Abstract
This paper introduces the concept of “skin bed preparation” prior to surgical procedures. Following the theory of chronic wound bed preparation and adapting the skin model to one of chronic wound changes related to extrinsic and intrinsic factors, a topical formulation aimed at recycling the extracellular matrix (ECM) from accumulated waste products is evaluated and discussed. The clearance of these products and stimulation of new replacements has the potential to change the regenerative milieu of the skin so that when procedures are carried out, cellular signaling and cross-talk at the dermal level are improved and healing is optimized. By introducing a combination of peptides and other synergistic active agents, a sequence of clearance, regeneration, and remodeling is initiated. This is confirmed and validated by a series of biopsies and clinical studies that demonstrate changes in the ECM as early as 2 to 3 weeks after application. Clinical studies related to resurfacing procedures show accelerated healing and improved symptomatic relief compared with standard of care by preconditioning the skin 2 weeks prior to the procedure. A similar approach is suggested as a potential advantage for invasive surgical procedures based on similar scientific principles elucidated on in the text.
The concept of prepping the canvas prior to surgery is not a new one. Plastic surgeons and dermatologists have utilized various techniques and agents prior to surgery, as well as treatment with laser/energy-based devices, in an attempt to influence the surgical outcome or final result. Whether the aim was to improve vascularity (ie, the delay phenomenon), influence pigmentation, or simply use oral supplements to improve immunity, the ultimate goal was to optimize the healing process. In recent years major strides have been made in our understanding and knowledge of the field of wound healing.
In particular, chronic wound management is governed by a set of principles that prepare the wound bed for healing prior to applying any therapeutics. This was deemed necessary as the accumulation of by-products from dysfunctional healing and excess inflammation created a wound bed milieu that obviated healing. Ridding the wound bed of these by-products thus became a priority in wound bed preparation. The preparation took the form of wound debridement, controlled inflammation, adequate moisture, and a host of techniques to encourage epithelialization—ie, the DIME principles, which were aimed at accelerating endogenous healing or facilitating the effectiveness of therapeutic measures.1
Advancing this concept and theory, and the principles to skin homeostasis, is a logical progression. When one considers that skin has been subjected to years of extrinsically induced degradation mainly in the form of photodamage, coupled with intrinsic changes brought about by aging, the similarity with the chronic wound model becomes apparent. Much the same as chronic wounds accumulate the by-products noted above, so the skin, in particular the extracellular matrix (ECM), accumulates waste products that hinder cellular and protein cross-talk and signaling, the very signaling that is paramount in normal wound healing.
The clearance of these products and stimulation of new replacements has the potential to change the regenerative milieu of the skin so that when procedures are carried out, cellular signaling and cross-talk at the dermal level are improved and healing is optimized.
ECM Changes Defined
