Abstract
Background
Wendan decoction (WDD) is one of the classical Chinese herb formulas used for psychotic symptoms. It is thought to be safe, accessible and inexpensive.
Objectives
To investigate the effects of WDD for treatment of people with schizophrenia or schizophrenia‐like illness compared with placebo, antipsychotic drugs and other interventions for outcomes of clinical importance.
Search methods
We searched the Cochrane Schizophrenia Group's Trials Register (February 2016), which is based on regular searches of CINAHL, BIOSIS, AMED, Embase, PubMed, MEDLINE, PsycINFO, China biomedical databases group (SinoMed, CNKI, VIP, Wanfang) and clinical trials registries. There are no language, date, document type, or publication status limitations for inclusion of records in the register. We also inspected references of identified studies and contacted relevant authors for additional information.
Selection criteria
Randomised controlled trials with useable data comparing WDD with antipsychotics, placebo or other interventions for people with schizophrenia.
Data collection and analysis
We extracted data independently. For binary outcomes, we calculated risk ratios (RR) and 95% confidence intervals (CIs), on an intention‐to‐treat basis. For continuous data, we estimated mean differences (MD) between groups and their 95% CIs. We employed a random‐effect model for analyses. We assessed risk of bias for included studies and created 'Summary of findings' tables using GRADE.
Main results
We included 15 randomised trials (1437 participants) of WDD for schizophrenia. There was a high risk of performance bias within the trials but overall, risk for selection, attrition and reporting bias was low or unclear.
Data showed WDD improved the short‐term global state of participants compared with placebo or no treatment (1 RCT n = 72, RR 0.53, 95% CI 0.39 to 0.73, low‐quality evidence).
When WDD was compared with antipsychotic drugs, such as chlorpromazine or risperidone, no difference in short‐term global state of participants was observed (2 RCTs n = 140, RR 1.18 95% CI 0.98 to 1.43, moderate‐quality evidence) and mental state (total endpoint Positive and Negative Syndrome Scale (PANSS): 2 RCTs, n = 140, MD 0.84, 95% CI ‐4.17 to 5.84, low‐quality evidence). However, WDD was associated with fewer people experiencing extrapyramidal effects (EPS) compared with other treatments (2 RCTs 0/70 versus 47/70, n = 140, RR 0.02, 95% CI 0.00 to 0.15, moderate‐quality evidence).
WDD is often used as an add‐on intervention alongside antipsychotics. When WDD + antipsychotic was compared to antipsychotic alone, the combination group had better global state (short‐term results, 6 RCTs, n = 684, RR 0.60, 95% CI 0.50 to 0.72, moderate‐quality evidence) and mental state (short‐term total endpoint PANSS: 5 RCTs, n = 580, MD ‐11.64, 95% CI ‐13.33 to ‐ 9.94, low‐quality evidence), fewer people with EPS (2 RCTs n = 308, RR 0.46, 95% CI 0.30 to 0.70, moderate‐quality evidence) and reduction of the mean use of risperidone (1 RCT n = 107, MD ‐0.70, 95% CI ‐0.87 to ‐0.53, low‐quality evidence). But, there was no effect on weight gain (1 RCT n = 108, RR 0.50, 95% CI 0.20 to 1.24, low‐quality evidence).
When WDD + low‐dose antipsychotic was compared with normal‐dose antipsychotic alone, the combination again showed benefits for short‐term global state (7 RCTs n = 522, RR 0.69, 95% CI 0.51 to 0.93, moderate‐quality evidence), mental state (total endpoint PANSS: 4 RCTs n = 250, MD ‐9.53, 95% CI ‐17.82 to ‐1.24, low‐quality evidence), and fewer participants with EPS (3 RCTS n = 280, RR 0.29, 95% CI 0.16 to 0.51, moderate‐quality evidence).
Across all comparisons, we found no data on outcomes directly reporting quality of life, hospital service use and economics.
Authors' conclusions
Limited evidence suggests that WDD may have some positive short‐term antipsychotic global effects compared to placebo or no treatment. However when WDD was compared with other antipsychotics there was no effect on global or mental state, but WDD was associated with fewer adverse effects. When WDD was combined with an antipsychotic, positive effects were found for global and mental state and the combination caused fewer adverse effects. The available evidence is not high quality. Better designed large studies are needed to fully and fairly test the effects of WDD for people with schizophrenia.
Plain language summary
Wendan decoction for schizophrenia
Review question
Is there trial‐based evidence that a traditional Chinese herbal medicine, Wendan decoction (WDD) is effective for treatment of people with schizophrenia?
Background
Schizophrenia is a severe mental illness, characterised by profound disruptions in thinking that affects language, perception, and sense of self. People with schizophrenia often hear voices or see things that are not present (hallucinations) and have strange beliefs (delusions). The main treatment for schizophrenia are antipsychotic drugs, However, antipsychotic drugs can cause unpleasant side effects, particularly movement disorders, which can be severe enough to stop people from continuing treatment. Experiences from China suggest some Traditional Chinese medicine (TCM ‐ a system of medicine originated in China and encompassing characteristics of traditional Chinese philosophy and culture) approaches can have an antipsychotic effect while causing fewer side effects. Wendan decoction is one of the classical TCM prescriptions for severe mental illness such as schizophrenia.
Searching for evidence
In Feburary 2016, the Information Specialist of the Cochrane Schizophrenia Group ran an electronic search for trials that randomised people with schizophrenia to receive either WDD, placebo/no treatment or antipsychotic drugs. We screened all records found in this search and included those that met our inclusion criteria and reported useful data.
Evidence found
Fifteen trials (with a total of 1437 participants) provided useable, but limited, data. Results showed that WDD may have some beneficial effects on short‐term global outcomes and mental state of people with schizophrenia compared to placebo or no treatment but did not show a benefit when compared to antipsychotics ‐ although WDD did cause fewer adverse effects. When WDD was combined with an antipsychotic, there were observed benefits for WDD on improving global state and reducing the side effects caused by antipsychotics.
Conclusions
Results of this review suggest WDD may be helpful for people with schizophrenia, but these results are based on low to moderate evidence and there is not enough high‐quality evidence to make firm conclusions. Better‐designed large studies are needed to fully and fairly test the effects of WDD for people with schizophrenia.
Summary of findings
Summary of findings for the main comparison. WENDAN DECOCTION versus PLACEBO OR NO TREATMENT for schizophrenia.
| WENDAN DECOCTION versus PLACEBO OR NO TREATMENT for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: hospital, in China Intervention: WENDAN DECOCTION versus PLACEBO OR NO TREATMENT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | WENDAN DECOCTION versus PLACEBO OR NO TREATMENT | |||||
| Global state: Clinically important change ‐ no improvement ‐ defined as PANSS < 50% reduction (short term) | 1000 per 1000 | 530 per 1000 (390 to 730) | RR 0.53 (0.39 to 0.73) | 72 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Mental state: Clinically important change, as defined by each study (short term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Adverse effects: Clinically important decline, as defined by each study (short term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Adverse effects: Metabolic ‐ weight change (medium term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Quality of life/satisfaction with care: Clinically important change in quality of life/satisfaction, as defined by each study (medium term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Economic outcomes: Costs due to treatment, as defined by each study (long term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Use of Western medicine: Reduced dose of Western medicine (short term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: rated 'serious' ‐ downgraded by 1 level ‐ no detail about how people were randomly allocated.
2 Imprecision: rated 'serious' ‐ downgraded by 1 level ‐ only one small trial reporting data.
Summary of findings 2. WENDAN DECOCTION versus ANTIPSYCHOTIC DRUG for schizophrenia.
| WENDAN DECOCTION versus ANTIPSYCHOTIC DRUG for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: hospital, in China Intervention: WENDAN DECOCTION versus ANTIPSYCHOTIC DRUG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | WENDAN DECOCTION versus ANTIPSYCHOTIC DRUG | |||||
| Global state: Clinically important change ‐ no improvement ‐ defined as PANSS < 50% reduction (short term) | Moderate1 | RR 1.18 (0.98 to 1.43) | 140 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 600 per 1000 | 690 per 1000 (576 to 828) | |||||
| Mental state: Average total endpoint PANSS score (short term)* | The mean mental state: average total score in the intervention groups was 0.84 higher (4.17 lower to 5.84 higher) | 140 (2 studies) | ⊕⊕⊝⊝ low1,2 | * More meaningful binary data ‐ Clinically important change data were not available, we used available continuous data. | ||
| Adverse effects: Movement disorders ‐ Extra Pyramidal Symptoms (TESS, short term)** | Moderate1 | RR 0.02 (0.00 to 0.15) | 140 (2 studies) | ⊕⊕⊕⊝ moderate1 | ** Data for pre‐stated ‐ Adverse effects: clinically important decline, as defined by each study (short term) were not available. EPS data were available. 1st adverse effect stipulated ‐ 'clinically significant change' ‐ we feel that EPS is important and have not downgraded because of use of proxy. |
|
| 700 per 1000 | 14 per 1000 (0 to 105) | |||||
| Adverse effects: Metabolic ‐ weight change (medium term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Quality of life/satisfaction with care: Clinically important in quality of life/satisfaction, as defined by each study (medium term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Economic outcomes: Costs due to treatment, as defined by each study (long term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Use of Western medicine: Reduced dose of Western medicine (short term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: rated serious ‐ downgraded by one level ‐ all trials were not explicit about randomisation or blinding.
2 Indirectness: rated serious ‐ downgraded by one level ‐ binary outcome assessing mental state is unavailable.
Summary of findings 3. WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC for schizophrenia.
| WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: hospital, in China Intervention: WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC | |||||
| Global state: Clinically important change ‐ no improvement ‐ defined as PANSS < 50% reduction (short term) | Moderate1 | RR 0.60 (0.50 to 0.72) | 684 (6 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 500 per 1000 | 300 per 1000 (250 to 360) | |||||
| Mental state: Average total endpoint PANSS score (short term)* | The mean mental state: average total score in the intervention groups was 11.64 lower (13.33 to 9.94 lower) | 580 (5 studies) | ⊕⊕⊝⊝ low2,3 | * More meaningful binary data ‐ Clinically important change data were not available, we used available continuous data. | ||
| Adverse effects: Movement disorders ‐ Extra Pyramidal Symptoms (TESS, short term)** | Moderate1 | RR 0.46 (0.30 to 0.70) | 308 (2 studies) | ⊕⊕⊕⊝ moderate2 | ** Data for pre‐stated ‐ Adverse effects: clinically important decline, as defined by each study (short‐term) were not available. EPS data were available. 1st adverse effect stipulated ‐ 'clinically significant change' ‐ we feel that EPS is important and have not downgraded because of use of proxy. |
|
| 400 per 1000 | 184 per 1000 (124 to 280) | |||||
| Adverse effects: Metabolic ‐ weight change (medium term) | Moderate1 | RR 0.50 (0.20 to 1.24) | 108 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 200 per 1000 | 100 per 1000 (40 to 248) | |||||
| Quality of life/satisfaction with care: Clinically important change in quality of life/satisfaction, as defined by each study (medium term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Economic outcomes: costs due to treatment, as defined by each study (long term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Use of Western medicine: Reduced dose of Western medicine (short‐term) | mean antipsychotic dose at the end point in the intervention groups was 0.7 lower (0.87 to 0.53 lower) | 107 (1) | ⊕⊕⊕⊝ low1,2 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Imprecision: rated serious ‐ downgraded by one level ‐ only one small trial reporting data 2 Risk of bias: rated serious ‐ downgraded by one level ‐ no detail about how people were randomly allocated.
3 Indirectness: rated serious ‐ downgraded by one level ‐ binary outcome assessing mental state is unavailable.
Summary of findings 4. WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC for schizophrenia.
| WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: hospital, in China Intervention: WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC | |||||
| Global state: Clinically important change ‐ no improvement ‐ defined as PANSS < 50% reduction (short term) | Moderate1 | RR 0.69 (0.51 to 0.93) | 522 (7 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 500 per 1000 | 345 per 1000 (265 to 450) | |||||
| Mental state: Average total endpoint PANSS score (short term)* | The mean mental state: average total score in the intervention groups was 9.53 lower (17.82 lower to 1.24 lower) | 250 (4 studies) | ⊕⊕⊝⊝ low1, 2 | * More meaningful binary data ‐ Clinically important change data were not available, we used available continuous data. | ||
| Adverse effects: Movement disorders ‐ Extra Pyramidal Symptoms (TESS, short term)** | Moderate1 | RR 0.29 (0.16 to 0.51) | 280 (3 studies) | ⊕⊕⊕⊝ moderate2 | ** Data for pre‐stated ‐ Adverse effects: clinically important decline, as defined by each study (short‐term) were not available. EPS data were available. 1st adverse effect stipulated ‐ 'clinically significant change' ‐ we feel that EPS is important and have not downgraded because of use of proxy. |
|
| 400 per 1000 | 104 per 1000 (60 to 176) | |||||
| Adverse effects: Metabolic ‐ weight change (medium term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Quality of life/satisfaction with care: Clinically important change in quality of life/satisfaction, as defined by each study (medium term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Economic outcomes: Costs due to treatment, as defined by each study (long term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| Use of Western medicine: Reduced dose of Western medicine (short term) | See comment | See comment | Not estimable | 0 (0) | See comment | No trial reported on this important outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Indirectness: rated serious ‐ downgraded by one level ‐ binary outcome assessing mental state is unavailable. 2 Risk of bias: rated 'serious' ‐ no detail about how people were randomly allocated.
Background
Description of the condition
Schizophrenia is a severe mental disorder, characterised by symptoms such as hallucinations, delusions, disorganised communication, poor planning, reduced motivation, and blunted affect. This illness, or group of illnesses, typically presents in early adulthood or late adolescence, affects around 0.30% to 0.66% of people at some point in their life (McGrath 2008), and causes approximately 1.1% of worldwide disability‐adjusted life years (DALYs) (Picchioni 2007). Schizophrenia is a multifactorial disorder, the genetic predisposition, in combination with perinatal, early childhood stress and/or suspected environmental factors are believed to lead to the disorder (van Os 2009). Schizophrenia is a treatable disorder (WHO 2016); it can be treated with medication, psychotherapy, social interventions and/or electroconvulsive therapy (ECT). Cost of treatment is expensive for the individual, family, and society (Initiative 2010). Antipsychotic drugs, which can partly relieve the symptoms of the patient, are considered to be the mainstay treatment option for schizophrenia. However, their adverse effects include somnolence, fatigue, insomnia, nausea, nervousness, dry mouth, movement disorders, weight gain and blurred vision, and can be severe enough to make people stop treatment (Peter 2007).
In Traditional Chinese medicine (TCM) there is not an exact equivalent disease to (what Western countries consider) schizophrenia. However, ancient Chinese doctors used several approaches, such as herb medicine, acupuncture, moxibustion (a therapy in which burning leaves of mugwort are applied on the skin at acupoints) and emotional therapies, to treat mental disorders. These approaches have been used in China for thousands of years.
Description of the intervention
Wendan decoction (WDD), also Wendan Tang or Warm Gallbladder decoction, is one of the classical Chinese herb formulae for schizophrenia‐like symptoms. WDD, was firstly recorded in Yao's Collection of Effective Prescriptions (A.D. 580), and then fully described in Valuable Prescriptions for Emergency ( A.D. 652) (Shi 2001). WDD is typically composed of Rhizoma Pinelliae (Qty: 6 g), Bambusae Caulis In Taeniam (6 g), Pericarpium Citri Reticulatae (9 g), Fructus Aurantii Immaturus (6 g), Poria Cocos (4.5 g), Rhizoma Zingiberis (five pieces), Jujube (one piece) and Radix Glycyrrhizae (3 g) (Deng 2003). The herbs and their dosages in the formula can be changed to treat different symptoms (modified WDD). As a herbal medicine, WDD is always prescribed by TCM doctors in hospital.
The contemporary indications for WDD and its variants include cardiovascular disease (premature ventricular contractions, bradycardia, viral myocarditis), digestive diseases (bile reflux gastritis, reflux oesophagitis, chronic atrophic gastritis) and mental disorders (epilepsy, sleep disorders, anxiety, stress, dizziness, schizophrenia) (Li 2013a; Mao 2013).
In China, Chinese herbal medicines are, we think, almost always combined with Western medicines to enhance their antipsychotic effects and/or reduce adverse effects. However, the true prevalence of the use of WDD remains unclear. Furthermore, we have not found any reports about limitations on the use of WDD – it seems likely that it is accessible to very wide groups of people including the elderly and less wealthy. Outside of China, WDD preparations would also be widely accessible through the global network of Chinese TCM practitioners.
How the intervention might work
There is an essential principle of treatment based on syndrome differentiation in TCM theory and clinical practice. This means that a single prescription can be used to treat different diseases as long as they show the same TCM clinical symptoms. TCM theory states that mental confusion by phlegm and reversed flow of Qi are the main causes of spirit disorders and the viscera of gallbladder and stomach are related to them (Zhou 2003). The target of WDD is to regulate Qi, resolve phlegm, purify the gallbladder and harmonise the stomach. In this way, WDD may be used to treat mental diseases (e.g. Dian, Kuang) such as those illness with schizophrenia‐like symptoms.
The chemical composition of the prescription is still a mystery. Several pharmacological analyses of WDD have been carried out in recent years. It has observed that WDD shows effects of sedation, hypnosis, improved memory and many other effects via the agents of IL‐2, oxygen‐free radicals ‐ monoamine neurotransmitters in the brain (Mao 2013).
It is widely believed that schizophrenia is a disorder of abnormal dopamine (DA) signalling (Howes 2016). Some studies have indicated that WDD can 'up‐regulate' the level of DA synthesis in the striatum of the rat (Xie 2004) and block the process of D2 receptor binding with DA (Luo 2009). One more extensive study showed that possible mechanisms of WDD include regulating brain glutamine and DA dysfunction and imbalances by blocking D2 receptors, enhancing synaptic plasticity of hippocampal cells, reducing oxygen free radical damage and increasing the body's immune function (Wan 2008).
Why it is important to do this review
Wendan decoction is a classical TCM prescription for spirit disorders and has been used to treat schizophrenia‐like symptoms for hundreds of years. WDD may be useful for individuals with schizophrenia as an alternative to typical antipsychotic drugs, which can have important side effects. People in China not only use it to treat schizophrenia‐like illnesses, but also to reduce unwanted effects induced by Western medicines. We are aware of randomised trials in this area and a published systematic review (Che 2016). This is an important area for which there should be a maintained review that can be updated in the light of new emerging evidence.
Objectives
To investigate the effects of WDD for treatment of people with schizophrenia or schizophrenia‐like illness compared with placebo, antipsychotic drugs and other interventions for outcomes of clinical importance.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials. If a trial was described as 'double‐blind' but implied randomisation, we included such trials in a sensitivity analysis (see Sensitivity analysis). We excluded quasi‐randomised studies, such as those allocating by alternate days of the week. Where people were given additional treatments within the WDD ( (including modified WDD formulae), we only included data if the adjunct treatment was evenly distributed between groups and it was only the WDD that was randomised.
Types of participants
Adults, however defined, with schizophrenia or schizophrenia‐like illness, including schizophreniform disorder, schizoaffective disorder and delusional disorder, again, by any means of diagnosis.
We are interested in making sure that information was as relevant to the current care of people with schizophrenia as possible so proposed to clearly highlight the current clinical state (acute, early post‐acute, partial remission, remission) as well as the stage (prodromal, first episode, early illness, persistent) and as to whether the studies primarily focused on people with particular problems (for example, negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Wendan decoction series
By Wendan decoction series, we mean the typical form (see Description of the intervention) and its modified versions, which change the specific blend of herbs and/or their dosages following the rules of TCM theory, such as Huanglian Wendan decoction, Shiwei Wendan decoction. An experienced TCM practitioner (review author HD) checked the prescriptions to decide whether they were really from the WDD family or not for each study. These decisions had been documented within the review. We tabulated the description that each study provided of the particular dosages and combinations employed. We considered those studies providing a clear and full description as being of higher quality. For any studies using a 'Wendan decoction' but not providing any description of the content of this treatment approach, we included these, but rated them as being of low quality. We presented data from studies using what seems to be full and unmodified WDD as a group and those that have modified the intervention separately (subgroup).
In addition, we proposed to include studies in which WDD was used as adjunct to atypical antipsychotics other than those studies in which WDD was used alone.
2. Placebo or no treatment
3. Antipsychotic drugs
Produced by pharmaceutical companies, any compound, dose, pattern or means of administration.
4. Any other treatments
We anticipated the following main comparisons:
WWD versus placebo or no treatment;
WWD versus antipsychotic drugs;
WWD versus other treatment;
WWD typical form versus modified WWD;
WWD usual technique delivery versus WDD other technique delivery.
For WDD ‐ usual technique of delivery, we mean a typical dosage form of decoction, which prepare the herbs of prescription by soaking in water, cooking and filtering for drinking, usually 200 mL/day, administered in the morning and evening.
For WDD ‐ other technique of delivery, dosage forms included, but not limited to powder, pill and tablet.
Types of outcome measures
We aimed to divide all outcomes into short term (less than three months), medium term (three to 12 months) and long term (over one year).
