Abstract
Chronic neuropathic pain is a debilitating condition that remains challenging to treat. Glutamate N-methyl-d-aspartate receptor (NMDAR) antagonists have been used to treat neuropathic pain, but the exact sites of their actions have been unclear until recently. Although conventionally postsynaptic, NMDARs are also expressed presynaptically, particularly at the central terminals of primary sensory neurons, in the spinal dorsal horn. However, presynaptic NMDARs in the spinal cord are normally quiescent and are not actively involved in physiological nociceptive transmission. In this review, we describe the emerging role of presynaptic NMDARs at the spinal cord level in chronic neuropathic pain and the implications of molecular mechanisms for more effective treatment. Recent studies indicate that presynaptic NMDAR activity at the spinal cord level is increased in several neuropathic pain conditions but not in chronic inflammatory pain. Increased presynaptic NMDAR activity can potentiate glutamate release from primary afferent terminals to spinal dorsal horn neurons, which is crucial for the synaptic plasticity associated with neuropathic pain caused by traumatic nerve injury and chemotherapy-induced peripheral neuropathy. Furthermore, α2δ-1, previously considered a calcium channel subunit, can directly interact with NMDARs through its C-terminus to increase presynaptic NMDAR activity by facilitating synaptic trafficking of α2δ-1–NMDAR complexes in neuropathic pain caused by chemotherapeutic agents and peripheral nerve injury. Targeting α2δ-1–bound NMDARs with gabapentinoids or α2δ-1 C-terminus peptides can attenuate nociceptive drive form primary sensory nerves to dorsal horn neurons in neuropathic pain.
Keywords: Calcineurin, K+–Cl− cotransporter-2, Synaptic plasticity, Dorsal root ganglion, Gabapentin, Pregabalin
Introduction
Chronic neuropathic pain, often caused by peripheral nerve injury, diabetic neuropathy, postherpetic neuralgia, and chemotherapy, can result in prolonged suffering and remains an enormous unmet medical need. Neuropathic pain is often associated with spontaneous pain, hyperalgesia (increased pain sensation in response to a painful stimulus), and allodynia (pain from normally non-painful stimuli). The cellular and molecular mechanisms responsible for neuropathic pain have not been fully delineated, and effective treatments for this condition are still limited.
Glutamate is the dominant excitatory neurotransmitter and facilitates fast excitatory synaptic transmission in the central nervous system. The glutamate N-methyl-d-aspartate receptor (NMDAR) is involved in numerous physiological functions, such as learning and memory. NMDARs, especially those in the dorsal horn of the spinal cord, play pivotal roles in synaptic plasticity and have long been targeted for the treatment of neuropathic pain. It was shown about 28 years ago that intrathecal injection of NMDAR antagonists reduces nociceptive behaviors and pain hypersensitivity caused by nerve injury [1, 2]. Because NMDARs were traditionally thought to be located postsynaptically in the central nervous system, NMDAR antagonists administered intrathecally were assumed to target postsynaptic NMDARs in the spinal cord [3].
NMDARs are now known to be also present at presynaptic nerve terminals and can powerfully shape synaptic transmission and neuronal plasticity in the amygdala [4], hypothalamus [5, 6], striatum [7, 8], and hippocampus [9]. Under physiological conditions, presynaptic NMDARs in the spinal dorsal horn are not functionally active in regulating neurotransmitter release. However, in opioid-induced hyperalgesia and chronic neuropathic pain conditions, presynaptic NMDARs in the spinal cord become tonically active and are stimulated by endogenous glutamate [10–14]. Here, we review the distribution and function of presynaptic NMDARs in the spinal dorsal horn and their prominent role in regulating nociceptive transmission in chronic neuropathic pain.
NMDAR components and distribution in the spinal dorsal horn
NMDARs are tetrameric protein complexes comprising two obligatory GluN1 subunits with either two GluN2 subunits or a combination of one GluN2 and one GluN3 subunits. There are eight GluN1 subunits generated by alternative splicing from a single gene (GRIN1), four GluN2 subunits (GluN2A-2D, encoded by GRIN2A-2D), and two GluN3 subunits (GluN3A and GluN3B, encoded by GRIN3A and GRIN3B). The GluN1 subunits are required for the formation of functional NMDARs, whereas the GluN2 and GluN3 subunits modify channel properties [15, 16]. GluN2 subunits are essential for glutamate-mediated NMDAR activation. The expression of different GluN2 subunits controls several NMDAR channel properties [16]. GluN2A-containing receptors show faster activation kinetics, higher open probability, and faster deactivation kinetics than GluN2B-containing receptors do [17, 18]. On the other hand, GluN2C-NMDARs exhibit relatively low conductance, low open probability, and low sensitivity to Mg2+ block [19]. GluN2D is typically expressed early in the developmental stage and is characterized by a very slow decay time (about 4–5 s). The GluN3 subunits function mainly as modulatory subunits that can reduce the vulnerability to Mg2+ block and attenuate the Ca2+ permeability of NMDARs [20]. GluN3A is primarily expressed early, whereas GluN3B levels increase over the development period [21].
Neurons in the dorsal root ganglion (DRG) are presynaptic because their central terminals synapse with second-order neurons in the spinal dorsal horn. Thus, many proteins are synthesized in DRG neurons and then transported to their central presynaptic terminals inside the spinal dorsal horn. GluN1 is expressed in large and small DRG neurons, including both peptidergic and nonpeptidergic neurons [22–24]. GluN2B and GluN2D are expressed at higher levels than GluN2A and GluN2C are in the DRG [25]. At the spinal cord level, NMDARs are abundant in the dorsal horn, with GluN1 and GluN2A subunits expressed throughout the gray matter, whereas GluN2B subunits are distributed mainly in laminae I–II [26]. The GluN1 immunoreactivity is present at the terminals of both unmyelinated and myelinated primary afferent nerves in the superficial dorsal horn [27, 28]. In addition, NMDARs are present on GABAergic terminals in the superficial dorsal horn of the spinal cord [29].
