Abstract
Objective:
Burn injury induces an acute hyperactive immune response followed by a chronic immune dysregulation that leaves those afflicted susceptible to multiple secondary infections. Many murine models are able to recapitulate the acute immune response to burn injury, yet few models are able to recapitulate long-term immune suppression and thus chronic susceptibility to bacterial infections seen in burn patients. This has hindered the field, making evaluation of the mechanisms responsible for these susceptibilities difficult to study. Herein we describe a novel mouse model of burn injury that promotes chronic immune suppression allowing for susceptibility to primary and secondary infections and thus allows for the evaluation of associated mechanisms.
Methods:
C57Bl/6 mice receiving a full-thickness contact burn were infected with Pseudomonas aeruginosa 14 days (primary infection) and/or 17 days (secondary infection) after burn or sham injury. The survival, pulmonary and systemic bacterial load as well as frequency and function of innate immune cells (neutrophils and macrophages) were evaluated.
Results:
Following secondary infection, burn mice were less effective in clearance of bacteria compared to sham injured or burn mice following a primary infection. Following secondary infection both neutrophils and macrophages recruited to the airways exhibited reduced production of anti-bacterial reactive oxygen and nitrogen species and the pro-inflammatory cytokineIL-12 while macrophages demonstrated increased expression of the anti-inflammatory cytokine interleukin-10 compared to those from sham burned mice and/or burn mice receiving a primary infection. In addition the BALF from these mice contained significantly higher level so of the anti-inflammatory cytokine IL-4 compared to those from sham burned mice and/or burn mice receiving a primary infection.
Conclusions:
Burn-mediated protection from infection is transient, with a secondary infection inducing immune protection to collapse. Repeated infection leads to increased neutrophil and macrophage numbers in the lungs late after burn injury, with diminished innate immune cell function and an increased anti-inflammatory cytokine environment.
Keywords: Burn, Granulocytes, Pseudomonas, neutrophils, macrophages
Introduction
Burn injury causes significant lengthy hospital stays among patients. Every year 486,000 patients in the United States seek medical attention for burn injury [1]. Burn injury commonly creates a significant wound that is slow to heal and causes significant systemic immune dysregulation and late stage immunosuppression. Patients that experience this immunosuppression are often susceptible to cutaneous, pulmonary and systemic colonization with nosocomial and opportunistic bacteria. Infection of burn patients is also commonly associated with increased morbidity and mortality. Studies have found that ventilated patients who have more than one incidence of bacterial infection during the course of their hospital stay can experience a 42% increase in mortality [2]. Thus, identification of mechanisms associated with this increased susceptibility are imperative to controlling mortality.
Bacterial pneumonia is a leading cause of mortality among burn patients [3]. One common source of bacterial pneumonia within the burn patient population is Pseudomonas aeruginosa, a gram-negative opportunistic infection [4, 5]. Full clearance of pseudomonal infections requires the activation and integration of the immune system [6, 7]. Studies have demonstrated that neutrophils are the primary innate immune cells responsible for preventing and clearing bacterial infections, and that a neutrophil response is both necessary and sufficient for clearance of P. aeruginosa infections [8].
Immediately after burn injury, patients experience a general activation of the immune response, with one model defining this as early systemic inflammatory response syndrome (SIRS) [9–11]. This response is typically associated with cytokine storm, immune cell proliferation and systemic immune cell recruitment [12]. Continual activation of the immune system leads to activation of a late compensatory anti-inflammatory response syndrome (CARS) [13]. Although many studies have demonstrated and examined the SIRS response in both human and animal models [14–19], few studies are able to recapitulate the CARS response seen in the human population using animal models. In addition, recent studies indicate that the SIRS/CARS paradigm may not accurately represent the complex immune response in burn patients because pro- and anti-inflammatory mediators are often detected simultaneously [20–25] and patients experience a mixed antagonist response syndrome (MARS) at all time points. We retain the SIRS/CARS terminology for this study to define the “early” and “late” phases after injury and net immune bias of the MARS response after burn injury.
Additional studies indicate that SIRS and CARS among burn patients leads to release of immune cytokines and alterations in the immune profile, and that poor outcomes following infection within patient populations can be predicted by production of the cytokines interleukin 10 (IL-10) and interleukins 12 (IL-12) and 4 (IL-4) [23, 26–29]. Additionally, murine studies have indicated that these cytokines play an important role in burn-associated responses to bacterial infection [14, 27, 30, 31]. Researchers have demonstrated that treatments resulting in decreased IL-10 production after burn injury lead to increased bacterial clearance and improved outcome [14, 32, 33] and that current therapeutic targets exist capable of altering cytokine production after burn injury [29]. These findings indicate that IL-10 and IL-12 are important markers and potential targets for therapeutic interventions.
Multiple studies in animal models indicate that after injury, burn mice are more capable of responding to infection than their sham counterparts [34–40] likely due to burn-induced upregulation of the innate arm of the immune system [41, 42] analogous to protective immune priming of lung mucosal innate immunity by bacterial pathogens against subsequent bacterial pathogens [43]. It is evident that there is an increased neutrophil presence in the lung vasculature early and late after burn injury [34–36]. Late after injury these neutrophils are part of an overall heightened immune response and have been shown to the key player in the improved outcome in burn mice following single infection [34, 35], an effect that is lost with the elimination of the protective neutrophil population using anti-Ly6G antibodies [36]. This has represented a significant paradox in the study of the late immune dysfunction after burn injury in which the accepted clinical picture of immune susceptibility to bacterial infection [44] in patients late after burn injury is not reflected in the animal model. We have demonstrated that in the presence of burn-associated comorbidities such as irradiation or smoke exposure, infection with bacterial exposure can result in a loss of the protective effect of burn injury [45, 46].
In a nosocomial environment, the skin microbiota of patients commonly changes to match that of their environment and the nurses with whom they commonly interact [47, 48]. Burn wounds represent a disrupted barrier to the environment, and burn patients have a high incidence of infection (39% in our burn unit) due to large burn wounds and necessary surgery, resulting in subsequent immune dysregulation [49–51]. In contrast, mice utilized in experimentation are housed in specific-pathogen free environments and are protected from bacterial exposure. We hypothesized that the pulmonary neutrophil population present after burn injury creates a protective environment in which mice are prepared to respond to an initial pulmonary infection with P. aeruginosa. However, we believe that this population represents a finite resource and that once activated to respond to a bacterial insult this short-lived population is unable to replenish and mount continual protection to repeated exposure to bacteria. We additionally predict that these cells may play an important role in the production of IL-10 and IL-12 in vivo.
Material and methods
Animals
Female C57BL/6 mice aged eight to twelve weeks old, weighing >18 grams were purchased (Taconic Farms) and used for this study. Mice were shaved dorsally, given a subcutaneous injection of morphine (3mg/kg body weight, West-Ward Pharmaceuticals) and underwent a 20% total body surface area burn as previously described [14, 45, 52, 53]. A full-thickness contact burn was achieved using a 65 gram copper rod (1.9 cm in diameter) that had been heated to 100°C and applied to the dorsum/flank for four applications, each lasting 10 seconds. Animals were then placed in individual cages, given food and water ad libitum and monitored twice daily. All sham animals underwent the same procedure with the exception of the application of the burn injury. All animals were housed in a specific pathogen-free environment and all procedures were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill in accordance with NIH guidelines for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Bacterial Inocula preparation and infection
A wildtype strain (PAK) of P. aeruginosa was utilized for all infections as previously described [14]. Bacteria from frozen cultures were grown overnight in Luria Broth (LB). The following morning cultures were diluted 50x and grown for approximately two hours until mid-log phase growth was achieved (OD600=0.6–1.2). Cultures were then centrifuged at 14000 rpm for 30 seconds, and the pellet was washed using 1mL PBS+1% Protease Peptone solution (PBS+1%PP). Bacterial pellets were re-suspended and diluted to the desired concentration and verified by optical density at 600nm. Bacterial concentration was then confirmed by serial dilution and plating on LB agar plates.
