Abstract
Background
Survivors of stroke due to intracerebral haemorrhage (ICH) are at risk of thromboembolism. Antithrombotic (antiplatelet or anticoagulant) treatments may lower the risk of thromboembolism after ICH, but they may increase the risks of bleeding.
Objectives
To determine the overall effectiveness and safety of antithrombotic drugs for people with ICH.
Search methods
We searched the Cochrane Stroke Group Trials Register (24 March 2017). We also searched the Cochrane Central Register of Controlled Trials (CENTRAL: the Cochrane Library 2017, Issue 3), MEDLINE Ovid (from 1948 to March 2017), Embase Ovid (from 1980 to March 2017), and online registries of clinical trials (8 March 2017). We also screened the reference lists of included trials for additional, potentially relevant studies.
Selection criteria
We selected all randomised controlled trials (RCTs) of any antithrombotic treatment after ICH.
Data collection and analysis
Three review authors independently extracted data. We converted categorical estimates of effect to the risk ratio (RR) or odds ratio (OR), as appropriate. We divided our analyses into short‐ and long‐term treatment, and used fixed‐effect modelling for meta‐analyses. Three review authors independently assessed the included RCTs for risks of bias and we created a 'Summary of findings' table using GRADE.
Main results
We included two RCTs with a total of 121 participants. Both RCTs were of short‐term parenteral anticoagulation early after ICH: one tested heparin and the other enoxaparin. The risk of bias in the included RCTs was generally unclear or low, with the exception of blinding of participants and personnel, which was not done. The included RCTs did not report our chosen primary outcome (a composite outcome of all serious vascular events including ischaemic stroke, myocardial infarction, other major ischaemic event, ICH, major extracerebral haemorrhage, and vascular death). Parenteral anticoagulation did not cause a statistically significant difference in case fatality (RR 1.25, 95% confidence interval (CI) 0.38 to 4.07 in one RCT involving 46 participants, low‐quality evidence), ICH, or major extracerebral haemorrhage (no detected events in one RCT involving 75 participants, low‐quality evidence), growth of ICH (RR 1.64, 95% CI 0.51 to 5.29 in two RCTs involving 121 participants, low‐quality evidence), deep vein thrombosis (RR 0.99, 95% CI 0.49 to 1.96 in two RCTs involving 121 participants, low quality evidence), or major ischaemic events (RR 0.54, 95% CI 0.23 to 1.28 in two RCTs involving 121 participants, low quality evidence).
Authors' conclusions
There is insufficient evidence from RCTs to support or discourage the use of antithrombotic treatment after ICH. RCTs comparing starting versus avoiding antiplatelet or anticoagulant drugs after ICH appear justified and are needed in clinical practice.
Plain language summary
Drugs to prevent clots after bleeding in the brain
Review question
What is the effectiveness and safety of medications used to prevent clots (antithrombotic treatments) both in the early stages and long‐term in people who have had a bleed within their brain (intracerebral haemorrhage)?
Background
People with stroke due to bleeding in the brain (also known as intracerebral haemorrhage: ICH) are more likely to develop clots in their blood vessels due to immobility (in the early stages) and due to other medical conditions (in the long term). Blood clots in the lungs, brain, or other organs can cause serious illness or death. Drugs that prevent clots (also known as 'antithrombotic drugs') might be useful to stop clot formation in people with ICH. However, these drugs can also cause serious bleeding complications.
Study characteristics
From extensive searches conducted on 8 March 2017, we identified two relevant randomised controlled trials (RCTs), which are the fairest tests of treatment. There were 121 participants in these two trials, which compared blood‐thinning 'anticoagulant' drugs (heparin in one and enoxaparin in the other) delivered by injections under the skin versus no anticoagulant drug soon after ICH.
Key results
The primary outcome of this review was the combined risk of several important clinical outcome events (such as another intracerebral haemorrhage, ischaemic stroke, or death from a cardiovascular cause). We were not able to calculate this outcome for the included studies. Neither RCT reported on recovery of independence or mental abilities. One RCT involving 46 participants reported on case fatality associated with short‐term antithrombotic treatment, and did not find a statistically meaningful effect. For the consequences of treatment that could be analysed, the risk estimates were imprecise and uncertain. Therefore, the potential benefits and harms of antithrombotic drugs soon after a stroke due to bleeding in the brain remain unclear. New high‐quality RCTs investigating the use of antithrombotic treatment after stroke due to ICH appear justified and are needed.
Quality of the evidence
The overall quality of the evidence was low. This is due to the way the included trials were conducted and reported, as well as the small number of participants, which may not have been high enough to detect small differences between the antithrombotic treatment and no antithrombotic treatment groups.
Summary of findings
Summary of findings for the main comparison. Antithrombotic treatment compared to no antithrombotic treatment for intracerebral haemorrhage.
| Short‐term antithrombotic treatment | ||||||
| Patient or population: people with intracerebral haemorrhage Setting: any Intervention: antithrombotic treatment (parenteral anticoagulation) Comparison: no antithrombotic treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no antithrombotic treatment | Risk with Antithrombotic treatment | |||||
| Composite vascular endpoint | Study population | not estimable | (0 studies) | ‐ | We were unable to calculate the composite endpoint because no included study reported all component endpoints. | |
| Death | Study population | RR 1.25 (0.38 to 4.07) | 46 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ‐ | |
| 174 per 1,000 | 217 per 1,000 (66 to 708) | |||||
| Growth of ICH | Study population | RR 1.64 (0.51 to 5.29) | 121 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ‐ | |
| 51 per 1,000 | 83 per 1,000 (26 to 269) | |||||
| ICH | Study population | not estimable | 75 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ‐ | |
| 0 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Major extracerebral haemorrhage | Study population | not estimable | 75 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ‐ | |
| 0 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Deep vein thrombosis | Study population | RR 0.99 (0.46 to 1.96) | 121 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ‐ | |
| 186 per 1,000 | 185 per 1,000 (86 to 365) | |||||
| Other major ischaemic events | Study population | RR 0.54 (0.23 to 1.28) | 121 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ‐ | |
| 186 per 1,000 | 101 per 1,000 (43 to 239) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1This included RCT/s has serious risk of bias due to it not describing random sequence generation or allocation concealment, as well as lack of blinding.
2The optimal information size (OIS) criterion is not met. The sample size of this study is probably lower than the minimum number of participants required for a trial adequately powered to identify a statistically significant difference for this outcome.
Background
Description of the condition
Stroke due to spontaneous (non‐traumatic) intracerebral haemorrhage (ICH) is caused by the extravasation of blood into brain parenchyma following rupture of a cerebral artery or arteriole. The Global Burden of Disease (GBD) Study estimated that in the world in 2010 there were 5.32 million incident (first ever) haemorrhagic strokes (i.e. ICH and subarachnoid haemorrhage), resulting in an incidence of 82 (95% confidence interval (CI) 72 to 93) per 100,000 person‐years (Krishnamurthi 2010). Hypertension is the strongest risk factor for ICH, with a population attributable risk of approximately 50% (O'Donnell 2016). Over recent decades, the prevalence of the use of antithrombotic (antiplatelet or anticoagulant) drug use at the time of ICH has increased to approximately 50% (Béjot 2013; Lovelock 2007). Population‐based case‐control studies report that, among people with ICH, about 20% were taking anticoagulants and 30% were taking antiplatelet agents at the onset of symptoms, whereas among populations free of ICH, about 6% were taking anticoagulants and about 23% were taking antiplatelet agents (Lauer 2013).
Survivors of ICH are at risk of recurrent ICH, particularly if the underlying cause of the incident ICH is not treated (e.g. hypertension, arteriovenous malformation). They are also at risk of ischaemic stroke, ischaemic heart disease, atrial fibrillation, and systemic embolism.
A review of observational studies found that the annual risk of recurrent ICH was between 1.8% and 7.4% (Poon 2014). Three studies compared outcomes after ICH in different locations within the brain, two of which found that the risk of recurrence after lobar ICH appeared higher than after deep ICH (Poon 2014). Most patients in the world have ICH diagnosis and location confirmed by computed tomography (CT). However, some patients have magnetic resonance imaging (MRI), on which brain microbleeds are biomarkers of a higher risk of both recurrent ischaemic stroke and ICH (Cordonnier 2007; Wilson 2016). In studies that quantified the risks of both recurrent ICH and ischaemic stroke after ICH, these risks appeared similar (Poon 2014).
Description of the intervention
Antithrombotic (anticoagulant and antiplatelet) drugs are effective for preventing thrombosis and embolism of clots in the brain, heart, limbs, and lungs. However, their effects are unknown in people who also have a past history of ICH, because they were excluded from the RCTs that demonstrated the benefits of antithrombotic drugs. There is uncertainty about whether to start these drugs after ICH (Al‐Shahi Salman 2014; Falcone 2014; Molina 2011; Steiner 2011).
Consequently, there is variation in clinical practice: the proportion of patients starting antithrombotic drugs after ICH varies from 11% to 45% in different countries (Pasquini 2014).
The indications for restarting antithrombotic drugs after ICH may include the presence of prevalent risk factors for thromboembolism (e.g. atrial fibrillation, valvular heart disease, and atherosclerosis). The indications for starting antithrombotic drugs for the first time after ICH may include new risk factors for thromboembolism (e.g. immobility, or new atrial fibrillation) or vaso‐occlusive events (e.g. deep vein thrombosis and pulmonary embolism). Antithrombotic drugs after ICH can be given for prophylaxis over the short term (e.g. immobility during the period of hospitalisation following ICH) with low doses of low‐molecular‐weight heparin or unfractionated heparin, or over the long term (e.g. atrial fibrillation, previous vaso‐occlusive disease) with oral antiplatelet or full‐dose oral anticoagulant drugs.
Why it is important to do this review
A previous systematic review identified three RCTs that included a subset of people who received antithrombotic treatment after ICH, but it did not identify any clear benefits or harms of doing so (Keir 2002).
Some observational studies have addressed the use of antithrombotic drugs after ICH, but did not identify associations with beneficial or adverse outcomes that were large enough to preclude the need for RCTs (Chao 2016; Chong 2012; Claassen 2008; De Vleeschouwer 2005; Flynn 2010a; Hawryluk 2010; Kuramatsu 2015; Nielsen 2015; Pennlert 2016; Poli 2014; Viswanathan 2006).
