Abstract
Background
Staphylococcus aureus is a leading cause of bacteremia, yet there remains a significant knowledge gap in the identification of relevant biomarkers that predict clinical outcomes. Heterogeneity in the host response to invasive S. aureus infection suggests that specific biomarker signatures could be utilized to differentiate patients prone to severe disease, thereby facilitating earlier implementation of more aggressive therapies.
Methods
To further elucidate the inflammatory correlates of poor clinical outcomes in patients with S. aureus bacteremia, we evaluated the association between a panel of blood proteins at initial presentation of bacteremia and disease severity outcomes using 2 cohorts of patients with S. aureus bacteremia (n = 32 and n = 124).
Results
We identified 13 candidate proteins that were correlated with mortality and persistent bacteremia. Prognostic modeling identified interleukin (IL)-8 and CCL2 as the strongest individual predictors of mortality, with the combination of these biomarkers classifying fatal outcome with 89% sensitivity and 77% specificity (P < .0001). Baseline IL-17A levels were elevated in patients with persistent bacteremia (P < .0001), endovascular (P = .026) and metastatic tissue infections (P = .012).
Conclusions
These results demonstrate the potential utility of selected biomarkers to distinguish patients with the highest risk for treatment failure and bacteremia-related complications, providing a valuable tool for clinicians in the management of S. aureus bacteremia. Additionally, these biomarkers could identify patients with the greatest potential to benefit from novel therapies in clinical trials.
Keywords: Staphylococcus aureus, bacteremia, endocarditis, prognostic biomarkers
Biomarkers identify S. aureus bacteremia patients at highest risk of treatment failure, with high circulating IL-17A levels at diagnosis prognostic for persistent bacteremia and chronic tissue infection foci while a combination of IL-8 and CCL2 identifies increased mortality risk.
Staphylococcus aureus is one of the leading and most fatal causes of bacteremia with an estimated mortality of 20% [1–3]. Of all patients with S. aureus bacteremia (SAB), at least 50% will develop complicated bacteremia [4, 5]. Patients with complicated SAB can have persistently positive blood cultures, deep-seated foci of infection including infective endocarditis (IE), vertebral osteomyelitis, septic arthritis, device-associated infections, and increased risk of recurrence [6–9]. The presence of these infectious complications is challenging to identify at the time of patient presentation and often necessitate multiple diagnostic and invasive procedures [1]. Although multiple clinical risk factors have been shown to be predictive of complicated bacteremia such as hemodialysis and immunosuppression [10], the clinical course of SAB is highly variable, and it remains unclear why only a subset of patients develop severe disease [4]. Outcomes and treatment for SAB are dependent on methicillin resistance and whether the infection is classified as uncomplicated or complicated [1–4, 11]. The latter distinction is critically important, as uncomplicated infections may qualify for short course antibiotic therapy and are associated with better outcomes, while complicated infections require at least 4–6 weeks of antibiotic therapy and are associated with significant morbidity and mortality [12–14]. Despite appropriate antibiotic therapy, unsatisfactory clinical response is observed in up to 50% of cases, characterized by persistent bacteremia, recurrence, development of secondary infections, or death [3, 15]. To help facilitate the management of patients at high risk for severe disease and poor outcomes, identification of other factors that contribute to the clinical course of SAB are needed [16].
There is growing evidence to support that differences in the host immune response may contribute to the heterogeneity in clinical outcomes in patients with SAB. Several studies have assessed the host immune response by measuring levels of circulating cytokines including interleukin (IL)-6, IL-10, IL-1β, and tissue necrosis factor in patients with SAB to identify correlates of poor clinical outcomes including increased mortality and duration of bacteremia [17–21]. Collectively, these studies have been vital in demonstrating the clinical relevance of the host immune response in SAB outcomes; however, an analysis that integrates a more comprehensive set of biomarkers is essential to assess comparative prognostic value in this patient population. The contribution of the immune response to disease severity and poor outcome in patients with complications of bacteremia and the relationship between host immune response and failure to clear bacteria from the blood remains poorly understood.
In this study, we evaluated a panel of 64 circulating proteins in an exploratory study of 32 serially enrolled SAB patients and selected 20 proteins that were superior to routine clinical metrics in identifying patients who are at greatest risk for mortality and/or persistent bacteremia. These candidate biomarkers were then evaluated in a case-control study of patients with complicated SAB selected to enrich for mortality and persistent bacteremia, which prioritized 13 candidate biomarkers. We observed distinct immune correlates of outcome: high IL-17A at presentation classified patients with persistently positive blood cultures and endovascular infection, while high baseline levels of the chemokines IL-8 and CCL2 were prognostic for mortality.