Mice infected intraperitoneally received a 1mL injection of P. aeruginosa at a concentration of 5×105 CFU/mL. Uninfected mice were given an intraperitoneal injection with 1mL of PBS+1%PP as a control. Mice infected intratracheally were anesthetized with an intraperitoneal injection of Avertin (0.475mg/g body weight: Sigma-Aldrich). Mice were then placed on an intubation platform, and infected by visualization of the vocal cords with a laryngoscope (Model LS-2, Penn Century Inc.), and inserting a MicroSprayer® Aerosolizer (Model 1A-1C and FMJ-250 High-Pressure Syringe Penn Century, Inc.) through the vocal cords, after which a 50uL volume was aerosolized into their lungs using either a bacterial innocula (2×107 CFU/ML in PBS+1%PP) or vehicle (PBS+1%PP).
Enumeration of bacteria
At time of sacrifice, the left lobe of the liver, the lungs, and the spleen were removed and placed in 0.5mL of LB broth on ice. Tissues were homogenized using a BulletBlender (Next Advance) and three 3.2mm stainless steel beads per tube of tissue. Tissue homogenate was serially diluted and plated on LB agar for quantification. Plates were incubated overnight at 37°C.
BAL and Whole Lung tissue collection
Bronchoalveolar lavage collection was performed on mice to obtain cells collected from the airway as previously described [54–56]. Mice were killed using administration of isoflurane and a catheter (22G x 1”, Exel) was placed into the trachea and tied off. A syringe with 1mL 0.6mM EDTA in PBS was connected to the catheter and 0.6 mL of the fluid was flushed into the lungs, the lungs were massaged, and then the fluid was withdrawn into the syringe to obtain a primary wash. This procedure was repeated three times. Two additional washes were performed. Samples were spun down for cellular analysis and the supernatant was collected for assay via Bradford assay and enzyme-linked immune-sorbent assay (ELISA). Lungs were removed from the animals and minced using sterile razor blades. Lungs were then placed in 4mL of PBS supplemented with 10% Fetal Bovine Serum (PBS+FBS), 0.1µg/mouse DNase, 1500 u/mouse collagenase and shaken at 250rpm at 37°C for 1h for digestion of tissue as previously described [14]. Samples were then filtered using a 100µm cell strainer and then pelleted. Pelleted cells then underwent ACK lysis for removal of red blood cells and then samples were washed and resuspended in PBS+FBS for staining for flow cytometric analysis. Cells collected from BAL and whole lung tissue were counted using a hemocytometer with 0.01% trypan blue viability dye.
Flow cytometric analysis
Cells were incubated with anti-mouse CD16/32 Block (eBiosciences) to block Fc receptors as previously described [14, 53]. Cells were then stained with antibodies against CD45, CD11c, CD11b, Ly6G, F4/80 and/or NOS2. Cells were then fixed in 1% paraformaldehyde and examined using a Dako CyAn (Beckmann-Coulter) and then data was analyzed using Summit software (Beckman-Coulter). Initial exclusion of CD45-cells was conducted, and then neutrophils (CD45+CD11b+CD11c-Ly6G+) and macrophages (CD45+CD11c+Ly6G-) were examined. To determine the potential of the neutrophils to produce reactive oxygen and nitrogen species (RONS), analysis was performed using dihydrorhodamine-123 (DHR123). Samples were stained using fluorochrome-conjugated antibodies as previously stated. Prior to fixation in paraformaldehyde, the samples were resuspended in DHR123 (1.875 mg/mL, Invitrogen). The samples were then split into stimulated and unstimulated samples. Samples were stimulated using 98nm Phorbol myristate acetate (PMA) for 30 minutes at 25˚C in the dark. All samples were then fixed in a 1% paraformaldehyde solution, and analyzed using flow cytometry. Reactive Oxygen and Nitrogen Species (RONS) expression was determined for each cell population present, as previously described [57].
Isolation and analysis of cytokine and chemokine levels by multiplex analysis.
BAL was collected as above, and we employed multiplex analysis of 33 chemokines and cytokines according to manufacturer’s instructions (Bio-Plex Pro™ Mouse Chemokine Panel 33-Plex #12002231, BioRad Inc).
Statistical Analysis
All data were visually displayed in GraphPad Prism Version 5.0 for Windows and analyzed by Student’s t-Test or One-Way Analysis of Variance (ANOVA) with a Tukey post-test; Kaplan-Meier survival plots were subject to Gehan-Breslow-Wilcoxon analysis. Data are represented as geometric mean, with statistical significance is indicated as * p < 0.05, ** p < 0.005, *** p < 0.001.
Results
Intratracheal bacterial infection leads to increased neutrophil and macrophage numbers in the lungs late after burn injury and increased immune protection.
To examine the underlying mechanism(s) of burn-dependent immune protection, we examined changes in immune cells present in infected lung tissue. We focused on the lungs because pulmonary infections are a common comorbidity and cause of mortality after burn injury. We examined the immune response to a single intratracheal (IT) inoculation of P. aeruginosa 14 days after administration of sham or burn injury. We observed a significant increase in the number of live immune cells in whole lung in sham and burn mice after IT infection (Figure 1A). Using specific flow cytometric quantification of innate immune cells in the lung (representative staining shown in Figure 1B), we found that there were no burn or infection-dependent changes in the total number of macrophages present in the BAL or whole lung tissue (Figure 1C–D). However, infection led to a significant increase in the numbers of neutrophils present in BAL collected from both sham and burn-injured mice (Figure 1E). Additionally, we found that infection led to an increased number of neutrophils present in the whole lung tissue collected in mice, a phenotype augmented by burn-injury (Figure 1F). Lung and spleen harvested 24 hours after infection had significantly less bacterial load in the burn mice than sham mice (Figure 1G). These data agree with earlier studies which demonstrate that an increased number of pulmonary neutrophils are responsible for burn-mediated immune protection from infection [14, 34, 36].
Figure 1: Single IT infection leads to increased neutrophil numbers in the lung BAL late after burn injury.
Mice underwent sham or burn injury and were infected (14 days after burn injury) IT with P. aeruginosa (ShamT or BurnT) or uninfected (Sham or Burn) and lungs harvested 24 hours later; A) total cells enumerated counted on a haemocytometer, B) representative flow cytometry of BAL or whole lung tissue used to identify neutrophils and macrophages; cells are shown after gating on live singlets and common leucocyte CD45+ marker; C–F) number of macrophages or neutrophils from either BAL or whole lung were quantified for each injury and infection group; G) lungs and spleen were harvested from mice 24 hours after final P. aeruginosa infection and bacterial load was quantified by colony forming unit (CFU) analysis. Data shown are *p<0.05, **p<0.01, with geometric mean highlighted and are representative of three repeated experiments.
