Abstract
Background
People with diabetes are at high risk for developing foot ulcers, which often become infected. These wounds, especially when infected, cause substantial morbidity. Wound treatments should aim to alleviate symptoms, promote healing, and avoid adverse outcomes, especially lower extremity amputation. Topical antimicrobial therapy has been used on diabetic foot ulcers, either as a treatment for clinically infected wounds, or to prevent infection in clinically uninfected wounds.
Objectives
To evaluate the effects of treatment with topical antimicrobial agents on: the resolution of signs and symptoms of infection; the healing of infected diabetic foot ulcers; and preventing infection and improving healing in clinically uninfected diabetic foot ulcers.
Search methods
We searched the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations), Ovid Embase, and EBSCO CINAHL Plus in August 2016. We also searched clinical trials registries for ongoing and unpublished studies, and checked reference lists to identify additional studies. We used no restrictions with respect to language, date of publication, or study setting.
Selection criteria
We included randomised controlled trials conducted in any setting (inpatient or outpatient) that evaluated topical treatment with any type of solid or liquid (e.g., cream, gel, ointment) antimicrobial agent, including antiseptics, antibiotics, and antimicrobial dressings, in people with diabetes mellitus who were diagnosed with an ulcer or open wound of the foot, whether clinically infected or uninfected.
Data collection and analysis
Two review authors independently performed study selection, 'Risk of bias' assessment, and data extraction. Initial disagreements were resolved by discussion, or by including a third review author when necessary.
Main results
We found 22 trials that met our inclusion criteria with a total of over 2310 participants (one study did not report number of participants). The included studies mostly had small numbers of participants (from 4 to 317) and relatively short follow‐up periods (4 to 24 weeks). At baseline, six trials included only people with ulcers that were clinically infected; one trial included people with both infected and uninfected ulcers; two trials included people with non‐infected ulcers; and the remaining 13 studies did not report infection status.
Included studies employed various topical antimicrobial treatments, including antimicrobial dressings (e.g. silver, iodides), super‐oxidised aqueous solutions, zinc hyaluronate, silver sulphadiazine, tretinoin, pexiganan cream, and chloramine. We performed the following five comparisons based on the included studies:
Antimicrobial dressings compared with non‐antimicrobial dressings: Pooled data from five trials with a total of 945 participants suggest (based on the average treatment effect from a random‐effects model) that more wounds may heal when treated with an antimicrobial dressing than with a non‐antimicrobial dressing: risk ratio (RR) 1.28, 95% confidence interval (CI) 1.12 to 1.45. These results correspond to an additional 119 healing events in the antimicrobial‐dressing arm per 1000 participants (95% CI 51 to 191 more). We consider this low‐certainty evidence (downgraded twice due to risk of bias). The evidence on adverse events or other outcomes was uncertain (very low‐certainty evidence, frequently downgraded due to risk of bias and imprecision).
Antimicrobial topical treatments (non dressings) compared with non‐antimicrobial topical treatments (non dressings): There were four trials with a total of 132 participants in this comparison that contributed variously to the estimates of outcome data. Evidence was generally of low or very low certainty, and the 95% CIs spanned benefit and harm: proportion of wounds healed RR 2.82 (95% CI 0.56 to 14.23; 112 participants; 3 trials; very low‐certainty evidence); achieving resolution of infection RR 1.16 (95% CI 0.54 to 2.51; 40 participants; 1 trial; low‐certainty evidence); undergoing surgical resection RR 1.67 (95% CI 0.47 to 5.90; 40 participants; 1 trial; low‐certainty evidence); and sustaining an adverse event (no events in either arm; 81 participants; 2 trials; very low‐certainty evidence).
Comparison of different topical antimicrobial treatments: We included eight studies with a total of 250 participants, but all of the comparisons were different and no data could be appropriately pooled. Reported outcome data were limited and we are uncertain about the relative effects of antimicrobial topical agents for each of our review outcomes for this comparison, that is wound healing, resolution of infection, surgical resection, and adverse events (all very low‐certainty evidence).
Topical antimicrobials compared with systemic antibiotics : We included four studies with a total of 937 participants. These studies reported no wound‐healing data, and the evidence was uncertain for the relative effects on resolution of infection in infected ulcers and surgical resection (very low certainty). On average, there is probably little difference in the risk of adverse events between the compared topical antimicrobial and systemic antibiotics treatments: RR 0.91 (95% CI 0.78 to 1.06; moderate‐certainty evidence ‐ downgraded once for inconsistency).
Topical antimicrobial agents compared with growth factor: We included one study with 40 participants. The only review‐relevant outcome reported was number of ulcers healed, and these data were uncertain (very low‐certainty evidence).
Authors' conclusions
The randomised controlled trial data on the effectiveness and safety of topical antimicrobial treatments for diabetic foot ulcers is limited by the availability of relatively few, mostly small, and often poorly designed trials. Based on our systematic review and analysis of the literature, we suggest that: 1) use of an antimicrobial dressing instead of a non‐antimicrobial dressing may increase the number of diabetic foot ulcers healed over a medium‐term follow‐up period (low‐certainty evidence); and 2) there is probably little difference in the risk of adverse events related to treatment between systemic antibiotics and topical antimicrobial treatments based on the available studies (moderate‐certainty evidence). For each of the other outcomes we examined there were either no reported data or the available data left us uncertain as to whether or not there were any differences between the compared treatments. Given the high, and increasing, frequency of diabetic foot wounds, we encourage investigators to undertake properly designed randomised controlled trials in this area to evaluate the effects of topical antimicrobial treatments for both the prevention and the treatment of infection in these wounds and ultimately the effects on wound healing.
Plain language summary
Topical antimicrobial agents (antibacterial products applied directly to wounds) for treating foot ulcers in people with diabetes
Review question
We reviewed the evidence about whether or not antimicrobial agents (antibacterial products) can prevent or treat foot infections in people with diabetes when they are applied topically (directly to the affected area). We wanted to find out if antibacterial treatments could help both infected and uninfected wounds to heal, and prevent infection in uninfected wounds.
Background
People with diabetes are at high risk of developing foot ulcers. These wounds can cause discomfort and often become infected. Diabetic foot ulcers that do not heal can result in amputation of part or all of the foot or even the lower leg. Antimicrobial agents, such as antiseptics and antibiotics, kill or prevent bacteria from growing, and are sometimes used to treat diabetic foot ulcers. Antimicrobials may be used either to reduce infection or promote healing in infected wounds, or to prevent infection or promote healing in wounds where infection has not been detected. We wanted to find out whether antimicrobial treatments were effective in either of these cases; which treatments were most effective; and if those treated experienced any harmful side effects.
Study characteristics
In August 2016 we searched for randomised controlled trials involving the use of any antimicrobial treatment on foot ulcers or other open wounds of the foot in people with diabetes. We found 22 trials involving a total of over 2310 adult participants (one trial did not report the number of participants). Participant numbers in each trial ranged from 4 to 317 and follow‐up times during and after treatment ranged from 4 to 24 weeks. Some trials included participants with ulcers that were infected, while other trials included participants with ulcers that were uninfected. The trials compared a variety of different antimicrobial dressings, solutions, gels, creams, or ointments.
Key results
Many of the trials did not report important data, which means the reliability of the results is uncertain. The results of five trials involving 945 participants suggest that use of some type of antimicrobial dressing may increase the number of ulcers healed in medium‐term follow‐up (4 to 24 weeks) when compared with a non‐antimicrobial dressing (low certainty evidence). Due to limited information, we were unable to assess the effectiveness of treatments in either preventing or resolving wound infection. Four trials involving 937 participants compared systemic antibiotics (given by mouth or via injection, distributed to the whole body by the bloodstream) with antimicrobial treatments applied directly to the wound. These trials did not provide data on healing or infection, but it appeared that there was no difference in the side effects experienced by participants whose ulcers were treated systemically or topically (moderate certainty evidence).
Quality of the evidence
Overall, the certainty of the evidence provided by the trials was too low for us to be certain of the benefits and harms of topical antimicrobial treatments for treating foot ulcers in people with diabetes. More, larger, and better‐designed randomised controlled trials should be carried out in this area.
Summary of findings
Background
Worldwide, there are currently over 415 million adults with diabetes mellitus (5 million of whom die of the disease annually), and the prevalence of diabetes is expected to reach over 640 million (1 in 10) by 2040 (IDF 2015). Furthermore, treating diabetes accounts for 12% of global health expenditure (USD 673 billion). Skin wounds, particularly chronic ulcers, commonly develop in the feet of people with diabetes mellitus, usually related to neuropathy (nerve damage), as well as arterial (blood vessel) disease or trauma (Davies 2007; Lipsky 2009). Peripheral neuropathy (damage to the nerves to the feet), peripheral arterial disease, or both develop over time in most people with diabetes (American Diabetes Association 2003). Many people with diabetes also have as‐yet poorly defined defects in immune responses that impair their ability to resist or overcome infection (Delamaire 1997). These factors put diabetic patients at high risk of developing foot ulcers, most of which become infected. The estimated lifetime risk of a foot ulcer in a person with diabetes is 25%, at a cost (in Europe in 2008) of EUR 10,000 for an uninfected ulcer and EUR 17,000 for an infected ischaemic ulcer (Markakis 2016). These wounds, especially those that become clinically infected, cause substantial morbidity. Estimates are that somewhere in the world a person with diabetes undergoes a lower extremity amputation every 20 seconds. (IWGDF 2016). Infection of a diabetic foot wound is defined as the presence of at least two of the classic signs or symptoms of inflammation (pain or tenderness, warmth, redness, swelling) or purulent secretions (pus). Foot problems, especially when complicated by infection, are now responsible for more days of hospitalisation than any other complication of diabetes (Pecoraro 1990; Singh 2005). Diabetic foot infections, in particular those that contiguously spread to underlying bone, are also the main precipitating factor for lower extremity amputation, which is associated with substantial financial cost, reduced quality of life, and early mortality (Lipsky 2012b; Lipsky 2016). To avoid these adverse outcomes it is crucial to prevent foot infections, or failing that, to optimally treat the infected wounds. Treatment of infection almost always requires antimicrobial therapy, which may be given systemically (to the whole body via the oral or parenteral (i.e. intravenous or intramuscular) route) or topically (i.e. locally, through application of antiseptic, antibiotic, or other antimicrobial preparations (e.g. solutions, creams, gels, ointments)). Sometimes it is difficult for the clinician to tell if a diabetic foot wound is infected, especially if the patient has peripheral neuropathy or arterial disease. Furthermore, the mere presence of micro‐organisms, especially if they are virulent or present in high numbers, may also impair wound healing in clinically uninfected wounds. Thus some advocate prescribing antimicrobial therapy (especially topically) for high‐risk clinically uninfected wounds to reduce the bacteria 'bioburden' and potentially accelerate healing or avoid overt infection.
Description of the condition
Micro‐organisms rapidly colonise virtually all open wounds; this usually has no apparent consequences in the absence of clinical evidence of infection, and healing occurs as expected (White 2006). However, some wounds exhibit a host response (usually manifested by inflammation or tissue damage) to the organisms they harbour, suggesting that they are clinically infected (Cutting 2005). The likelihood of a wound becoming infected increases directly with the size of its microbial inoculum, the virulence of the specific colonising organisms, and the level of diminution of the host’s local and systemic immunological resistance (Heinzelmann 2002). For the clinician, characterising a wound as infected or not is a key clinical challenge. Published studies show that almost half of all people with a diabetic foot ulcer have no clinical signs of infection; these people do not usually need to have cultures taken from their wound, as they generally do not require antimicrobial therapy (Lavery 2006; Prompers 2007).
Many classification schemes have been proposed for diabetic foot wounds, but most categorise infection only as being either 'present' or 'absent', and do not specify infection severity or how to define its presence. Classification systems that provide more information on infection have been developed by the International Working Group on the Diabetic Foot (IWGDF) and the Infectious Diseases Society of America (IDSA) (Table 6) (IWGDF 2015; Lipsky 2012b). These classifications, which are nearly identical, have been validated as predictive of the patient’s need for hospitalisation and for lower extremity amputation. As they also provide a way for a clinician to communicate key information to others caring for the wound, guidelines recommend that clinicians routinely use them to classify the presence and clinical severity of diabetic foot infections (Lipsky 2012b).
1. Infectious Diseases Society of America and International Working Group on the Diabetic Foot classification of diabetic foot infection.
| Clinical manifestation of infection | PEDIS grade | IDSA infection severity |
| No symptoms or signs of infection | 1 | Uninfected |
Infection present, as defined by the presence of at least 2 of the following items:
|
||
| Local infection involving only the skin and the subcutaneous tissue (without involvement of deeper tissues and without systemic signs as described below). If erythema, must be > 0.5 cm to ≤ 2 cm around the ulcer. Exclude other causes of an inflammatory response of the skin (e.g. trauma, gout, acute Charcot neuro‐osteoarthropathy, fracture, thrombosis, venous stasis) | 2 | Mild |
| Local infection (as described above) with erythema > 2 cm, or involving structures deeper than skin and subcutaneous tissues (e.g. abscess, osteomyelitis, septic arthritis, fasciitis), and no systemic inflammatory response signs (as described below) | 3 | Moderate |
Local infection (as described above) with the signs of SIRS, as manifested by ≥ 2 of the following:
|
4 | Severe* |
Abbreviations: IDSA, Infectious Diseases Society of America; PaCO2, partial pressure of arterial carbon dioxide; PEDIS, perfusion, extent/size, depth/tissue loss, infection, and sensation; SIRS, systemic inflammatory response syndrome
*Ischaemia may increase the severity of any infection, and the presence of critical ischaemia often makes the infection severe. Systemic infection may sometimes manifest with other clinical findings, such as hypotension, confusion, vomiting, or evidence of metabolic disturbances, such as acidosis, severe hyperglycaemia, and new‐onset azotaemia.
In light of the high prevalence of infection in foot wounds in people with diabetes, it is important for clinicians to consider this possibility when such patients present for care. Clinicians should generally define infection by the presence of at least two of the classic symptoms or signs of inflammation, that is erythema (redness), calor (warmth), tumour (swelling or induration), dolour (pain or tenderness), or purulent secretions (pus). As the presence of neuropathy or arterial or immunological diseases may obscure these findings, some authorities accept additional "secondary" or "intermediate" signs of infection (Cutting 2005; Gardner 2001; Lipsky 2012b).
Cultures of specimens from acutely infected wounds (especially in patients from high‐income Western countries who have not recently been on antibiotic therapy) usually grow bacteria classified as aerobic gram‐positive cocci. In this situation these are generally the only bacteria against which clinicians need target their antimicrobial therapy. However, in chronic wounds, or when a patient has recently been treated with antibiotics, other bacteria (especially aerobic gram‐negative rods and obligate anaerobes) often accompany these gram‐positive cocci, necessitating broader‐spectrum antibiotic therapy. Recently, molecular diagnostic studies of wounds have shown that they harbour an even greater variety of organisms than had previously been recognised (Davies 2004; James 2008), but the clinical importance of this finding is as yet unclear (Lipsky 2013). Furthermore, in many chronic wounds bacteria persist as so‐called "small colony variants" (von Eiff 2006), which are both more difficult to culture and to eradicate. Finally, micro‐organisms in chronic wounds often exist in states or communities that are particularly difficult to treat, such as in an adhesive, polymeric matrix called biofilm, which induces chronic inflammation, delays healing, and protects the organism from the effects of antimicrobial therapy (Rhoads 2008).
Given the problems associated with treating diabetic foot infections, treatment with topical antimicrobials has potential benefits, for example it could result in very high drug levels at the infected site (with little or none at other sites) and may allow the use of agents that cannot be given systemically (Lipsky 2009). These findings, combined with a wish to avoid systemic antibiotic therapy where possible, have led many clinicians to consider using topical antimicrobial therapy for open infected wounds, especially those that fail to heal despite apparently appropriate treatment. It was thus important to determine if this route of therapy is safe and effective.
Description of the intervention
Clinically infected wounds virtually always require antibiotic therapy, whereas clinically uninfected wounds that are healing normally do not (Lipsky 2009). Of note, some superficial infections (e.g. impetigo, fungal dermatitis) may respond to first‐line topical antimicrobial therapy alone, without recourse to systemic therapy. However, controversy exists over how to treat poorly healing wounds that display 'secondary' signs suggestive of infection and that may benefit from topical antimicrobial agents. The rationale for using a topical antimicrobial is to kill, or at least halt, the replication of pathogenic micro‐organisms on the skin, mucosae, or in a wound, without causing clinically significant damage to the host cells. Topical antimicrobials may be used on their own or in combination with other topical or systemic antimicrobial agents.
There are several classes of topical agents that inhibit or kill micro‐organisms (Lipsky 2009).
Disinfectants are non‐specific agents with activity against virtually all disease‐causing micro‐organisms, including those in a spore state. Since these may be toxic to host tissues, they are used primarily for sterilising inanimate surfaces and not for topical treatment of wounds.
Most topical antimicrobials for clinical use belong to one of two major groups:
Antiseptics: These are usually a type of disinfectant that can be used on intact skin and some open wounds to kill or inhibit micro‐organisms. They often have multiple microbial targets, a broad antimicrobial spectrum, and residual anti‐infective activity. Unfortunately, they may be toxic to one or more types of host cells or tissues (e.g. fibroblasts, keratinocytes, and possibly leukocytes). Topical antiseptic agents used in the past (e.g. hexachlorophene and iodines) are used less frequently today because of concerns about toxicity to host cells and the availability of safer agents. Chlorhexidine and povidone iodine are older agents that have been (and continue to be) widely used as wound antiseptics. Recently, a variety of products that release silver ions have been approved and are being promoted for management of wound micro‐organisms.
Antibiotics: These are chemicals produced either naturally (by a micro‐organism) or synthetically that, in dilute solution, inhibit or kill other micro‐organisms. They usually act on one specific cell target, have a narrower spectrum of activity than antiseptics, are relatively non‐toxic, and are more susceptible to losing their effectiveness as bacteria develop resistance. Most agents that are used exclusively as topical antibiotics have efficacy against gram‐positive bacteria (e.g. bacitracin, mupirocin, retapamulin), with a smaller number demonstrating efficacy against gram‐negative bacteria (e.g. neomycin, silver sulphadiazine). Some antibiotics that are used systemically (e.g. gentamicin, metronidazole, clindamycin) have also been formulated for topical use.
Below, we have provided a summary of the principal characteristics of currently available antiseptics (Table 7) and topical antibiotics (Table 8).
2. Topical antiseptic products available for treating chronic wounds.
| Product and formulations | Formulations | Bacterial spectrum | Advantages | Disadvantages | Costa | Indicationsb and comments |
| Acetic acid | 0.25%, 0.5%, and 1% solutions | Bactericidal against most gram‐positive and gram‐negative organisms, including Pseudomonas aeruginosa | Inexpensive; shown to eliminate P aeruginosa colonisation from burn | Cytotoxic in vitro although maybe not in vivo; limited activity against biofilm | $ | No longer as widely used as in the past |
| Cadexomer iodine | Gel,c ointment, and dressing | Polysaccharide starch lattice; active agent is slowly released free iodine; broad spectrum of activity (same as iodine) | Reduced local toxicity compared to iodine; elemental iodine released on exposure to exudate | Application may cause stinging and erythema, but less tissue damage than other iodine products; effect may not persist, and efficacy may be reduced in body fluids. | $$ | Indicated for use in cleaning wet ulcers and wounds and reducing microbial load in the wound environment |
| Cetrimide | Solution, 40% | Active against bacteria and fungi; not active against P aeruginosa | May be less toxic to wound tissues than other antiseptics | May be corrosive and is potentially harmful if swallowed | $ | Not available in the USA |
| Chlorhexidine gluconate |
Solution, 2% and 4%; liquid, 2% and 4%; hand rinse, 0.5%; wipes, 0.5%; sponge/brush, 4%; and foam, 4% | Active against gram‐positive bacteria (e.g. Staphylococcus aureus) and gram‐negative bacteria, including P aeruginosa | Persistent activity up to 6 h after application; few adverse effects | Hypersensitivity, including anaphylaxis, generalised urticaria, bronchospasm, cough, dyspnoea, wheezing, and malaise; may cause serious injury to the eye and middle ear; avoid contact with face or head; some resistance reported |
$ | 2% chlorhexidine indicated as surgical hand scrub, hand wash, skin and wound cleanser; polyhexanide is a similar, newer biguanide. |
| Hexachlorophene | Liquid, 3%; foam, 0.23% with 56% alcohol | Biguanide that is bacteriostatic against Staphylococcus species and other gram‐positive bacteria | May retain residual effect on skin for several days | Rapidly absorbed and may result in toxic blood levels; application to burns has resulted in neurotoxicity and death; may cause central nervous system stimulation and convulsions, dermatitis, and photosensitivity reactions | $$$ | Not recommended for routine use on wounds due to potential toxicity |
| Iodine compounds and iodine tincturec | Solution (aqueous) 2% and 2.4%; and tincture (44% to 50% alcohol) 2% and 2.4% | Microbicidal against bacteria, fungi, viruses, spores, protozoa, and yeasts | Broad spectrum | Highly toxic if ingested or significantly absorbed; do not use with occlusive dressings; causes pain and stains skin and clothing; use cautiously in people with thyroid disorders | $ | Iodine compounds are now rarely used for wound management; cadexomer iodine and povidone iodine products are less toxic. |
| Povidone iodinec | Ointment, 1%, 4.7%, 10%; solution, 1% and 10%; also wash, scrub, cleanser, gel, aerosol, gauze pad, swab, and other forms | Broad spectrum includes S aureus and enterococci; active ingredient is liberated free iodine; shares spectrum but is less potent than iodine | Less irritating to skin and allergenic than iodine. Can be covered with dressings. Clinically significant resistance very rare | Antibacterial action requires at least 2 min contact; may cause stinging and erythema; effect may not persist, and efficacy may be reduced in body fluids; prolonged use may cause metabolic acidosis; stains skin and clothing; possible interaction with starches in dressings | $ | Indicated for perioperative skin cleansing and for cleansing and prevention of infection in superficial burns, incisions, and other superficial wounds |
| Sodium hypochlorite (Dakin’s solution and EUSOL) |
Solution, 0.0125%, 0.125%, 0.25%, and 0.5% | Vegetative bacteria, viruses, and some spores and fungi | Inexpensive | No known systemic toxicity. May require prolonged contact for antibacterial action; inactivated by pus; toxic to fibroblasts and keratinocytes, and may cause pain or lyse blood clots | $ | A concentration of 0.025% is both bactericidal and non‐toxic to tissues (Heggers 1991). |
| Hydrogen peroxidec | Solution, 1% and 3%; and cream, 1% | Oxidizing agent active against many gram‐positive and gram‐negative bacteria | Broad‐spectrum, bactericidal, inexpensive; no known 1q11 | May cause some discomfort | $ | Commonly used, but few clinical studies |
| Silver nitrate | Solution 0.5%, 10%, 25%, and 50%; ointment, 10%; and swabs, 25% to 50% | Silver ions are bactericidal against a broad spectrum of gram‐positive and gram‐negative bacteria. | Low cost; easily applied | Painful on application; stains tissues; may delay healing; concentrations 10.5% cause cauterisation; inactivated by wound exudates and chlorine | $ | Previously widely used, but now largely replaced by other compounds, including newer silver dressings |
| Silver dressings | At least 6 approved products with different properties | Slowly released silver ions have broad spectrum, including MRSA and VRE. | Provide sustained levels of active silver ions; microbial resistance is rare; less painful and few adverse effects than silver nitrate; variety of products adaptable to different types of wounds; infrequent application required | Levels of silver ions at wound interface not well defined; may cause silver staining of tissues; may delay epithelialisation; relatively expensive; few published comparative trials | $$ | Should not substitute for non‐medicated dressings for uninfected wounds; may be useful for subclinically infected, highly colonised wounds or for wounds being prepared for skin grafting |
Abbreviations: EUSOL, Edinburgh University Solution of Lime; MRSA, methicillin‐resistant Staphylococcus aureus; VRE, vancomycin‐resistant enterococci.
aCosts are approximate in USD per day for treating 100‐square centimetre wound, as follows: $, < USD 3; $$, USD 3 to 15; and $$$, > USD 15. bUS Food and Drug Administration–approved indications. cAvailable without prescription. Modified from Lipsky 2009.
3. Topical antibiotic products available for treating chronic wounds.
| Product and formulations | Formulations | Bacterial spectrum | Advantages | Disadvantages | Costa | Indicationsb and comments |
| Bacitracin c | Ointment, 500 units/g; and powder combinations with neomycin, polymyxin B, and zinc | Many gram‐positive organisms, including aerobic staphylococci and streptococci, corynebacteria, anaerobic cocci, and clostridia; inactive against most gram‐negative organisms | Activity not impaired by blood, pus, necrotic tissue, or large bacterial inocula; resistance is rare but increasing among staphylococci; no cross‐resistance with other antibiotics; minimal absorption | May cause allergic reactions, contact dermatitis, and (rarely) anaphylactic reactions; may lead to overgrowth of drug‐resistant organisms, including fungi | $ | Widely used for many years; indicated for prevention of infection in minor skin wounds |
| Fusidic acid | Cream, 2%; ointment, 2%; and gel, 2% | Staphylococcus aureus, streptococci (in topical concentrations), corynebacteria, and clostridia | Penetrates intact and damaged skin as well as crust and cellular debris | Occasional hypersensitive reactions; resistance among staphylococci is emerging; must apply 3 times daily | $$ | Not available in the USA |
| Gentamicin | Cream, 0.1%; and ointment, 0.1% | Streptococci, staphylococci, Pseudomonas aeruginosa, Enterobacter aerogenes, Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae | Broad spectrum; inexpensive | Must be applied 3 to 4 times daily; may drive resistance to an agent used systemically | $ | Indicated for primary skin infections (pyodermas) and secondary skin infections, including infected excoriations, and for bacterial superinfections |
| Mafenide acetate | Solution, 5%; and cream, 85 mg/g | A sulfonamide that is bacteriostatic against many gram‐negative organisms, including P aeruginosa, and some gram‐positive organisms, but minimal activity against staphylococci and some obligate anaerobes | Remains active in the presence of pus and serum, and its activity is not affected by acidity of environment | Systemic absorption may occur; drug and metabolites may inhibit carbonic anhydrase, potentially causing metabolic acidosis; use cautiously in patients with renal impairment; pain on application; hypersensitive reactions. | $$$ | Indicated as adjunctive therapy in second‐ and third‐degree burns; may be used in rapidly progressing bacterial necrotising fasciitis; limited use in other wounds |
| Metronidazole | Cream, 0.75%; gel, 1%; lotion, 0.75% | Many clinically important anaerobic bacteria | May reduce odour associated with anaerobic infections; application only 1 to 2 times daily | Relatively expensive; systemic formulations available; could drive resistance to these | $–$$ | Indicated for inflammatory papules and pustules of rosacea |
| Mupirocin and mupirocin calcium | Ointment, 2%; for mupirocin calcium, cream, 2.15%; and nasal ointment, 2.15% (equivalent to 2% mupirocin) | Gram‐positive aerobes, including S aureus (most MRSA), Staphylococcus epidermidis, Staphylococcus saprophyticus, and streptococci (groups A, B, C, and G) but not enterococci, some gram‐negative aerobes (not P aeruginosa), corynebacteria, and obligate anaerobes | Minimal potential for allergic reactions | Rare local burning and irritation; applying ointment to large wounds in azotaemic patients can cause accumulation of polyethylene glycol; long‐term use can lead to resistance among staphylococci, which is increasing | $$ | Indicated for topical treatment of impetigo and eradication of nasal colonisation with S aureus |
| Neomycin sulfatec | Powder; cream, 0.5%; combinations with polymyxin B and pramoxine, and ointment, 0.5%; combinations with bacitracin, polymyxin B, lidocaine, and pramoxine | Good for gram‐negative organisms but not P aeruginosa; active against some gram‐positive bacteria, including S aureus, but streptococci are generally resistant; inactive against obligate anaerobes | Low cost; applied only 1 to 3 times daily; may enhance re‐epithelialisation | Topical powder in wound irrigating solution may cause systemic toxicity (FDA banned); use other formulations cautiously on large wounds, especially with azotaemia; hypersensitive reaction in 1% to 6%, often with chronic use or history of allergies. | $ | Use of topical powder alone or in solution is not recommended; cream and ointment, in combination with other agents, are indicated for prevention of infection in minor skin injuries. |
| Nitrofurazone | Solution, 0.2%; ointment, 0.2%; and cream, 0.2% | Broad gram‐positive and gram‐negative activity, including S aureus and streptococci, but not P aeruginosa | Used mainly for burn wounds | Hypersensitive reactions; polyethylene glycols (in some formulations) may be absorbed and can cause problems in azotaemic patients | $$ | Indicated as adjunctive to prevent infections in people with second‐ and third‐degree burns |
| Polymyxin Bc | Cream, 5000 units/g or 10,000 units/g, in combination with other agents | Bactericidal against many gram‐negative organisms, including P aeruginosa; minimal activity against gram‐positive bacteria; activity may be neutralised by divalent cations | Inexpensive | Some hypersensitive and neurological or renal adverse reactions reported; may show cross‐reaction with bacitracin. | $ | Only available in combination with other agents, including bacitracin and neomycin; indicated for prevention |
| Retapamulin | Ointment, 1% | Active against staphylococci (but uncertain for MRSA) and streptococci and some obligate anaerobes | May be active against some mupirocin‐resistant S aureus strains; broader activity than mupirocin | Not evaluated for use on mucosal surfaces; may cause local irritation | $$$ | Indicated for impetigo due to S aureus (methicillin‐susceptible only) or Streptococcus pyogenes |
| Silver sulphadiazine | Cream, 1% | A sulfonamide; the released silver ions are the primary active ingredient; active against many gram‐positive and gram‐negative organisms, including P aeruginosa . | Applied only once or twice daily; soothing application; low rate of hypersensitive reaction | Potential cross‐reaction with other sulphonamides; may rarely cause skin staining | $ | Indicated as adjunctive treatment to prevent infections in people with second‐ and third‐degree burns |
| Sulfacetamide Na+ | Lotion, 10% | Bacteriostatic against many gram‐positive and gram‐negative pathogens | Broad spectrum; can be combined with sulphur | Systemic absorption and rarely severe side effects occur with application to large, denuded areas; hypersensitive reactions may occur. | $$$ | Indicated for secondary bacterial skin infections due to susceptible organisms and for acne vulgaris in adults |
There are no published studies supporting the use of topical erythromycin, clindamycin, aminoglycosides other than neomycin, gramicidin, or tetracyclines for treating chronically infected wounds.
Abbreviations: FDA, US Food and Drug Administration; MRSA, methicillin‐resistant Staphylococcus aureus.
aCosts are approximate in USD per day for treating 100‐square centimetre wound, as follows: $, < USD 3; $$, USD 3 to 15; and $$$, > USD 15. bFDA‐approved indications. cAvailable without prescription.
