Abstract
Background
Cystic fibrosis is a multi‐system disease characterised by the production of thick secretions causing recurrent pulmonary infection, often with unusual bacteria. Intravenous antibiotics are commonly used in the treatment of acute deteriorations in symptoms (pulmonary exacerbations); however, recently the assumption that exacerbations are due to increases in bacterial burden has been questioned.
Objectives
To establish if intravenous antibiotics for the treatment of pulmonary exacerbations in people with cystic fibrosis improve short‐ and long‐term clinical outcomes.
Search methods
We searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched the reference lists of relevant articles and reviews and ongoing trials registers.
Date of last search of Cochrane trials register: 27 July 2015.
Selection criteria
Randomised controlled trials and the first treatment cycle of cross‐over studies comparing intravenous antibiotics (given alone or in an antibiotic combination) with placebo, inhaled or oral antibiotics for people with cystic fibrosis experiencing a pulmonary exacerbation.
Data collection and analysis
The authors assessed studies for eligibility and risk of bias and extracted data.
Main results
We included 40 studies involving 1717 participants. The quality of the included studies was largely poor and, with a few exceptions, these comprised of mainly small, inadequately reported studies.
When comparing treatment with a single antibiotic to a combined antibiotic regimen, those participants receiving a combination of antibiotics experienced a greater improvement in lung function when considered as a whole group across a number of different measurements of lung function, but with very low quality evidence. When limited to the four placebo‐controlled studies (n = 214), no difference was observed, again with very low quality evidence. With regard to the review's remaining primary outcomes, there was no effect upon time to next exacerbation and no studies in any comparison reported on quality of life. There were no effects on the secondary outcomes weight or adverse effects. When comparing specific antibiotic combinations there were no significant differences between groups on any measure. In the comparisons between intravenous and nebulised antibiotic or oral antibiotic (low quality evidence), there were no significant differences between groups on any measure. No studies in any comparison reported on quality of life.
Authors' conclusions
The quality of evidence comparing intravenous antibiotics with placebo is poor. No specific antibiotic combination can be considered to be superior to any other, and neither is there evidence showing that the intravenous route is superior to the inhaled or oral routes. There remains a need to understand host‐bacteria interactions and in particular to understand why many people fail to fully respond to treatment.
Plain language summary
The use of intravenous antibiotics to treat pulmonary exacerbations in people with cystic fibrosis
Review question
Do intravenous antibiotics (antibiotics given via a vein) given to treat 'flare ups' of lung disease (pulmonary exacerbations) in people with cystic fibrosis improve clinical outcomes in the short term and the long term?
Background
We wanted to evaluate the evidence for the current practice of using intravenous antibiotics to treat people with cystic fibrosis who have a pulmonary exacerbation. We wanted to discover if it is better to give two antibiotics than just a single antibiotic and wanted to consider if any particular antibiotic combination is better than any other. We also wanted to discover if intravenous antibiotics are any better than inhaled or oral antibiotics in treating pulmonary exacerbations in people with cystic fibrosis.
Search date
We last searched for evidence on 27 July 2015.
Study characteristics
The review included 40 studies with 1717 people with cystic fibrosis. Studies compared intravenous antibiotics with placebo (dummy drug with no active medication) and also one antibiotic compared to two antibiotics given together. Specific antibiotic combinations were also compared as were intravenous antibiotics with antibiotics that were breathed in (inhaled) and antibiotics that were swallowed (oral). The studies lasted from three to 15 days, although most of the studies lasted for two weeks.
Key results
In the comparison between those people who were given just one antibiotic and those who were given two, it appeared that those receiving two antibiotics experienced a greater improvement in lung function, but when limited to only those studies that included a dummy drug, we did not see any difference. There was no effect upon the amount of time until the next exacerbation, weight, or adverse effects. No combination of antibiotics was any better than any other. The outcomes for people were the same irrespective of whether they were treated by intravenous, oral or inhaled antibiotics. None of the studies reported on quality of life.
Quality of the evidence
The quality of the included studies was often poor and many were not properly reported. Some studies included volunteers more than once which made comparing treatments difficult. It was also often difficult to decide from the information given how well the studies were carried out ‐ particularly with respect to how volunteers were chosen and whether the volunteers or doctors could tell which treatment they were being given.
Summary of findings
Background
Description of the condition
Cystic fibrosis (CF) is a multi‐organ life‐limiting condition inherited in an autosomally recessive manner. It is characterised by viscid secretions of many organs, in particular the lungs and pancreas. In the lungs these viscid secretions interfere with the mechanisms responsible for clearing inhaled material, allowing opportunistic bacteria to establish infections. Important pathogens include Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S.aureus), Haemophilus influenzae, and many others, with S. aureus and P. aeruginosa being the most prevalent in childhood and adulthood respectively (Cystic Fibrosis Foundation Patient Registry 2011; Guss 2011). It is thought that early infection with P. aeruginosa may be eradicated by antibiotics (Gibson 2003; Langton Hewer 2009; Ratjen 2001), but eventually the infection becomes chronic and can no longer be eradicated. Chronic infection causes persistent symptoms of cough and sputum production and is associated with progressive loss of lung function (Ballmann 1998). In addition, people with CF experience pulmonary exacerbations, which are characterised by an increase in symptoms (Goss 2007) and reductions in lung function, weight and quality of life (Britto 2002). The causes of these exacerbations are not known, but it is suspected that they may be due, in part, to bacterial infection. A standard definition of what constitutes a pulmonary exacerbation has yet to be agreed, but there is reasonable consensus that a pulmonary exacerbation usually consists of people with CF reporting a decline in well‐being largely due to respiratory symptoms that prompts the commencement of a course of antibiotics (Bilton 2011).
Description of the intervention
Pulmonary exacerbations have long been treated with antibiotics and this is currently the recommendation in Europe (Doring 2000) and the USA (Flume 2009). An assumption underlying a cornerstone of CF care is that pulmonary exacerbations are associated with bacterial infection (in particular due to P. aeruginosa), leading to the conclusion that these exacerbations should be treated with antibiotics (Doring 2000). Observational data suggest that oral, intravenous (IV) and nebulised antibiotics are administered to treat a pulmonary exacerbation (Wagener 2013). When IV antibiotics are used, a combination of two or more different IV antibiotics are recommended; however, the optimal duration of IV antibiotic therapy is unknown (Flume 2009; Elphick 2014; Plummer 2013).
How the intervention might work
Although an accepted definition of what constitutes a pulmonary exacerbation has yet to be developed, pulmonary exacerbations are assumed to be caused (at least in part) by bacterial infection. Antibiotics are administered in order to reduce the amount of bacteria in the lungs that are presumed to be responsible for much of the decline in the individual's clinical condition (Flume 2009). These antibiotics may, or may not, reduce the amount of bacteria in the lungs. There may be many different species of bacteria present (Guss 2011) and these broad‐spectrum antibiotics may have variable activity against these bacteria. However, antibiotics may themselves be associated with considerable morbidity including selecting for antibiotic resistance (Rogues 2007) and causing renal toxicity and ototoxicity (Bertenshaw 2007; Smyth 2014). Prolonged courses of antibiotic treatment may also pose a significant burden of treatment for people with CF to endure.
Why it is important to do this review
Pulmonary exacerbations are responsible for an accelerated decline in lung function (de Boer 2011) and a significant proportion of people experiencing a pulmonary exacerbation who are treated with IV antibiotics, do not recover lung function to baseline (Sanders 2010). Potential reasons for this include:
the aetiology of the exacerbation (e.g. viral infection) and so prevention is the main goal;
the host (e.g. inflammation) so novel therapies might be developed to modulate the immune system;
factors related to the treatment, and so we must determine optimal treatments (and routes of treatment) which can include use of IV antibiotics.
Often, IV antibiotics are held to be the most effective form of antibiotic delivery. We wished not only to determine the efficacy of IV antibiotics in treating people with CF experiencing a pulmonary exacerbation, but also to determine the comparative effectiveness of the IV route compared against antibiotics administered via oral or inhaled routes.
We have reviewed the current evidence that treating such exacerbations with IV antibiotics improves short‐term and long‐term clinical outcomes in people with CF.
Objectives
To establish if IV antibiotics for the treatment of pulmonary exacerbations in people with CF improve short‐ and long‐term clinical outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We shall include randomised controlled trials (RCTs) and the first treatment cycle of cross‐over studies (seeUnit of analysis issues) in people with CF experiencing a pulmonary exacerbation.
Types of participants
We will consider people with CF as diagnosed using the Cystic Fibrosis Foundation consensus statement (Rosenstein 1998) of all ages and all degrees of disease severity. There are no universally agreed definitions for the diagnosis of a pulmonary exacerbation; some criteria are restrictive (Fuchs 1994) (since modified), while others only require an event needing hospitalisation and IV antibiotics due to worsening respiratory signs and symptoms (Brody 2005). We will therefore consider all studies that explicitly aim to trial an IV antibiotic for the treatment of a pulmonary exacerbation.
Types of interventions
We shall compare:
a single IV antibiotic versus placebo;
a combination of IV antibiotics versus placebo;
one regimen of IV antibiotics versus another IV regimen of antibiotics (with or without placebo);
an IV antibiotic regimen versus nebulised antibiotics; and
an IV antibiotic regimen versus oral antibiotics.
Studies that exclusively compare different doses of the same antibiotic will be excluded (post hoc change).
Types of outcome measures
Primary outcomes
-
Lung function
forced expiratory volume at one second (FEV1)
forced vital capacity (FVC)
Time to next exacerbation (although as there is no agreed definition of pulmonary exacerbations, we shall accept the individual clinicians' diagnosis, acknowledging the inherent difficulties that this poses)
Quality of life (e.g. Cystic Fibrosis Questionnaire‐Revised (CFQ‐R) (Quittner 2009))
Secondary outcomes
Symptom score using a validated tool (e.g. acute respiratory illness checklist (ARIC), respiratory and systemic symptoms questionnaire (RSS‐Q))
-
Nutritional status
body mass index (BMI) (all measures)
weight (all measures)
Adherence (all measures)
Mortality (CF‐related and all causes)
-
Adverse effects
toxicity and allergy ‐ e.g. idiosyncratic reaction, allergy, decline in renal function
microbiological ‐ isolation of antibiotic resistant strains, or new strains of bacteria
Search methods for identification of studies
Electronic searches
We identified relevant studies from the Group's Cystic Fibrosis Trials Register using the terms: antibiotics AND (intravenous OR not stated) AND (acute treatment [pulmonary exacerbations] OR unknown).
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group Module.
Date of the latest search: 27 July 2015.
We also searched the clinical trials registers: ClinicalTrials.gov; ISRCTN; and EudraCT for ongoing trials using the search terms: cystic fibrosis AND antibiotics.
Date of the latest search: 02 July 2014.
Searching other resources
We searched the reference lists of all included studies to identify other studies for potential inclusion.
Data collection and analysis
Selection of studies
One author (MH) reviewed the abstracts of studies identified by the literature search and excluded studies that did not consider the management of pulmonary exacerbations in CF with IV antibiotics. Two investigators (MH, AP) independently considered the full text reports of the remaining studies. They examined each study for potential inclusion and for consideration of multiple reporting by comparing reports using author names, study location, intervention details and date of the study as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). They resolved any disagreement through discussion and where necessary arbitration by the third author (PF).
Data extraction and management
Two authors (MH, AP) independently extracted the data from the included studies on a separate study report form and compared the output. They resolved any disagreement through discussion. Where one paper presented data graphically, the authors extracted the data they required using XYPLOT (a graphics‐based computer programme where data points may be extracted using the scale of axes as reference points) (XYPLOT 2010) and then entered data into the Review Manager software to be analysed (RevMan 2014).
Assessment of risk of bias in included studies
While interrogating each study report for the extraction of data, the authors also noted information regarding the conduct and design of each study in order to implement the Cochrane Collaboration's risk of bias tool (Higgins 2011b). This tool facilitates the assessment of biases introduced through inadequacies in random sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessments, reporting of incomplete outcome data and selective reporting. As with data extraction, the two investigators extracted this information with the same process for comparison and resolution of disparity. They were not blinded to the authors of each study. In some instances more information is required and in future the review authors will attempt to contact the study authors or sponsors for more detail to be included in updates of this review. All investigators agreed the final judgement regarding whether any individual bias or group of biases imposes a material bias impacting upon the results and conclusions of a study, informed by the empirical evidence, likely direction and magnitude of any bias.
Measures of treatment effect
The authors assessed continuous outcomes (lung function, nutritional status, quality of life and symptom scores) by the calculation of mean difference (MD) and 95% confidence intervals (CIs). Where trials reported multiple measures for the same outcome (e.g. absolute change FEV1 per cent (%) predicted, or absolute change of absolute FEV1 volumes) the review authors calculated standardised mean differences (SMDs). In the event that individual participant data had been available, the authors would have considered absolute changes in FEV1 in context of comparable data being available for each participant before and after the intervention so that a calculation of the effect size was possible.
If the data allowed, the authors would have extracted or calculated hazard ratios (HR) and 95% CIs for the outcome 'Time to next exacerbation'. Where possible, the authors evaluated dichotomous outcome data for death by the calculation of a risk ratio (RR) with 95% CIs. If it had been possible, they planned to assess adherence by calculating odds ratio (OR) with 95% CIs. Other dichotomous outcomes would have been reported by the calculation of ORs with 95% CIs.
Unit of analysis issues
The authors aimed to only consider the first pulmonary exacerbation for each participant, taking the measure of effect as the difference between baseline and the end of treatment. The optimal duration of therapy is yet to be established and so the authors considered the end of therapy measurement (as defined in the study) as the unit of analysis. In the case of cross‐over studies they aimed to consider only the first phase of participation, as participants may not recover lung function to baseline (Sanders 2010) and, in addition to introducing a unit of analysis error, multiple treatment episodes may not be comparable. In effect, these studies remain listed in Studies awaiting classification as the authors await data to include in the analysis. In order to compare interventions of differing durations the authors aimed, given sufficient comparable studies reporting these data, to combine outcome data for comparisons at two weeks, three months and one year after the exacerbation. If future studies consistently report this, they shall undertake this analysis in future updates.
Dealing with missing data
When possible the review authors aimed to contact the study authors for data that appeared to be missing. Where only mean values and standard deviations (SDs) before and after treatment were available, they imputed the SD of the mean change using a correlation coefficient as described in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For the calculation of a correlation coefficient for studies that reported FEV1 and FVC in terms of % predicted, the authors used data from the 1988 Bosso study, as this study reported the mean and SD for pre‐treatment and post‐treatment and also the SD of the mean change (Bosso 1988). Similarly, for studies that reported FEV1 and FVC in absolute change (litres) the authors used data from the 1987 Hodson study (Hodson 1987). When appropriate to do so, the authors may remedy missing data by the imputation of replacement values (e.g. using the mean value for a treatment group). Where possible they will conduct intention‐to‐treat analyses.
Assessment of heterogeneity
Where the review authors were able to perform a meta‐analysis with multiple studies suitable for inclusion, they attempted to identify statistical heterogeneity by calculating a Chi2 test and using this value to compute an I2 statistic (Higgins 2003). They interpreted this value based on thresholds as identified in theCochrane Handbook for Systematic Reviews of Interventions (Deeks 2011): 0% to 40% as probably not important; 30% to 60% as moderate heterogeneity; 50% to 90% as substantial heterogeneity; and 75% to 100% as considerable heterogeneity. The authors also considered sources of heterogeneity within the characteristics of the included studies.
Assessment of reporting biases
The review authors used multiple search methods to identify published studies and also aimed to contact the authors of all included studies in an attempt to identify studies that may not have been published. They also aimed to contact the authors of any study published only in abstract form for study data. Where available, they attempted to identify evidence of outcome reporting biases by the comparison of the published report to the study protocol. They further attempted to identify reporting biases by the construction and inspection of funnel plots for asymmetry and interpreting them in the context of study sizes, and methodological rigour (Sterne 2011).
Data synthesis
The authors conducted separate meta‐analyses for the grouped comparisons as detailed above (Types of interventions). They conducted a fixed‐effect method meta‐analysis to combine the measures of effect for the outcomes of study. In the case of at least substantial heterogeneity (as defined above), they would have employed a random‐effects method.
The authors used the inverse variance method of meta‐analysis for continuous data and the Mantel‐Haenzsel method for dichotomous data as the default fixed‐effect methods in RevMan. The authors would have used the generic inverse variance method for the outcome 'time to next pulmonary exacerbation' if data had been available for analysis.
Subgroup analysis and investigation of heterogeneity
In the case that the authors had identified a sufficient number of studies (i.e. 10), they would have undertaken subgroup analyses for the following groups:
bacteria isolated at time of exacerbation P. aeruginosa versus non P. aeruginosa;
severity of lung disease based on a composite of age and FEV1 to delineate 'severe' and 'mild' as classified by Schluchter (Schluchter 2006); and
age of participants (children (less than 18 years of age) and adults).
Sensitivity analysis
Again, if the authors had identified a sufficient number of studies for inclusion, they would have investigated the effect of arbitrary decisions made by the review team by undertaking sensitivity analyses of the affected components. In the case where they decided to manage missing data by the imputation of data, if possible they would have investigated the effect of these manipulations by repeating the analyses without these imputations. In the case of determining the effect of arbitrary decisions the authors made, for example by including all studies of pulmonary exacerbations instead of limiting selection only to those with stricter diagnostic criteria, they would have repeated the analyses limited to the stricter diagnostic criteria.
Summary of findings tables
In a post hoc change the authors have presented three summary of findings tables ‐ one comparing single and combination IV antibiotics, one comparing nebulised and IV antibiotics and one comparing oral and IV antibiotics (Table 1; Table 3; Table 2). The authors calculated the assumed risk as the mean of the effect size of the control group in each study; the corresponding risk being the result of the meta‐analysis as presented in the data tables. The authors determined the study quality using the GRADE approach, where quality was rated with regard to risk of bias or study limitations, directness, consistency of results, precision, publication bias and effect size. They downgraded the evidence by one level for serious (or by two for very serious) study limitations.
Summary of findings for the main comparison. Single versus combination IV antibiotics for pulmonary exacerbations in people with cystic fibrosis.
| Single versus combination IV antibiotics for pulmonary exacerbations in people with cystic fibrosis | ||||||
|
Patient or population: people with cystic fibrosis experiencing a pulmonary exacerbation
Settings: inpatient (hospital)
Intervention: single IV antibiotic (with or without a placebo) Comparison: combination IV antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Combination IV antibiotics | Single IV antibiotics (with or without placebo) | |||||
| FEV1 (% predicted) absolute change Follow up: 7 ‐ 14 days | The mean (range) absolute change in FEV1 (% predicted) in the control group was 11.82% (8% to 15%) | The mean absolute change in FEV1 (% predicted) in the intervention group was 1.14% lower (3.23 lower to 0.95 higher) | 265 (6 studies) (Bosso 1988; De Boeck 1989; Hyatt 1981; Master 2001; McLaughlin 1983; Smith 1999) |
⊕⊕⊝⊝ low1,2 | The assumed risk represents the mean of effect observed in the combination IV antibiotics group and the corresponding risk that of the result of the meta‐analysis with respect to the comparison group receiving single IV antibiotics (with or without placebo). Quality was determined by downgrading by one point based on participants re‐entering study more than once and so introducing bias; it was further downgraded due to the low numbers of events. |
|
| FVC (% predicted) absolute change Follow up: 7 ‐ 14 days | The mean absolute change in FVC (% predicted) in the control group was 11.70% (7% to 15.4%) | The mean absolute change in FVC (% predicted) in the intervention group was 1.37% lower (4.56 lower to 1.81 higher) | 146 (4 studies) (Bosso 1988; Hyatt 1981; McLaughlin 1983; Smith 1999) |
⊕⊕⊝⊝ low1,2 | The assumed risk represents the mean of effect observed in the combination IV antibiotics group and the corresponding risk that of the result of the meta‐analysis with respect to the comparison group receiving single IV antibiotics (with or without placebo). Quality was determined by downgrading by one point based on participants re‐entering study more than once and so introducing bias; it was further downgraded due to the low numbers of events. |
|
| Time to next exacerbation (weeks) | The mean time to next exacerbation in the control group was 24 weeks | The mean time to next exacerbation in the intervention group was 7.00 weeks lower (23.67 lower to 9.67 higher) | 34 (1 study) (McLaughlin 1983) | ⊕⊕⊝⊝ low1,2 | The assumed risk represents the mean of effect observed in the combination IV antibiotics group and the corresponding risk that of the result of the meta‐analysis with respect to the comparison group receiving single IV antibiotics (with or without placebo). Quality was determined by downgrading by one point based on participants re‐entering study more than once and so introducing bias; it was further downgraded due to the low numbers of events. |
|
| Quality of life | Not reported | |||||
| *The authors calculated the assumed risk as the mean of the effect size of the control group in each study. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1 : forced expiratory volume at one second; FVC: forced vital capacity; IV: intravenous | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level for risk of bias; the analysis did not account for multiple observations from the same participants.
2 Downgraded one level due to the low numbers of events observed in the studies.
Summary of findings 3. Oral antibiotics compared to IV antibiotics for pulmonary exacerbations in people with cystic fibrosis.
| Oral antibiotics compared to intravenous antibiotics for pulmonary exacerbations in people with cystic fibrosis | ||||||
| Patient or population: people with cystic fibrosis experiencing a pulmonary exacerbation Settings: inpatients (hospital) Intervention: oral antibiotics Comparison: intravenous antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IV antibiotics | Oral antibiotics | |||||
| FEV1 (% predicted) absolute change Follow up: 7 ‐ 14 days | The mean absolute change in FEV1 (% predicted) in the control group was 5.8% | The mean absolute change in FEV1 (% predicted) in the intervention group was 1.4% lower (7.23 lower to 4.43 higher) | 24 (1 study) (Bosso 1989) |
⊕⊕⊝⊝ low1,2 | The assumed risk represents the mean of effect observed in the IV antibiotics group and corresponding risk that of the result of the meta‐analysis with respect to the oral antibiotics group. Quality was determined by downgrading by one point based on the low numbers of events and downgraded further due to a lack of blinding. |
|
| FVC (% predicted) absolute change Follow up: 7 ‐ 14 days | The mean absolute change in FVC (% predicted) in the control group was 6.6%. | The mean absolute change in FVC (% predicted) in the intervention group was 2% higher (7.5 lower to 11.5 higher) | 24 (1 study) (Bosso 1989) |
⊕⊕⊝⊝ low1,2 | The assumed risk represents the mean of effect observed in the IV antibiotics group and corresponding risk that of the result of the meta‐analysis with respect to the oral antibiotics group. Quality was determined by downgrading by one point based on the low numbers of events and downgraded further due to a lack of blinding. |
|
| Time to next exacerbation | Not reported | |||||
| Quality of life | Not reported | |||||
| *The authors calculated the assumed risk as the mean of the effect size of the control group in each study. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1 : forced expiratory volume at one second; FVC: forced vital capacity; IV: intravenous | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level for risk of bias; the study was either unblinded or single blind. 2 Downgraded one level due to the low numbers of events observed in the studies.
Summary of findings 2. Nebulised antibiotics compared to IV antibiotics for pulmonary exacerbations in people with cystic fibrosis.
| Nebulised antibiotics compared to IV antibiotics for pulmonary exacerbations in people with cystic fibrosis | ||||||
| Patient or population: people with cystic fibrosis experiencing a pulmonary exacerbation Settings: inpatients (hospital) Intervention: nebulised antibiotics Comparison: IV antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IV antibiotics | Nebulised antibiotics | |||||
| FVC (% predicted) absolute change Follow up: 14 days |
The mean absolute change in FVC (% predicted) in the control group was 13% |
The mean absolute change in FVC (% predicted) in the intervention groups was not different 0% (3.94 lower to 3.94 higher) | 54 (1 study)(Schaad 1987) | ⊕⊝⊝⊝ very low1,2,3 | The assumed risk represents the mean of effect observed in the IV antibiotics group and corresponding risk that of the result of the meta‐analysis with respect to the nebulised antibiotics group. Quality was determined by downgrading by one point based on the low numbers of events and downgraded further due to a lack of blinding. |
|
| Time to next exacerbation | Not reported | |||||
| Quality of life | Not reported | |||||
| *The authors calculated the assumed risk as the mean of the effect size of the control group in each study. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; FVC: forced vital capacity; IV: intravenous | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level for risk of bias; the study was either unblinded or single blind. 2 Downgraded one level due to the low numbers of events observed in the studies. 3 Downgraded one level for risk of bias with no information on blinding or randomisation methods used.
Results
Description of studies
For study details please refer to the tables (Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification).
Results of the search
The original search (November 2011) identified 180 publications and a later search (July 2014) identified a further 12. The search of the Cystic Fibrosis and Genetic Disorders Group's Trials Register in 2015 did not identify any new publications. A search of the reference lists of these publications (and reviewing other publications included within the same supplementary journal issues) identified a further 17 published reports. Of these 209 published reports, of which some were duplicate reports of the same study, we identified 134 individual studies. We were able to exclude 82 studies after reviewing the abstracts or full reports (132 references) for the reasons stated below (Excluded studies). There were 40 trials (62 references) that met our inclusion criteria; 12 studies (15 references) await classification (see PRISMA diagram Figure 1).
1.
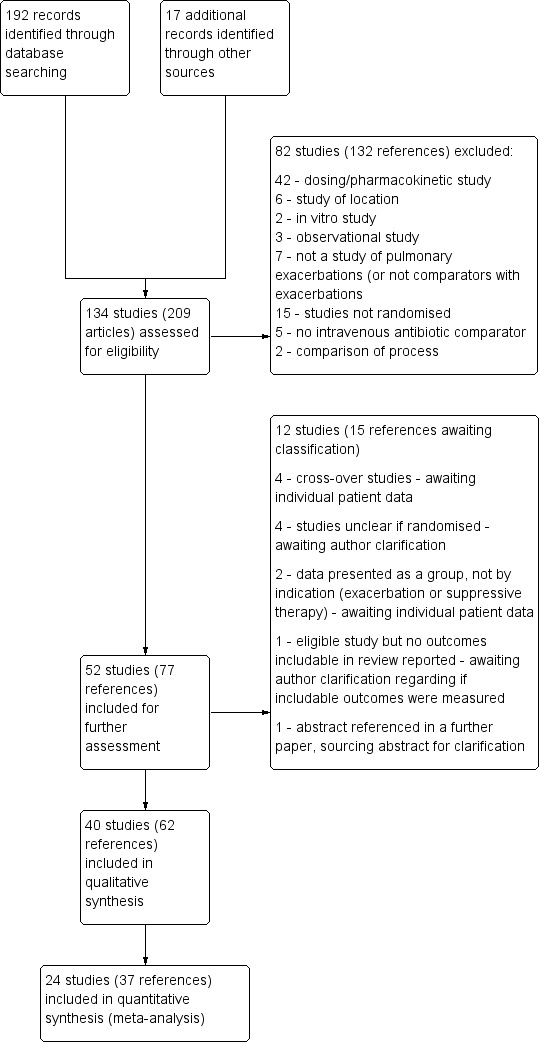
Study flow diagram.
Included studies
A total of 40 studies (n = 1717) were included in the review (Characteristics of included studies). Many of the included studies are older, with only three studies reporting since 2000 (Blumer 2005; Master 2001; Semykin 2010).
Trial design
A total of 14 studies were described as double‐blind and a further seven studies were single‐blind (an additional four involved a single assessment modality that was blinded); the remaining 18 studies were unblinded. All studies were of a parallel design; cross‐over studies are awaiting pending data for inclusion (see Characteristics of studies awaiting classification).
Most of the studies were single‐centre studies; however, one study had two centres (McLaughlin 1983) and a further five studies were multi‐centre (three or more centres) (Blumer 2005; BTS 1985; Church 1997; Richard 1997; Smith 1999). The majority of studies were conducted either in Western Europe (n = 17) or the USA (n = 15) with the remaining studies taking place in Canada (n = 4), Australia (n = 2), New Zealand (n = 1), Russia (n = 1) and one study was multinational (Richard 1997).
A total of 33 studies compared two groups of participants, five studies compared three groups (Costantini 1982; McLaughlin 1983; Padoan 1987; Semykin 2010; Wang 1988) and a further two studies had four arms (Macfarlane 1985) and seven arms (Agostini 1983). The duration of the interventions ranged from three days (Caplan 1984) to 15 days (Agostini 1983; Costantini 1982; Schaad 1987), although the vast majority of studies reported an administration of antibiotics of 14 days.
Participants
The included studies involved a total of 1717 participants. However, many of these studies each recruited only a small number of participants; indeed, 28 studies recruited fewer than 50 participants. The largest study randomised 147 participants (Agostini 1983), while the smallest recruited just 13 participants (Wesley 1988).
Five studies included only children (which we defined as younger than 18 years) (Church 1997; Knowles 1988; Padoan 1987; Semykin 2010; Wesley 1988) and four studies recruited only adults (Bosso 1989; Hodson 1987; Penketh 1984; Wang 1988). The remaining studies admitted participants of all ages. Where data were available regarding gender split, groups were largely equally split, except for five studies where males were predominant (Gold 1985; Penketh 1984; Regelmann 1990; Salh 1992; Stephens 1983) and three studies where females were predominant (Gold 1987; Huang 1983; Padoan 1987).
Few studies described the disease severity of the participants; those that did largely did so on the basis of FEV1 (Blumer 2005; Church 1997; Conway 1997), while two explicitly enrolled those with "severe" disease (Padoan 1987; Wesley 1988). Other studies actively excluded those with severe disease; two studies excluded those with protocol‐defined severe disease (Gold 1985; Schaad 1986), while a further two studies did so on the basis of lung function ‐ one excluded those with FEV1 less than 40% predicted (Master 2001) and the second excluded those with FEV1 less than 20% predicted (Penketh 1984). One study excluded those who had been admitted to hospital more recently than four months prior to the current admission (Schaad 1989).
Interventions
IV antibiotic versus placebo
We identified seven studies that investigated the activity of an IV antibiotic regimen with a placebo in the comparison (Gold 1987; Hyatt 1981; Macfarlane 1985; McLaughlin 1983; Regelmann 1990; Smith 1999; Wientzen 1980). While three of these were direct comparisons of an intervention versus placebo (Gold 1987; Regelmann 1990; Wientzen 1980), four trials involved a placebo drug as part of an IV antibiotic combination, thereby attempting to consider the effect of single active agent versus combination agent treatment and are further discussed in the section below (Single IV antibiotic versus combination IV antibiotic) (Hyatt 1981; Macfarlane 1985; McLaughlin 1983; Smith 1999).
Single IV antibiotic versus placebo
Two studies involved a direct comparison between a single antibiotic (ceftazidime and tobramycin respectively) and placebo (Gold 1987; Wientzen 1980).
Combination of IV antibiotics versus placebo
One further study compared an antibiotic combination (tobramycin and ticarcillin) and placebo (Regelmann 1990) .
IV antibiotic regimens compared
A total of 29 studies compared multiple IV antibiotic regimens.
Single IV antibiotic regimens compared
Six studies compared two (or more) different single antibiotic regimens (Agostini 1983; Caplan 1984; Costantini 1982; Elborn 1992; Huang 1983; Salh 1992). One study compared five different antibiotics ‐ azlocillin versus piperacillin versus ceftazidime versus cefsulodin versus cefoperazone (Agostini 1983); a further study compared cefsulodin to tobramycin or ticarcillin (Caplan 1984). The remaining three studies compared two single IV antibiotics: ceftazidime versus aztreonam (Elborn 1992); carbenicillin versus azlocillin (Huang 1983); and ceftazidime versus aztreonam (Salh 1992). Two arms of the Costantini trial compared carbenicillin alone to sisomycin alone (Costantini 1982).
Single IV antibiotic versus combination IV antibiotic
Four studies involved a placebo drug as part of an IV antibiotic combination, thereby attempting to consider the effect of single active agent versus combination agent treatment (Hyatt 1981; Macfarlane 1985; McLaughlin 1983; Smith 1999). Hyatt studied oxacillin in combination with placebo compared to a combination of oxacillin, sisomycin and carbenicillin (Hyatt 1981). MacFarlane considered two doses of piperacillin (50 mg/kg six times daily and 100 mg/kg three times daily) each in combination with tobramycin compared to tobramycin with placebo (Macfarlane 1985). McLaughlin compared the combination of ticarcillin and tobramycin to a combination of azlocillin and tobramycin and further compared these with azlocillin and placebo (McLaughlin 1983). In the final study, Smith considered the combination of azlocillin and tobramycin compared with azlocillin and placebo (Smith 1999).
A total of 12 separate studies compared a single IV antibiotic with a combination of two IV antibiotics; of these five investigated the use of combination antibiotic treatment by comparing the effect of a single antibiotic with the same antibiotic in combination with another agent (Conway 1997; Costantini 1982; Master 2001; McCarty 1988; Padoan 1987) and seven compared a single agent to two different antibiotics in combination (Bosso 1988; BTS 1985; De Boeck 1989; De Boeck 1999; Gold 1985; Padoan 1987; Wesley 1988). One study consisted of multiple comparison arms which fall into both these comparisons (Padoan 1987). Another, compared a combination of IV antibiotics with a different IV antibiotic followed by the same antibiotic in oral form (Church 1997).
The antibiotics and their combinations used were varied, none of the studies comparing a single IV antibiotic to combination IV antibiotics used the same single agent. One study compared colistin alone to a combination of colistin with either aztreonam, azlocillin, piperacillin, ceftazidime, imipinem, or ciprofloxacin (Conway 1997). A second study compared tobramycin to tobramycin with ceftazidime (Master 2001). A further study compared piperacillin to piperacillin with tobramycin (McCarty 1988). In addition to the two single‐agent comparison arms of the Costantini trial mentioned above (carbenicillin alone to sisomycin alone), the trial also compared each single agent alone to carbenicillin combined with sisomycin (Costantini 1982). A further trial with multiple comparison arms compared ceftazidime alone to ceftazidime with sisomycin (Padoan 1987). The seven studies comparing a single agent with a combination of two different antibiotics also used a range of different agents. Ceftazidime was used as the single agent in five studies, it was compared to: gentamicin plus carbenicillin (BTS 1985); tobramycin plus piperacillin (De Boeck 1989); tobramycin plus ticarcillin (Gold 1985; Wesley 1988) and, in addition to the comparison discussed above, to piperacillin plus sisomycin (Padoan 1987). Ceftazidime was also used in combination with tobramycin and compared to meropenem alone in one study (De Boeck 1999). The final study compared aztreonam to tobramycin plus azlocillin (Bosso 1988).
Combination IV antibiotic regimens compared
Nine studies compared two combinations of two different IV antibiotics (Blumer 2005; Conway 1985; McLaughlin 1983; Penketh 1983; Penketh 1984; Schaad 1986; Schaad 1989; Semykin 2010; Wang 1988). Again the IV antibiotics used were varied. Two studies administered a combination of netilmicin and ticarcillin, but compared these to tobramycin and ticarcillin (Conway 1985) and netilmicin and azlocillin (Schaad 1986). Two studies by the same lead author compared carbenicillin and gentamicin to other IV antibiotic combinations, firstly, carbenicillin plus gentamicin versus ticarcillin plus gentamicin (Penketh 1983) and secondly carbenicillin plus gentamicin versus azlocillin plus gentamicin (Penketh 1984). The remaining two studies compared meropenem plus tobramycin versus ceftazidime plus tobramycin (Blumer 2005) and aztreonam plus amikacin versus ceftazidime plus amikacin (Schaad 1989). Semykin compared IV cefepime plus IV amikacin to IV meropenem plus IV amikacin, in addition to a nebulised antibiotic arm (discussed below) (Semykin 2010). McLaughlin considered the combination of ticarcillin plus tobramycin compared with azlocillin plus tobramycin (as well as another arm with a placebo as discussed above) (McLaughlin 1983).
IV antibiotic regimen versus nebulised antibiotics
A total of five studies compared IV antibiotics to nebulised antibiotics.
One study compared an IV antibiotic regimen with an inhaled antibiotic regimen using IV tobramycin and ticarcillin compared to inhaled tobramycin and inhaled carbenicillin (Cooper 1985). Four studies investigated the effect of inhaled antibiotics as an adjunct to intravenous antibiotic use (Knowles 1988; Schaad 1987; Semykin 2010; Stephens 1983). Knowles compared IV piperacillin and IV tobramycin to IV piperacillin and IV tobramycin with the addition of these same antibiotics delivered by nebuliser (Knowles 1988). Similarly, Stephens compared IV ticarcillin and IV tobramycin to IV ticarcillin and IV tobramycin with the addition of inhaled tobramycin (Stephens 1983) and Schaad compared IV ceftazidime and IV amikacin to IV ceftazidime and IV amikacin with the addition of inhaled amikacin (Schaad 1987). The fourth study to compare IV antibiotics to nebulised antibiotics compared IV cefepime with IV amikacin to IV meropenem and IV amikacin and also to inhaled tobramycin given alongside IV ceftazidime and oral ciprofloxacin (Semykin 2010).
IV antibiotic regimen versus oral antibiotics
Six studies compared IV antibiotics to oral antibiotics.
Four studies compared oral ciprofloxacin with two‐agent IV combinations (Bosso 1989; Hodson 1987; Richard 1997; Wang 1988). One study compared oral ciprofloxacin to IV azlocillin with gentamicin (Hodson 1987) and another study compared it to IV ceftazidime with tobramycin (Richard 1997). A further study compared oral ciprofloxacin to IV tobramycin with azlocillin (Bosso 1989) and the remaining study had a three‐arm comparison of oral ciprofloxacin to IV tobramycin with azlocillin and to IV tobramycin and ticarcillin (Wang 1988).
A fifth study compared oral ciprofloxacin with oral ciprofloxacin cycled with IV tobramycin with azlocillin (Black 1990). One study compared IV ciprofloxacin followed by oral ciprofloxacin to IV tobramycin with IV ceftazidime (Church 1997).
Multiple comparisons
Among the studies described above, six included multiple comparisons within a single study (Church 1997; Costantini 1982; McLaughlin 1983; Padoan 1987; Semykin 2010; Wang 1988). Church compared single agent IV ciprofloxacin followed by single agent oral ciprofloxacin with combination treatment with IV tobramycin and IV ceftazidime with multiple reporting periods thus comparing both single with combination IV agents and oral compared with IV (Church 1997). Costantini compared IV carbenicillin to IV sisomycin each as single agents and to IV carbenicillin with sisomycin combined (Costantini 1982). McLaughlin compared IV ticarcillin with IV azlocillin each in combination with IV tobramycin and also with a third comparison group of IV azlocillin in combination with placebo (McLaughlin 1983).
Padoan compared IV ceftazidime to IV ceftazidime with sisomycin and to IV piperacillin with sisomycin (Padoan 1987). Semykin compared IV cefepime with IV amikacin to IV meropenem and IV amikacin and to inhaled tobramycin given alongside IV ceftazidime and oral ciprofloxacin (Semykin 2010). Wang studied oral ciprofloxacin compared to IV tobramycin with ticarcillin and to IV tobramycin with azlocillin (Wang 1988).
Outcomes
Some of the earlier studies reported clinical status in the form of a 'clinical score'. These were not standardised or validated and the components of the scores varied between studies. Many studies did not report absolute values of measures, instead detailing the results of a statistical comparison between groups.
Lung function was the most commonly reported outcome (32 studies); however, this was variably reported as either percentage change or absolute change in either % predicted or absolute values of FEV1 or FVC. This made comparing the results of similar studies difficult. In addition, few studies reported means and measures of distribution of all lung function measurements ‐ initial, end measurements and a measure of the change over time. Where necessary we imputed the SDs for change using a correlation coefficient that was calculated from those studies that did report the requisite information, namely for data reported as FEV1 % predicted, a correlation coefficient was calculated using data from the Bosso study (Bosso 1989) and for data reported as absolute values, a correlation coefficient was calculated using data in the Hodson study (Hodson 1987).
Studies infrequently reported nutritional status and again variously ‐ either in absolute terms or as a measure of % underweight. Time‐to‐next exacerbation was reported in only eight studies; and then with data suitable for use in a meta‐analysis only available from two studies (De Boeck 1989; McLaughlin 1983). Two studies report rates of re‐admission in the three months following the study (BTS 1985; Wesley 1988). Three studies report a statistical test without supporting data (Penketh 1983; Penketh 1984; Smith 1999).
Adverse effects were variably reported in 26 studies, consisting of either specific reports of toxicities (ototoxicity or nephrotoxicity) (Penketh 1983; Regelmann 1990; Schaad 1987; Schaad 1989), screens for general toxicities using serum markers of liver and renal function or reports of side effects (Agostini 1983; Black 1990; Bosso 1988; BTS 1985; Caplan 1984; Conway 1997; Costantini 1982; Huang 1983; Hyatt 1981; Macfarlane 1985; Master 2001; Padoan 1987; Penketh 1983; Schaad 1986; Schaad 1987; Wang 1988; Wesley 1988), or discussion of bacterial resistance patterns (Church 1997; Gold 1985; Hodson 1987; McLaughlin 1983; Penketh 1984; Salh 1992; Stephens 1983). Mortality was reported in seven studies (Caplan 1984; Conway 1985; De Boeck 1989; Hyatt 1981; McLaughlin 1983; Penketh 1984; Wientzen 1980).
Quality of life and adherence were not reported in any study.
Excluded studies
A total of 82 studies were excluded (Characteristics of excluded studies). Fifteen studies were not randomised (or were quasi‐randomised) (Cabezudo 1984; Hoogkamp‐Korstanje 1983; Jackson 1986; Jewett 1985; Krause 1979; Kuni 1992; Levy 1982a; Martin 1980; Michalsen 1981; Parry 1977; Popa 2001; Postnikov 2001; Postnikov 2001a; Rubio 1987; Shatunov 2001). Six studies related to treatment location, e.g. at home versus in hospital (Amelina 2000; Davis 1990; Donati 1987; Hjelte 1988; Klettke 1999; Wolter 1997). Seven studies were excluded since they did not include participants being treated for pulmonary exacerbations in both comparison arms (Brett 1992; Byrne 1995; Elborn 2000; Jensen 1987; Pedersen 1986; Permin 1983; Yasmin 1974). Five studies did not include an IV antibiotic comparator (Day 1988; Gold 1983; Heaf 1984; Levy 1982; Nikonova 2010). Two were excluded as the comparison was one of process; bronchoscopy‐guided management (Wainwright 2011) or pharmacist versus self‐care (Ramstrom 2000). Three studies were observational or non‐intervention studies (Dodge 1983; Hatziagorou 2013; Moss 1991) and two studies were in vitro studies (Aaron 2005; Balsamo 1986). The remaining 42 studies relate to dosing studies or pharmacokinetic or pharmacodynamic studies or toxicity studies (Adeboyeku 2011; Al‐Ansari 2006; Aminimanizani 2002; Beringer 2003; Beringer 2010; Burkhardt 2006; Canis 1998; Christensson 1992; Conway 1996a; Davis 1987; De Boeck 1998; Degg 1996; Eron 1983; Goldfarb 1987; Guglielmo 1996; Hamner 2006; Heininger 1993; Hubert 2009; Ivanov 1997; Jacobs 1985; Keel 2011; Kercsmar 1983; Kruger 2001; Kuzemko 1989; Labiris 2004; Li 1991; McCabe 2013; Mouton 1991; Nikolaizik 2005; Padoan 1988; Postnikov 2007; Powell 1983; Prayle 2013; Reed 1987; Reed 1987a; Riethmueller 2009; Roberts 1992; Smyth 2005; Turner 2013; Whitehead 2002; Winnie 1991; Wood 1996).
Studies awaiting classification
For 12 studies (15 references) we were unable to make a decision regarding inclusion or exclusion. For four of the studies it was unclear if the study was randomised (Crawley 2005; Huang 1979; Kapranov 1995; Vic 1997). We are awaiting data that report includable participant episodes in the case of the four cross‐over studies (Al‐Aloul 2005; Dinwiddie 1982; Döring 1995; Geborek 2003), one of which included a placebo arm (Döring 1995), and also the two studies that report multiple indications (e.g. exacerbations and suppressive regimens) (Latzin 2008; Parry 1978). The Beaudry study met our eligibility criteria, but did not report any outcomes listed in this review (Beaudry 1980). Finally, one abstract was cited in the reference list of a further article, we have not been able to access this abstract yet and as such details about participants, methods and interventions are still unclear (Harris 1984). In these cases we shall contact the study investigators for clarification or additional data and will make a decision regarding inclusion or exclusion based upon the responses received.
Risk of bias in included studies
The assessment of risk of bias in the included studies is summarised in the figures (Figure 2; Figure 3).
2.
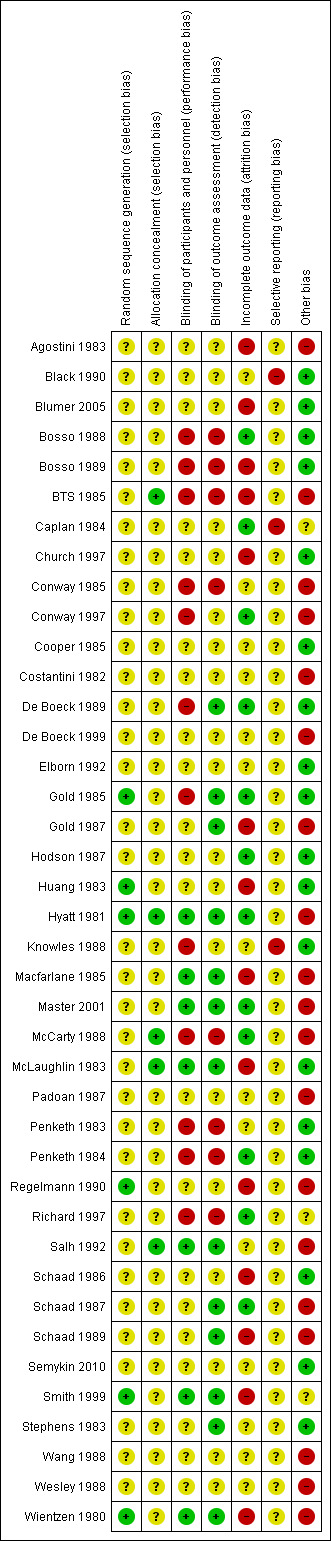
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
All studies were described as being randomised, although few described the method of generating the allocation sequence. Hence most included studies have been classified as being at an unclear risk of bias for this domain. In two studies, while it was stated that randomisation was carried out by pharmacists, the details of the method were not described and so the authors considered the risk of bias to be unclear (McLaughlin 1983; Salh 1992). A further study stratified randomisation by disease severity and age; however, again the method of randomisation used was not stated and so this study was also considered to be at an unclear risk of bias (Master 2001). Six studies were judged to be at a low risk of bias for sequence generation; four studies used a table of random numbers (Gold 1985; Hyatt 1981; Regelmann 1990; Wientzen 1980) and a further two were randomised by a randomisation code (Huang 1983; Smith 1999).
Allocation concealment
Four studies described either sequentially numbered envelopes or opaque envelopes as a mechanism for concealing allocation after randomisation (BTS 1985; McCarty 1988; McLaughlin 1983; Salh 1992). While no study used both sequential numbering or made clear that the envelopes were opaque, the authors regarded these studies to be at a low risk of bias with regard to allocation concealment. One study stated that both the antibiotics and placebo were prepared in the pharmacy and delivered in coded bottles; the code was not broken in case of 'treatment failure' (Hyatt 1981). This study was also considered to have a low risk of bias from allocation concealment. All other studies were considered to be at an unclear risk of bias due to insufficient information.
Blinding
Fourteen studies were described as double blind. Six of these studies involved the preparation of identical syringes or infusions prepared in pharmacy with adequate blinding of outcome assessment and so we felt them to be at a low risk of bias (Hyatt 1981; Macfarlane 1985; Master 2001; Salh 1992; Smith 1999; Wientzen 1980).
We judged the remaining eight studies which were described as double‐blind to have an unclear risk of bias. The Gold study attempted to blind both participants and outcome assessors; however, participant blinding was potentially compromised by participants being able to detect a characteristic odour from urine when they were treated with ceftazidime (Gold 1987). The effect of this has been classified as 'unclear' as it is unknown what proportion of participants had previously received ceftazidime and noticed the characteristic change (or otherwise); although it is noted that three participants who withdrew had correctly guessed that they were receiving placebo due to absence of urine odour. The Regelmann study involved the generation of sham drug levels, although no further detail was given and so we considered the study to also be at an unclear risk of bias (Regelmann 1990). The remaining six trials did not describe the method of participant blinding and so the risk of bias is also considered to be unclear (Agostini 1983; Church 1997; Huang 1983; McLaughlin 1983; Padoan 1987; Wesley 1988).
Eight studies were considered to be at a high risk of both performance and detection bias due to an open study design (Bosso 1988; Bosso 1989; BTS 1985; Conway 1985; McCarty 1988; Penketh 1983; Penketh 1984; Richard 1997).
Performance bias
Seven studies were considered to be at a low risk of performance bias due to adequate evidence of blinding of participants (Hyatt 1981; Macfarlane 1985; Master 2001; McLaughlin 1983; Salh 1992; Smith 1999; Wientzen 1980); 13 studies were considered to be at a high risk of performance bias due to no blinding of participants (Bosso 1988; Bosso 1989; BTS 1985; Caplan 1984; Conway 1985; Conway 1997; De Boeck 1989; Gold 1985; Knowles 1988; McCarty 1988; Penketh 1983; Penketh 1984; Richard 1997). With the remaining 20 studies we were unable to make an assessment due to insufficient information.
Detection bias
Thirteen studies were considered to be at a low risk of detection bias due to adequate evidence of outcome assessor blinding (De Boeck 1989; Gold 1985; Gold 1987; Hyatt 1981; Macfarlane 1985; Master 2001; McLaughlin 1983; Salh 1992; Schaad 1987; Schaad 1989; Smith 1999; Stephens 1983; Wientzen 1980). Eight studies were considered to be at a high risk of detection bias due to an open study design (Bosso 1988; Bosso 1989; BTS 1985; Conway 1985; McCarty 1988; Penketh 1983; Penketh 1984; Richard 1997). With the remaining 19 studies we were unable to make an assessment due to insufficient information.
Incomplete outcome data
Twelve studies were considered to be at a low risk of attrition bias; eight studies documented that there were no participants who withdrew during the study period and as such are considered to be at a low risk of bias (Bosso 1988; Caplan 1984; De Boeck 1989; Gold 1985; Hodson 1987; McCarty 1988; Penketh 1984; Schaad 1987). A further four studies reported an intention‐to‐treat analysis, or reported the last contributed data for those who withdrew and so are also considered to be at a low risk of bias (Conway 1997; Hyatt 1981; Master 2001; Richard 1997). Fourteen studies did not include participants who withdrew in the analysis (or where incomplete data are presented) and are considered to be at a high risk of bias (Agostini 1983; Blumer 2005; Bosso 1989; BTS 1985; Church 1997; Gold 1987; Huang 1983; Macfarlane 1985; McLaughlin 1983; Regelmann 1990; Schaad 1986; Schaad 1989; Smith 1999; Wientzen 1980). In some analyses this effect reaches a considerable proportion of the overall study group, as exemplified by the four studies contributing to the analysis of single IV antibiotics in combination with placebo versus combination IV antibiotics where 23% of participants do not contribute to the final analysis. Also in the comparison of a single agent (no placebo) versus an antibiotic combination, there were 10 studies (345 participants) reporting on FEV1; however only four studies with 152 participants contribute to the analysis.
Additionally, in the Wientzen study two participants died in the placebo group (one on Day 1 and the second on Day 4). Due to the small study size this is surprising and so suggests either a failure of random allocation or a difference in the characteristics of the comparator groups at baseline (Wientzen 1980). In the Regelmann study, one participant was withdrawn by the attending physician for "failing to improve rapidly enough". Furthermore of the 15 participants, only four in the placebo group and eight in the antibiotic group contribute data to the final analysis at two weeks (Regelmann 1990). Both studies are therefore considered to be at high risk of bias in the domain of incomplete outcome data.
The remaining 15 studies did not report details concerning withdrawals or adequacy of reporting to allow an assessment to be made.
Selective reporting
The inadequate reporting of studies made it difficult to reach a decision regarding selective reporting in the majority of instances. This was largely due to many of the included studies being undertaken prior to the establishment of trial registries and routine archiving of study protocols. In two studies it was clear that lung function data had been recorded but not reported (Black 1990; Caplan 1984) and another stated that time‐to‐next exacerbation data were recorded, but not reported (Knowles 1988). We considered these studies to have a high risk of bias. We were unable to retrieve the protocols for any study and so were unable to determine a study to be at a low risk of bias.
Other potential sources of bias
An additional issue with the outcomes addressed in this review is the heterogeneity with which they are reported, not only in terms of the wide variety of units for change in lung function (absolute change, percentage change in absolute values (litres), per cent change in % predicted values) but also the variety of measures reported (BMI, weight, proportion underweight) that make contributing studies to a meta‐analysis challenging.
A unit of analysis issue was introduced in 20 studies which involved people who participated on more than one occasion in a study (Agostini 1983; BTS 1985; Conway 1985; Conway 1997; Costantini 1982; De Boeck 1999; Gold 1987; Hyatt 1981; Macfarlane 1985; Master 2001; McCarty 1988; Padoan 1987; Regelmann 1990; Salh 1992; Schaad 1986; Schaad 1987; Schaad 1989; Wang 1988; Wesley 1988; Wientzen 1980). In some circumstances the proportion of re‐admittances to the study was considerable. In the Conway study, 18 out of 53 participants contributed multiple data points (34%). This was not only a considerable proportion within the study, but this also contributed a substantial degree of weight (47.1%) contributed to the analysis (Analysis 5.4).
5.4. Analysis.

Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 4 FEV1 (all measures).
In addition in the Penketh study, participants who isolated other (non‐pseudomonas) bacteria at baseline received "appropriate oral antibiotics" although the antibiotics, and distribution of administration are not noted (Penketh 1984).
We could identify no other sources of bias except in one study where a co‐author was affiliated to a pharmaceutical company that produced a drug in test (Richard 1997) and another study that was funded by a pharmaceutical company that produced a drug under test (Smith 1999). In neither case did we have enough information to reach a decision on the effect these relationships had toward bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Due to the large number of comparisons and outcomes measures, we have only reported below on those for which we either have data or narrative information.
IV antibiotic versus placebo
Single IV antibiotic versus placebo
Two studies with 48 participants compared a single IV antibiotic to placebo (Gold 1987; Wientzen 1980). Gold administered ceftazidime to 26 individuals (31 events) at a dose of 200 mg/kg/day (Gold 1987). Wientzen administered tobramycin to 22 individuals (24 events) at a dose of 2 mg/kg three times daily (Wientzen 1980). Both studies involve inherent attrition biases and unit of analysis issues (Figure 3).
Primary outcomes
1. Lung function
Gold reported both FEV1 and FVC data (see below) (Gold 1987). Wientzen reported the number of participants who demonstrated improved pulmonary function, which was defined as a greater than 15% improvement in two of FEV1, FVC or PEFR; four out of six participants in the tobramycin group improved, but none of the seven participants in the placebo group met this improvement criterion (Wientzen 1980). The study investigators found this difference significant (P < 0.05).
a. FEV1
Gold reported data for the percentage (relative) change in FEV1 % predicted; however, these are reported as means and 95% CIs with a typographical error in the CI for the placebo group, making the use of these data impossible. The study authors, however, conclude that "no significant differences were seen in any outcome measure" (Gold 1987).
b. FVC
Gold also reported the percentage (relative) change in % predicted FVC (Gold 1987). Values for SDs were calculated from the stated CIs; however, two of the participants had two episodes where they received ceftazidime and therefore contribute data to the intervention group twice. There was no difference detected between the two groups when data were analysed, MD 13.00% (95% CI ‐1.23 to 27.23) (Analysis 1.1).
1.1. Analysis.
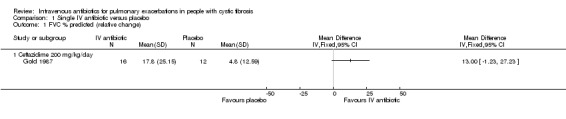
Comparison 1 Single IV antibiotic versus placebo, Outcome 1 FVC % predicted (relative change).
Secondary outcomes
2. Nutritional status
b. Weight
Gold reported mean weight gain as a percentage of initial weight (Gold 1987). Again, two of the participants had two episodes where they received ceftazidime and therefore contribute data to the intervention group twice.There was no difference between the two groups, MD 0.50% (95% CI ‐1.78 to 2.78) (Analysis 1.2).
1.2. Analysis.
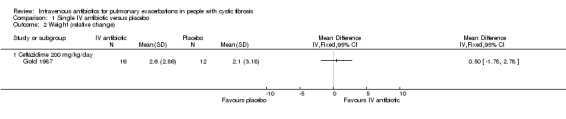
Comparison 1 Single IV antibiotic versus placebo, Outcome 2 Weight (relative change).
4. Mortality
In the Wientzen study, two participants in the placebo group died; one after 24 hours of treatment with placebo, the second participant received two days of placebo treatment then changed to antibiotic treatment and died two days later (Wientzen 1980). Gold reported no deaths (Gold 1987).
5. Adverse effects
a. toxicity and allergy
Wientzen only reported on nephrotoxicity, but did not detect this in either group (Wientzen 1980).
Combination of IV antibiotics versus placebo
One study with 15 participants compared a combination of IV antibiotics (tobramycin and ticarcillin) to placebo (Regelmann 1990). Data were presented graphically and so these were extracted using XYPLOT (XYPLOT 2010). By the end of the study only eight participants in the antibiotic group and four in the placebo group contributed data.
Primary outcomes
1. Lung function
a. FEV1
Data reported for absolute change in % predicted FEV1 demonstrated a statistically significant improvement in favour of IV antibiotics, MD 16.80% (95% CI 13.17 to 20.43) (Analysis 2.1).
2.1. Analysis.

Comparison 2 Combination IV antibiotic versus placebo, Outcome 1 FEV1 % predicted (absolute change).
b. FVC
Data reported for absolute change in % predicted FVC also demonstrated a statistically significant improvement in favour of IV antibiotics, MD 15.40% (95% CI 11.96 to 18.84) (Analysis 2.2).
2.2. Analysis.

Comparison 2 Combination IV antibiotic versus placebo, Outcome 2 FVC % predicted (absolute change).
Secondary outcomes
2. Nutritional status
Both groups reported increased weight with no difference between groups (no data presented).
5. Adverse effects
a. toxicity and allergy
No ototoxicity or blood parameter changes were noted in either group.
IV antibiotic regimens compared
A total of 29 studies with 1446 participants (reporting data from 1035 participants) compared one regimen of IV antibiotics to another IV regimen (either with or without placebo) (Agostini 1983; Blumer 2005; Bosso 1988; BTS 1985; Caplan 1984; Church 1997; Conway 1985; Conway 1997; Costantini 1982; De Boeck 1989; De Boeck 1999; Elborn 1992; Gold 1985; Huang 1983; Hyatt 1981; Macfarlane 1985; Master 2001; McCarty 1988; McLaughlin 1983; Padoan 1987; Penketh 1983; Penketh 1984; Salh 1992; Schaad 1986; Schaad 1989; Semykin 2010; Smith 1999; Wang 1988; Wesley 1988).
Single IV antibiotic regimens compared
Five studies with 251 participants compared two (or more) single antibiotic regimens (Agostini 1983; Caplan 1984; Elborn 1992; Huang 1983; Salh 1992). Agostini (n = 147) compared five different antibiotics ‐ azlocillin versus piperacillin versus ceftazidime versus cefsulodin versus cefoperazone (Agostini 1983); and Huang (n = 29) compared carbenicillin versus azlocillin (Huang 1983). Unfortunately in these two studies lung function was reported as part of a composite clinical score and so the only includable data relate to adverse effects (Agostini 1983; Huang 1983). Caplan (n = 29) compared cefsulodin to tobramycin or ticarcillin, but again the only includable data relate to adverse effects (Caplan 1984). The remaining two studies, Elborn (n = 24) and Salh (n = 22), both compared ceftazidime to aztreonam (Elborn 1992; Salh 1992).
Primary outcomes
1. Lung function
a. FEV1
Both Elborn and Salh report absolute change in FEV1 (Elborn 1992; Salh 1992); the SDs of mean change were imputed as discussed previously (Included studies). Neither study identified a significant difference between the two groups with the pooled MD also being statistically non‐significant, MD ‐0.12 litres (95%CI ‐1.08 to 0.84) (Analysis 3.1).
3.1. Analysis.
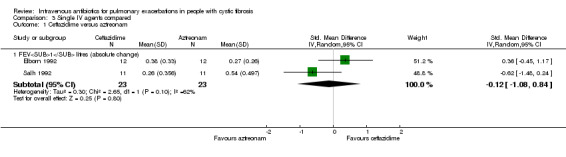
Comparison 3 Single IV agents compared, Outcome 1 Ceftazidime versus aztreonam.
Secondary outcomes
4. Mortality
In the Caplan study, two participants died; however, from the clinical data provided it would appear that these deaths could not be attributed to cefsulodin (Caplan 1984).
5. Adverse effects
a. toxicity and allergy
Agostini reported rates of symptoms of side effects and laboratory findings in two publications (Agostini 1983). The 1983 paper by Agostini reports more measures of adverse effects, but appears to report before the end of the study (Table 4); a further report of the same study by Mastella appears to report from the whole study for liver, renal and haematological parameters (Agostini 1983). These data are presented in the additional tables (Table 4; Table 5). It is difficult to attribute such reports with individual antibiotics with groups containing so few participants. The authors suggested that reports of nausea and vomiting with cefsulodin correlated with infusion rate and that fever and rash with piperacillin between 10 to 12 days was noticeable. They noted a transient rise in serum liver transaminases with all antibiotics except for azlocillin; and eosinophilia with all antibiotics, but particularly with ceftazidime and piperacillin.
1. Comparison of single antibiotics ‐ adverse effects.
| Agostini 1983 | Adverse effect |
Azlocillin n = 16 |
Piperacillin n = 23 |
Ceftazidime n = 28 |
Cefsulodin n = 19 |
Cefoperazone n = 15 |
| Reported symptoms | Fever | 3.6% | 18.7% | 0% | 2.6% | 0% |
| Rash | 3.6% | 12.5% | 0% | 10.3% | 3.8% | |
| Itching | 0% | 3.1% | 05 | 3.4% | 0% | |
| Nausea & vomiting | 0% | 3.1% | 3.1% | 34.5% | 0% | |
| Diarrhoea | 3.6% | 0% | 0% | 10.3% | 26.9% | |
| Vertigo | 0% | 3.1% | 0%% | 0% | 0% | |
| Laboratory findings | Raised AST (SGOT) | 0% | 12.5% | 9.3% | 6.8% | 3.8% |
| Raised ALT (SGPT) | 3.65 | 21.8% | 15.6% | 20.7% | 7.7% | |
| Leucopenia | 0% | 6.2% | 3.1% | 0% | 0% | |
| Eosinophilia | 28.6% | 34.4% | 43.7% | 20.7% | 26.9% | |
| Bleeding time increased | 05 | 6.2% | 0% | 0% | 0% | |
| Proteinuria | 0% | 0% | 0% | 0% | 0% | |
| Haematuria | 7.1% | 9.4% | 0% | 0% | 0% | |
| LAD 5th | 7.1% | 0% | 6.0% | 13.0% | 7.0% | |
| New bacterial species emerging after treatment | Achromobacter species | 0 | 0 | 2 | 0 | 0 |
| Candida species | 1 | 1 | 3 | 2 | 2 | |
| Enterobacter species | 3 | 3 | 0 | 0 | 1 | |
| Haemophilus species | 2 | 2 | 0 | 1 | 0 | |
| Pseudomonas alcaligenes | 0 | 0 | 1 | 0 | 0 | |
| Pseudomonas maltophilia | 1 | 0 | 3 | 0 | 0 | |
| Staphylococcus species | 2 | 3 | 0 | 0 | 0 |
ALT: alanine aminotransferase AST: aspartate aminotransferase SGOT: serum glutamic‐oxaloacetic transaminase SGPT: serum glutamic‐pyruvic transaminase
2. Data from Mastella 1983.
| Feature |
Azlocillin (%) |
Piperacillin (%) |
Ceftazidime (%) |
Cefsulodin (%) |
Cefoperazone (%) |
| Fever | 2.5 | 18.7 | 5.5 | ||
| Rash | 2.5 | 12.5 | 5.5 | 3 | |
| Itching | 3.1 | 2.7 | |||
| N&V | 3.1 | 3.1 | 30.5 | ||
| Diarrhoea | 2.5 | 8.3 | 24.2 | ||
| Vertigo | 3.1 |
N&V: nausea and vomiting
Caplan reported headache in one participant (7.1%) receiving cefsulodin and transiently raised liver enzymes in two participants (13%) receiving tobramycin (Caplan 1984).
Huang reported one case of rash in both of the groups (azlocillin or carbenicillin); also, cases of transient increase in serum liver enzymes were reported in six of the 14 participants receiving carbenicillin and in two of the 12 participants in the azlocillin group (Huang 1983).
b. microbiological ‐ isolation of antibiotic resistant strains, or new strains of bacteria
Agostini reported rates of emergent strains following treatment (Table 4). The numbers in each group are small and so it is difficult to determine trends except that it was uncommon for new strains to emerging following treatment (Agostini 1983). Caplan discussed an 'indication' of increasing resistance to cefsulodin but did not provide data (Caplan 1984). Both studies report rates of antibiotic sensitivity following treatment, but not rates of sensitivity at baseline. In the comparison of ceftazidime and aztreonam, Salh reported that from a baseline of sensitivity, two out of 12 participants receiving ceftazidime and three out of 14 participants receiving aztreonam developed an increase in minimum inhibitory concentration (MIC) above 16 mg/l; and concluded no difference in rates of resistance between the groups (Salh 1992).
Single IV antibiotic in combination with placebo versus combination IV antibiotics
Four studies with 189 participants, but reporting data for only 145 participants, considered a single IV antibiotic in combination with a placebo compared to an active two‐agent antibiotic combination (Macfarlane 1985; Master 2001; McLaughlin 1983; Smith 1999). MacFarlane considered two doses of piperacillin (50 mg/kg six times daily and 100 mg/kg three times daily) each in combination with tobramycin compared to tobramycin with placebo (Macfarlane 1985). Master compared tobramycin in combination with placebo with tobramycin combined with ceftazidime (Master 2001). McLaughlin considered the combination of ticarcillin plus tobramycin compared with azlocillin plus tobramycin and compared with azlocillin plus placebo (McLaughlin 1983). Smith considered the combination of azlocillin plus tobramycin compared with azlocillin plus placebo (Smith 1999).
A further study enrolled 15 participants to compare oxacillin in combination with placebo to a combination of oxacillin, sisomycin and carbenicillin (Hyatt 1981).
Each of these studies included attrition bias and two also had unit of analysis issues (Hyatt 1981; Macfarlane 1985) (see Characteristics of included studies).
Primary outcomes
1. Lung function
a. FEV1
Two further studies reported absolute change in FEV1 % predicted for comparisons between azlocillin plus tobramycin and azlocillin plus placebo (McLaughlin 1983; Smith 1999). A meta‐analysis of these two similar studies (I2 = 0%) demonstrated no significant difference between the two groups, pooled MD 1.37% (95% CI ‐1.50 to 4.23) (Analysis 4.1). Hyatt reported the absolute change in FEV1 % predicted in the comparison between oxacillin plus placebo versus oxacillin plus sisomycin plus carbenicillin (Hyatt 1981) and concluded there was a significant difference in favour of the three‐drug combination regimen, MD ‐9.54% (95% ‐15.98 to ‐3.10) (Analysis 4.1). Master reported the absolute change in FEV1 % predicted in the comparison between tobramycin plus placebo and tobramycin plus ceftazidime (Master 2001). There was no statistically significant difference between the two groups, MD ‐2.20% (95% CI ‐6.63 to 2.23) (Analysis 4.1). Analysis of a pooled estimate of FEV1 (% predicted) involving these four studies (Hyatt 1981; Master 2001; McLaughlin 1983; Smith 1999) yields no statistically significant difference between groups ‐0.89% (95% CI ‐3.14 to 1.36) (Analysis 4.1).
4.1. Analysis.
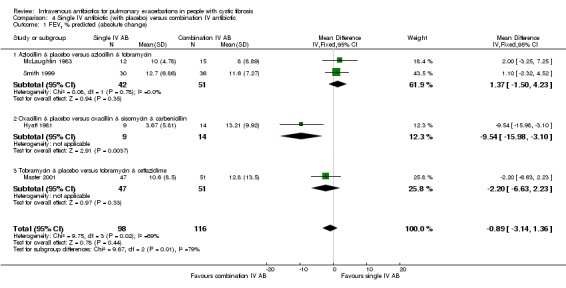
Comparison 4 Single IV antibiotic (with placebo) versus combination IV antibiotic, Outcome 1 FEV1 % predicted (absolute change).
MacFarlane reported data for percentage (relative) change in FEV1 % predicted for two doses of piperacillin: 50 mg/kg every four hours and 100 mg/kg every eight hours (Macfarlane 1985). There were improvements in lung function in all groups across the study period, but no significant differences between groups: piperacillin 50 mg/kg, MD ‐4.20 (95% CI ‐26.50 to 18.10); and piperacillin 100 mg/kg, MD 7.95 (95% CI ‐8.78 to 24.68). Even when piperacillin groups were combined (data not provided in paper but a pooled MD was computed in the meta‐analysis), the result was not statistically significant, MD ‐ 3.58% (95% CI ‐9.80 to 16.96) (Analysis 4.2).
4.2. Analysis.
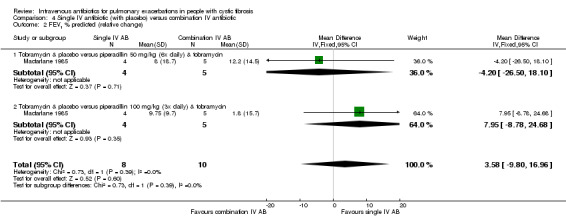
Comparison 4 Single IV antibiotic (with placebo) versus combination IV antibiotic, Outcome 2 FEV1 % predicted (relative change).
b. FVC
Two studies reported the absolute change in FVC % predicted for comparisons between azlocillin plus tobramycin compared with azlocillin plus placebo (McLaughlin 1983; Smith 1999). A meta‐analysis of these two studies demonstrated no significant difference between the two groups, pooled MD 1.18% (95% CI ‐2.53 to 4.89) (I2 = 0%) (Analysis 4.3).
4.3. Analysis.
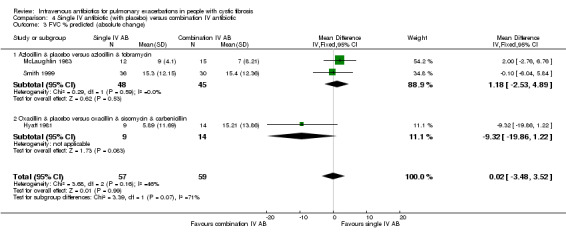
Comparison 4 Single IV antibiotic (with placebo) versus combination IV antibiotic, Outcome 3 FVC % predicted (absolute change).
Hyatt reported the absolute change in FVC % predicted in the comparison between oxacillin in combination with placebo compared with oxacillin, sisomycin and carbenicillin (Hyatt 1981), concluding no statistically significant difference between the two groups, MD ‐9.32% (95% CI ‐19.86 to 1.22) (Analysis 4.3). A meta‐analysis of each of these three studies yields no significant difference between the two groups, pooled MD 0.02% (95% CI ‐3.48 to 3.52) (Analysis 4.3).
MacFarlane reported the percentage (relative) change in % predicted FVC (Macfarlane 1985). There were improvements in lung function in all groups across the study period, but no significant differences between groups: piperacillin 50 mg/kg, MD ‐1.20% (95% CI ‐15.79 to 13.39); and piperacillin 100 mg/kg, MD ‐1.35% (95% CI ‐18.61 to 15.91) (Analysis 4.4). Even when piperacillin groups were combined (data not provided in paper but computed in meta‐analysis), the result was not statistically significant, pooled MD ‐1.26% (95% CI ‐12.40 to 9.88) (Analysis 4.4).
4.4. Analysis.

