Abstract
Uterine epithelial proliferation is regulated in a paracrine manner by a complex interplay between estrogen (E) and progesterone (P) signaling, in which E stimulates proliferation and P inhibits it. Perturbation of steroid hormone signaling within the uterine milieu could contribute to the development of endometrial hyperplasia and cancer. It is well established that bisphenol-A (BPA) is an endocrine-disrupting chemical with weak estrogenic effects, although little is known about how it affects steroid hormone signaling in the adult uterus. Because BPA acts as a weak E, we hypothesized that chronic exposure to BPA would create an imbalance between E and P signaling and cause changes in the uterus, such as aberrant epithelial proliferation. Indeed, exposure to an environmentally relevant dose of BPA had a uterotrophic affect. BPA-treated mice showed increased proliferation, notably in the glandular epithelium, which are sites of origin for endometrial hyperplasia and cancer. Increased proliferation appeared to be mediated through a similar mechanism as E-induced proliferation, via activation of the fibroblast growth factor receptor pathway and phosphorylation of the ERK1/2 mitogen-activated protein kinases in the epithelium. Interestingly, BPA reduced expression of heart and neural crest derivatives expressed 2 (HAND2), a known mediator of the antiproliferative effects of P. BPA also increased methylation of a CpG island in the Hand2 gene promoter, suggesting that BPA may promote epithelial proliferation through epigenetic silencing of antiproliferative factors like HAND2. Collectively, these findings establish that chronic exposure to BPA impairs steroid hormone signaling in the mouse uterus, and may contribute to the pathogenesis of uterine hyperplasia and cancer.
The endometrium is highly sensitive to stimulation by ovarian steroid hormones estrogen (E) and progesterone (P). These two hormones play critical roles both during the reproductive cycle and pregnancy to maintain normal uterine health. Imbalance in E and P signaling can lead to loss of pregnancy and a number of uterine pathologies, including endometrial hyperplasia and cancer. One of the physiological functions regulated by E and P is proliferation of the uterine epithelium. It is well established that E drives epithelial proliferation and P inhibits proliferation (1, 2). In the presence of E, uterine stromal cells produce a subset of fibroblast growth factors (FGFs) that act in a paracrine manner to activate FGF receptor (FGFR) signaling in the uterine epithelium, leading to subsequent proliferation through MAPK activation (3). When P is present, activation of its cognate receptor in the uterine stromal cells drives expression of heart and neural crest derivatives expressed 2 (HAND2), which inhibits the expression of these FGFs and acts as a mediator of P’s antiproliferative actions (3). When HAND2 is lost from the uterus through conditional deletion, FGF levels remain elevated and uterine epithelial proliferation persists.
Unchecked endometrial proliferation is a common feature of endometrial hyperplasia and leads to increased risk of endometrial cancer. Worldwide, endometrial cancer is the most common gynecological cancer, with an estimated 287,000 new cases developing annually (4). The majority of endometrial carcinomas are classified as endometrioid endometrial adenocarcinomas and originate in the epithelium lining the uterine glands (5). Women who are nulliparous, obese, diabetic, or experiencing prolonged/unopposed exposure to E face an increased risk of developing endometrial cancer (5). Endometrial carcinomas are preceded by endometrial hyperplasia, a precursor lesion associated with abnormal proliferation. Endometrial hyperplasia results from chronic E exposure, coupled with an insufficient counterbalance by P, which promotes the hyperproliferation and transformation of cells (6). The findings of the Postmenopausal Estrogen/Progestin Interventions trial support these observations, showing that administration of E-only hormone replacement therapy increased incidence of endometrial hyperplasia, whereas administration of E+P showed rates of hyperplasia similar to placebo (7). Moreover, findings from the Women’s Health Initiative show that combined E+P therapy in postmenopausal women decreased incidence of endometrial cancer compared with placebo (8). The findings of these trials highlight the importance of maintaining a proper balance of E and P for uterine health.
Endometrial hyperplasia is characterized by abnormal glandular proliferation resulting in an increase in the gland:stroma ratio and glandular remodeling, resulting in irregularities in gland shape and distribution throughout the endometrium (5, 9). Progestin therapy has been shown to be effective at regressing endometrial hyperplasia, demonstrating the importance of P as an antiproliferative agent (10). These observations highlight the importance of maintaining a balance of proproliferative E and antiproliferative P signaling in the uterus to maintain normal uterine health and function. In light of this, it is of increasing interest whether exposure to endocrine-disrupting chemicals having estrogenic effects can disrupt this balance to promote endometrial hyperplasia and cancer.
Endocrine disruptors are both natural and manmade substances that interfere with or inhibit the body’s normal endocrine function by mimicking endogenous hormones, interacting with the hormones’ cognate receptor, and/or altering endogenous hormone synthesis. Perhaps one of the most pervasive endocrine-disrupting chemicals is bisphenol-A (BPA), a component of polycarbonate plastics found in plastic food and drink containers, epoxy resins lining food cans, and thermal paper (11). Elevated serum or urine levels of BPA have been associated with several endocrine-related diseases, including obesity, diabetes, polycystic ovarian syndrome, and thyroid dysfunction (12–15). Although it possesses a lower binding affinity to the estrogen receptor (ER) than estradiol itself, BPA has been shown to exhibit estrogenic effects (16–19). Because the uterus is highly sensitive to E stimulation, it is possible that estrogenic compounds such as BPA may adversely affect E-regulated processes involved in normal uterine cyclicity and pregnancy.
In rodent models, BPA has been shown to dysregulate several reproductive parameters, including increased GnRH pulse frequency, reduced LH secretion, premature onset of puberty, and abnormal estrus cycle (20). Several animal studies have also demonstrated reduced fertility stemming from delayed embryo implantation and decreased number of implantation sites in rodents exposed to BPA (21–24). Other studies propose a transgenerational effect of BPA on fertility, wherein pregnant dams dosed with BPA give rise to filial generations 1–3 exhibiting reduced fertility, including reduced number of pregnancies and reduced number of pregnancies carried to term (25, 26). These observations suggest BPA exposure in the mother causes changes in the daughters and granddaughters through an epigenetic mechanism. In women, elevated levels of BPA in serum or urine have been associated with lower antral follicle counts, implantation failure, and increased risk of miscarriage (27–29). It is clear based on rodent studies and the human epidemiological studies that reproductive tissues are acutely sensitive to the endocrine-disrupting chemical BPA, and basic physiological processes, such as reproduction, are highly susceptible to BPA exposure.
Many studies have focused on the effects of BPA exposure during critical windows of development, such as during the perinatal period or during puberty, but relatively little is known about the consequences of BPA exposure during adulthood. Based on the endocrine-disrupting nature of BPA, we hypothesized that BPA exposure would disrupt the balance of steroid hormone signaling in the adult uterus and lead to dysregulation of the molecular pathway controlling endometrial proliferation. We used an experimental model wherein reproductively mature cycling female mice were exposed for long durations to a concentration of BPA comparable to the level established by the US Environmental Protection Agency (EPA) as the safe level for human exposure. Moreover, we used oral administration to better mimic the route of human exposure to BPA. We find that chronic exposure to BPA induces uterine glandular proliferation through suppression of HAND2, a known mediator of the antiproliferative effects of P.
