Abstract
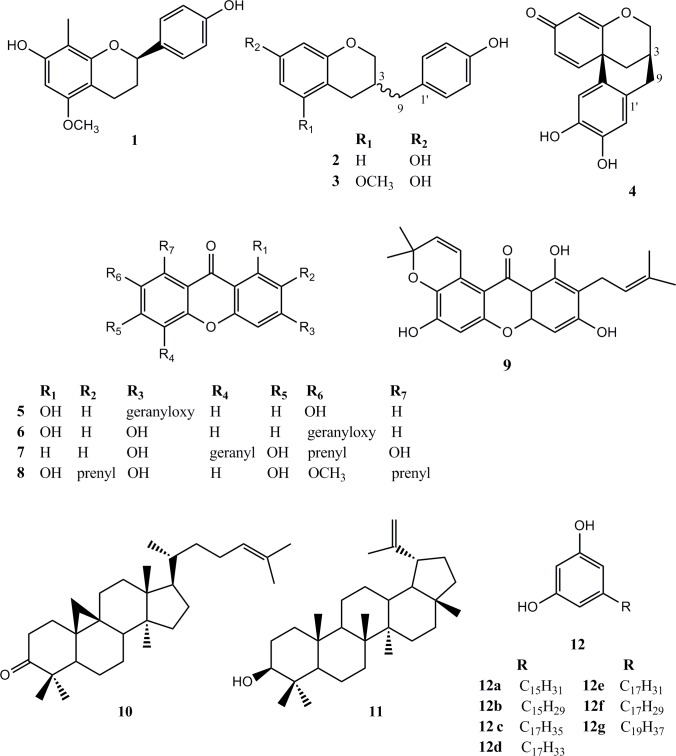
Propolis produced by the stingless bee Lisotrigona cacciae was studied for the first time. Using different chromatographic procedures, a total of eighteen constituents (phenols and triterpenes) were isolated, among which flavane 1, homoisoflavanes 2–4, and xanthones 5 and 6 were new for propolis. Propolis extract was also characterized by gas chromatography/mass spectrometry and other fifteen constituents were identified. The xanthone α-mangostin (8) demonstrated significant activity against Staphylococcus aureus with MIC and MBC 0.31 μg/ml, followed by 7,4'-dihydroxy-5-methoxy-8-methylflavane (1) with MIC 78 μg/ml and MBC 156 μg/ml. 10,11- Dihydroxydracaenone C (4), a component bearing ortho-hydroxyl groups, was the only compound displaying radical scavenging ability. Triple botanical origin of the sample was defined, consisting of Dracaena cochinchinensis, Cratoxylum cochinchinense and Mangifera indica. D. cochinchinensis is a new resin source of propolis.
Introduction
Propolis is a valuable beehive product containing plant secretions and beeswax. It is well known as a remedy with a wide range of biological and pharmacological properties, such as antibacterial, antioxidant, immunostimulating, antiviral, etc. [1,2]. Propolis chemistry depends on the geographical origin, plant species, and bee species, and thus various constituents contribute to its bioactivity [3]. Because of its chemical diversity, propolis has been classified into types based on the plants that bees have chosen as resin sources. At present, the majority of scientific information concerns propolis produced by the honey bee Apis mellifera (tribe Apini), which inhabit almost all ecosystems of the world, and over twenty propolis types have been formulated [3,4]. In tropical and southern subtropical regions, however, the native bee species are stingless bees (tribe Meliponini), which are also key pollinators and producers of beneficial honey, wax and propolis [5,6].
Unlike honey bees, stingless bees are a diverse group, and more than 500 species have been described [7]. Despite the traditional use of stingless bee products, a few studies concerning propolis have been published, particularly for propolis originating from Mainland Southeast Asia. Moreover, in this region bee species with unique behavior have been recorded such as the minute lachryphagous species of a rare genus Lisotrigona [8–11]. These bees have been investigated from biological and ecological point of view [11–14], while the products they manufacture are only scarcely analysed [15]. The first data on Lisotrigona spp. propolis appeared in 2018 [16–18]. Xanthones and triterpenes, new propolis constituents, were found in a sample collected by Lisotrigona furva in Vietnam.
In the present article, we report results of the phytochemical analysis of Vietnamese propolis produced by Lisotrigona cacciae, its antimicrobial and antioxidant activity. Using different chromatographic procedures, a total of 18 constituents were isolated, among which flavane 1, homoisoflavanes 2–4, and xanthones 5 and 6 were found in propolis for the first time. Crude propolis extract was also characterized by GC/MS after silylation, and other 15 constituents were identified. The botanical origin of the sample was defined, and a new plant source Dracaena cochinchinensis was suggested.
Materials and methods
Ethics statement
No specific permits were required for the described field studies. The field studies did not involve endangered or protected species, and were conducted on private land with owner permission.
General data
NMR spectra were recorded on a Bruker AVANCE II+ 600 NMR spectrometer operating at 600 MHz (150 MHz for 13C). Optical rotation was measured on a Jasco P-2000 polarimeter. Vacuum liquid chromatography (VLC) was performed on Silica gel 60H (Merck, 15 μm). Column chromatography (CC) was performed on Silica gel 60 (Merck, 63–200 μm) normal phase, and Sephadex LH-20 (Pharmacia Fine Chemicals, 25–100 μm). Low pressure liquid chromatography (LPLC) was carried out with LiChroprep Si 60 Merck column (40–63 μm). Preparative thin-layer chromatography (prep. TLC) was performed on silica gel 60 F254 glass plates (Merck, 20 x 20 cm; 0.25 mm). Detection of the spots was achieved under UV light at 254 and 366 nm, and by spraying with vanillin in sulfuric acid, followed by heating at 100°C. All solvents used were of analytical grade.
Propolis sample
The propolis sample was collected by scraping from stingless bees’ hives of L. cacciae from Binhdinh province in Vietnam’s South Central Coast region in July, 2017. The stingless bee species was identified by Dr. Nguyen Thi Phuong Lien, Department of Insect Ecology, Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology. The sample was characterized by a deep red color. The flow chart of the sample analysis is shown on S1 Fig.
