Abstract
Cardiolipin (CL) is a key phospholipid of the mitochondria. A loss of CL content and remodeling of CL’s acyl chains is observed in several pathologies. Strong shifts in CL concentration and acyl chain composition would presumably disrupt mitochondrial inner membrane biophysical organization. However, it remains unclear in the literature as to which is the key regulator of mitochondrial membrane biophysical properties. We review the literature to discriminate the effects of CL concentration and acyl chain composition on mitochondrial membrane organization. A widely applicable theme emerges across several pathologies, including cardiovascular diseases, diabetes, Barth syndrome, and neurodegenerative ailments. The loss of CL, often accompanied by increased levels of lyso-CLs, impairs mitochondrial inner membrane organization. Modest remodeling of CL acyl chains is not a major driver of impairments and only in cases of extreme remodeling is there an influence on membrane properties.
Keywords: Cardiolipin, Mitochondrial Structure-Function, Microdomains, Supercomplexes, Cardiolipin Content, Acyl Chain Remodeling
1.0. Cardiolipin structure and biosynthesis.
CL is a cone shaped anionic phospholipid that consists of two phosphatidic acid moieties linked via a central glycerol backbone. CL is predominately localized to the mitochondrial inner membrane where it plays an important role in bioenergetics, cristae morphology, supercomplex formation, and apoptosis (1–3). The functional importance of CL within the inner membrane is directly driven by its unique structure and highly specific acyl chain composition, which is suggested to be critical for optimal mitochondrial structure-function (4).
Cardiolipin comprises approximately 15–20% of the mitochondrial inner membrane. The other major phospholipids are phosphatidylcholine (PC: 40%) and phosphatidylethanolamine (PE: 30%). The remaining 10% of the inner mitochondrial phospholipidome consists of phosphatidylinositol (PI: 5%), phosphatidylserine (PS: 3–4%), and very small quantities of other lipids including phosphatidic acid (PA) and cholesterol (5). The acyl chains of CL are highly tissue specific. For example, in mammalian cardiac mitochondria, linoleic acid (LA, 18:2) constitutes nearly 80–90% of total CL acyl chains, rendering tetralinoleoyl-cardiolipin [(18:2)4CL] the most abundant CL species (6). However, this high degree of acyl chain specificity in CL is not observed across tissues (Table 1). Nevertheless, (18:2)4CL remains the most abundant molecular cardiolipin species in cardiac tissue across various organisms (Table 1).
Table 1:
Cardiolipin molecular species differ across organisms and tissues. Abundance is expressed as percent of total cardiolipin.
| Source of Cardiolipin | Abundance (% total CL) | Molecular Species | Ref. |
|---|---|---|---|
| Human Heart | 80 ± 2.0 | (18:2)4 | (6) |
| 12 ± 2.0 | (18:1)–(18:2)3 | ||
| Human Lymphoblast | 35 ± 3.0 | (16:1)–(18:1)3 | (6) |
| 32.0 ± 3.0 | (18:2)4 | ||
| Bovine Heart | 47.7 ± 0.4 | (18:2)4 | (7) |
| 21.0 ± 1.8 | (18:2)3–(18:3) | ||
| 14.6 ± 0.6 | (18:1)–(18:2)3 | ||
| Mouse Heart | 41.6 ± 0.5 | (18:2)4 | (8) |
| 17.7 ± 0.4 | (18:2)3–(22:6) | ||
| 14.3 ± 0.3 | (18:1)–(18:2)3 | ||
| Rat Liver | 55 ± 1.0 | (18:2)4 | (4) |
| 39 ± 1.0 | (18:1)–(18:2)3 | ||
| Mouse Liver | 52.3 ± 0.6 | (18:2)4 | (8) |
| (4 mo. male) | 19.2 ± 0.2 | (18:1)–(18:2)3 | |
| Mouse Liver | 80.6 ± 0.8 | (18:2)4CL | (9) |
| (6 mo. male) | 11.3 ± 0.1 | (18:2)2–(18:1)–(20:3) | |
| Mouse Brain | 7.1 ± 0.1 | (18:1)4 | (10) |
| 6.3 ± 0.1 | (18:1)3–(20:4) | ||
| 6.2 ± 0.0 | (18:1)3–(22:6) | ||
| S. cerevisiae | 40.0 ± 3.0 | (16:1)–(18:1)3 | (6) |
| 31.1 ± 2.0 | (18:1)4 | ||
| 20.0 ± 2.0 | (16:1)2–(18:1)2 | ||
| Mouse Gastrocnemius Muscle | 26.3 ± 0.3 | (18:2)3–(18:1) | (9) |
| 23.7 ± 0.2 | (18:2)4 | ||
| 23.7 ± 0.2 | (18:2)–(20:3)3 |
The high abundance of (18:2)4CL within cardiac mitochondria suggests an important role in regulating the molecular organization of the inner mitochondrial membrane. Indeed, (18:2)4CL is known to directly influence cristae formation due to CL’s proton buffering capacity and its propensity to form non-bilayer phases, which promotes negative membrane curvature (11,12). These distinctive structural properties of CL likely regulate membrane biophysical organization, which allows CL to bind a multitude of mitochondrial proteins, including key respiratory chain enzymes involved in oxidative phosphorylation (OXPHOS) (13).
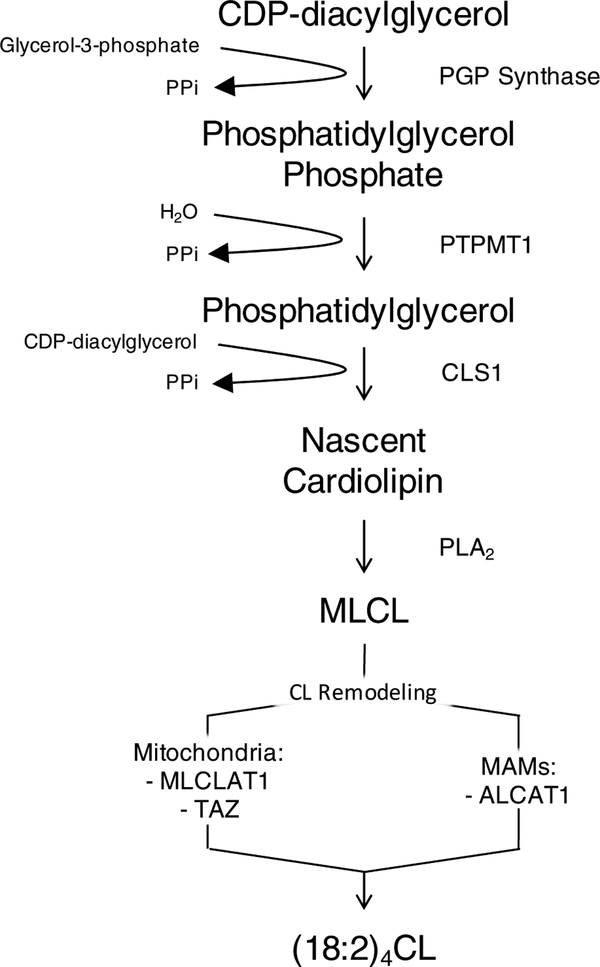
The initial synthesis of nascent CL is often characterized by the attachment of an assortment of acyl chains (Figure 1). Studies characterizing mammalian CL synthase establish that the enzyme is most abundantly expressed in tissues that are rich in mitochondria, such as cardiac, skeletal muscle, and liver (14–16). Subsequently, the tetralinoleoyl-rich composition of CL is attained through a series of specific transacylation steps (17). Nascent CL is transformed into its mature form by specific enzyme-dependent remodeling processes within the inner membrane, as well as at contact sites between the outer mitochondrial membrane and the endoplasmic reticulum membrane (18).
Figure 1: De novo biosynthesis of mature cardiolipin. (18:2)4CL is largely synthesized in the inner mitochondrial membrane.
Nascent CL is synthesized from PG through cardiolipin synthase (CLS1) using CDP-diacylglycerol as substrate. An acyl chain from nascent CL is then cleaved via phospholipase A2 (PLA2) to yield monolysocardiolipin (MLCL), which undergoes further remodeling. In mitochondria, MLCL is remodeled uniquely by tafazzin (TAZ) and monolyso-cardiolipin acyltransferase 1 (MLCLAT1) to yield mature cardiolipin. Additionally, a portion of mature CL [(18:2)4CL] is also synthesized by acyl-CoA:lysocardiolipin acyltransferase 1 (ALCAT1) within mitochondrial associated membranes (MAMs), a membrane bridge between the ER and outer mitochondrial membrane that serves as a major site for phospholipid synthesis and trafficking (19).
The final molecular composition of mature CL is characteristically defined by the symmetric inclusion of linoleic acid (18:2) (6). This remodeling process is initiated, in mammals, by phospholipase A2 (PLA2), which cleaves an acyl chain from CL and generates the intermediate monolyso-cardiolipin (MLCL) (20). This is followed by reacylation of CL, which can occur via the action of three distinct enzymes: acyl-CoA:lysocardiolipin acyltransferase 1 (ALCAT1), monolyso-cardiolipin acyltransferase 1 (MLCLAT1), and tafazzin (TAZ) (21–23). ALCAT1 and MLCLAT1 are transferase enzymes that use acyl-CoA as the acyl chain donor for the reacylation of MLCL (21,22). On the other hand, TAZ, a transacylase, removes an acyl chain from various phospholipids and attaches it to MLCL (23). Although the acyl chain specificity of these enzymes varies, it has been demonstrated that MLCLAT1 prefers acyl-CoA loaded with linoleoyl (18:2) species (22), whereas ALCAT1 utilizes acyl-CoA loaded with various longer polyunsaturated acyl chains (21).
The underlying mechanism by which TAZ elicits acyl chain specificity remains controversial. Schlame et al. have elegantly demonstrated that key physical and thermodynamic properties of phospholipids (i.e. the ability to form non-bilayer phases) play an important role in determining the acyl specificity of TAZ (24,25). Specifically, these studies suggest that TAZ catalyzes CL remodeling through a series of forward and reverse transacylations, which effectively creates an equilibrium distribution of acyl chain species (24). The thermodynamics involved in such reversible transacylations is extremely important to consider, and warrants further investigation, given that chemical equilibrium regulates which molecular CL species are synthesized, whereas enzymatic kinetics simply define how fast the reaction occurs (24).
Following de novo biosynthesis and remodeling, mature CL must be translocated and assembled into the outer and inner mitochondrial membranes. The mechanisms of CL translocation are not completely established. A few mechanisms have been identified and involve enzymes including phospholipase scramblase 3 (PLS3), mitochondrial creatine kinase (mtCK), and nucleoside diphosphate kinase (NDPK) (26,27). PLS3 has been demonstrated to facilitate translocation of CL between the outer and inner leaflets of the mitochondrial inner membrane (26,27). Furthermore, mtCK and NDPK reside in the intermembrane space and transfer phospholipids between the outer and inner membranes (26). The activity of these kinases is CL dependent and requires protein aggregation, which is known to induce CL segregation and thereby CL domain formation (28,29). CL domains are hypothesized to be critical for the regulation of mitochondrial membrane structure and the clustering of proteins (30), which is discussed in detail below.
2.0. Role of cardiolipin in mitochondrial structure-function
The structure of the mitochondrial inner membrane is considerably folded and compartmentalized. Cristae are dynamic bioenergetic compartments that give the inner membrane its characteristic wrinkled shape. The tubular structure of mitochondrial cristae provides a large amount of surface area for biochemical reactions to occur, such as cellular respiration and ATP synthesis via oxidative phosphorylation (2,31). Cristae contain significant amounts of non-bilayer phospholipids, such as CL and PE, which lessen torsional strain of the inner membrane by localizing into the negatively curved inner leaflet (2). The importance of CL in mitochondrial inner membrane structure-function is a direct consequence of its dimeric, anionic structure, which under standard physiological conditions, may only carry one negative charge at a time since the phosphates of CL are diastereotopically inequivalent (32,33). Therefore, ionization of these phosphates presumably occurs at two different pH levels (pK1= ~2.8, pK2= ~7.5–9.5) (32,33), which presumably allows CL to trap protons within its headgroup and thereby localize the proton pool near the surface of the inner membrane. In theory, highly mobile CL would laterally shuttle protons from protein complexes to the ATP synthase machinery.
The notion that CL contains two different pka’s is disputed. For example, studies monitoring CL’s phase behavior in lipid bilayers suggest that CL is fully ionized near neutral pH (34,35). Another study using FTIR analysis to examine the phosphate ionization properties of CL support the notion that the headgroup phosphates are fully ionized at neutral pH (36). Lastly, Sathappa et al. have shown using independent electrokinetic and spectroscopic approaches that both phosphates of CL’s headgroup exhibits strong ionization behavior typical of a strong acid (i.e. with pKa values near the first pKa of phosphoric acid) (37). These recent studies help shed light on a controversial topic and clearly implicate the need for further characterization of the ionization properties of cardiolipin under differing conditions. This is critical for understanding cardiolipin’s role in mitochondrial inner membrane molecular organization and bioenergetics.
CL is known to interact with many mitochondrial inner membrane enzymes and substrate carriers. The list of proteins that bind CL with high affinity is extensive and includes, but is not limited to, mitochondrial respiratory enzyme complexes, ADP/ATP carrier (AAC), and mitochondrial kinases (1,13,38). Indeed, CL has been shown to be required for the optimal function of complex I (NADH-ubiquinone oxidoreductase), complex III (ubiquinone-cytochrome c oxidoreductase), complex IV (cytochrome c oxidase), and complex V (ATP synthase) (39–41). Furthermore, crystallographic studies have shown tightly bound CL in the crystal structures of complex III and complex IV (42,43). This highlights the importance of CL as integral components of respiratory protein complexes and emphasizes the importance of specific CL-protein interactions for protein folding and clustering.
