Abstract
Introduction:
While the Accreditation Council for Graduate Medical Education limited first year resident-physicians to 16 consecutive work hours from 2011–2017, resident-physicians in their second year or higher were permitted to work up to 28 hours consecutively. This paper describes the Randomized Order Safety Trial Evaluating Resident-physician Schedules (ROSTERS) study, a clustered-randomized crossover clinical trial designed to evaluate the effectiveness of eliminating traditional shifts of 24 hours or longer for second year or higher resident-physicians in pediatric intensive care units (PICUs).
Methods:
ROSTERS was a multi-center non-blinded trial in 6 PICUs at US academic medical centers. The primary aim was to compare patient safety between the extended duration work roster (EDWR), which included shifts ≥24 hours, and a rapidly cycling work roster (RCWR), where shifts were limited to a maximum of 16 hours. Information on potential medical errors was gathered and used for classification by centrally trained physician reviewers who were blinded to the study arm. Secondary aims were to assess the relationship of the study arm to resident-physician sleep duration, work hours and neurobehavioral performance.
Results:
The study involved 6577 patients with a total of 38821 patient days (n=18749 EDWR, n=20072 RCWR). There were 413 resident-physician rotations included in the study (n=203 EDWR, n=210 RCWR). Resident-physician questionnaire data were over 95% complete.
Conclusions:
Results from data collected in the ROSTERS study will be evaluated for the impact of resident-physician schedule roster on patient safety outcomes in PICUs, and will allow for examination of a number of secondary outcome measures.
Keywords: randomized, sleep, work hours, medical errors, pediatric intensive care unit, patient safety
1. Introduction
Studies conducted in laboratory and occupational settings over the past several decades have established that sleep deficiency and circadian rhythm disruption degrade human alertness and performance [1]. These risk factors are particularly problematic for physicians-in-training, given their long work hours and irregular schedules, as well as the high consequences of medical errors [2]. Beginning in the early 2000s, a series of studies found that first-year resident-physicians [interns; Post Graduate Year 1 (PGY1)] working extended duration work rosters (EDWR) (≥24 hours) made more serious medical errors (SMEs) than those working shifts of ≤16 consecutive hours; moreover, PGY1s working EDWRs suffered more needle stick injuries, and had an increased risk of motor vehicle crashes (MVCs) on the drive home from work [2–8]. In 2009, after a year-long study, the Institute of Medicine concluded that while it remained unclear whether resident-physician sleep deficiency led to patient harm, “the scientific evidence base establishes that human performance begins to deteriorate after 16 hours of wakefulness”[10,11]. They consequently called for the elimination of resident-physician shifts without sleep over 16 consecutive hours.
In response, beginning in July 2011, the Accreditation Council for Graduate Medical Education (ACGME) limited interns to 16 consecutive hours of work; second year (PGY2) and higher resident-physicians were permitted to work up to 28 consecutive hours and 88 hours weekly, averaged over 4 weeks [12]. Despite extensive literature demonstrating the hazards of sleep deprivation, however, questions have persisted about whether the 2011 ACGME standards would be beneficial, as this unfunded mandate increased the number of handovers of care [13] (albeit without negative impact in earlier trials [2]), and may lead to decreases in staffing and physician-patient ratios. The ACGME limits were proposed without advice on how to operationalize such changes, and were generally left to hospital staff and administrators to apply, leading to mixed approaches of implementation. Studies evaluating the effects on the safety of the ACGME’s 2011 standards have shown mixed results [13–17]. Furthermore, the outcomes in these studies may not have been sufficiently sensitive to measure important adverse effects and did not rigorously capture the work hours, sleep, or neurobehavioral performance of the resident-physicians. In addition, most prior studies, have not assessed the effects of roster changes on PGY2 and higher resident-physicians.
The primary goal of the Randomized Order Safety Trial Evaluating Resident-physician Schedules (ROSTERS) study was to assess whether implementation of a schedule that eliminated shifts ≥24 hours for resident-physicians (PGY2+) would result in improved patient safety. The primary outcome was the rate of SMEs, defined as a preventable adverse event or near miss. Specifically, we sought to compare rates of SMEs when resident-physicians worked a traditional EDWR, compared with a rapidly cycling work roster (RCWR), during which shifts were limited to 16 consecutive hours. The novel study design included data from many levels (patient-level, resident-physician-level, hospital unit level). This allowed for assessment of additional secondary aims, including the relationship between schedules and resident-physician outcomes including sleep duration, MVC risk, work-related accidents, depression, quality of work experience, neurobehavioral performance, and sleepiness during tasks.
2. Methods
2.1. Study design
Medical error rates in pediatric intensive care units (PICUs) are among the highest in any hospital setting, making them a leading priority for implementation of safety efforts [18–20]. The ROSTERS study was a two arm multi-center cluster-randomized crossover clinical trial designed to evaluate the effectiveness of a RCWR on patient safety outcomes compared to an EDWR. The ROSTERS trial took place from July 2013 to March 2017 in six academic medical center sites in the United States. The data collected in the study are summarized in Table 1, and include resident-physician-related data [questionnaire data, sleep and work diaries, drive diaries, actigraphically measured objective sleep data, psychomotor vigilance tests (PVTs)], patient data (patient days, comorbid conditions index, gender, age), potential medical errors, physician observer shift data, and study tracking data.
Table 1.
Data collected throughout the study.
| Level | Data | Use | Description |
|---|---|---|---|
| Resident-physicians | Baseline questionnaire | Potential covariates | Demographics, lifestyle, medical history, Berlin Sleep Questionnaire, Morningness-Eveningness Questionnaire, commute information. |
| End of rotation survey | Secondary outcomes, potential covariates | Sleepiness during tasks, motor vehicle accidents or near misses, needle sticks and other bodily fluid exposures, lifestyle, height, weight, medication use, depression scale, quality of work experience. | |
| Drive diary | Secondary outcome | Information about drives to and from work. | |
| Sleep and work diary | Secondary outcome | Information about sleep, naps, and work schedules. | |
| Psychomotor vigilance task (PVT) testing | Secondary outcome | A 10 minute long test of sustained visual attention. Resident-physicians had tests during one shift per week, at the beginning and end of the shift and every 5 hours in between. | |
| Actigraphy | Secondary outcome | The actigraph is a small wrist-worn device worn for the duration of the rotation which measures activity and light exposure. It supplies information about sleep, light, activity. | |
| Tracking | Enrollment, screening, withdrawa l forms | Study tracking | Information about rotation, consent type, reason for withdrawal. |
| Serious adverse event | Resident-physician safety | Information about serious adverse events that occurred to a resident-physician | |
| Physician observer staff | Observer shift summary | Study tracking | Information about the observer shifts, including date and time, resident- physicians observed, procedures performed by resident-physicians. |
| Missed shift summary | Study tracking | Noted when there was no observer working, the patient census during the missed shift, reason why missed. | |
| Event | Potential medical error form | Primary outcome | Date, time, patient ID, source of error, description of possible event, harm level, incident category. |
| Patient | Patient days log | Used in primary analysis | Admit and discharge dates, number of days spent in the PICU, age and gender of patient. |
| ICD-9 or ICD-10 codes | Covariate | Used to create a comorbid conditions index for each patient stay. |
Abbreviations: PICU, pediatric intensive care unit; ICD, International Classification of Diseases.
2.1.1. Site requirements
Each site under consideration for study participation was required to have an individual willing to serve as the site principal investigator (PI), a Clinical and Translational Science Award program, and a PICU. Each site under consideration also had to have a current resident-physician work schedule of ≥24 hour shifts for PGY2s and PGY3s. To be eligible for participation, each site was required to obtain letters of support from administrative officials with decision-making authority, such as the president of the hospital, the chairman of pediatrics, the director of the PICU, the residency program director and a relevant dean, as appropriate, stating their willingness to implement the RCWR schedule. Many academic medical centers nationwide were not eligible to participate, as they had previously eliminated shifts over 16 hours for resident-physicians working in their PICUs. A convenience sample of seven sites under consideration met all of the entry criteria.
