Abstract
Influenza virus infection causes substantial morbidity and mortality worldwide. The limited efficacy of oseltamivir in delayed treatment, coupled with the increasing incidences of oseltamivir-resistant strains, calls for next-generation of antiviral drugs. In this study, we discovered NMS-873, an allosteric and specific p97 inhibitor, as a broad-spectrum influenza antiviral through forward chemical genomics screening. NMS-873 shows potent antiviral activity with low-nanomolar EC50s against multiple human influenza A and B viruses, including adamantine-, oseltamivir-, or double resistant strains. Our data further showed that silencing of p97 via siRNA or inhibiting p97 by NMS-873 both inhibited virus replication and retained viral ribonucleoproteins (vRNPs) in the nucleus, confirming p97 is the drug target. Mechanistic studies have shown that the nuclear retention of vRNP with NMS-873 treatment is a combined result of two effects: the reduced viral M1 protein level (indirect effect), and the disruption of p97-NP interactions (direct effect). Taken together, our results suggest that p97 could be a novel antiviral target and its inhibitor, NMS-873, is a promising antiviral drug candidate.
Keywords: Influenza virus, p97, NMS-873, broad-spectrum antiviral, host-targeting antiviral
1. Introduction
Influenza (also called flu) is a highly contagious respiratory illness with high morbidity and mortality rates in both human and animal worldwide [1]. In United States, it is estimated that seasonal influenza caused 140,000 to 710,000 hospitalizations since 2010, which resulted in 12,000 to 56,000 deaths in human (https://www.cdc.gov/flu/protect/keyfacts.htm). The recent 2017–18 influenza season was a high-severity season with record hospitalization rates and high numbers of influence-related pediatric deaths (https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm). Pandemic influenza occurs rarely but can cause high levels of mortality. For example, the 1918 Spanish influenza pandemic was responsible for the deaths of approximately 40 million people worldwide [2].
Both seasonal influenza and pandemic influenza are caused by influenza viruses, which are single-strand, segmented negative-sense RNA viruses that belong to Orthomyxoviruses family [3]. There are four types of influenza viruses: A, B, C and recently named type D. Among them, both human influenza A and B viruses can cause annual seasonal epidemics and influenza A viruses also can lead to sporadic influenza pandemics. Both influenza A and B viruses co-circulate in each influenza season although the constitution and ratio of influenza A and B viruses vary, which presents a grand challenge in developing influenza vaccines and antivirals [2].
There are two main strategies used to control influenza infections: vaccines and antiviral drugs [4]. Vaccination strategy is the most effect and widely used method to prevent influenza infections with an overall 60% effectiveness. However, vaccines have limited efficacy in immune-compromised patients and have to be reformulated every year due to the changes of the circulating influenza viruses in each influenza season. Despite intensive research for new influenza antivirals, only three classes antiviral drugs are approved by FDA: the M2 ion channel blockers (amantadine and rimantadine), which prevent viral uncoating [5], the neuraminidase (NA) inhibitors (oseltamivir, zanamivir, and peramivir), which block the release of newly formed viral particles [6], [7], and recently approved PA cap-dependent endonuclease inhibitor (baloxavir marboxil) (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624226.htm). For M2 inhibitors amantadine and rimantadine, more than 95% of currently circulating influenza A virus strains are drug resistant, and the use of amantadine and rimantadine for influenza prevention and treatment was no longer recommended by CDC [8, 9]. Although the drug binding site for the NA inhibitors is highly conserved, the number of reports of oseltamivir-resistant influenza strains keeps increasing [8, 10, 11]. Resistance to baloxavir marboxil was also reported during the clinical trials [12]. Therefore, there is an urgent and continuous need to develop next generation of broad-spectrum antiviral drugs with a novel mode of action to combat infections by both oseltamivir-sensitive and -resistant viruses. In addition to the virial proteins, host factors required for influenza replication are also valid antiviral drug targets. The advantage of host-targeting antivirals is that they are less likely than viral protein to mutate under drug selective pressure [13]. For this reason, several genome-wide screening had been conducted and a number of host factors required for influenza replication have been identified [14] [15] [16] [17] [18] [19] [20].
As an orthogonal approach, we employed a forward chemical genomics approach to identify novel anti-influenza drugs by screening of a library of bioactive compounds with known biological functions against A/WSN/33 (H1N1) virus using cytopathic (CPE) assay. Two of the screening hits with potent antiviral activity had been identified and their antiviral mechanism of action had also been characterized [21], [22]. In this study, we reported NMS-873 (Fig. 1A), an allosteric and specific p97 inhibitor [23], has potent and broad-spectrum antiviral activity against both influenza A and B viruses with low-nanomolar EC50 values. p97, also known as VCP (valosin-containing protein) or Cdc48p in yeast, is a ubiquitous, abundant and essential ATPase. It is highly conserved among eukaryotes [24], [25] and plays several essential roles in cellular processes, including protein degradation, membrane fusion, vesicular trafficking, and disassembly of stress granules [26]. It has been reported that p97 is involved in the replication of several viruses, such as picornaviruses, Sindbis virus, bronchitis virus, and West Nile virus [27] [28] [29] [30]. However, its role in influenza replication was unclear. In this study, we elucidated the roles of p97 in influenza virus replication by using the NMS-873 as a chemical probe. Overall, our study not only identified NMS-873 as a novel influenza antiviral, but also confirmed p97 as a promising antiviral drug target.