When viewing the skin in terms of the “chronic wound” paradigm it is necessary to first define the molecular changes occurring in the dermal (and to a lesser degree epidermal) layers. The ECM is the central orchestrator of communication between cells and a multitude of proteins. Changes within the matrix dictate outcomes in cellular function and protein production. Aging and photodamage directly affect these essential functions, creating inefficient communication between the constituents of the ECM. This ongoing conversation should continue uninterrupted to ensure healthy skin maintenance and renewal, but breakdown products tend to accumulate and disrupt communication in the following ways:
Photodamage causes cells to release enzymes (eg, MMP-1-collagenase) that fragment type 1 collagen from long healthy strands. These smaller collagen fragments prevent the fibroblast from “stretching out” its healthy spindle shape along the course of the fiber, resulting in a change of shape to a rounded fibroblast2
A rounded fibroblast is a senescent, inefficient fibroblast that produces limited amounts of collagen and elastin, impeding regeneration3
Acute bursts of sun exposure cause white blood cells to release neutrophil elastase, affecting the quality of existing elastin fibers, and breaking down fibrillin components3,4
The fragments of collagen that remain, known as gelatin, adhere to the elastin fragments, causing an agglutination of gelatinous clumps that interferes with normal ECM communication3
Intrinsic aging also results in accumulation of wear-and-tear waste products—intracellular protein fragments and glycation end products5
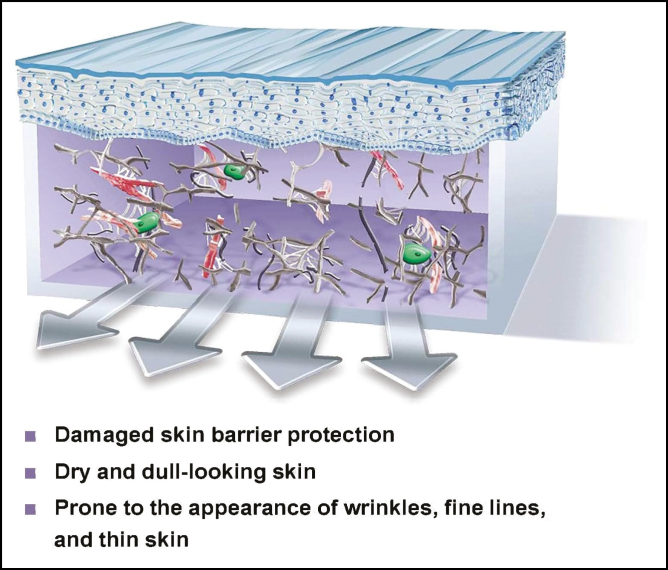
The dermis and the ECM is now filled with fragmented sticky clumps of collagen and elastin and the fibroblasts are senescent and unproductive. The epidermis is thinned and wrinkled, barrier protection is affected, and elasticity is diminished (Figure 1). This is normally the status of the skin prior to surgical intervention. What if the status could be changed and in a short period of time, a matter of weeks, the senescent milieu of the ECM could be transformed into a regenerative one? Would that not facilitate healing and outcomes irrespective of the procedure? This is skin bed preparation.
Figure 1.
Chronic sun damage and aging results in fragmented collagen, elastin, and senescent rounded fibroblasts in the ECM and thinned epidermal layer. ECM, extracellular matrix.
A key differentiator with TriHex Technology (ALASTIN Skincare, Inc., Carlsbad, CA) is its proprietary recycling mechanism to help remove aged, damaged fragments and restore the optimal environment for new, healthy collagen and elastin.
Effecting ECM Changes: Skin Bed Preparation
Analogous to wound bed preparation before therapeutic intervention for chronic wounds, “skin bed preparation” aims to optimize rejuvenation procedures and skin maintenance programs. This involves introducing agents that can combat stress-induced oxidation, proteasome dysfunction, and nonenzymatic cross-linkages involved in glycation end products, to collectively modulate this damaged ECM, and upregulate neocollagenesis and elastin production. Agents of particular interest are matrikines, peptides originating from the fragmentation of matrix proteins, which exhibit a wide range of biological activities relating to matrix modulation.6 Peptides of this type (tripeptides and hexapeptides) are incorporated in TriHex Technology, which is designed to target ECM modulation with the goal of optimizing results following invasive and noninvasive dermal rejuvenating procedures. Additionally, oleuropein (a lipoxygenase activity inhibitor) and phosphatidylserine (a signal enzyme activator) are included in the TriHex Technology formulation to decrease inflammation and stimulate cellular recycling of waste products.
