Abstract
Estradiol is known to play an important role in the developing human brain, but little is known on the entire network of potential regions which might be affected and on how these effects may vary from childhood to early adulthood, which in turn can explain sexually differentiated behaviors. Here we examined the relationship between estradiol, cortico-amygdalar structural covariance, and cognitive or behavioral measures typically showing sex differences (verbal/spatial skills, anxious-depressed symptomatology) in 152 children and adolescents (6–22 years old). Cortico-amygdalar structural covariance shifted from positive to negative across the age range. Estradiol was found to diminish the impact of age on cortico-amygdalar covariance for the pre-supplementary motor area/frontal eye field and retrosplenial cortex (across the age range), and for the posterior cingulate cortex (in older children). Moreover, the influence of estradiol on age-related cortico-amygdalar networks was associated with higher word identification and spatial working memory (across the age range), as well as higher reading comprehension (in older children), but did not impact anxious-depressed symptoms. There were no significant sex effects on any of the above relationships. These findings confirm the importance of developmental timing on estradiol-related effects and hint at the non-sexually dimorphic role of estradiol-related cortico-amygdalar structural networks in aspects of cognition distinct from emotional processes.
Keywords: Estrogen, Structural Covariance, Cognition, Puberty, Adolescence
INTRODUCTION
The amygdala and cortex undergo significant structural changes during critical developmental periods when there is a rise in steroid hormones, such as the prenatal and pubertal periods. In particular, endocrine disruption related to prenatal stress may influence amygdalar volumetric properties (1, 2) as well as alter behavioral outcomes such as aggression levels (3). Many steroid hormones may play an important role in brain development during these developmental periods, including but not limited to estrogens, androgens and corticosteroids (4). Here we are focusing on effects associated with the most potent estrogenic hormone, 17-beta estradiol during the transition from childhood to young adulthood (5). The amygdala appears to exhibit significant estradiol-related growth and sex differentiation during the pubertal and postpubertal periods in animal models (6, 7), and structural brain studies in human amygdalar volumes are related to estradiol levels during puberty (8). In addition to this role in modulating the structure of the amygdala, estradiol is thought to play a key role in sex-specific corticogenesis (9). For example, cortical decreases in gray matter volume have been associated with increasing estradiol levels across adolescence (8). These effects of estradiol on brain structure appear to play an important functional role, particularly during puberty (5, 10).
Structural covariance is a way to examine how different brain regions may follow similar developmental trajectories. The extent to which morphology in one brain region correlates with the morphology in other brain regions (i.e. covariance patterns) is thought to reflect underlying anatomical connectivity (32, 33) as well as functional changes in brain networks (2, 3). Cortical thickness is a component of cortical volume and, compared to the latter, is thought to be more closely related to the morphometry of cortical columns (31). Because of this, our group studied covariance patterns between the amygdala and whole-brain cortical thickness and found that amygdalar volume was inversely correlated with the thickness of several cortical regions involved in emotion regulation during childhood and adolescence (4, 5).
In addition, we have also described cortico-amygdalar and cortico-hippocampal structural networks associated with androgens such as dehydroepiandrosterone (DHEA) and testosterone (6–11). Together, these studies have contributed to the validation of steroid-sensitive structural covariance as a developmental marker relevant to cognition and behavior. For example, we found specific effects of testosterone on prefrontal-amygdalar and prefrontal-hippocampal structural covariance patterns that in turn predicted higher aggression and lower executive function, especially in boys (7, 9). In contrast, DHEA-related effects on cortico-amygdalar and cortico-hippocampal structural networks were associated with an optimization of cortical functions, i.e. better attention/working memory, particularly for visual information necessary for reading and writing (6, 11).
While we showed these effects to be independent from estradiol levels, the question remains as to whether estradiol would in itself impact structural covariance networks and if so, whether these effects would be sex-specific and overlap with those of testosterone. The latter is a definite possibility, since testosterone can be converted to estradiol by the enzyme aromatase both in the periphery and in the CNS, and is present at high levels in the amygdala (12, 13) and the cortex (14–16). In turn, DHEA is a precursor androgen hormone that can be eventually converted to testosterone and estradiol through a series of rate-limiting steps (17).
As such, estradiol may have effects on structural networks and cognition that are quite similar to those of testosterone and/or DHEA. However, given the specificity of estrogen-receptor signalling (18), estradiol is also likely to have effects on structural networks and cognition that are distinct from those of other steroid hormones, even though similar cognitive domains may be impacted. While there is prior evidence supporting the notion that effects of estradiol on cortico-amygdalar networks could lead to alterations in emotion regulation (19), spatial (20–22) and verbal skills (23–25), it is unclear whether estradiol relates to cognitive function in a way that is independent from emotional processes.
In sum, we are interested here in examining whether estradiol may regulate the structural relationship between the amygdala and the cortex, i.e. cortico-amygdalar structural covariance. We also tested whether estradiol-regulation of cortico-amygdala structural covariance may be associated with development of sexually-differentiated behaviors, such as anxious-depressed symptoms (as girls generally present higher levels of anxiety and depression compared to boys), as well as verbal (for which girls typically outperform boys) and spatial (for which boys typically outperform girls) skills (31–35). Because of the potential functional lateralization of the amygdala with regards to language processing, left and right amygdala were tested separately. Of note, estradiol-related cortico-hippocampal structural covariance is examined in a separate paper (under revision). We used data from the National Institutes of Health MRI Study of Normal Brain Development (NIHPD), a multi-site project that provides a normative database to characterize healthy brain maturation. The current study included 152 typically developing boys and girls matched for pubertal stage, 6 to 22 years old, with children followed from 1–3 times with repeated measurement of hormonal, cognitive and neuroimaging data every 2 years.
First, we tested for associations between estradiol and cortico-amygdalar structural covariance. Second, we examined the relationship between estradiol-related cortico-amygdalar covariance and internalizing behaviors (as measured by the Child Behavior Checklist (CBCL) Anxious-Depressed sub-scale (36), as well as visuo-spatial (as measured by the Cambridge Neuropsychological Test Automated Battery (CANTAB) test for Spatial Working Memory (37)), and language skills (as measured by the Woodcock-Johnson (WJ) tests for Letter-Word Identification and Passage Comprehension (38–40)). Age and sex effects were also considered. Finally, we tested whether the relationship between estradiol and cognition was mediated by cortico-amygdalar structural covariance. We hypothesized that: (1) estradiol would be significantly associated with developmental changes in the structural covariance between the amygdala and prefrontal as well as temporal regions implicated in emotion regulation as well as verbal and spatial skills; and (2) estradiol-related structural brain networks would favor the development of non-verbal spatial abilities over that of language abilities, as well as decrease the frequency of anxious-depressed symptoms across the age range examined.
METHODS AND MATERIALS
Sampling and Recruitment
In the context of the NIHPD study, 433 participants were recruited across the United States with a population-based sampling method seeking to achieve a representative sample in terms of income level and ethnicity (41). All experiments on human participants were conducted in accordance with the Declaration of Helsinki. All procedures were carried out with the adequate understanding and written parental consent, as well as assent of the participants (or consent, if >=18 years old). Participants underwent repeated magnetic resonance brain imaging (MRI) every 2 years. For the participants included in this study, age at first visit was between 6 and 18 years old, with each child then followed longitudinally for a maximum of 3 visits, over the course of 4 years (i.e. full age range 6–22 years). The sample was limited to developmentally healthy children with English speaking parents. Rigorous exclusion criteria included perinatal factors known to disrupt brain development, such as maternal smoking, drinking or drug use during pregnancy, obstetric complications, physical/medical or growth characteristics such as low height/weight, and history of neurological disorders or abnormal neurological exams. Behavioral/psychiatric assessments excluded any children with a current or past treatment for language disorder (simple articulation disorders not exclusionary); and a lifetime history of Axis I psychiatric disorder (except for simple phobia, social phobia, adjustment disorder, oppositional defiant disorder, enuresis, encopresis, nicotine dependency) were excluded from the study. Additional details are described elsewhere (41).
Because 1) there is a predominance of anovulatory -as opposed to ovulatory- cycles throughout adolescence (42); and because (2) estradiol levels follow a different trajectory in anovulatory vs. ovulatory cycles (43, 44), we aimed to increase sample homogeneity by restricting our analyses to girls with pubertal stage of 4 or less -thereby excluding older adolescents and young adults with pubertal stage 5 and a predominance of ovulatory cycles (see section Hormonal and Pubertal Measures for more details). To match the male sample to the female sample with regards to pubertal stage, we also restricted the sample to boys with pubertal stage of 4 or less. In addition to this criterion, we excluded MRI scans that did not meet strict quality control procedures (as described in detail in Method section Neuroimaging Measures), as well as scans with no paired hormonal measurements or behavioral parameters. The final sample included 152 participants for hormonal-related analyses (203 scans) and 149 participants (191–196 scans) for cognitive analyses, depending on data available for each cognitive test (age range 6–22 years old, see Table 1 for more details). Males and females were matched on pubertal stage. As a result of quality control procedures and missing data, the resulting sample represents a mix of cross-sectional and longitudinal data points (some subjects with only 1 data point, others with up to 3 data points).
Table 1.
Sample characteristics at each visit.
| Visit 1 (n=81, 51M, 30F) M+-SD [freq] (range) |
Visit 2 (n=64, 36M, 28F) M+-SD [freq] |
Visit 3 (n=58, 38M, 20F) M+-SD [freq] |
|
|---|---|---|---|
| Age (yrs) | 11.91±3.13 (6.09–18.13) | 12.27±3.40 (6.80–19.48) | 13.99±3.93 (9.09–22.10) |
| Testosterone (pg/mL) | 97.39±78.90 | 76.55±51.69 | 112.92± 119.91 |
| Estradiol (pg/mL) | 10.53±5.51 | 11.35±6.06 | 16.01±10.10 |
| DHEA (pg/mL) | 93.68±105.82 | 157.68± 149.93 | 162.37±139.65 |
| Season of sampling | [10 summer; 71 fall] | [63 fall, 1 winter] | [3 fall, 55 winter] |
| Collection Time (min after midnight) | 670.47±131.89 | 705.00±121.29 | 708.05±113.50 |
| Pubertal stage | 2.01±1.20 | 2.31±1.19 | 2.57±1.244 |
| Handedness | [L = 6, R = 75] | [L = 6, R = 58] | [L = 6, R = 52] |
| Total brain volume (cm3) | 1309.78±112.79 | 1316.37±118.42 | 1319.38±137.18 |
| Left amygdala (mm3) | 1087.11±132.73 | 1089.76±134.99 | 1124.09±135.24 |
| Right amygdala (mm3) | 1102.82±121.23 | 1115.94±123.64 | 1149.87±132.07 |
Note. Total number of scans=203 (78 females). 109 participants (54 females) were scanned only once, 35 (9 females) were scanned twice, and 8 (2 female) were scanned 3 times. Season of sampling was coded as Spring, Summer, Fall, or Winter. L: left; R: right. DHEA: dehydroepiandrosterone.