Whether and when to use antithrombotic drugs after ICH was identified as a dilemma in the European Stroke Organisation's recent ICH guideline (Steiner 2014), which was unable to make any strong recommendations about the use of these drugs.
Objectives
Primary objective
To determine the overall effectiveness and safety of antithrombotic drugs for people with ICH.
Secondary objective
To determine whether the effectiveness and safety of antithrombotic treatment differs in prespecified subgroups (see Subgroup analysis and investigation of heterogeneity).
Methods
Criteria for considering studies for this review
Types of studies
We sought all RCTs that made comparisons of starting versus avoiding antithrombotic drugs, or direct comparisons of different classes of antithrombotic drugs, for preventing thromboembolism after ICH. We included RCTs published in any language and planned to arrange translation where the language of publication was not English.
Types of participants
We sought RCTs whose participants survived a spontaneous ICH that had been diagnosed by CT or MRI. We included RCTs whether or not they had taken antithrombotic drugs at the time of ICH.
Types of interventions
We sought RCTs that compared the use of any antithrombotic drug against no antithrombotic treatment, as well as RCTs that compared different antithrombotic drugs. We placed no constraints on dosage, route of administration, or duration of administration. We separated RCTs investigating short‐term treatment (for example, with low‐molecular‐weight heparin or unfractionated heparin in the acute post‐stroke phase) and those investigating long‐term treatment (for example, with oral anticoagulants or antiplatelets).
We excluded RCTs in which the effects of antithrombotic drugs might have been confounded by the administration of another active drug to participants (e.g. if allocation to this additional treatment was not evenly distributed between groups in an RCT, or was not randomly allocated).
Types of outcome measures
Primary outcomes
Composite outcome of 'all serious vascular events' (ischaemic stroke, myocardial infarction, other major ischaemic event, ICH, major extracerebral haemorrhage, and vascular death) during the scheduled follow‐up period.
Secondary outcomes
Death during the scheduled follow‐up period.
The individual components of the composite vascular outcome: ischaemic stroke, myocardial infarction, other major ischaemic event, ICH, major extracerebral haemorrhage, and vascular death.
Growth of ICH.
Deep vein thrombosis.
Functional status (where measured using validated scales) at the end of the scheduled follow‐up period.
Cognitive status (where measured using validated scales) at the end of the scheduled follow‐up period.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for RCTs in any language and planned to arrange for the translation of relevant articles where necessary.
Electronic searches
We searched the Cochrane Stroke Group trials register in March 2017 and the following electronic databases, using search strategies that the Cochrane Stroke Group's Information Specialist helped us design:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 3) in the Cochrane Library (searched 8 March 2017) (Appendix 1);
MEDLINE Ovid (1946 to 8 March 2017) (Appendix 2);
Embase Ovid (1974 to 8 March 2017) (Appendix 3).
We also searched the following trials registers:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 2 March 2017) (Appendix 4);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch; searched 2 March 2017) (Appendix 4);
ISRCTN Registry (www.isrctn.com; searched 2 March 2017) (Appendix 4);
Stroke Trials Registry – the Internet Stroke Center (www.strokecenter.org/trials/; searched 2 March 2017) (Appendix 4).
Searching other resources
We screened the reference lists of relevant studies to identify further studies for potential inclusion in the review. We also used the Science Citation Index Cited Reference Search for forward tracking of relevant articles.
Data collection and analysis
Selection of studies
Three review authors (LAP, JB, EF) independently screened the titles and abstracts of the references obtained as a result of our searching activities, and excluded reports that were obviously irrelevant. We retrieved full‐text articles for the remaining references, and the same three review authors independently screened these full‐text articles to identify studies for inclusion, and identified and recorded reasons for the exclusion of ineligible studies. We resolved any disagreements through discussion or, if required, we planned on consulting a fourth review author (EB). We collated multiple reports of the same study so that each study, not each reference, is the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram (See Figure 1 in the Results of the search section).
Data extraction and management
Three review authors (LAP, JB, EF) independently extracted data from included studies using a preformulated data collection form.
Assessment of risk of bias in included studies
Three review authors (LAP, JB, EF) independently assessed risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (EB). We assessed the risks of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other potential bias
We graded the risk of bias for each domain as high, low or unclear and provide information from the study report, together with a justification for our judgement, in the 'Risk of bias' tables.
Measures of treatment effect
We intended to:
convert categorical estimates of effect to the risk ratio (RR) or odds ratio (OR), if required;
express measures of survival as a hazard ratio (HR); and
use summary measures obtained from univariate or, where RCTs used the same covariates for adjustment, multivariable analyses.
Unit of analysis issues
Repeated observations on participants
We planned to analyse functional and cognitive status at end of follow‐up, and not to use repeat observations during follow‐up. If one RCT (or only a few RCTs) had a much longer period of follow‐up than the majority of RCTs (for example, two years compared with six months in the majority of trials) we intended to perform a sensitivity analysis using the six‐month observations from all RCTs.
Events that may recur
We analysed the first event in all participants, and not later events.
Multiple intervention groups
Where a RCT contained multiple treatment groups that were all compared with just one control group, we intended to ensure that the placebo group was shared between the multiple treatment groups by dividing it into the appropriate number of subgroups and conducting separate, independent comparisons.
Dealing with missing data
We contacted RCT authors for unpublished data if relevant data were missing. If only a minority (less than half) of data were missing, we planned to ignore the missing data and perform a 'complete set analysis'. If more substantial amounts of data were missing, and there was a chance that data were not missing at random, we planned to perform sensitivity analyses assuming both a best‐case and a worst‐case scenario, or apply statistical imputation or models, or both, to account for the missing data. A best‐case scenario meant that we assumed that all missing data in the intervention group represent good outcomes and all missing data in the control group represent poor outcomes. A worst‐case scenario meant that we assumed that all missing data in the intervention group represent poor outcomes and all missing data in the control group represent good outcomes.
Assessment of heterogeneity
We investigated inconsistency between included RCTs using the I2 statistic. We interpreted this value using the guide provided in Chapter 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: represents considerable heterogeneity
If we observed substantial inconsistency in our data, we intended to use sensitivity analysis to elucidate which factors might explain the inconsistency.
Assessment of reporting biases
We included all published and unpublished data and secondary publications from RCTs. If we included a sufficient number of RCTs (more than 10), we planned to assess the likelihood of reporting biases through the use of a funnel plot.
Data synthesis
If RCTs were sufficiently similar, we planned to conduct a meta‐analysis by pooling the appropriate data using Review Manager 5 (RevMan 2014). We planned to calculate the risk ratio (RR), odds ratio (OR), or hazard ratio (HR) for each outcome from data extracted from included RCTs using the Peto fixed‐effect method, or a random‐effects model if there was significant heterogeneity between RCTs. We defined significant heterogeneity as substantial clinical or methodological diversity between RCTs such that the true effect measure is no longer uniform.
'Summary of findings' table
We created a ‘Summary of findings’ table using the GRADEpro Guideline Development Tool (GRADEpro GDT), and included the following outcomes for short‐term antithrombotic treatment: the composite outcome of all serious vascular events, death, growth of ICH, ICH, major extracerebral haemorrhage, deep vein thrombosis, other major ischaemic events, functional status, and cognitive status. We planned a 'Summary of findings' table for long‐term antithrombotic treatment which included the following outcomes: the composite outcome of all serious vascular events, death, functional status, and cognitive status. Two review authors (LAP, JB) independently classified the quality of the evidence as being 'high', 'moderate', 'low', or 'very low', based on the presence and extent of the following five criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
Limitations in the design and implementation of the contributing trials
Indirectness of evidence
Unexplained heterogeneity or inconsistency of results
Imprecision of results
High probability of publication bias
We provided justification in the footnotes when we downgraded the quality from 'high'.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses, if possible.
Participants with different age, sex, and stroke severity
Different classes of antithrombotic drugs
Different intensities of antithrombotic treatment
Different times of starting treatment (e.g. within one month of ICH versus later, or within 10 to 30 weeks or later)
Participants who were on antithrombotic treatment before ICH (re‐starters) versus participants who were not receiving these treatments (starters)
Different levels of risk for future ischaemic events (for example, because of differences in age, sex, history of hypertension, history of atrial fibrillation (with further stratification by the CHA2DS2‐Vasc score))
Different levels of risk for future ICH
Biomarkers of bleeding or clotting risk on brain CT or MRI (e.g. brain microbleeds on MRI)
Sensitivity analysis
If the results were heterogeneous, we planned to use sensitivity analysis to investigate how the results differed when we excluded RCTs that were found to have a high risk of bias. We planned to perform other sensitivity analyses to explore reasons for heterogeneity, for example, where there is an active therapy other than an antithrombotic drug that is not balanced by the randomisation process.
Results
Description of studies
We included two RCTs (see Included studies), excluded seven RCTs (see Excluded studies), and identified seven ongoing RCTs (see Ongoing studies). For comprehensive tabulated descriptions of included, excluded, and ongoing studies please refer to Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
Our searches of CENTRAL, Embase, and OVID MEDLINE identified 331, 2557, and 850 potentially relevant records, respectively. Automated de‐duplication identified 904 redundant records, leaving 2834 unique and potentially relevant records. We screened the titles and abstracts of these 2834 records and assessed the full texts of nine relevant records for inclusion (Boeer 1991; CAST 1997; Dickmann 1988; Frontera 2014; IST 1997; Li 2013; Orken 2009; Venturelli 2014; Yan 2014). We included two RCTs in our review (Dickmann 1988; Orken 2009). A reference search of included RCTs yielded no further relevant records. See Figure 1 for a graphical representation of our search.
1.
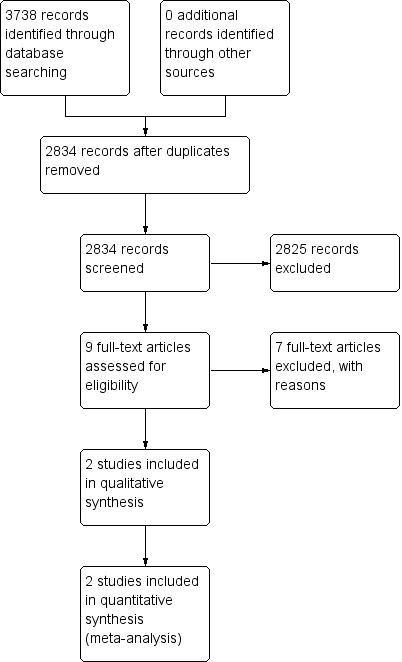
Study flow diagram.
