Abstract
Context
Insulin and leptin may increase growth and proliferation of thyroid cells, underlying an association between type 2 diabetes and papillary thyroid cancer (PTC). Patients with extreme insulin resistance due to lipodystrophy or insulin receptor mutations (INSR) are treated with high-dose insulin and recombinant leptin (metreleptin), which may increase the risk of thyroid neoplasia.
Objective
The aim of this study was to analyze thyroid structural abnormalities in patients with lipodystrophy and INSR mutations and to assess whether insulin, IGF-1, and metreleptin therapy contribute to the thyroid growth and neoplasia in this population.
Design
Thyroid ultrasound characteristics were analyzed in 81 patients with lipodystrophy and 11 with INSR (5 homozygous; 6 heterozygous). Sixty patients were taking metreleptin.
Results
The prevalence of thyroid nodules in children with extreme insulin resistance (5 of 30, 16.7%) was significantly higher than published prevalence for children (64 of 3202; 2%), with no difference between lipodystrophy and INSR. Body surface area–adjusted thyroid volume was larger in INSR homozygotes vs heterozygotes or lipodystrophy (10.4 ± 5.1, 3.9 ± 1.5, and 6.2 ± 3.4 cm2, respectively. Three patients with lipodystrophy and one INSR heterozygote had PTC. There were no differences in thyroid ultrasound features in patients treated vs not treated with metreleptin.
Conclusion
Children with extreme insulin resistance had a high prevalence of thyroid nodules, which were not associated with metreleptin treatment. Patients with homozygous INSR mutation had thyromegaly, which may be a novel phenotypic feature of this disease. Further studies are needed to determine the etiology of thyroid abnormalities in patients with extreme insulin resistance.
We evaluated thyroid abnormalities in extreme insulin resistance due to lipodystrophy or INSR mutation. Children in both groups had high prevalence of nodules. INSR−/− patients had thyromegaly.
Obesity is associated with increased risk of certain malignancies, including breast, endometrial, colon, rectal, and liver cancers (1–4). Both hyperinsulinemia and hyperleptinemia, which are associated with obesity and insulin resistance, have been proposed as causal links between obesity and cancer development (5–8). Although less well studied than other cancers, there is some evidence to link insulin resistance with thyroid proliferation and differentiated thyroid cancer (9–11). Patients with skin tags, a marker of hyperinsulinemia, have a higher prevalence of ultrasound (US)-detected thyroid nodules, size of thyroid nodules, and thyroid volume (12–14). Furthermore, the insulin sensitizer metformin reduced thyroid size in patients with type 2 diabetes mellitus (15), supporting the idea that hyperinsulinemia has a proliferative effect on the thyroid.
Insulin signaling activates two main signaling pathways: the insulin receptor substrate/phosphatidylinositol 3-kinase (PI3K) pathway, which is responsible for glucose uptake and most metabolic actions of insulin, and the Raf/mitogen-activated protein kinase kinase (MEK)/ERK (MAPK) pathway, which interacts with the PI3K pathway and controls cell growth and differentiation (16). Both signaling cascades can be activated by a wide range of stimuli in addition to insulin, such as leptin and IGF-1 (17–20). In most forms of insulin resistance, including obesity, insulin signaling is impaired in some intracellular insulin signaling pathways but remains intact in other pathways, referred to as “selective insulin resistance.” The pathways that are resistant to insulin vary in a tissue-specific manner (21). In general, it is thought that insulin signaling via insulin receptor substrate/PI3K pathways is impaired, whereas Raf/MEK/ERK signaling remains unaffected.
Syndromes of extreme insulin resistance include generalized or partial lipodystrophy, in which there is complete or partial absence of adipose tissue leading to selective insulin resistance, and insulin receptor mutation (22, 23) (INSR) syndromes, in which there is a primary insulin-signaling defect leading to impairment of all insulin signaling pathways, including the Raf/MEK/ERK pathway (24). Both high-dose insulin and metreleptin are used as treatments in both conditions (22, 23). There are no data regarding effects of known mitogenic stimuli such as hyperinsulinemia and leptin on thyroid proliferation in patients with extreme insulin resistance. Because a link between milder forms of insulin resistance and thyroid proliferation has already been shown, we hypothesized that severe hyperinsulinemia and/or leptin treatment in patients with extreme insulin resistance would lead to increased prevalence of proliferative thyroid changes. We further hypothesized that patients with lipodystrophy would have greater prevalence of thyroid proliferative changes compared with patients with homozygous or dominant-negative heterozygous INSR mutations, due to enhanced insulin-mediated stimulation of the proliferative MAPK pathway in this population.
The aims of this study were to (i) analyze thyroid structural abnormalities in patients with extreme insulin resistance due to lipodystrophy or INSR mutation, and (ii) to assess factors that might contribute to thyroid growth and neoplasia in these populations, including insulin, IGF-1, and metreleptin therapy.
Material and Methods
Patients
Patients with lipodystrophy or mutations of the insulin receptor participated in a natural history study of insulin resistance and/or prospective, open-label interventional studies of metreleptin at the National Institutes of Health Clinical Center (Bethesda, MD). Studies were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NCT00027456, NCT00085982, NCT00005905, NCT00025883, NCT01778556, NCT02262806, NCT02262832, NCT00001987). All subjects or their legal guardians provided written informed consent prior to participation. Minors provided assent when age appropriate. Eighty-one patients with lipodystrophy and 11 patients with INSR mutation had available thyroid US data.
Thyroid imaging
High-resolution thyroid US was performed using a linear array 10- to 12-MHz probe. Longitudinal and transverse scans were performed to measure depth, width, and length of each lobe. Thyroid lobe volume was calculated as 0.479 × depth × width × length (cm). Thyroid volume was calculated as the sum of the volumes of both lobes, excluding the isthmus. Thyroid volumes in subjects <20 years of age were compared with reference ranges for age and body surface area (BSA) as described by Suzuki et al. (25) based on assessment of 34,227 children from an iodine-sufficient area. Thyroid volume in adults (20 to 70 years of age) were compared with reference ranges described by Berghout et al. (26) based on analysis of 50 healthy volunteers (25 males and 25 females) from iodine-sufficient areas (normal ranges within inner limits of the percentiles 2.5 and 97.2 are 4.8 ± 15.1 mL for women and 7.7 ± 19.1 mL for men). Because adults with lipodystrophy and INSR mutation may have low BSA due to low weight or height, respectively, thyroid volume in adults was also analyzed according to BSA-based reference ranges described by Suzuki at al. (25) for healthy volunteers aged 0 to 19 years. Thyroid volume adjusted for BSA was calculated as total thyroid volume divided by BSA. BSA was calculated using the formula of Du Bois and Du Bois (27).
All thyroid ultrasonography studies were reviewed by one radiologist (S.K.) who was blinded to the patients’ diagnoses. Thyroid gland appearance on US was characterized based on echogenicity (homogeneous, heterogeneous), vascularity (decreased, normal, increased), and presence of cystic lesions and/or nodules. Nodules were characterized by size, composition (solid, solid-cystic, spongiform), echogenicity (hypoechogenic, isoechogenic, hyperechogenic), shape (taller than wide, wider than tall), margins (ill-defined, lobulated, smooth), and presence of calcified foci (peripheral/rim calcifications, microcalcifications).
Laboratory methods
Laboratory measurements were performed on blood samples obtained after an 8- to 12-hour fast. All laboratories were obtained within 1 week of the thyroid US with the exception of antithyroid antibodies. TSH, free T4, TT4, fT3, TT3, antithyroglobulin antibody (ATG2), thyroid peroxidase antibody (MTPO1), HbA1c, glucose, c-peptide, insulin, and IGF-1 were measured by the standard techniques of the National Institutes of Health Clinical Center Laboratory. IGF-2 was measured by a Quantikine® human IGF-2 immunoassay (R&D Systems, Minneapolis, MN) in stored serum samples. Sixty-eight samples were available for analysis (3 INSR−/−, 6 INSR+/−, and 59 lipodystrophy patients). Leptin was determined by radioimmunoassay (EMD Millipore, formerly Linco Research, St. Charles, MO). The intraassay and interassay coefficients of variation were 9.3% and 9.6% respectively. This assay measures both endogenous leptin and exogenous metreleptin. Prior studies have shown that most metreleptin-treated patients develop nonneutralizing antibodies that interfere with leptin assays, and thus leptin levels measured during metreleptin treatment may be falsely elevated (28). A 75-g (1.75 g/kg in patients weighing <43 kg) oral glucose tolerance test (OGTT) was performed with measurement of glucose and insulin at times −10, 0, 30, 60, 90, 120, and 180 minutes relative to the glucose load. Insulin and glucose area under the curve (AUC) were calculated using the trapezoidal method.