Functional significance of presynaptic NMDAR in the spinal dorsal horn
NMDARs are permeable to Na+, K+, and Ca2+. However, the channel permeability is normally blocked by extracellular Mg2+, and strong neuronal depolarization is needed to release the Mg2+ block. For this reason, NMDARs are viewed as a coincidence sensor for coordinating presynaptic and postsynaptic neuronal activities [30]. Nonetheless, presynaptic NMDARs can promote spontaneous neurotransmitter release in the absence of neuronal depolarization [31], suggesting that these NMDARs can be activated by endogenous glutamate even without relief of the Mg2+ block [32]. In contrast, most postsynaptic NMDARs are blocked by Mg2+ at rest and, therefore, require neuronal depolarization in concurrence with glutamate binding to become functionally active [33]. Activation of presynaptic NMDARs may cause calcium influx to directly trigger vesicle exocytosis, cause the depolarization of the terminals to activate voltage-gated calcium channels, and indirectly trigger neurotransmitter release through downstream intracellular signaling cascades independent of ion flux [9, 32, 34].
Although NMDARs are present in primary sensory neurons and their central terminals in the spinal dorsal horn [27, 35, 36], they are normally in a quiescent state, as evidenced by the observation that blocking NMDARs with the specific NMDAR antagonist 2-amino-5-phosphonopentanoic acid (AP5) has no effect on spontaneous or evoked synaptic glutamate release to spinal lamina I or lamina II neurons in normal rats [10, 11, 13, 14, 37]. This finding is consistent with reports showing that blocking NMDARs at the spinal cord level does not alter acute nociception in normal conditions [1, 11, 37].
However, there are some inconsistent reports about the function of spinal presynaptic NMDARs in the normal state in studies using exogenous NMDAR agonists. For example, intrathecal administration of NMDA can evoke the release of substance P from the central terminals of a subset of primary sensory neurons in the rat dorsal horn [35]. On the other hand, bath application of NMDA inhibits synaptic glutamate release evoked from primary afferent nerve stimulation [38]. Because the evoked glutamate release is likely mediated by voltage-gated Ca2+ channels, exogenous NMDA may induce calcium influx to activate calcineurin, which subsequently inhibits voltage-gated calcium channels [39, 40]. Indeed, the activation of presynaptic NMDARs can inhibit voltage-gated Ca2+ channels to attenuate evoked glutamate release at the calyx of Held synapse [41]. Intriguingly, it was reported that conditional knockout of GluN1 in DRG neurons in Advillin-Cre mice results in increased neuronal excitability and mechanical and thermal sensitivity [42]. This DRG neuronal hyperexcitability was shown to be due to the impairment of Ca2+-activated slow conductance K+ channels in GluN1 conditional knockout mice [42]. Others have shown that conditional knockout of GluN1 in DRG neurons in Peripherin-Cre mice does not affect normal nociception [36]. The report showing that removing NMDARs in DRG neurons paradoxically increases neuronal excitability and pain-like behaviors needs to be validated independently.
The first demonstration of increased activity of presynaptic NMDARs in the spinal cord is a study related to opioid analgesic tolerance, in which AP5 reduces the frequency of miniature excitatory postsynaptic currents (mEPSCs, a measure of quantal glutamate release from presynaptic terminals) and the amplitude of EPSCs of lamina I neurons evoked from the dorsal root (representing glutamate release from primary afferent nerves) in neonatal rat spinal cord slices prevously exposed to opioids [12]. Repeated opioid exposure in animal models and in patients can cause paradoxical hyperalgesia and analgesic tolerance, which are major obstacles to adequate pain management with opioids. Elevating endogenous glutamate levels using a glutamate transporter inhibitor produces an AP5-sensitive increase in the frequency of mEPSCs in opiate-naive spinal cord slices [43], suggesting that the lack of presynaptic NMDAR activity may be due to an inadequate level of endogenous NMDAR agonists in normal conditions. Furthermore, long-term morphine treatment can substantially increase the amplitude of monosynaptic EPSCs evoked from primary afferent nerves and the frequency of spontaneous EPSCs and mEPSCs, and these changes were reduced principally by blocking of NMDARs [13, 44]. Additionally, blocking NMDARs containing GluN2A or GluN2B significantly attenuates the frequency of spontaneous EPSCs and the amplitude of evoked EPSCs of lamina II neurons in morphine-treated rats [13]. In contrast to protein kinase C (PKC)- and mitogen-activated protein kinase-dependent activation of presynaptic NMDARs in the spinal cord, chronic morphine treatment diminishes postsynaptic NMDAR activity in the spinal dorsal horn [13, 44]. This reduction is likely caused by the persistent increase in glutamate release from transient receptor potential vanilloid type 1-expressing primary afferents [13]. Strikingly, even bath application of an opioid agonist for 3 min can increase presynaptic NMDAR activity in spinal lamina I and lamina II neurons to elicit a long-lasting increase in glutamate release from nociceptive primary afferents [45]. These findings highlight the critical role of spinal presynaptic NMDARs in opioid-induced hyperalgesia and analgesic tolerance.
Increased presynaptic NMDAR activity potentiates spinal nociceptive transmission in neuropathic pain induced by peripheral nerve injury
Ketamine, a clinically used NMDAR antagonist, has been used for many years to treat neuropathic pain [46, 47] and intrathecal injection of NMDAR antagonists, including MK-801 and AP5, effective reduce neuropathic pain behaviors in animals subjected to peripheral nerve injury [1, 48, 49]. Thus, NMDARs at the spinal cord level play a major role in the development of central sensitization and neuropathic pain after nerve injury. The majority of neurons in spinal lamina II are glutamatergic excitatory interneurons involved in nociceptive transmission [50, 51]. Consistent with the in vivo findings, bath application of AP5 rapidly and reversibly attenuates the increased frequency of mEPSCs in lamina II neurons in spinal cord slices from nerve-injured rats [10, 14, 52]. AP5 also normalizes the increased amplitude of monosynaptic EPSCs and the altered paired-pulse ratio (a measure of presynaptic effect) of evoked EPSCs in lamina II neurons in nerve-injured rats. These findings indicate that presynaptic NMDARs in the spinal dorsal horn are endogenously activated to potentiate glutamate release from nociceptive primary afferent terminals in neuropathic pain.
