Abstract
Background
People with abdominal aortic aneurysm who receive endovascular aneurysm repair (EVAR) need lifetime surveillance to detect potential endoleaks. Endoleak is defined as persistent blood flow within the aneurysm sac following EVAR. Computed tomography (CT) angiography is considered the reference standard for endoleak surveillance. Colour duplex ultrasound (CDUS) and contrast‐enhanced CDUS (CE‐CDUS) are less invasive but considered less accurate than CT.
Objectives
To determine the diagnostic accuracy of colour duplex ultrasound (CDUS) and contrast‐enhanced‐colour duplex ultrasound (CE‐CDUS) in terms of sensitivity and specificity for endoleak detection after endoluminal abdominal aortic aneurysm repair (EVAR).
Search methods
We searched MEDLINE, Embase, LILACS, ISI Conference Proceedings, Zetoc, and trial registries in June 2016 without language restrictions and without use of filters to maximize sensitivity.
Selection criteria
Any cross‐sectional diagnostic study evaluating participants who received EVAR by both ultrasound (with or without contrast) and CT scan assessed at regular intervals.
Data collection and analysis
Two pairs of review authors independently extracted data and assessed quality of included studies using the QUADAS 1 tool. A third review author resolved discrepancies. The unit of analysis was number of participants for the primary analysis and number of scans performed for the secondary analysis. We carried out a meta‐analysis to estimate sensitivity and specificity of CDUS or CE‐CDUS using a bivariate model. We analysed each index test separately. As potential sources of heterogeneity, we explored year of publication, characteristics of included participants (age and gender), direction of the study (retrospective, prospective), country of origin, number of CDUS operators, and ultrasound manufacturer.
Main results
We identified 42 primary studies with 4220 participants. Twenty studies provided accuracy data based on the number of individual participants (seven of which provided data with and without the use of contrast). Sixteen of these studies evaluated the accuracy of CDUS. These studies were generally of moderate to low quality: only three studies fulfilled all the QUADAS items; in six (40%) of the studies, the delay between the tests was unclear or longer than four weeks; in eight (50%), the blinding of either the index test or the reference standard was not clearly reported or was not performed; and in two studies (12%), the interpretation of the reference standard was not clearly reported. Eleven studies evaluated the accuracy of CE‐CDUS. These studies were of better quality than the CDUS studies: five (45%) studies fulfilled all the QUADAS items; four (36%) did not report clearly the blinding interpretation of the reference standard; and two (18%) did not clearly report the delay between the two tests.
Based on the bivariate model, the summary estimates for CDUS were 0.82 (95% confidence interval (CI) 0.66 to 0.91) for sensitivity and 0.93 (95% CI 0.87 to 0.96) for specificity whereas for CE‐CDUS the estimates were 0.94 (95% CI 0.85 to 0.98) for sensitivity and 0.95 (95% CI 0.90 to 0.98) for specificity. Regression analysis showed that CE‐CDUS was superior to CDUS in terms of sensitivity (LR Chi2 = 5.08, 1 degree of freedom (df); P = 0.0242 for model improvement).
Seven studies provided estimates before and after administration of contrast. Sensitivity before contrast was 0.67 (95% CI 0.47 to 0.83) and after contrast was 0.97 (95% CI 0.92 to 0.99). The improvement in sensitivity with of contrast use was statistically significant (LR Chi2 = 13.47, 1 df; P = 0.0002 for model improvement).
Regression testing showed evidence of statistically significant effect bias related to year of publication and study quality within individual participants based CDUS studies. Sensitivity estimates were higher in the studies published before 2006 than the estimates obtained from studies published in 2006 or later (P < 0.001); and studies judged as low/unclear quality provided higher estimates in sensitivity. When regression testing was applied to the individual based CE‐CDUS studies, none of the items, namely direction of the study design, quality, and age, were identified as a source of heterogeneity.
Twenty‐two studies provided accuracy data based on number of scans performed (of which four provided data with and without the use of contrast). Analysis of the studies that provided scan based data showed similar results. Summary estimates for CDUS (18 studies) showed 0.72 (95% CI 0.55 to 0.85) for sensitivity and 0.95 (95% CI 0.90 to 0.96) for specificity whereas summary estimates for CE‐CDUS (eight studies) were 0.91 (95% CI 0.68 to 0.98) for sensitivity and 0.89 (95% CI 0.71 to 0.96) for specificity.
Authors' conclusions
This review demonstrates that both ultrasound modalities (with or without contrast) showed high specificity. For ruling in endoleaks, CE‐CDUS appears superior to CDUS. In an endoleak surveillance programme CE‐CDUS can be introduced as a routine diagnostic modality followed by CT scan only when the ultrasound is positive to establish the type of endoleak and the subsequent therapeutic management.
Plain language summary
Ultrasonography versus computed tomography scan for endoleak detection after endoluminal abdominal aortic aneurysm repair
Background
An abdominal aortic aneurysm (AAA) is a localised swelling or widening of a major vessel that carries blood to the abdomen (tummy), pelvis, and legs. People with AAA are at risk from sudden death due to AAA rupture (bursting). Once detected, intervention (treatment) is recommended once the AAA is bigger than about 5 cm in diameter. Most repairs are now performed using a new vessel lining inside the aneurysm guided by x‐ray control (endovascular aneurysm repair or EVAR).
Once the new lining is in place, the seals at either end may leak or vessel branches arising from the aneurysm wall may bleed backwards into the AAA sac. These are collectively referred to as endoleaks. Endoleaks are common after EVAR, developing in about 40% of people during monitoring (follow‐up). Endoleaks can be associated with late aneurysm rupture and, therefore, detection and monitoring is essential. Ultrasound (uses high‐frequency sound waves), computed tomography (uses x‐rays), and magnetic resonance scans (uses strong magnetic fields and radio waves) have all been used to detect and monitor endoleaks. Sometimes, dye (contrast) is injected into a vein to improve the accuracy of ultrasound (contrast‐enhanced ultrasound).
Study characteristics
We collected the most recent evidence (to July 2016) and conducted a meta‐analysis according to the most appropriate methods for diagnostic tests. We included 42 studies with 4220 participants in the review.
Key results
The analyses measured sensitivity (how well a test identified people with endoleak correctly) and specificity (how well a test identified people without endoleak correctly). The summary accuracy estimates were sensitivity 82% (95% confidence interval 66% to 91%) and specificity 93% (95% confidence interval 87% to 96%) for ultrasonography without contrast; and sensitivity 94% (95% confidence interval 85% to 98%) and specificity 95% (95% confidence interval 90% to 98%) for ultrasonography with contrast. Use of contrast improved the sensitivity of ultrasound significantly. Based on these results, we would expect 94% of people with endoleaks will be correctly identified by contrast‐enhanced ultrasound.
Quality of the evidence
Studies that evaluated contrast‐enhanced ultrasound used better methods than the studies that evaluated ultrasound alone.
Summary of findings
Summary of findings'. 'Ultrasound for endoleak detection in participants who received endoluminal abdominal aortic aneurysm repair.
| Ultrasound for endoleak detection in participants who received endoluminal abdominal aortic aneurysm repair | |||||
| Population | Participants who received endovascular stent for abdominal aortic aneurysm. | ||||
| Index test | Ultrasound with or without contrast. | ||||
| Target condition | Endoleak (type I, II, III or IV). | ||||
| Reference standard | CT scan. | ||||
| Included studies | Cross‐sectional studies (studies that provided individual data only). | ||||
| Test | Number of studies (participants) | Prevalence % (median) | Summary accuracy | Implications | Quality |
| CDUS | 16 studies (1357) |
22% | Sensitivity: 0.82 (95% CI 0.66 to 0.91) Specificity: 0.93 (95% CI 0.87 to 0.96) |
Of 1000 people who receive CDUS, 35 people will have their endoleaks missed and 47 people will have an unnecessary CT scan. | Moderate/low: in 40% of studies, delay between tests was unclear (4/16) or > 4 weeks (2/16); in 50% of studies, blinding of either index test or reference standard not clearly reported or not performed; in 12%, interpretation of reference standard not clearly reported. |
| CE‐CDUS | 11 studies (947) |
25% | Sensitivity: 0.94 (95% CI 0.85 to 0.98) Specificity: 0.95 (95% CI 0.90 to 0.98) |
Of 1000 people who receive CE‐CDUS, 15 people will have their endoleaks missed and 47 people will have an unnecessary CT scan. | High/moderate: 5 studies (45%) fulfilled all QUADAS items; 4 (36%) studies did not report clearly blinding interpretation of reference standard; in 2 (18%) studies, the delay between the 2 tests not clearly reported. |
CDUS: colour duplex ultrasound; CE‐CDUS: contrast‐enhanced colour duplex ultrasound; CT: computed tomography.
Background
Target condition being diagnosed
Abdominal aortic aneurysm (AAA) is a localised dilation (of 3 cm or more) of the aorta. The prevalence of AAA increases with age and occurs much more frequently in men than women. The Tromsø Study, a population‐based study with 6386 participants, estimated an AAA prevalence of 8.9% in men and 2.2% in women (Singh 2001). In addition to gender, the following were strong risk factors for AAA: smoking, hypertension, hypercholesterolaemia (Forsdahl 2009), and family history (Hemminki 2006; Ogata 2005).
Most aneurysms are asymptomatic and once the AAA diameter exceeds 5 cm, rupture risk is considered to exceed the operative repair risk and therefore, elective repair is usually offered. The aim of endoluminal or endovascular abdominal aneurysm repair (EVAR) is to reach the target site via a remote vessel to deliver the stent, secure endograft fixation, and allow the formation of a haemostatic seal between the graft and the vessel wall. Several randomised controlled trials have documented the efficacy of EVAR. The anatomic suitability rate for EVAR varies between 15% (Wilson 2004) and 49% (Kristmundsson 2014) depending on multiple factors, including aortic anatomy and size, individual clinical judgement, and manufacturers' guidelines (Erbel 2014). Although, there is no advantage of EVAR in terms of long‐term mortality, the application EVAR technology is effective in reducing the 30‐day mortality rates, intensive care unit and hospital stay, and other complication rates (Adriaensen 2002; Brown 2012; Greenhalgh 2004; Prinssen 2004). Two randomised trials confirmed this with six years' (De Bruin 2010) and eight' years follow‐up (Greenhalgh 2010). EVAR is associated with significant long‐term complications such as late conversion to open repair, late rupture, and endoleaks (De Bruin 2010; Greenhalgh 2010; Leurs 2004).
Endoleak is the most common complication of the EVAR procedure and is characterized by persistent blood flow within the aneurysm sac. There are different types of endoleak (Table 2). Type II endoleaks are the most common, caused by back‐bleeding into the aneurysm sac from the lumber, inferior mesenteric, or other branch arteries. Persistent endoleak may cause enlargement and rupture of the aneurysm which may become the main indication for surgical late conversion (Becquemin 1999; Bush 2001; Hechelhammer 2005; Makaroun 1999; Zarins 2000). Estimates of the incidence rate of endoleak are highly variable and range from 10% to 50% (Cuypers 1999; Franco 2000; Gilling‐Smith 2000; Golzarian 1997; Gorich 2000; Schurink 1998). This variability may have different origins including the type of stent used or the sensitivity of the means used to perform the diagnosis. Moreover, the rate of complications does not diminish over time (Sampram 2003).
1. Classification scheme of endoleaks.
| Endoleak type | Description |
| Type I |
Attachment site leak‐proximal or distal. Type I endoleak are the most common that occur after endovascular repair. Typical in participants with complex arterial anatomy. |
| Type II |
Collateral vessel‐leak. Frequent type of endoleak characterized by retrograde blood flow through aortic branch vessels into the aneurism sac. |
| Type III |
Graft failure. Type III endoleaks are caused by a structural failure of the stent‐graft including fractures, holes of the device during production or junctional separations. Recurring stresses due to arterial pulsation or the aneurysmal pressure can be potential causes. Type III are infrequent. |
| Type IV |
Graft wall porosity. Type IV are caused by stent porosity. |
| Type V |
Endotension. This type of endoleak related to the expansion of the aneurysm. The cause is unknown. |
In contrast to open surgery, people with EVAR need lifetime surveillance with the purpose of controlling graft position and fixation, monitor aneurysmal sac diameter, and detect endoleak. Any enlarging aneurysm sac after EVAR can be an indicator of endoleak and this requires careful investigation. Identification of endoleak is critical because if left untreated, it can enhance the risk of aneurysmal rupture due to its progressive enlargement (Harris 2000; Hinchliffe 2001). In two randomised trials, the cumulative rate of reintervention for people who received EVAR was 30% (De Bruin 2010; Greenhalgh 2010). While some type II endoleaks can resolve spontaneously or result in aneurismal stability and shrinkage (Lawrence‐Brown 2009), most endoleak types need conversion to surgical repair or insertion of a new stent or graft. A variety of other methods to treat or repair endoleaks have been proposed: coil embolization, direct thrombin injection of the aneurysm sac, or direct surgical and laparoscopic ligation (Faries 2003; Rhee 2003).
Index test(s)
Different modalities exist for postoperative surveillance of aortic endograft including plain film radiograph, computed tomography (CT) scan, colour duplex ultrasound (CDUS) including contrast‐enhanced CDUS (CE‐CDUS), magnetic resonance (MR), and angiography.
The index test for the present review is ultrasound (US) (either CDUS or CE‐CDUS). US is a widely available instrument used in clinical practice for endoleak detection in people who have undergone EVAR and offers several potential advantages compared to CT: less invasive, lower cost, and easier to perform. In addition, factors such as the absence of the risk associated with ionising radiation, shorter scan times, and absence of nephrotoxicity make the CDUS an attractive alternative to CT scanning.
The main limitation of US is that it is highly dependent on operator skills. Another limitation is that, in a few circumstances, such as obesity or bowel gas, the aorta cannot be visualized.
Clinical pathway
The occurrence of endoleaks, migration of stent, or aneurysm enlargement following EVAR render the execution of a systematic surveillance programme mandatory for all patients.
There are no uniformly accepted guidelines for EVAR‐related complication surveillance. Generally, however, patients are scheduled for clinical and imaging visits at one, six, and 12 months postoperatively and, from the second year onwards, follow‐up every six or 12 months. In addition, any potential clinical pathway algorithm depends on the type of the endoleak. For example, Karch 1999 suggest CT scanning as a surveillance modality of choice in endografted patients, supplemented with angiography to localize the precise aetiology of any endoleak detected. After confirmation with angiography, their algorithm suggests surgical or endovascular repair for type I, III, and IV endoleaks and observation for type II endoleaks. This algorithm does not mention the use of US probably because the accuracy of US to detect endoleak was low.
The most recent clinical guideline provides a similar but simplified algorithm that indicates CT scan at 30 days and at 12 months followed thereafter by yearly US in addition to plain radiography. When type I and III endoleaks are detected, surgical treatment is usually recommended. In the event of type II endoleak, the guideline recommends CT scan plus plain radiography at six and 12 months (Moll 2011). However, in general, the role of US in a clinical pathway for people who require endoleak monitoring is unclear. We expect that the results from the present review may clarify the role of US as a triage test for people who received EVAR for AAA.
Alternative test(s)
Despite the availability of advanced equipment, abdominal plain radiography is a useful technique for endoleak surveillance. Radiographs are necessary for the confirmations of stent or to identify stent fracture or migration.
Gadolinium‐enhanced MR angiography (MRA) is an alternative test to detect endoleaks and may be particularly indicated for people who have contraindications to CT scan. MRA is as sensitive as CT in detecting endoleaks (Cejna 2002; Insko 2003; Van der Laan 2006). However, the image quality of MRA depends on the material composition of the graft. For example, nitinol stents are the best candidates for MRA surveillance while stainless steel or elgiloy stents produce significant artefacts (Engellau 1998; Haulon 2001). In addition, MRA has the disadvantages of high cost and may not be widely available.
Rationale
People with AAA who received EVAR need lifetime surveillance to detect potential endoleaks. CTA is considered the reference standard for endoleak surveillance due to its high sensitivity (Gorich 2001; Iezzi 2006; Stolzmann 2008). There is no agreement about the timing and the number of examinations to be performed, mainly in the presence of complications requiring further adjunctive surveillance. The European Collaborators on Stent/graft Techniques for Aortic Aneurysm Repair (EUROSTAR) Registry recommends CTA follow‐up at one, six, and 12 months after stent positioning (Vallabhaneni 2001). However, CT scans can be performed more frequently than expected, raising the possibility of radiation exposition concerns (Brenner 2007). In addition, the CT scan is associated with a cumulative risk of nephrotoxicity due to the use of contrast (Brenner 2007). US with or without the use of contrast agents is widely available, easy to use, and less expensive diagnostic tool for identifying endoleaks and can be a potential alternative to CT scan.
With the presence of false positives, the use of US may have no consequence since a suspected endoleak will always need a further investigation by a CT scan. With the presence of false negatives, people can be at risk of having a spontaneous abdominal rupture until the next examination is performed.
Objectives
To determine the diagnostic accuracy of colour duplex ultrasound (CDUS) and contrast‐enhanced‐colour duplex ultrasound (CE‐CDUS) in terms of sensitivity and specificity for endoleak detection after endoluminal abdominal aortic aneurysm repair (EVAR).
Secondary objectives
We aimed to explore several potential sources of heterogeneity by examining differences in diagnostic accuracy estimation according to technical differences of the imaging tests, US of different generations, and age of participants. We also aimed to explore heterogeneity related to methodological study quality items of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklists. We planned to explore further sources of heterogeneity concerning the size of the aneurysm, characteristics of patient population (concomitant disease, severity of aneurismal disease, location of aneurysm), and rupture of aneurysm.
Methods
Criteria for considering studies for this review
Types of studies
Any cross‐sectional diagnostic study was considered for inclusion if:
the participants were evaluated by both US (with or without contrast) and CT scan;
the assessments of both US and CT scan were performed at regular intervals during follow‐up.
Participants
People who received EVAR for AAA treatment and were under follow‐up for endoleak detection.
Index tests
The index test was Doppler US (either CDUS or CE‐CDUS) for the assessment of endoleak in people with EVAR.
The CDUS is a non‐invasive, non‐expensive, easy‐to‐use instrument for endoleak detection. CDUS depends on the experience of the operator and provides limited images for independent review by others. CE‐CDUS requires an intravenous injection with a contrast which consists of microbubbles that resonate when examined with sound of low intensity. The outcome of the index test is the presence of a leak from the endovascular graft that allows blood flow outside the stent but within the aneurysm sac.
Target conditions
Endoleak detected during follow‐up surveillance in people who received EVAR for AAA.
Reference standards
CT is the imaging technique of choice for follow‐up after EVAR.
Search methods for identification of studies
The review authors performed a comprehensive literature search to identify relevant studies. We did not use methodology filters when searching for diagnostic accuracy studies to maximise sensitivity. We sought translations for non‐English language studies.
Electronic searches
We searched the following trial databases were searched in June 2016:
MEDLINE (OvidSP) Appendix 1;
Embase (OvidSP) Appendix 2;
LILACS (lilacs.bvsalud.org/en/) Appendix 3;
ISI Conference Proceedings Citation Index ‐ Science; Appendix 4;
British Library Zetoc conference search (zetoc.mimas.ac.uk); Appendix 5.
We searched the following trial registries (June 2016) for details of ongoing and unpublished studies (see Appendix 6):
World Health Organization International Clinical Trials Registry (apps.who.int/trialsearch/);
ClinicalTrials.gov (ClinicalTrials.gov/);
ISRCTN Register (www.isrctn.com/).
Searching other resources
We contacted study authors for further details on the published studies when data were unclear.
We checked bibliographic citations of reviews for additional references.
We checked bibliographic citations in reports and in other reviews relevant to our topic for additional references.
We consulted the Science Citation Index to identify articles that have cited the studies included in the review.
Data collection and analysis
Selection of studies
Two pairs of review authors independently screened the title and abstract of all studies identified by the search strategy and obtained the full articles for all potentially relevant studies. We re‐assessed the full text of these reports independently and extracted data using a standardised form. When studies were excluded, we stated the reason of exclusion. A third review author resolved disagreements.
Data extraction and management
Two pairs of review authors extracted data independently and compared data. Another review author checked data for consistency.
We contacted authors of diagnostic accuracy studies for details when data from the reports were insufficient.
Unit of analysis issues
The primary unit of analysis was the number of individual participants included in the studies. The studies that provided accuracy data based on number of scans performed (and not on individual participant basis) were used for a secondary (explanatory only) analysis.
Assessment of methodological quality
Two review authors independently assessed the methodological quality of each included study using the QUADAS checklist (Whiting 2003). We classified each item as 'yes' (adequately addressed), 'no' (inadequately addressed), or 'unclear' (if insufficient information was reported) according to the criteria listed in Table 3. We resolved discrepancies by consensus.
2. QUADAS methodological items and operational definitions.
| Item definition | Item question | Assessment |
| 1. Representative spectrum (spectrum bias) | Was the spectrum of participants representative of the patients who will receive the test in practice? | Yes: if the study includes a consecutive series of participants referred for follow‐up to detect potential endoleaks. No: if the referred participants were not under follow‐up for endoleak detection. Unclear: insufficient information to make a judgement. |
| 2. Acceptable reference standard | Was the reference standard likely to classify the target condition correctly? | Yes: CT scan test with contrast agents performed and images evaluated by a radiologist. No: reference standard did not meet criteria outlined above. Unclear: insufficient information to make a judgement. |
| 3. Acceptable delay between tests | Was time period between reference standard and index test short enough to be reasonably sure that target condition did not change between the 2 tests? | Yes: time period between index test and reference standard ≤ 4 weeks. No: time period > 4 weeks. Unclear: insufficient information to make a judgement. |
| 4. Partial verification avoided | Did the whole sample or a random selection of the sample, receive verification using a reference standard of diagnosis? | Yes: all study participants accounted for and results of reference standard reported for all. No: not all participants who received index test received verification by reference standard. Unclear: insufficient information to make a judgement. |
| 5. Differential verification avoided | Did participants receive the same reference standard regardless of the index test result? | Yes: all participants who received index test were subjected to same reference standard. No: not all participants who received index test were subjected to same reference standard.; Unclear: insufficient information to make a judgement. |
| 6. Incorporation avoided | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes: index test was not part of reference standard. No: index test was clearly part of reference standard. Unclear: insufficient information was given to make a judgement. |
| 7. Reference standard results blinded | Were the index test results interpreted without knowledge of the results of the reference standard? | Yes: explicitly stated that index test was interpreted without knowledge of reference standard. No: if assessor of index test was aware of results of reference standard. Unclear: insufficient information to make a judgement. |
| 8. Index test results blinded | Was the execution of the reference standard described in sufficient detail to permit its replication? | Yes: explicitly stated that reference standard was interpreted without knowledge of index test. No: if assessor of reference standard was aware of results of index test. Unclear: insufficient information to make a judgement. |
| 9. Relevant clinical information | Were the same clinical data available when the index test results were interpreted as would be available when the test is used in practice? | Yes: clinical data (age, gender, symptoms, type of stent) would ordinarily be available in clinical practice when index test was being interpreted AND these same clinical data were available in this study when index test was being interpreted. No: above clinical data were not available when index test and reference standard were interpreted. Unclear: insufficient information to make a judgement. |
| 10. Uninterpretable results reported | Were uninterpretable/intermediate test results reported? | Yes: reported results for all study participants, including those with uninterpretable, indeterminate, or intermediate results of index test and reference standard. No: uninterpretable, indeterminate, or intermediate results of index test or reference standard were not reported OR results of index test and reference standard were not reported for all study participants. Unclear: insufficient information to make a judgement. |
| 11. Withdrawals explained | Were withdrawals from the study explained? | Yes: clear what happened to all participants who entered study, e.g. if a flow diagram of study participants reported explaining any withdrawals or exclusions, or numbers recruited match those in analysis. No: appeared that some participants who entered study did not complete study, i.e. did not receive both index test and reference standard, and these participants were not accounted for. Unclear: insufficient information to make a judgement. |
CT: computed tomography.
In addition to providing a methodological quality graph that shows the judgements for each QUADAS item of all the studies, we also generated overall graphical representation of the quality for each type of US that were included in the primary analysis (individual based data).
Statistical analysis and data synthesis
To perform analysis, studies differed in the use of the unit of analysis, that is, while in some studies the unit of analysis was the number of participants, in other studies the unit of analysis was the number of scans. This means that in the studies that used the number of scans some of the participants were counted more than once and this may introduce bias. Hence, in the primary analysis, we considered studies that used the number of participants as the unit of analysis and, in the secondary analysis, we considered studies that performed analysis based on the number of scans (the latter was used as an explanatory or corroborative to the primary analysis).
For both primary and secondary analyses, we carried out the statistical analyses following recommendations reported in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Macaskill 2010). We used Review Manager 5 software for analyses and plots (RevMan 2014). For studies that, in addition to the standard US, used different modalities (such as three‐ (3D) or four‐dimensional (4D) US) to assess the accuracy of US, we considered primarily the data based on standard US data in our analyses.
We generated a 2 × 2 table of true positive cases, false positive cases, false negative cases, and true negative cases. We calculated sensitivity and specificity with 95% confidence intervals (CIs) for each study. We performed meta‐analyses using the bivariate model (Reitsma 2005). Since fitting the model is too complex to implement within Review Manager 5, we used SAS statistical software (SAS 2008) and STATA 13 to generate parameter estimates (logit and variances). Parameter estimates from the bivariate model were transferred to Review Manager to produce the summary receiver operating characteristic (ROC) curve, the summary operating point (i.e. summary values for sensitivity and specificity), a 95% confidence region around the summary operating point, and a 95% prediction region.
We opted to employ the bivariate model as it is recommended for purely binary tests or when different studies report similar thresholds (Leeflang 2014).
We calculated positive (LR+) and negative (LR‐) likelihood ratios using summary sensitivity and specificity.
To determine the meaningfulness or clinical utility of US either with or without contrast we employed a Fagan plot as well as the likelihood ratio (LR) scatterplot matrix. Fagan plot is a graphical tool for estimating how much the result on a diagnostic test changes the probability that a person has a disease (Fagan 1975). LR ratio scatterplot matrix plots LR+ against LR‐ with 95% CIs and illustrates the distribution of accuracy estimates of individual studies. The matrix allows identification of outliers, as well as studies relevant for sensitivity analyses (Stengel 2003).
Investigations of heterogeneity
The factors that we proposed in the protocol to investigate for potential heterogeneity included:
characteristics of participant population (age, concomitant disease, severity of aneurismal disease, location of aneurysm);
size of the aneurysm (diameter and length);
technical differences of imaging tests (advanced, recent instruments versus older);
type of stent;
rupture of aneurysm.
We were able to investigate the following variables as a source of heterogeneity: use of contrast (CDUS versus CE‐CDUS), year of publication, characteristics of included participants (age and gender), direction of study (retrospective, prospective), methodological quality, country of origin, number of CDUS operators, and US manufacturer.
We investigated heterogeneity by visual inspection of forest plots and ROC plots. Moreover, we used a regression analysis to investigate the effects of the sources of heterogeneity on sensitivity and specificity by including the factors in the bivariate models.
For the covariate 'use of contrast,' we performed direct and indirect comparisons between CDUS and CE‐CDUS. The direct comparison refers to the studies that performed on the same occasion accuracy analysis before and after administration of contrast. For both comparisons, we performed a bivariate analysis including all the studies in one data set and inserting a binary covariate 'test type' in the model. Using the derived logit estimates of sensitivity and specificity and their respective covariances, we constructed summary ROC curves for CDUS and CE‐CDUS, with summary operating points for sensitivity and specificity on the curves and a 95% confidence region. The variance coefficients were assessed to investigate heterogeneity in sensitivities and specificities. The size of the prediction region on the summary receiver operator curve (SROC) plot can indicate the magnitude of potential heterogeneity. A regression test was used to assess the effects of the covariate 'use of contrast' on sensitivity and specificity.
Sensitivity analyses
We planned a sensitivity analysis based on type of study design (prospective versus retrospective study designs), type and generation of the index tests, and individual quality items.
Assessment of reporting bias
We did not assess reporting bias.
Results
Results of the search
See Figure 1.
1.
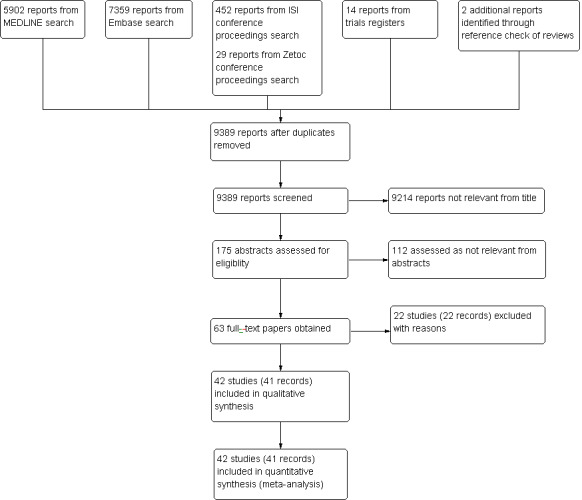
Study flow diagram.
The search strategy generated 9389 records for evaluation after removing duplicates. After screening the titles, we considered 175 abstracts relevant for investigation leaving 63 records for which a full‐text assessment was necessary. After excluding 22 studies with reasons, we included 42 studies in qualitative and quantitative analyses. We checked the reference lists of five reviews (Ashoke 2005a; Bakken 2010; Karthikesalingam 2012; Mirza 2010; Sun 2006) and identified and included two unpublished studies (Ashoke 2005b; Ashoke 2005c), both of which were reported in one review (Ashoke 2005a).
Characteristics of excluded studies
See Characteristics of excluded studies table.
Twenty‐two studies were excluded with the following reasons: in four studies the performance of one test depended on the results of the other (Chisci 2012; Collins 2007; Greenfield 2002; Harrison 2011); three studies included participants in the follow‐up programme based on the suspicion of endoleak (Clevert 2008a; Pfister 2009; Sommer 2012); four studies used US and CT scan for EVAR surveillance but did not evaluate endoleak (Almaroof 2013; Bredahl 2013; Clevert 2013; Han 2010); three studies reported insufficient data for 2 × 2 table production (Elkouri 2004; Hertault 2015; Nyheim 2013); two studies did not perform the two tests concurrently (Manning 2009; Napoli 2004); two studies evaluated a subset of participants with probable or possible endoleaks (Millen 2013: Yang 2015); one study used angiography as reference standard (Ormesher 2014); one study selected participants based on the presence of insurance coverage (Beeman 2009); one study was a follow‐up study of Beeman 2009 (Troutman 2014); and one study selected retrospectively participants with EVAR based on the presence of both tests (Sorrentino 2015).
Characteristics of included studies
See Characteristics of included studies table.
Forty‐two studies with 4220 participants were eligible for inclusion. Of these, 11 evaluated US with and without contrast (Bendick 2003; Cantisani 2011; Clevert 2008b; Clevert 2011; Costa 2013; Giannoni 2003; Heilberger 1997; Henao 2006; Iezzi 2009; McWilliams 1999; McWilliams 2002); 23 studies evaluated only CDUS (without contrast) (AbuRahma 2005; Arsicot 2014; Ashoke 2005b; Ashoke 2005c; Badri 2010; d'Audiffret 2001; Demirpolat 2011; França 2013; Golzarian 2002; Gray 2012; McLafferty 2002; Nagre 2011; Nerlekar 2006; Oikonomou 2012; Pages 2001; Parent 2002; Raman 2003; Sandford 2006; Sato 1998; Schmieder 2009; Thompson 1998; Wolf 2000; Zannetti 2000); and eight studies evaluated only CE‐CDUS (Abbas 2014; Gargiulo 2014; Giannoni 2007; Gurtler 2013; Motta 2012; Perini 2011; Perini 2012; Ten Bosch 2010). The distribution of the studies based on the unit of analysis is displayed in Appendix 7.
In terms of US, Gargiulo 2014 evaluated the accuracy of 4D and the standard two‐dimensional (2D) CE‐CDUS; Abbas 2014 compared 3D and 2D CE‐CDUS with CT scan; and Arsicot 2014 used 3D CDUS. All the studies provided sufficient detail about US image acquisition to replicate the index test except for McWilliams 1999; Nerlekar 2006; Thompson 1998; and Sandford 2006.
In terms of CT scan, Clevert 2011 and Gray 2012 reported insufficient details to replicate the reference test. Costa 2013; McLafferty 2002; Parent 2002; Sato 1998; and Zannetti 2000, despite reporting sufficient details of the reference standard, did not report the type of scanner used. Giannoni 2007 and Sandford 2006 reported the type of scanner used but reported no details about image acquisition. Gray 2012; Ashoke 2005b; and Ashoke 2005c reported no information about the use of contrast for CT scan.
The overall number of participants in the 42 studies was 4220 ranging from 10 to 445. The studies were performed in different geographical areas: 10 (24%) were performed in the USA, eight (19%) in the UK, seven (17%) in Italy, six (14%) in France, five (12%) in Germany, and one (2%) each in Australia, Belgium, Ireland, the Netherlands, Turkey, and Brazil.
Seven studies did not report any information related to the age of participants (McLafferty 2002; McWilliams 1999; Parent 2002; Perini 2011; Sandford 2006; Sato 1998; Wolf 2000). The mean age was 72 years across the remaining studies.
Ten studies did not report gender characteristics of the included participants (Giannoni 2007; McLafferty 2002; McWilliams 1999; Nerlekar 2006; Parent 2002; Perini 2011; Sandford 2006; Sato 1998; Thompson 1998; Wolf 2000). The percentage males in the remaining studies was 75% or greater; in four studies, all the included participants were males (Ashoke 2005c; Clevert 2008b; Henao 2006; Perini 2012).
Only 16 studies reported information about the aneurysm size. One study reported the range of aneurysm size (from 5.1 to 7.8 cm) (Golzarian 2002), whereas in 15 studies (Abbas 2014; Ashoke 2005b; Cantisani 2011; Costa 2013; Demirpolat 2011; França 2013; Gargiulo 2014; Giannoni 2007; Henao 2006; Nerlekar 2006; Oikonomou 2012; Pages 2001; Perini 2011; Perini 2012; Zannetti 2000), the aneurysmal mean size ranged from 5.0 (Zannetti 2000) to 6.4 cm (Abbas 2014).