Statistical methods
The following groups were compared: patients with lipodystrophy vs all patients with INSR mutation; patients with homozygous (INSR−/−) vs heterozygous (INSR+/−) INSR mutation and vs lipodystrophy; all patients (lipodystrophy and INSR mutation) treated with metreleptin vs all patients (lipodystrophy and INSR mutation) never treated with metreleptin. Because of age differences between patients with lipodystrophy vs INSR mutations, comparison between these groups were also performed in the subgroups of patients aged <19 years. Between-group comparisons were performed using a nonparametric t test, Mann–Whitney test, Wilcoxon matched-pairs signed rank test, and Fisher’s exact test depending on data type and distribution. Relationships between duration of metreleptin treatment in the combined group of lipodystrophy and INSR mutation patients and thyroid volume, as well as thyroid nodule size, were determined using Spearman correlation tests. Analyses were performed using GraphPad Prism version 6 (GraphPad Software, La Jolla, CA). Data are reported as mean ± SD unless otherwise indicated. A P value <0.05 was considered statistically significant.
Results
Demographic characteristics
Demographic characteristics of patients, type of INSR mutation, and phenotypic features are shown in Tables 1 and 2. Among 81 patients with lipodystrophy, 3 had atypical progeria, 9 had acquired generalized lipodystrophy, 27 had congenital generalized lipodystrophy, and 42 had familial partial lipodystrophy. Among 11 patients with INSR mutation, 5 had homozygous or compound heterozygous (INSR−/−) and six had heterozygous (INSR+/−) mutations in the INSR gene. Patients with INSR mutations were significantly younger than patients with lipodystrophy (18 ± 10 vs 32 ± 18 years, P = 0.01) with higher prevalence of males (45% vs 16%, P = 0.036) and lower BSA (1.34 ± 0.4 vs 1.67 ± 0.3 m2, P = 0.006). There was no difference in body mass index (BMI) between patients with lipodystrophy vs INSR mutation (Table 2). When only lipodystrophy and INSR patients <19 years old were compared, there were no differences in sex, BSA, BMI, or metreleptin treatment (Table 2). Sixty patients were on metreleptin therapy at the time of thyroid US for a mean of 6.1 ± 4.2 years (range, 0.9 to 16.7 years) for patients with lipodystrophy and 2.8 ± 2.5 years (range, 0.04 to 5.9 years) for patients with INSR mutation. Thirty-five patients with lipodystrophy and five with INSR mutation were on insulin therapy. Fifty-two patients with lipodystrophy and nine with INSR mutation were on metformin. Two patients with lipodystrophy were on pioglitazone in addition to metformin.
Table 1.
Characteristics of Patients With INSR Mutation
| Patient | Age, y | M/F | INSR Mutation, Location | Disease | HbA1c (%)a | DM | AN | Short Stature | Dysmorphic Featuresb | Nephrocalcinosis / Nephrolithiasis | Dyslipidemia |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | M | Homozygous, | RMS | 13.3 | Y | Y | Y | Y | Y | N |
| Ser635Leu | |||||||||||
| del exons 9 and 10 | |||||||||||
| 2 | 19.2 | F | Homozygous, | RMS | 12.9 | Y | Y | Y | Y | Y | N |
| Ile119Met | |||||||||||
| 3 | 18.4 | M | Homozygous, | RMS | 9.4 | Y | Y | N | N | Y | N |
| Tyr3X | |||||||||||
| Glu238Lys | |||||||||||
| 4 | 12.1 | M | Homozygous, | RMS | 10.8 | Y | Y | Y | Y | Y | N |
| Asn117Lys | |||||||||||
| del exon 3 | |||||||||||
| 5 | 6.8 | F | Homozygous, | RMS | 9.6 | Y | Y | Y | Y | Y | N |
| Cys293Arg | |||||||||||
| Gly142Asp | |||||||||||
| 6 | 22 | F | Heterozygous, | Type A IR | 5.1 | N | Y | Y | N | N | N |
| P1178L | |||||||||||
| 7 | 47.1 | M | Heterozygous, | Type A IR | 4.8 | N | Y | N | N | N | N |
| Val1029Gly | |||||||||||
| 8 | 12.1 | F | Heterozygous, | Type A IR | 9.2 | Y | Y | N | N | N | N |
| Pro1178Leu | |||||||||||
| 9 | 15 | F | Heterozygous, | Type A IR | 5.3 | N | Y | N | N | N | N |
| Pro1236Ala | |||||||||||
| 10 | 9.3 | F | Suspected heterozygous | Suspected type A IR | 5.9 | Y | Y | N | N | N | N |
| 11 | 15.4 | M | Heterozygous, | Type A IR | 7.2 | Y | Y | N | N | N | N |
| His1130Arg |
Abbreviations: AN, Acanthosis nigricans; DM, diabetes mellitus; F, female; IR, insulin resistance; M, male; N, no; RMS, Rabson– Mendenhall syndrome; Y, yes.
At the time of thyroid US.
Dysmorphic features included (but were not limited to) coarse face features, premature aging phenotype, prognathism, macroglossia, dental abnormalities such as premature dentition, macrodontia, and crowded/extra teeth.
Table 2.
Demographics and HbA1c of All Age Patients and >19 y of Age With Extreme Insulin Resistance at the Time of Thyroid Imaging
| Parameter/Disease | All Patients |
Patients <19 y of Age |
||||
|---|---|---|---|---|---|---|
| INSR Mutation (n = 11) | Lipodystrophy (n = 81) | P Value | INSR Mutation (n = 7) | Lipodystrophy (n = 23) | P Value | |
| Age, ya | 18.1 ± 10.6 (7–47) | 30.9 ± 18 (1–79) | 0.01 | 13.1 ± 3.9 (7–18) | 13.2 ± 4.6 (2–18) | 0.81 |
| % Male | 45 | 16 | 0.036 | 43 | 22 | 0.34 |
| BSA, m2 | 1.34 ± 0.36 | 1.67 ± 0.3 | 0.006 | 1.28 ± 0.34 | 1.42 ± 0.4 | 0.33 |
| BMI | 21.2 ± 4.4 | 23.3 ± 5 | 0.2 | 19.3 ± 3.2 | 19.6 ± 4.7 | 0.8 |
| % on Metreleptin | 36 | 67 | 0.048 | 29 | 70 | 0.08 |
| HbA1c, % (range) | 8.5 ± 3.1 (4.8–13.3); | 7.1 ± 2.1 (4.3–13.2); | 0.2 | 8.2 ± 2 (5.3–10.8): | 6.42 ± 2 (4.3–11); | 0.04 |
| n = 11 | n = 76 | n = 7 | n = 22 | |||
Boldface indicates statistically significant P value (P < 0.05).
Age is given as mean ± SD (range).
Thyroid function
There were no differences in TSH or thyroid hormone concentrations in patients with lipodystrophy vs INSR mutation (Table 3). Among patients with lipodystrophy, four were on thyroid hormone replacement for hypothyroidism and three did not have available thyroid function tests. Among the remaining 74 patients, four (5.4%) had subclinical hypothyroidism defined as elevated TSH with normal thyroid hormone levels; all others were biochemically euthyroid. Among patients with INSR mutation, none was taking thyroid hormone. One patient (9%) had hypothyroidism based on elevated TSH (5.1 μU/mL) with low T4 (0.8 ng/mL). One patient (9%) had subclinical hyperthyroidism with low TSH only. The remaining nine patients (82%) were biochemically euthyroid (Table 3).
