Of patients with pneumonia coming from the community, 18% are immunocompromised. Specific immunocompromised states are associated with specific microbiology, which has to be taken into consideration when choosing empirical therapy.
Keywords: pneumonia, multidrug-resistant pathogens, microbiology, MRSA, immunocompromise
Abstract
Background
The correct management of immunocompromised patients with pneumonia is debated. We evaluated the prevalence, risk factors, and characteristics of immunocompromised patients coming from the community with pneumonia.
Methods
We conducted a secondary analysis of an international, multicenter study enrolling adult patients coming from the community with pneumonia and hospitalized in 222 hospitals in 54 countries worldwide. Risk factors for immunocompromise included AIDS, aplastic anemia, asplenia, hematological cancer, chemotherapy, neutropenia, biological drug use, lung transplantation, chronic steroid use, and solid tumor.
Results
At least 1 risk factor for immunocompromise was recorded in 18% of the 3702 patients enrolled. The prevalences of risk factors significantly differed across continents and countries, with chronic steroid use (45%), hematological cancer (25%), and chemotherapy (22%) the most common. Among immunocompromised patients, community-acquired pneumonia (CAP) pathogens were the most frequently identified, and prevalences did not differ from those in immunocompetent patients. Risk factors for immunocompromise were independently associated with neither Pseudomonas aeruginosa nor non–community-acquired bacteria. Specific risk factors were independently associated with fungal infections (odds ratio for AIDS and hematological cancer, 15.10 and 4.65, respectively; both P = .001), mycobacterial infections (AIDS; P = .006), and viral infections other than influenza (hematological cancer, 5.49; P < .001).
Conclusions
Our findings could be considered by clinicians in prescribing empiric antibiotic therapy for CAP in immunocompromised patients. Patients with AIDS and hematological cancer admitted with CAP may have higher prevalences of fungi, mycobacteria, and noninfluenza viruses.
During initial evaluation of a patient coming from the community with pneumonia, the identification of possible risk factors for multidrug-resistant organisms or unusual pathogens is crucial [1–3]. Because a microbiological identification is found in about 30% of hospitalized patients with pneumonia coming from the community, and usually requires 24–48 hours to be available, most of patients are treated empirically [4]. Delay in initiation of appropriate empiric antibiotic therapy is a known risk factor for worse clinical outcomes [5–7]; therefore, it is relevant to promptly recognize patients at risk for specific pathogens, specially multidrug-resistant or atypical microbes [1–3].
The aging of the population and advancements in therapeutic protocols have led to an increase prevalence of chronic diseases as well as long-term treatments with immunosuppressive agents [8, 9]. Thus, among patients with pneumonia coming from the community and admitted to the hospital, the number who might not be fully immunocompetent is constantly increasing [8, 9]. Nevertheless, the real prevalence of immunocompromise among patients with pneumonia coming from the community is still unknown. Moreover, guidelines for community-acquired and hospital-acquired pneumonia did not address this topic—what is more, they specifically excluded patients with clinical characteristics determining immunocompromise [5–7], and current evidence in literature is also scarce.
To our knowledge, there are no studies addressing the clinical evaluation and initial empirical antibiotic coverage of patients coming from the community with pneumonia and immunocompromise. Moreover, specific risk factors to assess the causative microbiology and help clinicians choose more appropriate management for these patients have not been clearly identified. Thus, the aim of the current study was to identify the prevalence, type, microbiology, and intercorrelations between different risk factors for immunocompromise in hospitalized patients with pneumonia coming from the community.
MATERIALS AND METHODS
Study Design and Population
This is a secondary analysis of the Global Initiative for MRSA Pneumonia (GLIMP) database [10]. The GLIMP study was an international, multicenter, observational, point-prevalence study of adult patients hospitalized for community-onset pneumonia in 54 countries worldwide. Patients were enrolled on a single day during the months of March, April, May, and June 2015. The methods of the GLIMP study have been published elsewhere [10]. The coordinating center (University of Texas Health Science Center, San Antonio) received approval from its institutional review board (No. HSC20150184E).
All adult patients (aged >18 years old) coming from the community and hospitalized with pneumonia during study period were included. Pneumonia was defined as the presence of a new pulmonary infiltrate on chest radiograph at the time of hospitalization, associated with ≥1 of the following criteria: (1) new or increased cough with/without sputum production and/or purulent respiratory secretions, (2) fever or hypothermia, and (3) evidence of systemic inflammation (ie, abnormal white blood cell count or increased C-reactive protein or procalcitonin level). Hospitalized patients with a diagnosis of hospital-acquired or ventilator-associated pneumonia were excluded.
Data Collection
Data were collected from medical records at the time of hospital admission. Data gathered included demographics; respiratory and cardiovascular comorbid conditions; immunocompromised status and other chronic medical conditions; severity of pneumonia (defined as either intensive care unit admission, use of invasive or noninvasive mechanical ventilation, or use of vasopressors/inotropes during the first 24 hours after hospital admission); and specific risk factors for resistant pathogens infection, including chronic aspiration, being bedridden, malnutrition, presence of enteric tube feeding and indwelling catheters (including central venous and urinary catheters), previous infections, chronic microbial colonization, and previous healthcare exposures. The number and type of microbiological samples obtained within 24 hours after hospital admission were also collected. Culture-positive tests, kind of sample, and antibiotic resistance patterns were also gathered, along with empiric antibiotic treatment, given within 24 hours after hospital admission.
Microbiological Workup
Diagnostic testing was performed according to local standard operating procedures and included collection of respiratory and blood cultures and testing for urinary antigens. Microbiological examinations and susceptibility testing were performed according to local standard protocols within the first 24 hours after hospital admission [11]. Multivariable logistic regression models were performed for patients who had a positive culture, to identify specific risk factors for single pathogens.
Causative pathogens were stratified according to the coverage of standard therapy for community-acquired pneumonia (CAP) [5–7]. Those not covered by standard CAP therapy included the following: non–community-acquired bacteria (Acinetobacter baumanii, Enterococcus vancomycin-resistant, Nocardia spp.), mycobacteria, fungi (Aspergillus fumigatus, Coccidioides, Criptococcus, Pneumocystis jirovecii), and viruses other than influenza [5–7]. Those covered by standard CAP therapy included Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, methicillin-sensitive S. aureus, Enterobacter spp., Enterococcus spp., Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Proteus mirabilis, Serratia marcescens, Streptococcus pneumoniae, Chlamydia pneumoniae, Mycoplasma pneumoniae, Legionella pneumophilia, anaerobes bacteria, and influenza viruses. Atypical pathogens included C. pneumoniae, M. pneumoniae, and L. pneumophilia. CAP therapy was defined as β-lactams (ceftriaxone, ampicillin-sulbactam, amoxicillin-clavulanate, cefepime, ceftazidime, piperacillin-tazobactam) plus macrolide, or fluoroquinolones alone, and, eventually, in association with vancomycin, linezolid, or oseltamivir [5–7].
Definition of Immunocompromised and Study Groups
Immunocompromise was defined as the presence of ≥1 of the following risk factors: (1) AIDS, defined either as human immunodeficiency virus infection with CD4+ lymphocyte count <200/µL or by the occurrence of AIDS-defining conditions; (2) aplastic anemia; (3) asplenia; (4) hematological cancer, defined as lymphoma, acute or chronic leukemia, or multiple myeloma; (5) chemotherapy during the last 3 months; (6) neutropenia, defined as a neutrophil count <500/dL at complete blood cell count; (7) biological drug use (including trastuzumab and therapies for autoimmune diseases, eg, anti–tumor necrosis factor α, prescribed during ≥6 months before hospital admission); (8) lung transplantation; (9) chronic steroid use (>10 mg/d of prednisone or equivalent ≥3 months before hospital admission); (10) lung cancer with either neutropenia or chemotherapy; (11) other solid tumor with either neutropenia or chemotherapy; (12) other immunocompromise (any immunocompromised state, including congenital/genetic immunocompromise and immunosuppressive therapy due to hematological cancer/solid organ transplantation other than lung). Two study groups were identified: those with versus those without 1 risk factor for immunocompromise.
Statistical Analysis
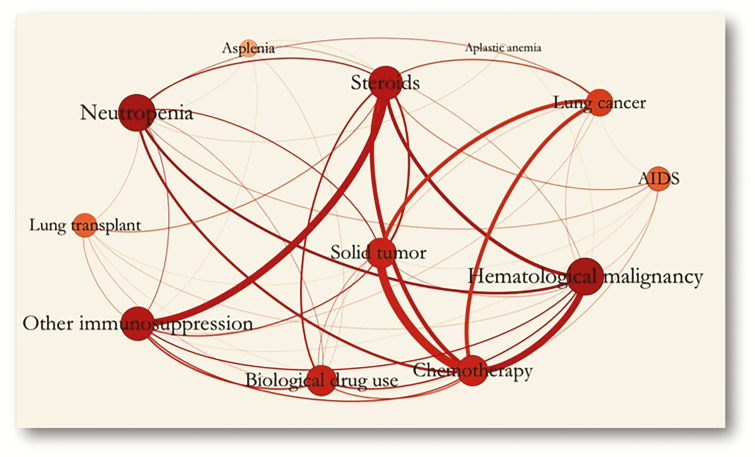
Categorical variables, expressed as counts (percentages), were compared using the χ2 test. Continuous variables were compared using the unpaired Student t test or the Mann-Whitney test, when appropriate. Statistical significance was defined as P < .05. A network analysis was conducted to represent the frequencies of all immunocompromise variables and their relationships. The size of the circles (the circles visible in Figure 4 [network analysis], each representing a single risk factor for immunocompromise) represents both prevalence of the risk factor and strength of association with other variables.