GC/MS analysis
The procedure of the GC/MS analysis was similar to the one described previously [19]. The propolis sample was grated after cooling, and extracted with 70% ethanol (1:10, w/v) at room temperature (2 x 24 h). A part (20 ml) of the crude extract was evaporated to dryness in vacuo. About 5 mg of the extract were mixed with 50 μl of dry pyridine and 75 μl of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and heated at 80°C for 20 min. The reference compounds, isolated from the sample, were subjected to the same silylation procedure as about 1 mg of the compound was mixed with 10 μl of dry pyridine and 15 μl of BSTFA. The GC/MS analysis of the silylated samples (TMS derivatives) was performed on Agilent 7820 GC System/5977B MSD instrument equipped with a 60 m long, 0.25 mm i.d., and 0.25 μm film thickness DB-5MS UI capillary column. The temperature was programmed from 100 to 325°C at a rate of 5°C/min, and a 30 min hold at 325°C. Helium was used as a carrier gas at a flow rate of 0.8 ml/min. The split ratio was 1:50, the injector temperature 300°C, the interface temperature 300°C, and the ionization voltage 70 eV. The identification of the compounds was performed using commercial libraries, literature data and/or comparison with mass spectra of reference compounds.
Extraction and isolation
Raw propolis sample (160 g) was grated after cooling and extracted with 70% ethanol (1:10, w/v) at room temperature (2 x 24 h). The combined ethanol extracts were concentrated and subjected to liquid-liquid extraction successively with petroleum ether (PE, 3 times) and diethyl ether (DEE, 3 times) to give 3 g PE and 2.5 g DEE dry residue. PE extract (2.5 g) was subjected to CC on Sephadex LH-20, eluted with CH3OH, and 10 fractions were obtained (A-J). Fraction A (1.3 g) was subjected to VLC on silica gel, eluted with PE–EtOAc (1:0 to 0:1), and 11 subfractions were obtained (A1-A11). Subfraction A1 (58 mg) was subjected to LPLC eluted with PE:Acetone to give cycloartenone 10 (17.7 mg) [20]. Fraction C (40 mg) was purified by prep. TLC with CH2Cl2:EtOAc (10:1) to yield lupeol 11 (4.3 mg) [21] and an inseparable mixture of resorcinols 12a-g (6.1 mg) [22]. Fraction F yielded cochinchinone A 7 (36.8 mg) [23]. From fraction H (52 mg), a mixture of 3-geranyloxy-1,7-dihydroxyxanthone (cochinchinone G) 5 and 7-geranyloxy-1,3-dihydroxyxanthone 6 (1.2 mg) [23] were obtained by prep. TLC with PE:EtOAc (3:2). Fraction J (31.1 mg) was purified by prep. TLC with CH2Cl2:EtOAc as a mobile phase (20:1) to yield α-mangostin 8 (15.2 mg) [24].
DEE extract (2 g) was subjected to VLC on silica gel using PE:EtOAc (1:0 to 0:1) as a mobile phase. Twenty two fractions were obtained (A-V). Fractions D and E were combined (242 mg) and subjected to CC on Sephadex LH-20, eluted with CH3OH to give 7 subfractions D1– D8. From subfractions D5 (80 mg), after LPLC eluted with CHCl3:CH3OH (1:0 to 9:1), seven subfractions (D5-1 - D5-7) were obtained. Subfraction D5-2 (39,2 mg) and D5-4 (19,2 mg) were purified by prep. TLC with CHCl3-EtOAc (9:1) to yield again compound 8 (6.0 mg), and (2R)-7,4'-dihydroxy-5-methoxy-8-methylflavane 1 (8.9 mg) +130.5° (c 0.26, CH3OH) [25], respectively. From fraction F (180 mg) after subsequent usage of silica gel CC, eluted with CHCl3:CH3OH (1:0 to 9:1) and prep. TLC with PE:EtOAc (7:3), a (3R)-7,4'-dihydroxyhomoisoflavane 2 (18 mg) +79.2° (c 0.26, CH3OH) [26,27] was isolated. Fraction G (240 mg) was subjected to CC on Sephadex LH-20, eluted with CH3OH to give 9 subfractions (G1-G7). Subfraction G5 (31 mg) was separated by prep. TLC with CHCl3:EtOAc (8:2) to yield garcinone B 9 (1.5 mg) [28], again compounds 1 (1.8 mg) and 2 (2 mg), and (3S)-7,4'-dihydroxy-5-methoxyhomoisoflavane 3 (9.3 mg) -44° (c 0.35, CH3OH) [27,29]. Fractions O-R (151 mg) were washed with CHCl3, and white crystals of a 10,11-dihydroxydracaenone C 4 (14.2 mg) -457° (c 0.12, CH3OH) [30] were obtained.
The compounds structures were elucidated using 1D and 2D NMR experiments, optical rotation data, and literature data comparison. The spectra and solvents used are indicated in the Supporting information (S2–S12 Figs).
Antimicrobial activity
Antimicrobial activity against Staphylococcus aureus SAIM 209 (collection of the Stephan Angeloff Institute of Microbiology, Bulgaria), Escherichia coli SAIM WF+ and Candida albicans SAIM 562 was evaluated in triplicate by the broth microdilution method according to Clinical Laboratory Standard Institute (CLSI) procedures [31] and as published before [32]. The bacterial inoculums with concentration 105 CFU/ml were added to microtitre trays containing Muller Hinton broth (MHB) loaded with extract or isolated compounds with concentrations in the range of 0.039 to 2.5 mg/ml. Plates were incubated at 37°C for 18 h. The negative control was prepared by spreading 10 μl of the inoculation-suspension on a nutrient agar plate and incubated at 37°C overnight. Minimal inhibitory concentration (MIC) was determined visually as the lowest concentration without visible growth. Minimal bactericidal concentration (MBC) was determined by overnight incubation on Muller Hinton agar (MHA) of 100 μl from the untreated control and samples treated with ½ x MIC, MIC and 2 x MIC for further 18 h at 37°C. MBC was read as concentrations where no bacterial growth occurred on the agar plates. The antibiotics Gentamicin and Amphotericin B were used as positive controls against bacteria and fungi, respectively.