Primary outcomes
1. Global state
1.1 Clinically important change, as defined by each study (by the short term)
2. Mental state
2.1 Clinically important change, as defined by each study (by the short term)
3. Adverse effects
3.1 Clinically important decline, as defined by each study (by the short term)
Secondary outcomes
1. Global state
1.1 Clinically important change, as defined by each study (by medium or long term) 1.2 Any improvement in global state 1.3 Average score/change in global state 1.4 Relapse
2. Mental state
2.1 Clinically important change, as defined by each study (by medium or long term) 2.2 Any improvement in mental state 2.3 Average score/change in mental state
3. Adverse effects/events
3.1 Death 3.2 Cardiovascular effects 3.3 Genitourinary effects 3.4 Gastrointestinal effects 3.5 Respiratory effects 3.6 Extrapyramidal side effects 3.7 Metabolic 3.8 Any abnormal laboratory tests 3.9 Any other specific adverse effects 3.10 Any serious adverse event/effect 3.11 Average endpoint/change adverse effects/event scale
4. Behaviour
4.1 Any clinically important change, as defined by each study 4.2 Average score/change in behaviour 4.3 Aggression/violence
5. Social functioning
5.1 Clinically important change, as defined by each study 5.2 Any improvement, as defined by each study 5.3 Average score/change in social functioning
6. Quality of life/satisfaction with care for either recipients of care or caregivers
6.1 Clinically important change in quality of life/satisfaction, as defined by each study 6.2 Average score/change in quality of life/satisfaction 6.3 Any change in employment status, as defined by each study
7. Acceptance of treatment
7. 1 Accepting treatment 7. 2 Average endpoint acceptance score 7. 3 Average change in acceptance score
8 Service utilisation outcomes
8.1 Hospital admission 8.2 Days in hospital
9. Economic outcomes
9.1 Costs due to treatment, as defined by each study 9.2 Savings due to treatment, as defined by each study
10. Use of Western medicine
10.1 Reduced dose of atypical antipsychotics
'Summary of findings' tables
We used the GRADE approach to interpret findings (Schünemann 2011) and used GRADE profiler (GRADEPRO) to import data from RevMan 5 (Review Manager) to create 'Summary of findings' tables. These tables provided outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision making. We aimed to select the following main outcomes for inclusion in the 'Summary of findings' tables
Global state: Clinically important change, as defined by each study (by the short term)
Mental state: Clinically important change, as defined by each study (by the short term)
Adverse effects: Clinically important decline, as defined by each study (by the short term)
Adverse effects: Metabolic ‐ weight change (by the medium‐term)
Quality of life/satisfaction with care for either recipients of care or caregivers: Clinically important change, as defined by each study (by the medium term)
Economic outcomes: Costs due to treatment, as defined by each study (by the long term)
Use of Western medicine: Reduced dose of Western medicine (by the short term)
These tables provided information concerning the overall quality of the evidence from the trial, the magnitude of effect of the interventions examined, and the sum of available data on all primary outcomes and on selected secondary outcomes. This summary was used to guide our conclusions and recommendations.
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Study‐Based Register of Trials
The Information Specialist searched this register using the following search strategy.
*Wendan* in Intervention Field of STUDY
In such a study‐based register, searching the major concept retrieves all the synonym and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics.
The register is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, Embase, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literatures, and conference proceedings (see Group's Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
We contacted the first author of each included study for information regarding unpublished trials. We noted the outcome of this contact in the included or awaiting assessment studies tables.
Data collection and analysis
Selection of studies
Review authors HD and JX independently inspected citations from the searches and identify relevant abstracts. HD independently re‐inspected a random 20% sample of these abstracts to ensure reliability. Where disputes arise, we acquired the full report for more detailed scrutiny. HD and JX then obtained and inspected full reports of the abstracts or reports meeting the review criteria. HD, again, re‐inspected a random 20% of these full reports in order to ensure reliable selection. Where it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study for clarification.
Data extraction and management
1. Extraction
Review authors HD and JX extracted data from all included studies. In addition, to ensure reliability, HD independently extracted data from a random sample of these studies, comprising 10% of the total. We attempted to extract data presented only in graphs and figures whenever possible, but included only if both review authors independently had the same result. If studies were multicentre, where possible, we extracted data relevant to each. We discussed any disagreement and document decisions. If necessary, we attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. With any remaining problems Clive Adams (Co‐ordinating Editor) helped clarify issues and we documented these final decisions.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if: a) the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and b) the measuring instrument had not been written or modified by one of the trialists for that particular trial. Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, in Description of studies, we noted if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We had decided primarily to use endpoint data, and only use change data if the former were not available. If necessary, we combined endpoint and changed data in the analysis as we preferred to use mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we planned to apply the following standards to relevant continuous data before inclusion.
Please note, we entered all relevant data from studies of > 200 participants in the analysis irrespective of the following rules, because skewed data pose less of a problem in large studies. We also entered all relevant change data as when continuous data were presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data were skewed or not.
For endpoint data from studies < 200 participants :
(a) when a scale starts from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). If this value was lower than 1, it strongly suggested a skew and we excluded these data. If this ratio was higher than 1 but below 2, there was suggestion of skew. We entered these data and tested whether their inclusion or exclusion changed the results substantially. Finally, if the ratio was larger than 2 we included these data, because skew is less likely (Altman 1996; Higgins 2011).
b) if a scale starts from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), (Kay 1986), which can have values from 30 to 210), we modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and 'S min' is the minimum score.
2.5 Common measure
To facilitate comparison between trials we intended, if necessary, to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for WDD. Where keeping to this makes it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'not un‐improved'), we reported data where the left of the line indicates an unfavourable outcome and noted this in the relevant graphs.
Assessment of risk of bias in included studies
Again, review authors HD and JX worked independently to assess risk of bias by using criteria described in the Cochrane Handbook for Systematic reviews of Interventions (Higgins 2011a) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If the raters disagreed, we made the final rating by consensus, with the involvement of another member of the editorial group. Where inadequate details of randomisation and other characteristics of trials were provided, we attempted to contact authors of the studies in order to obtain further information. We reported non‐concurrence in quality assessment, but if disputes arose as to which category a trial was to be allocated, again, we resolved by discussion.
We noted the level of risk of bias in both the text of the review, in 'Risk of bias' figures, and the 'Summary of findings' table/s.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). The number needed to treat/harm (NNT/H) statistic with its confidence intervals is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the 'Summary of findings' table/s, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference (SMD)). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and we calculated effect size and transform the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
Where clustering has been incorporated into the analysis of primary studies, we presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
Where clustering is not accounted for in primary studies, we presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. We sought to contact first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999).
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC is not reported, it was assumed to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. This occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we only used the data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we would simply add these and combine within the two‐by‐two table. If data are continuous, we would combine data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic reviews of Interventions (Higgins 2011). Where the additional treatment arms are not relevant, we would not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we did not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' table/s by down‐rating quality. Finally, we also downgraded quality within the 'Summary of findings' table/s should loss be 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, the rate of those who stay in the study ‐ in that particular arm of the trial ‐ were used for those who did not. We undertook a sensitivity analysis to test how prone the primary outcomes were to change when data only from people who complete the study to that point were compared to the ITT analysis using the above assumptions.
3. Continuous
3.1 Attrition
We reproduced and used data where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported.
3.2 Standard deviations (SDs)
If SDs were not reported, we first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals available for group means, and either 'P' value or 't' value available for differences in mean, we could calculate them according to the rules described in the Cochrane Handbook for Systematic reviews of Interventions (Deeks 2011). When only the SE was reported, SDs were calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic reviews of Interventions (Deeks 2011) present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges or other statistics. If these formulae did not apply, we calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers, others use the method of last observation carried forward (LOCF), while more recently, methods such as multiple imputation or mixed‐effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to somewhat better than LOCF (Leon 2006), we felt that the high percentage of participants leaving the studies early and differences in the reasons for leaving the studies early between groups was often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach used. However, we preferably used the more sophisticated approaches, e.g. we preferred to use MMRM or multiple imputation to LOCF and we only presented completer analyses if some kind of ITT data were not available at all. Moreover, we addressed this issue in the item "incomplete outcome data" of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise and discussed such situations or participant groups,
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise and discussed any such methodological outliers.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 method alongside the Chi2 'P' value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. 'P' value from Chi2 test, or a confidence interval for I2). We interpreted an I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic, as evidence of substantial levels of heterogeneity (Section 9.5.2 Cochrane Handbook for Systematic reviews of Interventions) (Deeks 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Cochrane Handbook for Systematic reviews of Interventions (Sterne 2011). We were aware that funnel plots might be useful in investigating reporting biases but were of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose random‐effects model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of WDD for people with schizophrenia in general. No subgroup analyses were anticipated, however, we planned to report data on subgroups of people in the same clinical state, stage and with similar problems should they be available.
2. Investigation of heterogeneity
We reported if inconsistency was high. First, we investigated whether data had been entered correctly. Second, if data were correct, we visually inspected the graph and we successively removed outlying studies to see if homogeneity was restored. For this review we had decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present data. If not, we would not pool these data but dicuss any issues. We known of no supporting research for this 10% cut‐off but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity were obvious, we simply stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. If their inclusion did not result in a substantive difference, they would remain in the analyses. If their inclusion did result in statistically significant differences, we would not add the data from these lower quality studies to the results of the better trials, but would present such data within a subcategory.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported results and discuss them, but continued to employ our assumption.
Where assumptions have to be made regarding missing SDs data (see Dealing with missing data), we compared the findings on primary outcomes when we used our assumption compared with complete data only. We undertook a sensitivity analysis to test how prone results were to change when 'completer' data only were compared to the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them, but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available), allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included relevant data from these trials.
4. Imputed values
We also undertook a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICCs in calculating the design effect in cluster‐randomised trials.
If substantial differences were noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome, but presented them separately.
5. Fixed‐effect and random‐effects
We synthesised data using a random‐effects model, however, we also synthesised data for the primary outcome using a fixed‐effect model to evaluate whether this alters the significance of the results.
Results
Description of studies
For substantive descriptions of studies please see Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The PRISMA table shows results of our search (Figure 1).
1.
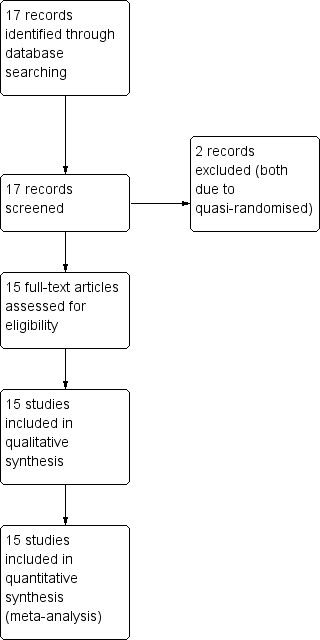
Study flow diagram.
In the search, we found 17 papers that were potentially relevant. After screening and inspecting abstracts, 17 full‐text papers were selected to be further assessed for inclusion. We had to exclude two (two reports) of these studies. So, at the end of the review we identified 15 reports of 15 trials.
Included studies
We included fifteen studies with a total of 1437 participants.
1. Allocation
All included studies were randomised controlled trials. Two trials contained descriptions of being "randomised by using a random allocation table" (Liu 2014; Shao 2014). None of the trials described allocation concealment and blindness.
2. Study duration
Study duration varied from 10 days (Huang 2005) to one year (Peng 2010). The majority of studies were short term (less than three months, see Types of outcome measures). One study was medium term (three to 12 months) and had six months and one‐year follow‐up after discharge (Peng 2010).
3. Setting
All studies were hospital‐based with inpatients.
7.4 Antipsychotic
When antipsychotics were used as the control group the doses were not different.
4. Country
All studies were undertaken and reported in China.
5. Participants
It was reported in all studies that the participants were people with schizophrenia. Seven of the 15 studies adopted the standards of Chinese Classification of Mental Disorder third edition (CCMD‐3, Chang 2009; Dou 2012; Huang 2005; Su 2015; Sun 2014; Wang 2008; Xu 2007); two adopted ICD‐10 (Guo 2013; Zhang 2014) and one used DSM‐IV (Lin 2010). Three studies diagnosed schizophrenia with both CCMD‐3 and DSM‐IV (Wang 2013), and "Integrative Chinese and Western Medicine Classification of Schizophrenia" (Li 2013), and "TCM Diagnostic criteria cited from the text book of 'TCM Internal Medicine'" (Shao 2014). One study stated "diagnostic criteria cited from book of 'Psychology'" (Peng 2010) and the other one did not mention the diagnostic standard used (Liu 2014).
The age of participants within the studies ranged from 15 to 71 years (derived from 11 trials). Four studies reported the mean age (Chang 2009; Dou 2012; Huang 2005; Wang 2008). All studies reported the ratios of men and women (810 men and 612 women in total, Sun 2014 and Zhang 2014 reported mistaken ratios). Twelve studies reported the duration of illness ranging from 1.8 to 20 years.
6. Study size
All studies reported the trial size. The number of participants ranged from 39 to 200 (96 mean SD 11, median 90).
7. Interventions
By 'Wendan decoction' we meant the typical form (see Description of the intervention) and its modified versions which change the specific blend of herbs and/or their dosages following rules of TCM theory. For each included study review author HD, an experienced TCM practitioner, collected and tabulated the details of prescriptions (Table 5). Then HD checked to decide whether they are really of the WDD family or not. All studies described the blend of herbs and their dosages (except Huang 2005; Li 2013; Zhang 2014 ‐ without dosages). Some judgement had to be used, but all prescriptions employed by included studies did seem to belong to the WDD series ‐ with two being 'typical' form (Li 2013; Zhang 2014) and others were 'modified' versions.
1. Table of WDDs.
| ID | Typical / Modified WDD | Compositions & Dosages | Note |
| Chang 2009 | Modified | 半夏 10g, 枳实 10g, 竹茹15g, 橘皮 6g, 甘草 3g, 朱砂 3g, 生铁落 6g, 黄连 5g, 大黄 15g, 栀子 10g, 龙胆草10g。 每日1剂,水煎分早晚饭后服用。 | |
| Dou 2012 | Modified | 半夏 10g, 枳实 10g, 竹茹15g, 橘皮 10g, 甘草 6g, 朱砂 3g, 生铁落 30g, 黄连 10g, 大黄 10g, 栀子 10g, 龙胆草 15g。每日1剂,水煎分早晚饭后服用。 | |
| Guo 2013 | Modified | 半夏 12g, 枳实 15g, 竹茹15g, 橘皮 9g, 甘草 5g, 朱砂 3g, 生铁落 6g, 黄连 5g, 大黄 10g, 茯苓 30g。每日1剂,水煎分早晚饭后服用。 | |
| Huang 2005 | Modified | 半夏, 枳实, 竹茹, 橘皮, 甘草, 生姜, 酸枣仁, 远志, 龙骨, 牡蛎。每日1剂,水煎, 中午及晚上分服。 | No dose stated. |
| Li 2013 | Typical | 半夏, 竹茹, 枳实, 陈皮, 生姜, 甘草. 煎药机煎药,每包200ml,分2次口服。 | No dose stated. |
| Lin 2010 | Modified | 半夏10g, 枳实10g, 竹茹15g, 茯苓10g, 陈皮6g, 甘草3g, 朱砂3g, 生铁落6g, 黄连5g, 大黄15g, 栀子10g, 龙胆草10g。 每日1剂,水煎分早晚饭后服用。 | |
| Liu 2014 | Modified | 生竹茹 12g、半夏 12g、茯苓 12g、枳实 6g、陈皮 9g、甘草5g龙胆草 9g、远志 6g、酸枣仁 12g、胆南星 6g、石菖蒲 15g. | |
| Meng 1998 | Modified | 黄连8g、竹茹30g、陈皮15g、法夏10g、枳实15g、茯神15g、龙胆草10g、远志10g、菖蒲15g、郁金15g,生龙牡各30g。 | Excluded |
| Peng 2010 | Modified | 生竹茹 12g、半夏 12g、茯苓 12g、枳实 6g、陈皮 9g、甘草5g、龙胆草 9g、远志 6g、酸枣仁 12g、胆南星 6g、石菖蒲 15g、黄连6g. | |
| Shao 2014 | Modified | 半夏12g、陈皮12g、竹茹18g、枳壳15g、远志15g、郁金12g、太子参20g、合欢皮15g、夜交藤15g、木香6g、胆南星10g、茯苓10g。每天一剂,水煎500ml,3次/d。 | |
| Shi 2010 | Modified | 半夏12g、陈皮15g、茯苓30g、枳实15g、竹茹15g、黄连10g、丹参30g、川芎15g、菊花15g、天竺黄10g、礞石60g、大黄10g。 | Excluded |
| Su 2015 | Modified | 黄芩、法半夏、石菖蒲、生石膏各10g,枳实、大黄各6g,茯苓、沙枣仁、远志各12g。 | |
| Sun 2014 | Modified | 半夏12g、黄连6g、竹茹15g、陈皮15g、枳实12g、茯苓15g、生铁落9g、甘草6g。 | |
| Wang 2008 | Modified | 法半夏10g、黄芩10g、枳实6g、茯苓12g、炒枣仁12g、远志12g、石菖蒲10g、大黄6g、生石膏10g等,每日1剂,水煎2次,取汁约200ml,早晚2次口服。 | |
| Wang 2013 | Modified | 半夏12g、酸枣仁12g、枳实10g、陈皮10g、生牡蛎15g,竹茹12g、甘草6g、茯苓13g、朱砂6g、石菖蒲10g、黄连6g、栀子10g、佛手9g、生铁落9g。 | |
| Xu 2007 | Modified | 法半夏10g、黄芩10g、枳实6g、茯苓12g、炒枣仁12g、远志12g、石菖蒲10g、大黄6g、生石膏10g等,每日1剂,水煎2次,取汁约200ml,早晚2次口服。 | |
| Zhang 2014 | Typical | 半夏、竹茹、枳实、陈皮、生姜、甘草、茯苓、大枣、生姜。煎药机煎药,每包200ml,分2次口服。 | No dose stated. |
Five intervention groups were employed within the relevant trials.
7.1 Wendan decoction
The four studies using WDD as sole treatment all employed modified prescriptions of WDD (Chang 2009; Dou 2012; Huang 2005; Wang 2013). Please see Table 5.
7.2 Wendan decoction plus normal‐dose antipsychotic
7.2.1 Wendan decoction
When WDD was added to normal doses of antipsychotic, two of the relevant studies employed the typical form of WDD (Li 2013; Zhang 2014), and four modified prescriptions of WDD (Lin 2010; Liu 2014; Shao 2014; Sun 2014). Again, for more details please see Table 5.
7.2.2 Normal‐dose antipsychotic
These studies used standard doses of comparison antipsychotic (Table 6).
2. Description of antipsychotic in Wendan decoction + normal dose antipsychotic group of studies.
| Included study | Drug | Dose |
| Li 2013; Lin 2010 | Risperidone | 6 mg/day, three times a day |
| Liu 2014 | Ziprasidone | 20 mg/day to 160 mg/day |
| Shao 2014 | Several antipsychotics based on symptoms,such as: exciting: quetiapine and olanzapine; looks dull, visual hallucinations, auditory hallucinations: perphenazine, risperidone, clozapine |
quetiapine: 300 ,mg to 600 mg, twice daily olanzapine: 10 mg to 30 mg, twice daily perphenazine: 20 mg to 40 mg, twice daily risperidone 3 mg to 6mg, twice daily clozapine 200 mg to 400 mg, twice daily. |
| Sun 2014 | Aripiprazole | Started from 5 mg/day, and increased individually, but did not exceed 30 mg/day. |
| Zhang 2014 | Olanzapine | olanzapine from 5 mg/day, range 5 mg/day to 20 mg/day. |
7.3 Wendan decoction plus low‐dose antipsychotic
7.3.1 Wendan decoction
The seven studies all employed modified prescriptions of WDD (Chang 2009; Dou 2012; Guo 2013; Peng 2010; Su 2015; Wang 2008; Xu 2007).
7.3.2 Low‐/Normal‐dose antipsychotic
These studies used WDD plus low‐dose antipsychotic together and compared this with the standard‐dose antipsychotic alone (Table 7).
3. Description of antipsychotic in Wendan decoction + low or normal dose antipsychotic group of studies.
| Included study | Drug | Low Dose | Normal Dose |
| Chang 2009 | chlorpromazine | 300 mg/day ± 7.65 mg/day, 60 days for a course | 600 mg/day ± 6.37 mg/day, 60 days for a course |
| Su 2015 | clozapine | 50 mg/day to 100 mg/day | 200 mg/day to 400 mg/day |
| Wang 2008 | clozapine | 100 mg/day to 200 mg/day | 250 mg/day to 550 mg/day |
| Xu 2007 | clozapine | 50 mg/day to 100 mg/day | 200 mg/day to 400 mg/day |
| Guo 2013 | quetiapine | 400 mg/day to 600 mg/day, 6 weeks | 600 mg/day to 700 mg/day, 6 weeks |
| Dou 2012 | risperidone | 3.10 mg/day ± 0.9 mg/day, 60 days for a course | 4.8 mg/day ± 1.2 mg/day, 60 days for a course |
| Peng 2010 | risperidone | Started at 1 mg/day, gradually increased to 2 mg/day to 6 mg/day in early two weeks, not more than 6 mg/day, then leave hospital 60 days later with maintenance dose of 2 mg/day | Started at 1 mg/day, gradually increased to 2 mg/day to 8 mg/day in early two weeks, not more than 8 mg/day, then leave hospital 60 days later with maintenance dose of 2 mg/day to 4 mg/day |
7.4 Antipsychotic/benzodiazepines
When antipsychotics/benzodiazepines were used as the control group, the doses were not different from what would be expected in clinical practice (Table 8).
4. Description of antipsychotic/benzodiazepine in direct Wendan decoction comparisons.
| Included study | Drug | Dose |
| Chang 2009 | chlorpromazine | 600 mg/day ± 6.37 mg/day, 60 days for a course |
| Dou 2012 | risperidone | 4.8 mg/day ± 1.2 mg/day, 60 days for a course |
| Huang 2005 | estazolam | 2 mg/day, 10 days |
7.5 No treatment
One study employed a 'no treatment' control group (Wang 2013).
8. Outcomes
8.1 General remarks
Most outcomes of global state and adverse effects were dichotomous but trials also used a variety of scales. All studies reported short term (< three months) outcomes, but only Peng 2010 reported medium term (three to 12 months) and long term (> one‐year) outcomes.
8.2 Outcomes scales from which it was possible to use data
8.2.1 Global state scales
All studies reported some form of global state outcome (Table 9).
