Abstract
Background
Intussusception is a common abdominal emergency in children with significant morbidity. Prompt diagnosis and management reduces associated risks and the need for surgical intervention. Despite widespread agreement on the use of contrast enema as opposed to surgery for initial management in most cases, debate persists on the appropriate contrast medium, imaging modality, pharmacological adjuvant, and protocol for delayed repeat enema, and on the best approach for surgical management for intussusception in children.
Objectives
To assess the safety and effectiveness of non‐surgical and surgical approaches in the management of intussusception in children.
Search methods
We searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library; Ovid MEDLINE (1950 to September 2016); Ovid Embase (1974 to September 2016); Science Citation Index Expanded (via Web of Science) (1900 to September 2016); and BIOSIS Previews (1969 to September 2016).
We examined the reference lists of all eligible trials to identify additional studies. To locate unpublished studies, we contacted content experts, searched the World Health Organization International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov (September 2016), and explored proceedings from meetings of the British Association of Paedatric Surgeons (BAPS), the American Soceity of Pediatric Surgery, and the World Congress of Pediatric Surgery.
Selection criteria
We included all randomised controlled trials comparing contrast media, imaging modalities, pharmacological adjuvants, protocols for delayed repeat enema, and/or surgical approaches for the management of intussusception in children. We applied no language, publication date, or publication status restrictions.
Data collection and analysis
Two review authors independently conducted study selection and data extraction and assessed risk of bias using a standardised form. We resolved disagreements by consensus with a third review author when necessary. We reported dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs). We analysed data on an intention‐to‐treat basis and evaluated the overall quality of evidence supporting the outcomes by using GRADE criteria.
Main results
We included six randomised controlled trials (RCTs) with a total of 822 participants. Two trials compared liquid enema reduction plus glucagon versus liquid enema alone. One trial compared liquid enema plus dexamethasone versus liquid enema alone. Another trial compared air enema plus dexamethasone versus air enema alone, and two trials compared use of liquid enema versus air enema. We identified three ongoing trials.
We judged all included trials to be at risk of bias owing to omissions in reported methods. We judged five of six trials as having high risk of bias in at least one domain. Therefore, the quality of the evidence (GRADE) for outcomes was low. Interventions and data presentation varied greatly across trials; therefore meta‐analysis was not possible for most review outcomes.
Enema plus glucagon versus enema alone
It is uncertain whether use of glucagon improves the rate of successful reduction of intussusception when compared with enema alone (reported in two trials, 218 participants; RR 1.09, 95% CI 0.94 to 1.26;low quality of evidence). No trials in this comparison reported on the number of children with bowel perforation(s) nor on the number of children with recurrent intussusception.
Enema plus dexamethasone versus enema alone
Use of the adjunct, dexamethasone, may be beneficial in reducing intussusception recurrence with liquid or air enema (two trials, 299 participants; RR 0.14, 95% CI 0.03 to 0.60; low quality of evidence). This equates to a number needed to treat for an additional beneficial outcome of 13 (95% CI 8 to 37). It is uncertain whether use of the adjunct, dexamethasone, improves the rate of successful reduction of intussusception when compared with enema alone (reported in two trials, 356 participants; RR 1.01, 95% CI 0.92 to 1.10;low quality of evidence).
Air enema versus liquid enema
Air enema may be more successful than liquid enema for reducing intussusception (two trials, 199 participants; RR 1.28, 95% CI 1.10 to 1.49; low quality of evidence). This equates to a number needed to treat for an additional beneficial outcome of 6 (95% CI 4 to 19). No trials in this comparison reported on the number of children with bowel perforation(s) or on the number of children with recurrent intussusception nor any intraoperative complications, such as bowel perforation, or other adverse effects. Only one trial reported postoperative complications, but owing to the method of reporting used, a quantitative analysis was not possible. We identified no studies that exclusively evaluated surgical interventions for management of intussusception.
Authors' conclusions
This review identified a small number of trials that assessed a variety of interventions. All included trials provided evidence of low quality and were subject to serious concerns about imprecision, high risk of bias, or both. Air enema may be superior to liquid enema for successfully reducing intussusception in children; however, this finding is based on a few studies including small numbers of participants. Dexamethasone as an adjuvant may be more effective in reducing intussusception recurrence rates following air enema or liquid enema, but these results are also based on a few studies of small numbers of participants. This review highlights several points that need to be addressed in future studies, including reducing the risk of bias and including relevant outcomes. Specifically, surgical trials are lacking, and future research is needed to address this evidence gap.
Keywords: Child, Humans, Air, Dexamethasone, Dexamethasone/therapeutic use, Enema, Enema/methods, Gastrointestinal Agents, Gastrointestinal Agents/therapeutic use, Glucagon, Glucagon/therapeutic use, Glucocorticoids, Glucocorticoids/therapeutic use, Intestinal Perforation, Intestinal Perforation/etiology, Intussusception, Intussusception/surgery, Intussusception/therapy, Postoperative Complications, Randomized Controlled Trials as Topic, Recurrence, Secondary Prevention, Secondary Prevention/methods
Plain language summary
Management of intussusception in children
Review question
How is intussusception best managed in children?
Background
Intussusception is a medical emergency that occurs in children when a part of the bowel 'telescopes' (folds) into another part of the bowel. This causes pain, vomiting, and obstruction, preventing passage. If left untreated, the bowel can perforate, resulting in passage of its contents into the abdominal cavity, causing further complications. In rare cases, these events can cause death. Prompt diagnosis and management reduces associated risks and the need for surgery.
Once intussusception is diagnosed, most doctors agree on the use of enema as initial treatment. This procedure involves introducing a substance (air or liquid) into the bowel, via the rectum, with a particular pressure that reduces the 'telescoped' bowel into its normal position.
Debate persists on specifics regarding what type of substance should be used for the enema, how the substance is visualised during the process, whether extra medications should be given to enhance treatment, and how one should deal with treatment failure, as well as the best approach to surgical management of intussusception in children.
Study characteristics
Evidence is current to September 2016. We identified six randomised studies, with a total of 822 participants, that explored the management of intussusception in children and assessed different types of interventions. We also identified three ongoing trials.
Main results
The main outcome was the number of children with a successfully reduced intussusception. Furthermore, outcomes included the number of children returning with a recurrent intussusception and evaluation of harms (adverse events) resulting from the interventions.
Evidence from two studies suggests that using air for the enema to reduce intussusception is superior to using liquid for the enema. Evidence from two studies also suggests that giving the child with intussusception a steroid medication, such as dexamethasone, may reduce the recurrence of intussusception, irrespective of whether liquid or air is used for the enema. We identified only sparse information on intraoperative and postoperative complications and on other adverse events.
Quality of the evidence
Of the six trials identified, we considered all to be potentially biased owing to lack of detail in reporting of how each study was undertaken. We found lack of consistency in how outcomes were defined and measured. All included studies were subject to serious concerns of imprecision based on few events, wide confidence intervals,or high risk of bias, Overall, we concluded that the quality of evidence provided by these studies was low, and that the real effects may differ significantly from those noted in these studies. Further research is needed to help doctors better understand the most effective way to manage intussusception in children.
Summary of findings
Summary of findings for the main comparison. Enema plus glucagon versus enema alone.
| Enema plus glucagon versus enema alone summary of findings table | |||||
| Patient or population: children with intussusception Setting: single centre, in‐patient setting Intervention: liquid enema plus glucagon Comparison: liquid enema alone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with liquid enema alone | Risk with liquid enema plus glucagon | ||||
| Successfully reduced intussusception | Study population | RR 1.09 (0.94 to 1.26) | 218 (2 studies) | Lowa | |
| 739 per 1000 | 805 per 1000 (694 to 931) | ||||
| Moderate | |||||
| 649 per 1000 | 707 per 1000 (610 to 818) | ||||
| Bowel perforation(s) | Outcome not reported in any studies | ||||
| Recurrent intussusception (follow‐up: 6 months) |
Outcome not reported in any studies | ||||
| Bowel resection | Outcome not reported in any studies | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
aDowngraded two levels for serious concerns for high risk of selection, attrition, and performance bias
Summary of findings 2. Enema plus dexamethasone versus enema alone.
| Enema plus dexamethasone versus enema alone summary of findings table | |||||
| Patient or population: children with intussusception Setting: single centre, in‐patient setting Intervention: enema plus dexamethasone Comparison: enema alone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with enema alone | Risk with enema plus dexamethasone | ||||
| Successfully reduced intussusception | Study population | RR 1.01 (0.92 to 1.10) | 356 (2 studies) | Lowa | |
| 157 per 1000 | 159 per 1000 (144 to 173) | ||||
| Moderate | |||||
| 771 per 1000 | 779 per 1000 (710 to 849) | ||||
| Bowel perforation(s) | Study population | RR 2.63 (0.11 to 62.66) | 75 (1 study) | Lowb,c | |
| 125 per 1000 | 329 per 1000 (14 to 1000) | ||||
| Moderate | |||||
| 125 per 1000 | 48 per 1000 (3 to 995) | ||||
| Recurrent intussusception (follow‐up: 6 months) |
Study population | RR 0.14 (0.03 to 0.60) | 299 (2 studies) | Lowa | |
| 69 per 1000 | 10 per 1000 (2 to 42) | ||||
| Moderate | |||||
| 370 per 1000 | 52 per 1000 (11 to 222) | ||||
| Bowel resection | Study population | RR 0.88 (0.19 to 4.06) | 75 (1 study) | Lowb,c | |
| 86 per 1000 | 75 per 1000 (16 to 348) | ||||
| Moderate | |||||
| 375 per 1000 | 330 per 1000 (71 to 1000) | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
aDowngraded two levels for serious concerns for high risk of attrition and performance bias
bDowngraded one level for serious imprecision (95% CI is wide and includes null effect)
cDowngraded one level for concerns for high risk of performance bias
Summary of findings 3. Air enema versus liquid enema.
| Air enema versus liquid enema summary of findings table | |||||
| Patient or population: children with intussusception Setting: single centre, in‐hospital setting Intervention: air enema Comparison: liquid contrast enema | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with liquid contrast enema | Risk with air enema | ||||
| Successfully reduced intussusception | Study population | RR 1.28 (1.10 to 1.49) | 199 (2 studies) | Lowa | |
| 677 per 1000 | 867 per 1000 (745 to 1000) | ||||
| Moderate | |||||
| 712 per 1000 | 911 per 1000 (783 to 1000) | ||||
| Bowel perforation(s) | Outcome not reported in any studies | ||||
| Recurrence of intussusception (follow‐up: 6 months) |
Outcome not reported in any studies | ||||
| Bowel resection | Outcome not reported in any studies | ||||
| Postoperative complication(s) | Outcome not reported in any studies | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
aDowngraded two levels for serious concerns for high risk of selection, performance, and detection bias
Background
Description of the condition
Intussusception in children is a medical emergency that requires prompt diagnosis and management. It occurs when a segment of bowel (the intussusceptum) invaginates or telescopes into the lumen of another segment of bowel (the intussuscipiens). Both small and large bowel can be involved, but the most common kind of intussusception arises at the junction between the ileum and the caecum and is called ileocaecal intussusception (Loukas 2011). When untreated, intussusception may cause bowel perforation, peritonitis, and shock (Ko 2007). Mortality is rare, with the USA reporting a stable mortality rate of 2.1 per 1 million live births between 1997 and 2007 (Buttery 2011; Davis 2003; Desai 2012; Parashar 2000). Case fatality rates are higher in developing countries, particularly in Africa (9.4%), than in other regions (< 1%). This may be due to delays in treatment, a higher incidence of non‐viable bowel, and lack of adequate medical care (Iwase 2010; Jiang 2013; Meier 1996).