MATERIALS AND METHODS
Experimental Design
The exploratory observational study was performed at San Francisco General Hospital, University of California, San Francisco, under a protocol approved by the Committee on Human Research (IRB protocol 14-13778). In sum, 32 adult patients with confirmed SAB infection were serially enrolled between July 2014 and November 2015. A convenience plasma sample was collected within 2 days of the start of empiric antibiotic therapy. Limited clinical histories were reviewed by a board-certified infectious disease physician and a clinical infectious disease fellow. Plasma samples from 6 healthy individuals obtained from BioreclamationIVT (Westbury, NY, USA) were used as controls. A case-control study of 124 patients with complicated SAB infections was designed using samples from a Duke University (Durham, NC, USA) biobank with associated clinical data, collected between 2002 and 2014 with informed consent under protocols approved by the Institutional Ethics Review Boards (IRB protocol Pro00008031). Subjects met the Infectious Diseases Society of America (IDSA) expert panel criteria for complicated SAB [11]. Subjects were selected to enrich for outcomes of persistent bacteremia and mortality, with a control SAB group enrolled over the same time period matched for demographic variables and infection source. Serum samples were collected within 1–3 days of empiric antibiotic therapy. Patient demographic and clinical characteristics are shown in Table 1 and Supplementary Table 2. Serum samples from 16 healthy volunteers were obtained through the Genentech Samples for Science program.
Table 1.
Characteristics of Clinical Studies
| Exploratory Cohort | Validation Cohort | |
|---|---|---|
| Number of patients | 32 | 124 |
| Age, years, median (range) | 53 (21–90) | 62 (22–91) |
| Female, no. (%) | 6 (19.8) | 54 (43.5) |
| Outcome, no (%) | ||
| Mortality | 4 (12.5) | 27 (21.8) |
| Attributable mortality | _ | 18 (14.5) |
| Persistent bacteremia | 4 (12.5) | 61 (49.2) |
| Complicated infection | 26 (81.3) | 124 (100) |
| Recurrence | _ | 15 (12.1) |
| LOS, days, median (range)a | 17 (5–58) | 15 (4–122) |
| Treatment duration (days), median (range) | 35 (7–86) | 43 (2–113) |
| MRSA, no. (%) | 13 (40.6) | 70 (56.7) |
| Infection foci, no. (%) | ||
| Endovascular | 10 (31.2) | 68 (54.3) |
| Extravascular osteoarticular | 7 (21.9) | 37 (29.8) |
| Extravascular soft tissue | 7 (21.9) | 37 (29.8) |
| Other | 8 (25.0) | 13 (10.5) |
| Comorbidities, no. (%) | ||
| Diabetes | 13 (40.6) | 58 (46.8) |
| Hemodialysis dependent | 4 (12.5) | 45 (36.3) |
| Cancer | _ | 22 (17.7) |
| Transplant recipient | _ | 11 (8.9) |
| HIV+ | _ | 5 (4.0) |
Abbreviations: HIV, human immunodeficiency virus; LOS, length of stay; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
aFatal cases and patients that left the hospital against medical advice were excluded from hospital LOS. Patients with more than one identified foci of infection were assigned to multiple infection foci categories in this table. The other foci category includes catheter-associated infections and unidentified sources of infection. The frequency of persistence was significantly different between infections caused by MRSA (40/70 = 57%) and MSSA (19/51 = 37%) whereas all-cause mortality was similar between MRSA (14/70 = 20%) or MSSA (12/51 = 24%) (P = .048, χ2 test).
Clinical Severity Definitions
All-cause mortality was measured as in-hospital mortality in the exploratory study and up to 90 days following hospital discharge in the validation study. Infection-related mortality was assessed by retrospective chart review by 2 Board-certified infectious disease physicians using the following criteria: culture positive and/or persistent infection focus and/or systemic signs and symptoms at time of death or attending physician considered death to be attributable to SAB. Persistent bacteremia was defined as a positive blood culture for ≥ 5 days on appropriate antibiotic therapy. Duration of positive blood culture was calculated as days to last positive blood culture.
Biomarker Measurements
Plasma concentrations of 64 proteins, the majority of which were previously reported to be associated with poor outcomes for SAB or sepsis (Supplementary Table 1), were measured by multiplex immunoassays (Ella platform, ProteinSimple/Bio-Techne Corporation, San Jose, CA, USA; Human Magnetic Luminex assays, R&D systems/Bio-Techne Corporation, Minneapolis, MN, USA).
Statistical Analysis
Cytokines and clinical variable levels that were significantly different between the outcome groups were identified using Wilcoxon rank-sum test. P values were adjusted for multiple testing using Benjamini & Hochberg procedure in Supplemental Figure 2. Spearman correlation coefficients were used to evaluate the association among the biomarker levels. Illustrative cutoffs were defined as sensitivity above 70% with the highest specificity possible. To identify the minimal set of variables in the prognosis of the binary patient outcomes while minimizing the possibility of overfitting, all measured variables were treated as covariates in a lasso model (with 10-fold cross-validation) using the R package glmnet [38], where we evaluated both the deviance and misclassification minimization approaches. We used random forest [39, 40] as an alternative approach to identify the main covariates driving patient classification. See Supplementary Methods for additional details.