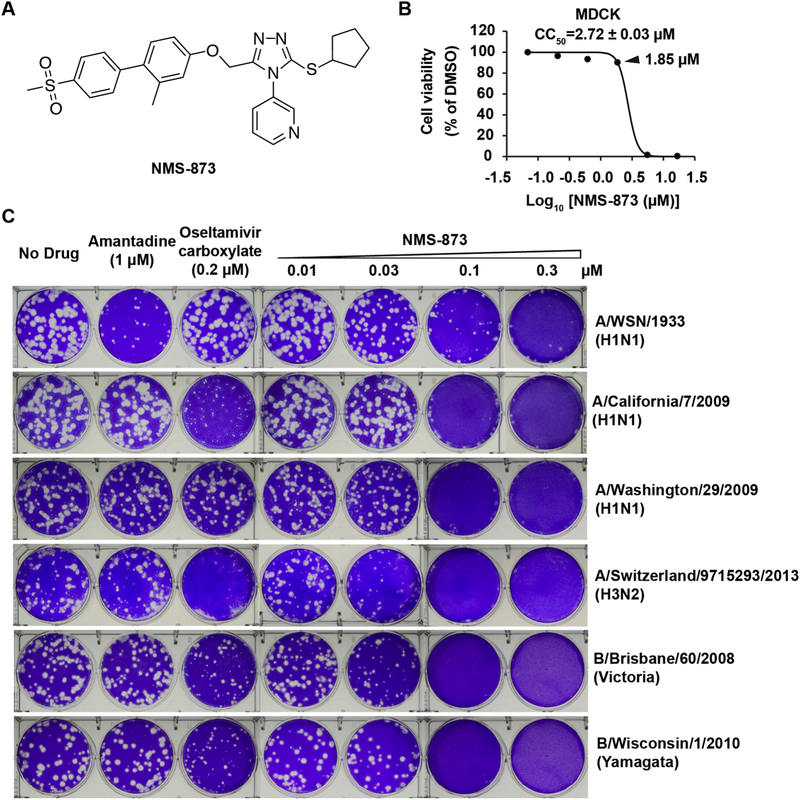
Fig. 1. NMS-873 inhibits both influenza A and B viruses.
(A) Chemical structure of NMS-873. (B) Cellular cytotoxicity of NMS-873 in MDCK cells. (C) Antiviral activity of NMS-873 against representative influenza A and B viruses. The antiviral activity of NMS-873 was determined in plaque reduction assay. Monolayers of MDCK cells overexpressing ST6Gal I were infected with indicated viruses and incubated with overlays containing a serial of concentrations of NMS-873, 1 μM of amantadine or 0.2 μM of oseltamivir carboxylate.
2. Materials and methods
2.1. Cell lines, viruses, and viral infection
Madin-Darby Canine Kidney cells (MDCK), MDCK cells overexpressing ST6Gal I and human alveolar basal epithelial cells A549 were cultured as previously described [21]. Human bronchoepithelial cells BEAS-2B were grown in complete medium RPMI1640 supplemented with 10% fetal bovine serum (FBS). Human lung epithelial primary cells were grown in human lung epithelial primary cell culture complete growth medium.
Influenza A virus strain A/WSN/1933 (H1N1) was obtained from Dr. Robert Lamb at the Northwestern University. A/Denmark/528/2009 (H1N1) was obtained from Dr. Elena Govorkova at St. Jude Children’s Research Hospital. A/Washington/29/2009 (H1N1) and A/Switzerland/9715293/2013 (H3N2) were obtained from CDC (Atlanta, GA, USA). Influenza A virus strains, A/California/07/2009 (H1N1) and A/Texas/04/2009 (H1N1), and influenza B virus strains, B/Brisbane/60/2008 (Victoria lineage) and B/Wisconsin/1/2010 (Yamagata lineage), were obtained from Dr. James Noah at the Southern Research Institute. Virus stocks were amplified in MDCK cells and their titers were determined in plaque assay.
2. 2. Cytotoxicity assay and CPE assay
Cellular cytotoxicity of compounds and the antiviral efficacy of compound against influenza virus induced CPE were determined in neutral red uptake assay or 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay [31]–[32].
2. 3. Plaque assay
MDCK cells expressing ST6Gal I were used to examine the effect of the compounds in plaque assay as previously described [33].
2. 4. Time-of-addition experiment
Time-of-addition experiment was performed as previously described [33]. NMS-873 (1.8 μM) was added at different time points either before, at, or after viral infection to test which stage(s) of viral replication was affected. Oseltamivir carboxylate (1 μM) was included as a control.
2. 5. Immunostaining assay
Influenza A/WSN/33 (H1N1) infected cells were fixed with 4% formaldehyde for 10 min followed by permeabilization with 0.2% Triton X-100 for another 10 min. After blocking with 10% bovine serum, cells were stained with mouse anti-NP antibody (Bio-Rad: MCA400) and rabbit anti-M1 (GeneTex: GTX125928) and followed by staining with anti-mouse secondary antibody conjugated to Alexa-488 and anti-rabbit secondary antibody conjugated to Alexa-594 (Thermo Scientific). Nucleus were stained with 300 nM DAPI (Thermo Scientific) after secondary antibodies incubation. Fluorescent images were acquired using a Leica SP5-II spectral Confocal Microscope (Leica).
2. 6. Western blotting
Total proteins were extracted from influenza infected cells using RAPI lysis buffer (50 mM Tris pH 8.0, 1% NP-40, 0.1% SDS, 150 mM NaCl, 0.5% Sodium deoxycholate, 5 mM EDTA, 10 mM NaF, 10 mM NaPPi, 1 mM phenyl-methylsulfonyl). Equal amount of extracted total proteins were separated by electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane. Target proteins were detected using the following antibodies: rabbit anti-PA (GeneTex: GTX125932); rabbit anti-PB1 (GeneTex: GTX125923); rabbit anti-HA (GeneTex: GTX127357); rabbit anti-NS1 (GeneTex: GTX125990); rabbit anti-NP (GeneTex: GTX125989), rabbit anti-M1 (GeneTex: GTX125928), mouse anti-GAPDH antibody (EMD Millipore: MAB374).
2. 7. Transfection of plasmid and small interfering RNA (siRNA)
Transfection of DNA plasmid was performed using Lipofectamine 3000 (Thermo Fisher Scientific) and OptiMEM (Invitrogen). siRNA transfection was performed using Hiperfect (Qiagen). p97 siRNAs, p97 siRNA#6 (SI03019618), p97 siRNA#7 (SI03019730), and control siRNA (Ctrl; 1027281) were purchased from Qiagen. NP siRNA (5’-GGAUCUUAUUUCUUCGGAGAA-3’) was included as a control.