The search of trials registries for ongoing RCTs identified three relevant RCTs: APACHE‐AF; PICASSO; RESTART, and we were aware of four additional RCTs from personal communication with investigators (NASPAF‐ICH; RESTART Nord de France; SoSTART; STATICH).
Included studies
Dickmann 1988 was a German single‐centre study that excluded patients if they had a bleeding diathesis, a sustained diastolic blood pressure higher than 120 mmHg or were in deep coma with signs of brain herniation, and retained 46 participants for inclusion.
Orken 2009 was a Turkish single‐centre study that started with 110 ICH patients and applied the following exclusion criteria: early death before heparin treatment (n = 13), death before day 7 investigations (n = 4), secondary ICH due to aneurysm, arteriovenous malformations, trauma, or tumour (n = 11), excessive anticoagulation (International normalised ratio (INR) > 2.0) (n = 5), and contraindication to contrast media (n = 2). This left 75 participants for inclusion.
Both of the included RCTs included participants with ICH diagnosed by CT. Orken 2009 specified that these were primary ICH, whereas Dickmann 1988 did not specify the type of ICH included. Dickmann 1988 used 5000 units of heparin (Liquemin‐Roche) delivered subcutaneously every eight hours starting at day four after ICH. Orken 2009 used 40 mg/day of enoxaparin sodium delivered subcutaneously after the first 48 hours from ICH onset. The intervention and comparator groups of both RCTs received graduated compression stockings. Dickmann 1988 reported on other major ischaemic events, and vascular death from other causes, although did not specifically define these outcomes. Orken 2009 reported on other major ischaemic events, ICH, and major extracerebral haemorrhage. Both of the included RCTs provided short‐term outcome data only. Dickmann 1988 had a follow‐up period of 10 days and Orken 2009 had a follow‐up period of 21 days.
Excluded studies
We excluded seven studies for not meeting the inclusion criteria of this review after having screened their full texts.
Boeer 1991 was an extension phase of Dickmann 1988 and randomisation was not described, leading to its exclusion from this review. CAST 1997 and IST 1997 were two RCTs investigating the use of antiplatelet treatment after acute ischaemic stroke, and included several hundred participants with ICH who were randomised before CT had been performed to establish the pathological sub‐type of stroke. Data on the ICH sub‐population of these studies were reported in a systematic review (Keir 2002) and the individual patient data from IST 1997 (Sandercock 2011). These sources reported that this population included primary ICH (52%) and haemorrhagic transformation of ischaemic stroke (48%), but unfortunately the chief investigator informed us that it would be impossible to isolate the participants with primary ICH (IST 1997). Frontera 2014 was excluded because it was not randomised. Li 2013 assessed transfusion of frozen apheresis platelets in patients with ICH and on aspirin. Venturelli 2014 was a post hoc analysis of a RCT in which antithrombotic treatment was not randomly assigned, making this analysis observational in nature. Yan 2014 included a population of patients with and without cerebral microbleeds after acute ischaemic stroke.
Risk of bias in included studies
We assessed risks of bias for both included RCTs (Dickmann 1988; Orken 2009). Please refer to Figure 2 and Figure 3 for graphical depictions of this analysis.
2.
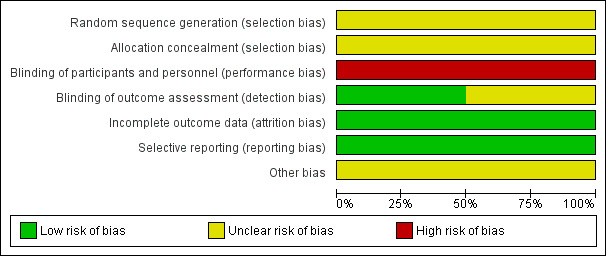
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
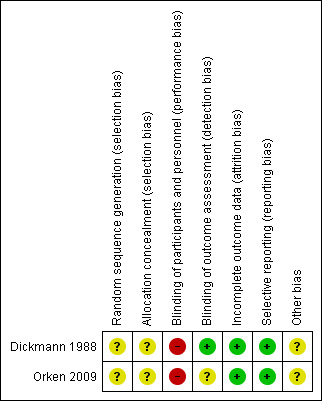
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both Dickmann 1988 and Orken 2009 were reported to be randomised but neither RCT described the method of randomisation, and neither RCT described the method of allocation concealment. We therefore determined both RCTs to be at unclear risk of selection bias.
Blinding
Due to the nature of the intervention (subcutaneous injection), and lack of placebo, blinding of participants and the personnel delivering the intervention was not possible. Both Dickmann 1988 and Orken 2009 were therefore at high risk of performance bias.
Dickmann 1988 explicitly mentioned blinding of outcome raters and was at low risk of detection bias. Orken 2009 reported that radiologists were blinded to the clinical baseline radiological findings, but did not explicitly mention blinding of treatment allocation and was therefore at unclear risk of detection bias.
Incomplete outcome data
Outcome data were complete in both included RCTs, which were both rated at low risk of attrition bias.
Selective reporting
Both Dickmann 1988 and Orken 2009 reported all outcomes planned in their respective Methods sections, and each reported most expected outcomes of interest. Both included RCTs were therefore at low risk of reporting bias.
Other potential sources of bias
Neither Dickmann 1988 nor Orken 2009 described conflicts of interest or sources of funding. Neither study was prospectively registered. Both were therefore at an unclear risk of other potential sources of bias.
Effects of interventions
See: Table 1
Short‐term antithrombotic treatment
We included two RCTs for this comparison (Dickmann 1988; Orken 2009). We were able to extract numerical data on some of the relevant outcomes.
Composite outcome of all serious vascular events
We were unable to calculate the composite vascular endpoint for either Dickmann 1988 or Orken 2009 (Analysis 1.1).
Death
Only one RCT reported death from all causes (Dickmann 1988). The causes of death in this RCT were rebleeding (4), bronchopneumonia (4), and heart failure (1). There was no statistically significant difference between the intervention and control groups for this outcome (risk ratio (RR) 1.25, 95% confidence interval (CI) 0.38 to 4.07; Analysis 1.2; 46 participants).
1.2. Analysis.
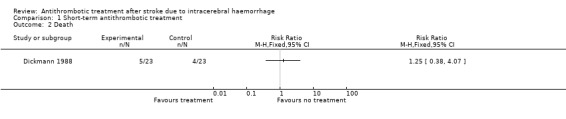
Comparison 1 Short‐term antithrombotic treatment, Outcome 2 Death.
Vascular death
Only one RCT reported deaths, but it did not adequately report in which group (control/intervention) instances of death from bronchopneumonia (4) and heart failure (1) occurred (Dickmann 1988). Because death from heart failure constitutes a vascular death and it was unclear in which group this death occurred, we were unable to include data for this outcome. However, it was reported that three deaths from expansion of the haematoma occurred in the control group and one such death occurred in the intervention group.
Ischaemic stroke
This outcome was not reported by either Dickmann 1988 or Orken 2009.
Myocardial infarction
This outcome was not reported by either Dickmann 1988 or Orken 2009.
Other major ischaemic event
Both included RCTs reported other major ischaemic events (Dickmann 1988; Orken 2009). All events for this outcome were pulmonary embolism detected by CT pulmonary angiogram (Orken 2009) or lung perfusion scintigraphy (Dickmann 1988). We combined results in a meta‐analysis (Analysis 1.6), and detected no statistically significant difference between groups (RR 0.54, 95% CI 0.23 to 1.28; 121 participants). The results of this analysis are limited by the different methods of detecting pulmonary emboli used by each study group.
1.6. Analysis.
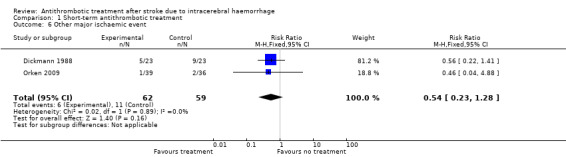
Comparison 1 Short‐term antithrombotic treatment, Outcome 6 Other major ischaemic event.
Intracerebral haemorrhage
One RCT involving 75 participants reported this outcome (Orken 2009). There were no recorded events in either group.
Major extracerebral haemorrhage
One RCT involving 75 participants reported this outcome (Orken 2009). There were no recorded events in either group.
Growth of ICH
Both of the included studies reported on ICH growth. Dickmann 1988 had an ambiguous definition for this outcome, 'rebleeding', which we considered to refer to ICH growth (whether this was due to continued bleeding, or recurrent ICH after cessation of the first ICH). This study did not describe a threshold of increased volume above which ICH growth was deemed to occur, and conducted brain CTs on days 1, 6, and 10 after ICH. Orken 2009 defined ICH growth as an increase in volume on CT of more than 33% or more than 12.5 mL using the ABC/2 method (Kothari 1996), and checked for this outcome on follow‐up brain CTs at 24 hours, 72 hours, day 7, and day 21 after ICH. We combined the results from these two RCTs in a meta‐analysis (Analysis 1.9), and observed no statistically significant differences between groups (RR 1.64, 95% CI 0.51 to 5.29; 121 participants).
1.9. Analysis.
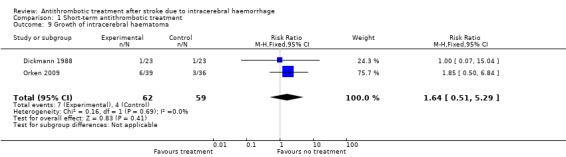
Comparison 1 Short‐term antithrombotic treatment, Outcome 9 Growth of intracerebral haematoma.
Deep vein thrombosis
Both included studies reported on the outcome of deep vein thrombosis (DVT). All events for this outcome were DVT detected by phleboscintigraphy at days 2 and 10 (Dickmann 1988) or by venous doppler ultrasound examination on day 7 (Orken 2009). All events reported in Orken 2009 were asymptomatic, whereas Dickmann 1988 did not specify whether events were symptomatic or not. We detected no statistically significant difference between groups for this outcome (RR 0.99, 95% CI 0.49 to 1.96; Analysis 1.10; 121 participants).
1.10. Analysis.
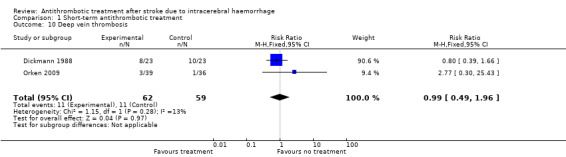
Comparison 1 Short‐term antithrombotic treatment, Outcome 10 Deep vein thrombosis.
