Abstract
Cancer patients are at lower risk of developing Parkinson’s disease (PD) compared with the general population. One explanation is the negative association between smoking and PD but PD risk is also lower for cancers not related to smoking. Another explanation is survival bias where death from cancer may act as a competing risk. We conducted a large population-based case-control study in Denmark and investigated whether cancer diagnosis reduced the risk of developing PD even after adjusting for important risk factors including smoking, physical activity, and lifetime estrogen status. Using probabilistic bias analysis we quantified the influence of survival bias. We estimated negative point estimates (Odds Ratios) between cancers and PD for all cancers except skin, female breast, and ill-defined and unspecified 0.85 (95%CI: 0.59, 1.21); smoking related cancers 0.75 (95%CI: 0.45, 1.23); and cancers not related to smoking 0.82 (95%CI: 0.49, 1.38) that are very similar to those previously reported for a much larger Danish register only based study, even though our confidence intervals include the null. These effect estimates shifted towards the null after accounting for survival bias but most bias-adjusted ORs remained below 1 within the range of priors considered in simulations. Overall, cancer patients have a lower risk of developing PD even after controlling for cancer related lifestyles factors and correcting for survival bias.
Keywords: Parkinson’s disease, cancer, survival, bias analysis
Introduction
Half a century ago Doshay (1954) stated that cancer was rare in “paralysis agitans” referring to Parkinson’s disease (PD). [1] Since then, further evidence accumulated from case series [2–4] and epidemiological studies [5–12] that cancer is rarer among PD patients compared with the general population except for melanoma and perhaps breast cancer. Cancer and PD have been attributed to distinct and opposing biological mechanisms with the former being characterized by uncontrolled cell proliferation, and the latter by abnormal neuronal cell loss that occurs progressively. [13] Cancer and PD may also share some genetic and biological pathways. [13–16] Nevertheless, the most obvious explanation for lower cancer incidence is the higher proportion of non- or former smokers among those who develop PD compared with the general population and smoking is a known risk factor for many cancers. [17–19] This still leaves unexplained why cancers that are not related to smoking - with a few exceptions - seem also to be under-represented among PD patients. Also, we recently suggested that quitting smoking is an early prodromal symptom signifying a loss of nicotine responsiveness that sets in years before motor symptoms and a PD diagnosis. [20] Similarly, there are other lifestyle related or biologic factors such as physical activity levels or estrogen status among women that have previously been associated with the developing PD [21, 22] and also affecting cancer risk [23–26] which may confound PD cancer associations.
An alternate explanation for the inverse association between most cancers and PD is survival or participation bias. When studying late onset diseases in the elderly, the increased mortality risk or the reluctance of individuals with multiple serious illnesses to participate in research studies has to be considered. If one disease selectively removes individuals at risk of developing the chronic disease of interest selection bias may affect the results. In other words, death or suffering from cancer may act as a competing risk since getting a PD diagnosis requires individuals to survive long enough to develop PD and, when active participation is needed, to be able to or willing to participate in research. [27]
Since PD and specific cancers are rare diseases, many interview-based case-control studies of PD that collected lifestyle data and controlled for confounding were small and many did not investigate specific types of cancer. [5–7, 9, 11] On the other hand, registry-based studies [8, 12, 33] with a relatively large number of PD cases while investigating specific cancers lacked information on individual-level lifestyle factors, such as smoking and physical activity that may confound cancer-PD associations. We conducted a population-based case-control study of Parkinson’s disease in Denmark (PASIDA) that was much larger than previous interview-based studies and collected detailed information on lifestyle factors. Importantly, the Danish national health register systems provided us with the necessary data for all subjects - whether or not they actively participated in our study - to quantitatively explore the influence of survival/participation bias. Thus, these unique resources enabled us to explore the potential influence of survival/participation bias on associations between specific cancers and PD quantitatively. It also allowed us to adjust our estimates for a number of possible confounding factors.
Methods
Study population
PASIDA is a population-based case-control study conducted in Denmark to identify environmental and genetic risk factors for PD. Study details have been described elsewhere. [20, 28] Patients over 35 years of age were identified from the Danish National Hospital Register files between 1996 and 2009 at 10 major neurological departments with a PD diagnosis (ICD-10 code: G20) assigned by at least one neurologist. Population controls were selected from the Danish Central Population Registry matched on birth year and sex, being alive and without a PD diagnosis at the time of case identification. Complete medical record review was conducted to verify idiopathic PD (iPD) diagnosis for all PD patients responding to our invitation and we excluded 378 non-iPD patients we identified. Among 2,718 eligible cases, we were able to enroll 1,813. Of 3,626 controls initially contacted, 1,887 were enrolled.
