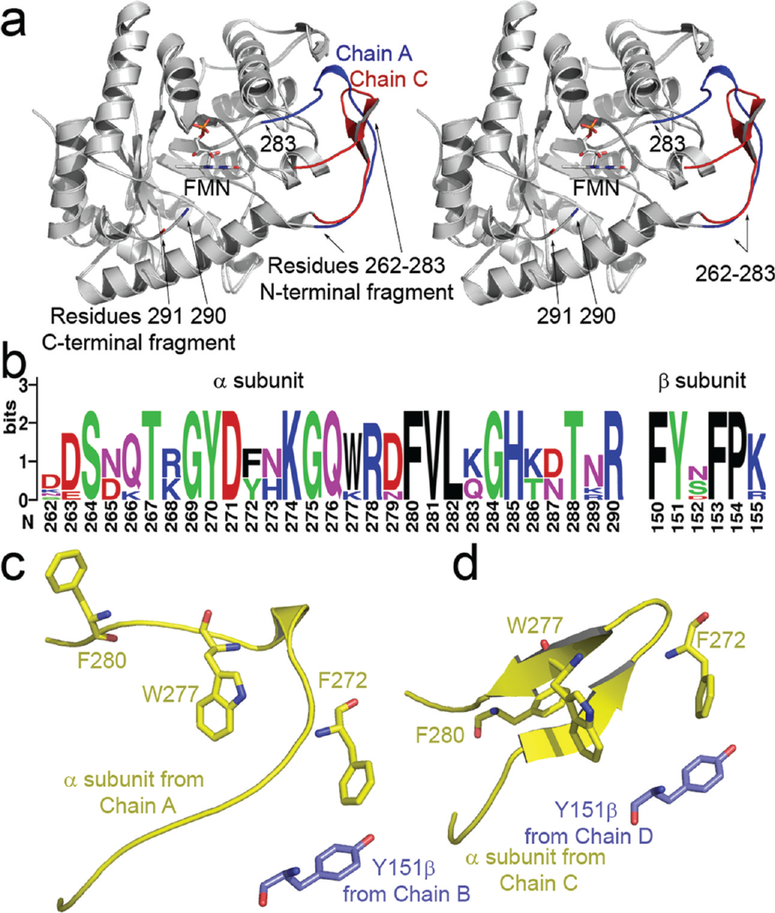
Figure 3:
(a) Stereoview of the two observed conformations of the mobile loop superimposed upon one another (R262–291). The location of bound FMN is indicated by an arrow. The α subunit observed in a complex with FMN is shown in red. The flavin-free α subunit is shaded in blue, note the location of residues 290 (bound) and 291 (flavin-free); unambiguous electron density for the backbone between residues 284 and 289 was not observed. (b) Multiple sequence alignment of residues in the mobile loop and the hydrophobic segment near βTyr151. Sequences were retrieved from eight species having complete luxA and luxB entries in the ExPASy database (62). Alignment was preformed using ClustalX prior to construction of the sequence logo (63, 64). (c, d) The position of residue 151 on the β (purple) subunit is indicated relative to hydrophobic residues in the mobile loop from the α subunit (yellow). The conformation of the α subunit bound to FMN (c) lacks the pair of antiparallel sheets formed in the flavin-free α subunit (d).