Table 3.
Thyroid Status in Patients With Extreme Insulin Resistance at the Time of Thyroid Imaging
| Parameter/Disease | All Patients |
Patients <19 y of Age |
||||
|---|---|---|---|---|---|---|
| INSR Mutation Group | Lipodystrophy Group | P Value | INSR Mutation Group | Lipodystrophy Group | P Value | |
| TSH, μIU/mL (range); n | 1.78 ± 1.38 | 1.89 ± 1.2 | 0.5 | 2 ± 1.7 | 2.3 ± 1.6 | 0.6 |
| (0.42–5.1); n = 11 | (0.37–6.1); n = 74 | (0.42–5.1); n = 7 | (0.58–6.08); n = 20 | |||
| fT4, ng/dL (range); n | 1.43 ± 0.46 | 1.22 ± 0.2 | 0.2 | 1.4 ± 0.6 | 1.27 ± 0.17 | 0.5 |
| (0.8–1.9); n = 4 | (0.9–1.8); n = 50 | (0.8–1.9); n = 3 | (1.1–1.6); n = 14 | |||
| TT4, μg/dL (range); n | 8.73 ± 1.7 | 7.34 ± 2.2 | 0.1 | 9.2 ± 2.3 | 7.46 ± 1.4 | 0.4 |
| (7–11.7); n = 7 | (4.7–15); n = 22 | (7.3–11.7); n = 3 | (5.3–9.2); n = 5 | |||
| TT3, ng/dL (range); n | 114 ± 33 | 120 ± 32.4 | 0.7 | 133.8 ± 35.4 | 142.2 ± 42 | 0.9 |
| (71–158.8); n = 6 | (75.7–219); n = 25 | (108.8–158.8); n = 2 | (86.2–219); n = 7 | |||
| Anti-Thyroglobulin antibodies,a % | n = 11 | n = 80 | 0.7 | n = 7 | n = 23 | 0.9 |
| Negative | 73 | 80 | 57.4 | 61 | ||
| Detectable | 9 | 6 | 14.3 | 13 | ||
| Unknown | 18 | 14 | 28.6 | 26 | ||
| Anti–thyroid peroxidase antibodies,a % | n = 11 | n = 80 | 0.9 | n = 7 | n = 23 | 0.7 |
| Negative | 64 | 61 | 43 | 52 | ||
| Detectable | 18 | 25 | 29 | 22 | ||
| Unknown | 18 | 14 | 29 | 26 | ||
Antithyroid antibodies were checked at any time point, including in patients on replacement therapy.
Insulin, glycemia, IGF-1, and IGF-2
Patients with INSR−/− and INSR+/− mutation had higher ambient insulin concentrations compared with lipodystrophy, as evidence by higher fasting insulin and higher insulin AUC during the OGTT (Table 4). There was no difference in HbA1c in patients of all ages with INSR mutation vs lipodystrophy; however, among patients <19 years of age, those with INSR mutation had higher HbA1c compared with those with lipodystrophy (8.2% ± 2% vs 6.4% ± 2%, P = 0.04). Patients with INSR−/− mutation had higher blood glucose concentrations compared with both INSR+/− and lipodystrophy as evidenced by higher HbA1C (11.2% ± 1.8% vs 6.3% ± 1.7% and 7.1% ± 2.1%, respectively; P = 0.0001); however, fasting glucose and glucose AUC during the OGTT did not differ between groups (Table 4). Patients with INSR−/− mutation had significantly lower serum IGF-1 (33.2 ± 19 ng/mL) in comparison with INSR+/− mutation (366.3 ± 218 ng/mL) and lipodystrophy (182.8 ± 96 ng/mL) (P < 0.0001). Eighty percent of patients with INSR−/− had low IGF-1 for age vs 0% in patients with INSR+/− mutation (P = 0.015) and 16% in lipodystrophy (P = 0.006). Patients with INSR−/− (n = 3) had significantly lower IGF-2 (136 ± 123 ng/mL) compared with lipodystrophy patients (449 ± 125 ng/mL; n = 59; P = 0.009) but were not statistically different from those with INSR+/− (347 ± 69 ng/mL; n = 6; P = 0.6) (Table 4).
Table 4.
Blood Glucose Parameters and IGF-1/IGF-2 Levels in Lipodystrophy and INSR Mutation Patients at the Time of Thyroid US
| INSR −/− | INSR +/− | Lipodystrophy | P Value (ANOVA) | |
|---|---|---|---|---|
| Fasting insulin, μU/mL | 626 ± 390 | 136 ± 124 | 64 ± 121 | 0.0005a |
| Fasting blood glucose, mg/dL | 135 ± 75 | 97 ± 14 | 125 ± 54 | 0.5a,b,c |
| AUC during OGTT for insulin, μU/mL/190 min | 1,103,313 ± 18,496 | 72,758 ± 48,780 | 28,596 ± 31,238 | 0.0005 a , c |
| AUC during OGTT for blood glucose, mg/dL/190 min | 50,966 ± 16,478 | 34,865 ± 13,239 | 39,873 ± 16,930 | 0.3a,b,c |
| HbA1c, % | 11.2 ± 1.8 | 6.3 ± 1.7 | 7.1 ± 2.1 | 0.003 a , b |
| IGF-1, ng/mL | 33 ± 19 | 366 ± 218 | 183 ± 96 | <0.0001a,b,c |
| IGF-2, ng/m) | 136 ± 123 | 347 ± 69 | 449 ± 125 | 0.0026 a |
P < 0.05 by post hoc Dunn multiple comparison. Boldface indicates statistically significant P value (P < 0.05).
Homozygous INSR mutation vs lipodystrophy group.
Homozygous vs heterozygous INSR mutation.
Heterozygous INSR mutation vs lipodystrophy group.
Thyroid imaging
Thyroid volume
There was no difference in thyroid volume in patients with lipodystrophy vs INSR mutation among patients of all ages (10.4 ± 6.3 and 9.3 ± 7.1 mL, respectively; P = 0.3) or those <19 years of age (8.2 ± 8.4 and 6.9 ± 3.7 mL, respectively; P = 0.9) (Table 5). As expected, thyroid volume correlated with BSA in patients <19 years of age (r = 0.65, P = 0.0001) but not in those ≥19 years of age (r = 0.13, P = 0.3). There was no difference in total thyroid volume corrected for BSA between lipodystrophy and INSR mutation among patients of all ages or those <19 years of age (Table 4). Among patients with INSR mutation, thyroid volume corrected for BSA was significantly larger in patients with INSR−/− vs INSR+/− [10.4 ± 5.1 cm2 (range, 4 to 16.6 cm2) vs 3.9 ± 1.5 cm2 (range, 1.8 to 6 cm2); P = 0.017; Fig. 1] as well as INSR−/− mutation vs lipodystrophy (6.2 ± 3.4 cm2; range, 1.3 to 21.8 cm2; P = 0.046).
Table 5.
Thyroid Volume in Patients With Extreme Insulin Resistance at the Time of Thyroid Imaging
| All Patients |
Patients <19 y of Age |
|||||
|---|---|---|---|---|---|---|
| INSR Mutation Group (n = 11) | Lipodystrophy Group (n = 70) | P Value | INSR Mutation Group (n = 7) | Lipodystrophy Group (n = 21) | P Value | |
| Right lobe, mL (range) | 5.1 ± 3.5 (1.3–13) | 5.8 ± 3.4 (1–20.1) | 0.3 | 3.9 ± 2.3 (1.3–7.8) | 4.4 ± 0.8 (1–20.1) | 0.9 |
| Left lobe, mL (range) | 4.3 ± 3.6 (0.9–13.7) | 4.6 ± 3.3 (0.4–21.15) | 0.5 | 2.9 ± 1.5 (0.9–5.1) | 3.8 ± 4.4 (0.4–21) | 0.9 |
| Total volume, mL (range) | 9.3 ± 7.1 (2.3–26.6) | 10.4 ± 6.3 (1.5–41.2) | 0.3 | 6.9 ± 3.7 (2.3–13) | 8.2 ± 8.4 (1.5–41.2) | 0.9 |
| Total volume per BSA, mL/m2 (range) | 6.8 ± 4.8 (1.8–16.6) | 6.2 ± 3.4 (1.3–22); (n = 64) | 0.9 | 5.7 ± 3.8 (1.8–13.6) | 5.4 ± 4.3 (1.3–22) | 0.7 |
| Not enlarged/enlarged thyroid volume based on age, %a | 91/9 | 83/17 | 0.7 | 100/0 | 81/19 | 0.3 |
| Not enlarged/enlarged thyroid volume based on BSA, %b | 70/30 (n = 10) | 88/12 (n = 51) | 0.07 | 86/14 | 95/5 (n = 20) | 0.3 |
Patients were excluded from the analysis when they had history of thyroid surgery, were taking thyroid hormone, or in whom thyroid volume could not be assessed due to limited US images.