The predictive value of each variable was categorized by quartiles and analyzed using a univariate regression logistic analysis. A multivariable model was obtained using a Cox regression analysis to identify independent predictors of specific pathogens, using an entry level of P value ≤0.05 and a removal level of P value ≥0.10. Hazard ratios and adjusted analyses were obtained. All statistical analyses were performed with IBM SPSS software (version 22, Statistics for Mac; version 22.0, IBM Crop), and Stata 13 software (StataCorp).
RESULTS
Prevalence of Risk Factors for Immunocompromise
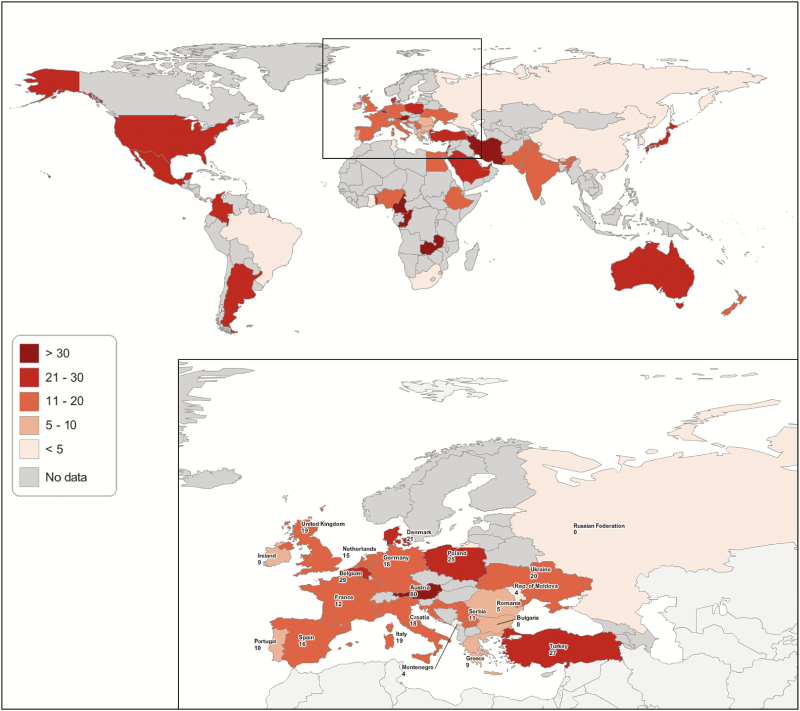
Among 3702 patients enrolled in the GLIMP database, ≥1 risk factor for immunocompromise was identified in 652 (17.6%). The prevalences of patients with pneumonia coming from the community and with ≥1 risk factor for immunocompromise differed among continents and countries, as depicted in Figure 1 and Supplementary Tables 1 and 2. The prevalence of immunocompromise was significantly higher in both North and South America than in the rest of the world (24.0% vs 16.5 [P < .001] and 24.8% vs 17.2 [P = .006], respectively) (Supplementary Table 1).
Figure 1.
Distribution of prevalence of immunocompromise among the different countries participating in the study, categorized as no data, <5%, 5%–10%, 11%–20%, 21%–30%, or >30% of total cases.
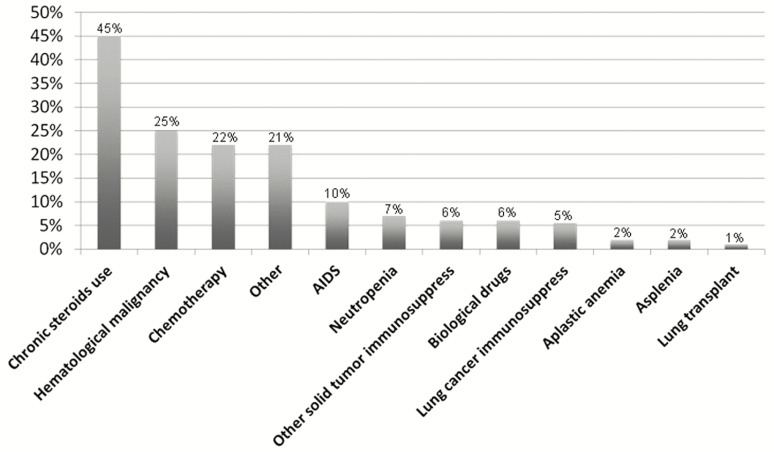
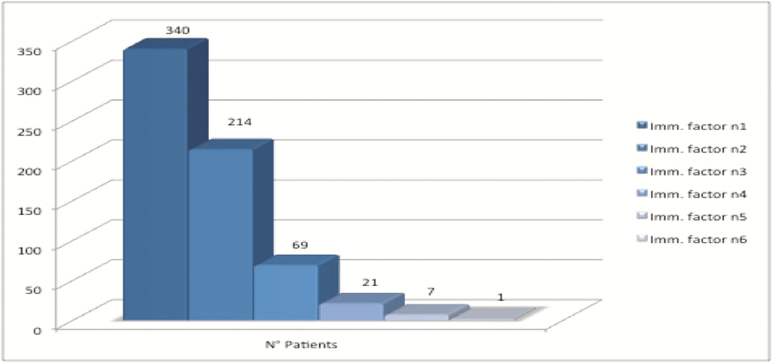
The prevalence of each risk factor for immunocompromise is depicted in Figure 2, with chronic steroid use (45.0%), hematological cancer (25.0%), and chemotherapy (22.0%) being the most frequent ones. A total of 312 patients (8.4%) had >1 risk factor for immunocompromise (Figure 3).
Figure 2.
Prevalence of each single risk factor for immunocompromise.
Figure 3.
Prevalence of the number of risk factors present simultaneously in a single patient.
Network Analysis Among Risk Factors for Immunocompromise
The results of the network analysis of all risk factors for immunocompromise are depicted in Figure 4. Relationships were identified between chemotherapy and solid tumor other than lung cancer, hematological cancer, and chronic steroid use, and between other immunocompromise and chronic steroid use.
Figure 4.
Network analysis between risk factors for immunocompromise.
Clinical and Microbiological Characteristics of Patients With Immunocompromise
Clinical features and disease severity of immunocompetent versus immunocompromised patients are shown in Table 1 and Supplementary Table 3. Immunocompromised patients were significantly younger and malnourished, had a higher frequency of comorbid conditions, previous infections, and colonization by resistant pathogens, and had more frequent contacts with the healthcare system. The prevalences of severe pneumonia did not differ among the 2 study groups.
Table 1.
Clinical and Severity Characteristics of the 2 Study Groups (Immunocompetent vs Immunocompromised)
| Variable | Patients, No. (%)a | P Value | |
|---|---|---|---|
| Immunocompetent (n = 3050) | Immunocompromised (n = 652) |
||
| Age, median (IQR) | 69 (54–81) | 65 (52–74) | <.001 |
| Underweight | 125 (6.5) | 41 (10.5) | .004 |
| Malnutrition | 243 (8.0) | 80 (12.3) | <.001 |
| Bedridden | 355 (11.6) | 60 (9.2) | .04 |
| Chronic aspiration | 224 (7.3) | 33 (5.1) | .02 |
| Bronchiectasis | 136 (4.5) | 42 (6.4) | .03 |
| Severe COPD | 72 (2.4) | 28 (4.3) | .006 |
| Interstitial lung disease | 60 (2.0) | 35 (5.4) | <.001 |
| Lung transplantation | 0 (0.0) | 7 (1.1) | <.001 |
| Tracheostomy | 37 (1.2) | 16 (2.5) | .02 |
| Hypertension | 1401 (45.9) | 254 (39.0) | .001 |
| Liver disease | 103 (3.4) | 37 (5.7) | .005 |
| Cirrhosis | 50 (1.6) | 20 (3.1) | .02 |
| Dementia | 372 (12.2) | 36 (5.5) | <.001 |
| Enteral tube feeding | 36 (1.2) | 16 (2.5) | .01 |
| Chronic renal failure | 315 (10.3) | 85 (13.0) | .04 |
| Hemodialysis | 34 (1.1) | 18 (2.8) | .001 |
| ICS use | 462 (15.2) | 128 (19.6) | .005 |
| PPI use | 777 (25.5) | 251 (38.5) | <.001 |
| Indwelling catheter | 52 (1.7) | 27 (4.1) | <.001 |
| Prior mycobacteria diseases | 70 (2.3) | 26 (4.0) | .01 |
| Prior ESBL | 39 (1.3) | 16 (2.5) | .02 |
| Prior Pseudomonas | 68 (2.2) | 33 (5.1) | <.001 |
| Severe CAP | 840 (27.5) | 190 (29.1) | .41 |
Abbreviations: CAP, community-acquired pneumonia; COPD, chronic obstructive pulmonary disease; ESBL, extended-spectrum β-lactamase; ICS, inhaled corticosteroids; IQR, interquartile range; PPI, proton pump inhibitors.
aData represent No. (%) unless otherwise specified.
Microbiological testing was performed in 91.0% (596 of 652) of immunocompromised and 86.0% (2626 of 3050) of immunocompetent patients (P < .001). Bacteremia was found in 6.0% (36 of 596) of immunocompromised and 5.5% (145 of 2626; P = .62) of immunocompetent patients. At least 1 positive culture was obtained in 40.0% (238 of 596) immunocompromised and 36.0% (935 of 2626) immunocompetent patients (P = .047). Microbiological findings are provided in Table 2 and Supplementary Table 4. Among pathogens covered by standard therapy, P. aeruginosa was more prevalent in immunocompromised patients (35 [5.9%] vs 98 [3.7%] patients; P < .02). Among pathogens not usually covered by standard therapy, immunocompromised patients were more likely to be infected by Nocardia spp. (4 [0.7%] vs 0 [0%] patients; P < .001), nontuberculous mycobacteria (NTM) (5 [0.8%] vs 2 [0.1%]; P < .002), A. fumigatus (8 [1.3%] vs 10 [0.4%]; P < .01), P. jirovecii (12 [2.0%] vs 5 [0.2%]; P < .02), and viruses, such as coronavirus (3 [0.5%] vs 3 [0.1%]; P < .047), and respiratory syncytial virus (6 [1.0%] vs 7 [0.3%]; P < .03).