Data collection
In PASIDA, information on lifestyle, including physical activity and lifelong smoking history, was obtained in structured telephone interviews or self-administered questionnaires if requested from 2007 and 2009. Pack-years were calculated for cigarette smoking up to the time of PD diagnosis for cases or index date for controls. Physical activity was defined as any vigorous leisure time physical activity during the young, adult, older adult periods (15–25, 25–50 or >50 years). An estrogen index was created based on age at menarche, use of estrogen dose oral contraceptives, parity, surgical menopause (both ovaries out), and hormone replacement therapy use before onset of motor symptoms representing relatively high or low estrogen status during a woman’s reproductive life course. [21] We estimated socioeconomic status (SES) based on job positions collected for Danish tax payers. Degree of urbanization was based on population density of the participants’ community. The Charlson Comorbidity Index was calculated from hospitalization files up to five years prior to PD diagnoses or index date. The Danish Cancer Registry is a nationwide population-based registry containing data on each primary cancer in all residents in Denmark since 1943. [29, 30] We used records from the Danish Cancer Registry based on an extended Danish version of ICD-7 (1943–1977), ICD-O (1978–2003) and ICD-10 (2004–2009) codes to identify cancer diagnoses prior to PD onset.
Standard Protocol Approvals, Registrations, and Patient Consents
We obtained written informed consent from all subjects, and the UCLA Institutional Review Board and the Ethical Committee of the Capital Region of Denmark approved the study protocol; the Danish Data Protection Agency (J.nr. 2006–41-7323 and 2013–41-145) approved the linkage of information.
Statistical analysis
Associations between cancer and PD were first evaluated for the most frequent cancer sites. Then we distinguished between smoking-related and not related cancers to optimize sample size and to allow for cross study comparisons. [31] ICD-codes used to identify smoking-related and not related to smoking cancers are listed in the footnotes of Table 2. We estimated odds ratios (ORs) and 95% confidence intervals (CIs) for cancers preceding PD using unconditional logistic regression models adjusting for age and sex and additionally covariates: i.e. in Model 1 we included age at PD index date (continuous in years), sex, SES (high/medium/low), urbanization (urban/rural), and Charlson Comorbidity Index; in Model 2 we additionally included two main lifestyle factors: smoking (continuous in pack-years) and high leisure time physical activity (yes/no). For female breast cancer, models were additionally adjusted for the estrogen score (continuous). For subjects with missing covariate data (25.2%) we used multiple imputation of missing covariates based on demographics and comorbidities assuming missing at random (MAR). Details on multiple imputation are provided in the appendix. To test the robustness of our results, we repeated the analyses using 3 categories of smoking: never/former/current smoker and also tested for interactions by adding multiplicative interaction terms between cancer and smoking into models.
Table 2.
Associations between cancer and risk of developing PD among participants in the PASIDA Study
| Site of cancer | cases/controls n (%) f | Model 1 c | Model 2 d | ||
|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | ||
| Both Genders | |||||
| No cancer | 1638/1731 | Ref | Ref | ||
| All cancers | 175/156 (9.6/8.3) | 1.14 | 0.89, 1.46 | 1.10 | 0.86, 1.41 |
| All cancers except skin, female breast, and ill-defined and unspecified | 69/79 (3.8/4.2) | 0.88 | 0.62, 1.25 | 0.85 | 0.59, 1.21 |
| Bladder | 9/11 (0.5/0.6) | 0.84 | 0.34, 2.05 | 0.88 | 0.35, 2.20 |
| Colon | 5/8 (0.3/0.4) | 0.59 | 0.18, 1.90 | 0.55 | 0.17, 1.81 |
| Melanoma | 14/9 (0.8/0.5) | 1.58 | 0.67, 3.71 | 1.47 | 0.62, 3.49 |
| All smoking-related a | 31/41 (1.2/2.2) | 0.75 | 0.46, 1.22 | 0.75 | 0.45, 1.23 |
| All not related to smoking b | 38/41 (2.1/2.2) | 0.97 | 0.61, 1.56 | 0.82 | 0.49, 1.38 |
| Male Only | |||||
| No cancer | 980/1039 | Ref | Ref | ||
| Prostate | 14/12 (1.4/1.1) | 1.22 | 0.55, 2.68 | 1.23 | 0.55, 2.74 |
| Female Only | |||||
| No cancer | 658/692 | Ref | Ref | ||
| Breast e | 24/26 (3.5/3.6) | 0.99 | 0.53, 1.85 | 0.96 | 0.51, 1.80 |
Includes buccal cavity and pharynx, esophagus, larynx, lung, bronchus and trachea, stomach, colorectal, liver, pancreas, nasal cavities, top ear and sinus, cervix uteri, ovary, fallopian tube and broad ligament, kidney, renal pelvis and ureter, urinary bladder, and myeloid leukemia.