From the aspect of matrix degradation and restructuring described above, a sequence of targeted approaches to repair and regenerate the ECM includes the following:
“Mopping-up” of excess collagen and elastin fragments in the ECM with balanced MMP production (MMP2- also known as gelatinase enzyme release)
Combating oxidative stress via reactive oxygen species end products generated in the ECM
Stimulation of procollagen and collagen, thereby increasing lysyl oxidase beneficial cross-linkages and improving fiber alignment
Stimulating elastin (particularly fibrillin) to regenerate ECM elasticity
Increasing the efficiency of the proteasome system by eliminating dysfunctional proteins
Reducing the destruction of normal collagen fragments by MMP-1
Decreasing glycation end products
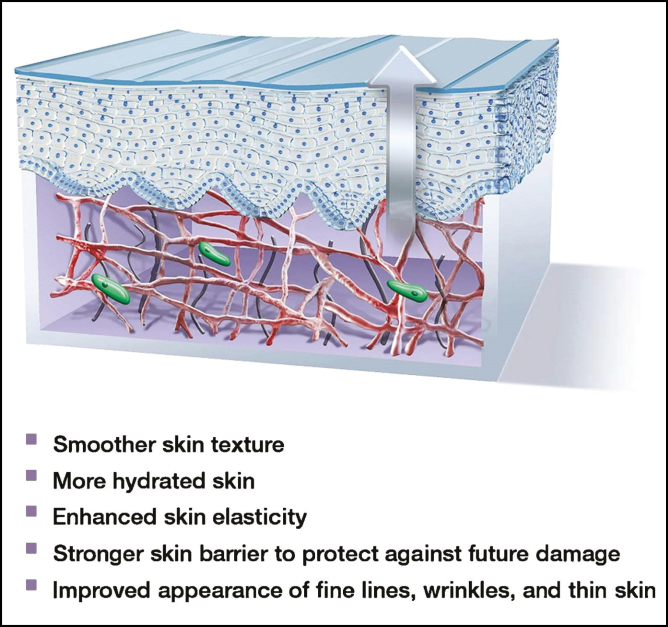
The select peptides and botanical extracts synergistically and sequentially aid in this ECM modulation to optimize and “prime” the skin for rejuvenating procedures (Figure 2).
Figure 2.
“Recycled” matrix where fragmented collagen and elastin have been replaced by new long-strand structural proteins, spindle-shaped active fibroblasts, a healthy basal stem cell layer, and a thickened epidermis.
Validating ECM Changes
The scientific narrative for skin bed preparation is a logical approach to consider prior to surgery if the skin is to be considered as akin to a chronic wound. However, validation of the narrative is imperative. First, in vitro testing of components and of the formulation itself was undertaken and expression of the genes involved in ECM remodeling was confirmed.7 Second, histologic examination of 5 subjects in their 60s was undertaken, assessing changes related to isolated topical application of product to the skin. The aim was to ascertain—from punch biopsy and examination of excision specimens—if topical application of product alone could initiate histologic changes in a short period of time.8 For each specimen, representative high-power fields of the superficial dermis and deep dermis were evaluated. The amount of collagen within the high-power field was quantified as a percentage of the total dermal ECM. In a similar manner, Verhoeff-Van Gieson (VVG) staining of elastic fibers was evaluated as a percentage of the connective tissue within a representative high-power field from both the superficial dermis and deep dermis. Overall, biopsy results revealed significant changes within the ECM in all subjects tested, who showed new collagen formation, increased elastin, a “healthier” epidermis with more cuboidal basal stem cells at the dermo-epidermal junction, and a thickened epidermis (Figures 3-5).8
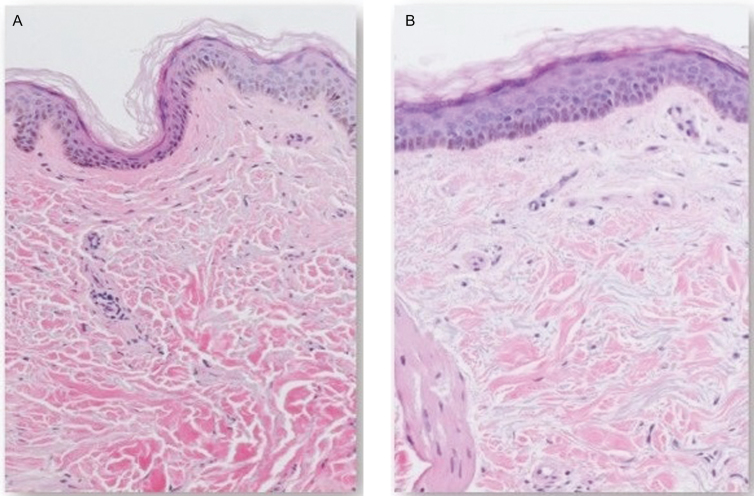
Figure 3.