Intussusception is one of the most common abdominal emergencies for children younger than age three (Applegate 2009). Its incidence varies from 0.24 to 2.4 per 1000 live births (Bines 2002; Eng 2012; Fischer 2004; Huppertz 2006; Samad 2014), although evidence suggests that this rate is higher in developing countries (Ugwu 2000). Boys are affected two to eight times more often than girls (Bines 2002), and peak incidence occurs between five and nine months of age (Daneman 2003; Samad 2012). Vaccination against rotavirus has been shown to increase the risk of intussusception. Currently, the monovalent rotavirus vaccine (Rotarix, GlaxoSmithKline, Abbott Park, North Carolina, USA) accounts for an increase of 5.3 cases of intussusception per 100,000 infants receiving the two doses of vaccine (Weintraub 2014). However, each year, rotavirus infection causes gastroenteritis, resulting in 592,000 deaths among children younger than five years of age, with 82% of deaths reported in developing countries (Parashar 2000). Hence, rotavirus vaccination is considered beneficial. A much stronger link between intussusception and an older rotavirus vaccine (RotaShield, Wyeth Laboratories, Marietta, Pennsylvania, USA) (Kramarz 2001; Murphy 2001; Peter 2002; Soares‐Weiser 2004) led to its worldwide withdrawal in 1999.
The cause of intussusception is often idiopathic (Staatz 1998), although any condition that produces pathological lead points (lesions in the bowel) can cause intussusception (Loukas 2011). Of these conditions, lymphoid hypertrophy seems to be the most common (Applegate 2009; Staatz 1998), implicating a viral or bacterial origin for most cases (Nylund 2010; Okimoto 2011; Parashar 2000; Staatz 1998). Other potential causes of pathological lead points include Meckel's diverticulum, duplication cyst, polyp, and lymphoma (Daneman 2003; Daneman 2004). Compared with idiopathic intussusception, intussusception caused by lead points is associated with poorer outcomes and may not be amenable to standard treatment owing to different intussusception locations (Applegate 2009; Loukas 2011).
Diagnosis is challenging because the symptoms of intussusception are wide‐ranging and non‐specific (Beasley 1988); the classic triad of symptoms associated with intussusception comprises vomiting, colicky abdominal pain, and bloody stool, but this triad is noted in less than half of cases (Blanch 2007; Lehnert 2009; Samad 2012). Three studies found that physicians correctly diagnosed intussusception in less than half of initial clinical encounters (Beasley 1988; Blanch 2007; Budwig 1994). Following successful reduction of the intussusception, early recurrence is rare, with rates ranging from 2.7% to 5.4% (Beres 2014; Gray 2014). Diagnostic delay increases the risk of surgical intervention (Lehnert 2009), thus emphasising the importance of prompt and effective management.
Description of the intervention
Non‐surgical management of intussusception in children consists of contrast enema (Applegate 2009; Daneman 2004; Ito 2012; Ko 2007), which involves instilling contrast medium (i.e. air, saline, or barium) into the rectum via a rectal tube to reduce the intussusceptum by increasing intraluminal pressure (Davis 2003). Fluoroscopy or, in the case of liquid contrast media, ultrasonography can guide the procedure and monitor the reduction. Ultrasonography avoids the radiation exposure associated with fluoroscopy and is an effective diagnostic tool (del‐Pozo 1999).
Pharmacological adjuvants can facilitate non‐surgical management, but their efficacy remains controversial. For example, glucagon is an antispasmodic adjuvant used by 10% to 21% of surveyed practitioners (Cachat 2012; Katz 1992; Meyer 1992; Rosenfeld 1999). It provides analgesia (Lappas 1995) and reduces colonic muscle tone (Skucas 1994). However, a recent narrative review suggests that glucagon does not improve the rate of reduction in the non‐surgical management of intussusception (Cachat 2012). Other adjuvants include antibiotics (Ein 2006; Moss 2000; Pepper 2012). One prospective study concluded that the actual risk of bacteraemia following fluoroscopically guided air reduction is low (Somekh 1996), although two other studies reported an elevated risk for intussusception following antibiotic administration (Hviid 2009; Spiro 2003).
Surgical management entails open laparotomy with manual reduction of the intussusception, although case series and retrospective studies show that laparoscopy may be safer and just as effective and may result in shorter hospitalisation (Bailey 2007; Bonnard 2008; Kia 2005; Sklar 2014). Surgical management is generally indicated only if peritonitis, bowel perforation, or shock occurs; when appropriate radiological facilities are unavailable; or when contrast enema fails (American College of Radiology 2007; Daneman 2004). However, because non‐surgical management may be associated with lower morbidity and shorter hospitalisation (Bruce 1987), delayed repeat attempts at contrast enema may be preferred to surgical management (Gonzalez‐Spinola 1999; Navarro 2004; Sandler 1999).
Why it is important to do this review
Intussusception is a common abdominal emergency in children with significant morbidity. Despite widespread agreement on the use of contrast enema for initial management, debate persists on the appropriate contrast medium, imaging modality, pharmacological adjuvant, and protocol to be used for delayed repeat enema (i.e. duration of delay and number of repeated attempts) (Beasley 1998; Daneman 2004; Davis 2003; del‐Pozo 1999; Littlewood 1998; Liu 1986; Schmit 1999). Debate also surrounds the best approach for its surgical management (i.e. open laparotomy vs laparoscopy). Prior reviews of non‐surgical management (Applegate 2009; Cachat 2012; Daneman 2003; Gray 2014; Ko 2007) are narrative in nature. In contrast to narrative reviews, systematic reviews use transparent, objective, and reproducible methods to locate and assess studies (Borenstein 2009). To the best of our knowledge, this is the first systematic review of non‐surgical and surgical approaches in the management of intussusception in children.
Objectives
To assess the safety and effectiveness of non‐surgical and surgical approaches in the management of intussusception in children.
Methods
Criteria for considering studies for this review
Types of studies
We considered for inclusion all randomised controlled trials (RCTs) comparing contrast media, imaging modalities, pharmacological adjuvants, protocols for delayed repeat enema, surgical approaches, or other curative techniques for the management of intussusception in children. Both quasi‐RCTs and cluster‐RCTs were eligible for inclusion.
Types of participants
Any child, younger than age 18, with a clinical diagnosis of intussusception as determined by study authors. For this review, we considered intussusception at any point in the gastrointestinal tract distal to the pylorus. Although the Brighton Collaboration established a validated and standardised case definition (Bines 2004a; Bines 2004b; Kohl 2008; Tapiainen 2006), this definition has been used only in the context of rotavirus vaccine post licensure monitoring. We have not used the Brighton Collaboration case definition in assessing eligibility of participants for inclusion in this review.
Types of interventions
We included all trials that compared different contrast media, imaging modalities, pharmacological adjuvants, protocols for delayed repeat enemas, and/or surgical approaches.
Types of outcome measures
When possible, we extracted the following primary and secondary outcome measures. We assessed outcomes at the time points reported by study authors unless otherwise noted. As recurring intussusception is associated with various outcomes (Applegate 2009), we conducted our assessment by using the participant as the unit of analysis. If we identified cluster trials, we planned to involve a statistician to ensure that we did not create unit of analysis errors.
Primary outcomes
Number of children with successfully reduced intussusception, characterised by radiologically confirmed passage of contrast media into the ileum
Number of children with radiologically confirmed or clinically suspected (intraoperative or endoscopic) bowel perforation(s)
Number of children with recurrent intussusception (recurrence is defined as occurring after a minimum of 12 hours following a successful reduction)
Secondary outcomes
Number of children who underwent a bowel resection (defined by any transection of the lumen, with removal of a segment of bowel)
Number of children with a diagnosis of sepsis (defined as life‐threatening organ dysfunction caused by a dysregulated host response to infection (Singer 2016))
Radiation exposure (measured in milli‐Sieverts (mSv)) resulting from intervention
Length of hospitalisation (measured in days) associated with intervention
Intraluminal pressure (measured in mm Hg) used to achieve reduction
Number of attempts required to achieve successful reduction
Length of operation (measured in minutes) in the case of surgical intervention
Number of intraoperative complications (as defined by study authors) in the case of surgical intervention
Number of postoperative complications (as defined by study authors) in the case of surgical intervention
Number of intraoperative conversions (i.e. open laparotomy required) in the case of laparoscopic intervention
Time to resumption of full diet (measured in hours), as defined by study authors
Search methods for identification of studies
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished randomised controlled trials with no language or date of publication restrictions. We searched the following electronic databases for relevant studies.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library (Appendix 1).
MEDLINE Ovid (1950 to 16 September 2016) (Appendix 2).
Embase Ovid (1974 to 16 September 2016) (Appendix 3).
Science Citation Index (via Web of Science) (1900 to 16 September 2016) (Appendix 4).
BIOSIS Previews (1969 to 16 September 2016) (Appendix 5).
Our subject search in MEDLINE followed the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE (Lefebvre 2011). Similarly, our subject search in Embase followed sensitivity‐maximising strategy as recommended by Cochrane (Wong 2006).
Searching other resources
Two review authors (SG and RGM) searched the reference lists of all eligible trials and contemporary reviews to identify further trials. To identify unpublished studies, we contacted content experts and searched the World Health Organization International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en) and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (http://www.clinicaltrials.gov) up to 16 September 2016. We also examined proceedings from meetings of the British Association of Paedatric Surgeons (BAPS), the American Society of Pediatric Surgery, and the World Congress of Pediatric Surgery (2009‐2015).
Data collection and analysis
Selection of studies
Two review authors (SG and RGM) screened titles and abstracts for study eligibility using the inclusion criteria of this review. When necessary, we read the full text of the paper or requested additional data from study authors. A third review author (ACW) adjudicated disagreements about study eligibility. We were not blinded to study details during this process.
Data extraction and management
Two review authors (SG and RGM) independently extracted data and assessed risk of bias using a standardised data extraction form. We resolved disagreements by consensus, involving a third review author (ACW) when required. Review authors were not blinded to study details during this process.
We extracted the following data.
General information: study author(s), title, source, contact address, country of study, language of publication, year of publication, any author conflicts of interest, study setting (e.g. hospital emergency department, specialised paediatric hospital).
Study characteristics and eligibility for review: study design, randomisation method, allocation concealment, recruitment method, duration of trial, study location, length of follow‐up, operator allocation, any obvious concerns of bias.
Participants: inclusion and exclusion criteria, age, gender, presence of pathological lead points, anatomical location of intussusception, criteria used to diagnose intussusception, total number of participants, country of origin, number of dropouts or withdrawals and reasons if recorded.
Interventions: number of participants for each intervention, a detailed description of interventions and comparison interventions including, when relevant, type, dose, concentration, and duration of application.
Outcomes: specific outcomes reported and rates of recurrence, perforation, resection, sepsis, and, when applicable, operative complications and intraoperative conversions.
We entered relevant data into Review Manager software (RevMan version 5.3) (RevMan 2014).
We contacted study authors via email when data were unclear or missing. Study authors provided no new information.