Studies have shown the requirement of CL for the assembly of mitochondrial respiratory proteins into higher order structures referred to as respiratory supercomplexes (44). For example, the Dowhan group demonstrated the requirement of CL for the organization of respiratory complexes III+IV into a supercomplex in vitro (45,46). Additionally, molecular dynamic simulations have shown the propensity of CL to selectively mediate protein–protein interactions, by which the formation of respiratory complexes III+IV into supercomplexes occurs (47). Although these studies have shown the necessity for CL in supercomplex assembly, it remains unclear how CL is incorporated and the mechanism by which CL modulates supercomplex stability and activity. Thus, further investigation into CL’s role in mitochondrial inner membrane dynamics is necessary.
3.0. Cardiolipin remodeling and loss of concentration across various diseases
The concept that CL is critical for cristae morphology and thereby mitochondrial inner membrane structure-function has emerged from studies where CL content is manipulated. For example, impairments in de novo cardiolipin biosynthesis have been shown to result in altered cristae morphology (48), decreased respiratory supercomplex formation (49), impaired membrane potential and reduced mitochondrial function (50). Thus, it is not surprising that various derangements in CL biosynthesis and/or metabolism are well documented to be causative factors in a variety of pathologies ranging from genetic disorders, such as Barth syndrome, to metabolic diseases, such as CVDs and type 2 diabetes (51,52) (Table 2). We briefly review the role of CL in select metabolic diseases below.
Table 2:
Functional consequence of CL alterations in various pathologies.
| Pathology | CL Alterations | Functional Consequence Attributed to CL Alterations | Ref. |
|---|---|---|---|
| Barth Syndrome | |||
| Human Tissues | ↓ CL content ↓ (18:2)4CL ↑ MLCL ↓ Rate of CL biosynthesis |
Dilated cardiomyopathy; Neutropenia; Impairments in respiratory chain activity. | (54,55,58,59) |
| Mouse Heart | ↓ (18:2)4CL ↑ MLCL |
Ultrastructural defects; Myofibrillar disarray; Mitophagy; LV dilation; LV mass reduction; Decreased fractional shortening and ejection fraction. | (60) |
| Decreased complex III activity; Destabilized ETC supercomplex assembly; Disruption between FAO-ETC interactions. | (61) | ||
| Mouse Cardiac and Skeletal Muscle | ↓ CL content by TAZKD |
Increased mitochondrial H2O2 emission; Whole tissue lipid peroxidation; Decreased mitochondrial respiration and protein abundance of OXPHOS complexes; Development of cardioskeletalmyopathy | (62) |
| Mouse Brain | ↓ CL content by TAZKD ↑ CL acyl chain heterogeneity |
19-fold increase in MLCL levels; Increased ROS; Increased complex I activity; Cognitive deficiency and derangement in hippocampal neurons. |
(63) |
| Decreased PUFA-containing CLs; Increased PUFA-containing MLCLs are increased in the brains of TAZ knockdown mice. | (64) | ||
| S. cerevisiae | ↓ CL content ↓ CL unsaturation ↑ MLCL |
Defective bioenergetic coupling; Destabilization of respiratory chain supercomplexes. | (57,65–67) |
| Ischemia | |||
| Rabbit Heart | ↓ CL content in SSM, not IFM | Lowered cytochrome c content; Decreased complex IV activity. | (68–70) |
| Ischemia-Reperfusion Injury | |||
| Rat Heart | ↓ CL content ↑ ox-CL |
Decreased complex I, III, IV activity; Restored by exogenous CL, but not other phospholipids. | (71–73) |
| Impaired state III respiration; Attenuated by lowered ROS. | (74,75) | ||
| Rat Brain | ↓ CL content ↑ ox-CL |
Decreased complex IV & V activity; Impaired mitochondrial respiration. | (76) |
| ↑ CL hydrolysis ↑ Lyso-CLs |
Activation of caspase 3/7; Motor and cognitive dysfunction. | (77) | |
| Heart Failure | |||
| Human Heart | ↓ CL content ↓ (18:2)4CL |
Decreased CL content in ischemic and dilated cardiomyopathy; Decreased (18:2)4CL in dilated cardiomyopathy. | (78,79) |
| Rat Heart | ↓ CL content ↓ (18:2)4CL | Cardiac hypertrophy and failure by aortic banding; Decreased complex IV activity. | (79–82) |
| Diabetes | |||
| Human Heart | ↓ CL content ↑ Incorporation of DHA into CL |
Increased mitochondrial polarization; Decreased mitochondrial mass; Altered mitochondrial morphology; Increased mitochondrial superoxide production. | (83) |
| Mitochondrial dysfunction associated with defective CL remodeling. | (84) | ||
| Mouse Heart | ↓ CL content ↓ (18:2)4CL ↑ Incorporation of DHA into CL |
Lowered PG content; Impaired PG biosynthesis; Lowered glycerol-3-phosphate protein content; Mitochondrial dysfunction associated with altered substrate utilization. DHA intervention decreased complex IV activity due to impaired lipid-protein interactions. | (85,86) |
| (87) | |||
| Mouse Liver | ↑ CL content ↑ CL acyl chain heterogeneity |
Western Diet intervention resulted in increased CL saturation; Increased CL heterogeneity; Decreased respiratory exchange ratio (dark). | (9) |
| TAZ knockdown results in normal hepatic mitochondrial supercomplex formation and elevated fatty acid oxidation, which prevents hepatic steatosis and diet induced obesity. | (88) | ||
| ALCAT1−/− mice exhibit hypermetabolism, which prevents the onset of diet induced obesity and enhances mitochondrial complex 1 activity, fatty acid oxidation, and insulin signaling. | (19) | ||
| Mouse Gastrocnemius Muscle | ↑ CL content ↑ CL acyl chain heterogeneity |
Western Diet intervention resulted in increased CL heterogeneity; Increased β-oxidation; Decreased respiratory exchange ratio (dark). | (9) |
| Aging | |||
| Rat Heart | ↓ CL content ↓ (18:2)4CL |
Decreased carnitine-acylcarnitine translocase and adenine nucleotide transporter activity; Decreased pyruvate carrier and phosphate carrier activity; | (89–92) |
| All restored by acyl-carnitine supplementation. Decreased complex IV activity; | (93) | ||
| Restored by exogenous CL, but not other phospholipids. Decreased oxidative phosphorylation due to impairments in complex III activity. | (94) | ||
| Rat Brain | ↓ CL content ↑ ox-CL |
Decreased complex I activity; Lowered state III respiration; Restored by exogenous CL, but not other phospholipids. Impaired mitochondrial membrane potential; Increase in H2O2 production. | (95,96) |
| Parkinson’s Disease | |||
| Mouse Brain | ↓ CL content ↓ (18:2)4CL ↓ Unsaturation of minor CL species |
Mutations in a-synuclein impairs CL biosynthesis; Reduced PG content; Decreased linked complex l-lll respiratory activity. | (97) |
| Oxidative Stress | |||
| Rat Brain | ↓ CL content | Elevated lipid peroxides; Loss of mitochondrial membrane potential; Impaired electron chain activity; Restored with BHT. | (98) |
| Bovine Heart | ↓ CL content | Decreased complex I, II, and IV activity; Restored by exogenous CL, but other mitochondrial phospholipids nor ox-CL. | (99–102) |
3.1. Barth syndrome
Barth syndrome was first described in 1983 by pediatric neurologist Dr. P.G. Barth as an X-linked recessive disease that is often clinically characterized by cardioskeletal myopathy and neutropenia associated with mitochondrial dysfunction (53). Barth syndrome is caused by mutations in the TAZ gene, which encodes tafazzin, the phospholipid transacylase intimately involved in the remodeling and maturation of mitochondrial CL (54). As a result, Barth syndrome patients often show abnormal CL profiles in a variety of tissues, such as a loss of total CL content and increased levels of monolyso-CL (MLCL) at the expense of (18:2)4CL (55). Being one of the first genetic disorders known to result directly from impairments in CL metabolism, the aforementioned observations have proven to be useful in diagnosing Barth syndrome, especially biochemical abnormalities in the MLCL-to-CL ratio, a very specific and sensitive biomarker (56).
Initial studies by Ma et al. using Δtaz1 mutant yeast have demonstrated that inactivation of taz1, a human orthologue of the TAZ gene, results in decreased CL levels, increased MLCL, and loss of acyl chain unsaturation (57). As a consequence, Δtaz1 mutant yeast exhibit defective bioenergetics and destabilization of respiratory chain protein complexes (57).
Furthermore, Acehan et al. demonstrated that shRNA-mediated knockdown of tafazzin, in mice, resulted in a dramatic increase of MLCL at the expense of decreased of (18:2)4CL in striated muscles (60). Additionally, tafazzin-deficient mice exhibit ultrastructural impairments, such as increased mitochondrial DNA content, myofibrillar disarray, increased mitochondrial deterioration and mitophagy, and abnormalities in mitochondrial membrane morphology (60). Other groups have focused on mitochondrial proteomics and cardiac respiratory function in a mouse model of Barth syndrome. For instance, Huang et al. identified and quantified changes in the cardiac proteome, where they found that a knockdown of tafazzin diminishes complex III activity, destabilizes supercomplexes, and disrupts cardiac metabolic pathways (61). Furthermore, studies with Barth syndrome patients reveal defects in respiratory chain function by either directly impacting enzymatic activity or by reducing the stability of individual respiratory chain complexes, and thereby respiratory supercomplexes (67,103). Alterations in the supramolecular organization of the mitochondria, particularly at the level of the inner membrane, would likely contribute to supercomplex dissociation, which would impair cristae stability and thereby promote membrane aggregation (60).
More recently, Cole et al. demonstrated that murine TAZ knockdown resulted in aberrant CL metabolism and was associated with cognitive deficiency (63). Specifically, TAZ knockdown mice exhibited reduced brain CL content, altered CL molecular species, and a 19-fold increase in MLCL levels relative to wild-type mice. This resulted in elevated mitochondrial complex I activity, which corresponded to increased state I respiration. Motor function was not perturbed although significant memory deficiency was observed based on novel object recognition test, which correlated with lowered synaptophysin levels in the hippocampus. Similarly, Chao et al. have used global lipidomics to demonstrate that PUFA-containing CLs are decreased and PUFA-containing MLCLs are increased in the brains of TAZ knockdown mice and mechanically injured rat brains (64). The accumulation of oxidized CL species was observed in mechanically injured rat brains, but not brains of TAZ knockdown mice, and was determined to precede CL hydrolysis and MLCL accumulation. Although these studies highlight the importance of TAZ-mediated CL remodeling in maintaining normal cognitive and mitochondrial function, many studies have failed to investigate the regulatory role of increased MLCL-to-CL ratio.
Other labs investigating the role of CL in models of Barth syndrome have focused on CL degradation, which may be a fundamental driver of pathologies. By using stable isotope techniques, Xu et al. have shown that CL turnover is indeed slower due to its compartmentalization within the membrane, specifically in proteins complexes, which is greatly compromised in Barth syndrome (104). This results in rapid CL degradation, which directly causes the accumulation of MLCL. Furthermore, they found that resveratrol induced supercomplex association, decreased the rate of CL turnover and thereby the concentration of monolyso-cardiolipin. These results provide a mechanistic explanation for previous studies showing that CL turnover is much slower than that of other phospholipids, as well as providing evidence for potential therapeutic targets in Barth syndrome patients (105).
3.2. Cardiac ischemia-reperfusion injury
Myocardial ischemia-reperfusion (I/R) injury is a complex pathophysiological process. Ischemia is often manifested as a result of impairments in the coronary blood supply that develop from acute alterations of atherosclerotic plaques. This results in increased intracellular levels of sodium, hydrogen, and calcium ions, causing a severe imbalance in pH and eventually culminating in tissue acidosis. In turn, reperfusion stimulates rapid alterations in ion flux, which unexpectedly leads to greater cell cytotoxicity due to renormalization of pH (106,107). Various pH regulatory mechanisms that are sodium-dependent become activated. Intracellular sodium accumulation dysregulates SERCA Ca2+−ATPase mechanisms (108,109) and impairs cytosolic Ca2+ exchange into sarcoplasmic reticulum. This imbalance in ion-flux subsequently results in myofibrillar hypercontractility, myocardial stunning, oxidative stress, compromised mitochondrial ultrastructure, and thereby ATP depletion (110,111).
It is well documented that reactive oxygen species (ROS) arising from I/R injury promote severe mitochondrial dysfunction driven by impairments in mitochondrial ultrastructure. Elevated ROS are considered to be an important factor in facilitating lethal cell injury and apoptosis during cardiac ischemia and reperfusion. Oxidative damage by mitochondrial ROS is typically observed at the level of mitochondrial membranes, where complexes of the respiratory chain and phospholipid constituents serve as major targets of ROS attack.