Thyroid volumes in subjects <20 y of age were compared with reference ranges for age (25). Thyroid volume in adults (20 to 70 y of age) were compared with adult reference ranges (26).
Thyroid volumes in both pediatric and adult subjects were compared with reference ranges for BSA derived from children <20 of age (25).
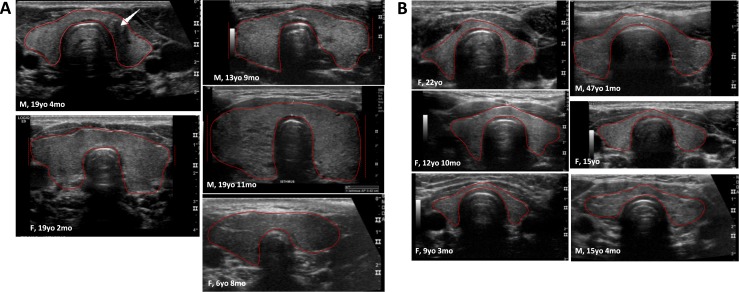
Figure 1.
Thyroid ultrasonography in patients with homozygous (−/−) (A) vs heterozygous (+/−) (B) INSR mutations. Patients with INSR−/− mutations had significantly larger thyroid volume adjusted for BSA (10.4 ± 5.1 vs 3.8 ± 1.5 mL, P = 0.017). One patient with INSR−/− mutation was diagnosed with PTC at age 18 [in (A); thyroid US with white arrow at the location of PTC]. Solid red lines show thyroid gland borders; dashed red lines indicate that a portion of the thyroid tissue was not captured by the US probe.
Patients with INSR mutation had a 30% frequency of enlarged thyroid volume for BSA compared with 12% in those with lipodystrophy, but the difference did not reach statistical significance (P = 0.07) (Table 5). Among INSR−/− patients, three (60%) had enlarged thyroid volume, one (20%) had thyroid volume at the upper limit of normal, and only one (20%) was within the normal range; in contrast, none of the INSR+/− patients had an enlarged thyroid gland.
Thyroid structure and vascularity
Echostructure of the thyroid gland was heterogeneous in 61% and 73% of patients with lipodystrophy and INSR mutation, respectively (P = 0.5). Most patients had normal thyroid vascularity with no difference between lipodystrophy and INSR mutation (96% vs 91%, P = 0.3).
Thyroid lesions
Cysts.
Among patients of all ages, pure cystic lesions (i.e., colloid cysts) were present in 60% and 73% of patients with lipodystrophy and INSR mutation, respectively (P = 0.5). Among patients with colloid cysts, the size of the largest cyst was 4.3 ± 2.6 mm (range, 1 to 8 mm) in patients with lipodystrophy and 3.3 ± 2.7 mm (range, 1 to 17 mm) in patients with INSR mutation (P = 0.2). In patients <19 years of age, colloid cysts were present in 36.4% and 71.4% of patients with lipodystrophy and INSR mutation, respectively (P = 0.2). Among patients <19 years of age with cysts, the size of the largest cyst was 2.7 ± 1.2 mm (range, 1 to 4 mm) in patients with lipodystrophy and 3.4 ± 3.1 mm (range, 1 to 8 mm) in patients with INSR mutation (P = 0.9).
There was no difference in the prevalence of colloid cysts in patients with extreme insulin resistance (13 of 29, 45%) compared with published prevalence for children aged 10 to 18 years (1924 of 3202, 60%) (29) (P = 0.1).
Nodules.
Thyroid nodule characteristics are shown in Table 6. Only one 18-year-old patient with INSR mutation had a thyroid nodule; thus, statistical comparisons of nodule characteristics in patients with lipodystrophy vs INSR mutation were not performed.
Table 6.
Thyroid Nodule Description in Lipodystrophy and INSR Mutation Groups
| Parameters | All Patients |
Patients <19 y of Age |
||
|---|---|---|---|---|
| INSR Mutation | Lipodystrophy | INSR Mutation | Lipodystrophy | |
| N | 11 | 80 | 7 | 23 |
| Patients with nodules, n (%) | 1 (9.1%) | 29 (36.3%) | 1 (14.3%) | 4 (17.4%) |
| Number of nodules | 1 | 55 | 1/1 | 4/4 |
| Size, cm | 0.9 | 0.86 ± 0.42 (0.3–2.7) | 0.9 | 1.2; 1; 0.4; 0.4 |
| Composition | ||||
| Solid | 1 | 22 | 1 | 2 |
| Solid–cystic/spongiform | 0 | 33 | 0 | 2 |
| Echogenicity | ||||
| Hyperechoic | 0 | 6 | 0 | 1 |
| Hypoechoic | 0 | 33 | 0 | 3 |
| Very hypoechoic | 1 | 12 | 1 | 0 |
| Isoechoic | 0 | 4 | 0 | 0 |
| Margins | ||||
| Smooth | 0 | 41 | 0 | 4 |
| Ill-defined | 1 | 12 | 1 | 0 |
| Lobulated/irregular | 0 | 2 | 0 | 0 |
| Extrathyroidal extension | 0 | 0 | 0 | 0 |
| Foci (calcifications) | ||||
| None | 0 | 15 | 0 | 4 |
| Microcalcifications | 1 | 40 | 1 | 0 |
| Macrocalcifications | 0 | 2 | 0 | 0 |
| Rim/peripheral | 0 | 15 | 0 | 0 |
In patients <19 years of age, thyroid nodules were detected in 4 of 23 (17%) patients with lipodystrophy and 1 of 7 patients (14%) with INSR mutation. The 0.9-cm nodule detected in the 18-year-old patient with INSR−/− mutation was found to be a papillary thyroid cancer (PTC) by fine-needle aspiration (FNA) and had typical US characteristics (very hypoechoic nodule with microcalcifications and ill-defined borders). Diagnosis was confirmed by pathology evaluation after hemithyroidectomy. In lipodystrophy patients <19 years of age, two thyroid nodules were subcentimeter (both 0.4 cm) and two measured 1.0 and 1.2 cm. All nodules had smooth margins, solid or cystic-solid structure, with hypoechoic echogenicity in two cases. The 1.2-cm thyroid nodule was spongiform with smooth margins, consistent with low risk for PTC. The prevalence of thyroid nodules in patients with extreme insulin resistance aged <19 years (5 of 30, 16.7%) was significantly higher than the published prevalence for children aged 10 to 18 years (64 of 3202, 2%) (29) (P = 0.0004).
In patients of all ages, thyroid nodules were detected in 29 of 80 (36.3%) patients with lipodystrophy. These 29 patients had 55 nodules with size 0.9 ± 0.4 cm (range, 0.3 to 2.7). US features of these nodules are described in Table 6. Most nodules had smooth margins (74.5%). None had extrathyroidal extension. Sixty percent of nodules were hypoechoic, 73% had calcifications, and 31% had macrocalcifications or peripheral calcifications. There were no nodules with a taller-than-wide shape.
Patients with lipodystrophy and INSR mutations with no thyroid lesions were significantly younger (16.5 ± 6.4 years) in comparison with patients with only colloid cysts (30.6 ± 15.4 years, P = 0.0002) and patients with thyroid nodules (43.4 ± 18.6 years, P < 0.0001). Patients with colloid cysts but no thyroid nodules were younger than patients with thyroid nodules (P = 0.0075).