RESULTS
Evaluation of Candidate Biomarkers in an Exploratory S. aureus Bacteremia Study
To identify biomarkers of mortality and persistent bacteremia in S. aureus bloodstream infections (Figure 1A), we selected a panel of 64 blood protein biomarker candidates based on their literature-supported associations with disease severity in SAB or sepsis (Supplementary Table 1). Circulating levels of these proteins were quantified in samples collected within 2 days of appropriate empiric antibiotic therapy from 32 serially enrolled patients with confirmed SAB (clinical characteristics are described in Table 1 and Supplementary Table 2). Proteins associated with 90-day all-cause mortality and/or persistent bacteremia (positive blood culture ≥5 days following antibiotics) are shown in Figure 1 and Supplementary Figure 1. These included members of the 3 broad biological categories of mediators that we assessed: immune response (ie, IL-8 and IL-10), endothelial function (ie, ANGPT2 and sE-selectin), and tissue damage and repair (ie, sFAS and TIMP-1). We prioritized 20 proteins that were associated with mortality or persistent bacteremia (Wilcoxon rank-sum test P < .1) to evaluate in a larger SAB cohort.
Figure 1.
Prognostic biomarker candidates prioritized in the exploratory study. A, Study overview. B, In sum, 31 of the 64 evaluated proteins were differentially expressed in patients with mortality or persistent bacteremia. Mortality: survivor vs. all-cause in-hospital mortality. Persistent bacteremia: clearance of blood culture within 5 days of initial diagnostic positive blood culture vs. positive blood culture for 5+ days. Wilcoxon rank sum test P < .1, n = 32. C, Heatmap of the 31 differentially expressed cytokines. Patients were grouped into 2 overall groups by unsupervised clustering as shown by the dendrograms. Abbreviations: IL, interleukin; SAB, S. aureus bacteremia.
Confirmation of Prioritized Biomarkers in a Study of Complicated S. aureus Bacteremia
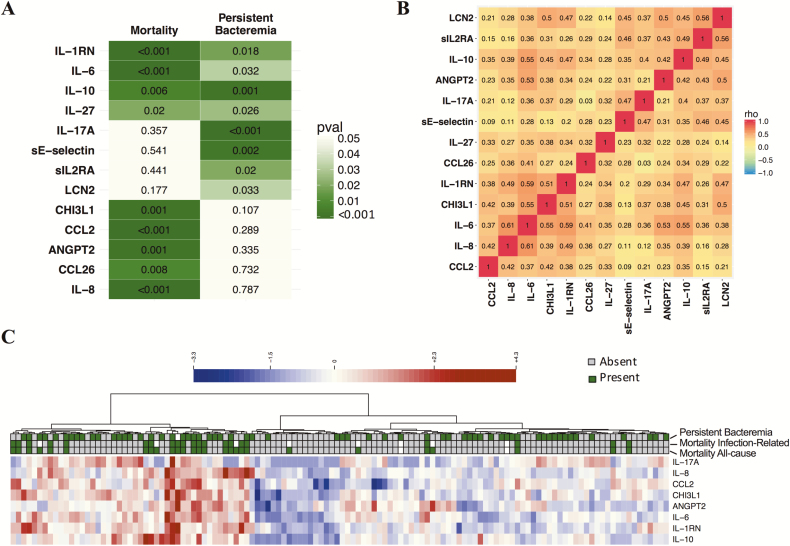
We designed a case-control study to evaluate the strength of association of prioritized biomarkers with mortality and persistent bacteremia and to examine relationships with infection source. We focused on complicated bacteremia patients because prognostic biomarkers would have the greatest clinical utility for these patients at greatest risk for poor outcomes. We selected samples from 124 patients from an observational cohort, with cases selected on all-cause mortality or persistent bacteremia outcomes, and a control group matched for demographics and infection source (cohort characteristics in Table 1 and Supplementary Table 2). Thirteen of the 20 prioritized serum proteins were significantly different at presentation in patients who developed persistent bacteremia or fatal outcomes and superior to patient demographic characteristics and clinical laboratory metrics (chemistry, complete blood counts) (Figure 2A; median fold change and false discovery rate [FDR] adjusted P values for the complete biomarker panel can be found in Supplementary Figure 2). We confirmed that IL-10 was associated with both mortality and persistent bacteremia [19] and observed that IL-1RN, IL-6, and IL-27 exhibited a similar prognostic relationship for both clinical outcomes. A group of 4 biomarkers (IL-17A, sE-selectin, sIL-2RA, and LCN2) were significantly higher in subjects who developed persistent bacteremia, whereas a group of 5 proteins (IL-8, CCL2, ANGPT2, CHI3L1, and CCL26) was associated with a fatal outcome (Figure 2A). There was only modest correlations observed between biomarkers, and coregulated protein expression did not explain the groups of biomarkers associated with different clinical outcomes (Figure 2B). An unsupervised clustering approach was used to reveal how inflammatory protein expression could group patients with fatal outcome (Figure 2C).
Figure 2.