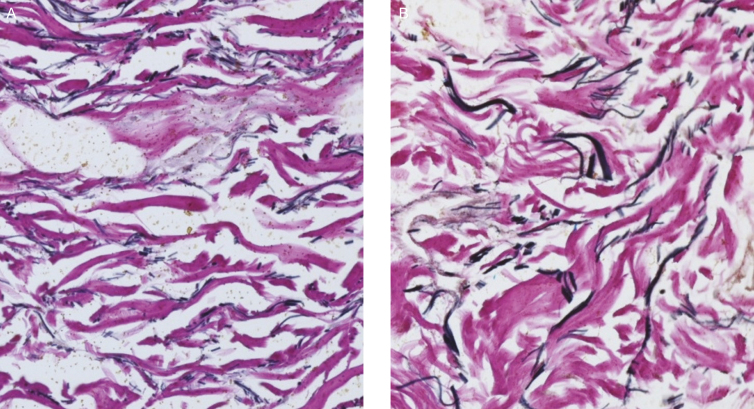
H&E staining (100×) showing ECM and epidermal changes following a 3-week topical application of product containing TriHex Technology peptides and botanicals to the forearm (65-year-old female patient). (A) Baseline H&E-stained sections from nontreated forearm skin shows thicker, mature collagen bundles, and thinner epidermis with flattened basal cells. (B) Post-treatment (right), the epidermis shows less atrophy, with increased number of layers, more cuboidal basal cells, and recovery of normal epidermal maturation. The ECM shows finer collagen bundles, likely representing neocollagenesis. This is a low-powered representation that clearly shows a good distribution of newer fresh collagen well spread over the entire frame of the ECM as opposed to the mature older collagen apparent in the baseline ECM of the slide in panel A. ECM, extracellular matrix; H&E, hematoxylin and eosin.
Figure 4.
H&E-stained sections (200×) from biopsies taken at baseline (left) and following a 3-week (right) topical application of TriHex Technology peptide gel to the preauricular region (62-year-old male patient). (A) Baseline H&E-stained sections from nontreated preauricular skin shows limited collagen in the upper dermis and basophilic solar elastotic changes in the dermal ECM. (B) Post topical treatment, there is less epidermal atrophy, with recovery of normal epidermal maturation. In the dermis, newly formed (pink) collagen is present, replacing some of the severe solar elastosis. An increased number of fibroblasts is easily identified among the newly formed ECM in the papillary dermis. Multiple views of these slides demonstrated 5% content of superficial collagen in baseline (A) vs 30% content of superficial collagen after 3 weeks (6-fold increase). ECM, extracellular matrix; H&E, hematoxylin and eosin.
Figure 5.
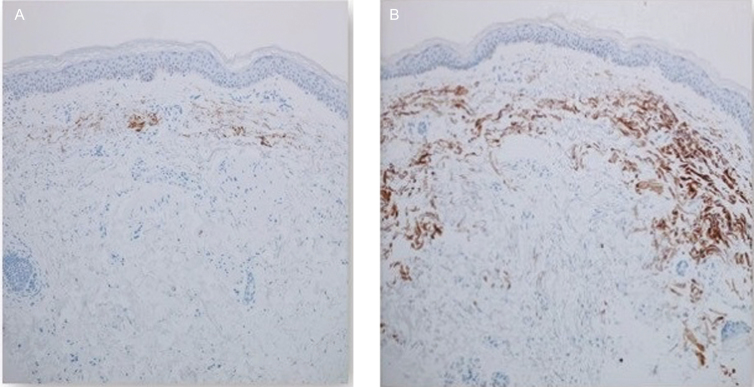
Elastin immunohistochemical staining of sections from biopsies taken at baseline and following a 3-week topical application of the TriHex Technology product to the forearm (63-year-old male patient) (100×) [Abcam anti-elastin mouse monoclonal antibody (BA-4), 1:100 dilution]. (A) Baseline shows minimal elastin staining in the dermis. (B) Post topical treatment, there is a striking increase in the amount of dermal elastin staining. This is a low-powered magnification which gives a good overall view; assessment of the complete slide confirmed 10% elastic fiber in superficial layers vs 90% elastin content in superficial dermis (extending to deep dermis) after treatment.
