Abstract
Background
One of the various ovarian stimulation regimens used for in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) cycles is the use of recombinant follicle‐stimulating hormone (rFSH) in combination with a gonadotrophin‐releasing hormone (GnRH) analogue. GnRH analogues prevent premature luteinizing hormone (LH) surges. Since they deprive the growing follicles of LH, the question arises as to whether supplementation with recombinant LH (rLH) would increase live birth rates. This is an updated Cochrane Review; the original version was published in 2007.
Objectives
To compare the effectiveness and safety of recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) for ovarian stimulation compared to rFSH alone in women undergoing in‐vitro fertilisation/intracytoplasmic sperm injection (IVF/ICSI).
Search methods
For this update we searched the following databases in June 2016: the Gynaecology and Fertility Group Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO and ongoing trials registers, and checked the references of retrieved articles.
Selection criteria
We included randomised controlled trials (RCTs) comparing rLH combined with rFSH versus rFSH alone in IVF/ISCI cycles.
Data collection and analysis
Two review authors independently selected studies, assessed risk of bias, and extracted data. We combined data to calculate odds ratios (ORs) and 95% confidence intervals (CIs). We assessed statistical heterogeneity using the I2 statistic. We assessed the overall quality of the evidence for the main comparisons using GRADE methods. Our primary outcomes were live birth rate and incidence of ovarian hyperstimulation syndrome (OHSS). Secondary outcomes included ongoing pregnancy rate, miscarriage rate and cancellation rates (for poor response or imminent OHSS).
Main results
We included 36 RCTs (8125 women). The quality of the evidence ranged from very low to moderate. The main limitations were risk of bias (associated with poor reporting of methods) and imprecision.
Live birth rates: There was insufficient evidence to determine whether there was a difference between rLH combined with rFSH versus rFSH alone in live birth rates (OR 1.32, 95% CI 0.85 to 2.06; n = 499; studies = 4; I2 = 63%, very low‐quality evidence). The evidence suggests that if the live birth rate following treatment with rFSH alone is 17% it will be between 15% and 30% using rLH combined with rFSH.
OHSS: There may be little or no difference between rLH combined with rFSH versus rFSH alone in OHSS rates (OR 0.38, 95% CI 0.14 to 1.01; n = 2178; studies = 6; I2 = 10%, low‐quality evidence). The evidence suggests that if the rate of OHSS following treatment with rFSH alone is 1%, it will be between 0% and 1% using rLH combined with rFSH.
Ongoing pregnancy rate: The use of rLH combined with rFSH probably improves ongoing pregnancy rates, compared to rFSH alone (OR 1.20, 95% CI 1.01 to 1.42; participants = 3129; studies = 19; I2 = 2%, moderate‐quality evidence). The evidence suggests that if the ongoing pregnancy rate following treatment with rFSH alone is 21%, it will be between 21% and 27% using rLH combined with rFSH.
Miscarriage rate: The use of rLH combined with rFSH probably makes little or no difference to miscarriage rates, compared to rFSH alone (OR 0.93, 95% CI 0.63 to 1.36; n = 1711; studies = 13; I2 = 0%, moderate‐quality evidence). The evidence suggests that if the miscarriage rate following treatment with rFSH alone is 7%, the miscarriage rate following treatment with rLH combined with rFSH will be between 4% and 9%.
Cancellation rates: There may be little or no difference between rLH combined with rFSH versus rFSH alone in rates of cancellation due to low response (OR 0.77, 95% CI 0.54 to 1.10; n = 2251; studies = 11; I2 = 16%, low quality evidence). The evidence suggests that if the risk of cancellation due to low response following treatment with rFSH alone is 7%, it will be between 4% and 7% using rLH combined with rFSH.
We are uncertain whether use of rLH combined with rFSH improves rates of cancellation due to imminent OHSS compared to rFSH alone. Use of a fixed effect model suggested a benefit in the combination group (OR 0.60, 95% CI 0.40 to 0.89; n = 2976; studies = 8; I2 = 60%, very low quality evidence) but use of a random effects model did not support the conclusion that there was a difference between the groups (OR 0.82, 95% CI 0.34 to 1.97).
Authors' conclusions
We found no clear evidence of a difference between rLH combined with rFSH and rFSH alone in rates of live birth or OHSS. The evidence for these comparisons was of very low‐quality for live birth and low quality for OHSS. We found moderate quality evidence that the use of rLH combined with rFSH may lead to more ongoing pregnancies than rFSH alone. There was also moderate‐quality evidence suggesting little or no difference between the groups in rates of miscarriage. There was no clear evidence of a difference between the groups in rates of cancellation due to low response or imminent OHSS, but the evidence for these outcomes was of low or very low quality.
We conclude that the evidence is insufficient to encourage or discourage stimulation regimens that include rLH combined with rFSH in IVF/ICSI cycles.
Plain language summary
Recombinant luteinizing hormone (rLH) and recombinant follicle‐stimulating hormone (rFSH) for ovarian stimulation in IVF/ICSI cycles
Review question
What is the effectiveness and safety of a combination of recombinant luteinizing hormone (rLH) and recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in women undergoing in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI)?
Background
In natural ovarian cycles, luteinizing hormone and follicle‐stimulating hormone (FSH) are necessary for the maturation of ovarian follicles. One of the various stimulation regimens in IVF or ICSI cycles is ovarian stimulation with rFSH in combination with a gonadotrophin‐releasing hormone (GnRH) analogue. GnRH analogues prevent premature luteinizing hormone surges. Since they deprive the growing follicles of luteinizing hormone, the question arises as to whether supplementation with recombinant luteinizing hormone (rLH) would increase live birth rates.
Study characteristics
We found 36 randomized controlled trials comparing rLH combined with rFSH versus rFSH alone among 8125 women undergoing IVF/ICSI. This is an update of a previous Cochrane Review, first published in 2007. The evidence is current to June 2016. Only seven of the 36 studies clearly stated that they were funded by government or research institutes. Six were funded by pharmaceutical companies and the rest did not state their source of funding.
Key results
We found no clear evidence of a difference between rLH combined with rFSH and rFSH alone in rates of live birth or OHSS. The evidence for these comparisons was of very low‐quality for live birth and low quality for OHSS. We found moderate quality evidence that the use of rLH combined with rFSH may lead to more ongoing pregnancies than rFSH alone. There was also moderate‐quality evidence suggesting little or no difference between the groups in rates of miscarriage. There was no clear evidence of a difference between the groups in rates of cancellation due to low response or imminent OHSS, but the evidence for these outcomes was of low or very low quality.
We conclude that the evidence is too limited to encourage or discourage stimulation regimens that include rLH combined with rFSH in IVF/ICSI cycles.
Quality of evidence
The quality of the evidence ranged from very low to moderate. The main limitations were risk of bias (associated with poor reporting of methods) and imprecision.
Summary of findings
Summary of findings for the main comparison. Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) versus rFSH alone.
| Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone | ||||||
| Population: women undergoing ovarian stimulation in IVF or ICSI treatment cycles Settings: assisted reproduction clinics Intervention: rLH combined with rFSH Comparison: rFSH alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| rFSH alone | rLH plus rFSH | |||||
| Live birth rate | 173 per 1000 | 217 per 1000 (151 to 302) | OR 1.32 (0.85 to 2.06) | 499 (4 studies) | ⊕⊕⊝⊝ very low1,2,4 | |
| OHSS incidence | 13 per 1000 | 5 per 1000 (2 to 13) | OR 0.38 (0.14 to 1.01) | 2178 (6 studies) | ⊕⊕⊝⊝ low3 | |
| Ongoing pregnancy rate | 206 per 1000 | 237 per 1000 (207 to 269) | OR 1.20 (1.01 to 1.42) | 3129 (19 studies) | ⊕⊕⊝⊝ moderate2 | |
| Miscarriage rate | 70 per 1000 |
65 per 1000 (45 to 93) |
OR 0.93 (0.63 to 1.36) |
1711 (13 studies) |
⊕⊕⊝⊝ moderate1 |
|
| Cancellation rate for low response | 67 per 1000 | 52 per 1000 (37 to 73) |
OR 0.77 (0.54 to 1.10) |
2251 (11 studies) |
⊕⊕⊝⊝ low1,2 | |
| Cancellation rate for imminent OHSS | 44 per 1000 | 27 per 1000 (18 to 40) |
OR 0.60 (0.40 to 0.89) |
2976 (8 studies) |
⊕⊕⊝⊝ very low2,4,5 | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICSI: intracytoplasmic sperm injection; IVF: in‐vitro fertilisation; OHSS: ovarian hyperstimulation syndrome; OR: Odds ratio; rFSH: recombinant follicle‐stimulating hormone;rLH: recombinant luteinizing hormone. | ||||||
| GRADE Working Group grades of evidence High‐quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to imprecision: effect estimate with wide confidence interval (wider than the interval 0.75 to 1.25) or low event rate. 2 Downgraded one level due to the presence of serious risk of bias in certain domains such as random sequence generation and allocation concealment. 3Downgraded two levels due to very serious imprecision with wide confidence interval (wider than the interval 0.75 to 1.25) and very low event rate.
4 Downgraded one level due to inconsistency (I2 >50%)
5Downgraded one level due to imprecision: findings are sensitive to choice of statistical model and are not statistically significant with use of a random effects model (OR 0.82, 95% CI 0.34 to 1.97)
Background
Description of the condition
About 15% of couples fail to achieve conception after a year of unprotected intercourse (Te Velde 2000). Such couples may choose to undergo an assisted reproductive technology procedure such as in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI).
Description of the intervention
One of the various stimulation regimens in IVF or ICSI consists of daily administration of subcutaneous injections of recombinant follicle‐stimulating hormone (rFSH) to induce multiple follicle growth in the ovaries. An integral part of this stimulation regimen is daily subcutaneous injections of a gonadotrophin‐releasing hormone (GnRH) analogue to prevent a premature luteinizing hormone (LH) surge. Two kinds of GnRH analogues are available, a GnRH agonist or a GnRH antagonist.
The intervention to be compared with this stimulation regimen is the addition of daily subcutaneous injections of recombinant luteinizing hormone (rLH) to rFSH.
How the intervention might work
Growing follicles become increasingly sensitive to, and ultimately dependent on, the presence of both luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) for their development. As described in the classic 'two cell ‐ two gonadotrophin' theory, LH is needed to provide the granulosa cells with androgen precursors for estradiol biosynthesis by FSH (Short 1962). LH is also needed for the resumption of meiosis and for progesterone production after ovulation to sustain the endometrium. The profound pituitary downregulation with GnRH agonists blocks the output of LH for at least 10 days after cessation of the GnRH agonist and deprives the growing follicles completely of LH stimulation during the entire stimulation phase (Broekmans 1992; Smitz 1988), while during downregulation with a GnRH antagonist, the output of LH remains present during the stimulation phase and the blockage of LH takes place periovulatory for only three to five days.
In view of the endocrinology of the normal menstrual cycle and the negative impact of the pituitary downregulation on folliculogenesis, the intervention of ovarian stimulation with rLH combined with rFSH in downregulated IVF/ICSI cycles may have beneficial effects for growing follicles and may lead to better pregnancy outcomes compared to rFSH alone. A meta‐analysis showed that urinary human menopausal gonadotrophins (HMG), a combination of FSH and hCG in a 1:1 ratio, leads to significantly higher rates in live birth and ongoing pregnancy than rFSH in IVF or ICSI cycles, emphasising a possible role for hCG/LH (van Wely 2011).
Why it is important to do this review
This is an update of a review first published in 2007 (Mochtar 2007). International guidelines do not specify a particular stimulation regimen for IVF or ICSI as regimen of first choice (European Society of Human Reproduction and Embryology (ESHRE), American Society for Reproductive Medicine (ASRM), National Institute for Health and Care Excellence (NICE), Nederlandse Vereniging voor Obstetrie en Gynaecologie (NVOG)). Since 2006 a substantial amount of new data on rLH combined with rFSH in comparison to rFSH became available. The continuing uncertainty regarding a role for rLH in ovarian stimulation is still ongoing due to conflicting results from a large number of trials.
Objectives
To compare the effectiveness and safety of recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) for ovarian stimulation compared to rFSH alone in women undergoing in‐vitro fertilisation/intracytoplasmic sperm injection (IVF/ICSI).
Methods
Criteria for considering studies for this review
Types of studies
Truly randomized controlled studies (RCTs) were eligible for inclusion. We excluded pseudo‐randomised studies as they are associated with a high risk of bias (Vail 2003).
Types of participants
Women undergoing in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI).
Types of interventions
We compared recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) to rFSH alone as stimulation protocols in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) followed by embryo transfer.
Types of outcome measures
Primary outcomes
1. Live birth rate; defined as delivery of a live foetus after 20 completed weeks of gestation.
2. Primary safety outcome: incidence of ovarian hyperstimulation syndrome (mild, moderate, or severe).
Secondary outcomes
3. Ongoing pregnancy rate; defined as foetal heartbeat at 12 weeks gestation.
4. Clinical pregnancy rate; defined as gestational sac at ultrasound, with or without foetal heartbeat, any time before 12 weeks gestation.
5. Miscarriage rate; defined as any pregnancy loss before 20 weeks of gestation.
6. Cancellation rate due to low response.
7. Cancellation rate due to imminent ovarian hyperstimulation syndrome.
Search methods for identification of studies
We searched for all relevant studies describing RCTs of women undergoing ovarian stimulation with recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) and rFSH alone for IVF or ICSI, without language restriction. The original search was performed in 2006 and updated in 2010 and 2012. In the latest update, we searched relevant studies from 2012 up to 9 June 2016.
We carried out all searches in consultation with the Gynaecology and Fertility Group (formerly Menstrual Disorders and Subfertility Group (MDSG)) Information Specialist.
Electronic searches
We searched the following electronic databases, trial registers and websites.
The Gynaecology and Fertility (formerly Menstrual Disorders and Subfertility) Group Specialised Register of Controlled Trials (from 2010 to June 2016) (Appendix 1); the Cochrane Central Register of Studies Online (CRSO) (from 2012 to June 2016) (Appendix 2); MEDLINE (from 2012 to June 2016) (Appendix 3); Embase (from 2012 to June 2016) (Appendix 4); and PsycINFO (from 2012 to June 2016) (Appendix 5). The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomized trials, which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). The Embase, PsycINFO and CINAHL searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters).
Other electronic sources of trials included:
trial registers for ongoing and registered trials;
www.ClinicalTrials.gov (a service of the US National Institutes of Health) (up to June 2016);
www.who.int/trialsearch (The World Health Organization International Trials Registry Platform search portal); (up to June 2016)
DARE (Database of Abstracts of Reviews of Effects) on the Cochrane Library at onlinelibrary.wiley.com (for reference lists from relevant non‐Cochrane reviews) (up to June 2016);
the Web of Knowledge (wokinfo.com) (another source of trials and conference abstracts) (June 2016);
OpenGrey ‐ (www.opengrey.eu) for unpublished literature from Europe (up to June 2016);
LILACS database (regional.bvsalud.org) (for trials from the Portuguese and Spanish speaking world) (up to June 2016);
PubMed and Google Scholar (for recent trials not yet indexed in MEDLINE) (up to June 2016).
Searching other resources
We handsearched reference lists of articles retrieved by the search. We also handsearched relevant journals and conference abstracts that are not covered in the Gynaecology and Fertility Group Register, in liaison with the Information Specialist.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, we retrieved the full‐text of all potentially eligible studies. Two review authors (ND and RA) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. Disagreements as to study eligibility were resolved by discussion with a third review author (MM). We documented the selection process with a PRISMA flow chart (Moher 2009; Figure 1).
1.
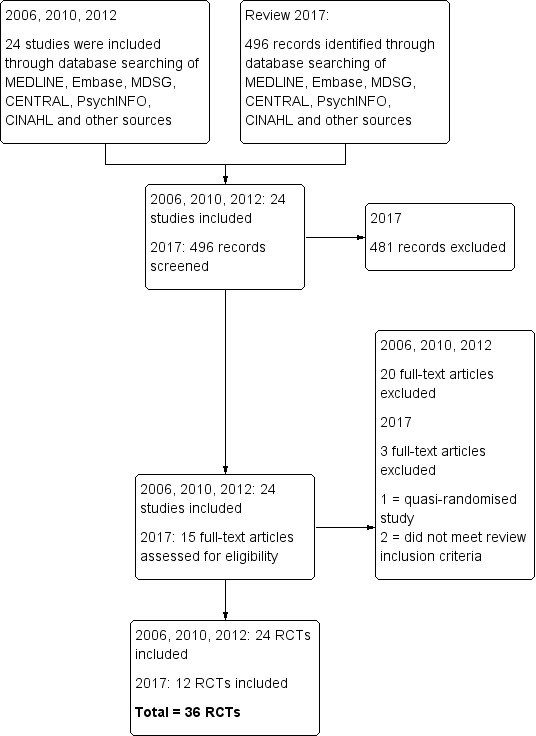
Study flow diagram.
Data extraction and management
Two review authors (ND and RA) independently extracted date from eligible studies using forms designed according to Cochrane guidelines. We resolved any disagreements by discussion or by a third review author (MM). We extracted study characteristics and have presented outcome data from the included studies in the Characteristics of included studies table.
Assessment of risk of bias in included studies
Two authors (ND and RA) independently assessed the included studies for risk of bias using the 'Risk of bias' assessment tool of Cochrane (Higgins 2011). Disagreements were resolved by discussion or by a third review author (MM). We assessed selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias, such as significant differences in demographic characteristics between treatment groups at baseline. We described all judgements and presented the conclusions in the 'Risk of bias' table.
(1) Random sequence generation
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
unclear risk of bias.
(2) Allocation concealment
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. web or telephone randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open list of random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel
No blinding is unlikely to introduce bias, so we assessed the methods at low risk of bias.
(3.2) Blinding of outcome assessment
No blinding is unlikely to introduce bias, so we assessed the methods at low risk of bias.
(4) Incomplete outcome data
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total number of randomized participants), reasons for attrition or exclusion, where reported, and whether missing data were balanced across groups or were related to outcomes.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure from intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias, where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported;
high risk of bias, where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; failure to include results of a key outcome that would have been expected to have been reported;
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
Measures of treatment effect
We performed statistical analyses in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We reported only dichotomous outcomes and for such outcomes; we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs). We presented 95% confidence intervals (CIs) for all outcomes and we used the Review Manager software for statistical analysis (RevMan 2014). For reporting purposes, we translated primary outcomes to absolute risks.
Unit of analysis issues
The primary analysis was ‘per woman randomized’.
Dealing with missing data
We analyzed the data on an intention‐to‐treat basis, as far as possible, and we made attempts to obtain missing data from the original trialists. If data were not obtainable from the trial authors, we assumed that live births had not occurred. For other outcomes, we analyzed only the available data.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I2 statistic. An I2 measurement greater than 50% was taken to indicate substantial heterogeneity (Higgins 2003; Higgins 2011).
Assessment of reporting biases
We aimed to minimise the impact of reporting biases by ensuring a comprehensive search for eligible studies, while being alert to duplication of data. If there were 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small study effects, since there is a tendency for estimates of the intervention effect to be more beneficial in smaller studies.
Data synthesis
If studies were sufficiently similar, we combined the data using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
Where there were sufficient data, we performed subgroup analyses for the following variables, for live birth, ovarian hyperstimulation syndrome, and ongoing pregnancy.
Downregulating agent used for oocyte maturation GnRH agonist, or GnRH antagonist.
Poor ovarian response, defined according to the Bologna criteria (Ferraretti 2011).
Women of advanced age, defined as above 35 years of age.
Where we detected substantial heterogeneity, we explored possible explanations in sensitivity analyses. We took any statistical heterogeneity into account when interpreting the results, especially where there was any variation in the direction of effect.
