Abstract
Background
Leprosy, caused by Mycobacterium leprae, affects over 200,000 people annually worldwide and remains endemic in the ethnically diverse, mountainous and underdeveloped southwestern provinces of China. Delayed diagnosis of leprosy persists in China, thus, additional knowledge to support early diagnosis, especially early diagnosis of paucibacillary (PB) patients, based on the host immune responses induced by specific M. leprae antigens is needed. The current study aimed to investigate leprosy patients and controls in Southwest China by comparing supernatants after stimulation with specific M. leprae antigens in an overnight whole-blood assay (WBA) to determine whether host markers induced by specific M. leprae antigens improve the diagnosis or discrimination of PB patients with leprosy.
Methodology/Principal findings
Leprosy patients [13 multibacillary (MB) patients and 7 PB patients] and nonleprosy controls [21 healthy household contacts (HHCs), 20 endemic controls (ECs) and 19 tuberculosis (TB) patients] were enrolled in this study. The supernatant levels of ten host markers stimulated by specific M. leprae antigens were evaluated by overnight WBA and multiplex Luminex assays. The diagnostic value in PB patients and ECs and the discriminatory value between PB patients and HHCs or TB patients were evaluated by receiver operator characteristics (ROC) analysis. ML2044-stimulated CXCL8/IL-8 achieved the highest sensitivity of 100%, with a specificity of 73.68%, for PB diagnosis. Compared to single markers, a 3-marker combination model that included ML2044-induced CXCL8/IL-8, CCL4/MIP-1 beta, and IL-6 improved the diagnostic specificity to 94.7% for PB patients. ML2044-stimulated IL-4 and CXCL8/IL-8 achieved the highest sensitivity (85.71% and 100%) and the highest specificity (95.24% and 84.21%) for discriminating PB patients from HHCs and TB patients, respectively.
Conclusions
Our findings suggest that the host markers induced by specific M. leprae antigens in an overnight WBA increase diagnostic and discriminatory value in PB patients with leprosy, with a particularly strong association with interleukin 8.
Author summary
Leprosy, caused by Mycobacterium leprae, affects over 200,000 people annually worldwide and remains endemic in the ethnically diverse, mountainous and underdeveloped southwestern regions of China. Although it is curable, delayed diagnosis of leprosy persists in China, with a disability rate as high as 20% nationwide. To identify the source of infection and block transmission more effectively, further knowledge about the diagnostic value of antigen-specific induced host immune responses in patients is needed. The current study aimed to evaluate the diagnostic value of an overnight whole-blood assay for the diagnosis of paucibacillary (PB) leprosy patients, and differences in the ability of specific M. leprae antigens to stimulate a panel of host markers was tested by an overnight whole-blood assay. Our findings suggest that host markers induced by specific M. leprae antigens in an overnight WBA have diagnostic value in leprosy patients and discriminatory value between leprosy patients and healthy household contacts (HHCs) or tuberculosis (TB) patients.
Introduction
Leprosy is a treatable infection that is caused by Mycobacterium leprae (M. leprae) and can result in skin lesions, nerve degeneration, and deformities. The current World Health Organization (WHO) directives for leprosy control programs encourage widespread administration of multidrug therapy (MDT) to treat patients and early diagnosis [1]. The implementation of WHO MDT treatment has drastically reduced the number of registered leprosy cases from the approximately 12 million reported cases in 1985 to fewer than 250,000 reported cases in 2006 [2]. In 2017, 210,671 new cases of leprosy were detected, and the registered prevalence was 192,713 cases [3]. Indeed, assays for detecting M. leprae-specific IgM antibodies against PGL-I have been developed successfully [4,5], and these assays are able to identify multibacillary (MB) leprosy patients (with strong humoral immunity against M. leprae) [6]. However, as only 1–5 skin lesions, 1–2 damaged nerves, and few bacteria are present, the diagnosis and discrimination of paucibacillary (PB) patients with leprosy remain challenging. Therefore, antigen-specific immune diagnostic tools, especially antigen-specific secretion of host markers in whole-blood assays (WBAs), have been an important topic in leprosy research.
Commercially available IFN-gamma (IFN-γ) release assays (IGRAs) such as Quanti-FERON-TB Gold have been developed successfully for specific detection of M. tuberculosis infection and discrimination from all (nonvirulent) BCG strains and most other nontuberculous mycobacteria (NTMs) [7], which has inspired research into the feasibility of developing similar peptide-based assays for the identification of asymptomatic leprosy [6]. Multiple M. leprae-specific antigens and host markers, including IFN-γ as a candidate host marker, have been studied widely [8–17].
Several studies have explored the immune response in M. leprae-stimulated WBAs [8–17]. Some of these studies have examined the supernatant levels of IFN-γ stimulated with multiple M. leprae antigens in infected patients [8–14], and others focused on a panel of multiple M. leprae antigen-induced host markers by WBA [15–17]. Sampaio et al. [13] reported previously that 9 of 33 M. leprae recombinant proteins could induce IFN-γ secretion in tuberculoid (TT)/borderline tuberculoid (BT) patients and HHCs by a WBA in a Brazilian population. However, our laboratory recently reported that IFN-γ secretion induced by stimulation with M. leprae antigens (LID-1, ML89, ML2044, and ML2028) achieved higher positive response rates in PB patients than in MB patients in Southwest China [14,15]. This marker could distinguish PB patients from tuberculosis (TB) patients after stimulation with ML2044 and ML2028, but it could not distinguish PB patients from healthy household contacts (HHC) or endemic controls (ECs) [15]. This result was consistent with those of a previous study in an Ethiopian population, in which M. leprae proteins did not distinguish patients from ECs in one leprosy endemic area based on IFN-γ [12]. Geluk et al. [12] reported that M. leprae and ML2478 induced significantly higher concentrations of MCP-1, MIP-1β and IL-1β in PB patients than in ECs in Ethiopia. These studies suggested that M. leprae antigen-specific IFN-γ secretion in a WBA had a limited ability to discriminate PB patients from nonleprosy controls; indeed, additional M. leprae-specific antigens and additional host biomarkers should be investigated for their ability to assist in the diagnosis and discrimination of PB patients.
The purpose of this study was to explore a new panel of host markers stimulated by specific M. leprae antigens in an overnight WBA to improve the diagnosis (PB patients vs. ECs) and discrimination (PB patients vs. HHCs or TB patients) of PB patients with leprosy in a hyperendemic area in China. The M. leprae antigens used for the WBA in this study were Leprosy IDRI diagnostic-1 (LID-1) and ML2044. Additional host markers, including tumor necrosis factor alpha (TNF-α), interleukin (IL)-4, interleukin 6, interleukin 10, CC chemokine ligand (CCL) 2, CCL4, CXC chemokine ligand (CXCL) 8, CXCL10, granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF), were also studied.