Cytopathology/histopathology diagnoses of thyroid nodules
Among 55 nodules in the lipodystrophy group, 48 (87%) lesions did not meet the criteria for FNA owing to small size or benign characteristics based on American Thyroid Association recommendations (30). Seven nodules in six patients had indications for FNA. PTC was proved by histopathology in four nodules in three patients; one was an adenomatoid nodule, and two showed atypia of unknown significance. The one thyroid nodule in a patient with INSR mutation was shown to be PTC by FNA and histology, as described above.
Effect of metreleptin on thyroid structure and function
Thyroid structural changes in patients treated and not treated with metreleptin
In the combined group with lipodystrophy or INSR mutation, 60 patients were treated and 32 were never treated with metreleptin. There were no differences in age, BSA, absolute or BSA-adjusted thyroid volume, echogenicity, or prevalence of nodules/cysts/calcifications in the treated vs untreated patients (Table 7). In the subgroup with lipodystrophy, there were no differences in these parameters in the treated (n = 25) vs not treated (n = 56) patients.
Table 7.
Thyroid Structural Features in Lipodystrophy and INSR Mutation Groups Based on Leptin Treatment
| Never on Leptin (n = 32) | On Leptina (n = 60) | P Value | |
|---|---|---|---|
| Age, y | 32 ± 18.5 (7–66) | 30 ± 18 (2–80) | 0.9 |
| BSA, m2 | 1.65 ± 0.3 (0.77–2.12) | 1.62 ± 0.35 (0.47–2.19) | 0.7 |
| Thyroid volume, mL | 9.4 ± 6 (2.3–28.2) | 11 ± 6.8 (1.5–41.2) | 0.2 |
| Echogenicity | 0.3 | ||
| Homogeneous, n, % | 12, 37.5 | 37, 62 | |
| Heterogeneous, n, % | 20, 62.5 | 23, 38 | |
| Cysts | 0.5 | ||
| Yes, n, % | 18, 56 | 38, 63 | |
| No, n, % | 14, 44 | 22, 38 | |
| Nodules | 0.1 | ||
| Yes, n, % | 7, 22 | 23, 38 | |
| No, n, % | 25, 78 | 37, 62 | |
| Number of nodules/patients | 11/7 | 45/23 | |
| Peripheral/rim calcifications, n, % | 2, 18.2 | 11, 24.4 | 0.9 |
One patient each in the INSR mutation and lipodystrophy groups were previously treated with leptin (duration of 1 to 4.3 y) but not taking leptin at the time of US.
Three patients with lipodystrophy and one with INSR mutation were diagnosed with PTC at the age of 18.4, 24, and 40 years, and one of them was never treated with metreleptin (age at the time of PTC diagnosis was 18.8 years).
In the subgroup with INSR mutation, there was no difference in age and BSA between metreleptin-treated and untreated patients. Of note, all four metreleptin-treated patients had INSR−/− mutation, whereas only one of seven nontreated patients had INSR−/− mutation. Thyroid volume was higher in the metreleptin-treated vs the nontreated group (15.4 ± 8.6 vs 5.9 ± 2.7 mL, respectively; P = 0.04). BSA adjusted thyroid volume was also higher in the treated vs untreated group (11.4 ± 5.4 vs 4.3 ± 1.7 mL, P = 0.042). All four metreleptin-treated patients with INSR mutation had cystic thyroid lesions, vs four of seven patients in the untreated group (P = 0.2). The only thyroid nodule in the INSR mutation group was found in patients with INSR−/− mutation.
In all patients with extreme insulin resistance (lipodystrophy plus INSR mutation), there was no significant association between the duration of metreleptin treatment and thyroid volume (P = 0.2, r = 0.7) as well as BSA-adjusted thyroid volume (P = 0.8, r = 0.04). Duration of treatment did not correlate with the size of thyroid nodules in a combined group (lipodystrophy and INSR mutation patients) (P = 0.2, r = −0.30).
Thyroid function in patients treated and not treated with metreleptin
In the combined group with lipodystrophy or INSR mutation, there was no statistical difference in TSH, thyroid hormone levels, or prevalence of patients with detectable antithyroid antibodies (antithyroglobulin or anti–thyroid peroxidase) between metreleptin-treated vs non–metreleptin-treated patients (Table 8). Similarly, in the subgroup with lipodystrophy, there were no differences in these parameters between metreleptin-treated vs untreated patients. In the subgroup with INSR mutation, there were no differences in thyroid parameters.
Table 8.
Thyroid Function Tests and HbA1c in Lipodystrophy and INSR Mutation Groups Based on Metreleptin Treatment
| Never on Leptin (n = 32) | On Leptin (n = 60) | P Value | |
|---|---|---|---|
| TSH, μIU/mL (range); n | 1.8 ± 1 (0.58–5.05); | 1.9 ± 1.3 (0.37–6.08); | 0.8 |
| n = 28 | n = 57 | ||
| fT4, ng/dL (range); n | 1.2 ± 0.4 (0.8–1.9); | 1.2 ± 0.2 (0.9–1.7); | 0.3 |
| n = 8 | n = 46 | ||
| TT4, μg/dL (range); n | 7.5 ± 2.3 (4.7–15); | 8 ± 1.9 (5.3–11.7); | 0.1 |
| n = 17 | n = 12 | ||
| TT3, ng/dL (range); n | 112 ± 27 (71–164); | 127 ± 37 (93–219); | 0.2 |
| n = 19 | n = 12 | ||
| Positive antithyroid antibodies,a %; n | 23; n = 22 | 36; n = 50 | 0.9 |
| HbA1c, % (range | 6.7 ± 1.8 (4.4–11); | 7.5 ± 2.5 (4.3–13.3); | 0.2 |
| n = 28 | n = 59 |
Antithyroid antibodies were checked at any time point.
Discussion
In this study, we hypothesized that enhanced insulin signaling through the mitogenic MAPK pathway would lead to increased thyroid growth and neoplasia in patients with selective insulin resistance due to lipodystrophy compared with those with nonselective insulin resistance (including MAPK pathways) in INSR mutation. Counter to our hypothesis, we found that only patients with INSR−/− mutation had elevated thyroid volume in comparison with patients with INSR+/− mutation, lipodystrophy, and healthy controls (25, 26). We further hypothesized that metreleptin treatment might contribute to thyroid proliferation (again through MAPK signaling), but counter to our hypothesis we did not observe an association between metreleptin treatment and thyroid size or nodularity.
In the general population, insulin resistance has been associated with increased thyroid volume and increased risk of benign and malignant thyroid lesions (10, 11, 31, 32), whereas treatment with the insulin sensitizer metformin has the opposite effects, including an antimitogenic effect on differentiated human thyroid cells and thyroid cancer cells (33), as well as reduced thyroid volume in patients with type 2 diabetes (15). The biology underlying these associations between insulin resistance and thyroid proliferation has not been fully elucidated. Although thyroid tissue is not a classical insulin sensitive tissue such as liver, fat, or muscle (34), insulin receptors are expressed in thyrocytes (31, 35). Insulin resistance leads to hyperinsulinemia, which leads to increased stimulation of insulin receptors. In most forms of insulin resistance, including obesity and type 2 diabetes, insulin stimulation of the MAPK signaling pathway via insulin receptors is preserved. Hence, hyperinsulinemia is thought to overstimulate MAPK pathways, leading to mitogenic effects. Furthermore, hyperinsulinemia can stimulate the IGF-1 receptor (IGF-1R), again resulting in overstimulation of MAPK pathways and mitogenic effects (36–39). These mitogenic effects of insulin via both the insulin receptor and IGF-1R are independent of TSH (40, 41). Additionally, insulin is permissive for the mitogenic actions of TSH in human thyroid cells in primary culture (40), and crosstalk between the TSH receptor and IGF-1R in regulation of thyroid function in human thyrocytes in vitro was demonstrated (42).