Materials and Methods
Animals and experimental protocol
C57BL6 mice were maintained in a University of Illinois–sponsored animal care facility and all procedures were approved by the University of Illinois Institutional Animal Care and Use Committee. Mice were housed in polysulfone cages containing corn cob bedding and fed Teklad 8664 rodent chow (Envigo) ad libitum. Water was provided ad libitum in glass bottles. For dosing experiments, reproductively mature (8-week-old) intact female mice were treated three times per day (9:00 am, 1:00 pm, and 5:00 pm) orally with BPA [60 µg/kg/d, cumulative, dissolved in ethanol, and suspended in tocopherol-stripped corn oil for 0.1% final ethanol concentration (Sigma)] or vehicle (tocopherol-stripped corn oil + 0.1% ethanol) (n = 4 per experimental group). Mice were fed these solutions by pipette and treatment continued for 60 days. At the end of the dosing period, vaginal cytology was used to determine stage of estrus, and animals were euthanized as they entered diestrus. Upon euthanization, uteri were collected, weighed, divided, and either fixed in 10% buffered formalin or flash frozen in liquid nitrogen for further analysis.
Immunohistochemistry
Formalin-fixed uterine segments were paraffin embedded and cross-sectioned to 5 µM thickness onto glass slides. Before staining, slides were deparaffinized in xylene and rehydrated in ethanol. Slides to be developed with Chromagen were incubated in 0.3% hydrogen peroxide in methanol for 15 minutes at room temperature to quench endogenous peroxidase activity. Slides were subjected to antigen retrieval, blocked with normal donkey serum, and incubated overnight at 4°C with primary antibody diluted in immunohistochemistry block A solution (0.25% BSA, 0.3% Triton X-100, sterile PBS). Primary antibodies used are as follows: cytokeratin 8 (Hybridoma Bank; RRID: AB_531826) (30), Ki67 (BD Pharmingen; RRID: AB_393778) (31), phosphorylated FGFR substrate 2 (FRS2; R&D Systems; RRID: AB_2106234) (32), phosphorylated ERK1/2 (Santa Cruz; RRID: AB_653182) (33), PR (Dako; RRID: AB_2315192) (34), and HAND2 (Santa Cruz; RRID: AB_2115995) (35). The next day, slides were washed in PBS and incubated with either biotinylated or Cy3/Dylite conjugated secondary antibody (Jackson Immunoresearch Laboratories, Inc.; RRID: AB_2340788, RRID: AB_2340398, RRID: AB_2315777, RRID: AB_2307443, and RRID: AB_2340616) (36–40) diluted in PBS and 1% BSA for 1 hour at room temperature. Immunofluorescence stained slides were washed and mounted with Prolong GOLD mounting medium (Promega) containing 4′,6-diamidino-2-phenylindole (DAPI). Biotinylated samples were incubated with streptavidin conjugated horseradish peroxidase (Invitrogen) for 30 minutes at room temperature. Samples were then stained with 3-amino-9-ethyl carbazole solution (Invitrogen) and counterstained with Mayer hematoxylin (Sigma). Slides were mounted with organic mounting medium before imaging. Imaging was performed using a Leica DM2500 light microscope (Leica Microsystems) fitted with a Qimaging Retiga 2000R camera (Teledyne Qimaging). Immunofluorescent images were captured on an Olympus BX51 fluorescence microscope (Olympus) fitted with an Olympus DP71 camera (Olympus). ImageJ software (National Institutes of Health) was used to perform postimaging analysis.
Uterine stromal cell isolation
Uteri were isolated from intact, reproductively mature mice in diestrus, as determined by vaginal cytology. Uterine horns were cut open longitudinally and divided into 3-mm pieces. This tissue was incubated in a solution of Hanks buffered salt solution (HBSS) containing 25 g/L of pancreatin (Sigma-Aldrich) and 6 g/L of dispase (Gibco) at room temperature for 1 hour and then 37°C for 15 minutes. The tissue was then washed with HBSS supplemented with 10% FBS and then HBSS alone. Tissue was then incubated in HBSS containing 0.5 g/L of collagenase (Gibco) for 1 hour at 37°C. HBSS containing 10% FBS was added to stop digestion; stromal cells were dissociated by pipetting. Cell suspensions were then passed through a 70-µm mesh cell strainer (VWR) to remove tissue debris. Stromal cells were plated in DMEM/F12 culture media (Gibco) containing 2% charcoal–stripped calf serum (Gibco) and treated with 5 µM of BPA or ethanol as vehicle for 48 hours. In a subset of experiments, cells were plated and treated with 5 µM of BPA in the presence or absence of 10 µM ICI 182,780 (Sigma) for 48 hours. In another subset of experiments, cells were plated and treated with ethanol vehicle or 10 µM S-adenosylmethionine (SAM; New England Biolabs) for 24 hours. To induce Hand2 expression, 1 µM of P (Sigma) was added to the treatment and cells were cultured for an additional 12 hours. Cells were then washed in PBS and harvested in TRIZOL for further RNA isolation and gene expression analysis.
RNA isolation and real-time quantitative PCR analysis
Frozen uteri were homogenized and RNA isolated using TRIZOL reagent (Life Technologies), according to the manufacturer's protocol. cDNA was prepared using the AffinityScript Multiple Temperature Reverse transcription, according to the manufacturer’s protocol (Agilent Technologies). The cDNA was amplified to quantify gene expression by real-time PCR, using gene-specific primers and Power SYBR Green PCR Master Mix (Applied Biosystems). The expression level of 36b4 was used as the internal control. For each treatment, the mean cycle threshold (Ct) and SD were calculated from individual Ct values obtained from three replicates of a sample. The normalized ΔCt in each sample was calculated as mean Ct of target gene subtracted by the mean Ct of internal control gene. ΔΔCt was then calculated as the difference between the ΔCt values of the vehicle and BPA treatment samples. The fold change of gene expression in each sample relative to a control was computed as 2−ΔΔCt. The mean fold induction and SEs were calculated from four independent experiments.
Methylation analysis
Methylation status was assessed using the EpiTect Methyl II PCR Assay (Qiagen). Briefly, genomic DNA was isolated from uteri of vehicle and BPA-treated mice using the DNeasy Blood and Tissue Kit (Qiagen), according to the manufacturer’s protocol. In a subset of methylation analysis experiments, DNA was isolated from primary murine uterine stromal cells that were treated with ethanol vehicle or SAM for 24 hours and then cotreated with P for 12 hours. Enzymatic treatment with the methyltransferase SssI and the methyl donor S-Adenosyl Methionine (New England Biolabs) was used to generate hypermethylated DNA as a positive control. Genomic DNA was then subjected to methyl-sensitive and methyl-dependent restriction enzyme digestion using the EpiTect II DNA Methylation Enzyme Kit (Qiagen). Methylation status of the isolated genomic DNA was assayed using the EpiTect Methyl II PCR Assay (Qiagen) with primer sets targeting two CpG islands in the mouse Hand2 gene. Ct values obtained from the different restriction enzyme conditions were used to calculate the percentage of methylated and unmethylated input DNA, as described in the manufacturer’s protocol. The mean percentage of methylated input DNA and SEs were calculated from four independent experiments.
Statistical analysis
Statistical significance between control and BPA-treated samples/tissues was determined using the Student t test. Asterisks denote significance with a P value <0.05.
Results
Chronic BPA treatment is uterotrophic and increases the gland:stroma ratio
To assess the risks of long-term exposure to the endocrine disruptor BPA in the uterus, we exposed reproductively mature cycling female mice three times a day orally to a cumulative dose of 60 µg/kg/d of BPA for 60 days. This concentration was chosen to reflect the reference dose established by the EPA and represents an estimate of the daily exposure likely to be without appreciable risk of deleterious effects (41). Oral route of administration was used to recapitulate the route of exposure in human populations. Uterine weight was measured upon euthanization, normalized to body weight, and expressed as fold change over vehicle [Fig. 1(a)]. A substantial increase in uterine wet weight was observed following long-term exposure to BPA compared with uteri from vehicle (corn oil only)-treated animals, indicating that chronic exposure to BPA has a uterotrophic effect. We next used hematoxylin and eosin staining to look at general uterine morphology [Fig. 1(b)]. Uteri from BPA-treated animals appeared to have increased number of uterine glands dispersed throughout the endometrium compared with vehicle-treated animals. To further investigate this observation, we subjected uterine sections to immunohistochemistry using cytokeratin 8 as an epithelial marker. Cytokeratin-positive structures were counted and expressed relative to stromal area, as determined by ImageJ (National Institutes of Health). We found that exposure to BPA increased the uterine gland:stroma ratio compared with the vehicle treatment [Fig. 1(c)]. Because increased gland:stroma ratios are often observed in incidences of hyperplasia, we further investigated into uterine abnormalities following BPA exposure.
