Abstract
Background
Bedaquiline is used as a substitute for second-line injectable (SLI) intolerance in the treatment of multidrug-resistant (MDR) tuberculosis, but the efficacy and safety of this strategy is unknown.
Methods
In this retrospective cohort study adults receiving bedaquiline substitution for MDR tuberculosis therapy, plus a matched control group who did not receive bedaquiline, were identified from the electronic tuberculosis register in the Western Cape Province, South Africa. The primary outcome measure was the proportion of patients with death, loss to follow-up, or failure to achieve sustained culture conversion at 12 months of treatment.
Results
Data from 162 patients who received bedaquiline substitution and 168 controls were analyzed; 70.6% were infected with human immunodeficiency virus. Unfavorable outcomes occurred in 35 of 146 (23.9%) patients in the bedaquiline group versus 51 of 141 (36.2%) in the control group (relative risk, 0.66; 95% confidence interval, .46 –.95). The number of patients with culture reversion was lower in those receiving bedaquiline (1 patient; 0.8%) than in controls (12 patients; 10.3%; P = .001). Delayed initiation of bedaquiline was independently associated with failure to achieve sustained culture conversion (adjusted odds ratio for every 30-day delay, 1.5; 95% confidence interval, 1.1–1.9). Mortality rates were similar at 12 months (11 deaths in each group; P = .97).
Conclusions
Substituting bedaquiline for SLIs in MDR tuberculosis treatment resulted in improved outcomes at 12 months compared with patients who continued taking SLIs, supporting the use of bedaquiline for MDR tuberculosis treatment in programmatic settings.
Keywords: bedaquiline, drug-resistant tuberculosis, HIV-associated tuberculosis
In this population with a high human immunodeficiency virus coinfection rate, bedaquiline substitution for second-line injectable agents was associated with fewer unfavorable treatment outcomes at 12 months than in a matched control group receiving standard multidrug-resistant tuberculosis treatment.
Multidrug-resistant (MDR) tuberculosis, defined as resistance to rifampicin and isoniazid, is associated with increased mortality rates and worse treatment outcomes than drug-susceptible tuberculosis [1]. Second-line injectables (SLIs), core agents used in the treatment of MDR tuberculosis [2], cause substantial toxicity, which leads to treatment discontinuation and contributes to the low success rates with conventional MDR tuberculosis treatment [3, 4].
There is a stepwise decline in the success of tuberculosis treatment as drug resistance patterns advance [5], and the presence of resistance to SLIs has been a significant predictor of poor long-term survival in some studies [5, 6]. Therefore, discontinuing SLIs from MDR tuberculosis regimens without replacement by an effective drug may put patients at risk of worse outcomes and ongoing transmission of drug-resistant tuberculosis.
The novel diarylquinoline, bedaquiline, improves culture conversion rates when added to conventional MDR tuberculosis treatment in clinical trials [7–9], and it has also been shown to improve treatment outcomes in observational studies [10]. However, there are safety concerns related to its effect on QT interval prolongation and the increased mortality rate associated with the bedaquiline arms in pooled data from phase 2 clinical trials [11]. The World Health Organization (WHO) has made a conditional recommendation for the use of bedaquiline in adults with MDR tuberculosis who have limited treatment options [12], which may occur in up to two-thirds of cases [13]. Bedaquiline is now being widely used as a substitute in MDR tuberculosis regimens for patients unable to tolerate SLIs [14], but the efficacy and safety of this strategy is unknown. We conducted a retrospective cohort study to determine outcomes for South African patients who received bedaquiline as a substitution for SLIs in conventional MDR tuberculosis therapy, with the hypothesis that this would not result in inferior outcomes at 12 months compared with patients who did not discontinue SLIs.
PATIENTS AND METHODS
Study Population and Eligibility Criteria
In September 2015, the Western Cape Provincial Department of Health expanded and decentralized bedaquiline access for adults who had confirmed MDR tuberculosis without additional second-line drug resistance who were unable to tolerate SLIs. Under this expanded program, which is ongoing, local clinicians made requests for bedaquiline access for individual patients to a Provincial Clinical Advisory Committee using a standardized application form. If the request was approved, bedaquiline was provided for a minimum of 24 weeks (with a loading dose of 400 mg once daily for the initial 2 weeks, followed by 200 mg 3 times per week for 22 weeks). Other drugs in the MDR tuberculosis regimen included moxifloxacin (which was replaced by levofloxacin at bedaquiline initiation, due to the greater QT-prolonging effect of moxifloxacin), pyrazinamide, ethionamide, high-dose isoniazid, ethambutol, and terizidone. Until late 2017, this standardized MDR tuberculosis regimen was generally administered for a total of 18–24 months, including the use of an SLI for 6 to 8 months, according to South African National Treatment Program guidelines (the WHO shorter MDR tuberculosis regimen was introduced in the Western Cape Province in late 2017, after the enrollment window for this study).