Thus, we had hypothesized that patients with INSR mutations would demonstrate less mitogenic effects of hyperinsulinemia vs those with lipodystrophy, as INSR mutation leads to a blockade of insulin signaling in the mitogenic MAPK pathway. Counter to our hypothesis, we observed significantly larger thyroid volume adjusted for BSA compared with patients with heterozygous INSR mutations and lipodystrophy, and most patients with homozygous INSR mutation had enlarged thyroid volume in comparison with published thyroid volume reference ranges in patients 0 to <19 years of age adjusted for BSA from an iodine-sufficient area (25).
A possible explanation for our finding is that increased thyroid proliferation in INSR−/− mutations may be mediated by hyperinsulinemia acting through IGF-1R. IGF-1 is a more potent stimulus for activation of MAPK pathways than insulin (18), and proliferative effects of insulin are at least partially mediated via IGF-1R. Knockout of the insulin receptor in different tissues, including the thyroid gland, led to overexpression of IGF-1R (35, 43). It has been also shown that insulin and IGF-1 acting through IGF-1R induce protein synthesis and cell hypertrophy in human thyroid cells (44). Thus, IGF-1R signaling might be involved in the development of thyroid morphological abnormalities. This may be particularly relevant in the presence of impaired INSR signaling, as increased activation of IGF-1R has been demonstrated in cultured skeletal muscle from mice with knockout of the insulin receptor in bone and thyroid tissue (35, 43, 45).
The larger thyroid size in INSR−/− patients was not secondary to direct mitogenic effects of IGF-1, as this group had markedly low serum IGF-1 levels. Insulin levels in patients with INSR−/− mutations were 10-fold above the IC50 of insulin at the IGF-1R, suggesting that insulin action at the IGF-1R could have stimulated thyroid growth (46). Additionally, low IGF-1 might upregulate expression of IGF-1R in patients with INSR−/− mutations, as has been demonstrated in vitro and in vivo. For comparison, insulin levels in patients with INSR+/− and lipodystrophy were approximately onefold to twofold the IC50 of insulin at the IGF-1R, making stimulation of IGF-1R by insulin less relevant as a mechanism for thyroid growth and proliferation.
Circulating and locally produced IGF-2 could also contribute to development of thyroid nodules and/or thyromegaly because both IGF-1 and IGF-2 are involved in cell proliferation and have high homology to each other and to insulin. Moreover, in insulin resistance, hyperinsulinemia can inhibit hepatic production of IGFBP-1 and IGFBP-2, leading to increased bioavailability of IGF-1 and IGF-2 (47). Unfortunately, free IGF-1 and IGF-2 levels were not available in this study. Analysis of IGF-2 in our cohorts showed low IGF-2 in patients with INSR−/−, suggesting that this is unlikely play a major role in thyroid proliferation in these patients.
Paracrine actions of IGF-1 and IGF-2 might also stimulate thyroid proliferation by binding to the A isoform of the insulin receptor (IR-A). IR-A is the major mediator of mitogenic effects of insulin, whereas the B isoform (IR-B) is the major mediator of metabolic effects of insulin (48). Relative expression of IR-A vs IR-B in humans or animals with INSR mutations has not been studied. However, in INSR mutations, both IR-A and IR-B isoforms would be expected to have reduced binding affinity for ligands or reduced signal transduction.
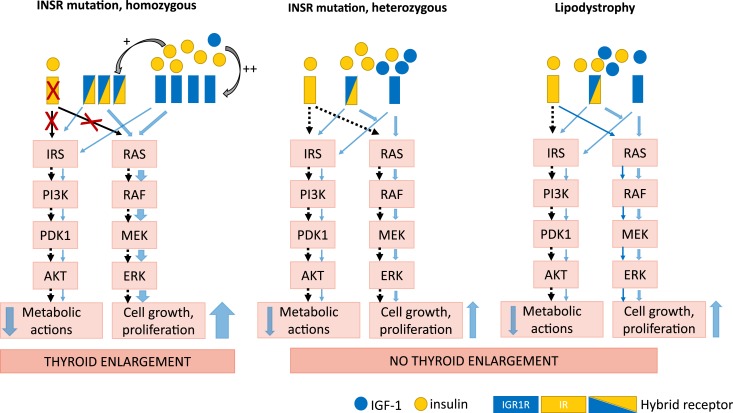
Another mechanism that could theoretically play a role in the thyroid enlargement in INSR−/− mutation patients is stimulation of hybrid receptors formed by an INSR αβ-hemireceptor and an IGF-1R αβ-hemireceptor. Formation of hybrid receptors is stimulated by hyperinsulinemia (49, 50), and hence they are likely to be more abundant in INSR−/− patients. Because the hybrid receptors behave as IGF-1R rather than insulin receptors, their stimulation by insulin may contribute to thyroid growth (49, 51). However, it is unclear how functional hybrid receptors are in patients with INSR−/− mutation. Thus, the combination of severe hyperinsulinemia with low IGF-1/IGF-2 may lead to thyroid growth in patients with INSR−/− by insulin binding to IGF-1R, by upregulation of IGF-1R expression, and by insulin binding to hybrid receptors (Fig. 2; Table 9).
Figure 2.
Proposed mechanism of thyroid enlargement in patients with homozygous INSR mutation. Solid blue arrows indicate intact signaling pathways. Dashed black arrows indicate inhibited signaling pathways. Red Xs indicate blocked receptors and downstream signaling. In patients with homozygous INSR mutation the proliferative effect on thyroid tissue might be mediated via the direct stimulation of IGF-1R by a high concentration of circulating insulin and/or hyperinsulinemia-mediated upregulation of hybrid receptors (+). A low level of IGF-1 can upregulate the number of IGFR1 receptors (++) and/or increase IGF-1R activity, resulting in increased insulin binding to IGF-1Rs with further stimulation of the MAPK pathway. MAPK-mediated proliferative signaling will lead to thyroid enlargement.
Table 9.
Thyroid Phenotypes and Growth Factors in Patients With Extreme Insulin Resistance
| INSR, Homozygous | INSR, Heterozygous | Lipodystrophy | |
|---|---|---|---|
| Insulin resistance | Nonselective | Nonselective | Selective |
| IGF-1 | ↓ | Normal | Normal |
| Fasting insulin | ↑↑↑ | ↑↑ | ↑ |
| Thyroid functional status | Euthyroid | Euthyroid | Euthyroid |
| Thyroid size | Enlarged | Normal | Normal |
| Risk for thyroid nodules | Probably increased | Probably increased | Probably increased |
| Risk of thyroid cancer | Unknown | Unknown | Unknown |
Patients with extreme insulin resistance had high detection rates of colloid cysts; however, this rate did not differ from published data and is thus unlikely to represent a pathologic finding (29, 52). Among patients of all ages, thyroid nodules were more prevalent (36%) in patients with lipodystrophy compared with those with INSR mutation (9%); however, this can be explained by significant demographic differences (age, sex) between groups. Interestingly, patients <19 years old with extreme insulin resistance had a high prevalence of thyroid nodules (17% and 14% for lipodystrophy and INSR mutation) in comparison with a published prevalence of ∼2% in children (29, 52). However, the size of our cohort is small, and these findings require verification in larger populations. All nodules in our pediatric cohort of extreme insulin resistance were diagnosed after age 9 years, and two patients had PTC diagnosed at age 18.4 and 18.8 years. Thus, thyroid US starting at a pubertal age might be considered for early detection of thyroid abnormalities in patients with extreme insulin resistance. We are currently conducting longitudinal follow-up in this cohort to better understand the risk of malignancy.
An unexpected finding was that 26.8% of nodules had rim/peripheral calcifications. This prevalence of rim calcification is substantially higher than the 1.78% reported in 2123 surgically removed thyroid nodules in 1498 patients (53). The pathogenesis of calcification in benign thyroid lesions is thought to begin with hyperplasia of fibrous thyroid tissue that affects follicular blood supply, resulting in hemorrhage and necrosis, resulting in calcification of the cyst wall (53). We speculate that in states of extreme insulin resistance, high endogenous insulin may stimulate growth of susceptible thyroid follicular cells, leading to rapid thyroid nodule enlargement causing ischemia and calcification.