2.1.2. Participating sites and institutions
Ultimately, six sites participated in the study: Boston Children’s Hospital; Children’s Hospital Colorado; University of Iowa Stead Family Children’s Hospital; Seattle Children’s Hospital; Cincinnati Children’s Hospital Medical Center; and University of Virginia Children’s Hospital. In presenting results, sites have been de-identified by assigning a reference letter (A through F) at random. In addition to the six sites, a Clinical Coordinating Center (CCC) was established at Brigham and Women’s Hospital (Boston, MA) and a Data Coordinating Center (DCC) was established at the San Francisco Coordinating Center at California Pacific Medical Center Research Institute (a Sutter Health affiliate, San Francisco, CA). The CCC was responsible for study design, site selection, protocol development and implementation, Institutional Review Board (IRB) management, site coordination, patient safety, and staff training. The DCC was responsible for developing standard operating procedures for data collection, processing and editing data, generating reports tracking data quality and completeness, statistical analysis and report generation for the Data Safety and Monitoring Board (DSMB) and statistical analysis of the primary aim of the study.
2.1.3. Site pairing and schedule design
The sites were assigned to pairs and the study was conducted in three waves to spread out the data collection efforts over the 5-year funding period. (Table 2) In each wave, PICUs were randomly assigned to start out with either the EDWR (i.e., overnight shifts of 24–28 hours scheduled every 4 or 5 nights, with shorter day shifts between) or to the RCWR (i.e., resident-physicians were limited to 16 consecutive work hours with night shifts scheduled every four to five days, sleep before night shifts was encouraged, time off after night shifts was arranged to allow for recovery sleep). Prior to data collection, there was a 4-month wash-in interval to allow the PICU to become accustomed to the schedule, after which 8 months of data collection took place. After these 8 months, the PICU crossed-over to the alternate schedule. Another 4-month wash-in interval followed, after which 8 months of data collection took place again (Table 2). The two sites within each wave gathered data during the same time of year to account for any seasonal differences in patient load. The nature of this study made it impossible to blind investigators, resident-physicians and study staff to schedule assignment, although all CCC staff and site PIs were blinded to interim analyses.
Table 2.
Study timeline by site and schedule type.
| Study Dates | ||||||
|---|---|---|---|---|---|---|
| Wave | Site | Schedule | Wash in | Data Collection | Days of data collection | Daysb without a Physician Observer |
| 1 | A | EDWR | July 2013–October 2013 | November 2013–June 2014 | 234 | 59.3c |
| RCWR | July 2014–October 2014 | November 2014–June 2015 | 240 | 42.5 | ||
| 1 | B | RCWR | July 2013–October 2013 | November 2013–June 2014 | 221 | 14.1 |
| EDWR | July 2014–October 2014 | November 2014–June 2015 | 224 | 19.2 | ||
| 2 | C | EDWR | September 2014–December 2014 | January 2015–September 2015 | 246 | 36.5 |
| RCWR | September 2015–December 2015 | January 2016–September 2016 | 253 | 38.4 | ||
| 2 | E | RCWR | March 2014–June 2014 | July 2014–March 2015 | 244 | 42.2c |
| EDWR | March 2015–June 2015 | July 2015–March 2016 | 245 | 45.6 | ||
| 3 | D | EDWR | February 2015–May 2015 | June 2015–January 2016 | 251 | 23.1c |
| RCWR | March 2016–June 2016 | July 2016–March 2017a | 226 | 0.5 | ||
| 3 | F | RCWR | February 2015–May 2015 | June 2015–January 2016 | 242 | 9.1c |
| EDWR | February 2016–May 2016 | June 2016–January 2017 | 244 | 14.5 | ||
Break in data collection from 12/22/16 to 1/13/17.
Time missed was summed, then divided by 24.
p<0.05 comparing the schedule within site.
Abbreviations: EDWR, extended duration work roster; RCWR, rapidly cycling work roster.
2.1.4. Sample size planning
Based on the previously conducted Intern Sleep and Patient Safety Study [2], during which 2,203 patient-days were accrued under both schedules over a total of 8.5 months at a single center with 2 ICUs totaling 20 beds, we estimated that approximately 46,080 patient-days would be accrued across the six sites in this study. Assuming that the error rates per-patient day on the EDWR were the same as those observed in the Intern Sleep and Patient Safety Study (136.0 per 1000 patient days), this current study would provide 90% power to detect relative rate reductions on the RCWR of 19.8% (a priori assuming a Poisson distribution) for the primary outcome of resident-physician-related SMEs. The actual reductions seen in the preliminary study (22%) were larger than this threshold. Thus, we expected to be adequately powered to draw definitive conclusions regarding the effectiveness of the RCWR.
2.2. Enrollment and resident-physician participation
Site PIs at each academic medical center made presentations to the pediatric resident-physicians describing the study and requesting voluntary participation. Incoming second-year resident-physicians were contacted about participation during their first year, and incoming third-year resident-physicians during their second year. All second- and third-year resident-physicians were invited to enroll in the study. Resident-physicians were given information about the data which would be collected, potential risks and benefits of participation, the timeline for participation, and offered an incentive (e.g., iPad® or cash equivalent) for participation. The duration of each resident-physician’s rotation was approximately one month. Resident-physicians could complete multiple rotations in the PICU. Therefore, they were allowed to enroll in the study multiple times, which makes statistical analyses more complex but is representative of the true work environment of the PICU. Resident-physicians could agree to full participation in the study (i.e., monitoring of sleep, work hours, and performance data collected as well as direct observation) or observation only, which meant they agreed to be directly observed by a physician observer while working in the PICU but did not agree to collection of other personal data. Resident-physicians could further indicate consent for the collection of salivary samples for DNA extraction. All resident-physicians in the PICU were assigned the work schedule under evaluation, regardless of their participation in the study. Data were collected on all PICU patients who were cared for by resident-physicians, regardless of the resident-physician’s participation in the study.
The IRB at each academic medical center, as well as at Sutter Health (DCC) and Partners Human Research Committee (CCC), approved the study. Data use agreements were established between each site and the DCC, and the trial was registered on ClinicalTrials.gov (NCT02134847). A DSMB was established and approved the study protocol prior to data collection. Study investigators obtained a Certificate of Confidentiality from the National Institutes of Health to protect the privacy of research subjects. Each site obtained a waiver of informed consent for the collection of patient data, and written informed consent was obtained from all resident-physicians enrolled in the study. PICU patients and their families were provided an information sheet with details of the study and were not considered as study participants.
2.3. Schedule implementation
On the EDWR, five of the six sites worked a 4 day rotation schedule consisting of 2 day shifts of approximately 12 hours long, followed by one overnight shift that started in the morning one day, ending in the morning the next day (about 28 hours long, Table 3). The PICU at one site worked a 5 day rotation with 2 day shifts of about 12 hours long, a day off, then one shift starting at 11:00AM that was about 24 hours long.
Table 3.
Summary of study implementation by site and schedule.
| Example of Shift Types | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Extended (day 3/4)a | ||||||||
| Site | Schedule | Start | End | Length (hrs) | Start | End | Length (hrs) | Start | End | Length (hrs) |
| Aa | EDWR | 6:00 | 18:00 | 12 | 6:00 | 18:00 | 12 | 11:00 | 11:00 | 24 |
| RCWR | 6:00 | 19:00 | 13 | 6:00 | 19:00 | 13 | 18:00 | 10:00 | 16 | |
| B | EDWR | 6:00 | 18:00 | 12 | 6:00 | 18:00 | 12 (or 6) | 6:00 | 10:00 | 28 |
| RCWR | 6:00 | 20:00 | 14 (or 6) | 6:00 | 17:00 | 11 (or 14) | 18:00 | 10:00 | 16 | |
| C | EDWR | 6:00 | 18:00 | 12 | 6:00 | 14:00 | 8 | 6:00 | 10:00 | 28 |
| RCWR | 7:00 | 20:00 | 13 | 6:00 | 18:00 | 12 | 19:00 | 11:00 | 16 | |
| D | EDWR | 6:00 | 18:00 | 12 | 6:00 | 18:00 | 12 | 6:00 | 10:00 | 28 |
| RCWR | 6:00 | 19:00 | 13 (or 14) | 6:00 | 17:00 | 11 (or 6) | 18:00 | 10:00 | 16 | |
| E | EDWR | 7:00 | 18:00 | 11 | 7:00 | 18:00 | 11 | 7:00 | 8:00 | 25 |
| RCWR | 7:00 | 21:00 | 14 | 7:00 | 18:00 | 11 | 19:00 | 11:00 | 16 | |
| F | EDWR | 6:00 | 15:00 | 9 (or 8) | 6:00 | 15:00 | 9 (or 8) | 6:00 | 10:00 | 28 |
| RCWR | 6:00 | 21:00 | 15 (or 8) | 6:00 | 21:00 | 15 (or 8) | 20:00 | 12:00 | 16 | |
Had a 5 day rotation, with day 3 scheduled as a day off, so the extended shift is day 4/5.