Figure 1.
Chronic BPA treatment increases uterine weight and increases the gland:stroma ratio. (a) Reproductively mature female mice (8 wk of age) were treated three times per day with vehicle (corn oil) or BPA (cumulative dose of 60 µg/kg/d in corn oil) for 60 d. Animals were euthanized in diestrus, as determined by vaginal cytology. Uterine wet weight was measured on collection. Weights were normalized to animal body weight, and data are represented as the mean fold change ± SEM; *P < 0.05. (b) Representative hematoxylin and eosin staining of uterine sections obtained from vehicle- and BPA-treated mice. (Magnification, ×10). (c) Uterine sections (n = 4 independent samples) were subjected to immunohistochemistry and stained with cytokeratin 8 as an epithelial marker. Glands were counted and expressed relative to stromal area using ImageJ software. Data are represented as the mean ± SEM; *P < 0.05. G, glands; L, lumen; S, stroma; veh, vehicle.
Changes in proliferation and glandular morphology following chronic BPA exposure
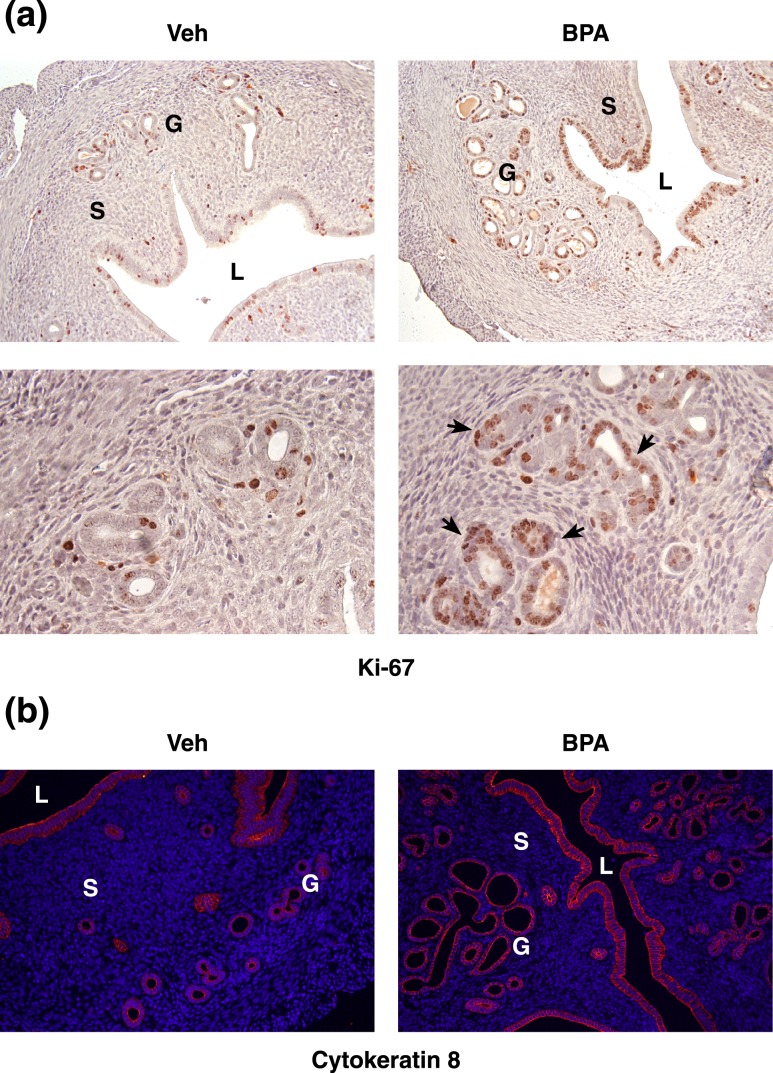
To determine if long-term exposure to BPA alters epithelial proliferation, we subjected uterine sections to immunohistochemistry using Ki67 as a marker for proliferation [Fig. 2(a)]. Indeed, we observed that uteri from mice exposed to BPA showed increased levels of proliferation, as indicated by Ki67+ staining in brown. The staining was particularly pronounced in the luminal epithelium and the uterine glands of BPA-exposed animals compared with the vehicle-treated controls. Because aberrant proliferative activity in glandular epithelium is one of the hallmarks of endometrial hyperplasia, we further looked at the glandular morphology in uteri of vehicle and BPA-treated animals using cytokeratin 8 [Fig. 2(b)]. Uteri of BPA-exposed animals exhibited densely packed clusters of glands, as well as elongated, irregularly shaped glands, compared with smaller, rounder, more evenly distributed glands found in uteri of vehicle-treated mice. These changes in proliferation, glandular architecture, and the gland:stroma ratio suggest that long-term BPA exposure could give rise to endometrial hyperplasia. We next determined the mechanism by which BPA alters endometrial proliferation.
Figure 2.
Changes in proliferation and glandular morphology following chronic BPA exposure. (a) Uterine sections obtained from vehicle- and BPA-treated (60 µg/kg/d for 60 d) mice were subjected to immunohistochemistry using Ki67 to indicate proliferating cells. Brown staining indicates Ki67+ cells. Arrows highlight actively proliferating uterine glands. [Magnification, ×20 (top), ×40 (bottom).] (b) Cytokeratin 8 (red) staining of uterine sections obtained from vehicle- and BPA-treated mice. DAPI was used as a nuclear counterstain. (Magnification, ×20.) G, glands; L, lumen; S, stroma; veh, vehicle.
BPA alters expression of the progesterone receptor target HAND2
It is well established that endometrial proliferation is controlled by a balance of proproliferative E and antiproliferative P signaling. Because we observed an increase in glandular proliferation following BPA exposure, we next wanted to investigate whether aspects of P signaling were impaired. We looked at expression of the progesterone receptor (PR) target HAND2, a stromal factor we have previously shown to mediate the antiproliferative effects of P (3). Gene expression analysis revealed that uterine Hand2 expression is reduced following chronic BPA exposure compared with vehicle-treated animals [Fig. 3(a)]. At the protein level, HAND2 is robustly expressed in the subepithelial stroma of mice treated with vehicle, as indicated by immunohistochemistry [Fig. 3(b)]. In BPA-treated animals, HAND2 is also detected in the subepithelial stroma, but to a lesser extent than in vehicle-treated animals. Indeed, when we quantify the number of HAND2+ stromal cells using ImageJ software, we see a substantial decrease in the number of HAND2+ cells in the subepithelial stromal compartment of BPA-treated mice, compared with vehicle [Fig. 3(b)]. This indicates that BPA exposure leads to downregulation of the downstream mediator of PR, resulting in persistent epithelial proliferation.
Figure 3.