2.8. Coimmunoprecipitation (CO-IP) assay
For performing CO-IP, mammalian expression constructs expressing Flag-tagged p97 (Flag-p97) or HA-tagged NP (NP-HA) were generated. Briefly, each gene was amplified using Platinum SuperFi DNA polymerase (ThermoFisher Scientific) and gene-specific primers (section 2.9). For Flag-p97 or NP-HA, digested PCR products were inserted into pCAGGS vector via Sac I and Bgl II or EcoR I and Bgl II sites. All the final constructs were confirmed by sequencing. HEK 293 cells transfected with Flag-p97 alone or along with NP-HA or A549 cells infected with influenza A/WSN/33 (H1N1) at MOI of 3 were used for coimmunoprecipitation assay. Harvested cells were lysed in RIPA buffer [50 mM Tris at pH 8.0, 1% Nonidet P-40, 0.1% SDS, 150 mM NaCl, 0.5% sodium deoxycholate, 5 mM EDTA, 10 mM NaF, 10 mM NaPPi, 2 mM phenylmethylsulfonyl, 5 mM benzamidine, and 1× protease inhibitor cocktail (ThermoFisher Scientific)]. After centrifugation, the supernatant was mixed with Protein A Sepharose beads (GE Healthcare) plus anti-Flag (GenScript, A00187) or anti-HA (Invitrogen, 71–5500) antibody and incubated overnight at 4 °C. Beads were pelleted and washed 3 times with RIPA buffer, then the beads was resuspended in 1× SDS gel loading buffer and boiled for 10 minutes. The samples were run in a 4–10% SDS-PAGE gel and used for detecting target proteins.
2.9. Primers used in this study
| vRNA-P | 5’-AGCAAAAGCAGG-3’ |
| cRNA-P | 5’-AGTAGAAACAAGG-3’ |
| oligo (dT)18 | 5′-TTTTTTTTTTTTTTTTTT-3' |
| NP-F | 5'-AGGGTCAGTTGCTCACAAGTCC-3' |
| NP-R | 5'-TTTGAAGCAGTCTGAAAGGGTCTA-3' |
| M1F | 5'-ATGGGAACGGAGATCCAAATAA-3' |
| MIR | 5'-TGCACCAGCAGAATAACTGAGTG-3' |
| GAPDH-F | 5'-ACACCCACTCCTCCACCTTTG-3' |
| GAPDH-R |
5'-CACCACCCTGTTGCTGTAGCC-3' 5'- |
| Flag-p97-F | CTAGAGCTCATGGACTACAAAGACGATGACGACAAGCTCGAGATGGC TTCTGGAGCCGAT-3' |
| p97-R | 5'-GGAAGATCTTTAGCCATACAGGTCATCATCATTG-3' |
| NP-F |
5'-GAATTCATGGCGACCAAAGGCACCAAAC-3' 5'- |
| NP-HA-R | AGATCTCTAAGCGTAATCTGGAACATCGTATGGGTACTCGAGATTGTC GTACTCCTCTGCATTGTCT -3' |
3. Results
3. 1. NMS-873 has potent activity against both influenza A and B viruses
In a screening of an in-house compound library including more than 1000 of known drugs or drug-like molecules, NMS-873 (Fig. 1A) was one of the screening hits showing potent antiviral activity against A/WSN/33 (H1N1) virus. To verify the screening result, we first tested the cellular cytotoxicity of NMS-873. When MDCK cells were treated with NMS-873 for 48 h, no cellular cytotoxicity was observed at concentrations up to 1.85 μM, and the calculated 50% cellular cytotoxicity (CC50) is 2.72 ± 0.03 μM (Fig. 1B). Therefore, nontoxic concentrations (0.01–0.3 μM) were used in the following plaque assay (Fig. 1C). NMS-873 actively inhibited A/WSN/33 (H1N1) virus replication in a dose-dependent manner and the EC50 value is 37 ± 2 nM.
Next, we tested whether NMS-873 also inhibits other influenza virus stains, in particular the drug-resistant strains, such as the amantadine-resistant, oseltamivir-sensitive strains A/California/07/2009 (H1N1), A/Switherland/9715293/2013 (H3N2), B/Wisconsin/1/2010 (Yamagata), and B/Brisbane/60/2008 (Victoria), and the oseltamivir- and amantadine-resistant strains A/Washington/29/2009 (H1N1), A/North Carolina/39/2009 (H1N1), A/Texas/4/2009 (H1N1), and A/Denmark/528/2009 (H1N1) (Fig. 1C and Table 1), which pose greater threat to public health [34]. Despite the diverse genetic background and drug sensitivity of these influenza viruses towards amantadine and oseltamivir, the replication of all the tested viruses was inhibited by NMS-873 in a dose-dependent manner, indicating a broad-spectrum antiviral activity of NMS-873. Of note, the EC50 values of NMS-873 against these viruses were 28–56 nM and the selectivity index (SI), ratio of CC50/EC50, ranges from 49 to 97 (Table 1), indicating an acceptable therapeutic window.
Table 1.
EC50 of NMS-873 against influenza A or B viruses.
| Influenza Strains | Drug sensitivity | EC50(nM)a | SIb |
|---|---|---|---|
| A/Washington/29/2009 (H1N1) | Amantadine Resistant Oseltamivir Resistant |
41 ± 5 | 66 |
| A/North Carolina/39/2009 (H1N1) | 35 ± 5 | 78 | |
| A/Texas/04/2009 (H1N1) | 38 ± 5 | 71 | |
| A/Denmark/528/2009 (H1N1) | 39 ± 4 | 70 | |
| A/WSN/1933 (H1N1) | Amantadine Resistant Oseltamivir Sensitive |
37 ± 2 | 74 |
| A/California/07/2009 (H1N1) | 56 ± 5 | 49 | |
| A/Switzerland/9715293/2013 (H3N2) | 21 ± 2 | 129 | |
| B/Wisconsin/1/2010 (Yamagata) | Amantadine Resistant Oseltamivir Sensitive |
35 ± 5 | 78 |
| B/Bris/60/2008 (Victoria) | 28 ± 8 | 97 |
, EC50 was determined in the plaque assay using the ST6Gal I–overexpressing MDCK cells (AX-4). Plaque areas were quantified for calculation of EC50 using Image J and Prism 5;
, SI is selectivity index, which was calculated by CC50/EC50.