Includes small intestine, gall bladder and biliary tract, other and unspecified digestive organs, thymus, heart and mediastinum, pleura, bones, joints and articular cartilage, mesothelium, non-pleural, peripheral nerves and autonomic nervous system, peritoneum and retroperitorium, other connective tissue, external female genital organs, corpus uteri, other and unspecified female genital organs, prostate, testis, other and unspecified male genital organs, male breast, other and unspecified urinary organs, eye, meninges, brain, spinal cord, cranial nerves and other unspecified parts of central nervous system, endocrine glands, Hodgkin lymphoma, non-Hodgkin lymphoma and malignant immunoproliferative disease, multiple myeloma, lymphatic leukemia, monocytes leukemia, other and unspecified leukemia, and other and unspecified cancer in lymphatic and hematopoietic tissue.
Model adjusted for age at PD diagnosis (cases) or age at index date (controls) (continuous in years), sex (except female breast cancer), urbanization (urban/rural), SES (high/medium/low), and Charlson Comorbidity Index (continuous).
Model adjusted for variables in model 1 plus smoking (pack-years, for colon cancer, bladder cancer, all cancers, and smoking-related cancers) and vigorous leisure time physical activity (yes/no).
Model 2 additionally adjusted for lifetime estrogen index score (continuous).
Percent of cancers among PD cases and controls, respectively.
To account for potential survival bias in PASIDA, we performed a two-step bias analysis. First, to address potential selection bias due to non-participation that may affect interview-based case-control studies and to compare our estimates to a previous Danish registry-based study, we assessed associations between cancers and PD combining participating and non-participating cases and controls. This was possible since the central person register provided us with basic demographic data on every eligible PD case and control and we could link everyone approached in PASIDA to the cancer registry. Second, to additionally adjust for survival bias that possibly exists in both interview-based and registry-based studies, we modeled survival status based on information available for all individuals, i.e. demographics, comorbidities, cancer and PD diagnosis, and lifestyle factors (imputed for non-participants), and estimated the association between cancer and PD while adjusting for survival bias with calculated selection probabilities as regression weight. We varied the assumed effect size of the bias parameters and reported medians and 95% simulation intervals (95%SI) for the estimated bias-adjusted ORs derived from these simulations. Details on the bias analysis are provided in the appendix.
All analyses and simulations were performed with SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
The average age at PD diagnosis was 62.4 and 59% of participants in PASIDA were male (Table1). More cases (49.6%) than controls (35.5%) were never smokers. Other demographic and lifestyle characteristics were similar in cases and controls. Demographic characteristics for non-participants are shown in Supplementary Table 1. Being younger, male, of higher SES, and having lower Charlson Comorbidity Index scores increased likelihood of participation in our interview-based case-control study.
Table 1.
Demographic and lifestyle characteristics among participants in the PASIDA Study
| Cases | Controls | |||
|---|---|---|---|---|
| n | % | n | % | |
| Total | 1813 | 100.0 | 1887 | 100.0 |
| Age at attempt to contact a (years), mean (SD) | 66.5±8.7 | 66.7±8.7 | ||
| PD duration at attempt to contact (years), mean (SD) | 4.1±4.1 | |||
| Age at index date b (years), mean (SD) | 62.4±9.3 | 62.6±9.4 | ||
| < 44 | 78 | 4.3 | 81 | 4.3 |
| 45–54 | 269 | 14.8 | 280 | 14.8 |
| 55–64 | 673 | 37.1 | 678 | 35.9 |
| 65–74 | 612 | 33.8 | 651 | 34.5 |
| > 75 | 181 | 10.0 | 197 | 10.4 |
| Sex | ||||
| Male | 1070 | 59.0 | 1121 | 59.4 |
| Female | 743 | 41.0 | 766 | 40.6 |
| SES | ||||
| Academic and top self-employed | 260 | 14.3 | 221 | 11.7 |
| High self-employed and high salaried | 275 | 15.2 | 281 | 14.9 |
| Low self-employed and mid-salaried | 341 | 18.8 | 363 | 19.2 |
| Skilled worker and low salaried | 384 | 21.2 | 422 | 22.4 |
| Unskilled worker | 149 | 8.2 | 203 | 10.8 |
| Unknown or unspecified | 404 | 22.3 | 397 | 21.0 |
| Degree of urbanization | ||||
| Capital | 443 | 24.4 | 564 | 29.9 |
| Provincial cities | 1118 | 61.7 | 965 | 51.1 |
| Rural areas | 167 | 9.2 | 209 | 11.1 |
| Peripheral regions | 82 | 4.5 | 148 | 7.8 |
| Unknown or unspecified | 3 | 0.2 | 1 | 0.1 |
| Physical activity c | ||||
| Yes | 1335 | 73.6 | 1455 | 77.1 |
| No | 396 | 21.8 | 361 | 19.1 |
| Unknown | 82 | 4.5 | 71 | 3.8 |
| Smoking | ||||
| Pack-years among ever smokers, mean (SD) | 16.4±16.6 | 20.0±17.8 | ||
| Never smokers | 900 | 49.6 | 667 | 35.3 |
| Former smokers | 753 | 41.5 | 837 | 44.4 |
| Current smokers | 147 | 8.1 | 376 | 19.9 |
| Unknown | 13 | 0.7 | 7 | 0.4 |
| Charlson Comorbidity Index 5 years prior to index date | ||||
| 0 | 1559 | 86.0 | 1664 | 88.2 |
| 1–3 | 247 | 13.6 | 215 | 11.4 |
| 4–10 | 7 | 0.4 | 8 | 0.4 |
Age at contact was defined as age at the time of attempt to contact and invite the individual to participate in the interview-based case-control study (2007–2009).