How the intervention might work
For millennia healers have applied various compounds to infected wounds, some of which (e.g. silver, honey) are still in use today. Use of a topical application has many potential advantages compared with giving systemic antibiotic therapy, including: a high and sustained concentration of the antimicrobial agent at the site of infection; the need to use only a limited amount of the antimicrobial at the selected site; avoidance of potential toxicity associated with systemic treatment; ability to use novel agents not available for systemic use; easy application in the outpatient setting; and potentially better patient adherence to treatment. Topical treatments may also prove helpful in addressing the globally increasing problem of multidrug‐resistant organisms that are now untreatable with most systemic agents. For example, a study of 47 organisms from burn wounds that were multidrug‐resistant to systemic antibiotics were susceptible to 11 commonly used topical antibiotics and antiseptics, although the rates of resistance were higher than in non–multidrug‐resistant organisms (Neely 2009).
Topical antimicrobial therapy also has some potential disadvantages: few agents have been proven to be effective in clinical trials; almost all have minimal penetration of intact skin or soft tissue, limiting use to open wounds that do not have either cellulitis or deep soft‐tissue infection; systemic absorption of some agents may occur if used on large wounds; agents may induce local hypersensitivity or contact dermatitis reactions; some agents may interfere with normal wound‐healing processes; treatment may produce an alteration of normal cutaneous flora that may lead to other problems; topical applications are difficult to dose accurately; topical agents may require frequent applications; agents may be difficult to apply or aesthetically unacceptable to some patients; and agents in multiuse containers can become contaminated during repeated use (Gelmetti 2008; Lio 2004).
Topical antimicrobials have traditionally been formulated in one of two ways. As ointments, they are more occlusive, often contain petrolatum, and are best used for dry lesions. As creams, they are less occlusive, wash off with water, are less messy, and are best for moist lesions. Newer technologies have allowed incorporation of antimicrobials into dressings, such as alginates, foams, collagen and sponges, potentially allowing controlled release at the wound surface. One major problem with topical therapies is that internationally no official oversight agency has standardised and approved specific tests to establish the efficacy and safety of these agents (Cooper 2004).
Why it is important to do this review
A recent Cochrane review summarised and analysed the data on the effectiveness of systemic antibiotic therapy for diabetic foot infections (Selva Olid 2015). To date, however, the lack of available data has made it difficult to assess the efficacy of topical antimicrobials for diabetic foot ulcers (Drucker 2012; Lipsky 2009; Peters 2012). A systematic review of antimicrobial agents for various chronic wounds (including diabetic foot ulcers) concluded that few systemic agents improved outcomes, but hastened healing was associated with use of several topical substances (O'Meara 2001). A Cochrane systematic review of treatment with antibiotics or antiseptics for healing venous leg ulcers found some evidence supporting the use of cadexomer iodine but not the routine use of honey‐ or silver‐based products (O'Meara 2014); further evidence was required before conclusions could be made about other agents. A systematic review of the effectiveness of various interventions for enhancing the healing of chronic diabetic foot ulcers found limited evidence of benefit of any agents for healing of diabetic foot wounds (Game 2016). Another Cochrane review of treatment with silver‐based wound dressings or topical agents for diabetic foot ulcers found no randomised controlled trials reporting outcomes on healing rates or infection resolution (Bergin 2006). Likewise, a Cochrane review of silver‐containing dressings or topical agents for treating infected or contaminated chronic wounds concluded there was insufficient evidence, on the basis of three randomised trials, to recommend these treatments (Vermeulen 2007). An updated Cochrane systematic review on topical honey for treating wounds concluded that it may reduce healing time for mild‐to‐moderate superficial and partial‐thickness burns and infected postoperative wounds, but did not significantly hasten leg ulcer healing (Jull 2015). Finally, a recent systematic review of the effectiveness of interventions in the management of diabetic foot infections found six studies that investigated the use of topical agents (Peters 2016), but the methods and results did not allow the authors to draw any definitive conclusions. Among the two studies of topical antibiotics, one found that an antimicrobial peptide, pexiganan cream, was similar in effectiveness to a systemic antibiotic (ofloxacin) in the treatment of mildly infected diabetic foot ulcers, while another study of adjunctive therapy with a gentamicin‐collagen sponge (along with systemic antibiotic therapy) was difficult to interpret because of methodological problems (Peters 2016).
Clearly, the currently available literature does not provide an adequate overview as to whether topical antimicrobial therapy is safe or effective for foot ulcers in people with diabetes. Given the high frequency of these wounds, their potentially serious adverse outcomes, and the possibility of benefit in preventing or curing infection or accelerating wound healing and of reducing unnecessary use of systemic antibiotics, we considered a systematic review of all the available evidence of the use of topical antimicrobial agents for preventing or treating infection in diabetic foot ulcers to be both timely and important.
Objectives
To evaluate the effects of treatment with topical antimicrobial agents on: the resolution of signs and symptoms of infection; the healing of infected diabetic foot ulcers; and preventing infection and improving healing in clinically uninfected diabetic foot ulcers.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) conducted in any setting (e.g. inpatient/institutional or outpatient/ambulatory).
Types of participants
People with diabetes mellitus (as defined by the study authors) diagnosed with an ulcer of the foot (i.e. below the malleoli, the bony prominences on each side of the ankle), whether clinically infected or uninfected. We only included a study that enrolled a mixed population of participants if some of those enrolled had a foot ulcer and diabetes, and if the randomisation to treatment was stratified by wound type. We otherwise excluded studies with partial trial data, as this approach is akin to a subgroup analysis. We also included studies that had a mixed population if more than 80% of participants were people with diabetes and a foot ulcer.
Types of interventions
We reviewed studies evaluating treatment with any type of solid (liquid, gel, ointment, cream) topical antimicrobial agent, including antiseptics and antibiotics. We did not include any studies of antimicrobial agents that were in a 'gaseous' form (e.g. local oxygen), or that relied on phototherapy.
Specific comparisons included one or more of the following:
a topical antimicrobial agent plus standard care (e.g. cleansing, debridement, wound dressings, pressure off‐loading) compared with standard care alone, or combined with a placebo;
two or more different topical antimicrobial agents;
a topical antimicrobial agent (with or without a systemic antimicrobial agent) compared with a systemic antimicrobial agent alone (or with a topical placebo).
Types of outcome measures
Our primary and secondary outcomes are listed below. If a study was otherwise eligible (i.e. it had the correct study design, population, and intervention/comparator) but did not report a listed outcome, we attempted to contact the study authors to establish whether or not they had measured an outcome of interest to us that they did not report.
We defined follow‐up as the time from participant randomisation to outcome measurement. We reported outcome measures at the latest time point available (assumed to be length of follow‐up if not otherwise specified) or the time point specified in the methods as being of primary interest to the authors (if this was different from latest time point available).
Primary outcomes
For studies of wounds that were clinically infected or clinically uninfected, our primary outcome was as follows.
-
Complete ulcer healing. We included this outcome (complete epithelialisation of the ulcer), seeking the following as measures:
time to complete ulcer healing (correctly analysed using survival, time‐to‐event approaches, ideally with adjustment for relevant covariates, such as baseline size);
the proportion of people with an ulcer that completely healed.
Where both of these outcomes were reported, our plan was to present all data in a summary outcome table for reference, but give 'time to complete ulcer healing' primacy; however, no study reported time‐to‐event data that was analysable. As planned, when time was analysed as a continuous measure, but it was not clear whether all ulcers had healed, we documented the use of this outcome in the study but did not extract, summarise, or otherwise use the data in any meta‐analysis.
For studies involving wounds that were clinically infected at baseline, a second primary outcome for this review was as follows.
Resolution of infection. We accepted the investigators' assessment of resolution of infection, e.g. diminution or disappearance of clinical findings such as erythema (redness), warmth, pain or tenderness, induration (swelling), or purulent secretions (Table 6).
For studies involving wounds that were clinically uninfected at baseline, a second primary outcome for the review was as follows.
Incidence of infection. We accepted the investigators' assessment of the development of infection in a diabetic foot wound, e.g. by the appearance of new clinical findings, such as erythema (redness), warmth, pain or tenderness, induration (swelling), or purulent secretions (Table 6) (Lipsky 2012b).
Secondary outcomes
For both clinically infected and clinically uninfected wounds, we reported the following outcomes, when available.
Microbial counts, usually defined as bacterial colony forming units/gram of tissue or semiquantitative counts of number of colonies on a culture plate (typically graded from 1 to 4).
Health‐related quality of life, if it was reported using global measures of a validated scale (e.g. SF‐36 or EQ‐5D) or a validated disease‐specific questionnaire (e.g. Cardiff Wound Impact Schedule). These reported data were adjusted for the baseline score. We did not include ad hoc measures of quality of life that are unlikely to be validated and would not be common to multiple trials.
Risk of surgical resection of the foot wound, including partial or complete lower limb amputation.
Adverse events, defined and grouped together, as 'adverse events' where the study provided a clear methodology for the collection of these data. This would include making it clear whether (i) events were reported at the participant level or if multiple events per person were reported; and (ii) that an appropriate adjustment was made for data clustering. Where available, we extracted data on all serious and all non‐serious adverse events. We anticipated that adverse events for topical treatments would be likely to be similar to those for conventional treatments (e.g. wound deterioration, maceration, pruritis). We also recorded information about study authors' assessment of the treatment‐related nature of adverse events. (Nebeker 2004).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant RCTs:
the Cochrane Wounds Specialised Register (searched 15 August 2016);
the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library) (2016, Issue 7, searched 15 August 2016);
Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations, MEDLINE Daily, and Epub Ahead of Print) (1946 to 15 August 2016);
Ovid Embase (1974 to 15 August 2016);
EBSCO CINAHL Plus (1937 to 15 August 2016).
The full search strategies for CENTRAL, Ovid MEDLINE, Ovid Embase, and EBSCO CINAHL Plus are shown in Appendix 1.
We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase randomised trials filter terms developed by the UK Cochrane Centre (Lefebvre 2011). We combined the Cumulative Index to Nursing and Allied Health Literature (CINAHL) search with the randomised trials filter terms developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). We used no restrictions with respect to an article's language, date of publication, or study setting.
We also searched the following clinical trials registries (19th December 2016) for additional eligible studies:
ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/Default.aspx);
EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
For studies that met our criteria we emailed any listed contact person to seek any available results of the study.
Searching other resources
In addition to the searches described above, we checked the reference lists of all relevant trials identified and retrieved by the above methods. We originally planned to contact other authors and trialists who work in the area, but did not do so.
Data collection and analysis
We summarised our data using standard Cochrane methodologies (Higgins 2011). Data collection and analysis were carried out according to methods stated in the published protocol (Lipsky 2014), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors independently assessed each reference identified by the search against our inclusion criteria. We retrieved full copies of those references that appeared potentially eligible, and two review authors independently assessed each of these papers. Any disagreements were resolved through discussion, or by consultation with a third review author if required.
Data extraction and management
One review author extracted data from the included trials using a piloted form, and another review author checked the entered data.
We extracted the following data when available:
trial identification (first author's surname and year of main publication);
setting of care;
participant eligibility criteria;
participant demographics (age, sex, country);
total number of participants recruited;
number of participants per group;
characteristics of the foot ulcers (e.g. anatomic site, size, number of ulcers, presence/absence of infection, duration of ulceration);
ulcer treatments (antimicrobial and other);
details of concurrent interventions (e.g. off‐loading, debridement);
duration of antimicrobial treatment;
duration of follow‐up;
outcomes, as defined above, at the end of therapy and at last follow‐up post‐therapy; and
withdrawals and losses to follow‐up, with reasons, by treatment group.
The review authors discussed any discrepancies and achieved a final consensus.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study following the domain‐based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions (Appendix 2) (Higgins 2011). They discussed any discrepancies and achieved consensus on the final assessment.
The Cochrane 'Risk of bias' tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting, and other issues relating to bias (Appendix 2).
We have presented our assessment of risk of bias using two 'Risk of bias' summary figures:
a summary of bias for each item across all studies; and
a cross‐tabulation of each trial by all of the 'Risk of bias' items. We classified studies judged to be at high risk of selection bias, detection bias, or attrition bias as being at overall high risk of bias (for the specified outcome for that study).
Measures of treatment effect
We reviewed the evidence separately for each of the different types of topical antimicrobial agents.
For each binary (yes/no) outcome (e.g. wound healed, lower extremity amputation, adverse event) we calculated the risk ratio (RR) with 95% confidence intervals (CI). In this review we only reported continuous data for the quality of life outcome, which we presented as mean differences (MD) with 95% CI. We were unable to present time‐to‐event data using hazard ratios with 95% CI, as these data were not available for any included study.
Unit of analysis issues
Our unit of analysis was the individual person: we collected and analysed a single measurement for each outcome from each participant. Where studies had unit of analysis issues that were not adequately handled, we noted this finding as part of our 'Risk of bias' assessment. We included three‐arm trials, but where possible we either combined control arms or included studies in multiple comparisons as required, but avoided double counting of data.
Dealing with missing data
Where data were missing that the review authors thought should be included in the analyses, we attempted to contact the relevant study authors to request any additional available data or information on the reasons for the missing data.
Where data remained missing for the primary outcome (proportion of ulcers healed and incidence/resolution of infection), we assumed participants did not achieve the outcome (i.e. they were considered in the denominator but not the numerator).
For continuous variables (e.g. quality of life), we presented available data from the study reports (and any additional information if provided by the study authors) and did not impute missing data.
For adverse events and all secondary dichotomous outcomes, we used an available‐case analysis, where possible. If this was not possible, we used whatever information the authors reported in the study.
Assessment of heterogeneity
To assess heterogeneity we did an initial assessment of clinical and methodological heterogeneity and then an assessment of the appropriateness of combining study results, that is the degree to which the included studies varied in terms of participants, interventions, outcomes, and characteristics such as length of follow‐up. We supplemented our assessment of clinical and methodological heterogeneity with information regarding statistical heterogeneity of the results, which we assessed using the Chi² test (at a significance level of P < 0.10) in conjunction with the I² measure (Higgins 2003). I² examines the percentage of total variation across RCTs that is due to heterogeneity rather than chance (Higgins 2003). In general, I² values of 40% or less may mean a low/unimportant level of heterogeneity (Higgins 2003), and values of 75% or more indicate very high heterogeneity (Deeks 2011).
Assessment of reporting biases
We assessed studies for reporting biases, including publication bias and small‐study effects. As we did not conduct any meta‐analyses with 10 or more RCTs, we could not assess the possibility of small‐study effects using funnel plots.
We also considered the publication status of the studies and any information provided on how they were funded.
Data synthesis
We combined details of the included studies in the narrative review according to the type of comparator, and then by outcomes. We considered clinical and methodological heterogeneity, and undertook pooling when studies appeared appropriately similar in terms of types of wounds, interventions, and outcomes.
Our default approach for undertaking a meta‐analysis was to use the random‐effects model. We only used a fixed‐effect approach when we considered clinical heterogeneity to be minimal and statistical heterogeneity was not statistically significant for the Chi² value and 0% for the I² measure (Kontopantelis 2012). We adopted this approach because statistical assessments can miss potentially important between‐study heterogeneity in small samples, making the more conservative random‐effects model preferable (Kontopantelis 2012). Where we considered clinical heterogeneity to be acceptable we undertook a meta‐analysis, even when statistical heterogeneity was high. We attempted to interpret the causes for this heterogeneity, but did not have enough data to use meta‐regression for this purpose.
Where possible, we have presented our data using forest plots. We have presented the summary estimate as a RR with 95% CI for dichotomous outcomes. Where we measured continuous outcomes in the same way across studies, we planned to present a pooled MD with 95% CI. We planned to pool standardised mean difference estimates where studies measured the same outcome, but had to use different methods. Unfortunately it was not possible for us to plot (and, if appropriate, to pool) estimates of hazard ratios and 95% CIs for time‐to‐event data, as there were insufficient data presented in the study reports. Where time to healing was analysed as a continuous measure, but it was not clear if all wounds had healed, we documented use of the outcome in the study, but did not summarise or use these data in any meta‐analysis.
We obtained pooled estimates of the treatment effect using Cochrane Review Manager 5 software (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
As we anticipated clinical heterogeneity in the effects of the interventions, we planned to conduct the following subgroup analyses where data were available.
Severity and depth of the wound, using whatever severity classification the authors used in each of the included RCTs; we were unable to do this.
Duration of follow‐up, using that provided in each included study. We defined short‐term follow‐up as 1 to 4 weeks, medium‐term follow‐up as from > 4 weeks to 24 weeks, and longer‐term follow‐up as > 24 weeks.
Stratifying studies according to overall risk of bias (Higgins 2011); we were unable to conduct this analysis due to limitations of the included studies.
Sensitivity analysis
Due to limitations of the data reported in the included studies, we were unable to conduct a planned sensitivity analysis using an alternative imputation assumption (such as available‐case analysis) to consider the effect on risk of bias where the percentage of missing data varied widely between groups.
'Summary of findings' tables
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes (Guyatt 2008), and constructed a 'Summary of findings' table using GRADEpro GDT software (GradePro GDT 2015).
These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach, which defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias (Schünemann 2011b). We have presented the following outcomes in the 'Summary of findings' tables:
complete ulcer healing;
infection (either incidence of developing, or resolution of established);
adverse events.
For relevant outcomes reported for comparisons not listed above, we presented GRADE assessment without a 'Summary of findings' table.
When evaluating the 'Risk of bias' domain, we downgraded the GRADE assessment only when we classified a study as being at high risk of bias for one or more domains, or when the 'Risk of bias' assessment for selection bias was unclear (this was classified as unclear for either the generation of the randomisation sequence or the allocation concealment domain). We did not downgrade for unclear 'Risk of bias' assessments in other domains.
We selected an informal optimal information size of 300 for binary outcomes, following the GRADE default value (Guyatt 2011). We also followed GRADE guidance and downgraded twice for imprecision when there were very few events and CIs around effects included both appreciable benefit and appreciate harm.
Results
Description of studies
Results of the search
The electronic and manual searches yielded a total of 665 citations (Figure 1). After excluding 590 records that were not relevant to the scope of this review, we assessed 75 records for eligibility and discarded 53 for various reasons (see Figure 1 and Characteristics of excluded studies). A total of 22 trials (reported in 21 individual papers) met our inclusion criteria (see Characteristics of included studies). Two studies are awaiting assessment as based on the available data we are unsure whether they are randomised controlled trials; we have contacted the study authors for further information. We will contact these authors again at the next update of this review.
1.
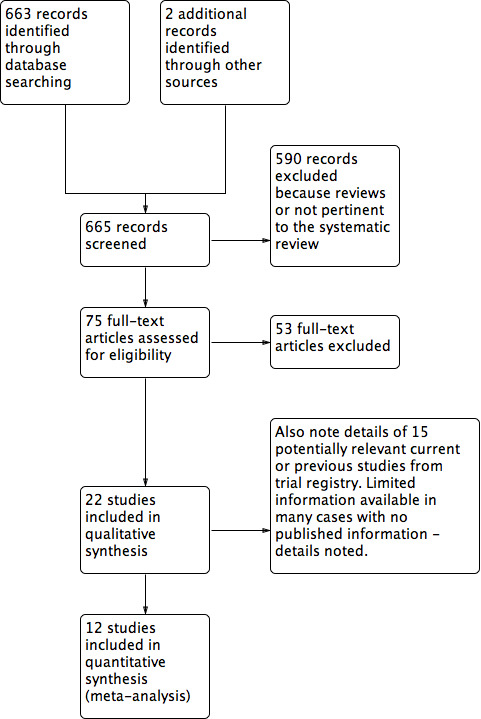
Study flow diagram.
We also located reports of 15 trials listed in various trial registries. Five studies were ongoing, but it was unclear if they met the inclusion criteria for this review. Eight studies were terminated or completed, but we were unable to locate any associated published data. We attempted to contact the designated person for each of these trials and succeeded with five trials; we obtained some information on these trials, but there were no published data. Based on the available information, we were unable to judge whether or not any of these studies might be eligible for the review (Table 9).
4. Information from trial registry.
| Title (comparator) | Current status | Relevant outcomes listed | Database | Results (# enrolled) | Listed contact | Company and any further information received |
| Phase IIa Randomised, Placebo Controlled Trial to Investigate Antimicrobial Photodynamic Therapy in Chronic Leg Ulcers and Diabetic Foot Ulcers (placebo = “cream”) | Prematurely ended (date unclear) | Photodynamic therapy using the combined effect of 3,7‐bis(N,N‐dibutylamino) phenothiazin‐5‐ium bromide (PPA904) and light; measure reduction of bacterial content of diabetic foot ulcers | ClincialTrialsRegister.eu EudraCT number: 2005‐001363‐58 |
None (not listed) | None listed. | Photopharmacia |
| Pexiganan Versus Placebo Control for the Treatment of Mild Infections of Diabetic Foot Ulcers (OneStep‐1 and 2) | Completed (August 2016) | 1°: clinical response (resolution of infection); 2°: microbiological response; safety |
ClinicalTrials.gov; NCT01594762 | No results (200 for each of the 2 trials) reported on website. | Robert Deluccia, Dipexium | Dipexium Pharmaceuticals, Inc. Multicentre study; all sites outpatient centre in USA |
| Comparison of Resin Salve and Octenidine in Patients with Neuropathic Diabetic Foot Ulcers (comparator: octenidine dihydrochloride‐impregnated gauze) | Completed (May 2015) | Investigate healing rate and healing time of neuropathic diabetic foot ulcer in people suffering from infected fore‐ or mid‐foot ulceration. 2°: eradication of bacteria; wound healing and infection | ClinicalTrials.gov; NCT02169167 |
No results on website (n = 35) (see addendum in “comments”) |
Janne J Jokinen | Salve prepared from Norway spruce (Repolar Ltd.) |
| Clinical Outcomes for Diabetic Foot Ulcers Treated With Clostridial Collagenase (SANTYL®) Ointment or With a Comparator Product Containing Silver (investigator choice of silver) | Running until January 2017 (last updated November 2016) | Randomly assigned to apply SANTYL or a topical treatment containing silver to their to foot ulcer. 1°: mean change in ulcer area at end of treatment; 2°: target ulcer infection rate | ClinicalTrials.gov; NCT02581488 | No results (102) | Jaime E Dickerson, PhD (Smith & Nephew) | (Smith & Nephew) Information from the sponsor received end of December 2016 stated that the trial is not yet complete but last participant out will be achieved in the next week. The trial enrolled its target number of participants, with the last participant completed December 2016. The evaluability will be carried out prior to the scheduled database lock in January 2017. As intention‐to‐treat is the analysis set for primary inference, it is anticipated that all participants will be included. Final study report is timed for April 2017 (15 December 2016). Waiting for further information to assess eligibility for review |
| Randomized, Controlled Study to Investigate the Efficacy and Safety of a Topical Gentamicin‐Collagen Sponge in Combination with Systemic Antibiotic Therapy in Diabetic Patients With a Moderate or Severe Foot Ulcer Infection | Recruiting (as of September 2013) | 1°: "clinical cure" at the test of cure; 2°: clinical response; time to clinical cure; eradication of baseline pathogen | ClinicalTrials.gov; NCT01951768 | No results (estimate 144) | Ilker Uckay, MD; Hospital of the University of Geneva | Innocoll, Inc. |
| Comparison of the Efficacy of Standard Treatment Associated with Phage Therapy Versus Standard Treatment Plus Placebo for Diabetic Foot Ulcers Monoinfected by Staphylococcus aureus: a Randomized, Multi‐centre, Controlled, 2‐parallel‐group, Double‐blind, Superiority Trial | Starting January 2017 | 1°: reduction in wound surface area; 2°: safety; changes in resistance and virulence of S aureus isolates; production of anti‐phage antibodies |
ClinicalTrials.gov; NCT026647401 | No results (estimate 60) | Albert Sotto, MD, PhD +33.(0)6.09.56.66.55 |
Centre Hospitalier Universitaire de Nīmes; Pherecydes Pharma. Per correspondence from Prof Sotto on 8 January 2017, National Agency for the Safety of Medicines and Health Products requested “pre‐clinical phase complements”, causing a postponement of the start of the clinical trial. |
| A Phase I/IIa, Randomized Double Blind, Placebo‐Controlled, Dose Escalating Study to Evaluate the Safety and Tolerability of Topically Applied Bisphosphocin Nu‐3 on Infected Diabetic Ulcers of Subjects With Type I or II Diabetes Mellitus (placebo) | Enrolling by invitation only (last verified April 2016) | Diabetic foot ulcers; infection localised to area of ulcer and mild. 1° outcome: treatment‐related adverse events, safety 2°: microbiological activity evaluated by wound assessments, presence of pathogenic bacteria |
ClinicalTrials.gov; NCT02737722 | No results (estimate 30) | Paul DiTullio, MSc | Lakewood‐Amedex, Inc. |
| A Phase II, Randomized, Parallel, Double‐blind, Placebo‐controlled Study to Assess Prevention of Infection Using a Topical Gentamicin‐Collagen Sponge in Diabetic Patients With Uninfected Lower Extremity Skin Ulcers (placebo sponge) | Terminated (last verified March 2012) | 1° outcome: uninfected diabetic foot ulcers that remain free of signs/symptoms of infection to end of study 2°: days to wound closure; time to any signs/symptoms of infection; decrease in wound area; pathogen burden in infected wounds |
ClinicalTrials.gov; NCT00658957 | No results (49) | David Prior, PhD; Chesapeake Foot and Ankle Center, Pasadena (MD), USA | Innocoll Pharmaceuticals |
| A Phase 3 Randomized, Placebo‐Controlled, Blinded Study to Investigate the Safety and Efficacy of a Topical Gentamicin‐Collagen Sponge in Combination With Systemic Antibiotic Therapy in Diabetic Patients With an Infected Foot Ulcer (COACT 1 and 2) (placebo is no sponge) | Last updated June 2016 | Sponge is adjunctive treatment to systemic antibiotic therapy. 1° outcome: per cent of participants with a clinical outcome of clinical cure (resolution of all clinical signs and symptoms of infection) ˜10 days after end of treatment; 2° outcomes: baseline pathogen eradication; re‐infection; time to clinical cure; amputation; ulcer closure |
ClinicalTrials.gov: NCT02447172 |
No results posted. | Nigel Jones, VP, Global Clinical Operations, Innocoll Pharmaceuticals | Innocoll Pharmaceuticals |
| Study of the Efficacy of Topical Application of Royal Jelly and Panthenol (PedyPhar® Ointment) on the Diabetic Foot Ulcers, an Open Label, Randomized, Non‐placebo‐controlled Study (active comparator panthenol ointment) | Terminated; (last updated February 2015) | Diabetic foot ulcers at any stage after proper surgical treatment (if needed) 1° outcome: healing of ulcer; 2°: reduction of infection in ulcer site; local reaction possibly related to study drug |
ClinicalTrials.gov; NCT01531517 | No results (estimate 120; 47 enrolled) | (?) | European Egyptian Pharmaceutical Industries |
| Platelet Rich Fibrin in Combination With Topical Antibiotics or Antiseptics in the Treatment of Chronic Wounds ‐ a Prospective, Randomized, Active Controlled, Double Blind Pilot Trial With an Observer‐blinded Control Group (3 platelet rich fibrin arms & 1 active comparator (Acticoat)) | Recruiting (last verified January 2016) | People with infected chronic wounds (unclear if diabetic foot) 1° outcome: reduction of wound area; 2°: number requiring systemic antimicrobial therapy; C‐reactive protein level; wound volume; occurrence of drug‐resistant bacteria |
ClinicalTrials.gov; NCT02652169 | No results (estimate 120) | Florian Thalhammer, Medical University of Vienna; 0043140400 ext 44400; florian.thalhammer@meduniwien.ac.at | Medical University of Vienna |
| Double Blind, Randomized, Placebo Controlled Clinical Trial for the Treatment of Diabetic Foot Ulcers, Using a Nitric Oxide Releasing Patch: PATHON | Completed (last verified November 2012) | 1° outcome: per cent reduction in ulcer size; 2°: complete cure of any infection; development of infection during treatment; adverse events |
ClinicalTrials.gov; NCT00428727 | No results (?) | Fundación Cardiovascular de Colombia | (?) |
| A Phase I/II, Open Label, Controlled Study to Evaluate the Safety and Efficacy of AppliGel‐G (Gentamicin Sulfate Topical Gel) for Treatment of Mild to Moderately Infected Diabetic Foot Ulcers in Patients With Type 1 and Type 2 Diabetes (comparator oral ciprofloxacin and doxycycline alone) | Terminated (last verified May 2015) | For mild to moderately infected diabetic foot ulcers 1°: complete wound clearing of infection 2°: incidence infection cleared; wound volume and area change |
ClinicalTrials.gov; NCT02036528 | No results | Royer Biomedical, Inc. | Royer Biomedical, Inc. |
| A Randomised, Double‐blind, Dose‐response, Placebo‐controlled, Multicenter, Phase IIA Clinical Study to Evaluate the Efficacy and Safety of Topical Application of G.68.y/EtOH in Patients with Type 1 or Type 2 Diabetes With Infected Foot Ulcers (placebo topical gel) | Completed | Enrolling patients with infected “grade 2 PEDIS” diabetic foot ulcers 1°: reduction of bacterial load 2°: maintenance of efficacy; tolerability and safety |
EudraCT number: 2010‐019598‐13 | No results (plan for 60) | I.CORTI@MOLTENIFARMA.IT | Molteni |
| Trial to Assess Safety and Efficacy of Topical MBN‐101 (BisEDT ) in Patients With Moderate/ Severe Diabetic Foot Infections (placebo – vehicle‐controlled) | Not yet open for participant recruitment (last update March 2016) | Part I, participants will be enrolled into 1 of 3 escalating dose cohorts at a ratio of 3:1 (active to placebo). In Part II, participants will be randomised in a 1:1 ratio (active to placebo) based on the optimal dose demonstrated in Part I. People with infected foot ulcer | ClinicalTrials.gov; NCT02723539 | No results (plan for 88) | Department of Vascular Surgery, Rigshospitalet Copenhagen, Denmark, 2100 |
Microbion Corporation |
Abbreviations: PEDIS, perfusion, extent/size, depth/tissue loss, infection, and sensation
Included studies
We have presented an overview of the 22 included trials in Table 10 and all outcome data in Table 11.