Methods
Ethics statement
This study was approved by the Medical Ethics Committee of Beijing Friendship Hospital, Capital Medical University, Beijing, P.R. China. Written informed consent was obtained from all adult participants. All of the procedures in this study that involved human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Study subjects
We recruited 13 MB patients, 7 PB patients, 21 HHCs, 20 ECs, and 19 TB patients from the Honghe Autonomous Prefecture in the Yunnan Province in the southwestern region of China during May 2016. The leprosy incidence in most provinces and regions of China was lower than 0.1 per 100,000 in 2010, and the case notification rate was up to 0.85 per 100,000 (190/4,470,000) from 2010–2014 in the communities in the Honghe Autonomous Prefecture [18]. The most common route of identification for the leprosy patients was out-patient (61/190), followed by clue investigation (53/190), family members (40/190), disease-reporting (13/190), epidemic area (12/190), self-reporting (10/190), and group survey (1/190). Leprosy diagnosis was established based on clinical signs and symptoms, skin smears, skin biopsy, and neurophysiologic examinations. The leprosy patients were classified into groups based on Ridley and Jopling [19]. The leprosy patients were also classified into two groups, PB and MB, according to the WHO operational classification [20]. HHCs had been living in the same house as an adult leprosy patient. ECs within the normal controls lived in the same community as leprosy patients. TB patients were referred to the Honghe Autonomous Prefecture Disease Prevention and Control Center (CDC).
Specific M. leprae antigens
The specific M. leprae antigens used for the WBA in this study were LID-1, a fusion protein developed by fusing the ML0405 and ML2331 genes [21,22], and ML2044, which were provided by Dr. M.S. Duthie from the Infectious Disease Research Institute (IDRI) in Seattle, USA. The list of accession numbers/ID numbers for genes and proteins that were mentioned in the text and included in the NCBI search is shown in S1 Table.
Overnight WBA
An undiluted WBA with overnight incubation was performed as previously described [23]. Briefly, undiluted venous whole blood was collected into a heparinized tube or green top BD vacutainer. A 48-well (flat-bottom) plate was set up with the antigens and controls in a final volume of 0.5 ml. The specific M. leprae antigens ML2044 and LID-1 (100 μg/ml)were used as stimuli. Phytohemagglutinin (PHA-M, Cat No: L2646, 750 μg/ml, Sigma-aldrich, Fluke, USA) was included as a positive control, and 0.01 mol/l PBS was included as a negative control. The antigens were added in a volume of 50 μl per well, followed by the addition of 450 μl of blood. The plate was sealed with micropore tape to avoid evaporation during incubation at 37°C with 5% CO2. After 24 hours of incubation, the supernatants were harvested and stored at −20°C until they were assayed for cytokines and chemokines by Luminex multiplex assays.
Luminex multiplex assays
The potential diagnostic value of host markers was analyzed with Luminex multiplex assays of supernatant samples collected from an overnight WBA; these markers included TNF-α, IL-4, IL-6, IL-10, CCL 2, CCL4, CXCL 8, CXCL10, G-CSF and GM-CSF. The concentrations of these cytokines were measured by a customized Human Premixed Multi-Analyte Kit (Cat. No. LXSAHM-10) on the Luminex-200™ system and the Xmap Platform (Luminex Corporation, Austin, TX). The list of accession numbers/ID numbers for genes and proteins of the host markers mentioned in the text and included in the HGNC and NCBI search is shown in S2 Table.
Statistical analysis
Statistical analysis was performed primarily with GraphPad Prism software version 5.0 (GraphPad Software Inc., San Diego, CA, USA). The nonparametric Mann-Whitney U test was used to analyze differences between two groups (PB patients vs. MB patients, HHCs, ECs, or TB patients). Probability (p) values less than 0.05 were considered significant. The diagnostic utility of individual M. leprae antigen-specific responses for leprosy, including sensitivity, specificity, p value, 95% confidence intervals (CI), cutoff value, area under the receiver operator characteristic curve (AUC), and receiver operator characteristics (ROC), was ascertained by ROC curve analysis based on the highest likelihood ratio.
Results
Study participants
A total of eighty participants, including leprosy patients (13 MB patients and 7 PB patients) and controls (21 HHCs, 20 ECs, and 19 TB patients) were included in the study. The basic characteristics of the participants are presented in Table 1. Both specific M. leprae antigens (ML2044 and LID-1) were evaluated in all 13 MB cases, 7 PB cases, 21 HHCs, 19 TB cases, and in different numbers of ECs (19 ECs for ML2044 and 20 ECs for LID-1). Newly diagnosed MB and PB leprosy patients undergoing MDT treatment. The median and interquartile range (IQR) of the treatment duration were 9 months (5–16 months) and 10 months (2–12 months) for MB and PB patients, respectively.
Table 1. Clinical characteristics of the leprosy patients enrolled in this study.
| Classification (n, %) | Number of cases | Gender ratio | Median & IQR of MDT | Bacterial index | ||
|---|---|---|---|---|---|---|
| WHO* | RJ** | (n, %) | (M/F) | (months) | (BI) | |
| Leprosy | MB | LL | 2 | 2/0 | 9(5–16) | 4–5.3 |
| BL | 11 | 6/5 | 0.8–4.3 | |||
| PB | BT | 7 | 2/5 | 10(2–12) | 0 | |
| Controls | HHC | 21 | 10/11 | / | / | |
| EC | 20 | 10/10 | / | / | ||
| TB | 19 | 10/9 | / | / | ||
Host immune markers induced by ML2044 and LID-1 in the overnight WBA
After stimulation with ML2044 (S1 Fig) and LID-1 (S2 Fig) in an overnight WBA, the concentrations of selected host markers were determined in each participant and compared between PB patients and MB patients, HCCs, ECs, and TB patients (Table 2).
Table 2. The median and IQR values of host markers detected in overnight culture supernatants from a WBA in leprosy patients (MB and PB patients) and non-leprosy controls (HHCs, ECs, and TB patients).