Table 2.
Pathogens in the 2 Study Groups
| Pathogen | Patients, No. (%) | P Value | |
|---|---|---|---|
| Immunocompetent (n = 2626) | Immunocompromised (n = 596) | ||
| Pathogens covered by CAP therapy | |||
| Streptococcus pneumoniae | 218 (8.3) | 50 (8.4) | >.99 |
| Atypical | 50 (1.9) | 13 (2.2) | .78 |
| Legionella | 21 (0.8) | 10 (1.7) | .08 |
| MRSA | 83 (3.2) | 12 (2.0) | .17 |
| MSSA | 73 (2.8) | 20 (3.4) | .53 |
| Pseudomonas aeruginosa | 98 (3.7) | 35 (5.9) | .02 |
| Haemophilus influenzae | 65 (2.5) | 10 (1.7) | .31 |
| Klebsiella pneumoniae | 89 (3.4) | 22 (3.7) | .81 |
| Influenza virus | 126 (4.8) | 28 (4.7) | >.99 |
| Pathogens not covered by CAP therapy | |||
| Non-CAP bacteria | |||
| Acinetobacter baumanii | 33 (1.3) | 7 (1.2) | >.99 |
| Nocardia spp. | 0 (0.0) | 4 (0.7) | <.001 |
| Mycobacteria | |||
| Mycobacterium tuberculosis | 21 (0.8) | 5 (0.8) | >.99 |
| NTM | 2 (0.1) | 5 (0.8) | .002 |
| Fungi | |||
| Aspergillus fumigatus | 10 (0.4) | 8 (1.3) | .01 |
| Actinomyces | 2 (0.1) | 0 (0.0) | >.99 |
| Cryptococcus | 3 (0.1) | 0 (0.0) | .94 |
| Pneumocystis jirovecii | 5 (0.2) | 13 (2.2) | <.001 |
| Viruses | |||
| Adenovirus | 5 (0.2) | 0 (0.0) | .62 |
| Coronavirus | 3 (0.1) | 3 (0.5) | .047 |
| Metapneumovirus | 3 (0.1) | 2 (0.3) | .51 |
| RSV | 7 (0.3) | 6 (1.0) | .03 |
| MDR pathogens | 231 (8.8) | 54 (9.0) | .54 |
Abbreviations: CAP, community-acquired pneumonia; MDR multidrug-resistant; MRSA methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S. aureus; NTM, nontuberculous mycobacteria; RSV, respiratory syncitial virus.
Once adjusted for confounders, no risk factors of immunocompromise have been recognized for P. aeruginosa infection. Likewise, pathogens not covered by usual CAP therapy were found to be associated not with immunocompromise but with chronic obstructive pulmonary disease (odds ratio [OR], 1.78; 95% confidence interval [CI], 1.07–2.99; P = .03), tracheostomy (2.91; 1.01–8.38; P = .048), and severe pneumonia (2.36; 1.42–3.93; P = .001) (Table 3).
Table 3.
Multivariable Logistic Regression Analysis
| Variable | OR (CI 95%) | ||||
|---|---|---|---|---|---|
| Pseudomonas aeruginosa | Non-CAP Bacteria | Fungi | Mycobacterium tuberculosis | Virus Other Than Influenza | |
| Severe COPD | 2.89 (1.34–6.22) | … | … | … | … |
| Tracheostomy | 6.95 (2.87–16.85) | 2.91 (1.01–8.38) |
… | … | … |
| ICS use | 1.76 (1.09–2.82) | … | … | … | … |
| Indwelling catheter | 2.49 (1.02–6.06) | … | … | … | … |
| Prior Pseudomonas | 19.20 (11.71–31.50) |
… | … | … | … |
| COPD | … | 1.78 (1.07–2.99) |
… | … | … |
| Severe CAP | … | 2.36 (1.42–3.93) |
… | … | 2.56 (1.27–5.19) |
| AIDS | … | … | 15.10 (6.36–35.88) |
… | … |
| Hematological cancer | … | … | 4.65 (1.85–11.69) |
… | 5.49 (2.20–13.70) |
| Malnutrition | … | … | … | 5.14 (2.21–11.93) | … |
Blank cells indicate no statistical significancy.
Abbreviations: CAP community-acquired pneumonia; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroids; OR, odds ratio.
Results showed that AIDS (OR, 15.10; 95% CI, 6.36–35.88; P ≤ .001) and hematological cancer (4.65; 91.85–11.69; P = .001) were independently associated with fungal infections; hematological cancer (5.49; 2.20–13.70; P < .001) and severe pneumonia (2.56; 1.27–5.19; P = .009) with infection by viruses other than influenza; and AIDS (4.41; 1.53–12.73; P = .006) and malnutrition (4.50; 2.08–9.72; P < .001) with mycobacterial infections. An additional analysis was conducted on mycobacteria, including M. tuberculosis and NTM. At multivariable analysis, M. tuberculosis was independently associated with malnutrition only (OR, 5.14; 95% CI, 2.21–11.93; P < .001). At univariate analysis, patients with AIDS were at higher risk for NTM (23.06; 4.39–121.12; P < .001).
A subanalysis was conducted among patients with chronic steroid use versus other risk factors for immunocompromise. Patients with chronic steroid use seemed to be more frequently affected by bacteria not covered by standard CAP therapy (10 [3.4%] vs 1 [0.3%] patients; P = .002), Nocardia spp. in particular (4 [0.4%] vs 0 [0.0%]; P = .03). No differences in the severity of the disease were found (see Supplementary Table 5).
DISCUSSION
The main findings of the present study are as follows: (1) 17.6% of patients admitted with pneumonia from the community have ≥1 risk factor for immunocompromise, with significant differences among continents and countries (ranging from 15.4% to 24.8% by continent and from 80.0% to 4.1% by country); (2) chronic steroid use is by far the most prevalent risk factor leading to immunocompromise, followed by hematological cancer and chemotherapy; (3) 1 of 2 immunocompromised patients has an overlap of ≥2 risk factors, which are also associated between one another in different ways; and (4) the 2 risk factors for immunocompromise independently associated with specific pathogens are AIDS (ie, fungal and mycobacterial infections) and hematological cancer (ie, fungal infection and viral infections other than influenza).
Almost 1 in 5 hospitalized patients with CAP are not immunocompetent. Therefore, it is mandatory to provide clinicians with recommendations or guidelines for the management of hospitalized patients with pneumonia coming from the community who have risk factors for immunocompromise. Currently, there are no guidelines for assessing pneumonia in immunocompromised patients coming from the community. Randomized controlled trials (RCTs) and observational prospective studies are missing owing to the fact that, generally, studies assessing management strategies for pneumonia exclude immunocompromised patients or take into account only a single specific risk factor [12–21]. This lack of information about immunocompromise could lead to both underestimation of the real prevalence with a higher rate of treatment failure and to overestimation and overuse of wide-spectrum antibiotics.
We found a 17.6% global prevalence of immunocompromise among patients coming from the community with pneumonia, with a significantly higher frequency in South and North America. This variability among continents and countries is probably attributable to different healthcare systems and rates of hospitalization of immunocompromised patients. Our analysis showed that the most frequent risk factor for immunocompromise is the chronic use of systemic steroids. Aging of the population and therapeutic advancements have favored the increased burden of chronic diseases and long-term therapies with immunosuppressive agents [8, 9]. In particular, steroids are the agents most frequently prescribed, for their wide spectrum of efficacy in several diseases [13, 17, 19]. Therefore, many patients presenting to the emergency room with pneumonia are receiving chronic steroid treatment. No data are available on this population group, and further studies are needed to characterize these patients and provide individualized management.
Hematological cancer and chemotherapy were other leading immunocompromised factors. These findings are consistent with those in previous studies; patients recruited in observational studies include patients with solid or hematological cancer and those who underwent chemotherapy with associated neutropenia [15–20, 22]. Dedicated guidelines and recommendations are available, especially on respiratory viruses, fungi, and P. jirovecii [23–26].
Our network analysis showed that several risk factors for immunocompromise show associations, especially chemotherapy, associated with hematological cancer and solid tumor, and other immunocompromise, associated with chronic steroid use. Moreover, neutropenic patients are well represented and mainly affected also by hematological cancer or under treatment with chemotherapy. Our results suggest that patients may have >1 risk factor characteristic and clinical assessment should be comprehensive, taking into consideration risk factors for immunocompromise and their associated biological mechanisms. In contrast, AIDS, lung transplantation, asplenia, and aplastic anemia seem to be less frequent at admission and to represent distinct clinical entities. Findings of previous studies seem to be in line with our results, with AIDS patients considered as a distinct patient population and with very few observational studies available on asplenia and aplastic anemia [21, 27–31]
In agreement with previous reports, S. pneumoniae is the leading microorganism in both immunocompromised and immunocompetent groups [32, 33]. Among pathogens covered by standard CAP therapy, only P. aeruginosa was more frequently isolated in immunocompromised compared with immunocompetent patients. These findings differ from microbiological results of previous studies. Gram-positive bacteria, especially S. aureus, were more frequently identified in patients with immunocompromise of different causes [22, 30, 34]. Only Li and coauthors [13] found patients with immunological disorders, treated with systemic steroids and cytotoxic agents, to have a higher incidence of infections caused by gram-negative bacteria, mainly P. aeruginosa. This similarity with our findings could be explained by the prevalence of patients exposed to chronic steroids in our cohort.