Overall, in most of the studies there was no information about participants' comorbidities except in four studies (Arsicot 2014; Costa 2013; d'Audiffret 2001; Nagre 2011). In these studies, common comorbidities were cardiovascular diseases, dyslipidaemia, diabetes, and overweight (see Characteristics of included studies table).
Type of stents
The description of the type of stent was not uniformly reported.
Perini 2012 stated that all participants received fenestrated grafts but did not provde other information. Costa 2013 and Perini 2011 reported that some participants received fenestrated stents. Abbas 2014; Ashoke 2005b; d'Audiffret 2001; Gurtler 2013; Iezzi 2009; Motta 2012; Parent 2002; and Thompson 1998 reported use of bifurcated and aorto‐uni‐iliac stents.
Twenty‐one studies reported the brand names of the stents (AbuRahma 2005; Arsicot 2014; Badri 2010; Cantisani 2011; Ashoke 2005b; d'Audiffret 2001; Gargiulo 2014; Giannoni 2007; Iezzi 2009; McLafferty 2002; McWilliams 1999; Motta 2012; Parent 2002; Raman 2003; Sato 1998; Schmieder 2009; Ashoke 2005c; Ten Bosch 2010; Thompson 1998; Wolf 2000; Zannetti 2000).
The most used type of stent was AneuRx (32.5%) followed by Ancure (27.1%), Talent (13.3), and Excluder (9.6%). Only six studies administered the same type of stent to all the included participants: Gargiulo 2014 used Advanta, Parent 2002 used Ancure, McLafferty 2002 and Wolf 2000 used AneuRx, Sato 1998 used Endovascular Technology, and Thompson 1998 used Talent.
The distribution of the stents used across the 21 studies that reported the brand names is displayed in Table 4. Two studies reported the types of stent used but did not provide the number of participants for each stent deployed (Oikonomou 2012; Perini 2011).
3. Distribution of the type of stent across the 21 included studies that reported the brand name.
| Type of stent | Anaconda | AneuRx | Talent Medtronic | Excluder | Ancure | Vanguard | Endovascular Technology | Zenith | Advanta | Powerlink | Jomed | Mintec | Stenford | Endurant | Powerlink | Stentor | Quantum | Low Profile |
| AbuRahma 2005 | ‐ | 55 | ‐ | 37 | 86 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ashoke 2005c | ‐ | 3 | 2 | 5 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Arsicot 2014 | 30 | 2 | ‐ | 8 | ‐ | ‐ | ‐ | 28 | ‐ | 2 | ‐ | ‐ | ‐ | 1 | ‐ | ‐ | ‐ | 4 |
| Ashoke 2005b | ‐ | 3 | ‐ | ‐ | ‐ | ‐ | ‐ | 13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Badri 2010 | ‐ | ‐ | 5 | ‐ | ‐ | ‐ | ‐ | 54 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cantisani 2011 | ‐ | ‐ | 55 | 50 | ‐ | ‐ | ‐ | ‐ | ‐ | 12 | 6 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| d'Audiffret 2001 | ‐ | 2 | 1 | ‐ | ‐ | 56 | 11 | ‐ | ‐ | ‐ | ‐ | 7 | 12 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Gargiulo 2014 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Giannoni 2007 | ‐ | ‐ | 3 | 24 | ‐ | 3 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Iezzi 2009 | ‐ | 1 | 46 | 28 | ‐ | 1 | ‐ | 8 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| McLafferty 2002 | ‐ | 79 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| McWilliams 1999 | ‐ | 3 | ‐ | ‐ | ‐ | 14 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 1 | ‐ | ‐ |
| Motta 2012 | ‐ | ‐ | 43 | 20 | ‐ | ‐ | ‐ | 6 | ‐ | ‐ | ‐ | ‐ | ‐ | 14 | ‐ | ‐ | ‐ | ‐ |
| Parent 2002 | ‐ | ‐ | ‐ | ‐ | 83 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Raman 2003 | ‐ | 34 | ‐ | ‐ | 247 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sato 1998 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 79 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Schmieder 2009 | ‐ | 160 | ‐ | 2 | 55 | ‐ | ‐ | 13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 5 | ‐ | 1 | ‐ |
| Ten Bosch 2010 | ‐ | ‐ | 83 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Thompson 1998 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 6 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 3 | ‐ | ‐ |
| Wolf 2000 | ‐ | 100 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Zannetti 2000 | ‐ | 144 | 1 | 9 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
Endoleaks: prevalence and types
Overall, there were 1208 endoleaks in 4220 participants. The median prevalence of endoleaks was 24.5% ranging from 5.4% (Gray 2012) to 56.7% (Abbas 2014).
Eleven studies did not report the type of endoleak (Arsicot 2014; Bendick 2003; Giannoni 2003; Heilberger 1997; McLafferty 2002; McWilliams 1999; Sandford 2006; Sato 1998; Thompson 1998; Wolf 2000; Zannetti 2000).
The number of endoleaks in the remaining studies was 975. Of these, 166 (17%) were type I, 736 (75%) were type II, 29 (3%) were type III, and three (0.3%) were type IV endoleaks.
Methodological quality of included studies
The following is the assessment of the quality of the 42 studies based on each QUADAS items as depicted in Figure 2.
2.
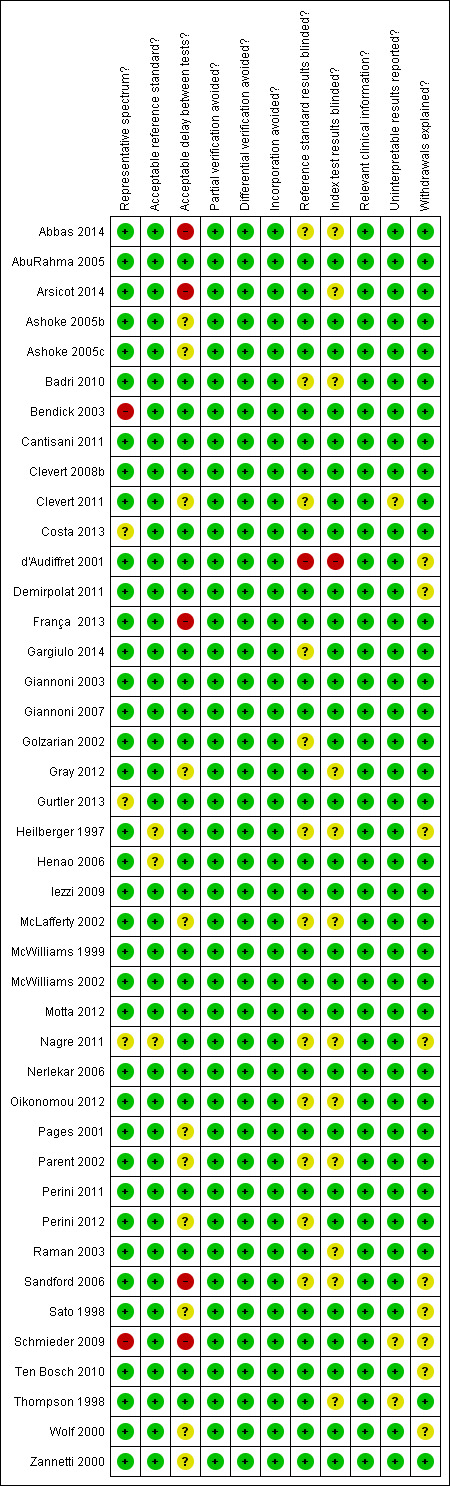
Methodological quality summary: review authors' judgements about each methodological quality item for all included study (42 participants).
Representative spectrum? All included studies considered a consecutive series of participants referred for follow‐up to detect potential endoleaks except for Costa 2013;Gurtler 2013; and Nagre 2011 who did not report sufficient information to make judgement; Bendick 2003 in which 10 of 20 participants were selected based on the participant's habitus or the presence of bowel gas; and Schmieder 2009, where from a cohort of 496 consecutive participants, 236 participants were identified with paired CDUS and CT scan.
Acceptable reference standard? All included studies reported the use of CT scan as a reference standard. However, three studies did not clearly report who interpreted the images (Heilberger 1997; Henao 2006; Nagre 2011).
Acceptable delay between tests? The time period between US and CT scan was four weeks or less in 27 studies and unclear in 11 studies (Ashoke 2005b; Ashoke 2005c; Clevert 2011; Gray 2012; McLafferty 2002; Pages 2001; Parent 2002; Perini 2012; Sato 1998; Wolf 2000; Zannetti 2000). Nerlekar 2006 and Ten Bosch 2010 considered the inclusion of participants with tests performed within one month and were at low risk of bias. Five studies were at high risk of bias: in Abbas 2014, the interval between the tests was (mean ± standard deviation) 3.9 ± 2.7 weeks; in Arsicot 2014, it was 48 ± 37 days; in Schmieder 2009, it was 18 days with a range between 0 and 90 days; in França 2013 concurrent scans were defined as having occurred within three months of each other; and in Sandford 2006 concurrent scans were defined as having occurred within six months of each other,
Partial verification avoided? In all included studies, all participants were accounted for and the results of the reference standard were reported for all.
Differential verification bias avoided? In all included studies, all participants who received US test were subjected to the same CT scan.
Incorporation avoided? In all included studies, the index test was not part of the reference standard.
Reference tests blinded? Twenty‐nine trials explicitly stated that the index test was interpreted without knowledge of the reference standard. Twelve studies reported insufficient information to make a judgement (Abbas 2014; Badri 2010; Clevert 2011; Gargiulo 2014; Golzarian 2002; Heilberger 1997; McLafferty 2002; Nagre 2011; Oikonomou 2012; Parent 2002; Perini 2012; Sandford 2006). In one study, the physician performing the US scan was not blinded to the results of the CT scan (d'Audiffret 2001).
Index test results blinded? In 12 studies, there was insufficient information to make a judgement (Abbas 2014; Arsicot 2014; Badri 2010; Gray 2012; Heilberger 1997; McLafferty 2002; Nagre 2011; Oikonomou 2012; Parent 2002; Raman 2003; Sandford 2006; Thompson 1998), whereas in one study, authors reported that the radiologist interpreting the results of the CT scan could have been aware of the results of the index test (d'Audiffret 2001).
Relevant clinical information? Appropriate clinical information was available in all included studies.
Uninterpretable results reported? Two studies did not report sufficient information to make any judgement (Clevert 2011; Thompson 1998); another study reported that inadequate examinations were excluded but did not provide detailed numbers (Schmieder 2009).
Withdrawals explained? Nine studies did not adequately explain the occurrence of withdrawals (d'Audiffret 2001; Demirpolat 2011; Heilberger 1997; Nagre 2011; Sandford 2006; Sato 1998; Schmieder 2009; Ten Bosch 2010; Wolf 2000).
Overall summary of quality of studies included in primary analysis
Figure 3 provides the overall summary of the quality of studies that evaluated CDUS and CE‐CDUS and used number of individuals as the unit of analysis.
3.
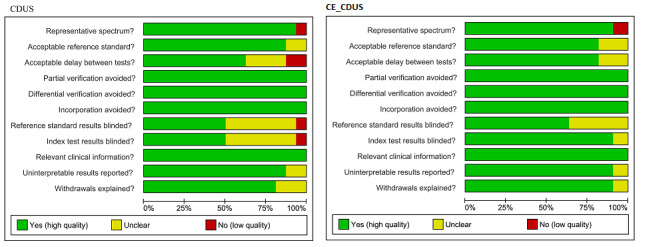
Risk of bias according to QUADAS 1: review authors' judgements about each domain presented as percentages for colour duplex ultrasound (CDUS) (n = 16) and contrast‐enhanced colour duplex ultrasound (CE‐CDUS) (n = 11) that were included in primary analysis. The unit of analysis was number of individuals (not number of scans).
Sixteen CDUS studies reported accuracy analysis based on individual data (Arsicot 2014; Bendick 2003; Cantisani 2011; Clevert 2008b; Clevert 2011; d'Audiffret 2001; Golzarian 2002; Heilberger 1997; Henao 2006; Iezzi 2009; McLafferty 2002; Oikonomou 2012; Parent 2002; Sandford 2006; Thompson 1998; Zannetti 2000). These studies were generally of moderate/low quality. Only three studies fulfilled all the QUADAS items (Cantisani 2011; Clevert 2008b; Iezzi 2009). In 6/16 (40%) studies, the delay between the tests was unclear (Clevert 2011; McLafferty 2002; Parent 2002; Zannetti 2000), or longer than four weeks (Arsicot 2014; Sandford 2006); in 50% of the studies, the blinding of either the index test or the reference standard was not clearly reported or was not performed; and in two studies (12%) the interpretation of the reference standard was not clearly reported (Heilberger 1997; Henao 2006).
Eleven CE‐CDUS studies reported accuracy analysis based on individual data (Bendick 2003; Cantisani 2011; Clevert 2008b; Clevert 2011; Gargiulo 2014; Giannoni 2007; Heilberger 1997; Henao 2006; Iezzi 2009; Perini 2011; Perini 2012). These studies were of better quality than the CDUS studies. Five studies (45%) fulfilled all the QUADAS items (Cantisani 2011; Clevert 2008b; Giannoni 2007; Iezzi 2009; Perini 2011). Four studies (36%) did not report the blinding interpretation of the reference standard clearly (Clevert 2011; Gargiulo 2014; Heilberger 1997; Perini 2012); in two (18%) studies, the delay between the two tests was not clearly reported (Clevert 2011; Perini 2012).
Findings
Diagnostic performance of colour duplex ultrasound and contrast‐enhanced colour duplex ultrasound (primary analysis)
Sixteen studies provided sufficient individual data on CDUS compared to CT to perform a meta‐analysis (Arsicot 2014; Bendick 2003; Cantisani 2011; Clevert 2008b; Clevert 2011; d'Audiffret 2001; Golzarian 2002; Heilberger 1997; Henao 2006; Iezzi 2009; McLafferty 2002; Oikonomou 2012; Parent 2002; Sandford 2006; Thompson 1998; Zannetti 2000). Individual estimates of sensitivity and specificity are shown in Figure 4. The sensitivities ranged between 33% and 100% while the specificities ranged between 64% and 100%. Using the bivariate model, the summary estimate of sensitivity was 0.82 (95% CI 0.66 to 0.91), and the summary estimate of specificity was 0.93 (95% CI 0.87 to 0.96). In Arsicot 2014, the accuracy estimates between standard US versus CT and 3D US versus CT were similar (equal rates of false/true positives or negatives).
4.
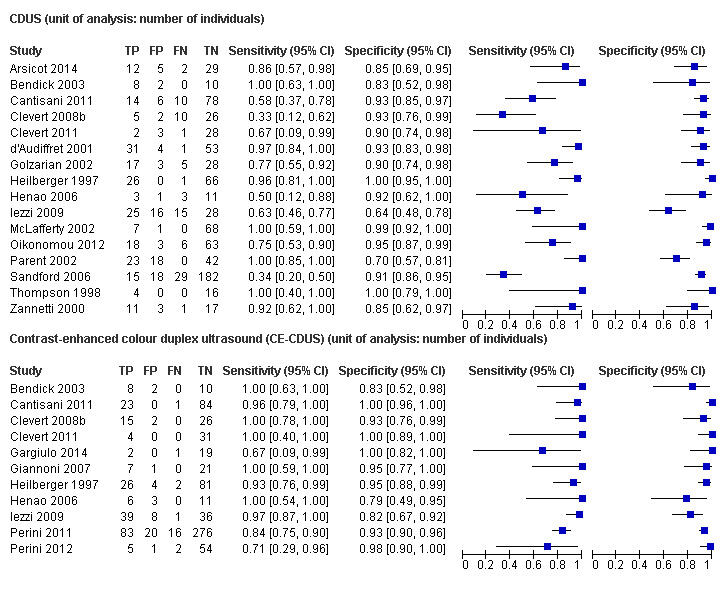
A forest plot of colour duplex ultrasound (CDUS) (n = 16) and contrast‐enhanced colour duplex ultrasound (CE‐CDUS) (n = 11) that were included in primary analysis.
Eleven CE‐CDUS studies provided individual data that allowed the performance of a meta‐analysis (Bendick 2003; Cantisani 2011; Clevert 2008b; Clevert 2011; Gargiulo 2014; Giannoni 2007; Heilberger 1997; Henao 2006; Iezzi 2009; Perini 2011; Perini 2012). The sensitivities ranged between 67% and 100% and the specificities ranged from 79% to 100% (Figure 4). The bivariate model meta‐analysis showed a sensitivity of 0.94 (95% CI 0.85 to 0.98) and a specificity of 0.95 (95% CI 0.90 to 0.98). In Gargiulo 2014, the accuracy estimates between standard US versus CT and 4D US versus CT were similar (equal rates of false/true positives or negatives).
Comparing the accuracy data between CDUS and CE‐CDUS based on the bivariate model, it appeared that sensitivity differed significantly with CE‐CDUS being superior to CDUS (LR Chi2= 5.08; P = 0.0242). Conversely, there was no statistical difference in the specificity estimates between CE‐CDUS and CDUS. Figure 5 shows the resulting SROC curves, with summary operating points for sensitivity and specificity on the curves and a 95% confidence region around these points.
5.
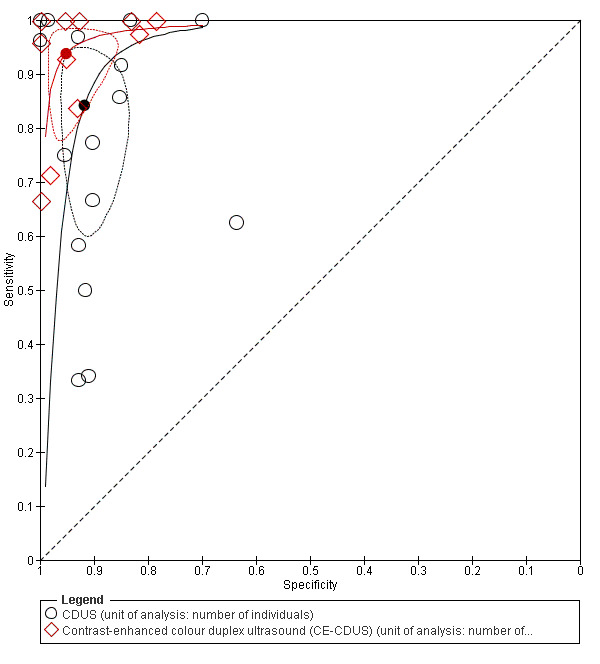
Summary receiver operating characteristic plot of studies assessing the accuracy of colour duplex ultrasound (CDUS) and contrast‐enhanced colour duplex ultrasound (CE‐CDUS) in discriminating endoleak (primary analysis). Each value of sensitivity and specificity is represented by a circle. The filled circle represents the summary point. Dotted closed line represent 95% confidence region of the summary point.
The diagnostic odds ratio (DOR), LR+, and LR‐ for CDUS and CE‐CDUS are reported in Table 5.
4. Diagnostic accuracy estimates for colour duplex ultrasound and contrast‐enhanced colour duplex ultrasound.
| Imaging method |
Summary sensitivity % (95% CI) |
Summary specificity % (95% CI) |
DOR (95% CI) |
LR+ (95% CI) | LR‐ (95% CI) |
| Studies with accuracy estimates based on number of individual participants | |||||
| CDUS (16 studies) | 82 (66 to 91) | 93 (87 to 96) | 56 (19 to 164) | 11.0 (6.0 to 20.0) | 0.19 (0.100 to 0.39) |
| CE‐CDUS (11 studies) | 94 (85 to 98) | 95 (90 to 98) | 299 (95 to 935) | 19.5 (9.1 to 41.7) | 0.07 (0.03 to 0.16) |
|
Subgroup analysis 1: diagnostic performance of studies that estimated accuracy before and after administration of contrast (7 studies; analyses based on number of individual participants) | |||||
| CDUS | 67 (47 to 83) | 94 (80 to 99) | 35 (5 to 246) | 12.0 (2.8 to 51.8) | 0.34 (0.18 to 0.63) |
| CE‐CDUS | 97 (92 to 99) | 95 (85 to 98) | 531 (131 to 2147) | 17.7 (6.0 to 51.6) | 0.03 (0.01 to 0.09) |
| Subgroup analysis 2: diagnostic performance of CE‐CDUS for type I and type III endoleaks | |||||
| CE‐CDUS (7 studies) | 97 (81 to 99) | 99 (96 to 100) | 7073 (254 to 196,804) | 220.7 (25.9 to 1875.5) | 0.031 (0.004 to 0.22) |
| Studies with accuracy estimates based on scan performed | |||||
| CDUS (18 studies) | 72 (55 to 85) | 95 (90 to 96) | 37 (16 to 87) | 11.1 (6.8 to 18.1) | 0.29 (0.17 to 0.51) |
| CE‐CDUS (8 studies) | 91 (68 to 98) | 89 (71 to 96) | 77 (9 to 605) | 8.2 (2.7 to 24.6) | 0.11 (0.03 to 0.43) |
| Studies with accuracy estimates based on scan performed (excluding outliersMcWilliams 2002 and Nagre 2011) | |||||
| CDUS (16 studies) | 77 (64 to 87) | 93 (89 to 96) | 48 (21 to 110) | 11.7 (6.8 to 20.0) | 0.24 (0.14 to 0.41) |
| CE‐CDUS (6 studies) | 93 (84 to 97) | 91 (70 to 98) | 125 (23 to 689) | 9.9 (2.7 to 36) | 0.08 (0.03 to 0.19) |
CDUS: colour duplex ultrasound; CE‐CDUS: contrast‐enhanced colour duplex ultrasound; CI: confidence interval; DOR: diagnostic odds ratio; LR+: positive likelihood ratio; LR‐: negative likelihood ratio; NE: not estimable.
To identify the implication of our results into clinical practice, using the Fagan's nomogram, we simulated three scenarios with low (5.4%), median (24.5%), and high (47.6%) prevalence of endoleak to obtain the post‐test probability. With low prevalence scenarios. if a person has a positive US test, the post‐test probability that the person has an endoleak would be 37% for CDUS and 53% for CE‐CDUS. In contrast, if a person has a negative US test, the post‐test probability that the person has an endoleak would be less than 1% for CDUS and 0% for CE‐CDUS. In a high prevalence scenario, the post‐test probability that the person has an endoleak would be 90% when a person has a positive CDUS and 95% with a positive CE‐CDUS; conversely, with a negative US result, the post‐test probability would be 14% for CDUS and 6% for CE‐CDUS (Figure 6).
6.
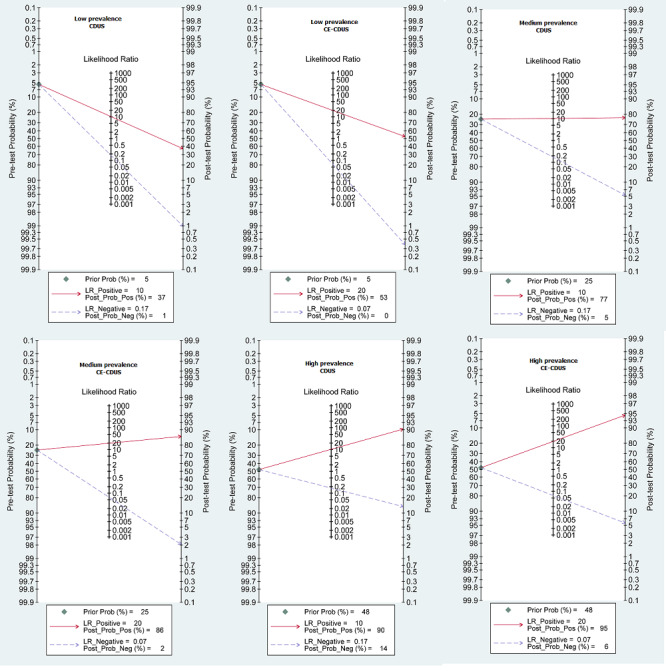
Fagan plot estimating changes in the probability that a person has an endoleak given a pre‐test probability: a presumed pre‐test probability at low (5.4%), median (24.5%) and high (47.6%) prevalence of endoleak for CDUS and CE‐CDUS. Left vertical axis represents the pre‐test probability, axis in the middle represents the likelihood ratio, and right vertical axis represents the post‐test probability (LR‐: negative likelihood ratio; LR+: positive likelihood ratio).
The LR scattergram shows that the summary point of LR+ and LR‐ for CDUS is located in the upper right quadrant (LR+ = 10; LR‐ = 0.17), suggesting that the accuracy is optimal for endoleak confirmation whereas the summary point of LR+ and LR‐ for CE‐CDUS is optimal for endoleak confirmation and exclusion (LR+ = 19; LR‐ = 0.06) (Figure 7).
7.
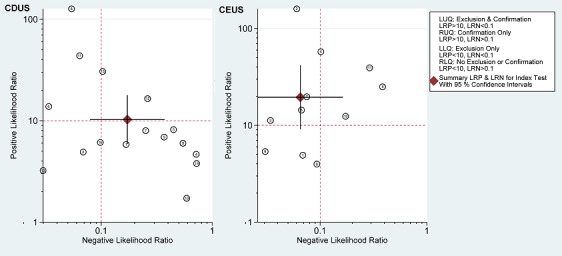
Likelihood ratio scatterplot matrix. Circles represent individual studies. The filled square circle shows the weighted summary likelihood ratios. Error bars represent 95% confidence intervals. The likelihood ratio profile shows that contrast‐enhanced colour duplex ultrasound (CE‐CDUS) is a potent tool for endoleak conformation or exclusion in people who received endovascular aneurysm repair (EVAR).
Subgroup analysis 1: direct comparison of colour duplex ultrasound and contrast‐enhanced colour duplex ultrasound
Seven studies provided accuracy data of US for endoleak detection before and after the administration of contrast (Bendick 2003; Cantisani 2011; Clevert 2008b; Clevert 2011; Heilberger 1997; Henao 2006; Iezzi 2009). Based on the confidence regions in Figure 8, there is evidence that the sensitivity varies with contrast, but not specificity. A regression analysis showed that CE‐CDUS was significantly superior to CDUS in terms of sensitivity (LR Chi2 = 13.47; P = 0.0002) but not specificity (LR Chi2 = 0.01; P = 0.9124). Table 5 compares the diagnostic accuracy estimates including DOR, LR+, and LR‐ between CDUS and CE‐CDUS.
8.
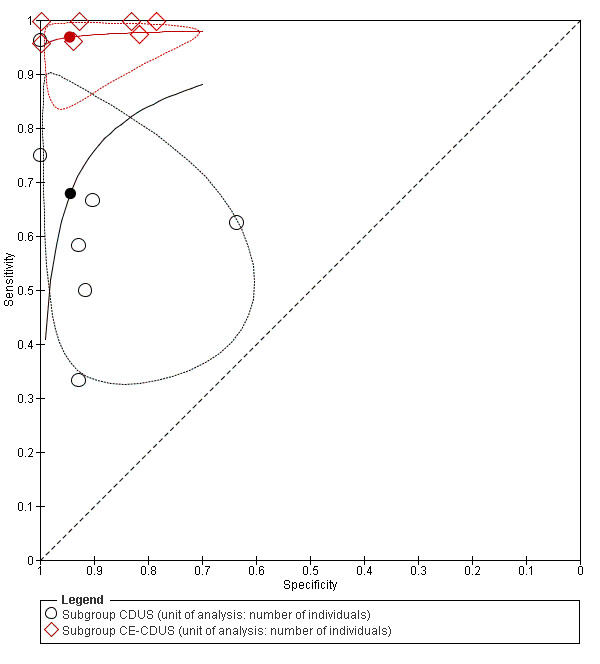
Summary Receiver Operating Characteristic Plot of studies (n = 7) that assessed accuracy measures for endoleak detection before and after administration of contrast. Studies used individual based analysis. The filled circle represents the summary point. Dotted closed line represent 95% confidence region of the summary point. CDUS: colour duplex ultrasound; CE‐CDUS: contrast‐enhanced colour duplex ultrasound.
Subgroup analysis 2: diagnostic performance of contrast‐enhanced colour duplex ultrasound for type I and type III endoleaks
We performed a posthoc subgroup analysis with seven CE‐CDUS studies that provided usable data regarding type I and type III endoleaks. Summary sensitivity estimate was 0.97 (95% CI 0.81 to 0.99) and specificity was 0.99 (95% CI 0.96 to 1.00) (Table 5).
Diagnostic performance of colour duplex ultrasound and contrast‐enhanced colour duplex ultrasound (secondary analysis)
Eighteen CDUS studies provided accuracy estimates based on the number of scans performed (AbuRahma 2005; Ashoke 2005b; Ashoke 2005c; Badri 2010; Costa 2013; Demirpolat 2011; França 2013; Giannoni 2003; Gray 2012; McWilliams 1999; McWilliams 2002; Nagre 2011; Nerlekar 2006; Pages 2001; Raman 2003; Sato 1998; Schmieder 2009; Wolf 2000). Meta‐analyses based on the bivariate model showed a sensitivity of 0.72 (95% CI 0.55 to 0.85) and specificity of 0.95 (95% CI 0.90 to 0.96).
Eight CE‐CDUS studies provided data based on number of scans performed (Abbas 2014; Costa 2013; Giannoni 2003; Gurtler 2013; McWilliams 1999; McWilliams 2002; Motta 2012; Ten Bosch 2010). The summary sensitivity was 0.91 (95% CI 0.68 to 0.98) and the specificity was 0.89 (95% CI 0.71 to 0.96). In Abbas 2014, the accuracy estimates between standard US versus CT and 3D US versus CT were similar (equal rates of false/true positives or negatives). In Abbas 2014, the accuracy estimates between standard US versus CT and 3D US versus CT were similar (equal rates of false/true positives or negatives).
An indirect comparison between CDUS and CE‐CDUS showed a higher sensitivity for CE‐CDUS but with no statistical difference. An ROC plot showed a wide confidence region for estimates of both index tests. The exclusion of two outliers (McWilliams 2002; Nagre 2011) reduced the CI, and the sensitivity estimates for CE‐CDUS were higher than those of CDUS with a statistical significant difference (LR Chi2= 5.40, 1 df, P = 0.0202). Figure 9 shows the reduction in the confidence region after exclusion of the outliers.
9.
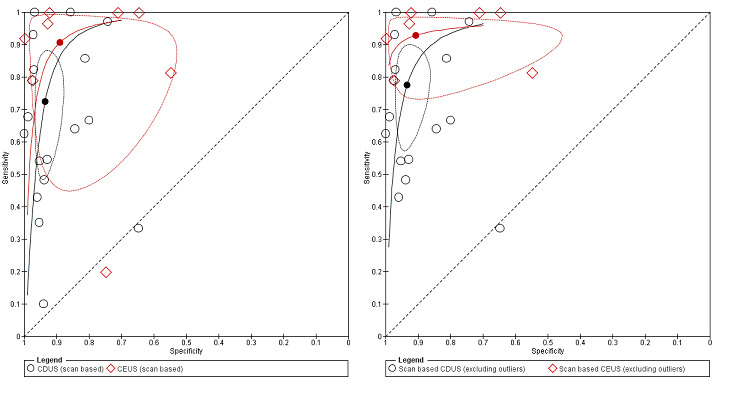
Figure A. Indirect comparison of summary estimates of studies assessing the accuracy of CDUS (colour duplex ultrasound) and CE‐CDUS (contrast‐enhanced colour duplex ultrasound) in discriminating endoleak (secondary analysis). Figure B. Summary Receiver Operating Characteristic plots of CDUS and CE‐CDUS (indirect) estimates excluding two outliers. The confidence region was significantly reduced when the outliers were excluded.
Details of accuracy estimates for both types of US modality (with and without outliers) are reported in Table 5.
Investigating heterogeneity
Due to absence of data, we were unable to explore sources of heterogeneity concerning the size of the aneurysm, characteristics of participant population (concomitant disease, severity of aneurismal disease, location of aneurysm), and rupture of aneurysm.
We were able to investigate the following potential sources of heterogeneity within the studies included in the primary analysis: year of publication, characteristics of included participants (age and gender), direction of study (retrospective, prospective), methodological quality (low quality versus unclear/high risk of bias), sample size, country of origin, number of US operators, and US manufacturer.
Regression testing showed evidence of statistically significant effect bias related to year of publication and study quality within participant‐based CDUS studies. Sensitivity estimates were higher in the studies published before 2006 than the estimates obtained from studies published in 2006 or later (P < 0.001); similarly, studies judged as low/unclear quality provided higher estimates of sensitivity. None of the remaining covariates were identified as a possible source of heterogeneity (Table 6). When regression testing was applied in the participant‐based CE‐CDUS studies, none of the items, namely direction of the study design, quality, and age, were identified as a source of heterogeneity.
