Abstract
Background
Myofascial pain (MP) is a painful condition characterized by pain transmitted from trigger points (TP) within myofascial structures (in the muscles), local or distant from the pain. TPs can produce a characteristic pattern of irradiated pain or autonomic symptoms when stimulated. Cyclobenzaprine, a muscle relaxant that suppresses muscle spasm without interfering with muscle function, is used in clinical management of MP to improve quality of sleep and reduce pain.
Objectives
To assess efficacy and safety of cyclobenzaprine in treating MP.
Search methods
The Pain Palliative and Supportive Care Review Group's Specialised Register, CENTRAL, PubMed, EMBASE, LILACS and Scielo were searched in February 2009.
Selection criteria
All RCTs and quasi‐RCTs reporting use of cyclobenzaprine for treating MP with pain assessment as a primary or secondary outcome.
Data collection and analysis
Two review authors independently screened studies identified, extracted data, assessed trial quality and analyzed results.
Main results
We identified two studies with a total of 79 participants. One study, with 41 participants, compared cyclobenzaprine with clonazepam and with placebo. Participants taking cyclobenzaprine had some improvement of pain intensity compared to those on clonazepam, mean difference (MD) ‐0.25 (95% CI, ‐0.41 to ‐0.09; P value 0.002) and placebo, MD ‐0.25 (95% CI, 0.41 to ‐0.09; P value 0.002). The other study, with 38 participants, compared cyclobenzaprine with lidocaine infiltration. Thirty days after treatment there were statistically non‐significant differences between comparison groups, favoring lidocaine infiltration, for the mean for global pain, MD 0.90 (95% CI ‐0.35 to 2.15, P value 0.16), and for the mean for pain at digital compression, MD 0.60 (95% CI ‐0.55 to 1.75, P value 0.30). There were no life‐threatening adverse events associated with the medications.
Authors' conclusions
There was insufficient evidence to support the use of cyclobenzaprine in the treatment of MP. We identified only two small studies in which a total of 35 participants were given cyclobenzaprine, and it was not possible to estimate risks for benefits or harms. Further high quality RCTs of cyclobenzaprine for treating MP need to be conducted before firm conclusions on its effectiveness and safety can be made. Experts in this area should elect cut‐off points for participants to identify whether a patient has achieved a clinically relevant reduction of pain (primary outcome), so that their results can be combined easily into future versions of this review.
Plain language summary
Cyclobenzaprine drug treatment for myofascial pain in adults
Myofascial pain (MP) is a painful condition of the muscles characterized by pain transmitted from trigger points (TP) within connective tissue surrounding and separating muscles (myofascial structures). TP can be located where the pain is felt, or can be at a distance from it. Cyclobenzaprine, one of the drugs used to treat MP, is taken as a pill. It is a muscle relaxant, particularly used to improve quality of sleep and to reduce pain. It suppresses muscle spasms ‐ and so may prevent pain caused by MP ‐ without interfering with muscle function. The purpose of this review was to assess how effective cyclobenzaprine is at reducing pain and improving sleep in patients with MP. We searched extensively through scientific publications and found two trials, with a total of 79 participants. These tested cyclobenzaprine against another drug called clonazepam, and fake medication (placebo), or against injections of a local anesthetic called lidocaine. A total of 35 of the 79 participants in the two trials were given cyclobenzaprine. Cyclobenzaprine was slightly better than clonazepam and placebo at reducing jaw pain, but was no better at improving sleep quality. The results from the other trial were not scientifically reliable because of the small number of participants involved, but lidocaine injections seemed to reduce pain slightly better than cyclobenzaprine pills. Despite this result, it is likely that, because it is uncomfortable to receive any form of injection, people who suffer from MP will prefer to be treated with cyclobenzaprine pills. There were no life‐threatening adverse events associated with any of the medications studied. Further studies are needed to show whether cyclobenzaprine really works for treating MP, but at the moment doctors cannot say whether it is really useful.
Background
Myofascial pain (MP) is a painful condition of the muscles characterized by pain transmitted from trigger points (TP) within myofascial structures (connective tissue surrounding and separating muscles). TP can be local to or distant from the pain (Fricton 1985; Fricton 1989; Manfredini 2006; Okeson 1998; Okeson 2006; Solberg 1986; Travell 1952). When stimulated, TP can produce a characteristic pattern of irradiated pain or autonomic symptoms (Fricton 1989; Okeson 1998; Solberg 1986). The pain can occur at rest or during function, and may be accompanied by muscle spasm. MP was initially described in 1952, although odontological and medical communities were very slow to understand it (Travell 1952). Physical examination of patients, and consideration of their medical history is required to make a diagnosis of MP, as well as the identification of the patients painful points by using digital palpation (Fricton 1985; Gerwin 1995). The face and jaw can be particularly affected by MP, and, in such temporomandibular disorders, MP can be associated with limitation of the mandibular opening (Dworkin 1992; Manfredini 2006; Okeson 2006). There are many diagnostic systems for temporomandibular disorders (TMD) of which MP is a part. Indeed, the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) was created to provide classification criteria for the condition that are universally accepted and validated. A diagnosis of MP by the RDC/TMD requires pain to be reported by the subject in response to palpation of three or more sites of the masticatory muscles (De Lucena 2006; Dworkin 1992; Manfredini 2006). Studies of prevalence show different results between Americans, Europeans and Asian populations for MP; these studies indicate that MP can occur in 21% to 93% of patients who complain of regional pain. MP shows an unusual distribution in the general population, with a predominance in females, and an age of onset ranging between 20 and 40 years (Fishbain 1986; Gerwin 1995; Graff‐Radford 1984; List 1996; List 1999; Manfredini 2006; Reitinger 1996; Schiffman 1990; Yap 2003).
The etiology (cause) of MP is complex and it is difficult to be specific about all etiological factors, however, some authors have described local (restricted to one part) and systemic (whole body) factors that seem to be associated, including: trauma, stress, emotional tension, deep pain impulse, hypovitaminosis (lack of one or more vitamins), infections, fatigue, and patients who are physically inactive and in a weak physical condition (Laskin 1969; Okeson 2006; Simons 2005).
Treatment options for MP include reassurance (patient education, self care and behavior therapy), physiotherapy (ultrasound, megapulse, short wave laser, heat exercises, biofeedback), acupuncture, splint therapy, occlusal adjustment of the teeth, dry needling (infiltration of the needle making movements, different of acupuncture), drug therapy and combined treatment (Al‐Ani 2005; Koh 2003; McMillan 1997; Shi 2003; Solberg 1986). Medicines used to treat MP include analgesics, non‐steroidal anti‐inflammatory drugs (NSAIDS), muscle relaxants and tricyclic antidepressants such as amitriptyline (Al‐Ani 2005; Koh 2003; Shi 2003).