| M. leprae antigens | Host marker | Median (IQR), pg/ml | P value (Mann-Whitney test) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MB | PB | HHC | EC | TB | PB vs. MB | PB vs. HHC | PB vs. EC | PB vs. TB | ||
| ML2044 | TNF-alpha | 8.84(2.96–12.74) | 8.43(2.84–20.10) | 2.96(2.90–2.96) | 2.96(2.84–2.96) | 2.96(0.83–5.53) | 0.78 | 0.10 | 0.08 | 0.04* |
| IL-4 | 46.00(21.91–62.79) | 46.00(46.00–62.79) | 5.24(5.24–21.91) | 5.24(5.24–21.91) | 5.24(5.24–21.91) | 0.46 | <0.01* | <0.01* | <0.01* | |
| IL-6 | 62.08(24.47–132.60) | 30.66(15.51–123.80) | 2.08(1.59–7.45) | 3.66(1.50–6.71) | 3.27(1.50–5.57) | 0.34 | 0.02* | 0.01* | 0.01* | |
| IL-10 | 1.20(1.06–1.20) | 1.20(0.24–1.20) | 1.20(1.20–1.20) | 1.20(1.20–1.20) | 1.20(1.20–1.20) | 0.22 | / | <0.01* | 0.22 | |
| CCL2/MCP-1 | 232.1(172.3–523.4) | 202.2(147–688.4) | 172.4(125–315.5) | 198.8(109.2–253.2) | 233.6(110.5–308.5) | 0.93 | 0.26 | 0.48 | 0.41 | |
| CCL4/MIP-1 beta | 3328(1471–4923) | 2414(1278–4291) | 470.9(221.8–759.6) | 470.9(374.6–827.8) | 193.9(118.7–337.4) | 0.52 | 0.01* | <0.01* | <0.01* | |
| CXCL8/IL-8 | 1060(991.3–1060) | 1060(1060–2040) | 708.5(346.3–1060) | 766.1(553.1–1060) | 222.7(144.8–553.7) | 0.16 | 0.01* | 0.01* | <0.01* | |
| CXCL10/IP-10 | 127.2(69.09–187) | 76.06(64.72–130.5) | 59.5(43.62–96.13) | 179.4(102–302.6) | 156.5(84.9–237.3) | 0.34 | 0.04* | <0.01* | 0.08 | |
| G-CSF | 61.9(24.7–185.8) | 77.56(56.38–180.5) | 17.33(7.572–35.33) | 17.33(8.16–32.06) | 17.33(7.57–56.38) | 0.36 | <0.01* | <0.01* | <0.01* | |
| GM-CSF | 1.65(0.14–3.97) | 3.97(3.97–3.97) | 3.97(3.97–3.97) | 3.97(0.93–3.97) | 3.97(1.27–3.97) | 0.14 | 0.72 | 0.61 | 0.41 | |
| LID-1 | TNF-alpha | 4.77(2.96–11.09) | 2.96(2.31–3.81) | 2.96(1.78–2.96) | 2.96(2.04–3.60) | 2.96(1.78–2.96) | 0.10 | 0.39 | 0.97 | 0.49 |
| IL-4 | 24.54(8.79–35.10) | 12.35(5.24–24.54) | 5.24(5.24–12.35) | 5.24(5.24–12.35) | 5.24(5.24–12.35) | 0.27 | 0.28 | 0.11 | 0.13 | |
| IL-6 | 29.07(12.09–109.10) | 21.95(4.10–45.82) | 4.742(1.70–15.43) | 5.79(2.34–13.97) | 4.528(2.34–11.90) | 0.15 | 0.06 | 0.15 | 0.09 | |
| IL-10 | 1.20(0.68–1.20) | 1.20(1.20–1.20) | 1.20(1.20–1.20) | 1.20(1.09–1.20) | 1.20(1.20–1.20) | 0.62 | 0.47 | 0.71 | 0.89 | |
| CCL2/MCP-1 | 95.12(78.94–187.10) | 97.45(79.79–179.30) | 122.2(96.23–175.90) | 90.25(62.64–145.80) | 87.77(57.21–195.70) | 0.93 | 0.45 | 0.48 | 0.62 | |
| CCL4/MIP-1 beta | 1472(804.5–2914) | 1379(505.8–1697) | 495.4(316.2–923.6) | 338(168.7–540.8) | 362.2(89.8–683.1) | 0.30 | 0.08 | 0.02* | 0.02* | |
| CXCL8/IL-8 | 1343(1006–1592) | 1325(429.4–1975) | 697.5(422.2–2333) | 349.8(155.1–972.7) | 520.6(252.6–776.4) | 0.93 | 0.71 | 0.03* | 0.09 | |
| CXCL10/IP-10 | 88.9(59.7–153.2) | 62.32(44.4–83.9) | 54.8(40.5–93.5) | 93.97(50.2–199.7) | 124.3(82.8–374.4) | 0.17 | 0.52 | 0.21 | 0.01* | |
| G-CSF | 44.15(31.62–113.20) | 70.75(58.22–82.31) | 51.43(27.07–58.22) | 44.15(29.35–67.62) | 58.22(27.07–82.31) | 0.45 | 0.02* | 0.07 | 0.23 | |
| GM-CSF | 3.97(3.97–3.97) | 3.97(3.97–3.97) | 3.97(3.97–3.97) | 3.97(3.97–3.97) | 3.97(3.97–3.97) | / | / | / | / | |
Whole blood was collected from all participates and stimulated overnight with specific M. leprae antigens (ML2044 and LID-1). The concentrations of cytokines and chemokines were determined with Luminex multiplex assays.
After stimulation with ML2044, the supernatants of PB patients had a higher level of IL-4 (median 46 pg/ml) than those of HHCs (median 5.24 pg/ml, p = 0.0004), ECs (median 5.24 pg/ml, p = 0.0026), or TB patients (median 5.24 pg/ml, p = 0.0005). The same trend was also found for IL-6 (PB patients vs. HHCs, ECs, and TB patients: median = 30.66 vs. 2.086, 3.661, and 3.271 pg/ml, p = 0.0225, 0.0109, and 0.0192, respectively); CCL4/MIP-1 beta (PB patients vs. HHCs, ECs, and TB patients: median = 2414 vs. 470.9, 470.9, and 193.9 pg/ml, p = 0.0101, 0.0066, and 0.0022, respectively); CXCL8/IL-8 (PB patients vs. HHCs, ECs, and TB patients: median = 1060 vs. 708.5, 766.1, and 222.7 pg/ml, p = 0.0139, 0.0185, and 0.0019, respectively); G-CSF (PB patients vs. HHCs, ECs, and TB patients: median = 77.56 vs. 17.33, 17.33, and 17.33 pg/ml, p = 0.0013, 0.0006, 0.0046, respectively); and TNF-α (PB patients vs. TB patients: median = 8.438 vs. 2.963 pg/ml, p = 0.0423). For ML2044-stimulated CXCL10/IP-10, the concentration detected in PB patients (median = 76.06 pg/ml) was higher than that detected in HHCs (median = 59.5 pg/ml, p = 0.0496) but lower than that detected in ECs (median = 179.4, p = 0.0093). Although the median of the ML2044-induced IL-10 concentration differed significantly between the PB and EC groups (median 1.207 vs. 1.207, IQR 0.2437–1.207 vs. 1.207–1.207, p = 0.0058), the ROC analysis precluded the determination of a discriminatory value for PB patients and ECs.
The concentration of CCL4/MIP-1 beta in whole blood cells during the response to LID-1 was elevated in PB patients compared with that in ECs or TB patients (median 1379 vs. 338, and 362.2 pg/ml, p = 0.0217, and 0.0242, respectively), as was the concentration of CXCL8/IL-8 between PB patients and ECs (median 1325 vs. 349.8 pg/ml, p = 0.0355) and the concentration of G-CSF between PB patients and HHCs (median 70.75 vs. 51.43 pg/ml, p = 0.0214). In contrast, the concentration of LID-1-induced CXCL10/IP-10 was lower in PB patients than in TB patients (median 62.32 vs. 124.3 pg/ml, respectively, p = 0.0129).
