Abstract
Background
Repetitive peripheral magnetic stimulation (rPMS) is a form of therapy that creates painless stimulation of deep muscle structures to improve motor function in people with physical impairment from brain or nerve disorders. Use of rPMS for people after stroke has been identified as a feasible approach to improve activities of daily living and functional ability. However, no systematic reviews have assessed the findings of available trials. The effect and safety of this intervention for people after stroke currently remain uncertain.
Objectives
To assess the effect of rPMS for improving activities of daily living and functional ability in people after stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (August 2016), the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library (August 2016), MEDLINE Ovid (November 2016), Embase Ovid (August 2016), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) in Ebsco (August 2016), PsycINFO Ovid (August 2016), the Allied and Complementary Medicine Database (AMED) Ovid (August 2016), Occupational Therapy Systematic Evaluation of Evidence (OTseeker) (August 2016), the Physiotherapy Evidence Database (PEDro) (October 2016), and ICHUSHI Web (October 2016). We also searched five ongoing trial registries, screened reference lists, and contacted experts in the field. We placed no restrictions on the language or date of publication when searching the electronic databases.
Selection criteria
We included randomised controlled trials (RCTs) conducted to assess the therapeutic effect of rPMS for people after stroke. Comparisons eligible for inclusion were (1) active rPMS only compared with 'sham' rPMS (a very weak form of stimulation or a sound only); (2) active rPMS only compared with no intervention; (3) active rPMS plus rehabilitation compared with sham rPMS plus rehabilitation; and (4) active rPMS plus rehabilitation compared with rehabilitation only.
Data collection and analysis
Two review authors independently assessed studies for inclusion. The same review authors assessed methods and risk of bias and extracted data. We contacted trial authors to ask for unpublished information if necessary. We resolved all disagreements through discussion.
Main results
We included three trials (two RCTs and one cross‐over trial) involving 121 participants. Blinding of participants and physicians was well reported in all trials, and overall risk of bias was low. We found no clear effect of rPMS on activities of daily living at the end of treatment (mean difference (MD) ‐3.00, 95% confidence interval (CI) ‐16.35 to 10.35; low‐quality evidence) and at the end of follow‐up (MD ‐2.00, 95% CI ‐14.86 to 10.86; low‐quality evidence). Investigators in one study with 63 participants observed no statistical difference in improvement of upper limb function at the end of treatment (MD 2.00, 95% CI ‐4.91 to 8.91) and at the end of follow‐up (MD 4.00, 95% CI ‐2.92 to 10.92). One trial with 18 participants showed that rPMS treatment was not associated with improved muscle strength at the end of treatment (MD 3.00, 95% CI ‐2.44 to 8.44). Another study reported a significant decrease in spasticity of the elbow at the end of follow‐up (MD ‐0.48, 95% CI ‐0.93 to ‐0.03). No studies provided information on lower limb function and death. Based on the GRADE approach, we judged the certainty of evidence related to the primary outcome as low owing to the small sample size of one study.
Authors' conclusions
Available trials provided inadequate evidence to permit any conclusions about routine use of rPMS for people after stroke. Additional trials with large sample sizes are needed to determine an appropriate rPMS protocol as well as long‐term effects. We identified three ongoing trials and will include these trials in the next review update.
Repetitive peripheral magnetic stimulation for improving everyday activities in people after stroke
Review question Is repetitive peripheral magnetic stimulation (rPMS) effective for improving everyday activities in people after stroke?
Background Stroke, the most common cause of disability, occurs when a blood clot blocks a blood vessel in the brain. Two types of stroke are known: ischaemic, due to lack of blood flow, and haemorrhagic, due to bleeding. Paralysis of the arm or leg after stroke causes problems with everyday activities and functions, including showering, dressing, and walking. Stroke patients with hemiparesis require physical rehabilitation: training of upper and lower limbs, exercise focused on activities of daily living, and fitting of walking aids (e.g. cane chosen appropriately). However, effective treatments are currently limited. rPMS is a painless method of stimulation that has been used to try to improve movement in people with brain or nerve disorders.
Study characteristics We found three trials of rPMS (two individual RCTs and one cross‐over trial) involving a total of 121 participants. One study compared rPMS against 'sham’ stimulation (a very weak form of stimulation or a sound only). Two studies compared rPMS plus rehabilitation versus rehabilitation alone.
Key results
We found limited evidence for use of rPMS to improve activities of daily living, muscle strength, upper limb function, and spasticity (unusual stiffness of muscles) in people after stroke. Although rPMS plus rehabilitation slightly reduced spasticity of the elbow compared with rehabilitation alone, it remains unclear what type of rPMS should be performed, and on which part of the body. No information was available on lower limb function. The included trials reported no impact on death.
Quality of the evidence
We classified the quality of the evidence as low for improving activities of daily living, mainly because one study had a small sample size. Additional trials with larger sample sizes are needed to determine a suitable rPMS protocol for treating people after stroke.
Summary of findings
Summary of findings for the main comparison.
rPMS compared with any type of control intervention in stroke
| rPMS compared with any type of control intervention in stroke | ||||||
|
Patient or population: people with stroke Intervention: rPMS Comparison: any type of control intervention Setting: Germany and Canada | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with any type of control intervention | Risk with rPMS | |||||
| Activities of daily living (ADLs) assessed with Barthel Index Scale, from 0 to 100 | Mean activities of daily living score was 50 | MD 3 lower (16.35 lower to 10.35 higher) | ‐ | 63 (1 RCT) | ⊕⊕⊝⊝ LOWa | |
| Upper limb function assessed with Fugl‐Meyer Assessment Scale, from 0 to 66 | Mean upper limb function score was 13 | MD 2 higher (4.91 lower to 8.91 higher) | ‐ | 63 (1 RCT) | ⊕⊕⊝⊝ LOWa | |
| Lower limb function ‐ not measured | ‐ | ‐ | See comments | ‐ | ‐ | No trials measured this outcome |
| Spasticity (elbow) assessed with Modified Tardieu Scale Scale, from 0 to 5 | Mean spasticity (elbow) score was 1.41 | MD 0.41 lower (0.89 lower to 0.07 higher) | ‐ | 63 (1 RCT) | ⊕⊕⊝⊝ LOWa | |
| Spasticity (wrist) assessed with Modified Tardieu Scale Scale, from 0 to 5 | Mean spasticity (wrist) score was 2.13 | MD 0.2 lower (0.76 lower to 0.36 higher) | ‐ | 63 (1 RCT) | ⊕⊕⊝⊝ LOWa | |
| Muscle strength assessed with dorsiflexion strength | Mean muscle strength was 10.44 kg | MD 3 kg higher (2.44 lower to 8.44 higher) | ‐ | 18 (1 RCT) | ⊕⊕⊝⊝ LOWa | |
| Death ‐ not reported | ‐ | ‐ | See comments | ‐ | ‐ | No trials reported this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aOne study with small sample size; 95% CI overlaps zero
Background
Description of the condition
Stroke is a serious healthcare problem that requires long‐term rehabilitation as a core component of recovery (Sacco 2013). Every year, around 16 million strokes occur throughout the world, causing 5.7 million deaths (Strong 2007). Approximately 88% of all strokes are of the ischaemic type; other types include haemorrhagic stroke and subarachnoid haemorrhage (Park 2012). The most common disability after stroke is motor impairment (Langhorne 2009), which adversely affects control of arm and leg movement and occurs in nearly 80% of people after stroke (De Vries 2007). At present, although post‐stroke functional recovery remains a high priority in health care, evidence on effective interventions for post‐stroke impairment is limited (McArthur 2011).
Description of the intervention
Repetitive peripheral magnetic stimulation (rPMS) is a unique non‐invasive stimulation method that was developed for therapeutic neuromodulation in movement disorders (Beaulieu 2013). In rPMS, a stimulation coil (magnetic field generator) is placed over the paralysed muscles of the arms, legs, or torso. The stimulation coil is attached to a stimulator (pulse generator), which provides an electrical current to the coil. The coil builds up a magnetic field as it passes through the skin, and it directs an electrical current into the neurons. Once the current achieves a certain value, an action potential is induced, which causes the neuron to depolarise and the muscles to eventually contract.
Treatment by rPMS allows painless stimulation of deep muscle structures that cannot be reached by electrical stimulation (Barker 1991; Ito 2013). People receiving rPMS do not need to remove their clothes because the procedure does not require placement of electrodes. Implanted medical devices, such as pacemakers or deep brain simulators, are contraindications for rPMS. However, the technology has no known negative side effects. Currently, rPMS is used to treat individuals with motor deficits resulting from brain or nerve disorders, although the high cost of rPMS devices precludes wide use of the technology. Nevertheless, rPMS can be performed to safely stimulate deeper regions of muscle without pain, and can potentially improve functional recovery in people after stroke (Han 2006).
How the intervention might work
Applying rPMS to the muscle induces a proprioceptive input to the central nervous system in two ways (Struppler 2004).
Direct activation of sensorimotor nerve fibres with an orthodromic and antidromic conduction.
Indirect activation of mechanoreceptors during rhythmical contraction and relaxation, as well as vibration of the muscles.
This afferent input elicits sensations and reaches higher levels of the central nervous system.