Age at index date was defined as age at first primary PD diagnosis for cases and age at the time of the matched case’s first diagnosis for controls.
Physical activity was defined as any vigorous leisure physical activity at any time in life.
Table 2 summarizes the associations between cancer and risk of developing PD in PASIDA. The overall prevalence of cancers was slightly lower among PD cases (3.8%) than in population controls (4.2%) and particularly for smoking-related cancers (1.7% in PD cases and 2.2% in controls). With adjustment for basic demographic factors only, the point estimates for PD and all cancers except skin, female breast, and ill-defined and unspecified (OR=0.88; 95%CI: 0.62, 1.25) or smoking-related cancers (OR=0.75; 95%CI: 0.46, 1.22) were below 1 but the confidence intervals were wide and included the null. We found no association for cancers not related to smoking (OR=0.97; 95%CI: 0.61, 1.56). The effect estimates changed minimally in models additionally adjusted for lifestyle factors. Results were similar when we adjusted for 3 categories of smoking instead of pack-years or when we added multiplicative interaction terms between cancer and smoking (results not shown).
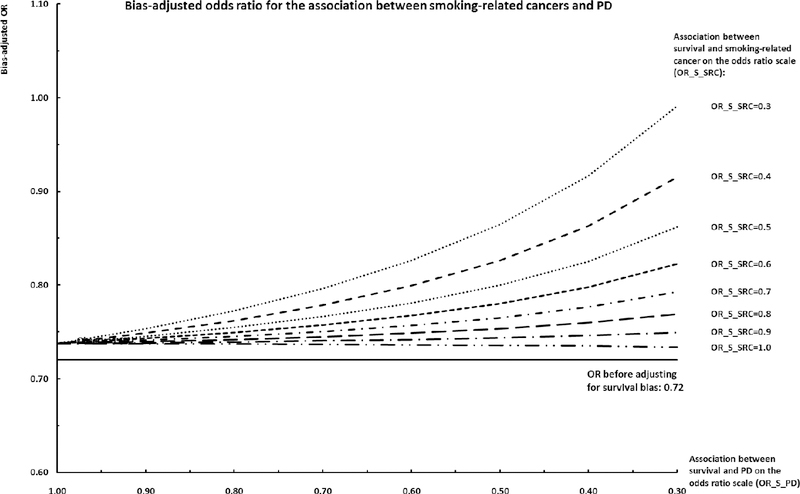
When adjusting for participation related selection bias by including non-participants, for most individual cancers both negative and positive associations became stronger and all estimates were more precise due to increased sample size (Table 3). Again, the effect estimates were similar comparing models with or without adjustment for lifestyle factors. Results of bias analyses that further adjusted for survival bias are shown in Figure 1 and 2 for smoking-related cancers and cancers not related to smoking, respectively. When the likelihood of survival was assumed to decrease for those affected by cancer and PD, the associations between cancer and PD generally shifted towards the null value. However, the bias-adjusted ORs for cancer and PD remained below 1 even when cancer and PD are presumed to have a moderate to strong impact on survival. Only in the most extreme scenario with a presumed 70% lower chance of survival among those affected by cancer and PD, the bias-adjusted OR became null for smoking-related cancers (OR=0.99; 95%SI: 0.75, 1.31) and crossed the null for cancers not related to smoking (OR=1.12 (95%SI: 0.85, 1.47).
Table 3.