Functional status
This outcome was not reported by either Dickmann 1988 or Orken 2009.
Cognitive status
This outcome was not reported by either Dickmann 1988 or Orken 2009.
Long‐term antithrombotic treatment
We did not find any completed RCTs that investigated long‐term antithrombotic treatment.
Discussion
Summary of main results
We found some evidence on the short‐term effects of antithrombotic drugs after ICH in two RCTs (Dickmann 1988; Orken 2009), but no evidence on the effects of long‐term antithrombotic treatment. We were unable to calculate the composite primary outcome of all serious vascular events due to incompleteness of reporting of the component outcomes in the included RCTs (Dickmann 1988; Orken 2009). None of the outcomes reported showed a statistically significant difference between groups, possibly because the two included RCTs were inadequately powered to detect significant differences for our outcomes. Neither included RCT reported outcomes for ischaemic stroke, myocardial infarction, functional status, or cognitive status. We were unable to extract data on any prespecified subgroups.
Overall completeness and applicability of evidence
The evidence available from completed RCTs of short‐term antithrombotic drug treatment after ICH is sparse. Data from the two included RCTs contribute moderately applicable evidence to this field; their inclusion criteria and settings are representative of typical ICH patients. The applicability of Dickmann 1988 is somewhat limited by waiting to start treatment until after day four following ICH onset; one implication might be that some of the potential effects of subcutaneous heparin, for example those on ICH growth, never manifested. There is no comparative evidence on the differences between distinct types of anticoagulants used in this setting. Dickmann 1988 investigated the effects of heparin, whereas Orken 2009 used enoxaparin sodium, a low‐molecular‐weight heparin.
However, we were encouraged to find that there are seven ongoing RCTs investigating long‐term antithrombotic treatment after ICH (APACHE‐AF; PICASSO; NASPAF‐ICH; RESTART; RESTART Nord de France; SoSTART; STATICH).
Quality of the evidence
The methodological quality of the included RCTs was limited by randomisation and allocation concealment not being described, failure to blind participants, and the optimal information size criterion not being met. The overall quality of evidence was therefore low. Both studies, despite investigating different drugs and starting treatment at different times, found no statistically significant difference between groups for outcomes relevant to this review. It is possible that this is due to these RCTs being inadequately powered to detect statistically meaningful differences, rather than there being no effect of the interventions.
Potential biases in the review process
None identified.
Agreements and disagreements with other studies or reviews
We identified two systematic reviews on short‐term antithrombotic treatment (Keir 2002; Paciaroni 2011). Keir 2002 included data from three RCTs (CAST 1997; Dickmann 1988; IST 1997) and included individual patient data from IST 1997 and CAST 1997 on participants with both primary ICH (52%) as well as haemorrhagic transformation of ischaemic infarcts (48%), but did not describe results for these groups separately. Keir 2002 preceded Orken 2009, a study which we included in this review. The second systematic review we identified included non‐randomised controlled studies (Paciaroni 2011). The non‐randomised studies included in this review were Wasay 2008 and Tetri 2008. This review concluded that early antithrombotic treatment was associated with a significant reduction in pulmonary embolism, a non‐significant reduction in mortality, and a non‐significant increase in haemorrhagic expansion (Paciaroni 2011). We identified one systematic review on long‐term antithrombotic use after ICH, but it could not draw any conclusions from the two epidemiological studies and several case series that it included (Flynn 2010b).
Authors' conclusions
Implications for practice.
The available evidence from two RCTs of short‐term parenteral anticoagulation after ICH neither support nor discourage the use of short‐term antithrombotic treatment after ICH.
There are no published RCTs on long‐term antithrombotic treatment after ICH, but seven RCTs are ongoing at the time of this review.
At the present time, clinicians will have to use other sources of information to support clinical judgements.
Implications for research.
Research on the use of antithrombotic drugs after ICH represents an area of unmet need for patients, clinicians, and other consumers. These data suggest that further high‐quality RCTs are needed. For short‐term antithrombotic use, future studies should consider functional outcome and quality of life in addition to clinical outcomes, such as ischaemic events, haemorrhagic events, or death. For long‐term antithrombotic use, future RCTs should consider investigating the relative effectiveness and safety of different antithrombotic drugs, for example warfarin, aspirin, and the direct oral anticoagulants.
Acknowledgements
We thank the Cochrane Stroke Group's Editorial Board for facilitating this review and providing editorial feedback.
We acknowledge and thank the Cochrane Stroke Group's Information Specialists Joshua Cheyne and his predecessor Brenda Thomas for their assistance in developing the search strategies for this review.
A special thanks to our two consumer reviewers, Odie Geiger and U Hla Htay, for generously providing their feedback on this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MESH DESCRIPTOR Basal Ganglia Hemorrhage EXPLODE ALL TREES #2 MESH DESCRIPTOR Intracranial Hemorrhages #3 MESH DESCRIPTOR Intracranial Hemorrhage, Hypertensive #4 MESH DESCRIPTOR Cerebral Hemorrhage #5 (brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or putaminal or putamen or hemispher* or stroke or apoplex*):TI #6 (basal and gangli*):TI #7 (posterior and fossa):TI,AB,KY #8 (haemorrhag* or hemorrhag* or haematoma* or hematoma* or bleed*):TI #9 #5 or #6 or #7 and #8 #10 (ICH or ICHs):TI #11 #1 or #2 or #3 or #4 or #9 or #10 #12 MESH DESCRIPTOR Anticoagulants EXPLODE ALL TREES #13 MESH DESCRIPTOR Pipecolic Acids EXPLODE ALL TREES WITH QUALIFIERS AE,TU #14 MESH DESCRIPTOR Vitamin K EXPLODE ALL TREES #15 MESH DESCRIPTOR Thrombin EXPLODE ALL TREES WITH QUALIFIERS AI #16 MESH DESCRIPTOR Factor Xa #17 MESH DESCRIPTOR Blood Coagulation Factors EXPLODE ALL TREES WITH QUALIFIERS AI #18 MESH DESCRIPTOR Blood Coagulation EXPLODE ALL TREES WITH QUALIFIERS DE #19 MESH DESCRIPTOR Antithrombins EXPLODE ALL TREES #20 MESH DESCRIPTOR Hirudin Therapy EXPLODE ALL TREES #21 (anticoagul* or antithromb*):TI,AB,KY #22 (Vitamin next K next antagonist*):TI,AB,KY #23 (VKA or VKAs):TI,AB,KY #24 #22 or #23 #25 (direct* NEAR5 thrombin):TI,AB,KY #26 "DTI":TI,AB,KY #27 (factor next Xa NEAR5 inhib*):TI,AB,KY #28 (factor next 10a NEAR5 inhib*):TI,AB,KY #29 (fXa NEAR5 inhib*):TI,AB,KY #30 (autoprothrombin NEAR5 inhib*):TI,AB,KY #31 (thrombokinase NEAR5 inhib*):TI,AB,KY
#32 (acenocoumarol* or dicoumarol* or ethyl next biscoumacetate* or phenprocoumon* or warfarin* or ancrod* or citric next acid* or coumarin* or chromonar* or coumestro* or esculi* or ochratoxin* or umbelliferone* or dermatan next sulfate* or dextran* or edetic next acid* or enoxaparin* or gabexate* or heparin* or lmwh* or nadroparin* or pentosan next sulfuric next polyester* or phenindione* or protein next c or protein next s or tedelparin*):TI,AB,KY #33 (tinzaparin or parnaparin or dalteparin or reviparin or danaparoid or lomoparan or org next 10172 or mesoglycan or polysaccharide next sulphate* or sp54 or sp‐54 or md805 or md‐805 or cy222 or cy‐222 or cy216 or cy‐216):TI,AB,KY #34 (Marevan or Fragmin* or Fraxiparin* or Klexane):TI,AB,KY #35 (argatroban or MD805 or MD‐805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin* or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil):TI,AB,KY #36 (xabans or antistasin or apixaban or betrixaban or du next 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym next 150 or ym150 or LY517717):TI,AB,KY #37 #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 #38 MESH DESCRIPTOR Platelet Glycoprotein GPIIb‐IIIa Complex EXPLODE ALL TREES WITH QUALIFIERS AI,DE #39 MESH DESCRIPTOR Platelet Activation EXPLODE ALL TREES WITH QUALIFIERS DE
#40 MESH DESCRIPTOR Blood Platelets EXPLODE ALL TREES WITH QUALIFIERS DE #41 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg* or (platelet* NEAR5 inhibit*) or (thrombocyt* NEAR5 inhibit*)):TI,AB,KY #42 (alprostadil* or aspirin* or acetylsalicylic next acid or (acetyl ADJ salicylic and acid*) or (acetyl‐salicylic and acid or epoprostenol* or ketanserin* or ketorolac next tromethamine* or milrinone* or mopidamol* or procainamide* or thiophen* or trapidil* or picotamide* or ligustrazine* or levamisol* or suloctidil* or ozagrel* or oky046 or oky‐046 or defibrotide* or cilostazol or satigrel or sarpolgrelate or kbt3022 or kbt‐3022 or isbogrel or cv4151 or cv‐ 4151)):TI,AB,KY #43 ((glycoprotein next iib* near/5 inhib*) or (glycoprotein next iib* near/5 antag*) or (gp next iib* near/5 inhib*) or (gp next iib* near/5 antag*) or GR144053 or GR‐144053 or triflusal):TI,AB,KY #44 (Argatroban or Beraprost or Cicaprost or Cilostazol or Clopidogrel or Dipyridamole or Iloprost or Indobufen or Lepirudin or Pentosan next Polysulfate or Pentoxifylline or Piracetam or Prostacyclin or Sulfinpyrazone or Sulphinpyrazone or Ticlopidine or Triflusal or Abciximab or Disintegrin or Echistatin or Eptifibatide or Lamifiban or Orbofiban or Roxifiban or Sibrafiban or Tirofiban or Xemilofiban or terutroban or picotamide or prasugrel):TI,AB,KY #45 (Dispril or Albyl* or Ticlid* or Persantin* or Plavix or ReoPro or Integrilin* or Aggrastat):TI,AB,KY #46 MESH DESCRIPTOR Platelet Aggregation Inhibitors EXPLODE ALL TREES #47 #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 #48 #37 or #47 #49 #48 and #11
Appendix 2. MEDLINE search strategy
1. exp basal ganglia haemorrhage/ or intracranial hemorrhages/ or cerebral haemorrhage/ or intracranial haemorrhage, hypertensive/
2. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
3. ((h?emorrhag$ or bleed$) adj5 (stroke or apoplex$)).tw.