Neuroimaging Measures
A three-dimensional T1-weighted (T1W) Spoiled Gradient Recalled (SPGR) echo sequence from 1.5 Tesla scanners was obtained on each participant, with 1mm isotropic data acquired sagittally from the entire head for most scanners. In addition, T2-weighted (T2W) and proton density-weighted (PDW) images were acquired using a two-dimensional (2D) multi-slice (2mm) dual echo fast spin echo (FSE) sequence.
Fully automated analysis of whole-brain cortical thickness was done through the CIVET pipeline, developed at the Montreal Neurological Institute (MNI). First, a multistage quality control process was implemented, as described previously (17, 19, 28), excluding participants with white or gray matter artifacts. All quality-controlled MR images were subsequently processed through the CIVET pipeline. Briefly, images were linearly registered to the ICBM152 template (45–47), corrected for intensity nonuniformity (48), and classified into gray matter, white matter and cerebrospinal fluid, and background using a neural net classifer (INSECT) (49). Next, images were fit with a deformable mesh model to extract the 2D inner (WM/GM interface) and outer (pial) cortical surfaces for each hemisphere, which generates 40 962 cortical points on each hemisphere (50–53), before doing a non-linear registration of both cortical surfaces for each hemisphere to the ICBM152 (47, 53, 54). Next, the reverse of the linear transformation was applied on the images of each subject to achieve cortical thickness estimations at each cortical point in the subject’s native space (55), before calculating cortical thickness (56) at each cortical point (57). A comprehensive description of these processing steps can be found in previous publications (17, 19, 28, 41).
Volumetric measures of the amygdala were obtained from MRI data using a fully automated segmentation method validated in human participants (58). This method utilizes a large MRI dataset (n = 80) of young healthy adults that serves as a template library for manually-labeled amygdalar volumes (59). The manual segmentation was done by four different raters, and intra-class intra-rater and inter-rater reliability varied between r=0.83 for the right and r=0.95 for the left amygdala (60). From this manual segmentation, a fully automated method was derived, characterized by label fusion techniques that combine segmentations from a subset of ‘n’ most similar templates. Specifically, each template is used to produce an independent segmentation of the participant using the ANIMAL pipeline (61), followed by a thresholding step to eliminate cerebrospinal fluid, which results in ‘n’ different segmentations. To fuse the segmentations at each voxel, a voting strategy is used; the label with the most votes from the ‘n’ templates is assigned to the voxel. Combining multiple segmentations minimizes errors and maximizes consistency between segmentations. When using n = 11 templates, the label fusion technique has been shown to yield an optimal median Dice Kappa of 0.826 and Jaccard similarity of 0.703 for the amygdala (58). Of note, even though the template library of manually labeled amygdalar volumes consisted of data from healthy young adults, using the ANIMAL pipeline combined with this template library results in a method fairly resistant to developmental volumetric deviations (particularly for ovoid structures such as the amygdala), as shown by its high Dice Kappa and Jaccard similarity values (58). In addition, previous comparisons between pediatric and adult structural MRI brain templates detected no systematic bias in comparisons between adults and children over 6 years of age in our NIHPD dataset (62).
Hormonal and Pubertal Measures
During each MRI visit, children provided two separate 1–3 cm3 samples of saliva, collected in the lab on the day of the scan, by instructing the subject to spit saliva into the tubes using a plastic funnel, before any scanning or neurocognitive procedures. Subjects were asked to continue to spit until at least 2–3 cc of saliva were collected (duration of sampling: approximately 1 min or less). These samples were subsequently assayed for steroid hormones by enzyme-linked immunosorbent assay (ELISA) methods, and the average results used as a measure of hormonal levels. The intra-assay and inter-assay coefficients of variation (COVs) were 4.1% and 9.1% for estradiol, 6.5% and 16.2% for DHEA, and 6.1% and 13.5% for testosterone, respectively (Salimetrics Salivary ELISA Kit, State College, PA). At the next MRI, a similar procedure was followed, and the child again provided two separate saliva samples for hormonal measurements. All available longitudinal hormonal measurements were included (with a maximum of 3 hormonal measurements, every 2 years, over the course of 4 years, for each individual child); however, because of the constraints of missing or poor-quality data that did not allow hormonal quantification, the resulting sample represents a mix of cross-sectional and longitudinal data.
Salivary sampling measures the unbound, biologically active portions of circulating hormonal levels, which freely crosses the blood-brain barrier and is therefore more relevant to studies of brain-hormone associations than total plasma hormonal levels (63, 64). Estradiol levels have been shown to follow diurnal patterns in response to the pulsatile release of gonadotropin-releasing hormone, with the trough occurring at different times depending on the sex of the child (65, 66). Other steroid hormones have been shown to exhibit seasonal patterns to a certain extent (67), although up to now there has been no conclusive evidence of this phenomenon for estradiol during puberty. Still, to avoid the confounding effects of sex, season (coded as a categorical variable: spring, summer, fall, winter), and time of collection (minutes after midnight) on hormonal levels, we have controlled for these variables in hormonal-related analyses (see Statistical Analyses and Table 2).
Table 2:
Description of statistical models
| Methods section | Statistical model |
|---|---|
|
2.5.1 Estradiol & Cortico-Amygdalar Structural Covariance |
(1) Whole-brain CTh = 1 + Estradiol*Amygdala + Estradiol+Amygdala +
Collection Time + Age + Sex + Scanner + Handedness + Total Brain Volume + random (id) + I (2) Whole-brain CTh = 1 + Estradiol*Amygdala*Sex + Estradiol*Amygdala + Amygdala*Sex + Estradiol*Sex + Estradiol +Amygdala + Sex + Collection Time + Age + Scanner + Handedness + Total Brain Volume + random (id) + I (3) Whole-brain CTh = 1 + Estradiol*Amygdala*Age + Estradiol*Amygdala + Amygdala*Age + Estradiol*Age + Estradiol +Amygdala + Age + Collection Time + Sex + Scanner + Handedness + Total Brain Volume + random (id) + I (4) Whole-brain CTh = 1 + Estradiol*Amygdala*Puberty + Estradiol*Amygdala + Amygdala*Puberty + Estradiol*Puberty + Estradiol +Amygdala + Puberty + Collection Time + Sex + Scanner + Handedness + Total Brain Volume + random (id) + I (5) Note that in order to limit the number of control variables per model: models (1), (2), (3) and (4) were retested while including testosterone, DHEA, pubertal stage, body mass index, or season of sampling as additional covariates (one at a time, in separate models) (6) Quadruple interactions were also tested, with terms of interest listed below: ‘Estradiol*Amygdala*Age*Sex’; ‘Estradiol*Amygdala*Puberty*Sex’ |
|
2.5.2 Cortico-Amygdalar Structural Covariance & Cognitive Tests |
(1) Given significant results with the ‘Estradiol*Amygdala*Age’ term in section 2.5.2: Cognitive Scores = 1 + CTh*Amygdala*Age + CTh*Amygdala + Amygdala*Age + CTh*Age + CTh + Amygdala + Age + Sex + Scanner + Handedness + Total Brain Volume + random (id) + I (2) Model (1) was also retested while including socioeconomic status as an additional covariate |
|
2.5.3 Mediation |
(1) Beta coefficients and p-values extracted from section 2.5.1 (2) Beta coefficients and p-values extracted from section 2.5.2 (3) Beta coefficients and p-values from (1), (2) and (3) were extracted from existing analyses, and entered in the Sobel-Goodman test calculator to formally test mediation effects of scans per participant (see ‘Methods’) |
The specific statistical term of interest is underlined in each model; the rest of the terms represent control variables.
‘id’ refers to a specific participant’s identification number: this term is included in order to identify and link all longitudinal data from the same participant
‘I’ to the identity matrix of the mixed effects model
‘CTh’ in section 2.5.2 refers to average cortical thickness of the brain regions found to be significant in section 2.5.1
Pubertal maturation was measured using the Pubertal Development Scale (PDS), which was administered by a physician to all participants included in this study, at each visit (68). This scale has been shown to have good reliability (Cronbach’s alpha coefficient: 0.77) (68). In addition, moderate to high correlations (r2=0.61–0.67) between PDS scores and physical examinations by a physician (i.e. the gold standard test for pubertal staging) have been reported, thereby establishing the validity of this scale (69). The PDS rates on a 4-point scale (1=no development to 4= development completed) the amount of change or development in five physical pubertal characteristics (pubic hair growth, skin changes, growth spurt, as well as facial hair and voice deepening in boys, and breast development and menarcheal status in girls). The total score was calculated by taking the sum of the five indicators and dividing by 5, to preserve the original 4-point metric (70). Total scores from the PDS were then converted to a puberty variable consisting of 5 stages, representing increasing levels of physical maturity similar to Tanner staging. The following recommended cut-offs (71) were used: Prepubertal (stage 1) = 0 to 1.7; Early Pubertal (stage 2) = 1.8 – 2.4; Midpubertal (stage 3) = 2.5 – 3; Late Pubertal (stage 4) = 3.1 – 3.6; Post pubertal (stage 5)= 3.7 – 4. As mentioned previously, we restricted the sample to data points collected in girls and boys with pubertal stage of 4 or less. No data points were included beyond pubertal stage 4. We found that excluding all girls who have had menarche was overly restrictive, as girls who are pubertal stage 4 or less tend to have anovulatory rather than regular, ovulatory menstrual cycles (72). At least half of the menstrual cycles in the 2 years post-menarche can represent anovulatory cycles, and it can take up to four years post-menarche for 90% of the menstrual cycles to be regular, though anovulatory cycles can still occur (72). Of note, pubertal stage does not match with chronological age, and some adults never reach pubertal stage 5 (73). All longitudinal pubertal measurements were included whenever available, and attempts were made to collect and include this data in statistical models for each child during each of their visits.