No differences in host immune markers induced by ML2044 or LID-1 stimulation were found between PB patients and MB patients.
Potential value of host markers induced by ML2044 and LID-1 in a WBA for the diagnosis of PB patients
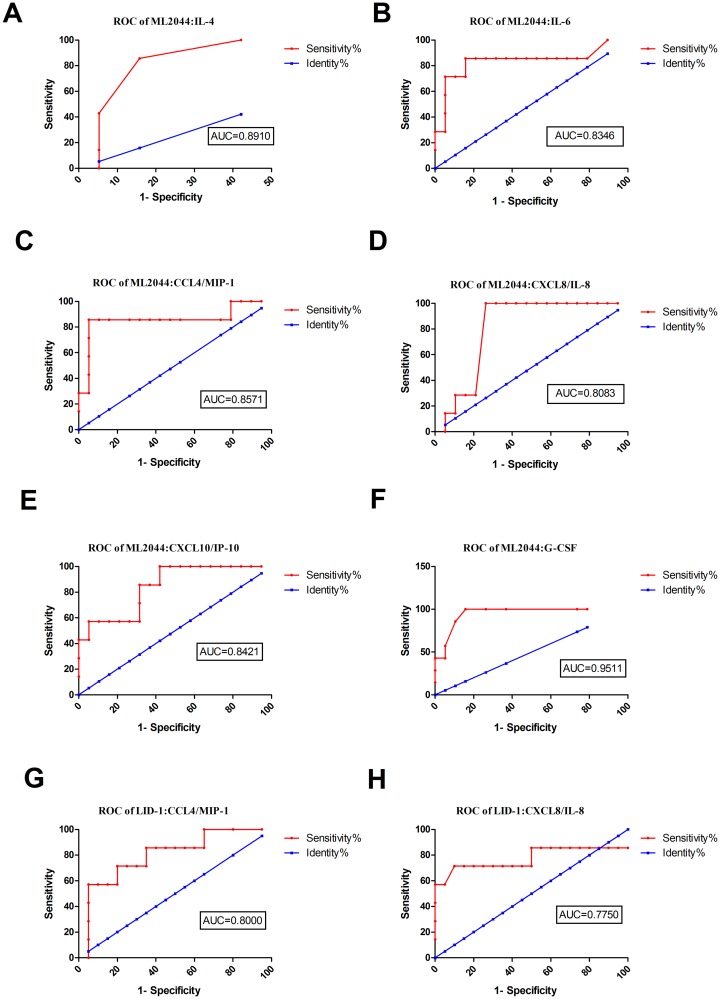
The levels of six out of the 10 markers evaluated in this study (IL-4, IL-6, CCL4/MIP-1 beta, CXCL8/IL-8, CXCL10/IP-10, and G-CSF) were significantly higher in ML2044-stimulated supernatants from PB patients than in those from ECs (S3 Table). The AUC values ranged from 0.81 to 0.95 (S3 Table, Fig 1A–1F). For LID-1, the levels of two markers (CCL4/MIP-1 beta and CXCL8/IL-8) were significantly higher in PB patients than in ECs. These two analytes discriminated between PB patients and ECs with AUC = 0.80, and 0.77, respectively, with a sensitivity of 57.14% and a specificity of 95.00% for both markers (S3 Table, Fig 1G and 1H). Combining the results of the host markers stimulated by the two M. leprae antigens, ML2044-stimulated CXCL8/IL-8 achieved the highest sensitivity of 100%, with a specificity of 73.68%, for PB diagnosis.
Fig 1. ROC curves showing the accuracy of host markers in discriminating between PB patients and ECs.
ROC curves for the accuracy of single markers [IL-4 (A), IL-6 (B), CCL4 (C), CXCL8 (D), CXCL10 (E), and G-CSF (F) induced by ML2044 and CCL4 (G) and CXCL-8 (H) induced by LID-1] in differentiating between PB patients and ECs. Only ROC curves for markers that differentiated between the two infection states with p value≤0.05 and AUC ≥ 0.73 are shown.
Potential value of host markers induced by ML2044 and LID-1 in a WBA for discriminating PB patients from HHCs
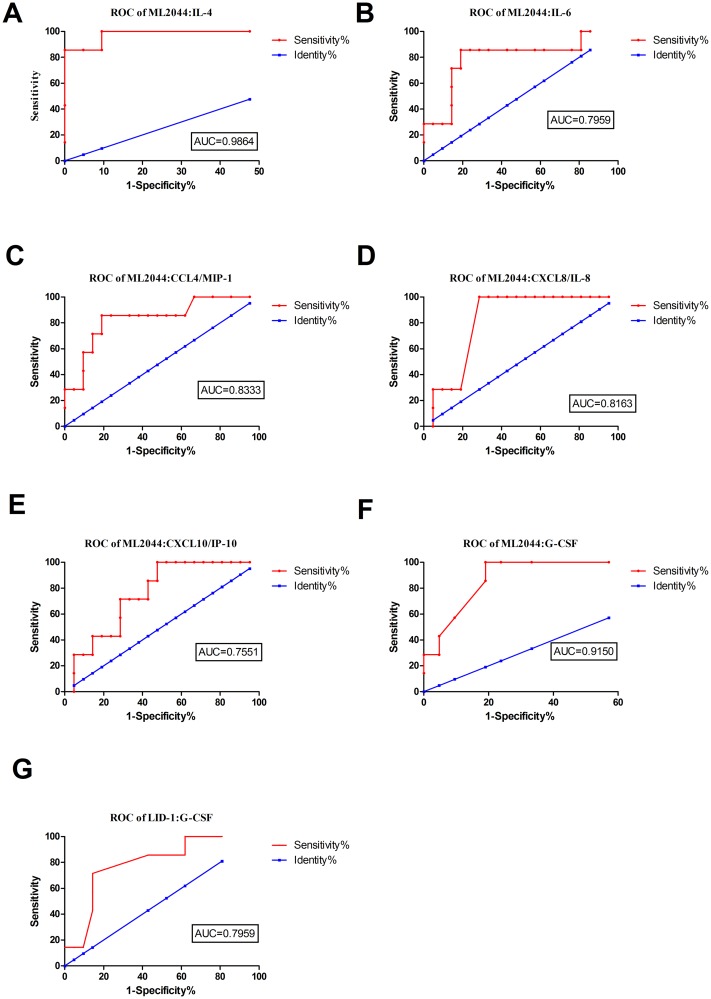
When ML2044-stimulated analyte levels were compared between the PB patients and HHCs, similar to the results obtained for PB patients vs. ECs, significant differences were obtained for IL-4, IL-6, CCL4/MIP-1 beta, CXCL8, CXCL10/IP-10, and G-CSF. The AUC values for all six of these markers were ≥0.73 by ROC analysis. The sensitivities of the six analytes for PB patients ranged from 28.75% to 85.71%, with specificities from 90.48% to 95.24% (Fig 2, S4 Table). For LID-1-stimulated supernatants, significant differences between the PB patients and HHCs were obtained for one host marker, G-CSF. After ROC analysis, the AUC for LID-1 stimulated G-CSF was 0.80. Among the host markers stimulated by the two M. leprae antigens, ML2044-stimulated IL-4 achieved the highest sensitivity (85.71%) and specificity (95.24%) for discriminating PB patients from HHCs.