We screened all applications to the Provincial Clinical Advisory Committee and included consecutive patients who received bedaquiline as a substitution for SLIs between October 2014 and October 2016. We also included a group of control patients with MDR tuberculosis who did not receive bedaquiline, matched 1:1 for clinic location and time of treatment initiation within a window of ±6 months. These patients were identified from the South African Electronic Drug-Resistant Tuberculosis Register, a Web-based network used in the surveillance and management of drug-resistant tuberculosis in South Africa. Patients <18 years old were excluded, as were those with Mycobacterium tuberculosis strains known to be resistant to aminoglycosides and/or fluoroquinolones (pre–extensively drug-resistant or extensively drug-resistant tuberculosis).
Outcome Measures
The primary outcome measure was the proportion of patients with unfavorable outcomes at 12 months, defined as a composite of death, loss to follow-up, or treatment failure (failure to achieve sustained culture conversion). Sustained culture conversion was defined as ≥2 consecutive negative cultures, with the last culture performed 12 months (±2 months) after starting antituberculosis treatment, including cultures from patients with negative or absent baseline sputum cultures. To account for missing data in the primary outcome measure, we created a secondary composite outcome of death, loss to follow-up, and a modified definition of treatment failure wherein any positive sputum culture result between 6 and 12 months after initiation of MDR tuberculosis treatment was regarded as treatment failure. Outcomes were censored at 12 months owing to limited availability of sputum culture data beyond that time.
Time to initial sputum culture conversion was defined as 2 consecutive negative cultures taken ≥30 days apart, in a patient with a positive baseline sputum culture, with the collection date of the first negative culture specimen reported as the conversion date. Culture reversion was defined as 2 positive cultures, taken ≥30 days apart, after initial sputum culture conversion at any time after starting MDR tuberculosis treatment, according to WHO criteria. Patients were considered lost to follow-up when there was a gap of >1 month in clinic visits or dispensing of antiretroviral therapy (ART) or antituberculosis treatment after the last recorded healthcare contact and no further contact by 12 months. Outcomes data at 18 months were collected for those patients with sufficient follow-up time.
Sample Size Estimation
The sample size estimation was calculated using death as an outcome. This was chosen because of the signal of excess mortality in the bedaquiline arm in a clinical trial [9], and because our composite primary end point of unfavorable outcomes at 12 months has not been previously assessed for bedaquiline to our knowledge. Mortality assumptions were based on a comparative mortality analysis from South Africa, published in the 2016 WHO Bedaquiline Guideline Development Group report [10]. With a sample size of 330 patients, we estimated that we would have sufficient power (>80%), at a 1-sided significance level of 2.5%, for a noninferiority margin of 10% in the proportion of deaths at 12 months between the bedaquiline group and the standard treatment group (estimated at 20%).
Analysis and Reporting
We calculated the proportions of case patients versus controls with the composite primary and secondary end points of unfavorable outcome at 12 months and compared these outcomes using the χ2 test. We also analyzed individual components of the composite outcome as binary variables, as well as the proportions with culture reversion and 18-month outcomes where data were available. Logistic regression analysis was performed to adjust for potential confounders in the primary outcome and to evaluate predictors for failure to achieve sustained culture conversion in the bedaquiline group. The time to initial sputum culture conversion and death was displayed with Kaplan-Meier plots and compared using the log-rank test; censoring was performed at 12 months, as well as for patients who were lost to follow-up or died, for the analysis of time to culture conversion. We used a Cox proportional-hazards model with adjustment for baseline smear positivity and human immunodeficiency virus (HIV) status to compare the time to culture conversion in the 2 study groups. Statistical analysis was performed using Stata software, version 14.2 (StataCorp).
Ethical Approval
This study was approved by the Human Research Ethics Committee at the University of Cape Town (reference 446/2016).