Cognitive Measures
Cognitive measures were administered each time the participant underwent a scan, and by extension, each time hormonal measures were collected. We measured: (1) anxious and depressed symptoms using the Child Behavior Checklist (CBCL) Anxious-Depressed sub-scale, which measures the frequency of anxious and depressed symptoms in the past 6 months via parental report; (2) verbal and language skills using the Woodcock-Johnson (WJ) Letter-Word Identification and Passage Comprehension tests, which features word identification skills and the understanding of written text, respectively (38–40); and (3) non-verbal spatial skills using the Cambridge Neuropsychological Test Automated Battery (CANTAB) Spatial Working Memory test, which features use of self-guided spatial search strategies and mental manipulations of spatial cues (37).
The CBCL is an age-appropriate standardized questionnaire completed by parents (74–77), and its Anxious-Depressed sub-scale is a clinically useful way to assess both depressive and anxious symptoms in children and adolescents (36). This sub-scale is a reliable measure with high stability over time (8-day test–retest reliability is r = 0.82, Cronbach’s α = 0.84; 12- and 24-month stabilities are r = 0.68 and r = 0.56, respectively) (78), has been validated in multiple cultures (74–77), and has been found to correlate with the diagnosis of adolescent depression (76, 79). The adult version of CBCL, the Young Adult Self Report (YASR) A/D syndrome provides similar information in subjects over 18 years of age, but is based on self-report rather than parental ratings (78).
The WJ is a cognitive battery measuring intelligence level and learning capacities, which uses interpretative scaling to predict how the individual would perform similar tasks in real-life, functional settings (38–40). It has been validated on large pediatric populations, with a median reliability coefficient of 0.9 for tests relating to verbal comprehension (38–40). The WJ Letter-Word Identification, and Passage Comprehension tests were selected (in contrast to other verbal items such as Spelling and Writing) because they directly test language skills without involving motor skills.
The CANTAB is a computerized battery that includes only nonverbal geometric designs or simple shapes, with minimal required language proficiency (37). The validity of the CANTAB for assessing brain-behavior relations in adults has been established, and results of tests in pediatric populations showed that children can be tested with the same item sets that are employed in adult studies (37). Reliability is high in pediatric populations (internal consistency coefficients=0.73 for reaction time, and 0.95 for performance on the spatial working memory test), and construct validity has been established in pediatric populations (37).. The CANTAB Spatial Working Memory subtest was selected (specifically, the CANTAB: Spatial working memory subtest, ‘use of strategy’), as opposed to other spatial-related items such as the Spatial Recognition Memory and Spatial Memory Span, because of its emphasis on self-guided and strategic spatial search (for which men typically outperform women), as opposed to reliance on spatial reference memory (for which women typically outperform men) (1).
There is a large literature documenting the existence of a gap between children from low and high socio-economic status (SES), with children with low SES backgrounds performing below children from higher SES backgrounds on tests of intellectual and academic achievement as well as emotion regulation (81, 82). To control for potential confounding effects of SES on intellectual and emotional development, we included SES as a control variable in all analyses related to cognition. SES was measured as a function of adjusted family income levels, according to methods established by the Department of Housing and Urban Development (HUD), which consider regional cost of living and family size. These “HUD-adjusted” incomes better equate income across sites and regions, thus providing a more meaningful indicator of socioeconomic status than unadjusted family income levels (83).
Statistical Analyses
Statistical analyses were done using SurfStat (Matlab toolbox: http://www.math.mcgill.ca/keith/surfstat/) and SPSS 21.0 (SPSS, Inc., Chicago, Illinois). Mixed effects analyses were used to take into account the within- and between- individual variances in this longitudinal sample, which allows the modeling of the trajectory of brain structural changes within a single child (within-individual) as well as the cumulative trajectory of the group of children over time (between-individuals), allowing for random variation of slopes between different subjects. For all analyses, we examined structural covariance between whole-brain CTh and both the right and left amygdalae. In the mixed effects models, all continuous variables were centered using their respective means. A correction for multiple comparisons across the whole brain, using random field theory (RFT, p<0.05), was applied to all analyses (84). RFT has been deemed to be preferable to a Bonferroni correction when handling neuroimaging data, and is widely used in the neuroimaging literature (85).
Estradiol-Related Cortico-Amygdalar Structural Covariance
To be more conservative, no a priori region of interest was identified for this section of the analyses, given the scarcity of the data available on estradiol-related cortico-amygdalar structural covariance. These mixed effects models were used to examine, first, the relationship between estradiol and amygdalar volume; then, the relationships between estradiol and whole-brain, native-space cortical thickness (CTh), and finally, the relationship between estradiol and covariance of the amygdala with CTh, controlling for the effects of age, sex, total brain volume, scanner, handedness, and time of salivary sampling. The primary statistical model of interest that was tested was:
Whole-brain CTh = 1 + Estradiol*Amygdala + Estradiol+Amygdala + Collection Time + Age + Sex + Scanner + Handedness + Total Brain Volume + id + I
Where “id” refers to the random effect of a specific participant’s identification number in order to identify and link all longitudinal data from the same participant, “I” refers to the identity matrix of the mixed effects model.
To examine associations between estradiol and structural covariance of the amygdala, we examined the interaction term ‘Estradiol*Amygdala’ on whole-brain cortical thickness, while controlling for all of the covariates described above. Because age, sex, and pubertal effects may also impact the relationship between estradiol and cortico-amygdalar structural covariance, we also tested the triple interactions ‘Estradiol*Amygdala*Age’, ‘Estradiol*Amygdala*Sex’, and ‘Estradiol*Amygdala*Puberty’, as well as quadruple interactions looking at ‘Estradiol*Amygdala*Age*Sex’; ‘Estradiol*Amygdala*Puberty*Sex’ effects, for significance.
In order to limit the number of control variables per model, additional separate models were conducted to examine any distinct effects of estradiol above and beyond other covariates (i.e., its precursor hormones DHEA and testosterone, pubertal stage, body mass index, and season of collection), by entering each of these covariates, one at a time, in separate models. Statistical models are summarized in Table 2.
Cortico-Amygdalar Structural Covariance and Cognition
To examine associations between estradiol-related cortico-amygdalar structural covariance and cognition, we averaged CTh of each brain cluster found to be significant in the estradiol-related cortico-amygdalar structural covariance models and examined the impact of cortico-amygdalar covariance on cognitive measures, while controlling for age, sex, total brain volume, scanner, and handedness. The statistical model that was tested was:
Where CTh refers to average cortical thickness of the brain regions found to be significant in the estradiol-related corticol-amygdala structural covariance models, “id” refers to a specific participant’s identification number in order to identify and link all longitudinal data from the same participant, “I” refers to the identity matrix of the mixed effects model.
In addition, because socioeconomic status has been previously shown to predict emotional and cognitive development (81, 82) as described in Method section Cognitive Measures, the same model was retested including SES as a control variable.
Mediation Effects of Cortico-Amygdalar Structural Covariance
We tested whether cortico-amygdalar structural covariance (covariance between the brain areas identified in the Estradiol-Related Cortico-Amygdalar Structural Covariance analyses and left/right amygdalar volumes) could mediate the relationship between estradiol and working memory, based on data collected within the same time point. First, we extracted the beta coefficients and standard errors for the relationship between estradiol and cortico-amygdalar covariance. Similarly, we extracted the beta coefficients and standard errors of the relationships between cortico-amygdalar covariance and the different cognitive tests. Beta coefficients and standard errors were then entered in the Sobel-Goodman test calculator to test mediation effects (http://quantpsy.org/sobel/sobel.htm).
To test mediation and moderation effects we used the Baron-Kenny criteria augmented by a formal Sobel’s test. This approach treats each relationship (between predictor and moderator, and then between moderator and outcome) separately, allowing us to model both the cross-sectional and longitudinal component of the data (i.e., with multiple scans per participants and different number of scans per participant). The same set of control variables (as listed in Methods, 2.5.1) was used for the mediation analyses.
RESULTS
Sample Characteristics
Table 1 details sample characteristics, including number of scans and covariates of interest. The sample included 152 participants (female=65) and 203 scans (female=78), with an age range of 6 to 22 years (mean = 11.91–13.99 years, SD = 3.13–3.93 years). Figure 1 shows estradiol levels by pubertal stage and by age. Samples below the lower limit of detection (LOD; 1*10^−5 ug/dL) were excluded from our analyses. The minimum estradiol level in our restricted sample was 2.13 ug/dL, with the majority of estradiol values between 5–20 ug/dL, for both boys and girls.
FIGURE 1. Variation of estradiol levels by pubertal stage, sex and age.