Fig 2. ROC curves showing the accuracy of host markers in discriminating between PB patients and HHCs.
ROC curves for the accuracy of single markers [IL-4 (A), IL-6 (B), CCL4 (C), CXCL8 (D), CXCL10 (E), and G-CSF (F) induced by ML2044 and G-CSF (G) induced by LID-1] for differentiating between PB patients and HHCs. Only ROC curves for markers that differentiated between the two infection states with p value≤0.05 and AUC ≥ 0.73 are shown.
Potential value of host markers induced by ML2044 and LID-1 in a WBA for discriminating PB patients from TB patients
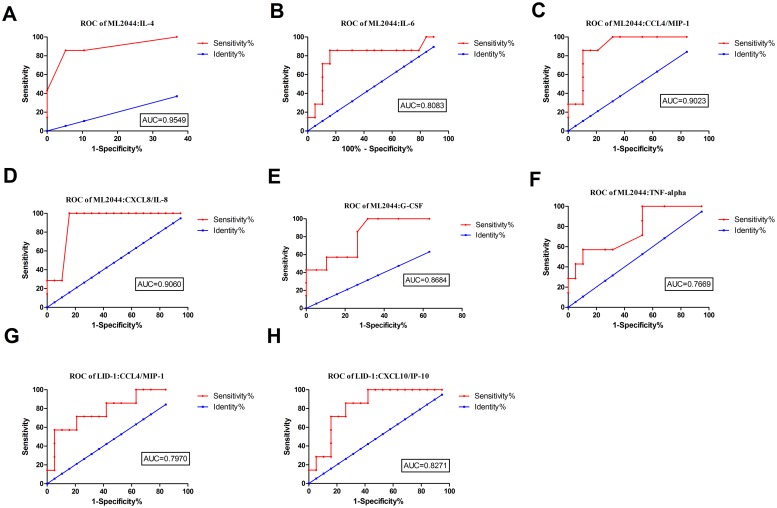
ML2044-induced IL-4, IL-6, CCL4/MIP-1 beta, CXCL8/IL-8, G-CSF and TNF-α levels were significantly higher in the PB patients than in the TB patients. After ROC analysis, the AUC values for all six analytes ranged from 0.7669 to 0.9549 (Fig 3, S5 Table). For LID-1-stimulated supernatants, significant differences between the PB patients and TB patients were obtained for two host markers, CCL4/MIP-1 beta and CXCL10/IP-10. After ROC analysis, the AUC values for these markers were 0.80 and 0.83, respectively. Among the host markers stimulated by the two M. leprae antigens, ML2044-stimulated CXCL8/IL-8 achieved the highest sensitivity of 100%, with a specificity of 84.21%, for discriminating PB patients from TB patients.
Fig 3. ROC curves showing the accuracy of host markers in discriminating between PB and TB patients.
ROC curves for the accuracy of single markers [IL-4 (A), IL-6 (B), CCL4 (C), CXCL8 (D), G-CSF (E), and TNF-α (F) induced by ML2044 and CCL4 (G) and CXCL10 (H) induced by LID-1] to differentiate between PB patients and TB patients. Only ROC curves for markers that differentiated between the two infection states with p value≤0.05 and AUC ≥ 0.73 are shown.
Diagnostic value of combination models for PB patients
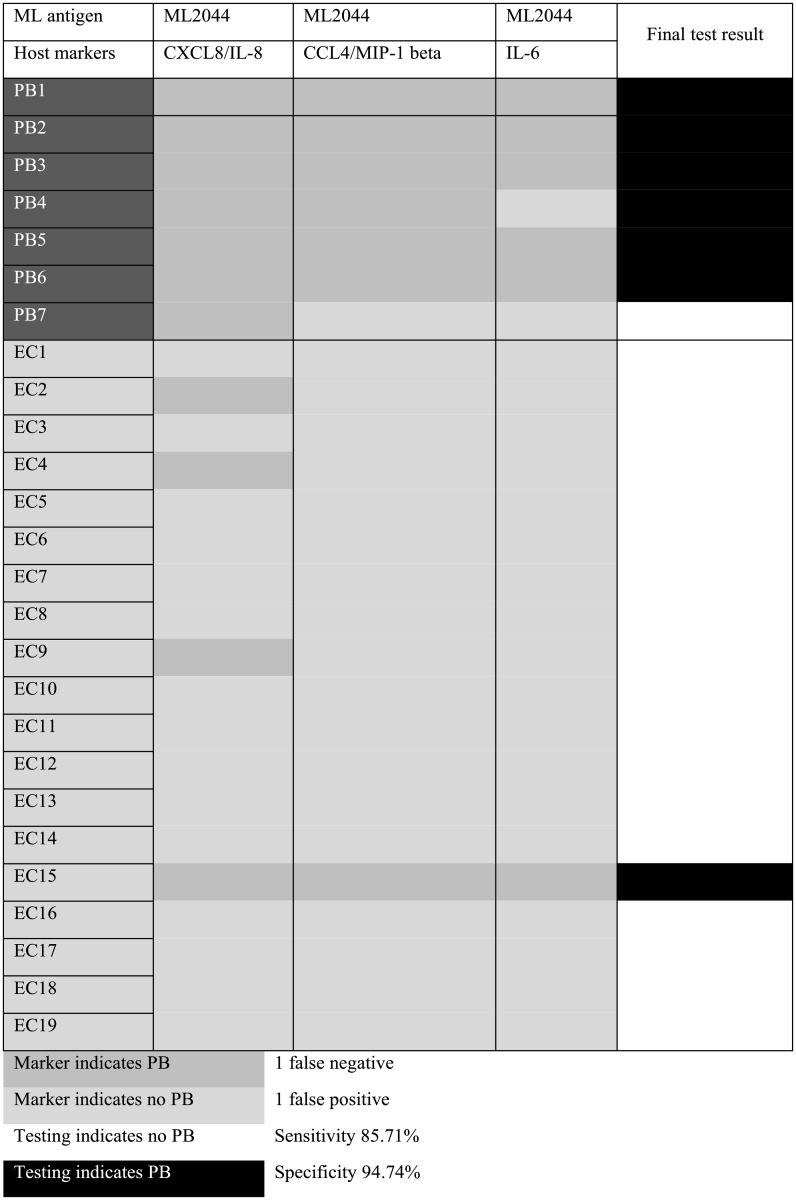
Although the sensitivity of ML2044-induced CXCL8/IL-8 (up to 100% for the discrimination of PB patients from ECs) predominated over any other single host marker induced by ML2044 or LID-1, the specificity of this marker reached only 73.68%, far lower than those of IL-6, CXCL10/IP-10, CCL4/MIP-1 beta, IL-4, and G-CSF induced by ML2044 (specificity = 94.74% for each marker) or CXCL8/IL-8 and CCL4/MIP-1 beta induced by LID-1 (specificity = 95% for each marker), as shown in S3 Table. To increase the specificity of diagnosis between PB patients and ECs, different combination models of 3–6 M. leprae-specific antigen-induced host markers were used to classify the participants. The cutoff value obtained using the ROC analysis described above was used to classify individuals into two groups. A 3-marker model (ML2044-induced CXCL8/IL-8, CCL4/MIP-1 beta, and IL-6) was the best model and was superior to the use of two M. leprae-specific antigens (ML2044 and LID-1) or 4, 5 or 6 host markers in combination. Participants were allocated to clinical groups according to the results of the majority of the individual marker tests (2/3 or 3/3). Accordingly, the ML2044-induced 3-host marker combination model classified 24 of the 26 participants (92.31%) in the correct clinical groups, with 85.71% sensitivity, which was lower than the sensitivity of ML2044-induced IL-8 as a single marker (100%), but the specificity of diagnosis for PB leprosy patients increased from 73.68% to 94.74%. Two (7.7%) participants were misclassified, with 1 false-negative and 1 false-positive classification. The classification of individual participants, sensitivity values and specificity values are shown in Fig 4. Despite the high cost and low efficiency, combined utilization of ML2044-induced CXCL8/IL-8 as a single marker and the 3-marker model (ML2044-induced CXCL8/IL-8, CCL4/MIP-1 beta, and IL-6) would help to both maintain high sensitivity and enhance specificity.