Abbreviations: EDWR, extended duration work roster; RCWR, rapidly cycling work roster.
During the RCWR, resident-physicians at five of the six sites were scheduled to work in a sequence of shifts in a repeating four day cycle. The approximate schedule was 2 days shifts (11–15 hours long) and one 16 hour long overnight shift that started in the evening and ended the next morning. The PICU at one site worked a 5 day rotation with 2 day shifts of about 13 hours long, a day off, then one shift starting at 6:00PM that was about 16 hours long.
When work shifts are shortened, by necessity patient care is transferred between physicians more frequently [2, 21]. PICUs were asked to use a structured handover of care during the evening shift change. This was in addition to the daily morning rounds, and the structure of the evening handover was not proscribed, varying from site to site. A fellow or faculty member typically oversaw the evening handover of care, which usually occurred between 8:00PM and 9:00PM.
The daily patient census was derived from the data collected on the patient days log form. The average daily resident-physicians present on unit was estimated using resident-physician schedules provided by each site, calculated during daytime hours between rounds and evening handover of care. ICU patients per resident-physician (IPRP) was calculated as the average daily patient census over the average resident-physicians present on the unit. These IPRPs varied considerably among PICUs, both on the EDWR and RCWR. On the EDWR, the mean daily resident-physicians on unit varied from 1.6 to 2.9, with IPRPs ranging from 4.1 ± 0.1 to 10.0 ± 0.2. In contrast, on the RCWR, the mean daily resident-physicians on unit varied from 1.3 to 3.0, with IPRPs ranging from 5.0 ± 0.1 to 13.0 ± 0.2.
With implementation of the RCWR, each PICU was asked to adjust its staffing as needed to preserve adequate staffing while implementing the reduced-hours schedule, but the manner in which each site adjusted staffing to accommodate the scheduling intervention varied. Daytime resident-physician staffing fell on many but not all PICUs, and workload as measured by IPRPs increased at all sites. To offset this, at least in part, some PICUs reallocated fellow-physician time to increase fellow supervision on the PICUs, or increased the use of nurse practitioners to cover some of the workload formerly handled by resident-physicians.
2.4. Site study staff
The research efforts at each participating academic medical center required, at a minimum, a site PI, a research nurse, five physician observers and a research assistant. The site PI and/or research assistant were responsible for obtaining IRB approval for the study and working out details of the RCWR implementation. They also were responsible for initial outreach to potential resident-physician enrollees.
Research assistants collected a saliva sample. Research assistants also provided instruction to the resident-physicians on the completion of daily sleep/work and driving diaries, examined diaries to ensure there were no inappropriate overlaps (e.g., a drive reported during a sleep episode) and that there was no missing information. The research assistants also provided demonstrations on the use of the actigraphs and PVT testing, and downloaded and transferred this data from the site to the DCC for processing.
Physician observers shadowed participating physician-residents and reported potential medical errors, and were responsible for collecting additional data on any other potential medical errors they observed while on the PICU. Physician observers were scheduled to ensure that at least one was on the PICU at all times. Each site developed an observation schedule to ensure that all resident-physicians were observed for approximately equal amounts of time during their rotations.
The research nurse was responsible for following up on and adding any additional detail to the potential medical errors identified by the physician observers, and completing forms for potential medical errors identified by other staff, found in the medical records or otherwise identified or recorded in the hospital database. The research nurse was also responsible for maintaining a patient log that tracked the number of patient days on the PICU during the trial.
2.5. Training
The CCC hosted webinars for the physician observers and research nurses during the wash-in intervals for each study wave. The webinars provided instruction on how to identify, record and classify potential medical errors. The training covered general information on patient safety, definitions of terms and reviewed a set of test cases. Links to the webinars were posted to the study website as a reference and a tool for new staff. The research assistant, research nurse and physician observers were trained by the DCC on the use of data collection forms and the study website, where they could address data edits, update data, and access study resources. The research assistant was also trained in the operation of the actigraph and PVT. Training was conducted by webinars or onsite as feasible. Site visits were performed by CCC and DCC staff on an as-needed basis to ensure correct implementation of the protocols.
2.6. Data collection
The ROSTERS study website and data system were designed and managed by the DCC. Electronic data entry and internet technology were utilized to provide real-time data access. Study data other than the diaries, actigraphy and PVT data were collected via REDCap™ and submitted electronically using online data collection instruments, accessed via a tablet, laptop/PC or smart phone [22]. The data collection forms were designed to prevent missing data, skip pattern errors, and inconsistent and out of range responses. Study data were subject to further daily error-checking programs following submission. Select research staff had permissions to resolve edits and update data as necessary via the website. These changes to the data were recorded in an audit trail. The study website was password protected and only accessible with a direct link. This website listed forms that were expected to have been submitted, which helped ensure data completeness. Diaries were collected electronically using a software program created by the CCC. Numerous reports were generated monthly to summarize data completeness and timeliness, track observer shift coverage, recruitment, event adjudication progress, and diary completeness. Monthly Steering Committee and Quality Assurance conference calls were held to review these reports with all study investigators and research assistants. This allowed for communication between all sites across the country, and helped to identify potential problems or site performance issues and determine solutions.
Resident-physicians were provided instructions on the collection of all resident-physician-level data entered. Resident-physicians entered responses to a baseline questionnaire within a week of the start of their rotation and an end-of-rotation survey within a week of the end of their rotation. This was done online on a secure resident-physician portal website. Resident-physicians also maintained an electronic sleep and work diary, completed daily. Drive diaries were completed online after each drive to or from work. Site staff provided notification via email or phone call as necessary to remind resident-physicians to complete these diaries.
Site staff entered data for resident-physician enrollment, actigraph tracking, PVT test information, and patient admission and discharge dates plus length of PICU stay. Site staff had two encrypted, password protected tablets for physician observers to collect data. These were linked to online data collection forms. Physician observers also recorded information about their shifts, and noted when a shift was missed.
2.7. Resident-physician safety
Weekly reports regarding resident-physician safety were generated, and included information on drowsy driving and accidental exposures (i.e. needle sticks, body fluid exposures). For each rotation, if a resident-physician had any MVCs, had two or more near misses while driving, fell asleep while driving, or had two or more episodes of drowsy driving the PI at the site discussed other transportation options or precautions to stay alert while driving. If a resident-physician had any accidental exposures, the PI insured that the resident-physician had followed hospital protocol for exposure, and discussed how to minimize subsequent exposures. If thoughts of suicide were reported, the PI discussed options for mental health counseling within 48 hours of the initial report. Sites reported serious adverse events (SAEs) that occurred to a resident-physician to the DCC within 24 hours and complied with their IRB reporting requirements.
2.8. Data safety and monitoring board (DSMB) and interim analyses
The DSMB was responsible for safeguarding the interests of study participants, assessing the safety and validity of study procedures, and for monitoring the overall conduct of the study and outcomes data. The DSMB members were an independent advisory group to the National Heart, Lung, and Blood Institute (NHLBI) Director and were required to provide recommendations about starting, continuing, and stopping the study. Strict procedures were in place to avoid any member conflict of interest, which was updated every year and at the start of each DSMB meeting. The DSMB was also asked to make recommendations, as appropriate, to the NHLBI about other aspects of the study. The study investigators did not communicate with DSMB members about the study directly, except when making presentations or responding to questions at DSMB meetings. The DSMB met approximately twice per year, and the DSMB recommended that the study continue at all interim meetings.
All interim and final analyses on the endpoints of resident-physician-related and unit-wide SMEs were performed by an unblinded statistician at the DCC. Interim analyses occurred after the completion of the first wave, and again after completion of the second and third wave. These results were reviewed by the DSMB for assessing benefit or harm. Lan-DeMets methods were used for defining symmetric stopping boundaries [23]. The DSMB was to consider stopping the study early for efficacy, safety or futility.
2.9. Classification of the primary outcome
All clinical types of potential medical errors were included in the study, including medication-related, procedure-related, diagnostic test-related, therapy-related, and nosocomial infections, among others.