Finally, clinical trials assessing the differences in patients preconditioned with the product versus standard of care were undertaken in a number of centers, with varied devices, procedures, and anatomic sites.9-11 In one trial under institutional review board (Chesapeake) supervision, 15 patients were evaluated after treatment with the product following fractionated CO2 laser resurfacing, and compared to treatment with a bland ointment and cream. A blinded investigator graded erythema, edema, crusting, exudation, and healing on postprocedure days 1, 3, 4, 7, 28, and 84. A photodamage/wrinkle scale was completed on days 28 and 84. Subjects performed symptomatology grading on days 1 to 14 and completed self-assessments at days 28 and 84. Data from 14 subjects were analyzed. Blinded-investigator-rated healing was better for the TriHex Technology system, reaching statistical significance at day 7. The TriHex group demonstrated less erythema and exudation during the first postprocedure week, reaching significance on day 3. On days 1 to 14, subjects treated with the test product reported less tenderness and burning/stinging, also reaching significance on day 3. On day 84, subjects treated with the TriHex system reported higher satisfaction and were more likely to recommend the treatment to others (Figures 6 and 7).9
Figure 6.
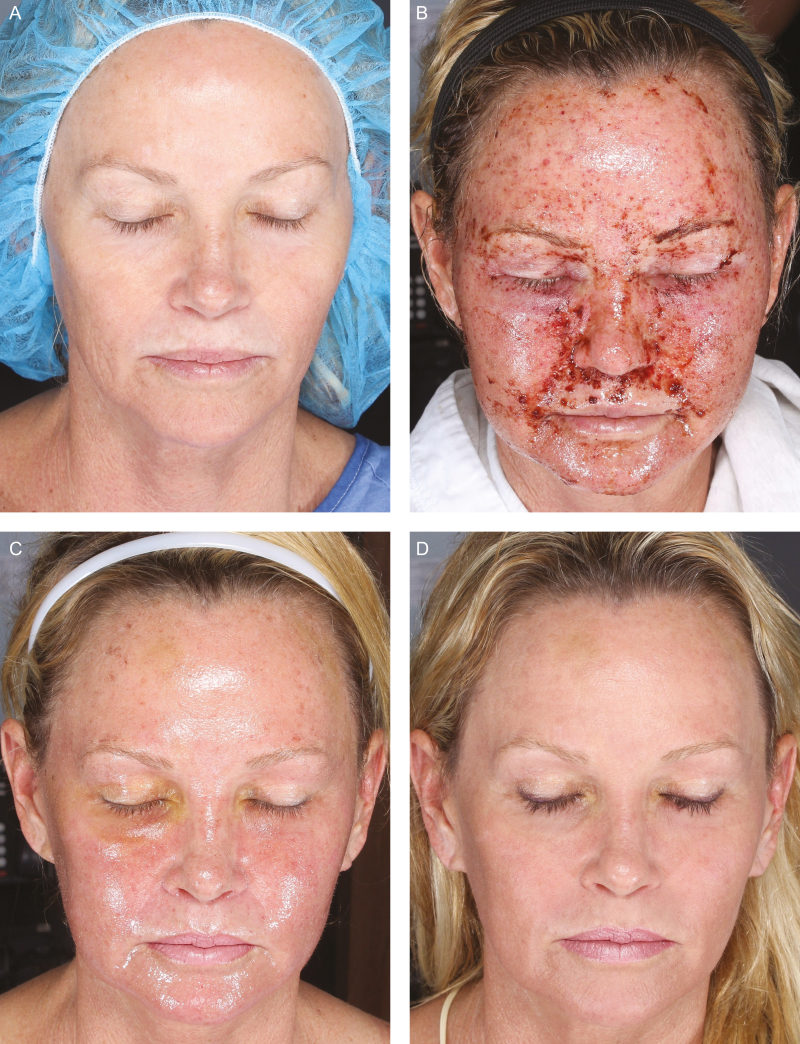
Baseline prior to treatment. (A) A 53-year-old female received TriHex Technology pretreatment and follow-up treatment. (B) Day 1 following moderately deep ablative resurfacing. (C) Day 4 following treatment showing good epithelialization and advanced healing. (D) Day 7 following treatment showing complete healing; able to apply cosmetics.