Among pathogens not covered by standard CAP therapy, immunocompromised patients were more frequently infected by Nocardia spp., NTM, P. jirovecii, A. fumigatus, and viruses other than influenza. Infections by P. jirovecii and NTM are frequently identified in patients with AIDS [35]. P. jirovecii is also frequent in other types of immunocompromise, such as solid or hematological cancer in patients who underwent chemotherapy [18, 19, 36]. Fungal infections (eg, Candida spp. and A. fumigatus) are highly incident in neutropenic hematological cancer patients [22, 37]. Viral infections other than influenza, especially respiratory syncytial virus, are more frequent in patients who underwent hematopoietic stem cell or lung transplantation [38, 39]. Conversely, Nocardia spp. infections are mainly described in solid organ transplant recipients [40]. These results, consistent with previous findings, suggest the need for a more in-depth microbiological workup, including community-acquired pathogens and microorganisms not covered by standard therapy.
Surprisingly, we found that risk factors for immunocompromise were not independently associated with P. aeruginosa or non–community-acquired bacteria; in contrast, AIDS and hematological cancer are both associated with fungal, mycobacterial, and noninfluenza viral pneumonia, respectively. Empirical therapy should include P. aeruginosa coverage, which is highly prevalent in immunocompromised patients. On the contrary, particular attention should be given to fungal, mycobacterial, and viral causes should be for patients admitted with AIDS and hematological cancer [21–29].
Finally, bacteremia rates did not differ between study groups. To our knowledge, there are no studies on bacteremia and immunocompromise in general. The majority of studies have focused on bacteremia in hematopoietic stem cell transplantation, with prevalences varying from 6% to 44% depending on the type of bacteria and host-related factors [41–43]. Few studies addressed this topic in kidney transplant recipients, reporting a prevalence of bacteremia ranging from 25% to 69% [44, 45]. Finally, few studies have addressed HIV and bacteremia, with prevalences ranging from 10% to 25%, depending on the pathogen and grade of immunosuppression [46, 47]. The prevalence of bacteremia in our study was 5T–6% in both immunocompetent and immunocompromised patients. Differences in the prevalence of bacteremia are due mainly to differences between the risk factors for immunocompromise in our study (chronic steroid use, hematological cancer, and chemotherapy) and those previously reported in the literature.
The current study has both limitations and strengths. First of all, to our knowledge, this is the first study showing a worldwide perspective on immunocompromise among patients coming from the community with pneumonia, with a large and diverse sample of patients enrolled across different countries in 6 continents. However, we were not able to involve many investigators from Asia and Africa, and most cases occurred in North America and Europe, thus limiting the generalizability of our findings. Another major limitation is the unfeasibility of grading the severity of immunocompromise and, therefore, stratifying patients and defining the physiopathological interaction between different risk factors, especially with regard to the use of biological drugs and chronic steroids. Furthermore, potentially important risk factors for an immunocompromised state, such as solid organ transplants other than lung, have not been specifically investigated. Finally, no outcome data have been collected, and this strongly limits our speculations as to the correct empiric antibiotic therapy for use in immunocompromised patients with CAP.
In conclusion, our study offers to the scientific community a perspective on immunocompromised patients coming from the community with pneumonia. Future prospective studies on patients with specific risk factors for immunocompromise could provide practical recommendations. In particular, it will be crucial to prepare guidelines on certain prevalent population groups, such as patients exposed to chronic steroids and those with hematological cancer.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. M. F. D. P., G. S., S. A., and M. I. R. participated in study design, analysis of data, and writing of the manuscript and take responsibility for the integrity of the work. A. G., D. R., S. T., L. F. R., J. R., J. G. d. C., and F. B. critically reviewed the final manuscript.
Acknowledgments. We thank the Asociacion Latinoamericana de Torax, European Respiratory Society, World Federation of Societies of Intensive and Critical Care Medicine, and American College of Chest Physicians for their support of this project. We also thank the following study contributors for their valuable collaboration. Argentina: Patricia Karina Aruj (Department of Internal Medicine, University Hospital Alfredo Lanari, Buenos Aires), Silvia Attorri (Hospital Luis Lago Maggiore, Mendoza), Enrique Barimboim (Hospital Central de Mendoza), Juan Pablo Caeiro and María I. Garzón (Hospital Privado Universitario, Córdoba), Victor Hugo Cambursano, A. Cazaux (Servicio de Neumologia, Hospital Rawson, Córdoba), Adrian Ceccato (Hospital Nacional Prof Alejandro Posadas), Julio Chertcoff, Florencia Lascar, and Fernando Di Tulio (Critical Care Unit and Respiratory Medicine, Buenos Aires British Hospital), Ariel Cordon Díaz (Hospital General Alvear, Ciudad, Mendoza), Lautaro de Vedia (Respiratory Intensive Care Unit, Hospital Muñiz, Buenos Aires) Maria Cristina Ganaha (Infectious Diseases Ward, Hospital Interzonal General de Agudos Vicente Lopez y Planes from General Rodriguez, Buenos Aires), Sandra Lambert (Hospital El Cruce–Alta Complejidad en Red), Gustavo Lopardo (Hospital Bernardo Houssay, Vicente López), Carlos M. Luna (Pulmonary Medicine Division, Department of Medicine, Hospital de Clínicas, Universidad de Buenos Aires), Alessio Gerardo Malberti (Hospital Nuestra Señora del Carmen), Nora Morcillo and Silvina Tartara (Hospital Zonal Especializado de Agudos y Crónicos), Antonio A. Cetrangolo (an individual who is being acknowledged for his contribution to the study), Claudia Pensotti (Infectious Diseases and Infection Control Department, Clinica Privada Monte Grande, Buenos Aires), Betiana Pereyra (Hospital San Roque, Córdoba), Pablo Gustavo Scapellato (Infectious Diseases Department, Hospital D. F. Santojanni), Juan Pablo Stagnaro (HZGA Mi Pueblo, Florencio Varela); Australia: Sonali Shah (Department of General Medicine, Austin Hospital, Heidelberg); Austria: Felix Lötsch and Florian Thalhammer (Division of Infectious Diseases and Tropical Medicine, Department of Medicine I, Medical University of Vienna); Belgium: Kurt Anseeuw (ZNA Campus Stuivenberg, Antwerp), Camille A. Francois (Anesthesia and Critical Care Department, Erasme University Hospital, Brussels), Eva Van Braeckel (Department of Respiratory Medicine, Ghent University Hospital), Jean Louis Vincent (Department of Intensive Care, Erasme University Hospital, Université Libre de Bruxelles, Brussels); Benin: Marcel Zannou Djimon, Jules Bashi and Roger Dodo (Centre Hospitalier Universitaire HKM of Cotonou); Brazil: Simone Aranha Nouér (Federal University of Rio de Janeiro); Bulgaria: Peter Chipev and Milena Encheva (Clinic of Pulmonary Diseases, Military Medical Academy, Sofia), Darina Miteva, (UMHAT St Marina, Varna), Diana Petkova (University Hospital Varna); Cameroon: Adamou Dodo Balkissou (Yaounde Jamot Hospital, Yaounde), Eric Walter Pefura Yone (Département de Médecine Interne, University of Yaounde), Bertrand Hugo Mbatchou Ngahane (Douala General Hospital); China: Ning Shen (Respiratory Medicine, Peking University Third Hospital, Beijing), Jin-fu Xu (Department of Respiratory Medicine, Shanghai Pulmonary Hospital, Tongji University); Colombia: Carlos Andres Bustamante Rico and Ricardo Buitrago (Clinica Shaio, Bogota), Fernando Jose Pereira Paternina (Las Americas Clinic, Medellin); Congo: Jean-Marie Kayembe Ntumba (Cliniques Universitaires de Kinshasa); Croatia: Vesna Vladic Carevic (Interne Medicine, Dubrovnik), Marko Jakopovic (Medical School, University of Zagreb, Department for Respiratory Diseases Jordanovac, University Hospital Centre Zagreb), Mateja Jankovic (University Hospital Center Zagreb, Department for Respiratory Diseases), Zinka Matkovic (University Hospital Dubrava, Zagreb), Ivan Mitrecic (Karlovac General Hospital); Denmark: Marie-Laure Bouchy Jacobsson (Emergency Department, North Zealand’s Hospital–Hillerød), Anette Bro Christensen (Department of Anaethesiology, Viborg Region Hospital), Uffe Christian Heitmann Bødtger (Department of Pulmonology, Naestved Hospital), Christian Niels Meyer (Department of Internal Medicine, Roskilde Hospital, Copenhagen University Hospital, Roskilde), Andreas Vestergaard Jensen, Gertrud Baunbæk-Knudsen, Pelle Trier Petersen, and Stine Andersen (Department