5. Covariate analyses for colour duplex ultrasound and contrast‐enhance colour duplex ultrasound studies (based on individual participants data).
| CDUS studies (n = 16) | CE‐CDUS studies (n = 11) | ||||
| Covariates |
Summary sensitivity % (95% CI) |
Summary specificity % (95% CI) |
Covariates |
Summary sensitivity % (95% CI) |
Summary specificity % (95% CI) |
| Age | Age | ||||
| < 72 years (6 studies) | 80 (50 to 95) | 94 (89 to 97) | < 72 years (4 studies) | 96 (87 to 99) | 96 (84 to 98) |
| ≥ 72 years (10 studies) | 86 (63 to 95) | 90 (81 to 95) | ≥ 72 years (7 studies) | 88 (73 to 95) | 94 (87 to 97) |
| P value | 0.65 | 0.25 | P value | 0.07 | 0.76 |
| Gender | Gender | ||||
| Men < 95 (7 studies) | 78 (64 to 88) | 90 (82 to 94) | Men < 95 (3 studies) | NE | NE |
| Men ≥ 95 (9 studies) | 91 (59 to 99) | 94 (85 to 98) | Men ≥ 95 (8 studies) | 92 (76 to 98) | 93 (89 to 96) |
| P value | 0.59 | 0.43 | P value | NE | NE |
| Study design (direction) | Study design (direction) | ||||
| Prospective (7 studies) | 68 (50 to 83) | 92 (82 to 96) | Prospective (7 studies) | 95 (83 to 99) | 94 (86 to 98) |
| Retrospective/unclear (9 studies) | 92 (73 to 98) | 91 (84 to 96) | Retrospective/unclear (4 studies) | 92 (74 to 98) | 96 (90 to 99) |
| P value | 0.09 | 0.99 | P value | 0.76 | 0.74 |
| Publication year | Publication year | ||||
| Before 2006 (8 studies) | 96 (87 to 99) | 94 (84 to 98) | Before 2006 (2 studies) | NE | NE |
| After 2005 (8 studies) | 58 (43 to 71) | 90 (83 to 94) | After 2005 (9 studies) | 94 (82 to 98) | 96 (89 to 99) |
| P value | < 0.001 | 0.42 | P value | NE | NE |
| Number of US operators | Number of US operators | ||||
| 1 operator (4 studies) | 71 (30 to 94) | 91 (87 to 94) | 1 operator (2 studies) | NE | NE |
| > 1 operators (8 studies) | 76 (60 to 87) | 92 (83 to 97) | > 1 operators (6 studies) | 95 (82 to 99) | 96 (84 to 99) |
| P value | 1 | 1 | P value | NE | NE |
| Country | Country | ||||
| Americas (4 studies) | 99 (03 to 99) | 91 (71 to 98) | Americas (2 studies) | NE | NE |
| Europe (12 studies) | 78 (61 to 89) | 92 (87 to 96) | Europe (9 studies) | 92 (83 to 97) | 97 (91 to 99) |
| P value | 0.85 | 0.88 | P value | NE | NE |
| Sample | Sample | ||||
| < 100 (5 studies) | 88 (67 to 96) | 90 (82 to 95) | < 100 (8 studies) | 96 (83 to 99) | 94 (86 to 98) |
| > 100 (11 studies) | 76 (46 to 92) | 95 (90 to 98) | > 100 (3 studies) | NE | NE |
| P value | 0.42 | 0.19 | P value | NE | NE |
| Quality | Quality | ||||
| High quality (4 studies) | 53 (40 to 66) | 88 (72 to 96) | High quality (5 studies) | 96 (88 to 99) | 97 (78 to 99) |
| Low/unclear quality (12 studies) | 91 (77 to 97) | 93 (87 to 96) | Low/unclear quality (6 studies) | 90 (77 to 96) | 93 (87 to 97) |
| P value | < 0.001 | 0.36 | P value | 0.19 | 0.94 |
CDUS: colour duplex ultrasound; CE‐CDUS: Contrast enhanced ultrasound; n: number of participants; NE: not estimable.
Sensitivity analysis
We carried out a sensitivity analysis based on type of study design (prospective versus retrospective study designs), generation of the index tests, and individual quality items.
Excluding retrospective studies in design, CDUS showed a lower sensitivity (0.68, 95% CI 0.50 to 0.83) than CE‐CDUS (0.95, 95% CI 0.83 to 0.99).
Similarly, the sensitivity of CDUS studies with unclear/low quality items was lower (0.53, 95% CI 0.40 to 0.66) than the corresponding sensitivity of CE‐CDUS studies (0.96, 95% CI 0.88 to 0.99).
There was no uniform use of the type of US across the studies. We performed sensitivity analysis based on the studies that used US produced by the same manufacturer but there were no significant differences (Table 6).
Discussion
Summary of main results
Using data from the largest set of published studies in the medical literature, this review summarised the evidence for the diagnostic accuracy of US for the detection of endoleak in people who received EVAR.
The results suggested that US provides clinically helpful information to rule in or rule out endoleak (Table 1). CDUS summary sensitivities ranged from 82% to 91% and specificities ranged from 93% to 96%. This means that, under a prevalence of endoleaks of 22%, for every 1000 people who receive a CDUS evaluation, endoleaks will be missed in 35 people and 47 people will undergo an unnecessary CT scan. However, accuracy estimates showed that CE‐CDUS has better sensitivity than CDUS with values ranging from 85% to 98%. Hence, for every 1000 people who will receive a CE‐CDUS evaluation, 15 people will have their endoleaks missed rather than 35, under similar prevalence of endoleaks. In conclusion, while CDUS usage is limited to ruling in endoleaks, CE‐CDUS has the advantage of use for both endoleak confirmation and exclusion and, therefore, it should be considered the primary alternative to CT scan for endoleak surveillance in people who have received EVAR.
Strengths and weaknesses of the review
The most relevant strength of our review is that our primary unit analysis was based on data derived from 20 primary studies. We provided separate summary estimates of sensitivity and specificity for CDUS (16 studies) and CE‐CDUS (11 studies) and, using appropriate statistical models, we compared the difference in accuracy between CDUS and CE‐CDUS. Results showed that sensitivity improves when contrast is used increasing from 82% to 94%. Figure 5 shows clearly that there is an important gain in sensitivity for CE‐CDUS, and that all the CE‐CDUS studies were distributed above the ROC curve of CDUS studies. Using the Fagan plot, we also demonstrated that a positive US raises significantly the probability of having an endoleak at different levels of disease prevalence (Figure 7).
In addition, based on the LR calculation we plotted the LR ratio in a four quadrant presentation (Figure 7). The weighted summary LRs for the CDUS studies lay within the upper right quadrant suggesting that the test should be indicated for confirmation only. However, the CIs for the LR+ crossed the lower right quadrant, which suggests no confirmation or exclusion ‐ in which half of the CDUS results were scattered. Conversely, the summary LR for the CE‐CDUS studies lay within the left upper quadrant suggesting that the test can be indicated for exclusion or confirmation. No CE‐CDUS study was located in the lower right quadrant.
We also performed accuracy estimates based on 22 studies that provided scan‐based analysis. This type of approach may have accounted for more than one endoleak in the same participant in some circumstances. Nerlekar 2006 included 121 participants but reported data about 243 pairs of scans. In this study, the number of people with endoleak was 20 whereas the number of endoleaks considered in the contingency table was 29. This discrepancy may affect the estimation of the prevalence and consequently the calculation of the positive and negative predictive values. However, the accuracy estimates in this secondary analysis provided similar results to those observed in the primary analysis.
Other strengths of our review include: transparent objectives and methods based on a prepublished protocol and comprehensive and systematic methods to search for and select eligible studies; thorough quality assessment of the primary studies; and a sensitivity analysis of studies with similar methodological features into a meta‐analytic summary based on recommended methods.
The most important limitation of our review concerns the issue of reproducibility. Unlike CT, the reliability of US measurement is highly dependent on the experience of the US operator. One systematic review that examined the potential observer bias and variability in US measurements in nine studies during AAA screening or surveillance programmes reported that six studies did not show a correlation between increasing standard deviation and increasing aortic diameter. In addition, five studies had repeatability coefficients lower than the 5‐mm level of acceptability (as suggested by the UK Abdominal Aortic Aneurysm Screening Programme), whereas two studies produced repeatability coefficients that were greater than 5 mm (Beales 2011). However, it should be emphasized that the studies used different US machines with no standardized measurement techniques (Beales 2011). In our review, most of the studies reported the operators performing US had good experience. In clinical practice, the operator/technician would likely be aware of an increasing aneurysm sac and, therefore, are likely to look more closely for an endoleak. However, we are unsure to what extent this would have affected the estimates of the diagnostic studies. Additionally, we found an US variability in the type of machine used and the protocol applied to acquire images that may have contributed to the heterogeneity especially in the CDUS studies.
Year of publication and study quality could be other potential sources of heterogeneity. However, comparing the two graphical representation of the accuracy estimates between CDUS and CE‐CDUS, we can conclude that heterogeneity was less in the CE‐CDUS studies.
We acknowledge that due to operational reasons blinding of one test operator to the results the other test especially in the presence of an enlarging sac may be difficult. However, 29 (69%) studies succeeded in interpreting the index test without knowledge of the reference standard and 31 (74%) studies succeeded in interpreting the reference standard without knowledge of the test.
Finally, we acknowledge that we used the bivariate model as it is recommended for purely binary tests or when different studies report similar thresholds (Leeflang 2014). In our analysis, the target condition was a dichotomous outcome (endoleak present or endoleak absent) and none of the included studies mentioned or defined any sort of threshold. However, by visual inspection of studies plotted in the ROC space, the presence of an implicit threshold effect seems unlikely, as the variation of sensitivity between studies seems to be unrelated to the variation of specificity (Figure 5). The studies with similar specificity have quite different sensitivities and the studies with similar sensitivity have quite different specificities, suggesting a random effect rather than a threshold effect.
Comparison with existing literature
Our results can be compared to four systematic reviews that have been published since 2005.
The most recent review was published in 2012 and compared CDUS or CE‐CDUS versus CT scan (Karthikesalingam 2012). Karthikesalingam 2012 searched MEDLINE and Embase and identified 25 studies that compared CDUS and 11 studies (961 paired scans) that compared CE‐CDUS with CT for all endoleaks. All these studies were included in the present review. With respect to the review from Karthikesalingam 2012, our review included 13 additional studies (Abbas 2014; Arsicot 2014; Badri 2010; Clevert 2011; Costa 2013; Demirpolat 2011; França 2013; Gargiulo 2014; Gray 2012; Gurtler 2013; Motta 2012; Oikonomou 2012; Perini 2012). The results of Karthikesalingam 2012 were similar to our results despite the fact that review by Karthikesalingam 2012 did not differentiate the results based on the unit of analysis (number of participants from number of scans): the sensitivity for CDUS was 0.74 (95% CI 0.62 to 0.83) and the specificity was 0.94 (95% CI 0.90 to 0.97); whereas the sensitivity for CE‐CDUS was 0.96 (95% CI 0.85 to 0.99) and the specificity was 0.85 (95% CI 0.76 to 0.92).
The second review searched MEDLINE, Embase, trial registries, and conference proceedings to identify studies comparing CDUS or CE‐CDUS with CT following EVAR (Mirza 2010). The review identified 21 studies for CDUS and provided a summary estimate of sensitivity of 0.77 (95% CI 0.64 to 0.86) and specificity of 0.94 of (95% CI 0.88); in addition, for CE‐CDUS, seven studies were meta‐analysed providing a sensitivity of 0.98 (95% CI 0.90 to 0.99) and a specificity of 0.88 (95% CI 0.78 to 0.94).
The third review was a single author review that performed a search in 2005 in MEDLINE only to identify studies that evaluated the accuracy of CDUS to detect endoleaks (Sun 2006). From 21 included studies, the summary estimates of sensitivity was 66% (95% CI 52% to 81%) and of specificity was 93% (95% 89% to 97%). Sun 2006 did not evaluate the accuracy of CE‐CDUS.
The fourth review searched MEDLINE, Embase, BioMED Central, and other databases in 2004 (Ashoke 2005a); it identified eight published and two unpublished studies (Ashoke 2005b; Ashoke 2005c) that evaluated the accuracy of CDUS in detecting endoleaks. Overall, 711 participants (1355 paired scans) were eligible for inclusion. Compared to CT scan, the summary estimates of CDUS were 69% (95% CI 52% to 87%) for sensitivity and 91% (95% CI 87% to 95%) for specificity. Ashoke 2005a did not consider studies that used contrast agents for image enhancement.
Applicability of findings to the review question
We identified a considerable number of studies with adequate number of participants enrolled to sufficiently address the diagnostic performance of US for endoleak detection in people who received EVAR for AAA. The characteristics of the participants included, clinical setting in which participants received the tests, and technical features of both index test and reference standard were appropriate in most of the studies. However, the main concern for applicability of the results from the present review was high heterogeneity mainly related to studies that used CDUS. Calculating the predictive values based on the results of CDUS, of 1000 subjects with EVAR who will undergo CDUS, 35 subjects will have their endoleaks missed and 47 will undergo unnecessary CT scan since they will be incorrectly classified as having endoleak. The number of missed endoleaks is significantly reduced to 15 when CE‐CDUS is used (Table 1). Hence, the results from the present review suggest that in a clinical pathway CE‐CDUS can be the first modality for monitoring people who receive EVAR. The proposed approach will permit people to avoid the risk of nephrotoxicity and the burden of ionising radiation from CT scans.
Knowledge of the type of endoleak is important for their management. Type II endoleaks are the most common leaks after EVAR, they are at low risk of rupture, and generally conservative management is not associated with increased risk of aneurysm rupture (Lawrence‐Brown 2009; Pippin 2016; Rayt 2009; Tolia 2005). In one retrospective‐prospective study of 77 endoleaks occurring in 369 participants who received EVAR for infrarenal AAA between March 1994 and June 2006, 41 (53%) were of type II. After a mean follow‐up period of four years, 48% had spontaneously sealed, another 48% remained under observation (with an enlarging or stable sac), whereas only 20% had an enlarging sac; no ruptured aneurysms occurred and no participant required conversion to open repair (Rayt 2009). These results were confirmed in a more recent retrospective study in which type II endoleak occurred in 66/163 (40%) people who received EVAR (40%). Over a median follow‐up of 24.7 months, the aneurysm size remained unchanged in 48.5%, decreased in 33.3%, and increased in 18.2% without aneurysm ruptures, conversion to open repair, or aneurysm‐related deaths (Pippin 2016). Hence, in light of our evidence, we can conclude that type II endoleaks could be monitored safely with CE‐CDUS without employing CT. In this regard, it is relevant to mention that US can offer the additional advantage of providing dynamic information (the ability to document flow velocity and direction in the aneurysm sac) that is not available with CT. For example, Arko 2003, by comparing intrasac Doppler velocities between sealed versus persistent type II endoleaks, concluded that US properties can be used to predict whether type II endoleaks will spontaneously seal. Further research is needed regarding this issue.
The management of type I and type III endoleak is quite different and the role of US needs to be addressed. Type I endoleak is characterized by a blood leak at the distal or proximal attachment site of the stent due to a poor cohesion between the stent and the aortic or iliac wall (Bashir 2009). A type I endoleak can occur immediately after stent deployment requiring an immediate correction. Type I endoleak can also develop later and it requires urgent intervention as it is characterized by high pressure with a risk of aneurysm rupture or tearing (Bashir 2009). Type III endoleak is characterized by leakage of blood through the body of a stent due to poor apposition or separation of the components of the stent, or it can be due to rupture or tear of the graft material. Similar to type I endoleaks, type III endoleaks are considered high‐pressure leaks, with a high rupture risk and require prompt management (Bashir 2009), although some suggest this complication is less frequent with technological improvements (Lawrence‐Brown 2009). Notwithstanding, in a subgroup analysis, our results showed that CE‐CDUS can accurately identify both types I and III endoleaks although specific accuracy studies for detection of type I and type III are required.
Authors' conclusions
Implications for practice.
Our findings support the use of contrast‐enhanced colour duplex ultrasound (CE‐CDUS) as a primary approach with which clinicians can allocate people to subsequent ultrasound (US) monitoring and subsequent therapeutic or diagnostic management of endoleaks in people who received endoluminal abdominal aortic aneurysm repair (EVAR) for the treatment of abdominal aortic aneurysm (AAA). When results of CE‐CDUS are negative, clinicians can appoint patients for a subsequent US monitoring while avoiding the performance of computed tomography (CT). When results are positive, the performance of CT scan becomes mandatory to establish the type of endoleak and the subsequent therapeutic management.
Implications for research.
Guidelines suggest endoleak monitoring at one, six, and 12 months and every year thereafter. Future studies may consider assessing the diagnostic performance of US for each time frame in which monitoring is performed and assess whether US accuracy may vary during follow‐up.
Acknowledgements
We thank Marlene Stewart and Cathryn Broderick for their support; Karen Welch for the search strategy and Rachel Forster for retrieving full‐text articles. We thank also the Biblioteca of the Istituto Zooprofilattico Sperimentale dell'Umbria e delle Marche, Perugia, Italy.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) <1946 to June Week 4 2016>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Aortic Aneurysm/ (46359)
2 Aorta, Abdominal/su [Surgery] (6353)
3 (abdom* adj3 aneurysm?).tw,kf. (15464)
4 (aort* adj3 aneurysm?).tw,kf. (28288)
5 (abdom* adj3 aort*).tw,kf. (27434)
6 (aort* adj3 morphol*).tw,kf. (688)
7 (EVAR or TEVAR or EVRAR).tw,kf. (2885)
8 AAA*.tw,kf. (11472)
9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (69611)
10 Blood Vessel Prosthesis/ (25694)
11 Blood Vessel Prosthesis Implantation/ (18366)
12 Endovascular Procedures/ (9182)
13 endovascular.tw,kf. (29367)
14 endoluminal*.tw,kf. (3584)
15 endoprosthe*.tw,kf. (5932)
16 exp stents/ (61420)
17 stent*.tw,kf. (68589)
18 graft*.tw,kf. (253134)
19 endograft*.tw,kf. (2351)
20 prosthe*.tw,kf. (99022)
21 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 (439973)
22 (aort* or abdominal).tw,kf. (419347)
23 21 and 22 (52737)
24 9 or 23 (100506)
25 postoperative complications/ or anastomotic leak/ or prosthesis failure/ (333258)
26 (endoleak* or endotension).tw,kf. (3100)
27 perigraft leak*.tw,kf. (59)
28 perigraft flow.tw,kf. (21)
29 follow‐up.tw,kf. (659655)
30 surveillance.tw,kf. (114489)
31 postoperative.tw,kf. (334207)
32 outcome.tw,kf. (658736)
33 evaluat*.tw,kf. (2336505)
34 effecti*.tw,kf. (1269598)
35 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 (4461903)
36 us.fs. (222575)
37 ultrasonics/ (22680)
38 ultrasonography/ (64866)
39 ultrasonography, Doppler/ (12929)
40 ultrasonography, Doppler, color/ (12269)
41 ultrasonography, Doppler, duplex/ (5659)
42 ultrasonography, Doppler, pulsed/ (1323)
43 ultrasound.tw,kf. (161980)
44 ultrasonic imaging.tw,kf. (810)
45 ultrasonogra*.tw,kf. (81040)
46 echograph*.tw,kf. (8722)
47 (USS or DUS or CDUS or CEUS).tw,kf. (2865)
48 (doppler or duplex).tw,kf. (107732)
49 sonograph*.tw,kf. (44251)
50 sonogram*.tw,kf. (3324)
51 (contrast adj4 US).tw,kf. (1441)
52 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (454360)
53 24 and 35 and 52 (5902)
Appendix 2. Embase search strategy
Database: Embase <1980 to 2016 Week 26>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp aorta aneurysm/ (47427)
2 abdominal aorta/su [Surgery] (2043)
3 exp aorta surgery/ (29048)
4 (abdom* adj3 aneurysm?).tw,kw. (20433)
5 (aort* adj3 aneurysm?).tw,kw. (36443)
6 (aort* adj3 endograft*).tw,kw. (915)
7 (aneurysm? adj3 repair).tw,kw. (9318)
8 (aort* adj3 morphol*).tw,kw. (1084)
9 (abdom* adj3 aort*).tw,kw. (35653)
10 (EVAR or TEVAR or EVRAR).tw,kw. (5136)
11 AAA*.tw,kw. (16173)
12 or/1‐11 (96271)
13 exp blood vessel prosthesis/ (14328)
14 exp blood vessel transplantation/ (93394)
15 endovascular surgery/ (18987)
16 endovascular.tw,kw. (49927)
17 endoluminal*.tw,kw. (5226)
18 endoprosthe*.tw,kw. (7086)
19 endograft*.tw,kw. (3479)
20 stents/ (70924)
21 (stent* or graft*).tw,kw. (449027)
22 prosthe*.tw,kw. (119669)
23 or/13‐22 (636877)
24 (aort* or abdominal).tw,kw. (580126)
25 23 and 24 (76044)
26 12 or 25 (141573)
27 Postoperative Complications/ (54233)
28 prosthesis failure/ or endoleak/ (20256)
29 (endoleak* or endotension).tw,kw. (4757)
30 perigraft leak*.tw,kw. (71)
31 perigraft flow.tw,kw. (24)
32 follow‐up.tw,kw. (1048410)
33 surveillance.tw,kw. (167616)
34 postoperative.tw,kw. (453187)
35 outcome.tw,kw. (1019612)
36 evaluat*.tw,kw. (3446507)
37 effecti*.tw,kw. (1807515)
38 or/27‐37 (6263385)
39 ultrasound/ (129176)
40 echography/ or doppler echography/ (283071)
41 ultrasound.tw,kw. (271703)
42 ultrasonic imaging.tw,kw. (1112)
43 ultrasonogra*.tw,kw. (124686)
44 echograph*.tw,kw. (11056)
45 (USS or DUS or CDUS or CEUS).tw,kw. (6070)
46 (doppler or duplex).tw,kw. (157018)
47 sonograph*.tw,kw. (62821)
48 sonogram*.tw,kw. (4204)
49 (contrast adj4 US).tw,kw. (2217)
50 or/39‐49 (645379)
51 26 and 38 and 50 (7359)
Appendix 3. LILACS search strategy
| Database : | LILACS |
| Search on : | endoleak [Subject descriptor] or endoleak [Words] or perigraft [Words] |
| Total of references : |
44 http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/ |
Appendix 4. ISI Conference Proceedings Citation Index search
| # 31 | 451 | #30 AND #23 AND #16 |
| Indexes=CPCI‐S Timespan=1950‐2016 | ||
| # 30 | 540,587 | #29 OR #28 OR #27 OR #26 OR #25 OR #24 |
| # 29 | 2,623 | TS=(contrast NEAR/4 US) |
| # 28 | 57,226 | TS=(sonograph* or sonogram*) |
| # 27 | 203,502 | TS=(doppler or duplex) |
| # 26 | 4,337 | TS=(USS or DUS or CDUS or CEUS) |
| # 25 | 5,344 | TS=echograph* |
| # 24 | 343,215 | TS=(ultrasound or ultrasonic) |
| # 23 | 3,564,463 | #22 OR #21 OR #20 OR #19 OR #18 OR #17 |
| # 22 | 1,583,294 | TS=evaluation |
| # 21 | 1,682,133 | TS=(surveillance or postoperative or outcome) |
| # 20 | 761,871 | TS=follow‐up |
| # 19 | 53 | TS=perigraft flow |
| # 18 | 111 | TS=perigraft leak* |
| # 17 | 3,473 | TS=(endoleak* or endotension) |
| # 16 | 79,264 | #15 OR #7 |
| # 15 | 46,235 | #14 AND #13 |
| # 14 | 406,141 | TS=(aort* or abdominal) |
| # 13 | 519,126 | #12 OR #11 OR #10 OR #9 OR #8 |
| # 12 | 96,378 | TS=prosthe* |
| # 11 | 408,581 | TS=(stent* or graft*) |
| # 10 | 8,742 | TS=(endoprosthe* or endograft*) |
| # 9 | 4,381 | TS=endoluminal |
| # 8 | 38,274 | TS=endovascular |
| # 7 | 45,797 | #6 OR #5 OR #4 OR #3 OR #2 OR #1 |
| # 6 | 16,206 | TS=AAA* |
| # 5 | 3,631 | TS=(EVAR or TEVAR or EVRAR) |
| # 4 | 28,911 | TS=(abdom* NEAR/3 aort*) |
| # 3 | 1,004 | TS=(aort* NEAR/3 endograft*) |
| # 2 | 13,331 | TS=(aort* NEAR/3 aneurysm?) |
| # 1 | 7,773 | TS=(abdom* NEAR/3 aneurysm?) |
Appendix 5. Zetoc search
29 for: any: endoleak
Appendix 6. Trials registries
Clinicaltrials.gov
10 studies found for: endoleak and ultrasound
WHO
4 records for 4 trials found for: endoleak and ultrasound
ISRCTN
0 results for endoleak
Appendix 7. Study distribution based on the type of ultrasound and the unit of analysis used
| Study ID | Type of ultrasound |
Unit of analysis number of individuals |
Unit of analysis number of scans |
| Abbas 2014 | CE‐CDUS | ‐ | ✔ |
| AbuRahma 2005 | CDUS | ‐ | ✔ |
| Arsicot 2014 | CDUS | ✔ | |
| Ashoke 2005b | CDUS | ‐ | ✔ |
| Ashoke 2005c | CDUS | ‐ | ✔ |
| Badri 2010 | CDUS | ‐ | ✔ |
| Bendick 2003 | CDUS/CE‐CDUS | ✔ | ‐ |
| Cantisani 2011 | CDUS/CE‐CDUS | ✔ | ‐ |
| Clevert 2008b | CDUS/CE‐CDUS | ✔ | ‐ |
| Clevert 2011 | CDUS/CE‐CDUS | ✔ | ‐ |
| Costa 2013 | CDUS/CE‐CDUS | ‐ | ✔ |
| d'Audiffret 2001 | CDUS | ✔ | |
| Demirpolat 2011 | CDUS | ‐ | ✔ |
| França 2013 | CDUS | ‐ | ✔ |
| Gargiulo 2014 | CE‐CDUS | ✔ | |
| Giannoni 2003 | CDUS/CE‐CDUS | ‐ | ✔ |
| Giannoni 2007 | CE‐CDUS | ✔ | ‐ |
| Golzarian 2002 | CDUS | ✔ | ‐ |
| Gray 2012 | CDUS | ‐ | ✔ |
| Gurtler 2013 | CE‐CDUS | ‐ | ✔ |
| Heilberger 1997 | CDUS/CE‐CDUS | ✔ | ‐ |
| Henao 2006 | CDUS/CE‐CDUS | ✔ | ‐ |
| Iezzi 2009 | CDUS/CE‐CDUS | ✔ | ‐ |
| McLafferty 2002 | CDUS | ✔ | ‐ |
| McWilliams 1999 | CDUS/CE‐CDUS | ‐ | ✔ |
| McWilliams 2002 | CDUS/CE‐CDUS | ‐ | ✔ |
| Motta 2012 | CE‐CDUS | ‐ | ✔ |
| Nagre 2011 | CDUS | ‐ | ✔ |
| Nerlekar 2006 | CDUS | ‐ | ✔ |
| Oikonomou 2012 | CDUS | ✔ | ‐ |
| Pages 2001 | CDUS | ‐ | ✔ |
| Parent 2002 | CDUS | ✔ | ‐ |
| Perini 2011 | CE‐CDUS | ✔ | ‐ |
| Perini 2012 | CE‐CDUS | ✔ | ‐ |
| Raman 2003 | CDUS | ‐ | ✔ |
| Sandford 2006 | CDUS | ✔ | ‐ |
| Sato 1998 | CDUS | ‐ | ✔ |
| Schmieder 2009 | CDUS | ‐ | ✔ |
| Ten Bosch 2010 | CE‐CDUS | ‐ | ✔ |
| Thompson 1998 | CDUS | ✔ | ‐ |
| Wolf 2000 | CDUS | ‐ | ✔ |
| Zannetti 2000 | CDUS | ✔ | ‐ |
| CDUS: colour duplex ultrasound; CE‐CDUS: contrast‐enhanced colour duplex ultrasound. | |||
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
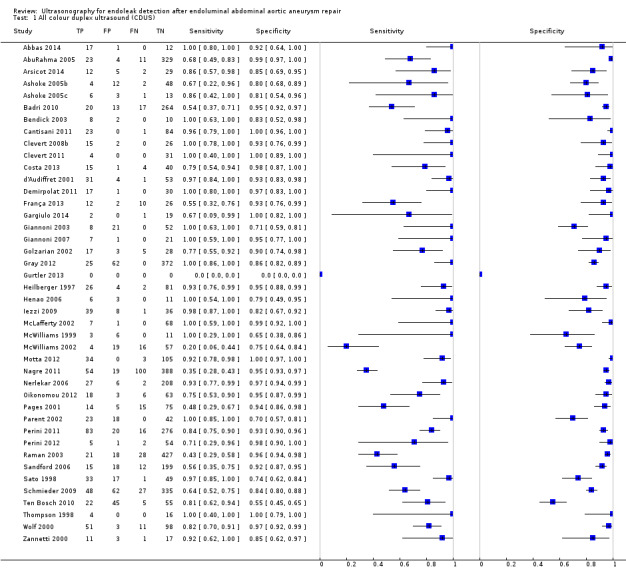
All colour duplex ultrasound (CDUS).
2. Test.
CDUS (unit of analysis: number of individuals).
3. Test.
Contrast‐enhanced colour duplex ultrasound (CE‐CDUS) (unit of analysis: number of individuals).
4. Test.
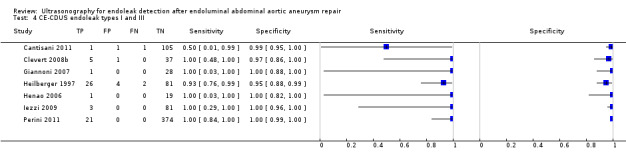
CE‐CDUS endoleak types I and III.
5. Test.
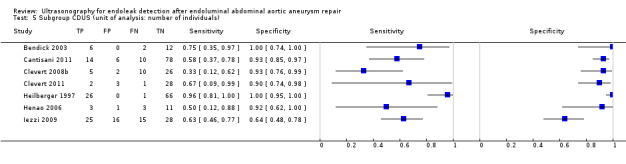
Subgroup CDUS (unit of analysis: number of individuals).
6. Test.
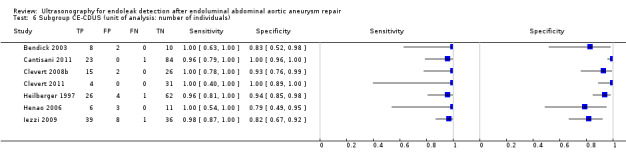
Subgroup CE‐CDUS (unit of analysis: number of individuals).
7. Test.
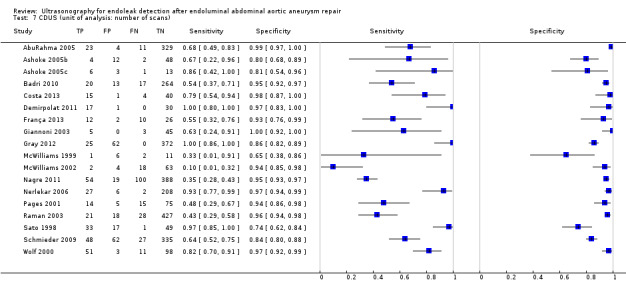
CDUS (unit of analysis: number of scans).
8. Test.
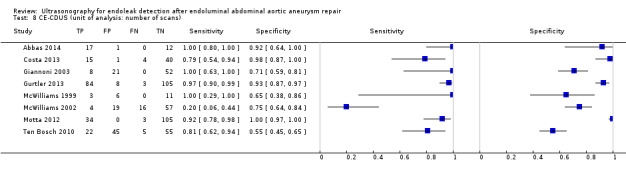
CE‐CDUS (unit of analysis: number of scans).
9. Test.
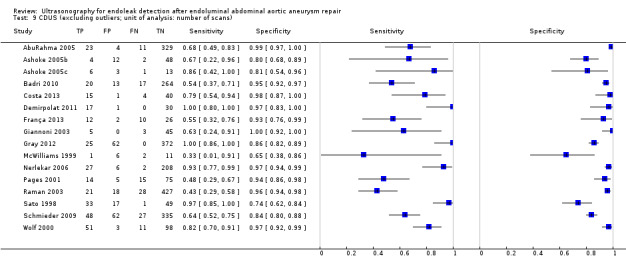
CDUS (excluding outliers; unit of analysis: number of scans).