Assessment of risk of bias in included studies
We assessed risk of bias using the 'Risk of bias' tool of the Cochrane Collaboration, as detailed in Section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (see Appendix 6). We assessed the following domains: selection bias (due to inadequate random sequence generation or allocation concealment); performance bias (due to inadequate blinding of participants or personnel); detection bias (due to inadequate blinding of outcome assessment and data analysis); attrition bias (due to incomplete outcome data); reporting bias (due to selective reporting); and other potential biases. We planned to assess publication bias by visually inspecting funnel plots and using Egger's linear regression (minimum 10 studies required). When we assessed studies as having 'unclear risk' in any domain, we attempted to contact study authors for clarification.
We planned to perform sensitivity analyses using risk of bias as one of the sensitivity factors (see Subgroup analysis and investigation of heterogeneity).
Summary of findings
Two review authors (SG and RGM) assessed the overall quality of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Schünemann 2008) and presented these results in 'Summary of findings' tables. We resolved disagreements by consensus, involving a third review author (ACW) when required. In the 'Summary of findings' tables, we included all primary outcomes, as well as secondary outcomes, reported by included studies for the following comparisons: enema plus glucagon versus enema alone; enema plus dexamethasone versus enema alone; and air enema versus liquid enema. We calculated baseline risk using the event rate in the control group.
The GRADE system classifies the quality of evidence as one of four grades.
| Grade | Definition |
| High | Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate | Further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate |
| Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| Very low | Any estimate of effect is very uncertain |
We judged the quality of evidence according to the following factors.
| Downgrades the evidence |
| Risk of bias |
| Inconsistency of results |
| Indirectness of evidence |
| Imprecision |
| Publication bias |
We described results while taking into account the quality of evidence and the importance (size) of the effect as follows.
| Important benefit or harm | Less important benefit or harm | No important benefit/harm or null effect | |
| High‐quality evidence | Improves/decreases/prevents/leads to [outcome] | Improves slightly/decreases slightly/leads to slightly fewer (more) [outcome] | Results in little or no difference in [outcome] |
| Moderate‐quality evidence | Probably improves/decreases/prevents/leads to [outcome] | Probably improves slightly/decreases slightly/leads to slightly fewer (more) [outcome] | Probably leads to little or no difference in [outcome] |
| Low‐quality evidence | May improve/decrease/prevent/lead to [outcome] | May slightly improve/slightly decrease/lead to slightly fewer (more) [outcome] | May lead to little or no difference in [outcome] |
| Very low‐quality evidence | It is uncertain whether [intervention] improves, decreases, prevents, leads to [outcome] because the quality of the evidence is very low | ||
| No data or no studies | [Outcome] was not measured or was not reported, or no studies were found that evaluated the impact of [intervention] on [outcome] | ||
Measures of treatment effect
We conducted our analysis according to the guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We presented results for dichotomous data as summary risk ratios (RRs) with 95% confidence intervals (CIs) and as number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) as appropriate. NNTB and NNTH reflect the numbers of participants who need to be treated for an additional beneficial and harmful outcome, respectively. For continuous data, we planned to present results as mean differences (MDs), if outcomes were measured in the same way between trials. We planned to use standardised mean differences (SMDs) to combine studies that measured the same outcome but used different methods. For rate data, we planned to present results as rate ratios with 95% CIs, and for survival data, we planned to present results as hazard ratios (HRs) with 95% CIs.
Unit of analysis issues
As recurring intussusception is associated with differing outcomes (Applegate 2009), when possible we conducted our assessment with the participant as the unit of analysis. If we had identified cluster‐randomised trials, we had planned to involve a statistician, to ensure that we did not create unit of analysis errors. However, we did not identify any cluster‐randomised trials for this systematic review.
Dealing with missing data
We analysed data for all participants in the group to which they were allocated, regardless of whether they received the allocated intervention. If in the original reports, participants were not analysed in the group to which they were randomised, and if information in the trial report was sufficient, we attempted to restore these participants to the correct group, that is, we conducted intention‐to‐treat analysis when it was possible to do so. When data were missing, we sought clarification from the authors of the trial. When intention‐to‐treat analysis was not possible, we conducted available‐case analysis or per‐protocol analysis.
Assessment of heterogeneity
To deal with clinical heterogeneity, we analysed studies of each intervention and presented them separately. We conducted subgroup analyses when required to deal with variations in the study population age (Subgroup analysis and investigation of heterogeneity).
To deal with statistical heterogeneity, we used the I² statistic and Chi² statistics to measure the proportion of total variation in estimates of treatment effect that was due to heterogeneity beyond chance (Borenstein 2009; Higgins 2003). We judged statistical heterogeneity to be substantial for I² values greater than 50% or Chi² P values less than 0.10. In the case of substantial statistical heterogeneity, we planned to perform prespecified subgroup and sensitivity analyses (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We planned to investigate publication bias by visually assessing funnel plots for the primary outcome if the number of identified and included trials exceeded 10. However, this review included only six trials.
Data synthesis
We analysed data using Review Manager software (RevMan Version 5.3) (RevMan 2014). For trials judged to have similar interventions, populations, and outcomes, we used fixed‐effect model meta‐analysis, as random‐effects models produce poor estimates with small numbers of studies (Higgins 2011), and we considered a P value of 0.05 or less to be statistically significant.
Subgroup analysis and investigation of heterogeneity
We expected the following areas to contribute to study heterogeneity, and we planned to conduct subgroup analyses of relevant models when necessary.
Care setting. Different care settings, such as tertiary care centres, are associated with differing outcomes (Bratton 2001; Calder 2001; Rosenfeld 1999).
Participants with confirmed presence of pathological lead point. The presence of lead points is associated with differing outcomes (Loukas 2011).
Participants with previous intussusceptions. Recurrence is associated with different patient characteristics and outcomes (Applegate 2009).
Bowel structures involved in the intussusception. Intussusception involving different bowel structures (e.g. ileocaecal vs ileoileal) are associated with different outcomes (Loukas 2011).
Studies with high risk of bias. We identified these studies as having one or more domains judged 'high risk' by the risk of bias tool, as suggested in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Quasi‐randomised trials. These studies by their design fail to implement optimal sequence generation and so are prone to bias (Higgins 2011).
Age. Children younger than one year of age or older than three years of age are more likely to possess pathological lead points (Applegate 2009).
Geographical region. Regional differences in epidemiology, equipment availability, and operator experience are known (Beasley 1998; Liu 1986; Schmit 1999; Ugwu 2000).
We planned to assess differences among subgroups using analysis of variance (Altman 1996).
However, we could not perform any of the planned subgroup analyses owing to the limited number of included studies.
Sensitivity analysis
We planned to conduct sensitivity analysis when unforeseen or arbitrary decisions were made, as per the guidance provided in Section 9.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, we were unable to perform the planned sensitivity analysis owing to the limited number of included studies.
Results
Description of studies
We included six RCTs with a total of 822 participants (Essa 2011; Franken 1983; Hadidi 1999; Lin 2000; Meyer 1993; Mortensson 1984), and we identified three ongoing trials (El Fiky 2016; Mehraeen 2011; Zhang 2015). Please see Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies.
Results of the search
We outlined in Figure 1 (study flow diagram) the process of identifying RCTs for inclusion in the review.
1.
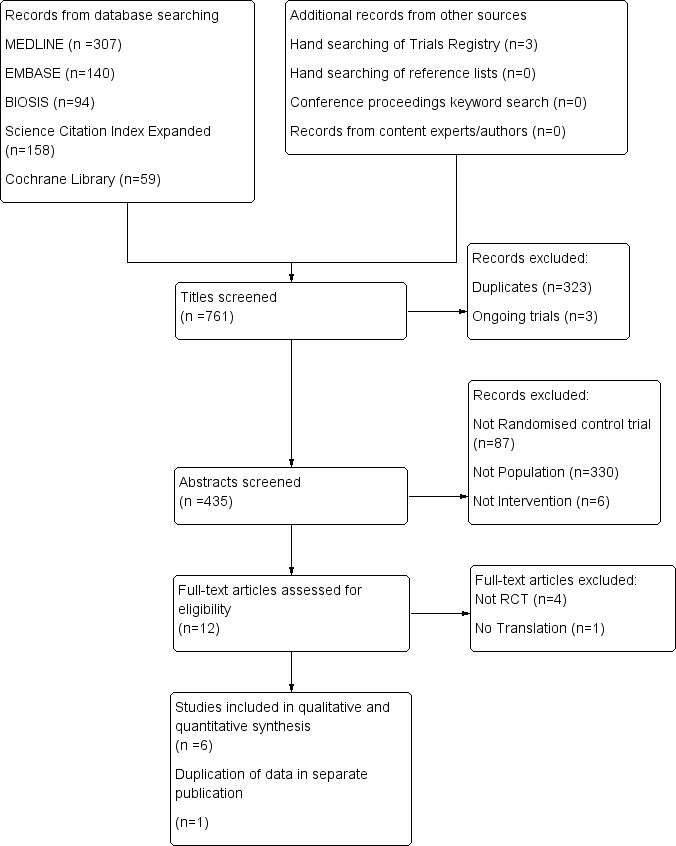
Study flow diagram for identification of randomised trials exploring management of intussusception in children.
Electronic searches of the Conchrane Central Register of Controlled Trials (n = 59), MEDLINE (n = 307), Embase (n = 140), BIOSIS (n = 94), and the Science Citation Index (n = 158) yielded a total of 758 publications. We identified three additional ongoing trials through trial registries and found no additional trials by searching conference proceedings and reference lists, or by contacting content experts. After exclusion of duplicates and ongoing trials, 435 unique records remained. Of these, we excluded 423 after reviewing titles and abstracts. We examined the full text of the remaining 12 publications and excluded five additional trials ‐ four because they were not RCTs (Diaz‐Aldagalan 2012; Guo 2010; Hsiao 1988; Morrison 2009) and one because we could not obtain a translation of the trial and classification is pending (Zhang 2014a). Two of the seven remaining publications were duplicates; thus we included them as one trial (Lin 2000). In summary, we included six RCTs (Essa 2011; Franken 1983; Hadidi 1999; Lin 2000; Meyer 1993; Mortensson 1984) in the review. These six completed trials were published in five different journals. Searches for ongoing trials revealed three (El Fiky 2016; Mehraeen 2011; Zhang 2015), for which no results were available.
No disagreements about trial selection among review authors required adjudication.
Included studies
Included trials assessed a wide range of treatments.
Essa 2011 compared use of saline enema plus dexamethasone versus saline enema alone in 75 participants.
Franken 1983 and Mortensson 1984 compared use of liquid contrast enema plus glucagon versus liquid contrast enema alone in 30 and 188 participants, respectively.
Hadidi 1999 and Meyer 1993 compared use of liquid contrast enemas versus air enemas in 147 and 101 participants, respectively.
Lin 2000 compared use of air enema plus dexamethasone versus air enema alone in 281 participants.
All six included trials recruited participants referred for management of intussusception in a hospital setting. Two of these studies were performed in the USA (Franken 1983; Meyer 1993). One trial was performed in Taiwan (Lin 2000), two in Egypt (Essa 2011; Hadidi 1999), and one in Sweden (Mortensson 1984).
Only one trial (Essa 2011) reported adverse outcomes for surgical interventions, including number of participants requiring manual reduction and number requiring bowel resection.
This review used subsets of data from two trials (Meyer 1993; Mortensson 1984). Meyer 1993 examined liquid enema versus air enema; however, not all participants who were initially randomised had intussusception at the time of intervention. Therefore, it was necessary to extrapolate data from those with confirmed intussusception. Mortensson 1984 conducted this study in three stages. We have included data only for the first stage, as this was the only stage that met our inclusion criteria (Characteristics of included studies).