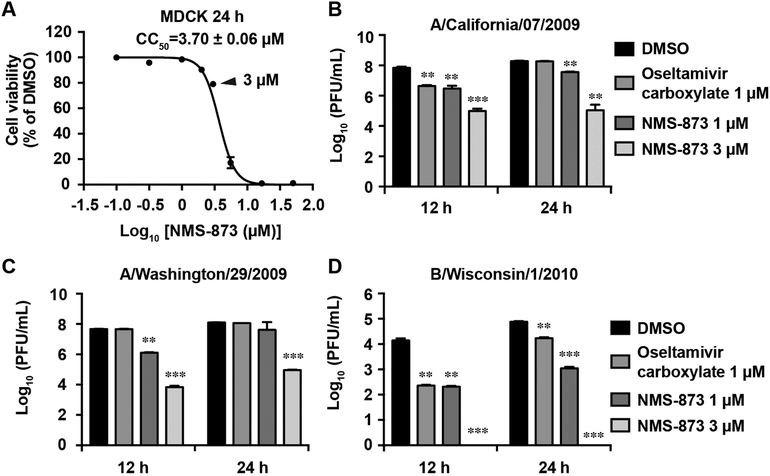
Infection with high MOI of influenza virus mimics the condition of late treatment post viral infection in the clinical setting. To test whether NMS-873 also inhibits viral replication under this condition, MDCK cells were infected with three of above viruses, A/California/7/2009 (H1N1), A/Washington/29/2009 (H1N1), and B/Wisconsin/1/2010 (Yamagata), at MOI of 1 and were treated with NMS-873 at non-cytotoxic drug concentrations (1–3 μM) (cell viability > 80%) (Fig. 2A). NMS-873 showed potent antiviral efficacy against all three viruses in a dose-dependent manner (Figs. 2B–2D). For instance, NMS-873 at 1 μM significantly reduced the viral titer of A/California/7/2009 (H1N1) by 1.3 and 0.7 log10 units at 12 and 24 h p.i., respectively, while 3 μM of NMS-873 significantly reduced the viral titer by 2.8 and 3.2 log10 units at 12 and 24 h p.i., respectively. Oseltamivir carboxylate (1 μM) only reduced the viral titer by 1.2 log10 units at 12 h p.i. and had no effect at 24 h p.i. (Fig. 2B). Similarly, NMS-873 also showed potent antiviral activity against the oseltamivir-resistant strain, A/Washington/29/2009 (H1N1), at both 12 and 24 h p.i., whereas oseltamivir did not (Fig. 2C). This result was consistent with the fact that A/Washington/29/2009 (H1N1) was resistant to oseltamivir due to the H275Y mutation in its NA gene (Fig. 1C). For influenza B/Wisconsin/1/2010 (Yamagata), NMS-873 at 1 μM reduced the viral titer by more than 1.8 log10 units at both 12 h p.i. and 24 h p.i., similar to the effect of oseltamivir treatment (1.7 log10 units of reduction). Noticeably, 3 μM of NMS-873 completely inhibited the influenza B/Wisconsin/1/2010 at both 12 h p.i. and 24 h p.i., (Fig. 2D), suggesting influenza B virus is more sensitive to NMS-873 treatment. In summary, the broad-spectrum antiviral activity, wide therapeutic window, and the lack of cross-resistance with the currently available anti-influenza drug oseltamivir, make NMS-873 as a promising antiviral drug candidate.
Fig. 2. Antiviral activity of NMS-873 against influenza viruses at a high MOI.
(A) Cellular cytotoxicity of NMS-873 in MDCK cell. The concentration of 3 μM was indicated with a triangle. (B-D) MDCK cells were infected with A/California/7/2009 (H1N1) (B), A/Washington/29/2009 (H1N1) (C) or B/Wisconsin/1/2010 (D) at MOI of 1. NMS-873 (1 or 3 μM) was added after viral infection. Viruses in supernatant were harvested at 12 or 24 h post infection for titration by plaque assay. Oseltamivir carboxylate (1 μM) was included as a control. Data represents mean ± SE from three independent experiments. **, p<0.01; *** p < 0.001 (student’s t-test).
3. 2. NMS-873 inhibits influenza virus in human lung epithelial cell lines
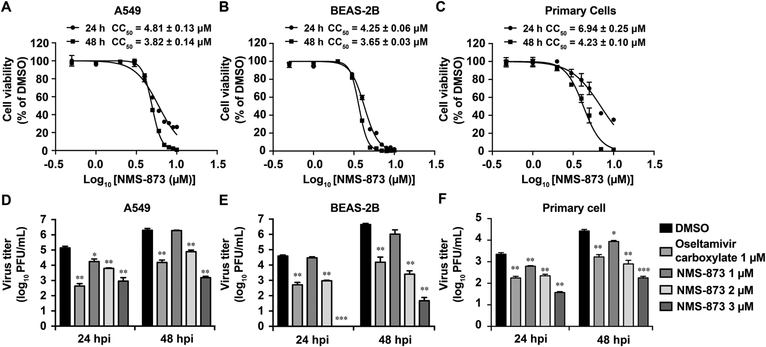
As NMS-873 is a host-targeting antiviral, it is important to rule out the possibility that the antiviral activity of NMS-873 might be cell-type dependent. For this, we tested the antiviral activity of NMS-873 in two human lung epithelial cell lines, A549 and BEAS-2B, as well as in human lung epithelial primary cells (Fig. 3). When A549 cells were infected with A/WSN/33 (H1N1) virus at a MOI of 0.01, NMS-873 significantly inhibited the viral replication at both 12 h p.i. and 24 h p.i. in a dose-dependent manner (Fig. 3D). The viral titer was significantly reduced 1.3–1.4 and 2.2–3.1 log10 units by 2 μM and 3 μM of NMS-873, respectively, while 2.1–2.5 log10 units of reduction was observed for oseltamivir (Fig. 3D). Of note, the highest drug concentration tested (3 μM) was not cytotoxic to A549 cells (Fig. 3A). For BEAS-2B and primary cells, a similar potent antiviral effect was observed (Fig. 3E and 3F). Taken together, these results confirmed the antiviral activity of NMS-873 in both human cell lines and primary cells and thus ruled out the possibility that the antiviral activity of NMS-873 is cell-type dependent.