DPPH radical scavenging activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was evaluated by colorimetric procedure [33]. In brief, 100 μl of the 70% EtOH extract and individual compounds at five concentrations (62.5; 125; 250; 500; 1000 μg/ml) were added to 2 ml of 100 μM DPPH ethanol solution. After 30 min, the absorbance at 517 nm was measured. The free radical scavenging activity was determined by comparison with the absorbance of blank (100%), containing only DPPH in ethanol. A graph plot percentage inhibition against concentration was used to calculate the concentration of the tested samples providing 50% inhibition (IC50). Caffeic acid was used as a positive control (6.25, 12.5; 25; 50; 100 μg/ml).
Results and discussion
Phytochemical analysis
Propolis is a resinous material with chemical diversity, which leads to formulation of propolis types. Most of the types formulated are well characterized by various analytical techniques, including GC/MS after silylation. The trends in propolis research using hyphenated techniques provide valuable information in respect to rapid identification of already known propolis constituents, and thus of propolis type dereplication [4,34]. That is why, the crude extract (70% ethanol) of L. cacciae propolis was firstly subjected to GC/MS analysis. Besides sugars and fatty acids, common propolis constituents, presence of alk(en)yl resorcinols, anacardic acids and triterpenes (Fig 1; Table 1) typical for propolis originating from Mangifera indica (Anacardiaceae) plants [35–38] was revealed. However, compounds corresponding to some of the most prominent peaks in the total ion current (TIC) chromatogram remained unidentified, which provoked us to proceed with isolation and identification of individual constituents.
Fig 1. GC chromatogram of 70% ethanol propolis extract.
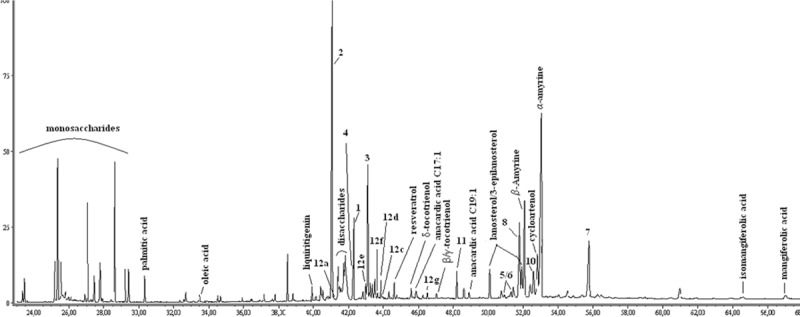
The numbers of compounds identified correspond to those of isolated compounds.
Table 1. Constituents identified in 70% ethanol propolis extract by GC/MS (TMS derivatives).
| Component | TIC, % | Component | TIC, % |
|---|---|---|---|
| Alk(en)ylresorcinolsa | 1.4 | Flavonoids | 18.7 |
| Alkylresorcinol C15H31 (12a) | Tr. | 7,4'-Dihydroxyflavanone (liquiritigenin) | 0,5 |
| Alkylresorcinol C15H29 (12b) | Tr. | (3R)-7,4'-Dihydroxyhomoisoflavane (2)b | 10,7 |
| Alkylresorcinol C17H35 (12c) | Tr. | (2R)-7,4'-Dihydroxy-5-methoxy-8-methylflavane (1)b | 3,4 |
| Alkylresorcinol C17H33 (12d) | 0.6 | 10,11-Dihydroxydracaenone C (4)b | |
| Alkylresorcinol C17H31 (12e) | 0.6 | (3S)-7,4'-Dihydroxy-5-methoxyhomoisoflavane (3)b | 4,1 |
| Alkylresorcinol C17H29 (12f) | Tr. | Xanthones | 8.1 |
| Alkylresorcinol C19H37 (12g) | 0.2 | 3-Geranyloxy-1,7-dihydroxyxanthone (5) | 4.7 |
| Anacardic acids | 0.5 | 7-Geranyloxy-1,3-dihydroxyxanthone (6) | 3.4 |
| Anacardic acid C17H33 | 0.3 | α-Mangostin (8)b | Tr. |
| Anacardic acid C19H37 | 0.2 | Cochinchinone A (7)b | Tr. |
| Triterpenes | 26.6 | Other phenols | 1.0 |
| α-Amyrine | 13.4 | Resveratrol | 0.5 |
| β-Amyrine | 4.8 | δ-Tocotrienol | 0.3 |
| Cycloartenol | 3.0 | β/γ-Tocotrienol | 0.2 |
| Cycloartenone (10) | 1.5 | Fatty acids and esters | 0.9 |
| Lupeol (11) | 1.0 | Palmitic acid | 0.7 |
| Mangiferolic acid | Tr. | Oleic acid | 0.2 |
| Isomangiferolic acid | Tr. | Sugars | 24.2 |
| Lanosterol | 1,3 | Monosaccharides | 17.3 |
| Lanosterol (3-epi) | 1,6 | Disaccharides | 6.9 |
Tr., traces (<0.1%TIC).
aIdentified by comparison with literature data and authentic sample (after isolation).
bIdentified by comparison with authentic samples (after isolation).
The crude propolis extract was extracted successively with PE and DEE. After repeated CC on silica gel and Sephadex, and prep. TLC flavanes, xanthones, triterpenes and a mixture of alk(en)yl recorcinols were isolated. The structures (Fig 2) were elucidated as: (2R)-7,4'-dihydroxy-5-methoxy-8-methylflavane 1, (3R)-7,4'-dihydroxyhomoisoflavane 2, (3S)-7,4'-dihydroxy-5-methoxyhomoisoflavane 3, 10,11-dihydroxydracaenone C 4, a mixture of 3-geranyloxy-1,7-dihydroxyxanthone (cochinchinone G) 5 and 7-geranyloxy-1,3-dihydroxyxanthone 6, 2,6,8-trihydroxy-5-geranyl-7-prenylxanthone (cochinchinone A) 7, α-mangostin 8, garcinone B 9, cycloartenone 10, lupeol 11, and a mixture of alk(en)yl resorcinols 12 by means of NMR (1D and 2D) experiments, optical rotation data, and literature data comparison. Mixture 12 was additionally characterized and confirmed by GC/MS as consisting of resorcinols 12a-g. Among the isolated compounds, six (1–6) were found in propolis for the first time.