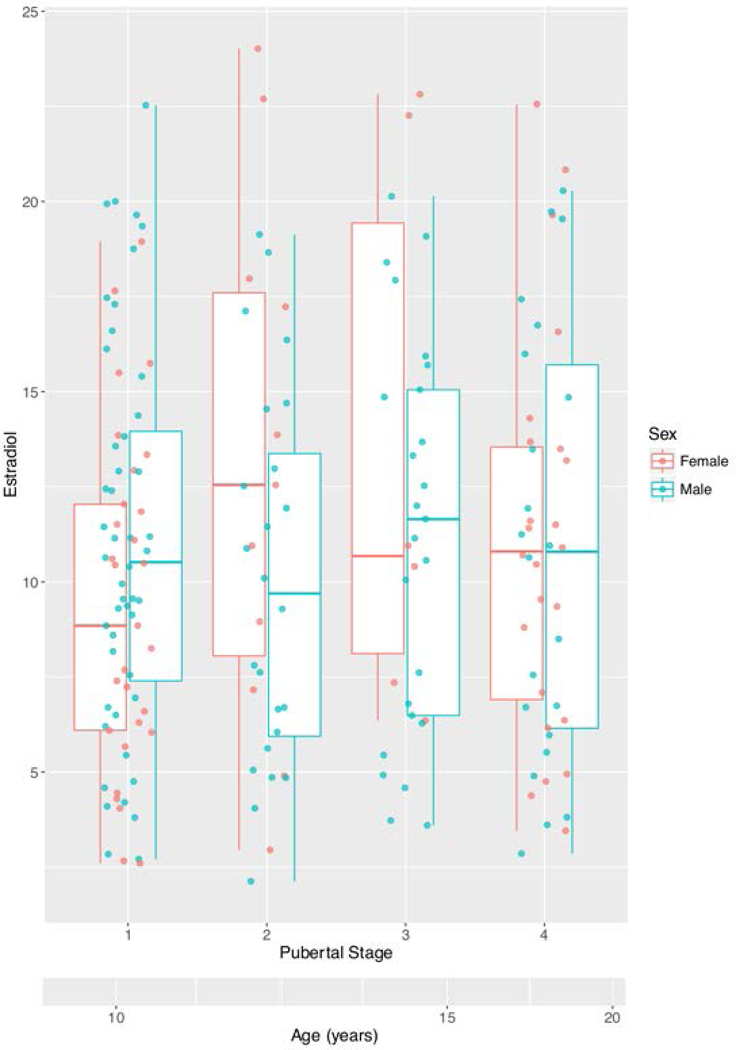
Because of concerns concerning the unreliability of the ELISA assay at the lower limit of detection (LOD; 1*10^−5 ug/d), none of the LOD samples was included in our analyses. The minimum estradiol level in our restricted sample was 2.13 ug/dL, with the bulk of estradiol values between 5–20 ug/dL, for both boys and girls. Mean age per pubertal stage are indicated on the scale at the bottom of the figure. Most children matured from pubertal stages 1–3 between ages 10–15 years old and from pubertal stages 3–4 between 15–20 years old. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
Estradiol-Related Cortico-Amygdalar Structural covariance
As shown in Figures 2, 3 and 4, whole-brain linear mixed models revealed an age effect on the relationship between estradiol levels and the structural covariance between left, as well as the right amygdala and the following brain regions (statistics for the ‘Estradiol*Age*Amygdala’ interaction listed below):
FIGURE 2. Age-related associations between estradiol, amygdala-frontal structural covariance and word identification.
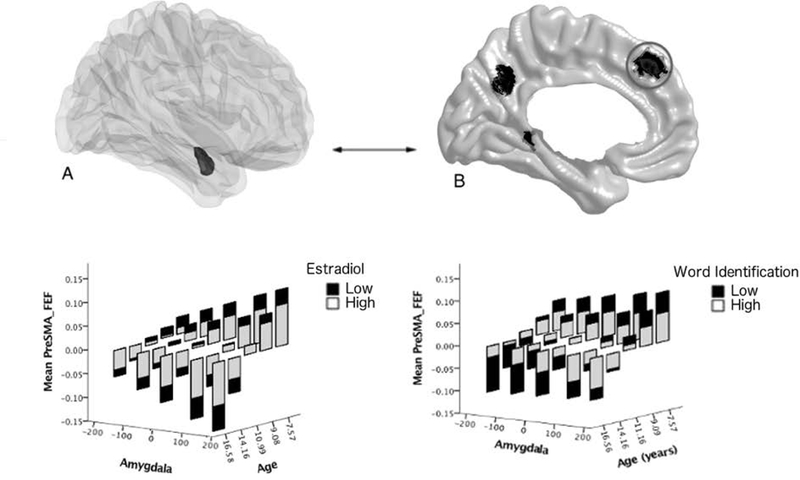
Brain figures A (the amygdala is displayed in the dark shaded region) and B (cortical regions are displayed in dark shaded regions) show the brain regions involved in the significant ‘Estradiol*Amygdala*Age’ interaction, with a focus in this figure on the left pre-supplementary motor area and frontal eye field (Pre-SMA and FEF). In order to visualize the interactions between estradiol, age, cortico-amygdalar structural covariance and cognition, graphs display estradiol and cognitive scores as dichotomous groups and each child’s longitudinal structural covariance trajectory is not included. Y axis represents residuals for cortical thickness (averaged over the significant region of the left SMA/FEF). The bars represent the range of expected cortical thickness residuals for each age bracket (e.g. 7–9 years) based on the parameters obtained from the mixed effect model. Age brackets were restricted to the ages for which there were the greatest number of data points (approximately 95% of the data were collected between 7–17 years of age, though the full age range of the sample is 6–22 years old). Note: The data are presented categorically for visualization purposes, i.e. continuous measures of estradiol, age, cortical thickness, amygdalar volumes, and cognitive scores were used in all analyses. (Full statistics are provided in the Results, sections Estradiol-Related Cortico-Amygdalar Structural Covariance and Cortico-Amygdalar Structural Covariance and Cognition). Data from the left amygdala are shown; similar results were present for the right amygdala. The lower left panel shows the age-specific relationship between estradiol and amygdala-frontal structural covariance. Amygdala-frontal covariance switched from positive to negative with increasing age. Higher estradiol levels tended to diminish this age effect such that there was a decrease in positive covariance in younger children and a decrease in negative covariance in older children. The lower right panel shows the age-specific relationship between estradiol-related amygdala-frontal covariance and word identification. The amygdala-frontal covariance related to higher estradiol levels was associated with higher word identification scores across the age range examined.
FIGURE 3. Age-related associations between estradiol, amygdala-posterior cingulate structural covariance and reading comprehension.
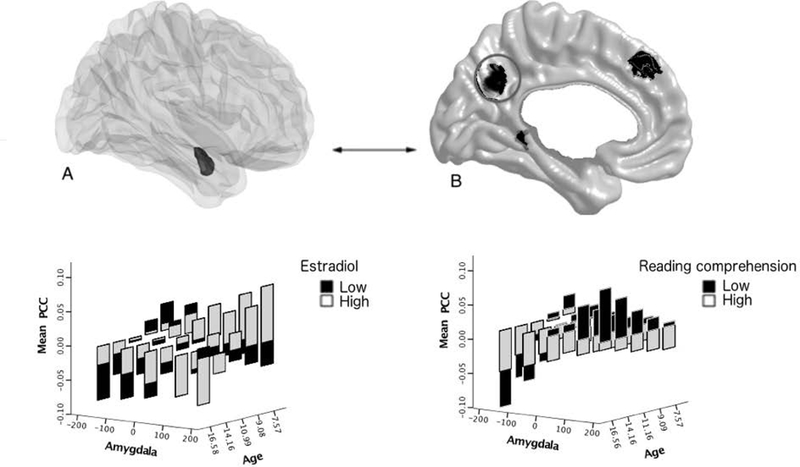
Brain figures A (the amygdala is displayed in the dark shaded region) and B (cortical regions of interest are displayed in dark shaded regions) show the brain regions involved in the significant ‘Estradiol*Amygdala*Age’ interaction, with a focus in this figure on the left posterior cingulate cortex. Similar to Figure 2, modifications were made to the data in order to visualize the interactions between estradiol, age, cortico-amygdalar covariance and cognition (see Figure 2 caption for more details). The graph shown in the lower left panel shows the age-specific relationship between estradiol and amygdala-posterior cingulate structural covariance. Amygdala-cingulate covariance switched from a positive to a negative relationship with age (from younger to older children). Higher estradiol levels enhanced this age effect in younger children, such that there was an increase in positive covariance, and diminished this effect in older children, with a decrease in negative covariance. The graph shown in the lower right panel shows the age-specific relationship between estradiol-related amygdala-posterior cingulate covariance and reading comprehension. The amygdala-cingulate covariance related to higher estradiol levels was associated with higher reading comprehension scores in older children, but this effect was reversed in younger children.
FIGURE 4. Age-related associations between estradiol, amygdala-retrosplenial structural covariance and spatial working memory.
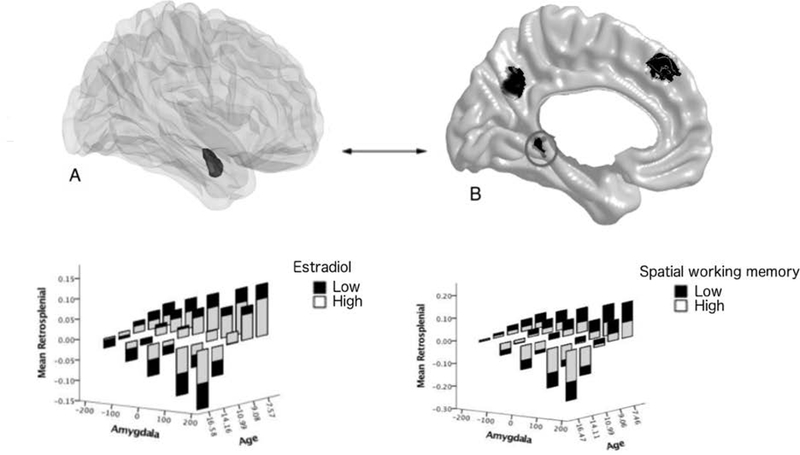
Brain figures A (the amygdala is displayed in the dark shaded region) and B (cortical regions of interest are displayed in dark shaded regions) show the brain regions involved in the significant ‘Estradiol*Amygdala*Age’ interaction, with a focus in this figure on the left retrosplenial cortex. Similar to Figure 2, modifications were made to the data in order to visualize the interactions between estradiol, age, cortico-amygdalar covariance and cognition (see Figure 2 legend for more details). The graph in the lower left panel shows the age-specific relationship between estradiol and amygdala-retrosplenial structural covariance. Amygdala-retrosplenial covariance switched from a positive to a negative relationship with age (from younger to older children). Higher estradiol levels diminished this age effect (such that there was a decrease in positive covariance in younger children, and a decrease in negative covariance in older children). The graph in the lower right panel shows the age-specific relationship between estradiol-related amygdala-retrosplenial covariance and spatial working memory. The amygdala-retrosplenial covariance related to higher estradiol levels was associated with higher spatial working memory scores across the age range examined.