Sensitivity analysis
Where we identified substantial heterogeneity, we conducted sensitivity analyses. The analyses included the use of a random‐effects model instead of a fixed‐effect model and the use of risk ratios (RRs) rather than ORs.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro GDT (GRADEpro GDT 2014). This table evaluates the overall quality of the body of evidence for all review outcomes (Table 1). We assessed the quality of the evidence using GRADE criteria (Atkins 2004): risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Two review authors working independently, made judgements about evidence quality (high, moderate, low or very low), with disagreements resolved by discussion. We justified, documented, and incorporated judgements into the reporting of results for each outcome.
Results
Description of studies
Results of the search
For the 2017 update, we identified 496 records. We retrieved 15 potentially eligible full‐text articles. Twelve studies met our inclusion criteria (these were in addition to the 24 studies included in the original review in 2007). We excluded three studies because they did not make the comparison of interest (Fei Yang 2013; Fermin 2013) or were not randomized (Barberi 2012). See Characteristics of included studies; Characteristics of excluded studies.
The screening and selection process is presented in a PRISMA flow chart (Moher 2009; Figure 1).
Included studies
Study design and setting
We included a total of 36 RCTs in this update, of which 20 were single‐centred (Abdelmassih 2006; Allegra 2011; Balasch 2001; Barrenetxea 2008; Berkkanoglu 2007; Bosch 2011; Demirol 2005; Fábreques 2006; Fernandez‐Ramirez 2006; Ferraretti 2004; Ferraretti 2014; Griesinger 2005; Humaidan 2004; Kovacs 2010; Levi‐Setti 2006; Lisi 2005; Lisi 2012; Matorras 2009; Razi 2014; Ruvolo 2007), and seven were multicentred (Caserta 2011; De Placido 2005; Van der Houwen 2011; Konig 2013; Musters 2012; Marrs 2003; Nyboe Andersen 2008). In the remaining nine studies this was not reported (Dravid 2015; Evangelio 2011; Fabregues 2011; Mohseni 2013; Nazzaro 2012; Pezzuto 2010; Tarlatzis 2006; Vuong 2015; Younis 2014).
Participants
We included a total of 8125 women undergoing in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) in these studies. Their mean age across studies ranged from 28 to 41 years.
Eight studies included poor responders (De Placido 2005; Demirol 2005; Dravid 2015; Evangelio 2011; Ferraretti 2004; Ferraretti 2014; Ruvolo 2007; Younis 2014). In five studies, poor responders were defined as women with a previous low response in an IVF/ICSI cycle in terms of follicle growth, which was not further specified (De Placido 2005; Ferraretti 2004; Ferraretti 2014; Ruvolo 2007; Younis 2014). One study defined poor responders as women with at least two cycles with one of the following criteria: three oocytes retrieved, three follicles of 16 mm diameter on hCG day and maximal E2 (estradiol) < 500 pg/ml (Demirol 2005). One study defined poor responders on the basis of their low AMH (anti‐mullerian hormone) levels and antral follicle count (Dravid 2015). One other study defined poor responders when they were 37 years or younger or had a basal follicle‐stimulating hormone (FSH) level of > 10 or had four or less follicles in a previous IVF/ICSI cycle (Evangelio 2011).
One study excluded poor responders (defined as having a previous unsuccessful IVF cycle due to two or less oocytes recovered) (Tarlatzis 2006).
Twelve studies included women of advanced age (Allegra 2011; Barrenetxea 2008; Bosch 2011; Fabregues 2011; Fábreques 2006; Konig 2013; Matorras 2009; Musters 2012; Nazzaro 2012; Van der Houwen 2011; Vuong 2015; Younis 2014). Definitions of advanced age varied amongst the studies. Three studies defined advanced age as 35 years or older (Van der Houwen 2011; Vuong 2015; Younis 2014); six studies as between 35 and 41 years of age (Fabregues 2011; Fábreques 2006; Konig 2013; Matorras 2009; Musters 2012; Nazzaro 2012); one study as between 38 and 44 years of age (Allegra 2011); one study as between 36 and 39 years of age (Bosch 2011); and one study as 40 years or older (Barrenetxea 2008).
Interventions
rLH combined with rFSH to rFSH alone in GnRH agonist downregulated cycles
Twenty‐five studies totalling 6100 women compared rLH combined with rFSH to rFSH alone in GnRH agonist downregulated IVF or ICSI cycles (Abdelmassih 2006; Allegra 2011; Balasch 2001; Barrenetxea 2008; Berkkanoglu 2007; Caserta 2011; De Placido 2005; Fabregues 2011; Fábreques 2006; Ferraretti 2004; Ferraretti 2014; Humaidan 2004; Kovacs 2010; Lisi 2005; Lisi 2012; Marrs 2003; Matorras 2009; Mohseni 2013; Musters 2012; Nazzaro 2012; Nyboe Andersen 2008; Pezzuto 2010; Razi 2014; Ruvolo 2007; Tarlatzis 2006).
Nineteen of 25 studies started the GnRH agonist downregulation in the mid luteal phase of the preceding cycle (Abdelmassih 2006; Allegra 2011; Balasch 2001; Caserta 2011; Fabregues 2011; Fábreques 2006; Ferraretti 2004; Ferraretti 2014; Humaidan 2004; Kovacs 2010; Lisi 2005; Lisi 2012; Marrs 2003; Matorras 2009; Mohseni 2013; Musters 2012; Nyboe Andersen 2008; Pezzuto 2010; Razi 2014); and six started in the follicular phase (Barrenetxea 2008; Berkkanoglu 2007; De Placido 2005; Nazzaro 2012; Ruvolo 2007; Tarlatzis 2006).
Seven of the 25 studies started with an initial dose of rFSH for ovarian stimulation of 150 IU with a dose of rLH of 37.5 IU, 75 IU, or 150 IU (Caserta 2011; Ferraretti 2004; Griesinger 2005; Kovacs 2010; Lisi 2005; Lisi 2012; Tarlatzis 2006). Twelve studies used an initial dose for ovarian stimulation of ≥ 225 IU rFSH (Abdelmassih 2006; Allegra 2011; Balasch 2001; Barrenetxea 2008; Berkkanoglu 2007; Fábreques 2006; Ferraretti 2014; Marrs 2003; Matorras 2009; Musters 2012; Nazzaro 2012; Pezzuto 2010); and a rLH dose of 75 IU (Abdelmassih 2006; Allegra 2011; Balasch 2001; Berkkanoglu 2007; Fabregues 2011; Kovacs 2010; Lisi 2005; Lisi 2012; Pezzuto 2010; Tarlatzis 2006); or 150 IU (Barrenetxea 2008; Ferraretti 2004; Humaidan 2004; Musters 2012; Nazzaro 2012). Four studies adjusted the initial rFSH dose (150 IU to 225 IU to 300 IU) and the dose of rLH (75 IU to 150 IU) according to the age of the patient (De Placido 2005; Ferraretti 2004; Humaidan 2004; Nyboe Andersen 2008). Three studies used a stepdown rFSH stimulation protocol: Balasch 2001 used 75 IU rLH or 150 IU rLH; Fábreques 2006 used 150 IU rLH; and Fabregues 2011 used 37.5 IU rLH or 75 IU rLH. In one study the FSH dose was unknown (Mohseni 2013). In four studies, the rLH was started on stimulation day six, two on stimulation day seven and two on stimulation day eight. In two studies the start of rLH depended on follicular response (Mohseni 2013; Tarlatzis 2006). All studies, except Tarlatzis 2006, continued rLH until hCG.
rLH combined with rFSH to rFSH alone in GnRH antagonist downregulated cycles
Eleven studies totaling 2025 women compared rLH combined with rFSH to rFSH alone in GnRH antagonist downregulated IVF or ICSI cycles (Bosch 2011; Demirol 2005; Dravid 2015; Evangelio 2011; Fernandez‐Ramirez 2006; Griesinger 2005; Konig 2013; Levi‐Setti 2006; Van der Houwen 2011; Vuong 2015; Younis 2014).
Ten studies started rLH combined with rFSH together with a GnRH antagonist and continued until day of hCG (Bosch 2011; Demirol 2005; Evangelio 2011; Fernandez‐Ramirez 2006; Griesinger 2005; Konig 2013; Levi‐Setti 2006; Van der Houwen 2011; Vuong 2015; Younis 2014). One study started the GnRH antagonist on stimulation day six (Dravid 2015). Four studies used an initial dose for ovarian stimulation of ≥ 225 IU rFSH (Fernandez‐Ramirez 2006; Konig 2013; Van der Houwen 2011; Younis 2014). Two studies used 225 IU rFSH in the rFSH alone group and 150 IU in the rLH combined with rFSH group (Bosch 2011; Levi‐Setti 2006). One study used 150 IU rFSH in both groups (Dravid 2015). One study used a step‐down rFSH stimulation protocol (Demirol 2005). Two studies adjusted the initial rFSH dose to the antral follicle count (Evangelio 2011; Vuong 2015). In four studies, 75 IU rLH was used (Bosch 2011; Dravid 2015; Fernandez‐Ramirez 2006; Levi‐Setti 2006), and in five studies, 150 IU rLH was used (Demirol 2005; Griesinger 2005; Konig 2013; Van der Houwen 2011; Younis 2014). One study adjusted the rLH dose to the individual patient characteristics in a 1:2 or 1:3 rate to rFSH (Evangelio 2011). Another study supplemented 75 IU rLH or 150 IU rLH (Vuong 2015).
Outcomes
Regarding the primary outcomes on effectiveness and safety, four of the included studies reported the live birth rate (Ferraretti 2004; Ferraretti 2014; Tarlatzis 2006; Vuong 2015), and six studies reported ovarian hyperstimulation syndrome (Bosch 2011; Caserta 2011; Fabregues 2011; Fábreques 2006; Levi‐Setti 2006; Tarlatzis 2006).
A total of 19 studies reported ongoing pregnancy (Balasch 2001, Barrenetxea 2008; Bosch 2011; Demirol 2005; De Placido 2005; Fernandez‐Ramirez 2006; Ferraretti 2004;Griesinger 2005; Van der Houwen 2011; Konig 2013; Kovacs 2010; Levi‐Setti 2006; Lisi 2005; Matorras 2009; Musters 2012; Nyboe Andersen 2008; Ruvolo 2007; Tarlatzis 2006); 23 studies reported on clinical pregnancy (Abdelmassih 2006; Allegra 2011; Balasch 2001, Caserta 2011; Dravid 2015; De Placido 2005; Fábreques 2006; Fabregues 2011; Fernandez‐Ramirez 2006; Ferraretti 2004;;Humaidan 2004; Van der Houwen 2011; Konig 2013; Kovacs 2010; ; Lisi 2005; Lisi 2012; Marrs 2003; Matorras 2009; Musters 2012; Nyboe Andersen 2008; Pezzuto 2010; Razi 2014;Vuong 2015); 13 studies reported on miscarriages (Balasch 2001,.De Placido 2005;Fábreques 2006; Fabregues 2011; Ferraretti 2004; Ferraretti 2014; Griesinger 2005; Humaidan 2004; Konig 2013; Musters 2012; Razi 2014;Tarlatzis 2006; Vuong 2015); 11 studies reported on the cancellation rate due to low response (Allegra 2011; Bosch 2011; De Placido 2005; Evangelio 2011; Fábreques 2006; Fabregues 2011; Ferraretti 2014; Konig 2013; Musters 2012; Tarlatzis 2006; Vuong 2015); and eight studies reported on the cancellation rate due to imminent ovarian hyperstimulation syndrome (Allegra 2011; Bosch 2011; Caserta 2011; Ferraretti 2004; Griesinger 2005; Konig 2013; Marrs 2003; Vuong 2015).
Excluded studies
We excluded 21 studies; 14 studies used interventions that were not relevant to the review, five used designs that were not relevant to the review, and two included participants who did not meet inclusion criteria.
Further information on the excluded studies is available in the Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 2 and Figure 3 for details.
2.
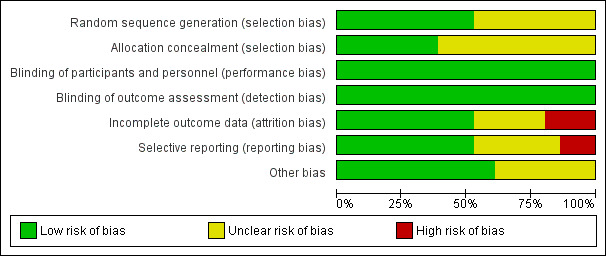
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
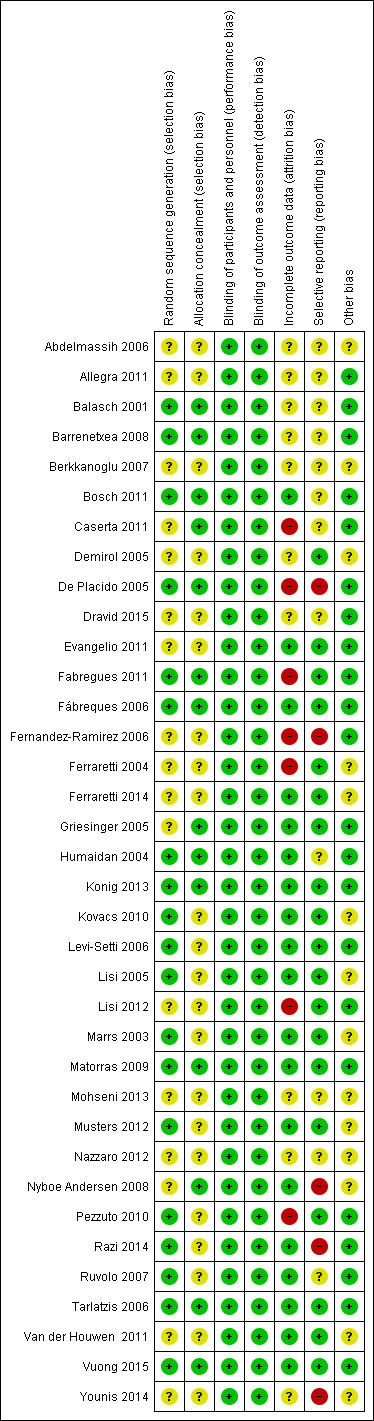
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We rated 19 studies at low risk of selection bias for sequence generation, since they used computer randomization or random number tables for sequence generation. For 17 studies the method used in sequence generation was not fully described and we rated them at unclear risk of selection bias in relation to sequence generation.
Allocation concealment
Fourteen studies used adequate methods in concealing the allocation, and we judged them at low risk of bias. In the remaining 22 studies, the process involved in concealing the allocation was not adequately described, and we rated them at unclear risk of bias.
Blinding
Perfomance bias
Clinicians and participants were not blinded to the interventions in some of the included studies, while others did not report sufficient information on whether or not clinicians and participants were blinded. Non‐blinding of clinicians and participants may not be likely to affect the outcomes of interest, as they are objectively assessed. We, therefore, judged all included studies to be at low risk of bias.
Detection bias
Outcome assessors were not blinded in some of the included studies while others did not report sufficient information on whether or not outcome assessors were blinded. Non‐blinding of outcome assessment may not be likely to affect some outcomes of interest as they are objectively assessed. We, therefore, judged all included studies to be at low risk of bias.
Incomplete outcome data
We rated 11 studies at low risk of incomplete outcome data either because there were no withdrawals or losses to follow‐up, or the proportions of withdrawals and reasons for withdrawals were similar across treatment groups and data were analyzed on the basis of intention‐to‐treat.
Eighteen studies did not report enough information to make conclusive judgements in respect to attrition bias, and thus we rated them at unclear risk of bias.
In the remaining seven studies, the proportions of withdrawals and reasons for withdrawals or losses to follow‐up differed significantly between the treatment groups, and not all women randomized at baseline were included in data analysis; we judged these studies at high risk of bias.
Selective reporting
We judged 20 studies to be at low risk of reporting bias since the methods were prespecified. We rated this domain as unclear in 11 studies because we found no sufficient information in the methods section. We rated reporting bias as high in the remaining five studies because there was evidence of selective reporting of outcomes, as data were not available on all the outcomes prespecified in the methods section.
Other potential sources of bias
With respect to other sources of bias, we assessed studies for significant differences in baseline demographic characteristics of participants. We rated 22 studies at low risk of bias, since there were no conflict of interests and there were no other potential sources of bias, such as differences in baseline demographic characteristics. We rated the risk of bias as unclear in 14 studies, because there was insufficient information on differences in baseline characteristics of participants.
Effects of interventions
See: Table 1
Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) versus rFSH alone in agonist or antagonist cycles
Primary outcomes
1. Live birth rate
Applying a fixed‐effect model to pool the data, there was no evidence of a difference in live birth rate between ovarian stimulation with rLH combined with rFSH and ovarian stimulation with rFSH alone (odds ratio (OR) 1.32, 95% confidence interval (CI) 0.85 to 2.06; n = 499; studies = 4; I2 = 63%, very low‐quality of evidence) (Table 1). The evidence suggests that if the live birth rate following treatment with rFSH alone is 17%, the range of live birth rate varies between 15% and 30% using rLH combined with rFSH (Analysis 1.1, Figure 4). Applying a random‐effects model to pool the data resulted in an OR of 1.43 (95% CI 0.85 to 2.06).
1.1. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 1 Live birth rate.
4.
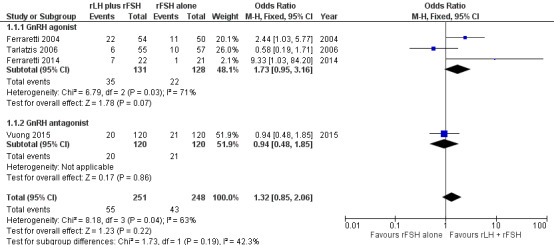
Forest plot of comparison: 1 rLH plus rFSH versus rFSH alone for OS in IVF or ICSI treatment cycles, outcome: 1.1 Live birth rate.
Subgroup analysis 1.1: Downregulating agent used
There was no good evidence that the effects of the intervention differed by type of analogue (test for subgroup differences: Chi² = 1.73, df = 1 (P = 0.19), I² = 42.3%), but there were too few studies to reach any conclusions. Analysis 1.1
Subgroup analysis 1.2: Ovarian response
When studies of women identified as low responders were compared with studies not restricted to women identified as low responders, the single study of low responders suggested a benefit in the intervention group, but there were too few studies to reach any firm conclusions and the test for subgroup differences was not statistically significant (Chi² = 3.33, df = 1 (P = 0.07), I² = 69.9%). Analysis 1.2
1.2. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 2 Subgroup analysis: Live birth by ovarian response.
Subgroup analysis 1.3: Advanced age
A single study was restricted to women of advanced age (Vuong 2015). There was no good evidence that the effects of the intervention differed between this study and the subgroup of studies not restricted to women of advanced age (test for subgroup differences: Chi² = 1.73, df = 1 (P = 0.19), I² = 42.3%), but there were too few studies to reach any conclusions. Analysis 1.3
1.3. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 3 Subgroup analysis: Live birth by advanced age.
2. Ovarian hyperstimulation syndrome
There was no evidence of a difference in ovarian hyperstimulation syndrome between ovarian stimulation with rLH combined with rFSH and ovarian stimulation with rFSH alone (OR 0.38, 95% CI 0.14 to 1.01; n = 2178; studies = 6; I2 = 10%, low‐quality evidence) (Table 1). The evidence suggests that if the risk of ovarian hyperstimulation syndrome following treatment with rFSH alone is 1%, the range of ovarian hyperstimulation syndrome varies between 0% and 1% using rLH combined with rFSH (Analysis 1.4).
1.4. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 4 OHSS.
Subgroup analysis 2.1: Downregulating agent used
There was no good evidence that the effects of the intervention differed by type of analogue (test for subgroup differences: Chi² = 2.15, df = 1 (P = 0.14), I² = 53.5%), but there were too few studies to reach any conclusions. Analysis 1.4
Subgroup analysis 2.2: Ovarian response
No conclusions could be reached as there were no studies reporting ovarian hyperstimulation syndrome in women with low ovarian response.