Association between cancer and risk of developing PD after inclusion of non-participants in the PASIDA Study
| Site of cancer | cases/controls n (%) f | Model 1 c | Model 2 d | ||
|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | ||
| Both Genders | |||||
| No cancer | 2450/3294 | Ref | Ref | ||
| All cancers | 268/332 (9.9/9.2) | 0.98 | 0.82, 1.18 | 0.95 | 0.79, 1.14 |
| All cancers except skin, female breast, and ill-defined and unspecified | 115/174 (4.2/4.8) | 0.79 | 0.61, 1.02 | 0.78 | 0.60, 1.01 |
| Bladder | 19/21 (0.7/0.6) | 1.11 | 0.59, 2.09 | 1.16 | 0.61, 2.22 |
| Colon | 12/21 (0.4/0.6) | 0.61 | 0.29, 1.28 | 0.61 | 0.29, 1.31 |
| Melanoma | 26/15 (1.0/0.4) | 1.96 | 1.02, 3.79 | 1.84 | 0.94, 3.60 |
| All smoking-related a | 54/91 (2.0/2.5) | 0.71 | 0.50, 1.02 | 0.72 | 0.50, 1.04 |
| All not related to smoking b | 62/87 (2.3/2.4) | 0.85 | 0.60, 1.19 | 0.81 | 0.57, 1.15 |
| Male Only | |||||
| No cancer | 1425/1815 | Ref | Ref | ||
| Prostate | 21/17 (1.5/0.9) | 1.53 | 0.80, 2.95 | 1.50 | 0.77, 2.91 |
| Female Only | |||||
| No cancer | 1025/1479 | Ref | Ref | ||
| Breast e | 35/61 (3.3/4.0) | 0.65 | 0.41, 1.03 | 0.61 | 0.38, 0.98 |
Included buccal cavity and pharynx, esophagus, larynx, lung, bronchus and trachea, stomach, colorectal, liver, pancreas, nasal cavities, top ear and sinus, cervix uteri, ovary, fallopian tube and broad ligament, kidney, renal pelvis and ureter, urinary bladder, and myeloid leukemia.
Included small intestine, gall bladder and biliary tract, other and unspecified digestive organs, thymus, heart and mediastinum, pleura, bones, joints and articular cartilage, mesothelium, nonpleural, peripheral nerves and autonomic nervous system, peritoneum and retroperitorium, other connective tissue, external female genital organs, corpus uteri, other and unspecified female genital organs, prostate, testis, other and unspecified male genital organs, male breast, other and unspecified urinary organs, eye, meninges, brain, spinal cord, cranial nerves and other unspecified parts of central nervous system, endocrine glands, Hodgkin lymphoma, non-Hodgkin lymphoma and malignant immunoproliferative disease, multiple myeloma, lymphatic leukemia, monocytes leukemia, other and unspecified leukemia, and other and unspecified cancer in lymphatic and hematopoietic tissue.
Model adjusted for age at PD diagnosis (cases) or age at index date (controls) (continuous in years), sex (except female breast cancer), urbanization (urban/rural), SES (high/medium/low), and Charlson Comorbidity Index (continuous).
Model adjusted for variables in model 1 plus smoking (pack-years, for colon cancer, bladder cancer, all cancers, and smoking-related cancers) and vigorous leisure time physical activity (yes/no).
Model 2 additionally adjusted for estrogen index score (continuous).
Percent of cancers among PD cases and controls, respectively.
Figure 1. Bias-adjusted odds ratio for the association between smoking-related cancers and PD.
Horizontal axis: Association between survival and PD on the odds ratio scale (OR_S_PD). Negative value represents scenarios where the likelihood of survival is decreased for those who are at risk of developing PD had they survived long enough; null value represents scenarios where the likelihood of survival is not impacted by risk of PD. Individual series: Association between smoking-related cancers and survival (OR_S_SRC). Negative value represents scenarios where the likelihood of survival is decreased for those who are diagnosed with cancer; null value represents scenarios where the likelihood of survival is not impacted by cancer status. Vertical axis: Bias-adjusted odds ratio given different combinations of the two bias parameters (OR_S_PD and OR_S_SRC).
Figure 2. Bias-adjusted odds ratio for the association between cancers not related to smoking and PD.
Horizontal axis: Association between survival and PD on the odds ratio scale (OR_S_PD). Negative value represents scenarios where the likelihood of survival is decreased for those who are at risk of developing PD had they survived long enough; null value represents scenarios where the likelihood of survival is not impacted by risk of PD. Individual series: Association between cancers not related to smoking and survival (OR_S_NRSC). Negative value represents scenarios where the likelihood of survival is decreased for those who are diagnosed with cancer; null value represents scenarios where the likelihood of survival is not impacted by cancer status. Vertical axis: Bias-adjusted odds ratio given different combinations of the two bias parameters (OR_S_PD and OR_S_NRSC).
Discussion
For PASIDA individuals who actively participated in our interview-based study, point estimates of effect for smoking related cancers and cancers not related to smoking were below one, even though they were estimated imprecisely due to the relatively small number of cancers we observed prior to PD. When including non-participants in the analysis, effect estimates strengthened and generally became more precise. Adjusting for lifestyle factors did not change the results. Our quantitative survival bias analyses suggested that survival bias alone was unlikely to explain away the negative association between cancer and PD in the PASIDA. In bias simulations, the negative associations between cancer and PD remained below 1 within the range of priors we set for both of these conditions affecting survival. Only under the most extreme assumptions with a very low chance of survival for those with cancer and at risk of developing PD would the estimates become null or even cross the null value.
Since PD cases stop smoking many years prior to PD diagnosis, one might say that “PD prevents smoking” and thus reduces the risk of smoking-related cancers and mortality. Negative confounding by smoking has been proposed as an explanation for the observed inverse association between cancer and a subsequent PD diagnosis. In PASIDA adjustment for pack-years of smoking did not change the observed associations between cancer and PD, which suggested that smoking is unlikely to be a strong confounder between cancer and PD.