5. Overview of included studies.
| Intervention 1 | Intervention 2 | Foot ulcer grade | Infection status at baseline | Follow‐up |
Review‐relevant outcomes with reportable data |
|
| Ahmed 2014 | Group 1: (n = 30) Pyodine bath and saline and vaseline gauze dressing | Group 2: (n = 30) Phenytoin powder | Grade I or II | Not reported | 8 weeks | None reported |
| Apelqvist 1996 | Group 1: (n = 19) Gentamicin solution | Group 2: (n = 22) Cadexomer iodine ointment | Grade I or II | Not reported | 12 weeks |
|
| Bergqvist 2016 | Group 1: (n = 19) Standard care | Group 2: (n = 21) Chloramine plus standard care | Not reported | Infected | 24 weeks |
|
| Bowling 2011 | Group 1: (n = 10) Saline solution | Group 2: (n = 10) Super‐oxidised aqueous solution | Grade I or II | Not infected | 4 weeks |
|
| Gottrup 2013 | Group 1: (n = 15) Foam dressing | Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing | Grade II or III | Not infected | 14 weeks |
|
| He 2016 | Group 1: (n = 40) Routine debridement plus standard care (including blood glucose control, nutritional support, improve microcirculation | Group 2: (n = 40) Silver ion dressing plus standard care | Not reported | Not reported | 4 weeks |
|
| Hwang 2010 | Group 1: (n = not reported) Iodine gauze | Group 2: (n = not reported) Hydrofiber dressing with silver | Ulcers with bone and tendon exposure | Not reported | Not reported | Not reported |
| Imran 2015 | Group 1: (n = 180) Saline dressing | Group 2: (n = 195) Honey dressing | Grade I or II | Not reported | 17 weeks |
|
| Jacobs 2008 | Group 1: (n = 20) Silver sulphadiazine cream | Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulphadiazine cream | Grade I or II | Not reported | 6 weeks |
|
| Jeffcoate 2009 | Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing |
Group 3: (n = 106) Iodine‐containing dressing | Not reported | Not reported | 24 weeks |
|
| Jude 2007 | Group 1: (n = 67) Calcium‐alginate dressing | Group 2: (n = 67) Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver | Grade I or II | Mixed infected and not infected | 8 weeks |
|
| Khandelwal 2013 | Group 1: (n = 20) Hyperbaric oxygen therapy (not considered further) Group 2: (n = 20) Recombinant human platelet‐derived growth factor |
Group 3: (n = 20) Antiseptic treatments (EUSOL, hydrogen peroxide, and povidone iodine) | Grade III or IV | Not reported | More than 8 weeks |
|
| Landsman 2011 | Group 1: (n = 21) Topical saline solution plus 750 mg levofloxacin once per day Group 2: (n = 21) Super‐oxidised aqueous solution (topical Microcyn) alone (not considered) |
Group 3: (n = 21) super‐oxidised aqueous solution (topical Microcyn) therapy plus 750 mg levofloxacin once per day | Eligible foot ulcers involved skin and deeper soft tissue | Infected | 4 weeks |
|
| Lipsky 2008a | Group 1: (n = 246) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream | Group 2: (n = 247) Topical pexiganan cream (1% or 2%) and placebo oral tablets | Not reported | Infected | Up to 42 days |
|
| Lipsky 2008b | Group 1: (n = 171) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream | Group 2: (n = 171) Topical pexiganan cream (1%) and placebo oral tablets | Full‐thickness wounds | Infected | Up to 42 days |
|
| Lipsky 2012a | Group 1: (n = 38) Systemic antibiotic therapy alone | Group 2: (n = 18) Daily topical application of the gentamicin‐collagen sponge combined with systemic antibiotic therapy | Not reported | Infected | Up to 42 days |
|
| Martinez‐De Jesus 2007 | Group 1: (n = 16) Povidone iodine and saline | Group 2: (n = 21) Neutral pH super‐oxidised aqueous solution | Not reported | Infected | 20 weeks |
|
| Ramos Cuevas 2007 | Group 1: (n = 25) Conventional treatment (no further details translated) | Group 2: (n = 25) Zinc hyaluronate | Not reported | Unclear | 20 weeks |
|
|
Shukrimi 2008 (30 participants randomised, but number in each group not specified) |
Group 1: Standard‐dressing group (povidone iodine solution 10%) (n not reported) | Group 2: Honey dressing group (n not reported) | Grade II | Not reported | Not reported | No useable data |
| Tom 2005 | Group 1: Normal saline solution, 11 ulcers (in 10 participants) | Group 2: Tretinoin group, 13 ulcers (in 12 participants) | Not reported | Not reported | 16 weeks |
|
| Ullal 2014 | Group 1: (n = 2) Povidone iodine and metronidazole 1% gel dressing | Group 2: (n = 2) Honey and metronidazole 1% gel dressing | Grade I and II | Not reported | Not reported |
|
| Viswanathan 2011 | Group 1: (n = 19) Polyherbal formulation | Group 2: (n = 19) silver sulphadiazine cream | Grade I, II, and III | Unclear | 20 weeks | No useable data |
Abbreviations: EUSOL, Edinburgh University Solution of Lime
6. Outcomes.
| Resolution of infection | Incidence of wound infection | Time to healing | Proportion of wounds healed | Microbial counts | Health‐related quality of life | Need for surgical resection, including partial or complete lower limb amputation | Safety (adverse events) | |
|
Ahmed 2014 Group 1: (n = 30) Povidone iodine bath and saline Vaseline gauze dressing Group 2: (n = 30) Phenytoin powder plus povidone iodine bath and saline Vaseline gauze dressing Not infected at baseline |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
|
Apelqvist 1996 Group 1: (n = 19) Gentamicin solution Group 2: (n = 22) Cadexomer iodine ointment Baseline infection status not reported. |
Not reported | Not reported | Not reported | Group 1: 2/18 Group 2: 5/17 |
Not reported | Not reported | Surgical resection was reported: Group 1: 5/19 Group 2: 3/22 |
Study reports that no adverse reactions related to the topical treatment were documented. |
|
Bergqvist 2016 Group 1: (n = 19) Standard care alone Group 2: (n = 21) Chloramine plus standard care Infected at baseline |
Group 1: 7/15 Group 2: 9/13 |
Not reported | Time‐to‐event data presented with no reported hazard ratio. Given the small number of participants and events, no further attempts were made to calculate time‐to‐event values. |
Healed at 24 weeks Group 1: 9/17 Group 2: 10/17 |
Not reported | Not reported | Vascular procedure or amputation Group 1: 3/17 Group 2: 5/17 |
Adverse event data reported but unable to get a per‐participant value, as it is noted that some participants had more than 1 event. |
|
Bowling 2011 Group 1: (n = 10) Saline solution Group 2: (n = 10) Super‐oxidised aqueous solution Not infected at baseline |
Not reported | Not reported | Not reported | Study notes that 15% of the study ulcers were healed, but this information not reported by group. |
The bacterial load in the wound bed was defined as scattered (0/+), light (+), medium (++), or heavy (+++). At week 4 there was a reduction of 33% in the bacterial load versus baseline. Figure presented but difficult to interpret data by group. |
Not reported | Not reported | No safety concerns were reported in either the super‐oxidised aqueous solution group or the saline group; no adverse reactions were recorded. |
|
Gottrup 2013 Group 1: (n = 15) Foam dressing Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing Not infected at baseline |
Not reported |
Wound infection Group 1: 4/13 Group 2: 0/23 |
Not reported |
Healed by week 14 Group 1: 4/13 Group 2: 12/23 |
Not reported | Not reported | Not reported | Limited details of adverse events (in addition to infection data already recorded). There were no reported adverse events related to the use of collagen/oxidised regenerated cellulose/silver dressing, and 5 cases of adverse events (no further details) related to foam dressing. |
|
He 2016 Group 1: (n = 40) Routine debridement plus standard care Group 2: (n = 40) Silver ion dressing plus standard care Baseline infection status not reported. |
Not reported | Not reported | Mean wound healing time in days: Group 1: 47.4 ± 11.5 Group 2: 31.3 ± 8.2 Mean granulation tissue occurrence time in days: Group 1: 10.8 ± 1.9 Group 2: 6.4 ± 0.72 |
Group 1: 15/40 Group 2: 24/40 |
Not reported | Not reported | Not reported | Not reported |
| Hwang 2010 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
|
Imran 2015 Group 1: (n = 180) Treated with normal saline dressing Group 2: (n = 195) Treated with honey dressing |
Not reported | Not reported | Median healing time in honey group is 18 days (IQR is 6 to 120), and in the saline group is 29 days (IQR 7 to 120). Data do not seem to have been calculated using correct time‐to‐event approaches and were not considered further. |
Group 1: 97/169 Group 2: 136/179 |
Not reported | Not reported | Not reported | Not reported |
|
Jacobs 2008 Group 1: (n = 20) Silver sulphadiazine Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulphadiazine cream Baseline infection status not reported. |
Not reported | Not reported | Not reported |
Healed by week 6 Group 1: 6/20 Group 2: 8/20 |
Not reported | Not reported | Not reported | Not reported |
|
Jeffcoate 2009 Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze (Johnson & Johnson) Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing (Aquacel, ConvaTec) Group 3: (n = 106) Iodine‐containing dressing (Inadine, Systagenix) Baseline infection status not reported. |
Not reported |
Number of infected ulcers at 24 weeks: not reported by group Study reports the number of episodes of infection listed as serious adverse events, but it is unclear if foot infections, and not clear how many people had how many infection events. |
Mean time to healing in days (SD) (fixed at max of 168 days)
Group 1: 130.7 (52.4) Group 2: 125.8 (55.9) Group 3: 127.8 (54.2) Not all ulcers healed, so mean is inappropriate measure of time to healing. |
Number of ulcers healed at 24 weeks:
Group 1: 41/108 Group 2: 46/103 Group 3: 48/106 |
Not reported |
Mean Cardiff Wound Impact Schedule score at 24 weeks (SD) Group 1: Physical functioning: 68.9 (19.1). Social functioning: 69.8 (23.5). Well‐being: 50.2 (21.1) Group 2: Physical functioning: 71.4 (19.5). Social functioning: 70.3 (25.4). Well‐being: 53.1 (19.9) Group 3: Physical functioning: 67.1 (23.6). Social functioning: 69.7 (24.1). Well‐being: 51.0 (22.3) Other Study also reports mean and SD for each of the 8 domains of the SF‐36. There was no significant difference between the groups for any domain. |
Minor amputations (below ankle):
Group 1: 1/108
Group 2: 3/103
Group 3: 1/106
Major amputations (above knee)
Group 1: 1/108 Group 2: 1/103 Group 3: 0/106 n not clear; assumed to be all participants |
Non‐serious adverse events
Group 1: 244/108 Group 2: 227/103 Group 3: 239/106 Serious adverse events Group 1: 35/108 Group 2: 28/103 Group 3: 37/106 Not clear how many participants had how many events, but seems to be more than 1 per person; data not analysed further |
|
Jude 2007 Group 1: (n = 67) Calcium‐alginate dressing Group 2: (n = 67) Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver Mixed wound infection status at baseline |
Not reported | Group 1: 11/67 Group 2: 8/67 |
Time to 100% healing also reported, but this is only for a subset of those that healed, so not a useful pan‐study measure. Not reported Mean time to healing in days Group 1: 52.6 ± 1.8 Group 2: 57.7 ± 1.7 |
Number of ulcers healed in 8 weeks Group 1: 15/67 Group 2: 21/67 | Not reported | Not reported | Not reported | Group 1: 26/67 participants experienced adverse event. Death = 1; Infection = 8. 13 participants discontinued treatment due to adverse event. Group 2: 25/67 participants experienced 1 or more events. Death = 1; Infection = 14. 8 participants discontinued treatment due to adverse event. |
|
Khandelwal 2013 Group 1: (n = 20) Hyperbaric oxygen therapy (not considered in review) Group 2: (n = 20) Recombinant human platelet‐derived growth factor Group 3: (n = 20) Antiseptic dressings |
Not reported | Not reported |
Mean time to healing in weeks (standard error) Group 1: 6.83 (2.5) Group 2: 7.6 (2.5) Group 3: 6.75 (2.7) Not all ulcers healed, so mean is inappropriate measure of time to healing. |
Number of ulcers healed Group 1: 12/20 Group 2: 16/20 Group 3: 8/20 Review authors calculated figures from graph. |
Not reported | Not reported | Not reported | Not recorded |
|
Landsman 2011 Group 1: (n = 21) Levofloxacin plus saline Group 2: (n = 21) Super‐oxidised aqueous solution alone (not considered) Group 3: (n = 25) Levofloxacin plus super‐oxidised aqueous solution Ulcers infected at baseline. |
Group 1: 6/21 Group 2: 11/21 Group 3: 11/25 |
Not reported | Not reported | Mentioned, but data not presented. | Not reported | Not reported | Not reported |
Adverse events (number of participants with 1 or more event) Group 1: 7/21 Group 2: 7/21 Group 3: 9/25 |
|
Lipsky 2008a Group 1: (n = 246) Ofloxacin Group 2: (n = 247) Pexiganan Ulcers infected at baseline. |
Not reported Resolution ("cure") and improvement data presented together, so unclear how many participants had resolution. |
Not reported | Not reported | Not reported | Not reported | Not reported | See below ‐ results presented by study authors cumulatively for these 2 studies only. |
Adverse events (number of participants with > 1 adverse event) Group 1: 109/246 Group 2: 98/247 |
|
Lipsky 2008b Group 1: (n = 171) Ofloxacin Group 2: (n = 171) Pexiganan Ulcers infected at baseline. |
Not reported Resolution ("cure") and improvement data presented together, so unclear how many participants had resolution. |
Not reported | Not reported | Not reported | Not reported | Not reported | Group 1: 9/417 Group 2: 11/418 (cumulative of two RCTs reported in single paper) |
Adverse events (number of participants with > 1 adverse event) Group 1: 84/171 Group 2: 76/171 |
|
Lipsky 2012a Group 1: (n = 18) Systemic antibiotic therapy alone Group 2: (n = 38) Topical application of the gentamicin‐collagen sponge + systemic antibiotic therapy Ulcers infected at baseline. |
Resolution of infection by 7 days Group 1: 7/18 Group 2: 22/38 |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
Adverse events (number of participants with 1 or more events) Group 1: 5/18 Group 2: 11/38 |
|
Martinez‐De Jesus 2007 Group 1: (n = 16) Standard management with chemical antiseptics such as soap or povidone iodine Group 2: (n = 21) Super‐oxidised aqueous solution |
Advances from infection to granulating tissue: Group 1: 10/16 Group 2: 19/21 |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
|
Ramos Cuevas 2007 Group 1: (n = 25) Conventional treatment (no further details translated) Group 2: (n = 25) Zinc hyaluronate |
Not reported/translated | Not reported/translated | Mean time to healing in weeks (not clear if standard deviation or standard error presented) Group 1: Only 2 ulcers healed; no time‐to‐event data reported Group 2: 7.80 (3.49) with all ulcers healing |
Group 1: 2/25 Group 2: 25/25 |
Not reported/translated | Not reported/translated | Not reported/translated | Not reported/translated |
|
Shukrimi 2008 Group 1: Standard‐dressing group (povidone iodine solution 10%) Group 2: Honey dressing group 30 participants randomised, but number in each group not specified. |
Not reported | Not reported | Time to healing in days Group 1: 15.4 days (range 9 to 36 days) Group 2: 14.4 days (range 7 to 26 days) Comment: mean and range, but no measure of variation provided. Unclear how many participants in each group and how many ulcers healed, thus if this measure is a valid time‐to‐healing measure |
Not reported | Not reported | Not reported | Not reported | Not reported |
|
Tom 2005 Group 1: Normal saline solution, 11 ulcers (in 10 participants) Group 2: Tretinoin group, 13 ulcers (in 12 participants) |
Not reported | Not reported | Data presented as time‐to‐event figure with no further data. Given the small number of participants and events, we have not tried to analyse further. |
16 weeks Group 1: 2/10 Group 2: 6/12 Unclear if ulcers were healed in the same or different participants; for the analysis we have assumed in different participants |
Not reported | Not reported | Not reported | Not reported |
|
Ullal 2014 Group 1: (n = 2) Povidone iodine and metronidazole 1% gel dressing Group 2: (n = 2) Honey and metronidazole 1% gel dressing |
Not reported | Not reported | Not reported | Group 1: 0/2 Group 2: 2/2 |
Not reported | Not reported | Not reported | Not reported |
|
Viswanathan 2011 Group 1: (n = 19) Polyherbal formulation Group 2: (n = 19) Silver sulphadiazine cream |
Not reported | Not reported | "Number of days taken for healing of the wound: Group 1: 43.1 ± 26.8 Group 2: 43.6 ± 30.7" Not clear what sort of analysis was conducted |
Healing was defined as complete epithelialisation either by secondary intention or by split skin graft. However, figures are not reported. | "the microbiological investigations were not done" | Not reported | Not reported | "There were no adverse events reported in both the groups." |
Abbreviations: IQR, interquartile range; SD, standard deviation
Trial design and location of conduct
The included trials had a combined total of 2310 participants; one trial did not report the total number of participants, so we did not consider the data from this study (Hwang 2010). The sample size of individual trials varied widely, ranging from 4 to 317; 17 (77%) of the trials had fewer than 100 participants or did not clearly report this number. The duration of follow‐up of the studies ranged from 4 to 24 weeks.
Three included trials were designed as three‐arm trials. One trial had two arms in which participants received a non‐antimicrobial treatment (Jeffcoate 2009); we combined these for analysis and compared them with the third trial arm, which was a topical antimicrobial treatment. The three‐arm trials of Khandelwal 2013 and Landsman 2011 had one arm that was not relevant to this review, so we did not consider it further.
The included trials were conducted in at least 10 countries:
China (He 2016);
Denmark (Gottrup 2013);
India (Khandelwal 2013; Ullal 2014; Viswanathan 2011);
Malaysia (Shukrimi 2008);
Mexico (Martinez‐De Jesus 2007; Ramos Cuevas 2007);
Pakistan (Ahmed 2014; Imran 2015);
Sweden Apelqvist 1996; Bergqvist 2016);
UK (Bowling 2011; Jeffcoate 2009; Jude 2007);
USA (Landsman 2011; Lipsky 2008a; Lipsky 2008b; Tom 2005);
UK and USA (Lipsky 2012a);
Not reported (Hwang 2010; Jacobs 2008).
Trial participants
We required that all trial participants had both diabetes mellitus and a foot wound. Eight studies noted that they included participants with grade I and II ulcers (using various assessment tools; see Characteristics of included studies) (Ahmed 2014; Apelqvist 1996; Bowling 2011; Imran 2015; Jacobs 2008; Jude 2007; Shukrimi 2008; Ullal 2014); one study included grade I to III ulcers (Viswanathan 2011); one study included grade II and III ulcers (Gottrup 2013); and one study included grade III and IV ulcers (Khandelwal 2013). The remaining 10 studies did not clearly report a grade, precluding the conduct of our planned subgroup analysis on severity of wound.
Nine trials reported the clinical infection status of the wound. Six trials included only ulcers that were reported by the study authors to be infected at baseline (Bergqvist 2016; Landsman 2011; Lipsky 2008a; Lipsky 2008b; Lipsky 2012a; Martinez‐De Jesus 2007). One study included both infected and uninfected ulcers (Jude 2007), and two trials included non‐infected ulcers at baseline (Bowling 2011; Gottrup 2013). The remaining 13 studies did not report the infection status of ulcers at baseline. We were unable to report data for infected and uninfected wounds in comparisons unless this information was specifically noted.
Interventions evaluated
The studies evaluated several different types of topical antimicrobial agents (see Characteristics of included studies and Table 10 for a full list) including antimicrobial dressings (Gottrup 2013; He 2016; Hwang 2010; Imran 2015; Jeffcoate 2009; Jude 2007; Shukrimi 2008; Ullal 2014), super‐oxidised aqueous solutions (Bowling 2011; Landsman 2011; Martinez‐De Jesus 2007), zinc hyaluronate (Ramos Cuevas 2007), tretinoin (Tom 2005), silver sulphadiazine (Viswanathan 2011), gentamicin‐collagen sponge (Lipsky 2012a), pexiganan cream (Lipsky 2008a; Lipsky 2008b), and chloramine (Bergqvist 2016).
Nine studies compared a topical antimicrobial agent with standard wound care or placebo (Bergqvist 2016; Bowling 2011; Gottrup 2013; He 2016; Imran 2015; Jeffcoate 2009; Jude 2007; Ramos Cuevas 2007; Tom 2005). Eight studies compared one topical agent against another (Ahmed 2014; Apelqvist 1996; Hwang 2010; Jacobs 2008; Martinez‐De Jesus 2007; Shukrimi 2008; Ullal 2014; Viswanathan 2011). Four studies compared a topical antimicrobial treatment with systemic antibiotic therapy (Landsman 2011; Lipsky 2008a; Lipsky 2008b; Lipsky 2012a). One trial compared a topical antimicrobial treatment with a growth factor cream (Khandelwal 2013).
Excluded studies
We excluded 53 of the assessed studies, most often because: the study was found not to be a RCT (n = 20); the intervention(s) being evaluated were not eligible (n = 14); and participants in the study population were not eligible (n = 14) (see Figure 1 and Characteristics of excluded studies).
Risk of bias in included studies
2.
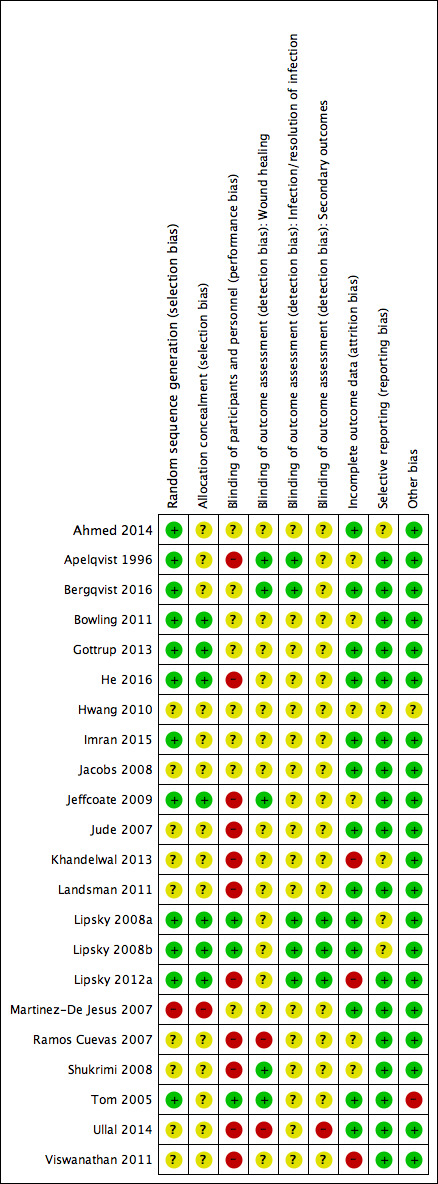
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
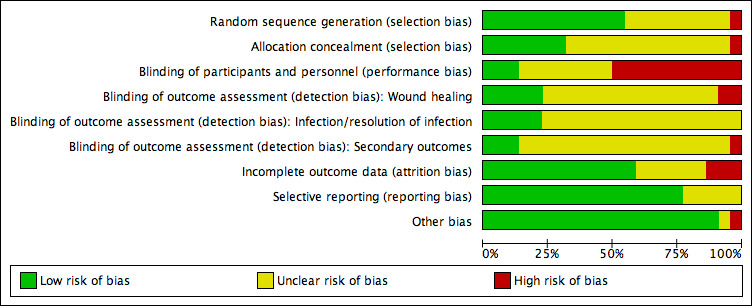
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We assessed no study as being at low risk of bias. We judged 12 studies (55%) as being at high risk of bias for one or more domains. We assessed the remaining 10 studies as being at unclear risk of bias for two or more domains.
Allocation
We assessed only one study as being at high risk of selection bias (Martinez‐De Jesus 2007), as the report's description of the randomisation process used was not clear and could have been alternation. We assessed seven studies as being at low risk of selection bias (Bowling 2011; Gottrup 2013; He 2016; Jeffcoate 2009; Lipsky 2008a; Lipsky 2008b; Lipsky 2012a), and the reports of the remaining studies were unclear for random sequence generation or allocation concealment, or both.
Blinding
We assessed 11 studies as being at high risk of performance bias (Apelqvist 1996; He 2016; Jeffcoate 2009; Jude 2007; Khandelwal 2013; Landsman 2011; Lipsky 2012a; Ramos Cuevas 2007; Shukrimi 2008; Ullal 2014; Viswanathan 2011). In three studies the participants and staff did not know which of the treatments was being delivered (Lipsky 2008a; Lipsky 2008b; Tom 2005). In all of the other studies blinding status was unclear.
Five studies reported blinded outcome assessment for healing (Apelqvist 1996; Bergqvist 2016; Jeffcoate 2009; Shukrimi 2008; Tom 2005), and in two studies outcome assessment for healing was not blinded (Ramos Cuevas 2007; Ullal 2014). Detection bias (for wound healing) for the remaining studies was either unclear or not relevant, as the outcome was not reported.
Five studies were at low risk of bias for the reporting of infection data (Apelqvist 1996; Bergqvist 2016; Lipsky 2008a; Lipsky 2008b; Lipsky 2012a). Detection bias (infection status) for the remaining studies was either unclear or not relevant, as the outcome(s) was not reported.
Three studies were at low risk of detection bias for secondary outcomes (Lipsky 2008a; Lipsky 2008b; Lipsky 2012a). Detection bias (for secondary outcomes) for the remaining studies was either unclear or not relevant, as relevant outcomes were not reported.
Incomplete outcome data
The risk of attrition bias was high in three studies (Khandelwal 2013; Lipsky 2012a; Viswanathan 2011), low in 13 studies, and unclear in the remaining studies.
Selective reporting
We judged five studies as at unclear risk of reporting bias, as we could not be certain if all outcomes had been reported (Ahmed 2014; Hwang 2010; Khandelwal 2013; Lipsky 2008a; Lipsky 2008b). We classified all other studies as being at low risk of reporting bias, although we did not obtain the full study protocol for any of these studies.
Other potential sources of bias
We judged all of the included studies as being at low risk of other sources of bias except for one, for which the limited available information precluded assessing this domain (Hwang 2010), and one judged at high risk of bias due to unit of analysis issues (Tom 2005).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Antimicrobial dressings compared with non‐antimicrobial dressings.
| Antimicrobial dressings compared with non‐antimicrobial dressings | ||||||
|
Patient or population: Foot ulcers in people with diabetes Settings: Mixed Intervention: Antimicrobial dressings Comparison: Standard dressings | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with standard dressings | Risk with antimicrobial dressings | |||||
|
Proportion of wounds healed Up to 24 weeks' follow‐up |
425 per 1000 | 544 per 1000 (476 to 616) | RR 1.28 (1.12 to 1.45) | 945 participants (5 studies) |
⊕⊕⊝⊝ low1 | On average, use of an antimicrobial dressing compared with a non‐antimicrobial dressing may increase the number of ulcers healed over a medium‐term follow‐up period. |
| Risk difference: 119 more healed wounds per 1000 (51 more to 191 more) | ||||||
|
Incidence of infection Up to 24 weeks' follow‐up |
183 per 1000 | 62 per 100 (7 to 567) | RR 0.34 (0.04 to 3.10) | 173 participants (2 studies) | ⊕⊝⊝⊝ very low2 | On average, it is unclear whether or not use of an antimicrobial dressing compared with a non‐antimicrobial dressing reduces the incidence of ulcer infection over a medium‐term follow‐up period. |
| Risk difference: 121 fewer infections per 1000 (176 fewer to 384 more) | ||||||
| Resolution infection | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
|
Adverse events Up to 24 weeks' follow‐up |
388 per 1000 | 373 per 1000 (241 to 574) | RR 0.96 (0.62 to 1.48) | 134 participants (1 study) |
⊕⊝⊝⊝ very low3 | It is uncertain whether use of an antimicrobial dressing affects the risk of adverse events compared with use of a non‐antimicrobial dressing over a medium‐term follow‐up period. |
| Risk difference: 16 fewer adverse events per 1000 (147 fewer to 186 more) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for risk of bias due to one study (with the highest weighting in the meta‐analysis) being at unclear risk of selection bias and three studies being at high risk of performance bias (36% weighting in analysis), although the studies were at unclear or low risk of detection bias for this outcome. 2Downgraded twice for imprecision due to sample size and low number of events. 95% CIs span both benefits and harms. Downgraded once due to inconsistency: I² = 60%. Downgraded once due to risk of performance bias. 3Downgraded twice for imprecision due to sample size and low number of events. 95% CIs span both benefits and harms. Downgraded once due to risk of performance bias.
Summary of findings 2. Topical antimicrobial agents (non‐dressing) compared with non‐antimicrobial topical agents (non‐dressing).
| Topical antimicrobial agents (non‐dressing) compared with non‐antimicrobial topical agents (non‐dressing) | ||||||
| Patient or population: Foot ulcers in people with diabetes Setting: Mixed Intervention: Topical antimicrobial agent Comparison: Non‐antimicrobial treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with non‐antimicrobial treatment | Risk with topical antimicrobial treatment | |||||
|
Proportion of wounds healed Up to 24 weeks' follow‐up |
241 per 1000 | 679 per 1000 (135 to 1000) | RR 2.82 (0.56 to 14.23) | 112 participants (3 studies) |
⊕⊝⊝⊝ very low1 | The average effect of antimicrobial agents compared with non‐antimicrobial treatment is uncertain over a medium‐term follow‐up period. |
| Risk difference: 438 more healed wounds per 1000 (106 fewer to 1000 more) | ||||||
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
|
Resolution of infection Up to 24 weeks' follow‐up |
368 per 1000 | 427 per 1000 (199 to 925) | RR 1.16 (0.54 to 2.51) | 40 participants (1 study) |
⊕⊕⊝⊝ low2 | It is unclear whether use of an antimicrobial topical agent has an effect on risk of infection over a medium‐term follow‐up period. |
| Risk difference: 59 more resolved infections per 1000 (169 fewer to 556 more) | ||||||
|
Adverse events Up to 24 weeks' follow‐up |
Not estimable | N/A | 81 participants (2 studies) |
⊕⊝⊝⊝ very low | 2 studies reported adverse event data. We were unable to extract per‐participant data for 1 study. The second study stated that no adverse events were reported in each arm. We judged this as very low‐certainty evidence. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for risk of bias with two studies at high risk of detection bias, which is of particular concern when healing is being assessed, and one study not accounting for a small number of participants with multiple ulcers in their trial. Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for inconsistency: one small study reported all wounds healed in one arm and few wounds healed in the other. These data are adding heterogeneity to the analysis. 2Downgraded twice for imprecision: small sample size and small number of events.