RESULTS
Patient Characteristics
Data from 330 patients with laboratory-confirmed pulmonary MDR tuberculosis (70.6% HIV infected) were analyzed; these included 162 case patients with bedaquiline substitution and 168 controls who did not receive bedaquiline. Demographic and clinical characteristics at the time of initiation of MDR tuberculosis therapy are summarized in Table 1. The groups were well matched other than for age, which was higher in the bedaquiline group, and CD4 cell count, which was lower among HIV-infected patients in the bedaquiline group.
Table 1.
Baseline Demographic and Clinical Characteristics
| Variable | Patients, No. (%)a | P Valueb | |
|---|---|---|---|
| Bedaquiline (n = 162) |
Control (n = 168) |
||
| Age, median (IQR), y | 42 (35–49) | 35 (28–42) | <.001 |
| Male sex | 93 (57.4) | 97 (58.1)c | .90 |
| Weight, median (IQR), kg | 54 (45–62) | ND | NA |
| Any comorbid condition | 44 (27.2) | ND | NA |
| HIV infection | 110 (67.9) | 94 (74.0)d | .26 |
| CD4 cell count, median (IQR), cells/µLe | 97 (45–201) | 205 (59–362) | .007 |
| Viral load below detectable limite | 46 (63.0)f | 50 (72.5)g | .23 |
| Receiving ARTe | 94 (85.5)h | ND | NA |
| Previous tuberculosis (any) | 88 (63.3)i | 95 (56.6) | .23 |
| Extrapulmonary tuberculosis | 18 (11.4)j | 13 (7.8)c | .27 |
| Positive sputum results | |||
| Culture | 142 (87.7) | 148 (88.1) | .90 |
| Xpert MTB/RIF | 111 (68.5) | 112 (66.7) | .72 |
| Smear | 98 (60.5) | 112 (66.7) | .24 |
| Isoniazid mutationk | |||
| inhA | 33 (55.9) | ND | NA |
| katG | 16 (27.1) | ND | NA |
| Both | 2 (3.4) | ND | NA |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not available; ND, no data.
aData represent No. (%) unless otherwise specified.
b P values calculated using Wilcoxon rank sum test for continuous variables and χ2 test for binary variables.
cDenominator: n = 167.
dDenominator: n = 127.
eThe data for CD4 cell count, viral load, and ART apply only to HIV-infected patients (n = 204) and were recorded at the start of multidrug-resistant tuberculosis or bedaquiline treatment.
fDenominator: n = 73.
gDenominator: n = 69.
hDenominator: n = 110.
iDenominator: n = 139.
jDenominator: n = 158.
kDenominator: n = 59.
Management in the Bedaquiline Group
Twenty-nine patients (18.6%) did not receive any SLI treatment and started bedaquiline a median of 29 days (interquartile range [IQR], 18–49 days; range, 0–161 days) after the start of MDR tuberculosis treatment. In the other 127 patients for whom this was documented, SLIs were stopped a median of 54 days (IQR, 25–82 days) after initiation of tuberculosis treatment. There was a 44-day delay (IQR, 29–70 days, range, 11–161 days) from SLI withdrawal to the start of bedaquiline. Hearing loss was the most common reason for SLI discontinuation, present in 115 (74%) of patients who switched. SLIs were also discontinued because of renal impairment in 28 patients (18%) and hypokalemia in 13 (8%).
Outcomes
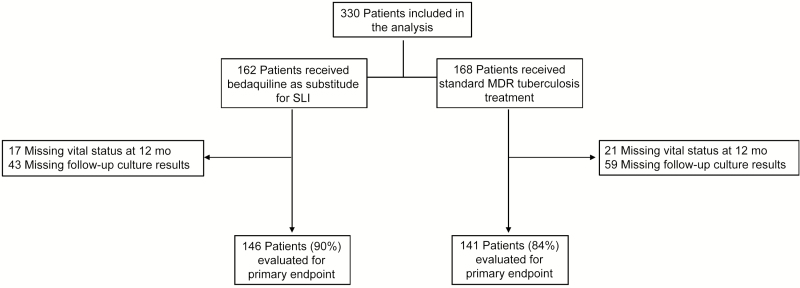
The number of patients assessed for the primary outcome is shown in Figure 1. Unfavorable outcome according to the primary composite measure was assessed in 288 patients (87%; 145 in the bedaquiline group and 143 controls). This outcome occurred in 35 patients (23.9%) in the bedaquiline group versus 51 (36.2%) in the control group (relative risk, 0.66; 95% confidence interval [CI], .46–.95; P = .02). The odds of unfavorable outcomes remained significantly lower in the bedaquiline group after adjustment for age, CD4 cell count, HIV status, and baseline smear positivity in a mutivariable logistic regression model (adjusted odds ratio [OR] 0.38; 95% CI, .18–.81). Bedaquiline use was associated with a protective effect of similar magnitude when almost the full cohort (n = 310) was assessed for the secondary composite outcome; 44 patients (27.9%) in the bedaquiline group versus 58 (38.2%) in the control group had unfavorable outcomes at 12 months (relative risk, 0.73; 95% CI, .53–1.0; P = .053).
