Abstract
Post-transplant lymphoproliferative disorder (PTLD) is a serious complication of organ transplantation that often manifests as Epstein-Barr virus (EBV)-associated B cell lymphomas. Current treatments for PTLD have limited efficacy and can be associated with graft rejection or systemic toxicities. The mTOR inhibitor, rapamycin, suppresses tumor growth of EBV+ B cell lymphoma cells in vitro and in vivo; however, the efficacy is limited and clinical benefits of mTOR inhibitors for PTLD are variable. Here, we show constitutive activation of multiple nodes within the PI3K/Akt/mTOR pathway in EBV+ PTLD-derived cell lines. Inhibition of either PI3K or Akt, with specific inhibitors CAL-101 and MK-2206, respectively, diminished growth of EBV+ B cell lines from PTLD patients in a dose-dependent manner. Importantly, rapamycin combined with CAL-101 or MK-2206 had a synergistic effect in suppressing cell growth as determined by IC50 isobolographic analysis and Loewe indices. Moreover, these combinations were significantly more effective than rapamycin alone in inhibiting tumor xenograft growth in NOD-SCID mice. Finally, both CAL-101 and MK-2206 also prolonged survival of heterotopic cardiac allografts in C57BL/6 mice. Thus, combination therapy with rapamycin and a PI3K inhibitor, or an Akt inhibitor, can be an efficacious treatment for EBV-associated PTLD, while simultaneously promoting allograft survival.
1. INTRODUCTION
Post-transplant lymphoproliferative disorder (PTLD) comprises a spectrum of pathologies ranging from reactive hyperplasia to malignant lymphoma that arise in the setting of immunosuppression. The vast majority of PTLD are associated with Epstein-Barr virus infection (EBV) (1). Current therapies for EBV+ PTLD, including withdrawal of immunosuppression, anti-B lymphocyte antibodies (rituximab), or conventional chemotherapy, have adverse effects including risk of graft loss, suppressed adaptive immunity, or systemic toxicities, and overall high mortality (2,3).
The mTOR inhibitor rapamycin (sirolimus), a potent immunosuppressant, has garnered interest as a therapy for malignancies, including EBV+ PTLD (4). Our laboratory has demonstrated that the PI3K/Akt/mTOR signaling pathway is constitutively active in EBV+ B lymphoma lines derived from PTLD patients (5,6). Activation of this pathway is triggered by latent membrane protein 1 (LMP1), a viral oncogene (1,7–9). Treatment with rapamycin inhibits lymphoma proliferation, in part through modulation of cell cycle proteins (5,10). Proteomic and immunohistochemical analyses of primary PTLD specimens also demonstrate dysregulation of the PI3K/mTOR pathway (8–10). Clinically, impressive responses to rapamycin have been reported in some PTLD cases (14) and approximately 30% of transplant centers in Europe routinely switch immunosuppression to rapamycin for transplant patients who exhibit EBV viremia (15). However, other reports indicate that rapamycin-based therapy has either no effect, or is associated with an increased incidence of PTLD (16,17). Thus, further studies are needed to determine the efficacy of targeting the PI3K/Akt/mTOR pathway in EBV+ PTLD.
Two mTOR complexes exist, mTORC1 and mTORC2. mTORC1 is activated downstream of Akt, and regulates mRNA translation, lipid biosynthesis, and metabolism. By contrast, mTORC2 acts upstream to phosphorylate Akt at serine residue 473, thereby increasing the activity of Akt. These biochemical differences suggest some possibilities to explain why rapamycin can have mixed efficacy in EBV+ PTLD. First, rapamycin only partially inhibits mTORC1 which results in ongoing cap-dependent protein translation (18,19). Second, there is an inhibitory feedback mechanism by which mTORC1 activation negatively regulates Akt via S6K (20). Consequently, inhibition of mTORC1 by rapamycin can result in reflex hyperactivation of Akt, which can stimulate other pro-growth pathways (21). Third, Akt is also directly activated by mTORC2, which contains a unique regulatory subunit, rictor, that confers specificity of mTORC2 towards Akt but renders mTORC2 resistant to rapamycin (22). Therefore, rapamycin is unable to suppress mTORC2 unless present at very high doses or for prolonged exposure times (23). Taken together, these mechanisms could explain why rapamycin, as a single agent, has shown only moderate success as an anti-cancer therapy in EBV+ PTLD, and suggest that combination therapies may be more effective.
In this study, we tested whether targeted inhibition of upstream nodes in the PI3K/Akt/mTOR pathway can augment rapamycin-mediated suppression of EBV+ B cell lymphomas. Our results suggest that combination therapy is significantly more effective at attenuating tumor growth than rapamycin alone, and that the upstream inhibitors of the PI3K/Akt/mTOR pathway can prolong allograft survival as well.
2. MATERIALS AND METHODS
2.1. Reagents
Small molecule inhibitors (rapamycin, CAL-101, MK-2206, AZD-2014) were obtained from Selleck Chemicals (Houston, TX). For in vitro studies, inhibitors were diluted in DMSO at the indicated concentrations. For in vivo studies, the following vehicles were used: 0.2% carboxymethylcellulose/0.25% Tween-80 for rapamycin, 30% PEG400/5% propylene glycol/0.5% Tween-80 for CAL-101, and 30% Captisol for MK-2206. All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO). Captisol was purchased from Ligand Pharmaceuticals (San Diego, CA). The following antibodies were purchased from Cell Signaling Technology (Danvers, MA): Akt, phospho-Akt Ser473, p70S6 kinase, phospho-p70 S6 kinase Thr389, STAT1, phospho-STAT1 Tyr701, p38 MAPK, phospho-p38 MAPK Thr180/Tyr182, ERK1/2, phospho-ERK1/2 Thr202/Tyr204, β-actin, and anti-rabbit IgG HRP-coupled secondary.
2.2. Cell lines and B cell isolation
The EBV-negative Burkitt’s lymphoma line, BL41, was provided by Dr. Elliot Kieff (Harvard). The spontaneously derived EBV+ B lymphoblastoid cell lines were established from peripheral blood (MF4, JB7, ZD3) or lymph nodes (AB5) of PTLD patients and have been extensively characterized previously (24). Cell lines were cultured as previously described (7, 24). B cells were isolated from healthy donors using the B Cell Isolation Kit II, human (GE Healthcare and Miltenyi Biotec, Sunnyvale, CA).
2.3. PI3K pathway analysis
Cell lysates were prepared with PathScan lysis buffer and analyzed on the PathScan Akt Signaling Antibody Array Kit using manufacturer’s protocols (Cell Signaling Technology). The array fluorescence was quantified using the Odyssey CLx (LI-COR Biosciences, Lincoln, NE) and normalized against the fluorescence values of lysates prepared from normal human B cells. Relative fluorescence values for phosphorylation sites in the PI3K pathway were analyzed using Cluster 3.0 (University of Tokyo, Human Genome Center). The data was log-transformed and proteins were centered by median, and the values were hierarchy-clustered using Euclidean distance. A heat map was generated using TreeView 3.0.