Fig 2. Chemical structures of isolated compounds.
Further, selected isolated compounds were used as standards and subjected to GC/MS analysis in order to obtain information for their mass spectral characteristics as TMS derivatives, and then to recognize them in the TIC chromatogram. The data obtained (Table 2) revealed that the fragmentation pathways of the flavonoids 1–4 are in accordance with those proposed by Su et al. [39] and Chen et al. [40], who studied Chinese dragon's blood and its constituents by means of IT-TOF HRMSn and UHPLC-QTOF-MS/MS, respectively. In the mass spectra of the homoisoflavans 2 and 3, the molecular ion was the most prominent peak (base peak), followed by B-ring and AC-rings fragments due to C3–C9 and C9–C1’ bond cleavages, which have been regarded as diagnostic for this type of flavonoids [40,41]. Compounds 4 and 1 gave fragmentation patterns identical to those of the homoisoflavanes and flavan-3-ols [42], respectively. The MS spectra of prenyl 8 and geranyl 7 xanthones showed a low intensity molecular ion peak and a base peak [M-15]+. Fragment ion [M-43]+ due to the subsequent loss of carbonyl group (M-CH3-CO) was also detected in both molecules. All these results could be useful in respect to further dereplication purposes, having in mind that the GC/MS (after silylation) is a rapid and very often used analytical technique for metabolite profiling of propolis and plant resins.
Table 2. Mass spectral data of isolated compounds (GC/MS, TMS derivatives).
| Compound | [M]+, m/z (%) | Fragments, m/z (%) |
|---|---|---|
| (2R)-7,4'-Dihydroxy-5-methoxy-8-methylflavane (1) | 430 (100) | 415 (29), 399 (12), 357 (10), 251 (8), 238 (95), 223(31), 207 (58), 192 (20), 165 (5), 73 (98) |
| (3R)-7,4'-Dihydroxyhomoisoflavane (2) | 400 (100) | 385 (12), 233 (18), 220 (31), 195 (8), 179 (69), 165 (10), 73 (60) |
| (3S)-7,4’-Dihydroxy-5-methoxyhomoisoflavane (3) | 430 (100) | 415 (16), 264 (15), 250 (50), 225 (9), 179 (45), 165 (7), 73 (74) |
| 10,11-Dihydroxydracaenone C (4) | 414 (100) | 399 (23), 357 (15) 341 (2), 267 (4), 253 (4), 179 (5), 147 (2), 73(44) |
| Cochinchinone A (7) | 664 (4) | 649 (100), 621 (6), 579 (32), 565 (24), 539 (9), 511 (18), 491 (16), 73 (26) |
| α-Mangostin (8) | 626 (15) | 611 (100), 595 (4), 583 (41), 553 (13), 73 (53) |
After the compounds were characterised as TMS derivatives, we succeeded in their reliable identification in TIC chromatogram. All six compounds corresponded to prominent peaks with relative abundance of 3–11% of TIC (Fig 1; Table 1). The compounds 3-geranyloxy-1,7-dihydroxyxanthone (5) and 7-geranyloxy-1,3-dihydroxyxanthone (6), isolated in small quantity, were identified in the chromatogram based on the mass spectral pattern of 7 and 8. In contrast, garcinone B (9) was not detected, probably because of overlapping peaks and/or its low concentration.
Since propolis constituents are potential chemical markers for the plants that bees have visited for resin collection [34,43,44], suggestions for the botanical origin of propolis sample analysed were made. The revealing of resin sources is of interest as the appropriate plants contribute to high quality of propolis as well as to the bee colony health [4,45]. In general, the compounds identified by NMR and/or GC/MS data (Table 1) can be divided into three groups, according to the plants from which they have been previously isolated.
The first group includes the flavonoids 1–4 and liquiritigenin, and the stilbene resveratrol, among which the flavanes are well known constituents of the stem and red resin of Dracaena cochinchinensis (Agavaceae) [23,27,29,30,39,46]. The red resinous material, known as Chinese dragon’s blood, is a popular folk medicine used for treatment of fractures, wounds, stomach ulcers, etc. [23,47]. In fact, Dragon’s blood is a common name for the deep red resin/latex obtained from injured trunk and branches of plant species of four genera (Dracaena, Croton, Pterocarpus and Daemonorops) [47,48], amongst which Dracaena spp., and D. cochinchinensis plants in particular, are exclusively distributed in southern China, Vietnam and Laos [49]. It is interesting to note that Chinese Dragon’s blood has been regarded as an induced plant defence against pathogens and pests [48], which is also the role of propolis in the beehive. These findings gave us the chance to suppose a Dracaena spp., most probably D. cochinchinensis, as plant visited by L. cacciae for resin collection. This was somehow supported by the fact that the raw propolis and its 70% ethanol extract were characterized by a deep red color. In addition, D. cochinchinensis is the first monocotyledonous plant showed as a source of propolis resins.
Xanthones 5–9 and the tocopherol derivatives δ-tocotrienol and β/γ-tocotrienol form a second group of taxonomic markers. Recently, prenylated xanthones have been isolated from propolis of the stingless bees Tetragonula leaviceps [50] and T. pagdeni [51,52] collected in Thailand with proven botanical origin Garcinia mangostana (Hypericaceae) [52]. In L. cacciae propolis, however, together with α-mangostin (8) and garcinone B (9), xanthones bearing a geranyl(oxy) group were isolated, and their simultaneous occurrence was found to be characteristic for Cratoxylum cochinchinense (Hypericaceae), a tropical medicinal plant distributed in Southeast Asia [53]. Numerous investigations have revealed that its stem, resin and green fruits are rich source of xanthones with antimicrobial, cytotoxic and antimalarial activities [23,54–56], and a source of vitamin E like compounds [57]. The above-mentioned data provide an evidence for the contribution of C. cochinchinense as a second plant source of the sample analysed. Moreover, C. cochinchinense seems to be the plant which is preferred by Lisotrigona bees’ species as it is the botanical source of propolis collected by L. furva in the same Vietnamese location [16].