Comparison 4 Single IV antibiotic (with placebo) versus combination IV antibiotic, Outcome 4 FVC % predicted (relative change).
2. Time to next exacerbation
McLaughlin reported on the time to next exacerbation with no significant difference between groups, MD ‐7.00 weeks (95% CI ‐23.67 to 9.67) (Analysis 4.5). Smith reported a survival analysis of data for the time to next exacerbation, concluding that at 80 days post admission, only 30% of those receiving the combination antibiotic regimen had been re‐admitted compared to 62% of those receiving azlocillin alone (ANOVA P < 0.01) (Smith 1999). Master did not reported any significant difference in the time to next exacerbation between the two groups: for the single‐agent treatment there were a mean of 173 days and a median (range) of 107 (44 to 476) days until the next exacerbation; and for the combination treatment, there was a mean of 153 days and a median (range) of 125 days (41 to 417) days until the next exacerbation (Master 2001).
4.5. Analysis.
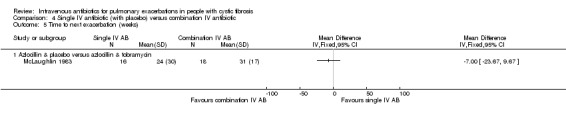
Comparison 4 Single IV antibiotic (with placebo) versus combination IV antibiotic, Outcome 5 Time to next exacerbation (weeks).
Secondary outcomes
2. Nutritional status
b. weight
Only McFarlane reported absolute changes in weight during treatment (Macfarlane 1985). There were no significant differences between groups, either individually, MD ‐0.72 kg (95% CI ‐2.65 to 1.21) and MD ‐0.07 kg (95% CI ‐1.83 to 1.69), or when both antibiotic groups and both placebo groups were combined, MD ‐0.36 kg (95% CI ‐1.66 to 0.93) (Analysis 4.6).
4.6. Analysis.
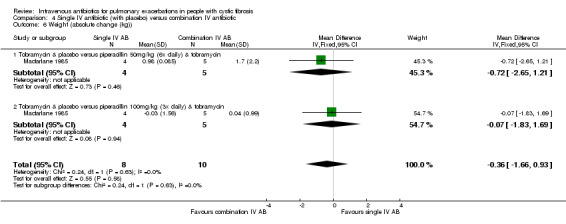
Comparison 4 Single IV antibiotic (with placebo) versus combination IV antibiotic, Outcome 6 Weight (absolute change (kg)).
4. Mortality
Two studies report there were no deaths (Hyatt 1981; McLaughlin 1983).
5. Adverse effects
a. toxicity and allergy
Macfarlane stated that during the 10 treatment periods with piperacillin, there were three episodes of sensitivity reactions (all in the higher‐dose group), consisting of nausea, vomiting, pruritic rashes, nocturnal fever, and facial oedema (Analysis 4.7); one participant withdrew as a result (Macfarlane 1985). Laboratory studies in all participants were normal throughout the study, except for one participant in the piperacillin group who had pyuria (Macfarlane 1985).
4.7. Analysis.
Comparison 4 Single IV antibiotic (with placebo) versus combination IV antibiotic, Outcome 7 Adverse effects ‐ sensitivity reaction.
A meta‐analysis of total adverse effects reported by two further studies demonstrated no significant difference between groups (McLaughlin 1983; Smith 1999), OR 1.08 (95% CI 0.50 to 2.37) (I2 = 0%) (Analysis 4.8). Two studies reported on ototoxicity with no cases in either group (Hyatt 1981; Smith 1999). The same two studies reported on nephrotoxicity with a non‐significant difference between groups, OR 0.63 (95% CI 0.05 to 7.27). Smith further reported on proteinuria and infusion site irritation, both which were non‐significant OR 0.21 (95% CI 0.02 to 1.89) and OR 1.62 (95% CI 0.26 to 10.08) respectively. Master reported on tinnitus on two occasions on one participant in each group (due on both occasions to inadvertent fast administration of tobramycin), OR 1.09 (95% CI 0.15 to 8.06) (Analysis 4.8). Master also reported on serum adverse effects in terms of serum creatinine (Master 2001), OR 4.00 (95% CI‐1.38 to 9.38), and serum NAG, OR 2.10 (95% CI 0.74 to 3.46) concluding that single‐agent treatment was less nephrotoxic (Analysis 4.9).
4.8. Analysis.
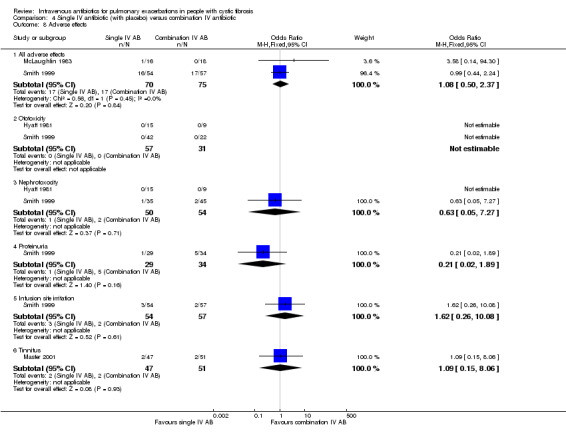
Comparison 4 Single IV antibiotic (with placebo) versus combination IV antibiotic, Outcome 8 Adverse effects.
4.9. Analysis.
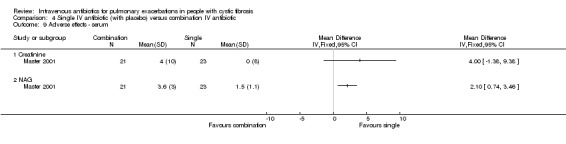
Comparison 4 Single IV antibiotic (with placebo) versus combination IV antibiotic, Outcome 9 Adverse effects ‐ serum.
b. microbiological ‐ isolation of antibiotic resistant strains, or new strains of bacteria
Five studies reported on this outcome (Hyatt 1981; Macfarlane 1985; Master 2001; McLaughlin 1983; Smith 1999). Hyatt examined sisomycin resistance and reported no significant change over time (Hyatt 1981). Macfarlane noted no change in susceptibility profiles in any group (Macfarlane 1985). Master reported increases in MIC in both treatment groups that reached significance in the single‐agent group (Master 2001). McLaughlin reported a high baseline rate of in vitro antibiotic resistance (40%) and an increase in the proportion of resistant isolates, but there were no differences between groups (P = 0.13; Fisher's exact test) (McLaughlin 1983). Smith reported a significant increase in the proportion of isolates resistant to either azlocillin, tobramycin or both in the combined azlocillin plus tobramycin‐treated group compared to those who received only azlocillin (ANOVA P < 0.001) (Smith 1999).
Single agent (no placebo) versus antibiotic combination
Ten studies (345 participants) compared a single IV antibiotic with a combination of two IV antibiotics. Of these six studies had unit of analysis issues (BTS 1985; Church 1997; De Boeck 1999; McCarty 1988; Padoan 1987; Wesley 1988). Three studies investigated the use of combination antibiotic treatment by comparing the effect of a single antibiotic with the same antibiotic in combination with another agent (Conway 1997; McCarty 1988; Padoan 1987) and six compared a single agent to two different antibiotics in combination (Bosso 1988; BTS 1985; De Boeck 1989; De Boeck 1999; Gold 1985; Wesley 1988).
The antibiotics and the combinations used were varied. Single agents compared with a combination including an additional antibiotic involved colistin with and without another antibiotic (Conway 1997) and piperacillin with and without tobramycin (McCarty 1988). The most commonly investigated antibiotic as the single agent was ceftazidime which was compared to: ceftazidime and sisomycin and piperacillin and sisomycin (Padoan 1987); gentamicin and carbenicillin (BTS 1985); tobramycin and piperacillin (De Boeck 1989); tobramycin and ticarcillin (Gold 1985; Wesley 1988). Other comparisons involved ceftazidime in combination with tobramycin compared to meropenem alone (De Boeck 1999) and aztreonam compared to tobramycin with azlocillin (Bosso 1988).
Unfortunately for one study clinical outcomes were reported in terms of a proprietary clinical score and so, although there appeared to be no significant differences between groups, includable data are not available to be presented in the review (Padoan 1987).
Primary outcomes
1. Lung function
One study did not report data for lung function outcomes in such a way that we could analyse them; however, it narratively reported no difference between groups in terms of change in peak expiratory flow rate (BTS 1985). A further study (in abstract form) narratively reported results with subgroups being classified as "exacerbation" or "electively" treated and stated a significant increase in vital capacity in both groups in the "exacerbation" group (De Boeck 1999). McCarty also narratively presented the lung function data from the study, reporting that both groups witnessed similar improvement in peak flow, FEV1 and FVC (McCarty 1988). Similarly, Wesley (another abstract) narratively reported the data in their study suggesting no difference in "pulmonary function" between groups at the end of 14 days of treatment (Wesley 1988). Church reported only mean change in lung function without a measure of distribution of the data and so we could not include data from this study. The duration of treatment in the two groups was also not reported making interpretation difficult (Church 1997).
a. FEV1
One study reported absolute change in absolute values (ml) for FEV1 finding a statistically significant effect favouring a combination regimen, MD ‐160.00 ml (95% CI ‐309.72 to ‐10.28) (Conway 1997) (Analysis 5.1). Two studies reported absolute change in FEV1 % predicted (De Boeck 1989; Bosso 1988) and one of these also reported percentage (relative) change in FEV1 % predicted FEV1 (Bosso 1988). Neither found any significant difference in absolute change between single and combination antibiotic regimens, MD 1.00 (95% CI ‐8.85 to 10.85) (De Boeck 1989) and MD ‐4.60 (95% CI ‐11.57 to 2.37) (Bosso 1988); when pooled there was also a non‐significant result, MD ‐2.73 (95% CI ‐8.42 to 2.95) (Analysis 5.2).
5.1. Analysis.
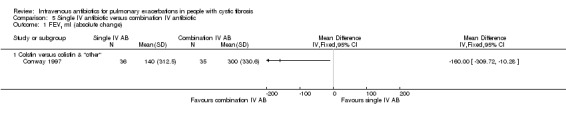
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 1 FEV1 ml (absolute change).
5.2. Analysis.
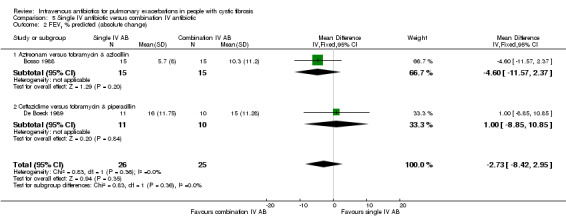
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 2 FEV1 % predicted (absolute change).
A further study reported the percentage (relative) change in absolute FEV1 with (contrary to the interpretation in the paper) a significant difference between groups favouring the combination agent regimen, MD ‐19.60 (95% CI ‐38.26 to ‐0.94) (Gold 1985) (Analysis 5.3).
5.3. Analysis.
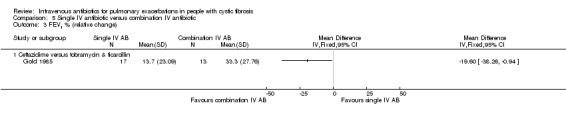
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 3 FEV1 % (relative change).
The remaining study reporting on this outcome found no difference in lung function tests when comparing ceftazidime with tobramycin and ticarcillin, but no data are presented (Wesley 1988).
A meta‐analysis using SMDs to analyse all measures of FEV1 in the three studies with a total of 122 participants demonstrates a statistically significant effect favouring combination antibiotic regimens (Bosso 1988; Conway 1997; De Boeck 1989), pooled SMD ‐0.38 (95% CI ‐0.74 to ‐0.02) with no heterogeneity (I2 = 0%) (Analysis 5.4).
b. FVC
Conway reported FVC in terms of absolute change of absolute values (Conway 1997) and found a significant difference between the two groups favouring the combination regimen, MD ‐470.00 ml (95% CI ‐695.76 to ‐244.24) (Analysis 5.5). Bosso reported FVC both in terms of absolute change and percentage (relative) change in FVC % predicted (Bosso 1988). Contrary to the findings in the paper, there was a statistically significant difference between the two groups favouring the combination antibiotic regimen in both absolute change, MD ‐8.10% (95% CI ‐15.79 to ‐0.41) (Analysis 5.6) and percentage (relative) change, MD ‐10.80% (95% CI ‐20.67 to ‐0.93) (Analysis 5.7).
5.5. Analysis.
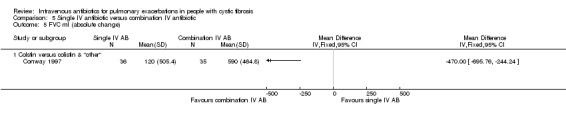
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 5 FVC ml (absolute change).
5.6. Analysis.
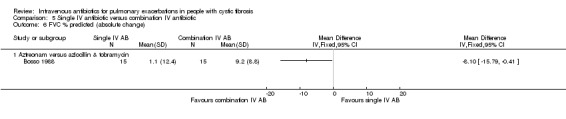
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 6 FVC % predicted (absolute change).
5.7. Analysis.
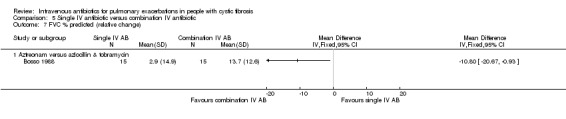
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 7 FVC % predicted (relative change).
A meta‐analysis using pooled SMDs to analyse all measures of FVC from the Bosso and Conway studies (101 participants) demonstrates a statistically significant effect favouring combination antibiotic regimens, pooled SMD ‐0.89 (95% CI ‐1.30 to ‐0.48) with no heterogeneity (I2 = 0%) (Analysis 5.8).
5.8. Analysis.
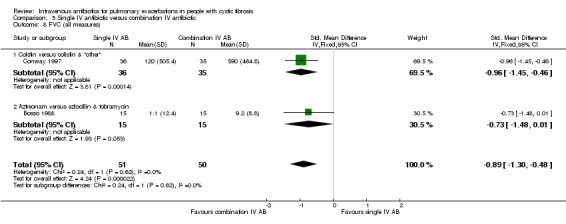
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 8 FVC (all measures).
2. Time to next exacerbation
De Boeck reported the time to re‐admission for all except four participants (one from each group who had not been re‐admitted at the time of the report and one from each group who died) (De Boeck 1989). There was no significant difference between the two groups, MD ‐1.00 months (95% CI ‐5.52 to 3.52) (Analysis 5.9). A second study reported the proportion of participants in each group who required re‐admission, IV antibiotics or who died in the three months following treatment (BTS 1985). Analysis showed a significant difference between the two groups favouring the single antibiotic group, OR 0.29 (95% CI 0.12 to 0.74) (Analysis 5.10). Wesley reported the proportion of participants who were re‐admitted within three months of treatment with no difference between the two groups, OR 1.40 (95% CI 0.26 to 7.58) (Wesley 1988). A pooled estimate for both studies suggests a significant effect in favour of single agents, OR 0.43 (95% CI 0.19 to 0.95), although largely influenced by one study (BTS 1985) and with substantial heterogeneity (I2 = 60%) (Analysis 5.10).
5.9. Analysis.

Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 9 Time to readmission (months).
5.10. Analysis.
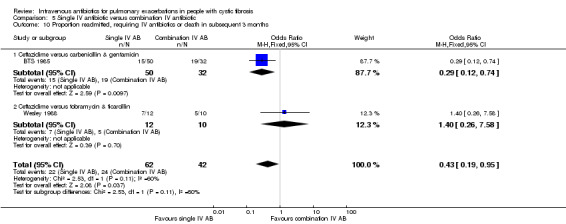
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 10 Proportion readmitted, requiring IV antibiotics or death in subsequent 3 months.
Secondary outcomes
2. Nutritional status
b. weight
Only one study reported data for percentage weight change which we could analyse (Gold 1985). There was no statistically significant difference between the two groups, MD ‐1.30% (95% CI ‐4.36 to 1.76) (Analysis 5.11).
5.11. Analysis.
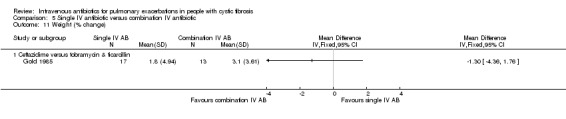
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 11 Weight (% change).
Conway noted absolute weight changes over time in the two groups; SDs were not stated and we are unable to impute them (Conway 1997). The study describes a statistically significant 8% weight gain from baseline in the combination IV antibiotic group (P < 0.01) compared to a 3% weight gain in the single IV antibiotic arm which was not statistically significant (P = 0.16). A second study reported significant mean (SEM) weight gain in both groups of 4 (1)%, with no difference between the two groups (De Boeck 1989). In the 1999 abstract the same authors narratively reported an ANOVA whereby only participants in the combination agent group gained "significant" weight (P < 0.05) (De Boeck 1999). A further study also reported weight data narratively, with similar improvements observed in both groups (McCarty 1988). Finally the BTS study stated there were no significant differences between groups although both treatment groups gained weight (BTS 1985).
3. Adherence (all measures)
Conway reported adherence to the two IV antibiotic regimens in hospitalised participants (Conway 1997). Four participants in the single antibiotic arm missed between one and five doses, while two participants in the combination treatment arm missed three doses of colistin and one participant missed four doses; reasons for missing doses were stated as the participant not being on the ward (n = 7), refusal of new IV line towards the end of treatment (n = 5), or leaving hospital on last day of treatment for work or school reasons. On five occasions no reason was given for missing doses and on two occasions it was unclear whether the doses had been given as this was not signed for on the drug chart.
4. Mortality
Three studies reported data we could analyse for this outcome (Conway 1997; De Boeck 1989; McCarty 1988). Conway reported the death of one participant in the combination antibiotic arm; this participant was understood to have severe "terminal CF" lung disease (Conway 1997). A second study reported one participant in each group who died; one in the single agent group died one month after treatment and one in the combination group died four months after treatment (De Boeck 1989). The McCarty study reported there were no deaths in either treatment arm (McCarty 1988). When pooled, results were not statistically significant, RR 0.62 (95% CI 0.09 to 4.37) (Analysis 5.12).
5.12. Analysis.
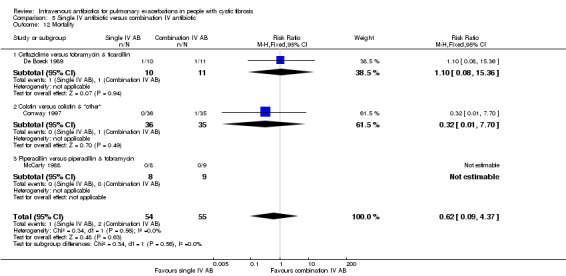
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 12 Mortality.
5. Adverse effects
a. toxicity and allergy
Five studies reported on liver enzymes and four had data for the analysis (Bosso 1988; BTS 1985; Gold 1985; Wesley 1988). Bosso reported changes in hepatic transaminases with 10 participants in the single agent group and five in the combination agent group experiencing elevated serum levels during treatment, but with no significant difference between groups, OR 4.00 (95% CI 0.88 to 18.26) (Bosso 1988). The BTS study reported the proportion of participants experiencing a rise in serum liver enzymes with no difference between the two groups, OR 0.58 (95% CI 0.18 to 1.85) (BTS 1985). Gold reported rises in AST for four out of 17 participants receiving single agent and two out of 13 participants receiving combination therapy, but no significant difference between the groups (Gold 1985). Wesley reported no change in liver enzymes in either group (Wesley 1988). A pooled analysis of liver effects demonstrate no significant difference between groups, pooled OR 1.00 (95% CI 0.48 to 2.07) (Analysis 5.13). Finally, Padoan reported narratively that 20% of participants in each treatment group experienced an increase in liver enzymes (Padoan 1987) (Table 6).
5.13. Analysis.
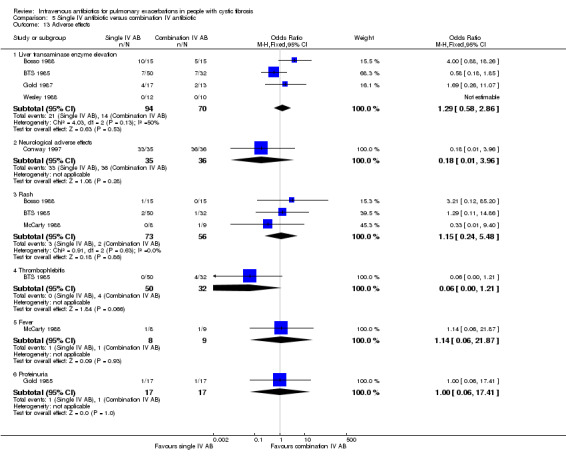
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 13 Adverse effects.
3. IV ceftazidime alone versus combination IV ceftazidime & IV sisomycin versus combination IV piperacillin & IV sisomycin: Adverse events.
| Padoan 1987 | Ceftazidime | Ceftazidime & sisomycin | Piperacillin & sisomycin |
| Adverse effect | |||
| Eosinophilia | 8/40 | 2/20 | |
| Raised liver enzymes | 20% | 20% | 20% |
| Fever | 7/20 | ||
| Renal impairment | 0 | 0 | 0 |
| Antibiotic resistance to agents at end of treatment | 30% | 37% & 40% | 40% & 32% |
Conway documented 37 neurological adverse events for 33 participants receiving the single antibiotic regimen, which resulted in one person withdrawing from the study (Conway 1997). In the combination antibiotic regimen arm 37 neurological adverse events were recorded for 36 participants; no change in treatment was needed as a result (Analysis 5.13).
Incidence of rash was reported by three studies (Bosso 1988; BTS 1985; McCarty 1988). Bosso reported one participant in the combination group who developed a rash which was thought to be due to azlocillin and as such administration of this was stopped (Bosso 1988). A second study also reported experience of rash with no difference between groups, OR 1.29 (95% CI 0.11 to 14.86) (BTS 1985). McCarty reported one participant in the single agent group who withdrew as a result of a rash (McCarty 1988). A pooled estimate of the comparison between groups reports no significant difference between groups, OR 1.15 (95% CI 0.24 to 5.48) (Analysis 5.13).
In two studies, the participants in the combination group who developed a rash also developed a fever and the participant in the BTS study additionally reported arthralgia (Bosso 1988; BTS 1985). McCarty reported that one participant in each group developed fever at day 13 (each resolved after cessation of antibiotic treatment), OR 1.14 (95% CI 0.06 to 21.87) (McCarty 1988). Finally, Padoan reported that seven of 20 participants who received piperacillin developed a fever between 9 and 14 days of treatment (Padoan 1987).
The BTS study reported that four participants in the combination group developed thrombophlebitis (and none in the single‐agent group); however, differences between groups did not reach statistical significance, OR 0.06 (95% CI 0.00 to 1.21) (Analysis 5.13). The BTS study also reported that one participant in the combination group "developed severe asthma" after one dose and so was withdrawn from the study (BTS 1985).
Proteinuria was reported in one participant in each group by Gold, OR 1.00 (95% CI 0.06 to 17.41) (Gold 1985).
In terms of renal toxicity, Conway noted statistically significant rises in blood urea and significant falls in creatinine clearance in both groups; however, there was no statistically significant difference between groups for either outcome (Analysis 5.14). Padoan reported that no changes in renal function were observed (Padoan 1987) (Table 6).
5.14. Analysis.

Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 14 Renal toxicity.
b. microbiological ‐ isolation of antibiotic resistant strains, or new strains of bacteria
One study reported that those participants with antibiotic‐resistant P. aeruginosa and B. cepacia responded as well to treatment as those with antibiotic‐sensitive strains (as determined by their proprietary clinical score) (Bosso 1988). The number of participants with resistant isolates increased with treatment with an additional five out of 14 participants, three out of 14 and none out of 14 participants isolating strains of P. aeruginosa resistant to aztreonam, tobramycin and azlocillin respectively (Analysis 5.15). The number of participants with resistant strains returned to baseline at follow up.
5.15. Analysis.
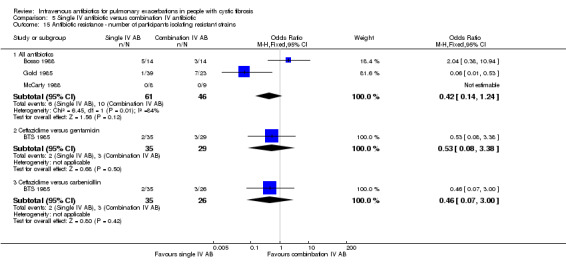
Comparison 5 Single IV antibiotic versus combination IV antibiotic, Outcome 15 Antibiotic resistance ‐ number of participants isolating resistant strains.
While Gold noted no correlation between clinical and bacteriological outcomes when reporting greater reductions in P. aeruginosa colony counts with the ceftazidime‐treated group (single agent), the emergence of antibiotic resistance, as defined by a greater than two‐fold increase in the MIC to the administered agents, occurred more frequently in those receiving the combination agent regimen (Gold 1985); one out of 39 participants in the single‐agent group and seven out of 23 participants in the combination‐agent group (Analysis 5.15). A pooled effect estimate including three studies (Bosso 1988; Gold 1985; McCarty 1988) suggests no statistically significant effect, OR 0.42 (95% CI 0.14 to 1.24), although with considerable heterogeneity (I2 = 84%) (Analysis 5.15).
The BTS study reported the acquisition of antibiotic resistance to each of the antibiotics administered (BTS 1985). Two out of 35 participants receiving ceftazidime, three out of 29 receiving gentamicin and three out of 26 receiving carbenicillin isolated antibiotic‐resistant P. aeruginosa strains following treatment. Comparing the proportion of participants isolating antibiotic‐resistant strains in the single‐agent (ceftazidime) group with the combination group consisting of gentamicin and carbenicillin there were no differences between the comparisons, OR 0.53 (95% CI 0.08 to 3.38) and OR 0.46 (95% CI 0.07 to 3.00) respectively (Analysis 5.15).
The development of antibiotic resistance was not witnessed in any participant in the McCarty study (McCarty 1988).
Padoan reported that the proportion of strains that were resistant to ceftazidime increased from 4% to 37% in both single and combination arms, although rather than returning to baseline after treatment, as was observed in the single‐agent arm, the proportion of isolates resistant to ceftazidime remained high (30%) in the combination group (Padoan 1987) (Table 6).
All‐group single agent versus combination treatment
1. Lung function
A meta‐analysis including eight studies with low heterogeneity for which we were able to present data comparing single‐agent with combination‐agent treatment did not show a statistically significant difference in FEV1, pooled SMD ‐0.21 (95% CI ‐0.42, 0.01) (Analysis 6.1). However, when we consider individual measures of reported FEV1 we find that the better quality studies reporting absolute and relative changes in FEV1 % predicted show no significant difference between single and combination treatment: absolute change in FEV1 % predicted, MD ‐1.14% (95% CI ‐3.23 to 0.95) (Analysis 6.3); relative change in FEV1 % predicted, MD 3.58% (95% CI ‐9.80 to 16.96) (Analysis 6.5); absolute change FVC % predicted MD ‐1.37 (95% CI ‐4.56 to 1.81) (Analysis 6.7). A meta‐analysis of five studies with moderate levels of heterogeneity favoured combination‐agent treatment for FVC, pooled SMD ‐0.44 (95% CI ‐0.71 to ‐0.16) (Analysis 6.6).
6.1. Analysis.
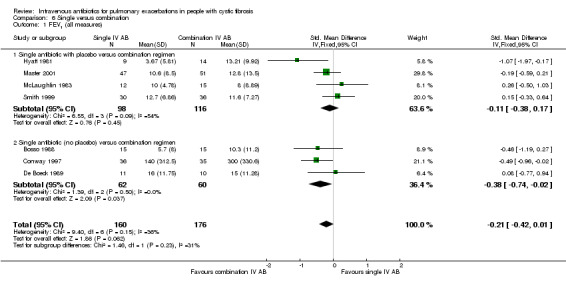
Comparison 6 Single versus combination, Outcome 1 FEV1 (all measures).
6.3. Analysis.
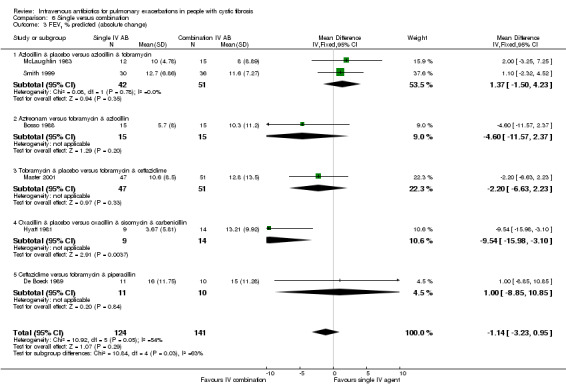
Comparison 6 Single versus combination, Outcome 3 FEV1 % predicted (absolute change).
6.5. Analysis.
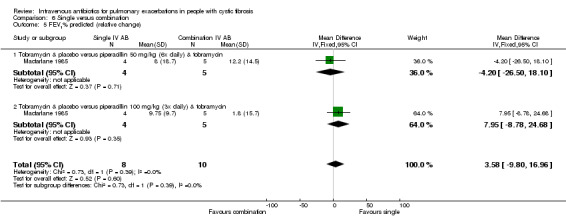
Comparison 6 Single versus combination, Outcome 5 FEV1% predicted (relative change).
6.7. Analysis.
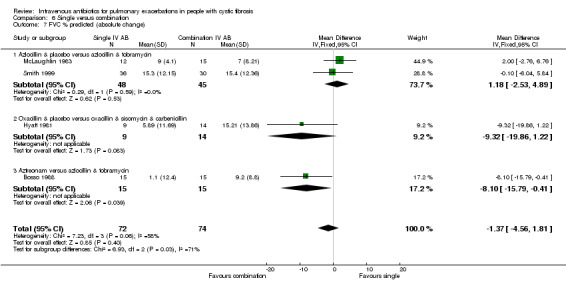
Comparison 6 Single versus combination, Outcome 7 FVC % predicted (absolute change).
6.6. Analysis.
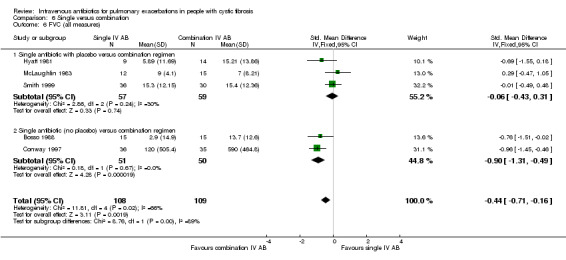
Comparison 6 Single versus combination, Outcome 6 FVC (all measures).
Combination IV antibiotic regimens compared
Nine studies, recruiting 417 participants, compared two combinations of two IV antibiotics (Blumer 2005; Conway 1985; McLaughlin 1983; Penketh 1983; Penketh 1984; Schaad 1986; Schaad 1989; Semykin 2010; Wang 1988). Again the IV antibiotics used were varied. Two studies administered a combination of netilmicin and ticarcillin, but used different comparators: tobramycin and ticarcillin (Conway 1985) and netilmicin and azlocillin (Schaad 1986). Two studies by the same lead author compared carbenicillin and gentamicin to other IV antibiotic combinations ‐ ticarcillin and gentamicin (Penketh 1983) and azlocillin and gentamicin (Penketh 1984). In one study, in addition to the two arms comparing azlocillin and placebo with azlocillin and tobramycin, McLaughlin also compared these with a combination of ticarcillin and tobramycin, a comparison we include in this section (McLaughlin 1983). A further study also undertook this comparison with an additional arm comprising of oral ciprofloxacin (Wang 1988). We include the azlocillin and tobramycin versus tobramycin and ticarcillin comparison in this section. One study with an arm involving the administration of oral and nebulised antibiotics, also compared cefipime and amikacin with meropenem and amikacin (Semykin 2010). The remaining two studies compared meropenem and tobramycin versus ceftazidime and tobramycin (Blumer 2005) and aztreonam and amikacin versus ceftazidime and amikacin (Schaad 1989).
Primary outcomes
1. Lung function
The Conway study narratively reported significant improvements in lung function, but did not report a comparison between groups, reporting only data for PEFR (Conway 1985). Similarly, Wang narratively reported "favourable results" in lung function in the two arms that consisted of combination intravenous antibiotics, but with no difference between the two groups (Wang 1988). A third study reported percentage (relative) changes in FEV1 and FVC in the two intravenous regimen groups; however, they do not report appropriate data for a comparison between groups (Semykin 2010).
a. FEV1
For the Blumer study, which compared tobramycin administered in combination with either ceftazidime or meropenem (Blumer 2005), we imputed change in terms of absolute change in % predicted FEV1 for potential future use in a meta‐analysis; the result was not statistically significant, MD 2.70% (95% CI ‐0.76 to 6.16) (Analysis 7.1). In the publication, Blumer reported the percentage (relative) change from baseline of % predicted FEV1; analysis showed that there was no significant difference between the two groups, MD 9.40% (95% CI ‐8.44 to 27.24) (Analysis 7.2).
7.1. Analysis.
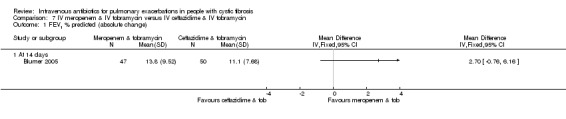
Comparison 7 IV meropenem & IV tobramycin versus IV ceftazidime & IV tobramycin, Outcome 1 FEV1 % predicted (absolute change).
7.2. Analysis.
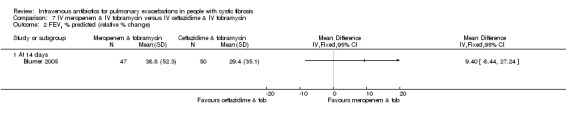
Comparison 7 IV meropenem & IV tobramycin versus IV ceftazidime & IV tobramycin, Outcome 2 FEV1 % predicted (relative % change).
Two studies reported the absolute change in FEV1 % predicted (McLaughlin 1983; Schaad 1989). McLaughlin compared tobramycin in combination with either azlocillin or ticarcillin and our analysis found no significant difference between the two groups, MD ‐3.00% (95% CI ‐8.75 to 2.75) (Analysis 8.1). Schaad compared aztreonam with amikacin to ceftazidime with amikacin and showed no significant difference between the two groups in absolute change in FEV1 % predicted at the end of intravenous therapy, MD 4.00% (95% CI ‐0.25 to 8.25) (Analysis 11.1).
8.1. Analysis.
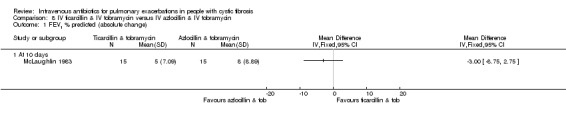
Comparison 8 IV ticarcillin & IV tobramycin versus IV azlocillin & IV tobramycin, Outcome 1 FEV1 % predicted (absolute change).
11.1. Analysis.

Comparison 11 IV aztreonam & IV amikacin versus IV ceftazidime & IV amikacin, Outcome 1 FEV1 % predicted (absolute change).
The later Penketh study reported absolute values in FEV1 (Penketh 1984). We imputed the SD of the mean change and detected no significant difference between the two groups in absolute change in FEV1, MD 51.00 ml (95% CI ‐358.68 to 460.68) (Analysis 9.1).
9.1. Analysis.

Comparison 9 IV azlocillin & IV gentamicin versus IV carbenicillin & IV gentamicin, Outcome 1 FEV1 ml (absolute change).
Penketh's 1983 study reported the change in FEV1, with statistically significant improvements from baseline in each group; however, they do not provide data to allow us to perform a between‐group comparison and they report no statistically significant differences between the groups (Penketh 1983).
b. FVC
The two studies by Schaad reported absolute change in FVC % predicted (Schaad 1986; Schaad 1989). Neither study found a significant difference between the two groups at the end of the IV therapy, MD 2.00% (95% CI ‐5.48 to 9.48) (Schaad 1986) (Analysis 10.1) and MD 2.00% (95% CI (‐5.17 to 9.17) (Schaad 1989) (Analysis 11.2).
10.1. Analysis.
Comparison 10 IV netilmicin & IV azlocillin versus IV netilmicin & IV ticarcillin, Outcome 1 FVC % predicted (absolute change).
11.2. Analysis.

Comparison 11 IV aztreonam & IV amikacin versus IV ceftazidime & IV amikacin, Outcome 2 FVC % predicted (absolute change).
The later Penketh study reported absolute change in FVC (Penketh 1984). Following the imputation of the SD of the change, we found no significant difference between the two groups, MD 74.00 ml (95% CI ‐410.48 to 558.48) (Analysis 9.2).
9.2. Analysis.
Comparison 9 IV azlocillin & IV gentamicin versus IV carbenicillin & IV gentamicin, Outcome 2 FVC ml (absolute change).
The earlier Penketh report does not provide data to allow us to analyse the change in absolute FVC. It reports statistically significant improvements from baseline in each group, but no data to allow us to perform a between‐group comparison; the differences between groups were not statistically significant (Penketh 1983).
2. Time to next exacerbation
One study reported an extended follow‐up period of two to four weeks after discontinuation of therapy and during this time 33 participants in the meropenem group and 38 participants in the ceftazidime group received treatment for an exacerbation (median period of 176 days and 207 days respectively) (Blumer 2005). Our analysis shows that this difference did not meet statistical significance, OR 0.72 (95% CI 0.31 to 1.67) (Analysis 7.3). McLaughlin reported time‐to‐next exacerbation data in weeks (McLaughlin 1983); there was no significant difference between the two groups, MD ‐6.00 weeks (95% CI ‐17.27 to 5.27) (Analysis 8.2).
7.3. Analysis.