5. Variety of global measures used in trials.
| Included study | Predefined global measure |
| Chang 2009; Dou 2012 | Global state: cure (binary PANSS scores ‐ reduced > 50%, 20% to 50%, no effect ‐ < 20%) |
| Guo 2013; Li 2013; Lin 2010; Liu 2014; Sun 2014; Wang 2008; Zhang 2014 | Global state: cure (binary PANSS scores ‐ reduced > 75%, 50% to 74%, 25% to 49%, no effect ‐ < 24%) |
| Lin 2010 | Global state: cure (binary TCMSS scores ‐ reduced > 95%, >75%, > 50%, no effect ‐ < 30%) |
| Huang 2005 | Global state: cure (binary sleep time ‐ > 8 hours, > 6 hours, < 6 hours every night, no effect ‐ no change) |
| Peng 2010; Shao 2014; Su 2015; Wang 2013 | Global state: cure (binary 'symptoms disappeared' ‐ all, partly, slightly, no change or even worse) |
| Xu 2007 | Global state: cure (binary BPRS scores ‐ reduced > 75%, 50% to 75%, 25% to 50%, no effect ‐ < 25%) |
BPRS: Brief Psychiatric Rating Scale PANSS: Positive and Negative Syndrome Scale TCMSS:Traditional Chinese Medicine Score
8.2.2 Mental state scales
8.2.2.1 Positive and Negative Syndrome Scale ‐ PANSS (Kay 1986) This is a 30‐item scale, each of which can be defined on a seven‐point scoring system from absent to extreme. It has three sub‐scales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P), and negative symptoms (PANSS‐N). A low score indicates lesser severity. Ten studies reported data using this scale (Chang 2009; Dou 2012; Guo 2013; Li 2013; Lin 2010; Liu 2014; Shao 2014; Sun 2014; Wang 2008; Zhang 2014).
8.2.2.2 Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962)
The BPRS is an 18‐item scale measuring positive symptoms, general psychopathology and affective symptoms. The original scale has 16 items, but a revised 18‐item scale is commonly used. Scores can range from zero to 126. Each item is rated on a seven‐point scale, with high scores indicating more severe symptoms. One study reported BPRS data (Shao 2014).
8.2.2.3 The Calgary Depression Scale for Schizophrenia ‐ CDSS (Addington 1990) The CDSS, developed by the University of Calgary, was specifically developed to assess the level of depression in schizophrenia. It has been extensively evaluated in both relapsed and remitted patients and appears sensitive to change. The CDSS depression score is obtained by adding each of the item scores. A score above six has an 82% specificity and 85% sensitivity for predicting the presence of a major depressive episode. Liu 2014 used this scale.
8.2.2.4 TCM Syndromes score ‐ TCMSS (SATCM 1994)
TCMSS created and published by the State Administration of TCM of the People's Republic of China, is a scale to assess the severity of TCM syndromes. TCMSS include some specific indicators that can be used to measure the mental disorders. Lin 2010 used this scale.
8.2.3 Adverse effects scales
8.2.3.1 Treatment Emergent Symptom Scale/Form ‐ TESS/F (Guy 1976) This checklist assesses a variety of characteristics for each adverse event, including severity, relationship to the drug, temporal characteristics (timing after a dose, duration and pattern during the day), contributing factors, course and action taken to counteract the effect. Symptoms can be listed a priori or can be recorded as observed by the investigator. Seven studies used this scale (Chang 2009; Dou 2012; Guo 2013; Su 2015; Sun 2014; Wang 2008; Xu 2007).
8.2.3.2 Rating Scale for Extrapyramidal Side Effects ‐ RESES (Simpson 1970) The Rating scale for Extrapyramidal Side Effects is a 10‐item scale relating to extrapyramidal side effects. The score of each item rates symptoms from zero to four. Zero means normal and high scores indicate severe side effects. The items are gait, arm dropping, shoulder shaking, elbow rigidity, wrist rigidity of fixation of position, pendulousness of legs, head dropping, glabella tap, tremor and salivation. Six studies used this scale (Chang 2009; Dou 2012; Shao 2014; Sun 2014; Wang 2008; Xu 2007).
9. Missing outcomes
No included study reported death, engagement with services, satisfaction with treatment, quality of life, or economic outcomes.
Excluded studies
We excluded two studies. Both studies listed in the Characteristics of excluded studies had to be inspected in hard copy in order to make the final decision. We had to exclude both Meng 1998 and Shi 2010 because they seem to be quasi‐randomised trials and the risk of bias was felt to be much too high.
Risk of bias in included studies
Overall, we judged the risk of bias for the included studies to be unclear or low (Figure 2; Figure 3).
2.
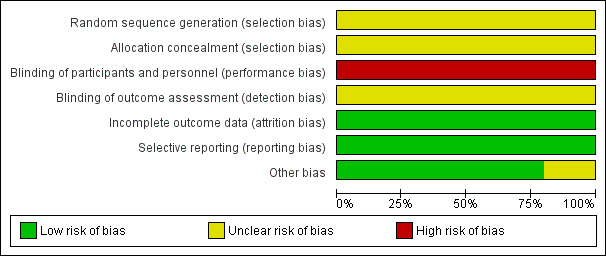
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
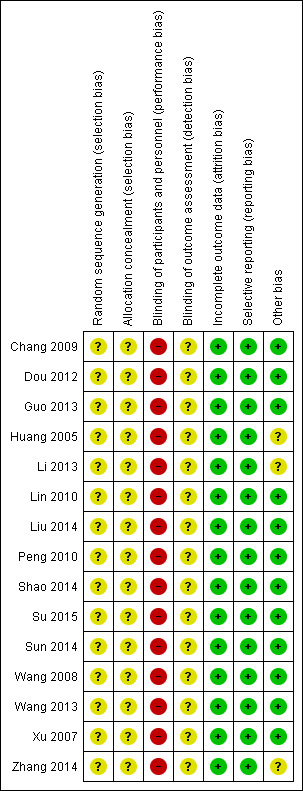
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All 15 included studies were stated to be randomised, two described being "randomised by using a random allocation table" (Shao 2014; Liu 2014). None stated allocation concealment.
Blinding
All included studies had no description of blinding. It seems that no included trials employed any form of blinding ‐ even trials carried out in recent years. Technically, blinding in these sorts of trials is possible to reduce the performance and detection biases. We, therefore, had to downgrade the quality of all trials.
Incomplete outcome data
We found no evidence of missing outcome data in any included study.
Selective reporting
The protocols of the studies were not available. We, therefore, could not compare outcomes in protocols with those in the published reports. However, all outcomes listed in the 'methods' section of the paper do appear to have been measured and reported.
Other potential sources of bias
All of 15 included studies were from the People's Republic of China and reported in Chinese. It is unclear if this represents a racial or cultural bias when applied to other regions. We admit both authors of this review ‐ HD and JX, are experienced TCM practitioners, hence these might have potential sources of bias. All authors of studies came from hospitals and were without any support from industry or institutes, except for two studies (Su 2015 and Liu 2014), which were funded by government. We were unclear whether these two studies had 'other potential sources' of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
For this review, we generated four comparisons and were able to extract numerical data from 15 randomised studies. We calculated risk ratios (RR) for dichotomous data and estimated mean differences (MD) for continuous data, with their respective 95% confidence intervals (CIs) throughout.
1. COMPARISON 1: WENDAN DECOCTION versus PLACEBO OR NO TREATMENT
In this comparison, there were two outcomes, both short term, from one study (Wang 2013, N = 72).
1.1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction)
One trial reported useable data for this outcome. There was evidence that clearly more people in the Wednon decoction (WDD) group had a clinically important improvement in global state (PANSS < 50% reduction) compared with those in the no treatment group (RR 0.53, 95% CI 0.39 to 0.73, low‐quality evidence, Analysis 1.1).
1.1. Analysis.
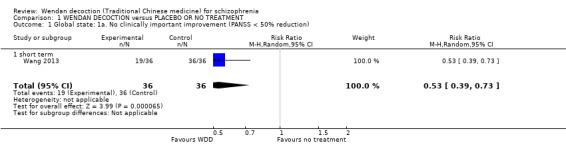
Comparison 1 WENDAN DECOCTION versus PLACEBO OR NO TREATMENT, Outcome 1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction).
1.2 Global state: 1b. No improvement (PANSS < 25% reduction)
One trial reported useable data for this outcome. There was evidence that clearly more people in the WDD group showed some improvement in global state (PANSS < 25%) compared with those in the no treatment group (RR 0.10, 95% CI 0.04 to 0.26, Analysis 1.2).
1.2. Analysis.
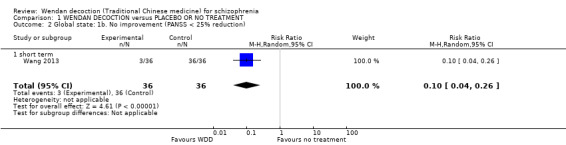
Comparison 1 WENDAN DECOCTION versus PLACEBO OR NO TREATMENT, Outcome 2 Global state: 1b. No improvement (PANSS < 25% reduction).
2. COMPARISON 2: WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG
Three trials (Chang 2009, Dou 2012, Huang 2005, N = 300) compared WDD with antipsychotic drugs (chlorpromazine, risperidone and estazolam). In this comparison, there were nine outcomes, all reported at short term.
2.1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction)
Two trials, (total n = 140), reported useable data for clinically important improvement. Overall, there was not a clear difference between WDD group and the antipsychotic groups (RR 1.18, 95% CI 0.98 to 1.43, moderate‐quality evidence, Analysis 2.1). There are two subgroups for this outcome.
2.1. Analysis.
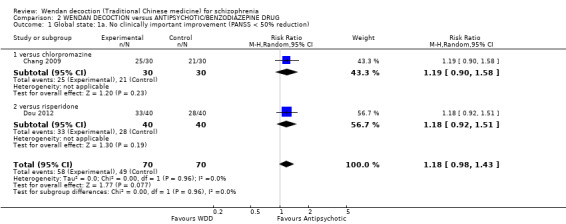
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction).
2.1.1 versus chlorpromazine
We found one trial comparing WDD with chlorpromazine (n = 60). There was not a clear difference between WDD and chlorpromazine (RR 1.19, 95% CI 0.90 to 1.58).
2.1.2 versus risperidone
We found one trial comparing WDD with risperidone, which included a total of 80 participants. There was not a clear difference between WDD and risperidone (RR 1.18, 95% CI 0.92 to 1.51).
2.2 Global state: 1b. No improvement (PANSS < 25% reduction)
Two trials, (total n = 140), reported useable data for improvement in global state. Overall, there was evidence that antipsychotics were better in their effects than WDD (RR 2.44, 95% CI 1.21 to 4.93, Analysis 2.2). There are two subgroups for this outcome.
2.2. Analysis.
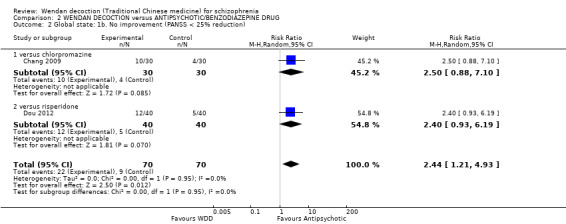
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 2 Global state: 1b. No improvement (PANSS < 25% reduction).
2.2.1 versus chlorpromazine
One trial, (total n = 60), compared WDD with chlorpromazine. There was no evidence of a clear difference between the two treatments (RR 2.50, 95% CI 0.88 to 7.10).
2.2.2 versus risperidone
One trial (total n = 80) compared WDD with risperidone. There was no evidence of a clear difference between the two treatments (RR 2.40, 95% CI 0.93 to 6.19).
2.3 Mental state: 1a. Average positive score (endpoint, PANSS, high score = bad)
Two trials (total n = 140) reported average positive endpoint scores on the PANSS (short term). Overall, there was no evidence that WDD was clearly different in its effects compared with antipsychotics (MD ‐1.25, 95% CI ‐4.97 to 2.48, Analysis 2.3, ). However, this outcome had important levels of heterogeneity (I2 = 82%), and the effects between comparing with chlorpromazine and risperidone were different.
2.3. Analysis.
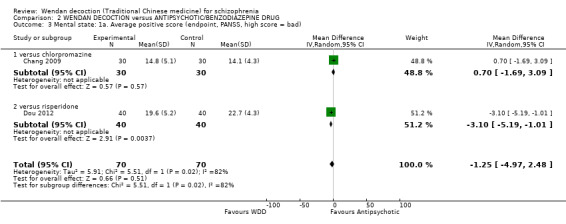
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 3 Mental state: 1a. Average positive score (endpoint, PANSS, high score = bad).
2.3.1 versus chlorpromazine
There was a single trial in this subgroup, with a total of 60 people. There was not a clear difference between WDD and chlorpromazine (MD 0.70, 95% CI ‐1.69 to 3.09).
2.3.2 versus risperidone
There was a single trial in this subgroup (total n = 80). We found evidence of a clear positive effect for WDD compared with risperidone (MD ‐3.10, 95% CI ‐5.19 to ‐1.01).
2.4 Mental state: 1b. Average negative score (endpoint, PANSS, high score = bad)
For this outcome, two short‐term trials (n = 140) reported useable data. Overall, there was no evidence that WDD was clearly different in its effects compared with antipsychotics (MD ‐0.58, 95% CI ‐2.08 to 0.91, Analysis 2.4). There are two subgroups for this outcome.
2.4. Analysis.
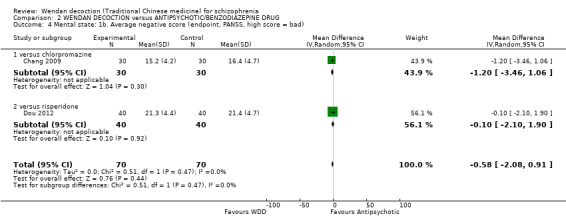
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 4 Mental state: 1b. Average negative score (endpoint, PANSS, high score = bad).
2.4.1 versus chlorpromazine
We found one trial to be relevant to this subgroup, which included a total of 60 participants. There was not a clear difference between WDD and chlorpromazine (MD ‐1.20, 95% CI ‐3.46 to 1.06).
2.4.2 versus risperidone
There was a single trial in this subgroup, which included a total of 80 participants. There was no evidence of a clear difference between WDD and risperidone (MD ‐0.10, 95% CI ‐2.10 to 1.90).
2.5 Mental state: 1c. Average total score (endpoint, PANSS, high score = bad)
For this outcome, two short‐term trials (n = 140) reported useable data. Overall, there was no evidence that WDD was clearly different in its effects compared with antipsychotics (MD 0.84; 95% CI ‐4.17 to 5.84, low‐quality evidence, Analysis 2.5). There are two subgroups for this outcome.
2.5. Analysis.
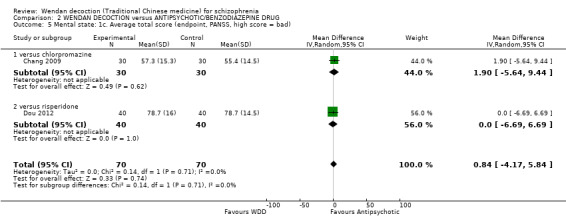
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 5 Mental state: 1c. Average total score (endpoint, PANSS, high score = bad).
2.5.1 versus chlorpromazine
There was a single trial in this subgroup (total n = 60). There was not a clear difference between WDD and chlorpromazine (MD 1.90, 95% CI ‐5.64 to 9.44).
2.5.2 versus risperidone
There was a single trial in this subgroup, with a total of 80 people. There was no evidence of a clear difference between WDD and risperidone (MD 0.00, 95% CI ‐6.69 to 6.69).
2.6 Adverse effect: 1. Anticholinergic ‐ Dry mouth (TESS)
One trial, n = 80, provided data for the number of participants reporting a dry mouth. There was evidence of a positive effect for WDD (i.e. clearly fewer people from the WDD group experiencing a dry mouth) compared with risperidone (RR 0.02, 95% CI 0.00 to 0.28, Analysis 2.6).
2.6. Analysis.
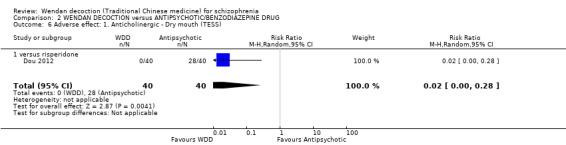
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 6 Adverse effect: 1. Anticholinergic ‐ Dry mouth (TESS).
2.7 Adverse effect: 2. Central Nervous System ‐ Insomnia (TESS)
Two trials, n = 140, provided data for the number of participants with the symptom of insomnia. Overall, clearly fewer people reported symptoms of insomnia in the WDD group than the antipsychotic groups (RR 0.23, 95% CI 0.11 to 0.50, Analysis 2.7). There were two subgroups for this outcome.
2.7. Analysis.
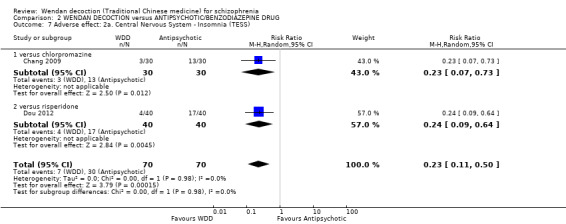
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 7 Adverse effect: 2a. Central Nervous System ‐ Insomnia (TESS).
2.7.1 versus chlorpromazine
A single trial, with a total of 60 people provided data. WDD had clearly fewer people reporting symptoms of insomnia than those in the chlorpromazine group (RR 0.23, 95% CI 0.07 to 0.73).
2.7.2 versus risperidone
A single trial with a total of 80 people provided data. Again, clearly fewer people in the WDD group reported symptoms of insomnia than the risperidone group (RR 0.24, 95% CI 0.09 to 0.64).
2.8 Adverse effect: 2b. Central nervous system ‐ Sleep time (< eight hrs)
2.8.1 versus estazolam
One trial (n = 90) reported the number of participants who slept for less than eight hours per night. We found one relevant trial. There was not a clear difference between WDD and estazolam (RR 0.81, 95% CI 0.42 to 1.58, Analysis 2.8).
2.8. Analysis.
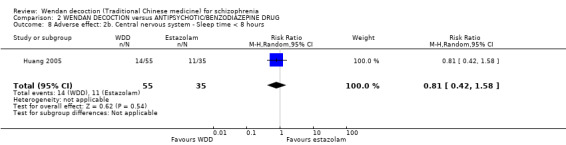
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 8 Adverse effect: 2b. Central nervous system ‐ Sleep time < 8 hours.
2.9 Adverse effect: 2c. Central nervous system ‐ No change in sleep time
2.9.1 versus estazolam
One trial (n = 90) reported the number of participants who did not experience any change in sleep time. There was not a clear difference between WDD and estazolam (RR 0.64, 95% CI 0.14 to 2.98, Analysis 2.9).
2.9. Analysis.
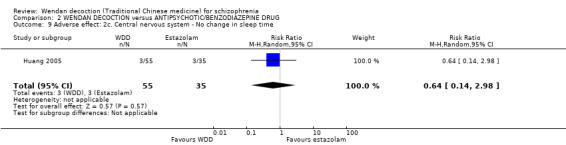
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 9 Adverse effect: 2c. Central nervous system ‐ No change in sleep time.
2.10 Averse effect: 3. Gastrointenstinal ‐ Constipation (TESS)
For this outcome, number of participants with symptom of constipation, we found a single trial (n = 60) reporting useable data. There was evidence that the number of people with constipation was clearly lower in the WDD group compared to chlorpromazine group (RR 0.02, 95% CI 0.00 to 0.37, Analysis 2.10).
2.10. Analysis.
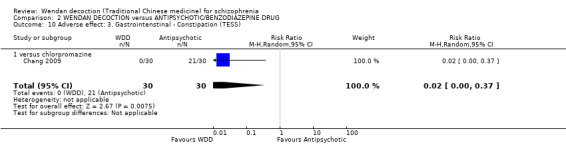
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 10 Adverse effect: 3. Gastrointenstinal ‐ Constipation (TESS).
2.11 Adverse effect: 4. Movement disorders ‐ Extrapyramidal symptoms (EPS) (TESS)
For this outcome, number of participants with symptom of EPS, we found two relevant trials involving 140 participants. Overall, there was a positive effect for WDD compared to antipsychotics (RR 0.02, 95% CI 0.00 to 0.15, moderate‐quality evidence, Analysis 2.11). There are two subgroups for this outcome.
2.11. Analysis.
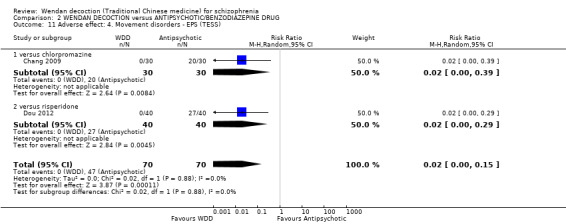
Comparison 2 WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG, Outcome 11 Adverse effect: 4. Movement disorders ‐ EPS (TESS).
2.11.1 versus chlorpromazine
There was a single trial in this subgroup (total n = 60). There was a clear effect, with fewer people with EPS in the WDD group compared to the chlorpromazine group (RR 0.02, 95% CI 0.00 to 0.39).
2.11.2 versus risperidone
There was a single trial in this subgroup, which included a total of 80 participants. There was no evidence of a clear effect for WDD compared to risperidone (RR 0.02, 95% CI 0.00 to 0.29, Analysis 2.11).
3. COMPARISON 3: WENDAN DECOCTION PLUS NORMAL‐DOSE ANTIPSYCHOTIC versus NORMAL‐DOSE ANTIPSYCHOTIC
We found six trials (Li 2013; Lin 2010; Liu 2014; Shao 2014; Sun 2014; Zhang 2014, N = 684) comparing WDD plus normal‐dose antipsychotic with normal dose antipsychotic drug alone. This comparison has 13 outcomes, all short term.
3.1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction)
Six trials, involving 684 participants, reported useable data for the outcome of clinically important improvement. There was evidence of an overall positive effect for WDD + antipsychotic when compared with antipsychotic alone (RR 0.60, 95% CI 0.50 to 0.72, moderate‐quality evidence, Analysis 3.1). There are five subgroups for this outcome.