A loss of CL content has been reported upon ischemia in rat and guinea pig cardiac mitochondria (70,71) with additional CL loss upon reperfusion (71,112). However, studies by Lesnefsky et al. (68,69) have indicated 20–25% loss of CL upon ischemia in isolated rabbit hearts, with no further CL loss during reperfusion. These observed losses in CL levels are strongly associated with concomitant increases in the levels of oxidized CL, which is dynamically associated with impairments in mitochondrial respiratory enzymatic activity. Several studies have shown that isolated mitochondria from I/R rat hearts exhibit mitochondrial dysfunction due to lowered enzymatic activities of respiratory chain complexes I, III, and IV (71–73). The addition of exogenous CL–containing liposomes to isolated mitochondria from I/R rat hearts almost completely restored the activity of respiratory chain complexes (71–73). Presumably, the defects in mitochondrial respiratory enzymatic activity could mostly be attributed to the loss in CL content, which further highlights the importance of CL concentration for the optimal function of these enzyme complexes. Although these studies have strong implications for the involvement of CL in the formation and higher-order organization of respiratory supercomplexes, it is possible that impaired mitochondrial biogenesis could simply be due to lowered mitochondrial content.
It is suggested that deceased CL levels following I/R, in part, results from increased peroxidation of CL due to is highly unsaturated nature. This renders the phospholipid more susceptible to degradation by PLA2, given PLA2 activation during I/R (113). PLA2 is well known to be involved in phospholipid hydrolysis during I/R, which is known to result in a loss of CL content in a variety of tissues including heart, brain, and liver (76,113–115). However, increased levels of oxidized CL are not reported in all studies (69), possibly due to elevated PLA2 activity or the unstable nature of oxidized acyl chains.
Oxidized CL is known to be tightly associated with Ca2+ regulation, cytochrome c (cyt c) release and thereby cellular apoptosis. Petrosillo et al. have demonstrated that oxidized CL lowers the threshold of Ca2+ for inducing and/or potentiating mitochondrial permeability transition pore (MPTP) opening and cytochrome c release in isolated rat heart mitochondria (116). Results from this study demonstrate that micromolar treatment of mitochondria with CL hydroperoxide caused mitochondrial matrix swelling, membrane potential collapse, release of pre-accumulated Ca2+ and release of cytochrome c (116). These abnormalities and ultimate detachment of cyt c from the mitochondrial inner membrane is considered one of the initial steps that activates the apoptotic cascade. Several reports have indicated that CL oxidation is required for cytochrome c release from the mitochondria into the cellular cytosol (117,118). These events have an important role in the etiology of mitochondrial dysfunction and serve as important therapeutic targets for the treatment of cardiac I/R injury and other cardiovascular diseases (30).
3.3. Heart failure
Heart failure is a complex pathology and is a multifactorial process involving advanced cardiovascular changes occurring at the molecular, cellular, and organ level. Impairments in myocardial mitochondrial energy metabolism are fundamentally important in the development and/or progression of the pathogenesis of heart failure. O’Rouke and Reibel provided early evidence for the role of CL in heart failure, where they reported a reduction in the levels of (18:2)4CL in rat hearts induced to rapid pressure-overload hypertrophy and failure (80,81). Similar observations have been made during the progression of heart failure in hamsters (119), as well as in patients with dilated or ischemic cardiomyopathies (78). Other studies have shown progressive alterations in CL composition occur in human and experimental models of hypertensive heart failure (82). These studies show that the pathogenesis of heart failure is characterized by the loss of total (18:2)4CL content, which contributes to mitochondrial respiratory dysfunction.
Studies have also reported decreased tafazzin mRNA levels, concomitant with compensatory increases in the activity of CL remodeling enzymes, such as MLCL acyltransferase, during the progression of heart failure (120). Interestingly, dietary supplementation of linoleic acid has been shown to preserve (18:2)4CL integrity and attenuate mitochondrial dysfunction in rats with hypertensive heart failure (121,122). However, studies investigating the role of the CL in heart failure remain controversial (122). A plausible explanation for lowered CL content, and thereby reduced activities of respiratory enzymes, in heart failure is the loss of mitochondrial mass (123). However, primary mitochondrial cardiomyopathy in human subjects results in mitochondrial proliferation in the heart (124). This has also been observed in mouse models where mitochondrial biogenesis is impaired by either ablation of ANT1 or mtTFA (125,126). Interestingly, in the myocardium of mtTFA knockout mice increased mitochondrial mass was accompanied by lowered mtDNA and impaired mitochondrial bioenergetics (125). Therefore, these studies suggest that increased mitochondrial content is not compensating for mitochondrial impairments arising from other underlying factors such as severe ATP depletion and changes in CL levels. Furthermore, Rosca et al. reported that the main phospholipid species, including CL, were unaffected in cardiac mitochondria during heart failure (127). Other studies in hypertensive rat hearts have in fact shown elevated CL content, relative to other phospholipids, as well as increased activity of CDPDAG synthase, suggesting that hypertension may actually amplify CL biosynthesis (128,129). Thus, the mechanism(s) of CL alterations in the failing heart are still poorly understood and remain an exciting area of investigation.
3.4. Diabetes
Diabetes is a common human metabolic disease and is characterized by insulin deficiency (type I) or insulin resistance (type II) due to pancreatic β-cell dysfunction and eventually failure. This results in chronic hyperglycemia and oxidative stress associated with a variety of complications, including neuropathies and cardiomyopathies (52). There is accumulating evidence implicating mitochondrial dysfunction as a primary contributor to pancreatic β-cell failure during the pathogenesis of type 2 diabetes (130–132). Pancreatic β-cell mitochondria from type II diabetic patients exhibit ultrastructure morphologies and functional abnormalities resulting in decreased ATP production and glucose-stimulated insulin secretion (133,134). It is hypothesized that extensive CL remodeling and CL oxidation in the diabetic myocardium drives impairments in mitochondrial structure-function and lead to cardiac dysfunction.
Pathological CL remodeling has been shown to be a contributing factor to the onset of mitochondrial dysfunction and metabolic complications associated with diabetes and thereby diabetic cardiomyopathy. Using shotgun lipidomics, Han et al. have demonstrated in the diabetic myocardium a dramatic decrease in the content of tetralinoleoyl-CL and PG, the direct metabolic precursor of CL (85). Furthermore, there is incorporation of longer polyunsaturated fatty acids into CL, such as docosahexaenoic acid (DHA, 22:6 n-3), (86,87). This is a direct consequence of aberrant CL remodeling, which is known to be an underlying cause of mitochondrial dysfunction (135). For example, when the acyl chain composition of rodent cardiac CL is enriched in DHA, the activity of cytochrome c oxidase is decreased by 50%, concomitant with decreased oxygen consumption (136).
Aberrant CL remodeling by specific enzymes, such as ALCAT1, has been implicated in the etiology of diet induced obesity and type II diabetes. Li et al. have shown that ALCAT1 is upregulated by oxidative stress and diet-induced obesity. This results in the abundance of CL species that are highly susceptible to oxidative damage, which promotes mitochondrial dysfunction, ROS production, and insulin resistance; all of which were rescued by rosiglitazone treatment (19). Alternatively, ALCAT1 deficient mice are resistant to diet-induced obesity and show significantly improved hepatic mitochondrial complex I activity, lipid oxidation, and insulin signaling. Other studies focused on the role of tetralinoleoyl-CL levels in the development of diet-induced obesity and insulin resistance have shown that impaired CL biosynthesis does not disrupt hepatic mitochondrial structure-function. Using a TAZ knockdown mouse model, Cole et al. demonstrated that impaired CL biosynthesis prevented the development of hepatic steatosis and obesity (88). As a result, TAZ knockdown mice exhibited normal hepatic mitochondrial supercomplex formation as well as elevated hepatic fatty acid oxidation. These studies strongly implicate hepatic CL remodeling in regulating susceptibility to insulin resistance, which may serve as a novel therapeutic target for diet-induced obesity.
Mitochondrial dynamics regulating fusion and/or fission are also fundamentally involved in the mechanisms underlying a variety of human cardiometabolic diseases. These processes are mediated through the action of GTPases. Mitochondrial fission occurs via dynamin-related protein 1 (Drp1), a cytosolic GTPase that translocates to the mitochondrial outer membrane upon activation. Mitochondrial fusion occurs via mitofusin-1 and mitofusin-2, located in the mitochondrial outer membrane, as well as optic atrophy 1 (OPA1), which resides within the mitochondrial inner membrane. Patients with obesity and type 2 diabetes exhibit reduced skeletal muscle mitochondrial fusion as demonstrated by lowered mitofusin-2 expression and decreased mitochondrial size (137). Furthermore, hepatic-specific ablation of mitofusin-2 in mice results in impaired insulin signaling in liver and muscle, which was characterized by glucose intolerance and enhanced hepatic gluconeogenesis (138). During I/R injury the regulatory role of Drp1 is compromised in both isolated neonatal murine cardiomyocytes and adult rat hearts. More specifically, dephosphorylation of Drp1 at serine 637 results in Drp1 mitochondrial translocation and increases mitochondrial fission, reactive oxygen species production, calcium concentrations, which weakens diastolic relaxation and left-ventricular function (139). However, inhibition of mitochondrial fission via mutant Drp1 variant protects the heart against I/R injury (140), thereby suggesting that mitochondrial morphology modulation and dynamics may serve as a valid pharmacological target for improving metabolic abnormalities and cardioprotection.
3.5. Neurodegenerative diseases
Oxidative stress and lipid oxidation have been implicated as contributing factors leading to neuronal loss and mitochondrial dysfunction in neurodegenerative diseases, including Parkinson’s and Alzheimer’s disease. Particularly, CL alterations have been reported to play a fundamental role in the brain with aging. Exposure of rat brain mitochondria to ROS decreased CL content due to enhanced lipid oxidation and membrane depolarization (98). Furthermore, studies by Petrosillo et al. have suggested that brain aging is associated with the oxidation/depletion of CL in mitochondria (95), which may disrupt the stability of supercomplexes and thereby mitochondrial bioenergetics. There is strong indication that the impairment of mitochondrial function in brain aging is mainly due to decreased activity in complexes I and IV, among others (95,141–146). However, some studies have reported no changes in CL profiles (147). Likewise, it appears that patients with Alzheimer’s do not display abnormal CL profiles (148).
In the brain of Parkinson’s subjects, mitochondrial dysfunction arises from decreased levels of complex I subunits and thereby reduction in oxidative phosphorylation activity (141,149,150). Furthermore, studies by Ellis et al. implicate a potential role of CL in the manifestation of Parkinson’s disease (97), involving α-synuclein, the major structural constituent of cytoplasmic inclusion bodies (Lewy bodies). Early reports by Ellis et al. have shown that mice lacking α-synuclein show defects in CL biosynthesis that results in decreased mitochondrial respiratory activity. More specifically, these mice display a 22% loss of CL mass, as well as a 25% reduction in the levels of CL species containing n-6 polyunsaturated fatty acids, including linoleic acid (18:2). There was also a 23% reduction in PG, without any other changes to other brain phospholipids. This resulted in diminished activity in linked mitochondrial respiratory enzymatic activity of complexes I-III, which is thought to be an underlying factor contributing to the development of Parkinson’s disease (97).
In vitro studies using model membranes have demonstrated preferential binding of α-synuclein to highly curved anionic mitochondrial membranes containing CL (151). Furthermore, CL-containing vesicles have been shown to decrease in size when exposed to recombinant α-synuclein, but not vesicles lacking CL (152). More recently it has been demonstrated that exposure of cardiolipin to the outer mitochondrial membrane modulates α-synuclein binding to facilitate folding of α-synuclein fibrils (153). Although there is little to no evidence supporting the role of CL remodeling and/or CL depletion in Parkinson’s disease, this does not exclude the possibility of CL abnormalities occurring in affected neurons. On the other hand, the interaction between CL and α-synuclein has strong implications for the role of CL in the pathogenesis of Parkinson’s disease, which warrants further investigation into their synergistic interrelationship (154).
4.0. The role of cardiolipin content versus cardiolipin acyl chain composition on mitochondrial inner membrane structure-function.
It is currently unknown as to which factor (i.e. CL content or CL acyl chain remodeling), or both, is responsible for disrupting inner mitochondrial membrane biophysical organization. In Table 3 we discriminate between the effects of lowered CL concentration and CL acyl chain remodeling on differing membrane biophysical properties, such as inner membrane ultrastructure and cristae morphology, CL microdomain organization, and protein-protein clustering.