4. (ICH or ICHs).tw.
5. 1 or 2 or 3 or 4
6. exp anticoagulants/
7. exp Vitamin K/ai or thrombin/ai or factor Xa/ai or exp Blood coagulation factors/ai
8. exp antithrombins/ or hirudin therapy/
9. (anticoagul$ or antithromb$).tw.
10. (Vitamin K antagonist$ or VKA or VKAs).tw.
11. (direct$ adj3 thrombin adj3 inhib$).tw.
12. DTI$1.tw.
13. ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) adj3 inhib$).tw.
14. (activated adj3 (factor X or factor 10) adj3 inhib$).tw.
15. (acenocoumarol$ or dicoumarol$ or ethyl biscoumacetate$ or phenprocoumon$ or warfarin$ or ancrod$ or citric acid$ or coumarin$ or chromonar$ or coumestro$ or esculi$ or ochratoxin$ or umbelliferone$ or dermatan sulfate$ or dextran$ or edetic acid$ or enoxaparin$ or gabexate$ or heparin$ or lmwh$ or nadroparin$ or pentosan sulfuric polyester$ or phenindione$ or protein c or protein s or tedelparin$).tw,nm.
16. (tinzaparin or parnaparin or dalteparin or reviparin or danaparoid or lomoparan or org 10172 or mesoglycan or polysaccharide sulphate$ or sp54 or sp‐54 or md805 or md‐805 or cy222 or cy‐222 or cy216 or cy‐216).tw,nm.
17. (Marevan or Fragmin$ or Fraxiparin$ or Klexane).tw,nm.
18. (argatroban or MD805 or MD‐805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin$ or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil).tw,nm.
19. (xabans or antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717).tw,nm.
20. exp platelet aggregation inhibitors/ or exp platelet glycoprotein gpiib‐iiia complex/ai
21. (antiplatelet$ or anti‐platelet$ or antiaggreg$ or anti‐aggreg$ or (platelet$ adj3 inhibit$) or (thrombocyt$ adj3 inhibit$)).tw.
22. (alprostadil$ or aspirin$ or acetylsalicylic acid or acetyl salicylic acid$ or acetyl?salicylic acid or epoprostenol$ or ketanserin$ or ketorolac tromethamine$ or milrinone$ or mopidamol$ or procainamide$ or thiophen$ or trapidil$ or picotamide$ or ligustrazine$ or levamisol$ or suloctidil$ or ozagrel$ or oky046 or oky‐046 or defibrotide$ or cilostazol or satigrel or sarpolgrelate or kbt3022 or kbt‐3022 or isbogrel or cv4151 or cv‐4151 or ((glycoprotein iib$ or gp iib$) adj5 (antagonist$ or inhibitor$)) or GR144053 or GR‐144053 or triflusal).tw,nm.
23. (Beraprost or Cicaprost or Cilostazol or Clopidogrel or Dipyridamole or Iloprost or Indobufen or Lepirudin or Pentosan Polysulfate or Pentoxifylline or Piracetam or Prostacyclin or Sulfinpyrazone or Sulphinpyrazone or Ticlopidine or Triflusal or Abciximab or Disintegrin or Echistatin or Eptifibatide or Lamifiban or Orbofiban or Roxifiban or Sibrafiban or Tirofiban or Xemilofiban or terutroban or picotamide or prasugrel).tw,nm.
24. (Dispril or Albyl$ or Ticlid$ or Persantin$ or Plavix or ReoPro or Integrilin$ or Aggrastat).tw,nm.
25. or/6‐24
26. Randomized Controlled Trials as Topic/
27. random allocation/
28. Controlled Clinical Trials as Topic/
29. control groups/
30. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
31. double‐blind method/
32. single‐blind method/
33. Placebos/
34. placebo effect/
35. randomised controlled trial.pt.
36. controlled clinical trial.pt.
37. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
38. (random$ or RCT or RCTs).tw.
39. (controlled adj5 (trial$ or stud$)).tw.
40. (clinical$ adj5 trial$).tw.
41. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
42. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
43. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
44. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
45. (placebo$ or sham).tw.
46. trial.ti.
47. (assign$ or allocat$).tw.
48. or/26‐47
49. 5 and 25 and 48
50. exp animals/ not humans/
51. 49 not 50
Appendix 3. EMBASE (Ovid) search strategy
1. anticoagulant agent/ or antivitamin k/ or exp blood clotting inhibitor/ or exp coumarin anticoagulant/ or defibrotide/ or dextran sulfate/ or fluindione/ or glycosaminoglycan polysulfate/ or exp heparin derivative/ or lupus anticoagulant/ or phenindione/ 2. (anticoagul$ or antithromb$).tw. 3. (Vitamin K antagonist$ or VKA or VKAs).tw. 4. (direct$ adj5 thrombin adj5 inhib$).tw. 5. DTI$1.tw. 6. ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) adj5 inhib$).tw. 7. (activated adj5 (factor X or factor 10) adj5 inhib$).tw. 8. (acenocoumarol$ or dicoumarol$ or ethyl biscoumacetate$ or phenprocoumon$ or warfarin$ or ancrod$ or citric acid$ or coumarin$ or chromonar$ or coumestro$ or esculi$ or ochratoxin$ or umbelliferone$ or dermatan sulfate$ or dextran$ or edetic acid$ or enoxaparin$ or gabexate$ or heparin$ or lmwh$ or nadroparin$ or pentosan sulfuric polyester$ or phenindione$ or protein c or protein s or tedelparin$).tw. 9. (tinzaparin or parnaparin or dalteparin or reviparin or danaparoid or lomoparan or org 10172 or mesoglycan or polysaccharide sulphate$ or sp54 or sp‐54 or md805 or md‐805 or cy222 or cy‐222 or cy216 or cy‐216).tw. 10. (Marevan or Fragmin$ or Fraxiparin$ or Klexane).tw. 11. (argatroban or MD805 or MD‐805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin$ or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil).tw. 12. (xabans or antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717).tw. 13. or/1‐12 14. exp antithrombocytic agent/ 15. fibrinogen receptor/dt [Drug Therapy] 16. (antiplatelet$ or anti‐platelet$ or antiaggreg$ or anti‐aggreg$ or (platelet$ adj5 inhibit$) or (thrombocyt$ adj5 inhibit$)).tw. 17. (alprostadil$ or aspirin$ or acetylsalicylic acid or acetyl salicylic acid$ or acetyl?salicylic acid or epoprostenol$ or ketanserin$ or ketorolac tromethamine$ or milrinone$ or mopidamol$ or procainamide$ or thiophen$ or trapidil$ or picotamide$ or ligustrazine$ or levamisol$ or suloctidil$ or ozagrel$ or oky046 or oky‐046 or defibrotide$ or cilostazol or satigrel or sarpolgrelate or kbt3022 or kbt‐3022 or isbogrel or cv4151 or cv‐4151 or ((glycoprotein iib$ or gp iib$) adj5 (antagonist$ or inhibitor$)) or GR144053 or GR‐144053 or triflusal).tw. 18. (Argatroban or Beraprost or Cicaprost or Cilostazol or Clopidogrel or Dipyridamole or Iloprost or Indobufen or Lepirudin or Pentosan Polysulfate or Pentoxifylline or Piracetam or Prostacyclin or Sulfinpyrazone or Sulphinpyrazone or Ticlopidine or Triflusal or Abciximab or Disintegrin or Echistatin or Eptifibatide or Lamifiban or Orbofiban or Roxifiban or Sibrafiban or Tirofiban or Xemilofiban or terutroban or picotamide or prasugrel).tw. 19. (Dispril or Albyl$ or Ticlid$ or Persantin$ or Plavix or ReoPro or Integrilin$ or Aggrastat).tw. 20. or/14‐19 21. 13 or 20 22. *basal ganglion hemorrhage/ or *brain hemorrhage/ or *brain ventricle hemorrhage/ or *cerebellum hemorrhage/ 23. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or stroke or apoplex$) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).ti. 24. 22 or 23 or (ICH or ICHs).ti. 25. randomized controlled trial/ or "randomized controlled trial (topic)"/ 26. Randomization/ 27. Controlled Study/ 28. control group/ 29. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/ 30. Double Blind Procedure/ 31. Single Blind Procedure/ or triple blind procedure/ 32. placebo/ 33. drug comparison/ or drug dose comparison/ 34. "types of study"/ 35. random$.tw. 36. (controlled adj5 (trial$ or stud$)).tw. 37. (clinical$ adj5 trial$).tw. 38. ((control or treatment or experiment$ or intervention or surgical) adj5 (group$ or subject$ or patient$)).tw. 39. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 40. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 41. placebo$.tw. 42. controls.tw. 43. or/25‐42 44. meta analysis/ or "meta analysis (topic)"/ or "systematic review"/ or "systematic review (topic)"/ 45. meta analy$.tw. 46. metaanaly$.tw. 47. (systematic adj (review$1 or overview$1)).tw. 48. literature/ 49. (cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or cinhal or science citation index or bids or medline or pubmed).ab. 50. (reference list$ or bibliograph$ or hand‐search$ or relevant journals or manual search).ab. 51. (selection criteria or data extraction).ab. 52. review.pt. or literature/ or review/ 53. 51 and 52 54. 44 or 45 or 46 or 47 or 48 or 49 or 50 or 53 55. (letter or editorial).pt. 56. 54 not 55 57. 43 or 56 58. 21 and 24 and 57 59. limit 58 to human
Appendix 4. Trials register search strategies
1. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
'Randomised' AND 'intracerebral haemorrhage'
2. World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch)
'Intracerebral haemorrhage' OR 'Intracerebral hemorrhage' OR 'ICH'
3. ISRCTN Registry (www.isrctn.com)
'Intracerebral haemorrhage' OR 'Intracerebral hemorrhage' OR 'ICH'
4. Stroke Trials Registry – the Internet Stroke Center (www.strokecenter.org/trials/)
'Intracerebral haemorrhage' OR 'Intracerebral hemorrhage' OR 'ICH'
Data and analyses
Comparison 1. Short‐term antithrombotic treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Other major ischaemic event | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.23, 1.28] |
| 7 Intracerebral haemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Major extracerebral haemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Growth of intracerebral haematoma | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.51, 5.29] |
| 10 Deep vein thrombosis | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.49, 1.96] |
1.7. Analysis.
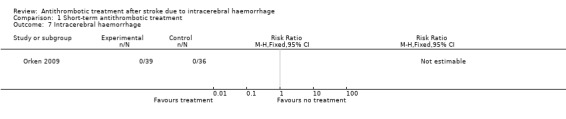
Comparison 1 Short‐term antithrombotic treatment, Outcome 7 Intracerebral haemorrhage.