Cyclobenzaprine, a muscle relaxant, is a centrally‐acting serotonin receptor antagonist that reduces muscle tone by inhibiting serotonergic descending systems in the spinal cord (Katz 1988; Kobayashi 1996). It suppresses muscle spasms without interfering with muscle function (Basmajian 1978; Lance 1972). Cyclobenzaprine is structurally related to amitriptyline. In fact, it was initially produced as an antidepressant, and tested in clinical trials for antidepressant activity at doses above those used now for muscle relaxation (De Lee 1980). The similarity to amitriptyline has raised concerns about side effects such as drowsiness, lethargy, sinus tachycardia (rapid heartbeat), agitation, and both hypertension and hypotension (Turturro 2003), but a five‐year multicenter review of its toxicity showed that cyclobenzaprine does not appear to produce life threatening cardiovascular and neurological effects (Kobayashi 1996). Also, in some trials, the incidence of troublesome side effects appears to be lower than that documented with amitriptyline (Lance 1964; Lance 1972). At present, cyclobenzaprine is used in the clinical management of MP in temporomandibular disorders to improve the quality of sleep and to reduce pain (Pertes 2005; Tofferi 2004), but its effectiveness has not been summarized in a systematic review.
Objectives
To assess the efficacy and safety of cyclobenzaprine in the treatment of myofascial pain.
To test the null hypothesis that there are no differences in outcomes between cyclobenzaprine versus other active treatments for treating myofascial pain.
We also sought to identify any adverse effects related to the treatment.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) and quasi‐randomized controlled trials (quasi‐RCTs) that fulfilled the criteria outlined below were included.
Types of participants
Participants of both sexes, aged over 18 years with a clinical diagnosis of MP, regardless of race, social and economical status, profession or residential location.
We excluded participants for whom cyclobenzaprine was contraindicated with conditions such as: heart problems, glaucoma, hyperthyroidism, and those who were pregnant or breastfeeding.
Types of interventions
Intervention group: cyclobenzaprine in any dose by any route. Control group: placebo, no intervention, physiotherapy, or other control.
Types of outcome measures
Primary outcomes
Intensity, frequency and duration of pain crises recorded using validated visual analogue scales (VAS) or categorical scales (Huskisson 1983).
Secondary outcomes
Sleep quality measured by the Pittsburg Sleep Quality Index (PSQI) (Buysse 1989; Melzack 1987).
Adverse events, such as drowsiness, xerostomia (dryness of the mouth), pain and swelling after infiltration, lethargy, sinus tachycardia, agitation, hypertension and hypotension.
Quality of life measured by OHIP (Slade 1994).
Search methods for identification of studies
Electronic searches
There were no language restrictions. To identify studies we searched the Pain Palliative and Supportive Care Review Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2009, Issue 1), PubMed (1966 to February 2009), EMBASE (1980 to February 2009), Literatura Latino‐Americana e do Caribe em Ciências da Saúde ‐ LILACS (1982 to February 2009), and the Scientific Electronic Library Online ‐ Scielo (to February 2009) to identify RCTs and quasi‐RCTs.
The search strategy was composed of terms for myofascial pain and cyclobenzaprine. As we searched with both subject headings and free text words, we expected to identify all studies of MP and cyclobenzaprine. The exhaustive list of synonyms for MP and cyclobenzaprine that we used can be seen in Appendix 2. Please see Appendix 3 for other bibliographic search strategies.
Searching other resources
Reference lists of the included studies were checked manually to identify any additional studies.
We contacted specialists in the field and authors of the included studies for information about unpublished data.
Data collection and analysis
Two review authors (FMGL, EJ) independently screened the studies identified by the literature search, extracted the data, assessed trial quality and analyzed the results. Other review authors were consulted when there was any disagreement or need for quality assessment of the process. If consensus was not reached, data from the studies in question were not included. The selection of titles and the methodological quality of the RCTs and quasi‐RCTs were assessed by two review authors (FMGL, EJ), using the kappa test.
Quality assessment
The methodological quality of the included studies in this review were measured using the Cochrane criteria described in The Cochrane Handbook (Higgins 2006), since scales and checklists are not a reliable method to assess the validity of a primary study (Jüni 1999).
Selection bias
The following questions were asked of each study identified.
-
Was the allocation sequence adequately generated?
Yes: adequate generation of allocation.
Unclear: not described, evaluator's blinding not reported, and not possible to obtain details from the authors of the primary study.
No: inadequate generation of allocation (i.e. alternate, sequential, by birth date, etc.)
-
Was allocation adequately concealed?
Yes: adequate concealment of allocation.
Unclear: method of allocation concealment not described, and not possible to obtain details through contact with the authors of the primary study.
No: inadequate allocation concealment, or allocation concealment not used.
Detection bias
-
Was there a blinded assessment of outcomes?
Yes: assessors unaware of the assigned treatment when collecting outcome measures.
Unclear: blinding of assessor not reported and could not be verified by contacting investigators.
No: assessors aware of the assigned treatment when collecting outcome measures.
Attrition bias
-
Were withdrawals described?
Yes: less than or equal to 20% for both groups.
Unclear: not reported in paper or by authors.
No: greater than or equal to 20% for both comparison groups.
Data extraction
Two review authors (FMGL, EJ) extracted data independently. There were no discrepancies between data extractors. A standard data extraction form was used to extract the following information: characteristics of the study (design, methods of randomization); participants; interventions; outcomes (types of outcome measures, timing of outcomes pain scores ‐ VAS, total pain scale etc, adverse events). The form was based on the recommendations made by the Pain, Palliative and Supportive Care Review Group and was used as a first step in the process of data extraction.
Data analysis
For dichotomous data, we had planned to use relative risk (RR) as the effect measure, but did not find dichotomous outcomes in the included studies. For continuous data, we used mean difference (MD).
When we found standard error of meaning (SEM) we changed it to Standard Deviation (SD), as appropriate.
We had planned to use intention‐to‐treat (ITT) analysis in instances where primary studies reported their findings as dichotomous data. Participants who dropped out of trials would be assumed to be non‐responders (Unnebrink 2001). ITT analysis will be used in future versions of this systematic review, where appropriate.
Heterogeneity
Inconsistency among the pooled estimates was quantified using the I2 statistic (Higgins 2003; Higgins 2006). We used a fixed‐effect model in the absence of significant heterogeneity and a random‐effects model if heterogeneity were found (DerSimonian 1986).
Subgroup analysis
Subgroup by type of intervention, dosage, follow‐up period, age and sex were planned. Subgroup analysis will be used in future versions of this systematic review, where appropriate.
Sensitivity analysis
If there were an adequate number of studies, a sensitivity analysis would be performed to explore causes of heterogeneity and the robustness of the results. Sensitivity analyses would evaluate the following: quality of allocation concealment (adequate, or unclear, or inadequate); method of double‐blinding (adequate, or unclear, or inadequate, or not performed); rates of withdrawal for each outcome; repeating the analysis excluding unpublished studies; and different study design (parallel versus cross‐over). In future versions of this systematic review, sensitivity analysis will be performed if we have an adequate number of studies.
Results
Description of studies
See 'Characteristics of included studies' table for full details of the studies included.
The search strategy identified two studies, with a total of 79 participants with MP, that were suitable for inclusion. Both studies used different diagnostic criteria. Furtado 2002 used the diagnostic criteria defined by Simons 2005 and Travell 1952, whilst Herman 2002 used those from the RDC/TMD (Dworkin 1992).