Fig. 3. Antiviral activity of NMS-873 in human lung cell lines and primary cells.
(A, B and C) Cellular cytotoxicity of NMS-873 in human lung epithelial cell lines. A549 (A), BEAS-2B (B) or primary cells (C) were incubated with a serial of concentrations of NMS-873 for 24 or 48 h, then cell viability was determined in neutral red uptake assay. (D, E and F) NMS-873 inhibited influenza virus growth in human lung epithelial cells. A549 (D), BEAS-2B (E), or primary cells (F) were infected with A/WSN/1933 at MOI of 0.01. Oseltamivir carboxylate (1 μM) or NMS-873 (1, 2, or 3 μM) was added after viral infection. Viruses in supernatant were harvested at 24 or 48 h post infection and titrated using the plaque assay. Data represents mean ± SE from three independent experiments. **, p<0.01; *** p < 0.001 (student’s t-test).
3. 3. Silencing the target of NMS-873, p97, inhibits influenza virus replication
Although NMS-873 is a specific p97 inhibitor, showing potent selectivity for p97 compared to a panel of other AAA ATPases, Hsp90, and 53 additional analyzed kinases [23], there is a possibility that its antiviral activity might due to its off-target effect. To rule out this possibility, we employed siRNA approach to specially knock down the expression of p97 in A549 cells and tested the effect of p97 knockdown on influenza replication. As shown in Fig. 4A, transfecting p97-specific siRNAs, siRNA6 or 7, largely reduced p97 protein level (Fig. 4A). Next, p97 silenced cells were mock infected or infected with A/WSN/1933 (MOI = 1). It was found that silencing p97 significantly reduced the virus titer by 0.7–1.6 log10 units at both 24 and 48 h p.i, without affecting cell viability (Fig. 4B and 4C), confirmed that p97 is the antiviral drug target of NMS-873.
Fig. 4. siRNA-medicated silencing of NMS-873 target, p97, inhibits influenza virus replication.
(A) Knockdown of p97 using p97-specific siRNAs. A549 cells were transfected with p97 siRNAs (6 and 7). Ctrl siRNA without targeting any host gene was used as a negative control. Total protein was extracted at 60 h post transfection for detection of p97 by western. GAPDH was served as a loading control. (B and C) Knockdown of p97 reduced influenza virus titer without affecting cell viability. A549 cells were transfected with p97 siRNAs and followed by mock (no virus) or A/WSN/33 (H1N1) infection (MOI = 1) at 60 h post transfection. Mock infected cells were used for cell viability determined at 48 h.p.i. by natural red uptake assay (B). Viruses in supernatant were collected at 24 or 48 h.p.i. and titrated using plaque assay (C). Ctrl siRNA without targeting any host gene was used as a negative control. NP siRNA targeting influenza NP segment were used as a positive control. Data represents mean ± SE from three independent experiments. *, p<0.05; **, p<0.01; *** p < 0.001 (student’s t-test).
3. 4. NMS-873 inhibits the intermediate stage of viral replication
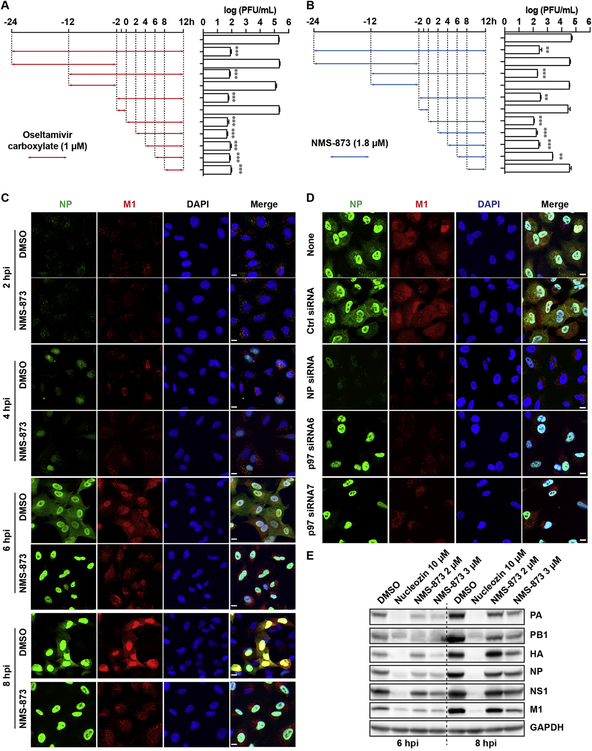
To dissect the antiviral mechanism of NMS-873, we first checked which stage(s) of the viral replication cycle was affected by NMS-873 in a drug time-of-addition experiment. In this experiment, NMS-873 was added at different time points before, at, or after virus infection and its effect on virus replication was determined by quantifying the titer of progeny virus in the cell culture supernatant. As shown in Fig. 5A, oseltamivir carboxylate, served as a control, had no effect on virus replication when it was present either before (−24 to −2 h or −12 to −2 h) or during (−2 to 0 h) the virus infection, but showed potent inhibition while it was present after virus infection, even when added as late as 8 h post infection (Fig. 5A). This result was consistent with the antiviral mechanism of oseltamivir which is inhibiting the release of progeny virions from host cell surface at the final stage of viral life cycle. When NMS-873 was present before or during the infection, no antiviral activity was observed. However, unlike oseltamivir, the antiviral efficacy of NMS-873 was dramatically decreased when it was added at 6 h post infection and the antiviral activity was completely abolished when it was added at 8 h post infection (Fig. 5B), suggesting that NMS-873 inhibits the intermediate stage of influenza virus replication before the virus egress step.