1.8. Analysis.
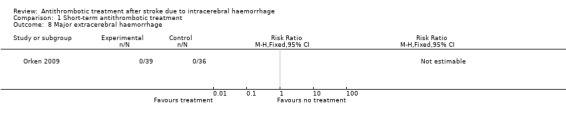
Comparison 1 Short‐term antithrombotic treatment, Outcome 8 Major extracerebral haemorrhage.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dickmann 1988.
| Methods | RCT Duration: 10 days Design: parallel Setting: inpatient unit in Germany Dates: not described |
|
| Participants | 46 participants Diagnosis: intracerebral haemorrhage Diagnosis model: cranial CT Eligibility criteria: diagnosis of ICH in the previous 24 hours before admission Exclusion criteria: bleeding diathesis, diastolic blood pressure higher than 120 mmHg, and deep coma with clinical signs of brain herniation Age (years, mean (SD)): 62 (SD not described) (intervention group), 60 (SD not described) (control group) Sex: 52% female (intervention group), 48% female (control group) |
|
| Interventions | 1. 5000 units of heparin administered subcutaneously 8‐hourly starting at day 4 (n = 23) 2. 5000 units of heparin subcutaneously 8‐hourly starting at day 10 (n = 23) Both groups had the same treatment otherwise, with compression stockings and physical treatment |
|
| Outcomes | Rebleeding occurring during the trial period Thrombosis of abdomen or legs at day 2 and day 10 Pulmonary embolism at day 10 Death during the trial period |
|
| Notes | Rebleeding is not defined by the authors of this study and could mean multiple things. Conflicts of interest and sources of funding were not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible due to the control group not receiving a placebo injection |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All scintigrams were read by the same blinded investigators" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were complete |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the Methods section were reported in full in the Results section. Additionally, most expected outcomes of interest were reported in this study |
| Other bias | Unclear risk | To our knowledge this study was not prospectively registered |
Orken 2009.
| Methods | RCT Duration: 21 days Design: parallel Setting: inpatient unit in Turkey Dates: between January 2006 and March 2008 |
|
| Participants | 75 participants Diagnosis: intracerebral haemorrhage Diagnosis model: cranial CT Eligibility criteria: diagnosis of primary ICH Exclusion criteria: early death before heparin treatment, death before 7th day investigations, secondary ICH due to aneurysm, arteriovenous malformations, traumas and tumours. Excessive anticoagulation (INR > 2.0). Contraindication to contrast agents Age (years, mean (SD)): 68.1 (11.98) (intervention group) 66.08 (9.55) (control group) Sex: 56% female (intervention group), 22% female (control group) Additonal details: mean National Institutes of Health Stroke Scale scores on admission for the LMWH group was 9.74 ± 6.06 and for the compression stocking group was 8.61 ± 6.87. 32/39 participants in the LMWH group and 29/36 participants in the compression stocking group had hypertension identified as a risk factor for ICH. 11 participants from the LMWH group and 6 from the compression stocking group had had a prior cerebrovascular accident |
|
| Interventions | 1. Enoxaparin sodium 48 mg/day (n = 39) 2. Compression stocking control (n = 36) Treatment began after the first 48 hours from hospital admission |
|
| Outcomes | Haematoma enlargement at 72 hours, 7 days and 21 days. Haematoma enlargement was defined as an increase in volume of > 33% or 12.5 mL Systemic bleeding complications DVT or PE based on CTPA and bilateral venous Doppler at 7 days |
|
| Notes | Conflicts of interest and sources of funding were not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible due to 1 group receiving a subcutaneous injection and the other group receiving compression stockings |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "All radiologic material was prospectively evaluated by 2 radiologists ... who were blinded to the clinical findings and cranial CTs of the patients". The methods did not explicitly state that the radiologists were also blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were complete |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the Methods section were reported in full in the Results section. Additionally, most expected outcomes of interest were reported in this study |
| Other bias | Unclear risk | To our knowledge this study was not prospectively registered |
CT: computed tomography CTPA: CT pulmonary angiography DVT: deep vein thrombosis ICH: intracerebral haemorrhage INR: International normalised ratio LMWH: low‐molecular‐weight heparin PE: pulmonary embolism RCT: randomised controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boeer 1991 | This is an extension phase of Dickmann 1988 that introduces non‐randomised data to the previously published data. |
| CAST 1997 | This trial included some people with ICH who were randomised prior to CT. These data were not available in the original manuscript. We had hoped that there would be useable data published in the Keir 2002 systematic review, but this did not separate primary ICH from haemorrhagic transformations of acute ischaemic stroke and we were therefore unable to include data from CAST 1997 in this review. |
| Frontera 2014 | This is a controlled clinical trial that was not randomised. |
| IST 1997 | This trial included several hundred participants with ICH who were randomised prior to CT. These data were not available in the original manuscript. We had hoped that data from the Keir 2002 systematic review or the published individual data forms (Sandercock 2011) would yield useable data for this review, but Keir 2002 did not report primary ICH separately from haemorrhagic expansion of an acute ischaemic stroke, and after contacting the chief investigator of IST 1997 we discovered that it was not possible to extract data on primary ICH separately. |
| Li 2013 | The intervention randomised in this study is transfusion of frozen apheresis platelets in participants on aspirin and with ICH. |
| Venturelli 2014 | This is a post hoc analysis of the INTERACT2 trial. The intervention that is the subject of this analysis, prophylactic subcutaneous heparin after ICH, was not randomly assigned in INTERACT2. Therefore, this analysis is observational in nature. |
| Yan 2014 | The participant group did not have primary ICH; it was a cohort of people with and without cerebral microbleeds and haemorrhagic transformation, with acute ischaemic stroke following rtPA treatment. |
CT: computed tomography ICH: intracerebral haemorrhage rtPA: Recombinant tissue plasminogen activator
Characteristics of ongoing studies [ordered by study ID]
APACHE‐AF.
| Trial name or title | Apixaban versus antiplatelet drugs or no antithrombotic drugs after anticoagulation‐associated intracerebral haemorrhage in patients with atrial fibrillation (APACHE‐AF) |
| Methods | Allocation: randomised Blinding: open‐label (none) Duration: 12 to 30 months Setting: multicentre trial across the Netherlands Dates: September 2014 and currently recruiting |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Participants will be randomised into 6 groups
Each of the groups will run in parallel |
| Outcomes | Primary outcome measures
Secondary outcome measures: number of participants who experience:
|
| Starting date | September 2014 |
| Contact information | Contact: Koen M van Nieuwenhuizen, MD. Tel: +31 88 757 4097; email: k.m.vannieuwenhuizen‐3@umcutrecht.nl Contact: H Bart van der Worp, MD PhD. Tel: +31 88 755 98 99; email: h.b.vanderworp@umcutrecht.nl |
| Notes |
NASPAF‐ICH.
| Trial name or title | Non‐VKA Anticoagulants for Stroke Prevention in Patients with Atrial Fibrillation and Previous IntraCerebral Hemorrhage Study (NASPAF‐ICH) |
| Methods | Allocation: randomised 2:1 (non‐vitamin K oral antagonist (agent at the discretion of the local investigator) vs aspirin) Blinding: open‐label, blinded adjudication of outcomes Duration: recruitment 24 months, participants will be followed to a common terminate date designated at 6 months following the end of recruitment. Average follow‐up per participant approximately 12 months (range: 12 to 30 months) Design (parallel, other): phase II, open‐label randomised controlled trial Setting: 10 high‐volume stroke centres across Canada Dates: January 2017 to June 2019 |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Non‐vitamin K oral antagonist (agent at the discretion of the local investigator) vs aspirin 81 mg Strict long‐term blood pressure control to target of < 130/80 mmHg for all participants |
| Outcomes | Primary outcome measure: the primary feasibility outcome will be recruitment rates Secondary outcomes will be refusal rates and retention rates |
| Starting date | January 2017 |
| Contact information | Co‐Prinicipal Investigator: Ashkan Shoamanesh, MD, FRCPC Assistant Professor of Medicine (Neurology) Director, Stroke Fellowship Program Marta and Owen Boris Chair in Stroke Research and Care McMaster University / Population Health Research Institute 237 Barton Street East HGH‐DBCVSRI C4‐118 Hamilton, Ontario, L8L 2X2, Canada Email: ashkan.shoamanesh@phri.ca Telephone: (905) 521‐2100 ext 41277 Fax: (905) 577‐1427 |
| Notes |
PICASSO.
| Trial name or title | PreventIon of CArdiovascular Events in iSchemic Stroke patients with high risk of cerebral hemOrrhage (PICASSO) |
| Methods | Allocation: randomisation Blinding: double‐blinding (participant, caregiver, investigator, outcome assessor) Duration: 1 to 5.5 years Setting: multicentre trial across China, South Korea and the Philippines Dates: June 2009 and ongoing |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | A factorial design across the following groups:
|
| Outcomes | Primary outcome measures: time to first occurrence of:
Secondary outcome measures: time to first occurrence of:
Other outcome measures
|
| Starting date | June 2009 |
| Contact information | Principal Investigator: Sun U Kwon, MD, PhD Departement of Neurology, Asan Medical Center |
| Notes |
RESTART.