Initial assessment of transcranial magnetic stimulation revealed an increase in corticomotor excitability after rPMS, and subsequent functional magnetic resonance imaging assessment showed focal activations within the sensorimotor cortex in healthy participants (Gallasch 2015). After stroke, rPMS can increase motor‐evoked potential amplitude (Flamand 2014) and motor cortex excitability (Heldmann 2000; Krause 2008). Further, rPMS can effectively suppress spasticity (Struppler 2003) and has a modulatory effect on motor performance (Struppler 2004). The technique is also thought to increase neural excitability of the cortex and to balance interactions between hemispheres, thereby contributing to functional improvement in people after stroke (Kerkhoff 2001).
Why it is important to do this review
Several clinical trials have examined the use of rPMS for people with functional disability (Heldmann 2000; Nielsen 1996; Struppler 2004; Struppler 2007). However, the peer‐reviewed literature includes no systematic review that has assessed the findings of available trials. It remains unclear what type of stimulation (high frequency, low frequency, or other) should be performed, and on which part of the body (upper limb, lower limb, or others). In addition, rPMS studies have tended to include small sample sizes. Therefore, a systematic review of trials is needed to evaluate the effectiveness of rPMS.
Objectives
To assess the effect of rPMS for improving activities of daily living and functional ability in people after stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included individual randomised controlled trials (RCTs), cluster‐RCTs, and cross‐over trials. We excluded quasi‐RCTs (trials in which the method of allocating participants to a treatment is not strictly random, e.g. by date of birth, hospital record number, or alternation).
Types of participants
We included people after stroke regardless of sex, age, and stroke severity and duration. Stroke is defined by the World Health Organization as a "neurological deficit of cerebrovascular cause that lasts more than 24 hours or leads to death within 24 hours" (World Health Organization 1989).
Types of interventions
We included trials comparing any type of active rPMS or rPMS plus rehabilitation for improving functional ability versus any type of control intervention (i.e. sham rPMS, sham rPMS plus rehabilitation for improving functional ability, or no intervention). Investigators conducted rPMS peripherally (not for central nervous system such as brain or spinal cord) and non‐invasively (without use of puncture needle or implantation techniques).
We investigated the following comparisons.
Active rPMS only compared with sham rPMS.
Active rPMS only compared with no intervention.
Active rPMS plus rehabilitation compared with sham rPMS plus rehabilitation.
Active rPMS plus rehabilitation compared with rehabilitation only.
Types of outcome measures
Primary outcomes
Activities of daily living (ADLs) at the end of treatment and at the end of scheduled follow‐up. ADLs refer to basic tasks of everyday life, including self‐care activities such as eating, bathing, dressing, and toileting. We preferentially used the Barthel Index (BI) or the Functional Independence Measure (FIM) but allowed the use of any validated ADL measures as follows.
Katz Index of Independence in Activities of Daily Living.
Frenchay Activities Index (FAI).
Secondary outcomes
We included the following secondary outcome measures.
-
Upper limb function.
Fugl‐Meyer Assessment.
Action Research Arm Test.
Wolf Motor Function Test (second).
-
Lower limb function.
Gait velocity (cm/s).
Timed Up and Go Test (seconds).
-
Spasticity.
(Modified) Tardieu Scale.
Modified Ashworth Scale (MAS).
-
Muscle strength.
Grip strength (kg).
Medical Research Council (MRC) Scale.
Death (as adverse event).
We explored secondary outcomes at the end of treatment and at the end of scheduled follow‐up. We treated these outcomes as continuous data.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged for translation of relevant articles when necessary.
Electronic searches
We searched the Cochrane Stroke Group trials register and the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11) in the Cochrane Library (searched August 2016) (Appendix 1).
MEDLINE in Ovid (1950 to August 2016) (Appendix 2).
Embase in Ovid (1980 to November 2016) (Appendix 3).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) in EBSCO (1937 to August 2016) (Appendix 4).
PsycINFO in Ovid (1806 to August 2016) (Appendix 5).
Allied and Complementary Medicine Database (AMED) in Ovid (1985 to August 2016) (Appendix 6).
Occupational Therapy Systematic Evaluation of Evidence (OTseeker; www.otseeker.com/) (searched August 2016) (Appendix 7).
Physiotherapy Evidence Database (PEDro; http://www.pedro.fhs.usyd.edu.au/) (1929 to October 2016) (Appendix 8).
Ichushi‐Web (Japan Medical Abstracts Society (JAMAS) (www.jamas.or.jp/) (searched October 2016) (Appendix 9).
We developed the MEDLINE search strategy (Appendix 2) with the help of the Cochrane Stroke Group Information Specialist and adapted it for use with the other databases.
We also searched the following ongoing trials registers.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched August 2016) (Appendix 10).
ISRCTN Registry (www.isrctn.com/; searched August 2016) (Appendix 11).
Stroke Trials Registry (www.strokecenter.org/trials/; searched 10 August 2016) (Appendix 12).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/; searched August 2016) (Appendix 13).
Japanese UMIN Clinical Trials Registry (UMIN‐CTR) (www.umin.ac.jp/ctr/; searched August 2016) (Appendix 14).
Searching other resources
To identify additional published and unpublished relevant studies for potential inclusion in the review, we:
contacted experts in the field;
screened reference lists of relevant trials; and
searched Google Scholar (http://scholar.google.co.uk/).
Data collection and analysis
Selection of studies
Two review authors (RM, NY) independently screened titles and abstracts of references obtained as a result of our searching activities and excluded obviously irrelevant reports. We retrieved full‐text articles for the remaining references, and two review authors (RM, NY) independently screened these to identify studies for inclusion. We identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion, or, if required, we consulted a third person (EO). We collated multiple reports of the same study, so that each study ‐ not each reference ‐ was the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram (Moher 2009). We included studies presented only as abstracts, if sufficient information was reported. We used Covidence software for reference handling (Covidence 2013).
Data extraction and management
Two review authors (RM, NY) independently extracted the following data from the included studies using Covidence (Covidence 2013).
Methods: study design, randomisation method, allocation concealment method, blinding methods.
Participants: diagnosis (type, severity, and location of stroke), number in each group, age, sex, baseline comparability between two groups, time from onset, losses to follow‐up.
Interventions: details of rPMS (frequency, intensity, duration, treatment session), target of stimulation, co‐exercise.
Outcomes: types of outcomes, assessment time points.
Other: setting, publication year, sources of funding, intention‐to‐treat analysis (ITT).
All review authors resolved disagreements by discussion.
Assessment of risk of bias in included studies
Two review authors (RM, NY) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) along with Covidence (Covidence 2013). We resolved disagreements by discussion or by consultation with another review author (EO). We assessed risk of bias according to the following domains.
Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions before assignment, and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the method as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the method used, if any, to blind study participants and personnel to which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes. We assessed the method as:
low, high, or unclear risk of bias for participants; and
low, high, or unclear risk of bias for personnel.
Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the method used, if any, to blind outcome assessors to which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed the method used to blind outcome assessment as:
low, high, or unclear risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the quantity, nature, and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported, or could be supplied by trial authors, we re‐included missing data in the analyses that we performed. We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis performed with a substantial departure of the intervention received from that assigned at randomisation); or
unclear risk of bias.
Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (when not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study failed to include results of a key outcome that was expected to have been reported); or
unclear risk of bias.
Other bias
For each included study, we described any important concerns that we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias by assigning:
low risk of other bias;
high risk of other bias; or
unclear whether risk of other bias is present.
Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to the above domains, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact study findings.
We graded the risk of bias for each domain and provided information from the study report together with a justification for our judgement in the 'Risk of bias' tables.
We used the GRADE approach to assess the quality of the body of evidence related to the following main outcomes at the end of treatment (Guyatt 2008).
ADLs.
Upper limb function.
Lower limb function.
Spasticity.
Muscle strength.
Death.
We used GRADEprofiler (GRADE 2014) to import data from Review Manager 5.3 (RevMan 2014) to create a 'Summary of findings' table. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach is based on five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) and is used to assess the quality of the body of evidence for each outcome. Evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as a summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome but used different measures.
Unit of analysis issues
Cluster‐randomised trials
In future updates, we will include cluster‐randomised trials in the analyses, along with individually randomised trials. We will adjust standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions on the basis of an estimate of the intracluster correlation coefficient (ICC) derived from the trial if possible, from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this and will conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we plan to synthesise relevant information.
In future updates, we will acknowledge heterogeneity in the randomisation unit and will perform a sensitivity analysis to investigate effects of the randomisation unit.
Cross‐over trials
We included cross‐over trials in the review. We analysed only data from the first phase of cross‐over trials.
Multi‐armed trials
When we identified trials with multiple intervention arms, we planned to include only directly relevant arms. If the trial included several relevant intervention arms, we planned to combine all relevant experimental intervention groups of the study into a single group and to combine all relevant control intervention groups into a single control group.
Dealing with missing data
We contacted trial authors to obtain missing data, if necessary. For included studies, we noted levels of attrition. We performed sensitivity analysis to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect.
