Abstract
Background
Purified thymus extracts (pTE) and synthetic thymic peptides (sTP) are thought to enhance the immune system of cancer patients in order to fight the growth of tumour cells and to resist infections due to immunosuppression induced by the disease and antineoplastic therapy.
Objectives
To evaluate the effectiveness of pTE and sTP for the management of cancer.
Search methods
We searched CENTRAL (The Cochrane Library 2010, Issue 3), MEDLINE, EMBASE, AMED, BIOETHICSLINE, BIOSIS, CATLINE, CISCOM, HEALTHSTAR, HTA, SOMED and LILACS (to February 2010).
Selection criteria
Randomised trials of pTE or sTP in addition to chemotherapy or radiotherapy, or both, compared to the same regimen with placebo or no additional treatment in adult cancer patients.
Data collection and analysis
Two authors independently extracted data from published trials. We derived odds ratios (OR) from overall survival (OS) and disease‐free survival (DFS) rates, tumour response (TR) rates, and rates of adverse effects (AE) related to antineoplastic treatments. We used a random‐effects model for meta‐analysis.
Main results
We identified 26 trials (2736 patients). Twenty trials investigated pTE (thymostimulin or thymosin fraction 5) and six trials investigated sTP (thymopentin or thymosin α1). Twenty‐one trials reported results for OS, six for DFS, 14 for TR, nine for AE and 10 for safety of pTE and sTP. Addition of pTE conferred no benefit on OS (RR 1.00, 95% CI 0.79 to 1.25); DFS (RR 0.97, 95% CI 0.82 to 1.16); or TR (RR 1.07, 95% CI 0.92 to 1.25). Heterogeneity was moderate to high for all these outcomes. For thymosin α1 the pooled RR for OS was 1.21 (95% CI 0.94 to 1.56, P = 0.14), with low heterogeneity; and 3.37 (95% CI 0.66 to 17.30, P = 0.15) for DFS, with moderate heterogeneity. The pTE reduced the risk of severe infectious complications (RR 0.54, 95% CI 0.38 to 0.78, P = 0.0008; I² = 0%). The RR for severe neutropenia in patients treated with thymostimulin was 0.55 (95% CI 0.25 to 1.23, P = 0.15). Tolerability of pTE and sTP was good. Most of the trials had at least a moderate risk of bias.
Authors' conclusions
Overall, we found neither evidence that the addition of pTE to antineoplastic treatment reduced the risk of death or disease progression nor that it improved the rate of tumour responses to antineoplastic treatment. For thymosin α1, there was a trend for a reduced risk of dying and of improved DFS. There was preliminary evidence that pTE lowered the risk of severe infectious complications in patients undergoing chemotherapy or radiotherapy.
Keywords: Adult; Female; Humans; Male; Adjuvants, Immunologic; Adjuvants, Immunologic/adverse effects; Adjuvants, Immunologic/therapeutic use; Disease‐Free Survival; Immune System; Immune System/drug effects; Immunocompromised Host; Neoplasms; Neoplasms/drug therapy; Neoplasms/immunology; Peptides; Peptides/adverse effects; Peptides/therapeutic use; Thymalfasin; Thymopentin; Thymopentin/therapeutic use; Thymosin; Thymosin/analogs & derivatives; Thymosin/therapeutic use; Thymus Extracts; Thymus Extracts/adverse effects; Thymus Extracts/therapeutic use; Thymus Gland; Thymus Gland/chemistry
Plain language summary
Thymic peptides for treatment of cancer patients in addition to chemotherapy or radiotherapy, or both
The immune system plays a key role in the body’s own defences against cancer cells. The thymus gland plays a central part in this and modifies T‐cells, a subset of lymphocytes. Studies with thymic peptides have shown a variety of effects on the immune system. There are two groups of thymic peptides available for use in treatment: purified extracts from animal (mostly calf) thymus glands and synthetically produced thymus gland peptides. This review aims to answer the question whether having thymic peptides can improve the response to and tolerability of standard chemotherapy or radiotherapy, or combined treatment. Further questions are whether the peptides inhibit or reduce the progression and recurrence of disease, whether they prolong the life of cancer patients and whether quality of life is improved.
This review looked at the evidence from 26 clinical trials with a total of 2736 adult cancer patients. Many of the trials were small and of moderate quality. Only three studies were less than 10 years old. Thymosin α1 is a synthetic peptide that shows some promise as a treatment option for patients with metastatic melanoma when used in addition to chemotherapy. Severe problems occur during chemotherapy and radiotherapy due to low white blood cell counts and infections. These were reduced by using purified thymus extracts. However, the use of purified thymus extracts should be investigated more thoroughly before the extracts are used routinely in patients. The findings were not conclusive and caution is advised. Overall, thymic peptides seem to be well tolerated.
Background
By the late 1950s and early 1960s the role of the thymus as a lymphoid organ became clearer based on observations of a decreased immune response and consequent lowered resistance to infectious disease that resulted from damage to or experimental removal of the gland (Seybold 1950). It is now well established that the thymus gland is a central lymphoid organ in which bone marrow‐derived T‐cell precursors undergo differentiation within the context of a specific cellular and extracellular microenvironment. The thymus gland is also responsible for the production of various peptides with hormone‐like activity and purified extracts from animal thymus glands have been used to treat primary immunodeficient states (Goldstein 2009).
The role of the immune system to recognize and destroy tumour cells has been hypothesized since the early 1950s and is now generally accepted (Dunn 2002). One of the approaches to treat cancer is via stimulation or modulation of the immune system with extracts and peptides from the thymus gland, which was first introduced in the 1970s (Costanzi 1977).
Thymus derived pharmaceuticals can be divided into two groups:
purified extracts from animal thymus glands containing peptide mixtures; and
synthetically produced single thymic peptides.
Historically these two groups represent two steps in the investigation of thymic peptides involved in T‐cell maturation and activation. The first step is to produce cell‐free extracts, the second is to characterize and analyse single components of these extracts.
Purified thymus extracts
Extracts from calf thymus glands were further processed in different steps of purification, fractionation and filtration to result in peptide mixtures. The exact composition and character of the peptides are not completely known and are subject to biological variation. Different preparations are not defined by their components but by the respective standardization of the extraction procedure. Two purified thymus extracts (pTE) were investigated in clinical trials and are included in this review, thymosin fraction 5 and thymostimulin (Table 1).
1. Type of interventional treatment.
| Type | Name | Ingredients | Provider | Applied in study |
| Purified thymus extracts | Thymosin fraction 5 | Peptide mixture, range 1‐15 kDa | Hoffmann‐La Roche | Bedikian 1984; Cohen 1979; Scher 1988; Wara 1981 |
| Thymostimulin | Peptide mixture, range 1‐12 kDa | Serono S.A. | Airoldi 1987; Canovas 1988; Canovas 1991; De Serdio 1997; Del Giacco 1988; Federico 1995; Gonnelli 1995; Guzman 1988; Iaffaioli 1994; Luzi 1984; Macchiarini 1989; Mantovani 1988; Mustacchi 1994; Pavesi 1993; Salvati 1984; Sanchiz 1996 | |
| Synthetic thymic peptides | Thymosin α1 | Polypeptide (28 amino acids) | SciClone Pharmaceuticals | Cheng 2004; Gish 2009; Maio 2010; Schulof 1985 |
| Thymopentin | Oligopeptide (5 amino acids) | Italfarmaco | Gebbia 1994; GISOT 1987 |
Thymosin fraction 5
Thymosin fraction 5 was produced by US investigators in 1966. Goldstein et al extracted a so called 'lymphocytopoietic factor' from calf thymus, referring to its capacity to stimulate proliferation of lymphocytes both in vitro and in animal models, and termed it thymosin, which was initially thought to be a single polypeptide (Goldstein 1966). A further 5‐step purification led to 'thymosin fraction 5', then identified as a mixture of 30 to 40 small polypeptide components with a molecular weight ranging from 1 to 15 kilodalton (Goldstein 1977).
Thymostimulin
Thymostimulin, also extracted from calf thymus, was first produced by Italian investigators in 1976. It consists of a group of peptides with molecular weights ranging from 1 to 12 kilodalton (Falchetti 1977). The way of processing differs from that of thymosin fraction 5 in several steps, which presumably results in a different composition of peptides (reviewed in Schulof 1985a).
Synthetic thymic peptides
Synthetically produced thymic peptides (sTP) are derivatives of peptides that have been isolated from thymus extracts and sequenced. Two synthetically produced thymic peptides were used in clinical trials included in this review, thymosin α1 and thymopentin (Table 1).
Thymosin α1
Thymosin α1 is a peptide of 28 amino acids that was first isolated from thymosin fraction 5 in 1977 (Goldstein 1977). It is highly conserved among species and the amino acid sequence of human and bovine thymosin α1 are identical (reviewed in Hannappel 2003). Thymosin α1 has been sequenced and produced synthetically. Nowadays it is approved, mainly in countries of Asia and South America, for the treatment of chronic hepatitis B and C as a vaccine enhancer and in few countries of Southeast Asia for the treatment of cancer (Billich 2002). Pharmacokinetic studies in healthy volunteers showed good absorption after subcutaneous injection with a peak serum level at between one and two hours and a half live of less than three hours (Rost 1999).
Thymopentin
Thymopentin is a fragment of a larger peptide called thymopoietin. Thymopoietin was initially isolated from calf thymus and consists of 49 amino acids. It had been shown to induce differentiation of T‐cell precursors both in vitro and in vivo (Schlesinger 1975). In the search for a smaller peptide with the same immunologic properties that was suitable for large‐scale synthesis, the five amino‐acid peptide thymopentin was identified (Goldstein 1979). Pharmacokinetic studies in humans showed a short half live of 30 seconds (reviewed in Singh 1998).
Preclinical and clinical studies with pTE and sTP
Preclinical studies with pTE and sTP showed a variety of modulatory effects on the immune system (Bodey 2000; Chretien 1978; Goldstein 2009; Schulof 1985a). They were tested with other substances in the Biological Response Modifiers Program of the National Cancer Institute for their efficacy in the treatment of human cancers in the 1980s (Schulof 1985a). Surveys from the late 1990s showed ample dissemination of information on the treatment of cancer with purified thymus extracts as part of a 'complementary and alternative treatment' of cancer (Grothey 1998; Hardell 1998; Kullmer 1999; Moschen 2001; Sehouli 2000; Soellner 1997). Clinical studies investigated the effects on various clinical endpoints as well as immunological effects in a broad range of malignant diseases. The findings of controlled trials of pTE and sTP in cancer have not been conclusive. The height of research activity was in the 1980s and early 1990s and then seemed to wane but very recently published studies with thymosin α1 indicate that it is still topical (Maio 2010).
The purpose of this review was to summarize the available evidence from clinical trials which investigated pTE and sTP in combination with chemotherapy or radiotherapy, or both, in order to determine whether the addition of thymic peptides had a beneficial effect on survival outcomes and quality of life in cancer patients as well as whether it improved the response to and tolerability of conventional cancer therapies. Given the diversity of pTE and sTP we also intended to elucidate their probable differential effects.
Objectives
To determine the effectiveness and tolerability of purified thymus extracts (pTE) and synthetically produced thymic peptides (sTP) for the treatment of cancer patients during chemotherapy or radiotherapy. The objectives of the review were to assess the following.
The effects of thymic peptides on:
overall survival (OS) and disease‐free survival (DFS) or progression‐free survival (PFS),
tumour response,
adverse effects of chemotherapy and radiotherapy,
patient‐reported quality of life.
Adverse effects of pTE and sTP;
and to make recommendations for future research.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs (for example trials which used alternation, allocation by date of birth, etc.).
Types of participants
Adult patients with histologically proven malignant diseases of all stages who were submitted to treatment with chemotherapy, chemo‐immunotherapy or radiotherapy (that is standard care).
Types of interventions
Intervention group
Standard care plus treatment with any kind of parenterally applied pTE or sTP.
Control group
Standard care plus placebo treatment or no additional treatment. Standard care was required to be similar between groups.
Types of outcome measures
The outcomes of interest were:
OS;
DFS and PFS;
tumour response (parameters for response had to be defined or follow standard criteria (WHO (Miller 1981), RECIST (Therasse 2000));
hematologic toxicities or infectious complications related to antineoplastic treatment (chemotherapy, radiotherapy) of at least grade 3, scored using standardized criteria (CTC version 2 or later) (CTC 2009);
adverse events related to pTE and sTP.
Quality of life (QoL), measured with validated instruments, was an outcome for which data were sought but no data for this outcome were found in any of the included RCTs. Trials which only reported physiological measures (for example immune parameters etc.) were excluded.
For a glossary of terms please see Appendix 1.
Search methods for identification of studies
The last systematic search was performed in February 2010.
Electronic searches
We searched the following databases without language restrictions: Cochrane Complementary Medicine Field Registry of randomised clinical trials and controlled clinical trials, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3), MEDLINE, EMBASE, PubMed, AMED, BIOETHICSLINE, BIOSIS, CATLINE, CISCOM, HEALTHSTAR, INTERNATIONAL HEALTH TECHNOLOGY ASSESSMENT, SOMED, LILACS. Synonyms of the specific terms were identified by looking up the thesaurus of each database, if available. Search strategies and terms are listed in Appendix 2. All databases were searched from inception to February 2010.
Duplicates were removed from the search results and bibliographies from retrieved articles were searched for additional studies. The search strategies used were developed and executed by the author team.
Searching other resources
To minimize the impact of publication bias, we searched conference abstracts and unpublished material. Inquiries were sent to the investigators or institutions of included studies and respective manufacturers of pTE and sTP requesting information on additional trials. Our own files were searched for further studies.
Data collection and analysis
All discrepancies between two authors in the process of data collection and analysis were discussed and, if not agreed upon, the opinion of a third review author was sought.
Selection of studies
All publications identified by the search were screened by one review author (SM), who excluded those that were clearly irrelevant (for example diseases other than cancer, reviews, etc.). The titles and abstracts of the remaining articles were independently checked by two review authors (KB, MH, SM, EW). When articles could not be excluded with certainty, full text material was obtained. At least two review authors (KB, MH, SM, EW) of the team independently assessed full text material by means of a standard eligibility form that applied the inclusion and exclusion criteria. All results of the selection process were documented and disagreements resolved by discussion with a third review author (MH, EW).
Data extraction and management
Data extraction was performed non‐blinded to the study authors and independently by at least two review authors using a pretested extraction form. For included studies, data were extracted as recommended in Higgins 2009. This included data on the following.
Author, year of publication (if published), journal citation and language.
Country.
Setting.
Study design, methodology.
Study population: total number enrolled, patient characteristics (inclusion and exclusion criteria, age, stage, histological cell type, co‐morbidity, previous treatment), number enrolled in each arm.
Intervention and control details: no treatment, composition of placebo.
Standard care: type of chemotherapy, number of cycles and dose; timing and dose of radiotherapy.
Risk of bias in study: see below.
Duration of follow up.
Deviations from protocol.
-
Outcomes, where data on all outcomes were extracted for:
time to event data, we extracted the median or mean survival times and their spread or confidence interval;
dichotomous outcomes (e.g. adverse events, deaths, disease recurrence, disease progression, tumour response), we extracted the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at the endpoint in order to estimate a risk ratio (RR). If necessary, data were extracted from Kaplan‐Meier curves;
adverse events, type of event and grade of toxicity.
The time points at which outcomes were collected and reported were noted. Data were entered from the forms into a Microsoft Access database and double‐checked using descriptive database methods and plausibility checks by two review authors (MH, EW).
If more than one report from a study was available, the most recent was considered as the primary publication and was used primarily for data extraction; information from other reports were extracted if not reported in the primary reference. Data from non‐english articles were extracted with the help of a native speaker.
Assessment of risk of bias in included studies
The assessment of risk of bias was carried out according to the approach of The Cochrane Collaboration (Higgins 2009). In a first step, information relating to study quality that was essential for the judgment of risk of bias was extracted onto a prespecified form. Two review authors (MH, EW) then independently judged the risk of bias for each criterion as being low, high or unclear. Disagreements were resolved by discussion. The 'blinding' item was split up in order to allow for differential assessment of the outcomes dependent or independent of outcome assessors. The risk of bias was scored 'low' to 'high' with three intermediates ('low to moderate', 'moderate', and 'moderate to high'), with 'high' indicating the highest risk of bias.
Dealing with missing data
Where information was missing in the study reports, lacked detail or there was a discrepancy between different reports, we tried to obtain the required information from the study authors. Contacting study authors helped to clarify our questions for only one publication (Maio 2010).
Assessment of heterogeneity
Heterogeneity was assessed according to the standard method using the I2 statistic, calculated for each comparison on each outcome. I2 values above 50% indicated high heterogeneity, between 25% and 50% moderate heterogeneity, and below 25% low heterogeneity.
Data synthesis
For both survival outcomes, OS and DFS, we analysed the number of patients in each treatment arm who experienced deaths from all causes or relapse or progression of their cancer disease at one year ± four months. Tumour response was analysed if studies reported events of complete or partial, or both, responses. Pooled random‐effects model estimates and their 95% confidence intervals (CI) were calculated. Analyses were run separately for pTE and sTP trials. A decision regarding whether to combine treatment‐related symptoms was made depending on how this information was collected in each trial. Results were expressed as relative risks or risk ratios (RR) with 95% CIs. In survival and tumour response analyses a RR higher than 1.0 favoured the intervention group, indicating that patients in the intervention group (pTE or sTP) had a greater chance of survival or for having a response to treatment. In the analysis of adverse effects of chemotherapy and radiotherapy, RR less than 1.0 favoured the intervention group, indicating that fewer patients experienced adverse events in the intervention groups than in the control group. In studies reporting the median survival time, we recalculated the number of events up to median survival time in the intervention group for both the intervention and the control group assuming one‐parametric exponential survival time. This assumption is equivalent to assuming a constant event hazard ʎ. Therefore, the formulae developed by Kirkwood 2003 were used (Appendix 3).