NMDAR activity is regulated predominantly by the phosphorylation status of NMDARs and/or their interacting proteins. NMDARs can be phosphorylated by many protein kinases, such as protein kinase A, PKC, casein kinase 2 (CK2), Ca2+/calmodulin-dependent protein kinase II, and tyrosine kinases Src and Fyn [16, 53–55]. Peripheral nerve injury in rats increases CK2α and CK2β protein levels in the spinal cord [56], and inhibition of CK2 or knockdown of CK2β with specific siRNA at the spinal cord level can reverse nerve injury-induced pain hypersensitivity. These findings indicate that CK2 plays a major role in the potentiation of synaptic NMDAR activity of spinal dorsal horn neurons [56]. Furthermore, treatment with a PKC inhibitor, GF109203X, significantly reduces the amplitude of monosynaptic EPSCs of dorsal horn neurons in nerve-injured rats [14]. Various protein kinases, including CK2, PKC, and Src, are likely responsible for the increased presynaptic and postsynaptic NMDAR activity in the spinal dorsal horn in nerve injury-induced neuropathic pain [14, 54, 56].
Regulating presynaptic NMDAR activity by KCC2 and α2δ-1 in nerve injury-induced neuropathic pain
Impaired synaptic inhibition in the spinal dorsal horn due to the downregulation of K+–Cl− cotransporter-2 (KCC2, encoded by the Slc12a5 gene) contributes substantially to the development of neuropathic pain [57]. Peripheral nerve injury impairs synaptic inhibition of spinal dorsal horn neurons through calpain-mediated KCC2 proteolysis resulting from the increased postsynaptic NMDAR activity [49]. Intrathecal Slc12a5 gene transfer completely reverses mechanical hyperalgesia, tactile allodynia, and thermal hypersensitivity induced by nerve injury [10]. Slc12a5 gene transfer at the spinal cord level via a lentiviral vector restores Cl− homeostasis disrupted by nerve injury in both the spinal dorsal horn and DRG neurons. KCC2 normally is not expressed in DRG neurons. Intriguingly, ectopic expression of Slc12a5 in the DRG and over-expression of Slc12a5 in the spinal cord via a lentiviral vector normalize both presynaptic and postsynaptic NMDAR activity increased by nerve injury [10], suggesting that disrupting Cl− homeostasis at the spinal cord level affects the synaptic NMDAR activity in the spinal dorsal horn in nerve injury-induced neuropathic pain.
α2δ-1 (encoded by the Cacna2d1 gene), commonly considered a calcium channel subunit, is expressed in the DRG, spinal cord, and discrete brain regions [58]. Recent studies indicate that α2δ-1 can form a protein complex with NMDARs and regulate NMDAR synaptic trafficking and activity in the brain [6, 8, 59, 60]. Nerve injury profoundly increases the expression level of α2δ-1 in the DRG and spinal dorsal horn [61], and genetic ablation of α2δ-1 reduces pain hypersensitivity induced by nerve injury [62]. In situ hybridization analysis shows that α2δ-1 is mainly present in small- and medium-sized neurons in the normal DRG and that sciatic nerve injury predominantly increases the α2δ-1 expression in medium and large DRG neurons [63]. There are no studies showing the NMDAR subunit co-localization with α2δ-1 in the DRG. However, their co-localization is highly likely because of the widespread expression of NMDAR subunits and α2δ-1 in various types of DRG neurons.
Gabapentinoids, including gabapentin and pregabalin, have been used clinically to treat neuropathic pain conditions such as postherpetic neuralgia and fibromyalgia [64, 65]. Although gabapentin and pregabalin bind to α2δ-1 [66], little has been known about how these drugs reduce neuropathic pain until recently. In a recent study, overexpression of Cacna2d1 at the spinal cord level via a lentiviral vector causes a large increase in presynaptic and postsynaptic NMDAR activity in the spinal dorsal horn [52]. Conversely, siRNA knockdown of Cacna2d1 or genetic knockout of Cacna2d1 abolishes nerve injury-induced increases in presynaptic and postsynaptic NMDAR activity in the spinal dorsal horn (Fig. 1). Further studies revealed that α2δ-1 can physically interact with NMDAR subunits in vivo and in vitro through its C-terminal domain, promoting the synaptic trafficking of NMDARs [52]. In addition, gabapentin treatment or disruption of the α2δ-1–NMDAR interaction with an α2δ-1 C-terminus peptide blocks nerve injury-induced potentiation of presynaptic and postsynaptic NMDAR activity in the spinal dorsal horn (Fig. 2). Thus, α2δ-1 can increase presynaptic NMDAR activity in neuropathic pain by increasing the synaptic targeting and trafficking of NMDARs at primary afferent terminals and/or by reducing the Mg2+ block of GluN2A-containing NMDARs [52]. These findings reveal that α2δ-1–bound NMDARs are the relevant molecular target responsible for the therapeutic action of gabapentinoids in treating neuropathic pain.
Fig. 1.

α2δ-1 is essential for the increased presynaptic and postsynaptic NMDAR activity of spinal dorsal horn neurons after nerve injury. a Original current traces and mean changes in NMDAR currents elicited by puff application of 100 µM NMDA to spinal dorsal horn neurons in wild-type (WT, n = 12 neurons in each group) and Cacna2d1 KO (n = 11 neurons in each group) mice 3 weeks after spared nerve injury (SNI) or sham surgery. Data are means ± s.e.m. *P < 0.05 (versus WT sham group). One-way ANOVA analysis followed by Tukey’s post hoc test. b Representative traces and mean changes in baseline values and the AP5 effect on the frequency and amplitude of mEPSCs of spinal dorsal horn neurons in wild-type (WT, n = 11 neurons in each group) and Cacna2d1 KO (n = 12 neurons in each group) mice subjected to spared nerve injury (SNI) or sham surgery. Data are means ± s.e.m. *P < 0.05 (versus baseline). #P < 0.05 (versus baseline in the WT sham group). One-way ANOVA analysis followed by Tukey’s post hoc test. Representative traces (c) and mean changes (d) in baseline values and the AP5 effect on the amplitude of EPSCs of spinal dorsal horn neurons monosynaptically evoked by dorsal root stimulation in wild-type (WT) and Cacna2d1 KO mice subjected to spared nerve injury (SNI, n = 11 neurons in each group) or sham surgery (n = 12 neurons in each group). Data are means ± s.e.m. *P < 0.05 (versus baseline). #P < 0.05 (versus baseline in the WT sham group). One-way ANOVA analysis followed by Tukey’s post hoc test
(Reproduced from [52])
Fig. 2.