Summary of findings 3. One topical antimicrobial agent compared with an alternative topical antimicrobial agent.
| One topical antimicrobial agent compared with another topical antimicrobial agent | ||||||
| Patient or population: Foot ulcers in people with diabetes Setting: Mixed Intervention: Topical antimicrobial agent Comparison: Alternative topical antimicrobial agent | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with topical antimicrobial agent | Risk with alternative topical antimicrobial agent | |||||
|
Proportion of wounds healed Up to 24 weeks' follow‐up |
Data were not pooled due to the 3 studies evaluating different interventions. | N/A | 85 participants (3 studies) | ⊕⊝⊝⊝ very low1 | It is generally uncertain whether 1 topical treatment has an increased likelihood of healing compared with the alternative treatment. We judged this as very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias. | |
|
Incidence of infection Up to 24 weeks' follow‐up |
Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
|
Resolution of infection Up to 24 weeks' follow‐up |
625 per 1000 | 906 per 1000 (606 to 1000) | RR 1.45 (0.97 to 2.17) | 37 participants (1 study) | ⊕⊝⊝⊝ very low2 | It is uncertain whether 1 specific type of topical antimicrobial agent has a different effect on resolution of infection than another over a medium‐term follow‐up period. |
| Risk difference: 281 more resolved infections per 1000 (19 fewer to 731 more) | ||||||
|
Adverse events Up to 24 weeks' follow‐up |
Not estimable | N/A | 41 participants (1 study) |
⊕⊝⊝⊝ very low3 | The 1 study noted that no events were reported in either group. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for imprecision: small sample size and small number of events. Downgraded for risk of performance and detection bias. 2Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for high risk of selection bias. 3Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for high risk of performance bias.
Summary of findings 4. Topical antimicrobial agent compared with systemic antimicrobial agent.
| Topical antimicrobial agent compared with systemic antimicrobial agent | ||||||
| Patient or population: Foot ulcers in people with diabetes Setting: Mixed Intervention: Topical antimicrobial agent Comparison: Systemic antibiotic | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with systemic antibiotic agent | Risk with topical antimicrobial agent | |||||
| Proportion of wounds healed | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Resolution of infection | 333 per 1000 | 503 per 1000 (303 to 830) | RR 1.51 (0.91 to 2.49) | 102 participants (2 studies) | ⊕⊝⊝⊝ very low1 | It is uncertain whether the effects of topical antimicrobial treatment on resolution of infection differ from those of systemic antibiotics. |
| Risk difference: 170 more resolved infections per 1000 (30 fewer to 497 more) | ||||||
| Adverse events | 450 per 1000 | 409 per 1000 (351 to 477) | RR 0.91 (0.78 to 1.06) | 937 participants (4 studies) |
⊕⊕⊕⊝ moderate2 |
On average, there is probably little difference in the risk of adverse events between the systemic antibiotics and topical antimicrobial treatments compared here. |
| Risk difference: 40 fewer adverse events per 1000 (99 fewer to 27 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for risk of performance bias. 2Downgraded once for risk of performance bias.
Summary of findings 5. Topical antimicrobial agent compared with growth factor.
| Topical antimicrobial agent compared with growth factor | ||||||
| Patient or population: Foot ulcers in people with diabetes Setting: Mixed Intervention: Topical antimicrobial agent Comparison: Growth factor | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with growth factor | Risk with topical antimicrobial | |||||
| Proportion of wounds healed | 800 per 1000 | 400 per 1000 (224 to 712) | RR 0.50 (0.28 to 0.89) | 40 participants (1 study) |
⊕⊝⊝⊝ very low1 | It is uncertain whether treatment with growth factor affects the risk of healing when compared with antiseptic dressing. |
| Risk difference: 400 fewer resolved infections 576 fewer to 88 fewer | ||||||
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Resolution of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Adverse events | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded once for imprecision: small sample size and small number of events ‐ optimal information size not met and results are fragile. Downgraded twice for risk of performance and attrition bias.
For each comparison we have only listed outcomes for which there were reported data.
Comparison 1: Antimicrobial dressings compared with non‐antimicrobial dressings (standard care or placebo, or both) (5 trials; 945 participants)
See Table 1.
Five trials met the criteria for this comparison: one with short‐term follow‐up, He 2016, and four with medium‐term follow‐up (Gottrup 2013; Imran 2015; Jeffcoate 2009; Jude 2007). Three studies evaluated silver‐containing dressings (Gottrup 2013; He 2016; Jude 2007), one a honey‐containing dressing (Imran 2015), and one an iodine‐containing dressing (Jeffcoate 2009). Wounds were not infected at baseline in one study (Gottrup 2013); mixed infected and not infected in one study (Jude 2007); and not reported in the remaining three studies.
Complete wound healing: proportion of ulcers healed (5 trials; 945 participants; 420 outcome events)
Using the average treatment effect from a random‐effects model, treatment with an antimicrobial dressing may increase the number of ulcers healed over a medium‐term follow‐up period compared with non‐antimicrobial dressings: risk ratio (RR) 1.28, 95% confidence interval (CI) 1.12 to 1.45 (I² = 0%; low‐certainty evidence ‐ downgraded twice due to risk of bias) Analysis 1.1. This corresponds to an absolute risk (based on a combined event rate in the control arms of 425 per 1000) of 119 healing events per 1000 (95% CI from 51 more to 191 more). Where reported, the grade of ulcer in the studies ranged from I to III.
1.1. Analysis.
Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 1 Proportion of wounds healed.
There was no evidence of a subgroup effect when studies were grouped based on their duration of follow‐up (test for subgroup differences P = 0.33, I² = 0%).
Incidence of infection (2 trials; 173 participants; 23 outcome events)
Using the average treatment effect from a random‐effects model, it is uncertain whether use of antimicrobial dressings reduces the incidence of an ulcer becoming clinically infected over a medium‐term follow‐up period when compared with non‐antimicrobial dressings: RR 0.34, 95% CI 0.04 to 3.10 (I² = 60%; very low‐certainty evidence ‐ downgraded twice due to imprecision, once due to inconsistency, and once due to risk of bias) Analysis 1.2.
1.2. Analysis.
Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 2 Incidence of infection: medium term follow‐up.
Health‐related quality of life (1 trial; 317 participants)
One study measured health‐related quality of life (using the Cardiff Wound Impact Schedule and the SF‐36 at 24 weeks), presenting the data for each domain, but with no global summary score (Jeffcoate 2009). The study reported no significant difference between groups across domains. We have presented these data narratively (Table 11), but have not analysed them further.
Surgical resection (1 trial; 317 participants; 7 outcome events)
Based on data from only one study (Jeffcoate 2009), it is uncertain whether treatment with an antimicrobial dressing reduces the risk of amputation (minor or major) compared with a non‐antimicrobial dressing over a medium‐term follow‐up period: RR 0.33, 95% CI 0.04 to 2.72 (very low‐certainty evidence ‐ downgraded twice due to imprecision and once for risk of bias) Analysis 1.3.
1.3. Analysis.
Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 3 Surgical resection: medium term follow‐up.
Adverse events (1 trial with data analysed; 134 participants; 51 outcome events)
Whilst three studies reported adverse event data (Gottrup 2013; Jeffcoate 2009; Jude 2007), we analysed only the data from the one study that clearly reported rates per participant (Jude 2007).
It is uncertain whether antimicrobial dressings affect the risk of adverse events compared with non‐antimicrobial dressings over a medium‐term follow‐up period: RR 0.96, 95% CI 0.62 to 1.48 (very low‐certainty evidence ‐ downgraded twice due to imprecision and once for risk of bias) Analysis 1.4.
1.4. Analysis.
Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 4 Adverse events.
Comparison 1: Summary
Low‐certainty evidence suggests antimicrobial dressings probably increase the number of healing events in the medium term compared with non‐antimicrobial dressings. However, the effect of antimicrobial dressings on the incidence of infection, other outcomes, and adverse events is unclear (Table 1).
Comparison 2: Topical antimicrobial agents (non‐dressing) compared with non‐antimicrobial topical agents (non‐dressing) (4 trials; 132 participants)
See Table 2.
Four studies met the criteria for this comparison (Bergqvist 2016; Bowling 2011; Ramos Cuevas 2007; Tom 2005). Each study investigated a different non‐dressing topical treatment: chloramine (Bergqvist 2016), super‐oxidised aqueous solution (Bowling 2011), zinc hyaluronate (Ramos Cuevas 2007), and tretinoin (Ullal 2014). All studies had medium‐term follow‐up.
Complete wound healing: proportion of ulcers healed (3 trials; 112 participants; 54 outcome events)
Using the average treatment effect from a random‐effects model, the relative effect of non‐dressing antimicrobial treatments compared with non‐dressing non‐antimicrobial treatments is uncertain over a medium‐term follow‐up period: RR 2.82, 95% CI 0.56 to 14.23 (I² = 86%; very low‐certainty evidence ‐ downgraded twice for imprecision, once for inconsistency, and twice for risk of bias) Analysis 2.1.
2.1. Analysis.
Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 1 Proportion of wounds healed: medium term follow‐up.
Resolution of infection (1 trial; 40 participants; 16 outcome events)
One study reported data on resolution of clinical evidence of infection of ulcers during treatment (Bergqvist 2016). Of note, over half the participants in both treatment groups also received systemic antibiotic therapy during the study. It is unclear whether use of a non‐dressing antimicrobial topical treatment compared with non‐dressing non‐antimicrobial treatment affects the resolution of infection over a medium‐term follow‐up period: RR 1.16, 95% CI 0.54 to 2.51 (low‐certainty evidence ‐ downgraded twice for imprecision) Analysis 2.2.
2.2. Analysis.
Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 2 Resolution of infection: medium term follow‐up.
Surgical resection (1 trial; 40 participants (data on 34 participants analysed); 8 outcome events)
One study reported data on the incidence of surgical resection (Bergqvist 2016). Data were missing in each arm for this outcome, although the report states that they conducted a complete‐case analysis. There was no clear evidence that use of a non‐dressing antimicrobial topical agent compared with non‐dressing non‐antimicrobial treatment affects the risk of surgical resection over a medium‐term follow‐up period: RR 1.67, 95% 0.47 to 5.90 (low‐certainty evidence ‐ downgraded twice for imprecision) Analysis 2.3.
2.3. Analysis.
Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 3 Surgical resection: medium term follow‐up.
Adverse events (2 trials; 81 participants; no trials reported data for analysis)
Two studies reported adverse event data over a medium‐term follow‐up period. We were unable to extract per‐participant values for one study (Bergqvist 2016), and the other study reported that no adverse events occurred in each arm (Bowling 2011). We considered this evidence to be of very low certainty.
Comparison 2: Summary
It is uncertain whether non‐dressing topical antimicrobial treatments affect wound healing, infection resolution, surgical resection, or adverse events compared with non‐dressing non‐antimicrobial treatments over a medium‐term follow‐up period. Data were available from only four small studies with limited outcome events, making them imprecise. The studies also evaluate a variety of treatment regimens (Table 2).
Comparison 3: One topical antimicrobial agent compared with an alternative topical antimicrobial agent (8 trials; 250 participants (1 trial did not report number of participants))
See Table 3.
Eight trials compared one topical antimicrobial agent with another (Ahmed 2014; Apelqvist 1996; Hwang 2010; Jacobs 2008Martinez‐De Jesus 2007; Shukrimi 2008; Ullal 2014; Viswanathan 2011). The comparisons varied, with no two comparisons the same (see Table 10). Reported outcome data were very limited, and we elected to present data from only four trials. All outcome data, including those that were not appropriate for analyses, are presented in Table 10.
Complete wound healing: proportion of ulcers healed (3 trials; 85 participants; 23 outcome events)
We included data from three studies in this analysis (Apelqvist 1996; Jacobs 2008; Ullal 2014). Due to the variation in treatments used in these studies, the data were not pooled.
Apelqvist 1996 (n = 41) compared treatment with a cadexomer iodine ointment to "standard treatment", which included a gentamicin solution, in people with a grade I or II ulcer and followed them for 12 weeks. It is uncertain whether there was a difference in the risk ratio of healing between these treatments: RR 2.16, 95% CI 0.47 to 9.88 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 3.1.
3.1. Analysis.
Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 1 Proportion of wounds healed.
Jacobs 2008 (n = 40) compared silver sulphadiazine cream with a formulation containing benzoic acid, salicylic acid, and oak bark in people with a grade I or II ulcer and followed them for six weeks. It is uncertain whether there was a difference in the risk of healing between these treatments: RR 1.33, 95% CI 0.57 to 3.14 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 3.1.
Ullal 2014 (n = 4) compared treatment with a povidone iodine and metronidazole 1% gel dressing with a honey and metronidazole 1% gel dressing; neither the types of participants nor the duration of follow‐up were clearly reported. It is uncertain whether there was a difference in the risk of healing between these treatments: RR 5.00, 95% CI 0.38 to 66.01 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 3.1.
Resolution of infection (1 trial; 37 participants; 29 outcome events)
One study compared povidone iodine treatment with super‐oxidised aqueous solution (Martinez‐De Jesus 2007). All participants in both groups also received oral antibiotic therapy. It is uncertain whether there was a difference in the risk of infection resolution (defined largely by reduction in periwound cellulitis) between these treatments over a medium‐term follow‐up period: RR 1.45, 95% 0.97 to 2.17 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 3.2.
3.2. Analysis.
Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 2 Resolution of infection: medium term follow‐up.
Surgical resection (1 trial; 41 participants; 8 outcome events)
One study that compared gentamicin solution with cadexomer iodine ointment reported data on the risk of surgical resection (Apelqvist 1996). It is uncertain whether there was a difference in the risk of surgical resection over a medium‐term follow‐up period: RR 1.93, 95% 0.53 to 7.03 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 3.3.
3.3. Analysis.
Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 3 Surgical resection: medium term follow‐up.
Adverse events (1 trial; 41 participants; no outcome events )
In the one study that reported this information there were no documented adverse reactions related to the topical treatment (Apelqvist 1996). We classified this as very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias.
Comparison 3: Summary
Whilst eight studies compared a variety of different antimicrobial topical agents against another, the outcome data were limited. Not all studies measured important outcomes, and the studies were small. We are uncertain about the relative effects of antimicrobial topical agents for all review outcomes, including wound healing and adverse events.
Comparison 4: Topical antimicrobial agents compared with systemic antimicrobials (4 trials; 937 participants)
See Table 4.
Four studies compared therapy with a systemic (in all cases administered orally) antibiotic versus a topical antimicrobial treatment (Landsman 2011; Lipsky 2008a; Lipsky 2008b; Lipsky 2012a). Landsman 2011 compared levofloxacin (750 mg) and topical saline versus levofloxacin (750 mg) and super‐oxidised aqueous solution. Two studies (reported in one paper) compared ofloxacin (200 mg) with topical pexiganan cream (1%) (Lipsky 2008a; Lipsky 2008b), and one study compared systemic antibiotic therapy (largely levofloxacin) versus a gentamicin‐collagen sponge (Lipsky 2012a). We considered pooling the data to be appropriate, but given the different interventions in the classes of treatment being compared, we used a random‐effects model (although no there was no statistical heterogeneity and an I² of 0%).
Resolution of infection (2 trials; 102 participants; 46 outcome events)
It is uncertain whether or not, on average, topical antimicrobial treatment affects resolution of infection compared with systemic antibiotics in those with infected ulcers: RR 1.51, 95% CI 0.91 to 2.49 (very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias) Analysis 4.1. There was no evidence of a subgroup difference between one study that had short‐term follow‐up and one that had medium‐term follow‐up (P = 0.96; I² = 0%). The protocol we used for this review defined resolution of infection as the equivalent of clinical "cure". As two studies included participants with "improvement" along with "cure" (Lipsky 2008a; Lipsky 2008b), we could not use them for this comparison.
4.1. Analysis.
Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 1 Resolution of infection.
Surgical resection (2 trials (reported in 1 paper); 835 participants; 20 outcome events)
On average, there is no clear difference in the risk of surgical resection between treatments over a medium‐term follow‐up period: RR 1.22, 95% CI 0.51 to 2.91 (low‐certainty evidence ‐ downgraded twice for imprecision) Analysis 4.2.
4.2. Analysis.
Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 2 Surgical resection: medium term follow‐up.
Adverse events (4 trials; 937 participants; 399 outcome events)
On average, there is probably little difference in the risk of adverse events between the systemic antibiotics and topical antimicrobial treatments that were compared: RR 0.91, 95% CI 0.78 to 1.06 (moderate‐certainty evidence ‐ downgraded once for inconsistency) Analysis 4.3. There was no evidence of a subgroup difference for studies with short‐ versus medium‐term follow‐up (P = 0.67; I² = 0%).
4.3. Analysis.
Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 3 Adverse events.
Comparison 4: Summary
There is no evidence from RCTs on the relative effects of systemic antibiotics compared with topical antimicrobial agents on wound healing. Data on resolution of infection in infected wounds and on the need for surgical resection are limited, as studies are small with limited outcome events, resulting in low statistical power. On average, there is probably no difference in adverse events between the systemic and topical treatments we evaluated.
Comparison 5: Topical antimicrobial agents compared with growth factor (1 trial; 40 participants)
See Table 5.
One study compared a topical application of growth factors versus antiseptic dressings (not described further in the study report) in people with a grade III or IV ulcer (Khandelwal 2013); the duration of follow‐up was described only as more than eight weeks.
Complete wound healing: proportion of ulcers healed (1 trial; 40 participants; 24 outcome events)
It is uncertain whether treatment with growth factors affects the risk of healing when compared with an antiseptic dressing: RR 0.50, 95% 0.28 to 0.89 (very low‐certainty evidence ‐ downgraded once for imprecision and twice for risk of bias) Analysis 5.1.
5.1. Analysis.
Comparison 5 Topical antimicrobial agent compared with growth factor, Outcome 1 Proportion of wounds healed: Medium term follow‐up.
Comparison 5: Summary
In terms of healing, the relative effect of topical antimicrobial agents compared with growth factors remains uncertain. No other RCT data were available concerning any of the other outcomes relevant to this review.
Discussion
Summary of main results
This review includes 22 RCTs with a cumulative total of over 2310 participants (one trial did not report the number of study participants). These studies were grouped into five comparisons, as summarised below. The certainty of the available evidence was largely of low or very low.
Pooled data for non‐antimicrobial dressings compared with antimicrobial dressings suggests (based on the average treatment effect from a random‐effects model) that more wounds in people treated with antimicrobial dressings may completely heal. In absolute terms, the results correspond to an additional 119 healing events in the antimicrobial‐dressing arm per 1000 (95% CI from 51 more to 191 more). This finding was based on low‐certainty evidence from 5 studies that enrolled a total of 945 participants and reported 420 outcome events. An assessment of low‐certainty evidence means that our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Data on adverse events or other outcomes produced very low‐certainty evidence, due to the limited number of included studies and their small sizes in terms of participants or events, or both.
Pooled data from non‐dressing non‐antimicrobial topical treatments compared with non‐dressing antimicrobial topical treatments produced low‐ and very low‐certainty evidence (based on the results from 4 trials with a total of 132 participants) for the proportion of wounds healed, resolution of infection, surgical resection, and adverse events.
Eight studies with a total of 250 participants compared different topical antimicrobial treatments (the comparisons varied, so these data were not pooled). Reported outcome data were limited, leading us to conclude that the evidence for the relative effects of any one antimicrobial topical treatment versus another was of very low certainty for all review outcomes, including wound healing and adverse events.
Four studies (937 participants) compared systemic antibiotics with topical antimicrobial treatments. No wound‐healing data were reported, and there was very low‐certainty evidence for the relative effects of the various agents on resolution of wound infection and need for surgical resection. Using an average treatment effect, it is possible that there is no difference in adverse events between the systemic and topical antimicrobial treatments evaluated here.
One included study (40 participants) compared the use of growth factors to topical antimicrobials. For the only outcome reported that was relevant to this review, that is the effect on the number of ulcers, the data were of very low certainty.
Overall completeness and applicability of evidence
Overall, the evidence for this review question was limited. Whilst we included 22 studies, these studies evaluated a wide range of treatment options, leading to a lack of homogeneity and limited data for specific comparisons of interest. It was also not clear how the evaluations undertaken in these studies relate to current practice, which is also likely to be varied. This variation is reflected in our analytical approach of often viewing non‐antimicrobial topical treatments and antimicrobial topical treatments as a 'class', despite the obvious variations within these treatments. For example, our 'class' of antimicrobial dressings contains trials of agents as varied as those using silver, iodine, and honey; readers should bear this in mind when interpreting our findings. We have frequently used random‐effects models, which allow for the treatment effects to vary from study to study following a normal distribution. We have thus assumed that there might be real variation between the relative effects of these classes of treatment, as well as random error.
In addition to the variation in treatments and types of wounds evaluated, the generally poor reporting of the included studies means that we have limited wound‐related baseline information in terms of the infection status and the severity of wounds. In studies in which authors reported an ulcer 'grade', they used different (and sometimes ill‐defined) measures. Specifically, 10 of the 22 studies (45%) did not report data on baseline ulcer severity, and 13 (59%) of the studies did not report baseline infection status. Nevertheless, we have summarised based on the general pattern that increasing wound grade denoted increased disease severity.
We also note that we have located details of eight trials from the trials registry that may be eligible for the review but that seem to be unpublished. We are continuing to try to obtain details of these studies.
Our review of this topic allowed us to identify several key issues that we think investigators should consider when planning future trials of topical antimicrobial agents for treating clinically infected or uninfected foot ulcers in people with diabetes. Firstly, it is essential to use a standard, and preferably validated, method of classifying the wounds, especially insofar as their infection status (for which the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (IDSA/IWGDF) classification seems most appropriate). While clinical definitions are imperfect, microbiological, imaging, and biomarker definitions of infection are less well validated. Secondly, investigators should be clear on how they define both the presence, and the resolution, of infection in the wounds. Thirdly, they should use consistent, and preferably validated, methods of measuring the healing of wounds (preferably using complete epithelialisation). Finally, the protocols used should clarify the primary and any secondary outcomes to be used for each type of wound (Clarke 2007). Following this approach may reduce the risk of reporting bias (Kirkham 2010). In addition, all trials should follow these key recommendations for good practice: include the robust generation of a randomisation sequence (e.g. by a computer‐generated randomisation schedule); use a robust method of allocation concealment (e.g. through the use of a telephone randomisation service); and ensure blinded outcome assessment where possible. Blinded outcome assessment is crucial for outcomes such as healing and infection, which are inherently subjective, thus introducing the risk of detection or observer bias (Hróbjartsson 2012).
Quality of the evidence
The quality of reporting in the included studies was limited. Not all trials reported the same outcomes, and many did not report key outcomes on infection prevention or resolution, or on wound healing. Over 75% of included studies were at unclear risk for selection bias and for detection bias, which are key domains. Many of the studies were also at risk of performance bias (which is avoided by blinding participants and healthcare professionals to treatments). While the risk of performance bias is not yet clear in wound care studies, the importance of detection bias (avoided by employing a blinded outcome assessment) is well recognised (Hróbjartsson 2012). The reporting of adverse events was poor or absent in the large majority of studies. It was thus difficult or impossible for us to make accurate assessments of the risk of adverse events that were specifically associated with the tested topical agents and their comparators.
In addition to the 'Risk of bias' issues, the included studies were also often small in terms of numbers of participants and numbers of documented outcome events. These factors are reflected in our assessment of the certainty of evidence, which was often low or very low.
Potential biases in the review process
Three of the included trials were led by one of the authors of this review (Lipsky 2008a; Lipsky 2008b; Lipsky 2012a); to overcome this potential bias, three other review authors (MC, MF, JD) conducted the data extractions, 'Risk of bias' assessments, and analyses.
We conducted a comprehensive search that included trial registries, and obtained translations to English as required, so we do not believe that language bias is an issue. We do not know the risk publication bias, as we were unable to explore this with the available studies. As we did not deviate from our original (and previously published) protocol for this review, with the exception of those changes highlighted in Differences between protocol and review, we do not believe we introduced bias in terms of selective outcome reporting.
It is noteworthy that we found 15 studies reported on a trial register that we are unable to link to published data. We emailed all available trial contacts to try to obtain additional data but were successful in only four cases. The risk of publication bias is increased if unpublished data exist that were not available for inclusion in the review.
Agreements and disagreements with other studies or reviews
There are few other reviews specifically examining the role of topical antimicrobial therapy for diabetic foot wounds or infections. One Cochrane review examined the role of systemic (but not topical) antibiotics for treating diabetic foot infections (Selva Olid 2015). Another Cochrane review examined data on silver‐based wound dressings and topical agents for treating diabetic foot ulcers (Bergin 2006), and concluded (as we did) that there are no randomised trials or controlled clinical trials evaluating their clinical effectiveness. Similarly, another Cochrane review concluded that there was insufficient evidence to establish whether or not silver‐containing dressings or topical agents promote wound healing or prevent wound infection (Vermeulen 2007). A review of the evidence for the use of topical antimicrobial agents in wound care concluded that despite limited data, judicious prophylactic use of antiseptics may prevent the development of infections while minimising antibiotic use, as well as promote faster healing (Cooper 2004). This review also noted that it was important to avoid misuse and abuse of topical antiseptics. In a review of the use of topical antimicrobial agents for treating various kinds of chronic wounds, the authors concluded that there are few proven indications for these agents (Lipsky 2009). Guidelines on management of diabetic foot infections from both the Infectious Diseases Society of America and the International Working Group on the Diabetic Foot (Lipsky 2012a; Lipsky 2016), recognising the scarce data, offered similar recommendations to ours on the current limited role of topical antimicrobial agents. A recent systematic review of the effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes included in their search papers on wound bed preparation using antiseptics, applications, and dressing products (Game 2016). They concluded that there is little published evidence to justify the use of any of these therapies.
Authors' conclusions
Implications for practice.
The meagre information identified to inform decision makers about the safety and efficacy of treating diabetic patients with a foot ulcer with topical antimicrobials is especially disappointing because diabetic foot infections are a large and growing problem worldwide. Low‐certainty evidence suggests that treatment with antimicrobial dressings may increase the likelihood of healing of these wounds. The limited and weak available evidence does not allow us to draw firm conclusions on the role of any topical antimicrobial in the treatment or prevention of wound infection in people with foot ulcers and diabetes.
Implications for research.
Foot ulcers in people with diabetes are becoming increasingly frequent in most countries throughout the world. The majority of these wounds are, or are at risk of becoming, infected.
In planning future research, we need to consider what constitutes the most appropriate approach to antimicrobial therapy for these difficult infections. Our findings highlight the lack of high‐certainty evidence that can inform this research question. Any future research needs to address information that is critically important to clinicians, administrators, and decision makers, as well as patients, as any investment in trials has an opportunity cost. Given the large number of treatment options, the investigators and funders need to consider which interventions are most crucial and potentially cost‐effective. Such planning means that research resources can be focused to address priorities. Where trials are conducted, they must follow good‐practice guidelines in their design, implementation, and reporting.
A key issue is that studies must make clear whether or not the diabetic foot ulcers are clinically infected, and whether the goal is to prevent or treat infection. As discussed above, studies should use a validated infection classification scheme based on clinical findings. Our review found low‐certainty evidence that treatment with antimicrobial dressings may increase the likelihood of wound healing; this may be a fruitful area for further research. Such research would need to carefully consider the types of interventions used and the study populations; current data are largely related to populations with 'grade I and II' (variably defined) ulcers. The duration of study follow‐up should also be clearly considered to allow adequate time for healing events to occur (ideally at least 24 weeks), and all outcome assessments should be by treatment‐blinded investigators.
Acknowledgements
The authors appreciate the contribution of the peer referees ( Jane Burch, Susan O’Meara, Marialena Trivella, Ann‐Marie Glenny, Laura Bolton, Malcolm Brewster and a clinical expert who wishes to remain anonymous) and the copy editors Elizabeth Royle and Lisa Winer. We also thank Carlo Mengoli, who played an important role in helping us develop the protocol.