We hypothesized that metreleptin therapy could contribute to thyroid growth and neoplasia. Although leptin and insulin receptors are not related, leptin signal transduction does act through some components of the insulin signaling cascade, including PI3K and MAPK pathways (19). To date, there is no direct evidence of a role of leptin in the development of thyroid cancer; however, patients with differentiated thyroid cancer have been reported to have BMI-independent elevation in leptin level (54). In this study, we did not observe any increase in thyroid nodules with metreleptin treatment, despite relatively long duration of metreleptin therapy (up to 16.7 years in lipodystrophy patients and 6 years in patients with INSR mutation). In patients with lipodystrophy, metreleptin reduces insulin resistance and hyperinsulinemia, hence the theoretical growth-promoting effects of metreleptin might be counteracted by the reduced effects of insulin. However, metreleptin is not expected to reduce insulin resistance in patients with INSR mutation, hence any effects of metreleptin to promote thyroid growth and neoplasia might be better observed in this cohort. We did observe that metreleptin-treated patients with INSR mutation, but not metreleptin-treated patients with lipodystrophy, had higher BSA-adjusted thyroid size vs untreated patients. Because this difference was only observed in the INSR mutation cohort, it likely relates to the fact that all metreleptin-treated INSR patients had homozygous mutations, rather being secondary to metreleptin treatment.
Based on our observations, patients with extreme insulin resistance have different thyroid phenotypes based on underlying genetic abnormalities, and thyromegaly might be a new phenotypical feature of extreme insulin resistance due to homozygous INSR mutation (Fig. 2; Table 8).
In conclusion, we found that patients with extreme insulin resistance have a high prevalence of thyroid nodules, metreleptin treatment was not associated with changes in thyroid size or nodularity, and patients with homozygous INSR mutation have significantly enlarged thyroid glands, which may be a novel phenotypic feature of this disease. We proposed the possible explanation for thyroid abnormalities in each group based on our findings (Figs. 1 and 2). Strengths of this study include analysis of a large cohort of rare patients with lipodystrophy and INSR mutations, with and without metreleptin treatment. Limitations include small sample size for patients with INSR mutation, comparison of thyroid volume and nodularity to published controls, rather than an internal control group, as well as a young patient population (especially among patients with INSR mutation). Prospective, longitudinal studies are needed to clarify the effects of extreme insulin resistance and/or metreleptin treatment on nodules/cancer development, and mechanistic studies are needed to determine the etiology of thyroid abnormalities in patients with extreme insulin resistance.
Acknowledgments
We thank Dr. Mary Walter and members of the National Institutes of Health Clinical Core Laboratory for support in performing assays.
Financial Support: This work was supported by the Intramural Research Program of the Diabetes, Endocrinology, and Obesity Branch of the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AUC
area under the curve
- BMI
body mass index
- BSA
body surface area
- FNA
fine-needle aspiration
- IGF-1R
IGF-1 receptor
- IR-A
A isoform of the insulin receptor
- IR-B
B isoform of the insulin receptor
- MEK
mitogen-activated protein kinase
- OGTT
oral glucose tolerance test
- PI3K
phosphatidylinositol 3-kinase
- PTC
papillary thyroid cancer
- US
ultrasound
References
- 1. Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;106(11):1911–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee MY, Lin KD, Hsiao PJ, Shin SJ. The association of diabetes mellitus with liver, colon, lung, and prostate cancer is independent of hypertension, hyperlipidemia, and gout in Taiwanese patients. Metabolism. 2012;61(2):242–249. [DOI] [PubMed] [Google Scholar]
- 3. Tanaka K, Tsuji I, Tamakoshi A, Matsuo K, Wakai K, Nagata C, Mizoue T, Inoue M, Tsugane S, Sasazuki S; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan . Diabetes mellitus and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2014;44(10):986–999. [DOI] [PubMed] [Google Scholar]
- 4. Weiderpass E, Gridley G, Persson I, Nyrén O, Ekbom A, Adami HO. Risk of endometrial and breast cancer in patients with diabetes mellitus. Int J Cancer. 1997;71(3):360–363. [DOI] [PubMed] [Google Scholar]
- 5. Noto H, Tsujimoto T, Sasazuki T, Noda M. Significantly increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Endocr Pract. 2011;17(4):616–628. [DOI] [PubMed] [Google Scholar]
- 6. Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24(2):68–72. [DOI] [PubMed] [Google Scholar]
- 7. van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. [DOI] [PubMed] [Google Scholar]
- 8. Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207(1):12–22. [DOI] [PubMed] [Google Scholar]
- 9. Gursoy A. Rising thyroid cancer incidence in the world might be related to insulin resistance. Med Hypotheses. 2010;74(1):35–36. [DOI] [PubMed] [Google Scholar]
- 10. Yeo Y, Ma SH, Hwang Y, Horn-Ross PL, Hsing A, Lee KE, Park YJ, Park DJ, Yoo KY, Park SK. Diabetes mellitus and risk of thyroid cancer: a meta-analysis. PLoS One. 2014;9(6):e98135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paulus YM, Riedel ER, Sabra MM, Tuttle RM, Kalin MF. Prevalence of diabetes mellitus in patients with newly evaluated papillary thyroid cancer. Thyroid Res. 2014;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sousa PA, Vaisman M, Carneiro JR, Guimarães L, Freitas H, Pinheiro MF, Liechocki S, Monteiro CM, Teixeira PF. Prevalence of goiter and thyroid nodular disease in patients with class III obesity. Arq Bras Endocrinol Metabol. 2013;57(2):120–125. [DOI] [PubMed] [Google Scholar]
- 13. Anil C, Akkurt A, Ayturk S, Kut A, Gursoy A. Impaired glucose metabolism is a risk factor for increased thyroid volume and nodule prevalence in a mild-to-moderate iodine deficient area. Metabolism. 2013;62(7):970–975. [DOI] [PubMed] [Google Scholar]
- 14. Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid. 2008;18(4):461–464. [DOI] [PubMed] [Google Scholar]
- 15. Ittermann T, Markus MR, Schipf S, Derwahl M, Meisinger C, Völzke H. Metformin inhibits goitrogenous effects of type 2 diabetes. Eur J Endocrinol. 2013;169(1):9–15. [DOI] [PubMed] [Google Scholar]
- 16. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. [DOI] [PubMed] [Google Scholar]
- 17. Szanto I, Kahn CR. Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc Natl Acad Sci USA. 2000;97(5):2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seto-Young D, Zajac J, Liu HC, Rosenwaks Z, Poretsky L. The role of mitogen-activated protein kinase in insulin and insulin-like growth factor I (IGF-I) signaling cascades for progesterone and IGF-binding protein-1 production in human granulosa cells. J Clin Endocrinol Metab. 2003;88(7):3385–3391. [DOI] [PubMed] [Google Scholar]
- 19. Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(Pt 1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am. 2012;41(2):425–443, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horita S, Nakamura M, Suzuki M, Satoh N, Suzuki A, Seki G. Selective insulin resistance in the kidney. BioMed Res Int. 2016;2016:5825170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cochran E, Musso C, Gorden P. The use of U-500 in patients with extreme insulin resistance. Diabetes Care. 2005;28(5):1240–1244. [DOI] [PubMed] [Google Scholar]