3.1. Analysis.
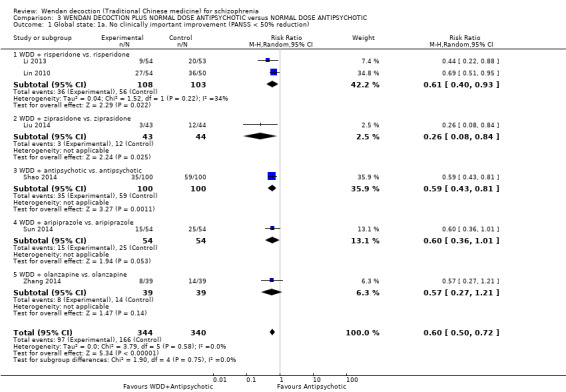
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction).
3.1.1 WDD + risperidone versus. risperidone
There were two relevant trials in this subgroup, with a total of 211 people. There was evidence that WDD + risperidone was clearly better than risperidone alone (RR 0.61, 95% CI 0.40 to 0.93). For this subgroup heterogeneity is moderately high (I2 = 34%)
3.1.2 WDD + ziprasidone versus ziprasidone
We found one trial to be relevant to this subgroup, which included a total of 87 participants. There was evidence that WDD + ziprasidone was clearly better than ziprasidone (RR 0.26, 95% CI 0.08 to 0.84).
3.1.3 WDD + antipsychotic versus antipsychotic
There was a single trial in this subgroup, with a total of 200 people. Within this subgroup, there was evidence that WDD + antipsychotic was clearly better than antipsychotic alone (RR 0.59, 95% CI 0.43 to 0.81).
3.1.4 WDD + aripiprazole versus aripiprazole
We found one trial to be relevant to this subgroup, with a total of 108 people. For this subgroup, there was no evidence of a clear difference between the two treatments (RR 0.60, 95% CI 0.36 to 1.01).
3.1.5 WDD + olanzapine versus olanzapine
We found one trial to be relevant to this subgroup (total n = 78). For this subgroup, there was no evidence of a clear difference between the two treatments (RR 0.57, 95% CI 0.27 to 1.21).
3.2 Global state: 1b. No improvement (PANSS < 25% reduction)
Six trials, involving 684 participants reported useable data for the outcome of improvement in global state. Overall, there was evidence that WDD + antipsychotic was clearly better compared with antipsychotic alone (RR 0.24, 95% CI 0.14 to 0.40, Analysis 3.2). There are five subgroups for this outcome.
3.2. Analysis.
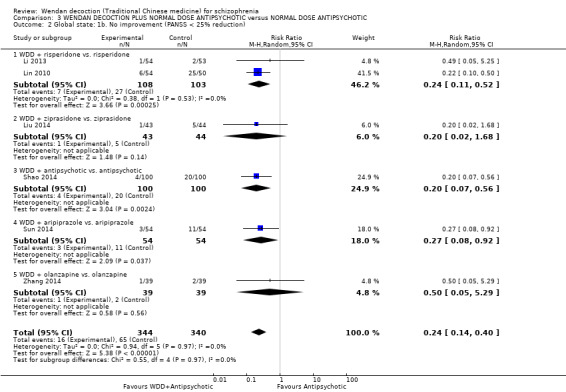
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 2 Global state: 1b. No improvement (PANSS < 25% reduction).
3.2.1 WDD + risperidone versus risperidone
Two trials reported useable data for this subgroup (total n = 211). There was evidence that WDD + risperidone was clearly better than risperidone alone (RR 0.24, 95% CI 0.11 to 0.52).
3.2.2 WDD + ziprasidone versus ziprasidone
We found one trial to be relevant to this subgroup, which included a total of 87 participants. There was not a clear difference between the two treatment groups (RR 0.20, 95% CI 0.02 to 1.68).
3.2.3 WDD + antipsychotic versus antipsychotic
There was a single trial in this subgroup, with a total of 200 people. There was evidence that WDD + antipsychotic was better than antipsychotic alone (RR 0.20, 95% CI 0.07 to 0.56).
3.2.4 WDD + aripiprazole versus aripiprazole
We found one trial to be relevant to this subgroup, which included a total of 108 participants. There was evidence that WDD + aripiprazole was better than aripiprazole alone (RR 0.27, 95% CI 0.08 to 0.92).
3.2.5 WDD + olanzapine versus olanzapine
There was a single trial in this subgroup (total n = 78). There was evidence that WDD + olanzapine was better than olanzapine alone (RR 0.50, 95% CI 0.05 to 5.29).
3.3 Global state: 2. Traditional Chinese Medicine syndromes ‐ no improvement (TCMSS < 30% reduction)
We identified one study with a total of 104 people reporting improvement using theTCMSS. There was evidence of a positive effect for WDD + risperidone compared with risperidone alone (RR 0.25, 95% CI 0.12 to 0.52, Analysis 3.3).
3.3. Analysis.
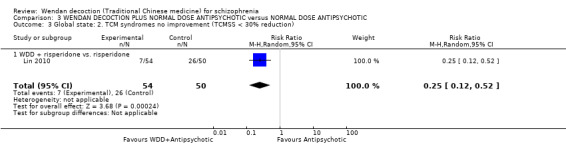
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 3 Global state: 2. TCM syndromes no improvement (TCMSS < 30% reduction).
3.4 Mental state: 1a. Average positive score (endpoint, PANSS, high score = bad)
One trial, n = 200 reported average endpoint scores for the PANSS (positive). There was evidence of a clear positive effect for WDD + antipsychotic compared to antipsychotic alone (MD ‐0.98, 95% CI ‐1.70 to ‐0.26, Analysis 3.4).
3.4. Analysis.
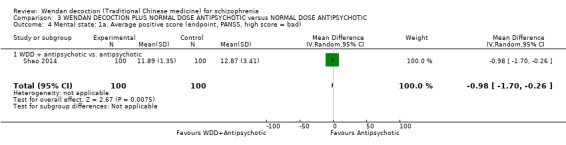
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 4 Mental state: 1a. Average positive score (endpoint, PANSS, high score = bad).
3.5 Mental state: 1b. Average negative score (endpoint, PANSS, high score = bad)
One trial with a total of 200 people reported average endpoint scores for the PANSS (negative). There was evidence of a positive effect for WDD + antipsychotic compared to antipsychotic alone (MD ‐4.47, 95% CI ‐5.05 to ‐3.89, Analysis 3.5).
3.5. Analysis.
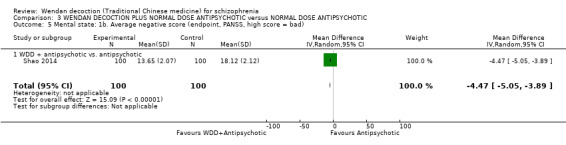
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 5 Mental state: 1b. Average negative score (endpoint, PANSS, high score = bad).
3.6 Mental state: 1c. Average total score (endpoint, PANSS, high score = bad)
Five trials with a total of 580 people reported average total endpoint PANSS scores. There was evidence of a positive effect for WDD + antipsychotic compared to antipsychotic alone (MD ‐11.64, 95% CI ‐13.33 to ‐9.94, low‐quality evidence, Analysis 3.6). For this outcome heterogeneity is (I2 = 61 %). There are five subgroups for this outcome.
3.6. Analysis.
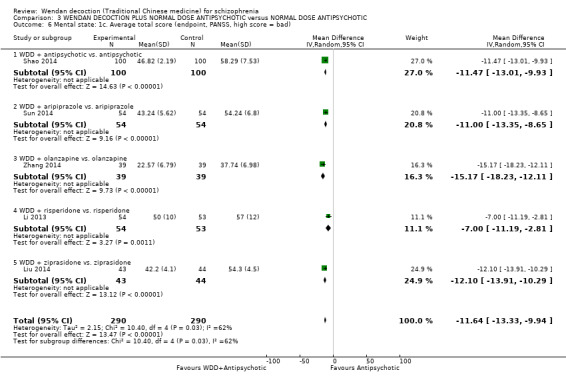
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 6 Mental state: 1c. Average total score (endpoint, PANSS, high score = bad).
3.6.1 WDD + antipsychotic versus antipsychotic
There was a single trial in this subgroup, (total n = 200). There was evidence that WDD + antipsychotic was better in reducing PANSS average total score than antipsychotic alone (MD ‐11.47, 95% CI ‐13.01 to ‐9.93).
3.6.2 WDD + aripiprazole versus aripiprazole
One trial, with a total of 108 people, reported data for this subgroup. There was evidence that WDD + aripiprazole was better in reducing PANSS average total score than aripiprazole alone (MD ‐11.00, 95% CI ‐13.35 to ‐8.65).
3.6.3 WDD + olanzapine versus olanzapine
One trial, which included a total of 78 participants, reported data for this subgroup. There was evidence that WDD + olanzapine was better in reducing PANSS average total score than olanzapine alone (MD ‐15.17, 95% CI ‐18.23 to ‐12.11).
3.6.4 WDD + risperidone versus risperidone
One trial, which included a total of 107 participants, reported data for this subgroup. There was evidence that WDD + normal‐dose risperidone was better in its effects compared with risperidone alone (MD ‐7.00, 95% CI ‐11.19 to ‐2.81).
3.6.5 WDD + ziprasidone versus ziprasidone
There was a single trial in this subgroup, which included a total of 87 participants. There was evidence that WDD + normal‐dose ziprasidone was better in its effects compared with ziprasidone alone (MD ‐12.10, 95% CI ‐13.91 to ‐10.29).
3.7 Mental state: 2. Average total score (endpoint, BPRS)
One trial, with a total of 200 participants, reported average total endpoint scores for the BPRS. There was evidence that WDD + antipsychotic was clearly better in its effects compared with antipsychotic alone (MD ‐4.92, 95% CI ‐6.18 to ‐3.66, Analysis 3.7).
3.7. Analysis.
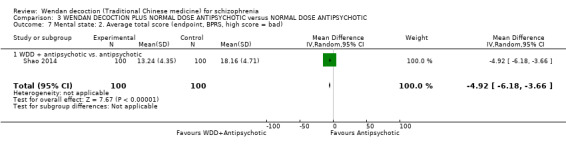
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 7 Mental state: 2. Average total score (endpoint, BPRS, high score = bad).
3.8 Mental state: 3. Average total score (endpoint, CDSS)
One trial involving 87 participants reported average total endpoint scores for the CDSS . There was evidence that WDD + ziprasidone was clearly better in its effects compared with ziprasidone alone (MD ‐1.50, 95% CI ‐2.11 to ‐0.89, Analysis 3.8).
3.8. Analysis.
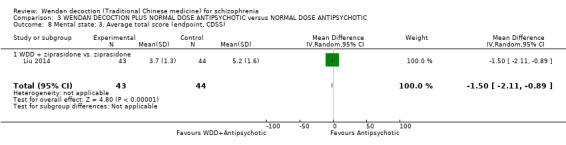
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 8 Mental state: 3. Average total score (endpoint, CDSS).
3.9 Adverse effect: 1. Movement disorders ‐ EPS (TESS)
Two trials, with a total of 308 people. reported useable data for this outcome. There was evidence of an overall clear difference between WDD + antipsychotic and antipsychotic alone (RR 0.46, 95% CI 0.30 to 0.70, moderate‐quality evidence, Analysis 3.9 ), whereby WDD had fewer people with EPS. There are two subroups for this outcome.
3.9. Analysis.
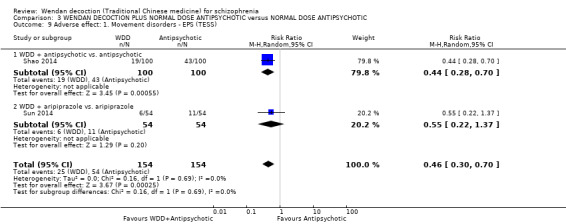
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 9 Adverse effect: 1. Movement disorders ‐ EPS (TESS).
3.9.1 WDD + antipsychotic versus antipsychotic
There was a single trial in this subgroup (total n = 200). There was evidence that WDD + antipsychotic had clearly fewer people with adverse effects of EPS than antipsychotic alone (RR 0.44, 95% CI 0.28 to 0.70).
3.9.2 WDD + aripiprazole versus aripiprazole
There was a single trial in this subgroup, with a total of 108 people. For this subgroup, there was no evidence of a clear difference between the two treatments (RR 0.55, 95% CI 0.22 to 1.37).
3.10 Adverse effect: 2a. Gastrointenstinal ‐ Abnormal Liver Function
For this outcome, number of people with abnormal liver function (by laboratory examination), we found a single study (total n = 200) There was no evidence of a difference between the two treatments (RR 0.14 CI 0.01 to 2.73, Analysis 3.10).
3.10. Analysis.
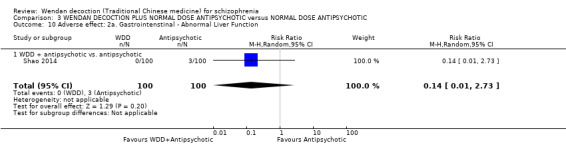
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 10 Adverse effect: 2a. Gastrointenstinal ‐ Abnormal Liver Function.
3.11 Adverse effect: 2b. Gastrointestinal ‐ Constipation (TESS)
Two trials (total n = 308) reported the number of participants with constipation. There was evidence of an overall clear difference favouring WDD + antipsychotic groups (RR 0.06, 95% CI 0.00 to 4.44, Analysis 3.11). This outcome had important levels of heterogeneity ( I2 = 88%). There are two subgroups for this outcome.
3.11. Analysis.
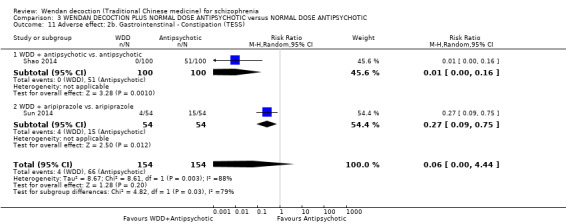
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 11 Adverse effect: 2b. Gastrointenstinal ‐ Constipation (TESS).
3.11.1 WDD + antipsychotic versus antipsychotic
There was a single trial in this subgroup, which included a total of 200 participants. There was evidence that fewer people in the WDD + antipsychotic group reported the side effect of constipation than those in the antipsychotic alone group (RR 0.01, 95% CI 0.00 to 0.16).
3.11.2 WDD + aripiprazole versus aripiprazole
There was a single trial in this subgroup, which included a total of 108 participants. There was evidence that WDD + aripiprazole group reported clearly fewer participants with constipation than the aripiprazole alone group (RR 0.27. 95% CI 0.09 to 0.75).
3.12 Adverse effect: 3. Metabolic ‐ Weight gain (TESS)
The number of participants reporting weight gain after treatment was provided by one trial involving 108 participants. There was no evidence of a clear difference between the WDD + aripiprazole and aripiprazole alone groups (RR 0.50, 95% CI 0.20 to 1.24, low‐quality evidence, Analysis 3.12).
3.12. Analysis.
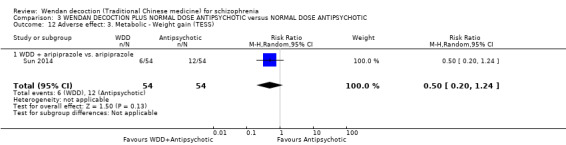
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 12 Adverse effect: 3. Metabolic ‐ Weight gain (TESS).
3.13 Use of antipsychotic: Drug dose at the endpoint
One trial, (n = 107), reported drug dose at endpoint. There was evidence that WDD + risperidone was clearly better at reducing drug dose than risperidone alone (MD ‐0.70, 95% CI ‐0.87 to ‐0.53, low‐quality evidence, Analysis 3.13).
3.13. Analysis.
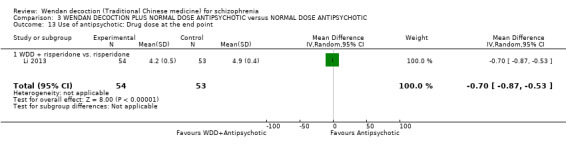
Comparison 3 WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 13 Use of antipsychotic: Drug dose at the end point.
4. COMPARISON 4: WENDAN DECOCTION PLUS LOW‐DOSE ANTIPSYCHOTIC versus NORMAL‐DOSE ANTIPSYCHOTICS
Seven trials (Chang 2009; Dou 2012; Guo 2013; Peng 2010; Su 2015; Wang 2008; Xu 2007, N = 591) compared WDD + low‐dose antipsychotic with normal‐dose antipsychotic drug alone. This comparison has 24 outcomes, all for short term except one trial (Peng 2010) which reported some medium‐ and long‐term outcomes.
4.1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction) ‐ short term
Seven trials, n = 522, reported useable data for clinically important improvement in global state at short term. Overall, there was evidence of a positive effect for WDD + low‐dose antipsychotic compared to normal‐dose antipsychotics (RR 0.69, 95% CI 0.51 to 0.93, moderate‐quality evidence, Analysis 4.1). There are six subgroups for this outcome.
4.1. Analysis.
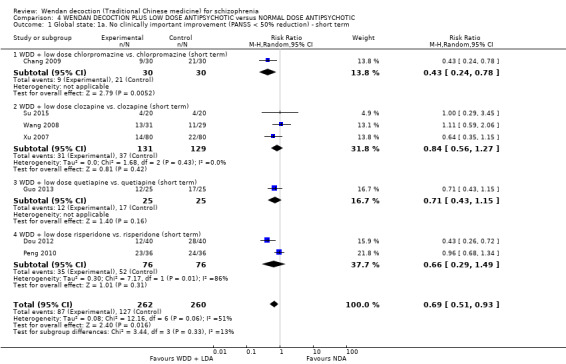
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction) ‐ short term.
4.1.1 WDD + low‐dose chlorpromazine versus chlorpromazine (short term)
There was a single trial in this subgroup (total n = 60). There was evidence that WDD + low‐dose chlorpromazine was better for important improvement in global state than normal‐dose chlorpromazine alone (RR 0.43, 95% CI 0.24 to 0.78).
4.1.2 WDD + low‐dose clozapine versus clozapine (short term)
There were three relevant trials in this subgroup (total n = 260). There was not a clear difference between WDD + low‐dose clozapine and clozapine alone (RR 0.84, 95% CI 0.56 to 1.27).
4.1.3 WDD + low‐dose quetiapine versus quetiapine (short term)
There was a single trial in this subgroup (total n = 50). There was not a clear difference between WDD + low‐dose quetiapine and quetiapine (RR 0.71, 95% CI 0.43 to 1.15).
4.1.4 WDD + low‐dose risperidone versus risperidone (short term)
There were two relevant trials in this subgroup, with a total of 152 people. There was no evidence of a clear difference between the two treatments (RR 0.66, 95% CI 0.29 to 1.49). This subgroup had important levels of heterogeneity (I2 = 86%, Analysis 4.1).
4.2 Global state: 1b. No clinically important improvement (PANSS < 50% reduction)
One trial, n = 72, reported clinically important improvement at medium and long term.
4.2.1 WDD + low‐dose risperidone versus risperidone (medium term)
There was not a clear difference between the two treatments (RR 0.87, 95% CI 0.59 to 1.27, Analysis 4.2).
4.2. Analysis.
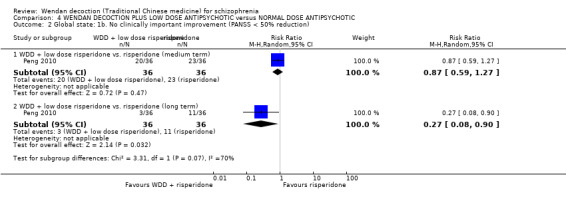
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 2 Global state: 1b. No clinically important improvement (PANSS < 50% reduction).
4.2.1 WDD + low‐dose risperidone versus risperidone (long term)
There was evidence that WDD + low‐dose risperidone was better than normal‐dose risperidone alone (RR 0.27, 95% CI 0.08 to 0.90, Analysis 4.2).
4.3 Global state: 1b. No improvement (PANSS < 25% reduction) ‐ short term
Seven trials (total n = 522) reported some improvement in global state. There was evidence that WDD + low‐dose antipsychotic was clearly better compared with normal‐dose antipsychotic alone (RR 0.62, 95% CI 0.43 to 0.90, Analysis 4.3).There are six subgroups for this outcome.
4.3. Analysis.
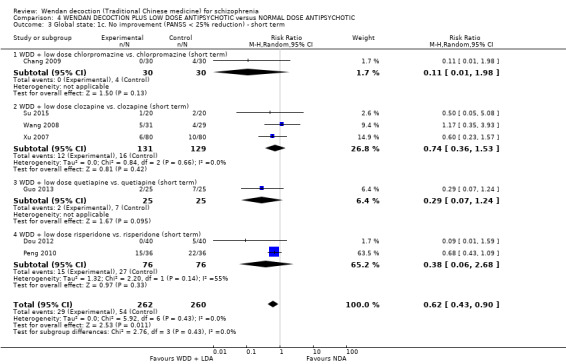
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 3 Global state: 1c. No improvement (PANSS < 25% reduction) ‐ short term.
4.3.1 WDD + low‐dose chlorpromazine versus chlorpromazine (short term)
There was a single trial in this subgroup (total n = 60). For this subgroup, There was no evidence of a clear difference between the two treatments (RR 0.11, 95% CI 0.01 to 1.98).
4.3.2 WDD + low‐dose clozapine versus clozapine (short term)
We found three trials to be relevant to this subgroup (total n = 260). There was no evidence of a clear difference between the two treatments (RR 0.74, 95% CI 0.36 to 1.53).
4.3.3 WDD + low‐dose quetiapine versus quetiapine (short term)
One trial reported data for this subgroup, which included a total of 50 participants. For this subgroup, There was no evidence of a clear difference between the two treatments (RR 0.29, 95% CI 0.07 to 1.24)
4.3.4 WDD + low‐dose risperidone versus risperidone (short term)
Two trials reported useable data for this subgroup (total n = 152). There was evidence that WDD + low‐dose risperidone, in the short‐term, was better in improving the global state than normal‐dose risperidone within this subgroup (RR 0.38, 95% CI 0.06 to 2.68). This subgroup had important levels of heterogeneity (I2 = 55%).
4.4 Global state: 1d. No improvement (PANSS < 25% reduction)
One trial, n = 72, reported improvement at medium and long term.