The observed negative effect size between cancers and PD in PASIDA was smaller than reported in previous studies. Since our PASIDA study was an interview-based case-control study and also did not entirely restrict its population to incident PD cases, our results could have been impacted by non-participation. In fact, some associations became stronger when we adjusted for selection bias due to non-participation by adding non-participants into the analysis. Our non-participation corrected effect estimates are very comparable in size to those reported in previous studies, [8, 11] especially those from a much larger previous Danish record linkage study based on all citizens with PD cases identified from the Danish National Hospital Register between 1986 to 1998 with an OR of 1.04 (95%CI: 0.96–1.12) for any site, 0.68 (95%CI: 0.58–0.81) for smoking-related cancers, and 0.99 (95%CI: 0.87–1.13) for other specified sites prior to PD index date. [8] Since this previous study was solely based on record linkage, selection bias from non-participation cannot occur. What our case-control study contributes and this previous study could not accomplish was 1) an extensive medical record review in which we verified iPD diagnoses; and 2) collecting smoking, physical activity, and estrogen status information in interviews, data that is not available in record linkage only studies. Thus, our estimates are less likely to be affected by outcome misclassification and potential (negative) confounding by smoking than the previous study. In our study we observed positive association for malignant melanoma, which was in line with previous studies. [6, 8, 12] Positive associations have been reported in previous Danish registry-based studies for breast cancer, [31, 32] opposite to what we saw in our study. However, this could be due to the difference in time sequence of disease diagnosis since the previous studies examined breast cancer after PD onset while in our study we focused on cancers prior to PD.
Our study has several strengths. Our study’s sample size was larger than previous interview-based case-control studies but smaller than previous linkage based studies that lacked covariates data such as for lifestyle factors and did not confirm PD diagnoses. In addition, individual-level information such as demographics, comorbidities, and cancer diagnosis was available from national registries regardless of participation status, which made it possible to adjust for selection by including non-participants in the analysis. Additional external data resources, e.g., Danish mortality statistics, made it possible for us to reasonably estimate the direction and magnitude of bias parameters and externally adjust for survivor bias. Our bias analysis suggested that negative associations between cancer and PD might still remain after adjusting for survival bias for most plausible priors regarding the effect size of PD and cancer on survival.
Our study has some limitations. For example, our analysis was under-powered to explore all individual cancer sites. Also, we did not have information on lifestyle factors for non-participants. Multiple imputation to generate smoking and physical activity for non-participants assumes missing at random. Incorrect predictions would result in residual confounding. However, our results also suggested that smoking and physical activity were unlikely to be strong confounders of the association between cancer and PD when we included PASIDA participants who provided complete information on lifestyle factors.
In conclusion, our study suggested a lower frequency of most cancers preceding PD diagnosis after adjustment for major lifestyle factors. Our bias analysis indicated that survival bias minimally impacts the observed associations between cancer and PD given the range of priors we considered plausible.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institute of Environmental Health/National Institutes of Health [R01ES013717 and R21ES022391]. Dr. Zeyan Liew was partly supported by the NIH/NIEHS Pathway to Independence Award [K99ES026729].
Appendix
Multiple imputation
We used multiple imputation with fully conditional specification to impute for subjects with missing values. Covariates including age at PD index date, sex, socioeconomic status, degree of urbanization, the Charlson Comorbidity Index, cancer diagnosis, PD status, smoking, physical activity, and estrogen score. The imputation was set to produce 5 separate imputed data sets. The final results were estimated using all 5 imputed data sets and 0.2 as the weight in logistic regression models for each data set.
Survival bias structure in PASIDA
We used directed acyclic graphs (DAG) to present the structural relationships between variables (34, 35) and proposed a bias structure based on the study design employed in PASIDA (Supplementary Figure 1). The causal relationship between cancer (CA) and Parkinson’s disease (PD) is the main focus of this analysis. Two levels of selection, survival (S1) and participation (S2), happen sequentially, i.e. the decision to participate can be made only by those who were alive during the study period when approached for interviews. The likelihood of both survival (S1) and participation (S2) are decreased among cancer sufferers – the latter due to diminished health status. Variable set Z represents a set of known and measured risk factors for cancer that also influence survival and risk of developing PD, which includes demographical characteristics, i.e. age (Z1), sex (Z2), SES (Z3), and urbanization (Z4), comorbidities (CM), and lifestyle factors such as smoking (SM) and physical activity (PA). In addition, a set of unknown or unmeasured variables (U) is also included in the causal diagram. Survival (S1) and PD are negatively related through U, which represents factors that make a person less likely to survive but more likely to subsequently have developed PD had they survived, hypothetically these factors may include behavioral changes due to insidious PD and prodromal dysfunctions such as depression or autonomous nervous system dysfunction in pre-clinical PD that may decrease survival chances. Conditioning on both survival (S1=1) and participation (S2=1) in an interview-based case-control study opens up a biasing path and induces biases.