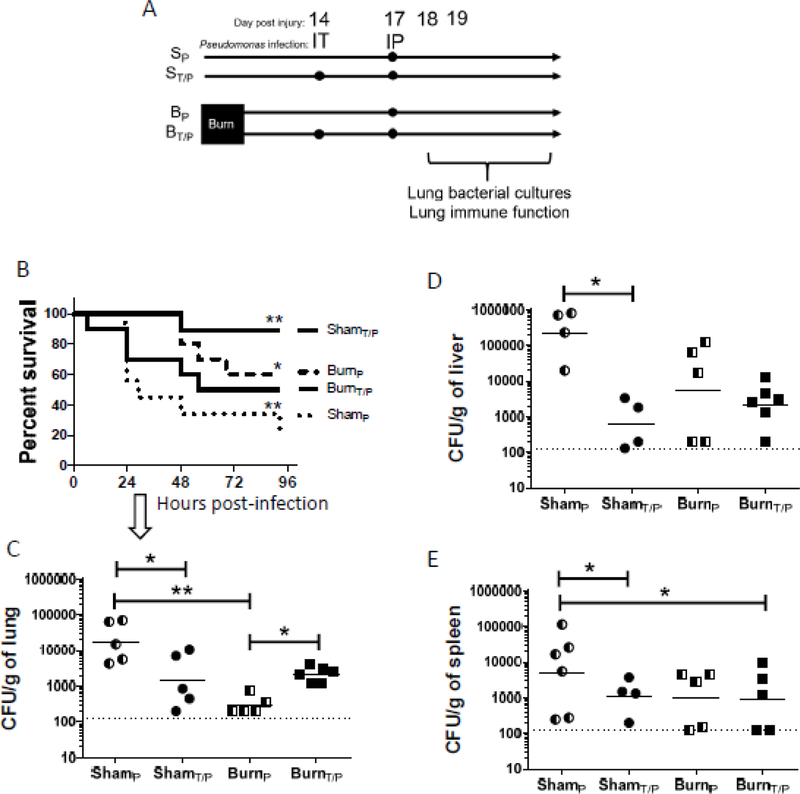
Burn-mediated protection from infection is transient, with a secondary infection inducing immune protection to collapse.
Hospital stays frequently result in changes in the microbiome of patients and these changes commonly lead to development of multiple hospital acquired infections [47, 49, 50]. Animal models of bacterial exposure often depend on a single exposure to bacteria, which is not representative of a nosocomial environment. To more closely mimic clinical conditions with repeated bacterial exposure, we employed an infection strategy in which mice were initially infected (14 days after burn injury) intratracheally (IT) with P. aeruginosa or not, and then received a high-dose intraperitoneal (IP) infection with P. aeruginosa (17 days after burn injury; described in Figure 2A). After a single IP infection, sham injured mice (ShamP) exhibited significant mortality (Figure 2B) compared to all other groups. Protective priming of the lung mucosal innate immunity by bacterial pathogens against bacterial pathogens has been previously described [43] and, as expected, administration of an initial IT infection in sham mice prior to the IP infection (ShamT/P) reversed this and protected the sham mice from death compared to singly infected sham mice (ShamP; Figure 2B). In contrast, after a single IP infection, burn-injured mice (BurnP) did not succumb to a dose of bacteria that was fatal to sham counterparts (ShamP; Figure 2B), as predicted by earlier studies [34–36, 39, 40, 53]. However, burn mice that had an initial IT infection followed by a second IP infection (BurnT/P) demonstrated increased mortality compared to singly infected burn (BurnP) and doubly infected sham mice (ShamT/P; Figure 2B). The survival data correlated well with bacterial load in the lungs with significantly higher bacterial burden in the lungs of doubly infected burn mice (BurnT/P) compared to singly infected burn mice (BurnP; Figure 2C). Figure 2C also highlights burn-induced protection against bacterial infection with single infected burn mice (BurnP) clearing bacteria significantly better than single infected sham mice (ShamP). A reduced bacterial burden in both the spleen and liver in double infected sham treated mice (ShamT/P) compared to single infected sham treated mice (ShamP)24 hours after final inoculation demonstrates a prior infection-induced protection against bacterial infection (Figure 2D–E). While there was no significant difference in liver bacterial burden between sham and burn mice regardless of infection protocol (Figure 2D), a lower splenic bacterial burden was observed in single infected burn mice compared to their sham counterparts (Figure 2E). Together these data suggest that the burn-mediated protection from infection is transient, with a secondary infection inducing immune protection to collapse. Burn injury also suppresses infection-induced priming protection from subsequent infection.
Figure 2: Repeated, but not single infection, leads to susceptibility to bacterial infection in a murine model of burn injury.
A) Mice underwent sham (Sham/S) or burn injury (Burn/B) and were infected with either intraperitoneal (IP) infection with P. aeruginosa 17 days after injury or intratracheal (IT) inoculum of P. aeruginosa 14 days after injury and a subsequent IP infection with P. aeruginosa 17 days after injury as described in Panel A. B) Survival was monitored for up to 96 hours after final infection. C) Lungs, liver and D) spleen were harvested from mice 24 hours after final P. aeruginosa infection and bacterial load was quantified by colony forming unit (CFU) analysis, with dashed line representing lower limit of detection for the CFU assay. Data shown are B) **p<0.01, ***p<0.005 as indicated, and †p<0.05, ††p<0.01 relative to BurnT/P after Kaplan-Meier and Gehan-Breslow-Wilcoxon analysis, representative of five repeated experiments, with numbers of initial mice; survival plots A–C, ShamP, n=9; ShamT/P, n=9; BurnP, n=10; BurnT/P, n=10.
C–D) *p<0.05, **p<0.01, with geometric mean highlighted and are representative of three repeated experiments.
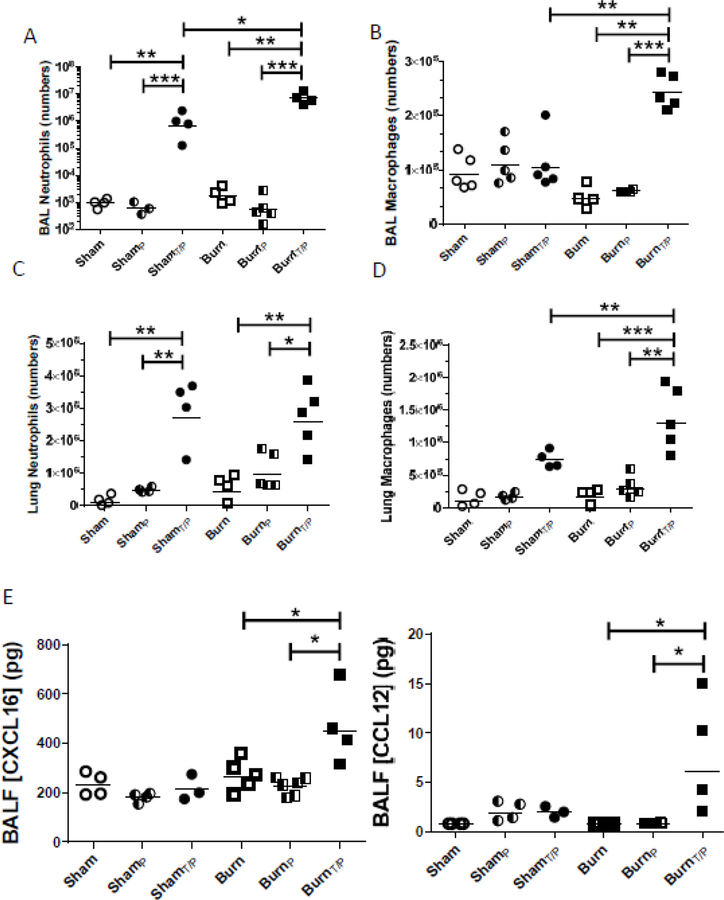
Repeated infection leads to increased neutrophil and macrophage numbers and increased chemokine responses in the lungs late after burn injury.