Serum proteins prognostic for mortality and persistent bacteremia in the validation study. A, Baseline serum proteins that were significantly different between all-cause mortality vs survivors or persistent bacteremia (positive blood cultures for 5+ days) vs microbiological clearance within 5 days; P < .05. B, Spearman correlation matrix of the biomarkers shown in panel A. C, Unsupervised clustering heat map of patients based on biomarkers prognostic for mortality and/or persistent bacteremia with P ≤ .001. Wilcoxon rank sum test P values are shown for n = 124.
Prognostic Biomarkers for Mortality in S. aureus Bacteremia
Receiver operator characteristics area under the curve (ROC AUC) >0.7 were calculated for IL-8, CCL2, IL-6, IL-1RN, CHI3L1, and ANGPT2 (Figure 3A and Supplementary Figure 3), with stronger prognostic value than routine clinical data (Supplementary Figure 2). ROC AUC were similar for both all-cause mortality and mortality assessed as related to infection for all biomarkers with adjusted P value < .1 (Supplementary Figure 2A and B). We utilized 2 modeling approaches to identify the minimal combination of protein biomarkers with clinical and demographic data (age, sex, race) for classifying all-cause mortality: lasso with 10-fold cross validation, and random forests with 500 decision trees. Both models identified the combination of IL-8 and CCL2 as the best classifier (Figure 3, Supplementary Figure 4 and Supplementary Table 3). Although there was high coordinate expression of most prognostic mortality biomarkers at admission (Figure 3C), CCL2 is the least correlated protein with IL-8. Some patients with fatal outcomes were high for either IL-8 or CCL2, resulting in the improved performance of the prognostic model that incorporates both biomarkers (Figure 3G).
Figure 3.
Mortality prognostic biomarkers in the validation study. A, Area under the ROC curve and Wilcoxon rank sum test P-values for the biomarker and clinical variables that differed between S. aureus patients who died or survived in the validation study with P < .05. B, ROC curves showing the power to discriminate fatal outcomes from survivors: age (AUC = 0.68; 95% CI: 0.57 to 0.79), the only demographic or clinical variable at baseline with discriminatory power; CCL2 (AUC = 0.81; 95% CI: 0.72 to 0.91); IL-8 (AUC = 0.83; 95% CI: 0.74 to 0.92), and the combined IL-8 and CCL2 prognostic model (AUC = 0.89; 95% CI: 0.82 to 0.96). C, Spearman correlation matrix of the 8 serum proteins that were prognostic for mortality with P < .01. Patients with fatal outcomes present with higher biomarker levels than survivors for (D) IL-8, (E) CCL2, and (F) the combined IL-8 and CCL2 model. Medians and interquartile ranges, Wilcoxon rank sum test P values, and the sensitivity and specificity of illustrative cutoff values from the S. aureus patient group ROC analysis (represented as dashed lines) are shown. Gray shaded area represents the healthy control range (n = 16). G, Scatter plot showing correlation of baseline IL-8 and CCL2 serum concentrations; Spearman rank correlation test, n = 124. Abbreviations: AUC, area under the curve; CI, confidence interval; IL, interleukin; ROC, receiver operating characteristic.
Prognostic Biomarkers for Persistent S. aureus Bacteremia
Elevated IL-17A levels at enrollment was the strongest discriminator between patients who went on to develop persistent bacteremia and those who cleared their blood cultures within 5 days, followed by IL-10 and sE-selectin, and correlated with duration of positive blood cultures (Figure 4). Using a similar prognostic modeling approach evaluating all combinations of protein biomarkers, clinical and demographic data, IL-17A alone was identified as the optimal predictor of persistent bacteremia (Supplementary Figure 4 and Supplementary Table 3). IL-17A was elevated in patients with persistent vs nonpersistent infections regardless of methicillin resistance of the pathogen (methicillin-resistant Staphylococcus aureus [MRSA] infections: P = .0006, methicillin-susceptible Staphylococcus aureus [MSSA] infections: P = .0085, Mann-Whitney). Importantly, when the cytokine levels were used to predict the outcomes, bacterial susceptibility to methicillin did not affect the modeling results for either outcome. The inclusion of additional clinical covariates such as acquisition route (hospital- or community-acquired infection), hemodialysis dependence, MRSA, diabetes, permanent implant, and steroid use, in addition to age, sex, and race, did not change the modeling results for either outcomes (data not shown).
Figure 4.
Persistent bacteremia prognostic biomarkers in the validation study. A, Area under the ROC curve and Wilcoxon rank sum test P-values for the serum proteins that differed between S. aureus patients who had persistent bacteremia vs. nonpersistent cases, P < .05. No single demographic or clinical data at baseline was significantly different between these 2 patient groups. B, ROC curves showing the optimal serum proteins with power to discriminate persistent bacteremia from nonpersistent cases: IL-17A (AUC = 0.73; 95% CI: 0.64 to 0.82); IL-10 (AUC = 0.68; 95% CI: 0.58 to 0.77), and sE-selectin (AUC = 0.66; 95% CI: 0.56 to 0.75). Patients with persistent bacteremia present with higher median biomarker levels than patients who clear their bloodstream infection within 5 days for: (C) IL17A, (D) IL-10, and (E) sE-selectin. Wilcoxon rank sum test P-values and the sensitivity and specificity of the illustrative cutoff value from ROC analysis represented as dashed lines. Scatter plots of baseline serum concentrations for (F) IL-17A, (G) IL-10, and (H) sE-selectin vs. days to last positive blood culture; n = 124. Spearman rank correlation test and gray shaded area represents the range of the healthy control values. Abbreviations: AUC, area under the curve; BC, blood cultures; CI, confidence interval; IL, interleukin; ROC, receiver operating characteristic.