Subgroup analysis 2.3: Advanced age
No conclusions could be reached as only two studies reported ovarian hyperstimulation syndrome in women of advanced age (Fabregues 2011; Fábreques 2006), and there were no cases of ovarian hyperstimulation syndrome in either study.
Secondary outcomes
3. Ongoing pregnancy rate
The use of rLH combined with rFSH was associated with a higher ongoing pregnancy rate than rFSH alone (OR 1.20, 95% CI 1.01 to 1.42; n = 3129; studies = 19; I2 = 2%, moderate‐quality evidence) (Table 1). The evidence suggests that if the ongoing pregnancy rate following treatment with rFSH alone is 21%, the range of ongoing pregnancy rate varies between 21% and 27% using rLH combined with rFSH (Analysis 1.5).
1.5. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 5 Ongoing pregnancy.
Subgroup analysis 3.1: Downregulating agent used
Effects did not appear to differ by type of analogue (test for subgroup differences: Chi² = 0.75, df = 1 (P = 0.39), I² = 0%) (Analysis 1.5).
Subgroup analysis 3.2: Ovarian response
When studies of women identified as low responders were compared with studies not restricted to women identified as low responders, the benefits of the intervention appeared to be stronger in women identified as low responders (OR 2.06, 95% CI 1.20 to 3.53, 79 women, 3 RCTs, I2=0%) and there was a significant difference between the subgroups (test for subgroup differences: Chi² = 4.33, df = 1 (P = 0.04), I² = 76.9%). This finding requires very cautious interpretation as the subgroup of low responders was very small (n = 79) and subgroup analyses should be regarded as exploratory, as they are not randomized comparisons (Analysis 1.6).
1.6. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 6 Subgroup analysis: ongoing pregnancy by ovarian response.
Subgroup analysis 3.3: Advanced age
When studies restricted to women of advanced age were compared with studies not restricted by age, effects did not appear to differ between the two subgroups (Chi² = 0.46, df = 1 (P = 0.50), I² = 0%) (Analysis 1.7).
1.7. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 7 Subgroup analysis: ongoing pregnancy by advanced age.
4. Clinical pregnancy rate
The use of rLH combined with rFSH was associated with a higher clinical pregnancy rate than rFSH alone (OR 1.18, 95% CI 1.03 to 1.34; n = 5071; studies = 23; I2 = 33%). The evidence suggests that if the clinical pregnancy rate following treatment with rFSH alone is 24%, the range of the clinical pregnancy rate varies between 23% and 29% using rLH combined with rFSH (Analysis 1.8). One study described higher but no significant clinical pregnancy rates in patients treated with rLH combined with rFSH compared to rFSH alone, without showing absolute numbers (Mohseni 2013).
1.8. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 8 Clinical pregnancy.
5. Miscarriage rate
The combination of rLH combined with rFSH was not associated with a difference in miscarriage rate compared to rFSH alone (OR 0.93, 95% CI 0.63 to 1.36; n = 1711; studies = 13; I2 = 0%, moderate‐quality evidence) (Analysis 1.9; Table 1). The evidence suggests that if the miscarriage rate following treatment with rFSH alone is 7%, the miscarriage rate following treatment with rLH combined with rFSH ranges between 4% and 9%.
1.9. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 9 Miscarriage rate.
6. Cancellation due to low response
There was no evidence of a difference in cancellation rate due to low response between rLH combined with rFSH and rFSH alone (OR 0.77, 95% CI 0.54 to 1.10; n = 2251; studies = 11; I2 = 16%) (Analysis 1.10). The evidence suggests that if the risk of cancellation due to low response following treatment with rFSH alone is 7%, the range of the cancellation rate due to low response varies between 4% and 7% using rLH combined with rFSH.
1.10. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 10 Adverse events (cancellation due to low response).
7. Cancellation due to imminent ovarian hyperstimulation syndrome
Applying a fixed‐effect model to pool the data, cancellation rates due to imminent ovarian hyperstimulation syndrome were lower in women who received rLH combined with rFSH than in those who received rFSH alone (OR 0.60, 95% CI 0.40 to 0.89; n = 2976; studies = 8; I2 = 60%) (Analysis 1.11). The evidence suggests that if the risk of cancellation due to imminent ovarian hyperstimulation syndrome following treatment with rFSH alone is 4%, the range of the cancellation due to imminent ovarian hyperstimulation syndrome varies between 2% and 4% using rLH combined with rFSH. However, heterogeneity was high (I2=60%) and applying a random‐effects model to pool the data resulted in an OR of 0.82 (95% CI 0.34 to 1.97).
1.11. Analysis.
Comparison 1 Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles, Outcome 11 Adverse events (cancellation due to imminent OHSS).
Investigation of publication bias
Visual scanning of funnel plots for clinical pregnancy (Analysis 1.8; Figure 5), and cancellation due to low response (Analysis 1.10; Figure 6), suggested a tendency towards publication bias, with smaller negative studies less likely to be included in the review. However, visual inspection of funnel plots for ongoing pregnancy (Analysis 1.5), and miscarriage (Analysis 1.9), did not reveal such a tendency towards publication bias in favour of larger studies with positive outcomes.
5.
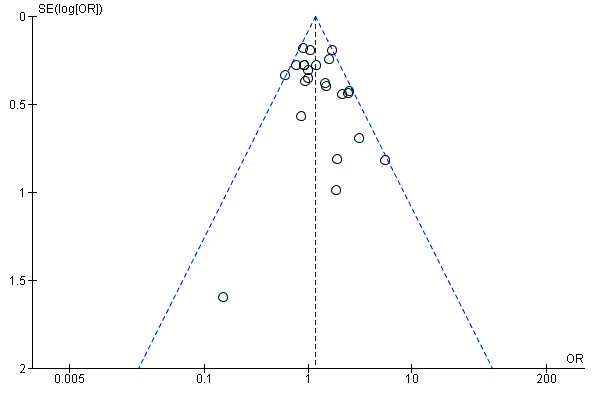
Funnel plot of comparison: 1 rLH plus rFSH versus rFSH alone for ovarian stimulation in IVF or ICSI treatment cycles, outcome: 1.8 Clinical pregnancy.
6.
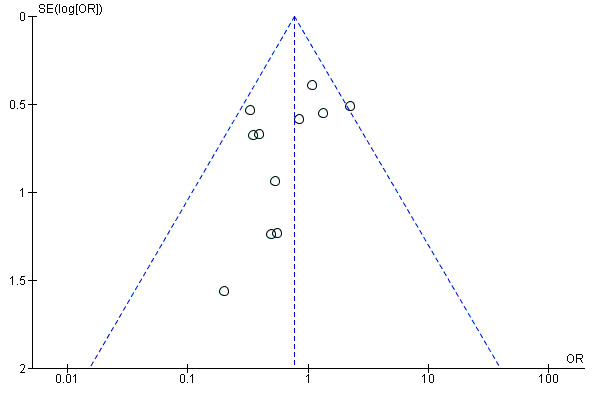
Funnel plot of comparison: 1 rLH plus rFSH versus rFSH alone for OS in IVF or ICSI treatment cycles, outcome: 1.10 Adverse events (cancellation due to low response).
Discussion
Summary of main results
There was no evidence of a difference in the live birth rate between women undergoing ovarian stimulation with recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) and women undergoing ovarian stimulation with rFSH alone, regardless of the type of downregulation.
There was no evidence of a difference in the ovarian hyperstimulation syndrome rate or the miscarriage rate following ovarian stimulation with rLH combined with rFSH compared to rFSH alone in gonadotrophin‐releasing hormone (GnRH) analogue downregulated in‐vitro fertilisation/intracytoplasmic sperm injection (IVF/ICSI) cycles. There was also no clear evidence of a difference between the groups in rates of cancellation due to low response or imminent OHSS.
However the evidence suggested a higher ongoing pregnancy rate in women treated with rLH combined with rFSH compared to rFSH alone in GnRH analogue downregulated IVF/ICSI cycles.
When studies of women identified as low responders were compared with studies not restricted to women identified as low responders, the ongoing pregnancy rate was higher in women identified as low responders. However the subgroup of low responders was very small (n = 79). This finding requires very cautious interpretation and should be regarded as exploratory.
Overall completeness and applicability of evidence
This Cochrane Review sought to evaluate the effectiveness of rLH combined with rFSH compared to rFSH alone for ovarian stimulation in downregulated IVF or ICSI cycles. We included 36 RCTs, totaling 8125 women. The sample sizes in the studies ranged between 30 and 999. Only four of the included studies, totalling 499 women had data on the primary outcome measure, live birth rate. To be able to show a difference of 5% compared to a standard live birth rate of 17%, one would require to include at least 1970 couples. Six of the included studies had data on the ovarian hyperstimulation syndrome rate. The evidence is generally applicable to women undergoing the conventional stimulation regimens in GnRH analogue downregulated IVF/ICSI cycles.
The sample size for the subgroup analysis in women with poor ovarian response and in women of advanced age was small, therefore there is insufficient evidence to make a conclusive judgement of any beneficial effect of rLH combined with rFSH in IVF or ICSI cycles compared to rFSH alone in these women.
Quality of the evidence
The overall quality of the evidence was very low for live birth, low for ovarian hyperstimulation syndrome and moderate for ongoing pregnancy and miscarriage. The main limitations in the evidence for the primary outcome live birth rate and for the secondary outcome miscarriage was imprecision, due to the small amount of data. We downgraded the quality of evidence of ovarian hyperstimulation syndrome and ongoing pregnancy because there was risk of bias associated with poor reporting of study methods.
Only seven of the 36 studies (19%) clearly stated that they were funded by government or research institutes. Six (17%) were funded by pharmaceutical companies and the rest (64%) did not state their source of funding.
Potential biases in the review process
The review authors minimised the risk of bias by conducting a search that was systematic and thorough and by having two review authors independently perform the data extraction, risk of bias assessment, and GRADE evaluation.
Agreements and disagreements with other studies or reviews
Our results are in agreement with those of a previous systematic review and meta‐analysis, comparing rLH combined with rFSH to rFSH alone in GnRH antagonist in downregulated IVF/ICSI cycles (Xiong 2014). This review identified four of the 11 studies that we included, and included one other study (Sauer 2004). We excluded Sauer 2004, since they randomized between using GnRH agonists (leuprolide) combined with rFSH versus using GnRH antagonists (cetrorelix) with or without rLH.
Our results were also in line with the results of another systematic review and meta‐analysis that compared the combination of rLH and rFSH to rFSH alone in women of advanced reproductive age undergoing IVF/ICSI (Hill 2012). This review identified the same studies that we identified.
Authors' conclusions
Implications for practice.
We found no clear evidence of a difference between rLH combined with rFSH and rFSH alone in rates of live birth or OHSS. The evidence for these comparisons was of very low‐quality for live birth and low quality for OHSS. We found moderate quality evidence that the use of rLH combined with rFSH may lead to more ongoing pregnancies than rFSH alone. There was also moderate‐quality evidence suggesting little or no difference between the groups in rates of miscarriage. There was no clear evidence of a difference between the groups in rates of cancellation due to low response or due to imminent OHSS, but the evidence for these outcomes was of low or very low quality.
We conclude that the evidence is too limited to encourage or discourage stimulation regimens that include rLH combined with rFSH in IVF/ICSI cycles.
Implications for research.
We suggest a systematic review and meta‐analysis addressing the head‐to‐head comparison of whether HP‐HMG or rLH combined with rFSH is the most effective and safe in GnRH analogue downregulated IVF/ICSI cycles. We suggest a cost‐effectiveness analysis on the combination of rLH and rFSH compared to rFSH alone in GnRH agonist downregulated IVF/ICSI cycles. In addition, we suggest an individual patient data analysis on the effectiveness of rLH combined with rFSH in women with poor ovarian response and in women of advanced age. All studies should clearly report their funding source.
What's new
| Date | Event | Description |
|---|---|---|
| 9 June 2016 | New citation required and conclusions have changed | New evidence has led to a change to the conclusions of this review. |
| 9 June 2016 | New search has been performed | We added 12 new studies (Allegra 2011; Dravid 2015; Ferraretti 2014; Konig 2013; Lisi 2012; Mohseni 2013; Musters 2012; Nazzaro 2012; Razi 2014; Van der Houwen 2011; Vuong 2015; Younis 2014). |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 20 September 2010 | Amended | Contact details updated. |
| 6 November 2008 | Amended | Converted to new review format. |
| 22 October 2007 | New citation required but conclusions have not changed | No changes. |
| 26 January 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the reviewers for their insightful comments on this review and the support of all the contributors from the Cochrane Gynaecology and Fertility Group.
Appendices
Appendix 1. Gynaecology and Fertility specialised register search strategy
From inception until 9 June 2016
PROCITE platform
Keywords CONTAINS "Luteinising hormone releasing hormone" or "luteinizing hormone" or "luteinizing hormone supplementation" or "Lutenising hormone releasing hormone" or "Luveris" or "lutropin alfa" or "recombinant LH" or "r‐hLH" or "r‐LH " or "Lh recombinant" or "LHRH" or "Lh" or "pergonal" or "pergonol" or Title CONTAINS "Luteinising hormone releasing hormone" or "luteinizing hormone" or "luteinizing hormone supplementation" or "Lutenising hormone releasing hormone" or "Luveris" or "lutropin alfa" or "recombinant LH" or "r‐hLH" or "r‐LH " or "Lh recombinant" or "LHRH" or "Lh" or "pergonal" or "pergonol"
AND
Keywords CONTAINS "IVF"or "ICSI" or "in vitro fertilisation" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "assisted reproduction techniques" or "assisted conception" or "ovulation induction"or "superovulation" or "superovulation induction" or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or "COH" or "ovarian stimulation" or "ovarian hyperstimulation" (622)
Appendix 2. CENTRAL search strategy
From inception until 9 June 2016
CENTRAL CRSO Web platform
#1 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES (886) #2 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES (1737) #3 MESH DESCRIPTOR Sperm Injections, Intracytoplasmic EXPLODE ALL TREES (437) #4 (embryo* adj2 transfer*):TI,AB,KY (1920) #5 (vitro fertili?ation):TI,AB,KY (1813) #6 ivf:TI,AB,KY (2828) #7 icsi:TI,AB,KY (1249) #8 (intracytoplasmic sperm injection*):TI,AB,KY (952) #9 (blastocyst* adj2 transfer*):TI,AB,KY (168) #10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 (4501) #11 MESH DESCRIPTOR Reproductive Techniques, Assisted EXPLODE ALL TREES (2652) #12 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES (886) #13 MESH DESCRIPTOR Ovulation Induction EXPLODE ALL TREES (1119) #14 MESH DESCRIPTOR Superovulation EXPLODE ALL TREES (57) #15 (ovulat* induc*):TI,AB,KY (1587) #16 superovulation:TI,AB,KY (164) #17 (ovar* adj2 stimulat*):TI,AB,KY (1116) #18 COH:TI,AB,KY (196) #19 (assisted reproducti*):TI,AB,KY (608) #20 (ovar* adj2 hyperstimulat*):TI,AB,KY (884) #21 (follic* stimulat*):TI,AB,KY (2445) #22 (follic* matur*):TI,AB,KY (154) #23 (IVF adj1 ICSI):TI,AB,KY (555) #24 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 (7560) #25 MESH DESCRIPTOR Luteinizing Hormone EXPLODE ALL TREES (1518) #26 (rLH or rec LH):TI,AB,KY (60) #27 (exogenous luteini?ing hormone*):TI,AB,KY (8) #28 lutropin:TI,AB,KY (15) #29 pergonal:TI,AB,KY (19) #30 (r‐hlh or r‐LH):TI,AB,KY (46) #31 (recLH or rhlh):TI,AB,KY (23) #32 (lhadi or luteoz?man):TI,AB,KY (1) #33 (recombinant adj2 luteini?ing hormone*):TI,AB,KY (78) #34 (recombinant human LH):TI,AB,KY (29) #35 (rec* adj2 luteini?ing hormone*):TI,AB,KY (102) #36 (recombinant LH):TI,AB,KY (73) #37 (LH supplement*):TI,AB,KY (37) #38 (recombinant HLH):TI,AB,KY (0) #39 (rec HLH):TI,AB,KY (1) #40 #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 (1667) #41 #24 AND #40 (1300)
Appendix 3. MEDLINE search strategy
Ovid MEDLINE(R) Epub Ahead of Print, In Process & Other Non‐Indexed Citations, Ovid MEDLINE (R) Daily, and Ovid MEDLINE (R) From 1946 until 9 June 2016
Ovid platform
1 exp Luteinizing Hormone/ (45058) 2 (rLH or rec LH).tw. (321) 3 rec luteini?ing hormone$.tw. (1) 4 exogenous luteini?ing hormone$.tw. (63) 5 lutropin.tw. (866) 6 pergonal.tw. (152) 7 (r‐hlh or r‐LH).tw. (90) 8 (recLH or rhlh).tw. (59) 9 (lhadi or luteoz?man).tw. (4) 10 (recombinant adj2 luteini?ing hormone$).tw. (113) 11 recombinant human LH.tw. (70) 12 (rec adj2 luteini?ing hormone$).tw. (2) 13 recombinant LH.tw. (140) 14 LH supplement$.tw. (78) 15 recombinant HLH.tw. (11) 16 or/1‐15 (45840) 17 exp reproductive techniques, assisted/ or exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp gamete intrafallopian transfer/ or exp zygote intrafallopian transfer/ (57984) 18 assisted reproductive technique$.tw. (1259) 19 in vitro fertili?ation.tw. (19022) 20 intracytoplasmic sperm injection$.tw. (5741) 21 (ivf or icsi).tw. (22056) 22 exp ovulation induction/ or exp superovulation/ (11273) 23 ovulat$ induc$.tw. (3700) 24 superovulation.tw. (1808) 25 controlled ovarian stimulation$.tw. (857) 26 COH.tw. (1334) 27 controlled ovarian hyperstimulation$.tw. (1522) 28 (ovari$ adj2 stimulat$).tw. (5607) 29 assisted reproducti$.tw. (11041) 30 (ovari$ adj2 hyperstimulat$).tw. (4339) 31 follicul$ stimulat$.tw. (484) 32 follicul$ maturation.tw. (1024) 33 (IVF adj1 ICSI).tw. (1376) 34 or/17‐33 (74373) 35 16 and 34 (3565) 36 randomized controlled trial.pt. (420793) 37 controlled clinical trial.pt. (91006) 38 randomized.ab. (358742) 39 placebo.tw. (178518) 40 clinical trials as topic.sh. (177498) 41 randomly.ab. (256600) 42 trial.ti. (156177) 43 (crossover or cross‐over or cross over).tw. (69506) 44 or/36‐43 (1065847) 45 (animals not (humans and animals)).sh. (4229204) 46 44 not 45 (981472) 47 35 and 46 (509)
Appendix 4. Embase search strategy
From 1980 until 9 June 2016
OVID platform
1 exp Luteinizing Hormone/ (50441) 2 (rLH or rec LH).tw. (441) 3 rec luteini?ing hormone$.tw. (1) 4 exogenous luteini?ing hormone$.tw. (54) 5 lutropin.tw. (831) 6 pergonal.tw. (1920) 7 (r‐hlh or r‐LH).tw. (131) 8 (recLH or rhlh).tw. (69) 9 (lhadi or luteoz?man).tw. (22) 10 (recombinant adj2 luteini?ing hormone$).tw. (133) 11 recombinant human LH.tw. (73) 12 (rec adj2 luteini?ing hormone$).tw. (3) 13 recombinant LH.tw. (192) 14 LH supplement$.tw. (110) 15 recombinant HLH.tw. (13) 16 or/1‐15 (52861) 17 assisted reproductive technique$.tw. (1961) 18 in vitro fertili?ation.tw. (23517) 19 intracytoplasmic sperm injection$.tw. (7360) 20 (ivf or icsi).tw. (34749) 21 ovulat$ induc$.tw. (4795) 22 superovulation.tw. (1969) 23 controlled ovarian stimulation$.tw. (1597) 24 COH.tw. (1820) 25 controlled ovarian hyperstimulation$.tw. (2210) 26 (ovari$ adj2 stimulat$).tw. (8272) 27 assisted reproducti$.tw. (16012) 28 (ovari$ adj2 hyperstimulat$).tw. (6102) 29 follicul$ stimulat$.tw. (635) 30 follicul$ maturation.tw. (1145) 31 (IVF adj1 ICSI).tw. (2926) 32 exp infertility therapy/ or exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ or exp ovulation induction/ (87734) 33 or/17‐32 (104125) 34 16 and 33 (6866) 35 Clinical Trial/ (858094) 36 Randomized Controlled Trial/ (404847) 37 exp randomization/ (70570) 38 Single Blind Procedure/ (22197) 39 Double Blind Procedure/ (128704) 40 Crossover Procedure/ (47202) 41 Placebo/ (275451) 42 Randomi?ed controlled trial$.tw. (136119) 43 Rct.tw. (20323) 44 random allocation.tw. (1528) 45 randomly allocated.tw. (24807) 46 allocated randomly.tw. (2113) 47 (allocated adj2 random).tw. (757) 48 Single blind$.tw. (17385) 49 Double blind$.tw. (162039) 50 ((treble or triple) adj blind$).tw. (555) 51 placebo$.tw. (232908) 52 prospective study/ (334857) 53 or/35‐52 (1574988) 54 case study/ (37932) 55 case report.tw. (305862) 56 abstract report/ or letter/ (961247) 57 or/54‐56 (1298029) 58 53 not 57 (1533942) 59 34 and 58 (1482)
Appendix 5. PsycINFO search strategy
From 1806 until 9 June 2016
OVID platform
1 exp Luteinizing Hormone/ (745) 2 (rLH or rec LH).tw. (17) 3 rec luteini?ing hormone$.tw. (0) 4 exogenous luteini?ing hormone$.tw. (2) 5 lutropin.tw. (2) 6 pergonal.tw. (2) 7 (r‐hlh or r‐LH).tw. (2) 8 (recLH or rhlh).tw. (1) 9 (lhadi or luteoz?man).tw. (0) 10 (recombinant adj2 luteini?ing hormone$).tw. (0) 11 recombinant human LH.tw. (0) 12 (rec adj2 luteini?ing hormone$).tw. (0) 13 recombinant LH.tw. (0) 14 LH supplement$.tw. (0) 15 recombinant HLH.tw. (0) 16 or/1‐15 (769) 17 assisted reproductive technique$.tw. (35) 18 in vitro fertili?ation.tw. (619) 19 intracytoplasmic sperm injection$.tw. (44) 20 (ivf or icsi).tw. (485) 21 ovulat$ induc$.tw. (27) 22 superovulation.tw. (4) 23 controlled ovarian stimulation$.tw. (4) 24 COH.tw. (84) 25 controlled ovarian hyperstimulation$.tw. (1) 26 (ovari$ adj2 stimulat$).tw. (53) 27 assisted reproducti$.tw. (719) 28 (ovari$ adj2 hyperstimulat$).tw. (11) 29 follicul$ stimulat$.tw. (16) 30 follicul$ maturation.tw. (6) 31 (IVF adj1 ICSI).tw. (12) 32 exp reproductive technology/ (1562) 33 or/17‐32 (2202) 34 16 and 33 (10)
Appendix 6. CINAHL search strategy
From 1961 until 9 June 2016
EBSCO platform
| # | Query | Results |
| S33 | S20 AND S32 | 37 |
| S32 | S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 | 1,053,818 |
| S31 | TX allocat* random* | 5,059 |
| S30 | (MH "Quantitative Studies") | 14,585 |
| S29 | (MH "Placebos") | 9,730 |
| S28 | TX placebo* | 38,559 |
| S27 | TX random* allocat* | 5,059 |
| S26 | (MH "Random Assignment") | 41,063 |
| S25 | TX randomi* control* trial* | 105,940 |
| S24 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 835,812 |
| S23 | TX clinic* n1 trial* | 186,430 |
| S22 | PT Clinical trial | 79,707 |
| S21 | (MH "Clinical Trials+") | 198,930 |
| S20 | S11 AND S19 | 94 |
| S19 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 | 4,335 |
| S18 | TX embryo* N3 transfer* | 913 |
| S17 | TX ovar* N3 hyperstimulat* | 381 |
| S16 | TX ovari* N3 stimulat* | 300 |
| S15 | TX IVF or TX ICSI | 1,559 |
| S14 | (MM "Fertilization in Vitro") | 1,611 |
| S13 | TX vitro fertilization | 3,319 |
| S12 | TX vitro fertilisation | 3,319 |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 | 1,349 |
| S10 | TX LH supplement* | 7 |
| S9 | TX recombinant LH | 6 |
| S8 | TX recombinant human LH | 3 |
| S7 | TX (recLH or rhlh) | 1 |
| S6 | TX (r‐hlh or r‐LH) | 3 |
| S5 | TX pergonal | 3 |
| S4 | TX lutropin | 7 |
| S3 | TX luteini?ing hormone | 1,307 |
| S2 | TX (rLH or rec LH) | 37 |
| S1 | (MM "Luteinizing Hormone") | 164 |
Appendix 7. Search strategies from previous versions of review
The following keywords were used.