Due to the variable study methods, all meta‐analyses were considered as being explorative and pooled effects sizes have to be interpreted with great caution.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were performed according to type of pTE or sTP if at least three studies reported data on the respective outcome and carried out sensitivity analyses as described below.
Sensitivity analysis
We performed sensitivity analyses taking account of different intervention treatments within one study (that is low dose or high dose of thymic peptides).
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
From electronic searches and handsearches we retrieved 326 relevant publications. Out of 326 publications, 23 publications were unclear or the abstracts were not retrievable and 251 publications were ineligible for this systematic review. Reasons for ineligibility were: trial design other than RCT (for example historical control group); participants other than adult cancer patients (for example children, other disease conditions); no thymic peptides; application mode other than subcutaneous or intramuscular (for example oral or topical) or combination with other substances; no control for thymic peptides; and no chemotherapy or radiotherapy or different regimes in the control and intervention groups.
Included studies
Twenty‐six randomised controlled trials were included in this review. Thirteen were conducted in Italy (Airoldi 1987; Del Giacco 1988; Federico 1995; Gebbia 1994; GISOT 1987; Gonnelli 1995; Iaffaioli 1994; Luzi 1984; Macchiarini 1989; Mantovani 1988; Mustacchi 1994; Pavesi 1993; Salvati 1984), six in the USA (Bedikian 1984; Cohen 1979; Gish 2009; Scher 1988; Schulof 1985; Wara 1981), four in Spain (Canovas 1988; Canovas 1991; De Serdio 1997; Sanchiz 1996), one in Argentina (Guzman 1988), one in China (Cheng 2004), and one study recruited patients from several countries (Maio 2010). Studies with thymosin fraction 5 were published between 1979 and 1988, with thymostimulin between 1984 and 1997, with thymopentin between 1987 and 1994, and with thymosin α1 between 1985 and 2010.
Participants
A total of 2931 adult patients were randomised (and 2744 evaluated) in the studies (median 49, range 28 to 650). The studies included the following number of randomised (and evaluated) cancer patients:
four studies with 427 (372 evaluated) breast cancer patients (Gonnelli 1995; Mantovani 1988; Pavesi 1993; Sanchiz 1996),
five with 314 (304) non‐small cell lung cancer patients (Bedikian 1984; Del Giacco 1988;Iaffaioli 1994; Luzi 1984; Schulof 1985),
four with 220 (192) small cell lung cancer patients (Cohen 1979; Macchiarini 1989; Salvati 1984; Scher 1988),
three with 236 (207) lymphoma patients (Canovas 1988; Canovas 1991; Federico 1995),
three with 160 (159) head and neck cancer patients (Airoldi 1987; De Serdio 1997; Wara 1981),
two with 267 (243) colorectal cancer (Guzman 1988; Mustacchi 1994),
two with 69 (66) hepatocellular carcinoma patients (Cheng 2004; Gish 2009),
two with 750 (705) patients with various types of cancer (Gebbia 1994; GISOT 1987), and
one with 488 (488) melanoma patients (Maio 2010).
Treatments
Intervention
In 20 studies pTE was used as interventional treatment: 16 used thymostimulin and four used thymosin fraction 5. Thymostimulin was applied intramuscularly and single doses ranged from 25 mg to 150 mg. Most study authors (n = 9) used a dose of 1 mg/kg body weight. Thymosin fraction 5 was applied subcutaneously with single doses of 60 mg in all trials. However, treatment schedules varied considerably among trials. Cohen 1979 had two interventional arms with different doses of thymosin fraction 5 (20 mg and 60 mg).
Six study authors used sTP as the interventional treatment: four used thymosin α1 and two used thymopentin. Thymosin α1 was applied subcutaneously with single doses of 1.6 mg in three trials and 0.9 mg/m² in one trial. Treatment schedules varied among these trials. Maio 2010 compared different doses of thymosin α1 (1.6, 3.2 and 6.4 mg); Schulof 1985 compared a 14‐day 'loading' dose of thymosin α1 with a maintenance therapy for up to one year; thymopentin was given intramuscularly in Gebbia 1994 and subcutaneously in GISOT 1987, both studies using single doses of 50 mg.
Control
Twenty studies had two arms and two of these studies used a placebo control (Iaffaioli 1994; Schulof 1985). Three studies had three arms (Cheng 2004; Cohen 1979; Schulof 1985). Cohen 1979 compared two different thymosin fraction 5 doses with no treatment; Cheng 2004 compared intrahepatic chemotherapy, with or without thymosin α1, with no intrahepatic chemotherapy; and Schulof 1985 compared two different regimen of thymosin α1 with placebo. Gebbia 1994 had four arms and compared thymopentin with or without granulocyte colony‐stimulating factor (G‐CSF) versus placebo and G‐CSF. Maio 2010 had five arms: three compared different doses of thymosin α1 in addition to chemotherapy plus interferon α, one arm had 3.2 mg thymosin α1 in addition to chemotherapy alone and the fifth arm had only chemotherapy plus interferon α. The comparison between 3.2 mg thymosin α1 and interferon α was not included in the analysis as interferon α was not a control treatment in accordance with our protocol.
Basic treatment
The chemotherapy or radiotherapy regimen was described in all but one of the studies (GISOT 1987). In 23 studies patients received chemotherapy alone, in combination with radiotherapy or immunotherapy, or applied as transcatheter arterial embolization. In two studies patients received radiotherapy alone (Schulof 1985; Wara 1981).
Outcomes
Survival
Twenty‐one studies reported OS. One author did not present estimates but described the results narratively (Iaffaioli 1994). Six studies reported DFS (Cohen 1979; De Serdio 1997; Federico 1995; Guzman 1988; Mantovani 1988; Scher 1988), although none gave a definition of how this was measured. Nine studies reported on PFS (Airoldi 1987; Cheng 2004; Macchiarini 1989; Maio 2010; Mustacchi 1994; Pavesi 1993; Salvati 1984; Schulof 1985; Wara 1981) although none gave a definition of how this was measured. Terminology of the measures of relapse and recurrence differed considerably between trials (Table 2, Table 3).
2. Purified thymus extracts: survival, response, toxicity.
| Study | Survival rates | Tumour response | Toxicity (no. of patients) | |||
| Overall survival (OS) | Disease‐/progression‐free survival (DFS/PFS) | Complete remission | Partial remission | Grade 3/4 neutropenia | Grade 3/4 infection | |
| Airoldi 1987 | After a median time of survival in IG of 7.9 months§: IG: 12/24 (50%) CG: 10/24 (42%) | After a median time of DFS in IG of 3.8 months§: IG: 7/14 (50%) CG: 2/6 (33%) | After 8 cycles of chemotherapy: IG: 3/24 (12.5%) CG: 1/24 (4%) | IG: 11/24 (46%) CG: 5/24 (21%) (p< 0.05 chi² test) | n.r. | |
| Bedikian 1984 | After 1 year#: IG: 8/46 (17%) CG: 16/53 (30%) (P = 0.14) | n.r. | IG: 0/46 CG: 2/53 (4%) | IG: 10/46 (22%) CG: 22/53 (42%) | n.r. | |
| Canovas 1988 | After 4 cycles of chemotherapy (approximately 3 to 4 months): IG: 23/23 CG: 20/23 (87%) | n.r. | n.r. | n.r. | ||
| Canovas 1991 | After 6 cycles of chemotherapy (approximately 4 to 6 months): IG: 19/20 (95%) CG:17/20 (85%) | n.r. | n.r. | n.r. | ("life threatening infections") IG: 2/20 (10%) CG: 4/20 (5%) | |
| Cohen 1979 | After 1 year#: IG1 (60 mg/m²): 10/18 (56%) IG2 (20 mg/m²): 2/12 (17%) CG: 3/16 (19%) | After 1 year (complete responders): IG1: 6/9 (67%) IG2: 1/2 (50%) CG: 2/8 (25%) | After 3 months of chemotherapy: IG1: 9/18 (50%) IG2: 2/12 (17%) CG: 8/16 (50%) | n.r. | n.r. | |
| De Serdio 1997 | n.r. | After a mean time of observation of 18 months: IG: 15/18 (83%) CG: 14/18 (78%) | After approximately 2 months of radiochemotherapy: IG: 17/18 (94%) CG: 17/18 (94%) | n.r. | n.r. | |
| Del Giacco 1988 | After 12 to 33 months observation time: IG: 8/25 (32%) CG: 8/23 (35%) | n.r. | After induction chemotherapy (only reported in preliminary publication): IG: 0/10 CG: 0/12 | IG: 0/10 CG: 0/12 | n.r. | (lethal infections) IG: 0/25 CG: 2/23 (9%) |
| Federico 1995 | After 1 year#: IG: 51/66 (72%) CG: 48/68 (71%) (P = 0.62) | Pats. with CR IG: 35/39 (90%) CG: 24/29 (83%) | After completion of chemotherapy (approximately 3 to 6 months): IG: 39/66 (59%) CG: 29/68 (43%) (P = 0.05 log‐rank) | IG: 14/66 (21%) CG: 22/68 (32%) | n.r. | |
| Gonnelli 1995 | n.r. | At 3 months of chemotherapy (40 patients evaluated): IG: 0/20 CG: 0/20 | IG: 1/20 (5%) CG: 0/20 | n.r. | ||
| At 6 months of chemotherapy (36 patients evaluated): IG: 0/19 CG: 0/17 | IG: 6/19 (32%) CG: 4/17 (24%) | |||||
| Guzman 1988 | After 18 to 42 months observation time: IG: 14/16 (87.5%) CG: 11/16 (69%) | IG: 11/16 (69%) CG: 10/16 (62.5%) | n.r. | n.r. | ||
| Iaffaioli 1994 | n.r. | n.r. | (grade 3/4) IG: 7/37 (19%) CG: 12/32 (37.5%) (P = 0.074) | n.r. | ||
| Luzi 1984 | After 1 year#: IG: 8/25 (32%) CG: 6/25 (24%) | n.r. | At 40 days (response was defined as CR, PR or radiologic improvement of atelectasis): IG: 13/23 (57%) CG: 21/24 (88%) | n.r. | ||
| Macchiarini 1989 | After 1 year#: IG: 10/15 (67%) CG: 2/11 (18%) (log rank p<0,0032) | After a median time of DFS/PFS in IG of 6 months§: IG: 7/15 (47%) CG: 3/11 (27%) | After approximately 6 months of chemotherapy: IG: 7/15 (47%) CG: 1/11 (9%) (P = 0.45, Fisher exact) | IG: 4/15 (27%) CG: 3/11 (27%) (n.s.) | (grade 3) IG: 0/15 CG: 3/11 (27%) (grade 4) IG: 0/15 CG: 1/11 (9%) | n.r. |
| Mantovani 1988 | After 1 or 2 years: IG: 18/20 (90%) CG: 16/17 (94%) (n.s.) | After 1 year: IG: 6/11 (55%) CG: 3/10 (30%) After 2 years: IG: 2/9 (22%) CG: 4/7 (57%) | n.r. | n.r. | ||
| Mustacchi 1994 | After a median time of survival in IG of 10 months§: IG: 53/106 (50%) CG: 60/105 (57%) | After a median time of DFS/PFS in IG of 6.5 months§: IG: 62/106 (58%) CG: 62/105 (59%) | IG: 6/106 (6%) CG: 3/105 (3%) | IG: 26/106 (25%) CG:16/105 (15%) (P = 0.02, chi²) | n.r. | |
| Pavesi 1993 | After a median time of survival in IG of approximately 16 to 17months§: IG: 74/148 CG: 85/148 | After a median time of survival in IG of 15 months§: IG: 74/148 CG: 86/148 | "Overall response" (not further described): IG: 77/148 (52%) CG: 88/148 (60%) | n.r. | ||
| Salvati 1984 | After a median time of survival in IG of 6 for extensive and 18 months for limited disease§: IG: 12/23 (52%) CG: 7/23 (30%) | After a median time of survival in IG of 2.1 for extensive and 2.8 months for limited disease§: IG: 11/23 (48%) CG: 3/23 (13%) | IG: 6/20 (32%) CG: 3/20 (16%) | IG: 6/20 (30%) CG: 9/20 (45%) | n.r. | |
| Sanchiz 1996 | n.r. | After 1 cycle of chemotherapy: IG: 0/27 CG: 0/27 | IG: 1/27 (3%) CG: 0/27 | (grade 4) IG: 20/27 (74%) CG: 27/27 (p<0.01) | (ANC <500/mm² and fever > 38°C) IG: 6/27 (22%) CG: 16/27 (59%) (P = 0.0119) | |
| Scher 1988 | After 1 year#: Limited disease: IG: 11/17 (65%) CG: 16/18 (89%) (P = 0.38, log rank) | After 1 year#: Limited disease: IG: 10/17 (59%) CG: 11/15 (73%) (P = 0.32, log rank) | After induction and consolidation radiochemotherapy (at approximately 6 months): IG:18/41 (44%) CG:17/39 (44%) | IG:23/41 (56%) CG: 20/39 (51%) | n.r. | (Admission for neutropenia and sepsis) Limited disease: IG: 5/17 (29%) CG: 11/15 (73%) |
| After 1 year#: Extensive disease: IG: 12/28 (43%) CG: 15/28 (54%) (P = 0.49, log rank) | After 1 year#:Extensive disease: approximately 28 to 60 months observation time#: IG: 8/23 (35%) CG: 10/24 (42%) (P = 0.49, log rank) | Extensive disease: IG: 12/24 (50%) CG: 15/24 (63%) | ||||
| Wara 1981 | n.r. | After 1 year#: IG: 22/33 (67%) CG: 25/42 (60%) (p<0.08) | n.r. | n.r. | ||
Abbreviations: # survival rates extracted from Kaplan‐Meier curves, § survival rates estimated from median survival times, CR: complete remission, PR: partial remission, SD: stable disease, NC: no change, PD: progressive disease, n.r.: not reported
3. Synthetic thymic peptides: survival, response, toxicity.
| Study | Survival rates | Tumour response | Toxicity (no. of patients) | |||
| OS | DFS | CR | PR | Grade 3/4 neutropenia | Grade 3/4 infection | |
| Maio 2010 |
At 1 year:
IG1 (IFN ɑ+1.6 mg thymosin α1): 39/97 (40%)
IG2 (IFN ɑ+3.2 mg thymosin α1): 36/97 (37%)
IG3 (IFN ɑ+6.4 mg thymosin α1): 45/98 (46%)
IG4 (3.2 mg thymosin α1)*: 38/99 (39%) total IG (IG1‐3): 120/292 (41%) CG (IFN ɑ): 33/97 (34%) |
At 1 year#: IG1: 4/97 (4%) IG2: 10/97 (10%) IG3: 3/98 (3%) IG4*: 10/99 (10%) total IG (IG1‐3): 17/292 (5%) CG: 0/97 |
Best response within 12 months (measured at various time points):
IG1: 2/97 (2%)
IG2: 3/97 (3%)
IG3: 2/98 (3%)
IG4*: 2/99 (2%) total IG (IG1‐3): 7/292 (2%) CG: 0/97 |
IG1: 5/97 (5%) IG2: 7/97 (7%) IG3: 4/98 (4%) IG4*: 10/99 (10%) total IG (IG1‐3): 16/292 (5%) CG: 4/97 (4%) | n.r. | |
| Cheng 2004 | After a median time of survival in IG of 10 months§: IG: 9/18 (50%) CG: 9/23 (39%) | At 1 year: IG: 3/18 (17%) CG: 3/23 (13%)(n.s.) | n.r. | n.r. | ||
| Gebbia 1994 | n.r. | n.r. | n.r. | (ANC<1,000/mm² and fever>38°C) IG1 (thymopentin): 12/23 (52%) IG2 (thymopentin+G‐CSF): 4/22 (18%) IG1+IG2: 16/45 (36%) CG1 (placebo): 18/28 (64%) CG2 (G‐CSF): 5/23 (22%) CG1+CG2: 23/51 (45%) | ||
| Gish 2009 | At 6 months IG: 12/14 (86%) CG: 7/11 (64%) | n.r. | Best response within 18 months (measured at various time points): IG: 0/14 CG: 0/11 | IG: 2/14 (14%) CG: 2/11 (18%) | n.r. | (severe bacterial infections) IG: 0/14 CG: 4/11 (36%) |
| At 12 months IG: 9/14 (64%) CG: 7/11 (64%) | ||||||
| At 2 years IG: 8/14 (57%) CG: 5/11 (45%) | ||||||
| GISOT 1987 | After 3 months mean observation time: IG: 432/447 (97%) CG:197/203 (97%) (P = 0,068, chi²) | n.r. | n.r. | n.r. | ||
| Schulof 1985 | After 1 year#: IG1 (maintenance therapy): 8/15 (53%) IG2 (loading dose): 4/13 (31%) CG: 1/13 (8%) | After 1 year#: IG1: 3/15 (20%) IG2: 4/13 (31%) CG: 0/13 | n.r. | n.r. | ||
Abbreviations: # survival rates extracted from Kaplan‐Meier curves, § survival rates estimated from median survival times, * not included in metaanalysis; CR: complete remission, PR: partial remission, SD: stable disease, NC: no change, PD: progressive disease
Tumour response
Seventeen studies reported on tumour response and 14 of them referred to defined response criteria mostly in accordance with standard criteria. In Iaffaioli 1994 tumour response data were not reported but the author summarised the results in the text. Pavesi 1993 reported data on an 'overall response rate' but did not define it any further and Sanchiz 1996 presented data on response referring to the first cycle of chemotherapy only (Table 2, Table 3).