Relationship Between High Early IL-17A and Endovascular Infection
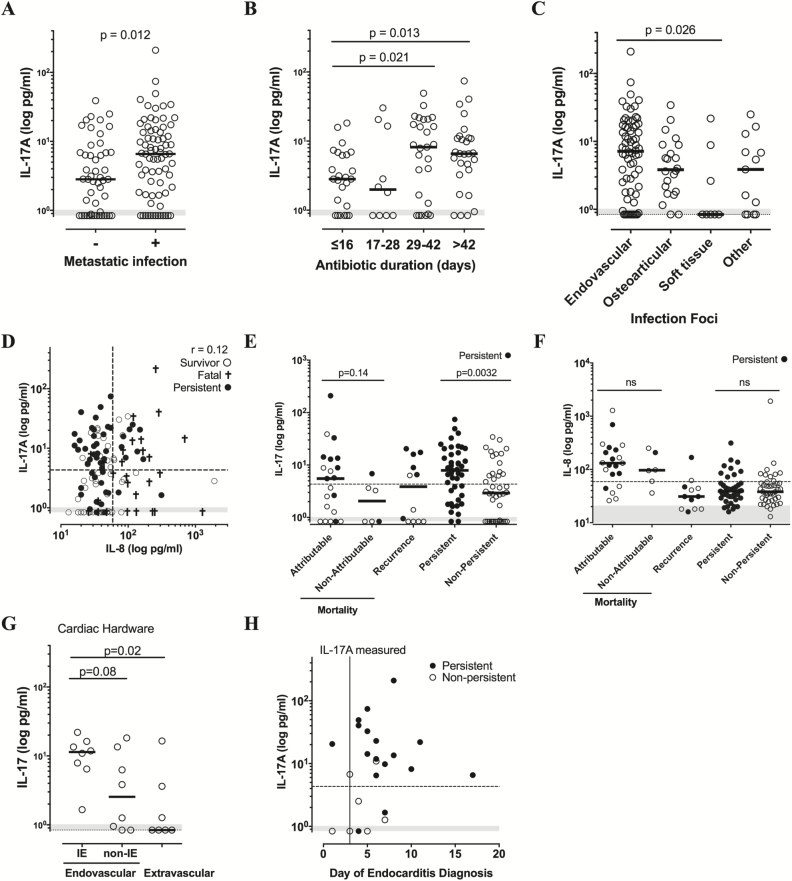
Persistent bacteremia commonly results from uncontrolled tissue infection reservoirs, and median IL-17A levels at presentation were higher in patients with diagnosed tissue metastasis (Figure 5A, P = .012). A longer duration of antibiotic treatment is recommended to clear underlying tissue foci of infection [22]. Baseline IL-17A was positively correlated with the duration of prescribed antibiotics (ρ = 0.35, P = .0003) and elevated in patients requiring ≥ 4 weeks compared with ≤ 2 weeks of antibiotic therapy (Figure 5B). Baseline IL-17A levels were highest in patients diagnosed with infective endocarditis and other endovascular infections over the course of treatment (Figure 5C), together confirming observations in the initial exploratory study (Supplementary Figure 5).
Figure 5.
IL-17A is associated with endovascular infections, longer duration of antibiotic therapy, and infection-related mortality. A, IL-17A is elevated at baseline in patients with identified metastatic foci of infection; n = 124. B, IL-17A levels by days of antibiotic treatment following negative blood culture, with fatal cases excluded; n = 90. C, Baseline IL-17A by infection foci in complicated SAB patients, n = 124. D, Scatter plot of baseline IL-17A vs. IL-8 levels; Spearman rank correlation test, n = 124. E–F, Baseline IL-17 or IL-8 levels in patient subset by disease outcome: mortality (infection-related vs. not attributed to the infection), recurrence of SAB within 90 days, or clinical cure (persistent vs. nonpersistent bacteremia). Fatal and recurrent cases with persistent bacteremia are shaded; n = 124. G, Baseline IL-17A levels in patients with cardiac hardware (implanted pacemakers or cardiac valves) subdivided by diagnosed endovascular (infective endocarditis (IE) vs. noninfective endocarditis) vs. extravascular infection. H, Measurement of high IL-17A precedes diagnosis of infective endocarditis. Baseline IL-17A levels measured within 3 days are plotted vs. time of infective endocarditis diagnosis by imaging of cardiac vegetation. Days from time of antibiotic therapy are shown. Patients with persistent bacteremia are shown by filled circles. For all panels, medians and Wilcoxon rank sum test P-value are shown, dashed line is an illustrative IL-17A cutoff with maximal sensitivity and specificity, LLOQ = 0.8 pg/mL IL-17A and 0.4 pg/mL IL-8, and gray shaded area represents the range of the healthy control values (n = 16). Abbreviations: IE, infective endocarditis; IL, interleukin; ns, not significant; SAB, S. aureus bacteremia.