Appendices
Appendix 1. Search strategies
The Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [Acetic Acid] explode all trees #2 ((acetic next acid*) or acetate* or acetamide*):ti,ab,kw #3 MeSH descriptor: [Antifungal Agents] explode all trees #4 ((therapeutic next fungicide*) or antifungal* or fungistatic*):ti,ab,kw #5 MeSH descriptor: [Antiviral Agents] explode all trees #6 (antiviral* or (anti next viral*) or idoxuridine*):ti,ab,kw #7 MeSH descriptor: [Bacitracin] explode all trees #8 MeSH descriptor: [Povidone‐Iodine] explode all trees #9 (bacitracin* or (povidone next iodine*) or betaisodona* or (polyvinylpyrrolidone next iodine*) or betadine* or disadine* or isodine* or pvpi or pharmadine*):ti,ab,kw #10 MeSH descriptor: [Cetrimonium Compounds] explode all trees #11 (cetyltrimethylammonium or cetrimide* or cetrimonium):ti,ab,kw #12 MeSH descriptor: [Chlorine Compounds] explode all trees #13 (chlorate* or (hydrochloric next acid*) or chloride* or (hypochlorous next acid*) or hypochlorite* or (perchloric next acid*) or (ruthenium next red*) or Dakin*):ti,ab,kw #14 MeSH descriptor: [Eosine Yellowish‐(YS)] explode all trees #15 (eusol or phenoxyethanol* or dextranomer* or (framycetin next sulphate*) or (mandelic next acid*) or tetrabromofluorescein* or eosin or eosine or chlortetracycline* or (chloroxylenol next solution*)):ti,ab,kw #16 ((edinburgh next university next solution) near/2 lime):ti,ab,kw #17 MeSH descriptor: [Framycetin] explode all trees #18 MeSH descriptor: [Mandelic Acids] explode all trees #19 (cyclandelate* or (vanilmandelic next acid*)):ti,ab,kw #20 MeSH descriptor: [Hexachlorophene] explode all trees #21 hexachloroph?ne*:ti,ab,kw #22 MeSH descriptor: [Triclosan] explode all trees #23 MeSH descriptor: [Polymyxins] explode all trees #24 (triclosan* or polymyxin* or polynoxylin*):ti,ab,kw #25 MeSH descriptor: [Silver] explode all trees #26 MeSH descriptor: [Silver Sulfadiazine] explode all trees #27 MeSH descriptor: [Gentian Violet] explode all trees #28 (violet or (methylrosaniline next chloride*) or (hexamethylpararosanine next chloride*)):ti,ab,kw #29 MeSH descriptor: [Potassium Permanganate] explode all trees #30 ((potassium next permanganate*) or (permanganic next acid*) or (potassium next salt*)):ti,ab,kw #31 {or #1‐#30} #32 MeSH descriptor: [Mupirocin] explode all trees #33 (mupirocin* or (pseudomonic next acid*) or bactroban*):ti,ab,kw #34 MeSH descriptor: [Neomycin] explode all trees #35 (neomycin* or fradiomycin* or neamin*):ti,ab,kw #36 MeSH descriptor: [Benzoyl Peroxide] explode all trees #37 ((benzyol next peroxide*) or (benzyol next superoxide*) or (diphenylglyoxal next superoxide*) or panoxyl*):ti,ab,kw #38 MeSH descriptor: [Hydrogen Peroxide] explode all trees #39 ((hydrogen next peroxide*) or hydroperoxide* or oxydol* or perhydrol* or superoxol* or (diphenylglyoxal next superoxide*) or panoxyl*):ti,ab,kw #40 MeSH descriptor: [Chlorhexidine] explode all trees #41 ((cadexomer next iodine*) or chlorhexidine* or novalsan* or sebidin* or tubulicid*):ti,ab,kw #42 MeSH descriptor: [Sucrose] explode all trees #43 MeSH descriptor: [Honey] explode all trees #44 MeSH descriptor: [Propolis] explode all trees #45 (sucrose or (sugar next paste*) or "granulated sugar" or propolis or honey or beebread* or (bee next bread*) or (bee next glue*)):ti,ab,kw #46 MeSH descriptor: [Plant Oils] explode all trees #47 MeSH descriptor: [Oils, Volatile] explode all trees #48 ((essential next oil*) or (plant next oil*) or "tea tree" or lavender or chamomile or camomile or rosemary):ti,ab,kw #49 MeSH descriptor: [Disinfectants] explode all trees #50 MeSH descriptor: [Anti‐Infective Agents, Local] explode all trees #51 (disinfect* or antiseptic* or anti‐septic*):ti,ab,kw #52 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees #53 (antibiotic* or antimicrobial*):ti,ab,kw #54 MeSH descriptor: [Penicillins] explode all trees #55 (penicillin* or amdinocillin* or amox?cillin* or ampicillin* or azlocillin* or carbenicillin* or carfecillin* or cloxacillin* or dicloxacillin* or floxacillin* or flucloxacillin* or methicillin* or mazlocillin* or nafcillin* or oxacillin* or (penicillanic next acid*) or (penicillic next acid*) or phenoxymethylpenicillin* or piperacillin* or pivampicillin* or sulbencillin* or talampicillin* or sultamicillin* or ticarcillin* or ticercillin*):ti,ab,kw #56 MeSH descriptor: [Cephalosporins] explode all trees #57 (cefaclor* or cefadroxil* or cefalexin* or cefazolin* or cefamandole* or cefixime* or cefotaxime* or cefoxitin* or cefpirome* or cefpodoxime* or cefprozil* or cefradine* or ceftazidime* or ceftizoxime* or ceftriaxone* or cefuroxime* or cefonicid* or cefmenoxine* or cefoperazone* or cefotiam* or cefsulodin* or cephacetrile* or cephalexin* or cephaloglycin* or cephaloridine or loracarbef* or cefotetan* or cefmetazole* or cefdinir* or cefditoren* or ceftibuten* or cefepime* or cefpirome* or ceftaroline* or ceftobiprole* or (cephalosporanic next acid*) or cephalothin* or cephapirin* or cephradine*):ti,ab,kw #58 MeSH descriptor: [Lactams] explode all trees #59 ((beta next lactam*) or aztreonam* or cilastin* or imipenem* or meropenem* or sulbactam* or tazobactam* or caprolactam* or clavulan* or moxalactam*):ti,ab,kw #60 MeSH descriptor: [Aminoglycosides] explode all trees #61 (Aminoglycoside* or anthracycline* or aclarubicin* or daunorubicin* or carubicin* or doxorubicin* or epirubicin* or idarubicin* or nogalamycin* or menogaril* or plicamycin*):ti,ab,kw #62 (gentamicin* or netilmicin* or tobramycin*):ti,ab,kw #63 {or #32‐#62} #64 MeSH descriptor: [Macrolides] explode all trees #65 (amphotericin* or antimycin* or candicidin* or roxithromycin* or josamycin* or leucomycin* or kitasamycin* or lucensomycin* or maytansine* or mepartricin* or miocamycin*):ti,ab,kw #66 (natamycin* or oleandomycin* or troleandomycin* or oligomycin* or rutamycin* or sirolimus* or tacrolimus* or tylosin* or propiolactone* or spironolactone* or venturicidin* or zearalenone* or zeranol*):ti,ab,kw #67 (azithromycin* or clarithromycin* or erythromycin* or spiramycin*):ti,ab,kw #68 MeSH descriptor: [Quinolones] explode all trees #69 (moxifloxacin* or quinolone* or ciprofloxacin* or clinafloxacin* or fluoroquinolone* or levofloxacin* or ofloxacin* or gatifloxacin*):ti,ab,kw #70 (fleroxacin* or enoxacin* or norfloxacin* or pefloxacin* or nalidixic acid* or nedocromil* or oxolinic acid* or quinpirole* or quipazine* or saquinavir*):ti,ab,kw #71 MeSH descriptor: [Sulfonamides] explode all trees #72 MeSH descriptor: [Trimethoprim] explode all trees #73 (dmso or sulfoxide* or sulphoxide* or sulfonamide* or sulphonamide* or trimethoprim* or sulfamethoxazole* or sulphamethoxazole* or co‐trimoxazole* or sulfadiazine* or sulphadiazine* or sulfametopyrazine* or sulfalene* or sulphametopyrazine* or sulphalene*):ti,ab,kw #74 (sulfachlorpyridazine* or sulfadimethoxine* or sulfadoxine* or sulfaguanidine* or sulfamerazine* or sulfameter* or sulfamethazine* or sulfamethoxypyridazine* or sulphachlorpyridazine* or sulphadimethoxine* or sulphadoxine* or sulphaguanidine* or sulphamerazine* or sulphameter* or sulphamethazine* or sulphamethoxypyridazine*):ti,ab,kw #75 (sulfamonomethoxine* or sulfamoxole* or sulfaphenazole* or sulfapyridine* or sulfaquinoxaline* or sulfathiazole* or sulfamethizole* or sulfisomidine* or sulfisoxazole* or sulfasalazine* or sumatriptan* or xipamide* or thioamide* or thioacetamide* or sulphamonomethoxine* or sulphamoxole* or sulphaphenazole* or sulphapyridine* or sulphaquinoxaline* or sulphathiazole* or sulphamethizole* or sulphisomidine* or sulphisoxazole* or sulphasalazine*):ti,ab,kw #76 MeSH descriptor: [Tetracyclines] explode all trees #77 (tetracycline* or demeclocycline* or doxycycline* or lymecycline* or minocycline* or oxytetracycline*):ti,ab,kw #78 (chlortetracycline* or methacycline* or rolitetracycline*):ti,ab,kw #79 MeSH descriptor: [Chloramphenicol] explode all trees #80 (cloranfenicol* or chloramphenicol*):ti,ab,kw #81 (thiamphenicol* or kloramfenikol* or levomycetin* or chlornitromycin* or chlorocid* or chloromycetin* or detreomycin* or ophthochlor* or syntomycin*):ti,ab,kw #82 MeSH descriptor: [Clindamycin] explode all trees #83 (clindamycin* or "dalacin c" or cleocin* or chlo?lincocin*):ti,ab,kw #84 MeSH descriptor: [Metronidazole] explode all trees #85 (linezolid* or trivazol* or vagilen* or clont* or danizol* or fagyl* or ginefavir* or metrogel* or metrodzhil* or satric* or trichazol* or trichopol*):ti,ab,kw #86 MeSH descriptor: [Fusidic Acid] explode all trees #87 ("granulocyte colony stimulating factor" or "granulocyte colony stimulating factors" or gcsf or ozone):ti,ab,kw #88 (fusidate* next (sodium or silver)):ti,ab,kw #89 (griseofulvin or synercid or dalfopristin or quinupristin):ti,ab,kw #90 MeSH descriptor: [Daptomycin] explode all trees #91 {or #64‐#90} #92 {or #31, #63, #91} #93 MeSH descriptor: [Foot Ulcer] explode all trees #94 MeSH descriptor: [Diabetic Foot] explode all trees #95 (diabet* near/3 ulcer*):ti,ab,kw #96 (diabet* near/3 (foot or feet)):ti,ab,kw #97 (diabet* near/3 wound*):ti,ab,kw #98 (diabet* near/3 amputat*):ti,ab,kw #99 (diabet* near/3 defect*):ti,ab,kw #100 {or #93‐#99} #101 {and #92, #100} in Trials
Ovid MEDLINE
1 exp Acetic Acid/ 2 (acetic acid* or acetate* or acetamide*).tw. 3 exp Antifungal Agents/ 4 (therapeutic fungicide* or antifungal* or fungistatic*).tw. 5 exp Antiviral Agents/ 6 (antiviral* or anti viral* or idoxuridine*).tw. 7 exp Bacitracin/ 8 exp Povidone‐Iodine/ 9 (bacitracin* or povidone iodine* or betaisodona* or polyvinylpyrrolidone iodine* or betadine* or disadine* or isodine* or pvpi or pharmadine*).tw. 10 exp Cetrimonium Compounds/ 11 (cetyltrimethylammonium or cetrimide* or cetrimonium).tw. 12 exp Chlorine Compounds/ 13 (chlorate* or hydrochloric acid* or chloride* or hypochlorous acid* or hypochlorite* or perchloric acid* or ruthenium red* or Dakin*).tw. 14 exp "Eosine Yellowish‐(YS)"/ 15 (eusol or phenoxyethanol* or dextranomer* or framycetin sulphate* or mandelic acid* or tetrabromofluorescein* or eosin or eosine or chlortetracycline* or chloroxylenol solution*).tw. 16 (edinburgh university solution adj2 lime).tw. 17 exp Framycetin/ 18 exp Mandelic Acids/ 19 (cyclandelate* or vanilmandelic acid*).tw. 20 exp Hexachlorophene/ 21 hexachloroph?ne*.tw. 22 exp Triclosan/ 23 exp Polymyxin/ 24 (triclosan* or polymyxin* or polynoxylin*).tw. 25 exp Silver/ 26 exp Silver Sulfadiazine/ 27 exp Gentian Violet/ 28 (violet or methylrosaniline chloride* or hexamethylpararosanine chloride*).tw. 29 exp Potassium Permanganate/ 30 (potassium permanganate* or permanganic acid* or potassium salt*).tw. 31 or/1‐30 32 exp Mupirocin/ 33 (mupirocin* or pseudomonic acid* or bactroban*).tw. 34 exp Neomycin/ 35 (neomycin* or fradiomycin* or neamin*).tw. 36 exp Benzoyl Peroxide/ 37 (benzyol peroxide* or benzyol superoxide* or diphenylglyoxal superoxide* or panoxyl*).tw. 38 exp Hydrogen Peroxide/ 39 (hydrogen peroxide* or hydroperoxide* or oxydol* or perhydrol* or superoxol* or diphenylglyoxal superoxide* or panoxyl*).tw. 40 exp Chlorhexidine/ 41 (cadexomer iodine* or chlorhexidine* or novalsan* or sebidin* or tubulicid*).tw. 42 exp Sucrose/ 43 exp Honey/ 44 exp Propolis/ 45 (sucrose or sugar paste* or granulated sugar or propolis or honey or beebread* or bee bread* or bee glue*).tw. 46 exp plant oils/ 47 exp oils, volatile/ 48 (essential oil* or plant oil* or tea tree or lavender or chamomile or camomile or rosemary).tw. 49 exp Disinfectants/ 50 exp Anti‐Infective Agents, Local/ 51 (disinfect* or antiseptic* or anti‐septic*).tw. 52 exp Antibiotics/ 53 (antibiotic* or antimicrobial*).tw. 54 exp Penicillins/ 55 (penicillin* or amdinocillin* or amox?cillin* or ampicillin* or azlocillin* or carbenicillin* or carfecillin* or cloxacillin* or dicloxacillin* or floxacillin* or flucloxacillin* or methicillin* or mazlocillin* or nafcillin* or oxacillin* or penicillanic acid* or penicillic acid* or phenoxymethylpenicillin* or piperacillin* or pivampicillin* or sulbencillin* or talampicillin* or sultamicillin* or ticarcillin* or ticercillin*).tw. 56 exp Cephalosporins/ 57 (cefaclor* or cefadroxil* or cefalexin* or cefazolin* or cefamandole* or cefixime* or cefotaxime* or cefoxitin* or cefpirome* or cefpodoxime* or cefprozil* or cefradine* or ceftazidime* or ceftizoxime* or ceftriaxone* or cefuroxime* or cefonicid* or cefmenoxine* or cefoperazone* or cefotiam* or cefsulodin* or cephacetrile* or cephalexin* or cephaloglycin* or cephaloridine or loracarbef* or cefotetan* or cefmetazole* or cefdinir* or cefditoren* or ceftibuten* or cefepime* or cefpirome* or ceftaroline* or ceftobiprole* or cephalosporanic acid* or cephalothin* or cephapirin* or cephradine*).tw. 58 exp Lactams/ 59 (beta lactam* or aztreonam* or cilastin* or imipenem* or meropenem* or sulbactam* or tazobactam* or caprolactam* or clavulan* or moxalactam*).tw. 60 exp Aminoglycosides/ 61 (Aminoglycoside* or anthracycline* or aclarubicin* or daunorubicin* or carubicin* or doxorubicin* or epirubicin* or idarubicin* or nogalamycin* or menogaril* or plicamycin*).tw. 62 (gentamicin* or netilmicin* or tobramycin*).tw. 63 or/32‐62 64 exp Macrolides/ 65 (amphotericin* or antimycin* or candicidin* or roxithromycin* or josamycin* or leucomycin* or kitasamycin* or lucensomycin* or maytansine* or mepartricin* or miocamycin*).tw. 66 (natamycin* or oleandomycin* or troleandomycin* or oligomycin* or rutamycin* or sirolimus* or tacrolimus* or tylosin* or propiolactone* or spironolactone* or venturicidin* or zearalenone* or zeranol*).tw. 67 (azithromycin* or clarithromycin* or erythromycin* or spiramycin*).tw. 68 exp Quinolones/ 69 (moxifloxacin* or quinolone* or ciprofloxacin* or clinafloxacin* or fluoroquinolone* or levofloxacin* or ofloxacin* or gatifloxacin*).tw. 70 (fleroxacin* or enoxacin* or norfloxacin* or pefloxacin* or nalidixic acid* or nedocromil* or oxolinic acid* or quinpirole* or quipazine* or saquinavir*).tw. 71 exp Sulfonamides/ 72 exp Trimethoprim/ 73 (dmso or sulfoxide* or sulphoxide* or sulfonamide* or sulphonamide* or trimethoprim* or sulfamethoxazole* or sulphamethoxazole* or co‐trimoxazole* or sulfadiazine* or sulphadiazine* or sulfametopyrazine* or sulfalene* or sulphametopyrazine* or sulphalene*).tw. 74 (sulfachlorpyridazine* or sulfadimethoxine* or sulfadoxine* or sulfaguanidine* or sulfamerazine* or sulfameter* or sulfamethazine* or sulfamethoxypyridazine* or sulphachlorpyridazine* or sulphadimethoxine* or sulphadoxine* or sulphaguanidine* or sulphamerazine* or sulphameter* or sulphamethazine* or sulphamethoxypyridazine*).tw. 75 (sulfamonomethoxine* or sulfamoxole* or sulfaphenazole* or sulfapyridine* or sulfaquinoxaline* or sulfathiazole* or sulfamethizole* or sulfisomidine* or sulfisoxazole* or sulfasalazine* or sumatriptan* or xipamide* or thioamide* or thioacetamide* or sulphamonomethoxine* or sulphamoxole* or sulphaphenazole* or sulphapyridine* or sulphaquinoxaline* or sulphathiazole* or sulphamethizole* or sulphisomidine* or sulphisoxazole* or sulphasalazine*).tw. 76 exp Tetracyclines/ 77 (tetracycline* or demeclocycline* or doxycycline* or lymecycline* or minocycline* or oxytetracycline*).tw. 78 (chlortetracycline* or methacycline* or rolitetracycline*).tw. 79 exp Chloramphenicol/ 80 (cloranfenicol* or chloramphenicol*).tw. 81 (thiamphenicol* or kloramfenikol* or levomycetin* or chlornitromycin* or chlorocid* or chloromycetin* or detreomycin* or ophthochlor* or syntomycin*).tw. 82 exp Clindamycin/ 83 (clindamycin* or dalacin c or cleocin* or chlo?lincocin*).tw. 84 exp Metronidazole/ 85 (linezolid* or trivazol* or vagilen* or clont* or danizol* or fagyl* or ginefavir* or metrogel* or metrodzhil* or satric* or trichazol* or trichopol*).tw. 86 exp Fusidic Acid/ 87 (granulocyte colony stimulating factor or gcsf or ozone).tw. 88 (fusidate* adj (sodium or silver)).tw. 89 (griseofulvin or synercid or dalfopristin or quinupristin).tw. 90 exp Daptomycin/ 91 or/64‐90 92 or/31,63,91 93 exp Foot Ulcer/ 94 exp Diabetic Foot/ 95 (diabet* adj3 ulcer*).tw. 96 (diabet* adj3 (foot or feet)).tw. 97 (diabet* adj3 wound*).tw. 98 (diabet* adj3 defect*).tw. 99 or/93‐98 100 and/92,99 101 randomized controlled trial.pt. 102 controlled clinical trial.pt. 103 randomi?ed.ab. 104 placebo.ab. 105 clinical trials as topic.sh. 106 randomly.ab. 107 trial.ti. 108 or/101‐107 109 exp animals/ not humans.sh. 110 108 not 109 111 and/100,110
Ovid Embase
1 exp Acetic Acid/ 2 (acetic acid* or acetate* or acetamide*).tw. 3 exp Antifungal Agents/ 4 (therapeutic fungicide* or antifungal* or fungistatic*).tw. 5 exp Antiviral Agents/ 6 (antiviral* or anti viral* or idoxuridine*).tw. 7 exp Bacitracin/ 8 exp Povidone‐Iodine/ 9 (bacitracin* or povidone iodine* or betaisodona* or polyvinylpyrrolidone iodine* or betadine* or disadine* or isodine* or pvpi or pharmadine*).tw. 10 exp Cetrimonium Compounds/ 11 (cetyltrimethylammonium or cetrimide* or cetrimonium).tw. 12 exp Chlorine Compounds/ 13 (chlorate* or hydrochloric acid* or chloride* or hypochlorous acid* or hypochlorite* or perchloric acid* or ruthenium red* or Dakin*).tw. 14 exp "Eosine Yellowish‐(YS)"/ 15 (eusol or phenoxyethanol* or dextranomer* or framycetin sulphate* or mandelic acid* or tetrabromofluorescein* or eosin or eosine or chlortetracycline* or chloroxylenol solution*).tw. 16 (edinburgh university solution adj2 lime).tw. 17 exp Framycetin/ 18 exp Mandelic Acids/ 19 (cyclandelate* or vanilmandelic acid*).tw. 20 exp Hexachlorophene/ 21 hexachloroph?ne*.tw. 22 exp Triclosan/ 23 exp Polymyxin/ 24 (triclosan* or polymyxin* or polynoxylin*).tw. 25 exp Silver/ 26 exp Silver Sulfadiazine/ 27 exp Gentian Violet/ 28 (violet or methylrosaniline chloride* or hexamethylpararosanine chloride*).tw. 29 exp Potassium Permanganate/ 30 (potassium permanganate* or permanganic acid* or potassium salt*).tw. 31 or/1‐30 32 exp Mupirocin/ 33 (mupirocin* or pseudomonic acid* or bactroban*).tw. 34 exp Neomycin/ 35 (neomycin* or fradiomycin* or neamin*).tw. 36 exp Benzoyl Peroxide/ 37 (benzyol peroxide* or benzyol superoxide* or diphenylglyoxal superoxide* or panoxyl*).tw. 38 exp Hydrogen Peroxide/ 39 (hydrogen peroxide* or hydroperoxide* or oxydol* or perhydrol* or superoxol* or diphenylglyoxal superoxide* or panoxyl*).tw. 40 exp Chlorhexidine/ 41 (cadexomer iodine* or chlorhexidine* or novalsan* or sebidin* or tubulicid*).tw. 42 exp Sucrose/ 43 exp Honey/ 44 exp Propolis/ 45 (sucrose or sugar paste* or granulated sugar or propolis or honey or beebread* or bee bread* or bee glue*).tw. 46 exp plant oils/ 47 exp oils, volatile/ 48 (essential oil* or plant oil* or tea tree or lavender or chamomile or camomile or rosemary).tw. 49 exp Disinfectants/ 50 exp Anti‐Infective Agents, Local/ 51 (disinfect* or antiseptic* or anti‐septic*).tw. 52 exp Antibiotics/ 53 (antibiotic* or antimicrobial*).tw. 54 exp Penicillins/ 55 (penicillin* or amdinocillin* or amox?cillin* or ampicillin* or azlocillin* or carbenicillin* or carfecillin* or cloxacillin* or dicloxacillin* or floxacillin* or flucloxacillin* or methicillin* or mazlocillin* or nafcillin* or oxacillin* or penicillanic acid* or penicillic acid* or phenoxymethylpenicillin* or piperacillin* or pivampicillin* or sulbencillin* or talampicillin* or sultamicillin* or ticarcillin* or ticercillin*).tw. 56 exp Cephalosporins/ 57 (cefaclor* or cefadroxil* or cefalexin* or cefazolin* or cefamandole* or cefixime* or cefotaxime* or cefoxitin* or cefpirome* or cefpodoxime* or cefprozil* or cefradine* or ceftazidime* or ceftizoxime* or ceftriaxone* or cefuroxime* or cefonicid* or cefmenoxine* or cefoperazone* or cefotiam* or cefsulodin* or cephacetrile* or cephalexin* or cephaloglycin* or cephaloridine or loracarbef* or cefotetan* or cefmetazole* or cefdinir* or cefditoren* or ceftibuten* or cefepime* or cefpirome* or ceftaroline* or ceftobiprole* or cephalosporanic acid* or cephalothin* or cephapirin* or cephradine*).tw. 58 exp Lactams/ 59 (beta lactam* or aztreonam* or cilastin* or imipenem* or meropenem* or sulbactam* or tazobactam* or caprolactam* or clavulan* or moxalactam*).tw. 60 exp Aminoglycosides/ 61 (Aminoglycoside* or anthracycline* or aclarubicin* or daunorubicin* or carubicin* or doxorubicin* or epirubicin* or idarubicin* or nogalamycin* or menogaril* or plicamycin*).tw. 62 (gentamicin* or netilmicin* or tobramycin*).tw. 63 or/32‐62 64 exp Macrolides/ 65 (amphotericin* or antimycin* or candicidin* or roxithromycin* or josamycin* or leucomycin* or kitasamycin* or lucensomycin* or maytansine* or mepartricin* or miocamycin*).tw. 66 (natamycin* or oleandomycin* or troleandomycin* or oligomycin* or rutamycin* or sirolimus* or tacrolimus* or tylosin* or propiolactone* or spironolactone* or venturicidin* or zearalenone* or zeranol*).tw. 67 (azithromycin* or clarithromycin* or erythromycin* or spiramycin*).tw. 68 exp Quinolones/ 69 (moxifloxacin* or quinolone* or ciprofloxacin* or clinafloxacin* or fluoroquinolone* or levofloxacin* or ofloxacin* or gatifloxacin*).tw. 70 (fleroxacin* or enoxacin* or norfloxacin* or pefloxacin* or nalidixic acid* or nedocromil* or oxolinic acid* or quinpirole* or quipazine* or saquinavir*).tw. 71 exp Sulfonamides/ 72 exp Trimethoprim/ 73 (dmso or sulfoxide* or sulphoxide* or sulfonamide* or sulphonamide* or trimethoprim* or sulfamethoxazole* or sulphamethoxazole* or co‐trimoxazole* or sulfadiazine* or sulphadiazine* or sulfametopyrazine* or sulfalene* or sulphametopyrazine* or sulphalene*).tw. 74 (sulfachlorpyridazine* or sulfadimethoxine* or sulfadoxine* or sulfaguanidine* or sulfamerazine* or sulfameter* or sulfamethazine* or sulfamethoxypyridazine* or sulphachlorpyridazine* or sulphadimethoxine* or sulphadoxine* or sulphaguanidine* or sulphamerazine* or sulphameter* or sulphamethazine* or sulphamethoxypyridazine*).tw. 75 (sulfamonomethoxine* or sulfamoxole* or sulfaphenazole* or sulfapyridine* or sulfaquinoxaline* or sulfathiazole* or sulfamethizole* or sulfisomidine* or sulfisoxazole* or sulfasalazine* or sumatriptan* or xipamide* or thioamide* or thioacetamide* or sulphamonomethoxine* or sulphamoxole* or sulphaphenazole* or sulphapyridine* or sulphaquinoxaline* or sulphathiazole* or sulphamethizole* or sulphisomidine* or sulphisoxazole* or sulphasalazine*).tw. 76 exp Tetracyclines/ 77 (tetracycline* or demeclocycline* or doxycycline* or lymecycline* or minocycline* or oxytetracycline*).tw. 78 (chlortetracycline* or methacycline* or rolitetracycline*).tw. 79 exp Chloramphenicol/ 80 (cloranfenicol* or chloramphenicol*).tw. 81 (thiamphenicol* or kloramfenikol* or levomycetin* or chlornitromycin* or chlorocid* or chloromycetin* or detreomycin* or ophthochlor* or syntomycin*).tw. 82 exp Clindamycin/ 83 (clindamycin* or dalacin c or cleocin* or chlo?lincocin*).tw. 84 exp Metronidazole/ 85 (linezolid* or trivazol* or vagilen* or clont* or danizol* or fagyl* or ginefavir* or metrogel* or metrodzhil* or satric* or trichazol* or trichopol*).tw. 86 exp Fusidic Acid/ 87 (granulocyte colony stimulating factor or gcsf or ozone).tw. 88 (fusidate* adj (sodium or silver)).tw. 89 (griseofulvin or synercid or dalfopristin or quinupristin).tw. 90 exp Daptomycin/ 91 or/64‐90 92 or/31,63,91 93 exp Foot Ulcer/ 94 exp Diabetic Foot/ 95 (diabet* adj3 ulcer*).tw. 96 (diabet* adj3 (foot or feet)).tw. 97 (diabet* adj3 wound*).tw. 98 (diabet* adj3 defect*).tw. 99 or/93‐98 100 and/92,99 101 Randomized controlled trials/ 102 Single‐Blind Method/ 103 Double‐Blind Method/ 104 Crossover Procedure/ 105 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab. 106 (doubl* adj blind*).ti,ab. 107 (singl* adj blind*).ti,ab. 108 or/101‐107 109 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 110 human/ or human cell/ 111 and/109‐110 112 109 not 111 113 108 not 112 114 and/100,113
EBSCO CINAHL Plus
S62 S48 AND S61 S61 S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 S60 TI allocat* random* or AB allocat* random* S59 MH "Quantitative Studies" S58 TI placebo* or AB placebo* S57 MH "Placebos" S56 TI random* allocat* or AB random* allocat* S55 MH "Random Assignment" S54 TI randomi?ed control* trial* or AB randomi?ed control* trial* S53 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S52 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S51 TI clinic* N1 trial* or AB clinic* N1 trial* S50 PT Clinical trial S49 MH "Clinical Trials+" S48 S39 and S47 S47 S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 S46 (MH "Foot Ulcer+") S45 (MH "Diabetic Foot") S44 TI (diabet* N3 defect*) or AB (diabet* N3 defect*) S43 TI (diabet* N3 wound*) or AB (diabet* N3 wound*) S42 TI ( diabet* N3 foot OR diabet* N3 feet ) or AB ( diabet* N3 foot OR diabet* N3 feet ) S41 AB diabet* N3 ulcer* S40 TI diabet* N3 ulcer* S39 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 S38 TI (Fusidic Acid*) or AB (Fusidic Acid*) S37 TI (Honey or plant oil* or disinfect* or antiseptic* or anti‐septic* or antibiotic* or antimicrobial* or penicillin* or Cephalosporin* or lactam* or Aminoglycoside* or Macrolide* or quinolone* or sulfonamide* or trimethoprim* or tetracycline* or cloranfenicol* or chloramphenicol* or clindamycin* or Metronidazole* or Daptomycin*) or AB (Honey or plant oil* or disinfect* or antiseptic* or anti‐septic* or antibiotic* or antimicrobial* or penicillin* or Cephalosporin* or lactam* or Aminoglycoside* or Macrolide* or quinolone* or sulfonamide* or trimethoprim* or tetracycline* or cloranfenicol* or chloramphenicol* or clindamycin* or Metronidazole* or Daptomycin*) S36 TI (Acetic Acid* or antifungal* or antiviral* or anti viral* or Bacitracin or povidone iodine* or Cetrimonium Compound* or Chlorine Compound* or Framycetin* or Mandelic Acid* or Hexachlorophene* or Triclosan* or Polymyxin* or silver* or silver sulphadiazine* or Gentian Violet* or Potassium Permanganate* or Mupirocin* or neomycin* or benzyol peroxide* or hydrogen peroxide* or chlorhexidine* or Sucrose) or AB (Acetic Acid* or antifungal* or antiviral* or anti viral* or Bacitracin or povidone iodine* or Cetrimonium Compound* or Chlorine Compound* or Framycetin* or Mandelic Acid* or Hexachlorophene* or Triclosan* or Polymyxin* or silver* or silver sulphadiazine* or Gentian Violet* or Potassium Permanganate* or Mupirocin* or neomycin* or benzyol peroxide* or hydrogen peroxide* or chlorhexidine* or Sucrose) S35 (MM "Daptomycin") S34 (MM "Fusidic Acid") S33 (MM "Metronidazole") S32 (MM "Clindamycin") S31 (MM "Chloramphenicol") S30 (MH "Tetracyclines+") S29 (MH "Trimethoprim+") S28 (MH "Sulfonamides+") S27 (MH "Quinolines+") S26 (MH "Antibiotics, Macrolide+") S25 (MH "Aminoglycosides+") S24 (MH "Antibiotics, Lactam+") S23 (MH "Cephalosporins+") S22 (MH "Penicillins+") S21 (MH "Antibiotics+") S20 (MH "Antiinfective Agents, Local+") S19 (MM "Disinfectants") S18 (MH "Plant Oils+") S17 (MM "Honey") S16 (MH "Sucrose+") S15 (MM "Chlorhexidine") S14 (MH "Hydrogen Peroxide") S13 (MM "Neomycin") S12 (MM "Mupirocin") S11 (MM "Gentian Violet") S10 (MM "Silver") OR (MM "Silver Sulfadiazine") S9 (MH "Polymyxins+") S8 (MM "Triclosan") S7 (MM "Hexachlorophene") S6 (MH "Chlorine Compounds+") S5 (MM "Povidone‐Iodine") S4 (MM "Bacitracin") S3 (MH "Antiviral Agents+") S2 (MH "Antifungal Agents+") S1 (MH "Acetic Acid") OR (MH "Acetic Acids+")
Appendix 2. Cochrane tool for assessing risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process is provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information is provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definitive judgement, for example if the use of assignment envelopes is described, but it is unclear whether envelopes were sequentially numbered, opaque, and sealed.
3. Blinding: was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded, and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following:
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following:
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
No missing outcome data.
Reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on the observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
Reasons for missing outcome data are likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce a clinically relevant bias in the observed effect size.