Fig. 5. NMS-873 inhibits the nuclear export of influenza vRNPs.
(A) MDCK cells were infected with A/WSN/33 (H1N1) (MOI = 0.01) at −2 h. After 1-hour incubation at 4 °C for attachment and another 1 h at 37 °C for viral entry, cells were washed with PBS buffer and incubated with DMEM at 37 °C for 12 h, then viruses in culture supernatant were harvested for titration by plaque assay. Oseltamivir carboxylate (1 μM) (A) or NMS-873 (1.8 μM) (B) was present during the time indicated by arrows. Data represents mean ± SE from three independent experiments. **, p<0.01; *** p < 0.001 (student’s t-test). (B) Retention of influenza vRNPs in nuclear by NMS-873 treatment. A549 cells were infected with A/WSN/33 (H1N1) virus at MOI of 30. DMSO or 3 μM of NMS-873 was added after viral infection, cells were fixed at 2, 4, 6 and 8 h p. i. and stained with mouse anti-influenza A NP antibody, rabbit anti-M1 and DAPI to determine the NP, M1, and nucleus, respectively. (C) Knockdown of p97 inhibited the nuclear export of influenza vRNPs. A549 cells were transfected with p97 siRNAs (6 and 7). After 60 h of incubation, cells were infected with A/WSN/33 (H1N1) virus (MOI = 30). Then cells were fixed at 6 h.p.i. and stained with mouse anti-influenza A NP antibody, rabbit anti-M1 and DAPI to determine the NP, M1, and nucleus, respectively. Ctrl siRNA without targeting any host gene was used as a negative control. NP siRNA targeting influenza NP segment were used as a positive control. (E) NMS-873 reduced viral protein levels. A549 cells were infected with A/WSN/33 (H1N1) virus (MOI = 1) and followed by treatment with DMSO, 2 or 3 μM of NMS-873 for 6 h and 8 h. Total protein was extracted for detection of viral protein by western blot. Nucleozin (10 μM) was included as a control.
To further dissect which step(s) of the viral replication was affected in detail, we next examined the subcellular localization of viral ribonucleoprotein (vRNP) complex during the time course of influenza replication with and without NMS-873 treatment (Fig. 5C). In this experiment, Nucleoprotein (NP), an indicator of vRNP, and M1 protein were visualized at 2, 4, 6 and 8 h p.i. by immunofluorescence staining assay. Similar to DMSO control, NMS-873 neither affected the virus entry nor NP nuclear import at 2 h p.i. or 4 h p. i. (Fig. 5C). However, NP largely accumulated in the nucleus in cells treated with NMS-873 at 6 h p.i. and even as late as 8 h p.i., whereas the DMSO treated control cells showed both cytoplasmic and nuclear distributions of NP (Fig. 5C). To further confirm these effects was due to the inhibition of p97, we examined the NP and M1 subcellular localization in p97 knockdown cells (Fig. 5D). Similarly, accumulation of NP in the nucleus and reduction of M1 in the nucleus were only observed in the cells transfected p97 siRNAs but not in control cells, consistent with the previous report [20]. Of note, the M1 protein level in the nucleus was significantly reduced by NMS-873 treatment or in p97 knockdown cells (Fig. 5C and 5D). It is known that M1 is synthesized at later stage of viral replication and shuttles between the nucleus and the cytoplasm [35, 36]. It has been suggested that M1 enters the nucleus and binds to nascent genomic RNP through the interactions with NP and possibly also with viral RNA and the NEP/NS2 complex, and the whole complex interacts with the nuclear export machinery and provides nuclear export signals for the export vRNP from nucleus to cytoplasm [37]. Therefore, the retention of vRNP in the nucleus with NMS-873 treatment might due to the reduced M1 protein level. To further confirm the reduction of M1 protein level as suggested by the immunofluorescence assay, we quantified M1 and other viral protein levels by western blot. Compared to DMSO control, NMS-873 significantly reduced the protein expression levels of M1 and NP at both 6 and 8 h p.i. in a dose dependent manner, suggesting that the reduced M1 and NP protein levels might account for the delayed vRNP nuclear export. In addition, NMS-873 also broadly reduced all the other tested influenza viral proteins, including PA, PB1, HA and NS1, in a dose dependent manner (Fig. 5E). Overall, the time-of-addition, immunofluorescence, and western blot assay results collectively suggest that p97 is essential for viral protein synthesis and vRNP nuclear export, and inhibiting p97 by NMS-873 led to inhibition of viral proteins as well as nuclear retention of vRNP.