10. Test.
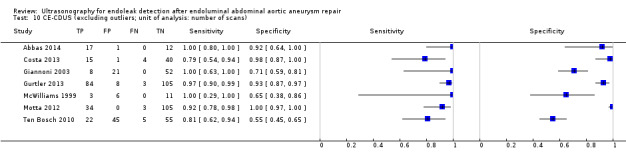
CE‐CDUS (excluding outliers; unit of analysis: number of scans).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbas 2014.
| Clinical features and settings | 23 consecutive participants attending for CTA and 3D CEUS imaging who were thought to have an endoleak following an EVAR. Type of stents received: bifurcated: 87% (n = 20); uni‐iliac: 13% (n = 3). Aneurysm diameter (mean ± SD): CTA measure: 6.6 ± 1.1 cm; US measure: 6.0 ± 0.97 cm. Setting: tertiary referral vascular centre. |
|
| Participants | 23 participants; 20 men; age (mean ± SD): 77.4 ± 6 years. Comorbidities: not reported. Geography: UK. |
|
| Study design | Retrospective cross‐sectional study; participants were consecutively enrolled. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: "The 3D reconstruction enables the operator to view the path of the endoleak. The ability to manoeuvre the 3D reconstruction and view the images in sagittal, coronal, and transverse planes simultaneously without moving the probe, enables the operator to accurately identify if flow is within or outside the aneurysm sac." Endoleak (absolute n): 17. Prevalence of endoleak: 56.7% (17/30). Reference standard: CTA. Image acquisition:
Type of CT scanner: Siemens Sensation (Siemens Medical, Germany). Use of contrast: 100 mL of iodinated contrast medium Omnipaque 240 (iohexol, 240 mg/mL) administered at flow rate of 3 mL/s. Operator: consultant interventional radiologist. |
|
| Index and comparator tests |
Index test: 3D CE‐CDUS. Image production: with participants supine, AAA and stent‐graft visualized and traced to proximal neck, which was measured in cross‐section and interrogated for potential endoleak using low colour flow velocity or power Doppler colour flow settings. Type of US: Curefab 3D system comprises tracking sensors installed in transducer of a high‐definition duplex Doppler US system (IU22‐C5) and an electromagnetic box. This technology uses motion tracking mini‐GPS with magnetic field emitter and 2 tracking sensors that transform standard 2D CEUS images into high‐definition 3D format (Curefab, Munich, Germany). Use of contrast: yes, "SonoVue, Bracco, Milan, Italy." Operator: all images reviewed by a research fellow and reported by either an accredited vascular or laboratory technologist. Comparator: 2D CE‐CDUS. Image production: with participants supine, AAA and stent‐graft visualized and traced to proximal neck, which was measured in cross‐section and interrogated for potential endoleak using low colour flow velocity or power Doppler colour flow settings. Type of US: DUS instrument (Philips IU22) with C5‐1 curved array transducer. Use of contrast: yes, "SonoVue, Bracco, Milan, Italy." Operator: all images reviewed by research fellow and reported by either an accredited vascular laboratory technologist. |
|
| Follow‐up | No loss to follow‐up, missing data, or adverse events. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants consecutively enrolled. |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | No | "The interval between paired images was 3.9 ± 2.7 (mean ± SD) weeks. Range: same day to 8 weeks." |
| Partial verification avoided? All tests | Yes | Appeared all participants received both tests. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test was not part of the reference standard. |
| Reference standard results blinded? All tests | Unclear | No description provided. |
| Index test results blinded? All tests | Unclear | No description provided. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | No apparent uninterpretable data occurred. |
| Withdrawals explained? All tests | Yes | No explicit report concerning loss to follow‐up, missing data, or adverse events. |
AbuRahma 2005.
| Clinical features and settings | 178 participants treated with aortic stent grafts for AAA. Type of stents received: 86 Ancure (Guidant Corporation, USA), 55 AneuRx (Medtronic,USA), and 37 Excluder (WL Gore & Associates, USA). Aneurysm diameter: not reported. Setting: vascular laboratory. |
|
| Participants | 156 men; mean age: 74 years; range: 49‐89 years. Comorbidities: not reported. Geography: USA. |
|
| Study design | Cross‐sectional study; participants were consecutively enrolled; all participants were without the target condition at the start of study. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: "An endoleak was determined using CT scans based on extravasation of contrast between the prosthesis and the aneurysm wall; by CDUS, endoleak was indicated by flow and spectral signals outside the prosthesis. Primary or early endoleak occurred within 30 days of the procedure; late endoleak referred to leaks observed beyond 30 days postoperatively." Endoleak (absolute n): 34. Prevalence of endoleak: 9.3% (34/367). Reference standard: helical CT imaging. Image acquisition:
Type of CT scanner: Philips Medical Systems, Inc (Shelton, CT, USA). Use of contrast: Optiray 350 (125 mL; Mallinkrodt Medical, USA). |
|
| Index and comparator tests |
Index test: CDUS. Image production: transverse and anteroposterior images obtained from level of suprarenal aorta above graft to distal iliac or femoral arteries. Type of US: HDI 5000 scanner, ATL‐Philips, USA. Use of contrast: no. Operator: registered vascular technologist and board‐certified vascular surgeon. |
|
| Follow‐up | No loss to follow‐up, missing data, or adverse events. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants consecutively enrolled. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Yes | "CT and CDUS scans were considered concurrent if they were done within 7 days." |
| Partial verification avoided? All tests | Yes | Appeared all participants that received both tests. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | Reference standard performed before CDUS. |
| Index test results blinded? All tests | Yes | "Neither the registered vascular technologist nor the reviewing surgeon was aware of the CT results during any portion of the CDUS examination." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | No apparent uninterpretable data ("no delayed imaging was performed to detect questionable endoleaks"). |
| Withdrawals explained? All tests | Yes | No explicit report concerning loss to follow‐up, missing data, or adverse events. |
Arsicot 2014.
| Clinical features and settings | 75 consecutive participants treated with EVAR for infrarenal AAA, representing 116 pairs of examinations (3D US and CTA). Type of stents received: Anaconda (Vascutek) 30, Low Profile (Cook) 4, Zenith (Cook) 28, Endurant (Medtronic) 1, Aneurx (Medtronic) 2, Excluder (WL Gore & Associates) 8, Powerlink (Edwards) 2. Aneurysm diameter: not reported. Setting: academic hospital. |
|
| Participants | 73 men; age (mean ± SD): 76.3 ± 9.2 years. Comorbidities: diabetes (n = 18), dyslipidaemia (n = 67), BMI ≥ 30 kg/m2 (n = 15), renal insufficiency (n = 24), supra‐aortic trunks lesions (n = 19), angina (n = 42), ASA score III‐IV (n = 56). Geography: France. |
|
| Study design | Retrospective cross‐sectional study; participants consecutively enrolled. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: not reported. Endoleak (absolute n): 14. Prevalence of endoleak: 29.2% (14/48). Reference standard: CTA. Image acquisition:
Type of CT scanner: Siemens Medical Solutions Inc (Somatom Definition AS+, Malvern). Use of contrast: "The protocol of imagery included an injection of 100 mL of iodised contrast of 250 concentration, with a 5 mL/s flow IV in the upper limb right." Operators: vascular surgeon. |
|
| Index and comparator tests |
Index test: 3D US Image production: "The examination was carried out by 1 of the 3 vascular sonographers of our institution with a transperitoneal approach. The ultrasound probe was placed over the umbilicus in the longitudinal axis of the infrarenal abdominal aorta, and side electronic scanning allowed a 3D acquisition of the infrarenal aneurysm to the aortic bifurcation." Type of US: Toshiba Aplio XG ultrasound system (Toshiba Medical Systems, Zoetermeer, the Netherlands) equipped with a marketed 3D 3.5‐MHz dedicated probe. Use of contrast: no. Operator: 1 vascular sonographer. |
|
| Follow‐up | No loss to follow‐up, missing data, or adverse events. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Patients were consecutively enrolled |
| Acceptable reference standard? All tests | Yes | The reference standard was CTA |
| Acceptable delay between tests? All tests | No | The average time interval between the achievements of CTA and 3DU for a given patient was 48.18 ± 36.52 days |
| Partial verification avoided? All tests | Yes | It appears that all patients that received both tests |
| Differential verification avoided? All tests | Yes | All patients who received the index test were subjected to the same reference standard |
| Incorporation avoided? All tests | Yes | The index test is not part of the reference standard |
| Reference standard results blinded? All tests | Yes | “Volumetric calculation by CTA was carried out by a single person, vascular surgeon of formation, by data processing of the native sections of the CTA (Siemens Medical Solutions Inc., Somatom Definition AS+, Malvern). He did not know the results of ultrasound measurements of volume” |
| Index test results blinded? All tests | Unclear | No description was reported |
| Relevant clinical information? All tests | Yes | Yes |
| Uninterpretable results reported? All tests | Yes | No apparent uninterpretable data occurred |
| Withdrawals explained? All tests | Yes | No explicit report concerning loss to follow‐up, missing data or adverse events |
Ashoke 2005b.
| Clinical features and settings | 30 participants undergoing EVAR recruited consecutively. 1 participant underwent EVAR as treatment for a pseudoaneurysm, and 4 did not have CT and CDUS scans within the required time intervals during follow‐up. Remaining 25 participants had a mean (± SD) original AAA diameter of 6.0 ± 0.6 cm. Type of stents received: AneuRx (n = 3), Zenith Bifurcated (n = 8), Zenith Trifab (n = 5), EVT (n = 1), and Nottingham‐style aortomonoiliac grafts with a femorofemoral crossover (n = 8). Aneurysm diameter: mean (± SD) original AAA diameter was 6.0 ± 0.6 cm. Setting: hospital. |
|
| Participants | 25 participants; 22 men; mean (± SD) age: 72.4 ± 6.9 years. Comorbidities: not reported. Geography: London, UK. |
|
| Study design | Cross‐sectional study; participants consecutively enrolled. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: "Presence or absence of flow within the aneurysm sac. …Endoleaks were defined by the presence of contrast within the aneurysm sac, and an attempt was made to determine the site of any leak" (Thompson 1998). Endoleak (absolute n): 6. Prevalence of endoleak: 9.1% (6/66). Reference standard: contrast‐enhanced CT. Image acquisition:
Type of CT scanner: Siemens HiQ scanner (Munich, Germany). Use of contrast: not reported. |
|
| Index and comparator tests |
Index test: CDUS. Image production: duplex imaging performed with participant supine. CDU utilized to image flow within graft, and any flow disturbance noted. Endoleaks specifically sought with colour Doppler set to detect low flow. CDUS scan times 35‐55 minutes. Type of US: 3.5‐MHz curved linear array transducer, HDI Ultramark 9 (ATL, Letchworth, UK). Use of contrast: no. Operator: not reported. |
|
| Follow‐up | "The 30 patients all received follow‐up scans at 1, 3, 12, 18, and 24 months postoperatively and annually thereafter, although not all follow‐up procedures were undertaken at the correct time intervals." | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants consecutively enrolled. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Unclear | Interval between the 2 tests not clearly stated: "At each visit, patients had a clinical examination followed by CT and duplex imaging." |
| Partial verification avoided? All tests | Yes | Appeared all participants received both tests. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "Diagnostic imaging was performed by investigators who were blinded of the result from the other imaging technique and previous scans." |
| Index test results blinded? All tests | Yes | "Diagnostic imaging was performed by investigators who were blinded of the result from the other imaging technique and previous scans." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "The CT scans were suboptimal in 2 cases due to calcification, but a diagnosis was still made." |
| Withdrawals explained? All tests | Yes | "One pair of scans was excluded because the CT scans were not archived or reported, and the films were lost from the hospital." |
Ashoke 2005c.
| Clinical features and settings | 78 people received regular follow‐up scans. 64 participants did not have CT and CDUS scans within required time intervals at any point during follow‐up. 10 remaining participants with paired scans were men. Type of stents received: AneuRx (n = 3), Excluder (n = 5), and Talent (n = 2). Aneurysm diameter: mean (± SD) original AAA diameter: 6.0 ± 0.6 cm. Setting: hospital. |
|
| Participants | 10 men; mean (± SD) age: 75.0 ± 5.2 years. Comorbidities: not reported. Geography: London, UK. |
|
| Study design | Cross‐sectional study; participants consecutively enrolled. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: "Presence or absence of flow within the aneurysm sac. … Endoleaks were defined by the presence of contrast within the aneurysm sac, and an attempt was made to determine the site of any leak" (Thompson 1998). Endoleak (absolute n): 7. Prevalence of endoleak: 30.4% (7/23). Reference standard: contrast‐enhanced CT. Image acquisition:
Type of CT scanner: Siemens HiQ scanner (Munich, Germany). Use of contrast: not reported. |
|
| Index and comparator tests |
Index test: CDUS. Image production: duplex imaging performed with participant supine. Colour Doppler ultrasonography utilized to image flow within graft, and any flow disturbance noted. Endoleaks specifically sought with colour Doppler set to detect low flow. CDUS scan times 35‐55 minutes. Type of US: 3.5‐MHz curved linear array transducer, HDI Ultramark 9 (ATL, Letchworth, UK). Use of contrast: no. Operator: not reported. |
|
| Follow‐up | "2 patients were lost to follow‐up, and CT films could not be located for 2 patients." | |
| Notes |
Data reported in review by Ashoke 2005a. |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants consecutively enrolled. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Unclear | Interval period between the 2 tests not clearly stated: "At each visit, patients had a clinical examination followed by CT and duplex imaging." |
| Partial verification avoided? All tests | Yes | It appeared all participants received both tests. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "Diagnostic imaging was performed by investigators who were blinded of the result from the other imaging technique and previous scans." |
| Index test results blinded? All tests | Yes | "Diagnostic imaging was performed by investigators who were blinded of the result from the other imaging technique and previous scans." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "The remaining endoleak was detected by CDU [CDUS] but unclassified because of suboptimal images due to patient habitus." |
| Withdrawals explained? All tests | Yes | "2 patients were lost to follow‐up, and CT films could not be located for 2 patients." |
Badri 2010.
| Clinical features and settings | People with AAA who underwent EVAR between April 1998 and December 2007. During this period, 93 procedures performed but complete records unavailable in 34 participants. Type of stents received: Cook‐Zenith (William A Cook, Australia; 54 participants), Talent Medtronic (Medtronic, UK) aortic stent graft (5 participants). Aneurysm diameter: not reported. Setting: Department of Vascular Surgery. |
|
| Participants | 59 participants; 50 males; mean age: 79 years. Comorbidities: not reported. Geography: UK. |
|
| Study design | Retrospective design; participants identified retrospectively based on availability of complete records ("93 procedures were performed but complete records were unavailable in 34 patients"). | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: not reported. Endoleak (absolute n): 14. Prevalence of endoleak: 11.8% (37/314). Reference standard: CTA (dual‐phase multidetector CT). Type of CT scanner: Philips MX80000 IDT or GE Prospeed SX. Image acquisition for Philips MX80000 IDT:
Image acquisition for GE Prospeed SX:
|
|
| Index and comparator tests |
Index test: DUS (2‐to 5‐MHz transducer, Philips IU22 scanner). Image production: anterior posterior and transverse aortic sac diameters measured in transverse and longitudinal section. Pulsed Doppler used to evaluate any colour Doppler signals exterior to graft. Type of US: 2‐ to 5‐MHz transducer (Philips IU22 scanner). Use of contrast: no. Operator: registered vascular technologist and board‐certified vascular surgeon. |
|
| Follow‐up | 1, 3, 6, 12, and 18 months, and then yearly afterward (total of 314 paired scans obtained over a follow‐up period from 3 days to 9 years); no apparent loss to follow‐up or missing data. | |
| Notes | Other endpoints: sac size: anterior posterior, transverse, and maximum diameter (Dmax); graft patency. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Although enrolled people with AAA who underwent EVAR, they were retrospectively studied and from 93 procedures performed complete records were available for only 59 participants. |
| Acceptable reference standard? All tests | Yes | CTA (dual‐phase multi‐detector CT on a Philips MX80000 IDT or GE Prospeed SX). |
| Acceptable delay between tests? All tests | Yes | "Three hundred and fourteen paired scans were studied. Paired scans and almost all scans took place on the same day. Single scans outside this timeframe were excluded." |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received US subjected to CT scan. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Unclear | "Two consultant interventional radiologists reported the CTA studies and 3 specialist vascular ultrasonographers performed and reported the DUS." |
| Index test results blinded? All tests | Unclear | "Two consultant interventional radiologists reported the CTA studies and 3 specialist vascular ultrasonographers performed and reported the DUS." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | All data interpreted. |
| Withdrawals explained? All tests | Yes | No apparent withdrawal occurred. |
Bendick 2003.
| Clinical features and settings | Overall population who received a stent composed of 63 male and 6 female participants, mean (± SD) age 72.6 ± 8.7 years (range 58‐87 years); Type of stents received: not reported. 64 grafts had modular design and 5 grafts had unibody bifurcated design. Aneurysm diameter: mean (± SD) aneurysm size: 5.6 ± 0.9 cm. Setting: vascular laboratory. |
|
| Participants | Included participants were 19 males and one female patient; mean (± SD) age: 74.5 ± 7.6 years; range: 65‐86 years. 18 of these participants had modular graft design and 2 participants had unibody bifurcated graft placed. Comorbidities: not reported. Geography: USA. |
|
| Study design | Unclear. Probably a retrospective study. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: any endoleaks that were seen with DUS were classified as being related to the stent graft itself (group l), at either proximal or distal attachment sites or at any graft module junctions or secondary to patent aortic branch vessels (group II), such as the inferior mesenteric artery or lumbar arteries, which showed collateral filing and back‐bleeding into the aneurysm sac. Endoleak classified as indeterminate if it could not be definitively identified as being in group I or group II. Endoleak (absolute n): 8. Prevalence of endoleak: 40% (8/20). Reference standard: CT scan. Image acquisition:
Type of CT scanner: not reported. Use of contrast: not clearly reported. |
|
| Index and comparator tests |
Index test: CDUS. Image production: aorta scanned in long axis and in cross‐sectional views from level of diaphragm distally to below attachment sites of iliac limbs of stent graft. Residual aneurysm sac size measured in both anterior‐posterior and transverse dimensions at its widest point, and arterial flow haemodynamics documented throughout stent graft with spectral Doppler velocity measurements. Suspected endoleak further documented for flow characteristics with spectral Doppler velocities. Type of US: standard DUS scan examination (LOGIQ 700, GE, Milwaukee, WI, USA) with CDI and spectral Doppler velocity measurements Use of contrast: yes (1‐mL bolus of US scan contrast agent (Optison, Mallinckrodt, St Louis, MO). Operator: unclear. |
|
| Follow‐up | No apparent missing data or adverse events. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | No | Of the whole sample who received stents, 20 participants included. While 10 participants selected at random, remaining 10 participants selected based on participant's habitus or presence of bowel gas. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Yes | "All CT angiography were read within a 2‐week period of the duplex ultrasound scan." |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received US subjected to CT scan. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "The operator was blinded to the results of any previous ultrasound scans and of any prior angiographic or CT angiography studies." |
| Index test results blinded? All tests | Yes | All CTA studies read independently for presence or absence of endoleaks. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "CT angiography identified eight endoleaks and classified two of them into group I and three into group II; three additional posterior sac endoleaks were seen, but their origin was not clearly identified." |
| Withdrawals explained? All tests | Yes | No withdrawals occurred. |
Cantisani 2011.
| Clinical features and settings | People who had undergone EVAR for AAA. Type of stents received: EVAR devices employed were Excluder (WL Gore & Associates, Flagstaff, AZ, USA) in 50 participants, Talent (Medtronic, Santa Rosa, CA, USA) in 55, Powerlink (Endologix, Irvine, CA, USA) in 12, and Jomed (Jomed International AB, Helsingborg, Sweden) in 6. Aneurysm diameter (mean ± SD): 5.4 ± 0.5 cm; range 3.9‐8.7 cm. Setting: vascular laboratory. |
|
| Participants | 123 participants; 92 males and 31 females; mean (± SD) age: 63.0 ± 7.3 years; who had undergone EVAR for AAA; aneurysm baseline mean (± SD) diameter: 5.4 ± 0.5 cm; range 3.9‐8.7 cm. Comorbidities: not reported. Geography: Italy. |
|
| Study design | Prospective, observational (cross‐sectional) study. | |
| Target condition and reference standard(s) |
Target condition: aneurysm sac size, attachment and integrity of prosthesis, and presence and type of any endoleak. Definition of endoleak: incomplete exclusion of aneurysm sac from circulation. Endoleak (absolute n): 24. Prevalence of endoleak: 22.2% (24/108). Reference standard: CTA. Image acquisition:
Type of CT scanner: Somaton Sensation (Siemens Medical Solutions, Erlangen, Germany). Use of contrast: non‐ionic contrast media: Iomeron, Bracco, Milan, Italy, flow 4 mL/s. |
|
| Index and comparator tests |
Index test: CDUS. Image production: 3‐ to 5‐MHz probe, with longitudinal and transversal scans with participant lying in dorsal or lateral position. Aneurysm sac size measured in both anterior‐posterior and transverse dimensions at widest point and mean of these measurements used for purposes of study. Arterial flow haemodynamics documented throughout stent graft with spectral Doppler velocity measurements. CE‐CDUS: 3‐ to 5‐MHz probe and with low mechanical index (varying from 0.06 to 0.10; about 35‐45 kPa), with real‐time tissue harmonic imaging and contrast harmonic imaging (pulse subtraction). Type of US: Aplio XV (Toshiba Vx, Zoetermeer, the Netherlands) and Technos MPX US (ESAOTE Biomedica, Genoa, Italy). Use of contrast: yes; second‐generation contrast agent (SonoVue, Bracco, Milan, Italy) consisting of sulphur hexafluoride gas microbubbles in a phospholipid membrane. Operator: 2 radiologists with 20 and 10 years of experience in this particular field. |
|
| Follow‐up | 15 participants excluded: 8 participants not undergo MRA (2 with claustrophobia; 6 had pace‐maker); 3 because of renal failure; 1 participant because of the allergy to iodine; 3 because of presence of severe comorbidity (heart failure and severe pulmonary disease). | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants consecutively enrolled for follow‐up to detect potential endoleaks. |
| Acceptable reference standard? All tests | Yes | CTA used to detect target disease. |
| Acceptable delay between tests? All tests | Yes | CDUS and CE‐CDUS performed on same day, and, within 1 week, CTA and MRA. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received US subjected to CT scan. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "The radiologists were blinded to the results of any previous examination." |
| Index test results blinded? All tests | Yes | Index test performed before reference standard. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | Yes. |
| Withdrawals explained? All tests | Yes | 15 participants excluded from study because of the following reasons: 8 participants could not undergo MRA (2 with claustrophobia; 6 had pace‐maker); 3 participants because of renal failure, 1 participant because of allergy to iodine, and 3 because of presence of severe comorbidity (heart failure and severe pulmonary disease). |
Clevert 2008b.
| Clinical features and settings | People who received a stent for AAA and were included in surveillance programme for endoleak detection. Type of stents received: not reported. Aneurysm diameter: not reported. Setting: radiology division. |
|
| Participants | 43 consecutive participants; 43 males; mean age: 63 years. Comorbidities: not reported. Geography: Germany. |
|
| Study design | Prospective recruitment of participants ("patients who were referred to our radiology division to have a MS‐CT using commercially available equipment, a supplementary ultrasound investigation with a 4 MHz multi‐frequency probe was conducted with the modalities of colour duplex ultrasound and CEUS"). | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: persistence of blood flow outside lumen of endoluminal graft but within an aneurysm sac or adjacent vascular segment being treated by the graft. Endoleak type I: If flow into aneurysm sac originated from around stent graft attachment site, it was classified as type I. Further categorization of type I endoleaks suggested as type IA (proximal), type IB (distal), or type IC, indicating persistent flow lateral to an iliac occlusion stent graft in contralateral limb after implantation of a mono‐iliac stent graft. Endoleak type II: Type II endoleaks represent retrograde blood flow through aortic branch vessels into aneurysm sac. They occur when blood travels through branches from unstented portion of aorta or iliac arteries that anastomose with vessels in direct communication with aneurysm sac. Typical sources include inferior mesenteric and lumbar arteries and Riolan's anastomosis. Endoleak type III: Type III endoleaks occur when there is structural failure with the stent graft such as stent‐graft fractures or holes that develop in fabric of device. In addition, category includes junctional separations seen with modular devices. Although type III endoleaks are currently rare, they will probably become more prevalent as stent grafts begin to age and long‐term follow‐up of these participants accrues. Endoleak (absolute n): 15. Prevalence of endoleak: 34.9% (15/43). Reference standard: 16‐ or 64‐detector CT scanner. Image acquisition:
Type of CT scanner: 16‐ or 64‐detector CT scanner (Somatom Sensation 16 or 64, Siemens Medical Systems, Forchheim, Germany). Use of contrast: 100‐120 mL Imeron (Altana Pharma, Germany) with iodine concentration 350 mg/mL. |
|
| Index and comparator tests |
Index test: CDUS/CE‐CDUS. Image production: transverse and sagittal imaging. In CDUS, colour gain selected just as high as necessary to avoid overwriting artefacts (i.e. colour pixels outside perfused lumen of vessel). Additionally, an automatic image gain optimisation could be selected. For CEUS examinations, Sequoia systems were equipped with CPS software which detects the microbubbles' fundamental non‐linear response. CEUS employed continuous low mechanical index (0.15‐0.19) real‐time tissue harmonic imaging (Cadence) CPS imaging. The US device featured a high‐performance processor and allowed documentation of dynamic image sequences in cine mode by a digital frame buffer. Type of US: 4‐MHz multifrequency transducer (curved array). Use of contrast: yes; contrast agent (SonoVue, Bracco, Milan, Italy) consisting of stabilised microbubbles of sulphur hexafluoride administered into antecubital vein through 18‐G needle and followed by flush of 10 mL saline solution (0.9% NaCl). Operator: 1 (sonographer was unaware of CT scan). |
|
| Follow‐up | No loss to follow‐up, missing data, or adverse events. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants consecutively enrolled for follow‐up to detect potential endoleaks. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Yes | "For the whole study population, contrast‐enhanced CT examinations were performed within 1 day before contrast‐enhanced sonography." |
| Partial verification avoided? All tests | Yes | All participants who received US were subjected to CT scan. |
| Differential verification avoided? All tests | Yes | Index test not part of reference standard. |
| Incorporation avoided? All tests | Yes | Two tests were not incorporated. |
| Reference standard results blinded? All tests | Yes | All CT examinations performed and read by experienced radiologists who were blinded to results of both sonography and contrast‐enhanced sonography. |
| Index test results blinded? All tests | Yes | Sonographer unaware of CT scan results during the examination and reading of duplex and CEUS examination. |
| Relevant clinical information? All tests | Yes | Relevant clinical information reported. |
| Uninterpretable results reported? All tests | Yes | All data were interpreted. ("In the follow up the two false positive endoleaks (types I and II) in CE‐CDUS were confirmed as true positive endoleaks by CE‐CDUS and MS‐CT (Figs 7‐9). The sensitivity of CE‐CDUS was therefore 100% and its specificity 100%; the positive and negative predictive values were 1 and 1, respectively.") |
| Withdrawals explained? All tests | Yes | No withdrawals occurred. |
Clevert 2011.
| Clinical features and settings | Participants referred to interdisciplinary ultrasound centre (Klinikum Grosshadern, Munich, Germany) for further diagnostic work‐up and follow‐up after EVAR for AAA. Type of stents received: not reported. Aneurysm diameter: not reported. Setting: interdisciplinary ultrasound centre. |
|
| Participants | 35 consecutive participants; 33 men; mean age: 73 years; range: 54‐83 years. Comorbidities: not reported. Geography: Germany. |
|
| Study design | Retrospective study; consecutively enrolled participants. | |
| Target condition and reference standard(s) |
Target condition: endoleak (not defined). Definition of endoleak: no definition provided. Endoleak (absolute n): 4. Prevalence of endoleak: 11.4% (4/35). Reference standard: CTS (not clearly defined); image fusion with CTA. No further information was reported. Image acquisition: not reported. Type of CT scanner: not reported. Use of contrast: not reported. |
|
| Index and comparator tests |
Index test: CDUS. Image production:‐ Type of US: curved array multi‐frequency probe: Siemens ACUSON S2000 (Siemens Healthcare, Erlangen, Germany) system or a GE Logic E9 (GE Healthcare, Milwaukee, WI, USA). Use of contrast: yes, IV bolus of 1.0 mL of a second‐generation blood pool contrast agent (SonoVue, Bracco, Milan, Italy) consisting of stabilized microbubbles of sulphur hexafluoride, followed by a second bolus injection of 10 mL saline solution (0.9% NaCl). Operator: US examinations performed by experienced sonographer and were later read by 2 blinded unbiased investigators with > 5 years of clinical US experience in consensus. |
|
| Follow‐up | No loss to follow‐up, missing data, or adverse events. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants consecutively referred for follow‐up to detect potential endoleaks. |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | Unclear | No information provided. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of CTA reported for all. ("All patients who were sent to our department for further diagnostic work‐up and follow‐up after EVAR, and who did not match any of the exclusion criteria were included in the study. We screened a total of thirty‐five patients. Each patient was examined using all diagnostic ultrasound and CT tools of the study.") |
| Differential verification avoided? All tests | Yes | All participants who received US subjected to CT scan. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Unclear | Information not clearly reported. |
| Index test results blinded? All tests | Yes | Examiner initially blinded to CT results. US examinations performed by experienced sonographer and later read by 2 blinded unbiased investigators with > 5 years of clinical US experience in consensus. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Unclear | Unclear. |
| Withdrawals explained? All tests | Yes | No apparent withdrawal. |
Costa 2013.
| Clinical features and settings | 40 people within the scope of stents monitoring protocol and risk of endoleaks type II followed from November 2010 to February 2013. Type of stents received: fenestrated. Aneurysm diameter (mean ± SD): 55 ± 8 mm in anteroposterior diameter. Setting: vascular surgery department at the University Hospital of Lyon. |
|
| Participants | 39 men; age (mean ± SD): 75 ± 8 years. Comorbidities: smoking habits, hypertension, dyslipidaemia, diabetes, overweight. Geography: France. |
|
| Study design | Cross‐sectional study. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: "Endoleaks (the most common complication) are responsible for feeding the aneurysm sac and is a breaking risk factor. The type I endoleak is a leak at a site proximal or distal attachment, type II is a reflux from the collateral of the aorta, the type III is a defect in the wall of the stent, and Type IV is a result of a porosity of the prosthesis." Endoleak (absolute n): 19. Prevalence of endoleak: 31.7% (19/60). Reference standard: CT. Image acquisition:
Type of CT scanner: not reported. Use of contrast: 120 mL of unspecified contrast medium. Operator: CT performed and interpreted in radiology. |
|
| Index and comparator tests |
Index test: echo‐Doppler with and without contrast injection. Contrast agent used in people at risk of endoleak and had no contraindications to product. Image production: participants supine. Type of US: General Electric equipment with a convex abdominal probe (4C‐A) and Siemens Acuson with a convex abdominal probe (C5‐2). Use of contrast: "The contrast agent used (the only available in France) was the Sonovue Braco Milan. The dose used was that recommended: 2.4 mL bolus followed by washing with 5 ml of isotonic saline." Operator: echo‐Doppler performed and interpreted by trained vascular physicians. |
|
| Follow‐up | 3 participants excluded from study because they had contraindication for contrast medium injection (severe pulmonary hypertension, heart failure stage III). | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Not clearly stated if the participants were consecutively enrolled. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Yes | "The Doppler with and without ultrasound contrast agent injection was made within less than 24 hours and CT less than ten days before or after." |
| Partial verification avoided? All tests | Yes | All participants received both tests. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "Ultrasound and CT analyzes were performed independently and blindly (vascular doctor did not know the results of the CT scan, and the radiologist did not know the results of echo‐Doppler)." |
| Index test results blinded? All tests | Yes | "Ultrasound and CT analyzes were performed independently and blindly (vascular doctor did not know the results of the CT scan, and the radiologist did not know the results of echo‐Doppler)." |
| Relevant clinical information? All tests | Yes | Age, gender, risk factors, and comorbidities of the participants reported. |
| Uninterpretable results reported? All tests | Yes | No apparent uninterpretable data occurred. |
| Withdrawals explained? All tests | Yes | "Three patients were excluded from the study because they had against‐indication for the contrast medium injection (severe pulmonary hypertension, heart failure stage III). |
d'Audiffret 2001.
| Clinical features and settings | People with AAA who underwent endoluminal exclusion with commercially available endoprosthesis and had a minimum follow‐up of 6 months. Type of stents received: devices included Mintec system (n = 7), Vanguard system (n = 56), Endovascular Technology (n = 11), Aneuryx (n = 2), Talent (n = 1), and Stenford system (n = 12). Prosthesis configurations were aortic tube grafts (n = 3), aorto‐uni‐iliac (n = 5), and bifurcated grafts (n = 81). Aneurysm diameter: mean aneurysm diameter: 53.2 mm; range: 45‐80 mm on preoperative CT. Setting: department of vascular medicine. |
|
| Participants | 89 participants; mean (± SD) age: 70 ± 5 years; female: 6, male: 83; ASA classification 1: 2, 2: 37, 3: 40, 4: 10. Comorbidities: risk factors: ischaemic heart disease 50 (56.2%), previous myocardial infarction 17 (19.2%), obesity 28 (31.3%), smoking 43 (49%), hypertension 53 (59.4%), pulmonary disease 25 (29%), diabetes mellitus 8 (9%), renal impairment 10 (11%), and hyperlipidaemia 27 (30.3%). Geography: France. |
|
| Study design | Retrospective study with consecutively selected participants ("A total of 89 patients were followed up with serial CT and DUS at 1, 3, 6, 12, and 24 months after endoluminal treatment. Special attention was directed toward the presence of endoleaks and aneurysm diameter evolution.") | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: presence of contrast between graft and arterial wall of aneurysm. Endoleak (absolute n): 32. Prevalence of endoleak: 36.0% (32/89). Reference standard: helical CT Twin scanner. Image acquisition:
Type of CT scanner: helical Elscint CT Twin scanner (Picker Marconi, Chatenay‐Malabry, France). Use of contrast: yes, type of contrast not reported ("100 to 150 mL of nonionic contrast agent"). |
|
| Index and comparator tests |
Index test: CDUS. Image production: "The aorta from the renal to the distal iliac arteries was examined with B‐mode imaging. The largest anteroposterior and transverse diameters were measured and recorded. Colour flow sampling within the aneurysm sac, outside the endoprosthesis, was used to detect endoleaks. When flow was detected, a Doppler waveform analysis completed the investigation." Type of US: 3.5‐MHz probe Esaote which included Doppler flow velocity measurement, CDUS, power Doppler, and B‐mode US (AU 4; Biomedica, Genoa, Italy). Use of contrast: no. Operator: physicians certified in vascular medicine and US. |
|
| Follow‐up | No loss to follow‐up, missing data, or adverse events. | |
| Notes | Secondary objective included value of transverse diameter preoperatively and at each follow‐up examination and variations of diameter from preoperative to latest available examination. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Spectrum of participants was representative of participants who receive test. |
| Acceptable reference standard? All tests | Yes | CTs performed with helical Elscint CT Twin scanner. |
| Acceptable delay between tests? All tests | Yes | "Comparisons were performed when both examinations were done within a 1‐month interval." |
| Partial verification avoided? All tests | Yes | After device implantation, participants followed up with serial CT and DUS at 1, 3, 6, 12, 18, and 24 months, then yearly. Current analysis performed on first 89 participants who underwent endoluminal exclusion and had minimum follow‐up of 6 months. |
| Differential verification avoided? All tests | Yes | All participants who received US subjected to CT scan. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | No | "The physicians performing the DU [DUS] or the CT may have been aware of the results of the examination, which was done first." |
| Index test results blinded? All tests | No | "The physicians performing the DU or the CT may have been aware of the results of the examination, which was done first." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "One false positive leak was detected with duplex ultrasound. The patient had an arteriogram that did not confirm the endoleak. The most likely explanation was a flow artefact due to the high position of a contralateral graft limb into the main graft body." |
| Withdrawals explained? All tests | Unclear | No apparent withdrawal occurred. |
Demirpolat 2011.