In order to identify the mechanism responsible for burn-mediated susceptibility to a repeat bacterial exposure in our mouse model, we examined changes in innate cellular compartments after repeated bacterial infection. Specifically, we analyzed immune cells present in BAL and found that there was an increase in the number of neutrophils in both sham and burn mice infected both IT and IP (ShamT/P and BurnT/P) versus the uninfected and singly infected counterparts (Sham, ShamP, Burn and BurnP; Figure 3A). In addition, the number of neutrophils was significantly higher in burn mice infected both IT and IP (BurnT/P) than observed in sham mice infected both IT and IP (ShamT/P; Figure 3A). While infection of sham mice regardless of infection protocol (ShamP and ShamT/P) did not alter macrophage levels in the BAL compared to uninfected sham mice (Sham), burn mice doubly infected (BurnT/P) had significantly increased BAL macrophage numbers compared to uninfected and singly infected counterparts (Burn and BurnP) as well as sham mice doubly infected (ShamT/P; Figure 3B). In addition, we sought to examine immune changes that took place within the lung tissue. Enumeration of single immune cell suspensions generated from whole lung tissue revealed that there was also a double infection-dependent increase in the number of neutrophils (ShamT/P and BurnT/P) when compared to uninfected and singly infected counterparts (Sham, ShamP, Burn and BurnP; Figure 3C). Similar to the BAL, burn mice doubly infected (BurnT/P) had significantly increased macrophage recruitment to lung tissue compared to uninfected and singly infected counterparts (Burn and BurnP) as well as sham mice doubly infected (ShamT/P; Figure 3D). We also characterized the effect of burn injury and infection on the expression of an array of chemokines chemotactic to neutrophils and macrophages in the BAL. We found that a subset of chemokines (including CXCL16 and CCL12; Figure 3E) were significantly increased in the burn with double infection compared to all other treatment groups which correlated well with macrophage accumulation (Figure 3B, D). Taken together, these data suggest that repeated infection following burn injury leads to induction of pulmonary chemokines that differentially recruit macrophage and neutrophil populations into the lungs, although the data do not explain loss of protection from infection.
Figure 3: Double infection leads to increased neutrophil and macrophage recruitmentin the lung late after burn injury.
Mice underwent sham or burn injury and were given IT inoculation with PBS+1%PP or with P. aeruginosa (ShamT/P or BurnT/P) 14 days after injury, followed by IP infection IP with P. aeruginosa (ShamP or BurnP) at 17 days after injury, or left uninfected (Sham or Burn). Lungs were harvested 24 hours later; A–D) number of macrophages or neutrophils from either BAL or whole lung were quantified for each injury and infection group. E) Whole BAL was subjected to multiplex chemokine analysis at the same time point. Data shown are *p<0.05, **p<0.01, ***p<0.005 with geometric mean highlighted and are representative of three repeated experiments.
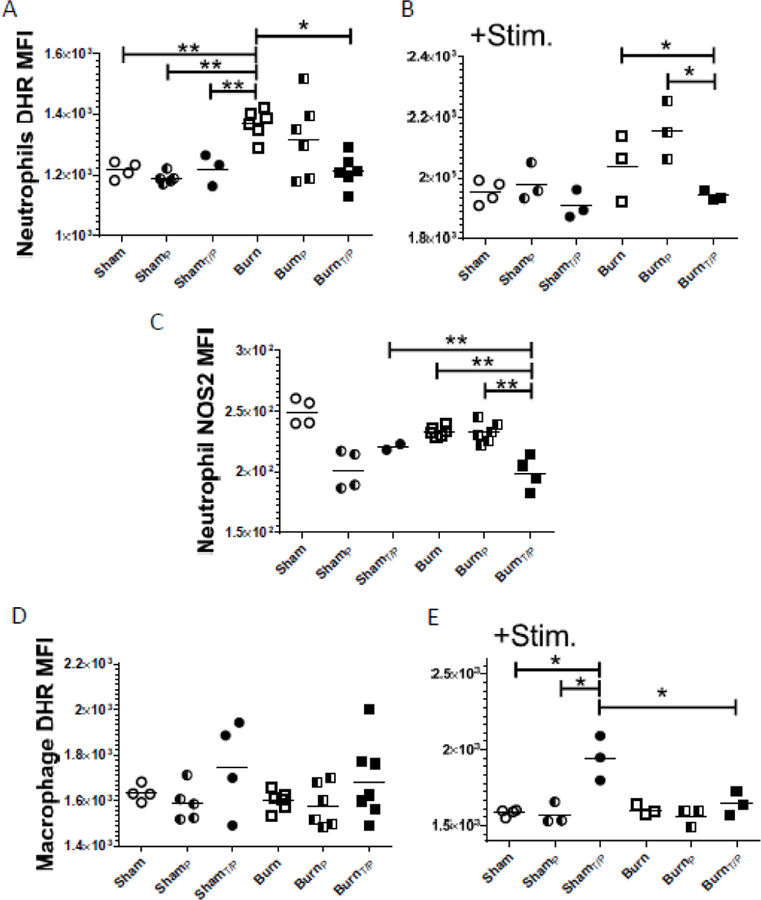
Loss of burn-mediated protection after double infection is associated with diminished neutrophil RONS production.
Protective priming of the lung mucosal innate immunity by bacterial pathogens against bacterial pathogens has been previously described [43], thus we examined function of macrophage and neutrophils isolated from lung tissue following each of our experimental paradigms to determine their microbicidal potential by oxidation. Initially, we utilized DHR123 to quantify the levels of RONS basally present in cells and their ability to produce RONS after in vitro stimulation. We found that burn injury alone results in increased basal neutrophil RONS production (Sham vs. Burn; Figure 4A). In addition, burn alone (Burn) results in higher basal neutrophil RONS production than that observed in neutrophils from singly or doubly infected sham mice (ShamP and ShamT/P; Figure 4A). Interestingly, we observed that if burn animals are doubly infected (BurnT/P), basal neutrophil RONS generation is significantly lower relative to uninfected burn injured mice (Burn, Figure 4A). Neutrophils from doubly-infected (BurnT/P) mice were also defective in producing RONS after in vitro stimulation relative to uninfected burn counterparts (Burn) as well as burn singly infected mice (BurnP; Figure 4B). Loss of RONS activity in neutrophils from doubly infected mice (BurnT/P) also correlated with a decrease in intracellular expression of inducible nitric oxide synthase (NOS2) compared to doubly infected sham (ShamT/P), uninfected burn (Burn) and singly infected burn mice (BurnT/P; Figure 4C).
Figure 4: Neutrophils from burn mice are not able to be activated after secondary infection to increase RONS production.
Mice underwent sham or burn injury and were infected (17 days after injury) either IP with P. aeruginosa (ShamP or BurnP) or IT with P. aeruginosa 14 days after injury followed by IP infection at 17 days after injury (ShamT/P or BurnT/P) or left uninfected (Sham or Burn). Lungs were harvested 24 hours later; (A, B, C,D) reactive oxygen / nitrogen oxidative (RONS) ability per cell was measured by flow cytometry in the absence or presence of Phorbol myristate acetate (PMA) stimulation (“+Stim.”), E) NOS2 expression per cell was quantified in neutrophils using flow cytometry. Data shown are *p<0.05, **p<0.01, with geometric mean highlighted and are representative of three repeated experiments.
While nominal differences in basal macrophage RONS production were observed in sham mice doubly infected compared to all other experimental groups (Figure 4D), in vitro stimulation induced a significant increase in RONS production by macrophages from these mice (ShamT/P) compared to those from uninfected sham (Sham) and singly infected sham mice (ShamP; Figure 4E). Burn injury suppressed the enhanced infection-dependent in vitro induction of RONS production by macrophages (ShamT/P vs BurnT/P; Figure 4E). We therefore hypothesize that diminished neutrophil RONS production is a key player in the loss of burn-mediated protection after double infection. Similarly, while increased macrophage RONS production is a mechanism for innate priming in sham mice, its burn-dependent suppression indicates another burn-mediated loss of protection after double infection.