Comparison 7 IV meropenem & IV tobramycin versus IV ceftazidime & IV tobramycin, Outcome 3 Participants experiencing an exacerbation.
8.2. Analysis.
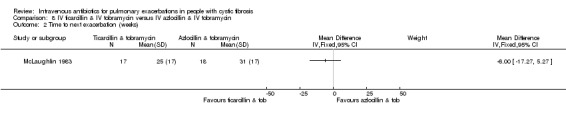
Comparison 8 IV ticarcillin & IV tobramycin versus IV azlocillin & IV tobramycin, Outcome 2 Time to next exacerbation (weeks).
In the 1983 study, Penketh reported a statistical test finding no significant difference between groups in the time to next admission due to an exacerbation; five participants in the carbenicillin group were re‐admitted on average 5.4 months following treatment, compared to three participants in the ticarcillin group being re‐admitted on average 3.5 months following treatment (Penketh 1983). Similarly, in the later study, Penketh reported the time to next exacerbation during the period of time since the study completed to the time of reporting (Penketh 1984). The study found that five participants in the azlocillin group were re‐admitted on average four months following treatment compared to five participants in the carbenicillin group being re‐admitted on average 3.6 months following treatment; this difference was not statistically significant.
Secondary outcomes
2. Nutritional status
b. weight
Conway reported percentage change in weight over the study period with significant gains in weight in both groups (3.2% and 3.1% netilmicin and tobramycin respectively), but does not comment upon differences between groups (Conway 1985). Both Schaad studies also report on weight as percentage underweight; in neither study were the differences significant (Schaad 1986; Schaad 1989).
4. Mortality
Two studies reported no deaths in in the first three months (Blumer 2005; McLaughlin 1983); although McLaughlin did further report 10 deaths in the subsequent 18 months (McLaughlin 1983). In the 1984 study, Penketh reported the death of one participant in the azlocillin group who did not respond sufficiently to go home and who later died (Penketh 1984), at 12‐month follow up RR 3.00 (95% CI 0.14 to 65.90) (Analysis 9.3).
9.3. Analysis.
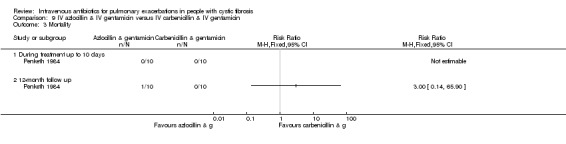
Comparison 9 IV azlocillin & IV gentamicin versus IV carbenicillin & IV gentamicin, Outcome 3 Mortality.
5. Adverse effects
a. toxicity and allergy
Two studies included general statements that they had observed no evidence of toxicity (Penketh 1984; Wang 1988). A further study reported treatment‐related adverse effects in 21 (40.4%) of participants in the ceftazidime group compared with 19 (38%) of those in the meropenem group (Blumer 2005).
Three studies commented on liver‐related adverse events (Penketh 1983; Schaad 1986; Schaad 1989). In the earlier study, Schaad reported significant reductions in serum alkaline phosphatase in both groups, but do not report a group‐wise analysis (Schaad 1986). They also reported no significant differences between groups in liver transaminase elevation, OR 1.58 (95% CI 0.24 to 10.60) (Analysis 10.2). In the 1989 study, Schaad reported analysable data for elevated liver enzymes, but this was not significant, OR 7.82 (95% CI 0.39 to 158.87) (Analysis 11.3). Penketh reported no evidence of hepatic toxicity in any participant (Penketh 1983).
10.2. Analysis.

Comparison 10 IV netilmicin & IV azlocillin versus IV netilmicin & IV ticarcillin, Outcome 2 Adverse effects.
11.3. Analysis.
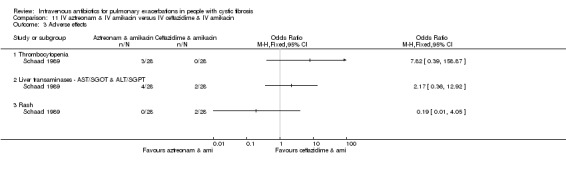
Comparison 11 IV aztreonam & IV amikacin versus IV ceftazidime & IV amikacin, Outcome 3 Adverse effects.
Four studies commented on renal toxicity (Conway 1985; Penketh 1983; Schaad 1986; Schaad 1989). Conway reported normal renal function (urea, creatinine and electrolytes) although serum NAG did suggest renal tubular damage in all participants (Conway 1985). Penketh reported no evidence of renal toxicity in any participant (Penketh 1983). Renal function was monitored in both Schaad studies; the earlier one stated there were no changes with treatment (Schaad 1986) and the later one reported no significant changes in renal function (Schaad 1989).
Three studies commented on ototoxicity (Conway 1985; Schaad 1986; Schaad 1989). The earlier Schaad study stated no incidences of regimen‐induced ototoxicity were found. The later study also examined hearing and found no change in any participants (Schaad 1989). Conway reported normal audiograms in all participants (Conway 1985).
One study reported phlebitis in three participants, but without detailing the groups to which these participants were assigned (Schaad 1986).
Both Schaad studies commented on skin rash; the earlier one stated that one participant in each group had urticaria (Schaad 1986) and the later one supplied data for the analysis, OR 0.19 (95% CI 0.01to 4.05) (Analysis 11.3).
The later Schaad study also reported no difference between groups in the proportions of participants experiencing thrombocytopenia, OR 7.82 (95% CI 0.39 to 158.87) (Schaad 1989) (Analysis 11.3).
b. microbiological ‐ isolation of antibiotic resistant strains, or new strains of bacteria
Blumer reported the isolation of antibiotic‐resistant strains to the antibiotics under examination (Blumer 2005). The study found a decrease from baseline in the number of participants isolating resistant strains at the end of treatment, but no significant difference between groups, OR 0.35 (95% CI 0.01 to 8.74) (Analysis 7.4). This decrease in isolation returned to baseline at follow up.
7.4. Analysis.
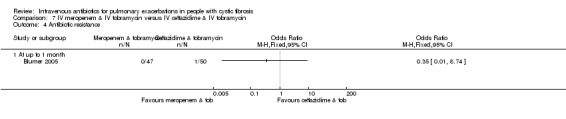
Comparison 7 IV meropenem & IV tobramycin versus IV ceftazidime & IV tobramycin, Outcome 4 Antibiotic resistance.
McLaughlin reported antibiotic susceptibility at baseline and at end of treatment with one participant acquiring ticarcillin resistance and two acquiring tobramycin resistance in the ticarcillin group compared with three participants acquiring azlocillin resistance and none acquiring tobramycin‐resistant strains during treatment in the azlocillin group (McLaughlin 1983). Analysed per regimen, there were no differences between the groups, OR 1.10 (95% CI 0.18 to 6.76) (Analysis 8.3).
8.3. Analysis.

Comparison 8 IV ticarcillin & IV tobramycin versus IV azlocillin & IV tobramycin, Outcome 3 Antibiotic resistance.
While the earlier Schaad study reported S. aureus and H. influenzae in pre‐treatment cultures, there were no non‐Pseudomonas isolates present at the end of treatment (Schaad 1986). In terms of P. aeruginosa resistance, resistance to netilmicin was not analysed by group but was found to have reduced during treatment; however, resistance to azlocillin increased from nine out of 37 to eight out of 25 strains in this group compared to nine out of 37 with ticarcillin resistance at baseline and five out of 25 at end of treatment in this group. This difference was not statistically significant, OR 0.91 (95% CI 0.40 to 2.09) (Analysis 10.3).
10.3. Analysis.
Comparison 10 IV netilmicin & IV azlocillin versus IV netilmicin & IV ticarcillin, Outcome 3 Adverse effects‐ antibiotic resistance.
Finally, the later Schaad study reported that the emergence of resistance with treatment was not significant, but that there was no significant association between bacteriologic response and clinical or laboratory findings (Schaad 1989).
IV antibiotic regimen versus nebulised antibiotics
A total of five studies with 235 participants compared an IV antibiotic regimen to nebulised antibiotics (Cooper 1985; Knowles 1988; Schaad 1987; Semykin 2010; Stephens 1983). Unfortunately one of these studies compared two IV regimens with a regimen consisting of a combination of an IV, an inhaled and an oral antibiotic and so it is difficult to attribute any change to the addition of either the oral or inhaled treatment (Semykin 2010).
Types of antibiotic varied among studies. One study compared an IV antibiotic regimen with an inhaled antibiotic regimen using IV tobramycin and ticarcillin compared to inhaled tobramycin and inhaled carbenicillin (Cooper 1985). Four studies investigated the effect of inhaled antibiotics as an adjunct to IV antibiotic use (Knowles 1988; Schaad 1987; Semykin 2010; Stephens 1983). Knowles compared IV piperacillin and IV tobramycin to IV piperacillin and IV tobramycin with the addition of these same antibiotics delivered by nebuliser (Knowles 1988). Similarly, Stephens compared IV ticarcillin plus IV tobramycin to IV ticarcillin plus IV tobramycin with the addition of inhaled tobramycin (Stephens 1983). Schaad compared IV ceftazidime and IV amikacin to IV ceftazidime and IV amikacin with the addition of inhaled amikacin (Schaad 1987). As mentioned above, the fourth study to compare IV antibiotics to nebulised antibiotics compared IV cefepime with IV amikacin to IV meropenem and IV amikacin and also to inhaled tobramycin given alongside IV ceftazidime and oral ciprofloxacin (Semykin 2010).
Primary outcomes
1. Lung function
a. FEV1
One study reported FEV1 in % predicted before and after treatment and a further study reported changes in FEV1 % predicted; neither study provided sufficient data to allow for contribution to a meta‐analysis, but stated that there were no significant differences between the two groups (Cooper 1985; Stephens 1983). A third study reported improvements in both groups during treatment, although there was no difference between groups in terms of FEV1 (Knowles 1988).
b. FVC
In the 1987 study, Schaad reported change in absolute values of FVC % predicted in their comparison of regimens with and without inhaled amikacin (Schaad 1987). Using imputed SDs of the measure of change, there were no significant differences between the two groups, MD 0.00% (95% CI ‐3.94 to 3.94) (Analysis 12.1).
12.1. Analysis.
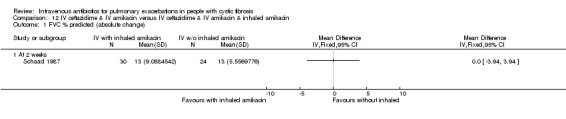
Comparison 12 IV ceftazidime & IV amikacin versus IV ceftazidime & IV amikacin & inhaled amikacin, Outcome 1 FVC % predicted (absolute change).
Two further studies commented on FVC, but did not provide data suitable to enter into an analysis (Cooper 1985; Knowles 1988). The first reported FVC in % predicted before and after treatment and found no significant difference between groups (Cooper 1985). The second study reported improvements in both groups during treatment, although there was no difference between groups (Knowles 1988).
Secondary outcomes
2. Nutritional status
b. weight
One study reported that only the group receiving IV and inhaled antibiotics significantly gained weight during treatment, although there were no differences between the groups (Knowles 1988). A second study reported change in body weight during treatment and cited no significant differences between the two groups (Stephens 1983). Schaad reported the effect of the compared regimens in terms of percentage underweight and suggested no significant difference between the two groups (Schaad 1987).
5. Adverse effects
a. toxicity and allergy
Schaad reported the proportions of participants experiencing raised liver enzymes (Schaad 1987), but there were no significant differences between the two groups, OR 0.60 (95% CI 0.16 to 2.27) (Analysis 12.2).
12.2. Analysis.
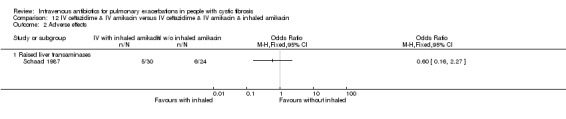
Comparison 12 IV ceftazidime & IV amikacin versus IV ceftazidime & IV amikacin & inhaled amikacin, Outcome 2 Adverse effects.
Two studies commented on renal toxicity; one stated that no renal toxicity was observed (Stephens 1983) and the second that there were no significant changes in renal function (Schaad 1987).
Schaad also stated that there were no significant changes in audiometry; but that transient haematologic abnormalities occurred in eight participants (eosinophilia, neutropenia and thrombocytopenia), although this was not analysed by group (Schaad 1987).
b. microbiological ‐ isolation of antibiotic resistant strains, or new strains of bacteria
Schaad noted an increase in ceftazidime‐ and amikacin‐specific antibiotic resistance in both groups under comparison, although from the data provided it is not possible to detect a significant difference between groups (Schaad 1987). The study noted no significant association between microbiology parameters and clinical response.
IV antibiotic regimen versus oral antibiotics
A total of seven studies with 450 participants compared an IV antibiotic regimen to a regimen that contained oral antibiotics (Black 1990; Bosso 1989; Church 1997; Hodson 1987; Richard 1997; Semykin 2010; Wang 1988). As already stated, Semykin compared two IV regimens with a regimen consisting of a combination of an IV, an inhaled and an oral antibiotic, and so it is difficult to attribute any change to the addition of either the oral or inhaled treatment (Semykin 2010).
Four studies compared oral ciprofloxacin with two‐agent IV combinations (Bosso 1989; Hodson 1987; Richard 1997; Wang 1988). Of these, one study compared oral ciprofloxacin to IV azlocillin with gentamicin (Hodson 1987); another compared it to IV ceftazidime with tobramycin (Richard 1997); a third compared it to IV tobramycin with azlocillin (Bosso 1989); and the remaining study had a three‐arm comparison of oral ciprofloxacin to IV tobramycin with azlocillin and to IV tobramycin plus ticarcillin (Wang 1988). A further study compared oral ciprofloxacin with oral ciprofloxacin cycled with IV tobramycin with azlocillin (Black 1990). Church compared single agent IV ciprofloxacin followed by single agent oral ciprofloxacin with combination treatment with IV tobramycin and IV ceftazidime with multiple reporting periods, thus comparing both single with combination IV agents and oral compared with IV (Church 1997).
Of the two studies comparing oral ciprofloxacin with tobramycin plus azlocillin, one was a more completely reported study in two publications (Bosso 1989) and the second was reported in abstract form only (Wang 1988). The Bosso publications contained some uncertainty regarding the reporting of those who withdrew from the study (Characteristics of included studies); the authors report an analysis suggesting that, for a number of variables, the characteristics at admission of those who completed the protocol were not statistically significantly different from compared to those that did not (Bosso 1989). There were also significant unit of analysis issues in the Wang study, the results of which are reported narratively with all three regimens improving, but no significant differences between the treatment arms.
Primary outcomes
1. Lung function
Black measured lung function but this was unfortunately not reported in detail, instead reporting that "clinical efficacy" was the same (Black 1990). Church reported only mean change in lung function without a measure of distribution of the data and so we could not include data from this study. The duration of treatment in the two groups was also not reported making interpretation difficult (Church 1997).
a. FEV1
Three studies reported on FEV1 (Bosso 1989; Hodson 1987; Richard 1997). Bosso (n = 24) reported the absolute change in FEV1 % predicted and found no significant difference between the two groups, MD 1.40% (95% CI ‐4.43 to 7.23) (Analysis 13.1). Hodson (n = 40) reported absolute changes in absolute values of FEV1, although this did not reach statistical significance, MD 0.11 litres (95% CI ‐0.14 to 0.37) (Analysis 14.1). Finally, Richard (n = 108) reported the percentage (relative) change in FEV1 without measures of distribution, but stated comparable changes from baseline in the two groups (Richard 1997). A pooled estimate of effect involving two studies yields no statistically significant difference, SMD ‐0.24 (95% CI ‐0.73 to 0.25) (Bosso 1989; Hodson 1987) (Analysis 16.1).
13.1. Analysis.
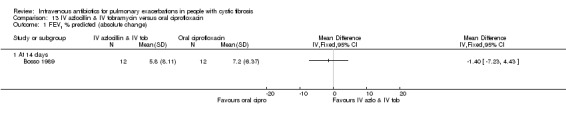
Comparison 13 IV azlocillin & IV tobramycin versus oral ciprofloxacin, Outcome 1 FEV1 % predicted (absolute change).
14.1. Analysis.
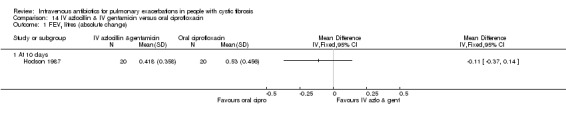
Comparison 14 IV azlocillin & IV gentamicin versus oral ciprofloxacin, Outcome 1 FEV1 litres (absolute change).
16.1. Analysis.
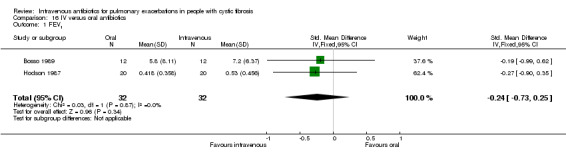
Comparison 16 IV versus oral antibiotics, Outcome 1 FEV1.
b. FVC
Three studies reported on FEV1 (Bosso 1989; Hodson 1987; Richard 1997). Bosso reported the absolute change in FVC % predicted and found no significant difference between the two groups, MD 2.00% (95% CI ‐7.50 to 11.50) (Analysis 13.2). Hodson reported the absolute changes in absolute values of FVC, reporting no statistically significant difference between the groups, MD 0.26 litres (95% CI ‐0.06 to 0.57) (Analysis 14.2). Similar to that with FEV1, Richard reported the percentage (relative) change in FVC without measures of distribution, reporting comparable changes from baseline in both groups (Richard 1997). A pooled estimate of effect involving two studies yields no statistically significant difference, SMD ‐0.24 (95% CI ‐0.74 to 0.26) (Bosso 1989; Hodson 1987) (Analysis 16.2) .
13.2. Analysis.

Comparison 13 IV azlocillin & IV tobramycin versus oral ciprofloxacin, Outcome 2 FVC % predicted (absolute change).
14.2. Analysis.

Comparison 14 IV azlocillin & IV gentamicin versus oral ciprofloxacin, Outcome 2 FVC litres (absolute change).
16.2. Analysis.
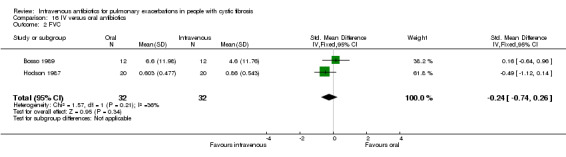
Comparison 16 IV versus oral antibiotics, Outcome 2 FVC.
2. Time to next exacerbation
Only Richard reported on this outcome (Richard 1997). There were nine participants in the ciprofloxacin group and five in the combined IV groups who had a pulmonary exacerbation between nine and 30 days after the end of initial treatment with no significant difference between the groups, OR 1.88 (95% CI 0.59 to 6.03) (Analysis 15.1).
15.1. Analysis.
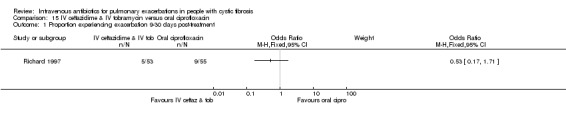
Comparison 15 IV ceftazidime & IV tobramycin versus oral ciprofloxacin, Outcome 1 Proportion experiencing exacerbation 9‐30 days post‐treatment.
Secondary outcomes
4. Mortality
Only Hodson (n = 40) reported on mortality at six weeks and at three months (Hodson 1987). One participant who received oral ciprofloxacin died within six weeks of treatment, one further participant in this group died within the first three months. One participant who received combination IV treatment also died within the first three months. Overall this was not significantly different across the two groups at either six weeks, RR 0.33 (95% CI 0.01, 7.72) or three months, RR 1.00 (95% CI 0.07 to 14.90) (Analysis 14.3).
14.3. Analysis.

Comparison 14 IV azlocillin & IV gentamicin versus oral ciprofloxacin, Outcome 3 Mortality.
5. Adverse effects
a. toxicity and allergy
Richard (n = 108) reported treatment‐related adverse effects with no significant differences between the two groups, OR 0.84 (95% CI 0.31 to 2.27) (Analysis 15.2). The study by Hodson (n = 40) reported one participant in the combination IV treatment group who developed mild anorexia and malaise while three participants in the oral ciprofloxacin group reported side effects ‐ tiredness, vague aches and pains and mild diarrhoea (one each) (Hodson 1987). A further study (n = 16) reported one participant withdrew from the study due to diarrhoea and three participants with photosensitivity (Black 1990). Two studies reported that no drug side effects were experienced in the study period (Bosso 1989; Wang 1988).
15.2. Analysis.
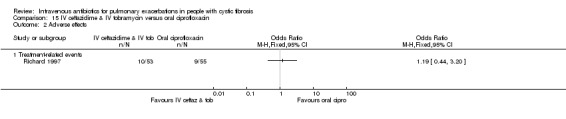
Comparison 15 IV ceftazidime & IV tobramycin versus oral ciprofloxacin, Outcome 2 Adverse effects.
b. microbiological ‐ isolation of antibiotic resistant strains, or new strains of bacteria
Hodson reported that three participants in the azlocillin plus gentamicin group had organisms resistant to these antibiotics at day 10 (for two of these participants later profiles returned to full sensitivity within six weeks), while two participants in the ciprofloxacin group had organisms resistant to this antibiotic (both of which returned to full sensitivity by six weeks) (Hodson 1987). One study reported that weekly sputum cultures did not identify emergence of resistance to ciprofloxacin (Wang 1988); and a further study reported no change in antibiotic susceptibility patterns (Richard 1997). Finally, Black did not report MIC breakpoints; however, they noted that MICs rose during treatment, but returned to pre‐treatment levels after treatment was stopped (Black 1990).
Discussion
We identified 40 studies that considered the role of intravenous (IV) antibiotics for the treatment of pulmonary exacerbations in people with cystic fibrosis (CF). In these studies 1717 participants were recruited, with data available for 1054 participants. The quality of these studies were variable; however, the majority were small studies with an unclear or high risk of bias. Eight studies were reported in conference abstract format only.
Summary of main results
Overall, antibiotics appear to be largely well‐tolerated; however, transient self‐limiting mild side effects are encountered by many participants.
IV antibiotics versus placebo
Three small studies (63 participants) considered the use of IV antibiotics compared to placebo. One study did not report sufficient data to allow for contribution to a meta‐analysis, the reporting of another contained a typographical error precluding its use and the final study required the use of graphical software to extract the data from graphs presented. These studies had conflicting results.There was no effect upon weight detected in the two studies which considered this outcome.
Single agent versus combination agent IV treatment
Four studies with a placebo‐containing group and 10 without, compared the effect of single versus combination IV antibiotic treatment (Table 1). A meta‐analysis of SMD for both measures of lung function for those studies which included a placebo agent suggested no difference between treatment groups, while the comparison of those studies without a placebo agent was statistically significant in favour of combination antibiotic treatment. A similar meta‐analysis considering FVC all those studies comparing a single versus a combination of agents also yielded a statistically significant effect favouring combination treatment. All studies which measured weight, found no difference between groups except for one which reported statistically significantly improved weight gain in those treated with combination IV antibiotics (De Boeck 1989). In terms of time to next exacerbation, all those studies which reported on this outcome found no significant difference except for one which reported a statistically significant effect in favour of single agent treatment (BTS 1985).
Combination agents compared
There is considerable variation in the combinations of agents compared, with nine studies recruiting 417 participants which administered eight antibiotics in nine different antibiotic combinations. However, no combination agent was found to have a statistically significantly different effect to any other for any outcome measure.
IV versus nebulised antibiotics
Five studies (235 participants) compared an IV antibiotic regimen to nebulised antibiotics with no significant differences between groups in terms of lung function or weight (Table 2).
IV versus oral antibiotics
Five studies (320 participants) compared an IV antibiotic regimen to a regimen that contained oral antibiotics with no statistically significant difference between the two treatment groups in terms of lung function, time to next exacerbation or mortality (Table 3).
Overall completeness and applicability of evidence
Many of the studies are old (28 studies reporting prior to 1990) with only three studies being reported since 2000. Many companion treatments have been introduced over this period and the outcomes of those with CF have improved considerably. The generalisability of the results of older studies to clinical practice encountered today could therefore be questioned.
It is disappointing that participant‐reported outcomes have been excluded from the literature to date (accepting that the evolution of such outcomes has been relatively recent compared to the era of these trials) and adherence was considered in only one study.
While we accept that there is no agreed definition of what constitutes a pulmonary exacerbation, we used a broad definition (and one that appears to be used in clinical practice) and do not think that this will impact the evidence found in the review. Also, we believe that each outcome is relatively equally represented in terms of participant mix, allowing the results to be applied to the wider CF population.
Quality of the evidence
Many studies include inherent methodological weaknesses, the classification of which is often difficult due to inadequate detail in the reporting of their methods. A significant challenge for this body of evidence and reasons for the determination of the low quality of evidence lies in the imprecision inherent in the large number of very small studies with low numbers of events.Many of which fail to report a power calculation. As such it is difficult to assess whether these studies were sufficiently powered to detect a difference, should one exist. Another common reason for downgrading the quality of evidence presented was due to risk of bias introduced by unit of analysis issues, whereby participants are re‐recruited to the study and, by definition, more unwell participants contribute data on numerous occasions. Intention‐to‐treat analyses with the participant as the unit of analysis would overcome these issues, but such analyses are rare in the studies eligible for inclusion in this review. It is therefore disappointing that we have been unable to perform subgroup analyses by severity of lung disease, as was planned, in order to consider whether the effect of treatment is greatest in those with most to gain or whether those with minimal lung disease are more sensitive to treatment.
Potential biases in the review process
No potential biases in the review process were identified. All authors documented their a priori opinions regarding the effectiveness of IV antibiotics for the treatment of pulmonary exacerbations:
MH: I think that IV antibiotics are likely to be beneficial in the treatment of some CF pulmonary exacerbations; however the fact that many people fail to regain their baseline lung function suggests that IV antibiotics are currently being used sub‐optimally and are perhaps not the whole answer for all people with CF.
AP: I think that IV antibiotics are beneficial for the treatment of pulmonary exacerbations. I think that teasing apart the effects of IV antibiotics (which are often delivered in hospital) and the effects of the hospital admission itself (frequent review, intensive physiotherapy dietetic and nursing input) will be difficult.
PF: I believe that antibiotics are beneficial in the treatment of CF pulmonary exacerbations. The need for IV antibiotics is primarily based upon the limitations of the drug formulation (i.e. only available in IV form). My prediction is that the literature will provide insufficient information regarding the benefit of IV antibiotics for several reasons:
there are few studies consisting of few participants;
we have a poor definition of a pulmonary exacerbation and must accept that there are different causes of worsening symptoms leading to the intervention;
any study will already be biased to demonstrate a benefit as those who are "less ill" will likely be treated with another form of therapy (e.g. oral antibiotics, inhaled antibiotics, dornase alfa).
Agreements and disagreements with other studies or reviews
No other systematic reviews addressing the study question have been identified.
Authors' conclusions
Implications for practice.
Due to the poor quality of evidence, the results are not conclusive. The principle of treating people with pulmonary exacerbations with IV antibiotics is based largely on clinical experience and current guidance largely recommends combination antibiotic treatment for reasons of antibiotic resistance. The quality of the studies included in this review is poor and while no effect on antibiotic resistance was observed, differences in lung function between combination treatment compared to single agent regimens were only seen in the inclusive meta‐analysis with no effect observed in the more restrictive analyses. There appears to be no evidence to recommend the use of any particular IV antibiotic combination over another; and no evidence to suggest that any route of antibiotic administration is superior to any other.
Implications for research.
Questions remain regarding the use of IV antibiotics for people with CF experiencing pulmonary exacerbations, an event that for many people results in a significant loss of lung function that is not regained (Sanders 2010). While there is unlikely to be equipoise in the use of antibiotics for pulmonary exacerbations per se, the appropriate route of such antibiotics may be open to enquiry, particularly for those with mild disease, with the potential attendant reductions in burden of treatment that would accompany a non‐parenteral route of administration. Ideally, to answer the question of the use of IV antibiotics to treat pulmonary exacerbations, a double‐blind randomised placebo‐controlled trial recruiting both adults and children with CF to receive combination antibiotics versus placebo would be needed. Debate is fierce regarding appropriate outcomes to be measured. As is the case in this review, lung function is an outcome of interest but is difficult to interpret and furthermore standardised timing of measurement is an issue. The time to next exacerbation is considered by many to be a meaningful outcome, but it is often distant in time from the intervention and so difficult to attribute exclusively. A more pragmatic outcome may be treatment failure rate (proportion of participants requiring an intervention within 28 days of the treatment under test), although this in itself is also open to confounding.
Recent research suggests that quantitative microbiological measures appear not to change prior to the onset of a pulmonary exacerbation (Stressmann 2011); and do not change considerably despite the administration of antibiotics (Stressmann 2012). With person‐to‐person differences in the number and diversity of infecting bacteria being considerable (Stressmann 2012) and an awareness that healthy lungs are host to a microbiome of their own (Blainey 2012), consideration of a more considered and personalised approach would appear to be of interest. With any effect of antibiotics administered during exacerbations perhaps not acting as originally expected, further understanding of the nature of exacerbations and investigation of the bacterial‐host interaction further detailing the nature of their effect is also needed.
The evolution of new agents targeting the specific molecular defect is likely to change the 'natural history' of CF, at least for some people. Nevertheless a significant proportion of people with CF do not adequately recover from their pulmonary exacerbation and females in particular experience less favourable outcomes than males. The significance of exacerbations for people with CF, and so the importance of further work to refine the treatment of these events, cannot be understated.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2017 | Amended | Contact details updated. |
History
Protocol first published: Issue 3, 2012 Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 9 September 2015 | Amended | Grade added to summary of finding table 'Single versus combination IV antibiotics'. |
Acknowledgements
We gratefully acknowledge the peer reviewers comments and especially the support of Nikki Jahnke for her critical review and management of the review process.
Appendices
Appendix 1. Glossary
For a full explanation of statistical and methodological terms please refer to the Cochrane Collaboration's online glossary available at www.cochrane.org/glossary.
| Term | Explanation |
| Antibiotic resistance | When bacteria develop the ability to withstand the effects of antibiotics that would have previously killed that type of bacteria. |
| Autosomal recessive inheritance | Children receive two sets of genes, one each from either parent. A condition is considered to be autosomal recessive if a faulty gene from both parents is required to inherit the condition. In this case 'carriers' have only one faulty gene and are usually healthy (or mildly affected). |
| Broad spectrum antibiotics | Antibiotics that are active against a wide variety of bacteria. |
| Intravenous antibiotics | Medication used to kill bacteria given through a device into a vein, directly into the bloodstream. |
| Pulmonary exacerbation | An acute deterioration in the well‐being of people with CF, usually characterised by an increase in respiratory symptoms (cough, sputum production), and usually prompting treatment with intravenous antibiotics. |
Data and analyses
Comparison 1. Single IV antibiotic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FVC % predicted (relative change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Ceftazidime 200 mg/kg/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Weight (relative change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Ceftazidime 200 mg/kg/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Combination IV antibiotic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Ticarcillin and tobramycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FVC % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Ticarcillin and tobramycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Single IV agents compared.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ceftazidime versus aztreonam | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 FEV1 litres (absolute change) | 2 | 46 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐1.08, 0.84] |
Comparison 4. Single IV antibiotic (with placebo) versus combination IV antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (absolute change) | 4 | 214 | Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐3.14, 1.36] |
| 1.1 Azlocillin & placebo versus azlocillin & tobramycin | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 1.37 [‐1.50, 4.23] |
| 1.2 Oxacillin & placebo versus oxacillin & sisomycin & carbenicillin | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐9.54 [‐15.98, ‐3.10] |
| 1.3 Tobramycin & placebo versus tobramycin & ceftazidime | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.63, 2.23] |
| 2 FEV1 % predicted (relative change) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.58 [‐9.80, 16.96] |
| 2.1 Tobramycin & placebo versus piperacillin 50 mg/kg (6x daily) & tobramycin | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐4.20 [‐26.50, 18.10] |
| 2.2 Tobramycin & placebo versus piperacillin 100 mg/kg (3x daily) & tobramycin | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | 7.95 [‐8.78, 24.68] |
| 3 FVC % predicted (absolute change) | 3 | 116 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐3.48, 3.52] |
| 3.1 Azlocillin & placebo versus azlocillin & tobramycin | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [‐2.53, 4.89] |
| 3.2 Oxacillin & placebo versus oxacillin & sisomycin & carbenicillin | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐9.32 [‐19.86, 1.22] |
| 4 FVC % predicted (relative change) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐1.26 [‐12.40, 9.88] |
| 4.1 Tobramycin & placebo versus piperacillin 50mg/kg (6x daily) & tobramycin | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐15.79, 13.39] |
| 4.2 Tobramycin & placebo versus piperacillin 100 mg/kg (3x daily) & tobramycin | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐18.61, 15.91] |
| 5 Time to next exacerbation (weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Azlocillin & placebo versus azlocillin & tobramycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Weight (absolute change (kg)) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐1.66, 0.93] |
| 6.1 Tobramycin & placebo versus piperacillin 50mg/kg (6x daily) & tobramycin | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐2.65, 1.21] |
| 6.2 Tobramycin & placebo versus piperacillin 100mg/kg (3x daily) & tobramycin | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐1.83, 1.69] |
| 7 Adverse effects ‐ sensitivity reaction | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Piperacillin (all regimens) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse effects | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 All adverse effects | 2 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.50, 2.37] |
| 8.2 Ototoxicity | 2 | 88 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Nephrotoxocity | 2 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.05, 7.27] |
| 8.4 Proteinuria | 1 | 63 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.02, 1.89] |
| 8.5 Infusion site irritation | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.26, 10.08] |
| 8.6 Tinnitus | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.15, 8.06] |
| 9 Adverse effects ‐ serum | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Creatinine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 NAG | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Single IV antibiotic versus combination IV antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 ml (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Colstin versus colistin & "other" | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEV1 % predicted (absolute change) | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐2.73 [‐8.42, 2.95] |
| 2.1 Aztreonam versus tobramycin & azlocillin | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐11.57, 2.37] |
| 2.2 Ceftazidime versus tobramycin & piperacillin | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐8.85, 10.85] |
| 3 FEV1 % (relative change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Ceftazidime versus tobramycin & ticarcillin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 FEV1 (all measures) | 3 | 122 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.74, ‐0.02] |
| 4.1 Aztreonam versus tobramycin & azlocillin | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.19, 0.27] |
| 4.2 Ceftazidime versus tobramycin & piperacillin | 1 | 21 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.77, 0.94] |
| 4.3 Colstin versus colistin & "other" | 1 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.96, ‐0.02] |
| 5 FVC ml (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Colstin versus colistin & "other" | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 FVC % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Aztreonam versus azlocillin & tobramycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 FVC % predicted (relative change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Aztreonam versus azlocillin & tobramycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 FVC (all measures) | 2 | 101 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐1.30, ‐0.48] |
| 8.1 Colstin versus colistin & "other" | 1 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.45, ‐0.46] |
| 8.2 Aztreonam versus azlocillin & tobramycin | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.48, 0.01] |
| 9 Time to readmission (months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Ceftazidime versus tobramycin & piperacillin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Proportion readmitted, requiring IV antibiotics or death in subsequent 3 months | 2 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.95] |
| 10.1 Ceftazidime versus carbenicillin & gentamicin | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.74] |
| 10.2 Ceftazidime versus tobramycin & ticarcillin | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.26, 7.58] |
| 11 Weight (% change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Ceftazidime versus tobramycin & ticarcillin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Mortality | 3 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.09, 4.37] |
| 12.1 Ceftazidime versus tobramycin & ticarcillin | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.08, 15.36] |
| 12.2 Colstin versus colistin & "other" | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.70] |
| 12.3 Piperacillin versus piperacillin & tobramycin | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Adverse effects | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Liver transaminase enzyme elevation | 4 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.58, 2.86] |
| 13.2 Neurological adverse effects | 1 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.96] |
| 13.3 Rash | 3 | 129 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.24, 5.48] |
| 13.4 Thrombophlebitis | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 1.21] |
| 13.5 Fever | 1 | 17 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.06, 21.87] |
| 13.6 Proteinuria | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 17.41] |
| 14 Renal toxicity | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Change in blood urea (mmol/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Change in serum creatinine (mol/l) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Antibiotic resistance ‐ number of participants isolating resistant strains | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 All antibiotics | 3 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.24] |
| 15.2 Ceftazidime versus gentamicin | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.08, 3.38] |
| 15.3 Ceftazidime versus carbenicillin | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.07, 3.00] |
Comparison 6. Single versus combination.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (all measures) | 7 | 336 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.42, 0.01] |
| 1.1 Single antibiotic with placebo versus combination regimen | 4 | 214 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.38, 0.17] |
| 1.2 Single antibiotic (no placebo) versus combination regimen | 3 | 122 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.74, ‐0.02] |
| 2 FEV1 ml (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Colstin versus colistin & "other" | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FEV1 % predicted (absolute change) | 6 | 265 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐3.23, 0.95] |
| 3.1 Azlocillin & placebo versus azlocillin & tobramycin | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 1.37 [‐1.50, 4.23] |
| 3.2 Aztreonam versus tobramycin & azlocillin | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐11.57, 2.37] |
| 3.3 Tobramycin & placebo versus tobramycin & ceftazidime | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.63, 2.23] |
| 3.4 Oxacillin & placebo versus oxacillin & sisomycin & carbenicillin | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐9.54 [‐15.98, ‐3.10] |
| 3.5 Ceftazidime versus tobramycin & piperacillin | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐8.85, 10.85] |
| 4 FEV1 litres (relative change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Ceftazidime versus tobramycin & ticarcillin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 FEV1% predicted (relative change) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 3.58 [‐9.80, 16.96] |
| 5.1 Tobramycin & placebo versus piperacillin 50 mg/kg (6x daily) & tobramycin | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐4.20 [‐26.50, 18.10] |
| 5.2 Tobramycin & placebo versus piperacillin 100 mg/kg (3x daily) & tobramycin | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | 7.95 [‐8.78, 24.68] |
| 6 FVC (all measures) | 5 | 217 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.71, ‐0.16] |
| 6.1 Single antibiotic with placebo versus combination regimen | 3 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.43, 0.31] |
| 6.2 Single antibiotic (no placebo) versus combination regimen | 2 | 101 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.31, ‐0.49] |
| 7 FVC % predicted (absolute change) | 4 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐1.37 [‐4.56, 1.81] |
| 7.1 Azlocillin & placebo versus azlocillin & tobramycin | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [‐2.53, 4.89] |
| 7.2 Oxacillin & placebo versus oxacillin & sisomycin & carbenicillin | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐9.32 [‐19.86, 1.22] |
| 7.3 Aztreonam versus azlocillin & tobramycin | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐8.1 [‐15.79, ‐0.41] |
| 8 FVC % predicted (relative change) | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐6.60 [‐13.99, 0.79] |
| 8.1 Tobramycin & placebo versus piperacillin 50mg/kg (6x daily) & tobramycin | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐15.79, 13.39] |
| 8.2 Tobramycin & placebo versus piperacillin 100 mg/kg (3x daily) & tobramycin | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐18.61, 15.91] |
| 8.3 Aztreonam versus azlocillin & tobramycin | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐10.80 [‐20.67, ‐0.93] |
| 9 FVC ml (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Colstin versus colistin & "other" | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.2. Analysis.