Because IL-17 is associated with complicated endovascular infections and these patients have higher mortality rates, we performed a post hoc analysis of the relationship between IL-17A and mortality cases that were clinically assessed as likely related to the S. aureus infection. Interestingly, there was a trend in higher IL-17A in mortality cases assessed as related to the infection vs. those that were not attributed to the infection (Figure 5E). This could be driven by the increased frequency of persistent bacteremia in infection-related fatalities, given the association between IL-17A and persistent bacteremia. Neither IL-8 nor the IL-8+CCL2 prognostic model could discriminate between infection-related mortality and non-infection-related mortality (Figures 5F and 3G).
Median IL-17A levels were not significantly elevated in the patient subset who developed recurrence of SAB over a 90-day follow-up window, with half of patients high for IL-17A and half near or at the assay detection limit (Figure 5E). Persistent bacteremia during the initial hospitalization was the only clinical feature that tracked with IL-17A levels in recurrent bacteremia patients, with most patients presenting with IL-17A levels above the median being unable to clear blood cultures within 5 days (Figure 5E, filled circles).
The observed relationship between IL-17A and endovascular infections prompted us to evaluate whether IL-17A may have clinical utility for identifying endovascular foci in patients with cardiac devices. This could aid clinical decision-making on whether a cardiac device should be surgically removed or spared. In the patients with cardiac devices, IL-17A was higher in patients with diagnosed endovascular infections, and highest in those with infective endocarditis (Figure 5G). Importantly, high IL-17A was measured within the first few days after the bacteremia diagnosis, which was prior to the diagnosis of infective endocarditis in the majority of patients (Figure 5H).
DISCUSSION
Clinicians currently lack precise tools to identify which patients with SAB will fail antibiotic therapy. In this study, we identified immune correlates of clinical failure in patients treated with appropriate antibiotics, which have potential clinical utility as well as identifying immune pathways that correlate with delayed clearance of bloodstream infection and mortality. This study identified 8 proteins that were significantly elevated in patients that were unable to subsequently clear their blood cultures within 5 days (P ≤ .001), with IL-17A, IL-10, and sE-selectin showing the strongest association. Increased circulating levels of sE-selectin (CD62E), shed by endothelial cells, has been reported in SAB patients [23] and is associated with organ failure and mortality in sepsis patients [24–26]. IL-10, a cytokine with immunosuppressive properties, can facilitate bacterial persistence in infected organs [27, 28]. IL-17A shapes neutrophilic antibacterial defense in infected tissues, through induction of G-CSF to promote granulopoeisis and neutrophil survival and neutrophil-recruiting chemokines such as IL-8 (reviewed in [29]). Patients genetically deficient for IL-17A are more susceptible to cutaneous and respiratory S. aureus infections [30–32]. High early levels of IL-17A showed the strongest association with multiple indices of the failure of antibiotic treatment to clear infection: persistently positive blood cultures, metastatic foci of infection, and a nonsignificant elevated trend in fatalities attributed to the bacterial infection. Other circulating metrics of neutrophilic inflammation such as blood neutrophils, IL-8, and IL-6 do not serve as a proxy for IL-17A.
There would be high clinical value of a blood biomarker that could quickly identify patients with potential complicated foci of infection to direct diagnostic work-up and the duration of antibiotic therapy. Patients with unidentified tissue foci of infection have poor clinical outcomes similar to patients with infective endocarditis [15]. Of note, high IL-17A was measured days earlier than the diagnosis of infective endocarditis in the majority of patients with implanted cardiac devices. These preliminary data support further evaluation in a larger cohort of patients with cardiac devices to see whether early IL-17A levels could guide diagnostic imaging of patients at greater risk for endovascular infections.
In this study, we evaluated whether biomarkers with value in sepsis cohorts are applicable to SAB, compared the relative prognostic value of biomarkers that have been identified individually in other studies, and developed a prognostic model. We confirmed the prognostic value of IL-8 and IL-10 for mortality in SAB patients [17–19, 33]. A broader set of circulating proteins prognostic for mortality has been identified across multiple sepsis cohorts, including IL-6, IL-1RN, IL-10, IL-8, CHI3L1, and ANGPT2 (Supplementary Table 1), and we measured significantly increased levels of these proteins in fatal S. aureus bacteremia. We also observed that high baseline levels of CCL26 and IL-27 were prognostic for mortality, which has not previously been reported. IL-8 had the strongest individual prognostic value, suggesting that the value of early IL-8 levels in predicting mortality across many sepsis cohorts (Supplementary Table 1) is applicable to SAB. Our prognostic model weights a combination of only levels of IL-8, a neutrophil-recruiting chemokine, and CCL2, a myeloid cell-recruiting chemokine, as the best classifier of fatal outcomes in SAB, which was superior to age and other clinical data routinely available at admission.