| Clinical features and settings | People with endovascular repair of AAA. Type of stents received: nitinol‐based grafts. Aneurysm diameter: mean (± SD): 64 6 ± 18.4 mm; range: 37‐103 mm. Participant with smallest aneurysm diameter (37 mm) was treated because of an associated iliac artery aneurysm. Setting: department of radiology. |
|
| Participants | 29 participants; 26 males; 3 females; mean age: 72.2 years; range: 47‐90 years. Comorbidities: not reported. Geography: Turkey. |
|
| Study design | Longitudinal study (consecutively selected participants, all received both tests). | |
| Target condition and reference standard(s) |
Target condition: type I and II endoleaks. Definition of endoleak: persistence of flow in aneurysm lumen after procedure; persistent flow can lead to increase in diameter of aneurysm, with subsequent risk of rupture. Endoleak (absolute n): 9. Prevalence of endoleak: 35.4% (17/48). Reference standard: CT scan. Image acquisition:
Type of CT scanner: 16‐detector‐row CT scanner (Toshiba Medical Systems, Tochigi‐ken, Japan). Use of contrast: yes, type not specified ("100 ml of nonionic contrast material was injected through an antecubital vein with an automated injector at a rate of 3 ml/sec"). |
|
| Index and comparator tests |
Index test: CDUS. Image production: "The aneurysm and the stent graft were evaluated in axial and longitudinal planes with B mode and CDUS. The transverse and sagittal outer to outer diameter of the aneurysm at the site of largest diameter was measured in the axial plane perpendicular to the axis of the aorta. The patency of the stent graft and iliac arteries was assessed and perigraft flow was searched for with CDUS. The colour box size was adjusted to encompass the entire aneurysm sac." Type of US: 3 types of duplex scanner, sector or linear scan heads with varying frequencies (9 to 4‐MHz linear or 4 to 1‐MHz sector) (Siemens Sonoline Antares or Siemens Ellegra, Siemens, Erlangen, Germany; or ATL HDI 5000, Advanced Technology Laboratories, Bothell, WA, USA). Use of contrast: no. Operator: a radiologist experienced in DU. |
|
| Follow‐up | 1 participant dropped out because of violation of study protocol: 1 stroke occurred during the time interval between the 2 investigations. | |
| Notes | Data analysis at 48 months. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Spectrum of participants was representative of participants who will receive test. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Yes | CTA and CDUS examinations were performed same day. |
| Partial verification avoided? All tests | Yes | Whole sample received reference standard test. |
| Differential verification avoided? All tests | Yes | All participants who received US subjected to CT scan. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "All CTA and CDUS exams were interpreted independently by two radiologists blinded to the results of the other study." |
| Index test results blinded? All tests | Yes | "All CTA and CDUS exams were interpreted independently by two radiologists blinded to the results of the other study." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | No apparent uninterpretable results. |
| Withdrawals explained? All tests | Unclear | Insufficient information. |
França 2013.
| Clinical features and settings | 33 participants who had undergone elective endovascular treatment of AAAs. Type of stents received: not reported. Aneurysm diameter: maximum transverse diameters (mean ± SD): 54.5 ± 12.6 mm for CTA; 52.5 ± 13.1 mm for US. Setting: vascular ultrasonography units. |
|
| Participants | 30 men; mean age (± SD): 73 ± 6.0 years. Comorbidities: not reported. Geography: Brazil. |
|
| Study design | Prospective study; participants were consecutively enrolled. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: "The transmission of flow and pressure into the aneurysm sac." Endoleak (absolute n): 12. Prevalence of endoleak: 44% (22/50). Reference standard: multidetector spiral CTA. Image acquisition:
Type of CT scanner: Elscint Twin Flash/Dual Slice Helical, Toshiba Multislice Aquilion, Siemens 64‐channel, Somatom Definition AS+/Multislice 128 channels. Use of contrast: iodinated non‐ionic contrast. |
|
| Index and comparator tests |
Index test: CDUS. Image production: "The vascular ultrasound protocol required 40 minutes to complete and followed the recommendations of Sato et al." Type of US: Philips EnVisor and Philips HD‐11 (Bothell, WA, USA). Use of contrast: no. Operator: 3 experienced vascular ultrasonographists certified, 3 radiologists specialising in diagnostic imaging for vascular studies. |
|
| Follow‐up | No loss to follow‐up, missing data, or adverse events. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants consecutively enrolled. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | No | Maximum delay between the 2 examinations 90 days. |
| Partial verification avoided? All tests | Yes | All participants received both tests. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "Exam interpretation was blinded for test information, even in patients with more than one test pair." |
| Index test results blinded? All tests | Yes | "Exam interpretation was blinded for test information, even in patients with more than one test pair." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | No apparent uninterpretable data occurred. |
| Withdrawals explained? All tests | Yes | No explicit report concerning loss to follow‐up, missing data, or adverse events. |
Gargiulo 2014.
| Clinical features and settings | 22 consecutive participants who underwent fenestrated EVAR follow‐up. Type of stents received: fenestrations were joined to native visceral vessels with a balloon‐expandable covered stent‐graft (Advanta V12, Atrium Medical, Hudson, NH, USA). Aneurysm diameter: mean (± SD) preoperative AAA diameter: 55 ± 7 mm; range: 48‐71 mm. Setting: ultrasound unit. |
|
| Participants | 21 men; mean (± SD) age: 74 ± 7 years; range: 54‐80 years. Comorbidities: all ASA ≥ III (ASA III/IV: 82%/18%). 5 (23%) participants had BMI ≥ 30 kg/m2. Geography: Italy. |
|
| Study design | Cross‐sectional study. Participants consecutively enrolled. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: "Endoleaks were detected and classified according to the White and May classification” (White GH, Yu W, May J, Chaufour X, Stephen MS. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. Journal of Endovascular Surgery 1997;4(2):152e68). Endoleak (absolute n): 3. Prevalence of endoleak: 14% (3/22). Reference standard: CTA. Image acquisition:
Type of CT scanner: 64‐slice CT scanner (GE Healthcare, Milwaukee, WI, USA). Use of contrast: "Iodinate contrast (100‐130 mL Iomeron 400; Bracco, Milan, Italy) was injected at 4 mL/second for the first 100 mL and 2 mL/second for the last 30 mL. Contrast injection was followed by saline solution (0.9% NaCl) at a rate of 2 mL/second." Operator: "CTA was performed by a radiologist with experience in vascular CTA evaluations (MD)." |
|
| Index and comparator tests |
Index test: 4D CE‐CDUS. Image production: "The US examination started with B‐mode evaluation of the aorta by live x‐plane imaging where the maximal aneurysm diameter and the stent‐graft were evaluated. The abdominal aorta was scanned from the diaphragm to the iliac arteries and the entire sac was analysed to detect possible colour flow within the aneurysm sac. Then, the blood flow in the visceral and renal arteries was analysed in colour flow and pulse‐wave modes." Type of US: iU22 system, software Q‐Lab (Philips Medical Systems, Bothell, WA, USA). Fully sampled matrix array with frequency 6.0‐1.0 MHz (x6‐1; Philips Medical Systems, Bothell, WA, USA). Use of contrast: "A sulfur hexafluoride‐filled microbubble contrast agent (SonoVue; BR1, Bracco)." Operator: 1 "one investigator (CS) who had more than 10 years of experience in contrast ultrasound and who was blinded to the CTA." |
|
| Follow‐up | No loss to follow‐up, missing data, or adverse events. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants consecutively enrolled. |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | Yes | "The interval between the two examinations was always ≤ 30 days." |
| Partial verification avoided? All tests | Yes | All participants received both tests. |
| Differential verification avoided? All tests | Yes | All participants who received index test were subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Unclear | Not clearly stated if CTA scans were performed before US scans or if reader of CTA scans was blinded to results of the other test. |
| Index test results blinded? All tests | Yes | "All US scanning was performed by one investigator […] who was blinded to the CTA." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | No apparent uninterpretable data occurred. |
| Withdrawals explained? All tests | Yes | No explicit report concerning loss to follow‐up, missing data, or adverse events. |
Giannoni 2003.
| Clinical features and settings | Participants scheduled to undergo endovascular repair of aortoiliac aneurysms. Type of stents received: not reported. Aneurysm diameter: not reported. Setting: department of vascular surgery. |
|
| Participants | 30 consecutive participants; 29 men; mean age: 69 years; range: 50‐82 years. Comorbidities: not reported. Geography: Italy. |
|
| Study design | Cross‐sectional study. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: endoleaks detected classified according to location of flow: type I at proximal or distal attachment sites, type II from patent lumbar or inferior mesenteric arteries, and type III at junction between graft and modular device extension. Endoleak (absolute n): 8. Prevalence of endoleak: 9.9% (8/81). Reference standard: CTA. Image acquisition:
Type of CT scanner: Somatom Plus‐S scanner (Siemens Medical Systems, Munich, Germany). Use of contrast: yes, used non‐ionic contrast agent (Omnipaque 300, Nycomed‐Amersham, Princeton, NJ, USA). |
|
| Index and comparator tests |
Index test: CDUS. unenhanced US imaging; enhanced US imaging. Image production: "The aorta was examined with transverse and longitudinal B‐mode imaging from the renal to the distal iliac arteries; the maximal external diameter of the aneurysm sac in any direction was measured…To assess proper stent‐graft placement, the distance between the graft and the renal arteries and the diameter of the aortic neck were measured; colour flow sampling inside and outside the stent‐graft was used to verify graft patency and to detect endoleaks. A Doppler waveform analysis completed the investigation." Type of US: B‐mode, 3.5‐MHz probe (Acuson 128 XP 10; Acuson, Mountain View, CA, USA). Use of contrast: US enhancer was galactose‐based microbubble suspension 300 mg/mL (Levovist SHU508A, Schering AG, Germany). Operator: not reported. |
|
| Follow‐up | "On day 1 after the endovascular repair, unenhanced and enhanced ultrasound scans were performed. At 3 and 12 months and annually thereafter, aortic endograft surveillance included ultrasound (unenhanced and enhanced) imaging, CTA or MRA comparable to the preoperative study, and plain biplanar abdominal radiography, all performed within a 7‐day period." | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Consecutive series of participants referred for follow‐up to detect potential endoleaks. |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | Yes | All performed within 7‐day period. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | 2 experienced radiologists blinded to results of the US jointly assessed CTAs and MRAs. |
| Index test results blinded? All tests | Yes | Index test performed before reference standard. |
| Relevant clinical information? All tests | Yes | Clinical data available at time of test interpretation. |
| Uninterpretable results reported? All tests | Yes | All data were interpretable. |
| Withdrawals explained? All tests | Yes | "Endovascular AAA repair was technically successful in 27 (90%) patients. Two (6.7%) patients died in the periprocedural period: one after conversion to an open repair and the other after an additional intervention. Of the remaining 28 patients, 26 (93%) reached the 24‐month follow‐up (mean 30 months, range 6‐60). One patient died 6 months after the endovascular procedure of thoracic aortic dissection; a broken femur immobilized the other patient after he had completed the 12‐month evaluation. Complications during follow‐up included 2 (6.6%) limb occlusions at 6 and 12 months, 2 (6.6%) extension detachments at 6 and 24 months, 1 (3.3%) proximal detachment due to neck dilatation at 24 months, and 1 (3.3%) kinked stent‐graft that displayed wire breakage, necessitating late conversion to open repair. Other than the wire breakage, which was identified only at radiography, all major complications were detected by unenhanced ultrasound and by CTA/MRA." |
Giannoni 2007.
| Clinical features and settings | Participants who received endovascular grafts for infrarenal aortic aneurysms; 13 aortic and 17 aortoiliac aneurysms, all previously treated in Department of Vascular Surgery. Type of stents received: 24 participants received Excluder (WL Gore & Associates, Flagstaff, AZ, USA); 3 received Vanguard (Boston Scientific, Natik, MA, USA); and 3 received Talent (Medtronic, Minneapolis, MN, USA). Aneurysm diameter: mean (±SD) transverse diameter: 53.19 ± 15.69 mm on contrast CT‐scan. Setting: division of vascular surgery. |
|
| Participants | 30 consecutive people; mean (± SD) age: 74.4 ± 5.4 years; range: 65‐84 years; BMI 22‐38 kg/m2. Comorbidities: not reported. Geography: Italy. |
|
| Study design | Cross‐sectional study; consecutively selected participants; all received both tests. | |
| Target condition and reference standard(s) |
Target condition: type II endoleaks. Definition of endoleak: persisting flow from patent lumbar or mesenteric arteries within aneurysm sac and outside endograft. Endoleak (absolute n): 7. Prevalence of endoleak: 24.1% (7/29). Reference standard: contrast CT scan performed with delayed triphasic sequences. No further information provided. Image acquisition: not reported. Type of CT scanner: Somatom Sensation Cardiac 64 (Siemens, Munich, Germany). Use of contrast: not reported. |
|
| Index and comparator tests |
Index test: CDUS. Image production: not reported. Type of US: convex probe (3‐4 MHz), equipped for Cadence CPS software (Sequoia Acuson Siemens, Mountain View, CA, USA). Use of contrast: echo‐contrast solution (Sono Vue, Bracco, Italy) injected in bolus by hand into antecubital vein and immediately followed by 10 mL of saline solution. Operator:> 1 ("The US examinations were performed by vascular doctors dedicated to US imaging (MD), blinded to the results of CT angiography.") |
|
| Follow‐up | "One patient dropped out because of violation of the study protocol: a stroke occurred in the time interval between the two investigations." | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Yes | "No more than 15 days elapsed between the two examinations." |
| Partial verification avoided? All tests | Yes | All participants received both tests except 1 who dropped‐out due to occurrence of stroke. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | US examinations performed by vascular doctors dedicated to US imaging, blinded to results of CTA. |
| Index test results blinded? All tests | Yes | US examinations performed by vascular doctors dedicated to US imaging, blinded to results of CTA. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "In one patient in which both investigations detected the increase of the diameter of aneurysm sac, CPS US (Video 1) (Fig. 2) demonstrated the type II endoleak not confirmed to CT‐scan (Fig. 3). The angiography disclosed a low flow type II endoleak from a lumbar artery." |
| Withdrawals explained? All tests | Yes | "One patient dropped out because of violation of the study protocol: a stroke occurred in the time interval between the two investigations." |
Golzarian 2002.
| Clinical features and settings | People who underwent transfemoral insertion of stent grafts (endoluminal graft; Corvita Europe, Brussels, Belgium) for AAA. In 21 participants, aneurysm was aortoiliac; remaining 34 participants had aortic aneurysms. Type of stents received: not reported. Aneurysm diameter: range 5.1‐7.8 cm. Setting: department of radiology. |
|
| Participants | 55 participants; 51 men; mean age: 73 years; range: 61‐87 years. Comorbidities: no comorbidities reported. Geography: Belgium. |
|
| Study design | Prospectively and consecutively enrolled participants ("All patients prospectively underwent colour Doppler sonography and biphasic helical CT within 7 days after stent‐graft implantation"). | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: Type I endoleak: direct flow into the aneurysmal sac related to the incomplete sealing of the stent‐graft to the aortic wall. Type II endoleak: retrograde filling of the aneurysm mainly from the lumbar arteries and the inferior mesenteric artery". Mentioned but not defined: transgraft endoleak, graft‐fabric degradation, and graft‐junction separation. Endoleak (absolute n): 22. Prevalence of endoleak: 41.5% (22/53). Reference standard: CT scan. Image acquisition:
Type of CT scanner: Somatom Plus S or a 4A scanner (Siemens Medical Systems, Erlangen, Germany). Use of contrast: 15 mL contrast medium or a bolus tracking system with threshold of 100 H used to determine optimal start delay. Participants received 80‐120 mL contrast medium at rate of 3.5 mL/s. Operators: "All helical CT examinations were performed by two experienced radiologists." |
|
| Index and comparator tests |
Index test: CDUS. Image production: "The aorta was first scanned transversally from the top of the stent‐graft to the femoral arteries, and the maximal transversal diameter was measured. Colour Doppler imaging was then performed in both the transverse and longitudinal axes." A leak considered present when signal associated with spectral Doppler signal observed outside aorta. In case of a perigraft leak, attempted to identify origin and direction of flow. Type of US: 2.5‐ and 3.75‐MHz curved array transducer SSH‐140A (Toshiba, Antwerp, Belgium). Use of contrast: no. Operator: 2 experienced operators (1 angiologist and 1 radiologist). |
|
| Follow‐up | 1 participant lost to follow‐up. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Yes | "All patients prospectively underwent colour Doppler sonography and biphasic helical CT within 7 days after stent‐graft implantation. The maximum time interval between helical CT and colour Doppler sonography was 48 hr (mean, 11.5 hr); however, 33 patients had both examinations on the same day. In all patients, colour Doppler sonography was performed before helical CT." |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Unclear | "All patients prospectively underwent colour Doppler sonography and biphasic helical CT within 7 days after stent‐graft implantation. The maximum time interval between helical CT and colour Doppler sonography was 48 hr (mean, 11.5 hr); however, 33 patients had both examinations on the same day. In all patients, colour Doppler sonography was performed before helical CT." |
| Index test results blinded? All tests | Yes | "In all patients, colour Doppler sonography was performed before helical CT." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "All helical CT scans were considered to be of good quality. Two colour Doppler sonographic examinations (3.6%) were considered to be uninterpretable because of patient obesity or intestinal gas. Six colour Doppler sonograms (10.9%) were evaluated as suboptimal because of excessive artifact caused by obesity, intestinal gas, or inappropriate gain (colour artifact completely filling the colour box)." |
| Withdrawals explained? All tests | Yes | No withdrawal occurred. |
Gray 2012.
| Clinical features and settings | People who underwent EVAR at the Mater Hospital from 1 June 2003 to 1 July 2010 retrospectively reviewed. Type of stents received: not reported. Aneurysm diameter: not reported. Setting: department of vascular surgery. |
|
| Participants | 145 participants; 122 (84.1%) male; mean (± SD) age: 77.1 ± 7.9 years. Comorbidities: not reported. Geography: Ireland. |
|
| Study design | Retrospective design. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: type I, evidence of high jet flow; type II, endoleak or low velocity flow within the old aneurysm sac demonstrating forward and reversed flow. Endoleak (absolute n): 25. Prevalence of endoleak: 5.4% (25/459). Reference standard: CT scan. Image acquisition: not reported.
Type of CT scanner: Somatom Definition AS 128‐slice scanner (Siemens AG, Erlangen, Germany). Use of contrast: not reported. |
|
| Index and comparator tests |
Index test: CDUS. Image production: all CDUS began with visualisation of aorta immediately inferior to diaphragm. Residual aneurysm imaged in B‐mode in both transverse and longitudinal planes from diaphragm to iliac bifurcation. Multiple measurements obtained of residual aneurysm sac in transverse plane. Maximum measurements of residual aneurysm sac recorded and compared to last scan report to ensure there was no significant increase in sac size. Careful note made in B‐mode of stent walls to ensure there was no evidence of obvious defects or kinking of metal exoskeleton. Iliac arteries imaged in B‐mode throughout entire length. Multiple transverse and anteroposterior measurements obtained and maximum of the 2 measurements recorded for follow‐up purposes. Type of US: 6‐mHz curvilinear broadband transducer: Sequoia 512 Ultrasound system and later in study an S200 Ultrasound system (Siemens AG, Erlangen, Germany). Use of contrast: no. Operator: 1 accredited vascular technologist. |
|
| Follow‐up | CDUS and CT scans of 31 (21.4%) participants not compared due to inconsistent timing of imaging modalities (scans performed > 90 days apart excluded), failure to attend and CT being contraindicated due to IV contrast allergy. | |
| Notes | After discharge, all participants CDUS scan at 1 month and then CDUS scan and CT scan at 6 months, 12 months, and annually thereafter provided there was no documented endoleak on either CDUS or CT. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Unclear | Number of participants with delays between the 2 test > 28 days unclear. However, study reported "scans performed greater than 90 days apart were excluded." |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | In all cases, technologist was blind to CT results. |
| Index test results blinded? All tests | Unclear | No clear information provided. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | Of the 2 participants who had type I endoleak on CDUS and not on CT, 1 was anatomical abnormality and misinterpreted on the CDUS scan. The second participant was documented as type II endoleak on CT. 4/5 participants who had type I endoleak detected on CDUS underwent further intervention. |
| Withdrawals explained? All tests | Yes | "The CDUS and CT scans of the remaining 31 (21.4%) patients were not compared due to inconsistent timing of imaging modalities (scans performed greater than 90 days apart were excluded), failure to attend and CT being contra‐indicated due to i.v. contrast allergy. Of the 426 CDUS scans carried out 26 (6.1%) scans were reported as limited, due to the presence of excess bowel gas and body habitus curtailing the determination of residual sac size and endoleak detection. The maximum residual aneurysm size was documented on the remaining 400 (93.9%) CDUS scans. Of the 289 CT’s performed 107 (37%) did not have the maximum residual aneurysm sac size documented in the report. The maximum residual aneurysm size was documented on the remaining 182 (63%) of CT scan reports." |
Gurtler 2013.
| Clinical features and settings | 171 people after EVAR who received 489 CE‐CDUS and 421 MS‐CT examinations during follow‐up. 39 participants withdrawn because of time mismatch between imaging studies. 200 contemporary examination pairs ± 30 days from 132 participants of the 489 CE‐CDUS and 421 MS‐CT examinations matched. Type of stents received: bi‐iliac or mono‐iliac stent graft. Aneurysm diameter: not reported. Setting: department for clinical radiology. |
|
| Participants | 151 men; mean (± SD) age: 70.4 ± 8.6 years; range: 34‐91 years. Comorbidities: not reported. Geography: Germany. |
|
| Study design | Cross‐sectional study. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: "An endoleak was defined as an extravasation of contrast between the aneurysm wall and the prosthesis." Endoleak (absolute n): 87. Prevalence of endoleak: 43.5% (87/200). Reference standard: MSCT. Image acquisition:
Type of CT scanner: Somaton Sensation 16‐, 64‐, or 128‐slice detector MS‐CT scanner (Siemens Medical Systems, Forchheim, Germany). Use of contrast: "A total of 100 to 120 mL Imeron (Bracco) with an iodine concentration of 350 mg/mL was administered, followed by 50 mL saline (0.9% NaCl)." Operator: 2 experienced radiologists. |
|
| Index and comparator tests |
Index test: CE‐CDUS. Image production: transverse and sagittal imaging. Type of US: ACUSON Sequoia 512 and a ACUSON S2000 (Siemens Healthcare, Erlangen, Germany) using a curved‐array 4‐MHz multi‐frequency transducer. Use of contrast: "an intravenous bolus injection of 1.0 mL SonoVue, a second‐generation blood pool contrast agent, consisting of stabilized microbubbles of sulfur hexafluoride, was administered into an antecubital vein through an 18‐gauge needle and was followed by a flush of 10 mL saline solution (0.9% NaCl)." Operator: 1 experienced sonographer. |
|
| Follow‐up | 39 participants withdrawn because of time mismatch between imaging studies. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Not clearly stated if the participants were consecutively enrolled. |
| Acceptable reference standard? All tests | Yes | Reference standard was MS‐CT scan. |
| Acceptable delay between tests? All tests | Yes | "We compared examinations that were performed on the same day or ≤30 days." |
| Partial verification avoided? All tests | Yes | All participants received both tests. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "Radiologists reading one test did not have access to the results of the other test." |
| Index test results blinded? All tests | Yes | "Radiologists reading one test did not have access to the results of the other test." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | No apparent uninterpretable data occurred. |
| Withdrawals explained? All tests | Yes | "39 patients were withdrawn because of time mismatch between imaging studies." |
Heilberger 1997.
| Clinical features and settings | People with aortic aneurysm who received EVAR. Indications for stent‐graft placement were symptomatic thoracic aortic aneurysm in 2 participants, suprarenal AAA in 1 participant, and infrarenal AAAs in remaining 110 participants. Types of endografts used: 9 tube and 34 bifurcate stent‐grafts; Chuter device, Stentor (MinTec, Freeport, Grand Bahama), and EGS aortic endograft (Endovascular Technologies, Menlo Park, CA, USA). Aneurysm diameter: mean: 45.5 mm; range: 32‐72 mm. Setting: department of vascular surgery, Klinikum Nurnberg. |
|
| Participants | 113 participants; 108 men; mean age: 67.3 years; range: 40‐83 years. Comorbidities: not reported. Geography: Germany. |
|
| Study design | Not clear description. Apparently participants were retrospectively identified. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: persistent blood flow within the aneurysm sac. Primary endoleaks defined as those noted during or immediately after procedure, whereas secondary leaks were detected at follow‐up examinations. Endoleaks that were disclosed only with contrast‐assisted contrast duplex sonography were deemed minor leaks; major endoleaks were those whose flow was detected by routine CDUS. Endoleak (absolute n): 28. Prevalence of endoleak: 24.8% (28/113). Reference standard: helical CT. Image acquisition:
Type of CT scanner: Somatom Plus S or a 4A scanner (Siemens Medical Systems, Erlangen, Germany). Use of contrast: "Either a bolus test injection of 15 mL of contrast medium or a bolus tracking system with a threshold of 100 H was used to determine the optimal start delay (unenhanced CT scans not obtained)." |
|
| Index and comparator tests |
Index test: CDUS. Image production: 4‐6 standard transverse images of aneurysm sac and stent graft to define their positions. Bifurcated stent‐grafts also examined using pulse Doppler frequency analysis to evaluate flow characteristics in both stent‐graft limbs. Type of US: unclear/not reported. Use of contrast: yes, Levovist: 99.9% of D‐galactose and 0.1% of palmitic acid. Operator: unclear. |
|
| Follow‐up | Excluded from follow‐up were:
Mean follow‐up time: 7.2 months; range: 1‐24 months. |
|
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Unclear | CT scan test with contrast agents performed, but not reported that images evaluated by a radiologist. |
| Acceptable delay between tests? All tests | Yes | "Computed tomography angiography was performed on the same day." |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Unclear | Unclear. |
| Index test results blinded? All tests | Unclear | Unclear. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "Among 5 endoleaks due to retrograde side‐branch perfusion, 3 were detected only with contrast‐enhanced duplex scanning; iliac artery occlusion was also documented using duplex, however, 2 stent fractures could not be seen with ultrasound…" "One endoleak originating from the distal iliac limb anchoring site was missed by duplex owing to bowel gas." "Two patients with retrograde aneurysm perfusion via the lumbar arteries remain under observation. In one, a minor leak was documented by duplex with Levovist only; it was not seen on CT scans, even with fractionated injection of contrast agent." |
| Withdrawals explained? All tests | Unclear | Excluded from follow‐up were 11 participants (9.7%) who required conversion to open surgical repair either during or shortly after endovascular procedure. Included in this number was 1 participant who died from haemorrhagic shock secondary to a retroperitoneal haematoma at the femoral puncture site. 5 participants died during follow‐up of causes unrelated to the procedure: 1 prostate cancer, 1 bronchial carcinoma, and 3 cardiopulmonary disease. |
Henao 2006.
| Clinical features and settings | People who underwent endovascular treatment for an infrarenal AAA. Type of stents received: not reported. Aneurysm diameter: 5.3 cm. Setting: unclear. |
|
| Participants | 20 men; mean age: 70.4 years; Mean height of group: 179 cm; range: 162‐200 cm; mean weight: 91 kg; range: 61‐137 kg; mean BMI: 28.2 kg/m2; mean aneurysm size: 5.27 cm at time of follow‐up. Comorbidities: not reported. Geography: USA. |
|
| Study design | Prospective study that included only men. "A prospective study, approved by the Institutional Review Board of Baylor College of Medicine, was conducted to evaluate the effectiveness of CE‐CDUS imaging to detect endoleaks in patients who underwent endovascular treatment for an infrarenal abdominal aortic aneurysm. Patients are typically followed after a successful endovascular aneurysm repair at 1, 6, 12, and 24 months, and annually thereafter. All men and postmenopausal women seen at these follow‐up intervals were asked to participate unless there was a documented contraindication to the use of ultrasound contrast, blood products, or albumin." |
|
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: presence of persistent intrasac flow outside stent‐graft. Endoleaks characterized in relation to endograft, aneurysm wall, and aortic side branches, and recorded in accordance to the White‐May classification. Endoleak (absolute n): 6. Prevalence of endoleak: 30% (6/20). Reference standard: CTA. Image acquisition: unclear.
Type of CT scanner: Lightspeed Ultra (GE Healthcare, Waukesha, WI, USA). Use of contrast: yes (type unknown; injection of 150 mL of contrast agent at rate of 2.5 mL/s). |
|
| Index and comparator tests |
Index test: CDUS. Image production: infrarenal aorta and native aneurysm sac scanned after Optison injection in longitudinal and transverse perspective from renal to distal iliac arteries. Flow evaluated within lumen of graft and its components, as well as the presence or absence of endoleaks. Type of US: 3.5‐MHz probe on a Phillips iU22 unit (Phillips Medical Systems, Bothell, WA, USA). Use of contrast: yes, Optison (Perflutren Protein Type A Microspheres for Injection, Amersham Health, Princeton, NJ, USA). Operator: 4 experienced vascular sonographers. |
|
| Follow‐up | No missing data at follow‐up. No adverse events secondary to CE‐CDUS. |
|
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Unclear | CT scan with contrast agency but not declared that images evaluated by a radiologist. |
| Acceptable delay between tests? All tests | Yes | CTA performed on same day before CE‐CDUS. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | 20 men underwent surveillance utilizing both CTA and contrast‐enhanced CDI. |
| Incorporation avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Reference standard results blinded? All tests | Yes | CTA performed on same day before CE‐CDUS. |
| Index test results blinded? All tests | Yes | Ultrasonographers blinded to results of previous angiographic or CTA results. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | No uninterpretable results occurred. "Colour Duplex ultrasound scans identified four (44%) endoleaks, including the type I endoleak. Six (67%) endoleaks were also identified with CTA. Three type II endoleaks found on CE‐CDUS were not confirmed on CTA (Fig 3). No endoleaks were seen on CTA that had not been found on CE‐CDUS." |
| Withdrawals explained? All tests | Yes | No withdrawals occurred. |
Iezzi 2009.
| Clinical features and settings | People who underwent endovascular repair of an unruptured infrarenal AAA. Type of stents received: 81 aorto‐bi‐iliac stent grafts, consisting of 43 Talent (Medtronic AVE), 28 Excluder (WL Gore & Associates), 8 Zenith (Cook), 1 Vanguard, and 1 AneuRx (Medtronic AVE); and 3 aortomonoiliac stent grafts (Talent, Medtronic, AVE). Aneurysm diameter: 5.3 cm. Setting: department of radiology. |
|
| Participants | 84 consecutive participants; 69 men; mean (± SD) age: 79.6 ± 5.2 years; range: 62‐89 years; mean (± SD) BMI: 27.4 ± 3.5 kg/m2; range: 22‐34.2 kg/m2. Comorbidities: not reported. Geography: Italy. |
|
| Study design | Prospective single centre cross‐sectional study. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: persistent perigraft flow within aneurysmal sac excluded by stent graft. Endoleaks classified according to size and aetiology. Endoleak (absolute n): 40. Prevalence of endoleak: 47.6% (40/84). Reference standard: multidetector row helical CT scanner. Image acquisition:
Type of CT scanner: Somatom Plus 4 Volume Zoom (Siemens, Forchheim, Germany). Use of contrast: 120 mL iodinated non‐ionic contrast medium (Iomeprol 300 mg/mL, Iomeron; Bracco). |
|
| Index and comparator tests |
Index test: CDUS. Image production: axial and longitudinal and acquisition scans used for US imaging. CEUS scans performed after administration of bolus of 2 different doses of contrast agent dissolved in 0.9% saline solution (1.2 mL and 2.4 mL), each followed by flushing with 5 mL bolus of saline solution through an 18‐ to 20‐gauge cannula placed in arm vein. A minimum interval of 10 minutes and complete bubble destruction, which was achieved by scanning entire abdominal aorta at a high mechanical index, required between the 2 injections to avoid carryover effects. Scanning started at beginning of contrast agent injection and sweep was usually completed within 5 minutes. Phases of CE‐CDUS defined as arterial (10‐40 s after contrast agent injection) and late (90‐300 s after injection). Type of US: convex multi‐frequency 5 to 2 MHz probe, Philips HDI 5000 scanner (Philips Medical Systems, Bothell, WA, USA). Use of contrast: second‐generation contrast agent (SonoVue, Bracco, Milan, Italy) made of sulphur hexafluoride‐filled microbubbles with flexible shells that allow real‐time imaging at low acoustic pressure (mechanical index range: 0.12‐0.14). Operator: 2. |
|
| Follow‐up | "All patients completed the protocol, and no adverse events were recorded during CEUS or multidetector CT examinations." | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients that after receiving EVAR are exposed to endoleak surveillance. "The study enrolled all patients treated with EVAR who underwent CTA as part of a routine surveillance programme at 1, 6, and 12 months after the procedure and annually thereafter. They underwent CTA and CDUS and CEUS imaging on the same day. To avoid selection bias in favor of patients who were 'easy to scan', patients were recruited before undergoing a baseline US scan. No patient was excluded on the basis of poor technical quality of the baseline US study." |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | Yes | Index test and reference standard performed on same day. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "US examinations were randomly reviewed independently by two radiologists not involved in the imaging, one radiologist specialized in vascular radiology (D. P. with 10 years of experience) and the other in CEUS (R. B. with 15 years of experience), and neither was aware of the CTA outcomes or dose of contrast used for CEUS." |
| Index test results blinded? All tests | Yes | "The radiologist was blinded to all other imaging findings at the time of examinations." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "None of the CTAs resulted in an uncertain diagnosis (score 2)". "Endoleaks classification: two large endoleaks were not clearly classified by CTA (differential diagnosis between type II and type III endoleak). These two patients underwent selective conventional angiography that detected two type II endoleaks due to retrograde flow into the aneurysm sac through lumbar arteries. Five small type II endoleaks were detected only on delayed phase and were classified as low‐flow leaks." |
| Withdrawals explained? All tests | Yes | "All patients completed the protocol, and no adverse events were recorded during CEUS or multidetector CT examinations." |
McLafferty 2002.