Figure 1.
Flow diagram showing screening and inclusion of study population. Culture results required ≥2 consecutive cultures with the last culture performed 12 months (±2 months) after the start antituberculosis treatment, per the definition of sustained culture conversion for this study; outcomes were recorded as missing in cases where culture results were insufficient to evaluate sustained culture conversion, per the prespecified definition. The proportions of patients with missing data were not different between groups (P = .09). For the totals of patients evaluated for the primary end points, the missing data do not sum to these values owing to overlap in outcomes (ie, any failure event contributes to the composite outcome, even if another component has a missing outcome). Abbreviations: MDR, multidrug-resistant; SLI, second-line injectable
As shown in Table 2, the proportion of deaths in the bedaquiline group (11 deaths; 7.6%) was noninferior to that in the control group (11 deaths; 7.5%) at 12 months (risk difference, 0.1%; 95% CI, −5.9 to 6.1; within the prespecified noninferiority limit of 10%). The reduction in unfavorable outcomes with bedaquiline use was mainly influenced by differences in sustained culture conversion rates: only 7 patients (5.9%) switched to bedaquiline failed to achieve sustained culture conversion at 12 months, compared with 19 (17.4%) in the control group (P = .006). The effect of bedaquiline on sustained culture conversion persisted at 18 months (Table 2). A total of 13 patients (5.4%; n = 241) with a positive baseline culture reverted to culture positive after initial culture conversion (ie, 2 consecutive negative sputum cultures), at a median time of 263 days (IQR, 217–296 days) from the start of treatment. The number of patients with culture reversion was significantly lower in the bedaquiline group (1 patient [0.8%] vs 12 [10.3%] in the control group; P = .001). The proportions of patients with missing culture reversion outcome data did not differ between the groups (P = .10).
Table 2.
Treatment Outcomes
| Variable | Patients, No./Total No. (%) | P Value | |
|---|---|---|---|
| Bedaquiline Group (n = 162) | Control Group (n = 168) | ||
| At 12 mo | |||
| Composite unfavorable outcome (primary)a | 35/146 (23.9) | 51/141 (36.2) | .02 |
| Composite unfavorable outcome (secondary)b | 44/158 (27.9) | 58/152 (38.2) | .053 |
| Death | 11/145 (7.6) | 11/147 (7.5) | .97 |
| Loss to follow-up | 17/162 (10.5) | 21/168 (12.5) | .57 |
| Treatment failurec | 7/119 (5.9) | 19/109 (17.4) | .006 |
| Modified treatment failured | 16/138 (11.6) | 29/131 (22.1) | .02 |
| At 18 mo | |||
| Death | 13/79 (16.5) | 15/100 (15.0) | .79 |
| Failure to achieve sustained culture conversion | 3/93 (3.2) | 16/81 (19.8) | <.001 |
aDefined as death, loss to follow-up, or treatment failure. Outcomes were recorded as missing in cases where there was no failure event and ≥1 of the components of the composite end point was absent.
bDefined as death, loss to follow-up, or modified definition of treatment failure. Outcomes were recorded as missing in cases where there were no data for all of the components of the composite end point. Note that the components of the secondary composite outcome do not sum in the bedaquiline group owing to overlap in outcomes in 2 patients (modified treatment failure plus death in 1 and modified treatment failure plus loss to follow-up in the other).
cDefined as failure to achieve sustained culture conversion (≥2 consecutive negative cultures with the last culture performed 12 months (±2 months) after the start of antituberculosis treatment). Outcomes were recorded as missing in cases where sustained culture conversion, per the prespecified definition, could not be assessed owing to missing sputum culture results. Proportions with missing sputum results were similar between the groups (P = .09).
dDefined as any positive sputum culture result between 6 and 12 months after initiation of multidrug-resistant tuberculosis treatment. Outcomes were recorded as missing in cases where there were no sputum culture results available after 6 months of therapy. Proportions with missing sputum results were similar between the groups (P = .09).