Figure 7.
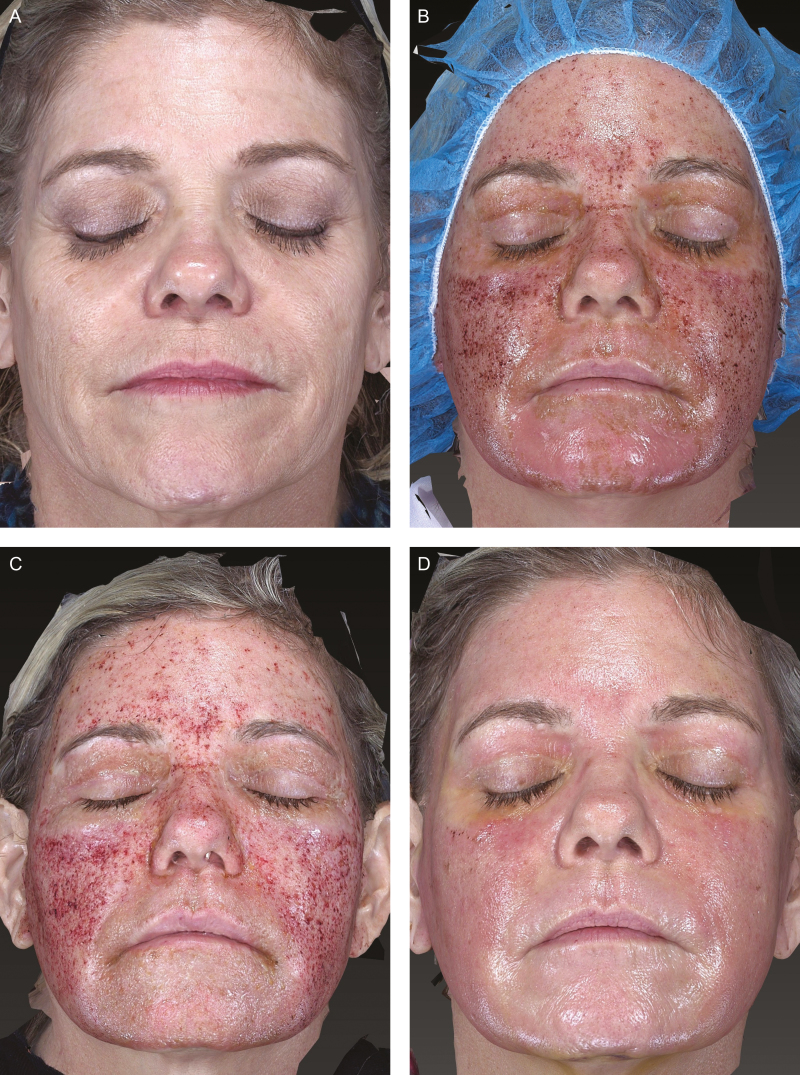
Baseline prior to treatment. (A) A 54-year-old female received (Vaniply, Pharmaceutical Specialties, Inc., Rochester, MN, USA) pretreatment and follow-up treatment. (B) Day 1 following moderately deep ablative resurfacing. (C) Day 4 following treatment showing minimal epithelialization and healing compared with the experimental group; day 4 is the day where the most obvious difference between groups was evident. (D) Day 7 following treatment showing some unresolved healing and more redness than comparator.