of Lung and Infectious Diseases, Nordsjællands Hospital-Hillerød); Egypt: Ibrahim El-Said Abd El-Wahhab (Thoracic Medicine, Faculty of Medicine, Mansoura University), Nesreen Elsayed Morsy (Pulmonary, Critical Care and Sleep Medicine, Faculty of Medicine, Mansoura University), Hanaa Shafiek (Chest Diseases Department, Faculty of Medicine, Alexandria University), Eman Sobh (Chest Diseases Department, Al-Azhar University, Cairo); Ethiopia: Kedir Abdella Abdulsemed (Department of Medical Laboratory Science and Pathology, College of Health Sciences, Mycobacteriology Research Centre, Institute of Biotechnology Research, Jimma University); France: Fabrice Bertrand (Critical Care Unit, Robert Ballanger Hospital, Aulnay sous Bois), Christian Brun-Buisson (University Hospital of Henri-Mondor, Créteil), Etienne de Montmollin (Intensive Care Unit, Hôpital Delafontaine, Centre Hospitalier de Saint-Denis), Muriel Fartoukh (Unité de Réanimation Médico-Chirurgicale, Pôle Thorax Voies Aériennes, Hôpital Tenon, Groupe Hospitalier Est Parisien), Jonathan Messika (Publique-Hôpital de Paris, Service de Réanimation Médico-chirurgicale, Hôpital Louis Mourier, Colombes, and Université Paris Diderot, IAME, UMR 1137, Sorbonne Paris Cité), Pierre Tattevin (Infectious Diseases and ICU, Pontchaillou University Hospital, Rennes), Abdo Khoury (Department of Emergency Medicine and Critical Care, University of Franche–Comté, Medical Center); Gambia: Bernard Ebruke (Medical Research Council Unit, Fajara, Gambia); Germany: Michael Dreher (Department of Cardiology, Pneumology, Vascular Medicine and Intensive Care Medicine, University Hospital Aachen), Martin Kolditz (Division of Pulmonology, Medical Department I, University Hospital Carl Gustav Carus, Technische Universität Dresden), Matthias Meisinger, Klinikum Niederlausitz, Klinik für Innere Medizin und Intensivmedizin, Senftenberg), Mathias W. Pletz and Stefan Hagel (Center for Infectious Diseases and Infection Control, Jena University Hospital), Jan Rupp (Department of Infectious Diseases and Microbiology, University of Lübeck), Tom Schaberg (Zentrum für Pneumologie, Agaplesion Diakonieklinikum Rotenburg), Marc Spielmanns (Internal Medicine Department, Pulmonary Rehabilitation and Department of Health, School of Medicine, University Witten-Herdecke, St Remigius Hospital, Leverkusen) Petra Creutz and Norton Suttorp (Department of Infectious Disease and Respiratory Medicine, Charité–University Medicine, Berlin); Ghana: Beatrice Siaw-Lartey (Komfo-Anokye Teaching Hospital, Kumasi); Greece: Katerina Dimakou (5th Respiratory Medicine Department, SOTIRIA Chest Hospital, Athens), Dimosthenis Papapetrou (Medical Group of Athens, Paleo Faliro Clinic, Athens), Evdoxia Tsigou and Dimitrios Ampazis (Agioi Anargiroi Hospital, Kifissia, Athens), Evangelos Kaimakamis (Intensive Care Unit, G. Papanikolaou General Hospital of Thessaloniki); India: Mohit Bhatia (S. S. Hospital IMS BHU Varanasi), Raja Dhar (Fortis Hospitals, Kolkata), George D’Souza (Department of Pulmonary Medicine, St John’s Medical College Hospital, Bangalore), Rajiv Garg (Department of Respiratory Medicine, King George’s Medical University UP, Lucknow), Parvaiz A. Koul (Department of Internal and Pulmonary Medicine, SheriKashmir Institute of Medical Sciences, Srinagar), P. A. Mahesh and B. S. Jayaraj (Department of Pulmonary Medicine, JSS Medical College, JSS University, Mysore), Kiran Vishnu Narayan (Pulmonary Medicine, Government Medical College Kozhikode, Kerala), Hirennappa B. Udnur and Shashi Bhaskara Krishnamurthy (Columbia Asia Hospital, Hebbal, Bengaluru, Karnataka), Surya Kant (Department of Respiratory Medicine, King George’s Medical University, Chowk, Lucknow, Uttar Pradesh), Rajesh Swarnakar (Getwell Hospital and Research Institute, Dhantoli, Nagpur), Sneha Limaye and Sundeep Salvi (on behalf of the Respiratory Research Network of India from the Chest Research Foundation in Pune); Iran: Keihan Golshani (Isfahan University of Medical Sciences); Ireland: Vera M. Keatings (Letterkenny General Hospital, County Donegal), Ignacio Martin-Loeches (Multidisciplinary Intensive Care Research Organization, St James’s University Hospital, Trinity Centre for Health Sciences Dublin); Israel: Yasmin Maor (Infectious Disease Unit, affiliated with Tel Aviv University, Wolfson Medical Center, Holon), Jacob Strahilevitz (Department of Clinical Microbiology and Infectious Diseases, Hadassah-Hebrew University, Jerusalem); Italy: Salvatore Battaglia, University of Palermo, Pneumologia DiBiMIS, Palermo), Maria Carrabba (Internal Medicine Department, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca’ Granda Ospedale Maggiore Policlinico, Milano), Piero Ceriana (Pulmonary Rehabilitation, IRCCS Fondazione Maugeri, Pavia), Marco Confalonieri (Department of Pulmunology, University Hospital, Trieste), Antonella d’Arminio Monforte (Department of Health Sciences, Clinic of Infectious Disease, San Paolo Hospital, University of Milan), Bruno Del Prato (Interventional Pneumology, Hospital Antonio Cardarelli, Naples), Marino De Rosa (Unità Operativa Complessa [UOC] Pneumologia PO San Filippo Neri Azienda Sanitaria Locale Roma E Roma), Riccardo Fantini (Respiratory Diseases Clinic, Policlinico di Modena, Modena), Giuseppe Fiorentino (UOC Fisiopatologia e Riabilitazione Respiratoria Azienda Ospedaliera Ospedali dei Colli PO Monaldi), Maria Antonia Gammino (Pulmonary Medicine Unit, San Martino Hospital, Sardegna), Francesco Menzella (Department of Cardiac-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS–Arcispedale Santa Maria Nuova, Reggio Emilia), Giuseppe Milani (Azienda Ospedaliera Sant Anna di Como, Presidio Ospedale S. Anna Nuovo, Unità Operativa di Pneumologia, Como), Stefano Nava (Alma Mater University of Bologna, DIMES, Respiratory and Critical Care Unit Sant’Orsola Malpighi Hospital), Gerardo Palmiero (Respiratory Unit, Versilia Hospital, Lido di Camaiore, Lucca), Roberta Petrino and Barbra Gabrielli (Emergency Medicine Unit, S. Andrea Hospital, Vercelli), Paolo Rossi (Internal Medicine Department, Azienda Ospedaliero–Universitaria S. Maria della Misericordia, Udine), Claudio Sorino, Pulmonology Unit, A. O. Sant’Anna di Como), Gundi Steinhilber (Spedali Civili Brescia, U. O. Pneumologia e Fisiopatologia Respiratoria, Brescia), Alessandro Zanforlin (ULSS 18 Rovigo, Ospedale San Luca, Trecenta), Fabio Franzetti, Manuela Carugati, Manuela Morosi, and Elisa Monge (Department of Biomedical and Clinical Sciences, Division of Infectious Diseases, Luigi Sacco Hospital, Università degli Studi di Milano), Mauro Carone, (Fondazione Salvatore Maugeri, IRCCS, Cassano Murge), Vincenzo Patella (Allergology and Clinical Immunology Unit, Department of Medical Sciences, Battipaglia Hospital, Battipaglia, Salerno), Simone Scarlata (Geriatrics, Unit of Respiratory Pathophysiology and Thoracic Endoscopy, Campus Bio Medico University and Teaching Hospital, Rome), Andrea Comel, UO Pneumologia, Ospedale Pederzoli, Peschiera del Garda); Japan: Kiyoyasu Kurahashi (Yokohama City University Medical Center); Lebanon: Zeina Aoun Bacha (Medicine School, St Joseph University, Beyrouth); Mexico: Daniel Barajas Ugalde (National Institute of Respiratory Diseases), Omar Ceballos Zuñiga (Hospital General de Mexicali, Mexicali, Baja California), José F. Villegas (Hospital Universitario Monterrey); Montenegro: Milic Medenica (Hospital for Lung Diseases–Brezovik, Niksic); Netherlands: E. M. W. van de Garde (Department of Clinical Pharmacy, St Antonius Hospital, Utrecht/Nieuwegein); Nepal: Deebya Raj Mihsra (Internal Medicine, BP Koirala Institute of Health Sciences), Poojan Shrestha (Oxford University Clinical Research Unit, Patan Hospital); New Zealand: Elliott Ridgeon (Medical Research Institute of New Zealand); Nigeria: Babatunde Ishola Awokola (Department of Family Medicine and Primary Care, Lily Hospitals Limited, Warri), Ogonna N. O. Nwankwo (University of Calabar Teaching Hospital), Adefuye Bolanle Olufunlola (Olabisi Onabanjo University Teaching Hospital, Sagamu, Ogun State), Segaolu Olumide (Department of Medicine, Pulmonary Unit, University College Hospital, Ibadan), Kingsley N. Ukwaja (Department of Medicine, Federal Teaching Hospital Abakaliki, Ebonyi State); Pakistan: Muhammad Irfan, Section of Pulmonary and Critical Care Medicine (Department of Medicine, Aga Khan University, Karachi); Poland: Lukasz Minarowski (Department of Lung Diseases and Tuberculosis, Medical University of Bialystok), Skoczyński Szymon (Department of Pneumology, School of Medicine in Katowice, Medical University of Silesia, Katowice, Institute of Occupational Medicine and Environmental Health, Sosnowiec); Portugal: Felipe Froes (Hospital Pulido Valente–CHLN, Lisboa), Pedro Leuschner, (Centro Hospitalar do Porto), Mariana Meireles, Cláudia Ferrão, Pedro Leuschner, and João Neves (Serviço de Medicina, Centro Hospitalar do Porto, Largo Prof Abel Salazar, Porto), Sofia B. Ravara (Faculty of Health Sciences, University