The prognostic model of IL-8 and CCL2 identified in this study needs to be evaluated in a larger unselected cohort of patients with SAB to assess its utility for informing clinical decision making and potential to enrich for patients with poor outcomes in clinical trials. The lower precision of blood cell count data routinely reported by automated blood cell counters (to the 100 of cells/μL) combined with some missing data for neutrophil counts, may have contributed to our observation that blood neutrophil composition was less informative about clinical severity than blood cytokines. Although there is literature support for the prognostic value of cytokines such as IL-8 for mortality across Gram-positive and Gram-negative bloodstream infections, a larger study will be required to evaluate whether the prognostic value of biomarkers identified in this study is generalizable to bacteremia caused by pathogens besides S. aureus. Some biomarkers with published prognostic value were deprioritized for validation due to weak associations with clinical outcome in the discovery cohort. For example, low IL-1β is prognostic for persistent S. aureus bacteremia [17] and clinical serotypes differ in virulence factor expression that can suppress inflammasome-mediated IL-1β production [34]. Because IL-1β can promote IL-17A production [35], it will be interesting to evaluate both cytokines in future studies. Although this study was limited to host correlates of disease severity, several bacterial clonal or virulence factors have been described to differentially stimulate cytokine responses and are associated with patient outcomes [36, 37]. Future studies integrating clinical, inflammatory, and microbial variables in prognostic modeling of poor bacteremia outcomes are warranted.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Xiaoying Yang for statistics advice and Michele Downing and Joann Volinski for recruiting subjects for the discovery cohort.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding. This study was funded by Genentech, Inc. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 1KL2TR002554 [S. A. M].
Potential conflicts of interests. A. O. G., Y. C., J. G., K. H., O. M., M. C.-T., M. P., J. B. D., A. B., and C. M. R. are currently employees of Genentech. V. G. F. served as Chair of the V710 Scientific Advisory Committee (Merck); has received grant support from Cerexa/Actavis/Allergan, Pfizer, Advanced Liquid Logics, the National Institutes of Health (NIH), MedImmune, Basilea Pharmaceutica, Karius, ContraFect, Regeneron Pharmaceuticals, and Genentech; has NIH STTR/SBIR grants pending with Affinergy, Locus, and Medical Surface, Inc; has been a consultant for Achaogen, AmpliPhi Biosciences, Astellas Pharma, Arsanis, Affinergy, Basilea Pharmaceutica, Bayer, Cerexa Inc., ContraFect, Cubist, Debiopharm, Durata Therapeutics, Grifols, Genentech, MedImmune, Merck, The Medicines Company, Pfizer, Novartis, NovaDigm Therapeutics Inc., Theravance Biopharma, Inc., XBiotech, and has received honoraria from Theravance Biopharma, Inc., and Green Cross, and has a patent pending in sepsis diagnostics. C. K. reports grants paid to her institution from Gilead Research Scholars Program in HIV, outside the submitted work. All other authors have no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Corey GR. Staphylococcus aureus bloodstream infections: definitions and treatment. Clin Infect Dis 2009; 48(Suppl 4):S254–9. [DOI] [PubMed] [Google Scholar]
- 2. Lindsay JA, Holden MT. Understanding the rise of the superbug: investigation of the evolution and genomic variation of Staphylococcus aureus. Funct Integr Genomics 2006; 6:186–201. [DOI] [PubMed] [Google Scholar]
- 3. van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25:362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–72. [DOI] [PubMed] [Google Scholar]
- 5. Casapao AM, Jacobs DM, Bowers DR, Beyda ND, Dilworth TJ; REACH-ID Study Group Early administration of adjuvant β-lactam therapy in combination with vancomycin among patients with methicillin-resistant Staphylococcus aureus bloodstream infection: a retrospective, multicenter analysis. Pharmacotherapy 2017; 37:1347–56. [DOI] [PubMed] [Google Scholar]
- 6. Hsu RB. Risk factors for nosocomial infective endocarditis in patients with methicillin-resistant Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 2005; 26:654–7. [DOI] [PubMed] [Google Scholar]
- 7. Jensen AG, Espersen F, Skinhøj P, Rosdahl VT, Frimodt-Møller N. Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark 1980–1990. J Infect 1997; 34:113–8. [DOI] [PubMed] [Google Scholar]
- 8. Rasmussen RV, Fowler VG Jr, Skov R, Bruun NE. Future challenges and treatment of Staphylococcus aureus bacteremia with emphasis on MRSA. Future Microbiol 2011; 6:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valente AM, Jain R, Scheurer M, et al. Frequency of infective endocarditis among infants and children with Staphylococcus aureus bacteremia. Pediatrics 2005; 115:e15–9. [DOI] [PubMed] [Google Scholar]
- 10. del Rio A, Cervera C, Moreno A, Moreillon P, Miró JM. Patients at risk of complications of Staphylococcus aureus bloodstream infection. Clin Infect Dis 2009; 48(Suppl 4):S246–53. [DOI] [PubMed] [Google Scholar]