In a split face study with the same product and protocol following fractional nonablative thulium-doped resurfacing treatment of the face or décolleté, the test product was found to be statistically superior to the basic regimen following laser treatment from day 4 based on healing, lentigines, texture, and global skin quality. Subjects also reported “better looking and feeling” skin on the test side. It was concluded that preconditioning with the TriHex test product improved healing post-nonablative thulium-doped resurfacing treatment to the face/décolleté in comparison with standard of care.10
Thus, in cases involving resurfacing of the face, preconditioning with TriHex Technology appears to speed up healing, and decrease redness, swelling, burning, and stinging; patients enjoy applying the treatment and feel it improves long-term outcome. No adverse events related to product use were reported in any of the studies referred to above.
The question now remains: how would preconditioning affect more invasive surgical procedures? To that end patients undergoing full face lift or upper blepharoplasties were selected. One side was pretreated for 2 to 4 weeks prior to surgery and specimens from both sides were submitted for histology at the time of the procedure. In the first 5 patients tested, biopsy results revealed increased collagen staining with healthier ECM and diminished solar elastosis (Figures 8 and 9).
Figure 8.
Elastin VVG staining 200× (58-year-old female patient). (A) Left side the of the face received no pretreatment; elastin staining evident. (B) Right side following 2 weeks of pretreatment demonstrating increased elastic fiber in the dermis with more prominent and deeper elastin staining. Overall multiview assessment demonstrated 10% elastin content in the superficial dermis and deep dermis on the untreated side vs 20% and 25%, respectively, on the treated side (at least a 2-fold increase plus changes in the nature of the fibers—fragmented smaller fibers untreated, more solid longer fibers after treatment). VVG, Verhoeff-Van Gieson.
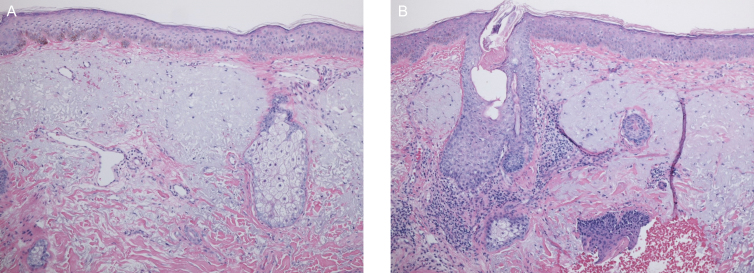
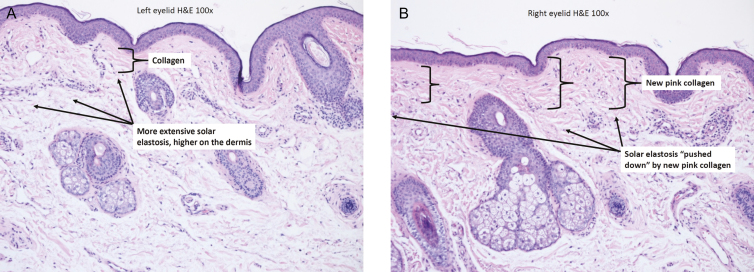
Figure 9.
Eyelid specimens post upper blepharoplasty (47-year-old female patient, H&E staining, 100×). (A) Left eyelid specimen; no treatment prior to procedure showing extensive solar elastosis and minimal collagen in the dermis. (B) Right eyelid specimen following a 4-week pretreatment showing improved solar elastosis and increased collagen production. There are well-demonstrated changes in this slide; quantitative reassessment showed a superficial collagen content of 40% on the nontreated side (A) vs a 90% content of superficial collagen on the treated side as well as significantly improved solar elastosis. H&E, hematoxylin and eosin.