of Beira Interior, Cova da Beira Hospital Center, Covilhã); Republic of Moldova: Victoria Brocovschii (Department of Pneumology and Allergology, State University of Medicine and Pharmacy Nicolae Testemitanu), Chesov Ion (Clinic of Anesthesia and Intensive Care Valeriu Ghrerg, Institute of Emergency Medicine, State University of Medicine and Pharmacy Nicolae Testemitanu, Chisinau), Doina Rusu (SMFU N. Testemitanu, Chisinau), Cristina Toma (Department of Pneumology and Allergology, State University of Medicine and Pharmacy Nicolae Testemitanu, Chisinau); Romania: Daniela Chirita (Hospital Sfantul Stefan, Bucharest), Carmen Mihaela Dorobat (Universitatea de Medicină şi Farmacie Gr T. Popa I a ş i Facultatea de Medicină Stomatologică, Spitalul Clinic de Boli Infecţioase Sfânta Parascheva I a ş i Iaşi); Russia: Alexei Birkun (Department of Anesthesiology, Critical Care and Emergency Medicine, Medical Academy named after S. I. Georgievsky), Anna Kaluzhenina (Volgograd State Medical University); Saudi Arabia: Abdullah Almotairi (King Fahad Medical City, Riyadh), Zakeya Abdulbaqi Ali Bukhary (College of Medicine, Taibah University, Medina), Jameela Edathodu (Al Faisal University, King Faisal Specialist Hospital, Riyadh), Amal Fathy (Pulmonary and Respiratory Critical Care Medicine, Mansoura University Egypt, Affiliate at Taibah University), Abdullah Mushira Abdulaziz Enani and Nazik Eltayeb Mohamed (Infectious Diseases Section, Medical Specialties Department, King Fahad Medical City, Riyadh), Jawed Ulhadi Memon (Pulmonology Division, Department of Internal Medicine, King Fahad Hospital, Hofuf, Al Ahasa), Abdelhaleem Bella (Dammam University–Saudi Arabia and King Fahad Hospital); Serbia: Nada Bogdanović (Pulmonary Department of KHC, Dragiša Mišović, Belgrade), Branislava Milenkovic (Clinic for Pulmonary Diseases, Clinical Centre of Serbia, Faculty of Medicine, University of Belgrade), Dragica Pesut (University of Belgrade School of Medicine, Teaching Hospital of Pulmonology, Clinical Centre of Serbia, Belgrade); Spain: Luis Borderìas (Respiratory and Sleep Unit, Hospital San Jorge, Huesca), Noel Manuel Bordon Garcia (Barcelona Policlínic and Moises Broggi Hospital at Sant Joan Despí), Hugo Cabello Alarcón (Sant Hospital Seu de Urgell, Catalonia), Catia Cilloniz and Antoni Torres (Department of Pneumology, Institut Clinic del Tórax, Hospital Clinic of Barcelona, Institut d’Investigacions Biomèdiques August Pi i Sunyer, University of Barcelona), Vicens Diaz-Brito and Xavier Casas (Infectious Diseases Unit and Pneumology Service, Parc Sanitari Sant Joan de Deu, Sant Boi, Barcelona), Alicia Encabo González (Hospital Complex of Pontevedra), Maria Luisa Fernández-Almira (Medicina Interna, Hospital Universitario Central de Asturias), Miguel Gallego (Department of Respiratory Medicine, Hospital de Sabadell, Institut Universitari Parc Taulí-UAB, Sabadell, and CIBER de Enfermedades Respiratorias, CIBERES, Bunyola), Inmaculada Gaspar-GarcÍa (Department of Respiratory Medicine, Hospital Costa del Sol, Marbella, Málaga), Juan González del Castillo (Emergency Department, Hospital Universitario Clínico San Carlos, Madrid), Patricia Javaloyes Victoria (Hospital General Universitario de Alicante, Alicante), Elena Laserna Martínez (Hospital Mollet, Barcelona), Rosa Malo de Molina (University Hospital Puerta de Hierro Majadahonda, Madrid), Pedro J. Marcos (Servicio de Neumología, Complejo Hospitalario Universitario de A Coruña, INIBIC, Sergas, Universidade de A Coruña), Rosario Menéndez (Pneumology Service, University and Polytechnic Hospital La Fe, Valencia), Ana Pando-Sandoval (Hospital Universitario Central de Asturias, Area de Gestion Clinica de Pulmon, Servicio de Neumologia, Oviedo), Cristina Prat Aymerich, Alicia Lacoma de la Torre, and Ignasi García-Olivé (Microbiology Department and Pneumology Department, Hospital Universitari Germans Trias i Pujol, Institut d’Investigació Germans Trias i Pujol, Badalona, and Universitat Autònoma de Barcelona, CIBER Enfermedades Respiratorias, Instituto de Salud Carlos III), Jordi Rello and Silvia Moyano (Critical Care Department, Hospital Vall d’Hebron, Barcelona), Francisco Sanz (Servicio de Neumología, Consorci Hospital General Universitari de Valencia, Valencia), Oriol Sibila and Ana Rodrigo-Troyano (Servei de Pneumologia, Hospital de la Santa Creu i Sant Pau, IIB-Sant Pau, Barcelona), Jordi Solé-Violán (Hospital Universitario de Gran Canaria Dr Negrín, Las Palmas de Gran Canaria), Ane Uranga (Pulmology Department, Hospital of Galdakao-Usansolo), Job F. M. van Boven (Hospital Universitari Son Espases, Palma de Mallorca), Ester Vendrell Torra and Jordi Almirall Pujol (Intensive Care Medicine, Hospital de Mataró); South Africa: Charles Feldman (Division of Pulmonology, Department of Internal Medicine, Charlotte Maxeke Johannesburg Academic Hospital, Faculty of Health Sciences, University of the Witwatersrand); South Korea: Ho Kee Yum (Inje University Seoul Paik Hospital); Togo: Arnauld Attannon Fiogbe (Pulmonology and Infectious Diseases Service/University Hospital of Sylvanus Olympio, Lomé); Tunisia: Ferdaous Yangui (Department of Pneumology, Hospital of Internal Forces Security, Marsa, Tunis); Turkey: Semra Bilaceroglu (Izmir Dr Suat Seren Training and Research Hospital for Thoracic Medicine and Surgery, Izmir), Levent Dalar (Pulmonary Medicine, Istanbul Bilim University, Istanbul), Ufuk Yilmaz (Suat Seren Chest Disease and Surgery Training and Research Hospital, İzmir); Ukraine: Artemii Bogomolov (Vinnitsa National Pirogov Memorial Medical University, Vinnitsa Regional Antituberculosis Hospital, Vinnitsa); United Arab Emirates: Naheed Elahi (Dubai Hospital); United Kingdom: Devesh J. Dhasmana (Victoria Hospital, Kirkcaldy, NHS Fife), Andrew Feneley, Rhiannon Ions, Julie Skeemer, and Gerrit Woltmann, University Hospitals of Leicester NHS Trust and University of Leicester), Carole Hancock (Royal Respiratory Research Team, Royal Liverpool University Hospital, Liverpool), Adam T. Hill (Royal Infirmary and University of Edinburgh), Banu Rudran (The Royal London Hospital, Barts Health Trust, London), Silvia Ruiz-Buitrago and Marion Campbell (Hairmyres Hospital, East Kilbride), Paul Whitaker (Department of Respiratory Medicine, St James’s Hospital, Leeds), Alexander Youzguin (Southport and Ormskirk Hospitals NHS Trust), Anika Singanayagam (Imperial College Healthcare NHS Trust, London); United States: Karen S. Allen (University of Oklahoma Health Sciences Center, Oklahoma City), Veronica Brito (Texas A&M Health Science Center, Division of Pulmonary, Critical Care, and Sleep Medicine, Baylor Scott & White Health, Temple); Jessica Dietz (Fargo VA Health Care System, Fargo, North Dakota), Claire E. Dysart and Susan M. Kellie (Clement J. Zablocki VA Medical Center, Milwaukee, Wisconsin; Division of Infectious Diseases, University of New Mexico School of Medicine; and Raymond G. Murphy VA Medical Center, Albuquerque, New Mexico), Ricardo A Franco-Sadud and Garnet Meier (Division of Hospital Medicine, Cook County Hospital, Chicago, Illinois), Mina Gaga (7th Respiratory Medical Department and Asthma Center, Athens Chest Hospital), Thomas L. Holland and Stephen P. Bergin (Department of Medicine, Duke University Medical Center and School of Medicine, Duke Clinical Research Institute, Durham), Fayez Kheir (Department of Pulmonary Diseases, Critical Care and Environmental Medicine, Tulane University Health Sciences Center, New Orleans, Louisiana), Mark Landmeier (Division of Pulmonary and Critical Care Medicine, Northwestern Memorial Hospital, Chicago, Illinois), Manuel Lois (John Peter Smith Hospital, Fort Worth, Texas), Girish B. Nair (Interstitial Lung Disease Program and Pulmonary Rehabilitation, SUNY Stony Brook, and Winthrop University Hospital, Mineola, New York), Hemali Patel (Department of Medicine, Division of General Internal Medicine, Hospital Medicine Group, University of Colorado, Denver), Katherine Reyes (Henry Ford Hospital, Detroit, Illinois), William Rodriguez-Cintron, (Pulmonary/Critical Care Medicine VA Caribbean Healthcare System, San Juan Puerto Rico), Shigeki Saito (Tulane University, New Orleans), Nilam J. Soni, Julio Noda, Cecilia I. Hinojosa, Stephanie M. Levine, Luis F. Angel, and Antonio Anzueto (Divisions of Hospital Medicine and Pulmonary/Critical Care Medicine, South Texas Veterans Health Care System, University of Texas Health Science Center San Antonio, San Antonio), K. Scott Whitlow, John Hipskind, Kunal Sukhija, and Vicken Totten (Kaweah Delta Health Care District, Department of Emergency Medicine, Visalia, California), Richard G. Wunderink and Ray D. Shah (Northwestern University Feinberg School of Medicine, Chicago, Illinois); Zambia: Kondwelani John Mateyo (Department of Internal Medicine, University Teaching Hospital, Lusaka). Other investigators: Lorena Noriega, Ezequiel Alvarado, Mohamed Aman, and Lucía Labra.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Veterans Affairs.