MEDLINE 1 (luveris or lhadi or reclh or rlh or rhlh).mp. 2 ((alpha or alfa or recombinant or rec or r or rh or r‐h) adj2 (lutropin or luteoz?man)).tw. 3 (recombinant adj3 ((luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 4 (rec adj2 ((luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 5 (r adj2 ((luteini?ing adj hormone$) or hlh or lhs or hlhs)).tw. 6 (r adj lh).mp. 7 ((recombinant adj2 gonadotropin$) and ((luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 8 ((exogenous or combination or (co adj (administrat$ or treatment))) adj2 (lutropin or luteoz?man or (luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 9 (added adj (lutropin or luteoz?man or (luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 10 exp Recombinant Proteins/ and exp Luteinizing Hormone/ 11 exp Luteinizing Hormone/ad, tu 12 FSH.mp. or exp follicle stimulating hormone/ 13 11 and 12 14 (or/1‐10) or 15 (clinical trial.mp. or randomi?ed.ti,ab. or placebo.ti,ab. or exp clinical trials/ or randomly.ti,ab. or trial.ti,ab.) not (animals/ not (animals/ and humans/)) 16 14 and 15
1 (luveris or lhadi or reclh or rlh or rhlh).mp. 2 ((alpha or alfa or recombinant or rec or r or rh or r‐h) adj2 (lutropin or luteoz?man)).tw. 3 (recombinant adj3 ((luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 4 (rec adj2 ((luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 5 (r adj2 ((luteini?ing adj hormone$) or hlh or lhs or hlhs)).tw. 6 (r adj lh).mp. 7 ((recombinant adj2 gonadotropin$) and ((luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 8 ((exogenous or combination or (co adj (administrat$ or treatment))) adj2 (lutropin or luteoz?man or (luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 9 (added adj (lutropin or luteoz?man or (luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 10 exp Recombinant Proteins/ and exp Luteinizing Hormone/ 11 exp Luteinizing Hormone/ad, tu 12 (or/1‐10)
13 limit 12 to (human and female and adult <18 to 64 years>) (489)
EMBASE 1 (luveris or lhadi or reclh or rlh or rhlh).mp. 2 ((alpha or alfa or recombinant or rec or r or rh or r‐h) adj2 (lutropin or luteoz?man)).tw. 3 (recombinant adj3 ((luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 4 (rec adj2 ((luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 5 (r adj2 ((luteini?ing adj hormone$) or hlh or lhs or hlhs)).tw. 6 (r adj lh).mp. 7 ((recombinant adj2 gonadotropin$) and ((luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 8 ((exogenous or combination or (co adj (administrat$ or treatment))) adj2 (lutropin or luteoz?man or (luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 9 (added adj (lutropin or luteoz?man or (luteini?ing adj hormone$) or lh or hlh or lhs or hlhs)).tw. 2 10 recombinant luteinizing hormone/ 11 Luteinizing Hormone/ad, cm, sc [Drug Administration, Drug Comparison, Subcutaneous Drug Administration] 12 Recombinant Follitropin/ or fsh.tw. 13 11 and 12 14 (randomized controlled trial or clinical trial or multicenter study or controlled study or crossover procedure or double blind procedure or single blind procedure or randomization or major clinical study or placebo or meta analysis or phase 2 clinical trial or phase 3 clinical trial or phase 4 clinical trial).mp. 15 (clin$ adj25 trial$).tw. 16 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).tw. 17 (placebo$ or random$ or control$).tw. 18 (cross?over or factorial or sham? or dummy).tw. 19 ABAB design$.tw. 20 or/14‐19 21 animals/ not (animals/ and humans/) 22 20 not 21 3394321 23 (or/1‐10) or 13 658 24 23 and 22
CENTRAL 1 (luveris or lhadi or reclh or rlh or rhlh) 2 (alpha or alfa or recombinant or rec or r or rh or r‐h) NEAR/2 (lutropin or luteoz*man) 3 (recombinant NEAR/3 ((luteini*ing NEXT hormone*) or lh or hlh or lhs or hlhs)) 4 (rec NEAR/2 ((luteini*ing NEXT hormone*) or lh or hlh or lhs or hlhs) ) 5 (r NEAR/2 ((luteini*ing NEXT hormone*) or hlh or lhs or hlhs)) 6 r NEXT lh 7 (recombinant NEAR/2 gonadotropin*) and ((luteini*ing NEXT hormone*) or lh or hlh or lhs or hlhs) 8 (exogenous or combination or (co NEXT (administrat* or treatment))) NEAR/2 (lutropin or luteoz*man or (luteini*ing NEXT hormone*) or lh or hlh or lhs or hlhs) 9 (adding OR added OR addition) NEAR/5 (lutropin or luteoz*man or (luteini*ing NEXT hormone*) or lh or hlh or lhs or hlhs) 10 MeSH descriptor Recombinant Proteins explode all trees 11 MeSH descriptor Luteinizing Hormone explode all trees 12 (#10 AND #11) 13 MeSH descriptor Luteinizing Hormone explode all trees with qualifier: AD 14 MeSH descriptor Luteinizing Hormone explode all trees with qualifier: TU 15 fsh 16 MeSH descriptor Follicle Stimulating Hormone explode all trees 17 (( #13 OR #14 ) AND ( #15 OR #16 )) 18 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #12 OR #17)
We searched:
1 The Cochrane Menstrual Disorders & Subfertility Group's Specialised Register and the Cochrane Central Register of Controlled Trials (CENTRAL) on the latest issue of The Cochrane Library. 2 MEDLINE database using the same key‐words (MeSH words) (1980 to June 2011) 3 EMBASE database using the same key‐words 4 CINAHL database using the same key‐words 5 Hand searching the reference lists of included studies, reviews and relevant textbooks. 6 Abstracts of The American Society for Reproductive Medicine and European Society for Human Reproduction and Endocrinology meetings. 7 Trial Register (www.controlled‐trials.com) (June 2011) 8 Abstracts of meetings such as ASRM, ESHRE (June 2010) There was no language restriction. When important information was lacking from the original publications the authors were contacted.
Data and analyses
Comparison 1. Recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone for ovarian stimulation in in‐vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 4 | 499 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.85, 2.06] |
| 1.1 GnRH agonist | 3 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.95, 3.16] |
| 1.2 GnRH antagonist | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.48, 1.85] |
| 2 Subgroup analysis: Live birth by ovarian response | 4 | 499 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.85, 2.06] |
| 2.1 Studies restricted to women with low response | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.33 [1.03, 84.20] |
| 2.2 Studies not restricted to women with low response | 3 | 456 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.72, 1.83] |
| 3 Subgroup analysis: Live birth by advanced age | 4 | 499 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.85, 2.06] |
| 3.1 Studies restricted to women of advanced age | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.48, 1.85] |
| 3.2 Studies not restricted to women of advanced age | 3 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.95, 3.16] |
| 4 OHSS | 6 | 2178 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.14, 1.01] |
| 4.1 GnRH agonist | 4 | 1418 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.88] |
| 4.2 GnRH antagonist | 2 | 760 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.21, 3.00] |
| 5 Ongoing pregnancy | 19 | 3129 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.01, 1.42] |
| 5.1 GnRH agonist | 12 | 1980 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.02, 1.57] |
| 5.2 GnRH antagonist | 7 | 1149 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.43] |
| 6 Subgroup analysis: ongoing pregnancy by ovarian response | 19 | 3129 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.01, 1.42] |
| 6.1 Studies restricted to women with low response | 3 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.20, 3.53] |
| 6.2 Studies not restricted to women with low response | 16 | 2853 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.94, 1.35] |
| 7 Subgroup analysis: ongoing pregnancy by advanced age | 19 | 3129 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.01, 1.42] |
| 7.1 Studies restricted to women of advanced age | 5 | 1170 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.84, 1.48] |
| 7.2 Studies not restricted to women of advanced age | 14 | 1959 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.00, 1.54] |
| 8 Clinical pregnancy | 23 | 5071 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.03, 1.34] |
| 9 Miscarriage rate | 13 | 1711 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.63, 1.36] |
| 10 Adverse events (cancellation due to low response) | 11 | 2251 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.10] |
| 11 Adverse events (cancellation due to imminent OHSS) | 8 | 2976 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelmassih 2006.
| Methods | Prospective randomised study, single centre. Randomisation method: not stated. Power analysis: not stated. Study period: not stated. Sample size: 206 women. Conflict of interest: not stated. |
|
| Participants | Normagonadotropic women with an indication for IVF/ICSI. Age < 35 years. No available baseline characteristics to compare. |
|
| Interventions | Luteal started pituitary downregulation with GnRH agonist. Standard treatment: from cycle day 2 onward daily 225 IU/L rFSH subcutaneous. Experimental treatment: from cycle day 7 onward daily additional 75 IU/L rLH until ovulation triggering with hCG. |
|
| Outcomes | Primary endpoints:
Secondary endpoint:
|
|
| Notes | Abstract only. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Those who administered the intervention were not blinded but non‐blinding of outcome assessment not likely to affect the outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only abstract available. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available, no live birth rates. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Allegra 2011.
| Methods | Prospective randomised study, single centre. Randomisation method: not stated. Power analysis: not stated. Study period: not stated. Sample size: 102 women. Conflict of interest: not stated. |
|
| Participants | Normagonadotropic women undergoing ICSI. Age 38 to 44 years. FSH ≥ 9 mIU/ml. Exclusion criteria: basal FSH ≥ 16 mIU/ml, women age ≥ 44 years, severe endometriosis, severe male factor, secondary infertility ≤ 3 years. Baseline characteristics to compare: age, BMI, menstrual cycle length, antral follicle count. |
|
| Interventions | Luteal started pituitary downregulation with GnRH agonist. The supplementation of the luteal phase was assured by the administration of progesterone. Standard treatment: rFSH alone, 225‐450 IU daily. Experimental treatment: rFSH, 225‐450 IU daily and rLH 75 IU daily from the day in which at least one follicle ≥ 14 mm was detected. |
|
| Outcomes | Primary endpoint:
Secondary endpoints:
|
|
| Notes | Abstract only. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was reported on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on blinding of outcome assessors but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although dropouts and reasons for withdrawals were given, proportions were not and reasons were not uniform between treatment groups and analysis was not on ITT basis. |
| Selective reporting (reporting bias) | Unclear risk | Methods section not detailed enough to make conclusive judgement. |
| Other bias | Low risk | Baseline demographic characteristics were similar between the two treatment groups. |
Balasch 2001.
| Methods | Prospective, randomized study, single centre. Randomisation: computer‐generated randomization table. Allocation: by opening a sealed envelope. Power analysis: not stated. Sample size: 30 women. Study period: not stated. Conflict of interest: not stated. |
|
| Participants | Normogonadotropic women with an indication for IVF/ICSI. Aged between 29‐40 years. Basal FSH < 11 IU/L, both ovaries present. Exclusion criteria: PCOS, more than two previous assisted reproductive technology attempts. Available baseline characteristic: mean age, BMI and duration of infertility were comparable between the two groups. |
|
| Interventions | Luteal started pituitary downregulation with GnRH agonist leuprolide 1 mg subcutaneous daily, reduced to 0.5 mg/day once ovarian arrest has been achieved i.e. serum estradiol < 30 pg/ml and absence of follicles > 10 mm. Standard treatment: rFSH was administered in a step‐down regimen: stimulation day 1 450 IU rFSH, stimulation day 2: 300 IU rFSH and stimulation day 3‐5: 150 IU rFSH, stimulation day 6 adjusted to ovarian response. Experimental treatment: from stimulation day 1 onward additional daily 75 IU rLH until ovulation triggering with hCG. |
|
| Outcomes | No primary endpoint stated. Endpoints: days of ovarian stimulation, rFSH dose used, number of (MII) oocytes retrieved, fertilisation rate, number and quality of retrieved and transferred embryos, poor fertilisation rate and total fertilisation failure clinical pregnancy rate (not defined), miscarriage rate in first trimester. | |
| Notes | Clinical pregnancy not defined. No data on live birth. Funding: rFSH and LH were provided by Ares‐Serono International S.A., Geneva Switzerland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to a computer‐generated randomization table. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Those who administered the intervention were not blinded but non‐blinding of outcome assessment is not likely to affect the outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No study protocol available. |
| Selective reporting (reporting bias) | Unclear risk | No data on live birth. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two groups. |
Barrenetxea 2008.
| Methods | Prospective randomized study, single centre. Randomisation: computer‐generated block randomization. Allocation: sealed envelopes. Power analyses: 10% difference in clinical pregnancy rate. Sample size: 84. Study period: January to June 2005. Conflict of interest: not stated. |
|
| Participants | Women with an indication for IVF and poor ovarian reserve. Age > 40 year. FSH cycle day 3 > 10. Available baseline characteristics to compare: mean age, BMI, duration of infertility, basal FSH. |
|
| Interventions | Follicular started pituitary downregulation with a GnRH agonist 0.5 mg/day leuprolide. Standard treatment: from cycle day 2 onward 375 rFSH. Experimental treatment from stimulation day 7 until stimulation day 10 150 IU rLH, from stimulation day 10 onward daily additional 75 IU rLH until ovulation triggering with hCG. |
|
| Outcomes | Primary endpoints:
Secondary endpoints:
|
|
| Notes | No data on live births. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Computer‐generated' block randomization. |
| Allocation concealment (selection bias) | Low risk | Allocations were concealed in 'sealed envelopes'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Those who administered the intervention were not blinded but non blinding of outcome assessment is not likely to affect the outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make a conclusive judgement. |
| Selective reporting (reporting bias) | Unclear risk | No data on live births. |
| Other bias | Low risk | Baseline demographic characteristics similar between groups. |
Berkkanoglu 2007.
| Methods | Prospective randomized study, single centre. Randomisation method: not stated. Power analyses: not stated. Sample size: 97. Study period: not stated. Conflict of interests: not stated. |
|
| Participants | Women undergoing ICSI, indication not stated, only first treatment cycle having more than 3 follicles on stimulation day 7. Aged < 42 AFC < 12 FSH < 12 Baseline characteristics to compare: age, AFC, basal FSH. |
|
| Interventions | Follicular started flare‐up GnRH agonist microdose 40 mg (twice daily) pre‐treated with OC. Standard treatment: from cycle day 3 onward 600 IU rFSH. Experimental treatment: from cycle day 3 onward daily additional 75 IU rLH until ovulation triggering with rhCG. |
|
| Outcomes | Primary outcome: not stated. Clinical effects: not defined. |
|
| Notes | No data on live births. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Those who administered the intervention were not blinded but non‐blinding of outcome assessment is not likely to affect the outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No study protocol available. |
| Selective reporting (reporting bias) | Unclear risk | No data on live births. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Bosch 2011.