4.4.1 WDD + low‐dose risperidone versus risperidone (medium term)
There was not a clear difference between the two treatments in medium term (RR 0.29, 95% CI 0.06 to 1.28, Analysis 4.4).
4.4. Analysis.
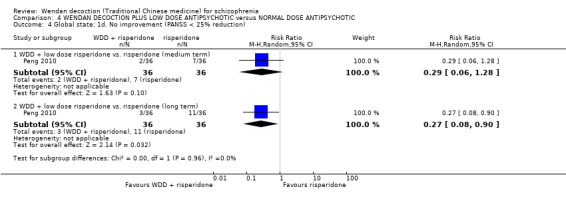
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 4 Global state: 1d. No improvement (PANSS < 25% reduction).
4.4.2 WDD + low‐dose risperidone versus risperidone (long term)
There was a single trial in this subgroup (total n = 72). There was evidence that, in the long term that WDD + low‐dose risperidone was better in improving the global state than normal‐dose risperidone within this subgroup (RR 0.27, 95% CI 0.08 to 0.9,Analysis 4.4.
4.5 Mental state: 1a. Average positive score (endpoint, PANSS, high score = bad)
We identified three studies, involving 190 participants, relevant to this outcome,. There was evidence of a clear difference (favouring WDD) between WDD + low‐dose antipsychotic and normal‐dose antipsychotic (MD ‐6.52, 95% CI ‐10.22 to ‐2.82, Analysis 4.5). For this outcome heterogeneity is high (I2 = 87%). There are three subgroups for this outcome.
4.5. Analysis.
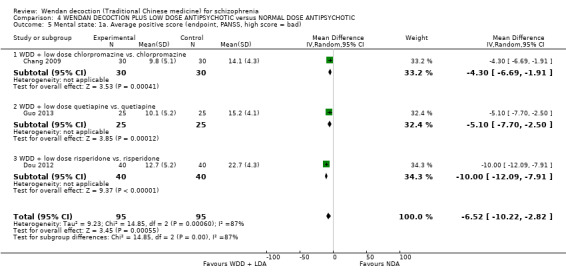
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 5 Mental state: 1a. Average positive score (endpoint, PANSS, high score = bad).
4.5.1 WDD + low‐dose chlorpromazine versus chlorpromazine
There was a single trial in this subgroup, which included a total of 60 participants. There was evidence that WDD + low‐dose chlorpromazine was better in reducing PANSS positive score than normal‐dose chlorpromazine alone (MD ‐4.30, 95% CI ‐6.69 to ‐1.91).
4.5.2 WDD + low‐dose quetiapine versus quetiapine
There was a single trial in this subgroup, with a total of 50 people. There was evidence that WDD + low‐dose quetiapine was better in reducing PANSS positive score than normal‐dose quetiapine alone (MD ‐5.10, 95% CI ‐7.70 to ‐2.50).
4.5.3 WDD + low‐dose risperidone versus risperidone
There was a single trial in this subgroup (total n = 80). For this outcome, within this subgroup, there was evidence that WDD + low‐dose risperidone was better in reducing PANSS positive score than normal‐dose risperidone alone (MD ‐10.00, 95% CI ‐12.09 to ‐7.91, Analysis 4.5).
4.6 Mental state: 1b. Average negative score (endpoint, PANSS, high score = bad)
We identified three studies, involving 190 participants, for this outcome. There was evidence that WDD + low‐dose antipsychotic was clearly different in its effects on improving mental state, while compared with normal‐dose antipsychotic (MD ‐4.64, 95% CI ‐7.45 to ‐1.82, Analysis 4.6 ). This outcome had important levels of heterogeneity (I2 = 68%). There are three subgroups for this outcome.
4.6. Analysis.
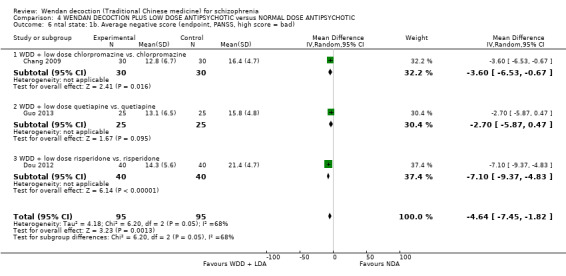
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 6 ntal state: 1b. Average negative score (endpoint, PANSS, high score = bad).
4.6.1 WDD + low‐dose chlorpromazine versus chlorpromazine
One trial reported data for this subgroup, which included a total of 60 participants. There was evidence that WDD + low‐dose chlorpromazine was better in reducing PANSS negative score than normal‐dose chlorpromazine alone (MD ‐3.60, 95% CI ‐6.53 to ‐0.67).
4.6.2 WDD + low‐dose quetiapine versus quetiapine
One trial reported data for this subgroup, with a total of 50 people. There was evidence that WDD + low‐dose quetiapine was better in reducing PANSS negative score than normal‐dose quetiapine alone (MD ‐2.70, 95% CI ‐5.87 to 0.47).
4.6.3 WDD + low‐dose risperidone versus risperidone
There was a single trial in this subgroup, which included a total of 80 participants. There was evidence that WDD + low‐dose risperidone was better in reducing PANSS negative score than normal‐dose risperidone alone (MD ‐7.10, 95% CI ‐9.37 to ‐4.83).
4.7 Mental state: 1c. Average total score (endpoint, PANSS, high score = bad)
We identified four studies (total n = 250) relevant to this outcome. There was evidence that WDD + low‐dose antipsychotic was clearly better compared with normal‐dose antipsychotic alone (MD ‐9.53, CI ‐17.82 to ‐1.24, low‐quality evidence, Analysis 4.7). This outcome had important levels of heterogeneity (I2 = 78%). There are four subgroups for this outcome.
4.7. Analysis.
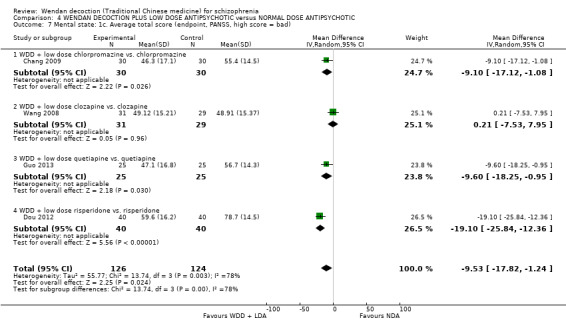
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 7 Mental state: 1c. Average total score (endpoint, PANSS, high score = bad).
4.7.1 WDD + low‐dose chlorpromazine versus chlorpromazine
One trial reported data for this subgroup (total n = 60). There was evidence that WDD + low‐dose chlorpromazine was better in reducing PANSS average total score than normal‐dose chlorpromazine alone (MD ‐9.10, 95% CI ‐17.12 to ‐1.08).
4.7.2 WDD + low‐dose clozapine versus clozapine
One trial reported data for this subgroup, which included a total of 60 participants. There was no evidence of a clear difference between the two treatments (MD 0.21, 95% CI ‐7.53 to 7.95).
4.7.3 WDD + low‐dose quetiapine versus quetiapine
One trial reported data for this subgroup, with a total of 50 people. There was evidence that WDD + low‐dose quetiapine was better in reducing PANSS average total score than normal‐dose quetiapine alone (MD ‐9.60, 95% CI ‐18.25 to ‐0.95).
4.7.4 WDD + low‐dose risperidone versus risperidone
There was a single trial in this subgroup, which included a total of 80 participants. There was evidence that WDD + low‐dose risperidone was better in reducing PANSS average total score than normal‐dose risperidone alone (MD ‐19.10, 95% CI ‐25.84 to ‐12.36).
4.8 Mental state: 2. Average total score (endpoint, BPRS, high score = bad)
For this outcome, we found a single study, n = 72. There was no evidence that WDD + low‐dose risperidone was clearly different in its effects compared with normal‐dose risperidone alone (MD 0.30, 95% CI ‐0.29 to 0.89, Analysis 4.8).
4.8. Analysis.
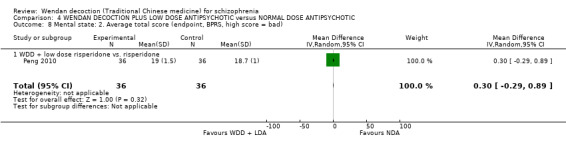
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 8 Mental state: 2. Average total score (endpoint, BPRS, high score = bad).
4.9 Adverse effect: 1a. Anticholinergic ‐ Dry mouth (TESS)
One study, n = 80 reported the number of participants with dry mouth. There was evidence that clearly fewer people in the WDD + low‐dose risperidone group experienced dry mouth than in the normal‐dose risperidone group (RR 0.43, 95% CI 0.26 to 0.72, Analysis 4.9).
4.9. Analysis.
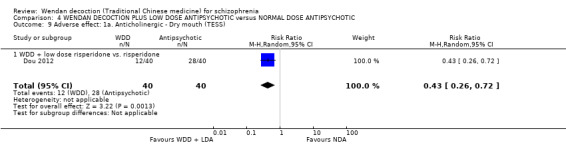
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 9 Adverse effect: 1a. Anticholinergic ‐ Dry mouth (TESS).
4.10 Adverse effect: 1b. Anticholinergic ‐ Salivation (TESS)
For this outcome, we found two relevant studies, (total n = 110). Overall, clearly fewer people in the WDD + low‐dose antipsychotic reported having salivation as a result of receiving treatment compared to normal‐dose antipsychotic groups (RR 0.10 CI 0.05 to 0.30, Analysis 4.10). There are five subgroups for this outcome.
4.10. Analysis.
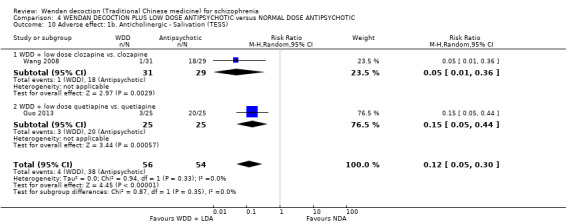
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 10 Adverse effect: 1b. Anticholinergic ‐ Salivation (TESS).
4.10.1 WDD + low‐dose clozapine versus clozapine
There was a single trial in this subgroup (total n = 60). There was evidence that WDD + low‐dose clozapine had fewer people with salivation compared with normal‐dose clozapine (RR 0.05, 95% CI 0.01 to 0.36).
4.10.2 WDD + low‐dose quetiapine versus quetiapine
One trial reported data for this subgroup, which included a total of 50 participants.There was evidence of a positive effect for WDD + low‐dose quetiapine compared with normal‐dose quetiapine alone (RR 0.15, 95% CI 0.05 to 0.44).
4.11 Adverse effect: 2a. Cardiovascular ‐ Drop in blood pressure
Two studies with a total of 100 people reported data for the number of people who had a drop in blood pressure,. There was no evidence of a clear difference between the WDD + low‐dose clozapine group and clozapine alone group (RR 0.37, 95% CI 0.10 to 1.34, Analysis 4.11).
4.11. Analysis.
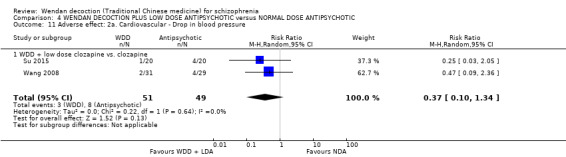
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 11 Adverse effect: 2a. Cardiovascular ‐ Drop in blood pressure.
4.12 Adverse effect: 2b. Cardiovascular ‐ ECG change
One trial ( total n = 40) reported data for the number of people with observed ECG change. There was no evidence of a clear difference between the WDD + low‐dose clozapine group and clozapine alone group (RR 0.33, 95% CI 0.01 to 7.72, Analysis 4.12).
4.12. Analysis.
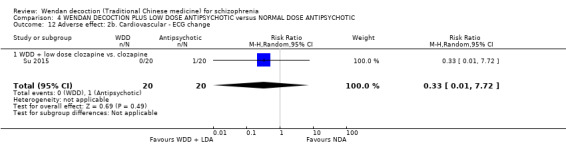
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 12 Adverse effect: 2b. Cardiovascular ‐ ECG change.
4.13 Adverse effect: 2c. Cardiovascular ‐ Tachycardia (TESS)
Two studies (total n = 90) reported the number of people experiencing tachycardia. Overall, there was evidence that WDD + plus low‐dose antipsychotic had clearly fewer people reporting this side effect compared to those in the normal‐dose antipsychotic groups (RR 0.33, 95% CI 0.12 to 0.94, Analysis 4.13). There are two subgroups for this outcome.
4.13. Analysis.
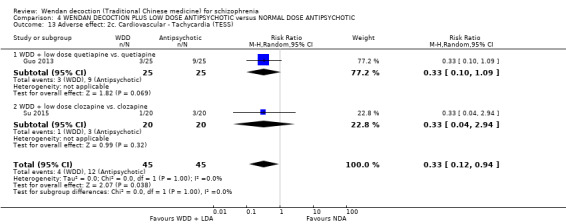
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 13 Adverse effect: 2c. Cardiovascular ‐ Tachycardia (TESS).
4.13.1 WDD + low‐dose quetiapine versus quetiapine
One trial reported data for this subgroup, which included a total of 50 participants. There was no evidence of a clear difference between the two treatments (RR 0.33, 95% CI 0.10 to 1.09).
4.13.2 WDD + low‐dose clozapine versus clozapine
One trial reported data for this subgroup (total n = 40). There was no clear difference between the two treatments (RR 0.33, 95% CI 0.04 to 2.94).
4.14 Adverse effect: 3a.i. Central Nervous System ‐ Arousal ‐ Anxious (TESS)
One trial (total n = 60) reported the number of people who became anxious. There was no evidence of a clear difference between WDD + low does clozapine and clozapine alone groups (RR 0.56, 95% CI 0.15 to 2.14, Analysis 4.14).
4.14. Analysis.
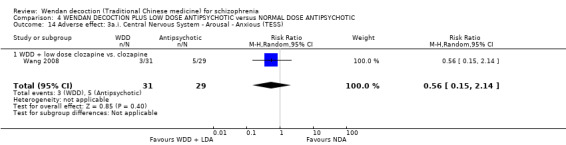
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 14 Adverse effect: 3a.i. Central Nervous System ‐ Arousal ‐ Anxious (TESS).
4.15 Adverse effect: 3a.ii. Central Nervous System ‐ Arousal ‐ Excitement (TESS)
One trial (total n = 60) reported the number of participants who showed excitement. There was no evidence of a clear difference between WDD + low‐dose clozapine and clozapine alone groups (RR 1.87, 95% CI 0.18 to 19.55, Analysis 4.15).
4.15. Analysis.
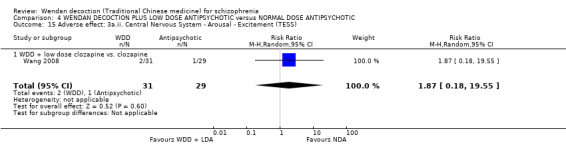
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 15 Adverse effect: 3a.ii. Central Nervous System ‐ Arousal ‐ Excitement (TESS).
4.16 Adverse effect: 3a.iii. Central Nervous System ‐ Arousal ‐ Insomnia (TESS)
Four trials (total n = 330) reported data for the number of people experiencing insomnia. Overall, there was evidence that WDD + plus low‐dose antipsychotic group had clearly fewer people experiencing insomnia compared to the antipsychotic alone groups (RR 0.53 CI 0.18 to 1.52, Analysis 4.16). This outcome had important levels of heterogeneity (I2 = 70%). There are three subgroups for this outcome.
4.16. Analysis.
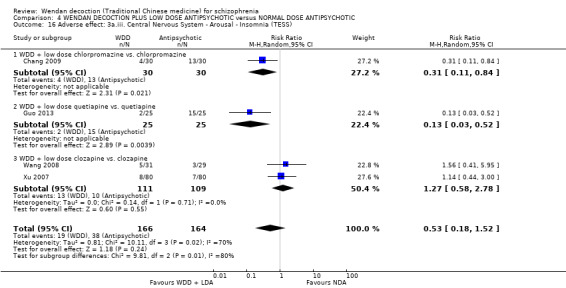
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 16 Adverse effect: 3a.iii. Central Nervous System ‐ Arousal ‐ Insomnia (TESS).
4.16.1 WDD + low‐dose chlorpromazine versus chlorpromazine
One trial reported data for this subgroup (total n = 60). There was evidence that WDD + low‐dose chlorpromazine had fewer people with insomnia than normal‐dose chlorpromazine alone (RR 0.31, 95% CI 0.11 to 0.84).
14.16.2 WDD + low‐dose quetiapine versus quetiapine
There was a single trial in this subgroup (total n = 50). There was evidence that WDD + low‐dose quetiapine group had fewer people with insomnia than normal‐dose quetiapine alone group (RR 0.13, 95% CI 0.03 to 0.52).
4.16.3 WDD + low‐dose clozapine versus clozapine
Two trials reported useable data for this subgroup, with a total of 220 people. There was no evidence of a clear difference between the two treatment groups (RR 1.27, 95% CI 0.58 to 2.78).
4.17 Adverse effect: 3a.iv. Central Nervous System ‐ Arousal ‐ Sleepiness (TESS)
One study ( n = 160) reported the number of people experiencing sleepiness. There was not a clear difference between the WDD + low‐dose clozapine group and clozapine alone group (RR 0.42, 95% CI 0.15 to 1.13, Analysis 4.17).
4.17. Analysis.
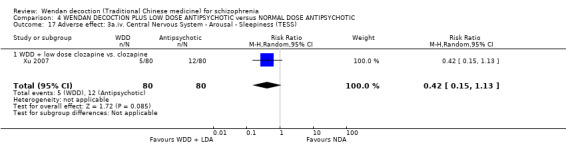
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 17 Adverse effect: 3a.iv. Central Nervous System ‐ Arousal ‐ Sleepiness (TESS).
4.18 Adverse effect: 3b. Central Nervous System ‐ Dizzy or headache(TESS)
Three trials ( n = 270) reported the number of people who became dizzy or experienced headache. Overall, there is no evidence of a clear difference between WDD + low‐dose antipsychotic and normal‐dose antipsychotic (RR 0.62, 95% CI 0.37 to 1.03, Analysis 4.18 ). There are two subgroups for this outcome.
4.18. Analysis.
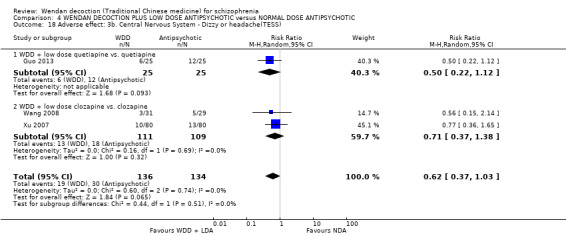
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 18 Adverse effect: 3b. Central Nervous System ‐ Dizzy or headache(TESS).
4.18.1 WDD + low‐dose quetiapine versus quetiapine
There was a single trial in this subgroup, with a total of 50 people. There was not a clear difference between WDD + low‐dose quetiapine and quetiapine alone (RR 0.50, 95% CI 0.22 to 1.12).
4.18.2 WDD + low‐dose clozapine versus clozapine
There were two relevant trials in this subgroup, with a total of 220 people. There was no evidence of a clear difference between WDD + low‐dose clozapine and clozapine alone (RR 0.71, 95% CI 0.37 to 1.38).
4.19 Adverse effect: 3c. Central Nervous System ‐ EEG change
One trial (total n = 160) reported the number of participants with observed EEG change. There was not a clear difference between the WDD + low‐dose clozapine and clozapine alone (RR 0.33, 95% CI 0.07 to 1.60, Analysis 4.19).
4.19. Analysis.
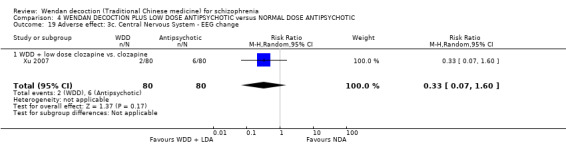
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 19 Adverse effect: 3c. Central Nervous System ‐ EEG change.
4.20 Adverse effect: 4a. Movement disorders ‐ EPS (TESS)
Three trials, (total n = 380), reported data for the number of participants experiencing EPS. Overall, there was evidence of a positive effect for WDD + low‐dose antipsychotic and normal‐dose antipsychotics (RR 0.29, 95% CI 0.16 to 0.51, moderate‐quality evidence, Analysis 4.20. There are two subgroups for this outcome.
4.20. Analysis.
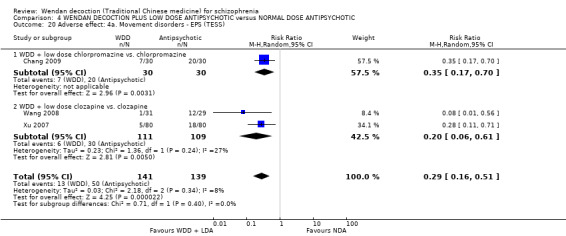
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 20 Adverse effect: 4a. Movement disorders ‐ EPS (TESS).
4.20.1 WDD + low‐dose chlorpromazine versus chlorpromazine
There was a single trial in this subgroup, with a total of 60 people. There was evidence that WDD + low‐dose chlorpromazine had fewer people with side effects of EPS than normal‐dose chlorpromazine alone (RR 0.35, 95% CI 0.17 to 0.70).
4.20.2 WDD + low‐dose clozapine versus clozapine
Two trials reported useable data for this subgroup, which included a total of 220 participants. There was evidence that WDD + low‐dose clozapine had fewer people with side effects of EPS than normal‐dose clozapine alone (RR 0.20, 95% CI 0.06 to 0.61).
4.21 Adverse effect: 4b. Movement disorders ‐ Tardive dyskinesia (TESS)
One trial ( n = 40) reported the number of people experiencing tardive dyskinesia as a result of treatment. There was not a clear difference between the WDD + low‐dose clozapine and clozapine alone groups (RR 0.33, 95% CI 0.01 to 7.72, Analysis 4.21).