Gabapentin treatment normalizes presynaptic and postsynaptic NMDAR activity of spinal dorsal horn neurons after nerve injury. a Original traces and mean effects of gabapentin (GBP, 100 µM for 30 min) on currents elicited by puff application of 100 µM NMDA or AMPA to spinal dorsal horn neurons in rats that had undergone sham surgery (n = 12 neurons in the vehicle group, n = 13 neurons in the gabapentin group) or spinal nerve ligation (SNL, n = 11 neurons in the vehicle group, n = 12 neurons in gabapentin group) 3 weeks after surgery. Data are means ± s.e.m. *P < 0.05 (versus sham rats treated with vehicle). One-way ANOVA analysis followed by Tukey’s post hoc test. Representative traces, cumulative probabilities (b) and mean changes (c) of baseline values and the AP5 effect on the frequency and amplitude of mEPSCs of spinal dorsal neurons recorded from rats subjected to sham surgery (n = 10 neurons) or SNL (n = 12 neurons in the vehicle group, n = 11 neurons in the gabapentin group). Data are means ± s.e.m. *P < 0.05 (versus respective baseline). #P < 0.05 compared with the baseline in sham group. One-way ANOVA analysis followed by Tukey’s post hoc test
(Reproduced from [52])
Spinal presynaptic NMDARs mediate increased nociceptive input to dorsal horn neurons in chemotherapy-induced neuropathic pain
Chemotherapeutic agents, including paclitaxel and bortezomib, can induce painful peripheral neuropathy, a major dose-limiting adverse effect, in many cancer patients. Intrathecal injection of the NMDAR antagonist AP5 significantly reverses pain hypersensitivity induced by paclitaxel or bortezomib. Moreover, paclitaxel-induced pain hypersensitivity can be reduced by systemic treatment with memantine, a clinically used NMDAR antagonist [11]. Although NMDARs are implicated in the development of chemotherapy-induced chronic pain, electrophysiological studies using spinal cord slices show that systemic treatment with paclitaxel or bortezomib does not affect postsynaptic NMDAR activity in spinal lamina II neurons [67, 68].
Subsequent studies using spinal cord slice recordings indicate that treatment with paclitaxel or bortezomib in rats significantly increases the frequency of mEPSCs and the amplitude of monosynaptic EPSCs evoked from the dorsal root [11, 68]. Bath application of AP5 quickly normalizes the frequency of mEPSCs and the amplitude of evoked EPSCs in dorsal horn neurons that had been increased by paclitaxel or bortezomib treatment [11, 68]. In addition, selective blocking of GluN2A-containing NMDARs decreases the frequency of mEPSCs and the amplitude of EPSCs evoked from the dorsal root in dorsal horn neurons from paclitaxel-treated rats [11], suggesting that chemotherapy primarily increases the activity of GluN2A-containing NMDARs at primary afferent terminals in the spinal cord.
Treatment with paclitaxel or bortezomib potentiates nociceptive input from primary afferent nerves via PKC-mediated tonic activation of presynaptic NMDARs as evidenced by the finding that inhibition of PKC with chelerythrine fully reverses the increased frequency of mEPSCs and amplitude of evoked EPSCs caused by paclitaxel and bortezomib [11, 68]. Furthermore, paclitaxel-induced painful neuropathy is associated with increased activity of presynaptic mGluR5, which is an upstream signaling mechanism for the activation of PKC and potentiation of presynaptic NMDAR activity in the spinal dorsal horn [69].
In a recent study, chronic treatment with paclitaxel in rats increases α2δ-1 expression levels in the DRG and spinal cord, α2δ-1–NMDAR interaction, and synaptic expression of α2δ-1–NMDAR complexes in the spinal dorsal horn [70]. In addition, treatment with pregabalin or an α2δ-1 C-terminus peptide fully blocks the increased presynaptic NMDAR activity of spinal lamina II neurons in paclitaxel-treated rats but has no effect on mEPSCs or EPSCs monosynaptically evoked by dorsal root stimulation in vehicle-treated rats. In Cacna2d1 knockout mice, chronic treatment with paclitaxel fails to significantly change the frequency of mEPSCs or the amplitude of EPSCs evoked by dorsal root stimulation. In addition, intrathecal injection of pregabalin or α2δ-1 C terminus–interfering peptide and Cacna2d1 knockout markedly attenuate paclitaxel-induced pain hypersensitivity [70]. Taken together, these findings clearly indicate that chemotherapy-induced α2δ-1–NMDAR coupling is fully responsible for the increased presynaptic NMDAR activity that potentiates nociceptive drive to the spinal dorsal horn to maintain chronic pain. This study provides further evidence that the therapeutic target of pregabalin in neuropathic pain is α2δ-1–bound NMDARs that are expressed presynaptically in the spinal dorsal horn.
Presynaptic NMDARs are involved in potentiated spinal nociceptive transmission in calcineurin inhibitor-induced chronic pain
Calcineurin is a serine/threonine protein phosphatase, and its activation is dependent on Ca2+/calmodulin. Calcineurin is highly expressed in the T cells of the immune system and in the nervous system, including neurons in the DRG and spinal cord [39, 40, 71]. Calcineurin inhibitors such as cyclosporin A and tacrolimus (FK506) are commonly used immunosuppressants and used for the preservation of allograft function in patients. However, these drugs can cause severe, persistent pain, frequently referred to as calcineurin inhibitor-induced pain syndrome (CIPS) [72]. CIPS is characterized by severe pain and pain hypersensitivity that mostly affects the lower limbs, and the pain is often agonizing during standing and walking [73].