| Clinical features and settings | People with AAAs who received stent graft. Type of stents received: AneuRx graft (Medtronics AVE, Sunnyvale, CA, USA). Aneurysm diameter: not reported. Setting: vascular laboratory (Intersocietal Commission for the Accreditation of Vascular Laboratories) at Memorial Medical Center (Springfield, IL, USA). |
|
| Participants | No further description about basic characteristics of participants provided. Comorbidities: not reported. Geography: USA. Period of recruitment: June 1997 to July 1999. |
|
| Study design | Prospective design. Whole sample included. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: "According to protocol, endoleaks were classified as arising from proximal, distal, or junctional graft attachment sites (types I and III), from branch vessel flow (Type II), or from undetermined source." Endoleak (absolute n): 7. Prevalence of endoleak: 9.2% (7/20). Reference standard: helical CT scan. Image acquisition:
Type of CT scanner: unclear. Use of contrast: yes (type not reported). |
|
| Index and comparator tests |
Index test: CDUS. Image production: "Imaging of the aorta was performed from the coeliac artery to the iliac bifurcation in the longitudinal and transverse axes. The iliac and common femoral arteries were scanned in a similar fashion. Transverse measurements, relative to the vessel, were made just proximal to the coeliac artery, at the level of the renal arteries, at the maximal aneurysm diameter, and just proximal to the iliac bifurcation. Measurement of the proximal, middle, and distal common and external iliac arteries in the transverse axis was performed. Similarly, Doppler scan waveforms and velocity measurements were obtained proximally, within, and distal to the endograft. Colour‐flow mode was used to help identify endoleaks with further focus on determining the origin." Type of US: "Low frequency transducers ranging from 2.0 to 3.5 MHz were used with either the Quantum 2000 scanner (Quantum Medical Systems, Issaquah, Wash) or the Philips P800 scanner (Philips, North American Corp, Itasca, Ill)." Use of contrast: no. Operator: registered vascular technologists. |
|
| Follow‐up | No missing data or adverse events. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represent average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | Unclear | Not reported. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Unclear | Not reported. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "Three patients (3.6%) had to have CT scan at the 1‐month follow‐up examination because the CFD scan could not be performed as a result of the presence of large body habitus or bowel gas. These patients had negative results for endoleak with CT scan at 1 month of follow‐up study." |
| Withdrawals explained? All tests | Yes | "Five patients who had negative results for endoleak at 1 month with CFD scan did not have CT scan at 6 months. One patient died of congestive heart failure before 6 months, one patient was unable to return, and three patients had lapses in scheduling. These remaining four patients had negative results for endoleak at 12 months. Four patients who had positive results for endoleak with CFD scan at 1 month did not have a CT scan at 3 months because of scheduling problems. All of these patients still had positive results for endoleak at 6 months with CT scan." |
McWilliams 1999.
| Clinical features and settings | People who received graft stents for AAA. Type of stents received: 14 participants had Vanguard devices (Boston Scientific Vascular, Natick, MA, USA), 1 participant a Stentor graft (Mintec, Freeport, Grand Bahama), and 3 participants an Aneurx graft (Medtronic, Eden Prairie, MN, USA). All grafts were modular bifurcated devices. Aneurysm diameter: not reported. Setting: department of radiology. |
|
| Participants | 18 participants. Comorbidities: not reported. Geography: UK. Recruitment period: May 1998 to October 1998. |
|
| Study design | Prospective design study ("From May 1998 to October 1998, patients who presented for follow‐ up scans after endovascular aortic aneurysm repair were invited to participate in the study. Eighteen patients were examined on 20 occasions. Levovist (Schering Health Care, Surrey, United Kingdom) is contraindicated in patients with galactosemia, and if there is a known or suspected right‐to‐left cardiac shunt. No patients were excluded because of such contraindications.") | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: persistence of blood flow outside lumen of endoluminal graft but within aneurysmal sac or adjacent vascular segment being treated with graft. Endoleak (absolute n): 3. Prevalence of endoleak: 15.0% (3/11). Reference standard: spiral CT. Image acquisition:
Type of CT scanner: spiral CT (HiSpeed Advantage; IGE Medical Systems, Slough, UK). Use of contrast:yes (type not reported). |
|
| Index and comparator tests |
Index test: CDUS. Image production: not reported. Type of US: 3.5‐MHz probe Diasonics Spectra machine (Sonotron Ltd, Bedford, UK). Use of contrast: yes, single dose of 300 mg/mL Levovist (Schering Health Care, Felbridge, UK). Operator: 1 vascular sonographer. |
|
| Follow‐up | Unclear whether missing data or an adverse event occurred. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients that after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard CTA. |
| Acceptable delay between tests? All tests | Yes | CT scan performed on same day. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | US performed before CT scan. |
| Index test results blinded? All tests | Yes | "The CT was reported as showing endoleak or no endoleak by a radiologist (D.A.G.) who was blinded to the results of ultrasound." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | Diagnostic confidence of scan was increased in 10 participants after Levovist injection by mean value of 15%. There was considerable difficulty in 1 participant in gaining venous access to inject Levovist after the unenhanced scan. The enhanced scan was much poorer because of bowel gas that was believed to be the result of participant swallowing air. |
| Withdrawals explained? All tests | Yes | No apparent withdrawal. |
McWilliams 2002.
| Clinical features and settings | People who received endovascular repair of an unruptured infrarenal AAA. Type of stents received: not reported (endografts were all bifurcated with either a modular or 1‐piece design except for 1 aortic tube device). Aneurysm diameter: not reported. Setting: department of radiology. |
|
| Participants | 53 participants; 44 men; mean age 70 years; mean height of group: 171 cm; range: 150‐183 cm); mean weight: 77 kg; range: 47‐107 kg). Comorbidities: not reported. Geography: UK. |
|
| Study design | Prospectively enrolled participants. "All patients seen at these follow‐up intervals were asked to participate unless there was a documented contraindication to the use of Levovist (e.g., galactosemia and a known or suspected right‐to‐left cardiac shunt)." |
|
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: presence of intrasac flow outside stent‐graft; characterized by its relationship to endograft, aneurysm wall, and aortic side branches and categorized using White/May classification. Endoleak (absolute n): 7. Prevalence of endoleak: 9.2% (7/20). Reference standard: contrast‐enhanced biphasic (arterial and delayed) CT. Image acquisition: not reported.
Type of CT scanner: HiSpeed Advantage (IGE Medical Systems, Slough, UK). Use of contrast: yes (type not reported). |
|
| Index and comparator tests |
Index test: CDUS. Image production: not reported. Type of US: 3.5‐MHz probe on either a Dyna‐View SSD‐1700 or a ProSound 5500 (Aloka Co Ltd, Tokyo, Japan). Use of contrast: yes, Levovist (Schering Health Care, Felbridge, UK). Operator: 1 experienced vascular sonographer. |
|
| Follow‐up | 2 participants excluded because radiology staff failed to follow protocol during 5 imaging sessions. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represents average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was biphasic enhanced CT. |
| Acceptable delay between tests? All tests | Yes | "Biphasic enhanced CT was performed on the same day using the same protocol and imager." |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "Either of two radiologists (R.M., D.A.G.), who were blinded to the ultrasound results, recorded all the CT studies." |
| Index test results blinded? All tests | Yes | US scan apparently performed before CT scan. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "Angiography in the patient with a 'definite' endoleak on the CT study confirmed a lumbar endoleak, which was treated. One patient with a 'probable' endoleak on the CT study showed lumbar vessels perfusing the sac margin at 3 levels but no endoleak. The other patient with a 'probable' lumbar endoleak on biphasic CT had increasing sac diameter, but no endoleak was seen on the arteriogram. Comparing each of the 4 ultrasound techniques with biphasic CT in the detection of endoleak, the number of nondiagnostic studies (flow detection too poor to allow diagnosis) was highest (n 12) in the unenhanced ultrasound group and lowest (n 4) with the enhanced power Doppler test." "One patient had nondiagnostic ultrasound examinations with all 4 test modalities due to bowel gas; the CT showed iliac limb dislocation." |
| Withdrawals explained? All tests | Yes | 2 participants excluded because radiology staff failed to follow protocol during 5 imaging sessions. |
Motta 2012.
| Clinical features and settings | People who received EVAR (initial transverse diameter not reported). Type of stents received: 87 bifurcated endografts and 1 aorto‐uni‐iliac endograft). Talent (Medtronic, Minneapolis, MN, USA): 43; Endurant (Medtronic): 14; Excluder (WL Gore & Associates, Flagstaff, AZ, USA): 20; Zenith (Cook Europe, Ireland): 6; Anaconda (Vascutek, Glasgow, UK): 3; E‐vita (JOTEC GmbH, Hechingen, Germany): 2. Aneurysm diameter: not reported. Setting: vascular medicine department. |
|
| Participants | 88 participants; 86 men (97.7%); mean age 75 years; range: 55‐95 years. Comorbidities: not reported. Geography: Italy. |
|
| Study design | Prospective single‐centre study enrolled consecutive participants who received both test (CTA and CEUS). | |
| Target condition and reference standard(s) |
Target condition: identification and characterization of endoleaks according to classification of standard guidelines; evaluation of graft patency; measurement of aneurysm maximum diameter. Definition of endoleak: not reported ("Endoleak CTA classification characteristics included: location and relation to the graft, density on delayed images, patency of the inferior mesenteric or lumbar arteries and appearance of endograft junctions"). Endoleak (absolute n): 154. Prevalence of endoleak: 27.5% (154/561). Reference standard: triple‐phase CTA. Image acquisition:
Acquisition parameters used for arterial phase were: collimation 64 × 0.6 mm, rotation time 0.5 s, automatic exposure modulation (Care‐Dose, Siemens Healthcare). Type of CT scanner: 64‐MDCT Somatom Sensation (Siemens Healthcare, Erlangen, Germany). Use of contrast: yes, Iohexol 350 mg/L, Omnipaque (GE Healthcare, Waukesha, WI, USA). |
|
| Index and comparator tests |
Index test: CDUS. Image production: from celiac to femoral arteries. Type of US: entire aorta scanned in longitudinal and transverse planes from diaphragm down to iliac limb attachment. Use of contrast: yes, second‐generation blood‐pool contrast agent (stabilised microbubbles of sulphur hexafluoride; SonoVue, Bracco, Milan, Italy) administered into antecubital vein, followed by flush of 10 mL saline solution (0.9% sodium chloride). Operator: 2 senior radiologists. |
|
| Follow‐up | During study period, 95 participants initially recruited. 7 excluded from participation because of severe allergy to iodinated contrast (n = 2) and severe renal failure (n = 5). All paired examinations were successful; during CEUS examinations, obesity, meteorism, and heavy sac calcifications were found in 21 cases but did not preclude correct evaluation. No adverse events recorded during examinations. No adverse interaction observed between the 2 contrast agents, which were administered within 2‐3 hours. |
|
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients that after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | Yes | Within a few hours, all participants underwent both CTA and CEUS. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | CEUS examinations and evaluations performed by 2 other senior radiologists (each with 10 years of experience in use of US contrast material) in consensus reading, masked to CTA findings. |
| Index test results blinded? All tests | Yes | CTA examinations performed by 2 senior radiologists (with 30 and 10 years of experience in vascular radiology and each with 10 years of experience in CTA), in consensus reading and blinded to CEUS results. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | All paired examinations successful; during CEUS examinations, obesity, meteorism, and heavy sac calcifications found in 21 cases but did not preclude correct evaluation. No adverse events recorded during examinations. No adverse interaction observed between the 2 contrast agents, which were administered within 2‐3 hours. |
| Withdrawals explained? All tests | Yes | 7 excluded from participation because of severe allergy to iodinated contrast (n = 2) and severe renal failure (n = 5). |
Nagre 2011.
| Clinical features and settings | People who received EVAR for AAA. Type of stents received: not reported. Aneurysm diameter: not reported. Setting: vascular surgery department. |
|
| Participants | 445 participants; 84.2% men; 91.2% white people; mean (± SD) age: 71.4 ± 8.5 years; range: 38‐93 years. Comorbidities: smoking (91%), coronary artery disease (51%), hypertension (64%), hyperlipidaemia (43%), stroke (10%), diabetes mellitus (14%), chronic obstructive pulmonary disease (21%), and end‐stage renal disease on dialysis (3%). Geography: USA. Recruitment period: October 1999 to June 2009. |
|
| Study design | Retrospective study. Review of prospectively maintained database designed to capture all EVAR procedures performed between October 1999 and June 2009. Participants routinely evaluated with CT and DUS imaging within 30 days after procedure and intermittently at 6‐ to 12‐month intervals after treatment. |
|
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: continuing blood flow around graft into aneurysm sac, thereby exposing participants to risk of rupture. "Endoleaks that were identified within 30 days of follow‐up were classified as early endoleaks, whereas those detected after 30 days were classified as late." Endoleak (absolute n): 154. Prevalence of endoleak: 27.5% (100/561). Reference standard: CTA. Image acquisition:
Type of CT scanner: GE LightSpeed 16 CT scanner (General Electric Medical Systems, Milwaukee, WI, USA). Use of contrast: yes, non‐ionic IV contrast. |
|
| Index and comparator tests |
Index test: CDUS. Image production: abdominal aorta and iliac arteries investigated in transverse, and antero‐posterior images obtained. Type of US: Sequoia 512 Acuson Sonography System (Siemens Medical Solutions, Mountain View, CA, USA). Use of contrast: no. Operator: 1 registered vascular technician. |
|
| Follow‐up | Missing data or loss to follow‐up unclear. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Sample derived from retrospective review of database in which data of people with AAA who received stent did not necessarily receive concomitant US and CTA. Participants included were those who had both tests available during follow‐up. ("A total of 1,062 EVARs were performed in 992 patients during this period. Medical records, vascular database records, and follow‐up images of these patients were reviewed in detail. National death indices were also reviewed for patients who were lost to follow‐up. A total of 3,120 postsurgical imaging encounters were recorded through the surveillance protocol. Of these 3,120 encounters, 1,729 were DUS encounters (1.86 per patient), whereas 2,001 were CTA scans (2.16 per patient), with 610 of these encounters recording a CTA and DUS at the same visit. Contrast material was not used in 49 CT scans, leaving 561 encounters in 455 patients, for comparing CTA imaging with DUS findings.") |
| Acceptable reference standard? All tests | Unclear | CT scan with contrast agents. No mention of expert who read and interpreted images provided. |
| Acceptable delay between tests? All tests | Yes | "Both studies should be recorded within 7 days of each other." |
| Partial verification avoided? All tests | Yes | All participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Unclear | Not reported. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "Of these 3,120 encounters, 610 had both CT scan and ultrasound at the same visit. Contrast material was not used in 49 CT scans, leaving 561 encounters for comparing contrast CT imaging with DUS results. CT and DUS detection of endoleaks correlated in 442 encounters. Discrepancies occurred in 119 encounters as follows: CT scan only endoleak in 17.8% (tot: 100; type I: 6, type II: 91 and type III: 3) and DUS only endoleak in 3.4% (N 19; type II: 19) encounters. Of these 119 encounters, 99 did not require secondary interventions. Eventually, 15 patients required intervention after 20 discrepancy encounters: 11 patients continued with the surveillance protocol through CT or DUS imaging, whereas four were observed by CT imaging only. Considering these 11 patients, DUS eventually detected an endoleak on subsequent visits in five patients, DUS identified an increase in aneurysm diameter in four patients, and DUS never identified the type II endoleaks in two patients. When the endoleak raised concern or the aneurysm enlarged, we undertook 19 secondary interventions in these 15 patients: vessel embolization (N 8), iliac extenders (N 5), graft relining (N 3), graft explants (N 2), and proximal cuff (N 1)." |
| Withdrawals explained? All tests | Unclear | "Of these 119 encounters, 99 did not require secondary interventions. Eventually, 15 patients required 19 re‐interventions after 20 discrepancy encounters (3.6%). Eleven patients continued with the surveillance protocol through CTA or DUS imaging, whereas four were followed up by CTA imaging only. One of these 15 patients had a type II endoleak that was missed by CTA and detected on DUS on subsequent follow‐up. Of these 15 patients, 12 were diagnosed with an early endoleak, whereas the remaining three were diagnosed with a late endoleak. There was no rupture, graft migration, limb occlusion, or structural failure in any of these 15 patients. Table III summarizes the secondary interventions in these 119 encounters." |
Nerlekar 2006.
| Clinical features and settings | People who received EVAR for AAA. Type of stents received: not reported. Aneurysm diameter: median: 52 (range 21‐75) mm using CT; 39 (38‐70) mm using US. Setting: department of surgery. |
|
| Participants | 121 participants enrolled; mean age: 73 years; median: 73 years; range: 52‐93 years. Comorbidities: not reported. Geography: Australia. Period of recruitment: 1995‐2003. |
|
| Study design | Retrospective review of prospectively collected data on people who received a stent for AAA. ("Between 1995 and 2003, 121 patients underwent EVR for an AAA. Their details regarding age, gender, and aneurysm morphology were entered into a prospective database. All patients were subjected to US and CT scan investigations. In addition, digital subtraction angiography was performed before surgery to assess vascular anatomy and to accurately determine aneurysm size." |
|
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: not provided. Endoleak (absolute n): 20. Prevalence of endoleak: 11.9% (29/243). Reference standard: CT scan. Image acquisition:
Type of CT scanner: high‐speed Advanced 2X spiral CT scanner (GE Medical Systems, Milwaukee, WI, USA). Use of contrast: yes, Ultravist 370 (Schering AC, Germany). |
|
| Index and comparator tests |
Index test: CDUS. Image production: not reported. Type of US: Sonoline Elegra Ultrasound lmaging System with colour flow Doppler (Siemens, New York, NY, USA). Use of contrast: no. Operator: 1 experienced ultrasonographer. |
|
| Follow‐up | People with modified device configurations (n = 5), pre‐existing grafts (n = 4), graft deployment failure (n = 1) and 3 participants who died before 1 month follow‐up from study. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients that after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | Yes | "There were 190 occasions in which US and CT were performed on the same day or within 1 month, and these results formed the basis of the study." |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "For the purpose of the study, all US and CT scan films were reviewed by two blinded reviewers." |
| Index test results blinded? All tests | Yes | "For the purpose of the study, all US and CT scan films were reviewed by two blinded reviewers." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | No uninterpretable results. |
| Withdrawals explained? All tests | Yes | "Excluded were patients with modified device configurations (n = 5), preexisting grafts (n = 4), graft deployment failure (n = 1) and three patients who died before 1 month follow up from the study." |
Oikonomou 2012.
| Clinical features and settings | People who underwent EVAR for infrarenal AAA. Type of stents received: (number of users not reported); Zenith (Cook Inc, Bloomington, IN, USA); Excluder and C3 (WL Gore & Associates, Flagstaff, AZ, USA); Powerlink (Endologix, Irvine, CA, USA). Aneurysm diameter: median: 5.8 cm; range: 48‐110 cm. Setting: vascular laboratory. |
|
| Participants | 100 participants; 85 men; median age: 73 years; range: 46‐91 years. Comorbidities: not reported. Geography: Germany. |
|
| Study design | Cross‐sectional study (consecutively selected participants, all received both tests). | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: endoleak on DUS defined as presence of persistent blood flow and spectral signal outside graft wall. Endoleak on CTA defined as presence of contrast agent outside graft within aneurysm sac. Endoleak (absolute n): 24. Prevalence of endoleak: 26.7% (24/90). Reference standard: CT scan. Image acquisition:
Type of CT scanner: contrast CT‐scan (Siemens Somatom scanner, Munich, Germany). Use of contrast: Solutrast 370 (ALTANA Pharma AG). |
|
| Index and comparator tests |
Index test: CDUS. Image production: abdominal aorta scanned from diaphragm to distal iliac arteries in longitudinal and transverse views using an anterior approach. Maximal aortic diameter identified, and DUS and spectral Doppler analysis performed to detect persistent flow outside graft wall. Type of US: CH4‐1 convex transducer (Acuson Antares Ultrasound System; Siemens Medical Solutions). Use of contrast: no. Operator: vascular surgeons (number not reported). |
|
| Follow‐up | 5 participants unsuitable for postoperative CTA due to severely impaired renal function. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Yes | Median interval between DUS and CTA: 9 days; range: 0‐25 days. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test was part of reference standard. |
| Reference standard results blinded? All tests | Unclear | No information reported. |
| Index test results blinded? All tests | Unclear | No information reported. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | Overall, 10 DUS examinations were inconclusive due to participant habitus (n = 6) or overlying bowel gas (n = 4) and were excluded from analysis. |
| Withdrawals explained? All tests | Yes | All participants received both tests. 5 participants unsuitable for postoperative CTA due to severely impaired renal function. 10 DUS examinations were inconclusive due to participant habitus (n = 6) or overlying bowel gas (n = 4) and were excluded from the analysis. |
Pages 2001.
| Clinical features and settings | People with infrarenal AAA who received EVAR. In 41 of these participants (21.6%), anatomical findings were compatible with stent‐graft placement. Type of stents received: not reported. Aneurysm diameter: mean (± SD) preoperative aneurysmal diameter determined by CT scan: 55 ± 9 mm; range: 40‐90 mm. Proximal neck of aneurysm located below renal arteries in all cases. Maximum proximal neck diameter 28 mm and minimal length 15 mm. Setting: unclear (department of surgery or department of radiology). |
|
| Participants | 41 participants; 39 men; mean age: 71 years; range: 50‐83 years. Comorbidities: not reported. Geography: France. |
|
| Study design | Prospectively consecutively enrolled study. Whole sample of participants who received stent considered. Recruitment period: November 1996 to September 1999. |
|
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: persistent blood flow or uptake of contrast between the stem graft and walls of the aneurysmal sac.
Endoleak (absolute n): 17. Prevalence of endoleak: 26.6% (29/109). Reference standard: spiral CT scan. Image acquisition:
Type of CT scanner: Somatom Plus S system (Siemens, Erlongen, Germany). Use of contrast: not reported. |
|
| Index and comparator tests |
Index test: CDUS. Image production: abdominal aorta visualized from celiac trunk to hypogastric arteries first in transverse plane and then in longitudinal plane in B‐mode and colour Doppler mode. Continuous spectral analysis performed if colour Doppler findings suggested presence of an endoleak. Type of US: 3.5‐MHz curved array transducer, Apogee 800PLUS ultrasound system (ATL, Philips, Eindhoven, the Netherlands). Use of contrast: no. Operator: 3 qualified angiologists. |
|
| Follow‐up | No loss to follow‐up, missing data, or adverse events. Uninterpretable data reported. | |
| Notes | Postoperative surveillance included plain abdominal roentgenography, CT scan, and CDUS. Procedures performed prior to discharge and at 3, 6, 12, 24, and 30 months. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Unclear | Not reported. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | CT scan and CDUS examination performed by different operators at different locations. Second operator had no knowledge of results of first examination. CT scans and videotaped CDUS procedures reviewed by independent radiologist. |
| Index test results blinded? All tests | Yes | CT scan and CDUS examination performed by different operators at different locations. Second operator had no knowledge of results of first examination. CT scans and videotaped CDUS procedures reviewed by independent radiologist. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "In six cases, B‐mode images were uninterpretable because of the presence of intestinal gas. In 55 cases, spectral study was necessary to confirm or deny suspicion of an endoleak based on colour Doppler findings." |
| Withdrawals explained? All tests | Yes | No apparent dropouts observed. |
Parent 2002.
| Clinical features and settings | People of Norfolk Surgical Group who underwent endovascular graft repair of AAA. Type of stents received: EVT‐EGS/Guidant ‐ Ancure product (Menlo Park, CA, USA) used in all cases. Bifurcated endograft: 63 participants, tube endograft: 12 participants, aortoiliac endograft in 8 participants. Aneurysm diameter: not reported. Setting: unclear. |
|
| Participants | 83 participants; age and gender not reported. Comorbidities: not reported. Geography: USA. |
|
| Study design | Retrospective study. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: absence of perigraft flow from the source vessel identified with prior study results. Endoleak (absolute n): 23. Prevalence of endoleak: 27.7% (23/83). Reference standard: CT scan. Image acquisition:
Type of CT scanner: not reported. Use of contrast: yes, 120 mL Omnipaque 350 (Nycomed, Inc, Princeton, NJ, USA). |
|
| Index and comparator tests |
Index test: CDUS. Image production: CDUS scan evidence of endoleak required identification of perigraft Doppler scan signals with colour flow and confirmed with spectral analysis and mapping of blood flow pattern. In addition, characterization of Doppler scan spectral analysis as biphasic, monophasic, or bidirectional (to/fro) obtained from CDUS scan studies. Type of US: not reported. Use of contrast: no. Operator: unclear. |
|
| Follow‐up | 8 examinations suboptimal because of gassy abdomen or large abdominal girth. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | Unclear | Information reported unclear although authors reported that CDUS and CT scan examinations were scheduled within 30 days and at 3, 6, and 12 months after surgery and then annually thereafter. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test was part of reference standard. |
| Reference standard results blinded? All tests | Unclear | No information provided. |
| Index test results blinded? All tests | Unclear | No information provided. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | 8 examinations suboptimal because of gassy abdomen or large abdominal girth. |
| Withdrawals explained? All tests | Yes | 42 (51%) participants never had an endoleak at any time in follow‐up period with CT and CDUS scan studies. Remaining 41 (49%) participants with endoleaks identified at any time in follow‐up period form basis of this analysis. |
Perini 2011.
| Clinical features and settings | People who underwent EVAR for AAA. Type of stents received: Zenith (Cook Medical, Bloomington, IA, USA), Talent (Medtronic, Santa Rosa, CA, USA), Anaconda (Vascutek, Glasgow, UK), fenestrated endografts (Cook Medical). All cases performed in dedicated operating theatre with OEC 9900 Elite MD Imaging System (GE Healthcare, Salt Lake City, UT, USA). Number of participants for each device not reported. Aneurysm diameter: mean (± SD): 5.5 ± 1.3. cm Setting: vascular surgery department. |
|
| Participants | 395 participants; basic characteristics not reported. Comorbidities: not reported. Geography: France. |
|
| Study design | Retrospective study. Recruitment period: January 2006 to December 2010. |
|
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: not reported. Endoleak (absolute n): 99. Prevalence of endoleak: 25.1% (99/395). Reference standard: 64‐slice CT scanner. Image acquisition:
Type of CT scanner: 64‐slice CT scanner (Philips Brilliance 64 CT scanner, Philips Healthcare, Amsterdam, the Netherlands). Use of contrast: yes, 2 types: Iomeron 350 (Bracco SA, Milano, Italy); Omnipaque 350 (Amersham Health, Princeton, NJ, USA). |
|
| Index and comparator tests |
Index test: CDUS. Image production: typical US examination started with standard B‐mode investigation to measure aneurysm sac diameter (outer wall to outer wall, dimensions recorded as the mean of 3 measurements). Then, blood flows from main body of endograft to femoral arteries analysed with pulse‐wave modality. In setting of fenestrated or multi‐branched endograft, visceral arteries also evaluated (feature not analysed in this study). Type of US: convex 3.5‐MHz probe, a Philips iE33 (Philips Healthcare, Amsterdam, the Netherlands), a Vivid 7 and a Vivid 9 (GE Healthcare, Salt Lake City, UT, USA) equipped with a convex 3.5‐MHz probe. Use of contrast: yes, SonoVue (Bracco, Milan, Italy). Operator:3 angiologists experienced in vascular ultrasonography. |
|
| Follow‐up | All participants completed follow‐up, and no adverse events recorded during these examinations. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | Yes | Time interval between the 2 examinations < 15 days. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test was part of reference standard. |
| Reference standard results blinded? All tests | Yes | CTAs analysed on independent dedicated workstation (Aquarius, TeraRecon, San Matteo, CA, USA) by both vascular surgeons and vascular radiologists (who were blinded to the results of CEUS, if already performed) to determine maximal aortic diameter by centre‐line measurements and to depict and characterise endoleaks. |
| Index test results blinded? All tests | Yes | All US scans performed by 3 angiologists experienced in vascular ultrasonography and use of US contrast material who were blinded to CTA findings at time of examination. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | All data were interpreted. |
| Withdrawals explained? All tests | Yes | All participants completed follow‐up, and no adverse events recorded during these examinations. |
Perini 2012.
| Clinical features and settings | People who underwent fenestrated EVAR for juxtarenal AAA. Type of stents received: all participants received a fenestrated stent‐graft. Aneurysm diameter: mean (± SD): 5.8 ± 0.9 cm. Setting: unclear (department of surgery or department of radiology). |
|
| Participants | 62 men; mean age: 72 years: underwent fenestrated EVAR follow‐up with both CTA and CEUS. Comorbidities: not reported. Geography: France. |
|
| Study design | Retrospective analysis. Recruitment period: January 2008 to April 2011. |
|
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: not defined but provided a bibliographic reference ("Endoleaks were identified and classified according to established reporting standards (Chaikof, J Vasc Surg. 2002;35:1048‐1060).") Endoleak (absolute n): 7. Prevalence of endoleak: 11.3% (7/62). Reference standard: 64‐slice CT scanner. Image acquisition:
Type of CT scanner: 64‐slice CT scanner (Philips Brillianee 64, Philips Healthcare, Amsterdam, the Netherlands). Use of contrast: yes, 100 mL lomeron 350 (Bracco SA, Milan, Italy) or Omnipaque 350 (Amersham Health, Princeton, NJ, USA). |
|
| Index and comparator tests |
Index test: CDUS. Image production: standard B‐mode investigation was performed to measure the maximal aneurysm sac diameter; blood flow in the visceral and renal arteries was analyzed in colour flow and pulse wave modes. A >50% stenosis of a stented vessel was considered significant and was identified using the peak systolic velocity and vessel/aortic systolic ratios. Type of US: convex 3.5‐MHz probe 3 machines, a Vivid 7 or a Vivid 9 (GE Healthcare, Salt Lake City, UT, USA) or a Philips IE33 (Philips Healthcare). Use of contrast: 2.5 mL bolus of SonoVue (Braceo, Milan, Italy) through an IV cannula, followed by a 5 mL bolus of isotonic saline solution. Operator: unclear. |
|
| Follow‐up | Of 81 participants remaining, 19 paired examinations excluded because CEUS was inadequate due to intervening bowel gas (n = 1) or ascites (n = 1) or interval between CEUS and CTA > 7 days (n = 17). | |
| Notes | All fenestrated EVAR procedures performed in a dedicated operating theatre with OEC 9900 Elite MD Imaging System (GE Healthcare, Salt Lake City, UT, USA). The fenestrated endografts were custom‐made by Cook Medical (Bloomington, IN, USA), and fenestrations were stented with balloon‐expandable covered grafts (Advanta V12; Atrium Medical, Hudson, NH, USA). | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Participants represented average patients who after receiving EVAR are exposed to endoleak surveillance. |
| Acceptable reference standard? All tests | Yes | Reference standard was CTA. |
| Acceptable delay between tests? All tests | Unclear | Unclear. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Unclear | No information provided. |
| Index test results blinded? All tests | Yes | "All CEUS scans were performed by 3 angiologists who had a minimum of 6 months of supervised training and experience in the use of ultrasound contrast material. All these physicians were blinded to the findings of the other study if already performed." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | No apparent uninterpretable results. |
| Withdrawals explained? All tests | Yes | No apparent missing data or withdrawals. |
Raman 2003.
| Clinical features and settings | People who underwent endovascular repair of AAA (between February 1996 and November 2002). Type of stents received: 247 participants received Ancure (Guidant, Menlo Park, CA; USA); 34 received AneuRX (Medtronic, Santa Rosa, CA, USA) endograft. Aneurysm diameter: not reported. Setting: hospital vascular laboratory, University of Pittsburgh Medical Centre. |
|
| Participants | 281 participants; 246 males, 35 females; mean (± SD) age: 73 ± 7 years; range: 47‐90 years. Comorbidities: not reported. Geography: USA. |
|
| Study design | Single‐centre, retrospective study (all participants received both tests). | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: perigraft flow into aneurysm sac. Endoleak (absolute n): 35. Prevalence of endoleak: 9.9% (49/494). Reference standard: helical CT. Image acquisition:
Type of CT scanner: helical CT, Lightspeed QXi multi‐detector‐row CT scanner (General Electric Medical Systems, Milwaukee, WI, USA). Use of contrast: yes, type not reported. |
|
| Index and comparator tests |
Index test: CDUS. Image production: protocol consisted of obtaining longitudinal and transverse views of proximal, mid, and distal aorta and iliac arteries. Peak systolic velocities obtained in graft and then compared with velocities in iliac vessels to assess for presence of limb flow anomalies including stenosis or occlusion. CFD scanning and Doppler interrogation of sac used to rule out presence of perigraft flow. Type of US: 3‐ to 5‐MHz transducers, Acuson 128 XP ultrasound machine (Mountain View, CA, USA). Use of contrast: no. Operator: registered vascular technologist. |
|
| Follow‐up | No loss to follow‐up, missing data, or adverse events reported ("All CT scans and CDU [CDUS] were technically satisfactory for determination of aneurysm size and presence of endoleak.") | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Retrospective review of all participants who underwent endovascular repair of AAAs between February 1996 and November 2002 and had same‐day CT and CDUS studies. |
| Acceptable reference standard? All tests | Yes | Reference standard was helical CT. |
| Acceptable delay between tests? All tests | Yes | Yes, participants received same‐day US and CT. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | US scanning technologist and surgeon reviewing tapes were both unaware of results of CT scan during any portion of US scan examination or review. |
| Index test results blinded? All tests | Unclear | Unclear. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "All CT scans and CDU [CDUS] were technically satisfactory for determination of aneurysm size and presence of endoleak." |
| Withdrawals explained? All tests | Yes | No withdrawal reported. |
Sandford 2006.