Toxicity (adverse effects of chemotherapy and radiotherapy)
The two outcomes which were reported in a way that allowed us to include them in our analyses were severe neutropenia and infectious complications. Nine studies (Canovas 1991; Del Giacco 1988; Federico 1995; Gebbia 1994; Gish 2009; Iaffaioli 1988; Macchiarini 1989; Sanchiz 1996; Scher 1988) reported on one or both of these outcomes according to the National Cancer Institute Common Toxicity Criteria (CTC 2009) or gave a sufficient description of the outcomes, which allowed us to apply grading criteria. Three of them reported on the incidence of grade 3 to 4 neutropenia per patient, one per chemotherapy cycle; and seven on the incidence of grade 3 to 4 infectious complications (Table 2, Table 3).
Safety (adverse effects of purified thymus extracts (pTE) and synthetic thymic peptides (sTP)
Ten trials commented on the 'tolerability' of pTE or sTP (Bedikian 1984; Canovas 1988; Canovas 1991; Cohen 1979; Del Giacco 1988; Gebbia 1994; Gish 2009; Luzi 1984; Salvati 1984; Sanchiz 1996). The numbers of patients with local or systemic adverse effects were given in three studies (Macchiarini 1989; Scher 1988; Schulof 1985) (Table 4, Table 5).
4. Purified thymus extract: safety.
| Study | Adverse effects of purified thymus extracts | |
| local | systemic | |
| Bedikian 1984 | Erythema and induration of site of injection | Generalized skin rash, febrile reaction |
| Canovas 1988 | Authors stated that thymostimulin was well tolerated, but 2 patients were excluded because of allergic reaction to TP‐1 | |
| Canovas 1991 | Authors stated that thymostimulin was well tolerated and no adverse reactions were observed | |
| Cohen 1979 | "Toxic effects of thymosin were confined to local irritation at the injection site manifested by greater or lesser degrees of pain and swelling. All reactions subsided within 12‐72 hours of injection." | |
| Del Giacco 1988 | "No side effects were observed with thymostimulin,[...]." | |
| Luzi 1984 | "(...) no allergic reactions or toxic effects were noted during TS treatment." | |
| Macchiarini 1989 | "No local or systemic thymostimulin‐related clinical toxicities were noted." | |
| Salvati 1984 | Authors stated that no toxic effects attributable to thymostimulin treatment were observed. | |
| Sanchiz 1996 | "(...) GCS‐F and TS were well tolerated without adverse events related to these drugs." | |
| Scher 1988 | Dose reduction because of local reactions (pain and inflammation at injection site): 9/45 patients | chills and fever within 24 h of injection in 5/45 patients |
5. Synthetic thymic peptides: safety.
| Study | Adverse effects of synthetic thymic peptides | |
| local | systemic | |
| Gebbia 1994 | "Thymopentin treatment did not cause any significant side effects." | |
| Gish 2009 | "Of the 23 adverse events the author judged possibly or probably related to thymalfasin, most were mild and resolved without sequelae. Only three of these events occurred in more than one patient: nausea (n=2), fatigue (n=2), and nipple pain (n=2).(...) Overall, thymalfasin was well tolerated." | |
| Schulof 1985 | mild burning at the injection site in 3 patients. | mild transient loss of muscle mass in1 patient. |
Quality of life
None of the included studies reported on patient‐reported QoL.
Excluded studies
Of the remaining 52 publications considered to be of possible relevance, nine papers were duplicates and 17 did not fulfil inclusion criteria. Reasons for exclusion of studies are described in the table Characteristics of excluded studies.
Risk of bias in included studies
The quality of the included studies and the subsequent risk of bias were assessed separately for the different outcomes of interest using the criteria defined in the Cochrane Handbook (Higgins 2009). The assessments and grades given are shown in Table 6 and Table 7. The studies are grouped below by the grades for risk of bias. The grading is a basic judgement and does not account for the complexity of many of the trials studied.
6. Purified thymus extracts: risk of bias.
| Study | Sequence generation | Allocation concealment | Blinding | Attrition | Selective reporting | Risk of Bias | |
| OS | DFS/Tox | ||||||
| Airoldi 1987 | Quote: "i pazienti sono stati stratificati (...) prima di essere randomizzati al trattamento (...)" Comment: sequence generation not reported; no earlier reports from the same investigators found that clearly describe the use of random sequences | Not reported; prognostic factors similarly distributed | No blinding reported | No dropouts or withdrawals | Comprehensive report of outcomes | moderate | moderate ‐ high |
| Bedikian 1984 | Quote: "Patients (...) were randomized (...)." Comment: sequence generation not reported | Probably not done: no concealment reported, dissimilarities in baseline prognostic factors | No blinding reported | Quote: Three of 49 thymosin patients have been excluded from the subsequent evaluation of response and survival of the thymosin group (...) Comment: differential loss in comparison groups, but extent of possible bias unclear | No indication for selective reporting | moderate ‐ high | high |
| Canovas 1988 | Quote: "La asignación de los pacientes.....se realizó mediante el sistema de numeros aleatorios." Comment: probably done, table of random numbers used | Not reported. Equal distribution of characteristics/prognostic factors stated in text, but no detailed data provided | No blinding reported | All patients analysed | All intended outcomes were reported | moderate | moderate ‐ high |
| Canovas 1991 | Quote: " (...) se realizó mediante la aplicación de la tabla de numeros aleatorios (...)." Comment: probably done, table of random numbers used | Not reported. Equal distribution of characteristics/prognostic factors stated in text, but no detailed data provided | No blinding reported | All patients analysed | All intended outcomes were reported | moderate | moderate ‐ high |
| Cohen 1979 | Quote: "(...) randomly received (...)" Comment: sequence generation not reported | No concealment reported. Dissimilarities in baseline characteristics; small sample size | No blinding reported | Quote:"Statistical analysis was also (...) of 55 patients. All results (..) in the 46 protocol‐eligible patients were also significant for (...) 55 patients." Comment: number of withdrawals/drop‐outs balanced, reasons for exclusions described, PP and ITT performed | Comprehensive report of outcomes | moderate | moderate ‐ high |
| De Serdio 1997 | Quote: "Las tablas de azar nos suministratron (...) siguiente esquema de randomización (...)" Comment: adequate sequence generation | No concealment reported. Detailed list of disease localisation and stage given; other patient related characteristics not reported | No blinding reported | All patients analysed | All intended outcomes were reported | Outcomes not assessed | moderate ‐ high |
| Del Giacco 1988 | Quote:" (...) patients were randomised between (...)" Comment: sequence generation not reported | No concealment reported. | No blinding reported | Quote: "31 could be randomised (...) but only 22 are completely evaluable (the other 9 having an incomplete follow‐up (..)" Comment: 9 patients lost to follow‐up, reasons not commented, distribution between intervention and control group unclear | Discrepancy between intended and reported outcome measures, results on quality of life were not reported, tumour response only reported in the preliminary publication | high | high |
| Federico 1995 | Quote: "(...) patients were randomised (...)." Comment: sequence generation not reported | No concealment reported. Dissimilarities in baseline prognostic factors, which were discussed by study authors as possibly having influenced the outcomes | No blinding reported | Equal numbers of drop‐outs/exclusions in both groups | Comprehensive report of outcomes | moderate ‐ high | high |
| Gonnelli 1995 | Quote: "(...) were randomly selected (...)" Comment: sequence generation not reported | No concealment reported. | No blinding reported | 4 patients inevaluable, 1 in IG, 3 in CG, reasons not stated, ITT for tumour response and rate of infection | Intended outcomes not stated | Outcome not assessed | high |
| Guzman 1988 | Quote: “(...) were randomised.” Comment: sequence generation not reported | No concealment reported. No data on distribution of risk factors | No blinding reported | All patients analysed | All intended outcomes were reported | moderate ‐ high | high |
| Iaffaioli 1994 | Quote: "(...) and randomised (...)" Comment: sequence generation not reported | No concealment reported; prognostic factors similarly distributed | No blinding reported | All patients analysed | Intended outcomes were not comprehensively reported | Outcome not assessed | moderate |
| Luzi 1984 | Quote: "(...) in a randomized controlled study; (...)" Comment: sequence generation not reported | No concealment reported. Slight imbalances in patient‐related factors. Small sample size. (direction of possible risk unclear) | No blinding reported | Three patients with adenocarcinoma were excluded afterwards for unknown reasons, (two in IG and on in CG) | Intended outcomes were not comprehensively reported | moderate ‐ high | high |
| Macchiarini 1989 | Quote: "The randomization was performed by assigning a prerandomized sequential number to each patient (...)" Comment: probably done, table of random numbers used | No concealment reported. Dissimilarities in disease stage. Small sample size.(possible risk of bias in favor of the intervention group) | No blinding reported | Two patients from the control group excluded because of death within the first 2 weeks of treatment; no ITT; (possible risk of bias concerning mortality outcomes in favor of the control group) | All intended outcomes were reported | high | high |
| Mantovani 1988 | Quote:"(...) enrolled for study and randomized (...)" Comment: sequence generation not reported | No concealment reported. Dissimilarities in disease characteristics. Small sample size.(possible risk of bias in favour of the control group) | No blinding reported | All patients analysed | All intended outcomes were reported | high | high |
| Mustacchi 1994 | Quote: "(...) entering this prospective randomized multicenter trial (...)"Comment: sequence generation not reported | Quote: "(...) were randomly allocated over the phone by the Central Office (...)"Comment: probably done (central allocation) | No blinding reported | Quote: "(...) 25 out of 235 patients were lost due to cancellation, ineligibility or protocol violations (..)"Comment: distribution between groups similar, outcome measure not likely to be influenced | All intended outcomes reported | low ‐ moderate | moderate |
| Pavesi 1993 | Quote: "(...) and randomly allocated (...)"Comment: sequence generation not reported | Quote: "(...) and randomly allocated over the phone (...)"Comment: probably done (central allocation) | No blinding reported | Quote: "(...) in 245 fully evaluable patients (..)"Comment: 51 randomised patients not included in analysis, reasons not reported, distribution between groups unclear | Intended outcomes were reported (only abstract publication available) | moderate | moderate ‐ high |
| Salvati 1984 | “(?) hanno ricevuto a random (?)”Comment: sequence generation not reported | No concealment reported. Distribution of possible risk factors/disease characteristics unclear. Small sample size | No blinding reported | Quote: "I pazienti valutabili sono stati 40 (..)"Comment: six patients not included in analysis, reasons not reported, distribution between groups unclear | Intended outcomes were reported (but report was not very detailed) | high | high |
| Sanchiz 1996 | Quote: "(...) were randomly assigned (by means of tables of random numbers) (...)"Comment: probably done, table of random numbers used | No concealment reported. Slight imbalances in possible risk factors/disease characteristics. Small sample size | No blinding reported | No dropouts/withdrawals reported but unclear whether all patients were included in the analyses | All intended outcomes were reported | Outcome not assessed | moderate ‐ high |
| Scher 1988 | Quote: "Randomization was by the method of random permuted blocks (...)"Comment: probably done | No concealment reported. Dissimilarities in prognostic factors between groups | No blinding reported | All randomised patients were included in the survival analysis and the reasons for the exclusion of three patients from the response analysis were reported and unlikely to introduce bias | Outcomes comprehensively reported | low ‐ moderate | moderate |
| Wara 1981 | Quote: “(?) were randomly assigned (?)"Comment: sequence generation not reported | No concealment reported. Dissimilarities in prognostic factors. | No blinding reported | All but one randomised patients included in the analyses, reason for exclusion not reported | All intended outcomes reported | Outcome not assessed | moderate ‐ high |
7. Synthetic thymic peptides: risk of bias.
| Study | Sequence generation | Allocation concealment | Blinding | Attrition | Selective reporting | Risk of Bias | |
| OS | DFS/Tox | ||||||
| Cheng 2004 | Quote: "(...) were randomly divided (...) based on the date of admission." Comment: Quasi‐randomisation | Probably not done; study authors did not use adequate sequence generation and baseline prognostic factors dissimilarly distributed. | No blinding reported | All patients analysed. | All intended outcomes were reported. | high | high |
| Gebbia 1994 | Quote: "(...) were randomised (...)" Comment: sequence generation not reported | Not reported. Similar distribution of age, gender, performance status, but no data on site of primary tumour for the placebo group | No blinding reported | Quote: "(...) 4 patients were excluded from final analysis due to major protocol violation." Comment: unclear distribution of drop‐outs between groups | Incomplete reporting of hematological and infectious outcomes | Outcome not assessed | moderate ‐ high |
| Gish 2009 | Quote: "Randomization was carried out centrally using a randomization table (...)" Comment: probably done, table of random numbers used | Quote: "Randomization was carried out centrally using a randomization table (...)" Comment: probably done (central allocation) | Quote: "tumour measurements and interpretation (...) performed centrally by radiologists blinded to treatment assignment | 28 randomised, 25 treated and evaluated, 3 withdrawals in CG before beginning of treatment | All intended outcomes reported | low ‐ moderate | low ‐ moderate |
| GISOT 1987 | Quote:"(...) per mezzo di una lista di randomizzazione;" Comment: probably done, table of random numbers used | Not reported. No data on characteristics/ risk factors | No blinding reported | Inconsistent numbers of drop‐outs/withdrawals | All intended outcomes were reported | moderate | moderate ‐ high |
| Maio 2010 | Quote: "The randomization list was produced by the Internal QualityControl Unit of Biostatistics and Data Management (...)" Comment: adequate sequence generation | Quote: "Randomization was blinded and centralized (...). Comment: probably done (central allocation) | Quote: "tumour response was evaluated (...) utilizing a central review." Comment: although central review performed, unclear whether assessor was blinded | For tumour response all patients were analyzed, authors assumed that this was the case for the outcomes OS and PFS as well | Comprehensive report of outcomes | low | low ‐ moderate |
| Schulof 1985 | Quote: "(...) was performed using a randomized, double‐blind design (...)"Comment: sequence generation not reported | No concealment reported. Dissimilarities in prognostic factors between groups (possibly in favour of intervention group). Small sample size | Quote: "The code did not have to be broken because of toxicity in any patient (...)"Comment: Successful blinding of patients and care provider likely Quote: "(...) and administered (...) for a period of up to 1 year or until relapse."Comment: Outcome was assessed during the blinded study phase in the majority of patients |
All but one randomised patients included in the analyses, reason for exclusion reported | All intended outcomes reported | moderate | moderate |
Mortality outcomes
Studies with pTE were judged as having the following risk of bias concerning OS:
low to moderate: Mustacchi 1994; Scher 1988,
moderate: Airoldi 1987; Canovas 1988; Canovas 1991; Cohen 1979; Pavesi 1993,
moderate to high: Bedikian 1984; Federico 1995; Guzman 1988; Luzi 1984,
high: Del Giacco 1988; Macchiarini 1989; Mantovani 1988; Salvati 1984.
Studies with sTP were judged as having the following risk of bias concerning OS:
low: Maio 2010,
low to moderate: Gish 2009,
moderate: GISOT 1987; Schulof 1985,
high: Cheng 2004.
Outcome assessor‐related outcomes
Studies with pTE were judged as having the following risk of bias concerning DFS and toxicity outcomes:
moderate: Iaffaioli 1994,
moderate to high: Airoldi 1987; Canovas 1988; Canovas 1991; Cohen 1979; De Serdio 1997; Gebbia 1994; Mustacchi 1994; Pavesi 1993; Sanchiz 1996; Scher 1988; Wara 1981,
high: Bedikian 1984; Del Giacco 1988; Federico 1995; Gonnelli 1995; Guzman 1988; Luzi 1984; Macchiarini 1989; Mantovani 1988; Salvati 1984.
Studies with sTP were judged as having the following risk of bias concerning DFS and toxicity outcomes:
moderate: Schulof 1985,
moderate to high: GISOT 1987,
high: Cheng 2004.
Overall, the reasons for higher grades of risk of bias were due to inadequate reporting of the methods used for random allocation, unbalanced risk factors for the outcome of interest and small sample sizes. For outcome assessor‐related outcomes, inadequate reporting of the methods used for blinding were an additional reason for assuming higher risk of bias.
Effects of interventions
Survival
Overall survival (OS)
Purified thymus extracts (pTE)
Fifteen trials with pTE reported OS data with observation periods ranging from three to over 60 months. Data for meta‐analysis of OS at one year could be obtained from eight trials. The analysis included a total of 705 patients and 355 events and the RR did not show a difference in the risk of survival between the thymic peptides regimen and no treatment or placebo (RR 1.00, 95% CI 0.79 to 1.25) (Analysis 1.1). Heterogeneity was moderate (I² = 44%).
1.1. Analysis.
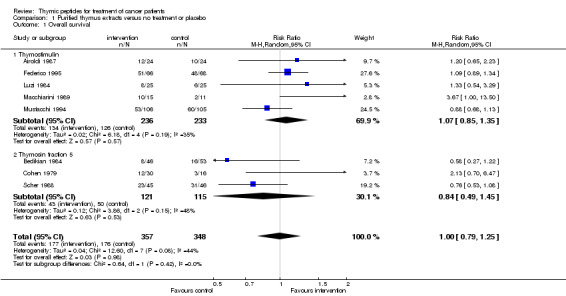
Comparison 1 Purified thymus extracts versus no treatment or placebo, Outcome 1 Overall survival.
Subgroup analysis
The thymostimulin group included five trials with 469 patients and the thymosin fraction 5 group had three trials including 236 patients. In the thymostimulin group, the pooled RR was above 1 (RR 1.07, 95% CI 0.85 to 1.35), whereas in the thymosin fraction 5 group the RR was 0.84 (95% CI 0.49 to 1.45).