Financial support. This project was not funded and relied solely on voluntary site and investigator participation. M. I. R.’s time is partially protected by the National Heart, Lung, and Blood Institute (award K23HL096054).
Potential conflicts of interest. F. B. reports personal fees from AstraZeneca, GlaxoSmithKline, Guidotti, Grifols, Menarini, and Novartis, and grants and personal fees from Bayer, Chiesi, Insmed, Pfizer, Teva, and Zambon, outside the submitted work. All other authors report no potential conflicts.
Contributor Information
GLIMP Investigators:
Patricia Karina Aruj, Silvia Attorri, Enrique Barimboim, Juan Pablo Caeiro, María I Garzón, Victor Hugo Cambursano, A Cazaux, Adrian Ceccato, Julio Chertcoff, Florencia Lascar, Fernando Di Tulio, Ariel Cordon Díaz, Lautaro de Vedia, Maria Cristina Ganaha, Sandra Lambert, Gustavo Lopardo, Carlos M Luna, Alessio Gerardo Malberti, Nora Morcillo, Silvina Tartara, Antonio A Cetrangolo, Claudia Pensotti, Betiana Pereyra, Pablo Gustavo Scapellato, Juan Pablo Stagnaro, Sonali Shah, Felix Lötsch, Florian Thalhammer, Kurt Anseeuw, Camille A Francois, Eva Van Braeckel, Jean Louis Vincent, Marcel Zannou Djimon, Jules Bashi, Roger Dodo, Simone Aranha Nouér, Peter Chipev, Milena Encheva, Darina Miteva, Diana Petkova, Adamou Dodo Balkissou, Eric Walter Pefura Yone, Bertrand Hugo Mbatchou Ngahane, Ning Shen, Jin-fu Xu, Carlos Andres Bustamante Rico, Ricardo Buitrago, Fernando Jose Pereira Paternina, Jean-Marie Kayembe Ntumba, Vesna Vladic Carevic, Marko Jakopovic, Mateja Jankovic, Zinka Matkovic, Ivan Mitrecic, Marie-Laure Bouchy Jacobsson, Anette Bro Christensen, Uffe Christian Heitmann Bødtger, Christian Niels Meyer, Andreas Vestergaard Jensen, Gertrud Baunbæk-Knudsen, Pelle Trier Petersen, Stine Andersen, Ibrahim El-Said Abd El-Wahhab, Nesreen Elsayed Morsy, Hanaa Shafiek, Eman Sobh, Kedir Abdella Abdulsemed, Fabrice Bertrand, Christian Brun-Buisson, Etienne de Montmollin, Muriel Fartoukh, Jonathan Messika, Pierre Tattevin, Abdo Khoury, Bernard Ebruke, Michael Dreher, Martin Kolditz, Matthias Meisinger, Klinikum Niederlausitz, Mathias W Pletz, Stefan Hagel, Jan Rupp, Tom Schaberg, Marc Spielmanns, Petra Creutz, Norton Suttorp, Beatrice Siaw-Lartey, Katerina Dimakou, Dimosthenis Papapetrou, Evdoxia Tsigou, Dimitrios Ampazis, Evangelos Kaimakamis, Mohit Bhatia, Raja Dhar, George D’Souza, Rajiv Garg, Parvaiz A Koul, P A Mahesh, B S Jayaraj, Kiran Vishnu Narayan, Hirennappa B Udnur, Shashi Bhaskara Krishnamurthy, Surya Kant, Rajesh Swarnakar, Sneha Limaye, Sundeep Salvi, Keihan Golshani, Vera M Keatings, Ignacio Martin-Loeches, Yasmin Maor, Jacob Strahilevitz, Salvatore Battaglia, Maria Carrabba, Piero Ceriana, Marco Confalonieri, Antonella d’Arminio Monforte, Bruno Del Prato, Marino De Rosa, Riccardo Fantini, Giuseppe Fiorentino, Maria Antonia Gammino, Francesco Menzella, Giuseppe Milani, Stefano Nava, Gerardo Palmiero, Roberta Petrino, Barbra Gabrielli, Paolo Rossi, Claudio Sorino, Gundi Steinhilber, Alessandro Zanforlin, Fabio Franzetti, Manuela Carugati, Manuela Morosi, Elisa Monge, Mauro Carone, Vincenzo Patella, Simone Scarlata, Andrea Comel, Kiyoyasu Kurahashi, Zeina Aoun Bacha, Daniel Barajas Ugalde, Omar Ceballos Zuñiga, José F Villegas, Milic Medenica, E M W van de Garde, Deebya Raj Mihsra, Poojan Shrestha, Elliott Ridgeon, Babatunde Ishola Awokola, Ogonna N O Nwankwo, Adefuye Bolanle Olufunlola, Segaolu Olumide, Kingsley N Ukwaja, Muhammad Irfan, Lukasz Minarowski, Skoczyński Szymon, Felipe Froes, Pedro Leuschner, Mariana Meireles, Cláudia Ferrão, Pedro Leuschner, João Neves, Sofia B Ravara, Victoria Brocovschii, Chesov Ion, Doina Rusu, Cristina Toma, Daniela Chirita, Carmen Mihaela Dorobat, Alexei Birkun, Anna Kaluzhenina, Abdullah Almotairi, Zakeya Abdulbaqi Ali Bukhary, Jameela Edathodu, Amal Fathy, Abdullah Mushira Abdulaziz Enani, Nazik Eltayeb Mohamed, Jawed Ulhadi Memon, Abdelhaleem Bella, Nada Bogdanović, Branislava Milenkovic, Dragica Pesut, Luis Borderìas, Noel Manuel Bordon Garcia, Hugo Cabello Alarcón, Catia Cilloniz, Antoni Torres, Vicens Diaz-Brito, Xavier Casas, Alicia Encabo González, Maria Luisa Fernández-Almira, Miguel Gallego, Inmaculada Gaspar-GarcÍa, Juan González del Castillo, Patricia Javaloyes Victoria, Elena Laserna Martínez, Rosa Malo de Molina, Pedro J Marcos, Rosario Menéndez, Ana Pando-Sandoval, Cristina Prat Aymerich, Alicia Lacoma de la Torre, Ignasi García-Olivé, Jordi Rello, Silvia Moyano, Francisco Sanz, Oriol Sibila, Ana Rodrigo-Troyano, Jordi Solé-Violán, Ane Uranga, Job F M van Boven, Ester Vendrell Torra, Jordi Almirall Pujol, Charles Feldman, Ho Kee Yum, Arnauld Attannon Fiogbe, Ferdaous Yangui, Semra Bilaceroglu, Levent Dalar, Ufuk Yilmaz, Artemii Bogomolov, Naheed Elahi, Devesh J Dhasmana, Andrew Feneley, Rhiannon Ions, Julie Skeemer, Gerrit Woltmann, Carole Hancock, Adam T Hill, Banu Rudran, Silvia Ruiz-Buitrago, Marion Campbell, Paul Whitaker, Alexander Youzguin, Anika Singanayagam, Karen S Allen, Veronica Brito, Jessica Dietz, Claire E Dysart, Susan M Kellie, Ricardo A Franco-Sadud, Garnet Meier, Mina Gaga, Thomas L Holland, Stephen P Bergin, Fayez Kheir, Mark Landmeier, Manuel Lois, Girish B Nair, Hemali Patel, Katherine Reyes, William Rodriguez-Cintron, Shigeki Saito, Nilam J Soni, Julio Noda, Cecilia I Hinojosa, Stephanie M Levine, Luis F Angel, Antonio Anzueto, K Scott Whitlow, John Hipskind, Kunal Sukhija, Vicken Totten, Richard G Wunderink, Ray D Shah, Kondwelani John Mateyo, Lorena Noriega, Ezequiel Alvarado, Mohamed Aman, and Lucía Labra
References
- 1. Aliberti S, Di Pasquale M, Zanaboni AM, et al. . Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis 2012; 54:470–8. [DOI] [PubMed] [Google Scholar]
- 2. Aliberti S, Cilloniz C, Chalmers JD, et al. . Multidrug-resistant pathogens in hospitalised patients coming from the community with pneumonia: a European perspective. Thorax 2013; 68:997–9. [DOI] [PubMed] [Google Scholar]
- 3. Shindo Y, Ito R, Kobayashi D, et al. . Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med 2013; 188:985–95. [DOI] [PubMed] [Google Scholar]
- 4. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014; 371:1619–28. [DOI] [PubMed] [Google Scholar]
- 5. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim WS, Baudouin SV, George RC, et al. . BTS guidelines for the management of community acquired pneumonia in adults. Thorax 2009; 64(suppl III):iii1–55. [DOI] [PubMed] [Google Scholar]
- 7. Woodhead M, Blasi F, Ewig S, et al. ; Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect 2011; 17(suppl 6):E1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Furman CD, Rayner AV, Tobin EP. Pneumonia in older residents of long-term care facilities. Am Fam Physician 2004; 70:1495–500. [PubMed] [Google Scholar]
- 9. Cillóniz C, Polverino E, Ewig S, et al. . Impact of age and comorbidity on cause and outcome in community-acquired pneumonia. Chest 2013; 144:999–1007. [DOI] [PubMed] [Google Scholar]
- 10. Aliberti S, Reyes LF, Faverio P, et al. ; GLIMP investigators Global Initiative for Meticillin-Resistant Staphylococcus aureus Pneumonia (GLIMP): an international, observational cohort study. Lancet Infect Dis 2016; 16:1364–76. [DOI] [PubMed] [Google Scholar]
- 11. Magiorakos AP, Srinivasan A, Carey RB, et al. . Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 12. Wan QJ, Hu HF, He YC, et al. . Severe pneumonia in mycophenolate mofetil combined with low-dose corticosteroids-treated patients with immunoglobulin A nephropathy. Kaohsiung J Med Sci 2015; 31:42–6. [DOI] [PubMed] [Google Scholar]
- 13. Li F, Jin HZ, Su F, Jia L, Sun QN. Pneumocystis pneumonia in patients with immunobullous dermatoses. Int J Dermatol 2011; 50:1144–9. [DOI] [PubMed] [Google Scholar]