Comparison 6 Single versus combination, Outcome 2 FEV1 ml (absolute change).
6.4. Analysis.
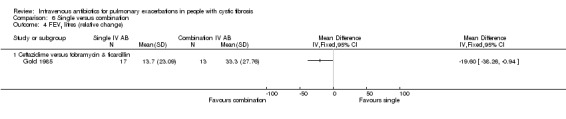
Comparison 6 Single versus combination, Outcome 4 FEV1 litres (relative change).
6.8. Analysis.
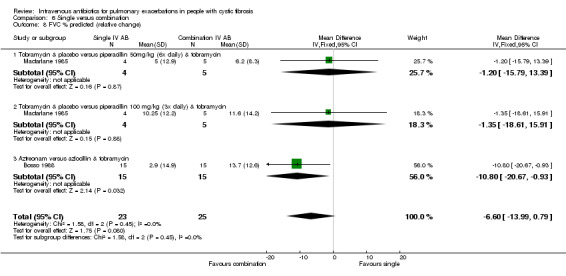
Comparison 6 Single versus combination, Outcome 8 FVC % predicted (relative change).
6.9. Analysis.
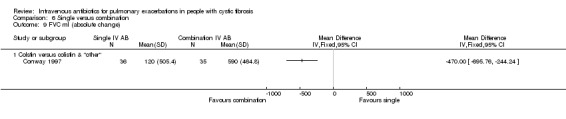
Comparison 6 Single versus combination, Outcome 9 FVC ml (absolute change).
Comparison 7. IV meropenem & IV tobramycin versus IV ceftazidime & IV tobramycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 14 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEV1 % predicted (relative % change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 14 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Participants experiencing an exacerbation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 At up to 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Antibiotic resistance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 At up to 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. IV ticarcillin & IV tobramycin versus IV azlocillin & IV tobramycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 10 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Time to next exacerbation (weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Antibiotic resistance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 4 weeks after end of treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. IV azlocillin & IV gentamicin versus IV carbenicillin & IV gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 ml (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 10 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FVC ml (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 10 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 During treatment up to 10 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 12‐month follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 During treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.4. Analysis.

Comparison 9 IV azlocillin & IV gentamicin versus IV carbenicillin & IV gentamicin, Outcome 4 Adverse effects.
Comparison 10. IV netilmicin & IV azlocillin versus IV netilmicin & IV ticarcillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FVC % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 10 to 17 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Liver transaminase elevation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects‐ antibiotic resistance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Azlocillin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Ticarcillin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. IV aztreonam & IV amikacin versus IV ceftazidime & IV amikacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FVC % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Thrombocytopenia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Liver transaminases ‐ AST/SGOT & ALT/SGPT | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Rash | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. IV ceftazidime & IV amikacin versus IV ceftazidime & IV amikacin & inhaled amikacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FVC % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Raised liver transaminases | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. IV azlocillin & IV tobramycin versus oral ciprofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 14 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FVC % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 14 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. IV azlocillin & IV gentamicin versus oral ciprofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 litres (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 10 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FVC litres (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 10 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Six weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. IV ceftazidime & IV tobramycin versus oral ciprofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion experiencing exacerbation 9‐30 days post‐treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Treatment‐related events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16. IV versus oral antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 2 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.73, 0.25] |
| 2 FVC | 2 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.74, 0.26] |
| 3 FEV1 litres (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 IV azlocillin & IV gentamicin versus oral ciprofloxacin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 FEV1 % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 IV azlocillin & IV tobramycin versus oral ciprofloxacin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 FVC litres (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 IV azlocillin & IV gentamicin versus oral ciprofloxacin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 FVC % predicted (absolute change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 IV azlocillin & IV tobramycin versus oral ciprofloxacin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
16.3. Analysis.

Comparison 16 IV versus oral antibiotics, Outcome 3 FEV1 litres (absolute change).
16.4. Analysis.

Comparison 16 IV versus oral antibiotics, Outcome 4 FEV1 % predicted (absolute change).
16.5. Analysis.