2.4. Western blot and cell proliferation assay
Cellular lysates were prepared and western blot performed as previously described (5). Cell proliferation was determined using the MTS assay as previously described (5).
2.5. In vivo xenograft tumor lymphoma studies
Six-week-old male NOD-SCID (NOD.CB17-Prkdcscid/J) mice (Jackson Laboratory, Bar Harbor, ME) were injected subcutaneously in the right flank with 7.5 × 106 cells of the EBV+ PTLD cell line, AB5, suspended in PBS. The mice were then randomized into six groups: 1) vehicle only (10 mL/kg of 30% PEG400/5% propylene glycol/0.5% Tween-80, daily, oral gavage), 2) rapamycin (1.5 mg/kg, dissolved in 0.2% carboxymethylcellulose/0.25% Tween-80, daily, intraperitoneal), 3) CAL-101 (30 mg/kg, in 30% PEG400/5% propylene glycol/0.5% Tween-80, daily, oral gavage), 4) MK-2206 (120 mg/kg, in 30% Captisol, three times per week, oral gavage), 5) rapamycin (1.5 mg/kg, daily) and CAL-101 (30 mg/kg, daily), and 6) rapamycin (1.5 mg/kg, daily) and MK-2206 (120 mg/kg, three times per week). Tumor sizes were measured three times per week using calipers and tumor volume was calculated as previously described (25).
2.6. Heterotopic cardiac allograft survival studies
Hearts from 6-week-old female BALB/c mice (H-2d) were transplanted into 6-week-old female C57BL/6 recipients (H-2b) (Jackson Labs) according to previously published methods (26,27). Starting the day of transplantation, the mice were randomized into three groups: 1) vehicle (10 mL/kg, daily, PO), 2) CAL-101 (30 mg/kg, daily, PO), or 3) MK-2206 (120 mg/kg, three times per week, PO). Hearts were palpated daily to assess for graft survival.
2.7. Statistics
Isobolograms were constructed using IC50 values from the cell growth assays. Each point in the isobologram represents a combination of the two inhibitors that produces 50% inhibition of cell growth. The line of additivity was created from the concentration of each individual drug needed to achieve 50% inhibition, and represents all the theoretical concentrations of inhibitors that would produce 50% inhibition if the inhibitors were purely additive. Drug-drug synergism was additionally assessed using the Loewe index. The Loewe index is a measure of synergism (additivity) where values less than 1 indicate synergism, a value of 1 indicates no synergism or antagonism, and values greater than 1 indicate antagonism. Loewe index estimates and confidence intervals follow from applying the delta method to parameter estimates and confidence intervals in a nonlinear mixed-effects model for the concentration-response curve. Loewe indices were estimated using the R package mixlow (28). For the in vivo tumor xenograft experiment, tumor growth between different treatment groups were compared using a linear mixed effect model (29). A random effect for mouse and fixed effects for time (in days), treatment group, and time by treatment group interaction were employed to assess for changes over time. Tumor size was square root transformed prior to modeling so that the assumption of linear change with time was plausible. Consequently, the treatment by time terms in the model allowed for different slopes by group. The null hypothesis of no time by treatment interaction term in the model (or that all groups had the same slope) was tested by an F-test with five degrees of freedom in the numerator and Kenward-Roger denominator degrees of freedom (30), using the R package lmerTest (31). When the F-test rejected this null hypothesis, differences in baseline to 7 week changes between groups were tested via the Westfall test (32) to account for multiple comparisons, as implemented in the R package multcomp (33). All statistical tests were two-tailed with type 1 error assumed to be 0.05. All analyses were conducted using R version 3.4.0 (R Core Team, 2017). For cardiac graft survival, a log-rank test was performed, with p<0.05 considered statistically significant.
2.8. Study approval
All animal studies were performed in accordance with the regulations set by the Stanford Administrative Panel on Laboratory Animal Care.
3. RESULTS
3.1. Multiple nodes of the PI3K/Akt/mTOR pathway are dysregulated in EBV+ PTLD B cell lines
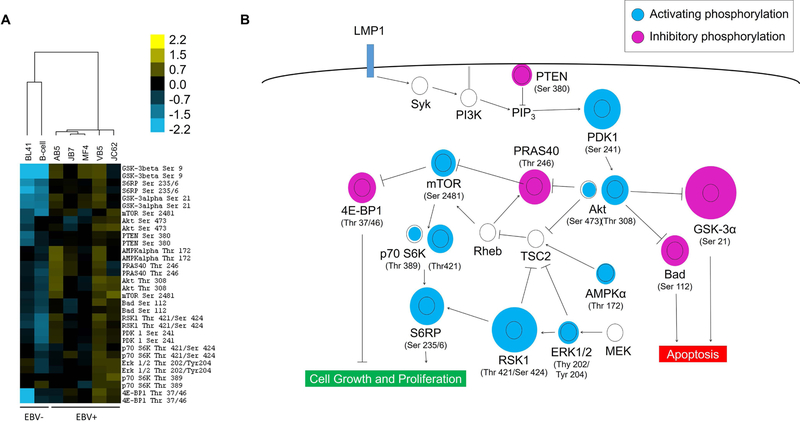
Cell lysates from EBV+ B cell lymphomas isolated from five patients with PTLD were analyzed by multiplex protein microarrays for relative phosphorylation levels of key nodes in the PI3K/Akt/mTOR pathway. Overall, the pathway was upregulated in EBV+ PTLD cell lines (Fig 1A). Figure 1B shows the relative phosphorylation levels of specific molecules within the PI3K/Akt/mTOR pathway, which we have previously shown to be triggered by the viral protein LMP1 (7,34). The dysregulation patterns in EBV+ cell lines strongly favor a pro-proliferation and anti-apoptotic phenotype. Most notably, there is hyperphosphorylation of the activating motifs of the central pathway that involves PDK1, Akt, mTOR, S6K, and S6RP, which lead to cell growth amd proliferation. At the same time, 4E-BP1 is hyperphosphorylated, which inhibits its repressor activity, leading to increased cap-dependent translation. In addition, GSK-3α and Bad, two pro-apoptotic proteins, are both hyperphosphorylated in the EBV+ cell lines, which lead to their suppression, thereby halting apoptosis.
Figure 1: EBV-positive B cell lines from PTLD patients have constitutive over-activation of the PI3K/Akt/mTOR pathway.