An intensive four-pronged approach was used to gather data on potential medical errors in a prospective manner. A team of physician observers conducted direct observation of resident-physicians, documenting all potential medical errors. Forms were made available for voluntary reporting of possible errors by nurses, residents, and other clinic staff. Formal hospital incident reports were collected and charts were reviewed. Information collected for each potential medical error included National Coordinating Council for Medication Error Reporting (NCCMERP) Index harm level, whether it was preventable, clinical category of the incident, and whether the potential medical error was made by a resident-physician. A detailed narrative description of the potential medical error was also gathered.
After these prospective data on potential medical errors were collected, final classification of all incidents was subsequently carried out by physician reviewers, who underwent centralized training. All potential medical errors were assigned by the DCC for examination by two physician reviewers, with 4 different pairings of reviewers. Physician reviewers received all information about the incident, including the harm level and whether the event was preventable based on the initial report. Physician reviewers also received information about the patient (age, gender, length of stay), and were blinded as to whether the incident happened during the EDWR or RCWR arm of the study.
Physician reviewers classified each potential medical error as one of the following:
adverse event/harm, defined as any harm due to medical care (or the absence of medical care)
A “near miss” (aka a potential adverse event), an error with potential for harm (error defined as something that goes wrong in the care delivery process, whether it causes harm or not)
error with little or no potential for harm
exclusion, defined as any incident reported by site staff that does not meet one of the above definitions (e.g., neither an adverse event nor an error)
If classified as adverse event/harm, the reviewer further classified the incident for harm level and whether or not the incident was preventable, as:
Harm level: (according to the modified NCC-MERP scale):
E. Temporary harm to the patient
F. Temporary harm to the patient and required prolonged hospitalization
G. Permanent patient harm
H. Intervention required to sustain life
I. Patient death
Was the incident preventable?
Definitely preventable
Probably preventable
Probably not preventable
Definitely not preventable
Discordant initial reviews were resolved via periodic teleconferences of paired reviewers. Agreement of the physician reviewers was examined on a monthly basis. Agreement of the initial reviews for event classification were moderate (>0.4 to 0.6) to substantial (>0.6 to 0.8). The weighted kappa (95% confidence interval) for the initial reviews of the 4 reviewer pairs were 0.59 (0.55, 0.63), 0.61 (0.58, 0.64), 0.67 (0.66, 0.69), and 0.52 (0.48, 0.56).
The primary outcome was the rate per 1000-patient days of resident-physician-related SMEs, with SME defined as a potential medical error classified as an adverse event determined to be definitely or probably preventable or a near miss. These SMEs were classified as resident-physician-related if the study staff member who initially reported the potential medical error (physician observer or research nurse) noted that the position of the provider who made the error was a resident-physician. A secondary outcome was the rate per 1000-patient days of all unit-wide SMEs, i.e., those that involved resident-physicians and those that did not.
2.10. Deviations from protocol or changes to methods after implementation
The resident-physician enrollment option of “observation only” was added during the first wave to allow resident-physicians to enroll in the study who were willing to be shadowed by physician observers but did not want to provide any other resident-physician-specific data, e.g., sleep/work diaries, actigraphy, or performance testing.
The tracking of missed physician observer shifts was added during wave 1, schedule 1 in July 2014. The missed shifts from wave 1, schedule 1 had to be entered retrospectively.
All data needed to compute IPRP was not collected in a database during the course of the study. The average daily resident-physicians on unit was estimated retrospectively, allowing only for a crude estimate per site and schedule rather than an estimate for each day of the schedule. This estimate did not include other ICU staff caregivers.
One of the original sites that had planned to participate in wave 2 of the study had a change of leadership before embarking on the study, and the new leadership did not support proceeding with the study. Another hospital was selected as the replacement site, but this change delayed the expected start time by 6 months (Table 2).
Although the sites were required to have a research nurse on staff, one site did not have this position filled for most of the first schedule (their EDWR arm). Other sites fell below the minimum of five physician observers at times. While each site was meant to have coverage from a physician observer at all times, there were some exceptions on a day to day basis and systematically some sites did not schedule coverage for some holidays. The lack of coverage of physician observers was tracked.
One PICU underwent a renovation during the first six months of their schedule 2 (their RCWR arm). During the renovation, the PICU beds were divided between two locations. Data collection was put on hold for approximately 3 weeks during this schedule over the winter holidays and while the PICU moved back into its renovated space. Schedule 2 was extended to account for the break in data collection. Prior to schedule 2, study investigators from the DCC and CCC visited the site to determine the impact of the PICU move, and felt satisfied that the study could proceed through the renovation period. The form capturing data regarding potential medical errors was modified to indicate the specific location of the event to allow investigators to later explore if the location of the temporary beds had an impact on potential medical error rates.
Drowsiness while driving to and from work was initially planned to be measured objectively using non-invasive, validated technology that uses infrared oculography to monitor alertness continuously. Resident-physicians who wore prescription eye glasses or had a commute shorter than 20 minutes were ineligible for this measurement. Too many resident-physicians met these exclusion criteria (75%), so use of this technology was discontinued during wave 2 and the drive diary was used to assess self-reported drowsiness while driving to and from work.
Initially, there were nine physician reviewer pairs who were to classify the potential medical errors. These physician reviewers were selected from each of the six sites. The larger number of reviewer pairs made it more difficult to manage data classification and to ensure timeliness and consistency. The levels of agreement of initial reviews within these nine reviewer pairs was low (weighted kappas in the 0.3 to 0.4 range). In December 2015, a new system for review was implemented. A set of four dedicated reviewers, all located in Boston, were trained. These reviewers re-adjudicated all prior potential medical error reviews as well as all new incoming potential medical errors. The agreement between reviewers improved (weighted kappas 0.52 to 0.67).
3. Results
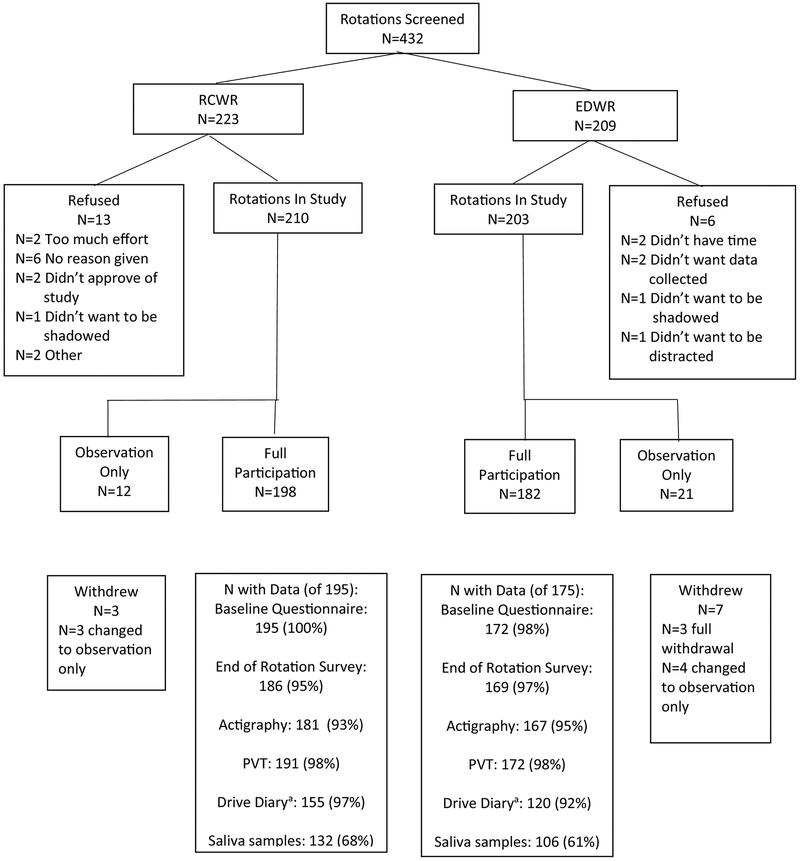
There were 413 individual resident-physician rotations included in data collection during the study, exceeding the goal of 300 (Figure 1). The majority of resident-physicians agreed to full participation in the study (n=380 rotations covered), and 33 (8%) rotations covered were observation only. These 413 rotations had 336 unique resident-physicians, with 58 enrolled twice, five enrolled three times and three enrolled four times (Table 4). The average rotation length was 28.2 days. Within site, the average rotation length did differ by schedule for three sites (p<0.05) but did not differ when all sites were combined (Table 5). The resident-physicians who participated were on average 29.4 ± 2.3 years of age, and predominantly white (81.2%) and female (62.3%) (Table 6). The majority noted their specialty as pediatrics (86.4%), and most (64.7%) were in their second year of residency. The nineteen resident-physicians who did not agree to participate were similar to those in the study in age, gender, race, ethnicity and specialty. Those who refused participation were less likely to be in their third year of residency than those who participated (10.5% vs. 35.3%, respectively, p=0.03).