In the bedaquiline group, the proportion of HIV-infected patients with unfavorable outcomes at 12 months (20 [20.0%]; n = 100) was not significantly different from that in HIV-uninfected patients (15 [32.6%]; n = 46; P = .14). This included mortality outcomes, with 5 (5.1%) deaths among HIV-infected and 6 (12.8%) deaths among HIV-uninfected patients (P = .18). At univariate analysis, shown in Table 3, the timing of initiation of bedaquiline from the start of MDR tuberculosis treatment was the only factor associated with failure to achieve sustained sputum culture conversion at 12 months (unadjusted OR for every 30-day delay, 1.4; 95% CI, 1.1–1.9). This remained an independent predictor after adjustment for comorbid conditions and HIV status (adjusted OR, 1.5; 95% CI, 1.1–1.9).
Table 3.
Predictors of Failure to Achieve Sustained Culture Conversion at 12 Months in the Bedaquiline Group
| Variable | Univariate OR (95% CI) |
P Value | Mutivariable OR (95% CI)a | P Value |
|---|---|---|---|---|
| Sputum smear positive at baseline | 1.4 (.3–7.8) | .67 | … | … |
| Comorbid illness | 2.1 (.5–10.2) | .34 | 1.6 (.3–9.5) | .61 |
| HIV infection | 0.3 (.06–1.4) | .12 | 0.3 (.5–1.7) | .17 |
| Per 30-d delay from start of treatment | 1.4 (1.1–1.9) | .007 | 1.5 (1.1–1.9) | .01 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
aGoodness-of-fit test P = .11 for final multivariate model (baseline smear status did not influence the effect size of the estimates in the mutivariable model and was dropped to improve fit).
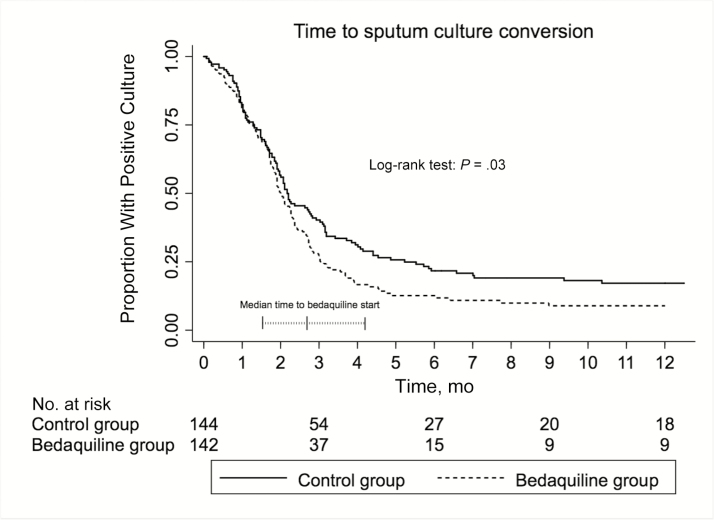
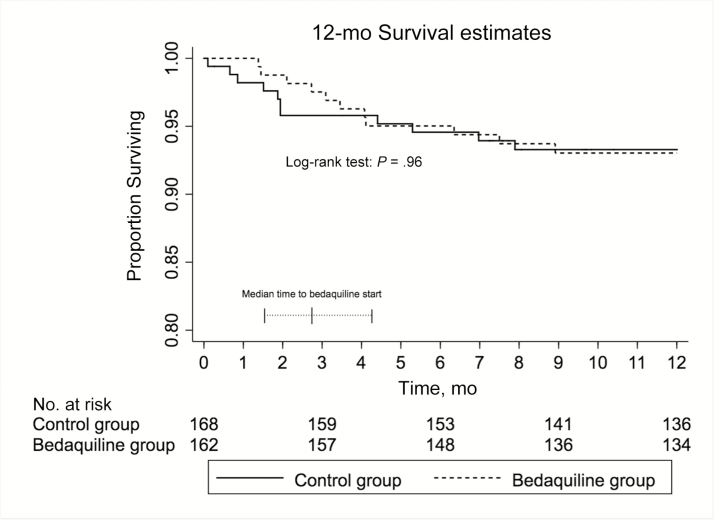
Among those with positive sputum cultures at baseline (n = 290), 87.4% (95% CI, 81.1%–92.4%) in the bedaquiline group had achieved sputum culture conversion by 6 months versus 78.3% (95% CI, 71.0%–85.0%) in the control group; the crude hazard ratio for culture conversion in the bedaquiline group was 1.32 (95% CI, 1.02–1.71; P = .03; Figure 2). This effect persisted after adjustment for HIV status and baseline sputum smear positivity (adjusted hazard ratio, 1.32; 95% CI, 1.00–1.76; P = .048). The median time to death within 12 months of initiation of tuberculosis treatment was not different between bedaquiline-exposed and bedaquiline-unexposed patients (P = .96; Figure 3).