Synthetic thymic peptides (sTP)
Five trials with sTP reported OS data with observation periods from three months to two years. Data for meta‐analysis of OS at one year could be obtained from four trials (Cheng 2004; Gish 2009; Maio 2010; Schulof 1985). All four trials used thymosin α1. The analysis included 496 patients and 200 events. The RR for OS was 1.21 (95% CI 0.94 to 1.56, P = 0.14) without statistical heterogeneity (I² = 0%).
Disease‐free survival (DFS)
Purified thymus extracts (pTE)
Twelve trials with pTE reported DFS data with observation periods ranging from three to over 60 months. Data for meta‐analysis of DFS at one year could be obtained from six trials (Cohen 1979; Federico 1995; Mantovani 1988; Pavesi 1993; Scher 1988; Wara 1981). The DFS analysis included a total of 511 patients and 308 events. The RR did not show a difference in the risk of DFS between the thymic peptides regimen and no treatment or placebo (RR 0.97, 95% CI 0.82 to 1.16) (Analysis 1.2). Heterogeneity was moderate (I² = 30%).
1.2. Analysis.
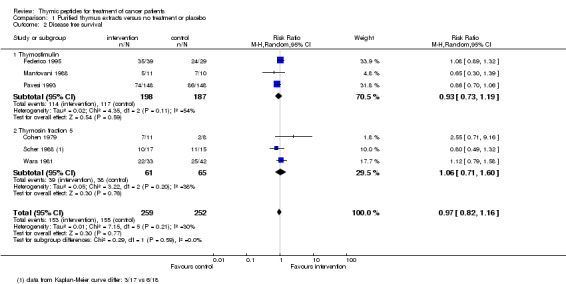
Comparison 1 Purified thymus extracts versus no treatment or placebo, Outcome 2 Disease free survival.
Subgroup analysis
The thymostimulin group included three trials with 385 patients and the thymosin fraction 5 group had three trials including 126 patients. The subgroup analysis showed no difference between the two subgroups (thymostimulin: RR 0.93, 95% CI 0.73 to 1.19; thymosin fraction 5: RR 1.06, 95% CI 0.71 to 1.60).
Synthetic thymic peptides (sTP)
Data were obtained for meta‐analysis of DFS at one year from three trials. All trials used thymosin α1. A total of 471 patients with 30 events were included in analysis. The RR was 3.37 (95% CI 0.66 to 17.30, P = 0.15) with moderate heterogeneity (I² = 37%) (Analysis 2.2).
2.2. Analysis.
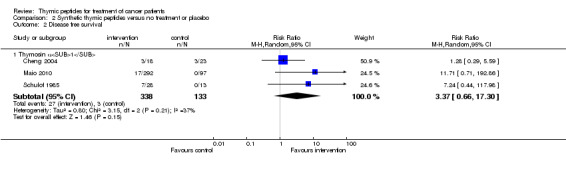
Comparison 2 Synthetic thymic peptides versus no treatment or placebo, Outcome 2 Disease free survival.
Tumour response
Purified thymus extracts (pTE)
Data could be obtained for response analysis from 11 trials with pTE. A total of 825 patients with 423 events were included in the analysis. There was no difference in the overall chance of achieving a complete or partial response between the intervention and the control groups (RR 1.07, 95% CI 0.92 to 1.25) (Analysis 1.3). Heterogeneity among the trials was rather high (I² = 53%).
1.3. Analysis.
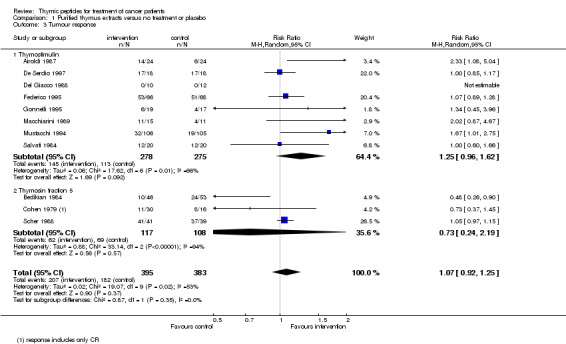
Comparison 1 Purified thymus extracts versus no treatment or placebo, Outcome 3 Tumour response.
Subgroup analysis
The thymostimulin group included eight trials with 553 patients and 258 events; the thymosin fraction 5 group had three trials including 225 patients with 131 events.
The pooled RR of trials using thymostimulin was 1.25 (95% CI 0.96 to 1.62, P = 0.09), whereas in the thymosin fraction 5 group the RR was below 1 (RR 0.73, 95% CI 0.24 to 2.19, P = 0.57).
Synthetic thymic peptides (sTP)
Only two trials with thymosin α1 reported data on tumour response (Gish 2009; Maio 2010). Therefore we did not pool data. Both trials showed no significant difference between the intervention and the control groups (RR 0.79, 95% CI 0.13 to 4.72; RR 1.91, 95% CI 0.68 to 5.39 respectively) (Analysis 2.3).
2.3. Analysis.
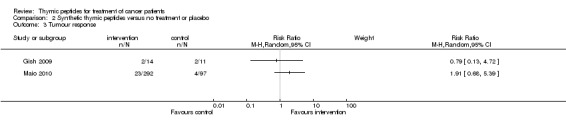
Comparison 2 Synthetic thymic peptides versus no treatment or placebo, Outcome 3 Tumour response.
Sensitivity analyses
Sensitivity analyses were performed using data from treatment arms with higher doses of thymic peptides (Cohen 1979; Maio 2010) or maintenance regime instead of the loading dose (Schulof 1985). Overall, no significant changes were found in risks for survival and tumour response and in statistical heterogeneity. Details are shown in Table 8.
8. Sensitivity analyses.
| Purified thymus extracts ‐ overall survival | ||
| Outcome | Random effects model | Single intervention groups in studies with more doses/regimes tested |
| pTE | RR 1.00, 95% CI 0.79 to 1.25, P = 0.98, I²=44% | 60 mg thymosin fraction 5 (Cohen 1979) RR 1.02, 95% CI 0.80 to 1.31, P = 0.87, I²=52% |
| Thymostimulin (subgroup) | RR 1.07, 95% CI 0.85 to 1.35, P = 0.57, I²=35% | not applicable |
| Thymosin fraction 5 (subgroup) | RR 0.84, 95% CI 0.49 to 1.45, P = 0.53, I²= 48% | 60 mg thymosin fraction 5 (Cohen 1979) RR 0.95, 95% CI 0.46 to 1.95, P = 0.89, I²=69% |
| Purified thymus extracts ‐ disease‐free survival | ||
| pTE | RR 0.97, 95% CI 0.82 to 1.16, P = 0.77, I²= 30% | 60 mg thymosin fraction 5 (Cohen 1979) RR 0.97, 95% CI 0.82 to 1.16, P = 0.78, I²=32% |
| Thymostimulin (subgroup) | RR 0.93, 95% CI 0.73 to 1.19, P = 0.59, I²= 54% | not applicable |
| Thymosin fraction 5 (subgroup) | RR 1.06, 95% CI 0.71 to 1.60, P = 0.76, I²= 38% | 60 mg thymosin fraction 5 (Cohen 1979) RR 1.07, 95% CI 0.70 to 1.64, P = 0.48, I²=41% |
| Purified thymus extracts ‐ tumour response | ||
| pTE | RR 1.07, 95% CI 0.92 to 1.25, P = 0.37, I²= 53% | 60 mg thymosin fraction 5 (Cohen 1979) RR 1.04, 95% CI 0.89 to 1.21, P = 0.64, I²=56% |
| Thymostimulin (subgroup) | RR 1.25, 95% CI 0.96 to 1.62, P = 0.09, I²=66% | not applicable |
| Thymosin fraction 5 (subgroup) | RR 0.73, 95% CI 0.24 to 2.19, P = 0.57, I²=94% | 60 mg thymosin fraction 5 (Cohen 1979) RR 0.81, 95% CI 0.32 to 2.07, P = 0.65, I²=92% |
| Synthetic thymic peptides ‐ overall survival | ||
| sTP | RR 1.21, 95% CI 0.94 to 1.56, P = 0.14, I²=0% | 6.4 mg thymosin α1(Maio 2010) and maintenance regime (Schulof 1985) RR 1.30, 95% CI 0.92 to 1.85, P = 0.14, I²=23% |
| Synthetic thymic peptides ‐ disease‐free survival | ||
| sTP | RR 3.37, 95% CI 0.66 to 17.30, P = 0.15, I²= 37% | 6.4 mg thymosin α1 (Maio 2010) and maintenance regime (Schulof 1985) RR 2.22, 95% CI 0.67 to 7.37, P = 0.19, I²=0% |
Toxicity
Purified thymus extracts (pTE)
Infectious complications Data could be obtained from four studies for pooled analysis of severe infections (at least CTC grade 3 or 4). Three investigated thymostimulin and one thymosin fraction 5. A total of 214 patients were included and 73 experienced a severe infectious complication at any site. The RR indicated a lower risk of severe infectious complications (RR 0.54, 95% CI 0.38 to 0.78, P = 0.0008) (Analysis 1.4). Heterogeneity among the trials was low (I² = 0%).
1.4. Analysis.
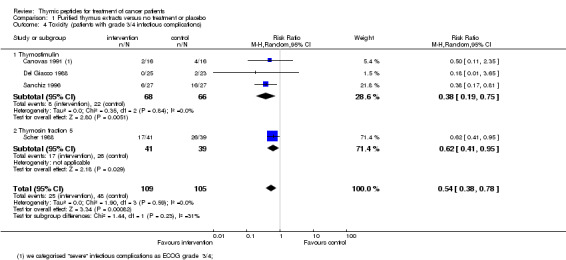
Comparison 1 Purified thymus extracts versus no treatment or placebo, Outcome 4 Toxicity (patients with grade 3/4 infectious complications).
Neutropenia Data for analysis of severe neutropenia (at least CTC grade 3 or 4) could be obtained from three trials, which all used thymostimulin. Overall, 72 of 149 patients experienced severe neutropenia. The RR was 0.55 (95% CI 0.25 to 1.23, P = 0.15) (Analysis 1.5) with high heterogeneity among the trials (I² = 63%).
1.5. Analysis.
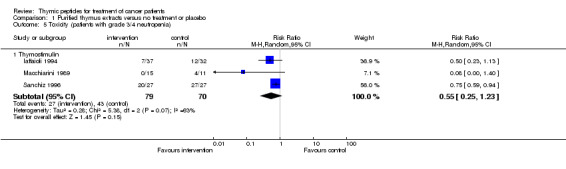
Comparison 1 Purified thymus extracts versus no treatment or placebo, Outcome 5 Toxicity (patients with grade 3/4 neutropenia).
Synthetic thymic peptides (sTP)
Only two trials with sTP reported data on infectious complications or neutropenia (Table 3). Therefore pooling of data was not feasible. Gebbia 1994 found a non‐significant reduction in the number of patients experiencing neutropenia during chemotherapy by treatment with thymopentin. Gish 2009 reported a non‐significant reduction in the rate of patients with severe bacterial infections by treatment with thymosin α1.
Safety
Ten out of 20 studies with pTE and three out of six trials with sTP reported on adverse effects of the interventional treatments. Seven authors reported that the interventional treatments were well tolerated. Adverse events reported by the other authors were mild, transient local reactions at the injection site with systemic reactions in few patients. Details are shown in Table 4 and Table 5.
Discussion
This review included data from 26 trials (2736 patients) investigating the treatment of various malignancies with pTE or sTP while receiving basic oncologic treatment consisting of chemotherapy alone or in combination with radiotherapy or immunotherapy, chemotherapy applied as transcatheter arterial embolization, or radiotherapy alone. These 26 studies included both published and unpublished trials and represented all RCTs matching the inclusion criteria at the time of the literature search. The last trial was identified in March 2010. Twenty studies used one of two pTE, thymostimulin or thymosin fraction 5, and six one of two sTP, thymopentin or thymosin α1, as investigational treatments.
We did not find evidence that the addition of pTE or sTP to antineoplastic treatment reduces the risk of death or disease progression, nor that it improves the rate of tumour response to antineoplastic treatment. However, there was preliminary evidence that pTE lowered the risk of severe infectious complications in patients undergoing chemotherapy or radiotherapy. There was no evidence of significant side effects either with pTE or sTP.
The pTE was used to treat 1436 patients, and 372 breast cancer patients from three studies was the largest group. There were 1300 participants treated with sTP, 488 patients with metastasised melanoma were from one study. There were sufficient numbers to assess treatment impact of both pTE and sTP on survival outcomes, and of pTE on tumour response. There were only a few trials with small numbers of patients that assessed the effects of pTE and sTP on adverse effects of chemotherapy or radiotherapy scored according to standardized criteria (CTC), therefore the trials in this review have low power to assess the impact of the intervention on this outcome. We had planned to perform subgroup analyses with respect to different types of cancer. After appraisal of the included studies, however, subgroup analysis was only possible for the different investigational drugs applied.
Other major problems for this review were the poor methodological quality of many of the included trials, variability in entry criteria, the nature and timing of outcomes, and poor reporting of both outcomes and methodology. In particular, there is a possibility of bias due to different treatment schedules and doses of both the investigational and the basic oncologic treatments across the trials, as well as a general failure to report data suitable for comparison of survival over time. Only four trials reported adequate methods of allocation concealment, which could have introduced bias. None of the trials with pTE and only one with sTP reported blinding of outcome assessors, which could have introduced bias in the assessment of DFS, tumour response and toxicity outcomes. Another limitation of this systematic review was the small sample size of many of the trials. In particular, two thirds of trials had a sample size of less than 60 participants and may have yielded inconclusive results because they were small and therefore did not have adequate statistical power. Only six trials included more than 100 participants.
The included trials were published over a 31‐year period, up to 2010, and mainly involved participants from Italy, Spain and the USA. Studies with pTE were conducted from 1979 until the late 1990s. Thereafter this treatment concept was seemingly abandoned and clinical investigations became orientated towards the application of sTP. All studies (n = 3) which were conducted after 1997 used the sTP thymosin α1.
Pooling of data was possible for a number of clinical outcomes of interest. For thymosin α1 there was a slight trend toward an overall reduction in the risk of dying (RR 1.21, 95% CI 0.94 to 1.56, P = 0.14) and improved DFS (RR 3.37, 95% CI 0.66 to 17.30, P = 0.15). Data from one large trial with low risk of bias on patients with metastatic melanoma mainly contributed to these results. Two trials with thymosin α1 compared either different doses (Maio 2010) or different regimes of application (Schulof 1985). Results from these individual studies indicated a possible dose‐dependent effect. A further finding from Maio's trial that was not included in our analysis but which could be of interest was that thymosin α1 added to chemotherapy seemed to be as effective as interferon α but better tolerated.
The different RR for tumour response of thymostimulin (above 1) and thymosin fraction 5 (below 1) might be regarded as a possible indication of differential effects of the two different pTE. However, such an interpretation should be made with caution because the suggested negative effect of thymosin fraction 5 is mainly caused by one study at high risk of selection bias that involved patients with advanced non‐small cell lung cancer (Bedikian 1984). Nevertheless, true opposite effects of different pTE, for instance caused by differences in the peptide composition, could not be ruled out based on our data. Thymostimulin and thymosin fraction 5 have dissimilar manufacturing processes and while there were little to no efforts to analyse the components of thymostimulin, those of thymosin fraction 5 came under scrutiny. One oligopeptide identified from the thymosin fraction 5 is thymosin ß4, which was recently discussed due to its possible stimulating effects on tumour metastasis by activating cell migration and angiogenesis (Cha 2003). The heterogeneity within the two groups might also be attributable to different reactions of the various cancer entities to pTE. Lack of sufficient studies with the same disease conditions hampers further evaluation of this aspect.
Pooled estimates of trials of pTE suggest an advantage on the risk of experiencing serious infectious complications or, as a trend, severe neutropenia during basic oncological treatment. Two trials with sTP reported similar findings on these outcomes but were not included in the meta‐analysis (Gebbia 1994; Gish 2009). Two of the four arms in Gebbia 1994 compared thymopentin with G‐CSF. Although there was some evidence that thymopentin might reduce the risk of infections, G‐CSF was significantly more effective (Gebbia 1994). Given the safety profile of sTP, they could still be of investigational interest for this indication.
All of these findings are subject to a potential publication bias. While we have made every effort to locate further unpublished data, it remains possible that this review is subject to a positive publication bias, with generally favourable trials more likely to achieve reporting. For instance Schulof referred, in a systematic review from 1985, to one trial of thymosin fraction 5 with negative effects on tumour response where the information was obtained by personal communication (Schulof 1985). We could not trace a publication.
Only one systematic review on thymic peptides in cancer patients has been published so far (Ernst 1997). The author addressed the question of clinical effectiveness of 'thymus therapy' in cancer patients and included 13 of 21 RCTs published between 1979 and 1996. Inclusion criteria were similar to those used in our review but additionally included oral thymus preparations as interventional treatments and immunologic parameters as outcomes. There was no tool for assessment of methodological quality and study results were interpreted narratively. The author criticised the trials because of low methodological quality, small sample sizes, heterogeneous study populations and statistical shortcomings. The overall conclusion saw no 'compelling' evidence for the efficacy of thymus extracts but regarded some results as 'promising' and deserving of further investigation. This overall conclusion is in accordance with the results of our review pertaining to the set of trials included in Ernst’s review.
Authors' conclusions
Implications for practice.