Bias analysis adjusting for selection bias due to non-participation
In PASIDA, since we have information on demographic characteristics and comorbidities available from the Danish national registries for every eligible and approached case and control, therefore we were able to extract individual level data for all selected cases and controls and include both participating (S2=1) and non-participating (S2=0) cases and controls in the analysis to adjust for potential selection bias due to non-participation assessing the association between cancer and PD. For non-participants, lifestyle factors were imputed using multiple imputation based on demographics and comorbidities assuming missing at random (MAR).
Bias analysis adjusting for survival bias
For survivors (S1=1), we then used Monte Carlo techniques to generate the conditional probability of survival using the logistic equation as:
| (1) |
Where survival is defined as binary (S1=1: survived; S1=0: dead); βS1 is the log odds of S1 = 1 when all predictors are set at the reference level; other βs are the log odds ratio (OR) relating S1 to each predictor holding others constant. Based on reported associations between cancer and PD (Supplementary Table 2) and annual national mortality statistics in Denmark [1] and reported cancer mortality rates by cancer site and stage for aging populations in European countries [2–4] we assumed values for each bias parameter on the OR scale reflecting the impact of each predictor on survival (Supplementary Table 3). Using equation (1) we generated the selection probabilities for those who were alive (S1=1) conditioning on their cancer, PD, smoking, physical activity, comorbidity, age, sex, SES, and urbanization values and the assumed bias parameters. We generated normal distributions with standard deviations of 0.10 for each bias parameter. Simulation of the bias parameters was repeated for 1000 iterations.
By using simulated priors, selection probabilities were generated for S1 and we estimated the OR adjusting for survival bias by using P(S1 = 1)/ P(S1 = 1| cancer, PD, SM, PA, CM, Z1, Z2, Z3, Z4) as the regression weight to estimate the association between cancer and PD after taking smoking, physical activity, comorbidities, age, sex, SES, urbanization, and selection into account.
References
- 1.Statistics Denmark. Death and Life Expectancy 2014. http://www.dst.dk/en/Statistik/emner/doedsfald-og-middellevetid.aspx
- 2.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377(9760):127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman MP, Gatta G, Verdecchia A, Esteve J, Sant M, Storm H, et al. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol. 2003;14(Sppl 5):v128–49. [DOI] [PubMed] [Google Scholar]
- 4.Jakobsen E, Rasmussen TR, Green A Mortality and survival of lung cancer in Denmark: Results from the Danish Lung Cancer Group 2000-2012. Acta Oncol. 2016. June;55 Suppl 2:2–9. [DOI] [PubMed] [Google Scholar]
Footnotes
Disclosures
All authors declared no conflicts of interest to disclose.
Contributor Information
Xin Cui, Email: cynthiacui1010@ucla.edu.
Zeyan Liew, Email: zeyanliew@ucla.edu.
Johnni Hansen, Email: johnni@cancer.dk.
Pei-Chen Lee, Email: peichen@ntunhs.edu.tw.
Onyebuchi A Arah, Email: arah@ucla.edu.
Beate Ritz, Email: britz@ucla.edu.
References
- 1.Doshay LJ, Problem situations in the treatment of paralysis agitans. Journal of the American Medical Association, 1954. 156(7): p. 680–684. [DOI] [PubMed] [Google Scholar]
- 2.Westlund K and Hougen A, Cancer as a cause of death among patients with other chronic diseases. Journal of the American Medical Association, 1956. 162(10): p. 1003–1003. [Google Scholar]
- 3.Barbeau A and Joly JG, Parkinsonism and cancer. Union Med Can, 1963. 92: p. 169–74. [PubMed] [Google Scholar]
- 4.Hoehn MM and Yahr MD, Parkinsonism: onset, progression and mortality. Neurology, 1967. 17(5): p. 427–42. [DOI] [PubMed] [Google Scholar]
- 5.Rajput AH, et al. , A case-control study of smoking habits, dementia, and other illnesses in idiopathic Parkinson's disease. Neurology, 1987. 37(2): p. 226–32. [DOI] [PubMed] [Google Scholar]
- 6.Elbaz A, et al. , Nonfatal cancer preceding Parkinson's disease: a case-control study. Epidemiology, 2002. 13(2): p. 157–64. [DOI] [PubMed] [Google Scholar]
- 7.D'Amelio M, et al. , Tumor diagnosis preceding Parkinson's disease: A case–control study. Movement Disorders, 2004. 19(7): p. 807–811. [DOI] [PubMed] [Google Scholar]
- 8.Olsen JH, Friis S, and Frederiksen K, Malignant Melanoma and Other Types of Cancer Preceding Parkinson Disease. Epidemiology, 2006. 17(5): p. 582–7. [DOI] [PubMed] [Google Scholar]