For all outcomes, we carried out analyses as far as possible on an ITT basis, that is, we attempted to include in the analyses all participants randomised to each group, and we analysed all participants in the group to which they were allocated, regardless of whether they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis by using T², I², and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either T² was greater than zero or the P value was low (< 0.10) in the Chi² test for heterogeneity. We used Review Manager to assess heterogeneity (RevMan 2014).
Assessment of reporting biases
In future updates, if appropriate, we will use funnel plots to detect reporting biases (such as publication bias). We will assess funnel plot asymmetry visually. If asymmetry is suggested by visual assessment, we will perform exploratory analyses to investigate this.
Data synthesis
Two review authors (RM, NY) independently extracted data from the included trials. One review author (RM) entered the data into RevMan, and the other review author (NY) checked the entries. We resolved disagreements through discussion, with reference to the original report.
We carried out statistical analysis using Review Manager (RevMan 2014). We used fixed‐effect meta‐analysis in combining data when it was reasonable to assume that studies were estimating the same underlying treatment effect, that is, when trials were examining the same intervention, and when trial populations and methods were judged sufficiently similar. If clinical heterogeneity was sufficient to expect that underlying treatment effects differ between trials, or if we detected substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects, and we discussed the clinical implications of differing treatment effects between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. If we used random‐effects analyses, we presented results as the average treatment effect with 95% confidence interval (CI), along with estimates of T² and I². If it was inappropriate or impossible to pool data quantitatively, we provided a narrative summary of study results.
Subgroup analysis and investigation of heterogeneity
When we identified substantial heterogeneity in the primary outcomes, we investigated this by conducting subgroup analyses. We considered whether an overall summary was meaningful, and if so, we used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses of primary outcomes, if sufficient data were available.
Location of stimulation: upper limb versus lower limb or trunk.
Type of stroke: cerebral infarction versus cerebral haemorrhage.
Duration of illness: acute to subacute phase (to six months after stroke) versus chronic phase (more than six months after stroke).
We assessed subgroup differences by performing interaction tests available within Review Manager (RevMan 2014). We reported the results of subgroup analyses by quoting the Chi² statistic and the P value, and results of the interaction test by providing the I² value.
Sensitivity analysis
If we identified an adequate number of studies, we planned to perform sensitivity analyses by excluding:
studies with inadequate allocation concealment and random sequence generation;
studies in which outcome evaluation was not blinded;
studies in which loss to follow‐up was not reported or was greater than 10%; and
unpublished studies.
Results
Description of studies
Results of the search
See Figure 1.
Figure 1.
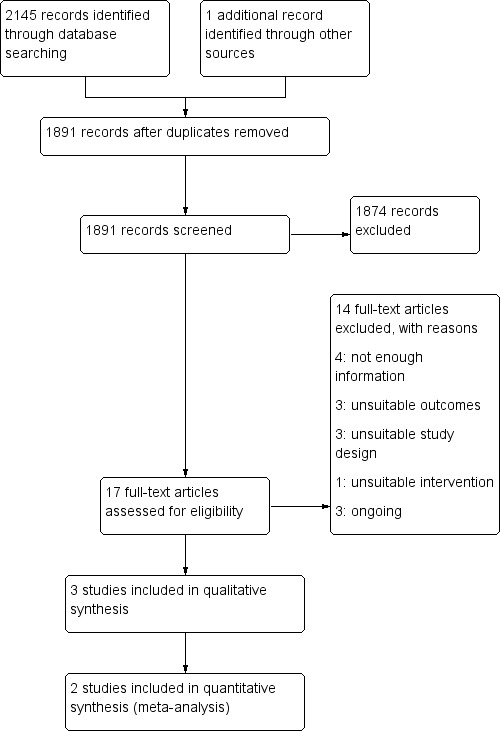
Study flow diagram.
After screening 2145 titles and abstracts, we identified 17 potentially relevant articles. After reviewing the full text of the 17 articles, we included in the review three trials involving a total of 121 participants (Beaulieu 2015; Krewer 2014; Werner 2016).
Included studies
See Characteristics of included studies.
Study design and study location
We included two parallel‐group trials (Beaulieu 2015; Krewer 2014) and one cross‐over trial (Werner 2016) in qualitative synthesis. Trials were reported from Germany and Canada.
Sample characteristics
The three included trials involved 121 participants. Individual sample sizes of identified trials ranged from 18 (Beaulieu 2015) to 63 (Krewer 2014). Mean ages of participants were less than 55 years (Beaulieu 2015; Krewer 2014; Werner 2016), and mean time from onset ranged from less than 26 weeks (Krewer 2014) to 83 months (Beaulieu 2015). Two studies included participants with stroke, and their elapsed time from onset was over 12 months (Beaulieu 2015; Werner 2016). Male participants (57%) exceeded female (43%) participants. Two studies included traumatic brain injury (Krewer 2014; Werner 2016), and one study included tetraparesis (Werner 2016). We could not exclude study participants with traumatic brain injury. We decided to include trials of mixed groups if more than half had a stroke diagnosis. We noted imbalances in time since onset (Beaulieu 2015; Krewer 2014) and in mean age (Werner 2016), but we considered these unlikely to affect outcomes. Groups in all studies were comparable in terms of assessed baseline characteristics.
Intervention approaches
The included studies used varied protocols of rPMS. Frequency of rPMS ranged from 5 Hz (Werner 2016) to 25 Hz (Krewer 2014). One study adopted theta‐burst frequency rPMS (Beaulieu 2015). Duration of stimulation (per session) ranged from 190 seconds (Beaulieu 2015) to 20 minutes (Krewer 2014), and number of stimulations (per session) ranged from 600 (Beaulieu 2015) to 5000 (Krewer 2014). Only one study conducted multiple stimulation sessions as part of the treatment regimen (two times a day, five times a week, for two weeks) (Krewer 2014). Targets of stimulation were the lower leg (Beaulieu 2015), upper and lower arm (Krewer 2014), and lower arm (Werner 2016). Co‐exercise included occupational therapy after each stimulation (Krewer 2014) and muscle stretching during stimulation (Werner 2016). Sham stimulation consisted of low‐intensity stimulation (Beaulieu 2015) or a clicking sound only (Krewer 2014; Werner 2016).
Outcomes
The included trials used several heterogeneous outcome measures. Only one study assessed our primary outcome (ADLs) as measured by the Barthel Index (Krewer 2014). Beaulieu 2015 measured dorsiflexion strength (kg) as a muscle strength evaluation. Krewer 2014 assessed upper limb function using the Fugl‐Meyer Assessment. Investigators measured spasticity by using the Modified Tardieu Scale (Krewer 2014) or the Modified Ashworth Score (Werner 2016). Two trials evaluated outcomes immediately after treatment (Beaulieu 2015; Werner 2016); one trial measured outcomes after two weeks of treatment and two weeks after the treatment phase (Krewer 2014). None of the included studies reported any deaths.
Excluded studies
Among 17 potentially relevant studies, we excluded 13 trials because they did not meet the inclusion criteria. We have listed reasons for exclusion in the Characteristics of excluded studies table. Three studies were not RCTs (Bernhardt 2007; Struppler 2002; Struppler 2009), and three studies measured outcomes that were different from those provided in our protocol (Heldmann 2000; Kuznetsova 2016; Momosaki 2014). Evidence was insufficient for review authors to determine inclusion eligibility for four trials (Kotchetkov 1999; Kuznetsova 2013; Samosiuk 2003; Zifko 2002), and we were unable to make contact with study authors.
Ongoing studies
We identified four ongoing trials that appeared to be relevant for inclusion (Izumi 2015; Pohl 2015a; Pohl 2015b; Suzuki 2015). See Characteristics of ongoing studies.
Risk of bias in included studies
Figure 2.
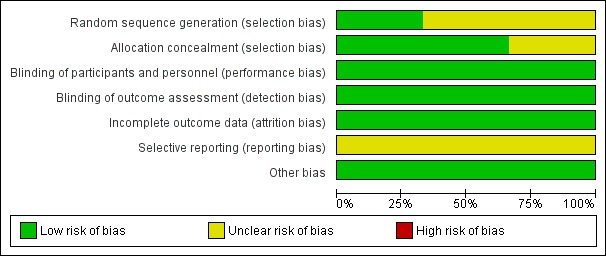
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Werner 2016 conducted sequence generation with the help of a computer‐generated lot (www.randomizer.at). As other studies did not report random sequence generation, we classified them as unclear.
Allocation concealment
Allocation concealment was adequate in two trials (Krewer 2014; Werner 2016); however, one study did not report on this (Beaulieu 2015).
Blinding
Participants and personnel
All trials provided blinding with regard to participants and personnel. Investigators conducted sham stimulations adequately, and we ranked these studies as having low risk of bias.
Outcome assessment
All trials provided blinding with regard to outcome assessors. All studies reported methods of blinding, and we ranked these studies as having low risk of bias.
Incomplete outcome data
Beaulieu 2015 and Werner 2016 reported no withdrawals or dropouts, so we classified these studies as having low risk of bias. Krewer 2014 reported that only three participants were lost to follow‐up (5%) and described no differences in the reasons why outcome data were missing. Krewer 2014 performed ITT analysis, and we classified this study as having low risk of bias.