Figure 2.
Kaplan–Meier graph of time to initial sputum culture conversion in each study group during the first 12 months of therapy. Superimposed on the graph is a plot of the median (interquartile range) time to initiation of bedaquiline after the start of multidrug-resistant tuberculosis therapy. This analysis includes only patients with a positive baseline culture. There was no difference between groups in the proportion of patients who were culture negative at baseline (P = .90). The median time to bedaquiline start was 2.7 months (interquartile range, 1.5–4.2 months).
Figure 3.
Kaplan-Meier graph of time to death in each study group during the first 12 months of therapy. Superimposed on the graph is a plot of the median (interquartile range [IQR]) time to initiation of bedaquiline after the start of multidrug-resistant tuberculosis therapy. Note the truncated scale on the y-axis. The median time to bedaquiline start was 2.7 months (IQR, 1.5–4.2 months).
DISCUSSION
In this population with MDR tuberculosis and a high burden of HIV coinfection, substituting bedaquiline for SLIs resulted in fewer unfavorable outcomes after 12 months of treatment compared with regimens containing an SLI for its full course. To our knowledge, this is the first study to specifically evaluate a strategy of bedaquiline substitution for SLIs in conventional MDR tuberculosis therapy.
Our results are consistent with those from other observational studies assessing the efficacy of bedaquiline in clinical practice [15, 16]. A WHO meta-analysis evaluating the use of bedaquiline among 391 patients with drug-resistant tuberculosis, including extensively drug-resistant tuberculosis, showed that almost 80% had culture converted at 6 months and that treatment success was achieved in 69% [10]. Importantly, in our study the number of patients with culture reversion was significantly lower in those switched to bedaquiline, suggesting a persistent effect after stopping the 6-month course, in keeping with its long terminal elimination half-life [17]. These findings lend support to the use of bedaquiline in shorter MDR tuberculosis regimens, although this needs to be evaluated in prospective studies with longer-term follow-up to assess true relapse.
The 12-month outcomes observed in the control arm of our study were better than the expected treatment success rates with conventional MDR tuberculosis therapy of approximately 54% in programmatic settings [1, 18]. However, the standard definition of treatment success involves a longer follow-up duration to treatment completion, which was not assessed in our cohort and could account for this discrepancy [19]. The external validity of our findings is supported by a 2017 systematic review, which found similar 6-month culture conversion rates (75%; 95% CI, 60%–90%) with the use of standardized treatment regimens for MDR tuberculosis [18].
The mortality rate associated with MDR tuberculosis is consistently about 15% [1, 20], similar to the proportion of deaths observed in our cohort at 18 months. The meta-analysis conducted by WHO found a 10.6% overall mortality rate with the use of bedaquiline [10], but with a large degree of heterogeneity between populations, ranging from about 6.8% in a French cohort [15] to about 20% in the South African Bedaquiline Clinical Access Program [10]. Unlike in our study, which included only patients with MDR tuberculosis, most patients in those cohorts had MDR tuberculosis with additional resistance to second-line agents, limiting conclusions that can be drawn from direct comparison.
It is reassuring that there were no differences in the 12- and 18-month mortality rates between bedaquiline-exposed and bedaquiline-unexposed patients in our study. In a phase 2b trial, which found a significantly higher mortality rate with bedaquiline use compared with placebo, almost all deaths occurred after 6 months, at a median time of 49 weeks after stopping bedaquiline [9]. Bedaquiline undergoes extensive tissue distribution with intracellular accumulation, resulting in an extremely long elimination half-life [17, 21]. The impact of these pharmacokinetic characteristics on QT prolongation and other toxic effects is unknown, and this is an important area for future research and pharmacovigilance.