Data provided by four small RCTs suggest that purified thymus extracts (pTE) might reduce the risk of infectious complications in patients undergoing chemotherapy or radiotherapy, or both. The effect of synthetic thymic petides on the same outcome is only supported by weak evidence. Findings that thymosin α1 might have beneficial effects on survival were mainly supported by one larger study with low risk of bias of patients with metastatic melanoma. Given the limited treatment options for this condition and the safety profile of thymosin α1, treatment with thymosin α1 could be considered assuming that the decision about its use was based on expert clinical judgement. This should be discussed with patients before they give their consent and, where possible, patients should be offered entry into well‐designed clinical trials.
Implications for research.
There is a case for well‐designed randomised trials to assess the possible value of the application of thymosin α1, suggested by one large trial in patients with metastasised melanoma. Future trials must employ up‐to‐date antineoplastic and supportive treatment regimens in both arms; should take into account a possible dose‐dependent effect of thymosin α1, evaluate appropriate sample sizes with power to detect expected differences and apply effective and explicit blinding of treatment allocation. Examined outcome measures should include QoL measured with validated instruments and careful elucidation of any adverse effects.
Clinical trials with purified thymus extracts should not be advocated in the management of cancer until the exact compositions of the extracts are scrutinized and components are identified that might confer possible effects on host immunity and tumour biology.
What's new
| Date | Event | Description |
|---|---|---|
| 15 May 2017 | Review declared as stable | Intervention is no longer clinically important. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 2, 2011
| Date | Event | Description |
|---|---|---|
| 24 February 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 12 January 2012 | Amended | Author details amended. |
| 1 October 2008 | New citation required and major changes | Authors: Reviewer team has changed Objectives: Text was rephrased and the population under study was restricted to cancer patients with thymus extracts during chemo‐ or radiotherapy Types of interventions: Interventional treatment under study was restricted to thymus extracts given during chemo‐ or radiotherapy and interventions in the control group were restricted to no treatment, or placebo treatment. Types of outcome measures: Text was rephrased Acknowledgments/Contributions of authors: the review team has changed and the text were rephrased/amended accordingly |
| 16 June 2008 | Amended | Converted to new review format. |
Notes
Intervention is no longer clinically important.
Acknowledgements
Erik Ritter and Gerd Büschel initially contributed to the development of the protocol and the first literature search but subsequently left the review team. Gerd Büschel kindly commented on the final draft of the manuscript. We would also like to thank Prof Dr Ewald Hannappel (Institut für Biochemie, Friedrich‐Alexander‐Universität, Erlangen‐Nürnberg) for his interest and his valuable suggestions.
Appendices
Appendix 1. Glossary of terms
| EORTC | European Organization for Research and Treatment of Cancer |
| Breslow thickness | Measuring of the depth of penetration of a melanoma into the skin in mm |
| Dukes | Staging score for Colorectal cancer |
| WHO | World Health Organization |
| RECIST | Response Evaluation Criteria In Solid Tumors: a set of published rules that define when malignant tumours respond ("respond"), stay the same ("stable") or worsen ("progression") during treatments |
| OS | Overall survival: denotes the chances of staying alive for a group of individuals suffering from a cancer. It denotes the percentage of individuals in the group who are likely to be alive after a particular duration of time |
| DFS | Disease‐free survival: denotes the chances of staying free of disease after a particular treatment for a group of individuals suffering from a cancer. It is the percentage of individuals in the group who are likely to be free of disease after a specified duration of time. |
| pTE | Purified extracts from animal thymus glands containing peptide mixtures |
| sTP | Synthetically produced single thymic peptides. |
Appendix 2. Search strategies
PubMed ‐ CENTRAL ‐ MEDLINE
These databases were searched with 37 terms that referred to Thymyc/Peptide extracts.
Search terms used to identify interventions were:
Thymostimulin or thymoxtimulin
TF5
Thymosin
Thymosin fraction 5
Tα1 or Talpha1 or Thymosin alfa one or thymalfasin or zadaxin
Thymic serum factors
Tβ4 or thymosin beta four
Tγ or thymosin gamma
TFX or thymomodulin or thymic factor x or TFX‐Polfa
TFX‐Jelfa
TP‐1
Thym‐uvocal or Thymuvocal
Thymoject/thymojekt
Biosin
Thymex‐L or thymex l
Thymophisin/Thymophysin
Zellmedin‐thymus or THX
Neytumourin Sol
NeyThymun
Thymuskin
Thymushydrolysate
Solcothymosin
Thymowied
Leucotrofina
FTS‐Zn
Thymulin
Thymic serum factor
THFγ
Thymic humoral factor
HTH or Homeostatic thymic hormone
Thymopoietin (I and II) or TP5 or Thymopentin
Prothymosin α
Thymus peptide
LSH
Lymphocytopoietic factor
Wobe‐Mugos
t‐activin or tactivin
PubMed limits to identify the type of study:
Humans
Type of Article: Clinical Trial OR Meta‐Analysis OR Randomized Controlled Trial OR Review
More Publication Types: Clinical Trial, Phase I OR Clinical Trial, Phase II OR Clinical Trial, Phase III OR Clinical Trial, Phase IV OR Controlled Clinical Trial OR Multicenter Study
Topics: Cancer OR Complementary Medicine OR Systematic Reviews OR Toxicology
Age : All Adult: 19+ years OR Young Adult: 19‐24 years OR Adult: 19‐44 years OR Middle Aged: 45‐64 years OR Middle Aged + Aged: 45+ years OR Aged: 65+ years80 and over: 80+ years
Example of search:
("thymostimulin"[Substance Name] OR "thymostimulin"[All Fields])
AND
("neoplasms"[MeSH Terms] OR "neoplasms"[All Fields] OR "cancer"[All Fields])
AND
("humans"[MeSH Terms])
AND
(Clinical Trial[ptyp] OR Meta‐Analysis[ptyp] OR Randomized Controlled Trial[ptyp] OR Review[ptyp] OR Clinical Trial, Phase I[ptyp] OR Clinical Trial, Phase II[ptyp] OR Clinical Trial, Phase III[ptyp] OR Clinical Trial, Phase IV[ptyp] OR Controlled Clinical Trial[ptyp] OR Multicenter Study[ptyp])
AND
(cancer[sb] OR cam[sb] OR systematic[sb] OR tox[sb] OR medline[sb] OR pubmed pmc local[sb]) AND ("adult"[MeSH Terms] OR "young adult"[MeSH Terms] OR "adult"[MeSH Terms:noexp] OR "middle aged"[MeSH Terms] OR ("middle aged"[MeSH Terms] OR "aged"[MeSH Terms]) OR "aged"[MeSH Terms] OR "aged, 80 and over"[MeSH Terms]))
EMBASE SEARCH:
The same 37 above mentioned PubMed terms were also searched in EMBASE.
EMBASE limits to identify the type of study:
human
article or "review"
adult <18 to 64 years> or aged <65+ years>
intramuscular or subcutaneous
Example of search:
Thymic extracts OR Thymus extracts OR Thymos* OR Thym*
AND
Cancer).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
OTHER DATABASE SEARCHES
The other databases were searched using the following specific terms as text words combined by the Boolean operator "OR":
thymus therapy; thymic peptide; thymic hormone; Thymustherapie; thymosin; thymosin fraction 5: thymosin fraction V; thymulin; thymusfactor; thymopentin; thymostimulin; thymic extracts; Ney‐Tumorin; Neythymun; Solcothymosin; Thymex; Thymowied; Thym‐Uvocal; Thymoject; thymophysin; Zellmedin‐Thymus; THX; TF5; TP‐1;THF;TFX;TP5.
These following terms were used to identify the study design:
"therapy"; "treatment"; clinical trial; randomised clinical trial as MeSH terms.
These following terms were used to identify cancer patients:
"cancer"; "tumours"; "neoplasms" as MeSH terms.
Date of last search 10.3.2010
All databases were searched from their inception until March 2010
Appendix 3. Kirkwood formulae
Survival Time (t)=S(t)=exp(‐ʎt). This transforms for the median survival time to Tmed = ‐ ln (0.5) / ʎ. The number ECG of events in the control group given NCG patients in the control group at median survival time of the intervention group Tmed(MIG) can now be calculated via ECG = NCG * (1‐EXP(‐Tmed(MIG)*ln(2)/ Tmed(MCG)). Obviously, the number of events in the intervention group is EIG= 0.5*NIG. Of note, these calculations assume no censoring of patients up to median follow‐up time. With these numbers a relative risk with an approximate 95% CI can be calculated as implemented in standard meta‐analysis software.
Data and analyses
Comparison 1. Purified thymus extracts versus no treatment or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 8 | 705 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.79, 1.25] |
| 1.1 Thymostimulin | 5 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.85, 1.35] |
| 1.2 Thymosin fraction 5 | 3 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.49, 1.45] |
| 2 Disease free survival | 6 | 511 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.82, 1.16] |
| 2.1 Thymostimulin | 3 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 2.2 Thymosin fraction 5 | 3 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.71, 1.60] |
| 3 Tumour response | 11 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.92, 1.25] |
| 3.1 Thymostimulin | 8 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.96, 1.62] |
| 3.2 Thymosin fraction 5 | 3 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.24, 2.19] |
| 4 Toxicity (patients with grade 3/4 infectious complications) | 4 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.38, 0.78] |
| 4.1 Thymostimulin | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.19, 0.75] |
| 4.2 Thymosin fraction 5 | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.41, 0.95] |
| 5 Toxicity (patients with grade 3/4 neutropenia) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Thymostimulin | 3 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.25, 1.23] |
Comparison 2. Synthetic thymic peptides versus no treatment or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 4 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.94, 1.56] |
| 1.1 Thymosin α1 | 4 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.94, 1.56] |
| 2 Disease free survival | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Thymosin α1 | 3 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 3.37 [0.66, 17.30] |
| 3 Tumour response | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
2.1. Analysis.
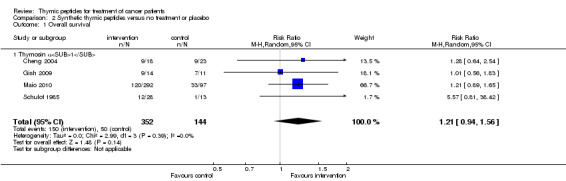
Comparison 2 Synthetic thymic peptides versus no treatment or placebo, Outcome 1 Overall survival.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Airoldi 1987.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group; stratification by type of pretreatment, location and grading of tumour, disease status (non‐responsive or recurrent) No of centres: 1 Recruitment and setting: Medical Clinic and Department of Radiotherapy, University of Turin, Italy Recruitment period: 01/84‐08/85 Observation period: median: 14 months, minimum: 11 months Ethical approval: unclear | |
| Participants | No of patients: 48 randomised, 48 evaluated Condition: squamous cell cancer of the oral cavity non‐responsive or recurrent after conventional therapy with surgery and/or irradiation Demographics: men: 39, women: 9; mean age (range): 58 (37‐71) years Informed consent: unclear | |
| Interventions | Interventional treatment: thymostimulin, dose/schedule: 1 mg/kg/day i.m. starting 7 days before chemotherapy treatment, thereafter 2x/week for 4 weeks and 1x/week until tumour progression Control treatment: no treatment Basic treatment: vincristine 1.2 mg/m² i.v. (d1), bleomycin 18 mg/m² i.m. (d1), methotrexate 30 mg/m² i.v. (d2); every week for 8 weeks | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), other | |
| Notes | Outcomes: side effects of chemotherapy were not scored using standardized criteria | |
Bedikian 1984.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group; stratification by histological type of disease and performance status No of centers: 1 Recruitment and setting: M.D. Anderson Cancer Center, University of Texas, USA Recruitment period: 01/79‐05/80 Observation period: max. 104 weeks Ethical approval: unclear | |
| Participants | No. of patients: 105 randomised, 99 evaluated Condition: advanced stage non‐small cell lung cancer (NSCLC) Demographics: men: 70, women: 29; median age (range): IG: 55 (37‐77), CG: 57 (35‐80) years Informed consent: yes | |
| Interventions | Interventional treatment: thymosin fraction 5; dose/schedule: 60 mg/m² s.c. every chemotherapy cycle (d1,4,8,12,16) Control treatment: no treatment Basic treatment: vindesine 3 mg/m² (d1), doxorubicin 50 mg/m² (d1), cisplatin 60 mg/m² (d1) every 3‐4 weeks | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), safety (AEs of thymic peptides), chemotherapy dose/schedule modifications, other | |
| Notes | Participants: first 13 patients were not randomised because of unavailability of thymosin fraction 5 and allocated to the no‐treatment arm, thereafter to equalise the two arms a randomisation scheme favouring the thymosin arm was used Outcomes: side effects of chemotherapy were not scored using standardized criteria Funding: sponsored by the National Cancer Institute (NCI) | |
Canovas 1988.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No of centres: 1 Recruitment and setting: outpatients department, Hospital de Cruces, Bilbao, Spain Recruitment period: unclear Observation period: 4 chemotherapy cycles Ethical approval: unclear | |
| Participants | No. of patients: 46 randomised, 41 evaluated Condition: multiple myeloma (28 patients), non‐Hodgkin lymphoma (NHL) (11 patients), Hodgkin lymphoma (2 patients) Demographics: unclear Informed consent: unclear | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 25 mg i.m., 6x within 2 weeks at beginning of the study, thereafter 4x within 2 weeks before each chemotherapy cycle Control treatment: no treatment Basic treatment: multiple myeloma: VCAP/VMCP, MP or M‐2; NHL: Promace MOPP, CVP or CHOP; Hodgkin lymphoma: MOPP/ABVD | |
| Outcomes | Outcome measures: survival, toxicity (AEs of chemo‐/radiotherapy), safety (AEs of thymic peptides/extracts), other | |
| Notes | Outcomes: side effects of chemotherapy were not scored using standardized criteria; pre/post analysis of performance status (ECOG) | |
Canovas 1991.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group; patients stratified by diagnosis No of centres: 1 Recruitment and setting: Hospital de Cruces, Bilbao, Spain Recruitment period: unclear Observation period: approximately 4‐6 months (6 cycles of chemotherapy) Ethical approval: unclear | |
| Participants | No. of patients: 40 randomised, 32 evaluated Condition: multiple myeloma (13 patients), NHL (11 patients), Hodgkin lymphoma (8 patients) Demographics: unclear Informed consent: unclear | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 1 mg/kg body weight i.m., daily for one week; thereafter 2x/week for 6 chemotherapy cycles Control treatment: no treatment Basic treatment: multiple myeloma: VCAP/VMCP, MP or M‐2; NHL: Promace MOPP, CVP, LSA2Ls, C‐MOPP or IMVP‐16; Hodgkin lymphoma: MOPP MOPP/ABVD | |
| Outcomes | Outcome measures: survival, toxicity (AEs of chemo‐/radiotherapy), safety (AEs of thymic peptides), other | |
| Notes | Outcomes: side effects of chemotherapy were not scored using standardized criteria; pre/post analysis of performance status (ECOG) | |
Cheng 2004.
| Methods | Design: 3‐arm parallel trial with a no‐treatment control group No. of centres: 1 Recruitment and setting: Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, China Recruitment period: 01/00‐12/02 Observation period: 6‐32 months Ethical approval: yes | |
| Participants | No. of patients: 57 for the whole trial, 41 randomised, 41 evaluated in the two relevant arms Condition: hepatocellular carcinoma after hepatectomy; Edmondson´s stage II‐IV Demographics: men: 34, women: 7; median age (range): 48 (30‐66) years for whole study population Informed consent: unclear | |
| Interventions | Interventional treatment: thymosin α1 (thymalfasin; Zadaxin) dose/schedule: 1.6 mg/day s.c., 2x/week from the first week after hepatectomy for 6 months Control treatment: no treatment Basic treatment: transcatheter hepatic arterial chemoembolisation (TACE) with carboplatin: 100 mg, epidoxorubicin 10 mg and mitomycin C 10 mg, starting 1.5 months after hepatectomy. In patients with recurrence, treatment was repeated max. four times | |
| Outcomes | Outcome measures: survival | |
| Notes | Design: 2 arms were relevant for this review, the third arm compares transcatheter hepatic arterial chemoembolisation with no treatment Participants: imbalance in stage of disease, with a higher proportion of patients with stage IV in the intervention group; distribution of patients with radical and palliative resection unclear Funding: supported by Shanghai Science and Technology Committee and Shanghai Hospital New Star Plan | |
Cohen 1979.