Comparison 16 IV versus oral antibiotics, Outcome 5 FVC litres (absolute change).
16.6. Analysis.
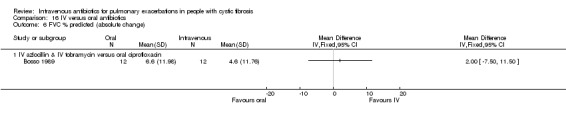
Comparison 16 IV versus oral antibiotics, Outcome 6 FVC % predicted (absolute change).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agostini 1983.
| Methods | Double‐blind, RCT with multiple arms comparing 7 different IV antibiotics. Parallel design. Duration: 10 ‐ 15 days. Single centre. Country: Italy. |
|
| Participants | People with CF with a pulmonary exacerbation with P. aeruginosa isolated in pure or predominant culture. Age range: 1 ‐ 24 years. 178 treatment episodes for 111 participants experiencing an acute or subacute exacerbation with a pure or predominant culture of P. aeruginosa identified as susceptible to the antibiotics under test in the trial. |
|
| Interventions | 10 ‐ 15 days IV treatment with azlocillin (20 participants), piperacillin (22 participants), cefoperazone (20 participants), ceftazidime (27 participants), cefsulodin (22 participants), cefotaxime or moxalactam. Ureidopenicillins (azlocillin, piperacillin): 400 mg/kg/day in 3 doses. Cephalosporins (cefoperazone, ceftazidime, cefsulodin, cefotaxime, moxalactam): 200 mg/kg/day in 3 doses. All participants continued with standard therapy (physiotherapy, mucolytic aerosol, pancreatic enzyme therapy). |
|
| Outcomes | A unique clinical score (for which lung function was a component), radiology, sputum bacterial count, blood and urine studies and adverse events. | |
| Notes | The study appears to be reported in 2 publications Agostini 1983 and Mastella 1983; Mastella 1983 appears to report the full trial with Agostini 1983 reporting before the end of the trial. Some cases randomly assigned to treatment before results of sputum culture known and not all subsequently fulfilled inclusion criteria ‐ these cases excluded from therapeutic trial but included for evaluation of side effects. The data are presented in terms of the unvalidated clinical score. We shall seek to contact the study authors for clarification and for study data to include in a meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomly assigned, but no method given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind but no further detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blind but no further detail given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals were not described. From the more complete analysis, data are presented for 111 participants while 113 infections were described as 'correctly assigned' in relation to in vitro susceptibility. Participants are described as being removed from the study if there was a failure in treatment after 7 days although this is not described. Some participants were randomised before the results of susceptibility testing were available. In some cases the results indicated that participants did not meet inclusion criteria and so were excluded (although remained in the analysis of side effects). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | High risk | UoA issues ‐ a higher number of infections than participants recruited. |
Black 1990.
| Methods | RCT. Parallel design. Duration: not stated. Single centre. Country: Northern Ireland. |
|
| Participants | 16 participants with CF experiencing an acute respiratory exacerbation. Age: 11 to 27 years. Intervention 1: 8 participants. Intervention 2: 8 participants. |
|
| Interventions | Intervention 1: oral ciprofloxacin alone over 4 exacerbations. Intervention 2: oral ciprofloxacin alternating with azlocillin and tobramycin over 4 exacerbations. |
|
| Outcomes | Adverse events. Lung function data measured but not reported. | |
| Notes | This study appears to be reported in a conference abstract and a discursive paper review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomly assigned' but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detail given. |
| Selective reporting (reporting bias) | High risk | Lung function data were measured but not reported. |
| Other bias | Low risk | No detail given. |
Blumer 2005.
| Methods | Investigator‐blinded RCT. Parallel design. Duration: expected duration 14 days, follow up 2 ‐ 4 weeks after discontinuation of therapy. Multicentre (16 centres). Country: USA. |
|
| Participants | 121 participants with a recent (usually < 1 month) culture of P. aeruginosa or B. cepacia complex recruited at a protocol‐defined exacerbation. 102 participants with P. aeruginosa infection susceptible to meropenem and ceftazidime recruited to randomised trial and stratified according to disease severity. 19 participants with B. cepacia or ceftazidime‐resistant P. aeruginosa recruited to open‐label study ‐ the open label study is analysed separately. Age: ≧ 5 years of age. Intervention group 1: 50 participants. Gender: 25 male; 25 female. Age: age 5 ‐ 12 years (n = 12), age >12 ‐ 16 years (n = 13), age > 16 to < 65 years (n = 25). Disease severity: FEV1 >70 % predicted (n = 11); FEV1 40% ‐ 69% predicted (n = 21); FEV1 < 40 % predicted (n = 18). Intervention group 2: 52 participants. Gender: 28 male, 24 female. Age: age 5 ‐ 12 years (n = 9), age > 12 ‐ 16 years (n = 11), age > 16 to < 65 years (n = 32). Disease severity: FEV1 > 70 % predicted (n = 11); FEV1 40% ‐ 69% predicted (n = 20); FEV1 < 40 % predicted (n = 21). |
|
| Interventions | Intervention 1: IV meropenem 40 mg/kg up to a maximum dose of 2 g and IV tobramcyin (given for a mean of 13.5 days). Intervention 2: IV ceftazidime 50 mg/kg up to a maximum dose of 2 g and IV tobramycin (given for a mean of 14.1 days). Tobramycin dose adjusted to give a peak serum concentration of ≧ 8 µg/mL and trough concentration of < 2 µg/mL. Each infusion given over a 30‐minute period. Standard physiotherapy and other supportive therapy continued. |
|
| Outcomes | Lung function (FEV1, FVC, FEV1/FVC ratio, peak expiratory flow, forced expiratory flow, FEV1 % predicted, FVC % predicted), acute change score, time to next exacerbation, microbiology, mortality and adverse effects. | |
| Notes | Sample size calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Investigator" blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The report indicates that the 'investigator' was blinded but that this did not include assessing all the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some 'evaluable' data for lung function and microbiology data are missing. 2 'clinically evaluable' participants (1 from each group) withdrew. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | Low risk | None identified. |
Bosso 1988.
| Methods | RCT. Parallel design. Duration: last measurement 7 ‐ 14 days after completion of treatment. Single centre. Country: USA. |
|
| Participants | 30 participants with CF experiencing protocol‐defined pulmonary exacerbation. Age: > 6 years. Intervention 1: 15 participants; mean age 14.1 years; 9 males, 6 females. Intervention 2: 15 participants; mean age 14.7 years; 6 males, 9 females. |
|
| Interventions | Intervention 1: IV aztreonam (50 mg/kg 4x daily) for a mean of 17.2 days. Intervention 2: IV azlocillin and IV tobramycin (azlocillin ‐ 350 mg/kg/day in 4 divided doses and tobramycin to reach target serum concentration) for a mean of 14.8 days. |
|
| Outcomes | Lung function (FEV1 / FVC), microbiology and adverse effects. | |
| Notes | No sample size calculation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'randomly assigned' but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None identified. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | Low risk | None identified. |
Bosso 1989.
| Methods | Open‐label RCT. Parallel design. Duration: 14 days. Single centre. Country: USA. |
|
| Participants | 25 adults with CF and a protocol‐defined pulmonary exacerbation. Intervention 1: 24 participants; mean (SD) age 22.9 (7.37) years. Intervention 2: 24 participants; mean (SD) age 23.1 (4.5) years. |
|
| Interventions | Intervention 1: oral ciprofloxacin (750 mg 2x daily). Intervention 2: IV tobramycin (to achieve peak 8 ‐ 10 µg/ml; trough < 2 µg/ml) and IV azlocillin (75 mg/kg 4x a day). |
|
| Outcomes | Lung function (FEV1, FVC), microbiology, adverse effects. | |
| Notes | The 1987 report details findings for the first 20 participants (of whom 7 withdrew). The 1989 report details findings for 25 participants of whom 24 were evaluable. Not possible to determine if the 7 withdrawals are included in the later report or if the cohort is a completely different recruitment. We shall seek to contact the authors for IPD to determine the ITT analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly assigned'. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 withdrawals are discussed and while a separate analysis is included to compare those who completed the protocol with those that did not, the withdrawals were not included in the final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | Low risk | None identified. |
BTS 1985.
| Methods | Open RCT. Parallel design. Duration: a minimum of 7 days, follow up for 3 months. Multicentre study. Country: UK. |
|
| Participants | 92 participants with CF and an acute exacerbation from whose sputum P. aeruginosa had been isolated on 2 occasions in the previous 6 months. Aged: > 5 years. Intervention 1: 42 participants; mean age (range) ‐ 15.5 (6 ‐ 28) years; 56% females. Intervention 2: 50 participants; mean age (range) ‐ 16.2 (5 ‐ 34) years; 52% females. |
|
| Interventions | Intervention 1: IV carbenicillin 5 g 6‐hourly (for under 14 years of age 10 mg/kg 3x daily) and IV gentamicin 80 mg 3x daily (for under 14 years of age 2 mg/kg 3x daily). Intervention 2: IV ceftazidime 2g 3x daily (for under 14 years of age 40 mg/kg 3x daily). |
|
| Outcomes | Lung function (PEFR), nutritional status (weight), adverse effects (including plasma urea, electrolytes and liver function). | |
| Notes | We shall contact the authors for data for inclusion in a meta‐analysis. Unit of analysis issues ‐ 9 participants had 2 courses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but details not given. |
| Allocation concealment (selection bias) | Low risk | Sequential envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 not evaluated for a number of reasons including lost records. |
| Selective reporting (reporting bias) | Unclear risk | No detail. |
| Other bias | High risk | UoA issues ‐ 9 participants had 2 courses. |
Caplan 1984.
| Methods | Mixed report with randomised and non‐randomised participants. Parallel design. Duration: at least 3 days (mean 11.4 days). Single centre. Country: USA. |
|
| Participants | 29 participants with CF and with P. aeruginosa as the 'significant infecting organism' randomised. Age: range 12 ‐ 30 years. Intervention 1: 14 participants; 8 females. Intervention 2: 15 participants; 8 females. |
|
| Interventions | Intervention 1: cefsulodin 100 mg/kg per day. Intervention 2: tobramycin 10 mg/kg day (14 participants) or ticarcillin (300 mg/kg/day) (1 participant). |
|
| Outcomes | Adverse effects and microbiology described. Lung function was measured but not reported. | |
| Notes | Randomised portion of the study reported separately, although there were 2 deaths ‐ of those who received cefsulodin, although it is not clear if they were in the randomised portion of the study. The study is described as ongoing, although we could find no complete report. We shall seek to contact the authors for IPD to quantify the change in lung function. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no method described. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals described. |
| Selective reporting (reporting bias) | High risk | Lung function was measured but not reported. |
| Other bias | Unclear risk | None identified. |
Church 1997.
| Methods | Double‐blind RCT. Parallel design. Duration: minimum of 10 days. Multicentre study. Country: USA. |
|
| Participants | 130 participants with CF enrolled and a protocol‐defined exacerbation and P. aeruginosa infection. Age: range 5 ‐ 17 years. Intervention 1: 41 participants; mean (SD) age 11.7 (3.1); 42% males; 18/41 had FEV1< 40%. Intervention 2: 43 participants; mean (SD) age 11.6 (3.3); 56% males; 17/43 had FEV1< 40%. |
|
| Interventions | Intervention 1: IV ciprofloxacin (10 mg/kg 3x daily) for 7 days followed by oral ciprofloxacin 20 mg/kg 2x daily for a minimum 3 days. Intervention 2: IV ceftazidime (50 mg/kg 3x daily) and IV tobramycin (3 mg/kg 3x daily) for a minimum 10 days. |
|
| Outcomes | Lung function (FEV1, FVC) and adverse effects. | |
| Notes | 130 recruited, 46 were not evaluable of whom only 25 are accounted for. No standard deviations are given for the change in lung function over time and the durations of treatment in the two groups are not reported. We shall seek to contact the authors for IPD to quantify the change in lung function. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly assigned' but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind but no detail. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blind but no description on outcome assessment, other than the estimation of antimicrobial sensitivity was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46 participants were not evaluable of whom 25 are accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | Low risk | None identified. |
Conway 1985.
| Methods | RCT. Parallel design. Single centre. Country: UK. |
|
| Participants | 17 participants with CF and chronic P. aeruginosa and a protocol‐defined exacerbation for whom bacterial sensitivity to the antibiotics allocated was confirmed (contributed 30 courses of treatment). Intervention 1: 15 participants, 12 females, 3 males. Intervention 2: 15 participants, 11 females, 4 males. |
|
| Interventions | Intervention 1: (median dose) IV netilmicin (10 mg/kg/day) and IV ticarcillin (468 mg/kg/day). Intervention 2: (median dose) IV tobramycin (9.2 mg/kg/day) and IV ticarcillin (586 mg/kg/day). Anti‐staphylococcal therapy also given. |
|
| Outcomes | Unvalidated clinical score, lung function (PEFR, FEV1, FVC), weight. | |
| Notes | UoA issues addressed narratively with a second analysis with the participant as the UoA, rather than the treatment episode. We shall seek to contact the authors for IPD to quantify the change in lung function to include in any meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomly assigned but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only radiographer was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on withdrawals given. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | High risk | UoA issues ‐ 17 participants contributed to 30 treatment courses. |
Conway 1997.
| Methods | Single‐blind RCT. Parallel design. Duration: 12 days. Single centre. Country: UK. |
|
| Participants | 53 participants with CF and chronic P. aeruginosa experiencing a protocol‐defined exacerbation. Intervention 1: 36 participants; mean (SD) age 21.7 (4.2) years; 17 females, 19 males; mean (SD) FEV1 % predicted 43.3 (16.6). Intervention 2: 35 participants; mean (SD) age 21.2 (4.25) years; 12 females, 23 males; mean (SD) FEV1 % predicted 45.8 (21.8). |
|
| Interventions | Intervention 1: IV colistin (2 MU 3x daily). Intervention 2: IV colistin (2 MU 3x daily) and a second anti‐pseudomonal antibiotic. |
|
| Outcomes | Lung function (FEV1, FVC), weight, mortality and adverse events. | |
| Notes | UoA issues ‐ 18 participants were enrolled twice. We shall seek to contact the authors for IPD to reconcile the UoA issues. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blind (outcome assessor). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Laboratory and radiology were blinded (unclear if physiological outcome assessors were blinded). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 withdrawals described and analysed as ITT. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ 18 participants enrolled 2x. |
Cooper 1985.
| Methods | RCT. Parallel design. Duration: unspecified. Single centre. Country: Canada. |
|
| Participants | 18 participants with CF and an exacerbation and P. aeruginosa infection. Intervention 1: 10 participants. Intervention 2: 8 participants. |
|
| Interventions | Intervention 1: IV tobramycin and IV ticarcillin (dose and regimen unstated). Intervention 2: inhaled tobramycin and inhaled carbenicillin (dose and regimen unstated). |
|
| Outcomes | Lung function (FEV1, FVC % predicted). | |
| Notes | We shall seek to contact the authors for IPD to include in any meta‐analysis. Currently SDs for the mean changes observed are not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomly allocated but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants in each group needed additional antibiotics. Unclear if this was analysed as ITT. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | Low risk | None identified. |
Costantini 1982.
| Methods | RCT. Parallel design. Duration: mean duration of 15 days. Single centre. Country: Italy. |
|
| Participants | 19 participants with CF colonised with P. aeruginosa experiencing an exacerbation over 28 exacerbation episodes. Intervention 1: 7 participants. Intervention 2: 10 participants. Invtervention 3: 11 participants. |
|
| Interventions | Intervention 1: IV carbenicillin 675 mg/kg/day (mean dosage). Intervention 2: IV sisomicin 10.5 mg/kg/day (mean dosage). Invtervention 3: IV carbenicillin 590 mg/kg/day AND IV sisomicin 10 mg/kg/day (mean dosage). |
|
| Outcomes | Unvalidated clinical score, microbiology and adverse effects. | |
| Notes | We shall seek to contact the authors for IPD to reconcile the UoA issues and quantify unpublished data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals described. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ 19 participants contributed 28 treatment episodes. |
De Boeck 1989.
| Methods | RCT. Parallel design. Duration: 14 days. Single centre. Country: Belgium. |
|
| Participants | 21 participants with CF and a protocol‐defined pulmonary exacerbation, chronically infected with P. aeruginosa that was sensitive to piperacillin, tobramycin and ceftazidime. Intervention 1: 10 participants. Intervention 2: 11 participants. |
|
| Interventions | Intervention 1: IV ceftazidime 50 mg/kg 3x daily. Intervention 2: IV piperacillin 75 mg/kg 4x daily and IV tobramycin 10 mg/kg/day in 3 doses. |
|
| Outcomes | Lung function (FEV1), time to next exacerbation, weight, mortality. | |
| Notes | We shall seek to contact the authors for IPD to include in any meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated but not described. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and clinicians were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Lung function undertaken by a technician blinded to regimen. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | None identified. |
| Other bias | Low risk | None identified. |
De Boeck 1999.
| Methods | RCT of those with exacerbation and those receiving elective treatment. Parallel design. Duration: 14 days. Single centre. Country: Belgium. |
|
| Participants | 40 participants with CF and chronic P. aeruginosa infection (sensitive to agent under test), 46 treatments were given for treatment of an exacerbation; 29 courses for elective or suppressive treatment. Mean (SD) age 14.8 (4.4) years. |
|
| Interventions | Intervention 1: IV meropenem 150 mg/kg/day. Intervention 2: IV ceftazidime 200 mg/kg/day and tobramycin 10 mg/kg/day. |
|
| Outcomes | Lung function, weight. | |
| Notes | We shall seek to contact the authors for IPD of those experiencing an exacerbation to reconcile the UoA issues (multiple enrolment). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals not described. |
| Selective reporting (reporting bias) | Unclear risk | Narrative data in the abstract. |
| Other bias | High risk | UoA issues ‐ 40 participants contribute 46 treatment episodes. |
Elborn 1992.
| Methods | RCT. Parallel design. Duration: 2 weeks. Single centre. Country: UK. |
|
| Participants | 24 participants with CF and chronic P. aeruginosa infection experiencing exacerbations. Mean (range) age 20 (14 ‐ 48) years. 12 male, 12 female. |
|
| Interventions | Intervention 1: IV ceftazidime 2 g 3x daily. Intervention 2: IV aztreonam 2 g 3x daily. |
|
| Outcomes | Lung function (FEV1), 'symptom scores', sputum weight, inflammatory markers (CRP, neutrophil elastase, TNF‐α, α‐1 antitrypsin complex C). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Desccribed as randomised but no method detailed. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals described. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | No other bias identified. |
Gold 1985.
| Methods | RCT. Parallel design. Duration: 10 ‐ 14 days. Single centre. Country: Canada. |
|
| Participants | 30 participants with CF and P. aeruginosa infection present at the previous clinic visit, experiencing an acute respiratory exacerbation. Participants deemed to be experiencing a severe exacerbation (protocol defined) were excluded. Intervention 1: 17 participants; mean (SE) age 18.9 (1.1) years; 15 males, 2 females. Intervention 2: 13 participants; mean (SE) age 17.8 (0.8) years; 9 males, 4 females. |
|
| Interventions | Intervention 1: IV ceftazidime 200 mg/kg/day in 4 doses. Intevention 2: IV ticarcillin 300 mg/kg/day in 4 doses and IV tobramycin 10 mg/kg/day in 3 doses. |
|
| Outcomes | Lung function (FEV1, FEF25‐75%), weight, microbiology, adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a table of random numbers used. |
| Allocation concealment (selection bias) | Unclear risk | No detail. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | No other bias identified. |
Gold 1987.
| Methods | Double‐blind placebo‐controlled RCT. Parallel design. Duration: 14 days treatment with 6 ‐ 24 months follow up. Single centre. Country: Canada. |
|
| Participants | 26 participants experiencing a protocol‐defined pulmonary exacerbation. Participants were excluded if they were considered to be experiencing a severe exacerbation (protocol‐defined). Intervention 1: 16 participants; mean (95% CI) age 17.9 (17 ‐ 18.8) years; 4 males, 12 females. Intervention 2: 15 participants; mean (95% CI) age 18.5 (17.3 ‐ 19.7) years; 9 males, 6 females. |
|
| Interventions | Intervention 1: IV ceftazidime 200 mg/kg/day in 4 doses. Intervention 2: IV placebo (colour‐matched vitamin B complex). |
|
| Outcomes | Lung function (FEV1 and VC), weight, microbiology, unvalidated clinical and symptom scores. | |
| Notes | UoA issues ‐ 5 participants were treated 2x. 3 participants in placebo group wished to withdraw as the lack of discolouration of their urine indicated to them that they were not in the intervention group. We shall seek to contact the authors for IPD to reconcile the UoA issues. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Colour‐matched placebo, although it is known that ceftazidime discolours the urine. 3 participants noted an absence of discolouration and wished to withdraw from the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The 3 participants who withdrew did not contribute to the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ 5 participants treated 2x. |
Hodson 1987.
| Methods | RCT. Parallel design. Duration: 10 days treatment with 6 weeks follow up. Single centre. Country: UK. |
|
| Participants | 40 participants with CF and chronic P. aeruginosa infection (over 6 months) admitted to hospital experiencing a pulmonary exacerbation. Mean (range) age 23 (18 ‐ 35) years. Intervention 1: 20 participants. Intervention 2: 20 participants. |
|
| Interventions | Intervention 1: oral ciprofloxacin 500 mg 3x daily. Intervention 2: IV azlocillin 5 g 3x daily and IV gentamicin 80 mg 3x daily. |
|
| Outcomes | Lung function (peak flow, FEV1, FVC), mortality, adverse effects, microbiology. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described a random allocation but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Lung function was tested by an 'investigator who was not involved in the study' although it is unclear if this assessor was truly blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all participants were available at 10 days, but only data for 15 participants in each group were available at 6 weeks. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | No additional bias noted. |
Huang 1983.
| Methods | Double‐blind RCT. Parallel design. Duration: 2 weeks. Single centre. Country: USA. |
|
| Participants | 29 participants with CF experiencing a protocol‐defined exacerbation with sputum bacteria that were sensitive to both azlocillin and carbenicillin. Intervention 1: 12 participants; median (range) age 12.25 (6.5 ‐ 24.5) years; 4 males, 8 females. Intervention 2: 14 participants; median (range) age 12.5 (5.75 ‐ 21) years; 7 males, 7 females. |
|
| Interventions | Intervention 1: IV azlocillin 250 mg/kg/day in 5 doses. Intervention 2: IV carbenicillin 500 mg/kg/day in 5 doses. |
|
| Outcomes | Lung function (unstated), microbiology, adverse effects and a scoring system. | |
| Notes | 3 participants were withdrawn from the study (2 had rashes and 1 developed cholecystitis) and did not contribute data to the analysis. The results are presented in terms of the clinical score and so we shall seek to contact the authors for IPD to contribute to a meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation code developed by statistician and kept in pharmacy. |
| Allocation concealment (selection bias) | Unclear risk | No detail other than "kept in pharmacy". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as 'double‐blind' but no detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as 'double‐blind' but no detail given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawn participants did not contribute to analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | No further biases identified. |
Hyatt 1981.
| Methods | Double‐blind RCT. Parallel design. Duration: 14 days. Single centre. Country: USA. |
|
| Participants | 15 participants with CF experiencing an acute exacerbation, each with P. aeruginosa isolated from 3 out of 4 most recent samples. Age: range 6 ‐ 21 years; gender split not detailed. Intervention 1: 9 participants. Intervention 2: 15 participants. |
|
| Interventions | Intervention 1: IV oxacillin 35 mg/kg 6x daily and IV 'placebo fluids' 6x daily. Intervention 2: IV oxacillin 35 mg/kg 6x daily and IV carbenicillin 65 mg/kg 6x daily and IV sisomicin 70 mg/m2 6x daily. |
|
| Outcomes | Lung function (FEV1, FVC, RV), microbiology, adverse effects, symptom score, treatment 'failure' (early withdrawal due to poor response, or additional treatment required at the end of the 14‐day period). | |
| Notes | UoA issues ‐ 9 participants contributed more than once to the study. The unequal assignment to the 2 groups (9 to the control group and 15 to the treatment group (intervention 2) occurred through chance alone. Oxacillin masked the odour of carbenicillin in the participants' urine. We shall seek to contact the authors for IPD to reconcile the UoA issues. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Antibiotics and placebo were prepared in pharmacy and delivered in coded bottles. Code was not broken in case of 'treatment failure'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as 'double‐blind'. Antibiotics and placebo were prepared in pharmacy and delivered in coded bottles. Code was not broken in case of 'treatment failure'. Sham serum levels of sisomicin given by (unblinded) pharmacists. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as 'double‐blind' with parents, participants and clinicians blinded as above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the case that participants were removed from the study due to "early failure" (control n = 3 out of 9; treatment n = 2 out of 15) the data from the last day of study participation contributed to the data analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data. |
| Other bias | High risk | UoA issues ‐ 9 participants recruited more than 1x. |
Knowles 1988.
| Methods | RCT. Parallel design. Duration: 12 ‐ 14 days, then 4‐week post‐hospitalisation visit. Country: USA. |
|
| Participants | 19 CF participants with an acute exacerbation. Intervention 1: 10 participants. Intervention 2: 9 participants. Analysis of baseline variables showed that Group 2 were "sicker". |
|
| Interventions | Intervention 1: IV piperacillin and tobramycin. Intervention 2: IV piperacillin and tobramycin plus aerosolised piperacillin and tobramycin. |
|
| Outcomes | Chest radiograph score, clinical score, weight, total WBC, absolute band count, pulmonary function tests (FVC, FEV1, PEFR), time in weeks to additional antibiotic use, time in months to next hospitalisation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | High risk | Time to next hospitalisation was recorded but not reported. |
| Other bias | Low risk | None identified. |
Macfarlane 1985.
| Methods | Double‐blind placebo‐controlled RCT. Parallel design. Duration: 14 days. Single centre. Country: Australia. |
|
| Participants | 19 participants aged over 8 years with CF with P. aeruginosa in sputum admitted to hospital for worsening respiratory status. Intervention 1: 4 participants; mean (SD) age 15.3 (3) years. Intervention 2: 5 participants; mean (SD) age 12.5 ( 2.9) years. Intervention 3: 4 participants; mean (SD) age 13.7 ( 2.6) years. Intervention 4: 5 participants; mean (SD) age 15.6 (3.4) years. Gender split not detailed. |
|
| Interventions | Intervention 1: IV piperacillin 50 mg/kg 4‐hourly. Intervention 2: IV placebo 5% dextrose 4‐hourly. Intervention 3: IV piperacillin 100 mg/kg 8‐hourly. Intervention 4: IV placebo 5% dextrose 8‐hourly. All participants received IV tobramycin 2.5 mg/kg 3x daily, oral flucloxacillin 25 mg/kg/day in 4 doses and oral probenecid (suggested to increase antibiotic concentrations) 250 ‐ 500 mg 3x daily. |
|
| Outcomes | Lung function (FEV1, VC, RV, TLC, FEF25‐75%), weight, symptom and clinical score, mortality, microbiology. | |
| Notes | UoA issues ‐ 1 participants received 2 courses. We shall seek to contact the authors for IPD to reconcile the UoA issues and for data to contribute to a meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomly assigned but no method given. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind. Identities of infusions known only to pharmacy personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants withdrew and did not contribute data to the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ 1 participant received 2 treatment courses. |
Master 2001.
| Methods | Double‐blind RCT. Parallel design. Duration: at least 10 days. Single centre. Country: Australia. |
|
| Participants | 51 participants with CF experiencing a protocol‐defined exacerbation with P. aeruginosa isolated from sputum. Participants with an FVC lower than 40% predicted were excluded. Intervention 1: 21 participants; mean (SD) age 16 (7) years. Intervention 2: 23 participants; mean (SD) age 15 (5) years. |
|
| Interventions | Intervention 1: IV ceftazidime 50 mg/kg/dose 3x daily and IV tobramycin 3 mg/kg/dose 3x daily. Intervention 2: IV tobramycin 9 mg/kg/day 1x daily. |
|
| Outcomes | Lung function (FEV1, FVC, FEF25‐75%), radiology, microbiology, adverse effects, time to next exacerbation. | |
| Notes | UoA issues ‐ each participant contributed on average 3 episodes. Study was suspended for 3 months after 1 participant committed suicide, data from this period were not included. We shall seek to contact the study authors for individual study data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, stratified for age and disease progression, but no method detailed. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double blind with medical and nursing staff and participants blinded with identical syringes and placebos. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were described and those participants who completed 10 days treatment but excluded for other reasons were included in an ITT analysis. The ITT analysis is described as not changing the effect of the short‐term analysis, but no data provided. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ each participant contributed multiple treatment episodes. |
McCarty 1988.
| Methods | Open‐label RCT. Parallel design. Duration: at least 10 days. Single centre. Country: USA. |
|
| Participants | 17 children with CF admitted for treatment of pulmonary exacerbations. Age: range 2 ‐ 12 years. Gender split not detailed. Intervention 1: 8 participants. Intervention 2: 9 participants. |
|
| Interventions | Intervention 1: IV piperacillin 600 mg/kg/day (regimen not detailed). Intervention 2: IV piperacillin 600 mg/kg/day and tobramycin 8 ‐ 10 mg/kg/day(regimen not detailed). |
|
| Outcomes | Lung function (PEFR, FEV1, FVC), weight, clinical score, microbiology, mortality, adverse effects. | |
| Notes | UoA issues ‐ 3 participants were included 2x. No data provided for lung function and nutritional status. We shall seek to contact the study authors for IPD to include in a meta‐analysis and reconcile the UoA issues. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomly assigned but no detail given. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered envelopes were used, although it is not clear if these were opaque and sealed. On balance, considered low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ 3 participants were included twice. |
McLaughlin 1983.
| Methods | Part‐placebo‐controlled double‐blind RCT. Parallel design. Duration: 10 days. Dual centre study. Country: USA. |
|
| Participants | 60 participants with CF experiencing an exacerbation requiring hospital admission. Age: mean (SD) 21 (5) years; range 11 ‐ 30 years. Gender split not detailed. Intervention 1: 17 participants. Intervention 2: 18 participants. Intervention 3: 16 participants. |
|
| Interventions | Intervention 1: IV ticarcillin 300 mg/kg/day in 6 doses and IV tobramycin 6 mg/kg/day in 3 doses. Intervention 2: IV azlocillin 300 mg/kg/day in 6 doses and IV tobramycin 6 mg/kg/day in 3 doses. Intervention 3: IV azlocillin 300 mg/kg/day in 6 doses and placebo 0.85% saline in 3 doses. |
|
| Outcomes | Lung function (FEV1, VC, maximal mid‐expiratory flow rate, RV), microbiology, antibiotic susceptibility, adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned by pharmacist, but no detail on method. |
| Allocation concealment (selection bias) | Low risk | Pharmacist used consecutively numbered sealed envelopes, but it is not clear if they were opaque, on balance considered low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Neither participants nor physicians know which regimen was prescribed", but no other detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double blind, but no other detail given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 participants withdrew, or data were not available for analysis. 3 participants excluded as they had incomplete lung function or bacteriology data available. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | No other bias identified. |
Padoan 1987.
| Methods | RCT. Parallel design. Duration: average of 14 days. Single centre. Country: Italy. |
|
| Participants | 30 participants with CF and moderate to severe lung disease with chronic P. aeruginosa infection experiencing an acute exacerbation. Intervention 1: 20 participants; mean (SD) age 12 years 2 months (5 years); 9 males, 11 females. Intervention 2: 20 participants; mean (SD) age 11 years (3 years); 5 males, 15 females. Intervention 3: 20 participants; mean (SD) age 10 years 3 months (4 years 11 months); 4 males 16 females. |
|
| Interventions | Intervention 1: IV ceftazidime 50 mg/kg 3x daily. Intervention 2: IV ceftazidime 50 mg/kg 3x daily and IV sisomicin 3 mg/kg 3x daily. Intervention 3: IV piperacillin 100 mg/kg 3x daily and IV sisomicin 3 mg/kg 3x daily. |
|
| Outcomes | Clinical score, microbiology, adverse effects. | |
| Notes | UoA issues ‐ 30 participants contributed 60 treatment episodes. As the data was collated and presented in the form of a clinical score, we shall seek to contact the study authors for IPD to include in a meta‐analysis and reconcile the UoA issues. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomly assigned but no method given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as a 'blind study' but little detail given other than participants likely to be blinded as given a saline infusion given instead of active drug in monotherapy group (although no detail on procedure/preparation). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as a 'blind study' but no detail given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ 30 participants contributed 60 treatment episodes. |
Penketh 1983.
| Methods | Single‐blind RCT. Parallel design. Duration: 10 days. Single centre. Country: UK. |
|
| Participants | 16 adults with CF and chronic P. aeruginosa infection admitted with deteriorating lung function and acute respiratory symptoms. Intervention 1: 8 participants; age range 16 ‐ 26 years; 5 males, 3 females. Intervention 2: 8 participants; age range 21 ‐ 33 years; 4 males, 4 females. |
|
| Interventions | Intervention 1: IV ticarcillin 5 g 6‐hourly and IV gentamicin 8‐hourly (dose adjusted to achieve peak serum level 8 ‐ 10 µg/ml). Intervention 2: IV carbenicillin 5 g 6‐hourly and IV gentamicin 8‐hourly (dose adjusted to achieve peak serum level 8 ‐ 10 µg/ml). |
|
| Outcomes | Lung function (PEFR, FEV1, FVC), time to next exacerbation and adverse effects. | |
| Notes | Means only (no SD) detailed for lung function and time to next exacerbation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only participants blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded healthcare personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals described. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | None identified. |
Penketh 1984.
| Methods | Single blind RCT. Parallel design. Duration: 10 days. Single centre. Country: UK. |
|
| Participants | 20 adults with CF and chronic P. aeruginosa infection admitted with a protocol‐defined exacerbation. People with severe disease (FEV1 < 20% predicted) were excluded. Intervention 1: 10 participants; age range 18 ‐ 25 years; 6 males, 4 females. Intervention 2: 10 participants; age range 17 ‐ 29 years; 9 males, 1 female. |
|
| Interventions | Intervention 1: IV azlocillin 5 g 3x daily and IV gentamicin (dose adjusted to achieve peak serum level 8 ‐ 10 µg/ml). Intervention 2: IV carbenicillin 5 g 4‐hourly and IV gentamicin (dose adjusted to achieve peak serum level 8 ‐ 10 µg/ml). |
|
| Outcomes | Lung function (PEFR, FEV1, FVC), time to next exacerbation, mortality and adverse effects. | |
| Notes | Participants identified as having other pathogens in sputum were given 'appropriate oral antibiotics'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'Single‐blind' study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals described. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | None identified. |
Regelmann 1990.
| Methods | Double‐blind placebo‐controlled RCT. Parallel study. Duration: 14 days. Single centre. Country: USA. |
|
| Participants | 15 participants with CF and chronic P. aeruginosa infection who had experienced a deterioration in FEV1 greater than 10% over previous 1 ‐ 6 months. Intervention 1; 8 participants; mean (SD) age 21 (6.5) years; 6 males, 2 females. Intervention 2: 5 participants; mean (SD) age 22 (7.2) years; 3 males, 2 females. |
|
| Interventions | Lead‐in period of 4 days consisting of chest physiotherapy and bronchodilators then those that did not deteriorate further were randomised to receive 1 of following. Intervention 1; IV tobramycin 3 mg 1x daily and IV ticarcillin 70 mg/kg with dose frequency adjusted to achieve target range. Intervention 2: IV placebo (dextrose water). |
|
| Outcomes | Lung function (FEV1, FVC, FEF25‐75%), weight, adverse effects. | |
| Notes | An improvement in weight and lung function was observed in the lead‐in period before antibiotic therapy was commenced. It is uncertain whether this improvement reduced the measured change in the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double blind. Little information given, sham dose adjustment of placebo was given a well as treatment groups. One investigator not involved in clinical care was unblinded and responsible for dosing and allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double blind but little detail provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant withdrew from the placebo arm on day 3 and did not contribute data. 4 participants contribute lung function data for the placebo group compared to 8 participants in the antibiotic group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ 1 participant re‐entered the study after a 2‐year gap. |
Richard 1997.
| Methods | Open‐label RCT. Parallel design. Duration: 14 days. Multicentre study. Countries: France, Germany, Greece, Hungary, Israel, Italy, Portugal, South Africa and Switzerland. |
|
| Participants | 108 children with CF and P. aeruginosa infection and experiencing a protocol‐defined pulmonary exacerbation. Intervention 1: oral ciprofloxacin ‐ mean age 10.2 years; 32 males, 23 females. Intervention 2: IV ceftazidime and IV tobramycin ‐ mean age 11.0 years; 27 males, 26 females. |
|
| Interventions | Intervention 1: oral ciprofloxacin 15 mg/kg 2x daily. Intervention 2: IV ceftazidime 50 mg/kg 3x daily and IV tobramycin 3 mg/kg 3x daily. |
|
| Outcomes | Lung function (FEV1, FVC), time to next exacerbation, adverse effects, microbiology, | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The efficacy and safety analysis were described as analysed on an ITT basis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | An author on the paper is affiliated to Pharma Research Center, Bayer AG. Bayer produced ciprofloxacin. |
Salh 1992.
| Methods | Double‐blind RCT. Parallel design. Duration: 2 weeks. Single centre. Country: UK. |
|
| Participants | 22 participants with CF and P. aeruginosa sensitive to the study drugs who were admitted to hospital due to an infective exacerbation. Age: 16 ‐ 32 years. Gender split: aztreonam ‐ 6 females, 8 males; ceftazidime ‐ 4 females, 8 males. |
|
| Interventions | Intervention 1; IV aztreonam 8 g/day in 4 doses. Intervention 2: IV ceftazidime 8 g/day in 4 doses. |
|
| Outcomes | Lung function (FEV1), symptom score, weight, adverse effects. | |
| Notes | UoA issues ‐ 4 participants received both drugs on separate occasions. We shall seek to contact the study authors for IPD to include in a meta‐analysis and reconcile the UoA issues. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in pharmacy using 'simple random allocation'. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes but unclear whether sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind infusions prepared in pharmacy and labelled with trial number. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear, but as the physicians and participants were blinded it is likely the outcome assessors were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 withdrew (3 of whom were treatment failures), it is unclear if these contributed to the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | High risk | UoA issues ‐ 4 participants contribute multiple treatment episodes. |
Schaad 1986.
| Methods | RCT. Parallel design. Duration: 2 weeks. Single centre. Country: Switzerland. |
|
| Participants | 29 participants with CF who were admitted for treatment of an exacerbation and had P. aeruginosa isolated on admission. Participants with severe disease were excluded. Intervention 1: 21 participants; mean (range) age 14.5 (4 ‐ 22) years; 11 males, 10 females. Intervention 2: 21 participants; mean (range) age 16.5 (5 ‐ 23) years; 9 males, 12 females. |
|
| Interventions | Intervention 1: IV netilmicin 11 mg/kg/day in 3 doses and IV azlocillin 500 mg/kg/day in 4 doses. Intervention 2: IV netilmicin 11 mg/kg/day in 3 doses and IV ticarcillin 500 mg/kg/day in 4 doses. |
|
| Outcomes | Lung function (VC, RV), nutritional status (relative underweight (%)), adverse effects. | |
| Notes | 29 participants received 42 courses of therapy, although only the 1st treatment course was used for analysis, thus negating a UoA issue. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Due to in vitro resistance pattern, 2 participants changed from azlocillin to ticarcillin and 2 participants changed from ticarcillin to azlocillin. It is unclear if these data feature in the final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | Low risk | UoA issues accommodated in analysis ‐ only the 1st treatment course was used for analysis. |
Schaad 1987.
| Methods | RCT. Parallel design. Duration: 15 days. Single centre. Country: Switzerland. |
|
| Participants | 62 participants with CF admitted with an acute pulmonary exacerbation who had P. aeruginosa isolated on admission. Those who had been admitted to hospital in the recent 6 months were excluded. Age: range 3 ‐ 24 years. Gender split: not detailed. Intervention 1: 24 participants. Intervention 2: 30 participants. |
|
| Interventions | Intervention 1: IV ceftazidime 250 mg/kg/day in 4 doses and IV amikacin 33 mg/kg/day in 3 doses. Intervention 2: IV ceftazidime 250 mg/kg/day in 4 doses and IV amikacin 33 mg/kg/day in 3 doses and nebulised amikacin 100 mg 2x daily. |
|
| Outcomes | Lung function (VC), nutritional status (degree of underweight (%)), adverse effects. | |
| Notes | UoA issues ‐ 62 participants received 87 courses of therapy. We shall seek to contact the study authors for IPD to include in a meta‐analysis and reconcile the UoA issues. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomly allocated but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical evaluator blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | High risk | UoA issues ‐ 13 participants enrolled 2x and 6 participants enrolled 3x. |
Schaad 1989.
| Methods | RCT. Parallel design. Duration: 2 weeks IV treatment, with oral treatment extended for a further 4 weeks in 1 group. Single centre. Country: Switzerland. |
|
| Participants | 42 participants with CF admitted with a protocol‐defined pulmonary exacerbation and P. aeruginosa isolated at admission. Those who had been admitted to hospital in previous 4 months were excluded. Age: mean (SD) 15.4 (6) years (range 2.3 ‐ 25.4 years). Gender split not detailed. Intervention 1: 28 participants. Intervention 2: 28 participants. |