Only 138 HIV-infected patients were included in the WHO meta-analysis, and it is of concern that these patients seemed to have a higher mortality rate than HIV-uninfected patients receiving bedaquiline (13% vs 9%, respectively) [10]. In our study, which included 110 HIV-infected patients receiving bedaquiline, we found no difference in 12-month mortality rate compared with those who were HIV uninfected. This finding may be related to the relatively high proportion of patients receiving ART (85%), and it is consistent with a previous report from the South African Bedaquiline Clinical Access Program that bedaquiline can be used successfully in HIV-infected patients receiving ART [16].
In our cohort, later initiation of bedaquiline after the start of MDR tuberculosis treatment was independently associated with failure to achieve sustained culture conversion at 12 months. Maintaining effective systems for decentralized bedaquiline implementation is challenging and will require continuous monitoring and review.
The current study has important limitations. The retrospective design introduces sources of bias, particularly in the selection of cases. For example, the process used by the Provincial Clinical Advisory Committee to evaluate applications may have systematically allocated patients with different disease characteristics to the bedaquiline group; this is possibly reflected by the older age and lower CD4 cell counts in those patients. However, this would tend to bias toward worse outcomes in the bedaquiline group, raising the possibility that bedaquiline could have an even larger effect on treatment efficacy in an unselected population. Adjustment for potential confounders did not change the effect of bedaquiline on reduction of unfavorable outcomes. We minimized selection bias by including consecutive applications for bedaquiline substitution and by matching cases with control patients for time of starting MDR tuberculosis treatment and for clinic location, which would tend to reduce confounding related to variations in quality of care between clinics. Although baseline characteristics were similar, our inability to perform matching for variables known to have prognostic significance (such as radiographic abnormalities and weight) is an additional limitation.
This study involves one of the largest published cohorts to describe the programmatic use of bedaquiline, but difficulties in ascertaining outcomes data retrospectively limited the power and accuracy of our primary end point. Data on the composite primary end point were missing for 43 patients (13%), mainly because of restricted access to the national death registry and incomplete follow-up culture results. However, the proportions of patients with missing outcomes data were similar between the groups, and we were able to verify the internal validity of the primary outcome by showing similar results with the use of a secondary outcome measure, which included a more conservative definition of treatment failure (any positive sputum culture result after month 6 of treatment) that evaluated almost the entire cohort (n = 310).
Another limitation is the possibility of immortal time bias conferring an early survival advantage on the bedaquiline group [22]. This is due to the initial period of observation time before SLI substitution when the primary outcome cannot occur in the bedaquiline group, as opposed to controls who entered the study from start of MDR tuberculosis treatment. However, the early mortality rate (as shown in Figure 3) was relatively low and did not differ significantly between the groups, suggesting limited bias toward survival in the bedaquiline group.
We were not able to obtain specific safety data related to bedaquiline use. Although pharmacovigilance is in place, the decentralization of bedaquiline use across many sites made obtaining electrocardiographic recordings unfeasible with the available resources for this study. Reassuringly, accumulating safety data from prospective observational studies suggest that the association with QT prolongation has not translated into adverse clinical outcomes [10, 23].
In conclusion, substituting bedaquiline for SLIs in the programmatic treatment of MDR tuberculosis is not associated with increased mortality rate and results in fewer unfavorable outcomes at 12 months than in patients who remain continue taking SLIs. The improved outcomes with bedaquiline use were driven by differences in sustained culture conversion, and reflected by the significantly lower rates of culture reversion among those patients. Notwithstanding the limitations of the study design, these findings provide additional evidence to support the routine inclusion of bedaquiline in MDR tuberculosis regimens [24].
Notes
Acknowledgments. We acknowledge the clinical, administrative, and managerial staff of the Western Cape Department of Health, the Provincial Health Data Centre at the Western Cape Department of Health, and colleagues involved in the national Bedaquiline Clinical Access Programme led by Norbert Ndjeka.
Disclaimer. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The opinions, findings, and conclusions expressed in this article reflect those of the authors alone.
Financial support. This work was supported by the Wellcome Trust (grants 098316 and 203135/Z/16/Z to G. M. and 203135/Z/16/ Z to S. W.), the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (grant 64787 to G. M.), National Research Foundation incentive funding (85858 to G. M.), the South African Medical Research Council through its TB and HIV Collaborating Centres Programme (TB/HIV/AIDS-01-2014 to G. M.), and the European & Developing Countries Clinical Trials Partnership (grant TMA2015 to S. W.).