| Methods | Design: 3‐arm parallel with placebo control No of centres: unclear Recruitment and setting: NCI‐VA Medical Oncology Branch, Veterans Administration Hospital, Washington, D.C.; Surgery Branch, National Cancer Institute, Bethesda, USA Recruitment period: 07/75‐01/77 Observation period: approximately 2 years for survival, 12 weeks for response Ethical approval: unclear | |
| Participants | No. of patients: 55 randomised, 46 evaluated Condition: small cell lung cancer (SCLC), limited (15) or extensive (31) disease Demographics: men: 34, women: 12; median age (range): IG1: 58 (49‐69), IG2: 61 (47‐69), CG: 53 (41‐67) years Informed consent: yes | |
| Interventions | Interventional treatment: thymosin fraction 5; dose/schedule: IG1: 60 mg/m² s.c., IG2: 20 mg/m² s.c., 2x/week during induction chemotherapy Control treatment: no treatment Basic treatment: induction therapy: cyclophosphamide 1500 mg/m² (d1), lomustine 100 mg/m² (d1), cyclophosphamide 1000 mg/m² (d22), methotrexate 15 mg/m² 2x/week for 10 doses; maintenance therapy: cyclically alternating two or three drug chemotherapy regimes for 2 years; starting on week 6 | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), safety (AEs of thymic peptides), other | |
| Notes | Participants: as stated by study authors patients in the IG2 tended to have a lower performance status Outcomes: side effects of chemotherapy were not scored using standardized criteria ITT analysis: was performed Funding: thymosin fraction 5 was provided by Hoffmann‐La Roche | |
De Serdio 1997.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: 1 Recruitment and setting: Hospital Nuestra Seňora de la Cruz, Santa Cruz de Tenerife, Spain Recruitment period: 03/93‐09/95 Observation period: mean 18 months, max. 30 months Ethical approval: unclear | |
| Participants | No. of patients: 36 randomised, 36 evaluated Condition: locally advanced head and neck cancer, stage III or IV Demographics: men: 35, women: 1; age (range): 30‐66 years (no median given) Informed consent: unclear | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 1.5 mg/kg/day i.m. for 7 days before radiochemotherapy, 1.5 mg/kg/day i.m. 2x/week during treatment, 1 mg/kg/day i.m. 2x/week for 2 years or until recurrence Control treatment: no treatment Basic treatment : radiochemotherapy with 1.15 Gy per fraction up to 80.5 Gy (cumultative dose), carboplatin 5 mg/m² per fraction up to 700 mg (cumulative dose); 2x/day, 5 days/week | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), other | |
| Notes | ||
Del Giacco 1988.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: 1 Recruitment and setting: Institute of Internal Medicine, University of Cagliari, Italy Recruitment period: starting 01/81; duration unclear Observation period: 12‐33 months Ethical approval: unclear | |
| Participants | No. of patients: 48 randomised, 48 evaluated (22 evaluated for tumour response) Condition: NSCLC or SCLC after incomplete resection or unresectable, classified as immunodepressed by various immunologic tests Demographics (only reported in the preliminary publication for 22 patients): men: all patients; mean age (SD): IG: 58 (±8), CG: 57 (±11) years Informed consent: yes | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 1.5 mg/kg i.m. daily between cycles for 2 months; on alternate days for 4 months; thereafter 2x/week Control treatment: no treatment Basic treatment: doxorubicin 50 mg/m² (d1), vincristine 1,2 mg/m² (d1), cyclophosphamide 400 mg/m² (d1), lomustine 30 mg/m² (d1); every 4 weeks; until demonstrable response for max 6 cycles; maintenance chemotherapy: NSCLC: cyclophosphamide 400 mg/m² (d1,d8), methotrexate 15 mg/m² (d1,d8), procarbazine 100 mg/m² (d1‐10); every 4 weeks; SCLC: cyclophosphamide 1000‐1500 mg/m²; every 3 weeks, methotrexate 10 mg/m² every 2 weeks, lomustine 50 mg/m² d1; thereafter VP16+adriamycin and methotrexate (no exact description given by authors) | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), safety (AEs of thymic peptides), other | |
| Notes | Various inconsistencies regarding inclusion criteria and dose of thymostimulin Participants: in an earlier publication (1984) preliminary results were published on 22 patients 1 year after terminating an enrolment phase of 21 months; patients with SCLC were not included at the beginning of the trial and inclusion criteria were later changed Interventions: there are differing doses stated in the two publications, the preliminary publication refers a dose of 1 mg/kg i.m. Outcomes: side effects of chemotherapy were not scored using standardized criteria | |
Federico 1995.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: 6 Recruitment and setting: 2 university hospitals (Modena and Pavia) and 4 other hospitals in Italy Recruitment period: 11/88‐12/90 Observation period: 4 years, median follow up 38 months Ethical approval: unclear | |
| Participants | No. of patients: 150 randomised, 134 evaluated Condition: intermediate‐ or high‐grade NHL, stage II‐IV and stage I with bulky disease Demographics: men: 73, women: 65 (mistake in publication); median age: IG: 52, CG: 51 years Informed consent: unclear | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 1 mg/kg i.m.; for pats. treated with MACOP‐B: (d22‐29, 50‐57, 77‐85), for pats. treated with ProMACE‐CytaBOM (d22‐28) of each chemotherapy cycle Control treatment: no treatment Basic treatment: comparative study of 2 chemotherapy regimes: MACOP‐B and ProMACE‐CytaBOM: in both regimes doxorubicin was replaced by a 20% higher dose of epidoxorubicin | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), other | |
| Notes | The study was designed by the Italian Lymphoma Study Group Participants: performance status significantly better in IG (P = 0.04) Funding: supported by public funding (MURST), the Associacione Italiana per la Ricerca sul Cancro (AIRC) and Serono, Italy | |
Gebbia 1994.
| Methods | Design: 4‐arm parallel trial with placebo control No. of centres: 1 Recruitment and setting: University of Palermo, Italy Recruitment period: unclear Observation period: unclear Ethical approval: unclear | |
| Participants | No. of patients: 100 randomised, 96 evaluated (51 relevant to this review) Condition: advanced breast cancer (26), advanced head and neck cancer (12), advanced gastric cancer (2), inoperable ovarian cancer (4), recurrent or metastatic endometrium cancer (4), SCLC (6) Demographics: women: 64, men: 36; mean age (range): 58.6 (40‐75) years Informed consent: yes | |
| Interventions | Interventional treatment: thymopentin; dose/schedule: 50 mg i.m. every other day starting two days after application of chemotherapy until the beginning of the next cycle Control treatment: placebo (sodium chloride solution) Basic treatment: breast cancer: 5‐FU 400 mg/m² (d1‐3), FA 100 mg/m² (d1‐3), mitoxantrone 24 mg/m² (d3) or 5‐FU 400 mg/m² (d1‐3), FA 100 mg/m² (d1‐3), cyclophosphamide 500 mg/m² (d1), epidoxorubicin 120 mg/m² (d1); SCLC, head and neck cancer, endometrium cancer: cisplatin 80 mg/m²(d1), vinorelbine 25‐30 mg/m² (d1, d8); gastric cancer: according to EAP regime; ovarian cancer: carboplatin 300 mg/m² (d1), cyclophosphamide 500 mg/m² (d1), epidoxorubicin 90 mg/m² (d1) | |
| Outcomes | Outcome measures: toxicity (AEs of chemo‐/radiotherapy), safety (AEs of thymic peptides) | |
| Notes | ||
Gish 2009.
| Methods | Design: 2‐arm open‐label trial with a no‐treatment control group No. of centres: 4 Recruitment and setting: California Pacific Medical Center, San Francisco; Henry Ford Health System, Detroit; University of Florida College of Medicine, Gainesville; Metropolitan Liver and Gastroenterology Center Fairfax Recruitment period: unclear Observation period: 72 weeks (24 weeks treatment and 48 weeks post‐treatment monitoring); 30 months for survival Ethical approval: unclear | |
| Participants | No. of patients: 28 randomised, 25 evaluated Condition: unresectable HCC, stage I‐III (Okuda), Demographics: women: 6, men: 22; mean age (SD): IG: 59 (±9.1), CG: 60 (±6.7) Informed consent: unclear | |
| Interventions | Interventional treatment: thymosin α1; dose/schedule: 1.6 mg s.c., 5x/week for 24 weeks Control treatment: no treatment Basic treatment: TACE with doxorubicin or cisplatin (according to participating site´s guidelines) | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), safety (AEs of thymic peptides), other | |
| Notes | Method: small pilot study, sample size calculation was performed based on tumour response, accordingly 18 patients would have been required in each arm Outcomes: side effects of chemotherapy were not scored using standardized criteria Funding: supported by SciClone Pharmaceuticals | |
GISOT 1987.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: 153 Recruitment and setting: inpatients of 153 hospitals, Italy Recruitment period: unclear Observation period: 3 months Ethical approval: unclear | |
| Participants | No. of patients: 650 randomised, 609 evaluated Condition: solid tumors Demographics: unclear Informed consent: unclear | |
| Interventions | Interventional treatment: thymopentin; dose/schedule: 50 mg s.c. 3x/week; for 4 weeks Control treatment: no treatment Basic treatment: chemotherapy or radiotherapy (not further specified by the author) | |
| Outcomes | Outcome measures: survival, toxicity (AEs of chemo‐/radiotherapy) | |
| Notes | Participants: the trial included in total three groups of patients at risk of infections: elderly people (> 65 years) affected by chronic bronchitis (n=519), patients with solid tumours undergoing chemo‐ or radiotherapy (n=650), patients with HIV infection and lymphoadenopathy syndrome (LAS) (n=250) Outcomes: side effects of chemotherapy were not scored using standardized criteria | |
Gonnelli 1995.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: 1 Recruitment and setting: Institute of Internal Medicine and Division of Medical Oncology, University of Siena, Italy Recruitment period: unclear Observation period: 6 months Ethical approval: yes | |
| Participants | No. of patients: 40 randomised, 36 evaluated Condition: breast cancer, patients with bone metastasis and at least one measurable osteolytic lesion Demographics: median age (range): IG: 59 (47‐71), CG: 61 (43‐70) years Informed consent: yes | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 50 mg i.m. daily for 6 months Control treatment: no treatment Basic treatment: 5‐fluoruracil 500 mg/m² (d1), epirubicin 50 mg/m² (d1), cyclophosphamide 500 mg/m² (d1), or: 5‐fluoruracil 400 mg/m² (d1‐5), folinic acid 200 mg/m² (d1‐5); mitomycin C 5 mg/m² (d3‐5); every 3 weeks | |
| Outcomes | Outcome measures: response, toxicity (AEs of chemo‐/radiotherapy), other | |
| Notes | Method: according to a sample size calculation 60 patients would have been required, but accrual was finished earlier due to loss of funding Funding: thymostimulin was supplied by Serono, Italy | |
Guzman 1988.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: unclear Recruitment and setting: Medical Institute Oncology Service and Guernes Center, Buenos Aires, Argentina Recruitment period: 12/83‐12/85 Observation period: up to 42 months Ethical approval: unclear | |
| Participants | No. of patients: 32 randomised, 32 evaluated Condition: colorectal cancer, Dukes B2, C1, C2 after surgery; colon cancer (29), rectal cancer (4) Demographics: women: 15, men: 17; mean age: women: 58.8, men: 61.8 years Informed consent: unclear | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 25 mg/m² i.m. every chemotherapy cycle (d9‐13,17,19, 24, 26) Control treatment: no treatment Basic treatment : 5‐fluoruracil 600 mg/m² i.v. (d1,d8), lomustine 60 mg/m² p.o. (d1); every 3 weeks; for 6 months | |
| Outcomes | Outcome measures: survival, toxicity (AEs of chemo‐/radiotherapy), other | |
| Notes | Participants: no reporting of distribution of risk factors between groups Outcomes: side effects of chemotherapy were not scored using standardized criteria | |
Iaffaioli 1994.
| Methods | Design: 2‐arm parallel with placebo control No. of centers: unclear Recruitment and setting: unclear Recruitment period: 04/89‐02/92 Observation period: approximately 5 months Ethical approval: yes | |
| Participants | No. of patients: 69 randomised, 69 evaluated Condition: NSCLC, stage IIIA and B Demographics: men: 51, women: 18; age: patients under or 65 years: 39, patients over 65 years: 30 Informed consent: yes | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 1 mg/kg; daily; after 2nd cycle: 3x/week, until end of treatment Control treatment: placebo (not further described) Basic treatment: radiochemotherapy: 24 fractions of 1.60 Gy 2x/day up to 38.4 G, followed within 14 days by one cycle of chemotherapy: carboplatin 250 mg/m² (d1), etoposide 100 mg/m² (d1), mitomycin C 8 mg/m² (d1‐3), followed within 14 days by radiotherapy: 12 fractions of 1.6 Gy 2x/day up to 19.2 Gy, thereafter 5 cycles of chemotherapy | |
| Outcomes | Outcome measures: toxicity (AEs of chemo‐/radiotherapy), other | |
| Notes | ||
Luzi 1984.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: 1 Recruitment and setting: unclear Recruitment period: unclear Observation period: 2 years Ethical approval: unclear | |
| Participants | No. of patients: 50 randomised, 47 evaluated Condition: unresectable NSCLC, stage II or III Demographics: men: 45, women: 5; mean age (range): 59 (35‐70) years Informed consent: unclear | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 0.5 mg/kg/day i.m. daily (starting 5 days before radiotherapy); thereafter 1x/week for 6 months Control treatment: no treatment Basic treatment: 3 Gy on alternate days for 5 weeks; bleomycin 8 mg/m² 2x/week during radiotherapy, after 18 days: doxorubicin 40 mg/m² (d1, 28), vincristine 1.4 mg/m² (d1, 28), lomustine 65 mg/m² (d2, 57) | |
| Outcomes | Outcome measures: survival; response, other | |
| Notes | Funding: the trial was supported by a grant of the national research institute (Consiglio Nazionale delle Ricerche); thymostimulin was supplied by Serono, Rome | |
Macchiarini 1989.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: unclear Recruitment and setting: unclear Recruitment period: 01/86‐05/87 Observation period: up to 32 months; median 26.5 months Ethical approval: unclear | |
| Participants | No. of patients: 28 randomised, 26 evaluated Condition: SCLC limited (20) or extensive (6) disease Demographics: men: 25, women: 1, median age: 61 years Informed consent: yes | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 1 mg/kg/day i.m.; every chemotherapy cycle (d7‐14); thereafter in pats. with complete remission, 2x/week Control treatment: no treatment Basic treatment : cyclophosphamide 1 g/m² (d1), epidoxorubicin 60 mg/m² (d1), etoposide 120 mg/m² (d1‐4), or: cisplatin 60 mg/m² (d1), etoposide 120 mg/m² (d1‐4); every 3‐4 weeks, alternating the two regimes; for 6 cycles | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), other | |
| Notes | ||
Maio 2010.
| Methods | Design: open label 5‐arm parallel, patients stratified by site of distant metastasis (M1a,b,c) and lactate dehydrogenase (LDH) level No of centres: 64 Recruitment and setting: multi‐centre study across 8 European countries Recruitment period: 08/02‐01/06 Observation period: 14.9‐56.5 months Ethical approval: yes | |
| Participants | No. of patients: 488 patients evaluated (389 relevant to this review) Condition: melanoma, stage IV without brain metastasis Demographics: men: 250, women: 238 Informed consent: yes | |
| Interventions | Interventional treatment: thymosin α1; dose/schedule: IG1: 1.6 mg s.c.; IG2: 3.2 mg s.c. or IG3: 6.4 mg s.c. (d8‐11 and d15‐18) of every chemotherapy cycle Control treatment: no treatment Basic treatment: dacarbazine 800 mg/m² i.v. every 4 weeks for a maximum of six cycles; interferon α (IFNα) 3 MIU s.c. (d11,18) of every chemotherapy cycle | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), safety (AEs of thymic peptides), other | |
| Notes | Method: sample size calculation was performed, accordingly 95 patients would be required in each arm; the original study design scheduled a four arm trial, but after preliminary analysis, which suggested a dose‐response relation the protocol was extended to integrate a fifth arm with a dose of thymosin α1 dose of 6.4 mg; sample size calculation was performed, accordingly 95 patients would be required in each arm Participants: only 4 of the 5 arms had a control group in accordance with the selection criteria of the review (the other arm was controlled by IFNα) Outcomes: AEs of thymic peptides not reported differentially; side effects of chemotherapy were not scored using standardized criteria Funding: supported by sigma‐tau and SciClone Pharmaceuticals | |
Mantovani 1988.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: 2 Recruitment and setting: university hospital and regional hospital, Cagliari, Italy Recruitment period: unclear Observation period: 1 (20 patients) or 2 years (16 patients) Ethical approval: unclear | |
| Participants | No. of patients: 37 randomised, 37 evaluated Condition: breast cancer, patients with positive axillary lymph nodes, after radical or modified radical mastectomy Demographics: mean/median age (range): IG: 47.8/47.5 (31‐60), CG: 45.8/47 (32‐57) years Informed consent: unclear | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 60 mg/m²/day i.m or s.c. starting within 1 month after termination of chemotherapy: 7x/week (d1‐15), 2x/week (d16‐30), 1x/week (d31‐60), repetition until d180 with a pause of 30 days in‐between Control treatment: no treatment Basic treatment : CMF regime; for 6 cycles | |
| Outcomes | Outcome measures: survival, toxicity (AEs of chemo‐/radiotherapy), other | |
| Notes | Funding: supported by the National Research Council C.N.R. | |
Mustacchi 1994.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: multicentre Recruitment and setting: various hospitals in Italy (Trieste, Pavia, Cagliari, Napoli, Sassari, Vigevano, Pinerolo, Savona, Rome, Turin) Recruitment period: 02/90‐12/92 Observation period: unclear Ethical approval: unclear | |
| Participants | No. of patients: 235 randomised, 211 evaluated Condition: colorectal cancer stage IV Demographics: men: 107, women: 128; median age: IG: 61, CG: 60 years Informed consent: yes | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 1 mg/kg i.m.; daily during chemotherapy treatment, 3x/week between cycles Control treatment: no treatment Basic treatment: 5‐fluoruracil 375 mg/m² i.v. (60 min. infusion) (d1‐5), folinic acid 200 mg/m² i.v. (60 min. infusion) (d1‐5); every 3 weeks | |
| Outcomes | Outcome measures: survival, response, safety (AEs of thymic peptides), other | |
| Notes | Method: sample size calculation was performed for incidence of side effects and tumour response and resulted in the requirement of 130 patients per group Outcomes: side effects of chemotherapy were not scored using standardized criteria | |
Pavesi 1993.