- 23. Brown RJ, Cochran E, Gorden P. Metreleptin improves blood glucose in patients with insulin receptor mutations. J Clin Endocrinol Metab. 2013;98(11):E1749–E1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Semple RK, Savage DB, Cochran EK, Gorden P, O’Rahilly S. Genetic syndromes of severe insulin resistance. Endocr Rev. 2011;32(4):498–514. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki S, Midorikawa S, Fukushima T, Shimura H, Ohira T, Ohtsuru A, Abe M, Shibata Y, Yamashita S, Suzuki S; Thyroid Examination Unit of the Radiation Medical Science Center for the Fukushima Health Management Survey . Systematic determination of thyroid volume by ultrasound examination from infancy to adolescence in Japan: the Fukushima Health Management Survey. Endocr J. 2015;62(3):261–268. [DOI] [PubMed] [Google Scholar]
- 26. Berghout A, Wiersinga WM, Smits NJ, Touber JL. Determinants of thyroid volume as measured by ultrasonography in healthy adults in a non-iodine deficient area. Clin Endocrinol (Oxf). 1987;26(3):273–280. [DOI] [PubMed] [Google Scholar]
- 27. Du Bois D, Du Bois EF. Clinical calorimetry. Tenth paper: a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med (Chic). 1916;XVII(6_2):863–871. [Google Scholar]
- 28. Chan JL, Koda J, Heilig JS, Cochran EK, Gorden P, Oral EA, Brown RJ. Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin Endocrinol (Oxf). 2016;85(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayashida N, Imaizumi M, Shimura H, Okubo N, Asari Y, Nigawara T, Midorikawa S, Kotani K, Nakaji S, Otsuru A, Akamizu T, Kitaoka M, Suzuki S, Taniguchi N, Yamashita S, Takamura N; Investigation Committee for the Proportion of Thyroid Ultrasound Findings . Thyroid ultrasound findings in children from three Japanese prefectures: Aomori, Yamanashi and Nagasaki. PLoS One. 2013;8(12):e83220. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malaguarnera R, Frasca F, Garozzo A, Gianì F, Pandini G, Vella V, Vigneri R, Belfiore A. Insulin receptor isoforms and insulin-like growth factor receptor in human follicular cell precursors from papillary thyroid cancer and normal thyroid. J Clin Endocrinol Metab. 2011;96(3):766–774. [DOI] [PubMed] [Google Scholar]
- 32. Tsatsoulis A. The role of insulin resistance/hyperinsulinism on the rising trend of thyroid and adrenal nodular disease in the current environment. J Clin Med. 2018;7(3):E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen G, Xu S, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab. 2012;97(4):E510–E520. [DOI] [PubMed] [Google Scholar]
- 34. Zhang H, Zhang C. Adipose “talks” to distant organs to regulate insulin sensitivity and vascular function. Obesity (Silver Spring). 2010;18(11):2071–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ock S, Lee SH, Ahn J, Lee TJ, Cho CH, Abel ED, Kimura S, Kim J. Conditional deletion of insulin receptor in thyrocytes does not affect thyroid structure and function. Endocr J. 2011;58(11):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu R, Hu LL, Sun A, Cao YJ, Tang T, Zhang XP, Zhang QH. mRNA expression of IGF-1 and IGF-1R in patients with colorectal adenocarcinoma and type 2 diabetes. Arch Med Res. 2014;45(4):318–324. [DOI] [PubMed] [Google Scholar]
- 37. Kasagi K, Shimatsu A, Miyamoto S, Misaki T, Sakahara H, Konishi J. Goiter associated with acromegaly: sonographic and scintigraphic findings of the thyroid gland. Thyroid. 1999;9(8):791–796. [DOI] [PubMed]
- 38. Dąbrowska AM, Tarach JS, Kurowska M, Nowakowski A. Thyroid diseases in patients with acromegaly. Arch Med Sci. 2014;10(4):837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reverter JL, Fajardo C, Resmini E, Salinas I, Mora M, Llatjós M, Sesmilo G, Rius F, Halperin I, Webb SM, Ricart V, Riesgo P, Mauricio D, Puig-Domingo M. Benign and malignant nodular thyroid disease in acromegaly. Is a routine thyroid ultrasound evaluation advisable? PLoS One. 2014;9(8):e104174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clément S, Refetoff S, Robaye B, Dumont JE, Schurmans S. Low TSH requirement and goiter in transgenic mice overexpressing IGF-I and IGF-Ir receptor in the thyroid gland. Endocrinology. 2001;142(12):5131–5139. [DOI] [PubMed] [Google Scholar]
- 41. Kimura T, Dumont JE, Fusco A, Golstein J. Insulin and TSH promote growth in size of PC Cl3 rat thyroid cells, possibly via a pathway different from DNA synthesis: comparison with FRTL-5 cells. Eur J Endocrinol. 1999;140(1):94–103. [DOI] [PubMed] [Google Scholar]
- 42. Morgan SJ, Neumann S, Marcus-Samuels B, Gershengorn MC. Thyrotropin and insulin-like growth factor 1 receptor crosstalk upregulates sodium-iodide symporter expression in primary cultures of human thyrocytes. Thyroid. 2016;26(12):1794–1803. [DOI] [PMC free article] [PubMed]
- 43. Irwin R, Lin HV, Motyl KJ, McCabe LR. Normal bone density obtained in the absence of insulin receptor expression in bone. Endocrinology. 2006;147(12):5760–5767. [DOI] [PubMed] [Google Scholar]
- 44. Deleu S, Pirson I, Coulonval K, Drouin A, Taton M, Clermont F, Roger PP, Nakamura T, Dumont JE, Maenhaut C. IGF-1 or insulin, and the TSH cyclic AMP cascade separately control dog and human thyroid cell growth and DNA synthesis, and complement each other in inducing mitogenesis. Mol Cell Endocrinol. 1999;149(1-2):41–51. [DOI] [PubMed] [Google Scholar]
- 45. Shefi-Friedman L, Wertheimer E, Shen S, Bak A, Accili D, Sampson SR. Increased IGFR activity and glucose transport in cultured skeletal muscle from insulin receptor null mice. Am J Physiol Endocrinol Metab. 2001;281(1):E16–E24. [DOI] [PubMed] [Google Scholar]
- 46. Kjeldsen T, Andersen AS, Wiberg FC, Rasmussen JS, Schäffer L, Balschmidt P, Møller KB, Møller NP. The ligand specificities of the insulin receptor and the insulin-like growth factor I receptor reside in different regions of a common binding site. Proc Natl Acad Sci USA. 1991;88(10):4404–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baxter RC, Bryson JM, Turtle JR. Somatogenic receptors of rat liver: regulation by insulin. Endocrinology. 1980;107(4):1176–1181. [DOI] [PubMed] [Google Scholar]
- 48. Belfiore A, Malaguarnera R, Vella V, Lawrence MC, Sciacca L, Frasca F, Morrione A, Vigneri R. Insulin receptor isoforms in physiology and disease: an updated view. Endocr Rev. 2017;38(5):379–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Federici M, Lauro D, D’Adamo M, Giovannone B, Porzio O, Mellozzi M, Tamburrano G, Sbraccia P, Sesti G. Expression of insulin/IGF-I hybrid receptors is increased in skeletal muscle of patients with chronic primary hyperinsulinemia. Diabetes. 1998;47(1):87–92. [DOI] [PubMed] [Google Scholar]
- 50. Federici M, Zucaro L, Porzio O, Massoud R, Borboni P, Lauro D, Sesti G. Increased expression of insulin/insulin-like growth factor-I hybrid receptors in skeletal muscle of noninsulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;98(12):2887–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Slaaby R, Schäffer L, Lautrup-Larsen I, Andersen AS, Shaw AC, Mathiasen IS, Brandt J. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J Biol Chem. 2006;281(36):25869–25874. [DOI] [PubMed] [Google Scholar]
- 52. Shimura H, Sobue T, Takahashi H, Yasumura S, Ohira T, Ohtsuru A, Midorikawa S, Suzuki S, Fukushima T, Suzuki S, Yamashita S, Ohto H; Thyroid Examination Unit of the Radiation Medical Center for the Fukushima Health Management Survey Group . Findings of thyroid ultrasound examination within 3 years after the Fukushima nuclear power plant accident: the Fukushima Health Management Survey. J Clin Endocrinol Metab. 2018;103(3):861–869. [DOI] [PubMed] [Google Scholar]
- 53. Lu Z, Mu Y, Zhu H, Luo Y, Kong Q, Dou J, Lu J. Clinical value of using ultrasound to assess calcification patterns in thyroid nodules. World J Surg. 2011;35(1):122–127. [DOI] [PubMed] [Google Scholar]
- 54. Rehem RA, Elwafa WA, Elwafa RA, Abdel-Aziz TE. Study of serum leptin in well-differentiated thyroid carcinoma: correlation with patient and tumor characteristics. World J Surg. 2014;38(10):2621–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]