Hadidi 1999 conducted a three‐arm trial to assess the efficacy of air, barium, and saline enemas. Review authors combined barium and saline into a liquid enema group for comparison with air enema.
Excluded studies
We excluded five full‐text articles (see Characteristics of excluded studies).
Risk of bias in included studies
Reporting of methods was incomplete for most trials, as shown in Figure 2 and Figure 3. We judged five trials as having at least one domain at high risk of bias, and we judged Franken 1983 as having unclear risk of bias.
2.
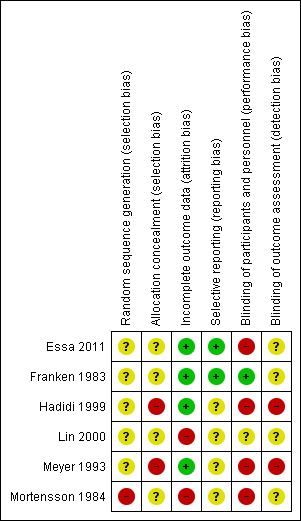
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
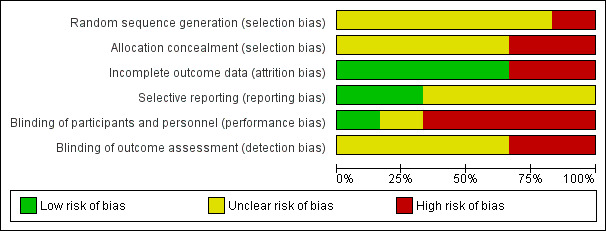
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
No study reported adequate sequence generation or adequate allocation concealment. Both Hadidi 1999 and Meyer 1993 used random number tables that may have allowed for prediction of intervention by participants. It is unclear whether this was adequate to ensure random sequence generation in Hadidi 1999; in Meyer 1993, the randomisation process was compromised by the need to extrapolate data for participants with confirmed intussusception; and Mortensson 1984 applied inadequate sequence generation by using birth dates to randomly allocate participants. The remaining studies (three studies for sequence generation and five for allocation concealment) used unclear methods. Both Essa 2011 and Franken 1983 referred to the random allocation used but provided no details.
Blinding
Owing to the nature of some treatments, blinding was not possible, for example, liquid versus air enema in Hadidi 1999 and Meyer 1993. Therefore, we reported four trials as having inadequate blinding of participants and personnel. One trial (Franken 1983) successfully blinded participants and personnel through the use of pre‐made identical appearing vials of drug and placebo. The remaining trial used unclear methods.
We judged two trials as having inadequate blinding of outcome assessors because treating personnel recorded the results and thus were unable to be blinded (Hadidi 1999; Meyer 1993). For the remaining four trials, it was unclear whether outcome assessors were study personnel (i.e. paediatricians, radiologists, or surgeons) or independent assessors.
Incomplete outcome data
Four studies adequately addressed incomplete outcome data (no missing data in the trials). Essa 2011 explicitly referred to reporting on all participants included in this trial, and Franken 1983, Hadidi 1999, and Meyer 1993 avoided attrition bias by randomising participants after completing an exclusion process. Two studies reported incomplete outcome data inadequately (Lin 2000; Mortensson 1984), when data were not available for unexplained reasons.
Selective reporting
We judged only two studies (Essa 2011; Franken 1983) as being free of selective reporting bias (all outcomes were reported). We judged the remaining four trials as having unclear risk. None of the trials included a protocol. Lin 2000 did not report how data were collected after participants were discharged, and Meyer 1993 and Mortensson 1984 did not report all expected outcomes.
Other potential sources of bias
No other biases were evident as judged by review authors (e.g. pharmaceutical funding).
We attempted to contact study authors to clarify all areas of unclear risk, but we received no replies and acquired no new information.
We could not assess publication bias as planned because of the small number of included studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Interventions and outcomes reported across trials varied greatly; therefore, meta‐analysis was not possible for many outcomes.
Enema plus glucagon versus enema alone
1. Primary outcomes
1.1 Number of children with successfully reduced intussusception
It is uncertain whether use of liquid enema plus glucagon improved the rate of successful reduction of intussusception when compared with enema alone because the quality of the evidence is low (reported in two trials, 218 participants; RR 1.09, 95% CI 0.94 to 1.26; I² = 0%; Analysis 1.1).
1.1. Analysis.
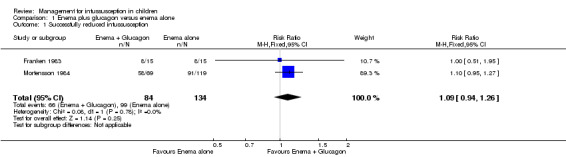
Comparison 1 Enema plus glucagon versus enema alone, Outcome 1 Successfully reduced intussusception.
1.2 Number of children with bowel perforation or perforations
This outcome was not reported for this comparison.
1.3 Number of children with recurrent intussusception
This outcome was not reported for this comparison.
2. Secondary outcomes
2.1 Number of children who undergo a bowel resection
This outcome was not reported for this comparison.
2.2 Number of children with a diagnosis of sepsis
This outcome was not reported in any trial.
2.3 Radiation exposure from intervention
This outcome was not reported in any trial.
2.4 Length of hospitalisation
This outcome was not reported in any trial.
2.5 Intraluminal pressure
This outcome was not reported in any trial.
2.6 Number of attempts required to achieve successful reduction
This outcome was not reported in any trial.
2.7 Length of operation, in the case of surgical intervention
This outcome was not reported in any trial.
2.8 Number of intraoperative complications
This outcome was not reported in any trial.
2.9 Number of postoperative complications
This outcome was not reported for this comparison.
2.10 Number of intraoperative conversions
This outcome was not reported in any trial.
2.11 Time to resumption of full diet
This outcome was not reported in any trial.
Enema plus dexamethasone versus enema alone
1. Primary outcomes
1.1 Number of children with successfully reduced intussusception
It is uncertain whether use of liquid enema plus dexamethasone improved the rate of successful reduction of intussusception when compared with enema alone because the quality of the evidence is low (reported in two trials, 356 participants; RR 1.01, 95% CI 0.92 to 1.10; I² = 0%; Analysis 2.1).
2.1. Analysis.
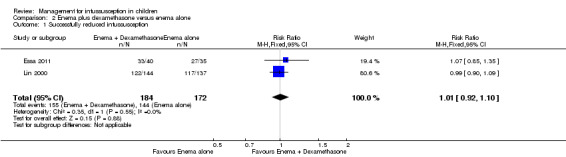
Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 1 Successfully reduced intussusception.
1.2 Number of children with bowel perforation or perforations
It is uncertain whether use of enema plus dexamethasone reduced the number of participants with bowel perforation or perforations because the quality of the evidence is low (reported in one trial, 75 participants; RR 2.63, 95% CI 0.11 to 62.66; Analysis 2.2).
2.2. Analysis.
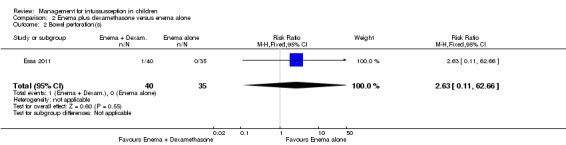
Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 2 Bowel perforation(s).
1.3 Number of children with recurrent intussusception
Treatment with enema plus dexamethasone compared with enema alone may reduce the recurrence rate of intussusception (reported in two trials, 299 participants; RR 0.14, 95% CI 0.03 to 0.60; I² = 0%; Analysis 2.3). This equates to an NNTB of 13 (95% CI 8 to 37).
2.3. Analysis.
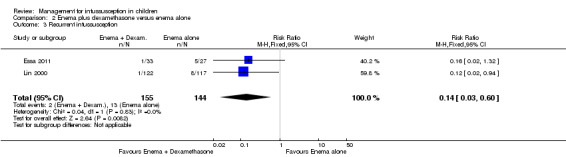
Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 3 Recurrent intussusception.
2. Secondary outcomes
2.1 Number of children who undergo a bowel resection
It is uncertain whether use of liquid enema plus dexamethasone reduced the number of participants who underwent bowel resection (an unwanted complication) (reported in one trial, 75 participants; RR 0.88, 95% CI 0.19 to 4.06; Analysis 2.4).
2.4. Analysis.
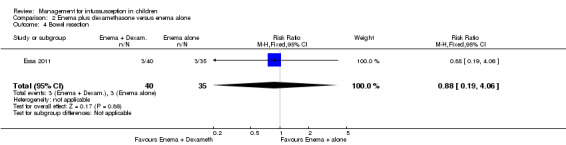
Comparison 2 Enema plus dexamethasone versus enema alone, Outcome 4 Bowel resection.
2.2 Number of children with a diagnosis of sepsis
This outcome was not reported for this comparison.
2.3 Radiation exposure from intervention
This outcome was not reported in any trial.
2.4 Length of hospitalisation
This outcome was not reported in any trial.
2.5 Intraluminal pressure
This outcome was not reported in any trial.
2.6 Number of attempts required to achieve successful reduction
This outcome was not reported in any trial.
2.7 Length of operation, in the case of surgical intervention
This outcome was not reported in any trial.
2.8 Number of intraoperative complications
This outcome was not reported in any trial.
2.9 Number of postoperative complications
Only one trial reported on postoperative complications (Essa 2011) when comparing use of enema plus dexamethasone versus enema alone. We did not perform a quantitative analysis of this outcome owing to poor reporting and high risk of bias. A small sample of 15 children underwent surgical intervention ‐ nine underwent manual reduction and the remaining six had a bowel resection. However, data on postoperative complications for the nine children undergoing manual reduction were not available. We contacted the study authors for clarification but received no response.
2.10 Number of intraoperative conversions
This outcome was not reported in any trial.
2.11 Time to resumption of full diet
This outcome was not reported in any trial.
Air enema versus liquid enema
1. Primary outcomes
1.1 Number of children with successfully reduced intussusception
Air enema may be superior to liquid enema for successfully reducing intussusception in children (reported in two trials, 199 participants; RR 1.28, 95% CI 1.10 to 1.49; I² = 0%; Analysis 3.1). This equates to an NNTB of 6 (95% CI 4 to 17).
3.1. Analysis.
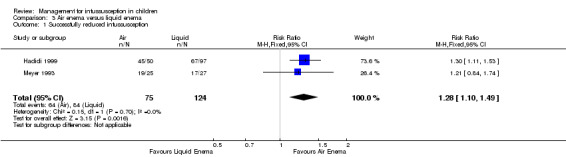
Comparison 3 Air enema versus liquid enema, Outcome 1 Successfully reduced intussusception.
1.2 Number of children with bowel perforation or perforations
This outcome was not reported for this comparison.
1.3 Number of children with recurrent intussusception
This outcome was not reported for this comparison.
2. Secondary outcomes
2.1 Number of children who undergo a bowel resection
This outcome was not reported for this comparison.
2.2 Number of children with a diagnosis of sepsis
This outcome was not reported in any trial.
2.3 Radiation exposure from intervention
This outcome was not reported in any trial.
2.4 Length of hospitalisation
This outcome was not reported in any trial.
2.5 Intraluminal pressure
This outcome was not reported in any trial.
2.6 Number of attempts required to achieve successful reduction
This outcome was not reported in any trial.
2.7 Length of operation, in the case of surgical intervention
This outcome was not reported in any trial.