Herman 2002 included 41 participants (33 female and eight male; mean age of 27 years) with MP as defined for axis I group 1 of the RDC/TMD. One group (15 participants) received 10 mg of oral cyclobenzaprine daily, the second group (13 participants) received 0.5 mg of oral clonazepam daily, while the third group (15 participants) received placebo (lactose filler). Jaw pain intensity, sleep quality and side effects were assessed at baseline and after three weeks. While jaw pain intensity and sleep quality were measured by mean difference (endpoint and change from baseline), side effects were reported by number of participants presenting with adverse events, irrespective of type.
Furtado 2002 included 38 participants (35 female and three male; mean age of 35 years) with trapezius myofascial syndrome (shoulder pain). One group (20 participants) received 10 mg of oral cyclobenzaprine for 15 days, while the other group (18 participants) received infiltration (injection) of lidocaine (xylocaine) 1% (a local anesthetic) in a maximum of six trigger‐points in the trapezius (a shoulder muscle) in a single session. Global pain, pain at digital compression and side effects were assessed at baseline, seven, 15 and 30 days after start of treatment. While global pain and pain at digital compression were measured by mean difference (endpoint) between comparison groups, side effects were reported by number of participants presenting with adverse events, irrespective of type.
Risk of bias in included studies
Both studies included in the review were described as randomized. Allocation concealment was adequate, and the methods of allocation concealment were described. Herman 2002 generated randomization by means of a randomized block design, with the blocking‐variable being the current use of psychotropic medication. Furtado 2002 generated randomization by means of a draw. Furtado 2002 could not withhold knowledge of the allocated interventions, as one group received infiltration and the other received tablets. Consequently, for this trial, performance bias was not considered, since the nature of the interventions was not compatible with this element of risk of bias. Both studies had no substantial dropouts/withdrawals in the sample as a whole, or substantial differences between comparison groups. Assessors were unaware of the assigned treatment when collecting outcome measures. The study reports were free of suggestion of selective outcome reporting, and were apparently free of features that could put them at a high risk of bias. For more details about each of the included studies, see the 'Characteristics of included studies' table.
Effects of interventions
1. Cyclobenzaprine versus clonazepam (Herman trial)
Mean for jaw pain intensity upon awakening (endpoint)
There was a statistically non‐significant difference between comparison groups for the mean for jaw pain intensity upon awakening (measured at endpoint), that favored cyclobenzaprine, as shown by the MD ‐0.11 (95% CI, ‐0.26 to 0.04; P value 0.15).
Mean for jaw pain intensity upon awakening (change from baseline)
The mean change from baseline between comparison groups for jaw pain intensity upon awakening was statistically significant, and favored cyclobenzaprine, as shown by MD ‐0.25 (95% CI, ‐0.41 to ‐0.09; P value 0.002).
Sleep quality (PSQI ‐ endpoint)
There was a statistically non‐significant difference between comparison groups for sleep quality (measured by the PSQ Index at endpoint), that favored clonazepam, as shown by MD 0.84 (95% CI, ‐1.35 to 3.03; P value 0.45).
Sleep quality (PSQI ‐ change from baseline)
There was a statistically non‐significant difference between comparison groups for the mean change from baseline for sleep quality (measured by the PSQ index), that favored cyclobenzaprine, as shown by MD ‐1.53 (95% CI, ‐3.50 to 0.44; P value 0.13).
Side effects (irrespective of type)
There was a statistically non‐significant difference between the comparison groups for the number of participants who reported side effects, that favored cyclobenzaprine, as shown by RR 1.60 (95% CI, 0.71 to 3.60; P value 0.26).
Quality of life
Neither of the included studies included quality of life as an outcome.
2. Cyclobenzaprine versus placebo (Herman trial)
Mean for jaw pain intensity upon awakening (endpoint)
There was a statistically non‐significant difference between comparison groups for mean jaw pain intensity upon awakening (measured at endpoint), that favored cyclobenzaprine, as shown by MD ‐0.13 (95% CI, ‐0.27 to 0.01; P value 0.07).
Mean for jaw pain intensity upon awakening (change from baseline)
The mean change from baseline between comparison groups for jaw pain intensity upon awakening was statistically significant, and favored cyclobenzaprine, as shown by MD ‐0.25 (95% CI, 0.41 to ‐0.09; P value 0.002).
Sleep quality (PSQI ‐ endpoint)
There was a statistically non‐significant difference between comparison groups for sleep quality (measured by the PSQ Index at endpoint), that favored cyclobenzaprine, as shown by MD ‐0.48 (95% CI, ‐2.27 to 1.31; P value 0.60).
Sleep quality (PSQI ‐ change from baseline)
There was a statistically non‐significant difference between comparison groups for the mean change from baseline for sleep quality (measured by the PSQ index), that favored cyclobenzaprine, as shown by MD ‐0.95 (95% CI, ‐2.89 to 0.99; P value 0.34).
Side effects (irrespective of type)
There was a statistically non‐significant difference between the comparison groups for the number of participants who reported side effects, that favored placebo, as shown by RR 3.08 (95% CI, 1.02 to 9.24; P value 0.05).
Quality of life
Neither of the included studies reported this outcome.
3. Cyclobenzaprine versus lidocaine infiltration (Furtado trial)
Mean for global pain after seven days (T7)
There was a statistically non‐significant difference between comparison groups for the mean for global pain (measured seven days after the intervention), that favored lidocaine infiltration, as shown by MD 0.90 (95% CI, ‐0.70 to 2.50; P value 0.27).
Mean for global pain after 15 days (T15)
There was a statistically non‐significant difference between comparison groups for the mean for global pain (measured 15 days after the intervention), that favored lidocaine infiltration, as shown by MD 0.50 (95% CI, ‐0.84 to 1.84; P value 0.46).
Mean for global pain after 30 days (T30)
There was a statistically non‐significant difference between comparison groups for the mean for global pain (measured 30 days after the intervention), that favored lidocaine infiltration, as shown by MD 0.90 (95% CI, ‐0.35 to 2.15; P value 0.16).
Mean pain at digital compression after seven days (T7)
There was a statistically non‐significant difference between comparison groups for the mean for pain at digital compression (measured seven days after the intervention), that favored lidocaine infiltration, as shown by MD 0.40 (95% CI, ‐1.06 to 1.86; P value 0.27).
Mean pain at digital compression after 15 days (T15)
There was a statistically non‐significant difference between comparison groups for the mean for pain at digital compression (measured 15 days after the intervention), that favored cyclobenzaprine, as shown by MD ‐0.10 (95% CI, ‐1.38 to 1.18; P value 0.88).
Mean pain at digital compression after 30 days (T30)
There was a statistically non‐significant difference between comparison groups for the mean for pain at digital compression (measured 30 days after the intervention), that favored lidocaine infiltration, as shown by MD 0.60 (95% CI, ‐0.55 to 1.75; P value 0.30).
Side effects (irrespective of type)
There was a statistically non‐significant difference between comparison groups for side effects, that favored lidocaine infiltration, as shown by RR 1.39 (95% CI, 0.92 to 2.10; P value 0.12).
Quality of life
None of the included studies reported this outcome.
NNT and NNH measures
It was not possible to calculate either NNT (number‐needed‐to‐treat‐to‐benefit) or NNH (number‐needed‐to‐treat‐to‐harm), since neither of the primary studies used dichotomous data.