(A) Cell lysates were prepared from B cells isolated from a healthy donor (first column), an EBV-negative Burkitt’s lymphoma line (BL41, second column), and five EBV-positive B cell lymphoma samples taken from patients with PTLD (AB5, JB7, JC62, MF4, VB5). The relative phosphorylation levels of the measured nodes for each sample is represented as a heat map, using hierarchical clustering based on Euclidean distances. The two EBV-negative cell samples are clustered separately from the EBV-positive lymphoma cell lines based on differences in the phosphorylation pattern. (B) The relative phosphorylation levels of each node is depicted within the context of the PI3K signaling pathway. The relative diameter of the blue or purple circle to the black ring is proportional to the fold-difference in average phosphorylation levels of the five EBV+ lymphoma cell lines compared to the normal B cell control. The constitutive phosphorylation patterns of the EBV+ lymphoma cell lines overall promote cell proliferation and inhibit apoptotic checkpoints.
3.2. The small molecule inhibitors CAL-101, MK-2206, and AZD-2014, but not rapamycin, inhibit Akt phosphorylation in EBV+ PTLD cell lines.
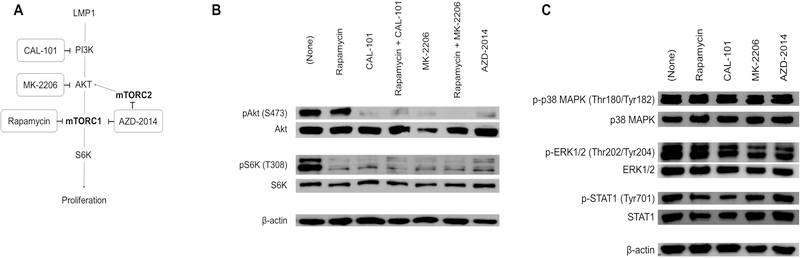
To identify therapeutic targets based on the signaling aberrations in EBV+ B cell lymphomas, we tested two small molecules: MK-2206, a highly selective inhibitor of Akt, and CAL-101, which targets PI3Kδ, an isoform of PI3K that is highly expressed in lymphocytes (Fig 2A). We evaluated the effect of these inhibitors on the phosphorylation of Akt and the mTORC1 substrate S6K in the EBV+ PTLD-derived cell line, AB5 (Fig 2b, first lane). Treatment with rapamycin, as expected, suppressed the phosphorylation of S6K, but did not affect phosphorylation of the upstream kinase Akt (Fig 2B, second lane). By contrast, treatment with either CAL-101 or MK-2206 led to inhibition of both Akt and S6K (Fig 2B, third and fifth lanes). Combining CAL-101 or MK-2206 with rapamycin did not substantially alter the dual suppression of both Akt and S6K (Fig 2B, four and sixth lanes). Akt and S6K phosphorylation was also inhibited by AZD-2014, a dual inhibitor of mTORC1 and mTORC2 (Fig 2B, seventh lane).
Figure 2: Inhibitors target specific nodes in the PI3K/Akt/mTOR pathway in EBV-positive B cell lymphoma (AB5).
(A) Inhibitors (in rectangles) were chosen which block specific nodes in the PI3K/Akt/mTOR pathway. Schematic shows intended targets of CAL-101 (PI3Kδ inhibitor), MK-2206 (Akt inhibitor), rapamycin (mTOR inhibitor), and AZD-2014 (mTORC1/mTORC2 dual inhibitor). (B) Western blot analysis of phosphoproteins (pAKT, pS6K) and total proteins (Akt, S6K, β-actin) in AB5 (EBV+ PTLD cell line) after treatment with rapamycin (100 nM) and with or without CAL-101 (1 µM) and MK-2206 (1 µM), and AZD-2014 (1 µM). β-actin was used as a loading control (representative image from 3 experiments). (C) Western blot of cell lysates from AB5 cultured without or with the indicated inhibitors (rapamycin, CAL-101, MK-2206, and AZD-2014) were probed for activation of alternative signaling pathways (p38 MAPK, p44/42 ERK1/2, and STAT1). β-actin was used as a loading control
To demonstrate that the selected inhibitors (CAL-101, MK-2206, AZD-2014) exerted their effect specifically on the PI3K/Akt/mTOR pathway, rather than through off-target effects, we analyzed their effect on the p38 MAPK, ERK1/2, and STAT1, as these pathways are known to be activated in EBV+ B cell lymphomas in PTLD. Indeed, CAL-101, MK-2206, and AZD-2014 all had minimal effect on the phosphorylation of p38 MAPK, ERK1/2, and STAT1 (Fig 2C).
3.3. Inhibitors of PI3Kδ and Akt decrease growth of EBV-positive B cell lymphomas
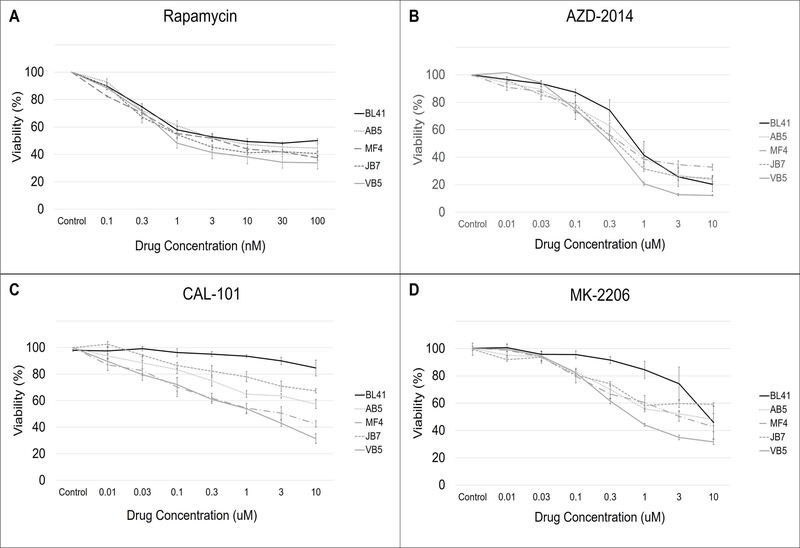
We next examined the effect of PI3Kδ and Akt inhibition on the growth of a panel of B cell lymphoma lines in vitro. In agreement with our previous finding (25), rapamycin inhibits growth of EBV+ B cell lymphoma lines in a dose-dependent manner (Fig 3A). However, the effect of rapamycin is partial, since even at high doses, the percentage of cells reaches a plateau (39.2% ± 3.26%, mean of n = 4 cell lines) and never achieves complete inhibition. Treatment of the EBV− cell line, BL41, with rapamycin resulted in a very similar dose-response curve compared with EBV+ cell lines, suggesting that rapamycin can also act on B cell lymphomas that are not EBV-infected.
Figure 3. Inhibitors of PI3Kδ, Akt, and mTOR suppress the growth of EBV-positive B cell lymphoma lines in vitro.
The EBV-negative Burkitt’s lymphoma line (BL41) and four EBV-positive B cell lymphoma lines from PTLD patients (AB5, MF4, JB7, VB5) were cultured in increasing concentrations of the small molecule inhibitors (A) rapamycin, (B) AZD-2014, (C) CAL-101 and (D) MK-2206 for 72 hours. Cell viability was measured after 72 hours and represented as a percentage relative to control. The error bars represent the SEM of three separate experiments for each condition and cell line. Each experiment was a mean of four technical replicates on the same plate.