'As‐treated' analysis done with a substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes is/are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes was/were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review is/are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information is provided to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Topical antimicrobial dressing compared with non‐antimicrobial dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds healed | 5 | 945 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.12, 1.45] |
| 1.1 Short term follow up | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.6 [1.00, 2.57] |
| 1.2 Medium term follow‐up | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.10, 1.44] |
| 2 Incidence of infection: medium term follow‐up | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.10] |
| 3 Surgical resection: medium term follow‐up | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.72] |
| 4 Adverse events | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.48] |
Comparison 2. Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds healed: medium term follow‐up | 3 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.56, 14.23] |
| 2 Resolution of infection: medium term follow‐up | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.54, 2.51] |
| 3 Surgical resection: medium term follow‐up | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.47, 5.90] |
Comparison 3. One topical antimicrobial agent compared with an alternative topical antimicrobial agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds healed | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Medium term follow‐up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Unknown follow‐up period | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Resolution of infection: medium term follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Surgical resection: medium term follow‐up | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.53, 7.03] |
Comparison 4. Topical antimicrobial agent compared with systemic antimicrobial agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resolution of infection | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.91, 2.49] |
| 1.1 Short‐term follow‐up | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.69, 3.45] |
| 1.2 Medium term follow‐up | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.79, 2.82] |
| 2 Surgical resection: medium term follow‐up | 1 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.51, 2.91] |
| 3 Adverse events | 4 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.05] |
| 3.1 Short‐term follow‐up | 3 | 891 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.05] |
| 3.2 Medium term follow‐up | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.49, 2.40] |
Comparison 5. Topical antimicrobial agent compared with growth factor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of wounds healed: Medium term follow‐up | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.28, 0.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2014.
| Methods | RCT Setting: Hospital, 1 centre Country: Pakistan Duration of follow‐up: 8 weeks Duration of treatment: Not noted Funding source: Not reported Unit of analysis: Participant |
|
| Participants | 60 participants Inclusion criteria: Grade I or II foot ulcer in person with diabetes (grading assessed using Meggitt‐Wagner scale and corresponds to absence of necrosis and osteomyelitis) of more than 4 weeks' duration. Adequate controlled diabetes with fasting blood sugar of 110 to 130 mg/dL on 2 consecutive days prior to recruitment in the study. Exclusion criteria: Patients with a history of hepatic or renal disease, those on corticosteroid therapy, and those with impalpable dorsalis pedis or posterior tibial arteries. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported) Group 1: All Wagner grade II ulcers; ulcer area 1107.53 SD: 486.5 Group 2: All Wagner grade II ulcers: ulcer area 1310.10 SD: 489.2 Infection status at baseline: Not reported |
|
| Interventions | Group 1: (n = 30) Pyodine bath and saline and Vaseline gauze dressing Group 2: (n = 30) Phenytoin powder (from capsules, no information on concentration) applied in a thin, uniform layer plus pyodine bath and saline/Vaseline gauze dressing as for Group 1. The amount of powder depended on ulcer area. Additional treatment information: Dressings were changed daily or on alternate days depending on need. |
|
| Outcomes | Primary review outcomes: None reported Secondary review outcomes: None reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study patients were divided in two equal groups randomly by lottery method" Comment: Whilst limited information is presented, we assumed that the a lottery approach refers to a random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "No information was provided on who conducted randomisation and if or how allocation was concealed" Comment: No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Comment: No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: Flow chart reports 0 lost to follow‐up in either group. Comment: Assumed all participants followed up |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained, all outcomes stated in methods reported. However, key outcomes not presented; unclear if these were measured. |
| Other bias | Low risk | None noted. |
Apelqvist 1996.
| Methods | RCT Setting: Hospital, 1 centre Country: Sweden Duration of follow‐up: 12 weeks Duration of treatment: Not reported Funding source: For‐profit organisation Unit of analysis: Participant |
|
| Participants | 41 participants Inclusion criteria: Caucasian > 40 years of age with previously known diabetes, an exudating cavity ulcer below the ankle (Wagner grade I or II) with an ulcer area > 1 cm² and systolic toe pressure > 30 mmHg or a systolic ankle pressure > 80 mmHg. Exclusion criteria: Patients with ulcers > 25 cm², deep abscess, osteomyelitis, or gangrene (Wagner grade III to IV). Patients undergoing investigation of the thyroid gland or unlikely to adhere to study protocol. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not reported |
|
| Interventions | Group 1: (n = 19) Gentamicin solution (Garamycin, Schering‐Plough); streptodornase/streptokinase (Varidase, Lederle); dry saline gauze. Group 2: (n = 22) Cadexomer iodine ointment (Iodosorb) changed once daily during the first week and daily or every second or third day in subsequent weeks. Additional comments: All participants were offered the same basic treatment during the study. Prior to inclusion footwear was corrected or special footwear provided whenever required to relieve local pressure. Oral antibiotics used in signs of infection. If the ulcer was infected, gentamicin solution (80 mg/mL) was prescribed twice daily, streptodornase/streptokinase was used for necrotic lesions. Dry saline gauze used as an absorptive dressing with Vaseline gauze used on dry wounds. |
|
| Outcomes | Primary review outcomes: Proportion of ulcers healed. Secondary review outcomes: Surgical resection; adverse events. |
|
| Notes | Stratifìcation was based on size and type of ulcer (Wagner grade I to II). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated list of randomly permuted blocks of patients, the size of the blocks was unknown to the investigator." Comment: Adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: No mention of allocation concealment process |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" Comment: Assumed staff and participants not blinded to treatment |
| Blinding of outcome assessment (detection bias) Wound healing | Low risk | Quote: "... with blinded photo evaluation ..." Comment: Blinded outcome assessment for healing |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Low risk | Quote: "... with blinded photo evaluation ..." Comment: Blinded outcome assessment for healing |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Comment: No information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Patients were withdrawn from the study in case [sic] of hospitalisation (n = 2), lack of compliance (n = 1), violation of inclusion criteria (n = 2)" Comment: Data presented for 35 participants, suggesting 6 dropped out (study started with 41 participants), for a loss of 15%. |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | None noted. |
Bergqvist 2016.
| Methods | Open‐label RCT Setting: Hospital, 4 centres Country: Gothenburg, Sweden Duration of follow‐up: Up to 24 weeks Duration of treatment: 12 weeks Funding source: Vinnova and RLS Global AB co funded the study Unit of analysis: Participant |
|
| Participants | 41 participants Inclusion criteria: Type 1 and 2 diabetes, age 18 years or older (67.5 ± 11.8 years in chloramine group; 74.5 ± 12.3 years in control group) and an infected foot for more than 4 weeks. Exclusion criteria: Patients with end‐stage renal disease, impaired blood circulation, or in need of vascular intervention, or a vascular intervention performed less than 3 months before the study, a history of kidney or pancreas transplant, treatment with cortisone > 60 mg daily, chemotherapy or any immune‐modulating agents during the past year, identified conditions, in the ulcer area (e.g. cancer), or generally poor health of the participant and at risk of requirement of hospitalisation. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Infected |
|
| Interventions | Group 1: (n = 19) Standard care alone. Ulcer was cleaned and debrided according to the guidelines of the International Working Group on the Diabetic Foot once weekly. The ulcer was dressed with foam, hydrocolloid, or alginate dressing. In a few cases an adjusted antiseptic agent, silver or polyhexamethylene biguanide, was used. Group 2: (n = 21) Chloramine plus standard care. Trialist applied a preparation containing sodium hypochlorite and amino acid, which are converted to chloramine by mixing the 2 components immediately prior to treatment. The gel was applied to the ulcer once a week. Debridement was done with the gel left on the ulcer surface to provide antibacterial protection for the exposed tissue. Additional comments: Nurses and podiatrist performed cleansing and debridement of the ulcer in both groups at least once weekly for 12 weeks. All participants were given standard care advice on the treatment of diabetes and risk factors. Oral antibiotic treatment was offered if signs of significant infection were observed, particularly affecting underlying tissues or bones. Appropriate off‐loading was considered in all participants. |
|
| Outcomes | Primary review outcomes: Proportion of ulcers healed; time‐to‐event data (partially reported); resolution of signs of infection Secondary review outcomes: Surgical resection; adverse events |
|
| Notes | The original study performed both ITT and PP analyses for efficacy outcomes, but did not state which analysis was used in the report, hence we assumed the numbers reported were completers only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised in blocks of 4 ..." Comment: Block randomisation is implied. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "in an explorative open randomised controlled multi‐centre study" Comment: No mention of allocation concealment process |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "open‐label" Comment: Assumed staff and participants not blinded to treatment |
| Blinding of outcome assessment (detection bias) Wound healing | Low risk | Quote: "a photo was taken every week after treatment ... the area of ulcer was subsequently measured by an independent observer" Comment: Adequate blinding |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Low risk | Quote: "a photo was taken every week after treatment ... the area of ulcer was subsequently measured by an independent observer" Comment: Adequate blinding |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Comment: It is unclear how the adverse events were assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Chloramine group: Violation of protocol (n = 1 had percutaneous angioplasty; n = 1 was accidentally included in 2 centres; n = 1 lower toe blood pressure < 30 mmHg), 2 ulcer coalesced and unable to assess (n = 1). Control group: lost to follow‐up (n = 1), withdrew informed consent (n = 1) Comment: The dropout rate did not differ significantly between groups; although more people dropped out of the intervention group, the reasons for dropout were mostly not related to the treatment. |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | |
Bowling 2011.
| Methods | RCT; prospective, 2‐centre, randomised, controlled, double‐blind, pilot study Setting: Hospital and community, 2 centres Country: UK Duration of follow‐up: 4 weeks Duration of treatment: Weekly treatment for 4 weeks Funding source: For‐profit organisation Unit of analysis: Participant |
|
| Participants | 20 participants Inclusion criteria: Chronic (4 weeks’ duration), non‐clinically infected foot ulcers (colonised) where necrotic tissue was present and mechanical debridement was indicated. A foot ulcer was defined as a full‐thickness break of the epithelium distal to the medial and lateral malleoli. Only 1 ulcer per participant was included. Exclusion criteria: Ulcers larger than 25 cm², ulcers defined as grade III in the University of Texas classification, osteomyelitis, peripheral arterial disease (absent pulses/ankle‐brachial index < 0.8), prescription use of anticoagulants, immunosuppressive drug treatment, or known allergies to chlorine (present in Dermacyn). Clinically infected wounds were excluded on the grounds of antibiotic use. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Group 1: Ulcer duration (weeks) 13.7 SD: 12.0 Group 2: Ulcer duration (weeks) 9.7 SD: 8.1 Infection status at baseline: Not infected |
|
| Interventions | Group 1 (n = 10): Saline solution Group 2 (n = 10): Super‐oxidised aqueous solution Additional comments: Both solutions used with the Versajet lavage system for removing necrotic tissue. Each solution was used with the Versajet lavage system in a clinic treatment room, followed by a wound rinse and a 10‐minute soak with the respective solution. After the soaking, all of the wounds were dressed with a hydrogel dressing that was changed at regular intervals of 3 to 4 days, as specified by the treating physician. Saline or super‐oxidised aqueous solution was applied at every dressing change. |
|
| Outcomes | Primary review outcomes: Proportion of wounds healed (partially reported) Secondary review outcomes: Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Ten subjects were randomised to each group using a computer‐generated block randomization scheme" Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Both medical centers were provided with sealed randomization envelopes for conducting the treatment assignment" Comment: It is unclear if the envelopes were opaque, but we assume it is a reporting issue. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This was a prospective, two‐center, randomised, controlled, double‐blind, pilot study." Comment: No further information was provided on who was blinded or how the blinding was achieved. |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Comment: No information was provided. |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Comment: No information was provided. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Comment: No information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not provided |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | None noted. |
Gottrup 2013.
| Methods | RCT Setting: Hospital, 2 centres Country: Denmark Duration of follow‐up: 14 weeks Duration of treatment: Not reported Funding source: For‐profit Unit of analysis: Participant |
|
| Participants | 39 participants Inclusion criteria: Diabetic patients aged 35 to 80 years with an ulcer of at least 30 days' duration. Ulcer defined as diabetic foot ulcer, Wagner grade II to III. No local or systemic signs of infection with normal leukocyte levels. Patient willing to return to centre for dressing changes and wound evaluation. Exclusion criteria: Known allergies to any of the contents of PROMOGRAN PRISMA (collagen, oxidised regenerated cellulose, or silver oxidised regenerated cellulose); clinical signs of infection; pregnancy or lactating; history of drug misuse or excessive alcohol consumption; currently undergoing chemotherapy; wound is considered to be malignant; peripheral arterial disease or toe pressure 45 mmHg, or both; patient is unable to walk; patient had haemolytic anaemia and/or iron deficiency anaemia and/or malnutrition, severe cardiac and/or hepatic and/or renal and/or pulmonary insufficiency or chronic administration of cortisone for chronic inflammatory disease and/or autoimmune disease. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Group 1: Ulcer duration (months) 16.9 SD: 36.6; wound area (cm²) 4.4 SD: 6.3 Group 2: Ulcer duration (months) 12.9 SD: 13.0; wound area (cm²) 2.1 SD: 3.1 Infection status at baseline: Not infected |
|
| Interventions | Group 1: (n = 15) Foam dressing (Biatain, Coloplast, Humlebæk, Denmark) for moderately exuding wounds and a more absorbent dressing (Mesorb, Mölnlycke Health Care, Gothenburg, Sweden) for highly secreting wounds. Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing (Promogran Prisma, Systagenix Wound Management Ltd., Gatwick, UK). Applied directly to wound. Where there was a low level of wound exudates, the dressing was pre‐wet before applying to the wound. The study protocol suggests that the control dressings were also used in the intervention group, but timing was unclear. Additional comments: The same type of dressings were used in the test and control group and consisted of a foam dressing (Biatain, Coloplast, Humlebæk, Denmark) for moderately exuding wounds and a more absorbent dressing (Mesorb, Mölnlycke Health Care, Gothenburg, Sweden) for highly secreting wounds. The dressings were changed at least twice a week according to the condition of the wound. Patients in both groups were treated with standard wound treatment protocol including debridement and off‐loading, based on specialist clinical evaluation. |
|
| Outcomes | Primary review outcomes: Proportion of wounds healed; wound infection (defined by a clinical specialist evaluation based on the classical infection signs, with no microbiological assessment) Secondary review outcomes: Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed independently of the research team using random number tables and group assignment was kept in sealed envelopes until the end of the study" Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed independently of the research team using random number tables and group assignment was kept in sealed envelopes until the end of the study" Comment: Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No mention of blinding |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Comment: No mention of blinding |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Comment: No mention of blinding |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Comment: No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Figure shows loss of 3 participants at 14 weeks' follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | None noted. |
He 2016.
| Methods | RCT Setting: Outpatients and inpatients admitted to the Department of Burn and Plastic Surgery of Dazhou Central Hospital Country: China Duration of follow‐up: 4 weeks Duration of treatment: 4 weeks Funding source: Not reported Unit of analysis: Not reported |
|
| Participants | Inclusion criteria: Patients with foot ulcers with over 2 years' history of diabetes and glycated haemoglobin > 6.5%. Exclusion criteria: Patients with severe heart or lung disease, high blood pressure, severe mental illness, required immediate amputation, malnutrition, severe sinusitis, detachment of retina, diabetic ketosis in the last 2 weeks, diabetic ketoacidosis, severe infection, and other patients at high risk of being non‐compliant. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Group 1: Ulcer area 12.34 SD: 3.42 (cm²) Group 2: Ulcer area 11.85 SD: 2.91 (cm²) Infection status at baseline: Not reported |
|
| Interventions | Group 1: (n = 40) Routine debridement plus standard care (including blood glucose control, nutritional support, improvement in microcirculation). Group 2: (n = 40) Silver ion dressing plus standard care (including blood glucose control, nutritional support, improve microcirculation). Additional information: Dressing was changed daily in Group 1; dressing was changed daily and silver ion was refreshed once a week in Group 2. |
|
| Outcomes | Primary review outcomes: Proportion of ulcers healed; time to healing (partially reported) Secondary review outcomes: None reported |
|
| Notes | English abstract; text translated from Chinese. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random numbers were generated with computer programme and managed by an assigned team member" Comment: Adequate method of generating random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "researchers, clinicians and patients do not know the allocation sequence before the trial" Comment: Although we do not know how the trialists concealed the allocation plan, we accept their statement quoted above as true. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "prospective, open, randomised controlled clinical trial" Comment: Open trial with no blinding |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Comment: Not stated |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: None obvious |
| Other bias | Low risk | Comment: None obvious |
Hwang 2010.
| Methods | RCT Setting: Not reported Country: Not reported Duration of follow‐up: Not reported Duration of treatment: Not reported Funding source: Not reported Unit of analysis: Not reported |
|
| Participants | Inclusion criteria: Patients with foot ulcers with bone and tendon exposure and diabetes. Exclusion criteria: None noted. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not reported |
|
| Interventions | Group 1: (n = not reported) Iodine gauze (in the control group, iodine gauze dressings were applied at the time of skin graft and changed 3 times a day thereafter). Group 2: (n = not reported) Hydrofiber dressing with silver (changed every 24 hours). Additional comments: All foot ulcers were surgically debrided prior to initiation of the Hydrofiber dressing with silver or gauze treatment. |
|
| Outcomes | Primary review outcomes: None reported. Secondary review outcomes: None reported. |
|
| Notes | Conference abstract; no outcome data clearly reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty patients were randomised into either the experimental hydrofibre dressing with silver* group or control iodine gauze group" Comment: Insufficient information to make a low‐risk assessment |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No information available |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Comment: No information available |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No information available |
| Selective reporting (reporting bias) | Unclear risk | Comment: No information available |
| Other bias | Unclear risk | Comment: No information available |
Imran 2015.
| Methods | RCT Setting: Department of General Surgery, Pakistan and Bhatti International Trust (BIT) Hospital Country: Pakistan Duration of follow‐up: 17 weeks Duration of treatment: Not reported Funding source: Not reported Unit of analysis: Not reported |
|
| Participants | Inclusion criteria: All patients > 18 years of age with diabetic foot ulcer (Wagner grade I or II) were selected. Exclusion criteria: Patients with Wagner grade III to V, ankle‐brachial pressure index < 7, venous ulcer or malignant ulcer, uncontrolled diabetes (glycated haemoglobin > 7%), patients with > 1 ulcers, patients with haemoglobin < 10 g/dL, and patients with local signs of infection (presence of pus, initial culture positive) in the wound were excluded from the study. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not reported |
|
| Interventions | Group 1: (n = 180) Treated with normal saline dressing. Group 2: (n = 195) Treated with honey dressing. |
|
| Outcomes | Primary review outcomes: Proportion of ulcers healed; time to wound healing (partially reported) Secondary review outcomes: Not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "grouping was done by simple randomization method (computer‐generated random numbers)" Comment: Adequate method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not reported |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Comment: Not reported |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Comment: Not reported |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Comment: Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 16 people dropped out of honey dressing group, and 11 dropped out of saline group. Although not included in the final analysis, the proportion of dropout was balanced between groups and did not compromise the statistical power to detect any potential difference between groups. |
| Selective reporting (reporting bias) | Low risk | Comment: None obvious |
| Other bias | Low risk | Comment: None noted. |
Jacobs 2008.
| Methods | RCT Setting: Office of study author (no further details) Country: Not reported Duration of follow‐up: 6 weeks Duration of treatment: 6 weeks Funding source: Not reported Unit of analysis: Participant |
|
| Participants | 40 participants Inclusion criteria: Wagner grade I or II ulcerations of the foot. Study authors note that all patients included in the study presented with ulcers that were 3 centimetres in diameter or less on the plantar aspect of the foot (unclear if inclusion criteria or not). Currently under care for diabetes. Exclusion criteria: Glycated haemoglobin greater than 10%. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not clearly reported Infection status at baseline: Not reported |
|
| Interventions | Group 1: (n = 20) Silver sulphadiazine cream (no further details). Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulfadiazine cream. Additional comments: All participants were treated by off‐loading of weight bearing and shoe pressure from the area of ulceration. Debridement with a scalpel was performed as determined for each participant. |
|
| Outcomes | Primary review outcomes: Proportion of ulcers healed (referred to as "resolved" by study authors) Secondary review outcomes: None reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A research coordinator randomly assigned patients to receive ..." Comment: No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: As above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This was a blinded study" Comment: No further details provided. |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Comment: As above |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: It seems from report that all participants were followed up, as results table contains data for all 40 randomised participants. |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | Comment: None noted. |
Jeffcoate 2009.
| Methods | RCT Setting: Hospital, 9 centres Country: UK Duration of follow‐up: 24 weeks Duration of treatment: 24 weeks or until healing Funding source: Not‐for‐profit Unit of analysis: Participant |
|
| Participants | 317 participants Inclusion criteria: Type 1 or 2 diabetes; 18 years of age or older; a foot ulcer present for at least 6 weeks with a cross‐sectional area of between 25 and 2500 mm²; able and willing to give informed consent; reasonably accessible by car to the hospital base; under routine review by the multidisciplinary clinic. Exclusion criteria: Those with a known allergy to any of the trial preparations (including iodine); any ulcer on either foot extending to tendon, periosteum, or bone, infection of bone, soft‐tissue infection requiring treatment with systemic antibiotics; an ulcer on a limb being considered for revascularisation; those chosen for management with a non‐removable cast without a dressing window; gangrene on the affected foot; eschar that was not removable by clinical debridement; those with evidence of a sinus or deep track; those in whom the hallux had been amputated on the affected side (preventing the measurement of toe pressure); those with an ankle‐brachial pressure index of less than 0.7 or toe systolic pressure less than 30 mmHg; ulceration judged to be caused primarily by disease other than diabetes; patients with any other serious disease likely to compromise the outcome of the trial; patients with critical renal disease (creatinine greater than 300 mmol/L); those receiving immunosuppressants, systemic corticosteroid therapy (other than by inhalation), or any other preparation that, in the opinion of the supervising clinician, could have interfered with wound healing; those living at such a distance (generally further than 10 miles) from the clinic as would have made frequent assessment visits inappropriately expensive or impractical, or both; those who withheld consent. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not clear |
|
| Interventions | Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze (Johnson & Johnson) Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing (Aquacel, ConvaTec) Group 3: (n = 106) Iodine‐containing dressing (Inadine, Systagenix) Additional comments: Dressings were changed daily, on alternate days or 3 times a week according to need or availability of professional staff, or both. Participants were advised to have a bath or shower as often as they wished, provided the ulcer could be redressed afterwards, and provided the ulcerated foot was not immersed in water for more than 5 minutes. |
|
| Outcomes | Primary review outcomes: Proportion of ulcers healed Secondary review outcomes: Health‐related quality of life (Cardiff Wound Impact Schedule and SF‐36); amputations (minor and major); adverse events (serious and non‐serious) | |
| Notes | Randomisation was stratified by both centre and size, using a block size of 9. Randomisation was stratified across the whole population by ulcer area into 3 groups: 25 to 100 mm², 101 to 250 mm², and 251 to 2500 mm². | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation lists were created using SPSS (SPSS Inc., Version 14), using blinded dressing codes." Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "The lists were held at Cardiff University and each recruiting centre telephoned a designated number during working hours. They were required to identify the centre and size of wound only." Comment: Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: The study was not blinded to personnel and participants. |
| Blinding of outcome assessment (detection bias) Wound healing | Low risk | Quote: "Dressings were removed prior to examination by assessors who were not involved in the conduct of the trial and who were blind to the randomisation group." |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Not clear if infection assessment was blinded |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Not clear if adverse event data collection was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Intention to treat analysis was carried out using the last value carried forward method, with strict adherence to the protocol such that only those who attended for a healing verification visit and reported as still healed at 28 days have been coded as ‘healed’ for the outcome classification." Comment: ITT analysis was done, but imputing missing data attributable to withdrawal of trial participants due to adverse events and protocol violations. |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | Comment: None noted. |
Jude 2007.
| Methods | RCT Setting: 18 centres ‐ settings not clear Country: UK, France, Germany, and Sweden Duration of follow‐up: 8 weeks Duration of treatment: 8 weeks or until healed Funding source: For‐profit Unit of analysis: Participant |
|
| Participants | 134 participants.
Inclusion criteria: Patients with Type 1 or Type 2 diabetes mellitus (glycated haemoglobin ≤ 12%); serum creatinine ≤ 200 mol/L; neuropathic or neuro‐ischaemic diabetic foot ulcers classed as Wagner grade I or II; all wounds > 1 cm² in area.
Exclusion criteria: Patients with known allergies to dressings being investigated; known or suspected malignancy near ulcer; taking systemic antibiotics > 7 days prior to enrolment; inadequate arterial perfusion defined by ankle‐brachial index < 0.8, or great toe systolic blood pressure < 40 mmHg or forefoot transcutaneous oxygen < 30 mmHg (participant supine) or < 40 mmHg (participant sitting). Ulcer characteristics at baseline (e.g. anatomic site, size, number of ulcers, presence of infection, duration of ulceration where reported): Group 1: Ulcer duration (years) 1.4 (SD 2.6); ulcer area (cm²) 4.2 (SD 7.8) Group 2: Ulcer duration (years) 1.2 (SD 2.1); ulcer area (cm²) 4.2 (SD 4.1) Infection status at baseline: Mixed: 22 participants had clinically infected ulcers at baseline, 13 in Group A and 9 in Group B. On enrolment antibiotics were prescribed to 8 participants in Group A and 13 in Group B. |
|
| Interventions | Group 1: (n = 67) Calcium‐alginate dressing (Algosteril, Smith & Nephew). Manufacturer's instructions were followed, and dressing was moistened before use on dry wounds, and changed on leakage or at evaluation or every 7 days as indicated (except for infected wounds, for which the dressing was changed daily). Group 2 (n = 67): Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver (Aquacel Ag, ConvaTec). Left in place and changed on leakage or at evaluation or every 7 days as indicated. In both groups, ulcers were cleansed using sterile saline; each dressing was covered with a sterile, non‐adherent foam dressing. Additional comments: Accommodative footwear for non‐plantar ulcers and off‐loading for plantar ulcers delivered as required. |
|
| Outcomes | Primary review outcomes: Proportion of ulcers healed (number of ulcers healed); time to healing (only partially reported) Secondary review outcomes: Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible individuals were randomly assigned to receive one of the two dressings according to instructions in a sealed envelope and stratified according to whether or not systemic antibiotics were being administered for treatment of the studied ulcer" Comment: No detailed information provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: See above Comment: It is unclear how allocation was conducted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" Comment: The study was not blinded to personnel and participants. |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | No information is provided. |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | No information is provided. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | No information is provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 65 out of the 67 participants in each study group were rated for wound condition at final evaluation. All included participants were evaluated for safety. |
| Selective reporting (reporting bias) | Low risk | Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | None noted. |
Khandelwal 2013.
| Methods | RCT Setting: Patients were managed initially on inpatient and then on outpatient basis Country: India Duration of follow‐up: Until healing > 8 weeks Duration of treatment: 10 weeks Funding source: Unclear: the study notes that "financial support was provided by dr. Ram Manohar Lohia Hospital, New Dehli" Unit of analysis: Participant |
|
| Participants | 60 participants Inclusion criteria: Diabetic foot ulcer of at least 8 weeks' duration ‐ stage III and IV, absence of vascular insufficiency involving large‐ and medium‐sized arteries proximal to the ulcer demonstrated by Doppler study, age ≥ 18 years with Type 1 or 2 diabetes. Exclusion criteria: Patients with uncontrolled diabetes, foot ulcer with established gangrene, compromised vascularity of the particular limb, associated osteomyelitis at site of ulcers, pregnant and lactating females, neoplasm at the local site, patients on any immunosuppressive agents, presence of multiple ulcers, patient HIV seropositive, patients with known drug allergy, presence of concomitant life‐threatening infections, chronic renal insufficiency (serum creatinine > 3 mg/dL), when ear cannot equalise the pressure when congested with cold/hay fever, patients with perforation of ear drum. High‐risk case, i.e. bronchial asthma/emphysema Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not reported |
|
| Interventions | Group 1: (n = 20) Hyperbaric oxygen therapy. Therapy delivered at 2.5 atmospheres absolute for 60 min per sitting for a total of 30 sittings or until the ulcer had healed. Sittings were distributed over a period of 10 weeks. Patients were given either daily or alternate‐day therapy depending on the availability of slot in the facility. The patients in this group were also debrided from time to time but dressed only with normal saline. No antiseptics were used (group not considered further in review). Group 2: (n = 20) Recombinant human platelet‐derived growth factor. The patients in this group were initially debrided surgically and subsequently as well when required. Group 3: (n = 20) Antiseptic treatments (Edinburgh University Solution of Lime (EUSOL), hydrogen peroxide, and povidone iodine). The foot was soaked in EUSOL for 30 min, followed by use of hydrogen peroxide and povidone iodine (no details about concentration). |
|
| Outcomes | Primary review outcome: Proportion of ulcers healed; time to healing (partially reported) Secondary outcomes: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomisation methods not specified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information is provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study Comment: Blinding unfeasible due to the differences in setting and formulation of the interventions. |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Comment: No information is provided. |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "6 patients in group 1 (30%), 5 in group 2 (25%) and 1 (5%) in group 3 lost to follow up" Comment: Systematic differences in withdrawal from the study among groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: The outcomes reported in the results are not the same as specified in the Material and Methods section. |
| Other bias | Low risk | Comment: None noted. |
Landsman 2011.
| Methods | RCT Setting: 16 centres, but outpatient or inpatient setting is not specified Country: USA Duration of follow‐up: 4 weeks Duration of treatment: 10 days Funding source: For‐profit funding; "This project was supported by a research grant from Oculus Innovative Sciences." Unit of analysis: Participant |
|
| Participants | 67 participants Inclusion criteria: > 18 years of age with diabetes mellitus (Type 1 or 2) and a mild diabetic foot infection. Eligible foot ulcers involved skin and deeper soft tissue and were classified by Infectious Diseases Society of America guidelines as mildly infected and by the University of Texas Classification as 1B. Ulcers could be located on the foot and malleolar areas, measured 1 to 9 cm², and were accessible for culture. Adequate circulation to the foot was required. Exclusion criteria: Antibiotic treatment for more than 24 hours within 72 hours of study entry; necrotising fasciitis, deep abscesses in the soft tissue, sinus tracts, gas gangrene, or infected burns, superinfected eczema or other chronic medical conditions; ulcers located on the stump of an amputated extremity; ulcers having a non‐diabetic aetiology; infections complicated by the presence of prosthetic materials and osteomyelitis; pregnancy or risk of pregnancy, breastfeeding; liver disease; neutropenia; hypersensitivity to chlorine or quinolones; patients receiving glucocorticoid or adjuvant therapy with hyperbaric oxygen or topical formulations containing growth factors, antimicrobials, enzymatic debriders, or granulation promoters; disorders of immune function and any medical condition that, in the investigator’s opinion, would require dose modification of levofloxacin to less than 750 mg/d or who had received an investigational agent within 1 month before the baseline evaluation. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Infected ulcers |
|
| Interventions | Group 1: (n = 21) Topical saline solution plus 750 mg levofloxacin once per day. Group 2: (n = 21) Topical Microcyn therapy once per day (not considered in review). Group 3 (n = 25) Topical Microcyn therapy plus 750 mg levofloxacin once per day. Additional comments: Wound cleaning and coverage was performed once a day with 30 mL of either Microcyn Rx or saline. Sterile gauze was saturated with approximately 25 mL of Microcyn Rx or saline, and the excess solution was wrung out. Working from the inside out, the wound was scrubbed gently to remove drainage and exudates. Once the wound bed was prepared, another sterile gauze pad was saturated with an additional 5 mL of Microcyn Rx or saline. Enough of the soaked gauze was applied to fill, but not tightly pack, the wound. The wound was covered with an occlusive dressing after each dressing change. Where necessary, off‐loading was achieved with fixed ankle boots or healing sandals, as indicated by the investigator. Debridement procedures were limited to 3 for the duration of the study. |
|
| Outcomes | Primary review outcomes: Resolution of infection (defined in the paper as "cure" ‐ resolution of all signs and symptoms, including the presence of culturable exudates, warmth, erythema, induration, tenderness, pain, swelling, and a healing wound (as determined by the investigator) after 5 or more days of treatment). Secondary review outcomes: Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was accomplished at each study site by using a manual system and stratified by site". "Envelopes containing group designations opened sequentially" Comment: It is not clear how the randomisation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Envelopes containing group designations opened sequentially" Comment: It is unclear if the allocation was foreseeable with this method; numbering envelopes would have added extra rigour, and it is not clear if this was done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label Comment: Blinding unfeasible due to the differences in setting and formulation of the interventions. |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Comment: No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: The study was conducted on an ITT basis with missing = failure; 66 out of 67 participants randomised were evaluated. |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not obtained, all outcomes stated in methods reported. |
| Other bias | Low risk | Comment: None noted. |
Lipsky 2008a.