BPA alters expression of the PR target HAND2. (a) RNA isolated from vehicle- and BPA-treated (60 µg/kg/d for 60 d) mice was subjected to qPCR using primers specific to Hand2. 36b4 was used to normalize RNA levels. Data are represented as the mean fold induction ± SEM; *P < 0.05; n = 4 independent samples. (b) Uterine sections obtained from vehicle- and BPA-treated mice were subjected to immunohistochemistry using an antibody specific for HAND2. HAND2 expression is indicated by the brown color. [Magnification, ×20 (top), ×40 (bottom)]. HAND2+ cells were counted using ImageJ software and expressed as a percentage of total cells in the counting field. Data are represented as the mean percentage ± SEM; *P < 0.05. (c) RNA isolated from vehicle- and BPA-treated mice was subjected to qPCR using primers specific to PR. 36b4 was used to normalize RNA levels. Data are represented as the mean fold induction ± SEM; n = 4 independent samples. (d) Uterine sections obtained from vehicle- and BPA-treated mice were subjected to immunohistochemistry using an antibody specific for PR (red) and counterstained with DAPI (blue). (Magnification, ×20.) G, glands; L, lumen; S, stroma; veh, vehicle.
We next determined whether PR expression itself is impaired in response to BPA exposure by using quantitative PCR (qPCR) [Fig. 3(c)]. Compared with vehicle, BPA-treated animals showed slightly higher expression of PR, although this was not statistically significant. Consistent with this RNA profile, we found that PR expression is maintained both in the epithelium and the stroma following BPA exposure [Fig. 3(d)]. These data suggest that reduced HAND2 expression following BPA exposure is due to changes in PR transcriptional activity rather than reduced PR expression.
Chronic BPA exposure alters FGF signaling in the glandular epithelium
Previously, we have shown that a primary function of HAND2 is to regulate uterine epithelial proliferation by suppressing expression of paracrine-acting FGFs in the uterine stroma (3). We next determined the consequences of BPA exposure on FGF levels by performing qPCR using uterine samples obtained from our BPA- or vehicle-exposed mice. We found that mice chronically exposed to BPA exhibited significantly increased uterine expression of Fgf9 and Fgf18 compared with vehicle-treated animals [Fig. 4(a)], indicating that reduced HAND2 expression corresponds with increased FGF production.
Figure 4.
Chronic BPA exposure alters FGF signaling in the glandular epithelium. (a) RNA isolated from vehicle- and BPA-treated (60 µg/kg/d for 60d) mice was subjected to qPCR using primers specific to Fgf9 and Fgf18. 36b4 was used to normalize RNA levels. Data are represented as the mean fold induction ± SEM; *P < 0.05; n = 4 independent samples. (b) Uterine sections obtained from vehicle- and BPA-treated mice were subjected to immunohistochemistry using an antibody specific to phosphorylated FRS2 (red, left) as an indicator of FGFR activation or phosphorylated ERK1/2 (green, right), and counterstained with DAPI (blue). (Magnification, ×40.) G, glands; S, stroma; veh, vehicle.
FGFs signal through membrane-bound FGFR. Binding of the ligand to the receptor triggers receptor activation and subsequent phosphorylation of FRS2. Using this phosphorylation event as a readout of FGFR activation, we performed immunohistochemistry using an antibody specific for phosphorylated FRS2 (pFRS2) [Fig. 4(b)]. We found that FGFR activation was greatly increased in the glandular epithelium following chronic BPA exposure compared with vehicle-treated animals, as indicated by pFRS2 staining in red. FGFR activation can lead to increased proliferation, which is mediated through activation of the MAPK cascade. Indeed, we have previously shown that loss of MAPK activation can attenuate proliferation in the uterine epithelium (3). To determine if BPA exposure leads to increased MAPK activation, we performed immunohistochemistry using an antibody specific to phosphorylated ERK1/2 [Fig. 4(b)]. We found that phosphorylation and activation of ERK1/2 was robustly increased in the uterine glands of animals exposed to BPA compared with vehicle-treated animals, as indicated by pERK1/2 staining in green. Together, these data establish a mechanism by which BPA induces expression of FGFs that bind FGFR in the glandular epithelium, leading to downstream MAPK activation and increased proliferation.
To further investigate how BPA modulates Fgf9 and Fgf18 expression in the uterus, we used an in vitro system in which stromal cells were isolated from the uterus and placed into culture. These cells were then stimulated with 5 µM of BPA for 48 hours; the resulting RNA was subjected to gene expression analysis using primers specific to Fgf9 and Fgf18. Indeed, cells exposed to BPA showed increased expression of Fgf9 and Fgf18 compared with vehicle-treated cells [Fig. 5(a)], indicating that BPA can drive expression of paracrine-acting FGFs in stromal cells. These observations are in agreement with those seen in vivo [Fig. 4(a)]. We next investigated the nature of this regulation and the involvement of ER. Previous groups have shown that FGF expression is positively regulated by E (42, 43). To determine if BPA is promoting FGF production through ER, we treated uterine stromal cells in vitro with BPA in the presence or absence of ER antagonist ICI 182,780 [Fig. 5(b)]. We observe marked reduction in BPA stimulated Fgf9 and Fgf18 levels with inclusion of ICI 182,780, indicating that functional ER is required for BPA-mediated induction of FGFs.
Figure 5.
BPA induces FGF expression in vitro via estrogen receptor. (a) Primary murine uterine stromal cells were isolated and stimulated in vitro with vehicle (ethanol) or 5 µM BPA for 48 h. RNA was isolated and subjected to qPCR using primers specific to Fgf9 and Fgf18. 36b4 was used to normalize RNA levels. Data are represented as the mean fold induction ± SEM; *P < 0.05; n = 3 independent samples. (b) Primary murine uterine stromal cells were isolated and stimulated in vitro with 5 µM BPA in the presence or absence of 10 µM ICI 182,780 for 48 h. RNA was isolated and subjected to qPCR using primers specific to Fgf9 and Fgf18. 36b4 was used to normalize RNA levels. Data are represented as the mean fold induction ± SEM; *P < 0.05; n = 3 independent samples. ICI, ICI 182,780; Veh, vehicle.