AZD-2014, a dual mTORC1 and mTORC2 inhibitor, was more effective than rapamycin in suppressing growth. The effect was also dose-dependent, and cell viability of the EBV+ cell lines was 23.5% (±5.98%, mean of n = 4 cell lines) at the maximum concentration of AZD-2014 tested (Fig 3B). However, like rapamycin, the effect of AZD-2014 plateaued and there was no significant difference in the dose-response curves between the EBV-negative BL41 and the EBV+ cell lines.
CAL-101 (PI3Kδ inhibitor) and MK-2206 (Akt inhibitor) also showed dose dependent inhibition of EBV+ B cell lymphomas (Figs 3C and 3D), with CAL-101 exhibiting a more variable effect. However, unlike the mTORC inhibitors rapamycin and AZD-2014, the EBV-negative BL41 was minimally affected by CAL-101 and MK-2206. Of note, the dose-response curves of CAL-101 and MK-2206 did not plateau at the concentrations tested.
3.4. Inhibitors of PI3Kδ and Akt act synergistically with rapamycin to suppress survival of EBV+ B cell lymphoma in vitro
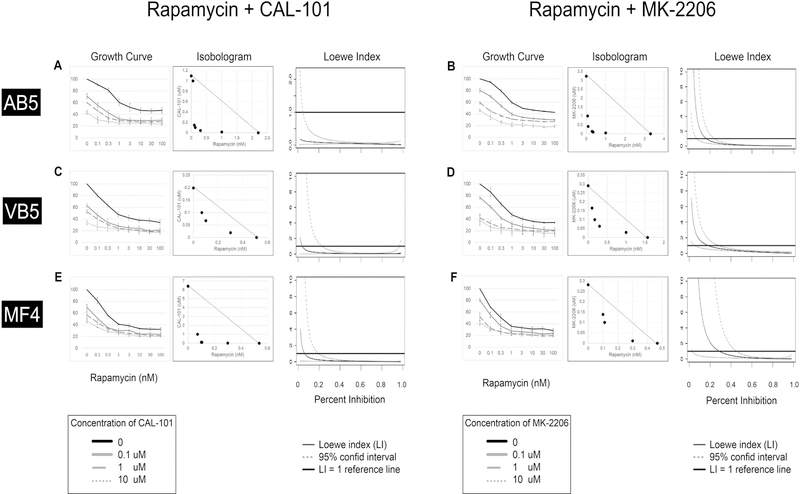
Given that both CAL-101 and MK-2206 target nodes upstream of mTOR and show efficacy in inhibiting EBV+ B cell lymphoma growth, we next asked whether combination treatment of these agents with rapamycin was superior to the individual inhibitors alone. EBV+ B cell lines were treated with rapamycin, plus either CAL-101 or MK-2206 over a range of concentrations. As shown in Figure 4 (A-F, left-most panels), increasing concentrations of both CAL-101 and MK-2206 further augmented the dose-response seen with rapamycin alone. In most cases, a low concentration of the second inhibitor (0.1 uM of CAL-101 or MK-2206) produced the greatest incremental shift in the dose-response curve. We interpret these finding to indicate that the combination of rapamycin and CAL-101, or rapamycin and MK-2206, are at least additive.
Figure 4. Inhibition of PI3Kδ and Akt potentiates the efficacy of rapamycin in suppressing growth of EBV-positive B cell lymphoma lines in vitro.
Three EBV-positive B cell lymphoma lines, AB5 (A, B), VB5 (C, D), and MF4 (E, F) were cultured over a range of concentrations of rapamycin plus either CAL-101 (A, C, E) or MK-2206 (B, D, F), for 72 hours. Cell survival (A–F, left panels) was measured and expressed as a percentage relative to control. Each experiment was repeated three times. Isobolograms (A–F, center panels) of two-drug combinations demonstrate that all tested concentrations of the two-drug combinations were below the line of additivity, suggesting synergy. Loewe indices (A–F, right panels) were calculated for each cell line (AB5, VB5, MF4) for both drug combinations (rapamycin + CAL-101 and rapamycin + MK-2206) using the MixLow Package for R. The index of 1.00 is shown (horizontal black line). The 95% confidence interval is shown in the dotted lines.
To determine whether these combinations are synergistic, we performed additional analyses. First, we constructed IC50 isobolograms such that different combinations of equi-effective concentrations of the inhibitor pairs were determined, using the same raw data from the dose-response curves. In this case, concentrations that produce an absolute decrease in growth to 50% (ie IC50) were plotted (Fig 4A-F, middle panels). As shown in the isobolograms for all three cell lines, the observed combination of drug concentrations all fell below the line of additivity, suggesting a synergistic (supra-additive) effect.
Finally, to analyze the quantitative degree of synergy between the combinations for each cell line, we calculated a Loewe index (LI) as a function of percent inhibition (Fig 4A-F, right panels). Any LI having an upper 95% confidence limit less than 1.00 was considered significant. For an inhibition level of 50% (to correspond with our IC50 isobologram analysis), the LIs of the rapamycin plus CAL-101 combination were 0.05 (0.03, 0.09, 95% CI), 0.07 (0.03, 0.16), and 0.07 (0.04, 0.15) for AB5, VF5, and MF4, respectively. For the rapamycin plus MK-2206 combination, the LIs were 0.12 (0.08, 0.16), 0.33 (0.23, 0.45), and 0.25 (0.09, 0.65), respectively. These data indicate that both combinations of inhibitors were synergistic in each of the three EBV+ B cell lymphoma lines tested.