4.21. Analysis.
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 21 Adverse effect: 4b. Movement disorders ‐ Tardive dyskinesia (TESS).
4.22 Adverse effect: 4c. Movement disorders ‐ Tremble (TESS)
One trial (total n = 50) reported the number of participants with 'tremble'. There was evidence that the WDD + low‐dose quetiapine group had clearly fewer people with 'tremble' than in the normal‐dose quetiapine alone group (RR 0.18, 95% CI 0.04 to 0.74, Analysis 4.22).
4.22. Analysis.
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 22 Adverse effect: 4c. Movement disorders ‐ Tremble (TESS).
4.23 Adverse effect: 5a. Gastrointestinal ‐ Constipation (TESS)
Four trials (total n = 330) reported the number of participants with constipation as a result of treatment. Overall, there was evidence that clearly fewer participants in the WDD + low‐dose antipsychotic groups experienced constipation compared to the antipsychotic alone groups (RR 0.26, 95% CI 0.09 to 0.78, Analysis 4.23). For this outcome heterogeneity is high (I2 = 64%). There are three subgroups for this outcome.
4.23. Analysis.
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 23 Adverse effect: 5a. Gastrointestinal ‐ Constipation (TESS).
4.23.1 WDD + low‐dose chlorpromazine versus chlorpromazine
There was a single trial in this subgroup, with a total of 60 people. There was evidence that WDD + low‐dose chlorpromazine had clearly fewer people with constipation than the normal‐dose chlorpromazine alone group (RR 0.43, 95% CI 0.24 to 0.78).
4.23.2 WDD + low‐dose quetiapine versus quetiapine
There was a single trial in this subgroup (total n = 50). There was evidence that WDD + low‐dose quetiapine had clearly fewer people with constipation than normal‐dose quetiapine alone group (RR 0.05, 95% CI 0.00 to 0.86).
4.23.3 WDD + low‐dose clozapine versus clozapine
Two trials reported useable data for this subgroup, with a total of 220 people. There was no evidence of a clear difference between the two treatment groups (RR 0.21, 95% CI 0.02 to 2.71).
4.24 Adverse effect: 5b. Gastrointestinal ‐ Indigestion (TESS)
One trial (total n = 60) reported the number of participants with indigestion. There was no evidence that WDD + low‐dose clozapine was any different in its effects compared to clozapine alone (RR 0.47, 95% CI 0.04 to 4.89, Analysis 4.24).
4.24. Analysis.
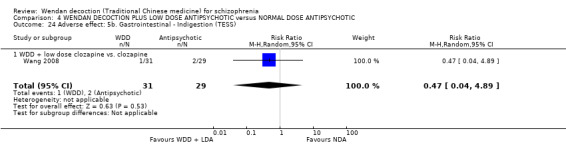
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 24 Adverse effect: 5b. Gastrointestinal ‐ Indigestion (TESS).
4.25 Adverse effect: 5c. Gastrointestinal ‐ Nausea and/or vomiting (TESS)
One trial (total n = 160) reported the number of participants with nausea and/or vomiting). There was no evidence that WDD + low‐dose clozapine was any different in its effects compared to clozapine alone (RR 0.75, 95% CI 0.27 to 2.06, Analysis 4.25).
4.25. Analysis.
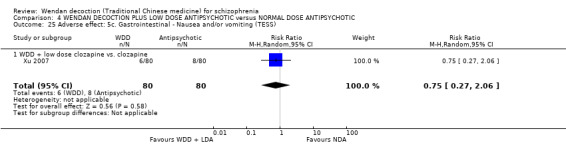
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 25 Adverse effect: 5c. Gastrointestinal ‐ Nausea and/or vomiting (TESS).
4.26 Adverse effect: 6. Other ‐ currently only for WDD + low‐dose clozapine versus clozapine (TESS)
Two trials (total n = 100) reported data for this outcome. Overall, there was no evidence that WDD + low‐dose clozapine was any different in its effects compared to clozapine alone (RR 0.65, 95% CI 0.19 to 2.14, Analysis 4.26,). There are two subgroups for this outcome.
4.26. Analysis.
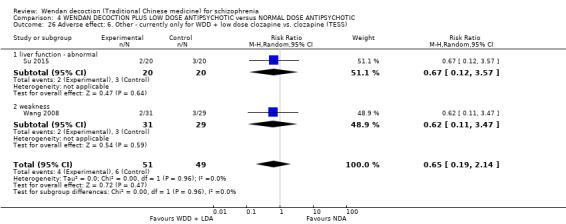
Comparison 4 WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC, Outcome 26 Adverse effect: 6. Other ‐ currently only for WDD + low dose clozapine vs. clozapine (TESS).
4.26.1 liver function ‐ abnormal
One trial reported data for this subgroup, which included a total of 40 participants. There was no evidence that WDD + low‐dose clozapine was any different in its effects compared to clozapine alone (RR 0.67, 95% CI 0.12 to 3.57).
4.26.2 weakness
There was a single trial in this subgroup, which included a total of 60 participants. There was no evidence that WDD + low‐dose clozapine was any different in its effects compared to clozapine alone (RR 0.62, 95% CI 0.11 to 3.47).
Discussion
Summary of main results
We were able to include 15 studies and all findings are, at the very least, of moderate quality. Two trials described the randomisation method in very limited terms and none their allocation concealment. No study stated that they used blinding. Most studies were short term and only one followed up participants to beyond six months. All studies were undertaken in China.
1. WENDAN DECOCTION versus PLACEBO OR NO TREATMENT for schizophrenia
Please see Table 1. We feel that this comparison does not often really represent usual care. People, if offered Wendan decoction (WDD), would usually be given it in the context of using other treatments such as antipsychotics.
1.1 Global state
It is interesting that there was the suggestion of some clinically important improvement over the effects of no treatment for this global outcome (RR 0.53 CI 0.39 to 0.73, low‐quality evidence), but these data are reported from one very small study (n = 72) with moderate risks of bias.
1.2 Other outcomes
We have no other data in the Table 1 for this comparison. We do not know if WDD really has any effect over no treatment or placebo on service use, quality of life, or even regarding whether it causes more adverse effects. Some people may not want Western medications and the value of WDD outside of Western treatments is certainly of interest but the data available give little clarity. The entirely unanswered questions are important, but, in our opinion, there are more important issues to be investigated first regarding this traditional Chinese medical approach for people with schizophrenia (please see below).
2. WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG for schizophrenia
Please see Table 2. Again, we feel that this comparison does not often really represent usual care. People, if offered WDD, would usually be given as an adjunct to other treatments.
2.1 Global state
For this outcome, it seems that treatment with WDD performed similarly to antipsychotics for people with schizophrenia. Two included trials employed different antipsychotics for control: Chang 2009 chlorpromazine; Dou 2012 risperidone. Because, the confidence intervals were quite tight and the global measure of a convincing degree of improvement (< 50% reduction in PANSS), this finding is interesting, with a moderate quality, needs replicated in a way that has potential to exclude more biases. Moreover, when the global measure employed a wide degree of improvement (< 25% reduction in PANSS), WDD seems to have had smaller overall effects than antipsychotics. Both findings suggested the complicated state of the treatment‐taking effects for people with schizophrenia, more evidence is needed to reach a robust and consistent result.
2.2 Mental state
There is low‐quality evidence to suggest that there is no difference in the change of average PANSS total scores observed between WDD (modified) and antipsychotics (total n = 140). Again, this finding is based on relatively few people over a short period of time in trials that are ‐ at the very least ‐ at moderate risk of inclusion of bias. These two outcomes, however, do suggest that the WDD approach, in itself, may be antipsychotic.
2.3 Adverse effects
Extrapyramidal symptoms (EPS) are drug‐induced movement disorders that include acute and tardive symptoms, and are most commonly caused by antipsychotic drugs that antagonise dopamine D2 receptors. In these trials, WDD showed a potential for less EPS than antipsychotic drugs and this result is based on moderate‐quality evidence. WDD caused no EPS while antipsychotics (chlorpromazine and risperidone) induced 67% of people to experience and report them. This finding ‐ again with a moderate quality ‐ is based on two small studies (total N = 140), so has to be treated with a degree of caution.
2.4 Other outcomes
We have no more data to assess the effects on service use, quality of life and economic outcomes of WDD for people with schizophrenia.
Should WDD be both as effective as antipsychotics in improving symptoms and not cause movement disorders, this could be most important. This type of study also should be replicated and expanded using highest standards of trial conduct.
3. WENDAN DECOCTION PLUS NORMAL‐DOSE ANTIPSYCHOTIC versus NORMAL‐DOSE ANTIPSYCHOTIC for schizophrenia
Please see Table 3. Studies included in this comparison added WDD to a standard dose of antipsychotic drugs and compared this combination with the same drugs alone.
3.1 Global state
Several trials (six RCTs, n = 684) supported that WDD could enhance the effects of antipsychotics such as risperidone, ziprasidone, aripiprazole, olanzapine, on global state (PANSS < 50% reduction) of patients of schizophrenia (RR 0.6 CI 0.50 to 0.72, moderate‐quality evidence). This is an interesting and encouraging finding, which suggests that the clinical effects of the current first‐line drugs are not perfect and some added approaches, such as traditional Chinese medicine, could be used to improve efficacy and reduce adverse effects.
3.2 Mental state
Also, WDD showed similar enhancement in the effects of drugs on mental state of average PANSS total score (five RCTs, N = 580, MD ‐11.64 CI ‐13.33 to ‐9.94, moderate‐quality evidence). These findings are encouraging ‐ although what exactly a 12‐point reduction means is difficult to put into words ‐ but the high heterogeneity (61%) weakens the findings; the latter probably being due to the different antipsychotic drugs being used.
3.3 Adverse effects
The antipsychotics listed above do cause extrapyramidal effects. Thirty five per cent of people given aripiprazole (Sun 2014) or one of a series of antipsychotic drugs (quetiapine, olanzapine, perphenazine, risperidone and clozapine, Shao 2014) do experience these problematic adverse effects. Limited evidence suggested that WDD, when used as companion to antipsychotics, the Chinese medicine may reduce these effects. We realise that this finding is based only on two relatively small studies (n = 308) (moderate‐quality evidence), but it does remain an important finding. This needs to be replicated, but is genuinely encouraging that WDD can be of value not only for enhancing global/mental state but also for offsetting the adverse effects.
Wendan Decoction showed no effect on the effect of weight gain induced by antipsychotics. However, any subsequent trial should, nevertheless, report on this again to add to the total data on this topic.
3.4 Use of antipsychotic: Drug dose at the endpoint
Wendan decoction reduced drug dose of antipsychotic. This could be that the enhancement of the antipsychotic effect allowed the reduction and, again, this is, potentially an important finding that is well worth replication.
3.5 Other outcomes
We have no more data to assess the effects on service use, quality of life and economic outcomes of WDD for people with schizophrenia.
4. WENDAN DECOCTION PLUS LOW‐DOSE ANTIPSYCHOTIC versus NORMAL‐DOSE ANTIPSYCHOTIC for schizophrenia
Please see Table 4. These studies used various antipsychotics to compare adding WDD to around half the standard dose of the antipsychotic drug with use of the standard dose of the same antipsychotic alone. These trials address a common question for how WDD is used for people with schizophrenia in China.
4.1 Global state
Wendan decoction plus low‐dose drugs performed better than full‐dose antipsychotic drugs for improving the global state (PANSS < 50% reduction) of schizophrenia at short term (n = 522, 7 RCTs, RR 0.69 CI 0.51 to 0.93, moderate‐quality evidence), and one study found an effect at long term (n = 72, 1 RCT, RR 0.27 CI 0.08 to 0.90). This does point to the possibility that WDD could be used to reducing use of Western antipsychotic drugs.
4.2 Mental state
Again, WDD plus low‐dose drugs does seem to reduce measures of disturbed mental state from between 18 and two points on the PANSS score (moderate‐quality evidence). It is hard to know what this really means clinically. It could be a considerable improvement if the person got this improvement right where they needed it. However, if spread right across the mental state, small declines here and there could well have little clinical impact and be only detectable by use of fine‐grain measures. However, these small trials do suggest that WDD is of some value. There are so few things that really are of value for treatment of this difficult illness or set of illnesses that such a finding must generate more scientific curiosity.
4.3 Adverse effects
Wendan decoction plus low‐dose drugs caused less EPS effects (13/141, 9%) compared with full‐dose drugs (50/139, 36%), but it is difficult to know if this is induced by the WDD or by the reduction of drug doses. No new adverse effects seem to be added by the WDD. Again, we felt these findings to be of moderate quality ‐ meaning further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
4.4 Other outcomes
We have no more data to assess the effects on service use, quality of life and economic outcomes of WDD for people with schizophrenia.
Overall completeness and applicability of evidence
1. Completeness
Fifteen studies were included in this review with a total of 1437 participants. WDD has been compared with no treatment, with antipsychotic drug, and WDD plus normal‐/low‐dose antipsychotic with normal‐dose antipsychotic. We found that many important outcomes were not reported by the trialists ‐ such as death, engagement with services, satisfaction with treatment, quality of life or economic outcomes. Most of these missing outcomes are participant‐oriented data. Only one study reported the specific outcome of use of antipsychotic ‐ drug dose at the endpoint (Li 2013), which is a key issue when considering the use of WDD. There is a place for wider agreement about the need for specific and clinically important outcomes, and how to report these in some way that is consistent across studies (COMET).
2. Applicability
All 15 included studies were complete and published in the Chinese language between 2005 and 2015, in China. Also, although the great majority of people with schizophrenia are in the community, all included studies were undertaken in hospital settings. Certainly, findings of this review may be more applicable to people in hospital in China, where care can be more regulated. However, it is difficult to see how findings would be inapplicable to much wider groups of people in the community. Also millions of Chinese people are dispersed around the globe and, when unwell, consult practitioners of TCM, perhaps especially more often before doctors working in Western medicine when it comes to mental disorders. TCM practitioners are likely to use some form of WDD, even first line, for schizophrenia‐like illnesses.
Quality of the evidence
Overall, the quality was not strong (Figure 3 and Figure 4). No study adhered to the CONSORT statement; all but two of the included studies did not describe how they undertook the randomisation procedure and none reported blindness. Schizophrenia is a chronic illness but most studies reported short‐term data. We have probably been generous in our judgements regarding quality and there is a risk that we, in doing this, have downplayed an additional real risk of bias in this group of studies, and therefore in this review.
Potential biases in the review process
1. Our methods
We used thorough search strategies and followed review protocol in the process of study selection, data extraction and analyses. We did employ only published reports and could not entirely avoid the potential for publishing bias for negative results and small studies. We tried to contact the authors to find more details about their studies, and, to date, had not one reply. We will update this review in the light of any new evidence.
We also recognise that using WDD within the Western paradigm of the randomised trial for such a specific condition as a Western diagnosis of schizophrenia is not really how this treatment is supposed to be used. This narrow application and confining methodology may underplay a broader value of WDD in the 'real world' ‐ although, conversely, it also does tell Western doctors something about the modest but intriguing effects for people with schizophrenia.
2. The authors
Both review authors have a strong affiliation to the idea of the traditional Chinese medicines being effective. This could have biased our view of the data. As an effort try to resolve this, we adhered strictly to the review protocol. This protocol, along with working closely with the Cochrane Schizophrenia Group's editorial base, protected the review from inclusion of personal biases.
Agreements and disagreements with other studies or reviews
We identified one other review of WDD for schizophrenia (Che 2016). This other review concluded that WDD appears to be effective on improving symptoms in people with schizophrenia. It also remarked that, due to poor methodological quality in the majority of the included trials, the potential benefit from WDD needs to be confirmed in rigorous trials. Finally, it stated that the design and reporting of trials should follow international standards. We fully agree with this view.
Authors' conclusions
Implications for practice.
We would like to reiterate that all the conclusions and recommendations are based on the limited and not very robust evidence currently available to us.
1. For people with schizophrenia
The level of evidence is clearly not as good as it could be. We really do not know if Wendan decoction (WDD) has any effect on important outcomes of daily functioning. There are hints from proxy measures of mental state that there may be positive effects on functioning and we have no evidence at all that there are negative effects. It may well be that those people with schizophrenia negotiate with their carer to see if this approach works for them over an agreed time period. One step further is that it does not seem that unreasonable that people with schizophrenia or their families expect their professional carers to generate better evidence about the effects of their care ‐ and could assist them in doing so.
It may be that the best group to target with WDD would be people who have schizophrenia but also troubling adverse effects.
2. For clinicians
Probably the most encouraging findings were when WDD was added to the Western medicines and seemed to increase the antipsychotic effect as well as reducing the adverse results of these drugs. Certainly, more research does need to be undertaken in this area. However, currently the best available evidence does encourage doctors wanting to increase the antipsychotic effects of the drugs whilst reducing the antipsychotic drugs' adverse effects to try WDD ‐ if, available and acceptable to patients and their families.
For doctors not familiar with traditional Chinese medicines, should a person with schizophrenia already be treated with WDD, the authors of this review see no clear reason for this treatment to be discontinued.
3. For policymakers
Traditional Chinese medicine (TCM) as a valuable complementary medicine is gradually affecting global medical treatment systems. This traditional treatment originating from ancient Chinese culture needs more real‐word trials to evaluate the treatment method objectively and completely, not limited to assessing treatment effects and adverse effects, but also focusing on social and economic conditions.
Implications for research.
1. General
All studies should now comply with the Consolidated Standards of Reporting Trials (CONSORT, Moher 2001). More transparency in the reporting of randomised controlled trials, would enable readers to understand the design, conduct, analysis and interpretation, and to assess the validity of results. Binary data are easier to interpret. Where continuous data are used, some measure of variance should be provided. Data presented in graphs should be accompanied by exact numbers and standard deviations in the text.
2. Specific
2.1 Reviews
We had thought that perhaps excluded trials might suggest other similar reviews to be undertaken. This was not the case as the search was so specific. However, we did discover that use of WDD has generated ‐ at least ‐ four comparisons, all of which we included in this one review. Should more evidence come to light across time there may be a place for fragmentation of this review into several reviews.
2.2 Trials
TCM is an important treatment approach that is likely to be widely used, at least, in China or on the huge Chinese Diaspora. Considering the limited data in this review, we do think that further well‐designed trials are required. We think, until now, the best place to carry out more studies in this field is China. There are, however, specific issues to consider.
2.2.1 Large, good trials
The number of participants reported in 15 included studies averaged 96 (range 39 to 200). Considering the potential importance of this topic, both in the 'East' and 'West' it would seem that there could be far larger studies. The cumulative total in some of the meta‐analyses in this review do include hundreds of people but all studies are limited as regards their risk of bias. One good study with 150 people in each arm, reporting clear outcomes in a unbiased way, would be of great value. Today's China does not suffer from the lack of basic conditions that was a problem even in the recent past. Funding and patient recruitment to allow the conduct of large sample clinical trials is possible to find. However, clinical researchers and policy makers have to be interested and supportive. Other topics may be considered more important and of global interest but the questions posed in this review do suggest that there is genuine clinical value to be gained by setting the issues regarding WDD.
2.2.2 More interesting outcomes, and longer duration follow‐up
Currently, limited evidence has suggested that TCM (WDD) may have some antipsychotic effects as measured on global and mental state, and has shown good features and potential in reducing the side effects induced by antipsychotic drugs. However, we could not find sufficient data to assess the effects on service use, quality of life and economic outcomes of WDD for people with schizophrenia. Meanwhile, there is only one study that reported medium‐ and long‐term follow‐up, which looks odd under the background of schizophrenia, which is a disease that routinely requires lifelong medication.
2.2.3 Investigate the differences between various forms of WDD
We had planned to explore the different effects of various forms of WDD in this review, but failed to find any relevant data focusing on that specific question. This resulted in us considering why this should be? One possible reason, based on TCM thinking, is that 'changeable' should be considered, in keeping with the nature of every prescription in TCM. The review authors (both experienced TCM practitioners) consider that little attention has been focused on the various forms of TCM. This reason may be hard to understand and accepted by those who are not familiar with TCM theory. We are anticipating further studies that will investigate the differences between various forms of WDD in the treatment of schizophrenia in the future.
We do realise that much time and effort has to be invested in designing a good trial. However, we have invested some of this time in considering those studies which have been undertaken. Considering the limited data in this review, we do think that further large simple trials are indicated. We suggest an outline for a trial in Table 10.
6. Suggested design for a trial.
| Methods | Allocation: randomised, clearly described, concealed.
Blindness: double, described and tested.
Duration: 4‐week washout period + 24 weeks treatment period. Follow‐up: 2 years. |
| Participants | Diagnosis: schizophrenia (DSM V) with one TCM type according to TCM diagnosis standard. N = 300*. Age: any. Sex: both. History: duration of schizophrenia over 1 years. |
| Interventions | 1. Wendan decoction: N = 150.
2. Sham decoction**: N = 150. The blend of herbs and their dosages clearly described. |
| Outcomes | 1. Global state: CGI ‐ clinically important changes. 2. Mental state: BPRS ‐ changes in mental state. 3. Behaviour: clinically important changes in general behaviour. 4. Service outcomes: hospitalisation, time in hospital, time until readmission. 5. Adverse effects: clinically important general adverse effects. 6. Engagement with service: clinically important engagement. 7. Satisfaction with treatment: recipient of care not satisfied with treatment. 8. Quality of life: clinically important changes in quality of life. 9. Economic outcome: costs of care. |
| Notes | * Powered to be able to identify a difference of ˜20% between groups for primary outcome with adequate degree of certainty. **In case of administering WDD in the form of decoction, the sham decoction should be mixed with flavourings and edible pigments. Further, we suggested WDD administered in the form of powder into capsule, and placebo in capsule as control, which should be a better way to blind. |
BPRS: Brief Psychiatric Rating Scale CGI: Clinical Global Impression Scale DSM: Diagnostic and Statistical Manual TCM: Traditional Chinese medicine WDD: Wendan decoction
Acknowledgements
We are grateful to Professor Clive E Adams and Ms. Jun Xia from University of Nottingham for their help in methodology and translating. We would like to thank Timothy Kariotis and Winnie Wing Sze Mak for peer reviewing this version of the review.