|
| Interventions | Intervention 1: IV aztreonam 300 mg/kg/day in 4 doses and IV amikacin 36 mg/kg/day in 3 doses. Intervention 2: IV ceftazidime 300 mg/kg/day in 4 doses and IV amikacin 36 mg/kg/day in 3 doses for 2 weeks followed by oral ciprofloxacin 30 mg/kg/day for 4 weeks. |
|
| Outcomes | Lung function (FEV1, VC), nutritional status (degree underweight (%)), adverse effects. | |
| Notes | UoA issues ‐ 42 participants received 56 courses of treatment. We shall seek to contact the study authors for IPD to include in a meta‐analysis and reconcile the UoA issues. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear ‐ no detail given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical evaluation undertaken by 2 investigators without knowledge of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Clinical outcomes available for about 50% of participants only. Some participants are young children (and so would be able to perform lung function tests) but the mean age is 15.4 years and so there are data missing for many participants for whom lung function testing would have been possible. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence. |
| Other bias | High risk | UoA issues ‐ 42 participants received 56 courses of treatment. |
Semykin 2010.
| Methods | RCT. Parallel design. Duration: 14 days. Single centre. Country: Russia. |
|
| Participants | 108 participants with CF and chronic P. aeruginosa infection and acute pulmonary exacerbations. Intervention 1: 32 participants; age range 4 ‐ 16 years. Intervention 2: 39 participants; age range 6 ‐ 17 years. Intervention 3: 37 participants; age range 4 ‐ 17 years. Gender split not detailed. |
|
| Interventions | Intervention 1: inhaled tobramycin (TOBI or Bramitob) 300 mg 2x daily and IV ceftazidime (regimen not detailed) and oral ciprofloxacin (regimen not detailed). Intervention 2: IV cefepime (regimen not detailed) and IV amikacin (regimen not detailed). Intervention 3: IV meropenem (regimen not detailed) and IV amikacin (regimen not detailed). |
|
| Outcomes | Lung function (FEV1, FVC), microbiology. | |
| Notes | Abstract only. We shall contact the authors for more detail and data to allow contribution to a meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detail given. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient detail. |
| Other bias | Low risk | None identified. |
Smith 1999.
| Methods | Double‐blind RCT. Parallel design. Duration: 14 days. Multicentre (9 centres). Country: USA. |
|
| Participants | 111 participants with CF experiencing a protocol‐defined pulmonary exacerbation at which time the predominant P. aeruginosa morphotype was susceptible to azlocillin and tobramycin. Intervention 1: 33 participants; mean (SD) age 16.07 (7.4) years; 19 males, 14 females. Intervention 2: 43 participants; mean (SD) age 16.53 (6.9) years; 18 males, 25 females. |
|
| Interventions | Intervention 1: IV azlocillin 450 mg/kg/day in 6 doses and placebo (5% dextrose) in 4 doses. Intervention 2:IV azlocillin 450 mg/kg/day in 6 doses and IV tobramycin 240 mg/m2/day in 4 doses. |
|
| Outcomes | Lung function (FEV1, FVC, PEFR, FRC, FEF25‐75%), time to next exacerbation. | |
| Notes | 35 withdrawals are described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by the core centre pharmacist with a code generated at that centre. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as placebo‐controlled double‐blind with unblinded third parties adjusting tobramycin dosages and dummy adjusting placebo dosages by study pharmacist. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 35 withdrawals are described, although not analysed as ITT. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Funded by a grant from Miles Pharmaceuticals who manufactured azlocillin, however azlocillin not a comparator as both study groups received azlocillin. |
Stephens 1983.
| Methods | RCT. Parallel design. Duration: 14 days. Single centre. Country: Canada. |
|
| Participants | 28 participants with CF experiencing a pulmonary exacerbation. Intervention 1: 12 participants; mean (SD) age 15.1 (4.7) years; 9 males, 3 females. Intervention 2: 16 participants; mean (SD) age 15.3 (3.5) years; 9 males, 7 females. |
|
| Interventions | Intervention 1: IV tobramycin 10 mg/kg/day in 3 doses and IV ticarcillin 300 mg/kg/day in 3 doses. Intervention 2: IV tobramycin 10 mg/kg/day in 3 doses and IV ticarcillin 300 mg/kg/day in 3 doses and nebulised tobramycin 80 mg 3x daily. |
|
| Outcomes | Lung function (FEV1, FEV25‐75%), weight, adverse effects. | |
| Notes | 3 participants were unable to be discharged at the end of treatment due to slow resolution of symptoms, although it is unclear if these participants contributed to the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Technician performing lung function was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 participants required a longer admission, it is unclear if they contributed to the data analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | None identified. |
Wang 1988.
| Methods | RCT. Parallel design. Duration: 2 weeks. Single centre. Country: USA |
|
| Participants | 23 participants with CF experiencing pulmonary exacerbations. Age: over 18 years. Gender split: no details given. |
|
| Interventions | Intervention 1: oral ciprofloxacin 750 mg 2x daily. Intervention 2: IV tobramycin and IV ticarcillin (dose not stated). Intervention 3: IV tobramycin and IV azlocillin (dose not stated). |
|
| Outcomes | Lung function (specific tests not stated), adverse effects, laboratory tests (blood counts, blood chemistries, blood gases, sputum cultures), chest x‐ray. | |
| Notes | UoA issues ‐ many participants received more than 1 treatment allocation. We shall seek to contact the study authors for IPD to include in a meta‐analysis and reconcile the UoA issues. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as random, but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detail given. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ many participants received more than 1 treatment allocation. |
Wesley 1988.
| Methods | RCT. Parallel design. Duration: 14 days. Single centre. Country: New Zealand. |
|
| Participants | 13 children with CF and severe chest disease. Age range 9 ‐ 15 years. Gender split: not detailed. Intervention 1: 13 participants. Intervention 2: 10 participants. |
|
| Interventions | Intervention 1: IV ceftazidime 150 mg/kg/day (regimen not detailed). Intervention 2: IV tobramycin 7.5 mg/kg/day and IV ticarcillin 300 mg/kg/day (regimen not detailed). |
|
| Outcomes | Lung function (not detailed), adverse effects. | |
| Notes | UoA issues ‐ 13 participants received 23 courses of treatment. We shall seek to contact the study authors for IPD to include in a meta‐analysis and reconcile the UoA issues. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no detail given. |
| Allocation concealment (selection bias) | Unclear risk | No detail given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double blind but no detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double blind but no detail given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detail available. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ 13 participants received 23 courses of treatment. |
Wientzen 1980.
| Methods | Double‐blind placebo‐controlled RCT. Parallel design. Duration: not detailed. Single centre. Country: USA |
|
| Participants | 22 participants with CF admitted to hospital due to an acute pulmonary exacerbation. Intervention 1: 12 participants; mean age (range) ‐ 10.5 years (9 months ‐ 27 years); 7 males, 5 females. Intervention 2: 12 participants; mean age (range) ‐ 8.5 years (3 ‐ 16 years); 6 males, 6 females. |
|
| Interventions | Intervention 1: IV tobramycin 2 mg/kg 3x daily. Intervention 2: IV placebo (lactose solution). |
|
| Outcomes | Lung function (FEV1, PEFR, VC), adverse effects, mortality. | |
| Notes | UoA issues ‐ 2 participants treated 2x. We shall contact the authors for data for inclusion in a meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double blind with adequate evidence of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate evidence of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not include participants who withdrew in the analysis. 2 participants died in the placebo group (one on day 1 and the second on day 4). Due to the small study size this is surprising and so suggests either a failure of random allocation or a difference in the characteristics of the comparator groups at baseline. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk | UoA issues ‐ 2 participants treated 2x. |
B. cepacia: Burkholderia cepacia CF: cystic fibrosis CI: confidence interval CRP: C‐reactive protein FEV1: forced expiratory volume at one second FEV25‐75%: mid peak expiratory flow FRC: functional residual capacity FVC: forced vital capacity IPD: individual patient data ITT: intention to treat IV: intravenous P. aeruginosa: Pseudomonas aeruginosa PEFR: peak expiratory flow rate RCTL randomised controlled trial RV: residual volume SD: standard deviation TLC: total lung capacity TNF‐α: tumour necrosis factor alpha UoA: unit of analysis VC: vital capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaron 2005 | In vitro susceptibility testing trial. |
| Adeboyeku 2011 | Dosing study. |
| Al‐Ansari 2006 | Dosing study. |
| Amelina 2000 | Study of antibiotic location (home IVs). |
| Aminimanizani 2002 | Dosing study. |
| Balsamo 1986 | In vitro study. |
| Beringer 2003 | Pharmacodynamics study. |
| Beringer 2010 | Study of anti‐inflammatory effect of doxycyline. |
| Brett 1992 | Trial of chronic/maintenance therapy. |
| Burkhardt 2006 | Dosing study. |
| Byrne 1995 | Maintenance therapy, not exacerbation. |
| Cabezudo 1984 | Non‐randomised, no comparison group. |
| Canis 1998 | Dosing study. |
| Christensson 1992 | Pharmacokinetic/pharmacodynamic study. |
| Conway 1996a | Dosing study. |
| Davis 1987 | Pharmacokinetic study. |
| Davis 1990 | Study of antibiotic location (home vs hospital). |
| Day 1988 | Comparison of inhaled therapies. |
| De Boeck 1998 | Dosing study. |
| Degg 1996 | Effect of antibiotics upon hearing (non‐CF comparison group). |
| Dodge 1983 | Observational study, no comparator. |
| Donati 1987 | Non‐randomised, study of antibiotic location (home vs hospital). |
| Elborn 2000 | Study of antibiotic usage by indication (elective vs symptomatic). |
| Eron 1983 | Randomised dosing study (multiple indications; not reported by indication). |
| Gold 1983 | No comparator. |
| Goldfarb 1987 | Single arm pharmacokinetic, toxicity and microbiology monitoring study. |
| Guglielmo 1996 | Dosing study. |
| Hamner 2006 | Pharmacokinetic study, no comparator. |
| Hatziagorou 2013 | Non‐randomised observational study of lung clearance index. |
| Heaf 1984 | Comparison of two inhaled regimens. |
| Heininger 1993 | Once vs. thrice‐daily study. |
| Hjelte 1988 | Study of treatment location ‐ home vs hospital study. |
| Hoogkamp‐Korstanje 1983 | Non‐randomised study. |
| Hubert 2009 | Dosing study. |
| Ivanov 1997 | Dosing study. |
| Jackson 1986 | Non‐randomised study. |
| Jacobs 1985 | Pharmacokinetic study. |
| Jensen 1987 | Study of maintenance/elective therapy. |
| Jewett 1985 | Quasi‐randomised by alternate participant selection. |
| Keel 2011 | Pharmacokinetic study, not exacerbations. |
| Kercsmar 1983 | Pharmacokinetic study, no comparator. |
| Klettke 1999 | Study of antibiotic location (home vs hospital). |
| Krause 1979 | Quasi‐randomised as allocation by birth month. |
| Kruger 2001 | Dosing study. |
| Kuni 1992 | Non‐randomised, no comparator, deposition study. |
| Kuzemko 1989 | Dosing study. |
| Labiris 2004 | Toxicity study, no comparator. |
| Levy 1982 | No comparison group. |
| Levy 1982a | Non‐randomised study. |
| Li 1991 | Computerised dosing study. |
| Martin 1980 | Non‐randomised study. |
| McCabe 2013 | Dosing study ‐ twice vs thrice daily tobramycin. |
| Michalsen 1981 | Non‐randomised study. |
| Moss 1991 | Immunology/desensitisation study. |
| Mouton 1991 | Dosing study. |
| Nikolaizik 2005 | Dosing study. |
| Nikonova 2010 | Inhaled medications only. |
| Padoan 1988 | Dosing study. |
| Parry 1977 | Non‐randomised study. |
| Pedersen 1986 | Maintenance therapy, not exacerbation. |
| Permin 1983 | Maintenance therapy, not exacerbation. |
| Popa 2001 | Non‐randomised study. |
| Postnikov 2001 | Non‐randomised study. |
| Postnikov 2001a | Non‐randomised study. |
| Postnikov 2007 | Dosing study. |
| Powell 1983 | Dosing study. |
| Prayle 2013 | Pharmacokinetic study only. |
| Ramstrom 2000 | Trial of drug preparation (patient vs pharmacist). |
| Reed 1987 | Reports an open, uncontrolled study and a randomised study of dosing. |
| Reed 1987a | Dosing study. |
| Riethmueller 2009 | Dosing study. |
| Roberts 1992 | Pharmacokinetic study. |
| Rubio 1987 | Non‐randomised study. |
| Shatunov 2001 | Non‐randomised. |
| Smyth 2005 | Dosing study. |
| Turner 2013 | Dosing study ‐ continuous vs intermittent dosing regimens. |
| Wainwright 2011 | Trial of bronchoscopy‐guided antibiotic therapy. |
| Whitehead 2002 | Dosing study. |
| Winnie 1991 | Dosing study. |
| Wolter 1997 | Study of antibiotic location (home vs hospital). |
| Wood 1996 | Dosing study. |
| Yasmin 1974 | Maintenance therapy, not exacerbations. |
CF: cystic fibrosis IVs: intravenous antibiotics vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Al‐Aloul 2005.
| Methods | Cross‐over RCT comparing intravenous and nebulised tobramycin. |
| Participants | 13 or 14 adults with CF and chronically infected with Liverpool epidemic strain P. aeruginosa. Age: mean (SD) 22 (7) years. Disease severity: mean (SD) FEV1 % predicted 65 (22). |
| Interventions | Participants were randomised to receive, either nebulised or IV antibiotics during consecutive exacerbations over four successive exacerbations (mean (SD) interval between exacerbations was 7.8 (5.5) months). Intervention 1: TOBI (nebulised tobramycin) 300 mg 2x daily. Intervention 2: IV tobramycin (mean daily dose 8.2 mg/kg in 2 or 3 divided doses). In both arms IV colomycin 2 megaunits 3x daily was also given. |
| Outcomes | Lung function, quantitative microbiology and renal toxicity adverse effects. |
| Notes | 2 references appear to report data from the same study. Both reports have the same mean and SD values for FEV1, BMI and mean daily dose of tobramycin. The 2005 report is noted to include 13 participants and the 2004 report noted to include 14 participants. Due to all other similarities we have considered the reports to be two from the same study. We shall seek to contact the authors to request data for the first treatment allocation for each participant. |
Beaudry 1980.
| Methods | RCT. |
| Participants | Children with severe CF and signs of acute infection. |
| Interventions | Cloxacillin or carbenicillin plus gentamicin administered intravenously for 10 days. |
| Outcomes | Clinical improvement, radiology. |
| Notes | We shall seek to contact the authors for data relating to outcomes includable in the review. |
Crawley 2005.
| Methods | Unclear if randomised. |
| Participants | Unclear number of participants included. |
| Interventions | Subcutaneous infusions of either meropenem or ceftazidime (plus TOBI in 3 cases). |
| Outcomes | Lung function, adverse effects. |
| Notes | We shall seek to contact the authors for details of study design and individual participant data. |
Dinwiddie 1982.
| Methods | Cross‐over RCT. |
| Participants | 9 children with chronic P. aeruginosa infection. |
| Interventions | 14 days treatment with IV azlocillin or IV gentamicin. |
| Outcomes | Lung function, weight, adverse effects. |
| Notes | We shall seek to contact the authors for individual participant data in order to determine the first treatment episode for each participant. |
Döring 1995.
| Methods | Double‐blind, placebo‐controlled cross‐over trial. |
| Participants | 10 participants with P. aeruginosa infection. |
| Interventions | Individualised IV antibiotic (not detailed) therapy versus placebo for 2 weeks. |
| Outcomes | Lung function, immunology. |
| Notes | No data in the abstract ‐ we shall seek to contact the authors for individual participant data to include the 1st treatment event data in the analysis. |
Geborek 2003.
| Methods | Open cross‐over RCT. |
| Participants | Unstated number of participants with chronic P. aeruginosa infection, experiencing an exacerbation. |
| Interventions | Nebulised TOBI and an IV ß‐lactam versus IV tobramycin and a ß‐lactam for 10 days. |
| Outcomes | Lung function, adverse effects, time to next exacerbation. |
| Notes | We shall seek to contact the authors for individual participant data in order to use the 1st episode for each participant in the meta‐analysis. There also appears to be some typographical errors in the data table and so shall clarify the correct data. |
Harris 1984.
| Methods | Methods not clear. Duration: 10 ‐ 14 days. |
| Participants | Participants with CF. |
| Interventions | IV antibiotics and aggressive pulmonary treatment. |
| Outcomes | Pulmonary function, PWC, and VO2max. |
| Notes |
Huang 1979.
| Methods | Double‐blind study (part placebo‐controlled) not clear if randomised. Duration: 10 days. |
| Participants | 25 participants experiencing an acute exacerbation. |
| Interventions | Intervention 1: IV ticarcillin 300 mg/kg/day or placebo. Intervention 2: IV ticarcillin 300 mg/kg/day and IV tobramycin 6 mg/kg/day. Intervention 3: IV carbenicillin 500 mg/kg/day and IV tobramycin 6 mg/kg/day. |
| Outcomes | Scoring system (including lung function, and clinical assessment), time to next exacerbation and microbiology. |
| Notes | Difficulty in obtaining informed consent to study with placebo arm (3 participants recruited to this arm) and so placebo group was replaced with ticarcillin group. 25 participants contributed 29 treatment episodes. We shall seek to contact the authors for clarification of randomisation and for IPD to contribute to a meta‐analysis and resolve the UoA issues. |
Kapranov 1995.
| Methods | Unclear if randomised. |
| Participants | 41 participants with CF with severe or very severe disease aged 3 ‐ 16 years. |
| Interventions | Ciprofloxacin (n = 31) versus ofloxacin (n = 9). |
| Outcomes | "Clinical remission" and side effects. |
| Notes | Unclear if symptomatic or elective treatment. We plan to contact authors to clarify randomisation. Given the imbalance in number of participants receiving each intervention, this is probably not a randomised trial. |
Latzin 2008.
| Methods | Open‐label RCT for 3 indications: (1) suppression therapy for those with chronic P. aeruginosa not experiencing an exacerbation; (2) acute exacerbation in those with chronic P. aeruginosa infection; and (3) eradication of first detection of P. aeruginosa infection. |
| Participants | 127 participants enrolled, of whom 34 were recruited as they had chronic P. aeruginosa infection and were experiencing an acute exacerbation. |
| Interventions | Intervention 1: IV meropenem 120 mg/kg/day in 3 doses and IV tobramycin 9 ‐ 12 mg/kg/day in 2 doses. Intervention 2: IV ceftazidime 200 ‐ 400 mg/kg/day in 2 or 3 doses and tobramycin 9 ‐ 12 mg/kg/day in 2 doses. |
| Outcomes | Lung function, adverse effects, microbiology. |
| Notes | While a subgroup analysis consisting of the 3 indications is described for lung function, data are presented as a whole. Participants could be recruited 2x although this is not described. We shall seek to contact the authors for IPD to reconcile the UoA issues and for data to contribute to a meta‐analysis. |
Parry 1978.
| Methods | RCT. Duration: a minimum of 10 days. |
| Participants | 88 participants of whom 51 had CF. |
| Interventions | Intervention 1: IV ticarcillin 300 mg/kg/day and IV tobramycin 4.5 mg/kg/day. Intervention 2: IV carbenicillin 450 mg/kg/day and IV gentamicin 4.5 mg/kg/day. |
| Outcomes | Lung function, clinical score, microbiology, adverse effects. |
| Notes | A pooled analysis is presented combining treatment for all indications. We shall seek to contact the authors for IPD for those with CF to contribute to a meta‐analysis. |
Vic 1997.
| Methods | Comparison study ‐ unclear if randomised. Duration: 14 days. |
| Participants | 38 participants with chronic P. aeruginosa infection presenting with a protocol‐defined pulmonary exacerbation. |
| Interventions | Intervention 1: IV ceftazidime 200 mg/kg/day and IV amikacin 35 mg/kg/day. Intervention 2: IV ceftazidime 200 mg/kg/day and IV tobramycin 15 mg/kg/day. |
| Outcomes | Nutritional status. |
| Notes | Need to clarify if randomised. |
% predicted: per cent of lung function measure compared to someone of the same age, height and ethnicity BMI: body mass index CF: cystic fibrosis CI: confidence intervals FEV1: forced expiratory volume at one second FVC: forced vital capacity IPD: individual patient data IV: intravenous P. aeruginosa: Pseudomonas aeruginosa PEFR: peak expiratory flow rate PWC: physical work capacity RCT: randomised controlled trial SD: standard deviation UoA: unit of analysis VO2max: maximum volume of oxygen WBC: white blood cell
Differences between protocol and review
In a post hoc change, we decided to exclude any studies that exclusively compared different doses of the same antibiotic.
In a further post hoc change, we have presented summary of findings tables for single versus combination intravenous antibiotics, for oral versus intravenous antibiotics and for nebulised versus intravenous antibiotics.
Contributions of authors
| Roles and responsibilities | |
| TASK | WHO WILL UNDERTAKE THE TASK? |
| Protocol stage: draft the protocol | MH, AP, PF |
| Review stage: select which trials to include (2 + 1 arbiter) | MH, AP, PF |
| Review stage: extract data from trials (2 people) | MH, AP |
| Review stage: enter data into RevMan | MH |
| Review stage: carry out the analysis | MH |
| Review stage: interpret the analysis | MH |
| Review stage: draft the final review | MH with comments from PF & AP |
| Update stage: update the review | MH, AP, PF |
Sources of support
Internal sources
No sources of support supplied
External sources
-
Wellcome Trust, UK.
MH was funded by a Wellcome Trust Clinical Research Training Fellowship (Grant number WT092295AIA).
-
National Institute for Health Research, UK.
AP is funded by an NIHR Doctoral Research Fellowship (Grant number DRF‐2009‐02‐112).
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Matt Hurleyis a Clinical Research Fellow at the University of Nottingham. He has received funding from the Wellcome Trust by way of a Clinical Research Training Fellowship (Grant Number WT092295AIA) and a People Award (Grant Number WT098643AIA People Award ‐ "Cystic Fibrosis Unite") and grant funding from the UK CF Trust (Grant Number PJ563 ‐ Anti‐staphylococcal antibiotic prophylaxis for young children with CF; travel award poster prize). MH has received an honorarium for writing a review article on antibiotic strategies against Pseudomonas aeruginosa biofilms (Smyth AR, Hurley MN. Targeting the Pseudomonas aeruginosa biofilm to combat infections in patients with cystic fibrosis. Drugs of the Future. 2010;35(12):1007‐14).
Andrew Prayle undertakes research in intravenous tobramycin funded by the National Institute of Health Research (DRF 2009‐02‐112). He received travel expenses from the North American Cystic Fibrosis Conference to speak at the NACFC 2012 on the subject of ototoxicity in cystic fibrosis.
PF declares no known potential conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Agostini 1983 {published data only}
- Agostini M, Barlocco G, Bonomi U, Cappelletti LM, Castellani L, Conforti M, et al. Alternative antibiotics against Pseudomonas infections in cystic fibrosis. In vitro activity, pharmacokinetics, and double‐blind randomized clinical trial with azlocillin, piperacillin, cefoperazone, ceftazidime, cefsulodin, cefotaxime and moxalactam. Preliminary results. Drugs Under Experimental and Clinical Research 1983;98(9):671‐86. [CFGD Register: PI25b] [Google Scholar]
- Mastella G, Agostini M, Barlocco G, Bonomi U, Borgo G, Bozzino L, et al. Alternative antibiotics for the treatment of Pseudomonas infections in cystic fibrosis. Journal of Antimicrobial Chemotherapy 1983;12(Suppl A):297‐311. [CFGD Register: PI25a] [DOI] [PubMed] [Google Scholar]
Black 1990 {published data only}
- Black A, Redmond A, Scott E. Randomised study comparing courses of oral ciprofloxacin alone with intermittent courses of intravenous azlocillin plus tobramycin or oral ciprofloxacin in the treatment of acute exacerbations of respiratory infection in cystic fibrosis [abstract]. 16th Annual Meeting of the European Working Group for Cystic Fibrosis; 1989; Prague, Czechoslovakia. 1989:31. [CFGD Register: PI93a]
- Black A, Redmond AO, Steen HJ, Oborska IT. Tolerance and safety of ciprofloxacin in paediatric patients. Journal of Antimicrobial Chemotherapy 1990;26(Suppl F):25‐9. [CFGD Register: PI93c] [DOI] [PubMed] [Google Scholar]
- Black A, Stevenson M, Scott E, Redmond A. Randomised study comparing oral Ciprofloxacin alone with Ciprofloxacin alternating with intravenous therapy in cystic fibrosis [abstract]. 17th European Cystic Fibrosis Conference; 1991 June 18‐21; Copenhagen, Denmark. 1991:80. [CFGD Register: PI93b]
Blumer 2005 {published data only}
- Blumer JL, Minkwitz M, Saiman L, San Gabriel P, Iaconis J, Melnick D. Meropenem (MEM) compared with ceftazidime (CAZ) in combination with tobramycin (TOB) for treatment of acute pulmonary exacerbations (APE) in patients with cystic fibrosis (CF) infected with Pseudomonas aeruginosa (PA) or burkholderia cepacia (BC) [abstract]. Pediatric Pulmonology 2003;36 Suppl 25:294. [MEDLINE: ] [Google Scholar]
- Blumer JL, Saiman L, Konstan MW, Melnick D. The efficacy and safety of meropenem and tobramycin vs ceftazidime and tobramycin in the treatment of acute pulmonary exacerbations in patients with cystic fibrosis. Chest 2005;128:2336‐46. [CFGD Register: PI179b; DOI: 10.1378/chest.128.4.2336] [DOI] [PubMed] [Google Scholar]
Bosso 1988 {published data only}
- Bosso JA, Black PG. A comparative trial of aztreonam and tobramycin plus azlocillin [abstract]. Excerpta Medica, Asia Pacific Congress Series 1988;74:R(c)17. [CFGD Register: PI59a] [Google Scholar]
- Bosso JA, Black PG. Controlled trial of aztreonam vs. tobramycin and azlocillin for acute pulmonary exacerbations of cystic fibrosis. Pediatric Infectious Disease Journal 1988;7(3):171‐6. [CFGD Register: PI159b] [DOI] [PubMed] [Google Scholar]
Bosso 1989 {published data only}
- Bosso JA. Use of ciprofloxacin in cystic fibrosis patients. American Journal of Medicine 1989;87(Suppl 5a):123S‐7S. [CFGD Register: PI64] [DOI] [PubMed] [Google Scholar]
- Bosso JA, Black PG, Matsen JM. Ciprofloxacin versus tobramycin plus azlocillin in pulmonary exacerbations in adult patients with cystic fibrosis. American Journal of Medicine 1987;82(4A):180‐4. [CFGD Register: PI47] [PubMed] [Google Scholar]
BTS 1985 {published data only}
- British Thoracic Society Research Committee. Ceftazidime compared with gentamicin and carbenicillin in patients with cystic fibrosis, pulmonary pseudomonas infection, and an exacerbation of respiratory symptoms. Thorax 1985;40(5):358‐63. [CFGD Register: PI37a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack BHR, Geddes DM, Williams KJ, Dinwiddie R, Selkon JB, Godfrey RC. Ceftazidime compared with a combination of gentamicin and carbenicillin in cystic fibrosis patients with persistent pulmonary pseudomonas infection and an acute exacerbation of respiratory symptoms [abstract]. 9th International Cystic Fibrosis Congress; 1984 June 9‐15; Brighton, England. 1984:4.16. [MEDLINE: ]
Caplan 1984 {published data only}
- Caplan DB, Buchanan CN. Treatment of lower respiratory tract infections due to Pseudomonas aeruginosa in patients with cystic fibrosis. Reviews of Infectious Diseases 1984;6(Suppl 3):S705‐10. [CFGD Register: PI32] [DOI] [PubMed] [Google Scholar]
Church 1997 {published data only}
- Church DA, Kanga JF, Kuhn RJ, Rubio TT, Spohn WA, Stevens JC, et al. Sequential ciprofloxacin therapy in pediatric cystic fibrosis: comparative study vs. ceftazidime/tobramycin in the treatment of acute pulmonary exacerbations. The Cystic Fibrosis Study Group. Pediatric Infectious Disease Journal 1997;16(1):97‐105. [CFGD Register: PI115; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Conway 1985 {published data only}
- Conway SP, Miller MG, Ramsden C, Littlewood JM. Intensive treatment of pseudomonas chest infection in cystic fibrosis: a comparison of tobramycin and ticarcillin, and netilmicin and ticarcillin. Acta Paediatrica Scandinavica 1985;74(1):107‐13. [CFGD Register: PI34b] [DOI] [PubMed] [Google Scholar]
- Conway SP, Miller MG, Ramsden CH, Littlewood JM. Comparison of netilmicin‐plus ticarcillin and tobramycin plus ticarcillin in exacerbations of pseudomonas chest infection in cystic fibrosis [abstract]. 12th Annual Meeting European Working Group for Cystic Fibrosis; 1983 October; Athens, Greece. 1983:271. [CFGD Register: PI34a]
Conway 1997 {published data only}
- Conway SP, Pond MN, Watson A, Etherington C, Robey HL, Goldman MH. Intravenous colistin sulphomethate in acute respiratory exacerbations in adult patients with cystic fibrosis. Thorax 1997;52(11):987‐93. [CFGD Register: PI103c; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway SP, Pond MN, Watson AJ, Robey E, Goldman MH. A safety profile of intravenous colomycin in adult CF care [abstract]. 20th European Cystic Fibrosis Conference; 1995 June 18‐21; Brussels, Belgium. 1995:P3. [CFGD Register: PI103a]
- Conway SP, Pond MN, Watson AJ, Robey H, Goldman MH. Colistin alone or in combination with a second antibiotic is effective in the treatment of respiratory exacerbations in cystic fibrosis [abstract]. Pediatric Pulmonology 1996;22 Suppl 13:296. [CFGD Register: PI103b] [Google Scholar]
Cooper 1985 {published data only}
- Cooper DM, Harris M, Mitchell I. Comparison of intravenous and inhalation antibiotic therapy in acute pulmonary deterioration in cystic fibrosis [abstract]. American Review of Respiratory Disease 1985;131:A242. [CFGD Register: PI129; MEDLINE: ] [Google Scholar]
Costantini 1982 {published data only}
- Costantini D, Padoan R, Brienza A, Lodi G, Assael BM, Giunta A. Clinical evaluation of carbenicillin and sisomicin alone or in combination in CF patients with pulmonary exacerbations [abstract]. 11th European Cystic Fibrosis Conference; 1982; Brussels, Belgium. 1982:227. [CFGD Register: PI122]
De Boeck 1989 {published data only}
- Boeck K, Smet M, Eggermont E. Treatment of Pseudomonas lung infection in cystic fibrosis with piperacillin plus tobramycin versus ceftazidime monotherapy: preliminary communication. Pediatric Pulmonology 1989;7(3):171‐3. [CFGD Register: PI61] [DOI] [PubMed] [Google Scholar]
De Boeck 1999 {published data only}
- Boeck K, Sauer K, Vandeputte S. Meropenem versus ceftazidime plus tobramycin for pulmonary disease in CF patients [abstract]. The Netherlands Journal of Medicine 1999;54(Suppl):S39. [CFGD Register: PI150; MEDLINE: ] [Google Scholar]
Elborn 1992 {published data only}
- Elborn S, Colville A, Cordon S, Hiller EJ, Shale D. A comparison of intravenous ceftazidime and aztreonam in the treatment of respiratory exacerbations in cystic fibrosis [abstract]. 11th International Cystic Fibrosis Congress; 1992; Dublin, Ireland. 1992:TP17. [CFGD Register: PI97]
Gold 1985 {published data only}
- Gold R, Overmeyer A, Knie B, Fleming PC, Levison H. Controlled trial of ceftazidime vs. ticarcillin and tobramycin in the treatment of acute respiratory exacerbations in patients with cystic fibrosis. Pediatric Infectious Disease 1985;4(2):172‐7. [CFGD Register: PI35] [DOI] [PubMed] [Google Scholar]
Gold 1987 {published data only}
- Gold R. Mild to moderate chest exacerbations: Do antibiotics help? [abstract]. Pediatric Pulmonology 1987;3:38‐9. [CFGD Register: PI67] [Google Scholar]
- Gold R, Carpenter S, Heurter H, Corey M, Levison H. Randomized trial of ceftazidime versus placebo in the management of acute respiratory exacerbations in patients with cystic fibrosis. Journal of Pediatrics 1987;111(6 Pt 1):907‐13. [CFGD Register: PI57] [DOI] [PubMed] [Google Scholar]
Hodson 1987 {published data only}
- Hodson ME, Roberts CM, Butland RJ, Smith MJ, Batten JC. Oral ciprofloxacin compared with conventional intravenous treatment for Pseudomonas aeruginosa infection in adults with cystic fibrosis. Lancet 1987;1(8527):235‐7. [CFGD Register: PI45c] [DOI] [PubMed] [Google Scholar]
- Hodson ME, Roberts CM, Butland RJA, Batten JC. Ciprofloxacin compared with intravenous azlocillin and gentamicin in the treatment of pseudomonas aeruginosa infection in cystic fibrosis (CF) [abstract]. Pediatric Pulmonology 1987;3:126. [CFGD Register: PI45b] [Google Scholar]
- Hodson ME, Roberts CM, Butland RJA, Batten JC. Ciprofloxacin compared with intravenous azlocillin and gentamicin in the treatment of pseudomonas infection in cystic fibrosis (CF) [abstract]. 14th Annual Meeting of the European Working Group for Cystic Fibrosis; 1986 1‐2 September; Budapest. 1986:20. [CFGD Register: PI45a]
Huang 1983 {published data only}
- Huang NN, Palmer J, Keith H, Schidlow D, Braverman S, Goldberg M. Comparative efficacy and tolerance study of azlocillin and carbenicillin in patients with cystic fibrosis: a double blind study. Journal of Antimicrobial Chemotherapy 1983;11(Suppl B):205‐14. [CFGD Register: PI28] [DOI] [PubMed] [Google Scholar]
Hyatt 1981 {published data only}
- Hyatt AC, Chipps BE, Kumor KM, Mellits ED, Lietman PS, Rosenstein BJ. A double‐blind controlled trial of anti‐Pseudomonas chemotherapy of acute respiratory exacerbations in patients with cystic fibrosis. Journal of Pediatrics 1981;99(2):307‐11. [CFGD Register: PI23] [DOI] [PubMed] [Google Scholar]
Knowles 1988 {published data only}
- Knowles NE, Hallberg TK, Colombe JL. Efficacy of aerosolized antibiotics combined with intravenous treatment of cystic fibrosis pulmonary exacerbation [abstract]. Pediatric Pulmonology 1988;5 Suppl 2:120‐1. [CFGD Register: PI87] [Google Scholar]
Macfarlane 1985 {published data only}
- Macfarlane PI, Hughes DM, Landau LI, Olinsky A. The role of piperacillin therapy in pulmonary exacerbations of cystic fibrosis: a controlled study. Pediatric Pulmonology 1985;1(5):249‐55. [CFGD Register: PI39] [DOI] [PubMed] [Google Scholar]
Master 2001 {published data only}
- Master V, Martin AJ, Holmes M, Roberts G, Coulthard K, Kennedy D, et al. Once daily tobramycin monotherapy versus conventional antibiotic therapy for the treatment of pseudomonal pulmonary exacerbations in cystic fibrosis patients [abstract]. European Respiratory Journal 1997;10(Suppl 25):162S. [CFCD Register: PI148a] [Google Scholar]
- Master V, Roberts GW, Coulthard KP, Baghurst PA, Martin A, Roberts ME, et al. Efficacy of once‐daily tobramycin monotherapy for acute pulmonary exacerbations of cystic fibrosis: a preliminary study. Pediatric Pulmonology 2001;31(5):367‐76. [CFGD Register: PI148b] [DOI] [PubMed] [Google Scholar]
McCarty 1988 {published data only}
- McCarty JM, Tilden SJ, Black P, Craft JC, Blumer J, Waring W, et al. Comparison of piperacillin alone versus piperacillin plus tobramycin for treatment of respiratory infections in children with cystic fibrosis. Pediatric Pulmonology 1988;4(4):201‐4. [CFGD Register: PI58] [DOI] [PubMed] [Google Scholar]
McLaughlin 1983 {published data only}
- McLaughlin FJ, Matthews WJ Jr, Strieder DJ, Sullivan B, Goldmann DA. Randomized, double‐blind evaluation of azlocillin for the treatment of pulmonary exacerbations of cystic fibrosis. Journal of Antimicrobial Chemotherapy 1983;11(Suppl B):195‐203. [CFGD Register: PI26a] [DOI] [PubMed] [Google Scholar]
- McLaughlin FJ, Matthews WJ Jr, Strieder DJ, Sullivan B, Taneja A, Murphy P, et al. Clinical and bacteriological responses to three antibiotic regimens for acute exacerbations of cystic fibrosis: ticarcillin‐tobramycin, azlocillin‐tobramycin, and azlocillin‐placebo. Journal of Infectious Diseases 1983;147(3):559‐67. [CFGD Register: PI26b] [DOI] [PubMed] [Google Scholar]
Padoan 1987 {published data only}
- Padoan R, Cambisano W, Costantini D, Crossignani R, Giunta A. Pseudomonas pulmonary infections in cystic fibrosis: Ceftazidime vs ceftazidime plus sisomicin vs piperacillin plus sisomicin [abstract]. 9th International Cystic Fibrosis Congress; 1984 June 9‐15; Brighton, England. 1984:Poster 4.21. [CFGD Register: PI52a]
- Padoan R, Cambisano W, Costantini D, Crossignani RM, Danza ML, Trezzi G, et al. Ceftazidime monotherapy vs. combined therapy in Pseudomonas pulmonary infections in cystic fibrosis. Pediatric Infectious Disease Journal 1987;6(7):648‐53. [CFGD Register: PI52b] [DOI] [PubMed] [Google Scholar]
Penketh 1983 {published data only}
- Penketh AR, Hodson ME, Batten JC. Ticarcillin compared with carbenicillin in the treatment of exacerbations of bronchopulmonary infection in cystic fibrosis. British Journal of Diseases of the Chest 1983;77(2):179‐84. [CFGD Register: PI27b] [DOI] [PubMed] [Google Scholar]
- Penketh ARL, Hodson ME, Batten JC. Ticarcillin compared with carbenicillin in the treatment of exacerbations of bronchopulmonary infection due to Pseudomonas aeruginosa in cystic fibrosis [abstract]. 11th European Cystic Fibrosis Conference. 1982:250, Abstract No.: 140. [CFGD Register: PI27c]
- Penketh ARL, Hodson ME, Batten JC. Ticarcillin compared with carbenicillin in the treatment of exacerbations of bronchopulmonary infection in cystic fibrosis [abstract]. Research Conference. 1982:Poster Abstract 3. [CFGD Reference: PI27a] [DOI] [PubMed]
Penketh 1984 {published data only}
- Penketh ARL, Hodson ME, Gaya H, Batten JC. Azlocillin compared with carbenicillin in the treatment of bronchopulmonary infection due to Pseudomonas aeruginosa in cystic fibrosis. Thorax 1984;39(4):299‐304. [CFGD Register: PI31b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penketh ARL, Hodson ME, Gaya H, Batten JC. Azlocillin compared with carbenicillin in the treatment of bronchopulmonary infection due to Pseudomonas aeruginosa in cystic fibrosis [abstract]. Research Conference. 1982:1. [CFGD Register: PI31a] [DOI] [PMC free article] [PubMed]
Regelmann 1990 {published data only}
- Regelmann WE, Elliott GR, Clawson CC, Warwick WJ. Reduction of sputum Ps. Aeruginosa density by antibiotics improves lung function in CF more than bronchodilators and chest physiotherapy alone [abstract]. Pediatric Pulmonology 1988;Suppl 2:97. [CFGD Reference: PI65a] [DOI] [PubMed] [Google Scholar]
- Regelmann WE, Elliott GR, Warwick WJ, Clawson CC. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. American Review of Respiratory Disease 1990;141(4 Pt 1):914‐21. [CFGD Register: PI65b] [DOI] [PubMed] [Google Scholar]
Richard 1997 {published data only}
- Richard DA, Nousia Arvanitakis S, Sollich V, Hampel BJ, Sommerauer B, Schaad UB. Oral ciprofloxacin vs. intravenous ceftazidime plus tobramycin in pediatric cystic fibrosis patients: comparison of antipseudomonas efficacy and assessment of safety with ultrasonography and magnetic resonance imaging. cystic fibrosis Study Group. Pediatric Infectious Disease Journal 1997;16(6):572‐8. [CFGD Register: PI127; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salh 1992 {published data only}
- Salh B, Bilton D, Dodd M, Abbot J, Webb K. A comparison of aztreonam and ceftazidime in the treatment of respiratory infections in adults with cystic fibrosis. Scandinavian Journal of Infectious Diseases 1992;24(2):215‐8. [CFGD Register: PI72] [DOI] [PubMed] [Google Scholar]
Schaad 1986 {published data only}
- Schaad UB, Desgrandchamps D, Kraemer R. Antimicrobial therapy of Pseudomonas pulmonary exacerbations in cystic fibrosis. A prospective evaluation of netilmicin plus azlocillin versus netilmicin plus ticarcillin. Acta Paediatrica Scandinavica 1986;75(1):128‐38. [CFGD Register: PI43] [DOI] [PubMed] [Google Scholar]
Schaad 1987 {published data only}
- Schaad UB, Wedgwood Krucko J, Suter S, Kraemer R. Efficacy of inhaled amikacin as adjunct to intravenous combination therapy (ceftazidime and amikacin) in cystic fibrosis. Journal of Pediatrics 1987;111(4):599‐605. [CFGD Register: PI56] [DOI] [PubMed] [Google Scholar]
Schaad 1989 {published data only}
- Schaad UB, Wedgwood Krucko J, Guenin K, Buehlmann U, Kraemer R. Antipseudomonal therapy in cystic fibrosis: aztreonam and amikacin versus ceftazidime and amikacin administered intravenously followed by oral ciprofloxacin. European Journal of Clinical Microbiology & Infectious Diseases 1989;8(10):858‐65. [CFGD Register: PI63] [DOI] [PubMed] [Google Scholar]
Semykin 2010 {published data only}
- Semykin SY, Polikarpova SV, Dubovik LG, Kashirskaya NY. Efficiency of the inhalational tobramycin therapy in complex antibacterial therapy of lung exacerbation in cystic fibrosis children with chronic pseudomonas aeruginosa infection [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S55, Abstract no: 214. [CFGD Register: PI246; MEDLINE: ] [Google Scholar]
Smith 1999 {published data only}
- Smith AL, Doershuk C, Goldmann D, Gore E, Hilman B, Marks M, et al. Comparison of a beta‐lactam alone versus beta‐lactam and an aminoglycoside for pulmonary exacerbation in cystic fibrosis. Journal of Pediatrics 1999;134(4):413‐21. [CFGD Register: PI152] [DOI] [PubMed] [Google Scholar]
Stephens 1983 {published data only}
- Stephens D, Garey N, Isles A, Levison H, Gold R. Efficacy of inhaled tobramycin in the treatment of pulmonary exacerbations in children with cystic fibrosis. Pediatric Infectious Disease 1983;2(3):209‐11. [CFGD Register: PI105] [DOI] [PubMed] [Google Scholar]
Wang 1988 {published data only}
- Wang CI, Inderlied CB, Armer C, Roldan MA, Osher AB. Comparison of the efficacy and safety of oral ciprofloxacin with that of i.v. tobrarmycin plus azlocillin and/or tobramycin plus ticarcillin in patients with cystic fibrosis [abstract]. Excerpta Medica, Asia Pacific Congress Series 1988;74:R(c)19. [CFGD Register: PI89] [Google Scholar]
Wesley 1988 {published data only}
- Wesley AW, Quested C, Edgar BW, Lennon DR. A double‐blind comparison of ceftazidime with tobramycin and ticarcillin in the treatment of exacerbations of pseudomonas chest infection in children with cystic fibrosis [abstract]. Excerpta Medica, Asia Pacific Congress Series 1988;74:R(c)13. [CFGD Register: PI90] [Google Scholar]
Wientzen 1980 {published data only}
- Wientzen R, Prestidge CB, Kramer RI, McCracken GH, Nelson JD. Acute pulmonary exacerbations in cystic fibrosis. A double‐blind trial of tobramycin and placebo therapy. American Journal of Diseases of Children 1980;134(12):1134‐8. [CFGD Register: PI22] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aaron 2005 {published data only}
- Aaron S. Clinical evidence for combination antibiotic susceptibility testing (synergy testing) [abstract]. Pediatric Pulmonology 2008;43 Suppl 31:157. [MEDLINE: ] [Google Scholar]
- Aaron S, Vandemheen K, Ferris W, Tullis E, Haase D, Berthiaume Y, et al. Treatment of CF exacerbations based on multiple combination antibiotic susceptibility testing‐a randomized, double‐blind, controlled clinical trial [abstract]. Pediatric Pulmonology 2005;40 Suppl 28:304. [CFGD Register: PI198a] [Google Scholar]
- Aaron SD, Vandemheen KL, Ferris W, Fergusson D, Tullis E, Haase D, et al. Combination antibiotic susceptibility testing to treat exacerbations of cystic fibrosis associated with multiresistant bacteria: a randomised, double‐blind, controlled clinical trial. Lancet 2005;9484(366):463‐71. [CFGD Register: PI198b] [DOI] [PubMed] [Google Scholar]
Adeboyeku 2011 {published data only}
- Adeboyeku D, Jones AL, Hodson ME. Twice vs three‐times daily antibiotics in the treatment of pulmonary exacerbations of cystic fibrosis. Journal of Cystic Fibrosis 2011;10(1):25‐30. [CFGD Register: PI250; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Al‐Ansari 2006 {published data only}
- Al Ansari NA, Foweraker J, Mackeown D, Bilton D. Evaluation of once daily tobramycin versus the traditional three times daily for the treatment of acute pulmonary exacerbations in adult cystic fibrosis patients [abstract]. Qatar Medical Journal 2006;15(1):34‐8. [CFGD Register: PI173b; MEDLINE: ] [Google Scholar]
- Al‐Ansari N, McKeon D, Parmer J, Gunn E, Foweraker J, Bilton D. Efficacy of once daily tobramycin for acute pulmonary exacerbations of cystic fibrosis (CF) ‐ a microbiological perspective [abstract]. Thorax 2001;56(Suppl 3):iii85. [CFGD Register: PI173a] [Google Scholar]
Amelina 2000 {published data only}
- Amelina E, Senkevich N, Cherniak A, Cherniaev A, Chuchalin A. Home intravenous therapy in adult cystic fibrosis patients. The impact on lung function and quality of life [abstract]. European Respiratory Journal 2000;16(Suppl 31):123s. [CFGD Register: PI181] [Google Scholar]
Aminimanizani 2002 {published data only}
- Aminimanizani A, Beringer PM, Kang J, Tsang L, Jelliffe RW, Shapiro BJ. Distribution and elimination of tobramycin administered in single or multiple daily doses in adult patients with cystic fibrosis. Journal of Antimicrobial Chemotherapy 2002;50(4):553‐9. [CFGD Register: PI158b] [DOI] [PubMed] [Google Scholar]
- Tsang L, Aminimanizani A, Beringer PM, Jelliffe R, Shapiro B. Pharmacokinetics of once‐daily tobramycin in adult cystic fibrosis patients [abstract]. Pediatric Pulmonology 2000;30 Suppl 20:284. [CFGD Register: PI158a; MEDLINE: ] [Google Scholar]
Balsamo 1986 {published data only}
- Balsamo V, Bragion E, Iapichino L, Natoli D, Pardo F. Clinical efficacy and "in vitro" activity of some antibiotics, ceftazidime aztreonam or carbenicillin with aminoglycosides against pseudomonas in cystic fibrosis patients [abstract]. 14th Annual Meeting of the European Working Group for Cystic Fibrosis; 1986 Sept 1‐2; Budapest, Hungary. 1986:63. [CFGD Register: PI84]
Beringer 2003 {published data only}
- Beringer P, Shapiro B, Han E, Louie S, Gill M, Rao A. Pharmacodynamics (PD) of continuous infusion (CI) cefepime in adult cystic fibrosis (CF) patients [abstract]. Pediatric Pulmonology 2003;36 Suppl 25:297‐8. [CFGD Register: PI178] [Google Scholar]
Beringer 2010 {published data only}
- Beringer P, Owens H, Nguyen A, Benitez D, Boyd‐King A, Rao AP. Safety, pharmacokinetics and preliminary evaluation of the antiinflammatory effect of doxycycline in CF. Pediatric Pulmonology 2010;45(Suppl 33):370. [Google Scholar]
Brett 1992 {published data only}