Fig 4. Utility of a 3-host marker combination model for the diagnosis of paucibacillary (PB) leprosy patients.
Seven PB patients, as defined by Jopling, and 19 HCCs were analyzed. The concentrations and the best cutoff were determined for each cytokine or chemokine, as described in S3 Table. The cutoff was used to define whether the concentrations of cytokines and chemokines indicated that the participant was a PB patient or an EC. Each cytokine or chemokine was used as an independent marker. Prediction of PB patients is shown in dark gray, and prediction of ECs is shown in light gray, for 3 different phenotypic markers. When ≥ 2 phenotypes supported one of the diagnoses, a final diagnosis of either PB (black) or EC (white) was made.
Subsequently, we explored a model with 3 or more markers for distinguishing PB patients from HHCs or TB patients in the same way. The sensitivity and the specificity of a single marker (ML2044-induced IL-4) reached 85.71% and 100% for distinguishing PB patients from HHCs. Only the 3-marker model (ML2044-induced IL-4, LID-1-induced G-CSF, and ML2044-induced CCL4/MIP-1 beta) achieved the same sensitivity and specificity as the single marker (ML2044-induced IL-4), as shown in S3 Fig.
In addition, the sensitivity and the specificity of a single marker (ML2044-induced CXCL8) reached 100% and 94.74%. Only the 3-marker model (ML2044-induced CXCL8, CCL4/MIP-1 beta, and IL-4) was found to have the same sensitivity and specificity as ML2044-induced CXCL8, as shown in S4 Fig.
Discussion
The use of the M. leprae antigens ML2044 and LID-1 to induce host immune responses in a WBA for the diagnosis of PB patients with leprosy and discrimination between PB patients and MB patients, HHCs, or TB patients were evaluated in this study. The key findings are presented below. (1) ML2044-stimulated CXCL8/IL-8 reached the highest sensitivity of 100%, with a specificity of 73.68%, for PB diagnosis. A 3-marker combination model that included ML2044-induced CXCL8/IL-8, CCL4/MIP-1 beta, and IL-6 improved the diagnostic specificity to 94.7% in comparison to that of the single marker. (2) ML2044- stimulated IL-4 and ML2044-stimulated CXCL8/IL-8 reached the highest sensitivity (85.71% and 100%, respectively) and the highest specificity (95.24% and 84.21%, respectively) for discriminating PB patients from HHCs and TB patients, respectively. No combination model with 3 or more markers had more promising diagnostic utility than analysis of this single marker for discriminating PB patients from HHCs and TB patients. (3) Although no selected host markers showed potential value for discriminating PB patients from MB patients, MB patients and PB patients showed similar but not identical M. leprae-induced immune responses by WBA.
None of the single markers could correctly classify all participants into their respective groups; however, ML2044-stimulated CXCL8/IL-8 reached the highest sensitivity of 100%, with a moderate specificity of 73.68%, for PB diagnosis. Indeed, the 3-marker model (ML2044-induced CXCL8/IL-8, CCL4/MIP-1 beta, and IL-6) enhanced the specificity to 94.74%. As ML2044 induced more host markers and had higher sensitivity than LID-1, immune responses induced by the M. leprae-specific antigen ML2044 were superior to LID-1-induced immune responses for the diagnosis and discrimination of PB patients from ECs, HHCs, and TB patients.
It was reported that at diagnosis, PB patients produce IFN-γ, and MB patients exhibit a weak/absent response. Shortly after MDT, IFN-γ production in PB patients decreases, except in response to LID-1; MB patients produced IFN-γ in response to LID-1. Almost 2 years after MDT, IFN-γ levels decreased in PB and MB patients [9]. This finding implied that cytokines and chemokines, such as IFN-γ, may fluctuate during immune responses to M. leprae antigens. In this study, the median and IQR of the treatment duration were 9 months (5–16 months) and 10 months (2–12 months) for enrolled MB patients and PB patients, respectively. It remained unknown whether the kinetics of the response of other host immune markers to M. leprae antigens during MDT treatment also fluctuated.
Interleukin 8 (CXCL8) is a chemoattractant and a regulator of white blood cell production, which can affect the pathogenesis of intense infectious diseases, such as TB, by suppressing the normal immune response to M. tuberculosis that can lead to granuloma formation. Although monocytes and macrophages infected with M. tuberculosis are the main sources of CXCL8 production, this chemokine can also be produced by neutrophils and respiratory epithelial cells [24]. However, the relationship between M. leprae and CXCL8 remained unclear. IL-8 may assume a pivotal role in cell recruitment in leprosy patients with disseminated mycobacterial infections [25]. The presence of the neutrophil chemoattractant IL-8 in leprosy lesions, which do not contain neutrophils, strongly suggests a role of IL-8 in monocyte and lymphocyte recruitment in leprosy lesions [26]. TNF-induced IL-8 can be produced by Schwann cells (SCs), indicating involvement of this factor in leprosy-associated nerve damage [27].