-
(1)
CTh of the left pre-supplementary motor area and frontal eye field (Brodmann area 8, left amygdala: r (for the age interaction)=3.4 × 10−1, SE=8.4 × 10−2, RFT cluster-level p (controlled for multiple comparisons across the whole brain)=3.3*10−2, peak vertex id 28066 [x=−4.5, y=39.8, z=37.1, 70 voxels; right amygdala: r=3.3 × 10−1, SE=7.0 × 10−2, cluster-level p=4.7*10−4, peak vertex id 28219 [x=−6.0, y=32.0, z=36.7], 106 voxels)
-
(2)
CTh of the left posterior cingulate cortex (Brodmann area 31, left amygdala: r (for the age interaction)=2.8 × 10−1, SE=7.1 × 10−2, RFT cluster-level p (controlled for multiple comparisons across the whole brain)=6.0*10−3, peak vertex id 30497 [x=−4.3, y=−62.6, z=33.0], 146 voxels; right amygdala: r=3.1 × 10−1, SE=7.3 × 10−2, cluster-level p=5.0*10−4, peak vertex id 31196 [x=−5.9, y=−61.9, z=40.5], 198 voxels)
-
(3)
CTh of the left retrosplenial cortex (Brodmann area 30, left amygdala: r (for the age interaction)=3.0 × 10−1, SE=7.2 × 10−2, RFT cluster-level p (controlled for multiple comparisons across the whole brain)=3.2*10−3, peak vertex id 40624 [x=−9.8, y=−44.3, z=−1.7], 108 voxels; right amygdala: r=3.3 × 10−1, SE=8.2 × 10−2, cluster-level p=2.8*10−3, peak vertex id 38886 [x=−20.3, y=−48.0, z=−0.3], 123 voxels)
More specifically, age-related changes in cortico-amygdalar structural covariance tended to switch from a positive covariance in younger children to a negative relationship in older children. Higher estradiol levels tended to diminish this age effect (a decrease in positive covariance in younger children and a decrease in negative covariance in older children) for the left pre-supplementary motor area/frontal eye field and retrosplenial cortex (across the age range examined) and for the posterior cingulate cortex (only in older children).
Post-hoc probing testing for moderational effects of age on the relationship between estradiol, amygdalar volumes and cortical thickness showed that:
-
1)
for the pre-supplementary motor area and frontal eye field, only the effect in older children was significant (5th age quintile >16 years, for the 3rd quintile of amygdalar volumes, p=3.98*10−2);
-
2)
for the posterior cingulate cortex, both the effects in younger children and older children were significant (1st quintile 6-<8 years, for the 3rd-5th quintiles of amygdalar volumes, p<0.00001; 2nd quintile 8-<10 years, for the 3rd-5th quintiles of amygdalar volumes, p<0.00001; 3rd quintile 10->=13 years, for the 3rd-5th quintiles of amygdalar volumes, p=0.0014–0.0051; 5th quintile >=16 years, for the 3rd-5th quintiles of amygdalar volumes, p=0.05–0.0002);
-
3)
for the retrosplenial area, no single age quintile emerged as significant (all p-values>0.05).
No other brain region met the threshold for significance (RFT, p<0.05). Adding DHEA, testosterone, pubertal stage, body mass index, and season of sampling as control variables (one at a time, to limit decreases in power) did not alter the significance of the findings. Finally, there were no significant sex or pubertal interactions between estradiol and cortico-amygdalar structural covariance, i.e. no significant effects of ‘Estradiol*Amygdala*Sex’; ‘Estradiol*Amygdala*Sex*Age’; ‘Estradiol*Amygdala*Puberty’; and ‘Estradiol*Amygdala*Puberty*Sex’ on CTh. Of note, we found no significant associations (age-dependent or otherwise) between estradiol and cortical thickness, or between estradiol and amygdalar volume. Therefore, estradiol only affects the developmental relationship, or covariance, between amygdalar volume and the cortical thickness of specific areas of the frontal, parietal and occipital lobes, rather than the development of any of these brain structures in isolation.
Cortico-Amygdalar Structural covariance and Cognition
As shown in Figures 2, 3 and 4, we found an age effect on the relationship between estradiol-related cortico-amygdalar structural covariance and the cognitive tests. Specifically, we found that age interacted with: (1) the amygdalar-frontal covariance on the WJ-Letter Word Identification test (interaction with age: left amygdala: r=−3.0 × 10−5, SE=1.1 × 10−5, p=5.0 × 10−3, right amygdala: r=−3.2 × 10−5, SE=1.1 × 10−6, p=4.0 × 10−3, adjusted for SES: p=2.6*10−2); (2) the amygdalar-posterior cingulate covariance on the WJ-Passage Comprehension test (interaction with age: left amygdala: r=−8.6 × 10−5, SE=2.5 × 10−5, p=1.0 × 10−3; right amygdala: r =−1.5 × 10−5, SE=6.0 × 10−6, p=1.2 × 10−2, adjusted for SES: p<0.0001); and (3) the amygdalar-retrosplenial covariance on the CANTAB Spatial Working Memory test (interaction with age: left amygdala: r=−2.6 × 10−5, SE=8.0 × 10−6, p=2.0 × 10−3; right amygdala: r=−3.5 × 10−5, SE=9.0 × 10−6, p<1.0 × 10−3; adjusted for SES: p=2.6*10−2).
More specifically, the amygdalar-frontal and amygdalar-retrosplenial covariance related to higher estradiol levels was associated with higher word identification and spatial working memory across the age range examined, while the amygdala-cingulate covariance related to higher estradiol levels was associated with higher reading comprehension only in older children. In contrast, there were no significant age interactions between estradiol-related cortico-amygdalar structural covariance and the CBCL Anxious-Depressed subscale.
Mediation Effects of Cortico-Amygdalar Structural covariance
As shown in Figure 5, age-related differences in cortico-amygdalar covariance were found to significantly mediate the age-specific relationship between estradiol and word identification (age interaction, left amygdala: p=1.6 × 10−2; right amygdala: p=1.3 × 10−2), estradiol and reading comprehension (age interaction, left amygdala: p=8.8 × 10−3; right amygdala: p=3.3 × 10−2), and estradiol and spatial working memory (age interaction: left amygdala: p=9.9 × 10−3; right amygdala: p=5.1 × 10−3) throughout the age range examined.
FIGURE 5. Age-related differences in cortico-amygdalar structural covariance mediate the developmental relationship between estradiol and cognition.
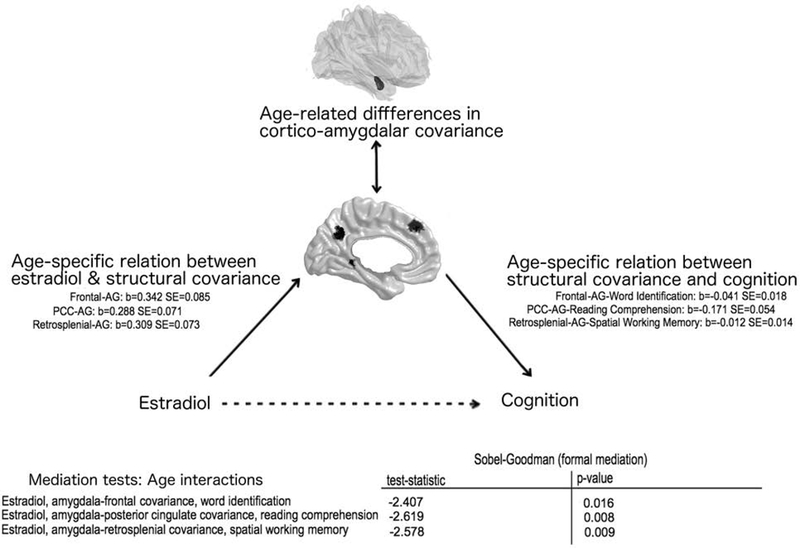
The beta coefficients and standard errors are displayed on the left, for the age-specific relationships between estradiol and cortico-amygdalar covariance, and on the right, for the age-specific relationship between cortico-amygdalar covariance and cognition. Test statistics and p-values for the formal Sobel-Goodman mediation tests are displayed in the bottom right table. Data from the left amygdala are shown; similar results were present for the right amygdala. AG: Amygdala
DISCUSSION
Results from this study show age-specific effects on the relationship between estradiol, cortico-amygdalar structural covariance and cognition. Estradiol was found to diminish the impact of age on cortico-amygdalar structural covariance for the pre-supplementary motor area/frontal eye field and retrosplenial cortex (across the age range examined), and for the posterior cingulate cortex (in older children). Estradiol-related cortico-amygdalar covariance (i.e. the covariance pattern seen at higher estradiol levels) was associated with higher performance on cognitive tasks (higher verbal skills and spatial skills), but not to frequency and severity of anxious-depressed symptoms. There were no significant sex effects of estradiol on either brain structure or cognition.
Estradiol-related cortico-amygdalar structural covariance networks partially overlapped with structural covariance networks between the amygdala and fronto-parietal regions outlined in a prior study (13). However, in contrast to our group’s prior findings regarding cortical-amygdalar structural networks, estradiol-related cortico-amygdalar covariance was found to be age-sensitive. Thus, by accounting for estradiol, we are uncovering novel developmentally-sensitive patterns of cortico-amygdalar covariance. Further, we show that these estradiol-related structural cortico-amygdalar covariance patterns have functional relevance as they are associated with significant alterations in cognitive ability. Interestingly, these results are consistent, at least in part, with prior studies suggesting that estradiol may be closely associated with the development of long-range white matter tracts and cortico-limbic functional connectivity (37, 38).
These results confirm the importance of developmental timing on the effects of estradiol, by outlining specific estradiol-related cortico-amygdalar structural networks during development that are not traditionally associated with cognitive function. Interestingly, there were no age interactions between estradiol-related cortico-amygdala covariance and anxious/depressed symptoms measured with the CBCL, perhaps due to the fact that our sample only included typically developing children, with relatively little variance in CBCL scores. Importantly, the data add to the growing literature supporting an important role for the amygdala beyond that of an emotional processing center, i.e. in the development of verbal (25–27) and spatial abilities (22–24).
Our observations of an age-related change in the relationship between estradiol, cortico-amygdalar networks and cognition during the pubertal period are consistent with prior reports of estradiol’s variable effects during the prenatal and perinatal period (5). For example, while estradiol is involved in masculinizing the preoptic area during the rat perinatal period, it induces cell death in the anteroventral periventricular region of the hypothalamus but prevents cell death in the preoptic area during the rat’s pubertal period. In addition, the lack of an interaction between estradiol and pubertal stage on cortico-amygdalar structural covariance suggests that the developmental effects of estradiol may be modulated, at least in part, by age-related processes independent from the effects of other pubertal steroid hormones.