Discussion
Despite the prevalence of MP, and the widespread use of cyclobenzaprine, searches for evidence revealed only two RCTs that evaluated the drug for MP. The internal and external validity of the evidence provided by these studies was limited by sample sizes that were too small to detect real differences between cyclobenzaprine, clonazepam and lidocaine infiltration in people with different profiles (age range, co‐morbidities, etc). The other methodological limitation was that each trial reported outcomes in different ways. Moreover, both studies included in this review used different diagnostic criteria for MP.
Although nearly all the results from the studies included in this review were statistically non‐significant, generally there was a slight improvement in the participants treated with cyclobenzaprine compared to clonazepam. Although comparison of cyclobenzaprine with lidocaine produced no statistically significant results, but generally favored lidocaine, it may, nonetheless, be sensible to use cyclobenzaprine as the therapeutic option, because of the invasive nature of lidocaine infiltration. Moreover, the small amount of evidence available showed cyclobenzaprine to be clinically and statistically similar to placebo and clonazepam with regard to safety data (e.g. morning drowsiness, dry mouth, nightmares, headache, pain and swelling), but it is important to state that neither cyclobenzaprine nor clonazepam are indicated primarily for use in sleep disturbances, where a higher dose may be required to be effective, as the dosages specified for the study were intended to combat pain.
Fair‐quality studies corroborate the results of this review (Basmajian 1978; De Lee 1980; Katz 1988; Tofferi 2004; Turturro 2003). Thus, there is evidence that medications that help in muscle relaxation may be effective for treating the pain of myogenic origin, and also that medications that do not allow muscle relaxation, may not offer adequate pain relief (Basmajian 1978). For example, cyclobenzaprine has been shown to be highly effective and well tolerated for the relief of muscle spasm, when compared to placebo and other drugs in trials, for patients with traumatic strains of the neck and lower back (De Lee 1980; Katz 1988). Despite this, a short‐term and well‐conducted study (Turturro 2003), found no difference in pain relief provided by a combination of cyclobenzaprine and ibuprofen versus ibuprofen and placebo, although this study confirmed the higher prevalence of side effects in patients receiving cyclobenzaprine. In another trial, patients with chronic musculoskeletal pain who were treated with cyclobenzaprine, reported overall improvement and moderate reductions in individual symptoms, particularly with regard to sleep (Tofferi 2004).
In agreement with the studies included in this review and previous studies, we would like to highlight the importance of a home‐care regimen and patient education (cognitive‐behaviour therapies) as another option to manage patients with MP (Al‐Ani 2005; Herman 2002).
Future studies should elect cut‐off points for participants involved to assume that a patient has or has not achieved a clinically relevant reduction of pain (primary outcome). In this way, their results can be more easily combined in future versions of this review as it will be easier to calculate sample sizes. Moreover, we would recommend that the authors of future trials compare cyclobenzaprine with other available treatment options, on its own or in combination with them (e.g. patient education, ibuprofen, clonazepam, etc).
Authors' conclusions
Implications for practice.
The limited available evidence, involving a total of 79 participants in two studies, suggests that cyclobenzaprine is similar to clonazepam and placebo for effectiveness and safety. Lidocaine infiltration showed mild superiority (not statistically significant) when compared to cyclobenzaprine, although it was associated with a higher ‐ but not significant ‐ number of adverse effects (pain and swelling). It is probable that the invasive characteristics of the infiltration procedure would encourage the use of cyclobenzaprine. In these circumstances, there is insufficient evidence either to support or refute the use of cyclobenzaprine for the treatment of MP.
Implications for research.
The two included studies provided positive, but weak, evidence for using cyclobenzaprine to treat MP. Some methodologic flaws influenced validity and reproducibility of the conclusion. In addition, different diagnostic criteria were used in the included clinical studies. Therefore, more RCTs, especially multicenter trials with sufficient sample sizes, and more than one diagnostic criterion (in order to increase the external validity of their findings (applicability)) should be performed. Moreover, the diversity of ways of reporting estimate effects should be standardized by using clinically relevant and objective outcome variables, including quality of life. These are needed to establish cyclobenzaprine's true therapeutic effects, and, if that is shown, further studies would be required to establish the best dose and type of use of the drug.
What's new
| Date | Event | Description |
|---|---|---|
| 5 June 2017 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 18 April 2012 | Amended | The plain language summary in this review was corrected and the risk of bias tables were brought up to date for this amendment. |
| 8 February 2011 | Amended | Contact details updated. |
| 9 November 2009 | Amended | Contact details updated. |
| 12 August 2009 | Amended | Contact details updated. |
| 14 March 2008 | Amended | Converted to new review format. |
| 12 February 2008 | Amended | The protocol has been updated in order to broaden the scope. The use of cyclobenzaprine for the treatment of myofascial pain in temporomandibular disorders will now be analyzed as a subgroup. |
Notes
We performed a full search in May 2015 and August 2016 intending to complete a full update, but we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
Jessica Thomas for her help during our work.
Sylvia Bickley, PaPas Trials Search Co‐ordinator for her comments on the search strategy presented in the protocol.
Consumer referee comments: Jayne Harrison, Anne Peticolas and Amy Zelmer.
Peer referee comments: Helen Worthington, Michael A Turturro, Andrew Moore.
Appendices
Appendix 1. Answer by Author of Furtado 2002
| De: | "Rita Furtado" |
| Para: | "FREDERICO MOTA" |
| Assunto: | RE: Revisão Ciclobenzaprina |
| Data: | Wed, 27 Feb 2008 01:59:18 +0000 |
Olá Frederico, a minha gestação está nas últimas 4 semanas e estou pela "bola sete", louca para que acabe logo, risos... Quanto á randomização, foi feita por sorteio com sigilo de alocação. Foram colocados 40 papéis (metade ciclo, metade infiltração) em uma grande caixa e esses papéis iam sendo retirados conforme os pacientes iam sendo captados. Não foi realizada através de tábua de randomização. Um abraço, Rita.
Translation:
Dear Frederico,
My pregnancy is in the last 4 weeks. As regards to the randomization, it was done by draw with concealment of allocation. There were 40 papers (half with infiltration, half with cyclobenzaprine) in a big box and these papers were taken as the patients were captured. There was not performed board of randomization. Yours, Rita
| De: | "FREDERICO MOTA" |
| Para: | "Rita Furtado" |
| Assunto: | RE: Revisão Ciclobenzaprina |
| Data: | Mon, 25 Feb 2008 23:57:37 ‐0300 |
Prezada Rita,
Tudo bem?
E o neném? Já está crescendo né?
Estou incluindo os dados de seu estudo em minha revisão sistemática, porém, preciso saber como foi feita a randomização para a criação dos dois grupos. Seria possível vc me informar?
Aguardo seu contato.
Devo precisar de outras informações mas somente no decorrer do trabalho.
Grande abraço e mais uma vez obrigado.
Frederico
Translation:
Dear Rita,
How is everything?
I am including the data of your study in my systematic review, but, I need to know how was the randomization done for the creation of the two groups.
I should probably need further information during the work.