- 9.Driver JA, et al. , Prospective case–control study of nonfatal cancer preceding the diagnosis of parkinson’s disease. Cancer Causes & Control, 2007. 18(7): p. 705–711. [DOI] [PubMed] [Google Scholar]
- 10.Fois AF, et al. , Cancer in patients with motor neuron disease, multiple sclerosis and Parkinson's disease: record linkage studies. Journal of Neurology, Neurosurgery & Psychiatry, 2010. 81(2): p. 215. [DOI] [PubMed] [Google Scholar]
- 11.Lo RY, et al. , Comorbid cancer in Parkinson's disease. Movement Disorders, 2010. 25(12): p. 1809–1817. [DOI] [PubMed] [Google Scholar]
- 12.Wirdefeldt K, et al. , Parkinson's Disease and Cancer: A Register-based Family Study. American Journal of Epidemiology, 2014. 179(1): p. 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inzelberg R and Jankovic J, Are Parkinson disease patients protected from some but not all cancers? Neurology, 2007. 69(15): p. 1542–50. [DOI] [PubMed] [Google Scholar]
- 14.Driver JA, Inverse association between cancer and neurodegenerative disease: review of the epidemiologic and biological evidence. Biogerontology, 2014. 15(6): p. 547–557. [DOI] [PubMed] [Google Scholar]
- 15.West AB, Dawson VL, and Dawson TM, To die or grow: Parkinson's disease and cancer. Trends in Neurosciences, 2005. 28(7): p. 348–352. [DOI] [PubMed] [Google Scholar]
- 16.Staropoli JF, Tumorigenesis and neurodegeneration: two sides of the same coin? BioEssays, 2008. 30(8): p. 719–727. [DOI] [PubMed] [Google Scholar]
- 17.Hernan MA, et al. , A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol, 2002. 52(3): p. 276–84. [DOI] [PubMed] [Google Scholar]
- 18.Kiyohara C and Kusuhara S, Cigarette smoking and Parkinson's disease: a meta-analysis. Fukuoka Igaku Zasshi, 2011. 102(8): p. 254–65. [PubMed] [Google Scholar]
- 19.Gorell JM, et al. , Smoking and Parkinson's disease: a dose-response relationship. Neurology, 1999. 52(1): p. 115–9. [DOI] [PubMed] [Google Scholar]
- 20.Ritz B, et al. , Parkinson disease and smoking revisited: Ease of quitting is an early sign of the disease. Neurology, 2014. 83(16): p. 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene N, et al. , Reproductive factors and Parkinson's disease risk in Danish women. European Journal of Neurology, 2014. 21(9): p. 1168–e68. [DOI] [PubMed] [Google Scholar]
- 22.Shih IF, et al. , Occupational and recreational physical activity and Parkinson’s disease in Denmark. Scandinavian Journal of Work, Environment & Health, 2017(3): p. 210–216. [DOI] [PubMed] [Google Scholar]
- 23.Lemanne D, Cassileth B, and Gubili J, The role of physical activity in cancer prevention, treatment, recovery, and survivorship. Oncology, 2013. 27(6): p. 580–5. [PubMed] [Google Scholar]
- 24.Brown JC, et al. , Cancer, Physical Activity, and Exercise. Comprehensive Physiology, 2012. 2(4): p. 2775–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo J, et al. , Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. The Journal of Steroid Biochemistry and Molecular Biology, 2003. 87(1): p. 1–25. [DOI] [PubMed] [Google Scholar]
- 26.Russo J and Russo IH, The role of estrogen in the initiation of breast cancer. The Journal of Steroid Biochemistry and Molecular Biology, 2006. 102(1): p. 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keyes KM, et al. , How healthy are survey respondents compared with the general population? Using survey-linked death records to compare mortality outcomes. Epidemiology, 2018. 29(2):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenborg L, et al. , Lifestyle, Family History, and Risk of Idiopathic Parkinson Disease: A Large Danish Case-Control Study. American Journal of Epidemiology, 2015. 181(10): p. 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gjerstorff ML, The Danish Cancer Registry. Scandinavian Journal of Public Health, 2011. 39(7_suppl): p. 42–45. [DOI] [PubMed] [Google Scholar]
- 30.Storm HH, et al. , The Danish Cancer Registry--history, content, quality and use. Dan Med Bull, 1997. 44(5): p. 535–9. [PubMed] [Google Scholar]
- 31.Rugbjerg K, et al. , Malignant melanoma, breast cancer and other cancers in patients with Parkinson's disease. International Journal of Cancer, 2012. 131(8): p. 1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen JH, et al. , Atypical cancer pattern in patients with Parkinson's disease. Br J Cancer, 2004. 92(1): p. 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peretz C, et al. , Cancer incidence among Parkinson's disease patients in a 10-yrs time-window around disease onset: A large-scale cohort study. Parkinsonism Relat Disord, 2016. 28:68–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.