| Clinical features and settings | People who underwent EVAR were referred to Leicester Royal Infirmary for endoleak follow‐up (over 11‐year period between 30 March 1994 and 8 October 2005). Type of stents received: not reported. Aneurysm diameter: not reported. Setting: department of cardiovascular sciences. |
|
| Participants | 310 participants who underwent EVAR. No information reported related to age and gender. Comorbidities: not reported. Geography: UK. |
|
| Study design | Single‐centre, retrospective design, participants consecutively enrolled (all participants received both tests). | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: not described. Endoleak (absolute n): 44 (unclear). Prevalence of endoleak: 18.0% (44/244). Reference standard: contrast CT scan performed using IV contrast and Phillips Secura single‐slice spiral CT. Image acquisition: no details reported. Type of CT scanner: Phillips Secura single‐slice spiral CT. Use of contrast: yes. |
|
| Index and comparator tests |
Index test: CDUS. Image production: not reported. Type of US: Phillips HDI 5000. Use of contrast: no. Operator: a trained vascular technician. Comparator test: CT scan. Image acquisition: not reported. Type of CT scanner: GE Lightspeed plus 16‐slice scanner (GE Healthcare, Waukesha, WI, USA). Use of contrast: yes, Omnipaque 350 contrast 120 mL (GE Healthcare, Milwaukee, WI, USA). CT scan surveillance performed using GE Lightspeed plus 16‐slice scanner (GE Healthcare, Waukesha, WI, USA) using 2.5‐mm acquisition slice. Omnipaque 350 contrast 120 mL (GE Healthcare, Milwaukee, WI, USA) was injected at rate of 4‐5 mL/s using SmartPrep software (GE Healthcare, Milwaukee, WI, USA). Arterial phase acquisition obtained by a mean delay of 25 s after injection. Delayed phase obtained at 70 s after completion of first scan. CT scan reconstruction used 0.625‐mm format. Index test: colour flow DUSS performed by a trained vascular technician using Phillips HDI 5000 ultrasound machine. No contrast used. If abnormality found on DUSS, or views were inadequate, participants underwent CT scan, using IV contrast and a Phillips Secura single‐slice spiral CT. All participants having undergone concurrent DUSS and CT scans were included in analysis. Concurrent scans defined as having occurred within 6 months of each other. |
|
| Follow‐up | Follow‐up period: 60 months; however, number of participants eventually lost to follow‐up unclear. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Imaging was retrospectively reviewed for 310 consecutive patients undergoing endovascular aneurysm repair at a single. Patients were followed up after EVAR in a nurse led clinic and underwent six monthly clinical examination and duplex ultrasound scan |
| Acceptable reference standard? All tests | Yes | The reference standard was CT scan |
| Acceptable delay between tests? All tests | No | No: concurrent scans were defined as having occurred within 6 months of each other |
| Partial verification avoided? All tests | Yes | All study participants are accounted for and results of the reference standard are reported for all |
| Differential verification avoided? All tests | Yes | All patients who received the index test were subjected to the same reference standard |
| Incorporation avoided? All tests | Yes | The index test was not part of the reference standard |
| Reference standard results blinded? All tests | Unclear | Unclear |
| Index test results blinded? All tests | Unclear | Unclear |
| Relevant clinical information? All tests | Yes | Yes |
| Uninterpretable results reported? All tests | Yes | "Of the 1352 CDUS performed, 151 (11%) reported difficult views due to either increased bowel gas or obesity. The proportion of scans which reported poor views was higher immediately post operatively than subsequent scans, affecting 19 of 99 (19%) pre‐discharge scans" |
| Withdrawals explained? All tests | Unclear | Unclear |
Sato 1998.
| Clinical features and settings | People who were implanted with the Endovascular Technologies stent graft device. Type of stents received: not reported. Aneurysm diameter: not reported. Setting: division of vascular surgery. |
|
| Participants | 79 participants; no demographic information provided. Comorbidities: not reported. Geography: USA. |
|
| Study design | Retrospective review of records. "The EnACT Core Laboratory records were reviewed from CDU [CDUS] and CT studies that were performed in patients who were implanted with the Endovascular Technologies stent graft device. All of the studies were evaluated by the Core Laboratory for endoleak and interpreted as having an endoleak present or absent or recorded as an indeterminate study as a result of technical factors. Data were entered into a computerized database and analyzed for diagnostic accuracy of CDU studies as compared with CTs." |
|
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: not reported. Endoleaks have been reported at a rate of 13‐44%. Endoleak (absolute n): unclear. Prevalence of endoleak: 34.0% (34/100). Reference standard: contrast‐enhanced CT scan. "The CTs were obtained according to study protocol. A scout CT was obtained without contrast to identify the superior mesenteric artery. This was followed by a contrast‐enhanced CT with 3‐mm‐thick slices from above the superior mesenteric artery to the level of the profunda femoris and the superficial femoral artery. No delayed images were required with the study protocol. The CDUs were performed according to the study protocol, which included the evaluation of the flow through the endograft, the perigraft flow, the renal and the iliac arterial flow, the maximum diameter of aneurysm, and the presence of branch vessel flow." Type of scanner: not reported. Use of contrast: yes (type not reported). |
|
| Index and comparator tests |
Index test: CDUS. Image production: B‐mode image performed to assess graft, proximal and distal stents, and AAA sac and size measurements of the AAA sac. Colour Doppler scan added, and settings optimized to avoid excessive overgain. Doppler scan may be added to assist in detection of perigraft flow. Type of US:low‐frequency (range: 2.25‐5 MHz), curved array, phased array or mechanical sector, and pulsed Doppler scan transducers. Use of contrast: not used. Operator: > 1. |
|
| Follow‐up | Unclear. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Yes. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Unclear | No information provided. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | Interpretation of all CDUS and CT scans blinded to all concurrent and prior studies. |
| Index test results blinded? All tests | Yes | Interpretation of all CDUS and CT scans blinded to all concurrent and prior studies. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | Of 117 studies, 103 CDUs (88%) and 114 CTs (97%) were recorded as having presence or absence of an endoleak and 14 CDUs (12%) and 3 CTs (3%) were indeterminate. The 14 indeterminate CDUs caused by suboptimal imaging technique. The 3 indeterminate CTs caused by unsatisfactory contrast administration in 2 studies and by extensive calcification in the third study. In 18 studies (18%), CTs and CDUs were conflicting for endoleaks. |
| Withdrawals explained? All tests | Unclear | Unclear whether missing data or adverse events occurred. |
Schmieder 2009.
| Clinical features and settings | People with AAAs who underwent elective treatment with EVAR from 11 July 1996 to 31 March 2007. Analysis excluded people with symptomatic or ruptured AAA and isolated iliac aneurysms. Type of stents received: commercially available and investigational devices, including 160 AneuRx (Medtronic, Santa Rosa, CA, USA), 55 Ancure/EVT (Guidant, Indianapolis, IN, USA), 13 Zenith (Cook, Bloomington, IN, USA), 5 Powerlink (Endologix, Irvine, CA, USA), 2 Excluder (WL Gore & Associates, Flagstaff, AZ, USA), and 1 Quantum (Cordis, New Brunswick, NJ, USA). Aneurysm diameter: not reported. Setting: division of vascular surgery. |
|
| Participants | 236 participants;202 (86%) men; mean age at the time EVAR: 72 years; range: 51‐90 years. Study population: 211 (89%) white, 20 (8%) African‐American, 4 (2%) Asian, and 1 Hispanic. Comorbidities: not reported. Geography: USA. |
|
| Study design | Retrospective longitudinal study (from cohort of 496 consecutive participants, 236 participants had paired CDUS and CT scan). | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: not reported ("Endoleaks were categorized as type I, type II, type III or indeterminate.") Endoleak (absolute n):75. Prevalence of endoleak: 15.9% (75/472). Reference standard: contrast CT scan. Image acquisition:
Type of scanner: GE Light‐speed plus 16‐slice scanner (GE Healthcare, Waukesha, WI, USA). Use of contrast: yes, Omnipaque 350 contrast (120 mL; GE Healthcare, Milwaukee, WI, USA). |
|
| Index and comparator tests |
Index test: CDUS. Image production: endograft, proximal and distal fixation points, and AAA sac imaged in B‐mode. Size of AAA sac measured. Type of US: range: 2.5‐5 MHz curved array, phased array, or mechanical sector, and pulsed Doppler scan transducer. Use of contrast: no. Operator: 1 ("The US examinations were performed by vascular doctors dedicated to US imaging.") |
|
| Follow‐up | Unclear. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | No | From cohort of 496 consecutive participants, 236 participants had paired CDUS and CT scan. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | No | Mean interval between CDUS and CT scans 18 days; range: 0‐90 days, and 33% of paired studies performed 4 days from each other. CT scan obtained before CDUS scan 69% of time (n = 325), CDUS study obtained before CT scan 15% of time (n = 71), and both studies obtained on the same day 16% of time (n = 76). |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | US examinations performed by vascular doctors dedicated to US imaging, blinded to results of CTA. |
| Index test results blinded? All tests | Yes | Interpretation of CT scan results performed by radiology staff, whereas vascular surgery staff interpreted CDUS results. |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Unclear | Uninterpretable data of participants excluded. No specific numbers reported. |
| Withdrawals explained? All tests | Unclear | No information provided. |
Ten Bosch 2010.
| Clinical features and settings | People who underwent endovascular repair for infrarenal AAA. Type of stent received: Talent (Medtronic, Minneapolis, MN. USA). Aneurysm diameter: not reported. Setting: department of vascular surgery. |
|
| Participants | 83 participants consecutively enrolled for CEUS and CTA imaging during surveillance after EVAR. Comorbidities: not reported. Geography: the Netherlands. |
|
| Study design | Prospectively enrolled consecutive participants; all had target condition; all received both tests. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: presence of persistent intrasac flow outside graft. Endoleaks classified as type IA/B, II, III, or IV. Endoleak (absolute n): unclear. Prevalence of endoleak: 21.3% (27/127). Reference standard: triple‐phase (unenhanced and contrast‐enhanced in arterial and delayed phases) CTA. Image acquisition:
CTA performed from diaphragm to common femoral arteries after continuous IV administration of iodinated contrast agent (Xenetix 300; Laboratoire Andre Guerbet, Aulnaysous Bois, France). Parameters: high‐speed mode capability, rotation time 0.5 s, table speed 24 mm per rotation, collimation of 1.5 mm, and slice thickness 3 mm. Type of CT scanner: multidetector 16‐slice spiraI CT scanner (Somatom Sensation; Siemens, Forchheim, Germany). Use of contrast: yes, iodinated contrast agent (Xenetix 300; Laboratoire Andre Guerbet, Aulnaysous Bois, France). |
|
| Index and comparator tests |
Index test: CDUS. Image production: continuous real‐time tissue harmonic imaging for endoleak detection performed for 15 minutes during sonographic contrast agent infusion at mechanical index of 0.4‐0.5 and at low acoustic power. Type of US: 3.5‐MHz curved array transducer (Aloka 550‐5000; Biomedic, Almere, the Netherlands). Use of contrast: yes, 5 mL SonoVue (Bracco, Milan, Italy) containing 8 μL sulphur hexafluoride microbubbles per millilitre with 55 mL saline solution. Operator: 3 ("three well trained vascular technicians dedicated to US imaging"). |
|
| Follow‐up | "Seven of the 113 patients were excluded from participation in the study because of severe iodinated contrast allergy (n = 3) or severe renal insufficiency (n = 4), which precluded CT angiography. The remaining 106 patients who were eligible for the study were prospectively enrolled far dual‐modality imaging after consent. Overall, 62 of 189 potential paired examinations were excluded from comparative analysis for one of the following reasons: time interval between CT angiography and US examinations exceeding 30 days as a consequence of logistic problems (n = 53), failure to perform US because of obesity (n = 2) or bowel gas (n = 1), and failure to receive CT angiography as a result of study protocol violation (n = 6)." | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | 106 participants who were eligible were prospectively enrolled for dual‐modality imaging after consent. Overall, 127 of 189 potential paired examinations in 83 participants available for comparative analysis. |
| Acceptable reference standard? All tests | Yes | CTA triple‐phase (unenhanced and contrast‐enhanced in arterial and delayed phases) performed. |
| Acceptable delay between tests? All tests | Yes | Participants with time interval between CTA and US examinations > 30 days were excluded. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "Each of the three vascular technicians independently measured AAA sac diameters and reported the presence or absence of endoleak at the end of each contrast‐enhanced US examination; technicians were blinded to the results of CT angiography. AAA dimensions on contrast‐enhanced US were recorded as the means of the three measurements." |
| Index test results blinded? All tests | Yes | "Each of the three vascular technicians independently measured AAA sac diameters and reported the presence or absence of endoleak at the end of each contrast‐enhanced US examination; technicians were blinded to the results of CT angiography. AAA dimensions on contrast‐enhanced US were recorded as the means of the three measurements." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | Failure to perform US because of obesity (n = 2) or bowel gas (n = 1), and failure to receive CTA due to study protocol violation (n = 6). |
| Withdrawals explained? All tests | Unclear | 7/113 participants enrolled were excluded because of severe iodinated contrast allergy (n = 3) or severe renal insufficiency (n = 4), which precluded CTA. Overall, 62/189 potential paired examinations were excluded from comparative analysis for 1 of the following reasons: time interval between CTA and US examinations > 30 days as consequence of logistic problems (n = 53), failure to perform US because of obesity (n = 2) or bowel gas (n = 1), and failure to receive CTA due to study protocol violation (n = 6). |
Thompson 1998.
| Clinical features and settings | 20 people who received endovascular grafts for infrarenal aortic aneurysms. Type of stent received: 6 aortic tube endografts (Endovascular Technologies EGS, Menlo Park, CA, USA), 3 bifurcated systems (Stentor; Mintec, Freeport, Bahamas), and 11 tapered aorto‐uni‐iliac graft. Aneurysm diameter: median transverse diameter: 5.2 cm; range: 4.8‐7.3 cm measured by CT. Setting: department of surgery. |
|
| Participants | Median age: 72 years; range: 68‐84 years; median aneurysm diameter: 5.2 cm; range: 4.8‐7.3 cm measured by CT; 5.0 cm; range: 4.3‐7.0 cm measured by duplex imaging; median follow‐up: 14 months; range: 6‐31 months. Comorbidities: not reported. Geography: UK. |
|
| Study design | All participants who had a technically successful EVAR were entered into a standard prospective surveillance programme at 3, 6, 12, 18, and 24 months following repair. | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: to presence or absence of flow within aneurysm sac. Endoleak (absolute n):75. Prevalence of endoleak: 15.9% (49/494). Reference standard: contrast CT scan. Image acquisition:
Type of CT scanner: Siemens HiQ scanner (Munich, Germany). Use of contrast: yes (type unclear). |
|
| Index and comparator tests |
Index test: CDUS. Image production: "Duplex imaging was performed with the patient supine using a 3.5 MHz curved linear array transducer, HDI Ultramark 9 (ATL, Letchworth, UK). Colour Doppler ultrasonography was utilized to image flow within the graft, and any flow disturbance was noted. Endoleaks were specifically sought with the colour Doppler set to detect low flow." Type of US: 3.5‐MHz curved linear array transducer, HDI Ultramark 9 (ATL, Letchworth, UK). Use of contrast: no. Operator: 2 ("Diagnostic imaging was performed by investigators.") |
|
| Follow‐up | Unclear. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Up to December 1996, EVAR was attempted in 48 participants, with primary success in 43. 20 of these participants followed for ≥ 6 months and formed group for study. Median age: 72 years; range: 68‐84 years; median aneurysm diameter: 5.2 cm; range: 4.8‐7.3 cm as measured by CT and 5.0 cm; range: 4.3‐7.0 cm as measured by duplex imaging; median follow‐up: 14; range: 6‐31 months. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Yes | Yes: CT and US examinations performed on same day. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "Diagnostic imaging was performed by investigators (G.F. and T.H.) who were blinded of the result from the other imaging technique and previous scans." |
| Index test results blinded? All tests | Unclear | "Diagnostic imaging was performed by investigators (G.F. and T.H.) who were blinded of the result from the other imaging technique and previous scans." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Unclear | Unclear. |
| Withdrawals explained? All tests | Yes | No withdrawals. |
Wolf 2000.
| Clinical features and settings | People who received endovascular repair of AAA with bifurcated endograft. Type of stent received: AneuRx (Medtronic). Aneurysm diameter: not reported. Setting: vascular surgery department, Stanford University Hospital. |
|
| Participants | 100 consecutive participants (age and gender not reported). Comorbidities: not reported. Geography: USA. |
|
| Study design | Prospective cross‐sectional study (consecutively selected participants, all received both tests). | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: no definition reported. Endoleak (absolute n): unclear. Prevalence of endoleak: 38.0% (62/163). Reference standard: CTA. Image acquisition:
Type of CT scanner: CT scanner (both General Electric Medical Systems, Milwaukee, WI, USA). Use of contrast: yes (type not reported). |
|
| Index and comparator tests |
Index test: CDUS. Image production: "The protocol included transverse and sagittal imaging and peak systolic diameter measurements at the largest region of the proximal, mid, and distal segments of the abdominal aorta. Visible segments of the iliac arteries were also measured. Close attention was given to the stent device in gray scale and in colour Doppler scanning to rule out endoleak and graft compression." Type of US: Sequoia 512 ultrasound scanning system (Acuson, Mountain View, CA, USA) and sector V4 transducer. Use of contrast: no. Operator: 1 ("the vascular technologist was not aware of the CT scan"). |
|
| Follow‐up | No apparent loss to follow‐up, missing data, or adverse events. | |
| Notes |
|
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Enrolled participants who "underwent endovascular repair for AAA with the AneuRx (Medtronic) bifurcated endograft at Stanford University Hospital from October 1996 to May 1999. Follow‐up protocol included CT angiography before discharge, duplex scan at 1 month, and CT angiography at 6 months, 1 year, and yearly thereafter." |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Unclear | Delay between 2 tests in 24 participants unclear. "To compare CT and duplex scans, we obtained both studies, whenever possible, within a period of 7 days from each other. CT and duplex scans were obtained concurrently (within 7 days of each other) in 166 instances in 76 patients (1‐6 scan pairs per patient). These concurrent scan pairs form the basis for the comparison between the tests." |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "During the examination and the reading of the duplex scan, the vascular technologist was not aware of the CT scan results." |
| Index test results blinded? All tests | Yes | "In addition to a formal reading by a radiologist who was unaware of the duplex scan result, CT angiograms were reviewed by a panel of radiologists and vascular surgeons to confirm the presence or absence of an endoleak." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "All CT scans were technically satisfactory. Delayed scans, which were obtained routinely after September 1998, were performed in 57% of CT scans. Sixteen (7%) duplex scans in 10 patients were technically inadequate for determination of aneurysm size and presence of endoleak." |
| Withdrawals explained? All tests | Unclear | Unclear. |
Zannetti 2000.
| Clinical features and settings | People who underwent EVAR. Type of stents received: "The AneuRxstent graft was employed in 144 procedures, the Gore Excluder in 9 procedures, and the Talent graft in one." Aneurysm diameter: mean (± SD) AAA diameter: 50.2 ± 8.3 mm. Setting: unit of vascular surgery. |
|
| Participants | 108 participants; mean (± SD) age: 70.1 ± 6.7 years; ASA IV: n = 19 (19%); Eurostar classification A: n = 18 (17%); B: n = 62 (61%); C: n = 7 (7%); D: n = 7 (7%); E: n= 8 (8%). Comorbidities: not reported. Geography: Italy. |
|
| Study design | Consecutively enrolled participants. "After surgery, patients were entered in a follow‐up protocol consisting of colour‐duplex and CT scan examinations scheduled at 1, 6, 12 months after surgery, and every 6 months thereafter. Mean follow up of the study cohort was 8.5 months." | |
| Target condition and reference standard(s) |
Target condition: endoleak. Definition of endoleak: flow "outside the endograft and within the aneurysmal sac." Endoleak (absolute n): 12. Prevalence of endoleak: 37.5% (12/32). Reference standard: contrast‐enhanced CT. Image acquisition:
Type of CT scanner: not reported. Use of contrast: mean 140 mL of iso‐osmotic, non‐ionic iodinated contrast media injected 25 s before imaging acquisition. |
|
| Index and comparator tests |
Index test: CDUS. Image production: "The scanhead was applied in both the transverse and longitudinal views to obtain colour and Doppler optimisation. The entire AAA sac, proximal and distal necks, the aorta, iliac and femoral arteries were systematically imaged and measurements were performed on both sagittal and transverse views. The presence of perigraft endoleaks was suspected when a reproducible colour signal outside the endograft and within the aneurysmal sac was visualised. All suspected endoleaks were further evaluated with the Doppler signal to avoid colour artefacts." Type of US: C4‐2‐MHz curved array transducer (ATL 3000 HDI system, Advanced Technology Laboratory). Use of contrast: no. Operator: 2 ("All duplex scan examinations were performed by two vascular surgeons with the same machine (ATL HDI).") |
|
| Follow‐up | "Compliance with the study protocol was not achieved in 51 patients for different reasons including perioperative death (two patients), conversion to open repair (four patients), duplex scan performed in a different centre in patients from out of town and refusal. Three patients (2%) were excluded from the study protocol because of inadequate duplex visualisation of the abdominal aortic aneurysm (AAA) sac due to obesity or intestinal gas." |
|
| Notes | 198 concurrent all duplex‐scan examinations performed. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Consecutive participants who received EVAR received both index test and reference standard. |
| Acceptable reference standard? All tests | Yes | Reference standard was CT scan. |
| Acceptable delay between tests? All tests | Unclear | Not reported. |
| Partial verification avoided? All tests | Yes | All study participants accounted for and results of reference standard reported for all. |
| Differential verification avoided? All tests | Yes | All participants who received index test subjected to same reference standard. |
| Incorporation avoided? All tests | Yes | Index test not part of reference standard. |
| Reference standard results blinded? All tests | Yes | "The interpretation of all colour‐duplex and CT scans was blinded to all concurrent and prior studies." |
| Index test results blinded? All tests | Yes | "The interpretation of all colour‐duplex and CT scans was blinded to all concurrent and prior studies." |
| Relevant clinical information? All tests | Yes | Yes. |
| Uninterpretable results reported? All tests | Yes | "With respect to the presence of endoleak, CT and colour‐duplex scans were conflicting in 4 cases (2%). In detail, duplex scan failed to show one endoleak (1 false negative) and revealed 3 endoleaks not confirmed by CT examination (3 false positives). Digital subtraction angiography performed on these 4 patients revealed the absence of endoleak in all cases. With respect to type of endoleak, of the 11 endoleaks detected both by CT and colour‐duplex scan, there was discordance in 2 cases. Based on the 2 colour duplex‐scan examinations, reperfusion of the aneurysmal sac appeared in continuity with the inferior mesenteric artery and the 2 endoleaks were classified as non‐graft‐related. Inversely, in these 2 patients CT scan revealed accumulation of the majority of contrast media in the area of the proximal implant zone, suggesting the presence of a graft‐related endoleak in accordance with digital subtraction angiography obtained subsequently." |
| Withdrawals explained? All tests | Yes | "Compliance with the study protocol was not achieved in 51 patients for different reasons including perioperative death (two patients), conversion to open repair (four patients), duplex scan performed in a different centre in patients from out of town and refusal. Three patients (2%) were excluded from the study protocol because of inadequate duplex visualisation of the abdominal aortic aneurysm (AAA) sac due to obesity or intestinal gas." |
2D: two‐dimensional; 3D: three‐dimensional; 4D: four‐dimensional; AAA: abdominal aortic aneurysm; ASA: American Society of Anesthesiology; BMI: body mass index; CCDS: colour‐coded duplex sonography; CDI: colour Doppler imaging; CDUS: colour duplex ultrasound; CE‐CDUS: contrast‐enhanced colour duplex ultrasound; CEUS: contrast‐enhanced ultrasound; CFD: colour flow duplex; CI: confidence interval; CPS: contrast pulse sequences; CT: computed tomography; CTA: computed tomography angiography; DSA: digital subtraction angiography; DUS: duplex ultrasound; DUSS: duplex ultrasound sonography; erAAA: emergency abdominal aortic aneurysm; EVAR: endovascular aneurysm repair; GPS: global positioning system; IV: intravenous; LDCE: low‐dose contrast‐enhanced; MRA: magnetic resonance angiography; MS‐CT: multi‐slice computer tomography; n: number of participants; s: second; SD: standard deviation; SMA: superior mesenteric artery; US: ultrasound.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almaroof 2013 | Performed EVAR surveillance using US and CT scan but did not evaluate endoleaks. |
| Beeman 2009 | People without insurance coverage could not receive US test and were excluded. |
| Bredahl 2013 | Target conditions: volume estimation of residual sac after EVAR. |
| Chisci 2012 | Study was a follow‐up protocol based on colour DUS + plain abdominal radiography and CTA on demand. |
| Clevert 2008a | 36 included participants were with known or suspected treated and untreated aortic lesions detected by CTA. |
| Clevert 2013 | Target condition was not endoleak (time‐to‐peak i.e. time between point where contrast agent was first seen in stent graft until it was first seen in aneurysmal sac, of all digitally stored CEUS video sequences showing an endoleak, to confirm type of endoleak in uncertain cases. |
| Collins 2007 | CT scan performed only when US was positive (for 160 screening only 35 CT scans performed). |
| Elkouri 2004 | Data insufficient to perform a contingency table. |
| Greenfield 2002 | Not all participants included received both tests: US performed only when CT scan results were positive. |
| Han 2010 | Study limited to diameter measurements after endovascular aortic aneurysm repair. |
| Harrison 2011 | Study considered participants from a registry where participants who received EVAR required CT scan only when DUS was not diagnostic. In addition, data reported were insufficient. |
| Hertault 2015 | Study did not sufficiently provide data for 2 × 2 table production. |
| Manning 2009 | Ultrasonography and CT not performed concurrently. |
| Millen 2013 | Study evaluated a subset of participants with CEUS with unresolved issues. |
| Napoli 2004 | US not performed concurrently to CT scan. |
| Nyheim 2013 | Study did not report sufficient data for inclusion. |
| Ormesher 2014 | Reference standard angiography (not CTA). |
| Pfister 2009 | Participants included based on suspect of endoleak. |
| Sommer 2012 | Not consecutive participants. Included people with suspect of endoleak at previous imaging study, or with postoperative endoleaks. |
| Sorrentino 2015 | Retrospective study that was not performed for purpose of performing an accuracy study. |
| Troutman 2014 | Follow‐up study of Beeman 2009; study included subsequent participants who never received CT scan. |
| Yang 2015 | Included participants were a selected population with endoleaks. |
CEUS: colour enhanced ultrasound; CT: computed tomography; CTA: computed tomography angiography; DUS: duplex ultrasound; EVAR: endovascular aneurysm repair; US: ultrasound.
Differences between protocol and review
The current unit of analysis was not specified in the protocol (Abraha 2013).
Contributions of authors
IA conceived the idea for the review and wrote the protocol with input from GC, PD, BP, FC, and AM. IA, RDF, AG, MO, and MLL screened the search results and selected the papers for inclusion. IA, RDF, MLL, FC, AG, MO, and RDF appraised the included papers and performed the data extractions. IA, GC, PE, and PD conducted the analyses. All the authors contributed to the writing of the review and approved the final manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding to Cochrane Vascular (10/4001/14). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
The review authors have no financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the review.
IA: none known. MLL: none known. RDF: none known. FC: none known. GC: none known. PD: none known. BP: none known. MO: none known. AG: none known. PE: none known. AM: none known.
New
References
References to studies included in this review
Abbas 2014 {published data only}
- Abbas A, Hansrani V, Sedgwick N, Ghosh J, McCollum CN. 3D contrast enhanced ultrasound for detecting endoleak following endovascular aneurysm repair (EVAR). European Journal of Vascular and Endovascular Surgery 2014;47(5):487‐92. [PUBMED: 24618331] [DOI] [PubMed] [Google Scholar]
AbuRahma 2005 {published data only}
- AbuRahma AF, Welch CA, Mullins BB, Dyer B. Computed tomography versus color duplex ultrasound for surveillance of abdominal aortic stent‐grafts. Journal of Endovascular Therapy 2005;12(5):568‐73. [PUBMED: 16212456] [DOI] [PubMed] [Google Scholar]
Arsicot 2014 {published data only}
- Arsicot M, Lathelize H, Martinez R, Marchand E, Picquet J, Enon B. Follow‐up of aortic stent grafts: comparison of the volumetric analysis of the aneurysm sac by ultrasound and CT. Annals of Vascular Surgery 2014;28(7):1618‐28. [PUBMED: 24709403] [DOI] [PubMed] [Google Scholar]
Ashoke 2005b {published data only}
- Ashoke R, Brown LC, Rodway A, Choke E, Thompson MM, Greenhalgh RM, et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. Journal of Endovascular Therapy 2005;12(3):297‐305. [DOI] [PubMed] [Google Scholar]
Ashoke 2005c {published data only}
- Ashoke R, Brown LC, Rodway A, Choke E, Thompson MM, Greenhalgh RM, et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. Journal of Endovascular Surgery 2005;12(3):297‐305. [DOI] [PubMed] [Google Scholar]
Badri 2010 {published data only}
- Badri H, Haddad M, Ashour H, Nice C, Timmons G, Bhattacharya V. Duplex ultrasound scanning (DUS) versus computed tomography angiography (CTA) in the follow‐up after EVAR. Angiology 2010;61(2):131‐6. [PUBMED: 19825870] [DOI] [PubMed] [Google Scholar]
Bendick 2003 {published data only}
- Bendick PJ, Bove PG, Long GW, Zelenock GB, Brown OW, Shanley CJ. Efficacy of ultrasound scan contrast agents in the noninvasive follow‐up of aortic stent grafts. Journal of Vascular Surgery 2003;37(2):381‐5. [PUBMED: 12563210] [DOI] [PubMed] [Google Scholar]
Cantisani 2011 {published data only}
- Cantisani V, Ricci P, Grazhdani H, Napoli A, Fanelli F, Catalano C, et al. Prospective comparative analysis of colour‐Doppler ultrasound, contrast‐enhanced ultrasound, computed tomography and magnetic resonance in detecting endoleak after endovascular abdominal aortic aneurysm repair. European Journal of Vascular and Endovascular Surgery 2011;41(2):186‐92. [PUBMED: 21095141] [DOI] [PubMed] [Google Scholar]
Clevert 2008b {published data only}
- Clevert DA, Minaifar N, Weckbach S, Kopp R, Meimarakis G, Clevert DA, et al. Color duplex ultrasound and contrast‐enhanced ultrasound in comparison to MS‐CT in the detection of endoleak following endovascular aneurysm repair. Clinical Hemorheology and Microcirculation 2008;39(1‐4):121‐32. [PUBMED: 18503118] [PubMed] [Google Scholar]
Clevert 2011 {published data only}
- Clevert DA, Helck A, D'Anastasi M, Gürtler V, Sommer WH, Meimarakis G, et al. Improving the follow up after EVAR by using ultrasound image fusion of CEUS and MS‐CT. Clinical Hemorheology and Microcirculation 2011;49(1‐4):91‐104. [DOI] [PubMed] [Google Scholar]
Costa 2013 {published data only}
- Costa P, Bureau Du Colombier P, Lermusiaux P. Duplex ultrasound detection of type II endoleaks by after endovascular aneurysm repair: interest of contrast enhancement [Detection echo‐Doppler des endofuites de type II apres endoprotheses aortiques: interet des agents de contraste.]. Journal des Maladies Vasculaires 2013;38(6):352‐9. [PUBMED: 24113391] [DOI] [PubMed] [Google Scholar]
d'Audiffret 2001 {published data only}
- d'Audiffret A, Desgranges P, Kobeiter DH, Becquemin JP. Follow‐up evaluation of endoluminally treated abdominal aortic aneurysms with duplex ultrasonography: validation with computed tomography. Journal of Vascular Surgery 2001;33(1):42‐50. [PUBMED: 11137922] [DOI] [PubMed] [Google Scholar]
Demirpolat 2011 {published data only}
- Demirpolat G, Ozturk N, Parildar M, Posacioglu H, Tamsel S. Duplex ultrasound evaluation of endoluminally treated aortic aneurysms with emphasis on diameter measurement: a comparison with computed tomography. Journal of Clinical Ultrasound 2011;39(5):263‐9. [PUBMED: 21425274] [DOI] [PubMed] [Google Scholar]
França 2013 {published data only}
- França GJ, Baroncini LAV, Oliveira A, Vidal EA, Miyamotto M, Toregeani JF, et al. Evaluation with Doppler vascular ultrasound in postoperative endovascular treatment of abdominal aortic aneurysm: a prospective comparative study with angiotomography. Jornal Vascular Brasileiro. Sociedade Brasileira de Angiologia e Cirurgia Vascular (Avenida Venezuela 131 grupo 509/510, Cep 20081‐311 Rio de Janeiro, Brazil), 2013; Vol. 12, issue 2:102‐9.