With kind regards,
Frederico
Appendix 2. Basic search strategy
((Myofascial Pain Syndromes) OR (Myofascial Pain Syndrome) OR (Myofascial Trigger PointS) OR (Myofascial Trigger Point)) AND (cyclobenzaprine OR (cyclobenzaprine hydrochloride) OR Flexeril OR Lisseril OR Dibenzocycloheptenes OR Propylamines OR (Tricyclic Antidepressive Agents) OR (Central Muscle Relaxants) OR (Tranquilizing Agents) OR Miosan OR Mirtax OR Fexmid OR Flexeril OR Flexiban OR Flexitec OR Gen‐Cyclobenzaprine OR Masterelax OR Medarex OR Med Cyclobenzaprine OR Miosan OR Mirtax OR Nostaden OR Novo‐Cycloprine OR Amrix OR Apo‐Cyclobenzaprine OR Ciclamil OR Ratio‐Cyclobenzaprine OR Reflexan OR Relexil OR Riva‐Cycloprine OR Tensamox OR Tensiomax OR Tensodox OR Tonalgen OR Yuredol OR Yurelax)
Appendix 3. Search strategy for other databases
| PUBMED | EMBASE | LILACS | CENTRAL |
| #1 MYOFASCIAL PAIN SYNDROMES [MeSH] #2 ("myofascial pain") #3 (myofascial AND ("trigger point" or trigger‐point*)) #4 #1 or #2 or #3 #5 (cyclobenzaprine OR "cyclobenzaprine hydrochloride" OR Aripiprazole OR "Dorixina relax" OR Flexeril OR Lisseril OR Miosan OR Mirtax OR Fexmid OR Flexeril OR Flexiban OR Flexitec OR Gen‐Cyclobenzaprine OR Masterelax OR Medarex OR Med Cyclobenzaprine OR Miosan OR Mirtax OR Nostaden OR Novo‐Cycloprine OR Amrix OR Apo‐Cyclobenzaprine OR Ciclamil OR Ratio‐Cyclobenzaprine OR Reflexan OR Relexil OR Riva‐Cycloprine OR Tensamon OR Tensamox OR Tensiomax OR Tensodox OR Tonalgen OR Yuredol OR Yurelax OR Ziclob)) #6 #4 AND #5 | 1. MYOFASCIAL PAIN SYNDROME [EMTREE term] 2. ("myofascial pain") 3. (myofascial AND ("trigger point$" or trigger‐point$)) 4.OR/1‐3 5. (cyclobenzaprine OR "cyclobenzaprine hydrochloride" OR Aripiprazole OR "Dorixina relax" OR Flexeril OR Lisseril OR Dibenzocycloheptenes OR Miosan OR Mirtax OR Fexmid OR Flexeril OR Flexiban OR Flexitec OR Gen‐Cyclobenzaprine OR Masterelax OR Medarex OR Med Cyclobenzaprine OR Miosan OR Mirtax OR Nostaden OR Novo‐Cycloprine OR Amrix OR Apo‐Cyclobenzaprine OR Ciclamil OR Ratio‐Cyclobenzaprine OR Reflexan OR Relexil OR Riva‐Cycloprine OR Tensamon OR Tensamox OR Tensiomax OR Tensodox OR Tonalgen OR Yuredol OR Yurelax OR Ziclob) 6. 4 AND 5 | (Mh random allocation) OR (Mh double blind method) OR (Mh single blind method) AND NOT (Ct animal) AND NOT (Ct human and Ct animal) OR (Pt clinical trial) OR (Ex E05.318.760.535$) OR (Tw clin$) AND (Tw trial$) OR (Tw ensa$) OR (Tw estud$) OR (Tw experim$) OR (Tw investiga$) OR (Tw singl$) OR (Tw simple$) OR (Tw doubl$) OR (Tw doble$) OR (Tw duplo$) OR (Tw trebl$) OR (Tw trip$) AND (Tw blind$) OR (Tw cego$) OR (Tw ciego$) OR (Tw mask$) OR (Tw mascar$) OR (Mh placebos) OR (Tw placebo$) OR (Tw random$) OR (Tw randon$) OR (Tw casual$) OR (Tw acaso$) OR (Tw azar) OR (Tw aleator$) OR (Mh research design) AND NOT (Ct animal) AND NOT (Ct human and Ct animal) OR (Ct comparative study) OR (Ex E05.337$) OR (Mh follow‐up studies) OR (Mh prospective studies) OR (Tw control$) OR (Tw prospectiv$) OR (Tw volunt$) OR (Tw volunteer$) AND NOT ((Ct animal) AND NOT (Ct human and Ct animal)) | #1 Exp MYOFASCIAL PAIN SYNDROMES #2 ("myofascial pain") #3 (myofascial AND ("trigger point" or trigger‐point*)) #4 #1 or #2 or #3 #5 (cyclobenzaprine OR "cyclobenzaprine hydrochloride" OR Aripiprazole OR "Dorixina relax" OR Flexeril OR Lisseril OR Dibenzocycloheptenes OR Miosan OR Mirtax OR Fexmid OR Flexeril OR Flexiban OR Flexitec OR Gen‐Cyclobenzaprine OR Masterelax OR Medarex OR Med Cyclobenzaprine OR Miosan OR Mirtax OR Nostaden OR Novo‐Cycloprine OR Amrix OR Apo‐Cyclobenzaprine OR Ciclamil OR Ratio‐Cyclobenzaprine OR Reflexan OR Relexil OR Riva‐Cycloprine OR Tensamon OR Tensamox OR Tensiomax OR Tensodox OR Tonalgen OR Yuredol OR Yurelax OR Ziclob) #6 #4 AND #5 |
Data and analyses
Comparison 1. Cyclobenzaprine versus clonazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean for jaw pain intensity upon awakening (endpoint) | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.26, 0.04] |
| 2 Mean for jaw pain intensity upon awakening (change from baseline) | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.41, ‐0.09] |
| 3 Sleep quality (PSQI ‐ endpoint) | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐1.35, 3.03] |
| 4 Sleep quality (PSQI ‐ change from baseline) | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐3.50, 0.44] |
| 5 Side effects (irrespective of type) | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.71, 3.60] |
1.1. Analysis.
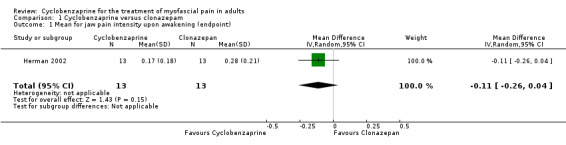
Comparison 1 Cyclobenzaprine versus clonazepam, Outcome 1 Mean for jaw pain intensity upon awakening (endpoint).
1.2. Analysis.
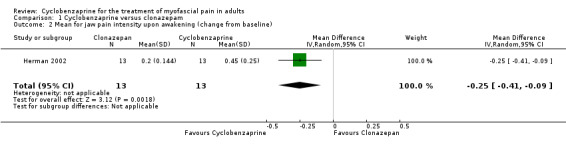
Comparison 1 Cyclobenzaprine versus clonazepam, Outcome 2 Mean for jaw pain intensity upon awakening (change from baseline).
1.3. Analysis.
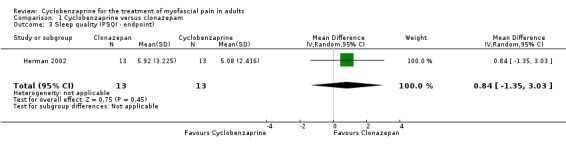
Comparison 1 Cyclobenzaprine versus clonazepam, Outcome 3 Sleep quality (PSQI ‐ endpoint).