Table 3:
Summary of studies to show how a loss of CL abundance compared to remodeling of CL acyl chains influences key membrane biophysical properties across model systems.
| CL Alteration | Model System | Biophysical Consequence Attributed to CL Alteration | Ref. |
|---|---|---|---|
| Lowered CL Content | |||
| Biomimetic mitochondrial lipid monolayer | ↓ (18:2)4CL promotes lipid-lipid miscibility on subphase of 10 mM sodium phosphate buffer (pH 7.4). | (155) | |
| Egg phosphatidylcholi ne(EPC) monolayer | ↓ (18:2)4CL promotes lipid-lipid miscibility on subphase of 150 mM NaCI. | (156) | |
| Δcrd1 in S. cerevisiae | ↓ (18:2)4CL results in impairments in mitochondrial membrane potential, defective ADP/ATP carrier (AAC) activity, and ATP synthesis. | (50) | |
| Δcrd1 in S. cerevisiae | ↓ (18:2)4CL results in mitochondrial membrane depolarization and increased proton leak. | (157) | |
| Phb2−/− in mouse embryonic fibroblasts | ↓ (18:2)4CL; Impaired cell growth, defective cristae morphogenesis. | (158) | |
| 4:1 DOPC:CL lipid bilayers | Replacement of CL with DOPG promotes membrane morphological changes induced by cyt c association. | (159) | |
| 9:1 EPC:CL lipid bilayers | Replacement of CL with DOPG promotes cristae-like tubule formation induced by a localized transmembrane pH gradient. | (160) | |
| Biomimetic mitochondrial lipid bilayers | ↓ (18:2)4CL impairs lipid-lipid miscibility and cyt c-CL proteolipid domain organization. | (161) | |
| ΔCLS in Drosophila | ↓ (18:2)4CL impairs lateral cristae morphology and disrupted the density and organization of ATP synthase molecules. | (162) | |
| Δcrd1 in S. cerevisiae | ↓ (18:2)4CL impairs cyt c pool activity and destabilizes supercomplexes containing CIII-CIV; CL is required for CIII-CIV supercomplex formation. | (44,45) | |
| CL Acyl Chain Remodeling | |||
| Biomimetic mitochondrial lipid monolayer | (18:1)4CL and (22:6)4CL minimally impact lipid-lipid miscibility, relative to (18:2)4CL; (14:0)4CL promotes lipid-lipid immiscibility on subphase of 10 mM sodium phosphate buffer (pH 7.4). | (155) | |
| DPPG lipid bilayers | (14:0)4CL promotes packing inconsistencies and lipid-lipid immiscibility. | (163) | |
| Δcld1 and/or Δtaz1 in S. cerevisiae | Increased CL acyl chain saturation does not impact mitochondrial morphology, cristae size, and mitochondrial respiration relative to wild type cells. | (157) | |
| Acsl1T−/− in murine cardiac mitochondria | (18:2)4CL is remodeled by 83% which impairs calcium uptake and promotes mitochondrial respiratory dysfunction. | (164) | |
| Δaim24 + Mic 12-His6 or Mic26-His6 modifications in S. cerevisiae | Increased CL acyl chain saturation results in smaller mitochondria lacking inner mitochondrial membrane cristae ultrastructure and impaired oxidative phosphorylation; Cellular inviability under respiratory conditions. | (165) | |
| Phb2−/− in mouse embryonic fibroblasts | Accumulation of MLCL and CL species with longer, and more unsaturated acyl chains; Impaired cell growth; Defective cristae morphogenesis. | (158) | |
| Biomimetic mitochondrial lipid bilayers | cyt c-CL proteolipid domains form in the presence of (18:2)4CL, (18:1)4CL, and MLCL; (22:6)4CL inhibited cyt c-CL domain formation driven by favorable lipid-lipid miscibility. | (87,161) | |
| Pure CL lipid monolayers | mtCK-CL proteolipid domains formed in the presence of (18:2)4CL, (18:1)4CL, andPG; (14:0)4CL inhibited mtCK-CL domain formation. | (166) | |
| High fat (HF) diet intervention in mice | HF diet modestly remodels murine cardiac and hepatic mitochondrial phospholipidome; Tissue specific | (167) | |
| impairments in respiratory enzyme activity; No overall effect on respiratory function. | |||
| DHA diet intervention in mice | DHA diet intervention remodels murine cardiac mitochondrial phospholipidome; Accumulation of (22:6)4CL species lowers the specific enzymatic activities of respiratory complexes 1, IV, V, and l+lll. | (87) | |
| Human BTHS lymphoblasts | Accumulation of MLCL; Increased CL acyl chain turnover; Decreased abundance of respiratory supercomplexes, ATP synthase, and AAC. | (104) | |
4.1. The role of cardiolipin in mitochondrial inner membrane organization
A considerable amount of work has been aimed at understanding how CL regulates membrane microviscosity, lipid packing, and phase behavior in model membranes (33,168–171). For instance, cardiolipin-containing membranes are more fluid in nature which results in decreased mechanical stability, and presumably, decreased lipid packing. Other studies have determined that various biomimetic mixtures modeling the inner mitochondrial membrane display different structural properties that regulate thermodynamics of coexisting lipid domains. Our lab has shown that the microviscosity of CL-containing membranes is dependent on the surrounding lipid environment (172). These results support the notion that surrounding phospholipids strongly regulate membrane microviscosity and thereby lipid packing in CL-containing membranes.
To exemplify, studies investigating distinct membrane biophysical properties using Langmuir lipid monolayers, demonstrate that the addition of increasing amounts of CL increase the average phospholipid molecular area, which disrupts monolayer packing properties. Using a simple phospholipid monolayer mixture containing CL and dipalmitoylphosphatidylcholine (DPPC), Phan and Shin have shown that increasing levels of CL abolish DPPC’s unique liquid expanded (LE) to liquid condensed (LC) phase transition (171). This indicates that CL promotes the LE phase in phospholipid monolayers and presumably ‘liquefies’ the LC domains of DPPC at high enough concentrations. Their results suggest that the addition of CL results in overall expansion of the lipid films, which enhances monolayer elastic properties. It was hypothesized that the enhancement in elasticity allows CL-rich regions within the monolayer to stretch out of the membrane plane to create a stable folding structure.
Studies using simple mitochondrial biomimetic membranes have also shown that CL plays modulates the biophysical properties of monolayers. Nichols-Smith et al. originally suggested that cardiolipin plays a dual role in modulating membrane properties by expanding lateral interactions between phospholipids and regulating the surrounding phospholipid microenvironment (156). The presence of CL decreases the cohesiveness within the membrane, which is suggested to promote lipid-lipid miscibility under physiologically relevant conditions (156). This is in disagreement with currents studies indicating CL’s propensity to promote lipid-lipid immiscibility in an inner-membrane mimicking mitochondrial phospholipid mixture (155). These discrepancies could simply be due to differences in the complexity between the CL-containing phospholipid mixtures used in each study.
Extreme changes to CL acyl chain composition heavily modulate the biophysical properties of membranes. For example, replacement of (18:2)4CL acyl chains with tetramyristoyl [(14:0)4] CL promotes lipid-lipid immiscibility in simple and complex phospholipid monolayers. This strongly agrees with previous studies suggesting that (14:0)4CL (TMCL) does not ideally mix in binary phospholipid mixtures at physiologically relevant membrane surface pressures (163). Conversely, using grazing incidence X-ray diffraction techniques, Etienne et al. concluded that TMCL promotes phospholipid mixing within DPPC monolayers, which increases order by a tight packing of lipid acyl chains (173). Extreme deviations in carbon-chain length and acyl chain saturation would presumably influence such biophysical properties of membranes. However, results showing TMCL’s ability to disrupt phospholipid packing have limited eukaryotic biological relevance given that (14:0)4CL is predominantly found in sea urchin sperm and in bacteria (174–176).
4.2. Mitochondrial inner membrane ultrastructure and cristae morphology
Studies focused on bridging lipid dynamics to membrane ultrastructure have focused on determining how CL regulates properties of mitochondrial membrane curvature and cristae morphology in vivo and in vitro. It has been hypothesized that the organization and regulation of cristae ultrastructural morphologies are driven by (i) the local pH gradient and (ii) the distribution of macromolecular protein complexes. Studies by Khalifat et al. have elegantly demonstrated that lipid packing variations in CL-containing giant vesicles are modulated by a transmembrane pH gradient, which leads to the formation of tubules that have morphologies similar to mitochondrial cristae (160). Presumably CL is critical for these phenomena to occur, given CL’s propensity for trapping protons at the membrane surface and inducing negative membrane curvature. However, Khalifat et al. have demonstrated that replacement of CL with its biosynthetic precursor, phosphatidylglycerol (PG), promotes tubule formation induced by a localized transmembrane pH gradient (160). This sheds light on how the molecular properties of anionic phospholipids, and their ability to bind or release a proton, can modulate complex changes in the mitochondrial membrane potential and thereby mitochondrial bioenergetics.
Although PG may behave similarly relative to CL, it is clear that the loss of CL has strong effects on mitochondrial function. For instance, the absence of CL synthase (Δcrd1) in yeast results in impairments in mitochondrial membrane potential, which results in mitochondrial dysfunction due to defective ADP/ATP carrier (AAC) activity and ATP synthesis (50). When PG was substituted for CL, the decrease in membrane potential was found the be less pronounced relative to membranes lacking both PG and CL (50). This indicates that the lack of CL potentially impairs the inner membrane proton gradient by either directly disrupting inner membrane microviscosity, and thereby membrane ultrastructure, or by lowering the activity of respiratory chain enzymes and inner membrane protein carriers. Another study recently suggested that elevating mitochondrial phosphatidylethanolamine (PE) may compensate for CL deficiency (177). Ball and Baker found that ethanolamine supplementation rescued the respiratory growth of CL-deficient Δtaz1 and Δcrd1 yeast cells, independent of PE incorporation. The rescue was only sensitive to ethanolamine and not choline or serine. Furthermore, ethanolamine supplementation resulted in improved mitochondrial function by increasing the expression of respiratory complex proteins and thereby supercomplex organization in Δtaz1 cells, but not Δcrd1 yeast cells (177). These results have great implications into how CL content versus CL acyl chain remodeling impact various endpoints of cellular and subcellular processes. Although, any perturbations in CL’s profile would likely impact biophysical properties of the inner mitochondrial membrane that heavily rely on the presence of CL, such as inner membrane cristae morphology and protein clustering and activity.
Conversely, Baile et al. have demonstrated that mitochondrial morphology, cristae size, and mitochondrial respiration are indistinguishable in mutant yeast (Δcld1 and/or Δtaz1) where CL’s acyl chains are significantly remodeled, relative to wild type cells (157). However, yeast with mutated CL synthase (Δcrd1), and thereby decreased CL content, displayed severe mitochondrial membrane depolarization, which resulted in increased proton leak. Suggesting that CL acyl chain remodeling may not be essential for optimal bioenergetics in yeast, while lowered CL levels may be a critical regulator of the mitochondrial membrane potential. These results are in agreement with the work of Grevengoed et al. (164) where they show that mice with an inducible, tissue specific, knockout of long-chain acyl-CoA synthetase 1 (ACSL1T−/−) displayed substantial CL acyl chain remodeling concomitant with no changes in overall CL abundance. The wild type ACSL1 enzyme is known to exhibit a strong substrate preference for linoleate. Therefore, ACSL1T−/− hearts were determined to be remodeled by 83% from tetralinoleoyl-cardiolipin (CL) to aberrant CL species, which promoted impaired calcium uptake and severe mitochondrial respiratory dysfunction (164). Although CL is substantially remodeled in mutant yeast (Δcld1 and/or Δtaz1) and in ACSL1T−/− murine models (157,164,178,179), it is tough to definitively conclude that CL acyl chain remodeling has no effect on aspects of mitochondrial structure-function given that other pathways may compensate for the loss of mature CL species.
Phospholipids that are stable in an elastically strained environment such as CL and PE, are critical for maintaining inner mitochondrial membrane structure-function, particularly at cristae sites. Given, the unique phospholipid composition of the inner membrane it is highly likely that proteins, such as the mitochondrial contact site and cristae organizing system (MICOS), play an essential role in regulating the biogenesis and structure of cristae. MICOS is a complex of proteins that function to assemble and maintain cristae ultrastructure, particularly at crista junctions where individual crista tubules extend from the mitochondrial inner membrane (2). In addition to MICOS, a variety of specialized proteins and enzymes have been identified that regulate crista morphology, CL content, and membrane fusion/fission events. These proteins include Opa1, tafazzin, Aim24, MICOS proteins 10, 12, 26, 27, and 60, as well as prohibitins and stomatin like protein-2 (2). In many cases, overexpression or deletion of these proteins modifies CL content and thereby inner mitochondrial membrane structure-function, which is discussed in detail below.
Studies in yeast where Aim24, an ancillary MICOS interacting protein, was deleted demonstrate that this gene is necessary for respiratory growth, OXPHOS supercomplex stability and mitochondrial ultrastructure (165,180). Although yeast with a deletion in Aim24 exhibit mitochondrial deficiencies, destabilized MICOS complexes, and inner mitochondrial membrane defects, these cells do not display decreased levels of cardiolipin. However, when Aim24 deletion is combined with minor genetic modifications to MICOS proteins, MIC12 or MIC26, an altered cardiolipin acyl chain composition is observed, where longer, more saturated acyl chains predominate (165). This leads to the presence of particularly smaller mitochondria that lack inner mitochondrial membrane ultrastructure and cristae, which is accompanied by impaired oxidative phosphorylation and cellular inviability under respiratory conditions.
Silencing prohibitin (PHB) expression in mouse embryonic fibroblasts leads to distinctive changes in CL’s acyl chain composition as well as the accumulation of monolyso-cardiolipin (158). Consistent with this, a deletion of stomatin like protein-2 (SLP-2) (181,182) correlates with abnormal CL compartmentalization and thereby decreased supercomplex formation. It has been hypothesized that SLP-2 functions to stabilize cristae morphology and mitochondrial inner membrane structure by serving as a platform for supercomplex assembly, and thereby the organization of CL-enriched microdomains (183). As more information emerges about proteins involved in regulating cristae dynamics, it is clear that many of these proteins directly involve cardiolipin and require its association for optimal function, which absolutely warrants future studies aimed at better understanding the importance of CL and its acyl chains in cristae morphology and mitochondrial membrane ultrastructure.
4.3. Mitochondrial membrane microdomain organization
The unique ability of CL to induce negative membrane curvature and form non bilayer phases, such as the inverted-hexagonal phase, implicate its potential role in the formation of mitochondrial microdomains (5,184–188). Indeed, studies have indirectly suggested that CL-enriched microdomains exist in mitochondria as essential activating platforms for mitochondrial fusion/fission and apoptosis (187,188). The overall notion that mitochondria contain enriched regions of CL is still in its infancy, although such microdomains may cluster proteins, including respiratory protein complexes, for optimal oxidative phosphorylation and enhanced mitochondrial signaling. However, these studies repeatedly fail to discriminate between the role of decreased CL content and CL acyl chain composition on mitochondrial microdomain organization.
