysical regulation of tumor cell invasion: Moving beyond matrix stiffness,” Integr. Biol. 3, 267–278 (2011). 10.1039/c0ib00095g [DOI] [PubMed] [Google Scholar]
- 88. Schedin P. and Keely P. J., “Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression,” Cold Spring Harb. Perspect. Biol. 3, a003228 (2011) 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schrader J. et al. , “Matrix stiffness modulates proliferation, chemotherapeutic response and dormancy in hepatocellular carcinoma cells,” Hepatology 53, 1192–1205 (2011). 10.1002/hep.24108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ulrich T. A., Pardo E. M., de J., and Kumar S., “The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells,” Cancer Res. 69, 4167–4174 (2009). 10.1158/0008-5472.CAN-08-4859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yoon A. R. et al. , “COX-2 dependent regulation of mechanotransduction in human breast cancer cells,” Cancer Biol. Ther. 16, 430–437 (2015). 10.1080/15384047.2014.1003004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McGrail D. J., Kieu Q. M. N., and Dawson M. R., “The malignancy of metastatic ovarian cancer cells is increased on soft matrices through a mechanosensitive Rho–ROCK pathway,” J. Cell Sci. 127, 2621–2626 (2014). 10.1242/jcs.144378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang T., Chen J., Zhang Q., Dou J., and Gu N., “Poly(ethylene glycol)-cross linked poly(methyl vinyl ether-co-maleic acid)hydrogels for three-dimensional human ovarian cancer cell culture,” Colloids Surf. Physicochem. Eng. Asp. 422, 81–89 (2013). 10.1016/j.colsurfa.2013.01.030 [DOI] [Google Scholar]
- 94. Loessner D. et al. , “Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells,” Biomaterials 31, 8494–8506 (2010). 10.1016/j.biomaterials.2010.07.064 [DOI] [PubMed] [Google Scholar]
- 95. Guo Z. et al. , “The effects of macroporosity and stiffness of poly[(methyl vinyl ether)-alt-(maleic acid)] cross-linked egg white simulations of an aged extracellular matrix on the proliferation of ovarian cancer cells,” RSC Adv. 6, 43892–43900 (2016). 10.1039/C6RA05134K [DOI] [Google Scholar]
- 96. Mou H. Z., Xu S. H., and Zhang Y. Y., “The establishment of human ovarian carcinoma cell line HO-8910 and its characteristics,” Zhonghua Fu Chan Ke Za Zhi 29(164), 162–191 (1994). [PubMed] [Google Scholar]
- 97. Möbus V. et al. , “Morphological, immunohistochemical and biochemical characterization of 6 newly established human ovarian carcinoma cell lines,” Int. J. Cancer 52, 76–84 (1992). 10.1002/ijc.2910520115 [DOI] [PubMed] [Google Scholar]
- 98. Dupont S. et al. , “Role of YAP/TAZ in mechanotransduction,” Nature 474, 179–183 (2011). 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 99. Yuan Y., Zhong W., Ma G., Zhang B., and Tian H., “Yes-associated protein regulates the growth of human non-small cell lung cancer in response to matrix stiffness,” Mol. Med. Rep. 11, 4267–4272 (2015). 10.3892/mmr.2015.3231 [DOI] [PubMed] [Google Scholar]
- 100. Jabbari E., Sarvestani S. K., Daneshian L., and Moeinzadeh S., “Optimum 3D matrix stiffness for maintenance of cancer stem cells is dependent on tissue origin of cancer cells,” PLoS One 10, e0132377 (2015). 10.1371/journal.pone.0132377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Spill F., Rynolds D. S., Kamm R. D., and Zaman M. H., “Impact of the physical microenvironment on tumor progression and metastasis,” Curr. Opin. Biotechnol. 40, 41–48 (2016). 10.1016/j.copbio.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. van Dijik M., Goeransson S. A., and Stroemblad S., “Cell to extracellular matrix interactions and their reciprocal nature in cancer,” Exp. Cell Res. 319, 1663–1670 (2013). 10.1016/j.yexcr.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 103. Bordeleau F. et al. , “Tissue stiffness regulates serine/arginine-rich protein-mediated splicing of the extra domain B-fibronectin isoform in tumors,” Proc. Natl. Acad. Sci. U. S. A. 112, 8314–8319 (2015). 10.1073/pnas.1505421112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kamai T. et al. , “Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer,” Clin. Cancer Res. 9, 2632–2641 (2003). [PubMed] [Google Scholar]