1.4. Analysis.
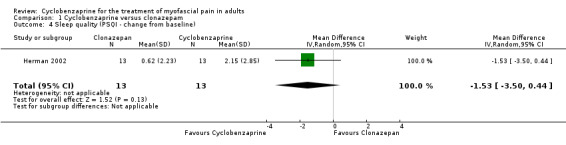
Comparison 1 Cyclobenzaprine versus clonazepam, Outcome 4 Sleep quality (PSQI ‐ change from baseline).
1.5. Analysis.
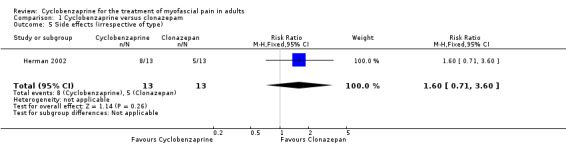
Comparison 1 Cyclobenzaprine versus clonazepam, Outcome 5 Side effects (irrespective of type).
Comparison 2. Cyclobenzaprine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean for jaw pain intensity upon awakening (endpoint) | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.27, 0.01] |
| 2 Mean for jaw pain intensity upon awakening (change from baseline) | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.41, ‐0.09] |
| 3 Sleep quality (PSQI ‐ endpoint) | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐2.27, 1.31] |
| 4 Sleep quality (PSQI ‐ change from baseline) | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐2.89, 0.99] |
| 5 Side effects (irrespective of type) | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [1.02, 9.24] |
2.1. Analysis.
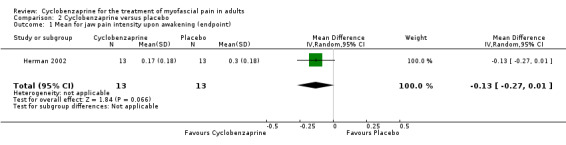
Comparison 2 Cyclobenzaprine versus placebo, Outcome 1 Mean for jaw pain intensity upon awakening (endpoint).
2.2. Analysis.
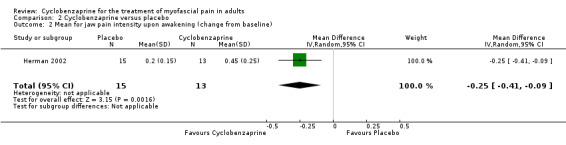
Comparison 2 Cyclobenzaprine versus placebo, Outcome 2 Mean for jaw pain intensity upon awakening (change from baseline).
2.3. Analysis.
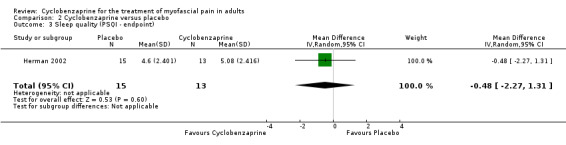
Comparison 2 Cyclobenzaprine versus placebo, Outcome 3 Sleep quality (PSQI ‐ endpoint).
2.4. Analysis.
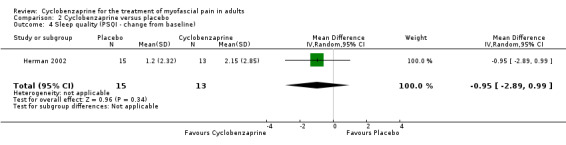
Comparison 2 Cyclobenzaprine versus placebo, Outcome 4 Sleep quality (PSQI ‐ change from baseline).
2.5. Analysis.
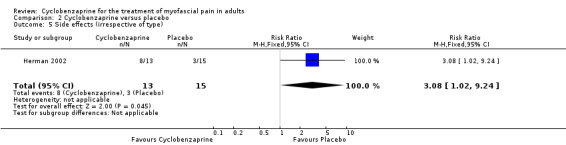
Comparison 2 Cyclobenzaprine versus placebo, Outcome 5 Side effects (irrespective of type).
Comparison 3. Cyclobenzaprine versus lidocaine infiltration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean for global pain ‐ T7 | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.70, 2.50] |
| 2 Mean for global pain ‐ T15 | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.84, 1.84] |
| 3 Mean for global pain ‐ T30 | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.35, 2.15] |
| 4 Mean pain at digital compression ‐ T7 | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐1.06, 1.86] |
| 5 Mean pain at digital compression ‐ T15 | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.38, 1.18] |
| 6 Mean pain at digital compression ‐ T30 | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.55, 1.75] |
| 7 Side effects (irrespective of type) | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.92, 2.10] |
3.1. Analysis.
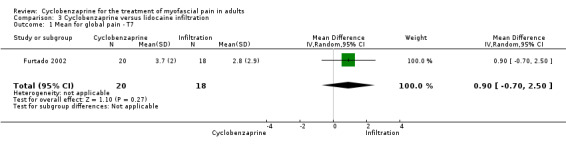
Comparison 3 Cyclobenzaprine versus lidocaine infiltration, Outcome 1 Mean for global pain ‐ T7.
3.2. Analysis.
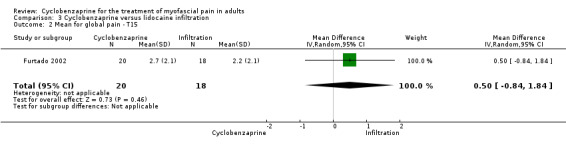
Comparison 3 Cyclobenzaprine versus lidocaine infiltration, Outcome 2 Mean for global pain ‐ T15.
3.3. Analysis.
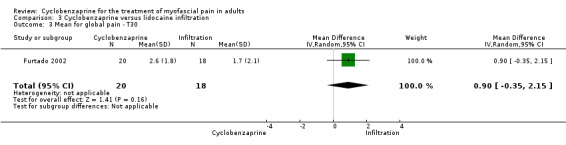
Comparison 3 Cyclobenzaprine versus lidocaine infiltration, Outcome 3 Mean for global pain ‐ T30.
3.4. Analysis.
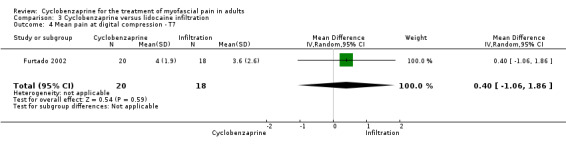
Comparison 3 Cyclobenzaprine versus lidocaine infiltration, Outcome 4 Mean pain at digital compression ‐ T7.
3.5. Analysis.
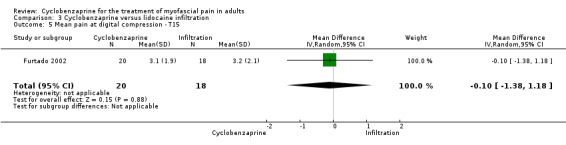
Comparison 3 Cyclobenzaprine versus lidocaine infiltration, Outcome 5 Mean pain at digital compression ‐ T15.
3.6. Analysis.
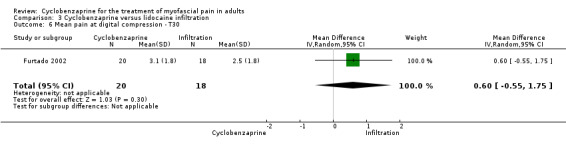
Comparison 3 Cyclobenzaprine versus lidocaine infiltration, Outcome 6 Mean pain at digital compression ‐ T30.