Several mechanisms may underlie these findings. For example, there is evidence of an age-related variation in the CNS density and distribution of estrogen receptors alpha (ESR1) and beta (ESR2) (86, 87). ESR2 knockout mice display both neuronal hypocellularity and glial proliferation within cortical and limbic regions, respectively, and carry an increased risk of age-related neurodegeneration compared with wild-type mice (88, 89). ESR1 knockout mice display comparatively little brain pathology, though some studies have described decreased hypothalamic volumes in the pre-optic area (90) as well as decreased expression of a neuronal calcium-binding gene, potentially reflecting neuronal hypocellularity in the frontal cortex and cerebellum (91). In addition, ESR1 goes from relatively low to high levels in the human cortex across the age range examined (87), while staying at relatively stable (and high) levels in the amygdala (92, 93). In contrast, ESR2 tends to show relatively stable, high expression in the cortex across the age range targeted in this study (87).
Thus, although much remains to be clarified with regards to their actions in the CNS, it is possible that these differences in estrogen receptor trajectories may have supported more apoptotic and anti-proliferative actions of estradiol in the cortex of younger children (dampening the age-related positive covariance between the cortex and the amygdala) and more neuroprotective actions in older children (dampening the age-related negative covariance between the cortex and amygdala). Indeed, age was associated with a shift from a positive to an increasingly negative cortico-amygdalar covariance over time, with younger children tending to show more positive cortico-amygdalar covariance and older children tending to show more negative cortico-amygdalar covariance. In contrast, higher estradiol levels tended to limit or oppose the effect of age within each quintile, such that higher estradiol levels were associated with a positive –> negative shift in covariance for younger children, as well as a negative -> positive shift in covariance for older children. Post-hoc analyses suggest that these age-related effects of estradiol may be more prominent in the posterior cingulate cortex than in the frontal and retrosplenial cortex. One potential explanation for this could be the existence of other influences, such as androgen levels, that can also significantly alter cortical thickness in the posterior cingulate cortex. For example, our group previously documented competing influences of DHEA and testosterone on cortical thickness of that area, particularly in younger, pre-pubertal children (19).
Another potential mechanism that may underlie age-specific effects of estradiol is the variation in membrane-initiated, rapid vs. slower genomic signaling (requiring nuclear receptor translocation) that occurs with age (97). Neuroprotective actions of estrogens may predominantly rely on membrane-initiated signaling (98). Thus, age-related shifts in estrogen signaling may lead to a relatively more prominent effect of rapid estrogen signaling (and perhaps, neuroprotective effects) in older children (99). Still, our study was not designed to answer mechanistic questions, and therefore definitive confirmation of the pathophysiology underlying estradiol-related effects will require further investigation.Our current findings support the concept that the developmental effects of testosterone and estradiol on the human CNS can be differentiated from one another. Indeed, our group has previously found testosterone to be associated with prefrontal-amygdalar and prefrontal-hippocampal covariance, in regions distinct from those involved in estradiol-related cortico-amygdalar networks (16, 18). In turn, testosterone-related cortico-limbic structural covariance predicted aggression levels and lower executive function, particularly in boys (16, 18). In contrast, the age-specific effects of estradiol in this study were not found to vary by sex, and showed no clear masculinization effects, supporting the notion that estradiol plays a less prominent role in sexual differentiation in humans than in lower animals (100, 101). Together these findings suggest that during puberty, influences other than estradiol (e.g. androgens) may be involved in brain masculinization in humans (100, 101), and may be more important in sexual differentiation of cortico-limbic structural covariance.
In fact, estradiol-sensitive neural networks were more similar to those related to DHEA than those related to testosterone. Indeed, prior studies from our group regarding DHEA-related structural covariance also showed non-sexually dimorphic relationships between the amygdala and different areas of the frontal, parietal and occipital areas (6, 11). These areas did not perfectly overlap with those of estradiol-related networks but are certainly closely interconnected. These DHEA-sensitive cortico-amygdalar networks were associated with reading, visual decoding, and visuo-motor abilities, again similar to the effects of estradiol on verbal and spatial abilities described in the present study.
We have hypothesized that findings from these prior studies supported a beneficial effect of DHEA on cognition through an inhibition of bottom-up influences, i.e. an inhibition of amygdalar afferents on cortical function. Similarly, results of the present study support the notion that estradiol may alter structural brain development in a way that optimizes verbal and spatial abilities. Thus, estradiol may be conceptualized in this context as a DHEA ‘metabolite’ or ‘end-product’ that may further enhance its beneficial effect on cognitive function, perhaps by altering the balance between top-down and bottom-up influences. However, in contrast to DHEA-related effects, which did not interact with age, there is a shift in estradiol-related structural networks that may be interpreted as increased pruning/apoptosis in younger children vs. growth/neuroprotective effects in older children.
Interestingly, these opposing structural covariance patterns related to estradiol levels both predicted higher verbal and spatial skills -albeit at different ages-, highlighting the dynamic nature of the relationship between brain structure and cognition during childhood and adolescence. Taking these findings together, one could speculate that: (1) in younger children, estradiol enhances cognition by inhibiting the effect of amygdalar afferents on cortical function, perhaps by decreasing the emotional salience of distracting stimuli; (2) in older children, estradiol enhances cognition by increasing the effect of amygdalar afferents on cortical function, perhaps by increasing the emotional salience of relevant stimuli.
A sex difference was not detected, which was unexpected but may be explained by the methodological approach and the age range studied. The key aim of this study was to assess estradiol-related cortico-amygdala covariance- thus, because estradiol levels vary widely across the menstrual cycle at advanced stages of pubertal development when regular cycles occur (as opposed to a predominance in anovulatory cycles during earlier pubertal stages) (72), we restricted our sample range to include only participants with pubertal stage 4 or less. Future studies should examine whether sex differences exist in estradiol-related cortico-amygdala covariance in individuals at full sexual maturity, as well as how the structural covariance can differ with endogenous estradiol levels (e.g., across the menstrual cycle, or pre- post-menopause) and with exogenous administration (e.g., various forms of contraception in the reproductive years, as well as the effects of hormone replacement therapy in post-menopausal women).
Finally, the relationships seen here between estradiol-related structural covariance and higher verbal and spatial skills are compatible with previous investigations into the beneficial effects of estradiol on cognition. Indeed, studies of estrogen administration have reported improved oral reading and overall executive function (102, 103). There may also be beneficial effects of estradiol on visuospatial perception, retinal sensitivity and smooth pursuit eye movements (104–107). Interestingly, the latter is regulated by a set of cortical regions known as the cortical pursuit system, and which includes the pre-supplementary motor area and frontal eye fields, among other structures (108, 109). Finally, the association between estradiol-related structural covariance and improved reading comprehension (at least in older children) replicates other reports of ERT-induced metabolic and cholinergic activity in the posterior cingulate cortex (110, 111) and ERT-induced improvements in oral reading (102).
4.1. Strengths and Limitations
Strengths of our study include the large developmental dataset, including the repeated collection of hormonal, neuroimaging and cognitive data, and the pubertal matching of the boys and girls in this sample. Because of issues related to missing data and MRI or hormonal data quality, a significant proportion of the longitudinal data was lost, resulting in data that is partly cross-sectional and partly longitudinal in nature. Future studies will be needed to confirm that the developmental effects seen in this study can be replicated within individuals followed longitudinally across the entire age span of this study (6–22 years old).
Because the CNS has the capacity to produce local amounts of estradiol (5, 10), the question remains open as to what extent findings of studies examining peripheral estradiol levels (as measured in this study) can be generalized to those examining CNS estradiol levels. During fetal life, most of the peripheral production of estradiol is bound to alpha-fetoprotein and cannot cross the blood-brain barrier (BBB) (112). However, after birth levels of alpha-fetoprotein drop precipitously, allowing peripheral estradiol to cross the BBB and enter the CNS during subsequent developmental periods such as puberty (113, 114). Studies comparing peripheral and central estradiol levels showed significant correlations between estradiol levels in plasma, cerebral cortex, cerebellum, spinal cord and sciatic nerve (115), supporting the concept that peripheral estradiol levels can represent a valid approximation of CNS levels and in turn, may have functional relevance in the context of brain-hormone studies.
An additional concern in hormone-brain association studies is the presence of within- or between-individual hormonal variation due to confounding factors. In that regard, the lack of menstrual cycle information (i.e. number of days passed since the last menstrual period) could be construed as a significant limitation. However, we also know that there is a predominance of anovulatory -as opposed to ovulatory- cycles throughout adolescence (42). As such, duration of menstrual cycle varies widely in this population, and many of these cycles cannot be meaningfully divided into follicular or luteal phases (42), thereby rendering any information about the number of days passed since the last menstrual period less relevant with regards to hormonal levels.
The lack of significant pubertal interactions in the current study does not preclude an influence of other pubertal hormones on estradiol-related cortico-amygdalar structural covariance. Rather, it suggests that age, when entered as a continuous variable (6–22 years old), likely represents a more precise estimate of physical maturation than pubertal staging (more crudely accounted by 5 stages). Finally, as opposed to prior findings indicating lateralization of language skills (with left>right amygdala) (25–27), no difference in the direction of findings was found between structural covariance of the left vs. right amygdala, though findings related to the left amygdala were, as a whole, more significant than those related to the right amygdala.
Finally, although the child (CBCL) and adult (YASR) versions of this scale show significant stability over time, the significant qualitative difference between the parental CBCL ratings and the self-report required through the YASR represents a limitation and may have restricted our power to detect age-related effects of estradiol on anxious-depressed symptoms.
4.2. Conclusions
In this study, we found age-specific effects on the relationship between estradiol-related cortico-amygdalar structural covariance and verbal/spatial skills, but not anxious-depressed symptoms. These findings add to our understanding of the complex relationship between estradiol, brain structure and cognition by confirming the importance of developmental timing on the CNS effects of estradiol and the non-sexually dimorphic role of estradiol and cortico-amygdalar structural networks in cognitive functions distinct from emotional processes. As such, these results support the concept that, as opposed to lower animals, influences other than estradiol (e.g. androgens) may have a more important impact on sexual differentiation of the brain in humans.