A previous study showed that CXCL10 could discriminate PB patients from ECs in an ML0276 + LID-1 WBA and did not discriminate active disease (PB) from M. leprae-infected (HHC) individuals in Brazil [8]. Another study also focused on M. leprae antigens (ML0276, ML1623, ML0405, ML1632, 92f, and ML1011) that stimulated host markers (eotaxin, IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-15, IL-17A, IL-23, IL-31, IP-10, and TNF-α) in a WBA in a Brazil population, which demonstrated that IFN-γ is currently the best indicator of an antigen-specific cellular immune response and that none of the biomarkers tested could discriminate leprosy patients from HHCs [17]. In this study, CXCL10 could discriminate not only PB patients from ECs in an ML2044 WBA but also PB patients from HHCs in an ML2044 WBA and PB patients from TB patients in a LID-1 WBA. Heterogeneity among individuals, different M. leprae antigen characteristics, and different time points of sample collection may represent influencing factors.
The present study has some limitations. The number of PB patients was rather low, and the findings should be interpreted with caution. Further investigation in larger populations will give more confidence to the diagnostic and discriminatory value of the identified host markers, such as ML-2044-induced IL-8. We also lack data on cytokine concentrations in biopsies of skin lesions. Moreover, associations do not necessarily indicate causal relationships, and further mechanistic studies are needed to elucidate the role of the immune response during M. leprae infection.
Conclusion
In conclusion, we identified a biosignature of a single M. leprae-specific host marker in antigen-stimulated overnight WBAs (ML2044-induced CXCL8/IL-8) that showed potential for the diagnosis of PB disease, with accurate prediction of 100% of PB cases and 73.68% of EC cases. The sensitivity of this analyte model was better than that of any other single host marker, but the specificity was relatively low. A 3-marker model of ML2044-induced CXCL8/IL-8, CCL4/MIP-1 beta, and IL-6 improved the specificity of diagnosis between PB patients and ECs to 94.74%. Moreover, ML2044-induced CXCL8/IL-8 and ML2044-induced IL-4 dominated in discriminating PB patients from TB patients and HHCs, respectively. However, both diagnostic performance at the time of screening and assessment of immunological changes in host markers during MDT therapy need to be further evaluated before these diagnostic approaches can be recommended for routine clinical practice.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Each dot represents the analyte level of one participant in the study, and horizontal lines represent the median and IQR values. The cytokine and chemokine levels [TNF-α (A), IL-4 (B), IL-6 (C), IL-10 (D), CCL2 (E), CCL4 (F), CXCL8 (G), CXCL10 (H), G-CSF (I) and GM-CSF (J)] obtained in the supernatant after overnight stimulation with the specific M. leprae antigen ML2044 by WBA.
(TIF)
Each dot represents the analyte level of one participant in the study, and horizontal lines represent the median and IQR values. The cytokine and chemokine levels [TNF-α (A), IL-4 (B), IL-6 (C), IL-10 (D), CCL2 (E), CCL4 (F), CXCL8 (G), CXCL10 (H), G-CSF (I) and GM-CSF (J)] obtained in supernatants after overnight stimulation with the specific M. leprae antigen LID-1 by WBA.
(TIF)
Seven PB patients, as defined by the WHO, and 21 HCCs were analyzed. The concentration and the best cutoff were determined for each cytokine or chemokine, as described in S4 Table. The cutoff was used to define whether the concentrations of cytokines and chemokines indicated that the participant was a PB patient or an HHC. Each cytokine or chemokine was used as an independent marker. Prediction of PB patients is shown in dark gray, and prediction of HHCs is shown in light gray for 3 different phenotypic markers. When ≥ 2 phenotypes supported one of the diagnoses, a final diagnosis of either PB (black) or HHC (white) was made.
(PDF)
Seven PB patients, as defined by the WHO, and 21 HCCs were analyzed. The concentration and the best cutoff were determined for each cytokine or chemokine, as described in S5 Table. The cutoff was used to define whether the concentrations of cytokines and chemokines indicated that the participants was a PB patient or a TB patient. Each cytokine or chemokine was used as an independent marker. Prediction of PB patients is shown in dark gray, and prediction of TB patients is shown in light gray for 3 different phenotypic markers. When ≥ 2 phenotypes supported one of the diagnoses, a final diagnosis of either PB (black) or TB (white) was made.
(PDF)
Acknowledgments
We thank Dr. Malcolm S. Duthie, Infectious Disease Research Institute, 1616 Eastlake Avenue East, Suite 400, Seattle, WA 98102 USA for providing the M. leprae recombinant antigens LID-1 and ML2044.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Duthie MS, Sampaio LH, Oliveira RM, Raman VS, O'Donnell J, Bailor HR, et al. Development and pre-clinical assessment of a 73 kD chimeric fusion protein as a defined sub-unit vaccine for leprosy. Vaccine. 2013;31: 813–819. 10.1016/j.vaccine.2012.11.073 [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global leprosy situation. Wkly Epidemiol Rec. 2007;82: 225–232. [PubMed] [Google Scholar]
- 3.WHO. Global leprosy update, 2017: reducing the disease burden due to leprosy. Wkly Epidemiol Rec. 2017;93: 444–456. [Google Scholar]
- 4.Oskam L, Slim E, Buhrer-Sekula S. Serology: recent developments, strengths, limitations and prospects: a state of the art overview. Lepr Rev. 2003;74: 196–205. [PubMed] [Google Scholar]
- 5.Spencer JS, Kim HJ, Wheat WH, Chatterjee D, Balagon MV, Cellona RV, et al. Analysis of antibody responses to Mycobacterium leprae phenolic glycolipid I, lipoarabinomannan, and recombinant proteins to define disease subtype-specific antigenic profiles in leprosy. Clin Vaccine Immunol. 2011;18: 260–267. 10.1128/CVI.00472-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobosha K, van der Ploeg-van Schip JJ, Esquenazi DA, Guimarães MM, Martins MV, Bekele Y, et al. Peptides derived from Mycobacterium leprae ML1601c discriminate between leprosy patients and healthy endemic controls. J Trop Med. 2012;2012: 132049 10.1155/2012/132049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara G, Losi M, D'Amico R, Roversi P, Piro R, Meacci M, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet. 2006;367: 1328–1334. 10.1016/S0140-6736(06)68579-6 [DOI] [PubMed] [Google Scholar]
- 8.Hungria EM, Freitas AA, Pontes MA, Goncalves HS, Sousa AL, Costa MB, et al. Antigen-specific secretion of IFNgamma and CXCL10 in whole blood assay detects Mycobacterium leprae infection but does not discriminate asymptomatic infection from symptomatic leprosy. Diagn Microbiol Infect Dis. 2017;87: 328–334. 10.1016/j.diagmicrobio.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 9.Freitas AA, Oliveira RM, Hungria EM, Cardoso LP, Sousa AL, Costa MB, et al. Alterations to antigen-specific immune responses before and after multidrug therapy of leprosy. Diagn Microbiol Infect Dis. 2015;83: 154–161. 10.1016/j.diagmicrobio.2015.06.021 [DOI] [PubMed] [Google Scholar]