Figure 1.
Resident-physician Rotation Recruitment and Data Completeness
a Only required of those who drove to or from work.
Table 4.
Description of resident-physicians enrolled in the study multiple times.
| Total | A | B | C | D | E | F | |
|---|---|---|---|---|---|---|---|
| Total Rotations | 413 | 74 | 57 | 71 | 76 | 68 | 67 |
| Repeat enrollments, n (%) | 77 (19) | 16 (22) | 3 (5) | 8 (11) | 13 (17) | 30 (44) | 7 (11) |
| Unique resident-physicians | 336 | 58 | 54 | 63 | 63 | 38 | 60 |
| Unique resident-physicians enrolled >1 time | 66 | 16 | 3 | 8 | 12 | 20 | 7 |
| Times resident-physicians enrolled | |||||||
| 1 | 270 | 42 | 51 | 55 | 51 | 18 | 53 |
| 2 | 58 | 16 | 3 | 8 | 11 | 13 | 7 |
| 3 | 5 | 0 | 0 | 0 | 1 | 4 | 0 |
| 4 | 3 | 0 | 0 | 0 | 0 | 3 | 0 |
| Post Graduate Year (if known), n(%)a | |||||||
| With the same PGY | 26 (39) | 15 (94) | 2 (67) | 0 | 2 (17) | 7 (35) | 0 |
| With a different PGY | 32 (49) | 0 | 0 | 8 (100) | 6 (50) | 12 (60) | 6 (86) |
| By Schedule, n (%)a | |||||||
| With different schedules | 27 (41) | 0 | 0 | 3 (38) | 7 (58) | 13 (65) | 4 (57) |
| All EDWR schedule | 23 (35) | 7 (44) | 3 (100) | 4 (50) | 4 (33) | 3 (15) | 2 (29) |
| All RCWR schedule | 16 (24) | 9 (56) | 0 | 1 (12) | 1 (8) | 4 (20) | 1 (14) |
| Participation type, n (%)a | |||||||
| All full participation | 58 (88) | 15 (94) | 2 (67) | 8 (100) | 8 (67) | 19 (95) | 6 (86) |
| All observation only | 2 (3) | 0 | 1 (33) | 0 | 0 | 1 (5) | 0 |
| Both types | 6 (9) | 1 (6) | 0 | 0 | 4 (33) | 0 | 1 (14) |
Abbreviations: PGY, post graduate year; EDWR, extended duration work roster; RCWR, rapidly cycling work roster.
For those n=66 resident-physicians with repeat enrollment.
Abbreviations: PGY, post graduate year; EDWR, extended duration work roster; RCWR, rapidly cycling work roster.
Table 5.
Recruitment by site and schedule.
| Rotations | Rotations | Rotation | ||
|---|---|---|---|---|
| Screened | Enrolled | Length | ||
| Site | Schedule | N | N (%) | mean ± SD |
| A | EDWR | 36 | 31 (86.1)a | 29.9 ± 1.9b |
| RCWR | 43 | 43 (100) | 28.0 ± 4.2 | |
| B | EDWR | 32 | 32 (100)a | 27.8 ± 0.4b |
| RCWR | 37 | 25 (67.6) | 28.8 ± 1.9 | |
| C | EDWR | 36 | 35 (97.2) | 28.6 ± 2.7 |
| RCWR | 36 | 36 (100) | 28.3 ± 3.4 | |
| D | EDWR | 37 | 37 (100) | 26.5 ± 4.8 |
| RCWR | 39 | 39 (100) | 24.1 ± 7.2 | |
| E | EDWR | 34 | 34 (100) | 27.3 ± 1.8b |
| RCWR | 35 | 34 (97.1) | 28.9 ± 2.2 | |
| F | EDWR | 34 | 34 (100) | 30.9 ± 2.9 |
| RCWR | 33 | 33 (100) | 30.7 ± 2.1 | |
| All | EDWR | 209 | 203 (97.1) | 28.5 ± 3.2 |
| Sites | RCWR | 223 | 210 (94.2) | 28.0 ± 4.6 |
| Total | 432 | 413 (95.6) | 28.2 ± 3.9 |
p<0.05 comparing refusal rate between schedules (chi-square test or a Fisher’s exact test).
p<0.05 comparing rotation length between schedules (t-test).
Abbreviations: EDWR, extended duration work roster; RCWR, rapidly cycling work roster; SD, standard deviation.
Table 6.
Resident-physician rotation characteristics by participation.
| Refused | Enrolled | P-value | |
|---|---|---|---|
| Characteristic | (N=19) | (N=380) | |
| Gender | 0.59 | ||
| Female | 13 (68.4) | 233 (62.3) | |
| Male | 6 (31.6) | 141 (37.7) | |
| Age, years | 30.4 ± 4.4 | 29.4 ± 2.3 | 0.54 |
| Race | 0.10 | ||
| White | 7 (70.0) | 303 (81.2) | |
| Black | 2 (20.0) | 15 (4.0) | |
| Asian | 0 | 31 (8.3) | |
| More than one race | 1 (10.0) | 15 (4.0) | |
| Other | 0 | 9 (2.4) | |
| Ethnicity | 0.46 | ||
| Hispanic/Latino | 1 (111) | 23 (6.4) | |
| Not Hispanic/Latino | 8 (88.9) | 338 (93.6) | |
| Year of residency program | 0.03 | ||
| PGY2 | 17 (89.5) | 242 (64.7) | |
| PGY3 | 2 (10.53) | 132 (35.3) | |
| Specialty | 0.11 | ||
| Pediatrics | 16 (84.2) | 323 (86.4) | |
| Internal medicine/pediatrics | 2 (10.5) | 8 (2.1) | |
| Family practice | 0 | 0 | |
| Anesthesiology | 0 | 14 (3.7) | |
| Emergency medicine | 0 | 21 (5.6) | |
| Other | 1 (5.3) | 8 (2.1) | |
| Marital statusa | |||
| Married | 207 (55.4) | ||
| Separated | 0 | ||
| Divorced | 1 (0.3) | ||
| Widowed | 0 | ||
| Never married | 166 (44.4) | ||
| Body mass index, kg/m2a | 23.2 ± 3.4 |
Data shown as n(%) or mean ± SD
P-values from a chi-square test, Fisher’s exact test, or t-test.
Not gathered among those who refused.
Abbreviations: PGY, post graduate year; SD, standard deviation.
Of the 380 rotations with full participation, seven withdrew from full participation but agreed to remain in the study as observation only, and three withdrew completely from the study (Figure 1). The most common reasons for withdrawal were unwillingness to complete the diaries (n=2) and unwillingness to complete the PVT testing (n=3). There were two SAEs that occurred to resident-physicians, both of which were determined to be unrelated to the study (one on EDWR, one on RCWR). Three resident-physicians reported thoughts of suicide during the RCWR, none during the EDWR. Almost all questionnaire data was completed (99% baseline questionnaire, 96% end-of-rotation survey). Almost all had some actigraphy (94%) and PVT (98%) data collected. Fewer agreed to saliva collection (64%). The completion of the drive diary differed by schedule type (97% RCWR, 92% EDWR, p=0.046). Rates of refusals, full participation, withdrawals, and all other types of data completeness did not differ by schedule type (p>0.05).
The study involved 6577 patients (3267 during the EDWR, 3310 during the RCWR), representing 7099 admissions (3508 during the EDWR, 3591 during the RCWR). (Table 7). Data were collected over a total of 2870 days among six PICUs, for a total of 38821 patient-days (18749 during the EDWR, 20072 during the RCWR). Patients were on average 7.2 ± 6.7 years of age, with slightly more male than female patients (53.5% vs. 46.5%, respectively) (Table 7). The median length of unit stay was 2 days (range 1 to 244 days). There were no statistically significant differences of patient characteristics across schedule type.
Table 7.