ACKNOWLEDGEMENTS:
This work was supported by Federal funds from the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02–3343, N01-MH9–0002, and N01-NS-9–2314, −2315, −2316, −2317, −2319 and −2320). Tuong-Vi Nguyen and Sherri Lee Jones were funded by the Fonds de recherche Québec – santé.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES
- 1.Weinstock M Prenatal stressors in rodents: Effects on behavior. Neurobiol Stress. 2017; 63–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. P Natl Acad Sci USA. 2012; 109(20): E1312–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen TV, Jones SL, Elgbeili G, Monnier P, Yu C, Laplante DP, King S. Testosterone-cortisol dissociation in children exposed to prenatal maternal stress, and relationship with aggression: Project Ice Storm. Dev Psychopathol. 2018; 30(3): 981–94. [DOI] [PubMed] [Google Scholar]
- 4.Herting MM, Sowell ER. Puberty and structural brain development in humans. Front Neuroendocrinol. 2017; 44 122–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy MM. Estradiol and the developing brain. Physiological reviews. 2008; 88(1): 91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008; 11(9): 995–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Castilhos J, Forti CD, Achaval M, Rasia-Filho AA. Dendritic spine density of posterodorsal medial amygdala neurons can be affected by gonadectomy and sex steroid manipulations in adult rats: A Golgi study. Brain research. 2008; 1240 73–81. [DOI] [PubMed] [Google Scholar]
- 8.Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER. The Role of Testosterone and Estradiol in Brain Volume Changes Across Adolescence: A Longitudinal Structural MRI Study. Hum Brain Mapp. 2014; 35(11): 5633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denley MCS, Gatford NJF, Sellers KJ, Srivastava DP. Estradiol and the Development of the Cerebral Cortex: An Unexpected Role? Front Neurosci. 2018; 12 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010; 62(2): 155–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raznahan A, Lerch Jason P, Lee N, Greenstein D, Wallace Gregory L, Stockman M, Clasen L, Shaw Phillip W, Giedd Jay N. Patterns of Coordinated Anatomical Change in Human Cortical Development: A Longitudinal Neuroimaging Study of Maturational Coupling. Neuron. 2011; 72(5): 873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander-Bloch A, Raznahan A, Bullmore ET, Giedd J. The Convergence of Maturational Change and Structural Covariance in Human Cortical Networks. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013; 33(7): 2889–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albaugh MD, Ducharme S, Collins DL, Botteron KN, Althoff RR, Evans AC, Karama S, Hudziak JJ, Grp BDC. Evidence for a cerebral cortical thickness network anti-correlated with amygdalar volume in healthy youths: Implications for the neural substrates of emotion regulation. Neuroimage. 2013; 71 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ameis SH, Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Lepage C, Zhao L, Khundrakpam B, Collins DL, Lerch JP, Wheeler A, Schachar R, Evans AC, Karama S. Cortical thickness, cortico-amygdalar networks, and externalizing behaviors in healthy children. Biol Psychiatry. 2014; 75(1): 65–72. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen TV, Gower P, Albaugh MD, Botteron KN, Hudziak JJ, Fonov VS, Collins L, Ducharme S, McCracken JT. The developmental relationship between DHEA and visual attention is mediated by structural plasticity of cortico-amygdalar networks. Psychoneuroendocrino. 2016; 70122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen TV, Lew J, Albaugh MD, Botteron KN, Hudziak JJ, Fonov VS, Collins DL, Ducharme S, McCracken JT. Sex-specific associations of testosterone with prefrontal-hippocampal development and executive function. Psychoneuroendocrino. 2017; 76 206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S. Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 2013; 23(6): 14 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TV, McCracken JT, Albaugh MD, Botteron KN, Hudziak JJ, Ducharme S. A testosterone-related structural brain phenotype predicts aggressive behavior from childhood to adulthood. Psychoneuroendocrino. 2016; 63 109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen TV, McCracken JT, Ducharme S, Cropp BF, Botteron KN, Evans AC, Karama S. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J Neurosci. 2013; 33(26): 10840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen TV, Wu M, Lew J, Albaugh MD, Botteron KN, Hudziak JJ, Fonov VS, Collins DL, Campbell BC, Booij L, Herba C, Monnier P, Ducharme S, McCracken JT. Dehydroepiandrosterone impacts working memory by shaping cortico-hippocampal structural covariance during development. Psychoneuroendocrino. 2017; 86 110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005; 48(2): 175–87. [DOI] [PubMed] [Google Scholar]
- 22.McGaugh JL. Memory--a century of consolidation. Science. 2000; 287(5451): 248–51. [DOI] [PubMed] [Google Scholar]
- 23.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004; 271–28. [DOI] [PubMed] [Google Scholar]
- 24.Packard MG, Cahill L. Affective modulation of multiple memory systems. Curr Opin Neurobiol. 2001; 11(6): 752–6. [DOI] [PubMed] [Google Scholar]
- 25.Ortiz-Mantilla S, Choe MS, Flax J, Grant PE, Benasich AA. Associations between the size of the amygdala in infancy and language abilities during the preschool years in normally developing children. Neuroimage. 2010; 49(3): 2791–9. [DOI] [PubMed] [Google Scholar]
- 26.Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, Hollander E. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiat. 2000; 157(12): 1994–2001. [DOI] [PubMed] [Google Scholar]
- 27.Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, Shaw D, Artru A, Dager SR. Amygdalar volume and behavioral development in autism. Arch Gen Psychiat. 2006; 63(6): 686–93. [DOI] [PubMed] [Google Scholar]
- 28.Ducharme S, Albaugh MD, Nguyen TV, Hudziak JJ, Mateos-Perez JM, Labbe A, Evans AC, Karama S, Brain Development Cooperative G. Trajectories of cortical thickness maturation in normal brain development--The importance of quality control procedures. Neuroimage. 2016; 125267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albaugh MD, Ducharme S, Collins DL, Botteron KN, Althoff RR, Evans AC, Karama S, Hudziak JJ. Evidence for a cerebral cortical thickness network anti-correlated with amygdalar volume in healthy youths: implications for the neural substrates of emotion regulation. Neuroimage. 2013; 71 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006; 31(3): 993–1003. [DOI] [PubMed] [Google Scholar]
- 31.Halpern DF, LaMay ML. The smarter sex: A critical review of sex differences in intelligence. Educ Psychol Rev. 2000; 12(2): 229–46. [Google Scholar]
- 32.Kimura D Sex-Differences in the Brain. Sci Am. 1992; 267(3): 119–25. [DOI] [PubMed] [Google Scholar]
- 33.Kimura D Neural and Hormonal Mechanisms Mediating Sex-Differences in Cognition. Int J Psychol. 1992; 27(3–4): 333–. [Google Scholar]
- 34.Kimura D Sex-Differences in Cognition - Biological Influences. Int J Psychol. 1992; 27(3–4): 332–. [Google Scholar]
- 35.Wizemann TM, Pardue M. Exploring the biological contributions to human health: Does sex matter? J Women Health Gen-B. 2001; 10(5): 433–9. [DOI] [PubMed] [Google Scholar]
- 36.Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. 2000; 21(8): 265–71. [DOI] [PubMed] [Google Scholar]
- 37.Luciana M Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB). Journal of child psychology and psychiatry, and allied disciplines. 2003; 44(5): 649–63. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds MR, Niileksela CR, Schrank FA, McGrew KS, Mather N. Woodcock- Johnson IV Tests of Cognitive Abilities. J Psychoeduc Assess. 2015; 33(4): 381–90. [Google Scholar]
- 39.Villarreal V, Schrank FA, Mather N, McGrew KS. Woodcock-Johnson IV Tests of Achievement. Rolling. J Psychoeduc Assess. 2015; 33(4): 391–8. [Google Scholar]
- 40.Schrank FA. Unique Contributions of the Woodcock-Johnson Psychoeducational Battery-Revised to Psychoeducational Assessment. J Psychoeduc Assess. 199371–9. [Google Scholar]
- 41.Evans AC. The NIH MRI study of normal brain development. Neuroimage. 2006; 30(1): 184–202. [DOI] [PubMed] [Google Scholar]
- 42.Rosenfield RL. Adolescent Anovulation: Maturational Mechanisms and Implications. Journal of Clinical Endocrinology & Metabolism. 2013; 98(9): 3572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed BG, Carr BR. The Normal Menstrual Cycle and the Control of Ovulation. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al. , eds. Endotext; South Dartmouth (MA) 2000. [Google Scholar]
- 44.Hambridge HL, Mumford SL, Mattison DR, Ye A, Pollack AZ, Bloom MS, Mendola P, Lynch KL, Wactawski-Wende J, Schisterman EF. The influence of sporadic anovulation on hormone levels in ovulatory cycles. Human Reproduction. 2013; 28(6): 1687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain : 3-dimensional proportional system : an approach to cerebral imaging Stuttgart; New York: G. Thieme; New York: : Thieme Medical Publishers, 1988. [Google Scholar]
- 46.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of computer assisted tomography. 1994; 18(2): 192–205. [PubMed] [Google Scholar]
- 47.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage. 1995; 2(2): 89–101. [DOI] [PubMed] [Google Scholar]
- 48.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE transactions on medical imaging. 1998; 17(1): 87–97. [DOI] [PubMed] [Google Scholar]
- 49.Zijdenbos AP, Forghani R, Evans AC. Automatic “Pipeline” Analysis of 3-D MRI Data for Clinical Trials: Application to Multiple Sclerosis. . IEEE transactions on medical imaging. 2002; 21 1280–91. [DOI] [PubMed] [Google Scholar]
- 50.Kabani N, Le Goualher G, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001; 13(2): 375–80. [DOI] [PubMed] [Google Scholar]
- 51.Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005; 27(1): 210–21. [DOI] [PubMed] [Google Scholar]
- 52.MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000; 12(3): 340–56. [DOI] [PubMed] [Google Scholar]
- 53.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007; 34(4): 1535–44. [DOI] [PubMed] [Google Scholar]
- 54.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2006; 9(Pt 2): 58–66. [DOI] [PubMed] [Google Scholar]
- 55.Ad-Dab’bagh Y, Singh V, Robbins S, Lerch J, Lyttelton O, Fombonne E, Evans AC. Native space cortical thickness measurement and the absence of correlation to cerebral volume. Proceedings of the 11th Annual Meeting of the Organization for Human Brain Mapping, Toronto, 2005. [Google Scholar]