CL microdomains were first visualized in bacterial membranes and have been determined to localize near regions of negative membrane curvature (184,185). Since then, studies have utilized giant vesicles as a model for visualizing and quantitatively analyzing various properties of CL microdomains. For instance, Beales et al. employed ‘raft-like’ giant vesicles in order to investigate CL-cytochrome c interactions (159). This study found that cytochrome c (cyt c) binds very strongly to domains enriched in CL, which causes severe morphological changes within these regions of the membrane. Over time, these CL-enriched domains start to form small buds, eventually aggregating protein and folding into a collapsed state. The formation of the CL-cyt c complex is driven by cytochrome c binding to the surface of the lipid membrane. This association may lead to the organization of either catalytically active ‘bubbles’ inside the bilayer, formed by the CL-cyt c complex, or the appearance of hydrophilic pores (189,190). Although these studies highlight the importance of cyt c in regulating the localized cardiolipin microenvironment, it should be noted that CL is not absolutely required for these phenomena to occur. Specifically, Beales at el. verified that folding and collapse of domains induced by the CL-cyt complex c was also observed in vesicles where the anionic lipid dioleoylphosphatidylglycerol (DOPG) had been substituted for CL (159). It is not surprising that DOPG is able to regulate cyt c-induced microdomain organization given that these interactions are initially driven by the electrostatic association of lipids and proteins.
Similar studies have shown that other proteins, such as dynamin related protein 1 (Drp1) and mitochondrial creatine kinase (mtCK), are capable of strongly interacting with CL and inducing proteolipid microdomain formation (188,191). In vesicles mimicking the mitochondrial outer membrane, Stepanyants et al. show preferential association between Drp1 and CL at a high spatial density within the membrane bilayer (188). It is suggested that this increases CL’s propensity to transition from a lamellar bilayer arrangement to an inverted hexagonal, nonbilayer configuration, resulting in the organization of localized proteolipid membrane domains. Calorimetric studies investigating the relationship between mitochondrial kinases and CL have determined that ubiquitous mtCK promotes the segregation and clustering of CL phospholipids in mixed membranes (28). This was recently visualized in a study using giant vesicles mimicking the mitochondrial inner mitochondrial membrane as a model for studying CL-mtCK interactions (191). This study by Cheniour et al. determined that these proteolipid microdomains only formed when CL was present in the lipid mixture, which was accompanied by changes in both vesicle curvature and membrane fluidity (191). Other studies using lipid monolayers, have shown that binding between mtCK and CL is fundamentally driven by CL’s acyl chain composition (166). Maniti et al. found that mtCK-CL domains were observed with unsaturated CL species, including tetralinoleoylcardiolipin, but also with phosphatidylglycerol (PG), CL’s biosynthetic precursor (166). However, when tetramyristoylcardiolipin was introduced to the phospholipid monolayer, mtCK was homogeneously distributed underneath the lipid film and mtCK binding was not observed (166).
It is essential to better understand how changes in CL’s profile regulate mitochondrial microdomains given that CL abnormalities would effectively impair mitochondrial inner membrane ultrastructure-function. Using a complex mitochondrial inner membrane mimicking model, our lab has also shown that cyt c and mtCK promote proteolipid microdomain formation. These domains are highly sensitive to a decreased CL levels but not substitution of MLCL or (18:1)4CL, suggesting that CL content is the more important factor in regulating mitochondrial proteolipid microdomains (161). However, extreme remodeling by the complete incorporation of DHA into CL’s acyl chains [(22:6)4CL] abolishes proteolipid microdomain organization, which is driven by changes in the Gibbs free energy of lipid mixing (87). The inability of (22:6)4CL to induce proteolipid domains may simply be due to the fact that a longer, more unsaturated acyl chain is unable to sterically fit within a specific binding pocket of cyt c. Of course, there are major limitations of this type of approach, as studies in biomimetic membranes need to be linked with genetic murine models and importantly, connected to results from human studies.
4.4. Mitochondrial lipid-protein interactions and supercomplex organization
As mentioned earlier, specific CL binding sites have been identified and determined to be structurally and functionally important for complexes I, III, and IV (13,47). Specifically, bovine complex I contains nine binding sites, complex III has six binding sites, and complex IV has four binding sites. Structural studies suggest that CL binding to complex III assists in proton uptake (42), where CL binding to complex IV is associated with structural integrity of dimerization and regulation of electron activity (43). Furthermore, dimers of complex V require CL for the recruitment of individual complexes and thus the assembly into larger oligomeric structures (39).
Given the aforementioned requirement of CL for optimal structure-function of proteins involved in mitochondrial bioenergetics, CL is often considered to be the “glue” that holds respiratory supercomplexes together (46). Studies involving blue-native gel electrophoresis and electron microscopy have been fundamental in providing evidence for the presence of large protein complexes into higher ordered molecular structures termed supercomplexes (192). Supercomplexes compromising C-I, C-III and C-IV have been extracted from various organisms including, bovine heart (193,194), potato (195,196) and from yeast (197,198). CL is critical in the formation and stability of such supercomplexes (44,46,49). However, reduced levels of (18:2)4CL in the mitochondrial inner membrane leads to supercomplex dissociation, and results in decreased ATP production. Studies in Drosophila flight muscle mitochondria reveal that mutations in CL synthase leads to destabilization in the supramolecular organization of ATP synthase dimers (162). Results from these studies suggest that CL regulates the lateral organization and morphology of the cristae membrane. Similarly, studies using mutant yeast demonstrate a direct correlation between CL levels and supercomplex organization (45,199), such that the reconstitution of purified yeast supercomplexes containing complexes III and IV was entirely dependent on the presence of CL (44,45).
Alterations in CL’s acyl chain composition have been shown to differentially impact various aspects of mitochondrial bioenergetics and supercomplex organization. For instance, in murine models of diet-induced obesity, high-fat diet intervention modestly remodels mitochondrial CL and exerts tissue specific impairments of respiratory enzyme activity concomitant with no effect on overall respiratory function (167). Although, hepatic CL remodeling was accompanied by diminished complex I to III respiratory enzyme activity. These results have also been recently observed in murine cardiac tissue, where CL remodeling by DHA intervention lowered the specific enzymatic activities of respiratory complexes I, IV, V, and I+III. The observed reduction in enzymatic activities was determined to not be mechanistically driven by a dramatic reduction in the abundance of supercomplexes, but potentially by extensive remodeling of the mitochondrial phospholipidome (87).
Although the destabilization of mitochondrial respiratory supercomplexes seems to be exclusively driven by CL levels, the underlying mechanisms by which this occurs has yet to be determined. One potential mechanism may involve CL turnover, and the rate at which CL biosynthesis and subsequent degradation occur. To exemplify, Xu et al. demonstrated in lymphoblasts that CL is protected from degradation by associating with select inner mitochondrial membrane proteins. However, loss of this protection resulted in increased CL degradation and turnover (104). These studies highlight the ability of lipids to selectively mediate protein–protein interactions by strongly binding specific proteins that require specific lipid microenvironments. Given CL’s unique ability to bind specific mitochondrial proteins for regulating bioenergetics, future studies are necessary to investigate mechanisms by which CL biosynthesis and turnover regulate mitochondrial structure-function.
5.0. Conclusion
CL has a critical role in maintaining the structural organization of the inner mitochondrial membrane. Due to its unique cone shaped structure, CL is critical for mitochondrial membrane structure, cristae morphology, lipid-protein and protein-protein interactions, and thereby mitochondrial bioenergetics and ATP synthesis. The manifestation of decreased CL content and/or CL acyl chain remodeling severely disrupts the aforementioned properties of mitochondrial structure-function. A systematic review of the literature indicates that CL content is a greater driver of impairments in various biophysical properties of the mitochondrial inner membrane, and that extreme CL acyl chain remodeling to species including tetramyristoyl- and tetradocosahexaenoyl- CL species likely play a contributing role. Future studies should be aimed at better understanding how CL concentration and CL’s acyl chain composition modulate other biophysical parameters of mitochondria such as CL-protein binding kinetics and mitochondrial fusion/fission. Furthermore, it is clear that CL content is a valid therapeutic target for improving mitochondrial dysfunction throughout various metabolic disease, including CVDs and Barth syndrome. Thus, studies aimed at investigating aberrant CL turnover as a therapeutic target is an area for future reference.
Highlights.
Cardiolipin (CL) is a unique mitochondrial phospholipid
In many diseases, CL levels are decreased and CL acyl chains are remodeled
Alterations to CL are associated with aberrant mitochondrial structure-function
Loss of CL content often impairs inner mitochondrial membrane properties
Only extreme CL acyl chain remodeling influences mitochondrial membrane properties
ACKNOWLEDGEMENTS:
This work was supported by the National Institutes of Health R01DK107397 (K.F.), R03DK109888 (K.F.), R01HL123647 (S.R.S. and D.B.), and R01AT008375 (S.R.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Paradies G, Paradies V, De Benedictis V, Ruggiero FM, and Petrosillo G (2014) Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 1837, 408–17. [DOI] [PubMed] [Google Scholar]
- 2.Ikon N, and Ryan RO (2017) Cardiolipin and mitochondrial cristae organization. Biochim Biophys Acta 1859, 1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kagan VE, Bayır HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, and Borisenko G (2009) Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med 46, 1439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlame M, Rua D, and Greenberg ML (2000) The biosynthesis and functional role of cardiolipin. Prog Lipid Res 39, 257–88. [DOI] [PubMed] [Google Scholar]
- 5.Osman C, Voelker DR, and Langer T (2011) Making heads or tails of phospholipids in mitochondria. J Cell Biol 192, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlame M, Ren M, Xu Y, Greenberg ML, and Haller I (2005) Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids 138, 38–49. [DOI] [PubMed] [Google Scholar]
- 7.Schlame M, and Otten D (1991) Analysis of cardiolipin molecular species by high-performance liquid chromatography of its derivative 1,3-bisphosphatidyl-2-benzoyl-sn-glycerol dimethyl ester. Anal Biochem 195, 290–5. [DOI] [PubMed] [Google Scholar]
- 8.Han X, Yang K, Yang J, Cheng H, and Gross RW (2006) Shotgun lipidomics of cardiolipin molecular species in lipid extracts of biological samples. J Lipid Res 47, 864–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song H, Wohltmann M, Bao S, Ladenson JH, Semenkovich CF, and Turk J (2010) Mice deficient in group VIB phospholipase A2 (iPLA2gamma) exhibit relative resistance to obesity and metabolic abnormalities induced by a Western diet. Am J Physiol Endocrinol Metab 298, E1097–E1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiebish MA, Han X, Cheng H, Chuang JH, and Seyfried TN (2008) Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J Lipid Res 49, 2545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlame M, and Ren M (2009) The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim Biophys Acta 1788, 2080–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalifat N, Puff N, Bonneau SP, Fournier J-B, and Angelova MI (2008) Membrane deformation under local pH gradient: mimicking mitochondrial cristae dynamics. Biophys J 95, 4924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planas-Iglesias J, Dwarakanath H, Mohammadyani D, Yanamala N, Kagan VE, and Klein-Seetharaman J (2015) Cardiolipin interactions with proteins. Biophys J 109, 1282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu B, Xu FY, Jiang YJ, Choy PC, Hatch GM, Grunfeld C, and Feingold KR (2006) Cloning and characterization of a cDNA encoding human cardiolipin synthase (hCLS1). J Lipid Res 47, 1140–5. [DOI] [PubMed] [Google Scholar]