The third group of compounds is the combination of the phenolic lipids resorcinols 12a-g and anacardic acids together with the triterpenes, which are found in propolis containing resin of M. indica, as mentioned above. M. indica propolis type has been revealed for honey bee propolis in many regions of Asia, such as Oman [37], Thailand [38], Myanmar [35] and Indonesia [36]. Interestingly, M. indica was also suggested to be a source of Vietnamese propolis from the stingless bee Trigona minor [58,59].
The results showed that the propolis sample analysed is of triple botanical origin. Mixed propolis types have also been established for Apis mellifera propolis collected in different geographical regions [37,60,61].
Antimicrobial activity
The crude extract and some of the isolated compounds were evaluated in vitro for antimicrobial potency against S. aureus, E. coli, and C. albicans. The results (Table 3) showed that the compounds inhibited all three microorganisms, while the crude extract was inactive against C. albicans. For α-mangostin (8), significant activity was observed against S. aureus with MIC and MBC 0.31 μg/ml, followed by 7,4’-dihydroxy-5-methoxy-8-methylflavane (1) with MIC 78 μg/ml and MBC 156 μg/ml. The other constituents displayed low activity, as 4, 7, 10 and 12a-g inhibited the Gram negative bacteria E. coli at lower concentration.
Table 3. Antimicrobial activities of 70% ethanol extract and isolated compounds.
| Sample |
S. aureus SAIM 209 |
E. coli SAIM WF+ |
C. albicans SAIM 562 |
|||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| μg/ml | ||||||
| 70% ethanol extract | 156 | 156 | 156 | 156 | NA | NA |
| 1 | 78 | 156 | 156 | 156 | 156 | 156 |
| 2 | 156 | 313 | 156 | 156 | 156 | 156 |
| 3 | 156 | 156 | 156 | 156 | 156 | 313 |
| 4 | 313 | 625 | 156 | 156 | 313 | 625 |
| 7 | 313 | 625 | 156 | 156 | 313 | 625 |
| 8 | 0.31 | 0.31 | 156 | 156 | 156 | 156 |
| 10 | 313 | 313 | 156 | 156 | 313 | 313 |
| 12a-g | 313 | 625 | 156 | 156 | 313 | 625 |
| Gentamicina | 0.03 | 0.03 | 0.5 | 0.5 | NT | NT |
| Amphotericin Ba | NT | NT | NT | NT | 0.125 | 0.125 |
MIC, minimal inhibitory concentration; MBC, minimal bactericidal concentration; SAIM, collection of the Stephan Angeloff Institute of Microbiology. NA, no activity (> 2.5 mg/ml); NT, not tested
aPositive control.
It is interesting to note that α-mangostin (8) and garcinone B (9) are the major compounds contributing to the antibacterial activity of Thai stingless bee propolis [49], and 8 is one of the most active principles in C. cochinchinense [23]. Boonnak et al. [23] also found a significantly greater antibacterial effect for α-mangostin as compared to cochinchinone A, as well as a higher potency for the mixture of geranyloxy xanthones 5 and 6 in comparison to that of the individual compounds against a panel of Gram positive bacteria.
Free radical scavenging activity
DPPH radical scavenging activity was tested for the crude extract and isolated phenols 1–9, and 12a-g. All tested samples, except for compound 4, were inactive (IC50 >1000 μg/ml). The 10,11-dihydroxydracaenone C (4) displayed good antioxidant ability with IC50 116.0 μg/ml, vs. IC50 69.3 μg/ml of the positive control caffeic acid. Moreover, 4 is the only isolated component bearing ortho-hydroxyl groups, which is in agreement with the fact that ortho-hydroxyl phenols exhibit enhanced antioxidant activity [62]. The lack of antioxidant activity of the crude extract could be explained by the findings that 4 is also the only ortho-hydroxylated molecule identified in the complex mixture of compounds. No ability to scavenge the free DPPH radicals for the homoisoflavans and xanthones has been observed by several research groups [51,62,63].