Potential conflicts of interest. All authors: No reported funding conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 2. World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis, 2016 update. Annex 6. Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 3. Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis 2004; 8:1382–4. [PubMed] [Google Scholar]
- 4. Shean K, Streicher E, Pieterson E, et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One 2013; 8:e63057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falzon D, Gandhi N, Migliori GB, et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 2013; 42:156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim DH, Kim HJ, Park SK, et al. Treatment outcomes and survival based on drug resistance patterns in multidrug-resistant tuberculosis. Am J Respir Crit Care Med 2010; 182:113–9. [DOI] [PubMed] [Google Scholar]
- 7. Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009; 360:2397–405. [DOI] [PubMed] [Google Scholar]
- 8. Diacon AH, Donald PR, Pym A, et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother 2012; 56:3271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diacon AH, Pym A, Grobusch MP, et al. ; TMC207-C208 Study Group Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 2014; 371:723–32. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. Report of the Guideline Development Group Meeting on the use of bedaquiline in the treatment of multidrug-resistant tuberculosis: a review of available evidence. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 11. Fox GJ, Menzies D. A review of the evidence for using bedaquiline (TMC207) to treat multi-drug resistant tuberculosis. Infect Dis Ther 2013; 2:123–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization. WHO best-practice statement on the off-label use of bedaquiline and delamanid for the treatment of multidrug-resistant tuberculosis. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 13. Bonnet M, Bastard M, du Cros P, et al. Identification of patients who could benefit from bedaquiline or delamanid: a multisite MDR-TB cohort study. Int J Tuberc Lung Dis 2016; 20:177–86. [DOI] [PubMed] [Google Scholar]
- 14. Guglielmetti L, Hewison C, Avaliani Z, et al. Examples of bedaquiline introduction for the management of multidrug-resistant tuberculosis in five countries. Int J Tuberc Lung Dis 2017; 21:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guglielmetti L, Le Dû D, Jachym M, et al. ; MDR-TB Management Group of the French National Reference Center for Mycobacteria and the Physicians of the French MDR-TB Cohort Compassionate use of bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: interim analysis of a French cohort. Clin Infect Dis 2015; 60:188–94. [DOI] [PubMed] [Google Scholar]
- 16. Ndjeka N, Conradie F, Schnippel K, et al. Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. Int J Tuberc Lung Dis 2015; 19:979–85. [DOI] [PubMed] [Google Scholar]
- 17. van Heeswijk RP, Dannemann B, Hoetelmans RM. Bedaquiline: a review of human pharmacokinetics and drug-drug interactions. J Antimicrob Chemother 2014; 69:2310–8. [DOI] [PubMed] [Google Scholar]
- 18. Bastos ML, Lan Z, Menzies D. An updated systematic review and meta-analysis for treatment of multidrug-resistant tuberculosis. Eur Respir J 2017; 49:1600803. [DOI] [PubMed] [Google Scholar]
- 19. Günther G, Lange C, Alexandru S, et al. Treatment outcomes in multidrug-resistant tuberculosis. N Engl J Med 2016; 375:1103–5. [DOI] [PubMed] [Google Scholar]
- 20. Ahuja SD, Ashkin D, Avendano M, et al. ; Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9153 patients. PLoS Med 2012; 9:e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Svensson EM, Dosne AG, Karlsson MO. Population pharmacokinetics of bedaquiline and metabolite M2 in patients with drug-resistant tuberculosis: the effect of time-varying weight and albumin. CPT Pharmacometrics Syst Pharmacol 2016; 5:682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ho AM, Dion PW, Ng CS, Karmakar MK. Understanding immortal time bias in observational cohort studies. Anaesthesia 2013; 68:126–30. [DOI] [PubMed] [Google Scholar]
- 23. Pym AS, Diacon AH, Tang SJ, et al. ; TMC207-C209 Study Group Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J 2016; 47:564–74. [DOI] [PubMed] [Google Scholar]
- 24. Dheda K, Cox H, Esmail A, Wasserman S, Chang KC, Lange C. Recent controversies about MDR and XDR-TB: global implementation of the WHO shorter MDR-TB regimen and bedaquiline for all with MDR-TB?Respirology 2018; 23:36–45. [DOI] [PubMed] [Google Scholar]