3.5. Combination therapy is significantly more effective than rapamycin alone in suppressing growth of PTLD-derived tumor xenografts in vivo
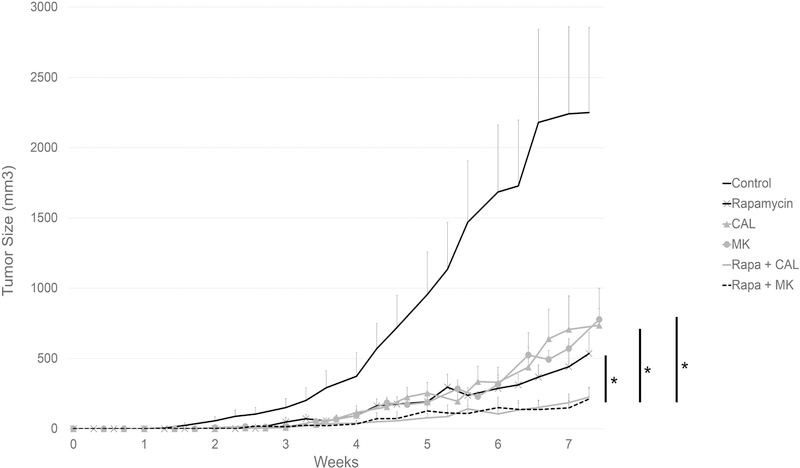
We utilized a PTLD tumor xenograft model (25) to investigate whether the synergy between rapamycin and either CAL-101 or MK-2206 could also be observed in vivo. We inoculated immunodeficient NOD-SCID mice with an EBV+ PTLD B cell line, AB5, and randomized the mice to one of six treatment groups. As expected, mice treated with vehicle alone developed large tumors (> 500 mm3) before week 5 (Fig 5). By week 7, the average tumor size in the untreated group was 2193.2 ± 583.8 mm3. Treatment with rapamycin alone significantly suppressed tumor growth rate (p < 0.001). Mice treated with either CAL-101 or MK-2206 alone had similar tumor growth curves compared with the rapamycin-treated group. There was no statistical difference in the tumor growth rates between mice treated with rapamycin, CAL-101, or MK-2206 as single agents over the seven weeks of the study, with the final tumor sizes for the three groups being 440.3 ± 111.2, 706.9 ± 237.6, and 570.9 ± 69.6 mm3, respectively.
Figure 5: Inhibition of PI3Kδ or Akt potentiates the effect of rapamycin in suppressing the growth of an EBV-positive B cell lymphoma xenograft.
Six-week-old male NOD-SCID mice were injected subcutaneously in right flank with AB5 (7.5 × 106). Mice were randomized into six groups (n = 8 per group): control (200 uL vehicle by oral gavage, daily), rapamycin (1.5 mg/kg by intraperitoneal injection, daily), CAL-101 (30 mg/kg by oral gavage, daily), MK-2206 (120 mg/kg by oral gavage, three times weekly), rapamycin plus CAL-101 (30 mg/kg by oral gavage, daily), and rapamycin plus MK-2206 (120 mg/kg by oral gavage, three times weekly). Tumor sizes were measured three times per week using caliper. (* denotes p < 0.001).
Mice treated with a combination regimen, either rapamycin plus CAL-101, or rapamycin plus MK-2206, had significantly diminished tumor growth compared to mice treated with single agents only (p < 0.001). The mean tumors sizes by week 7 in the two combination treatment groups were 193.8 ± 59.6 and 185.1 ± 71.5 mm3, respectively. These data indicate that combination treatments targeting PI3Kδ and mTOR, or Akt and mTOR, are superior to targeting either mTOR, PI3Kδ, or Akt alone in inhibiting the growth of EBV+ B cell lymphomas in vivo.
3.6. Inhibition of PI3Kδ or Akt significantly prolong graft survival in a heterotopic heart transplant model
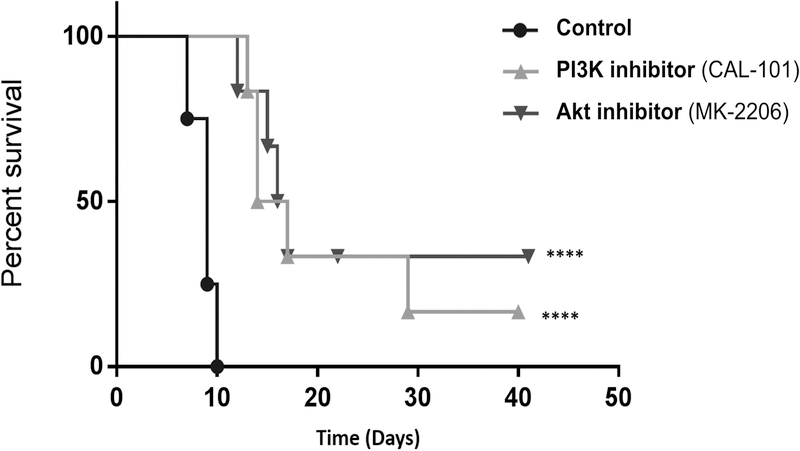
Having established their anti-tumor properties, we assessed the effect of CAL-101 and MK-2206 on allograft survival. C57Bl/6 mice transplanted with a BALB/c cardiac allograft were randomized to treatment with CAL-101, MK-2206, or control, at the same doses used in the tumor xenograft experiments. As expected, control allograft recipients showed evidence of graft rejection starting on day 7 post-transplant. In contrast, graft rejection was not observed in the CAL-101 or MK-2206-treated mice until day 13 and 12, respectively (Fig 6). The median graft survival was 15.5 ± 5.2 and 16.5 ± 6.4 (SEM) days for CAL-101 and MK-2206, respectively. Overall graft survival was significantly prolonged in mice treated with either CAL-101 or MK-2206, compared with control mice (p < 0.001). Thus, in addition to demonstrating anti-tumor efficacy, PI3Kδ or Akt inhibition can prolong cardiac allograft survival in fully MHC mismatched donor-recipient pairs.
Figure 6. Inhibition of PI3Kδ or Akt in vivo prolongs graft survival in a heterotopic cardiac transplant model.
Kaplan-Meier curve for survival of allogeneic heterotopic heart allografts in control (black circle), CAL-101 (light gray triangle) or MK-2206 (dark gray triangle)-treated recipients. Hearts from 6-week old female Balb/c mice (H-2d) were transplanted into 6-week old female C57BL/6 recipients (H-2b). Mice were randomized into three groups (n = 4–6 per group): control (200 uL vehicle by oral gavage, daily), CAL-101 (30 mg/kg by oral gavage, daily), and MK-2206 (120 mg/kg by oral gavage, three times weekly). Graft survival was monitored by daily transabdominal palpation and significance between groups was determined by log rank test. Rapamycin given at 1.5 mg/kg IP daily prolonged graft survival indefinitely (not shown). **** denotes p < 0.001.
4. DISCUSSION
Treatment of EBV-associated PTLD remains a clinical challenge, and is hindered by the absence of rational approaches that specifically consider the pathogenesis of the virus. The current regimen includes reduction of immunosuppression (RI), anti-CD20 immunotherapy (rituximab), conventional cytotoxic chemotherapy, or adoptive T cell transfer. Many of these therapies modulate the immune system without a targeted approach towards EBV. For example, RI allows host T cells to recover and mediate a response against the lymphoma, but this can also lead to acute rejection of the allograft. In one study, pediatric kidney recipients with PTLD, of whom 90.2% received RI, had shorter graft survival compared to matched recipients without PTLD or RI (35). Rituximab can suppress the growth of CD20-positive B cell-derived PTLD lesions, but it globally depletes B cells, and has been observed to increase the rate of bacterial infection and overall mortality compared with match-controlled PTLD patients not receiving rituximab (36). Systemic chemotherapy has well-studied organ toxicities and can be particularly harsh for the allograft. Adoptive T cell transfer is a strategy that has shown promise, but requires specialized centers and lengthy preparation time, which may not be suitable for patients with fulminant lymphomas. Finally, locoregional therapies such as surgery or radiation are inappropriate for most cases of PTLD that are disseminated. Altogether, our current armamentarium of treatments is both suboptimal in efficacy and toxic to the host and allograft.