2.8 Number of intraoperative complications
This outcome was not reported in any trial.
2.9 Number of postoperative complications
This outcome was not reported for this comparison.
2.10 Number of intraoperative conversions
This outcome was not reported in any trial.
2.11 Time to resumption of full diet
This outcome was not reported in any trial.
GRADE analysis indicated that the quality of evidence supporting all reported outcomes was low (see Table 1, Table 2, and Table 3).
Although none of the included trials reported Outcome 2.3, four trials made use of fluoroscopy (Franken 1983; Hadidi 1999; Meyer 1993; Mortensson 1984), and one trial made use of ultrasound guidance alone (Essa 2011). One trial did not stipulate whether fluoroscopy or ultrasound guidance was used (Lin 2000).
Discussion
Summary of main results
We identified six completed trials of 822 participants in which all children had presented for management of intussusception. Investigators used a wide range of treatments, and this prevented meta‐analysis for most of our outcomes. In particular, many review outcomes related to adverse effects (e.g. number of intraoperative complications) were not reported.
We could make few direct comparisons of interventions. However, air enema may be superior to liquid enema for successfully reducing intussusception in children. Use of dexamethasone as an adjunct may reduce the rate of recurrence of intussusception. No other results were statistically significant. See summary of findings tables (Table 1; Table 2; Table 3). Of note, we downgraded many of the recommendations provided in these tables owing to the small numbers of included trials and the small participant numbers.
It is important to note that surgical intervention was not the primary study question for any of the included studies. Lack of trials on surgical management might reflect the nature of treatment of children with intussusception, and might suggest that cases are managed largely by non‐surgical means, although this suggestion does not seem to be based on trial evidence.
Overall completeness and applicability of evidence
The internal validity of the design, conduct, and analysis of included studies was difficult to assess because important methodological details were omitted from the study reports. No single study adequately reported all domains of the risk of bias assessment (Figure 2). We judged most trials as having high risk of bias in at least one domain, and omissions in methods were evident in all included studies. Selection bias was generally addressed adequately.Although detection and performance biases are difficult to mitigate for researchers in this field, it may be possible to overcome such biases, for example, Franken 1983 used identical appearing vials for injection in both intervention and control groups. Reporting bias was also difficult to address, although with adequate reporting of protocols and reporting of all expected outcomes, as in Essa 2011 and Franken 1983, this may be mitigated. Although postoperative complications were reported (Outcome 2.9), Essa 2011 presented data in such a way that analysis was not possible. Data for one subgroup of children, specifically those undergoing manual reduction, were not available. We attempted to contact study authors but received no response. Thus data were provided by only one study, and for only a subgroup of children receiving surgical intervention, and data were not sufficient to permit an analysis of this outcome. Again, trials infrequently reported data related to adverse events and harms. These situations might reflect missing data, which may have implications for analysis.
Included trials largely assessed participants of varied ethnic and cultural backgrounds from single centres; this fact may influence the comparability of results between studies. However, given the small quantity of evidence and our inability to perform a meta‐analysis, we could not assess the implications of population differences for applicability of the evidence.
Again we wish to highlight the lack of evidence on surgical interventions and on different imaging modalities and protocols used for delayed repeat enemas.
Quality of the evidence
We have summarised the quality of evidence for each outcome in summary of findings tables (Table 1; Table 2; Table 3), which present evidence of low quality for all outcomes examined. We obtained only data for the outcomes 'liquid enema plus glucagon versus liquid enema alone' and 'air enema versus liquid enema' from two trials each, and data for all other outcomes from single trials only, most with small sample sizes. In Table 1, Table 2, and Table 3, we downgraded quality of trial evidence for serious to very serious concerns of imprecision or wide confidence intervals, or because trials were subject to serious to very serious concerns of high risk of bias. This limits the strength of our conclusions and our ability to investigate both clinical and statistical heterogeneity. The limited number of included studies and the heterogeneity between them precluded performance of sensitivity and subgroup analyses.
As all examined outcomes were subject to a GRADE assessment of low quality, the true effect of outcomes measured may be substantially different from the estimates; therefore, these estimates can be accepted only with limited confidence.
Potential biases in the review process
We undertook an extensive literature search to examine different aspects of surgical and non‐surgical management of intussusception in children, and we sought data from each identified study. In particular, we attempted to contact study authors to gain further information, and we identified ongoing trials. Two independent review authors undertook searching and data extraction and analysis, and a third review author provided arbitration. However, we could not contact study authors to obtain the data that we required. Individuals who apply the results of this review need to acknowledge the limitations of available data derived from few trials.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the only systematic review of RCTs related to this topic, including unpublished data and ongoing trials. However, several narrative reviews have included comparative studies and RCTs (Applegate 2009; Cachat 2012; Daneman 2003; Ko 2007; Sadigh 2015). Applegate 2009 included comparative trials as well as RCTs to examine the role of ultrasonography, air versus liquid enema for reduction, and risk of bowel perforation in children with intussusception. This review concurred that air enema was superior to liquid enema for successful reduction of intussusception in children ‐ a fact that review authors attribute to speed, cost of the procedure, and safety. Daneman 2003 similarly included comparative studies and RCTs, highlighting in their review the ongoing debate regarding fluoroscopy versus ultrasound‐guided enema reduction, suggesting that greater accuracy can be afforded with ultrasound‐guided reduction. Ko 2007 also examined the role of fluoroscopy versus ultrasound‐guided enema reduction by examining both comparative studies and RCTs; these review authors concluded that ultrasound‐guided reduction is superior to fluoroscopy owing to its greater accuracy, lack of ionising radiation, lower costs, and no need for sedation. The authors of the current review could not perform the comparison offered in both Daneman 2003 and Ko 2007 but agree with the findings of Ko 2007, which suggest that lack of standardisation among single studies makes objective comparison difficult. Cachat 2012 performed a meta‐analysis of studies examining children with radiologically confirmed intussusception, including RCTs and retrospective comparative studies, to compare rates of recurrence. Although Cachat found that dexamethasone was beneficial in reducing rates of recurrence of intussusception among children, review findings suggest that risk of recurrence of intussusception is low, and that regardless of the technique used for successful reduction, it is safe to discharge a patient after performing successful reduction. Sadigh 2015 compared the efficacy of air versus liquid enema for reduction of intussusception in children and found that air enema was superior to liquid enema. These results are similar to those of the current review. Applegate 2009, Cachat 2012, Daneman 2003, Ko 2007, and Sadigh 2015 included no relevant randomised trial that was not included in our review.
Authors' conclusions
Implications for practice.
Low‐quality evidence suggests that air enema may be more effective than liquid enema for reducing intussusception in children. Evidence is insufficient to show whether adjuncts such as glucagon or dexamethasone influenced intussusception reduction rates. Low‐quality evidence suggests that use of dexamethasone as an adjunct may be associated with lower rates of recurrent intussusception when compared with enema alone. Evidence on any of the interventions examined was insufficient to allow us to draw any conclusions regarding rates of bowel perforation or other adverse effects.
We found no data on surgical interventions that were suitable for analysis, and no evidence regarding the relative effectiveness and safety of different imaging modalities or protocols for delayed repeat enemas.
Implications for research.
The evidence base for this topic is lacking and must be developed further. Clinical trials in children present specific challenges, although randomised controlled trials in surgery are well documented. Researchers must address these concerns. Populations studied should include people in low‐ and middle‐income countries, where the burden of disease is greatest; and trials should be more adequately powered. Interventions utilised must be standardised and clearly defined. In particular, research on the surgical management of intussusception is needed. Outcomes should be standardised and data related to safety and harm should be included. In addition, future investigators should consider how blinding of participants and personnel might be achieved to minimise bias. Further clinical research is needed to determine the most effective and least harmful non‐surgical and surgical approaches to management of intussusception in children.
History
Protocol first published: Issue 2, 2007 Review first published: Issue 6, 2017
| Date | Event | Description |
|---|---|---|
| 4 August 2015 | New search has been performed | New search performed. One new trial included |
| 28 October 2013 | Amended | New review author team. Major changes and new citation |
| 5 October 2013 | Amended | Copy edited and ready for publication |
Acknowledgements
Jooly Joseph and Madan Mohan Palliyil wrote an initial protocol for this review in 2007. We would like to thank Tanvir Kapoor for helping to draft the updated protocol. We would also like to thank the Cochrane Colorectal Cancer Review Group (Managing editor, Information specialist, editors and referees) for support and guidance provided.
Appendices
Appendix 1. CENTRAL search strategy
CENTRAL (2016, Issue 8)
#1 MeSH descriptor Intussusception explode all trees #2 ((intestin* and invagination*) or intususcep* or intussuscep*):ti,ab,kw #3 (#1 OR #2) #4 MeSH descriptor Infant explode all trees #5 MeSH descriptor Child explode all trees #6 (infant* or child* or newborn*):ti,ab,kw #7 (#4 OR #5 OR #6) #8 (#3 AND #7)
Appendix 2. MEDLINE search strategy
MEDLINE (Ovid 1950 to September 2016)
1. exp Intussusception/ 2. ((intestin* and invagination*) or intususcep* or intussuscep*).mp.
3. 1 or 2 4. exp Infant/ 5. exp Child/ 6. (infant* or child* or newborn*).mp.
7. 4 or 5 or 6 8. 3 and 7 9. randomized controlled trial.pt. 10. controlled clinical trial.pt. 11. randomized.ab. 12. placebo.ab. 13. drug therapy.fs.
14. Randomly.ab. 15. trial.ab. 16. groups.ab. 17. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp animals/ not humans.sh. 19. 17 not 18 20. 8 and 19
Appendix 3. Embase search strategy
Embase (Ovid, 1974 to September 2016)
1. exp intussusception/ 2. ((intestin* and invagination*) or intususcep* or intussuscep*).mp. 3. 1 or 2 4. exp child/ 5. (infant* or child* or newborn*).mp. 6. 4 or 5 7. 3 and 6 8. CROSSOVER PROCEDURE.sh. 9. DOUBLE‐BLIND PROCEDURE.sh. 10. SINGLE‐BLIND PROCEDURE.sh. 11. (crossover* or cross over*).ti,ab. 12. placebo*.ti,ab. 13. (doubl* adj blind*).ti,ab. 14. allocat*.ti,ab. 15. trial.ti. 16. RANDOMIZED CONTROLLED TRIAL.sh. 17. random*.ti,ab. 18. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.) 20. 18 not 19 21. 7 and 20
Appendix 4. Science Citation Index search strategy
Science Citation Index Expanded (via Web of Science) (1900 to September 2016)
#1 Topic=(((intestin* and invagination*) or intususcep* or intussuscep*)) #2 Topic=((infant* or child* or newborn*)) #3 Topic=((controlled trial or controlled clinical trial or placebo or clinical trial or random* or trial or cct or rct)) #4 (#3 AND #2 AND #1)
Appendix 5. Biosis Previews search strategy
Biosis Previews (via Web of Science) (1969 to September 2016)
#1 Topic=(((intestin* and invagination*) or intususcep* or intussuscep*)) #2 Topic=((infant* or child* or newborn*)) #3 Topic=((controlled trial or controlled clinical trial or placebo or clinical trial or random* or trial or cct or rct)) #4 (#3 AND #2 AND #1)
Appendix 6. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Criteria for a judgement of ‘Low risk’ of bias | The investigators describe a random component in the sequence generation process such as:
*Minimization may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of ‘High risk’ of bias. | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorization of participants, for example:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgement of ‘High risk’ of bias. | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following:
|
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of the allocated interventions by outcome assessors. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following: · No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; · Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following:
|
|
INCOMPLETE OUTCOME DATA Attrition bias due to amount, nature or handling of incomplete outcome data. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following:
|
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any of the following:
|
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. It is likely that the majority of studies will fall into this category. |
|
OTHER BIAS Bias due to problems not covered elsewhere in the table. | |
| Criteria for a judgement of ‘Low risk’ of bias. | The study appears to be free of other sources of bias. |
| Criteria for the judgement of ‘High risk’ of bias. | There is at least one important risk of bias. For example, the study:
|
| Criteria for the judgement of ‘Unclear risk’ of bias. | There may be a risk of bias, but there is either:
|
Data and analyses
Comparison 1. Enema plus glucagon versus enema alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Successfully reduced intussusception | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.26] |
Comparison 2. Enema plus dexamethasone versus enema alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Successfully reduced intussusception | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.10] |
| 2 Bowel perforation(s) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [0.11, 62.66] |
| 3 Recurrent intussusception | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.60] |
| 4 Bowel resection | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.19, 4.06] |
Comparison 3. Air enema versus liquid enema.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Successfully reduced intussusception | 2 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.10, 1.49] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Essa 2011.