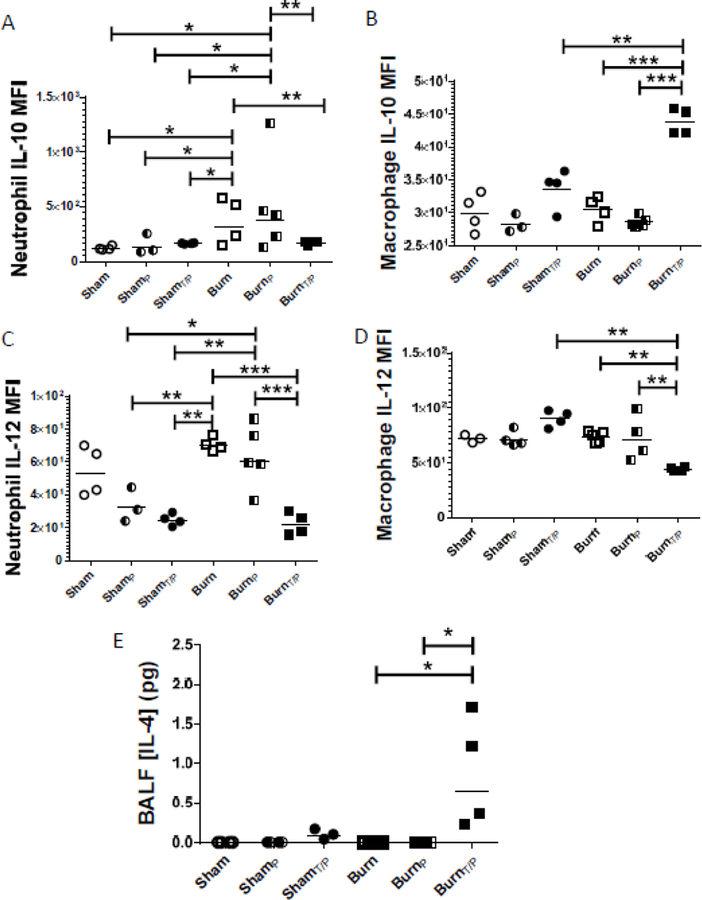
Neutrophil and macrophage IL-10 and IL-12 are differentially expressed after single and double infections in burn mice.
We have previously demonstrated that aberrant production of the cytokines increased IL-10 and reduced IL-12 is likely a major factor contributing to the immunopathology responsible for susceptibility to infection in burn patients [14, 23, 26, 27, 29]. We examined the expression of the anti-inflammatory cytokine IL-10 and the pro-inflammatory IL-12 in neutrophil and macrophage populations isolated from the BAL. Using flow cytometry, we found that intracellular IL-10 levels in neutrophils were significantly higher in burn injury mice (Burn) and burn injury mice that received a single infection (BurnP) compared to burn mice receiving a double infection (BurnT/P), as well as all sham groups (Sham, ShamP and ShamT/P; Figure 5A). In contrast, only burn mice receiving a double infection (BurnT/P) presented with significantly higher levels of IL-10 in the macrophage population when compared to burn mice (Burn), burn mice receiving a single infection (BurnP) and all sham groups (Sham, ShamP and ShamT/P; Figure 5B).
Figure 5: Neutrophil and macrophage IL-10 and IL-12 are differentially expressed after single and double infections in burn mice.
Mice underwent sham or burn injury and were infected (17 days after injury) either IP with P. aeruginosa (ShamP or BurnP) or IT with P. aeruginosa 14 days after injury followed by IP infection at 17 days after injury (ShamT/P or BurnT/P), or left uninfected (Sham or Burn). Lungs were harvested 24 hours later; A–D) intracellular IL-10 and IL-12 expression per cell was measured by flow cytometry (MFI). E) IL-4 was measured in whole BAL at the same points. Data shown are *p<0.05, **p<0.01, ***p<0.005 with geometric mean highlighted and are representative of three repeated experiments.
When we examined intracellular levels of IL-12 we found that neutrophils from burn alone and burn mice singly infected (BurnP) exhibited significantly higher levels of IL-12 compared to all sham groups (Sham, ShamP and ShamT/P; Figure 5C). Importantly, this elevated expression was abolished in burn and doubly infected animals (BurnT/P; Figure 5C). Within the macrophage compartment, there was significantly lower level of IL-12 expression observed in burn mice receiving a double infection (BurnT/P) compared to burn alone, burn mice receiving a single infection (BurnP) and sham mice receiving double infections (ShamT/P; Figure 5D). While we were unable to find detectable levels of IL-10 in whole BAL at the level of bacterial infection used, we were able to detect significantly increased levels of the anti-inflammatory cytokine IL-4 in burn mice that underwent double infection compared to all other experimental groups (Figure 5E). Taken together, these data highlight that there is differential expression of pro- and anti-inflammatory cytokines by macrophage and neutrophil populations following secondary bacterial challenges.
Discussion
Murine models are frequently utilized to examine molecular and cellular mechanisms of burn-associated immune dysfunction [14, 53, 58–60]. However, clinically relevant animal model of injury should recapitulate phenotypes seen among human patients throughout the entire course of dysfunction. Multiple studies have indicated that current murine models of burn injury are insufficient to elicit immunosuppression late after burn injury [34, 61]. In our model burn mice required repeated exposure to bacterial insult for burn-mediated immunosuppression to become apparent. This phenotype is similar to previously reported clinical observations. A previous study investigating critically ill trauma patients that experienced multiple episodes of bacterial ventilator-associated pneumonia indicated that mortality is significantly increased only among a patient population after a secondary bacterial challenge [2]. These clinical data in conjunction with our data imply that the immune compartment is sufficient to protect patients against a single bacterial insult, but that a second infection will result in immunosuppression.
We hypothesized that immune cells that respond to repeated infection would exhibit immune cell exhaustion and that they would therefore be unable to mount appropriate immune responses to subsequent infection. To characterize immune exhaustion, we examined immune cells frequencies as well as cellular function. We found that burn injury resulted in increased numbers of neutrophils present in whole lung tissue collected from mice. Neutrophils are recruited to the lungs after injury and represent a pool of innate immune cells that rapidly respond to any bacterial insult in the lung compartment. Neutrophils production of multiple mediators, including RONS, result in the destruction of bacterial pathogens and have been found to be necessary and sufficient to resolve infection with P. aeruginosa [6, 8]. We postulated that the recruited neutrophil population would represent a finite resource, and that after initial immune activation and use of the neutrophil reservoir any innate immune cells later recruited to the compartment would experience immune cell exhaustion. Upon initial examination we found a single intratracheal infection resulted in comparable movement of cells (both neutrophils and macrophages) to the airspace in both sham and burn-treated animals. We also found that intratracheal infection resulted in recruitment of these cells to the lung tissue, with significantly higher numbers of neutrophils, but not macrophages present in the whole lung tissue of burn mice following intratracheal infection. Increased neutrophil numbers in the lungs correlated with improved bacterial clearance in burn mice after a single infection when compared to their sham counterparts. Upon subsequent infection, sham mice are able to “catch up” recruiting more neutrophils to combat infection, where there is no difference in the ability of these cells to produce RONS or cytokine whether they are isolated from animals receiving a single or double infection. In contrast, the neutrophils recruited to combat a second infection after burn injury produce less RONS, and less IL-12 than observed in those neutrophils recruited after the first bacterial hit. Similarly, macrophages isolated from animals receiving a double infection after burn also produce less RONS and IL-12 concomitant with increased IL-10 production. Finally, elevated BALF IL-4 levels were also observed in animals receiving a double infection after burn, although the cellular source of the IL-4 was not evaluated. These results support our hypothesis that despite burn-induced protection, due to innate immune cell exhaustion a successful immune response to a secondary bacterial exposure is inhibited.