BPA induces differential methylation at the Hand2 promoter and increased expression of methylation-related factors
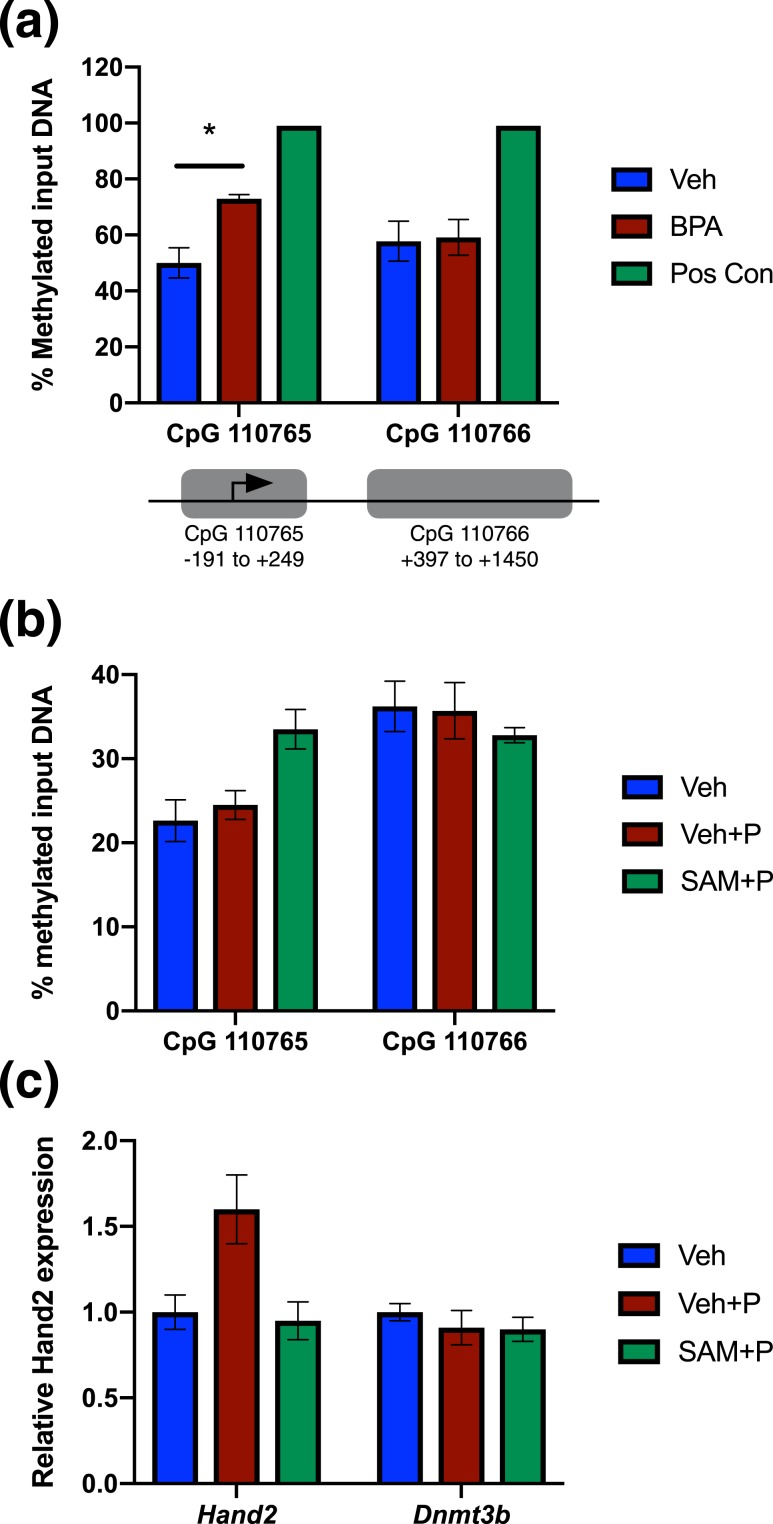
Chronic BPA exposure reduces HAND2 expression, but does not alter the expression of PR, the upstream regulator of HAND2. This suggests that BPA may be interfering with the ability of PR to recognize and stably bind to its downstream targets as opposed to decreasing the levels of PR available to signal. Previous studies have shown that BPA exposure causes differential methylation of certain gene promoters that can then lead to altered expression of that gene (44–47). To determine if BPA downregulates HAND2 in the uterus via hypermethylation and gene silencing, we looked at the methylation status of the Hand2 promoter following chronic BPA exposure [Fig. 6(a)]. Our analysis focused on methylation within two different CpG islands proximal to the Hand2 transcription start site [Fig. 6(a)]. We observed a substantial increase in DNA methylation in uterine samples following chronic BPA exposure at the first CpG island assayed, flanking the Hand2 transcription start site. No differential methylation was observed in the second CpG island, coding for the 5′-UTR and exon1 of Hand2. MatInspector software by Genomatix was used to identify transcription factor binding sites within this first CpG island. We identified several potential transcription factor binding sites within this region, including response elements for Kruppel-like factors, signal transducer and activator of transcription, GATA binding proteins, and hypoxia inducible factors. Further investigation is needed to determine what regulatory elements located in this region may be required for proper Hand2 expression.
Figure 6.
BPA induces differential methylation at the Hand2 promoter. (a) Genomic DNA isolated from vehicle- and BPA-treated (60 µg/kg/d for 60 d) uteri was subjected to enzymatic digestion as described in “Materials and Methods”; SssI- and SAM-treated genomic DNA was used to generate hypermethylated DNA as a positive control. Digested DNA was subjected to qPCR using primers targeting the indicated Hand2 CpG islands. Data are represented as the mean percent methylated input DNA ± SEM; *P < 0.05; n = 4 independent samples. (b) Mouse uterine stromal cells were placed in culture and treated with 10 µM SAM or vehicle for 24 h. The treatment was supplemented with 1 μM of progesterone for 12 h to induce Hand2 expression. Genomic DNA was isolated and subjected to enzymatic digestion as described in “Materials and Methods” and subjected to qPCR using primers targeting the Hand2 CpG islands 110765 or 110766, shown in (a). Representative data are shown as mean percent methylated input DNA; n = 2. (c) RNA isolated from mouse uterine stromal cells treated as described in (b) was isolated and subjected to qPCR using primers specific to Hand2 and Dnmt3b. 36b4 was used to normalize RNA levels. Representative data are shown as mean fold induction; n = 2. Pos con, positive control; Veh, vehicle.
We next used our in vitro system to further investigate the link between methylation at the Hand2 promoter and how this affects its expression. SAM is a methyl donor that supplies DNA methyltransferases with the methyl group for transfer to cytosine residues in DNA. We exposed uterine stromal cells to 10 μM of the methyl donor SAM and then stimulated Hand2 expression with P. We observed increased DNA methylation at the CpG island 110765 flanking the Hand2 transcription start site following treatment with P and SAM, compared with vehicle or P alone [Fig. 6(b)]. We saw no change in DNA methylation at CpG island 110766, consistent with our in vivo observations [Fig. 6(a)]. We next analyzed gene expression changes and observed increased Hand2 expression following stimulation with P. This induction was blocked by treatment with SAM [Fig. 6(c)]. Moreover, expression of a DNA methyltransferase, Dnmt3b, is unchanged, indicating that reduced gene expression in response to SAM is not a global effect. Together, these in vitro data suggest that increased methylation at the Hand2 promoter can impair HAND2 expression.
DNA methylation and gene silencing are mediated by DNA methyltransferases and methylated DNA binding proteins. A growing body of literature suggests that BPA exposure modulates expression of these factors in a variety of tissue types (44–47). We observed increased uterine expression of several of these factors, including DNA methyltransferases Dnmt1 and Dnmt3b and methylated DNA binding protein Mbd2, in vivo following chronic oral exposure to BPA [Fig. 7(a)]. We also observed increased Dnmt1, Dnmt3b, and Mbd2 expression in primary uterine stromal cells treated with BPA in vitro [Fig. 7(b)]. Previous studies have shown Dnmt expression is induced by E acting through ER (48, 49). Inclusion of the ER antagonist ICI 182,780 to BPA-treated uterine stromal cells in vitro reduces the expression of Dnmt1 and Dnmt3b, compared with BPA alone, suggesting that BPA acts via ER to upregulate Dnmt expression [Fig. 7(c)].
Figure 7.
BPA induces expression of DNA methylation factors. (a) RNA isolated from vehicle- and BPA-treated mice was subjected to qPCR using primers specific to Dnmt1, Dnmt3b, Mbd2, Mbd3, and Mecp2; 36b4 was used to normalize RNA levels. n = 4 independent samples. (b) Primary murine uterine stromal cells were isolated and stimulated in vitro with vehicle (ethanol) or 5 µM BPA for 48 h. RNA was isolated and subjected to qPCR using primers specific to Dnmt1, Dnmt3b, and Mbd2. 36b4 was used to normalize RNA levels. n = 3 independent samples. (c) Mouse uterine stromal cells were placed in culture and stimulated with 5 μM BPA in the presence or absence of 10 μM ICI 182,780 for 48 hours. RNA was isolated and subjected to qPCR using primers specific to Dnmt1 and Dnmt3b; 36b4 was used to normalize RNA levels. n = 3 independent samples. (a)–(c) Data are represented as the mean fold induction ± SEM; *P < 0.05. ICI, ICI 182,780; Veh, vehicle.
Based on these data, we propose a mechanism by which BPA exposure induces methylation at the Hand2 promoter that obstructs transcription factor binding and recruitment of transcription machinery, leading to reduced Hand2 expression. Reduced expression alleviates antagonism of FGF production by HAND2, allowing for increased FGF stimulation of the uterine glandular epithelium and subsequent proliferation.