Selective reporting
Study protocols were not available for any of the included studies, and so we judged selective reporting bias as low.
Other potential sources of bias
We identified no other information associated with other potential sources of bias.
Effects of interventions
See: Table 1
See Table 1.
We contacted the authors of included studies to request missing outcome data. However, we could not obtain data from the first phase of the cross‐over trial (Werner 2016), so we excluded this study from the quantitative synthesis (meta‐analysis). We included two studies in the quantitative analysis (Beaulieu 2015; Krewer 2014). As Krewer 2014 evaluated spasticity at both the elbow and the wrist, we analysed these data separately.
Comparison 1: rPMS versus sham
Primary outcome
Activities of daily living
We found no studies examining the effect of rPMS on the primary outcome in stroke patients.
Secondary outcomes
Muscle strength
Only one small study assessed our secondary outcome of muscle strength at the end of treatment (Beaulieu 2015). This trial included a total of 18 participants and showed that rPMS treatment was not associated with a significant improvement in muscle strength at the end of treatment (mean difference (MD) 3.00, 95% CI ‐2.44 to 8.44; see Analysis 1.1). This study did not report muscle strength at the end of follow‐up.
Analysis 1.1.
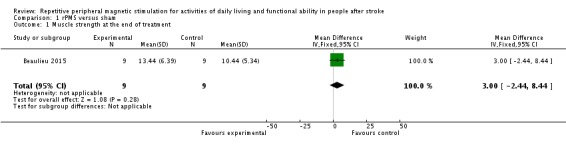
Comparison 1 rPMS versus sham, Outcome 1 Muscle strength at the end of treatment.
Others
Included trials did not report adverse events including death associated with rPMS.
Comparison 2: rPMS plus rehabilitation versus rehabilitation only
Primary outcome
Activities of daily living
Krewer 2014 provided data on activities of daily living as a Barthel Index score at the end of treatment and at the end of follow‐up. Data show no significant differences between the rPMS plus rehabilitation group and the rehabilitation only group (end of treatment: MD ‐3.00, 95% CI ‐16.35 to 10.35; see Analysis 2.1; end of follow‐up: MD ‐2.00, 95% CI ‐14.86 to 10.86; see Analysis 2.2).
Analysis 2.1.
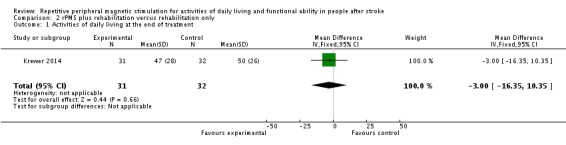
Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 1 Activities of daily living at the end of treatment.
Analysis 2.2.
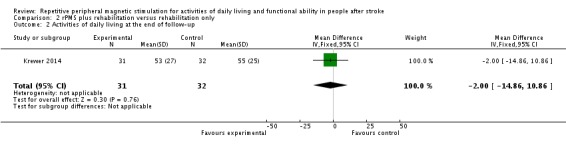
Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 2 Activities of daily living at the end of follow‐up.
Secondary outcomes
Upper limb function
Only Krewer 2014 reported the Fugl‐Meyer Assessment as an outcome measure of upper limb function. Results of this study show that rPMS plus rehabilitation did not increase upper limb function compared with rehabilitation only at the end of treatment and at the end of follow‐up (end of treatment: MD 2.00, 95% CI ‐4.91 to 8.91; see Analysis 2.3; end of follow‐up: MD 4.00, 95% CI ‐2.92 to 10.92; see Analysis 2.4).
Analysis 2.3.
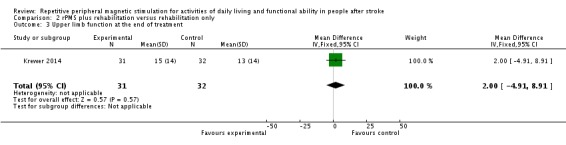
Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 3 Upper limb function at the end of treatment.
Analysis 2.4.
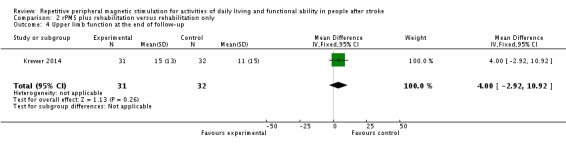
Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 4 Upper limb function at the end of follow‐up.
Spasticity
Krewer 2014 evaluated spasticity at the elbow and wrist using the Modified Tardieu Scale. We separately evaluated results related to the elbow and the wrist. We found no significant differences in spasticity between the rPMS plus rehabilitation group and the rehabilitation group at the end of treatment (elbow: MD ‐0.41, 95% CI ‐0.89 to 0.07; wrist: MD ‐0.20, 95% CI ‐0.76 to 0.36; see Analysis 2.5). rPMS plus rehabilitation slightly reduced spasticity of the elbow compared with rehabilitation only at the end of follow‐up (MD ‐0.48, 95% CI ‐0.93 to ‐0.03). We found no differences between the rPMS plus rehabilitation group and the rehabilitation group in spasticity of the wrist at the end of follow‐up (MD ‐0.13, 95% CI ‐0.67 to 0.41; see Analysis 2.6).
Analysis 2.5.
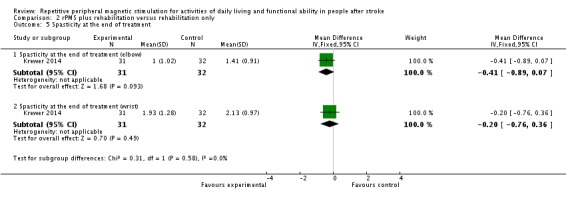
Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 5 Spasticity at the end of treatment.
Analysis 2.6.
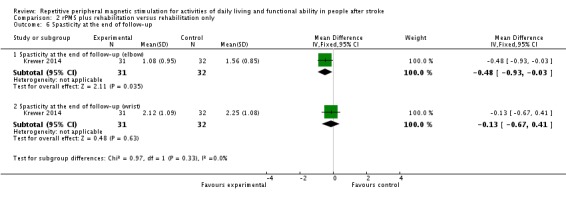
Comparison 2 rPMS plus rehabilitation versus rehabilitation only, Outcome 6 Spasticity at the end of follow‐up.
Other outcomes
No study reported lower limb function as an outcome. None of the included trials reported adverse events, including death, associated with rPMS.
Discussion
Summary of main results
We found three trials (121 participants) that were eligible for inclusion in the review. We did not find high risk of bias across these trials, and we determined that the overall risk of bias was low. Only one randomised controlled trial (RCT) (63 participants) reported the effect of repetitive peripheral magnetic stimulation (rPMS) on activities of daily living and showed that rPMS was not associated with a significant increase in the Barthel Index score (see Table 1). One study compared rPMS versus sham (Beaulieu 2015). Another two studies compared rPMS plus rehabilitation versus rehabilitation (Krewer 2014; Werner 2016). Only one study conducted multiple stimulation sessions as part of treatment (Krewer 2014). Investigators reported spasticity, muscle strength, and upper limb function as secondary outcomes. One study reported significant reduction in spasticity of the elbow at the end of follow‐up (mean difference (MD) ‐0.48, 95% confidence interval (CI) ‐0.93 to ‐0.03) but noted no significant differences in the other outcomes. None of the included studies reported death.
Overall completeness and applicability of evidence
Included trials did not provide sufficient information for review authors to address the aim of our review. Only three trials contributed data to our review, and only two of these were individual RCTs. We identified one cross‐over placebo‐controlled trial. We contacted the authors of this cross‐over trial, but as none of them responded, we could not include this study in our analysis. Stimulation parameters (frequency, intensity, pulses) also varied across studies. Sample sizes of the studies were small, ranging from 18 to 63 participants, which may have led to insufficient statistical power to detect differences. Large‐scale RCTs are needed to verify the efficacy of rPMS. Most of the included trials assessed the outcome at the end of the treatment period or within several weeks after treatment. Whether rPMS had long‐term effects on functional recovery is unclear.
Quality of the evidence
Overall risk of bias was low. All studies clearly reported blinding of participants and physicians, so we were able to make a clear decision about performance bias. However, all included studies had relatively small sample sizes: 18 in Beaulieu 2015, 63 in Krewer 2014, and 40 in Werner 2016. We downgraded the quality of evidence related to the primary outcome, mainly because one study had a small sample size and the 95% CI overlaps zero (Table 1).
Potential biases in the review process
Despite our extensive literature search, selection bias may have occurred. Although two review authors independently assessed eligibility of studies for inclusion along with risks of bias to minimise potential bias in this review, several subjective judgements were required during the review process. A different review team may judge risk of bias differently.
Agreements and disagreements with other studies or reviews
Two previous reviews have investigated the effectiveness of rPMS treatment (Beaulieu 2013; Beaulieu 2015b). Beaulieu 2013 summarised the results of 13 studies that used different types of outcomes (neurophysiological, biomechanical, clinical) in healthy individuals and in people with stroke or a spinal disorder. This review included quasi‐experimental studies and case studies and conducted no pooled analysis. Review authors reported that owing to limited evidence, they could reach no conclusion. Beaulieu 2015b dealt with stimulation parameters reported in any scientific research that applied rPMS as an intervention to improve somatosensory or motor disorders. The literature search yielded 24 studies on various pathological disorders. Review authors conducted no pooled analysis and concluded that future studies require a more structured design and larger samples. Similarly, our review assessed RCTs with small sample sizes that focused on clinical outcomes after stroke and found lack of sufficient evidence for effectiveness of rPMS.