Patient characteristics by schedule.
| Characteristic | Total | EDWR | RCWR | P-value |
|---|---|---|---|---|
| Number of patients | 6577 | 3267 | 3310 | 0.60 |
| Number of unit admissions | 7099 | 3508 | 3591 | 0.32 |
| Number of patient-days | 38821 | 18749 | 20072 | 0.20 |
| age, year, mean ± SD | 7.2 ± 6.7 | 7.3 ± 6.7 | 7.1 ± 6.6 | 0.14 |
| Gender, n (%) | ||||
| Female | 3303 (46.5) | 1655 (47.2) | 1648 (45.9) | 0.27 |
| Male | 3796 (53.5) | 1853 (52.8) | 1943 (54.1) | |
| Length of unit stay, days | ||||
| mean ± SD | 5.5 ± 11.9 | 5.3 ± 11.4 | 5.6 ± 12.3 | 0.20 |
| median (inter quartile range) | 2 (2, 5) | 2 (2, 5) | 2 (2, 5) | |
| Patient Chronic Condition Indexa, range 0–18 | ||||
| mean ± SD | 2.6 ± 2.0 | 2.6 ± 1.9 | 2.6 ± 2.0 | |
| median (inter quartile range) | 2 (1, 4) | 2 (1, 4) | 2 (1, 4) | 0.53 |
p-values from a chi-square test or Wilcoxon rank-sum test.
Developed by Agency for Healthcare Research and Quality. Higher scores denote more chronic conditions.
Abbreviations: EDWR, extended duration work roster; RCWR, rapidly cycling work roster; SD, standard deviation.
Of the 2870 days covered by the study at all sites, a total of 345 days (12%) were not covered by a physician observer (summing hours with lack of coverage/24). (Table 2) Of these, 198.2 were in the EDWR, 146.8 in the RCWR (p<0.001).
4. Discussion
Results from data collected in the ROSTERS study will be evaluated for the impact of changes in pediatric resident-physician work schedules on patient outcomes in PICUs, together with intermediary variables such as sleep, performance, health and safety of individual resident-physicians. The study will also allow for a number of secondary analyses, such as further examination of SME rates across the variety of schedule types that were implemented, examination of specific types of harm level and category of events (i.e. medication-related, procedure-related). The data collected allow for adjustment of analyses for potential confounding factors, including resident-physician characteristics, such as experience level, and volume characteristics, such as the number of patients per resident-physician. Analyses can also be adjusted for patient-level characteristics, such as comorbidity index.
The secondary outcomes collected allow for examination of the impact of schedule on additional resident-physician-level outcomes, such as depression, MVCs, vigilance, sleepiness, and sleep duration and work quality.
Including six sites of varying size from around the country will improve the generalizability of findings to other PICUs, particularly in the academic setting.
Strengths of the study include the large sample size, the cross over design to minimize local effects, the geographic variation of the sites, and the collection of data on a number of levels (patient, resident-physician, medical errors). Limitations of the study include the lack of data collection on handovers of care, retrospective measurement of patient load (IPRP), and limited data gathered on patient complexity (ICD-9 and ICD-10 codes). The lack of these data limits the measurement of the impact of resident-physician workload and stress level on patient outcomes. Physician reviewers were given the harm level and whether or not the potential event was considered preventable by the initial reporter, which may have biased their decisions for classification of the primary outcome.
There were challenges to conducting this study. As discussed above, the difference in the EDWR scheduling and staffing of the study sites led to variations in the manner in which the RCWR schedule was implemented across sites. The frequency of resident-physician extended shifts, resident-physician staffing, IPRP, and the manner in which handovers of care were conducted varied. Any of these factors could potentially have modified the effects of the RCWR schedule. While efforts were made to standardize certain features of the implementation schedule (e.g., avoiding recurrent night shifts, ensuring sufficient days off each month), the differences in site characteristics, as well as the manner in which site PIs and program directors chose to implement the schedule within CCC and DCC guidelines could have had important effects. Analyzing site-specific differences in the performance of the RCWR schedule, and exploring possible contributors to any differences, will thus be of importance in interpreting the results of the trial.
The intensive data collection requirements of the study also posed challenges. These requirements made it difficult to find sites that were willing and able to participate in the study. The data collection was done in three waves due to annual budget restrictions, which required additional staff time from the DCC and CCC. Given that potential medical errors were collected by a combination of methods (direct observation, chart review, etc.) there was a possibility for duplicate reporting requiring additional diligence and training of physician observers and DCC staff to detect and remove duplicates from the database. There were some failures with the actigraph equipment leading to loss of data, although in most cases back-up devices were available to minimize data loss. While almost all resident-physicians have some PVT recording data, difficulty scheduling resident-physician test times led to some incomplete data collection. The design of the electronic diary data collection website allowed for duplicate data entry, so additional time was spent processing these data to remove overlapping or duplicate information. About 12% of study time was not covered by a physician observer.
The data collection was conducted over four years. In that time a few studies have examined 2011 work hour reforms for interns (PGY1). An examination of the medical records of Medicare beneficiaries the year before the 2011 ACGME work hour reforms and a year after revealed no significant difference in 30-day mortality or readmission rates by level of intensity of teaching [15]. Another study enrolled 2323 interns at 14 teaching hospitals before and after the 2011 reforms. Interns after the reforms did report working fewer hours but did not report any increases in sleep or decreases in rates of depressive symptoms. The rate of self-reported concern about making medical errors increased after the reform [14]. A study comparing a 2003-compliant model to the 2011 model among 43 interns at one teaching hospital did show increased sleep duration along with an increase in handovers of care and less educational opportunities [13]. These studies did not include any PGY2 or PGY3 resident-physicians, and did not have adjudicated medical errors or objectively measured sleep. One large trial found no difference in operative mortality and major complication rates when surgical resident-physicians abided by the 2011 ACGME rules, as compared with when they did not. This study did include PGY2+ resident-physicians (73%), but results for patient-related outcomes were not presented stratified by PGY [16]. The iCOMPARE trial (Individualized Comparative Effectiveness of Models Optimizing Patient Safety and Resident Education trial) was conducted in 63 internal medicine residency programs in the United States during 2015–2016. Residency programs underwent cluster randomization to a schedule following the 2011 ACGME standards, or a schedule that permitted more flexible duty hours (removing the 16-hour restriction on shift length). Comparing outcomes from the prior year to the trial year, allowing flexible schedules did not adversely affect 30-day mortality or several other measured outcomes of patient safety [24].
5. Conclusions
Data collection for the ROSTERS trial was successfully completed in March 2017, including resident-physician level data and patient level data. Classification of potential medical errors was completed in October 2017. Resident-physician enrollment exceeded the goal of 300. Number of patient-days observed were less than expected (84%) based on projections. The study design allows for analyses on a number of aims, which are ongoing.
Acknowledgements
We thank the Data Safety and Monitoring Board members for their oversight: Donald L. Bliwise, PhD; Barry Markovitz, MD, MPH; Eva Petkova, PhD; Wasima N. Rida, PhD; and Ramesh Sachdeva, MD, PhD, MBA, JD.
We thank the National Heart, Lung, and Blood Institute for their support: Carol J. Blaisdell, MD; Peyvand Ghofrani, MDE, CCRA; Lora A. Reineck, MD, MS; Robert A. Smith, PhD, FCCM; Michael Twery, PhD; Gail G. Weinmann, MD; and Colin O. Wu, PhD.
Thank you also to the resident physicians, attending physicians, nurses, and clinical pharmacists of the participating Pediatric Intensive Care Units for their ongoing support.
Thank you to the following ROSTERS team members:
Clinical Coordinating Center: Justin D. Buie, BS; and Joshua T. Stephens, BS.
Data Coordinating Center: Lynn Harvey, BS and Vicki Li, BS.
Colorado: Gentle Arnez; Bradley Brainard, MBA, MHA, PMP; Tristan Bakke Dear, MS, MD; Rui Fang; Tondeleyo Gonzalez, MA, BSN, RN; Jonathan D. Haywood, MD, MPH; Omar W. Hendi; Heather Hoch, MD, MSCS; Brian M. Jackson, MD, MA; Ayoub Lahlou, MD, MBA; Kathryn J. Yucha, MSN, BSN; Karen Meyer, RN, BSN, CPN; Maryam Nuriyeva; Tolulope Oyewumi, MD, MPH; Kimberly Ralston, MPH, BSN, RN; Nabeel Sawaged, MD; Beth E. Smith, MSN, RN, WHNP-BC; Vanitha K. Varre, MBBS, MPH, MPS; and Gaurav Yadav.