| Methods | Design: 4‐arm parallel (2 chemotherapy regimes) with no treatment control No. of centres: 13 Recruitment and setting: 13 centres all over Italy Recruitment period: 01/90‐12/92 Observation period: unclear Ethical approval: unclear | |
| Participants | No. of patients: 296 randomised, 245 evaluated Condition: metastatic breast cancer (presumably stage IV) Demographics: unclear Informed consent: unclear | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: IG1/IG2: 1 mg/kg i.m. daily (during chemotherapy treatment), thereafter 3x/week (until progression or withdrawal) Control treatment: no additional treatment Basic treatment: 5‐fluoruracil 500 mg/m² i.v. (d1), epidoxorubicin 75 mg/m² i.v. (d1), cyclophosphamide 500 mg/m² i.v. (d1); every three weeks or: folinic acid 200 mg/m² (d1‐5), 5‐fluoruracil 370 mg/m² (d1‐5), epidoxorubicin 75 mg/m² (d1), cyclophosphamide 500 mg/m² (d1); every three weeks | |
| Outcomes | Outcome measures: survival; response, other | |
| Notes | This study has not been published in full text (February 2010) and was performed by the Italian Cooperative Trials Group Participants: unclear how many patients were allocated to which arm | |
Salvati 1984.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: 1 or 2 Recruitment and setting: Hospital C. Forlanini and Clinic of Respiratory Diseases, University of Rome, Italy Recruitment period: unclear Observation period: 6 months Ethical approval: unclear | |
| Participants | No. of patients: 46 randomised, 40 evaluated Condition: SCLC, limited (34) or extensive (12) disease Demographics: men: 42, women: 4; median age (range): 57 (46‐71) years Informed consent: unclear | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 1 mg/kg/day; 1st cycle (d4‐10), 2nd‐4th cycle (d4‐6), 5th‐9th cycle (d4, 5) Control treatment: no treatment Basic treatment: methotrexate 40 mg/m² i.v. (d1), doxorubicin 40 mg/m² i.v. (d1), cyclophosphamide 400 mg/m² i.v. (d1), nitrosourea 30 mg/m² i.v., (d1); every 3 weeks; for 6 months or until progression | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), other | |
| Notes | Participants: distribution of risk factors between groups not reported Funding: thymostimulin supplied by Serono, Rome | |
Sanchiz 1996.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group No. of centres: 1 Recruitment and setting: Department of Radiotherapy and Oncology, Clinica Platon, Barcelona, Spain Recruitment period: 06/92‐12/93 Observation period: one cycle of chemotherapy Ethical approval: unclear | |
| Participants | No. of patients: 54 randomised, 54 evaluated Condition: metastatic breast cancer Demographics: median age (range): IG: 46 (38‐54), CG: 46 (32‐54) years Informed consent: yes | |
| Interventions | Interventional treatment: thymostimulin; dose/schedule: 50 mg/day i.m.; every cycle (d2‐16) Control treatment: no treatment Basic treatment: mitoxantrone 28 mg/m² i.v., supportive treatment: G‐CSF 5 µg/kg s.c. (d2‐16) | |
| Outcomes | Outcome measures: response, toxicity (AEs of chemo‐/radiotherapy) | |
| Notes | ||
Scher 1988.
| Methods | Design: 2‐arm parallel trial with a no‐treatment control group; patients stratified by performance status and disease extent No. of centres: 2 Recruitment and setting: Memorial Sloan‐Kettering Cancer Center, Cornell University Medical College, New York, USA Recruitment period: 05/79‐ 05/82 Observation period: approximately 25 to 60 months Ethical approval: unclear | |
| Participants | No. of patients: 91 randomised, 80 evaluated Condition: SCLC limited (32) or and extensive disease (48) Demographics: men: 59, women: 32; median age (range): IG: 59 (35‐73), CG: 53 (32‐72) years Informed consent: unclear | |
| Interventions | Interventional treatment: thymosin fraction 5; dose/schedule: 60 mg/m² s.c.; 2x/week from the start of induction therapy through the completion of radiotherapy Control treatment: no treatment Basic treatment: induction therapy: 1st and 3rd cycle: cyclophosphamide 1200 mg/m² (d1), doxorubicin 50 mg/m² (d1), vincristine 1.2 mg/m² (d1, 8); 2nd and 4th cycle: cisplatin 60 mg/m² (d1) and etoposide 120 mg/m² (d4, 6, 8); consolidation therapy: cyclophosphamide 500 mg/m² (d1,14), vincristine 1,4 mg/m² (d1,14) along with radiation therapy in patients with limited disease; maintenance therapy was started 10 weeks after completion of radiotherapy in patients who had achieved complete remission, others started after hematologic recovery: 1st cycle: lomustine 60 mg/m² p.o. (d1), methotrexate 30 mg/week for 4 weeks, procarbazine 100 mg/m² p.o. (d1‐14); 2nd cycle cyclophosphamide 1000 mg/m² (d42) and doxorubicin 30 mg/m²(d42); 3rd cycle: vincristine 1,2 mg/m² d63, cisplatin 50 mg/m² d63 and etoposide 120 mg/m² (d67, 69, 71); radiotherapy with 2.5 Gy/day up to 45 Gy to primary site and anterior mediastinum (patients with LD); 3 Gy/day up to 30 Gy whole brain radiation (all patients) | |
| Outcomes | Outcome measures: survival, response, toxicity (AEs of chemo‐/radiotherapy), safety (AEs of thymic peptides), other | |
| Notes | Method: sample size calculation was performed, accordingly 80 patients would be required in order to detect a 25% increase in complete remission rate Funding: supported in part by the American Cancer Society and the National Institutes of Health (NIH); thymosin fraction 5 was supplied by Hoffmann‐La Roche | |
Schulof 1985.
| Methods | Design: double blind 3‐arm parallel with placebo control No. of centres: 1 Recruitment and setting: Washington University Medical Center, Washington D.C., USA Recruitment period: 11/80‐01/83 Observation period: 1 year for relapse; all patients were followed up until death; median 40 weeks (8‐108) Ethical approval: unclear | |
| Participants | No. of patients: 42 randomised, 41 evaluated Condition: locally advanced NSCLC, patients who had received radiotherapy because of either an unresectable tumour or incomplete resection (R1 or R2); patients with progression under radiotherapy were not included Demographics: men: 26, women: 15; mean age (SD): IG1: 57.3 (± 9.2), IG2: 52.8 (± 8.5), CG: 55.6 (± 10.5) years Informed consent: yes | |
| Interventions | Interventional treatment: thymosin α1; dose/schedule: IG1: placebo daily for 14 days; thereafter 900 µg/m²/day s.c., 2x/week as maintenance therapy; IG2: 900µg/m²/day, for 14 days as loading dose, thereafter placebo 2x/week as maintenance therapy; administration was initiated one week after completion of radiotherapy for a period of up to 1 year or until relapse Control treatment: placebo (mannitol powder reconstituted in bicarbonate diluent, provided in same coded vials as thymosin α1): daily for 14 days; maintenance therapy: 2x/week Basic treatment: radiotherapy: 2 Gy/day 5x/week for 6‐8 weeks to mediastinum and primary lesion, patients with prior resection of tumour received irradiation only to mediastinum | |
| Outcomes | Outcome measures: survival, safety (AEs of thymic peptides), other | |
| Notes | Participants: imbalance in gender distribution and proportion of patients with resection of primary (IGs 11/28, CG 1/13) which was also discussed by the authors Funding: supported by the National Cancer Institute (NCI) and Hoffmann‐La Roche | |
Wara 1981.
| Methods | Design: open label 2‐arm parallel No. of centres: 1 Recruitment and setting: Department of Radiation Oncology and Pediatrics, University of California, San Francisco, USA Recruitment period: 4 years before publication Observation period: min. 8 months, median 2 years. Ethical approval: unclear | |
| Participants | No. of patients: 76 randomised, 75 evaluated Condition: squamous cell cancer of head and neck, stage II‐IV Demographics: unclear Informed consent: unclear | |
| Interventions | Interventional treatment: thymosin fraction 5; dose/schedule: 60 mg/m²: daily for 10 days; thereafter 2x/week for 50 weeks Control treatment: no treatment Basic treatment: radiotherapy with 50‐60 Gy | |
| Outcomes | Outcome measures: survival, other | |
| Notes | Interventions: thymosin fraction 5 was supplied by Hoffmann‐La Roche | |
Outcomes which were not relevant to this review are indicated as 'other'. This includes immunologic parameters, dose modifications of chemotherapy or radiotherapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Azizi 1984 | Patients received neither chemotherapy nor radiotherapy |
| Bernengo 1983 | No sufficient outcome data reported |
| Cartia 1990 | No sufficient outcome data reported |
| Chen 2000 | Outcome assessment not according to eligibility criteria of the review |
| De Maria 1993 | Only immune parameters reported |
| Denaro 1994 | Only immune parameters reported |
| Holowiecki 1984 | Not randomised for purified thymic extract |
| Iaffaioli 1988 | Outcome assessment not according to eligibility criteria of the review |
| Kreuser 1998 | Registered randomised controlled trial, yet unpublished; manufacturer contacted, but no data provided |
| Liberati 1998 | Only immune parameters reported |
| Mantovani 1995 | Only immune parameters reported |
| Migeod 1985 | Only immune parameters reported |
| Munno 1995 | Only immune parameters reported |
| Quang‐Xing 2001 | Outcome assessment not according to eligibility criteria of the review |
| Shoham 1988 | No concomitant chemotherapy or radiotherapy |
| Surico 1992 | Outcome assessment not according to eligibility criteria of the review |
| Tetti 1987 | Outcome assessment not according to eligibility criteria of the review |
Characteristics of studies awaiting assessment [ordered by study ID]
Dollinger 2010.
| Methods | Prospective randomised, placebo‐controlled, double blind, multicentre clinical phase III trial |
| Participants | 135 patients with locally advanced or metastasised HCC (Karnofsky >=60% ‐ Child‐Pugh <=12) |
| Interventions | Thymostimulin 75mg s.c. 5x per week or placebo |
| Outcomes | Primary endpoint was 12‐month survival, secondary endpoints overall survival (OS), time to progression (TTP), tumour response, safety and quality of life |
| Notes | Current Controlled Trials ISRCTN64487365 |
Contributions of authors
MH and EW had full access to all data in the review and takes responsibility for the integrity of the data and the accuracy of the analysis. Study concept and design: KB, MH, SM, EW. Inclusion and exclusion of studies: KB, MH, SM, EW. Acquisition of data: KB, MH, SM, EW. Data entry and plausibility check: EW, MH. Analysis and interpretation of data: MH, SM, EW. Drafting of the manuscript: MH, EW with contributions from all other review authors. Statistical analysis: MH, SM, EW, MZ. Study supervision: MH.
Sources of support
Internal sources
None, Other.
External sources
AG Biologische Krebstherapie, Deutsche Krebshilfe (70‐301), Germany.
Dr. Ernst und Anita Bauer‐Stiftung, Nürnberg, Germany.
Cochrane Gynecologic Cancer Group, Bath, UK.
Declarations of interest
None known. It is certified that there are no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (for example employment, consultancy, stock ownership, honoraria).
Deceased
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Airoldi 1987 {published data only}
- Airoldi M, Gabriele P, Penani F, Brando V, Melano A. Chemioterapia versus chemioimmunoterapia con Timostimulina nei carcinomi avanzati del cavo orale: risultati clinici di uno studio randomizzato. Recenti Progressi in Medicina 1987;78 Suppl 6:67‐70. [Google Scholar]
Bedikian 1984 {published data only}
- Bedikian AY, Patt YZ, Murphy WK, Umsawasadi T, Carr DT, Hersh EM, et al. Prospective evaluation of thymosin fraction V immunotherapy in patients with non‐small cell lung cancer receiving vindesine, doxorubicin, and cisplatin (VAP) chemotherapy. American Journal of Clinical Oncology 1984;7(5):399‐404. [DOI] [PubMed] [Google Scholar]
Canovas 1988 {published data only}
- Canovas Fernandez A, Gonzalez de Zarate P, Alonso Alonso J, Aguirre Errasti C. [Thymostimulin as an adjuvant to antineoplastic chemotherapy. Experience with patients with myeloma and lymphoma]. Revista Clinica Espanola 1988;183(7):340‐3. [PubMed] [Google Scholar]
Canovas 1991 {published data only}
- Canovas Fernandez A, Alonso Alonso J, Gonzalez de Zarate P, Garcia Masdevall MD, Rinon Martinez‐Gallo M, Aguirre Errasti C. [The use of thymostimulin in lymphoma and myeloma patients]. Anales de Medicina Interna 1991;8(2):69‐73. [PubMed] [Google Scholar]
Cheng 2004 {published data only}
- Cheng S, Mengchao W, Han C, Feng S, Jiahe Y, Wenming C, et al. Combination transcatheter hepatic arterial chemoembolization with thymosin alpha1 on recurrence prevention of hepatocellular carcinoma. Hepatogastroenterology 2004;51(59):1445‐7. [PubMed] [Google Scholar]
Cohen 1979 {published data only}
- Chretien PB, Lipson SD, Makuch R, Kenady DE, Cohen MH, Minna JD. Thymosin in cancer patients: in vitro effects and correlations with clinical response to thymosin immunotherapy. Cancer Treatment Reports 1978;62(11):1787‐90. [PubMed] [Google Scholar]
- Cohen MH, Chretien PB, Ihde DC, Fossieck BE, Makuch R, Bunn PA, et al. Thymosin fraction V and intensive combination chemotherapy. Journal of the American Medical Association 1979;241:1813‐5. [DOI] [PubMed] [Google Scholar]
- Lipson SD, Chretien PB, Makuch R, Kenady DE, Cohen MH. Thymosin immunotherapy in patients with small cell carcinoma of the lung: correlation of in vitro studies with clinical course. Cancer 1979;43(3):863‐70. [DOI] [PubMed] [Google Scholar]
Del Giacco 1988 {published data only}
- Giacco GS, Mantovani G, Cengiarotti L, Puxeddu G, Pischedda A, Tucci A, et al. Secondary immunodeficiency in advanced lung cancer: effects of chemotherapy plus thymostimulin in immunodepressed patients: updating results. International Journal of Tissue Reactions 1984;6(6):499‐504. [PubMed] [Google Scholar]
- Giacco GS, Mantovani G, Pilidi G, et al. Thymic factors in lung cancer. In: Nagel G, Schioppacassi G, Schuff‐Werner P editor(s). Thymic hormones in oncology. Roma: Ares Serono Symposia, 1988:149‐56. [Google Scholar]
De Serdio 1997 {published data only}
- Serdio JL, Villar A, Alvarez IE, Gil‐Cubelo JA, Suner M, Hernandez R, et al. [The effects of thymostimulin in a protocol of concurrent hyperfractionated carboplatin and irradiation]. Acta Otorrinolaringologica Espanola 1997;48(6):487‐92. [PubMed] [Google Scholar]
Federico 1995 {published data only}
- Federico M, Gobbi PG, Mretti G, Avanzini P, Renzo N, Cavanna L, et al. Effects of thymostimulin with combination chemotherapy in patients with aggressive Non‐Hodgkin lymphoma. A report from the Italian Lymphoma Study Group (GISL). American Journal of Clinical Oncology 1995;18(1):8‐14. [DOI] [PubMed] [Google Scholar]
Gebbia 1994 {published data only}
- Gebbia V, Valenza R, Testa A, Cannata G, Borsellino N, Gebbia N. A prospective randomized trial of thymopentin versus granulocyte‐colony stimulating factor with or without thymopentin in the prevention of febrile episodes in cancer patients undergoing highly cytotoxic chemotherapy. Anticancer Research 1994;14(2B):731‐4. [PubMed] [Google Scholar]
Gish 2009 {published data only}
- Gish RG, Gordon SC, Nelson D, Rustgi V, Rios I. A randomized controlled trial of thymalfasin plus transarterial chemoembolization for unresectable hepatocellular carcinoma. Hepatology International 2009;3:480‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
GISOT 1987 {published data only}
- GISOT (Gruppo Italiano di Studio Sugli Ormoni Timici). [Efficacy and tolerability of thymopoietin in patients at risk of infection]. Recenti Progressi in Medicina 1987;78(7‐8):358‐64. [PubMed] [Google Scholar]
Gonnelli 1995 {published data only}
- Gonnelli S, Petrioli R, Cepollaro C, Palmieri R, Aquino A, Gennari C. Thymostimulin in association with chemotherapy in breast cancer patients with bone metastases. Clinical Drug Investigation 1995;9(2):79‐87. [Google Scholar]
Guzman 1988 {published data only}
- Guzman Machado CA, Verruno L, Ribas MC. Adjuvant therapy with thymic factors in solid tumours. In: Nagel G, Schioppacassi G, Schuff‐Werner P editor(s). Thymic Hormones in Oncology. Roma: Ares serono Symposia, 1988:121‐32. [Google Scholar]
Iaffaioli 1994 {published data only}
- Iaffaioli RV, Caponigro F, Facchini G, Tortoriello A, Panelli G, Muto P, et al. Immunological and clinical effects of thymostimulin in patients with locally advanced non‐small‐cell lung cancer treated with combined chemoradiotherapy. Drug Investigation 1994;7:209‐14. [Google Scholar]
Luzi 1984 {published data only}
- Luzi G, Tropea F, Siminara R, et al. Clinical and immunological evaluation in non‐resectable lung cancer patients treated with thymostimulin. Serono Symposia Publications 1984;16:309‐20. [Google Scholar]
Macchiarini 1989 {published data only}
- Macchiarini P, Danesi R, Tacca M, Angeletti CA. Effects of thymostimulin on chemotherapy‐induced toxicity and long‐term survival in small cell lung cancer patients. Anticancer Research 1989;9:193‐6. [PubMed] [Google Scholar]
Maio 2010 {published and unpublished data}
- Camerini R, Mackiewicz A, Testori A, Trefzer U, Jassem J, Ferraresi V, et al. A large first‐line, randomized, dose‐finding, phase II study on thymosin‐alpha 1 (T‐alpha 1) plus dacarbazine (DTIC) with or without interferon‐alpha (IFN‐alpha) compared to DTIC plus IFN‐alpha in stage IV melanoma. Tumor response and survival results. Journal of Clinical Oncology 2007;25(18S):8535. [Google Scholar]
- Camerini R, Mackiewicz A, Testori A, Trefzer U, Jassem J, Ferraresi V, et al. A large first‐line, randomized, dose‐finding, phase II study on thymosin‐alpha 1 (T‐alpha 1) plus dacarbazine (DTIC) with or without interferon‐alpha (IFN‐alpha) compared to DTIC plus IFN‐alpha in stage IV melanoma. Tumor response and survival results. Powerpoint presentation ASCO Annual Meeting June 1‐5, 2007, Chicago.