3.7. Analysis.
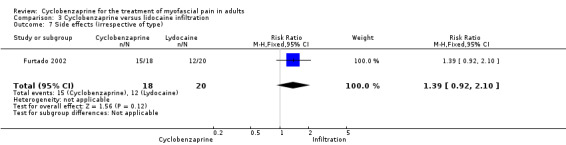
Comparison 3 Cyclobenzaprine versus lidocaine infiltration, Outcome 7 Side effects (irrespective of type).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Furtado 2002.
| Methods | RCT. Randomization: by draw with concealment of allocation. Design: parallel. Blindness: observer blind. Duration: 30 days. Analysis: no ITT. Location: Brazil. |
|
| Participants | Diagnosis: trapezius myofascial syndrome according to Simons & Travell (Travell 1983). Number of participants: 38. Gender: 35 female, 3 male. Mean age: 34.6 years. Setting: clinic of physical medicine and rehabilitation of Universidade Federal de São Paulo. Participants lived at home. |
|
| Interventions | Group 1. Xylocaine (lidocaine): 1% infiltration in a maximum of 6 trigger‐points in the trapezius in a single session (n = 18). Group 2. Cyclobenzaprine: 10 mg at night for 15 days (n = 20). |
|
| Outcomes | A. Global pain score (VAS). B. Pain at digital compression (VAS). C. Side effects: Group 1: xerostomia and drowsiness. Group 2: pain and swelling after infiltration. |
|
| Notes | No dropouts or withdrawals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was generated adequately by draw. |
| Allocation concealment (selection bias) | Low risk | The allocation was concealed adequately. |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no way to prevent the participants from having knowledge of their allocated interventions, as Group 1 received infiltration and Group 2 received tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The reports of the study were free of suggestion of selective outcome reporting. |
| Other bias | Low risk | It appears that the study was free of other problems that could put it at a high risk of bias. |
| Attrition Bias | Low risk | There were no substantial dropouts/withdrawals in the sample as a whole, or substantial differences between comparison groups. |
| Detection Bias | Low risk | Assessors were unaware of the assigned treatment when collecting data on outcome measures. |
Herman 2002.
| Methods | RCT. Randomization: by means of a randomized block design with the blocking variable being the current use of psychotropic medication. Design: parallel. Blindness: double‐blind. Duration: 3 weeks. Analysis: no ITT. Location: United States of America. |
|
| Participants | Diagnosis: myofascial pain as defined for axis I group 1 of the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD). Number of participants: 41. Gender: 33 female and 8 male. Mean age: 27.06 years. Setting: not reported. Participants lived at home. |
|
| Interventions | Group 1. Clonazepam 0.5 mg daily (n = 13). Group 2. Placebo (lactose filler) (n = 15). Group 3. Cyclobenzaprine 10 mg (n = 13). |
|
| Outcomes | A. Jaw‐pain intensity. B. Sleep quality. C. Side effects. |
|
| Notes | No dropouts or withdrawals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial report stated: "Subjects were allocated to their treatment group by means of a randomised block design with the blocking variable being the current use of psychotropic medication" Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | The allocation was concealed adequately by pharmacy‐controlled randomization. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Trial report stated: "The capsules were formulated to have the same appearance, ... Neither the treating doctor nor the subject was aware of the treatment assignment until completion of the intervention". Comment:the allocated interventions were adequately concealed during the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The reports of the study were free of suggestion of selective outcome reporting. |
| Other bias | Low risk | It appears that the study was free of other problems that could put it at a high risk of bias. |
| Attrition Bias | Low risk | Trial report stated: "The final sample consisted of 33 women and 8 men with no subject dropouts or withdrawals". Comment: there were no substantial dropouts/withdrawals in the sample as a whole, or substantial differences between comparisons groups. |
| Detection Bias | Low risk | Trial report stated: "The capsules were formulated to have the same appearance, ... Neither the treating doctor nor the subject was aware of the treatment assignment until completion of the intervention". Comment: assessors were unaware of the assigned treatment when collecting data on outcome measures. |
Abbreviations
VAS = visual analogue scale
Contributions of authors
Conceived the review: Frederico Mota Gonçalves Leite (FMGL) Co‐ordinating the review: Álvaro Nagib Atallah (ANA) Undertaking manual searches and screening results: FMGL and Édina da Silva (EK) Organising retrieval of studies: FMGL and EK Screening retrieved studies: FMGL and EK Appraising quality of studies: FMGL and EK Abstracting data from studies: FMGL, EJ and Regina El Dib (RED) Writing to authors of studies for additional information: FMGL Providing additional data about studies: FMGL, EJ and Eduardo Grossmann (EG) Obtaining and screening data on unpublished studies: FMGL, RED, EK Data management for the review: FMGL Entering data into Review Manager (RevMan 5): FMGL RevMan statistical data: FMGL, Régis Andriolo (RA), RED Other statistical analysis not using RevMan: FMGL, RA and RED Double entry of data: (data entered by one person: FMGL; data entered by second person: EK) Interpretation of data: FMGL, EK, RA and RED Statistical analysis: FMGL, RED and ANA Writing the review: FMGL Performing previous research that was the foundation of the present review: FMGL Guarantor for the review: FMGL Responsible for reading and checking review before submission: FMGL, ANA, EK, RA, EJ and RED Responsible for updating: FMGL, RA, RED
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Furtado 2002 {published data only}
- Furtado RNV, Carazzato S, Farias CA, Chamlian TR, Masiero D. Myofascial syndrome: comparison between infiltration of trigger points treatment and oral medication (cyclobenzaprine) [Síndrome miofascial: comparação entre o tratamento com infiltração de trigger points e medicação oral (ciclobenzaprina)]. Acta Fisiátrica 2002;9(3):117‐26. [Biblioteca Central: BR734.1] [Google Scholar]
Herman 2002 {published data only}
- Herman CR, Schiffman EL, Look JO. The effectiveness of adding pharmacologic treatment with clonazepam or cyclobenzaprine to patient education and self‐care for the treatment of jaw pain upon awakening: A randomized clinical trial. Journal of Orofacial Pain 2002 Winter;16(1):64‐70. [PUBMED: 11889661] [PubMed] [Google Scholar]
Additional references
Al‐Ani 2005
- Al‐Ani Z, Gray RJ, Davies SJ, Sloan P, Glenny AM. Stabilization splint therapy for the treatment of temporomandibular myofascial pain: a systematic review. Journal of Dental Education 2005;69(11):1242‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Basmajian 1978
- Basmajian JV. Cyclobenzaprine hydrochloride effect on skeletal muscle spasm in the lumbar region and neck: Two double‐blind controlled clinical and laboratory studies. Archives of Physical Medicine and Rehabilitation 1978;59:58‐63. [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989 May;28(2):V. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Lee 1980