Upon infection of a host, bacterial agents such as P. aeruginosa will release pathogen associated molecular patterns (PAMPS) that are able to alter the immune response. In addition, we and others have previously reported that cells from burn-injured animals exhibit altered signaling of toll-like receptors (TLRs), the receptors responsible for detection of PAMPS and subsequent activation of the immune system [14, 27, 51, 62, 63]. Alteration of TLR function after burn injury have additionally been shown to lead to alterations in cytokine production in a model of burn injury [64]. It is possible that alterations in innate immune cell TLR levels result in alterations in the immune response, production of cytokines, and subsequent response to bacterial infection. Future studies will establish the link between TLR signaling and antimicrobial activity in our model of burn injury and repeated infection. To this end, multiple studies have indicated that the presence of PAMPS in a host is sufficient to result in altered immune cell signaling, cytokine production and changes in RONS-associated machinery and expression [65, 66]. Studies have additionally indicated that burn injury leads to increased RONS and pro-inflammatory cytokine production in immune cells which can mediate additional tissue injury [67–70]. Some studies have indicated that it is possible to decrease RONS activity shortly after burn injury and decrease damage to lungs while still permitting successful clearance of subsequent infection [69], and other studies have implicated neutrophils as the only innate cell type responsible for burn-mediated hyperactivity [34, 36]. In this study we report increased total numbers of macrophages and neutrophils present in the whole lung tissue after burn injury and repeated infection. We additionally found that burn injury increased basal RONS along with pro- and anti- inflammatory cytokine expression of neutrophils, but that upon repeated infection these anti-microbial functions were lost. We also found that upon each incidence of infection these cells decreased their baseline production of RONS. Thus, as stated above, it is plausible that repeated incidence of infection would cause cells to be repeatedly exposed to PAMPS and result in intracellular signaling changes leading to alterations in RONS production (reviewed in [71]).
We have previously demonstrated that increased production of IL-10 and reduced IL-12 cytokines from several pulmonary and systemic cell types, including macrophages and neutrophils, is a major factor contributing to the immunopathology responsible for susceptibility to infection in burn patients [14, 23, 26, 27, 29]. We have also demonstrated the prognostic potential of IL-10/IL-12 protein ratios within bronchial washes on predicting lung and systemic infections following severe burn trauma [14, 23, 29]. We report here that that there is differential expression of pro- and anti- inflammatory cytokines by innate immune cells which are significantly different following secondary bacterial challenges. Specifically, neutrophils from burn mice receiving a double infection presented with decreased RONS and IL-12 expression as well as IL-10 expression. Conversely, macrophages from burn mice did not have any apparent change in RONS production after burn or infections and exhibited a significant increase in IL-10 production after a secondary bacterial infection. In addition, BALF from these mice had significantly higher levels of IL-4 compared to all other experimental groups. IL-4 is responsible for induction of polarization of pulmonary macrophages and neutrophils into IL-10hiIL-12lo cells that we hypothesize are responsible for the reduced clearance of bacterial pathogens after burn injury in the presence or absence of other co-morbidities [14, 23, 72]. These data suggest that macrophages might “guide” subsequent immune responses in the pulmonary micro-environment and warrants further investigation. Excess neutrophils found in the lungs of burn mice may suggest that macrophage/monocyte efferocytosis of apoptotic neutrophils as a mechanism leading to the induction of higher levels of IL-10, IL-4 and reduced IL-12 [73]. Multiple studies have indicated that cytokines play an important role in patient immune responses, and we have found that levels of IL-10 and IL-12 are predictive of outcomes after burn injury, although we have not evaluated the predictive value of IL-4 [23, 29]. Our study indicates that burn and infection alter innate immune cell production of IL-10, IL-4 and IL-12. Moreover, our unpublished data from human studies suggest a relationship between peripheral blood IL-10/IL-12 and arginase 1 gene (ARG1)/NOS2 gene expression ratios and indicate that they have a powerful predictive ability for susceptibility to infection in burn patients. When we examined neutrophil levels of NOS2 we found that infection of sham-injured mice resulted in decreased NOS2 expression (Figure 4C). This phenotype was not found in burn injured-mice after a single exposure to P. aeruginosa, however loss of NOS2 levels took place after repeated infection, indicative of exhaustion arising in burn- injured mice and associated loss of burn-mediated protection.
In summary, we have found that the pulmonary neutrophil population recruited after burn injury creates a protective environment in which mice are prepared to respond to an initial pulmonary bacterial infection. However, this population is unable to mount continued protection to repeated infection due to a reduced oxidative and pro-inflammatory function which is supported and complemented by an anti-inflammatory microenvironment supported in part by less effective macrophage populations.
Highlights.
Burn patients commonly develop late immunosuppression associated with bacterial infections.
Animal studies have not been able to recapitulate the late immunosuppression.
We describe a novel mouse model of repeated bacterial infection after burn injury.
Immunosuppression was associated with perturbations in pulmonary innate cells.
Anti-bacterial reactive O/N species were reduced and increased production IL10.
Acknowledgements
All studies utilizing flow cytometry were conducted in the UNC Flow Cytometry Core Facility, which is partially supported by the P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center (LCCC). Studies were funded through the NIH R01 GM076250-01A2 grant awarded to the Cairns lab. Work conducted by Laurel Kartchner was additionally supported through the NSF GRFP DGE-1144081 training grant.
Funding Sources: All studies utilizing flow cytometry were conducted in the UNC Flow Cytometry Core Facility, which is partially supported by the P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center (LCCC). Studies were funded through the NIH R01 GM076250-01A2 grant awarded to the Burn Lab. Work conducted by Laurel Kartchner was additionally supported through the NSF GRFP DGE-1144081 training grant.