| Methods | Prospective, randomised study, open‐label, single centre. Randomisation method: computer‐generated lists. Power analysis: A sample size of 311 women were needed to detect a difference of 10% in implantation rate (25% to 35%). Study period: From January 2005 to December 2007. Sample size: 720. Conflict of interests: not stated. |
|
| Participants | Normo‐ovulatory women with an indication for IVF or ICSI in good health, without uterine abnormalities or recurrent miscarriages. Aged ≥ 36 years < 40. BMI < 30. Basal serum FSH < 12 IU/L. Exclusion criteria: history of recurrent pregnancy loss, any significant systemic disease or endocrine or metabolic disorder, a low response to gonadotropin stimulation in a previous cycle, a basal LH/FSH ratio > 2, any indication for preimplantation genetic diagnosis or screening, or concomitant medication interfering with the purposes of the study. Baseline characteristics to compare: age, BMI, indication IVF/ICSI, basal FSH. |
|
| Interventions | Short pituitary downregulation with antagonist protocol, pre‐treated with OC on the second day of the withdrawal bleeding. Standard treatment: 300 IU rFSH was started. Experimental treatment: rFSH and rLH group, 225 IU/l rFSH was started with daily 75 IU rLH. On the stimulation day 6 women received 0.25 mg Cetrorelix was started until ovulation triggering with hCG. |
|
| Outcomes | Primary endpoint: implantation rate (gestational sacs at 4 weeks gestation per 100 embryos transferred). Secondary endpoints: clinical (gestational sac with positive heartbeat at 5 weeks gestation) and ongoing (viable foetus at 20 weeks gestation) pregnancy rates, total amount of retrieved oocytes and the incidence of OHSS (not defined). |
|
| Notes | Power analysis based on implantation rate. Further information sought after. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated lists. |
| Allocation concealment (selection bias) | Low risk | Centrally allocated to treatment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessment is not likely to affect the outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Outcome measures were not prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Caserta 2011.
| Methods | Prospective, randomized, controlled, open, multicentric, group comparative clinical trial. Randomisation method: randomly assigned by sealed envelopes. Power analysis: not stated. Study period: from 2005 to April 2010. Sample size: 999 women. Conflict of interests: no. |
|
| Participants | Women with an indication for IVF or ICSI. Age: ≤ 40 years. Basal FSH ≤ 12 mIU/Ml. Exclusion criteria: > 3 previous unsuccessful assisted reproduction technique attempts, previous poor response to gonadotropin stimulation defined as < 3 preovulatory follicle, history of OHSS, polycystic ovarian syndrome, abnormal uterine cavity as evaluated by ultrasonography, presence of clinically significant system disease. Baseline characteristics to compare: mean age, body mass index, duration of sterility, primary infertility. |
|
| Interventions | Luteal started pituitary downregulation with an GnRH agonist. Standard treatment: rFSH dose of 150 IU (Gonal F1, Serono, SP, Italy) from day 2. Experimental treatment: rFSH fixed‐dose (150 IU); at the 7th day of stimulation 75 IU of rLH were added and the dose of rFSH customised according to response. |
|
| Outcomes | Primary endpoint: not stated. Secondary endpoints: number of oocytes (met II), mean number of 2 PN eggs, mean number of developed embryos, number of embryos transferred, number of patients with b‐hCG positive, number of clinical pregnancies (not defined), number of clinical developed OHSS (not defined). |
|
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used in random sequence generation not reported, study stated that: 'The randomization process was conducted by drawing sealed envelopes.....' |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This was an open trial but non‐blinding of outcome assessment is not likely to affect the outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in the proportions of withdrawals/losses to follow‐up between the two treatment groups and analysis was not on the basis of ITT. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes were not reported. |
| Other bias | Low risk | Baseline demographic characteristics were comparable between the two treatment groups. |
De Placido 2005.
| Methods | Prospective, randomized study, multicentred (7 centres). Randomisation method: in blocks of four using computer‐generated random number tables. Power analysis: a sample size of 55 patients in each group would have 80% power to detect a mean difference of 2.0. in mean number of retrieved oocytes. Study period: from February to December 2003. Sample size: 260. Conflict of interests: this study was realised with grants from the Ministero dell'Istruzione, dell'Università e della Ricerca. |
|
| Participants | Normo‐ovulatory women with an indication for IVF/ICSI and hysteroscopic evidence of a normal uterine cavity within the last 6 months. Age 18‐37 years. Basal FSH <= 9 IU/l. Exclusion criteria: BMI < 18 or > 28 kg/m2, biochemical and/or ultrasonographic evidence of polycystic ovarian syndrome, stage III‐IV endometriosis, chromosomal abnormalities, endocrinological and/or autoimmune disorders, more than two previously unsuccessful IVF or ICSI cycles, the presence of only one ovary. Baseline characteristics to compare: age, BMI, duration of infertility, basal FSH and indication for IVF/ICSI. |
|
| Interventions | Follicular started pituitary downregulation with GnRH agonist triptorelin 3.75 mg depot. 150‐300 IU rFSH. On stimulation day 5 women with an inadequate response (serum E2 levels < 180 pg/ml and ultrasound evidence of at least six follicles with a mean diameter between 6 mm and 10 mm, but with no follicle with a mean diameter of > 10 mm). Standard treatment: receive from stimulation day 6 onward rFSH in a step‐up protocol (daily increasing the dose with 150 IU/L) alone. Experimental treatment: rFSH in combination with 150 IU/L rLH until ovulation triggering with hCG. |
|
| Outcomes | Primary endpoint: the mean number of oocytes. Secondary endpoints: cumulative pregnancy rate (positive pregnancy test), cumulative ongoing pregnancy rate (pregnancies reaching 12 weeks of gestation), cumulative abortion rate (not defined), duration of stimulation, number MII oocytes, fertilisation rate, and cancellation rate. |
|
| Notes | Only data of the two truly randomized groups were included. Funding: This study was realised with grants from the Minestero dell'Istruzione, dell'Universita e della Ricerca |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done in blocks of four using computer‐generated random number tables. |
| Allocation concealment (selection bias) | Low risk | Adequate, realised via a central telephone number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportions of withdrawals and reasons for withdrawals differ between the two treatment groups and data were not analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | High risk | Outcome measures were not prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Demirol 2005.
| Methods | Prospective, randomized study, single centre. Randomisation method: not stated. Power analysis: not stated. Study period: not stated. Sample size: 106 patients. Conflict of interests: not stated. |
|
| Participants | Women with previous failed IVF cycle due to poor response (number of oocytes < 3 maximal E2 < 500 pg/ml). Age: not stated. FSH: not stated. Exclusion criteria: not stated. Baseline characteristics to compare: age, BMI, FSH, type of infertility. |
|
| Interventions | Short GnRH antagonist cetrorelix protocol. On cycle day 2 450 IU rFSH alone was started in a step‐down protocol with 150 IU rLH and on stimulation day 6 cetrorelix until ovulation triggering with HCG. | |
| Outcomes | Primary outcome: not stated. Secondary outcome: cancellation rate (defined), duration of stimulation, number of follicles and oocytes, fertilisation rate, implantation rate (not defined) and pregnancy rate (not defined). |
|
| Notes | Abstract only. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | insufficient information to make a conclusive judgement. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Dravid 2015.
| Methods | Prospective, randomized study, single centre. Randomisation method: not stated. Power analysis: not stated. Study period: between 2012 and 2014. Sample size: 106 patients. Conflict of interests: not stated. |
|
| Participants | Women with poor ovarian response classified on the basis of low AMH levels and antral follicle count. Age: not stated. FSH: not stated. Exclusion criteria: not stated. Baseline characteristics to compare: demographic or clinical differences. |
|
| Interventions | Short GnRH antagonist protocol. Standard treatment: rFSH only, 150 IU. Experimental treatment: addition of LH 75 IU from stimulation day 6. |
|
| Outcomes | Primary endpoint:
Secondary endpoints:
|
|
| Notes | Abstract only. Funding: Funded by hospital/clinic(s) – self funded by our own IVF clinic: Vaunshdhara Clinic and assisted Conception Centre, Nagpur |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information. |
| Selective reporting (reporting bias) | Unclear risk | No information. |
| Other bias | Low risk | It was reported that 'There were no demographic or clinical differences between the two study groups'. |
Evangelio 2011.
| Methods | Randomised prospective study. Randomisation method: not stated. Power analysis: not stated. Study period: June 2007 to January 2009. Sample size: 90 women. Conflict of interests: not stated. |
|
| Participants | Patients who met at least one of these low response criteria: > 37 years, basal FSH > 10, history of previous cycle cancelled by low response or < 4 follicles on the day of the puncture. Baseline characteristics to compare: age, body mass index (BMI) and basal hormonal parameters (FSH, LH and E2). Exclusion criteria: azoospermia. |
|
| Interventions | Short GnRH antagonist cetrorelix protocol. Standard treatment: rFSH only, fixed‐dose calculated according to expected response. Experimental treatment: addition of LH to rFSH during ovarian stimulation phase (in a 1: 2 0 1: 3 to FSH). |
|
| Outcomes | Primary endpoints:
Secondary endpoints:
|
|
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Fabregues 2011.
| Methods | Prospective, randomized parallel‐group study. Randomisation method: computer‐generated simple randomization table. Power analysis: provide power of 80% to detect this magnitude of treatment effect was calculated as 52 patients per group, using a two‐tailed analysis with a detection limit of 5% of avoiding a type I error in hypothesis testing. Study period: between January and June 2006. Sample size: 187 patients. Conflict of interests: not stated. |
|
| Participants | Normogonadotrophic infertile patients. Age: 35‐41 years. BMI: range 19.8‐27.6 kg/m2. FSH: ≤ 12 IU/l on day 2‐4. Exclusion criteria: receiving any hormone therapy, including gonadotrophins, for at least 6 months preceding the study. Baseline characteristics to compare: age, BMI, duration of infertility, infertility factor, basal FSH, basal LH, basal E2. |
|
| Interventions | Pituitary downregulation with GnRH agonist. Standard treatment: rFSH alone (Group 1). Experimental treatment: rFSH in combination with rLH in one of two daily doses: 37.5 IU (Group 2) or 75 IU (Group 3). |
|
| Outcomes | Primary endpoint: pregnancy rate. Secondary endpoints: the number of developing follicles, plasma E2 level on the day of hCG administration, total FSH dose, numbers of metaphase II oocytes and embryos, cancellation rate, implantation rate. |
|
| Notes | Funding: This work was supported, in part, by a grant from the Agència de Gestió d́Ajuts Universitaris i de Recerca ‐ Generalitat de Catalunya | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22 losses to follow‐up and no information given on whether or not the analysis was on an ITT basis. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Fernandez‐Ramirez 2006.
| Methods | Prospective, randomized study, single centre. Randomisation method: Power analysis: not stated. Study period: between January and June 2006. Sample size: 34 patients. Conflict of interests: not stated. |
|
| Participants | Women in good health with a regular menstrual cycle and both ovaries present. Age: < 37 years. BMI < 30. Exclusion criteria: known HIV, HBV or HCV, prolactin serum level of > 25 ng/ml, suffering any clinically significant systemic disease mind, hypothalamic or pituitary tumour, ovarian, uterine or breast cancer, endocrine disease and/or medical, biochemical or hematological disorders, to have followed more than 3 previous cycles of assisted reproduction, have cryopreserved embryos with the same partner, presence of vaginal bleeding of unknown cause, PCOS, known allergy to gonadotropins, drug abuse, drug abuse or alcoholism in the past five years. Baseline characteristics to compare: age, BMI, years infertility, cause of infertility. |
|
| Interventions | Short pituitary downregulation with GnRH antagonist cetrorelix. Standard treatment: on second or third day of the menstrual cycle 300 rFSH or 400 rFSH was started. Experimental treatment: rFSH and 75 IU rLH twice daily and when the leading follicle reached 14 mm. |
|
| Outcomes | Primary endpoint: not stated. Secondary endpoints: number of punctured follicles oocytes, number of metaphases II, IVF fertilisation rate and ICSI fertilisation rate, progesterone levels, E2, FSH, LH. |
|
| Notes | Article in Spanish. Funding: this study is a part of Serono Laboratories |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was given on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information was given on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29.4% cancellation rate. |
| Selective reporting (reporting bias) | High risk | Outcome measures were not prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Ferraretti 2004.
| Methods | Prospective, randomized study, single centre. Randomisation method: not stated. Power analysis: Study period: January 2002 to April 2004. Sample size: 1009. Conflicts of interest: not stated. |
|
| Participants | Normo‐ovulatory women with inadequate response on COS and no previous ovarian stimulation within 6 months, normal uterine cavity, presence of both ovaries, normal karyotypes in both partners. Age < 37 years. BMI < 27 kg/m2. AFC > 10. Baseline characteristics to compare: age and indication IVF. Exclusion criteria: not stated. |
|
| Interventions | Luteal started pituitary downregulation with an GnRH agonist. 150 IU rFSH was started for ovarian stimulation in patients < 30 years, 225 IU 30‐37, and 300 IU >= 38 years. Standard treatment: increasing the dosage of rFSH to 450 IU alone. Experimental treatment: rFSH in combination with 75‐150 IU rLH until ovulation triggering with HCG. |
|
| Outcomes | Primary endpoints: pregnancy rate (not defined) per embryo transfer, implantation rate (number of gestational sacs per total number of embryos transferred), live birth rate per started cycles. Secondary endpoints: rFSH dose used, mean number of oocytes, fertilisation rate, cleavage rate, number of cryopreserved oocytes for OHSS, number of fresh embryo transfer's, number of pregnancies after 2PN thawing, abortion rate. |
|
| Notes | BMI and duration of infertility not stated. Miscarriage rate not stated. Incidence of multiple pregnancies was not stated. The data the third group C is not included. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information given on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportions of withdrawals/losses to follow‐up were imbalanced between the two treatment groups (Group A: 0/54; Group B: 4/54) and analysis was not based on ITT. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Ferraretti 2014.
| Methods | Prospective, randomized study, single centre. Randomisation method: not stated. Power analysis: not stated. Study period: between 2008 and 2010. Sample size: 43 patients. Conflicts of interest: no conflict of interests. |
|
| Participants | Women with normo‐ovulatory cycles, both ovaries, normal uterine cavity, normal karyotype and a history of repeated poor responses. Age: ≤ 38 years. FSH: not stated. AFC: not stated. Exclusion criteria: not stated. Baseline characteristics to compare: age, infertility factor, mean base level of FSH. |
|
| Interventions | Pituitary downregulation with GnRH agonist or antagonist. Standard treatment: maximal stimulation with 400 IU of rFSH per day. Experimental treatment: pre‐treatment with rLH (150 IU/day for 4 days) preceding the administration of 400 IU/day of rFSH. |
|
| Outcomes | Primary endpoints: the incidence of cycle cancellation and the live birth rate per started cycle. Secondary endpoints: the number of collected eggs, the cleavage rate, and the implantation rate. |
|
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was given on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants dropped out of the study and all participants were included in data analysis. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Fábreques 2006.
| Methods | Prospective, randomized study, single centre. Randomisation method: by means of a computer‐generated randomization table, in sealed envelopes Power analysis: a sample size of 104 patients was needed to detect a difference of 23.3% in pregnancy rate (gestational sac seen by ultrasound) with a power of 80%. Study period: November 2003 to September 2004. Sample size: 120 patients. Conflicts of interest: not stated. |
|
| Participants | Normagonadotropic women with an indication for IVF/ICSI with both ovaries present and normal, no previous ovarian surgery, basal FSH < 12 IU/l. Age: > 35 < 42. BMI: between 19‐28. Exclusion criteria: hormone therapy in the past 6 months. Baseline characteristics to compare: age, BMI, duration of infertility and baseline FSH. |
|
| Interventions | Luteal started pituitary downregulation with an GnRH agonist 0.1 mg daily triptoreline reduced to 0.5 mg once ovarian arrest have been achieved i.e. serum estradiol < 30 pg/ml and absence of follicles > 10 mm. Standard treatment: 450 IU/l rFSH was started in a step‐down regimen (stimulation day 2 300 IU/l, stimulation day 3 and 4 150 IU/l and from stimulation day 5 onward adjusted according to ovarian response) with rFSH alone until ovulation triggering with hCG. Experimental treatment: rFSH in combination with 150 IU/L rLH daily from stimulation day 6 onward until ovulation triggering with hCG. |
|
| Outcomes | Primary endpoints: not stated. Secondary endpoints: rFSH dose used, number of oocytes (MII) retrieved, total number and quality of embryos, clinical pregnancy rate (not defined), implantation rate (not defined) number of twin pregnancies, miscarriage rate (not defined). |
|
| Notes | Incidence of cryo‐survival was not mentioned. Clinical pregnancy was not defined. Funding: This research was supported in part by grants from the Instituto de Salud Carlos III (RCMN C03/08) and the Comissionat per a Universitat i Recerca‐Generalitat de Catalunya |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes for the randomization list were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the outcomes reported were prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Griesinger 2005.
| Methods | Prospective, randomized study, open‐label, single centre. Randomisation method: by means of opening a sealed envelope, before the start of the treatment. Power analysis: in order to detect a difference of 1 day in the number of gonadotropin treatment days, with a power of 81%, a total of 94 patients were needed. Study period: not stated. Sample size: 127. Conflicts of interest: not stated. |
|
| Participants | Normo‐ovulatory women with an indication for IVF/ICSI. Age: Between 20 and 39 years. AFC: not stated. FSH: not stated. BMI: > 18 < 35. Exclusion criteria: more than 3 previous unsuccessful IVF attempts, previous poor response to gonadotropin stimulation defined as < 3 preovulatory follicles; history of ovarian hyperstimulation syndrome grade II‐III; polycystic ovarian syndrome; any other endocrine disorder; no natural luteal phase prior to treatment cycle; abnormal uterine cavity as evaluated by ultrasonography; presence of a clinically significant systemic disease. Baseline characteristics to compare: age, BMI, duration of infertility. |
|
| Interventions | Short pituitary downregulation protocol with GnRH antagonist with 150 IU rFSH started on cd 2. Standard treatment: After 5 days of stimulation a GnRH antagonist was started (cetrotide 0.25 mg/day subcutaneous) alone. Experimental treatment: GnRH antagonist or in combination with 75 IU rLH subcutaneous. On stimulation day 6 the rFSH dosage was increased to 300 IU, and the rLH dose to 150 IU. |
|
| Outcomes | Primary endpoint: number of stimulation days. Secondary endpoints: rFSH dose used, total number of retrieved MII oocytes (in ICSI cases), fertilisation rate, total number of embryos, biochemical pregnancy (defined as HCG > 10 mIU/L 14 days after embryo transfer, clinical pregnancy rate (defined as an ongoing pregnancy at 12 weeks of gestation), implantation rate (defined as the number of gestational sacs per number of embryos transferred, miscarriage rate (defined as pregnancy loss before 12 weeks of gestation). |
|
| Notes | No data on cryo‐survival. No data on multiple pregnancies. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up; ITT analysis. |
| Selective reporting (reporting bias) | Low risk | All the outcomes reported were prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Humaidan 2004.