- Maio M, Mackiewicz A, Testori A, Trefzer U, Ferraresi V, Jassem J, et al. Large randomized study of thymosin {alpha} 1, interferon alfa, or both in combination with dacarbazine in patients with metastatic melanoma. Journal of Clinical Oncology 2010;[Epub ahead of print]:1780‐7. [DOI] [PubMed] [Google Scholar]
Mantovani 1988 {published data only}
- Mantovani G, Coiana A, Zucca MV, Sanna GP, Floris C, Sulis E, et al. [Results of a controlled clinical protocol in the treatment of patients with operable breast carcinoma with positive axillary lymph nodes. Comparison of a chemotherapeutic regimen with a chemo‐immunotherapeutic regimen]. Recenti Progressi in Medicina 1988;79(12):513‐8. [PubMed] [Google Scholar]
Mustacchi 1994 {published data only}
- Mustacchi G, Pavesi L, Milani S, Iaffaioli V, Caraco A, Comella G, et al. High‐dose folinic acid (FA) and fluorouracil (FU) plus or minus thymostimulin (TS) for treatment of metastatic colorectal cancer: results of a randomized multicenter clinical trial. Anticancer Research 1994;14:617‐20. [PubMed] [Google Scholar]
Pavesi 1993 {published data only}
- Pavesi L. Fluorouracil (F), with and without high‐dose folinic acid (HDFA) plus epirubicin (E) and cyclophosphamide (C): FEC versus HDFA‐FEC plus or minus thymostimulin (TS) in metastatic breast cancer: results of a multicenter study. European Journal of Cancer 1993; Vol. 29 A Suppl:77.
Salvati 1984 {published data only}
- Salvati F, Pallotta G, Antilli A, Nunziati F, Marinis F, Lucchesi M. [MACC plus Thymostimulin (TP‐1 Serono) therapy of small cell bronchogenic carcinoma. Clinico‐immunologic evaluation of the results of a randomized trial]. Giornale Italiano di Chemiotherapia 1984;31:185‐9. [PubMed] [Google Scholar]
Sanchiz 1996 {published data only}
- Sanchiz F, Milla A. A randomised study comparing granulocyte‐colony stimulating factor (G‐CSF) with G‐CSF plus thymostimulin in the treatment of heamatological toxicity in patients with advanced breast cancer after high dose mitoxantrone therapy. European Journal of Cancer 1996;32 A:52‐6. [DOI] [PubMed] [Google Scholar]
Scher 1988 {published data only}
- Scher HI, Shank B, Geller N, Pinsky C, Gralla R, Kelsen D, et al. Randomized trial of combined modality therapy with and without thymosin fraction V in the treatment of small cell lung cancer. Cancer Research 1988;48(6):1663‐70. [PubMed] [Google Scholar]
Schulof 1985 {published data only}
- Schulof RS, Lloyd MJ, Cleary PA, Palaszynski SR, Mai DA, Cox JW, et al. A randomized trail to evaluate the immunorestorative properties of synthetic thymosin‐a in patients with lung cancer. Journal of Biological Response Modifiers 1985;4(2):147‐58. [PubMed] [Google Scholar]
Wara 1981 {published data only}
- Wara WM, Neely MH, Amman AJ, Wara DW. Biologic modifications of immunologic parameters in head and neck cancer patients with thymosin fraction V. In: Goldstein AL, Chirigos MA editor(s). Lymphokines and Thymic Hormones: their potential utilization in cancer therapeutics. Raven Press, New York, 1981:257‐62. [Google Scholar]
References to studies excluded from this review
Azizi 1984 {published data only}
- Azizi E, Brenner HJ, Shoham J. Postoperative adjuvante behandlung von patienten mit malignem melanom durch die thymusfaktor thymostimulin. Arzneimittelforschung 1984;34(II):1043‐6. [Google Scholar]
Bernengo 1983 {published data only}
- Bernengo MG, Fra P, Lisa F, Meregalli M, Zina G. Thymostimulin therapy in melanoma patients: correlation of immunologic effects with clinical course. Clinical Immunology and Immunopathology 1983;28(3):311‐24. [DOI] [PubMed] [Google Scholar]
- Bernengo MG, Lisa F, Meregalli M, Matteis A, Zina G. The prognostic value of T‐lymphocyte levels in malignant melanoma. A five‐year follow‐up. Cancer 1983;52(10):1841‐8. [DOI] [PubMed] [Google Scholar]
Cartia 1990 {published data only}
- Cartia GL. [Evaluation of the effects of thymopentin on the incidence of leucopenia in patients treated with chemotherapy for breast carcinoma] Valutazione degli effetti di Timopentina sull`incidenza di leucopenia in pazienti trattate con chemioterapia per carcinom mammario. Minerva Medica 1990;81(11):815‐7. [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen J, Huang F, Zheng X, Chen L, Qin S, Li G, et al. Thymosin alpha 1 positively alters quality of life in chemotherapy treated patients. Proceedings American Society of Clinical Oncology 2000; Vol. 1.
De Maria 1993 {published data only}
- Maria D, Falchi AM, Armaroli L, Balli M, Bortolus R, Busutti L, et al. T‐lymphocyte subsets in cancer patients undergoing radiotherapy and their recovery after thymostimulin treatment. A cooperative study. RAYS 1993;18:438‐53. [PubMed] [Google Scholar]
Denaro 1994 {published data only}
- Denaro A, Stivala F, Mazzarino MC. [Immunologic study on patients with head and neck cancer treated with thymopentin associated with surgery, chemotherapy and radiotherapy]. Acta Otorhinolaryngologica Italica 1994;14:611‐25. [PubMed] [Google Scholar]
Holowiecki 1984 {published data only}
- Holowiecki J, Duraj M, Rudzka E, Jarczok K, Krawczyk M, Holowiecka B, et al. Cooperative randomized studies on the treatment of adult acute leukemia in Poland. A comparison of two remission induction regimes and two maintenance regimes for AML. Folia Haematologica 1984;111(2):201‐7. [PubMed] [Google Scholar]
Iaffaioli 1988 {published data only}
- Iaffaioli RV, Frasci G, Tortora G, Ciardiello F, Nuzzo F, Scala S, et al. Effect of thymic extract "thymostimulin" on the incidence of infections and myelotoxicity during adjuvant chemotherapy for breast cancer. Thymus 1988;12:69‐75. [PubMed] [Google Scholar]
Kreuser 1998 {published data only}
- Kreuser ED. [Randomised, double‐blind trial to compare low‐molecular thymic peptides, 5‐fluorouracil and folic acid with placebo, 5‐fluorouracil and folic acid in patients with metastasised colorectal cancer] [Randomisierter, doppelblinder Therapievergleich von niedermolekularen Thymus‐ Peptiden, 5‐Fluorouracil und Folinsäure versus Placebo, 5‐Fluorouracil und Folinsäure bei Patienten mit metastasiertem kolorektalen Karzinom im Stadium UICC IV (pT1‐4, N0‐2, M1)]. Internet ("This trial was identified via internet databases. In this multicenter study patients were randomised to receive thymuvocal or placebo. The pharmaceutical company (DR Schmidt) supposed to be in charge of did not provide information about this study".) 1998.
Liberati 1998 {published data only}
- Liberati AM, Ballatori E, Fizzotti M, Schippa M, Cini L, Cinieri S, et al. A randomized trial to evaluate the immunorestorative properties of thymostimulin in patients with Hodgkin`s disease in complete remission. Cancer Immunology, Immunotherapy 1998;26:87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantovani 1995 {published data only}
- Mantovani G, Ghiani M, Bianchi A, Curreli L, Contini L, Maccio A, et al. [The immunological assessment of a combined therapeutic approach (chemo‐ + radiotherapy +/‐ surgery) including immunotherapy in neoplasms of the head and neck area at an advanced stage] [Valutazione immunologica di un approccio terapeutico combinato (chemio + radioterapia +/‐ chirurgia) includente l'immunoterapia nelle neoplasie del distretto cervico‐facciale in stadio avanzato.]. Recenti Progressi in Medicina 1995;86:8‐16. [PubMed] [Google Scholar]
Migeod 1985 {published data only}
- Migeod F. [Results of a prospective randomised study on the immunologic effects of the xenogenic organ lysates Ney Tumorin‐Sol and Neythymun compared to placebo] [Ergebnisse einer prospektiv randomisierten Studie über immunologische Wirkungen der xenogenen Organlysate Ney Tumorin‐Sol und Neythymun in Vergleich zu einem Placebo]. Therapiewoche 1985;35, 26A:115‐20. [Google Scholar]
Munno 1995 {published data only}
- Munno I, Marinaro M, Gesario A, Cannuscio B, Michel Y, Paulling E. Immunomodulatory effects of alpha interferon and thymostimulin in patients with neoplasias. Clinical and Diagnostic Laboratory Immunology 1995;2:503‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quang‐Xing 2001 {published data only}
- Quang‐Xing Ni, Fu DI, Yu XJ, Xu J, Hua JM, Chen W, et al. Efficacy of thymalfasin on cellular immune function and chemotherapy induced toxicity in pancreatic cancer. Proceedings American Society of Clinical Oncology 2001.
Shoham 1988 {published data only}
- Shoham J. The effect of the thymic factor thymostimulin on survival rate, immune function and resistance to infection in experimental and clinical cancer. Thymic Hormones in Oncology. Ares Serono Symposia, 1988:45‐57. [Google Scholar]
Surico 1992 {published data only}
- Surico N, Tavassoli K. Effect of immunostimulating therapy on the immunocompetent system in breast carcinoma. Panminerva Medica 1992;34:172‐80. [PubMed] [Google Scholar]
Tetti 1987 {published data only}
- Tetti M, Tomassone W, Milani R, Paste M, Vaudano G, Rolfo A, et al. Uso di un farmaco immunomodulatore nella prevenzione della comparsadi episodi infettivi a carico delle vie urinarie in pazienti affette da neoplasie ginecologiche e sottoposte a ciclo TCT. Recenti Progressi in Medicina 1987;78 Suppl 6:51‐3. [Google Scholar]
References to studies awaiting assessment
Dollinger 2010 {published data only}
- Dollinger MM, Lautenschlaeger C, Lesske J, Tannapfel A, Wagner AD, Schoppmeyer K, et al. Thymostimulin versus placebo for palliative treatment of locally advanced or metastasised hepatocellular carcinoma: a phase III clinical trial. BMC Cancer 2010;10:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Billich 2002
- Billich A. Thymosin alpha1. SciClone Pharmaceuticals. Current Opinion in Investigational Drugs 2002;3(5):698‐707. [PubMed] [Google Scholar]
Bodey 2000
- Bodey B, Bodey BJr, Siegel SE, Kaiser HE. Review of thymic hormones in cancer diagnosis and treatment. International Journal of Immunopharmacology 2000;22(4):261‐73. [DOI] [PubMed] [Google Scholar]
Cha 2003
- Cha HJ, Jeong MJ, Kleinman HK. Role of thymosin beta4 in tumor metastasis and angiogenesis. Journal of the National Cancer Institute 2003;95(22):1674‐80. [DOI] [PubMed] [Google Scholar]
Chretien 1978
- Chretien PB, Lipson SD, Makuch R, Kenady DE, Cohen MH, Minna JD. Thymosin in cancer patients: in vitro effects and correlations with clinical response to thymosin immunotherapy. Cancer Treatment Reports 1978;62(11):1787‐90. [PubMed] [Google Scholar]
Costanzi 1977
- Costanzi JJ, Gagliano RG, Delaney F, Harris N, Thurman GB, Sakai H, et al. The effect of thymosin on patients with disseminated malignancies. A phase I study. Cancer 1977;40(1):14‐9. [DOI] [PubMed] [Google Scholar]
CTC 2009
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) ‐ Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/About.html May 28, 2009.
Dunn 2002
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology 2002;3(11):991‐8. [DOI] [PubMed] [Google Scholar]
Ernst 1997
- Ernst E. Thymus therapy for cancer? A criteria‐based, systematic review [see comments]. European Journal of Cancer 1997;33(4):531‐5. [DOI] [PubMed] [Google Scholar]
Falchetti 1977
- Falchetti R, Bergesi G, Eshkol A, Cafiero G, Adorini L, Caprino L. Pharmacological and biological properties of a calf thymus extract (TP‐1). Drugs Under Experimental and Clinical Research 1977;3:39‐46. [Google Scholar]
Goldstein 1966
- Goldstein AL, Slater FD, White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proceedings of the National Academy of Sciences of the United States of America 1966;56(3):1010‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 1977
- Goldstein AL, Low TL, McAdoo M, McClure J, Thurman GB, Rossio J, et al. Thymosin alpha1: isolation and sequence analysis of an immunologically active thymic polypeptide. Proceedings of the National Academy of Sciences of the United States of America 1977;74(2):725‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 1979
- Goldstein G, Scheid MP, Boyse EA, Schlesinger DH, Wauwe J. A synthetic pentapeptide with biological activity characteristic of the thymic hormone thymopoietin. Science 1979;204(4399):1309‐10. [DOI] [PubMed] [Google Scholar]
Goldstein 2009
- Goldstein AL, Goldstein AL. From lab to bedside: emerging clinical applications of thymosin à1. Expert Opinion on Biological Therapy 2009;9(5):593‐608. [DOI] [PubMed] [Google Scholar]
Grothey 1998
- Grothey A, Düppe J, Hasenburg A, Voigtmann R. Anwendung alternativmedizinischer methoden durch onkologische patienten. Deutsche Medizinische Wochenschrift 1998;123:923‐9. [DOI] [PubMed] [Google Scholar]
Hannappel 2003
- Hannappel E, Huff T. The thymosins prothymosin à, parathymosin, and á‐thymosins: structure and function. Vitamins and Hormones. Academic press, 2003:2‐296. [DOI] [PubMed] [Google Scholar]
Hardell 1998
- Hardell L, Nordenstam M, Moqvist I, Lindberg L. A new study of patients with cancer in Umea alternative medicine is no alternative [Ny studie av cancerpatienter i Umea: alternativmedicin inget alternativ.]. Lakartidningen 1998;95(18):2092‐5. [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. Vol. available from www.cochrane‐handbook.org, The Cochrane Collaboration, 2009. [Google Scholar]
Kirkwood 2003
- Kirkwood BR, Sterne JAC. Essential medical statistics. Essential medical statistics. Oxford: Blackwell Science Ltd, 2003:Chapter 22. [Google Scholar]
Kullmer 1999
- Kullmer U, Stenger K, Milch W, Zygmunt M, Sachsse S, Münstedt K. Self‐concept, body image, and use of unconventional therapies in patients with gynaecological malignancies in the state of complete remission and recurrence. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1999;82(1):101‐6. [DOI] [PubMed] [Google Scholar]
Miller 1981
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting Results of Cancer Treatment. Cancer 1981;47(1):207‐14. [DOI] [PubMed] [Google Scholar]
Moschen 2001
- Moschen R, Kemmler G, Schweigkofler H, Holzner B, Dnser M, Richter R, et al. Use of alternative / complementary therapy in breast cancer patients ‐ a psychological perspective. Supportive Care in Cancer 2001;9:267‐74. [DOI] [PubMed] [Google Scholar]
Rost 1999
- Rost KL, Wierich W, Masayuki F, Tuthill CW, Horwitz DL, Herrmann WM. Pharmacokinetics of thymosin alpha1 after subcutaneous injection of three different formulations in healthy volunteers. International Journal of Clinical Pharmacology and Therapeutics 1999;37(1):51‐7. [PubMed] [Google Scholar]
Schlesinger 1975
- Schlesinger DH, Goldstein G, Scheid MP, Boyse EA. Chemical synthesis of a peptide fragment of thymopoietin II that induces selective T cell differentiation. Cell 1975;5(4):367‐70. [DOI] [PubMed] [Google Scholar]
Schulof 1985a
- Schulof RS. Thymic peptide hormones: basic properties and clinical applications in cancer. Critical Reviews in Oncology/Hematology 1985;3(4):309‐76. [DOI] [PubMed] [Google Scholar]
Sehouli 2000
- Sehouli J. How frequently do female patients with gynecological cancers use unconventional cancer therapies?. Gynecologic Oncology 2000;79(2):336‐7. [DOI] [PubMed] [Google Scholar]
Seybold 1950
- Seybold WD, McDonald JR, Clagett OR, Good CA. Tumors of the thymus. Journal of Thoracic Surgery 1950;20(2):195‐215. [PubMed] [Google Scholar]
Singh 1998
- Singh VK, Biswas S, Mathur KB, Haq W, Garg SK, Agarwal SS. Thymopentin and splenopentin as immunomodulators. Current status. Immunologic Research 1998;17(3):345‐68. [DOI] [PubMed] [Google Scholar]
Soellner 1997
- Soellner W, Zingg‐Schir M, Rumpold G, Fritsch P. Attitude toward alternative therapy, compliance with standard treatment, and need for emotional support in patients with melanoma. Archives of Dermatology 1997;133(3):316‐21. [DOI] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205‐16. [DOI] [PubMed] [Google Scholar]