Discussion
BPA exposure in the general population is ubiquitous, and numerous biomonitoring studies have shown measurable BPA levels in serum and urine of children and adults all over the world (11). These observations highlight the importance in understanding what adverse health outcomes are associated with BPA exposure. A recent study demonstrated that a single oral exposure to 50 μg/kg led to BPA accumulation in the uteri of both cycling and pregnant rats and mice within an hour of exposure (50). Moreover, ∼72% of the BPA recovered from uterine samples was in the aglycone form, which is capable of interacting with steroid receptors. In this study, we report that BPA has uterotrophic effects in the cycling mouse uterus [Fig. 1(a)]. This uterotrophic effect is in agreement with observations made by other groups (51, 52). However, these studies use doses delivered via injection or continuous exposure via ALZET pump for short treatment periods, and may not adequately represent the common route of exposure in humans. In the current study, we demonstrate that oral exposure to a lower concentration of BPA for a longer treatment period can also induce a uterotrophic response. Currently, the reference dose for BPA is 50 µg/kg/d, as established by the EPA. This represents an estimate of the daily exposure in humans likely to be without appreciable risk of deleterious effects. This study establishes that BPA exposure comparable to what the EPA deems safe for human exposure can lead to undesirable uterine pathologies.
Aberrant proliferation is a hallmark of hyperplasia, a condition characterized by an increase in cell number without changes to cell morphology. Hyperplasia is not itself cancer, but can develop into cancer with further genetic mutations or environmental insults. Here we report that BPA exposure leads to aberrant proliferation of the uterine epithelium, as indicated by Ki67 staining [Fig. 2(a)]. Notably, proliferation was particularly robust in the epithelium of the uterine glands, which are predominantly the site of origin for endometrial hyperplasia. A previous study demonstrated that gestational exposure to low doses of BPA (ng/kg/d) led to increased 5-bromo-2′-deoxyuridine incorporation and DNA synthesis in the glandular epithelium of the pups when euthanized at 3 months of age (53). These findings support the observations made in this study; however, because a different dosing paradigm and age of exposure was used, different mechanisms may be at play compared with the current study. In addition to increased proliferation, we observed changes in uterine glandular architecture suggestive of endometrial hyperplasia, including increased gland:stroma ratio [Fig. 1(c)], densely clustered glands, and enlarged, irregularly shaped glands [Fig. 2(b)]. Several rodent studies have shown that BPA exposure during the perinatal period increased incidence of uterine gland abnormalities later in life, including signs of cystic hyperplasia and squamous metaplasia (54, 55). Again, these data support our observations, but our study demonstrates that BPA exposure outside of the critical windows of development can also have deleterious effects in the uterine glands, perhaps through an alternative mechanism. Another study reported changes in uterine architecture following BPA exposure in adults, including increased epithelial height and increased stromal and myometrial thickness (56). However, this study dosed mice acutely (4 days) and does not provide insight into the effects of chronic BPA exposure. Interestingly, one study looking at serum BPA levels in women with endometrial hyperplasia showed that BPA levels were reduced in patients with complex endometrial hyperplasia and endometrial cancer compared with healthy patients (57). The authors hypothesize that the reduced BPA levels may be a result of altered metabolism of BPA or that BPA exerts antiestrogenic effects. Endocrine-disrupting chemicals such as BPA have been shown to exhibit nonmonotonic dose responses, meaning that the dose response curve generated for a specific response resembles a “U” shape, showing a greater magnitude of response at lower and higher doses, and a lesser magnitude of response at intermediate doses (58). In light of this, perhaps the lower concentrations observed in women with endometrial hyperplasia and cancer correspond with a greater response (i.e., hyperplasia compared with healthy patients with higher serum BPA levels).
We report here that BPA causes aberrant Fgf production and FGFR activation in uterine tissue. The FGF family consists of 18 secreted proteins that serve as ligands to 4 membrane-bound tyrosine kinase receptors (59). FGFs bind to membrane-bound FGFRs and trigger receptor dimerization, activation, and autophosphorylation that will recruit the adaptor protein FRS2. FRS2 is phosphorylated on several tyrosine residues that serve as docking sites for downstream signaling molecules that lead to activation of the MAPK pathway and the phosphatidylinositol 3-kinase/AKT pathway. FGF signaling orchestrates multiple biological responses, including cell proliferation, cell migration, and angiogenesis (60). In the uterus, FGFs produced in the stroma act in a paracrine fashion to induce proliferation in the epithelium (3). In a previous study, we have observed using a delayed implantation model that prepubertal exposure to BPA leads to impaired implantation (24). This is accompanied by increased stromal FGF production, epithelial FGFR activation, and MAPK activity that maintains the luminal epithelium in a proliferative state and therefore unreceptive to blastocyst implantation. Similar to what is observed in the pregnant mouse uterus, here we show BPA increases Fgf production [Figs. 4(a) and 5(a)] and leads to FGFR activation and increased MAPK activity in the adult nonpregnant uterine glands, as indicated by pFRS2 and pERK1/2 staining [Fig. 4(b)]. Aberrant activation of this signaling pathway is likely a driver for epithelial proliferation, increased gland:stroma ratio, and changes in glandular morphology observed in BPA-treated mice. Aberrant FGF signaling has been previously observed in human endometrial cancer, where activating mutations in FGFR2 have been reported in 10% to 12% of primary uterine tumors (61, 62). Inhibition of FGFR2 expression or activity both resulted in decreased endometrial tumor cell survival and transformation, indicating that excessive FGFR2 activation contributes to endometrial cancer progression (62). Pharmacological agents targeting FGF signaling are currently being explored for use in the treatment of endometrial cancers (63). Here, we show that BPA exposure leads to a robust increase in Fgf9 expression and corresponding activation of FGFR in the glandular epithelium. FGF9 has a greater binding affinity for FGFR2 and FGFR3 than FGFR1 and 4 (64). We hypothesize that increased production of FGF9 by BPA acts on FGFR2 in the glandular epithelia to promote proliferation. Further experiments are needed to determine which FGFR isoform is responsible for mediating the proliferative effects of FGF9 in the uterine glands.
In the current study, we show that chronic exposure to BPA increases methylation of a CpG island in the murine Hand2 promoter and correlates with reduced uterine Hand2 transcript levels compared with vehicle-treated animals [Figs. 6(a) and 3(a), respectively]. This linkage between methylation at the Hand2 promoter and reduced Hand2 expression was also observed in vitro using the methyl donor SAM [Fig. 6(b) and 6(c)]. CpG islands represent short stretches of DNA that are rich in CpG dinucleotides. Many of these areas are associated with gene promoters, and CpGs in these regions are generally protected from methylation. In the incidence of cancer, global methylation analysis reveals that cancer genome in tissues such as breast, colon, and prostate tend to be hypomethylated in the intergenic and intronic regions relative to normal healthy tissue, whereas specific CpG islands tend to be concurrently hypermethylated with the incidence of cancer (65, 66). Other groups have shown that CpGs located within promoters of genes associated with tumor suppression, DNA repair, and hormone receptors are hypermethylated in tumor samples and cancer cell lines, and that demethylation of these regions with 5-aza-2′-deoxycytidine could restore gene expression (67–69). There is a growing body of literature suggesting that BPA modifies gene expression through an epigenetic mechanism, including modulating CpG methylation at target gene promoters and regulatory regions that correlates with reduced gene expression (44–47, 70). A number of studies also show that exposure to BPA increases the expression of DNA methyltransferases in various tissues such as heart, liver, and testis, and suggest this may be a mechanism by which BPA mediates hypermethylation and gene silencing (44–47). Similarly, in our study in the uterus, we observed that BPA induces increased mRNA expression of Dnmt1, a maintenance methylase, Dnmt3b, a de novo methylase, as well as increased expression of Mbd2, a protein that binds methylated DNA and mediates transcriptional repression at methylated gene promoters [Fig. 7(a)]. We have further shown that, in regard to Dnmt1 and Dnmt3b, this action is mediated through ER [Fig. 7(c)]. It is possible that elevated expression levels of these factors may be mediating the hypermethylation and transcriptional repression of Hand2 in the uterus of BPA-treated mice.