Calcineurin inhibition prolongs NMDAR channel opening [74]. Systemic administration of FK506 in rats leads to long-lasting nociceptive and mechanical hypersensitivity [37]. Blocking spinal NMDARs with AP5 can reverse the tactile allodynia and mechanical hyperalgesia caused by FK506 treatment. In addition, systemic administration of the NMDAR antagonist memantine reduces pain hypersensitivity in a dose-dependent manner in FK506-treated rats [37]. Moreover, FK506 treatment increases the amplitude of NMDAR-mediated monosynaptic EPSCs of dorsal horn neurons evoked from primary afferent nerves and profoundly increases the frequency of mEPSCs in lamina II neurons. The increase in synaptic glutamate release can be readily normalized by AP5, suggesting that presynaptic NMDAR activity is augmented the calcineurin inhibitor, thereby potentiating glutamate release from primary afferent nerves in CIPS [37, 75]. CK2 inhibition completely normalizes the amplitude of evoked NMDAR-EPSCs and the frequency of mEPSCs in dorsal horn neurons increased by FK506 treatment, suggesting that CK2 mediates the increased synaptic NMDAR activity in the spinal cord and the pain hypersensitivity associated with CIPS. In addition, intrathecal injection of CK2 inhibitors reverses tactile allodynia and mechanical hyperalgesia in FK506-treated rats [75]. Therefore, calcineurin and CK2 can reciprocally control NMDAR activity through phosphorylation, and inhibition of endogenous CK2 reverses calcineurin inhibitor-induced pain hypersensitivity by normalizing synaptic NMDAR activity in the spinal cord. The potential role of α2δ-1–bound NMDARs in CIPS-related synaptic NMDAR hyperactivity in the spinal dorsal horn is still being investigated.
Chronic inflammatory pain is not associated with increased presynaptic NMDAR activity in the spinal cord
Unlike neuropathic pain, there is currently no evidence about the involvement of spinal presynaptic NMDARs in inflammatory pain. A recent study examined changes in presynaptic and postsynaptic NMDAR activity of spinal dorsal horn neurons in rats 10–16 days after induction of tissue inflammation with complete Freund’s adjuvant (CFA). Interestingly, the glutamatergic input, measured by the frequency of mEPSCs and spontaneous EPSCs, is markedly increased in lamina I, but not lamina II, neurons in CFA-treated rats [76]. However, bath application of AP5 has no effect on the CFA-induced increase in the frequency of spontaneous EPSCs in lamina I neurons. Furthermore, the amplitude of puff NMDA currents in lamina I neurons is similar in vehicle- and CFA-treated rats [76]. These findings suggest that chronic inflammatory pain is not associated with an increase in presynaptic or postsynaptic NMDAR activity in the spinal dorsal horn. Instead, presynaptic TRPA1 and TRPV1 channels play a major role in the augmented glutamatergic input to spinal lamina I neurons in chronic inflammatory pain [76]. It has been shown that CFA-induced pain hypersensitivity in the hindpaw is attenuated only for the first 2 days in mice in which spinal cord GluN1 is ablated [77]. Thus, although spinal NMDARs may play a role in the initial induction of inflammatory pain, the NMDAR activity in the spinal dorsal horn is not required for the maintenance of chronic inflammatory pain.
Conclusions and perspectives
Recent studies clearly indicate that presynaptic NMDARs in the spinal dorsal horn become tonically activated in neuropathic pain conditions. At the spinal cord level, presynaptic NMDARs are present mainly at the first sensory synapse, which is the interface between primary sensory nerves and spinal dorsal horn neurons, and can “gate” peripheral nociceptive input to the spinal cord in neuropathic pain. In neuropathic pain caused by traumatic nerve injury, chemotherapy, or calcineurin inhibitors, presynaptic NMDAR activity in the spinal cord is consistently increased. Consequently, presynaptic NMDARs may play a greater role than postsynaptic NMDARs in initiating and maintaining chronic neuropathic pain.
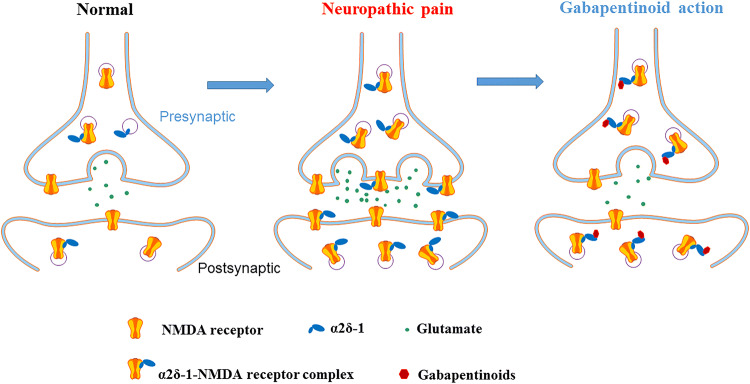
The increase in presynaptic NMDAR activity at the spinal cord level is associated primarily with α2δ-1–bound NMDARs in neuropathic pain (Fig. 3) [52, 70]. Thus, α2δ-1–bound NMDARs act mainly as a pathological form of NMDARs and represent an opportune target for treating neuropathic pain. Systemic administration of general NMDAR antagonists, such as ketamine, often produces various adverse effects in patients due to the interference of these antagonists with normal NMDAR function in the central nervous system. We have learned from recent studies that gabapentinoids reduce nociceptive input from primary sensory neurons to spinal dorsal horn neurons by inhibiting synaptic trafficking and activity of α2δ-1–bound NMDARs [52, 70]. The α2δ-1Tat peptide that targets the C-terminus of α2δ-1 can disrupt the α2δ-1–NMDAR interaction and normalize the aberrant synaptic NMDAR activity in neuropathic pain. Thus, peptides and small molecules targeting the C-terminal domain of α2δ-1 may be developed clinically to treat chronic neuropathic pain.
Fig. 3.
Schematic showing the potential role of α2δ-1 in the regulation of synaptic NMDARs in the spinal dorsal horn in neuropathic pain. Under normal conditions, most synaptic NMDARs are not associated with α2δ-1 in the spinal dorsal horn. In the neuropathic pain condition, α2δ-1 expression is increased and physically interacts with NMDARs, at presynaptic and postsynaptic sites, to promote synaptic trafficking of α2δ-1–NMDAR complexes. Gabapentinoids restore the normal activity of presynaptic and postsynaptic NMDARs by inhibiting synaptic trafficking of α2δ-1–bound NMDARs in the spinal dorsal horn, thereby reducing neuropathic pain
Despite the progress being made, our knowledge about the role of presynaptic NMDARs in the development of neuropathic pain is still fragmentary. Many important questions remain: What signaling mechanisms prevent the tonic activation of spinal presynaptic NMDARs under normal conditions? Are any changes in presynaptic NMDARs in chronic pain associated with diabetic neuropathy and postherpetic neuralgia? Are separate protein kinases involved in regulating the function of presynaptic and postsynaptic NMDARs in neuropathic pain? What is the relationship between NMDAR phosphorylation/glycosylation and the protein–protein interaction between α2δ-1 and NMDARs in neuropathic pain? Do presynaptic NMDARs similarly regulate the glutamatergic input to excitatory and inhibitory dorsal horn neurons in neuropathic pain? Forthcoming studies will undoubtedly improve our understanding of the cellular and molecular mechanisms of chronic neuropathic pain and enable the design of effective therapies for this debilitating condition.