| Trial name or title | REstart or STop Antithrombotics Randomised Trial (RESTART) |
| Methods | Allocation: randomised Blinding: treatment is open‐label, but outcome assessors are blinded Duration: at least 6 months Design: parallel Setting: UK National Health Service (NHS) secondary care (inpatient and outpatient services in stroke, neurology and neurosurgery) and primary care Dates: May 2013 to May 2018 (recruitment), November 2018 |
| Participants | Inclusion criteria:
Exclusion criteria:
Brain magnetic resonance imaging (MRI) sub‐study: MRI done after ICH but before randomisation. No claustrophobia. MRI not contraindicated |
| Interventions | Participants will be randomised to either 'start antiplatelet medication' (restricted to the use of 1 or more of aspirin, dipyridamole or clopidogrel at the investigator's discretion) or "avoid antiplatelet medication" |
| Outcomes | Primary outcome measure: recurrent symptomatic ICH Secondary outcome measures:
|
| Starting date | April 2013 |
| Contact information | UK Chief Investigator: Rustam Al‐Shahi Salman; Trial Manager: Karen Innes RESTART.trial@ed.ac.uk. |
| Notes |
RESTART Nord de France.
| Trial name or title | RESTART Nord de France |
| Methods | Allocation: randomisation 1:1 ‐ restart vs no antiplatelet Blinding: PROBE design Duration: each participant will be followed up during year 2 Design (parallel, other): RCT 1:1 Setting: region North of France Dates: start October 2016, recruitment 3 years, overall: 5 years |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Restart antiplatelet agent vs no antiplatelet agent |
| Outcomes | Primary outcome measure: recurrent ICH (fatal and non fatal) Secondary outcome measures:
|
| Starting date | October 2016 |
| Contact information | Contact information: Professor Charlotte Cordonnier charlotte.cordonnier@chru‐lille.fr |
| Notes |
SoSTART.
| Trial name or title | Start or STop Anticoagulants Randomised Trial |
| Methods | Investigator‐led, multicentre, randomised, open, assessor‐masked, parallel group, clinical trial of investigational medicinal product (CTIMP) prescribing strategies. We plan for a pilot phase, followed by a main phase. |
| Participants | Inclusion criteria: spontaneous intracranial haemorrhage, AF and a CHA2DS2‐VASc score ≥ 2 Exclusion criteria:
Brain MRI substudy: MRI must be done after intracranial haemorrhage but before randomisation. Substudy participants must not have contraindications to MRI. |
| Interventions |
|
| Outcomes | Primary outcome: all symptomatic serious vascular events (i.e. major adverse cardiac or cerebrovascular events: MACCE) including non‐fatal stroke, non‐fatal acute coronary syndrome, vascular death, sudden death, or death of unknown cause Secondary outcomes:
|
| Starting date | 2017 |
| Contact information | Professor Rustam Al‐Shahi Salman, University of Edinburgh, UK (Rustam.Al‐Shahi@ed.ac.uk) |
| Notes | Funded by Chest Heart and Stroke Scotland |
STATICH.
| Trial name or title | Study of Antithrombotic Treatment after Intracerebral Haemorrhage (STATICH) |
| Methods | Allocation: randomised Blinding: open, blinded endpoint Duration: 2 years Design : parallel Setting: multicenter in Norway, Denmark, and Sweden Dates: start up 2017 |
| Participants | Inclusion criteria:
Exclusion criteria:
For MRI substudy: contraindication for MRI |
| Interventions | Interventions for each group: the intervention is starting antithrombotic drug. The comparator is avoiding antithrombotic drugs |
| Outcomes | Primary outcome measure: recurrent symptomatic ICH Secondary outcome measures:
|
| Starting date | |
| Contact information | Trial co‐ordinating investigator: Eivind Berge, MD, PhD, email: eivind.berge@medisin.uio.no Trial manager: Elisabeth Forfang, MD, email: elisabeth.forfang@medisin.uio.no |
| Notes |
AF: atrial fibrillation CCA: common carotid artery CT: computed tomography DBP: diastolic blood pressure DOAC: direct oral anticoagulant ICH: intracerebral haemorrhage MRI: magnetic resonance imaging mRS: modified Rankin Scale NOAC: novel oral anticoagulant OAC: oral anticoagulation SBP: systolic blood pressure TIA: transient ischaemic stroke VKA: vitamin K antagonist
Differences between protocol and review
We separately grouped RCTs investigating short‐term and long‐term treatment, instead of performing subgroup analyses as our protocol had specified.
We added two new outcomes: deep vein thrombosis and ICH growth, as we believe that they are of potential interest to consumers of this review. The addition of the outcome deep vein thrombosis allowed us to include it as a secondary outcome in this review without including it in another category (e.g. major ischaemic events). The addition of the ICH growth outcome is relevant to short‐term antithrombotic treatment and was considered after we decided to divide our analysis into two groups according to the duration of antithrombotic drug use. The addition of these new outcomes did not result in any additional RCTs being included in this review.
We optimised the 'Summary of findings' tables to better reflect the outcomes of interest related to short‐ and long‐term treatment.
Contributions of authors
All authors, Luke A Perry, Eivind Berge, Joshua Bowditch, Elisabeth Forfang, Ole Morten Rønning, Graeme J Hankey, Elmer Villanueva, Rustam Al‐Shahi Salman, contributed to planning, writing, and editing the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Medical Research Council, UK.
Senior clinical fellowship to RA‐SS
Declarations of interest
Luke A Perry: none known Eivind Berge: co‐ordinating investigator of the ongoing STATICH trial. Joshua Bowditch: none known Elisabeth Forfang: managing investigator of the ongoing STATICH trial. Ole Morten Rønning: none known Graeme J Hankey: in the past three years, GJH has received honoraria from AC Immune for chairing the data safety monitoring committee of two clinical trials of vaccines for Alzheimer’s disease, from Bayer for lecturing about stroke prevention in atrial fibrillation at sponsored scientific symposia, and from Medscape, Web MD for participating in a discussion about stroke prevention in atrial fibrillation for theheart.org. Elmer Villanueva: none known Rustam Al‐Shahi Salman: Chief investigator of the UK REstart or STop Antithrombotics Randomised Trial (RESTART, www.RESTARTtrial.org, ISRCTN71907627), which is funded by a special project grant from the British Heart Foundation.
Joint lead author
Joint lead author
New
References
References to studies included in this review
Dickmann 1988 {published data only}
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep‐vein thrombosis and pulmonary embolism after intracerebral haemorrhage. Klinische Wochenschrift 1988;66(23):1182‐3. [DOI: 10.1007/BF01727666; PUBMED: 3062268] [DOI] [PubMed] [Google Scholar]
Orken 2009 {published data only}
- Orken DN, Kenangil G, Ozkurt H, Guner C, Gundogdu L, Basak M, et al. Prevention of deep venous thrombosis and pulmonary embolism in patients with acute intracerebral haemorrhage. Neurologist 2009;15(6):329‐31. [DOI: 10.1097/NRL.0b013e3181a93bac; PUBMED: 19901711] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Boeer 1991 {published data only}
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. Journal of Neurology, Neurosurgery, and Psychiatry 1991;54(5):466‐7. [DOI: 10.1136/jnnp.54.5.466] [DOI] [PMC free article] [PubMed] [Google Scholar]
CAST 1997 {published data only}
- CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomised placebo‐controlled trial of early aspirin use in 20 000 patients with acute ischaemic stroke; CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997;349(9066):1641‐9. [DOI: 10.1016/S0140-6736(97)04010-5; PUBMED: 9186381] [DOI] [PubMed] [Google Scholar]
Frontera 2014 {published data only}
- Frontera JA, Jovine M, Zach V, Gordon E. Safety of venous thromboembolism prophylaxis in intracranial hemorrhage patients with external ventricular drains. Stroke 2014;45:236. [Google Scholar]
IST 1997 {published data only}
- International Stroke Trial Collaborative Group. The International Stoke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19 435 patients with acute ischaemic stroke. Lancet 1997;349(9065):1569‐81. [DOI: 10.1016/S0140-6736(97)04011-7; PUBMED: 9186381] [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li X, Zhaosheng S, Zhao W, Zhang J, Chen J, Li Y, et al. Effect of acetylsalicylic acid usage and platelet transfusion on postoperative hemorrhage and activities of daily living in patients with acute intracerebral hemorrhage. Journal of Neurosurgery 2013;118:94‐103. [DOI: 10.3171/2012.9.JNS112286] [DOI] [PubMed] [Google Scholar]
Venturelli 2014 {published data only}
- Venturelli PA, Wang X, Arima H, Heeley E, Delcourt C, Lavados P, et al. Safety of prophylactic heparin in acute intracerebral haemorrhage: post‐hoc analysis of the INTERACT2 study. International Journal of Stroke 2014;9 Suppl 1:38. [Google Scholar]
Yan 2014 {published data only}
- Yan L, Li YD, Li YH, Li MH, Zhao JG, Chen SW. Outcomes of antiplatelet therapy for haemorrhage patients after thrombolysis: a prospective study based on susceptibility‐weighted imaging. La Radiologica Medica 2014;119(3):175‐82. [DOI: 10.1007/s11547-013-0328-1] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
APACHE‐AF {published data only}
- Nieuwenhuizen KM, Worp HB, Algra A, Kappelle LJ, Rinkel GJ, Gelder IC, et al. Apixaban versus antiplatelet drugs or no antithrombotic drugs after anticoagulation‐associated intracerebral haemorrhage in patients with atrial fibrillation (APACHE‐AF): study protocol for a randomised controlled trial. Trials 2015;16:393. [DOI: 10.1186/s13063-015-0898-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
NASPAF‐ICH {published data only}
- NCT02998905. NOACs for stroke prevention in patients with atrial fibrillation and previous ICH (NASPAF‐ICH). ClinicalTrials.gov/NCT02998905 (first received: 25 November 2016). [NCT02998905]
PICASSO {published data only}
- Hong KS, Kim BJ, Lee JY, Kwon SU, PICASSO Investigators. Rationale and design of the preventIon of cardiovascular events in ischemic stroke patients with high risk of cerebral hemorrhage (PICASSO) study: a randomized controlled trial. International Journal of Stroke 2015;10(7):1153‐8. [10.1111/ijs.12519; PUBMED: 26044566] [DOI] [PubMed] [Google Scholar]
RESTART {published data only}
- Al‐Shahi Salman R, Innes K, RESTART Trial Collaborators. Restart or stop antithrombotics randomised trial (RESTART). International Journal of Stroke 2015;10 Suppl 2:426. [10.1186/ISRCTN71907627] [Google Scholar]
RESTART Nord de France {published data only}
- Cordonnier C. RESTART Nord de France [personal communication]. Email to: LA Perry. 26 September 2016.
SoSTART {unpublished data only}
- Start or STop Anticoagulants Randomised Trial. Ongoing study 2017.
STATICH {published data only}
- Berge E. STATICH [personal communication]. Email to: LA Perry. 7 September 2016.