Based on previous findings that EBV activates the PI3K/Akt/mTOR pathway in its host B cells as a mechanism of oncogenesis in PTLD (5,8,10, 24,25), and the mixed clinical efficacy of rapamycin which targets this pathway, we sought to investigate whether combination therapy serially targeting the PI3K/Akt/mTOR pathway would be more effective than rapamycin alone. The constitutively activated pathway favors a pro-growth, anti-apoptotic phenotype for the host cells, and Akt, a master regulator of this pathway, is insensitive to rapamycin. Therefore, an ideal rational therapy should capitalize on the potency and clinical familiarity and profile of rapamycin, while supplementing its deficiencies.
By combining CAL-101 or MK-2206 with rapamycin, we demonstrated that the suppressive effect of the combination was greater than rapamycin alone. Moreover, our pharmacodynamic analyses suggest that the effect is synergistic, exceeding the additive effect expected from the two drugs alone. Often, inhibiting a single node within a complex pathway can trigger a feedback mechanism to dampen its effect. Indeed, previous studies have demonstrated that such compensatory feedback loops exist within the mTOR pathway, and that dual blockade can in part overcome this compensation (20,37). Dual blockade of the mTOR pathway has been shown to be synergistic for other cancer types, such as hepatocellular carcinoma (38,39). In addition, dual blockade can overcome certain resistances that arise in cancer cell lines, as demonstrated in neuroblastoma cells (40). Therefore, the observed synergy of dual PI3K/Akt/mTOR blockade in our PTLD model has precedence in other cancer types, and is a rational strategy for overcoming the modest efficacy of rapamycin monotherapy in PTLD patients.
The ability of rapamycin to suppress tumor growth in vivo has been demonstrated for several tumor types (41,42). Our lab and others have previously demonstrated the efficacy of rapamycin in suppressing PTLD B cell lymphoma xenografts in immunodeficient mice (43,25). Combination therapy in vivo is more difficult to study, and has never been investigated in PTLD. Here, we demonstrate using a mixed effect model that while rapamycin alone had a sizeable effect on tumor growth when compared with mice receiving vehicle control, adding a second agent (CAL-101 or MK-2206) had a significant effect on further suppressing tumor growth. Although the combination therapy could not completely halt tumor growth, these findings suggest that it may be possible to administer lower doses of the two drugs in combination and achieve the same efficacy as a higher dose of rapamycin alone, thereby reducing drug-related toxicity. In the case of rapamycin, observed clinical toxicities include poor wound healing, diabetes, and pneumonitis (44–47).
On the other hand, one of the potential benefits of utilizing mTOR inhibitors for PTLD is their dual anti-neoplastic and anti-rejection properties. Previous murine studies have demonstrated that rapamycin is able to promote graft survival while suppressing melanoma and colorectal carcinoma xenografts (48). Everolimus, an mTOR inhibitor derived from rapamycin, has been shown to have both anti-tumor and anti-rejection properties for PTLD (49). Since T cells that mediate graft rejection also utilize the PI3K/Akt/mTOR pathway for activation (50,51), we reasoned that mechanistically, CAL-101 and MK-2206 should suppress allograft rejection. Indeed, we showed that both CAL-101 and MK-2206 significantly prolong allograft survival. Our finding with CAL-101 are in concordance with a recent report demonstrating that mice carrying a p110δ mutation show prolonged survival of cardiac allografts (52). Interestingly, there were some mice in our CAL-101 and MK-2206-treated groups that maintain their graft function long-term (beyond day 45) in the absence of ongoing treatment. Further studies are required to dissect the mechanisms by which CAL101 and MK-2206 promote graft survival.
PI3K/Akt/mTOR is one of the most commonly altered pathways associated with human malignancies (53,54). The next generation of PI3Kδ inhibitors are being studied for various lymphoid malignancies, while MK-2206 is being evaluated for a wide range of carcinomas and sarcomas (clinicaltrials.gov). This progress can potentially facilitate the translation of the findings in this study to the treatment of PTLD, especially for refractory cases when established therapies are not efficacious or threaten graft survival.
This study was not designed to specifically confirm an association between EBV and the mTOR pathway. Indeed, the mTOR inhibitors rapamycin and AZD-2014 have similar effects on the EBV+ and EBV− B cell lines we tested. It was instead intended to elucidate how the pathway can be targeted by small molecule inhibitors. Our study focused on the effects of the small molecule inhibitors on PI3K/Akt pathway proteins and did not examine the effects on apoptosis or cell cycle progression. However, we have previously shown that rapamycin induces an arrest in the G1 to S transition in the EBV+ B cell lines (25) and others have shown that CAL-101 and MK-2206 induce G1 arrest in leukemia cells (55, 56). Collectively, our results indicate that inhibition of PI3Kδ and Akt, with CAL-101 and MK-2206, respectively, is synergistic with mTOR inhibition in the treatment of EBV+ PTLD B cell lymphomas in vitro, and that combination therapy in vivo is significantly more efficacious than any single agent in suppressing the growth of PTLD-derived tumor xenografts. Moreover, all three agents have both anti-tumor as well as anti-rejection properties. Altogether, these data provide preclinical evidence that dual inhibition of serial nodes in the PI3K/Akt/mTOR pathway can be a viable therapeutic approach for patients with PTLD.
ACKNOWLEDGMENTS
AXS was funded by the Ernest and Amelia Gallo Endowed Postdoctoral Fellowship, as part of the Child Health Research Institutes at Stanford University, and the Stanford NIH-NCATS-CTSA grant (no. UL1 TR001085). Funding was provided by grant 5R01AI113130 (OMM).