Acknowledgements
Work conducted in the authors’ laboratory was funded by the National Institutes of Health (Grants R01 GM120844 and R01 NS101880).
Abbreviations
- AP5
2-Amino-5-phosphonopentanoic acid
- CFA
Complete Freund’s adjuvant
- CIPS
Calcineurin inhibitor-induced pain syndrome
- DRG
Dorsal root ganglion
- EPSC
Excitatory postsynaptic current
- mEPSC
Miniature excitatory postsynaptic current
- NMDAR
N-Methyl-d-aspartate receptor
- PKC
Protein kinase C
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yamamoto T, Yaksh TL. Spinal pharmacology of thermal hyperesthesia induced by constriction injury of sciatic nerve. Excitatory amino acid antagonists. Pain. 1992;49(1):121–128. doi: 10.1016/0304-3959(92)90198-K. [DOI] [PubMed] [Google Scholar]
- 2.Seltzer Z, Cohn S, Ginzburg R, Beilin B. Modulation of neuropathic pain behavior in rats by spinal disinhibition and NMDA receptor blockade of injury discharge. Pain. 1991;45(1):69–75. doi: 10.1016/0304-3959(91)90166-U. [DOI] [PubMed] [Google Scholar]
- 3.Zhou HY, Chen SR, Pan HL. Targeting N-methyl-d-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol. 2011;4(3):379–388. doi: 10.1586/ecp.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humeau Y, Shaban H, Bissiere S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426(6968):841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- 5.Ye ZY, Li DP, Li L, Pan HL. Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J Neurosci. 2011;31(22):8271–8279. doi: 10.1523/JNEUROSCI.1147-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma H, Chen SR, Chen H, Zhou JJ, Li DP, Pan HL. alpha2delta-1 couples to NMDA receptors in the hypothalamus to sustain sympathetic vasomotor activity in hypertension. J Physiol. 2018;596(17):4269–4283. doi: 10.1113/JP276394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park H, Popescu A, Poo MM. Essential role of presynaptic NMDA receptors in activity-dependent BDNF secretion and corticostriatal LTP. Neuron. 2014;84(5):1009–1022. doi: 10.1016/j.neuron.2014.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou JJ, Li DP, Chen SR, Luo Y, Pan HL. The alpha2delta-1-NMDA receptor coupling is essential for corticostriatal long-term potentiation and is involved in learning and memory. J Biol Chem. 2018;293(50):19354–19364. doi: 10.1074/jbc.RA118.003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuinness L, Taylor C, Taylor RD, Yau C, Langenhan T, Hart ML, Christian H, Tynan PW, Donnelly P, Emptage NJ. Presynaptic NMDARs in the hippocampus facilitate transmitter release at theta frequency. Neuron. 2010;68(6):1109–1127. doi: 10.1016/j.neuron.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Chen SR, Chen H, Wen L, Hittelman WN, Xie JD, Pan HL. Chloride homeostasis critically regulates synaptic NMDA receptor activity in neuropathic pain. Cell Rep. 2016;15(7):1376–1383. doi: 10.1016/j.celrep.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie JD, Chen SR, Chen H, Zeng WA, Pan HL. Presynaptic N-methyl-d-aspartate (NMDA) receptor activity is increased through protein kinase C in paclitaxel-induced neuropathic pain. J Biol Chem. 2016;291(37):19364–19373. doi: 10.1074/jbc.M116.732347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng J, Thomson LM, Aicher SA, Terman GW. Primary afferent NMDA receptors increase dorsal horn excitation and mediate opiate tolerance in neonatal rats. J Neurosci. 2006;26(46):12033–12042. doi: 10.1523/JNEUROSCI.2530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao YL, Chen SR, Chen H, Pan HL. Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-d-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. J Biol Chem. 2012;287(30):25073–25085. doi: 10.1074/jbc.M112.378737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan X, Jiang E, Gao M, Weng HR. Endogenous activation of presynaptic NMDA receptors enhances glutamate release from the primary afferents in the spinal dorsal horn in a rat model of neuropathic pain. J Physiol. 2013;591(7):2001–2019. doi: 10.1113/jphysiol.2012.250522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004(255):re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 16.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanz-Clemente A, Nicoll RA, Roche KW. Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist Rev J Bringing Neurobiol Neurol Psychiatry. 2013;19(1):62–75. doi: 10.1177/1073858411435129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehoe LA, Bernardinelli Y, Muller D. GluN3A: an NMDA receptor subunit with exquisite properties and functions. Neural Plast. 2013;2013:145387. doi: 10.1155/2013/145387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994;368(6469):335–339. doi: 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- 20.Cavara NA, Orth A, Hicking G, Seebohm G, Hollmann M. Residues at the tip of the pore loop of NR3B-containing NMDA receptors determine Ca2+ permeability and Mg2+ block. BMC Neurosci. 2010;11:133. doi: 10.1186/1471-2202-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukaya M, Hayashi Y, Watanabe M. NR2 to NR3B subunit switchover of NMDA receptors in early postnatal motoneurons. Eur J Neurosci. 2005;21(5):1432–1436. doi: 10.1111/j.1460-9568.2005.03957.x. [DOI] [PubMed] [Google Scholar]
- 22.Shigemoto R, Ohishi H, Nakanishi S, Mizuno N. Expression of the mRNA for the rat NMDA receptor (NMDAR1) in the sensory and autonomic ganglion neurons. Neurosci Lett. 1992;144(1–2):229–232. doi: 10.1016/0304-3940(92)90756-W. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. NeuroReport. 1993;4(11):1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Willcockson H, Valtschanoff J. AMPA and NMDA glutamate receptors are found in both peptidergic and non-peptidergic primary afferent neurons in the rat. Cell Tissue Res. 2008;334(1):17–23. doi: 10.1007/s00441-008-0662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marvizon JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-d-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446(4):325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- 26.Nagy GG, Watanabe M, Fukaya M, Todd AJ. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci. 2004;20(12):3301–3312. doi: 10.1111/j.1460-9568.2004.03798.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-d-aspartate autoreceptors in the spinal cord dorsal horn. Proc Natl Acad Sci USA. 1994;91(18):8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu CR, Hwang SJ, Phend KD, Rustioni A, Valtschanoff JG. Primary afferent terminals that express presynaptic NR1 in rats are mainly from myelinated, mechanosensitive fibers. J Comp Neurol. 2003;460(2):191–202. doi: 10.1002/cne.10632. [DOI] [PubMed] [Google Scholar]