Interestingly, we see that uterine PR expression appears to be unchanged between vehicle- and BPA-treated mice [Fig. 3(c) and 3(d)]. This is in contrast to our previous study showing the effects of BPA exposure during pregnancy, in which BPA reduced not only HAND2, but also PR expression (24). P, through the activation of PR, induces HAND2 expression in the uterine stroma. This suggests that during pregnancy, the reduction in HAND2 expression in response to BPA is due to reduced levels of PR. In the current study, our data indicate that in the nonpregnant uterus BPA is interfering with PR transcriptional activity by inhibiting its ability to interact with the target gene rather than through inhibition of PR expression itself.
HAND2 is a critical mediator of the antiproliferative effects of P in the uterus (3). We have previously observed that BPA reduces HAND2 expression during early pregnancy (24). A pervasive consequence of HAND2 loss is persistent epithelial proliferation, which would suggest that HAND2 may play a role in preventing uterine hyperplasia and cancer. Indeed, mice lacking uterine HAND2 exhibit signs of complex atypical hyperplasia in their uterine glands, including changes in glandular morphology and an increase in the gland:stoma ratio, compared with their age-matched controls (71). In humans, we have shown that HAND2 is the most commonly hypermethylated and silenced gene in endometrial cancer (71). Moreover, HAND2 hypermethylation and silencing is observed in endometrial precancerous lesions, indicating that loss of HAND2 occurs early on in the progression of endometrial cancer. Interestingly, HAND2 hypermethylation correlates with failure to respond to P therapies to treat endometrial cancer, indicating that HAND2 is the primary mediator of the antiproliferative effects of P. With chronic BPA treatment in nonpregnant adult mice, we observe decreased HAND2 expression and increased glandular proliferation, suggesting that the repression of HAND2 plays a critical role in driving the hyperplastic effects of BPA in the uterus.
Conclusion
Our studies demonstrate that chronic oral exposure to a low concentration of BPA during adulthood impedes transcriptional activation of the antiproliferative factor HAND2, likely through an epigenetic mechanism involving hypermethylation at the Hand2 promoter. Reduction of HAND2 alleviates its repression of FGFs, increasing FGF levels and promoting FGF-induced epithelial proliferation in the uterine glands and altered glandular morphology. This is mediated through increased MAPK activity downstream of glandular FGFR activation. This study offers further insight into the endocrine-disrupting nature of BPA and provides a potential mechanism by which BPA may influence endometrial hyperplasia and other uterine disorders.
Acknowledgments
The authors thank Jason Neff for creating the figures.
Financial Support: This work was supported by National Institutes of Health Grants R21HD078983 (to M.K.B. and I.C.B.) and T32ES007326 (to A.M.N.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BPA
bisphenol-A
- Ct
cycle threshold
- DAPI
4′,6-diamidino-2-phenylindole
- E
estrogen
- ER
estrogen receptor
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- FRS2
fibroblast growth factor receptor substrate 2
- HAND2
heart and neural crest derivatives expressed 2
- HBSS
Hanks buffered salt solution
- MBD2
methylated DNA–binding protein 2
- P
progesterone
- pFRS2
phosphorylated fibroblast growth factor receptor substrate 2
- PR
progesterone receptor
- qPCR
quantitative PCR
- SAM
S-adenosylmethionine
References
- 1. Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol. 1973;56(1):133–144. [DOI] [PubMed] [Google Scholar]
- 2. Pan H, Deng Y, Pollard JW. Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci USA. 2006;103(38):14021–14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331(6019):912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 5. Trimble CL, Method M, Leitao M, Lu K, Ioffe O, Hampton M, Higgins R, Zaino R, Mutter GL; Society of Gynecologic Oncology Clinical Practice Committee. Management of endometrial precancers. Obstet Gynecol. 2012;120(5):1160–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275(5):370–375. [DOI] [PubMed] [Google Scholar]
- 8. Chlebowski RT, Anderson GL, Sarto GE, Haque R, Runowicz CD, Aragaki AK, Thomson CA, Howard BV, Wactawski-Wende J, Chen C, Rohan TE, Simon MS, Reed SD, Manson JE. Continuous combined estrogen plus progestin and endometrial cancer: the Women’s Health Initiative Randomized Trial. J Natl Cancer Inst. 2015;108(3):djv350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanderson PA, Critchley HO, Williams AR, Arends MJ, Saunders PT. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update. 2017;23(2):232–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandra V, Kim JJ, Benbrook DM, Dwivedi A, Rai R. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol. 2016;27(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118(8):1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308(11):1113–1121. [DOI] [PubMed] [Google Scholar]
- 13. Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab. 2011;96(12):3822–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, Palimeri S, Panidis D, Diamanti-Kandarakis E. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96(3):E480–E484. [DOI] [PubMed] [Google Scholar]
- 15. Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ Health Perspect. 2011;119(10):1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. [DOI] [PubMed] [Google Scholar]
- 17. vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113(8):926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singleton DW, Feng Y, Yang J, Puga A, Lee AV, Khan SA. Gene expression profiling reveals novel regulation by bisphenol-A in estrogen receptor-alpha-positive human cells. Environ Res. 2006;100(1):86–92. [DOI] [PubMed] [Google Scholar]
- 19. Sekar TV, Foygel K, Massoud TF, Gambhir SS, Paulmurugan R. A transgenic mouse model expressing an ERα folding biosensor reveals the effects of bisphenol A on estrogen receptor signaling. Sci Rep. 2016;6(1):34788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernández M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect. 2009;117(5):757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berger RG, Foster WG, deCatanzaro D. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod Toxicol. 2010;30(3):393–400. [DOI] [PubMed] [Google Scholar]
- 22. Xiao S, Diao H, Smith MA, Song X, Ye X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod Toxicol. 2011;32(4):434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan X, Wang X, Sun Y, Dou Z, Li Z. Inhibitory effects of preimplantation exposure to bisphenol-A on blastocyst development and implantation. Int J Clin Exp Med. 2015;8(6):8720–8729. [PMC free article] [PubMed] [Google Scholar]
- 24. Li Q, Davila J, Kannan A, Flaws JA, Bagchi MK, Bagchi IC. Chronic exposure to bisphenol A affects uterine function during early pregnancy in mice. Endocrinology. 2016;157(5):1764–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang W, Hafner KS, Flaws JA. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276(2):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ziv-Gal A, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol Appl Pharmacol. 2015;284(3):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, Hauser R. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol. 2013;42:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, Ye X, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. 2012;120(7):978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lathi RB, Liebert CA, Brookfield KF, Taylor JA, vom Saal FS, Fujimoto VY, Baker VL. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil Steril. 2014;102(1):123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.RRID:AB_531826, https://scicrunch.org/resolver/AB_531826.
- 31.RRID:AB_393778, https://scicrunch.org/resolver/AB_393778.
- 32.RRID:AB_2106234, https://scicrunch.org/resolver/AB_2106234.
- 33.RRID:AB_653182, https://scicrunch.org/resolver/AB_653182.
- 34.RRID:AB_2315192, https://scicrunch.org/resolver/AB_2315192.
- 35.RRID:AB_2115995, https://scicrunch.org/resolver/AB_2115995.
- 36.RRID:AB_2340788, https://scicrunch.org/resolver/AB_2340788.