| Methods | Prospective randomized study, open‐label, single centre. Randomisation method: by means of a computer programme generating random numbers in sealed unlabelled envelopes. Power analysis: 100 cycles were needed to obtain a statistical significant difference of 10% in pregnancy rates (not defined) in favour of rLH. Study period: From November 2001 to October 2002. Sample size: 231. Conflict of interests: not stated. |
|
| Participants | Normo‐ovulatory women undergoing IVF or ICSI. Age: < 40 AFC: not stated FSH: < 10 IU/l Baseline characteristics to compare: age, BMI, the number of previous IVF attempts. |
|
| Interventions | Luteal started pituitary downregulation with a GnRH agonist Suprefact 0.5 mg subcutaneous daily for 14 days, then to 0.2 mg subcutaneous. The rFSH dose depended on age (< 35 150 IU/l > 35 225 IU/l), BMI and ovarian volume. On stimulation day 8 patients were randomized. Standard treatment: rFSH alone (adjusted if necessary). Experimental treatment: rFSH in combination with LH in a 2:1 ratio. |
|
| Outcomes | Primary endpoint: clinical pregnancy rate (defined as positive foetal heart beat 5 weeks after embryo transfer) Secondary endpoints: rFSH dose used, number of oocytes retrieved total days of stimulation, implantation rate. |
|
| Notes | No data on ongoing pregnancy or life birth rate, no data on cryo‐survival or multiple pregnancies. No data on cancellation rate. The duration of infertility not stated. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed, unlabelled envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessment is not likely to affect the outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Outcome measures were prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics were similar between the two treatment groups. |
Konig 2013.
| Methods | Prospective, randomized, multicentre, controlled trial. Randomisation method: serially numbered, opaque, sealed envelopes and was stratified per centre. Power analysis: 275 patients per group was required to prove that treatment with rLH yields 10% more ongoing pregnancies than without rLH treatment. Study period: January 2004 and September 2010. Sample size: a total 250 patients. Conflict of interests: None to declare. |
|
| Participants | Normo‐ovulatory women undergoing IVF/ICSI. Age: between 35 and 43 years. AFC: Not stated. FSH: Not stated. Exclusion criteria: history of a high (> 15 oocytes) ovarian response, polycystic ovary syndrome, stage III–IV endometriosis. Baseline characteristics to compare: age, BMI, duration of infertility, diagnosis, AFC, E2, FSH, LH, progesterone, testosterone. |
|
| Interventions | rFSH (Gonal‐F 225 IU/day) starting from cycle day 3 and GnRH antagonist (Cetrotide 0.25 mg/day) from stimulation day 6. Standard treatment: rFSH alone. Experimental treatment: rFSH and rLH (Luveris 150 IU/day). |
|
| Outcomes | Primary endpoint: clinical pregnancy rate and implantation rate. Secondary endpoints: ongoing pregnancy rate, cancellation rate, number of developed follicles 15 mm on the day of hCG administration, number of retrieved oocytes. |
|
| Notes | Funding: This study was supported by the Foundation for Gynecological Research and Education, Amsterdam, The Netherlands. The funding sources did not influence the design, collection, management, analysis or interpretation of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was achieved using random permutation table. |
| Allocation concealment (selection bias) | Low risk | Allocations were concealed using sealed opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was reported on the blinding of outcome assessment; however, non‐blinding of outcome assessors is unlikely to affect outcomes of interest as they are objective in nature. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawal and rates of withdrawal were fairly similar between treatment groups, data were analyzed using ITT. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two groups. |
Kovacs 2010.
| Methods | Prospective randomized study, single centre. Randomisation method: in two blocks, method not stated. Power analysis: not stated. Study period: not stated. Sample size: 50. Conflicts of interest: nothing to disclose. |
|
| Participants | Women with normal ovarian function undergoing their first or second IVF attempt. Age: < 40. AFC: not stated. FSH < 10 IU/l. Baseline characteristics to compare: age, baseline FSH and E2, suppression LH and E2, amount of gonadotropin. |
|
| Interventions | Long luteal started pituitary downregulation with GnRH agonist with Suprefact 0.5 mg subcutaneous for 10 to 12 days until ovarian suppression was achieved than 75 IU rLH daily was started (cd 1) followed by 150 IU rFSH on cd 2. rLH was administered for 4 days and rFSH for 5 days. On cd 5 rFSH was adjusted if necessary and continued until ovulation triggering with rhCG. Standard treatment: 75 IU of rLH daily for 4 days and recombinant FSH (rFSH, Gonal F, Merck‐Serono) at a fixed starting dose of 150 IU for the first 5 days was started a day later, on day 2 of rLH. Experimental treatment: rFSH at a fixed‐dose of 150 IU for the first 5 days at suppression. |
|
| Outcomes | Primary endpoint: effect on ovarian stimulation. Secondary endpoints: number of follicles, oocytes, high‐quality embryos, cryo‐preserved embryos and biochemical pregnancies (defined as serum bhCG) clinical pregnancies (defined as gestational sac two weeks after embryo transfer) and ongoing pregnancy rate (defined as positive heartbeat at 4 weeks after embryo transfer). |
|
| Notes | No power analysis. Duration of infertility not stated. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization (blocks of two). |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Levi‐Setti 2006.
| Methods | Prospective randomized study, single centre. Randomisation method: by computer‐generated list. Power analysis: a sample size of 38 women was necessary to detect a difference of 2 oocytes with a power of 80%. Study period: not stated. Sample size: 40. Conflict of interests: not stated. |
|
| Participants | Women with an indication for ICSI, male factor, normal menstrual cycle 25‐35 days, BMI < 25, no more than 3 previous cycles. Age: < 37. AFC: not stated. FSH < 12. Basal characteristics to compare: age, basal FSH, BMI, duration of infertility and number of previous cycles. |
|
| Interventions | Pituitary downregulation with GnRH antagonist in short protocol. Standard treatment: rFSH alone. Experimental treatment: rFSH and rLH combined. |
|
| Outcomes | Primary endpoint: mean number of retrieved MII oocytes. Secondary endpoints: serum oestrogen mean total number of oocytes, fertilisation rate, embryo‐quality, ongoing pregnancy rate (defined as pregnancies > 12 weeks gestation), implantation rate. |
|
| Notes | No data on cryo‐survival. No data on multiple pregnancies. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two groups. |
Lisi 2005.
| Methods | Prospective, randomized, trial, single centre, in a private setting.
Randomisation method: with a computer‐generated random number programme.
Power analysis: not stated. Study period: not stated. Sample size: 428. Conflicts of interest: not stated. |
|
| Participants | Women undergoing IVF who had a body mass index > 18 or < 35 and no abnormal karyotype, anovulation, oligomenorrhoea, or any known endocrinopathy/illness. Age: not stated. Exclusion criteria: BMI <18 or >35, an abnormal karyotype, anovulation, oligomenorrhoea, or any known endocrinopathy illness. Baseline characteristics to compare: age, indication for IVF/ICSI basal FSH or number of previous cycles were comparable. |
|
| Interventions | Long luteal started pituitary downregulation with GnRH agonist. Ovarian stimulation was started with 150 IU rFSH. Standard treatment: no further supplementation. Experimental treatment: daily with 37.5 IU rLH from day 7. Experimental treatment: daily 75 IU rLH from day 7. |
|
| Outcomes | Primary endpoint: number of clinical pregnancies, defined as patients who are in their third trimester and in whom a foetal heartbeat had been monitored, or who have already delivered, proportions of embryos by grade. Secondary endpoints: multiple pregnancy rate. |
|
| Notes | No data on cryo‐survival. Patients BMI and duration of infertility not stated. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number programme. |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Unclear risk | Although patients were initially selected on the basis of randomization by allocation of treatment at consultation (approximately half for rLH and half for rFSH with rLH), the final division for those receiving treatment during the study period was 56%, 25%, and 18% for rFSH only, 37.5 IU rLH, and 75 IU rLH, respectively (groups A, B, and C, respectively). |
Lisi 2012.
| Methods | A prospective, randomized, open‐label, multicentre study.
Randomisation method: using block randomization (Block of 1:1).
Power analysis: not stated. Study period: June 2009 to December 2010. Sample size: 150 patients. Conflicts of interest: the authors declare no conflict of interest. |
|
| Participants | Women with infertility caused by tubal factors, male factors or of unknown cause, at their first or second attempt of IVF or ICSI. Age: < 40 years old. FSH: < 10 IU/l on day 3 of their cycle. AFC: not stated. Exclusion criteria: patients with endometriosis or polycystic ovarian syndrome and patients with a body mass index above 28.0 or below 18.0. Baseline characteristics to compare: age, FSH, LH, E2. |
|
| Interventions | Long luteal started pituitary downregulation with GnRH agonist. Standard treatment: rFSH (Gonal F, Merck‐Serono, Geneva, Switzerland) at a starting dose of 150 IU for 6 days and at the 7th day of rFSH the dose was adjusted according to individual response. Experimental treatment: 75 IU of rLH daily for 4 days (total dose 300 IU) and rFSH (starting from day 2 of rLH administration) at a fixed starting dose of 150 IU for the first 6 days and, at the 7th day of rFSH dose of rFSH was adjusted according to the individual response. |
|
| Outcomes | Primary endpoint: not stated. Endpoints: oocytes retrieved per patient (total), oocytes metaphase II insemination/per patient (total), 2PN oocytes (fertilisation rate) embryos, total (cleavage rate), embryos, total grades I and II (%), no. of patients receiving embryos (%), no. of embryos transferred per starting patients (total), no. of hCG positive (% of patients receiving embryos), no. of clinical pregnancies (% of patients receiving embryos), no. of foetal hearts (implantation rate). |
|
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence was said to have been generated using block randomization but what was used in generating the block was not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although there were no losses to follow‐up in the trial, not all participants were analyzed for the outcome of interest in this review (clinical pregnancy rate). |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Marrs 2003.
| Methods | Prospective randomized, open‐label study, multicentred.
Randomisation method: a computer‐generated randomization sequence.
Power analysis: a group of 280 patients would give 80% power to detect the expected a difference of 8.2% in obtained number of metaphase II oocytes. Study period: not stated. Sample size: 431. Conflict of interests: not stated. |
|
| Participants | Patients with normal ovulatory cycles, the presence of both ovaries, a male partner and an ICSI indication. Age: between 18 and 40 years. FSH: < 11.2. Exclusion criteria: clinically significant systemic disease; smoking more than 10 cigarettes a day; any contraindication to pregnancy; serum/plasma LH; FSH ratio > 2; more than 2 previous ICSI cycles in which gonadotrophin stimulation was used. Baseline characteristics to compare: Age, BMI, duration of infertility, previous assisted reproduction cycles rFSH+rLH. |
|
| Interventions | Luteal started pituitary downregulation with GnRH agonist.When serum E2 < 75 pg/ml 225 IU rFSH was started. Standard treatment: rFSH dosage alone. Experimental treatment: rFSH in combination with 150 IU rLH until hCG. |
|
| Outcomes | Primary endpoint: number of metaphase II oocytes retrieved. Secondary endpoints: cancellation rate, fertilisation rate, rFSH dose used, number of embryo's obtained, biochemical, clinical pregnancy rate, implantation rate and live birth rate. |
|
| Notes | No data on multiple pregnancies. No data on cryo‐survival. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers of women analyzed at the end of study were the same as those randomized at the beginning. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Unclear risk | Baseline demographic characteristics were similar between the two groups. |
Matorras 2009.
| Methods | Single centre, randomized, parallel group, comparative study. Randomisation method: not stated. Power analysis: power of 80% to detect a significant difference of 20% in the number of MII oocytes retrieved and provided for a significance level of 0.05. The resulting calculation required a total of 124 enrolled patients. Study period: January 2005 and November 2006. Sample size: 138. Conflict of interests: not stated. |
|
| Participants | Normo‐ovulatory women with an uterine cavity capable of sustaining a pregnancy and presence of both ovaries. Age: between 35 and 39 years. FSH: < 10 IU/L. Exclusion criteria: (i) human immunodeficiency virus or hepatitis B virus/hepatitis C virus positive; (ii) clinically significant condition preventing them from undergoing gonadotrophin treatment; (iii) more than two previous assisted cycles; (iv) cancellation of two previous cycles; (v) cryopreserved embryos available from previous assisted reproduction treatment; (vi) unexplained gynaecological bleeding; (vii) polycystic ovary or an ovarian cyst of unknown aetiology; (viii) pregnancy contraindication; (ix) active substance abuse; (x) simultaneous participation in another trial or reentry in the current trial; and (xi) refusal or inability to comply with the procedures set forth in the protocol. Baseline characteristics to compare: age, BMI, infertility duration, FSH, LH, oestradiol, no. previous children, tubal factor, male factor, endometriosis, mixed cause, unknown cause, no. of previous IVF/ICSI cycles, sperm parameters. |
|
| Interventions | Luteal started pituitary downregulation with Gnrh‐agonist Decapeptyl 0.1 mg/day. When serum E2 < 30 pg/ml ovarian stimulation was started with 300‐450 IU rFSH, at a fixed‐dose until stimulation day 6. After randomization 150 IU rLH was administrated until ovulation triggering with hCG. | |
| Outcomes | Primary endpoint: number of metaphase II oocytes retrieved. Secondary endpoints: Cancellation rate, fertilisation rate, rFSH dose used, number of embryo's obtained, biochemical, clinical pregnancy rate, implantation rate and live birth rate. |
|
| Notes | Funding: This study was partially supported by a grant from Merck Farma y Química, SL, an affiliate of Merck, Darmstadt, Germany. The authors thank Hannah Wills and Carol Cooper of Caudex Medical (supported by Merck Serono, Geneva) for their editorial assistance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers of women analyzed at the end of study were the same as those randomized at the beginning. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were prespecified in the methods section. |
| Other bias | Low risk | Baseline characteristics were similar between the two groups. |
Mohseni 2013.
| Methods | Randomised study. Randomisation method: not stated. Power analysis: not stated. Study period: January 2005 and November 2006. Sample size: 40. Conflict of interests: not stated. |
|
| Participants | Normoresponder patients. Age: mean age 31.5. FSH: not stated. Exclusion criteria: not stated. Baseline characteristics to compare: age. |
|
| Interventions | Long luteal GnRH agonist protocol. Standard treatment: rFSH alone, dose not stated. Experimental treatment: rFSH and rLH, dose not stated. |
|
| Outcomes | Primary endpoint:
Secondary endpoints:
|
|
| Notes | Abstract only. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on whether or not outcome assessors were blinded but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information. |
| Selective reporting (reporting bias) | Unclear risk | No information. |
| Other bias | Unclear risk | Abstract only. |
Musters 2012.
| Methods | Randomised controlled trial. Randomisation method: central web‐based randomization was performed prior to the start of ovarian stimulation using a computer programme minimisation procedure with stratification according to study centre. Power analysis: 520 embryos per treatment arm. Assuming a mean number of five embryos are available per woman this means that 104 women would have to be included per arm. Study period: August 2008 and April 2010. Sample size: 116 women to the rLH group and 128 allocated to the control group. Conflict of interests: The authors thank Merck Serono for the donation of the rLH (Luverisw) and the HCG (Ovitrellew). |
|
| Participants | Women who were scheduled for their first IVF or ICSI in the Academic Medical Center or the Onze Lieve Vrouwe Gasthuis in Amsterdam. Age: 35‐41 years old or younger than 35 years. FSH: 12 IU/ml. AFC: ≤ 5. Exclusion criteria: any endocrinopathological disease: Cushing’s syndrome, adrenal hyperplasia, hyperprolactinaemia, acromegaly, hypothalamic amenorrhoea, hypothyroidism and diabetes mellitus type I or polycystic ovary syndrome. |
|
| Interventions | Women underwent OS after downregulation with the GnRH agonist triptorelin (Decapeptylw) in a long protocol with a midluteal start. Standard treatment: OS was started on cycle day 5 with rFSH (GONAL‐fw, MerckSerono). Experimental treatment: rFSH with addition of rLH. Depending on the AFC, women started with different doses of gonadotrophins. If the AFC was three or lower on cycle day 5, women started with a maximal stimulation of 450 IU rFSH and 225 IU rLH or 450 IU rFSH alone. If the AFC was between 4 and 14 follicles on cycle day 5, women started with 300 IU rFSH and 150 IU rLH or 300 IU rFSH alone. If the AFC was 15 or higher on cycle day 5, women started with 150 IU rFSH and 75 IU rLH or 150 IU rFSH alone. |
|
| Outcomes | Primary endpoint: proportion of top‐quality embryos per woman on the day of transfer. Secondary endpoints: number of stimulation days until hCG administration, number of follicles 17 mm on the day of hCG administration, number of oocytes, the fertilisation rate, the number of women with top‐quality embryos, the biochemical pregnancy rate (defined as an increase in serum HCG 3, 14 days after follicle aspiration), clinical pregnancy rate (defined as positive heartbeat on transvaginal sonography in week 8 of pregnancy), miscarriage rate and ongoing pregnancy rate (defined as a positive heartbeat at 12 weeks gestational age). |
|
| Notes | Funding: The authors thank Merck Serono for the donation of the rLH (Luverisw) and the HCG (Ovitrellew). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes of interest are objective in nature and non blinding of outcome assessors is not likely to affect their measurement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers of women analyzed at the end of study were the same as those randomized at the beginning. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were specified in the methods section. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Nazzaro 2012.
| Methods | Randomised controlled trial. Randomisation method: not stated. Power analysis: not stated. Study period: not stated. Sample size: 422 patients. Conflict of interests: not stated. |
|
| Participants | Women without ovulatory dysfunction at their first IVF/ICSI cycle. Age: 35‐42 years. FSH: not stated. AFC: maximum 3 per ovary. Exclusion criteria: not stated. Baseline characteristics to compare: not stated. |
|
| Interventions | Pituitary downregulation with GnRH‐a starting on day two of the menstrual cycle. Standard treatment: rFSH administration (225 IU/day). Experimental treatment: rFSH (225 IU/day) + rLH (rLH 150 IU/day). |
|
| Outcomes | Primary endpoint: not stated. Endpoints: number of (MFII) oocytes, fertilisation rate, mean high grade embryos, mean number of frozen embryos, implantation rate (fresh+thawed embryos) and clinical pregnancy rate. |
|
| Notes | Abstract only. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was reported on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided on the blinding of outcome assessors but non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information was reported on withdrawals/dropouts and methods used in data analysis. |
| Selective reporting (reporting bias) | Unclear risk | Methods section not detailed enough to make a definite judgement on selective outcome reporting. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Nyboe Andersen 2008.
| Methods | Prospective randomized, open‐label study, multicentred. Randomisation: sealed envelopes in blocks of 10 and sequentially numbered. Power analysis: with a power of 77% and P < 0.05, 400 patients were needed per arm. Study period: August 2003 to November 2004. Sample size: 526. Conflicts of interest: not stated. |
|
| Participants | Women with a regular menstrual cycle, undergoing their first, second or third cycle of IVF or ICSI. Age: < 40. FSH (cd2‐5) < 10 IU/l. Exclusion criteria: not stated. Baseline characteristics to compare: age, duration of infertility, cycle number, indication IVF/ICSI. |
|
| Interventions | Luteal started pituitary downregulation with a long agonist protocol. Standard treatment: rFSH alone. Experimental treatment: rFSH with rLH from Day 6 of stimulation. |
|
| Outcomes | Primary endpoint: ongoing pregnancy rate. Secondary endpoints: total dose rFSH, number of stimulation days, number of oocytes retrieved. |
|
| Notes | The planned sample size was not reached. BMI was not stated. Funding: Serono Nordic provided Luveris (rLH), and funded the central measurements of serum LH. The statistical Unit, Serono International, Switzerland, did the statistical analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The process used in random sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessors is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | High risk | Only the primary endpoint is stated in the methods section. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Pezzuto 2010.