| Methods |
|
|
| Participants |
Exclusion criteria: pathological lead points, late neglected intestinal obstruction, bowel perforation or shock |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
Procedure details: The technique of ultrasound‐guided saline enema reduction involved the following: "a reservoir filled with warm, normal saline was placed at a maximum height of 120cm above the table, with its upper end opened connected to a 10‐18‐Fr Foley's catheter." The enema could be repeated twice more, after a 30‐minute rest, if the initial attempt failed (i.e. lack of reduction within 5 minutes) The ratio of participants requiring non‐surgical reduction to those requiring surgical reduction was 60:15. In other words, 4/5 participants had successful reduction achieved with non‐surgical techniques |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The cases were randomly classified into two groups…”. No further details supplied |
| Allocation concealment (selection bias) | Unclear risk | “The cases were randomly classified into two groups…”. No further details supplied |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 75 cases were reported, including those that failed initial intervention: "Cases who failed ultrasound guided saline enema reduction underwent surgical exploration, with operative details and postoperative complications also reported" |
| Selective reporting (reporting bias) | Low risk | Study includes all expected outcomes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo treatment used in control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified who assessed outcomes |
Franken 1983.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
Procedure details: The enema consisted of barium sulphate suspension of approximately 20% w/v concentration, with the enema bag 1 metre above the table top. The enema could be repeated twice more if the initial attempt failed (i.e. lack of reduction within 5 minutes) Other details: Glucagon and placebo were supplied by the Eli Lilly Company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The injections were given in randomized, double‐blind fashion"; no further details supplied |
| Allocation concealment (selection bias) | Unclear risk | "The injections were given in randomized, double‐blind fashion"; no further details supplied |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data (randomisation post exclusion) |
| Selective reporting (reporting bias) | Low risk | Reporting included all outcomes and explained outcomes that were unexpected: "eight of 15 intussusceptions...were successfully reduced" ‐ "two patients in the study suffered complications of intussusception...before full recovery ensued" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The injections were given in randomized, double‐blind fashion...Glucagon and the placebo were supplied in identical vials" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified who assessed outcomes |
Hadidi 1999.
| Methods |
|
|
| Participants |
Exclusion criteria: more than 48 hours of symptoms, general or abdominal signs of toxicity, peritonism or peritonitis, or unreasonable electrolyte levels |
|
| Interventions | Participants were prepared in the same manner. Preparation included a nasogastric tube with drainage of the stomach, intravenous fluid deficit replacement, and intravenous metronidazole and cefotaxime All air insufflations were performed by the paediatric surgeon, who was experienced in the technique, and all barium and saline reductions were done by the radiologist, who was experienced in those 2 techniques Treatment 1
Treatment 2
|
|
| Outcomes |
|
|
| Notes |
Procedure details: Diagnosis and treatment were provided by a dedicated “intussusception clinical team,” consisting of a single paediatric surgeon, a single paediatric radiologist, and 3 residents; all data were recorded on a specially designed protocol sheet The study protocol allowed a maximum of 3 attempts at reduction for each participant. An attempt was defined as pneumatic or hydrostatic pressure for 5 minutes Barium enemas were prepared by routine methods During enemas administered with liquid contrast material, the top of the bag of liquid contrast agent could be raised to a maximum of 1.5 m above the table top. For air insufflation, the maximum pressure used was 120 mmHg. After 3 unsuccessful attempts, the examination was considered a failure. No sedation was used Sonographic criteria for successful reduction were disappearance of intussusception and visualisation of passage of fluid and air bubbles from the caecum well into the terminal ileum. After successful reduction, saline solution was replaced by gastrografin, and a single abdominal radiograph was taken again to document the successful reduction Other details: All air reductions and barium reductions were performed with a GE DRS Prestilix 1600X x‐ray machine. All saline reductions were done under sonographic guidance with Tochiba SSA 140 ultrasound machine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomisation was based on a table of random numbers, wherein 15 consecutive random numbers were selected and assigned to cases 1 through 15. This list of 15 cases was used repeatedly throughout the study (10 times) with random sequence every time.” This allocation sequence is predictable |
| Allocation concealment (selection bias) | High risk | "...randomisation was based on a table of random numbers, wherein 15 consecutive random numbers were selected and assigned to cases 1 through 15. This list of 15 cases was used repeatedly throughout the study (10 times) with random sequence every time.” This allocation sequence is predictable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data (randomisation post exclusion). “Only 76 patients came to follow up examinations”; however these data were not used in this review |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | By definition, the paediatric surgeon or radiologist was aware of the procedure each was conducting |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment involved a treating surgeon or radiologist capable of interpreting sonographic criteria for successful reduction (disappearance of intussusception and visualisation of the passage of fluid and air bubbles from the caecum well into the terminal ileum) |
Lin 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Procedure details: no details on procedure provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "We designed a randomised, double‐blind study" |
| Allocation concealment (selection bias) | Unclear risk | "We designed a randomised, double‐blind study" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on participants lost to follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Method of data collection post discharge not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "We designed a randomised, double‐blind study” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not specified who assessed outcomes |
Meyer 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
Procedure details: During the first 1.5 years of the study, barium was the only liquid contrast agent used. During the final year, owing to evolving concepts in intussusception management and changes in personnel, the type of liquid contrast material (water‐soluble or barium) used was determined at the radiologist's discretion The study protocol allowed a maximum of 3 attempts at reduction for each participant. An attempt was defined as pneumatic or hydrostatic pressure applied for a total of 5 minutes. After 3 unsuccessful attempts, the examination was considered a failure The concentration of barium used in individual cases was not recorded Cysto‐Conray II (Iothalamate meglumine 17.2%; Mallinckrodt Medical, St Louis, Missouri) was the water‐soluble enema administered The air insufflation device included an electronic pop‐off valve that could be set to pressure of 60, 80, or 120 mmHg Other details: This study examined the accuracy of diagnosis with air versus liquid enema, and thus included participants who did not have intussusception. It was necessary to extrapolate the data of those who did have confirmed intussusception for our review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ”Randomization was based on a table of random numbers, wherein 20 consecutive random numbers were selected and assigned to cases 1 through 20. Cases assigned even random numbers were to undergo examination with liquid contrast material and those assigned odd random numbers were to be examined with air.” This allocation sequence is predictable and is compromised by the need to extrapolate data for participants with confirmed intussusception |
| Allocation concealment (selection bias) | High risk | Central randomisation table (n = 20) Used repeatedly throughout the study. Repetative use of the random number table may have allowed prediction of intervention for participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data (randomisation post exclusion). Successful diagnosis of intussusception not significantly different between air and liquid contrast groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Not all expected outcomes were reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | By definition, the radiologist was aware of the procedure he was conducting |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Radiologist who conducted the procedure recorded results |
Mortensson 1984.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
Procedure details: The pressure of the enema employed was kept as uniform as possible, corresponding to 100 to 120 cm of barium suspension Reduction was considered a failure when an intussusception could no longer be moved in an oral direction after several minutes of effective pressure 5 minutes after a first attempt had failed, a second and later a third attempt was made. Failure after this point meant that the participant was prepared for operation. Participants in the control group with 3 failed attempts were administered IV glucagon, as in the treatment group, and an attempt at hydrostatic reduction was repeated. After a fourth attempt, these participants were prepared for operation Other details: Study was undertaken in 3 steps (Step 1: initial 3 attempts at reduction as per treatment group; Step 2: participants belonging to the control group were administered glucagon and a fourth attempt was made at reduction; participants with reduction regarded as a failure were prepared for surgery; Step 3: all other participants otherwise not reduced were given a final attempt at reduction before they were prepared for surgery). We have included data only for Step 1, as this step pertains to our outcomes and criteria for inclusion |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation of material to a test group and a reference group according to date of birth; participants born on an even calendar date were given an intramuscular injection of 0.05 mg glucagon/kg body weight |
| Allocation concealment (selection bias) | Unclear risk | Unclear who allocated participants, and who administered treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not stated why some participants from reference group or control group progressed to steps 2 and 3, and why others were excluded |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Not all expected outcomes were reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo given Treatment group given intramuscular injection; control group given no injection |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not specified who assessed outcomes |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Diaz‐Aldagalan 2012 | Not a randomised controlled trial |
| Guo 2010 | Not a randomised controlled trial |
| Hsiao 1988 | Not a randomised controlled trial |
| Morrison 2009 | Not a randomised controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
Zhang 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Translation pending |
Characteristics of ongoing studies [ordered by study ID]
El Fiky 2016.
| Trial name or title | Effect of hydrocortisone on improving outcome of pneumatic reduction of infantile intussusception |
| Methods |
|
| Participants |
|
| Interventions |
Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | April 2015 |
| Contact information | Mahmoud El Fiky, Lecturer of Pediatric Surgery, Cairo University |
| Notes | ClinicalTrials.gov Identifier:NCT02691858 |
Mehraeen 2011.
| Trial name or title | The effect of midazolam in decreasing time of hydrostatic reduction of childhood intussusceptions |
| Methods |
|
| Participants |
|
| Interventions |
Treatment group
Control group
|
| Outcomes |
|
| Starting date | Date of registration: 26 August, 2011 |
| Contact information | Dr. Raheleh Mehraeen sany_monzavi@yahoo.com |
| Notes |
Zhang 2015.
| Trial name or title | Open reduction of paediatric intussusception through inferior umbilical skin fold incision |
| Methods | Study design: randomised single‐blind controlled; 2‐arm study Study duration: 1 May 2014 until 30 June 2015 |
| Participants |
|
| Interventions |
Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | Date of registration: 26 April 2014 |
| Contact information | Wen Zhang wenzhang09@126.com |
| Notes | Randomisation procedure involves flipping a coin |
Differences between protocol and review
In contrast to our published protocol, we decided to conduct the analysis using fixed‐effect meta‐analysis because it is more conservative in the presence of heterogeneity and small‐study effects. Although not specifically stipulated in the protocol, we saw both quasi‐RCTs and cluster RCTs as fit for inclusion in this review.
When data were missing, and intention‐to‐treat analysis was not possible, we planned to use available‐case or per‐protocol analysis.
Although we did not discuss these matters in the protocol, we used the GRADE approach and 'Summary of findings' tables to summarise our findings.