- 29.Lu CR, Willcockson HH, Phend KD, Lucifora S, Darstein M, Valtschanoff JG, Rustioni A. Ionotropic glutamate receptors are expressed in GABAergic terminals in the rat superficial dorsal horn. J Comp Neurol. 2005;486(2):169–178. doi: 10.1002/cne.20525. [DOI] [PubMed] [Google Scholar]
- 30.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 31.Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci. 2015;16(1):5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- 32.Corlew R, Brasier DJ, Feldman DE, Philpot BD. Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist. 2008;14(6):609–625. doi: 10.1177/1073858408322675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen RS, Corlew RJ, Henson MA, Roberts AC, Mishina M, Watanabe M, Lipton SA, Nakanishi N, Perez-Otano I, Weinberg RJ, Philpot BD. NR3A-containing NMDARs promote neurotransmitter release and spike timing-dependent plasticity. Nat Neurosci. 2011;14(3):338–344. doi: 10.1038/nn.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banerjee A, Larsen RS, Philpot BD, Paulsen O. Roles of presynaptic NMDA receptors in neurotransmission and plasticity. Trends Neurosci. 2016;39(1):26–39. doi: 10.1016/j.tins.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386(6626):721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 36.McRoberts JA, Ennes HS, Marvizon JC, Fanselow MS, Mayer EA, Vissel B. Selective knockdown of NMDA receptors in primary afferent neurons decreases pain during phase 2 of the formalin test. Neuroscience. 2011;172:474–482. doi: 10.1016/j.neuroscience.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen SR, Hu YM, Chen H, Pan HL. Calcineurin inhibitor induces pain hypersensitivity by potentiating pre- and postsynaptic NMDA receptor activity in spinal cords. J Physiol. 2014;592(1):215–227. doi: 10.1113/jphysiol.2013.263814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J Neurosci. 2004;24(11):2774–2781. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu ZZ, Chen SR, Pan HL. Transient receptor potential vanilloid type 1 activation down-regulates voltage-gated calcium channels through calcium-dependent calcineurin in sensory neurons. J Biol Chem. 2005;280(18):18142–18151. doi: 10.1074/jbc.M501229200. [DOI] [PubMed] [Google Scholar]
- 40.Wu ZZ, Chen SR, Pan HL. Signaling mechanisms of down-regulation of voltage-activated Ca2+ channels by transient receptor potential vanilloid type 1 stimulation with olvanil in primary sensory neurons. Neuroscience. 2006;141(1):407–419. doi: 10.1016/j.neuroscience.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Oshima-Takago T, Takago H. NMDA receptor-dependent presynaptic inhibition at the calyx of Held synapse of rat pups. Open Biol. 2017 doi: 10.1098/rsob.170032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagadala P, Park CK, Bang S, Xu ZZ, Xie RG, Liu T, Han BX, Tracey WD, Jr, Wang F, Ji RR. Loss of NR1 subunit of NMDARs in primary sensory neurons leads to hyperexcitability and pain hypersensitivity: involvement of Ca(2+)-activated small conductance potassium channels. J Neurosci. 2013;33(33):13425–13430. doi: 10.1523/JNEUROSCI.0454-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson LM, Zeng J, Terman GW. Differential effect of glutamate transporter inhibition on EPSCs in the morphine naive and morphine tolerant neonatal spinal cord slice. Neurosci Lett. 2006;407(1):64–69. doi: 10.1016/j.neulet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Deng M, Chen SR, Chen H, Luo Y, Dong Y, Pan HL. Mitogen-activated protein kinase signaling mediates opioid-induced presynaptic NMDA receptor activation and analgesic tolerance. J Neurochem. 2019;148(2):275–290. doi: 10.1111/jnc.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou HY, Chen SR, Chen H, Pan HL. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci. 2010;30(12):4460–4466. doi: 10.1523/JNEUROSCI.5857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Correll GE, Maleki J, Gracely EJ, Muir JJ, Harbut RE. Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain medicine (Malden, Mass) 2004;5(3):263–275. doi: 10.1111/j.1526-4637.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- 47.Kiefer RT, Rohr P, Ploppa A, Dieterich HJ, Grothusen J, Koffler S, Altemeyer KH, Unertl K, Schwartzman RJ. Efficacy of ketamine in anesthetic dosage for the treatment of refractory complex regional pain syndrome: an open-label phase II study. Pain Med (Malden, Mass) 2008;9(8):1173–1201. doi: 10.1111/j.1526-4637.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- 48.Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280(2):829–838. [PubMed] [Google Scholar]