- 56.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005; 24(1): 163–73. [DOI] [PubMed] [Google Scholar]
- 57.Chung MK, Worsley KJ, Taylor J, Ramsay JO, Robbins S, Evans AC. Diffusion Smoothing on the Cortical Surface. . NeuroImage. 2001; 13S 95. [Google Scholar]
- 58.Collins DL, Pruessner JC. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. Neuroimage. 2010; 52(4): 1355–66. [DOI] [PubMed] [Google Scholar]
- 59.Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001; 21(1): 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000; 10(4): 433–42. [DOI] [PubMed] [Google Scholar]
- 61.Collins DL, Evans AC. Animal: Validation and Applications of Nonlinear Registration-Based Segmentation. International Journal of Pattern Recognition and Artificial Intelligence. 1997; 11(8): 1271–94. [Google Scholar]
- 62.Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011; 54(1): 313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan-Dawood FS, Choe JK, Dawood MY. Salivary and plasma bound and “free” testosterone in men and women. American journal of obstetrics and gynecology. 1984; 148(4): 441–5. [PubMed] [Google Scholar]
- 64.Worthman CM, Stallings JF, Hofman LF. Sensitive salivary estradiol assay for monitoring ovarian function. Clinical chemistry. 1990; 36(10): 1769–73. [PubMed] [Google Scholar]
- 65.Ankarberg-Lindgren C, Norjavaara E. Twenty-four hours secretion pattern of serum estradiol in healthy prepubertal and pubertal boys as determined by a validated ultra-sensitive extraction RIA. BMC Endocrine Disorders. 2008; 8(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janfaza M, Sherman TI, Larmore KA, Brown-Dawson J, Klein KO. Estradiol levels and secretory dynamics in normal girls and boys as determined by an ultrasensitive bioassay: a 10 year experience. J Pediatr Endocrinol Metab. 2006; 19. [DOI] [PubMed] [Google Scholar]
- 67.Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiol Int. 2007; 24(5): 969–90. [DOI] [PubMed] [Google Scholar]
- 68.Petersen A, Crockett L, Richards M, Boxer A. A Self-Report Measure of Pubertal Status: Reliability, Validity, and Initial Norms. J Youth Adolescence. 1988; 17(2): 117–33 [DOI] [PubMed] [Google Scholar]
- 69.Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child development. 1987; 58(3): 829–41. [PubMed] [Google Scholar]
- 70.Dorn LD, Crockett L, Petersen A. The Relations of Pubertal Status to Intrapersonal Changes in Young Adolescents. Journal of Early Adolescence. 1988; 8(4): 405–19. [Google Scholar]
- 71.Wichstrom L The emergence of gender difference in depressed mood during adolescence: The role of intensified gender socialization. Developmental psychology. 1999; 35(1): 232–45. [PubMed] [Google Scholar]
- 72.Rosenfield RL. Clinical review: Adolescent anovulation: maturational mechanisms and implications. J Clin Endocrinol Metab. 2013; 98(9): 3572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenbloom AL, Tanner JM. Misuse of Tanner puberty stages to estimate chronologic age. Pediatrics. 1998; 102(6): 1494. [DOI] [PubMed] [Google Scholar]
- 74.Crijnen AA, Achenbach TM, Verhulst FC. Comparisons of problems reported by parents of children in 12 cultures: total problems, externalizing, and internalizing. J Am Acad Child Adolesc Psychiatry. 1997; 36(9): 1269–77. [DOI] [PubMed] [Google Scholar]
- 75.Compas BE, Oppedisano G, Connor JK, Gerhardt CA, Hinden BR, Achenbach TM, Hammen C. Gender differences in depressive symptoms in adolescence: comparison of national samples of clinically referred and nonreferred youths. J Consult Clin Psychol. 1997; 65(4): 617–26. [DOI] [PubMed] [Google Scholar]
- 76.Hinden BR, Compas BE, Howell DC, Achenbach TM. Covariation of the anxious-depressed syndrome during adolescence: separating fact from artifact. J Consult Clin Psychol. 1997; 65(1): 6–14. [DOI] [PubMed] [Google Scholar]
- 77.Stanger C, Achenbach TM, Verhulst FC. Accelerated longitudinal comparisons of aggressive versus delinquent syndromes. Dev Psychopathol. 1997; 9(1): 43–58. [DOI] [PubMed] [Google Scholar]
- 78.Achenbach TM. Manual for the young adult self-report and young adult behavior checklist Burlington, VT: University of Vermont, 2001. [Google Scholar]
- 79.Rey JM, Morris-Yates A. Diagnostic accuracy in adolescents of several depression rating scales extracted from a general purpose behavior checklist. J Affect Disord. 1992; 26(1): 7–16. [DOI] [PubMed] [Google Scholar]
- 80.Hamson DK, Roes MM, Galea LAM. Sex Hormones and Cognition: Neuroendocrine Influences on Memory and Learning. Compr Physiol. 2016; 6(3): 1295–337. [DOI] [PubMed] [Google Scholar]
- 81.Raizada RDS, Kishiyama MM. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front Hum Neurosci. 2010; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raver CC. Placing emotional self-regulation in sociocultural and socioeconomic contexts. Child Dev. 2004; 75(2): 346–53. [DOI] [PubMed] [Google Scholar]
- 83.Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, Leonard G, Milovan D, Paus T, Rumsey J, Brain Development Cooperative G. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. Journal of the International Neuropsychological Society : JINS. 2007; 13(5): 729–46. [DOI] [PubMed] [Google Scholar]
- 84.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1992; 12(6): 900–18. [DOI] [PubMed] [Google Scholar]
- 85.Brett M An introduction to random field theory. 2003. [Google Scholar]
- 86.Mccarthy MM. Estradiol and the developing brain. Physiol Rev. 2008; 88(1): 91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez M, Cabrera-Socorro A, Perez-Garcia CG, Fraser JD, Lopez FJ, Alonso R, Meyer G. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol. 2007; 503(6): 790–802. [DOI] [PubMed] [Google Scholar]
- 88.Wang L, Andersson S, Warner M, Gustafsson JA. Morphological abnormalities in the brains of estrogen receptor beta knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2001; 98(5): 2792–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proceedings of the National Academy of Sciences of the United States of America. 2003; 100(2): 703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCarthy MM, Schlenker EH, Pfaff DW. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology. 1993; 133(2): 433–9. [DOI] [PubMed] [Google Scholar]
- 91.Abel JM, Witt DM, Rissman EF. Sex differences in the cerebellum and frontal cortex: roles of estrogen receptor alpha and sex chromosome genes. Neuroendocrinology. 2011; 93(4): 230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: High levels in the amygdaloid complex. Neuroscience. 2000; 95(2): 333–42. [DOI] [PubMed] [Google Scholar]
- 93.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997; 389(1): 81–93. [DOI] [PubMed] [Google Scholar]
- 94.Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav Ecol. 2008; 19(3): 690–3. [Google Scholar]
- 95.Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. J Pediatr Psychol. 2002; 27(1): 87–96. [DOI] [PubMed] [Google Scholar]
- 96.Lee JJ, Rubin DB. Evaluating the Validity of Post-Hoc Subgroup Inferences: A Case Study. Am Stat. 2016; 70(1): 39–46. [Google Scholar]
- 97.Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011; 31(45): 16056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fiocchetti M, Ascenzi P, Marino M. Neuroprotective effects of 17beta-estradiol rely on estrogen receptor membrane initiated signals. Front Physiol. 2012; 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front Neuroendocrin. 2012; 33(4): 342–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008; 53(5): 613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A. 2004; 101(6): 1673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shaywitz SE, Naftolin F, Zelterman D, Marchione KE, Holahan JM, Palter SF, Shaywitz BA. Better oral reading and short-term memory in midlife, postmenopausal women taking estrogen. Menopause. 2003; 10(5): 420–6. [DOI] [PubMed] [Google Scholar]
- 103.Krug R, Born J, Rasch B. A 3-day estrogen treatment improves prefrontal cortex-dependent cognitive function in postmenopausal women. Psychoneuroendocrino. 2006; 31(8): 965–75. [DOI] [PubMed] [Google Scholar]
- 104.Eisner A, Burke SN, Toomey MD. Visual sensitivity across the menstrual cycle. Visual neuroscience. 2004; 21(4): 513–31. [DOI] [PubMed] [Google Scholar]
- 105.Andreescu CE, Milojkovic BA, Haasdijk ED, Kramer P, De Jong FH, Krust A, De Zeeuw CI, De Jeu MT. Estradiol improves cerebellar memory formation by activating estrogen receptor beta. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007; 27(40): 10832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gyenes A, Hoyk Z, Csakvari E, Siklos L, Parducz A. 17beta-estradiol attenuates injury-induced microglia activation in the oculomotor nucleus. Neuroscience. 2010; 171(3): 677–82. [DOI] [PubMed] [Google Scholar]
- 107.Currie E The Effect of Cycling Female Estradiol Levels on Retinal-Based Smooth Pursuit Eye Movements and Post-Target Offset Persistence. Psychology: Lakehead 2015. [Google Scholar]
- 108.Ilg UJ, Thier P. The neural basis of smooth pursuit eye movements in the rhesus monkey brain. Brain and cognition. 2008; 68(3): 229–40. [DOI] [PubMed] [Google Scholar]
- 109.Ono S, Mustari MJ. Smooth pursuit-related information processing in frontal eye field neurons that project to the NRTP. Cereb Cortex. 2009; 19(5): 1186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rasgon NL, Silverman D, Siddarth P, Miller K, Ercoli LM, Elman S, Lavretsky H, Huang SC, Phelps ME, Small GW. Estrogen use and brain metabolic change in postmenopausal women. Neurobiology of aging. 2005; 26(2): 229–35. [DOI] [PubMed] [Google Scholar]
- 111.Smith YR, Bowen L, Love TM, Berent-Spillson A, Frey KA, Persad CC, Reame NK, Koeppe RA, Zubieta JK. Early initiation of hormone therapy in menopausal women is associated with increased hippocampal and posterior cingulate cholinergic activity. The Journal of clinical endocrinology and metabolism. 2011; 96(11): E1761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrin. 2008; 29(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andrews GK, Dziadek M, Tamaoki T. Expression and Methylation of the Mouse Alpha-Fetoprotein Gene in Embryonic, Adult, and Neoplastic Tissues. J Biol Chem. 1982; 257(9): 5148–53. [PubMed] [Google Scholar]
- 114.Raynaud JP. Influence of Rat Estradiol Binding Plasma-Protein (Ebp) on Uterotrophic Activity. Steroids. 1973; 21(2): 249–58. [DOI] [PubMed] [Google Scholar]
- 115.Caruso D, Pesaresi M, Abbiati F, Calabrese D, Giatti S, Garcia-Segura LM, Melcangi RC. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrino. 2013; 38(10): 2278–90 [DOI] [PubMed] [Google Scholar]