Iowa: Rasha Abdalla, MD; Safa Abukhalil, MD; Ihab Ahmed, MBBS, MPH; Safa Ahmed, MBBS; Shilpa C. Balikai, DO; Maria Ana C. Canaya-Voskov, MD; Alaa Hamed, MD, MPH, MBA; Janice M. Jeter, RN, CCRC; Sameer Kamath, MBBS; Crystal Tuley, RN; Jessica G. Moreland, MD; Vani C. Movva, MBBS; Geoffrey Ounda Obel, MPH, MD; Angie Platt, BSN, RN; Thomas D. Scholz, MD; Ruthann Schrock, BSN, RN; Amy Stier, MD, MME; Alexandra Paige Davis Volk, MD; and Jin Zhou, RN, MNHP.
Massachusetts: Oluwafunmilola Alabi, MD; Joseph Asemota, MD, MPH; Abimbola Chris-Olaiya, MD, MPH; Virginia Leon, BSN, MEd, CCRN; Alexandra Male, MPH; Siyu Ma, MS; Adeolu O. Oladunjoye, MBChB, MPH; Olubunmi O. Oladunjoye, MBBS, MPH; Saki Onda, MD, MPH; Kimberly Ralston, MPH, BSN, RN; Bhavya Atul Shah, MBBS; Lisa Tse, MPH; and Sandra Wooldridge, BSN, RN.
Ohio: Juanita Dudley, BSN, RN; Tatiana Elson, BS; Michael Jarman, MD; Narinderpal Kaur, MD; Samuel Lee, MD, MBA; Najima Mwase, MD; Narissa Puran, MD, MS, MPH; Jennifer Ross, MD; Ndidi Unaka, MD, MEd; Andrew M. Warner, MD; Robin Widing, RN, MSN, CCRP; and Hector R. Wong, MD.
Virginia: Indu Aggarwal, MD; Fatimah Begom, MBBS; Kateryna Bilanovych, MD, ND, PhD, BCMAS; Ashley C. Eason, MD; Nasir Farhat, MD; David Finkler, MD; Nicole M. Frank, PA-C; Farida Ibrahim, MD; Robin L. Kelly, BSN, RN; Muhammad Khan, MD; Jan-Pablo Kollmar, MD; Evan B. Kudron, MD; Abigail V.W. Kumral, MD; Jeffrey Li, MD; Brock D. Libby, MD; Jules Mukunde Katotola, MD; Linda Okai, MD; Trevor Pollock, MD; Justin Rizer, MD; Isaac A. Shields, MD; Benjamin Simson, MD; Terrell D. Smith, BS; Carolyn Spilman, MS, RN, CPNP; Albert T. Tang, MD; Linlin Wang, MD, PhD; Stephanie Waterhouse, MD; Weonpo Yarl, MD; Hong Zhu, RN, CCRC; and Jenna V. Zschaebitz, BS.
Washington: Mouammar M. Abouagila, MBBS; Ibrahim K. Abukenda, MBChB, MPH; Canan Akture, MD, CCRC; Jennifer Jane Gile, BS; Richa Kashayap, MD; Carol Mendivil, MD; Anas L. Najjar, MS; Gowri Rajendran, MBBS; Shahar Robinzon, MD, MSc; Erin M. Sullivan, MPH; and Nastassya West, BS.
Other: David Gozal, MD; Leila Kheirandish-Gozal, MD; and Sharon M. Unti, MD.
Funding Support
Randomized Order Safety Trial Evaluating Resident-physician Schedules (ROSTERS) is supported by National Heart, Lung, and Blood Institute (U01-HL-111478 and U01-HL-111691). Drs. Barger, Lockley, and Czeisler were supported in part by funding from the National Institute of Occupational Safety and Health R01-OH-010300.
Role of the Funder
The funder was not involved in the study design, data collection, or analysis of results.
Financial Disclosures for Authors
Dr. Barger is on the scientific advisory board for CurAegis Technologies. She has received consulting fees from University of Pittsburgh, Sygma, Insight and Puget Sound Pilots.
Ms. Blackwell reports grants from NIH during the conduct of the study; grants from Merck Sharp & Dohme Corp, outside the submitted work.
Dr. Czeisler reports grants from Cephalon Inc., Ganésco Inc., Jazz Pharmaceuticals Plc., Inc., National Football League Charities, Optum, Philips Respironics, Inc., Regeneron Pharmaceuticals, ResMed Foundation, San Francisco Bar Pilots, Sanofi S.A., Sanofi-Aventis, Inc, Schneider Inc., Sepracor, Inc, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Sysco, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries, Ltd., and Wake Up Narcolepsy; and personal fees from Bose Corporation, Boston Celtics, Boston Red Sox, Cephalon, Inc., Columbia River Bar Pilots, Institute of Digital Media and Child Development, Klarman Family Foundation, Samsung Electronics, Quest Diagnostics, Inc, Vanda Pharmaceuticals, Washington State Board of Pilotage Commissioners, Zurich Insurance Company, Ltd. In addition, Dr. Czeisler holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker), and holds an equity interest in Vanda Pharmaceuticals, Inc. Since 1985, Dr. Czeisler has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms including those involving the following commercial entities: Casper Sleep Inc., Comair/Delta Airlines, Complete General Construction Company, FedEx, Greyhound, HG Energy LLC, Purdue Pharma, LP, South Carolina Central Railroad Co., Steel Warehouse Inc., Stric-Lan Companies LLC, Texas Premier Resource LLC and United Parcel Service (UPS). Dr. Czeisler receives royalties from the New England Journal of Medicine, McGraw Hill, Houghton Mifflin Harcourt/Penguin, and Philips Respironics, Inc. for the Actiwatch-2 and Actiwatch-Spectrum devices. Dr. Czeisler’s interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Dr. Halbower reports she has a patent In-Ear Sensing Systems and Methods for Biological Signal Monitoring pending.
Mrs. Kriesel reports no conflicts.
Dr. Landrigan reports grants from Patient-Centered Outcomes Research Institute, during the conduct of the study; personal fees and other from I-PASS Patient Safety Institute, personal fees from Children’s Hospital Association, personal fees from Virgin Pulse, outside the submitted work.
Dr. Lockley reports commercial interests from the last 3 years (2015–2018), unrelated to the work, that are listed below. Dr. Lockley has received consulting fees from the Atlanta Falcons, Atlanta Hawks, Delos Living LLC, Noble Insights, OpTerra Energy Services Inc., Pegasus Capital Advisors LP, Serrado Capital, Slingshot Insights and Team C Racing. He has current consulting contracts with Akili Interactive, Apex 2100 Ltd, BHP Billiton, Consumer Sleep Solutions, Headwaters Inc., Hintsa Performance AG, Light Cognitive, Lighting Science Group Corporation, Mental Workout, McCullough Hill Leary PS, Paul, Weiss, Rifkind, Wharton & Garrison LLP, PlanLED, Six Senses, Stantec and Wyle Integrated Science and Engineering. Dr. Lockley has received unrestricted equipment gifts from Biological Illuminations LLC, Bionetics Corporation and F.LUX Software LLC; has equity in iSLEEP, Pty; royalties from Oxford University Press; honoraria plus travel, accommodation and/or meals for invited seminars, conference presentations or teaching from BHP Billiton, Estee Lauder, Informa Exhibitions (USGBC), and Teague; travel, accommodation and/or meals only (no honoraria) for invited seminars, conference presentations or teaching from IES, Lightfair, USGBC, DIN and SLTBR. Dr. Lockley has completed investigator-initiated research grants from Biological Illumination LLC and has an ongoing investigator-initiated grant from F. Lux Software LLC; He is a Program Leader for the non-profit CRC for Alertness, Safety and Productivity, Australia, through an adjunct faculty position at Monash University and unpaid Member of the Scientific Advisory Board for the non-profit Midwest Lighting Institute. Dr. Lockley holds a process patent for ‘Systems and methods for determining and/or controlling sleep quality’, which is assigned to the Brigham and Women’s Hospital per Hospital policy. Dr. Lockley has also served as a paid expert in legal proceedings related to light and health.
Mr. O’Brien reports no conflicts.
Dr. Poynter reports no conflicts.
Dr. Rahman holds patents for prevention of circadian rhythm disruption by using optical filters and improving sleep performance in subjects exposed to light at night. Dr. Rahman owns equity in Melcort Inc. and has provided paid consulting services to Sultan & Knight Limited, Bambu Vault LLC. Dr. Rahman has received honoraria as an invited speaker and travel funds from Starry Skies Lake Superior, University of Minnesota Medical School, PennWell Corp., Seoul Semiconductor Co. LTD.