| Methods | Prospective randomized study. Randomisation:computer‐generated randomization list. Power analysis: not stated. Study period: March 2004 to October 2007. Sample size: 80. Conflict of interests: No conflicts of interest. |
|
| Participants | Healty woman undergoing IVF, with a regular mens. cycle and a normal uterine cavity after hysteroscopy. Age: between 20 and 39 years. FSH: < 10 IU/L. Exclusion criteria: not stated. Baseline characteristics to compare: mean age, BMI, baseline serum FSH, baseline serum LH, number of tubal factors, male factors, unexplained factors and endometriosis. |
|
| Interventions | Long GnRH agonist protocol. Standard treatment: 14 days after downregulation with leuprorelin, ovarian stimulation was initiated only with rFSH 225 IU. Experimental treatment: at the same time stimulation was initiated with rFSH 225 IU associated with rLH 75 IU on cycle day 6 of stimulation. |
|
| Outcomes | Primary endpoint: oocytes quality, classified into four maturation stages depending on the maturity of the oocyte‐cumulus‐corona complex. Secondary endpoints: duration of stimulation, FSH dose, serum E2 levels, follicular fluid VEGF levels, fertilisation rate, pregnancy rate. |
|
| Notes | Funding: not stated, but the authors declare that they are alone responsible for the content and writing of the paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessors is not likely to affect the outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The reported outcomes were not specified in the methods section. |
| Selective reporting (reporting bias) | Low risk | No dropouts. |
| Other bias | Low risk | Baseline characteristics similar between the two treatment groups. |
Razi 2014.
| Methods | Prospective single centre randomized control trial. Randomisation method: Randomisation was done with random numbers table. Power analysis: Not stated. Study period: 2012. Sample size: a total of 40 patients. Conflict of interests: The authors declare no conflict of interest. |
|
| Participants | Infertile women with male infertility or unexplained infertility. Age: younger than 35 years old. FSH: day 3 serum levels < 10 U/L. BMI: less than 30. Exclusion criteria: azoospermia, uterine myoma, mild endometriosis, hydrosalpinx, history of previous IVF (successful or unsuccessful), history of endocrine diseases such as diabetes or thyroid disorders, and patients who had hysteroscopic surgery due to intrauterine lesions such as uterine submucosal myoma or intrauterine adhesions. Baseline characteristics to compare: mean age, mean duration of infertility, basal LH and FSH, kind and cause of infertility. |
|
| Interventions | Pituitary downregulation with Buserelin (Cinnafact, Laboratory, Cinnagen, Iran), using a daily dose of 500 mg, subcutaneous, according to the long agonist protocol, starting on day 21 of the cycle preceding gonadotrophin treatment and continued 250 mg/daily with start of menstruation until the day of hCG administration. Standard treatment: standard long protocol (GnRH agonist) and rFSH alone. Experimental treatment: standard long protocol (GnRH agonist) and rFSH with rLH. |
|
| Outcomes | Primary endpoint: not stated. Secondary endpoints: number of retrieved oocytes, mature oocytes, cleaved embryos, transferred embryos, estradiol levels in hCG administration day, implantation rate and clinical pregnancy rate. |
|
| Notes | Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was achieved using random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessors is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants dropped out. |
| Selective reporting (reporting bias) | High risk | None of the reported outcomes were specified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Ruvolo 2007.
| Methods | Prospective randomized trial, single centre. Randomisation: computer‐generated randomization list. Power analysis: the sample size of 30 patients in each treatment group was calculated to have 80% power to detect a mean difference of 2.0, with a significance level of 0.01. Study period: from September 2004 to February 2005. Sample size: 60. Conflict of interests: not stated. |
|
| Participants | Women with low response in a failed previous IVF cycle. Age: not stated. FSH: < 12 IU/mL. Baseline characteristics to compare: mean age, BMI. |
|
| Interventions | Patients undergoing assisted fertilisation programmes treated with a GnRH agonist. Standard treatment: rLH and rFSH. Experimental treatment: rFSH alone. |
|
| Outcomes | Primary endpoint:
Secundary endpoints:
|
|
| Notes | Funding: this study is supported in part by the Italian Ministero Istruzione Universita Ricerca, Roma, Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was realised in blocks of three, using computer‐generated random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported although non blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers of women analyzed at the end of study were the same as those randomized at the beginning. |
| Selective reporting (reporting bias) | Unclear risk | Outcome measures were specified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Tarlatzis 2006.
| Methods | Prospective, randomized, placebo‐controlled trial.
Randomisation: by means of a computer programme generated by Serono International S.A.
Power analysis: In order to detect a difference of 2.5 in the mean number of MII oocytes between the two groups a 100 patients were needed. Study period: Not stated. Sample size: 123 patients. Conflict of interests: Not stated. |
|
| Participants | Patients with a normal uterus and two ovaries with an indication for IVF or ICSI.
Age: between 18 and 37 years. FSH: maximum 12 IU/l. Exclusion criteria: previous poor respondents. Baseline characteristics to compare: mean age, BMI, duration of infertility, primary/secondary infertility, cause of infertility: tubal factor, male factor, Semen characteristics: normal, abnormal. |
|
| Interventions | Follicular started, pituitary downregulation with a GnRH agonist buserelin 200 mg subcutaneous until serum E2 < 200 pmol/l and no follicle > 15 mm. 150 IU rFSH was started adjusted if necessary on stimulation day 5. Once the leading follicle reached > 14 mm, patients were randomized. Standard treatment: r‐hLH (lutropin alfa; Luveris, Laboratoires Serono S.A.), 75 IU subcutaneously for a maximum of 10 days. Experimental treatment: placebo. |
|
| Outcomes | Primary endpoint: mean number of MII oocytes. Secondary endpoints: duration of stimulation, the dose of rFSH required for stimulation, the number of fertilised embryo's, the number of cleaved oocytes, the pregnancy rate and live birth rate. | |
| Notes | No data on cryo‐survival. No data on multiple pregnancies. All IVF cycles were converted to ICSI. Funding: this study was supported by Serono. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Adequate, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was reported on blinding of outcome assessment although non‐blinding of outcome assessment is not likely to affect the outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up; ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were specified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Van der Houwen 2011.
| Methods | Prospective randomized multicentre study. Randomisation method: Not stated. Power analysis: Not stated. Study period: Not stated. Sample size: A total of 249 patients. Conflicts of interest: Not stated |
|
| Participants | Women who were undergoing IVF or ICSI. Age: 35 years or older. FSH: not stated. AFC: not stated. Exclusion criteria: not stated. Baseline characteristics to compare: not specified. |
|
| Interventions | Short pituitary downregulation protocol with GnRH antagonist with 225 IU rFSH started on cd 3. Standard treatment: rFSH alone. Experimental treatment: rFSH and rLH (Luveris 150 IU/day). |
|
| Outcomes | Primary endpoint: Not stated. Endpoints: Implantation rate (the chance of an individual embryo to implant), clinical pregnancy rate (defined as hCG > 50 IU) and ongoing pregnancy rate (defined as a pregnancy diagnosed by ultrasonographic visualisation with at least one foetus of 12 or more weeks of gestational age). |
|
| Notes | Abstract only. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was reported on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It was stated that data analysis was on ITT basis. |
| Selective reporting (reporting bias) | Low risk | All the outcomes reported were prespecified in the methods section. |
| Other bias | Unclear risk | No sufficient information was reported to make a conclusive judgement. |
Vuong 2015.
| Methods | Prospective, randomized trial.
Randomisation: by means of a computer programme.
Power analysis: 109 patients in each group would be required. Study period: 1 October 2012 to 30 June 2014. Sample size: 240 patients. Conflict of interests: No conflicts of interest. |
|
| Participants | All patients undergoing routine assisted cycles during the trial period were invited to participate. Age: ≥ 35 years. FSH: not stated. Exclusion criteria: participation in another interventional clinical trial, had PCOS, were WHO group 1, had uterine abnormalities such as uterine bicornuate, uterine cavity adhesion, and/or had endocrine disorders such as hyperprolactinaemia and thyroid disorders. Baseline characteristics to compare: age, number of treatment cycles, BMI, AMH, AFC, Cycles with reduced ovarian reserve. |
|
| Interventions | Ovarian stimulation was performed by using a GnRH antagonist protocol; rFSH was administered on day 2 or day 3 of the menstrual cycle. The first rFSH dose was individualised for each patient based on the following criteria: AFC ≤ 6, dose 300 IU/day; AFC7‐15, dose 225 IU/day; and AFC ≥ 16, dose 150 IU/day. Standard treatment: continued to receive rFSH. Experimental treatment: rLH was supplemented from day 6, 150/75 IU/day. |
|
| Outcomes | Primary endpoint: live birth rate. Secondary endpoints: clinical pregnancy rate, embryo implantation rate, miscarriage rate, duration of stimulation, total number of rFSH units used, estradiol concentrations on the hCG‐administered day, endometrial thickness on the hCG‐administered day, premature LH surge rate (>10 IU/l), number of oocytes retrieved, number of embryos, number of good embryos, number of patients with a premature rise in progesterone (>1.5 ng/ml) on the day of hCG administration, ovarian hyperstimulation syndrome, cycle cancellation due to poor response. |
|
| Notes | Funding: This study was supported by the Research Center for Genetics and Reproductive Health, School of Medicine, Vietnam National University HCMC. The authors state that they have no financial or commercial conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers of women analyzed at the end of study were the same as those randomised at the beginning). |
| Selective reporting (reporting bias) | Low risk | Outcome measures were specified in the methods section. |
| Other bias | Low risk | Baseline demographic characteristics similar between the two treatment groups. |
Younis 2014.
| Methods | Prospective randomised controlled trial. Randomisation: not stated. Power analysis: not stated. Study period: not stated. Sample size: 63 patients. Conflict of interests: not stated. |
|
| Participants | Infertile women above 35 years of age and/or with a previous low ovarian response admitted for IVF/ICSI. FSH: not stated. Exclusion criteria: not stated. Baseline characteristics to compare: not defined. |
|
| Interventions | recombinant FSH (Gonal‐F) 300 IU/day and the flexible GnRH antagonist (Cetrotide) 0.25 mg/day protocol. Standard treatment: only rFSH. Experimental treatment: on the same day of the antagonist start, rLH(Luveris) 150 IU/day was added and continued until the hCG day. |
|
| Outcomes | Primary endpoint: not stated. Endpoints: serum FSH, LH, E2 and P, follicular phase duration, number of > 14 mm follicles, oocytes, MII oocytes, 2PN zygotes, embryos and top graded embryos, endometrial thickness, implantation and pregnancy rates. |
|
| Notes | Abstract only. Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding is not likely to affect the outcomes of interest as they are objectively assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Non‐blinding of outcome assessment is not likely to affect outcomes of interest as they are objectively assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information was provided on withdrawals or losses to follow up or how the data were analyzed. |
| Selective reporting (reporting bias) | High risk | None of the reported outcomes were specified in the methods section. |
| Other bias | Unclear risk | Insufficient information reported on participants demographic characteristics to make a conclusive judgement. |
BMI: body mass index FSH: follicle‐stimulating hormone GnRH: gonadotrophin‐releasing hormone hCG: human chorionic gonadotropin HIV: human immunodeficiency virus ICSI: intracytoplasmic sperm injection ITT: intention‐to‐treat IU: international unit IVF: in‐vitro fertilisation L: litre LH: luteinizing hormone OHSS: ovarian hyperstimulation syndrome PCOS: polycystic ovary syndrome rFSH: recombinant follicle‐stimulating hormone rLH: recombinant luteinizing hormone WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acévedo 2004 | Oocyte donation programme, donors and recipients. |
| Barberi 2012 | A quasi‐randomised controlled trial. |
| Baruffi 2006 | Study design (literature review) not relevant. |
| Cedrin‐Durnerin 2004 | Pseudo‐randomised trial, according to women's birthdays. |
| Cedrin‐Durnerin 2008 | rLH was administered before OS with rFSH (as pre‐treatment). |
| De Placido 2004 | Comparison of two different rLH doses. |
| De Placido 2006 | Comparison of GnRH antagonist versus GnRH agonist. |
| Drakakis 2005 | Comparison of hMG versus rFSH. |
| Fei Yang 2013 | Compared rLH/hCG/rFSH versus HP‐hMG/rFSH/HCG versus HP‐HMG/rFSH. |
| Fermin 2013 | Compared rFSH/rLH versus rFSH/rLH using 2 different doses. |
| Garcia‐Velasco 2007 | Interventions not relevant; GnRH agonist versus cetrorelix (GnRH antagonist). |
| Gomez‐Palomares 2005 | comparison of hMG versus rFSH and rLH. |
| Hugues 2005 | Inclusion of WHO group II anovulatory women. |
| Lahoud 2010 | Study design not relevant. |
| Lisi 2003 | Not a randomised trial. |
| Motta 2006 | Use of GnRH agonist versus antagonist protocols for downregulation. |
| Papanikolaou 2010 | rLH is used as luteal support. |
| Sauer 2004 | Interventions not relevant: GnRH agonist (leuprolide) + rFSH versus GnRH antagonist (cetrorelix) with or without rLH. |
| Sills 1999 | Comparison of HP‐FSH versus rFSH. |
| Tesarik 2002 | Comparison of hMG versus rFSH. |
| Topercerová 2005 | Interventions not relevant. |
Abbreviations:
AFC: antral follicle count
AMH: anti‐Mullerian hormone
b‐hCG: beta hCG
OS: ovarian stimulation
E2: estradiol
FSH: follicle ‐stimulating hormone
GnRH: gonadotrophin‐releasing hormone
HBV: hepatitis B
HCV: hepatitis C
hCG: human chorionic gonadotrophins
hMG: human menopausal gonadotrophin
HP‐hMG: highly purified hMG
HMG: human menopausal gonadotrophins
mIU: milli‐international units
OC: oral contraceptive
PN: pronucleus
PCOS: polycystic ovary syndrome
rhCG: recombinant hCG
rLH: recombinant luteinizing hormone
rFSH: recombinant luteinizing hormone
IU: international units
VEGF: vascular endothelial growth factor
WHO: World Health Organization
Differences between protocol and review
Methods: In this updated review (2017) we have utilized current Cochrane methods (including use of GRADE, summary of findings tables and searching of clinical trials registers) that we did not plan at protocol stage.
Other changes since the protocol was published include the following:
1. The title. The protocol title was ‘Recombinant luteinizing hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles’
2. The authors
3. The outcome measures:
In this update (2017), in order to focus on the most clinically relevant outcomes, we decided not to include the following intermediate outcomes that were planned in the protocol:
Gonadotrophin total dose used per treatment in units
Number of oocytes retrieved per treatment
Number of grade I, II and III embryos per treatment
Number of frozen embryos and cryo‐survival after thawing
For the same reason we added ongoing pregnancy rate to the review, and split cancellation rate into two separate outcomes.
4. Subgroup analyses:
In this update we performed subgroup analyses to evaluate the effectiveness of recombinant luteinizing hormone (rLH) combined with recombinant follicle‐stimulating hormone (rFSH) compared to rFSH alone in IVF/ICSI cycles in women with poor ovarian response and in women of advanced age. A beneficial effect of rLH combined with rFSH in women with poor ovarian response (Placido 2001), and in women of advanced age (Hill 2012), was suggested earlier.
Contributions of authors
M Mochtar and M v Wely wrote the protocol and the original review.
In the 2017 update, N Danhof and R Ayekele independently selected eligible studies and extracted data. N Danhof and R Ayeleke contributed to data entry, interpretation of results and discussion of review findings. Differences of opinion were registered and resolved by consensus together with M Mochtar.
M Mochtar, F vd Veen, and M v Wely took part in interpretation of the data and writing of the review.
Sources of support
Internal sources
None detailed by the review authors, Other.
External sources
No sources of support supplied
Declarations of interest
Monique H Mochtar: none known
Nora A Danhof: none known
Reuben Olugbenga Ayeleke: none known
Fulco van der Veen: none known
Madelon van Wely: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abdelmassih 2006 {published data only}
- Abdelmassih V, Salgueiro R, Abdelmassih C, Carizza C. Less miscarriage rate using LH (rLH) in GnRH agonists long protocols. Twenty‐Second Annual Meeting of the European Society of Human Reproduction and Embryology; 2006 June 18‐21. Prague: ESHRE, 2006.
Allegra 2011 {published data only}
- Allegra A, Pane A, Marino A, Scaglione P, Ruvolo G, Volpes A. Is rLH useful in the treatment of infertile poor prognosis patients undergoing IVF cycles? A preliminary report of a controlled trial. Abstracts of the 27th Annual Meeting of the European Society of Human Reproduction and Embryology (ESHRE); 2011 July 3‐6; Stockholm. 2011:i298‐9.
Balasch 2001 {published data only}
- Balasch J, Creus M, Fabregues F, Civico S, Carmona F, Puerto B, et al. The effect of exogenous luteinizing hormone (LH) on oocyte viability: Evidence from a comparative study using recombinant human follicle‐stimulating hormone (FSH) alone or in combination with recombinant LH for ovarian hyperstimulation in pituitary‐suppressed women undergoing assisted reproduction. Journal of Assisted Reproductive Genetics 2001;18(5):250‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrenetxea 2008 {published data only}
- Barrenetxea G, Agirregoika JA, Jimenez MR, Lopez de Larruzea A, Ganzabal T, Carbonero K. Ovarian response and pregnancy outcome in poor‐responder women: a randomised controlled trial on the effect of luteinizing hormone supplementation on in vitro fertilization cycles. Fertility and Sterility 2008;89(3):546‐53. [DOI] [PubMed] [Google Scholar]
Berkkanoglu 2007 {published data only}
- Berkkanoglu M, Isikoglu M, Aydin D, Ozgur K. Clinical effects of ovulation induction with recombinant follicle‐stimulating hormone supplemented with recombinant luteinizing hormone or low‐dose recombinant human chorionic gonadotropin in the midfollicular phase in microdose cycles in poor responders. Fertilility and Sterility 2007;88(3):665‐9. [DOI] [PubMed] [Google Scholar]
Bosch 2011 {published data only}
- Bosch E, Labarta E, Crespo J, Simon C, Remohi J, Pellicer A. Impact of luteinizing hormone administration on gonadotropin‐releasing hormone antagonist cycles: An age‐adjusted analysis. Fertility and Sterility 2011;95(3):1031‐6. [DOI] [PubMed] [Google Scholar]
Caserta 2011 {published data only}
- Caserta D, Lisi F, Marci R, Ciardo F, Fazi A, Lisi R, et al. Does supplementation with recombinant luteinizing hormone prevent ovarian hyperstimulation syndrome in down regulated patients undergoing recombinant follicle stimulating hormone multiple follicular stimulation for IVF/ET and reduces cancellation rate for high risk of hyperstimulation?. Gynecological Endocrinology 2011;27:862‐6. [DOI] [PubMed] [Google Scholar]
Demirol 2005 {published data only}
- Demirol A, Gurgan T, Girgin B. Supplementation of rec‐LH for poor responder patients. Abstracts of the 21st Annual Meeting of the European Society of Human Reproduction and Embryology (ESHRE); 2005 June 19‐22; Copenhagen. 2005:i74.
De Placido 2005 {published data only}
- Placido G, Alviggi C, Perino A, Strina I, Lisi F, Fasolino A, et al. Recombinant human LH supplementation versus recombinant human FSH (rFSH) step‐up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. A multicentre, prospective, randomized controlled trial. Human Reproduction 2005;20(2):390‐6. [DOI] [PubMed] [Google Scholar]
Dravid 2015 {published data only}
- Dravid RM, Chimote BN, Chimote AN, Chimote NN, Chimote NM. Supplementation of recombinant LH to poor responders in mid‐follicular phase along with recombinant FSH results in better blastocyst formation and implantation rate in antagonist IVF cycles. 31st European Society of Human Reproduction and Embryology (ESHRE); 2015 June 14‐17; Lisbon. 2015:165.