- 37.RRID:AB_2340398, https://scicrunch.org/resolver/AB_2340398.
- 38.RRID:AB_2315777, https://scicrunch.org/resolver/AB_2315777.
- 39.RRID:AB_2307443, https://scicrunch.org/resolver/AB_2307443.
- 40.RRID:AB_2340616, https://scicrunch.org/resolver/AB_2340616.
- 41. US Environmental Protection Agency National Center for Environmental Assessment. Bisphenol A; CASRN 80-05-7. Available at: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=356. Accessed 20 March 2019. [Google Scholar]
- 42. Tsai SJ, Wu MH, Chen HM, Chuang PC, Wing LY. Fibroblast growth factor-9 is an endometrial stromal growth factor. Endocrinology. 2002;143(7):2715–2721. [DOI] [PubMed] [Google Scholar]
- 43. Šućurović S, Nikolić T, Brosens JJ, Mulac-Jericevic B. Spatial and temporal analyses of FGF9 expression during early pregnancy. Cell Physiol Biochem. 2017;42(6):2318–2329. [DOI] [PubMed] [Google Scholar]
- 44. Doshi T, Mehta SS, Dighe V, Balasinor N, Vanage G. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology. 2011;289(2-3):74–82. [DOI] [PubMed] [Google Scholar]
- 45. Patel BB, Raad M, Sebag IA, Chalifour LE. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci. 2013;133(1):174–185. [DOI] [PubMed] [Google Scholar]
- 46. Ma Y, Xia W, Wang DQ, Wan YJ, Xu B, Chen X, Li YY, Xu SQ. Hepatic DNA methylation modifications in early development of rats resulting from perinatal BPA exposure contribute to insulin resistance in adulthood. Diabetologia. 2013;56(9):2059–2067. [DOI] [PubMed] [Google Scholar]
- 47. Jiang Y, Xia W, Yang J, Zhu Y, Chang H, Liu J, Huo W, Xu B, Chen X, Li Y, Xu S. BPA-induced DNA hypermethylation of the master mitochondrial gene PGC-1α contributes to cardiomyopathy in male rats. Toxicology. 2015;329:21–31. [DOI] [PubMed] [Google Scholar]
- 48. Cui M, Wen Z, Yang Z, Chen J, Wang F. Estrogen regulates DNA methyltransferase 3B expression in Ishikawa endometrial adenocarcinoma cells. Mol Biol Rep. 2009;36(8):2201–2207. [DOI] [PubMed] [Google Scholar]
- 49. Shi JF, Li XJ, Si XX, Li AD, Ding HJ, Han X, Sun YJ. ERα positively regulated DNMT1 expression by binding to the gene promoter region in human breast cancer MCF-7 cells. Biochem Biophys Res Commun. 2012;427(1):47–53. [DOI] [PubMed] [Google Scholar]
- 50. Pollock T, deCatanzaro D. Presence and bioavailability of bisphenol A in the uterus of rats and mice following single and repeated dietary administration at low doses. Reprod Toxicol. 2014;49:145–154. [DOI] [PubMed] [Google Scholar]
- 51. Papaconstantinou AD, Fisher BR, Umbreit TH, Brown KM. Increases in mouse uterine heat shock protein levels are a sensitive and specific response to uterotrophic agents. Environ Health Perspect. 2002;110(12):1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect. 2001;109(1):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72(6):1344–1351. [DOI] [PubMed] [Google Scholar]
- 54. Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 2007;24(2):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vigezzi L, Bosquiazzo VL, Kass L, Ramos JG, Muñoz-de-Toro M, Luque EH. Developmental exposure to bisphenol A alters the differentiation and functional response of the adult rat uterus to estrogen treatment. Reprod Toxicol. 2015;52:83–92. [DOI] [PubMed] [Google Scholar]
- 56. Papaconstantinou AD, Umbreit TH, Fisher BR, Goering PL, Lappas NT, Brown KM. Bisphenol A-induced increase in uterine weight and alterations in uterine morphology in ovariectomized B6C3F1 mice: role of the estrogen receptor. Toxicol Sci. 2000;56(2):332–339. [DOI] [PubMed] [Google Scholar]
- 57. Hiroi H, Tsutsumi O, Takeuchi T, Momoeda M, Ikezuki Y, Okamura A, Yokota H, Taketani Y. Differences in serum bisphenol a concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocr J. 2004;51(6):595–600. [DOI] [PubMed] [Google Scholar]
- 58. Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, Guerbet M, Rousselle C. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ Health. 2015;14(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J. 2011;437(2):199–213. [DOI] [PubMed] [Google Scholar]
- 61. Pollock PM, Gartside MG, Dejeza LC, Powell MA, Mallon MA, Davies H, Mohammadi M, Futreal PA, Stratton MR, Trent JM, Goodfellow PJ. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene. 2007;26(50):7158–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C, Nicoletti R, Winckler W, Grewal R, Hanna M, Wyhs N, Ziaugra L, Richter DJ, Trovik J, Engelsen IB, Stefansson IM, Fennell T, Cibulskis K, Zody MC, Akslen LA, Gabriel S, Wong KK, Sellers WR, Meyerson M, Greulich H. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci USA. 2008;105(25):8713–8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee PS, Secord AA. Targeting molecular pathways in endometrial cancer: a focus on the FGFR pathway. Cancer Treat Rev. 2014;40(4):507–512. [DOI] [PubMed] [Google Scholar]
- 64. Hecht D, Zimmerman N, Bedford M, Avivi A, Yayon A. Identification of fibroblast growth factor 9 (FGF9) as a high affinity, heparin dependent ligand for FGF receptors 3 and 2 but not for FGF receptors 1 and 4. Growth Factors. 1995;12(3):223–233. [DOI] [PubMed] [Google Scholar]
- 65. Wilson AS, Power BE, Molloy PL. DNA hypomethylation and human diseases. Biochim Biophys Acta. 2007;1775(1):138–162. [DOI] [PubMed] [Google Scholar]
- 66. Sproul D, Meehan RR. Genomic insights into cancer-associated aberrant CpG island hypermethylation. Brief Funct Genomics. 2013;12(3):174–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lopez-Serra L, Ballestar E, Fraga MF, Alaminos M, Setien F, Esteller M. A profile of methyl-CpG binding domain protein occupancy of hypermethylated promoter CpG islands of tumor suppressor genes in human cancer. Cancer Res. 2006;66(17):8342–8346. [DOI] [PubMed] [Google Scholar]
- 68. Carter JA, Górecki DC, Mein CA, Ljungberg B, Hafizi S. CpG dinucleotide-specific hypermethylation of the TNS3 gene promoter in human renal cell carcinoma. Epigenetics. 2013;8(7):739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang WT, Zheng PS. Promoter hypermethylation of KLF4 inactivates its tumor suppressor function in cervical carcinogenesis. PLoS One. 2014;9(2):e88827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang HQ, Zhang XF, Zhang LJ, Chao HH, Pan B, Feng YM, Li L, Sun XF, Shen W. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol Biol Rep. 2012;39(5):5651–5657. [DOI] [PubMed] [Google Scholar]
- 71. Jones A, Teschendorff AE, Li Q, Hayward JD, Kannan A, Mould T, West J, Zikan M, Cibula D, Fiegl H, Lee SH, Wik E, Hadwin R, Arora R, Lemech C, Turunen H, Pakarinen P, Jacobs IJ, Salvesen HB, Bagchi MK, Bagchi IC, Widschwendter M. Role of DNA methylation and epigenetic silencing of HAND2 in endometrial cancer development. PLoS Med. 2013;10(11):e1001551. [DOI] [PMC free article] [PubMed] [Google Scholar]