Conclusion
In the present article, a phytochemical study of propolis produced by the stingless bee Lisotrigona cacciae was described for the first time. The results add new knowledge in the field of propolis research in terms of new constituents and a new plant source. The study also reveals that the propolis of L. cacciae, and stingless bee propolis in general, is a valuable product as well as a promising source of biologically active compounds.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors are grateful to Maya Tavlinova-Kirilova for assistance in measuring Optical Rotations, and to Daniela Antonova for running the GC/MS. The authors are grateful to Prof. Georgi Petkov for editing the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The study was funded by Bulgarian Academy of Sciences and Vietnamese Academy of Science and Technology (VAST.HTQT.Bulgaria.02/17-18) through a bilateral project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lotfy M. Biological Activity of Bee Propolis in Health and Disease. Asian Pac J Cancer Prev. 2006;7: 22–31. Available from: http://journal.waocp.org/article_24421.html [PubMed] [Google Scholar]
- 2.Silva-Carvalho R, Baltazar F, Almeida-Aguiar C. Propolis: a complex natural product with a plethora of biological activities that can be explored for drug development. Evid Based Complement Alternat Med. 2015;2015: 206439 Available from: 10.1155/2015/206439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salatino A, Fernandes-Silva CC, Righi AA, Salatino MaLF. Propolis research and the chemistry of plant products. Nat Prod Rep. 2011; 28(5): 925–936. 10.1039/c0np00072h [DOI] [PubMed] [Google Scholar]
- 4.Bankova V, Popova M, Trusheva B. The phytochemistry of the honeybee. Phytochemistry. 2018;155: 1–11. 10.1016/j.phytochem.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 5.Cortopassi-Laurino M, Imperatriz-Fonseca VL, Roubik D, Dollin A, Heard T, Aguilar I, et al. Global meliponiculture: Challenges and opportunities. Apidologie. 2006;37(2): 275–292. 10.1051/apido:2006027 [DOI] [Google Scholar]
- 6.Choudhari MK, Punekar SA, Ranade RV, Paknikar KM. Antimicrobial activity of stingless bee (Trigona sp.) propolis used in the folk medicine of Western Maharashtra. India. J Ethnopharmacol. 2012;141(1): 363–7. 10.1016/j.jep.2012.02.047 [DOI] [PubMed] [Google Scholar]
- 7.Michener CD. The Meliponini In: Vit P, Pedro SRM, Roubik DW, editors. Pot-Honey: A Legacy of Stingless Bees. New York: Springer; 2013. pp. 3–17. [Google Scholar]
- 8.Bänziger H, Boongird S, Sukumalanand P, Bänziger S. Bees (Hymenoptera: Apidae) That Drink Human Tears. J Kansas Entomological Society. 2009;2(2): 13–151. 10.2317/JKES0811.17.1 [DOI] [Google Scholar]
- 9.Engel MS. A review of the Indo-Malayan meliponine genus Lisotrigona, with two new species (Hymenoptera: Apidae). Oriental Insects 2000;34: 229–237. 10.1080/00305316.2000.10417261 [DOI] [Google Scholar]
- 10.Bänziger H, Bänziger S. Mammals, birds and reptiles as hosts of Lisotrigona bees, the tear drinkers with the broadest host range (Hymenoptera, Apidae). Mitt Schweiz Entomol Ges. 2010;83: 271–282. Available from: https://www.e-periodica.ch/digbib/view?pid=seg-001:2010:83#6 [Google Scholar]
- 11.Karunaratne WAIP, Edirisinghe JP, Engel MS. First record of a tear drinking stingless bee Lisotrigona cacciae (Nurse)(Hymenoptera: Apidae: Meliponini), from the central hills of Sri Lanka. J Natn Sci Foundation Sri Lanka. 2017;45(1): 79–81. 10.4038/jnsfsr.v45i1.8042 [DOI] [Google Scholar]
- 12.Michener CD. Lisotrigona in Thailand, and the Male of the Genus (Hymenoptera: Apidae: Meliponini). J. of the Kansas Entomological Society. 2007;80: 130–136. 10.2317/0022-8567(2007)80[130:LITATM]2.0.CO;2 [DOI] [Google Scholar]
- 13.Rasmussen CA. Stingless bees (Hymenoptera:Apidae:Meliponini) of the Indian subcontinent: Diversity, taxonomy and current status of knowledge. Zootaxa 2013;3647(3): 401–428. 10.11646/zootaxa.3647.3.1 [DOI] [PubMed] [Google Scholar]
- 14.Jongjitvimol T. Poolprasert P. Pollen Sources of Stingless Bees (Hymenoptera: Meliponinae) in Nam Nao National Park, Thailand. Int J Sci. 2014;11(2): 1–10. Available from: http://www.sci.nu.ac.th/sciencejournal/index.php/journal/article/view/278 [Google Scholar]
- 15.Chuttong B, Chanbang Y, Sringarm K, Burgett M. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South East Asia (Thailand). Food Chem. 2016;192: 149–55. 10.1016/j.foodchem.2015.06.089 [DOI] [PubMed] [Google Scholar]
- 16.Thanh LN, Oanh VTK, Thoa HT, Phuong DTL, Lien NTP, Giap TH, et al. Isolated Triterpenes from Stingless Bee Lisotrigona furva Propolis in Vietnam. Journal of Apitherapy and Nature 2018;1(3): 73–73. Available from: http://dergipark.gov.tr/download/article-file/584803 [Google Scholar]
- 17.Thanh LN, Thoa HT, Oanh VTK, Phuong DTL, Vah HT, Chi NQ, et al. Isolated Xanthones from Lisotrigona furva Propolis in Vietnam. Journal of Apitherapy and Nature 2018;1(3): 74–74. Available from: http://dergipark.gov.tr/download/article-file/584809 [Google Scholar]
- 18.Thoa HT, Van HT, Oanh VTK, Phuong DTL, Chi NQ, Thanh LN. [Xanthones isolated from the propolis of the stingless bee Lisotrigona furva Engel]. Tạp chí Dược học. 2010;58(10): 22–24. Vietnamese. [Google Scholar]
- 19.Popova M, Graikou K, Chinou I, Bankova V. GC-MS Profiling of diterpene compounds in Mediterranean propolis from Greece. J Agric Food Chem. 2010;58: 3167–3176. 10.1021/jf903841k [DOI] [PubMed] [Google Scholar]
- 20.Ragasa CY, Jorvina K, Rideout JA. Antimicrobial Compounds from Artocarpus heterophyllus. Philipp J Sci. 2004;133: 97–101. [Google Scholar]