Abbreviations:
- 4EBP
4E-binding protein
- DMSO
Dimethyl sulfoxide
- EBV
Epstein-Barr virus
- ERK
Extracellular signal-regulated kinase
- IC50
Half maximal Inhibitory concentration
- LI
Loewe index
- LMP1
Latent membrane protein 1
- MAPK
Mitogen-activated protein kinase
- mTOR
Mammalian target of rapamycin
- mTORC
Mammalian target of rapamycin complex
- NOD-SCID
Non-obese diabetic/severe combined immunodeficiency
- PI3K
Phospho-inositide 3-kinase
- PTLD
Post-transplant lymphoproliferative disorder
- RI
Reduction of immunosuppression
- SEM
Standard error of the mean
- STAT
Signal transducer and activator of transcription
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S. Post-transplant lymphoproliferative disorders. Nat Rev Dis Primer 2016. January 28;15088. [DOI] [PubMed]
- 2.Evens AM, David KA, Helenowski I, Nelson B, Kaufman D, Kircher SM, et al. Multicenter Analysis of 80 Solid Organ Transplantation Recipients With Post-Transplantation Lymphoproliferative Disease: Outcomes and Prognostic Factors in the Modern Era. J Clin Oncol 2010. February 20;28(6):1038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mansour Z, Nelson BP, Evens AM. Post-Transplant Lymphoproliferative Disease (PTLD): Risk Factors, Diagnosis, and Current Treatment Strategies. Curr Hematol Malig Rep 2013. September;8(3):173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muthukkumar S, Ramesh TM, Bondada S. Rapamycin, a potent immunosuppressive drug, causes programmed cell death in B lymphoma cells. Transplantation 1995. August 15;60(3):264–70. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa S, Wei L, Krams SM, Esquivel CO, Martinez OM. PI3Kδ Inhibition Augments the Efficacy of Rapamycin in Suppressing Proliferation of Epstein−Barr Virus (EBV)+ B Cell Lymphomas: PI3Kδ, mTOR and EBV+ PTLD. Am J Transplant 2013. August;13(8):2035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatton O, Lambert SL, Phillips LK, Vaysberg M, Natkunam Y, Esquivel CO, et al. Syk-Induced Phosphatidylinositol-3-Kinase Activation in Epstein-Barr Virus Posttransplant Lymphoproliferative Disorder: Role of Syk-Induced PI3K in EBV+ PTLD. Am J Transplant 2013. April;13(4):883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert SL, Martinez OM. Latent Membrane Protein 1 of EBV Activates Phosphatidylinositol 3-Kinase to Induce Production of IL-10. J Immunol 2007. December 15;179(12):8225–34. [DOI] [PubMed] [Google Scholar]
- 8.Hatton O, Lambert SL, Krams SM, Martinez OM. Src Kinase and Syk Activation Initiate PI3K Signaling by a Chimeric Latent Membrane Protein 1 in Epstein-Barr Virus (EBV)+ B Cell Lymphomas. Ernberg IT, editor. PLoS ONE 2012. August 3;7(8):e42610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatton O, Martinez OM, Esquivel CO. Emerging therapeutic strategies for Epstein-Barr virus+ post-transplant lymphoproliferative disorder: Emerging therapeutic strategies for EBV+PTLD. Pediatr Transplant 2012. May;16(3):220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaysberg M, Balatoni CE, Nepomuceno RR, Krams SM, Martinez OM. Rapamycin Inhibits Proliferation of Epstein-Barr Virus Positive B-cell Lymphomas Through Modulation of Cell-Cycle Protein Expression: Transplantation 2007. April;83(8):1114–21. [DOI] [PubMed] [Google Scholar]
- 11.El-Salem M, Raghunath PN, Marzec M, Wlodarski P, Tsai D, Hsi E, et al. Constitutive activation of mTOR signaling pathway in post-transplant lymphoproliferative disorders. Lab Invest 2007;87(1):29. [DOI] [PubMed] [Google Scholar]
- 12.Alsayed Y, Leleu X, Leontovich A, Oton AB, Melhem M, George D, et al. Proteomics analysis in post-transplant lymphoproliferative disorders. Eur J Haematol 2008. October;81(4):298–303. [DOI] [PubMed] [Google Scholar]
- 13.Nelson BP, Wolniak KL, Evens A, Chenn A, Maddalozzo J, Proytcheva M. Early posttransplant lymphoproliferative disease: clinicopathologic features and correlation with mTOR signaling pathway activation. Am J Clin Pathol 2012. October;138(4):568–78. [DOI] [PubMed] [Google Scholar]
- 14.Cullis B, D’Souza R, McCullagh P, Harries S, Nicholls A, Lee R, et al. Sirolimus-Induced Remission of Posttransplantation Lymphoproliferative Disorder. Am J Kidney Dis 2006. May;47(5):e67–72. [DOI] [PubMed] [Google Scholar]
- 15.San-Juan R, Manuel O, Hirsch HH, Fernández-Ruiz M, López-Medrano F, Comoli P, et al. Current preventive strategies and management of Epstein–Barr virus-related post-transplant lymphoproliferative disease in solid organ transplantation in Europe. Results of the ESGICH Questionnaire-based Cross-sectional Survey. Clin Microbiol Infect 2015. June;21(6):604.e1–604.e9. [DOI] [PubMed] [Google Scholar]
- 16.Kirk AD, Cherikh WS, Ring M, Burke G, Kaufman D, Knechtle SJ, et al. Dissociation of Depletional Induction and Posttransplant Lymphoproliferative Disease in Kidney Recipients Treated With Alemtuzumab. Am J Transplant 2007. November;7(11):2619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sampaio MS, Cho YW, Shah T, Bunnapradist S, Hutchinson IV. Association of immunosuppressive maintenance regimens with posttransplant lymphoproliferative disorder in kidney transplant recipients. Transplantation 2012. January 15;93(1):73–81. [DOI] [PubMed] [Google Scholar]
- 18.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-Site Inhibitors of mTOR Target Rapamycin-Resistant Outputs of mTORC1 and mTORC2. Hunter T, editor. PLoS Biol 2009. February 10;7(2):e1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive Mammalian Target of Rapamycin Inhibitor Reveals Rapamycin-resistant Functions of mTORC1. J Biol Chem 2009. March 20;284(12):8023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest [Internet] 2008. August 1 [cited 2017 Jul 9]; Available from: http://www.jci.org/articles/view/34739 [DOI] [PMC free article] [PubMed]
- 21.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 2007;26(13):1932. [DOI] [PubMed] [Google Scholar]
- 22.Liao Y, Hung M-C. Physiological regulation of Akt activity and stability. Am J Transl Res 2010;2(1):19. [PMC free article] [PubMed] [Google Scholar]
- 23.Sarbassov DD, Ali SM, Sengupta S, Sheen J-H, Hsu PP, Bagley AF, et al. Prolonged Rapamycin Treatment Inhibits mTORC2 Assembly and Akt/PKB. Mol Cell 2006. April;22(2):159–68. [DOI] [PubMed] [Google Scholar]
- 24.Beatty PR, Krams SM, Martinez OM, Immunol J. Involvement of IL-10 in the autonomous growth of EBV-transformed B cell lines 2007;158(9):4045–51. [PubMed] [Google Scholar]
- 25.Nepomuceno RR, Balatoni CE, Natkunam Y, Snow AL, Krams SM, Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res 2003;63(15):4472–4480. [PubMed] [Google Scholar]