Abbreviations:
- CARS
Compensatory anti-inflammatory response syndrome
- CFU
Colony Forming Units
- DHR
Dihydrorhodamine 123
- FBS
Fetal bovine serum
- LB
Luria Broth
- MARS
mixed antagonist response syndrome
- MFI
Mean Fluorescence Intensity
- NO
Nitric Oxide
- PAK
Pseudomonas aeruginosa strain PAK
- RONS
Reactive oxygen and nitrogen species
- SIRS
Systemic inflammatory response syndrome
- TBSA
Total Body Surface Area
- TLR
Toll-like Receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- [1].Branch TAaHCS. National Hospital Ambulatory Medical Care Survey: 2011. Emergency Department Summary Tables CDC/National Center for Health Statistics: CDC; 2011. [Google Scholar]
- [2].Mueller EW, Hanes SD, Croce MA, Wood GC, Boucher BA, Fabian TC. Effect from multiple episodes of inadequate empiric antibiotic therapy for ventilator-associated pneumonia on morbidity and mortality among critically ill trauma patients. The Journal of trauma 2005;58:94–101. [DOI] [PubMed] [Google Scholar]
- [3].Shupp JW, Pavlovich AR, Jeng JC, Pezzullo JC, Oetgen WJ, Jaskille AD, et al. Epidemiology of bloodstream infections in burn-injured patients: a review of the national burn repository. Journal of burn care & research : official publication of the American Burn Association 2010;31:521–8. [DOI] [PubMed] [Google Scholar]
- [4].Sousa D, Ceniceros A, Galeiras R, Pertega-Diaz S, Gutierrez-Urbon JM, Rodriguez-Mayo M, et al. Microbiology in burns patients with blood stream infections: trends over time and during the course of hospitalization. Infectious diseases (London, England) 2017:1–8. [DOI] [PubMed]
- [5].van Duin D, Strassle PD, DiBiase LM, Lachiewicz AM, Rutala WA, Eitas T, et al. Timeline of health care-associated infections and pathogens after burn injuries. American journal of infection control 2016;44:1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rada B Interactions between Neutrophils and Pseudomonas aeruginosa in Cystic Fibrosis. Pathogens (Basel, Switzerland) 2017;6. [DOI] [PMC free article] [PubMed]
- [7].Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. American journal of physiology Lung cellular and molecular physiology 2014;306:L591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lavoie EG, Wangdi T, Kazmierczak BI. Innate immune responses to Pseudomonas aeruginosa infection. Microbes and infection 2011;13:1133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fontaine M, Lepape A, Piriou V, Venet F, Friggeri A. Innate danger signals in acute injury: From bench to bedside. Anaesthesia, critical care & pain medicine 2016;35:283–92. [DOI] [PubMed] [Google Scholar]
- [10].Rogobete AF, Sandesc D, Papurica M, Stoicescu ER, Popovici SE, Bratu LM, et al. The influence of metabolic imbalances and oxidative stress on the outcome of critically ill polytrauma patients: a review. Burns & trauma 2017;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dahiya P Burns as a model of SIRS. Frontiers in bioscience (Landmark edition) 2009;14:4962–7. [DOI] [PubMed] [Google Scholar]
- [12].Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, et al. Role of cytokines as a double-edged sword in sepsis. In vivo (Athens, Greece) 2013;27:669–84. [PMC free article] [PubMed] [Google Scholar]
- [13].Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clinics in chest medicine 2008;29:617–25, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Neely CJ, Kartchner LB, Mendoza AE, Linz BM, Frelinger JA, Wolfgang MC, et al. Flagellin treatment prevents increased susceptibility to systemic bacterial infection after injury by inhibiting anti-inflammatory IL-10+ IL-12- neutrophil polarization. PLoS One 2014;9:e85623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rae L, Fidler P, Gibran N. The Physiologic Basis of Burn Shock and the Need for Aggressive Fluid Resuscitation. Critical care clinics 2016;32:491–505. [DOI] [PubMed] [Google Scholar]
- [16].O’Dea KP, Porter JR, Tirlapur N, Katbeh U, Singh S, Handy JM, et al. Circulating Microvesicles Are Elevated Acutely following Major Burns Injury and Associated with Clinical Severity. PloS one 2016;11:e0167801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu YW, Yang T, Zhao L, Ni Z, Yang N, He F, et al. Activation of Adenosine 2A receptor inhibits neutrophil apoptosis in an autophagy-dependent manner in mice with systemic inflammatory response syndrome. Scientific reports 2016;6:33614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu YC, Luo CQ, Li X. Systemic inflammatory response syndrome following burns is mediated by brain natriuretic peptide/natriuretic peptide A receptor-induced shock factor 1 signaling pathway. Clinical and experimental pharmacology & physiology 2016;43:921–9. [DOI] [PubMed] [Google Scholar]
- [19].Burmeister DM, McIntyre MK, Baker BA, Rizzo JA, Brown A, Natesan S, et al. Impact of Isolated Burns on Major Organs: A Large Animal Model Characterized. Shock (Augusta, Ga) 2016;46:137–47. [DOI] [PubMed] [Google Scholar]
- [20].Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, et al. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Molecular medicine 2008;14:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One 2011;6:e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jones SW, Zhou H, Ortiz-Pujols SM, Maile R, Herbst M, Joyner BL, Jr., et al. Bronchoscopy-derived correlates of lung injury following inhalational injuries: a prospective observational study. PloS one 2013;8:e64250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maile R, Jones S, Pan Y, Zhou H, Jaspers I, Peden DB, et al. Association between early airway damage-associated molecular patterns and subsequent bacterial infection in patients with inhalational and burn injury. American journal of physiology Lung cellular and molecular physiology 2015;308:L855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mendoza AE, Maile LA, Cairns BA, Maile R. Burn injury induces high levels of phosphorylated insulin-like growth factor binding protein-1. International journal of burns and trauma 2013;3:180–9. [PMC free article] [PubMed] [Google Scholar]
- [25].Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A genomic storm in critically injured humans. The Journal of experimental medicine 2011;208:2581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen W, Lian J, Ye JJ, Mo QF, Qin J, Hong GL, et al. Ethyl pyruvate reverses development of Pseudomonas aeruginosa pneumonia during sepsis-induced immunosuppression. International immunopharmacology 2017;52:61–9. [DOI] [PubMed] [Google Scholar]
- [27].Rani M, Nicholson SE, Zhang Q, Schwacha MG. Damage-associated molecular patterns (DAMPs) released after burn are associated with inflammation and monocyte activation. Burns : journal of the International Society for Burn Injuries 2017;43:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tsurumi A, Que YA, Ryan CM, Tompkins RG, Rahme LG. TNF-alpha/IL-10 Ratio Correlates with Burn Severity and May Serve as a Risk Predictor of Increased Susceptibility to Infections. Frontiers in public health 2016;4:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eitas TK, Stepp W, Sjeklocha L, Long C, Riley C, Callahan J, et al. Differential regulation of innate immune cytokine production through pharmacological activation of Nuclear Factor-Erythroid-2-Related Factor 2 (NRF2) in burn patient immune cells and monocytes. PloS one 2017;12:e0184164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Souza HR, de Azevedo LR, Possebon L, Costa SS, Iyomasa-Pilon MM, Oliani SM, et al. Heterogeneity of mast cells and expression of Annexin A1 protein in a second degree burn model with silver sulfadiazine treatment. PloS one 2017;12:e0173417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Alexis A, Carrer DP, Droggiti DI, Louis K, Pistiki A, Netea MG, et al. Immune responses in relation to the type and time of thermal injury: an experimental study. Injury 2015;46:227–32. [DOI] [PubMed] [Google Scholar]
- [32].Argenta A, Satish L, Gallo P, Liu F, Kathju S. Local Application of Probiotic Bacteria Prophylaxes against Sepsis and Death Resulting from Burn Wound Infection. PloS one 2016;11:e0165294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Laghaei P, Hashemi FB, Irajian G, Korpi F, Amirmozafari N, Behrouz B. Immunogenicity and protective efficacy of Pseudomonas aeruginosa type a and b flagellin vaccines in a burned mouse model. Molecular immunology 2016;74:71–81. [DOI] [PubMed] [Google Scholar]