Evangelio 2011 {published data only}
- Evangelio PM, Buendicho IE, Yeste AM, Gustem MRB, Egea IB, Hernandez NC. Randomized prospective study on the effect of the addition of the effect of the addition of business cycles exogenous LH IVF/ICSI GnRH antagonist predictors in patients with low response. Revista Iberoamericana de Fertilidad y Reproduccion Humana 2011;28(3):3. [Google Scholar]
Fabregues 2011 {published data only}
- Fabregues F, Iraola A, Casals G, Creus M, Carmona F, Balasch J. Evaluation of two doses of recombinant human luteinizing hormone supplementation in down‐regulated women of advanced reproductive age undergoing follicular stimulation for IVF: a randomized clinical study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2011;158:56‐61. [DOI] [PubMed] [Google Scholar]
Fábreques 2006 {published data only}
- Fabregues F, Creus M, Penarrubia J, Manau D, Vanrell JA, Balasch J. Effects of recombinant human luteinizing hormone supplementation on ovarian stimulation and the implantation rate in down‐regulated women of advanced reproductive age. Fertility and Sterility 2006;85:925‐31. [DOI] [PubMed] [Google Scholar]
Fernandez‐Ramirez 2006 {published data only}
- Fernández Ramírez MJ, Monzó A, García‐Gimeno T, Rubio JM, Montañana V, Duque C. Role of LH administration during the follicular phase in women with risk of low response in ovarian stimulation with FSH and cetrorelix for IVF [Papel de la administracion de LH en el curso de la fase folicular en mujeres con riesgo de pobre respuesta en ciclos de estimulacion con FSH y cetrorelix para FIV]. Revista Iberoamericana de Fertilidad y Reproduccion Humana 2006;23(5):281‐90. [Google Scholar]
Ferraretti 2004 {published data only}
- Ferraretti AP, Gianaroli L, Magli MC, D'Angelo A, Farfalli V, Montanaro N. Exogeneous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproductive techniques. Fertility and Sterility 2004;82(6):1521‐6. [DOI] [PubMed] [Google Scholar]
Ferraretti 2014 {published data only}
- Ferraretti AP, Gianaroli L, Motrenko T, Feliciani E, Tabanelli C, Magli MC. LH pretreatment as a novel strategy for poor responders. BioMed Research International 2014 Aug 12 [Epub ahead of print]. [http://dx.doi.org/10.1155/2014/926172] [DOI] [PMC free article] [PubMed]
Griesinger 2005 {published data only}
- Griesinger G, Schultze‐Mosgau A, Dafopoulos K, Schroeder A, Schroer A, Otte S, et al. Recombinant luteinizing hormone supplementation to recombinant follicle‐stimulating hormone induced ovarian hyperstimulation in the GnRH‐antagonist multiple‐dose protocol. Human Reproduction 2005;20(5):1200‐6. [DOI] [PubMed] [Google Scholar]
Humaidan 2004 {published data only}
- Humaidan P, Bungum M, Bungum L, Yding Andersen C. Effects of recombinant LH supplementation in women undergoing assisted reproduction with GnRH agonist down‐regulation and stimulation with recombinant FSH: an opening study. Reproductive Biomedine 2004;8(6):635‐43. [DOI] [PubMed] [Google Scholar]
Konig 2013 {published data only}
- König TE, Houwen LE, Overbeek A, Hendriks ML, Beutler‐Beemsterboer SN, Kuchenbecker WK, et al. Recombinant LH supplementation to a standard GnRH antagonist protocol in women of 35 years or older undergoing IVF/ICSI: a randomized controlled multicentre study. Human Reproduction 2013;28(10):2804‐12. [DOI] [PubMed] [Google Scholar]
Kovacs 2010 {published data only}
- Kovacs P, Kovats T, Kaali SG. Results with early follicular phase recombinant luteinizing hormone supplementation during stimulation for in vitro fertilization. Fertility and Sterility 2010;93(2):475‐9. [DOI] [PubMed] [Google Scholar]
Levi‐Setti 2006 {published data only}
- Levi‐Setti PE, Cavagna M, Bulletti C. Recombinant gonadotrophins associated with GnRH antagonist (cetrorelix) in ovarian stimulation for ICSI: comparison of rFSH alone and in combination with rLH. European Journal of Obstetrics and Gynecology and Reproductive Biology 2006;126:212‐16. [DOI] [PubMed] [Google Scholar]
Lisi 2005 {published data only}
- Lisi F, Rinaldi L, Fishel S, Caserta D, Lisi R, Campbell A. Evaluation of two doses of recombinant luteinizing hormone supplementation in an unselected group of women undergoing follicular stimulation for in vitro fertilization. Fertility and Sterility 2005;83(2):309‐15. [DOI] [PubMed] [Google Scholar]
Lisi 2012 {published data only}
- Lisi F, Caserta D, Montanino M, Berlinghieri V, Bielli W, Carfagna P, et al. Recombinant luteinizing hormone priming in multiple follicular stimulation for in‐vitro fertilization downregulated patients. Gynecological Endocrinology 2012;28(9):674‐7. [DOI: 10.3109/09513590.2011.652716] [DOI] [PubMed] [Google Scholar]
Marrs 2003 {published data only}
- Marrs R, Meldrum D, Muasher S, Schoolcraft W, Werlin L, Kelly E. Randomized trial to compare the effect of recombinant FSH (follitropin alfa) with or without recombinant human LH in women undergoing assisted reproduction treatment. Reproductive Biomedicine Online 2003;8(2):175‐82. [DOI] [PubMed] [Google Scholar]
Matorras 2009 {published data only}
- Matorras R, Prieto B, Exposito A, Mendoza R, Crisol L, Herranz P, et al. Mid‐follicular LH supplementation in women aged 35‐39 years undergoing ICSI cycles: a randomized controlled study. Reproductive Biomedicine Online 2009;19(6):879‐87. [DOI] [PubMed] [Google Scholar]
Mohseni 2013 {published data only}
- Mohseni F, Dehgani Firouzabadi R, Yari N, Etebary S. Results of adding recombinant LH in normoresponder patients for assisted reproductive technology treatment: a prospective randomized control trial. International Journal of Fertility and Sterility 2013;7:112. [Google Scholar]
Musters 2012 {published data only}
- Musters AM, Wely M, Mastenbroek S, Kaaijk EM, Repping S, Veen F, et al. The effect of recombinant LH on embryo quality: a randomized controlled trial in women with poor ovarian reserve. Human Reproduction 2012;27(1):244‐50. [DOI] [PubMed] [Google Scholar]
Nazzaro 2012 {published data only}
- Nazzaro A, Salemo A. Recombinant LH supplementation to recombinant FSH during induced ovarian stimulation in the GnRH‐antagonist protocol improves implantation and pregnancy rates. Fertility and Sterility 2012;98(3):S280. [Google Scholar]
Nyboe Andersen 2008 {published data only}
- Nyboe Andersen A, Humaidan P, Fried G, Hausken J, Antila L, Bangsbøll S, et al. Nordic LH study group. Recombinant LH supplementation to recombinant FSH during the final days of controlled ovarian stimulation for in vitro fertilization. A multicentre,prospective, randomized, controlled trial. Human Reproduction 2008;23(2):427‐34. [DOI] [PubMed] [Google Scholar]
Pezzuto 2010 {published data only}
- Pezzuto A, Ferrari B, Coppola F, Nardelli GB. LH supplementation in down‐regulated women undergoing assisted reproduction with baseline low serum LH levels. Gynecological Endocrinology 2010;26(2):118‐24. [DOI] [PubMed] [Google Scholar]
Razi 2014 {published data only}
- Razi MH, Mohseni F, Dehghani Firouzabadi R, Janati S, Yari N, Etebary S. Results from adding recombinant LH for assisted reproductive technology treatment: A randomized control trial. Iranian Journal of Reproductive Medicine 2014;12(2):111‐6. [PMC free article] [PubMed] [Google Scholar]
Ruvolo 2007 {published data only}
- Ruvolo G, Bosco L, Pane A, Morici G, Cittadini E, Roccheri MC. Lower apoptosis rate in human cumulus cells after administration of recombinant luteinizing hormone to women undergoing ovarian stimulation for in vitro fertilization procedures. Fertility and Sterility 2007;87(3):542‐6. [DOI] [PubMed] [Google Scholar]
Tarlatzis 2006 {published data only}
- Tarlatzis B, Tavmergen E, Szamatowicz M, Barash A, Amit A, Levitas E, et al. The use of recombinant human LH (lutropin alfa) in the late stimulation phase of assisted reproduction cycles: a double‐blind, randomized, prospective study. Human Reproduction 2006;21(1):90‐4. [DOI] [PubMed] [Google Scholar]
Van der Houwen 2011 {published data only}
- Houwen L, Konig TE, Overbeek A, Hendriks ML, Beemsterboer SN, Kuchenbecker WK, et al. The influence of LH substitution for GnRH antagonist blocked endogenous LH in older IVF patients. Human Reproduction 2011; Vol. 26:i314‐5.
Vuong 2015 {published data only}
- Vuong TNL, Phung HT, Ho MT. Recombinant follicle‐stimulating hormone and recombinant luteinizing hormone versus recombinant follicle‐stimulating hormone alone during GnRH antagonist ovarian stimulation in patients aged ≥35 years: a randomized controlled trial. Human Reproduction 2015;30(5):1188‐95. [DOI] [PubMed] [Google Scholar]
Younis 2014 {published data only}
- Younis JS, Izhaki I, Ben‐Ami M. The effect of LH supplementation following GNRH antagonist administration in advanced reproductive ageing women undergoing IVF‐ET: A prospective randomized controlled study. Fertility and Sterility 2014;102(3):e23. [Google Scholar]
References to studies excluded from this review
Acévedo 2004 {published data only}
- Acevedo B, Sanchez M, Gomez JL, Cuadros J, Ricciarelli E, Hernandez ER. Luteinizing hormone supplementation increases pregnancy rates in gonadotropin‐releasing hormone antagonist donor cycles. Fertility and Sterility 2004;82(2):343. [DOI] [PubMed] [Google Scholar]
Barberi 2012 {published data only}
- Barberi M, Ermini B, Morelli MB, Ermini M, Cecconi S, Canipari R. Follicular fluid hormonal profile and cumulus cell gene expression in controlled ovarian hyperstimulation with recombinant FSH: Effects of recombinant LH administration. Journal of Assisted Reproduction and Genetics 2012;29(12):1381‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baruffi 2006 {published data only}
- Baruffi RLR, Mauri AL, Petersen CG, Felipe V, Martins AMC, et al. The use of recombinant luteinizing hormone in addition to recombinant follicle‐stimulating hormone in ovarian stimulation with the GnRH‐antagonist protocol. Jornal Brasileiro de Reproducao Assistida 2006;10(4):9‐16. [Google Scholar]
Cedrin‐Durnerin 2004 {published and unpublished data}
- Cedrin‐Durnerin I, Grange‐Dujardin D, Laffy A, Parneix I, Massin N, Galey J, et al. Recombinant human LH supplementation during GnRH antagonist administration in IVF/ICSI cycles: a prospective randomized study. Human Reproduction 2004;19(9):1979‐84. [DOI] [PubMed] [Google Scholar]
Cedrin‐Durnerin 2008 {published data only}
- Durnerin CI, Erb K, Fleming R, Hillier H, Hillier SG, Howles CM, et al. Luveris Pretreatment Group. Effects of recombinant LH treatment on folliculogenesis and responsiveness to FSH stimulation. Human Reproduction 2008;23(2):421‐6. [DOI] [PubMed] [Google Scholar]
De Placido 2004 {published data only}
- Placido G, Alviggi C, Mollo A, Strina I, Ranieri A, Alviggi E, et al. Effects of recombinant LH (rLH) supplementation during controlled ovarian hyperstimulation (COH) in normogonadotropic women with an initial inadequate response to recombinant FSH (rFSH) after pituitary downregulation. Clinical Endocrinology 2004;60:637‐43. [DOI] [PubMed] [Google Scholar]
De Placido 2006 {published data only}
- Placido G, Mollo A, Clarizia R, Strina I, Conforti S, Alviggi C. Gonadotropin‐releasing hormone (GnRH) antagonist plus recombinant luteinizing hormone vs. a standard GnRH agonist short protocol in patients at risk for poor ovarian response. Fertility and Sterility 2006;85(1):247‐50. [DOI] [PubMed] [Google Scholar]
Drakakis 2005 {published data only}
- Drakakis P, Loutradis D, Kallianidis K, Liapi A, Milingos S, Makrigiannakis A, et al. Small doses of LH activity are needed early in ovarian stimulation for better quality oocytes in IVF‐ET. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2005;121(1):77‐80. [DOI] [PubMed] [Google Scholar]
Fei Yang 2013 {published data only}
- Fei Yang D, Jia Yin L. Different LH add‐back and luteal phase supplement influence clinical outcome in GnRH antagonist protocol‐a prospective RCT study in fresh and frozen transfer cycles. Fertility and Sterility 2013;100(3):S270. [Google Scholar]
Fermin 2013 {published data only}
- Fermin A, Crisol L, Exposito A, Prieto B, Mendoza R, Matorras R. Influence of the fsh/lh ratio in ovarian stimulation in ivf results in women aged over 35 years: a randomized study. Human Reproduction 2013;28:i311‐56. [Google Scholar]
Garcia‐Velasco 2007 {published data only}
- Garcia‐Velasco J, Coelingh Bennink HJ, Epifanio R, Escudero E, Pellicer A, Simón C. High‐dose recombinant LH add‐back strategy using high‐dose GnRH antagonist is an innovative protocol compared with standard GnRH antagonist. Reproductive Biomedicine Online 2007;15(3):280‐7. [DOI] [PubMed] [Google Scholar]
Gomez‐Palomares 2005 {published data only}
- Gomez‐Palomares JL, Acevedo‐Martin B, Andres L, Ricciarelli E, Hernandez ER. LH improves early follicular recruitment in women over 38 years old. Reproductive BioMedicine Online 2005;11(4):409‐14. [DOI] [PubMed] [Google Scholar]
Hugues 2005 {published data only}
- Hugues JN, Soussis J, Calderon I, Balasch J, Anderson RA, Romeu A, Recombinant LH Study Group. Does the addition of recombinant LH in WHO group II anovulatory women over‐responding to FSH treatment reduce the number of developing follicles? A dose‐finding study. Human Reproduction 2005;20(3):629‐35. [DOI] [PubMed] [Google Scholar]
Lahoud 2010 {published data only}
- Lahoud R, Foley J, Ryan J, Costello M, Quinn F, Illingworth P. Recombinant LH supplementation in patients with a relative reduction in lh levels during IVF/ICSI cycles: A prospective randomised controlled trial. Human Reproduction 2010;25(6):i90 (Abstract no. O‐227). [DOI] [PubMed] [Google Scholar]
Lisi 2003 {published data only}
- Lisi F, Rinaldi L, Fishel S, Lisi R, Pepe G, Picconeri MG. Use of recombinant follicle‐stimulating hormone (Gonal F) and recombinant luteinizing hormone (Luveris) for multiple follicular stimulation in patients with a suboptimal response to in vitro fertilization. Fertility and Sterility 2003;79(4):1037‐8. [DOI] [PubMed] [Google Scholar]
Motta 2006 {published data only}
- Motta ELA, Maia V, Massaguer A, Fassolas G, Rocca T, Rossi LM, et al. Administration of either recombinant LH or increasing dose of recombinant FSH in late follicular phase of patients with suboptimal response. Abstracts of the 22nd Annual Meeting of the European Society of Human Reproduction and Embryology (ESHRE); 2006 June 18‐21; Prague. 2006:O‐141, i55.
Papanikolaou 2010 {published data only}
- Papanikolaou E, Werpoest W, Fatemi H, Polyzos N, Humaidan P, Tarlatzis B, et al. Recombinant LH as luteal supplementation method after agonist triggering in IVF. A proof of concept study. 26th Annual Meeting of the European Society of Human Reproduction and Embryology (ESHRE); 2010 June 14‐17; Lisbon. 2010:i167‐8.
Sauer 2004 {published data only}
- Sauer MV, Thornton MH 2nd, Schoolcraft W, Frishman GN. Comparative efficacy and safety of cetrorelix with or without mid‐cycle recombinant LH and leuprolide acetate for inhibition of premature LH surges in assisted reproduction. Reproductive Biomedicine Online 2004;9(5):487‐93. [DOI] [PubMed] [Google Scholar]
Sills 1999 {published data only}
- Sills ES, Levy DP, Moomjy M, McGee M, Rosenwaks Z. A prospective, randomized comparison of ovulation induction using highly purified follicle‐stimulating hormone alone and with recombinant human luteinizing hormone in in‐vitro fertilization. Human Reproduction 1999;14(9):2230‐5. [DOI] [PubMed] [Google Scholar]
Tesarik 2002 {published data only}
- Tesarik J, Mendoza C. Effects of exogenous LH administration during ovarian stimulation of pituitary down‐regulated young oocyte donors on oocyte yield and developmental competence. Human Reproduction 2002;17(12):3129‐37. [DOI] [PubMed] [Google Scholar]
Topercerová 2005 {published data only}
- Toporcerová S, Hredzák R, Ostró A, Zdilová V, Adam J, Potoceková D. Influence of serum levels of luteinizing hormone during controlled ovarian hyperstimulation on the results of IVF cycle [Vplyv exogénnej suplementácie luteinizacného hormónu pocas kontrolovaney ovariálnej hyperstimulácie na výsledky cyklu IVF]. Ceska Gynekologie 2005;70(4):247‐53. [PubMed] [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Broekmans 1992
- Broekmans F, Bernardus R, Berkhout G, Schoenmaker J. Pituitary and ovarian suppression after early follicular and mid‐luteal administration of LHRH agonist in a depot formulation: decapeptyl CR. Gynecoligal Endocrinology 1992;6:153‐61. [DOI] [PubMed] [Google Scholar]
Ferraretti 2011
- Ferraretti AP, Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Human Reproduction 2011;26(7):1616‐24. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to 12 November 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2003
- Higgins JP, Tompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration. [www.cochrane‐handbook.org.]
Hill 2012
- Hill MJ, Levens ED, Levy G, Ryan ME, Csokmay JM, DeCherney AH, et al. The use of recombinant luteinizing hormone in patients undergoing assisted reproductive techniques with advanced reproductive age: a systematic review and meta‐analysis. Fertility and Sterility 2012;97(5):1108‐14. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Placido 2001
- Placido G, Mollo A, Alviggi C, Strina I, Varricchio MT, Ranieri A, et al. Rescue of IVF cycles by HMG in pituitary down‐regulated normogonadotropic young women characterized by a poor initial response to recombinant FSH. Human Reproduction 2001;16:1875‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Short 1962
- Short RV. Steroids in the follicular fluid and the corpus luteum of the mare. A 'two‐cell type' theory of ovarian steroid synthesis. Journal of Endocrinology 1962;24:59‐63. [DOI] [PubMed] [Google Scholar]
Smitz 1988
- Smitz J, Devroey P, Camus M, Deschacht J, Khan I, Staessen C, et al. The luteal phase and early pregnancy after combined GnRH‐agonist/HMG treatment for superovulation in IVF or GIFT. Human Reproduction 1988;3(5):585‐90. [DOI] [PubMed] [Google Scholar]
Te Velde 2000
- Velde ER, Eijkemans R, Habbema HD. Variation in couple fecundity and time to pregnancy, an essential concept in human reproduction. Lancet 2000;355(9219):1928‐9. [DOI] [PubMed] [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18:1000‐4. [DOI] [PubMed] [Google Scholar]
van Wely 2011
- Wely M, Kwan I, Burt AL, Thomas J, Vail A, Veen F, et al. Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD005354.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiong 2014
- Xiong Y, Bu Z, Dai W, Zhang M, Bao X, Sun Y. Recombinant luteinizing hormone supplementation in women undergoing in vitro fertilization/ intracytoplasmic sperm injection with gonadotropin releasing hormone antagonist protocol: a systematic review and meta‐analysis. Reproductive Biology and Endocrinology 2014;12:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Mochtar 2005
- Mochtar MMH, Wely M, Veen F. Recombinant luteinizing hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD005070] [DOI] [PubMed] [Google Scholar]
Mochtar 2007
- Mochtar MH, Veen F, Ziech M, Wely M. Recombinant Luteinizing Hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005070.pub2] [DOI] [PubMed] [Google Scholar]