Authors' conclusions
To date, evidence is insufficient to allow generalisable conclusions about the effect of rPMS for people with stroke. Routine use of rPMS for stroke cannot be supported by the results of our review.
Future studies with large sample sizes are needed to validate rPMS in people after stroke. Also, the most optimal rPMS protocol (intensity, duration, and frequency) and long‐term effects of rPMS should be investigated. We found several ongoing RCTs on this topic; findings of these studies could change assessment of the quality of evidence in the future.
Acknowledgements
We thank Hazel Fraser from the Cochrane Stroke Group for providing relevant information, Joshua David Cheyne for helping to develop the search strategy and for conducting searches, Louis‐David Beaulieu and Carmen Krewer for performing re‐analysis and providing unpublished data, and Emma Barber for providing editorial support. Portions of the methods section in this review protocol are based on the text template of the Pregnancy and Childbirth Review Group.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial arteriovenous malformations"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"stroke, lacunar"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"] or [mh ^"brain injuries"] or [mh ^"brain injury, chronic"] #2 (stroke or poststroke or post‐stroke or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH):ti,ab,kw (Word variations have been searched) #3 ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab,kw (Word variations have been searched) #4 ((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) near/5 (h?emorrhag* or h?ematoma$ or bleed*)):ti,ab,kw (Word variations have been searched) #5 [mh ^hemiplegia] or [mh paresis] #6 (hempar* or hemipleg* or paresis or paraparesis or paretic):ti,ab,kw (Word variations have been searched) #7 {or #1‐#6} #8 [mh ^"magnetic field therapy"] #9 [mh ^magnetics] #10 [mh ^"electromagnetic fields"] or [mh ^"electromagnetic phenomena"] or [mh ^"magnetic fields"] #11 ((magnet* or electromagnet* or electro‐magnet*) near/5 (field* or coil* or induction)):ti,ab,kw (Word variations have been searched) #12 ((peripher* or nerv* or musc* or spine or spinal) near/5 (magnet* or electromagnet* or electro‐magnet*) near/5 (stimulat* or neurostimulat*)):ti,ab,kw (Word variations have been searched) #13 (PMS or rPMS or PrMS):ti,ab,kw (Word variations have been searched) #14 {or #8‐#13} #15 #7 and #14
Appendix 2. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebral small vessel diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiplegia/ or exp paresis/ or exp Gait Disorders, Neurologic/
6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw.
7. or/1‐6
8. magnetic field therapy/
9. magnetics/
10. electromagnetic fields/ or electromagnetic phenomena/ or magnetic fields/
11. ((magnet$ or electromagnet$ or electro‐magnet$) adj5 (field$ or coil$ or induction)).tw.
12. ((peripher$ or nerv$ or musc$ or spine or spinal) adj5 (magnet$ or electromagnet$ or electro‐magnet$) adj5 (stimulat$ or neurostimulat$)).tw.
13. (PMS or rPMS or PrMS).tw.
14. 8 or 9 or 10 or 11 or 12 or 13
15. 7 and 14
16. Randomized Controlled Trials as Topic/
17. random allocation/
18. Controlled Clinical Trials as Topic/
19. control groups/
20. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
21. double‐blind method/
22. single‐blind method/
23. Placebos/
24. placebo effect/
25. cross‐over studies/
26. randomized controlled trial.pt.
27. controlled clinical trial.pt.
28. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
29. (random$ or RCT or RCTs).tw.
30. (controlled adj5 (trial$ or stud$)).tw.
31. (clinical$ adj5 trial$).tw.
32. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
33. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
34. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
35. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
36. (cross‐over or cross over or crossover).tw.
37. (placebo$ or sham).tw.
38. trial.ti.
39. (assign$ or allocat$).tw.
40. controls.tw.
41. or/16‐40
42. 15 and 41
43. exp animals/ not humans.sh.
44. 42 not 43
Appendix 3. Embase (Ovid) search strategy
1. cerebrovascular disease/ or brain disease/ or exp basal ganglion hemorrhage/ or exp brain hemangioma/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or exp cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or exp vertebrobasilar insufficiency/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. exp hemiplegia/ or exp paresis/
6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw.
7. or/1‐6
8. magnetotherapy/
9. exp magnetic field/ or exp magnetism/
10. ((magnet$ or electromagnet$ or electro‐magnet$) adj5 (field$ or coil$ or induction)).tw.
11. ((peripher$ or nerv$ or musc$ or spine or spinal) adj5 (magnet$ or electromagnet$ or electro‐magnet$) adj5 (stimulat$ or neurostimulat$)).tw.
12. (PMS or rPMS or PrMS).tw.
13. or/8‐12
14. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/
15. Randomization/
16. Controlled clinical trial/ or "controlled clinical trial (topic)"/
17. control group/ or controlled study/
18. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/
19. Crossover Procedure/
20. Double Blind Procedure/
21. Single Blind Procedure/ or triple blind procedure/
22. placebo/ or placebo effect/
23. (random$ or RCT or RCTs).tw.
24. (controlled adj5 (trial$ or stud$)).tw.
25. (clinical$ adj5 trial$).tw.
26. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw
27. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
28. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
29. (cross‐over or cross over or crossover).tw.
30. (placebo$ or sham).tw.
31. trial.ti.
32. (assign$ or allocat$).tw.
33. controls.tw.
34. or/14‐33
35. 7 and 13 and 34
36. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
37. 35 not 36
Appendix 4. CINAHL (EBSCO) search strategy
S1 (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR ( (MH "Intracranial Embolism and Thrombosis") ) OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR (MH "Stroke Patients") OR (MH "Stroke Units")
S2 TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH)
S3 TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral)
S4 TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)
S5 S3 AND S4
S6 TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid)
S7 TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)
S8 S6 AND S7
S9 S1 OR S2 OR S5 OR S8
S10 (MH "Magnetics+") OR (MH "Magnet Therapy+") OR (MH "Magnets")
S11 TI ((magnet* or electromagnet* or electro‐magnet*) N5 (field* or coil* or induction)) OR AB ((magnet* or electromagnet* or electro‐magnet*) N5 (field* or coil* or induction))
S12 TI ((peripher* or nerv* or musc* or spine or spinal) N5 (magnet* or electromagnet* or electro‐magnet*) N5 (stimulat* or neurostimulat*)) or AB ((peripher* or nerv* or musc* or spine or spinal) N5 (magnet* or electromagnet* or electro‐magnet*) N5 (stimulat* or neurostimulat*))
S13 TI (PMS or rPMS or PrMS) or AB ( PMS or rPMS or PrMS)
S14 S10 OR S11 OR S12 OR S13
S15 (MH "Randomized Controlled Trials") or (MH "Random Assignment") or (MH "Random Sample+")
S16 (MH "Clinical Trials") or (MH "Intervention Trials") or (MH "Therapeutic Trials")
S17 (MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies")
S18 (MH "Control (Research)") or (MH "Control Group") or (MH "Placebos") or (MH "Placebo Effect")
S19 (MH "Crossover Design") OR (MH "Quasi‐Experimental Studies")
S20 PT (clinical trial or randomized controlled trial)
S21 TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs)
S22 TI (controlled N5 (trial* or stud*)) or AB (controlled N5 (trial* or stud*))
S23 TI (clinical* N5 trial*) or AB (clinical* N5 trial*)
S24 TI ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) or AB ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*))
S25 ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) or AB ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*))
S26 TI ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) or AB ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*))
S27 TI (cross‐over or cross over or crossover) or AB (cross‐over or cross over or crossover)
S28 TI (placebo* or sham) or AB (placebo* or sham)
S29 TI trial
S30 TI (assign* or allocat*) or AB (assign* or allocat*)
S31 TI controls or AB controls
S32 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31
S34 S9 AND S14 AND S32
Appendix 5. PsycINFO (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiparesis/ or hemiplegia/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. or/1‐6
8. exp magnetism/
9. ((magnet$ or electromagnet$ or electro‐magnet$) adj5 (field$ or coil$ or induction)).tw.
10. ((peripher$ or nerv$ or musc$ or spine or spinal) adj5 (magnet$ or electromagnet$ or electro‐magnet$) adj5 (stimulat$ or neurostimulat$)).tw.
11. (PMS or rPMS or PrMS).tw.
12. or/8‐11
13. clinical trials/ or treatment effectiveness evaluation/ or placebo/
14. (random$ or RCT or RCTs).tw.
15. (controlled adj5 (trial$ or stud$)).tw.
16. (clinical$ adj5 trial$).tw.
17. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw
18. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
19. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
20. (cross‐over or cross over or crossover).tw.
21. (placebo$ or sham).tw.
22. trial.ti.
23. (assign$ or allocat$).tw.
24. controls.tw.
25. or/13‐24
26. 7 and 12 and 25
Appendix 6. AMED (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ or brain injuries/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiplegia/
6. (hemipleg$ or hemipar$ or paresis or paretic or brain injur$).tw.