- Lee JC, Rockwood CA. Skeletal muscle spasm and a review of muscle relaxants. Current Therapeutic Research 1980;27(1):64‐74. [Google Scholar]
De Lucena 2006
- Lucena LB, Kosminsky M, Costa LJ, Goes PS. Validation of the Portuguese version of the RDC/TMD Axis II questionnaire. Pesquisa Odontológica Brasileira ‐ Brazilian Oral Research 2006;20(4):312‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;4(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dworkin 1992
- Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. Journal of Craniomandibular Disorders: Facial & Oral Pain 1992;6(4):301‐55. [MEDLINE: ] [PubMed] [Google Scholar]
Fishbain 1986
- Fishbain DA, Goldberg M, Meagher BR, Steele R, Rosomoff H. Male and female chronic pain patients categorized by DSM‐III psychiatric diagnostic criteria. Pain 1986;26:181‐97. [DOI] [PubMed] [Google Scholar]
Fricton 1985
- Fricton JR, Kroening R, Haley D, Siegert R. Myofascial pain syndrome of the head and neck: A review of clinical characteristics of 164 patients. Oral Surgery, Oral Medicine, and Oral Pathology 1985;60:615‐23. [DOI] [PubMed] [Google Scholar]
Fricton 1989
- Fricton JR, Schiffman EL. Research in temporomandibular disorders. Northwest Dentistry 1989;68(4):29‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Gerwin 1995
- Gerwin RDA. Study of 96 subjects examined both for fibromyalgia and myofascial pain. Journal of Musculoskeletal Pain 1995;3 (suppl 1):121. [Google Scholar]
Graff‐Radford 1984
- Graff‐Radford B. Myofascial trigger‐points: their importance and diagnosis in dental office. Journal of the Dental Association in South Africa 1984;39:237‐40. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. British Medical Journal 2003;327:555‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Assessment of study quality. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; section 6. The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd, 2007. [Google Scholar]
Huskisson 1983
- Huskisson EC. Visual analogue scale for global pain for the trapezius region. In: Melzack R editor(s). Visual analogue scales. New York: Reven Press, 1983:33‐7. [Google Scholar]
Jüni 1999
- Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. The Journal of the American Medical Association 1999;282(11):1054‐60. [DOI] [PubMed] [Google Scholar]
Katz 1988
- Katz WA, Dube J. Cyclobenzaprine in the treatment of acute muscle spasm: Review of a decade of clinical experience. Clinical Therapeutics 1988;10(2):216‐28. [PubMed] [Google Scholar]
Kobayashi 1996
- Kobayashi H, Hasegawa Y, Ono H. Cyclobenzaprine, a centrally acting muscle relaxant, acts on descending serotonergic systems. European Journal of Pharmacology 1996;311(1):29‐35. [DOI] [PubMed] [Google Scholar]
Koh 2003
- Koh H, Robinson PG. Occlusal adjustment for treating and preventing temporomandibular joint disorders. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003812] [DOI] [PubMed] [Google Scholar]
Lance 1964
- Lance JW, Curran DA. Treatment of chronic tension headache. Lancet 1964;42:1236‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lance 1972
- Lance JW, Anthony M. Cyclobenzaprine in the treatment of chronic tension headache. The Medical Journal of Australia 1972;2(25):1409‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laskin 1969
- Laskin DM. Etiology of the pain‐dysfunction syndrome. Journal of the American Dental Association 1969;79(1):147‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
List 1996
- List T, Dworkin SF. Comparing TMD diagnoses and clinical findings at Swedish and US TMD centers using research diagnostic criteria for temporomandibular disorders. Journal of Orofacial Pain 1996;10(3):240‐53. [MEDLINE: ] [PubMed] [Google Scholar]
List 1999
- List T, Wahlund K, Wenneberg B, Dworkin SF. TMD in children and adolescents: prevalence of pain, gender differences, and perceived treatment need. Journal of Orofacial Pain 1999;13(1):9‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Manfredini 2006
- Manfredini D, Chiappe G, Bosco M. Research diagnostic criteria for temporomandibular disorders (RDC/TMD) axis I diagnoses in an Italian patient population. Journal of Oral Rehabilitation 2006;33(8):551‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McMillan 1997
- McMillan AS, Nolan A, Kelly PJ. The efficacy of dry needling and procaine in the treatment of myofascial pain in the jaw muscles. Journal of Orofacial Pain 1997;11(4):307‐14. [MEDLINE: ] [PubMed] [Google Scholar]
Melzack 1987
- Melzack R. The short‐form McGill Pain Questionnaire. Pain 1987;30(2):191‐7. [DOI] [PubMed] [Google Scholar]
Okeson 1998
- Okeson JP. Orofacial Pain: Guidelines for assessment, diagnosis and management. Quintessence Publishing Co Inc, 1998. [Google Scholar]
Okeson 2006
- Okeson JP. Bell's Orofacial Pain. 6. São Paulo: Quintessence publishing Co, Inc, 2006. [Google Scholar]
Pertes 2005
- Pertes RA, Gross SG. Clinical management of temporomandibular disorders and orofacial pain. Quintessence Publishing Co. Inc., 2005. [Google Scholar]
Reitinger 1996
- Reitinger A, Radner H, Tilscher H. Morphologic study of trigger‐points. Manuelle Medizin 1996;34:256‐62. [Google Scholar]
Schiffman 1990
- Schiffman EL, Fricton JR, Haley DP, Shapiro BL. The prevalence and treatment needs of subjects with temporomandibular disorders. Journal of the American Dental Association 1990;120:295‐303. [DOI] [PubMed] [Google Scholar]
Shi 2003
- Shi Z, Guo C, Awad M. Hyaluronate for temporomandibular joint disorders. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002970; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simons 2005
- Simons DG, Travell JG, Simons LS. Travell & Simons Myofascial Pain and Dysfunction: The Trigger Point Manual. 2. Vol. 1, Sao Paulo: ArtMed, 2005. [Google Scholar]
Slade 1994
- Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dental Health 1994;11(1):3‐11. [PubMed] [Google Scholar]
Solberg 1986
- Solberg WK. Temporomandibular disorders: masticatory myalgia and its management. British Dental Journal 1986;160(10):351‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tofferi 2004
- Tofferi JK, Jackson JL, O'Malley PG. Treatment of fibromyalgia with cyclobenzaprine: A meta‐analysis. Arthritis and Rheumatism (Arthritis Care and Research) 2004;51(1):9‐13. [DOI] [PubMed] [Google Scholar]
Travell 1952
- Travell J, Rinzler SH. The myofascial genesis of pain. Postgraduate Medicine 1952;11(5):425‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Travell 1983
- Travell JG, Simons DG. Myofascial pain and dysfunction: The trigger point manual.. Baltimore: willians and Wilkins, 1983. [Google Scholar]
Turturro 2003
- Turturro MA, Frater CR, D'Amico FJ. Cyclobenzaprine with ibuprofen versus ibuprofen alone in acute myofascial strain: A randomized double‐blind clinical trial. Annals of Emergency Medicine 2003;41(6):818‐26. [DOI] [PubMed] [Google Scholar]
Unnebrink 2001
- Unnebrink K, Windeler J. Intention‐to‐treat methods for dealing with missing values in clinical trials of progressively deteriorating diseases. Statistics in Medicine 2001;20(24):3931‐46. [DOI] [PubMed] [Google Scholar]
Yap 2003
- Yap AU, Dworkin SF, Chua EK, List T, Tan KB, Tan HH. Prevalence of temporomandibular disorder subtypes, psychologic distress, and psychosocial dysfunction in Asian patients. Journal of Orofacial Pain 2003;17(1):21‐8. [MEDLINE: ] [PubMed] [Google Scholar]