- 49.Zhou HY, Chen SR, Byun HS, Chen H, Li L, Han HD, Lopez-Berestein G, Sood AK, Pan HL. N-Methyl-d-aspartate receptor- and calpain-mediated proteolytic cleavage of K+–Cl− cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J Biol Chem. 2012;287(40):33853–33864. doi: 10.1074/jbc.M112.395830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santos SF, Rebelo S, Derkach VA, Safronov BV. Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol. 2007;581(Pt 1):241–254. doi: 10.1113/jphysiol.2006.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Chen SR, Ma H, Chen H, Hittelman WN, Pan HL. Regulating nociceptive transmission by VGluT2-expressing spinal dorsal horn neurons. J Neurochem. 2018;147(4):526–540. doi: 10.1111/jnc.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Li L, Chen SR, Chen H, Xie JD, Sirrieh RE, MacLean DM, Zhang Y, Zhou MH, Jayaraman V, Pan HL. The α2δ-1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018;22(9):2307–2321. doi: 10.1016/j.celrep.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 54.Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14(12):1325–1332. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu XM, Salter MW. Src, a molecular switch governing gain control of synaptic transmission mediated by N-methyl-d-aspartate receptors. Proc Natl Acad Sci USA. 1999;96(14):7697–7704. doi: 10.1073/pnas.96.14.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen SR, Zhou HY, Byun HS, Chen H, Pan HL. Casein kinase II regulates N-methyl-d-aspartate receptor activity in spinal cords and pain hypersensitivity induced by nerve injury. J Pharmacol Exp Ther. 2014;350(2):301–312. doi: 10.1124/jpet.114.215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424(6951):938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 58.Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA, Gu G. Differential distribution of voltage-gated calcium channel alpha-2 delta (alpha2delta) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. J Comp Neurol. 2005;491(3):246–269. doi: 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- 59.Luo Y, Ma H, Zhou JJ, Li L, Chen SR, Zhang J, Chen L, Pan HL. Focal cerebral ischemia and reperfusion induce brain injury through alpha2delta-1-bound NMDA receptors. Stroke. 2018;49(10):2464–2472. doi: 10.1161/STROKEAHA.118.022330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma H, Chen SR, Chen H, Li L, Li DP, Zhou JJ, Pan HL. alpha2delta-1 is essential for sympathetic output and NMDA receptor activity potentiated by angiotensin II in the hypothalamus. J Neurosci. 2018;38(28):6388–6398. doi: 10.1523/JNEUROSCI.0447-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci Off J Soc Neurosci. 2001;21(6):1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel R, Bauer CS, Nieto-Rostro M, Margas W, Ferron L, Chaggar K, Crews K, Ramirez JD, Bennett DL, Schwartz A, Dickenson AH, Dolphin AC. alpha2delta-1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. J Neurosci Off J Soc Neurosci. 2013;33(42):16412–16426. doi: 10.1523/JNEUROSCI.1026-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newton RA, Bingham S, Case PC, Sanger GJ, Lawson SN. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res. 2001;95(1–2):1–8. doi: 10.1016/S0169-328X(01)00188-7. [DOI] [PubMed] [Google Scholar]
- 64.Hauser W, Bernardy K, Uceyler N, Sommer C. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145(1–2):69–81. doi: 10.1016/j.pain.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 65.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280(21):1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 66.Fuller-Bicer GA, Varadi G, Koch SE, Ishii M, Bodi I, Kadeer N, Muth JN, Mikala G, Petrashevskaya NN, Jordan MA, Zhang SP, Qin N, Flores CM, Isaacsohn I, Varadi M, Mori Y, Jones WK, Schwartz A. Targeted disruption of the voltage-dependent calcium channel alpha2/delta-1-subunit. Am J Physiol Heart Circ Physiol. 2009;297(1):H117–124. doi: 10.1152/ajpheart.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen SR, Zhu L, Chen H, Wen L, Laumet G, Pan HL. Increased spinal cord Na(+)–K(+)–2Cl(−) cotransporter-1 (NKCC1) activity contributes to impairment of synaptic inhibition in paclitaxel-induced neuropathic pain. J Biol Chem. 2014;289(45):31111–31120. doi: 10.1074/jbc.M114.600320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie JD, Chen SR, Chen H, Pan HL. Bortezomib induces neuropathic pain through protein kinase C-mediated activation of presynaptic NMDA receptors in the spinal cord. Neuropharmacology. 2017;123:477–487. doi: 10.1016/j.neuropharm.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie JD, Chen SR, Pan HL. Presynaptic mGluR5 receptor controls glutamatergic input through protein kinase C-NMDA receptors in paclitaxel-induced neuropathic pain. J Biol Chem. 2017;292(50):20644–20654. doi: 10.1074/jbc.M117.818476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Chen SR, Chen H, Zhang J, Pan HL. Increased alpha2delta-1-NMDA receptor coupling potentiates glutamatergic input to spinal dorsal horn neurons in chemotherapy-induced neuropathic pain. J Neurochem. 2019;148(2):252–274. doi: 10.1111/jnc.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strack S, Wadzinski BE, Ebner FF. Localization of the calcium/calmodulin-dependent protein phosphatase, calcineurin, in the hindbrain and spinal cord of the rat. J Comp Neurol. 1996;375(1):66–76. doi: 10.1002/(SICI)1096-9861(19961104)375:1<66::AID-CNE4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 72.Kakihana K, Ohashi K, Murata Y, Tsubokura M, Kobayashi T, Yamashita T, Sakamaki H, Akiyama H. Clinical features of calcineurin inhibitor-induced pain syndrome after allo-SCT. Bone Marrow Transplant. 2012;47(4):593–595. doi: 10.1038/bmt.2011.120. [DOI] [PubMed] [Google Scholar]
- 73.Noda Y, Kodama K, Yasuda T, Takahashi S. Calcineurin-inhibitor-induced pain syndrome after bone marrow transplantation. J Anesth. 2008;22(1):61–63. doi: 10.1007/s00540-007-0574-2. [DOI] [PubMed] [Google Scholar]
- 74.Lieberman DN, Mody I. Regulation of NMDA channel function by endogenous Ca(2 +)-dependent phosphatase. Nature. 1994;369(6477):235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- 75.Hu YM, Chen SR, Chen H, Pan HL. Casein kinase II inhibition reverses pain hypersensitivity and potentiated spinal N-methyl-d-aspartate receptor activity caused by calcineurin inhibitor. J Pharmacol Exp Ther. 2014;349(2):239–247. doi: 10.1124/jpet.113.212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Y, Chen SR, Chen H, Pan HL. Endogenous TRPA1 and TRPV1 activity contributes to potentiated glutamatergic input to spinal lamina I neurons in inflammatory pain. J Neurochem. 2019 doi: 10.1111/jnc.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weyerbacher AR, Xu Q, Tamasdan C, Shin SJ, Inturrisi CE. N-Methyl-d-aspartate receptor (NMDAR) independent maintenance of inflammatory pain. Pain. 2010;148(2):237–246. doi: 10.1016/j.pain.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]