- 26.Wei L, Wang M, Qu X, Mah A, Xiong X, Harris AGC, et al. Differential Expression of MicroRNAs During Allograft Rejection: MicroRNAs in Allograft Rejection. Am J Transplant 2012. May;12(5):1113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei L, Kaul V, Qu X, Xiong X, Lau AH, Iwai N, et al. Absence of miR-182 Augments Cardiac Allograft Survival: Transplantation 2017. March;101(3):524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boik JC, Newman RA, Boik RJ. Quantifying synergism/antagonism using nonlinear mixed-effects modeling: a simulation study. Stat Med 2008. March 30;27(7):1040–61. [DOI] [PubMed] [Google Scholar]
- 29.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw [Internet] 2015. [cited 2017 Oct 15];67(1). Available from: http://www.jstatsoft.org/v67/i01/ [Google Scholar]
- 30.Kenward MG, Roger JH. Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics 1997;53(3):983–97. [PubMed] [Google Scholar]
- 31.Kuznetsova A, Brockhoff P, Christensen RHB. LmerTest: Tests in linear mixed effects models Vol. 2 2015. [Google Scholar]
- 32.Westfall PH. Multiple Testing of General Contrasts Using Logical Constraints and Correlations. J Am Stat Assoc 1997. March 1;92(437):299–306. [Google Scholar]
- 33.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J Biom Z 2008. June;50(3):346–63. [DOI] [PubMed] [Google Scholar]
- 34.Hatton O, Phillips LK, Vaysberg M, Hurwich J, Krams SM, Martinez OM. Syk Activation of Phosphatidylinositol 3-Kinase/Akt Prevents HtrA2-dependent Loss of X-linked Inhibitor of Apoptosis Protein (XIAP) to Promote Survival of Epstein-Barr Virus+ (EBV+) B Cell Lymphomas. J Biol Chem 2011. October 28;286(43):37368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dharnidharka VR, Martz KL, Stablein DM, Benfield MR. Improved Survival with Recent Post-Transplant Lymphoproliferative Disorder (PTLD) in Children with Kidney Transplants: Improved Survival with Recent Post-transplant Lymphoproliferative Disorder. Am J Transplant 2011. April;11(4):751–8. [DOI] [PubMed] [Google Scholar]
- 36.Petropoulou AD, Porcher R, de Latour RP, Xhaard A, Weisdorf D, Ribaud P, et al. Increased Infection Rate After Preemptive Rituximab Treatment for Epstein-Barr Virus Reactivation After Allogeneic Hematopoietic Stem-Cell Transplantation: Transplant J 2012. October;94(8):879–83. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Hawk N, Yue P, Kauh J, Ramalingam SS, Fu H, et al. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors’ anticancer efficacy. Cancer Biol Ther 2008. December;7(12):1952–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grabinski N, Ewald F, Hofmann BT, Staufer K, Schumacher U, Nashan B, et al. Combined targeting of AKT and mTOR synergistically inhibits proliferation of hepatocellular carcinoma cells. Mol Cancer 2012;11(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ewald F, Nörz D, Grottke A, Bach J, Herzberger C, Hofmann BT, et al. Vertical Targeting of AKT and mTOR as Well as Dual Targeting of AKT and MEK Signaling Is Synergistic in Hepatocellular Carcinoma. J Cancer 2015;6(12):1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi L, Toyoda H, Xu D, Zhou Y, Sakurai N, Amano K, et al. PDK1-mTOR signaling pathway inhibitors reduce cell proliferation in MK2206 resistant neuroblastoma cells. Cancer Cell Int [Internet] 2015. December [cited 2018 Apr 16];15(1). Available from: http://www.cancerci.com/content/15/1/91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García-Echeverría C. Allosteric and ATP-competitive kinase inhibitors of mTOR for cancer treatment. Bioorg Med Chem Lett 2010. August;20(15):4308–12. [DOI] [PubMed] [Google Scholar]
- 42.Gupta M, Hendrickson AEW, Yun SS, Han JJ, Schneider PA, Koh BD, et al. Dual mTORC1/mTORC2 inhibition diminishes Akt activation and induces Puma-dependent apoptosis in lymphoid malignancies. Blood 2012. January 12;119(2):476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majewski M, Korecka M, Kossev P, Li S, Goldman J, Moore J, et al. The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: A potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc Natl Acad Sci 2000. April 11;97(8):4285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller LW. Cardiovascular Toxicities of Immunosuppressive Agents. Am J Transplant 2002. October;2(9):807–18. [DOI] [PubMed] [Google Scholar]
- 45.Stallone G, Infante B, Grandaliano G, Gesualdo L. Management of Side Effects of Sirolimus Therapy: Transplantation 2009. April;87(Supplement):S23–6. [DOI] [PubMed] [Google Scholar]
- 46.Barlow AD, Nicholson ML, Herbert TP. Evidence for Rapamycin Toxicity in Pancreatic β-Cells and a Review of the Underlying Molecular Mechanisms. Diabetes 2013. August;62(8):2674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bootun R Effects of immunosuppressive therapy on wound healing. Int Wound J 2013. February;10(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koehl GE, Andrassy J, Guba M, Richter S, Kroemer A, Scherer MN, et al. Rapamycin protects allografts from rejection while simultaneously attacking tumors in immunosuppressed mice. Transplantation 2004;77(9):1319–1326. [DOI] [PubMed] [Google Scholar]
- 49.Majewski M, Korecka M, Joergensen J, Fields L, Kossev P, Schuler W, et al. Immunosuppressive TOR kinase inhibitor everolimus (RAD) suppresses growth of cells derived from posttransplant lymphoproliferative disorder at allograft-protecting doses: Transplantation 2003. May;75(10):1710–7. [DOI] [PubMed] [Google Scholar]
- 50.Law BK. Rapamycin: An anti-cancer immunosuppressant? Crit Rev Oncol Hematol 2005. October;56(1):47–60. [DOI] [PubMed] [Google Scholar]
- 51.So L, Fruman DA. PI3K signalling in B− and T-lymphocytes: new developments and therapeutic advances. Biochem J 2012. March 15;442(3):465–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uehara M, McGrath MM, Ohori S, Solhjou Z, Banouni N, Routray S, et al. Regulation of T cell alloimmunity by PI3Kγ and PI3Kδ. Nat Commun [Internet] 2017. December [cited 2018 Apr 2];8(1). Available from: http://www.nature.com/articles/s41467-017-00982-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia P, Xu X-Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res 2015;5(5):1602–9. [PMC free article] [PubMed] [Google Scholar]
- 54.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K Pathway in Human Disease. Cell 2017. August;170(4):605–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiron D, Martin P, Liberto M, Huang X, Ely S, Lannutti BJ, Leonard JP, Mason CE, Chen-Kiang S. Induction of prolonged early G1 arrest by CDK4/CDK6 inhibition reprograms lymphomas for durable PI3Kδ inhibition through PIK3IPI. Cell Cycle 2013. 103 June; 12(12) 1892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu JW, Lin YM, Lai YL, Chen CY, Hu CY, Tien HF, Ou DL, Lin LI. MK-2206 induces apoptosis of AML cells and enhances the cytotoxicity of cytarabine. Med Oncol 2015. July 32 (7): 206. [DOI] [PubMed] [Google Scholar]