- 10.Freitas AA, Hungria EM, Costa MB, Sousa AL, Castilho ML, Goncalves HS, et al. Application of Mycobacterium Leprae-specific cellular and serological tests for the differential diagnosis of leprosy from confounding dermatoses. Diagn Microbiol Infect Dis. 2016;86: 163–168. 10.1016/j.diagmicrobio.2016.07.024 [DOI] [PubMed] [Google Scholar]
- 11.Oliveira RM, Hungria EM, de Araujo FA, de Sousa AL, Costa MB, Reed SG, et al. Synergistic antigen combinations for the development of interferon gamma release assays for paucibacillary leprosy. Eur J Clin Microbiol Infect Dis. 2014;33: 1415–1424. 10.1007/s10096-014-2077-z [DOI] [PubMed] [Google Scholar]
- 12.Geluk A, Bobosha K, van der Ploeg-van SJJ, Spencer JS, Banu S, Martins MV, et al. New biomarkers with relevance to leprosy diagnosis applicable in areas hyperendemic for leprosy. J Immunol. 2012;188: 4782–4791. 10.4049/jimmunol.1103452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampaio LH, Stefani MMA, Oliveira RM, Sousa ALM, Ireton GC, Reed SG, et al. Immunologically reactive M. leprae antigens with relevance to diagnosis and vaccine development. BMC Infect Dis. 2011;11: 26 10.1186/1471-2334-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen Y, You YG, Yuan L-C, Yuan YH, Zhang Y, Duthie MS, et al. Evaluation of novel tools to facilitate the detection and characterization of leprosy patients in China. Biomed Res Int. 2014;2014: 371828 10.1155/2014/371828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, You YG, Yuan YH, Yuan LC, Zhang Y, Yan W. Evaluation of antigen-specific immune responses for leprosy diagnosis in a hyperendemic area in China. PLoS Negl Trop Dis. 2018;12: e0006777 10.1371/journal.pntd.0006777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobosha K, Fat EMTK, van den Eeden SJ, Bekele Y, van der Ploeg-van Schip JJ, de Dood CJ, et al. Field-evaluation of a new lateral flow assay for detection of cellular and humoral immunity against Mycobacterium leprae. PLoS Negl Trop Dis. 2014;8: e2845 10.1371/journal.pntd.0002845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sampaio LH, Sousa AL, Barcelos MC, Reed SG, Stefani MM, Duthie MS. Evaluation of various cytokines elicited during antigen-specific recall as potential risk indicators for the differential development of leprosy. Eur J Clin Microbiol Infect Dis. 2012;31: 1443–1451. 10.1007/s10096-011-1462-0 [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Li J. Analysis of leprosy epidemic situation in in Honghe prefecture (1930 to 2014). Chinese Community Doctors. 2016;32: 179–180. [Google Scholar]
- 19.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34: 255–273. [PubMed] [Google Scholar]
- 20.WHO. WHO expert commitee on leprosy: Seventh report. Geneva: World Health Organization; 1998.
- 21.Rada E, Duthie MS, Reed SG, Aranzazu N, Convit J. Serologic follow-up of IgG responses against recombinant mycobacterial proteins ML0405, ML2331 and LID-1 in a leprosy hyperendemic area in Venezuela. Mem Inst Oswaldo Cruz. 2012;107 Suppl 1: 90–94. [DOI] [PubMed] [Google Scholar]
- 22.Duthie MS, Goto W, Ireton GC, Reece ST, Sampaio LH, Grassi AB, et al. Antigen-specific T-cell responses of leprosy patients. Clin Vaccine Immunol. 2008;15: 1659–1665. 10.1128/CVI.00234-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiong-Hua P, Zhong-Yi Z, Jun Y, Yan W, Lian-Chao Y, Huan-Ying L, et al. Early revelation of leprosy in China by sequential antibody analyses with LID-1 and PGL-I. J Trop Med. 2013;2013: 352689 10.1155/2013/352689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aryanpur M, Mortaz E, Masjedi MR, Tabarsi P, Garssen J, Adcock IM, et al. Reduced phagocytic capacity of blood monocyte/macrophages in tuberculosis patients is further reduced by smoking. Iran J Allergy Asthma Immunol. 2016;15: 174–182. [PubMed] [Google Scholar]
- 25.Hasan Z, Mahmood A, Zafar S, Khan AA, Hussain R. Leprosy patients with lepromatous disease have an up-regulated IL-8 response that is unlinked to TNF-alpha responses. Int J Lepr Other Mycobact Dis. 2004;72: 35–44. [DOI] [PubMed] [Google Scholar]
- 26.Kirkaldy AA, Musonda AC, Khanolkhar-Young S, Suneetha S, Lockwood DN. Expression of CC and CXC chemokines and chemokine receptors in human leprosy skin lesions. Clin Exp Immunol. 2003;134: 447–453. 10.1111/j.1365-2249.2003.02306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrade PR, Jardim MR, da Silva AC, Manhaes PS, Antunes SL, Vital R, et al. Inflammatory cytokines are involved in focal demyelination in leprosy neuritis. J Neuropathol Exp Neurol. 2016;75: 272–283. 10.1093/jnen/nlv027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Each dot represents the analyte level of one participant in the study, and horizontal lines represent the median and IQR values. The cytokine and chemokine levels [TNF-α (A), IL-4 (B), IL-6 (C), IL-10 (D), CCL2 (E), CCL4 (F), CXCL8 (G), CXCL10 (H), G-CSF (I) and GM-CSF (J)] obtained in the supernatant after overnight stimulation with the specific M. leprae antigen ML2044 by WBA.
(TIF)
Each dot represents the analyte level of one participant in the study, and horizontal lines represent the median and IQR values. The cytokine and chemokine levels [TNF-α (A), IL-4 (B), IL-6 (C), IL-10 (D), CCL2 (E), CCL4 (F), CXCL8 (G), CXCL10 (H), G-CSF (I) and GM-CSF (J)] obtained in supernatants after overnight stimulation with the specific M. leprae antigen LID-1 by WBA.
(TIF)
Seven PB patients, as defined by the WHO, and 21 HCCs were analyzed. The concentration and the best cutoff were determined for each cytokine or chemokine, as described in S4 Table. The cutoff was used to define whether the concentrations of cytokines and chemokines indicated that the participant was a PB patient or an HHC. Each cytokine or chemokine was used as an independent marker. Prediction of PB patients is shown in dark gray, and prediction of HHCs is shown in light gray for 3 different phenotypic markers. When ≥ 2 phenotypes supported one of the diagnoses, a final diagnosis of either PB (black) or HHC (white) was made.
(PDF)
Seven PB patients, as defined by the WHO, and 21 HCCs were analyzed. The concentration and the best cutoff were determined for each cytokine or chemokine, as described in S5 Table. The cutoff was used to define whether the concentrations of cytokines and chemokines indicated that the participants was a PB patient or a TB patient. Each cytokine or chemokine was used as an independent marker. Prediction of PB patients is shown in dark gray, and prediction of TB patients is shown in light gray for 3 different phenotypic markers. When ≥ 2 phenotypes supported one of the diagnoses, a final diagnosis of either PB (black) or TB (white) was made.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.