- [34].Gardner JC, Noel JG, Nikolaidis NM, Karns R, Aronow BJ, Ogle CK, et al. G-CSF drives a posttraumatic immune program that protects the host from infection. J Immunol 2014;192:2405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huber NL, Bailey SR, Schuster R, Ogle CK, Lentsch AB, Pritts TA. Prior thermal injury accelerates endotoxin-induced inflammatory cytokine production and intestinal nuclear factor-kappaB activation in mice. Journal of burn care & research : official publication of the American Burn Association 2012;33:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Noel G, Wang Q, Schwemberger S, Hanson C, Giacalone N, Haar L, et al. Neutrophils, not monocyte/macrophages, are the major splenic source of postburn IL-10. Shock 2011;36:149–55. [DOI] [PubMed] [Google Scholar]
- [37].Alexander JW. Effect of thermal injury upon the early resistance to infection. The Journal of surgical research 1968;8:128–37. [DOI] [PubMed] [Google Scholar]
- [38].Noel G, Wang Q, Osterburg A, Schwemberger S, James L, Haar L, et al. A ribonucleotide reductase inhibitor reverses burn-induced inflammatory defects. Shock (Augusta, Ga) 2010;34:535–44. [DOI] [PubMed] [Google Scholar]
- [39].Stieritz DD, Holder IA. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. The Journal of infectious diseases 1975;131:688–91. [DOI] [PubMed] [Google Scholar]
- [40].Pinto M, Zehavi-Willner T. Thermal injury-induced non-specific resistance to fatal Pseudomonas aeruginosa burn-infection in mice. The Japanese journal of experimental medicine 1989;59:189–96. [PubMed] [Google Scholar]
- [41].Mattick JS, Yang Q, Orman MA, Ierapetritou MG, Berthiaume F, Gale SC, et al. Impact of burn priming on immune and metabolic functions of whole Liver in a rat cecal ligation and puncture model. International journal of burns and trauma 2013;3:55–65. [PMC free article] [PubMed] [Google Scholar]
- [42].Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, et al. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol 2003;171:1473–83. [DOI] [PubMed] [Google Scholar]
- [43].Clement CG, Evans SE, Evans CM, Hawke D, Kobayashi R, Reynolds PR, et al. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am J Respir Crit Care Med 2008;177:1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].van Duin D, Strassle PD, DiBiase LM, Lachiewicz AM, Rutala WA, Eitas T, et al. Timeline of health care-associated infections and pathogens after burn injuries. Am J Infect Control 2016. [DOI] [PMC free article] [PubMed]
- [45].Mendoza AE, Neely CJ, Charles AG, Kartchner LB, Brickey WJ, Khoury AL, et al. Radiation combined with thermal injury induces immature myeloid cells. Shock (Augusta, Ga) 2012;38:532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Murphy TJ, Paterson HM, Kriynovich S, Zang Y, Kurt-Jones EA, Mannick JA, et al. Linking the “two-hit” response following injury to enhanced TLR4 reactivity. Journal of leukocyte biology 2005;77:16–23. [DOI] [PubMed] [Google Scholar]
- [47].Lax S, Sangwan N, Smith D, Larsen P, Handley KM, Richardson M, et al. Bacterial colonization and succession in a newly opened hospital. Science translational medicine 2017;9. [DOI] [PMC free article] [PubMed]
- [48].Sanon MA, Watkins S. Nurses’ uniforms: How many bacteria do they carry after one shift? Journal of public health and epidemiology 2012;4:311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Posluszny JA Jr., Conrad P, Halerz M, Shankar R, Gamelli RL. Surgical burn wound infections and their clinical implications. Journal of burn care & research : official publication of the American Burn Association 2011;32:324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hazeldine J, Hampson P, Lord JM. The diagnostic and prognostic value of systems biology research in major traumatic and thermal injury: a review. Burns & trauma 2016;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].D’Arpa P, Leung KP. Toll-Like Receptor Signaling in Burn Wound Healing and Scarring. Advances in wound care 2017;6:330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dunn JL, Hunter RA, Gast K, Maile R, Cairns BA, Schoenfisch MH. Direct detection of blood nitric oxide reveals a burn-dependent decrease of nitric oxide in response to Pseudomonas aeruginosa infection. Burns 2016. [DOI] [PMC free article] [PubMed]
- [53].Linz BM, Neely CJ, Kartchner LB, Mendoza AE, Khoury AL, Truax A, et al. Innate Immune Cell Recovery Is Positively Regulated by NLRP12 during Emergency Hematopoiesis. J Immunol 2017;198:2426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, et al. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. The Journal of infectious diseases 2012;205:807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Livraghi A, Grubb BR, Hudson EJ, Wilkinson KJ, Sheehan JK, Mall MA, et al. Airway and lung pathology due to mucosal surface dehydration in {beta}-epithelial Na+ channel-overexpressing mice: role of TNF-{alpha} and IL-4R{alpha} signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. Journal of immunology (Baltimore, Md : 1950) 2009;182:4357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yamada M, Gomez JC, Chugh PE, Lowell CA, Dinauer MC, Dittmer DP, et al. Interferon-gamma production by neutrophils during bacterial pneumonia in mice. American journal of respiratory and critical care medicine 2011;183:1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Leutner S, Eckert A, Muller WE. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. Journal of neural transmission (Vienna, Austria : 1996) 2001;108:955–67. [DOI] [PubMed] [Google Scholar]
- [58].Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cellular and molecular life sciences : CMLS 2014;71:3241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Williams FN, Herndon DN, Jeschke MG. The hypermetabolic response to burn injury and interventions to modify this response. Clinics in plastic surgery 2009;36:583–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sayeed MM. Inflammatory/cardiovascular-metabolic responses in a rat model of burn injury with superimposed infection. Shock (Augusta, Ga) 2005;24 Suppl 1:40–4. [DOI] [PubMed] [Google Scholar]
- [61].Maung AA, Fujimi S, MacConmara MP, Tajima G, McKenna AM, Delisle AJ, et al. Injury enhances resistance to Escherichia coli infection by boosting innate immune system function. Journal of immunology (Baltimore, Md : 1950) 2008;180:2450–8. [DOI] [PubMed] [Google Scholar]
- [62].Kaufman T, Magosevich D, Moreno MC, Guzman MA, D’Atri LP, Carestia A, et al. Nucleosomes and neutrophil extracellular traps in septic and burn patients. Clinical immunology (Orlando, Fla) 2017. [DOI] [PubMed]
- [63].Cairns B, Maile R, Barnes CM, Frelinger JA, Meyer AA. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. The Journal of trauma 2006;61:293–8; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- [64].Cairns BA, Barnes CM, Mlot S, Meyer AA, Maile R. Toll-like receptor 2 and 4 ligation results in complex altered cytokine profiles early and late after burn injury. The Journal of trauma 2008;64:1069–77; discussion 77–8. [DOI] [PubMed] [Google Scholar]
- [65].Okamoto T, Gohil K, Finkelstein EI, Bove P, Akaike T, van der Vliet A. Multiple contributing roles for NOS2 in LPS-induced acute airway inflammation in mice. American journal of physiology Lung cellular and molecular physiology 2004;286:L198–209. [DOI] [PubMed] [Google Scholar]
- [66].Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxidants & redox signaling 2014;20:1126–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Han S, Cai W, Yang X, Jia Y, Zheng Z, Wang H, et al. ROS-Mediated NLRP3 Inflammasome Activity Is Essential for Burn-Induced Acute Lung Injury. Mediators of inflammation 2015;2015:720457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns : journal of the International Society for Burn Injuries 2008;34:6–17. [DOI] [PubMed] [Google Scholar]
- [69].Miyazaki H, Kinoshita M, Ono S, Seki S, Saitoh D. Burn-Evoked Reactive Oxygen Species Immediately After Injury are Crucial to Restore the Neutrophil Function Against Postburn Infection in Mice. Shock (Augusta, Ga) 2015;44:252–7. [DOI] [PubMed] [Google Scholar]
- [70].Kasten KR, Goetzman HS, Reid MR, Rasper AM, Adediran SG, Robinson CT, et al. Divergent adaptive and innate immunological responses are observed in humans following blunt trauma. BMC immunology 2010;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Prince LR, Whyte MK, Sabroe I, Parker LC. The role of TLRs in neutrophil activation. Curr Opin Pharmacol 2011;11:397–403. [DOI] [PubMed] [Google Scholar]
- [72].Dunn JLM, Kartchner LB, Stepp WH, Glenn LI, Malfitano MM, Jones S, et al. Blocking CXCL1-dependent neutrophil recruitment prevents immune damage and reduces pulmonary bacterial infection after inhalation injury. Am J Physiol Lung Cell Mol Physiol 2018. [DOI] [PMC free article] [PubMed]
- [73].Filardy AA, Pires DR, Nunes MP, Takiya CM, Freire-de-Lima CG, Ribeiro-Gomes FL, et al. Proinflammatory clearance of apoptotic neutrophils induces an IL-12(low)IL-10(high) regulatory phenotype in macrophages. J Immunol 2010;185:2044–50. [DOI] [PubMed] [Google Scholar]