| Methods | RCT Setting: Predominantly outpatients (with some inpatients) (from study author) Country: USA Duration of follow‐up: 28 to 42 days Duration of treatment: 14 to 28 days Funding source: For‐profit; "Magainin Pharmaceuticals sponsored the studies" Unit of analysis: Participant |
|
| Participants | 493 participants Inclusion criteria: Men or women aged > 18 years with diabetes mellitus and an infected wound below the malleoli that exceeded 0.5 cm² in area after appropriate debridement. Wounds had to be full‐thickness (i.e. through the epidermis and into or through the dermis, but not involving tendon, bone, or joint capsule). Exclusion criteria: Patients were excluded if they had: an abscess; extensive gangrene; an imminently limb‐threatening infection; evidence of systemic infection (e.g. fever, chills, or hypotension); plain radiograph findings suggestive of osteomyelitis; no palpable dorsalis pedis or posterior tibial pulse or a pedal systolic pressure (by Doppler) of ≤ 40 mmHg on the affected limb; requirement for renal dialysis; need for immunosuppressive medication; or hypersensitivity to either study medication. Infection was defined by the presence of purulent drainage or ≥ 2 of the following: erythema, warmth, pain or tenderness, or oedema or induration. The diabetic foot infection had to be severe enough to require antibiotic therapy, but it had to be amenable to outpatient treatment. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Group 1: Ulcer area (mm²) 131.5 (no SD reported) Group 2: Ulcer area (mm²) 117.3 (no SD reported) Infection status at baseline: Infected ulcers |
|
| Interventions | Group 1: (n = 246) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream. Additional comments: Investigators performed appropriate local wound care, including any necessary debridement and pressure off‐loading of the infected site, and they obtained wound tissue specimens for aerobic and anaerobic culture at enrolment, and at follow‐up, when material was available. Group 2: (n = 247) Topical pexiganan cream (1% or 2%) and placebo oral tablets. Each treatment administered twice daily. |
|
| Outcomes | Primary outcomes: None reported in useable format Secondary outcomes: Surgical resection; adverse events |
|
| Notes | Some information about study methods was available from corresponding author. Classed as study 303 in paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Computer generated random sequence" (from study author) Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "After completion of required screening procedures eligible patients received a sequentially assigned randomization number. Each investigational centre received a unique set of randomization numbers." (from study author) Comment: Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind. Patients were instructed to take 2 tablets (either 200 mg of active ofloxacin orally twice daily and to apply a cream (either active pexiganan acetate or placebo, sufficient to form a dime thick layer) twice daily directly onto the ulcer and to dress the wound with sterile, dry gauze. Patients randomised to treatment with pexiganan received placebo tablets, and those randomised to ofloxacin treatment received placebo cream" Comment: Blinded |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Figure in paper shows that all participants had clinical data analysed in ITT. There were more missing data for microbial analysis, but we did not consider these in the review. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Healing data did not seem to be reported. |
| Other bias | Low risk | Comment: None noted. |
Lipsky 2008b.
| Methods | RCT Setting: Predominantly outpatients (with some inpatients) (from study author) Country: USA Duration of follow‐up: 28 to 42 days Duration of treatment: 14 to 28 days Funding source: For‐profit Unit of analysis: Participant |
|
| Participants | 342 participants Inclusion criteria: Men or women aged > 18 years with diabetes mellitus and an infected wound below the malleoli that exceeded 0.5 cm² in area after appropriate debridement. Wounds had to be full‐thickness (i.e. through the epidermis and into or through the dermis, but not involving tendon, bone, or joint capsule). Exclusion criteria: Patients were excluded if they had an abscess, extensive gangrene, an imminently limb‐threatening infection, evidence of systemic infection (e.g. fever, chills, or hypotension), plain radiograph findings suggestive of osteomyelitis, no palpable dorsalis pedis or posterior tibial pulse or a pedal systolic pressure (by Doppler) of ≤ 40 mmHg on the affected limb, requirement for renal dialysis, need for immunosuppressive medication, or hypersensitivity to either study medication. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Infected ulcers |
|
| Interventions | Group 1: (n = 171) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream. Group 2: (n = 171) Topical pexiganan cream (1%) and placebo oral tablets. Additional comments: Investigators performed appropriate local wound care, including any necessary debridement and pressure off‐loading of the infected site, and they obtained wound tissue specimens for aerobic and anaerobic culture at enrolment, and at follow‐up, when material was available. Each treatment administered twice daily. |
|
| Outcomes | Primary outcomes: None reported in useable format Secondary outcomes: Surgical resection; adverse events |
|
| Notes | Some information about study methods was available from corresponding author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Computer generated random sequence" (from study author) Comment: Adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "20 or more clinical study centres received sufficient randomization numbers to complete approximately 224 patients at each site. The investigators were blinded as to the treatment group assignment throughout the study. After completion of required screening procedures eligible patients received a sequentially assigned randomization number. Each investigational centre received a unique set of randomization numbers." (from study author) Comment: Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind. Patients were instructed to take 2 tablets (either 200 mg of active ofloxacin orally twice daily and to apply a cream (either active pexiganan acetate or placebo, sufficient to form a dime thick layer) twice daily directly onto the ulcer and to dress the wound with sterile, dry gauze. Patients randomised to treatment with pexiganan received placebo tablets, and those randomised to ofloxacin treatment received placebo cream" Comment: Blinded |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Figure in paper shows that all participants had clinical data analysed in ITT. There were more missing data for microbial analysis, but we did not consider these in the review. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Healing data did not seem to be reported. |
| Other bias | Low risk | Comment: None noted. |
Lipsky 2012a.
| Methods | RCT (2:1 randomisation ratio) Setting: Diabetic foot clinics (mainly inpatients) (from study author) Country: USA and UK Duration of follow‐up: Outcome assessment planned for 2 weeks after cessation of treatment for a total study duration of 42 days Duration of treatment: At least 7 days and for a maximum of 28 days Funding source: For‐profit; "This study was funded in whole by Innocoll Technologies Ltd." Unit of analysis: Participant |
|
| Participants | 56 participants Inclusion criteria: Diabetic patients aged 18 to 80 years with a single, moderately infected lower extremity ulcer. Exclusion criteria: Patients who had received any antimicrobial therapy in the preceding 2 weeks; those with ischaemia of the lower limb; and, at institutional review board request, patients with a glycated haemoglobin level of ≥ 10.0%. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Infected ulcers |
|
| Interventions | Group 1: (n = 38) Systemic antibiotic therapy alone (a daily oral or intravenous dose of 750 mg of levofloxacin or alternative antimicrobial therapy, as determined by susceptibility testing). Group 2: (n = 18) Daily topical application of the gentamicin‐collagen sponge combined with systemic antibiotic therapy (a daily oral or intravenous dose of 750 mg of levofloxacin or alternative antimicrobial therapy, as determined by susceptibility testing). Additional comments: Participants in both arms also received standard diabetic wound management, including sharp surgical debridement at each visit where appropriate, pressure off‐loading as applicable, and daily dressing changes using a non‐adherent, moisture‐permeable gauze dressing followed by a second saline‐moistened gauze dressing. |
|
| Outcomes | Primary review outcomes: Resolution of infection Secondary review outcomes: Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: No information presented. |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomised in a 2:1 ratio to the treatment or control group using a interactive voice response system" Comment: Confirmed centralised randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" Comment: The authors chose not to administer placebo collagen sponges to participants in the control group due to the concern that a placebo sponge could potentially harbour bacteria and bias the results in favour of the active treatment. Consequently, to reduce the complexity of this pilot study, they chose an open‐label design. |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Quote: "assessors were also blinded to treatment" (from study author) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote "of the 56 randomised subjects, 20 (12 in group 1 and 8 in group 2) were deemed ineligible; three more subjects in the study group discontinued (1 because of adverse events, 1 because of protocol non‐compliance and 1 lost to follow up)." "we defined a modified ITT population to use of efficacy analyses to include only the 36 eligible patients" Comment: 41% of participants were not included in analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported as outlined in methods. Protocol not obtained. |
| Other bias | Low risk | Comment: None noted. |
Martinez‐De Jesus 2007.
| Methods | RCT Setting: Outpatient clinic Country: Mexico Duration of follow‐up: 20 weeks Duration of treatment: Minimum of 10 days Funding source: Not reported Unit of analysis: Participant |
|
| Participants | 45 participants Inclusion criteria: Type 2 diabetes; older than 18 years of age; infected, deep wounds at or distal to the malleoli; presence of malodour, active periwound cellulites; loss of protective sensation; and at least 1 Dopplerable pedal pulse. Exclusion criteria: Severe arterial disease; ankle‐brachial index below 0.5; a diagnosis of osteomyelitis; total gangrene of the study foot or forefoot; severe cardiovascular or renal failure; and severe neurological problems. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Group 1: Ulcer duration (weeks) 15.1 (SD 16.3) Group 2: Ulcer duration (weeks) 13.7 (SD 24.0) Infection status at baseline: Infected ulcers |
|
| Interventions | Group 1: (n = 16) Povidone iodine and saline. Povidone iodine was used after debridement. When the infection resolved and formation of granulation tissue was observed, the participant was switched to a surgical soap (Dermo Clean) with saline rinse to minimise the cytotoxic effects of povidone iodine. If clinical signs of infection returned, the use of povidone iodine was resumed. Group 2: (n = 21) Neutral pH super‐oxidised aqueous solution. Participants received an initial 15‐ to 20‐minute immersion of the affected foot. Following appropriate debridement, the affected foot soak was repeated either weekly or biweekly. Additional comments: All participants were treated using an outpatient ambulatory model, which included appropriate surgical debridement, administration of aggressive parenteral/intramuscular broad‐spectrum antibiotic therapy, appropriate off‐loading, and strict glycaemic control. Systemic antibiotics were given for a minimum of 10 days to all participants in both groups. Antibiotics were used for more that 10 days if clinical signs of infection continued to be present. All participants received pentoxyphylline at a dose of 1200 mg/day as a haemorheologic. All participants in both groups were instructed to reduce weight bearing on the affected foot by using a wheelchair or crutches and by resting as much as possible. |
|
| Outcomes | Primary review outcomes: Resolution of infection Secondary review outcomes: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "patient randomised using randomly alternate assignment" Comment: It was not clear whether process was random ‐ could also describe alternation. |
| Allocation concealment (selection bias) | High risk | Quote: "patient randomised using randomly alternate assignment" Comment: The description is not entirely clear, but allocation could have been foreseeable. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients were blinded about [sic] the differences in treatment." Comment: Adequate blinding for participants but not personnel |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Outcomes reported for all participants. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported as outlined in methods. Protocol not obtained. |
| Other bias | Low risk | Comment: None noted. |
Ramos Cuevas 2007.
| Methods | RCT Setting: Diabetic foot clinic Country: Mexico Duration of follow‐up: 20 weeks Duration of treatment: Until healing Funding source: Indas S. A. Laboratory, distributor of Cicactiv (zinc hyaluronate) Unit of analysis: Participant |
|
| Participants | 50 participants Inclusion criteria: People with foot ulcer and diabetes Exclusion criteria: Not reported Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported as a summary measure Infection status at baseline: Not reported (based on translated material) |
|
| Interventions | Group 1 (n = 25): Conventional treatment (no further details provided) Group 2 (n = 25): Zinc hyaluronate Additional comments: Not reported (based on translated material) |
|
| Outcomes | Primary review outcomes: Proportion of ulcers healed; time to healing (partially reported) Secondary review outcomes: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... were assigned randomly ..." Comment: No further information reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label" |
| Blinding of outcome assessment (detection bias) Wound healing | High risk | Quote: "open label" |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Figure in paper shows that all participants had clinical data analysed in ITT. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported as outlined in methods. Protocol not obtained. |
| Other bias | Low risk | Comment: None obvious |
Shukrimi 2008.
| Methods | RCT Setting: Hospital university centre Country: Malaysia Duration of follow‐up: Not stated Duration of treatment: Between 7 and 26 days Funding source: Not reported Unit of analysis: Participant |
|
| Participants | 30 participants. Non‐insulin‐dependent diabetes mellitus patients with Wagner grade II ulcers admitted to the hospital for surgery Inclusion criteria: age between 35 and 65 years, transcutaneous oxygen tension of more than 30 mmHg, and serum albumin level of more than 35 g/dL. Exclusion criteria: Multiple medical comorbidity, corticosteroid therapy, neutrophil count less than 2000/mm₃. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not stated Infection status at baseline: Not reported |
|
| Interventions | Group 1 (n = not stated): Standard dressing group (povidone iodine solution 10%). Group 2 (n = not stated): Honey dressing group. Additional comments: 30 consecutive patients were randomised, but number of participants in each group not reported. |
|
| Outcomes | Primary review outcomes: Time to healing (partially reported) Secondary review outcomes: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised into two study arms" Comment: Randomisation methods were not specified. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomised into two study arms" Comment: Randomisation methods were not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Not blinded |
| Blinding of outcome assessment (detection bias) Wound healing | Low risk | Quote: "all the wounds were assessed every other day by a surgeon blinded to the material of dressing" |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 30 participants were randomised, but no further information on the number of participants in each group and for each outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported as outlined in methods. Protocol not obtained. |
| Other bias | Low risk | Comment: None noted. |
Tom 2005.
| Methods | RCT; prospective, randomised, double‐blind, placebo‐controlled clinical trial Setting: Foot clinic at the Veterans Affairs Medical Center, San Diego Country: USA Duration of follow‐up: 16 weeks Duration of treatment: 4 weeks Funding source: Supported by OrthoNeutrogena Unit of analysis: Some participants had more than 1 ulcer (22 participants with 24 ulcers) |
|
| Participants | 24 participants Inclusion criteria: Lower extremity ulcer and a diagnosis of diabetes mellitus Exclusion criteria: Patients unable to give informed consent; had a known bleeding disorder; were pregnant at the time of enrolment; had infected ulcers or nearby tissues; or had lower extremity ulcers due to large artery disease (by clinical examination or abnormal ankle‐brachial index, or both) Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not infected |
|
| Interventions | Group 1 (n = 11): Placebo (normal saline solution that was coloured the same as the topical tretinoin). Group 2 (n = 13): Topical 0.05% tretinoin solution (Retin‐A; Ortho Pharmaceutical Corp, Raritan, NJ). The randomly assigned solution was applied directly to the wound bed and left in contact for 10 minutes every day; it was then rinsed off with normal saline. |
|
| Outcomes | Primary review outcomes: Proportion of ulcers healed; time to healing (partially reported) Secondary review outcomes: None |
|
| Notes | 24 participants included, 22 participants analysed (13 + 11 ulcers) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by an uninvolved third party who used a computer‐generated random sequence to balance the numbers of the 2 treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each newly enrolled patient was assigned a topical solution in ascending order" Comment: Not clear if the sequence was foreseeable with this method |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all dispensed bottles of solutions were identical in appearance (identified by number only), and neither the investigators nor the patients were aware of the treatment group to which patients were assigned until the study was completed" Comment: The double‐blind appears to have been respected. |
| Blinding of outcome assessment (detection bias) Wound healing | Low risk | As above |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All outcomes reported as outlined in methods. Protocol not obtained. |
| Selective reporting (reporting bias) | Low risk | Comment: 24 participants were randomised; 22 completed the study and were considered for the outcomes, 20 with 1 foot ulcer and 2 with 2 foot ulcers. |
| Other bias | High risk | Comment: Some participants had multiple ulcers, but this was not accounted for. |
Ullal 2014.
| Methods | RCT; randomised, controlled, open study Setting: Unclear Country: India Duration of follow‐up: Not reported (not clear) Duration of treatment: 2 months Funding source: Not stated Unit of analysis: Not stated |
|
| Participants | 4 participants Inclusion criteria: People with diabetes having grade I or II foot ulcer Exclusion criteria: Unclear Infection at baseline: Unclear |
|
| Interventions | Group 1 (n = 2): Povidone iodine and metronidazole 1% gel dressing. Group 2 (n = 2): Honey and metronidazole 1% gel dressing. |
|
| Outcomes | Primary outcomes: Proportion of ulcers healed Secondary outcomes: Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a randomised, controlled, open study" Comment: Unclear how randomisation was achieved |
| Allocation concealment (selection bias) | Unclear risk | Quote: "open study" Comment: No mention of allocation concealment process |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Open study, no blinding |
| Blinding of outcome assessment (detection bias) Wound healing | High risk | Comment: Open study, no blinding |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Secondary outcomes | High risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: None obvious |
| Other bias | Low risk | Comment: None observed. |
Viswanathan 2011.
| Methods | RCT; single‐centre, open‐label, phase III, comparative study Setting: Diabetes Research Centre Country: India Duration of follow‐up: 20 weeks Duration of treatment: Not clear Funding source: Cholayil Products and Services, Koyambedu, Chennai, India provided the polyherbal cream with their formulation. Unit of analysis: Participant Participants enrolled between August 2008 and February 2009 |
|
| Participants | 40 participants Inclusion criteria: Consecutive Type 2 diabetes patients who presented with an ulcer up to Wagner's grade III classification (grade I, superficial ulcer; grade II, deep ulcer probing to tendon, capsule, or bone; grade III, deep ulcer with abscess, osteomyelitis, or joint sepsis). Exclusion criteria: People who had clinical signs of severe infection; wound that had exposed bone; unwillingness to participate in the study were excluded. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): "There was no significant difference in the location of the wound between the groups. The distribution of ulcers according to Wagner's grade was also similar in both the study groups. Wagner grade I and II foot ulcers were viable and grade III ulcers were non‐viable tissues" Infection status at baseline: Unclear; severe infections were excluded |
|
| Interventions | Group 1 (n = 20): Polyherbal formulation wound cream: Glycyrrhiza glabra, Musa × paradisiaca,Curcuma longa,Pandanus,Aloe vera,Cocos nucifera oil. Group 2 (n = 20): Silver sulphadiazine cream. |
|
| Outcomes | Primary review outcomes: Proportion of ulcers healed (partially reported); time to healing (partially reported) Secondary review outcomes: Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Not blinded |
| Blinding of outcome assessment (detection bias) Wound healing | Unclear risk | Comment: No information provided. |
| Blinding of outcome assessment (detection bias) Infection/resolution of infection | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Rate of ulcer healing not reported. |
| Selective reporting (reporting bias) | Low risk | Quote: "Of the 40 patients enrolled in this study, 38 adhered to the protocol (group 1; n = 19 and group 2; n = 19). One patient in group 1 was excluded from the study because of severe infection and one patient in group 2 died during the study period (unrelated cause)" |
| Other bias | Low risk | Comment: None noted. |
ITT: intention‐to‐treat PP: per protocol RCT: randomised controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abidia 2003 | Not relevant intervention (hyperbaric oxygen therapy) |
| Ajmeer 2015 | Not relevant study population (mixed wounds) |
| Al‐Ebous 2005 | Not relevant intervention (all antibiotics were administered intervenously) |
| Alzahrani 2013 | Not relevant study population |
| Bahar 2015 | Not RCT |
| Belcaro 2010 | Not relevant study population (mixed wound types) |
| Braumann 2008 | Not RCT |
| Braumann 2011 | Not RCT |
| Dalla Paola 2005 | Not RCT |
| Della Marchina 1997 | Not relevant study population (mixed wound types) |
| Driver 2015 | Not relevant intervention |
| Dwivedi 2007 | Not RCT |
| Gao 2007 | No outcome data available on request |
| Gibbons 2015 | Not RCT |
| Hadi 2007 | Not relevant study population |
| Kamaratos 2014 | Not RCT |
| Kapur 2011 | Not relevant study population |
| Kastelan 1998 | Not RCT |
| Li 2004 | Not relevant intervention |
| Li 2008 | Not relevant intervention (no topical treatment tested) |
| Li 2011 | Not relevant intervention |
| Lipsky 2015 | Not relevant intervention (no topical treatment tested) |
| Lishner 1985 | Not RCT |
| Londahl 2013 | Not relevant intervention (BioLight, combination of pulsating monochromatic light) |
| Lázaro‐Martínez 2014 | Not relevant intervention (no topical treatment tested) |
| Mahmoud 2008 | Not RCT |
| Martinez‐Sanchez 2005 | Not relevant intervention (ozone therapy) |
| Mikhaloĭko 2014 | Not relevant intervention (no topical treatment tested) |
| Minatel 2009 | Not relevant intervention (phototherapy) |
| Monami 2012 | Not relevant intervention (photosensitiser compound) |
| Morley 2012 | Not relevant intervention (cationic photosensitisers) |
| Motta 2004 | Not relevant study population (mixed wound types) |
| Münter 2006 | Not RCT |
| Otvos 2015 | Not RCT |
| Panahi 2015 | Not relevant study population (mixed population; only 18 diabetic patients included, separate data not available) |
| Paquette 2001 | Not RCT |
| Piaggesi 2010 | Not relevant study population |
| Reyzelman 2009 | Not relevant study population |
| Rhaiem 1998 | Not relevant study population (mixed population, number with diabetes and foot ulcers not reported) |
| Santomauro 2015 | Not relevant intervention |
| Scalise 2003 | Not RCT |
| Siavash 2011 | Not RCT |
| Siavash 2015 | Not RCT |
| Sibbald 2011 | Not relevant study population (mixed population, number with diabetes and foot ulcers not reported) |
| Song 2009 | Not RCT |
| Tardivo 2014 | Not RCT |
| Tauro 2013 | Not RCT |
| Tran 2014 | Not RCT |
| Trial 2010 | Not relevant study population (mixed population, data for people with diabetes and foot ulcers not available) |
| Uribe 2007 | Not RCT |
| Vandeputte 1997 | Antimicrobial treatment not the only systematic difference between trial arms |
| Varga 2014 | Not relevant study population |
| Wainstein 2011 | Not relevant intervention (ozone therapy) |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Fazal 2012.
| Methods | RCT Setting: Hospital Country: India Duration of follow‐up: Not reported Duration of treatment: 14 days Funding source: Not reported Unit of analysis: Participant |
| Participants | 50 participants Inclusion criteria: Diabetic ulcer of the foot Exclusion criteria: Not reported Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported): Not reported Infection status at baseline: Not reported |
| Interventions | Group 1: (n = 25) 5% w/v povidone iodine solution twice daily for 14 days. Group 2: (n = 25) Phenytoin‐soaked suspension (20 mg/cm²) total body surface area; frequency not reported. Additional comments: After 14 days all participants were subject to split‐thickness skin graft. |
| Outcomes | Primary review outcomes: Time to healing Secondary review outcomes: None reported |
| Notes | Available only as a conference abstract (authors contacted for further information; awaiting response) |
Rehman 2013.
| Methods | RCT Setting: Surgical unit, hospital, 1 centre Country: Pakistan Duration of follow‐up: 2 weeks Duration of treatment: 2 weeks Funding source: Not reported Unit of analysis: Participant |
| Participants | 60 participants Inclusion criteria: Wagner grade I or II diabetic ulcers. Exclusion criteria: Patients not consenting to study, having features of systemic infection and other comorbidities. Ulcer characteristics at baseline (size of ulcer, number of ulcers, duration of ulceration where reported)): Size of ulcer after surgical debridement measured at baseline, but data not reported. Infection status at baseline: Not reported |
| Interventions | Group 1: Honey‐soaked dressing (local honey used ‐ no further information). Group 2: Povidone iodine/normal saline dressing. Additional comments: Dressing changed once a day. |
| Outcomes | Primary review outcomes: Proportion of ulcers healed Secondary review outcomes: None |
| Notes | Contacted author for randomisation methods |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Heybeck 2012.
| Trial name or title | |
| Methods | Double‐blind, multicentre trial |
| Participants | Diabetic patients with foot ulcers |
| Interventions | Normal diabetic socks versus (experimental group) copper‐impregnated socks |
| Outcomes | Quality of life, healing, odour |
| Starting date | |
| Contact information | |
| Notes | ABSTRACT ONLY, ONGOING |
NCT01594762.
| Trial name or title | A randomized, double‐blind, multicenter, superiority, placebo‐controlled phase 3 study of pexiganan cream 0.8% applied twice daily for 14 days in the treatment of adults with mild infections of diabetic foot ulcers |
| Methods | Interventional RCT |
| Participants | Mild infected ulcer in diabetic patients, full‐ or partial‐thickness ulcer on the foot distal to the malleoli with a surface area ≥ 1 cm² after the wound has undergone appropriate debridement |
| Interventions | Pexiganan versus placebo |
| Outcomes | 28 days clinical response, 28 days microbiological response, incidence and severity of adverse events |
| Starting date | 2014 |
| Contact information | |
| Notes |
Differences between protocol and review
We revised the Methods section in keeping with standard Cochrane methods. We also restructured the Types of outcome measures section to improve readability and to add more detailed definitions of assessment. We added 'proportion of ulcers healed' as well as 'time to wound healing', which is standard in wounds review but had been omitted at the protocol stage. We also added details not previously included about how outcome data at different time points would be managed. We renamed the safety outcome 'adverse events' and added a fuller description in line with other Cochrane Wounds reviews.
In the protocol the Types of participants section noted that: "Studies with a mixed population of participants with foot ulcers who do, as well as those who do not, have diabetes will be included if the results for the diabetic patient subset are separately provided." As this approach is essentially a subgroup analysis of an included trial, we revised this approach to consider the use of separately reported data only when stratification by wound type had been used at randomisation, or when the majority of wounds were ulcers of the foot in people with diabetes.
We also clarified the interventions that were not relevant to this review (i.e. those not considered to be topical).
We added GRADE assessment and 'Summary of findings' tables; updated sections with more detailed analytical information; and edited the wording. We made no changes that fundamentally altered the review as planned or in its implementation and do not believe we have biased the review in any way. Changes were made prior to data extraction and the writing of the current draft of the review.
Contributions of authors
Jo Dumville: co‐ordinated the review; extracted data; checked the quality of data extraction; analysed and interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; contributed to writing and editing the review; made an intellectual contribution to the review; approved the final review prior to submission; advised on the review; secured funding; and is a guarantor of the review.
Benjamin Lipsky: conceived, designed, and co‐ordinated the review; checked the quality of data extraction; analysed and interpreted data; checked quality assessment; produced the first draft of the review; contributed to writing and editing the review; made an intellectual contribution to the review; approved the final review prior to submission; advised on the review; performed previous work that was the foundation of the current review; wrote to study author/experts/companies; provided data; and is a guarantor of the review.
Christopher Hoey: extracted data; contributed to writing or editing of the review; made an intellectual contribution to the review; approved the final review prior to submission; advised on the review; performed previous work that was the foundation of the current review; and provided data.
Mario Cruciani: conceived, designed, and co‐ordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing or editing of the review; made an intellectual contribution to the review; approved the final review prior to submission; and advised on the review.
Marta Fiscon: extracted data; checked the quality of data extraction; contributed to writing or editing of the review; made an intellectual contribution to the review; and approved the final review prior to submission.
Jun Xia: extracted data; checked the quality of data extraction; and approved the final review prior to submission.
Contributions of editorial base
Nicky Cullum (Editor): edited the protocol and the review; advised on methodology, interpretation, and content; approved the final protocol and review prior to submission.
Sally Bell‐Syer and Gill Rizzello (Managing Editors): co‐ordinated the editorial process; advised on interpretation, and content; edited the protocol and review.
Ruth Foxlee and Reetu Child (Information Specialists): designed the search strategy, ran the searches and edited the search methods section.
Ursula Gonthier (Editorial Assistant): checked and edited the Plain Language Summary and reference sections of the review.
Sources of support
Internal sources
Division of Nursing, Midwifery and Social Work, School of Health Sciences, University of Manchester, UK.
External sources
-
National of Institute of Health Research, UK.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure and Programme Grant funding (NIHR Cochrane Programme Grant 13/89/08 ‐ High Priority Cochrane Reviews in Wound Prevention and Treatment) to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health
-
National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester Centre, UK.
Jo Dumville was partly funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester. The funder had no role in the design of the studies, data collection and analysis, decision to publish, or preparation of the manuscript. However, the review may be considered to be affiliated to the work of the NIHR CLAHRC Greater Manchester. The views expressed herein are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health.
Declarations of interest
Jo C Dumville: has received research funding from the National Institute for Health Research (NIHR) for the production of systematic reviews focusing on high‐priority Cochrane reviews in the prevention and treatment of wounds. This work was also partly funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester.
Benjamin A Lipsky: has been a member of an advisory board or speakers bureau for KCI and Affinium and has served as a consultant to, or received research funding or lecture fees from: Innocoll, Dipexium and Merck. He recused himself from data extraction and from discussions on papers for which he was an author. He asserts that none of his industry‐related activities presented any conflict of interest for this review.
Christopher Hoey: none known
Mario Cruciani: received payments for consultancy work from ViiV Healthcare and Bristol‐Myers Squibb and payment for lectures from Abbott, Gilead Sciences and Merck but states that these were not related to his work with Cochrane or the subject of this review.
Marta Fiscon: none known.
Jun Xia: none known.
Laura Bolton (specialist peer reviewer) states: 'Before I retired in 2006, as a scientist for Johnson & Johnson (13 years), then R&D manager then director of Scientific Affairs for ConvaTec (19 years) I was aware of details of some studies cited, though I never participated directly as investigator or monitor in any of these studies. As a volunteer guideline developer and Cochrane Wounds reviewer I have searched for related study information on prior occasions'.