- 15.Houtkooper RH, Akbari H, van Lenthe H, Kulik W, Wanders RJA, Frentzen M, and Vaz FM (2006) Identification and characterization of human cardiolipin synthase. FEBS Lett 580, 3059–64. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Zhang X-Y, and Shi Y (2006) Identification and functional characterization of hCLS1, a human cardiolipin synthase localized in mitochondria. Biochem J 398, 169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mejia EM, Nguyen H, and Hatch GM (2014) Mammalian cardiolipin biosynthesis. Chem Phys Lipids 179, 11–6. [DOI] [PubMed] [Google Scholar]
- 18.Heden TD, Neufer PD, and Funai K (2016) Looking beyond structure: membrane phospholipids of skeletal muscle mitochondria. Trends Endocrinol Metab 27, 553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Romestaing C, Han X, Li Y, Hao X, Wu Y, Sun C, Liu X, Jefferson LS, Xiong J, LaNoue KF, Chang Z, Lynch CJ, Wang H, and Shi Y (2010) Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab 12, 154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zachman DK, Chicco AJ, McCune SA, Murphy RC, Moore RL, and Sparagna GC (2010) The role of calcium-independent phospholipase A2 in cardiolipin remodeling in the spontaneously hypertensive heart failure rat heart. J Lipid Res 51, 525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao J, Liu Y, Lockwood J, Burn P, and Shi Y (2004) A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J Biol Chem 279, 31727–34. [DOI] [PubMed] [Google Scholar]
- 22.Taylor WA, and Hatch GM (2003) Purification and characterization of monolysocardiolipin acyltransferase from pig liver mitochondria. J Biol Chem 278, 12716–21. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Malhotra A, Ren M, and Schlame M (2006) The enzymatic function of tafazzin. J Biol Chem 281, 39217–24. [DOI] [PubMed] [Google Scholar]
- 24.Schlame M, Xu Y, and Ren M (2017) The basis for acyl specificity in the tafazzin reaction. J Biol Chem 292, 5499–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlame M, Acehan D, Berno B, Xu Y, Valvo S, Ren M, Stokes DL, and Epand RM (2012) The physical state of lipid substrates provides transacylation specificity for tafazzin. Nat Chem Biol 8, 862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlattner U, Tokarska-Schlattner M, Rousseau D, Boissan M, Mannella C, Epand R, and Lacombe M-L (2014) Mitochondrial cardiolipin/phospholipid trafficking: the role of membrane contact site complexes and lipid transfer proteins. Chem Phys Lipids 179, 32–41. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Dai Q, Chen J, Durrant D, Freeman A, Liu T, Grossman D, and Lee RM (2003) Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol Cancer Res 1, 892–902. [PubMed] [Google Scholar]
- 28.Epand RF, Tokarska-Schlattner M, Schlattner U, Wallimann T, and Epand RM (2007) Cardiolipin clusters and membrane domain formation induced by mitochondrial proteins. J Mol Biol 365, 968–80. [DOI] [PubMed] [Google Scholar]
- 29.Schlattner U, and Wallimann T (2000) Octamers of mitochondrial creatine kinase isoenzymes differ in stability and membrane binding. J Biol Chem 275, 17314–20. [DOI] [PubMed] [Google Scholar]
- 30.Brown DA, Sabbah HN, and Shaikh SR (2013) Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion injury. Pharmacol Ther 140, 258–66. [DOI] [PubMed] [Google Scholar]
- 31.Gilkerson RW, Selker JML, and Capaldi RA (2003) The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. FEBS Lett 546, 355–8. [DOI] [PubMed] [Google Scholar]
- 32.Olofsson G, and Sparr E (2013) Ionization constants pKa of cardiolipin. PLoS One 8, e73040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis RNAH, and McElhaney RN (2009) The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim Biophys Acta 1788, 2069–79. [DOI] [PubMed] [Google Scholar]
- 34.Lewis RN, and McElhaney RN (2000) Surface charge markedly attenuates the nonlamellar phase-forming propensities of lipid bilayer membranes: Calorimetric and (31)P-nuclear magnetic resonance studies of mixtures of cationic, anionic, and zwitterionic lipids. Biophys J 79, 1455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarahovsky YS, Arsenault AL, MacDonald RC, McIntosh TJ, and Epand RM (2000) Electrostatic control of phospholipid polymorphism. Biophys J 79, 3193–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malyshka D, Pandiscia LA, and Schweitzer-Stenner R (2014) Cardiolipin containing liposomes are fully ionized at physiological pH. An FT-IR study of phosphate group ionization. Vib Spectrosc 75, 86–92. [Google Scholar]
- 37.Sathappa M, and Alder NN (2016) The ionization properties of cardiolipin and its variants in model bilayers. Biochim Biophys Acta 1858, 1362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klingenberg M (2009) Cardiolipin and mitochondrial carriers. Biochim Biophys Acta 1788, 2048–58. [DOI] [PubMed] [Google Scholar]
- 39.Eble KS, Coleman WB, Hantgan RR, and Cunningham CC (1990) Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J Biol Chem 265, 19434–40. [PubMed] [Google Scholar]
- 40.Fry M, and Green DE (1981) Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem 256, 1874–80. [PubMed] [Google Scholar]
- 41.Robinson NC (1993) Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr 25, 153–63. [DOI] [PubMed] [Google Scholar]
- 42.Arnarez C, Mazat J-P, Elezgaray J, Marrink S-J, and Periole X (2013) Evidence for cardiolipin binding sites on the membrane-exposed surface of the cytochrome bc1. J Am Chem Soc 135, 3112–20. [DOI] [PubMed] [Google Scholar]
- 43.Arnarez C, Marrink SJ, and Periole X (2013) Identification of cardiolipin binding sites on cytochrome c oxidase at the entrance of proton channels. Sci Rep 3, 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mileykovskaya E, and Dowhan W (2014) Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem Phys Lipids 179, 42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bazán S, Mileykovskaya E, Mallampalli VKPS, Heacock P, Sparagna GC, and Dowhan W (2013) Cardiolipin-dependent reconstitution of respiratory supercomplexes from purified saccharomyces cerevisiae complexes III and IV. J Biol Chem 288, 401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Mileykovskaya E, and Dowhan W (2002) Gluing the respiratory chain together: cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem 277, 43553–6. [DOI] [PubMed] [Google Scholar]
- 47.Arnarez C, Marrink SJ, and Periole X (2016) Molecular mechanism of cardiolipin-mediated assembly of respiratory chain supercomplexes. Chem Sci 7, 4435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y, Sutachan JJ, Plesken H, Kelley RI, and Schlame M (2005) Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab Invest 85, 823–30. [DOI] [PubMed] [Google Scholar]
- 49.Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, and Schägger H (2003) Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem 278, 52873–80. [DOI] [PubMed] [Google Scholar]
- 50.Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, and Greenberg ML (2000) Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J Biol Chem 275, 22387–94. [DOI] [PubMed] [Google Scholar]
- 51.Claypool SM, and Koehler CM The complexity of cardiolipin in health and disease. Trends Biochem Sci 37, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chicco AJ, and Sparagna GC (2007) Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 292, C33–44. [DOI] [PubMed] [Google Scholar]
- 53.Barth PG, Scholte HR, Berden JA, Van Der Klei-Van Moorsel JM, Luyt-Houwen IEM, Van’T Veer-Korthof ET, Van Der Harten JJ, and Sobotka-Plojhar MA (1983) An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci 62, 327–55. [DOI] [PubMed] [Google Scholar]
- 54.Barth PG, Valianpour F, Bowen VM, Lam J, Duran M, Vaz FM, and Wanders RJA (2004) X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am J Med Genet 126A, 349–54. [DOI] [PubMed] [Google Scholar]
- 55.Schlame M, Kelley RI, Feigenbaum A, Towbin JA, Heerdt PM, Schieble T, Wanders RJA, DiMauro S, and Blanck TJJ (2003) Phospholipid abnormalities in children with Barth syndrome. J Am Coll Cardiol 42, 1994–9. [DOI] [PubMed] [Google Scholar]
- 56.Kulik W, van Lenthe H, Stet FS, Houtkooper RH, Kemp H, Stone JE, Steward CG, Wanders RJ, and Vaz FM (2008) Bloodspot assay using HPLC–tandem mass spectrometry for detection of Barth syndrome. Clin Chem 54, 371–8. [DOI] [PubMed] [Google Scholar]
- 57.Ma L, Vaz FM, Gu Z, Wanders RJA, and Greenberg ML (2004) The human TAZ gene complements mitochondrial dysfunction in the yeast taz1Δ mutant. Implications for Barth syndrome. J Biol Chem 279, 44394–9. [DOI] [PubMed] [Google Scholar]
- 58.Schlame M, Towbin JA, Heerdt PM, Jehle R, DiMauro S, Blanck TJ (2002) Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann Neurol 51, 634–7. [DOI] [PubMed] [Google Scholar]
- 59.Vreken P, Valianpour F, Nijtmans LG, Grivell LA, Plecko B, Wanders RJA, and Barth PG (2000) Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem Biophys Res Commun 279, 378–82. [DOI] [PubMed] [Google Scholar]
- 60.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, and Khuchua Z (2011) Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem 286, 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang Y, Powers C, Madala SK, Greis KD, Haffey WD, Towbin JA, Purevjav E, Javadov S, Strauss AW, and Khuchua Z (2015) Cardiac metabolic pathways affected in the mouse model of Barth syndrome. PLoS One 10, e0128561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson JM, Ferrara PJ, Verkerke ARP, Coleman CB, Wentzler EJ, Neufer PD, Kew KA, de Castro Brás LE, and Funai K (2018) Targeted overexpression of catalase to mitochondria does not prevent cardioskeletal myopathy in Barth syndrome. J Mol Cell Cardiol 121, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cole LK, Kim JH, Amoscato AA, Tyurina YY, Bayır H, Karimi B, Siddiqui TJ, Kagan VE, Hatch GM, and Kauppinen TM (2018) Aberrant cardiolipin metabolism is associated with cognitive deficiency and hippocampal alteration in tafazzin knockdown mice. Biochim Biophys Acta 1864, 3353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chao H, Anthonymuthu TS, Kenny EM, Amoscato AA, Cole LK, Hatch GM, Ji J, Kagan VE, and Bayır H (2018) Disentangling oxidation/hydrolysis reactions of brain mitochondrial cardiolipins in pathogenesis of traumatic injury. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen S, He Q, and Greenberg ML (2008) Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol Microbiol 68, 1061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu Z, Valianpour F, Chen S, Vaz FM, Hakkaart GA, Wanders RJA, and Greenberg ML (2004) Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth syndrome. Mol Microbiol 51, 149–58. [DOI] [PubMed] [Google Scholar]
- 67.Brandner K, Mick DU, Frazier AE, Taylor RD, Meisinger C, and Rehling P (2005) Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: Implications for Barth syndrome. Mol Biol Cell 16, 5202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, and Hoppel CL (2004) Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem 279, 47961–7. [DOI] [PubMed] [Google Scholar]
- 69.Lesnefsky EJ, Chen Q, Slabe TJ, Stoll MSK, Minkler PE, Hassan MO, Tandler B, and Hoppel CL (2004) Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Phys Heart Circ Physiol 287, H258–67. [DOI] [PubMed] [Google Scholar]
- 70.Lesnefsky EJ, Slabe TJ, Stoll MSK, Minkler PE, and Hoppel CL (2001) Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Phys Heart Circ Physiol 280, H2770–8. [DOI] [PubMed] [Google Scholar]
- 71.Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Serena D, and Ruggiero FM (1999) Lipid peroxidation and alterations to oxidative metabolism in mitochondria isolated from rat heart subjected to ischemia and reperfusion. Free Radic Biol Med 27, 42–50 [DOI] [PubMed] [Google Scholar]
- 72.Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, and Ruggiero FM (2004) Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res 94, 53–9. [DOI] [PubMed] [Google Scholar]
- 73.Petrosillo G, Ruggiero FM, Di Venosa N, and Paradies G (2003) Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J 17, 714–6. [DOI] [PubMed] [Google Scholar]
- 74.Petrosillo G, Venosa ND, Pistolese M, Casanova G, Tiravanti E, Colantuono G, Federici A, Paradies G, and Ruggiero FM (2006) Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia- reperfusion: role of cardiolipin. FASEB J 20, 269–76. [DOI] [PubMed] [Google Scholar]
- 75.Petrosillo G, Di Venosa N, Ruggiero FM, Pistolese M, D’Agostino D, Tiravanti E, Fiore T, and Paradies G (2005) Mitochondrial dysfunction associated with cardiac ischemia/reperfusion can be attenuated by oxygen tension control. Role of oxygen-free radicals and cardiolipin. Biochim Biophys Acta 1710, 78–86. [DOI] [PubMed] [Google Scholar]
- 76.Nakahara I, Kikuchi H, Taki W, Nishi S, Kito M, Yonekawa Y, Goto Y, and Ogata N (1992) Changes in major phospholipids of mitochondria during postischemic reperfusion in rat brain. J Neurosurg 76, 244–50. [DOI] [PubMed] [Google Scholar]
- 77.Ji J, Baart S, Vikulina AS, Clark RSB, Anthonymuthu TS, Tyurin VA, Du L, St Croix CM, Tyurina YY, Lewis J, Skoda EM, Kline AE, Kochanek PM, Wipf P, Kagan VE, and Bayır H (2015) Deciphering of mitochondrial cardiolipin oxidative signaling in cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 35, 319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heerdt PM, Schlame M, Jehle R, Barbone A, Burkhoff D, and Blanck TJJ (2002) Disease-specific remodeling of cardiac mitochondria after a left ventricular assist device. Ann Thorac Surg 73, 1216–21. [DOI] [PubMed] [Google Scholar]
- 79.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, and Moore RL (2007) Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res 48, 1559–70. [DOI] [PubMed] [Google Scholar]