- Brett MM, Simmonds EJ, Ghoneim AT, Littlewood JM. The value of serum IgG titres against Pseudomonas aeruginosa in the management of early pseudomonal infection in cystic fibrosis Comment in: Arch Dis Child 1993 Mar;68(3):432. Archives of Disease in Childhood 1992;67(9):1086‐8. [CFGD Register: PI73] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burkhardt 2006 {published data only}
- Burkhardt O, Lehmann C, Madabushi R, Kumar V, Derendorf H, Welte T. Once‐daily tobramycin in cystic fibrosis: better for clinical outcome than thrice‐daily tobramycin but more resistance development?. Journal of Antimicrobial Chemotherapy 2006;58(4):822‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lehmann C, Heilmann M, Stichtenoth D, Weissbrodt H, Hohlfeld J. Once‐daily versus thrice‐daily tobramycin in adult cystic fibrosis patients chronically infected with pseudomonas aeruginosa [abstract]. Pediatric Pulmonology 2004;38 Suppl 27:288. [CFGD Register: PI185a] [Google Scholar]
Byrne 1995 {published data only}
- Byrne S, Maddison J, Connor P, Doughty I, Dodd M, Jenney M, et al. Clinical evaluation of meropenem versus ceftazidime for the treatment of Pseudomonas spp. infections in cystic fibrosis patients. Journal of Antimicrobial Chemotherapy 1995;36(Suppl A):135‐43. [CFGD Register: PI99b; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- David TJ, Byrne S, Maddison J, Webb AK, Jenney M, Connor P. Meropenem versus ceftazadime for pseudomonas infection in cystic fibrosis [abstract]. 18th European Cystic Fibrosis Conference; 1993 May 21‐26; Madrid, Spain. 1993:PD40. [CFGD Register: PI99a]
Cabezudo 1984 {published data only}
- Cabezudo I, Thompson RL, Selden RF, Guenthner SH, Wenzel RP. Cefsulodin sodium therapy in cystic fibrosis patients. Antimicrobial Agents and Chemotherapy 1984;25(1):4‐6. [CFGD Register: PI136] [DOI] [PMC free article] [PubMed] [Google Scholar]
Canis 1998 {published data only}
- Canis F, Trivier D, Vic P, Ategbo S, Turck D, Husson MO. Comparison of the pharmacokinetics of tobramycin administered in a single daily dose or in 3 doses in cystic fibrosis [Comparaison de la pharmacocinetique de la tobramycine administree en dose unique journaliere ou en trois doses dans la mucoviscidose.]. Pathologie‐Biologie (Paris) 1998;46(6):449‐51. [CFGD Register: PI107c; MEDLINE: ] [PubMed] [Google Scholar]
- Vic P, Ategbo S, Turck D, Husson MO, Launay V, Loeuille GA, et al. Efficacy, tolerance and pharmacokinetics of once‐daily (OD) versus thrice‐daily (TD) tobramycin (TBM) for pseudomonas aeruginosa (PA) pulmonary exacerbations [abstract]. Pediatric Pulmonology 1996;22 Suppl 13:293. [CFGD Register: PI107a] [Google Scholar]
- Vic P, Ategbo S, Turck D, Husson MO, Launay V, Loeuille GA, et al. Efficacy, tolerance, and pharmacokinetics of once daily tobramycin for pseudomonas exacerbations in cystic fibrosis. Archives of Disease in Childhood 1998;78(6):536‐9. [CFGD Register: PI107b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensson 1992 {published data only}
- Christensson BA, Nilsson‐Ehle I, Ljungberg B, Lindblad A, Malmborg AS, Hjelte L, et al. Increased oral bioavailability of ciprofloxacin in cystic fibrosis patients. Antimicrobial Agents and Chemotherapy 1992;36(11):2512‐7. [CFGD Register: PI119b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandvik B, Hjelte L, Lindblad A, Ljungberg B, Malmborg AS, Nilsson Ehle I. Comparison of efficacy and tolerance of intravenously and orally administered ciprofloxacin in cystic fibrosis patients with acute exacerbations of lung infection. Scandinavian Journal of Infectious Diseases 1989;60:84‐8. [CFGD Register: PI119a] [PubMed] [Google Scholar]
Conway 1996a {published data only}
- Conway SP. Ceftazidime 3G BD is as effective as ceftazidime 2G TDS in the treatment of respiratory exacerbations in cystic fibrosis [abstract]. Israel Journal of Medical Sciences 1996; Vol. 32, issue Suppl:S256. [CFGD Register: PI78]
Davis 1987 {published data only}
- Davis RL, Koup JR, Williams Warren J, Weber A, Heggen L, Stempel D, et al. Pharmacokinetics of ciprofloxacin in cystic fibrosis. Antimicrobial Agents and Chemotherapy 1987;31(6):915‐9. [CFGD Register: PI50] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 1990 {published data only}
- Davis S, Davis P, Mather F, Tankersly P, Waring W. A randomized trial of home intravenous antibiotic therapy (HIVAT) in cystic fibrosis (CF) : Short‐term safety and efficacy [abstract]. Pediatric Pulmonology 1990;9 Suppl 5:245. [CFGD Register: PI91a] [Google Scholar]
- Davis S, Davis P, Mather F, Waring W, Home IV Antibiotic Study Group. A randomized trial of home intravenous antibiotic therapy (HIVAT) in cystic fibrosis (CF): Short‐term psychological effects [abstract]. Pediatric Pulmonology 1990;9 Suppl 5:281‐2. [CFGD Register: PI91b] [Google Scholar]
Day 1988 {published data only}
- Day AJ, Williams J, McKeown C, Bruton A, Weller PH. Evaluation of inhaled colomycin in children with cystic fibrosis. Excerpta Medica, Asia Pacific Congress Series. 1988:74 R(c)3.
De Boeck 1998 {published data only}
- Boeck K//Breysem L. Treatment of Pseudomonas aeruginosa lung infection in cystic fibrosis with high or conventional doses of ceftazidime. Journal of Antimicrobial Chemotherapy 1998;41(3):407‐9. [CFGD Register: PI133; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Degg 1996 {published data only}
- Degg C, Mulheran M. The effect of frequent exposure to gentamicin on distortion product OAEs in patients with cystic fibrosis. British Journal of Audiology 1996;30(2):99‐100. [CFGD Register: PI167] [Google Scholar]
Dodge 1983 {published data only}
- Dodge J, Zamiri I, Goodchild M, Ingram P. Experience with ceftazidime in cystic fibrosis. J. Antimicrob. Chemother. 1983;12(suppl A):325‐329. [DOI: 10.1093/jac/12.suppl_A.325] [DOI] [PubMed] [Google Scholar]
Donati 1987 {published data only}
- Donati MA, Guenette G, Auerbach H. Prospective controlled study of home and hospital therapy of cystic fibrosis pulmonary disease. Journal of Pediatrics 1987;111(1):28‐33. [CFGD Register: PI143; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elborn 2000 {published data only}
- Elborn JS, Prescott R, Stack B, Shale DJ, Pantin C, Ali N, et al. A comparison of the clinical outcome of symptomatic and elective regular intravenous antibiotics in the treatment of cystic fibrosis [abstract]. Thorax 1997;52(Suppl 6):A4. [CFGD Register: PI155b] [Google Scholar]
- Elborn JS, Prescott RJ, Stack BH, Goodchild MC, Bates J, Pantin C, et al. Elective versus symptomatic antibiotic treatment in cystic fibrosis patients with chronic Pseudomonas infection of the lungs. Thorax 2000;55(5):355‐8. [CFGD Register: PI155c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborn JS, Prescott RJ, Stack BH, Goodchild MC, Bates J, Pantin C, et al. Elective versus symptomatic antibiotic treatment in cystic fibrosis patients with chronic Pseudomonas infection of the lungs [abstract]. Pediatric Pulmonology 2000;30 Suppl 5:434. [CFGD Register: PI155d] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborn JS, Prescott RJ, Stack BHR. The British Thoracic Society study of elective versus symptomatic treatment of Pseudomonas aeruginosa infection in patients with cystic fibrosis: 5 year results [abstract]. 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:172. [CFGD Register: PI155a]
Eron 1983 {published data only}
- Eron EJ, Park CH, Hixon DL, GoMenberg RI, Poretz DM. Ceftazidime in patients with pseudomonas infectio. J. Antimicrob. Chemother. 1983;12(suppl A):161‐169. [DOI: 10.1093/jac/12.suppl_A.161] [DOI] [PubMed] [Google Scholar]
Gold 1983 {published data only}
- Gold R, Jin E, Levison H, Isles A, Fleming PC. Ceftazidime alone and in combination in patients with cystic fibrosis: lack of efficacy in treatment of severe respiratory infections caused by Pseudomonas cepacia. J. Antimicrob. Chemother. 1983;12(suppl A):331‐336. [DOI: 10.1093/jac/12.suppl_A.331] [DOI] [PubMed] [Google Scholar]
Goldfarb 1987 {published data only}
- Goldfarb J, Stern RC, Reed MD, Yamashita TS, Myers CM, Blumer JL. Ciprofloxacin monotherapy for acute pulmonary exacerbations of cystic fibrosis.. The American Journal of Medicine 1987;82(4A):174‐179. [PUBMED: 3555032] [PubMed] [Google Scholar]
Guglielmo 1996 {published data only}
- Guglielmo BJ, Quan LA, Stulbarg MS. Pharmacokinetics of once‐daily versus thrice daily tobramycin in cystic fibrosis patients [letter]. Journal of Antimicrobial Chemotherapy 1996;37(5):1040‐2. [CFGD Register: PI112; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hamner 2006 {published data only}
- Hamner JR, Poppy A, Ebaugh S, Reiter PD, Pan Z, Wagener J, et al. Pharmacokinetics and safety of once‐daily tobramycin in children with cystic fibrosis [abstract]. Pediatric Pulmonlogy 2006;41 Suppl 29:326. [CFGD Register: PI200] [Google Scholar]
Hatziagorou 2013 {published data only}
- Hatziagorou E, Avramidou V, Kirvassilis F, Tsanakas J. Lung clearance index: A tool to assess the response to intravenous treatment among children with cystic fibrosis. Journal of cystic fibrosis 2013;12(Suppl 1):S23. [PMC free article] [PubMed] [Google Scholar]
Heaf 1984 {published data only}
- Heaf DP, Tyson S, Dinwiddie R, Matthew D. A comparison of inhaled therapies in children with cystic fibrosis. 9th International Cystic Fibrosis Congress, June 9th ‐ 15th 1984, Brighton, England. 1984:274. Poster no: 4.08.
- Stroobant J, Heaf DP, Tyson S, Matthew DJ. Effect of inhaled azlocillin, mistabron and combination therapy in children with cystic fibrosis. 13th Annual Meeting of the European Working Group for Cystic Fibrosis. 1985:47.
- Stroobant J, Heaf DP, Tyson S, Matthew DJ. Effect of inhaled azlocillin, mistabron and combination therapy in children with cystic fibrosis. Pediatric research 1985;19:1099. [Google Scholar]
Heininger 1993 {published data only}
- Heininger U, Bowing B, Stehr K, Solbach W. Aminoglycosides in patients with mucoviscidosis and pulmonary exacerbation. Comparison of once or three times daily administration [Aminoglykoside bei Patienten mit Mukoviszidose und pulmonaler Exazerbation: Vergleich von Einmal‐ und Dreimalgabe.]. Klinische Padiatrie 1993;205(1):18‐22. [CFGD Register: PI74] [DOI] [PubMed] [Google Scholar]
Hjelte 1988 {published data only}
- Hjelte L, Widen B, Malmborg AS, Freyschuss U, Strandvik B. Intravenous administration of antibiotics at home in patients with cystic fibrosis improves quality of life. Lakartidningen 1988;85(18):1614‐7. [PubMed] [Google Scholar]
Hoogkamp‐Korstanje 1983 {published data only}
- Hoogkamp‐Korstanje JA, Laag J. Piperacillin and tobramycin in the treatment of Pseudomonas lung infections in cystic fibrosis. Journal of Antimicrobial Chemotherapy 1983;12(2):175‐83. [CFGD Register: PI140; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hubert 2009 {published data only}
- Hubert D, Roux E, Lavrut T, Wallaert B, Scheid P, Manach D, et al. Continuous versus intermittent infusions of ceftazidime for treating exacerbation of cystic fibrosis. Antimicrobial Agents and Chemotherapy 2009;53(9):3650‐6. [CFGD Register: PI180b; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert D, Wallaert B, Scheid P, Ramel S, Grenet D, Sermet‐Gaudelus I, et al. Continuous infusion versus intermittent administration of ceftazidime in cystic fibrosis patients [abstract]. Pediatric Pulmonology 2003;36 Suppl 25:294. [CFGD Register: PI180a] [Google Scholar]
Ivanov 1997 {published data only}
- Ivanov V, Shabalova L, Holmes C, Hill C, Illangovan P, O'Hara S, et al. Ceftazidime bid vs tid in the treatment of bacterial respiratory exacerbations in Russian cystic fibrosis [abstract]. Pediatric Pulmonology 1997;25 Suppl 14:292. [CFGD Register: PI125] [Google Scholar]
Jackson 1986 {published data only}
- Jackson MA, Kusmiesz H, Shelton S, Prestidge C, Kramer RI, Nelson JD. Comparison of piperacillin vs. ticarcillin plus tobramycin in the treatment of acute pulmonary exacerbations of cystic fibrosis. Pediatric Infectious Disease 1986;5(4):440‐3. [CFGD Register: PI38b] [DOI] [PubMed] [Google Scholar]
- Nelson JD. Management of acute pulmonary exacerbations in cystic fibrosis: a critical appraisal. Journal of Pediatrics 1985;106(6):1030‐4. [CFGD Register: PI38a] [DOI] [PubMed] [Google Scholar]
Jacobs 1985 {published data only}
- Jacobs RF, Trang JM, Kearns GL, Warren RH, Brown AL, Underwood FL, et al. Ticarcillin/clavulanic acid pharmacokinetics in children and young adults with cystic fibrosis. Journal of Pediatrics (ST LOUIS) 1985;106(6):1001‐7. [CFGD Register: PI142] [DOI] [PubMed] [Google Scholar]
Jensen 1987 {published data only}
- Jensen T, Pedersen SS, Hoiby N, Koch C. Efficacy of oral fluoroquinolones versus conventional intravenous antipseudomonal chemotherapy in treatment of cystic fibrosis. European Journal of Clinical Microbiology 1987;6(6):618‐22. [CFGD Register: PI55c] [DOI] [PubMed] [Google Scholar]
- Jensen T, Pedersen SS, Nielsen CH, Hoiby N, Koch C. The efficacy and safety of ciprofloxacin and ofloxacin in chronic Pseudomonas aeruginosa infection in cystic fibrosis. Journal of Antimicrobial Chemotherapy 1987;20(4):585‐94. [CFGD Register: PI55a] [DOI] [PubMed] [Google Scholar]
- Pedersen SS, Jensen T, Hvidberg EF. Comparative pharmacokinetics of ciprofloxacin and ofloxacin in cystic fibrosis patients. Journal of Antimicrobial Chemotherapy 1987;20(4):575‐83. [CFGD Register: PI155b] [DOI] [PubMed] [Google Scholar]
Jewett 1985 {published data only}
- Jewett CV, Ledbetter J, Lyrene RK, Brasfield DM, Tiller RE. Comparison of cefoperazone sodium vs methicillin, ticarcillin, and tobramycin in treatment of pulmonary exacerbations in patients with cystic fibrosis. Journal of Pediatrics 1985;106(4):669‐72. [CFGD Register: PI36] [DOI] [PubMed] [Google Scholar]
Keel 2011 {published data only}
- Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, et al. Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrobial agents and chemotherapy 2011;55(7):3393‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kercsmar 1983 {published data only}
- Kercsmar CM, Stern RC, Reed MD, Myers CM, Murdell D, Blumer JL. Ceftazidime in cystic fibrosis: pharmacokinetics and therapeutic response. Journal of Antimicrobial Chemotherapy 1983;12(Suppl A):289‐95. [DOI: 10.1093/jac/12.suppl_A.289] [DOI] [PubMed] [Google Scholar]
Klettke 1999 {published data only}
- Klettke U, Magdorf K, Staab D, Bisson S, Paul K, Wahn U. Ambulatory vs. inpatient intravenous antibiotic therapy in mucoviscidosis patients‐‐a controlled study [Ambulante vs. stationare intravenose antibiotische Therapie bei Mukoviszidosepatienten‐‐Eine kontrollierte Studie]. Pneumologie 1999;53(1):31‐6. [CFGD Register: PI108b] [PubMed] [Google Scholar]
- Klettke U, Magdorf K, Staab D, Paul K, Wahn U. Can home therapy replace hospital intravenous antibiotic therapy in patients with cystic fibrosis in Germany? [abstract]. Pediatric Pulmonology 1996;22 Suppl 13:340. [CFGD Register: PI108a] [Google Scholar]
- Magdorf K, Klettke U, Staab D, Paul K, Wahn U. Home intravenous antibiotic therapy vs. hospital therapy ‐ an alternative for patients with cystic fibrosis? [abstract]. Monatsschrift fur Kinderheilkunde 1996;144:1047. [CFGD Register: PI108d] [Google Scholar]
- Schulenburg JM, Greiner W, Klettke U, Wahn U. Economic aspects in treatment of cystic fibrosis with chronic pulmonary pseudomonas infection. Ambulatory intravenous therapy in comparison with inpatient treatment [Okonomische Aspekte der Behandlung zystischer Fibrose mit chronischer pulmonaler Pseudomonasinfektion. Ambulante intravenose Therapie im Vergleich zur stationaren Behandlung]. Medizinische Klinik 1997;92(10):626‐9. [CFGD Register: PI108c] [DOI] [PubMed] [Google Scholar]
Krause 1979 {published data only}
- Krause PJ, Young LS, Cherry JD, Osher AB, Spencer MJ, Bryson YJ. The treatment of exacerbations of pulmonary disease in cystic fibrosis: netilmicin compared with netilmicin and carbenicillin. Current Therapeutic Research, Clinical and Experimental 1979;25:609‐17. [CFGD Register: PI166] [Google Scholar]
Kruger 2001 {published data only}
- Junge S, Kruger K, Schonweiler R, Ptok M, Ballmann M. Once daily dosage of intravenous tobramycin ‐ increased risk for cochlea damage in children with cystic fibrosis (CF)? [abstract]. Pediatric Pulmonology 2001;32 Suppl 22:291. [CFGD Register: PI160b] [Google Scholar]
- Kruger K, Junge S, Schonweiler R, Ptok M, Ballmann M. Once daily dosage of intravenous tobramycin in patients with cystic fibrosis ‐ increased risk for cochlea damage? [abstract]. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P191. [CFGD Register: PI160a]
Kuni 1992 {published data only}
- Kuni CC, Regelmann WE, duCret RP, Boudreau RJ, Budd JR. Aerosol scintigraphy in the assessment of therapy for cystic fibrosis. Clinical Nuclear Medicine 1992;17(2):90‐3. [CFGD Register: PI236] [DOI] [PubMed] [Google Scholar]
Kuzemko 1989 {published data only}
- Kuzemko J, Crawford C. Continuous infusion of ceftazidime in cystic fibrosis [letter]. Lancet 1989;2(8659):385. [CFGD Register: PI16; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Labiris 2004 {published data only}
- Labiris R, Freitag A, Pratt B, Efthimiadis A, Hargreave F, Dolovich M. Does inhalation of preservatives from IV tobramycin preparations (TOB) cause airway inflammation? [abstract]. American Journal of Respiratory and Critical Care Medicine 2004;169(7):A307. [CFGD Register: PI182] [Google Scholar]
Levy 1982 {published data only}
- Levy J, Baran D, Klastersky J. Anti‐pseudomonas activity of azlocillin during pulmonary infection in patients with cystic fibrosis. Journal of Antimicrobial Chemotherapy 1982;10(3):235‐8. [CFGD Register: PI141; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levy 1982a {published data only}
- Levy J, Baran D, Klastersky J. Comparative study of the antibacterial activity of amikacin and tobramycin during Pseudomonas pulmonary infection in patients with cystic fibrosis. Journal of Antimicrobial Chemotherapy 1982;10(3):227‐34. [CFGD Register: PI134; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 1991 {published data only}
- Bowes G, Li SC, Williams TJ, Spicer WJ, McLean AJ. Computer‐aided tobramycin in adults with cystic fibrosis [abstract]. Pediatric Pulmonology 1988;Suppl 2:92. [CFGD Register: PI71a] [Google Scholar]
- Li SC, Bowes G, Ioannides Demos LL, Spicer WJ, Hooper RE, et al. Dosage adjustment and clinical outcomes of long‐term use of high‐dose tobramycin in adult cystic fibrosis patients. Journal of Antimicrobial Chemotherapy 1991;28(4):561‐8. [CFGD Register: PI71b] [DOI] [PubMed] [Google Scholar]
Martin 1980 {published data only}
- Martin AJ, Smalley CA, George RH, Gealing DE, Anderson CM. Gentamicin and tobramycin compared in the treatment of mucoid pseudomonas lung infections in cystic fibrosis. Archives of Disease in Childhood 1980;55:604‐7. [CFGD Register: PI20] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCabe 2013 {published data only}
- McCabe D, Rodgers HC. Evaluation of a twice daily tobramycin regimen in adult cystic fibrosis patients. Journal of cystic fibrosis 2013;12(Suppl 1):S71. [Google Scholar]
Michalsen 1981 {published data only}
- Michalsen H, Bergan T. Azlocillin with and without an aminoglycoside against respiratory tract infections in children with cystic fibrosis. Scandinavian Journal of Infectious Disease. Supplementum 1981;29:92‐97. [PubMed] [Google Scholar]
Moss 1991 {published data only}
- Moss RB. Sensitization to aztreonam and cross‐reactivity with other beta‐lactam antibiotics in high‐risk patients with cystic fibrosis. Journal of Allergy and Clinical Immunology 1991;87(1 Pt 1):78‐88. [CFGD Register: PI163] [DOI] [PubMed] [Google Scholar]
Mouton 1991 {published data only}
- Mouton JW, Horrevorts AM, Overbeek S, Kerrebijn KF, Michel MF. Pharmacokinetics of Ceftazidime during continuous and intermittent infusion in cystic fibrosis patients [abstract]. 17th European Cystic Fibrosis Conference; 1991 June 18‐21; Copenhagen, Denmark. 1991:88. [CFGD Register: PI96]
Nikolaizik 2005 {published data only}
- Nikolaizik WH, Vietzke D, Ratjen F. A pilot study to compare tobramycin 80 mg injectable preparation with 300 mg solution for inhalation in cystic fibrosis patients. Canadian Respiratory Journal 2008;15(5):259‐62. [CFGD Register: PI191c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaizik WH, Vietzke D, Ratjen F. Comparison of tobramycin 80 mg (IV‐ Preparation) and 300mg solution for inhalation in cystic fibrosis patients [abstract]. European Respiratory Journal 2005;26(Suppl 49):620s. [CFGD Register: PI191b] [Google Scholar]
- Nikolaizik WH, Vietzke D, Ratjen F. Comparison of tobramycin 80mg (IV‐preparation) and 300mg solution inhaled twice daily for chronic P. aeruginosa infection [abstract]. Journal of Cystic Fibrosis 2005;4 Suppl:S53. [CFGD Register: PI191a] [Google Scholar]
Nikonova 2010 {published data only}
- Nikonova VS, Kashirskaya NY, Kapranov NI. Efficacy and safety of tobramycin and colistin for inhalation in children with cystic fibrosis from Moscow region. Journal of cystic fibrosis 2010;9(Suppl 1):S54. [Google Scholar]
Padoan 1988 {published data only}
- Padoan R, Costantini D, Crossignani RM, Colavita G, Cambisano W, Cesana B, et al. Ceftazidime once a day vs t.i.d. in pseudomonas lung infection in cystic fibrosis patients [abstract]. Excerpta Medica, Asia Pacific Congress Series 1988;74:R(c)12. [CFGD Register: PI88] [Google Scholar]
Parry 1977 {published data only}
- Parry MF, Neu HC, Merlino M, Gaerlan PF, Ores CN, Denning CR. Treatment of pulmonary infections in patients with cystic fibrosis: a comparative study of ticarcillin and gentamicin. Journal of Pediatrics 1977;90(1):144‐8. [CFGD Register: PI17] [DOI] [PubMed] [Google Scholar]
Pedersen 1986 {published data only}
- Pedersen SS, Pressler T, Pedersen M, Hoiby N, Friis Moller A, Koch C. Immediate and prolonged clinical efficacy of ceftazidime versus ceftazidime plus tobramycin in chronic Pseudomonas aeruginosa infection in cystic fibrosis. Scandinavian Journal of Infectious Diseases 1986;18(2):133‐7. [CFGD Register: PI42] [DOI] [PubMed] [Google Scholar]
Permin 1983 {published data only}
- Permin H, Koch C, Hoiby N, Christensen HO, Moller AF, Moller S. Ceftazidime treatment of chronic Pseudomonas aeruginosa respiratory tract infection in cystic fibrosis. Journal of Antimicrobial Chemotherapy 1983;12(Suppl A):313‐23. [CFGD Register: PI29] [DOI] [PubMed] [Google Scholar]
Popa 2001 {published data only}
- Popa I, Pop L, Moldoban R, Popa Z, Liker M, Popa ZL. The comparative efficiency of ceftazidime and meropenem within the pseudomonas aeruginosa infection in cystic fibrosis [abstract]. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P207. [CFGD Register: PI161]
Postnikov 2001 {published data only}
- Postnikov SS, Semykin SJ, Najimov VP. Safety of fluoroquinolones in children [abstract]. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P213. [CFGD Register: PI162]
Postnikov 2001a {published data only}
- Postnikov SS, Semiakin SI, Nazhimov VP, Kapranov NI. Comparative yearly growth rate of children with mucoviscidosis treated and not treated with ciprofloxacin:clinicomorphological comparisons [Cravnitel'naiia godovaia skorost' rosta u detei s mukovistsidozom, poluchavshikh i nepolychavshikh tsiprofloksatsin:klinicomorfologicheskie copostavleniia.]. Antibiotiki I Khimioterapiia 2001;46(10):11‐3. [CFGD Register: PI169] [PubMed] [Google Scholar]
Postnikov 2007 {published data only}
- Postnikov SS, Semykin SY, Polikarpova SV, Dubovik LG, Gracheva LA, Sagatelyan RM. A prospective trial on the efficacy and tolerability of twice‐daily dosing (TDD) versus once‐daily dosing (ODD) amikacin in cystic fibrosis patients [abstract]. Journal of Cystic Fibrosis 2007;6 Suppl 1:S34. [CFGD Register: PI204] [Google Scholar]
Powell 1983 {published data only}
- Powell SH, Stern RC, Thompson WL. Safety of once daily therapy with high‐dose tobramycin [abstract]. 20th Annual Meeting Cystic Fibrosis Club Abstracts; 1979 May 1; Atlanta, Georgia. 1979:74. [CFGD Register: PI139b]
- Powell SH, Thompson WL, Luthe MA, Stern RC, Grossniklaus DA, Bloxham DD, et al. Once‐daily vs. continuous aminoglycoside dosing: efficacy and toxicity in animal and clinical studies of gentamicin, netilmicin, and tobramycin. Journal of Infectious Diseases 1983;147(5):918‐32. [CFGD Register: PI139a; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prayle 2013 {published data only}
- Prayle A, Jain K, Watson A, Smyth AR. Are morning doses of intravenous tobramycin less nephrotoxic than evening? Evidence from urinary biomarkers in the critic study. Pediatric Pulmonology 2013;48(Suppl 36):299. [Google Scholar]
Ramstrom 2000 {published data only}
- Ramstrom H, Erwander I, Mared L, Kornfalt R, Seiving B. Pharmaceutical intervention in the care of cystic fibrosis patients. Journal of Clinical Pharmacy and Therapeutics 2000;25(6):427‐34. [CFGD Register: PI159] [DOI] [PubMed] [Google Scholar]
Reed 1987 {published data only}
- Reed MD, Stern RC, Myers CM, Klinger JD, Yamashita TS, Blumer JL. Therapeutic evaluation of piperacillin for acute pulmonary exacerbations in cystic fibrosis. Pediatric Pulmonology 1987;3(2):101‐9. [CFGD Register: PI46] [DOI] [PubMed] [Google Scholar]
Reed 1987a {published data only}
- Reed MD, Stern RC, O'Brien CA, Crenshaw DA, Blumer JL. Randomized double‐blind evaluation of ceftazidime dose ranging in hospitalized patients with cystic fibrosis. Antimicrobial Agents and Chemotherapy 1987;31(5):698‐702. [CFGD Register: PI49] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riethmueller 2009 {published data only}
- Riethmueller J, Ballmann M, Schroeter TW, Franke P, Butler R, Claass A, et al. Tobramycin once‐ vs thrice‐daily for elective intravenous antipseudomonal therapy in pediatric cystic fibrosis patients. Infection 2009;37(5):424‐31. [CFGD Register: PI154f; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Riethmueller J, Busch A, Franke P, Ziebach R, Butler R, Stern M. Pharmacodynamic variation of intravenous antibiotic treatment with ceftazidime and tobramycin in CF‐patients is equally effective [abstract]. 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:166. [CFGD Register: PI154a]
- Riethmueller J, Franke P, Schroeter TW, Claass A, Busch A, Ziebach R, et al. Optimised intravenous antibiotic treatment with ceftazidime (thrice daily vs continuous) and tobramycin (thrice vs once daily) in CF patients [abstract]. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P192. [CFGD Register: PI154b]
- Riethmueller J, Junge S, Schroeter TW, Kuemmerer K, Franke P, Ballmann M, et al. Continuous vs thrice‐daily ceftazidime for elective intravenous antipseudomonal therapy in cystic fibrosis. Infection 2009;37(5):418‐23. [CFGD Register: PI154e; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schroeter T, Eggert P, Riethmueller J, Stern M, Claass A. Acute‐phase nephrotoxicity of tobramycin in CF patients: A prospective randomized trial of once‐versus thrice‐daily dosing [abstract]. Pediatric Pulmonology 2002;34 Suppl 24:289. [CFGD Register: PI154d] [Google Scholar]
- Schroeter TW, Eggert P, Riethmueller J, Stern M, Claass A. A prospective randomized trial on the nephrotoxicity of thrice‐daily versus once‐daily tobramycin in cystic fibrosis patients [abstract]. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P190. [CFGD Register: PI154c]
Roberts 1992 {published data only}
- Roberts GW, Nation RL, Jarvinen AO. Measurement of serum tobramycin in the presence of ticarcillin or piperacillin. Australian Journal of Hospital Pharmacy 1992;22(2):152‐4. [CFGD Register: PI132b] [Google Scholar]
- Roberts GW, Nation RL, Jarvinen AO, Martin AJ. An in vivo assessment of the tobramycin/ticarcillin interaction in cystic fibrosis patients. British Journal of Clinical Pharmacology 1993;36(4):372‐5. [CFGD Register: PI134a] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubio 1987 {published data only}
- Rubio TT. Ciprofloxacin: comparative data in cystic fibrosis. American Journal of Medicine 1987;82(Suppl 4A):185‐8. [CFGD Register: PI137; MEDLINE: ] [PubMed] [Google Scholar]
Shatunov 2001 {published data only}
- Shatunov SM. Comparative efficacy of different methods of ceftazidime administration in children with cystic fibrosis [abstract]. 11th European Respiratory Society Annual Congress; 2001 Sept 22‐26; Berlin. 2001:860. [CFGD Register: PI164]
Smyth 2005 {published data only}
- Mulheran M, Hyman‐Taylor P, Tan KH, Lewis S, Stableforth D, Knox A, et al. Absence of cochleotoxicity measured by standard and high‐frequency pure tone audiometry in a trial of once‐ versus three‐times‐daily tobramycin in cystic fibrosis patients. Antimicrobial Agents and Chemotherapy 2006;50(7):2293‐9. [CFGD Register: PI172e] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth A, Doherty C, Govan J, Tan K, Hyman‐Taylor P, Stableforth D, et al. Once daily tobramycin achieves cidal levels against Pseudomonas Aeruginosa in patients with cystic fibrosis [abstract]. Thorax 2001;56(Suppl 3):iii84. [CFGD Register: PI172a] [Google Scholar]
- Smyth A, Tan K, Hyman‐Taylor P, Mulheran M, Lewis S, Stableforth D, et al. A randomised controlled trial of once vs three times daily tobramycin for pulmonary exacerbations of cystic fibrosis (the TOPIC study) [abstract]. Pediatric Pulmonology 2003;36 Suppl 25:292. [CFGD Register: PI172b] [Google Scholar]
- Smyth A, Tan KH, Hyman‐Taylor P, Mulheran M, Lewis S, Stableforth D, et al. Once versus three‐times daily regimens of tobramycin treatment for pulmonary exacerbations of cystic fibrosis‐‐the TOPIC study: a randomised controlled trial. Lancet 2005;365(9459):573‐8. [CFGD Register: PI172c] [DOI] [PubMed] [Google Scholar]
- Touw DJ, Knox AJ, Smyth A. Population pharmacokinetics (PK) of tobramycin administered thrice daily (TD) and once daily (OD) in children and adults with CF [abstract]. Journal of Cystic Fibrosis 2007;6 Suppl 1:S16. [CFGD Register: PI172f] [DOI] [PubMed] [Google Scholar]
- Touw DJ, Knox AJ, Smyth A. Population pharmacokinetics of tobramycin administered thrice daily and once daily in children and adults with cystic fibrosis. Journal of Cystic Fibrosis 2007;6(5):327‐33. [CFGD Register: PI172g] [DOI] [PubMed] [Google Scholar]
- Vandenbussche HL, Klepser ME. Single daily tobramycin dosing in cystic fibrosis: is it better for the patients or the bugs? [comment]. Lancet 2005;365(9459):547‐8. [CFGD Register: PI172d] [DOI] [PubMed] [Google Scholar]
Turner 2013 {published data only}
- Turner R, Biondo LR, Slain D, Phillips U, Cardenas SC, Moffett K. A randomized pilot study of continuous versus intermittent infusion piperacillin‐tazobactam for the treatment of pulmonary exacerbations in patients with cystic fibrosis.. Pediatric pulmonology 2013;48(Suppl 36):326. [Google Scholar]
Wainwright 2011 {published data only}
- Byrnes CA, Vidmar S, Cheney JL, Carlin JB, Armstrong DS, Cooper PJ, et al. Prospective evaluation of respiratory exacerbations in children with cystic fibrosis from newborn screening to 5 years of age. Thorax 2013;68(7):643‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney J, Vidmar S, Grimwood K, Carlin JB, Wainwright C, on behalf of ACFBAL Study Group. Interim outcomes of a Pseudomonas aeruginosa (Pa) eradication protocol in young children in the Australian Cystic Fibrosis Bronchoalveolar Lavage (ACFBAL) Study [abstract]. Journal of Cystic Fibrosis 2009;8(Suppl 2):S39, Abstract no:157. [CFGD Register: PE167c] [Google Scholar]
- Cheney J, Wainwright C, ACFBAL Study Group. Trials, tribulations and triumphs of a cystic fibrosis study ‐ a behind the scenes look at the workings of an international multi‐centre study [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S118, Abstract no: 452. [CFGD Register: PE167g; ] [Google Scholar]
- Wainwright C, Carlin J, Cooper P, Byrnes C, Martin J, Grimwood K, et al. Australasian cystic fibrosis BAL study interim analysis [abstract]. Pediatric Pulmonology 2006;41 Suppl 29:317. [CFGD Register: PE167a] [Google Scholar]
- Wainwright CE, Carlin J, Cooper P, Byrnes C, Whitehead B, Martin J, et al. Early infection with pseudomonas aeruginosa can be cleared in young children with cystic fibrosis [abstract]. Pediatric Pulmonology 2002;34 Suppl 24:300‐1. [CFGD Register: PI167d] [Google Scholar]
- Wainwright CE, Kidd TJ, Ramsey KA, Bell SC, Grimwood K. Australasian CF bronchoalveolar lavage (ACFBAL) study: P.Aeruginosa (PA) genotypes in pre‐school CF children [abstract]. Pediatric Pulmonology 2011;46 Suppl 34:320, Abstract no: 299. [CFGD Register: PE167h; ] [Google Scholar]
- Wainwright CE, Vidmar S, Armstrong DS, Byrnes CA, Carlin JB, Cheney J, et al. Effect of bronchoalveolar lavage‐directed therapy on Pseudomonas aeruginosa infection and structural lung injury in children with cystic fibrosis: a randomized trial. JAMA 2011;306(2):163‐71. [CFGD Register: PE167e] [DOI] [PubMed] [Google Scholar]
- Wainwright CE, Vidmar S, Armstrong DS, Byrnes CA, Carlin JB, Cheney J, et al. Online Supplement to 'Effect of bronchoalveolar lavage‐directed therapy on Pseudomonas aeruginosa infection and structural lung injury in children with cystic fibrosis: a randomized trial' [online]. JAMA 2011;306(2):163‐171 Online. [CFGD Register: PE167f; ] [DOI] [PubMed] [Google Scholar]
- Wainwright CE, Grimwood K, Carlin JB, Vidmar S, Cooper PJ, Francis PW, et al. Safety of bronchoalveolar lavage in young children with cystic fibrosis. Pediatric Pulmonology 2008;43(10):965‐72. [CFGD Register: PE167b] [DOI] [PubMed] [Google Scholar]
Whitehead 2002 {published data only}
- Watson A, Whitehead A, Conway SP, Etherington C. Efficacy and safety of once daily tobramycin in treating acute respiratory exacerbations in adult patients [abstract]. Pediatric Pulmonology 1999;28 Suppl 19:262‐3. [CFGD Register: PI149b] [Google Scholar]
- Whitehead A, Conway SP Etherington C, Dave J. Efficacy and safety of once daily tobramycin in treating acute respiratory exacerbations in adult patients [abstract]. The Netherlands Journal of Medicine 1999;54(Suppl):S36‐7. [CFGD Register: PI149a] [Google Scholar]
- Whitehead A, Conway SP, Etherington C, Caldwell NA, Setchfield N, Bogle S. Once‐daily tobramycin in the treatment of adult patients with cystic fibrosis. European Respiratory Journal 2002;19(2):303‐9. [CFGD Register: PI149c] [DOI] [PubMed] [Google Scholar]
Winnie 1991 {published data only}
- Winnie GB, Cooper JA, Witson J, Cowan RG, Mayer D, Lepow M. Comparison of 6 and 8 hourly tobramycin dosing intervals in treatment of pulmonary exacerbations in cystic fibrosis patients. Pediatric Infectious Disease Journal 1991;10(5):381‐6. [CFGD Register: PI69] [DOI] [PubMed] [Google Scholar]
Wolter 1997 {published data only}
- Wolter JM, Bowler SD, McCormack JG, Nolan PJ. Home versus hospital therapy including intravenous antibiotics in cystic fibrosis [abstract]. 11th Annual Scientific Meeting of the Thoracic Society of Australia and New Zealand (TSANZ). 1994:148. [CFGD Register: PI130a]
- Wolter JM, Bowler SD, Nolan PJ, McCormack JG. Home intravenous therapy in cystic fibrosis: a prospective randomized trial examining clinical, quality of life and cost aspects. European Respiratory Journal 1997;10(4):896‐900. [CFGD Register: PI130b; MEDLINE: ] [PubMed] [Google Scholar]
Wood 1996 {published data only}
- Wood PJ, Ioannides Demos LL, Li SC, Williams TJ, Hickey B, Spicer WJ, et al. Minimisation of aminoglycoside toxicity in patients with cystic fibrosis. Thorax 1996;51(4):369‐73. [CFGD Register: PI109; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yasmin 1974 {published data only}
- Yasmin N, Laraya‐Cuasay LR, Mueller S, Liberi P, Braverman S, Capitanio M, et al. A critical evaluation of antibiotic aerosol in patients with cystic fibrosis [abstract]. 15th Annual Meeting Cystic Fibrosis Club Abstracts; 1974. 1974. [CFGD Register: PI79]
References to studies awaiting assessment
Al‐Aloul 2005 {published data only}
- Al‐Aloul M, Miller H, Browning P, Ledson MJ, Walshaw MJ. A randomised cross over trial of TOBI® vs IV tobramycin in acute pulmonary exacerbations in CF [abstract]. Pediatric Pulmonology 2004;38 Suppl 27:249. [CFGD Register: PI186a] [Google Scholar]
- Al‐Aloul M, Miller H, Ledson MJ, Walshaw MJ. Tobramycin nebuliser solution in the treatment of cystic fibrosis pulmonary exacerbations: effect on sputum pseudomonas aeruginosa density [abstract]. Thorax 2005;2(Suppl 2):ii92. [CFGD Register: PI186b; MEDLINE: ] [Google Scholar]
Beaudry 1980 {published data only}
- Beaudry PH, Marks MI, McDougall D, Desmond K, Rangel R. Is anti‐Pseudomonas therapy warranted in acute respiratory exacerbations in children with cystic fibrosis?. Journal of Pediatrics 1980;97(1):144‐7. [CFGD Register: PI121a] [DOI] [PubMed] [Google Scholar]
- Beaudry PH, Marks MI, Rangel R, McDougall D, Desmond K. Is anti‐pseudomonas therapy warranted in acute respiratory exacerbations in children with cystic fibrosis? [abstract]. 20th Annual Meeting Cystic Fibrosis Club Abstracts; 1979 may 1; Atlanta, Georgia, USA.. 1979 May:1. [CFGD Register: PI121b] [DOI] [PubMed]
Crawley 2005 {published data only}
- Crawley J, Ledson MJ, Walshaw MJ. Use of subcutaneous antibiotics to treat pseudomonas infection in an adult CF centre [abstract]. Journal of Cystic Fibrosis 2005;4 Suppl 1:S52. [CFGD Register: PI94] [Google Scholar]
Dinwiddie 1982 {published data only}
- Dinwiddie R, Hindmarsh P, Lock P. Azlocillin compared to gentamicin in the treatment of pseudomonas infection in cystic fibrosis [abstract]. 11th European Cystic Fibrosis Conference; 1982; Brussels, Belgium. 1982:229. [CFGD Register: PI123]
Döring 1995 {published data only}
- Döring G, Stiller T, Magdorf K, Wahn U. Impact of antibiotic therapy on neutrophil elastase release in cystic fibrosis patients [abstract]. American Journal of Respiratory and Critical Care Medicine 1995;151(4):A248. [CFGD Register: PI60; MEDLINE: ] [Google Scholar]
Geborek 2003 {published data only}
- Geborek A, Hjelte L, Lindblad A, Mared L, Eriksson L, Johannesson M, et al. Cross‐over study of TOBI® vs. intravenous tobramycin in combination treatment of pulmonary exacerbations in cystic fibrosis patients [abstract]. Journal of Cystic Fibrosis 2003;2 Suppl 1:S22. [CFGD Register: PI176] [Google Scholar]
Harris 1984 {published data only}
- Harris M, Cooper DM, Mitchell I. Exercise testing in assessing therapy for respiratory infections [abstract]. Proceedings of the 9th International Cystic Fibrosis Congress, Brighton, England 1984;‐:240. [Google Scholar]
Huang 1979 {published data only}
- Huang N, Palmer J, Schidlow D, Hsuan F, Hsu C, Goldberg M, et al. Evaluation of antibiotic therapy in patients with cystic fibrosis [abstract]. Chest 1979;76(3):354‐5. [CFGD Register: PI113] [Google Scholar]
- Huang NN, Palmer J, Braverman S, Keith HH, Schidlow D. Therapeutic efficacy of ticarcillin and carbenicillin in patients with cystic fibrosis: a double blind study [abstract]. 23rd Annual Meeting Cystic Fibrosis club abstracts; 1982 May 14; Washington D.C, USA. 1982:124. [CFGD Register: PI114]
Kapranov 1995 {published data only}
- Kapranov NI, Belousov YB, Kashyrskaya NY, Smirnova EY. Quinoline therapy in children with cystic fibrosis [abstract]. 20th European Cystic Fibrosis Conference; 1995 June 18‐21; Brussels, Belgium. 1995:P19. [CFGD Register: PI104]
Latzin 2008 {published data only}
- Latzin P, Fehling M, Bauernfeind A, Reinhardt D, Kappler M, Griese M. Efficacy and safety of intravenous meropenem and tobramycin versus ceftazidime and tobramycin in cystic fibrosis. Journal of Cystic Fibrosis 2008;7(2):142‐6. [CFGD Register: PI209; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parry 1978 {published data only}
- Parry MF, Neu HC. A comparative study of ticarcillin plus tobramycin versus carbenicillin plus gentamicin for the treatment of serious infections due to gram‐negative bacilli. American Journal of Medicine 1978;64(6):961‐6. [CFGD Register: PI18] [DOI] [PubMed] [Google Scholar]
Vic 1997 {published data only}
- Vic P, Ategbo S, Gottrand F, Launay V, Loeuille GA, Elian JC, et al. Nutritional impact of antipseudomonas intravenous antibiotic courses in cystic fibrosis. Archives of Disease in Childhood 1997;76(5):437‐40. [CFGD Register: PI126; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ballmann 1998
- Ballmann M, Rabsch P, Hardt H. Long term follow up of changes in FEV1 and treatment intensity during Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Thorax 1998;53(9):732‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bertenshaw 2007
- Bertenshaw C, Watson A, Lewis S, Smyth A. Survey of acute renal failure in patients with cystic fibrosis in the UK. Thorax 2007;62(6):541‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bilton 2011
- Bilton D, Canny G, Conway S, Dumcius S, Hjelte L, Proesmans M, et al. Pulmonary exacerbation: Towards a definition for use in clinical trials. Report from the EuroCareCF Working Group on outcome parameters in clinical trials. Journal of Cystic Fibrosis 2011;10(Suppl 2):S79‐81. [DOI] [PubMed] [Google Scholar]
Blainey 2012
- Blainey PC, Milla CE, Cornfield DN, Quake SR. Quantitative analysis of the human airway microbial ecology reveals a pervasive signature for cystic fibrosis. Science Translational Medicine 2012;4(153):153ra130. [DOI: 10.1126/scitranslmed.3004458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Britto 2002
- Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 2002;121(2):64‐72. [DOI: 10.1378/chest.121.1.64] [DOI] [PubMed] [Google Scholar]
Brody 2005
- Brody AS, Sucharew H, Campbell JD, Millard SP, Molina PL, Klein JS, et al. Computed tomography correlates with pulmonary exacerbations in children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2005;172(9):1128‐32. [DOI: 10.1164/rccm.200407-989OC] [DOI] [PubMed] [Google Scholar]
Cystic Fibrosis Foundation Patient Registry 2011
- Cystic Fibrosis Foundation. Patient Registry Annual Data Report 2010. www.cff.org/LivingWithCF/CareCenterNetwork/PatientRegistry/ (accessed 06 February 2011).
de Boer 2011
- Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011;66(8):680‐5. [DOI: 10.1136/thx.2011.161117] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins J, Altman D. Chapter 9 Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Doring 2000
- Doring G, Conway SP, Heijerman HG, Hodson ME, Hoiby N, Smyth A, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. European Respiratory Journal 2000;16(4):749‐67. [PUBMED: 11106223] [DOI] [PubMed] [Google Scholar]
Elphick 2014
- Elphick HE, Jahnke N. Single versus combination intravenous antibiotic therapy for people with cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD002007.pub3] [DOI] [PubMed] [Google Scholar]
Flume 2009
- Flume PA, Mogayzel PJ Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. American Journal of Respiratory and Critical Care Medicine 2009;180(9):802‐8. [DOI: 10.1164/rccm.200812-1845PP] [DOI] [PubMed] [Google Scholar]
Fuchs 1994
- Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. New England Journal of Medicine 1994;331(10):637‐42. [DOI: 10.1056/NEJM199409083311003] [DOI] [PubMed] [Google Scholar]
Gibson 2003
- Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2003;167(6):841‐9. [DOI] [PubMed] [Google Scholar]
Goss 2007
- Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax 2007;62(4):360‐7. [DOI: 10.1136/thx.2006.060889] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guss 2011
- Guss AM, Roeselers G, Newton ILG, Young CR, Klepac‐Ceraj V, Lory S, et al. Phylogenetic and metabolic diversity of bacteria associated with cystic fibrosis. International Society for Microbial Ecology Journal 2011;5(1):20‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Langton Hewer 2009
- Langton Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004197.pub3] [DOI] [PubMed] [Google Scholar]
Plummer 2013
- Plummer A, Wildman M. Duration of intravenous antibiotic therapy in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD006682.pub4] [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratjen 2001
- Ratjen F, Doring G, Nikolaizik WH. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet 2001;358(9286):983‐4. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogues 2007
- Rogues AM, Dumartin C, Amadéo B, Venier AG, Marty N, Parneix P. Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus aureus and Pseudomonas aeruginosa isolates from 47 French hospitals. Infection Control and Hospital Epidemiology 2007;28(12):1389‐95. [DOI: 10.1086/523280] [DOI] [PubMed] [Google Scholar]
Rosenstein 1998
- Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: A consensus statement. Journal of Pediatrics 1998;132(4):589‐95. [DOI: 10.1016/S0022-3476(98)70344-0] [DOI] [PubMed] [Google Scholar]
Sanders 2010
- Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. American Journal of Respiratory and Critical Care Medicine 2010;182(5):627‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schluchter 2006
- Schluchter MD, Konstan MW, Drumm ML, Yankaskas JR, Knowles MR. Classifying severity of cystic fibrosis lung disease using longitudinal pulmonary function data. American Journal of Respiratory and Critical Care Medicine 2006;174(7):780‐6. [DOI: 10.1164/rccm.200512-1919OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smyth 2014
- Smyth AR, Bhatt J. Once‐daily versus multiple‐daily dosing with intravenous aminoglycosides for cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD002009.pub5] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne J, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook forSystematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stressmann 2011
- Stressmann FA, Rogers GB, March P, Lilley AK, Daniels TW, Carroll MP, et al. Does bacterial density in cystic fibrosis sputum increase prior to pulmonary exacerbation?. Journal of Cystic Fibrosis 2011;10(5):357‐65. [ISSN: 1569‐1993] [DOI] [PubMed] [Google Scholar]
Stressmann 2012
- Stressmann FA, Rogers GB, Gast CJ, Marsh P, Vermeer LS, Carroll MP, et al. Long‐term cultivation‐independent microbial diversity analysis demonstrates that bacterial communities infecting the adult cystic fibrosis lung show stability and resilience. Thorax 2012;67(10):867‐73. [DOI: 10.1136/thoraxjnl-2011-200932] [DOI] [PubMed] [Google Scholar]
Wagener 2013
- Wagener JS, Rasouliyan L, Devanter DR, Pasta DJ, Regelmann WE, Morgan WJ, et al. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatric Pulmonology 2013;48:666‐73. [DOI: 10.1002/ppul.22652] [DOI] [PMC free article] [PubMed] [Google Scholar]
XYPLOT 2010 [Computer program]
- Creative Consulting for Research and Education. XYPLOT. Creative Consulting for Research and Education, 2010. [CCREweb.org]