- 105. Li B. et al. , “Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells,” FEBS Lett. 580, 4252–4260 (2006). 10.1016/j.febslet.2006.06.056 [DOI] [PubMed] [Google Scholar]
- 106. Zohrabian V. M., Forzani B., Chau Z., Murali R., and Jhanwar-Uniyal M., “Rho/ROCK and MAPK signaling pathways are involved in glioblastoma cell migration and proliferation,” Anticancer Res. 29, 119–123 (2009). [PubMed] [Google Scholar]
- 107. Malik R., Lelkes P. I., and Cukierman E., “Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer,” Trends Biotechnol. 33, 230–236 (2015). 10.1016/j.tibtech.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lin H. H. et al. , “Mechanical phenotype of cancer cells: Cell softening and loss of stiffness sensing,” Oncotarget 6, 20946–20958 (2015) 10.18632/oncotarget.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu W. R. et al. , “Caveolin-1 promotes tumor growth and metastasis via autophagy inhibition in hepatocellular carcinoma,” Clin. Res. Hepatol. Gastroenterol. 40, 169–178 (2016). 10.1016/j.clinre.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 110. Goetz J. G., Lajoie P., Wiseman S. M., and Nabi I. R., “Caveolin-1 in tumor progression: The good, the bad and the ugly,” Cancer Metastasis Rev. 27, 715–735 (2008). 10.1007/s10555-008-9160-9 [DOI] [PubMed] [Google Scholar]
- 111. Wang Z. et al. , “Caveolin-1, a stress-related oncotarget, in drug resistance,” Oncotarget 6, 37135–37150 (2015) 10.18632/oncotarget.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wiechen K. et al. , “Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene,” Am. J. Pathol. 159, 1635–1643 (2001). 10.1016/S0002-9440(10)63010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lu Z., Ghosh S., Wang Z., and Hunter T., “Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion,” Cancer Cell 4, 499–515 (2003). 10.1016/S1535-6108(03)00304-0 [DOI] [PubMed] [Google Scholar]
- 114. Davidson B. et al. , “Caveolin-1 expression in advanced-stage ovarian carcinoma—A clinicopathologic study,” Gynecol. Oncol. 81, 166–171 (2001). 10.1006/gyno.2001.6156 [DOI] [PubMed] [Google Scholar]
- 115. Bagnoli M. et al. , “Downmodulation of caveolin-1 expression in human ovarian carcinoma is directly related to α-folate receptor overexpression,” Oncogene 19, 4754–4763 (2000). 10.1038/sj.onc.1203839 [DOI] [PubMed] [Google Scholar]
- 116. Yousif N. G., “Fibronectin promotes migration and invasion of ovarian cancer cells through up‐regulation of FAK–PI3K/Akt pathway,” Cell Biol. Int. 38, 85–91 (2013) 10.1002/cbin.10184. [DOI] [PubMed] [Google Scholar]
- 117. Rubashkin M. G. et al. , “Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3,4,5)-triphosphate,” Cancer Res. 74, 4597–4611 (2014). 10.1158/0008-5472.CAN-13-3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chin L., Xia Y., Discher D. E., and Janmey P. A., “Mechanotransduction in cancer,” Curr. Opin. Chem. Eng. 11, 77–84 (2016). 10.1016/j.coche.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. He J., Zhu L., Liu Y., Li D., and Jin Z., “Sequential assembly of 3D perfusable microfluidic hydrogels,” J. Mater. Sci. Mater. Med. 25, 2491–2500 (2014). 10.1007/s10856-014-5270-9 [DOI] [PubMed] [Google Scholar]
- 120. Eyre R. et al. , “Reversing paclitaxel resistance in ovarian cancer cells via inhibition of the ABCB1 expressing side population,” Tumor Biol. 35, 9879–9892 (2014). 10.1007/s13277-014-2277-2 [DOI] [PubMed] [Google Scholar]