- 80.O’Rourke B, and Reibel DK (1992) Effects of adrenoceptor blockade on cardiac hypertrophy and myocardial phospholipids. Proc Soc Exp Biol Med 200, 95–100. [DOI] [PubMed] [Google Scholar]
- 81.Reibel DK, O’Rourke B, Foster KA, Hutchinson H, Uboh CE, and Kent RL (1986) Altered phospholipid metabolism in pressure-overload hypertrophied hearts. Am J Physiol 250, H1–6. [DOI] [PubMed] [Google Scholar]
- 82.Sparagna GC, Johnson CA, McCune SA, Moore RL, and Murphy RC (2005) Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res 46, 1196–204. [DOI] [PubMed] [Google Scholar]
- 83.Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ, Kluge MA, Weihrauch D, Gutterman DD, and Vita JA (2010) Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res 156, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watkins SM, Reifsnyder PR, Pan H. j., German JB, and Leiter EH (2002) Lipid metabolome-wide effects of the PPARγ agonist rosiglitazone. J Lipid Res 43, 1809–17. [DOI] [PubMed] [Google Scholar]
- 85.Han X, Yang J, Cheng H, Yang K, Abendschein DR, and Gross RW (2005) Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry 44, 16684–94. [DOI] [PubMed] [Google Scholar]
- 86.Han X, Yang J, Yang K, Zhao Z, Abendschein DR, and Gross RW (2007) Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry 46, 6417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sullivan EM, Pennington ER, Sparagna GC, Torres MJ, Neufer PD, Harris M, Washington J, Anderson EJ, Zeczycki TN, Brown DA, and Shaikh SR (2018) Docosahexaenoic acid lowers cardiac mitochondrial enzyme activity by replacing linoleic acid in the phospholipidome. J Biol Chem 293, 466–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cole LK, Mejia EM, Vandel M, Sparagna GC, Claypool SM, Dyck-Chan L, Klein J, and Hatch GM (2016) Impaired cardiolipin biosynthesis prevents hepatic steatosis and diet-induced obesity. Diabetes 65, 3289–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paradies G, Ruggiero FM, Petrosillo G, Gadaleta MN, and Quagliariello E (1994) Effect of aging and acetyl-l-carnitine on the activity of cytochrome oxidase and adenine nucleotide translocase in rat heart mitochondria. FEBS Lett 350, 213–5. [DOI] [PubMed] [Google Scholar]
- 90.Paradies G, Ruggiero FM, Gadaleta MN, and Quagliariello E (1992) The effect of aging and acetyl-l-carnitine on the activity of the phosphate carrier and on the phospholipid composition in rat heart mitochondria. Biochim Biophys Acta 1103, 324–6. [DOI] [PubMed] [Google Scholar]
- 91.Paradies G, Petrosillo G, Gadaleta MN, and Ruggiero FM (1999) The effect of aging and acetyl-l-carnitine on the pyruvate transport and oxidation in rat heart mitochondria. FEBS Letters 454, 207–209 [DOI] [PubMed] [Google Scholar]
- 92.Paradies G, Ruggiero FM, Petrosillo G, Gadaleta MN, and Quagliariello E (1995) Carnitine-acylcarnitine translocase activity in cardiac mitochondria from aged rats: the effect of acetyl-l-carnitine. Mech Ageing Dev 84, 103–12. [DOI] [PubMed] [Google Scholar]
- 93.Paradies G, Ruggiero FM, Petrosillo G, and Quagliariello E (1997) Age-dependent decline in the cytochrome c oxidase activity in rat heart mitochondria: role of cardiolipin. FEBS Lett 406, 136–8. [DOI] [PubMed] [Google Scholar]
- 94.Lesnefsky EJ, and Hoppel CL (2008) Cardiolipin as an oxidative target in cardiac mitochondria in the aged rat. Biochim Biophys Acta 1777, 1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petrosillo G, Matera M, Casanova G, Ruggiero FM, and Paradies G (2008) Mitochondrial dysfunction in rat brain with aging: involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int 53, 126–31. [DOI] [PubMed] [Google Scholar]
- 96.Petrosillo G, De Benedictis V, Ruggiero FM, and Paradies G (2013) Decline in cytochrome c oxidase activity in rat-brain mitochondria with aging. Role of peroxidized cardiolipin and beneficial effect of melatonin. J Bioenerg Biomembr 45, 431–40. [DOI] [PubMed] [Google Scholar]
- 97.Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barceló-Coblijn GC, and Nussbaum RL (2005) Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking α-synuclein. Mol Cell Biol 25, 10190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sen T, Sen N, Tripathi G, Chatterjee U, and Chakrabarti S (2006) Lipid peroxidation associated cardiolipin loss and membrane depolarization in rat brain mitochondria. Neurochem Int 49, 20–7. [DOI] [PubMed] [Google Scholar]
- 99.Paradies G, Petrosillo G, Pistolese M, and Ruggiero FM (2000) The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome c oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles. FEBS Lett 466, 323–6. [DOI] [PubMed] [Google Scholar]
- 100.Paradies G, Petrosillo G, Pistolese M, and Ruggiero FM (2002) Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 286, 135–141. [DOI] [PubMed] [Google Scholar]
- 101.Paradies G, Petrosillo G, Pistolese M, and Ruggiero FM (2001) Reactive oxygen species generated by the mitochondrial respiratory chain affect the complex III activity via cardiolipin peroxidation in beef-heart submitochondrial particles. Mitochondrion 1, 151–9 [DOI] [PubMed] [Google Scholar]
- 102.Paradies G, Ruggiero FM, Petrosillo G, and Quagliariello E (1998) Peroxidative damage to cardiac mitochondria: cytochrome oxidase and cardiolipin alterations. FEBS Lett 424, 155–8. [DOI] [PubMed] [Google Scholar]
- 103.McKenzie M, Lazarou M, Thorburn DR, and Ryan MT (2006) Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. J Mol Biol 361, 462–9. [DOI] [PubMed] [Google Scholar]
- 104.Xu Y, Phoon CKL, Berno B, D’Souza K, Hoedt E, Zhang G, Neubert TA, Epand RM, Ren M, and Schlame M (2016) Loss of protein association causes cardiolipin degradation in Barth syndrome. Nat Chem Biol 12, 641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu Y, and Schlame M (2014) The turnover of glycerol and acyl moieties of cardiolipin. Chem Phys Lipids 179, 17–24. [DOI] [PubMed] [Google Scholar]
- 106.Bond JM, Herman B, and Lemasters JJ (1991) Protection by acidotic pH against anoxia/reoxygenation injury to rat neonatal cardiac myocytes. Biochem Biophys Res Commun 179, 798–803. [DOI] [PubMed] [Google Scholar]
- 107.Lemasters JJ, Bond JM, Chacon E, Harper IS, Kaplan SH, Ohata H, Trollinger DR, Herman B, and Cascio WE (1996) The pH paradox in ischemia-reperfusion injury to cardiac myocytes. EXS 76, 99–114. [DOI] [PubMed] [Google Scholar]
- 108.Kaplan P, Hendrikx M, Mattheussen M, Mubagwa K, and Flameng W (1992) Effect of ischemia and reperfusion on sarcoplasmic reticulum calcium uptake. Circ Res 71, 1123–30. [DOI] [PubMed] [Google Scholar]
- 109.Krause S, and Hess M (1984) Characterization of cardiac sarcoplasmic reticulum dysfunction during short-term, normothermic, global ischemia. Circ Res 55, 176–84. [DOI] [PubMed] [Google Scholar]
- 110.Nayler WG (1981) The role of calcium in the ischemic myocardium. Am J Pathol 102, 262–70. [PMC free article] [PubMed] [Google Scholar]
- 111.Kusuoka H, Porterfield JK, Weisman HF, Weisfeldt ML, and Marban E (1987) Pathophysiology and pathogenesis of stunned myocardium. Depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts. J Clin Invest 79, 950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gadicherla AK, Stowe DF, Antholine WE, Yang M, and Camara AKS (2012) Damage to mitochondrial complex I during cardiac ischemia reperfusion injury is reduced indirectly by anti-anginal drug ranolazine. Biochim Biophys Acta 1817, 419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Adibhatla RM, Hatcher JF, and Dempsey RJ (2003) Phospholipase A2, hydroxyl radicals, and lipid peroxidation in transient cerebral ischemia. Antioxid Redox Signal 5, 647–54. [DOI] [PubMed] [Google Scholar]
- 114.De Windt LJ, Reneman RS, Van der Vusse GJ, and Van Bilsen M (1998) Phospholipase A2-mediated hydrolysis of cardiac phospholipids: the use of molecular and transgenic techniques. Mol Cell Biochem 180, 65–73. [PubMed] [Google Scholar]
- 115.Okayasu T, Curtis MT, and Farber JL (1985) Structural alterations of the inner mitochondrial membrane in ischemic liver cell injury. Arch Biochem Biophys 236, 638–45. [DOI] [PubMed] [Google Scholar]
- 116.Petrosillo G, Casanova G, Matera M, Ruggiero FM, and Paradies G (2006) Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: induction of permeability transition and cytochrome c release. FEBS Lett 580, 6311–6. [DOI] [PubMed] [Google Scholar]
- 117.Petrosillo G, Ruggiero FM, and Paradies G (2003) Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J 17, 2202–8. [DOI] [PubMed] [Google Scholar]
- 118.Petrosillo G, Ruggiero FM, Pistolese M, and Paradies G (2004) Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J Biol Chem 279, 53103–8. [DOI] [PubMed] [Google Scholar]
- 119.Okumura K, Yamada Y, Kondo J, Hashimoto H, Ito T, and Kitoh J (1991) Decreased 1,2-diacylglycerol levels in myopathic hamster hearts during the development of heart failure. J Mol Cell Cardiol 23, 409–16. [DOI] [PubMed] [Google Scholar]
- 120.Saini-Chohan HK, Holmes MG, Chicco AJ, Taylor WA, Moore RL, McCune SA, Hickson-Bick DL, Hatch GM, and Sparagna GC (2009) Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res 50, 1600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chicco AJ, Sparagna GC, McCune SA, Johnson CA, Murphy RC, Bolden DA, Rees ML, Gardner RT, and Moore RL (2008) Linoleate-rich high-fat diet decreases mortality in hypertensive heart failure rats compared with lard and low-fat diets. Hypertension 52, 549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mulligan CM, Sparagna GC, Le CH, De Mooy AB, Routh MA, Holmes MG, Hickson-Bick DL, Zarini S, Murphy RC, Xu FY, Hatch GM, McCune SA, Moore RL, and Chicco AJ (2012) Dietary linoleate preserves cardiolipin and attenuates mitochondrial dysfunction in the failing rat heart. Cardiovasc Res 94, 460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, and Hoppel CL (2008) Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res 80, 30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sebastiani M, Giordano C, Nediani C, Travaglini C, Borchi E, Zani M, Feccia M, Mancini M, Petrozza V, Cossarizza A, Gallo P, Taylor RW, and d’Amati G (2007) Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J Am Coll Cardiol 50, 1362–9. [DOI] [PubMed] [Google Scholar]
- 125.Hansson A, Hance N, Dufour E, Rantanen A, Hultenby K, Clayton DA, Wibom R, and Larsson N-G (2004) A switch in metabolism precedes increased mitochondrial biogenesis in respiratory chain-deficient mouse hearts. Proc Natl Acad Sci USA 101, 3136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, and Wallace DC (1997) A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet 16, 226–34. [DOI] [PubMed] [Google Scholar]
- 127.Rosca M, Minkler P, and Hoppel CL (2011) Cardiac mitochondria in heart failure: normal cardiolipin profile and increased threonine phosphorylation of complex IV. Biochim Biophys Acta 1807, 1373–82. [DOI] [PubMed] [Google Scholar]
- 128.Vorbeck ML, Malewski EF, Erhart LS, and Martin AP (1975) Membrane phospholipid metabolism in the isoproterenol-induced cardiomyopathy of the rat. Recent Adv Stud Cardiac Struct Metab 6, 175–81. [PubMed] [Google Scholar]
- 129.Chi Y, and Gupta RK (1998) Alterations in heart and kidney membrane phospholipids in hypertension as observed by 31P nuclear magnetic resonance. Lipids 33, 1023–30. [DOI] [PubMed] [Google Scholar]
- 130.Lowell BB, and Shulman GI (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–7. [DOI] [PubMed] [Google Scholar]
- 131.Parish R, and Petersen KF (2005) Mitochondrial dysfunction and type 2 diabetes. Curr Diab Rep 5, 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Højlund K, Mogensen M, Sahlin K, and Beck-Nielsen H (2008) Mitochondrial dysfunction in type 2 diabetes and obesity. Endocrinol Metab Clin North Am 37, 713–31. [DOI] [PubMed] [Google Scholar]
- 133.Ma ZA, Zhao Z, and Turk J (2012) Mitochondrial dysfunction and β-cell failure in type 2 diabetes mellitus. Exp Diabetes Res 2012, 703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marchetti P, Lupi R, Del Guerra S, Bugliani M, Marselli L, and Boggi U (2010) The β-cell in human type 2 diabetes. Adv Exp Med Biol 654, 501–14. [DOI] [PubMed] [Google Scholar]
- 135.He Q, and Han X (2014) Cardiolipin remodeling in diabetic heart. Chem Phys Lipids 179, 75–81. [DOI] [PubMed] [Google Scholar]
- 136.Yamaoka S, Urade R, and Kito M (1988) Mitochondrial function in rats is affected by modification of membrane phospholipids with dietary sardine oil. J Nutr 118, 290–6. [DOI] [PubMed] [Google Scholar]
- 137.Zorzano A, Liesa M, and Palacín M (2009) Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int J Biochem Cell Biol 41, 1846–54. [DOI] [PubMed] [Google Scholar]
- 138.Sebastián D, Hernández-Alvarez MI, Segalés J, Sorianello E, Muñoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P, Orešič M, Pich S, Burcelin R, Palacín M, and Zorzano A (2012) Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci USA 109, 5523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, and Archer SL (2014) Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J 28, 316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ong S-B, Subrayan S, Lim SY, Yellon DM, Davidson SM, and Hausenloy DJ (2010) Inhibiting Mitochondrial Fission Protects the Heart Against Ischemia/Reperfusion Injury. Circulation 121, 2012–22. [DOI] [PubMed] [Google Scholar]
- 141.Paradies G, Petrosillo G, Paradies V, and Ruggiero FM (2011) Mitochondrial dysfunction in brain aging: role of oxidative stress and cardiolipin. Neurochem Int 58, 447–57. [DOI] [PubMed] [Google Scholar]