- 21.Imam S, Azhar I, Hasan M, Ali MS, Ahmed W. Two triterpenes lupanone and lupeol isolated and identified from Tamarindus indica Linn. Pak J Pharm Sci. 2007;20(2): 125–127. [PubMed] [Google Scholar]
- 22.Silva MSS, de Lima SG, Oliveira EH, Lopes JAD, Chaves MH, Reis FAM, et al. Anacardic acid derivatives from Brazilian propolis and their antibacterial activity. Ecl Quim. 2008;33(3): 53–58. Available from: http://www.scielo.br/scielo.php?pid=S0100-46702008000300008&script=sci_arttext [Google Scholar]
- 23.Boonnak N, Karalai C, Chantrapromma S, Ponglimanont C, Fun H-K, Kanjana-Opas A, et al. Anti-Pseudomonas aeruginosa xanthones from the resin and green fruits of Cratoxylum cochinchinense. Tetrahedron. 2009;65: 3003–3013. 10.1016/j.tet.2009.01.083 [DOI] [Google Scholar]
- 24.Ahmat N, Azmin NFN, Ghani NA, Aris SR, Sidek NJ, Abdullah S, et al. Bioactive Xanthones from the Pericarp of Garcinia mangostana. Middle-East J Sci Res. 2010;6(2): 123–7. [Google Scholar]
- 25.Awale S, Miyamoto T, Linn TZ, Li F, Win NN, Tezuka Y, et al. Cytotoxic constituents of Soymida febrifuga from Myanmar. J Nat Prod. 2009;72: 1631–1636. 10.1021/np9003323 [DOI] [PubMed] [Google Scholar]
- 26.Meksuriyen D, Cordell GA, Ruangrungsi N, Tantivatana P. Traditional Medicinal Plants of Thailand, IX. 10-Hydroxy-11-methoxydracaenone and 7,10-Dihydroxy-11-methoxydracaenone from Dracaena loureiri. J Nat Prod. 1987;50(6): 1118–1125. 10.1021/np50054a018 [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Cheng K, Cheng W, Zhou T, Jian M, Xu J. Isolation and chatacterization of homoisoflavonoids from Dracaena cochinchinensis and their osteogenic activities in mouse mesenchymalstem cells. J Pharm Biomed Anal. 2016;129: 466–472. 10.1016/j.jpba.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 28.Sen AK, Sarkar KK, Mazumder PC, Banerji N, Uusvuori R, Hase TA. The structures of garcinones a, band c: Three new xanthones from Garcinia mangostana. Phytochemistry. 1982; 21(7): 1747–1750. 10.1016/S0031-9422(82)85052-8 [DOI] [Google Scholar]
- 29.Zheng Q-A, Li H-Z, Zhang Y-J, Yang C-R. Flavonoids from the Resin of Dracaena cochinchinensis. Helv Chim Acta. 2004;87: 1167–1171. 10.1002/hlca.200490106 [DOI] [Google Scholar]
- 30.Zheng Q-A, Li H-Z, Zhang Y-J, Yang C-R. A new meta-homoisoflavane from the fresh stems of Dracaena cochinchinensis. J Asian Nat Prod Res. 2006;8(6): 571–577. 10.1080/1028602042000204126 [DOI] [PubMed] [Google Scholar]
- 31.Watts JL (2008) Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: approved standard. CLSI 3:M31–A33 [Google Scholar]
- 32.Dimitrova L, Zaharieva MM, Popova M, Kostadinova N, Tsvetkova I, Bankova V, et al. Antimicrobial and antioxidant potential of different solvent extracts of the medicinal plant Geum urbanum L. Chem Cent J. 2017;11: 113 10.1186/s13065-017-0343-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murthy NK, Pushpalatha KC, Joshi CG. Antioxidant activity and phytochemical analysis of endophytic fungi isolated from Lobelia nicotianifolia. J Chem Pharm Res. 2011;3(5): 218–225. Available from: www.jocpr.com [Google Scholar]
- 34.Bankova V, Bertelli D, Borba R, Conti BJ, da Silva Cunha IB, Danert C, et al. Standard methods for Apis mellifera propolis research. In: Dietemann V, Ellis JD, Neumann P, editors. The COLOSS BEEBOOK, Volume III: standard methods for Apis mellifera hive products research. J. Apicult. Res. England: Taylor & Francis; 2017. 56g(SI3). Available from: 10.1080/00218839.2016.1222661 [DOI] [Google Scholar]
- 35.Li F, Awale S, Zhang H, Tezuka Y, Esumi H, Kadota S. Chemical constituents of propolis from Myanmar and their preferential cytotoxicity against a human pancreatic cancer cell line. J Nat Prod. 2009;72(7): 1283–1287. 10.1021/np9002433 [DOI] [PubMed] [Google Scholar]
- 36.Trusheva B, Popova M, Koendhori EB, Tsvetkova I, Najdenski H, Bankova V. Indonesian propolis: chemical composition, biological activity and botanical origin. Nat Prod Res. 2011;25(6): 606–613. [DOI] [PubMed] [Google Scholar]
- 37.Popova M, Dimitrova R, Al-Lawati HT, Tsvetkova I, Najdenski H, Bankova V. Omani propolis: chemical profiling, antibacterial activity and new propolis plant sources. Chem Cent J. 2013;7: 158 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3851436/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanpa S, Popova M, Tunkasiri T, Eitssayeam S, Bankova V, Chantawannakul P. Chemical Profiles and Antimicrobial Activities of Thai Propolis Collected from Apis mellifera. Chiang Mai J Sci. 2017;44: 438–448. [Google Scholar]
- 39.Su X-Q, Song Y-L, Zhang J, Huo H-X, Huang Z, Zheng J, et al. Dihydrochalcones and homoisoflavanes from the red resin of Dracaena cochinchinensis (Chinese dragon's blood). Fitoterapia. 2014;99: 64–71. 10.1016/j.fitote.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 40.Chen Q, He L, Mo C, Zhang Z, Long H, Gu X, et al. Rapid Evaluation of Chemical Consistency of Artificially Induced and Natural Resina Draconis Using Ultra-Performance Liquid Chromatography Quadrupole-Time-of-Flight Mass Spectrometry-Based Chemical Profiling. Molecules 2018;23(8): 1850 Available from: 10.3390/molecules23081850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang WZ, Ye M, Qiao X, Wang Q, Bo T, Guo DA. Collision-induced dissociation of 40 flavonoid aglycones and differentiation of the common flavonoid subtypes using electrospray ionization ion-trap tandem mass spectrometry and quadrupole time-of-flight mass spectrometry. Eur J Mass Spectrom. 2012;18(6): 493–503. 10.1255/ejms.1206 [DOI] [PubMed] [Google Scholar]