Limitations
Much of this scientific narrative is based on a working hypothesis that the documented histologic molecular changes seen in many cases following topical application may be beneficial to surgical outcomes by changing the nature of the ECM and thus the potential for improved cell to cell and cell to matrix cross-talk. The limitations here are small numbers and limited data related to the clinical studies and biopsies presented; however, all preliminary findings do appear to indicate healing advantages and symptomatic relief related to the preconditioning. More work is definitely required to validate this concept, but this is in keeping with the introduction of a new “hot topic” concept.
CONCLUSIONS
This concept of preparing skin and its molecular complexities for surgery has a sound scientific basis. The final outcome of all surgeries, especially those with aesthetic objectives, relies on optimized healing and scarring. To that end previous work has demonstrated that controlled inflammation, adequate hydration, and an accelerated healing process all contribute to good scar outcomes.12,13 Additionally, we have shown in our resurfacing cases that redness, exudate, burning, and stinging can be reduced by preconditioning, but perhaps more importantly, that healing can be hastened. Taking these data and expanding them to objectively assess outcomes following more complex surgical procedures is a natural next step in the process. The foundation for pursuing this endeavor appears to be well established.
Disclosures
Dr Widgerow is Chief Medical Officer of Alastin Skincare, Inc. The offices of Drs Fagien and Cohen will be remunerated for studies performed for Alastin Skincare, Inc. Products were supplied by Alastin SkinCare, Inc.
Funding
This article was supported by Alastin Skincare (Carlsbad, CA), who co-funded the development of this supplement.
REFERENCES
- 1. Sibbald RG, Woo KY, Ayello E. Wound bed preparation: DIM before DIME. Wound Healing Southern Africa. 2008;1(1):29-34. [Google Scholar]
- 2. Fisher GJ, Quan T, Purohit T, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174(1):101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144(5):666-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age (Dordr). 2009;31(4):305-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lohwasser C, Neureiter D, Weigle B, Kirchner T, Schuppan D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J Invest Dermatol. 2006;126(2):291-299. [DOI] [PubMed] [Google Scholar]
- 6. Maquart FX, Bellon G, Pasco S, Monboisse JC. Matrikines in the regulation of extracellular matrix degradation. Biochimie. 2005;87(3-4):353-360. [DOI] [PubMed] [Google Scholar]
- 7. Widgerow AD, Fabi SG, Palestine RF, et al. Extracellular matrix modulation: optimizing skin care and rejuvenation procedures. J Drugs Dermatol. 2016;15(Suppl 4):S63-S71. [PubMed] [Google Scholar]
- 8. Calame A, Widgerow AD. Histological changes associated with extracellular matrix-remodeling topical therapy. Dermatol Case Rep. 2017;2(2):1000126. [Google Scholar]
- 9. Vanaman Wilson MJ, Bolton J, Fabi SG. A randomized, single-blinded trial of a tripeptide/hexapeptide healing regimen following laser resurfacing of the face. J Cosmet Dermatol. 2017;16(2):217-222. [DOI] [PubMed] [Google Scholar]
- 10. Robinson DM, Frulla AP. Randomized, split-face/décolleté comparative trial of procedure enhancement system for fractional non-ablative laser resurfacing treatment. J Drugs Dermatol. 2017;16(7):707-710. [PubMed] [Google Scholar]
- 11. Widgerow A. Topical skin restoration technology—advances in age management strategies. Modern Aesthetics. 2016:1-8. [Google Scholar]
- 12. Widgerow AD. Cellular/extracellular matrix cross-talk in scar evolution and control. Wound Repair Regen. 2011;19(2):117-133. [DOI] [PubMed] [Google Scholar]
- 13. Widgerow AD. Current concepts in scar evolution and control. Aesthetic Plast Surg. 2011;35(4):628-635. [DOI] [PubMed] [Google Scholar]