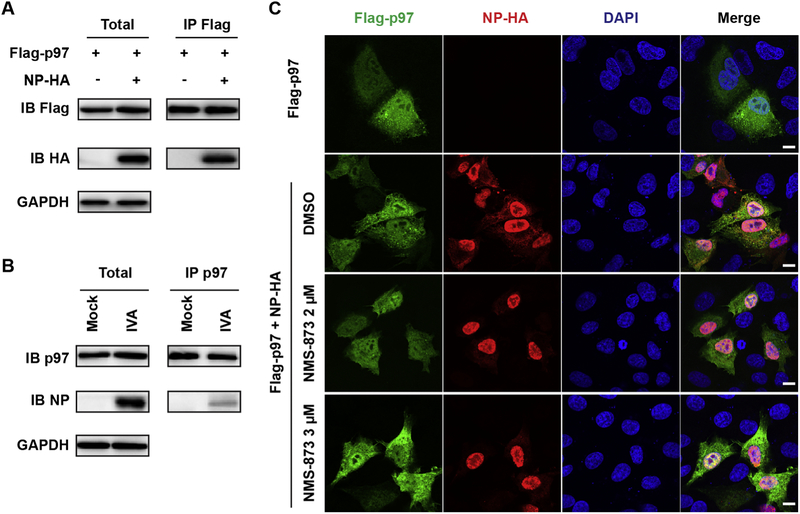
3. 5. p97 interacts with NP and NMS-873 retains NP protein in the nucleus
Apart from the reducing the protein levels of M1 and NP by NMS-873 treatment, which affects NP nuclear export, it was reported that p97 co-precipitates with multiple influenza viral proteins, including the NP protein [20], implying a possible role of p97 in influenza NP nuclear export. To test this hypothesis, we first confirmed the interactions between p97 and NP protein. A Flag-tagged p97 was expressed alone or along with a HA-tagged NP in HEK293 cells. In Co-IP assay, the anti-Flag antibody successfully pulled down NP-HA, indicating an interaction between p97 and NP (Fig. 6A). The p97-NP interaction was also detected in the influenza A/WSN/33-infected A549 cells (Fig. 6B). Next, we tested the effect of NMS-873 treatment on the subcellular localization of NP. We expressed Flag-p97 alone or along with NP-HA in A549 cells. As shown in Fig. 6C, Flag-p97 ubiquitously distributed in both the nucleus and cytoplasm and its subcellular localization was not affected by presence of NP-HA or NMS-873 treatment. In contrast, the NP-HA distributed majorly in the nucleus and partially in cytoplasm without NMS-873 treatment, whereas majority of NP-HA retained in the nucleus with NMS-873 treatment (Fig. 6C). Similar phenotype was observed in the A/WSN/1933 infected cells with NMS-873 treatment or in p97 knocking down cells (Fig. 5B and C). Taken together, the mechanistic studies showed that NMS-873 inhibited vRNP nuclear export not only through reducing viral M1 proton level (indirect effect), but also inhibited NP nuclear export through disrupting p97-NP interactions (direct effect).
Fig. 6. p97 interacts with NP and NMS-873 retains NP in the nucleus.
(A) Co-IP of Flag-p97 and NP-HA. HEK 293 cells were transfected with Flag-p97 alone or along with NP-HA. After 24 h post transfection, cell lysates were subjected to IP using anti-Flag antibody (IP Flag), followed by Western blotting using anti-HA (IB HA) or anti-His (IB His) anti-bodies, respectively. (B) Co-IP of p97 and NP. A549 cells infected with influenza A/WSN/33 (H1N1) at MOI of 3 were used for co-immunoprecipitation assay. Cell lysates were subjected to IP using anti-p97 antibody (IP p97), followed by Western blotting using anti-p97 (IB p97) or anti-NP (IB NP) antibodies, respectively. (C) NMS-873 retains NP in the nuclear. A549 cells were transfected with Flag-p97 alone or along with NP-HA. At 2 h post transfection, compound was added into the culture medium. At 24 h post transfection, cells were fixed and stained with mouse anti-Flag antibody, rabbit anti-HA and DAPI to determine the Flag-p97, NP-HA, and nucleus, respectively.
4. Discussion and conclusion
Influenza, in its seasonal epidemic and pandemic forms, remains a substantial global public health threat. Anti-influenza treatment with current direct-acting antivirals often leads to the drug resistance problem. Therefore, there is an urgent need to find new antiviral targets and developing new antiviral agents with a novel mode of action.
Compared to targeting viral protein, drugs targeting host factors are less likely to develop mutations under drug selective pressure [13], thus representing a promising antiviral strategy. In this study, we reported that NMS-873, a host p97 inhibitor, inhibits both influenza A and B viruses with low-nanomolar efficacy (Fig. 1 and Table 1). We confirmed that the antiviral drug target of NMS-873 is p97 as siRNA mediated silencing of p97 gave similar results as that of NMS-873 treatment (Figs. 4 and 5D). Our p97 knockdown results were also consistent with the previous reports: knocking down p97 blocked NP nuclear export and decreased influenza viral replication in A549 cells [20] and silencing TER94, a p97 ortholog, reduced influenza viral replication in Drosophila cells [15], indicating a role of p97 in influenza replication. Previous report showed that p97 co-precipitates with multiple influenza viral protein, including HA, NA, M1, M2, NP and two polymerase subunits, PB1 and PA [20, 38], here we confirmed the p97-NP interaction in the cells expressing p97 and NP or influenza virus infected cells. Strikingly, NMS-873 treatment could retain the NP protein in the nucleus when expressing NP protein only (Fig. 6), suggesting a role of p97 in NP’s subcellular trafficking. In addition, M1 protein level was significantly reduced by NMS-873 treatment or p97 knockdown (Fig. 5), which also affects vRNPs nuclear export.
In conclusion, the broad-spectrum antiviral activity and novel mechanism of action of NMS-873 render it a promising antiviral drug candidate, and designing more potent and selective p97 inhibitors appears to be an appealing antiviral strategy.