Additional references
Al‐Shahi Salman 2014
- Al‐Shahi Salman R, Dennis M. Antiplatelet therapy may be continued after intracerebral hemorrhage. Stroke 2014;45(10):3149‐50. [DOI: 10.1161/STROKEAHA.114.005786; PUBMED: 25205310] [DOI] [PubMed] [Google Scholar]
Béjot 2013
- Béjot Y, Cordonnier C, Durier J, Aboa‐Eboulé C, Rauaud O, Giroud M. Intracerebral haemorrhage profiles are changing: results from the Dijon population‐based study. Brain 2013;136(Pt 2):658‐64. [DOI: 10.1093/brain/aws349] [DOI] [PubMed] [Google Scholar]
Chao 2016
- Chao TF, Liu CJ, Liao JN, Wang KL, Lin YJ, Chang SL, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation 2016;133(16):1540‐7. [DOI: 10.1161/CIRCULATIONAHA.115.019794] [DOI] [PubMed] [Google Scholar]
Chong 2012
- Chong BH, Chan KH, Pong V, Lau KK, Chan YH, Zuo ML, et al. Use of aspirin in Chinese after recovery from primary intracranial haemorrhage. Thrombosis and Haemostasis 2012;107(2):241‐7. [DOI: 10.1160/TH11-06-0439] [DOI] [PubMed] [Google Scholar]
Claassen 2008
- Claassen DO, Kazemi N, Zubkov AY, Wijdicks EF, Rabinstein AA. Restarting anticoagulation therapy after warfarin‐associated intracerebral hemorrhage. Archives of Neurology 2008;65(10):1313. [DOI: 10.1001/archneur.65.10.1313; PUBMED: 18852344] [DOI] [PubMed] [Google Scholar]
Cordonnier 2007
- Cordonnier C, Salman RAS, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 2007;130(8):1988‐2003. [DOI: 10.1093/brain/awl387] [DOI] [PubMed] [Google Scholar]
De Vleeschouwer 2005
- Vleeschouwer S, Calenbergh F, Loon J, Nuttin B, Goffin J, Plets C. Risk analysis of thrombo‐embolic and recurrent bleeding events in the management of intracranial haemorrhage due to oral anticoagulation. Acta Chirurgica Belgica 2005;105(3):268‐74. [DOI: 10.1080/00015458.2005.11679715; PUBMED: 16018519] [DOI] [PubMed] [Google Scholar]
Falcone 2014
- Falcone G, Rosand J. Aspirin should be discontinued after lobar intracerebral hemorrhage. Stroke 2014;45(10):3151‐2. [PUBMED: 10.1161/STROKEAHA.114.00578; PUBMED: 25205309] [DOI] [PubMed] [Google Scholar]
Flynn 2010a
- Flynn RW, MacDonald TM, Murray GD, MacWalter RS, Doney AS. Prescribing antiplatelet medicine and subsequent events after intracerebral hemorrhage. Stroke 2010;41(11):2606. [DOI: 10.1161/STROKEAHA.110.589143; PUBMED: 20947854] [DOI] [PubMed] [Google Scholar]
Flynn 2010b
- Flynn RWV, MacDonald TM, Murray GD, Doney ASF. Systematic review of observational research studying the long‐term use of antithrombotic medicines following intracerebral haemorrhage. Cardiovascular Therapeutics 2010;28:177‐84. [DOI: 10.1111/j.1755-5922.2009.00118.x] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University. GRADEpro Guideline Development Tool. McMaster University, 2015.
Hawryluk 2010
- Hawryluk GW, Austin JW, Furlan JC, Lee JB, O'Kelly C, Fehlings MG. Management of anticoagulation following central nervous system hemorrhage in patients with high thromboembolic risk. Journal of Thrombosis and Haemostasis 2010;8(7):1500. [DOI: 10.1111/j.1538-7836.2010.03882.x; PUBMED: 20403088] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Keir 2002
- Keir SL, Wardlaw JM, Sandercock PA, Chen Z. Antithrombotic therapy in patients with any form of intracranial haemorrhage: a systematic review of the available controlled studies. Cerebrovascular Diseases 2002;14(3‐4):197‐206. [DOI: 10.1177/2042098611415457] [DOI] [PubMed] [Google Scholar]
Kothari 1996
- Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27(8):1304‐5. [DOI: 10.1161/01.STR.27.8.1304] [DOI] [PubMed] [Google Scholar]
Krishnamurthi 2010
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. GBD Stroke Experts Group. Global and regional burden of first‐ever ischaemic and haemorrhagic stroke during 1990‐2010: findings from the Global Burden of Disease Study 2010. Lancet Global Health 2013;1(5):259‐81. [DOI: 10.1016/S2214-109X(13)70089-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuramatsu 2015
- Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation‐related intracerebral hemorrhage. JAMA 2015;313(8):824‐36. [DOI: 10.1001/jama.2015.0846] [DOI] [PubMed] [Google Scholar]
Lauer 2013
- Lauer A, Pfeilschifter W, Schaffer CB, Lo EH, Foerch C. Intracerebral haemorrhage associated with antithrombotic treatment: translational insights from experimental studies. Lancet 2013;12(4):394‐405. [DOI: 10.1016/S1474-4422(13)70049-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lovelock 2007
- Lovelock CE, Molyneux AJ, Rothwell PM, Oxford Vascular Study. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population‐based study. Lancet Neurology 2007;6(6):487‐93. [DOI: 10.1016/S1474-4422(07)70107-2] [DOI] [PubMed] [Google Scholar]
Molina 2011
- Molina CA, Selim MH. The dilemma of resuming anticoagulation after intracranial hemorrhage. Stroke 2011;42(12):3665‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Nielsen 2015
- Nielsen PB, Larsen TB, Skjøth F, Gorst‐Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial haemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality and bleeding: a nationwide cohort study. Circulation 2015; Vol. 132, issue 6:517‐25. [DOI: 10.1161/CIRCULATIONAHA.115.015735] [DOI] [PubMed]
O'Donnell 2016
- O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case‐control study. Lancet 2016;388(10046):761‐75. [DOI: 10.1016/S0140-6736(16)30506-2] [DOI] [PubMed] [Google Scholar]
Paciaroni 2011
- Paciaroni M, Agnelli G, Venti A, Alberti A, Acciaressi M, Caso V. Efficacy and safety of anticoagulants in the prevention of venous thromboembolism in patients with acute cerebral hemorrhage: a meta‐analysis of controlled studies. Journal of Thrombosis and Haemostasis 2011;9:893‐8. [DOI: 10.1111/j.1538-7836.2011.04241.x] [DOI] [PubMed] [Google Scholar]
Pasquini 2014
- Pasquini M, Charidimou A, Asch CJ, Baharoglu MI, Samarasekera N, Werring DJ, et al. Variation in restarting antithrombotic drugs at hospital discharge after ICH. Stroke 2014;45(9):2643‐8. [DOI: 10.1161/STROKEAHA.114.006202] [DOI] [PubMed] [Google Scholar]
Pennlert 2016
- Pennlert J, Overholser R, Asplund K, Carlberg B, Rompaye BV, Wiklund PG, et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke 2016;48(2):1‐7. [DOI: 10.1161/STROKEAHA.116.014643] [DOI] [PubMed] [Google Scholar]
Poli 2014
- Poli D, Antonucci E, Dentali F, Erba N, Testa S, Tiraferri E, Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014;82(12):1020. [DOI: 10.1212/WNL.0000000000000245; PUBMED: 16434655] [DOI] [PubMed] [Google Scholar]
Poon 2014
- Poon MT, Fonville AF, Al‐Shahi Salman R. Long‐term prognosis after intracerebral haemorrhage: systematic review and metaanalysis. Journal of Neurology, Neurosurgery, and Psychiatry 2014;85(6):660‐7. [DOI: 10.1136/jnnp-2013-306476] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sandercock 2011
- Sandercock PAG, Niewada M, Czlonkowska A, the International Stroke Trial Collaborative Group. The International Stroke Trial database. Trials 2011;12:101. [DOI: 10.1186/1745-6215-12-101] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steiner 2011
- Steiner T. Resumption of oral anticoagulation after warfarin‐associated intracerebral hemorrhage. Stroke 2011;42(12):3661‐2. [DOI: 10.1161/STROKEAHA.111.621797] [DOI] [PubMed] [Google Scholar]
Steiner 2014
- Steiner T, Al‐Shahi Salman R, Beer R, Christensen H, Cordonnier, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. International Journal of Stroke 2014;9:840‐55. [DOI: 10.1111/ijs.12309] [DOI] [PubMed] [Google Scholar]
Tetri 2008
- Tetri S, Hakala J, Juvela S, Saloheimo P, Pyhtinen J, Rusanen H, et al. Safety of low‐dose subcutaneous enoxaparin for the prevention of venous thromboembolism after primary intracerebral haemorrhage. Thrombosis Research 2008;123(2):206‐12. [DOI: 10.1016/j.thromres.2008.01.018; PUBMED: 18420258] [DOI] [PubMed] [Google Scholar]
Viswanathan 2006
- Viswanathan A, Rakich SM, Engel C, Snider R, Rosand J, Greenberg SM, et al. Antiplatelet use after intracerebral hemorrhage. Neurology 2006;66(2):206. [DOI: ; PUBMED: 16434655] [DOI] [PubMed] [Google Scholar]
Wasay 2008
- Wasay M, Khan S, Zaki K, Khealani BA, Kamal A, Azam I, et al. A non‐randomized study of safety and efficacy of heparin for DVT prophylaxis in intracerebral haemorrhage. Journal of the Pakistan Medical Association 2008;58(7):362‐4. [PUBMED: 18988406] [PubMed] [Google Scholar]
Wilson 2016
- Wilson D, Charidimou A, Ambler G, Fox ZV, Gregoire S, Rayson P, et al. Recurrent stroke risk and cerebral microbleed burden in ischaemic stroke and TIA: a meta‐analysis. Neurology 2016;87(14):1‐10. [DOI: 10.%E2%80%8B1212/%E2%80%8BWNL.%E2%80%8B0000000000003183] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Perry 2016
- Perry LA, Berge E, Bowditch J, Forfang E, Rønning OM, Hankey GJ, et al. Antithrombotic treatment after intracerebral haemorrhage. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012144] [DOI] [PMC free article] [PubMed] [Google Scholar]