- 11. Liu C, Bayer A, Cosgrove SE, et al. ; Infectious Diseases Society of America Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 12. Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mitchell DH, Howden BP. Diagnosis and management of Staphylococcus aureus bacteraemia. Intern Med J 2005; 35:S17–24. [DOI] [PubMed] [Google Scholar]
- 14. Lautenschlager S, Herzog C, Zimmerli W. Course and outcome of bacteremia due to Staphylococcus aureus: evaluation of different clinical case definitions. Clin Infect Dis 1993; 16:567–73. [DOI] [PubMed] [Google Scholar]
- 15. Kaasch AJ, Barlow G, Edgeworth JD, et al. ; ISAC, INSTINCT, SABG, UKCIRG, and Colleagues Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 2014; 68:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 2012; 206:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rose WE, Shukla SK, Berti AD, et al. Increased endovascular Staphylococcus aureus inoculum is the link between elevated serum interleukin 10 concentrations and mortality in patients with bacteremia. Clin Infect Dis 2017; 64:1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minejima E, Bensman J, She RC, et al. A dysregulated balance of proinflammatory and anti-inflammatory host cytokine response early during therapy predicts persistence and mortality in Staphylococcus aureus bacteremia. Crit Care Med 2016; 44:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rhee C. Cytokine imbalance predicts poor outcomes in Staphylococcus aureus bacteremia–but what can we do about it?Crit Care Med 2016; 44:846–7. [DOI] [PubMed] [Google Scholar]
- 21. McNicholas S, Talento AF, O’Gorman J, et al. Cytokine responses to Staphylococcus aureus bloodstream infection differ between patient cohorts that have different clinical courses of infection. BMC Infect Dis 2014; 14:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, et al. ; UK Clinical Infection Research Group Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis 2011; 11:208–22. [DOI] [PubMed] [Google Scholar]
- 23. Soderquist B, Sundqvist KG, Vikerfors T. Adhesion molecules (E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)) in sera from patients with Staphylococcus aureus bacteraemia with or without endocarditis. Clin Exp Immunol 1999; 118:408– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cummings CJ, Sessler CN, Beall LD, Fisher BJ, Best AM, Fowler AA 3rd. Soluble E-selectin levels in sepsis and critical illness: correlation with infection and hemodynamic dysfunction. Am J Respir Crit Care Med 1997; 156:431–7. [DOI] [PubMed] [Google Scholar]
- 25. Cowley HC, Heney D, Gearing AJ, Hemingway I, Webster NR. Increased circulating adhesion molecule concentrations in patients with the systemic inflammatory response syndrome: a prospective cohort study. Crit Care Med 1994; 22:651–7. [DOI] [PubMed] [Google Scholar]
- 26. Zonneveld R, Martinelli R, Shapiro NI, Kuijpers TW, Plötz FB, Carman CV. Soluble adhesion molecules as markers for sepsis and the potential pathophysiological discrepancy in neonates, children and adults. Crit Care 2014; 18:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heim CE, Vidlak D, Kielian T. Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. J Leukoc Biol 2015; 98:1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leech JM, Lacey KA, Mulcahy ME, Medina E, McLoughlin RM. IL-10 plays opposing roles during Staphylococcus aureus systemic and localized infections. J Immunol 2017; 198:2352–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol 2017; 18:612–21. [DOI] [PubMed] [Google Scholar]
- 30. Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011; 332:65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am 2008; 28:277–291, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minegishi Y, Saito M, Nagasawa M, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 2009; 206:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. West TE, Wikraiphat C, Tandhavanant S, et al. Patient characteristics, management, and predictors of outcome from severe community-onset Staphylococcal sepsis in Northeast Thailand: a prospective multicenter study. Am J Trop Med Hyg 2017; 96:1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hancz D, Westerlund E, Bastiat-Sempe B, et al. Inhibition of inflammasome-dependent interleukin 1β production by streptococcal NAD+-glycohydrolase: evidence for extracellular activity. MBio 2017; 8:e00756–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maher BM, Mulcahy ME, Murphy AG, et al. Nlrp-3-driven interleukin 17 production by γδT cells controls infection outcomes during Staphylococcus aureus surgical site infection. Infect Immun 2013; 81:4478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Recker M, Laabei M, Toleman MS, et al. Clonal differences in Staphylococcus aureus bacteraemia-associated mortality. Nat Microbiol 2017; 2:1381–8. [DOI] [PubMed] [Google Scholar]
- 37. Niebuhr M, Gathmann M, Scharonow H, et al. Staphylococcal alpha-toxin is a strong inducer of interleukin-17 in humans. Infect Immun 2011; 79:1615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010; 33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 39. Liaw A, Wiener M. Classification and regression by randomForest. R News 2002; 2:18. [Google Scholar]
- 40. R Core Team (R Foundation for Statistical Computing). R: a language and environment for statistical computing. 2013. Available at: https://www.r-project.org/. Accessed 2 March 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.