Contributions of authors
SG: running searches, selecting studies, extracting data, analysing results, and writing the main review.
JK: drafting the protocol, identifying studies, and providing content area advice.
ACW: drafting the protocol and providing methodological advice.
RGM: drafting the protocol, running searches, selecting studies, extracting data, analysing results, and writing the main review.
Declarations of interest
Review authors have no conflicts of interest to declare.
New
References
References to studies included in this review
Essa 2011 {published data only}
- Essa AE, Eltayeb AA, Mansour E. Evaluation of the role of dexamethasone in decreasing early recurrence of intussusception: using ultrasound‐guided saline enema for reduction. Surgical Practice 2011;15(4):114‐9. [Google Scholar]
Franken 1983 {published data only}
- Franken, EA, Smith WL, Chernish SM, Campbell JB, Fletcher BD, Goldman HS. The use of glucagon in hydrostatic reduction of intussusception: a double‐blind study of 30 patients. Radiology 1983;146(3):687‐9. [Accession Number: CN‐00030376; PUBMED: 6828682] [DOI] [PubMed] [Google Scholar]
Hadidi 1999 {published data only}
- Hadidi AT, Shal N. Childhood intussusception: a comparative study of nonsurgical management. Journal of Pediatric Surgery 1999;34(2):304‐7. [DOI] [PubMed] [Google Scholar]
Lin 2000 {published data only}
- Lin SL, Kong MS, Houng DS. Decreasing early recurrence rate of acute intussusception by the use of dexamethasone. European Journal of Pediatrics 2000;159(7):551‐2. [PUBMED: 10923238] [DOI] [PubMed] [Google Scholar]
- Lin SL, Kong MS, Huong D. Decrease early recurrence rate of childhood acute intussusception by dexamethasone. Proceedings from The 11th Asia Pacific Congress of Doppler Echocardiography and 8th APCDE. New Dehli, India, November 26‐27, 2005.
Meyer 1993 {published data only}
- Meyer JS, Dangman BC, Buonomo C, Berlin JA. Air and liquid contrast agents in the management of intussusception: a controlled, randomized trial. Radiology 1993;188(2):507‐11. [PUBMED: 8327705] [DOI] [PubMed] [Google Scholar]
Mortensson 1984 {published data only}
- Mortensson W, Eklof O, Laurin S. Hydrostatic reduction of childhood intussusception. The role of adjuvant glucagon medication. Acta Radiologica: Diagnosis 1984;25(4):261‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Diaz‐Aldagalan 2012 {published data only}
- Diaz‐Aldagalan GR, Perez‐Martinez A, Pison‐Chacon J, Ayuso‐Gonzalez L, Salcedo‐Munoz B, Goni‐Orayen C. Rescue by pneumoenema under general anaesthesia of apparently non‐reducible intestinal intussusception. European Journal of Pediatrics 2012;171(1):189‐91. [DOI] [PubMed] [Google Scholar]
Guo 2010 {published data only}
- Guo WL, Zhou M, Wang J, Sheng M. Analyses of air enema and radiographic film for acute intussusception in children. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2010;90(47):3359‐61. [PubMed] [Google Scholar]
Hsiao 1988 {published data only}
- Hsiao JY, Kao HA, Shih SL. Intravenous glucagon in hydrostatic reduction of intussusception: a controlled study of 63 patients. Chung‐Hua Min Kuo Hsiao Erh Ko i Hsueh Hui Tsa Chih 1988;29(4):242‐7. [PubMed] [Google Scholar]
Morrison 2009 {published data only}
- Morrison J, Lucas N, Gravel J. The role of abdominal radiography in the diagnosis of intussusception when interpreted by pediatric emergency physicians. Journal of Pediatrics 2009;155(4):556‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Zhang 2014 {published data only}
- Zhang PJ, Wang LY, Zhang Z. Ultrasound guided hydrostatic enema in the treatment of pediatric intussusception. Journal of Dallan Medical University 2014;36(4):365‐6, 379. [Google Scholar]
References to ongoing studies
El Fiky 2016 {published data only}
- Fiky M. Effect of hydrocortisone on improving outcome of pneumatic reduction of infantile intussusception. clinicaltrials.gov/show/NCT02691858.
Mehraeen 2011 {unpublished data only}
- Mehraeen R. The effect of midazolam in decreasing time of hydrostatic reduction of childhood intussusceptions. Iranian Registry of Clinical Trials.
Zhang 2015 {published data only}
- Zhang W, Ning L. Open reduction of pediatric intussusception through inferior umbilical skin fold incision. Chinese Clinical Trials Registry [Prospective Registration] 26 April 2014.
Additional references
Altman 1996
- Altman DG, Bland JM. Comparing several groups using analysis of variance. BMJ 1996;312(7044):1472‐3. [PMID: 8664633] [DOI] [PMC free article] [PubMed] [Google Scholar]
American College of Radiology 2007
- American College of Radiology. ACR practice guideline for the performance of paediatric fluoroscopic contrast enema examinations. http://www.acr.org (last accessed 15 May 2012). 2007 [Revised 2011].
Applegate 2009
- Applegate KE. Intussusception in children: evidence‐based diagnosis and treatment. Pediatric Radiology 2009;39:140‐3. [PMID: 19308373] [DOI] [PubMed] [Google Scholar]
Bailey 2007
- Bailey KA, Wales PW, Gerstle JT. Laparoscopic versus open reduction of intussusception in children: a single‐institution comparative experience. Journal of Pediatric Surgery 2007;42(5):845‐8. [DOI] [PubMed] [Google Scholar]
Beasley 1988
- Beasley SW, Auldist AW, Stokes KB. The diagnostically difficult intussusception: its characteristics and consequences. Pediatric Surgery International 1988;3:135‐8. [DOI: 10.1007/BF00182768] [DOI] [Google Scholar]
Beasley 1998
- Beasley SW, Myers NA. Intussusception: current views. Pediatric Surgery International 1998;14(3):157‐62. [DOI: 10.1007/s003830050473] [DOI] [PubMed] [Google Scholar]
Beres 2014
- Beres AL, Baird R, Fung E, Hsieh H, Abou‐Khalil M, Ted Gerstle J. Comparative outcome analysis of the management of pediatric intussusception with or without surgical admission. Journal of Pediatric Surgery 2014;49(5):750‐2. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bines 2002
- Bines JE, Ivanoff B. Acute intussusception in infants and children: a global perspective. A report prepared for the Steering Committee on Diarrhoeal Disease Vaccines, Vaccine Development,Vaccines and Biologicals, World Health Organization, Geneva, Switzerland. Vaccines and Biologicals 2002; Vol. WHO V & B:02.19.
Bines 2004a
- Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, Hansen J, et al. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine 2004;22(5‐6):569‐74. [PMID: 14741146] [DOI] [PubMed] [Google Scholar]
Bines 2004b
- Bines JE, Ivanoff B, Justice F, Mulholland K. Clinical case definition for the diagnosis of acute intussusception. Journal of Pediatric Gastroenterology and Nutrition 2004;39(5):511‐8. [PMID: 15572891] [DOI] [PubMed] [Google Scholar]
Blanch 2007
- Blanch AJ, Perel SB, Acworth JP. Paediatric intussusception: epidemiology and outcome. Emergency Medicine Australasia 2007;19(1):45‐50. [PMID: 17305660] [DOI] [PubMed] [Google Scholar]
Bonnard 2008
- Bonnard A, Demarche M, Dimitriu C, Podevin G, Varlet F, François M, et al. Indications for laparoscopy in the management of intussusception: a multicenter retrospective study conducted by the French Study Group for Pediatric Laparoscopy (GECI). Journal of Pediatric Surgery 2008;43(7):1249‐53. [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges L, Higgins JPT, Rothstein HR. Introduction to Meta‐analysis. Chichester, U.K.: John Wiley & Sons, 2009. [Google Scholar]
Bratton 2001
- Bratton SL, Haberkern CM, Waldhausen JH, Sawin RS, Allison JW. Intussusception: hospital size and risk of surgery. Pediatrics 2001;107(2):299‐303. [PMID: 11158462] [DOI] [PubMed] [Google Scholar]
Bruce 1987
- Bruce J, Huh YS, Cooney DR, Karp MP, Allen JE, Jewett TC Jr. Intussusception: evolution of current management. Journal of Pediatric Gastroenterology and Nutrition 1987;6:663‐4. [PMID: 3320323] [PubMed] [Google Scholar]
Budwig 1994
- Budwig K, Rothrock SG, Sudhipong V, Falk JL, Molpus B, Perlman R. Misdiagnosis of pediatric intussusception. Annals of Emergency Medicine 1994;23(3):611‐2. [DOI: 10.1016/S0196-0644(94)80309-9] [DOI] [Google Scholar]
Buttery 2011
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: post‐marketing surveillance in the National Immunization Program in Australia. Vaccine 2011;29(16):3061‐6. [PMID: 21316503] [DOI] [PubMed] [Google Scholar]
Cachat 2012
- Cachat F, Ramseyer P. Question 3: Does the administration of glucagon improve the rate of radiological reduction in children with acute intestinal intussusception?. Archives of Disease in Childhood 2012;97:389‐91. [DOI: 10.1136/archdischild-2012-301763] [DOI] [PubMed] [Google Scholar]
Calder 2001
- Calder FR, Tan S, Kitteringham L, Dykes EH. Patterns of management of intussusception outside tertiary centres. Journal of Pediatric Surgery 2001;36(2):312‐5. [PMID: 11172423] [DOI] [PubMed] [Google Scholar]
Daneman 2003
- Daneman A, Navarro O. Intussusception, Part 1: A review of diagnostic approaches. Pediatric Radiology 2003;33(2):79‐85. [PMID: 12557062] [DOI] [PubMed] [Google Scholar]
Daneman 2004
- Daneman A, Navarro O. Intussusception, Part 2: An update on the evolution of management. Pediatric Radiology 2004;34(2):97‐108. [PMID: 14634696] [DOI] [PubMed] [Google Scholar]
Davis 2003
- Davis CF, McCabe AJ, Raine PA. The ins and outs of intussusception: history and management over the past fifty years. Journal of Pediatric Surgery 2003;38:60‐4. [DOI] [PubMed] [Google Scholar]
del‐Pozo 1999
- del‐Pozo G, Albillos JC, Tejedor D, Calero R, Rasero M, de‐la‐Calle U, et al. Intussusception in children: current concepts in diagnosis and enema reduction. Radiographics 1999;19(2):299‐319. [PMID: 10194781] [DOI] [PubMed] [Google Scholar]
Desai 2012
- Desai R, Curns AT, Patel MM, Parashar UD. Trends in intussusception‐associated deaths among US infants from 1979‐2007. Journal of Pediatrics 2012;160(3):456‐60. [PMID: 21925681] [DOI] [PubMed] [Google Scholar]
Ein 2006
- Ein SH, Daneman A. Intussusception. In: Grosfeld L, O'Neill JA, Fonkalsrud EW, Coran AG editor(s). Pediatric Surgery. Philadelphia: Mosby‐Elsevier, 2006:1313–41. [Google Scholar]
Eng 2012
- Eng PM, Mast TC, Loughlin J, Clifford CR, Wong J, Seeger JD. Incidence of intussusception among infants in a large commercially insured population in the United States. Pediatric Infectious Disease Journal 2012;31(3):287‐91. [PMID: 22173141] [DOI] [PubMed] [Google Scholar]
Fischer 2004
- Fischer TK, Bihrmann K, Perch M, Koch A, Wohlfahrt J, Kåre M, et al. Intussusception in early childhood: a cohort study of 1.7 million children. Pediatrics 2004;114(3):782‐5. [PMID: 15342854] [DOI] [PubMed] [Google Scholar]
Gonzalez‐Spinola 1999
- Gonzalez‐Spinola J, Pozo G, Tejedor D, Blanco A. Intussusception: the accuracy of ultrasound guided saline enema and the usefulness of a delayed attempt at reduction. Journal of Pediatric Surgery 1999;34:1016‐20. [DOI] [PubMed] [Google Scholar]
Gray 2014
- Gray MP, Li SH, Hoffman RG, Gorelick MH. Recurrence rates after intussusception enema reduction: a meta‐analysis. Pediatrics 2014;134(1):110‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. handbook.cochrane.org.