Acknowledgements
We thank Dr. Donna Zhang for providing p97 siRNAs. This work was supported by the University of Arizona startup fund, the Arizona Biomedical Research Centre Young Investigator grant, and the NIH AI 119187 and AI 144887 to J.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Miller M, Viboud C, Simonsen L, Olson DR, Russell C. Mortality and morbidity burden associated with A/H1N1pdm influenza virus: Who is likely to be infected, experience clinical symptoms, or die from the H1N1pdm 2009 pandemic virus ? PLoS Curr 2009;1:RRN1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105–115. [DOI] [PubMed] [Google Scholar]
- [3].Braciale TJ, Webster RG. Textbook of Influenza. Textbook of Influenza, 2nd Edition 2013:479–482. [Google Scholar]
- [4].Loregian A, Mercorelli B, Nannetti G, Compagnin C, Palu G. Antiviral strategies against influenza virus: towards new therapeutic approaches. Cell Mol Life Sci. 2014;71:3659–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang J, Li F, Ma CL. Recent Progress in Designing Inhibitors That Target the Drug-Resistant M2 Proton Channels From the Influenza A Viruses. Biopolymers. 2015;104:291–309. [DOI] [PubMed] [Google Scholar]
- [6].Monto AS, Fleming DM, Henry D, de Groot R, Makela M, Klein T, et al. Efficacy and safety of the neuraminidase inhibitor zanamivirin the treatment of influenza A and B virus infections. J Infect Dis. 1999;180:254–261. [DOI] [PubMed] [Google Scholar]
- [7].Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, Mercier CH, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–1850. [DOI] [PubMed] [Google Scholar]
- [8].Bloom JD, Gong LI, Baltimore D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science. 2010;328:1272–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA. 2006;295:891–894. [DOI] [PubMed] [Google Scholar]
- [10].Hay AJ, Hayden FG. Oseltamivir resistance during treatment of H7N9 infection. Lancet. 2013;381:2230–2232. [DOI] [PubMed] [Google Scholar]
- [11].Hurt AC. The epidemiology and spread of drug resistant human influenza viruses. Curr Opin Virol. 2014;8:22–29. [DOI] [PubMed] [Google Scholar]
- [12].Hayden FG, Sugaya N, Hirotsu N, Lee N, de Jong MD, Hurt AC, et al. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N Engl J Med. 2018;379:913–923. [DOI] [PubMed] [Google Scholar]
- [13].Shaw ML. The host interactome of influenza virus presents new potential targets for antiviral drugs. Rev Med Virol. 2011;21:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brass AL, Huang IC, Benita Y, John SP, Krishnan MN, Feeley EM, et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009;139:1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hao LH, Sakurai A, Watanabe T, Sorensen E, Nidom CA, Newton MA, et al. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature. 2008;454:890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Karlas A, Machuy N, Shin Y, Pleissner KP, Artarini A, Heuer D, et al. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. 2010;463:818–822. [DOI] [PubMed] [Google Scholar]
- [17].Konig R, Stertz S, Zhou Y, Inoue A, Hoffmann HH, Bhattacharyya S, et al. Human host factors required for influenza virus replication. Nature. 2010;463:813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shapira SD, Gat-Viks I, Shum BO, Dricot A, de Grace MM, Wu L, et al. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell. 2009;139:1255–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sui B, Bamba D, Weng K, Ung H, Chang S, Van Dyke J, et al. The use of Random Homozygous Gene Perturbation to identify novel host-oriented targets for influenza. Virology. 2009;387:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Watanabe T, Kawakami E, Shoemaker JE, Lopes TJS, Matsuoka Y, Tomita Y, et al. Influenza Virus-Host Interactome Screen as a Platform for Antiviral Drug Development. Cell Host Microbe. 2014;16:795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hu YM, Zhang JT, Musharrafieh R, Hau R, Ma CL, Wang J. Chemical Genomics Approach Leads to the Identification of Hesperadin, an Aurora B Kinase Inhibitor, as a Broad-Spectrum Influenza Antiviral. Int J Mol Sci. 2017;18, E1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hu YM, Zhang JT, Musharrafieh RG, Ma CL, Hau R, Wang J. Discovery of dapivirine, a nonnucleoside HIV-1 reverse transcriptase inhibitor, as a broad-spectrum antiviral against both influenza A and B viruses. Antivir Res. 2017;145:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Magnaghi P, D’Alessio R, Valsasina B, Avanzi N, Rizzi S, Asa D, et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat Chem Biol. 2013;9:548–556. [DOI] [PubMed] [Google Scholar]
- [24].Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol. 2012;14:117–123. [DOI] [PubMed] [Google Scholar]
- [25].Wolf DH, Stolz A. The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochim Biophys Acta. 2012;1823:117–124. [DOI] [PubMed] [Google Scholar]
- [26].Meyer H, Weihl CC. The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J Cell Sci. 2014;127:3877–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Arita M, Wakita T, Shimizu H. Valosin-containing protein (VCP/p97) is required for poliovirus replication and is involved in cellular protein secretion pathway in poliovirus infection. J Virol. 2012;86:5541–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Panda D, Rose PP, Hanna SL, Gold B, Hopkins KC, Lyde RB, et al. Genome-wide RNAi screen identifies SEC61A and VCP as conserved regulators of Sindbis virus entry. Cell Rep. 2013;5:1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Phongphaew W, Kobayashi S, Sasaki M, Carr M, Hall WW, Orba Y, et al. Valosin-containing protein (VCP/p97) plays a role in the replication of West Nile virus. Virus Res. 2017;228:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wong HH, Kumar P, Tay FP, Moreau D, Liu DX, Bard F. Genome-Wide Screen Reveals Valosin-Containing Protein Requirement for Coronavirus Exit from Endosomes. J Virol. 2015;89:11116–11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3:1125–1131. [DOI] [PubMed] [Google Scholar]
- [32].Morgan DM. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol Biol. 1998;79:179–183. [DOI] [PubMed] [Google Scholar]
- [33].Ma C, Li F, Musharrafieh RG, Wang J. Discovery of cyclosporine A and its analogs as broad-spectrum anti-influenza drugs with a high in vitro genetic barrier of drug resistance. Antiviral Res. 2016;133:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang W, Webster RG. Can we beat influenza? Science. 2017;357:111. [DOI] [PubMed] [Google Scholar]
- [35].Bui M, Whittaker G, Helenius A. Effect of M1 protein and low pH on nuclear transport of influenza virus ribonucleoproteins. J Virol. 1996;70:8391–8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Whittaker G, Bui M, Helenius A. Nuclear trafficking of influenza virus ribonuleoproteins in heterokaryons. J Virol. 1996;70:2743–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hutchinson EC, Fodor E. Transport of the influenza virus genome from nucleus to nucleus. Viruses. 2013;5:2424–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bhowmick S, Chakravarty C, Sellathamby S, Lal SK. The influenza A virus matrix protein 2 undergoes retrograde transport from the endoplasmic reticulum into the cytoplasm and bypasses cytoplasmic proteasomal degradation. Arch Virol. 2017;162:919–929. [DOI] [PubMed] [Google Scholar]