Dr. Stone reports grants from Merck & Co., outside the submitted work.
Mr. Sullivan reports no conflicts.
Dr. Wright Jr. reports grants from National Institutes of Health, during the conduct of the study; personal fees from Circadian Therapeutics, LTD, grants, personal fees, non-financial support and other from CurAegis Technologies, personal fees from Kellogg’s, non-financial support from Somalogic, Inc, personal fees from American Academy of Sleep Medicine, personal fees from American College of Chest Physicians, personal fees from American College of Sports Medicine, personal fees from, American Diabetes Association, personal fees from Associated Professional Sleep Societies, grants from Philips Inc, personal fees from Obesity Medicine Association, outside the submitted work.
Ms. Viyaran reports no conflicts.
Dr. Vittinghoff reports personal fees from California Pacific Medical Center, during the conduct of the study.
Dr. Yu reports no conflicts.
Dr. Zee reports grants from National Institutes of Health, during the conduct of the study; personal fees from Merck, grants and personal fees from Eisai, grants and personal fees from Philips, personal fees from Sanofi, grants from Jazz, grants from Technogel, grants and personal fees from Harmony Biosciences, grants from Apnimed, grants from X – a Division of Alphabet, Inc., other from Teva, outside the submitted work; In addition, Dr. Zee has a patent U.S. Serial Nos: 62/038,700 & PCT/US2015/045273 pending, and a patent U.S. Serial No: 62/515,361 pending.
List of abbreviations:
- ACGME
Accreditation Council for Graduate Medical Education
- CCC
Clinical Coordinating Center
- DCC
Data Coordinating Center
- DSMB
Data Safety and Monitoring Board
- EDWR
Extended duration work roster
- ICD
International Classification of Diseases
- IRB
Institutional Review Board
- MVC
Motor vehicle crash
- NCC-MERP
National Coordinating Council for Medication Error Reporting
- NHLBI
National Heart, Lung, and Blood Institute
- PGY
Post graduate year
- PI
Principal Investigator
- PICU
Pediatric intensive care unit
- PVT
Psychomotor vigilance test
- RCWR
Rapidly cycling work roster
- ROSTERS
Randomized Order Safety Trial Evaluating Resident-physician Schedules
- SAE
Serious adverse event
- SME
Serious medical errors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT02134847
References
- [1].Goel N, Basner M, Rao H, Dinges DF. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013;119:155–90. doi: 10.1016/B978-0-12-396971-2.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Landrigan CP, Rothschild JM, Cronin JW, Kaushal R, Burdick E, Katz JT, Lilly CM, Stone PH, Lockley SW, Bates DW, Czeisler CA. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004. October 28;351(18):1838–48. [DOI] [PubMed] [Google Scholar]
- [3].Barger LK, Cade BE, Ayas NT, Cronin JW, Rosner B, Speizer FE, Czeisler CA; Harvard Work Hours, Health, and Safety Group. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005. January 13;352(2):125–34. [DOI] [PubMed] [Google Scholar]
- [4].Lockley SW, Cronin JW, Evans EE, Cade BE, Lee CJ, Landrigan CP, Rothschild JM, Katz JT, Lilly CM, Stone PH, Aeschbach D, Czeisler CA; Harvard Work Hours, Health and Safety Group. Effect of reducing interns’ weekly work hours on sleep and attentional failures. N Engl J Med. 2004. October 28;351(18):1829–37. [DOI] [PubMed] [Google Scholar]
- [5].Ayas NT, Barger LK, Cade BE, Hashimoto DM, Rosner B, Cronin JW, Speizer FE, Czeisler CA. Extended work duration and the risk of self-reported percutaneous injuries in interns. JAMA. 2006. September 6;296(9):1055–62. [DOI] [PubMed] [Google Scholar]
- [6].Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Laparoscopic performance after one night on call in a surgical department: prospective study. BMJ. 2001. November 24;323(7323):1222–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barger LK, Ayas NT, Cade BE, Cronin JW, Rosner B, Speizer FE, Czeisler CA. Impact of extended-duration shifts on medical errors, adverse events, and attentional failures. PLoS Med. 2006. December;3(12):e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. JAMA. 2005. September 7;294(9):1025–33. [DOI] [PubMed] [Google Scholar]
- [9].Philibert I Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Sleep. 2005. November;28(11):1392–402. [DOI] [PubMed] [Google Scholar]
- [10].Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. Washington, DC: Institute of Medicine; 2008. [Google Scholar]
- [11].Iglehart JK. Revisiting duty-hour limits--IOM recommendations for patient safety and resident education. N Engl J Med. 2008. December 18;359(25):2633–5. doi: 10.1056/NEJMp0808736. [DOI] [PubMed] [Google Scholar]
- [12].Accreditation Council for Graduate Medical Education Common Program Requirements. 2011; https://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed 10/4/2018.
- [13].Desai SV, Feldman L, Brown L, Dezube R, Yeh HC, Punjabi N, Afshar K, Grunwald MR, Harrington C, Naik R, Cofrancesco J Jr. Effect of the 2011 vs 2003 duty hour regulation-compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013. April 22;173(8):649–55. doi: 10.1001/jamainternmed.2013.2973. [DOI] [PubMed] [Google Scholar]
- [14].Sen S, Kranzler HR, Didwania AK, Schwartz AC, Amarnath S, Kolars JC, Dalack GW, Nichols B, Guille C. Effects of the 2011 duty hour reforms on interns and their patients: a prospective longitudinal cohort study. JAMA Intern Med. 2013. April 22;173(8):657–62; discussion 663. doi: 10.1001/jamainternmed.2013.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patel MS, Volpp KG, Small DS, Hill AS, Even-Shoshan O, Rosenbaum L, Ross RN, Bellini L, Zhu J, Silber JH. Association of the 2011 ACGME resident duty hour reforms with mortality and readmissions among hospitalized Medicare patients. JAMA. 2014. December 10;312(22):2364–73. doi: 10.1001/jama.2014.15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bilimoria KY, Chung JW, Hedges LV, Dahlke AR, Love R, Cohen ME, Hoyt DB, Yang AD, Tarpley JL, Mellinger JD, Mahvi DM, Kelz RR, Ko CY, Odell DD, Stulberg JJ, Lewis FR. National Cluster-Randomized Trial of Duty-Hour Flexibility in Surgical Training. N Engl J Med. 2016. February 25;374(8):713–27. doi: 10.1056/NEJMoa1515724. [DOI] [PubMed] [Google Scholar]
- [17].Rajaram R, Chung JW, Jones AT, Cohen ME, Dahlke AR, Ko CY, Tarpley JL, Lewis FR, Hoyt DB, Bilimoria KY. Association of the 2011 ACGME resident duty hour reform with general surgery patient outcomes and with resident examination performance. JAMA. 2014. December 10;312(22):2374–84. doi: 10.1001/jama.2014.15277. Erratum in: JAMA. 2015 Jan 27;313(4):422. [DOI] [PubMed] [Google Scholar]
- [18].Donchin Y, Gopher D, Olin M, Badihi Y, Biesky M, Sprung CL, Pizov R, Cotev S. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995. February;23(2):294–300. [DOI] [PubMed] [Google Scholar]
- [19].Rothschild JM, Landrigan CP, Cronin JW, Kaushal R, Lockley SW, Burdick E, Stone PH, Lilly CM, Katz JT, Czeisler CA, Bates DW. The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med. 2005. August;33(8):1694–700. [DOI] [PubMed] [Google Scholar]
- [20].Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, Goldmann DA. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001. April 25;285(16):2114–20. [DOI] [PubMed] [Google Scholar]
- [21].DeRienzo CM, Frush K, Barfield ME, Gopwani PR, Griffith BC, Jiang X, Mehta AI, Papavassiliou P, Rialon KL, Stephany AM, Zhang T, Andolsek KM; Duke University Health System Graduate Medical Education Patient Safety and Quality Council. Handoffs in the era of duty hours reform: a focused review and strategy to address changes in the Accreditation Council for Graduate Medical Education Common Program Requirements. Acad Med. 2012. April;87(4):403–10. doi: 10.1097/ACM.0b013e318248e5c2. [DOI] [PubMed] [Google Scholar]
- [22].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. April;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:649–653. [Google Scholar]
- [24].Silber JH, Bellini LM, Shea JA, et al. Patient safety outcomes under flexible and standard resident duty-hour rules. N Engl J Med 2019;380:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]