Huppertz 2006
- Huppertz HI, Soriano‐Gabarró M, Grimprel E, Franco E, Mezner Z, Desselberger U, et al. Intussusception among young children in Europe. Pediatric Infectious Disease Journal 2006;25(1 Suppl):S22‐9. [PMID: 16397426] [DOI] [PubMed] [Google Scholar]
Hviid 2009
- Hviid A, Svanström H. Antibiotic use and intussusception in early childhood. Journal of Antimicrobial Chemotherapy 2009;64(3):642‐8. [DOI] [PubMed] [Google Scholar]
Ito 2012
Iwase 2010
- Iwase H, Motani H, Yajima D, Hayakawa M, Kobayashi K, Sato K, et al. Two infant deaths linked to intussusception without peritonitis. Japanese Society of Legal Medicine 2010;12(3):151‐3. [PMID: 20304696] [DOI] [PubMed] [Google Scholar]
Jiang 2013
- Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS One 2013;8(7):e68482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 1992
- Katz ME, Kolm P. Intussusception reduction 1991: an international survey of pediatric radiologists. Pediatric Radiology 1992;22:318‐22. [DOI] [PubMed] [Google Scholar]
Kia 2005
- Kia KF, Mony VK, Drongowski RA, Golladay ES, Geiger JD, Hirschl RB, et al. Laparoscopic vs open surgical approach for intussusception requiring operative intervention. Journal of Pediatric Surgery 2005;40(1):281‐4. [DOI] [PubMed] [Google Scholar]
Ko 2007
- Ko HS, Schenk JP, Tröger J, Rohrschneider WK. Current radiological management of intussusception in children. European Radiology 2007;17(9):2411‐21. [PMID: 17308922] [DOI] [PubMed] [Google Scholar]
Kohl 2008
- Kohl KS, Magnus M, Ball R, Halsey N, Shadomy S, Farley TA. Applicability, reliability, sensitivity, and specificity of six Brighton Collaboration standardized case definitions for adverse events following immunization. Vaccine 2008;26(50):6349‐60. [PMID: 18805456] [DOI] [PubMed] [Google Scholar]
Kramarz 2001
- Kramarz P, France EK, Destefano F, Black SB, Shinefield H, Ward JI, et al. Population‐based study of rotavirus vaccination and intussusception. Pediatric Infectious Disease Journal 2001;20(4):410‐6. [PMID: 11332666] [DOI] [PubMed] [Google Scholar]
Lappas 1995
- Lappas JC, Maglinte DD, Chernish SM, Hage JP, Kelvin FM. Discomfort during double‐contrast barium enema examination: a placebo‐controlled double‐blind evaluation of the effect of glucagon and diazepam. Radiology 1995;197:95–9. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Lehnert 2009
- Lehnert T, Sorge I, Till H, Rolle U. Intussusception in children ‐ clinical presentation, diagnosis and management. The International Journal of Colorectal Disease 2009 ;24(10):1187‐92. [PMID: 19418060] [DOI] [PubMed] [Google Scholar]
Littlewood 1998
- Littlewood Teele R, Vogel SA. Intussusception: the paediatric radiologist's perspective. Pediatric Surgery International 1998;14(3):158‐62. [PMID: 9880736] [DOI] [PubMed] [Google Scholar]
Liu 1986
- Liu KW, MacCarthy J, Guiney EJ, Fitzgerald RJ. Intussusception ‐ current trends in management. Archives of Disease in Childhood 1986;61:75‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loukas 2011
- Loukas M, Pellerin M, Kimball Z, Garza‐Jordan J, Tubbs RS, Jordan R. Intussusception: an anatomical perspective with review of the literature. Clinical Anatomy 2011;24:552‐61. [DOI] [PubMed] [Google Scholar]
Meier 1996
- Meier DE, Coln CD, Rescorla FJ, OlaOlorun A, Tarpley JL. Intussusception in children: international perspective. World Journal of Surgery 1996;20(8):1035‐9. [PMID: 8798362] [DOI] [PubMed] [Google Scholar]
Meyer 1992
- Meyer JS. The current radiologic management of intussusception: a survey and review. Pediatric Radiology 1992;22:323–5. [DOI] [PubMed] [Google Scholar]
Moss 2000
- Moss RL, Skarsgard ED, Kosloske AM, Smith BM. Case Studies in Pediatric Surgery. New York: McGraw‐Hill, 2000. [Google Scholar]
Murphy 2001
- Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. New England Journal of Medicine 2001;344(8):564‐72. [PMID: 11207352] [DOI] [PubMed] [Google Scholar]
Navarro 2004
- Navarro OM, Daneman A, Chae A. Intussusception: the use of delayed, repeated reduction attempts and the management of intussusceptions due to pathologic lead points in pediatric patients. Pediatric Radiology 2004;182(5):1169‐76. [PMID: 15100113] [DOI] [PubMed] [Google Scholar]
Nylund 2010
- Nylund CM, Denson LA, Noel JM. Bacterial enteritis as a risk factor for childhood intussusception: a retrospective cohort study. Journal of Pediatrics 2010;156(5):761‐5. [PMID: 20138300] [DOI] [PubMed] [Google Scholar]
Okimoto 2011
- Okimoto S, Hyodo S, Yamamoto M, Nakamura K, Kobayashi M. Association of viral isolates from stool samples with intussusception in children. International Journal of Infectious Diseases 2011;15(9):641‐5. [PMID: 21757385] [DOI] [PubMed] [Google Scholar]
Parashar 2000
- Parashar UD, Holman RC, Cummings KC, Staggs NW, Curns AT, Zimmerman CM. Trends in intussusception‐associated hospitalizations and deaths among US infants. Pediatrics 2000;106(6):1413‐21. [PMID: 11099597] [DOI] [PubMed] [Google Scholar]
Pepper 2012
- Pepper VK, Stanfill AB, Pearl RH. Diagnosis and management of pediatric appendicitis, intussusception, and meckel diverticulum. Surgical Clinics of North America 2012;92(3):505‐26. [PMID: 22595706 ] [DOI] [PubMed] [Google Scholar]
Peter 2002
- Peter G, Myers MG. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics 2002;110:e67. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenfeld 1999
- Rosenfeld K, McHugh K. Survey of intussusception reduction in England, Scotland and Wales: how and why we could do better. Clinical Radiology 1999;54(7):452‐8. [PMID: 10437697] [DOI] [PubMed] [Google Scholar]
Sadigh 2015
- Sadigh G, Zou KH, Razavi SA, Khan R, Applegate KE. Meta‐analysis of air versus liquid enema for intussusception reduction in children. American Journal of Roentgenology 2015;205(5):542‐9. [DOI] [PubMed] [Google Scholar]
Samad 2012
- Samad L, Marven S, Bashir H, Sutcliffe AG, Cameron JC, Lynn R, et al. Prospective surveillance study of the management of intussusception in UK and Irish infants. British Journal of Surgery 2012;99(3):411‐5. [DOI: 10.1002/bjs.7821] [DOI] [PubMed] [Google Scholar]
Samad 2014
- Samad L. Epidemiology of intussusception in children: national surveillance and use of record linkage to validate the incidence, and study of incidence trends [Doctoral Thesis]. London (UK): University College London, 2014. [Google Scholar]
Sandler 1999
- Sandler AD, Ein SH, Connolly B, Daneman A, Filler RM. Unsuccessful air‐enema reduction of intussusception: is a second attempt worthwhile?. Pediatric Surgery International 1999;15(3‐4):214‐6. [DOI] [PubMed] [Google Scholar]
Schmit 1999
- Schmit P, Rohrschneider WK, Christmann D. Intestinal intussusception survey about diagnostic and non‐surgical therapeutic procedures. Pediatric Radiology 1999;29(10):752‐61. [PMID: 10525783] [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreteing results and drawing conclusions. Higgins JP T, GreenS editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Singer 2016
- Singer M, Deutschman CS, Seymour CW. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA 2016;315(8):801‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sklar 2014
- Sklar CC, Nasr EA. Laparoscopic versus open reduction of intussusception in children: a retrospective review and meta‐analysis. Journal of Laparoendoscopic & Advanced Surgical Techniques 2014;24(7):518‐22. [DOI: 10.1089/lap.2013.0415] [DOI] [PubMed] [Google Scholar]
Skucas 1994
- Skucas J. The use of antispasmodic drugs during barium enemas. American Journal of Roentgenology 1994;162:1323–5. [DOI] [PubMed] [Google Scholar]
Soares‐Weiser 2004
- Soares‐Weiser K, Goldberg E, Tamimi G, Pitan OC, Leibovici L. Rotavirus vaccine for preventing diarrhoea. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002848.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Somekh 1996
- Somekh E, Serour F, Goncalves D, Gorenstein A. Air enema for reduction of intussusception in children: risk of bacteremia. Radiology 1996;200(1):217‐8. [DOI] [PubMed] [Google Scholar]
Spiro 2003
- Spiro DM, Arnold DH, Barbone F. Association between antibiotic use and primary idiopathic intussusception. Archives of Pediatrics & Adolescent Medicine 2003;157(1):54‐9. [DOI] [PubMed] [Google Scholar]
Staatz 1998
- Staatz G, Alzen G, Heimann G. Intestinal infection, the most frequent cause of invagination in childhood: results of a 10‐year clinical study [Darminfektion, die häufigste Invaginationsursache im Kindesalter: Ergebnisse einer 10jährigen klinischen Studie]. Klinische Pädiatrie 1998;210(2):61‐4. [PMID: 9561958] [DOI] [PubMed] [Google Scholar]
Tapiainen 2006
- Tapiainen T, Bär G, Bonhoeffer J, Heininger U. Evaluation of the Brighton Collaboration case definition of acute intussusception during active surveillance. Vaccine 2006;24(9):1483‐7. [PMID: 16226829 ] [DOI] [PubMed] [Google Scholar]
Ugwu 2000
- Ugwu BT, Legbo JN, Dakum NK, Yiltok SJ, Mbah N, Uba FA. Childhood intussusception: a 9‐year review. Annals of Tropical Paediatrics 2000;20(2):131‐5. [PMID: 10945064] [DOI] [PubMed] [Google Scholar]
Weintraub 2014
- Yin K, Lieu T, Kulldorff M, Martin D, McMahill‐Walraven C, Platt R, et al. Intussusception risk after rotavirus vaccination in U.S. infants. The New England Journal of Medicine 2014;370:503‐12. [DOI: 10.1056/NEJMoa1303164] [DOI] [PubMed] [Google Scholar]
Wong 2006
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMID: 16404468 ] [PMC free article] [PubMed] [Google Scholar]