7. or/1‐6
8. exp magnetics/
9. exp electromagnetics/ or exp electromagnetic fields/
10. ((magnet$ or electromagnet$ or electro‐magnet$) adj5 (field$ or coil$ or induction)).tw.
11. ((peripher$ or nerv$ or musc$ or spine or spinal) adj5 (magnet$ or electromagnet$ or electro‐magnet$) adj5 (stimulat$ or neurostimulat$)).tw.
12. (PMS or rPMS or PrMS).tw.
13. or/8‐12
14. clinical trials/ or randomized controlled trials/ or random allocation/
15. research design/ or comparative study/
16. double blind method/ or single blind method/
17. placebos/
18. (random$ or RCT or RCTs).tw.
19. (controlled adj5 (trial$ or stud$)).tw.
20. (clinical$ adj5 trial$).tw.
21. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
22. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
23. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
24. (cross‐over or cross over or crossover).tw.
25. (placebo$ or sham).tw.
26. trial.ti.
27. (assign$ or allocat$).tw.
28. controls.tw.
29. or/14‐28
30. 7 and 13 and 29
Appendix 7. OTseeker (Occupational Therapy Systematic Evaluation of Evidence) search strategy
[Any Field] like 'stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH' AND [Any Field] like 'magnet* or electromagnet* or electro‐magnet*' AND [Method] like 'Randomised controlled trial'
Appendix 8. PEDro (physiotherapy evidence database) search strategy
<electrotherapies, heat, cold> in “Therapy” field, <muscle weakness> in “Problem” field. <neurology> in "Subdiscipline" field, and <clinical trial> in "Method" field
Appendix 9. Ichushi‐Web (Japanese medical database) search strategy
(脳卒中/AL or 脳梗塞/AL or 脳出血/AL or クモ膜下出血/AL or 脳血管障害/AL) and (磁気/AL) and (臨床試験/AL or 比較試験/AL or ランダム化比較試験/AL or 準ランダム化比較試験/AL or 第I相試験/AL or 第II相試験/AL or 第III相試験/AL or 第IV相試験/AL or 盲検/AL or ランダム/AL or プラセボ/AL or 対照群/AL or コントロール群/AL)
(We used Japanese characters in the search.)
Appendix 10. US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov
(stroke* OR poststroke OR apoplex* OR "cerebral vascular" OR "brain vascular*" OR cerebrovascular* OR "transient ischemic" OR tia OR cva* OR SAH) AND (magnetic OR electromagnetic OR electro‐magnetic OR PMS OR rPMS OR PrMS) | Interventional Studies
Appendix 11. ISRCTN Registry
(cerebrovascular OR stroke OR TIA OR SAH OR "transient ischemic attack" OR (cerebral AND (ischemia OR ischemia OR embolism OR infarction OR haematoma OR hematoma OR haemorrhage OR hemorrhage))) AND magnet*
Appendix 12. Stroke Trials Registry
Intervention ; Clinical Trials:“Magnetic”
Appendix 13. World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)
stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or transient isch?emic or tia or cva* or SAH – Title AND magnetic OR electromagnetic OR electro‐magnetic OR PMS OR rPMS OR PrMS ‐ Intervention
Appendix 14. Japanese UMIN Clinical Trials Registry (UMIN‐CTR)
Study type: Intervention:“Magnetic”
Data and analyses
Comparison 1.
rPMS versus sham
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Muscle strength at the end of treatment | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐2.44, 8.44] |
Comparison 2.
rPMS plus rehabilitation versus rehabilitation only
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living at the end of treatment | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐16.35, 10.35] |
| 2 Activities of daily living at the end of follow‐up | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.86, 10.86] |
| 3 Upper limb function at the end of treatment | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐4.91, 8.91] |
| 4 Upper limb function at the end of follow‐up | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐2.92, 10.92] |
| 5 Spasticity at the end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Spasticity at the end of treatment (elbow) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.89, 0.07] |
| 5.2 Spasticity at the end of treatment (wrist) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.76, 0.36] |
| 6 Spasticity at the end of follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Spasticity at the end of follow‐up (elbow) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.93, ‐0.03] |
| 6.2 Spasticity at the end of follow‐up (wrist) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.67, 0.41] |
Differences between protocol and review
We divided our evaluation of spasticity into parts of the body (elbow and wrist), although this was not specified in the protocol. We used Covidence software (Covidence 2013) for selection of studies, data extraction, and assessment of risk of bias. We included ADLs, upper limb function, lower limb function, muscle strength, spasticity, and death in the 'Summary of findings' table.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: RCT Study grouping: parallel group |
|
| Participants | Inclusion criteria: chronic unilateral, first‐ever stroke more than 12 months before the start of the study. Participants with stroke presented with paretic ankle muscles with spasticity (medical records), had a CT or MRI scan taken within the previous 5 years, and were able to walk independently (i.e. with no physical assistance) more than 10 m with or without an assistive device Exclusion criteria: use of antispastic medication; past vertebral surgery; major circulatory, respiratory, or cardiac disease; neurological disease/deficit other than stroke; severe lower limb orthopaedic condition; or cognitive disorder Baseline characteristics rPMS (n = 9)
Sham (n = 9)
Baseline comparability between 2 groups: rPMS group was earlier from onset than sham group Loss of follow‐up: 0% |
|
| Interventions | Intervention characteristics rPMS
Sham
Sham stimulation was applied using the same parameters but at a very low intensity |
|
| Outcomes | Muscle strength: dorsiflexion strength (kg)
|
|
| Identification | Sponsorship source: Canadian Foundation for Innovation (CS) and studentships from the Fondsde la Recherche en Sante du Quebec (LDB, HMA) and the Canadian Institutes for Health Research (LDB, HMA) Country: Canada Setting: n/a Authors' names: Louis‐David Beaulieu, Hugo Masse‐Alarie, Brenda Brouwer, Cyril Schneider Institution: Laboratoire de Neurostimulation et Neurosciences Cliniques Email: cyril.schneider@rea.ulaval.ca Address: Centre de recherche du CHU de Quebec, Axe Neurosciences RC‐9800, 2705 Boulevard Laurier, Quebec, QC G1V 4G2, Canada |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To ensure blinding, all participants were informed at enrolment that they could receive real rPMS or sham stimulation over the paretic lower limb, but they were not provided with information about the location of the coil or sensations induced by stimulation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Experimenters performing pre‐ and post‐intervention measures and analysis had to leave the room during the intervention and remained blind to group allocation during the experiments and to times of measurement during analysis (i.e. pre‐ or post‐intervention) until completion of analyses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 0% |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available |
| Other bias | Low risk | No other biases |
| Methods | Study design: RCT Study grouping: parallel group |
|
| Participants | Inclusion criteria: hemiparesis caused by stroke or traumatic brain injury; spasticity of an upper extremity, with a score of 1 to 3 on the Tardieu scale; ages between 18 and 75 years Exclusion criteria: metal implant in the head or within the stimulation area; medically implanted device (cardiac pacemaker, cochlear implant, or medication pump); pregnancy; comorbidity with other neurodegenerative disorders or other neurological or orthopaedic disorders; increased intracranial pressure; unstable fracture of the paretic upper extremity Baseline characteristics rPMS (n = 31)
Sham (n = 32)
Baseline comparability between 2 groups: only rPMS groups included traumatic brain injury; rPMS group earlier from onset than sham group Loss to follow‐up: 0.05%; ITT analysis was performed |
|
| Interventions | Intervention characteristics rPMS
Sham
|
|
| Outcomes | Activities of daily living: Barthel Index (scores range from 0 to 100)
Upper limb function: Fugl‐Meyer Assessment (scores range from 0 to 66)
Spasticity: Modified Tardieu Scale of elbow and wrist (scores range from 0 to 5)
|
|
| Identification | Sponsorship source: Cambridge Electronic Design Limited, Unit 4, Science Park, Milton Rd, Cambridge, CB4 0FE, UK. MAG&More GmbH,Geisenhausenerstrasse 11A, 81379 Munich, Germany Country: Germany Setting: neurological rehabilitation hospital Authors' names: Carmen Krewer, Sandra Hartl, Friedemann Muller, Eberhard Koenig Institution: Schoen Klinik Bad Aibling, Motor Research Department, Bad Aibling, Germany Email: CKrewer@schoen‐kliniken.de Address: Schoen Klinik Bad Aibling, Kolbermoorer Strasse 72, D‐83043 Bad Aibling, Germany |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Randomised allocation was done by an individual not involved in any other part of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active coil makes typical discharge noises. Blinding of participants and personnel was enough |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained therapists, blinded for treatment allocation, assessed each participant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 5%; no differences in reasons why outcome data were missing |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available |
| Other bias | Low risk | No other biases |
| Methods | Study design: cross‐over trial | |
| Participants | Inclusion criteria: single history of CNS lesion due to stroke or traumatic brain injury; lesion interval > 12 months; increased muscle tone, i.e. 1, 2, 3, or 4 in the Modified Ashworth Score (0‐5) in affected wrist or finger joints; no volitional distal motor function of the affected arm, except for mass flexion; no metal implants or open wounds in the stimulation area; no deep vein thrombosis; no relevant oedema; no pacemaker; no preceding botulinum toxin injection within previous 6 months; signed written informed consent (approved by local ethics committee) Exclusion criteria: n/a Baseline characteristics Group 1 (rPMS‐sham) (n = 20)