The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required.
Parts of this review were generated using RevManHAL v 4.2. You can find more information about RevManHAL here.
The search strategy was developed by the Information Specialist of the Cochrane Schizophrenia Group.
Data and analyses
Comparison 1. WENDAN DECOCTION versus PLACEBO OR NO TREATMENT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction) | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.39, 0.73] |
| 1.1 short term | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.39, 0.73] |
| 2 Global state: 1b. No improvement (PANSS < 25% reduction) | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.04, 0.26] |
| 2.1 short term | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.04, 0.26] |
Comparison 2. WENDAN DECOCTION versus ANTIPSYCHOTIC/BENZODIAZEPINE DRUG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction) | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.98, 1.43] |
| 1.1 versus chlorpromazine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.90, 1.58] |
| 1.2 versus risperidone | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.92, 1.51] |
| 2 Global state: 1b. No improvement (PANSS < 25% reduction) | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.21, 4.93] |
| 2.1 versus chlorpromazine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.5 [0.88, 7.10] |
| 2.2 versus risperidone | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.4 [0.93, 6.19] |
| 3 Mental state: 1a. Average positive score (endpoint, PANSS, high score = bad) | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐4.97, 2.48] |
| 3.1 versus chlorpromazine | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.69, 3.09] |
| 3.2 versus risperidone | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐5.19, ‐1.01] |
| 4 Mental state: 1b. Average negative score (endpoint, PANSS, high score = bad) | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐2.08, 0.91] |
| 4.1 versus chlorpromazine | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐3.46, 1.06] |
| 4.2 versus risperidone | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.10, 1.90] |
| 5 Mental state: 1c. Average total score (endpoint, PANSS, high score = bad) | 2 | 140 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐4.17, 5.84] |
| 5.1 versus chlorpromazine | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐5.64, 9.44] |
| 5.2 versus risperidone | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐6.69, 6.69] |
| 6 Adverse effect: 1. Anticholinergic ‐ Dry mouth (TESS) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.28] |
| 6.1 versus risperidone | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.28] |
| 7 Adverse effect: 2a. Central Nervous System ‐ Insomnia (TESS) | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.11, 0.50] |
| 7.1 versus chlorpromazine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.07, 0.73] |
| 7.2 versus risperidone | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.09, 0.64] |
| 8 Adverse effect: 2b. Central nervous system ‐ Sleep time < 8 hours | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.42, 1.58] |
| 9 Adverse effect: 2c. Central nervous system ‐ No change in sleep time | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.14, 2.98] |
| 10 Adverse effect: 3. Gastrointenstinal ‐ Constipation (TESS) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.37] |
| 10.1 versus chlorpromazine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.37] |
| 11 Adverse effect: 4. Movement disorders ‐ EPS (TESS) | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.15] |
| 11.1 versus chlorpromazine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.39] |
| 11.2 versus risperidone | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.29] |
Comparison 3. WENDAN DECOCTION PLUS NORMAL DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction) | 6 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.50, 0.72] |
| 1.1 WDD + risperidone vs. risperidone | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.93] |
| 1.2 WDD + ziprasidone vs. ziprasidone | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.08, 0.84] |
| 1.3 WDD + antipsychotic vs. antipsychotic | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.81] |
| 1.4 WDD + aripiprazole vs. aripiprazole | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.36, 1.01] |
| 1.5 WDD + olanzapine vs. olanzapine | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.27, 1.21] |
| 2 Global state: 1b. No improvement (PANSS < 25% reduction) | 6 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.14, 0.40] |
| 2.1 WDD + risperidone vs. risperidone | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.11, 0.52] |
| 2.2 WDD + ziprasidone vs. ziprasidone | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.68] |
| 2.3 WDD + antipsychotic vs. antipsychotic | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.07, 0.56] |
| 2.4 WDD + aripiprazole vs. aripiprazole | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.92] |
| 2.5 WDD + olanzapine vs. olanzapine | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.29] |
| 3 Global state: 2. TCM syndromes no improvement (TCMSS < 30% reduction) | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.12, 0.52] |
| 3.1 WDD + risperidone vs. risperidone | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.12, 0.52] |
| 4 Mental state: 1a. Average positive score (endpoint, PANSS, high score = bad) | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.70, ‐0.26] |
| 4.1 WDD + antipsychotic vs. antipsychotic | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.70, ‐0.26] |
| 5 Mental state: 1b. Average negative score (endpoint, PANSS, high score = bad) | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐4.47 [‐5.05, ‐3.89] |
| 5.1 WDD + antipsychotic vs. antipsychotic | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐4.47 [‐5.05, ‐3.89] |
| 6 Mental state: 1c. Average total score (endpoint, PANSS, high score = bad) | 5 | 580 | Mean Difference (IV, Random, 95% CI) | ‐11.64 [‐13.33, ‐9.94] |
| 6.1 WDD + antipsychotic vs. antipsychotic | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐11.47 [‐13.01, ‐9.93] |
| 6.2 WDD + aripiprazole vs. aripiprazole | 1 | 108 | Mean Difference (IV, Random, 95% CI) | ‐11.0 [‐13.35, ‐8.65] |
| 6.3 WDD + olanzapine vs. olanzapine | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐15.17 [‐18.23, ‐12.11] |
| 6.4 WDD + risperidone vs. risperidone | 1 | 107 | Mean Difference (IV, Random, 95% CI) | ‐7.0 [‐11.19, ‐2.81] |
| 6.5 WDD + ziprasidone vs. ziprasidone | 1 | 87 | Mean Difference (IV, Random, 95% CI) | ‐12.10 [‐13.91, ‐10.29] |
| 7 Mental state: 2. Average total score (endpoint, BPRS, high score = bad) | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐4.92 [‐6.18, ‐3.66] |
| 7.1 WDD + antipsychotic vs. antipsychotic | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐4.92 [‐6.18, ‐3.66] |
| 8 Mental state: 3. Average total score (endpoint, CDSS) | 1 | 87 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐2.11, ‐0.89] |
| 8.1 WDD + ziprasidone vs. ziprasidone | 1 | 87 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐2.11, ‐0.89] |
| 9 Adverse effect: 1. Movement disorders ‐ EPS (TESS) | 2 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.30, 0.70] |
| 9.1 WDD + antipsychotic vs. antipsychotic | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.28, 0.70] |
| 9.2 WDD + aripiprazole vs. aripiprazole | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.22, 1.37] |
| 10 Adverse effect: 2a. Gastrointenstinal ‐ Abnormal Liver Function | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.73] |
| 10.1 WDD + antipsychotic vs. antipsychotic | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.73] |
| 11 Adverse effect: 2b. Gastrointenstinal ‐ Constipation (TESS) | 2 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 4.44] |
| 11.1 WDD + antipsychotic vs. antipsychotic | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.16] |
| 11.2 WDD + aripiprazole vs. aripiprazole | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.09, 0.75] |
| 12 Adverse effect: 3. Metabolic ‐ Weight gain (TESS) | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.20, 1.24] |
| 12.1 WDD + aripiprazole vs. aripiprazole | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.20, 1.24] |
| 13 Use of antipsychotic: Drug dose at the end point | 1 | 107 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.87, ‐0.53] |
| 13.1 WDD + risperidone vs. risperidone | 1 | 107 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.87, ‐0.53] |
Comparison 4. WENDAN DECOCTION PLUS LOW DOSE ANTIPSYCHOTIC versus NORMAL DOSE ANTIPSYCHOTIC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically important improvement (PANSS < 50% reduction) ‐ short term | 7 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 1.1 WDD + low dose chlorpromazine vs. chlorpromazine (short term) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.24, 0.78] |
| 1.2 WDD + low dose clozapine vs. clozapine (short term) | 3 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.56, 1.27] |
| 1.3 WDD + low dose quetiapine vs. quetiapine (short term) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.43, 1.15] |
| 1.4 WDD + low dose risperidone vs. risperidone (short term) | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.29, 1.49] |
| 2 Global state: 1b. No clinically important improvement (PANSS < 50% reduction) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 WDD + low dose risperidone vs. risperidone (medium term) | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.59, 1.27] |
| 2.2 WDD + low dose risperidone vs. risperidone (long term) | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.90] |
| 3 Global state: 1c. No improvement (PANSS < 25% reduction) ‐ short term | 7 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.43, 0.90] |
| 3.1 WDD + low dose chlorpromazine vs. chlorpromazine (short term) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.98] |
| 3.2 WDD + low dose clozapine vs. clozapine (short term) | 3 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.36, 1.53] |
| 3.3 WDD + low dose quetiapine vs. quetiapine (short term) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.07, 1.24] |
| 3.4 WDD + low dose risperidone vs. risperidone (short term) | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.06, 2.68] |
| 4 Global state: 1d. No improvement (PANSS < 25% reduction) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 WDD + low dose risperidone vs. risperidone (medium term) | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.28] |
| 4.2 WDD + low dose risperidone vs. risperidone (long term) | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.90] |
| 5 Mental state: 1a. Average positive score (endpoint, PANSS, high score = bad) | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐6.52 [‐10.22, ‐2.82] |
| 5.1 WDD + low dose chlorpromazine vs. chlorpromazine | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐6.69, ‐1.91] |
| 5.2 WDD + low dose quetiapine vs. quetiapine | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐5.1 [‐7.70, ‐2.50] |
| 5.3 WDD + low dose risperidone vs. risperidone | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐12.09, ‐7.91] |
| 6 ntal state: 1b. Average negative score (endpoint, PANSS, high score = bad) | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐4.64 [‐7.45, ‐1.82] |
| 6.1 WDD + low dose chlorpromazine vs. chlorpromazine | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐6.53, ‐0.67] |
| 6.2 WDD + low dose quetiapine vs. quetiapine | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐5.87, 0.47] |
| 6.3 WDD + low dose risperidone vs. risperidone | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐7.10 [‐9.37, ‐4.83] |
| 7 Mental state: 1c. Average total score (endpoint, PANSS, high score = bad) | 4 | 250 | Mean Difference (IV, Random, 95% CI) | ‐9.53 [‐17.82, ‐1.24] |
| 7.1 WDD + low dose chlorpromazine vs. chlorpromazine | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐9.10 [‐17.12, ‐1.08] |
| 7.2 WDD + low dose clozapine vs. clozapine | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐7.53, 7.95] |
| 7.3 WDD + low dose quetiapine vs. quetiapine | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐9.60 [‐18.25, ‐0.95] |
| 7.4 WDD + low dose risperidone vs. risperidone | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐19.1 [‐25.84, ‐12.36] |
| 8 Mental state: 2. Average total score (endpoint, BPRS, high score = bad) | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.29, 0.89] |
| 8.1 WDD + low dose risperidone vs. risperidone | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.29, 0.89] |
| 9 Adverse effect: 1a. Anticholinergic ‐ Dry mouth (TESS) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.72] |
| 9.1 WDD + low dose risperidone vs. risperidone | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.72] |
| 10 Adverse effect: 1b. Anticholinergic ‐ Salivation (TESS) | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.05, 0.30] |
| 10.1 WDD + low dose clozapine vs. clozapine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.36] |
| 10.2 WDD + low dose quetiapine vs. quetiapine | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.05, 0.44] |
| 11 Adverse effect: 2a. Cardiovascular ‐ Drop in blood pressure | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.10, 1.34] |
| 11.1 WDD + low dose clozapine vs. clozapine | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.10, 1.34] |
| 12 Adverse effect: 2b. Cardiovascular ‐ ECG change | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.72] |
| 12.1 WDD + low dose clozapine vs. clozapine | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.72] |
| 13 Adverse effect: 2c. Cardiovascular ‐ Tachycardia (TESS) | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.12, 0.94] |
| 13.1 WDD + low dose quetiapine vs. quetiapine | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.10, 1.09] |
| 13.2 WDD + low dose clozapine vs. clozapine | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.94] |
| 14 Adverse effect: 3a.i. Central Nervous System ‐ Arousal ‐ Anxious (TESS) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.15, 2.14] |
| 14.1 WDD + low dose clozapine vs. clozapine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.15, 2.14] |
| 15 Adverse effect: 3a.ii. Central Nervous System ‐ Arousal ‐ Excitement (TESS) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.18, 19.55] |
| 15.1 WDD + low dose clozapine vs. clozapine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.18, 19.55] |
| 16 Adverse effect: 3a.iii. Central Nervous System ‐ Arousal ‐ Insomnia (TESS) | 4 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.18, 1.52] |
| 16.1 WDD + low dose chlorpromazine vs. chlorpromazine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.84] |
| 16.2 WDD + low dose quetiapine vs. quetiapine | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.03, 0.52] |
| 16.3 WDD + low dose clozapine vs. clozapine | 2 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.58, 2.78] |
| 17 Adverse effect: 3a.iv. Central Nervous System ‐ Arousal ‐ Sleepiness (TESS) | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.15, 1.13] |
| 17.1 WDD + low dose clozapine vs. clozapine | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.15, 1.13] |
| 18 Adverse effect: 3b. Central Nervous System ‐ Dizzy or headache(TESS) | 3 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.03] |
| 18.1 WDD + low dose quetiapine vs. quetiapine | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.22, 1.12] |
| 18.2 WDD + low dose clozapine vs. clozapine | 2 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.38] |
| 19 Adverse effect: 3c. Central Nervous System ‐ EEG change | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.07, 1.60] |
| 19.1 WDD + low dose clozapine vs. clozapine | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.07, 1.60] |
| 20 Adverse effect: 4a. Movement disorders ‐ EPS (TESS) | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.16, 0.51] |
| 20.1 WDD + low dose chlorpromazine vs. chlorpromazine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.17, 0.70] |
| 20.2 WDD + low dose clozapine vs. clozapine | 2 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.06, 0.61] |
| 21 Adverse effect: 4b. Movement disorders ‐ Tardive dyskinesia (TESS) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.72] |
| 21.1 WDD + low dose clozapine vs. clozapine | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.72] |
| 22 Adverse effect: 4c. Movement disorders ‐ Tremble (TESS) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.74] |
| 22.1 tremble (WDD + low dose quetiapine vs. quetiapine) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.74] |
| 23 Adverse effect: 5a. Gastrointestinal ‐ Constipation (TESS) | 4 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.78] |
| 23.1 WDD + low dose chlorpromazine vs. chlorpromazine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.24, 0.78] |
| 23.2 WDD + low dose quetiapine vs. quetiapine | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.86] |
| 23.3 WDD + low dose clozapine vs. clozapine | 2 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.02, 2.71] |
| 24 Adverse effect: 5b. Gastrointestinal ‐ Indigestion (TESS) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 4.89] |
| 24.1 WDD + low dose clozapine vs. clozapine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 4.89] |
| 25 Adverse effect: 5c. Gastrointestinal ‐ Nausea and/or vomiting (TESS) | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.27, 2.06] |
| 25.1 WDD + low dose clozapine vs. clozapine | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.27, 2.06] |
| 26 Adverse effect: 6. Other ‐ currently only for WDD + low dose clozapine vs. clozapine (TESS) | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.19, 2.14] |
| 26.1 liver function ‐ abnormal | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.57] |
| 26.2 weakness | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.11, 3.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chang 2009.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 60 days. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3, PANSS> = 60). N = 90. Age: average ˜ 40 years SD ˜ 6. Sex: 55 men 35 women. History: average duration ill ˜ 20 years (SD ˜ 6); average length in hospital ˜ 8 years (SD ˜ 5). Inclusion criteria: not stated. Exclusion criteria: not stated. |
|
| Interventions | 1. WDD (please see details in Table 5): 60 days for a course. N = 30.
2. WDD + chlorpromazine: dose chlorpromazine 300 mg/d ± 7.65 mg/d, 60 days for a course. N = 30.
3. Chlorpromazine: dose chlorpromazine 600 ± 6.37 mg/d, 60 days for a course. N = 30. Anticholinergic drugs and benzodiazepines drugs used only for adverse effects, no other antipsychotic drugs. |
|
| Outcomes | Global state: cure (binary PANSS scores ‐ reduced > 50%, 20% to 50%, no effect ‐ < 20%). Mental state: PANSS negative subscale score, positive subscale score, PANSS total.* Adverse events: EPS, insomnia, constipation (TESS). Outcomes unable to use: Drug compliance: no numerical data. |
|
| Notes | * PANSS at 7, 14 and 28 days after treatment not used in this review (short‐term data used 42‐day outcomes). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group I, II, and control group…" We accept trialists' report of randomisation as true and accurate, hence rated this as unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods’ section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Dou 2012.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 60 days. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3, PANSS> = 60). N = 120. Age: average ˜ 40 years SD ˜ 8. Sex: 74 men 46 women. History: average duration ill ˜ 18 years (SD ˜ 7); average length in hospital ˜ 7 years (SD ˜ 5). Inclusion criteria: not stated. Exclusion criteria: not stated. |
|
| Interventions | 1. WDD (please see details in Table 5): 60 days for a course. N = 40.
2. WDD + risperidone: dose risperidone 3.10 mg/d ± 0.9 mg/d, 60 days for a course. N = 40.
3. Risperidone: dose risperidone 4.8 mg/d ± 1.2 mg/d, 60 days for a course. N = 40. Anticholinergic drugs and benzodiazepines drugs used only for adverse effects, no other antipsychotic drugs. |
|
| Outcomes | Global state: cure (binary PANSS scores ‐ reduced > 50%, 20% to 50%, no effect ‐ < 20%). Mental state: PANSS negative subscale score, positive subscale score, PANSS total.* Adverse events: EPS, insomnia, dry mouth (TESS). Outcomes unable to use: Drug compliance: no numerical data. |
|
| Notes | * PANSS at 1, 2 and 4 weeks after treatment not used in this review (short‐term data used 6‐week outcomes). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group I, II, and control group…" Comment: we accept trialists' report of randomisation as true and accurate, hence rated this as unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Guo 2013.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 6 weeks. |
|
| Participants | Diagnosis: schizophrenia (ICD‐10, PANSS> = 60). N = 50. Age: 18‐55 years. Sex: 24 men 26 women. History: not stated. Inclusion criteria: not stated. Exclusion criteria: not stated. |
|
| Interventions | 1. WDD (please see details in Table 5) + Quetiapine: dose quetiapine 400 mg/d to 600 mg/d, 6 weeks. N = 25.
2. Quetiapine: dose quetiapine 600 mg/d to 700 mg/d, 6 weeks. N = 25. Anticholinergic drugs and benzodiazepines drugs used only for adverse effects, no other antipsychotic drugs. |
|
| Outcomes | Global state: cure (binary PANSS scores ‐ reduced >75%, 50 % to 74%, 25% to 49%, no effect ‐ < 24%). Mental state: PANSS negative subscale score, positive subscale score, PANSS total.* Adverse events: salivation, insomnia, dizzy, tachycardia, trembling of hands, constipation (TESS). Outcomes unable to use: Drug compliance: no numerical data. |
|
| Notes | * PANSS at 1, 2 and 4 weeks after treatment not used in this review (short‐term data used 6‐week outcomes). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control group…" Comment: we accept trialists' report of randomisation as true and accurate, hence rated this as unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Huang 2005.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 10 days. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 90. Age: average ˜ 28 years SD ˜ 3. Sex: 62 men 28 women. History: average duration ill ˜ 4 years (SD ˜ 3). Inclusion criteria: CCMD‐3; Hard to fall into sleep or difficult to keep sleep or poor quality sleep, at least 3 times a week for more than one month. Exclusion criteria: Exclude patients with alcohol, drugs addicted and serious physical illness. |
|
| Interventions | 1. WDD (please see details in Table 5), 10 days. N = 55.
2. Estazolam: dose estazolam 2 mg/d, 10 days. N = 35. Both groups were treated with antipsychotics, equivalent chlorpromazine dose: 250 mg ± 150 mg (WDD group), 260 mg ± 180 mg (estazolam group). |
|
| Outcomes | Adverse effect: central nervous system (sleep). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control group…" Comment: we accept trialists' report of randomisation as true and accurate, hence rated this as unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Unclear risk | No dose stated. |
Li 2013.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 8 weeks. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3;Integrative Chinese and Western Medicine Classification of Schizophrenia). N = 107. Age: 18‐60 years. Sex: 53 men 54 women. History: average duration ill ˜ 1.8 years (SD ˜ 1.2). Inclusion criteria: CCMD‐3;Integrative Chinese and Western Medicine Classification of Schizophrenia; Aged 18 to 60, no sex limitations; Junior high school or higher educated; First onset; Without systemic antipsychotic treatment; PANSS > = 60. Exclusion criteria: Been treated with systemic antipsychotics; suffer serious physical illness and organic brain diseases, and alcohol or drug abuse; pregnancy, women who were lactating; high risk of self‐injury, violence, suicide or extremely excited person. |
|
| Interventions | 1. WDD (please see details in Table 5) + Risperidone. N = 54.