- 42.Cren-Olive C, Deprez S, Lebrun S, Coddeville B, Rolando C. Characterization of methylation site of monomethylflavan-3-ols by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2000;14: 2312–2319. [DOI] [PubMed] [Google Scholar]
- 43.Inui S, Hosoya T, Kumazawa S. Hawaiian propolis: Comparative analysis and botanical origin. Nat Prod Commun. 2014;9(2): 165–166. [PubMed] [Google Scholar]
- 44.Drescher N, Klein A-M, Schmitt T, Leonhardt SD. A clue on bee glue: New insight into the sources and factors driving resin intake in honeybees (Apis mellifera). PLoS ONE. 2019;14(2): e0210594 Available from: 10.1371/journal.pone.0210594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simone-Finstrom M, Borba RS, Wilson M, Spivak M. Propolis counteracts some threats to honey bee health. Insects. 2017;8(2): 46 Available from: 10.3390/insects8020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang Y, Su G, Li N, Li W, Chen G, Chen R, et al. Preventive agents for neurodegenerative diseases from resin of Dracaena cochinchinensis attenuate LPS‑induced microglia over-activation. J Nat Med. 2019;73: 318 10.1007/s11418-018-1266-y [DOI] [PubMed] [Google Scholar]
- 47.Gupta D, Bleakley B, Gupta RK. Dragon’s blood: Botany, chemistry and therapeutic uses. J Ethnopharmacol. 2008;115: 361–380. 10.1016/j.jep.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 48.Jura-Morawiec J, Tulik M. Dragon’s blood secretion and its ecological significance. Chemoecology. 2016;26: 101–105. 10.1007/s00049-016-0212-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan J-Y, Yi T, Sze-To C-M, Zhu L, Peng W-L, Zhang Y-Z, et al. A Systematic Review of the Botanical, Phytochemical and Pharmacological Profile of Dracaena cochinchinensis, a Plant Source of the Ethnomedicine “Dragon’s Blood”. Molecules 2014;19(7): 10650–10669. Available from: 10.3390/molecules190710650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanpa S, Popova M, Bankova V, Tunkasiri T, Eitssayeam S, Chantawannakul P. Antibacterial compounds from propolis of Tetragonula laeviceps and Tetrigona melanoleuca (Hymenoptera: Apidae) from Thailand. PLoS ONE. 2015;10(5): e0126886 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4436274/ 10.1371/journal.pone.0126886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vongsak B, Kongkiatpaiboon S, Jaisamut S, Machana S, Pattarapanich C. In vitro alpha glucosidase inhibition and free-radical scavenging activity of propolis from Thai stingless bees in mangosteen orchard. Rev Bras Farmacogn. 2015;25: 445–450. Available from: https://www.sciencedirect.com/science/article/pii/S0102695X15001258 [Google Scholar]
- 52.Ishizu E, Honda S, Vongsak B, Kumazawa S. Identification of plant origin of propolis from Thailand stingless bees by comparative analysis. Nat Prod Commun. 2018;13: 973–975. [Google Scholar]
- 53.Laphookhieo S, Maneerat W, Buatip T, Syers JK. New xanthones from Cratoxylum cochinchinense. Can J Chem. 2008;86: 757–760. 10.1139/V08-076 [DOI] [Google Scholar]
- 54.Laphookhieo S, Maneerat W, Koysomboon S. Antimalarial and Cytotoxic Phenolic Compounds from Cratoxylum maingayi and Cratoxylum cochinchinense. Molecules. 2009;14: 1389–1395. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6254373/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahabusarakam W, Rattanaburi S, Phongpaichit S, Kanjana-Opas A. Antibacterial and cytotoxic xanthones from Cratoxylum cochinchinense. Phytochem Lett. 2008;1: 211–214. 10.1016/j.phytol.2008.09.012 [DOI] [Google Scholar]
- 56.Ren Y, Matthew S, Lantvit DD, Ninh TN, Chai H, Fuchs JR, et al. Cytotoxic and NF-κB Inhibitory Constituents of the Stems of Cratoxylum cochinchinense and Their Semisynthetic Analogues. J Nat Prod. 2011;74: 1117–1125. 10.1021/np200051j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chailap B, Nuanyai T1, Puthong S, Buakeaw A. Chemical Constituents of Fruits and Leaves of Cratoxylum cochinchinense and Their Cytotoxic Activities. Naresuan University Journal: Science and Technology 2017;25(3): 22–30. Availale from: http://www.journal.nu.ac.th/NUJST/article/view/1892 [Google Scholar]
- 58.Nguyen HX, Nguyen MTT, Nguyen NT, Awale S. Chemical Constituents of Propolis from Vietnamese Trigona minor and Their Antiausterity Activity against the PANC-1 Human Pancreatic Cancer Cell Line. J Nat Prod. 2017;80(8): 2345–2352. 10.1021/acs.jnatprod.7b00375 [DOI] [PubMed] [Google Scholar]
- 59.Nguyen HX, Do TNV, Nguyen MTT, Dang PH, Tho LH, Awale S, Nguyen NT. A New Alkenylphenol from the Propolis of Stingless Bee Trigona minor. Nat Prod Commun. 2018; 13(1): 69–70. [Google Scholar]
- 60.Cuesta-Rubio O, Piccinelli AL, Fernandez MC, Hernández IM, Rosado A, Rastrelli L. Chemical Characterization of Cuban Propolis by HPLC−PDA, HPLC−MS, and NMR: the Brown, Red, and Yellow Cuban Varieties of Propolis. J Agric Food Chem. 2007;55(18): 7502–7509. 10.1021/jf071296w [DOI] [PubMed] [Google Scholar]
- 61.Popova M, Trusheva B, Khismatullin R, Gavrilova N, Legotkina G, Lyapunov J, et al. The Triple Botanical Origin of Russian Propolis from the Perm Region, Its Phenolic Content and Antimicrobial Activity. Nat Prod Commun. 2013;8(5): 617–620. [Google Scholar]
- 62.Luo Y, Wang H, Xu X, Mei W, Dai H. Antioxidant Phenolic Compounds of Dracaena Cambodiana. Molecules. 2010;15: 8904–8914. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6259265/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Udomchotphruet S, Phuwapraisirisan P, Sichaem J, Tip-pyang S. Xanthones from the stems of Cratoxylum cochinchinense. Phytochemistry. 2012;73: 148–151. 10.1016/j.phytochem.2010.04.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.