New
References
References to studies included in this review
Ahmed 2014 {published data only}
- Ahmed A, Ahmed MI. A comparison of efficacy of topical use of phenytoin and Vaseline gauze dressing with Vaseline gauze dressing alone in healing of diabetic foot ulcers. Journal of Postgraduate Medical Institute 2014;28(3):297‐302. [Google Scholar]
Apelqvist 1996 {published data only}
- Apelqvist J, Ragnarson‐Tennvall G. Cavity foot ulcers in diabetic patients: a comparative study of cadexomer iodine ointment and standard treatment. An economic analysis alongside a clinical trial. Acta Dermato‐Venereologica 1996;76(3):231‐5. [DOI] [PubMed] [Google Scholar]
Bergqvist 2016 {published data only}
- Bergqvist K, Almhöjd U, Herrmann I, Eliasson B. The role of chloramines in treatment of diabetic foot ulcers: an exploratory multicentre randomised controlled trial. Clinical Diabetes and Endocrinology 2016;2(6):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowling 2011 {published data only}
- Bowling FL, Crews RT, Salgami E, Armstrong DG, Boulton AJ. The use of superoxidized aqueous solution versus saline as a replacement solution in the Versajet lavage system in chronic diabetic foot ulcers. Journal of the American Podiatric Medical Association 2011;101(2):124‐6. [DOI] [PubMed] [Google Scholar]
Gottrup 2013 {published data only}
- Gottrup F, Cullen BM, Karlsmark T, Bischoff‐Mikkelsen M, Nisbet L, Gibson MC. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair and Regeneration 2013;21:1‐10. [DOI] [PubMed] [Google Scholar]
He 2016 {published data only}
- He WQ, Luo WH, Li L, Jiang L. Effects of silver ions dressing for diabetic foot ulcers: a randomized controlled trial [银离子敷料治疗糖尿病足溃疡的疗效观察]. Chinese Journal of Evidence‐Based Medicine 2016;5:510‐2. [Google Scholar]
Hwang 2010 {published data only}
- Hwang JH, Kim KS, Lee SY. Hydrofibre dressings with silver in the management of diabetic foot ulcers. EWMA Journal 2010;10(2):136. [Abstract P57] [Google Scholar]
Imran 2015 {published data only}
- Imran M, Hussain MB, Baig M. A randomized, controlled clinical trial of honey‐impregnated dressing for treating diabetic foot ulcer. Journal of the College of Physicians and Surgeons ‐ Pakistan 2015;25:721‐5. [DOI] [PubMed] [Google Scholar]
Jacobs 2008 {published data only}
- Jacobs AM, Tomczak R. Evaluation of Bensal HP for the treatment of diabetic foot ulcers. Advances in Skin & Wound Care 2008;21(10):461‐5. [DOI] [PubMed] [Google Scholar]
Jeffcoate 2009 {published data only}
- Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technology Assessment 2009;13(54):1‐86. [DOI] [PubMed] [Google Scholar]
Jude 2007 {published data only}
- Jude EB, Apelqvist J, Spraul M, Martini J, Silver Dressing Study Group. Prospective randomized controlled study of Hydrofiber dressing containing ionic silver or calcium alginate dressings in non‐ischaemic diabetic foot ulcers. Diabetic Medicine 2007;24(3):280‐8. [DOI] [PubMed] [Google Scholar]
Khandelwal 2013 {published data only}
- Khandelwal S, Chaudhary P, Poddar DD, Saxena N, Singh RA, Biswal UC. Comparative study of different treatment options of grade III and IV diabetic foot ulcers to reduce the incidence of amputations. Clinics and Practice 2013;3(1):20‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Landsman 2011 {published data only}
- Landsman A, Blume PA, Jordan DA, Vayser D, Gutierrez A. An open‐label, three‐arm pilot study of the safety and efficacy of topical Microcyn Rx wound care versus oral levofloxacin versus combined therapy for mild diabetic foot infections. Journal of the American Podiatric Medical Association 2011;101(6):484‐96. [DOI] [PubMed] [Google Scholar]
Lipsky 2008a {published data only}
- Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double‐blinded, multicenter trial of Pexiganan cream. Clinical Infectious Diseases 2008;47(12):1537‐45. [DOI] [PubMed] [Google Scholar]
Lipsky 2008b {published data only}
- Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double‐blinded, multicenter trial of Pexiganan cream. Clinical Infectious Diseases 2008;47(12):1537‐45. [DOI] [PubMed] [Google Scholar]
Lipsky 2012a {published data only}
- Lipsky BA, Kuss M, Edmonds M, Reyzelman A, Sigal F. Topical application of a gentamicin‐collagen sponge combined with systemic antibiotic therapy for the treatment of diabetic foot infections of moderate severity: a randomized, controlled, multicenter clinical trial. Journal of the American Podiatric Medical Association 2012;102(3):223‐32. [DOI] [PubMed] [Google Scholar]
Martinez‐De Jesus 2007 {published data only}
- Martinez‐De Jesus FR, Ramos‐De la Medina A, Remes‐Troche JM, Armstrong DG, Wu SC, Lazaro Martinez JL, et al. Efficacy and safety of neutral pH superoxidised solution in severe diabetic foot infections. International Wound Journal 2007;4:353‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramos Cuevas 2007 {published data only}
- Ramos Cuevas F, Velazquez Mendez AA, Castaneda Andrade I. Zinc hyaluronate effects on ulcers in diabetic patients. Gerokomos 2007;18(2):91‐101. [Google Scholar]
Shukrimi 2008 {published data only}
- Shukrimi A, Sulaiman AR, Halim AY, Azril A. A comparative study between honey and povidone iodine as dressing solution for Wagner Type II diabetic foot ulcers. Medical Journal of Malaysia 2008;63(1):44‐6. [PubMed] [Google Scholar]
Tom 2005 {published data only}
- Tom WL, Peng DH, Allaei A, Hsu D, Hata TR. The effect of short‐contact topical tretinoin therapy for foot ulcers in patients with diabetes. Archives of Dermatology 2005;141(11):1373‐7. [DOI] [PubMed] [Google Scholar]
Ullal 2014 {published data only}
- Ullal S, Adhikari P. Efficacy of honey in healing diabetic ulcers: a pilot study. Diabetes Research and Clinical Practice 2014;106(1 Suppl):S63‐4. [Poster PO037] [Google Scholar]
Viswanathan 2011 {published data only}
- Viswanathan V, Kesavan R, Kavitha KV, Kumpatla S. A pilot study on the effects of a polyherbal formulation cream on diabetic foot ulcers. Indian Journal of Medical Research 2011;134(2):168‐73. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abidia 2003 {published data only}
- Abidia A, Laden G, Kuhan G, Johnson BF, Wilkinson AR, Renwick PM, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double‐blind randomised‐controlled trial. European Journal of Vascular and Endovascular Surgery 2003;25(6):513‐8. [DOI] [PubMed] [Google Scholar]
Ajmeer 2015 {published data only}
- Ajmeer AS, Dudhamal TS, Gupta SK. Management of Madhumehajanya Vrana (diabetic wound) with Katupila (Securinega leucopyrus (Willd) Muell.) Kalka. AYU 2015;36(3):351‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Ebous 2005 {published data only}
- Al‐Ebous AD, Hiasat B, Sarayrah M, Al‐Jahmi M, Al‐Zuriqat AN. Management of diabetic foot in a Jordanian hospital. Eastern Mediterranean Health Journal 2005;11(3):490‐3. [PubMed] [Google Scholar]
Alzahrani 2013 {published data only}
- Alzahrani H, Bedir Y, Al‐Hayani A. Efficacy of shellac, a natural product, for the prevention of wet gangrene. Journal of International Medical Research 2013;41(3):795–803. [DOI] [PubMed] [Google Scholar]
Bahar 2015 {published data only}
- Bahar A, Saeedi M, Kshi Z, Davoodi M. The effect of Aloe vera and honey gel in healing diabetic foot ulcers. Journal of Mazandaran University of Medical Sciences 2015;25(128):110‐4. [Google Scholar]
Belcaro 2010 {published data only}
- Belcaro G, Cesarone MR, Errichi BM, Ricci A, Dugall M, Pellegrini L, et al. Venous and diabetic ulcerations: management with topical multivalent silver oxide ointment. Panminerva Medica 2010;52(2 Suppl 1):37‐42. [PubMed] [Google Scholar]
Braumann 2008 {published data only}
- Braumann C, Pirlich M, Menenakos C, Lochs H, Mueller JM. Implementation of the clean and close concept for treatment of surgical and chronic wounds in three university centres in Berlin Germany. old.ewma.org/fileadmin/user_upload/EWMA/pdf/conference_abstracts/2008/oral/44._pdf.pdf (accessed 3 February 2017).
Braumann 2011 {published data only}
- Braumann C, Guenther N, Menenakos C, Muenzberg H, Pirlich M, Lochs H, et al. Clinical experiences derived from implementation of an easy to use concept for treatment of wound healing by secondary intention and guidance in selection of appropriate dressings. International Wound Journal 2011;8(3):253‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalla Paola 2005 {published data only}
- Dalla Paola L, Brocco E, Senesi A, Ninkovic S, Merico M, Vido D. Use of Dermacyn, a new antiseptic agent, for the local treatment of diabetic foot ulcers. Journal of Wound Healing 2005;2:201. [Google Scholar]
Della Marchina 1997 {published data only}
- Della Marchina M, Renzi G. A new antiseptic preparation used for the disinfection of cutaneous distrophic ulcers. Chronica Dermatologica 1997;7(6):873‐85. [Google Scholar]
Driver 2015 {published data only}
- Driver VR, Lavery LA, Reyzelman AM, Dutra TG, Dove CR, Kotsis SV, et al. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair and Regeneration 2015;23(6):891‐900. [DOI] [PubMed] [Google Scholar]
Dwivedi 2007 {published data only}
- Dwivedi KN, Shukla VK, Ojha JK. Role of plant extract in non‐healing diabetic foot ulcers. 10th Conference of the European Wound Management Association; 2000 May 18‐20; Stockholm, Sweden. 2000:27.
Gao 2007 {published data only}
- Gao L, Xu GX, Zhou HB. Efficacy evaluation of diabetic foot ulceration treated with iodophors dressings therapy. Journal of Clinical Nursing 2007;6:21‐2. [Google Scholar]
Gibbons 2015 {published data only}
- Gibbons GW. Grafix, a cryopreserved placental membrane, for the treatment of chronic/stalled wounds. Advances in Wound Care 2015;4(9):534‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadi 2007 {published data only}
- Hadi SF, Khaliq T, Bilal N, Sikandar I, Saaiq M, Aurangzeb S. Treating infected diabetic wounds with superoxidized water as antiseptic agent: a preliminary experience. Journal of the College of Physicians and Surgeons ‐ Pakistan 2007;17(12):740‐3. [PubMed] [Google Scholar]
Kamaratos 2014 {published data only}
- Kamaratos AV, Tzirogiannis KN, Iraklianou SA, Panoutsopoulos GI, Kanellos IE, Melidonis AI. Manuka honey‐impregnated dressings in the treatment of neuropathic diabetic foot ulcers. International Wound Journal 2014;11(3):259‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapur 2011 {published data only}
- Kapur V, Marwaha AK. Evaluation of effect and comparison of superoxidised solution (Oxum) vs povidone iodine (Betadine). Indian Journal of Surgery 2011;73(1):48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kastelan 1998 {published data only}
- Kastelan M, Brajac I, Gruber F. Treatment of leg ulcers with autologous heparinized blood. Acta Dermato‐Venereologica 1998;7:30‐4. [Google Scholar]
Lázaro‐Martínez 2014 {published data only}
- Lázaro‐Martínez JL, Aragón‐Sánchez J, García‐Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: a randomized comparative trial. Diabetes Care 2014;37(3):789‐95. [DOI] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li HM, Liang ZQ, Meng CY, Li DH. The efficacy and safety of recombinant human epidermal growth factor in 4 types of wounds. Chinese Journal of New Drugs 2004;13(11):1048‐50. [Google Scholar]
Li 2008 {published data only}
- Li Y, Song Z‐Q, Ren H‐Z. Alprostadil combined with deproteinized calf blood extractives injection for treatment of diabetic foot. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12(50):9970‐2. [Google Scholar]
Li 2011 {published data only}
- Li FL, Deng H, Wang HW, Xu R, Chen J, Wang YF, et al. Effects of external application of Chinese medicine on diabetic ulcers and the expressions of β‐catenin, c‐myc and K6. Chinese Journal of Integrative Medicine 2011;17(4):261‐6. [DOI] [PubMed] [Google Scholar]
Lipsky 2015 {published data only}
- Lipsky BA, Cannon CM, Ramani A, Jandourek A, Calmaggi A, Friedland HD, et al. Ceftaroline fosamil for treatment of diabetic foot infections: the CAPTURE study experience. Diabetes/Metabolism Research and Reviews 2015;31(4):395‐401. [DOI] [PubMed] [Google Scholar]
Lishner 1985 {published data only}
- Lishner M, Lang R, Kedar I, Ravid M. Treatment of diabetic perforating ulcers (mal perforant) with local dimethylsulfoxide. Journal of the American Geriatrics Society 1985;33(1):41‐3. [DOI] [PubMed] [Google Scholar]
Londahl 2013 {published data only}
- Londahl M, Sjoberg S, Apelqvist J. Monochromatic phototherapy enhances healing rate in diabetic foot ulcers. EWMA Journal 2013; Vol. 13, issue 1 Suppl:54. [Abstract 70]
Mahmoud 2008 {published data only}
- Mahmoud SM, Mohamed AA, Mahdi SE, Ahmed ME. Split‐skin graft in the management of diabetic foot ulcers. Journal of Wound Care 2008;17(7):303‐6. [DOI] [PubMed] [Google Scholar]
Martinez‐Sanchez 2005 {published data only}
- Martínez‐Sánchez G, Al‐Dalaina SM, Menéndez S, Rec L, Giuliani A, Candelario‐Jalila E, et al. Therapeutic efficacy of ozone in patients with diabetic foot. European Journal of Pharmacology 2005;523:151‐61. [DOI] [PubMed] [Google Scholar]
Mikhaloĭko 2014 {published data only}
- Mikhaloĭko II, Sabadosh RV, Kovalenko AL, Skripko VD. Rationale of application of the drug Cytoflavin in complex treatment of patients with diabetic foot syndrome with mediacalcification arteries. Khirurgiia 2014;12:36‐40. [PubMed] [Google Scholar]
Minatel 2009 {published data only}
- Minatel DG, Frade MA, França SC, Enwemeka CS. Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies. Lasers in Surgery and Medicine 2009;41(6):433‐41. [DOI] [PubMed] [Google Scholar]
Monami 2012 {published data only}
- Monami M, Genovese S, Anichini R, Fondelli C, Romagnoli F, Bartoli N, et al. Randomised, double‐blind versus placebo, proof of concept clinical trial to evaluate efficacy and safety of g.68.y/etoh in diabetic infected foot ulcers (DANTE study). Diabetologia 2012;55(1 Suppl):S476. [Google Scholar]
Morley 2012 {published data only}
- Morley S, Griffiths J, Philips G, Moseley H, O'Grady C, Mellish K, et al. Phase IIa randomized, placebo‐controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: a new approach to antimicrobial therapy. British Journal of Dermatology 2013;168(3):617‐24. [DOI] [PubMed] [Google Scholar]
Motta 2004 {published data only}
- Motta GJ, Milne CT, Corbett LQ. Impact of antimicrobial gauze on bacterial colonies in wounds that require packing. Ostomy/Wound Management 2004;50(8):48‐62. [PubMed] [Google Scholar]
Münter 2006 {published data only}
- Münter KC, Beele H, Russell L, Crespi A, Gröchenig E, Basse P, et al. Effect of a sustained silver‐releasing dressing on ulcers with delayed healing: the CONTOP study. Journal of Wound Care 2006;15(5):199‐206. [DOI] [PubMed] [Google Scholar]
Otvos 2015 {published data only}
- Otvos L, Ostorhazi E. Therapeutic utility of antibacterial peptides in wound healing. Expert Review of Anti‐infective Therapy 2015;13(7):871‐81. [DOI] [PubMed] [Google Scholar]
Panahi 2015 {published data only}
- Panahi Y, Izadi M, Sayyadi N, Rezaee R, Jonaidi‐Jafari N, Beiraghdar F, et al. Comparative trial of Aloe Vera/olive oil combination cream versus phenytoin cream in the treatment of chronic wounds. Journal of Wound Care 2015;24(10):459‐65. [DOI] [PubMed] [Google Scholar]
Paquette 2001 {published data only}
- Paquette D, Badiavis E, Falanga V. Short‐contact topical tretinoin therapy to stimulate granulation tissue in chronic wounds. Journal of the American Academy of Dermatology 2001;45(3):382‐6. [DOI] [PubMed] [Google Scholar]
Piaggesi 2010 {published data only}
- Piaggesi A, Goretti C, Mazzurco S, Tascini C, Leonildi A, Rizzo L, et al. A randomized controlled trial to examine the efficacy and safety of a new super‐oxidized solution for the management of wide postsurgical lesions of the diabetic foot. International Journal of Lower Extremity Wounds 2010;9(1):10‐5. [DOI] [PubMed] [Google Scholar]
Reyzelman 2009 {published data only}
- Reyzelman A, Crews RT, Moore JC, Moore L, Mukker JS, Offutt S, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. International Wound Journal 2009;6(3):196‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rhaiem 1998 {published data only}
- Rhaiem BB, Ftouhi B, Brahim SB, Mekaouer A, Kanoun F, Abde'nnebi A, et al. A comparative study of saccharose use in the treatment of cutaneous lesions in diabetic patients: about 80 cases [Essai comparatif de l'utilisation du saccharose dans le traitement des lésions cutanées chez le diabétique à propos de 80 cas]. Tunisie Médicale 1998;76(3):19‐23. [PubMed] [Google Scholar]
Santomauro 2015 {published data only}
- Santomauro AC Jr, Tardivo JP, Mascarenhas BM, Ramiro RE, Santomauro AT, Correa JA. A new proposal for the treatment of patients with diabetic foot ‐ photodynamic therapy. Diabetes 2015;64(1 Suppl):A185‐6. [Google Scholar]
Scalise 2003 {published data only}
- Scalise A, Forma O, Happe M, Hahn TW. The CONTOP study: real life experiences from an international study comparing a silver containing hydro‐activated foam dressing with standard wound care. old.ewma.org/fileadmin/user_upload/EWMA/pdf/conference_abstracts/2003/Poster/Poster_81.pdf (accessed 3 February 2017).
Siavash 2011 {published data only}
- Siavash M, Shokri S, Haghighi S, Mohammadi M, Shahtalebi MA, Farajzadehgan Z. The efficacy of topical royal jelly on diabetic foot ulcers healing: a case series. Journal of Research in Medical Sciences 2011;16(7):904‐9. [PMC free article] [PubMed] [Google Scholar]
Siavash 2015 {published data only}
- Siavash M, Shokri S, Haghighi S, Shahtalebi MA, Farajzadehgan Z. The efficacy of topical royal jelly on healing of diabetic foot ulcers: a double‐blind placebo‐controlled clinical trial. International Wound Journal 2015;12(2):137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sibbald 2011 {published data only}
- Sibbald RG, Coutts P, Woo KY. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing: clinical trial results. Advances in Skin & Wound Care 2011;24(2):78‐84. [DOI] [PubMed] [Google Scholar]
Song 2009 {published data only}
- Song M, Li X, Zhong X. Clinical observation and nursing care of patients with diabetic foot treated with sodium humate in combination with insulin external applying. Chinese Nursing Research 2009;23(10):862‐4. [Google Scholar]
Tardivo 2014 {published data only}
- Tardivo JP, Adami F, Correa JA, Pinhal MA, Baptista MS. A clinical trial testing the efficacy of PDT in preventing amputation in diabetic patients. Photodiagnosis and Photodynamic Therapy 2014;11(3):342‐50. [DOI] [PubMed] [Google Scholar]
Tauro 2013 {published data only}
- Tauro LF, Shetti P, Dsouza NT, Mohammed S, Sucharitha S. A comparative study of efficacy of topical phenytoin vs conventional wound care in diabetic ulcers. International Journal of Molecular Medical Science 2013;3(8):65‐71. [Google Scholar]
Tran 2014 {published data only}
- Tran TD‐X, Le PT‐B, Pham P. Diabetic foot ulcer treatment by activated platelet rich plasma: a clinical study. Biomedical Research and Therapy 2014;1(2):37‐42. [Google Scholar]
Trial 2010 {published data only}
- Trial C, Darbas H, Lavigne JP, Sotto A, Simoneau G, Tillet Y, et al. Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: a RCT. Journal of Wound Care 2010;19(1):20‐6. [DOI] [PubMed] [Google Scholar]
Uribe 2007 {published data only}
- Uribe F. Effect of a neutral pH superoxidized solution in the healing of diabetic foot ulcers. International Journal of Lower Extremity Wounds 2007;6(3):224. [Abstract 117] [Google Scholar]
Vandeputte 1997 {published data only}
- Vandeputte J, Gryson L. Diabetic foot infection controlled by immuno‐modulating hydrogel containing 65% glycerine. Presentation of a clinical trial. Journal of Wound Care 1996; Vol. 5, issue 8:9.
Varga 2014 {published data only}
- Varga M, Sixta B, Bem R, Matia I, Jirkovska A, Adamec M. Application of gentamicin‐collagen sponge shortened wound healing time after minor amputations in diabetic patients: a prospective, randomised trial. Archives of Medical Science 2014;10(2):283‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wainstein 2011 {published data only}
- Wainstein J, Feldbrin Z, Boaz M, Harman‐Boehm I. Efficacy of ozone–oxygen therapy for the treatment of diabetic foot ulcers. Diabetes Technology & Therapeutics 2011;13(12):1255‐60. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fazal 2012 {published data only}
- Fazal F. Comparative evaluation of the beneficial effects of phenytoin in diabetic ulcers. Australasian Annals of Medicine 2012;5(1):55. [Google Scholar]
Rehman 2013 {published data only}
- Rehman EU, Afzal OM, Ali A, Qureshi RA, Rashid M. Comparison between honey and povidone‐iodine/normal saline dressing for management of Wagner's grade I & II diabetic foot ulcers. Pakistan Journal of Medical and Health Sciences 2013;7(4):1082‐5. [Google Scholar]
References to ongoing studies
Heybeck 2012 {published data only}
- Heybeck T, Rothenberg G. Utilizing cupron antimicrobial therapeutic socks to help prevent lower limb and foot ulcers in the diabetic patients: a double blind trial of safety and efficacy. Foot 2012;22(2):108‐9. [Google Scholar]
NCT01594762 {unpublished data only}
- NCT01594762. Pexiganan versus placebo control for the treatment of mild infections of diabetic foot ulcers (Onestep‐2). clinicaltrials.gov/ct2/show/NCT01594762 (first received 7 May 2012).
Additional references
American Diabetes Association 2003
- American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care 2003;26(12):3333‐41. [DOI] [PubMed] [Google Scholar]
Bergin 2006
- Bergin SM, Wraight P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005082.pub2] [DOI] [PubMed] [Google Scholar]
Cooper 2004
- Cooper R. A review of the evidence for the use of topical antimicrobial agents in wound care. World Wide Wounds. February 2004. www.worldwidewounds.com/2004/february/Cooper/Topical‐Antimicrobial‐Agents.html (accessed 3 February 2017).
Cutting 2005
- Cutting KF, White RJ. Criteria for identifying wound infection: revisited. Ostomy/Wound Management 2005;51:28‐34. [PubMed] [Google Scholar]
Davies 2004
- Davies CE, Hill KE, Wilson MJ, Stephens P, Hill CM, Harding KG, et al. Use of 16S ribosomal DNA PCR and denaturing gradient gel electrophoresis for analysis of the microfloras of healing and nonhealing chronic venous leg ulcers. Journal of Clinical Microbiology 2004;42(8):3549‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2007
- Davies CE, Hill KE, Newcombe RG, Stephens P, Wilson MJ, Harding KG, et al. A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Repair and Regeneration 2007;15(1):17‐22. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Delamaire 1997
- Delamaire M, Maugendre D, Moreno M, Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabetic Medicine 1997;14(1):29‐34. [DOI] [PubMed] [Google Scholar]
Drucker 2012
- Drucker CR. Update on topical antibiotics in dermatology. Dermatologic Therapy 2012;25:6‐11. [DOI] [PubMed] [Google Scholar]
Game 2016
- Game FL, Apelqvist J, Attinger C, Hartemann A, Hinchliffe RJ, Löndahl M, et al. International Working Group on the Diabetic Foot (IWGDF). Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes/Metabolism Research and Reviews 2016;32(1 Suppl):154‐68. [DOI] [PubMed] [Google Scholar]
Gardner 2001
- Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair and Regeneration 2001;9(3):178‐86. [DOI] [PubMed] [Google Scholar]
Gelmetti 2008
- Gelmetti C. Local antibiotics in dermatology. Dermatologic Therapy 2008;21:187‐95. [DOI] [PubMed] [Google Scholar]
GradePro GDT 2015 [Computer program]
- GRADEpro GDT. GRADEpro Guideline Development Tool. McMaster University, 2015 (developed by Evidence Prime, Inc.).
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. What is 'quality of evidence' and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman A, Kunz R, Brozek J, Alonso‐Coello P, Rind D. GRADE guidelines 6. Rating the quality of evidence: imprecision. Journal of Clinical Epidemiology 2011;64:1283‐93. [DOI] [PubMed] [Google Scholar]
Heggers 1991
- Heggers JP, Sazy JA, Stenberg BD, Strock LL, McCauley RL, Herndon DN, et al. Bactericidal and wound‐healing properties of sodium hypochlorite solutions: the 1991 Lindberg Award. Journal of Burn Care and Rehabilitation 1991;12(5):420‐4. [DOI] [PubMed] [Google Scholar]
Heinzelmann 2002
- Heinzelmann M, Scott M, Lam T. Factors predisposing to bacterial invasion and infection. American Journal of Surgery 2002;183(2):179‐90. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hróbjartsson 2012
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ 2012;344:e1119. [DOI] [PubMed] [Google Scholar]
IDF 2015
- International Diabetes Foundation (IDF). Diabetes Atlas. 7th edition. 2015. www.diabetesatlas.org (accessed 10 February 2017).
IWGDF 2015
- International Working Group on the Diabetic Foot (IWGDF). IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Prepared by the IWGDF Working Group on Foot Infections 2015. iwgdf.org/guidelines/guidance‐on‐infection (accessed 3 February 2017).
IWGDF 2016
- International Working Group on the Diabetic Foot (IWGDF). iwgdf.org/ (accessed 10 February 2017).
James 2008
- James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair and Regeneration 2008;16(1):37‐44. [DOI] [PubMed] [Google Scholar]
Jull 2015
- Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD005083.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kontopantelis 2012
- Kontopantelis E, Springate DA, Reeves D. A re‐analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta‐analyses. PLoS ONE 2013;26:e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavery 2006
- Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29(6):1288‐93. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lio 2004
- Lio PA, Kaye ET. Topical antibacterial agents. Infectious Disease Clinics of North America 2004;18:717‐33. [DOI] [PubMed] [Google Scholar]
Lipsky 2009
- Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clinical Infectious Diseases 2009;49(10):1541‐9. [DOI] [PubMed] [Google Scholar]
Lipsky 2012b
- Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clinical Infectious Diseases 2012;54(12):e132‐73. [DOI] [PubMed] [Google Scholar]
Lipsky 2013
- Lipsky BA, Richard JL, Lavigne JP. Diabetic foot ulcer microbiome: one small step for molecular microbiology: one giant leap for understanding diabetic foot ulcers?. Diabetes 2013;62(3):679‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipsky 2016
- Lipsky BA, Aragón‐Sánchez J, Diggle M, Embil J, Kono S, Lavery L, et al. International Working Group on the Diabetic Foot (IWGDF). IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes/Metabolism Research and Reviews 2016;32(1 Suppl):45‐74. [DOI] [PubMed] [Google Scholar]
Markakis 2016
- Markakis K, Bowling FL, Boulton AJ. The diabetic foot in 2015: an overview. Diabetes/Metabolism Research and Reviews 2016;32(1 Suppl):169‐78. [DOI] [PubMed] [Google Scholar]
Nebeker 2004
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Annals of Internal Medicine 2004;140(10):795‐801. [DOI] [PubMed] [Google Scholar]
Neely 2009
- Neely AN, Gardner J, Durkee P, Warden GD, Greenhalgh DG, Gallagher JJ, et al. Are topical antimicrobials effective against bacteria that are highly resistant to systemic antibiotics?. Journal of Burn Care and Research 2009;30(1):19‐29. [DOI] [PubMed] [Google Scholar]
O'Meara 2001
- O’Meara SM, Cullum NA, Majid M, Sheldon TA. Systematic review of antimicrobial agents used for chronic wounds. British Journal of Surgery 2001;88:4‐21. [DOI] [PubMed] [Google Scholar]
O'Meara 2014
- O’Meara S, Al‐Kurdi D, Ologun Y, Ovington LG, Martyn‐St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD003557.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pecoraro 1990
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care 1990;13:513‐21. [DOI] [PubMed] [Google Scholar]
Peters 2012
- Peters EJ, Lipsky BA, Berendt AR, Embil JM, Lavery LA, Senneville E, et al. A systematic review of the effectiveness of interventions in the management of infection in the diabetic foot. Diabetes/Metabolism Research and Reviews 2012;28(1 Suppl):142‐62. [DOI] [PubMed] [Google Scholar]
Peters 2016
- Peters EJ, Lipsky BA, Aragón‐Sánchez J, Boyko EJ, Diggle M, Embil JM, et al. International Working Group on the Diabetic Foot (IWGDF). Interventions in the management of infection in the foot in diabetes: a systematic review. Diabetes/Metabolism Research and Reviews 2016;32(1 Suppl):145‐53. [DOI] [PubMed] [Google Scholar]
Prompers 2007
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007;50(1):18‐25. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhoads 2008
- Rhoads DD, Wolcott RD, Percival SL. Biofilms in wounds: management strategies. Journal of Wound Care 2008;17:502‐8. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Selva Olid 2015
- Selva Olid A, Solà I, Barajas‐Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD009061.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2015
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html (accessed 8 July 2016).
Singh 2005
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293(2):217‐28. [DOI] [PubMed] [Google Scholar]
Vermeulen 2007
- Vermeulen H, Hattem JM, Storm‐Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005486.pub2] [DOI] [PubMed] [Google Scholar]
von Eiff 2006
- Eiff C, Peters G, Becker K. The small colony variant (SCV) concept: the role of staphylococcal SCVs in persistent infections. Injury 2006;37(2 Suppl):S26‐33. [DOI] [PubMed] [Google Scholar]
White 2006
- White RJ, Cutting K, Kingsley A. Topical antimicrobials in the control of wound bioburden. Ostomy/Wound Management 2006;52(8):26‐58. [PubMed] [Google Scholar]
References to other published versions of this review
Lipsky 2014
- Lipsky BA, Hoey C, Cruciani M, Mengoli C. Topical antimicrobial agents for preventing and treating foot infections in people with diabetes. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD011038] [DOI] [PMC free article] [PubMed] [Google Scholar]