Group 2 (sham‐rPMS) (n = 20)
Baseline comparability between 2 groups: group 1 was younger than group 2 Loss to follow‐up: 0% |
|
| Interventions | Intervention characteristics rPMS
Sham
|
|
| Outcomes | Spasticity: Modified Ashworth Score of wrist and finger (scores range from 0 to 4)
|
|
| Identification | Sponsorship source: n/a Country: Germany Setting: n/a Comments: The Verein zur Förderung der Hirnforschung und Rehabilitation, e.V., Berlin Authors' names: Werner C, Schrader M, Wernicke S, Bryl B, Hesse S Institution: Medical Park Berlin Humboldtmühle, Neurological Rehabilitation, Charité, University Medicine Berlin, Germany Email: c.werner@medicalpark.de Address: Medical Park Berlin Charité – University Medicine Berlin An der Mühle 2‐9, Berlin 13507, Germany |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was conducted with the help of a computer‐generated lot (www.randomizer.at) |
| Allocation concealment (selection bias) | Low risk | Before start of therapy, the sub‐investigator of the study attached the rPMS or sham coil according to group assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This study used a sham coil delivered with an atypical clicking sound. Therapists who applied stimulation and muscle stretch were not aware of whether the coil used was the one intended for rPMS or sham |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A rater, blinded to treatment allocation, assessed participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 0% |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available |
| Other bias | Low risk | No other biases |
CT: computed tomography ITT: intention‐to‐treat MRI: magnetic resonance imaging RCT: randomised controlled trial rPMS: repetitive peripheral magnetic stimulation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bernhardt 2007 | Unsuitable study design |
| Heldmann 2000 | Unsuitable outcomes |
| Kuznetsova 2016 | Unsuitable outcomes |
| Momosaki 2014 | Unsuitable outcomes |
| Rossini 2005 | Unsuitable intervention |
| Struppler 2002 | Unsuitable study design |
| Struppler 2009 | Unsuitable study design |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | n/a |
| Participants | Participants with stroke |
| Interventions | Low‐frequency magnetic fields |
| Outcomes | Spasticity |
| Notes |
| Methods | Comparative study |
| Participants | 42 participants with stroke (mean age 64 ± 1.0 years) |
| Interventions | 10 daily sessions of 1 Hz repetitive transcranial magnetic stimulation and repetitive peripheral magnetic stimulation |
| Outcomes | Motor Club Assessment Scale |
| Notes |
| Methods | Comparative study |
| Participants | 121 participants with ischaemic stroke in the acute period |
| Interventions | Technique of frequency‐modulated magnetolaser therapy |
| Outcomes | n/a |
| Notes |
| Methods | Study design: RCT Study grouping: parallel group |
| Participants | 18 participants with stroke and spastic hemiparesis (mean age 60.8 years; 9 females, 9 males; 3 to 12 months after stroke) |
| Interventions | Daily (5 times a week) session with repetitive peripheral magnetic stimulation over a period of 4 weeks, consisting of 12 repetitions of 4000 stimuli with 12‐second breaks between serials |
| Outcomes | Range of motion of wrist, Action Reach Arm Test, Bard and Hirschberg score, Ashworth Score, Gerstenbrand Spasticity Rating Scale |
| Notes |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Effect of pairing peripheral and transcranial magnetic stimulations triggered by actual movement on motor plasticity |
| Methods | Cross‐over trial |
| Participants | Inclusion criteria: people with chronic stroke (more than 3 months after onset) who were inpatients and outpatients of Tohoku University Hospital Exclusion criteria: people with metal in cranium, trauma or operation of brain, intracardiac lines, increased intracranial pressure, pregnancy, childhood, heart disease, cardiac pacemaker, medication pump, tricyclic antidepressants, neuroleptics, febrile convulsion, epilepsy, family history of epilepsy |
| Interventions | Subthreshold peripheral and transcranial magnetic stimulations with actual movement |
| Outcomes | Direction of transcranial magnetic stimulation‐induced upper limb movement of the paretic side, excitability of corticospinal tract |
| Starting date | 1 October 2015 |
| Contact information | Akihiko Asao, Tohoku University Graduate School of Medicine, Department of Physical Medicine and Rehabilitation, 2‐1 Seiryo‐machi, Aoba‐ku, Sendai, Miyagi, Japan email: a3omail@gmail.com |
| Notes | UMIN000019106 |
| Trial name or title | The effect of repetitive peripheral magnetic stimulation in stroke‐rehabilitation: a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: subacute stroke (occurred no longer than 6 months previously), spastic hemiparesis of the upper limb (at least modified Ashworth Scale 1); slight function in the fingers or hand (at least 1 point on the Fugl‐Meyer Test in subscore C) Exclusion criteria: epilepsy, implanted metal in the stimulation area, implanted medical devices, dysfunctional speech comprehension, pregnancy |
| Interventions | Stimulation intensity is adjusted individually for each participant, so that a joint movement results from the muscle contraction. Muscles of the upper arm and forearm are stimulated with a butterfly coil; the participant takes a sitting position with raised feet in the wheelchair or on a chair with backrest; the arm is placed to be stimulated or maintained by the therapist |
| Outcomes | Primary outcome is group difference in the Fugl‐Meyer‐Score 3 weeks post stimulation. Secondary outcome is group difference in the Katz Index of Independence Activities of Daily Living scale (ADL) score after 6 months |
| Starting date | 23 September 2014 |
| Contact information | Kristin Pohl, Moritz Klinik Bad Klosterlausnitz, Hermann‐Sachse‐Straße 46 07639 Bad Klosterlausnitz Germany email: kristin.pohl@moritz‐klinik.de |
| Notes | DRK00007722 |
| Trial name or title | The effects of repetitive peripheral magnetic stimulation in patient with spastic hemiparesis after stroke: a randomised‐controlled study |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: subacute stroke (occurred no longer than 6 months previously); spastic hemiparesis of the upper limb (at least modified Ashworth Scale 1); slight function in the fingers or hand (at least 1 point on the Fugl‐Meyer Test in subscore C) Exclusion criteria: epilepsy, implanted metal in the stimulation area, implanted medical devices, dysfunctional speech comprehension, pregnancy |
| Interventions | Stimulation is 15 minutes daily for 3 weeks for a total of 15 sessions. Stimulation intensity is adjusted individually for each participant, so that a joint movement results from the muscle contraction. Muscles of the upper arm and forearm are stimulated with a butterfly coil. Participant takes a sitting position with raised feet in a wheelchair or on a chair with a backrest. The arm is then placed to be stimulated or maintained by the therapist |
| Outcomes | Primary outcome is the Fugl‐Meyer Test of the upper extremity ‐ a test that evaluated the function of the affected upper extremity. This test will be performed directly after the end of the 3 weeks of intervention/control intervention. Secondary outcome is determined with a questionnaire ‐ the Katz Index of Independence Activities of Daily Living. That questionnaire aims to identify dependence on performance of activities of daily living and will be performed 6 months after the end of the intervention/control intervention |
| Starting date | 15 April 2015 |
| Contact information | Kristin Pohl, Moritz Klinik Bad Klosterlausnitz, Hermann‐Sachse‐Straße 46 07639 Bad Klosterlausnitz, Germany email: kristin.pohl@moritz‐klinik.de |
| Notes | DRKS00007899 |
| Trial name or title | Repetitive peripheral magnetic stimulation for patients with hemiplegia |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: cerebral hemisphere damage, people who could walk independently, modified Rankin Scale between 0 and 2 before onset Exclusion criteria: severe dementia, people with contraindications outlined in the guidelines for repetitive transcranial magnetic stimulation |
| Interventions | Repetitive peripheral magnetic stimulation + standard physical therapy Repetitive peripheral magnetic stimulation: While participants are participating in this study, they receive repetitive peripheral magnetic stimulation on the day of performing physical therapy Repetitive peripheral magnetic stimulation is performed on the quadriceps femoris at 30 Hz for 10 minutes. Standard physical therapy is performed according to the standard schedule of the authors' hospital |
| Outcomes | Knee extension strength, evaluation time: at the time of starting physical therapy, 1 week later, 2 weeks later, 1 month later, 2 months later Stroke Impairment Assessment Set, 10 meter walking speed, Functional Independence Measure, quadriceps muscle thickness, acceleration during walking, Berg Balance Scale, Timed Up and Go Test, biochemical blood test, number of days until gait reacquisition, hospitalisation |
| Starting date | 1 October 2015 |
| Contact information | Keita Suzuki, Kawasaki University of Medical Welfare, Department of Rehabilitation, 288 Matsushima, Kurashiki, Okayama, Japan email: suzuki@mw.kawasaki‐m.ac.jp |
| Notes | UMIN000018750 |
Contributions of authors
Ryo Momosaki prepared the protocol and drafted the review with support from Erika Ota. Ryo Momosaki and Naoki Yamada contributed to literature selection, data extraction, and analyses. Masahiro Abo provided critical revisions on intellectual content. All review authors approved the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
This research is supported by the Project for Baby and Infant in Research of Health and Development to Adolescent and Young adult (BIRTHDAY) from the Japan Agency for Medical Research and Development, AMED, Japan.