- 14. Gruson D, Vargas F, Hilbert G, et al. . Predictive factors of intensive care unit admission in patients with haematological malignancies and pneumonia. Intensive Care Med 2004; 30:965–71. [DOI] [PubMed] [Google Scholar]
- 15. Garcia JB, Lei X, Wierda W, et al. . Pneumonia during remission induction chemotherapy in patients with acute leukemia. Ann Am Thorac Soc 2013; 10:432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JO, Kim DY, Lim JH, et al. . Risk factors for bacterial pneumonia after cytotoxic chemotherapy in advanced lung cancer patients. Lung Cancer 2008; 62:381–4. [DOI] [PubMed] [Google Scholar]
- 17. Lim KH, Yoon HI, Kang YA, et al. . Severe pulmonary adverse effects in lymphoma patients treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen plus rituximab. Korean J Intern Med 2010; 25:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waks AG, Tolaney SM, Galar A, et al. . Pneumocystis jiroveci pneumonia (PCP) in patients receiving neoadjuvant and adjuvant anthracycline-based chemotherapy for breast cancer: incidence and risk factors. Breast Cancer Res Treat 2015; 154:359–67. [DOI] [PubMed] [Google Scholar]
- 19. Kim T, Choi SH, Kim SH, et al. . Point prevalence of Pneumocystis pneumonia in patients with non-Hodgkin lymphoma according to the number of cycles of R-CHOP chemotherapy. Ann Hematol 2013; 92:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aliberti S, Myers JA, Peyrani P, et al. . The role of neutropenia on outcomes of cancer patients with community-acquired pneumonia. Eur Respir J 2009; 33:142–7. [DOI] [PubMed] [Google Scholar]
- 21. Sogaard OS, Lohse N, Gerstoft J, et al. . Hospitalization for pneumonia among individuals with and without HIV infection, 1995-2007: a Danish population-based, nationwide cohort study. Clin Infect Dis 2008; 47:1345–53. [DOI] [PubMed] [Google Scholar]
- 22. Kuehnhardt D, Hannemann M, Schmidt B, Heider U, Possinger K, Eucker J. Therapeutic implication of BAL in patients with neutropenia. Ann Hematol 2009; 88:1249–56. [DOI] [PubMed] [Google Scholar]
- 23. Maschmeyer G, Beinert T, Buchheidt D, et al. . Diagnosis and antimicrobial therapy of lung infiltrates in febrile neutropenic patients: guidelines of the Infectious Diseases Working Party of the German Society of Haematology and Oncology. Eur J Cancer 2009; 45:2462–72. [DOI] [PubMed] [Google Scholar]
- 24. Alanio A, Hauser PM, Lagrou K, et al. ; 5th European Conference on Infections in Leukemia (ECIL-5), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and the European LeukemiaNet (ELN) ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 2016; 71:2386–96. [DOI] [PubMed] [Google Scholar]
- 25. Freifeld AG, Bow EJ, Sepkowitz KA, et al. ; Infectious Diseases Society of America Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:e56–93. [DOI] [PubMed] [Google Scholar]
- 26. Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2014; 59(suppl 5):S344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buchacz K, Lau B, Jing Y, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA Incidence of AIDS-defining opportunistic infections in a multicohort analysis of HIV-infected persons in the United States and Canada, 2000-2010. J Infect Dis 2016; 214:862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madeddu G, Porqueddu EM, Cambosu F, et al. . Bacterial community acquired pneumonia in HIV-infected inpatients in the highly active antiretroviral therapy era. Infection 2008; 36:231–6. [DOI] [PubMed] [Google Scholar]
- 29. Park DR, Sherbin VL, Goodman MS, et al. ; Harborview CAP Study Group The etiology of community-acquired pneumonia at an urban public hospital: influence of human immunodeficiency virus infection and initial severity of illness. J Infect Dis 2001; 184:268–77. [DOI] [PubMed] [Google Scholar]
- 30. Torres HA, Bodey GP, Rolston KV, Kantarjian HM, Raad II, Kontoyiannis DP. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer 2003; 98:86–93. [DOI] [PubMed] [Google Scholar]
- 31. Gil-Prieto R, Pascual-Garcia R, Walter S, Álvaro-Meca A, Gil-De-Miguel Á. Risk of hospitalization due to pneumococcal disease in adults in Spain: the CORIENNE study. Hum Vaccin Immunother 2016; 12:1900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Putot A, Tetu J, Perrin S, et al. . Impact of microbiological samples in the hospital management of community-acquired, nursing home-acquired and hospital-acquired pneumonia in older patients. Eur J Clin Microbiol Infect Dis 2016; 35:489–95. [DOI] [PubMed] [Google Scholar]
- 33. Polverino E, Dambrava P, Cillóniz C, et al. . Nursing home-acquired pneumonia: a 10 year single-centre experience. Thorax 2010; 65:354–9. [DOI] [PubMed] [Google Scholar]
- 34. Kohli R, Lo Y, Homel P, et al. ; HER Study Group Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis 2006; 43:90–8. [DOI] [PubMed] [Google Scholar]
- 35. Benito N, Rañó A, Moreno A, et al. . Pulmonary infiltrates in HIV-infected patients in the highly active antiretroviral therapy era in Spain. J Acquir Immune Defic Syndr 2001; 27:35–43. [DOI] [PubMed] [Google Scholar]
- 36. Mori H, Ohno Y, Ito F, et al. . Polymerase chain reaction positivity of Pneumocystis jirovecii during primary lung cancer treatment. Jpn J Clin Oncol 2010; 40:658–62. [DOI] [PubMed] [Google Scholar]
- 37. Boersma WG, Erjavec Z, van der Werf TS, de Vries-Hosper HG, Gouw AS, Manson WL. Bronchoscopic diagnosis of pulmonary infiltrates in granulocytopenic patients with hematologic malignancies: BAL versus PSB and PBAL. Respir Med 2007; 101:317–25. [DOI] [PubMed] [Google Scholar]
- 38. Mikulska M, Del Bono V, Gandolfo N, et al. . Epidemiology of viral respiratory tract infections in an outpatient haematology facility. Ann Hematol 2014; 93:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beaird OE, Freifeld A, Ison MG, et al. . Current practices for treatment of respiratory syncytial virus and other non-influenza respiratory viruses in high-risk patient populations: a survey of institutions in the Midwestern Respiratory Virus Collaborative. Transpl Infect Dis 2016; 18:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poonyagariyagorn HK, Gershman A, Avery R, et al. . Challenges in the diagnosis and management of Nocardia infections in lung transplant recipients. Transpl Infect Dis 2008; 10:403–8. [DOI] [PubMed] [Google Scholar]
- 41. Tavadze M, Rybicki L, Mossad S, et al. . Risk factors for vancomycin-resistant Enterococcus bacteremia and its influence on survival after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2014; 49:1310–6. [DOI] [PubMed] [Google Scholar]
- 42. Bock AM, Cao Q, Ferrieri P, Young JA, Weisdorf DJ. Bacteremia in blood or marrow transplantation patients: clinical risk factors for infection and emerging antibiotic resistance. Biol Blood Marrow Transplant 2013; 19:102–8. [DOI] [PubMed] [Google Scholar]
- 43. Herbers AH, de Haan AF, van der Velden WJ, Donnelly JP, Blijlevens NM. Mucositis not neutropenia determines bacteremia among hematopoietic stem cell transplant recipients. Transpl Infect Dis 2014; 16:279–85. [DOI] [PubMed] [Google Scholar]
- 44. Wu SW, Liu KS, Lin CK, et al. . Community-acquired urinary tract infection in kidney transplantation: risk factors for bacteremia and recurrent infection. J Formos Med Assoc 2013; 112:138–43. [DOI] [PubMed] [Google Scholar]
- 45. Daskalaki E, Koukoulaki M, Bakalis A, et al. . Blood stream infections in renal transplant recipients: a single-center study. Transplant Proc 2014; 46:3191–3. [DOI] [PubMed] [Google Scholar]
- 46. Huson MA, Stolp SM, van der Poll T, Grobusch MP. Community-acquired bacterial bloodstream infections in HIV-infected patients: a systematic review. Clin Infect Dis 2014; 58:79–92. [DOI] [PubMed] [Google Scholar]
- 47. Taramasso L, Tatarelli P, Di Biagio A. Bloodstream infections in HIV-infected patients. Virulence 2016; 7:320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.