- 121. Baba T. et al. , “Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells,” Oncogene 28, 209–218 (2009). 10.1038/onc.2008.374 [DOI] [PubMed] [Google Scholar]
- 122. Yue P. et al. , “Hyperactive EGF receptor, Jaks and Stat3 signaling promote enhanced colony-forming ability, motility and migration of cisplatin-resistant ovarian cancer cells,” Oncogene 31, 2309 (2012). 10.1038/onc.2011.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Klymenko Y., Kim O., and Stack M. S., “Complex determinants of epithelial: Mesenchymal phenotypic plasticity in ovarian cancer,” Cancers 9, 104 (2017) 10.3390/cancers9080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gil O. D. et al. , “Lysophosphatidic acid (LPA) promotes E-cadherin ectodomain shedding and OVCA429 cell invasion in an uPA-dependent manner,” Gynecol. Oncol. 108, 361–369 (2008). 10.1016/j.ygyno.2007.10.027 [DOI] [PubMed] [Google Scholar]
- 125. Al-Alem L. and Curry T. E., “Ovarian cancer: Involvement of the matrix metalloproteinases,” Reproduction 150, R55–R64 (2015). 10.1530/REP-14-0546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Steg A. D. et al. , “Stem cell pathways contribute to clinical chemoresistance in ovarian cancer,” Clin. Cancer Res. 18, 869–881 (2012). 10.1158/1078-0432.CCR-11-2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Skubitz A. et al. , “Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression,” Gynecol. Oncol. 130, 579–587 (2013). 10.1016/j.ygyno.2013.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Curley M. D., Garrett L. A., Schorge J. O., Foster R., and Rueda B. R., “Evidence for cancer stem cells contributing to the pathogenesis of ovarian cancer,” Front. Biosci. 16, 368–392 (2011). 10.2741/3693 [DOI] [PubMed] [Google Scholar]
- 129. Zhang S. et al. , “Identification and characterization of ovarian cancer-initiating cells from primary human tumors,” Cancer Res. 68, 4311–4320 (2008). 10.1158/0008-5472.CAN-08-0364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kuo C. T. et al. , “Modeling of cancer metastasis and drug resistance via biomimetic nano-cilia and microfluidics,” Biomaterials 35, 1562–1571 (2014). 10.1016/j.biomaterials.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 131. Gu L. and Mooney D. J., “Biomaterials and emerging anticancer therapeutics: Engineering the microenvironment,” Nat. Rev. Cancer 16, 56–66 (2016). 10.1038/nrc.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mehta G., Hsiao A. Y., Ingram M., Luker G. D., and Takayama S., “Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy,” J. Controlled Release 164, 192–204 (2012) 10.1016/j.jconrel.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Mehta P., Novak C., Raghavan S., Ward M., and Mehta G., “Self-renewal and CSCs in vitro enrichment: Growth as floating spheres,” in Cancer Stem Cells: Methods and Protocols, edited by Papaccio G. and Desiderio V. ( Springer, New York, 2018), pp. 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Raghavan S. et al. , “Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity,” Oncotarget 7, 16948–16961 (2016) 10.18632/oncotarget.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Raghavan S. et al. , “Personalized medicine based approach to model patterns of chemoresistance and tumor recurrence using ovarian cancer stem cell spheroids,” Clin. Cancer Res. 23, 6934–6945 (2017) 10.1158/1078-0432.CCR-17-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Raghavan S. et al. , “Formation of stable small cell number three-dimensional ovarian cancer spheroids using hanging drop arrays for preclinical drug sensitivity assays,” Gynecol. Oncol. 138, 181–189 (2015). 10.1016/j.ygyno.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Baker A. F. et al. , “Evaluation of a hypoxia regulated gene panel in ovarian cancer,” Cancer Microenviron. 8, 45–56 (2015). 10.1007/s12307-015-0166-x [DOI] [PMC free article] [PubMed] [Google Scholar]