Gargiulo 2014 {published data only}
- Gargiulo M, Gallitto E, Serra C, Freyrie A, Mascoli C, Bianchini Massoni C, et al. Could four‐dimensional contrast‐enhanced ultrasound replace computed tomography angiography during follow up of fenestrated endografts? Results of a preliminary experience. European Journal of Vascular and Endovascular Surgery. W.B. Saunders Ltd, 2014; Vol. 48, issue 5:536‐42. [DOI] [PubMed]
Giannoni 2003 {published data only}
- Giannoni MF, Palombo G, Sbarigia E, Speziale F, Zaccaria A, Fiorani P. Contrast‐enhanced ultrasound imaging for aortic stent‐graft surveillance. Journal of Endovascular Therapy 2003;10(2):208‐17. [PUBMED: 12877601] [DOI] [PubMed] [Google Scholar]
Giannoni 2007 {published data only}
- Giannoni MF, Fanelli F, Citone M, Cristina Acconcia M, Speziale F, Gossetti B. Contrast ultrasound imaging: the best method to detect type II endoleak during endovascular aneurysm repair follow‐up. Interactive Cardiovascular and Thoracic Surgery 2007;6(3):359‐62. [PUBMED: 17669866] [DOI] [PubMed] [Google Scholar]
Golzarian 2002 {published data only}
- Golzarian J, Murgo S, Dussaussois L, Guyot S, Said KA, Wautrecht JC, et al. Evaluation of abdominal aortic aneurysm after endoluminal treatment: comparison of color Doppler sonography with biphasic helical CT. American Journal of Roentgenology 2002;178(3):623‐8. [PUBMED: 11856687] [DOI] [PubMed] [Google Scholar]
Gray 2012 {published data only}
- Gray C, Goodman P, Herron CC, Lawler LP, O'Malley MK, O'Donohoe MK, et al. Use of colour duplex ultrasound as a first line surveillance tool following EVAR is associated with a reduction in cost without compromising accuracy. European Journal of Vascular and Endovascular Surgery 2012;44(2):145‐50. [PUBMED: 22717670] [DOI] [PubMed] [Google Scholar]
Gurtler 2013 {published data only}
- Gurtler VM, Sommer WH, Meimarakis G, Kopp R, Weidenhagen R, Reiser MF, et al. A comparison between contrast‐enhanced ultrasound imaging and multislice computed tomography in detecting and classifying endoleaks in the follow‐up after endovascular aneurysm repair. Journal of Vascular Surgery 2013;58(2):340‐5. [PUBMED: 23591188] [DOI] [PubMed] [Google Scholar]
Heilberger 1997 {published data only}
- Heilberger P, Schunn C, Ritter W, Weber S, Raithel D. Postoperative color flow duplex scanning in aortic endografting. Journal of Endovascular Surgery 1997;4(3):262‐71. [PUBMED: 9291051] [DOI] [PubMed] [Google Scholar]
Henao 2006 {published data only}
- Henao EA, Hodge MD, Felkai DD, McCollum CH, Noon GP, Lin PH, et al. Contrast‐enhanced Duplex surveillance after endovascular abdominal aortic aneurysm repair: improved efficacy using a continuous infusion technique. Journal of Vascular Surgery 2006;43(2):259‐64; discussion 264. [PUBMED: 16476596] [DOI] [PubMed] [Google Scholar]
Iezzi 2009 {published data only}
- Iezzi R, Basilico R, Giancristofaro D, Pascali D, Cotroneo AR, Storto ML. Contrast‐enhanced ultrasound versus color duplex ultrasound imaging in the follow‐up of patients after endovascular abdominal aortic aneurysm repair. Journal of Vascular Surgery 2009;49(3):552‐60. [PUBMED: 19135839] [DOI] [PubMed] [Google Scholar]
McLafferty 2002 {published data only}
- McLafferty RB, McCrary BS, Mattos MA, Karch LA, Ramsey DE, Solis MM, et al. The use of color‐flow duplex scan for the detection of endoleaks. Journal of Vascular Surgery 2002;36(1):100‐4. [PUBMED: 12096265] [DOI] [PubMed] [Google Scholar]
McWilliams 1999 {published data only}
- McWilliams RG, Martin J, White D, Gould DA, Harris PL, Fear SC, et al. Use of contrast‐enhanced ultrasound in follow‐up after endovascular aortic aneurysm repair. Journal of Vascular and Interventional Radiology 1999;10(8):1107‐14. [PUBMED: 10496715] [DOI] [PubMed] [Google Scholar]
McWilliams 2002 {published data only}
- McWilliams RG, Martin J, White D, Gould DA, Rowlands PC, Haycox A, et al. Detection of endoleak with enhanced ultrasound imaging: comparison with biphasic computed tomography. Journal of Endovascular Therapy 2002;9(2):170‐9. [PUBMED: 12010096] [DOI] [PubMed] [Google Scholar]
Motta 2012 {published data only}
- Motta R, Rubaltelli L, Vezzaro R, Vida V, Marchesi P, Stramare R, et al. Role of multidetector CT angiography and contrast‐enhanced ultrasound in redefining follow‐up protocols after endovascular abdominal aortic aneurysm repair. La Radiologia Medica 2012;117(6):1079‐92. [PUBMED: 22430681] [DOI] [PubMed] [Google Scholar]
Nagre 2011 {published data only}
- Nagre SB, Taylor SM, Passman MA, Patterson MA, Combs BR, Lowman BG, et al. Evaluating outcomes of endoleak discrepancies between computed tomography scan and ultrasound imaging after endovascular abdominal aneurysm repair. Annals of Vascular Surgery 2011;25(1):94‐100. [PUBMED: 21172584] [DOI] [PubMed] [Google Scholar]
Nerlekar 2006 {published data only}
- Nerlekar R, Warrier R, Ryke R, Miller R, Hewitt PM, Scott A. A comparative study of ultrasound and computed tomography scan for the follow‐up of abdominal aortic aneurysms after endovascular repair. Journal of Vascular Ultrasound 2006; Vol. 30, issue 2:81‐5.
Oikonomou 2012 {published data only}
- Oikonomou K, Ventin FC, Paraskevas KI, Geisselsoder P, Ritter W, Verhoeven EL. Early follow‐up after endovascular aneurysm repair: is the first postoperative computed tomographic angiography scan necessary?. Journal of Endovascular Therapy 2012;19(2):151‐6. [PUBMED: 22545878] [DOI] [PubMed] [Google Scholar]
Pages 2001 {published data only}
- Pages S, Favre JP, Cerisier A, Pyneeandee S, Boissier C, Veyret C. Comparison of color duplex ultrasound and computed tomography scan for surveillance after aortic endografting. Annals of Vascular Surgery 2001;15(2):155‐62. [PUBMED: 11265078] [DOI] [PubMed] [Google Scholar]
Parent 2002 {published data only}
- Parent FN, Meier GH, Godziachvili V, LeSar CJ, Parker FM, Carter KA, et al. The incidence and natural history of type I and II endoleak: a 5‐year follow‐up assessment with color duplex ultrasound scan. Journal of Vascular Surgery 2002;35(3):474‐81. [PUBMED: 11877694] [DOI] [PubMed] [Google Scholar]
Perini 2011 {published data only}
- Perini P, Sediri I, Midulla M, Delsart P, Mouton S, Gautier C, et al. Single‐centre prospective comparison between contrast‐enhanced ultrasound and computed tomography angiography after EVAR. European Journal of Vascular and Endovascular Surgery 2011;42(6):797‐802. [PUBMED: 21962588] [DOI] [PubMed] [Google Scholar]
Perini 2012 {published data only}
- Perini P, Sediri I, Midulla M, Delsart P, Gautier C, Haulon S. Contrast‐enhanced ultrasound vs. CT angiography in fenestrated EVAR surveillance: a single‐center comparison. Journal of Endovascular Therapy 2012;19(5):648‐55. [PUBMED: 23046331] [DOI] [PubMed] [Google Scholar]
Raman 2003 {published data only}
- Raman KG, Missig‐Carroll N, Richardson T, Muluk SC, Makaroun MS. Color‐flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. Journal of Vascular Surgery 2003;38(4):645‐51. [PUBMED: 14560207] [DOI] [PubMed] [Google Scholar]
Sandford 2006 {published data only}
- Sandford RM, Bown MJ, Fishwick G, Murphy F, Naylor M, Sensier Y, et al. Duplex ultrasound scanning is reliable in the detection of endoleak following endovascular aneurysm repair. European Journal of Vascular and Endovascular Surgery 2006;32(5):537‐41. [PUBMED: 16875850] [DOI] [PubMed] [Google Scholar]
Sato 1998 {published data only}
- Sato DT, Goff CD, Gregory RT, Robinson KD, Carter KA, Herts BR, et al. Endoleak after aortic stent graft repair: diagnosis by color duplex ultrasound scan versus computed tomography scan. Journal of Vascular Surgery 1998;28(4):657‐63. [PUBMED: 9786261] [DOI] [PubMed] [Google Scholar]
Schmieder 2009 {published data only}
- Schmieder GC, Stout CL, Stokes GK, Parent FN, Panneton JM. Endoleak after endovascular aneurysm repair: duplex ultrasound imaging is better than computed tomography at determining the need for intervention. Journal of Vascular Surgery 2009;50(5):1012‐7; discussion 1017‐8. [PUBMED: 19878784] [DOI] [PubMed] [Google Scholar]
Ten Bosch 2010 {published data only}
- Bosch JA, Rouwet EV, Peters CT, Jansen L, Verhagen HJ, Prins MH, et al. Contrast‐enhanced ultrasound versus computed tomographic angiography for surveillance of endovascular abdominal aortic aneurysm repair. Journal of Vascular and Interventional Radiology 2010;21(5):638‐43. [PUBMED: 20363153] [DOI] [PubMed] [Google Scholar]
Thompson 1998 {published data only}
- Thompson MM, Boyle JR, Hartshorn T, Maltezos C, Nasim A, Sayers RD, et al. Comparison of computed tomography and duplex imaging in assessing aortic morphology following endovascular aneurysm repair. British Journal of Surgery 1998;85(3):346‐50. [PUBMED: 9529490] [DOI] [PubMed] [Google Scholar]
Wolf 2000 {published data only}
- Wolf YG, Johnson BL, Hill BB, Rubin GD, Fogarty TJ, Zarins CK. Duplex ultrasound scanning versus computed tomographic angiography for postoperative evaluation of endovascular abdominal aortic aneurysm repair. Journal of Vascular Surgery 2000;32(6):1142‐8. [PUBMED: 11107086] [DOI] [PubMed] [Google Scholar]
Zannetti 2000 {published data only}
- Zannetti S, Rango P, Parente B, Parlani G, Verzini F, Maselli A, et al. Role of duplex scan in endoleak detection after endoluminal abdominal aortic aneurysm repair. European Journal of Vascular and Endovascular Surgery 2000;19(5):531‐5. [PUBMED: 10828236] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Almaroof 2013 {published data only}
- Almaroof BH, Ahanchi SS, Chen BL, Day JD, Nelms CR, Panneton JM. Comparison of duplex ultrasound and computed tomography surveillance after endovascular aneurysm repair in determining proximal endograft location. Journal for Vascular Ultrasound 2013;37(1):9‐13. [Google Scholar]
Beeman 2009 {published data only}
- Beeman BR, Doctor LM, Doerr K, McAfee‐Bennett S, Dougherty MJ, Calligaro KD. Duplex ultrasound imaging alone is sufficient for midterm endovascular aneurysm repair surveillance: a cost analysis study and prospective comparison with computed tomography scan. Journal of Vascular Surgery 2009;50(5):1019‐24. [PUBMED: 19656651] [DOI] [PubMed] [Google Scholar]
Bredahl 2013 {published data only}
- Bredahl K, Long A, Taudorf M, Lonn L, Rouet L, Ardon R, et al. Volume estimation of the aortic sac after EVAR using 3‐D ultrasound ‐ a novel, accurate and promising technique. European Journal of Vascular and Endovascular Surgery 2013;45(5):450‐5; discussion 456. [PUBMED: 23433497] [DOI] [PubMed] [Google Scholar]
Chisci 2012 {published data only}
- Chisci E, Setacci F, Iacoponi F, Donato G, Cappelli A, Setacci C. Surveillance imaging modality does not affect detection rate of asymptomatic secondary interventions following EVAR. European Journal of Vascular and Endovascular Surgery 2012;43(3):276‐81. [PUBMED: 22240330] [DOI] [PubMed] [Google Scholar]
Clevert 2008a {published data only}
- Clevert DA, Weckbach S, Kopp R, Meimerakis G, Clevert DA, Jauch KW, et al. Imaging of aortic lesions with color coded duplex sonography and contrast‐enhanced ultrasound versus multislice computed tomography (MS‐CT) angiography. Clinical Hemorheology and Microcirculation 2008;40(4):267‐79. [PUBMED: 19126989] [PubMed] [Google Scholar]
Clevert 2013 {published data only}
- Clevert DA, Gurtler VM, Meimarakis G, D'Anastasi M, Weidenhagen R, Reiser MF, et al. Classification of endoleaks in the follow‐up after EVAR using the time‐to‐peak of the contrast agent in CEUS examinations. Clinical Hemorheology and Microcirculation 2013;55(1):183‐91. [PUBMED: 23455839] [DOI] [PubMed] [Google Scholar]
Collins 2007 {published data only}
- Collins JT, Boros MJ, Combs K. Ultrasound surveillance of endovascular aneurysm repair: a safe modality versus computed tomography. Annals of Vascular Surgery 2007;21(6):671‐5. [PUBMED: 17980791] [DOI] [PubMed] [Google Scholar]
Elkouri 2004 {published data only}
- Elkouri S, Panneton JM, Andrews JC, Lewis BD, McKusick MA, Noel AA, et al. Computed tomography and ultrasound in follow‐up of patients after endovascular repair of abdominal aortic aneurysm. Annals of Vascular Surgery 2004;18(3):271‐9. [PUBMED: 15354627] [DOI] [PubMed] [Google Scholar]
Greenfield 2002 {published data only}
- Greenfield AL, Halpern EJ, Bonn J, Wechsler RJ, Kahn MB. Application of duplex US for characterization of endoleaks in abdominal aortic stent‐grafts: report of five cases. Radiology 2002;225(3):845‐51. [PUBMED: 12461270] [DOI] [PubMed] [Google Scholar]
Han 2010 {published data only}
- Han SM, Patel K, Rowe VL, Perese S, Bond A, Weaver FA. Ultrasound‐determined diameter measurements are more accurate than axial computed tomography after endovascular aortic aneurysm repair. Journal of Vascular Surgery 2010;51(6):1381‐7; discussion 1387‐9. [PUBMED: 20488320] [DOI] [PubMed] [Google Scholar]
Harrison 2011 {published data only}
- Harrison GJ, Oshin OA, Vallabhaneni SR, Brennan JA, Fisher RK, McWilliams RG. Surveillance after EVAR based on duplex ultrasound and abdominal radiography. European Journal of Vascular and Endovascular Surgery 2011;42(2):187‐92. [PUBMED: 21546278] [DOI] [PubMed] [Google Scholar]
Hertault 2015 {published data only}
- Hertault A, Maurel B, Pontana F, Martin‐Gonzalez T, Spear R, Sobocinski J, et al. Benefits of completion 3D angiography associated with contrast enhanced ultrasound to assess technical success after EVAR. European Journal of Vascular and Endovascular Surgery 2015; Vol. 49, issue 5:541‐8. [DOI] [PubMed]
Manning 2009 {published data only}
- Manning BJ, O'Neill SM, Haider SN, Colgan MP, Madhavan P, Moore DJ. Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: a comparison with computed tomography aortography. Journal of Vascular Surgery 2009;49(1):60‐5. [PUBMED: 18829237] [DOI] [PubMed] [Google Scholar]
Millen 2013 {published data only}
- Millen A, Canavati R, Harrison G, McWilliams RG, Wallace S, Vallabhaneni SR, et al. Defining a role for contrast‐enhanced ultrasound in endovascular aneurysm repair surveillance. Journal of Vascular Surgery 2013;58(1):18‐23. [PUBMED: 23490295] [DOI] [PubMed] [Google Scholar]
Napoli 2004 {published data only}
- Napoli V, Bargellini I, Sardella SG, Petruzzi P, Cioni R, Vignali C, et al. Abdominal aortic aneurysm: contrast‐enhanced US for missed endoleaks after endoluminal repair. Radiology 2004;233(1):217‐25. [PUBMED: 15454621] [DOI] [PubMed] [Google Scholar]
Nyheim 2013 {published data only}
- Nyheim T, Staxrud LE, Rosen L, Slagsvold CE, Sandbaek G, Jorgensen JJ. Review of postoperative CT and ultrasound for endovascular aneurysm repair using Talent stent graft: can we simplify the surveillance protocol and reduce the number of CT scans?. Acta Radiologica (Stockholm, Sweden: 1987) 2013;54(1):54‐8. [PUBMED: 23377874] [DOI] [PubMed] [Google Scholar]
Ormesher 2014 {published data only}
Pfister 2009 {published data only}
- Pfister K, Rennert J, Uller W, Schnitzbauer AA, Stehr A, Jung W, et al. Contrast harmonic imaging ultrasound and perfusion imaging for surveillance after endovascular abdominal aneurysm repair regarding detection and characterization of suspected endoleaks. Clinical Hemorheology and Microcirculation 2009;43(1‐2):119‐28. [PUBMED: 19713606] [DOI] [PubMed] [Google Scholar]
Sommer 2012 {published data only}
- Sommer WH, Becker CR, Haack M, Rubin GD, Weidenhagen R, Schwarz F, et al. Time‐resolved CT angiography for the detection and classification of endoleaks. Radiology 2012;263(3):917‐26. [PUBMED: 22623699] [DOI] [PubMed] [Google Scholar]
Sorrentino 2015 {published data only}
- Sorrentino K, Shah PS, Bendick P. Ultrasonographic surveillance of abdominal aortic endovascular aneurysm stent graft repair in a dedicated vascular laboratory. Journal of Diagnostic Medical Sonography. SAGE Publications Inc., 2015; Vol. 31, issue 1:40‐51.
Troutman 2014 {published data only}
Yang 2015 {published data only}
- Yang X, Chen Y‐X, Zhang B, Jiang Y‐X, Liu C‐W, Zhao R‐N, et al. Contrast‐enhanced ultrasound in detecting endoleaks with failed computed tomography angiography diagnosis after endovascular abdominal aortic aneurysm repair. Chinese Medical Journal. Chinese Medical Association, 2015; Vol. 128, issue 18:2491‐7. [DOI] [PMC free article] [PubMed]
Additional references
Adriaensen 2002
- Adriaensen ME, Bosch JL, Halpern EF, Hunink MGM, Gazelle GS. Elective endovascular versus open surgical repair of abdominal aortic aneurysms: systematic review of short‐term results. Radiology 2002;224(3):739‐47. [PUBMED: 12202708] [DOI] [PubMed] [Google Scholar]
Arko 2003
- Arko FR, Filis KA, Siedel SA, Johnson BL, Drake AR, Fogarty TJ, et al. Intrasac flow velocities predict sealing of type II endoleaks after endovascular abdominal aortic aneurysm repair. Journal of Vascular Surgery 2003;37(1):8‐15. [PUBMED: 12514572] [DOI] [PubMed] [Google Scholar]
Ashoke 2005a
- Ashoke R, Brown LC, Rodway A, Choke E, Thompson MM, Greenhalgh RM, et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. Journal of Endovascular Therapy 2005;12(3):297‐305. [PUBMED: 15943504] [DOI] [PubMed] [Google Scholar]
Bakken 2010
- Bakken AM, Illig KA. Long‐term follow‐up after endovascular aneurysm repair: is ultrasound alone enough?. Perspectives in Vascular Surgery and Endovascular Therapy 2010;22(3):145‐51. [PUBMED: 21098495] [DOI] [PubMed] [Google Scholar]
Bashir 2009
- Bashir MR, Ferral H, Jacobs C, McCarthy W, Goldin M. Endoleaks after endovascular abdominal aortic aneurysm repair: management strategies according to CT findings. American Journal of Roentgenology 2009;192(4):W178‐86. [PUBMED: 19304678] [DOI] [PubMed] [Google Scholar]
Beales 2011
- Beales L, Wolstenhulme S, Evans JA, West R, Scott DJ. Reproducibility of ultrasound measurement of the abdominal aorta. British Journal of Surgery 2011;98(11):1517‐25. [PUBMED: 21861264] [DOI] [PubMed] [Google Scholar]
Becquemin 1999
- Becquemin JP, Lapie V, Favre JP, Rousseau H. Mid‐term results of a second generation bifurcated endovascular graft for abdominal aortic aneurysm repair: the French Vanguard trial. Journal of Vascular Surgery 1999;30(2):209‐18. [PUBMED: 10436440] [DOI] [PubMed] [Google Scholar]
Brenner 2007
- Brenner DJ, Hall EJ. Computed tomography ‐ an increasing source of radiation exposure. New England Journal of Medicine 2007;357(22):2277‐84. [PUBMED: 18046031] [DOI] [PubMed] [Google Scholar]
Brown 2012
Bush 2001
- Bush RL, Lumsden AB, Dodson TF, Salam AA, Weiss VJ, Smith RB 3rd, et al. Mid‐term results after endovascular repair of the abdominal aortic aneurysm. Journal of Vascular Surgery 2001;33(2 Suppl):S70‐6. [PUBMED: 11174815] [DOI] [PubMed] [Google Scholar]
Cejna 2002
- Cejna M, Loewe C, Schoder M, Dirisamer A, Holzenbein T, Kretschmer G, et al. MR angiography vs CT angiography in the follow‐up of nitinol stent grafts in endoluminally treated aortic aneurysms. European Radiology 2002;12(10):2443‐50. [PUBMED: 12271383] [DOI] [PubMed] [Google Scholar]
Cuypers 1999
- Cuypers P, Buth J, Harris PL, Gevers E, Lahey R. Realistic expectations for patients with stent‐graft treatment of abdominal aortic aneurysms. Results of a European multicentre registry. European Journal of Vascular and Endovascular Surgery 1999;17(6):507‐16. [PUBMED: 10377023] [DOI] [PubMed] [Google Scholar]
De Bruin 2010
- Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. Long‐term outcome of open or endovascular repair of abdominal aortic aneurysm. New England Journal of Medicine 2010;362(20):1881‐9. [PUBMED: 20484396] [DOI] [PubMed] [Google Scholar]
Engellau 1998
- Engellau L, Larsson EM, Albrechtsson U, Jonung T, Ribbe E, Thörne J, et al. Magnetic resonance imaging and MR angiography of endoluminally treated abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery 1998;15(3):212‐9. [DOI] [PubMed] [Google Scholar]
Erbel 2014
- Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo R, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. European Heart Journal 2014;35(41):2873‐926. [DOI] [PubMed] [Google Scholar]
Fagan 1975
- Fagan TJ. Letter: nomogram for Bayes theorem. New England Journal of Medicine 1975;293(5):257. [PUBMED: 1143310] [DOI] [PubMed] [Google Scholar]
Faries 2003
- Faries PL, Cadot H, Agarwal G, Kent KC, Hollier LH, Marin ML. Management of endoleak after endovascular aneurysm repair: cuffs, coils, and conversion. Journal of Vascular Surgery 2003;37(6):1155‐61. [PUBMED: 12764258] [DOI] [PubMed] [Google Scholar]
Forsdahl 2009
- Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7‐year prospective study: the Tromso Study, 1994‐2001. Circulation 2009;119(16):2202‐8. [PUBMED: 19364978] [DOI] [PubMed] [Google Scholar]
Franco 2000
- Franco TJ, Zajko AB, Federle MP, Makaroun MS. Endovascular repair of the abdominal aortic aneurysm with the Ancure endograft: CT follow‐up of perigraft flow and aneurysm size at 6 months. Journal of Vascular and Interventional Radiology 2000;11(4):429‐35. [PUBMED: 10787200] [DOI] [PubMed] [Google Scholar]
Gilling‐Smith 2000
- Gilling‐Smith GL, Martin J, Sudhindran S, Gould DA, McWilliams RG, Bakran A, et al. Freedom from endoleak after endovascular aneurysm repair does not equal treatment success. European Journal of Vascular and Endovascular Surgery 2000;19(4):421‐5. [PUBMED: 10801377] [DOI] [PubMed] [Google Scholar]
Golzarian 1997
- Golzarian J, Struyven J, Abada HT, Wery D, Dussaussois L, Madani A, et al. Endovascular aortic stent‐grafts: transcatheter embolization of persistent perigraft leaks. Radiology 1997;202(3):731‐4. [PUBMED: 9051026] [DOI] [PubMed] [Google Scholar]
Gorich 2000
- Gorich J, Rilinger N, Sokiranski R, Kramer SC, Ermis C, Schutz A, et al. Treatment of leaks after endovascular repair of aortic aneurysms. Radiology 2000;215(2):414‐20. [PUBMED: 10796918] [DOI] [PubMed] [Google Scholar]
Gorich 2001
- Gorich J, Rilinger N, Sokiranski R, Soldner J, Kaiser W, Kramer S, et al. Endoleaks after endovascular repair of aortic aneurysm: are they predictable? ‐ initial results. Radiology 2001;218(2):477‐80. [PUBMED: 11161165] [DOI] [PubMed] [Google Scholar]
Greenhalgh 2004
- Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30‐day operative mortality results: randomised controlled trial. Lancet 2004;364(9437):843‐8. [PUBMED: 15351191] [DOI] [PubMed] [Google Scholar]
Greenhalgh 2010
- United Kingdom EVAR Trial Investigators. Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. Endovascular versus open repair of abdominal aortic aneurysm. New England Journal of Medicine 2010;362(20):1863‐71. [PUBMED: 20382983] [DOI] [PubMed] [Google Scholar]
Harris 2000
- Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, Marrewijk C, Laheij RJ. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. Journal of Vascular Surgery 2000;32(4):739‐49. [PUBMED: 11013038] [DOI] [PubMed] [Google Scholar]
Haulon 2001
- Haulon S, Lions C, McFadden EP, Koussa M, Gaxotte V, Halna P, et al. Prospective evaluation of magnetic resonance imaging after endovascular treatment of infrarenal aortic aneurysms. European Journal of Vascular and Endovascular Surgery 2001;22(1):62‐9. [DOI] [PubMed] [Google Scholar]
Hechelhammer 2005
- Hechelhammer L, Wildermuth S, Lachat ML, Pfammatter T. Endovascular repair of inflammatory abdominal aneurysm: a retrospective analysis of CT follow‐up. Journal of Vascular and Interventional Radiology 2005;16(5):737‐41. [PUBMED: 15872330] [DOI] [PubMed] [Google Scholar]
Hemminki 2006
- Hemminki K, Li X, Johansson SE, Sundquist K, Sundquist J. Familial risks of aortic aneurysms among siblings in a nationwide Swedish study. Genetics in Medicine 2006;8(1):43‐9. [PUBMED: 16418598] [DOI] [PubMed] [Google Scholar]
Hinchliffe 2001
- Hinchliffe RJ, Singh‐Ranger R, Davidson IR, Hopkinson BR. Rupture of an abdominal aortic aneurysm secondary to type II endoleak. European Journal of Vascular and Endovascular Surgery 2001;22(6):563‐5. [PUBMED: 11735209] [DOI] [PubMed] [Google Scholar]
Iezzi 2006
- Iezzi R, Cotroneo AR, Filippone A, Fabio F, Quinto F, Colosimo C, et al. Multidetector CT in abdominal aortic aneurysm treated with endovascular repair: are unenhanced and delayed phase enhanced images effective for endoleak detection?. Radiology 2006;241(3):915‐21. [PUBMED: 17032909] [DOI] [PubMed] [Google Scholar]
Insko 2003
- Insko EK, Kulzer LM, Fairman RM, Carpenter JP, Stavropoulos SW. MR imaging for the detection of endoleaks in recipients of abdominal aortic stent‐grafts with low magnetic susceptibility. Academic Radiology 2003;10(5):509‐13. [PUBMED: 12755539] [DOI] [PubMed] [Google Scholar]
Karch 1999
- Karch LA, Henretta JP, Hodgson KJ, Mattos MA, Ramsey DE, McLafferty RB, et al. Algorithm for the diagnosis and treatment of endoleaks. American Journal of Surgery 1999;178(3):225‐31. [PUBMED: 10527444] [DOI] [PubMed] [Google Scholar]
Karthikesalingam 2012
- Karthikesalingam A, Al‐Jundi W, Jackson D, Boyle JR, Beard JD, Holt PJ, et al. Systematic review and meta‐analysis of duplex ultrasonography, contrast‐enhanced ultrasonography or computed tomography for surveillance after endovascular aneurysm repair. British Journal of Surgery 2012;99(11):1514‐23. [PUBMED: 23001681] [DOI] [PubMed] [Google Scholar]
Kristmundsson 2014
- Kristmundsson T, Sonesson B, Dias N, Malina M, Resch T. Anatomic suitability for endovascular repair of abdominal aortic aneurysms and possible benefits of low profile delivery systems. Vascular 2014;22(2):112‐5. [PUBMED: 23518837] [DOI] [PubMed] [Google Scholar]
Lawrence‐Brown 2009
- Lawrence‐Brown MM, Sun Z, Semmens JB, Liffman K, Sutalo ID, Hartley DB. Type II endoleaks: when is intervention indicated and what is the index of suspicion for types I or III?. Journal of Endovascular Therapy 2009;16 Suppl 1:I106‐18. [PUBMED: 19317572] [DOI] [PubMed] [Google Scholar]
Leeflang 2014
- Leeflang MM. Systematic reviews and meta‐analyses of diagnostic test accuracy. Clinical Microbiology and Infection 2014;20(2):105‐13. [PUBMED: 24274632] [DOI] [PubMed] [Google Scholar]
Leurs 2004
- Leurs LJ, Hobo R, Buth J. EUROSTAR Collaborators. The multicenter experience with a third‐generation endovascular device for abdominal aortic aneurysm repair. A report from the EUROSTAR database. Journal of Cardiovascular Surgery 2004;45(4):293‐300. [PUBMED: 15365511] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
Makaroun 1999
- Makaroun M, Zajko A, Sugimoto H, Eskandari M, Webster M. Fate of endoleaks after endoluminal repair of abdominal aortic aneurysms with the EVT device. European Journal of Vascular and Endovascular Surgery 1999;18(3):185‐90. [PUBMED: 10479624] [DOI] [PubMed] [Google Scholar]
Mirza 2010
- Mirza TA, Karthikesalingam A, Jackson D, Walsh SR, Holt PJ, Hayes PD, et al. Duplex ultrasound and contrast‐enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: systematic review and bivariate meta‐analysis. European Journal of Vascular and Endovascular Surgery 2010;39(4):418‐28. [PUBMED: 20122853] [DOI] [PubMed] [Google Scholar]
Moll 2011
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. European Journal of Vascular and Endovascular Surgery 2011;41 Suppl 1:S1‐S58. [PUBMED: 21215940] [DOI] [PubMed] [Google Scholar]
Ogata 2005
- Ogata T, MacKean GL, Cole CW, Arthur C, Andreou P, Tromp G, et al. The lifetime prevalence of abdominal aortic aneurysms among siblings of aneurysm patients is eightfold higher than among siblings of spouses: an analysis of 187 aneurysm families in Nova Scotia, Canada. Journal of Vascular Surgery 2005;42(5):891‐7. [PUBMED: 16275443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pippin 2016
- Pippin K, Hill J, He J, Johnson P. Outcomes of type II endoleaks after endovascular abdominal aortic aneurysm (AAA) repair: a single‐center, retrospective study. Clinical Imaging 2016;40(5):875‐9. [PUBMED: 27179957] [DOI] [PubMed] [Google Scholar]
Prinssen 2004
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. New England Journal of Medicine 2004;351(16):1607‐18. [PUBMED: 15483279] [DOI] [PubMed] [Google Scholar]
Rayt 2009
- Rayt HS, Sandford RM, Salem M, Bown MJ, London NJ, Sayers RD. Conservative management of type 2 endoleaks is not associated with increased risk of aneurysm rupture. European Journal of Vascular and Endovascular Surgery 2009;38(6):718‐23. [PUBMED: 19767222] [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [PUBMED: 16168343] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhee 2003
- Rhee SJ, Ohki T, Veith FJ, Kurvers H. Current status of management of type II endoleaks after endovascular repair of abdominal aortic aneurysms. Annals of Vascular Surgery 2003;17(3):335‐44. [PUBMED: 12712372] [DOI] [PubMed] [Google Scholar]
Sampram 2003
- Sampram ES, Karafa MT, Mascha EJ, Clair DG, Greenberg RK, Lyden SP, et al. Nature, frequency, and predictors of secondary procedures after endovascular repair of abdominal aortic aneurysm. Journal of Vascular Surgery 2003;37(5):930‐7. [PUBMED: 12756335] [DOI] [PubMed] [Google Scholar]
SAS 2008 [Computer program]
- SAS Institute Inc.. SAS. Version 9.2. Cary, NC: SAS Institute Inc., 2008.
Schurink 1998
- Schurink GW, Aarts NJ, Wilde J, Baalen JM, Chuter TA, Schultze Kool LJ, et al. Endoleakage after stent‐graft treatment of abdominal aneurysm: implications on pressure and imaging ‐ an in vitro study. Journal of Vascular Surgery 1998;28(2):234‐41. [PUBMED: 9719318] [DOI] [PubMed] [Google Scholar]
Singh 2001
- Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population‐based study: the Tromso Study. American Journal of Epidemiology 2001;154(3):236‐44. [PUBMED: 11479188] [DOI] [PubMed] [Google Scholar]
Stengel 2003
- Stengel D, Bauwens K, Sehouli J, Ekkernkamp A, Porzsolt F. A likelihood ratio approach to meta‐analysis of diagnostic studies. Journal of Medical Screening 2003;10(1):47‐51. [PUBMED: 12790315] [DOI] [PubMed] [Google Scholar]
Stolzmann 2008
- Stolzmann P, Frauenfelder T, Pfammatter T, Peter N, Scheffel H, Lachat M, et al. Endoleaks after endovascular abdominal aortic aneurysm repair: detection with dual‐energy dual‐source CT. Radiology 2008;249(2):682‐91. [PUBMED: 18780822] [DOI] [PubMed] [Google Scholar]
Sun 2006
- Sun Z. Diagnostic value of color duplex ultrasonography in the follow‐up of endovascular repair of abdominal aortic aneurysm. Journal of Vascular and Interventional Radiology 2006;17(5):759‐64. [PUBMED: 16687740] [DOI] [PubMed] [Google Scholar]
Tolia 2005
- Tolia AJ, Landis R, Lamparello P, Rosen R, Macari M. Type II endoleaks after endovascular repair of abdominal aortic aneurysms: natural history. Radiology 2005;235(2):683‐6. [PUBMED: 15758193] [DOI] [PubMed] [Google Scholar]
Vallabhaneni 2001
- Vallabhaneni SR, Harris PL. Lessons learnt from the EUROSTAR registry on endovascular repair of abdominal aortic aneurysm repair. European Journal of Radiology 2001;39(1):34‐41. [PUBMED: 11439229] [DOI] [PubMed] [Google Scholar]
Van der Laan 2006
- Laan MJ, Bartels LW, Viergever MA, Blankensteijn JD. Computed tomography versus magnetic resonance imaging of endoleaks after EVAR. European Journal of Vascular and Endovascular Surgery 2006;32(4):361‐5. [PUBMED: 16630731] [DOI] [PubMed] [Google Scholar]
Whiting 2003
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2004
- Wilson WR, Fishwick G, Bell PRG, Thompson MM. Suitability of ruptured AAA for endovascular repair. Journal of Endovascular Therapy 2004;11(6):635‐40. [PUBMED: 15615554] [DOI] [PubMed] [Google Scholar]
Zarins 2000
- Zarins CK, White RA, Hodgson KJ, Schwarten D, Fogarty TJ. Endoleak as a predictor of outcome after endovascular aneurysm repair: AneuRx multicenter clinical trial. Journal of Vascular Surgery 2000;32(1):90‐107. [PUBMED: 10876210] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abraha 2013
- Abraha I, Florio R, Casazza G, Duca P, Parente B, Cozzolino F, et al. Ultrasonography for endoleak detection after endoluminal abdominal aortic aneurysm repair. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010296] [DOI] [PMC free article] [PubMed] [Google Scholar]