2. Risperidone. N = 53. Both groups risperidone started from 1 mg/d, and variable dose (range 2 mg/d to 6 mg/d) during the observation. |
|
| Outcomes | Global state: cure (binary PANSS scores ‐ reduced >75%, 50% to 74%, 25% to 49%, no effect ‐ < 24%).* Mental state: PANSS total scores.* Use of Western medicine: risperidone dose at end of 8 weeks. Outcomes unable to use: TESS: no numerical data. |
|
| Notes | *: Data at 2, 4 and 6 weeks after treatment not used in this review (short‐term data used 8‐week outcomes). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control group…" Comment: we accept trialists' report of randomisation as true and accurate, hence rated this as unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Unclear risk | No dose stated. |
Lin 2010.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 60 days. |
|
| Participants | Diagnosis: schizophrenia (DSM‐IV). N = 104. Age: 18‐60 years. Sex: 72 men 32 women. History: average duration ill ˜ 4.4 years (SD ˜ 1.9). Inclusion criteria: Aged 18‐60, no sex limitations; more than 3 years duration; without taking any antipsychotic or having took one antipsychotic less than two weeks; PANSS total score > = 60 points; CGI‐S > = 4 points; obtained permission of patients and their families. Exclusion criteria: Patients treated with other antipsychotics, or long‐acting antipsychotics, or antidepressants and/or anti‐manic drugs, 30 days before randomised; observed in any other drug clinical trials, 30 days before randomised; suffered serious or unstable physical illness; alcohol or drug abuse; alanine aminotransferase > = 60U/L; pregnancy, women who were lactating. |
|
| Interventions | 1. WDD (please see details in Table 5) + risperidone: dose risperidone, 6 mg/d, three times daily. N = 54. 2. Risperidone: dose risperidone, 6 mg/d, three times daily. N = 50. | |
| Outcomes | Global state: cure (binary PANSS scores ‐ reduced >75%, 50% to 74%, 25% to 49%, no effect ‐ <24%). Global state: TCM Syndromes score (TCMSS) cure (binary TCMSS scores ‐ reduced > 95%, > 75%, > 50%, no effect ‐ < 30%). Outcomes unable to use: Global state: CGI – no numerical data. Mental state: PANSS – no numerical data. Mental state: BPRS – no numerical data. Adverse effect: TESS – no numerical data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control group…" Comment: we accept trialists' report of randomisation as true and accurate, hence rated this as unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Liu 2014.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 6 weeks. |
|
| Participants | Diagnosis: schizophrenia (CDSS> = 6; PANSS> = 60). N = 87. Age: 18‐60 years. Sex: 55 men 32 women. History: average duration ill ˜ 4 years (SD ˜ 1.5). Inclusion criteria: Aged 18‐60, no sex limitations; CDSS> = 6; PANSS> = 60; without taking any antipsychotic 2 weeks before randomised. Exclusion criteria: Patients with drug allergy; serious suicidal tendencies; intellectual disability; serious physical illness; serious impulse; pregnancy, women who were lactating; psychotropic substances abuse; organic brain disease. |
|
| Interventions | 1. WDD (please see details in Table 5) + ziprasidone: dose ziprasidone, 20 mg/d to 160 mg/d. N = 43. 2. Ziprasidone: dose ziprasidone, 20 mg/d to 160 mg/d. N = 44. | |
| Outcomes | Global state: cure (binary PANSS scores ‐ reduced >75%, 50% to 74%, 25% to 49%, no effect ‐ < 24%). Mental state: PANSS total score.* Mental state: CDSS.* |
|
| Notes | *: Data at 2, 4 weeks after treatment not used in this review (short‐term data used 6‐week outcomes). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control group according to random number table…" |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Peng 2010.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 1 years. |
|
| Participants | Diagnosis: schizophrenia (Diagnostic criteria cited from book of 'Psychology'). N = 72. Age: 15‐53 years. Sex: 37 men 35 women. History: average duration ill ˜ 3 years (SD ˜ 1). Inclusion criteria: Same to diagnostic criteria. Exclusion criteria: Organic brain disease; psychoactive substances or non‐addictive substance induced psychotic disorder. |
|
| Interventions | 1. WDD (please see details in Table 5) + risperidone: dose risperidone started at 1 mg/d, gradually increased to 2 mg/d to 6 mg/d in early two weeks, not more than 6 mg/d, then leave hospital 60 days later with maintenancemaintain dose of 2 mg/d. N = 36. 2. Risperidone: dose risperidone started at 1 mg/d, gradually increased to 2 mg/d to 8 mg/d in early two weeks, not more than 8 mg/d, then leave hospital 60 days later with maintenance dose of 2 mg/d to 4mg/d). N = 36. | |
| Outcomes |
Short term (<3 months) Global state: cure (binary 'symptoms disappeared' ‐ all, partly, slightly, no change or even worse) (60 days). Mental state: BPRS total scores. Medium‐term (3‐12 months) Global state: cure (binary 'symptoms disappeared' ‐ all, partly, slightly, no change or even worse) (6 months). Long‐term (> 1 year) Global state: cure (binary 'symptoms disappeared' ‐ all, partly, slightly, no change or even worse) (1 year). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control …" |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Shao 2014.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 6 weeks. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3; TCM Diagnostic criteria cited from text book of “TCM Internal Medicine”). N = 200. Age: 17‐62 years. Sex: 107 men 93 women. History: average duration ill ˜ 4 years (SD ˜ 0.8). Inclusion criteria: Aged 17‐62; Patients and their families signed the informed consent. Exclusion criteria: Other diseases or complications; accepted related treatments, and may affect observations; accompanied with situations that may affect observations; serious heart, liver and kidney damage, affect drug metabolism; special groups (pregnant women, lactating women, infants, minors, the elderly, critical condition, terminally ill); patients who were receiving drug treatment, could be included after a washout period. |
|
| Interventions | 1. WDD (please see details in Table 5) + antipsychotics (Exciting: quetiapine 300 mg to 600 mg twice daily and olanzapine 10 mg to 30 mg twice daily; looks dull, visual hallucinations, auditory hallucinations: perphenazine 20 mg to 40 mg twice daily, risperidone 3 mg to 6 mg twice daily, clozapine 200 mg to 400 mg twice daily.). N = 100. 2. Antipsychotics (Exciting: quetiapine 300 mg to 600 mg twice daily and olanzapine 10 mg to 30 mg twice daily; looks dull, visual hallucinations, auditory hallucinations: perphenazine 20 mg to 40 mg twice daily, risperidone 3 mg to 6 mg twice daily, clozapine 200 mg to 400 mg twice daily). N = 100. | |
| Outcomes | Global state: cure (binary 'symptoms disappeared' ‐ all, partly, slightly , no change). Mental state: BPRS total scores. Mental state: PANSS negative subscale score, positive subscale score, and PANSS total score. Adverse effects: RSESE, constipation (dry stool), abnormal liver function. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control according to random number table…" |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Su 2015.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 8 weeks. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 39. Age: 16‐45 years. Sex: 17 men 22 women. History: not stated. Inclusion criteria: as diagnosis. Exclusion criteria: Brain organic mental disorders; mental disorders induced by drugs or poisoned. |
|
| Interventions | 1. WDD (please see details in Table 5) + clozapine: dose clozapine 50 mg/d to 100 mg/d. N = 20. 2. Clozapine: dose clozapine 200 mg/d to 400 mg/d. N = 19. | |
| Outcomes | Global state: cure (binary 'symptoms disappeared' ‐ all, partly, slightly , no change). Adverse effects: TESS. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control …" |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Sun 2014.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 2 months. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3; PANSS > = 60). N = 108. Age: 18‐71 years. Sex: 51 men 43 women. History: average duration ill ˜ 6 years (SD ˜ 3). Inclusion criteria: not stated. Exclusion criteria: not stated. |
|
| Interventions | 1. WDD (please see details in Table 5) + aripiprazole (dose stared from 5 mg/d, and increased individually, but did not exceed 30 mg/d.). N = 54. 2. Aripiprazole (dose stared from 5 mg/d, and increased individually, but did not exceed 30 mg/d). N = 54. | |
| Outcomes | Global state: cure (binary PANSS scores ‐ reduced >75%, 50% to 74%, 25% to 49%, no effect ‐ <24%). Mental state: PANSS total. Adverse events: EPS, weight gain, constipation (dry stool) (TESS). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control …" |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Wang 2008.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 6 weeks. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 60. Age: average ˜ 28 years (SD ˜ 9). Sex: 29 men 31 women. History: average duration ill ˜ 7 years (SD ˜ 5). Inclusion criteria: With permission from patients and their families. Exclusion criteria: Serious physical diseases; brain organic mental disorders; drug abuse; pregnancy and women who were lactating; drug allergy. |
|
| Interventions | 1. WDD (please see details in Table 5) + clozapine: dose clozapine, 100 mg/d to 200 mg/d. N = 31. 2. Clozapine: dose clozapine, 250 mg/d to 550 mg/d. N = 29. | |
| Outcomes | Global state: cure (binary PANSS scores ‐ reduced >75%, 50% to 74%, 25% to 49%, no effect ‐ <24%). Mental state: PANSS total.* Adverse events: EPS, salivation, constipation, drop in blood pressure, headache and dizziness, weakness, exciting, indigestion, insomnia (TESS). |
|
| Notes | *: PANSS at 2 and 4 weeks after treatment not used in this review (short‐term data used 6‐week outcomes). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control …" |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Wang 2013.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 60 days. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3; DSM‐IV‐TR). N = 72. Age: 21‐54. Sex: 32 men 40 women. History: average duration ill ˜ 8 years. Inclusion criteria: With permission from patients and their families. Exclusion criteria: Organic mental disorders; Non‐addictive substance or psychoactive substances induced mental disorders. |
|
| Interventions | 1. WDD (please see details in Table 5). N = 36. 2. No treatment. N = 36. | |
| Outcomes | Global state: PANSS < 50% reduction (binary 'symptoms disappeared' ‐ all, partly, no change, worse). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control …" |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Xu 2007.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 8 weeks. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 160. Age: 16‐50. Sex: 88 men 72 women. History: not stated. Inclusion criteria: Blood, urine and biochemical tests normal. Exclusion criteria: Brain organic mental disorders; drug induced mental disorders. |
|
| Interventions | 1. WDD (please see details in Table 5) + clozapine: dose clozapine 50 mg/d to 100 mg/d. N = 80. 2. Clozapine: dose clozapine 200‐400mg/d. N = 80. | |
| Outcomes | Global state: cure (binary BPRS scores ‐ reduced >75%, 50% to 75%, 25% to 50%, no effect ‐ < 25%). Adverse events: insomnia, sleepiness, nausea or vomiting, constipation, dizzying or headache, EPS, EEG abnormal, liver function abnormal (TESS). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control …" |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Low risk | No obvious other bias. |
Zhang 2014.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 8 weeks. |
|
| Participants | Diagnosis: schizophrenia (ICD‐10). N = 78. Age: 27‐53. Sex: 54 men 23 women. History: average duration ill ˜ 8 years (SD ˜ 5). Inclusion criteria: PANSS> = 60; no serious physical diseases. Exclusion criteria: not stated. |
|
| Interventions | 1. WDD (please see details in Table 5) + olanzapine: dose olanzapine from 5 mg/d, range 5 mg/d to 20 mg/d. N = 39. 2. Olanzapine: dose olanzapine from 5 mg/d, range 5 mg/d to 20 mg/d. N = 39. | |
| Outcomes | Global state: cure (binary PANSS scores ‐ reduced >75%, 50% to 74%, 25% to 49%, no effect ‐ <24%)*. Mental state: PANSS total.** Outcomes ‐ unable to use Adverse effects: TESS – no numerical data reported. |
|
| Notes | *: Global state: cure 4 weeks after treatment not used in this review (short‐term data used 8‐week outcomes). **: PANSS at 4 weeks after treatment not used in this review (short‐term data used 8‐week outcomes). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided in to intervention group and control …" |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | We were unable to locate original study protocol, however, all outcomes listed in the 'methods' section of the paper appear to have been measured and reported. |
| Other bias | Unclear risk | No dose stated. |
Diagnostic tools and scales: DSM: Diagnostic and Statistical Manual. ICD: International Classification of Diseases. CCMD: Chinese Classification of Mental Disorders.
Global state: CGI: Clinical Global Impression Scale.
Mental state: BPRS: Brief Psychiatric Rating Scale. PANSS: Positive and Negative Syndrome Scale. CDSS: Calgary Depression Scale for Schizophrenia.
Adverse effects: TESS: Treatment Emergent Symptom Scale. EPS: Extrapyramidal syndrome. RESES: Rating Scale for Extrapyramidal Side Effects.
Test: EEG: electroencephalogram.
Others: WDD: Wendan decoction. TCM: Traditional Chinese medicine. SD: Standard deviation. mg/d: mg per day.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Meng 1998 | Allocation: quote: "randomised according to sequence of coming for treatment" ‐ quasi‐randomised. Participants: people with schizophrenia. N = 63. Interventions: WDD + sulpiride (100 mg/d, twice daily) vs sulpiride (200 mg/d, three times daily). |
| Shi 2010 | Allocation: quote: "randomised according to sequence of coming for treatment" ‐ quasi‐randomised. Participants: people with schizophrenia. N = 78. Interventions: WDD + sulpiride (start with 0.4 g/d, add 0.2 g/d every 2 days, until 0.8 g/d to 1.2 g/d in 2 weeks) vs haloperidol (start with 6 mg/d, add 4 mg/d every 2 days, until 20 mg/d to 40 mg/d in 2 weeks). |
WDD: Wendan decoction
Differences between protocol and review
We have changed the wording of the quality of life 'Summary of findings' outcome from significant change to clinically important change ‐ in line with the other outcomes.
Contributions of authors
Hongyong Deng ‐ selected studies, extracted data, undertook analysis and drafted the full review.
Ji Xu ‐ selected studies, extracted data, contacted the authors and helped with drafting of the full review.
Sources of support
Internal sources
-
Shanghai University of Traditional Chinese Medicine, China.
Employs review authors
-
University of Nottingham, UK.
Teaching and training
External sources
-
Shanghai Municipal Education Commission (SMEC), China.
To undertake Cochrane review writing training
Declarations of interest
HD ‐ none known. JX ‐ none known.
New
References
References to studies included in this review
Chang 2009 {published data only}
- Chang W. The clinical research of wendan decoction combined with chlorpromazine on schizophrenia patients [温胆汤联合氯丙嗪治疗精神分裂症临床研究]. Journal of Henan University of Chinese Medicine 2009;24(142):54‐5. [Google Scholar]
Dou 2012 {published data only}
- Dou N. 40 cases report of modified wendan decoction combined with risperidone on schizophrenia patients [加味温胆汤联合利培酮治疗精神分裂症40例]. Guangming Journal of Chinese Medicine 2012;27(7):1411‐2. [Google Scholar]
Guo 2013 {published data only}
- Guo Y. Effect of wendan decoction in collaborative treatment of schizophrenia [温胆汤加减协同治疗精神分裂症的疗效]. China Modern Medicine 2013;20(3):112‐3. [Google Scholar]
Huang 2005 {published data only}
- Huang L. 55 cases report of wendan decoction effects on insomnia of schizophrenia patients [温胆汤加减治疗精神分裂症失眠55例疗效观察]. Journal of Chinese Medicine Research 2005;5(2):167‐8. [Google Scholar]
Li 2013 {published data only}
- Li L. Clinical research of wendan decoction combined with risperidone treating schizophrenia [温胆汤联合利培酮治疗精神分裂症的临床观察]. Journal of Practical Medical Techniques 2013;20(2):187‐9. [Google Scholar]
Lin 2010 {published data only}
- Lin S. Effects of wendan decoction combined with risperidone on the first onset of schizophrenia [温胆汤加减联合利培酮治疗首发精神分裂症的临床疗效分析]. Contemporary Medicine 2010;16(20):156‐7. [Google Scholar]
Liu 2014 {published data only}
- Liu F, Zheng D, Li Y. The clinical effect of wendan tang combined with ziprasidone in treating schizophrenia with depressive symptoms [温胆汤联合齐拉西酮治疗精神分裂症抑郁症状的临床疗效观察]. China Journal of Health Psychology 2014;22(8):1158‐60. [Google Scholar]
Peng 2010 {published data only}
- Peng D. 36 cases of integrative Chinese and western medicine in treating schizophrenia [中西医结合治疗精神分裂症36例临床疗效观察]. Guilding Journal of Traditional Chinese Medicine and Pharmacy 2010;16(11):50‐1. [Google Scholar]
Shao 2014 {published data only}
- Shao H, Yang Z. Wendan decoction combined with western medicine in the treatment of schizophrenia research random parallel control [温胆汤联合西药治疗精神分裂症随机平行对照研究]. Journal of Practical Traditional Chinese Internal Medicine 2014;28(1):84‐6. [Google Scholar]
Su 2015 {published data only}
- Su B, Yao Z, Meng Y, Zhang L, Bai S. Clinical effect of integrated Chinese and western medicine on schizophrenia accompanied with cardiovascular disease [中西医结合治疗精神分裂症伴心血管病的疗效观察]. Cardiovascular Disease Journal of Intergrated Traditional Chinese and Western Medicine 2015;3(9):144‐5. [Google Scholar]
Sun 2014 {published data only}
- Sun Z. Clinical effect of integration of Chinese and western medicine on schizophrenia [中西医结合治疗精神分裂症临床疗效分析]. Asia‐Pacific Traditional Medicine 2014;10(7):74‐5. [Google Scholar]
Wang 2008 {published data only}
- Wang R. Control trial of wendan decoction combined with low dose chlorpromazine on schizophrenia [中药温胆汤合并小剂量氯丙嗪治疗精神分裂症对照研究]. Medical Journal of Chinese People's Health 2008;20(11):1159‐76. [Google Scholar]
Wang 2013 {published data only}
- Wang W. Clinical test of Ningshen wendan decoction in treating schizophrenia [宁神温胆汤中药治疗精神分裂症的实验与临床分析]. Journal of Medicine Theory and Practice 2013;26(11):1448‐9. [Google Scholar]
Xu 2007 {published data only}
- Xu Y, Lu Y. 80 cases report integration of Chinese and western medicine in treating schizophrenia [中西医结合治疗精神分裂症80例的临床观察]. Medical Journal of Chinese Prople's Health 2007;19(3):229‐31. [Google Scholar]
Zhang 2014 {published data only}
- Zhang G. Clinical observation of wendan decoction combined with olanzapine in treating schizophrenia [温胆汤联合奥氮平治疗精神分裂症的临床观察]. Medical Journal of Chinese Prople's Health 2014;26(9):23‐4. [Google Scholar]
References to studies excluded from this review
Meng 1998 {published data only}
- Meng Z. Clinical research of Huanglian wendan decoction treating schizophrenia with phlegm and heat [黄连温胆汤加味治疗精神分裂症痰火内扰型的临床观察]. Journal of Guiyang College of Traditional Chinese Medicine 1998;20(3):25‐6. [Google Scholar]
Shi 2010 {published data only}
- Shi L. 39 cases report of wendan decoction combined with sulpiride in treating first onset schizophrenia [加味温胆汤联合舒比利治疗首发精神分裂症39例]. Henan Traditional Chinese Medicine 2010;30(4):386‐7. [Google Scholar]
Additional references
Addington 1990
- Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophrenia Research 1990;3:247‐51. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Che 2016
- Che YW, Yao KY, Xi YP, Chen ZJ, Li YL, Yu N, et al. Wendan decoction for treatment of schizophrenia: a systematic review of randomized controlled trials. Chinese Journal of Integrative Medicine 2016;22(4):302‐10. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Deng 2003
- Deng Z. [方剂学(第七版)]. Prescriptions of Chinese Materia Medica. 7th Edition. Beijing: China TCM Publising House, 2003. [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology (DOTES: Dosage Record and Treatment Emergent Symptom Scale). Rockville: National Institute of Mental Health, 1976. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Howes 2016
- Howes OD, Nour MM. Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry 2016;15(1):3‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [PUBMED: 19438480] [DOI] [PubMed] [Google Scholar]
Initiative 2010
- The Internet Mental Health Initiative. Schizophrenia Facts and Statistics. www.schizophrenia.com/szfacts.htm [accessed date 19/2/2016] 2010; Vol. (accessed 19 February 2016).
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Leon 2006
- Leon AC, Mallinckrodt CH, Chuang‐Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001‐5. [PUBMED: 16905632] [DOI] [PubMed] [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Li 2013a
- Li Y, Guo G, Guo Y. Research progress of wendan decoction's clinical application and pharmacological experiment [温胆汤临床应用及药理实验研究进展]. Inner Mongol Journal of Traditional Chinese Medicine 2013;32(23):114‐5. [Google Scholar]
Luo 2009
- Luo D, He YS, Yuan Z, Chen J. Study on the effects of wendan decoction and its compositions on the dopamine receptor of the rat [温胆汤的不同配伍对大鼠纹状体多巴胺受体的影响]. Lishizhen Medicine and Materia Medica Research 2009;20(6):1416‐7. [Google Scholar]
Mao 2013
- Mao M, Song Y, Sun H, Li F, Liu Y, Bai S. Application and thinking of Wendan Tang in treating mind disease [温胆汤在神志病治疗中的应用与思考]. Journal of Chinese Medicine 2013;28(179):517‐9. [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
McGrath 2008
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiologic Reviews 2008;30:67–76. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Peter 2007
- Peter MH, Sonu GS. Adverse effects of atypical antipsychotics: differential risk and clinical implications. CNS Drugs 2007;21(11):911‐36. [DOI] [PubMed] [Google Scholar]
Picchioni 2007
- Picchioni MM, Murray RM. Schizophrenia. BMJ 2007;335(7610):91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
SATCM 1994
- SATCM. TCM Syndrome Diagnostic and Efficacy Standards. Nanjing: Nanjing University Press, 1994. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shi 2001
- Shi L. A discussion on origin, indications and regularities of modification of wendan decoction [温胆汤类方的源流、方证及加减规律探讨]. Jiangsu Journal of Traditional Chinese Medicine 2001;22(1):33‐4. [Google Scholar]
Simpson 1970
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychoiatrica Scandinavica 1970;212:11‐9. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
van Os 2009
- Os J, Kapur S. Schizophrenia. Lancet 2009;374(9690):635–45. [DOI] [PubMed] [Google Scholar]
Wan 2008
- Wan H. [温胆汤对实验性大鼠精神分裂症的作用及其机制研究]. Study on Effects and Mechanisms of Wendan Decoction in Rats with Schizophrenia [PhD Thesis]. Changsha: Hunan University of TCM, 2008. [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
Xie 2004
- Xie H, He Y. An experimental study on the effects of wendan decoction and its compositions on the dopamine synthesis of the rat's striatum [温胆汤及其配伍对大鼠纹状体DA合成的影响]. Hunan Journal of Traditional Chinese Medicine 2004;20(3):66‐70. [Google Scholar]
Zhou 2003
- Zhou Z. [中医内科学(第七版)]. Chinese Traditional Internal Medicine. 7th Edition. Beijing: China TCM Publising House, 2003. [Google Scholar]
References to other published versions of this review
Deng 2016
- Deng H, Xu J. Wendan decoction (Traditional Chinese medicine) for schizophrenia. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012217] [DOI] [PMC free article] [PubMed] [Google Scholar]