- 142.Ferrándiz ML, Martínez M, De Juan E, Díez A, Bustos G, and Miquel J (1994) Impairment of mitochondrial oxidative phosphorylation in the brain of aged mice. Brain Res 644, 335–8. [DOI] [PubMed] [Google Scholar]
- 143.Navarro A, and Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292, C670–86. [DOI] [PubMed] [Google Scholar]
- 144.Navarro A (2004) Mitochondrial enzyme activities as biochemical markers of aging. Mol Aspects Med 25, 37–48. [DOI] [PubMed] [Google Scholar]
- 145.Cocco T, Sgobbo P, Clemente M, Lopriore B, Grattagliano I, Di Paola M, and Villani G (2005) Tissue-specific changes of mitochondrial functions in aged rats: effect of a long-term dietary treatment with N-acetylcysteine. Free Radic Biol Med 38, 796–805. [DOI] [PubMed] [Google Scholar]
- 146.Jones TT, and Brewer GJ (2009) Critical age-related loss of cofactors of neuron cytochrome c oxidase reversed by estrogen. Exp Neurol 215, 212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kwong LK, and Sohal RS (2000) Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys 373, 16–22. [DOI] [PubMed] [Google Scholar]
- 148.Guan ZZ, Söderberg M, Sindelar P, and Edlund C (1994) Content and fatty acid composition of cardiolipin in the brain of patients with Alzheimer’s disease. Neurochem Int 25, 295–300. [DOI] [PubMed] [Google Scholar]
- 149.Schapira AHV, Cooper JM, Dexter D, Clark JB, Jenner P, and Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54, 823–7. [DOI] [PubMed] [Google Scholar]
- 150.Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, and Kagawa Y (1989) Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun 163, 1450–5. [DOI] [PubMed] [Google Scholar]
- 151.Pranke IM, Morello V, Bigay J, Gibson K, Verbavatz J-M, Antonny B, and Jackson CL (2011) α-Synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding. J Cell Biol 194, 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, Sesaki H, Cheng Y, Finkbeiner S, Nussbaum RL, Masliah E, and Edwards RH (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein α-synuclein. J Biol Chem 286, 20710–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ryan T, Bamm VV, Stykel MG, Coackley CL, Humphries KM, Jamieson-Williams R, Ambasudhan R, Mosser DD, Lipton SA, Harauz G, and Ryan SD (2018) Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat Commun 9, 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nakamura K (2013) α-Synuclein and mitochondria: partners in crime? Neurotherapeutics 10, 391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pennington ER, Fix A, Sullivan EM, Brown DA, Kennedy A, and Shaikh SR (2017) Distinct membrane properties are differentially influenced by cardiolipin content and acyl chain composition in biomimetic membranes. Biochim Biophys Acta Biomembr 1859, 257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nichols-Smith S, Teh S-Y, and Kuhl TL (2004) Thermodynamic and mechanical properties of model mitochondrial membranes. Biochim Biophys Acta 1663, 82–8. [DOI] [PubMed] [Google Scholar]
- 157.Baile MG, Sathappa M, Lu Y-W, Pryce E, Whited K, McCaffery JM, Han X, Alder NN, and Claypool SM (2014) Unremodeled and remodeled cardiolipin are functionally indistinguishable in yeast. J Biol Chem 289, 1768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Richter-Dennerlein R, Korwitz A, Haag M, Tatsuta T, Dargazanli S, Baker M, Decker T, Lamkemeyer T, Rugarli Elena I., and Langer T (2014) DNAJC19, a mitochondrial cochaperone associated with cardiomyopathy, forms a complex with prohibitins to regulate cardiolipin remodeling. Cell Metab 20, 158–71. [DOI] [PubMed] [Google Scholar]
- 159.Beales PA, Bergstrom CL, Geerts N, Groves JT, and Vanderlick TK (2011) Single vesicle observations of the cardiolipin-cytochrome c interaction: induction of membrane morphology changes. Langmuir 27, 6107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khalifat N, Rahimi M, Bitbol A-F, Seigneuret M, Fournier J-B, Puff N, Arroyo M, and Angelova Miglena I. (2014) Interplay of packing and flip-flop in local bilayer deformation. How phosphatidylglycerol could rescue mitochondrial function in a cardiolipin-deficient yeast mutant. Biophys J 107, 879–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pennington ER, Sullivan EM, Fix A, Dadoo S, Zeczycki TN, DeSantis A, Schlattner U, Coleman RA, Chicco AJ, Brown DA, and Shaikh SR (2018) Proteolipid domains form in biomimetic and cardiac mitochondrial vesicles and are regulated by cardiolipin concentration but not monolyso-cardiolipin. J Biol Chem 293, 15933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Acehan D, Malhotra A, Xu Y, Ren M, Stokes David L., and Schlame M (2011) Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J 100, 2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Prossnigg F, Hickel A, Pabst G, and Lohner K (2010) Packing behaviour of two predominant anionic phospholipids of bacterial cytoplasmic membranes. Biophys Chem 150, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grevengoed TJ, Martin SA, Katunga L, Cooper DE, Anderson EJ, Murphy RC, and Coleman RA (2015) Acyl-CoA synthetase 1 deficiency alters cardiolipin species and impairs mitochondrial function. J Lipid Res 56, 1572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Harner ME, Unger A-K, Izawa T, Walther DM, Ozbalci C, Geimer S, Reggiori F, Brügger B, Mann M, Westermann B, and Neupert W (2014) Aim24 and MICOS modulate respiratory function, tafazzin-related cardiolipin modification and mitochondrial architecture. eLife 3, e01684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maniti O, Cheniour M, Lecompte M-F, Marcillat O, Buchet R, Vial C, and Granjon T (2011) Acyl chain composition determines cardiolipin clustering induced by mitochondrial creatine kinase binding to monolayers. Biochim Biophys Acta 1808, 1129–39. [DOI] [PubMed] [Google Scholar]
- 167.Sullivan EM, Fix A, Crouch MJ, Sparagna GC, Zeczycki TN, Brown DA, and Shaikh SR (2017) Murine diet-induced obesity remodels cardiac and liver mitochondrial phospholipid acyl chains with differential effects on respiratory enzyme activity. J Nutr Biochem 45, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lewis RNAH, Zweytick D, Pabst G, Lohner K, and McElhaney RN (2007) Calorimetric, x-ray diffraction, and spectroscopic studies of the thermotropic phase behavior and organization of tetramyristoyl cardiolipin membranes. Biophys J 92, 3166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Frias M, Benesch MGK, Lewis RNAH, and McElhaney RN (2011) On the miscibility of cardiolipin with 1,2-diacyl phosphoglycerides: binary mixtures of dimyristoylphosphatidylethanolamine and tetramyristoylcardiolipin. Biochim Biophys Acta 1808, 774–83. [DOI] [PubMed] [Google Scholar]
- 170.Unsay JD, Cosentino K, Subburaj Y, and García-Sáez AJ (2013) Cardiolipin effects on membrane structure and dynamics. Langmuir 29, 15878–87. [DOI] [PubMed] [Google Scholar]
- 171.Phan Minh D., and Shin K (2015) Effects of cardiolipin on membrane morphology: a langmuir monolayer study. Biophys J 108, 1977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zeczycki TN, Whelan J, Hayden WT, Brown DA, and Shaikh SR (2014) Increasing levels of cardiolipin differentially influence packing of phospholipids found in the mitochondrial inner membrane. Biochem Biophys Res Commun 450, 366–71. [DOI] [PubMed] [Google Scholar]
- 173.Etienne F, Roche Y, Peretti P, and Bernard S (2008) Cardiolipin packing ability studied by grazing incidence X-ray diffraction. Chem Phys Lipids 152, 13–23. [DOI] [PubMed] [Google Scholar]
- 174.Schlame M (2008) Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J Lipid Res 49, 1607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lopes SC, Neves CS, Eaton P, and Gameiro P (2012) Improved model systems for bacterial membranes from differing species: the importance of varying composition in PE/PG/cardiolipin ternary mixtures. Mol Membr Biol 29, 207–17. [DOI] [PubMed] [Google Scholar]
- 176.Hoch FL (1992) Cardiolipins and biomembrane function. Biochim Biophys Acta 1113, 71–133. [DOI] [PubMed] [Google Scholar]
- 177.Basu Ball W, Baker CD, Neff JK, Apfel GL, Lagerborg KA, Žun G, Petrovič U, Jain M, and Gohil VM (2018) Ethanolamine ameliorates mitochondrial dysfunction in cardiolipin-deficient yeast cells. J Biol Chem 293, 10870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Beranek A, Rechberger G, Knauer H, Wolinski H, Kohlwein SD, and Leber R (2009) Identification of a cardiolipin-specific phospholipase encoded by the gene CLD1 (YGR110W) in yeast. J Biol Chem 284, 11572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ye C, Lou W, Li Y, Chatzispyrou IA, Hüttemann M, Lee I, Houtkooper RH, Vaz FM, Chen S, and Greenberg ML (2014) Deletion of the cardiolipin-specific phospholipase Cld1 rescues growth and life span defects in the tafazzin mutant: implications for Barth syndrome. J Biol Chem 289, 3114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Deckers M, Balleininger M, Vukotic M, Römpler K, Bareth B, Juris L, and Dudek J (2014) Aim24 stabilizes respiratory chain supercomplexes and is required for efficient respiration. FEBS Lett 588, 2985–92. [DOI] [PubMed] [Google Scholar]
- 181.Christie DA, Mitsopoulos P, Blagih J, Dunn SD, St-Pierre J, Jones RG, Hatch GM, and Madrenas J (2012) Stomatin-like protein 2 deficiency in T cells is associated with altered mitochondrial respiration and defective CD4+ T cell responses. J Immunol 189, 4349–60. [DOI] [PubMed] [Google Scholar]
- 182.Christie DA, Lemke CD, Elias IM, Chau LA, Kirchhof MG, Li B, Ball EH, Dunn SD, Hatch GM, and Madrenas J (2011) Stomatin-like protein 2 binds cardiolipin and regulates mitochondrial biogenesis and function. Mol Cell Biol 31, 3845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mitsopoulos P, Chang Y-H, Wai T, König T, Dunn SD, Langer T, and Madrenas J. n. (2015) Stomatin-like protein 2 is required for in vivo mitochondrial respiratory chain supercomplex formation and optimal cell function. Mol Cell Biol 35, 1838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Renner LD, and Weibel DB (2011) Cardiolipin microdomains localize to negatively curved regions of escherichia coli membranes. Proc Nat Acad Sci USA 108, 6264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mileykovskaya E, and Dowhan W (2009) Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta 1788, 2084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.de Kruijff B, Verkleij AJ, van Echteld CJA, Gerritsen WJ, Noordam PC, Mombers C, Rietveld A, de Gier J, Cullis PR, Hope MJ, and Nayar R (1981) Non-bilayer lipids and the inner mitochondrial membrane. Int Cell Biol 1980–1981, 559–71. [Google Scholar]
- 187.Sorice M, Manganelli V, Matarrese P, Tinari A, Misasi R, Malorni W, and Garofalo T (2009) Cardiolipin-enriched raft-like microdomains are essential activating platforms for apoptotic signals on mitochondria. FEBS Lett 583, 2447–50. [DOI] [PubMed] [Google Scholar]
- 188.Stepanyants N, Macdonald PJ, Francy CA, Mears JA, Qi X, and Ramachandran R (2015) Cardiolipin’s propensity for phase transition and its reorganization by dynamin-related protein 1 form a basis for mitochondrial membrane fission. Mol Biol Cell 26, 3104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bergstrom CL, Beales PA, Lv Y, Vanderlick TK, and Groves JT (2013) Cytochrome c causes pore formation in cardiolipin-containing membranes. Proc Natl Acad Sci USA 110, 6269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Marchenkova MA, Dyakova YA, Tereschenko EY, Kovalchuk MV, and Vladimirov YA (2015) Cytochrome c complexes with cardiolipin monolayer formed under different surface pressure. Langmuir 31, 12426–36. [DOI] [PubMed] [Google Scholar]
- 191.Cheniour M, Brewer J, Bagatolli L, Marcillat O, and Granjon T (2017) Evidence of proteolipid domain formation in an inner mitochondrial membrane mimicking model. Biochim Biophys Acta 1861, 969–76. [DOI] [PubMed] [Google Scholar]
- 192.Schägger H, and Pfeiffer K (2000) Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J 19, 1777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schäfer E, Seelert H, Reifschneider NH, Krause F, Dencher NA, and Vonck J (2006) Architecture of Active Mammalian Respiratory Chain Supercomplexes. J Biol Chem 281, 15370–5. [DOI] [PubMed] [Google Scholar]
- 194.Althoff T, Mills DJ, Popot J-L, and Kühlbrandt W (2011) Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J 30, 4652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eubel H, Heinemeyer J, and Braun H-P (2004) Identification and characterization of respirasomes in potato mitochondria. Plant Physiol 134, 1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bultema JB, Braun H-P, Boekema EJ, and Kouřil R (2009) Megacomplex organization of the oxidative phosphorylation system by structural analysis of respiratory supercomplexes from potato. Biochim Biophys Acta 1787, 60–7. [DOI] [PubMed] [Google Scholar]
- 197.Heinemeyer J, Braun H-P, Boekema EJ, and Kouril R (2007) A structural model of the cytochrome c reductase/oxidase supercomplex from yeast mitochondria. J Biol Chem 282, 12240–8. [DOI] [PubMed] [Google Scholar]
- 198.Mileykovskaya E, Penczek PA, Fang J, Mallampalli VKPS, Sparagna GC, and Dowhan W (2012) Arrangement of the respiratory chain complexes in Saccharomyces cerevisiae supercomplex III2IV2 revealed by single particle cryo-electron microscopy. J Biol Chem 287, 23095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang M, Mileykovskaya E, and Dowhan W (2005) Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J Biol Chem 280, 29403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]