Declarations of interest
Ryo Momosaki: none known. Naoki Yamada: none known. Erika Ota: none known. Masahiro Abo: none known.
New
References
References to studies included in this review
- Beaulieu L‐D, Masse‐Alarie H, Brouwer B, Schneider C. Noninvasive neurostimulation in chronic stroke: a double‐blind randomized sham‐controlled testing of clinical and corticomotor effects. Topics in Stroke Rehabilitation 2015;22(1):8‐17. [DOI] [PubMed] [Google Scholar]
- Krewer C, Hartl S, Muller F, Koenig E. Effects of repetitive peripheral magnetic stimulation on upper‐limb spasticity and impairment in patients with spastic hemiparesis: a randomized, double‐blind, sham‐controlled study. Archives of Physical Medicine and Rehabilitation 2014;95(6):1039‐47. [DOI] [PubMed] [Google Scholar]
- Werner C, Schrader M. Repetitive peripheral magnetic stimulation (rpMS) in combination with muscle stretch decreased the wrist and finger flexor muscle spasticity in chronic patients after CNS lesion. International Journal of Physical Medicine & Rehabilitation 2016;4:4. [Google Scholar]
References to studies excluded from this review
- Bernhardt M, Angerer B, Buss M, Struppler A. Isometric muscle contraction induced by repetitive peripheral magnetic stimulation (RPMS)‐modeling and identification. Biomedical Signal Processing and Control 2007;2(3):180‐90. [Google Scholar]
- Heldmann B, Kerkhoff G, Struppler A, Havel P, Jahn T. Repetitive peripheral magnetic stimulation alleviates tactile extinction. NeuroReport 2000;11(14):3193‐8. [DOI] [PubMed] [Google Scholar]
- Kuznetsova S, Kuznetsov V, Skachkova N. Enhancement of cortical excitability and motor function in stroke patients after combined repetitive transcranial and peripheral magnetic stimulation. European Journal of Neurology 2016;23:223. 26041498 [Google Scholar]
- Momosaki R, Abo M, Watanabe S, Kakuda W, Yamada N, Mochio K. Functional magnetic stimulation using a parabolic coil for dysphagia after stroke. Neuromodulation 2014;17(7):637‐641. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Johnston CS. Facilitating acute stroke recovery with magnetic fields?. Neurology 2005;65(3):353‐4. [DOI] [PubMed] [Google Scholar]
- Struppler A, Havel P, Muller‐Barna P. Facilitation of skilled finger movements by repetitive peripheral magnetic stimulation (RPMS) ‐ a new approach in central paresis. NeuroRehabilitation 2002;18(1):69‐82. [PubMed] [Google Scholar]
- Struppler A, Angerer B, Gebhard B. Repetitive peripheral magnetic stimulation (RPMS) as a method for the rehabilitation of sensorimotor deficits of hand and arm following cerebral lesions. Neurologie und Rehabilitation 2009;15(1):28‐38. [Google Scholar]
References to studies awaiting assessment
- Kotchetkov A Gorbunov F, Streltsova N, Fillina T. Spasticity modulation using low frequency magnetic fields (LFMF) in stroke patients. 2nd World Congress in Neurological Rehabilitation 1999;42:42. [Google Scholar]
- Kuznetsova S, Kuznetsov V, Skachkova N. Combined central and peripheral magnetic stimulation to facilitate motor recovery after stroke. Cerebrovascular Diseases 2013;35 Suppl 3:548. [Google Scholar]
- Samosiuk NI. Magnetic and laser therapy of acute ischemic stroke. Voprosy Kurortologii, Fizioterapii, i Lechebnoi Fizicheskoi Kultury 2003;2:19‐20. [PubMed] [Google Scholar]
- Zifko U, Morph M, Diem K, Havel P, Struppler A. Repetitive peripheral magnetic stimulation is effective in the rehabilitation of the paretic arm. Neurorehabilitation and Neural Repair 2002;16(1):18‐9. [Google Scholar]
References to ongoing studies
- Effect of pairing peripheral and transcranial magnetic stimulations triggered by actual movement on motor plasticity. UMIN‐CTR (accessed August 2016). [UMIN000019106]
- The effect of repetitive peripheral magnetic stimulation in stroke‐rehabilitation: a randomized controlled trial. German Clinical Trials Register (accessed August 2016). [DRKS00007722]
- The effects of repetitive peripheral magnetic stimulation in patient with spastic hemiparesis after stroke: a randomized‐controlled study. German Clinical Trials Register (accessed August 2016). [DRKS00007899]
- Repetitive peripheral magnetic stimulation for patients with hemiplegia. UMIN‐CTR (accessed August 2016). [UMIN000018750]
Additional references
- Barker AT. An introduction to the basic principles of magnetic nerve stimulation. Journal of Clinical Neurophysiology 1991;8:26‐37. [DOI] [PubMed] [Google Scholar]
- Beaulieu LD, Schneider C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Clinical Neurophysiology 2013;43(4):251‐60. [DOI] [PubMed] [Google Scholar]
- Beaulieu LD, Schneider C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: a literature review on parameters of application and afferents recruitment. Neurophysiologie Clinique 2015;45(3):223‐37. [DOI] [PubMed] [Google Scholar]
- Melbourne, Australia: Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2013.
- Vries S, Mulder T. Motor imagery and stroke rehabilitation: a critical discussion. Journal of Rehabilitation Medicine 2007;39(1):5‐13. [DOI] [PubMed] [Google Scholar]
- Flamand VH, Schneider C. Noninvasive and painless magnetic stimulation of nerves improved brain motor function and mobility in a cerebral palsy case. Archives of Physical Medicine and Rehabilitation 2014;95(10):1984‐90. [DOI] [PubMed] [Google Scholar]
- Gallasch E, Christova M, Kunz A, Rafolt D, Golaszewski S. Modulation of sensorimotor cortex by repetitive peripheral magnetic stimulation. Frontiers in Human Neuroscience 2015;9:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton (ON): McMaster University. GRADEpro. Hamilton (ON): McMaster University, 2014.
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, et al. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TR, Shin HI, Kim IS. Magnetic stimulation of the quadriceps femoris muscle: comparison of pain with electrical stimulation. American Journal of Physical Medicine & Rehabilitation 2006;85(7):593‐9. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
- Ito T, Tsubahara A, Watanabe S. Use of electrical or magnetic stimulation for generating hip flexion torque. American Journal of Physical Medicine & Rehabilitation 2013;92:755‐61. [DOI] [PubMed] [Google Scholar]
- Kerkhoff G, Heldmann B, Struppler A, Havel P, Jahn T. The effects of magnetic stimulation and attentional cueing on tactile extinction. Cortex 2001;37(5):719‐23. [DOI] [PubMed] [Google Scholar]
- Krause P, Straube A. Peripheral repetitive magnetic stimulation induces intracortical inhibition in healthy subjects. Neurological Research 2008;30(7):690‐4. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurology 2009;8(8):741‐54. [DOI] [PubMed] [Google Scholar]
- McArthur KS, Quinn TJ, Higgins P, Langhorne P. Post‐acute care and secondary prevention after ischaemic stroke. BMJ 2011;342:d2083. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic reviews and Meta‐Analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9. [DOI] [PubMed] [Google Scholar]
- Nielsen JF, Sinkjaer T, Jakobsen J. Treatment of spasticity with repetitive magnetic stimulation: a double‐blind placebo‐controlled study. Multiple Sclerosis Journal 1996;2(5):227‐32. [DOI] [PubMed] [Google Scholar]
- Park JH, Razuk A, Saad PF, Telles GJ, Karakhanian WK, Fioranelli A, et al. Carotid stenosis: what is the high‐risk population?. Clinics (Sao Paulo) 2012;67(10):1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(7):2064‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurology 2007;6(2):182‐7. [DOI] [PubMed] [Google Scholar]
- Struppler A, Havel P, Müller‐Barna P. Facilitation of skilled finger movements by repetitive peripheral magnetic stimulation (RPMS) ‐ a new approach in central paresis. NeuroRehabilitation 2003;18:69‐82. [PubMed] [Google Scholar]
- Struppler A, Angerer B, Gündisch C, Havel P. Modulatory effect of repetitive peripheral magnetic stimulation on skeletal muscle tone in healthy subjects: stabilization of the elbow joint. Experimental Brain Research 2004;157:59‐66. [DOI] [PubMed] [Google Scholar]
- Struppler A, Binkofski F, Angerer B, Bernhardt M, Spiegel S, Drzezga A, et al. A fronto‐parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: a PET‐H2O15 study. Neuroimage 2007;36(2):T174‐86. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Stroke‐1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 1989;20(10):1407‐31. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Momosaki R, Yamada N, Ota E, Abo M. Repetitive peripheral magnetic stimulation for activities of daily living and functional ability in people after stroke. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011968] [DOI] [PMC free article] [PubMed] [Google Scholar]


